- 1College of Animal Science and Technology, Inner Mongolia Minzu University, Tongliao, China
- 2Medical College, Inner Mongolia Minzu University, Tongliao, China
- 3Inner Mongolia Academy of Agriculture and Animal Husbandry Science, Hohhot, China
Caragana korshinskii is a forage shrub species with high-protein content that has been extensively used to alleviate feed shortages for ruminants in northern China. Herein, we investigated the effects of Lactobacillus rhamnosus and Lactobacillus buchneri on the fermentation quality, aerobic stability, and microbiome composition and the predicted functional characteristics of C. korshinskii silage. C. korshinskii silages were inoculated with and without L. rhamnosus or L. buchneri. After 14 and 56 days of ensiling, the aerobic stability was determined. The results revealed that after 14 and 56 days of ensiling, L. rhamnosus- and L. buchneri-inoculated silage exhibited increased acetic acid and lactic acid contents, whereas the pH and 2,3-butanediol and butyric acid contents were decreased compared with those of the control silage. The control silages that were opened at 14 and 56 d, deteriorated during the aerobic stability test, whereas silages inoculated with L. rhamnosus and L. buchneri did not exhibit any aerobic deterioration. The control silage showed an increased Clostridium and Bacillus abundance, whereas Lactobacillus abundance decreased compared with L. rhamnosus- and L. buchneri-inoculated silages, following the 7 days of aerobic exposure. The fermentation parameters were associated with microbial communities, including Lactobacillus, Pedicoccus, Weissella, Clostridium, and Bacillus. Carbohydrate and amino acid metabolisms in the control silage decreased after 7 days of aerobic exposure compared with lactic acid bacteria-inoculated silages. To conclude, next-generation sequencing combined with 16S ribosomal RNA gene-predicted functional analyses might provide new information about the silage quality during fermentation and the aerobic stability.
1. Introduction
Caragana korshinskii is a type of leguminous shrub widely planted in the arid and semi-arid areas of China (Bai et al., 2023). According to a statistical report, >40 million tons of the existing leguminous C. korshinskii in China is produced via stumping and has a crude protein (CP) content of 15%, which is equivalent to 17 million tons of soybean CP content (Li et al., 2021). The untimely stump can drastically age and degrade C. korshinskii, resulting in increased resource wastage (Liu et al., 2023). Therefore, rational and effective utilization of C. korshinskii is important, which can effectively alleviate the present forage shortage and improve ecosystem stability.
The branches, leaves, flowers, and fruits of C. korshinskii are rich in nutrients, with high contents of protein, crude fat, and mineral elements, with a high essential amino acid content compared with those of the general straw feed, making it a good unconventional feed resource (Deng et al., 2017). The high degree of lignification accounts for a critical factor restricting C. korshinskii development as a feed resource, and suitable processing methods are conducive to reducing its fiber content. A previous study showed that C. korshinskii harvested at the flowering stage has a high nutritional value, and its palatability can be improved by the rubbing processing method (Zhong et al., 2014). Additionally, silage can effectively preserve the nutritional components of C. korshinskii, acting as a storage method to preserve the shrub feed and alleviate feed shortage in arid areas. The poor fermentation quality of leguminous plants is mainly due to characteristics such as low lactic acid bacteria (LAB) count and water-soluble carbohydrate (WSC) content, along with a high buffering capacity (You et al., 2022). Therefore, LAB addition is one of the effective ways to improve the poor fermentation ability of the C. korshinskii silage.
Usually, a LAB count of ≥105 cfu/g in fresh material is required in high-quality silage. Exogenous LAB inoculation can meet the initial LAB count required for the fermentation of the leguminous silage (Zhang et al., 2023). We isolated several LAB strains with good fermentation parameters from L. chinensis, alfalfa, and corn silage. We found that the LAB additives in the L. chinensis silage promoted its fermentation quality while inhibiting the growth of harmful microorganisms (Wu et al., 2022). Nonetheless, those LAB strains do not affect the fermentation quality, bacterial community, or aerobic stability of the C. korshinskii silage. The information regarding the characteristics of Lactobacillus rhamnosus and Lactobacillus buchneri, isolated from the C. korshinskii silage, along with their effects on the fermentation product, microbial community, and aerobic stability of the C. korshinskii silage is lacking. Therefore, L. rhamnosus and L. buchneri strains were isolated from a high-quality C. korshinskii silage and identified using their phenotypes and 16S ribosomal RNA (rRNA) gene sequence and then were cultured. Investigating the microbial changes caused by LAB addition to the C. korshinskii silage during fermentation and after aerobic exposure is of great necessity. The high quality of silage depends on the succession of the microbial community and microbial metabolites during the fermentation process (Zou et al., 2023). Investigation of the microbial community during ensiling and after aerobic exposure can provide insights for improving the preservation of the C. korshinskii silage. During the past decade, culture-independent/dependent methods, including 16S rRNA gene sequencing, real-time polymerase chain reaction (PCR), and denaturing gradient gel electrophoresis, have been applied to investigate the epiphytic microbiota within fresh materials together with microbiota within the ensiled feed (Stevenson et al., 2006; Pang et al., 2011; Wu et al., 2014). Although studies have reported the microbiome composition during the fermentation stage, their results only identify various operational taxonomic units (OTUs), rather than providing the classification details of microorganisms in the silage. Next-generation sequencing (NGS) can be applied in evaluating LAB effectiveness in promoting the fermentation quality and aerobic stability of silage (Wang et al., 2020).
Herein, we investigated the effects of L. rhamnosus and L. buchneri inoculant on the fermentation product, microbial community, function prediction of 16 S rRNA gene, and the aerobic stability of the C. korshinskii silage during the fermentation process and after aerobic exposure through high throughput sequencing technology.
Materials and methods
Ensiling and bacterial inoculants
On June 10, 2022, boot-stage C. korshinskii was collected from an experimental field of the Jarud Banner Fengyi Agriculture and Animal Husbandry Company from the Inner Mongolia Autonomous Region, China. Following 3-h wilting, C. korshinskii was cut into 15–20 mm-long segments using the crop cutter for 326 g/kg dry matter (DM). L. rhamnosus (CGMCC No. 134426) and L. buchneri (CGMCC No. 187961) were isolated from C. korshinskii silage in Inner Mongolia, China. Cultures of L. rhamnosus and L. buchneri were made by anaerobic incubation in MRS broth at 37°C for 48 h. cultures of L. rhamnosus and L. buchneri were diluted with sterile physiological saline as additives. The C. korshinskii silage was inoculated using L. rhamnosus and L. buchneri at 1 × 106 cfu/g of fresh matter (FM). The control group was sprayed with an equivalent volume of sterile physiological saline. The inoculated and uninoculated samples (300 g each) were tightly packaged in plastic pouches (BN-10, 250 × 350 mm; Wangnuo, Beijing, China) using the commercially available vacuum sealer (ZK-320; Ouxin, Beijing, China). Every treatment was performed in triplicate. Additionally, the silos were preserved for 14 and 56 days under ambient temperature.
Aerobic stability test
After completely opening the silos stored for 14 and 56 days, silage (150 g) was placed into an uncompacted polyethylene bottle (500 mL) and exposed to air for 7 days under ambient temperature.
Chemical composition and quantitative analysis of the microbiome
The DM levels in the freshly collected samples and silages were determined through 48-h drying under 65°C. Following this, the samples were pulverized in a 1-mm-sieve Wiley mill (ZM200, Retsch GmbH). The CP level was analyzed according to the standard procedures from the Association of Official Analytical Chemists (AOAC, 1990). Previously reported approaches were adopted for measuring the acid detergent fiber and neutral detergent fiber (Van Soest et al., 1991), and WSCs (Wu and Nishino, 2016).
The fresh samples (20 g each) were homogenized in a blender using sterile water (180 mL) for a 1-min period, and the homogenized mix was filtered with a 0.22-μm membrane to prepare the silage sample water extracts, used for determining the pH, alcohol, lactic acid, and short-chain fatty acid levels. The pH value of the extract was analyzed using the glass electrode pH meter (SX-620, Sanxin, Shanghai, China). Ion-exclusion polymer high-performance liquid chromatography with a refractive index detector was employed for detecting the fermentation products.
A serial dilution (range: 10−1 to 10−6) was performed on a clean bench for quantifying different microbes. For quantifying enterobacteria, the violet red bile agar was used (CM0107B, Oxoid Ltd., United Kingdom), whereas LAB was quantified using the de Man, Rogosa, and Sharpe agar (CM0361B, Oxoid Ltd., UK). Mold and yeast were quantified on the potato dextrose agar (CM0139B, Oxoid Ltd., UK) spread plates with a 3.5 pH (maintained using sterile lactic acid). The number of colonies in FM (cfu/g) was used for calculating the number of living microorganisms.
Microbiome sequencing and analysis
The refrigerated silage (10 g) was blended with 40 mL of sterile phosphate-buffered saline (pH 7.4) using an electronic oscillator at 120 rpm for 2 h, and the sample was filtered through two layers of gauze. The filtrate was centrifuged for 10 min at 13,000 × g and 4°C, and the supernatants were removed, whereas the pellets were maintained on the dry ice. PCR amplification, DNA extraction, and metagenomic sequencing were performed at the Majorbio Bio-Pharm Technology, Shanghai, China. Thereafter, the Illumina MiSeq sequencing and processing of sequenced data were also performed. OTUs were clustered using UPARSE version 7.1 (Edgar, 2013) at a similarity threshold of 97%. After identifying and eliminating the chimeric sequences, taxonomic analysis was conducted on the characteristic OTU sequences (0.7 confidence level), based on 16S rRNA databases (including Silva v138) using the Ribosomal Database Project Classifier (version 2.2) (Yang et al., 2019). Raw sequence data were deposited in the NCBI Sequence Read Archive (SRA) under the accession number PRJNA990987.
Kyoto encyclopedia of genes and genomes (KEGG) enrichment
The KEGG database was employed for the functional enrichment of bacterial communities with Tax4Fun (version 0.3.1) (Aßhauer et al., 2015). The KEGG pathways were compared and displayed using bubble charts.
Statistical analyses
Data were statistically analyzed using the John’s Macintosh Project software (version 13; SAS Institute, Tokyo, Japan). The effect of LAB was assessed using the one-way analysis of variance along with subsequent multiple comparisons based on Tukey’s honestly significant difference test. The value of p of <0.05 was considered statistically significant for comparing means among the various treatments. Additionally, correlation analysis of the bacterial community with fermentation products and KEGG metabolic pathways was performed using the Majorbio Cloud Platform.
Results and discussion
Chemical and microbial components in pre-ensiling Caragana korshinskii
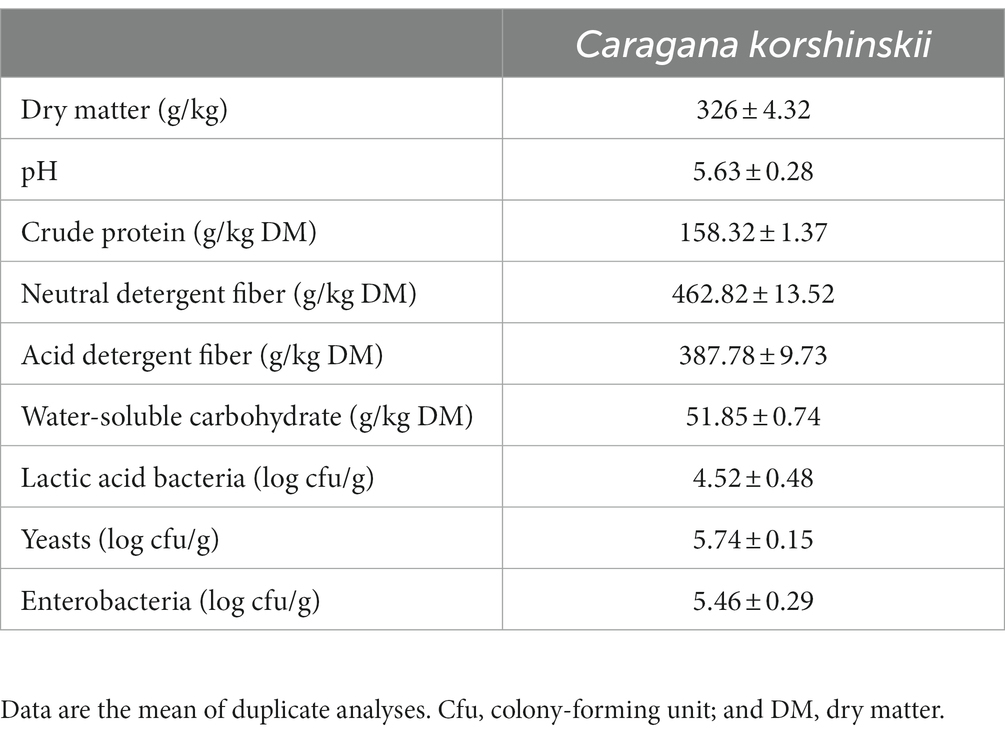
Table 1 presents the chemical and microbial components in pre-ensiling C. korshinskii. DM content of C. korshinskii was determined to be 326 g/kg of FM, which was relatively low compared with the large needlegrass and Chinese leymus silages (Zhang et al., 2016). CP level was 158.32 g/kg of DM, which was relatively high compared with the DM level (102.53 g/kg), as reported by Bai et al. (2023). WSC of fresh samples is an important silage fermentation substrate, and a WSC level of >6% of DM can ensure the expected fermentation. In this study, the WSC content in the pre-ensiling C. korshinskii was 51.85 g/kg of DM, suggesting it is a limitation in achieving high-quality silage with no additives. Epiphytic LAB, yeast, and enterobacteria counts in pre-ensiling C. korshinskii were 4.52, 5.74, and 5.46 log cfu/g of FM, respectively, based on the culturable method. Epiphytic LAB count in freshly harvested crops is often recognized as a critical factor for determining the requirement of LAB inoculation within the fresh silage material and estimating the silage fermentation quality. The LAB count of <105 cfu/g in the raw material can cause poor fermentation during the ensiling process (Cai et al., 1998). LAB addition is necessary to improve the silage fermentation quality because of the low LAB count and the presence of many harmful microorganisms in the raw materials of C. korshinskii.
Roles of Lactobacillus rhamnosus and Lactobacillus buchneri in fermentation products, microbial counts, and aerobic stability
After 14 days of storage, lactic and acetic acid levels in the untreated silage were 9.60 and 4.77 g/kg of DM, respectively (Table 2). With prolonged storage time, lactic acid and acetic acid levels increased, whereas propionic acid was not detected in both 14- and 56-day silage. The ethanol and 1,2-propanediol contents in the untreated silage were 2.70 and 0.62 g/kg of DM, respectively. According to Muck (2010), ethanol and 1,2-propanediol can be produced from sugar by heterofermentative LAB species such as Lactobacillus hilgardii, Lactobacillus brevis, and L. buchneri. L. rhamnosus, a homofermentative LAB strain, have been used to improve the fermentation quality of the whole-crop corn silage (Li and Nishino, 2011b). In the L. rhamnosus-inoculated group, the LAB count and the lactic and acetic acid levels were increased, whereas the pH value and the butyric acid, ethanol, and 2,3-butanediol contents were reduced compared with those of the untreated group. In the L. buchneri-inoculated group, the 1,2-propanediol and acetic acid contents were increased after 56 and 120 days of storage (Li and Nishino, 2011a). Herein, L. buchneri addition increased the 1,2-propanediol, acetic acid, and lactic acid levels, whereas the pH value, butyric acid, and 2,3-butanediol levels decreased after 14 and 56 days. This result indicated that regardless of ensiling time, metabolism of L. buchneri is known to be more active in C. korshinskii silage. The enterobacteria and yeast counts in the untreated silage were high during the ensiling process. The L. buchneri- and L. rhamnosus-inoculated silage exhibited fast antagonistic and acidification functions, which inhibited the enterobacteria count, resulting in the ethanol content efficiency was limited.
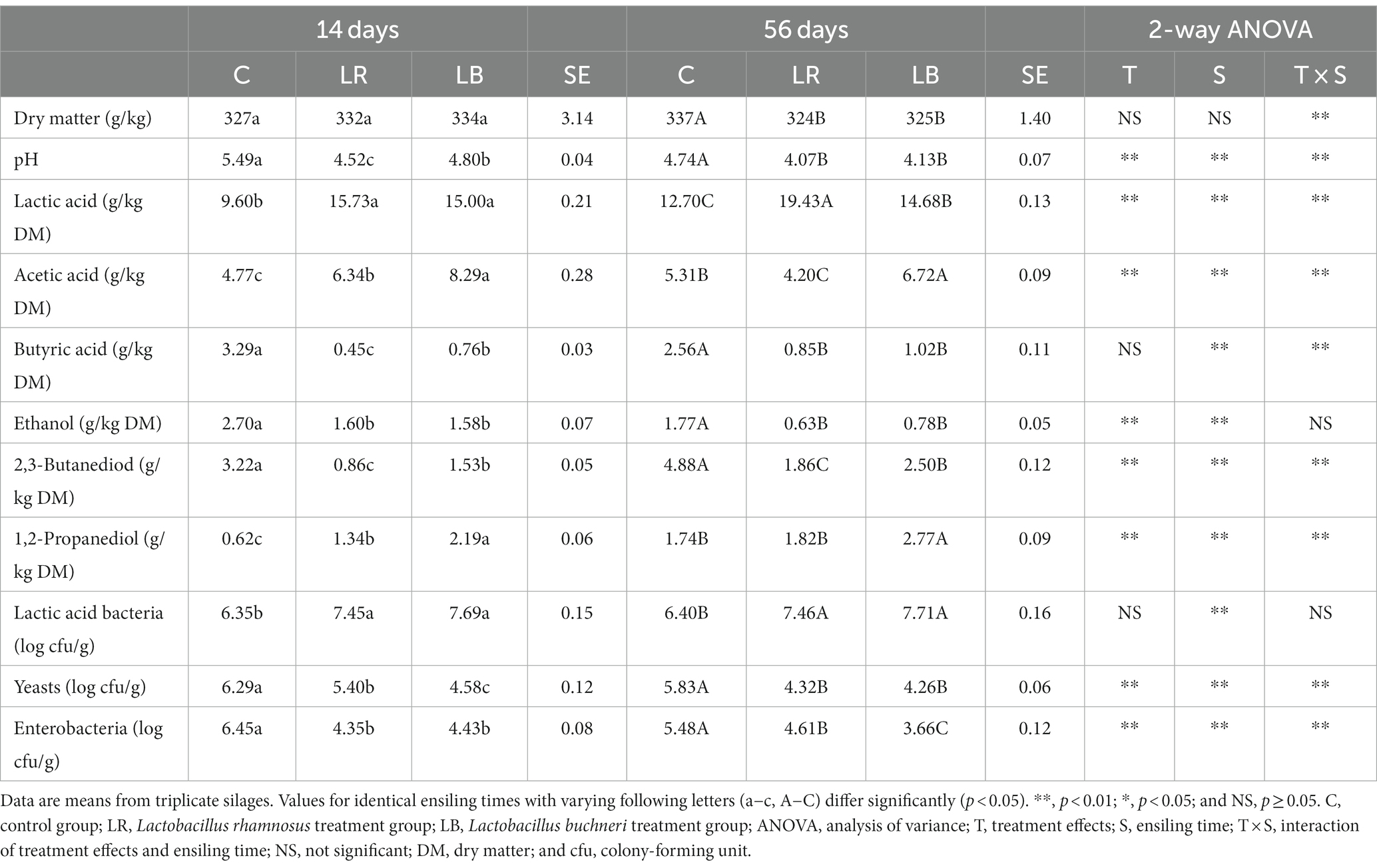
Table 2. Fermentation products and microbial counts of Caragana korshinskii silage stored with/without Lactobacillus rhamnosus or Lactobacillus buchneri for 14 and 56 days.
The heating in the uninoculated silage was observed on day 2 following the aerobic exposure, regardless of the ensiling time. Yeast count and pH value in the uninoculated silage increased to >107 cfu/g and > 6.0, respectively (Table 3). At yeast count of >105 cfu/g, the silage shows reduced aerobic stability (Muck et al., 2018). The homofermentative/heterofermentative LAB-inoculated whole-crop corn silage caused aerobic deterioration during the 7 days of aerobic exposure due to yeast and mold count rapidly increased. However, no heating was observed in the LAB-inoculated silage during the 7 days of aerobic exposure. Although the L. rhamnosus-inoculated silage showed an improved fermentation quality after 56 d, Li and Nishino (2011a) found that L. rhamnosus inoculation had no significant effect on yeast inhibition, resulting in the aerobic deterioration of the Italian ryegrass silage. However, Zhao et al. (2020) reported that L. rhamnosus produced the bacteriocins, along with improving the fermented food quality, which inhibited the growth of harmful microorganisms, including mold, enterobacteria, and yeast, and increased the aerobic stability of the fermented food. These studies showed that different LAB species added silage had different effects on improving fermentation quality and inhibiting spoilage. The L. buchneri-inoculated silage exhibited increased 1,2-propanediol and acetic acid contents compared with the uninoculated silage, following the 7 days of aerobic exposure. Acetic acid improved the aerobic stability of silage by inhibiting the growth of undesirable microorganisms after air exposure. Hence, L. buchneri-inoculated silage had improved aerobic stability.
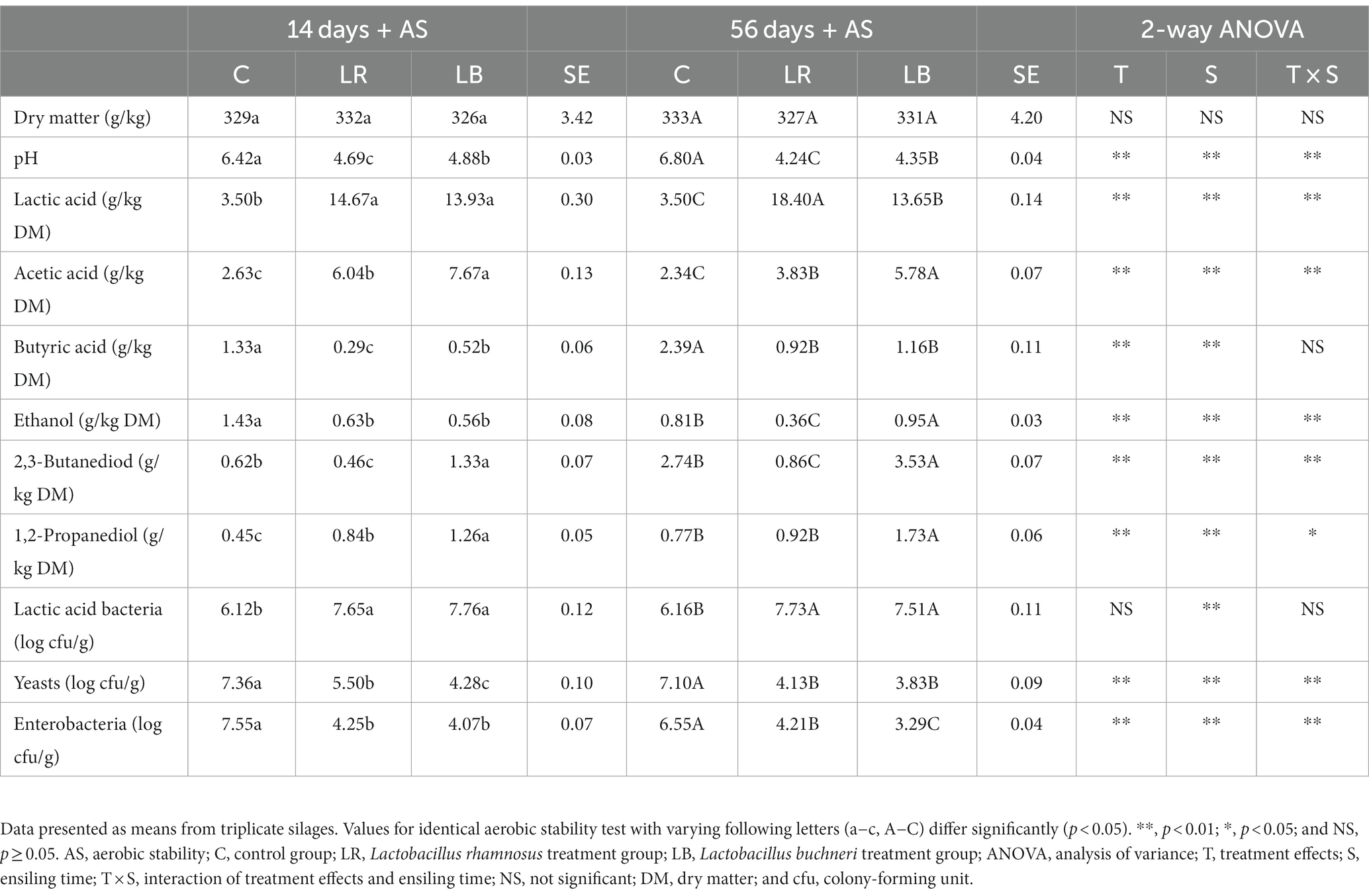
Table 3. Fermentation products and microbial counts of Caragana korshinskii silage subjected to aerobic stability test for 7 days.
Roles of Lactobacillus rhamnosus and Lactobacillus buchneri in bacterial diversity during ensiling and after aerobic exposure
Through NGS, we sequenced the 16S rRNA gene of 39 samples (3 fresh materials and 36 silages). A total of 5,920,574 high-quality sequence readings were generated, with an average of 151,810 readings per sample (Table 4). All the readings were clustered in 7,234 OTUs at a sequence similarity of 97%. The coverage values in every sample were approximately 0.99, suggesting a high bacterial flora diversity. We also utilized Chao/Shannon index and OTUs for assessing the bacterial diversity and abundance within the feed. After 14 days of ensiling, the OTUs, abundance-based coverage estimator (ACE), and Chao 1 levels in L. rhamnosus and L. buchneri inoculation were higher than those in the untreated silage, indicating that LAB addition increased the bacterial abundance. The OTUs, ACE, and Chao 1 levels were lower in the L. buchneri- and L. rhamnosus-inoculated samples compared with those in non-inoculated samples after 56 days of ensiling. Consistent with the study by Guo et al. (2018), OTUs, ACE, and Chao1 levels increased in the L. buchneri and L. rhamnosus-treated silage compared with those in untreated silage. The Shannon index in LAB-inoculated C. korshinskii silage was the lowest on day 56 possibly because of the low pH value, which caused a rapid decrease in the bacterial diversity.
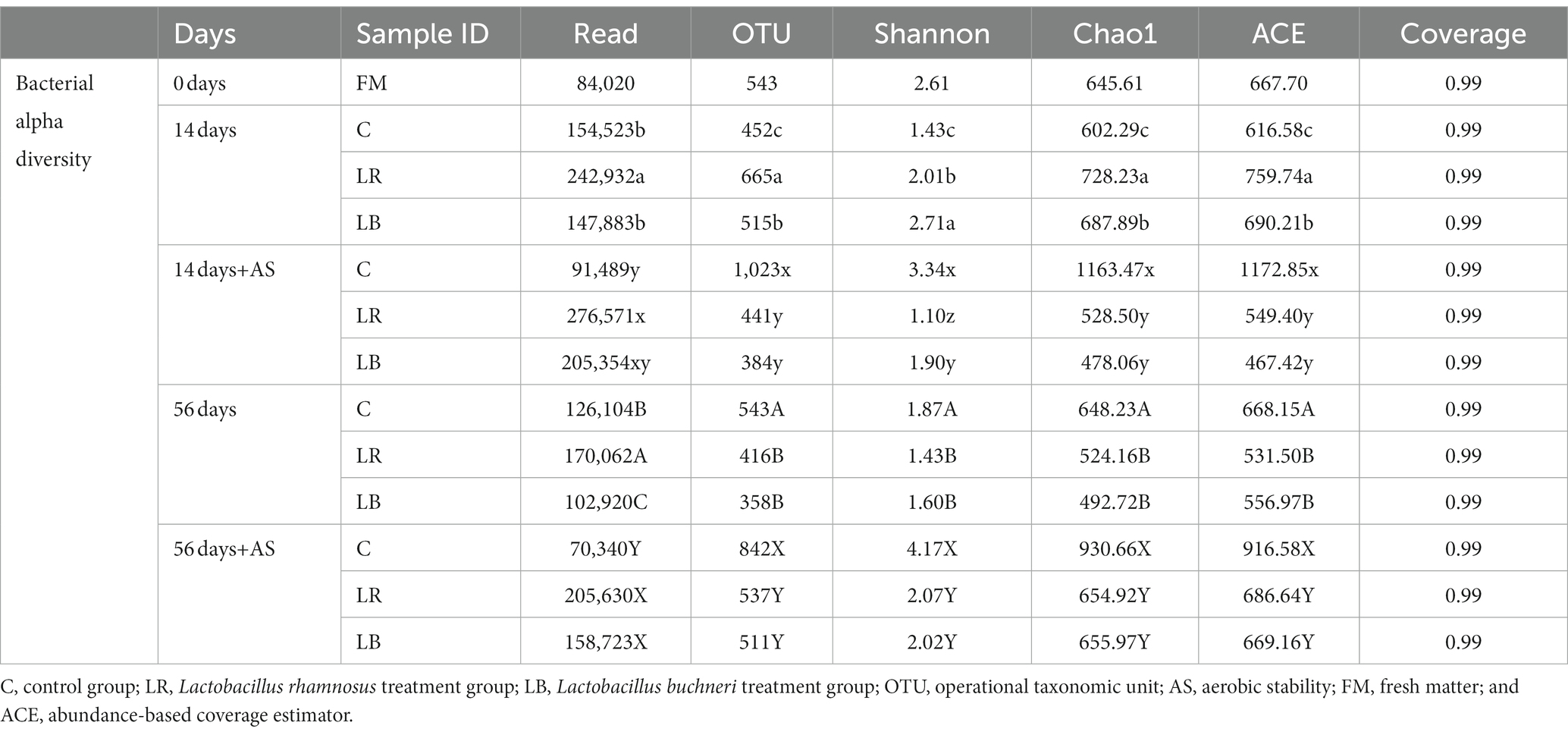
Table 4. The bacterial alpha diversity of Caragana korshinskii silage with/without Lactobacillus rhamnosus or Lactobacillus buchneri during ensiling process and after aerobic exposure.
After the aerobic deterioration test, OTUs, ACE, and Chao 1 index in the spoiled silage were decreased, indicating the proliferation of low-acid-resistant aerobic microorganisms during the aerobic stability test (Wilkinson and Davies, 2013). However, as revealed by denaturing gradient gel electrophoresis analysis, frequent observation of predominant bacteria within alfalfa silage indicated a less diverse bacterial community (Wu and Nishino, 2016).
The Venn plot shows the different groups with common and unique OTUs (Figure 1A). All groups contained 65 common OTUs and 1,150 exclusive OTUs, which were distributed as follows: fresh material, 222; 14-day silos, 201; 56-day silos, 369; 7 days of air-exposed 14-day silos, 143; and 7 days of air-exposed 56-day silos, 215. Figure 1B illustrates the nonmetric multidimensional scaling analysis of the bacterial community in the C. korshinskii silage with/without L. rhamnosus or L. buchneri inoculation. There was an obvious separation of bacterial communities in the pre-ensiling crop compared to the silage sample. In a previous study, native grass silage after Ephedra sinica inoculation exhibited similar results (Du et al., 2022). Because aerobic spoilage was measured in the CAS and CAE groups, following 7 days of aerobic exposure, the alterations among the bacterial communities in the CAS and CAE groups cause aerobic spoilage, separating them from others.
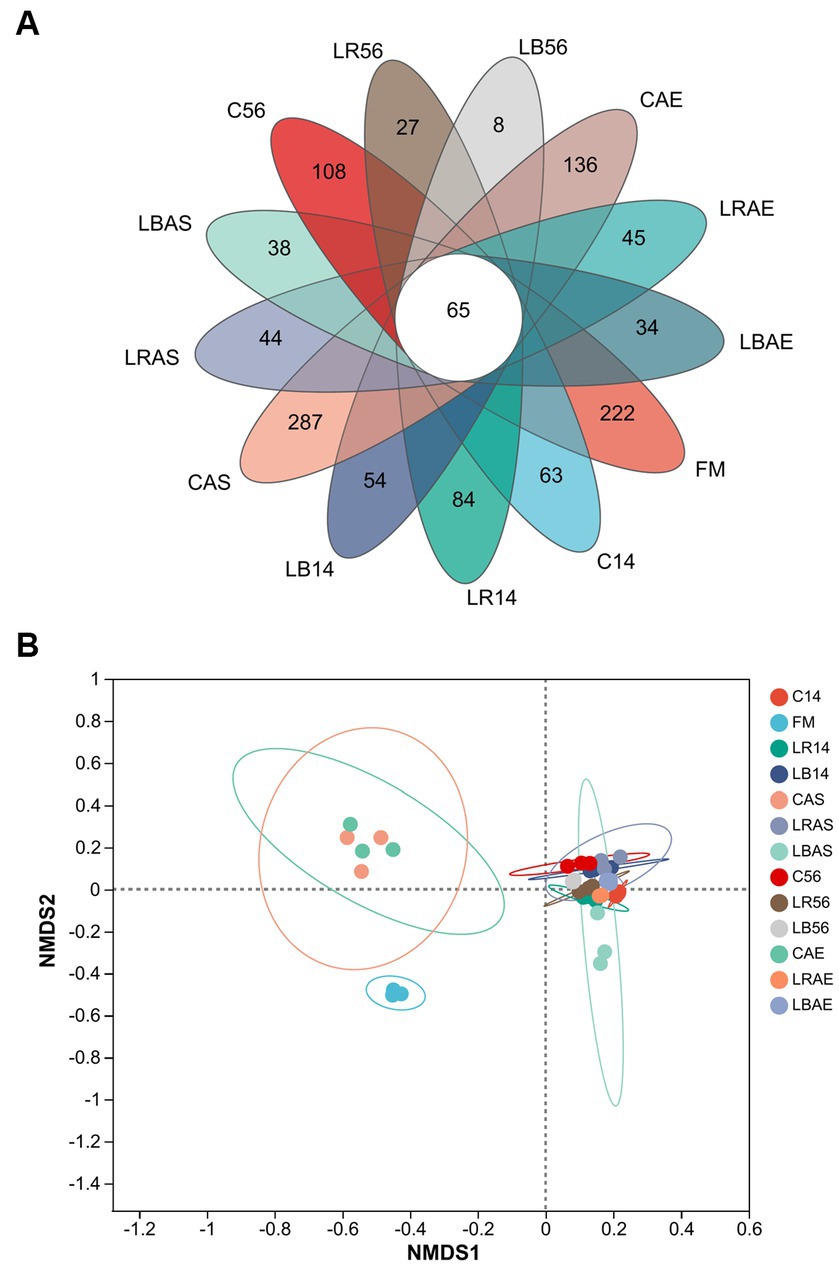
Figure 1. Venn diagram (A) and nonmetric multidimensional scaling (B) of the bacterial community at the operational taxonomic unit level. FM, fresh material; C, control group; LR, Lactobacillus rhamnosus treatment group; and LB, Lactobacillus buchneri treatment group. Numbers after C, LR, and LB denote storage periods. AS and AE indicates aerobic stability test parameters.
Roles of Lactobacillus rhamnosus and Lactobacillus buchneri in the dynamics of bacterial communities during ensiling and after aerobic exposure
Figure 2 illustrates the bacterial communities in the fresh material and silage at the phylum level. In the fresh forage, Firmicutes were the bacteria with the highest phylum level, followed by Proteobacteria, Cyanobacteria, Bacteroidota, and Actinobacteriota. However, in every sample during ensiling process and after aerobic exposure, Firmicutes and Proteobacteria were the most dominant phyla, accounting for >83.3% of the analyzed sequences. Pseudomonadaceae, Erwiniaceae, and Leuconostocaceae were the predominant families and could be detected within pre-ensiling crops, including alfalfa, whole-crop corn, and Leymus chinensis silage (Figure 3A) (Ni et al., 2017; Wu et al., 2022). Though some microorganisms died during the fermentation of C. korshinskii silage with/without L. rhamnosus and L. buchneri inoculation, Lactobacillacae and Leuconostocaceae became the predominant families, regardless of short or long storage period. Li P. et al. (2022) and Li X. et al. (2022) found that the total proportion of Lactobacillacae and Leuconostocaceae reached 65% in the 30- and 60-day silos (Li P. et al., 2022). Lactobacillacae proportion was lower within the uninoculated silage compared with that in the L. rhamnosus- and L. buchneri-inoculated silage after 14 and 56 days of ensiling. L. plantarum-inoculated oat silage has shown similar results (Li X. et al., 2022). However, after the 7 days of spoilage test, the microbiome structure of the LAB-treated silage showed considerable differences compared with that of the untreated silage. The control silage exhibited an increased abundance of Peptostreptococcaceae, Clostridiaceae, and Bacillaceae, whereas a decrease in Lactobacillaceae abundance compared with the L. rhamnosus- and L. buchneri-inoculated silage during the 7 days of spoilage test following 14 and 56 days of ensiling.
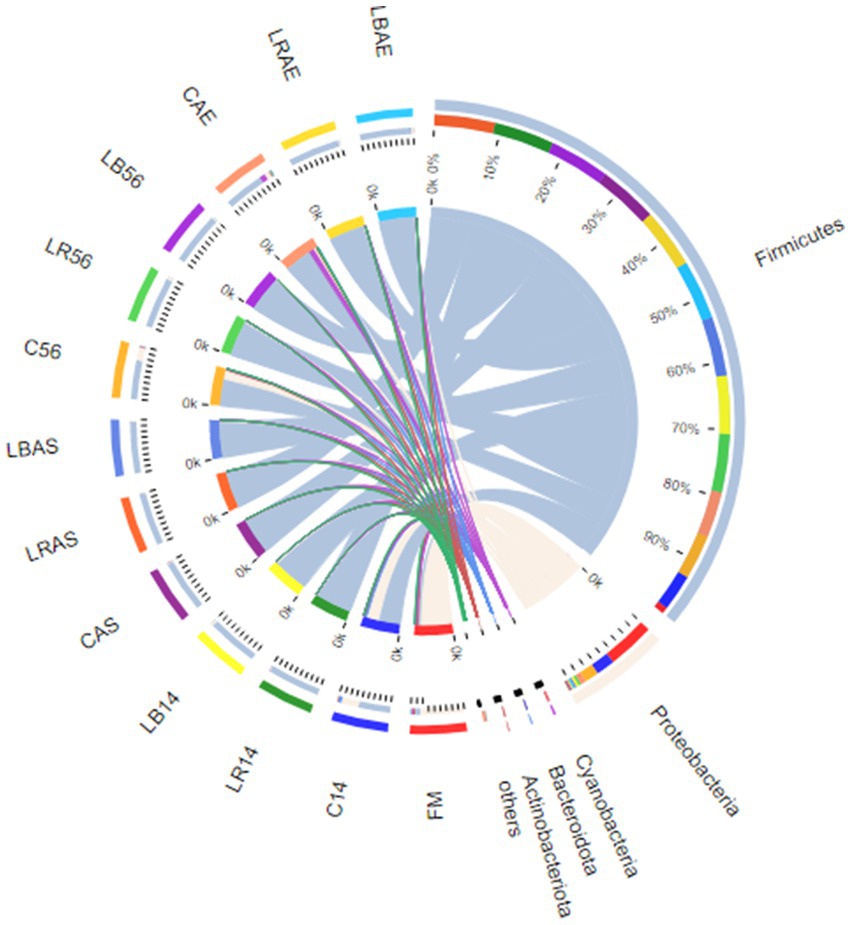
Figure 2. Bacterial community at the phylum level in silage with/without Lactobacillus rhamnosus and Lactobacillus buchneri inoculation. FM, fresh material; C, control group; LR, Lactobacillus rhamnosus treatment group; and LB, Lactobacillus buchneri treatment group. Numbers after C, LR, and LB denote storage periods. AS and AE indicates aerobic stability test parameters.
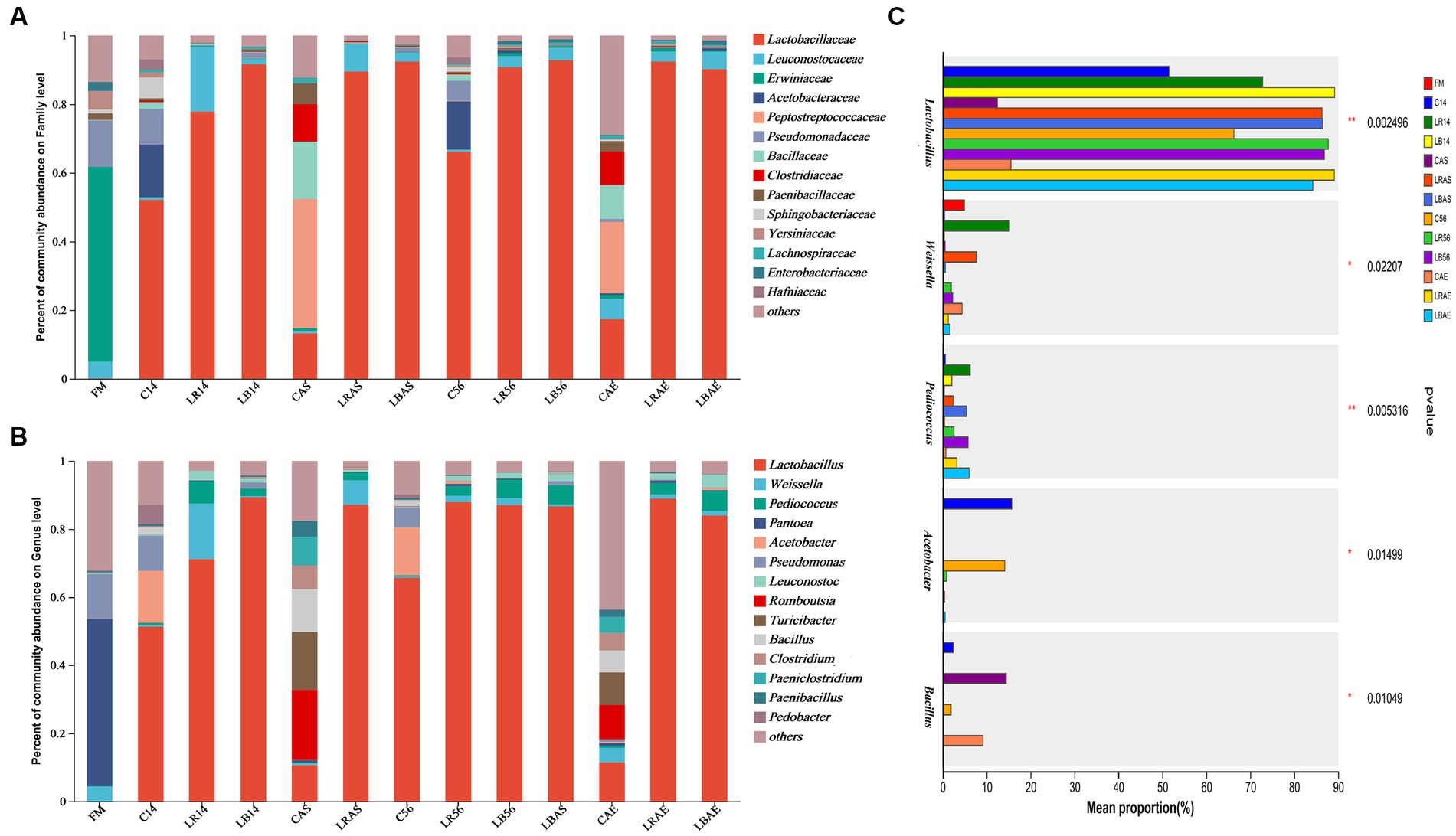
Figure 3. Bacterial community at the family level (A), genus level (B), and error bar plot (C) in silage with/without Lactobacillus rhamnosus and Lactobacillus buchneri inoculation. FM, fresh material; C, control group; LR, Lactobacillus rhamnosus treatment group; and LB, Lactobacillus buchneri treatment group. Numbers after C, LR, and LB denote storage periods. AS and AE indicates aerobic stability test parameters.
Figure 3B illustrates bacterial communities in the fresh material and silage at a genus level. The main genera in the fresh material included Pseudomonas, Pantoea, and Weissella. Our previous study showed similar results that Pantoea and Pseudomonas were the predominant genera in the pre-ensiling crop in L. chinensis (Wu et al., 2022). Although they are different raw materials, sometimes the microbes attached to the surface of raw materials are similar in Inner Mongolian Plateau. Lactobacillus had the highest abundance within the 14- and 56-day silage, which was consistent with the findings of Wang et al. (2020). After 14 and 56 days of ensiling, the control silage exhibited an increased Acetobacter and Bacillus abundance, whereas a decreased Lactobacillus abundance compared with that in the L. rhamnosus- and L. buchneri-treated silage (Figure 3C). Acetobacter species can induce aerobic deterioration of silage, which has been detected within the 14-, 56-, and 120-day whole-crop corn silage (Li and Nishino, 2011b). However, after the aerobic stability test of the 14- and 56-day silage, the control silage exhibited an increased Clostridium and Bacillus abundance, whereas a decreased Lactobacillus abundance compared with that in the L. rhamnosus- and L. buchneri-treated silage. Before aerobic deterioration, Bacillus count in the air-exposed silage increases (Okatsu et al., 2019). Liu et al. (2013) discovered that Serratia nematodiphila, Lysinibacillus fusiformis, Myroides odoratimimus, Stenotrophomonas maltophilia, Acinetobacter soli, and Bacillus pumilus present in the 65-day ensiled corn stalk silage, and the aerobic spoilage caused by them during the 7 days of aerobic exposure. Clostridium species are obligate anaerobic bacteria, and their presence in the whole-crop maize silage has been related to the anaerobic stability during the ensiling phase but not to aerobic spoilage in the feed-out phase (Driehuis and Oude Elferink, 2000). Jonsson (1991) reported that in the Clostridium tyrobutyricum-inoculated silage, the Clostridium count increased from 105 cfu/g at the silo opening to 107 cfu/g after the aerobic deterioration. Additionally, Vissers et al. (2007) reported that the Clostridium count in the grass and whole-crop corn silages must be above 105 cfu/g, especially on the surface of bunker silos and in the top of tower silos. Therefore, we speculated that deterioration of silage in control group may be related to the existence of Clostridium species.
Relation between the bacterial community and pH and fermentation products
Natural silage fermentation and aerobic exposure of silage are mostly dependent on the complex processes that involve interactions of main bacteria with various fermentation products. Pahlow et al. (2003) reported that LAB produced acetic acid, lactic acid, carbon dioxide, and ethanol by fermenting sugar. Clostridium butyricum and Clostridium tyrobutyricum ferment WSCs and some sugars into acetic acid, butyric acid, and carbon dioxide, whereas Bacillus species ferment organic acids into water and carbon dioxide during aerobic exposure to silage (Muck, 2010). After 14- and 56-day ensiling, Spearman’s correlations between the bacterial community and pH and fermentation products were predicted (Figure 4). According to Spearman’s correlation results, Lactobacillus, Pedicoccus, and Methylobacterium genera were positively related (p < 0.01) to the lactic acid and acetic acid levels. However, Weissella and Lactobacillus richness was negatively correlated (p < 0.01) with the pH and the ethanol, 2,3-butanediol, and butyric acid levels. Clostridium, Paenibacillus, Romboutsia, Bacillus, and Paeniclostridium genera were negatively correlated (p < 0.01) with the 1,2-propanediol and lactic acid levels, whereas positively correlated to pH (p < 0.01), with the correlation values being 0.46, 0.40, 0.57, 0.65, and 0.73, respectively. The butyric acid content exhibited a positive relation (p < 0.01) to Clostridium (R = 0.48). These findings contradict a previous study (Wu et al., 2022) because different silages have different microbial compositions and produce different metabolites during the fermentation process and aerobic exposure.
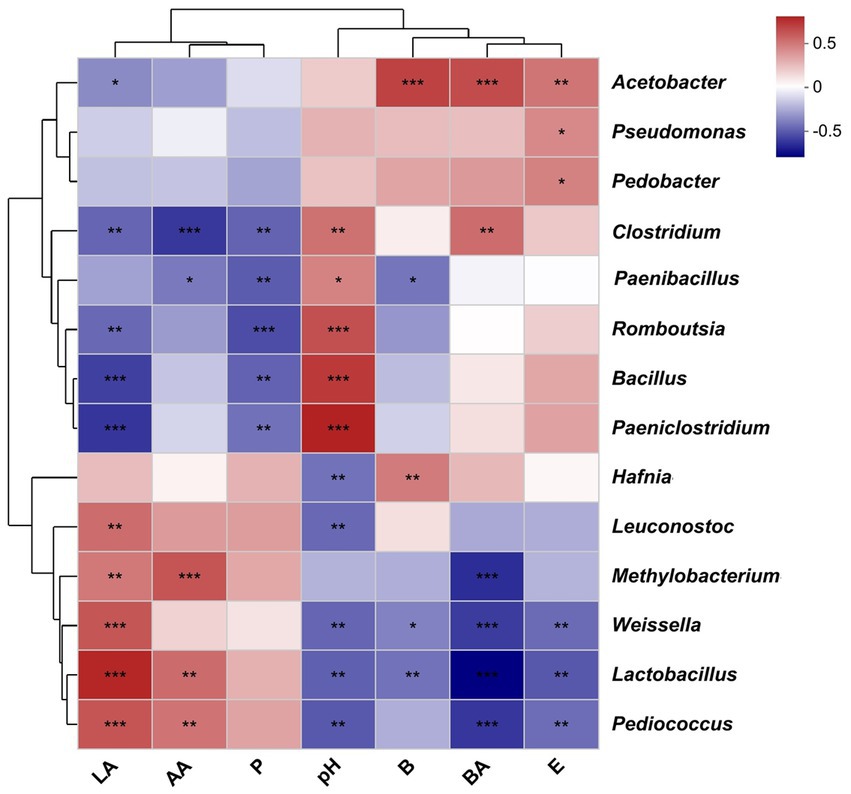
Figure 4. Correlation between the bacterial community and pH and fermentation products. Heatmap analysis was completed with Spearman’s rho and p-values. *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; and ***, p < 0.001. BA, butyric acid; AA, acetic acid; LA lactic acid; E, ethanol; B, 2,3-butanediol; and P, 1,2-propanediol.
KEGG functional annotation of bacterial communities
The KEGG function profiles on the first and second pathway levels are illustrated in Figures 5A,B. The predicted functions under the first pathway level in all silages were mainly related to metabolism. Following the 7 days of aerobic exposure, the untreated silage showed a lower abundance regarding metabolism compared with the L. rhamnosus- and L. buchneri-treated silage. The functional profiles at the second pathway level exhibited a primary association with the amino acid and carbohydrate metabolisms, followed by energy, cofactors, vitamins, and nucleotide metabolisms, and glycan biosynthesis and metabolism, which was similar to the findings of Zhao et al. (2022). The relative abundance of the common metabolism differed in all silage samples. L. buchneri-treated silage exhibited a lower abundance of amino acid and carbohydrate metabolisms compared with that in the control silage after 14 and 56 days of ensiling, whereas after the aerobic stability test, the control silage had a lower abundance of amino acid and carbohydrate metabolisms compared with that in the LAB-treated silage. Ensiling primarily ferments WSCs within the silage into organic acids, including acetic acid, butyric or lactic acid, via LAB in an anaerobic environment, which explains the cause of increased carbohydrate metabolism during the ensiling process. Amino acids are essential substances in crops and have a critical effect on increasing protein synthesis and primary metabolism, which accounts for the increased amino acid metabolism level within the control silage, whereas inhibition of amino acid metabolism within L. buchneri-inoculated silage may be due to a decrease in the pH value after 14 and 56 days of ensiling.
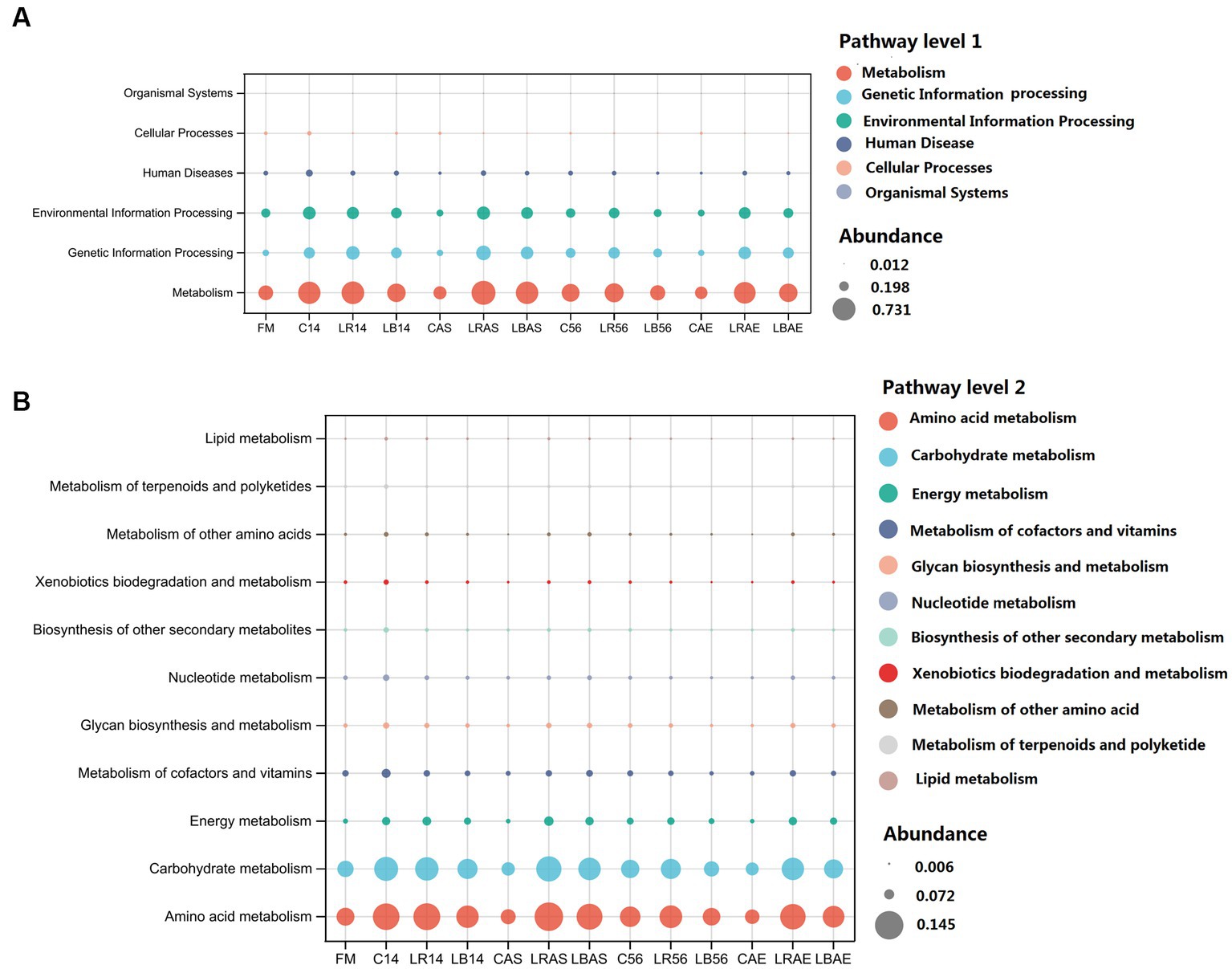
Figure 5. 16S ribosomal RNA gene estimated Kyoto encyclopedia of genes and genomes functional profiles at first (A) and second (B) pathway levels. FM, fresh material; C, control group; LR, Lactobacillus rhamnosus treatment group; and LB, Lactobacillus buchneri treatment group. Numbers after C, LR, and LB denote storage periods. AS and AE indicates aerobic stability test parameters.
Figure 6 illustrates the functional profiles at the third pathway level. After 7 days of aerobic exposure, the carbohydrate metabolism, including glycolysis/gluconeogenesis, starch and sucrose metabolism, amino sugar and nucleotide sugar metabolism, pyruvate metabolism, fructose, and mannose metabolism, pentose phosphate pathway, propanoate metabolism, galactose metabolism, glyoxylate and dicarboxylate metabolism, butanoate metabolism, pentose and glucuronate interconversions, citrate cycle; and amino acid metabolism, including alanine, aspartate, and glutamate metabolism, glycine, serine, and threonine metabolism, cysteine and methionine metabolism, lysine biosynthesis, and tyrosine metabolism in the control silage decreased compared with those in the LAB-inoculated silage. Most amino acid and carbohydrate metabolisms in the control silage were considerably inhibited after 7 days of aerobic exposure, which was consistent with the aerobic spoilage findings in the control silage. This was probably because of the rapid increase in pH values after 7 days of air exposure, which restricted LAB-induced carbohydrate and protein catabolism (Mcdonald et al., 1991).
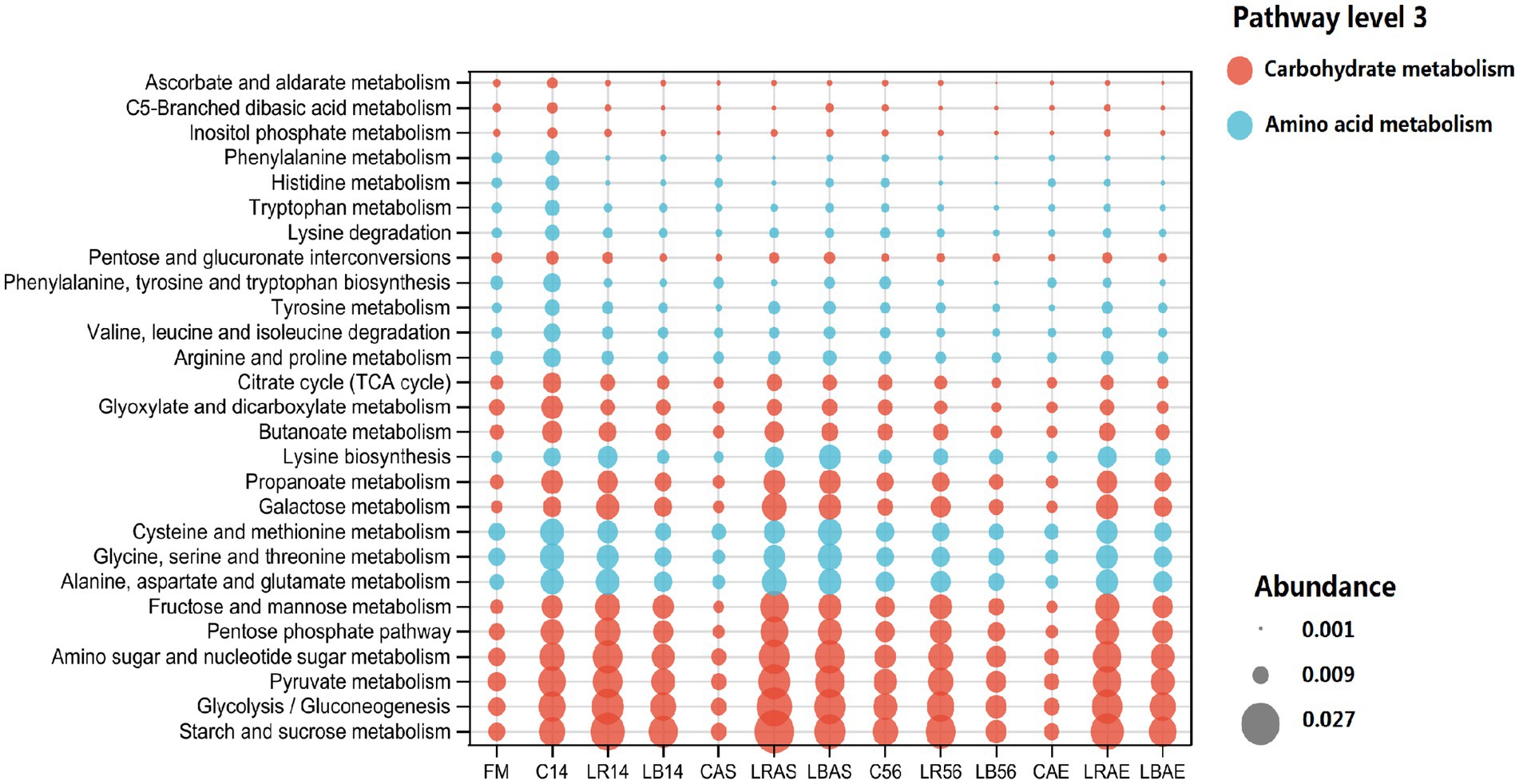
Figure 6. 16S ribosomal RNA gene estimated Kyoto encyclopedia of genes and genomes functional profiles at the third pathway level. FM, fresh material; C, control group; LR, Lactobacillus rhamnosus treatment group; and LB, Lactobacillus buchneri treatment group. Numbers after C, LR, and LB denote storage periods. AS and AE indicates aerobic stability test parameters.
Conclusion
After 14 and 56 days of ensiling, L. rhamnosus- and L. buchneri-inoculated silage exhibited increased lactic and acetic acid contents, whereas a decrease in the pH and 2,3-butanediol and butyric acid contents compared with those in the control silage. Heating could be observed on day 2 of the aerobic stability test in 14- and 56-day uninoculated silages; however, heating was not observed during the 7 days of air exposure of L. rhamnosus and L. buchneri-treated silages. The control silage presented with increased Clostridium and Bacillus abundance, whereas a decreased Lactobacillus abundance compared with that in L. rhamnosus- and L. buchneri-treated silages subjected to 7 days of air exposure. The fermentation parameters were associated with the microbial communities, especially for Lactobacillus, Pedicoccus, Weissella, Clostridium, and Bacillus. Following 7 days of aerobic exposure, carbohydrate and amino acid metabolisms decreased within the control silage compared with that in the LAB-inoculated silage. In conclusion, L. rhamnosus or L. buchneri inoculation improved the fermentation quality, aerobic stability, and microbiome structure and function of the C. korshinskii silage.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA990987.
Author contributions
BW: Conceptualization, Writing – original draft, Funding acquisition, Supervision, Writing – review & editing. JA: Data curation, Formal analysis, Writing – original draft. TL: Conceptualization, Data curation, Writing – original draft. WQ: Conceptualization, Data curation, Writing – original draft. ZH: Data curation, Formal analysis, Writing – original draft. TS: Conceptualization, Data curation, Writing – original draft. TW: Conceptualization, Data curation, Writing – original draft. CW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. HN: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Program for Young Talents of Science and Technology of the Universities of Inner Mongolia Autonomous Region [grant number NJYT22054]; Scientific and Technological Innovation Guidance Project of Inner Mongolia [grant number KCJL-202211]; Scientific Research Project of Inner Mongolia University Minzu University [grant number NMDYB20031]; Doctoral Funding of the Inner Mongolia Minzu University [grant number BS610]; Natural Science Foundation of Inner Mongolian [grant number 2022QN03027, 2020BS03025, 2021BS03016].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aßhauer, K. P., Bernd, W., Rolf, D., and Peter, M. (2015). Tax4fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31, 2882–2884. doi: 10.1093/bioinformatics/btv287
Bai, B., Qiu, R., Wang, Z., Liu, Y., Bao, J., Sun, L., et al. (2023). Effects of Cellulase and lactic acid Bacteria on ensiling performance and bacterial community of Caragana korshinskii silage. Microorganisms 11:337. doi: 10.3390/microorganisms11020337
Cai, Y., Benno, Y., Ogawa, M., Ohmomo, S., and Nakase, T. (1998). Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 64, 2982–2987. doi: 10.1128/AEM.64.8.2982-2987.1998
Deng, L., Han, Q. S., Zhang, C., Tang, Z. S., and Shangguan, Z. P. (2017). Above-ground and below-ground ecosystem biomass accumulation and carbon sequestration with Caragana korshinskii Kom plantation development. Land Degrad. Dev. 28, 906–917. doi: 10.1002/ldr.2642
Driehuis, F., and Oude Elferink, S. J. W. H. (2000). The impact of the quality of silage on animal health and food safety: a review. Vet. Q. 22, 212–216. doi: 10.1080/01652176.2000.9695061
Du, S., You, S., Jiang, X., Li, Y., and Jia, Y. (2022). Dynamics of the fermentation quality and microbiotsa in Ephedra sinica treated native grass silage. J. Appl. Microbiol. 133, 3465–3475. doi: 10.1111/jam.15779
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Guo, X. S., Ke, W. C., Ding, W. R., Ding, L. M., Xu, D. M., Wang, W. W., et al. (2018). Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 8:357. doi: 10.1038/s41598-017-18348-0
Jonsson, A. (1991). Growth of Clostridium tyrobutyricum during fermentation and aerobic deterioration of grass silage. J. Sci. Food Agric. 54, 557–568. doi: 10.1002/jsfa.2740540407
Li, X., Chen, F., Xiong, Y., Guo, L., Xu, J., Lin, Y., et al. (2022). Perilla frutescens as potential antimicrobial modifier to against forage oat silage spoilage. Front. Microbiol. 13:1053933. doi: 10.3389/fmicb.2022.1053933
Li, Y., and Nishino, N. (2011a). Bacterial and fungal communities of wilted Italian ryegrass silage inoculated with and without Lactobacillus rhamnosus or Lactobacillus buchneri. Lett. Appl. Microbiol. 52, 314–321. doi: 10.1111/j.1472-765X.2010.03000.x
Li, Y., and Nishino, N. (2011b). Effects of inoculation of Lactobacillus rhamnosus and Lactobacillus buchneri on fermentation, aerobic stability and microbial communities in whole crop corn silage. Grassl. Sci. 57, 184–191. doi: 10.1111/j.1744-697X.2011.00226.x
Li, W., Zhang, S., Zhang, T., Shen, Y., Han, L., Peng, Z., et al. (2021). Bacterial cellulose production from ethylenediamine pretreated Caragana korshinskii Kom. Ind. Crop. Prod. 164:113340. doi: 10.1016/j.indcrop.2021.113340
Li, P., Zhao, W., Yan, L., Chen, L., Chen, Y., Gou, W., et al. (2022). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan plateau. Bioresour. Technol. 347:126347. doi: 10.1016/j.biortech.2021.126347
Liu, L., Guo, Y., Liu, X., Yao, Y., and Qi, W. (2023). Relationship between the roots of Hippophae rhamnoides at different stump heights and the root microenvironment in feldspathic sandstone areas. PeerJ 11:e14819. doi: 10.7717/peerj.14819
Liu, Q. H., Shao, T., and Zhang, J. G. (2013). Determination of aerobic deterioration of corn stalk silage caused by aerobic bacteria. Anim. Feed Sci. Technol. 183, 124–131. doi: 10.1016/j.anifeedsci.2013.05.012
Mcdonald, P., Henderson, A. R., and Heron, S. J. E. (1991). The biochemistry of silage, 2nd. Lincon: Chalcombe Publications.
Muck, R. E. (2010). Silage microbiology and its control through additives. Rev. Bras. Zootec. 39, 183–191. doi: 10.1590/S1516-35982010001300021
Muck, R. E., Nadeau, E. M. G., McAllister, T. A., Contreras-Govea, F. E., Santos, M. C., and Kung, L. Jr. (2018). Silage review: recent advances and future uses of silage additives. J. Dairy Sci. 101, 3980–4000. doi: 10.3168/jds.2017-13839
Ni, K., Minh, T. T., Tu, T. T., Tsuruta, T., Pang, H., and Nishino, N. (2017). Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 101, 1385–1394. doi: 10.1007/s00253-016-7900-2
Okatsu, Y., Swanepoel, N., Maga, E. A., and Robinson, P. H. (2019). Impacts of some factors that effect spoilage of silage at the periphery of the exposed face of corn silage piles. Anim. Feed Sci. Technol. 247, 234–247. doi: 10.1016/j.anifeedsci2018.11.018
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S. J. W. H. O., and Spoelstra, S. F. (2003) in Microbiology of ensiling. eds. D. R. Buxton, R. E. Muck, and R. E. Harrison, Am. Soc. Agron., Crop Sci. Soc. Am., Soil Sci. Soc. Am (Madison, WI: Silage Science and Technology), 31–93.
Pang, H., Qin, G., Tan, Z., Li, Z., Wang, Y., and Cai, Y. (2011). Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 34, 235–241. doi: 10.1016/j.syapm.2010.10.003
Stevenson, D. M., Muck, R. E., Shinners, K. J., and Weimer, P. J. (2006). Use of real time PCR to determine population profiles of individual species of lactic acid bacteria in alfalfa silage and stored corn Stover. Appl. Microbiol. Biotechnol. 71, 329–338. doi: 10.1007/s00253-005-0170-z
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022–0302(91)78551–2
Vissers, M. M. M., Driehuis, F., Giffel, M. C. T., Jong, P. D., and Lankveld, J. M. G. (2007). Concentrations of butyric acid Bacteria spores in silage and relationships with aerobic deterioration. J. Dairy Sci. 90, 928–936. doi: 10.3168/jds.S0022-0302(07)71576-X
Wang, T., Teng, K., Cao, Y., Shi, W., Xuan, Z., Zhou, J., et al. (2020). Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 312:123600. doi: 10.1016/j.biortech.2020.123600
Wilkinson, J. M., and Davies, D. R. (2013). The aerobic stability of silage: key findings and recent developments. Grass Forage Sci. 68, 1–19. doi: 10.1111/j.1365-2494.2012.00891.x
Wu, B., Hu, Z., Wei, M., Yong, M., and Niu, H. (2022). Effects of inoculation of Lactiplantibacillus plantarum and Lentilactobacillus buchneri on fermentation quality, aerobic stability, and microbial community dynamics of wilted Leymus chinensis silage. Front. Microbiol. 13:928731. doi: 10.3389/fmicb.2022.928731
Wu, B., and Nishino, N. (2016). Identification and isolation of Lactobacillus fructivorans from wilted alfalfa silage with and without molasses. J. Appl. Microbiol. 120, 543–551. doi: 10.1111/jam.13031
Wu, B., Zhang, Q., Liu, Z., Yu, Z., and Nishino, N. (2014). Bacterial communities in alfalfa and corn silages produced in large-scale stack and bunker silos in China. Grassl. Sci. 60, 247–251. doi: 10.1111/grs.12063
Yang, L., Yuan, X., Li, J., Dong, Z., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi: 10.1016/j.biortech.2018.12.067
You, J., Zhang, H., Zhu, H., Xue, Y., Cai, Y., and Zhang, G. (2022). Microbial community, fermentation quality, and in vitro degradability of ensiling Caragana with lactic acid Bacteria and Rice bran. Front. Microbiol. 13:804429. doi: 10.3389/fmicb.2022.804429
Zhang, Y., Wang, M., Usman, S., Li, F., Bai, J., Zhang, J., et al. (2023). Lignocellulose conversion of ensiled Caragana korshinskii Kom. Facilitated by Pediococcus acidilactici and cellulases. Microb. Biotechnol. 16, 432–447. doi: 10.1111/1751-7915.14130
Zhang, Q., Wu, B., Nishino, N., Wang, X., and Yu, Z. (2016). Fermentation and microbial population dynamics during the ensiling of native grass and subsequent exposure to air. Anim. Sci. J. 87, 389–397. doi: 10.1111/asj.12427
Zhao, R., Lu, Y., Ran, J., Li, G., Lei, S., Zhu, Y., et al. (2020). Purification and characterization of bacteriocin produced by Lactobacillus rhamnosus zrx01. Food Biosci. 38:100754. doi: 10.1016/j.fbio.2020.100754
Zhao, J., Yin, X. J., Wang, S. R., Li, J. F., Dong, Z. H., and Shao, T. (2022). Changes in the fermentation products, taxonomic and functional profiles of microbiota during high-moisture sweet sorghum silage fermentation. Front. Microbiol. 13:967624. doi: 10.3389/fmicb.2022.967624
Zhong, C., Sun, Z., Zhou, Z., Jin, M. J., Tan, Z. L., and Jia, S. R. (2014). Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. J. Agric. Food Chem. 62, 3217–3222. doi: 10.1021/jf500349s
Keywords: Caragana korshinskii silage, fermentation products, Lactobacillus rhamnosus, Lactobacillus buchneri, microbial community, microbial function characteristics
Citation: Wu B, Ai J, Li T, Qin W, Hu Z, Siqin T, Wu T, Wang C and Niu H (2023) Fermentation quality, aerobic stability, and microbiome structure and function of Caragana korshinskii silage inoculated with/without Lactobacillus rhamnosus or Lactobacillus buchneri. Front. Sustain. Food Syst. 7:1255936. doi: 10.3389/fsufs.2023.1255936
Edited by:
Cristobal N. Aguilar, Autonomous University of Coahuila, MexicoReviewed by:
Guadalupe Virginia Nevárez-Moorillón, Autonomous University of Chihuahua, MexicoLiliana Londono Hernandez, National Open and Distance University, Colombia
Copyright © 2023 Wu, Ai, Li, Qin, Hu, Siqin, Wu, Wang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Wang, d2FuZ2NoYW9AaW1hYWFocy5hYy5jbg==; Huaxin Niu, bml1aHhAaW11bi5lZHUuY24=
 Baiyila Wu
Baiyila Wu Juanjuan Ai2
Juanjuan Ai2 Zongfu Hu
Zongfu Hu Huaxin Niu
Huaxin Niu