
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Sustain. Food Syst., 20 October 2023
Sec. Agro-Food Safety
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1232992
 Getachew Dinede1*
Getachew Dinede1* Kebede Amenu1,2
Kebede Amenu1,2 Silvia Alonso1
Silvia Alonso1 Lina Gazu1
Lina Gazu1 Florence Mutua3
Florence Mutua3 Kristina Roesel3
Kristina Roesel3 Johanna F. Lindahl3,4,5
Johanna F. Lindahl3,4,5 Filipe Maximiano Sousa6
Filipe Maximiano Sousa6 Pattama Ulrich7
Pattama Ulrich7 Tadesse Guadu8
Tadesse Guadu8 Michel Dione9
Michel Dione9 Guy Ilboudo10
Guy Ilboudo10 Theodore J. D. Knight-Jones1
Theodore J. D. Knight-Jones1 Delia Grace1,11
Delia Grace1,11Background: Foodborne diseases impose substantial public health burden and jeopardize socio-economic development worldwide. While accurate information on foodborne hazards is needed for informed decision in food safety interventions, such information is scarce in developing countries such as Burkina Faso. We conducted a systematic review and meta-analysis of studies reporting foodborne hazards in foods in Burkina Faso to describe the present knowledge of the situation.
Methods: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to conduct this review. Abstracts were searched in PubMed and CAB direct between 1 January 1990 to 30 September 2019. We used random-effects models to estimate pooled prevalence and I2 values to measure heterogeneity between studies.
Results: 188 articles were identified, of which 14 are included in this review: 12 were on bacterial hazards (Salmonella, Campylobacter, Staphylococcus, E. coli, Shigella), three on fungal hazards and one on parasitic hazards (Toxoplasma gondii). The overall pooled prevalence of Salmonella spp. was 13% (95% CI: 8–21), the highest in lettuce: 50% (95% CI: 30–70) and the lowest in milk: 1.2% (95% CI: 0–5), demonstrating substantial variation among the studies (I2 = 85, 95% CI: 79–90%, p < 0.01). Campylobacter spp. was reported in chicken carcass, with 50% of the samples being positive. The overall pooled microbial load of Staphylococcus in the studied food samples was 3.2 log (95% CI: 2.8–3.6) CFU per g or ml of food, the highest in poultry samples: 4.5 log (95% CI: 2.8–6.2) CFU per g or ml of food. The overall pooled prevalence of Escherichia coli (E. coli) was 40% (95% CI: 29–51), the highest in beef intestines: 62% (95% CI: 22–91) and the lowest in dairy products: 31% (95% CI: 17–50), showing substantial variation across the studies (I2 = 86, 95% CI: 80–90%, p < 0.01).
Conclusion: Our results showed widespread contamination of foods with foodborne hazards across various food value chains indicating poor hygienic handling of foods, raising consumers’ health risk due to foodborne illnesses from the foods. We recommend promotion of awareness creation in food safety and improved monitoring of hazards in food.
Consumption of foods contaminated with foodborne hazards causes more than 200 diseases, ranging from diarrhea to cancers (World Health Organization Food safety, n.d.). In 2010, 31 foodborne hazards caused 600 million foodborne illnesses worldwide-almost one in ten people in the world-and 420,000 deaths every year, resulting in the loss of 33 million healthy life years, with the greatest per capita burden falling on the subregions in Africa. Children under the age of five years bore 40% of the burden (World Health Organization, 2015). The burden of foodborne illness is higher in the subregion of Africa with high child and adult mortality (AFR D), to which Burkina Faso belongs, compared with other subregions (Havelaar et al., 2015).
Food-producing animals (e.g., cattle, poultry, pigs and fish) are the main reservoirs for several important foodborne pathogens (Gal-Mor et al., 2014; Ferrari et al., 2019). Vegetables, water and beverages are also high risk foods for foodborne pathogens (Berger et al., 2010; Paudyal et al., 2017; Dinede et al., 2020). Moreover, produce and animal source foods consumption is expected to markedly increase in low-and middle-income countries (LMICs), raising food safety concerns unless appropriate food safety management is implemented (Grace et al., 2020).
Although consumers’ concern about food safety has been increasing in LMICs, food safety management is neglected, with especially low compliance with food safety regulations in the traditional or informal markets, where most people buy food (Grace, 2015). Risk factors for unsafe food include lack of clean water used for cleaning and processing of food; poor food-production processes and food-handling (including inappropriate use of agricultural chemicals); inadequate food storage infrastructures; and inadequate enforcement of regulations (World Health Organization, 2015).
Several studies show that food handlers in Burkina Faso have poor knowledge about food safety (Barro et al., 2002; Ilboudo et al., 2009; Somda et al., 2018). Poor sanitary practices during food production, sale, preparation, cooking and servicing leads to microbial contamination. Studies in Burkina Faso have shown that foods obtained from retail markets are often contaminated with foodborne hazards, with food handlers in these retail markets frequently having poor hygienic practices while handling foods, including using unclean water for cleaning and processing of foods and storing food at inappropriate temperatures (Kagambèga et al., 2012a,b; Somda et al., 2018).
Food safety authorities are responsible for food safety monitoring to help safeguard consumers’ health. In high-income regions, for example in the European Union (EU), responsible bodies for food safety issues set out sampling strategies and acceptable standards for microbiological hazards in foods sold, with removal from market and potential prosecution if these standards are exceeded (European Commission Regulation, 2005). Such monitoring schemes need comprehensive information on food safety, including the risk of different foodborne hazards, to inform public health policies and implement interventions that contribute to reducing foodborne disease burden. Systematic reviews reporting food hazards have been conducted in some African countries to provide synthesized information on food contamination in these countries, which help inform policy and decision making by different stakeholders (Alonso et al., 2016; Paudyal et al., 2017; Oduori and Kwoba, 2022; Gazu et al., 2023). However, such information is lacking in Burkina Faso. Given the paucity of food hazards information in this country, this Systematic Literature Review (SLR) was conducted to summarize the evidence on hazards in foods in Burkina Faso from 1990 to 2019 through systematic review of available literature reporting foodborne hazards in foods. Specific objectives of the review include describing: (1) the trends of food safety research in the review period; (2) the hazards studied including their types and associated contamination levels in the food samples and (3) to estimate a pooled contamination levels (prevalence, microbial load) of the hazards in different food samples through meta-analysis, where applicable.
This systematic review and meta-analysis aimed to address the following research questions related to hazards in foods in Burkina Faso during the review period:
a. What potential foodborne hazards (biological and chemical hazards) have been reported in foods in Burkina Faso?
b. What is the contamination level (proportion of foods with hazards or microbial loads of the hazards in foods) of these hazards in foods in Burkina Faso?
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Page et al., 2021) was used to conduct this systematic review. We searched for abstracts published in English or French in PubMed and CAB Direct databases from January 1, 1990 to September 30, 2019. We used different search terms including, but not limited to, the following keywords: “foodborne,” OR “food borne” OR “food-borne,” OR “food safety,” OR “food related,” OR “food associated,” OR “food derived,” “food* illness” OR “food* disease*” OR “food* intoxica*” OR “food* poison*,” “food pathogen” OR “food* microb*” OR “food* vir*” OR “food parasit*” OR “food toxin,” AND “Burkina Faso.” Search terms kept broad and more general to allow retrieving all the literature reporting hazards in different aspects: proportion of foods with hazards, microbial loads of hazards in foods and so on.
We included studies (that followed probabilistic approach in their study design) that reported foodborne hazards in foods (prevalence or microbial load) in Burkina Faso. We excluded studies focusing exclusively on non-foodborne disease hazards and antimicrobial resistance. We also excluded studies reporting hazards exclusively in non-foods items, such as in animal feces, animal serology, or carriage in vectors. Studies outside Burkina Faso and papers on basic science were also excluded. Furthermore, studies reporting literature review findings were excluded.
Searched abstracts were imported to Mendeley to remove duplicates. Two reviewers then independently screened the titles and abstracts of the articles against the given inclusion and exclusion criteria. Articles considered relevant by both reviewers were kept and those considered relevant by just one reviewer were reviewed again by a third reviewer, to decide on their eligibility. Then, full text articles of relevant studies were retrieved.
Full text articles were assessed for their quality against the set criteria, which includes use of (1) scientifically sound methods, (2) appropriate laboratory procedures, (3) appropriate data analysis and, (4) reporting of accurate results. Articles were rated ‘good’, ‘medium’ and ‘poor’ using detail criteria indicated in Table 1. Only studies rated good, and medium were considered for inclusion.
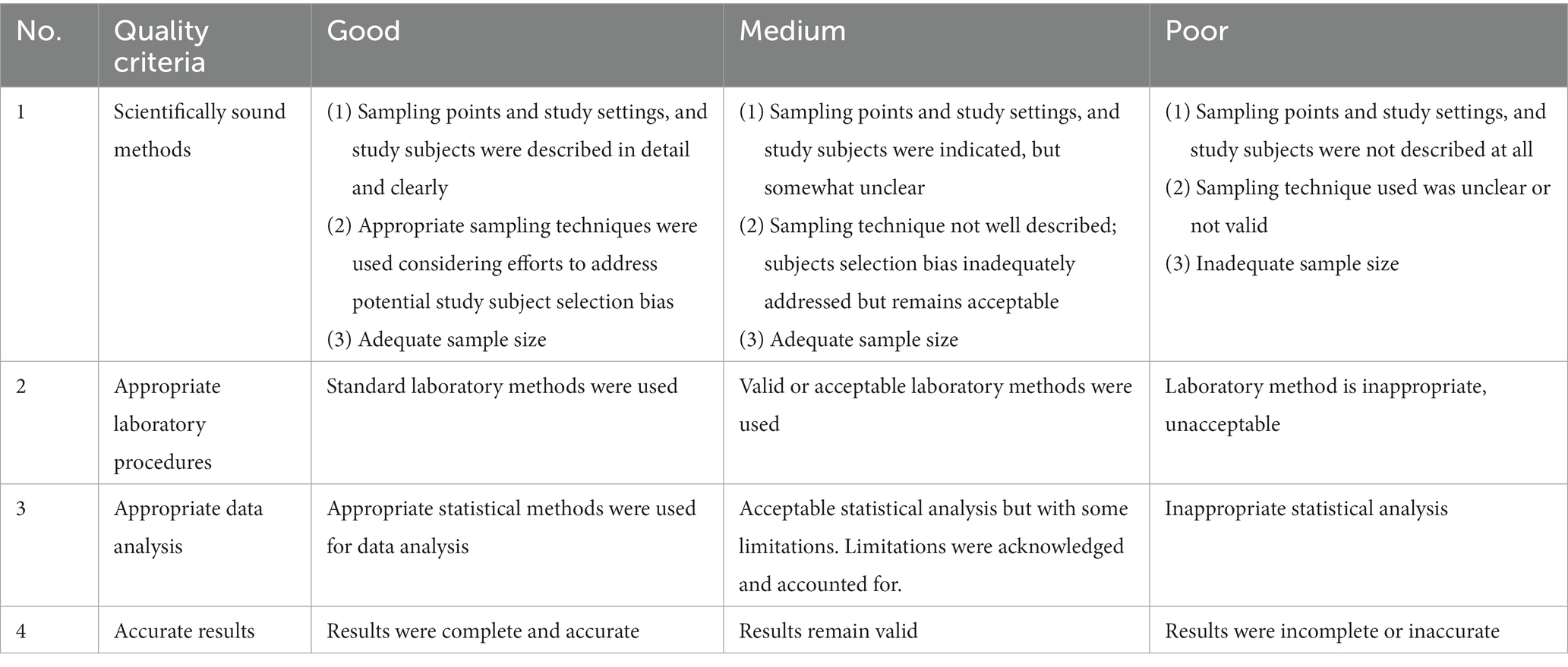
Table 1. Quality assessment checklist for articles included in the systematic review and meta-analysis of food hazards in Burkina Faso, 1990–2019.
One of the two reviewers extracted the required data which included authors, publication year, title, study design, study site, sampling points, food sample types, sample size, hazard group, and specific hazards.
We described study characteristics using descriptive statistics (frequencies). A random-effects model (DerSimonian–Laird method) was used to estimate a pooled prevalence and microbial load, and the corresponding 95% confidence intervals (95% CI). Heterogeneity across the studies was assessed using the Higgins I2 statistic, with 25, 50, and 75% values of I2 showing low, moderate and high heterogeneity, respectively (Higgins et al., 2003). Sources of heterogeneity across the studies were assessed using subgroup analysis, a value of p for this test of less than 0.1 indicates a statistically significant subgroup effect (Richardson et al., 2019). Studied food samples were grouped as chicken, cattle, sheep, fish, pig, dairy products, plant, vegetable, fruit, cereal, legume, and water samples, with this classification being used for our subgroup meta-analysis, where applicable. But samples from chicken were classified as chicken meat and those from cattle and sheep were classified as meat for subgroup meta-analysis purpose. That is hazard contamination level reported in one of these subgroups was summarized as subgroup pooled estimate, which contribute to the overall pooled estimate. A study reporting a given hazard in multiple sample types was used as different records (studies) for the meta-analysis-multiple entry of a given study in the meta-analysis. Forest plot was used to visualize outputs of meta-analysis. Prevalence estimate from a single study was kept in the meta-analysis (shown in forest plots) as it would contribute to the overall pooled estimate. The analysis was done using R (version 4.1.3, The R Foundation, Vienna, Austria), with meta package (version: 5.5–0) (Team, R.D.C, 2022).
A total of 188 unique records were identified from PubMed and CAB direct databases. Of these, 171 records were excluded based on the screening of the titles and abstracts. Full texts of the remaining 17 articles were retrieved and three articles among these were excluded during further assessment for eligibility and quality. Of these 14 articles, 36% (5/14) was rated good and 64% (9/14) rated medium using the quality assessment criteria, with 14 of them being included in the qualitative synthesis (Figure 1).
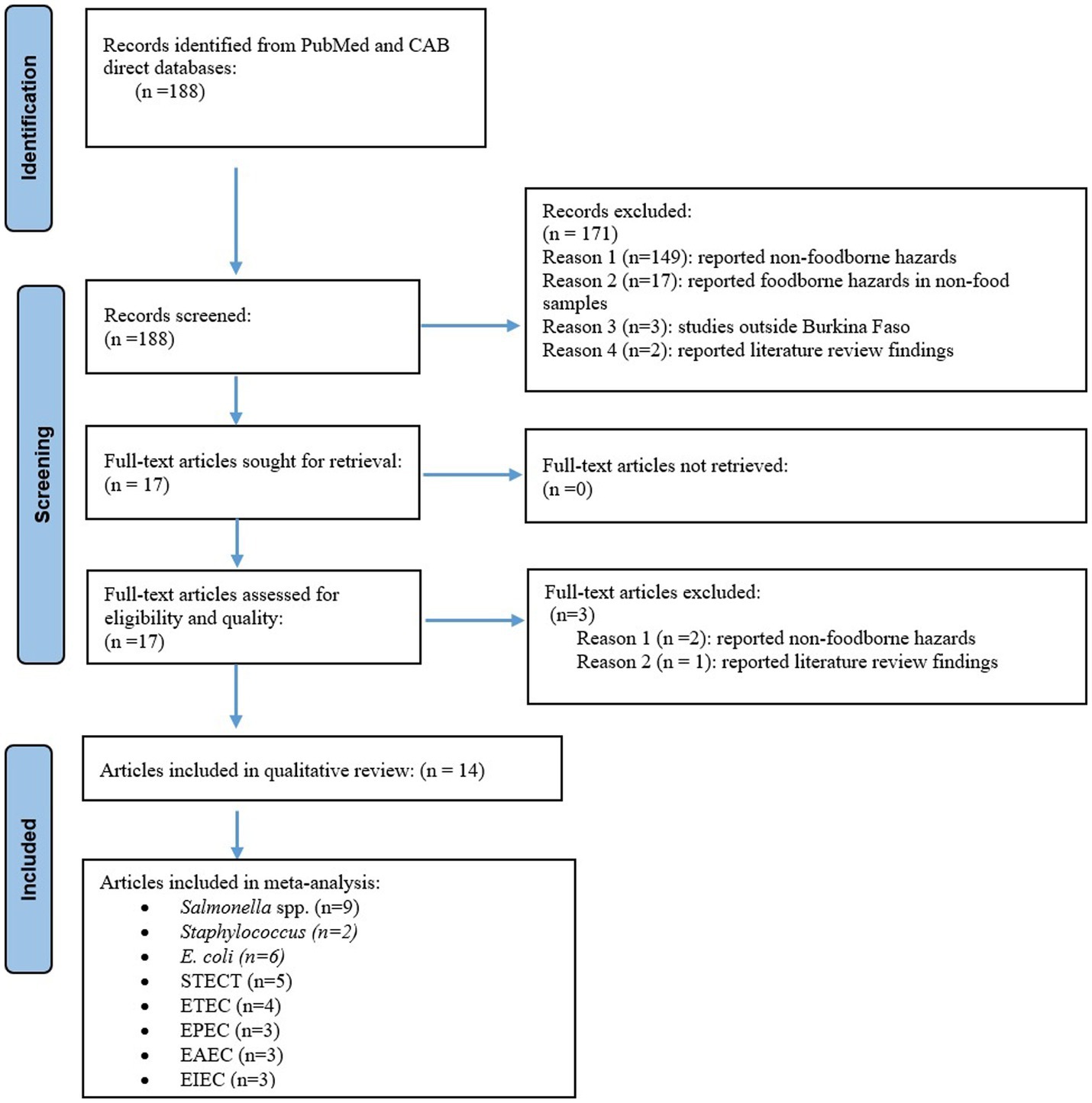
Figure 1. PRISMA flowchart showing abstract search, screening, and inclusion of eligible articles reporting foodborne hazards in Burkina Faso in the systematic review and meta-analysis, 1990–2019.
Eighty-six percent (12/14) of the studies were conducted between 2010 and 2019, and the remaining two studies were between 2000 and 2009, with no earlier studies found. About 71% (10/14) of the studies covered Ouagadougou, the capital city. While samples were collected from different sampling points, 57% (8/14) of the studies had retail markets as, at least, one of their sampling points. About 86% (12/14), 21%(3/14) and 7%(1/14) of the studies investigated bacteria (Ilboudo et al., 2009; Kagambèga et al., 2011, 2018; Martikainen et al., 2012; Kagambèga et al., 2012a,b; Touwendsida et al., 2017; Somda et al., 2018; Waré et al., 2018; Cissé et al., 2019), fungi (Ssepuuya et al., 2018; Waré et al., 2018; Cissé et al., 2019) and parasites (Bamba et al., 2016), respectively. In addition to the food hazards, the studies also assessed hygiene indicator bacteria including Enterobacterales (Supplementary material S1) (Barro et al., 2002), aerobic mesophilic bacteria (Supplementary material S2) (Barro et al., 2002; Somda et al., 2018) and thermotolerant coliforms (Supplementary material S3) (Somda et al., 2018; Cissé et al., 2019). The number of articles that reported bacteria, fungi and parasite seem to exceed the number of articles included in the review when added up because some articles reported multiple hazards.
Studies included in this review assessed foodborne hazards in different food samples:
– Chicken samples (chicken meat, whole chicken carcass, grilled chicken, flamed chicken, fumed chicken, chickens prepared around fire);
– Cattle samples (beef, beef intestine);
– Sheep samples (fresh mutton, grilled mutton);
– Fish samples (fish);
– Pig samples (diaphragm, heart, fresh pork);
– Dairy products (raw milk, pasteurized milk, yoghurt, degue, Lait cailléc);
– Plant samples (maari-baobab seed fermented product);
– Vegetable and fruit samples (lettuce, Bissap, zoom-koom, Limburgui, fresh mango);
– Cereal and legume samples (sorghum, infant flours, boiled millet, rice sauce, fatty rice, cooked bean, peanut paste, terracotta peas); and.
– Water samples (tap water, channel water, reservoir water, well water).
In this article, we classified chicken samples as chicken meat while beef and mutton as meat. Of the samples mentioned above, chicken samples were most investigated (43% (6/14)) by the reviewed studies (Table 2).
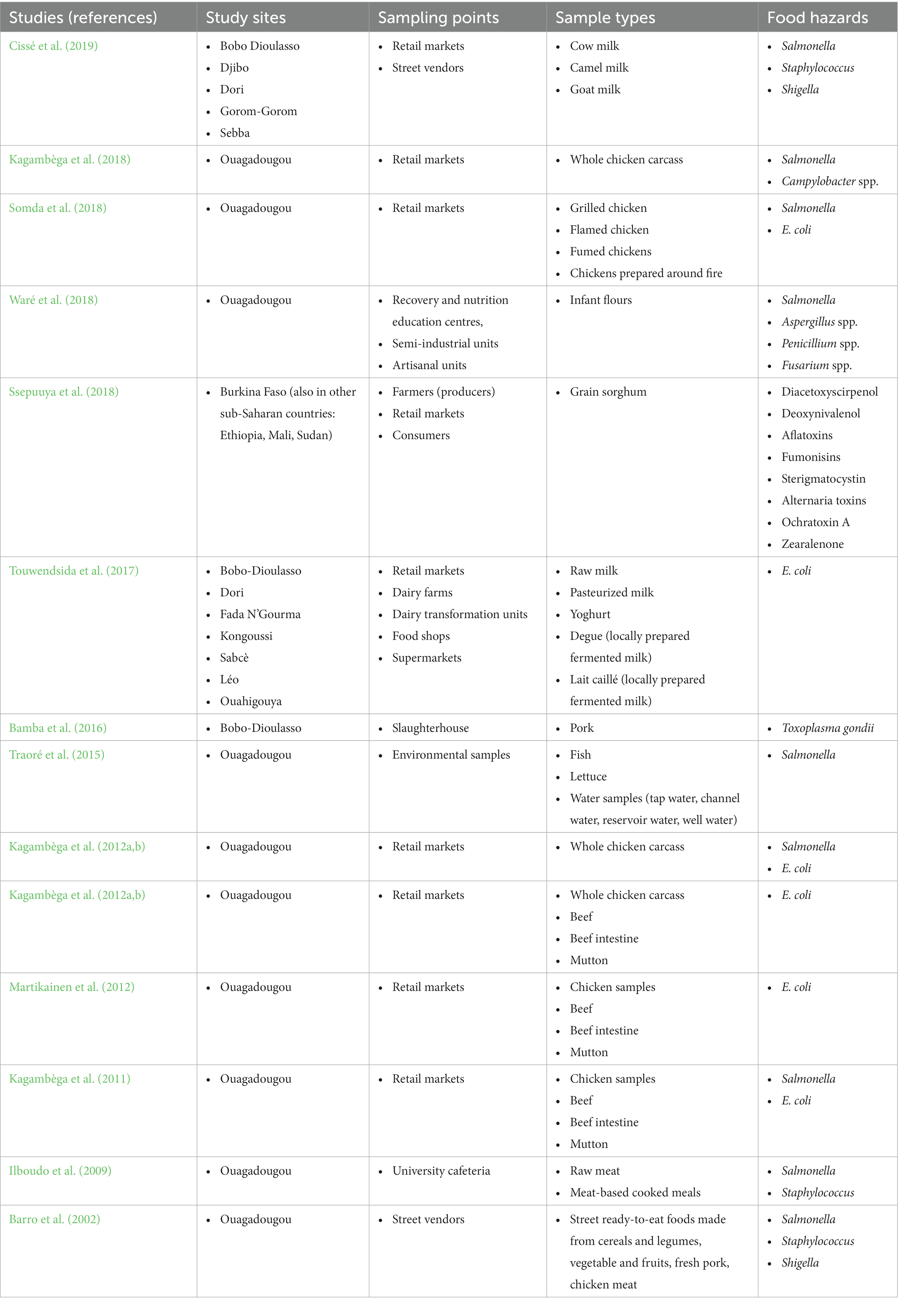
Table 2. Reviewed studies in the systematic review and meta-analysis of food hazards in Burkina Faso (1990–2019) with their study sites, sampling points, sample types and food hazards investigated.
About 75% (9/14) of the articles reported Salmonella spp. in different food matrices with information on prevalence, serotypes and antimicrobial sensitivity of the serotypes (Barro et al., 2002; Ilboudo et al., 2009; Kagambèga et al., 2012a,b; Traoré et al., 2015; Kagambèga et al., 2018; Somda et al., 2018; Waré et al., 2018; Cissé et al., 2019). We included the nine Salmonella studies (1,201 samples) in meta-analysis to estimate its pooled prevalence. Although individual studies reported Salmonella prevalence ranging from 0–90%, the meta-analysis found an overall pooled prevalence of 13% (95% CI: 8–21). Chicken samples accounted for about 30.3% of the pooled Salmonella prevalence found in this meta-analysis. We observed substantial variation of Salmonella prevalence across the studies (I2 = 85, 95% CI: 79–90%, p < 0.01). The subgroup analysis suggested that Salmonella prevalence varied by the sample types (Test for subgroup differences: χ27 = 41, df = 7, p < 0.01) (Figure 2).
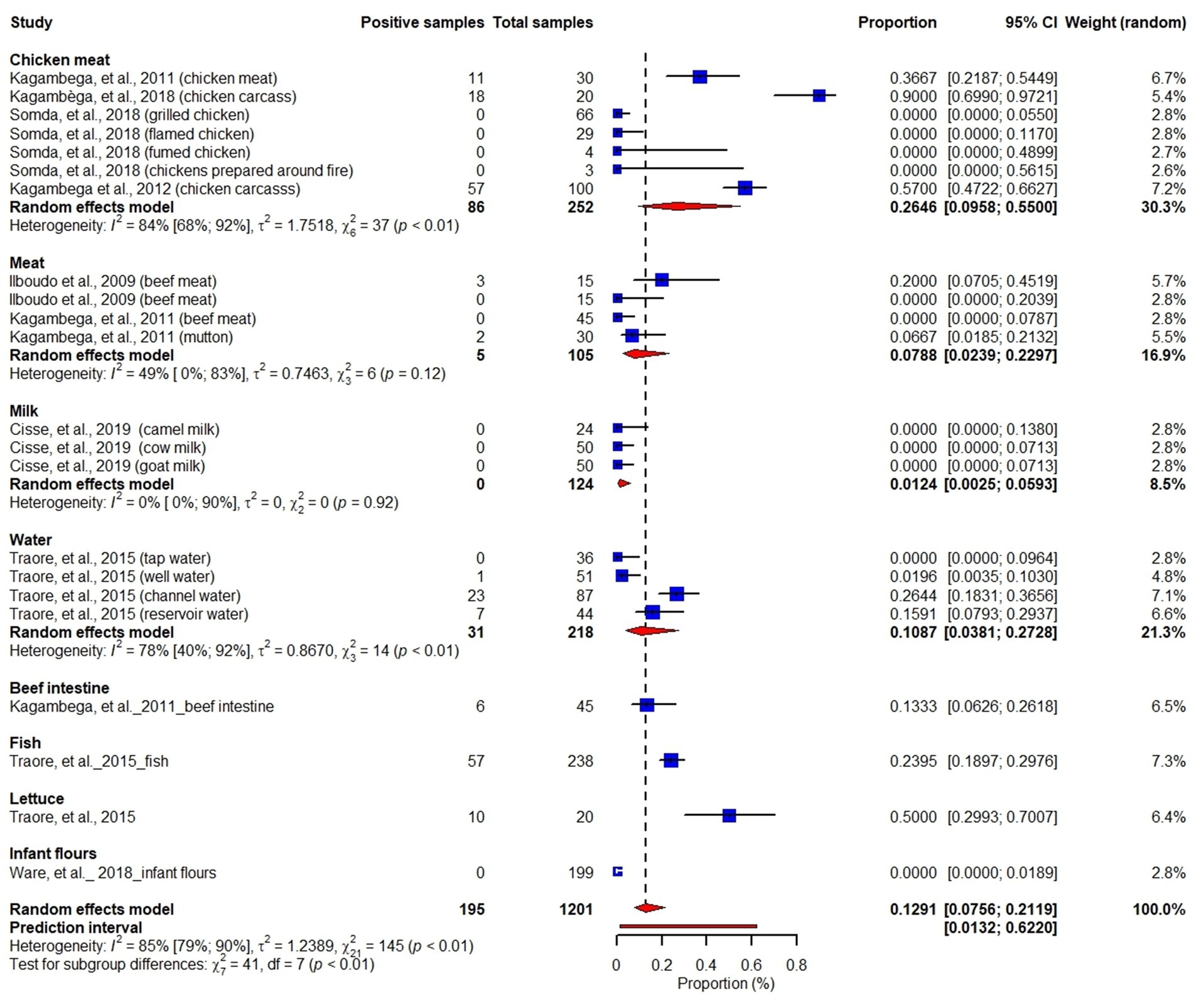
Figure 2. Forest plot showing prevalence of Salmonella in chicken meat, meat, milk, water, beef intestine, fish, lettuce and infant flours.
Of the studies included in our review, one article (Kagambèga et al., 2018) reported Campylobacter spp. in poultry feces and carcasses collected from retail markets in Ouagadougou. The study showed that 68% (70/103) of fecal samples and 50% (10/20) of carcass samples were positive for Campylobacter.
Of the reviewed studies, three studies (Barro et al., 2002; Ilboudo et al., 2009; Cissé et al., 2019) reported Staphylococcus in chicken, meat, dairy products, fruits, cereals and legumes. The overall pooled microbial load of Staphylococcus in the studied food samples was 3.2 log (95% CI: 2.8–3.6) CFU per g or ml of food, with the highest microbial load being in chicken samples: 4.5 log (95% CI: 2.8–6.2) CFU per g or ml of food (Figure 3). Our meta-analysis revealed substantial variation of microbial load of Staphylococcus across the studies (I2 = 100%, p < 0.01).
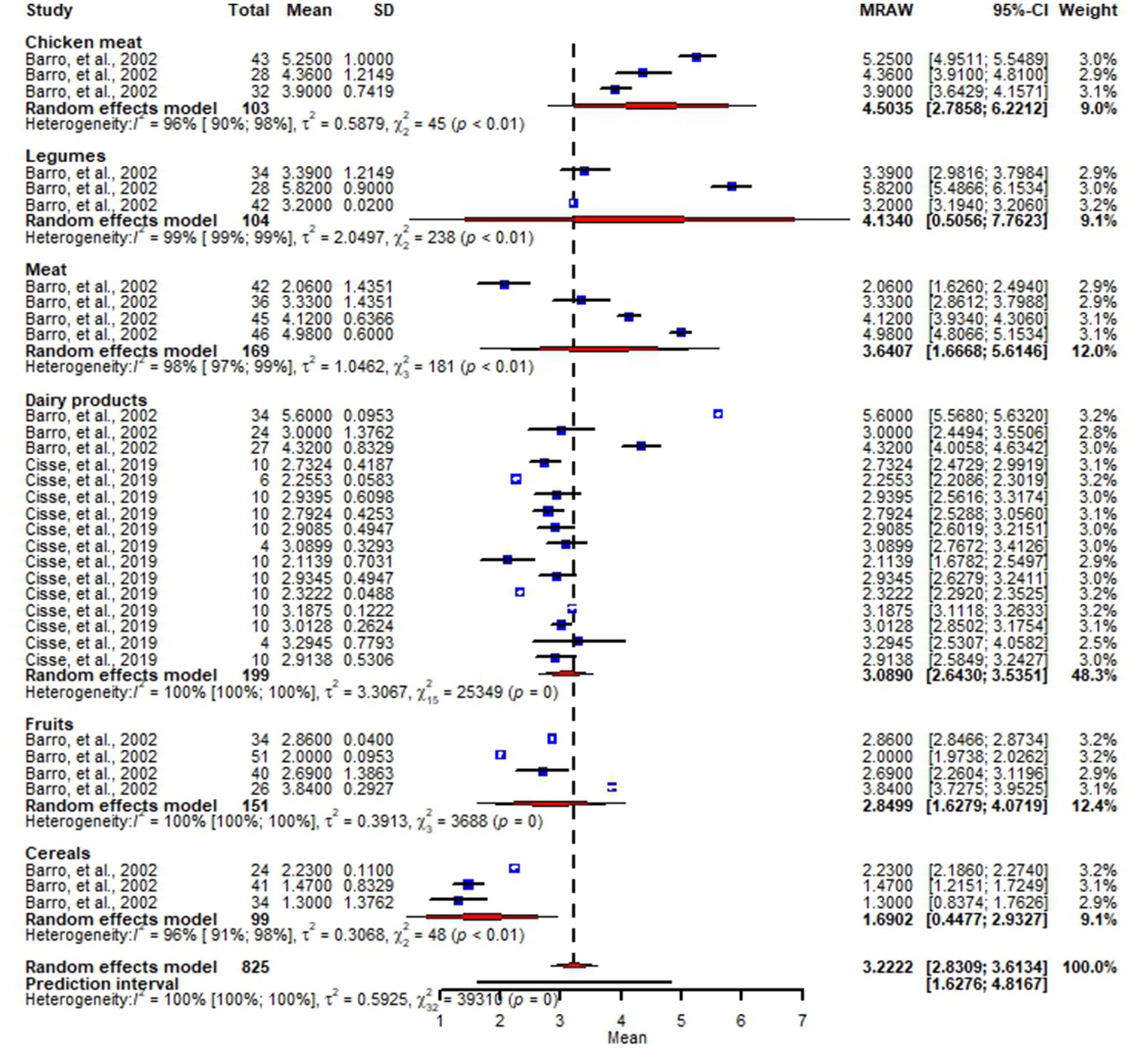
Figure 3. Forest plot showing microbial loads of Staphylococcus in chicken meat, legumes, meat, dairy products, fruits and cereals.
About 43% (6/14) of the articles included in our review (Kagambèga et al., 2011, 2012a,b; Martikainen et al., 2012; Touwendsida et al., 2017; Somda et al., 2018) investigated Escherichia coli (E. coli) in beef intestines, meat (beef, mutton), chicken meat and dairy products with information on prevalence, pathotypes and antimicrobial sensitivity of the strains. We included these six studies reporting E. coli with 1,143 total samples in our meta-analysis. While individual studies reported an E. coli prevalence ranging from 0 to 100%, our meta-analysis showed an overall pooled prevalence of 40% (random-effects model, 95% CI: 29–51), with the highest prevalence being in beef intestines: 62% (95% CI: 22–91) and the lowest in dairy products: 31% (95% CI: 17–50). We observed substantial variation of E. coli prevalence across the studies (I2 = 86, 95% CI: 80–90%, p < 0.01) (Figure 4). However, subgroup analysis of our meta-analysis showed weak evidence for variation of E. coli prevalence among sample types (Test for subgroup differences: χ23 = 41, df = 3, p = 0.44).
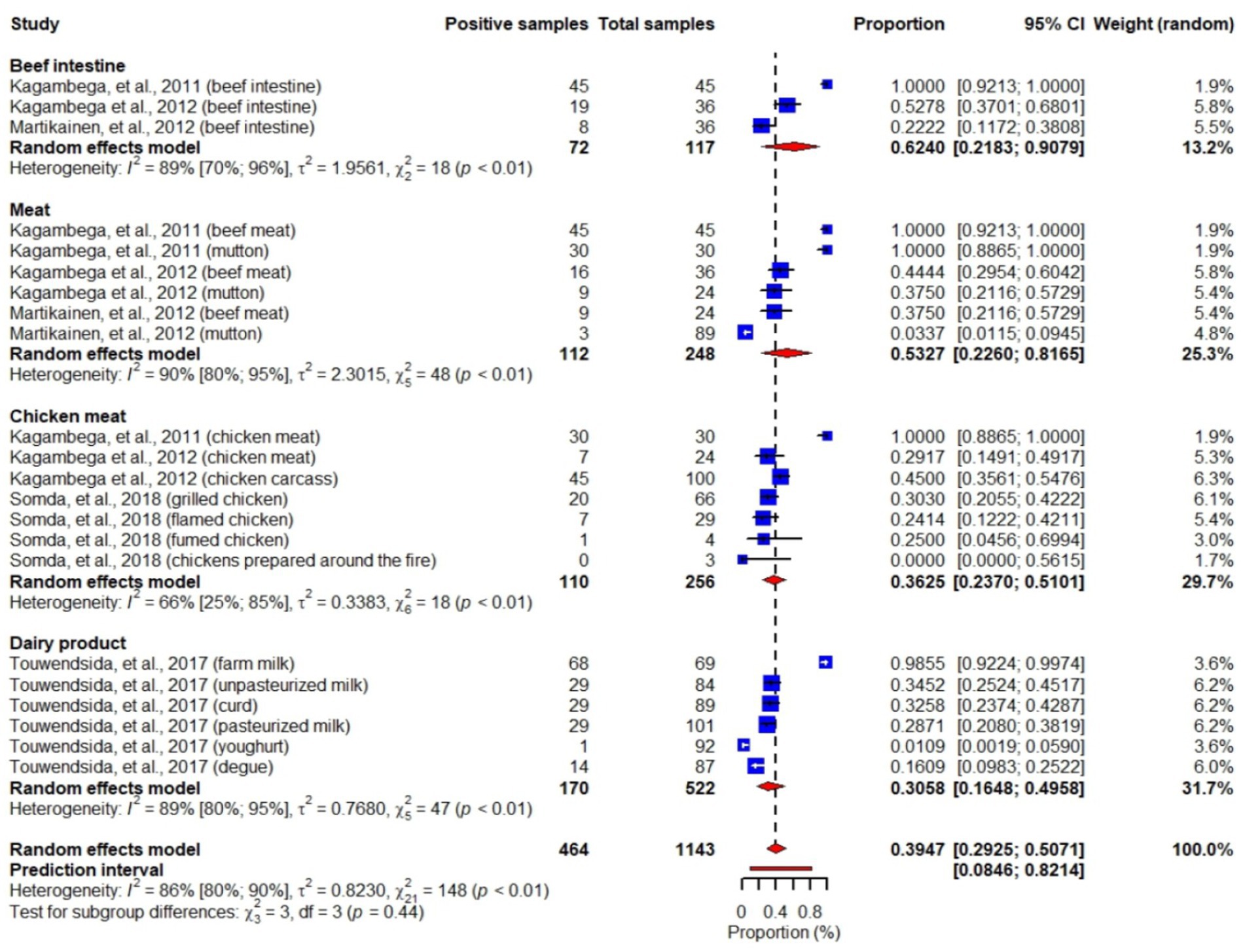
Figure 4. Forest plot showing E. coli prevalence in beef intestines, meats (beef, mutton), chicken meats and dairy products.
Studies included in this review also identified different pathotypes of E. coli including Shiga toxin-producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), and enteroaggregative E. coli (EAEC) (Martikainen et al., 2012; Kagambèga et al., 2012a,b; Somda et al., 2018). While STEC, ETEC, EPEC and EAEC were reported in meats, beef intestines, chicken meats and dairy products, EIEC was reported in chicken meat and dairy products. An overall pooled prevalence of STEC in the studied samples was 6% (95% CI: 3–11), highest in beet intestines: 25% (95% CI: 16–36) and lowest in dairy products: 0.6% (95% CI: 0.2–1.8) (Figure 5). We also found that pooled prevalence of ETEC, EPEC and EAEC were 3.3% (95% CI: 2.2–5.2) (Figure 6), 4.5% (95% CI: 1.7–11) (Figure 7) and 2% (95% CI: 1–4) (Figure 8), respectively. Reviewed studies also reported EIEC in chicken meat and dairy products with an overall contamination proportion of 1.1% (95% CI: 0.5–2.4) (Figure 9), higher in chicken meat: 2% (95% CI: 0.6–6.3).
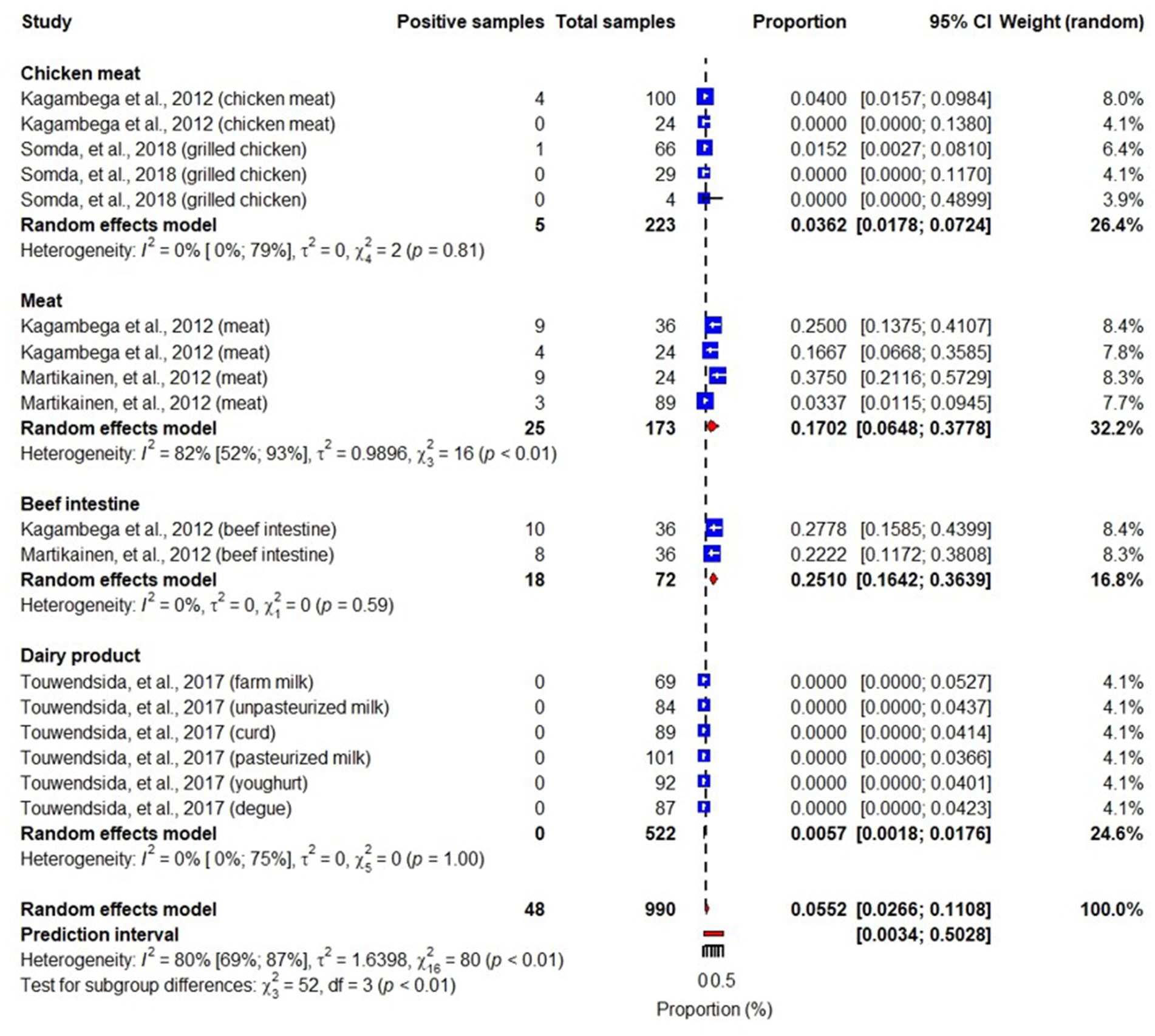
Figure 5. Forest plot showing STEC prevalence in meats, beef intestines, chicken meats and dairy products.
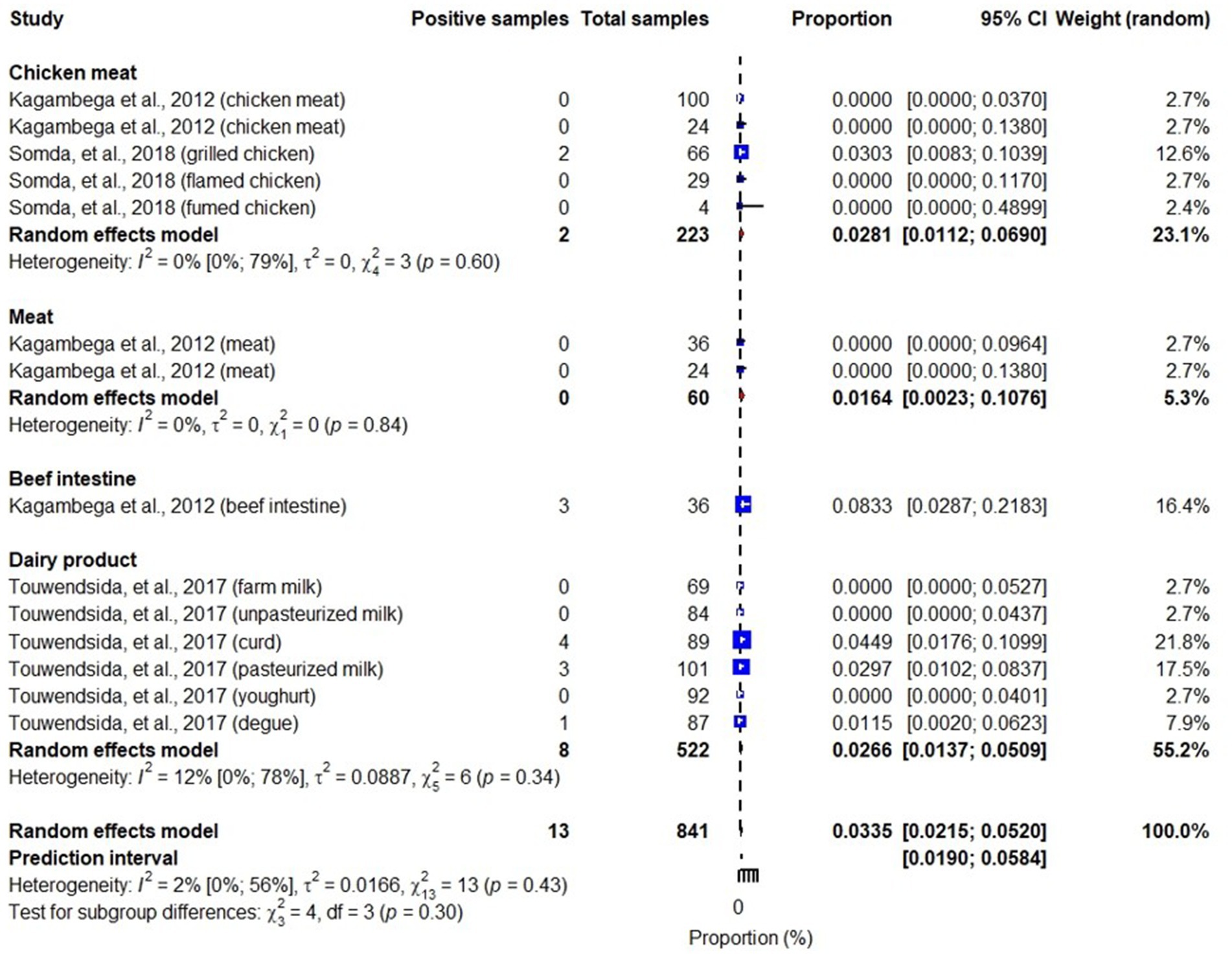
Figure 6. Forest plot showing ETEC prevalence in meats, beef intestines, chicken meats and dairy products.
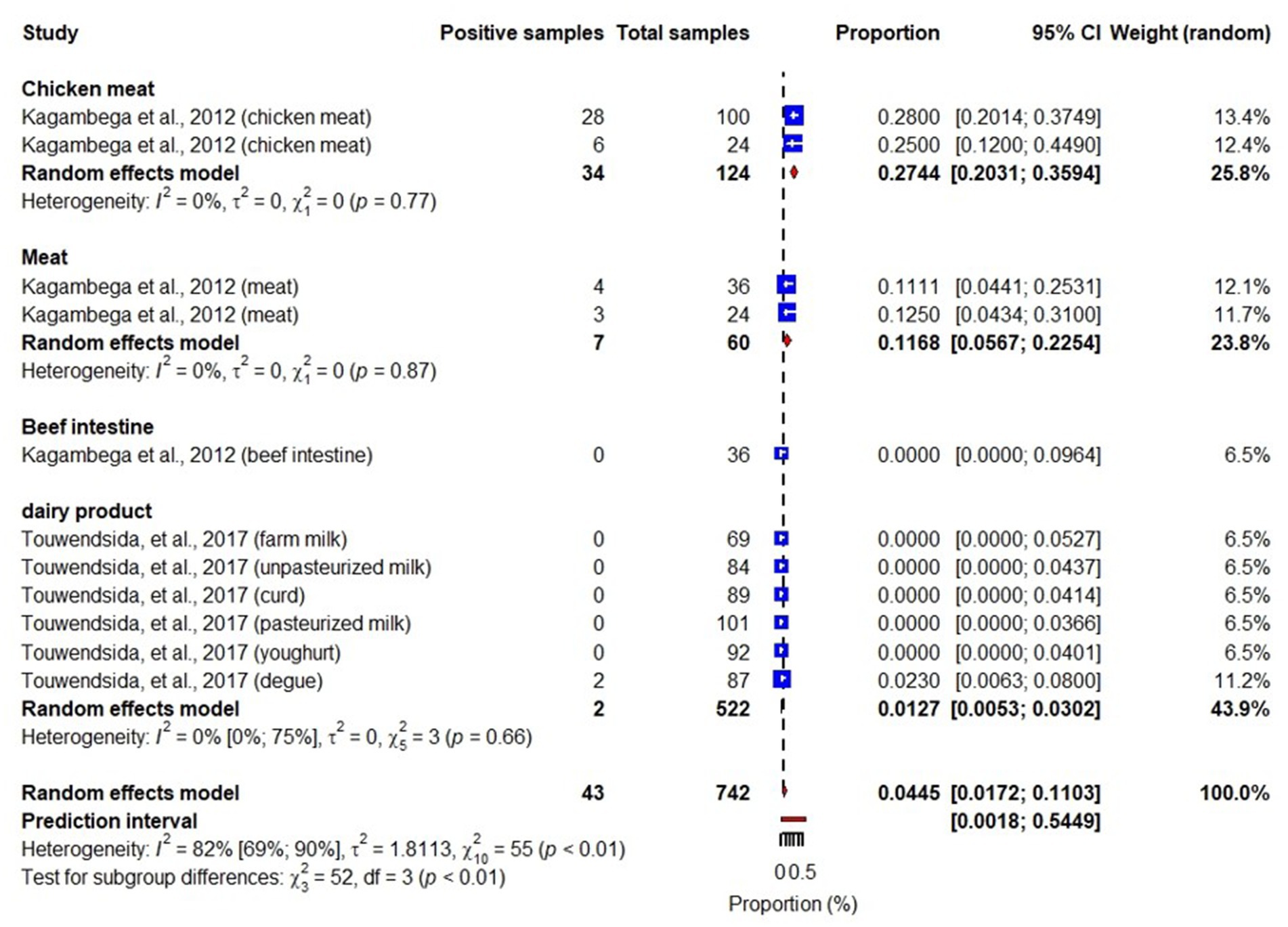
Figure 7. Forest plot showing EPEC prevalence in meats, beef intestines, chicken meats and dairy products.
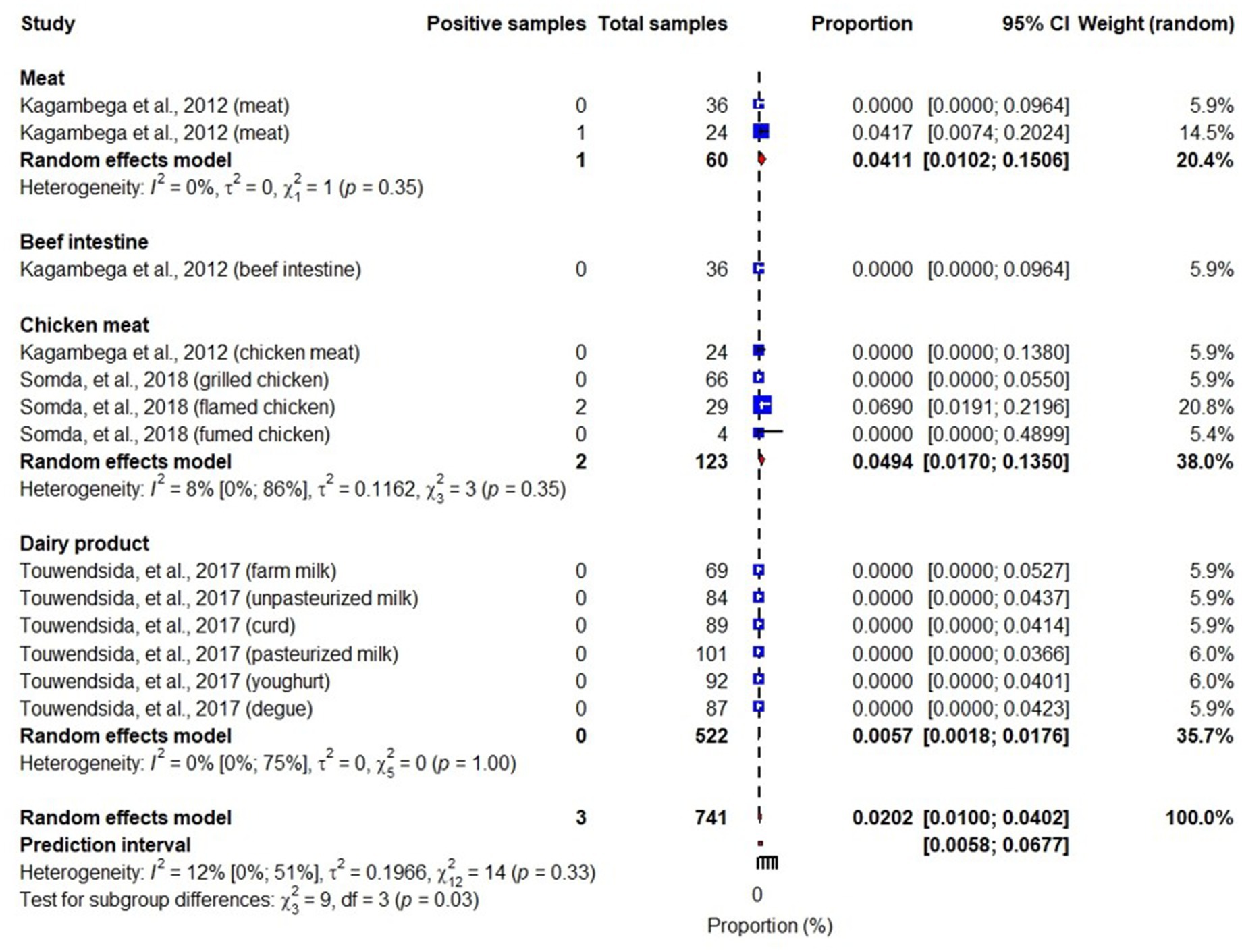
Figure 8. Forest plot showing EAEC prevalence in meat, beef intestines, chicken meat and dairy products.
Waré et al. (2018) reported fungal spp. prevalences ranging from 3 to 70% in food samples with a pooled prevalence of 17.3% (95% CI: 1.6–73). They reported higher Aspergillus spp. prevalence (70% (95% CI: 63–76)) in infant flours than Penicillium spp. and Fusarium spp. Another study showed that 10% (95% CI: 7–14) of sorghum samples were contaminated with mycotoxins. This study also indicated that children below 6 years old in Burkina Faso have the highest mean consumption of sorghum per kg body weight in sub-Saharan African, implicating that they are a group at risk for mycotoxin exposure (Ssepuuya et al., 2018) (Figure 10).
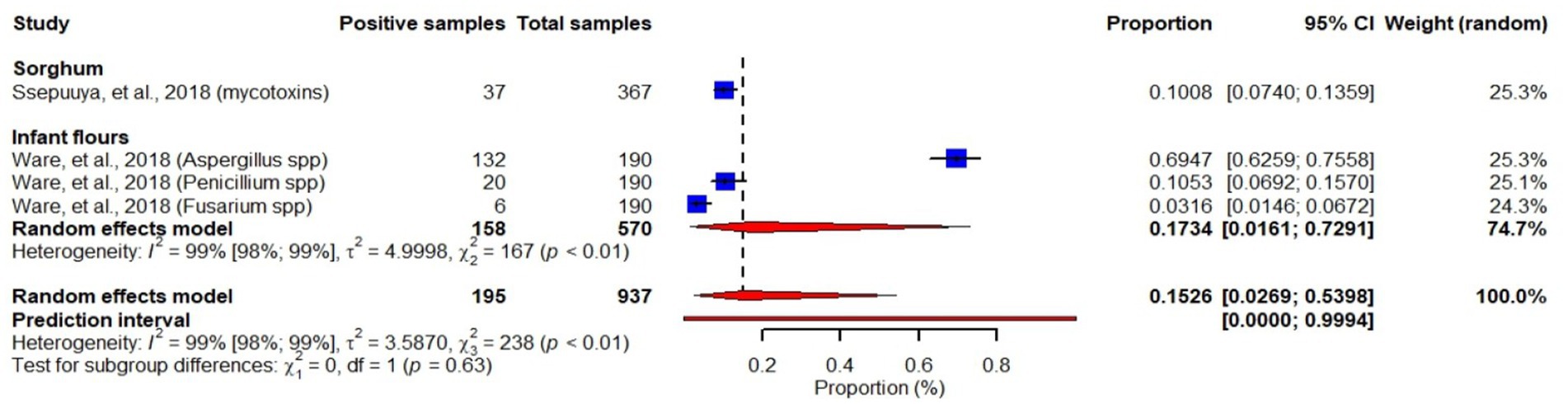
Figure 10. Forest plot showing prevalence of fungi and mycotoxins in infant flours and sorghum. Forest plot was just used to visualize the proportions as reported in the individual studies and the overall pooled prevalence was not considered in our report since mycotoxins reported by Ssepuuya et al. (2018) and fungal species reported by Waré et al. (2018) are not comparable.
Of the studies included in this review, one article reported a 29% (87/300) Toxoplasma gondii seropositivity in pig carcasses sampled from slaughterhouse of Bobo-Dioulasso, the second largest city of Burkina Faso (Bamba et al., 2016).
Our systematic review finding suggests increasing activities in food safety research in the country because we found a steady rise of the studies in food safety during the review period (1990–2019). However, most of these studies were conducted in the capital city of the country and assessed a small number of food hazards reflecting that the research is still inadequate in the country. Our review also implies widespread contamination of foods with different foodborne hazards including bacteria (Salmonella, Campylobacter, Staphylococcus, E. coli), fungi and parasites (Toxoplasma gondii), with bacteria the most studied hazard. In addition to the food hazards, the studies also investigated microbial loads of hygiene indicator bacteria such as Enterobacterales, aerobic mesophilic bacteria and thermotolerant coliforms in the food samples. Even if we reviewed and used the information of these hygiene indicator bacteria to support and interpret our findings on food hazards, we had not presented detail reports of these bacteria as the primary interest of this review was in foodborne hazards, but results are still reported as supplementary materials associated with this article (Supplementary materials S1–S3). The presence of these hygiene indicator bacteria in food samples suggests poor hygienic practices in food handling in Burkina Faso. Our review also indicated that foods have been contaminated with hazards along various food value chains such as beef, poultry, pork, fish, vegetables and crops, suggesting the extent of food safety problems in the country.
We found that most of the identified studies were conducted in the later decades of the review period (2010–2019), which indicates increasing efforts in food safety research. However, the sites of the studies were mostly geographically limited to the capital city of the country and only a few of the many foodborne hazards were assessed, showing that research remains inadequate. Furthermore, the studies investigated foodborne hazards in different food samples of chicken, cattle, sheep, fish, pig, dairy products, plants, vegetables and fruits, cereals and legumes, with most of the articles studying hazards in chicken meat. However, the studies often assessed bacteria, indicating limited focus on other foodborne hazards (although bacterial hazards are responsible for most of the foodborne disease burden). Consistent with our findings, food safety is often under-invested in Africa, especially in the dominant informal markets (GFSP, 2019). Food safety studies in Africa often have limited capacity-covering limited geographic areas of the study country and only a few foodborne hazards in their investigation scope (Paudyal et al., 2017).
Our present meta-analysis revealed contamination of chicken meat, meat, milk, water, beef intestine, fish, lettuce and infant flours with Salmonella, with higher pooled prevalence in chicken meats than meats and milk samples. Our review finding illustrated that meat products including-beef, mutton, chicken meat-appear to account for 44% of the pooled Salmonella prevalence reported in this review. Furthermore, subgroup analysis of our meta-analysis demonstrated that prevalence of Salmonella could be significantly affected by sample types. Salmonella have often been isolated from the gastrointestinal tract of animals, but with varying colonization levels in different hosts (Ferrari et al., 2019; Thomas et al., 2020). Chickens are asymptomatic carriers of Salmonella, leading to cross-contamination of the carcass during or after slaughtering, causing higher prevalence in poultry products (Dione et al., 2009). Poultry is the most common source of foodborne salmonellosis in the United States of America, with chicken, turkey and egg products attributing to nearly seven out of ten human cases (Thomas et al., 2020). Higher Salmonella prevalence in foods has been reported in Africa (Paudyal et al., 2017), with poultry in Africa having a higher prevalence of Salmonella than other food animals (Thomas et al., 2020). One study found that Salmonella were significantly more likely to be isolated or detected from western (including Burkina Faso) and central African food animal samples than samples from northern Africa (Thomas et al., 2020).
One paper reported Campylobacter spp. in chicken carcass, with 50% of the carcass samples being positive for the bacteria. However, relatively lower prevalence of Campylobacter in chicken meat was reported in different developing countries: 32.8% in Benin (Kouglenou et al., 2020), 38.8% in Ghana (Asuming-Bediako et al., 2022) and 26.6% in Malaysia (Sinulingga et al., 2020). Variation in Campylobacter prevalence could be due to animal species, season of the study and sample types (Ozbey and Tasdemir, 2014; Thomas et al., 2020).
Studies in this review found Staphylococcus in food products made from chicken meat, meat, dairy products, fruits, cereals and legumes, with an overall pooled bacterial load of 3.2 log (95% CI: 2.8–3.6) CFU per ml or g of food, highest load being in chicken samples: 4.5 log (95% CI: 2.8–6.2) cfu per ml or g of food. Buzon-Duran, et al. found a slightly higher bacterial loads of Staphylococcus in poultry meat, 4.07 ± 0.80 log10 cfu/g and Tsehayneh et al. (2021) reported comparable Staphylococcus bacterial loads in Ethiopia in raw meat from butcher shops, 3.40 ± 0.63 (log10 cfu/g). In general, the bacterial load counts requirement for Staphylococcus in food products should be below 20 cfu/g (Health Protection Agency, 2009), reflecting that finding of this meta-analysis demonstrated an exceedance of the maximum microbiological limit of the hazard in foods for human consumption.
This systematic review demonstrated presence of E. coli in beef intestines, meat (beef, mutton), chicken meat and dairy products, with the highest prevalence in beef intestines and the lowest in dairy products. Our research found a 40% overall pooled prevalence of E. coli in the studied food products. Previous studies reported comparable E. coli overall prevalence values in foods, such as 35% in Africa (Paudyal et al., 2017) and 34% in developing countries (Mengistu and Tolera, 2020). However, other studies reported lower prevalence values, such as 15% in Ethiopia (Assefa and Bihon, 2018) and 4% in China (Paudyal et al., 2018). Differences could be due to variations in food products as, for example, in our meta-analysis we found that while meat products (beef, beef intestines, mutton, chicken meat) accounted for about 70% of E. coli pooled prevalence, the rest was contributed by dairy products. However, sub-group analysis of E. coli prevalence in the present meta-analysis indicated that sample types have weak effects on E. coli prevalence. Although E. coli is a common inhabitant of gastrointestinal tract of animals and humans and not all strains are pathogenic, some E. coli are pathogenic capable of causing illness in humans such as diarrhea or illness outside of the intestinal tract (Levine, 1987; Kaper et al., 2004). Five out of the six diarrheagenic E. coli pathotypes including STEC, EPEC, ETEC, EIEC, and EAEC were reported by the reviewed studies included in the recent review. In addition, a previous study has illustrated that an estimated burden of ETEC associated with beef, dairy, poultry meat, and vegetables was found increasing in Burkina Faso from 2010 to 2017 (Havelaar et al., 2022). The presence of E. coli in foods is an indicator of both poor hygienic handling practices of foods and of presence of other fecal pathogens in foods (Health Protection Agency, 2009). Food can become contaminated with E. coli at all stages of food production and retail. Evisceration during slaughtering and defecation during milking are critical events where E. coli are likely to enter food products destined for human consumption (Hussein, 2007).
Several factors seem to contribute to the higher contamination levels of studied food samples with the identified food hazards in Burkina Faso. Poultry is one of the main asymptomatic carriers of Campylobacter and Salmonella (Thomas et al., 2020), which might cause the higher contamination level of chicken samples with these hazards especially during slaughtering due to carcass cross-contamination. Studies in Burkina Faso have provided evidence that chicken retailers slaughter chickens in a traditional way-they themselves slaughter chickens in marketplaces, executing bleeding, plucking, evisceration, and cutting on the same table; rinse carcasses in the same bucket of water and sold off a table at ambient temperature without any type of protection from dust and pests at any point during the day. Moreover, poultry vaccinations are not mandatory in the country which leads to higher pathogen prevalence in the poultry population, causing higher carcass cross-contamination during slaughtering (Kagambèga et al., 2018). Food retailers in Burkina Faso have inadequate safe food handling knowledge and practices (Barro et al., 2002; Ilboudo et al., 2009; Kagambèga et al., 2018). Furthermore, we found, in our meta-analysis, higher microbial loads of hygiene indicator bacteria including Enterobacterales, aerobic mesophilic bacteria and thermotolerant coliforms in different food commodities representing poor hygienic practices related to food handling (Health Protection Agency, 2009), which might contribute to the higher contamination of studied food commodities with the hazards in the country.
One of the unique strengths of this systematic review is its ability to do a meta-analysis, providing pooled contamination levels of the hazards in the studied food samples. To the best of our knowledge, this is the first systematic review of foodborne hazards in Burkina Faso using a meta-analysis. A meta-analysis generates pooled estimate results through combining individual studies’ results which is more informative compared to narrative reviews’ findings. Such pooled estimates from a meta-analysis would enable informed policy-and decision-making (Van Wely, 2014).
However, this review was subjected to two main limitations. First, high heterogeneity was observed among some studies included in this meta-analysis, although, combining studies with low heterogeneity is recommended to be sure that the studies’ findings are comparable. However, as systematic reviews synthesize results from studies that are diverse in different aspects such as sampling techniques, laboratory methods, sample size and so on, it is almost inevitable to see some heterogeneity across the studies (Higgins et al., 2002). We conducted subgroup analysis by categorizing food products that share common characteristics such as meat, chicken meat, intestines, dairy product and so on to understand the sources of heterogeneity. We found that the prevalence of some hazards such as Salmonella could be affected by sample types, reflecting that even if studies are comparable in many ways heterogeneity could arise from the sample types investigated. Strict inclusion and exclusion criteria were also used to include only relevant studies in the review. Second, results of few studies were combined in the recent meta-analysis, for example, only two studies were used in the meta-analysis of Staphylococcus. Although results from two primary studies can be combined in a meta-analysis, combining findings of more studies would improve the quality of meta-analysis findings. It is therefore recommended to cautiously interpret the findings based on the limitations.
Our review found more food safety studies in recent years, indicating growing awareness of this problem. However, many important hazards receive little research attention and there is sparse information on food hazards outside the capital of Burkina Faso. In addition, most studies were at the retail point and there was a lack of information from other important nodes of the food value chains (e.g., production, processing, consumption).
Our review findings also demonstrated high prevalence of contamination of foods with hazards, including Salmonella spp., Campylobacter spp., Staphylococcus spp., toxigenic E. coli, fungi and Toxoplasma gondii. The presence of hygiene indicator bacteria such as Enterobacterales, aerobic mesophilic bacteria and thermotolerant coliforms in foods is indicative of poor hygiene practices while handling foods. These may include undercooking, cross contamination from raw food especially meat, food handlers or food contact surfaces as well as poor temperature and time control. Our findings indicated that a variety of food samples were reported contaminated with food hazards reflecting the need to target different food value chains-including beef, poultry, pork, vegetables, cereals, fruits and water-for food safety interventions. Consistent with our findings, a review on food hazards in Ethiopia showed that food contamination with hazards is common in beef, poultry and vegetable food value chains (Gazu et al., 2023). The widespread contamination of foods with hazards in the country raises public health concerns, especially for vulnerable groups such as children, the elderly, pregnant women and the immunocompromised.
Our results suggest that interventions are urgently needed to improve the safety of food retailed in the capital city of the country. More information is needed on food safety in outside of the capital city of the country; in different food value chains; at the different points along the food value chains and on a variety of food hazards. We suggested food safety interventions targeting different food value chains, and improved monitoring of hazards in food in the country.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
DG conceived, designed, supervised and managed the project and received funding for the study. SA, KA, and LG searched and screened abstracts, retrieved eligible studies. GD assessed relevant studies quality, extracted data, conducted meta-analysis and wrote original draft of the manuscript. TK-J, SA, KA, LG, FM, KR, JL, FS, PU, TG, GI, and DG reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Bill & Melinda Gates Foundation [INV-008430 (OPP1195588)]. Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The work was also supported by the German Federal Ministry for Economic Cooperation and Development (BMZ) through the One Health Research, Education and Outreach Centre in Africa (OHRECA) led by ILRI. Additional support was received from CGIAR Research Program on Agriculture for Nutrition and Health (A4NH). Support was also provided by UK aid from the UK Foreign, Commonwealth and Development Office.
This activity is part of the urban food markets in Africa: incentivizing food safety using a pull-push approach which is supported by the Bill & Melinda Gates Foundation, German Federal Ministry for Economic Cooperation and Development (BMZ) through the One Health Research, Education and Outreach Centre in Africa (OHRECA) led by ILRI, the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH) led by the International Food Policy Research Institute and the Foreign, Commonwealth and Development Office (FCDO) of the UK Government. We also acknowledge the CGIAR Fund Donors (https://www.cgiar.org/funders).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1232992/full#supplementary-material
Alonso, S., Lindahl, J., Roesel, K., Traore, S. G., Yobouet, B. A., Ndour, A. P. N., et al. (2016). Where literature is scarce: observations and lessons learnt from four systematic reviews of zoonoses in African countries. Anim. Health Res. Rev. 17, 28–38. doi: 10.1017/S1466252316000104
Assefa, A., and Bihon, A. (2018). A systematic review and meta-analysis of prevalence of Escherichia coli in foods of animal origin in Ethiopia. Heliyon 4:e00716. doi: 10.1016/j.heliyon.2018.e00716
Asuming-Bediako, N., Kunadu, A. P.-H., Jordan, D., Abraham, S., and Habib, I. (2022). Prevalence and antimicrobial susceptibility pattern of Campylobacter jejuni in raw retail chicken meat in metropolitan Accra Ghana. Int. J. Food Microbiol. 376:109760. doi: 10.1016/j.ijfoodmicro.2022.109760
Bamba, S., Halos, L., Tarnagda, Z., Alanio, A., Macé, P., Moukoury, S., et al. (2016). Seroprevalence of toxoplasma gondii and direct genotyping using minisequencing in free-range pigs in Burkina Faso. Int. J. Food Microbiol. 230, 10–15. doi: 10.1016/j.ijfoodmicro.2016.04.016
Barro, N., Ouattara, C., Nikiema, P., and Ouattara, A. (2002). Microbial quality assessment of some street food widely consumed in Ouagadougou, Burkina Faso. Sante :12.
Berger, C. N., Sodha, S. V., Shaw, R. K., Griffin, P. M., Pink, D., Hand, P., et al. (2010). Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 12, 2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x
Cissé, H., Muandze-Nzambe, J. U., Somda, N. S., Sawadogo, A., Drabo, S. M., Tapsoba, F., et al. (2019). Assessment of safety and quality of fermented milk of camels, cows, and goats sold and consumed in five localities of Burkina Faso. Vet. World 12, 295–304. doi: 10.14202/vetworld.2019.295-304
Dinede, G., Abagero, A., and Tolosa, T. (2020). Cholera outbreak in Addis Ababa, Ethiopia: a case-control study. PLoS One 15:e0235440. doi: 10.1371/JOURNAL.PONE.0235440
Dione, M. M., Ieven, M., Garin, B., Marcotty, T., and Geerts, S. (2009). Prevalence and antimicrobial resistance of Salmonella isolated from broiler farms, chicken carcasses, and street-vended restaurants in Casamance. Senegal. J. Food Prot. 72, 2423–2427. doi: 10.4315/0362-028X-72.11.2423
European Commission Regulation (2005). On microbiological criteria for foodstuffs (text with EEA relevance), 2005.
Ferrari, R. G., Rosario, D. K. A., Cunha-Neto, A., Mano, S. B., Figueiredo, E. E. S., and Conte-Juniora, C. A. (2019). Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl. Environ. Microbiol. 85, 1–21. doi: 10.1128/AEM.00591-19
Gal-Mor, O., Boyle, E. C., and Grassl, G. A. (2014). Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 5, 1–10. doi: 10.3389/fmicb.2014.00391
Gazu, L., Alonso, S., Mutua, F., Roesel, K., Lindahl, J. F., Amenu, K., et al. (2023). Foodborne Disease Hazards and Burden in Ethiopia : A Systematic Literature Review. Front. Sustain. Food Syst. 7:1058977. doi: 10.3389/fsufs.2023.1058977
GFSP Global food safety partnership.Food Safety in Africa. PAST ENDEAVORS AND FUTURE DIRECTIONS. (2019).
Grace, D. (2015). Food safety in low and middle income countries. Int. J. Environ. Res. Public Health 12, 10490–10507. doi: 10.3390/ijerph120910490
Grace, D., Wu, F., and Havelaar, A. H. (2020). MILK symposium review: foodborne diseases from milk and milk products in developing countries—review of causes and health and economic implications. J. Dairy Sci. 103, 9715–9729. doi: 10.3168/jds.2020-18323
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12:e1001923. doi: 10.1371/journal.pmed.1001923
Havelaar, A. H., Sapp, A. C., Amaya, M. P., Nane, G. F., Morgan, K. M., Devleesschauwer, B., et al. (2022). Burden of foodborne disease due to bacterial hazards associated with beef, dairy, poultry meat, and vegetables in Ethiopia and Burkina Faso, 2017. Front. Sustain. Food Syst. 6:1024560. doi: 10.3389/fsufs.2022.1024560
Health Protection Agency. (2009). Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market.
Higgins, J., Thompson, S., Deeks, J., and Altman, D. (2002). Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J. Heal. Serv. Res. Policy 7, 51–61. doi: 10.1258/1355819021927674
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hussein, H. S. (2007). Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85, E63–E72. doi: 10.2527/jas.2006-421
Ilboudo, A. J., Savadogo, A., Barro, N., Ouedraogo, M., and Traore, A. S. (2009). Hygienic quality of meat used in institutional food services: university cafeterias in Ouagadougou (Burkina Faso). Cahiers d’études et de recherches francophones/Santé, 19, 195–199.
Kagambèga, A., Barro, N., Traoré, A. S., Siitonen, A., and Haukka, K. (2012b). Characterization of Salmonella enterica and detection of the virulence genes specific to diarrheagenic escherichia coli from poultry carcasses in Ouagadougou. Burkina Faso. Foodborne Pathog. Dis. 9, 589–593. doi: 10.1089/fpd.2011.1071
Kagambèga, A., Haukka, K., Siitonen, A., Traoré, A. S., and Barro, N. (2011). Prevalence of Salmonella enterica and the hygienic indicator Escherichia coli in raw meat at markets in Ouagadougou. Burkina Faso. J. Food Prot. 74, 1547–1551. doi: 10.4315/0362-028X.JFP-11-124
Kagambèga, A., Martikainen, O., Lienemann, T., Siitonen, A., Traoré, A. S., Barro, N., et al. (2012a). Diarrheagenic Escherichia coli detected by 16-plex PCR in raw meat and beef intestines sold at local markets in Ouagadougou. Burkina Faso. Int. J. Food Microbiol. 153, 154–158. doi: 10.1016/j.ijfoodmicro.2011.10.032
Kagambèga, A., Thibodeau, A., Trinetta, V., Soro, D. K., Sama, F. N., Bako, É., et al. (2018). Salmonella spp. and Campylobacter spp. in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Sci. Nutr. 6, 1601–1606. doi: 10.1002/fsn3.725
Kaper, J. B., Nataro, J. P., and Mobley, H. L. T. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Kouglenou, S. D., Agbankpe, A. J., Dougnon, V., Djeuda, A. D., Deguenon, E., Hidjo, M., et al. (2020). Prevalence and susceptibility to antibiotics from Campylobacter jejuni and Campylobacter coli isolated from chicken meat in southern Benin. West Africa. BMC Res. Notes 13, 1–6. doi: 10.1186/s13104-020-05150-x
Levine, M. M. (1987). Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 155, 377–389. doi: 10.1093/infdis/155.3.377
Martikainen, O., Kagambèga, A., Bonkoungou, I. J., Barro, N., Siitonen, A., and Haukka, K. (2012). Characterization of shigatoxigenic escherichia coli strains from Burkina Faso. Foodborne Pathog. Dis. 9, 1015–1021. doi: 10.1089/fpd.2012.1228
Mengistu, D. A., and Tolera, S. T. (2020). Prevalence of microorganisms of public health significance in ready-to-eat foods sold in developing countries: systematic review and Meta-analysis. Int. J. Food Sci. 2020, 1–9. doi: 10.1155/2020/8867250
Oduori, D. O., and Kwoba, E. (2022). Assessment of foodborne disease hazards in beverages consumed in Nigeria: a systematic literature review. Assessment of Foodborne Disease Hazards in Beverages Consumed in Nigeria 19, 1–18. doi: 10.1089/fpd.2021.0043
Ozbey, G., and Tasdemir, B. (2014). Variazioni stagionali e resistenza ad antibiotici del Campylobacter in campioni di carne di pollame in turchia. Vet. Ital. 50, 277–283. doi: 10.12834/VetIt.170.2543.1
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an updated guideline for reporting systematic reviews. BMJ 88:372. doi: 10.1136/bmj.n71
Paudyal, N., Anihouvi, V., Hounhouigan, J., Matsheka, M. I., Sekwati-Monang, B., Amoa-Awua, W., et al. (2017). Prevalence of foodborne pathogens in food from selected African countries – a meta-analysis. Int. J. Food Microbiol. 249, 35–43. doi: 10.1016/j.ijfoodmicro.2017.03.002
Paudyal, N., Pan, H., Liao, X., Zhang, X., Li, X., Fang, W., et al. (2018). A Meta-analysis of major foodborne pathogens in Chinese food commodities between 2006 and 2016. Foodborne Pathog. Dis. 15, 187–197. doi: 10.1089/fpd.2017.2417
Richardson, M., Garner, P., and Donegan, S. (2019). Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin. Epidemiol. Glob. Heal. 7, 192–198. doi: 10.1016/j.cegh.2018.05.005
Sinulingga, T. S., Aziz, S. A., Bitrus, A. A., Zunita, Z., and Abu, J. (2020). Occurrence of Campylobacter species from broiler chickens and chicken meat in Malaysia. Trop. Anim. Health Prod. 52, 151–157. doi: 10.1007/s11250-019-01995-y
Somda, N. S., Bonkoungou, O. J. I., Zongo, C., Kagambèga, A., Bassolé, I. H. N., Traoré, Y., et al. (2018). Safety of ready-to-eat chicken in Burkina Faso: microbiological quality, antibiotic resistance, and virulence genes in Escherichia coli isolated from chicken samples of Ouagadougou. Food Sci. Nutr. 6, 1077–1084. doi: 10.1002/fsn3.650
Ssepuuya, G., Van Poucke, C., Ediage, E. N., Mulholland, C., Tritscher, A., Verger, P., et al. (2018). Mycotoxin contamination of sorghum and its contribution to human dietary exposure in four sub-Saharan countries. Food Addit. Contam. – Part A Chem. Anal. Control. Expo. Risk Assess. 35, 1384–1393. doi: 10.1080/19440049.2018.1461253
Team, R.D.C. R Core team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2022).
Thomas, K. M., de Glanville, W. A., Barker, G. C., Benschop, J., Buza, J. J., Cleaveland, S., et al. (2020). Prevalence of Campylobacter and Salmonella in African food animals and meat: a systematic review and meta-analysis. Int. J. Food Microbiol. 315:108382. doi: 10.1016/j.ijfoodmicro.2019.108382
Touwendsida, S. B., Bissoume, S.-B., Hadiza, B. I., Gertrude, B. T., Rene, D., Abdoul, A. W., et al. (2017). Isolation and characterization of enteropathogenic and enterotoxinogenic Escherichia coli from dairy products consumed in Burkina Faso. African J. Microbiol. Res. 11, 537–545. doi: 10.5897/ajmr2017.8485
Traoré, O., Nyholm, O., Siitonen, A., Bonkoungou, I. J. O., Traoré, A. S., Barro, N., et al. (2015). Prevalence and diversity of Salmonella enterica in water, fish and lettuce in Ouagadougou. Burkina Faso. BMC Microbiol. 15, 1–7. doi: 10.1186/s12866-015-0484-7
Tsehayneh, B., Yayeh, T., and Agmas, B. (2021). Evaluation of bacterial load and antibiotic resistance pattern of staphylococcus aureus from ready-to-eat raw beef in Bahir Dar City. Ethiopia. Int. J. Microbiol. 2021. doi: 10.1155/2021/5560596
Van Wely, M. (2014). The good, the bad and the ugly: Meta-analyses. Hum. Reprod. 29, 1622–1626. doi: 10.1093/humrep/deu127
Waré, L., Nikièma, P., Meile, A. C., Kaboré, J., Fontana, S., Durand, A., et al. (2018). Microbiological safety of flours used in follow up for infant formulas produced in Ouagadougou, Burkina Faso. AIMS Microbiol. 4, 347–361. doi: 10.3934/microbiol.2018.2.347
World Health Organization (2015). WHO estimates of the global burden of foodborne diseases: executive summary. WHO Exec. Summ. :257.
World Health Organization Food safety. (n.d.) Available at: https://www.who.int/news-room/fact-sheets/detail/food-safety.
Keywords: foods, foodborne disease, foodborne hazard, food poisonings, food safety, Burkina Faso
Citation: Dinede G, Amenu K, Alonso S, Gazu L, Mutua F, Roesel K, Lindahl JF, Sousa FM, Ulrich P, Guadu T, Dione M, Ilboudo G, Knight-Jones TJD and Grace D (2023) Foodborne hazards in food in Burkina Faso, 1990–2019: a systematic review and meta-analysis. Front. Sustain. Food Syst. 7:1232992. doi: 10.3389/fsufs.2023.1232992
Received: 01 June 2023; Accepted: 05 October 2023;
Published: 20 October 2023.
Edited by:
Joshua B. Gurtler, Eastern Regional Research Center, Agricultural Research Service (USDA), United StatesReviewed by:
X. Deng, University of Georgia, United StatesCopyright © 2023 Dinede, Amenu, Alonso, Gazu, Mutua, Roesel, Lindahl, Sousa, Ulrich, Guadu, Dione, Ilboudo, Knight-Jones and Grace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Getachew Dinede, ZGluZWRlZ2VjaEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.