- Department of Agricultural Sciences, University of Sassari, Viale Italia, Sassari, Italy
The reuse and valorization of agri-food by-products is a pivotal activity in the pursuit of a circular model that can improve sustainability and efficiency of agri-food production. During artichoke processing, 60–80% of the biomass produced by the plant consists of inedible fractions, which nevertheless represent a natural source of high value-added compounds, such as phenolics. In this study, response surface methodology was applied to investigate and optimize the amount of ethanol and the reduction of extraction time to achieve the maximum yield of polyphenols and flavonoids from artichoke stems, leaves, and bracts, by using two extraction methods, namely maceration and ultrasound-assisted extraction. Overall, phenolic compounds were most concentrated in extracts obtained from the stems, followed by those derived from the bracts and leaves, with the percentage of ethanol being the most influential factor. After applying the optimization criteria, the best factor setting to achieve maximum extraction yields and strong antioxidant capacities was: 53% ethanol for stems, 45% for leaves, and 50% for bracts and 60 min for all by-products in the case of maceration; 10 min for stems and leaves with 42 and 20% of ethanol, respectively, and 41 min and 64% ethanol for bracts in the case of ultrasound-assisted extraction. Comparison between the two techniques evidenced that maceration was significantly more efficient, but similar recoveries were obtained with ultrasound-assisted extraction in shorter extraction time and lower ethanol consumption. Therefore, using this unconventional method to convert Spinoso Sardo artichoke by-products into bioactive ingredients with interesting industrial applications could be a viable strategy to reduce food losses and mitigate related environmental impacts.
1. Introduction
The agri-food industry generates a huge amount of by-products and waste every year, resulting in increasing disposal problems, environmental pollution, and sustainability issues. The Food and Agriculture Organization (FAO) found that around 14% of the food produced worldwide is lost between harvest and retail. Fruits and vegetables have the second highest wastage rate among the different commodity groups after roots and tubers (Méndez et al., 2021). In this sense, reducing food processing wastes is one of the most important goals, but it is not the only way that can be achieved to improve and promote environmental sustainability and food security. In fact, food by-products are an extraordinary source of bioactive compounds, such as phenolics, as well as proteins, alkaloids, carbohydrates, and lipids (Fernández et al., 2018). Most agro-industrial wastes are allocated to the production of animal feed, fuel, or organic fertilizers. However, there is growing interest in the valorization of fruit and vegetable by-products as a natural source of high value-added compounds that may be used as food and cosmetic ingredients (Trigo et al., 2020; Taghian Dinani and van der Goot, 2022).
The globe artichoke (Cynara cardunculus L. subsp. scolymus L.), which is a perennial herbaceous plant belonging to the Asteraceae family, is cultivated worldwide, and appreciated for its taste and health-promoting benefits. Its cultivation is considered an important agro-economy activity for Mediterranean region, especially for Italy, France, Spain, Egypt, and Morocco, that have an annual production of about 770,000 tons. The edible part of this plant is the core (inner bracts and receptacle) of the inflorescence called “capitula,” harvested in the early stage. So, artichoke processing, which is directed to production of minimally, frozen, or canned items, generates several fractions of by-products (mainly leaves, outer bracts and stems) that represent about 60–80% of the total biomass, which amounts approximately to 460,000 tons of waste per year (Lattanzio et al., 2009; López-Salas et al., 2021). This non-edible part, however, is still a source of constituents with high biological value, such as inulin and phenolic compounds, which are secondary metabolites known for their functional properties (hypocholesterolemic, antimicrobial, antioxidant, anticancer, anti-inflammatory, etc.). The main phenolics in artichoke tissues are caffeic acid derivatives, including chlorogenic acid, and a wide range of caffeoylquinic acid derivatives. Flavonoids, such as apigenin and luteolin, and several cyanidin caffeoylglucoside derivatives have also been identified (Lattanzio et al., 2009). Several studies showed that artichoke by-products are still a rich source of easily extractable phenolic compounds (Zuorro, 2014; Zuorro et al., 2014, 2016; Jiménez-Moreno et al., 2019). These bioactive compounds can be extracted by different solid–liquid conventional and non-conventional methods. The existing classical techniques, such as Soxhlet extraction, maceration and hydrodistillation, are based on the extracting power of different solvents and on the application of heat and/or stirring. The main limitations of conventional methods are longer extraction time, usage of expensive solvents, low extraction selectivity, and thermal decomposition of thermolabile compounds. Non-conventional techniques were introduced to overcome these limitations. One of these methods is the ultrasound-assisted extraction (UAE), which causes a phenomenon called cavitation, that intensifies mass transfer and accelerates access of solvent to plant tissues (Reche et al., 2021). The benefits of UAE include a reduction of extraction time, amount of energy and solvent (Azmir et al., 2013). Organic solvents such as methanol, ethanol and acetone are generally used to extract phenolic compounds from plant matrices, often in combination with different proportions of water (Dai and Mumper, 2010). Ethanol is widely used because, in addition to being a good extraction solvent, is a food grade solvent, thus it is safe for human consumption (Dai and Mumper, 2010). Moreover, mixtures of alcohol solvents with water have been found to be much more efficient in extracting phenolics than when used individually (Garcia-Castello et al., 2022).
Besides the type of solvent used, other factors can affect the recovery of phenolic compounds, such as extraction time, temperature, and solid–solvent ratio (Živković et al., 2018). In general, shorter extraction times and smaller amounts of solvent ensure lower cost processes (Panja, 2018). With reference to artichoke by-products, other papers are present in the literature that deal with the use of UAE compared to maceration. While Reche et al. (2021, 2022) studied a mathematical model to simulate the extraction curves of total phenolic and chlorogenic acid content, as well as the effects on microstructural changes by using different temperatures and ultrasound power density in the stem fraction, Quispe et al. (2021) applied the Box-Wilson design to study the effect of ethanol concentration (40–60%), extraction time (5–15 min) and radiation amplitude (80–100%) on the total phenolic content and antioxidant activity in the artichoke outer bracts. To the best of the authors’ knowledge, however, there are no studies comparing the effect of the two extraction methods (maceration and UAE) on each individual fraction of artichoke by-products (stems, bracts, and leaves).
Therefore, in this work, the response surface methodology (RSM) with a Central Composite Design (CCD) was applied to optimize the extraction of phenolics and flavonoid compounds from three different artichoke discards—namely stems, leaves, and bracts—by finding the proper amount of ethanol and reducing the extraction time. This multivariate statistic technique is widely used for development, improvement and optimization of products and processes in which one or more responses are influenced by different variables. Furthermore, CCD, which is suitable to study factors with three to five levels, allows a large amount of information to be obtained from a limited number of experiments, but without neglecting the relationship among parameters (Yolmeh and Jafari, 2017). The experimental design was conducted using both maceration and UAE. Subsequently, the optimized extracts obtained for each fraction by both extraction methods were carried out to maximize the phenolic and flavonoid content and the antioxidant capacity. The phenolic profile was also investigated by HPLC-DAD.
The artichoke by-products used in this study belong to an important ecotype—Spinoso sardo—cultivated in Sardinia (Italy), which obtained the Protected Designation of Origin (PDO) in the year 2011 and, to date, is the only artichoke PDO in Europe. The cultivation of Spinoso sardo is currently undergoing a significant reduction due to increased irrigation volumes resulting from drought and global warming and to its seasonality. Therefore, the reduction of the huge amount of wastes produced might render more sustainable and competitive the artichoke industry.
2. Materials and methods
2.1. Plant material and chemicals
By-products of Spinoso sardo artichoke (Cynara cardunculus L. var. scolymus Fiori) cultivar were provided by North Sardinia companies of consortium “Carciofo Spinoso di Sardegna DOP” and collected in the 2019. Outer bracts, leaves, and stems were individually stored at − 20°C, freeze-dried and then finely ground with an ultracentrifugal rotor mill (WX Ultra Series, Thermo scientific, Waltham, MA, United States). The residual moisture content of artichoke samples was determined according to the official method AACC 44-15A. Dry powders were kept at − 20°C until analysis and extraction procedure.
Solvents like absolute ethanol and methanol, were purchased from VWR Chemicals BHD (Milan, Italy). The radical DPPH (2,2-diphenyl-1-picrylhydrazyl), the radical cation ABTS 2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were obtained from Sigma-Aldrich (Milan, Italy). The standards used for identification and quantification of phenolic acids and flavonoids (apigenin 7-glucoside, luteolin 7-glucoside, caffeic acid, cynarin, 3,5-Di-O-caffeoylquinic acid, chlorogenic acid, neochlorogenic acid) were purchased from Extrasynthese (Genay, France). HPLC methanol was purchased from Carlo Erba (Milan, Italy).
2.2. Experimental designs
Two different Central Composite Designs (CCDs) were set with 13 randomized runs and 5 replicated central points (to evaluate the pure error) using the Design Expert software 10 (Stat-Ease Inc. Minneapolis, MN, United States). Specifically, a central composite rotatable design was used to optimize maceration, while a face-centered design was employed for the UAE. CCDs were used to investigate the effect of two independent factors at three levels (low: − 1, central: 0, and high: + 1), i.e., ethanol percentage (X1) and extraction time (X2), on the recovery of bioactive compounds; as well as to evaluate the most influential of the 3 levels chosen for each factor. The levels of the selected factors were determined by preliminary trials. The experimental layout of the design utilized for maceration and UAE are displayed in Tables 1, 2, respectively.
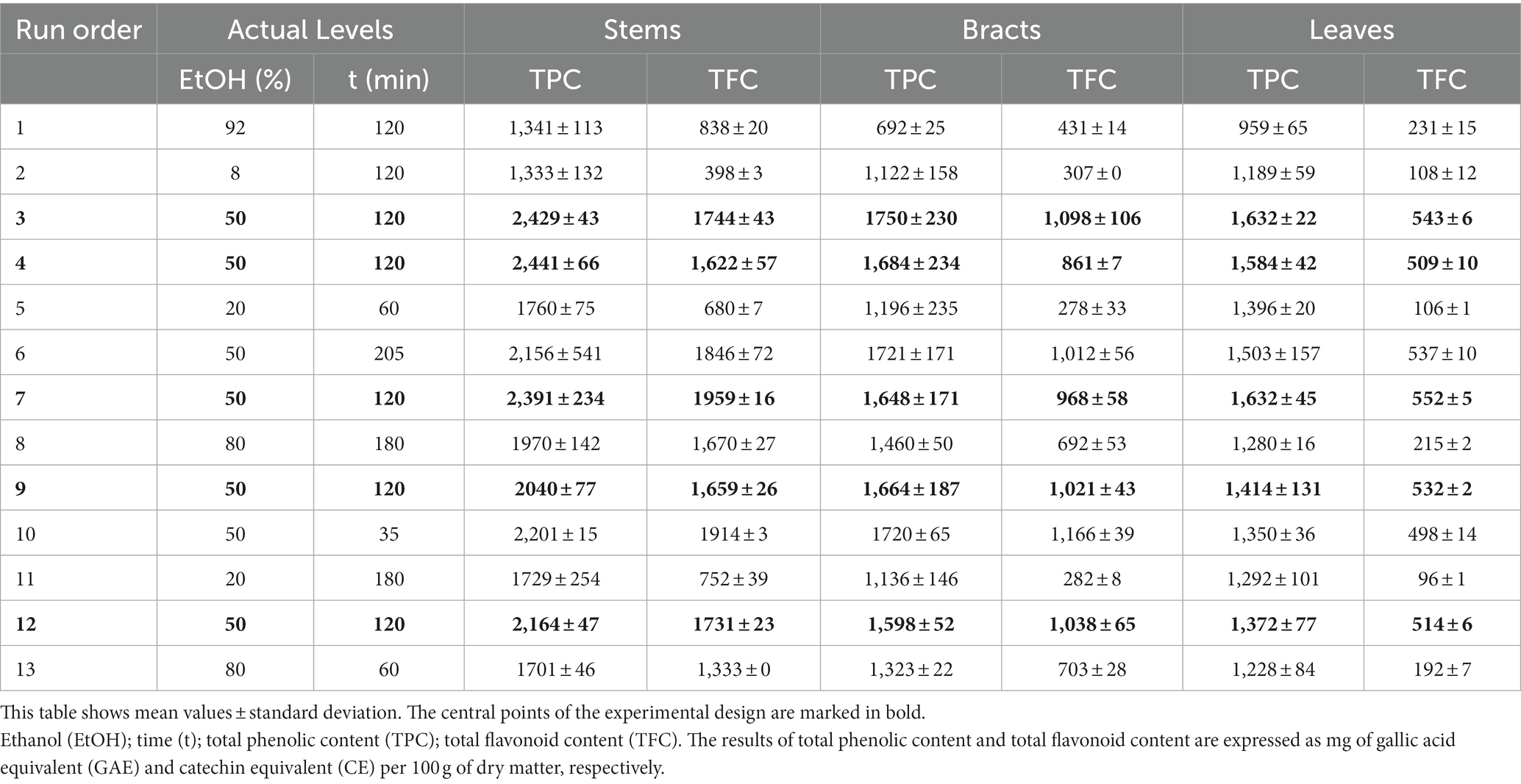
Table 1. Central composite design used for maceration extraction (variable levels are presented in actual values) and experimental results of each by-product fraction.
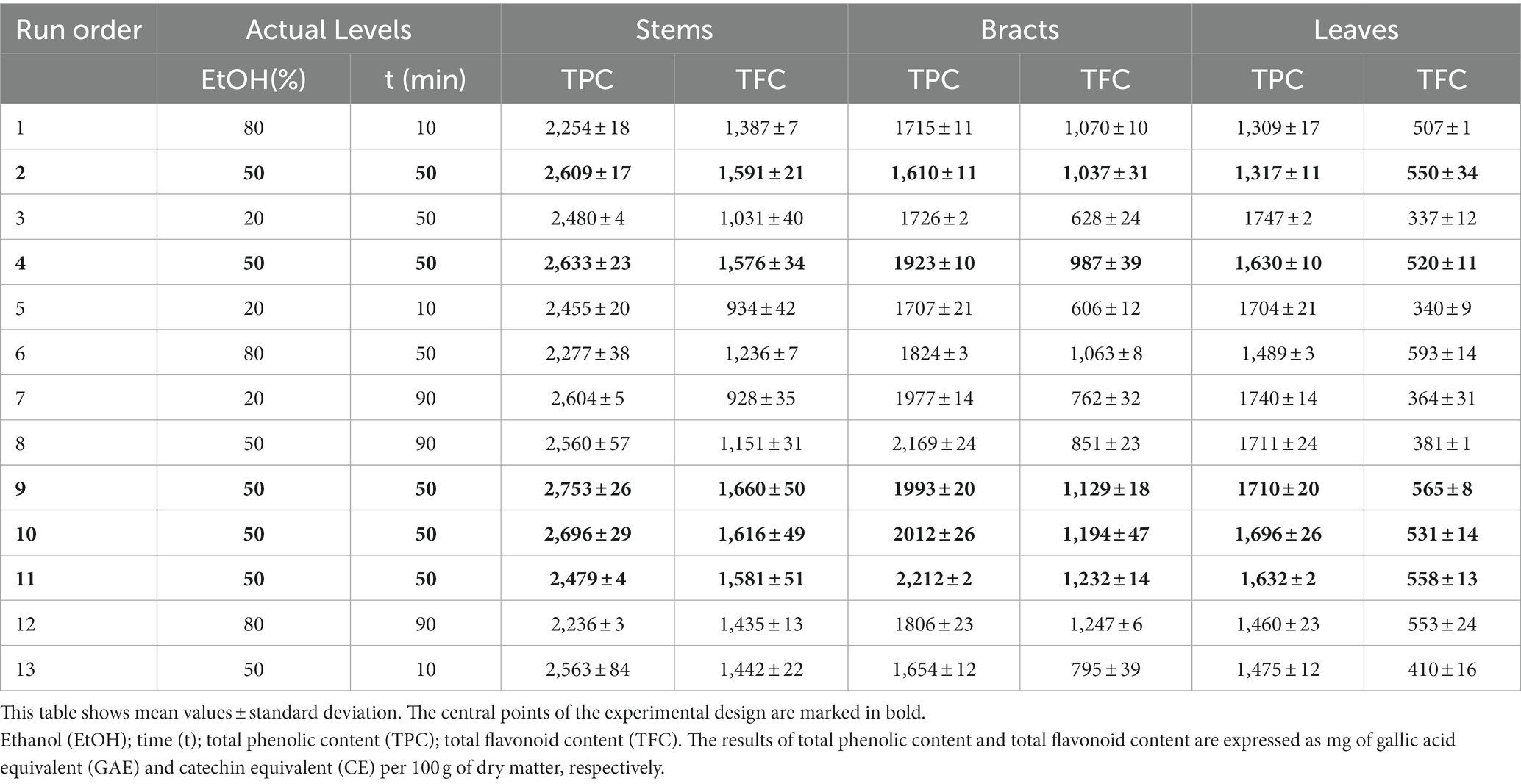
Table 2. Central composite design used for ultrasound assisted extraction (variable levels are presented in actual values) and experimental results of each by-product fraction.
Total phenolic content (TPC) and total flavonoid content (TFC) measured on each extract were used as outcome variables (Yn).
2.3. Extraction and determination of total phenolic and flavonoid content
Bioactive compounds recovery from the different fractions of artichoke by-products were performed using maceration and UAE. Both extraction methods were carried out on 1 g of lyophilized sample by using 20 mL of ethanol/water food-grade solution, at different percentages (20, 50, 80%) according to the two factorial designs described above.
Maceration was conducted by shaking samples in a thermostatic water bath (model WB-MF24, FALC Instruments, Bergamo, Italy) set at 400 rpm and 38 ± 2°C, for a period of time varying from 60 to 180 min. UAE was performed using an ultrasonic bath (ARGO Lab, model DU-100, Carpi, Italy) at constant frequency of 40 kHz and a power of 144 W (parameters established by a previous experimental design), for times varying from 10 to 90 min. Then, the obtained mixtures were centrifuged at 9,000 rpm for 10 min at 22°C. Supernatants were collected, filtered by cellulose acetate syringe filter (0.45 μm pore-size), and stored at − 20°C until analysis.
The determination of the TPC of artichoke extracts (bracts, leaves, and stems) was carried out following the Folin–Ciocalteu method proposed by Noriega-Rodríguez et al. (2020), with slight modifications. Briefly, in a test tube containing 7.5 mL of distilled water, 1 mL of sample diluted 1:10 (v/v) with the extraction solution, 0.5 mL of of Folin Ciocalteau reagent (50%) and 1 mL of sodium carbonate (10%) were added. Samples were kept in the dark at room temperature for 1 h and then measured in a spectrophotometer (Agilent, model Cary 3500, Cernusco, Milan, Italy) at a wavelength of 765 nm. Results were expressed as mg of Gallic Acid Equivalent (GAE) per 100 g of dry matter (d.m.).
The TFC of the artichoke extracts was obtained by applying a spectrophotometric method (Dabbou et al., 2017) and expressed as mg of Catechin Equivalent (CE) per 100 g of d.m. An aliquote of 1 mL of extract, diluted 1:10 (v/v) with the extraction solution, was mixed with 5 mL distilled water and 0.3 mL of 5% NaNO2 solution. Six minutes later, 0.6 mL of 10% AlCl3 solution was added and allowed to react for another 5 min. Then, 2 mL of 1 M NaOH solution was added, and the total volume was made up to 10 mL with distilled water. The absorbance was measured at 510 nm. The analyses were conducted in triplicate.
2.4. Determination of antioxidant capacity
The antioxidant capacity was determined on the optimized artichoke extracts by both extraction methods using two different spectrophotometric assays (ABTS• + and DPPH•) according to Prior et al. (2005), with some modifications.
DPPH• method. The DPPH• solution used was adjusted adding methanol to reach an initial absorbance of 1.0 ± 0.2. In this assay, aliquots of 70 μL of samples diluted with the extraction solution (1:10 v/v for bracts and stems and 1:4 v/v for leaves) were made to react, for 30 min in darkness, with 2.03 mL of a DPPH• methanol solution (1 mM). The decrease in absorbance of the radical DPPH was monitored using a spectrophotometer set at 517 nm, and results were compared to the concentration-response curve of the standard Trolox and expressed as μmol of Trolox equivalents per 1 g of d.m.
ABTS• + method. The ABTS radical cation was produced by the reaction of 7.4 mM of ABTS• + stock solution with 2.6 mM potassium persulfate (which were dissolved in phosphate buffer) in darkness at room temperature for 12 h. Before use, ABTS• + was diluted with phosphate buffer to obtain a working solution with an initial absorbance of 1.0 ± 0.2 at 734 nm. After the addition of 40.8 μL of diluted sample to 2 mL of ABTS• + solution, absorbance values were taken after 6 min of incubation at 22°C in the dark at 734 nm. Standard solutions of Trolox were used to calculate the antioxidant capacity and the results were expressed as μmol of Trolox equivalent (TE) per 1 g of d.m. The assays were carried out in triplicate.
2.5. HPLC-DAD analysis
To analyze the phenolic fraction of the studied artichoke bracts, leaves, and stems, 5 mL of ethanol-water extracts that had given the best results in spectrophotometric analysis were concentrated to dryness in a rotary evaporator (Buchi, model Rotavapor R-200, Flawil, Switzerland), resuspended in 5 mL of a methanol–water solution (50:50), and filtered with 0.45 μm acetate cellulose syringe filters before HPLC analysis. The determination was performed by using an Agilent 1260 (Santa Clara, CA 95051, United States) equipped with a quaternary pump, an autosampler and a photodiode array detector (DAD). A reversed phase column Luna C18, 250 × 4.6 mm i.d., particle size 5 μm (Phenomenex, Torrance, CA, United States), set at 40°C was used. The mobile phase consisted of solvent A (methanol) and solvent B (water/acetic acid 95:5, v/v), as reported in D’Antuono et al. (2015). The following gradient was used: 85 to 60% B (0–25 min), 60% B (25–30 min), 60 to 37% B (30–45 min), 37% B (45–47 min), 37 to 0% B (47–52). The flow rate was set at 1.0 mL·min−1 and the injection volume at 25 μL. The photodiode array detection was performed at the absorbances of 280, 325 and 360 nm. Phenolics were identified by retention time and spectra of pure available standards. Additionally, when the standards were not available, chlorogenic acid and 3,5-O-dicaffeoylquinic acid were used for the quantification of mono and dicaffeoylquinic acids, respectively identified following the classification of Lattanzio et al. (2009).
2.6. Statistical analysis
Design Expert 10 Software (Stat-Ease Inc. Minneapolis, MN, United States) was used to analyze the results. To define predictive models, the experimental data were transformed, through a RSM analysis, into the following second-order polynomial model:
where Yn are the responses, X1 and X2 are the actual values of the independent variables (ethanol percentage and extraction time), is the intercept, , are the linear coefficients, while , and are the quadratic and interaction regression coefficient terms, respectively. To obtain the most appropriate model, a backward regression technique was used to select only those independent variables and relevant interactions that showed significance (p < 0.05) at Analysis of Variance (ANOVA), based on the value of p (p < 0.05) and the Lack of Fit (LOF) test. p-Values less than 0.05 indicate that model terms are significant, while a non-significant lack of fit means that the model is fitting well. Model reliability was also evaluated with the coefficients of determination and adequate precision, which is a measure of the signal-to-noise ratio. If this ratio is greater than 4 the model can be used for the purpose of prediction and optimization (Fadjare Frempong et al., 2021). The R2 values close to 1 are desirable; adjusted R2 coefficients are useful when models have a great number of terms. Only significant regression coefficients should be considered in the equation and contribute to model development. Terms required to support the hierarchy are not removed from the model. Therefore, the models used in RSM are not always of second order (quadratic models), sometimes reduced models (linear, 2FI) can also be obtained (Yolmeh and Jafari, 2017). Design Expert software was also used to perform numerical optimization of parameters and model validation. The optimal levels of the studied factors were found for both extraction method via desirability function (D) that ranges from 0 to 1, where 1 is the most desirable condition for the maximization of the response variable (Garcia-Castello et al., 2022).
Response surface methodology was employed to optimize both extraction methods, by locating the best combination of parameters to minimize extraction time and maximize the responses for each by-product fraction.
The validation of the models was done by performing the UAE and maceration at the optimal parameters obtained and comparing the experimental values with those predicted.
Furthermore, the measured data on the extracts obtained by applying the optimal conditions were analyzed using the Statistica 12.0 software (StatSoft, Inc., Tulsa, OK, United States). First, the optimized extracts were subjected to the t-test and ANOVA to find statistical differences between the two extraction methods and among the by-products. Next, a two-way ANOVA was conducted to evaluate the effect of the waste fraction, the extraction method used and their interaction on the optimized data of TPC, TFC, DPPH• and ABTS•+. Moreover, Pearson’s correlation test was performed to assess the relationship between the results of antioxidant capacity, TPC, and TFC.
3. Results and discussion
3.1. Effects of maceration parameters on TPC extraction
The results obtained for all by-product fractions from the CCD used for maceration are listed in Table 1. In general, stems showed a higher TPC, followed by bracts and leaves. In fact, TPC varied for stems from 1,333 to 2,441 mg GAE·100 g−1, for bracts from 692 to 1,750 mg GAE·100 g−1, while for leaves ranged from 959 to 1,632 mg GAE·100 g−1. These results are in good agreement with those reported by Fadda et al. (2018) in stems, outer bracts, and rosette and peduncle leaves of the Spinoso sardo ecotype, not only in terms of total concentration (2,230, 1,980, and 1,830 mg GAE·100 g−1 for stems, outer bracts, and leaves, respectively), but also in terms of distribution of these compounds in the various organs of the plant. Zuorro et al. (2014) reported similar ranges in artichoke stems (1,266–2,802 mg GAE·100 g−1) and outer bracts (1,123–1,944 mg GAE·100 g−1). Reche et al. (2021), while observing the same distribution of polyphenols in stems and bracts of an artichoke variety grown in Spain, registered significantly higher values than those found in the present study for both fractions (4,570 and 2,740 mg GAE·100 g−1 for stems and bracts, respectively). On the contrary, findings reported by Colantuono et al. (2018) in artichoke by-products of the Tondo di Paestum varietal type, while confirming the stems as the plant organs with the highest polyphenol accumulation (3,470 mg GAE·100 g−1), revealed a higher TPC in the leaves (2,160 mg GAE·100 g−1) than in the bracts (880 mg GAE·100 g−1). On the other hand, Rejeb et al. (2020), when analyzing the inedible parts of two Tunisian artichoke varieties, found that bracts (1,526 mg GAE·100 g−1) were richer source of total polyphenols than leaves (1,159 mg GAE·100 g−1) and floral stems (1,069 mg GAE·100 g−1). The inconsistency on the different distribution and concentration of total polyphenols in the various organs of the globe artichoke plant that emerged in these studies, however, could be due to the genetic background, the environmental conditions, and the harvest period, as previously confirmed in the literature (Pandino et al., 2011a,b).
The regression coefficients of the mathematical models obtained from the data of TPC collected for each fraction examined for maceration are reported in Table 3.
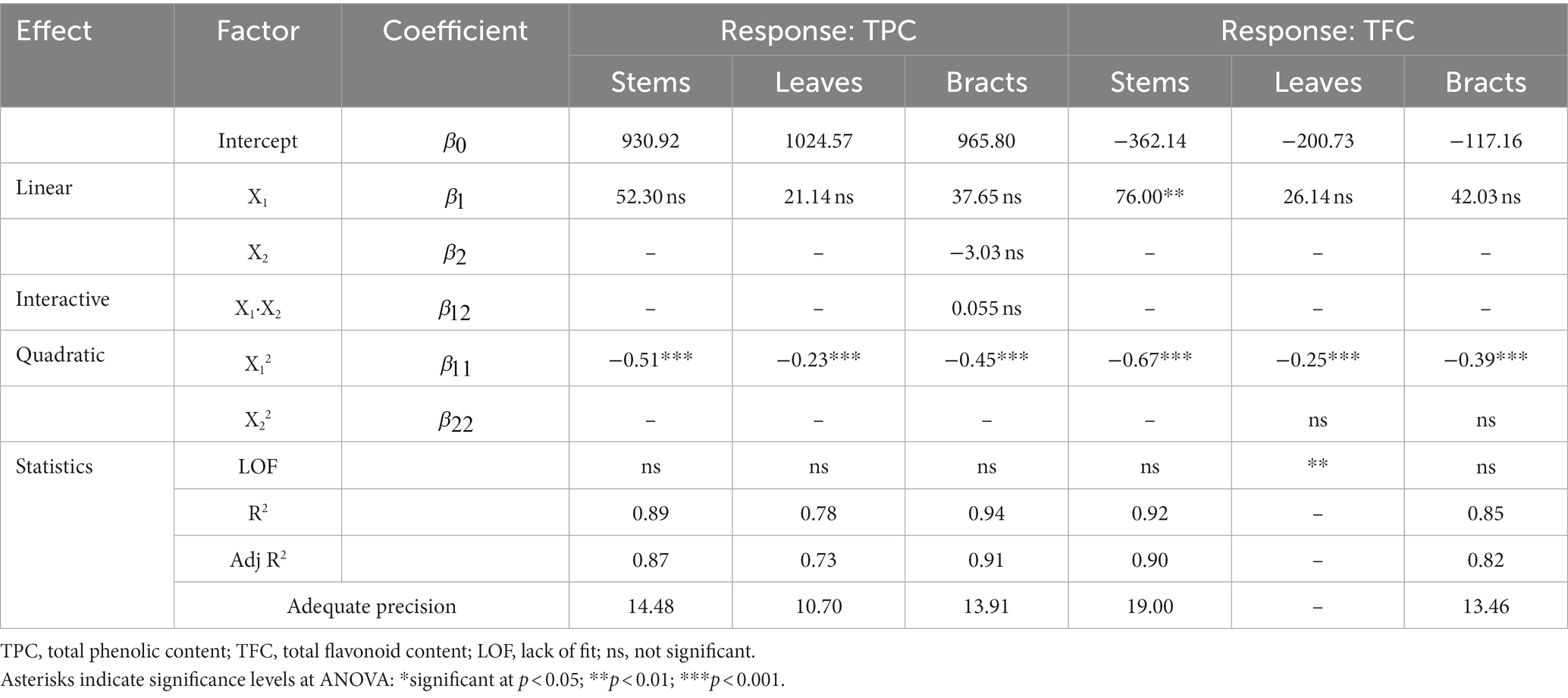
Table 3. Estimated regression coefficients of mathematical models (final equations in terms of actual factors) obtained for each response for different by-product fractions with the maceration and statistical criteria used to assess model accuracy.
ANOVA analysis and fit statistics showed that the selected reduced quadratic models were significant (p < 0.05) and well fitted to the TPC for all three fractions analyzed, as evidenced by the satisfactory levels of R2 and Adj R2 that varied from 0.78 to 0.94 and from 0.73 to 0.91, respectively (Table 3). Moreover, the adequate precision values were greater than 4—indicating that the model can be used to navigate the design space—and the LOF tests resulted in a non-significant F-value, denoting that the models are sufficiently accurate for predicting the TPC of the experimental by-products. As it can be seen in Table 3, the TPC of all three by-products was only affected by the negative quadratic regression coefficient of the ethanol percentage factor, while the effect of the extraction time was not significant (p < 0.05). This means that the extraction yield of total polyphenols increased gradually as the percentage of ethanol raised, reaching the highest values around 50% ethanol, and then decreased considerably with increasing levels of alcohol used, regardless of the extraction times chosen. This tendency can also be noted in the response surface plots displayed in Figures 1A–C, where it is evident that the extraction efficiency is reduced at small and high ethanol ratios, especially in stem and bract samples. In particular, the highest extraction efficiency values were reached at 45–55% ethanol in stems, 40–50% in leaves, and 40–60% in bracts. However, it should be noted that, although the linear regression coefficient of extraction time (X2) included in the model of the bracts was not statistically significant, it tended to negatively influence TPC extraction, suggesting that shorter extraction times might lead to a better extraction efficiency with lower ethanol concentrations (Figure 1C). It is known from the literature that polyphenolic compounds are present in the artichoke plant—as well as in most fruits and vegetables—mainly in the free and soluble conjugated form rather than in the insoluble-bound form (Domínguez-Fernández et al., 2021). Therefore, the lack of influence of the extraction time on the recovery of the phenolic fraction could be related to the relative solubility of the phenolics present in the plant, which, together with the solubilization capacity of the solvent and its polarity, influences their extractability and distribution coefficient (Gil-Martín et al., 2022). Probably, in a conventional extraction method, such as maceration, in which the release of polyphenols from plant matrices occurs according to their solubility (Gil-Martín et al., 2022), the equilibrium of diffusion of the solute had already been reached at the lowest level of the independent factor (within 60 min), making further extension of the extraction time irrelevant.
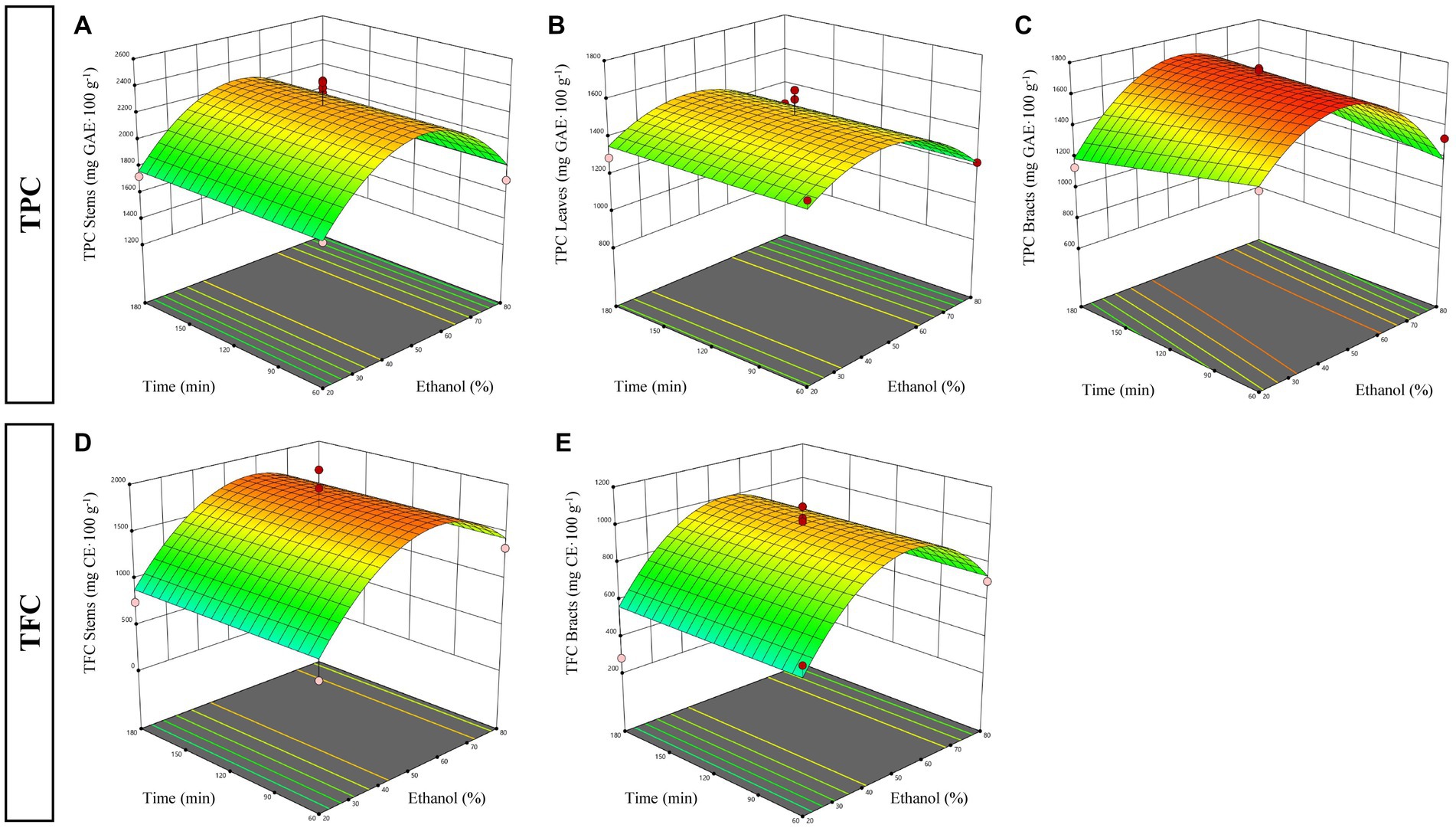
Figure 1. Response surfaces plots explaining the effect of time (X1) and ethanol (X2) factors during maceration on total phenolic content (TPC) in stems (A), leaves (B), bracts (C) and total flavonoid content (TFC) in stems (D) and bracts (E).
3.2. Effects of maceration parameters on TFC extraction
Flavonoids are a group of constituents that are included in the class of polyphenols, secondary metabolites found in vegetables, fruits, and some alcoholic beverages (Panche et al., 2016; Chávez-González et al., 2020). Within artichoke tissues, flavonoids are present in fewer amounts than caffeoylquinic acid derivatives, nevertheless they are important for their antioxidant properties and for their essential role in appearance of plant-based foods and, thus, in food acceptance (Lattanzio et al., 2009).
The results obtained for all by-product fractions from the CCD used for maceration are listed in Table 1. As with TPC, stems showed a higher TFC, followed by bracts and leaves. Specifically, TFC varied from 398 to 1,959 mg CE·100 g−1 for stems, from 278 to 1,098 mg CE·100 g−1 for bracts, and between 96 and 552 mg CE·100 g−1 for leaves. Fadda et al. (2018) reported the same distribution of flavonoids in stems, outer bracts, and rosette and peduncle leaves of the Spinoso sardo ecotype and a similar TFC in the floral stems (2,180 mg CE·100 g−1), but also higher concentration values than those found in the present study in both bract (1,770 mg CE·100 g−1) and leaf (1,220–1,500 mg CE·100 g−1) fractions. Rejeb et al. (2020), despite a similar TFC observed in the stems of two Tunisian accessions (1,113–1,417 mg CE·100 g−1), recorded the highest concentration values in the leaves for both cultivars (5,225–5,823 mg CE·100 g−1), evidencing a wide variability in both flavonoid content and distribution depending on the plant organs and cultivar analyzed.
The regression coefficients of the mathematical models obtained from the data of TFC collected for each fraction examined for maceration are reported in Table 3. ANOVA results and fit statistics evidenced that the reduced quadratic models were significant (p < 0.05) and adequately accurate in predicting the TFC for stem and bract fractions only, as evidenced by the high levels of R2 (0.85–0.92) and Adj R2 (0.82–0.90), desirable values of adequate precision, and non-significant LOF tests. In contrast, the model selected to fit the experimental data for the leaf fraction, while significant (p < 0.05), was discarded because the LOF test resulted in a significant F-value, indicating that the model is not reliable and, consequently, does not allow adequate prediction of the data. As shown in Table 3, the ethanol percentage, as already observed for the recovery of the total polyphenols, was the only factor that affected the flavonoids extraction efficiency in both stems and bracts, while the effect of the extraction time was not significant (p < 0.05). Specifically, regarding the stem fraction, although the model analysis showed that the ethanol percentage had positive linear and negative quadratic effects on the TFC, values of regression coefficients revealed a greater influence of the linear term on the quadratic term. This means that regardless of the extraction time chosen, as the percentage of ethanol used increased, extraction yields also increased, reaching maximum efficiency at intermediate rates of ethanol concentration (40–70%). However, as ethanol percentage raised further, flavonoid yields began to decrease, although to a lesser extent than that observed at the lowest ethanol levels (Figure 1D). A similar tendency was also observed for the TFC of the bract fraction, which was found to be negatively affected only by the quadratic term of the ethanol factor (Figure 1E). In this case, however, increasing the level of ethanol used led to an improvement in extraction efficiency, but only up to intermediate percentages, beyond which flavonoid yield strongly decreased. Although it is not possible to identify a single solvent (or aqueous formulation thereof) capable of maximizing extraction yields of phytochemicals from plant matrices of different origins, the results obtained in this study seem to confirm the high efficacy of using equivolumetric water/ethanol solutions in artichoke by-products. Similar conclusions were drawn by Zuorro et al. (2014) who reported that mixture composed of an equal proportion of low molecular weight polar compounds, such as ethanol and water, was more effective than other solvents in extracting phenolic compounds from artichoke stems and bracts. This could be due to the development of a synergistic effect between the two solvents when used in combination. Probably, water, which is a strongly polar solvent, acts as a swelling agent by increasing the contact surface area between the solvent and the plant sample, while ethanol promotes solubility and diffusion of phenolic compounds due to its lower polarity (Medina-Torres et al., 2017).
3.3. Effects of UAE parameters on TPC extraction
The results obtained for all by-product fractions from the CCD used for UAE are listed in Table 2. As already observed for maceration, stems showed a higher TPC, followed by bracts and finally by leaves. In this case, however, the data obtained exhibited lower variability probably in relation to the different extraction system, which, by allowing better solvent penetration even in a short time, resulted in greater leaching of phenolics. In fact, UAE relies on acoustic cavitation, which increases the permeability of the solvent within the plant matrix, particularly in the cell walls, and enhances the release of bioactive compounds (Medina-Torres et al., 2017). Specifically, in the present study, TPC ranged from 2,236 to 2,753 mg GAE·100 g−1 for stems, from 1,610 to 2,212 mg GAE·100 g−1 for bracts, while varied from 1,309 to 1,747 mg GAE·100 g−1 for leaves. Reche et al. (2022), when performing an UAE for 35 min at 25°C in the stems of an artichoke variety grown in Spain, observed lower (1,290 mg GAE·100 g−1) or similar (2,370 mg GAE·100 g−1) yields of total polyphenols depending on the ultrasound power density applied. Quispe et al. (2021) extracted similar amount of total polyphenols from the outer bracts of an artichoke variety grown in Peru, using water/ethanol in different proportions as extraction solution. In contrast to the present study, Kollia et al. (2017) reported a lower total polyphenols concentration in both artichoke stems (330 mg GAE·100 g−1) and bracts (410 mg GAE·100 g−1), whereas Stumpf et al. (2020), applying the UAE and using 40% methanol as extraction solution, recovered higher yields of total polyphenols from the leaf fraction (2,860 mg GAE·100 g−1). However, to the best of the authors’ knowledge, the latter is the only study that carried out the extraction of phenolic compounds from artichoke leaves by using this unconventional extraction technique. Therefore, comparison of the data obtained with the literature is difficult.
The regression coefficients of the mathematical models obtained from the data of TPC collected for each fraction examined for UAE are reported in Table 4. As it can be seen, the relationship between the two independent variables and the selected response fitted well with the reduced quadratic model for stems and bracts and with a linear model for leaves. All regression models were found to be statistically significant (p < 0.05) and valid for the studied response within the selected range of factor’s levels, as confirmed by the adequate R2 and Adj R2, the desirable values of adequate precision, and the non-significant F-value of the LOF test (Table 4). It is worth noting that the model selected to fit the experimental data for the leaf fraction, while showing an intermediate R2 that explained 59% of the variability in the data, was considered reliable since all the other statistical parameters, such as adequate precision and LOF test (as well as the final model validation) proved its effectiveness.
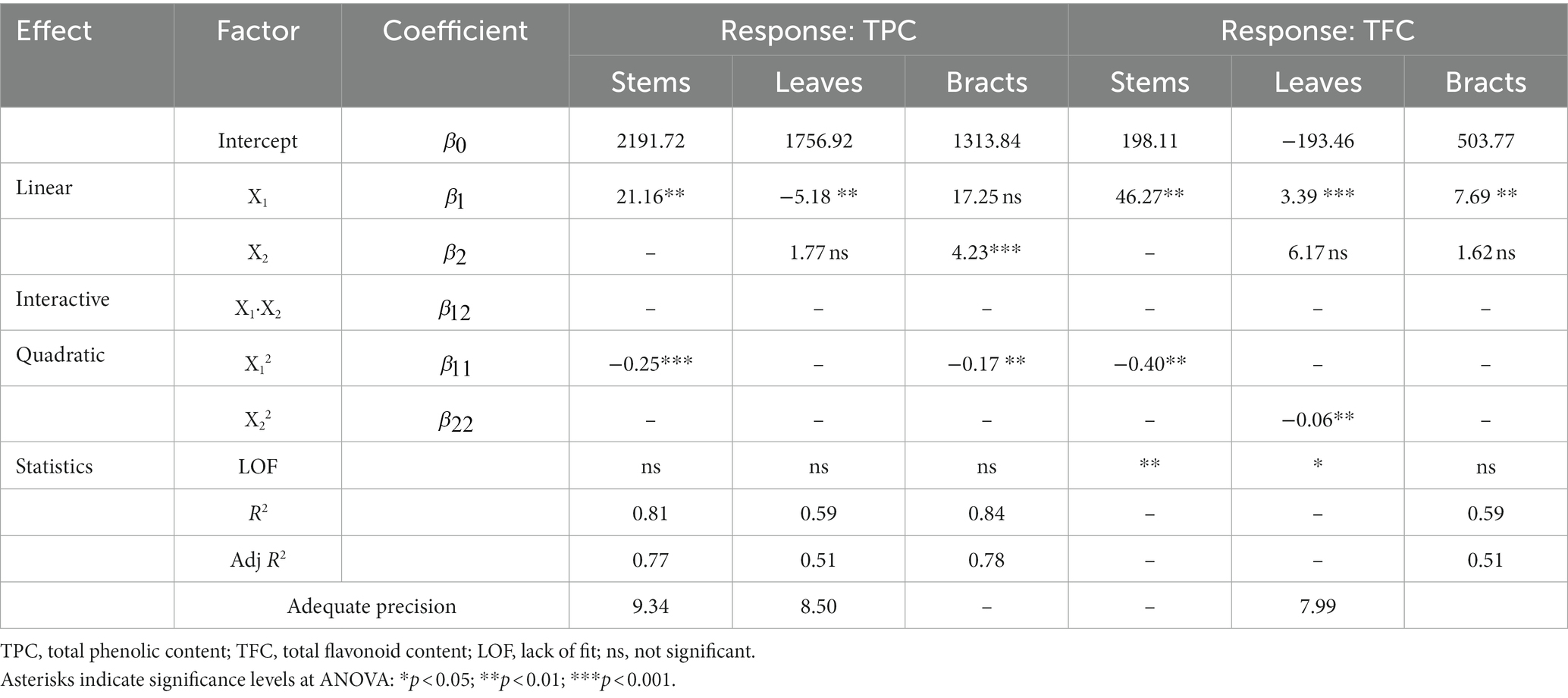
Table 4. Estimated regression coefficients of mathematical models obtained for each response for different by-product fractions with the ultrasound assisted extraction and statistical criteria used to assess model accuracy.
The selected fitting models revealed that polyphenols extraction efficiency was affected differently by the two independent factors depending on the by-product analyzed. Indeed, while in stem and leaf fractions TPC yield was influenced only by the change in ethanol percentage, in the bract fraction it was also affected by the extraction time. Specifically, regarding stems, model analysis showed that the ethanol percentage had positive linear and negative quadratic effects on the TPC, with values of regression coefficients revealing a greater influence of the linear term on the quadratic term. This means that the total polyphenol yields increased with increasing ethanol percentage until a maximum level of recovery is reached, beyond which additional alcohol increments led to a reduction in the total amount recovered. This behavior can be also observed in the response surface plot displayed in Figure 2A, where the highest (2,753 mg GAE·100 g−1) and the lowest (2,236 mg GAE·100 g−1) TPC were reached at 50 and 80% ethanol, respectively. In contrast, the extraction of polyphenols from artichoke leaves, being negatively affected only by the linear term of the ethanol percentage factor, followed a different trend. In fact, in this case, the recovery of total polyphenols decreased linearly as the level of the alcohol used increased (Figure 2B). Moreover, although the linear regression coefficient of extraction time (X2) included in the model was not statistically significant, it tended to positively influence TPC extraction, suggesting that prolonged extraction times might lead to a better extraction efficiency. In fact, the maximum polyphenols yield, which amounted for 1,747 mg GAE·100 g−1, was achieved at the lowest level of ethanol percentage and at intermediate to high extraction times. To better understand the different behavior observed in the two by-product fractions, it must be born in mind that, normally, free phenolic compounds are contained in cell vacuoles, whereas insoluble phenols are covalently bound to structural components of the cell and to rod-shaped structural proteins in the cell wall (Acosta-Estrada et al., 2014). Protein denaturation caused by the use of high concentrations of ethanol, may have hindered the dissolution of polyphenols, impairing their extraction efficiency (Chen et al., 2013). Probably, in this study, such effect was more pronounced in the leaves than in the stems due to the higher amount (at about 2.5-fold) of protein contained in this fraction (data not shown).
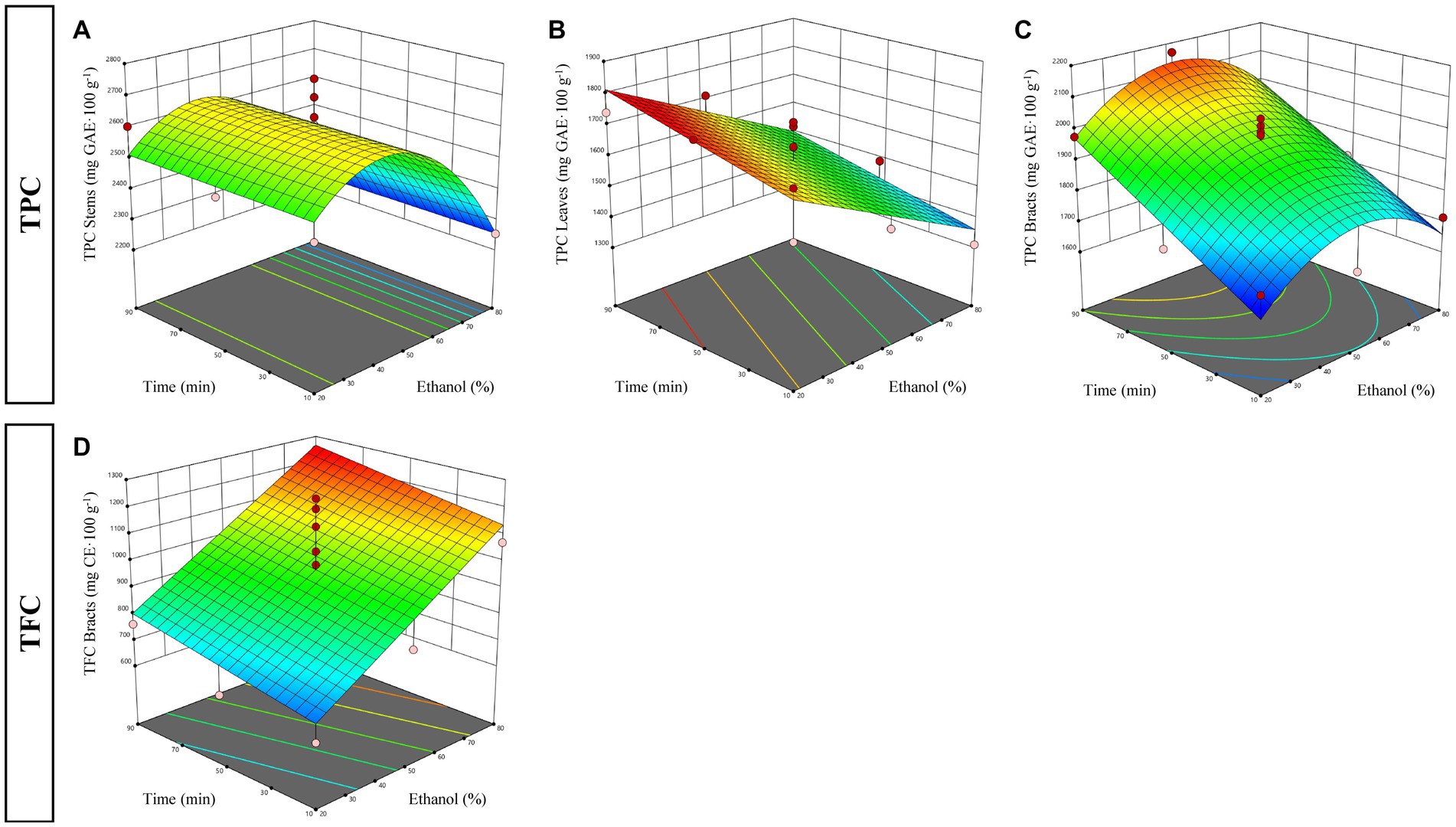
Figure 2. Response surfaces plots explaining the effect of time (X1) and ethanol (X2) factors during ultrasound assisted extraction on total phenolic content (TPC) in stems (A), leaves (B), bracts (C) and total flavonoid content (TFC) in bracts (D).
Unlike stems and leaves, model analysis for the bract fraction evidenced that the TPC extraction was positively affected by the linear term of the extraction time and negatively influenced by the quadratic term of the ethanol percentage (Table 4). This suggests that the highest TPC was achieved at intermediate ethanol percentages (40–60%), but longer extraction times (70–90 min), as also evidenced by the response surface plot depicted in Figure 2C. These findings are consistent with those reported by Ghafoor et al. (2009), who observed higher yield of total polyphenols in grape seeds when UAE was done for a longer time.
3.4. Effects of UAE parameters on TFC extraction
The results obtained for all by-product fractions from the CCD used for UAE are listed in Table 2. Consistent with the results previously reported for TPC, stems showed a higher TFC, followed by bracts and leaves. Specifically, TFC varied from 928 to 1,660 mg CE·100 g−1 for stems, from 606 to 1,247 mg CE·100 g−1 for bracts, and between 337 and 593 mg CE·100 g−1 for leaves. The polyphenols concentration observed in this study in the leaf fraction is similar to that recorded by Stumpf et al. (2020) in UAE extracts (at about 600 mg·100 g−1) obtained using 40% methanol as extraction solution. A more in-depth comparison of data is unfortunately difficult because, to the best of the authors’ knowledge, there are no studies in the literature reporting the yield of TFC from artichoke by-products using the ultrasound-assisted technique.
The regression coefficients of the mathematical models obtained from the data of TFC collected for each fraction examined for UAE are shown in Table 4. The results of the ANOVA analysis and fit statistics evidenced that the only reliable regression model was the one obtained for the bract fraction. In fact, the models selected to fit the experimental data of both stems and leaves, while significant (p < 0.05), were discarded because the LOF test resulted in a significant F-value, indicating that the models do not allow adequate prediction of the data. Regarding the bract fraction, the relationship between the two independent variables and the TFC fitted well with a linear model in which only the regression coefficient of the ethanol factor was highly significant (p < 0.01). On the other hand, the linear coefficient of the extraction time, although not significant, was included in the model, suggesting it may have a positive influence on the extraction efficiency. This trend is clearly visible in the response surface plot shown in Figure 2D, in which the yield of total flavonoids increased as the concentration of ethanol used increased, with a more pronounced effect for extraction times from 30 min onward (Figure 2D).
3.5. Optimal parameters and their validation
The numerical optimization was performed considering the mathematical models gained, the significance of terms of the regression equations and the statistical parameters. Design Expert software, through the desirability function, allowed simultaneous optimization involving both factors and responses to achieve the desired goals. In the present study, the optimization was conducted for all by-product fractions and for both extraction methods with the aim of maximize the responses (TPC and TFC), keeping the value of X1 factor in its range (20–80%) and specifying the value of X2 factor as the minimum desirable. Specifically, while the former choice was made considering the greater impact exerted by the percentage of ethanol on the extraction efficiency of the bioactive compounds from all three by-products studied, the second was aimed at achieving the dual objectives of making the process more sustainable—through the reduction of energy consumption and, consequently, costs—and increasing the competitiveness of the industries. Accordingly, several combinations of optimal parameters were obtained for each fraction and extraction method under consideration. The best combination was found by using, when applicable, the combined maximum desirability of the models of the two responses (TPC and TFC). In fact, the desirability function (D) was applied to models that were able to predict well, with a not significant LOF. As a result of maceration optimization, the following parameters were obtained: 53% of ethanol and 60 min of extraction (D = 0.91) for stems, 45% and 60 min (D = 0.90) for leaves, and 50% and 60 min (D = 0.92) for bracts.
Whereas, with UAE optimization the succeeding combinations were reached: 42% of ethanol and 10 min of extraction (D = 0.87) for stems, 20% and 10 min (D = 0.91) for leaves, and 64% and 41 min (D = 0.60) for bracts.
Therefore, the optimized results obtained evidenced that the maximum extraction efficiency can be achieved at intermediate ethanol concentrations for both extraction method and for all three by-product fractions—except for the sonicated leaves—probably due to the aforementioned synergistic effect established between the two polar solvents in equivolumetric solution. The optimized extraction time, on the other hand, coincided with the lowest level of the factor-selected range for both type of extraction, except for sonicated bracts where the time required to maximize the recovery of bioactive compounds was at intermediate value.
The optimal extraction conditions predicted by the designs were then used to perform additional experiments needed to validate the models and confirm the accuracy of their predictive ability. To this end, TPC and TFC were redetermined on the extracts obtained at the optimal factor settings from both the extraction methods and for each artichoke by-product (Table 5). Specifically, the extracts produced by maceration showed the following TPC values: 2603.5 ± 10.33 mg GAE·100 g−1 for stems, 1863.26 ± 5.81 mg GAE·100 g−1 for leaves, and 1,865 ± 4.93 mg GAE·100 g−1 for bracts. The TFC values obtained were as follows: 1845.80 ± 72.06 mg CE·100 g−1 in stems, 873.08 ± 4.83 mg CE·100 g−1 in leaves, and 1464.64 ± 18.15 mg CE·100 g−1 in bracts. On the other hand, the optimized UAE process enabled the following amount of TPC to be extracted: 2516.03 ± 4.35 mg GAE·100 g−1 for stems, 1723.10 ± 20.03 mg GAE·100 g−1 for leaves, 2014.40 ± 31.13 mg GAE·100 g−1 for bracts; while the amount of TFC was of 1947.75 ± 4.67, 754.55 ± 29.07, 1380.39 ± 4.53 mg GAE·100 g−1, for stems, leaves, and bracts, respectively. All the values were within the 95% prediction intervals, confirming that the models have a good fit to the experimental data and high predictive performance.
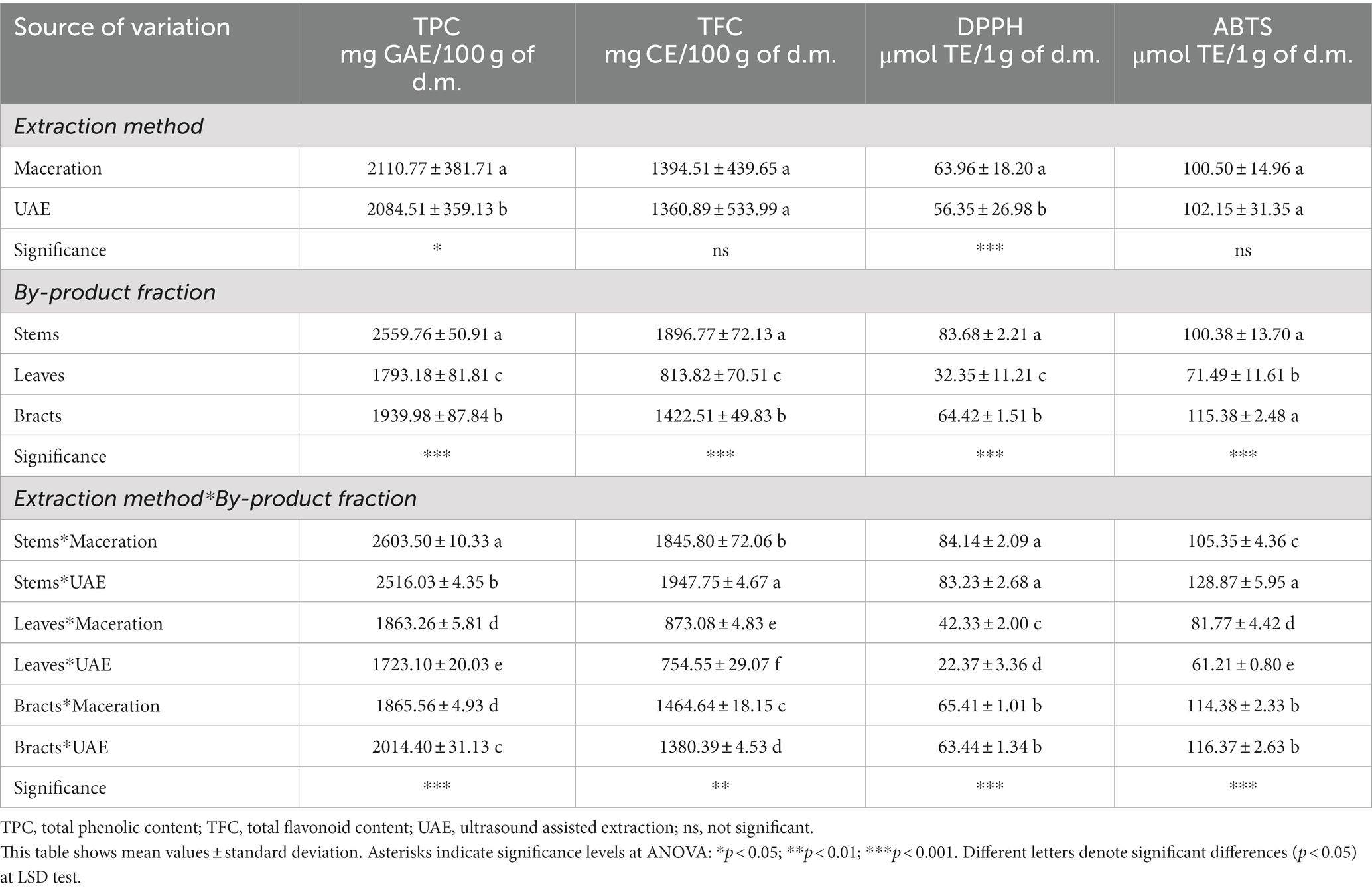
Table 5. Results of two-way ANOVA performed on total phenolic and flavonoid content, and on DPPH and ABTS results of the optimized extracts.
From the outputs of the two-way ANOVA shown in Table 5, it can be observed that for both TPC and TFC a highly significant effect (p < 0.001) of the by-product fraction was found. In fact, the highest values were obtained in the stems, regardless of the extraction method used, closely followed by the bracts and then the leaves. The gap in phenolic content among different parts of the globe artichoke plant is confirmed by the literature, as already reported above (Pandino et al., 2011a,b).
The two-way ANOVA also revealed that the effect of the extraction method was significant only for TPC (p < 0.05), with maceration being more effective than UAE, and that there was a highly significant interaction (p < 0.01) between the two simple effects (by-product fraction and extraction method) on both TPC and TFC.
The results revealed that significant differences were found in stems between the two extraction methods and that higher TPC were obtained with maceration, while UAE allowed better recovery of TFC. In leaves the maceration conducted to a significant higher yield extraction for both TPC and TFC. Concerning bracts, the maceration significantly increased the TFC content, but UAE was found more effective for TPC extraction (p < 0.05).
As it was possible to note, in most cases, contrary to what is usually found in the literature, maceration resulted in a more effective extraction of phenolic compounds. Generally, alternative extraction methods allow higher yields than conventional methods (Osorio-Tobón, 2020). In previous studies conducted on mango and kinnow peel, UAE proved to be a more efficient technique and resulted in higher phenolic content than maceration (Safdar et al., 2017a,b). Comparative investigations conducted on citrus peel and fresh olives also indicated the greater efficiency of UAE in extracting phenolics, both in terms of yield and antioxidant properties, with respect to maceration (Deng et al., 2017; Saini et al., 2019). The contrasting results obtained in the present study are probably due to the different operating conditions (sample preparation, state of the raw material, solvents, extraction times, instrumentation etc.) and matrices used.
3.6. Antioxidant capacity
The complex nature of phytochemicals in plant extracts hinders accurate assessment of total antioxidant capacity by a single method, therefore two commonly used assays were employed in this study: DPPH• and ABTS•+. Both methods are based on electron transfer and reduction of colored oxidants by various antioxidant species in the extracts, which could react in different ways with the two radicals used. In fact, the values achieved with the ABTS• + assay were always higher than those recorded with the DPPH• method (Table 5).
The following values were obtained from the DPPH• assay for maceration and UAE, respectively: in stems 84.14 ± 2.09 and 83.23 ± 2.68 μmol of TE·g−1; in leaves 42.33 ± 2.00 and 22.37 ± 3.36 μmol of TE·g−1; in bracts 65.41 ± 1.01 and 63.44 ± 1.34 μmol of TE·g−1. Regarding the ABTS• + assay, the results measured for maceration and UAE in artichoke by-products were as follow: in stems 105.35 ± 4.36 and 128.87 ± 5.95 μmol of TE·g−1; in leaves 81.77 ± 4.42 and 61.21 ± 0.80 μmol of TE·g−1; while in bracts 114.38 ± 2.33 and 116.37 ± 2.63 μmol of TE·g−1.
In Table 5, the two-way ANOVA indicated a highly significant (p < 0.01) effect of extraction method only for DPPH•. Instead, the by-product fraction effect affected significantly both the DPPH• and ABTS• + results (p < 0.01). In fact, the values measured on each fraction were different. The two-way ANOVA also pointed out the presence of a significant interaction (p < 0.01) between the effects of the extraction type and the by-product fraction. Therefore, it was noted that overall, the antioxidant capacity was highest in stems, followed by bracts and then leaves. In the DPPH• assay, the extraction method significantly influenced only the leaves fraction, where maceration yielded a higher extraction of antioxidant compounds. Besides, the ABTS• + method put in evidence that maceration was more efficient for the leaves respect to UAE. On the contrary, in the stems the UAE led to higher results than maceration.
An additional aspect that emerged was that the antioxidant capacity values were consistent with the phenolic content of the extracts. As a matter of fact, Pearson’s correlation test showed that TFC was significantly correlated with the antioxidant capacity, both in extracts obtained by maceration (DPPH• r = 0.99 p < 0.001; ABTS• + r = 0.83 p < 0.05) and by UAE (DPPH• r = 0.98 p < 0.01; ABTS• + r = 0.95 p < 0.01). With reference to TPC, a significant correlation with the values acquired from the DPPH• assay (r = 0.84; p < 0.05) was found for the extraction with maceration, while in the UAE the TPC resulted significantly correlated with both DPPH• and ABTS• + values (r = 0.93; p < 0.01 and r = 0.88; p < 0.05, respectively). This correlation between the phenolic content and antioxidant capacity, in extracts obtained from artichoke residues, appears consistent with previous findings reported by other authors (Jiménez-Moreno et al., 2019; Rejeb et al., 2020). Moreover, the highest correlation values between TFC and ABTS• + and DPPH• results, suggested that the antioxidant capacity of these extracts is closely related to their flavonoid content, probably due to the fact that flavonoids act as good hydrogen donors (Brown and Rice-Evans, 1998; Jiménez-Moreno et al., 2019).
3.7. Phenolics screening
Stems, bracts, and leaves extracts obtained by optimization of maceration and UAE were analyzed by HPLC-DAD to investigate their phenolic profiles. The concentration of the 16 identified compounds (flavones and caffeoylquinic acids) is displayed in Table 6. As it is conceivable to note, the two extraction methods allowed the extraction of the same compounds.
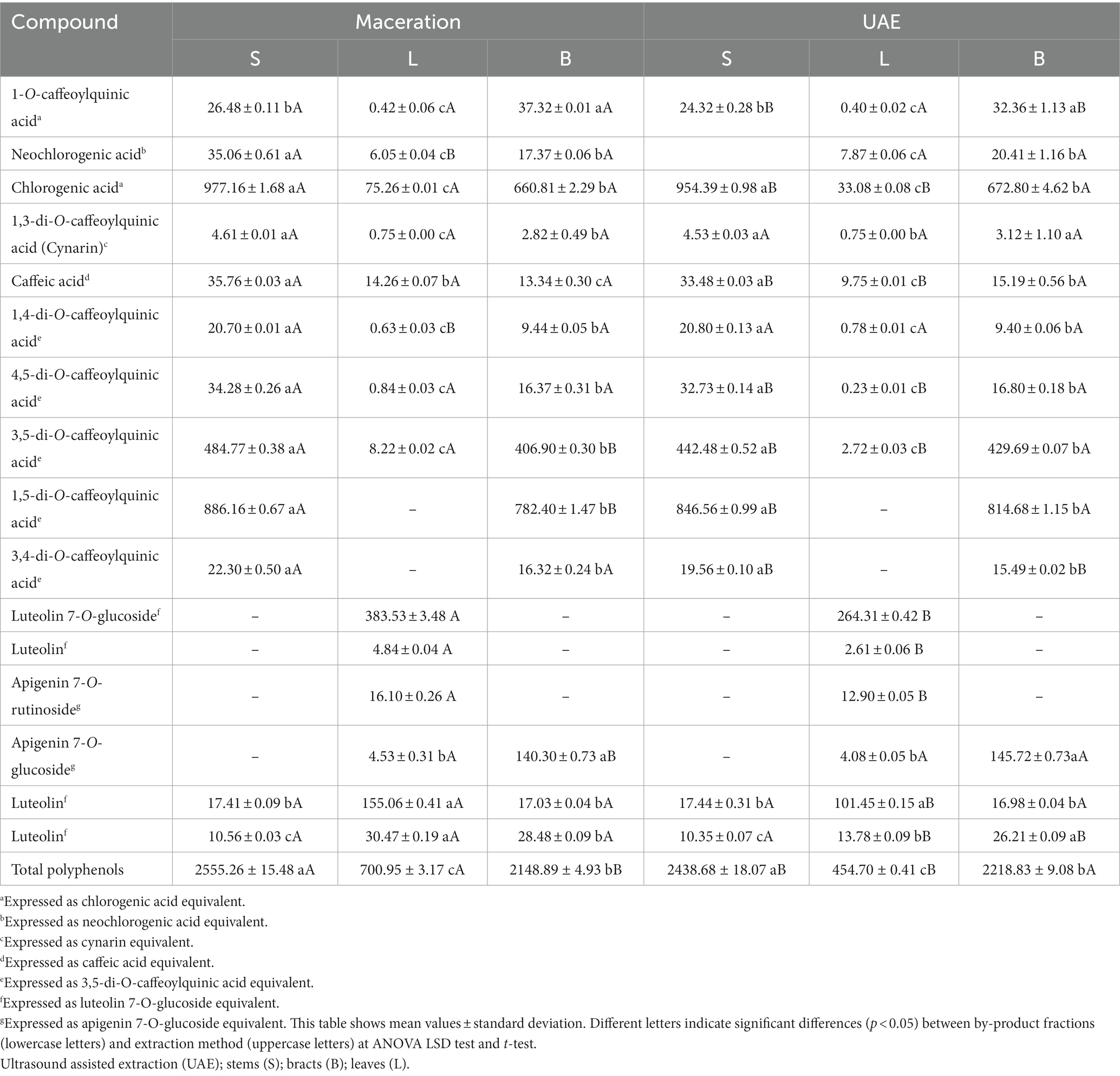
Table 6. Phenolic composition of the different artichoke by-products fractions optimized extracts, obtained with maceration and UAE, expressed in mg •100 g−1 of lyophilized material.
In both cases (UAE and maceration), stems and bracts showed similar phenolic composition, in which predominant constituents were caffeoylquinic acid derivatives, such as chlorogenic acid, 1,5-di-O-caffeoylquinic acid, and 3,5-di-O-caffeoylquinic acid, in order of prevalence. Otherwise, in leaves there was a predominance of flavonoid compounds, with a high concentration of luteolin 7-O-glucoside. Apigenin was found in leaves extracts as both glucoside and rutinoside. These flavonoids were poorly represented or absent in extracts obtained from bracts and stems. In agreement with previous works, these results confirmed that phenolic compounds are distributed differently in distinct parts of the plant, probably depending on their specific biological role (Fratianni et al., 2007; Lombardo et al., 2010; Pandino et al., 2011a). In fact, as these flavonoids are also deputed to protect cells from oxidative damage by ultraviolet light, they are mainly concentrated in leaves, which are the parts of the plant most exposed to sunlight (Lombardo et al., 2010; Samanta et al., 2011; Pandino et al., 2011a). Whereas in the stems there is a higher content of caffeoylquinic acids, probably because these compounds are involved in structural support within plant cell walls (Pandino et al., 2011a).
Overall, the total phenolics amount, obtained from the sum of all compounds quantified by HPLC-DAD, was highest in stems, followed by bracts and leaves (Table 6). Additionally, the t-test revealed that maceration was significantly more effective in stems and leaves, while for bracts UAE allowed for higher performance. The extraction method did not affect the amount of cynarin (1,3-di-O-caffeoylquinic acid) taken out from all the three fractions, while it affected the quantity of the other phenolic compounds. This aspect becomes important if the aim is to obtain an extract with characteristics related to the specific phytochemicals.
4. Conclusion
Achieving the sustainable development goal of halving food losses and waste by 50% by 2030, set by the United Nations in 2015, inevitably comes through the upcycling and valorization of plants by-products, which are known to be rich in bioactive compounds with potential interest for the food industry. In the present study, the effect of maceration and ultrasound assisted extraction process variables on the green recovery of phenolic compounds from artichoke stems, leaves, and bracts were evaluated by RSM. The investigation and optimization of the independent variables influencing the extraction efficiency within the studied design space revealed that maximum polyphenol and flavonoid yields were maintained at the lowest levels of extraction time for both maceration (60 min) and UAE (10 min)—except for sonicated bracts, which took about 41 min—and at intermediate percentages of ethanol for both techniques (42–64%)—except for sonicated leaves (20%). Under these optimal conditions, although maceration led to higher extraction efficiency, UAE resulted in very tight recoveries in shorter times and with lower ethanol consumption (except for bracts). Therefore, the use of UAE, in addition to being a competitive advantage for companies, can have a positive impact on the environment, economy, and society, allowing for a reduction in the amount of food waste generated, the energy consumption required during the recovery and valorization processes, and, consequently, process costs and the environmental impact.
Further research is needed to quantify the cost reduction associated with process optimization and the development on an industrial scale, as well as to evaluate the feasibility of incorporating these extracts into food products.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MC, PC, and ADC: methodology. MC: formal analysis and writing—original draft. PC, AP, and ADC: supervision and writing—review and editing. AP: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the MUR-PRIN research project protocol number 2017JTNK78_002 “GOOD-BY-WASTE. Obtain GOOD products—exploit BY-products—reduce WASTE.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acosta-Estrada, B. A., Gutiérrez-Uribe, J. A., and Serna-Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chem. 152, 46–55. doi: 10.1016/j.foodchem.2013.11.093
Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F., et al. (2013). Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 117, 426–436. doi: 10.1016/j.jfoodeng.2013.01.014
Brown, J. E., and Rice-Evans, C. A. (1998). Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Pad. Res 29, 247–255. doi: 10.1080/10715769800300281
Chávez-González, M. L., Sepúlveda, L., Verma, D. K., Luna-García, H. A., Rodríguez-Durán, L. V., Ilina, A., et al. (2020). Conventional and emerging extraction processes of flavonoids. PRO 8:434. doi: 10.3390/PR8040434
Chen, X. X., Wu, X. B., Chai, W. M., Feng, H. L., Shi, Y., Zhou, H. T., et al. (2013). Optimization of extraction of phenolics from leaves of Ficus virens. J. Zhejiang Univ. Sci. B 14, 903–915. doi: 10.1631/jzus.B1200365
Colantuono, A., Ferracane, R., and Vitaglione, P. (2018). Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 245, 838–844. doi: 10.1016/j.foodchem.2017.11.099
D’Antuono, I., Garbetta, A., Linsalata, V., Minervini, F., and Cardinali, A. (2015). Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): in vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 6, 1268–1277. doi: 10.1039/c5fo00137d
Dabbou, S., Dabbou, S., Flamini, G., Peiretti, P. G., Pandino, G., and Helal, A. N. (2017). Biochemical characterization and antioxidant activities of the edible part of globe artichoke cultivars grown in Tunisia. Int. J. Food Prop. 20, S810–S819. doi: 10.1080/10942912.2017.1315131
Dai, J., and Mumper, R. J. (2010). Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352. doi: 10.3390/molecules15107313
Deng, J., Xu, Z., Xiang, C., Liu, J., Zhou, L., Li, T., et al. (2017). Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 37, 328–334. doi: 10.1016/j.ultsonch.2017.01.023
Domínguez-Fernández, M., Irigoyen, Á., Vargas-Alvarez, M. D. L. A., Ludwig, I. A., De Peña, M. P., and Cid, C. (2021). Influence of culinary process on free and bound (poly)phenolic compounds and antioxidant capacity of artichokes. Int. J. Gastron. Food Sci. 25:100389. doi: 10.1016/j.ijgfs.2021.100389
Fadda, A., Virdis, A., Barberis, A., and Melito, S. (2018). Variation in secondary metabolites contents of spinoso sardo artichoke (Cynara cardunculus l.) under different day lengths. Turk. J. Agric. For. 42, 372–381. doi: 10.3906/tar-1711-27
Fadjare Frempong, T., Owusu Boadi, N., and Badu, M. (2021). Optimization of extraction conditions for polyphenols from the stem bark of Funtumia elastica (Funtum) utilizing response surface methodology. AAS Open Res. 4:46. doi: 10.12688/aasopenres.13284.1
Fernández, M. D. L. Á., Espino, M., Gomez, F. J. V., and Silva, M. F. (2018). Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 239, 671–678. doi: 10.1016/j.foodchem.2017.06.150
Fratianni, F., Tucci, M., Palma, M.De, Pepe, R., and Nazzaro, F. (2007). Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 104, 1282–1286. doi: 10.1016/j.foodchem.2007.01.044
Garcia-Castello, E. M., Mayor, L., Calvo-Ramirez, A., Ruiz-Melero, R., and Rodriguez-Lopez, A. D. (2022). Response surface optimization of inulin and polyphenol extraction from artichoke (Cynara scolymus (L.)) solid wastes. Appl. Sci. 12:7957. doi: 10.3390/app12167957
Ghafoor, K., Choi, Y. H., Jeon, J. Y., and Jo, I. H. (2009). Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 57, 4988–4994. doi: 10.1021/jf9001439
Gil-Martín, E., Forbes-Hernández, T., Romero, A., Cianciosi, D., Giampieri, F., and Battino, M. (2022). Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 378:131918. doi: 10.1016/j.foodchem.2021.131918
Jiménez-moreno, N., Cimminelli, M. J., Volpe, F., Ansó, R., Esparza, I., Mármol, I., et al. (2019). Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 11:1723. doi: 10.3390/nu11081723
Kollia, E., Markaki, P., Zoumpoulakis, P., and Proestos, C. (2017). Αntioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Nat. Prod. Res. 31, 1163–1167. doi: 10.1080/14786419.2016.1219864
Lattanzio, V., Kroon, P. A., Linsalata, V., and Cardinali, A. (2009). Globe artichoke: a functional food and source of nutraceutical ingredients. J. Funct. Foods 1, 131–144. doi: 10.1016/j.jff.2009.01.002
Lombardo, S., Pandino, G., Mauromicale, G., Knödler, M., Carle, R., and Schieber, A. (2010). Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 119, 1175–1181. doi: 10.1016/j.foodchem.2009.08.033
López-Salas, L., Borrás-Linares, I., Quintin, D., García-Gomez, P., Giménez-Martínez, R., Segura-Carretero, A., et al. (2021). Artichoke by-products as natural source of phenolic food ingredient. Appl. Sci. (Switzerland) 11:3788. doi: 10.3390/app11093788
Medina-Torres, N., Ayora-Talavera, T., Espinosa-Andrews, H., Sánchez-Contreras, A., and Pacheco, N. (2017). Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 7:47. doi: 10.3390/agronomy7030047
Méndez, D. A., Fabra, M. J., Falcó, I., Sánchez, G., Aranaz, P., Vettorazzi, A., et al. (2021). Bioactive extracts from persimmon waste: influence of extraction conditions and ripeness. Food Funct. 12, 7428–7439. doi: 10.1039/d1fo00457c
Noriega-Rodríguez, D., Soto-Maldonado, C., Torres-Alarcón, C., Pastrana-Castro, L., Weinstein-Oppenheimer, C., and Zúñiga-Hansen, M. E. (2020). Valorization of globe artichoke (Cynara Scolymus) agro-industrial discards, obtaining an extract with a selective EECT on viability of cancer cell lines. PRO 8:715. doi: 10.3390/pr8060715
Osorio-Tobón, J. F. (2020). Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 57, 4299–4315. doi: 10.1007/s13197-020-04433-2
Panche, A. N., Diwan, A. D., and Chandra, S. R. (2016). Flavonoids: An overview. J. Nutr. Sci. 5:e47. doi: 10.1017/jns.2016.41
Pandino, G., Lombardo, S., Mauromicale, G., and Williamson, G. (2011a). Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 126, 417–422. doi: 10.1016/j.foodchem.2010.11.001
Pandino, G., Lombardo, S., Mauromicale, G., and Williamson, G. (2011b). Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compost. Anal. 24, 148–153. doi: 10.1016/j.jfca.2010.04.010
Panja, P. (2018). Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 23, 173–182. doi: 10.1016/j.cofs.2017.11.012
Prior, R. L., Wu, X., and Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53, 4290–4302. doi: 10.1021/jf0502698
Quispe, M. A., Valenzuela, J. A. P., de la Cruz, A. R. H., Silva, C. R. E., Quiñonez, G. H., and Cervantes, G. M. M. (2021). Optimization of ultrasound-assisted extraction of polyphenols from globe artichoke (Cynara scolymus L.) bracts residues using response surface methodology. Acta Sci. Pol. Technol. Aliment. 20, 277–290. doi: 10.17306/J.AFS.0937
Reche, C., Rosselló, C., Dalmau, E., Eim, V., and Simal, S. (2022). Quantification of microstructural changes in artichoke by-products by image analysis after high-power ultrasound-assisted extraction of bioactive compounds. LWT 171:114127. doi: 10.1016/j.lwt.2022.114127
Reche, C., Rosselló, C., Umaña, M. M., Eim, V., and Simal, S. (2021). Mathematical modelling of ultrasound-assisted extraction kinetics of bioactive compounds from artichoke by-products. Foods 10:931. doi: 10.3390/foods10050931
Rejeb, I. B., Dhen, N., Gargouri, M., and Boulila, A. (2020). Chemical composition, antioxidant potential and enzymes inhibitory properties of globe artichoke by-products. Chem. Biodivers. 17:e2000073. doi: 10.1002/cbdv.202000073
Safdar, M. N., Kausar, T., Jabbar, S., Mumtaz, A., Ahad, K., and Saddozai, A. A. (2017a). Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 25, 488–500. doi: 10.1016/j.jfda.2016.07.010
Safdar, M. N., Kausar, T., and Nadeem, M. (2017b). Comparison of ultrasound and maceration techniques for the extraction of polyphenols from the mango Peel. J. Food Process. Preserv. 41:e13028. doi: 10.1111/jfpp.13028
Saini, A., Panesar, P. S., and Bera, M. (2019). Comparative study on the extraction and quantification of polyphenols from citrus peels using maceration and ultrasonic technique. Curr. Res. Nut. Food Sci. 7, 678–685. doi: 10.12944/CRNFSJ.7.3.08
Samanta, A., Das, G., and Das, S. K. (2011). Roles of flavonoids in plants. Int. J. Pharm. Sci. Tech. 6, 12–35. ISSN: 0975-0525
Stumpf, B., Künne, M., Ma, L., Xu, M., Yan, F., Piepho, H. P., et al. (2020). Optimization of the extraction procedure for the determination of phenolic acids and flavonoids in the leaves of globe artichoke (Cynara cardunculus var. scolymus L.). J. Pharm. Biomed. Anal. 177:112879. doi: 10.1016/j.jpba.2019.112879
Taghian Dinani, S., and van der Goot, A. J. (2022). Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: a review. Crit. Rev. Food Sci. Nutr., 62, 1–23. doi: 10.1080/10408398.2022.2049692
Trigo, J. P., Alexandre, E. M. C., Saraiva, J. A., and Pintado, M. E. (2020). High value-added compounds from fruit and vegetable by-products–characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 60, 1388–1416. doi: 10.1080/10408398.2019.1572588
Yolmeh, M., and Jafari, S. M. (2017). Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 10, 413–433. doi: 10.1007/s11947-016-1855-2
Živković, J., Šavikin, K., Janković, T., Ćujić, N., and Menković, N. (2018). Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 194, 40–47. doi: 10.1016/j.seppur.2017.11.032
Zuorro, A. (2014). Response surface methodology analysis of polyphenol recovery from artichoke waste. Am. J. Appl. Sci. 11, 1463–1471. doi: 10.3844/ajassp.2014.1463.1471
Zuorro, A., Maffei, G., and Lavecchia, R. (2014). Effect of solvent type and extraction conditions on the recovery of phenolic compounds from artichoke waste. Chem. Eng. Trans. 39, 463–468. doi: 10.3303/CET1439078
Keywords: artichoke by-products, green recovery, phenolics, antioxidant activity, response surface methodology
Citation: Cannas M, Conte P, Piga A and Del Caro A (2023) Green recovery optimization of phenolic compounds from “Spinoso sardo” globe artichoke by-products using response surface methodology. Front. Sustain. Food Syst. 7:1215809. doi: 10.3389/fsufs.2023.1215809
Edited by:
Carlos Enrique Ochoa-Velasco, Meritorious Autonomous University of Puebla, MexicoReviewed by:
Paola Hernández-Carranza, Benemérita Universidad Autónoma de Puebla, MexicoSelin Şahin Sevgili, Istanbul University, Türkiye
Pasquale Crupi, University of Bari Aldo Moro, Italy
Copyright © 2023 Cannas, Conte, Piga and Del Caro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Conte, cGNvbnRlQHVuaXNzLml0
 Michela Cannas
Michela Cannas Paola Conte
Paola Conte Antonio Piga
Antonio Piga Alessandra Del Caro
Alessandra Del Caro