
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 18 May 2023
Sec. Sustainable Food Processing
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1189149
This article is part of the Research Topic The Aquatic Product Processing and By-product Utilization View all 10 articles
 Weiwen Duan1
Weiwen Duan1 Hui Qiu1
Hui Qiu1 Kyi Kyi Htwe1
Kyi Kyi Htwe1 Shuai Wei1
Shuai Wei1 Yang Liu1
Yang Liu1 Zefu Wang1
Zefu Wang1 Qinxiu Sun1
Qinxiu Sun1 Zongyuan Han1
Zongyuan Han1 Qiuyu Xia1
Qiuyu Xia1 Shucheng Liu1,2,3*
Shucheng Liu1,2,3*Changes in protein structure are closely related to gel strength. Dense phase carbon dioxide (DPCD) treatment is an excellent non-thermal food processing method that can be used to induce gel formation in surimi. The sensory, water holding capacity and gel strength of DPCD induced gels are superior to heat-induced gels. Fourier-transform infrared spectroscopy was used to investigate the role of DPCD in the quality of golden pompano surimi gels and changes in protein structure. The intermolecular forces of surimi gels were analyzed in terms of ionic and hydrogen bonds, disulfide covalent and non-disulfide covalent bonds, as well as hydrophobic interactions. Correlation analysis was used to investigate the relationship between the changes in advanced protein structure and gel strength during DPCD-induced gel formation in golden pompano surimi. The results showed that the α-helix and random coil levels of surimi gel were significantly decreased (p < 0.05), while the β-sheet and β-turn content was significantly increased (p < 0.05). The number of ionic and hydrogen bonds in gel proteins decreased significantly (p < 0.05), while the hydrophobic interactions, and disulfide and non-disulfide covalent bonds increased significantly (p < 0.05) after DPCD treatment. Correlation analysis showed that β-sheets, β-turns, hydrophobic interactions, and disulfide and non-disulfide covalent bonds were strongly positively correlated with gel strength, whereas α-helices, random coils, and ionic and hydrogen bonds were strongly negatively correlated with gel strength. Therefore, the α-helix and random coil structures of surimi gels were transformed into β-sheet and β-turn structures after DPCD treatment. Hydrophobic interactions, and disulfide and non-disulfide covalent bonds were the main intermolecular forces during the DPCD-induced gel formation of surimi. Ionic and hydrogen bonds were not the main intermolecular forces. The results provide fundamental data for elucidating the mechanism of DPCD-induced protein gel formation.
Golden pompano (Trachinotus ovatus) is an important commercial marine fish inhabiting the coastal areas of southern China (Liu et al., 2019). It is a delicious, nutritious and fast-growing fish. The recent increase in market demand and the widespread application of cage culture technology, have increased the yield of golden pompano over the years (Bureau of Fisheries of the Ministry of Agriculture, 2021). As an important aquatic food, surimi is popular among consumers because of its convenience, high protein and low fat content. With the rapid development of the surimi industry, challenges such as unstable quality of surimi products, single processing method, severe homogenization of products, and lack of healthy new products have emerged (Jaziri et al., 2021; Monto et al., 2021). Therefore, effective improvement of the quality of surimi products and development of healthy surimi products is an important issue in the field of aquatic processing.
Currently, the processing of surimi products is mainly based on heat treatment. However, excessive heat treatment may result in the loss of heat-sensitive substances such as fatty acids, amino acids, and bioactive peptides in surimi. The poor heating and heat transfer rates, which prolong the temperatures treatment, result in gel degradation. Compared with a few non-thermal processing methods, heat treatment is associated with high energy consumption, and the waste generated during the treatment is not conducive to environmental protection (Nakamura et al., 2021). Dense phase carbon dioxide (DPCD) treatment is a new non-thermal processing method that couples CO2 at a specific temperature (< 60°C) and pressure (< 50 MPa). DPCD treatment generates high-pressure acidic environment due to the pressure of carbon dioxide and its molecular action (Guo et al., 2017). Compared with conventional heat treatment, DPCD can be used under mild processing conditions. It is widely used for sterilization, enzyme inactivation, and improvement of textural and nutritional properties of food products (Damar et al., 2006). In addition, due to its low viscosity and high diffusivity, CO2 penetrates bacterial cell membranes. Thus, DPCD can be used for maximum preservation of food quality while avoiding thermal damage.
As an elastic protein concentrate, the gel strength of surimi represents an important index for the evaluation of surimi products (Duan et al., 2023). During the gelation, the change in protein structure is closely related to the change in gel strength because the gelation is the result of protein denaturation and aggregation. Under the action of external factors, such as heat, acid, salt, and high pressure, the protein is first denatured. The intermolecular forces (hydrogen bonds, ionic bonds, hydrophobic interactions, disulfide and non-disulfide covalent bonds) that maintain the protein structure are disrupted. The secondary structures (α-helix, β-sheet, β-turn, and random coil) are also changed and the groups are exposed. The protein then aggregates and forms a stable gel three-dimensional gel network under the influence of covalent and non-covalent chemical interactions within the protein molecule (Liu et al., 2014). As reported previously by our team, DPCD treatment can denature proteins and form gels, while maintaining the color and water-holding qualities of food products. It can be used as an alternative to traditional thermal processing methods (Zheng et al., 2022; Duan et al., 2023). Accordingly, this study investigated the effects of DPCD treatment on the secondary structure and intermolecular forces of surimi gel proteins, and analyzed the correlation between the effects of DPCD on protein conformation and gel strength of golden pompano surimi. It provides fundamental data to elucidate the mechanism of DPCD-induced gel formation in pompano surimi, and thereby provides a theoretical basis for the development of aquatic gel products using DPCD technology.
Golden pompano fish of average weight (750 ± 50 g) were purchased from Dongfeng Seafood Market (Zhanjiang, China). The fish were stored in oxygenated water and immediately transported to the laboratory within 1 h. The fish were rapidly immersed in ice water. The surimi was prepared according to the method proposed by Liu et al. (2021). Briefly, fresh fish were minced, washed and dewatered, following by chopping, mixing with salt, setting, cooking, and cooling. The prepared surimi was evacuated using a vacuum packaging machine (DZ500/2D, Wenzhou, China) and refrigerated at − 35°C. Pure carbon dioxide (99.99%) was obtained from the Zhanjiang Oxygen Plant. All the chemicals and solvents used in this study were of analytical grade.
The process of DPCD treatment is based on methods previously reported by our team (Zhang et al., 2011). The surimi was placed in a custom-made cylindrical mold, before being processed by the DPCD equipment (Figure 1), this equipment diagram is taken from Duan et al. (2023). The main steps include equipment heating, sample placement, sealing, venting, pressurizing, pressure holding, and pressure release sampling. The treated samples are placed in sealed bags and the test indices are measured after 12 h at 4°C.
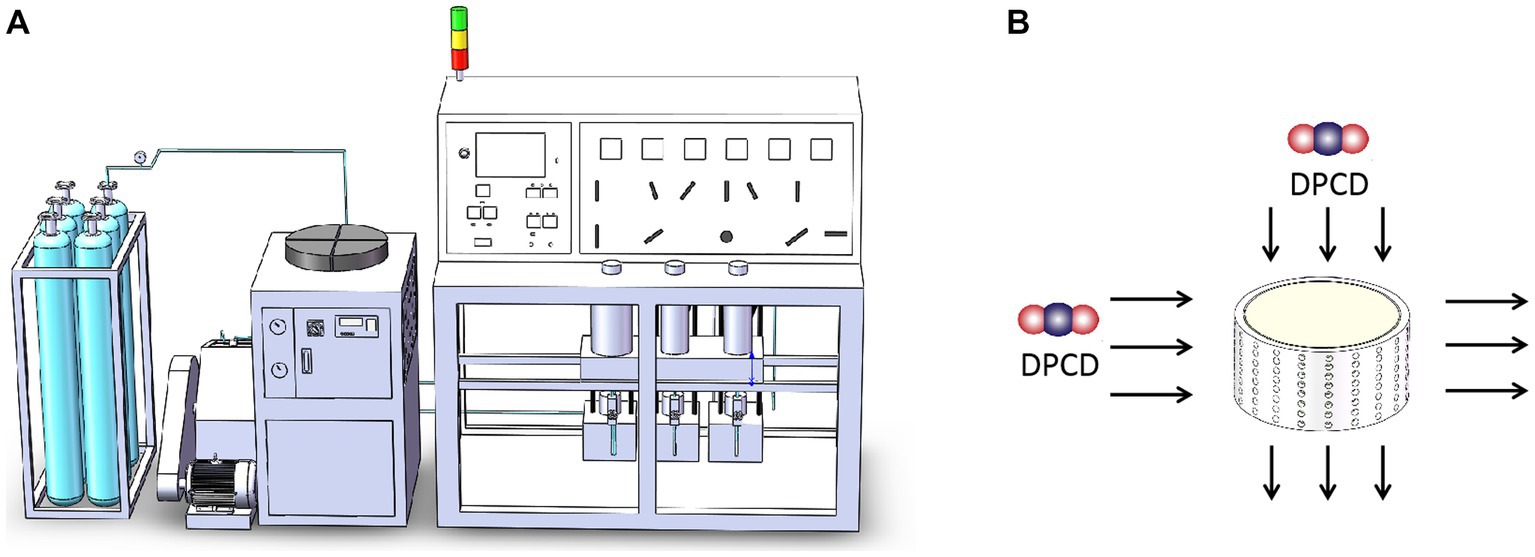
Figure 1. Dense phase carbon dioxide (DPCD) processing unit diagram (A) and mold drawing (B): colorful balls is dense phase carbon dioxide.
A single-factor experimental design was used. The detailed experimental conditions are shown in Table 1. Two control groups were set up: the untreated group representing the raw surimi group (Control), and the heat-treated group (WB) heated in a two-stage water bath at 40°C for 30 min (pre-gelation), followed by heating at 90°C for 30 min (gelation).
The vacuum freeze-dried sample was thoroughly mixed with anhydrous potassium bromide, and ground and pressed into transparent flakes. It was scanned at the full wavelength (400 ~ 4,000 cm−1) using an infrared spectrometer (FTIR Bruker Tensor 27; Ettlingen, Germany) with a resolution of 4 cm−1; the scans were accumulated 32 times and repeated 3 times. Then, Omnic 9.2 and Peakfit V4.12 software programs were used to analyze the characteristic spectral peaks of the amide I band in the 1,600 ~ 1,700 cm−1 band. First, the baseline was corrected, followed by Gaussian deconvolution and fitting with second-order derivatives to maximize the residuals. The number of sub-peaks were obtained and the relative percentage of each sub-peak area was calculated to determine the levels of protein secondary structure.
The intermolecular forces of the protein were analyzed using a protein solubility method with slight modifications (Tan et al., 2010). The 2 g sample was treated with 10 mL B1 (0.6 mol/L NaCl), homogenized at 5,000 rpm/min for 5 min, transferred to 4°C for 1 h, centrifuged at 4°C and 18,600 × g for 25 min, and filtered through 1,000 mesh filter cloth, the supernatant S1 was stored at 4°C. Add 10 mL of B2 (1.5 mol/L urea, 0.6 mol/L NaCl) to the precipitate from B1, homogenized at 3,700 rpm/min for 2 min, transferred to 4°C for 1 h, centrifuged at 4°C, 18,600 × g for 25 min, stored the supernatant S2 at 4°C. Add 10 mL of B3 (8 mol/L urea, 0.6 mol/L NaCl) to the precipitate from B2, homogenized at 5,000 rpm/min for 5 min, transferred to 4°C for 1 h, centrifuged at 4°C, 18,600 × g for 25 min, stored the supernatant S3 at 4°C (repeated twice). Add 10 mL of B4 (0.5 mol/L β-mercaptoethanol, 0.6 mol/L NaCl, 8 mol/L urea) to the precipitate from B3, homogenized at 5,000 rpm/min for 5 min, transferred to 4°C for 1 h, centrifuged at 4°C, 18,600 × g for 25 min, store the supernatant S4 at 4°C. The final precipitate was dissolved in 10 mL B5 (1 mol/L NaOH solution). The supernatant obtained after centrifugation was added into the same volume (5 mL) of 20% trichloroacetic acid, and centrifuged at 3,950 × g for 15 min. The supernatant was discarded, and 1 mL of 1 mol/L NaOH solution was added into the precipitation and placed at 4°C (S5). The protein content of each supernatant was determined via Bradford method. The protein content of S1, S2, S3, S4, and S5 were accounted for the percentage of the total protein content that represented ionic bonds, hydrogen bonds, hydrophobic interactions, disulfide bonds, and non-disulfide covalent bonds in the whole system, respectively.
The gel strength of the surimi was determined using a TMS-Pro analyzer (FTC Co., Ltd., Vienna, Virginia, United States). The probe = P/0.5 s, trigger force = − 5 g; pre-test speed = 5 mm s−1; test speed = 1 mm·s−1; compression deformation, 75% were set. The gel strength (g × mm) was obtained by multiplying the breaking strength (g) and the breaking distance (mm).
Experimental data were expressed as the mean ± standard deviation. Variance and Tukey’s HSD multiple comparisons (with a 95% confidence interval) were obtained using JMP 16.0 software. The correlation between advanced protein structure and gel strength during DPCD-induced gel formation in surimi was analyzed via Pearson’s correlation analysis using Origin 2022 software (Origin Lab, Hampton, NH, United States). Three batches of experiments were performed, with each batch containing three parallel samples.
Fourier-transform infrared spectroscopy is easy to operate. Independent of functional groups, it can be used to quickly and accurately detect small structural changes in complex material systems. In addition, its low sample requirement and reproducible detection facilitate its widespread application for structural identification of materials in complex systems such as food (Wan et al., 2022). In general, protein infrared spectra consist of amides I, II, III, A and B. They can be used to determine the secondary structure of complex proteins because they are almost independent of the nature of the side chains (Leng et al., 2022). They strongly contribute to hydrogen bonding, dipole–dipole interactions and the geometry of the protein peptide skeleton.
The effects of different DPCD treatments on the secondary structure of surimi are presented in Figure 2. With the increase in the intensity of different DPCD treatments, surimi proteins show typical absorptions at all major wave numbers in the FT-IR, with distinctive absorptions near the bands of amide A (~3,300 cm−1), amide B (~3,100 cm−1), amide I (1,700–1,600 cm−1), amide II (1,600–1,500 cm−1), and amide III (1,220–1,330 cm−1; Xie et al., 2020). The red shift of the amide A band peak indicates N–H stretching and hydrogen bond formation. It suggests reduction of the intramolecular and intermolecular N–H stretching vibrations of surimi protein or weakening of hydrogen bonds by DPCD treatment. The amide I–III bands are strongly sensitive to the secondary structure of protein molecules, especially the amide I band located at 1,700–1,600 cm−1, which is often used to analyze the secondary structure of proteins. Compared with the control group, the wave positions of the characteristic absorption peaks of amides B and II of surimi protein after DPCD treatment did not shift significantly after DPCD treatment. However, the wave peaks of the amide I band shifted to lower wave numbers, indicating a further decrease in the α-helical structure of surimi protein with the increased treatment intensity (Andonegi et al., 2020).
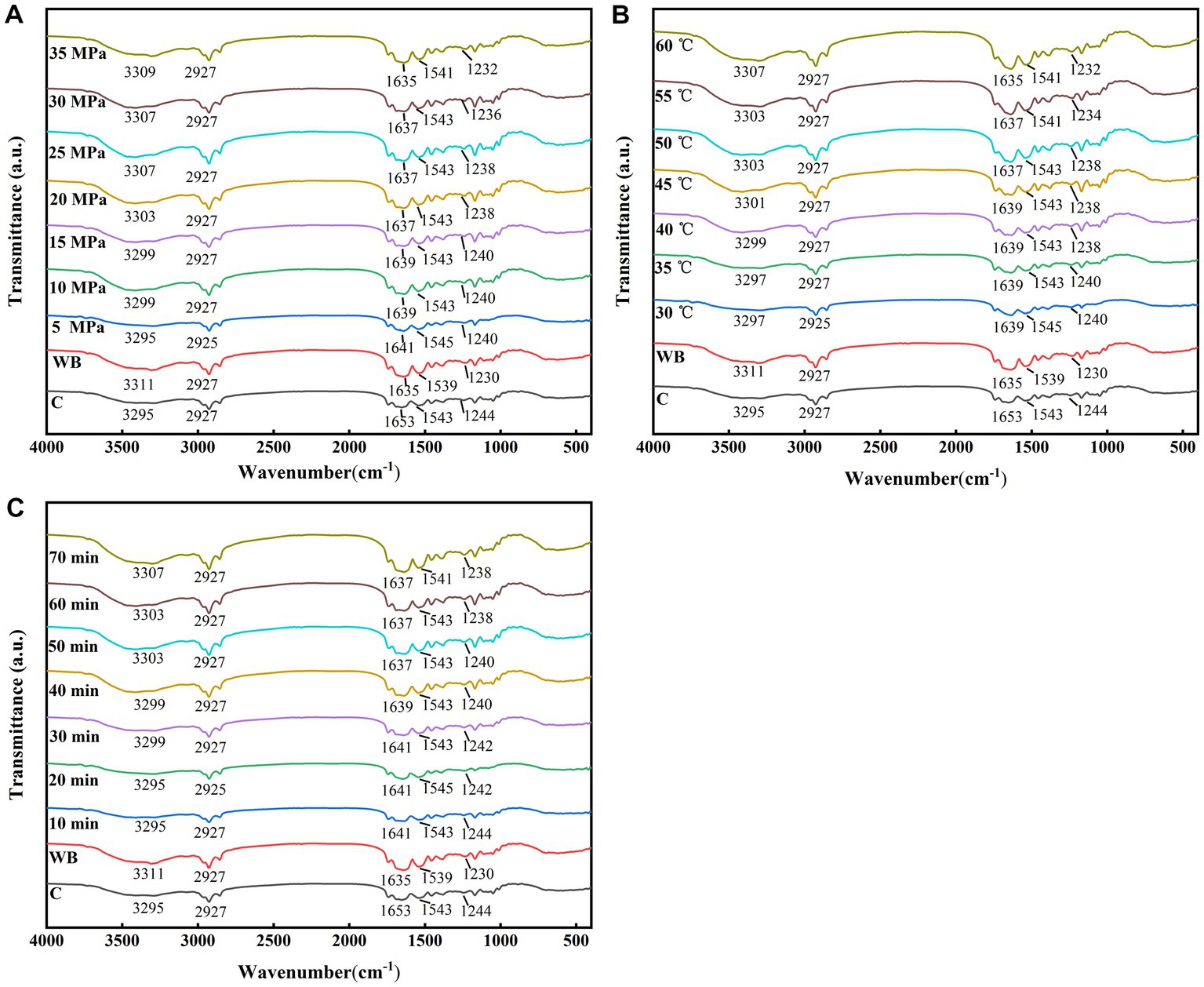
Figure 2. Fourier-transform infrared spectroscopy (FT-IR) spectra of protein in surimi with different DPCD treatments (A: Pressure, B: Temperature, C: Time).
The protein secondary structure includes four main forms: α-helix, β-sheet, β-turn, and random coil, which correspond to the FT-IR wave number ranges of 1,650 ~ 1,660, 1,600 ~ 1,640, 1,660 ~ 1700, and 1,640 ~ 1,650 cm−1, respectively (Yang et al., 2022). The effects of different DPCD treatments on the secondary structure levels of surimi protein are shown in Figure 3, based on the Gaussian fit of the protein secondary structure in the range of 1,700 ~ 1,600 cm−1. The results showed a significant difference in the effect of different DPCD treatments on the secondary structure of surimi protein compared with the control group (p < 0.05). With increased intensity of DPCD treatment, the levels of α-helix and random coil decreased significantly (p < 0.05), and the content of β-sheet and β-turn increased significantly (p < 0.05). As the treatment intensity continued to increase, the concentration of each component gradually decreased. During the gelation of surimi protein, the formation of β-sheet is often accompanied by the decrease of α-helix. The β-sheet structure promotes the formation of a more ordered and compact network structure (Wei et al., 2018). This indicates that the protein structure of surimi protein undergoes reconstruction of secondary structure under the action of DPCD, mainly from α-helix and random coil to β-sheet and β-turn.
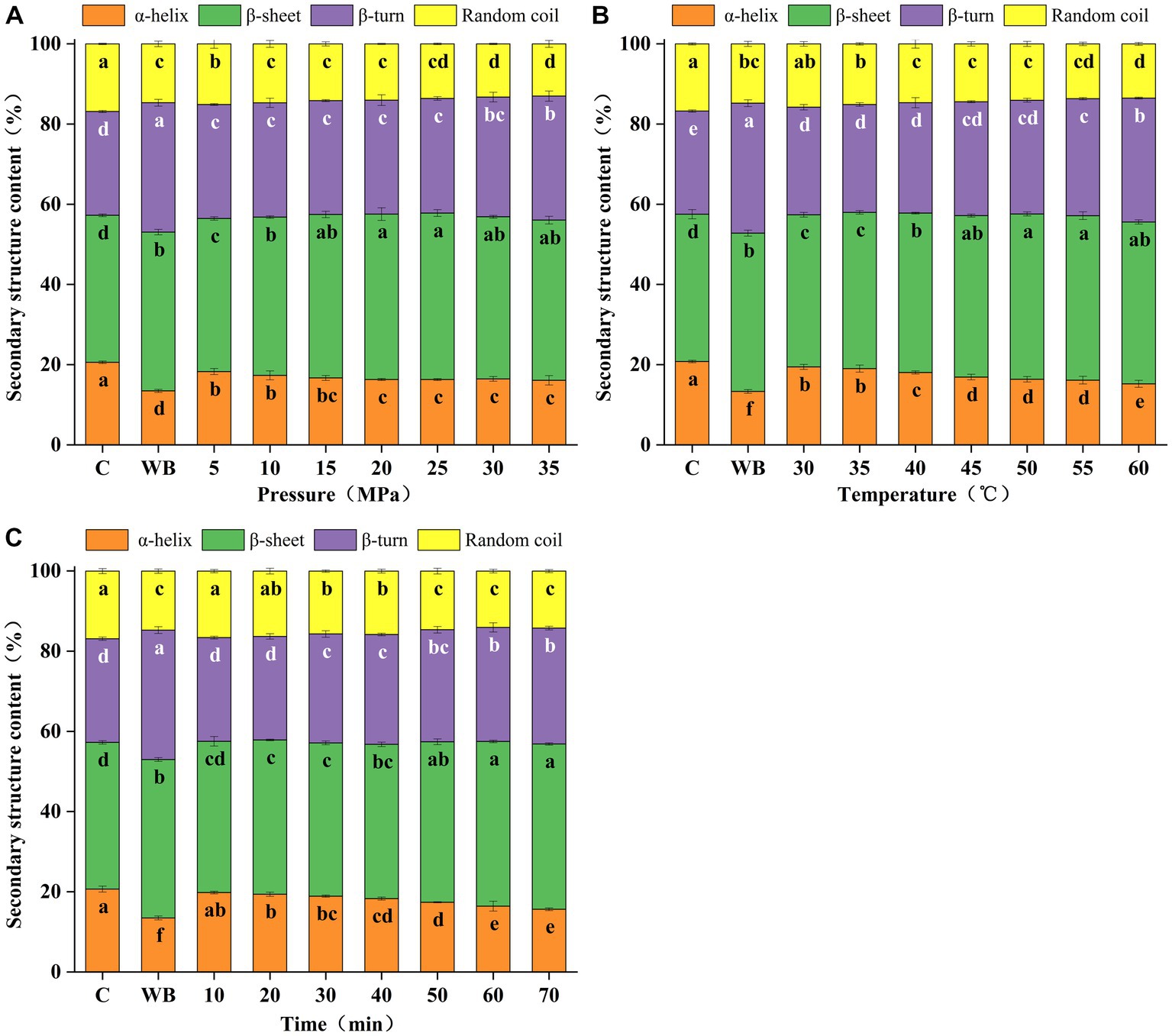
Figure 3. Effect of different DPCD treatments on the secondary structure content of surimi (A: Pressure, B: Temperature, C: Time). Different letters indicate statistically significant differences (p < 0.05).
The secondary structure of surimi protein has a significant effect on its processing characteristics. The content of α-helical structure is closely related to the gel strength. The α-helix is the major conformation of the secondary structure in natural proteins. During the processing of surimi into gels, the α-helix structure is partially uncoiled and transformed into β-sheet, β-turn or random coil. The β-sheet structure contributes to the hardness of surimi gels, while the β-turn and random coils cannot induce the formation of an ordered gel network (Ding et al., 2019). As shown in Figure 3, the content of α-helix and random coil was significantly lower (p < 0.05) and the level of β-sheet and β-turn was significantly higher (p < 0.05) in thermally-induced gel samples (13.3%) compared with the control group (20.8%). In addition, the α-helices were significantly fewer (p < 0.05) and the β-turns were significantly higher (p < 0.05) in the heat-treated gel samples than in the other sample groups. This indicates the destruction of additional α-helical structures and their transformation into β-turns under heat treatment.
Under normal pH of surimi (near neutral), the ionic bond is the main force contributing to the natural structure of myogenic fibronectin. However, the ionic bond between protein molecules can be broken by external factors, such as heat, salt, and high pressure, resulting in protein aggregation and gelation (Hiromoto et al., 2022). As shown in Figure 4, compared with the control group (16.4%), the ionic bond of golden pompano surimi showed different degrees of decrease under different DPCD treatment intensities. The ionic bond content decreased significantly (p < 0.05) when the treatment pressure reached 5 MPa (9.3%). No significant change in the ionic bond content was detected when the treatment pressure continued to increase above 20 MPa. It indicates that at lower treatment pressure, the reduced pH of CO2 and molecular effects break the ionic bonds between protein molecules, as well as between protein and salt ions (Ohashi et al., 1991). The ionic bond content of the surimi samples under different DPCD treatment times was similar to that of the different DPCD treatment pressure samples, with a significant decrease (p < 0.05) when the treatment time ranged between 10 min (14.1%) and 50 min (7.1%). No significant change in ionic bond content was found when the treatment time was extended beyond 50 min. The number of ionic bonds of the surimi samples at different DPCD treatment temperatures first decreased and then increased. The ionic bonds decreased significantly (p < 0.05) with increasing temperature below 50°C (6.7%), indicating that heating can disrupt the repulsion between protein molecules (Yang et al., 2020). However, when the temperature was above 50°C, the ionic bond content of the surimi increased slightly. Due to the addition of salts such as KCl and NaCl during the preparation of surimi, the ionic bonds between protein molecules were broken and new ionic bonds were formed between proteins and salt ions when the treatment temperature reached 60°C. Two key factors contribute to the effect of DPCD on the ionic bonding of surimi. First, the dissolution of CO2 in water decreases the pH of the system, which affects the ionization of proteins and the net charge value by adjusting the pH. Second, the heating facilitates the free movement of the ions without binding in a fixed lattice.
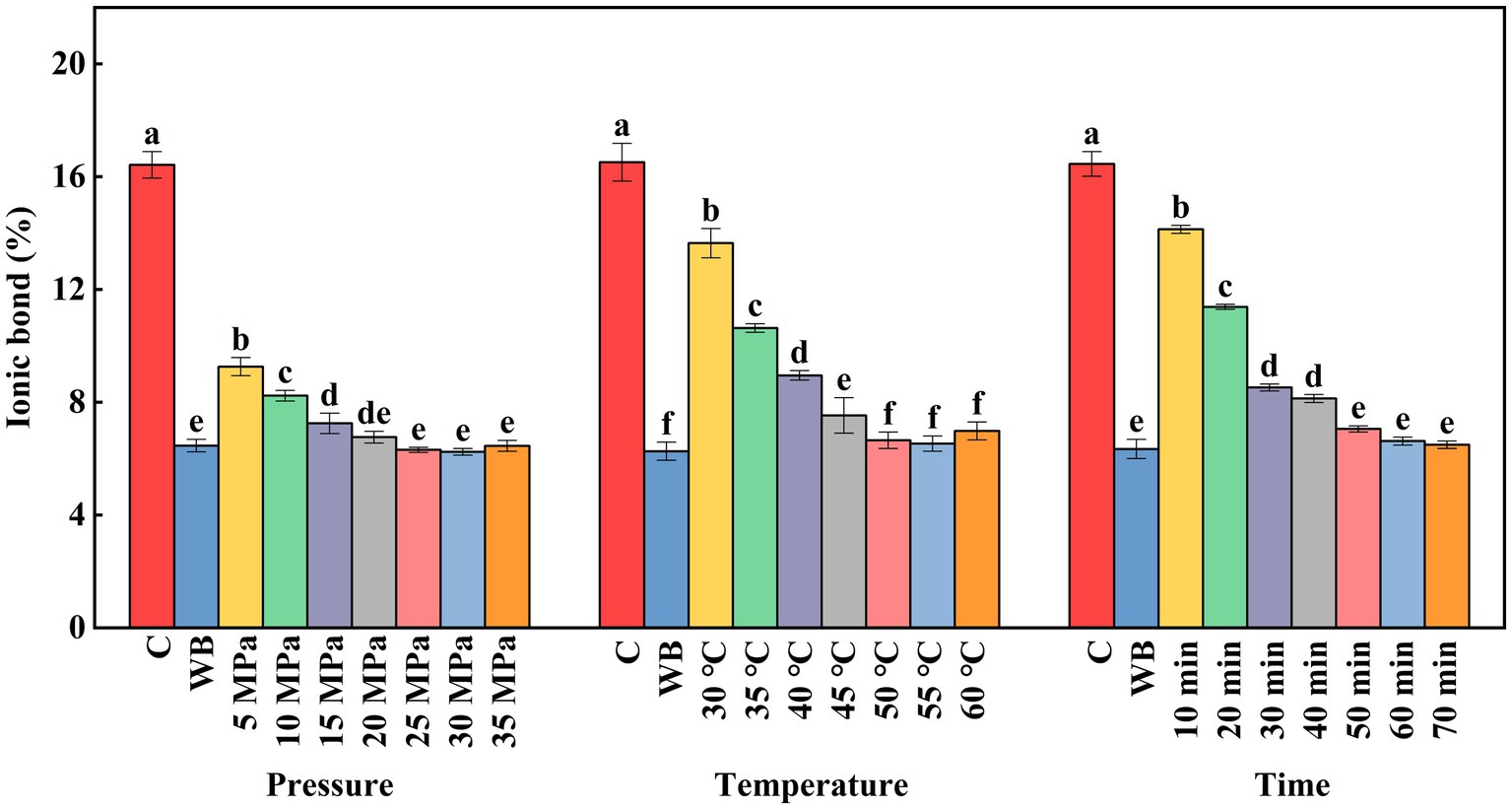
Figure 4. Effect of DPCD treatment on ionic bonds of golden pompano surimi. Different letters indicate statistically significant differences (p < 0.05).
Hydrogen bond is a weak dipole bond and the main chemical force that maintains the secondary structure of natural proteins. As shown in Figure 5, the hydrogen bond content in untreated surimi was (15.6%). The different DPCD treatments significantly reduced the hydrogen bond content in surimi compared with the untreated group (p < 0.05). The hydrogen bonds of surimi showed a significant decrease with increasing pressure during DPCD treatment, especially below 15 MPa (p < 0.05). The DPCD denatures the surimi due to decreased pH, resulting in disruption of hydrogen bonds. When the DPCD treatment pressure was higher than 20 MPa, the hydrogen bonds decreased slowly, which may be attributed to the steady state after the prior disruption of a large number of hydrogen bonds in surimi (Xu et al., 2011). The trend of the hydrogen bond content of the surimi samples under different DPCD treatment times was similar to that of the stress group, which decreased significantly (p < 0.05) when the treatment time was less than 50 min. It did not change significantly when the treatment time was greater than 50 min. The hydrogen bonds in the surimi samples under different DPCD treatment temperatures decreased significantly (p < 0.05) below 50°C, because hydrogen bonding is more sensitive to temperature, and the higher the temperature, the weaker the hydrogen bonding (Wang et al., 2020). However, there was no significant change in the hydrogen bond content of the surimi when the treatment temperature was higher than 50°C. The thermal effect already induced severe damage in the protein. When the denaturation temperature was exceeded, the hydrogen bonds in surimi did not change significantly.
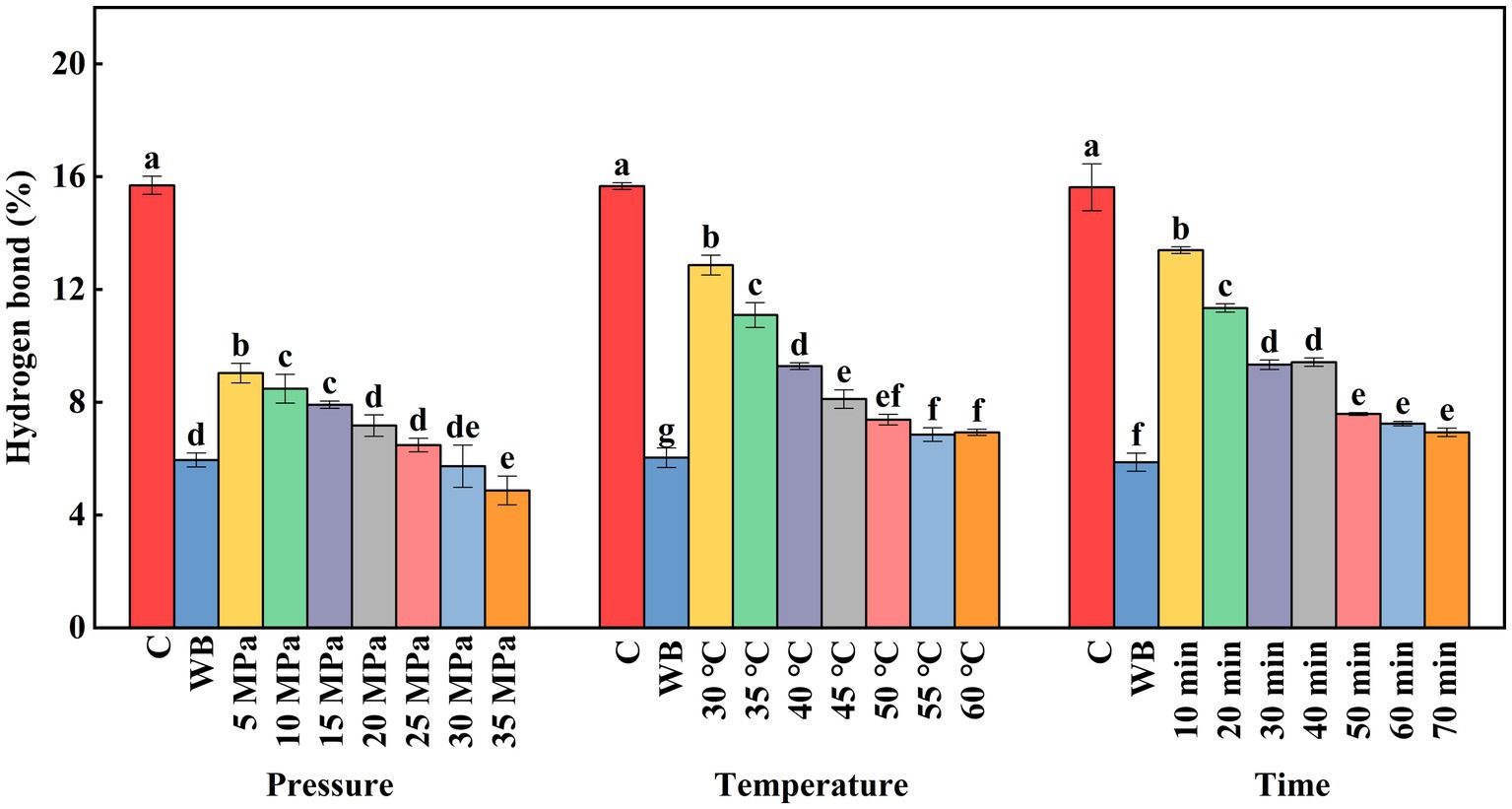
Figure 5. Effect of DPCD treatment on hydrogen bonds of golden pompano surimi. Different letters indicate statistically significant differences (p < 0.05).
Hydrophobic interaction occurs when natural proteins are exposed to external factors, such as heat, high pressure, and ions. Hydrophobic groups on the molecular surface are exposed and the hydrophobic components of neighboring proteins are bound tightly. Hydrophobic interactions cause protein aggregation, which is one of the factors facilitating gel formation in surimi (Gilleland et al., 1997). The hydrophobic interactions between protein molecules can be increased by heating. The hydrophobic interactions promote protein–protein binding to form an ordered three-dimensional gel network. As shown in Figure 6, all DPCD treatments significantly increased the hydrophobic interactions of surimi compared with the control group (p < 0.05). DPCD treatment induced denaturation of surimi, which exposed hydrophobic groups and increased hydrophobic interactions. CO2 is a non-polar molecule, which promotes hydrophobic interactions between protein molecules. The solubility of CO2 in water increases under pressure, which also promotes molecular effects of CO2 (Duba and Fiori, 2016). However, the hydrophobic effect decreases at treatment pressures exceeding 20 MPa, which is attributed to the contraction of the protein structure under high pressure, resulting in buried hydrophobic groups. The hydrophobic interactions were increased by treatment temperatures in the range of 30–50°C. The thermal effects at this treatment temperature alter the protein structure slowly. The hydrophobic groups are gradually exposed, which increases hydrophobic interactions. In contrast, the hydrophobic interactions decreased significantly (p < 0.05) when the temperature was increased to 60°C. This is due to the weakening of the hydrophobic interactions of proteins in the gel under an acidic environment during DPCD treatment.
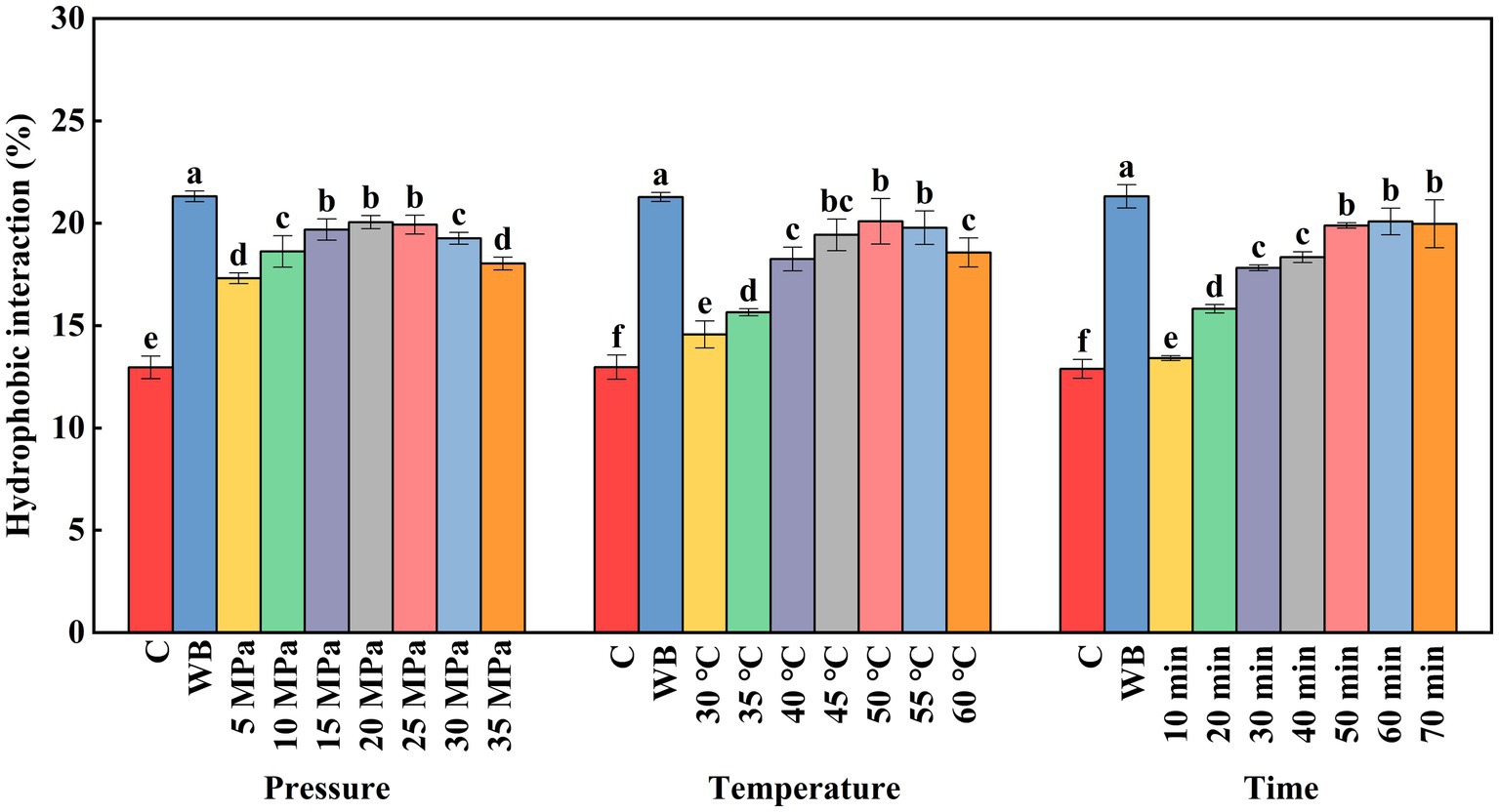
Figure 6. Effect of DPCD treatment on hydrophobic interaction of golden pompano surimi. Different letters indicate statistically significant differences (p < 0.05).
The intermolecular disulfide covalent bond is formed by the oxidation of two cysteine molecules with reactive sulfhydryl groups located on adjacent protein peptide chains. It is the most important covalent bond in thermally-induced protein gel (Chen et al., 2023). As shown in Figure 7, all DPCD treatments significantly increased the disulfide covalent bond levels of surimi compared with the control group (p < 0.05). Under treatment pressure below 15 MPa and treatment duration less than 50 min, the content of disulfide covalent bonds increased significantly, without additional changes following further increase in treatment intensity. This is because DPCD treatment has both thermal and molecular effects of CO2, which can completely denature and stretch the protein to expose highly reactive sulfhydryl groups, resulting in the formation of additional disulfide covalent bonds. Compared with the control group, the number of disulfide covalent bonds gradually increased when the treatment temperature was increased to 45°C. The bond number did not change significantly when the treatment temperature exceeded 50°C. This is due to the limited total number of active sulfhydryl groups contained in the surimi, which are fully exposed and oxidized to disulfide covalent bonds when heated at 50°C. Their levels remained stable.
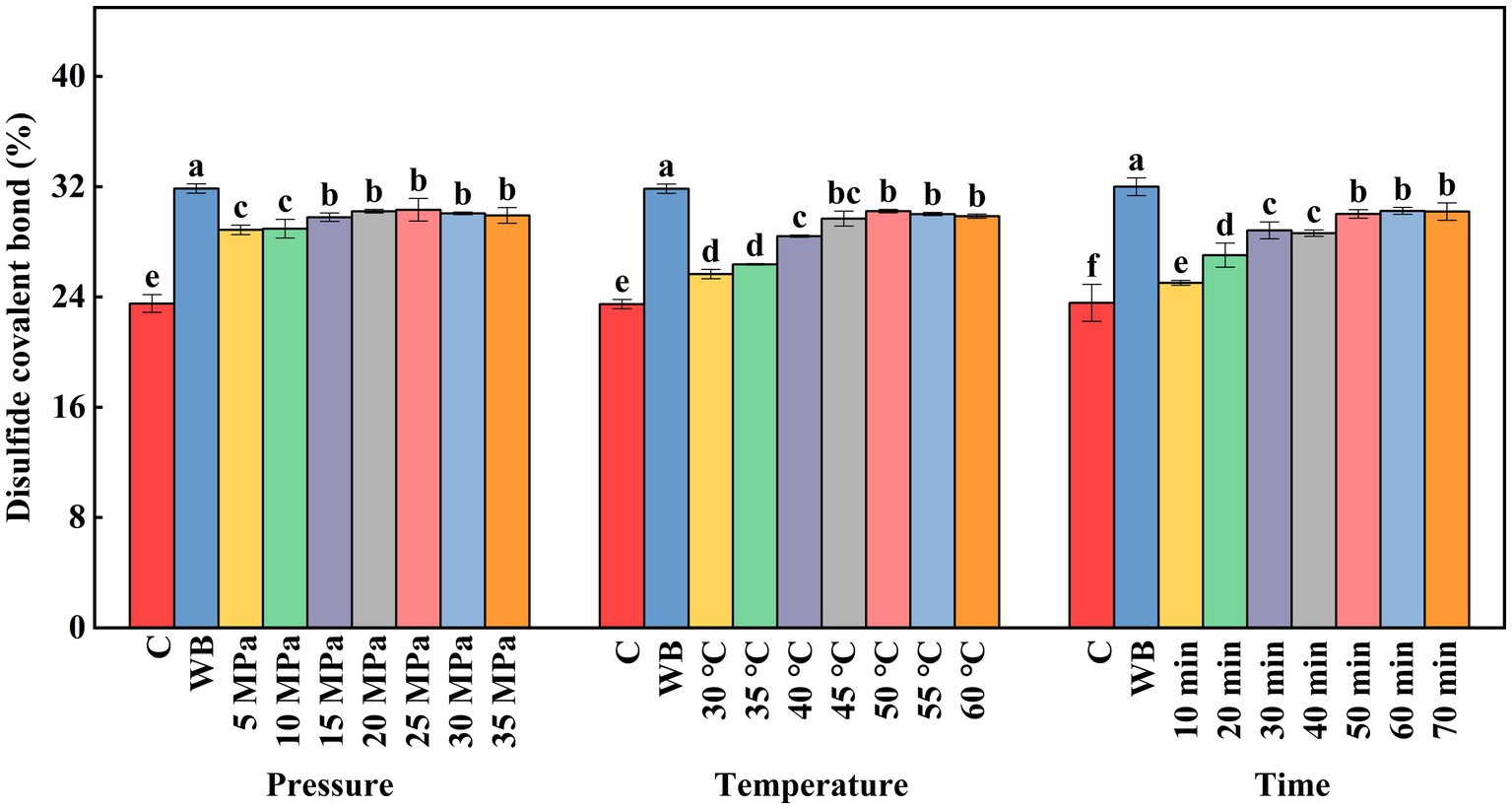
Figure 7. Effect of DPCD treatment on disulfide covalent bonds of golden pompano surimi. Different letters indicate statistically significant differences (p < 0.05).
During protein gel formation, covalent cross-linking between proteins occurs mainly via non-disulfide covalent bonds. Non-disulfide covalent bonds are not only involved in protein gelation, but also play an important role in stabilizing the protein gel network (Du et al., 2022). As shown in Figure 8, compared with the control group, the increase of DPCD treatment pressure and treatment time can significantly increase the number of non-disulfide covalent bonds. The decreased pH of the system under high pressure and the molecular effect of CO2 induced the formation of new non-disulfide covalent bonds in the surimi. The non-disulfide covalent bonds showed differential increase with the increase of DPCD treatment intensity. When the treatment pressure was above 25 MPa and the treatment time was greater than 30 min, there was no significant change in the non-disulfide covalent bond content. It is possible that within this treatment range, the molecular effect of CO2 can induce the formation of a large number of non-disulfide covalent bonds in the gel. The non-disulfide covalent bond content increased with the increase in DPCD treatment temperature, which may be due to the increased molecular motion under increased thermal energy and the formation of a relatively large number of new covalent bonds.
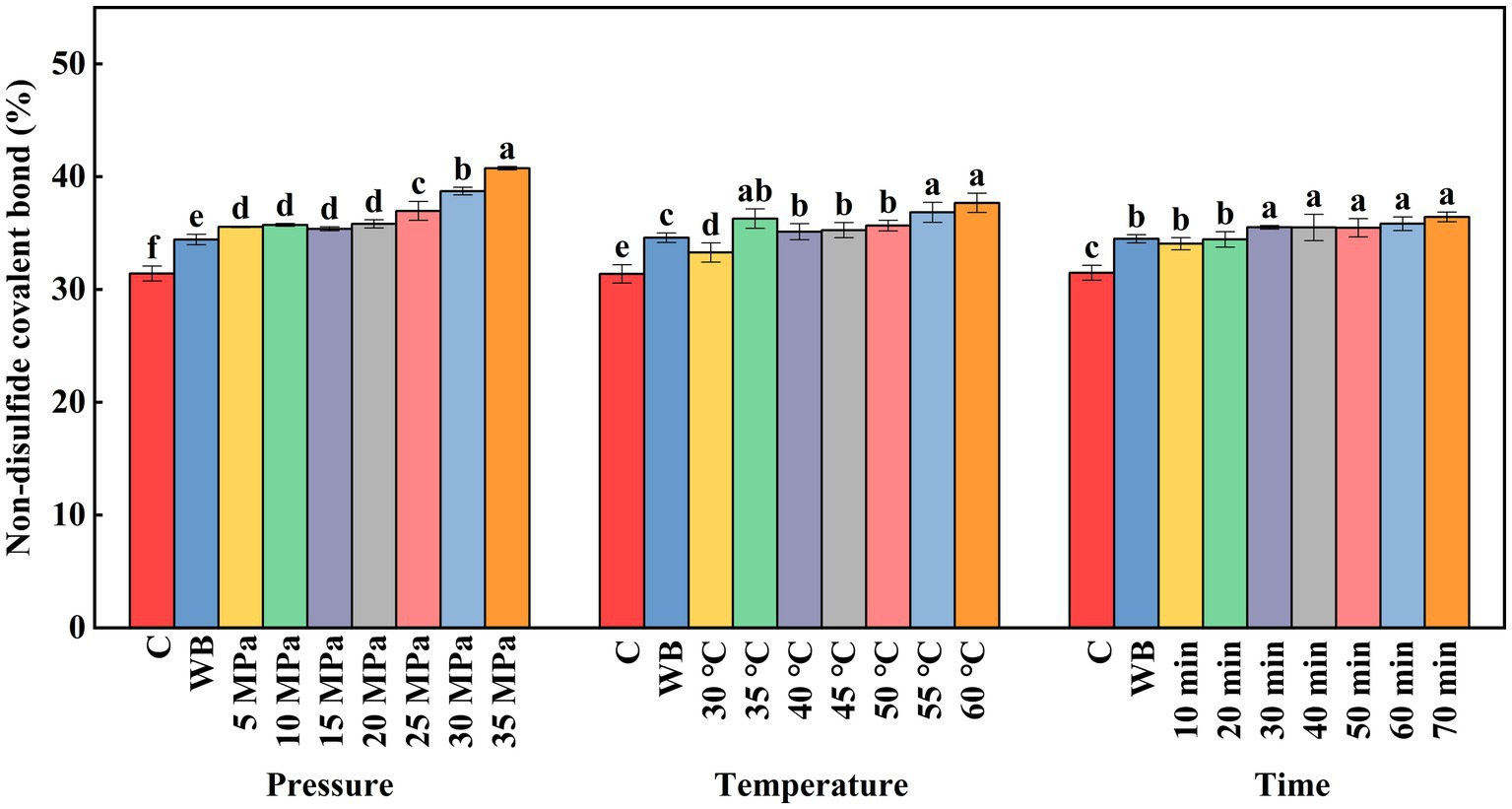
Figure 8. Effect of DPCD treatment on non-disulfide covalent bonds of golden pompano surimi. Different letters indicate statistically significant differences (p < 0.05).
The protein structure and intermolecular forces and gel strength during DPCD-induced gel formation in golden pompano surimi were correlated with gel strength. The effect of DPCD treatment on the gel strength of golden pompano surimi is shown in Appendix 1. As shown in Figure 9, the correlation heat map was color-coded using different intensities of red and blue. Red indicates positive correlation, blue denotes negative correlation. The intensity of the color is expressed by the Pearson correlation coefficient (P). Depending on the magnitude of P, the correlation can be classified as strong (0.6 ~ 1.0), medium (0.2 ~ 0.6), and weak (0.0 ~ 0.19; Marín et al., 2021).
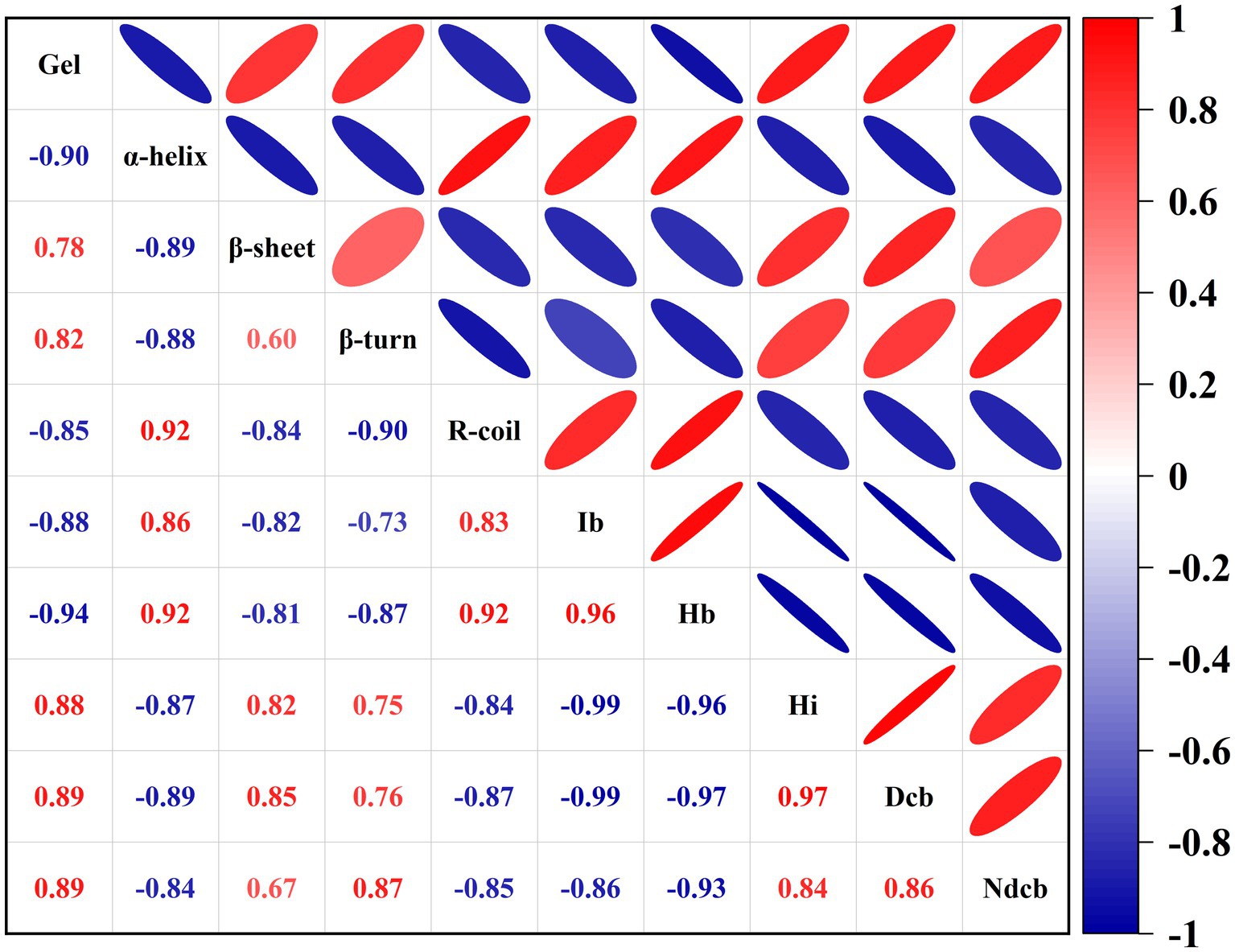
Figure 9. Correlation between protein structure and gel strength during DPCD-induced gel formation (Ib: Ionic bond, Hb: Hydrogen bond, Hi: Hydrophobic interaction, Dcb: Disulfide covalent bond, NDcb: Non-disulfide covalent bond).
As shown in Figure 9, β-sheets and β-turns exhibit a strong positive correlation with gel strength, whereas α-helix and R-coil showed a strong negative correlation with gel strength. Thus, as the treatment intensity of DPCD increases, the content of α-helix decreases, the content of β-sheet increases, resulting in enhanced gel strength. The β-sheet structure in proteins most likely interacts with CO2 than the α-helix, because the β-sheet structure has fewer intramolecular hydrogen bonds and additional exposed side chain groups, which facilitate the reaction with CO2. The interaction of CO2 with the gel was further enhanced by increasing DPCD treatment pressure and temperature. Hydrophobic interactions, disulfide and non-disulfide covalent bonds were strongly positively correlated with gel strength. The ionic and hydrogen bonds were strongly negatively correlated with gel strength. Thus, the higher the number of hydrophobic interactions, disulfide and non-disulfide covalent bonds, the higher is the strength of DPCD-induced surimi gels. Therefore, hydrophobic interactions, disulfide and non-disulfide covalent bonds are the main intermolecular forces during the DPCD-induced gel formation in golden pompano surimi.
The structure of proteins determines the properties of gels. The variation in protein secondary structure is closely related to the gel strength. The secondary structure of surimi protein forms specific spatial structures such as coils and folds by the side chain groups under the joint action of intermolecular forces (ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide and non-disulfide covalent bonds). The surimi gels are depolymerized, cross-linked, and aggregated to form a three-dimensional gel network structure (Ai et al., 2022; Xu et al., 2022). However, the intermolecular forces contribute to different stages of protein gel formation and induce protein gels in different ways.
The variation of advanced protein structure during DPCD-induced gel formation of golden pompano surimi are shown in Figure 10. Treatment of surimi with DPCD induces gelation. CO2 in DPCD dissolves in water to form carbonic acid, which in turn dissociates to form H+, HCO3− and CO32− ions, lowering the pH of the system and thereby altering the advanced structure of proteins. The α-helix content in the secondary structure decreases and the β-sheet content increases. The β-sheet structure is a prerequisite for protein aggregation during the formation of gel network. The conversion of the α-helix structure to a more ordered β-sheet facilitates the formation of the gel network (Ju et al., 2022). The molecular effect of CO2 in the temperature synergistic high-pressure state decreases the α-helix content of surimi gels and disrupts the hydrogen bonding. As a result, the protein is in a stretched state, which promotes the interaction between protein molecules (Sikka et al., 2008; Roy and Bandyopadhyay, 2023). The reduction in the number of hydrogen bonds also exposes additional hydrophobic groups. However, CO2, as a hydrophobic solvent, interacts with the hydrophobic groups in the amino-terminal residues of surimi proteins, changing the aqueous environment around the protein (Duba and Fiori, 2016). Likewise, the hydrophobic groups are exposed, which further enhances the hydrophobic interactions. As the treatment intensity increases, additional sulfhydryl groups inside the protein are exposed and the free sulfhydryl groups oxidize to disulfide covalent bonds, resulting in protein polymerization (Chen et al., 2020). This indicates that the denaturation of proteins in the presence of DPCD may be induced by the combination of hydrophobic interactions, and disulfide and non-disulfide covalent bonds.
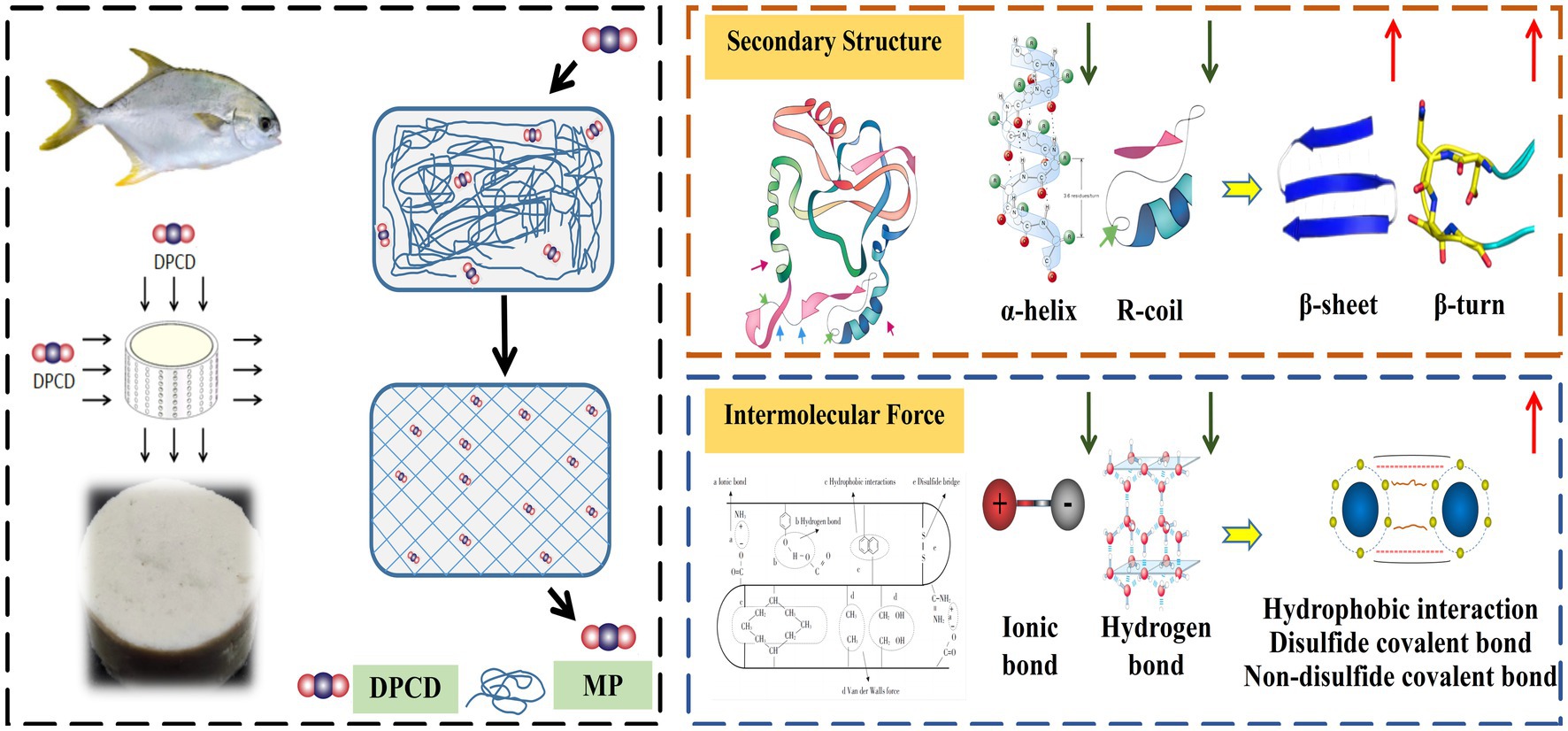
Figure 10. The variation of advanced protein structure during DPCD-induced gel formation of golden pompano surimi.
Dense phase carbon dioxide treatment induced gel formation in golden pompano surimi. Compared with the control group, different DPCD treatment conditions had significant effects on the protein secondary structure content and intermolecular forces in golden pompano surimi. The contents of α-helix and random coil were significantly decreased (p < 0.05), while the levels of β-sheet and β-turn increased significantly (p < 0.05). The number of ionic and hydrogen bonds decreased significantly (p < 0.05), and hydrophobic interactions, disulfide and non-disulfide covalent bonds increased significantly (p < 0.05). Correlation analysis showed that β-sheet, β-turn, hydrophobic interactions, disulfide covalent bonds and non-disulfide covalent bonds of surimi protein under DPCD treatment were strongly positively correlated with gel strength. The α-helix, random coil, ionic and hydrogen bonds were strongly negatively correlated with gel strength. Therefore, the secondary structure and intermolecular forces can be used as effective indicators of altered gel strength during gel formation induced by DPCD treatment in pompano surimi. In subsequent studies, we hope to develop novel techniques to evaluate gel quality based on such protein structure indicators. Meanwhile, the relationship between gel strength and protein structure can be explored at the microscopic level for rapid, efficient and comprehensive monitoring of surimi freshness.
The data that support the findings of this study will be available upon request to the corresponding author.
WD and SL: conceptualization and methodology. WD and HQ: software and formal analysis. ZW and QX: validation. HQ and KH: investigation. SW: resources. QS and KH: data curation. WD: writing—original draft preparation and writing—review and editing. YL: visualization. ZH: supervision. SL: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by the Guangdong Innovation Team of Seafood Green Processing Technology (2019KCXTD011), the Modern Agro-industry Technology Research System of China [CARS-48], the National Natural Science Foundation of China (Youth Program; 32202086), the Scientific and Technological Innovation Strategy of Guangdong Province (2022A05036), the Guangdong General Universities Young Innovative Talents Project (2020KQNCX028), the General transfer payment fund project of fishery development support policy in Guangdong Province in 2022 (2022-440000-45060100-9680).
We thank all the other members of Marine Food Processing New Technology Team at Guangdong Ocean University for their helpful suggestions and constructive criticism.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1189149/full#supplementary-material
Ai, M., Xiao, N., Zhou, Q., Tian, X., Guo, S., Chen, W., et al. (2022). The relationship between acylation degree and gelling property of NaOH-induced egg white gel: efficient is better? Food Res. Int. 160:111668. doi: 10.1016/j.foodres.2022.111668
Andonegi, M., Heras, K. L., Santos-Vizcaíno, E., Igartua, M., Hernandez, R. M., de la Caba, K., et al. (2020). Structure-properties relationship of chitosan/collagen films with potential for biomedical applications. Carbohyd Polym. 237:116159. doi: 10.1016/j.carbpol.2020.116159
Bureau of Fisheries of the Ministry of Agriculture. (2021). 2022 China Fishery Statistical Yearbook. Beijing: China Agriculture Press.
Chen, B., Liu, X., Zhou, K., Xie, Y., Wang, Y., Zhou, H., et al. (2023). Differentiating the effects of hydrophobic interaction and disulfide bond on the myofibrillar protein emulsion gels at the high temperature and the protein interfacial properties. Food Chem. 412:135472. doi: 10.1016/j.foodchem.2023.135472
Chen, Y., Xu, A., Yang, R., Jia, R., Zhang, J., Xu, D., et al. (2020). Chemical interactions and rheological properties of hairtail (Trichiurus haumela) surimi: effects of chopping and pressure. Food Biosci. 38:100781. doi: 10.1016/j.fbio.2020.100781
Damar, S., and Balaban, M. (2006). Review of dense phase CO2 technology: microbial and enzyme inactivation, and effects on food quality. J. Food Sci. 71, R1–R11. doi: 10.1111/j.1365-2621.2006.tb12397.x
Ding, H., Li, X., Li, R. Z., Yi, S., Xu, Y., Mi, H., et al. (2019). Changes of water state and gel characteristics of Hairtail (Trichiurus lepturus) surimi during thermal processing. J. Texture Stud. 25, 332–340. doi: 10.1111/jtxs.12393
Du, J., Cao, J., Zhou, C., Pan, D., Geng, F., and Wang, Y. (2022). Insight into the mechanism of myosin-fibrin gelation induced by non-disulfide covalent cross-linking. Food Res. Int. 156:111168. doi: 10.1016/j.foodres.2022.111168
Duan, W., Qiu, H., Htwe, K. K., Wang, Z., Liu, Y., Wei, S., et al. (2023). Correlation between water characteristics and gel strength in the gel formation of Golden pompano Surimi induced by dense phase carbon dioxide. Foods 12:1090. doi: 10.3390/foods12051090
Duba, K. S., and Fiori, L. (2016). Solubility of grape seed oil in supercritical CO2: experiments and modeling. J. Chem. Thermodyn. 100, 44–52. doi: 10.1016/j.jct.2016.04.010
Gilleland, G. M., Lanier, T. C., and Hamann, D. D. (1997). Covalent bonding in pressure-induced fish protein gels. J. Food Sci. 62, 713–733. doi: 10.1111/J.1365-2621.1997.TB15442.X
Guo, M., Liu, S., Ismail, M., Farid, M. M., Ji, H., Mao, W., et al. (2017). Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem. 227, 219–226. doi: 10.1016/j.foodchem.2017.01.050
Hiromoto, T., Ikura, T., Honjo, E., Blaber, M., Kuroki, R., and Tamada, T. (2022). Creation of cross-linked crystals with intermolecular disulfide bonds connecting symmetry-related molecules allows retention of tertiary structure in different solvent conditions. Front. Mol. Biosci. 9:908394. doi: 10.3389/fmolb.2022.908394
Jaziri, A. A., Shapawi, R., Mohd Mokhtar, R. A., Noordin, M., and Huda, N. (2021). Tropical marine fish Surimi by-products: utilisation and potential as functional food application. Food Rev. Int. 37, 1–26. doi: 10.1080/87559129.2021.2012794
Ju, Q., Wu, C., Yuan, Y., Hu, Y., Zhou, S., and Luan, G. (2022). Insights into the mechanism on Glucono-delta-lactone induced gelation of soybean protein at subunit level. Food Hydrocolloid. 125:107402. doi: 10.1016/j.foodhyd.2021.107402
Leng, L., Zou, H., Wang, Y., Yu, C., and Qi, H. (2022). Seaweed slurry improved gel properties and enhanced protein structure of silver carp (Hypophthalmichthys molitrix) Surimi. Foods 11:3115. doi: 10.3390/foods11193115
Liu, H., Gao, L., Ren, Y., and Zhao, Q. (2014). Chemical interactions and protein conformation changes during silver carp (Hypophthalmichthys Molitrix) Surimi gel formation. Int. J. Food Prop. 17, 1702–1713. doi: 10.1080/10942912.2012.700538
Liu, B., Guo, H.-Y., Zhu, K., Guo, L., Liu, B. S., Zhang, N., et al. (2019). Growth, physiological, and molecular responses of golden pompano Trachinotus ovatus (Linnaeus, 1758) reared at different salinities. Fish Physiol. Biochem. 45, 1879–1893. doi: 10.1007/s10695-019-00684-9
Liu, Y., Sun, Q., Pan, Y., Wei, S., Xia, Q., Liu, S., et al. (2021). Investigation on the correlation between changes in water and texture properties during the processing of surimi from golden pompano (Trachinotus ovatus). J. Food Sci. 86, 376–384. doi: 10.1111/1750-3841.15581
Marín, A., Bindelle, J., Zubieta, Á. S., Correa, G. A., Arango, J., Chirinda, N., et al. (2021). In vitro fermentation profile and methane production of kikuyu grass harvested at different Sward Heights. Front. Sustain Food Syst. 5:682653. doi: 10.3389/fsufs.2021.682653
Monto, A. R., Li, M., Wang, X., Wijaya, G. Y. A., Shi, T., Xiong, Z., et al. (2021). Recent developments in maintaining gel properties of surimi products under reduced salt conditions and use of additives. Crit. Rev. Food Sci. Nutr. 62, 8518–8533. doi: 10.1080/10408398.2021.1931024
Nakamura, Y., Takahashi, S., and Takahashi, K. (2021). Long-term suppression of suwari phenomenon for improvement in the manufacturing process of surimi gel product. LWT 150:111934. doi: 10.1016/j.lwt.2021.111934
Ohashi, S., Ura, F., Takeuchi, M., Iida, H., Sakaue, K., Ochi, T., et al. (1991). Interaction of thaumatin with carrageenans. II. Effects of pH, temperature and competing cations studied by circular dichroism. Food Hydrocolloid. 4, 379–394. doi: 10.1016/S0268-005X(09)80133-1
Roy, U., and Bandyopadhyay, P. (2023). Correlation between protein conformations and water structure and thermodynamics at high pressure: a molecular dynamics study of the bovine pancreatic trypsin inhibitor (BPTI) protein. J. Chem. Phys. 158:095102. doi: 10.1063/5.01248371589
Sikka, S., and Sharma, S. (2008). The hydrogen bond under pressure. Phase Transit. 81, 907–934. doi: 10.1080/01411590802098864
Tan, F. J., Lai, K. M., and Hsu, K. C. (2010). A comparative study on physical properties and chemical interactions of gels from tilapia meat pastes induced by heat and pressure. J. Texture Stud. 41, 153–170. doi: 10.1111/j.1745-4603.2010.00219.x
Wan, Y. T., Chen, Y., Zhang, W. J., Liu, B. C., Tiem, L. Y., Mun, F. Y. M. F., et al. (2022). Application of FT-IR spectroscopy and chemometric technique for the identification of three different parts of Camellia nitidissima and discrimination of its authenticated product. Front. Pharmacol. 13:931203. doi: 10.3389/fphar.2022.931203
Wang, X., Xia, M., Zhou, Y., Wang, L., Feng, X., Yang, K., et al. (2020). Gel properties of myofibrillar proteins heated at different heating rates under a low-frequency magnetic field. Food Chem. 321:126728. doi: 10.1016/j.foodchem.2020.126728
Wei, W., Hu, W., Zhang, X.-Y., Zhang, F., Sun, S., Liu, Y., et al. (2018). Analysis of protein structure changes and quality regulation of surimi during gelation based on infrared spectroscopy and microscopic imaging. Sci. Rep. 8:5566. doi: 10.1038/s41598-018-23645-3
Xie, J., Yan, Y., Pan, Q. N., Shi, W. Z., Gan, J. H., Lu, Y., et al. (2020). Effect of frozen time on Ctenopharyngodon idella surimi: with emphasis on protein denaturation by tri-step spectroscopy. Mol Struct. 1217:128421. doi: 10.1016/j.molstruc.2020.128421
Xu, L., Wang, J., Lv, Y., Su, Y., Chang, C., Gu, L., et al. (2022). Influence of konjac glucomannan on the emulsion-filled/non-filled chicken gel: study on intermolecular forces, microstructure and gelling properties. Food Hydrocolloid. 124:107269. doi: 10.1016/j.foodhyd.2021.107269
Xu, D., Yuan, F., Jiang, J., Wang, X., Hou, Z., and Gao, Y. (2011). Structural and conformational modification of whey proteins induced by supercritical carbon dioxide. Innov Food Sci Emerg. 12, 32–37. doi: 10.1016/j.ifset.2010.10.001
Yang, X., Su, Y., and Li, L. (2020). Study of soybean gel induced by Lactobacillus plantarum: protein structure and intermolecular interaction. LWT 119:108794. doi: 10.1016/j.lwt.2019.108794
Yang, S., Zhang, Q., Yang, H., Shi, H., Dong, A., Wang, L., et al. (2022). Progress in infrared spectroscopy as an efficient tool for predicting protein secondary structure. Int. J. Biol. Macromol. 206, 175–187. doi: 10.1016/j.ijbiomac.2022.02.104
Zhang, L., Liu, S., Ji, H., Zhang, C., Deng, C., Cao, W., et al. (2011). Inactivation of polyphenol oxidase from Pacific white shrimp by dense phase carbon dioxide. Innov Food Sci Emerg 12, 635–641. doi: 10.1016/j.ifset.2011.05.004
Keywords: surimi processing, dense phase carbon dioxide, protein structure, gel strength, golden pompano
Citation: Duan W, Qiu H, Htwe KK, Wei S, Liu Y, Wang Z, Sun Q, Han Z, Xia Q and Liu S (2023) Changes in advanced protein structure during dense phase carbon dioxide induced gel formation in golden pompano surimi correlate with gel strength. Front. Sustain. Food Syst. 7:1189149. doi: 10.3389/fsufs.2023.1189149
Received: 18 March 2023; Accepted: 02 May 2023;
Published: 18 May 2023.
Edited by:
Paula Jauregi, Centro tecnológico experto en innovación marina y alimentaria (AZTI), SpainReviewed by:
Esther Sanmartín, Centro tecnológico experto en innovación marina y alimentaria (AZTI), SpainCopyright © 2023 Duan, Qiu, Htwe, Wei, Liu, Wang, Sun, Han, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shucheng Liu, bGl1c2NAZ2RvdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.