- 1Department of Environmental Health, Food Safety Division, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran
- 3Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 4CEO at Isfahan Chocolate Company (R&D), Isfahan, Iran
- 5Faculty of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
Introduction: The gut microbiota may be altered following changes in diet or exposure to drugs. Humans can be exposed to antibiotic residue in food. People may be exposed to these compounds for years. But in determining the maximum residue level (MRL), the effects of antibiotic residue on the intestinal microbiota are not investigated. Some evidence suggests that antibiotics in small amounts also lead to changes in the intestinal microbiota. Therefore, a systematic study was conducted with the aim of investigating the effect of antibiotic residues in food on the intestinal microbiota.
Method: The main criterion of this research was to investigate the effects of antibiotics at low doses. For this purpose, a search was made in the databases with keywords antibiotic, veterinary antibiotic, food, residue, microbiome, and microbiota. The investigated doses of each of the antibiotics in the studies were compared with their MRL in food.
Results: The most significant change in the structure and function of the microbiota was made by tetracycline, sulfamethoxazole, cefquinome, florfenicol and tylosin. The lowest observed effect was related to the antibiotics fosfomycin and amoxicillin.
Discussion: Exposure to antibiotic residues through food is usually a long-term exposure. In vivo studies, changes in the intestinal microbiota were observed. Therefore, it is necessary to inform the breeders and competent authorities in order to comply with the principles of treatment. The gut microbiota may be altered following changes in diet or exposure to drugs.
Introduction
The human intestinal microbiota includes all kinds of microorganisms, including fungi, protozoa, bacteria, and viruses (Piñeiro and Cerniglia, 2021). The intestinal microbiota has several physiological roles, including in host immunity and metabolic processes and in the integrity of the intestines and gut epithelium (Ahn et al., 2021; Ma X. et al., 2021; Sadighara et al., 2022). Colonic bacteria are gram-negative anaerobic bacteria that perform several metabolic activities, including carbohydrate fermentation, production of vitamins B and K, and polysaccharide hydrolysis (Riley et al., 2013; Jung et al., 2018). The dysbiosis of the intestinal microbiota causes metabolic disease (Chen et al., 2022). Antibiotics are one of the most effective drugs in the treatment of diseases, but their excessive use will lead to changes in the intestinal microbiota and antibiotic resistance (Li et al., 2019; Rahman et al., 2021). In children, it leads to complications, such as obesity and an increased risk of allergies, caused by changes in the microbiota of the intestines (Li et al., 2019). Exposure to antibiotic residues in childhood interferes with the maturation and colonization of intestinal microbiota (Chen et al., 2022). Furthermore, disruption of microbiota homeostasis leads to neurological disorders, such as anorexia, depression, Alzheimer's, autism, inflammatory bowel diseases, and Parkinson's disease (Van den Abbeele et al., 2013; Piñeiro and Cerniglia, 2021). According to the reports of the American Food and Drug Administration, 80% of antimicrobials are used to treat livestock that produce food products of animal origin (Rahman et al., 2021). In addition to treating diseases, they are used as a growth stimulant in livestock (Qian et al., 2021; Chen et al., 2022). In the USA, since 1951, the use of antibiotics as a growth promoter for livestock has been allowed (Castanon, 2007). Foods of animal origin may contain antibiotic residues. In a study, four veterinary antibiotics, enrofloxacin, chlortetracycline, sulfachlorpyridazine, and sulfadimethoxine, were isolated in the intestines of healthy people, which indicates that they were ingested through food products of animal origin (Duan et al., 2020). Food of animal origin include edible tissue, muscle, liver, kidneys, and animal products, including eggs and milk (Subirats et al., 2019). In a study conducted in India, ~11.3% of raw milk samples contained antibiotic residues. In this study, enrofloxacin and oxytetracycline were the most detected antibiotics in milk samples (Moudgil et al., 2019). In another study, 45% of chicken samples contained antibiotic residues. Amoxicillin, enrofloxacin, and sulfamethoxazole were reported more than other antibiotics (Lee et al., 2018). The residue of veterinary antibiotics was also observed in food products of marine origin, as in the case of the residues of the antibiotic trimethoprim that were isolated in shrimp samples (Willis et al., 1999).
Furthermore, some antibiotics prescribed in poultry and livestock are usually not absorbed from the intestines and are mostly excreted without being converted into metabolites (Rafiq et al., 2022). Some sulfonamide antibiotics, including sulfadimidine, sulfamethazine, and sulfadiazine, are completely metabolized and removed from the animal's body. However, some other sulfonamide antibiotics are less likely to undergo degradation in the gut and are excreted unchanged through the feces and urine of humans and animals (Li et al., 2021), entering the environment via this route. Pharmaceuticals are similar to organic compounds found in the environment and are currently classified as contaminants (Abed and Faisal, 2022). Therefore, these components may enter the human food chain from the environment (Rafiq et al., 2022). Some antibiotics in the environment are absorbed by plants, and even some antibiotics accumulate in the plant body (Riley et al., 2013). Therefore, it is also possible to be exposed to antibiotics through food products of plant origin.
There is evidence that the residues of some antibiotics in food, such as enrofloxacin, remain active in the human colon (Ahn et al., 2012). These components disturb the microbial balance of the intestines and cause the colonization of pathogenic bacteria (Subirats et al., 2019). This systematic study aimed to investigate the effects caused by residual doses of antibiotics in food on the structure and physiological function of the intestinal microbiota.
Methods
This systematic review was done based on the Prisma checklist. First, a proposal with specific aims and inclusion and exclusion criteria was determined by the research team. To avoid bias, all procedures were performed by two authors. Disagreements regarding inclusion and exclusion criteria were resolved by the corresponding author.
Search strategy
The search formulation was defined as follows: (antibiotic OR “veterinary antibiotic”) AND (food) AND (residue) AND (microbiome OR microbiota). The search was done on 7 January 2023 in the PubMed, Web of Science, and Scopus databases. To prevent bias, the search was conducted by two authors (P.S and Y.M). The results of both searches were the same.
Inclusion and exclusion criteria
The study design was defined based on PICO (populations, interventions, comparators, and outcomes). The selected population in this systematic review was the intestinal microbiota. The intervention included interventions that were investigated by veterinary drugs in the doses found in food. Studies that investigated the effects of therapeutic doses were excluded from this research. The outcome also included the changes made to the structure and physiology of the microbiota. Furthermore, similar to systematic studies, review articles and chapters of books were excluded.
Extraction of data
The necessary data according to the research objectives were extracted from the articles after the final selection by two authors (P.S and SH. R). These data included the name of the first author, year of publication, country, type of antibiotic, selected doses, the unit of doses, type of trial and study, and the most important achievements of the manuscript.
Results
The process of search
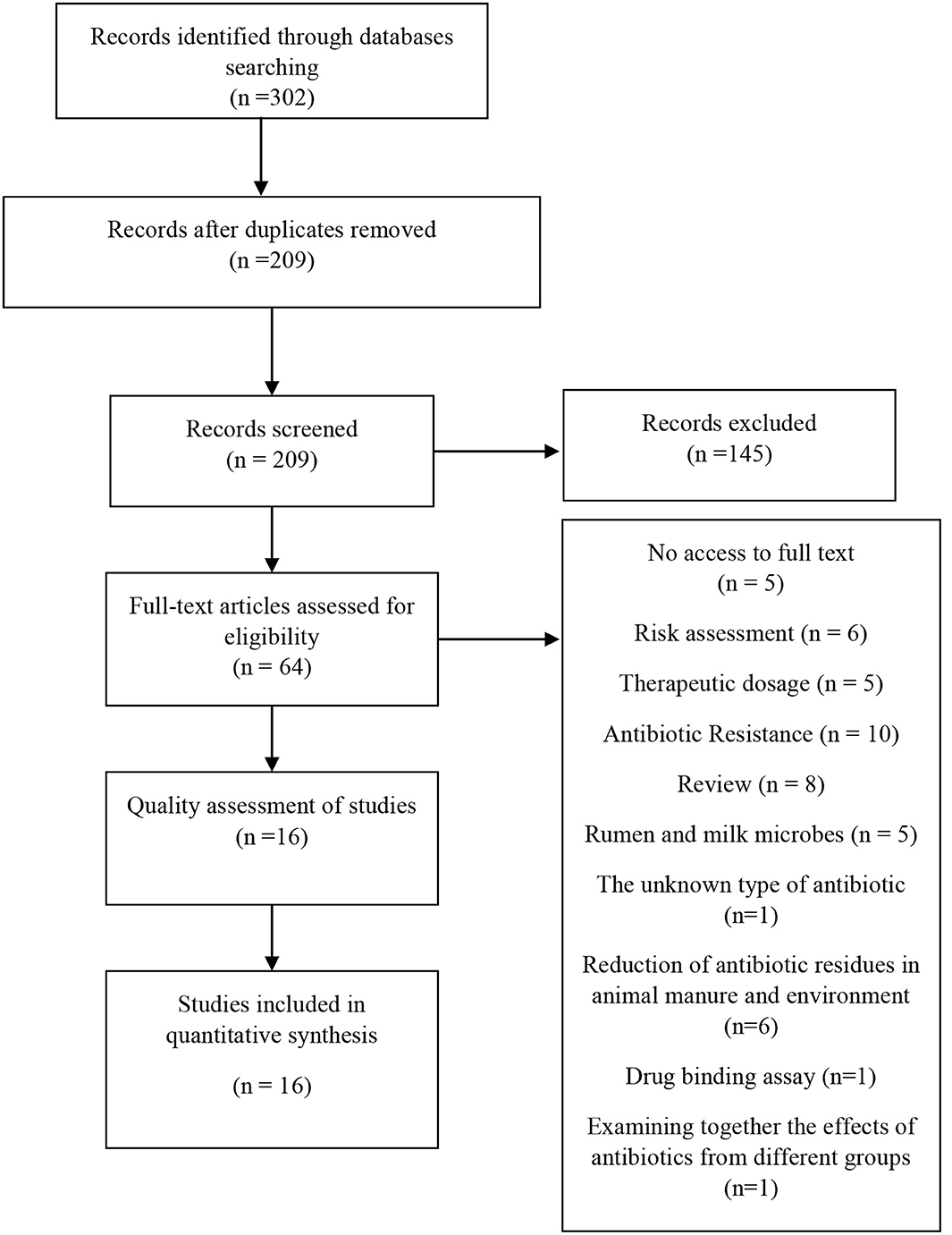
A total of 302 articles were obtained after removing the duplicate manuscripts, and 209 articles were selected for the initial screen. Review and congress articles and articles that were about antibiotic alternatives, risk assessment, and diagnostic methods were excluded from the initial screen. In the next step, 64 original scientific articles were selected to fully evaluate the text. The full text of five articles was not available, so the remaining 59 articles were carefully evaluated. Five points were considered for the qualitative evaluation of the articles. The articles that received three points or more were selected as final and their data was extracted. This step was carried out by two of the authors of this study (A.S and Y.M). In case of disagreement, the corresponding author's opinion was obtained. Finally, 16 manuscripts were selected (Figure 1).
The data extracted from selected manuscripts
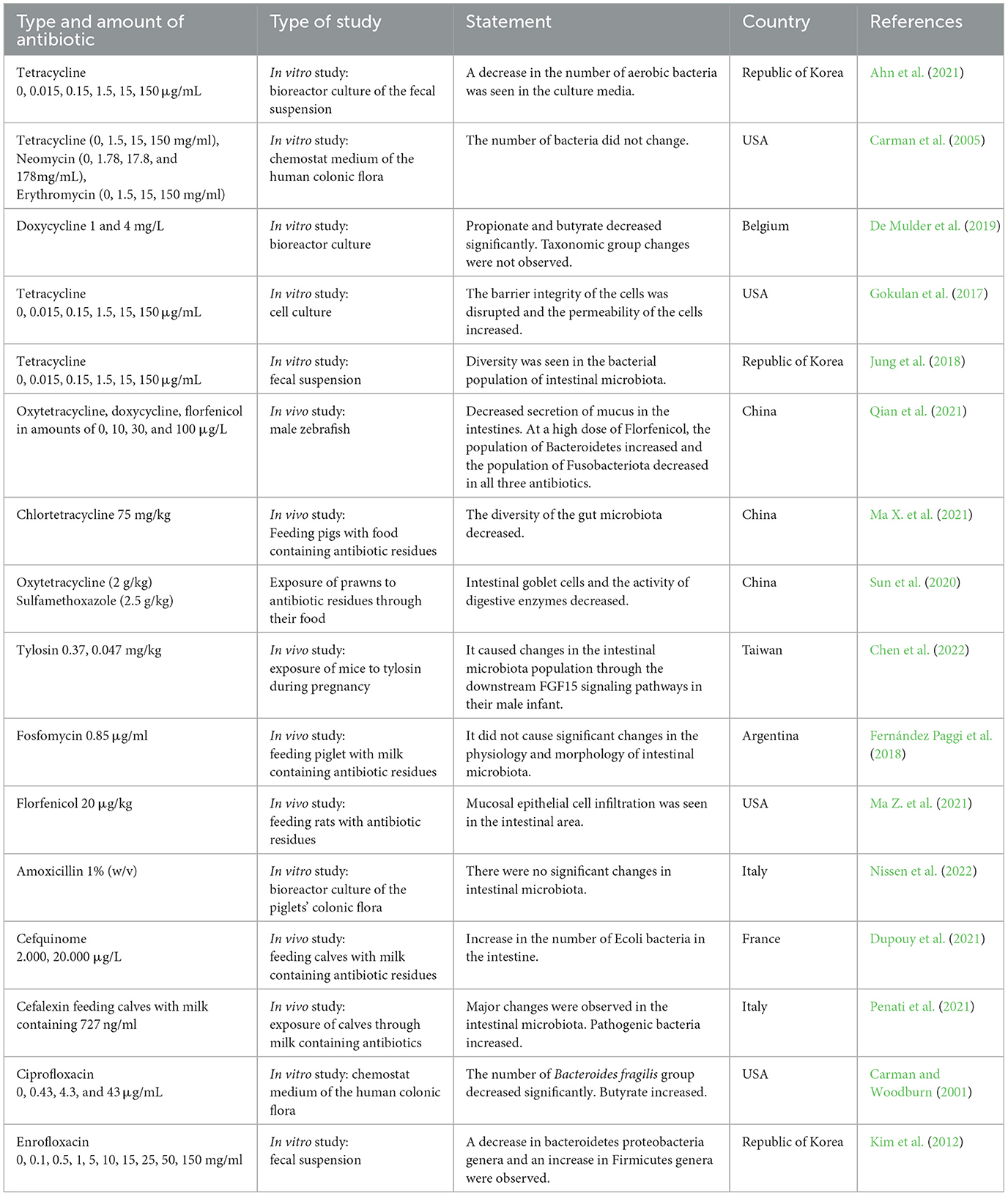
This systematic review contained 16 articles. The necessary data were extracted from articles in accordance with the purpose of this study. The data are summarized in Table 1. The studies were both in vitro and in vivo models, with half of the studies being in vitro and the other half being in vivo. Most studies were carried out in recent years, which indicates that this issue is new and recently important to researchers. As seen in Table 1, the antibiotics were inserted based on their category. A total of 40% of studies were related to the tetracycline antibiotic. After tetracycline, most research was conducted on cephalosporin. In the case of the country where the research was conducted, the highest share was of the US (25%), followed by China and the Republic of Korea with equal shares (18.75%). The number of chosen doses was higher in the in vitro studies. In most in vivo studies, one or two doses were selected. Most of the in vivo studies were carried out on farm animals (62.5%), whereas 30% of the in vivo studies were related to laboratory animals. According to the extracted data, the selected laboratory animals included mice, rats, and zebrafish. The important findings of the studies are mentioned in Table 1.
Discussion
In this study, we considered residues that are usually reported in food. The effects of therapeutic doses of antibiotics on the intestinal microbiota were not considered. The Joint Expert Committee on Food Additives (JECFA) has developed a maximum residue level (MRL) for each antibiotic in food of animal origin (Ahn et al., 2021). In tests to determine the MRL of these compounds in food, the effect of these compounds on the microbiota of the intestines has not been investigated (Wagner et al., 2008).
The human intestines have 1,000 species of bacteria (Jung et al., 2018). Factors affecting the intestinal microbiota are genetics, diet, age, and antimicrobial drugs (Ricker et al., 2020; Ma X. et al., 2021). Diets are the most important factors affecting the growth and maturation of intestinal microbiota (Chen et al., 2022). Antibiotic residues also play an important role in disrupting and changing the diversity and abundance of intestinal surface microbes (Ma X. et al., 2021).
In this systematic review, most research was done on tetracycline, a broad-spectrum antibiotic against gram-positive and gram-negative bacteria, which is used both in human and veterinary medicine (Gokulan et al., 2017). Oral absorption of tetracycline is poor, so higher amounts of it will remain in the digestive tract (Gokulan et al., 2017). In particular, a relatively high concentration of this antibiotic is found in the cecum and colon of pigs following the intake of food containing its residue (Peeters et al., 2016). This antibiotic interferes with calcium absorption (Gokulan et al., 2017). In Gokulan's study, which was conducted in intestinal cell culture, the permeability of cells exposed to tetracycline decreased. The study was conducted in five doses 0.015, 0.15, 1.5, 15, and 150 μg/mL, and changes were seen in doses 15 and 150 μg/mL (Gokulan et al., 2017). The integrity of the intestinal cells is made by a series of proteins that are expressed by some genes. Claudin is one of the components of tight junctions that seal the space between epithelial and endothelium cells (Watanabe et al., 2021). The claudin-15 gene is responsible for the tight junction of intestinal cells and the expression of this gene decreases in cells exposed to tetracycline (Gokulan et al., 2017). Furthermore, gap junctions are the channels between cells that allow the passage of substances between cells; in cells exposed to tetracycline, the expression of the gap junction gene decreases (Gokulan et al., 2017). In Jung et al. (2018)'s study, suspensions were prepared from human feces and exposed to concentrations of tetracycline for 40 days. The human fecal suspensions were exposed to five doses: 0.015, 0.15, 1.5, 15, and 150 μg/mL. Changes in the microbial population living in the intestines were seen: the clostridium family XI population increased after 40 days and these changes were observed even in low doses (Jung et al., 2018). A fecal culture medium was also used in Ahn's study. Stool samples were collected from a healthy man who had not received antibiotics in the past 6 months. The samples were transferred to an anaerobic chamber hood and the culture medium was exposed to five doses (0.015, 0.15, 1.5, 15, and 150 μg/mL) of tetracycline (Ahn et al., 2021). At the highest dose of tetracycline (150 μg/mL), the number of anaerobic bacteria increased. With sequence analysis, it was observed that the number of Enterobacteriaceae bacteria also increased (Ahn et al., 2021). In vitro laboratory models provide the possibility to evaluate the effect of residual antibiotics on the production of fatty acids and enzymes by bacteria (Wagner et al., 2008). The most important fatty acids produced in this study include n-butyric acid, acetic acid, and propionic acid (Ayesh et al., 1999). With increasing the dose of tetracycline, two fatty acids, acetate and propionate, also increased (Ahn et al., 2021). A long-term study was also conducted with chlortetracycline in an animal model. Pigs were exposed to this antibiotic through feeding for 90 days, and changes in their intestinal microbiota and its function were observed, with lipid biosynthesis, vitamin B6 metabolism, and oxidative phosphorylation decreasing (Ma X. et al., 2021). However, it is worth mentioning that, in these abovementioned studies, the selected doses for the three antibiotics, chlortetracycline, oxytetracycline, and tetracycline, were more than the standard limit of Codex. Codex standard has set 100 μg/L for milk and 200 μg/kg for beef, fish, and chicken.
In another study, shrimps were exposed to two antibiotics, oxytetracycline and sulfamethoxazole, and were divided into three groups: a control group, a group with antibiotic oxytetracycline at a dose of 2 g/kg, and a group with antibiotic sulfamethoxazole at a dose of 2.5 g/kg. The shrimps were exposed to different doses of antibiotics through their food (Sun et al., 2020). The advantage of this study was that antioxidant parameters in the gastrointestinal tract were also evaluated. A decrease in antioxidant activity will lead to oxidative damage (Sadighara et al., 2016). Antioxidant activity was evaluated in superoxide dismutase and catalase enzymes, glutathione, and malondialdehyde. It was observed that glutathione and enzyme activity decreased and malondialdehyde increased (Sun et al., 2020). This evidence indicates that antibiotic residues can lead to oxidative stress. Of course, this study was conducted in shrimps and the MRL for these two antibiotics in shrimps has not been determined.
There were two studies on doxycycline. One was done in vivo on zebrafish and the other was done in vitro in a bioreactor culture (De Mulder et al., 2019; Qian et al., 2021). Taxonomic changes were seen in the fish model, but not in the bioreactor culture model. In the bioreactor culture model, two concentrations of 1 and 4 mg/L of doxycycline were selected. Changes were not seen at these concentrations. Probably, the difference in these results is due to the type of designed model. As a rule, the in vivo models are more valid. Furthermore, in the zebrafish model, the duration of exposure was long, lasting for 21 days (Qian et al., 2021). The observed symptoms will be more with the increase in the duration of exposure. Residual exposures to antibiotics through food are usually long-term and considered chronic exposures. The doses designed in the study of the bioreactor culture model were higher than the MRL, but in the fish model, the highest dose was equivalent to the MRL, which should be considered.
Florfenicol is a broad-spectrum antibiotic in veterinary medicine (Yang et al., 2020) and the effects of its residues in food were investigated in two animal model studies (Ma Z. et al., 2021; Qian et al., 2021). Rat pups were exposed to doses of florfenicol for 15 days with a dose of 20 μg/kg by oral gavage. In the rat animal model, necrosis and degeneration of intestinal epithelial cells and increased mucus secretions were observed. Changes in the diversity of bacteria were also seen in the zebrafish model (Table 1). In this study, three doses of 10, 30, and 100 μg/L were examined in zebrafish. Dysbiosis was observed in the intestinal microbiota at the dose of 100 μg/L. Codex has not established an MRL for florfenicol. The American Food and Drug Administration has set a range of 0.3–3.7 ppm for different tissues of cattle (Anderson et al., 2016). The small changes observed in the dose of 100 μg/L were lower than the approved MRL, which should be taken into consideration.
Tylosin is an antibiotic used as a growth promoter in livestock. It both reduces infection and increases weight, so it has an economic advantage (Chen et al., 2022). Tylosin residue in milk is a global health problem (Gomes Marques de Freitas et al., 2021). An amount of 71.80 μg/kg of this antibiotic has been detected in sheep meat (Sallam et al., 2022). In one study, pregnant rats were exposed to tylosin at doses of 0.37 and 0.047 mg/kg. This exposure continued for their male infants as well. Changes in the intestinal microbiota population were made through the downstream FGF15 (fibroblast growth factor 15) signaling pathway in these mice (Chen et al., 2022). Suppression of this pathway causes metabolic abnormalities and obesity (Chen et al., 2022). Furthermore, this factor plays a role in liver regeneration (Kong et al., 2018). The selected doses in this study were theoretical maximal daily intake (TMDI) and the Maximum Residue Limits (MRL) for Tylosin are set at 100 μg/kg for muscle and 100 μg/L for milk according to the Codex standard in different animal species. Therefore, this issue is important in tylosin, which leads to changes even in low doses.
Fosfomycin is a broad-spectrum antibiotic and, in the study, the effects of fosfomycin were investigated on the anatomical and physiological structure of the intestines in piglets exposed through their milk. High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS/MS) was used to evaluate how much antibiotics were in the calves and 0.85 μg/ml was measured. No significant difference was observed between the exposed and control groups (Fernández Paggi et al., 2018). The investigated factors were the number of bacteria, the amount of pH, the amount of absorption from the intestines, the production of volatile fatty acids, and the activity of the disaccharidase enzyme (Fernández Paggi et al., 2018). A decrease in the number of goblet cells was seen only in some areas (Fernández Paggi et al., 2018). The MRL for fosfomycin has not been defined by Codex and the European Union, only the Food Chemical Research Foundation of Japan has determined 0.5 ppm for animal tissues (Pérez et al., 2014).
A study on the effects of enrofloxacin, an antibiotic that has been widely used in veterinary medicine since 2005, was done in vitro. Ten concentrations of 0, 0.1, 0.5, 1, 5, 10, 15, 25, 50, and 150 mg/ml were selected. Bacteroidetes and Proteobacteria decreased significantly with increasing concentration of enrofloxacin exposure. However, the proportions of Firmicutes increased (Kim et al., 2012). In inflammatory bowel diseases and obesity, the ratio of Firmicutes/Bacteroidetes increases (Strati et al., 2017). Therefore, these changes caused by antibiotic residues are likely to be related to such conditions. Furthermore, observing changes in a dose-dependent manner is a confirmation of changes due to antibiotic residues. The most changes were observed in the dose of 150 mg/ml. This dose is more than the determined MRL. According to the European Union standards, the residual amount of the antibiotic enrofloxacin in meat is 100 μg/kg.
In another study, a fecal culture medium was placed with three antibiotics: tetracycline, neomycin, and erythromycin. For each antibiotic, three consecutive doses that were lower than the therapeutic dose and with residues that are normally detected in food were selected. The number of bacteria did not change. Neomycin led to an increase in propionate at the highest dose (Table 1) (Carman et al., 2005). The selected doses for neomycin in this study were 0, 1.78, 17.8, and 178 mg/mL. The MRL for milk and beef is 1,500 and 500 μg/kg, respectively. The doses in this study were lower than the MRL of the Codex standard. Some changes have been observed in this and other in vitro studies.
In Nissen's study, the effect of milk with residual antibiotic amoxicillin (1%) on the microbiota of piglets was investigated with a bioreactor model. Receiving milk containing amoxicillin residues did not lead to significant changes in the piglets' intestinal microbiota (Nissen et al., 2022).
Cephalosporins are an important class of antibiotics that are widely used in veterinary and human medicine. The use of eight of these antibiotics has been approved in the treatment of animals producing human food (Baeza et al., 2016). In the extracted data, there were two antibiotics of this category, cefalexin and cefquinome. In Penati's study, conducted in Italy, calves were fed with milk containing the antibiotic cefalexin. These calves were fed with the milk of cows that had received an intramammary injection of 210 mg of cephalexin monohydrate. Major changes in the intestinal microbiota were observed; beneficial bacteria such as Faecalibacterium decreased and pathogenic bacteria such as Campylobacter, Pseudomonas, and Chlamydophila spp. increased (Penati et al., 2021). Codex has not set an MRL for this antibiotic. The European Union has set 100 and 200 μg/kg for milk and muscle, respectively (Rageh et al., 2019). In the study, calves were fed with milk containing 727 ng/ml of antibiotics, an amount that is higher than the MRL determined for this antibiotic. In the experimental study by Dupouy et al. (2021), calves were fed with milk containing residual antibiotic cefquinome with concentrations of 2,000, 20,000 μg/L. This treatment continued for 3 days. According to the rules of the European Union, the MRL for cefquinome in milk is 20 ng/g (Bachmann et al., 2018). The doses investigated in this study were higher than this limit. By examining the bacterial feces of calves, it was observed that the number of Escherichia bacteria increased. The observed changes were not dose-dependent (Dupouy et al., 2021). A fecal medium was used in a study to evaluate the residual effect of the ciprofloxacin antibiotic on the intestinal microbiota. For this purpose, the feces of healthy people were transferred to an anaerobic chamber. Ciprofloxacin was added at three concentrations (0.43, 4.3, and 43 μg/mL). Bacteroides fragilis bacteria decreased and, among short-chain fatty acids, butyrate increased at 4.3 and 43 μg/mL. In this study, it was observed that lower doses of acceptable daily intake (ADI) led to changes in the intestinal microbiota (Carman and Woodburn, 2001). It has been observed that the administration of ciprofloxacin reduces Bacteroides fragilis group bacteria in other studies (Wagner et al., 2008).
The survival of bacteria depends on the intestines' conditions. Bacteria face many stressors in the intestines, including antibiotics (Sharma et al., 2023). As a rule, this makes the bacteria living in the gut more likely to survive. The altered gut environment will lead to adaptive mechanisms. Adaptive mechanisms lead to the survival of bacteria and the development of new infections (Alreshidi et al., 2023). Therefore, for future research, it is necessary to consider the residual effects of antibiotics on the adaptation mechanism of pathogenic bacteria.
Conclusion
The residual amount of antibiotics in food of animal origin is low but, usually, exposure to these compounds through food is considered long-term exposure. The studies reviewed were done both in vivo and in vitro. In vitro studies showed no significant changes in the intestinal microbiota. Whereas, several side effects, including changes in the diversity of resident bacteria, disruption of intestinal cell integrity, decreased mucus secretion, decreased activity of goblet cells, decreased activity of digestive enzymes, and decreased antioxidant activity of the intestines, were observed in various types of in vivo studies. The major problem observed in this systematic review was the selected doses of the studies. In choosing doses, the determined MRL of antibiotics in food was not taken into account. Some studies selected doses higher than the MRL, which should be considered for future research. Furthermore, the majority of the studies were about antibiotic resistance, and in this regard, it is suggested that a systematic review is conducted for future studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
The search was conducted and manuscript was written by YM and PS. The extract of data was performed by SR and AS. All authors contributed to the article and approved the submitted version.
Conflict of interest
AS was employed by CEO at Isfahan Chocolate Company (R&D), Isfahan, Iran.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abed, M. F., and Faisal, A. A. H. (2022). Calcium/iron-layered double hydroxides-sodium alginate for removal of tetracycline antibiotic from aqueous solution. Alexand. Eng. J. 63, 127–142. doi: 10.1016/j.aej.2022.07.055
Ahn, Y., Jung, J. Y., Chung, Y. H., Chae, M., Jeon, C. O., and Cerniglia, C. E. (2012). In vitro analysis of the impact of enrofloxacin residues on the human intestinal microbiota using H-NMR spectroscopy. J. Mol. Microbiol. Biotechnol. 22, 317–325. doi: 10.1159/000345147
Ahn, Y., Jung, J. Y., Kweon, O., Veach, B. T., Khare, S., Gokulan, K., et al. (2021). Impact of chronic tetracycline exposure on human intestinal microbiota in a continuous flow bioreactor model. Antibiotics 10, 886. doi: 10.3390/antibiotics10080886
Alreshidi, M., Dunstan, H., MacDonald, M., Saeed, M., Elkahoui, S., and Roberts, T. (2023). Significant changes in cytoplasmic amino acid composition occur in the transition between mid-exponential and stationary phases of growth of staphylococcus aureus: an example of adaptive homeostasis in response to nutrient limitations. Microorganisms. 11, 1–10. doi: 10.3390/microorganisms11010147
Anderson, S. C., Subbiah, S., Gentles, A., Austin, G., Stonum, P., Brooks, T. A., et al. (2016). Qualitative and quantitative drug residue analyses: florfenicol in white-tailed deer (Odocoileus virginianus) and supermarket meat by liquid chromatography tandem-mass spectrometry. J. Chromatogr. B 1033–1034, 73–79. doi: 10.1016/j.jchromb.2016.08.014
Ayesh, R., Weststrate, J. A., Drewitt, P. N., and Hepburn, P. A. (1999). Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem. Toxicol. 37, 1127–1138.
Bachmann, J., Helmschrodt, C., Richter, A., Heuwieser, W., and Bertulat, S. (2018). Residue concentration of cefquinome after intramammary dry cow therapy and short dry periods. J. Dairy Sci. 101, 7540–7550. doi: 10.3168/jds.2017-13826
Baeza, A. N., Urraca, J. L., Chamorro, R., Orellana, G., Castellari, M., and Moreno-Bondi, M. C. (2016). Multiresidue analysis of cephalosporin antibiotics in bovine milk based on molecularly imprinted polymer extraction followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1474, 121–129. doi: 10.1016/j.chroma.2016.10.069
Carman, R. J., Simon, M. A., Petzold, H. E., Wimmer, R. F., Batra, M. R., Fernandez, A. H., et al. (2005). Antibiotics in the human food chain: Establishing no effect levels of tetracycline, neomycin, and erythromycin using a chemostat model of the human colonic microflora. Regulat. Toxicol. Pharmacol. 43, 168–180. doi: 10.1016/j.yrtph.2005.06.005
Carman, R. J., and Woodburn, M. A. (2001). Effects of low levels of ciprofloxacin on a chemostat model of the human colonic microflora. Regulat. Toxicol. Pharmacol. 33, 276–284. doi: 10.1006/rtph.2001.1473
Castanon, J. I. (2007). History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86, 2466–2471. doi: 10.3382/ps.2007-00249
Chen, R. A., Wu, W. K., Panyod, S., Liu, P. Y., Chuang, H. L., Chen, Y. H., et al. (2022). Dietary exposure to antibiotic residues facilitates metabolic disorder by altering the gut microbiota and bile acid composition. mSystems 7, e0017222. doi: 10.1128/msystems.00172-22
De Mulder, T., Rasschaert, G., Van Coillie, E., Van den Meersche, T., Haegeman, A., Ruttink, T., et al. (2019). Impact of cross-contamination concentrations of doxycycline hyclate on the microbial ecosystem in an ex vivo model of the pig's cecum. Microb. Drug Resist. 25, 304–315. doi: 10.1089/mdr.2018.0034
Duan, Y., Chen, Z., Tan, L., Wang, X., Xue, Y., Wang, S., et al. (2020). Gut resistomes, microbiota and antibiotic residues in Chinese patients undergoing antibiotic administration and healthy individuals. Sci. Total Environ. 705, 135674. doi: 10.1016/j.scitotenv.2019.135674
Dupouy, V., Madec, J. Y., Wucher, J., Arpaillange, N., Métayer, V., Roques, B., et al. (2021). Selection of ESBL-producing Escherichia coli in the gut of calves experimentally fed with milk containing antibiotic residues. Vet. Microbiol. 257, 109049. doi: 10.1016/j.vetmic.2021.109049
Fernández Paggi, M. B., Martínez, G., Diéguez, S. N., Pérez Gaudio, D. S., Decundo, J. M., Riccio, M. B., et al. (2018). Fosfomycin residues in colostrum: impact on morpho-physiology and microbiology of suckling piglets. J. Vet. Pharmacol. Ther. 41, 415–427. doi: 10.1111/jvp.12480
Gokulan, K., Cerniglia, C. E., Thomas, C., Pineiro, S. A., and Khare, S. (2017). Effects of residual levels of tetracycline on the barrier functions of human intestinal epithelial cells. Food Chem. Toxicol. 109(Pt 1), 253–263. doi: 10.1016/j.fct.2017.09.004
Gomes Marques de Freitas, A., Almir Cavalcante Minho, L., Elizabeth Alves de Magalhães, B., Nei Lopes dos Santos, W., Soares Santos, L., and Augusto de Albuquerque Fernandes, S. (2021). Infrared spectroscopy combined with random forest to determine tylosin residues in powdered milk. Food Chem. 365, 130477. doi: 10.1016/j.foodchem.2021.130477
Jung, J. Y., Ahn, Y., Khare, S., Gokulan, K., Pineiro, S. A., and Cerniglia, C. E. (2018). An in vitro study to assess the impact of tetracycline on the human intestinal microbiome. Anaerobe 49, 85–94. doi: 10.1016/j.anaerobe.2017.12.011
Kim, B.-S., Kim, J. N., Yoon, S.-H., Chun, J., and Cerniglia, C. E. (2012). Impact of enrofloxacin on the human intestinal microbiota revealed by comparative molecular analysis. Anaerobe 18, 310–320. doi: 10.1016/j.anaerobe.2012.01.003
Kong, B., Sun, R., Huang, M., Chow, M. D., Zhong, X. B., Xie, W., et al. (2018). Fibroblast growth factor 15-dependent and bile acid-independent promotion of liver regeneration in mice. Hepatology 68, 1961–1976. doi: 10.1002/hep.30041
Lee, H. J., Cho, S. H., Shin, D., and Kang, H. S. (2018). Prevalence of antibiotic residues and antibiotic resistance in isolates of chicken meat in Korea. Korean J. Food Sci. Anim. Resour. 38, 1055–1063. doi: 10.5851/kosfa.2018.e39
Li, J. H., Yousif, M. H., Li, Z. Q., Wu, Z. H., Li, S. L., Yang, H. J., et al. (2019). Effects of antibiotic residues in milk on growth, ruminal fermentation, and microbial community of preweaning dairy calves. J. Dairy Sci. 102, 2298–2307. doi: 10.3168/jds.2018-15506
Li, R., Zhou, T., Khan, A., Ling, Z., Sharma, M., Feng, P., et al. (2021). Feed-additive of bioengineering strain with surface-displayed laccase degrades sulfadiazine in broiler manure and maintains intestinal flora structure. J. Hazard. Mater. 406, 124440. doi: 10.1016/j.jhazmat.2020.124440
Ma, X., Yang, Z., Xu, T., Qian, M., Jiang, X., Zhan, X., et al. (2021). Chlortetracycline alters microbiota of gut or faeces in pigs and leads to accumulation and migration of antibiotic resistance genes. Sci. Total Environ. 61, 796. doi: 10.1016/j.scitotenv.2021.148976
Ma, Z., Lin, L., Yang, X., Yuan, Y., Fu, X., Chen, S., et al. (2021). Effects of florfenicol exposure during early life on toxicity, gut microbiota, and fecal metabolome in SD rats. Ecotoxicol. Environ. Saf. 228, 113038. doi: 10.1016/j.ecoenv.2021.113038
Moudgil, P., Bedi, J. S., Aulakh, R. S., and Gill, J. P. S. (2019). Antibiotic residues and mycotoxins in raw milk in Punjab (India): a rising concern for food safety. J. Food Sci. Technol. 56, 5146–5151. doi: 10.1007/s13197-019-03963-8
Nissen, L., Aniballi, C., Casciano, F., Elmi, A., Ventrella, D., Zannoni, A., et al. (2022). Maternal amoxicillin affects piglets colon microbiota: microbial ecology and metabolomics in a gut model. Appl. Microbiol. Biotechnol. 106, 7595–7614. doi: 10.1007/s00253-022-12223-3
Peeters, L. E., Daeseleire, E., Devreese, M., Rasschaert, G., Smet, A., Dewulf, J., et al. (2016). Residues of chlortetracycline, doxycycline and sulfadiazine-trimethoprim in intestinal content and feces of pigs due to cross-contamination of feed. BMC Vet. Res. 12, 1–9. doi: 10.1186/s12917-016-0803-8
Penati, M., Sala, G., Biscarini, F., Boccardo, A., Bronzo, V., Castiglioni, B., et al. (2021). Feeding pre-weaned calves with waste milk containing antibiotic residues is related to a higher incidence of diarrhea and alterations in the fecal microbiota. Front. Vet. Sci. 8, 675. doi: 10.3389/fvets.2021.650150
Pérez, D. S., Tapia, M. O., and Soraci, A. L. (2014). Fosfomycin: uses and potentialities in veterinary medicine. Open Vet. J. 4, 26–43. doi: 10.5455/OVJ.2014.v4.i1.p26
Piñeiro, S. A., and Cerniglia, C. E. (2021). Antimicrobial drug residues in animal-derived foods: potential impact on the human intestinal microbiome. J. Vet. Pharmacol. Ther. 44, 215–222. doi: 10.1111/jvp.12892
Qian, M., Wang, J., Ji, X., Yang, H., Tang, B., Zhang, H., et al. (2021). Sub-chronic exposure to antibiotics doxycycline, oxytetracycline or florfenicol impacts gut barrier and induces gut microbiota dysbiosis in adult zebrafish (Daino rerio). Ecotoxicol. Environ. Saf. 221, 112464. doi: 10.1016/j.ecoenv.2021.112464
Rafiq, K., Hossain, M. T., Ahmed, R., Hasan, M. M., Islam, R., Hossen, M. I., et al. (2022). Role of different growth enhancers as alternative to in-feed antibiotics in poultry industry. Front. Vet. Sci. 8, 1675. doi: 10.3389/fvets.2021.794588
Rageh, A. H., Abdel-Rahim, S. A., Askal, H. F., and Saleh, G. A. (2019). Hydrophilic-interaction planar chromatography in ultra-sensitive determination of α-aminocephalosporin antibiotics. Application to analysis of cefalexin in goat milk samples using modified QuEChERS extraction technique. J. Pharmaceut. Biomed. Anal. 166, 421–434. doi: 10.1016/j.jpba.2019.01.001
Rahman, M. S., Hassan, M. M., and Chowdhury, S. (2021). Determination of antibiotic residues in milk and assessment of human health risk in Bangladesh. Heliyon 7, e07739. doi: 10.1016/j.heliyon.2021.e07739
Ricker, N., Trachsel, J., Colgan, P., Jones, J., Choi, J., Lee, J., et al. (2020). Toward antibiotic stewardship: route of antibiotic administration impacts the microbiota and resistance gene diversity in swine feces. Front. Vet. Sci. 7, 255. doi: 10.3389/fvets.2020.00255
Riley, L. W., Raphael, E., and Faerstein, E. (2013). Obesity in the United States–dysbiosis from exposure to low-dose antibiotics? Front. Public Health 1, 69. doi: 10.3389/fpubh.2013.00069
Sadighara, P., Pirhadi, M., Safta, M., and Abbaszadeh, S. (2022). The gut microflora changes during the COVID-19 pandemic due to exposure to disinfectants. Anaesth. Pain Intens. Care 25, 2. doi: 10.35975/apic.v25i5.1639
Sadighara, P., Godarzi, S., Bahmani, M., and Asadi-Samani, M. (2016). Antioxidant activity and properties of walnut brown seed coat extract. J. Global Pharma Technol. 8, 26–30.
Sallam, K. I., Saad, F. S. S., and Abdelkhalek, A. (2022). Health risk assessment of antimicrobial residues in sheep carcasses marketed in Kuwait. Food Chem. 383, 132401. doi: 10.1016/j.foodchem.2022.132401
Sharma, R., Thakur, A., Saini, A., Giri, S. K., Kumar, A., Priya, K., et al. (2023). “Antibiotics stress response of bacteria as mechanism of development of drug resistance,” in Microbial Stress Response: Mechanisms and Data Science (Washington, DC: ACS Publications), 23–42. doi: 10.1021/bk-2023-1434.ch002
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 24. doi: 10.1186/s40168-017-0242-1
Subirats, J., Domingues, A., and Topp, E. (2019). Does dietary consumption of antibiotics by humans promote antibiotic resistance in the gut microbiome? J. Food Prot. 82, 1636–1642. doi: 10.4315/0362-028X.JFP-19-158
Sun, S., Korheina, D. K., Fu, H., and Ge, X. (2020). Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (Macrobrachium nipponense), and provokes human health risk. Sci. Total Environ. 720, 137478. doi: 10.1016/j.scitotenv.2020.137478
Van den Abbeele, P., Belzer, C., Goossens, M., Kleerebezem, M., De Vos, W. M., Thas, O., et al. (2013). Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 7, 949–961. doi: 10.1038/ismej.2012.158
Wagner, R. D., Johnson, S. J., and Cerniglia, C. E. (2008). In vitro model of colonization resistance by the enteric microbiota: effects of antimicrobial agents used in food-producing animals. Antimicrob Agents Chemother. 52, 1230–1237. doi: 10.1128/AAC.00852-07
Watanabe, M., Higashi, T., Ozeki, K., Higashi, A. Y., Sugimoto, K., Mine, H., et al. (2021). CLDN15 is a novel diagnostic marker for malignant pleural mesothelioma. Sci. Rep. 11, 12554. doi: 10.1038/s41598-021-91464-0
Willis, C., Booth, H., Westacott, S., and Hawtin, P. (1999). Detection of antibacterial agents in warm water prawns. Commun. Dis. Public Health 2, 210–214.
Yang, F., Yang, F., Wang, G., Kong, T., Wang, H., and Zhang, C. (2020). Effects of water temperature on tissue depletion of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus gibelio) following multiple oral doses. Aquaculture 515, 734542. doi: 10.1016/j.aquaculture.2019.734542
Keywords: veterinary antibiotics, microbiota, gut, bacteria, risk
Citation: Sadighara P, Rostami S, Shafaroodi H, Sarshogi A, Mazaheri Y and Sadighara M (2023) The effect of residual antibiotics in food on intestinal microbiota: a systematic review. Front. Sustain. Food Syst. 7:1163885. doi: 10.3389/fsufs.2023.1163885
Received: 11 February 2023; Accepted: 23 March 2023;
Published: 18 April 2023.
Edited by:
Clement Ngun, ITMO University, RussiaReviewed by:
Aneliya Milanova, Trakia University, BulgariaAshenafi Feyisa Beyi, Iowa State University, United States
Copyright © 2023 Sadighara, Rostami, Shafaroodi, Sarshogi, Mazaheri and Sadighara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parisa Sadighara, c2FkaWdoYXJhQGZhcmFiaS50dW1zLmFjLmly
 Parisa Sadighara
Parisa Sadighara Shahrbano Rostami2
Shahrbano Rostami2