
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Sustain. Food Syst. , 09 May 2023
Sec. Sustainable Food Processing
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1161153
This article is part of the Research Topic Fermentation and Enzymatic Processes for the Production of Functional Food View all 6 articles
Emotional neglect (EN) during childhood is a worldwide problem compromising cognitive functions and mental health. Its scars can be life-lasting and often associated with community violence. Therefore, different approaches are mandatory to reduce its detrimental effects. This review discusses the EN's negative impact on the hypothalamic-pituitary-adrenal axis, its consequences on the immune system, and its subsequent impact on the limbic system. On the other hand, growing evidence shows that gut microbiota affects mental health and vice versa; mental disorders affect microbiota leading to dysbiosis and triggering other metabolic malfunctions. Production of functional fermented foods containing targeted probiotic strains and neuroactive compounds released during fermentation may aid to modulate inflammation via immune processes alleviating anxiety and depressive symptoms and improving cognitive function. Therefore, we propose that tailored probiotic-containing fermented food can improve the mental health of EN victims via immune system modulation.
Child abuse (sexual abuse, physical abuse, and emotional abuse) is a problem that lacerates the entire culture and worldwide society. Particularly, negligence is a type of abuse defined as an act of omission by parents or caregivers, which can be harmful to the child (Leeb, 2008) and affects the health, survival, development, and dignity in the context of a relationship of responsibility, trust or power (World Health Organization, 1999). In emotional neglect, caregivers might address physical, academic, or medical needs, however, there exists a lack of psychological (emotional) availability from parents to their children (Stoltenborgh et al., 2013).
Almost 75% of the 6.6 million allegations of child maltreatment have been related to neglectful practices (U.S. Department of Health & Human Services, 2016). The clinical affectations are manifested in different domains of the individual and given the risk it represents to society, the magnitude of this problem is a serious concern, and therefore suitable solutions are still needed.
On the other hand, fermentation is an ancient biotechnology used to enhance the shelf life, nutritional value, and acceptability of food. Part of the microbiome in fermented food may act as probiotics, which are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). In this sense, probiotics that deliver a benefit to patients suffering from psychiatric illness are classified as psychobiotics (Dinan et al., 2013). Furthermore, fermented food may also contain prebiotics, which are defined as substrates, such as oligosaccharides, that are selectively utilized by host microorganisms conferring a health benefit (Gibson et al., 2017). Additionally, a synbiotic is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Swanson et al., 2020). Finally, biogenics are molecules produced by microorganisms that benefit the host, including the neurotransmitters gamma-aminobutyric acid (GABA), tryptophane (a serotonin precursor), and short-chain-fatty-acids (SCFAs) such as butyrate and acetate, neuroactive peptides, and vitamins (Aslam et al., 2020). Recent evidence shows that these fermented food components positively affect the microbiota-gut-brain axis, improving mental health in different mental disorders (Wastyk et al., 2021; Berding and Cryan, 2022). For examples of studies of probiotics on mental disorders see Supplementary Table 1. In this perspective article, we discuss the pathological basis of EN and how anti-inflammatory factors in probiotic fermented food may act as a treatment for the deleterious process of mental disorders in EN victims.
Emotional neglect (EN) is the most common type of abuse (Hildyard and Wolfe, 2002; Stoltenborgh et al., 2013; Cohen et al., 2017), especially when the increase of technology is becoming common in our societies and parents are less emotionally available to their children (Sundqvist et al., 2020; Nabi and Wolfers, 2022; Yang et al., 2023).
Clinical manifestations observed in neglected children are anxious attachment (Egeland and Sroufe, 1981), affectation on their ability to discriminate emotions (Pollak et al., 2000), negative affective reactions to tasks, and less presentation of positive reactions to the Peek-a-boo task (Smyke et al., 2007). Moreover, EN children showed more negative attitudes compared to other types of abuse (Egeland et al., 1983) and worse language development scores compared to the other maltreatment groups (Egeland and Sroufe, 1981). Also, it is registered with internalizing (anxiety disorders and depressive disorders) and externalizing symptoms and disorders like attention-deficit/hyperactivity disorder [ADHD], oppositional defiant disorder, and conduct disorder (Zeanah et al., 2009) and with depression, and anxiety in adolescents (Hevia-Orozco and Sanz-Martín, 2017).
In adulthood, EN is related to countless affectations like psychiatric conditions such as depression, dysthymia, and social phobia (Spinhoven et al., 2010; Cohen et al., 2017), post-traumatic stress disorder (PTSD) (Grassi-Oliveira and Stein, 2008; Salokangas et al., 2020), as well as an increased risk of the onset of schizophrenia (Cancel et al., 2015), paranoid and avoidant personality (Johnson et al., 2000), having trouble identifying emotional expressions (Maheu et al., 2010) and differentiating them from one another (Kim and Cicchetti, 2009). Concerning cognitive affectations, detrimental effects on language and different cognitive functions have been described (Tarullo et al., 2007; Loman et al., 2009). For a review of psychiatric affectations, the reader is recommended to refer to Bos et al. (2011); and Dozier et al. (2012).
It has been postulated that the absence or lack of socio-emotional stimulation in the child is a stressful environment. This can be explained through the mechanism described as “serve and return” (National Scientific Council on the Developing Child, 2012). In this experience-expectant social interaction, the caregiver addresses the child's socio-emotional needs through sounds and movements that the caregiver makes to attract the child's attention and allow them to develop emotional, attentional, and cognitive skills. The impossibility of having these expected and significant interactions in children triggers a metabolic response, which influences the development of the stress response system. In the case of lacking social and emotional stimuli, this response is represented by lower levels of cortisol. This happens in opposition to the higher cortisol levels found in other types of maltreatment (Doom et al., 2022).
In the face of a stress response, the activity of the hypothalamus-pituitary-adrenal (HPA) axis increases triggering a series of reactions that involves both the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) by the paraventricular nucleus of the hypothalamus (Serra et al., 2005). Those hormones stimulate the anterior pituitary gland to produce a release of adrenocorticotropic hormone (ACTH), which in turn stimulates the adrenal gland, located at the upper pole of the renal glands, for the final release of cortisol. The activation of the HPA axis derived from cognitive and emotional stressors occurs through the activation of the limbic system, which is in charge of detecting signals with an emotional charge from the environment, involving the participation of brain regions such as the amygdala (Bogdan et al., 2012) and the insula (Kim-Spoon et al., 2021).
Hypercortisolemia is a common finding in depression (Murphy, 1997; Gillespie and Nemeroff, 2005). Conversely, hypocortisolemia has been found in atypical types of depression (Fries et al., 2005). This type of depression is clinically featured by mood reactivity and two or more of the following features: increased appetite or weight gain, hypersomnia, leaden paralysis, and long-standing interpersonal rejection sensitivity (Parker et al., 2002). In the case of EN, hypocortisolemia has also been reported, derived from an affectation both in the corticotropin-releasing system and in the corticotropin-releasing hormone and adrenocorticotropic hormone. This was found in Rhesus monkeys that were separated from their mothers for 3 h per day that exhibit a flattened diurnal cortisol rhythm and a delayed cortisol peak in response to stress (Feng et al., 2011). This same pattern has been found in adolescents raised in shelters where socio-emotional deprivation is high (Koss et al., 2016). In adolescents with a history of institutionalization, it has been found that the normal cortisol peak in the morning (30–45 after waking up) is flattened (Leneman et al., 2018). This response is typical of adolescents under a scheme of parental care neglect but not parental harshness as previously reported (Gunnar and Donzella, 2002; Doom et al., 2022). Thus, neglectful experiences shape how the HPA axis functions and responds to stressors that the child will face in the following years of life (Koss et al., 2016).
Humans, as a social specie, perceive the lack of social stimuli as a stressor. This has been shown in animal models in social isolation in the early stages of life, and the natural response to this type of stressor is an increase in blood cortisol (Cacioppo et al., 2011). However, the continuous increases in glucocorticoids associated with acute stress over time cause the GR receptors found in the immune system to become less responsive to the influence of cortisol, inducing resistance to glucocorticoids, perhaps as a protective mechanism (Fries et al., 2005). Consequently, this causes the immune system activity to be gradually less regulated (Hawkley et al., 2012). The result of this lower sensitization of the immune system to the cortisol effect is the increment of different inflammation biomarkers such as C-reactive protein (CRP), interleukin-6 (IL−6), and tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL-1β), among others (Nobis et al., 2020; Kerr et al., 2021).
Concerning the effect of the immune system on the brain, it has been postulated that some peripheral cytokines, such as IL-1β, IL-6, and TNF-α, may gain access to the central nervous system through active transport or may cross the blood-brain barrier (BBB) (Mapunda et al., 2022). Peripheral cytokines can also reach the central nervous system through their concentration with receptors of afferent vagal fibers, which project to the limbic system through the nucleus of the solitary tract, knowing this immuno-neuronal communication as the neuro-immune circuit (Irwin and Cole, 2011), affecting regions such as the limbic system and the structures that comprise it (Harrison, 2009). In this way, we can establish a link between negligence and affectations in the central nervous system, through alterations in the immune system.
What are the regions affected by EN through damage to the immune system that leads to changes in moods and subsequent psychiatric disorders? One of the main regions affected by EN is the limbic system (Sciarrino et al., 2018). This finding may be closely associated with the impact of some cytokines on these brain regions. For example, in a study carried out by Harrison (2009), the level of IL-6 in the nervous system was increased through the application of typhoid vaccination, and emotionally charged facial expression perception tasks were carried out during the acquisition of functional magnetic resonance imaging, in addition to applying questionnaires on the state of mind. The authors found a decrease in mood 3 hours after the application of the vaccine. Furthermore, these mood findings were related to decreased functional connectivity of the anterior cingulate, amygdala, medial prefrontal cortex, nucleus accumbens, and superior temporal sulcus. This finding confirms previous studies about the impact of the immune system on the structures of the limbic system (Haas and Schauenstein, 1997). It is likely that this lower cortico-limbic connectivity in children with a history of neglect and the subsequent hyperreactivity of the limbic system to emotional stimuli (Inagaki et al., 2012), was behind the psychopathological behaviors described in this population group (Callaghan et al., 2020).
Regarding the analysis of mental health pathophysiology, one of the main agents that have gained importance is the gut-brain axis. A milestone on the gut microbiota (GM) influence on the gut-brain axis and mental health was Zheng et al. (2016) study where they demonstrated that fecal microbiota transplantation (FMT) of subjects with depression into healthy germ-free mice triggered depressive-like symptoms. GM involves a vast diversity of microorganisms, which is defined by the host's diet, medication, and health. Evidence shows that the communication between the gut and the brain is in a bidirectional sense, mental disorders may impact GM diversity and quality, whereas GM diversity plays an important role in maintaining mental health (Jiang et al., 2015; Dinan and Cryan, 2017; Winter et al., 2018). This bidirectional communication has also revealed great avenues of research to find the bases that support alterations in the anatomy and physiology of the brain that affect the behavior or cognition of people (Dinan and Cryan, 2012). Child abuse is not an exception (Callaghan et al., 2020). GM is altered in adult patients with a history of abuse and anxiety scores measured by the Hamilton scale (Zhang et al., 2022). In the same study, variability in the microbiota was found as an important mediator between abuse and depression in these participants (Zhang et al., 2022). Other authors have found similar results in patients with a history of sexual abuse (Chuang et al., 2019).
GM diversity often marks the difference between the health-promoting microbiome and pro-inflammatory dysbiosis (Galley et al., 2023). A systematic review comparing the GM taxa diversity in individuals with anxiety and or depression found that certain disorders are related to the abundance of pro-inflammatory taxa such as Enterobacteriaceae and Desulfovibrio (Simpson et al., 2021). Moreover, abundance in taxa such as UCG-002, Ruminococcus_torques_group, and Ruminococcus, were correlated to anxiety and depression symptoms in individuals that suffered stress in confined spaces, as a human anxiety model (Chen et al., 2022). These findings represent a great opportunity to explore new treatment pathways, such as fermented food containing probiotics or synbiotics to restore the GM eubiosis that may lead to alternative treatments for mental disorders.
Fermented food (FF) is associated with decreased inflammatory markers and an increase in GM diversity (Wastyk et al., 2021). FF products can be engineered using different approaches to produce probiotic-containing functional food to aid mental disorders in EN victims related to chronic inflammation: (1) enrichment with butyrate-producing probiotic, either directly (Tamanai-Shacoori et al., 2017) or induced by diet (Bach Knudsen et al., 2018; Wastyk et al., 2021), (2) production of neuroactive compounds during fermentation, such as GABA (Cui et al., 2020), and (3) microbiota-targeted composition (Foster et al., 2021; Berding and Cryan, 2022).
SCFAs, such as butyrate, bind to free fatty acid receptors hindering inflammatory response (Rooks and Garrett, 2016). Butyrate regulates the production of cytokines in T-cells through histone deacetylase (HDACs) inhibition acting as an anti-inflammatory and antitumoral agent (Koh et al., 2016). Previous studies have shown that probiotic strains could hinder IL-6 production, a pro-inflammatory cytokine tied to cognitive development in children (Rasmussen et al., 2019), by generating butyrate (Pham et al., 2021). Moreover, evidence shows that the induction of cytokines production by probiotics is strain dependent. For instance, Bifidobacterium (B.) infantis CCUG52486, isolated from elderly subjects, showed a higher increase in the IL-10/IL-12 ratio compared to Lacticaseibacillus rhamnosus GG, Lacticaseibacillus casei Shirota or B. longum SP 07/3 in PBMC (You and Yaqoob, 2012). Although evidence suggests the benefit of butyrate-producing probiotics in mental disorders (Mörkl et al., 2023), more clinical studies are needed to test the efficacy in individuals with EN and their effect on inflammatory biomarkers.
Another anti-inflammatory mechanism of probiotics is their antipathogenic activity. The presence of pathogens can activate microglia inducing inflammation that in turn may lead to CNS cell damage and consequently to neurodegeneration (Rodríguez et al., 2022). Conversely, probiotics attenuate the pro-inflammatory effect of pathogenic bacteria induced by lipoproteins saccharides (LPS) (Vemuri et al., 2017). Their impact on the GM and the subsequent regulation of the immune system to reduce proinflammatory cytokines (Park et al., 2018) are summarized in Figure 1.
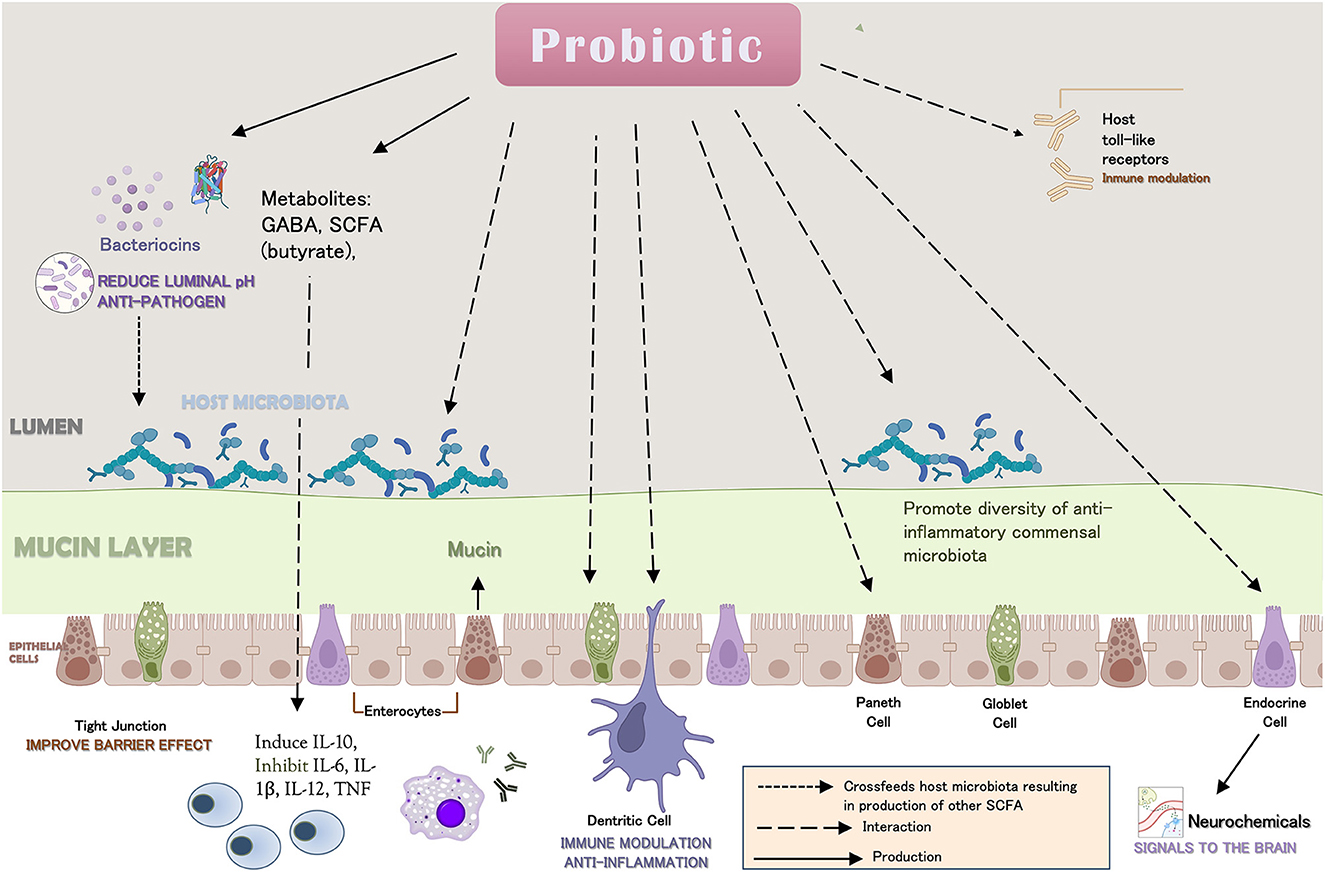
Figure 1. Mechanisms described of probiotics modulation on the immune system leading to anti-inflammatory activity.
GABA can be a mediator to modulate inflammatory processes in EN mental disorders. GABA has been found naturally occurring in ripened cheese and lactic acid bacteria have been isolated with the capability to produce GABA in high yields by optimization of milk fermentation (Gonzalez-Gonzalez et al., 2019; Santos-Espinosa et al., 2020). Additionally, by adding L-monosodium glutamate, GABA production can be optimized by substrate induction during fermentation (Zhuang et al., 2018). Although the capability of peripheral GABA to cross the BBB is still in debate, a study demonstrated that it could regulate cytokine content in CD4+ T cells in peripheral blood (Bhandage et al., 2018). Therefore, the intake of GABA can act as an anti-inflammatory coadjutant in a dose-dependent- manner. Further studies are needed to establish an effective concentration in psychiatric patients.
Probiotics may also intervene in the GM improving microbiota alpha diversity and modulating the immune system improving cognitive function and mental health. For instance, intervention with Lactobacillus acidophilus DDS-1 in aging mice modulated the GM increasing levels of SCFA producer Akkermansia and Lactobacillus spp and lowering levels of mucin degraders Bacteroides acidifaciens and Ruminococcus gnavus (Vemuri et al., 2019). Besides DDS-1 increased levels of butyrate, downregulating the production of pro-inflammatory cytokines (Vemuri et al., 2019). Lactiloplantibacillus plantarum probiotic strains have also shown the capacity to increase Veillonella, Bifidobacterium, Akkermansia, and Lactobacillus genus, in human interventions, helping to restore the eubiosis (Echegaray et al., 2023). Moreover, tailored microbiota-targeted therapies based on microbiome-based biomarkers are promising (Foster et al., 2021). However, this approach is still in its cradle. More studies are necessary to associate host phenotypes to the GM that aid to define biomarkers, particularly in mental disorders (Manor et al., 2020).
A systematic review of clinical trials from 2016 to 2022 in patients with depression reports that consumption of probiotics or synbiotics showed improvement with a short margin of effect compared to placebo. However, this was not the case for prebiotics, which did not show a significant difference (Alli et al., 2022). This indicates that prebiotics alone cannot always improve health conditions in patients with dysbiosis, possibly due to the insufficient presence of beneficial microbiota to promote significant changes. Thus, intervention with tailored microbiome composition, including probiotics may improve eubiosis and mental health in individuals (Kang et al., 2014; Dash et al., 2015; Bear et al., 2020; Manor et al., 2020). Finally, cognitive-behavioral interventions will increase the opportunities for synbiotic therapy success. Those types of intervention need to cover the lack of personal stimulation through individual, long - term and affective support, for instance, an after-school program to teach virtues and values to EN children.
In summary, evidence shows that the immune system is affected by the HPA axis disturbed by EN chronic stress. This disturbance has been associated with GM dysbiosis in EN individuals. Due to the bidirectional nature of gut-brain axis communication, this affectation can be reversibly modulated using probiotics' capability to act as an anti-inflammatory agent through the production of bioactive compounds during fermentation. When probiotics are administered in functional FF with a prebiotic matrix as synbiotics, they can improve the GM to achieve a healthier microbiota that aims to regulate the immune system may improve EN symptoms, and reverse its psychosocial detrimental effects, as proposed in Figure 2. Hence, we propose tailored fermented food, such as fermented milk, yogurt, or cheese, containing probiotic strains able to produce SFCAs including butyrate, and/or GABA, as means to promote anti-inflammatory activity that may contribute to other types of intervention, such as cognitive-behavioral therapy, for people with anxiety and depression with antecedents of EN.
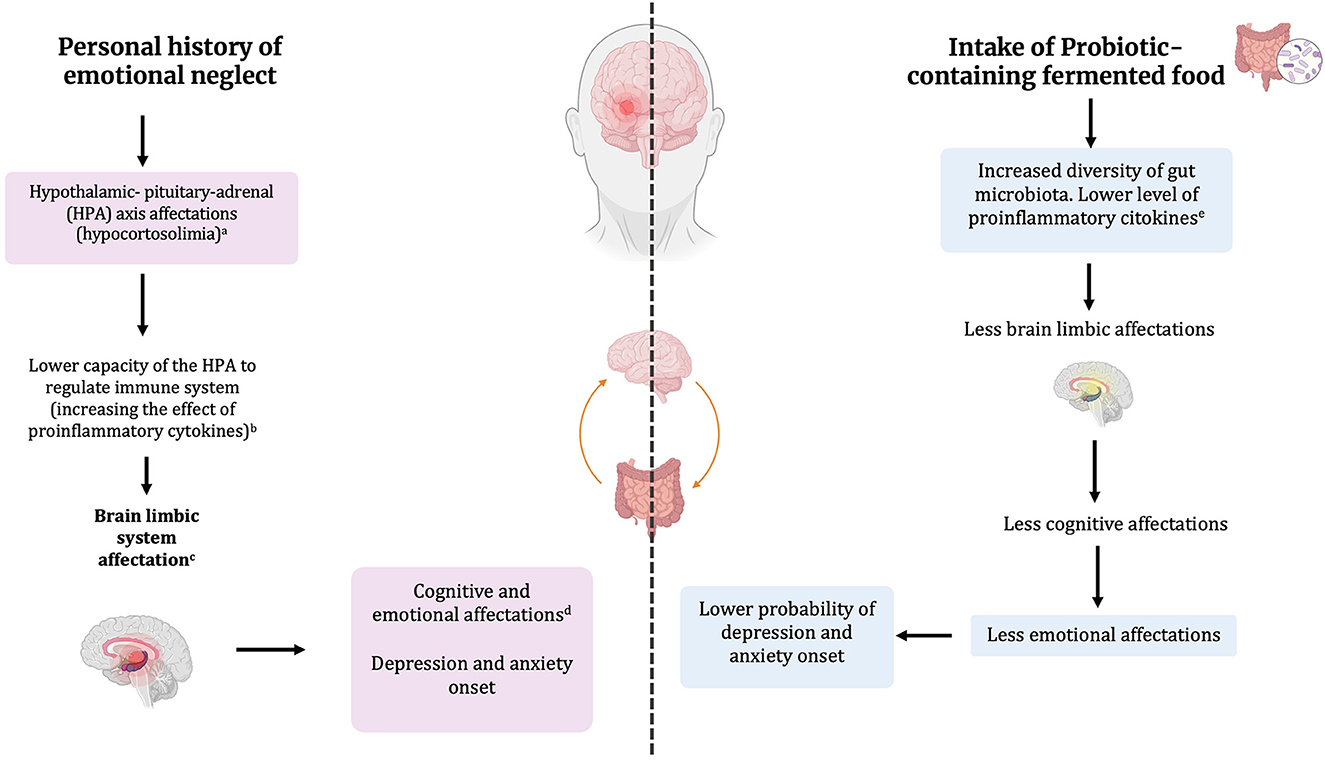
Figure 2. Overview of emotional neglect affectations in the HPA axis and the limbic systems are countered by probiotic-containing fermented food intake reducing the probability of depression and anxiety via immune system modulation. (a) Reilly and Gunnar (2019); (b) Elenkov and Chrousos (2002); (c) Harrison (2009); (d) Sciarrino et al. (2018); (e) Simpson et al. (2021).
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
We thank Anahuac Mayab University and the Tecnológico Nacional de Mexico campus Acayucan for providing the funding for writing this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1161153/full#supplementary-material
Alli, S. R., Gorbovskaya, I., Liu, J. C., Kolla, N. J., Brown, L., and Müller, D. J. (2022). The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 23, 4494. doi: 10.3390/ijms23094494
Aslam, H., Green, J., Jacka, F. N., Collier, F., Berk, M., Pasco, J., et al. (2020). Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutri. Neurosci. 23, 659–671. doi: 10.1080/1028415X.2018.1544332
Bach Knudsen, K. E., Lærke, H. N., Hedemann, M. S., Nielsen, T. S., Ingerslev, A. K., Gundelund Nielsen, D. S., et al. (2018). Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10, 1499. doi: 10.3390/nu10101499
Bear, T. L., Dalziel, J. E., Coad, J., Roy, N. C., Butts, C. A., and Gopal, P. K. (2020). The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutri. 11, 890–907. doi: 10.1093/advances/nmaa016
Berding, K., and Cryan, J. F. (2022). Microbiota-targeted interventions for mental health. Curr. Opin. Psychiatr. 35, 3. doi: 10.1097/YCO.0000000000000758
Bhandage, A. K., Jin, Z., Korol, S. V., Shen, Q., Pei, Y., Deng, Q., et al. (2018). GABA regulates release of inflammatory cytokines from peripheral blood mononuclear cells and CD4+ T cells and is immunosuppressive in type 1 diabetes. EBioMedicine. 30, 283–294. doi: 10.1016/j.ebiom.2018.03.019
Bogdan, R., Williamson, D. E., and Hariri, A. R. (2012). Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatr. 169, 515–522. doi: 10.1176/appi.ajp.2011.11060855
Bos, K., Zeanah, C. H., Fox, N. A., Drury, S. S., McLaughlin, K. A., and Nelson, C. A. (2011). Psychiatric outcomes in young children with a history of institutionalization. Harvard Rev. Psychiatr. 19, 15–24. doi: 10.3109/10673229.2011.549773
Cacioppo, J. T., Hawkley, L. C., Norman, G. J., and Bernston, G. G. (2011). Social isolation. Annal. NY Acad. Sci. 1231, 17–22. doi: 10.1111/j.1749-6632.2011.06028.x
Callaghan, B. L., Fields, A., Gee, D. G., Gabard-Durnam, L., Caldera, C., Humphreys, K. L., et al. (2020). Mind and gut: associations between mood and gastrointestinal distress in children exposed to adversity. Develop. Psychopathol. 32, 309–328. doi: 10.1017/S0954579419000087
Cancel, A., Comte, M., Truillet, R., Boukezzi, S., Rousseau, P. F., Zendjidjian, X. Y., et al. (2015). Childhood neglect predicts disorganization in schizophrenia through gray matter decrease in dorsolateral prefrontal cortex. Acta Psychiatrica Scandinavica 132, 244–256. doi: 10.1111/acps.12455
Chen, Z., Wang, Z., Li, D., Zhu, B., Xia, Y., Wang, G., et al. (2022). The gut microbiota as a target to improve health conditions in a confined environment. Front. Microbiol. 13, 1067756. doi: 10.3389/fmicb.2022.1067756
Chuang, L. C., Chung, Y. C. E., Chen, H. C., Chou, H. C. L., Lee, M. S., Ni, Y. H., et al. (2019). Microbiota features in participants with childhood trauma among the major depressive disorder and healthy controls. Euro. Neuropsychopharmacol. 29, S890–S891. doi: 10.1016/j.euroneuro.2017.08.197
Cohen, J. R., Menon, S. V., Shorey, R. C., Le, V. D., and Temple, J. R. (2017). The distal consequences of physical and emotional neglect in emerging adults: a person-centered, multi-wave, longitudinal study. Child Abuse Neglect. 63, 151–161. doi: 10.1016/j.chiabu.2016.11.030
Cui, Y., Miao, K., Niyaphorn, S., and Qu, X. (2020). Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int. J. Mol. Sci. 21, 995. doi: 10.3390/ijms21030995
Dash, S., Clarke, G., Berk, M., and Jacka, F. N. (2015). The gut microbiome and diet in psychiatry: focus on depression. Curr. Opin. Psychiatr. 28, 1. doi: 10.1097/YCO.0000000000000117
Dinan, T. G., and Cryan, J. F. (2012). Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378. doi: 10.1016/j.psyneuen.2012.03.007
Dinan, T. G., and Cryan, J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterol. Clinic. Nam. 46, 77–89. doi: 10.1016/j.gtc.2016.09.007
Dinan, T. G., Stanton, C., and Cryan, J. F. (2013). Psychobiotics: a novel class of psychotropic. Biologic. Psychiatr. 74, 720–726. doi: 10.1016/j.biopsych.2013.05.001
Doom, J. R., Speltz, M. L., DeKlyen, M., and Endriga, M. C. (2022). Differential associations of parental harshness and parental disengagement with overall cortisol output at 15 years: Implications for adolescent mental health. Develop. Psychopathol. 34, 129–146. doi: 10.1017/S0954579420000954
Dozier, M., Zeanah, C. H., Wallin, A. R., and Shauffer, C. (2012). Institutional care for young children: review of literature and policy implications: institutional care for children. Soc. Issues Policy Rev. 6, 1–25. doi: 10.1111/j.1751-2409.2011.01033.x
Echegaray, N., Yilmaz, B., Sharma, H., Kumar, M., Pateiro, M., Ozogul, F., et al. (2023). A novel approach to Lactiplantibacillus plantarum: From probiotic properties to the omics insights. Microbiologic. Res. 268, 127289. doi: 10.1016/j.micres.2022.127289
Egeland, B., and Sroufe, A. (1981). Developmental sequelae of maltreatment in infancy. New Direct. Child Adolesc. Develop. 1981, 77–92. doi: 10.1002/cd.23219811106
Egeland, B., Sroufe, L. A., and Erickson, M. (1983). The developmental consequence of different patterns of maltreatment. Child Abuse Neglect. 7, 459–469. doi: 10.1016/0145-2134(83)90053-4
Elenkov, I. J., and Chrousos, G.P. (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N.Y. Acad. Sci. 966, 290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x
Feng, X., Wang, L., Yang, S., Qin, D., Wang, J., Li, C., et al. (2011). Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proceed. Nat. Acad. Sci. 108, 14312–14317. doi: 10.1073/pnas.1010943108
Foster, J. A., Baker, G. B., and Dursun, S. M. (2021). The relationship between the gut microbiome-immune system-brain axis and major depressive disorder. Front. Neurol. 12, 721126. doi: 10.3389/fneur.2021.721126
Fries, E., Hesse, J., Hellhammer, J., and Hellhammer, D. H. (2005). A New View of Hypocortisolism. Psychoendocrinology 30, 1010–1016. doi: 10.1016/j.psyneuen.2005.04.006
Galley, J. D., Mashburn-Warren, L., Blalock, L. C., Lauber, C. L., Carroll, J. E., Ross, K. M., et al. (2023). Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav. Immun. 10, 05. doi: 10.1016/j.bbi.2022.10.005
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Gillespie, F., and Nemeroff, B. (2005). Hypercortisolemia and depression. Psychosomatic Med. 67, 26–S28. doi: 10.1097/01.psy.0000163456.22154.d2
Gonzalez-Gonzalez, C. R., Machado, J., Correia, S., McCartney, A. L., Elmore, J. S., and Jauregi, P. (2019). Highly proteolytic bacteria from semi-ripened Chiapas cheese elicit angiotensin-I converting enzyme inhibition and antioxidant activity. LWT. 111, 449–456. doi: 10.1016/j.lwt.2019.05.039
Grassi-Oliveira, R., and Stein, L. M. (2008). Childhood maltreatment associated with PTSD and emotional distress in low-income adults: The burden of neglect. Child Abuse Neglect 32, 1089–1094. doi: 10.1016/j.chiabu.2008.05.008
Gunnar, M. R., and Donzella, B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. doi: 10.1016/S0306-4530(01)00045-2
Haas, H. S., and Schauenstein, K. (1997). Neuroimmunomodulation via limbic structures—the neuroanatomy of psychoimmunology. Progr. Neurobiol. 51, 195–222. doi: 10.1016/S0301-0082(96)00055-X
Harrison, N. A. (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biologic. Psychiatr. 66, 407–414. doi: 10.1016/j.biopsych.2009.03.015
Hawkley, L. C., Cole, S. W., Capitanio, J. P., Norman, G. J., and Cacioppo, J. T. (2012). Effects of social isolation on glucocorticoid regulation in social mammals. Horm. Behav. 62, 314–323. doi: 10.1016/j.yhbeh.2012.05.011
Hevia-Orozco, H., and Sanz-Martín, A. (2017). EEG Correlation during social decision-making in institutionalized adolescents. Abnorm. Behav. Psychol. 03, 01. doi: 10.4172/2472-0496.1000131
Hildyard, K. L., and Wolfe, D. A. (2002). Child neglect: developmental issues and outcomes?. Child Abuse and Neglect 26, 679–695. doi: 10.1016/S0145-2134(02)00341-1
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Inagaki, T. K., Muscatell, K. A., Irwin, M. R., Cole, S. W., and Eisenberger, N. I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage 59, 3222–3226. doi: 10.1016/j.neuroimage.2011.10.090
Irwin, M. R., and Cole, S. W. (2011). Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632. doi: 10.1038/nri3042
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Johnson, J. G., Smailes, E. M., Cohen, P., Brown, J., and Bernstein, D. P. (2000). Associations between four types of childhood neglect and personality disorder symptoms during adolescence and early adulthood: findings of a community-based longitudinal study. J. Personal. Disord. 14, 171–187. doi: 10.1521/pedi.2000.14.2.171
Kang, S. S., Keraldo, P. R., Kurti, A., Miller, M. E. B., Cook, M. D., Whitlock, K., et al. (2014). Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Neurodegeneration 9, 1–12. doi: 10.1186/1750-1326-9-36
Kerr, D. M., McDonald, J., and Minnis, H. (2021). The association of child maltreatment and systemic inflammation in adulthood: a systematic review. PLOS ONE 16, e0243685. doi: 10.1371/journal.pone.0243685
Kim, J., and Cicchetti, D. (2009). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology: pathways linking maltreatment, emotion regulation, and psychopathology. J. Child Psychol. Psychiatr. 51, 706–716. doi: 10.1111/j.1469-7610.2009.02202.x
Kim-Spoon, J., Herd, T., Brieant, A., Peviani, K., Deater-Deckard, K., Lauharatanahirun, N., et al. (2021). Maltreatment and brain development: the effects of abuse and neglect on longitudinal trajectories of neural activation during risk processing and cognitive control. Develop. Cogn. Neurosci. 48, 100939. doi: 10.1016/j.dcn.2021.100939
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Koss, K. J., Mliner, S. B., Donzella, B., and Gunnar, M. R. (2016). Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology 66, 31–38. doi: 10.1016/j.psyneuen.2015.12.018
Leeb, R. T. (2008). Child Maltreatment Surveillance: uniform definitions for public health and recommended data elements. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Available online at: https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
Leneman, K. B., Donzella, B., Desjardins, C. D., Miller, B. S., and Gunnar, M. R. (2018). The slope of cortisol from awakening to 30 min post-wake in post-institutionalized children and early adolescents. Psychoneuroendocrinology 96, 93–99. doi: 10.1016/j.psyneuen.2018.06.011
Loman, M. M., Wiik, K. L., Frenn, K. A., Pollak, S. D., and Gunnar, M. R. (2009). Postinstitutionalized children's development: growth, cognitive, and language outcomes. J. Develop. Behav. Pediatr. 30, 426–434. doi: 10.1097/DBP.0b013e3181b1fd08
Maheu, F. S., Dozier, M., Guyer, A. E., Mandell, D., Peloso, E., Poeth, K., et al. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 10, 34–49. doi: 10.3758/CABN.10.1.34
Manor, O., Dai, C. L., Kornilov, S. A., Smith, B., Price, N. D., Lovejoy, J. C., et al. (2020). Health and disease markers correlate with gut microbiome composition across thousands of people. Nature Commun. 11, 5206. doi: 10.1038/s41467-020-18871-1
Mapunda, J. A., Tibar, H., Regragui, W., and Engelhardt, B. (2022). How does the immune system enter the brain?. Front. Immunol. 13, 805657. doi: 10.3389/fimmu.2022.805657
Mõrkl, S., Butler, M. I., and Lackner, S. (2023). Advances in the gut microbiome and mood disorders. Curr. Opin. Psychiatry 36, 1–7. doi: 10.1097/YCO.0000000000000829
Murphy, B. E. P. (1997) Antiglucocort therapies in depression: a review. Psychoneuroendocrinology 22, 125–132. doi: 10.1016/S0306-4530(97)00021-8
Nabi, R. L., and Wolfers, L. N. (2022). Does digital media use harm children's emotional intelligence? a parental perspective. Media Commun. 10, 350–360. doi: 10.17645/mac.v10i1.4731
National Scientific Council on the Developing Child. (2012). The Science of Neglect: The Persistent Absence of Responsive Care Disrupts the Developing Brain, Working Paper 12. Available online at: http://www.developingchild.harvard.edu
Nobis, A., Zalewski, D., and Waszkiewicz, N. (2020). Peripheral markers of depression. J. Clinic. Med. 9, 3793. doi: 10.3390/jcm9123793
Park, C., Brietzke, E., Rosenblat, J. D., Musial, N., Zuckerman, H., Ragguett, R. M., et al. (2018). Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism?. Brain Behav. Immun. 73, 115–124. doi: 10.1016/j.bbi.2018.07.006
Parker, G., Roy, K., Mitchell, P., Wilhelm, K., and Malhi, G. (2002). Atypical depression: a reappraisal. Am. J. Psychiatr. 159, 1470–1479. doi: 10.1176/appi.ajp.159.9.1470
Pham, M. T., Yang, A. J., Kao, M. S., Gankhuyag, U., Zayabaatar, E., Jin, S. L. C., et al. (2021). Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein. J. Nutri. Biochemistr. 98, 108821. doi: 10.1016/j.jnutbio.2021.108821
Pollak, S. D., Cicchetti, D., Hornung, K., and Reed, A. (2000). Recognizing emotion in faces: Developmental effects of child abuse and neglect. Develop. Psychol. 36, 679–688. doi: 10.1037/0012-1649.36.5.679
Rasmussen, J. M., Graham, A. M., Entringer, S., Gilmore, J. H., Styner, M., Fair, D. A., et al. (2019). Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. NeuroImage 185, 825–835. doi: 10.1016/j.neuroimage.2018.04.020
Reilly, E. B., and Gunnar, M. R. (2019). Neglect, HPA axis reactivity, and development. Int. J. Dev. Neurosci. 78, 100–108. doi: 10.1016/j.ijdevneu.2019.07.010
Rodríguez, A. M., Rodríguez, J., and Giambartolomei, G. H. (2022). Microglia at the crossroads of pathogen-induced neuroinflammation. ASN Neuro 14, 175909142211045. doi: 10.1177/17590914221104566
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Salokangas, R. K. R., Schultze-Lutter, F., Schmidt, S. J., Pesonen, H., Luutonen, S., Patterson, P., et al. (2020). Childhood physical abuse and emotional neglect are specifically associated with adult mental disorders. J. Mental Health 29, 376–384. doi: 10.1080/09638237.2018.1521940
Santos-Espinosa, A., Beltrán-Barrientos, L. M., Reyes-Díaz, R., Mazorra-Manzano, M. Á., Hernández-Mendoza, A., González-Aguilar, G. A., et al. (2020). Gamma-aminobutyric acid (GABA) production in milk fermented by specific wild lactic acid bacteria strains isolated from artisanal Mexican cheeses. Annal. Microbiol. 70, 12. doi: 10.1186/s13213-020-01542-3
Sciarrino, N. A., Hernandez, T. E., and Davidtz, J. (2018). “The effects of childhood neglect on neurological development,” in Understanding Child Neglect, eds Sciarrino, N. A., Hernandez, T. E., and Davidtz, J. (Cham: Springer International Publishing, SpringerBriefs in Psychology), 11–18.
Serra, M., Pisu, M. G., Floris, I., and Biggio, G. (2005) Social isolation-induced changes in the hypothalamic–pituitary– adrenal axis in the rat. Stress 8, 259–264. doi: 10.1080/10253890500495244
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. (2021). The gut microbiota in anxiety and depression—a systematic review. Clinic. Psychol. Rev. 83, 101943. doi: 10.1016/j.cpr.2020.101943
Smyke, A. T., Koga, S. F., Johnson, D. E., Fox, N. A., Marshall, P. J., Nelson, C. A., et al. (2007). The caregiving context in institution-reared and family-reared infants and toddlers in Romania. J. Child Psychol. Psychiatr. 48, 210–218. doi: 10.1111/j.1469-7610.2006.01694.x
Spinhoven, P., Elzinga, B. M., Hovens, J. G., Roelofs, K., Zitman, F. G., van Oppen, P., et al. (2010). The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J. Affect. Disord. 126, 103–112. doi: 10.1016/j.jad.2010.02.132
Stoltenborgh, M., Bakermans-Kranenburg, M. J., and van IJzendoorn, M. H. (2013). The neglect of child neglect: a meta-analytic review of the prevalence of neglect. Soc. Psychiatr. Psychiatr. Epidemiol. 48, 345–355. doi: 10.1007/s00127-012-0549-y
Sundqvist, A., Heimann, M., and Koch, F.-S. (2020). Relationship between family technoference and behavior problems in children aged 4–5 years. Cyberpsychol. Behav. Soc. Network. 23, 371–376. doi: 10.1089/cyber.2019.0512
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, G., et al. (2020). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701. doi: 10.1038/s41575-020-0344-2
Tamanai-Shacoori, Z., Smida, I., Bousarghin, L., Loreal, O., Meuric, V., Fong, S. B., et al. (2017). roseburia spp. : a marker of health?. Fut. Microbiol. 12, 157–170. doi: 10.2217/fmb-2016-0130
Tarullo, A. R., Bruce, J., and Gunnar, M. R. (2007). False belief and emotion understanding in post-institutionalized children. Soc. Develop. 16, 57–78. doi: 10.1111/j.1467-9507.2007.00372.x
U.S. Department of Health & Human Services (2016). “Administration for children and families, administration on children, youth and families, children's Bureau,” in Child Maltreatment 2014. Available online at: https://www.acf.hhs.gov/cb/report/child-maltreatment-2018
Vemuri, R., Gundamaraju, R., and Eri, R. (2017) Role of lactic acid probiotic bacteria in IBD. Curr. Pharmaceutic. Des. 23, 2352–2355. doi: 10.2174/1381612823666170207100025
Vemuri, R., Gundamaraju, R., Shinde, T., Perera, A. P., Basheer, W., Southam, B., et al. (2019). Lactobacillus acidophilus DDS-1 Modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients 11, 1297. doi: 10.3390/nu11061297
Wastyk, H. C., Fragiadakis, G. K., Perelman, D., Dahan, D., Merrill, B. D., Feiqiao, B. Y., et al. (2021). Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153. doi: 10.1016/j.cell.2021.06.019
Winter, G., Hart, R. A., Charlesworth, R. P. G., and Sharpley, C. F. (2018). Gut microbiome and depression: what we know and what we need to know. Rev. Neurosci. 29, 629–643. doi: 10.1515/revneuro-2017-0072
World Health Organization (1999). Report of the consultation on child abuse prevention. WHO/HSC/PVI/99.1: Geneva. Available online at: https://apps.who.int/iris/handle/10665/65900.
Yang, X., Jiang, P., and Zhu, L. (2023). Parental problematic smartphone use and children's executive function: the mediating role of technoference and the moderating role of children's age. Early Childhood Res. Q> 63, 219–227. doi: 10.1016/j.ecresq.2022.12.017
You, J., and Yaqoob, D (2012). Evidence of immunomodulatory effects of a novel probiotic, Bifidobacterium longum bv. infantis CCUG 52486. FEMS Immunol. Med. Microbiol. 66, 353–362. doi: 10.1111/j.1574-695X.2012.01014.x
Zeanah, C. H., Egger, H. L., Smyke, A. T., Nelson, C. A., Fox, N. A., Marshall, P. J., et al. (2009). Institutional rearing and psychiatric disorders in romanian preschool children. Am. J. Psychiatr. 166, 777–785. doi: 10.1176/appi.ajp.2009.08091438
Zhang, Y., Zhang, R., Liu, P., Wang, J., Gao, M., Zhang, J., et al. (2022). Characteristics and mediating effect of gut microbiota with experience of childhood maltreatment in major depressive disorder. Front. Neurosci. 16, 926450. doi: 10.3389/fnins.2022.926450
Zheng, P., Elsayed, M., Margineanu, M., Boury-Jamot, B., Fragnière, L., and Meylan, E. M. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 21, 786–796. doi: 10.1038/mp.2016.44
Keywords: emotional neglect, hypothalamic-pituitary-adrenal axis, probiotics, fermented food, mental health
Citation: Hevia-Orozco J and González-González CR (2023) Designing probiotic-containing fermented food to improve mental disorders derived from childhood emotional neglect. Front. Sustain. Food Syst. 7:1161153. doi: 10.3389/fsufs.2023.1161153
Received: 08 February 2023; Accepted: 11 April 2023;
Published: 09 May 2023.
Edited by:
Raul Avila-Sosa, Benemérita Universidad Autónoma de Puebla, MexicoReviewed by:
Humberto Hernandez-Sanchez, National Polytechnic Institute (IPN), MexicoCopyright © 2023 Hevia-Orozco and González-González. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cid R. González-González, Y2lkZ29uemFsZXpAaXRzYWNheXVjYW4uZWR1Lm14
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.