
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 21 April 2023
Sec. Agro-Food Safety
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1155543
This article is part of the Research Topic Ensuring the Quality and Safety of Livestock Products in Sustainable Food Systems View all 4 articles
 Jiaming Li1,2†
Jiaming Li1,2† Liwen Yan1,2†
Liwen Yan1,2† Xuehai Cao1,2
Xuehai Cao1,2 Yueming Luo1,2
Yueming Luo1,2 Xintong Peng1,2
Xintong Peng1,2 Zirui Wang1,2
Zirui Wang1,2 Tiande Zou1,2
Tiande Zou1,2 Jun Chen1,2*
Jun Chen1,2* Jinming You1,2*
Jinming You1,2*This study aimed to investigate the effects of dietary rare earth (RE) supplementation on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. A total of 960 Lohmann Pink laying hens (380 d old) were randomly assigned to 1 of 5 dietary treatments in a 21-day feeding trial. There were 6 replicates in each treatment, with 32 hens per replicate. The five experimental diets were supplemented with 0, 150, 300, 450, and 600 g/t RE in the basal diet. Compared with the control group, hens fed the 150 g/t RE diet had a greater average egg weight during the third week of the experimental period (p < 0.05). However, dietary 150, 300, or 600 g/t RE supplementation decreased the eggshell thickness of laying hens compared to that of the control group (p < 0.05). No differences were observed in the serum biochemical parameters of laying hens among treatments except for the HDL-C concentration, which was higher in the 300 or 450 g/t RE-supplemented group than in the control group (p < 0.05). However, GSH-Px activity increased when hens were fed the 600 vs. 0 g/t RE diet (p < 0.05). But dietary supplementation with 600 g/t RE increased the ileum’s crypt depth in laying hens compared to the control group (p < 0.05). There were significant differences in beta diversity of cecum microbiota in laying hens fed a 600 g/t RE diet in place of the other 4 experimental diets (p < 0.05). Compared with the control diet, dietary 600 g/t RE supplementation significantly decreased the relative abundance of Fusobacteriota (phylum) and Fusobacterium (genus) while markedly increasing the relative abundance of Ruminococcus (genus) and Subdoligranulum (genus) (p < 0.05). A high RE dosage negatively affects egg quality and intestinal morphology and alters gut microbiota diversity and composition. In contrast, a moderate RE dosage has beneficial effects on production performance in late-phase laying hens. Further research is warranted regarding eggshell thickness to investigate whether dietary calcium levels must be adjusted when 150 g/t RE is supplemented for late-phase laying hens.
The application of antibiotics in livestock feed has been banned in the European Union, America, and China due to antibiotic resistance spread and drug-resistant bacterial issues (Zeng et al., 2021). Under tremendous pressure, livestock nutritionists and farmers urgently seek new growth-promoting additives for livestock production. The rare earth elements had been reported as a new type of safe feed additive due to their beneficial effects on body weight gain, egg production, milk production, and feed conversion rate in livestock and poultry production (Tariq et al., 2020). In this context, an increasing number of researchers and livestock producers have started to notice rare earth elements. Rare earth elements, normally known as rare earths, are made up of 15 lanthanides mostly in the sixth period of the periodic table of the elements, and 17 elements with similar chemical properties, such as scandium and yttrium. Most of the rare earth (RE) resources, production, processing, and supply are concentrated in Asia-Pacific. In this regard, China dominates the RE industry by producing more than 90% of current rare earth requirements (Dushyantha et al., 2020). The development of rare earth elements as livestock and poultry feed additives has enormous development potential, socioeconomic benefits, and environmental safety benefits.
Among rare earth elements, lanthanum and cerium, commonly utilized as feed additives for livestock production, have been demonstrated to be growth-promoting additives and environmentally friendly (Durmuş and Bölükbaşı, 2015; Bölükbaşı et al., 2016; Abdelnour et al., 2019). It has been demonstrated that dietary RE supplementation improved nutrient digestibility and growth performance in pigs (Förster et al., 2008; Cai et al., 2018; Xiong et al., 2019). For poultry, feeding lanthanum oxide (100–400 mg/kg) for 10 weeks had beneficial effects on the feed efficiency, egg production, and serum oxidative stress status of 22-wk-old laying hens (Durmuş and Bölükbaşı, 2015). Additionally, Bölükbaşı et al. (2016) found that dietary supplementation with 100–400 mg/kg cerium oxide for 10 weeks increased feed efficiency, egg production, and egg shelf life of brown Lohman LSL laying hens (Bölükbaşı et al., 2016). In a 28-day feeding trial of a 1-day-old broiler, dietary supplementation with 1,500 mg/kg rare earth element-enriched yeast improved the nutrient digestibility and meat quality of broilers fed an antibiotic-free diet (Cai et al., 2015). Overall, RE has great potential to be developed as functional feed additive for animal production. However, the research and application of RE (lanthanum and cerium combination) in laying hens is scant, especially for laying hens during the late laying phase. It has been demonstrated that laying performance, egg quality, antioxidant capacity, and intestinal health are compromised with the increasing age of laying hens after the laying peak phase (Katz et al., 2004; Liu et al., 2013; Molnár et al., 2017; Zhu et al., 2019). In light of current knowledge, it is hypothesized that RE could be utilized as functional feed supplement to improve productivity of late-phase layers, including production performance and egg quality. Also, the beneficial effects of RE supplementation may be due to the healthy implications regarding the antioxidant-enhancing and intestine-regulating properties of RE.
Therefore, this study was conducted to investigate the effects of dietary rare earth (150–600 mg/kg) supplementation on the production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota of laying hens during the late laying phase.
A total of 960 Lohmann Pink laying hens (380 d old) were randomly assigned to 1 of 5 dietary treatments. There were 6 replicates in each treatment, with 32 hens per replicate. The 5 experimental diets were supplemented with 0, 150, 300, 450, and 600 g/t RE in a basal diet. All diets were presented in mash form. The RE product is a commercial RE feed additive, which is provided by Jiangxi Pengda biotechnology Co., Ltd. (Jiangxi, China). The RE product contains 2.92% cerium and 5.02% lanthanum (analyzed values). The basal diet was corn-soybean-based, and the ingredient composition and nutritional level of the basal diet are presented in Table 1. The feeding trial lasted for 21 days.
This study was conducted in Jiangxi Province (China) between April and May, 2021. Laying hens were reared in three-layer cages (4 hens per cage; cage size: 50 cm length × 45 cm width × 43 cm height) in a ventilated room with illumination 16 h/d (20 lx) and darkness for 8 h/d. Temperature and humidity were recorded every half hour using an automatic temperature and humidity recorder (the instrument was from Miaoxin Technology Co., Ltd., Th10R). The average temperature and humidity every half hour were 25.50 ± 0.28°C and 75.92 ± 0.75, respectively. During the whole experimental period, hens were fed twice daily for ad lithium feed intake and had free freshwater access. All eggs were collected daily to measure egg production and egg weight, while feed residues were recorded weekly to calculate the average daily feed intake and feed conversion ratio.
Four eggs per replicate were collected at the end of the feeding trial to analyze egg quality and yolk antioxidant indices. Six hens (1 hen per replicate) per treatment were sampled for blood collection via the wing vein. Serum samples were separated using a centrifuge set at 3,000 × g for 10 min. After blood sampling, those birds were killed, and the liver was sampled for antioxidant index measurement. For intestinal morphology analysis, the duodenum, jejunum, and ileum were sampled and fixed in 10% formaldehyde phosphate buffer. The cecal microbiota was sampled, frozen in liquid nitrogen, and stored at −80°C until gut microbiota analysis.
All eggs were collected daily to measure egg production and egg weight, while feed residual quantity were recorded weekly to calculate the average daily feed intake and feed conversion ratio.
Twelve eggs per treatment were measured for egg quality, with six replicates per treatment and 2 eggs per replicate. The egg diameter was measured longitudinally and transversely using a caliper (Guilin Guanglu Measuring Instrument Co., Ltd., 111-101B). The longitudinal diameter was divided by the transverse diameter to calculate the shape index. Eggshell thickness was measured at 3 different positions (equator, air cell end, and small end position), and the averaged value was considered eggshell thickness (Dongguan Sanliang Measuring Instrument Co., Ltd). Eggshell strength was measured with an Egg Force Reader (Tenovo International Co., Ltd., Produced KQ-1A Egg Shell tester). Haugh units, albumen height, and yolk color were measured with a multifunctional egg quality tester (EMT-5200, Robotmation Co., Ltd., Tokyo, Japan). The yolk color was determined using a Roche color fan (6 to 15 color grades).
The concentrations of total protein, calcium, high-density liptein cholesterol (HDL-C), low-density liptein cholesterol (LDL-C), total cholesterol (T-CHO), triglycerides, urea nitrogen (BUN), glucose, and phosphorus were measured using commercial kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
The malondialdehyde (MDA) concentration, total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px) activity, superoxide dismutase (SOD) activity, and glutathione (GSH) concentration were measured using commercial assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Take a certain amount of liver (0.1 g) with saline in a 1:9 ratio, add 2–3 steel balls, then 3,500 r/min centrifuge for 10 min; take the supernatant for indicator detection. The liver and yolk protein concentrations were tested using a BCA protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Intestinal morphology was evaluated using the method described in our previous study (Zeng et al., 2021). Briefly, samples were dehydrated, followed by paraffin embedment. Then, the embedded samples were sectioned and stained with hematoxylin and eosin. Motic Images Advanced 3.2 software (Motic, Xiamen, China) analyzed the villus height and crypt depth. At least 15 well-oriented intact villi and their associated crypts were measured in each intestinal section of each hen. The villus height was divided by crypt depth to calculate the villus height/crypt depth ratio.
Gut microbiota analysis was performed as described in our previous study (Zeng et al., 2021). Briefly, genomic DNA was extracted, followed by bacterial 16S rRNA gene amplification. PCR products were mixed in equidensity ratios and purified. 16S rRNA sequencing was performed on an Illumina HiSeq2500 PE250 platform (Illumina Technologies, San Diego, CA, United States). Sequencing data were analyzed according to the results of our previous study (Zeng et al., 2021). Briefly, the Qiime software (Version 1.9.1) was used to calculate the Observed, Chao1, Shannon, Simpson, ACE, Goods-coverage, PD_whole_tree indices. Dilution curves, rank abundance curves, species accumulation curves, and inter-group variation analysis of alpha diversity indices were performed using R software (Version 2.15.3). Analysis of differences between groups in beta diversity index were carried out using R software with and without parametric tests, respectively. ADONIS was used to analyze the explanatory degree of different grouping factors to sample differences, and permutation test was used to analyze the statistical significance of grouping. All of the sequencing data are available in the Sequence Read Archive (SRA) database at NCBI under accession number of SRP421449.
Statistical analysis was conducted using SPSS 22.0 (SPSS, INC., Chicago, IL, United States). All raw data were statistically analyzed by one-way analysis of variance (ANOVA). Duncan’s multiple comparison test was performed to analyze the differences among treatments for significant effects. Linear and quadratic effects were tested by SPSS 22.0. The results are described as means and SEM. p < 0.05 was significant.
As shown in Table 2, the egg production, average daily feed intake, and feed conversion ratio of laying hens were unaffected by dietary treatments (p > 0.05). Compared with the control group, hens fed a 150 g/t rare earth (RE) diet had a greater average egg weight during the third week of the experimental period (p < 0.05). However, dietary supplements with 300, 450, or 600 g/t RE did not influence the average egg weight compared to the control group (p > 0.05).
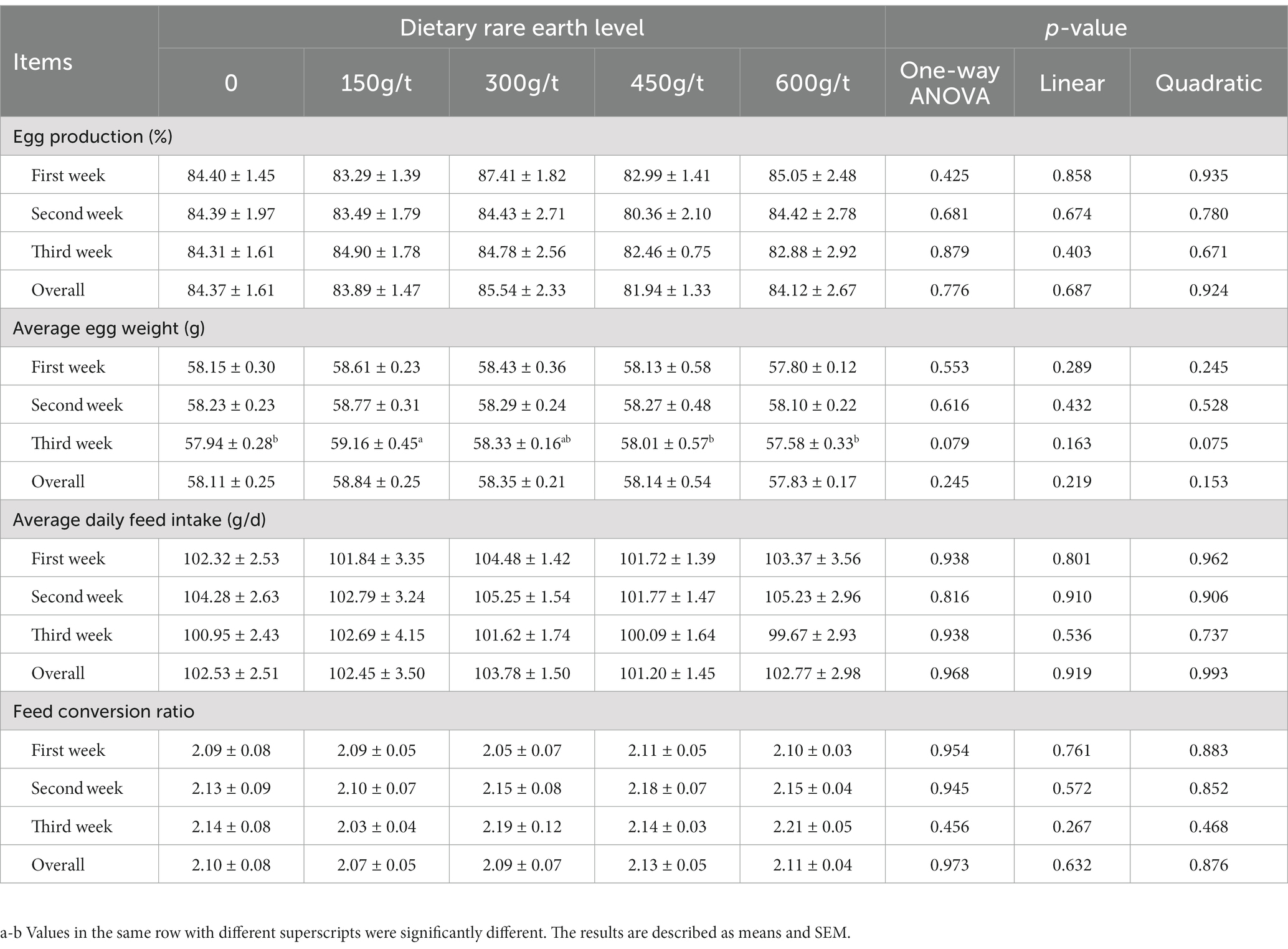
Table 2. Effects of dietary rare earth supplementation on production performance in laying hens during the late laying stage (n = 6).
The effects of dietary RE supplementation on egg quality and the yolk antioxidant index in laying hens are summarized in Table 3. There were no significant differences in egg shape index, eggshell strength, albumen height, yolk color, or Haugh unit among dietary treatments (p > 0.05). However, dietary 150, 300, or 600 g/t RE supplementation decreased the eggshell thickness of laying hens compared to the control group (p < 0.05).
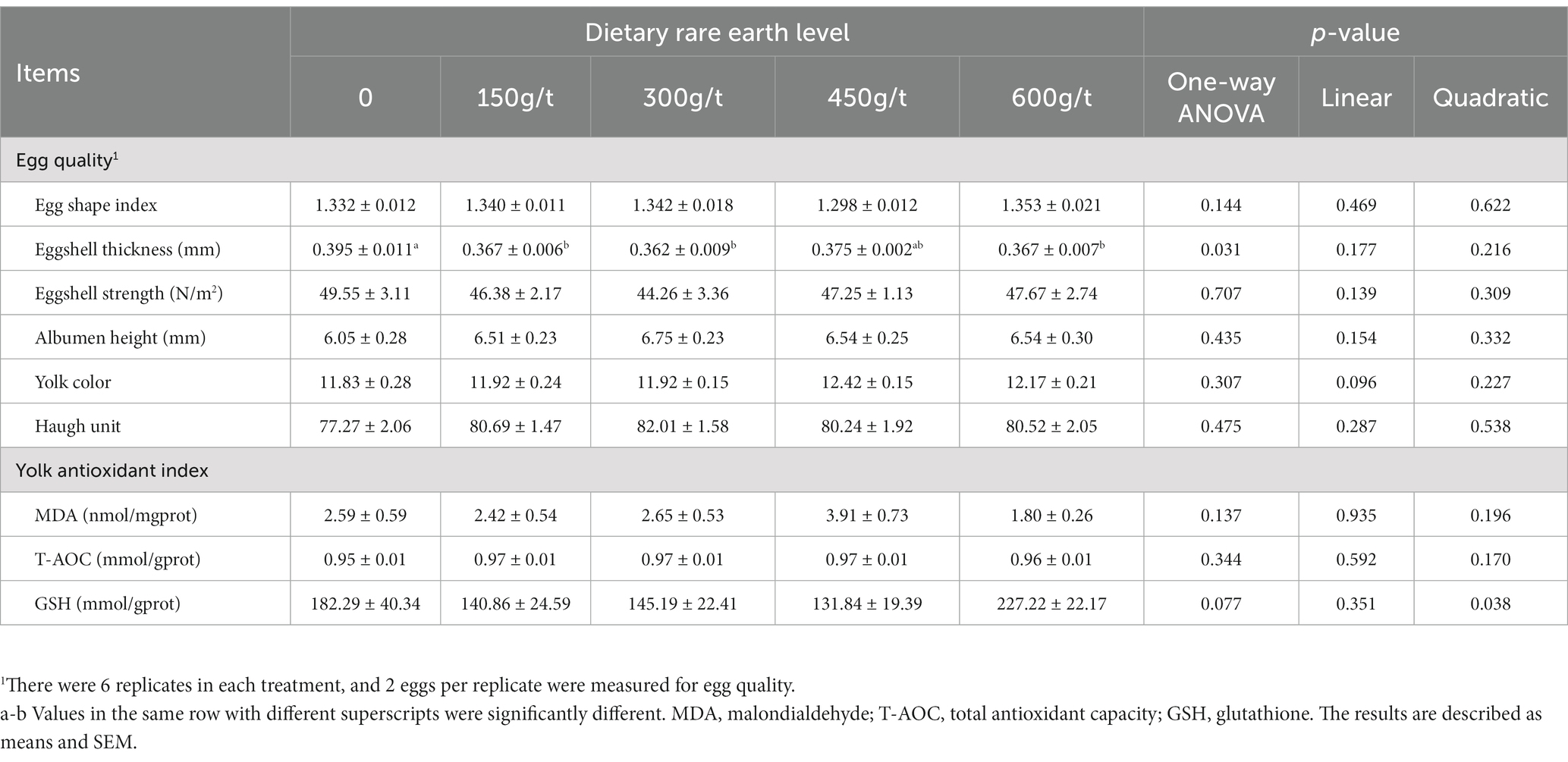
Table 3. Effects of dietary rare earth supplementation on egg quality and yolk antioxidant index in laying hens during the late laying stage (n = 6).
The effects of dietary RE supplementation on serum biochemical parameters are displayed in Table 4. No differences were observed in the serum biochemical parameters of laying hens among treatments except for the HDL-C concentration, higher in the 300 or 450 g/t RE-supplemented group than in the control group (p < 0.05).
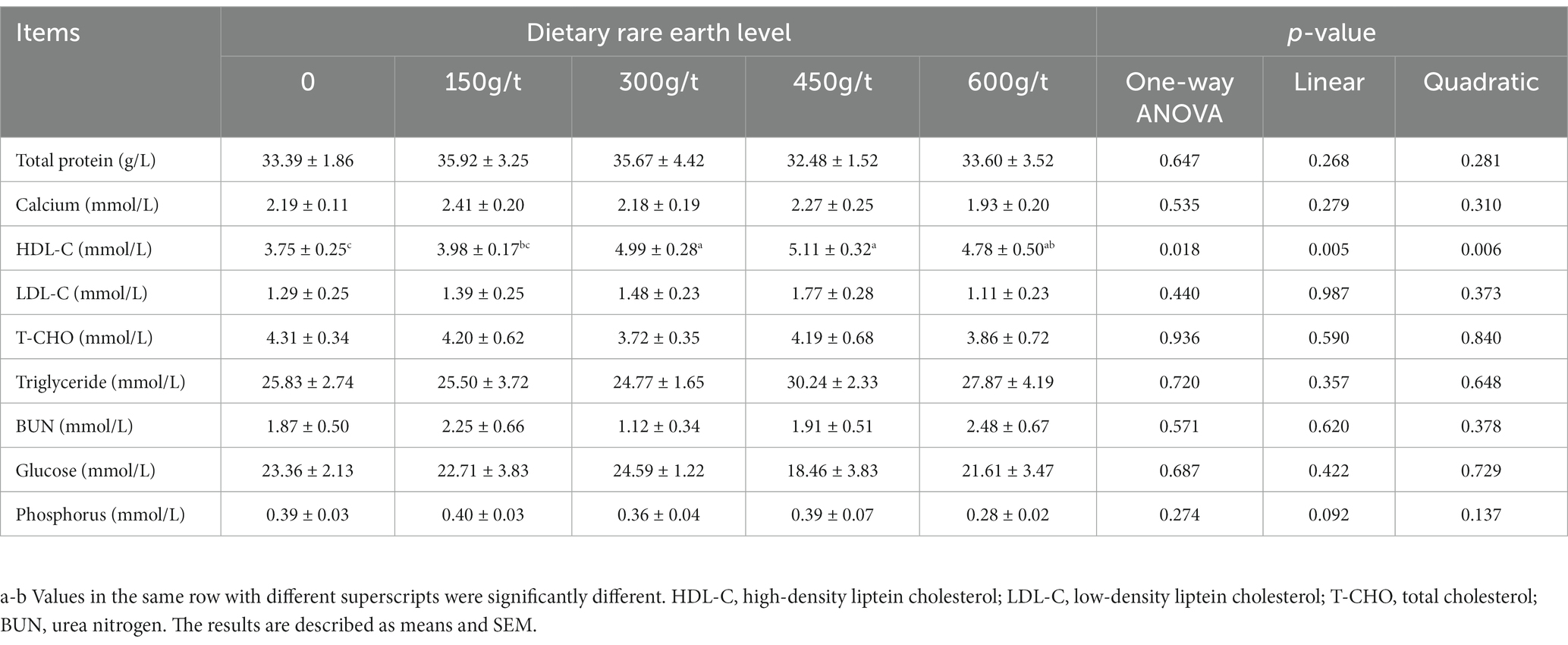
Table 4. Effects of dietary rare earth supplementation on serum biochemical parameters in laying hens during the late laying stage (n = 6).
Dietary RE supplementation had no significant effect on the concentrations of MDA and GSH or T-AOC and SOD activity in the serum of laying hens (p > 0.05) (Table 5). However, GSH-Px activity increased when hens were fed the 600 vs. 0 g/t RE diet (p < 0.05).
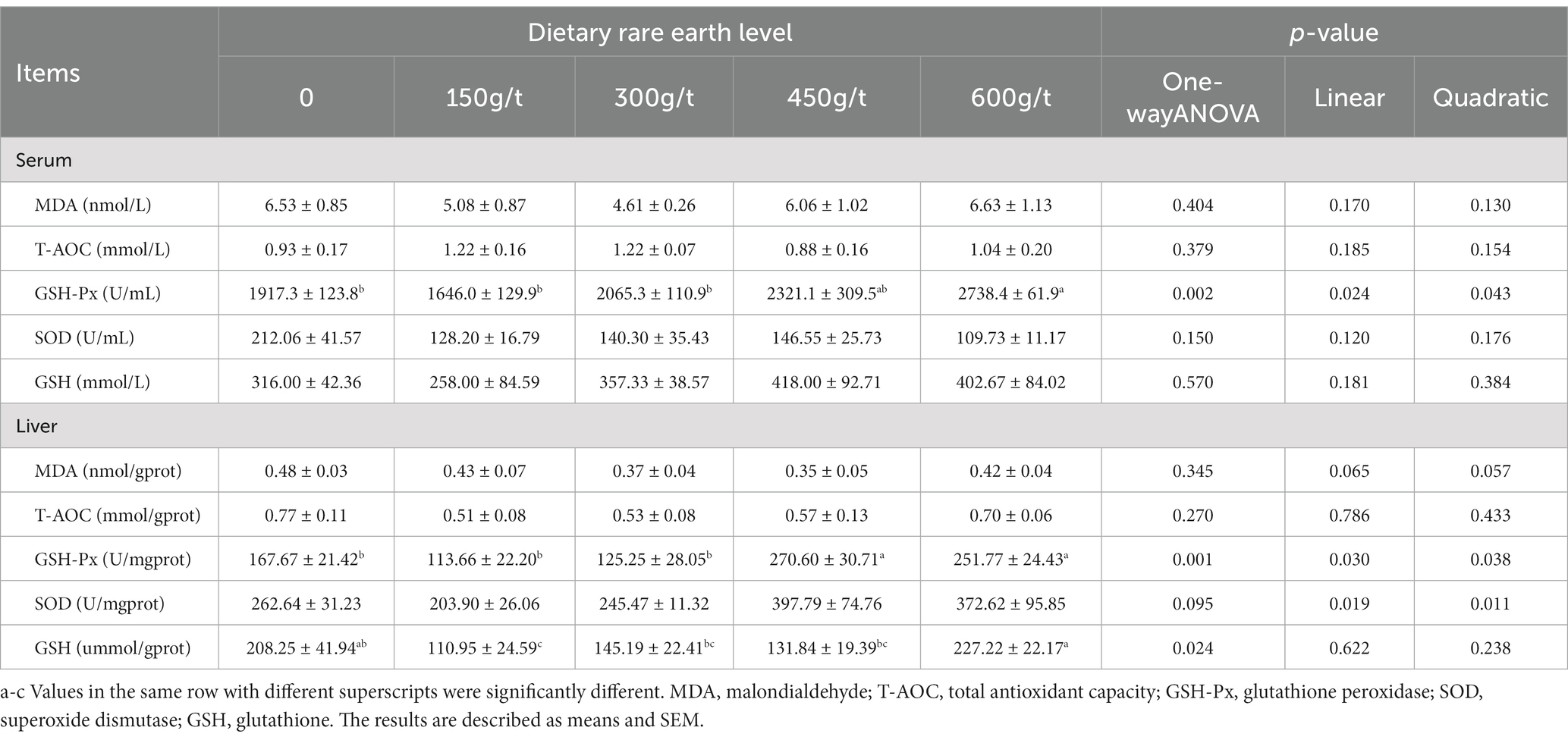
Table 5. Effects of dietary rare earth supplementation on the antioxidant index in the serum and liver of laying hens during the late laying stage (n = 6).
Dietary RE supplementation did not affect the MDA concentration, T-AOC, or SOD activity in the liver of laying hens (p > 0.05). However, the hepatic GSH-Px activity was more significant when hens were fed 450 or 600 g/t RE diet instead of the control diet (p < 0.05). In addition, the hepatic GSH concentration was decreased when hens were fed the 600 vs. 150 g/t RE diet (p < 0.05).
No differences were found in yolk antioxidant indices of laying hens among dietary treatments, including MDA concentration, T-AOC, and GSH concentration (p > 0.05).
As displayed in Table 6, dietary RE supplementation did not impact the morphology of the duodenum and jejunum of laying hens, including villus height, crypt depth, and the ratio of villus height to crypt depth (p > 0.05). However, dietary supplementation with 600 g/t RE increased the ileum’s crypt depth in laying hens compared to the control group (p < 0.05).
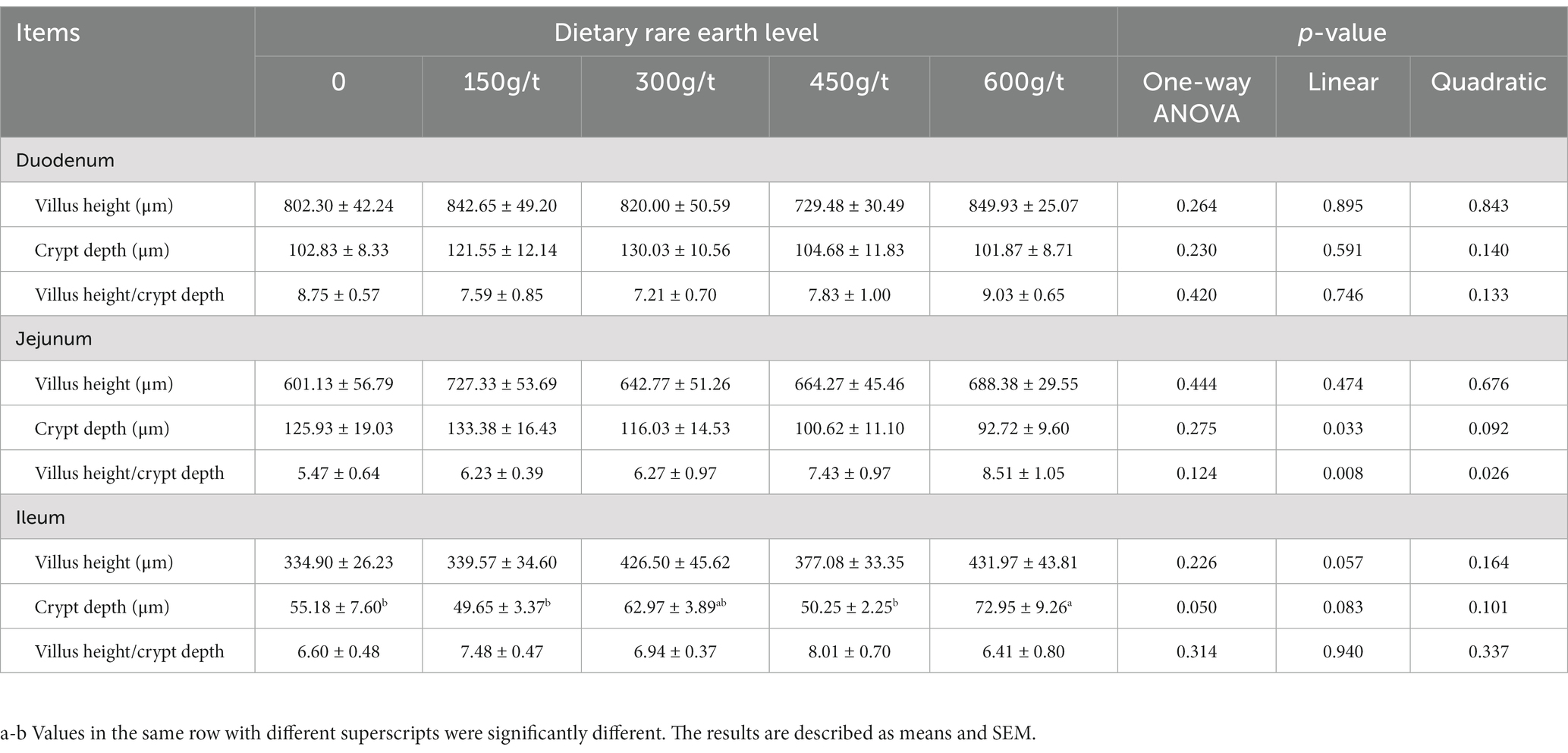
Table 6. Effects of dietary rare earth supplementation on the intestinal morphology of laying hens during the late laying stage (n = 6).
The effect of dietary RE supplementation on the alpha diversity indices of the cecum microbiota in laying hens is shown in Table 7. No significant differences were observed for the alpha diversity indices of the cecum in laying hens among dietary treatments (p > 0.05). As shown in Figure 1, principal coordinate analysis (PCoA) based on unweighted UniFrac was performed to visualize the beta diversity of the cecum microbiota in laying hens fed the 5 experimental diets. There were significant differences in beta diversity of cecum microbiota in laying hens fed a 600 g/t RE diet in place of the other 4 experimental diets (p < 0.05, adonis analysis).
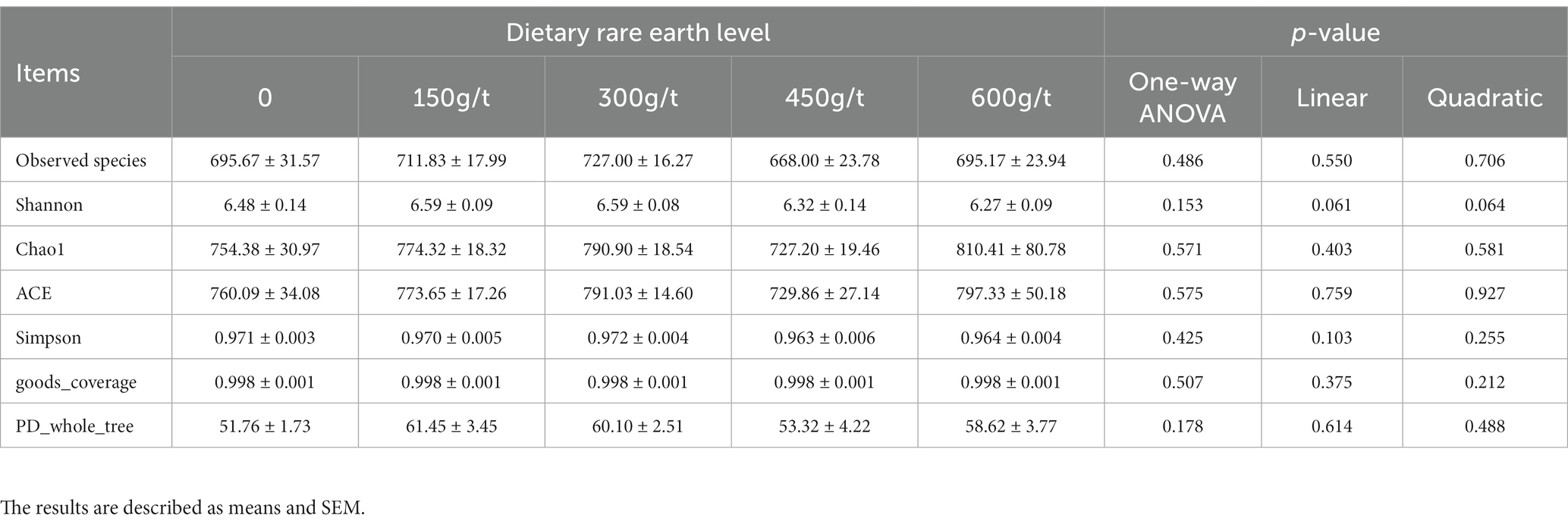
Table 7. Effects of dietary rare earth supplementation on alpha diversity indices of cecum microbiota in laying hens during the late laying stage (n = 6).
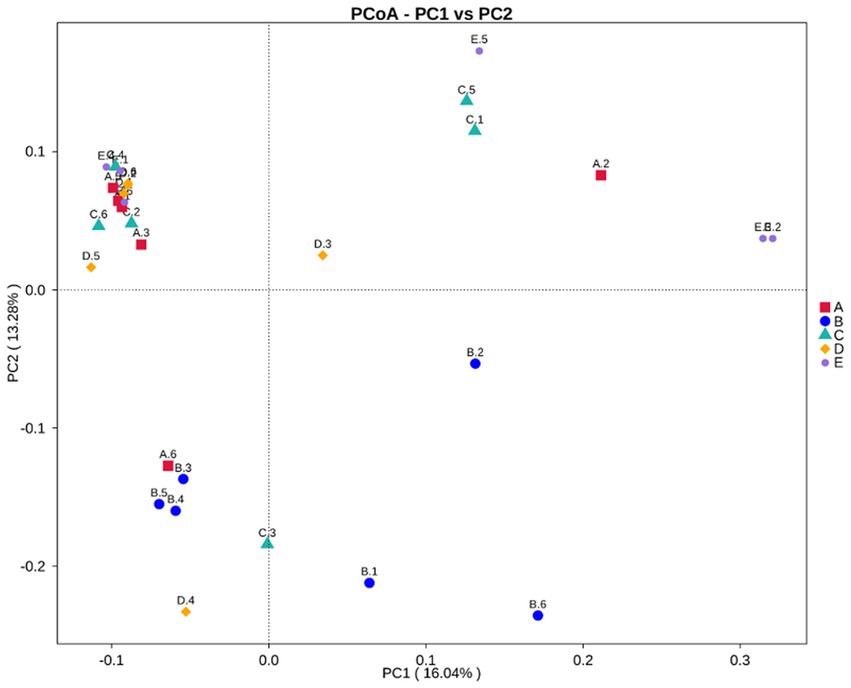
Figure 1. The beta diversity of the cecal microbiota in laying hens fed the 5 experimental diets using principal component analysis (PCoA). There were 6 replicates (birds) in each treatment (n = 6). Group A (A.1-A.6): 0 g/t rare earth diet; Group B (B.1-B.6): 150 g/t rare earth diet; Group C (C.1-C.6): 300 g/t rare earth diet; Group D (D.1-D.6): 450 g/t rare earth diet; Group E (E.1-E.6): 600 g/t rare earth diet. To test the significant differences in beta diversity of bacterial communities among treatment groups, PERMANOVA (Adonis procedure with 999 permutations) was conducted to calculate p values. There were significant differences between Group A and Group E (R2 = 0.191, p = 0.002), Group B and Group E (R2 = 0.201, p = 0.004), Group C and Group E (R2 = 0.153, p = 0.008), and Group D and Group E (R2 = 0.166, p = 0.006).
The relative microbial abundance (phylum level, top 10; genus level, top 30) of the cecum microbiota in laying hens fed the 5 experimental diets is presented in Figure 2. The most abundant phyla were Bacteroidetes, followed by Firmicutes in all treatment groups. Compared with the control diet, dietary 600 g/t RE supplementation significantly decreased the relative abundance of Fusobacteriota (phylum) and Fusobacterium (genus) while markedly increasing the relative abundance of Ruminococcus (genus) and Subdoligranulum (genus) (p < 0.05). Furthermore, Figure 3 displays the significantly differentiated bacterial taxa among treatments using linear discriminant analysis coupled with effect size (LEfSe) with the default parameters (threshold >3.0).

Figure 2. The relative abundance of cecal microbiota in laying hens fed the 5 experimental diets at the phylum level (top 10, A) and genus level (top 30, B). (C–F) shows the changes in 4 district genera in the gut microbiota composition of laying hens, including Fusobacteriota (phylum), Fusobacterium (genus), Ruminococcus (genus), and Subdoligranulum (genus). Six replicates (birds) were analyzed in each treatment (n = 6). Group A (A.1-A.6): 0 g/t rare earth diet; Group B (B.1-B.6): 150 g/t rare earth diet; Group C (C.1-C.6): 300 g/t rare earth diet; Group D (D.1-D.6): 450 g/t rare earth diet; Group E (E.1-E.6): 600 g/t rare earth diet.
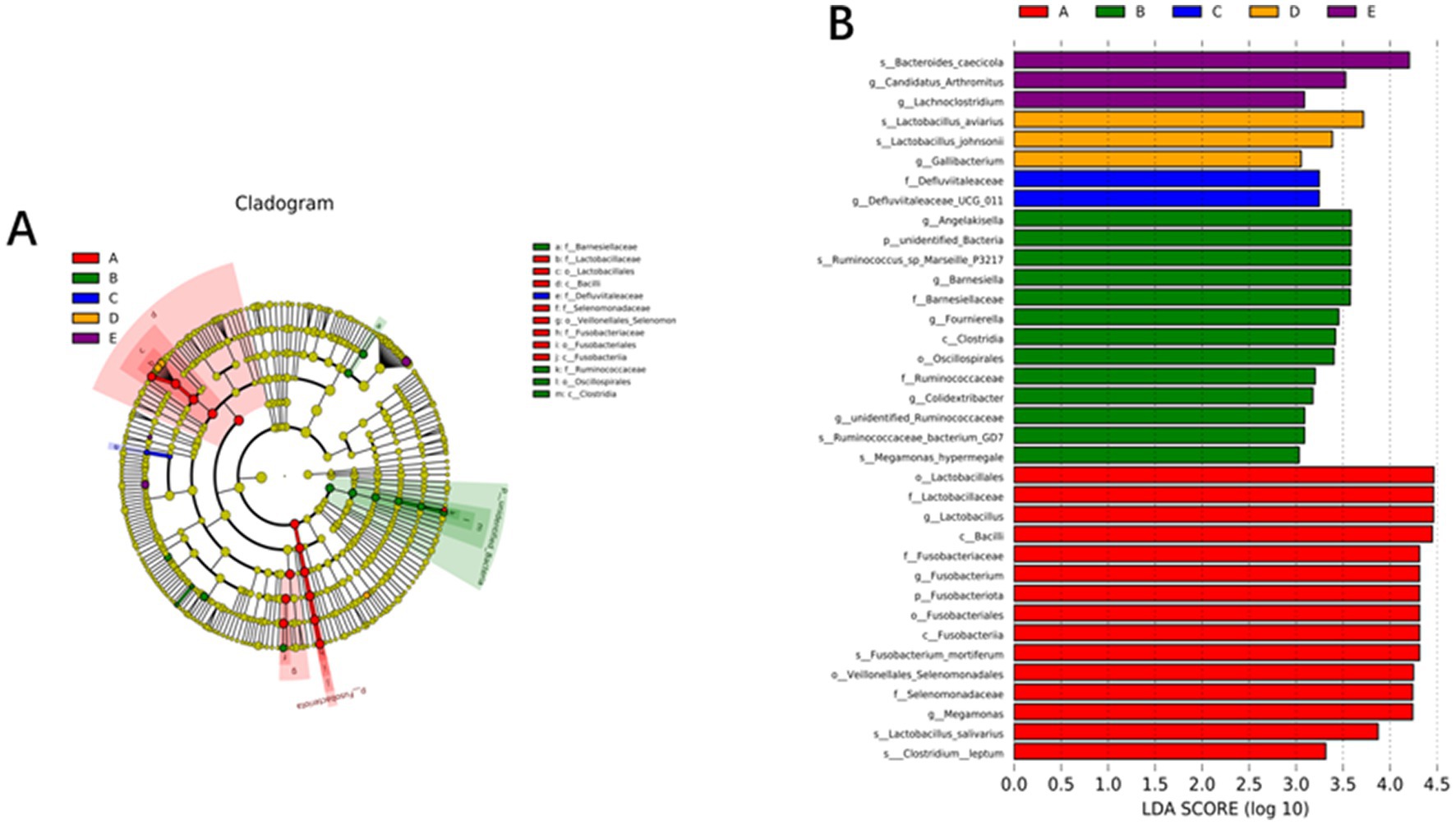
Figure 3. Cladogram (A) and LDA value distribution histogram (B). The bacterial taxa were significantly differentiated among treatments using linear discriminant analysis coupled with effect size (LEfSe) with the default parameters (threshold >3.0).
The primary objective of this study was to evaluate the effects of dietary RE supplementation on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. In this study, the egg production, average daily feed intake, and feed conversion ratio of laying hens were unaffected by dietary treatments. However, compared with the control group, hens fed the 150 g/t RE diet had a greater average egg weight during the third week of the experimental period, which indicates that a moderate dosage of RE could improve egg performance. Still, a high dosage of RE did not affect the egg performance of laying hens. The best explanation is that a moderate RE dosage could contribute to nutrient absorption and utilization, thus resulting in a greater productivity performance of poultry (He et al., 2010; Cai et al., 2015). He et al. (2010) also found that feeding 70 mg/kg RE to broilers for 5 weeks increased broilers’ feed intake and body weight gain from Days 22 to 35 and Days 1 to 35 of the experimental period, respectively. In laying hens, Cai et al. (2016) reported that dietary supplementation with rare earth element-enriched yeast (0, 500, and 1,000 mg/kg) linearly increased egg production in ISA brown late-phase laying hens in a 5-week feeding trial (Cai et al., 2016). Like our results, Ham et al. (2006) reported that dietary supplementation with 300 and 600 mg/kg RE slightly increased the egg weight of 158-day-old Ross broiler breeder hens during a 13-week experimental period (Ham et al., 2006). Therefore, dietary supplementation with 150 mg/kg RE had beneficial effects on the egg performance of laying hens during the late-laying phase.
There were no significant differences in the egg shape index, eggshell strength, albumen height, yolk color, Haugh unit, or yolk antioxidant indices among dietary treatments in the present study. Dietary supplementation with 150, 300, or 600 g/t RE decreased the eggshell thickness of laying hens compared to the control group. However, Durmuş and Bölükbaşı (2015) found that dietary addition of 100, 200, 300, or 400 mg/kg lanthanum oxide for 6 weeks did not impact the eggshell thickness of 22-week-old brown Lohman laying hens (Durmuş and Bölükbaşı, 2015). Additionally, Bölükbaşı et al. (2016) reported no significant effects of 100–400 mg/kg cerium oxide on the eggshell thickness of laying hens in a 10-week study (Bölükbaşı et al., 2016). It is most likely that RE (especially lanthanum, La3+) is competing with Ca2+ for binding sites in biological systems, inhibiting calcium absorption and metabolism (Flachowsky et al., 2019; Malhotra et al., 2020) and thus resulting in decreased eggshell thickness by RE supplementation in the diet of laying hens. For instance, it had been demonstrated that a Ln (III) ion will readily replace the Ca (II) ion at site with the concomitant expulsion of its double site Ca (II) partner (Horrocks et al., 1979). In this regard, further research is warranted to investigate whether dietary calcium levels need to be adjusted when 150 g/t RE is supplemented for late-phase laying hens.
Serum biochemical indices can reflect the function and metabolism of nutrients (Hu et al., 2016). No differences were observed in serum biochemical parameters of laying hens among treatments except for the concentration of high-density liptein cholesterol (HDL-C), which was higher in the 300 or 450 g/t RE-supplemented group than in the control group. These results indicate that dietary RE supplementation positively affected lipid metabolism. The increased serum HDL-C content could accelerate cholesterol transportation and improve excretion and metabolism (März et al., 2017). For calcium and phosphorus, similar results were observed by Durmuş and Bölükbaşı (2015). They reported that dietary RE supplementation had no significant effect on calcium and phosphorus concentrations in the serum of livestock (Durmuş and Bölükbaşı, 2015). However, inconsistent with our results, Bölükbaşı et al. (2016) found that the calcium and phosphorus contents in the serum of laying hens were increased when hens were fed 100 mg/kg cerium oxide (Bölükbaşı et al., 2016). The discrepancy may be due to environmental factors, animal species, or supplemental duration and dosage of RE elements.
Aging is a natural and irreversible physiological process, which will result in harmful reactive oxygen species overproduction (Lee et al., 2004). When endogenous antioxidants are not enough to neutralize excessive free radicals in the body, the redox balance in the body will be destroyed, resulting in oxidative stress (Estevez, 2015). In the antioxidant defense system, SOD detoxifies superoxide radicals into hydrogen peroxide, which is then converted into water by CAT or into nontoxic hydroxyl compounds by glutathione peroxidase (GSH-Px) (He et al., 2017). In the current study, serum GSH-Px activity was increased when laying hens were fed a 600 vs. 0 g/t RE diet. In addition, hepatic GSH-Px activity was more significant when hens were fed a 450 or 600 g/t RE diet in place of the control diet. GSH-PX is an important antioxidant enzyme that converts hydrogen peroxide into nontoxic water. The increase in GSH-Px activity may be interpreted as a defense mechanism against stress environment by high dosage supply of RE. Similarly, Durmuş and Bölükbaşı (2015) found that dietary RE addition with lanthanum oxide markedly reduced oxidative stress status, as indicated by decreased MDA content in the serum of laying hens (Durmuş and Bölükbaşı, 2015). These results suggest that high-dosage RE supplementation could enhance the antioxidant status of laying hens.
The intestinal villus is the primary nutrient absorption, while villus epithelial cells are characterized by digestion and absorption functions (Hu et al., 2016). The villus height, crypt depth, and the ratio of villus height to crypt depth are usually measured as meaningful markers to reflect the absorption ability of the small intestine of livestock (Zeng et al., 2021). In the present study, dietary RE supplementation did not impact the intestinal morphology of the duodenum and jejunum in laying hens, including villus height, crypt depth, and the ratio of villus height to crypt depth. However, dietary supplementation with 600 g/t RE increased the crypt depth of the ileum in laying hens compared to that of the control group, which suggests that a high dosage of RE could lead to intestinal morphology injury. As reported by Cheng et al. (2022), dietary supplementation of RE-chitosan chelate improved intestinal immune status, as reflected by reduced pro-inflammatory factor levels. Interestingly, dietary addition with 2.5–5.0 g/kg Azomite has been found to elevate the height, width, and density of intestinal villus in tilapia (Xu et al., 2021). The villus height and width were also increased in largemouth bass fed diets supplemented with 2.0–4.0 g/kg Azomite (Xu et al., 2021). Still, according to our results, a high dosage of RE is not suggested to be added to the diet of laying hens from the perspective of intestinal morphology.
The gut microbiota plays an essential role in maintaining intestinal health and normal physical functions. A balanced microbiota population provides a healthy intestinal tract environment, resulting in better control of gut pathogens (Song et al., 2019). In this study, no significant differences were observed for the alpha diversity indices of the cecum in laying hens among dietary treatments. However, there were significant differences in beta diversity of cecum microbiota in laying hens fed a 600 g/t RE diet in place of the other 4 experimental diets. These results indicate that a high RE dosage altered the microbiota diversity of laying hens. For cecum microbiota composition, compared with the control diet, dietary 600 g/t RE supplementation significantly decreased the relative abundance of Fusobacteriota (phylum) and Fusobacterium (genus) while markedly increasing the relative abundance of Ruminococcus (genus) and Subdoligranulum (genus). Fusobacterium contributes to butyric acid production and bile acid metabolism (Khan et al., 2020). Ruminococcus is beneficial bacteria that can break down cellulose in the intestinal tract and obtain decomposed nutrients. These results suggest that a high dosage of RE supplementation altered the diversity and composition of the cecum microbiota of late-phase laying hens. In contrast, a low or moderate dosage of RE supplementation had no effects on the cecum microbiota of hens. The gut microbiota results confirmed that a high dosage of RE is not recommended for supplementation in the diet of late-phase laying hens.
In conclusion, a high RE dosage negatively affects egg quality and intestinal morphology. It alters gut microbiota diversity and composition, while a moderate RE dosage has beneficial effects on production performance in late-phase laying hens. In terms of eggshell thickness, further research is necessary to investigate whether dietary calcium levels must be adjusted when 150 g/t RE is supplemented for late-phase laying hens.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/-PRJNA932582.
The animal study was reviewed and approved by Jiangxi Agricultural University Institutional Animal Care and Use Committee.
JC and JY: conceptualization. YL and XP: methodology. XC and ZW: validation. JL: writing—original draft preparation. TZ and JY: writing—review and editing. JY: funding acquisition. All authors contributed to the article and approved the submitted version.
This research was supported by the Jiangxi Agriculture Research System (JXARS-03), and Natural Science Foundation of Jiangxi Province (20212BAB215015; 20224BAB215035), China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BUN, urea nitrogen; GSH, glutathione; GSH-Px, glutathione peroxidase; HDL-C, high-density liptein cholesterol; LDL-C, low-density liptein cholesterol; MDA, malondialdehyde; RE, rare earth; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; T-CHO, total cholesterol.
Abdelnour, S. A., Abd el-Hack, M. E., Khafaga, A. F., Noreldin, A. E., Arif, M., Chaudhry, M. T., et al. (2019). Impacts of rare earth elements on animal health and production: highlights of cerium and lanthanum. Sci. Total Environ. 672, 1021–1032. doi: 10.1016/j.scitotenv.2019.02.270
Bölükbaşı, S. C., Al-Sagan, A. A., Ürüşan, H., Erhan, M. K., Durmuş, O., and Kurt, N. (2016). Effects of cerium oxide supplementation to laying hen diets on performance, egg quality, some antioxidant enzymes in serum and lipid oxidation in egg yolk. J. Anim. Physiol. Anim. Nutr. 100, 686–693. doi: 10.1111/jpn.12429
Cai, L., Nyachoti, C. M., Hancock, J. D., Lee, J. Y., Kim, Y. H., Lee, D. H., et al. (2016). Rare earth element-enriched yeast improved egg production and egg quality in laying hens in the late period of peak egg production. J. Anim. Physiol. Anim. Nutr. 100, 492–498. doi: 10.1111/jpn.12376
Cai, L., Nyachoti, C. M., and Kim, I. H. (2018). Impact of rare earth element-enriched yeast on growth performance, nutrient digestibility, blood profile, and fecal microflora in finishing pigs. Can. J. Anim. Sci. 98, 347–353. doi: 10.1139/cjas-2017-0089
Cai, L., Park, Y. S., Seong, S. I., Yoo, S. W., and Kim, I. H. (2015). Effects of rare earth elements-enriched yeast on growth performance, nutrient digestibility, meat quality, relative organ weight, and excreta microflora in broiler chickens. Livest. Sci. 172, 43–49. doi: 10.1016/j.livsci.2014.11.013
Cheng, Y., Xie, Y., Shi, L., Xing, Y., Guo, S., Gao, Y., et al. (2022). Effects of rare earth-chitosan chelate on growth performance, antioxidative and immune function in broilers. Ital. J. Anim. Sci. 21, 303–313. doi: 10.1080/1828051x.2022.2028589
Durmuş, O., and Bölükbaşı, Ş. C. (2015). Biological activities of lanthanum oxide in laying hens. J. Appl. Poult. Res. 24, 481–488. doi: 10.3382/japr/pfv052
Dushyantha, N., Batapola, N., Ilankoon, I. M. S. K., Rohitha, S., Premasiri, R., Abeysinghe, B., et al. (2020). The story of rare earth elements (REEs): occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 122:103521. doi: 10.1016/j.oregeorev.2020.103521
Estevez, M. (2015). Oxidative damage to poultry: from farm to fork. Poult. Sci. 94, 1368–1378. doi: 10.3382/ps/pev094
Flachowsky, G., Bampidis, V., Zhao, G., Grün, M., and Meyer, U. (2019). Rare earth elements (REE) as feed additives in animal nutrition. CABI Rev. 14:46. doi: 10.1079/PAVSNNR2019.14.046
Förster, D., Berk, A., Hoppen, H. O., Rambeck, W. A., and Flachowsky, G. (2008). A note on the effect of rare earth elements on the performance and thyroid hormone status of rearing piglets. J. Anim. Feed Sci. 17, 70–74. doi: 10.1016/j.still.2007.01.010
Ham, S. K., Song, T. H., Zhang, G. Q., Hur, S. N., and Park, H. S. (2006). Effect of feeding rare earth on egg production and hatchability broiler growth. Korean J. Poult. Sci. 33, 225–231.
He, L., He, T., Farrar, S., Ji, L., Liu, T., and Ma, X. (2017). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 44, 532–553. doi: 10.1159/000485089
He, M. L., Wehr, U., and Rambeck, W. A. (2010). Effect of low doses of dietary rare earth elements on growth performance of broilers. J. Anim. Physiol. Anim. Nutr. 94, 86–92. doi: 10.1111/j.1439-0396.2008.00884.x
Horrocks, W. D., Jr, H., and Sudnick, D. R. (1979). Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 101, 334–340. doi: 10.1021/ja00496a010
Hu, Y., Wang, Y., Li, A., Wang, Z., Zhang, X., Yun, T., et al. (2016). Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric. Immunol. 27, 182–193. doi: 10.1080/09540105.2015.1079592
Katz, J. M., Plowden, J., Renshaw-Hoelscher, M., Lu, X., Tumpey, T. M., and Sambhara, S. (2004). Immunity to influenza: the challenges of protecting an aging population. Immunol. Res. 29, 113–124. doi: 10.1385/IR:29:1-3:113
Khan, S., Moore, R. J., Stanley, D., and Chousalkara, K. K. (2020). The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 86, e00600–e00620. doi: 10.1128/AEM
Lee, J., Koo, N., and Min, D. B. (2004). Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 3, 21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x
Liu, Y., Li, Y., Liu, H. N., Suo, Y. L., Hu, L. L., Feng, X. A., et al. (2013). Effect of quercetin on performance and egg quality during the late laying period of hens. Br. Poult. Sci. 54, 510–514. doi: 10.1080/00071668.2013.799758
Malhotra, N., Hsu, H. S., Liang, S. T., Roldan, M. J. M., Lee, J. S., Ger, T. R., et al. (2020). An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 10:1663. doi: 10.3390/ani10091663
März, W., Kleber, M. E., Scharnagl, H., Speer, T., Zewinger, S., Ritsch, A., et al. (2017). HDL cholesterol: reappraisal of its clinical relevance. Clin. Res. Cardiol. 106, 663–675. doi: 10.1007/s00392-017-1106-1
Molnár, A., Maertens, L., Ampe, B., Buyse, J., Zoons, J., and Delezie, E. (2017). Supplementation of fine and coarse limestone in different ratios in a split feeding system: effects on performance, egg quality, and bone strength in old laying hens. Poult. Sci. 96, 1659–1671. doi: 10.3382/ps/pew424
Song, D., Wang, Y. W., Lu, Z. X., Wang, W. W., Miao, H. J., Zhou, H., et al. (2019). Effects of dietary supplementation of microencapsulated enterococcus fecalis and the extract of Camellia oleifera seed on laying performance, egg quality, serum biochemical parameters, and cecal microflora diversity in laying hens. Poult. Sci. 98, 2880–2887. doi: 10.3382/ps/pez033
Tariq, H., Sharma, A., Sarkar, S., Ojha, L., Pal, R. P., and Mani, V. (2020). Perspectives for rare earth elements as feed additive in livestock - a review. Asian-Australas. J. Anim. Sci. 33, 373–381. doi: 10.5713/ajas.19.0242
Xiong, Y., Pang, J., Lv, L., Wu, Y., Li, N., Huang, S., et al. (2019). Effects of maternal supplementation with rare earth elements during late gestation and lactation on performances, health, and fecal microbiota of the sows and their offspring. Animals 9:738. doi: 10.3390/ani9100738
Xu, X., Li, X., Xu, Z., Yao, W., and Leng, X. (2021). Dietary Azomite, a natural trace mineral complex, improved the growth, immunity response, intestine health and resistance against bacterial infection in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 108, 53–62. doi: 10.1016/j.fsi.2020.11.016
Zeng, Y., Wang, Z., Zou, T., Chen, J., Li, G., Zheng, L., et al. (2021). Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 8:623899. doi: 10.3389/fvets.2021.623899
Keywords: egg quality, gut microbiota, late-phase laying hens, production performance, rare earth
Citation: Li J, Yan L, Cao X, Luo Y, Peng X, Wang Z, Zou T, Chen J and You J (2023) Effects of rare earth as feed additive on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. Front. Sustain. Food Syst. 7:1155543. doi: 10.3389/fsufs.2023.1155543
Received: 31 January 2023; Accepted: 10 April 2023;
Published: 21 April 2023.
Edited by:
Li Min, Guangdong Academy of Agricultural Sciences (GDAAS), ChinaReviewed by:
Wu Shugeng, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2023 Li, Yan, Cao, Luo, Peng, Wang, Zou, Chen and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen, anVuY2hlbkBqeGF1LmVkdS5jbg==; Jinming You, eW91amlubUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.