- 1State Key Laboratory for Managing Biotic and Chemical Threats to Quality and Safety of Agro-products, Key Laboratory of Biotechnology in Plant Protection of MOA of China and Zhejiang Province, Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences, Hangzhou, China
- 2College of Plant Protection, Nanjing Agricultural University, Nanjing, China
- 3Lishui Academy Institute of Agricultural and Forestry Sciences, Lishui, Zhejiang, China
Introduction: Tuta absoluta is currently considered one of the most devastating invasive pests of solanaceous plants worldwide, causing severe damage to the tomato industry. Insects use volatile organic compounds (VOCs) to locate host plant for feeding and oviposition. Those VOCs could be developed as lures for pest monitoring and control.
Methods: In this study, the differentially accumulated VOCs between the preferred host (tomato) and non-preferred host (eggplant) were analyzed by GC–MS method, and their roles on female T. absoluta host selection and egg laying behaviors were investigated using electroantennography (EAG), olfactometer and cage experiments.
Results: A total of 39 differentially accumulated VOCs were identified in tomato and eggplant. Strong EAG signals were obtained in 9 VOCs, including 5 VOCs highly accumulated in tomato and 4 VOCs highly accumulated in eggplant. Further olfactometer bioassays showed that 4 compounds (1-nonanol, ethyl heptanoate, ethyl octanoate and o-nitrophenol) were attractive to T. absoluta females, while 5 compounds (2-phenylethanol, 2-pentylfuran, trans,trans-2,4-nonadienal, 2-ethyl-5-methylpyrazine and trans-2-nonenal) were repellent, indicating that VOCs from host plants play important roles in host plant preferences. The attractive activities of 1-nonanol and ethyl octanoate, as well as the repellent activities of trans,trans-2,4-nonadienal and trans-2-nonenal, were further confirmed in cage experiments.
Discussion: In this study, two attractants and two repellents for T. absoluta were developed from plant released VOCs. Our results could be useful to enhance the development of eco-friendly and sustainable pest management strategies for T. absoluta.
1. Introduction
The South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae), an invasive pest native to Peru, South America, has become one of the most devastating pests of solanaceous plants worldwide (Biondi et al., 2018). It can infest host plants at all developmental stages, with the larvae mining and feeding on leaves, stems and fruits, causing crop losses up to 80–100% in the area without timely control measures (Desneux et al., 2010). After invading Europe in 2006, this pest spread quickly to Afro-Eurasian supercontinent, and now has been recorded in more than 90 countries and regions outside South America (Biondi et al., 2018; Desneux et al., 2022). In Asia, T. absoluta has been found in many countries (Campos et al., 2017; Han et al., 2019), including countries on the northwestern and southwestern border of China, e.g., Tajikistan, Kyrgyzstan, India, Nepal, etc. (Campos et al., 2017; Sankarganesh et al., 2017; Uulu et al., 2017; Saidov et al., 2018). Recently, this pest was found in northwest China in Xinjiang (Li D. et al., 2019; Li X. W. et al., 2019; Zhang et al., 2019) and southwest China in Yunnan (Zhang et al., 2020), and has quickly spread to 14 provinces in China mainland, poses a significant threat to China’s tomato production (Zhang et al., 2021).
Currently, chemical control is still the main control method for T. absoluta implemented in its native ranges and invaded countries to counter its great threat to agricultural production (Guedes et al., 2019). It has been reported that the application of insecticides could prevent the occurrence and spread of T. absoluta to some extent (Silvério et al., 2009). However, the control effects of insecticides is limited because of the larval endophytic feeding behavior which makes T. absoluta a difficult target for insecticide sprays (Guedes and Siqueira, 2012). In addition, due to the intensive use of insecticides, T. absoluta has become resistant to many chemical classes of insecticides, including organophosphates (Siqueira et al., 2000b; Lietti et al., 2005; Haddi et al., 2017; Barati et al., 2018), pyrethroids (Haddi et al., 2012; Biondi et al., 2015), spinosyn (Campos et al., 2014, 2015), avermectins (Siqueira et al., 2001; Silva et al., 2016), cartap (Siqueira et al., 2000a), benzoylureas (Silva et al., 2011), indoxacarb (Roditakis et al., 2018) and diamides (Silva et al., 2019). Alternative control strategies should therefore be used within the context of integrated pest management (IPM) for this destructive pest.
In response to insect herbivory, plants release volatile compounds that may serve as protective substances as well as mediators of interactions with other plants, microbes, and animals. Plant-released semiochemicals are promising eco-friendly pest management methods that has been widely used as a sustainable alternative for synthetic insecticides (Dudareva et al., 2006; Shrivastava et al., 2010; Beck et al., 2017). In the process of co-evolution between insects and plants, there is a corresponding interaction between insects and plants. The most primitive ecological relationship is that insects select their compatible host plants, while the phytoconstituents of host plants are one of the direct causes of host plant-insect interaction (Thompson, 1988). Plants could release different classes of volatile organic compounds (VOCs) into the external environment during their growth and development, which enables plants to generate defense signals and communicate with each other (Baldwin et al., 2006; Heil and Silva Bueno, 2007). Plant VOCs also play important roles in plant-insect interactions, affecting insect feeding, mating and egg-laying (Bruce et al., 2005). Insects use plant volatiles to locate plant hosts for feeding and oviposition (Kuhnle and Muller, 2011; Wynde and Port, 2012). Those VOCs could be developed as lures for pest monitoring and control (Shrivastava et al., 2010). On the other hand, many plants have developed counter strategies to defend themselves against these insects, including repellent VOCs, which could be developed into repellents to reduce pest populations on target crops (War et al., 2012).
The use of plant chemicals (VOCs and non-volatile secondary metabolites) for pest control has been reported for T. absoluta. For instance, it has been reported that the extracts of jojoba, Simmondsia chinensis, can effectively control T. absoluta (Abdel-Baky and Al-Soqeer, 2017). Essential oils of three Satureja species, S. khuzestanica, S. bachtiarica, and S. rechingeri, had fumigant toxicity on T. absoluta, with geraniol the main component of all essential oils (Rahmani and Azimi, 2021). Shared volatile compounds from different hosts [a blend consisting of limonene (16.64%), β-ocimene (1.84%), α-terpinene (12.17%),δ-eIemene (4.29%) and (E)-β-caryophyllene (6.78%)] could attract female T. absoluta (Msisi et al., 2021). Consequently, understanding the plant chemicals involved in T. absoluta-host plant interactions could be useful for the development of a new strategy for the control of this pest.
Tuta absoluta is oligophagous and can survive and reproduce normally on potatoes, tobacco and other Solanaceae crops (Arnó et al., 2019). Nevertheless, it has been found that T. absoluta had a strong preference for tomatoes among host plants, and volatile chemical signals played important roles in its host plant preferences (Subramani et al., 2021). Similarly, in our previous study, it has been found that the number of eggs laid by T. absoluta was significantly higher on tomatoes than on eggplants (Chen et al., 2021). In this study, we further analyzed the differentially accumulated VOCs between these two host plants, and their roles on female host selection and egg laying behaviors were investigated by using electroantennography (EAG), olfactometer and cage experiments. The VOCs with attractive and repellent activities could be used to develop new control strategies for this pest.
2. Materials and methods
2.1. Plant materials and insects
Seeds of tomato (variety Zhefen 202) and eggplant (variety Zheqie NO.1) were sown in the coconut coir for germination. After the two-leaf stage, seedlings were individually transplanted into plastic pots (7 cm long, 7 cm wide and 9 cm high) and placed in an insect-free greenhouse (26 ± 5°C, 60% ± 5% RH, 16 L:8 D photoperiod). The plants were watered at regular intervals and 1 g of water-soluble fertilizer containing 18 macro-elements (OMEX, 18–18-18) was applied to each plant. Plants at the 5-leaf stage were used for host plant VOC collection and egg-laying experiments.
Tuta absoluta populations were collected in 2018 from tomato fields in Yili, Xinjiang, and were continuously reared in an artificial climate chamber (25 ± 1°C, 60% ± 5% RH, 16 L:8 D photoperiod) on tomato plants.
2.2. Headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry (HS-SPME/GC–MS)
When the healthy host plants (tomato and eggplant) were at the 5-leaf stage, the third leaf from the top was selected, the veins were removed, approximately 1 g of each sample was lyophilized in liquid nitrogen (LN) and subsequently stored in a − 80°C freezer. Samples were later pulverized in liquid nitrogen and vortexed to mix evenly. The samples were moved into a headspace bottle with fully automatic headspace solid-phase microextraction (HS-SPME) (Lee et al., 2007). The gas chromatography-mass spectrometer (GC–MS) was used to identify terpenoids, benzene ring types and phenylpropanoids, fatty acid derivatives and other volatiles. The volatile content was determined by the headspace collection method or extraction method. The SPME readings were taken at 250°C aging temperature; 5 min aging time; 60°C heating temperature; 10 min heating time; 20 min adsorption time; 5 min desorption time; and 5 min aging time after sample injection. The original data file obtained by GC–MS analysis was first extracted using the MassHunter software (Agilent) (Yuan et al., 2022). Three samples were collected and tested both for tomato and eggplant.
2.3. Electroantennographic (EAG) responses of T. absoluta females to VOCs
To test whether differentially accumulated VOCs between tomato and eggplant contribute to host plant preference of T. absoluta, EAG responses of T. absoluta females and males to 20 differentially accumulated VOCs were determined using the EAG detection system (Stimulus Air Controller CS-55 and SYNTECH IDIC2; Syntech, Hilversum, the Netherlands). The 20 VOCs, which were selected according to the principal component analysis and the characteristics of VOCs, were as follows: 1-nonanol, 2-phenylethanol, 2-isopropyl-3-methoxypyrazine, ethyl heptanoate, ethyl octanoate, 1,4-diethylbenzene, o-nitrophenol, which were highly accumulated in tomato; benzyl alcohol, 2-pentylfuran, benzaldehyde, trans,cis-2,6-nonadienal, trans,trans-2,4-nonadienal, furfural, trans-2-hexen-1-al, trans,trans-2,4-heptandienal, isophorone, 2-s-butylphenol, 4-hexen-3-one, 2-ethyl-5-methylpyrazine, and trans-2-nonenal, which were highly accumulated in eggplant. The synthetic standards of the above VOCs were purchased from Merck and Shanghai Aladdin Biochemical Technology Co.
The standard compounds were diluted in a gradient with paraffin oil to four concentrations (0.1, 1, 10, and 100 mg/mL), 10 μL of each was applied to a piece of filter paper (5 mm × 2 cm), which was placed into Pasteur pipette 10 min before testing. 10 μL of paraffin oil was used as a control stimulus. The stimulus was made by introducing the test volatile to the antenna at a flow rate of 25 mL/min for 2 s and with an interval of 1 min for the next stimulus. For each test chemical, paraffin oil was used as control. The test order was paraffin oil, the test compound, and paraffin oil. The test compound of each concentration was performed on five females and males. Relative EAG values of T. absoluta were reported as the percentage of the response to paraffin oil.
2.4. Olfactometer bioassay
The responses of T. absoluta females and males to 9 volatile compounds with strong EAG responses, including 5 highly accumulated in tomato (1-nonanol, 2-phenylethanol, ethyl heptanoate, ethyl octanoate and o-nitrophenol) and 4 highly accumulated in eggplant (2-pentylfuran, trans,trans-2,4-nonadienal, 2-ethyl-5-methylpyrazine and trans-2-nonenal), were tested by using Y-tube olfactometer. The glass Y-tube is with a 3-arm structure, which consists of a 60-mm-long base tube and two 60-mm-long arms. The two arms were separated from each other at an angle of 90°. Teflon tubes were used to connect the components of the olfactometer apparatus. Air was pumped into the apparatus by an electromagnetic air pump, filtered by activated carbon, and split into two air streams at a flow rate of 60 ml/s. Before each test, the apparatus was rinsed with pure ethanol and dried in an oven (120°C).
Tuta absoluta females and males of mixed ages (2–4 days) were used for Y-tube olfactometer bioassays. The bioassays were conducted in a dark room at 25 ± 1°C and 60% ± 5% RH. The light was provided by an LED lamp located in the ceiling directly above the Y-tube. Solutions of each VOC compounds were prepared in paraffin oil at a gradient of four concentrations (0.1, 1, 10, and 100 mg/mL), and 10 μL was pipetted onto a piece of clean filter paper (1 × 1 cm), which was then transferred to a glass flask as the test odor source. Filter paper with 10 μl of paraffin oil in a glass flask was used as a control odor source.
Tuta absoluta females and males were individually transferred to the base tube of the Y-tube and their choice was recorded within 5 min. When the tested individual crossed half-length of either arm, the “effective choice” was recorded. If the tested individual did not cross half-length of either arm within 5 min, the “no choice” was recorded. To prevent the effects of light, the Y-tube arms were swapped after every 5 insects. The experiment was repeated five times, with 20 individuals each time.
2.5. Cage experiments
Cage experiments were conducted in a climate chamber (25 ± 1°C, 60% ± 5% RH, 16 L: 8 D photoperiod) to test the responses of T. absoluta females to 4 VOCs (1-nonanol, ethyl octanoate, trans,trans-2,4-nonadienal and trans-2-nonenal) that showed the highest attractive or repellent activities to T. absoluta females in olfactometer bioassays. In each cage, six five-leaf stage tomato plants were placed equally along two opposite sides of the cage, with three plants along each side. The concentrations of standard compounds that showed the highest attractive or repellent activities to T. absoluta were used in cage experiments. The standard compounds of selected VOCs were dissolved in hexane at the required concentration. A Rubber septum with 10 μl diluted standard compounds of selected VOCs was hung on each of the three plants on one side of the cage. Rubber septa with 10 μl of hexane were used as control, and were hung on plants on the other side of the cage. Thirty 2 to 4 days old females of T. absoluta were released from the middle of the cage. After 48 h, the number of T. absoluta eggs on all leaves of each tomato plant was counted. The experiment was repeated twice for each tested VOC compound.
2.6. Statistical analysis
Quality control (QC) analysis was conducted before data were obtained from GC–MS to confirm the reliability of the data before the overall analyses. The QC sample was prepared by mixing sample extracts for insertion into every three samples to monitor the changes in repeated analyses. Data matrices with the intensity of the metabolite features from the samples were uploaded to the Analyst software (version 1.6.1; AB Sciex, Canada) for statistical analyses. The partial least squares discriminant analysis (PLS-DA) was performed to maximize the metabolome differences between sample pairs. The relative importance of each metabolite to the PLS-DA model was tested using the variable importance in projection (VIP) as a parameter. Metabolites with VIP ≥ 1 and fold change ≥2 or fold change ≤0.5 were considered differential metabolites for group discrimination (Chong et al., 2018). PCA and Ward’s hierarchical clustering heatmap were performed using R software (version 3.3.2).1 Consequently, a metabolic pathway was constructed according to KEGG2; and pathway analysis was performed using MetaboAnalyst3 based on the change in metabolite concentration compared with the corresponding controls.
EAG data were analyzed using one-way ANOVA followed by Turkey’s Highest Significant Difference. Olfactometer data was analyzed using the Chi square test. The null hypothesis was that T. absoluta had 50: 50 distributions across the two arms of the olfactometer. Differences in the number of eggs on tomato plants with VOC compounds and solvent control were analyzed using Student t-test. Data analyses were performed by using SPSS (SPSS Inc., 2007, Chicago, IL) with p ≤ 0.05.
3. Results
3.1. Analysis of differentially accumulated VOCs in tomato and eggplant
VOCs released from the two host plants (tomato and eggplant) collected by HS-SPME were identified by GC–MS. In total, one hundred and forty VOCs predominantly from alkanes (24), heterocyclic compounds (20), alcohol (16), aldehyde (16), terpenes (14), ketone (13), ester (11), aromatics (10), phenol (8), olefin (3), acid (2), ether (1), amine (1) and other (1) classes were detected in this study (Figure 1A; Supplementary Table S1). Thirty-nine differentially accumulated VOCs were identified between these two different host plants (Supplementary Table S2), of which, 15 VOCs were accumulated higher in tomato than in eggplant, which belong to alcohol (3), heterocyclic compound (3), terpenes (2), aromatics (2), ester (2), alkanes (1), aldehyde (1) and phenol (1). These VOCs might contribute to the higher attraction of tomato plants to T. absoluta females for host selection and oviposition. While 24 VOCs were accumulated higher in eggplant than in tomato, which belong to aldehyde (9), heterocyclic compound (5), alcohol (3), ketone (3), phenol (2), terpenes (2). These VOCs might have repellent activities to T. absoluta.
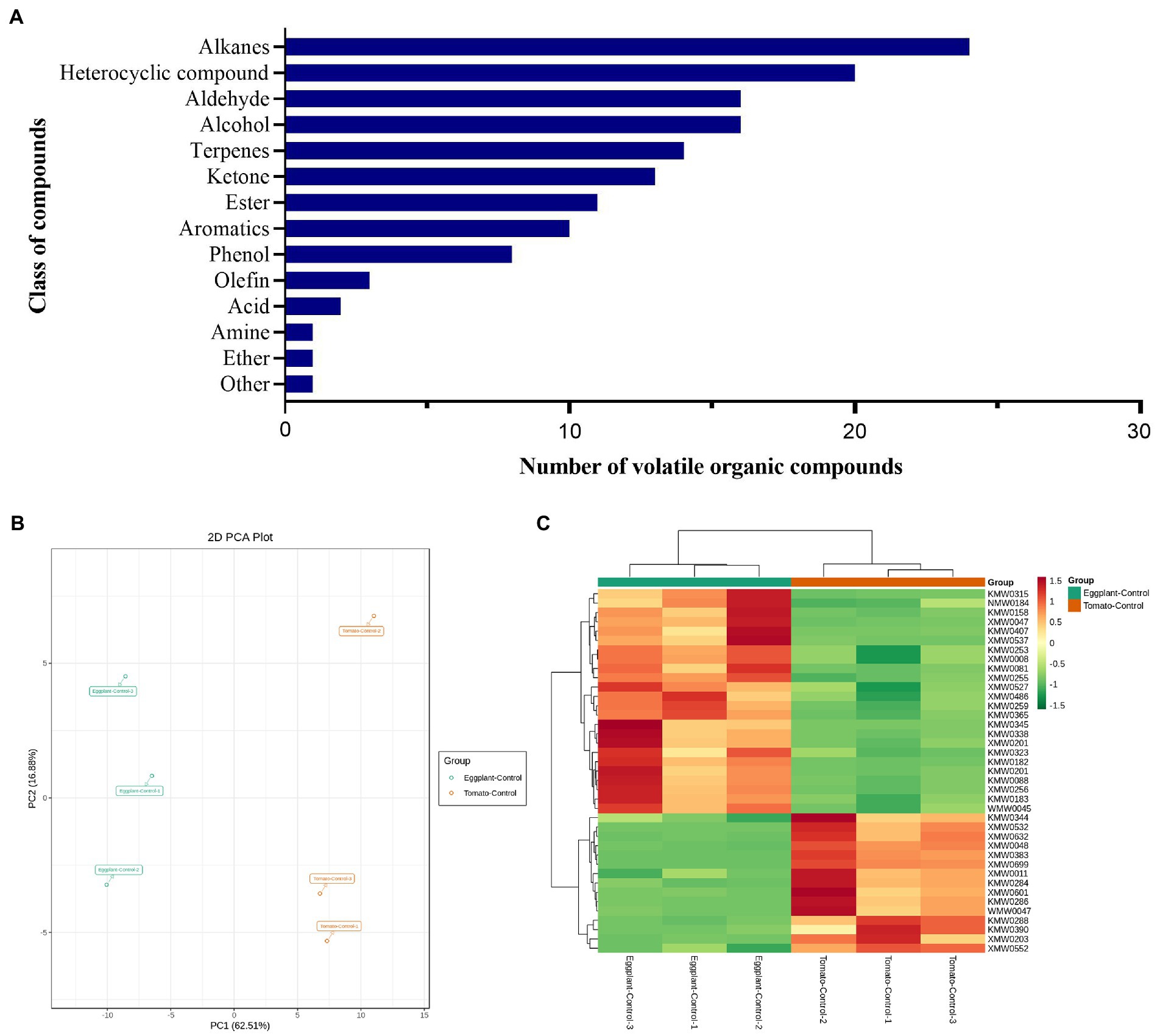
Figure 1. (A) Classes of volatile organic compounds identified of tomato and eggplant. (B) Principal component analysis (PCA) of differentially accumulated VOCs identified from tomato and eggplant leaves by headspace solid-phase microextraction (HS-SPME). (C) Heatmap clustering of 39 differentially accumulated VOCs identified from tomato and eggplant leaves.
The 39 differentially accumulated VOCs were further evaluated using principal component analysis (PCA) to clarify that the differentially accumulated VOCs detected could be used to distinguish between the two host plants. PCA analysis (Figure 1B) indicated that the detected VOCs were divided into two groups, with significant differences between tomato and eggplant, suggesting significant differences in VOCs between the two host plants. Although tomato and eggplant could not be distinguished in the PC2 (vertical axis) principal component, a significant distinction between tomato and eggplant could be found in the PC1 (horizontal axis) principal component. The PC1 (horizontal axis) principal component explained 62.51% of the total variance between samples, while PC2 (vertical axis) explained only 16.88%. The variability between sample groups and the similarity within sample groups confirmed the differential accumulation of VOCs in the two host plants.
We performed a hierarchical clustering analysis of the differentially accumulated VOCs detected in tomato and eggplant samples (Figure 1C), which showed a high degree of similarity between the biological replicates within each host plant and significant differences between the tomato and eggplant samples. These results indicate the high quality of data from both sets of samples and the presence of significant differences in VOCs in the two host plants.
3.2. EAG responses of T. absoluta females to VOCs
To demonstrate that the differential accumulation of VOCs on the two host plants identified in this study does have effects on the egg-laying behavior of T. absoluta females, the EAG response of T. absoluta females and males to 20 differentially accumulated VOCs were initially screened. These 20 VOCs included 7 VOCs that were higher accumulated in tomatoes than in eggplants, namely 1-nonanol, 2-phenylethanol, 2-isopropyl-3-methoxypyrazine, ethyl heptanoate, ethyl octanoate, 1,4-diethylbenzene, o-nitrophenol, and 13 VOCs that were higher accumulated in eggplants namely benzyl alcohol, 2-pentylfuran, benzaldehyde, trans,cis-2,6-nonadienal, trans,trans-2,4-nonadienal, furfural, trans-2-hexen-1-al, trans,trans-2,4-heptandienal, isophorone, 2-S-butylphenol, 4-hexen-3-one, 2-ethyl-5-methylpyrazine, trans-2-nonenal.
The results showed that all the 20 VOCs triggered certain EAG responses of T. absoluta females (Figure 2), confirming that the selected VOCs might have some effects on host plant selection and egg-laying behavior of T. absoluta females. It is also noteworthy that among the 20 compounds tested, 9 VOCs, including 5 highly accumulated in tomato (1-nonanol, 2-phenylethanol, ethyl heptanoate, ethyl octanoate and o-nitrophenol) and 4 highly accumulated in eggplant (2-pentylfuran, trans,trans-2,4-nonadienal, 2-ethyl-5-methylpyrazine and trans-2-nonenal), caused significantly higher EAG responses in T. absoluta females. The EAG responses of T. absoluta males to 20 VOCs (Supplementary Figure S1) were roughly the same as females.
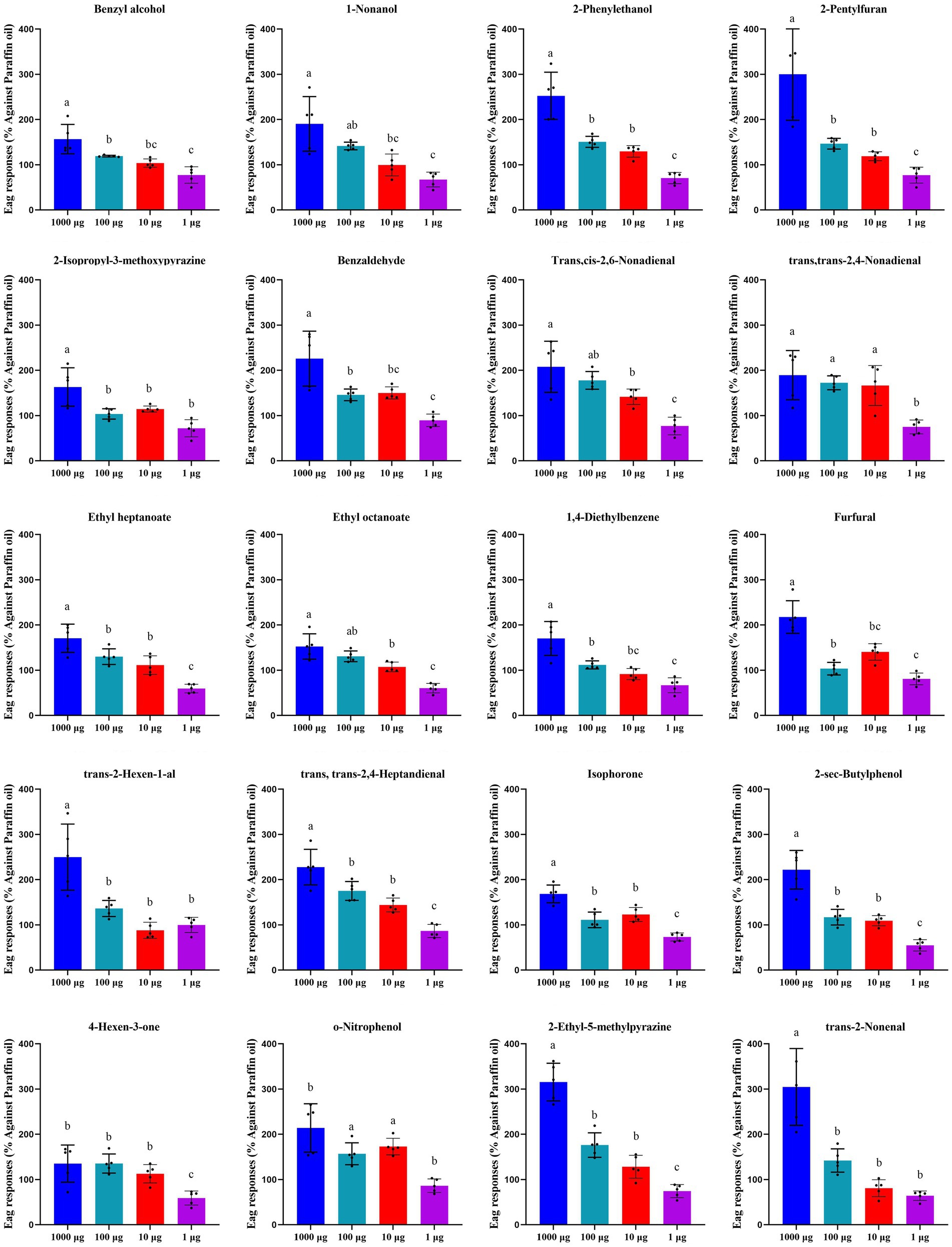
Figure 2. Electroantennographic (EAG) responses of T. absoluta females to 20 volatile compounds. The bar represents the standard error, and the different letters above each bar indicate Turkey’s highest significant difference at p < 0.05.
3.3. Olfactory responses of T. absoluta females to VOCs
Among the 9 VOCs in the olfactometer bioassays, 4 compounds (all higher in tomatoes than in eggplants) were attractive to T. absoluta females and 5 compounds (four of them were higher in eggplant than in tomato) were repellent (Figure 3). Specifically, 1-nonanol was shown to be attractive to T. absoluta females at doses of 1 and 10 μg, but had no significant attractive effect at doses of 100 and 1,000 μg. Ethyl heptanoate at a dose of 10 μg showed an attractive effect on T. absoluta females, but no significant effects at 1, 100 and 1,000 μg. Ethyl octanoate was attractive to T. absoluta females at 1, 10 and 100 μg, but there was no significant effect at 1000 μg. O-nitrophenol at a dose of 1 μg produced an attractive effect on T. absoluta females, but there were no significant effects at doses of 10, 100 and 1,000 μg. By contrast, 2-phenylethanol at 100 and 1,000 μg produced repellent effects on T. absoluta females, but no significant repellent activities were found at 1 and 10 μg doses. 2-pentylfuran at 1000 μg produced a repellent effect on T. absoluta females, but there was no significant repellent effect at 1, 10 and 100 μg. The trans,trans-2,4-nonadienal produced repellent effects on T. absoluta females at doses of 10, 100 and 1,000 μg, but no significant effect at 1 μg. 2-ethyl-5-methylpyrazine produced repellent effects on T. absoluta females at doses of 100 and 1,000 μg, but not repellent at 1 and 10 μg. The trans-2-nonenal produced repellent effects on T. absoluta females at four doses of 1, 10, 100 and 1,000 μg. Notably, 1-nonanol and ethyl octanoate, which were more abundant in tomatoes compared to eggplants, showed significant attractive activities to T. absoluta females, while trans,trans-2,4-nonadienal and trans-2-nonenal, which were more abundant in eggplants than tomatoes, showed significant repellent activities to T. absoluta females. The behavioral responses of T. absoluta males to these nine VOCs were highly consistent with females (Supplementary Figure S2).
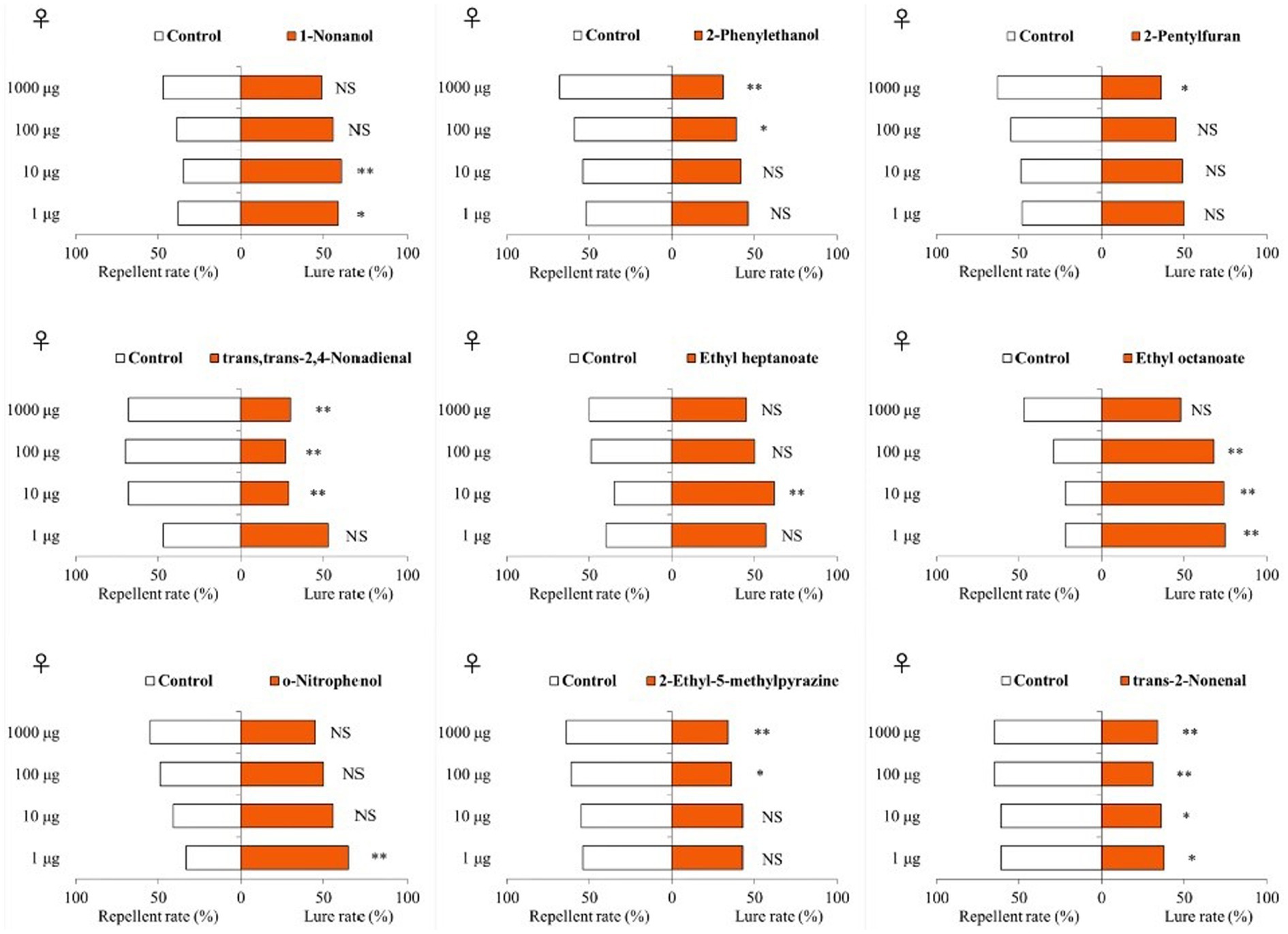
Figure 3. Responses of female T. absoluta to nine VOCs in a Y-tube olfactometer. NS indicates no significant difference; asterisks indicate significant differences (*p < 0.05; **p < 0.01).
3.4. Effects of VOCs on the oviposition behavior of T. absoluta
Results from cage experiments showed that 1-nonanol, ethyl octanoate, trans,trans-2,4-nonadienal and trans-2-nonenal could significantly influence the number of eggs laid by T. absoluta on the host plants (Figure 4). Specifically, T. absoluta females laid significantly more eggs on tomato plants with 1-nonanol by 91.6% compared to control plants with hexane. Similarly, T. absoluta females produced significantly more eggs on tomato plants with ethyl octanoate by 245.2% compared to control plants with hexane. By contrast, T. absoluta females produced 71.9% fewer eggs on tomato plants with trans,trans-2,4-nonadienal compared to control plants with hexane. T. absoluta females produced 35.8% fewer eggs on tomato plants with trans-2-nonenal compared to control with hexane. These results suggest that 1-nonanol and ethyl octanoate had significant attractive effects on the oviposition choice of T. absoluta females. On the contrary, trans,trans-2,4-nonadienal and trans-2-nonenal had repellent effects on the oviposition choice of T. absoluta females.
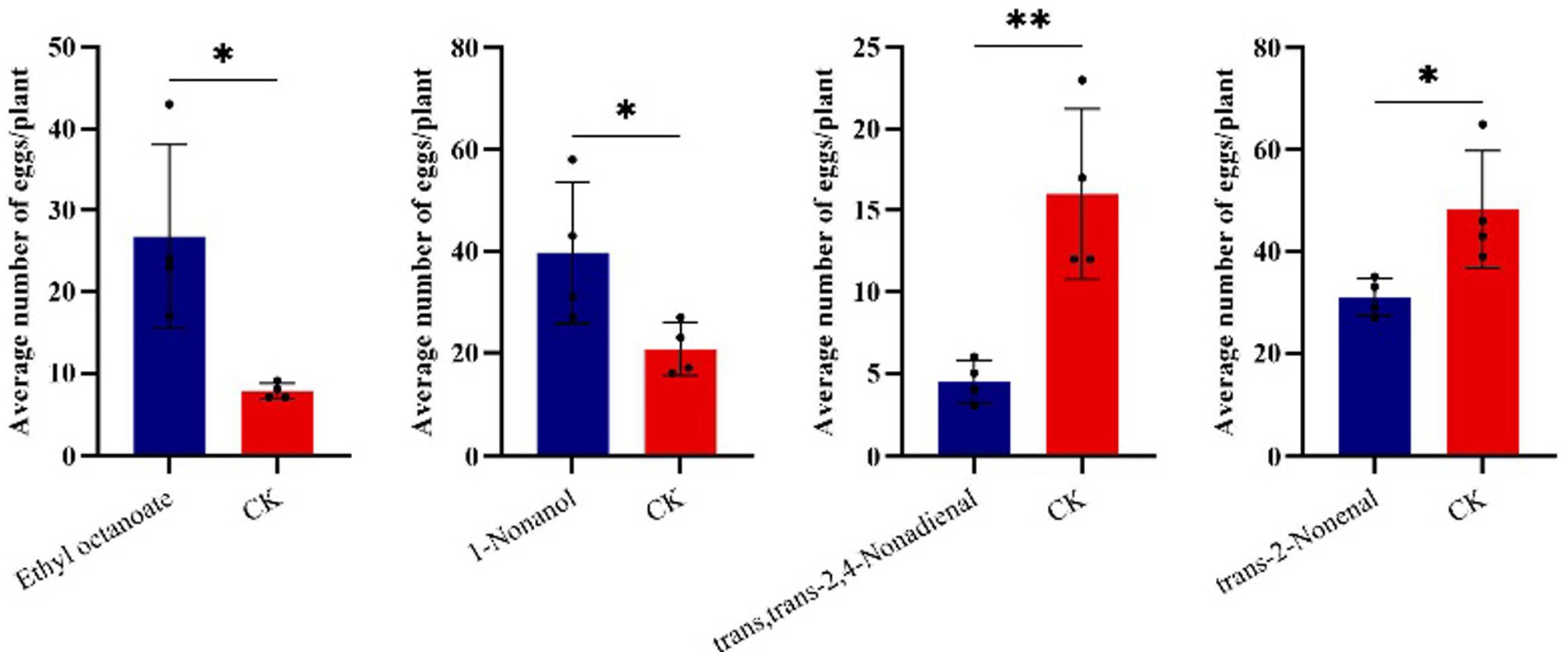
Figure 4. The effect of four VOCs on the oviposition behavior of T. absoluta. The bar represents the standard error, and asterisks indicate significant differences (*p < 0.05; **p < 0.01).
4. Discussion
Compared with polyphagous insects, oligophagous insects usually have a much stronger preference for selecting suitable host plants (Gripenberg et al., 2010). The dispersal ability of T. absoluta larvae is limited, consequently, host plant selection of T. absoluta females often determines the food source of their offspring at the larval stage (Silva et al., 2021). Results from our previous study showed that T. absoluta females showed significant oviposition preference to tomatoes compared to eggplants (Chen et al., 2021). This phenomenon is consistent with the “preference performance hypothesis” (Jaenike, 1978; Thompson, 1988; Mayhew, 1997; Gripenberg et al., 2010). In response to the host plant preference behavior of T. absoluta, we supposed that one or more specific plant VOCs released by tomato plants may have attractive effects on T. absoluta females, facilitating their rapid localization to tomato plants and preferred oviposition on tomato leaves.
The results from this study showed that 39 differentially accumulated VOCs were identified between the preferred host (tomato) and non-preferred host (eggplant) by headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME/GC–MS). Specifically, 15 VOCs were highly accumulated in tomato, with alcohol and heterocyclic compounds the most abundant. These results were different from the previous study, which reported that tomato had a higher number of terpenes and acid compounds than other host plants (including eggplant) (Msisi et al., 2021; Subramani et al., 2021). Our results showed that 24 VOCs were accumulated higher in eggplant with aldehyde and heterocyclic compounds the most abundant. However, some highly accumulated compounds reported in eggplant compared with tomato, such as 1,2,3,5-tetramethylcyclohexane, cyclooctene, 3-(1-methylethenyl), 1,2,3,5-tetramethylcyclohexane, etc. (Subramani et al., 2021), were not found in our study. Similar to previous studies (Proffit et al., 2011; Msisi et al., 2021; Subramani et al., 2021), these differentially accumulated VOCs might contribute to the oviposition preference differences between tomato and eggplant. A previous study reported that the high composition of terpenes in tomato contributed to the attractive activity of tomato volatiles to female T. absoluta, while highly constituted green leaves volatiles (GLVs) in watermelon contributed to the repellent activity of watermelon volatiles to female T. absoluta (Msisi et al., 2021). Subramani et al. reported that volatiles from tomato, such as p-quinone, 2-carene, δ-curcumene, and 1,2-diethylbenzene, could serve as oviposition stimulants to T. absoluta, whereas the presence of 1-fluorododecane in host plants such as datura, eggplant, ashwagandha, and black nightshade, might deterred T. absoluta from ovipositing (Subramani et al., 2021). It has also been reported that plant volatiles from different tomato cultivars contributed to the oviposition choice of T. absoluta (Proffit et al., 2011). 2-tridecanone, 2-undecanone, and zingiberene, which are compounds not detected in susceptible tomato varieties, were detected in wild tomato varieties resistant to T. absoluta (Leite et al., 1999; Azevedo et al., 2003). Consequently, we suspected the VOCs accumulated higher in tomato might contributed to the higher attraction of tomato plants to T. absoluta females for host selection and oviposition, while the VOCs accumulated higher in eggplant might be account for the repellency for egg laying.
To confirm the behavioral effects of the differentially accumulated VOCs on T. absoluta, 20 differentially accumulated VOCs had been selected for electroantennographic tests. The results showed 9 VOCs, including 5 highly accumulated in tomato (1-nonanol, 2-phenylethanol, ethyl heptanoate, ethyl octanoate and o-nitrophenol) and 4 highly accumulated in eggplant (2-pentylfuran, trans,trans-2,4-nonadienal, 2-ethyl-5-methylpyrazine and trans-2-nonenal), caused significant higher EAG responses of T. absoluta females (Figure 2) and males (Supplementary Figure S1). Further olfactometer bioassays showed that 4 compounds (1-nonanol, ethyl heptanoate, ethyl octanoate and o-nitrophenol) were attractive to T. absoluta females, while 5 compounds (2-phenylethanol, 2-pentylfuran, trans,trans-2,4-nonadienal, 2-ethyl-5-methylpyrazine and trans-2-nonenal) were repellent (Figure 3). These results showed that, except for 2-phenylethanol, VOCs that were highly accumulated in tomato elicited attractive activities to T. absoluta, while VOCs that were highly accumulated in eggplant elicited repellent activities, and the results for males were highly consistent with those of females (Supplementary Figure S2). These results proved that volatile chemical signals played important roles in the host plant preferences of this pest. The VOCs identified in this study were different from the VOCs with oviposition selection behavior effects on T. absoluta in previous studies (Smith et al., 1996; Anastasaki et al., 2018), which could provide new candidate compounds for the development of bisexual attractants and repellents for this pest.
Results from cage experiments confirmed that 1-nonanol and ethyl octanoate were attractive to T. absoluta for oviposition. The attractive activities of these two volatiles have been reported in other pests. For instance, 1-nonanol could induce attraction response in sandfly Lutzomyia longipalpis (Magalhaes-Junior et al., 2014), melon fly Bactrocera cucurbitae (Siderhurst and Jang, 2010), and parasitic wasp Campsomeris tasmaniensis (Allsopp, 1992). However, this compound has been reported to be an oviposition deterrent for codling moth, Cydia pomonella (Yokoyama and Miller, 1991). Ethyl octanoate itself or synthetic compounds blend containing ethyl octanoate were attractive to fruit flies, such as Bactrocera dorsalis, Anastrepha ludens and A. obliqua (Robacker et al., 1992; Cruz-Lopez et al., 2006; Jayanthi et al., 2012). Ethyl octanoate is also one of the major volatile compounds of fermented sugar baits, which are commonly used for mass trapping of lepidopteran species (El-Sayed et al., 2005). Our results also showed that trans,trans-2,4-nonadienal and trans-2-nonenal could repel T. absoluta from oviposition. Trans,trans-2,4-nonadienal has been frequently reported to be a repellent against stored product insects, such as granary weevil Sitophilus granarius (Germinara et al., 2015), cigarette beetle Lasioderma serricorne and booklouse Liposcelis bostrychophila (Wei et al., 2018). Trans-2-nonenal was reported to be repellent to Culicoides biting midges (Isberg et al., 2017), and this compound was also effective in inactivating pathogenic bacteria (Cho et al., 2004). Further study should be conducted to test the effects of the identified attractive and repellent VOCs on field populations of T. absoulta.
In conclusion, our results identified 39 differentially accumulated VOCs between the preferred host (tomato) and non-preferred host (eggplant). Then the behavioral effects of these VOCs on the host selection and oviposition of T. absoluta were further investigated by using electroantennography and olfactometer tests. Almost all the selected VOCs that were highly accumulated in tomato showed attractive activities to T. absoluta, while VOCs highly accumulated in eggplants showed repellent activities, indicating that VOCs from host plants play important roles in host plant preferences. The attractive activities of 1-nonanol and ethyl octanoate, as well as the repellent activities of trans,trans-2,4-nonadienal and trans-2-nonenal, were further confirmed in cage experiments. These VOCs will enhance the development of semiochemicals-based eco-friendly control strategies for this pest.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LC and XL: conceptualization and writing—review and editing. TC and YLi: methodology. TC and JW: software. TC, JC, and SY: validation. MH: formal analysis. SZ: investigation. XL: resources. YLi: data curation. YLu: writing—original draft preparation. LC: visualization. TC: supervision. XL: project administration. XL and YLu: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (2022C04016), the National Natural Science Foundation of China (32272524), the Primary Research & Development Plan of Lishui (No. 2020ZDYF02), the China Postdoctoral Science Foundation (2021M702906), and the Zhejiang Provincial Natural Science Foundation of China (No. LQ22C140004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1155317/full#supplementary-material
SUPPLEMENTARY FIGURE S1
Electroantennographic (EAG) responses of T. absoluta males to 20 volatile compounds. The bar represents the standard error, and the different letters above each bar indicate Turkey’s highest significant difference at p < 0.05.
SUPPLEMENTARY FIGURE S2
Responses of male T. absoluta to nine VOCs in a Y-type olfactometer. NS indicates no significant difference; asterisks indicate significant differences (*p < 0.05; **p < 0.01).
Footnotes
References
Abdel-Baky, N. F., and Al-Soqeer, A. A. (2017). Controlling the 2nd instar larvae of Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) by simmondsin extracted from jojoba seeds in KSA. J. Entomol. 14, 73–80. doi: 10.3923/je.2017.73.80
Allsopp, P. G. (1992). Volatile compounds as attractants for Campsomeris tasmaniensis (Saussure) (Hymenoptera: Scoliidae). Austr. Entomol. Magaz. 19, 107–110. doi: 10.3316/informit.098199888946445
Anastasaki, E., Drizou, F., and Milonas, P. G. (2018). Electrophysiological and oviposition responses of Tuta absoluta females to herbivore-induced volatiles in tomato plants. J. Chem. Ecol. 44, 288–298. doi: 10.1007/s10886-018-0929-1
Arnó, J., Gabarra, R., Molina, P., Godfrey, K. E., and Zalom, F. G. (2019). Tuta absoluta (Lepidoptera: Gelechiidae) success on common Solanaceous species from California tomato production areas. Environ. Entomol. 48, 1394–1400. doi: 10.1093/ee/nvz109
Azevedo, S., Faria, M. V., Maluf, W. R., Oliveira, A., and Freitas, J. (2003). Zingiberene-mediated resistance to the south American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica 134, 347–351. doi: 10.1023/b:euph.0000005007.14924.d2
Baldwin, I. T., Halitschke, R., Paschold, A., von Dahl, C. C., and Preston, C. A. (2006). Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311, 812–815. doi: 10.1126/science.1118446
Barati, R., Hejazi, M. J., and Mohammadi, S. A. (2018). Insecticide susceptibility in Tuta absoluta (Lepidoptera: Gelechiidae) and metabolic characterization of resistance to diazinon. J. Econ. Entomol. 111, 1551–1557. doi: 10.1093/jee/toy134
Beck, J. J., Torto, B., and Vannette, R. L. (2017). Eavesdropping on plant-insect-microbe chemical communications in agricultural ecology: a virtual issue on semiochemicals. J. Agric. Food Chem. 65, 5101–5103. doi: 10.1021/acs.jafc.7b02741
Biondi, A., Guedes, R. N. C., Wan, F. H., and Desneux, N. (2018). Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annu. Rev. Entomol. 63, 239–258. doi: 10.1146/annurev-ento-031616-034933
Biondi, A., Zappala, L., Desneux, N., Aparo, A., Siscaro, G., Rapisarda, C., et al. (2015). Potential toxicity of alpha -cypermethrin-treated nets on Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 108, 1191–1197. doi: 10.1093/jee/tov045
Bruce, T. J. A., Wadhams, L. J., and Woodcock, C. M. (2005). Insect host location: a volatile situation. Trends Plant Sci. 10, 269–274. doi: 10.1016/j.tplants.2005.04.003
Campos, M. R., Biondi, A., Adiga, A., Guedes, R. N. C., and Desneux, N. (2017). From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest. Sci. 90, 787–796. doi: 10.1007/s10340-017-0867-7
Campos, M. R., Rodrigues, A. R. S., Silva, W. M., Silva, T. B. M., Silva, V. R. F., Guedes, R. N. C., et al. (2014). Spinosad and the tomato borer Tuta absoluta: a bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS One 9:e103235. doi: 10.1371/journal.pone.0103235
Campos, M. R., Silva, T. B. M., Silva, W. M., Silva, J. E., and Siqueira, H. A. A. (2015). Spinosyn resistance in the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest. Sci. 88, 405–412. doi: 10.1007/s10340-014-0618-y
Chen, L., Li, X., Zhang, J., He, T., Huang, J., Zhang, Z., et al. (2021). Comprehensive metabolome and volatilome analyses in eggplant and tomato reveal their differential responses to Tuta absoluta infestation. Front. Plant Sci. 12:757230. doi: 10.3389/fpls.2021.757230
Cho, M. J., Buescher, R. W., Johnson, M., and Janes, M. (2004). Inactivation of pathogenic bacteria by cucumber volatiles (E,Z)-2,6-nonadienal and (E)-2-nonenal. J. Food Prot. 67, 1014–1016. doi: 10.4315/0362-028X-67.5.1014
Chong, J., Soufan, O., Li, C., Caraus, I., Li, S., Bourque, G., et al. (2018). MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46, W486–W494. doi: 10.1093/nar/gky310
Cruz-Lopez, L., Malo, E. A., Toledo, J., Virgen, A., Del Mazo, A., and Rojas, J. C. (2006). A new potential attractant for Anastrepha obliqua from Spondias mombin fruits. J. Chem. Ecol. 32, 351–365. doi: 10.1007/s10886-005-9006-7
Desneux, N., Han, P., Mansour, R., Arno, J., Brevault, T., Campos, M. R., et al. (2022). Integrated pest management of Tuta absoluta: practical implementations across different world regions. J. Pest. Sci. 95, 17–39. doi: 10.1007/s10340-021-01442-8
Desneux, N., Wajnberg, E., Wyckhuys, K. A. G., Burgio, G., Arpaia, S., Narvaez-Vasquez, C. A., et al. (2010). Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J. Pest. Sci. 83, 197–215. doi: 10.1007/s10340-010-0321-6
Dudareva, N., Negre, F., Nagegowda, D. A., and Orlova, I. (2006). Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440. doi: 10.1080/07352680600899973
El-Sayed, A. M., Heppelthwaite, V. J., Manning, L. M., Gibb, A. R., and Suckling, D. M. (2005). Volatile constituents of fermented sugar baits and their attraction to lepidopteran species. J. Agric. Food Chem. 53, 953–958. doi: 10.1021/jf048521j
Germinara, G. S., Cristofaro, A., and Rotundo, G. (2015). Repellents effectively disrupt the olfactory orientation of Sitophilus granarius to wheat kernels. J. Pest. Sci. 88, 675–684. doi: 10.1007/s10340-015-0674-y
Gripenberg, S., Mayhew, P. J., Parnell, M., and Roslin, T. (2010). A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393. doi: 10.1111/j.1461-0248.2009.01433.x
Guedes, R. N. C., Roditakis, E., Campos, M. R., Haddi, K., Bielza, P., Siqueira, H. A. A., et al. (2019). Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J. Pest. Sci. 92, 1329–1342. doi: 10.1007/s10340-019-01086-9
Guedes, R. N. C., and Siqueira, H. A. A. (2012). The tomato borer Tuta absoluta: insecticide resistance and control failure. CAB Rev. 2012, 1–7. doi: 10.1079/PAVSNNR20127055
Haddi, K., Berger, M., Bielza, P., Cifuentes, D., Field, L. M., Gorman, K., et al. (2012). Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem. Mol. Biol. 42, 506–513. doi: 10.1016/j.ibmb.2012.03.008
Haddi, K., Berger, M., Bielza, P., Rapisarda, C., Williamson, M. S., Moores, G., et al. (2017). Mutation in the ace-1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. J. Appl. Entomol. 141, 612–619. doi: 10.1111/jen.12386
Han, P., Bayram, Y., Shaltiel-Harpaz, L., Sohrabi, F., Saji, A., Esenali, U. T., et al. (2019). Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. J. Pest. Sci. 92, 1317–1327. doi: 10.1007/s10340-018-1062-1
Heil, M., and Silva Bueno, J. C. (2007). Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. U. S. A. 104, 5467–5472. doi: 10.1073/pnas.0610266104
Isberg, E., Bray, D. P., Hillbur, Y., and Ignell, R. (2017). Evaluation of host-derived volatiles for trapping Culicoides biting midges (Diptera: Ceratopogonidae). J. Chem. Ecol. 43, 662–669. doi: 10.1007/s10886-017-0860-x
Jaenike, J. (1978). On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 14, 350–356. doi: 10.1016/0040-5809(78)90012-6
Jayanthi, P. D. K., Woodcock, C. M., Caulfield, J., Birkett, M. A., and Bruce, T. J. A. (2012). Isolation and identification of host cues from mango, Mangifera indica, that attract gravid female oriental fruit fly, Bactrocera dorsalis. J. Chem. Ecol. 38, 361–369. doi: 10.1007/s10886-012-0093-y
Kuhnle, A., and Muller, C. (2011). Relevance of visual and olfactory cues for host location in the mustard leaf beetle Phaedon cochleariae. Physiol. Entomol. 36, 68–76. doi: 10.1111/j.1365-3032.2010.00763.x
Lee, J. M., Kim, D. H., Chang, P. S., and Lee, J. H. (2007). Headspace-solid phase microextraction (HS-SPME) analysis of oxidized volatiles from free fatty acids (FFA) and application for measuring hydrogen donating antioxidant activity. Food Chem. 105, 414–420. doi: 10.1016/j.foodchem.2006.12.059
Leite, G. L. D., Picanco, M., Della Lucia, T. M. C., and Moreira, M. D. (1999). Role of canopy height in the resistance of Lycopersicon hirsutum f. glabratum to Tuta absoluta (Lep., Gelechiidae). J. Appl. Entomol. 123, 459–463. doi: 10.1046/j.1439-0418.1999.00385.x
Li, X. W., Li, D., Guo, W. C., and Lu, Y. B. (2019). Host-plant suitability of South America tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on four solanaceous plants. Plant Quar. 33, 1–5. doi: 10.19662/j.cnki.issn1005-2755.2019.03.002
Li, D., Li, X. W., Ma, L., Fu, K. Y., Ding, X. H., Guo, W. C., et al. (2019). Effects of temperature on the growth, development and reproduction of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae). Acta Entomol. Sin. 62, 1416–1426. doi: 10.16380/j.kcxb.2019.12.008
Lietti, M. M. M., Botto, E., and Alzogaray, R. A. (2005). Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 34, 113–119. doi: 10.1590/S1519-566X2005000100016
Magalhaes-Junior, J. T., Barrouin-Melo, S. M., Correa, A. G., Silva, F. B., Machado, V. E., Govone, J. S., et al. (2014). A laboratory evaluation of alcohols as attractants for the sandfly Lutzomyia longipalpis (Diptera:Psychodidae). Parasit. Vect. 7. doi: 10.1186/1756-3305-7-60
Mayhew, P. J. (1997). Adaptive patterns of host-plant selection by Phytophagous insects. Oikos 79, 417–428. doi: 10.2307/3546884
Msisi, D., Matojo, N. D., and Kimbokota, F. (2021). Attraction of female tomato leaf miner,Tuta absoluta(Meyrick, 1917) (Lepidoptera:Gelechiidae) to shared compounds from hosts. Phytoparasitica 49, 153–162. doi: 10.1007/s12600-020-00848-x
Proffit, M., Birgersson, G., Bengtsson, M., Reis, R., Witzgall, P., and Lima, E. (2011). Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 37, 565–574. doi: 10.1007/s10886-011-9961-0
Rahmani, S., and Azimi, S. (2021). Fumigant toxicity of three Satureja species on tomato leafminers, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Toxin Rev. 40, 724–735. doi: 10.1080/15569543.2020.1767651
Robacker, D. C., Warfield, W. C., and Flath, R. A. (1992). A four-component attractant for the Mexican fruit fly, Anastrepha ludens (Diptera: Tephritidae), from host fruit. J. Chem. Ecol. 18, 1239–1254. doi: 10.1007/BF00980077
Roditakis, E., Vasakis, E., Garcia-Vidal, L., Martinez-Aguirre, M., Rison, J. L., Haxaire-Lutun, M. O., et al. (2018). A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J. Pest. Sci. 91, 421–435. doi: 10.1007/s10340-017-0900-x
Saidov, N., Ramasamy, S., Mavlyanova, R., and Qurbonov, Z. (2018). First report of invasive South American tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Tajikistan. Fla. Entomol. 101, 147–149. doi: 10.1653/024.101.0129
Sankarganesh, E., Firake, D. M., Sharma, B., Verma, V. K., and Behere, G. T. (2017). Invasion of the South American tomato pinworm, Tuta absoluta, in northeastern India: a new challenge and biosecurity concerns. Entomol. Gener. 36, 335–345. doi: 10.1127/entomologia/2017/0489
Shrivastava, G., Rogers, M., Wszelaki, A., Panthee, D. R., and Chen, F. (2010). Plant volatiles-based insect pest management in organic farming. Crit. Rev. Plant Sci. 29, 123–133. doi: 10.1080/07352681003617483
Siderhurst, M. S., and Jang, E. B. (2010). Cucumber volatile blend attractive to female melon fly, Bactrocera cucurbitae (Coquillett). J. Chem. Ecol. 36, 699–708. doi: 10.1007/s10886-010-9804-4
Silva, G. A., Picanço, M. C., Bacci, L., Crespo, A. L. B., Rosado, J. F., and Guedes, R. N. C. (2011). Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 67, 913–920. doi: 10.1002/ps.2131
Silva, G. A., Queiroz, E. A., Arcanjo, L. P., Lopes, M. C., Araújo, T. A., Galdino, T. S., et al. (2021). Biological performance and oviposition preference of tomato pinworm Tuta absoluta when offered a range of solanaceous host plants. Sci. Rep. 11:1153. doi: 10.1038/s41598-020-80434-7
Silva, J. E., Ribeiro, L. M., Vinasco, N., Guedes, R. N. C., and Siqueira, H. Á. A. (2019). Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: inheritance, cross-resistance profile, and metabolism. J. Pest. Sci. 92, 1421–1431. doi: 10.1007/s10340-018-1064-z
Silva, T. B. M., Silva, W. M., Campos, M. R., Silva, J. E., Ribeiro, L. M. S., and Siqueira, H. A. A. (2016). Susceptibility levels of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) to minor classes of insecticides in Brazil. Crop Prot. 79, 80–86. doi: 10.1016/j.cropro.2015.10.012
Silvério, F. O., de Alvarenga, E. S., Moreno, S. C., and Picanço, M. C. (2009). Synthesis and insecticidal activity of new pyrethroids. Pest Manag. Sci. 65, 900–905. doi: 10.1002/ps.1771
Siqueira, H. A. A., Guedes, R. N. C., Fragoso, D. B., and Magalhaes, L. C. (2001). Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Pest Manag. 47, 247–251. doi: 10.1080/09670870110044634
Siqueira, H. A. A., Guedes, R. N. C., and Picanco, M. C. (2000a). Cartap resistance and synergism in populations of Tuta absoluta (Lep., Gelechiidae). J. Appl. Entomol. 124, 233–238. doi: 10.1046/j.1439-0418.2000.00470.x
Siqueira, H. A. A., Guedes, R. N. C., and Picanco, M. C. (2000b). Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2, 147–153. doi: 10.1046/j.1461-9563.2000.00062.x
Smith, R. M., Marshall, J. A., Davey, M. R., Lowe, K. C., and Power, J. B. (1996). Comparison of volatiles and waxes in leaves of genetically engineered tomatoes. Phytochemistry 43, 753–758. doi: 10.1016/0031-9422(96)00364-0
Subramani, V., Damodaram, K. J. P., Krishnegowda, R. G., Parepally, S. K., Kempraj, V., Thimmappa, R., et al. (2021). Volatile chemical signals underlying the host plant preferences of Tuta absoluta. Entomol. Exp. Appl. 169, 997–1007. doi: 10.1111/eea.13099
Thompson, J. N. (1988). Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 47, 3–14. doi: 10.1111/j.1570-7458.1988.tb02275.x
Uulu, T. E., Ulusoy, M. R., and Calskan, A. F. (2017). First record of tomato leafminer Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in Kyrgyzstan. Bull. OEPP/EPPO Bull. 47, 285–287. doi: 10.1111/epp.12390
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hussain, B., Ignacimuthu, S., et al. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7, 1306–1320. doi: 10.4161/psb.21663
Wei, X. M., Guo, S. S., Yan, H., Cheng, X. L., Wei, F., and Du, S. S. (2018). Contact toxicity and repellency of the essential oil from Bupleurum bicaule Helm against two stored product insects. J. Chem. 2018:5830864. doi: 10.1155/2018/5830864
Wynde, F. J. H., and Port, G. R. (2012). The use of olfactory and visual cues in host choice by the capsid bugs Lygus rugulipennis Poppius and Liocoris tripustulatus Fabricius. PLoS One 7:e46448. doi: 10.1371/journal.pone.0046448
Yokoyama, V. Y., and Miller, G. T. (1991). A plum volatile, 1-nonanol: an oviposition deterrent for codling moth [Cydia pomonella]. Can. Entomol. 123, 711–712. doi: 10.4039/Ent123711-3
Yuan, H., Cao, G., Hou, X., Huang, M., Du, P., Tan, T., et al. (2022). Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant 15, 189–202. doi: 10.1016/j.molp.2021.09.003
Zhang, G. F., Ma, D. Y., Liu, W. X., Wang, Y. S., Fu, W. J., Wang, Y. H., et al. (2019). The arrival of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), China. J. Biosaf. 28, 200–203. doi: 10.3969/j.issn.2095-1787.2019.03.007
Zhang, G. F., Xian, X. Q., Zhang, Y. B., Liu, W. X., Liu, H., Feng, X. D., et al. (2021). Outbreak of the south American tomato leafminer, Tuta absoluta, in the Chinese mainland: geographic and potential host range expansion. Pest Manag. Sci. 77, 5475–5488. doi: 10.1002/ps.6588
Zhang, G. F., Zhang, Y. B., Zhang, J., Liu, W. X., Wang, Y. S., Wan, F. H., et al. (2020). Evaluation of indoor toxicity and field control effect of biopesticide Bacillus thuringiensis G033A on the South American tomato leafminer Tuta absoluta (Meyrick), a newly invaded alien insect pest in China. Chin. J. Biol. Control 36, 175–183. doi: 10.16409/j.cnki.2095-039x.2020.02.003
Keywords: Tuta absoluta , tomato, eggplant, VOCs, oviposition preferences, attractants, repellents
Citation: Chen T, Chen L, Wang J, Cheng J, Yi S, Hafeez M, Zhou S, Li Y, Li X and Lu Y (2023) Development of attractants and repellents for Tuta absoluta based on plant volatiles from tomato and eggplant. Front. Sustain. Food Syst. 7:1155317. doi: 10.3389/fsufs.2023.1155317
Edited by:
Fang Ouyang, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Huipeng Pan, South China Agricultural University, ChinaWendy-Ann P. Isaac, The University of the West Indies St. Augustine, Trinidad and Tobago
Copyright © 2023 Chen, Chen, Wang, Cheng, Yi, Hafeez, Zhou, Li, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaobin Lu, bHV5YmNuQDE2My5jb20=; Xiaowei Li, bGl4aWFvd2VpMTAwNUAxNjMuY29t
†These authors have contributed equally to this work
 Tingting Chen
Tingting Chen Limin Chen
Limin Chen Jinchao Wang1
Jinchao Wang1 Muhammad Hafeez
Muhammad Hafeez Yuanxi Li
Yuanxi Li Xiaowei Li
Xiaowei Li Yaobin Lu
Yaobin Lu