
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 06 July 2023
Sec. Nutrition and Sustainable Diets
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1154757
This article is part of the Research Topic Nutrition and Sustainable Development Goal 15: Life on Land View all 9 articles
The processing of edible mushrooms generates a large amount of mushroom residue. How to handle this mushroom residue in a way that avoids environmental pollution and maximizes effective utilization is a current issue that needs to be explored. This study aimed to investigate the effects of substituting dietary corn with Hypsizygus marmoreus mushroom stem waste (HSW) in the diet of geese. The control group was fed with a basal diet (BD), and the other groups were fed the basal diet to which 12% (HSW12 group), 24% (HSW24 group), or 32% (HSW32 group) of HSW were added to replace the equivalent proportion of corn. The test lasted 28 days. The results showed that the average daily feed intake (ADFI) of the HSW12 and HSW24 groups at 35–49 d, and the HSW12 and HSW32 groups at 35–63 d, was significantly higher compared to the BD group (p<0.05). The average daily gain (ADG) of the HSW12 group was significantly higher than BD at 35–49 d (p<0.05), but there was no significant difference in the feed/gain (F/G) among the groups. The levels of serum total protein (TP), albumin (ALB), globulin (GLOB), glutathione peroxidase (GSH-Px), and catalase (CAT) in HSW24 group were significantly higher than those in the BD group (p<0.05). Total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and malondialdehyde (MDA) decreased significantly, and there were no significant differences in carcass traits and meat quality. As 24% HSW supplementation had the best overall effect on the growth performance, serum biochemical indicators, meat quality, and carcass traits of geese, gut microbiota analysis was only performed on this group. The microbiota α-diversity of the cecum and ileum did not differ significantly between the BD and HSW24 groups. Principal coordinate analysis (PCoA) indicated that the difference in the cecum was significant in the β-diversity (p<0.05). Short chain fatty acid-producing bacteria and decomposing protein and carbohydrate bacteria (Prevotella) were enriched in the cecum in the HSW24 group. Gut immune regulating and nutritional bacteria, Lactococcus and Bacillus, respectively, were enriched in the ileum in HSW24 group. Spearman’s analysis indicated that Bacillus, Prevotella, and Clostridium were positively associated with serum protein and lipid metabolism. These results indicate that 24% HSW substitution of corn could improve goose serum ALB and fat metabolism, and increase serum antioxidant capacity, which may becaused by the improvement of goose cecal microbiota.
Corn is nutritionally balanced and an important part of animal husbandry (Limba et al., 2019; Williams, 2022). However, owing to the impact of COVID-19 and wars in recent years, the international price of corn has increased sharply (Lv and Xu, 2022; Xu and Zhang, 2022). Simultaneously, the supply of corn has recently been insufficient, increasing the cost of animal husbandry and limiting its development (Jim, 2020; Jacqueline, 2021). Therefore, it has become necessary to find alternate, unconventional feeds to replace corn, depending on the available local resources. Studies have found that corn in animal diets can be partially replaced without changing growth performance (Kim et al., 2021; Jeong et al., 2022), while improving the gut microbiome (Gheorghe et al., 2017).
The production and export of edible fungi in China has always been in the forefront of the world, ranking among the top five in terms of agricultural output (Li and Xu, 2022). H. marmoreus accounts for a large proportion of the edible fungus industry, and is widely planted worldwide (Yamanaka, 1997). The cultivation substrate of H. marmoreus mainly consists of corn cobs, rice bran, wheat bran, soybean husks, and sorghum flour. The production cycle of H. marmoreus is 110 to 120 days. In 2019, the total daily production capacity of H. marmoreus in China’s commercial mushroom farms reached 930 tons. Its roots, stems, and caps have similar nutritional components, and are rich in crude protein, crude fiber, amino acids, and umami peptides, making it rich in nutrients and giving it a high edibility value. However, during processing or picking, the lower half of the stem is usually treated as waste, which causes environmental pollution while being wasted (Wu et al., 2019). Mushroom waste stems are considered a type of prebiotic and contain hemicellulose and polysaccharides (Singdevsachan et al., 2016). Studies have shown that adding mushroom waste stems to the diet of pigs is beneficial for growth, and can increase short-chain fatty acids in the gut (Liu et al., 2020). Studies have also shown that mushroom waste stems can improve the antioxidant capacity, immunity, and apparent digestibility of animals (Chen et al., 2018; Sun and Li, 2021). The in vitro immune activity of mushroom polysaccharides can provide a theoretical basis for determining the growth and health status of animals (Guo et al., 2003). Mushrooms contain high levels of cellulose (Liu et al., 2020), which cannot be directly digested and absorbed by many animals. However, geese, compared to other poultry, have well-developed ceca (Zhang et al., 2022), which provide a larger volume and longer retention time, allowing more time for interaction between the digesta and the microorganisms in the ceca. Research has shown that geese rely on cellulose-degrading bacteria in the ceca to decompose cellulose (Volk and Lacy, 2017), thereby releasing nutrients such as organic acids and short-chain fatty acids.
Therefore, in this experiment, the HWS were used as an unconventional feed to replace part of the corn-based diet of geese, and its growth performance, serum biochemical indices, slaughter performance and gut microbiome were evaluated.
Animal care and procedures were performed according to the Chinese Animal Welfare Guidelines. This research was approved by the Laboratory Animal Ethics Committee of the Shanghai Academy of Agricultural Sciences (SAASPZ0522046).
HMS were provided by the Zhuanghang Comprehensive Experimental Station of Shanghai Academy of Agriculture Sciences (Shanghai, China) and processed at Shengwang Feed Co., Ltd. (Shanghai, China). Specifically, fresh HMS was dried using a three-stage rotary drum dryer (Lunji, Shandong, China) at 65°C, then pulverized using a grinder (Jishun, Shandong, China) and sieved through a 2-millimeter mesh to obtain a powdered form, which was subsequently incorporated into the daily ration. The same batch of HMS was used for all experiments. The nutritional and amino acid composition of HMS are presented in Table 1.
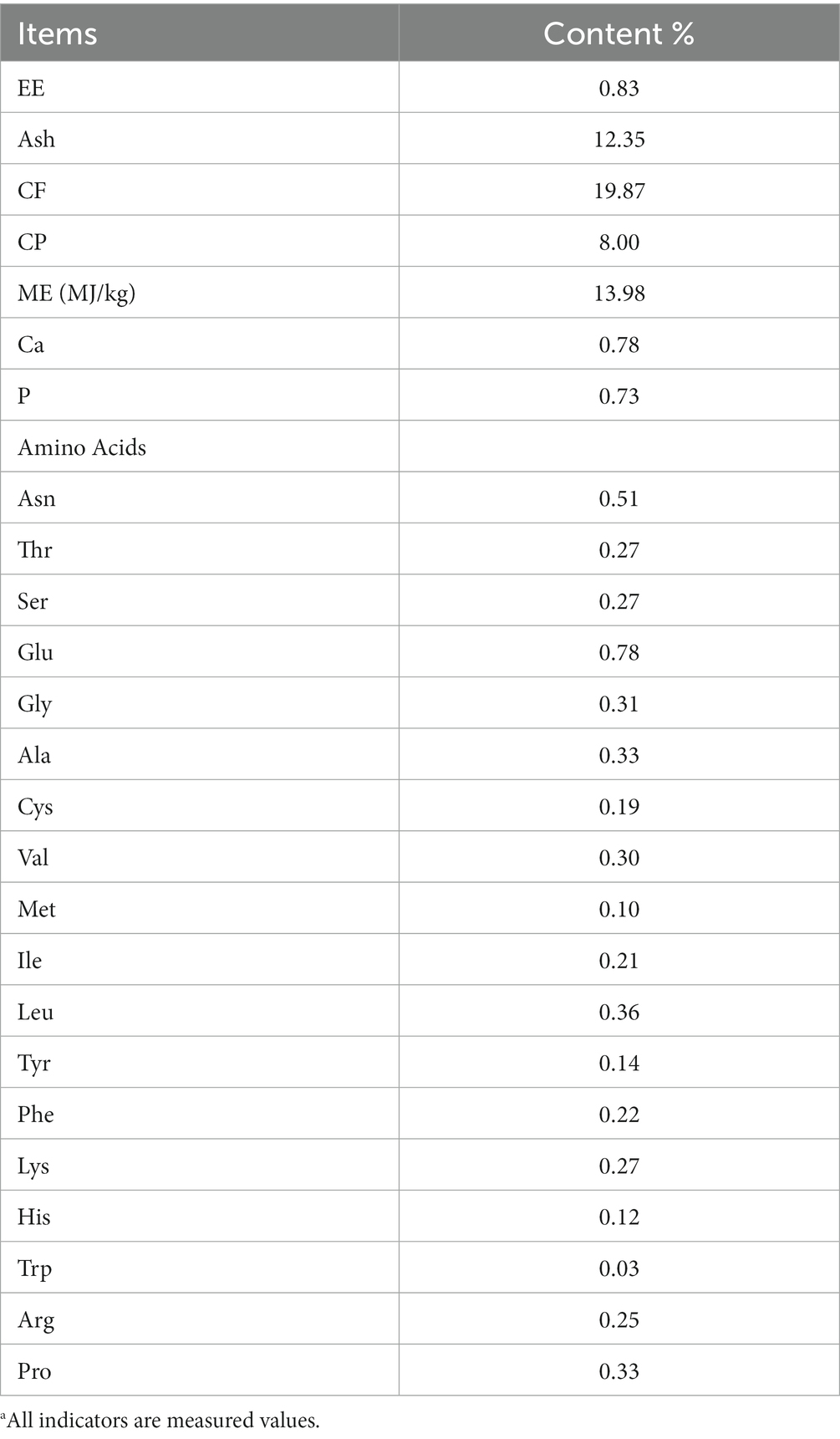
Table 1. Nutrient level of HSW (air-dried basis) %a.
The geese used in the experiment were purchased from the Xiangtiange Family Farm, Ma’anshan City, Anhui Province, China. A total of 192 35-day-old Hordobagy geese from the same batch were randomly allocated into four groups. Each group had six replicates with eight geese (half males and half females). The control group was fed a basal diet (BD) comprising corn, the other groups were fed a mixture of the basal diet with 12% (HSW12 group), 24% (HSW24 group), or 32% (HSW32 group) dry mass of HSW, included to replace the same proportion of corn. The test period was 28 days. The basal diet group was based on the NRC1994 standard and adjusted according to the nutritional status of Chinese geese (Table 2). Each replicate of eight geese was housed in an individual mesh cage (80 × 80 × 80 cm) in a naturally ventilated windowed shed at a temperature between 10–15°C. Only natural light was provided, and the light time of each cage was the same throughout the experiment. Feed and water were provided ad libitum. Disinfection and administration of vaccines progressed on schedule, as for other geese on the farm.
At the beginning (35 d), middle (49 d), and end (63 d) of the experiment, the body weight and feed consumption of each goose were measured, and its ADG, ADFI and F/G were calculated. The geese were fasted for 12 h before weighing.
Blood samples were collected at the end of the experiment.One male goose from each cage was chose and draw 4 mL of blood from the wing vein, stored in vacuum-sealed blood collection tubes at 37°C for 1 h, and centrifuged at 4,500 rpm for 15 min. Then collected the serum for further analysis. Serum biochemical parameters, including total protein (TP), albumin (ALB), globulin (GLOB), glucose (GLU), burea nitrogen (BUN), total cholesterol (TC), Triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (AKP) were detected using an automatic biochemical analyzer (HITACHI 7180, Japan). Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and total antioxidant capacity (T-AOC) were detected by ELISA (DENLEY DRAGON Wellscan MK 3, Finland), with the appropriate test kit purchased from Nanjing Jiancheng Biotechnology Co., Ltd., China.
After the experiment, geese were fasted for 12 h and deprived of water for 2 h. One goose was selected closed to the average body weight of each replicate. After weighing, the jugular vein was cut and the goose bled to death.The measurement and calculation of carcass traits is reference to Zhai et al. (2020).
According to the method in “Determination of Meat Quality of Livestock and Poultry” (NY/T 823–2004), a colorimeter (Chroma Meter CR-410, Konica Metanon Corporation, Japan) was used to measure the chest muscle and leg muscle. The tissue pH was measured using a Testo-205 pH-measuring instrument.
After slaughtering, the ileum and cecum were retrieved. Using cotton thread, both ends were ligated. Approximately 1 g of digesta from the middle portion was collected in a 2 mL centrifuge tube. The samples were rapidly frozen using liquid nitrogen and stored at-80°C.The DNA of microorganisms in ileal and cecal digesta was extracted using a DNA extraction kit (Tiangen, Beijing, China). The concentration of DNA was measured with a UV spectrophotometer (NanoDrop 2000, ThermoFisher, MA, United States). The purity of the DNA was checked by 1% agarose gel electrophoresis. DNA is used as a template for PCR amplification and the products are purified, quantified and normalized to form a sequencing library. The library was sequenced after dilution and quantification (NovaSeq 6,000, San Diego, United States), and the amplicon sequence variants, diversity and difference analysis was performed on the sequence information.
The raw data on growth performance, serum biochemical indicators, and slaughter performance were organized using Microsoft Excel 2007, and one-way ANOVA analysis of variance and Duncan’s multiple range test were conducted using SPSS 25.0 software (IBM, New York, United States). Significant differences were indicated by p < 0.05. The raw gut microbiota sequencing data were quality controlled using QIIME 2.0 software. Optimized sequences were obtained by sequence trimming, filtering, and chimera removal. Clustering analysis of amplicon sequence variants (ASV) was performed using USEARCH 7.0 software. Classification analysis of ASV sequences was conducted using the greengene gene database, and the community composition of each sample was analyzed at the phylum, class, order, family, genus, and species levels. Principal coordinate analysis (PCoA) of the cecal microbiota was performed using R language, and data visualization was conducted using STAMP 2.1.3 software. -diversity indices were calculated using Mothur 1.2 software. Spearman analysis was performed using GeneCloud.1
Compared with the BD group, there was no difference in body weight between groups at 49 d and 63 d (Table 3), but the ADFI of the HSW12 and HSW24 groups at 35–49 d, and of the HSW12 and HSW32 groups at 35–63 d, increased significantly (p < 0.05). The ADG of the HSW12 group increased significantly compared to the control at 35–49 d (p < 0.05), but there was no significant difference in the F/G between groups.
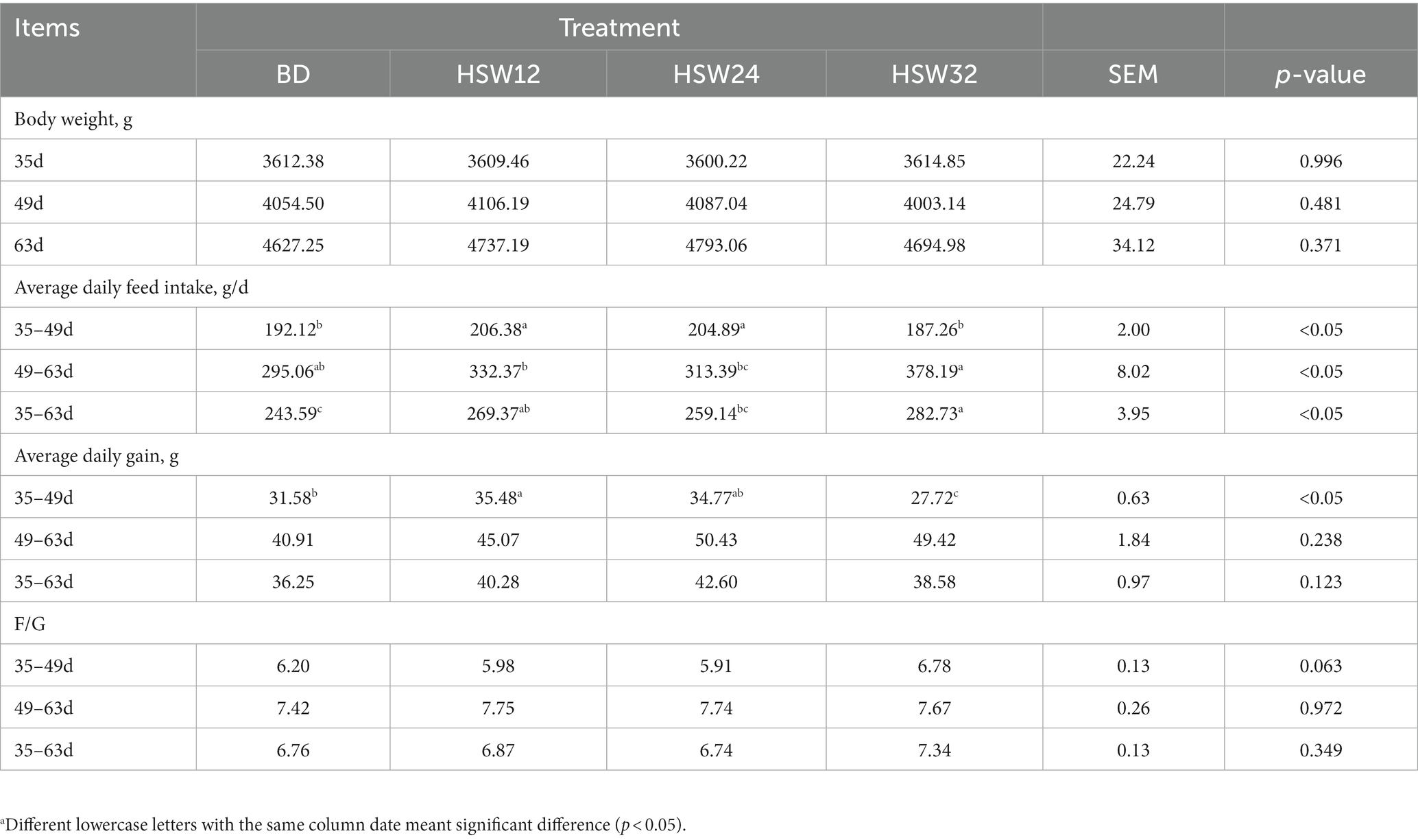
Table 3. Effect of HSW on growth performancea.
Compared with the BD group, there was no significant difference in the levels of GLU, BUN, UA, HDL-C, ALT, AST, and AKP (Table 4). However, the levels of TP, ALB, and GLOB in the HSW24 group increased significantly. In contrast, the levels of TG, TC, and LDL-C decreased significantly (p < 0.05).
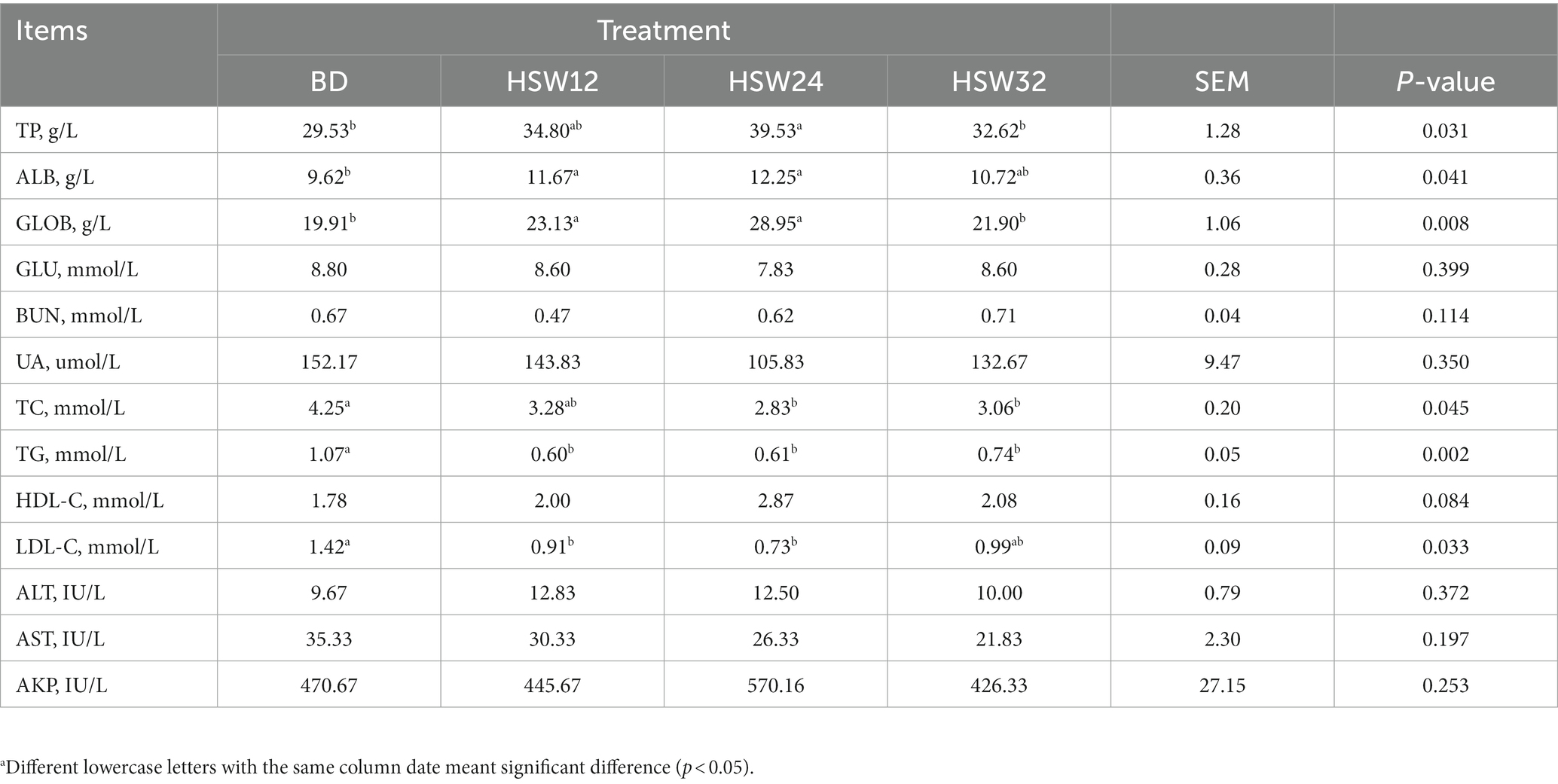
Table 4. Effect of HSW on serum biochemical indexesa.
There were significant differences among the other indexes between the groups: MDA levels decreased significantly in the HSW24 and HSW32 groups (p < 0.05), the levels of CAT and GSH-Px increased significantly in the HSW12 and HSW24 groups (p < 0.05), and the T-AOC levels were significantly higher in the HSW32 group (Table 5).
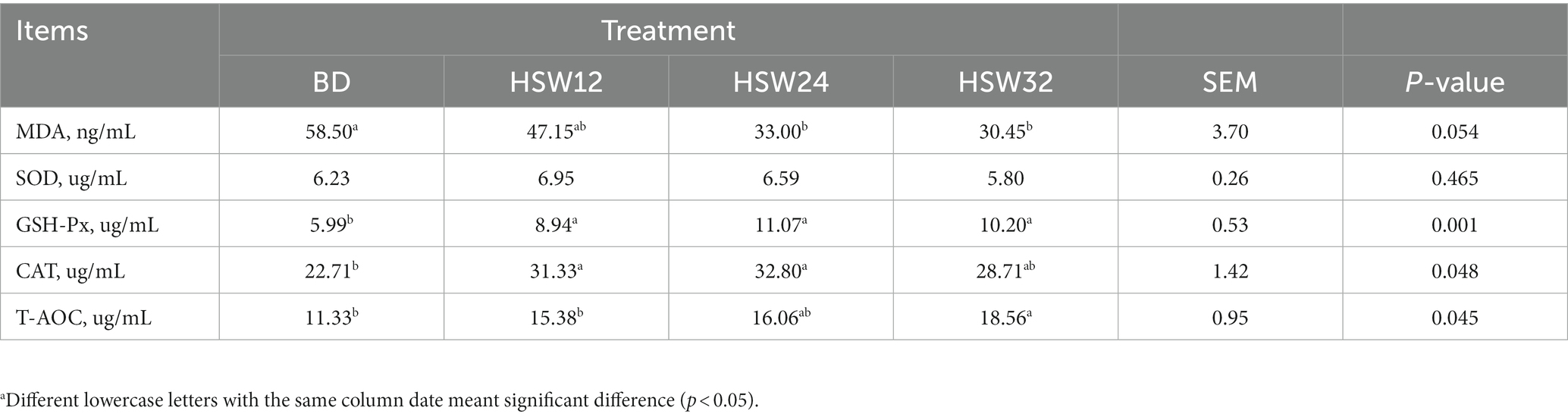
Table 5. Effect of HSW on serum antioxidant indexesa.
There were no significant differences in carcass traits or meat quality (Table 6). However, the pH of the breast muscle in the HSW24 group tended to increase slightly, but not significantly (p < 0.10).
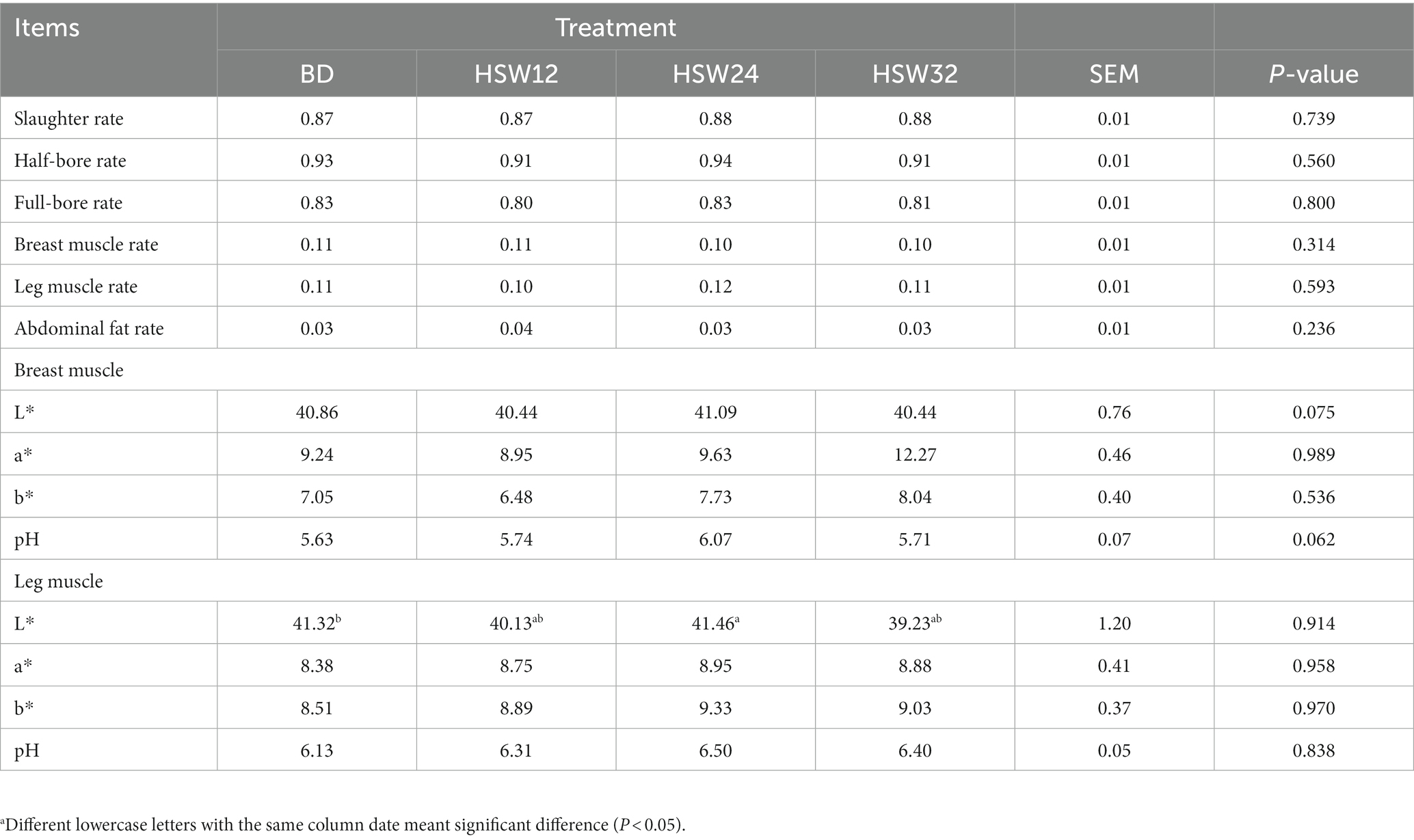
Table 6. Effect of HSW on carcass traits and meat qualitya.
In summary, we believe that adding 24% HSW to the diet had the best overall effect on growth performance, serum biochemical indicators, meat quality, and carcass traits of geese. Therefore, we further investigated the differences in gut microbiota composition between the BD and HSW24 groups.
In the ileum (Figure 1A), the dominant phyla (top 10) were Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria (Thermi), Cyanobacteria, Tenericutes, Acidobacteria, Verrucomicrobia, Planctomycetes, and the dominant genera (top 10) were Cupriavidus, Lactobacillus, Ochrobactrum, Sphingomonas, Pseudomonas, Acinetobacter, Lachnospiraceae_Clostridium, Pelomonas, Subdoligranulum, and Enterococcus. The dominant phyla in the cecum (top 10) are Firmicutes, Proteobacteria, Bacteroidetes, Verrucomicrobia, Actinobacteria, Tenericutes, Cyanobacteria, Synergistetes, Elusimicrobia, Fusobacteria, with the dominant genera (top 10) being Desulfovibrio, Bacteroidaceae_Bacteroides, Subdoligranulum, Oscillospira, Phascolarctobacterium, Akkermansia, (Prevotella), Faecalibacterium, Barnesiella, and Ruminococcus (Figure 1B).
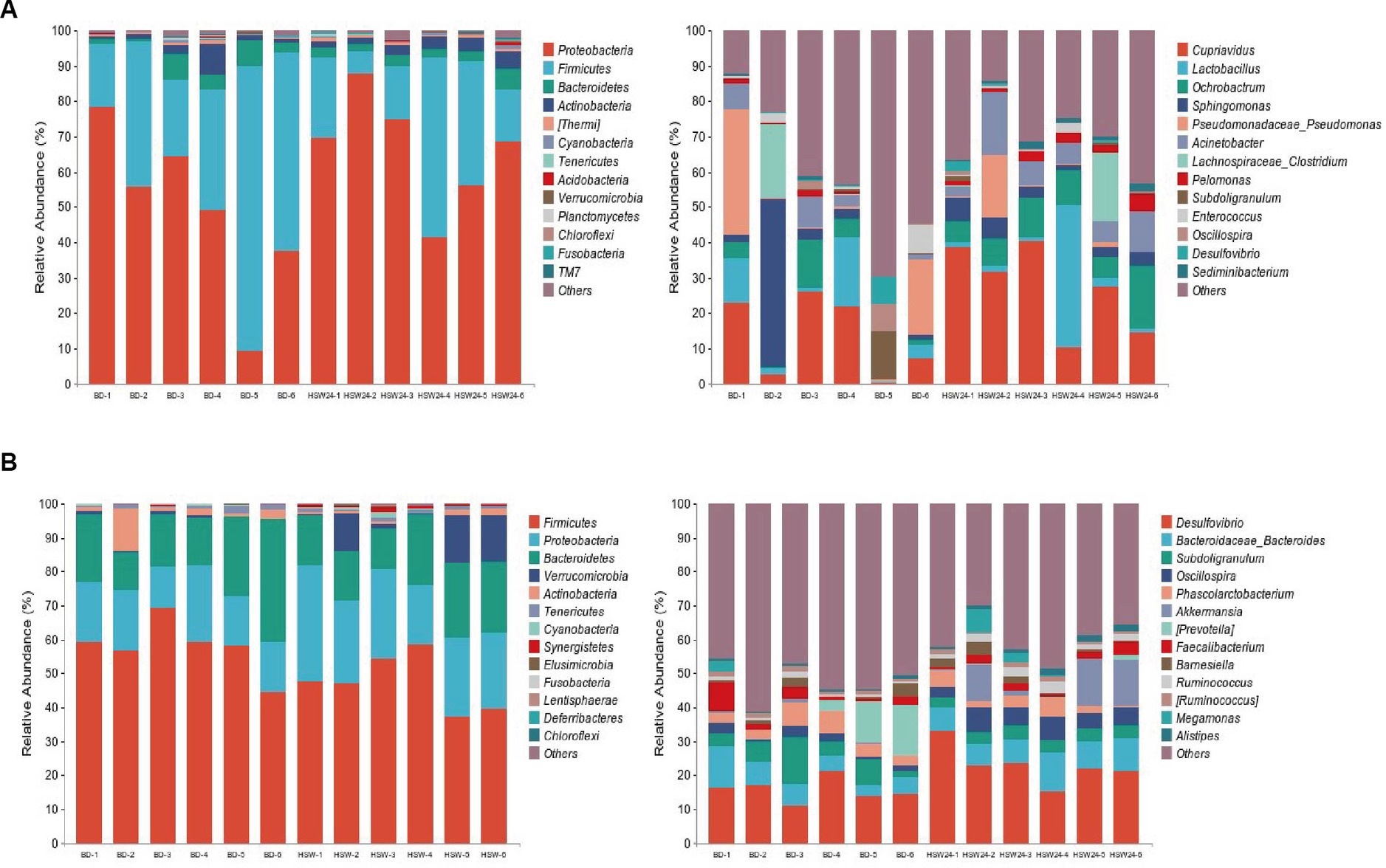
Figure 1. Effects of HMS on changes in microbiota of geese. In the ileum (A) and cecum (B), the classification composition and relative abundance of microbiota in each group.
There was no significant difference in the microbial α-diversity index between the ileum and cecum (p > 0.05), as shown in Figure 2. The β-diversity based on PCoA showed that the flora of the BD group and the HSW24 group, there was no obvious clustering in the ileum (p > 0.05) (Figure 3A), but the clustering was more obvious in the cecum (p < 0.05) (Figure 3B), indicating that the diversity of the flora was more similar in the ileum and more dissimilar in the cecum.

Figure 2. Effects of HMS on the ɑ-diversity of microbiota in geese. Boxplots show the differences in Chao1, Observed species, Shannon, Simpson, Faith’s PD, and Pielou’s evenness indices between groups.
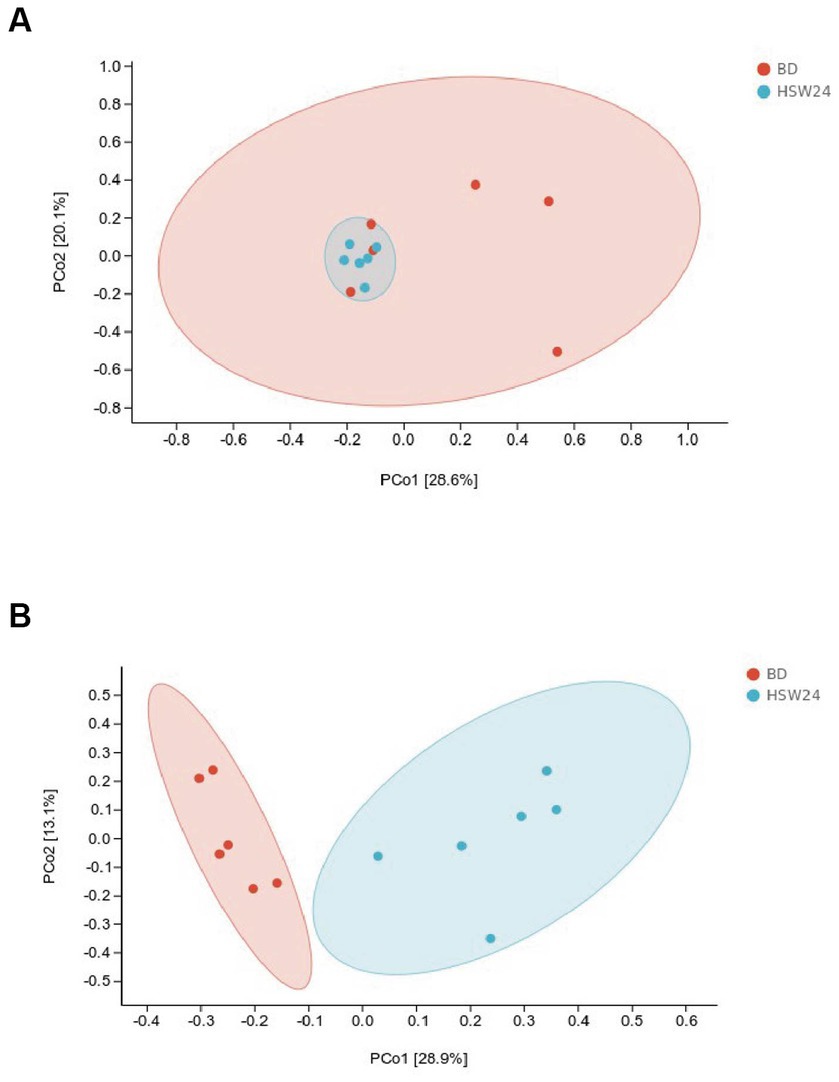
Figure 3. Effects of HMS on the β-diversity of microbiota in geese. The β-diversity analysis of the ileum (A) and cecum (B) microbiota among groups. PCoA and analyses were performed based on Bray-Curtis distance, and different colors and shapes represent samples from different groups.
The biomarkers of different groups were determined by Linear discriminant analysis Effect Size (LEfSe). Hymenobacter, Bacillus, Tetragenococcus, Lactococcus, Asticcacaulis, Ochrobactrum, Agrobacterium, Sphingobium, Acidovorax, Aquabacterium, Pelomonas, Ralstonia, and Bdellovibrio were the biomarkers of the ileum of the HSW24 group, and g_Butyricimonas was the biomarker of the ileum of the BD group (Figure 4A). In the cecum, Prevotella, AF12, Alistipes, f-Ruminococcaceae, g-Clostridium, Oscillospira, Ruminococcus, Sutterella, Desulfovibrio were the biomarkers of the HSW24 group (Figure 4B), while YRC22, Megasphaera, Lactobacillus, Dorea, Turicibacter were biomarkers for the BD group.
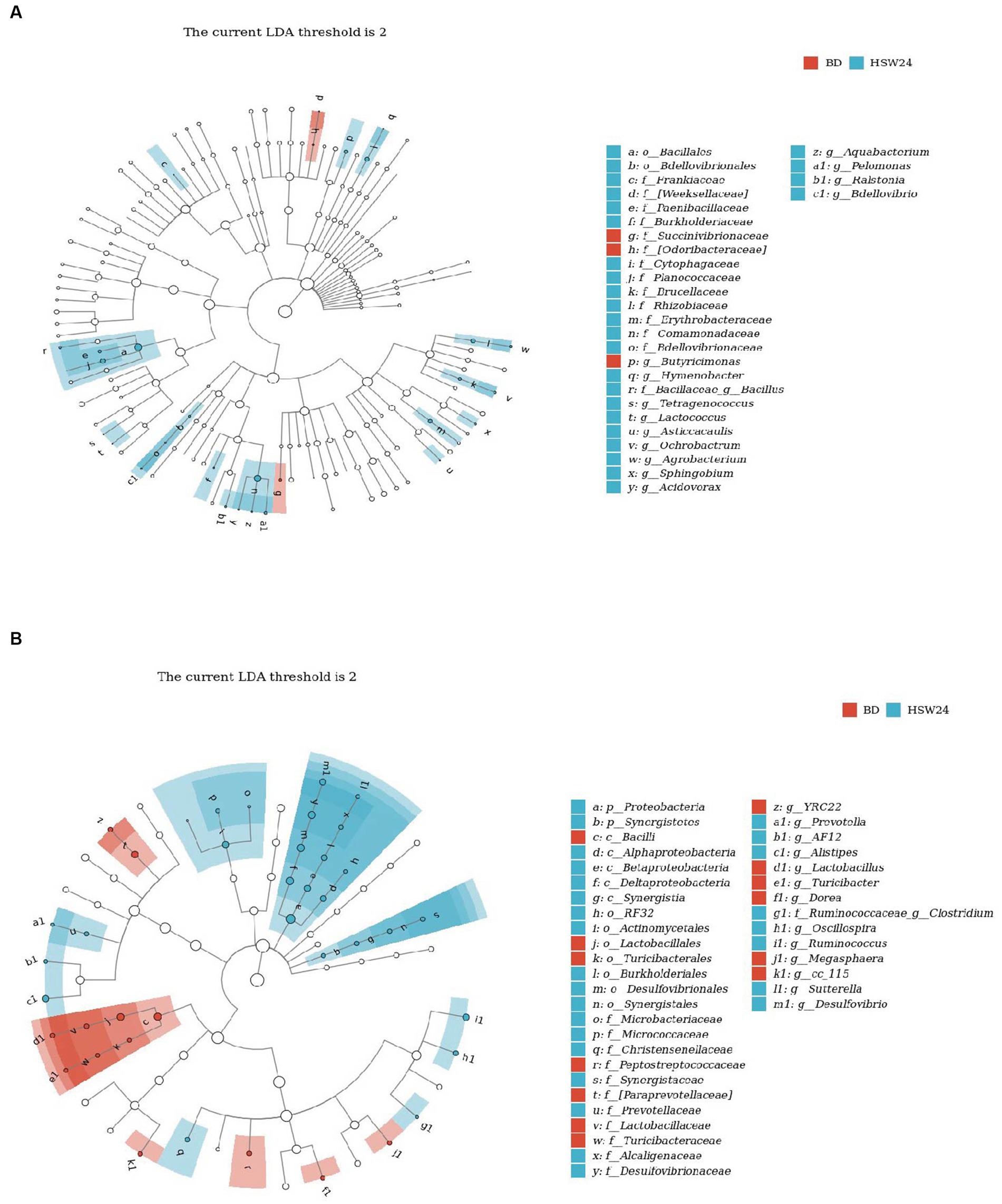
Figure 4. Effects of HMS on changes in ileum (A) and cecum (B) microbiota of geese. Linear discriminant analysis (LDA) (LDA > 2.0, p < 0.05) of cecal microbiomes.
By Spearman analysis, we found among the ileum biomarkers (Figure 5A), Ochrobactrum was positively associated with ADG and CAT, and negatively associated with ALT and MDA. Pelomonas was positively associated with ADG, CAT, GSH-Px, and T-AOC, and negatively associated with ALT. Agrobacterium was positively associated with ADG, CAT, and T-AOC, and negatively associated with ALT and MDA. Butyricicoccus was negatively associated with BUN and AST; Lactococcus was negatively associated with TG; Bacillus was positively associated with ADG, TP, ALB, GLOB, HDL-C, and AKP, and negatively associated with TC. Sphingobium was positively associated with ADFI, GLOB, CAT, GSH-Px, and T-AOC. Among the biomarkers of the cecum (Figure 5B), Desulfovibrio was positively associated with ADG, ALB, GLOB, and HDL-C, and negatively associated with UA, TC, TG, and LDL-C. Oscillospira was positively associated with GSH-Px and CAT, and negatively associated with UA; Alistipes was positively associated with CAT; Butyricicoccus was positively associated with TG. YRC22 was positively associated with TG and AST, and negatively associated with GSH-Px and T-AOC. Megasphaera was positively associated with TG, LDL-C, and MDA, and negatively associated with GLOB, CAT, GSH-Px, and T-AOC. Prevotella was positively associated with ADFI, GSH-Px, and CAT, and negatively associated with LDL-C levels. Dorea was negatively associated with GSH-Px, and positively associated with TG. AF12 was positively associated with TP, CAT, GSH-Px, and T-AOC and negatively associated with TG. Sutterella was positively associated with T-AOC and negatively associated with UA, ALT, and MDA. Clostridium was positively associated with ADG, ADFI, TP, ALB, GLOB, HDL-C, CAT, GSH-Px, and T-AOC, and negatively associated with TC, TG, and LDL-C.
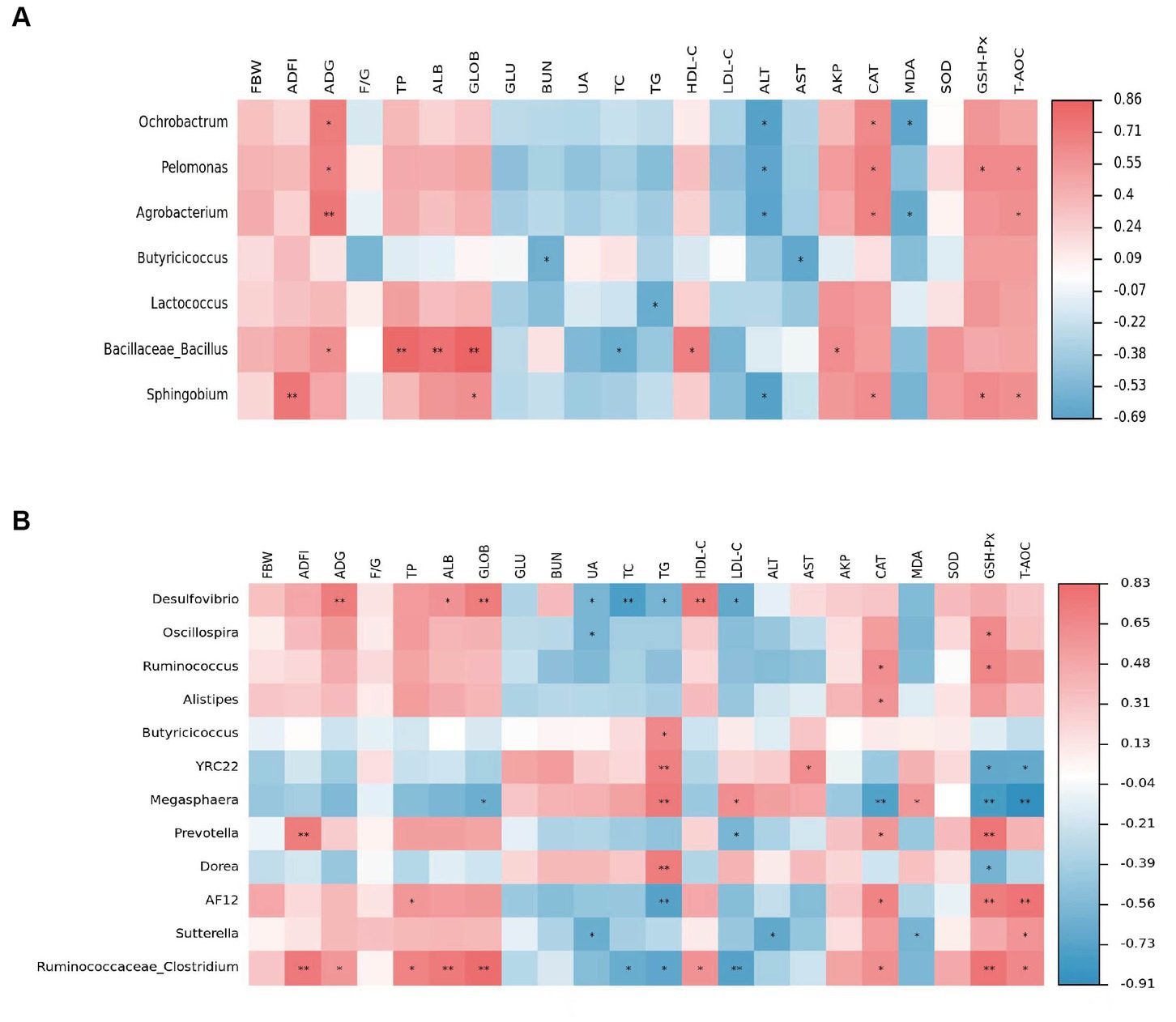
Figure 5. Relationships between main parameters and genera in the ileum (A) and cecum (B). Red represents positive correlation, blue represents negative correlation, and *p < 0.05 and **p < 0.01 represent significant correlations.
Palatability refers to the positive response of animals to the taste of particular foods. In animal husbandry, the average daily feed intake is the main index reflecting food palatability, which is usually used to evaluate feed quality (Kondo et al., 1988; Brown et al., 2016). Animal feed intake is mainly affected by the degree of feed crushing and crude fiber level (Ginindza et al., 2017; Sagols et al., 2019). In poultry, smell and taste are important factors affecting feed intake (Niknafs and Roura, 2018). Mushroom aroma is mainly derived from volatile aldehydes, acids, ketones, and esters. The umami taste of mushrooms originates from guanylates, taste amino acids, and umami peptides (Shen et al., 2023). Some studies have found that using waste stems from edible fungi (Hwangbo, 2014; Zheng et al., 2022) can improve the feed intake of animals, which is similar to the results of the present study, possibly because mushrooms contain different polysaccharides, such as chitin, hemicellulose, mannan, α-glucan, β-glucan, galactan, and xylan (Singdevsachan et al., 2016). The gut contains various bacteria that are capable of hydrolyzing polysaccharides and converting them from macromolecular polymers to small molecules, thereby promoting host health (Shang et al., 2018; Sun et al., 2018). Although adding HSW can improve feed intake and average daily gain from 35 to 49 days, we also noticed that there was no difference in the final body mass among the groups, and the feed-to-weight ratio of the HSW32 group was 8.6% higher than that of the BD group. This could be attributed to the crude fiber levels of 8.39% in the HSW32 group and 2.56% in the BD group. The production performance of geese is best when the crude fiber level is 4–7% (Li et al., 2022; Zheng et al., 2022). Although the goose has a more developed cecum compared to other poultry, its ability to decompose cellulose is not well developed, therefore we think that adding 32% HSW has a negative effect on the F/G.
TP, ALB, and serum levels are related to liver physiological function and body protein metabolism (Tasharofi et al., 2018). GLOB is an important indicator of the immune ability of the body. An increase in its content is indicative of the body’s immunity (Tsang et al., 2011). In this study, we found that TP, ALB, and GLOB increased with an increase in HSW addition, and the effect was the best when the HSW addition was 24%. Previous studies have found that adding fermented Flammulina velutipes mycelia can improve crude protein digestibility (Lee et al., 2014), and that adding mushroom powder to diets can improve nitrogen digestibility. This may be the reason for the increased serum protein content. Simultaneously, we found that HSW had a positive effect on reducing TC and TG levels in goose serum. It is possible that HSW has a higher level of crude fiber, which improves lipid metabolism and lowers TC levels (Massa et al., 2022). An appropriate amount of crude fiber can improve serum TC levels, and studies have shown that 10% crude fiber in broiler diets can cause a significant reduction (Sethi and Sikka, 2013). Elevated levels of serum TG and LDL-C have been shown associated with an increased risk of disease, such as increased abdominal fat and liver fat (Sun et al., 2007). Adding mushroom powder to the diet of broilers can reduce serum TC content (Daneshmand et al., 2012; Shang et al., 2014; Fanhani et al., 2016), which is consistent with the results of this study.
Mushrooms contain various phenolic compounds, which are generally believed to be related to antioxidant activity (Bayram and Karabacak, 2022; Moazzen et al., 2022), with phenolic compounds in mushrooms reportedly possessing the ability to remove LDL-C (Tang et al., 2016), Mushrooms also contain vitamin C and selenium, which have antioxidant properties (Lauridsen et al., 2021; Rathore et al., 2022). Six antioxidants can be extracted from H. marmoreus (Cai et al., 2020), and polysaccharides extracted from H. marmoreus can improve the antioxidant status by enhancing CAT, SOD, GSH-Px, and T-AOC, and reducing MDA (Liu et al., 2018). Nowadays, the antioxidant properties of mushrooms are widely used in animal husbandry. For example, adding mushrooms (Hericium caput-medusae) to the diet of broiler chickens can increase the levels of SOD, GSH-PX, and CAT in serum, liver, and breast muscle, and reduce MDA (Shang et al., 2016). Adding Agaricus bisporus stem residues to the diet of laying hens can increase the levels of SOD and GSH-PX in the serum (Yang et al., 2021). Adding mushroom residues to broiler diets resulted in higher expression of the Nrf2 and SOD-1 antioxidant genes (Chuang et al., 2020, 2021). Therefore, we believe that adding HSW to the diet improves the antioxidant activity of the serum.
Carcass traits and meat quality are important indicators of animal health and breeding benefits, and diet composition and nutritional level are important factors (Meel et al., 2021; Sallam et al., 2021). Adding A. bisporus dry powder to broiler diets does not improve carcass traits (Kavyani et al., 2012), and the addition of F. velutipes residues to the diets of finishing pigs had no significant effect on their meat color (Liu et al., 2020). The results of our experiments showed that adding 32% HSW to the diet did not affect the quality of breast and leg muscles of geese.
The intestinal microbes of animals play important roles in nutrient digestion and absorption, immune status and gut homeostasis (Peng et al., 2021; Kogut and Fernández-Miyakawa, 2022; Norozi et al., 2022). The cecum is an ideal habitat for the microbes and has the highest density in the goose gut. The ileum has a unique microbiome, with fewer microbiota than the cecum. However, they can shape epithelial gene expression, gut physiology, nutrition, and molecular exchange between microbes (Yilmaz et al., 2022). In this study, Firmicutes, Bacteroidetes, and Proteobacteria were the dominant phyla in the goose cecum and ileum, which is similar to previous studies (Li et al., 2018; Xu et al., 2022). Many members of Firmicutes were highly positively associated with blood lipid levels and fat storage capacity (Turnbaugh et al., 2006; Zhang et al., 2020), which is also consistent with the TC and TG levels in serum. We noticed a decrease in the ratio of Firmicutes to Bacteroidetes in both the ileum and cecum, suggesting that F/B may be a factor in obesity in animals (Indiani et al., 2018). Lactobacillus and Bacteroides were the dominant bacterial genera in the ileum and cecum, and the abundance of the HSW24 group was higher than that of the BD group. These bacteria can break down complex polysaccharides, proteins, and lipids, thereby supporting the growth of other bacteria and maintaining intestinal homeostasis (Brown et al., 2019). Lactobacillus has a strong ability to metabolize carbohydrates to produce acid, synthesize glucan and heteropolysaccharides, and ferment sugars to produce lactic acid and acetic acid (Moestedt et al., 2020). Furthermore, they antagonize pathogenic bacteria and help maintain immune function at the same time (Wang et al., 2022).
Through LEfSe analysis, we found biomarkers in the ileum and cecum of the HSW24 group. Prevotella has been extensively studied for its ability to break down the cellulosic hemicellulose, secreting cellulase enzymes that help the host gut digest fiber (Dao et al., 2021). This may explain why its abundance was positively related with the ADFI in this study. Prevotella is a bacterium associated with health status (Du et al., 2022). Adding mushrooms or selenium-enriched mushroom powder to pig diets increased the abundance of Prevotella, which is the main SCFA-producing bacterium that metabolizes succinate and acetate (Shkoporov et al., 2015). Although Alistipes was not associated with serum lipid metabolism in this experiment, it is fatty acid utilizable (Radka et al., 2020) and inversely associated with obesity (Dai et al., 2022). Desulfovibrio is a sulfate-reducing bacterium, it was positively associated with serum immune-related GLOB in this study, probably due to its bioremedial potential (Rowan et al., 2010). In the gut of pigs, Desulfovibrio was also positively associated with the health state and growth performance (Jiang et al., 2022). Oscillospira are widely found in the digestive tracts of herbivores (Mackie et al., 2003; Ren et al., 2017), and from metagenomic and metabolic characterization, this organism was able to mediate butyrate kinase-mediated, which was indicated that Oscillospira are butyrate producers. Like Alistipes, Oscillospira are negatively associated with obesity (Shen et al., 2021). It is the dominant bacterium in the intestinal of chicken, which is a manifestation of animal production performance and intestinal function (Keerqin et al., 2021). We observed that although Oscillospira is not directly related to protein metabolism in the serum in this sudy, it is negatively associated with serum UA level, which is the main metabolite of birds and reptiles. High levels of UA in serum respond to abnormal kidney and liver metabolism in animals (Yustisia et al., 2022).
Bacillus has a variety of probiotic functions, similar to Bacillus amyloliquefaciens and Bacillus subtilis, which are widely used as probiotics in animal husbandry (Jiang et al., 2022; Xu et al., 2022). Bacillus can induce Oscillospira to become a dominant genus (Keerqin et al., 2021) and can be used to treat diarrhea in pigs (Jinno et al., 2022). In this experiment, Bacillus was positively associated with growth performance, serum protein content, and antioxidant level, and negatively associated with serum fat and TC, as consistent with previous studies. The addition of B. subtilis to broiler diets can reduce TC and LDL-C levels (Mohamed et al., 2022). Ruminococcus obtains nutrients by decomposing cellulose in the host’s digestive system, making it is one of the most efficient bacterial genera for decomposing carbohydrates (Iakiviak et al., 2016). It is the key bacterium for degrading resistant starch (Sun et al., 2016), and members of the genus Sutterella are important commensals in the gut. Sutterella, which is abundant in the duodenum of healthy adults, has been found to increase the abundance of feces in mice fed prebiotics (Nowak et al., 2018). Therefore, we conclude that HSW-supplemented diets have a positive gut response in geese.
Partial substitution of corn with HSW in geese diets had no negative effects on growth performance, carcass traits, and meat quality. The addition of 24% HSW improved serum lipid metabolism and antioxidant levels, which may be related to the increased abundance of probiotics such as Prevotella, Oscillospira, Alistipes, and Ruminococcus in the cecum. However, the functions of these bacteria require further investigation to verify the links between HSW and intestinal bacteria. Further research is also needed to determine the underlying mechanism of HSW in lipid metabolism in geese.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA935563.
The animal study was reviewed and approved by the Laboratory Animal Ethics Committee of the Shanghai Academy of Agricultural Sciences.
GL, YL, XW, DH, and HW contributed to the design of this study. GL, YL, CW, YY, and SG participated in the sample collection and data analysis. DH and HW provided the funding and analytical tools. GL wrote the original draft. All authors contributed to the article and approved the submitted version.
This research was funded by the Climbing Plan of the Shanghai Academy of Agricultural Sciences (PG21171), SAAS Program for Excellent Research Team (2022–021), and China Agriculture Research System of MOF and MARA (CARS-42-35).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bayram, Y., and Karabacak, C. E. (2022). Characterization of unripe grapes (Vitis vinifera L.) and its use to obtain antioxidant phenolic compounds by green extraction. Front. Sustain. Food. Sys. 6:909894. doi: 10.3389/fsufs.2022.909894
Brown, E. M., Ke, X., Hitchcock, D., Jeanfavre, S., Avila-Pacheco, J., Nakata, T., et al. (2019). Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host. Microbe. 25, 668–680.e7. doi: 10.1016/j.chom.2019.04.002
Brown, D., Ng'ambi, J. W., and Norris, D. (2016). Voluntary intake and palatability indices of Pedi goats fed different levels of Acacia karroo leaf meal by cafeteria method. Indian J. Anim. Res. 50, 41–47. doi: 10.18805/ijar.5542
Cai, X., Xiao, M., Zou, X., Tang, J., Huang, B., and Xue, H. (2020). Separation of six antioxidants from Hypsizygus marmoreus by high-speed countercurrent chromatography utilizing an approach based upon the polarity parameter model. J. Chromatogr. A 1633:461650. doi: 10.1016/j.chroma.2020.461650
Chen, S. X., Li, B., Pan, Q. M., Xiao, M. X., and Dong, Y. F. (2018). Effects of Flammulina velutipes mushroom residue on production performance, calcium metabolism, immune performance and antibody titer of laying hens. China Feed. 14, 88–92. doi: 10.15906/j.cnki.cn11-2975/s.20181420
Chuang, W. Y., Lin, L. J., Hsieh, Y. C., Chang, S. C., and Lee, T. T. (2021). Effects of Saccharomyces cerevisiae and phytase co-fermentation of wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Anim Biosci. 34, 1157–1168. doi: 10.5713/ajas.20.0399
Chuang, W. Y., Liu, C. L., Tsai, C. F., Lin, W. C., Chang, S. C., Shih, H. D., et al. (2020). Evaluation of waste mushroom compost as a feed supplement and its effects on the fat metabolism and antioxidant capacity of broilers. Animals 10:445. doi: 10.3390/ani10030445
Dai, K., Song, Y., Zhang, D., Wei, Y., Jiang, S., Xu, F., et al. (2022). Thinned peach polyphenols alleviate obesity in high fat mice by affecting gut microbiota. Food Res. Int. 157:111255. doi: 10.1016/j.foodres.2022.111255
Daneshmand, A., Sadeghi, G. H., and Karimi, A. (2012). The effects of a combination of garlic, oyster mushroom and propolis extract in comparison to antibiotic on growth performance, some blood parameters and nutrients digestibility of male broilers. Braz. J. Poult. Sci. 14, 141–147. doi: 10.1590/S1516-635X2012000200009
Dao, T. K., Do, T. H., Le, N. G., Nguyen, H. D., Nguyen, T. Q., Le, T. T. H., et al. (2021). Understanding the role of Prevotella genus in the digestion of lignocellulose and other substrates in Vietnamese native goats rumen by metagenomic deep sequencing. Animals 11:3257. doi: 10.3390/ani11113257
Du, S., Bu, Z., You, S., Bao, J., and Jia, Y. (2022). Diversity of growth performance and rumen microbiota vary with feed types. Front. Sus. Food Sys. 6:1004373. doi: 10.3389/fsufs.2022.1004373
Fanhani, J. C., Murakami, A. E., Guerra, A. F. Q. G., do Nascimento, G. R., Pedroso, R. B., and Alves, M. C. F. (2016). Effect of Agaricus blazei in the diet of broiler chickens on immunity, serum parameters and antioxidant activity. Semina 37, 2235–2246. doi: 10.5433/1679-0359.2016v37n4p2235
Gheorghe, A., Habeanu, M., Tabuc, C., Dumitru, M., and Lefter, N. A. (2017). Blood parameters, digestive organ size and intestinal microflora of broiler chicks fed Sorghum as partial substitute of corn. Bulletin of the University of Agricultural Sciences & veterinary medicine Cluj-Napoca. Anim. Sci. Biotechnol. 74, 162–168. doi: 10.15835/buasvmcn-asb:0026
Ginindza, M. M., Ng'Ambi, J. W., and Norris, D. (2017). Effect of dietary crude fibre level on intake, digestibility and productivity of slow-growing indigenous Venda chickens aged one to 91 days. Indian J. Anim. Res. 51, 1073–1079. doi: 10.18805/ijar.v0iOF.7255
Guo, F. C., Savelkoul, H. F. J., Kwakkel, R. P., Williams, B. A., and Verstegen, M. W. A. (2003). Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World Poult. Science. J. 59, 427–440. doi: 10.1079/WPS20030026
Hwangbo, S. (2014). Effects of Total mixed fermentations with spent mushroom (Flammuliua velutipes) and wet Brewer's grain on growth performance, feed intake and nutrient digestibility in Korean black goats. J. Korean Soc. Grassland Forage. Sci. 34, 45–51. doi: 10.5333/KGFS.2014.34.1.45
Iakiviak, M., Devendran, S., Skorupski, A., Moon, Y. H., Mackie, R. I., and Cann, I. (2016). Functional and modular analyses of diverse endoglucanases from Ruminococcus albus 8, a specialist plant cell wall degrading bacterium. Sci. Rep-UK. 6, 1–13. doi: 10.1038/srep29979
Indiani, C. M. D. S. P., Rizzardi, K. F., Castelo, P. M., Ferraz, L. F. C., Darrieux, M., and Parisotto, T. M. (2018). Childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: a systematic review. Child. Obes. 14, 501–509. doi: 10.1089/chi.2018.0040
Jeong, I., Na, S. W., Kang, H. J., Park, S. J., Jung, D. J. S., Beak, S. H., et al. (2022). Partial substitution of corn grain in the diet with beet pulp reveals increased ruminal acetate proportion and circulating insulin levels in Korean cattle steers. Animals 12:1419. doi: 10.3390/ani12111419
Jiang, Z., Su, W., Li, W., Wen, C., Du, S., He, H., et al. (2022). Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim Nutr. 12, 116–127. doi: 10.1016/j.aninu.2022.09.006
Jinno, C., Li, X., and Liu, Y. (2022). Dietary supplementation of Bacillus subtilis or antibiotics modified intestinal microbiome of weaned pigs under enterotoxigenic Escherichia coli infection. Front. Microbiol. 13:1064328. doi: 10.3389/fmicb.2022.1064328
Kavyani, A., Zare, S. A., PorReza, J., and Jalali, H. A. S. A. (2012). Evaluation of dried powder of mushroom (Agaricus bisporus) as an antibiotic growth promoter substitution on performance, carcass traits and humoral immune responses in broiler chickens. J. Med. Plants Res. 6, 94–100. doi: 10.5897/JMPR11.1168
Keerqin, C., Rhayat, L., Zhang, Z. H., Gharib-Naseri, K., Kheravii, S. K., Devillard, E., et al. (2021). Probiotic Bacillus subtilis 29,784 improved weight gain and enhanced gut health status of broilers under necrotic enteritis condition. Poult. Sci. 100:100981. doi: 10.1016/j.psj.2021.01.004
Kim, S., Cho, J. H., Kim, Y., Kim, H. B., and Song, M. (2021). Effects of substitution of corn with ground brown rice on growth performance, nutrient digestibility, and gut microbiota of growing-finishing pigs. Animals 11:375. doi: 10.3390/ani11020375
Kogut, M., and Fernández-Miyakawa, M. E. (2022). Functional mechanisms at the avian gut microbiome-intestinal immunity interface and its regulation of avian physiological responses. Front. Physiol. 13:1063102. doi: 10.3389/fphy5.20221063102
Kondo, S., Maeda, T., Nishino, S., and Asahida, Y. (1988). Relationships among voluntary intake, eating rate and palatability of forage in sheep. Jpn. J. Lives. Manag. 24, 57–61. doi: 10.20652/kachikunokanri.24.2_57
Lauridsen, C., Schönherz, A. A., and Højsgaard, S. (2021). Effect of maternal dietary redox levels on antioxidative status and immunity of the suckling off-spring. Antioxidants. 10:478. doi: 10.3390/antiox10030478
Lee, S., Im, J. T., Kim, S., Kim, Y., Kim, M., Lee, J., et al. (2014). Effects of dietary fermented Flammulina velutipes mycelium on performance and egg quality in laying hens. Int. J. Poult. Sci. 13, 637–644. doi: 10.3923/ijps.2014.637.644
Li, H., Liu, Y., Wei, L., Lin, Q., and Zhang, Z. (2022). Effects of feeding fermented Medicago sativa (plus soybean and DDGS) on growth performance, blood profiles, gut health, and carcass characteristics of Lande (meat) geese. Front. Physiol. 13:902802. doi: 10.3389/fphys.2022.902802
Li, C., and Xu, S. (2022). Edible mushroom industry in China: current state and perspectives. Appl. Microbiol. Biotechnol. 106, 3949–3955. doi: 10.1007/s00253-022-11985-0
Li, Y., Yang, H., Xu, L., Wang, Z., Zhao, Y., and Chen, X. (2018). Effects of dietary fiber levels on cecal microbiota composition in geese. Asian Austral. J. Anim. Sci. 31, 1285–1290. doi: 10.5713/ajas.17.0915
Limba, A. K., Dhuria, R. K., Sharma, T., and Nehra, R. (2019). Effect of feeding of hydroponic maize fodder on milk production in Rathi cows. Anim. Nutr. Feed. Techn. 19, 517–522. doi: 10.5958/0974-181X.2019.00047.7
Liu, M., Li, S., Wang, X., Zhu, Y., Zhang, J., Liu, H., et al. (2018). Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 111, 121–128. doi: 10.1016/j.ijbiomac.2018.01.010
Liu, X., Zhang, B., Liu, H., Zhang, G., Zhao, J., Liu, L., et al. (2020). Determination of the available energy values and amino acid digestibility of Flammulina velutipes stem waste and its effects on carcass trait and meat quality fed to growing-finishing pigs. J. Anim. Sci. Biotechnol. 11, 1–20. doi: 10.1186/s40104-020-00449-y
Liu, X., Zhao, J., Zhang, G., Hu, J., Liu, L., Piao, X., et al. (2020). Dietary supplementation with Flammulina velutipes stem waste on growth performance, fecal short chain fatty acids and serum profile in weaned piglets. Animals 10:82. doi: 10.3390/ani10010082
Lv, J., and Wu, X. (2022). Corn Price prediction in China's futures market during COVID-19. Acad. J. Bus. Manag. 4, 7–12. doi: 10.25236/AJBM.2022.041102
Mackie, R. I., Aminov, R. I., Hu, W., Klieve, A. V., Ouwerkerk, D., Sundset, M. A., et al. (2003). Ecology of uncultivated Oscillospira species in the rumen of cattle, sheep, and reindeer as assessed by microscopy and molecular approaches. Appl. Environ. Microbiol. 69, 6808–6815. doi: 10.1128/AEM.69.11.6808-6815.2003
Massa, M., Compari, C., and Fisicaro, E. (2022). On the mechanism of the cholesterol lowering ability of soluble dietary fibers: interaction of some bile salts with pectin, alginate, and chitosan studied by isothermal titration calorimetry. Front. Nutr. 9:968847. doi: 10.3389/fnut.2022.968847
Meel, M. S., Sharma, T., Joshi, M., Gurjar, M. L., Sharma, S. K., and Kumari, M. (2021). Effect of feeding Moringa oleifera leaf meal with multienzyme on performance, carcass characteristics and economics of production of broiler chicks. Asian J. Dairy Food Res. 40, 118–122. doi: 10.18805/ajdfr.DR-1612
Moazzen, A., Öztinen, N., Ak-Sakalli, E., and Koşar, M. (2022). Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 8:e10467. doi: 10.1016/j.heliyon.2022.e10467
Moestedt, J., Müller, B., Nagavara Nagaraj, Y., and Schnürer, A. (2020). Acetate and lactate production during two-stage anaerobic digestion of food waste driven by Lactobacillus and Aeriscardovia. Front. Energy Res. 8:105. doi: 10.3389/fenrg.2020.00105
Mohamed, T. M., Sun, W., Bumbie, G. Z., Dosoky, W. M., Rao, Z., Hu, P., et al. (2022). Effect of dietary supplementation of Bacillus subtilis on growth performance, organ weight, digestive enzyme activities, and serum biochemical indices in broiler. Animals 12:1558. doi: 10.3390/ani12121558
Niknafs, S., and Roura, E. (2018). Nutrient sensing, taste and feed intake in avian species. Nutr. Res. Rev. 31, 256–266. doi: 10.1017/S0954422418000100
Norozi, M., Rezaei, M., and Kazemifard, M. (2022). Effects of acid-hydrolyzed soybean meal on growth performance, jejunal morphology, digestive enzyme activities, nutrient utilization, and intestinal microbial population in broiler chickens. Trop Anim. Health Pro. 54, 162–110. doi: 10.1007/s11250-022-03167-x
Nowak, R., Nowacka-Jechalke, N., Juda, M., and Malm, A. (2018). The preliminary study of prebiotic potential of polish wild mushroom polysaccharides: the stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 57, 1511–1521. doi: 10.1007/s00394-017-1436-9
Peng, S., Wang, X., Wang, Y., Lv, T., Zhao, H., Wang, Y., et al. (2021). Effects of dietary Bacillus and non-starch polysaccharase on the intestinal microbiota and the associated changes on the growth performance, intestinal morphology, and serum antioxidant profiles in ducks. Front. Microbiol. 12:786121. doi: 10.3389/fmicb.2021.786121
Radka, C. D., Frank, M. W., Rock, C. O., and Yao, J. (2020). Fatty acid activation and utilization by Alistipes finegoldii, a representative Bacteroidetes resident of the human gut microbiome. Mol. Microbiol. 113, 807–825. doi: 10.1111/mmi.14445
Rathore, S. S., Hanumappa, S. M., Yusufzai, S. I., Suyani, N. K., Abdullah-Al-Mamun, M., Nasren, S., et al. (2022). Dietary Administration of Engineered Nano-selenium and Vitamin C Ameliorates Immune Response, nutritional physiology, oxidative stress, and resistance against Aeromonas hydrophila in Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 201, 4079–4092. doi: 10.1007/s12011-022-03473-3
Ren, T., Boutin, S., Humphries, M. M., Dantzer, B., Gorrell, J. C., Coltman, D. W., et al. (2017). Seasonal, spatial, and maternal effects on gut microbiome in wild red squirrels. Microbiome. 5, 163–114. doi: 10.1186/s40168-017-0382-3
Rowan, F., Docherty, N. G., Murphy, M., Murphy, B., Coffey, J. C., and O‘Connell, P. R. (2010). Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon Rectum 53, 1530–1536. doi: 10.1007/DCR.0b013e3181f1e620
Sagols, E., Hours, M. A., Daniel, I., Feugier, A., Flanagan, J., and German, A. J. (2019). Comparison of the effects of different kibble shape on voluntary food intake and palatability of weight loss diets in pet dogs. Res. Vet. Sci. 124, 375–382. doi: 10.1016/j.rvsc.2019.04.023
Sallam, E. A., Mohammed, L. S., Elbasuni, S. S., Azam, A. E., and Soliman, M. M. (2021). Impacts of microbial based therapy on growth performance, intestinal health, carcass traits and economic efficiency of Clostridium perfringens-infected cobb and arbor acres broilers. Vet. Med. Sci. 7, 773–791. doi: 10.1002/vms3.412
Sethi, A. P. S., and Sikka, S. S. (2013). Effect of fiber without and with enzyme supplementation on the productive parameters and egg cholesterol content in laying hens. Indian J. Poult. Sci. 48, 390–392.
Shang, H. M., Song, H., Jiang, Y. Y., Ding, G. D., Xing, Y. L., Niu, S. L., et al. (2014). Influence of fermentation concentrate of Hericium caput-medusae (bull.: Fr.) Pers. on performance, antioxidant status, and meat quality in broilers. Anim. Feed Sci. Technol. 198, 166–175. doi: 10.1016/j.anifeedsci.2014.09.011
Shang, H. M., Song, H., Xing, Y. L., Niu, S. L., Ding, G. D., Jiang, Y. Y., et al. (2016). Effects of dietary fermentation concentrate of Hericium caput-medusae (bull.: Fr.) Pers. on growth performance, digestibility, and intestinal microbiology and morphology in broiler chickens.J. Sci Food Agric. 96, 215–222. doi: 10.1002/jsfa.7084
Shang, Q., Wang, Y., Pan, L., Niu, Q., Li, C., Jiang, H., et al. (2018). Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 16:167. doi: 10.3390/md16050167
Shen, H., Huang, L., Dou, H., Yang, Y., and Wu, H. (2021). Effect of trilobatin from lithocarpus polystachyus rehd on gut microbiota of obese rats induced by a high-fat diet. Nutrients 13:891. doi: 10.3390/nu13030891
Shen, Q., Sun, L., He, Z., Xie, J., and Zhuang, Y. (2023). Isolation, taste characterization and molecular docking study of novel umami peptides from Lactarius volemus (Fr.). Food Chem. 401:134137. doi: 10.1016/j.foodchem.2022.134137
Shkoporov, A. N., Chaplin, A. V., Khokhlova, E. V., Shcherbakova, V. A., Motuzova, O. V., Bozhenko, V. K., et al. (2015). Alistipes inops sp. nov. and Coprobacter secundus sp. nov., isolated from human faeces. Int. J. Syst. Evol. Micr. 65, 4580–4588. doi: 10.1099/ijsem.0.000617
Singdevsachan, S. K., Auroshree, P., Mishra, J., Baliyarsingh, B., Tayung, K., and Thatoi, H. (2016). Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: a review. Bioact. Carb. Diet Fibre 7, 1–14. doi: 10.1016/j.bcdf.2015.11.001
Sun, X., Duan, M., Liu, Y., Luo, T., Ma, N., Song, S., et al. (2018). The beneficial effects of Gracilaria lemaneiformis polysaccharides on obesity and the gut microbiota in high fat diet-fed mice. J. Funct. Foods 46, 48–56. doi: 10.1016/j.jff.2018.04.041
Sun, T., and Li, W. (2021). Effects of mushroom residue on production performance, nutrient digestion and serum antioxidant performance of laying hens. China Feed. 18, 113–116. doi: 10.15906/j.cnki.cn11-2975/s.20211829
Sun, Q., Ma, J., Campos, H., Hankinson, S. E., Manson, J. E., Stampfer, M. J., et al. (2007). A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation 115, 1858–1865. doi: 10.1161/CIRCULATIONAHA.106.679985
Sun, Y., Su, Y., and Zhu, W. (2016). Microbiome-metabolome responses in the cecum and colon of pig to a high resistant starch diet. Front. Microbiol. 7:779. doi: 10.3389/fmicb.2016.00779
Tang, C., Hoo, P. C. X., Tan, L. T. H., Pusparajah, P., Khan, T. M., Lee, L. H., et al. (2016). Golden needle mushroom: a culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 7:474. doi: 10.3389/fphar.2016.00474
Tasharofi, S., Mohammadi, F., Amiri, N., and Nazem, M. N. (2018). Effects of intra-yolk-sac injection of dextrose and albumin on performance, jejunum morphology, liver and pectoral muscle glycogen and some serum metabolites of broilers. J. Anim. Physiol. Anim. Nutr. 102, 917–923. doi: 10.1111/jpn.12882
Tsang, C. L., Fan, Y. K., Chang, W. C., and Ju, J. C. (2011). Hematological characteristics and their correlation coefficients in adult New Zealand white rabbits. J. Anim. Diagn. Med. 1, 1–6.
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Volk, N., and Lacy, B. (2017). Anatomy and physiology of the small bowel. Gastrointest. Endosc. Clin. N. Am. 27, 1–13. doi: 10.1016/j.giec.2016.08.001
Wang, K. K., He, K. Y., Yang, J. Y., Liu, M. J., Guo, J. R., Liang, J. Y., et al. (2022). Lactobacillus suppresses tumorigenesis of oropharyngeal Cancer via enhancing anti-tumor immune response. Front. Cell Dev. Biol. 10:842513. doi: 10.3389/fcell.2022.842153
Williams, P. (2022). Corn fermented protein a commercially viable alternative protein for aquaculture and livestock. Anim-Sci Proc. 13, 87–88. doi: 10.1016/J.ANSCIP.2022.03.118
Wu, L., Nong, J., Zeng, D., and Li, Y. (2019). Effects of an integrated carbide slag-mushroom dreg-calcium superphosphate amendment on the stabilization process of Pb, cu, Zn and cd in contaminated soils. Sustainability 11:4957. doi: 10.3390/su11184957
Xu, P., Hong, Y., Chen, P., Wang, X., Li, S., Wang, J., et al. (2022). Regulation of the cecal microbiota community and the fatty liver deposition by the addition of brewers spent grain to feed of Landes geese. Front. Microbiol. 13:970563. doi: 10.3389/fmicb.2022.970563
Xu, S., Wang, F., Zou, P., Li, X., Jin, Q., Wang, Q., et al. (2022). Bacillus amyloliquefaciens SC06 in the diet improves egg quality of hens by altering intestinal microbiota and the effect is diminished by antimicrobial peptide. Front. Nutr. 9:999998. doi: 10.3389/fnut.2022.999998
Xu, X., and Zhang, Y. (2022). Commodity price forecasting via neural networks for coffee, corn, cotton, oats, soybeans, soybean oil, sugar, and wheat. Intel. Syst. Account, Finance Manag. 29, 169–181. doi: 10.1002/isaf.1519
Yamanaka, K. (1997). I. Production of cultivated edible mushrooms. Food Rev. Int. 13, 327–333. doi: 10.1080/87559129709541113
Yang, B., Zhao, G., Wang, L., Liu, S., and Tang, J. (2021). Effects of the Agaricus bisporus stem residue on performance, nutrients digestibility and antioxidant activity of laying hens and its effects on egg storage. Anim. Biosci. 34, 256–264. doi: 10.5713/ajas.19.0853
Yilmaz, B., Fuhrer, T., Morgenthaler, D., Krupka, N., Wang, D., Spari, D., et al. (2022). Plasticity of the adult human small intestinal stoma microbiota. Cell Host Microbe 30, 1773–1787.e6. doi: 10.1016/J.CHOM.2022.10.002
Yustisia, I., Tandiari, D., Cangara, M. H., Hamid, F., and Nu'man, A. S. (2022). A high-fat, high-fructose diet induced hepatic steatosis, renal lesions, dyslipidemia, and hyperuricemia in non-obese rats. Heliyon. 8:e10896. doi: 10.1016/j.heliyon.2022.e10896
Zhai, S. S., Li, M. M., Li, M. M., and Yang, L. (2020). Effect of dietary Moringa stem meal level on growth performance, slaughter performance and serum biochemical parameters in geese. J. Anim. Physiol. An. 104, 126–135. doi: 10.1111/jpn.13209
Zhang, J., Cheng, Y. T., Wang, F., Yuan, Y. C., Liu, A. F., Wan, K., et al. (2022). Effect of dietary yeast culture supplementation on the cecal microbiota modulation of geese. J. Appl. Poult. Res. 31:100271. doi: 10.1016/j.japr.2022.100271
Zhang, Y., Liu, Y., Li, J., Xing, T., Jiang, Y., Zhang, L., et al. (2020). Dietary corn-resistant starch suppresses broiler abdominal fat deposition associated with the reduced cecal Firmicutes. Poult. Sci. 99, 5827–5837. doi: 10.1016/j.psj.2020.07.042
Zheng, J., Liang, S., Zhang, Y., Sun, X., Li, Y., Diao, J., et al. (2022). Effects of compound Chinese herbal medicine additive on growth performance and gut microbiota diversity of Zi goose. Animals 12:2942. doi: 10.3390/ani12212942
Keywords: diet, growth, lipids, microorganisms, mushrooms, nutrition, proteins
Citation: Li G, Liu Y, Wang X, Gong S, Yang Y, Wang C, Wang H and He D (2023) Assessment of the influence on Hypsizygus marmoreus stem waste as a sustainable alternative to corn in Holdobagy geese dietary. Front. Sustain. Food Syst. 7:1154757. doi: 10.3389/fsufs.2023.1154757
Received: 31 January 2023; Accepted: 22 June 2023;
Published: 06 July 2023.
Edited by:
Maghsoud Besharati, University of Tabriz, IranReviewed by:
Doriana Eurosia Angela Tedesco, University of Milan, ItalyZhihua Ren, Sichuan Agricultural University, ChinaCopyright © 2023 Li, Liu, Wang, Gong, Yang, Wang, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiying Wang, eWpzaHl3YW5nQHNpbmEuY29t; Daqian He, ZGFxaWFuaGVAYWxpeXVuLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.