
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 02 February 2023
Sec. Agroecology and Ecosystem Services
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1119717
This article is part of the Research Topic Crop Pest Control and Pollination, volume II View all 14 articles
Introduction: Plant-associated microbes critically shape the dynamics of plant-and insect-associated communities. In previous studies, we reported that the yellow peach moth Conogethes punctiferalis (YPM) preferred to Penicillium digitatum-infected apples (PDA) for oviposition. However, the underlying mechanisms remains unclear.
Methods: In the present study, the behavioral and physiological experiments were conducted to determine how P. digitatum affects the oviposition selection of mated YPM females via altering host plant volatile organic compounds (VOCs).
Results: Mated YPM females were attracted to and laid more eggs on PDA than on non-infected apples (NIA), mechanically damaged apples (MDA), and P. digitatum in potato dextrose agar medium (PPD) in the oviposition selection experiments. Four-arm olfactometer assays further confirmed that odors in PDA were responsible for the attractiveness of mated YPM females. Further analyses showed that 38 VOCs were collected and identified from all treatments by GC-MS, with five specific VOCs (methyl 2-methylbutyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane) emitting from PDA. A principal component analysis (PCA) based on the absolute contents of 38 VOCs revealed a clear separation of PDA from NIA, MDA, and PPD. Moreover, when P. digitatum-induced specific VOCs were added to apples in individual or synthetic blends, there was a significantly higher percentage of mated YPM females to apples with individual or synthetic blends consisting of methyl 2-methylbutyrate, butyl caprylate, or n-tetradecane in Y-tube olfactometer experiments, suggesting that these three specific VOCs acted as predominant olfactory signals for mated YPM females to PDA.
Discussion: Taken together, the microbe P. digitatum was an important driver of the interactions between YPMs and host plants by altering plant volatiles. These findings may form the basis for developing attractant baits for field trapping YPMs in the future.
Plant-associated microbes are widely reported as important but overlooked drivers of host plant-herbivorous insect interactions, either direct effects of plant-associated microbes on herbivorous insects via the ingestion of microbes and/or microbial metabolites or indirect effects of plant-associated microbes via altering the host plant biochemistry (Eberl et al., 2018, 2019, 2020). The impacts of plant-associated microbes on herbivorous insects could further cascade up and down multiple trophic levels in the arthropod community at spatial scales ranging from patterns within single host plants to entire landscapes (Tack and Dicke, 2013). Consequently, it is meaningful to investigate tripartite interactions among plant-associated microbes, host plants, and herbivorous insects for improving our knowledge on the ecology and evolution of plant-microbe-insect interactions and designing more effective management strategies to control herbivorous insects in agroecosystems.
Plant-associated microbes exhibit diverse effects on the herbivorous insects' foraging behavior, such as the location and selection of host plants, including detrimental, beneficial, and neutral (Kopper et al., 2004; Witzgall et al., 2012). Furthermore, the changes in host plant volatile organic compounds (VOCs) caused by plant-associated microbes infection could be responsible for the alteration of herbivorous insects' foraging behavior (Groen et al., 2016; Rizvi et al., 2016; Grunseich et al., 2019). For example, the bacteria on the egg-surface, such as Providencia sp. and Klebsiella sp., increase the relative content of β-caryophyllene in host plant VOCs, and result in deterring the oviposition of Bactrocera dorsalis (Li et al., 2020). Interestingly, a recent study shows that Lymantria dispar L. are attracted to volatiles from rust spores (Melampsora laricipopulina) (Eberl et al., 2018), suggesting that VOCs emitted by both host plants and the microbes themselves are important for establishing tripartite interactions among herbivorous insects, host plants, and plant-associated microbes (Tasin et al., 2012; Fernandez-Conradi et al., 2018). Our previous results also found that mated yellow peach moth (Conogethes punctiferalis, YPM) females preferred to Penicillium fungi-infected apples, including Penicillium sumatrense-infected apples, Penicillium citrinum-infected apples, Penicillium digitatum-infected apples, and the components and proportions of apples' VOCs were changed by Penicillium fungi-infection (Shi et al., 2019; Guo et al., 2022). Furthermore, P. digitatum-infected apples were more attraction to mated YPM females than other two Penicillium fungi-infected apples, triggering us to explore which components of VOCs in P. digitatum-infected apples or P. digitatum itself were crucial for mediating the foraging preference of YPM females to Penicillium-infected apples.
The YPM is a generalist herbivorous insect and a serious pest in tropical and eastern Asia, and Australia. Damage caused by the tunneling of YPM larvae into fruits results in serious loss of apples, corns, chestnut, and other crops (Li et al., 2015; Du et al., 2016). Recent efforts for controlling this pest have been focused on modulating male behavior by sex pheromones (Xiao et al., 2012; Du et al., 2014). However, the current strategies of interrupting their normal mating with sex pheromones are useless for mated YPM females. Other strategies, including plant-derived attractants and/or repellents as allelochemicals that selectively manipulate the behavior of YPM females, demand for the integrated pest management of YPMs (Xiao et al., 2012; Luo and Honda, 2015a). P. digitatum, an important and common phytopathogen of citrus fruits in the postharvest period around the world, causes citrus green mold disease with the deterioration and rotting of citrus fruits (Bhatta, 2022). Furthermore, the orange-originated P. digitatum fungus could infect apple fruits with the typical symptom described in other studies (Shi et al., 2019), suggesting the infection capacity of the orange-originated P. digitatum fungus on apple fruits.
Therefore, further understanding the principle of chemical ecology about the effects of plant-associated microbes on YPMs might be meaningful for developing attractants based on bioactive host plant VOCs to trap YPM females. Keeping the above in view, we determined the effect of P. digitatum on the VOCs of apples, and the cascading effects on the host preference of mated YPM females. Our specific objectives were to determine (1) the oviposition selection and behavioral responses of YPM females among the non-infected apples (NIA), mechanically damaged apples (MDA), P. digitatum-infected apples (PDA), and P. digitatum in potato dextrose agar medium (PPD); (2) the differences of VOCs from NIA, MDA, PDA, as well as PPD; (3) which VOCs were key components for affecting oviposition behavior of YPMs.
A colony of YPMs was established and had been maintained for about 25 generations on maize in climate incubators (RTOP-B, Zhejiang Top Instrument Co., Ltd.) at 23 ± 1°C, RH 75 ± 2%, 16L/8D photoperiod, and 3, 500 lux light intensity (Guo et al., 2021). Adult moths were provided with 5–8% honey solution after emergence. Apples covered with gauze pieces were provided for the oviposition of mated YPM females in the cage.
The P. digitatum isolated from orange fruits were purified using the traditional tissue separation method (Shi et al., 2019). The symptomatic–asymptomatic junction tissue was cut into segments (about 0.5 × 0.5 cm2), which was immersed completely into 2% sodium hypochlorite for 3 min and flushed with sterilized water three times, and then were immersed completely into 75% ethanol for 1 min and flushed with sterilized water for three times. Finally, the segments were incubated onto potato dextrose agar (containing (g/L): potato 200; dextrose 20; agar 18) medium in Petri dishes and placed in a constant temperature incubator at 28°C. After repeated purification for four times, the pure culture of P. digitatum was obtained. Potato dextrose agar medium (7 mm diameter) with fully grown P. digitatum (PPD) was also prepared and incubated at 28°C for 6 d before behavioral assays.
The P. digitatum was cultured on potato dextrose agar at 25°C to prepare the conidial suspension. The potato dextrose agar culture of P. digitatum bearing 7-d-old conidia was gently rinsed in 1.5 mL sterilized distilled water and the density of conidial suspension was adjusted to 8 × 107 conidia/mL.
Apple (Malus pumila, Red fuji variety) fruits with uniform size (7–9 cm diameter) and shape were bought from the supermarket of Beijing University of Agriculture (Beijing, China) and were stored at 4°C in a refrigerator. Apple fruits with uniform size were firstly sterilized using 75% alcohol for 1 min, and then 1% sodium hypochlorite for 3 min, at last washed with sterilized distilled water under the horizontal-laminar airflow clean bench for further experiments.
Apple fruits with uniform size were sterilized as NIA treatment. Two holes (7 mm diameter) at the opposite sides of each apple were punched and immediately stuffed using sterilized fungus-free potato dextrose agar medium.
Apple fruits with uniform size were sterilized as NIA treatment. Two holes were punched as MDA, and then stuffed using potato dextrose agar medium with fully grown P. digitatum.
After treatments, each apple was placed into a sterilized plastic box (25 × 18 × 12 cm) and incubated at 28°C for 2, 4, 6, and 8 d before used for following behavioral assays. Considering to the fact that the apples infected by P. digitatum for 8 d or longer would become rotten in the following days, the apples infected for 6 d were, therefore, used in the later experiments.
To test the effects of P. digitatum on the oviposition behavior of mated YPM females, four treatments (NIA, MDA, PDA, and PPD) were simultaneously offered in a wood-frame cage (35 × 27 × 25 cm) with plastic gauzes on side walls to allow the oviposition of mated YPM females. The experimental materials (NIA, MDA, PDA, and PPD) were individually put into two opposite plastic bowls that were punched holes in advance. The materials were renewed every day and their positions were randomly changed. Ten newly emerged naive females and 15 males (no exposure to natural or synthetic sources of apples or fungi-infected apples) had been released into a cage to copulate for 3–4 days before the oviposition selection experiments. Each experiment was replicated 20 times with a total number of 200 females. The egg numbers on each sheet were counted separately and the data were statistically treated on the basis of average number of eggs by 10 females.
Four-arm olfactometer was used to test two bioassay experiments about the behavioral responses of mated YPM females, one is to the odors from NIA, MDA, PDA, and PPD, the other is to different concentrations (10−1, 10−2, 10−3, and 10−4 (v/V)) of the same specific VOCs from P. digitatum-infected apples. According to our previous study, the four-arm olfactometer consists of four glass chambers (2.5 cm diameter, 10.0 cm long), each with terminal end coupling to a cap (3.0-cm-long arms, 2.0 cm diameter) and central converging into a 10-cm-long common arm (2.5 cm diameter) (Shi et al., 2019). Moistened and charcoal-filtered air was pumped through each odor and then respectively went into one of the caps at a rate of 250 ml/min controlled by flow meters (LZYIA Instrument Co. Ltd, China). One moth was introduced into the entrance of the common arm of the olfactometer using a glass vial, and its behavioral response was observed under a 25-W red light lamp. The test for each moth lasted 3 min, and the behavioral response was classified as a choice if the moth passed over 1/3 length of the arm associated with one of the four odors and stayed there for more than 30 s. Conversely, no-choice was assigned if the test moth remained in the common arm for 3 min. The position of the lateral chambers along with the olfactometer was systematically exchanged after testing 2 moths to avoid positional bias. The olfactometer was flushed following Du et al. (2016). The moths used for test were allowed to acclimatize to the test conditions for 2 h before the start of the test. 3- to 4-day-old mated females were used and each individual moth was used only once. The selection rate in the four-arm olfactometer experiment was defined as the number of females that made a selection for the odor divided by the total number of females that made a selection for any odors offered simultaneously.
The experiment 1: the behavioral responses of mated YPM females to the odors from NIA, MDA, PDA, and PPD. Three apples of each treatment (NIA, MDA, PDA) and 4 pieces of PPD (diameter 7 mm) were placed into an oven bag (Reynolds Kitchens, Richmond, VA, USA) respectively. Each oven bag was tightened and connected with a Teflon tube to one of the caps of the four-arm olfactometer for behavioral tests. A total of 211 individual mated YPM females were tested.
The experiment 2: the behavioral responses of mated YPM females to different concentrations of the five specific VOCs from P. digitatum-infeced apples. For each VOC, four concentrations (10−1, 10−2, 10−3, and 10−4 (v/V)) were prepared with mineral oil as solvent. And then, an aliquot of 10 μl test solution for each of the four concentrations of the same compound was applied onto a 1 × 5 cm filter paper, which was thereafter placed into one chamber of the four-arm olfactometer for behavioral tests. Totally, 80 mated YPM females (styrene), 86 mated YPM females (methyl 2-methylbutyrate), 80 mated YPM females (methyl caproate), 85 mated YPM females (butyl caprylate), and 80 mated YPM females (n-tetradecane) were tested.
All chemicals (purities ≥ 95%) were purchased from commercial companies, which methyl 2-methyl butyrate and styrene were from J & K Chemical Ltd. (Shanghai, China), methyl caproate, butyl caprylate, and n-tetradecane from TCI Development Co., Ltd. (Shanghai, China).
Five apples of each treatment (NIA, MDA, PDA) and correspondingly similar size of PPD that were placed into a 48.2 × 59.6 cm oven bag respectively were used to collected VOCs according to dynamic headspace collection method reported by Guo et al. (2021).
The bag mouth was tightened with a twist tie around a glass tube (6 mm diameter, 10 cm long) filled with 50 mg of Porapark Q adsorbent (80–100 mesh, Waters Corporation). The humidified and purified air was pushed at a rate of 450 ml/min by a QC-1S pump (Labor Protection Science Research Institute of Beijing) into the bag through the Teflon tube and was pulled out through the glass tube filled with Porapark Q adsorbent. VOCs were trapped by the Porapark Q adsorbent when they passed through the glass tube.
After collection, the trapped VOCs were eluted using chromatography-grade n-hexane (99.9%) and then were analyzed using an Agilent 6,890 gas chromatograph (GC) coupled to an Agilent 5,975 Mass Spectrometer (MS). The procedures for the GC-MS analysis were the same as described in Du et al. (2016) with the exceptions that the GC was equipped with a DB-5MS column (60 m × 0.25 mm × 0.15 μm, Agilent, USA) rather than a HP-5MS column (30 m × 0.25 mm × 0.25 μm) and the injector temperature was 250°C other than 210°C. Following injection, the column temperature was maintained at 37°C for 6 min, followed by an increase in temperature of 2°C/min to 70°C for 5 min, and then an increase of 5°C/min up to 200°C, at last maintained at 200°C for 5 min. Compounds were tentatively identified by comparing mass spectra with NIST Standard Reference Database 98 (Agilent Technologies, Palo Alto, CA, USA). Compounds were quantified by their total ion abundance relative to that of the internal standard (n-nonyl acetate).
Five specific VOCs from PDA, including methyl 2-methyl butyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane, were chosen for electroantennogram (EAG) measurements. All chemicals (purities ≥ 95%) were purchased from commercial companies, which methyl 2-methyl butyrate and styrene were from J & K Chemical Ltd. (Shanghai, China), methyl caproate, butyl caprylate, and n-tetradecane from TCI Development Co., Ltd. (Shanghai, China). Four concentrations [10−1, 10−2, 10−3, and 10−4 (v/V)] of five individual compounds were prepared as the four-arm olfactometer experiment 2. The test solutions were stored at −20°C for further EAG analyses.
EAG recordings were performed on 3- to 4-day-old mated YPM females that the moths at this stage were eggs-loading and sensitive to signals used for oviposition location (Belmain et al., 2002). The method of EAG recordings was the same as that described by Du et al. (2016). Stimulus was delivered and tested in increasing doses on the antennae of mated YPM females with mineral oil and n-hexanol being used as control and standard stimuli, respectively. EAG test was run for a variable number of replicates per day, and each compound at each concentration was tested on 15 antennae. In each test, the control and standard stimuli were applied subsequently after four successive stimulations. Normalization was achieved by dividing the peak EAG amplitude of the test puff with the average EAG amplitude of the two nearest standard stimulations after subtracting the amplitude recorded in response to the mineral oil.
The preference of mated YPM females to apples with or without five specific VOCs (methyl 2-methylbutyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane) from PDA was tested using Y-tube olfactometer. For Y-tube olfactometer assays, apples with and without five specific VOCs from PDA [the amount was referred to the concentrations in fungi-infected apple fruits (Supplementary Table S1)] were separately placed into the chambers of the Y-tube. The test procedure was similar to that in our previous study (Guo et al., 2022). Each individual moth was used only once, and totally 80 mated female moths were tested for each treatment. The selection rate in the Y-tube olfactometer experiment was defined as the number of females that made a selection for apples with exogenous compounds divided by the total number of females that made a selection between apples with and without exogenous compounds.
Data obtained from oviposition selection experiments, behavioral assay in four-arm olfactometers, EAG tests, and the absolute content of host plant VOCs were subjected to analysis of variance (ANOVA) using Tukey-HSD test (P < 0.05). The data of Y-tube olfactometer experiments were analyzed using non-parametric Chi-square analysis (Females with no choice were excluded from statistical analyses, P < 0.05). The quantification of VOCs measured as the absolute content of each compound was analyzed using principal component analysis (PCA) by the software program SIMCA P+ 11.0 (Umetrics AB, Umeå, Sweden) (P < 0.05). All statistics except the PCA analysis were performed using the SPSS16.0 statistical software. Graphs were generated in the program of Graphpad Prism 9.0.
In order to assess the effects of P. digitatum on the oviposition selection of mated YPM females, the apples infected with P. digitatum for 2, 4, 6, and 8 d were simultaneously provided to allow the oviposition selection of mated YPM females. The results showed that the number of eggs on the 8d-PDA was significantly higher than those on the 2d- and 4d-PDA, and was larger (but not significant) than that on the 6d-PDA (F3, 79 = 11.699, P < 0.01; Figure 1A). When NIA, MDA, PDA, and PPD were simultaneously offered in a cage to allow for oviposition, the average number of eggs laid by 10 mated YPM females (173.2) was significantly higher on PDA than those on MDA (22.3), NIA (22.2), and PPD (5.7) (F3, 79 = 111.413, P < 0.01; Figure 1B), respectively.
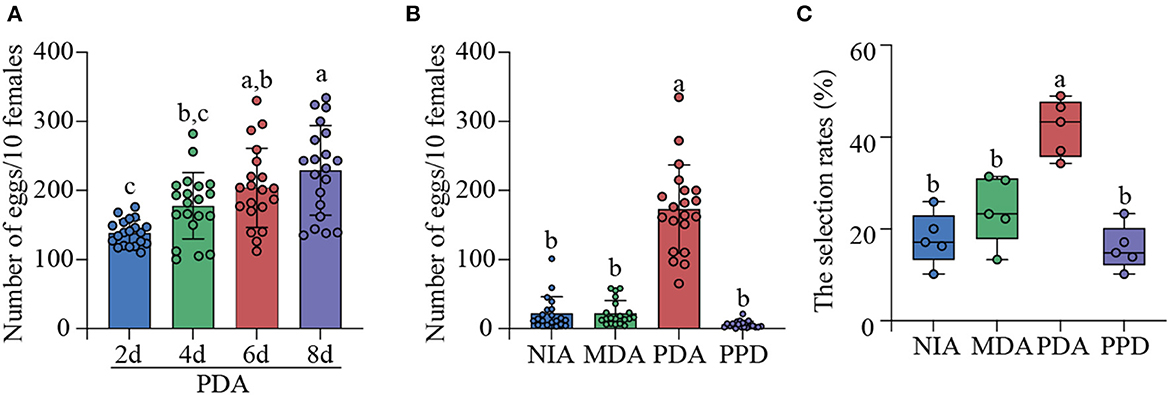
Figure 1. The selection behavior of mated YPM females. (A) The average egg numbers of mated YPM females on P. digitatum-infected apples among different infection time (2, 4, 6, and 8 d, respectively). It was repeated five times. (B) The average egg numbers of mated YPM females among PPD, NIA, MDA, and PDA. It was repeated five times. (C) The selection rates of four-arm olfactometer experiments among the odors from PPD, NIA, MDA, and PDA. A total of 211 mated YPM females were tested for four-arm olfactometer experiments. Different letters indicated significant difference among different treatments (Tukey-HSD test after ANOVA, P < 0.05). Non-infected apples (NIA), Mechanically damaged apples (MDA), P. digitatum-infected apples (PDA), and P. digitatum in potato dextrose agar medium (PPD).
The behavioral responses of mated YPM females were tested in a four-arm olfactometer. The selection rate of mated YPM females to the odor of PDA (42.03%) was significantly higher than those to the odor of NIA (17.91%), the odor of MDA (24.17%), and the odor of PPD (15.89%) (F 3, 19 = 18.948, P < 0.01; Figure 1C), suggesting that mated YPM females preferred to the odor of PDA.
In total, 38 volatile compounds were detected from the emissions of NIA, MDA, PDA, and PPD, including 24 compounds in NIA, 22 compounds in MDA, 24 compounds in PDA, and 1 compound in PPD (Supplementary Figure S1; Table 1). The results showed that α-farnesene was the most abundant compound in NIA, MDA, and PDA. Compared with MDA and NIA, five compounds, including methyl 2-methylbutyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane, were specifically detected in PDA. Only one compound, ethyl butyrate, was found in PPD (Table 1).
A principal component analysis (PCA) based on the absolute content of the 38 compounds was then performed to determine the major sources of variations in the four treatments (NIA, MDA, PDA, and PPD). The biplot depicted by graphical PCA expounded the first two principal components (PCs) with an explication of 70.61% of the total variance in the four treatments. The first component (PC1), which explained 50.68% of the total variance, was clearly isolated NIA. The second component (PC2) accounted for 19.93% of total variance (Figure 2A). Further analyses indicated that the major loadings of PC1 were for n-butyl butyrate (-0.995), isoamyl 2-methylbutyrate (−0.995), hexyl hexanoate (-0.989), amyl hexanoate (-0.989) and major loadings of PC2 were for styrene (0.887), ethyl octanoate (0.887), α-farnesene (0.868), ethyl caproate (0.865). And then, PC scores were subjected to one-way ANOVA. The results showed that NIA was significantly higher than PDA and MDA in PC1 scores, and PDA had the highest PC2 scores among four treatments (Figures 2B, C).
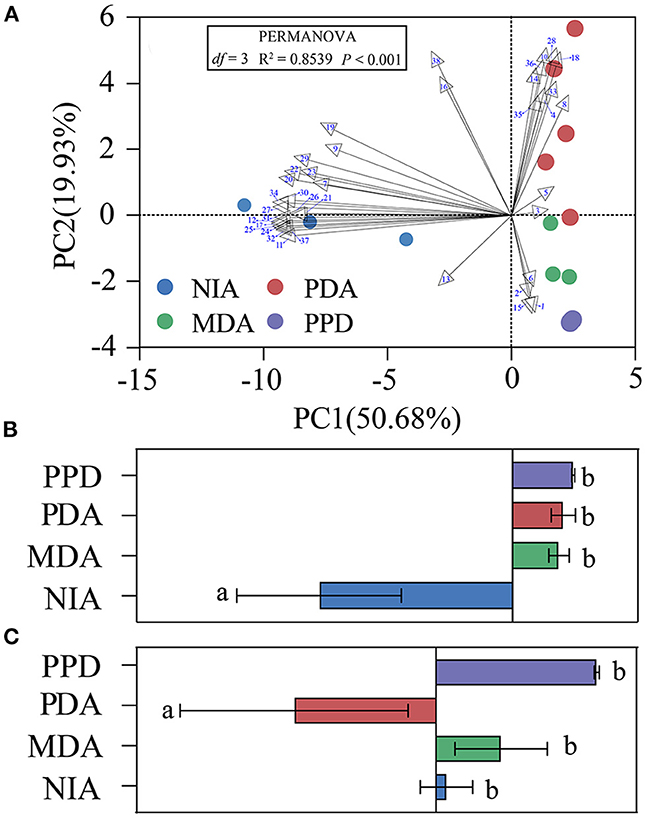
Figure 2. Principal component analysis based on 38 compounds which were obtained from NIA, MDA, PDA, and PPD. (A) The percentage of total variance explained by the first two principal components (PCs). (B) The overall distribution of PC1 scores within four treatments. (C) The overall distribution of PC2 scores within four treatments. Different letters indicated significant difference among four treatments (Tukey-HSD test after ANOVA, P < 0.05). The numbers in the graph were the same as those in Table 1. Non-infected apples (NIA), Mechanically damaged apples (MDA), P. digitatum-infected apples (PDA), and P. digitatum in potato dextrose agar medium (PPD). The VOCs of PDA were identified in five repetitions. The VOCs of NIA, MDA, PPD were identified in three repetitions.
Five specific VOCs emitted from PDA, including methyl 2-methylbutyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane, were selected for EAG and behavioral tests. Each of the five synthetic compounds could trigger significant EAG responses of mated YPM females, with dose-dependent decreasing from 10−1 to 10−4 (v/V) (Figure 3). Of which, the strongest EAG response was elicited by methyl caproate at 10−1 (v/V) (EAG value = 1.314 μA) (Figure 3C), followed by methyl 2-methylbutyrate at 10−1 (v/V) (EAG value = 0.995 μA) (Figure 3B) and butyl caprylate at 10−1 (v/V) (EAG value = 0.790 μA) (Figure 3D). The compound methyl 2-methylbutyrate at concentration of 10−2 (v/V) (EAG value = 0.620 μA) (Figure 3B) could trigger stronger EAG responses than styrene at 10−1 (v/V) (EAG value = 0.602 μA) (Figure 3A) and, butyl caprylate at 10−2 (v/V) (EAG value = 0.398 μA) (Figure 3D) and styrene at 10−2 (v/V) (EAG value = 0.374 μA) (Figure 3A) both trigger stronger EAG responses than n-tetradecane at 10−1(v/V) (EAG value = 0.347 μA) (Figure 3E).
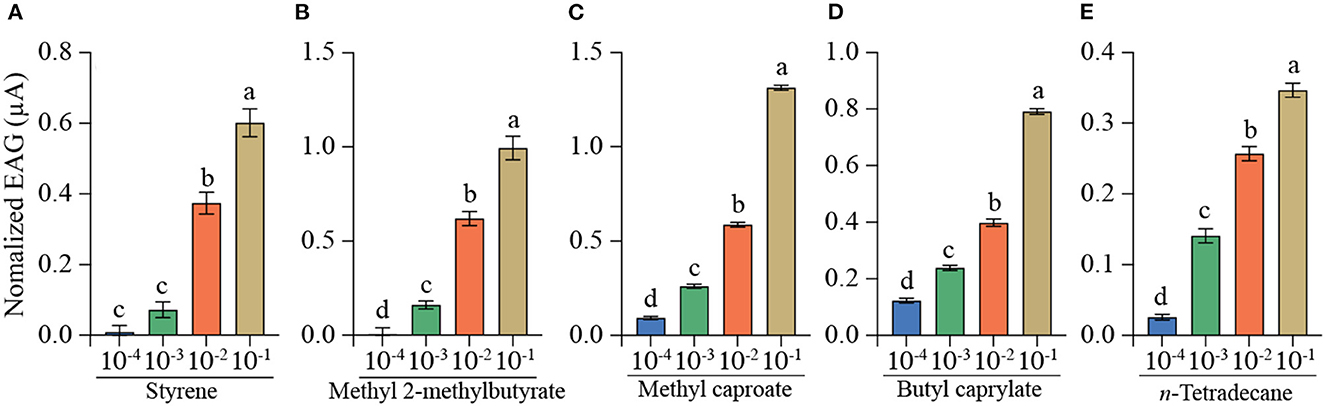
Figure 3. EAG responses of mated YPM females to five individual compounds. Bars represented mean ± SE (n = 15). Different letters on the bars indicated significant difference among the four concentrations of the same compounds (Tukey-HSD test after ANOVA, P < 0.05). (A) styrene, (B) methyl 2-methylbutyrate, (C) methyl caproate, (D) butyl caprylate, (E) n-tetradecane. Each compound at each concentration was tested on 15 antennae, and a total of 300 antennae were tested for all treatments.
To further predict the optimal concentration for attraction, the four-arm olfactometer was further used to determine the simultaneous selection of C. punctiferalis mated females among four concentrations [10−1, 10−2, 10−3, and 10−4 (v/V)] of each same compound. The behavioral responses indicated that the five individual compounds all showed the strongest attractiveness to the mated YPM females at 10−2 (v/V) in the four-arm olfactometer experiments (Figure 4). For styrene, the selection rates of mated YPM females were 35.89% at 10−3 (v/V) and 39.56% at 10−2(v/V), which was significantly higher than those of 14.19% at 10−4 (v/V) and 10.35% at 10−1 (v/V) (Figure 4A). The selection rate of mated YPM females was highest at 10−2 (v/V) (41.82%) among four concentrations of methy1 2-methylbutyrate, followed by those at 10−1 (v/V) (25.58%) and 10−4 (v/V) (17.48%), and the lowest was at 10−3 (v/V) (15.13%) (Figure 4B). The selection rate of mated YPM females was significantly higher at 10−2 (v/V) (34.24% of methyl caproate and 36.97% of n-tetradecane) than those at 10−1 (v/V) (13.64% of methyl caproate and 17.79% of n-tetradecane), while it was intermediate at 10−3 (v/V) (24.72% of methyl caproate and 24.55% of n-tetradecane) and 10−4 (v/V) (27.39% of methyl caproate and 20.69% of n-tetradecane) (Figures 4C, E). For butyl caprylate, the selection rate of mated YPM females at 10−2 (v/V) (37.88%) was the highest, followed by those at 10−3 (v/V) (24.24%), 10−4 (v/V) (19.70%), and 10−1 (v/V) (16.67%) (Figure 4D).
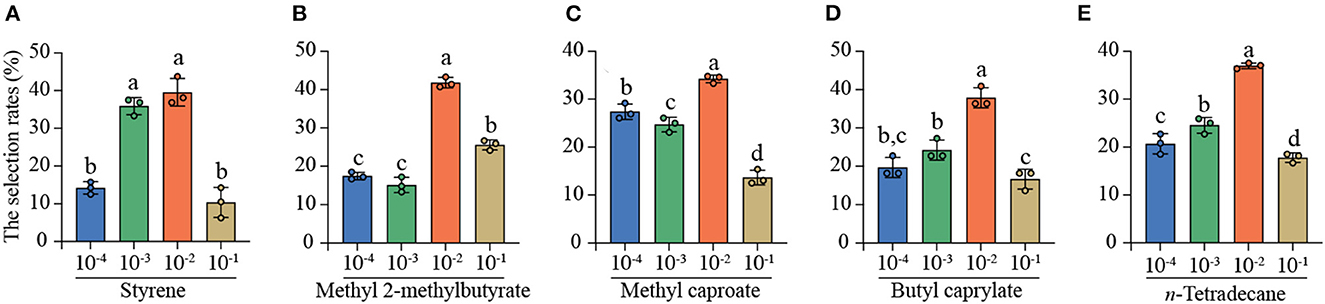
Figure 4. The selection rates of mated YPM females among the four concentrations [10−1, 10−2, 10−3, and 10−4 (v/V)] of the same compounds in four-arm olfactometer. Different letters on the bars indicated significant difference among the four concentrations of the same compounds (Tukey-HSD test after ANOVA, P < 0.05). (A) styrene, (B) methyl 2-methylbutyrate, (C) methyl caproate, (D) butyl caprylate, (E) n-tetradecane. 80 mated YPM females (styrene), 86 mated YPM females (methyl 2-methylbutyrate), 80 mated YPM females (methyl caproate), 85 mated YPM females (butyl caprylate), and 80 mated YPM females (n-tetradecane) were tested.
To address the critical synergist in attracting mated YPM females to fungi-infected apples, the individual or mixed blends of five specific VOCs were added onto the mechanically damaged apples (MDA) respectively to test the behavioral responses, with MDA as control (Figure 5; Supplementary Table S1). For individual compounds, mated YPM females preferred to apples with methyl 2-methylbutyrate (the selection rate of 66.25%, χ2= 8.45, P = 0.004) (Figure 5B), butyl caprylate (the selection rate of 61.25%, χ2= 4.05, P = 0.044) (Figure 5D), and n-tetradecane (the selection rate of 66.25%, χ2= 8.45, P = 0.004) (Figure 5E). However, mated YPM females had significant repellence to apples with styrene (the selection rate of 37.5%, χ2= 5.00, P = 0.025) (Figure 5A). For mixed compounds, mated YPM females had significant preference to apples with methyl 2-methylbutyrate and butyl caprylate (the selection rate of 62.5%, χ2 = 5.00, P = 0.025) (Figure 5K), methyl 2-methylbutyrate and n-tetradecane (the selection rate of 63.75%, χ2 = 6.05, P = 0.014) (Figure 5L), butyl caprylate and n-tetradecane (the selection rate of 70%, χ2 = 12.8, P < 0.001) (Figure 5O), styrene, methyl 2-methylbutyrate, and n-tetradecane (the selection rate of 62.5%, χ2 = 5.00, P = 0.025) (Figure 5R), methyl 2-methylbutyrate, methyl caproate, and butyl caprylate (the selection rate of 67.5%, χ2 = 9.80, P = 0.002) (Figure 5V), styrene, methyl 2-methylbutyrate, methyl caproate, and butyl caprylate (the selection rate of 61.25%, χ2 = 4.05, P = 0.044) (Figure 5Z), methyl 2-methylbutyrate, methyl caproate, butyl caprylate, and n-tetradecane (the selection rate of 61.25%, χ2 = 4.05, P = 0.044) (Figure 5AD), styrene, methyl 2-methylbutyrate, methyl caproate, butyl caprylate, and n-tetradecane (the selection rate of 63.75%, χ2 = 6.05, P = 0.014) (Figure 5AE).
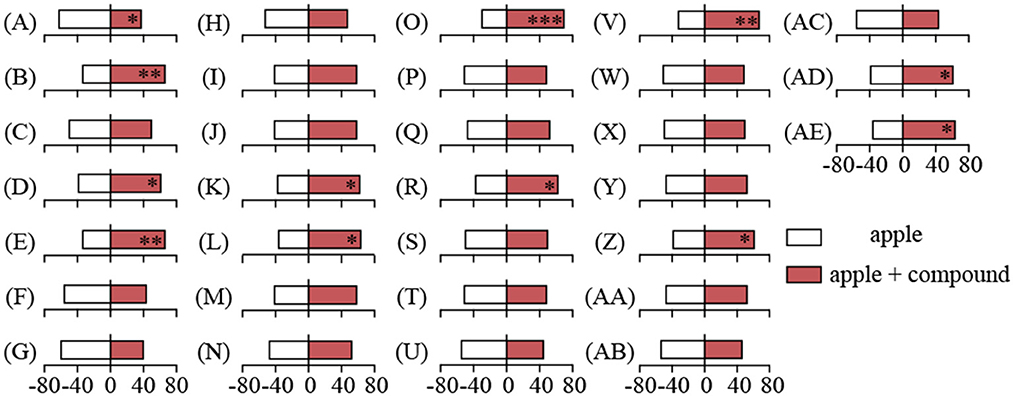
Figure 5. Behavioral responses of mated YPM females to apples with and without compounds in Y-tube olfactometer. The bars represented the percentage of the responding mated YPM females to apples with or without compounds. Stars indicated significant difference in Y-tube olfactometer assays using χ2 test (*P < 0.05, **P < 0.01, ***P < 0.001). 1. Individual compound: (A) compound 1, (B) compound 2, (C) compound 3, (D) compound 4; (E) compound 5; 2. compounds' mixture: (F) compounds (1+5); (G) compounds (1+2); (H) compounds (1+4); (I) compounds (1+3); (J) compounds (2+3); (K) compounds (2+4); (L) compounds (2+5); (M) compounds (3+4); (N) compounds (3+5); (O) compounds (4+5); 3. compounds' mixture: (P) compounds (1+2+3); (Q) compounds (1+2+4); (R) compounds (1+2+5); (S) compounds (1+3+4); (T) compounds (1+3+5); (U) compounds (1+4+5); (V) compounds (2+3+4); (W) compounds (2+3+5); (X) compounds (2+4+5); (Y) compounds (3+4+5); 4. compounds' mixture: (Z) compounds (1+2+3+4); (AA) compounds (1+2+3+5); (AB) compounds (1+2+4+5); (AC) compounds (1+3+4+5); (AD) compounds (2+3+4+5); 5. compounds' mixture: (AE) compounds (1+2+3+4+5). Compound 1: styrene; Compound 2: methyl 2-methylbutyrate; Compound 3: methyl caproate; Compound 4: butyl caprylate; Compound 5: n-tetradecane. 80 mated YPM females were tested for each compound, and a total of 2,480 mated YPM females were tested for all compounds.
Plant-associated microbes and herbivorous insects often co-occur on the same host plant. It has indicated that plant-associated microbes have significantly cascading effects on host preference of herbivorous insects via affecting host plant VOCs (Fernandez-Conradi et al., 2018; Grunseich et al., 2019). In the present study, P. digitatum infection changed the VOCs profile of apple fruits, including five specific VOCs that were methyl 2-methylbutyrate, styrene, methyl caproate, butyl caprylate, and n-tetradecane, and consequently attracted the oviposition of YPM females. These findings implied that the roles of plant-associated microbes should be taken into account in the interactions between YPMs and host plants.
Plant associated microbes could alter the oviposition and foraging behaviors of subsequent herbivorous insects. However, there are no uniform effects of plant-associated microbes on insects' behavior. For example, Botrytis cinerea has an avoidance response of Lobesia botrana for laying eggs on the grape plants (Tasin et al., 2012). On the contrary, YPM females laid more eggs on PDA than on NIA and MDA, as well as than on PPD in the current study. Furthermore, the number of eggs laid by YPM females increased along P. digitatum infection time. The positive effects of plant-associated microbes on the preference of herbivorous insects for host plants have also been reported in other studies (Cardoza et al., 2002, 2003), confirming the important roles of plant-associated microbes in the host selection of herbivorous insects. Moreover, a meta-analysis of 1,113 case studies gathered from 101 primary papers suggests that the concept of tripartite interactions among host plants, plant-associated microbes, and herbivorous insects is dependent on microbes lifestyle (biotrophic or necrotrophic pathogens), herbivorous insects feeding guild (sap-sucking or chewing insects), and the spatial scale of the interaction (local or distant) (Fernandez-Conradi et al., 2018), suggesting that different factors remain to be explored in the overall effects of P. digitatum on the performance and host preference of YPMs in the further experiments.
Chemical communication is an ancient and ubiquitous channel to mediate species interactions, and host plant VOCs is defined as olfactory cues in host location, recognition and selection of herbivorous insects. The YPM females were attracted to and laid eggs on artificial substrates that released host plant odors (Luo and Honda, 2015a,b; Du et al., 2016). In the present study, the VOCs profile of PDA was significantly different from NIA, MDA and PPD. Furthermore, mated YPM females had an obvious preference for PDA odors to NIA, MDA, and PPD odors in the four-arm olfactometer, which was not only in line with the oviposition preference of YPM females for PDA, but also further indicated the potential role of host plant VOCs for the preference of YPM females. Moreover, our experiments revealed a clear separation between VOC profiles of PDA (including five P. digitatum-induced specific VOCs) vs. MDA or NIA via PCA analysis, implying that these specific VOCs could be served as signals for the oviposition and foraging behaviors of YPMs to PDA. These results are agree with recent studies that host plant VOCs could be frequently altered by plant-associated microbes' infection and consequently have impacts on the host selection of herbivorous insects (Groen et al., 2016; Rizvi et al., 2016; Grunseich et al., 2019). Thus, it is not uncommon for herbivorous insects to employ fungi-induced kairomones for host location.
Some specific VOCs are emerged as attractants in the oviposition and foraging behavior of herbivorous insects (Turlings and Erb, 2018). For example, styrene is reported to be a spoilage marker of decayed apples after infection by Penicillium expansum and elicits strong electrophysiological antennal activity for Ips typographus at very low levels (Kim et al., 2018, 2019; Schiebe et al., 2019). As in our study, styrene was specifically emitted in a relatively ample amount from PDA and triggered significant EAG responses. However, mated YPM females showed obvious repellence to the styrene-supplemented apples in Y-tube olfactometer assay, which is the same as a previous study that styrene significantly reduces pine weevils' attraction to cut pieces of Scots pine twigs (Azeem et al., 2013). Indeed, YPM females exhibited significant preference to apples with three other P. digitatum-induced specific VOCs, including methyl 2-methylbutyrate, butyl caprylate, and n-tetradecane, individually or together, suggesting that it was the mixed blends, but not one specific VOCs, that served as olfactory cues for the host orientation and oviposition selection of YPM females to PDA. This is consistent with the evidence that changes in the overall composition and relative ratios of the host plant VOCs make plants co-infested by Nilaparvata lugens (Stål) and Chilo suppressalis (Walker) unattractive to Anagrus nilaparvatae females (Hu et al., 2020). One possible explanation is that, compared with individual components, the quantitative as well as qualitative differences in the blend of plant VOCs have a significant effect on herbivorous insects' behaviors (Bruce and Pickett, 2011). Collectively, these P. digitatum induced specific VOCs functioned together as olfactory cues for the interactions between YPM females and apples.
In summary, a battery of experiments were carried out to gain further insight into the hypothetical roles of the plant-associated microbes (P. digitatum) in mediating the host plant location and oviposition selection of YPMs via host plant VOCs. Current study found that YPM females preferred to P. digitatum-infected apples for oviposition in the oviposition behavioral experiments. Odors from P. digitatum-infected apples were responsible for the attractiveness of YPM females as demonstrated in the four-arm olfactometer assay. Furthermore, three of five P. digitatum-induced specific VOCs, including methyl 2-methylbutyrate, butyl caprylate, and n-tetradecane, might serve as key olfactory cues for YPM females to P. digitatum-infected apples via Y-tube olfactometer assay. These findings shed light on the underlying mechanisms of the attraction of YPMs by P. digitatum-infected apples, and might form the basis for the development of attractant formulations for field trapping YPM moths. In the future, field experiments will be carried out to prove the semiochemical roles of individuals as well as blends of P. digitatum-infected apple volatiles in attracting YPMs.
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
YLD and HGG conceived the idea, designed the experiments, and directed the implementation. PPA and SZM performed the experiments. SZM, PPA, HGG, and YLD conducted statistical analyses. MZZ contributed to the conception and design of the study. ZY prepared the materials and lab facilities necessary for this work. HGG and YLD wrote the first draft of the manuscript. All authors read and approved the final manuscript.
This study was supported by the science and technology fund of Beijing Municipal Commission of Education (Grant Number: KZ202210020027), Beijing University of Agriculture Science and Technology Innovation Sparkling Support Plan (Grant Number: BUA-HHXD2022004), Science Fund for Young Scholars from the Beijing University of Agriculture to HGG (Grant Number: QNKJ202103), and 2022 Research and Innovation Ability Promotion Fund for Young Scholars from the Beijing University of Agriculture to HGG (Grant Number: QNKC2022001).
We are grateful to Prof. Long Zhang (China Agricultural University) for his advice on behavioral experiment, Mrs. Jun Wang and Deyu Li for breeding insects, and Prof. Zeng Guang Yan (Ministry of Ecology and Environment of the People's Republic of China).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1119717/full#supplementary-material
Azeem, M., Rajarao, G. K., Nordenhem, H., Nordlander, G., and Borg-Karlson, A. K. (2013). Penicillium expansum volatiles reduce pine weevil attraction to host plants. J. Chem. Ecol. 39, 120–128. doi: 10.1007/s10886-012-0232-5
Belmain, S. R., Simmonds, M. S., and Blaney, W. M. (2002). Influence of odor from wood-decaying fungi on host selection behavior of deathwatch beetle, Xestobium rufovillosum. J. Chem. Ecol. 28, 741–754. doi: 10.1023/a:1015284625697
Bhatta, U. K. (2022). Alternative management approaches of Citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 12, 833328. doi: 10.3389/fpls.2021.833328
Bruce, T. J., and Pickett, J. A. (2011). Perception of plant volatile blends by herbivorous insects-finding the right mix. Phytochemistry. 72, 1605–1611. doi: 10.1016/j.phytochem.2011.04.011
Cardoza, Y. J., Alborn, H. T., and Tumlinson, J. H. (2002). In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J. Chem. Ecol. 28, 161–174. doi: 10.1023/a:1013523104853
Cardoza, Y. J., Lait, C. G., Schmelz, E. A., Huang, J., and Tumlinson, J. H. (2003). Fungus induced biochemical changes in peanut plants and their effect on development of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae) larvae. Environ. Entomol. 32, 220–228. doi: 10.1043/0046-225X(2003)032(0220:FIBCIP)2.0.CO
Du, Y. L., Zhang, J. X., Yan, Z. G., Ma, Y. Q., Yang, M. M., Zhang, Z. Y., et al. (2016). Host preference and performance of the yellow peach moth (Conogethes punctiferalis) on chestnut cultivars. PLoS One. 11, e0157609. doi: 10.1371/journal.pone.0157609
Du, Y. L., Zhang, M. Z., Ma, Y. Q., Sun, S. l., Wang, J. B., and Liu, J. L. (2014). Formulation screening of sex pheromones and field trapping tests for the yellow peach moth, Conogethes punctiferalis (Guenée) (Lepidoptera: Pyralidae). Acta Phytophy. Sin. 41, 187–191. doi: 10.13802/j.cnki.zwbhxb.2014.02.011
Eberl, F., Bobadilla, M. F. D., Reichelt, M., Hammerbacher, A., and Unsicker, S. B. (2020). Herbivory meets fungivory: insect herbivores feed on plant pathogenic fungi for their own benefit. Ecol. Lett. 23, 1073–1084. doi: 10.1111/ele.13506
Eberl, F., Hammerbacher, A., Gershenzon, J., and Unsicker, S. B. (2018). Leaf rust infection reduces herbivore-induced volatile emission in black poplar and attracts a generalist herbivore. New. Phytol. 220, 760–772. doi: 10.1111/nph.14565
Eberl, F., Uhe, C., and Unsicker, S. B. (2019). Friend or foe? The role of leaf-inhabiting fungal pathogens and endophytes in tree-insect interactions. Fungal. Ecol. 38, 104–112. doi: 10.1016/j.funeco.2018.04.003
Fernandez-Conradi, P., Jactel, H., Robin, C., Tack, A. J. M., and Castagneyrol, B. (2018). Fungi reduce preference and performance of insect herbivores on challenged plants. Ecol. 99, 300–311. doi: 10.1002/ecy.2044
Groen, S. C., Jiang, S., Murphy, A. M., Cunniffe, N. J., Westwood, J. H., and Davey, M. P. (2016). Virus infection of plants alters pollinator preference: a payback for susceptible hosts? PLoS Pathog. 12, e1005790. doi: 10.1371/journal.ppat.1005790
Grunseich, J. M., Thompson, M. N., Aguirre, N. M., and Helms, A. M. (2019). The role of plant-associated microbes in mediating host-plant selection by insect herbivores. Plants (Basel, Switzerland). 9, 6. doi: 10.3390/plants9010006
Guo, H., Meng, L., Zhang, M., Ren, Z., Qin, X., and Du, Y. (2021). Binding specificity of OBPs in the yellow peach moth Conogethes punctiferalis (Guenée). J. Appl. Entomol. 145, 1001–1014. doi: 10.1111/jen.12922
Guo, H. G., Han, C. Y., Zhang, A. H., Yang, A. Z., Qin, X. C., Zhang, M. Z., et al. (2022). Penicillium fungi mediate behavioral responses of the yellow peach moth, Conogethes punctiferalis (Guenée) to apple fruits via altering the emissions of host plant VOCs. Arch. Insect. Biochem. Physiol. 110, e21895. doi: 10.1002/arch.21895
Hu, X., Su, S., Liu, Q., Jiao, Y., Peng, Y., Li, Y., et al. (2020). Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. Elife. 9, e55421. doi: 10.7554/eLife.55421
Kim, H. W., Lee, S. M., Seo, J. A., and Kim, Y. S. (2019). Effects of pH and cultivation time on the formation of styrene and volatile compounds by Penicillium expansum. Molecules. 24, 1333. doi: 10.3390/molecules24071333
Kim, S. M., Lee, S. M., Seo, J., and Kim, Y. (2018). Changes in volatile compounds emitted by fungal pathogen spoilage of apples during decay. Postharvest. Biol. Technol. 146, 51–59. doi: 10.1016/j.postharvbio.2018.08.003
Kopper, B. J., Klepzig, B. J., and Raffa, K. F. (2004). Components of antagonism and mutualism in Ips pini–fungal interactions: relationship to a life history of colonizing highly stressed and dead trees. Environ. Entomol. 33, 28–34. doi: 10.1603/0046-225X-33.1.28
Li, D. Y., Ai, P. P., Du, Y. L., Sun, S. L., and Zhang, M. Z. (2015). qEffects of different host plants on the development and reproduction of yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Austral. Entomol. 54, 149–153. doi: 10.1111/aen.12105
Li, H. J., Ren, L., Xie, M. X., Gao, Y., He, M. Y., Hassan, B., et al. (2020). Egg-surface bacteria are indirectly associated with oviposition aversion in Bactrocera dorsalis. Curr. Biol. 30, 4432–4440. doi: 10.1016/j.cub.2020.08.080
Luo, Z. X., and Honda, H. (2015a). Olfactory and biophysical assessment of the oviposition stimulating potential of host and non-host plants for the yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 50, 183–189. doi: 10.1007/s13355-014-0320-9
Luo, Z. X., and Honda, H. (2015b). Function of plant odors in oviposition behaviors of the yellow peach moth Conogethes punctiferalis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 50, 347–353. doi: 10.1007/s13355-015-0341-z
Rizvi, S. Z., Raman, A., Wheatley, W. M., and Cook, G. (2016). Oviposition preference and larval performance of Epiphyas postvittana (Lepidoptera: Tortricidae) on Botrytis cinerea (Helotiales: Sclerotiniaceae) infected berries of Vitis vinifera (Vitales: Vitaceae). Insect. Sci. 23, 313–325. doi: 10.1111/1744-7917.12191
Schiebe, C., Unelius, C. R., Ganji, S., Binyameen, M., Birgersson, G., and Schlyter, F. (2019). Styrene, (+)-trans-(1R,4S,5S)-4-thujanol and oxygenated monoterpenes related to host stress elicit strong electrophysiological responses in the bark beetle Ips typographus. J. Chem. Ecol. 45, 474–489. doi: 10.1007/s10886-019-01070-8
Shi, X., Ai, Y. T., Shi, R. Z., Zhang, M. Z., Wang, H. X., and Du, Y. L. (2019). Oviposition selection and behavioral tendency of yellow peach moth Conogethes punctiferalis (Guenée) to Penicillium-inoculated apples. J. Plant Prot. 46, 4. doi: 10.13802/j.cnki.zwbhxb.2019.2018127
Tack, A. J. M., and Dicke, M. (2013). Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct. Ecol. 27, 633–645. doi: 10.1111/1365-2435.12087
Tasin, M., Knudsen, G. K., and Pertot, I. (2012). Smelling a diseased host: grapevine moth responses to healthy and fungus-infected grapes. Anim. Behav. 83, 555–562. doi: 10.1016/j.anbehav.2011.12.003
Turlings, T. C. J., and Erb, M. (2018). Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452. doi: 10.1146/annurev-ento-020117-043507
Witzgall, P., Proffit, M., Rozpedowska, E., Becher, P. G., Andreadis, S., Coracini, M., et al. (2012). “This is not an apple”-yeast mutualism in codling moth. J. Chem. Ecol. 38, 949–957. doi: 10.1007/s10886-012-0158-y
Keywords: Conogethes punctiferalis, Penicillium digitatum, plant-microbe-insect interactions, chemical communication, host plant volatile organic compounds
Citation: Guo HG, Miao SZ, Ai PP, Zhang MZ, Yan Z and Du YL (2023) Bioactive volatile compounds from Penicillium digitatum-infected apples: Oviposition attractants for yellow peach moth Conogethes punctiferalis (Lepidoptera: Crambidae). Front. Sustain. Food Syst. 7:1119717. doi: 10.3389/fsufs.2023.1119717
Received: 09 December 2022; Accepted: 13 January 2023;
Published: 02 February 2023.
Edited by:
Fang Ouyang, Institute of Zoology (CAS), ChinaReviewed by:
Huai-Jun Xue, Nankai University, ChinaCopyright © 2023 Guo, Miao, Ai, Zhang, Yan and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li Du,  eWFubGlkdUAxMjYuY29t
eWFubGlkdUAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.