
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 24 May 2023
Sec. Agro-Food Safety
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1110915
This article is part of the Research TopicFood Safety in Low and Middle Income CountriesView all 25 articles
 Yoshiharu Sugino1
Yoshiharu Sugino1 James Bugeza2
James Bugeza2 David Bahame3
David Bahame3 Joseph Byaruhanga1,4
Joseph Byaruhanga1,4 Haruka Shimazaki1
Haruka Shimazaki1 Masahiko Anzai1
Masahiko Anzai1 Taishi Kayano1
Taishi Kayano1 William Mwebembezi3
William Mwebembezi3 Andrew Akashaba3
Andrew Akashaba3 Taku Shimada3
Taku Shimada3 Yasukazu Muramatsu5
Yasukazu Muramatsu5 Kohei Makita1*
Kohei Makita1*In Uganda, informal raw milk sales dominate for domestic dairy consumption. This study was implemented to identify the structure of the dairy value chain starting from farms that participated in the Japan International Cooperation Agency Safe Milk Promotion in Mbarara project conducted between 2016 and 2019, to assess the hygiene conditions along the chain, and thereby identify the bottleneck of dairy hygiene intervention. A longitudinal study was conducted in 30 dairy farms in Mbarara District to compare the practice, prevalence of sub-clinical mastitis, and level of milk hygiene in 2016–2017 and 2019, before and after the milking hygiene intervention in 2018. California Mastitis Test was used for diagnosis with sub-clinical mastitis. Bulk milk samples were collected and a checklist was used to examine hygiene practices by observation. A cross-sectional study was conducted in 15 milk collecting centers using a structured questionnaire to quantify the dairy value chain, and to sample milk from cooler tanks in 2020. Microbiological examinations of bulk milk from farms and collection centers were conducted using six-point blood agar scoring and 3M Petri film, respectively. Participatory online appraisals with farmers and dairy cooperatives union were conducted to better understand the overall dairy value chains. The cooperatives sold milk to both formal and informal chains, but the sale of raw milk to Kampala was conducted by independent private traders. Within-herd prevalence of sub-clinical mastitis significantly decreased from 72.3% before the intervention to 25.8% after (p < 0.001). However, the farm bulk milk score did not change (3.3 vs. 3.2, p = 0.418). A significant increase in the total bacterial count was observed in the milk from collection centers (mean: 6.50 log10 CFU/ml) when compared to farm bulk milk (mean: 3.79 log10 CFU/ml; p < 0.001). Only 13.3% of the samples from the centers met the microbiological criteria for processing for human consumption. Our findings suggest that intervention targeted only at mastitis does not lead to better public health due to the low level of hygiene in transportation and milk handling in milk collection centers. Systematic interventions are needed to improve post-harvest dairy hygiene in Uganda.
Even during the coronavirus disease pandemic, Uganda's economy was estimated to have grown by 2.9% in the 2019/2020 fiscal year (Uganda Bureau of Statistics, 2021). Domestic milk production increased from 2.08 billion liters in 2015 to 2.81 billion liters in 2021 (Dairy Development Authority, 2021). Revenue from the export of dairy products has risen from United States Dollars (USD) 131.5 million [1 USD is ~3,650 Uganda Shillings (Shs)] in 2018 to USD 205 million in 2020 (Ministry of Agriculture, 2021), and accounted for 5.0% of the total export revenue of Shs 15,126 billion in the 2019/2020 fiscal year (Uganda Revenue Authority, 2021). While dairy exports significantly contribute to Uganda's economy, dairy farming provides three main benefits to more than 2.5 million farming households, i.e., nutritious food, additional income, and a productive labor force, and it plays a significant role in reducing Uganda's food insecurity and poverty (Staal and Kaguongo, 2003; Balikowa, 2011; Wangalwa et al., 2016). Pasteurized milk can safely provide nutrition to the population, but the marketing of untreated milk is popular in Uganda; it has been reported that 72.1% of milk is sold through formal channels [cooperative union purchases (38.06%) and milk collection centers (MCCs) owned by licensed raw milk traders (34.05%)] while 27.9% of milk is sold through informal channels (vendors and restaurants) in Kiruhura District (Nkwasibwe et al., 2015).
Contaminated and spoiled raw milk affects the entire dairy industry, ultimately resulting in raw milk of reduced quality, dairy products with less flavor, and a shorter shelf life (Roberts, 1993; Barbano et al., 2006). In particular, raw milk may contain microorganisms that are dangerous to human health, and the consumption of unsafe milk is known to cause many milk-borne diseases (Dhanashekar et al., 2012). Poor animal husbandry and practices, such as udder and handwashing with unsafe water, adulteration, and poor equipment cleaning, have been identified to potentially cause the spread of harmful pathogens in milk (Oliver et al., 2009). It is crucial to ensure that high-quality raw milk is produced from healthy animals under hygienic conditions, and that quality control measures are applied in the value chain.
Mbarara District, located in southwest Uganda, is one of Uganda's most crucial milk production and market areas. In this region, a project called the “Japan International Cooperation Agency (JICA) Safe Milk Promotion in Mbarara (Safe Milk) Project” was implemented from September 2016 to September 2019 in the dairy herds of Mbarara District (Rakuno Gakuen University, 2019). The project aimed to improve the dairy productivity in intensive dairy production areas of Uganda. However, even though the quality of milk at the time of production has improved, dairy hygiene measures need to be maintained throughout the value chain to deliver good quality milk to consumers. The purposes of this study were to quantitatively understand the structure of the dairy cooperative value chains, starting from JICA project farms, in Mbarara, an intensive dairy production area of the country, and to assess the hygiene levels along the value chains, and thereby identify the bottleneck of dairy hygiene intervention in Uganda.
The study was conducted in Mbarara District, the Republic of Uganda, located between 0°12′20.6″ and 0°50′36.5″ south in latitude, and between 30°18′47.5″ and 30°49′12.8″ east in longitude (Figure 1). The topography of the district includes a mixture of shallow valleys and flat land. The average annual rainfall is 1,200 mm, rainy seasons last from February to May and September to December, temperatures range from 17 to 30°C, and humidity levels range from 80 to 90% (Mbarara District Local Government, 2021). In Uganda, the Western Region, which includes Mbarara District, is the region with the second highest number of cattle in the country, following the Northern Region (Uganda Bureau of Statistics, 2021). According to the Mbarara District Veterinary Office, the estimated total number of cattle in Mbarara District at the time of project implementation was 185,680, and there were 10,200 dairy farmers (Miyama et al., 2020).
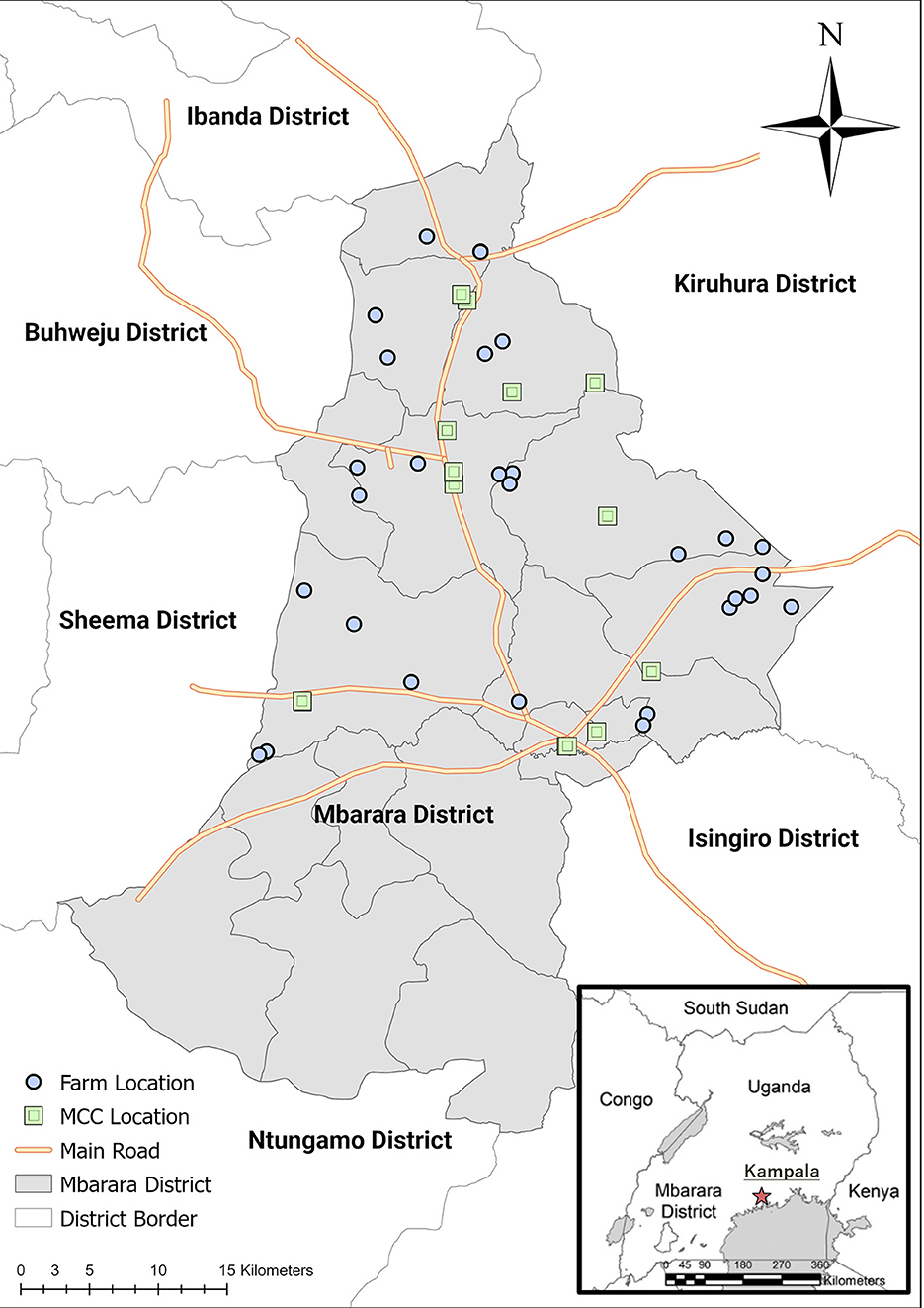
Figure 1. Map of Mbarara District with the locations of the JICA project farms and milk collection centers (MCCs) studied.
A longitudinal study was conducted in the 30 dairy farms that participated in the JICA Safe Milk project, to compare the practice and level of milk hygiene between in 2016–2017 and 2019, before and after the dairy hygiene intervention, which took place in 2018. The intervention involved frequent visits to the farms for demonstration of hygienic milking using proper hand milking and one towel for a cow. All the participating farms employed hand milking. The longitudinal study in the dairy farms also aimed to determine the association between farm bulk milk hygiene and hygiene practices.
The target farms were the 30 farms in Mbarara District that participated in the JICA project. For the JICA project, farmers were purposively selected in collaboration with the Mbarara District Veterinary Officer (DVO) according to the following criteria: (a) herd size, five farms with a small herd (<10 adult cows, including milking and dry cows), 20 farms with a medium herd (10–40 adult cows), and five farms with a large herd (>40 adult cows); (b) herd management type; (c) accessibility, the farms had to be located within driving distance for regular visits; (d) farm distribution, two–five farms per sub-county and five–six farms per MCC; and (e) commitment, the farms were anticipated to continue participating in the JICA project. The criteria a, b, and d were decided, so that the selected farms are representative of the dairy farms in Mbarara District. The 30 study farms were selected from 10 sub-counties in the district, namely Biharwe, Bubaare, Bukiro, Kagongi, Kakiika, Kakoba, Kashare, Rubaya, Rubindi, and Rwanyamahembe.
In 2020, a cross-sectional study was conducted in cooperatives' MCCs to better understand the structure of dairy value chains, starting from the JICA project participating dairy farms in Mbarara District, and the level of hygiene in bulk milk at the MCCs. The targeted MCCs were all of the 17 milk collection stations of the producers' cooperatives in Mbarara District; they were located in eight sub-counties: Bubaare, Kakiika, Kakoba, Kamukuzi, Kashare, Rubaya, Rubindi, and Rwanyamahembe.
In addition, online participatory appraisals with dairy stakeholders were carried out to ensure the representativeness of this study in relation to wider dairy value chains.
The research protocol was approved by the School of Veterinary Medicine and Animal Resources (SVAR) Research Ethics Committee of Makerere University (reference number SVARREC/09/2018). Informed consent was obtained from all farms studied.
Field studies of the milking hygiene practices and the level of hygiene on the 30 dairy farms was conducted between October 2016 and May 2017, and in January 2019. The milking hygiene practices were checked during afternoon milking on each farm in both studies using a check list (Table 1). For the seven farms that did not milk cows in the afternoon, milking practice information was collected through interviews with the owners/managers in both periods. The checklist questioning, accompanied by observation, was performed in English, the official language of Uganda, and the judgment criteria for the contents were confirmed among assessors before the surveys, to avoid information bias during checklist administration. During the surveys in 2016–2017 and 2019, the California Mastitis Test (CMT) was performed for individual milking cows to detect sub-clinical mastitis. The results of CMT were classified as negative, trace, or score 1, 2, or 3, depending on the amount of gel formed (Ruegg, 2005). A quarter was defined as CMT positive if it had a score 1 or above, and a cow was defined as CMT positive if it had at least one CMT-positive quarter. In addition, during the both surveys, farm bulk milk was aseptically sampled for microbiological tests. In addition, swabs of empty cans before milking (dry swabs), empty cans after washing (wet swabs), and samples of the water used to clean milk cans were collected from the farms in 2019. Information on the milk yield at each farm and the shipping destination was collected in 2019. These surveys were conducted during the JICA project, and data on the geographical locations of the farms were obtained from the project.
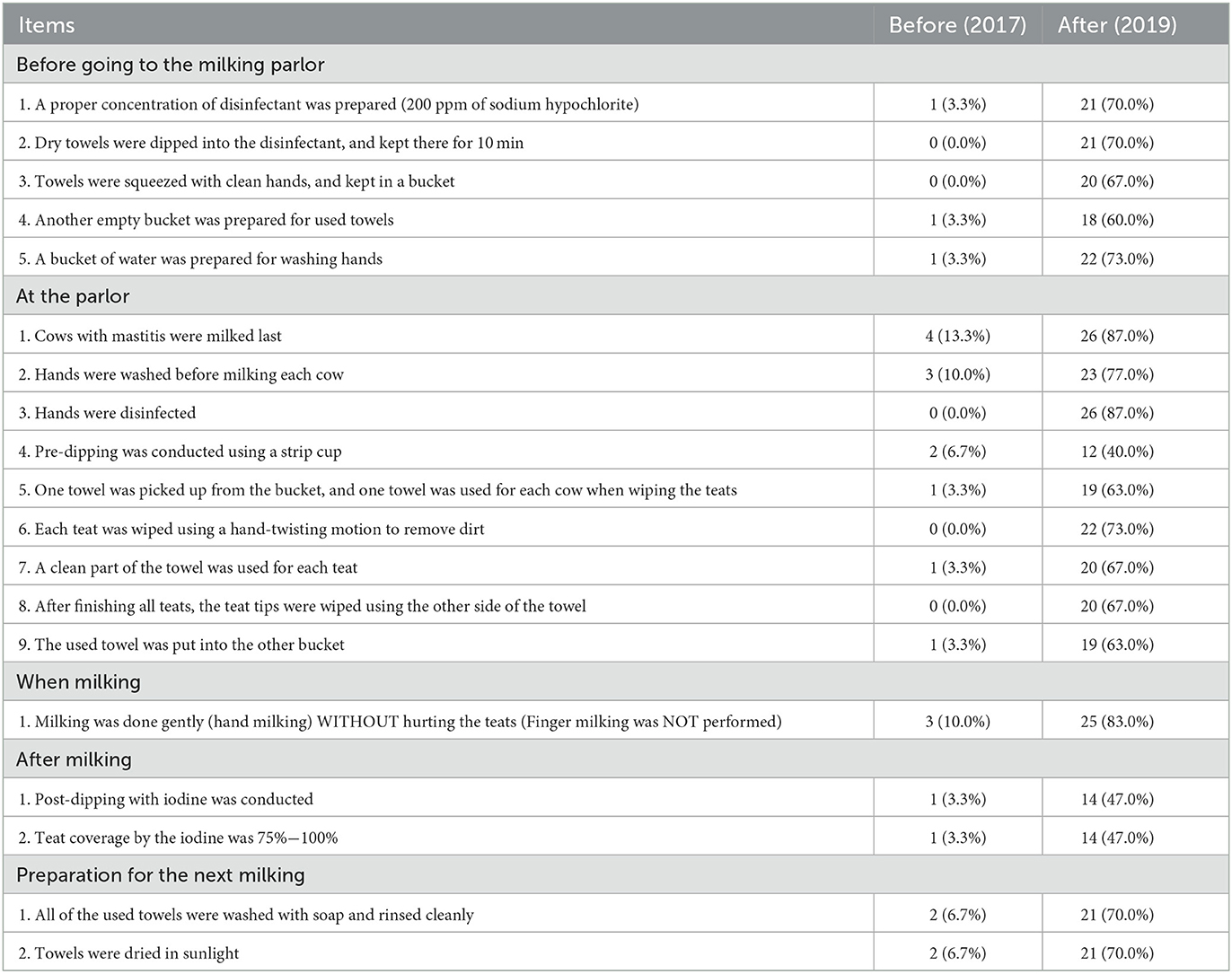
Table 1. A comparison in the frequency and percentage of milking hygiene practices conducted at 30 dairy farms in Mbarara District, Uganda, between before and after the intervention.
In January 2020, a field study of the value chain and milk hygiene was conducted in all of the 17 MCCs that were owned by dairy cooperatives and registered in Mbarara District. Of the 17 MCCs, two MCCs had stopped doing business. A structured questionnaire regarding the testing of milk upon the receipt of raw milk, the sources and sales destinations of milk, and the volumes of milk was administered through interviews with MCC managers. The purchase of milk from the 30 dairy farms participating in the JICA project was also investigated in the MCCs. Bulk milk was aseptically sampled from milk coolers in these MCCs.
All milk, water, and swab samples were transported to the Mbarara DVO laboratory in a cooling box immediately after sampling.
In the JICA project, the microbiological level of milk hygiene in dairy farm bulk milk was semi-quantitatively assessed by the observation of 5% sheep blood agar with a six-point scoring system (Supplementary Figure 1). This test was selected by the project because it is sustainable for use in the field for identification of causal bacteria for sub-clinical mastitis at the teat level; this scoring system has also been used by the Japanese Dairy Association (Ministry of Agriculture, 1997). For this method, 0.1 ml of raw milk was inoculated onto 5% sheep blood agar and incubated at 37°C for 48 h. The bacterial counts were estimated on a scale of 1–6: (1) <3,000 colony forming units (CFU)/ml; (2) 3,000 to <4,500 CFU/ml; (3) 4,500 to <6,000 CFU/ml; (4) 6,000 to <13,000 CFU/ml; (5) 13,000 to <20,000 CFU/ml; and (6) ≥20,000 CFU/ml (Ministry of Agriculture, 1997).
For the bulk milk samples collected from the MCCs, and the wet and dry swabs and tap water samples from the farms, the total bacterial counts were determine using 3M Petri film according to the manufacturer's instructions (3M Japan Limited. Shinagawa, Japan). Serial dilutions of 10, 103, and 105 were prepared, and the total bacterial count (CFU/ml) was determined by direct counting. Plates with ≥400 colonies were categorized as “too numerous to count” (TNTC), indicating a high level of bacterial contamination in the sample.
All of the data collected from the survey, scoring, and diagnostic tests were digitized using Microsoft Excel spreadsheets (Microsoft Office 365, USA) and imported into Microsoft Access (Microsoft Office 365) for assembly; datasets for the farm level and MCC level were then built. ArcGIS software (ESRI Japan, Tokyo, Japan) was used to map the milk contamination levels, milk handling volumes, and value chain status.
Descriptive analysis was conducted for all data collected at the farms and the MCCs. The means, medians, and ranges were calculated for numerical variables, and the number of responses and percentages were calculated for categorical variables. The dairy value chain through cooperatives was described by summarizing the questionnaire survey results from the farms and the MCCs. The Wilcoxon rank sum test was used to compare the milk sales volume between MCCs that accepted milk from JICA project farms and those that did not, and between MCCs that sold milk to processing plants and those that did not.
The within-farm prevalence of sub-clinical mastitis diagnosed by CMT was compared between before and after the intervention using mixed-effects model with binomial errors, selecting identity of farms as a random effect, in lme4 package (Bates et al., 2023). The six-point farm bulk milk hygiene scores were compared between before and after the intervention using Wilcoxon signed rank test, matching the scores of respective farms. The proportions of the farms with bulk milk score <4 were compared between before and after the intervention using a chi-squared test.
Sub-clinical mastitis is generally caused by bacterial infection, which can increase the risk of bacterial contamination in farm bulk milk. Spearman's correlation test was performed for the six-point farm bulk milk hygiene score and the within-farm prevalence of sub-clinical mastitis by CMT, for before and after the intervention. A detailed study on the risk factors for sub-clinical mastitis on these 30 farms has been published elsewhere (Miyama et al., 2020).
To evaluate the effect of hygiene practices during milking on the milk quality in farm bulk milk, first, the six-point bulk milk hygiene scores were compared using the Wilcoxon rank sum test between the farms that conducted or did not conduct each of the hygiene practice items on the checklist. Second, the number of hygiene practice items on the checklist that were conducted was counted as the hygiene practice score. Spearman's correlation test was performed for the six-point farm bulk milk hygiene score and the hygiene practice score. These analyses for the effect of hygiene practices on the quality of farm bulk milk were conducted for before and after the intervention. The proportions of hygiene practice items conducted were compared between before and after the intervention using mixed-effects model with binomial errors, selecting identity of farms as a random effect.
The relationship between the hygiene practice score and herd size was analyzed using a generalized linear model with Poisson errors with the hygiene practice score as an outcome variable and herd size and cattle breed as explanatory variables to test the hypothesis that milking hygiene is higher on farms with intensified milk production, for before and after the intervention. The relationship between the herd size and average milk yield per cow was analyzed using linear regression after checking the normality of the milk yield distribution, selecting the average milk yield as an outcome variable.
As many dry swabs and tap water samples had TNTC total bacterial counts (see Section 3), the farm bulk milk hygiene scores were compared using the Wilcoxon rank sum test between the farms with and without heavy contamination (TNTC) in the empty cans, and between the farms with or without heavy contamination (TNTC) in tap water.
To characterize the level of hygiene of bulk cooler milk in MCCs, a Spearman's correlation test was performed for the relationship between the average daily milk volume shipped from the MCCs and the logarithm of the total bacterial count (log10 CFU/ml). To test the hypothesis that milk quality is higher if a MCC sells milk to a milk processing plant, the mean log10 total bacterial counts were compared using a t-test between MCCs that sold milk to processing plants and those that did not (i.e., they sold milk to milk shops and milk vendors in towns only).
To evaluate the magnitude of post-harvest increases in the level of bacterial contamination in raw milk, a series of simulation was applied. This was because (1) the level of bacterial contamination in farm bulk milk was measured with the six-point hygiene score while that of MCCs was enumerated using 3M Petri film, and (2) the sample size of MCCs was only 15, and resampling of these values would provide robust view by Bayesian approach. To implement this analysis, the log10 total bacterial counts of farm bulk milk and bulk cooler milk in MCCs were first simulated, then the simulated log10 total bacterial counts were compared. For the simulation of the log10 total bacterial counts in farm bulk milk, a random number was sampled from a uniform distribution of the logarithm range of the bacterial counts of the six-point scores for each of 29 farms (a farm was not included since farm bulk milk was not sampled from that farm), and one value was resampled from these 29 values to obtain a total sample number of 30. To estimate the mean log10 total bacterial count in farm bulk milk, calculation of a mean of randomly sampled these 30 values was iterated 1,000 times. For the simulation of the log10 total bacterial count in bulk cooler milk of MCCs, 30 values were randomly sampled from a set of log10 total bacterial counts from the 15 MCCs studied. A comparison using a t-test of these 30 simulated values each from farm bulk milk and MCCs was repeated for 1,000 iterations to obtain a Bayesian simulation of the p-values. This comparison between farm bulk milk and MCCs was performed for the farm bulk milk samples both before and after the intervention.
The simulated log10 total bacterial count in farm bulk milk and MCC bulk milk were compared with the log10 acceptable limit of bacterial count for human consumption, 105 CFU/ml, according to the International Microbiological Criteria for Dairy Products for raw bovine milk intended for processing for human consumption [Institute of Medicine (US) and National Research Council (US) Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food, 2003].
All statistical analyses were conducted using R statistical software version 4.2.1 (R Core Team, Vienna, Austria).
To understand the relationships between dairy cooperative value chains in Mbarara and wider dairy value chains interconnecting with the capital, Kampala, and other formal and informal dairy value chains, online participatory appraisals were conducted with the 30 farmers who participated in the JICA project, the Uganda Crane Creameries Cooperatives Union (UCCCU), and the Mbarara DVO via social networking service and online meetings in November 2022. The research team created an online chat group in a social networking service in 2017, and invited the 30 farmers, UCCCU personnel and Mbarara DVO officers to enhance the project communication. The farmers still regularly use the online group chat to exchange information on dairy farming.
For the online participatory appraisal, first, the research team had an online meeting using Webex system (Cisco, San Jose, USA) with a representative farmer, to construct the first draft of the dairy value chain. There were disruptions in connection several times, but this exercise successfully produced a draft figure in 40 min. Second, the draft figure was shared in the online chat group, and the group members were invited for comments. A number of comments were provided by the group members in a few days. The draft figure was improved based on the comments, and was shared in the group chat again for additional comments. This procedure was repeated for three times, and the figure was finalized at the third round with the approval from the members.
Figure 2 shows the dairy cooperative value chains connecting the 30 dairy farms studied. The mean herd size of the 30 farms was 73.6 cows (median: 60.5, quartile range: 30–79, range: 7–547). The mean milk yield per cow per day at the farms was 9.2 l (median = 8.3, range: 1.5–20.1), and the mean farm milk yield per day was 193.2 l (median = 162.5, quartile range: 67.0–247.2, range: 3.0–808.5) in 2019. There was no significant linear relationship between the log herd size and average milk yield per cow [slope = −0.01, standard error (SE) = 0.01, p = 0.270].
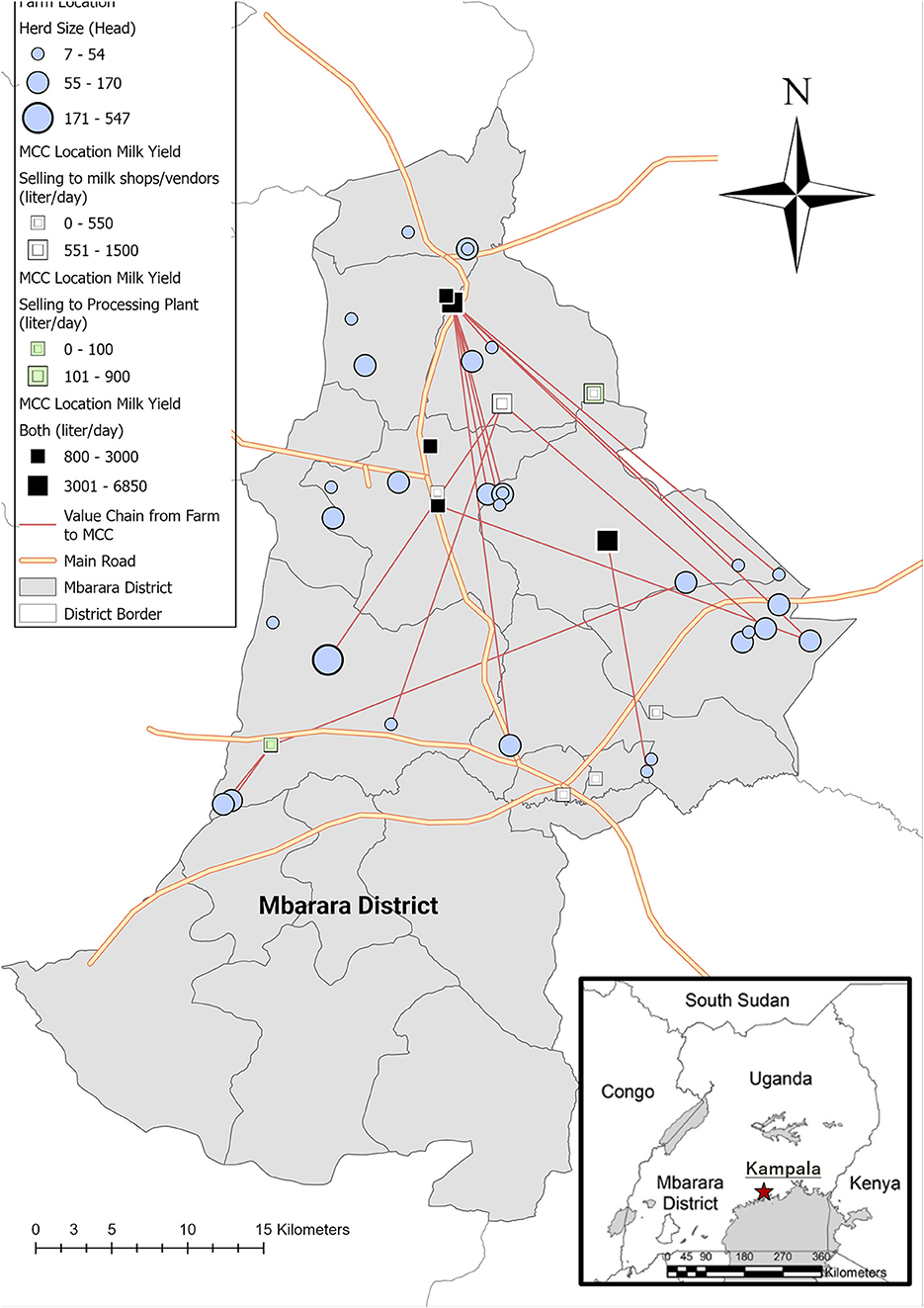
Figure 2. The structure of dairy cooperative value chains from the studied dairy farms to milk collection centers (MCCs) in Mbarara District. The blue circles indicate the locations and herd sizes of the dairy farms. The green squares indicate the locations and milk yields in liters of the MCCs that sold milk to processing plants only. The black squares indicate the locations and milk yields in liters of the MCCs that sold milk to both processing plants and milk shops/vendors in town. The white squares indicate the locations and milk yields in liters of the MCCs that sold milk to shops/vendors in town only.
The mean daily milk sales volume at 15 MCCs was 1,669 l (median = 900, quartile range: 450–2,250, range: 100–6,850). Among the 14 MCCs that provided a response, 6 MCCs (42.9%) bought milk from JICA project farms, and the mean number of JICA project farms that sold milk to an MCC was 3.2 (median = 2.5, range: 1–9, Figure 2). Out of the 30 JICA project farms, 16 farms (53.3%) sold milk to the six MCCs. The mean milk sales volume was significantly higher in the MCCs that accepted milk from JICA project farms (2,933.3 l) than in those that did not (866.3 l, p = 0.033). Out of 15 MCCs, nine MCCs sold milk to processing plants (60.0%), and the mean milk sales volume was marginally higher in the MCCs that sold milk to processing plants (2,386.7 l) than in those that specialized in selling raw milk to local towns (591.7 l, p = 0.052). Of the nine MCCs that sold milk to processing plants, seven MCCs (77.8%) also sold milk to local raw milk sales: milk shops and vendors, and even to local individual customers. Among the MCCs that sold milk to both processing plants and local customers, the mean proportion of local raw milk sales was 25.8% (median = 10.0%, range: 1.8%−70.6%).
Figure 2 shows the geographical structure of the dairy cooperative value chains in Mbarara District. There were three hub dairy cooperative MCCs that accepted milk from JICA project farms. Farmers did not always sell milk to neighboring MCCs; they sometimes chose to transport the milk over a long distance to the hub collection centers. Regardless the sales destinations (processing plants or milk shops/vendors), the majority (12/15, 80.0%) were located on the main tarmac roads.
Figure 3 shows the structure of dairy value chains starting from farms in Mbarara District that were identified in the participatory online appraisals. The field surveys in this study targeted the dairy value chains of dairy cooperatives belonging to the UCCCU. The participatory appraisals identified two other types of MCCs: MCCs owned by dairy processors and those by independent centers of traders. Dairy cooperatives distributed raw milk to both formal and informal value chains, as can be described quantitatively, but MCCs of independent traders distributed entirely to information value chains, which included a large volume of long distance supply to Kampala, the capital of Uganda. The milk collected by foreign investors was processed for export. There were several MCCs of domestic dairy processors of pasteurized and packaged milk, which were distributed to both Mbarara town and other cities, including Kampala. Ghee, cheese, yogurt, and butter were produced by foreign and domestic processors, dairy cooperatives, and home-based cottage industries. Consumers in Mbarara District bought raw milk directly from farm gates, MCCs, milk shops, and milk vendors.
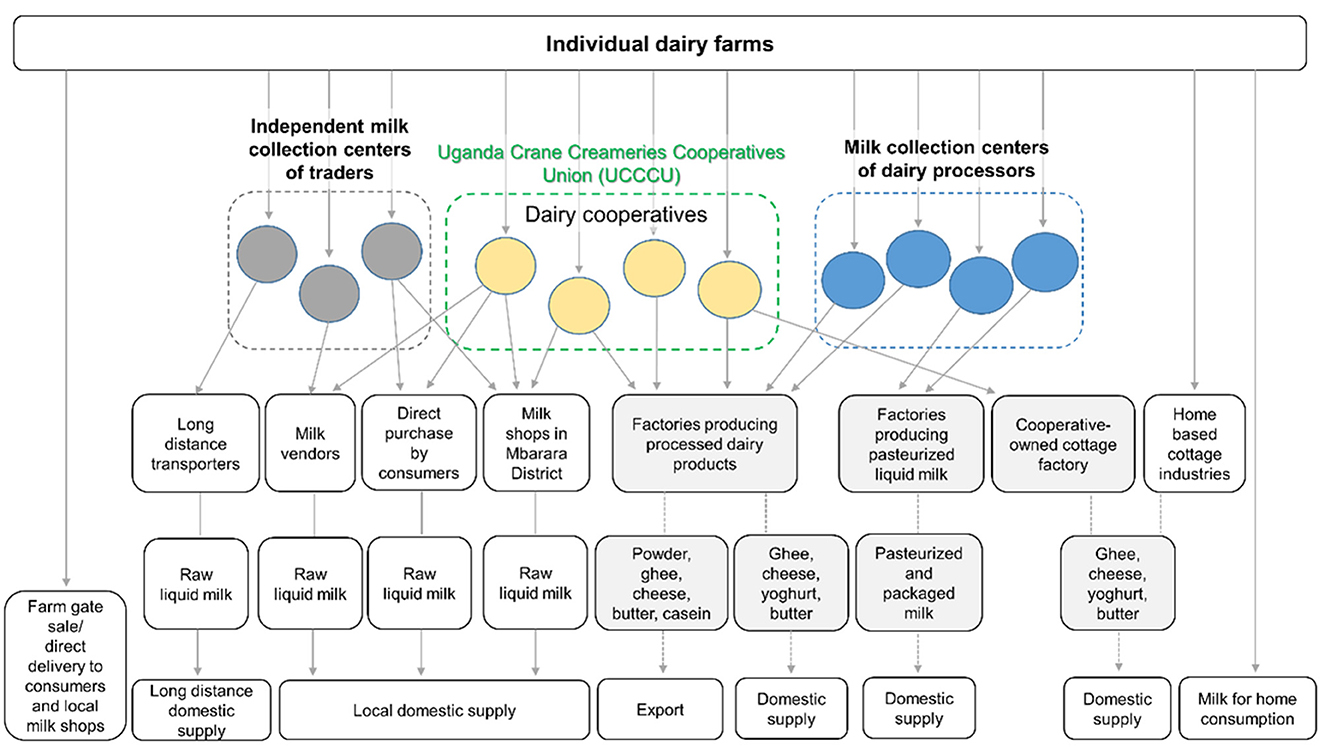
Figure 3. The structure of dairy value chains starting from individual dairy farms in Mbarara District, Uganda. Gray squares show the formal value chains, which export or sell treated products, and white squares show the informal value chains of raw liquid cow milk.
The mean farm bulk milk contamination score was not significantly different between before (3.3, median: 4; range: 1–4) and after (3.2, median: 3; range: 2–5) the intervention (p = 0.418). However, the proportion of hygienic farm bulk milk samples with score <4 significantly increased from before the intervention (37.9%, 11/29 farms) to after (69.0%, 20/29 farms, x2 = 4.4, df = 1, p = 0.035). Based on the scores, the mean log10 total bacterial count of farm bulk milk was simulated to be 3.69 (95% credible interval: 3.60–3.78) and 3.79 (95% credible interval: 3.76–3.81), before and after the intervention, respectively. Both total bacterial counts of farm bulk milk before and after the intervention were below the acceptable limit for human consumption as source milk intended for processing (p < 0.001).
The mean within-herd prevalence of sub-clinical mastitis significantly reduced from 72.3% before the intervention to 25.8% after [difference in logit = −2.29, SE = 0.160, p < 0.001, random effect: standard deviation (SD) = 0.775]. Based on the CMT results, there was no significant relationship between the farm bulk milk score and the within-farm prevalence of sub-clinical mastitis either before the intervention (rho = 0.09, p = 0.646) or after (rho = 0.25, p = 0.199).
Table 1 shows a comparison of the frequency and percentages of milking hygiene practice items conducted before and after the intervention. The mean proportion of the 19 items on the milking hygiene practice conducted significantly increased from before the intervention (4.2%) to after (67.4%, difference in logit = 7.13, SE = 0.636, p < 0.001, random effect SD = 2.498). The practices most conducted after the intervention were the milking of cows with mastitis last, and the disinfection of hands at the parlor (87.0%); the least conducted was pre-dipping using a strip cup (40.0%). Post-milking care (post-dipping, 47.0%) was often not conducted.
Table 2 shows the results of comparisons of mean farm bulk milk hygiene scores between the farms conducting milk hygienic items and not conducting among 29 dairy farms before the intervention. The mean bulk milk hygiene score was significantly or marginally lower (less bacteria contamination) in the farms conducting gentle hand milking (2.33), washing used towel with soap and rinsing cleanly (2.00), and drying a towel in sunlight (2.00) than the farms not conducting (3.36, p = 0.036; 3.41, p = 0.053, and 3.41, p = 0.053). In contrast, there was no milk hygiene practice item with significant difference in milk hygiene score between the farms conducting and not conducting after the intervention (Table 3). There was no significant relationship between the milk hygiene practice score and the farm bulk milk hygiene score both before the intervention (rho = −0.29, p = 0.121) and after (rho = 0.06, p = 0.787). The number of hygiene practice items conducted was not associated with the herd size either before the intervention (slope in log = −0.008, SE = 0.017, p = 0.656) or after (slope in log = 0.003, SE = 0.017, p = 0.859).
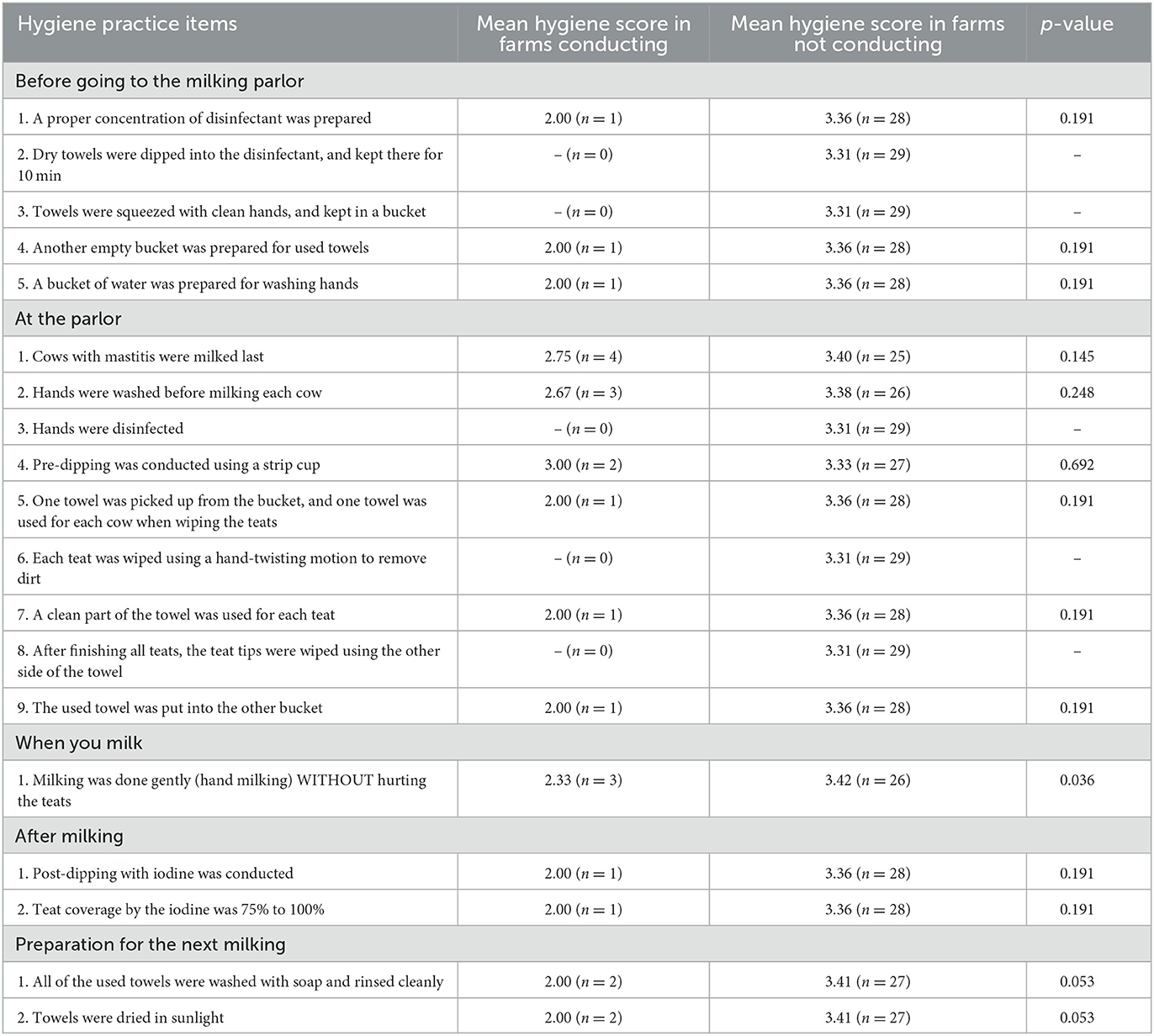
Table 2. Comparisons of mean farm bulk milk hygiene scores between the farms conducting milk hygiene practice items and not conducting among 29 dairy farms before the intervention.
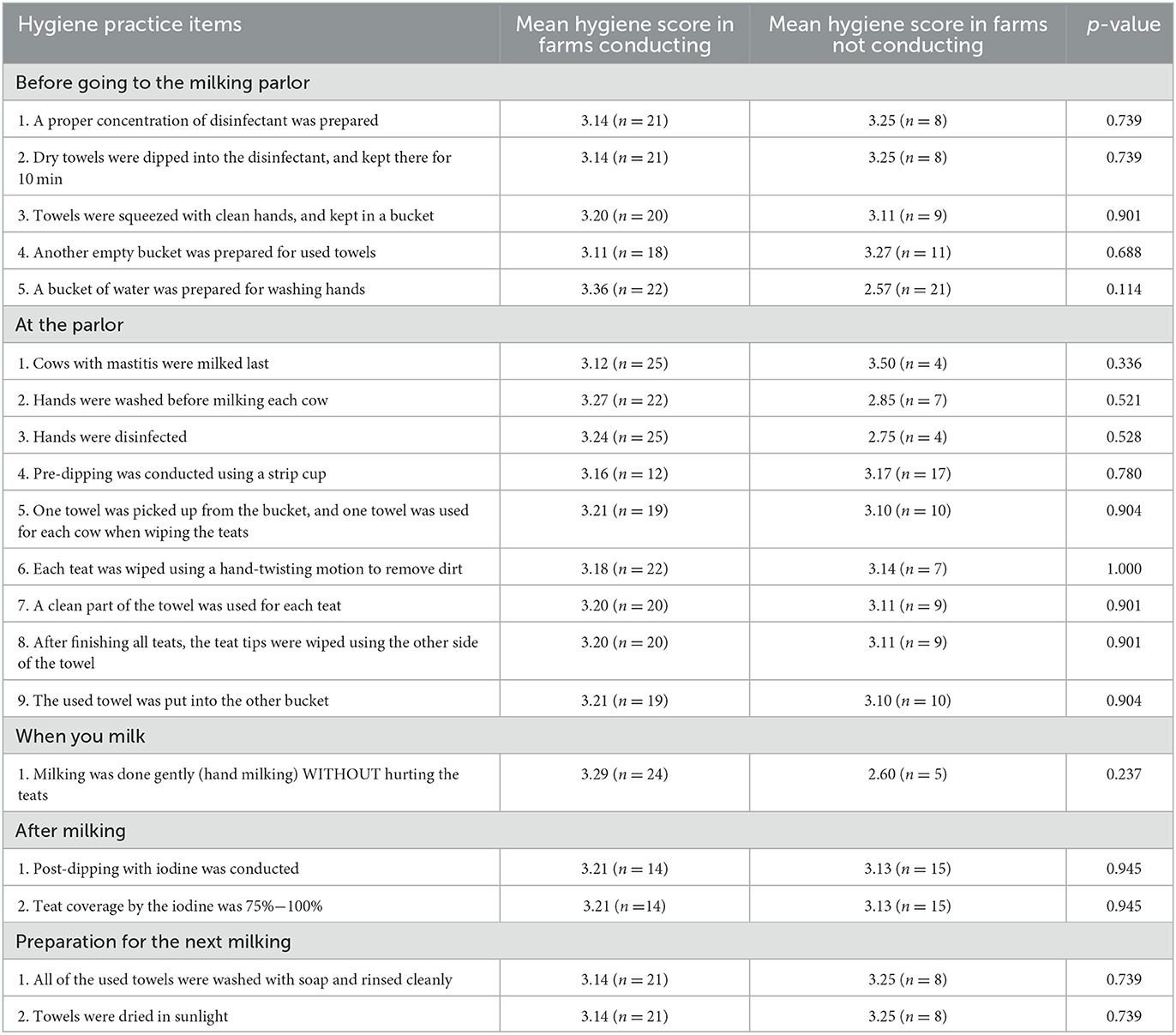
Table 3. Comparisons of mean farm bulk milk hygiene scores between the farms conducting milk hygiene practice items and not conducting among 29 dairy farms after the intervention.
The proportions of samples with a TNTC bacterial count among the dry swab and wet swab samples were 73.3% (22/30) and 80.0% (24/30), respectively. Water used for cleaning was also contaminated, with 56.7% (17/30) of the samples having a TNTC bacterial count. There were no significant differences in the mean bulk milk hygiene scores between farms with or without TNTC contamination in the pre-milking dry collecting cans (3.1 and 3.3, respectively, p = 0.957), between farms with or without TNTC contamination in the collecting cans after washing (3.3 and 2.8, respectively, p = 0.467), and between farms with or without TNTC contamination in the water used for cleaning (3.4 and 2.9, respectively, p = 0.234).
The log10 mean total bacterial count of bulk cooler milk in MCCs was estimated to be 6.50 (95% credible interval: 4.15–7.68). The proportion of milk samples with a total bacterial count below 105 CFU/ml, which is the limit for human consumption [Institute of Medicine (US) and National Research Council (US) Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food, 2003], was 13.3% (2/15). When receiving milk, 100.0% (14/14), 92.8% (13/14), and 7.1% (1/14) of the MCCs (n = 14) conducted regular alcohol tests, used a lactometer, and conducted resazurin tests. There was no significant correlation between the log10 total bacterial count in sold bulk cooler milk and the milk sales volume in MCCs (rho = 0.30, p = 0.277). The log10 total bacterial counts of bulk cooler milk were not significantly different between the MCCs that shipped milk to the processing plants of foreign investors (mean = 6.6) and those that shipped milk to local milk shops and vendors (mean = 6.3, t = 0.51, df = 13, p = 0.621).
In the evaluation of the magnitude of post-harvest contamination/bacterial multiplication, all of the 1,000 iterations of Wilcoxon rank sum tests comparing the log10 total bacterial counts between farm bulk milk (mean, before the intervention: 3.69, and after: 3.79, respectively) and bulk cooler milk in MCCs (mean: 6.50) produced p-values < 0.001, suggesting that the level of bacterial contamination is significantly higher in MCCs than in farm bulk milk.
This study was conducted to quantitatively understand the structure of the dairy cooperative value chains in an intensive dairy production area of Uganda, Mbarara District, and to assess the hygiene along the chain, and thereby identify the bottleneck of dairy hygiene intervention.
The JICA project targeted dairy farms that belong to the UCCCU, because the union comprised dairy cooperatives involving 18,000 farmers as of 2016, and was the largest dairy farmers' association in Uganda (Rakuno Gakuen University, 2019). Southwestern Uganda, including its historical center, Mbarara, the capital of the Ankole Kingdom, has been reported to be the largest source of raw milk in the value chain in Kampala, the capital of Uganda (Makita et al., 2010). However, the present study found that raw milk collected at the MCCs of dairy cooperatives under UCCCU was not transported to Kampala, but was targeted at formal value chains connected to export and domestic trades and the local domestic raw milk supply in Mbarara District. Although the relative contributions of entire dairy value chains were not quantified, the additional participatory online appraisals characterized entire formal and informal dairy value chains starting from Mbarara District. The entire picture indicated that the findings of the present study are representative of the milk hygiene situation only in dairy cooperative value chains. Therefore, the hygiene of dairy value chains of MCCs owned by independent traders and dairy processors should be separately studied. The UCCCU is preparing to open its own factory that produces pasteurized and packaged milk for domestic supply in Uganda (personal communications). This factory will bring direct revenue from consumers to the UCCCU, and will contribute to formalizing dairy value chains, and improve public health in Uganda. It has been estimated that the pasteurization of milk in intensive dairy production areas would have the greatest impact on reducing milk-borne diseases, such as brucellosis, in Kampala, if introduced (Makita et al., 2010).
The JICA project participating farms adopted the milk hygiene practice items very well, and the behavioral changes may have occurred because of the provision of epidemiological evidence and frequent visits for demonstration of intervention packages (Rakuno Gakuen University, 2019; Miyama et al., 2020). Before the intervention, there were significant associations between conduct of gentle hand milking and hygiene in the towels used for wiping teat and better farm bulk milk quality. The findings on the effect of these hygiene practices were similar to those on sub-clinical mastitis in these farms (Miyama et al., 2020), suggesting some potential effect of reducing sub-clinical mastitis on reducing bacterial contamination in bulk milk. However, our results showed that the level of bulk milk hygiene was not improved by the intervention in the health of cows. After the intervention, there was no milk hygiene practice item or handling of equipment which was associated with bulk milk hygiene. This may be due to the effects of several other hygiene practices introduced, and also contamination by the failure in hygienic handling of milk and equipment. The high levels of contamination of the water and milk cans before and after milking were of concern, and they indicated that the cleaning process was ineffective. The dry swab culture results suggested that sun-drying may have little effect against bacterial contamination if the washing process was inadequate inside a can. Regarding the issue of contaminated water, there have been reports of microbial contamination during the handling and storage after water collection in sub-Saharan Africa (Harris et al., 2013; Owusu-Kwarteng et al., 2020). It has also been reported that water contamination can occur when water storage containers are placed outdoors (Amenu et al., 2016). Although such contamination may be present, the level of hygiene in farm bulk milk is acceptable as a source milk intended for processing for human consumption [Institute of Medicine (US) and National Research Council (US) Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food, 2003].
A critical challenge identified was that post-harvest dairy hygiene still needs to be improved even though milk hygiene at the farm level is below the acceptable limit for processing. This study showed a significant increase in the bacterial counts at the MCCs when compared to the dairy farms. Only 13.3% of the MCCs in Mbarara District met this standard of the International Microbiological Criteria for Dairy Products [Institute of Medicine (US) and National Research Council (US) Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food, 2003]. Inadequate dairy hygiene practices by dairy farmers may contribute to increases in the bacterial counts in the value chain from dairy farmers to MCCs (Food and Agriculture Organization, 2019; Majalija et al., 2020; Miyama et al., 2020). Although the farms examined in the present study were trained for hygiene in the JICA project and used metal milk cans, there were still farmers using plastic buckets for milking in Mbarara District (Daburon and Ndambi, 2019). Dairy farms with poor hygiene management may cause more contamination in the MCCs. Even more problematic is the use of plastic jerry cans for transport. These cans cannot be adequately cleaned, and their surfaces are easily scratched, which promotes bacterial contamination. Although it has been reported that more farmers and MCCs in Mbarara District use milk cans when compared to other districts (Van Campenhout et al., 2019), the high contamination level of bulk milk in the MCCs studied may have been due to less hygienic milking equipment and contaminated water from farms that were not trained for hygiene in the JICA project (Van Campenhout et al., 2019; Majalija et al., 2020). The present study did not examine other factors associated with the transportation of milk from farms to MCCs, such as the milk temperature, mode of transportation, and the amount of time in transport, nor the environmental conditions at the dairy farms and MCCs. As shown in Figure 2, sometimes, farms did not transport milk to the nearest MCCs, but to distant ones. Some farms used a truck, motorcycle, or even a bicycle for transporting milk without cooling equipment. A prolonged transportation period without cooling may lead to increased bacterial counts (Mogotu et al., 2022). All of these factors might have contributed to the high bacterial count in bulk milk in the MCCs. As this study has shown, milk is transported to Kampala in both formal (packaged pasteurized milk) and informal value chains. A report has suggested that informal marketing systems generally lack proper sanitation, and raw milk is traded without regard to international standards for quality, pasteurization, or cold chain facilities (Majalija et al., 2020). Another study in Kampala reported that all of the 50 milk samples collected from milk outlets had total aerobic counts exceeding the limits of the World Health Organization and Uganda National Bureau of Standards (Kateregga et al., 2019). There is another limitation of the study—the sampling framework of dairy farms. To capture entire dairy value chain starting from Mbarara, larger sample size involving the dairy farms which do not belong to UCCCU would be necessary.
To improve the quality of milk in dairy value chains, the Netherlands Development Organization conducted a pilot project on a Quality Based Milk Payment System (QBMPS) in Mbarara between 2016 and 2019 (Daburon and Ndambi, 2019). The project introduced QBMPS to three processors (a large, a medium, and a small processor), and the large processor succeeded in developing QBMPS in the sourcing network, while the medium and small processors have yet to finish the pilot project (Daburon and Ndambi, 2019). The QBMPS needs to be introduced at least at the MCC level, as the processor examines the milk quality at that level. A pilot QBMPS project in Kenya has shown that the milk quality improved as well (Njiru and 3R Kenya Project, 2018). Sub-clinical mastitis can decrease the milk yield without being noticed (Blowey and Edmondson, 2010), and rejection of low-quality milk affects both dairy farmers and the MCCs. The present study showed the bottleneck of dairy hygiene intervention in Uganda. Awareness of the importance of milk quality must be urgently raised among health and agricultural authorities and private sectors to shift to quality-based thinking along entire dairy value chains throughout the country.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
KM, DB, JBu, WM, and AA conceptualized the study. KM, JBu, DB, JBy, MA, TK, and YM designed the study. JBu, DB, JBy, MA, TS, TK, and KM conducted field surveys. WM and AA facilitated the field surveys. YS, HS, JBy, and KM conducted the formal analyses. YS and KM drafted the manuscript. All authors edited and approved the final manuscript.
This study was supported by the JICA Partnership Program, Safe Milk Promotion in Mbarara Project, Hokkaido University World-Leading Innovative & Smart Education (WISE) Program for One Health Frontier, and Rakuno Gakuen University Graduate School of Veterinary Medicine.
We thank dairy farmers, Mbarara DVO extension workers, the MCCs, and the UCCCU for participating in the surveys and online participatory appraisals, particularly Paul Nyakairu of Rubyerwa Dairy Investment and Kharm Kamuntu of the UCCCU. We also thank The Inclusive Dairy Enterprise (TIDE) project of the Netherlands Development Organization for collaborations during the implementation of the JICA project. JICA Japan Overseas Cooperation Volunteers dispatched to Mbarara Veterinary Office collaborated in the follow up field activities in 2022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1110915/full#supplementary-material
Amenu, K., Shitu, D., and Abera, M. (2016). Microbial contamination of water intended for milk container washing in smallholder dairy farming and milk retailing houses in southern Ethiopia. SpringerPlus 5, 1195. doi: 10.1186/s40064-016-2841-x
Balikowa, D. (2011). Dairy development in Uganda: a review of Uganda's dairy industry. Dairy Dev. Authority Uganda. 3202, 65–66.
Barbano, D. M., Ma, Y., and Santos, M. (2006). Influence of raw milk quality on fluid milk shelf life. J. Dairy Sci. 89, E15–9. doi: 10.3168/jds.S0022-0302(06)72360-8
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., et al (2023). Linear mixed-effects models using ‘Eigen' and S4. Package ‘lme4' version 1.1-32. Available online at: https://github.com/lme4/lme4/ (accessed April 2, 2023).
Blowey, R. W., and Edmondson, P. (2010). Mastitis Control in Dairy Herds: Wallingford: Cabi. doi: 10.1079/9781845935504.0000
Daburon, A., and Ndambi, A. (2019). Assessment of the Quality Based Milk Payment System Pilot Supported by TIDE in Mbarara Milkshed, Uganda. Wageningen: Wageningen Centre for Development Innovation Wageningen University and Research. doi: 10.18174/497041
Dairy Development Authority (2021). Annual Report FY 2020/2021. Available online at: https://dda.go.ug/assets/files/DDA-annulareport20-21.pdf (accessed November 15, 2022).
Dhanashekar, R., Akkinepalli, S., and Nellutla, A. (2012). Milk-borne infections. An analysis of their potential effect on the milk industry. Germs 2, 101. doi: 10.11599/germs.2012.1020
Food Agriculture Organization (2019). The Future of Livestock in Uganda: Opportunities and Challenges in the Face of Uncertainty. Available online at: https://www.fao.org/3/ca5420en/ca5420en.pdf (accessed August 21, 2022).
Harris, A. R., Davis, J., and Boehm, A. B. (2013). Mechanisms of post-supply contamination of drinking water in Bagamoyo, Tanzania. J. Water Health 11, 543–554. doi: 10.2166/wh.2013.023
Institute of Medicine (US) National Research Council (US) Committee on the Review of the Use of Scientific Criteria Performance Standards for Safe Food (2003). Scientific Criteria to Ensure Safe Food. Washington, DC: National Academies Press. Appendix F, International Microbiological Criteria for Dairy Products. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK221563/ (accessed October 21, 2022).
Kateregga, J. N., Atuheire, C., Nabatta, E., Majalija, S., and Ndukui, J. G. (2019). Microbial load in unpasteurized milk obtained from selected milk outlets in Kawempe Division of Kampala, Uganda. Ann. Biotechnol. 2, 1018. doi: 10.13140/RG.2.2.11680.43526
Majalija, S., Tumwine, G., Kiguli, J., Bugeza, J., Ssemadaali, M. A., Kazoora, H. B., et al. (2020). Pastoral community practices, microbial quality and associated health risks of raw milk in the milk value chain of Nakasongola District, Uganda. Pastoralism 10, 1–11. doi: 10.1186/s13570-020-0158-4
Makita, K., Fevre, E. M., Waiswa, C., Eisler, M. C., and Welburn, S. C. (2010). How human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally-marketed milk. PLoS ONE 5, e14188. doi: 10.1371/journal.pone.0014188
Mbarara District Local Government (2021). Draft Third District Development Plan 2020/2021 – 2024/2025 (DDPIII). Available online at: https://www.mbarara.go.ug/sites/default/files/downloads/Mbarara%20draft%20DDPIII%20FEB%202021.pdf (accessed November 15, 2022).
Ministry of Agriculture Animal Industry Fisheries. (2021). Dairy Exports Jump 63 Percent in Three Years. Available online at: https://www.agriculture.go.ug/2021/07/06/dairy-exports-jump-63-per-cent-in-three-years (accessed November 15, 2022).
Ministry of Agriculture Forestry and Fisheries. (1997). Food Safety and Consumer Affairs Bureau Guidelines for Clinical Pathology Tests in Livestock Insurance Scheme. Tokyo: Ministry of Agriculture, Forestry and Fisheries, 493–505.
Miyama, T., Byaruhanga, J., Okamura, I., Nagahata, H., Murata, R., Mwebembezi, W., et al. (2020). Prevalence of sub-clinical mastitis and its association with milking practices in an intensive dairy production region of Uganda. J. Vet. Med. Sci. 82, 488–493. doi: 10.1292/jvms.19-0588
Mogotu, M. W., Abong, G. O., Mburu, J., and Ndambi, O. A. (2022). Assessment of hygiene practices and microbial safety of milk supplied by smallholder farmers to processors in selected counties in Kenya. Trop. Anim. Health Prod. 54, 1–13. doi: 10.1007/s11250-022-03214-7
Njiru, R., and 3R Kenya Project (2018). Costs and Benefits of a Quality-based Milk Payment System (QBMPS): A Private Good Perspective. The Case of Happy Cow Ltd. 3R Kenya Research Report 002. Available online at: http://www.3r-kenya.org/wp-content/uploads/2018/06/Cost-Benefit-Analysis-of-QBMPS.pdf (accessed November 21, 2022).
Nkwasibwe, A., Mugisha, J., Elepu, G., and Kaneene, J. (2015). Increasing the efficiency of the dairy value chain in Uganda: determinants of choice of milk marketing channels by dairy farmers in Kiruhura District, Uganda. Livest. Res. Rural. Dev. 27, 168.
Oliver, S. P., Boor, K. J., Murphy, S. C., and Murinda, S. E. (2009). Food safety hazards associated with consumption of raw milk. Foodborne Pathog. Dis. 6, 793–806. doi: 10.1089/fpd.2009.0302
Owusu-Kwarteng, J., Akabanda, F., Agyei, D., and Jespersen, L. (2020). Microbial safety of milk production and fermented dairy products in Africa. Microorganisms 8, 752. doi: 10.3390/microorganisms8050752
Rakuno Gakuen University (2019). The JICA Partnership Program, Safe Milk Promotion in Mbarara Project Final Report. Available online at: https://rakuno-woahcentre.org//wp-content/uploads/Safe-milk-final-report_20190916_final-1.pdf (accessed August 20, 2022).
Roberts, H. A. (1993). Raw milk quality - milk flavor. Kansas Agricultural Experiment Station Research Reports. Manhattan, 57–60. doi: 10.4148/2378-5977.2966
Ruegg, P. (2005). California Mastitis Test (CMT) Fact Sheet 1. Available online at: http://milkquality.wisc.edu/wp-content/uploads/2011/09/california-mastitis-test-factsheet.pdf (accessed on February 20, 2018).
Staal, S. J., and Kaguongo, W. (2003). The Ugandan Dairy Sub-sector: Targeting Development Opportunities. Nairobi: International Livestock Research Institute(ILRI). Available online at: https://hdl.handle.net/10568/2089 (accessed November 15, 2022).
Uganda Bureau of Statistics (2021). 2020 Statistical Abstract. Available online at: https://www.ubos.org/wp-content/uploads/publications/11_2020STATISTICAL__ABSTRACT_2020.pdf (accessed November 15, 2022).
Uganda Revenue Authority (2021). URA Annual Data Book Fiscal Year 2020/2021. Available online at: https://www.ura.go.ug/openFileController/execute?path=//webupload//upload//download//staticContent//TOPMENU//924//10586_URA_ANNUAL_DATA_BOOK_-FY_2020-21.pdf (accessed November 15, 2022).
Van Campenhout, B., Minten, B., and Swinnen, J. (2019). Domestic versus Export-led Agricultural Transformation: Evidence from Uganda's Dairy Value Chain. Washington, DC: International Trade eJournal. doi: 10.2499/p15738coll2.133502
Wangalwa, R., Tolo, C. U., Rugunda Kagoro, G., and Matofari, J. W. (2016). Assessment of on-Farm Milk Handling Practices in Mbarara District Southwestern Uganda. Available online at: http://ir.must.ac.ug/xmlui/bitstream/handle/123456789/697/Assessmentofon-farmmilkhandlingpracticesinMbararaDistrictSouthwesternUganda_BIOLOGY.pdf?sequence=1andisAllowed=y (accessed November 15, 2022).
Keywords: dairy value chain, milk hygiene, dairy cooperative, hygiene management, milk contamination
Citation: Sugino Y, Bugeza J, Bahame D, Byaruhanga J, Shimazaki H, Anzai M, Kayano T, Mwebembezi W, Akashaba A, Shimada T, Muramatsu Y and Makita K (2023) Structure and milk hygiene of dairy cooperative value chains in an intensive production area of Uganda—A bottleneck of intervention. Front. Sustain. Food Syst. 7:1110915. doi: 10.3389/fsufs.2023.1110915
Received: 29 November 2022; Accepted: 05 May 2023;
Published: 24 May 2023.
Edited by:
Hung Nguyen-Viet, International Livestock Research Institute (ILRI), KenyaReviewed by:
Kebede Amenu, Addis Ababa University, EthiopiaCopyright © 2023 Sugino, Bugeza, Bahame, Byaruhanga, Shimazaki, Anzai, Kayano, Mwebembezi, Akashaba, Shimada, Muramatsu and Makita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kohei Makita, a21ha2l0YUByYWt1bm8uYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.