- 1Indian Council of Agricultural Research (ICAR)-Central Research Institute for Dryland Agriculture, Hyderabad, Telangana, India
- 2All India Coordinated Research Project on Dryland Agriculture Center, Ballowal Saunkhri, Punjab Agricultural University, Ludhiana, Punjab, India
Plant beneficial microorganisms are being used to improve soil health and crop yield in different cropping systems. Maize is an important crop grown around the world for food, feed and raw material for various industries. The aim of the present study was to evaluate two microbial consortia viz., microbial consortia 1 (Pseudomonas putida P7 + Paenibacillus favisporus B30) and microbial consortia 2 (Pseudomonas putida P45 + Bacillus amyloliquefaciens B17) under field conditions for their suitability in improving maize yield under rainfed situations at Ballowal Saunkhri (Punjab) having sub-humid (Hot Dry) climatic conditions. Pooled analysis of three years field experiments data showed that, seed + soil application of microbial consortia 1 and 2 led to enhancement in grain yield of kharif maize by 27.78 and 23.21% respectively over uninoculated control. Likewise, significant increase in Benefit:Cost ratio as well as straw yield was also observed. The present investigation suggests that, microbial consortia would help in significantly improving the yield and economics of maize grown on inceptisols under rainfed conditions.
Introduction
Human population is expected to reach around 10 billion by 2050 and global food demand is projected to rise by 62% (van Dijk et al., 2021). It is estimated that, agricultural production needs to be increased by 60–70% to meet food demand of human population in 2050 (Silva, 2018). Maize is cultivated globally being the 3rd most important cereal crop after rice and wheat. Climate change significantly affects the crop production (Zhao et al., 2017). It is estimated that, climate change negatively affects maize production and decrease its yield by 24% (Jägermeyr et al., 2021). In India, it is being grown in about 9.3 m ha with a production of 30 m tons during the year 2020–21 (www.agricoop.nic.in). Kharif season accounts for about 83% of area under maize cultivation. Out of which more than 70% area is under rainfed condition.
Beneficial bacteria are extensively used to improve soil health, crop growth and yield (Srinivasarao and Manjunath, 2017; Efthimiadou et al., 2020). They extend necessary ecosystem services that help to enhance soil health and plant growth (Santos et al., 2019; Rouphael and Colla, 2020). The need for sustainable soil health and agricultural production worldwide led scientists to investigate different kinds of beneficial bacteria for their effectiveness. This has led to generation of lot of scientific evidence on beneficial interaction between plants and useful bacteria improving yield and productivity of cropping systems (Pereg and McMillan, 2015; Efthimiadou et al., 2020). Nowadays, instead of single culture combination of two or more cultures as consortia are being used to improve plant growth and development (Olanrewaju and Babalola, 2019). Use of microbial consortia improved the plant growth and yield of various crops (Saikia et al., 2018; Silambarasan et al., 2019; Jain et al., 2021). Hence, we hypothesized that, microbial consortia increase crop growth and yield of maize. Since combination of microbial cultures carries out multiple functions, which are not possible for a single species. Further, consortia are not easily get affected by environmental alterations as individual microbial cultures (Brenner et al., 2008; Lindemann et al., 2016). Bacillus and Pseudomonas species have been reported to improve growth and yield crop plants (Radhakrishnan and Lee, 2016; Costa-Gutierrez et al., 2020). Application of microbial consortium comprising of Rhizoglomus irregulare and Pseudomonas putida increased P use efficiency and maize productivity (Pacheco et al., 2021). Use of beneficial microorganisms along with farm yard manure improved maize growth (Hussain et al., 2021). Consortia of beneficial bacteria stimulated maize growth and development (Walker et al., 2012; Olanrewaju and Babalola, 2019). The bacterial isolates used in the present study were tested individually for their ability improve plant growth under pot conditions (Sandhya et al., 2009, 2011; Ali et al., 2011). The Bacillus amyloliquefaciens B17 and Paenibacillus favisporus B30 were Gram positive with irregular margin, tested positive for citrate, catalase, and oxidase activity. The former was able to produce siderophores but not the latter. The Pseudomonas putida P7 and Pseudomonas putida P45 were Gram negative and posses the PGP characteristics such as P-solubilization, synthesis of indole acetic acid, gibberellins and siderophore. Further, they were also evaluated in pot experiments for plant growth promotion in maize, sunflower and wheat under moisture stress conditions. They have increased proline, sugar and protein contents in leaves, improved relative water content (RWC) and reduced leaf water loss (LWL) of inoculated plants under moisture stress conditions (Sandhya et al., 2009, 2011; Ali et al., 2011).
Hence, the aim of the present study was to evaluate the open field effectiveness of two microbial consortia for improving yield of maize under rainfed conditions at Ballowal Saunkhri (Punjab) having sub-humid (Hot dry) climatic conditions.
Materials and methods
Experimental site
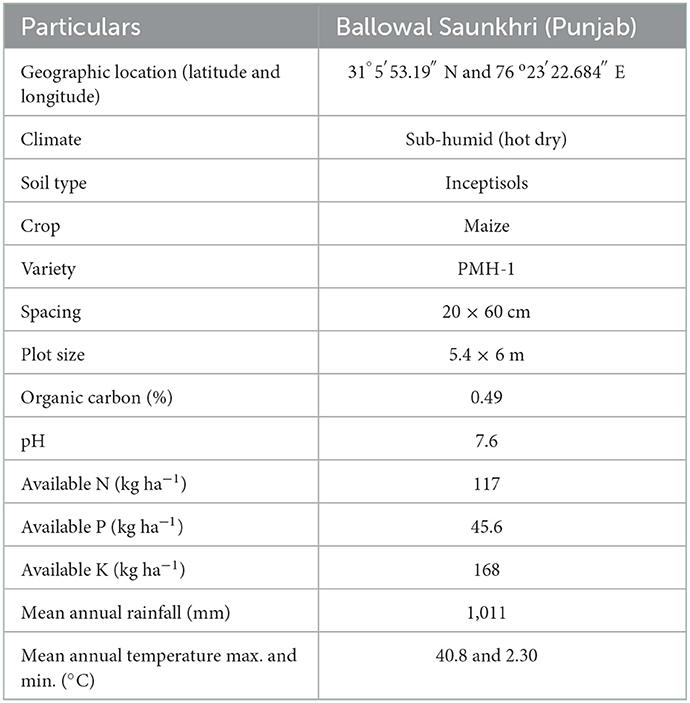
Starting from 2018–19 to 2020–21, 3 years consecutive field experiments were conducted at the research farm of All India Coordinated Research Project on Dryland Agriculture (AICRPDA) center, Ballowal Saunkhri (Punjab). A summary of geographic, edaphic and climatic characteristics of the site has been given in Table 1.
Microbial consortia
The microbial consortia developed by ICAR-Central Research Institute for Dryland Agriculture, Hyderabad were used for the evaluation. They include Pseudomonas putida P7 + Paenibacillus favisporus B30 (Microbial consortia 1) and Pseudomonas putida P45 + Bacillus amyloliquefaciens B17 (Microbial consortia 2). The bacteria used in the consortia development have been deposited at a national repository i.e., National Agriculturally Important Microbial Culture Collection (NAIMCC), Kushmaur, Mau Nath Bhanjan−275103, Uttar Pradesh (India) with the following accession numbers NAIMCC-B-00922 (Pseudomonas putida P7); NAIMCC-B-01801 (Paenibacillus favisporus B30); NAIMCC-B-00923 (Pseudomonas putida P45); and NAIMCC-B-00921 (Bacillus amyloliquefaciens B17). The isolates used in consortia development were tested for compatibility through confrontation studies. They were also tested for PGPR activities such as exopolysaccharide production, thermotolerance, ammonia production, P solubilization, synthesis of indole acetic acid, gibberellins, siderophore, and hydrogen cyanide (Sandhya et al., 2009, 2011; Ali et al., 2011).
Preparation of talc formulations
The Kings' B broth was used to grow Pseudomonas putida P7 and Pseudomonas putida P45 whereas, Paenibacillus favisporus B30 and Bacillus amyloliquefaciens B17 were grown in nutrient broth media. The cultures were kept on a shaker (120 rpm) at 28 ± 2°C for 48 h. The cell density of all the isolates was adjusted spectrophotometrically to 108 cells mL−1 by measuring optical density (0.8) at 600 nm. Talc formulations were made by mixing equal volumes of Pseudomonas putida P45 + Bacillus amyloliquefaciens B17 and Pseudomonas putida P7 + Paenibacillus favisporus B30 (Vidhyasekaran and Muthuamilan, 1995).
Treatment details
T1: Uninoculated control, T2: Seed treatment of microbial consortia 1 (@30 g/kg seeds), T3: Soil application of microbial consortia 1 [@2.5 kg of talc formulation mixed with 50 kg of well-decomposed Farmyard manure (FYM)/ha], T4: Seed + Soil application of microbial consortia1 (T1 + T2), T5: Seed treatment of microbial consortia 2 (@30 g/kg seeds) T6: Soil application of microbial consortia 2 (@2.5 kg of talc formulation mixed with 50 kg of well-decomposed FYM/ha), T7: Seed + Soil application of microbial consortia 2 (T4 + T5). For seed treatment, a slurry of talc formulation was prepared with 1% carboxy methyl cellulose, the slurry was coated onto the surface of seeds, shade dried for 30 min and used for sowing. For soil application, the dosage used was 2.5 kg of talc formulation was mixed with 50 kg of well-decomposed FYM/ha. The talc formulation was properly mixed with FYM and kept overnight, applied to soil by broadcasting before sowing. The experiment was conducted by following randomized block design with three replications. The crop was raised by following recommended agronomic practices and fertilizer doses. Maize variety, PMH-1 was sown on 7th July in 2018 and 2019 and on 6th July in 2020 after the onset on monsoon rains entirely as a rainfed crop. The crop was sown at a spacing of 60 × 20 cm by using 20 kg seeds ha−1. The sowing was done by dibbling seeds 3–5 cm deep. The size of each plot was 5.4 × 6 m with a buffer zone of 1 m between the plots with 120 plants in net plot area (4 × 3.6 m). The recommended dose of 80 kg N ha−1 was applied through urea (180 kg ha−1), 40 kg P2O5 through single superphosphate (250 kg ha−1) and 20 kg K2O through muriate of potash (37.5 kg ha−1). The half of N (90 kg urea per hectare) and entire doses of P2O5 and K2O were applied as basal and remaining half dose of N was top dressed uniformly at knee high stage in all the treatments. It was on done 3rd October, 27th and 22nd September in 2018, 2019, and 2020, respectively. All other cultural operations were followed as per the package of practices of Punjab Agricultural University (Anonymous, 2018). The crop was harvested manually and grain yield was calculated on 14% moisture basis. The rows of maize from the net plot and border strips (14.4 m2) were harvested separately to record the grain and straw yield. Ten cobs were randomly selected from each net plot area to evaluate cob length, and number of grains per cob. For 1,000-grain weight, all the cobs from each net plot were thrashed and one thousand grains were counted from the yield of each net plot and then weighed. Cost of cultivation, net monetary returns and benefit: cost ratio were calculated on the basis of prevailing market price of inputs and outputs.
Soil sampling and analysis
Before sowing of maize crop, three random soil samples were collected 0–15 cm depth (from top surface) and composited. The soil samples were hand crushed and passed through 2 mm sieve and used for the analysis. The available nitrogen (Subhiah and Asija, 1956), available phosphorus (Olsen et al., 1954), available potassium (Piper, 1966) and soil organic carbon (Walkley and Black, 1934) contents were determined. Soil moisture was measured by following gravimetric method at sowing, flowering and harvest stages (Black, 1965). Net Returns, Benefit: Cost and Rain Water Use Efficiency (RWUE) were calculated by following formulae viz.
respectively.
Statistical analysis
Each year data was analyzed using triplicate sets of data, by ANOVA using the Statistical Package for Social Sciences 16.0 (SPSS, 2016) for windows. The pooled data analysis was done by taking mean value of each year for the parameters analyzed. The p-value of each parameter obtained through the analysis is given in Table 3 and Supplementary Tables S1–S3.
Results
Rainfall received and dryspells occurred during the cropping season
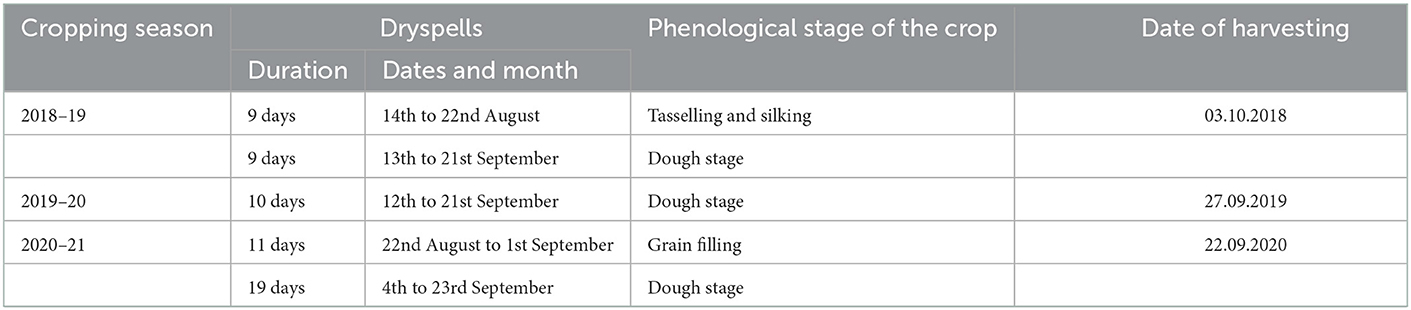
An amount of 894.5, 732.4, and 493.9 mm of rainfall received at Ballowal Saunkhri during the cropping seasons of 2018, 2019, and 2020, respectively. Year wise rainfall received during the cropping season has been presented as Supplementary Figure 1. During the year 2018, two dryspells of 09 days each occurred at tasselling and silking stage as well as dough stage, respectively. One dryspell of 10 days occurred at dough stage during the year 2019. In the year, 2020 two dryspells of 11 and 19 days occurred at grain filling and dough stage, respectively (Table 2).
Effect of seed and soil application of microbial consortia on grain and straw yield of maize
Three years of field experiments have showed that, the treatments with seed + soil application of microbial consortia 1 (3,858.66 kg/ha) and microbial consortia 2 (3,720.66 kg/ha) significantly increased maize grain yield as compared to uninoculated control (3,019.66 kg/ha). The straw yield was also significantly improved due to seed + soil application of microbial consortia 1 (9,110 kg/ha) as well as microbial consortia 2 (8,827.33 kg/ha) compared to uninoculated control which recorded lowest straw yield of 6,934.33 (Table 3). Year wise data on the influence of microbial consortia on grain and straw yield of maize has been provided in Supplementary Tables S1–S3.
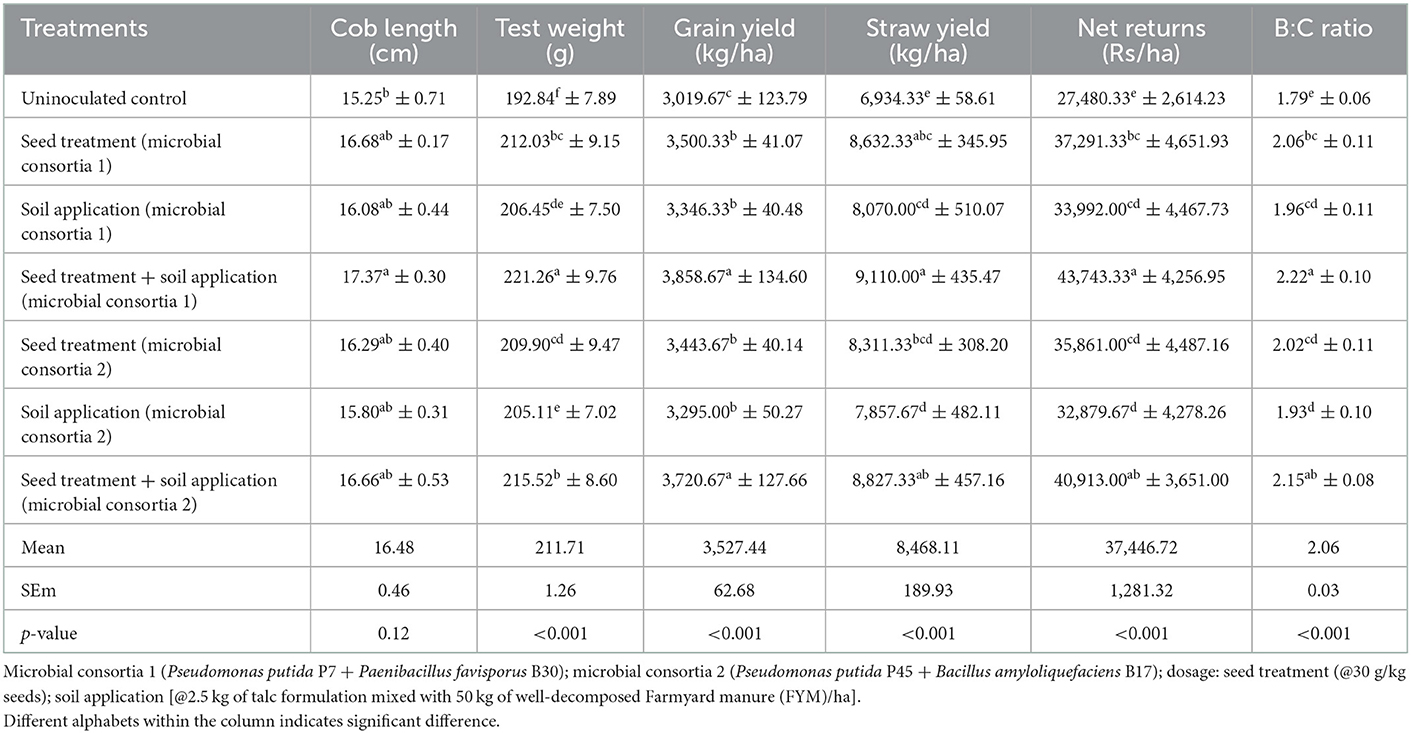
Table 3. Influence of seed and soil application of microbial consortia on yield attributes, yield, and economics of maize.
Effect of microbial consortia on net returns and Benefit:Cost ratio
Significant increase in net returns was observed due to the use of microbial consortia. The treatments with seed + soil application of microbial consortia 1 (Rs. 43,743.33) and microbial consortia 2 (Rs.40,913.00) recorded significantly higher net returns (p < 0.001) as compared to uninoculated control (Rs. 27,480.33). Likewise, significantly (p < 0.001) higher B:C ratios were also recorded due to the use of microbial consortia 1 (2.22) and microbial consortia 2 (2.14) vis-a-vis uninoculated control, which recorded lowest B:C ratio of 1.79 (Table 3). Year wise data on net returns and B:C ratio has been given as Supplementary Tables S1–S3.
Effect of microbial consortia on soil moisture content and rain water use efficiency
Soil moisture content was measured at sowing, flowering and harvest stages. Relatively higher soil moisture content was observed at flowering stage between uninoculated control and microbial consortia 1 and 2 (Figure 1). Year wise soil moisture content data has been provided as Supplementary Figures S2, S4, S6. Use of microbial consortia particularly seed + soil application of microbial consortia 1 (5.73 kg/ha/mm) and microbial consortia 2 (5.53 kg/ha/mm) significantly enhanced the rain water use efficiency of maize compared to uninoculated control (4.52 kg/ha/mm) (Figure 2). Year wise data on rain water use efficiency has been presented in Supplementary Figures S3, S5, S7.
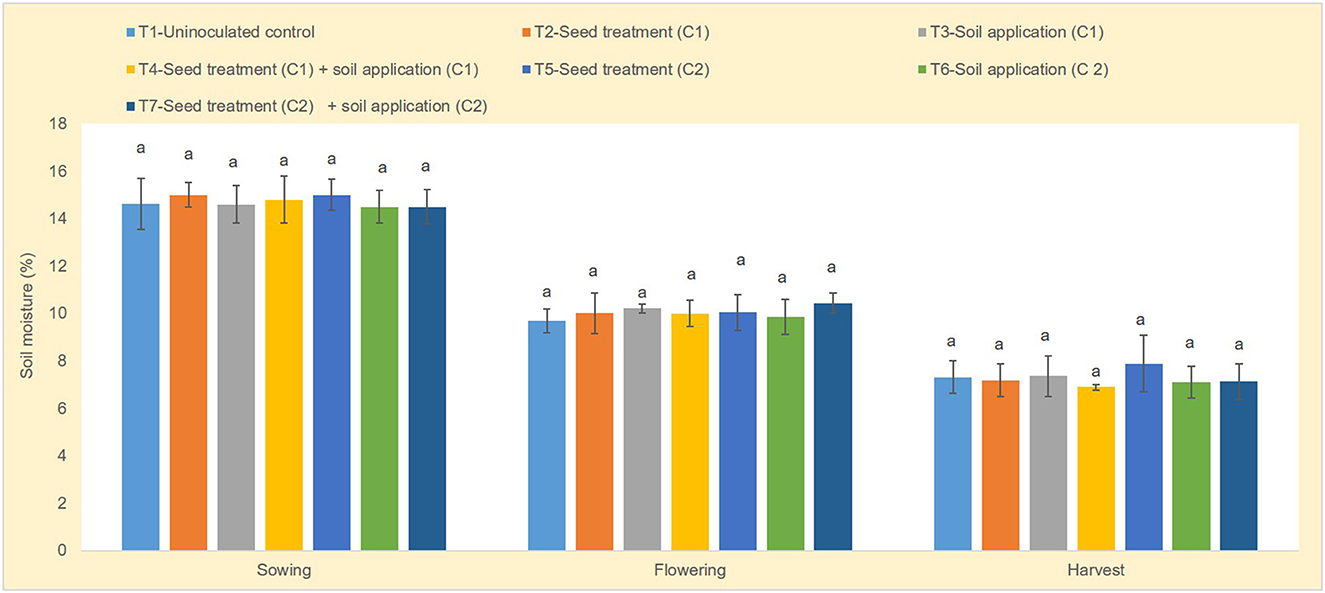
Figure 1. Soil moisture (%) at different stages of crop growth at Ballowal Saunkhri (Punjab). Sowing (p = 0.92), flowering (p = 0.53), and harvest (p = 0.67); error bars indicate standard error. The different alphabets on the top of error bars indicate the significant difference.
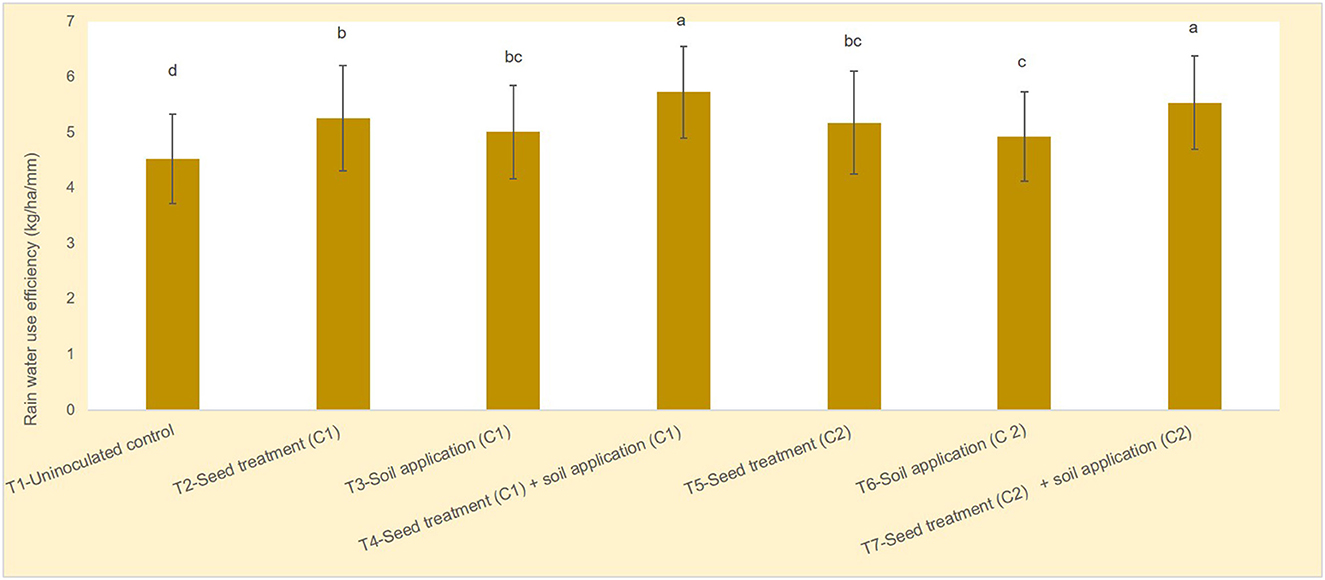
Figure 2. Effect of microbial consortia on rain water use efficiency of maize. p = 0.00; error bars indicate standard error. The different alphabets on the top of error bars indicate the significant difference.
Discussion
Beneficial microorganisms have been used to improve growth and yield of wheat (Kumar et al., 2014), sunflower (Alami et al., 2000), chickpea (Ogola et al., 2021), maize (Chahal et al., 2022), okra (Manjunath et al., 2016), and many other crops. The microbial technologies offer ecofriendly and affordable means for sustainable soil health and crop production (Kumar et al., 2014; Manjunath et al., 2016). Plant associated microorganisms help to improve plant growth and development through mobilization, solubilization and transformation of plant nutrients, production of plant hormones, exopolysaccharides and regulation of biochemical compounds like sugars and proline.
In the present investigation, seed + soil application of microbial consortia 1 and 2 recorded significantly higher grain as well as straw yield of maize as compared to uninoculated control (Table 3). This may be due to beneficial effects exerted by the microbial consortia on maize plants. The bacteria used in the present study viz., Pseudomonas putida P7 + Paenibacillus favisporus B30 (Microbial consortia 1) and Pseudomonas putida P45 + Bacillus amyloliquefaciens B17 (Microbial consortia 2) were previously tested for plant growth promoting characteristics. They were able to produce phytohormones such as IAA, solubilize phosphorus, synthesize exopolysaccharides (EPS) and increased proline, sugar and protein contents in leaves under moisture stress conditions. Also improved relative water content (RWC) and reduced leaf water loss (LWL) of inoculated plants (Sandhya et al., 2009, 2010, 2011; Ali et al., 2011). Both seed as well as soil application might have resulted in providing more inoculum for improved colonization as compared to seed or soil application alone. Fitriatin et al. (2021) reported that, seed and soil application of biofertilizers improved growth of maize compared to single method of application.
Kaur and Reddy (2014) evaluated phosphate-solubilizing bacteria such as Pantoea cypripedii and Pseudomonas plecoglossicida at three different agroclimatic regions and reported that, these two bacteria significantly improved the phosphorous (P) uptake, soil organic carbon, activity of soil enzymes, grain yield of maize and wheat at three agroclimatic regions. Notable increase in maize productivity under field conditions was observed due to the use of microbial consortium which resulted in improved phosphorus use efficiency (Pacheco et al., 2021). Likewise, IAA producing bacteria promoted plant growth through improved root growth leading to increased water and nutrient uptake (Mantelin and Touraine, 2004; Myo et al., 2019). Inoculation of plant growth promoting bacteria viz., Pseudomonas spp. and Azotobacter spp. in maize significantly improved leaf area and relative water content as compared to uninoculated control under moisture stress conditions (Osama et al., 2020). Seed treatment of maize with Pseudomonas fluorescens strain 153 caused enhancement in proline content and improved the tolerance of plants to water deficit stress conditions (Ansary et al., 2012). Proline helps to maintain osmotic potential of cell, scavenge reactive oxygen species, stabilizes proteins and cell membrane leading to prevention of electrolytes leakage (Chun et al., 2018). EPS producing Pantoea aglomerans NAS206 improved root colonization, soil aggregation and thereby moisture stress tolerance of wheat (Amellal et al., 1998). In the present study, dryspells of 09–19 days duration were observed during the cropping seasons. Application of microbial consortia 1 and 2 resulted in relatively higher soil moisture content at flowering stage as compared to uninoculated control (Figure 1). This may be attributed to improved water holding capacity as a consequence of increased soil aggregation due to exopolysaccharide synthesis ability of bacteria used in the consortia. Inoculation of wheat with EPS producing microbial consortia consisting of Planomicrobium chinense strain P1 and Bacillus cereus strain P2 significantly improved the soil moisture availability as well as chlorophyll, sugar and leaf protein contents under rainfed conditions at different locations (Khan and Bano, 2019). Further, significant increase in rain water use efficiency was recorded due to seed + soil application of microbial consortia 1 and 2 as compared uninoculated control (Figure 2).
Conclusions
Evaluation of microbial consortia under field conditions for 3 years suggested that, both microbial consortia 1 (Pseudomonas putida P7 + Paenibacillus favisporus B30) and microbial consortia 2 (Pseudomonas putida P45 + Bacillus amyloliquefaciens B17) significantly increased yield and economics of kharif maize under rainfed conditions at Ballowal Saunkhri (Punjab) having sub-humid (Hot Dry) climatic conditions. Since microbial consortia are ecofriendly and affordable, they can be conveniently used to improve maize production under rainfed farming.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MM, GC, SY, and KG: designed research. AK: performed research. MM and AK: analyzed data. MM, NJ, and KS: wrote the manuscript. VS, MS, and MP: resources. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely acknowledge the help received from ICAR-CRIDA, AICRPDA, Hyderabad; ICAR Network project on National Innovations in Climate Resilient Agriculture (NICRA); and AICRPDA center, Ballowal Saunkhri (PAU), Punjab toward undertaking this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1108492/full#supplementary-material
Supplementary Figure 1. Rainfall received during the cropping seasons 2018 (a), 2019 (b), and 2020 (c).
Supplementary Figure 2. Soil moisture (%) at different stages of maize growth during the year 2018. Sowing (p = 0.07), flowering (p = 0.73), and harvest (p = 0.85); error bars indicate standard error.
Supplementary Figure 3. Effect of microbial consortia on rain water use efficiency of maize during the year 2018. p = 0.05; error bars indicate standard error.
Supplementary Figure 4. Soil moisture (%) at different stages of maize growth during the year 2019. Sowing (p = 0.20), flowering (p = 0.94), and Harvest (p = 0.11); error bars indicate standard error.
Supplementary Figure 5. Effect of microbial consortia on rain water use efficiency of maize during the year 2019. p = 0.04; error bars indicate standard error.
Supplementary Figure 6. Soil moisture (%) at different stages of maize growth during the year 2020. Sowing (p = 0.92), flowering (p = 0.15), and Harvest (p = 0.75); error bars indicate standard error.
Supplementary Figure 7. Effect of microbial consortia on rain water use efficiency of maize during the year 2020 p = 0.02; error bars indicate standard error.
Supplementary Table 1. Influence of seed and soil application of microbial consortia on yield attributes, yield and economics of maize during the year 2018 under rainfed conditions of India.
Supplementary Table 2. Influence of seed and soil application of microbial consortia on yield attributes, yield and economics of maize during the year 2019 under rainfed conditions of India.
Supplementary Table 3. Influence of seed and soil application of microbial consortia on yield attributes, yield and economics of maize during the year 2020 under rainfed conditions of India.
References
Alami, Y., Achouak, W., Marol, C., and Heulin, T. (2000). Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 66, 3393–3398. doi: 10.1128/AEM.66.8.3393-3398.2000
Ali, S. K. Z., Sandhya, V., Grover, M., Rao, L. V., and Venkateswarlu, B. (2011). Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Int. 6, 239–246. doi: 10.1080/17429145.2010.545147
Amellal, N., Burtin, G., Bartoli, F., and Heulin, T. (1998). Colonization of wheat rhizosphere by EPS producing Pantoea agglomerans and its effect on soil aggregation. Appl. Environ. Microbiol. 64, 3740–3747. doi: 10.1128/AEM.64.10.3740-3747.1998
Anonymous (2018). Package of Practices for Kharif Crops of Punjab. Ludhiana: Punjab Agricultural University.
Ansary, M. H., Rahmani, H. A., Ardakani, M. R., Paknejad, F., Davood Habibi, D., and Mafakheri, S. (2012). Effect of Pseudomonas fluorescens on proline and phytohormonal status of maize (Zea mays L.) under water deficit stress Ann. Biol. Res. 3, 1054–1062.
Black, C. A. (1965). Methods of Soil Analysis: Part I Physical and Mineralogical Properties. Madison, WI: American Society of Agronomy.
Brenner, K., You, L., and Arnold, F. H. (2008). Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotech. 26, 483–489. doi: 10.1016/j.tibtech.2008.05.004
Chahal, G. K., Kaur, A., and Ghai, N. (2022). Mitigation of salt stress with Azospirillium and Azotobacter inoculation in maize (Zea mays L.). Cereal Res. Comm. 50, 915–927.
Chun, S. C., Paramasivan, M., and Chandrasekaran, M. (2018). Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 9, 2525. doi: 10.3389/fmicb.2018.02525
Costa-Gutierrez, S. B., Lami, M. J., Santo, M. C. C. D., Zenoff, A. M., Vincent, P. A., Molina-Henares, M. A., et al. (2020). Plant growth promotion by Pseudomonas putida KT2440 under saline stress: role of eptA. Appl. Microbiol. Biotech. 104, 4577–4592. doi: 10.1007/s00253-020-10516-z
Efthimiadou, A., Katsenios, N., Chanioti, S., Giannoglou, M., Djordjevic, N., and Katsaros, G. (2020). Effect of foliar and soil application of plant growth promoting bacteria on growth, physiology, yield and seed quality of maize under Mediterranean conditions. Sci. Rep. 10, 21060. doi: 10.1038/s41598-020-78034-6
Fitriatin, B. N., Yusuf, M. I. M., Sofyan, E. T., and Nurbaity, A. A. (2021). Biofertilizers application to improve growth of maize and soil nutrients. E3S Web Conf. 316, 03020. doi: 10.1051/e3sconf/202131603020
Hussain, I., Khan, A., and Akbar, H. (2021). Maize growth in response to beneficial microbes, humic acid and farmyard manure application. Sarh. J. Agric. 37, 1426–1435. doi: 10.17582/journal.sja/2021/37.4.1426.1435
Jägermeyr, J., Müller, C., Ruane, A. C., Elliott, J., Balkovic, J., Castillo, O., et al. (2021). Climate impacts on global agriculture emerge earlier in new generation of climate and crop models. Nat. Food 2, 873–885. doi: 10.1038/s43016-021-00400-y
Jain, D., Meena, R. H., Choudhary, J., Sharma, S. K., Chauhan, S., Bhojiya, A. A., et al. (2021). Effect of microbial consortia on growth and yield of wheat under typic haplustepts. Plant Physiol. Rep. 26, 570–580. doi: 10.1007/s40502-021-00607-y
Kaur, G., and Reddy, M. (2014). Influence of P-solubilizing bacteria on crop yield and soil fertility at multilocational sites. Eur. J. Soil Biol. 61, 35–40. doi: 10.1016/j.ejsobi.2013.12.009
Khan, N., and Bano, A. (2019). Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 14, e0222302. doi: 10.1371/journal.pone.0222302
Kumar, A., Maurya, B. R., and Raghuwanshi, R. (2014). Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 3, 121–128. doi: 10.1016/j.bcab.2014.08.003
Lindemann, S., Bernstein, H., Song, H. S., Fredrickson, J. K., Fields, M. W., Shou, W., et al. (2016). Engineering microbial consortia for controllable outputs. ISME J. 10, 2077–2084. doi: 10.1038/ismej.2016.26
Manjunath, M., Kanchan, A., Ranjan, K., Venkatachalam, S., Prasanna, R., Ramakrishnan, B., et al. (2016). Beneficial cyanobacteria and eubacteria synergistically enhance bioavailability of soil nutrients and yield of okra. Heliyon. 2, e00066. doi: 10.1016/j.heliyon.2016.e00066
Mantelin, S., and Touraine, B. (2004). Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J. Exp. Bot. 55, 27–34. doi: 10.1093/jxb/erh010
Myo, E. M., Ge, B., Ma, J., Cui, H., Liu, B., Shi, L., et al. (2019). Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol. 19, 155. doi: 10.1186/s12866-019-1528-1
Ogola, J. B. O., Macil, P. J., and Odhiambo, J. J. O. (2021). Biochar application and rhizobium inoculation increased intercepted radiation and yield of chickpea in contrasting soil types. Int. J. Plant Prod. 15, 219–229. doi: 10.1007/s42106-021-00141-9
Olanrewaju, O. S., and Babalola, O. O. (2019). Bacterial consortium for improved maize (Zea mays L.) production. Microorganisms 7, 519. doi: 10.3390/microorganisms7110519
Olsen, S. R., Cole, G. V., Watnable, F. S., and Dean, L. A. (1954). Estimation of available phosphorus in soil by extraction with sodium bicarbonate. USDA Cir. 15, 25–28.
Osama, A. R., Alsaedi, A., and Bassam, A. A. (2020). Leaf area, proline and relative water content in the leaves of maize plant under the effect of soil bacteria, organic matter and water stress. Plant Arch. 20, 405–412.
Pacheco, A. R., Osborne, M. L., and Segrè, D. (2021). Non-additive microbial community responses to environmental complexity. Nat. Comm. 12, 2365. doi: 10.1038/s41467-021-22426-3
Pereg, L., and McMillan, M. (2015). Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 80, 349–358. doi: 10.1016/j.soilbio.2014.10.020
Radhakrishnan, R., and Lee, I. J. (2016). Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Biochem. 109, 181–189. doi: 10.1016/j.plaphy.2016.09.018
Rouphael, Y., and Colla, G. (2020). Editorial: biostimulants in agriculture. Front. Plant Sci. 11, 40. doi: 10.3389/fpls.2020.00040
Saikia, J., Sarma, R. K., Dhandia, R., Yadav, A., Bharali, R., Gupta, V. K., et al. (2018). Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 8, 1–16. doi: 10.1038/s41598-018-21921-w
Sandhya, V., Ali, S. K. Z., Grover, M., Reddy, G., and Venkateswarlu, B. (2009). Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fert. Soils 46, 17–26. doi: 10.1007/s00374-009-0401-z
Sandhya, V., Ali, S. K. Z., Grover, M., Reddy, G., and Venkateswarlu, B. (2010). Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 62, 21–30. doi: 10.1007/s10725-010-9479-4
Sandhya, V., Ali, S. K. Z., Grover, M., Reddy, G., and Venkateswarlu, B. (2011). Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress J. Plant Int. 6, 1–14. doi: 10.1080/17429145.2010.535178
Santos, M. S., Nogueira, M. A., and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Exp. 9, 1–13. doi: 10.1186/s13568-019-0932-0
Silambarasan, S., Logeswari, P., Cornejo, P., and Kannan, V. R. (2019). Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 26, 27647–27659. doi: 10.1007/s11356-019-05939-9
Silva, G. (2018). Feeding the World in 2050 and Beyond–Part 1: Productivity Challenges. Michigan State University Extension−3. Available online at: https://www.canr.msu.edu/news/feeding-the-world-in-2050-~and-beyond-part-1 (accessed August, 2022).
Srinivasarao, C., and Manjunath, M. (2017). “Potential of beneficial bacteria as eco-friendly options for chemical free alternative agriculture,” in Plant-Microbe Interactions in Agro-Ecological Perspectives, eds D. Singh, H. Singh, and R. Prabha (Singapore: Springer), 473–493.
Subhiah, B. V., and Asija, G. L. (1956). A rapid procedure for estimation of available N in soil. Curr. Sci. 25, 259–260.
van Dijk, M., Morley, T., Rau, M. L., and Saghai, Y. (2021). A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2, 494–501. doi: 10.1038/s43016-021-00322-9
Vidhyasekaran, P., and Muthuamilan, M. (1995). Development of formulation of Pseudomonas flourescens for control of chickpea wilt. Plant Dis. 79, 780–782. doi: 10.1094/PD-79-0782
Walker, V., Couillerot, O., Von Felten, A., Bellvert, F., Jansa, J., Maurhofer, M., et al. (2012). Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356, 151–163. doi: 10.1007/s11104-011-0960-2
Walkley, A., and Black, C. A. (1934). Estimation of organic carbon by chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Keywords: agricultural production, maize, microbial consortia, rainfed, yield
Citation: Manjunath M, Khokhar A, Chary GR, Singh M, Yadav SK, Gopinath KA, Jyothilakshmi N, Srinivas K, Prabhakar M and Singh VK (2023) Microbial consortia enhance the yield of maize under sub-humid rainfed production system of India. Front. Sustain. Food Syst. 7:1108492. doi: 10.3389/fsufs.2023.1108492
Received: 26 November 2022; Accepted: 12 January 2023;
Published: 16 February 2023.
Edited by:
Mohamed Ait-El-Mokhtar, University of Hassan II Casablanca, MoroccoReviewed by:
Raja Ben-Laouane, Cadi Ayyad University, MoroccoSowmyalakshmi Subramanian, McGill University, Canada
Copyright © 2023 Manjunath, Khokhar, Chary, Singh, Yadav, Gopinath, Jyothilakshmi, Srinivas, Prabhakar and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mallappa Manjunath,  bWFuanVtYmxAZ21haWwuY29t
bWFuanVtYmxAZ21haWwuY29t
 Mallappa Manjunath
Mallappa Manjunath Anil Khokhar
Anil Khokhar Gajjala Ravindra Chary1
Gajjala Ravindra Chary1 Manmohanjit Singh
Manmohanjit Singh Kodigal A. Gopinath
Kodigal A. Gopinath Karlapudi Srinivas
Karlapudi Srinivas