- 1International Institute of Tropical Agriculture (IITA), Olusegun Obasanjo Research Campus, Bukavu, Democratic Republic of Congo
- 2Department of Animal Science, Jomo Kenyatta University of Agriculture and Technology (JKUAT), Nairobi, Kenya
- 3Department of Animal Production, Faculty of Agriculture and Environmental Sciences, Université Evangélique en Afrique (UEA), Bukavu, Democratic Republic of Congo
- 4Tanzania Livestock Research Institute (TALIRI), Tanga, Tanzania
- 5International Institute of Tropical Agriculture (IITA), Bujumbura, Burundi
Dairying is one of the new promising economic sectors in eastern Democratic Republic of Congo (DRC), but still not explored enough to ensure consumers' safety. This study aimed to assess the health risks and nutritional profile of milk products along the value chain in South-Kivu and Tanganyika provinces. A total of 288 milk actors, including 160 producers, 35 collectors and 93 vendors, were concerned for interview and milk samples collection. A total of 302 milk samples (159 raw, 44 pasteurized, 76 fermented and 19 white cheese so-called “Mashanza”) were collected for physicochemical [pH, fat, non-fat dry matter (NFDM), lactose, protein, freezing point, density] and microbiological (total Aerobic Mesophilic Flora, Escherichia coli, Total Coliforms, Fecal Coliforms, Salmonella and Staphylococci) analyses. Results revealed that the physicochemical characteristics of the milk mostly varied according to the type of milk and the regions. The pasteurized milk from Tanganyika presented the best physicochemical parameters [crude protein (CP) = 4.36%, Fat = 4.06%, NFDM = 12%, lactose = 5.4%, density = 1.02 and pH = 6.59] compared to other types of milk. For microbiology, no E. coli was recorded but Salmonella and Staphylococci were found in all the milk types with the values not exceeding 3 × 104 CFU ml−1 and 3 × 103 CFU ml−1, respectively. This implies a long-term consumers' health issue if appropriate measures are not taken by milk actors along the value chain. The microbiological quality was influenced by the ecologies of production axis (representing the production zones) and by handling methods and infrastructures used by the actors involved along the value chain. Factors related to animal husbandry, milking method, milk processing and packaging had no significant effect on the physicochemical parameters under study. These results indicated that health risks for milk consumers are accrued by production practices and handling by milk actors due to shortage of required skills and appropriate equipment along the milk value chain. Observance of hazard analysis critical control point (HACCP) measures is carefully required along the milk value chain nodes to improve the quality of milk produced and sold and thus reduce the risks among consumers in South-Kivu and Tanganyika provinces.
1. Introduction
Milk is defined by the United States Code of Federal Regulations as “the lacteal secretion, practically free from colostrum, obtained by the complete milking of cow and containing more than 8.25% of milk solids-not-fat and more than 3.25% of milk fat” (Goff and Hill, 1992). It is considered as one of the most important food sources and is a compensatory component of daily diets for people of all categories, mainly due to its high nutrients content (Abate and Addis, 2015). In Sub-Saharan Africa (SSA), milk production has social and nutritional considerations (Mutwedu et al., 2018; Ahikiriza et al., 2021).
In SSA, an increase in annual milk consumption was observed in the last two decades and a significant increase is expected by 2025 (Kabui et al., 2015). This indicates that good management of the dairy sector could serve as a powerful tool for poverty alleviation and wealth creation, especially in developing countries (FAO, 2021).
In the Democratic Republic of Congo (DRC), 9.2% of GDP comes from the livestock sector, which also plays a crucial role in the livelihoods of the rural farmers. Yet, cattle are the most important livestock contributing more than 80% of the total protein consumed and an estimated annual milk production of 1.18 billion liters (SNSA: Service National des Statistiques Agricoles, 2020). However, despite its economic, food and nutritional importance, the produced milk is reported to be of marginal qualities and marketed through traditional or informal networks. Up to 80–90% of the locally produced milk is handled in these informal markets while < 10% is processed (Staal et al., 2001). Milk is produced mostly in rural areas by unorganized smallholder farmers and usually supplied to consumers in urban and rural areas by milk vendors or groceries (Ayagirwe and Mutwedu, 2021). Therefore, milking, handling, distribution to the consumers and conservation remains challenging.
This is a common situation in most African countries whereas informal milk value chain is predominant and represent more than 70% of milk traded (Nyokabi S. et al., 2021). The products traded in these informal value chains are traditionally processed and mainly concern mashanza and pasteurized or fermented milk. By traditional processing, we refer to small processing units that are typically carried out by isolated individuals or groups of individuals using local equipment such as jerricans, pans, calabashes, and local ferments (lactoserum, filtered local drink, lemon juice, etc.), without necessarily considering the classic norms to process food products. Informal value chains include licensed and unlicensed entities selling milk or dairy products directly to consumers through milk-bars, milk vending machines, corner-shops, street vendors and mobile vendors on bicycle or motorbike (Chepkoech, 2010; Odero-Waitituh, 2017; Alonso et al., 2018). The proportion of pasteurized milk traded in the informal value chains has been increasing due to growing demand for safe milk (Alonso et al., 2018; Bebe et al., 2018). However, milk is often re-contaminated after pasteurization due to unhygienic milk handling practices impairing its nutritional quality (Lindahl et al., 2018).
Taken together, the present state of milk handling and marketing may pose health risks to the public. These risks are linked to contamination of milk, growth and survival of harmful pathogens in the milk and increasing number of other micro-organisms caused by storage time and conditions such as temperature and humidity. Mutwedu et al. (2018) highlighted the implication of pasteurization process in physicochemical and microbiology characteristics of milk. Birali et al. (2019) observed a high variability of raw milk materials and the variation in processing procedures as source of milk product variability, composition, and shelf life. In fact, factors influencing milk composition (e.g., diet, breed and lactation stage) have been studied individually, while the interactions between several factors have been largely ignored. For example, the fatty acid profile of milk reacts quickly and is very sensitive to changes in diet (Schwendel et al., 2015). The milk handling practices and microbial health risks along the milk value chain in South-Kivu and Tanganyika provinces, Eastern of DRC are not known to ensure consumers' safety.
Therefore, the purpose of this study was to assess the health risks and nutritional profile of milk products as well as underlying factors along their value chain. Specifically, the current study sought to assess the physicochemical and microbiological composition of milk products as well as the risk factors associated with milk quality in South-Kivu and Tanganyika provinces.
2. Materials and methods
2.1. Study area
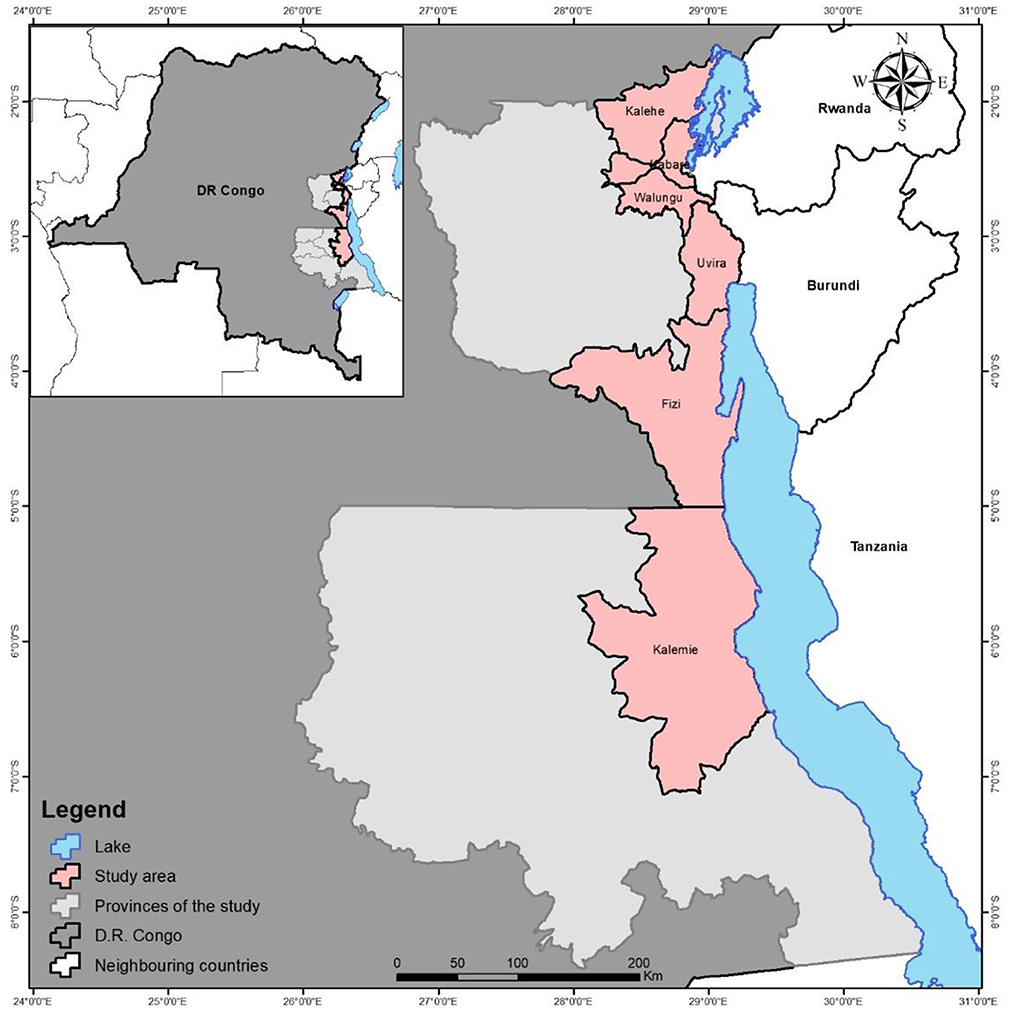
This study was carried out in the provinces of South-Kivu (territories of Kabare, Kalehe, Walungu, Uvira, Fizi and the city of Bukavu) and Tanganyika (territory of Kalemie), located in Eastern of DRC (Figure 1). The selection of these sites was motivated by the high livestock population and therefore milk production (Defailly, 2000).
In South-Kivu, 3 axis were selected including Bukavu and its hinterland (Bukavu+) (villages of Miti, Mudaka, Kavumu, Katana, Kabamba, Bitale, Mulumemunene and Walungu center), the Ruzizi plain (Ruzizi) (Kamanyola, Luvungi, Bwegera, Luberizi, Sange, Kiliba) and the Fizi axis (Mboko, Baraka, Katanga, Fizi villages). In the context of the study area, ax means main production zone, mostly defined based on agroecological zones and livestock abundance.
In Tanganyika, 2 axis were concerned including the Kalemie-Malia axis (KLM-Ma) and the Kalemie-Kisondja axis (KLM-Ki). In KLM-Ma, 8 villages were selected including Kichanga, Kahengele, Tabac Congo, Kabutonga, PRP, Batumba and Malia; while in KLM-Ki, Kalemie town and the villages of Tundwa and Kisondja were concerned.
2.2. Data collection
2.2.1. Sampling strategy and survey
structured questionnaire was developed and formalized using the Kobo collect tool to gather information from three categories of milk actors, including milk producers, milk collectors and milk vendors. A cross-sectional study design was used where respondents were selected based on their experience in the specific category. All clients were asked to participate voluntarily, and confidentiality of information was ensured to respondents before giving their verbal consent to respond to questions. Direct and structured questions were used to obtain targeted qualitative information.
The questionnaire was designed to capture the information on demography, livestock and milk components. The questionnaire was developed after reviewing published papers in the same field (Dehinenet et al., 2013; Nyokabi S. et al., 2021; Nyokabi S. N. et al., 2021). Once the questionnaire was developed, it was submitted to a dairy expert from the Tanzania Livestock Research Institute (TALIRI) for review and validation. A field trip was therefore organized to test the questionnaire to ensure that respondents understood the different questions and the answers given were consistent with the objectives of the study. A total of 288 persons were interviewed including 160 milk producers, 35 milk collectors and 93 milk vendors. Respondents were identified based on existing database of actors intervening in the livestock value chain, retrieved at the provincial divisions of livestock and fishery in South-Kivu and Tanganyika provinces. From the lists, respondents were selected randomly and personally identified on the ground though their cooperatives or associations. In Eastern DRC, almost all milk actors are organized into associations or cooperatives.
2.2.2. Collection of milk samples
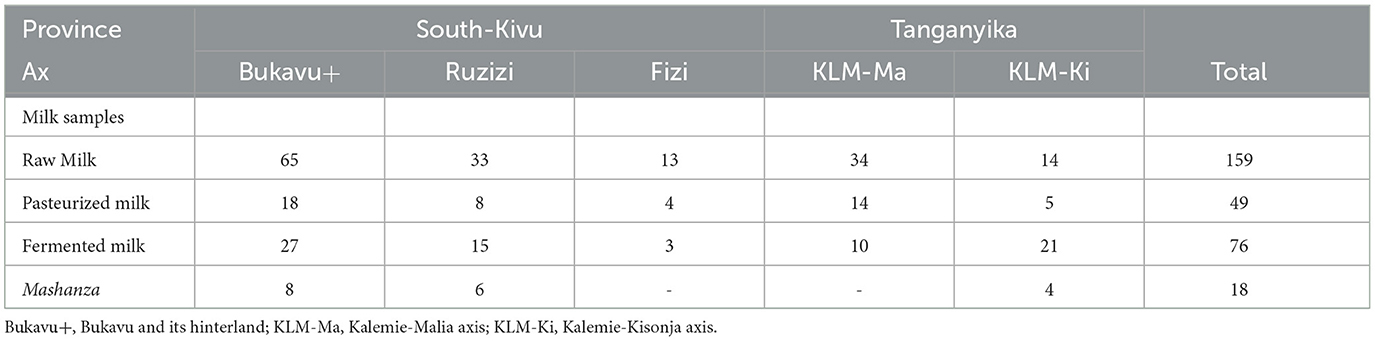
A sample of 302 milk products were collected from the 288 actors and consisted of raw milk, pasteurized milk, fermented milk, and white cheese so-called “Mashanza” (Table 1).
The milk sampling considered 100 ml of each milk product, then homogenized using hermetically sealed white jars, labeled, and placed in cooling containers to maintain the temperature at +4°C and transferred to the lab within 4–6 h before being processed for lab analyses. After then, samples were stored in a freezer at −20°C while waiting for physicochemical analyses. All the analyses were carried out in the laboratory of food science and technology of the Université Evangélique en Afrique (UEA) in Bukavu, DRC. For liquid samples such as raw milk, they were directly analyzed. Fermented milk sometimes contains curds and therefore requires homogenization before analysis. However, a dilution was made for white cheese (so-called Mashanza), followed by a post-analysis homogenization.
2.2.3. Physico-chemical analyses
The physicochemical analyses evaluated the content in fat (%), protein (%), non-nitrogenous solid (%), freezing point (°C), density (kg/m3) and lactose (%) by spectrophotometric method using a Lactostar as milk analyzer (Hoxha and Mara, 2012; Hossain and Dev, 2013). For this purpose, 5 ml of each sample was taken and sucked by the Lactostar. The pH value of each milk sample was determined by a pH meter brand 315i.
2.2.4. Microbiological analysis
The selective media were prepared in advance; dilutions and isolations were performed directly upon arrival of the samples in the lab. Reductase test was performed using the blue methylene to assess bacterial activity as described by Guiraud (2003). Decimal dilution was then elaborated according to Haas (1989) method.
The total mesophilic aerobic flora was quantified by counting colonies after growth on Plate Count Agar (PCA) inoculated and incubated for 72 h at 30°C. Colonies quantification was done on the boxes of 30–300 colonies and the number of microorganisms per ml was calculated using the formula developed by Houaria and Zohra (2018):
N = ∑c/v (n1+0,1n2) d
∑c: total number of counted colonies.
n1: number of boxes scored in the first dilution.
n2: number of boxes scored in the second dilution.
V: volume of applied solution (1 ml).
d: the dilution factor from which the first counts will be observed.
Enumeration of total and fecal coliforms was performed. Coliforms are revealed in the presence of neutral red by the appearance of pink or red colonies on Mac Conkey Agar media. Separation between total coliforms (TC) and fecal coliforms (FC) was based on the incubation temperature of 37°C for 24 h for total coliform enumeration and 44°C for fecal coliforms (Yetis and Selek, 2015). The inoculation was performed in depth dilutions from 10−1 to 10−3.
Staphylococcus aureus count was enumerated as bacteria that appear like black colonies resulting from the reduction of tellurite to tellurium, which are surrounded by a transparent halo indicating the presence of lipoproteinases. The medium used was Baird-Parker associated to egg yolk and potassium tellurite. A Gram stain test was used afterwards for confirmation.
For enumeration of Salmonella, Eosin Methylene Blue was used to determine the presence/absence of Salmonella and Hektoen agar was used as recommended by the Association of Official Analytical Chemists (1998) and the American Public Health Association (2001) for enumeration. It is a differential selective medium for enteropathogenic bacteria, particularly Salmonella. The composition of the medium allows the differentiation of colonies that ferment rapidly one of the three sugars (salicin, sucrose and lactose) by changing from blue to salmon-red and/or producing H2S (black center). Salmonella which does not attack any of these carbohydrates, are able to produce H2S from thiosulfate in the medium. This result in blue-green colonies with a black center. Pre-enrichment was performed by suspending 25 ml of the sample in 225 ml of buffered peptone water (BPW). This broth was incubated at 37°C for 16–20 h, followed by enrichment on Selenite-Cysteine Broth (SCB) for 24 h at 37°C (Bachtarzi et al., 2015). The enumeration and isolation were performed on Hektoen medium by inoculating 1ml on the surface in the petri dish after incubation for 24 h at 37°C (Law et al., 2015).
For enumeration of Escherichia coli, Lysogeny media or Luria Broth (LB) was used. 1 ml of each sample from the selected decimal dilutions, was aseptically transferred to a sterile screw tube to which 15 ml of enrichment medium were added. These tubes were placed in the incubator at 37°C for 24 h. Tubes that color turned black were then considered positive. These tubes were isolated on Urea indole for Escherichia that had been melted. The inoculated Petri dishes were incubated at 37°C for 48 h. Counting was done considering the result of the incubation of the tubes as well as the presence of colonies. For E coli enumeration, the most probable number procedure was applied. It is a statistical method based upon the probability theory. Samples were serially diluted to the point of extinction.
2.3. Statistical analysis
The data were summarized by means and standard deviations. In order to find the factors that induce statistically significant effects on the analyzed parameters, Wilcoxon-Mann-Whitney test or Student's T test were used to compare two samples' means while the analysis of variance or Kruskal-Wallis's test were used to compare more than two samples' means. In both cases, the choice of the statistical method was based on the results of the assessment of assumptions of normality and homoskedasticity. When these assumptions were met parametric methods (Student T test for two samples or analysis of variance for more than two samples) were used. Otherwise, non-parametric methods were used. All these analyses were performed using threshold α = 0.05. In case of significant differences between more than two means, Dunn's test and Tukey HSD test were employed to determine homogeneous groups. The Redundancy analysis (RDA) technique was used to summarize the linear relationships between components of physicochemical and microbiological variables of milk products that are assumed to be redundant with their production, storage, and conservation conditions. All these statistical analyses were performed in R software version 4.0.5 (R Core Team, 2022).
3. Results
3.1. Nutritional profile of milk products in South-Kivu and Tanganyika provinces
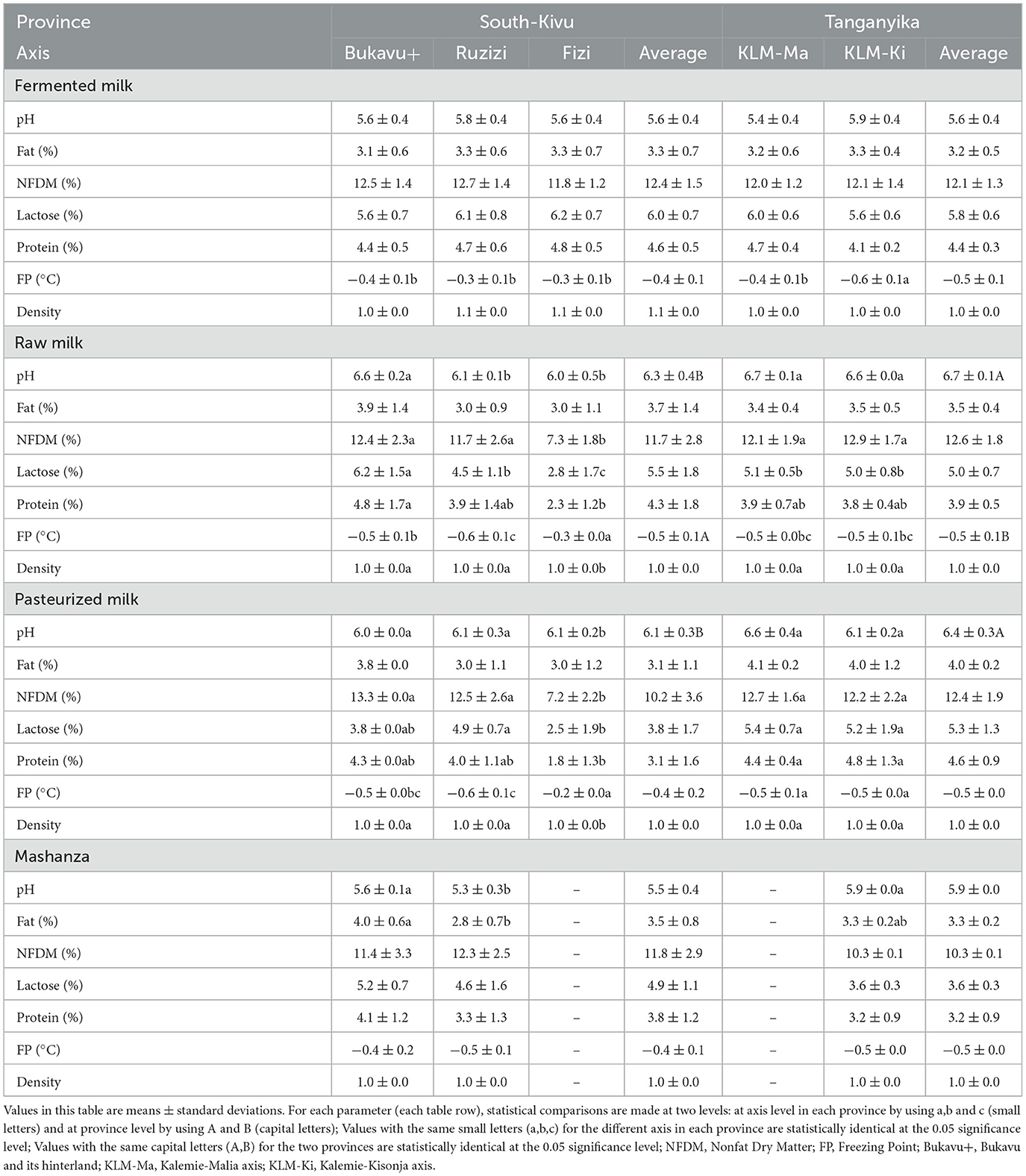
3.1.1. Physicochemical parameters of collected milk samples according to the production axis
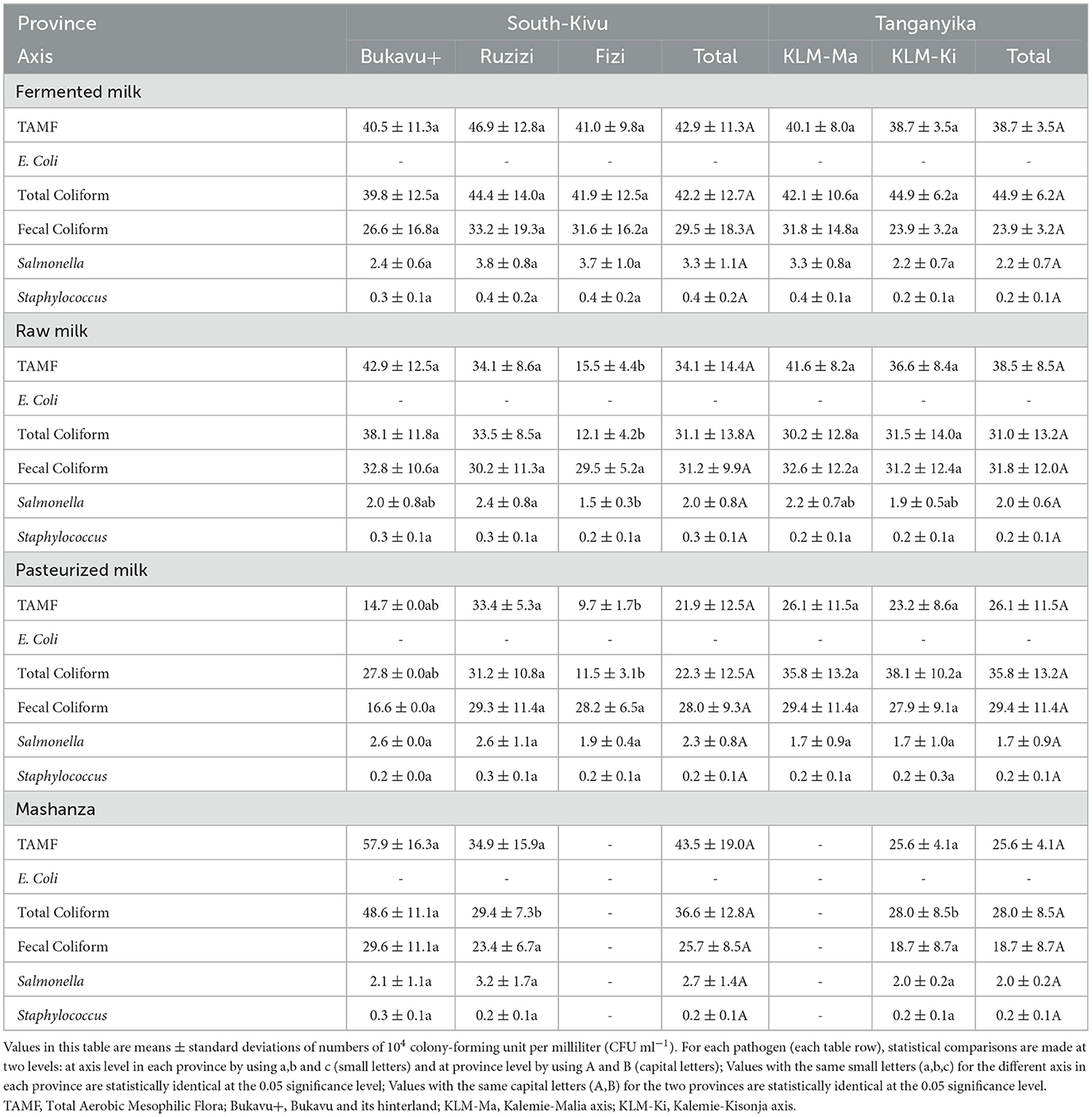
Table 2 shows that the evaluated physicochemical parameters for fermented milk did not vary according to the different axis, except the freezing point, which was higher (p < 0.05) in fermented milk from Kisondja than the other axis.
In raw milk, pH was lower (p < 0.05) in the milk collected in Fizi (6.0 ± 0.5) followed by that of Ruzizi plain (6.1 ± 0.1); the samples from Bukavu and its hinterland (6.6 ± 0.2), Kalemie-Kisondja (6.6 ± 0.0) and Kalemie-Malia (6.7 ± 0.1) had the highest pH. NFDM was lower (p < 0.05) in samples collected in Fizi (7.3 ± 1.8) compared to other sites. Lactose content was lowest (p < 0.05) in samples from Fizi (2.8 ± 1.7) followed by those from the Ruzizi plain (4.5 ± 1.1) and those from the Kalemie-Kisondja (5.0 ± 0.8) and Kalemie-Malia (5 ± 0.5) axis, while samples from the Bukavu and its hinterland had the highest lactose content (6.2 ± 1.5). Protein content was higher in samples from Bukavu and its hinterland (4.3 ± 0.0), followed by samples from the Kalemie-Kisondja (3.8 ± 0.4) and Kalemie-Malia (3.9 ± 0.7) axis and Ruzizi plain (3.9 ± 1.4), while samples from Fizi had the lowest protein content (2.3 ± 1.2). The freezing point was lower (p < 0.05) in Fizi samples (−0.3 ± 0.0) but higher in the Ruzizi Plain samples (−0.6 ± 0.1) while the density was lower (p < 0.05) in milk samples collected in Fizi (1.0 ± 0.0) compared to those from other sites.
Results obtained in pasteurized milk indicate that pH, NFDM and lactose were low in samples collected in Fizi (6.1 ± 0.2; 7.2 ± 2.2 and 2.5 ± 1.9 respectively) compared to samples collected in Bukavu and its hinterland, Ruzizi plain and Kalemie-Kisondja. Protein content was low (p < 0.05) in milk samples from Fizi (1.8 ± 1.3) followed by those from the Ruzizi plain (4 ± 1.1) and Bukavu and its hinterland (4.3 ± 0.0) while samples from the Kalemie-Malia axis presented the highest protein content (4.4 ± 0.4). The freezing point was lower in milk collected in Fizi (−0.2 ± 0.0) but higher in the samples from the Ruzizi plain (−0.6 ± 0.1) while the density was lower in the samples from Fizi (1.0 ± 0.0) than in those from other axis.
For Mashanza, beside the pH which was low in the Ruzizi plain samples (5.3 ± 0.3) compared to those from Bukavu and its hinterland (5.6 ± 0.1) and Kalemie-Kisondja axis (5.9 ± 0. 0), as well as the fat content which was higher in the samples collected in Bukavu and its hinterland (4.0 ± 0.6) and Kalemie-Kisondja axis (3.3 ± 0.2) than in the Plaine de la Ruzizi axis (2.8 ± 0.7), any other parameter varied following the axis.
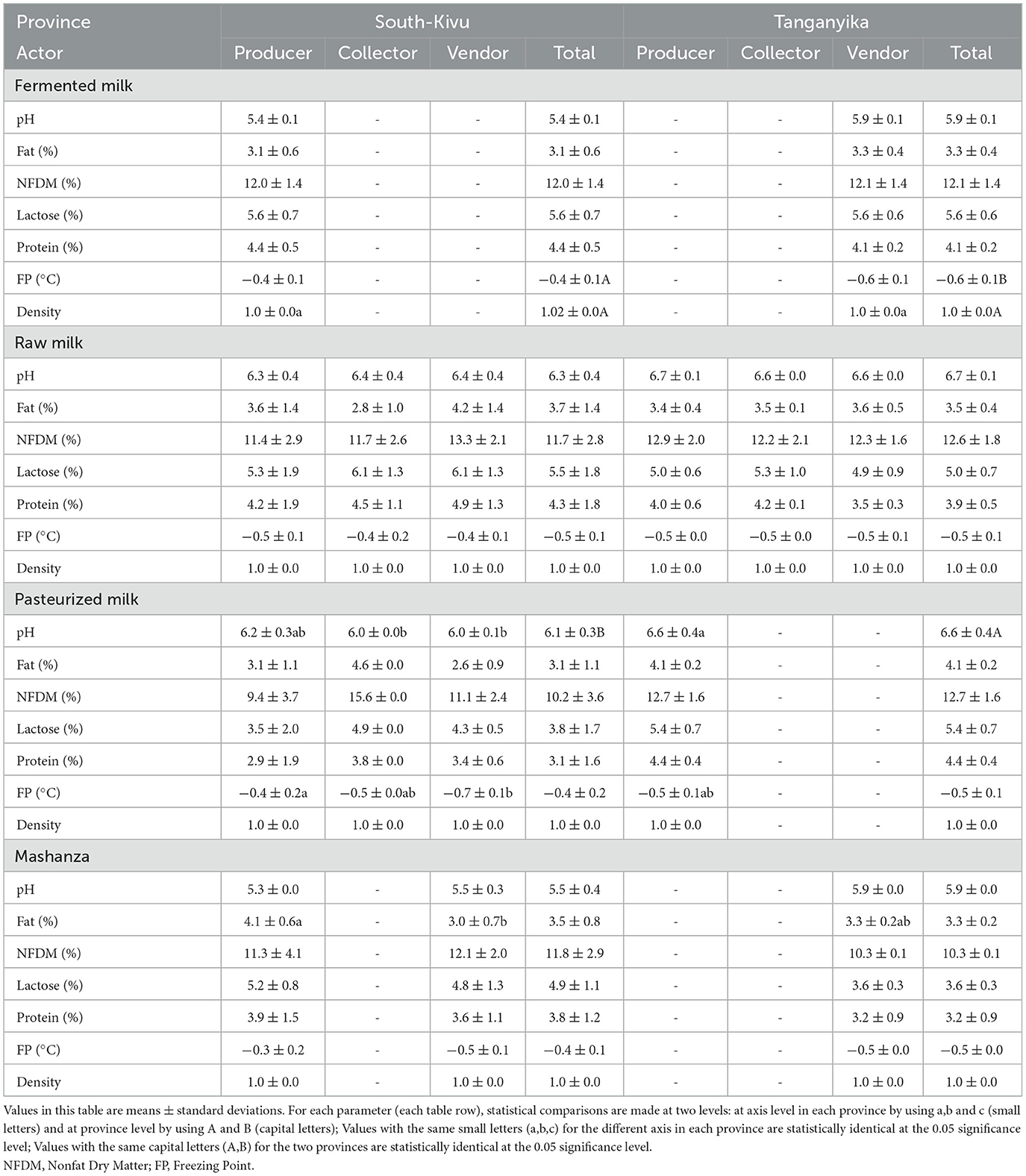
3.1.2. Physicochemical parameters of milk products according to the value chain actors
Table 3 presents the findings on physicochemical parameters as influenced by actors along the milk value chain. For fermented milk, only the freezing point was statistically lower (p < 0.05) among milk producers in South-Kivu (−0.4 ± 0.1) compared to milk vendors in Tanganyika (−0.6 ± 0.1), where fermented milk is only produced. For raw milk, no significant difference was recorded (p > 0.05) among the different actors along the milk value chain in both South-Kivu and Tanganyika provinces.
Concerning the pasteurized milk, pH was high in the milk from producers in Tanganyika (6.6 ± 0.4) than those in South-Kivu (6.1 ± 0.3). The freezing point was high in samples from sellers of South-Kivu (−0.7 ± 0.1) but very low in South-Kivu farmers (−0.4 ± 0.2). For Mashanza, only the fat content was high in samples collected from South-Kivu farmers (4.1 ± 0.6) compared to those from sellers in Tanganyika (3.3 ± 0.2) and South-Kivu (3.0 ± 0.7).
3.2. Microbiological profile of milk products in South-Kivu and Tanganyika provinces
3.2.1. Microbiological parameters of milk products according to production axis
Table 4 indicates that, for fermented milk, all microbiological parameters did not vary significantly according to the different axis. For the raw milk, TAMF, total coliform and Salmonella counts were significantly lower (p < 0.05) in the milk samples collected in Fizi (155 ± 44 × 103 CFU/ml; 121 ± 42 × 103 CFU/ml and 15 ± 3 × 103 CFU/ml, respectively) compared to other axis. The fecal coliforms and Staphylococci counts did not vary significantly following the different axis under study. For pasteurized milk, the TAMF (97 ± 17 × 103 CFU/ml) and total coliform (115 ± 31 × 103 CFU/ml) counts were lower (p < 0.05) in milk from Fizi than in the other axis, while there was no significant difference in fecal coliforms, Salmonella and Staphylococcus counts following axis. For Mashanza, apart from the total coliform load, which was lower in samples from the Ruzizi plain (294 ± 73 × 103 CFU/ml) and Kalemie-Kisondja (280 ± 85 × 103 CFU/ml) compared to Bukavu and its hinterland (486 ± 111 × 103 CFU/ml), no other parameter varied significantly across axis. Escherichia coli was not found in any of the milk samples.
3.2.2. Microbiological parameters of milk products according to the value chain actors
Table 5 indicates that, for fermented milk, only the Staphylococcus count was statistically higher at producers' level in South-Kivu (3 ± 1 × 103 CFU/ml) compared to milk samples collected from vendors in Tanganyika (2 ± 1 × 103 CFU/ml). For raw and pasteurized milk, the pathogens germs count did not significantly vary among the value chain actors (p > 0.05). In Mashanza, only TAMF count was statistically higher in samples collected from South-Kivu vendors (441 ± 105 × 103 CFU/ml) compared to vendors of Tanganyika (256 ± 41 × 103 CFU/ml). Escherichia coli was not found in any of these milk samples.
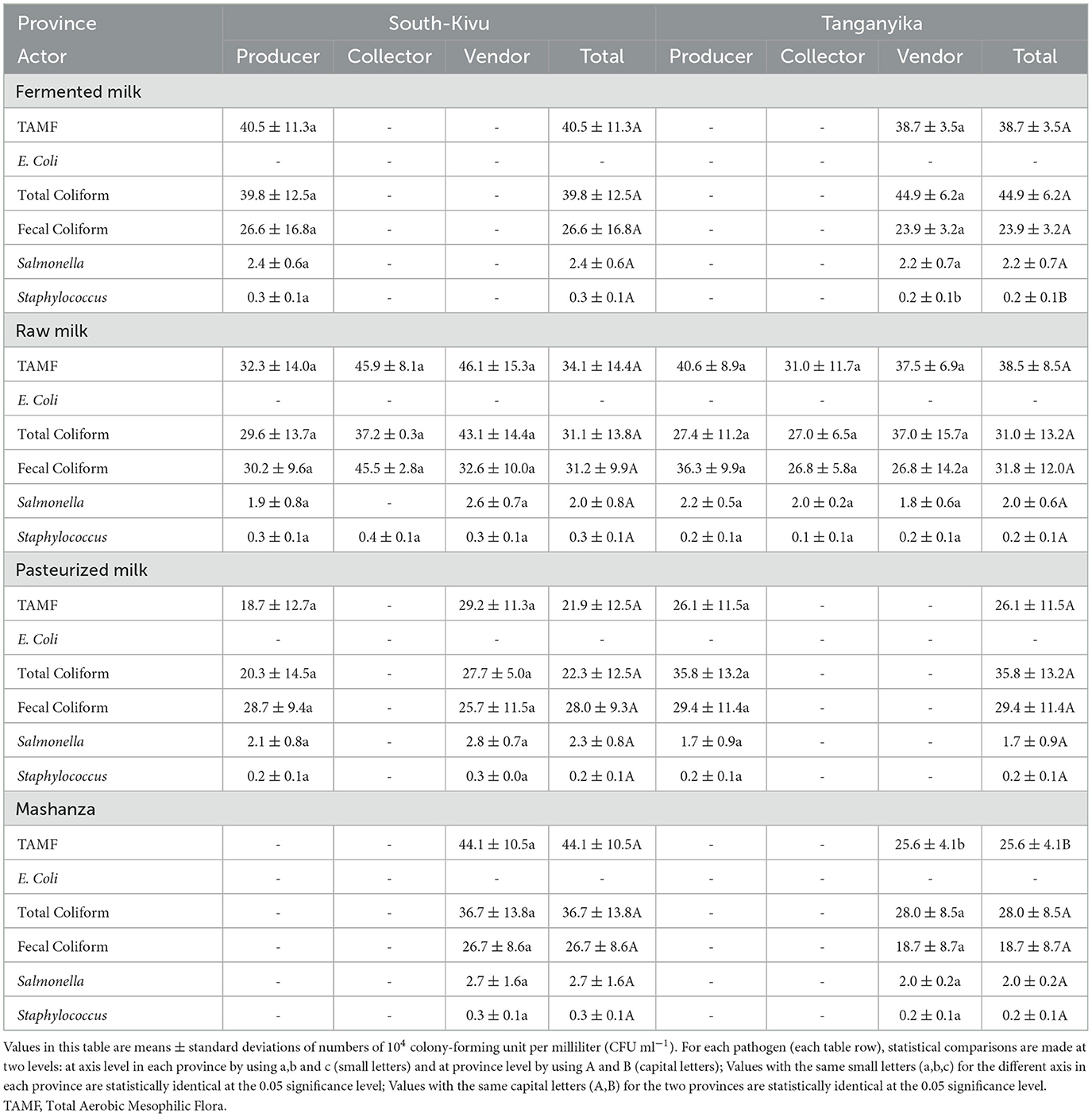
Table 5. Microbiological parameters of milk products according to the value chain actors (in × 104).
3.3. Factors influencing the quality of milk products in South-Kivu and Tanganyika provinces
3.3.1. Factors influencing the microbiological quality of milk products from producers
Results obtained with the CRA design indicate that pathogens tend to be more abundant in milk stored for a long time, even though during interviews farmers reported that most of them clean the milk storage equipment before use (92%) and wash their hands before milking (89%). In the Ruzizi plain, where plastic containers are the main packaging material (66.7%), Salmonella germs are much more prevalent in milk samples. It was found a lower abundance of pathogens in milk collected from producers in Fizi axis compared to the other axis because all the farmers usually pasteurize the milk before delivery (100%). Moreover, in Tanganyika province, where raw milk is the main dairy product (83.7%), fecal coliforms are more abundant in milk.
3.3.2. Factors influencing the nutritionnal quality of milk products from producers
Figure 2 shows that milk products in the Kalemie-Malia and Kalemie-Kisondja axis have similar physico-chemical characteristics. These are milk products with high concentration in fat, non-fat dry matter, protein, and lactose contents. However, milk products from Fizi are mainly characterized by high freezing point and low contents of other physicochemical parameters. It was also noticed that when cattle are fed with concentrate as a supplement to pasture, the milk produced is of high density. This is particularly the case for milk from stall-feeding farms with crossbreds or improved dairy breeds compared to local breeds where a very low milk density was reported. It was also noticed that milk nutritional quality is influenced by the calving rank. In fact, milk collected from cows in their first lactation stage had less concentrated milk compared to cows in the second lactation stage (see Figure 3).
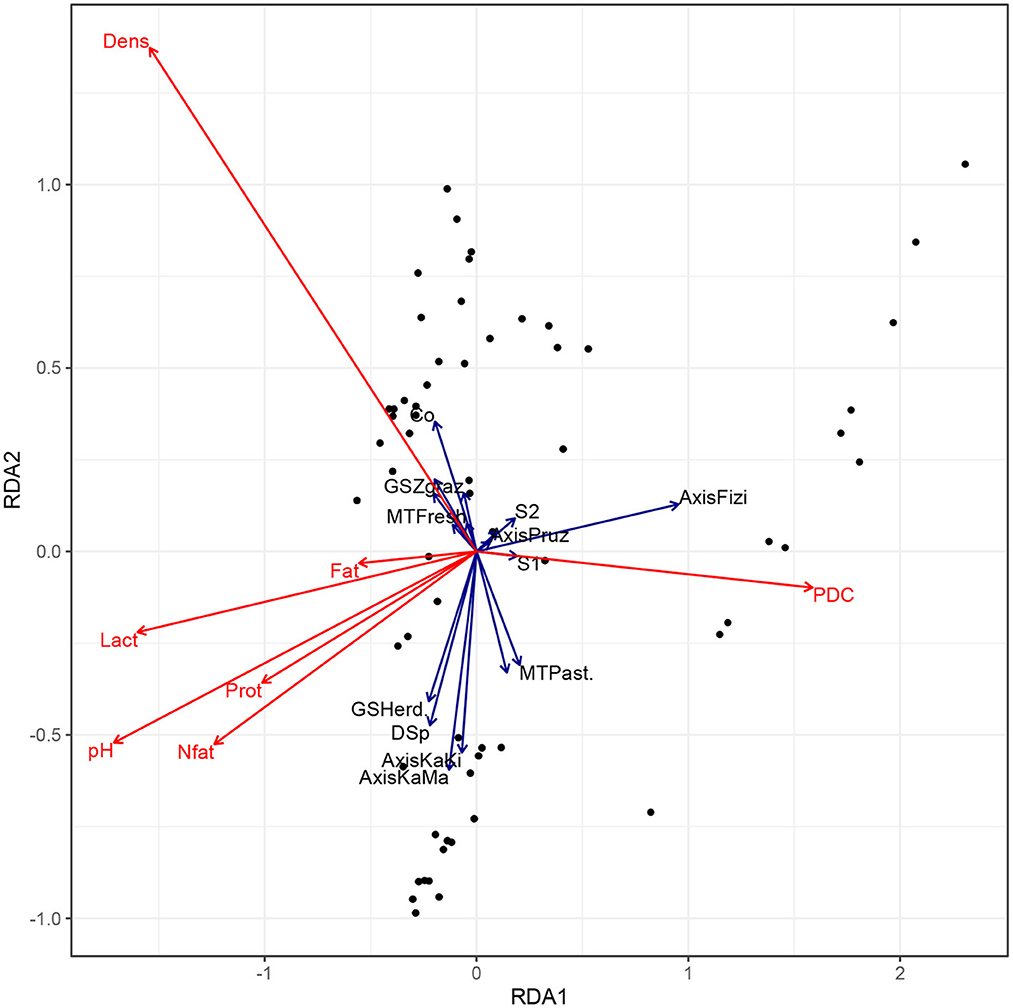
Figure 2. Biplot of Redundancy Analysis (RDA) of the nutritional quality of milk collected from farmers. MT, Milk type; GS, Grazing system; Co, feeding with concentrate; DSp, Dietary supplements provision; S1, First lactation rank; S2, Second lactation rank; S3, Third lactation rank; PDC, Freezing point; Lact, lactose; Prot, protein; Nfat, Nonfat dry matter.
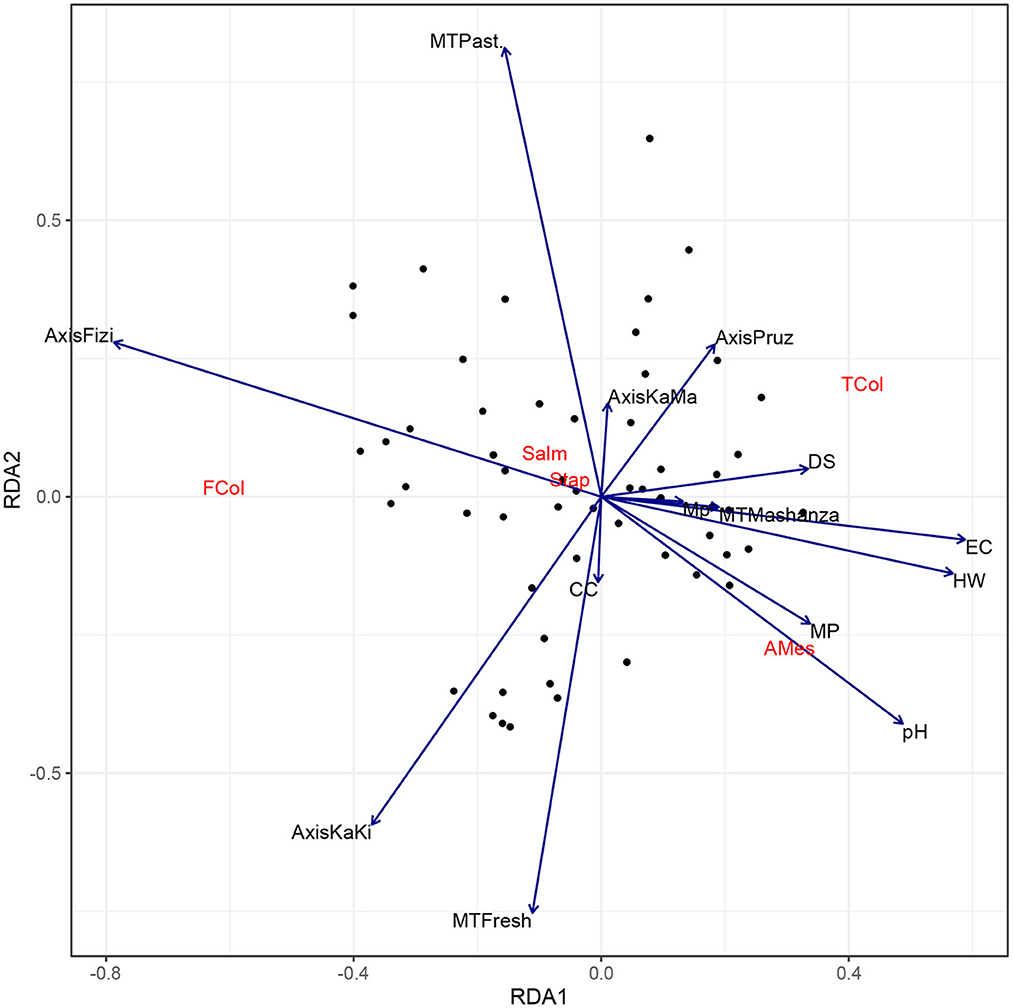
Figure 3. Biplot of Redundancy Analysis (RDA) of the encountered pathogens in milk collected from farmers. MT, Milk type; HW, Hand washing; EC, Equipment cleaning; CC, Cold chain equipment; MP, Milk processing; DS, Duration of stock depletion; AMes, Aerobic Mesophilic; TCol, Total Coliforms; FCol, Fecal Coliforms; Salm, Salmonella; Stap, Staphylococcus.
3.3.3. Factors influencing the microbiological quality of milk products from collectors
Figure 4 indicates that transportation of milk by foot (71.4%), using plastic containers (82.9%) as well as the long time taken before milk stock disposal (around 4 days) are the main factors promoting the proliferation of fecal coliforms and staphylococci in milk samples. TAMF and total coliforms are common in Fizi axis, where collectors are supplied in milk directly at farm level (100%). However, in the Kalemie-Kisondja axis, where all collectors are used to clean their equipment for milk transportation and packaging (100%) and the cold chain management equipment is mostly available (72.73%), the milk collected had less pathogen counts. Globally, the cold chain and cleaning the equipment are the main factors that seem to reduce the abundance of pathogens in milk products at the collector's level.
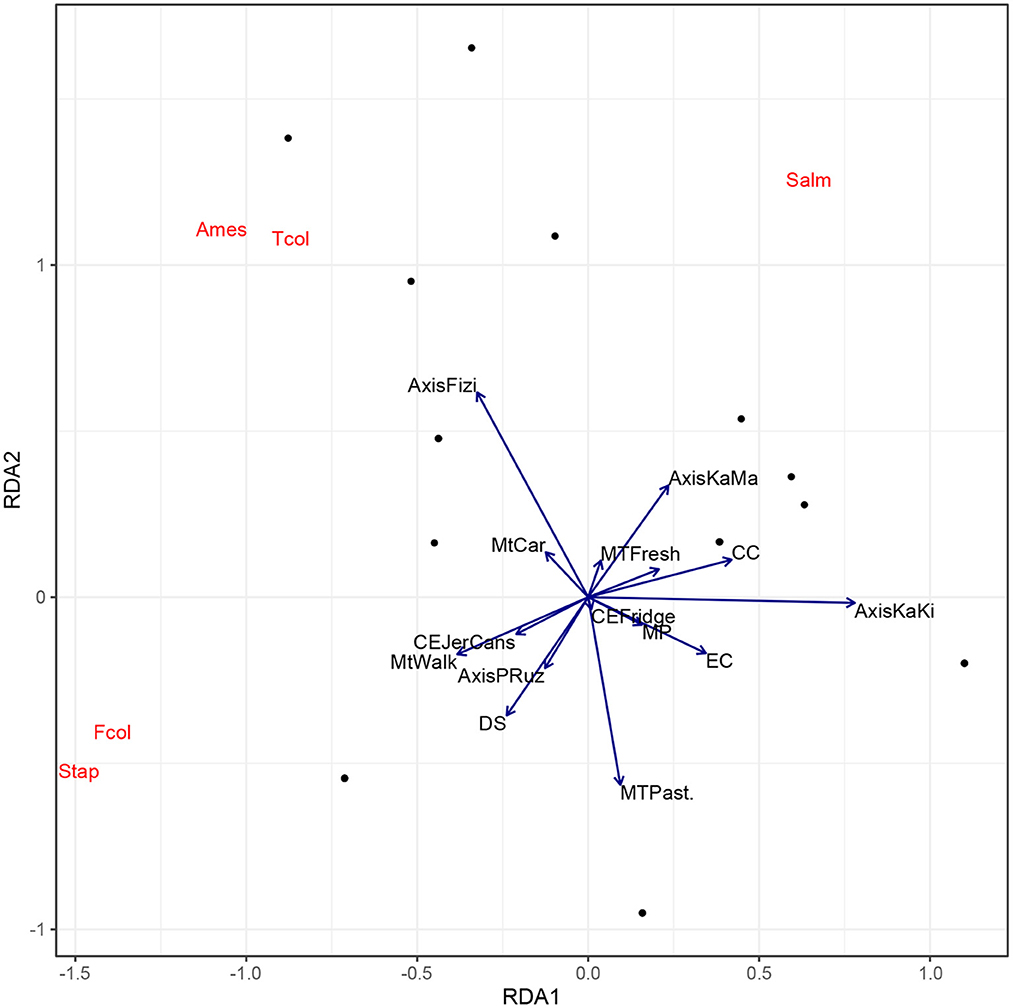
Figure 4. Biplot of Redundancy Analysis (RDA) of the encountered pathogens in collectors' milk. MT, Milk type; Mt, Means of transportation; CE, Conditioning equipment; EC, Equipment cleaning; CC, Cold chain equipment; MP, Milk processing; DS, Duration of stock depletion; AMes, Aerobic Mesophilic; TCol, Total Coliforms; FCol, Fecal Coliforms; Salm, Salmonella; Stap, Staphylococcus.
3.3.4. Factors influencing the nutritionnal quality of milk products from collectors
Figure 5 shows that at the collectors' level, pasteurized milk is richer in fat and non-fat dry matter but with a low density and tend to be much acidic. Moreover, raw milk possesses a high quantity of lactose, with a high density and less acidity. Other parameters related to milk transportation, packaging and delivery did not impact the milk nutritional quality at collector's level.
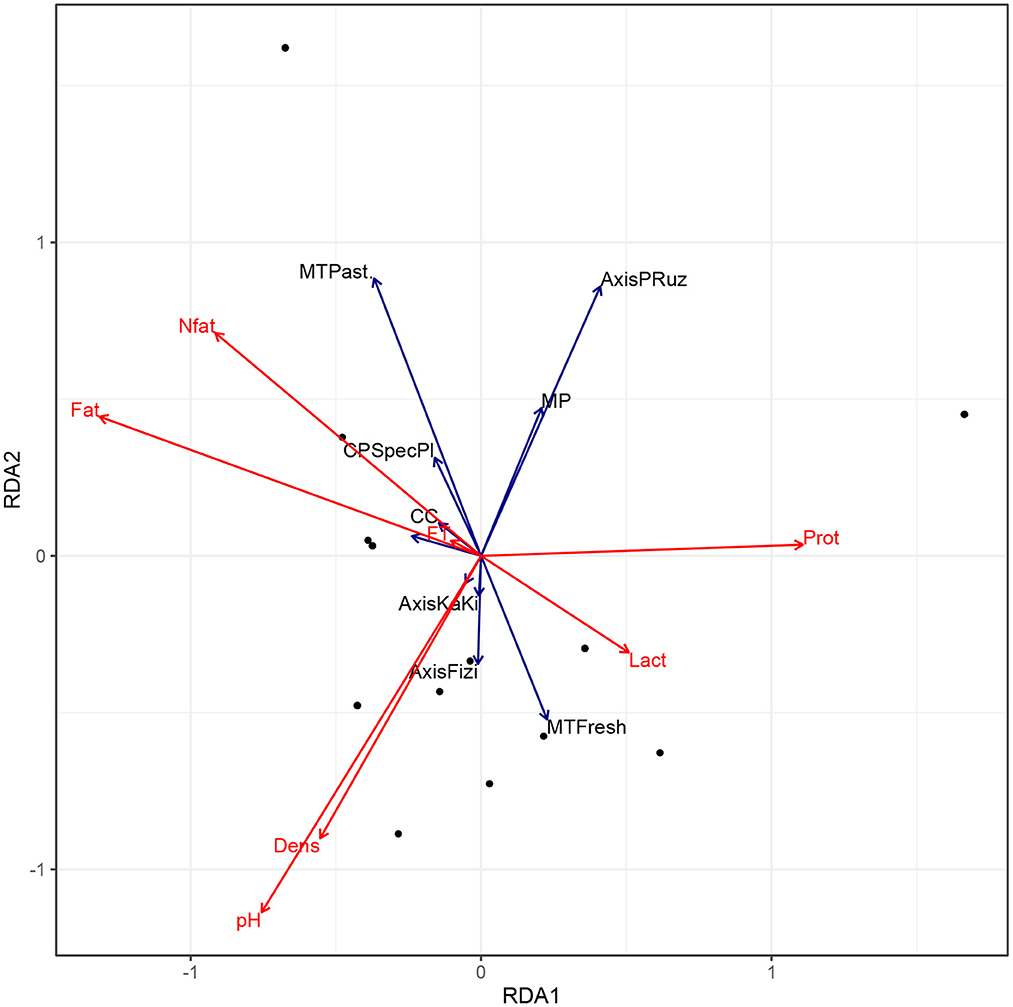
Figure 5. Biplot of Redundancy Analysis (RDA) of the nutritional quality of milk at collectors' level. MT, Milk type; CP, Collection place; CC, Cold chain equipment; MP, Milk processing; FT, Freezing point; Lact, lactose; Prot, protein; Nfat, Nonfat dry matter; PRuz, Ruzizi plain.
3.3.5. Factors influencing the microbiological quality of milk products from vendors
Figure 6 indicates that the milk collected from vendors contains a low quantity of pathogens count when pasteurized and this was much found in milk products in the Fizi axis. The Mashanza samples collected from vendors are more characterized by a high accumulation of pathogen count mostly due to contamination during processing of raw milk into the Mashanza product. However, parameters related to milk transportation, packaging and delivery did not impact the milk nutritional quality at vendor's level mostly due to the short time taken before milk stock disposal (< 1 days) and measures taken to avoid milk contamination during the sale, including the control by hygiene service, covering plastics containers with sachet, and placing it in water.
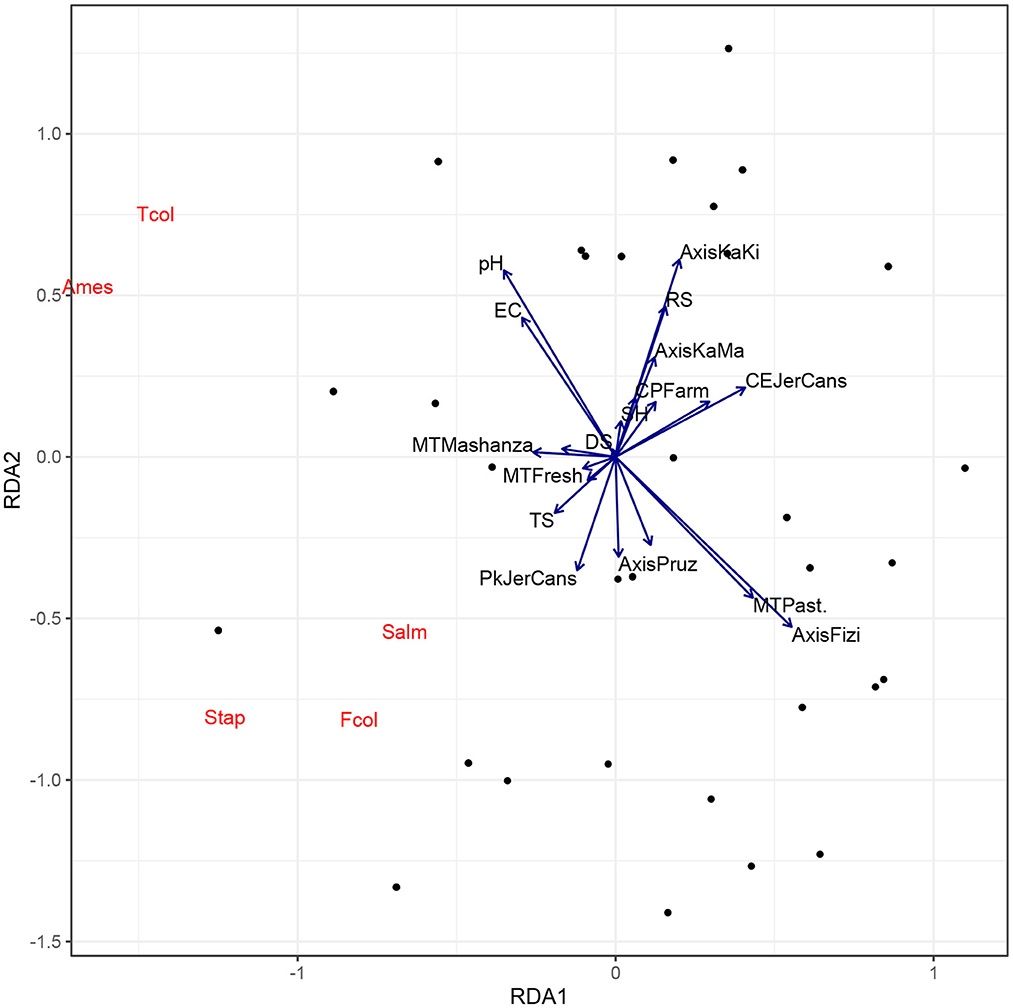
Figure 6. Biplot of Redundancy Analysis (RDA) of the encountered pathogens in sellers' milk. MT, Milk type; CP, Collection place; Pk, Pack; SH, Sale at home; RS, Roadside sale; TS, Time spent on the way; EC, Equipment cleaning; DS, Duration of stock depletion; Ames, Aerobic Mesophilic; Tcol, Total Coliforms; Fcol, Fecal Coliforms; Salm, Salmonella; Stap, Staphylococcus.
3.3.6. Factors influencing the nutritionnal quality of milk products from vendors
Figure 7 indicates that the milk products collected from vendors in Fizi and in Kalemie-Kisondja axis have very different physico-chemical characteristics. In fact, milk from Fizi have a high freezing point and low fat, non-fat dry matter, protein, and lactose contents and are more acidic. Other parameters related to milk transportation, packaging and delivery did not impact the milk nutritional quality at collector's level.
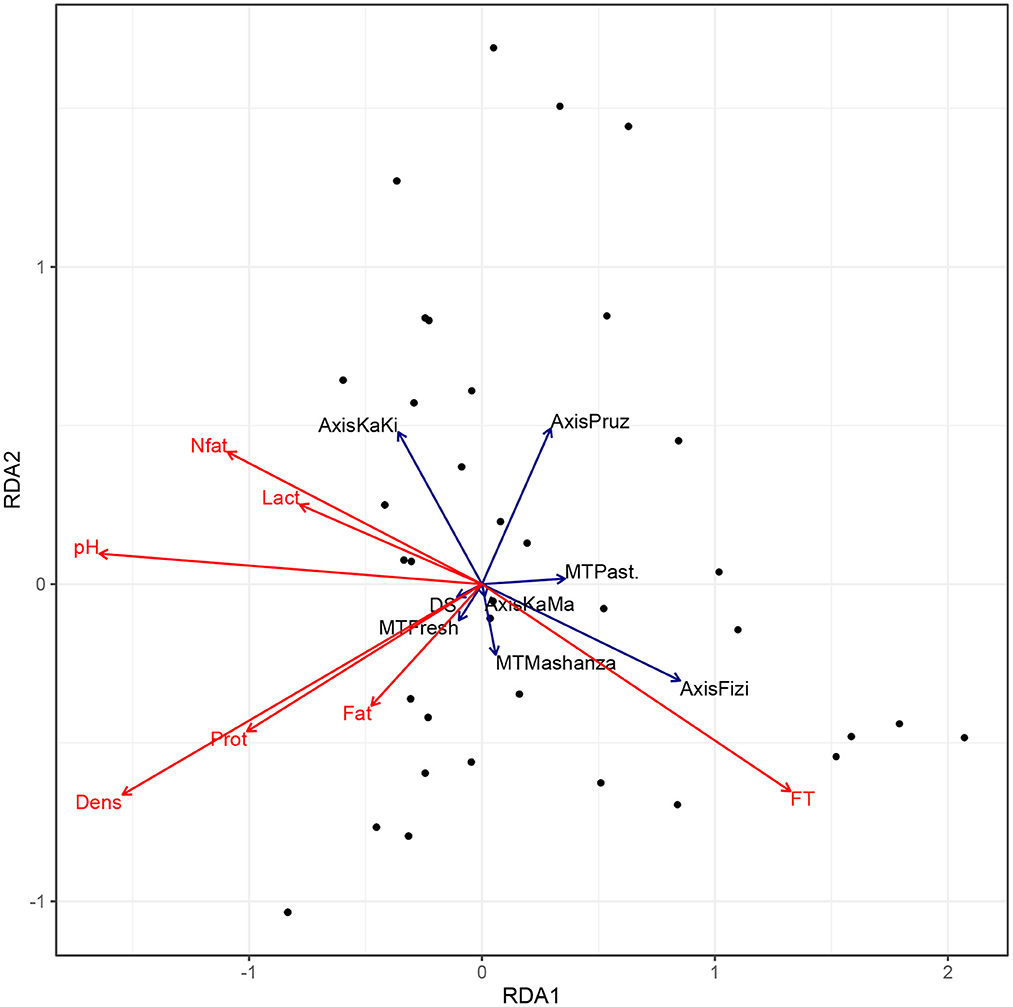
Figure 7. Biplot of Redundancy Analysis (RDA) of the nutritional quality of milk at sellers' level. MT, Milk type; EC, Equipment cleaning; CC, Cold chain equipment; DS, Duration of stock depletion; KaMa, Kalemie-Malia; PRuz, Ruzizi plain; KaKi, Kalemie-Kisondja; Past, Pasteurized milk; FT, Freezing point; Lact, lactose; Prot, protein; Nfat, Nonfat dry matter.
4. Discussion
In the two provinces, pH was lower in fermented milk and Mashanza compared to the raw and pasteurized milk. This result is in accordance with results found by Mutwedu et al. (2018) in Mashanza produced in South-Kivu province of DRC and Mukisa et al. (2020) in Bongo, a popular beverage in western and central Uganda produced traditionally by fermenting unpasteurized cows' milk. The diminution of the pH is desirable for fermented dairy products since it facilitates flavor development, coagulum formation and prevents growth of pathogenic and spoilage microbes (Downes and Ito, 2001; Mukisa et al., 2020). The fat content was high in Mashanza produced in South-Kivu compared to Tanganyika province. This result could be explained by the fact that the fat content in processed milk is mostly due to several factors including raw milk used, ferment used, condition of use (fermentation time, equipment), draining process (Fidler et al., 2001; Birali et al., 2019). The situation can also be explained by the fact that there more cows fed on concentrates and improved forages in South-Kivu than in Tanganyika. Fat is the main caloric-energetic source of milk but is considered the most variable changeable constituent of milk (Shanler, 1985). Besides that, the repeated processing and ferment used can alter the fat globule structure in milk. This fact, associated with the use of plastic facilities to deliver milk to consumers, especially when this delivery process takes a long time, might be an explanation for the intense decline of fat content in the samples that were delivered by intensive biochemical and enzymatic processes (Metha et al., 1998; Thomaz et al., 1999). A decrease in lactose was observed in pasteurized milk compared to fermented, raw milk and Mashanza. The observed lactose values are in range with findings of Elbagermi et al. (2021) in cows' milk. Lactose is the major carbohydrate in milk and its reasonable concentration helps to maintain osmotic pressure of the milk by regulating its water content (Martin et al., 2016). In addition, lactose helps the absorption of calcium and other minerals contained in milk (Kalyanasundaram et al., 2021). In each milk type, protein content was not significantly influenced (P<0.05) by the production region. However, protein content was higher in raw and fermented milk compared to pasteurized milk and Mashanza. The decrease in protein content in pasteurized milk could be due to long time taken for the heat treatment and storage temperature and time, as reported in the present study. Lowe et al. (2004) reported that the degree of proteolysis depends on the intensity of heat treatment of milk. The decrease in protein content in Mashanza could be associated with the partial hydrolysis of proteins during its fabrication process. This hydrolysis could be the result of dripping during the processing period of Mashanza, native milk enzyme activity (plasmin) and/or proteases originating from incidental milk microflora (Ismail and Nielsen, 2010; Yasser et al., 2010).
Escherichia coli was not found in any of the milk samples. This should encourage efforts to preserve safe milk since Escherichia coli indicates a possible presence of enteropathogenic and/or toxigenic microorganisms which constitute a public health hazard and are therefore known as pathogenic bacteria causing severe intestinal and extra intestinal diseases for men (Kaper et al., 2004). Total Bacterial Counts (TBC) or Total Aerobic Mesophyll Flora (TAMF) reported in this study are in accordance with results of Mpatswenumugabo et al. (2019) who assessed milk bacterial loads and micro-organisms associated with milk handling practices from dairy farmers, milk hawkers, milk collection centers and milk kiosks in the North-western region of Rwanda. Indeed, the observed TAMF varied from 14.7 × 104 CFU/ml (for pasteurized milk) to 57.9 × 104 CFU/ml (for Mashanza). These results concur with the EAC standards (106 CFU/ml), the AFNOR (France standard) (5 × 105 CFU/ml) and the American standard (3 × 103CFU/ml) (Mutwedu et al., 2018; Hoffmann et al., 2022). This contamination in the milk samples collected in the two provinces at farmers, collectors, vendors' level is influenced by different factors, including the hygienic condition of the milker and the mammary gland while milking (Mutwedu et al., 2018), storage and transport in unclean milk containers, prolonged time for milk storage and uncontrolled temperature during milk transportation (Mpatswenumugabo et al., 2019). In the study area, some farmers milked their cows in the morning hours and stored milk for about five hours at ambient temperature while waiting for milk collectors to take it for distribution to different customers like individual consumers, milk kiosks/restaurants. These collectors also had tendency of selling milk in the afternoon hours on public roads or milk “markets”. Indeed, the milk market in eastern DRC is often designated as “informal” because most of the milk coming from smallholder farmers does not enter the regulatory food chain (Ayagirwe and Nfuamba, 2021), thus increasing the risks of delivering contaminated milk products to consumers.
In this study, coliforms were found abundant in all collected milk samples. All pasteurized milk samples were assessed to be out of the EAC standard (more than 10 CFU/ml). The same observation is made for non-pasteurized milk whose values are above the EAC accepted standard of 5 × 104 CFU/ml in almost all evaluated samples (Hoffmann et al., 2022). In fact, milk from emerging economies has been reported to contain very high coliform. For insistence, in Tanzania, mean coliform counts of 3-14 × 106 CFU/ml has been reported in milk that was examined along the informal value chain (Swai and Schoonman, 2011). In Zimbabwe, total coliform counts of 1.56–6.22 log10 CFU/ml was reported in milk samples (Chimuti et al., 2016). Coliforms are a subset of bacteria that can grow at higher temperatures of 44.5-45.5°C, indicating that hot climate is one of the major ways of its proliferation (Wanjala et al., 2018). The principal sources of coliform contamination have been associated to sanitary infrastructures and establishments (Lues et al., 2003), non-hygienic milking equipment and udder (Miseikiene et al., 2015), containers (Wafula et al., 2016) and animal mastitis (Torkar and Teger, 2005).
Results of this study revealed the contamination in Salmonella in the tested milk products across axis. Salmonella is known to occur in raw milk, but pasteurized milk can be contaminated after heating and therefore transfer the pathogen to consumers (Holschbach and Peek, 2018), leading to salmonellosis, one of the most common causes of diarrhea globally (Sánchez-Vargas et al., 2011). The presence of Salmonella and Staphylococcus in food is typically prohibited (AFNOR, 1986; JORA, 1998). However, they are commonly found in contaminated food, especially when processing procedures are not adhered to. In this study, pasteurized milk samples were collected from local markets mainly characterized by non-observance of aseptic standards during milk processing and the storage and marketing conditions of milk products. This explains partly why pasteurized milk still contain Salmonella and Staphylococcus. Also, in the study area, milk buyers prefer to inspect the milk quality by opening the container or tasting the savor before they decide to purchase or not. This practice force vendors to open their products every time, exposing them to new contaminations. As the containers are not hermetically sealed and the products are not marketed under a cold chain, the ambient temperature accelerates the development of microorganisms and degrades the quality of the products.
The presence of Staphylococcus in tested milk samples is in accordance with findings of Kilango (2011) in milk samples collected in Dar es Salaam and of Addis et al. (2011) in milk samples collected in Ethiopia. Milk may carry a potential risk of poisoning with Staphylococcus along the value chain if the milk is subject to conditions and storage temperatures conducive to the multiplication of the pathogen, with subsequent production of enterotoxins (Nádia et al., 2012). Inappropriate handling of milk could result in bacterial growth and substantially increase the potential risk to consumers of milk products. Thus, vigilance in maintaining hygienic conditions in milking and in infrastructure along the milk value chain is of crucial importance (Van Kessel et al., 2004).
Generally, it was found that animal housing and feeding, animal health and management, practices of milk harvesting, storage, transportation, and retailing predisposed the milk to microbial contamination. The general hygiene at milking is known to affect the numbers of microorganisms in the milk (Kivaria et al., 2006). It is recommended that before milking, the animal house should be cleaned, the udder washed and dried before milking. After milking, teat dipping in suitable disinfectant is necessary to control entry of microorganisms through the teat canal (Shija, 2013).
From this study, occurrence of microorganisms at farm level could be associated with the fact that some farmers did not clean their hands, wash cow teats and clean animal houses before milking. Hand-milking using unwashed hands practiced by famers may transmit microorganisms to the milk (Shija, 2013). In addition, it was observed that milking was done either in the cowsheds or in a kraal with very dirty floor under traditional rearing system. This could be another risk practice that contributed to high microbial contamination of milk at producers' level. Worse enough, the longtime of milk storage at farmers, collectors and vendors' level was associated to the increase of microorganisms loads in collected milk in both South-Kivu and Tanganyika provinces. Indeed, storage and handling of milk under room temperature favors bacteria multiplication. The contamination of the milk stored in the freezer, especially at vendors' level, could be related to the irregularity of the electric power in the two provinces which can be unavailable for several hours per day, thus causing the bacterial multiplication when the cold chain is interrupted. Previous study by Swai and Schoonman (2011) in Tanga reported similar observations. Furthermore, other studies in Zimbabwe, Tanzania and Ghana reported that unhygienic practices and cold chain issues along the milk value chain predisposed milk to high bacterial load (Gran et al., 2001; Omore et al., 2009).
The general microbial contamination in milk from vendors could be associated with the source of milk, bulking, cleanliness of the selling points and storage conditions. Dirty selling environment, lack of cold chain facilities and bulking were all together regarded among main risk factors that contributed to the high bacterial contamination of the milk products from vendors. These findings are in line with the study done in Dar es Salaam city by Kivaria et al. (2006).
It was noticed that the containers used during milking, transportation, storage and distribution were the wide and narrow necked plastic containers which sometimes are difficult to wash. The inner corners of narrow necked plastic containers are not easy to wash, this led to sticking of milk residues. In such a situation, microorganisms can rapidly build up in milk residues and may contaminate the milk products on subsequent uses. Similar observations were made by Bukuku (2013) who reported that plastic containers increased microbial count in milk. The plastic containers can thus be a source of several types of bacteria in milk. It is therefore not surprising that the milk storage containers played a significant role in the contamination of milk.
In the present study, milk collected from regions where farmers practice pasteurization had fewer numbers of pathogens compared to regions where most farmers deliver raw milk to collectors. In the latter, pathogens counts were higher such as fecal coliforms. In fact, pasteurization was reported to be positive effect on microbial contents in milk, which reduces the total bacteria count, Coliform bacteria count and pathogens (El Zubeir et al., 2007). Pasteurization can extend shelf life of milk while reducing its microbial load with no effect on chemical composition (AbdElrahman et al., 2013).
5. Conclusion
From the findings of this study, it can be concluded that physicochemical parameters in pasteurized, raw, fermented milk as well as in Mashanza depend on the feeding system, cattle breed kept by farmers and the calving rank. These parameters were not affected when milk was transferred from one stakeholder to another (producer, collector and, vendor). Nevertheless, microbiological parameters were highly affected by the types of milk products, production zone and stakeholders in the value chain. These microbiological parameters were mostly influenced by storage time, cleaning of equipment and hands, type of material for storage, the presence of the cold chain, contamination during milk transformation. However, in view of these results, the milk products sold along the milk value chain in South-Kivu and Tanganyika provinces are of poor quality and susceptible to negatively influence consumer's safety. It is therefore important to provide training on the health risk related to poor hygienic condition of marketed milk among actors along the milk value chain. Appropriate milk handling, conservation facilities and quality control services should be encouraged for consumers' safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: SB and RA. Methodology: SB, RA, VM, and ZN. Software, data curation, and analysis: YM. Validation: MF and JM. Data collection: SB, RA, and VM. Laboratory analysis: RA, VM, and JZM. Writing—original draft preparation: SB and VM. Writing—review and editing: RA, ZN, PU, and JM. Supervision and funding acquisition: MF and JM. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was from the Integrated Project for Agriculture Growth in the Great Lakes Region (PICAGL, Project ID: P143307) funded by the World Bank Group via the Government of the Democratic Republic of the Congo and implemented by the International Institute of Tropical Agriculture (IITA) in South-Kivu and Tanganyika provinces.
Acknowledgments
The Université Evangélique en Afrique (UEA) is thanked for have provided laboratory facilities. Dr. B. Vanlauwe, DDG R4D IITA, is thanked for reviewing an earlier draft of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1105515/full#supplementary-material
References
Abate, M. R., and Addis, A. H. (2015). Microbiological quality assessment of raw and pasteurized milk. Int. J. Food Sci. Microbiol. 2, 87–91.
AbdElrahman, A. M. S., Ahmed, M. M. A., El Zubeir, M. E. I., El Owni, O. A. O., and Ahmed, A. K. M. (2013). Effect of storage temperature on the microbiological and physicochemical properties of pasteurized milk. Annals Food Sci. Tech. 14, 1 www.afst.valahia.ro
Addis, M., Pal, M., and Kyule, M. N. (2011). Isolation and Identification of Staphylococcus species from Raw Bovine Milk in Debre Zeit, Ethiopia. Med. J. Vet. Res. 4, 45–49.
AFNOR (1986). Contrôle de la qualité des produits laitiers, ISTV, 3ème edition. Paris: AFNOR; ISTV. p. 303.
Ahikiriza, E., Wesana, J., Gellynck, X., Van Huylenbroeck, G., and Lauwers, L. (2021). Context specificity and time dependency in classifying Sub-Saharan Africa dairy cattle farmers for targeted extension farm advice: the case of Uganda. Agriculture. 11, 836. doi: 10.3390/agriculture11090836
Alonso, S., Muunda, E., Ahlberg, S., Blackmore, E., and Grace, D. (2018). Beyond food safety: Socio- conomic effects of training informal dairy vendors in Kenya. Global Food Sec. 18, 86–92. doi: 10.1016/j.gfs.2018.08.006
American Public Health Association (2001). Compendium of Methods for the Microbiological Examination of Foods. Washington DC: APHA Inc.
Association of Official Analytical Chemists (1998). Bacteriological Analytic Manual. 5th Edn. Washington DC: AOAC.
Ayagirwe, R. B. B., and Mutwedu, V. B. (2021). Evaluation du profil nutrionnel du lait le long de la chaine de valeur dans les provinces du Sud Kivu et Tanganyika, Est de la RD Congo. Université Evangélique en Afrique (UEA) et International Institute of Tropical Agriculture. p. 71.
Ayagirwe, R. B. B., and Nfuamba, F. (2021). Analyse diagnostic des systèmes de production laitière du Sud-Kivu et du Tanganyika, Est de la RD Congo. Université Evangélique en Afrique (UEA), Vétérinaire Sans Frontière (VSF) et International Institute of Tropical Agriculture. p. 113.
Bachtarzi, N., Amourache, L., and Dehkal, G. (2015). Qualité du lait cru destiné à la fabrication d'un fromage à pâte molle type Camembertdans une laiterie de Constantine (Est algérien). Int. J. In. Sci. Res. 17, 34–42.
Bebe, B. O., Lee Van Der, J., Kilelu, C. W., Van Der Lee, J., and Kilelu, C. W. (2018). Milk Retailing Innovation in Kenya and Consumers Perceptions of Safety (No. 010; 3R Kenya Project Practice Brief). Wageningen Livestock Research, Wagenigen, Netherland. p. 6. doi: 10.18174/413390
Birali, M., Sumbu, Z., Walangululu, J., Busime, M., and Cirhuza, M. (2019). Analyse des pratiques d'hygiène et de fabrication et évaluation de la qualité du Mashanza dans 12 unités de production au Sud-Kivu. J. Anim. Plant Sci. 42, 7314–7329. doi: 10.35759/JAnmPlSci.v42-3.4
Bukuku, J. N. (2013). Health Risks Awareness Due to Raw Milk Consumption in Arusha City and Meru District, Tanzania. Unpublished Dissertation for Award of MSc. Morogoro, Tanzania: Degree at Sokoine University of Agriculture. p. 91.
Chepkoech, B. (2010). “Milk quality control and regulation in dairy production: A case of dairy producers in Kikuyu division, Kabete district, central province - Kenya,” in 3rd African Association of Agricultural Economists (AAAE) and 48th Agricultural Economists' Association of South Africa (AEASA) Conference. Cape Town. p. 7. doi: 10.22004/ag.econ.97077
Chimuti, S., Midzi, N., Njage, P. K., and Mugadza, D. T. (2016). Microbial species of safety concern in milk from informal processors in Harare, Zimbabwe. Afr. J. Microbiol. Res. 10, 1257–126.
Defailly, D. (2000). L'économie du Sud-Kivu 1990–2000: Mutations profondes cachées par une panne. Rapport, Sud Kivu, RD Congo. p. 8. Available online at: http://www.ua.ac.be/objs/00111067
Dehinenet, G., Mekonnen, H., Ashenafi, M., and Emmanuelle, G. (2013). Determinants of raw milk quality under a smallholder production system in selected areas of Amhara and Oromia National Regional States, Ethiopia. Am J Agric Biol Sci. 4, 84–90. doi: 10.5251/abjna.2013.4.1.84.90
Downes, F. P., and Ito, K. (2001). Compendium of Methods for the Microbiological Examination in Foods. Washington, D.C.: American Public Health Association (APHA). doi: 10.2105/9780875531755
El Zubeir, I. E. M., Gabriechise, V., and Johnson, Q. (2007). Study on some quality control measures of pasteurized milk of the Western Cape, South Africa. Int. J. Dairy Sci. 2, 372–379 doi: 10.3923/ijds.2007.372.379
Elbagermi, M. A., Alajtal, A. I., and Edwards, H. G. M. (2021). A comparative study on the physicochemical parameters and trace elements in raw milk samples collected from Misurata- Libya. Sop Trans. Analy. Chem. 1, 15–23. doi: 10.15764/ache.2014.02002
FAO (2021). World Food and Agriculture – Statistical Yearbook. Rome: Food and Agriculture Organization of the United Nations.
Fidler, N., Sauerwald, T. U., Demmelmair, H., and Koletzko, B. (2001). Fat content and fatty acid composition of fresh, pasteurized, or sterilized human milk. Adv. Exp. Med. Biol. 501, 485–495. doi: 10.1007/978-1-4615-1371-1_60
Goff, H. D., and Hill, A. R. (1992). “Chemistry and physics,” in Dairy Science And Technology Handbook, ed Y. H. Hui (Wiley-VCH Publication), 2–4.
Gran, H. M., Mutukumira, A. N., Wetlesen, A., and Narvhus, J. A. (2001). Smallholder dairy processing in Zimbabwe: hygiene practices during milking and the microbiological quality of the milk at the farm and on delivery. Food Control. 13:41–47. doi: 10.1016/S0956-7135(01)00082-2
Haas, C. N. (1989). Estimation of microbial densities from dilution count experiments. Appl. Environ. Microbiol. 55, 1934–1942. doi: 10.1128/aem.55.8.1934-1942.1989
Hoffmann, V., Simiyu, S., Sewell, D. K., Tsai, K., Cumming, O., Mumma, J., et al. (2022). Milk product safety and household food hygiene influence bacterial contamination of infant food in Peri-Urban Kenya. Front. Public Health. 9, 772892. doi: 10.3389/fpubh.2021.772892
Holschbach, C. L., and Peek, S. F. (2018). Salmonella in dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 34, 133–154. doi: 10.1016/j.cvfa.2017.10.005
Hossain, M. B., and Dev, S. R. (2013). Physiochemical characteristics of various raw milk samples in a selected dairy plant of Bangladesh. Int. J. Engin. Appl. Sci. 1, 91–96.
Houaria, J., and Zohra, H. (2018). Determination of aerobic mesophilic bacteria and coliforms in raw milk in the region of Kosovo. Albanian J. Agric. Sci. 17, 37–40.
Hoxha, M., and Mara, V. (2012). Impact of physical-chemical properties on milk coagulation ability for some albanian breeds of cow, sheep and goat. Int. J. Lat. Res. Sci. Tech. 1, 234–238. Available online at: https://www.mnkjournals.com/journal/ijlrst/pdf/temp/Volume_1_3/10063.pdf
Ismail, B., and Nielsen, S. S. (2010). Invited review: plasmin protease in milk: current knowledge and relevance to dairy industry. J. Dairy Sci. 93, 4999–5009. doi: 10.3168/jds.2010-3122
JORA (1998). Arrêté interministériel du 25 Ramadhan 1418 correspondant au 24janvier 1998 modifiant et complétant l'arrêté du 14 Safar 1415 correspondant au 23 juillet 1994 relatif aux spécifications microbiologiques de certaines denrées alimentaires. Algérie. p. 18
Kabui, K. K., Arimi, S. M., Kang'ethe, E. K., Omore, A., Makokha, S., Nduhiu, G., et al. (2015). A determination of raw milk quality and the most suitable microbiological test at the milk collection level in two regions of Kenya. Int. J. Vet. Sci. 5, 44–47.
Kalyanasundaram, S., Narayanan, V. K., and Krishnamurthy, K. (2021). The role of lactose in milk and an overview of intolerance to lactose. J. Pub. Health and Nutr. 4, 300–301. Available online at: https://www.alliedacademies.org/articles/the-role-of-lactose-in-milk-and-an-overview-of-intolerance-to-lactose.pdf
Kaper, J. B., Nataro, J. P., and Mobley, H. L. T. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Kilango, K. G. (2011). Food safety in milk markets of smallholder farmers in Tanzania: A case of peri urban wards in temeke municipality (Ph.D. Dissertation). Sokoine University of Agriculture, Morogoro, Tanzania. p. 58.
Kivaria, F. M., Noordhuizen, J. P. T. M., and Kapanga, A. M. (2006). Evaluation of the hygienic quality and associated public health hazards of raw milk marketed by smallholder dairy producers in the Dar es Salaam region, Tanzania. J. Trop. An. Health Prod. 38, 185–194. doi: 10.1007/s11250-006-4339-y
Law, J. W. -F., Ab Mutalib, N. -S., Chan, K.-G., and Lee, L.-H. (2015). An insight into the isolation, enumeration, and molecular detection of Listeria monocytogenes in food. Front. Microbiol. 6, 1227. doi: 10.3389/fmicb.2015.01227
Lindahl, J. F., Deka, R. P., Asse, R., Lapar, L., and Grace, D. (2018). Hygiene knowledge, attitudes, and practices among dairy value chain actors in Assam, North-East India and the impact of a training intervention. Inf. Ecol. Epidemiol. 8, 10. doi: 10.1080/20008686.2018.1555444
Lowe, E. K., Anema, S. G., Bienvenue, A., Roland, M. J., Creamer, L. K., and Jiménez-Flores, R. (2004). Heat-induced redistribution of disulfide bonds in milk proteins. 2. Disulfide bonding patterns between bovine β-lactoglobulin and κ-casein. J. Agr. Food Chem. 52(25), 7669–7680 doi: 10.1021/jf0491254
Lues, J. F. R., Venter, P., and Van Der Westhuizen, H. (2003). Enumeration of potential microbiological hazards in milk from a marginal urban settlement in central South Africa. Food Microbiol. 20, 321–326. doi: 10.1016/S0740-0020(02)00128-4
Martin, C. R., Ling, P. R., and Blackburn, G. L. (2016). Review of infant feeding: key features of breast milk and infant formula. Nutrients. 8, 279. doi: 10.3390/nu8050279
Metha, N. R., Hamosh, M., Bitman, J., and Wood, D. L. (1998). Adherence of medium-chain fatty acids to tube feeding of human milk during gavage feeding. J. Pediatr.112, 474–476 doi: 10.1016/S0022-3476(88)80341-X
Miseikiene, R., Rudejeviene, J., and Gerulis, G. (2015). Effect of pre-milking antiseptic treatment on the bacterial contamination of cow teats' skin. Bulg. J. Vet. Med. 18, 159–166. doi: 10.15547/bjvm.833
Mpatswenumugabo, J. P. M., Bebora, L. C., Gitao, G. C., Mobegi, V. A., Iraguha, B, and Shumbusho, B. (2019). Assessment of bacterial contamination and milk handling practices along the raw milk market chain in the north-western region of Rwanda. Afr. J. Microbiol. Res. 13, 640–648. doi: 10.5897/AJMR2018.8919
Mukisa, I. M., Ssendagala, G. W., and Byakika, S. (2020). Microbiological safety and physicochemical composition of Bongo, a traditional fermented milk product from Lyantonde district, Uganda. Sci. Afr. 10, e00583. doi: 10.1016/j.sciaf.2020.e00583
Mutwedu, V. B., Ayagirwe, R. B. B., Mugumaarhahama, Y., Bahindwa, G., Barume, A., Matendo, R., et al. (2018). Effects des techniques de transformation sur la qualité du fromage blanc traditionnel ≪Mashanza≫ produit au Sud-Kivu, RD Congo. J. Anim. Plant Sci. 38, 6097–6111.
Nádia, M., Diane, S., Débora, O., and Mirlei, R. E. (2012). Evaluation of Microbiological quality of Raw Milk produced at two properties in the Far West of Santa Catarina, Brazil. J. Food Pub. Health. 2, 79–84. doi: 10.5923/j.fph.20120203.04
Nyokabi, S., Luning, P. A., De Boer, I. J. M., Korir, L., Muunda, E., Bebe, B. O., et al. (2021). Milk quality and hygiene: knowledge, attitudes and practices of smallholder dairy farmers in central Kenya. Food Control. 130, 108303, doi: 10.1016/j.foodcont.2021.108303
Nyokabi, S. N., De Boer, I. J. M., Luning, P. A., Korir, L., Lindahl, J., Bett, B., et al. (2021). Milk quality along dairy farming systems and associated value chains in Kenya: An analysis of composition, contamination and adulteration. Food Control. 119, 107482. doi: 10.1016/j.foodcont.2020.107482
Omore, A. O., Staal, S. J., Wanyoike, F., Osafo, E. L. K., Kurwijila, L., Barton, D., et al. (2009). “Market Mechanisms and Efficiency in Urban Dairy Products Markets in Ghana and Tanzania,” in ILRI Research Report 19. Nairobi, Kenya: IRIL (International Livestock Research Institute). p. 51.
R Core Team (2022). A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available online at: http://www.r-project.org
Sánchez-Vargas, F. M., Abu-El-Haija, M. A., and Gómez-Duarte, O. G. (2011). Salmonella infections: an update on epidemiology, management, and prevention. Trav. Med. Infect. Dis. 6 263–277. doi: 10.1016/j.tmaid.2011.11.001
Schwendel, B. H., Wester, T. J., Morel, P. C., Tavendale, M. H., Deadman, C., Shadbolt, N. M., et al. (2015). Invited review: Organic and conventionally produced milk- an evaluation of factors influencing milk composition. J. Dairy Sci. 98, 721–746. doi: 10.3168/jds.2014-8389
Shanler, R. J. (1985). Suitability of human milk for the low-birthweight infant. Clin. Perinatol. 22, 207–222 doi: 10.1016/S0095-5108(18)30309-9
Shija, F. (2013). Assessment of milk handling practices and bacterial contaminations along the dairy value chain in Lushoto and Handeni Districts, Tanzania (Ph.D. Dissertation). Sokoine University of Agriculture, Morogoro, Tanzania. p. 85.
SNSA: Service National des Statistiques Agricoles. (2020). Plan national d'investissement agricole (PNIA) 2014–2020. Kinshasa: Service National des Statistiques Agricoles - Ministère National de l'agriculture.
Staal, S. J., Owango, M., Muriuki, H., Lukuyu, B., Njoroge, L., Njubi, D., et al (2001). Dairy Systems Characterization of the Greater Nairobi Milk Shed. Smallholder Dairy (Research & Development) Project. Kenya Agricultural Research Institute (KARI), Ministry of Agriculture (MoA) and International Livestock Research Institute (ILRI) Collaborative Dairy Research Program, Nairobi, Kenya. p. 73. Available online at: http://www.smallholderdairy.org/collaborative%20res%20reports.htm
Swai, E. S., and Schoonman, L. (2011). Microbial quality and associated health risks of raw milk marketed in the Tanga region of Tanzania. Asian Pacific J. Trop. Biomed. 1, 217–222. doi: 10.1016/S2221-1691(11)60030-0
Thomaz, A. C. P., Gonçalves, A. L., and Martinez, F. E. (1999). Effects of human milk homogenization on fat absorption in very low birth weight infants. Nutr. Res. 19, 483–492. doi: 10.1016/S0271-5317(99)00015-9
Torkar, K. G., and Teger, S. G. (2005). The microbiological quality of raw milk after introducing the two day's milk collecting system. Acta Agriculturae Slovenica. 92, 61–74.
Van Kessel, J. S., Karns, J. S., Gorski, L., McCluskey, B. J., and Perdue, M. L. (2004). Prevalence of Salmonellae, Listeria monocytogenes and Fecal Coliforms in Bulk Tank Milk on US Dairies. J. Dairy Sci. 87, 2822–2830. doi: 10.3168/jds.S0022-0302(04)73410-4
Wafula, W. N., Matofari, W. J., Nduko, M. J., and Lamuka, P. (2016). Effectiveness of the sanitation regimes used by dairy actors to control microbial contamination of plastic jerry cans'surfaces. Int. J. Food Contam. 3, 1–8. doi: 10.1186/s40550-016-0032-8
Wanjala, W. N., Nduko, J. M., and Mwende, M. C. (2018). Coliforms contamination and hygienic status of milk chain in emerging economies. J. Food Qual. Hazards Contr. 5, 3–10 doi: 10.29252/jfqhc.5.1.3
Yasser, H. H., Khemais, K., and Max, J. P. (2010). Effect of plasmin, milk somatic cells and psychotrophic bacteria on casein fractions of ultra-high temperature treated milk. Food Sci. Tech. Res. 16, 79–86. doi: 10.3136/fstr.16.79
Keywords: dairy products, health risk, microbiological aspects, milk value chain, nutritional profile, physicochemical characteristics
Citation: Bacigale SB, Ayagirwe RB, Mutwedu VB, Mugumaarhahama Y, Mugisho JZ, Nziku Z, Fofana M, Udomkun P and Mignouna J (2023) Assessing milk products quality, safety, and influencing factors along the dairy value chain in eastern Democratic Republic of the Congo. Front. Sustain. Food Syst. 7:1105515. doi: 10.3389/fsufs.2023.1105515
Received: 22 November 2022; Accepted: 06 March 2023;
Published: 27 March 2023.
Edited by:
Delia Grace, University of Greenwich, United KingdomReviewed by:
Marina Mozgovoj, Instituto Nacional de Tecnología Agropecuaria, ArgentinaThomas Matthew Taylor, Texas A&M University, United States
Copyright © 2023 Bacigale, Ayagirwe, Mutwedu, Mugumaarhahama, Mugisho, Nziku, Fofana, Udomkun and Mignouna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samy Bashizi Bacigale, cy5iYWNpZ2FsZUBjZ2lhci5vcmc=
†ORCID: Samy Bashizi Bacigale orcid.org/0000-0001-8766-6494
Rodrigue Basengere Ayagirwe orcid.org/0000-0002-5019-0653
Valence Bwana Mutwedu orcid.org/0000-0002-7360-2374
Yannick Mugumaarhahama orcid.org/0000-0002-4690-502X
Zabron Nziku orcid.org/0000-0001-7414-7329
Patchimaporn Udomkun orcid.org/0000-0002-3777-9252
 Samy Bashizi Bacigale
Samy Bashizi Bacigale Rodrigue Basengere Ayagirwe
Rodrigue Basengere Ayagirwe Valence Bwana Mutwedu
Valence Bwana Mutwedu Yannick Mugumaarhahama
Yannick Mugumaarhahama Janvier Zirhumana Mugisho
Janvier Zirhumana Mugisho Zabron Nziku4†
Zabron Nziku4†