- 1School of Public Health, University of Pennsylvania, Philadelphia, PA, United States
- 2International Livestock Research Institute (ILRI), Nairobi, Kenya
- 3Department of Obstetrics and Gynecology, Duke University Medical Center, School of Medicine, Duke University, Durham, NC, United States
- 4Independent Research and Evaluation Consultant, Guelph, ON, Canada
- 5Center for Public Health and Ecosystem Research, Hanoi University of Public Health, Hanoi, Vietnam
Introduction: African swine fever in Vietnam is contributing to existing concerns over zoonotic disease transmission from sick pigs to humans. While slaughterhouses are key sites of occupational hazards to workers and contamination of meat, the specific slaughtering practices contributing to zoonotic occupational and foodborne disease risks remain under-researched. Our objective is to identify and characterize aspects of pig slaughtering processes that contribute to such risks.
Methods: We draw on qualitative observations, photos, and videos from three mobile slaughterhouses and seven abattoirs in Hung Yen, Vietnam.
Results: Based on our analysis, areas likely leading to zoonotic disease risks include slaughtering procedures, personal hygiene of workers, equipment sanitation, and facility sanitation. Within the small-scale swine industry, slaughtering practices are long-standing and difficult to change.
Conclusion: Our study underscores the importance of hygiene training of workers, improvements to equipment and facilities, and awareness-building activities targeting consumers to reduce the burden of zoonotic disease risks in small-scale pig slaughter settings.
1. Introduction
Since 2019, the impacts of African swine fever—exacerbated by the COVID-19 pandemic—have been contributing to concerns over zoonotic disease transmission from sick pigs to humans in Vietnam (Nguyen-Thi et al., 2021). These concerns are developing against the backdrop of already high existing worries about food safety in Vietnam among consumers, where repeated episodes of unsafe food practices receive widespread media attention (Nguyen-Viet et al., 2017). In response, the Vietnamese government has focused efforts on centralizing swine husbandry, based on the assumption that large-scale and regulated production will not only enhance productivity but also disease control (Nguyen et al., 2021).
Despite efforts to centralize Vietnam's swine industry, most pork production continues to occur among an informally defined network of small-scale actors from production to retail (Nguyen Thi Thuy et al., 2020). For example, at the point of slaughter, facilities of all types exist, from household slaughterhouses to intermediate-sized abattoirs and centralized facilities, though household slaughterhouses dominate the domestic pork supply. There also exists mobile slaughtering, particularly in rural areas, where farmers hire workers to slaughter pigs directly on farms. Importantly, no formal government oversight exists for defining, guiding, and monitoring small-scale pig slaughtering operations (Dang-Xuan et al., 2016; Pham and Dinh, 2020). Occupational exposure to sick pigs and consumption of contaminated pork are important risk factors for zoonotic diseases associated with swine such as bacteria (e.g., Salmonella spp., Streptococcus suis), parasites (e.g., Trichinella), and viruses (e.g., swine influenza) (Van De et al., 2015; Takemae et al., 2017; Rayanakorn et al., 2018). While slaughterhouses are key sites for occupational hazards to workers and contamination of meat, the specific slaughtering practices contributing to zoonotic disease risks in Vietnam remain underexplored (Hoa et al., 2011; Ho et al., 2011; Nguyen et al., 2021). This paper aims to identify and characterize risky pig slaughtering practices, and in doing so, inform interventions to decrease zoonotic disease transmission and address food safety concerns.
2. Methods
2.1. Context
This research was conducted as part of PigRISK, a 5-year program (2012–2017) that aimed to reduce disease risks and improve food safety in smallholder pig value chains in Vietnam (Lam et al., 2016). The core objectives of PigRISK were: (1) To assess the impacts of pork-borne diseases on human health and the livestock sector and to identify opportunities for risk management; (2) To develop incentive-based innovations to improve the management of human and animal health risks in smallholder pig value chains; and, (3) To improve capacity to assess and manage risks in smallholder pig value chains by engaging stakeholders. This study contributes to the first objective of PigRISK by characterizing risky pig slaughtering practices.
PigRISK was guided by One Health, a conceptual framework considered helpful for tackling food safety issues in low- and middle-income countries (Grace, 2017). One Health calls for different disciplines to work together on complex challenges at the intersection of human, animal, and environmental health (Zinsstag et al., 2011; Nguyen-Viet et al., 2022). Responding to this call, PigRISK mobilized researchers from different fields and institutions including public health (Hanoi University of Public Health), agricultural economics (Vietnam National University of Agriculture), and veterinary epidemiology (International Livestock Research Institute). Our multidisciplinary research team also worked closely with program participants (local authorities and slaughterhouse workers) to shape the direction of PigRISK activities.
2.2. Study site
We selected small-scale slaughterhouses and mobile slaughter settings in Hung Yen Province, where the PigRISK research team had developed longstanding relationships with local authorities. Hung Yen is 47 km southeast of Hanoi, the capital city of Vietnam; its proximity to urban markets has contributed to the province experiencing rapid, unplanned, and demand-driven development for pork (Dang-Xuan et al., 2016). Hung Yen has a population of around 1.2 million people across 10 districts (Hung Yen Portal, 2021). It is characterized by primarily rural areas with small-scale (<10 pigs/day) and medium-scale (11–50 pigs/day) pork processing settings (Dang-Xuan et al., 2017). Hung Yen represents a more intensive pork production system compared to other provinces considering pork in other provinces is mostly supplied by small-scale producers (Nguyen-Viet et al., 2019). We focused on two districts in Hung Yen (Van Giang and Van Lam) which have representation from different scales of pork production. We selected slaughterhouses that met the criteria of (1) operating every day and (2) operating at either small- or medium-scale. These criteria applied to both abattoir and mobile slaughter settings. To obtain a glimpse into traditional pig slaughter practices, only a subset of slaughterhouse settings that met the selection criteria in the two districts were included; these slaughter settings were randomly selected.
2.3. Data collection and analysis
We applied an observational study design to identify and characterize risky pig slaughtering practices because of its potential to enable researchers to visualize and contextualize behaviors most people likely would not describe in an interview (Harvey, 2018). Specifically, we documented pig slaughtering practices primarily through field notes. We also took photos and recorded videos to provide richer data on such practices (Asan and Montague, 2014). The process involved visiting one to two slaughter settings in the early mornings (e.g., around 3 am). On average, two to three slaughter settings were visited per week over the course of four weeks in July 2013. Two researchers conducted observations and interacted with workers. Around two to three workers were present at each slaughter setting and were all engaged in our study. While we did not ask workers about their education and training, previous research suggested workers mainly acquired knowledge and experience of pork handling practices through “learning by doing” rather than a specific training program (Dang-Xuan et al., 2016).
Then, through regular discussions among our multidisciplinary team, we generated hypotheses for practices that could contribute to (1) human infection from occupational exposure and (2) the potential for contamination of pork products. To guide this process, we drew on standard procedures for slaughterhouse operations in developing countries (Mann et al., 1983; Heinz, 2008) while remaining open to other practices that might not be documented in written procedures. Specifically, we documented practices that contradicted what was recommended—or was not covered—in standard procedures. After sharing observations among the team, we drew from our own experiences working in food safety contexts to highlight potential risks and consulted the literature to corroborate our findings.
We conducted a thematic analysis to identify patterns or themes in the data (Braun et al., 2018). Specifically, we applied three levels of sequential deductive coding. First, we examined the flow of the pig slaughtering process (e.g., from livestock receiving to bleeding and transport). Secondly, we focused on contextual factors influencing the slaughtering process (e.g., facility, employee hygiene). Finally, we explored opportunities for zoonotic occupational and foodborne disease risks (e.g., from handling carcasses, from contamination of pork). NVivo© software (QSR International, Burlington, MA, USA) was used to facilitate the coding of fieldnotes and descriptions of photos and still-frames from video footage. As a way of assuring research quality for this study, we wrote reflective memos alongside our notes to document contextual information and reflections on data collection. We also held regular discussions among the authors to co-develop the themes.
3. Results
A total of seven abattoirs and three mobile slaughter sites were visited. Slaughter practices were largely consistent across and within abattoirs and mobile slaughter sites, from pig restraint to transport to markets. Workers processed pigs one at a time. Where variations were observed were in the number of pigs processed per night, the number of employees, and the physical layout of facilities. For example, at the abattoirs, 6–15 swine were processed per day at a fixed site, often requiring two to three workers to complete the entire slaughter process. At mobile slaughter sites, a similar number of pigs were slaughtered on farm sites by mobile butchers who often worked in pairs (e.g., husband-wife team) and with additional help from their clients. Farms provided water and space, while slaughter workers brought knives, hooks, and motorbikes.
In reviewing pig slaughtering practices and evaluating their potential for health risks, we developed four overarching themes: slaughter process, personnel hygiene, equipment sanitation, and facility sanitation.
3.1. Overview of the slaughter process
3.1.1. Transport from holding pens to the restraint area
In both abattoirs and mobile slaughter sites, pigs were first moved from holding pens to restraint areas with a pointed metal rod placed deep in the ventral inter-mandibular space. Pigs were then hoisted onto a metal restraint device or a concrete block that restricted them to lateral recumbency (Figure 1A). Of note, animals with signs of disease were not separated from healthy animals in the abattoir setting. Antemortem and post-mortem examinations of pigs and carcasses were also not consistently conducted. There were no protocols for the identification and disposal of sick animals or diseased tissues, resulting in the potential for infected products to enter the food supply.

Figure 1. Photos of the pork processing process at a mobile slaughter site. (A) Transport from holding pens to the restraint area at a household site. (B) Exsanguination at a household site. (C) Carcass dressing at a household site. (D) Transport to market from household site.
3.1.2. Bleeding
Workers performed exsanguination by a puncture wound through the skin at the base of the neck to sever the carotid arteries and jugular veins, with blood collected in a metal bowl that contained anti-coagulation salts (Figure 1B). Before bleeding, pigs were not stunned, resulting in a violent procedure. In the peri-mortem phase, the animals were often thrashing, with reflexive movements and high intravascular pressure contributing to a projectile expulsion of blood from the laceration site.
3.1.3. Dressing
Carcasses were moved from the restraint device directly onto the floor and to the processing area where all dressing procedures were performed (Figure 1C). Carcasses were not disinfected before further processing. These procedures included scalding and manual hair removal. The water temperature in abattoirs and household sites was not controlled, however, boiling water was used for scalding to facilitate hair removal by knife scraping. Then, workers proceeded with head removal, evisceration (gastrointestinal tract, urogenital tract, liver, respiratory tract, and heart), trimming (lymph nodes and kidneys), and lastly, splitting of the carcass along the spine. While organs were removed en bloc, and evisceration did not result in organ perforation or leads in gastrointestinal contents, the knives used were not disinfected between carcasses. Cold-water rinses were applied once organs were fully removed. Viscera was rinsed in the same water used for washing other parts of the carcass, representing another possible pathway toward contamination. Fabric cloths were then used to dry the internal and external surfaces. They were reused between carcasses without disinfection.
3.1.4. Transport to the market
Each carcass half was then weighed and transported to nearby markets by motorbike with two to three carcasses and heads per vehicle (Figure 1D). Carcasses were uncovered and un-chilled during transportation. The time for carcasses to reach the market varied from as soon as possible or after a couple of carcasses have been processed. Slaughterhouses operated from around 2 am until 6 am while markets opened from 5 am to 11 am.
3.2. Overview of personnel hygiene
The use of personal protective equipment (PPE) is often considered necessary to minimize the transmission of diseases and contamination of products, which include goggles, face masks, aprons, overalls, gloves, and boots. Although abattoir employees and mobile butchers wore some PPE, no participants wore PPE that offered complete protection of dermal, ocular, or mucous membrane surfaces. Employees at abattoirs were not provided uniforms or guidelines for PPE. In both abattoirs and at mobile slaughtering sites, we observed that employees wore personal attire at work and did not change their garments throughout the working period. Hand, arm, or face protection was not worn by any person participating in slaughter activities at any of the observed sites. At the mobile slaughter sites, five out of the six butchers wore rubber boots, and one wore plastic open-toed slippers. Three of the six had complete leg coverage which included rubber boots and plastic overalls. Similar observations were noted in abattoirs; for example, one abattoir employee donned a plastic overall (lower body only) to transport carcasses to the market (Figure 2). And while most abattoirs had employees that were fully clothed, in one abattoir one employee did not have a shirt on.

Figure 2. Abattoir employee wearing a plastic overall (lower body only) to transport pork from the slaughterhouse to the market.
3.3. Overview of equipment sanitation
In abattoir and mobile slaughtering sites, the equipment consisted of a knife (general-purpose, axe, or a large butcher knife) to process pigs, a metal bowl to collect blood, a concrete block (or metal device) to restrain pigs, and a kettle for dehairing. There were also waste buckets, food storage containers, and woven nylon bags for viscera storage and transport. To store blood, employees used plastic containers for the storage of blood (Figure 3A). Mobile butchers brought their equipment, while additional receptacles for storage and waste were provided by the households. A single knife was employed throughout the slaughter process. Equipment disinfection was not performed between the different stages of the pig slaughter process.

Figure 3. Abattoir setting. (A) Equipment storage in an abattoir. (B) Drainage system in an abattoir.
3.4. Overview of facility sanitation
In the abattoirs observed in this study, pigs arriving from different households or farms were held in available pens for several hours before slaughter. There were no biosafety measures in place to prevent the co-mingling of pigs from different origins or of different species. Abattoirs were in one-story buildings that contained (1) a holding area; (2) a restraining, slaughtering, and bleeding area; (3) a carcass and viscera processing area; and, (4) a weighing and transportation area. The roof was made of corrugated metal while the walls were comprised of brick. Inside, the interior floors were paved concrete. Slaughter processes occurred in separate, closely neighboring zones, with no functional barriers to physically delineate them likely due to space limitations. To move between zones, two workers lifted the carcass and moved them manually. In two of the sites, we observed live pigs wandering through the slaughter areas while carcasses were being processed, increasing the probability of meat contamination. In other sites, we observed inadequate physical separation of dogs from the pig slaughter processes which creates additional avenues for disease transmission. All abattoirs had indoor drainage systems with a drain in the floor feeding into roadside channels that emptied into rice paddies. The manual removal of waste was often necessary as drains were not located in gravity-dependent parts of the facilities and were not built to accommodate the high volume of liquid (e.g., blood, wastewater) and solid waste (e.g., hair, bones) (Figure 3B).
In households, mobile slaughter sites were established in the outdoor foyer or backyard of residences within the vicinity of swine pens. Areas used for slaughtering/bleeding, dressing, and transport were paved, and finished pork products were placed on nylon tarps for clients. As within abattoirs, the slaughter process largely occurred within the same area. Wastewater and materials were swept directly into roadside channels that emptied into the rice paddies.
There were no hand washing stations for employees at abattoirs and slaughtering sites in the vicinity of the slaughtering and processing areas. However, workers had access to household bathrooms for handwashing, although we did not observe this practice performed throughout the slaughter process. There were no established sanitation protocols to guide the use of soap/detergents, the temperature of the water, and the frequency of cleaning or disinfection. Cleaning and disinfection were not performed between the slaughter of individual animals but only at the very end of the slaughter process.
4. Discussion
While infected pigs are considered an important source of human infections with zoonotic disease (Hoa et al., 2011), the specific routes of zoonotic transmission remain unclear. For example, while S. suis carriage among healthy pigs entering slaughter facilities is common, how infected pork could affect workers and contaminate uninfected pork remains unclear (Nguyen et al., 2021). Proposed routes of human infection include exposure through skin injuries, mucous membranes, inhalation, and ingestion of contaminated food, though some instances of swine-related human disease have also been reported without known pork exposure (Rayanakorn et al., 2019; Nguyen et al., 2021). Given that occupational exposure and ingestion of contaminated foods are the most likely sources of infection (Rayanakorn et al., 2018), this study aimed to identify gaps in pig slaughterhouse activities which could result in zoonotic occupational exposure for workers and foodborne disease risk for consumers.
4.1. Gaps in the slaughter process
4.1.1. Transport from holding pens to the restraint area
During slaughtering, there were several opportunities for human contact with live pig secretions and infected areas, increasing the possibility of disease transmission from swine to humans. For instance, S. suis has a particular predilection for the upper respiratory tract (specifically, the tonsils and nasopharynx) and the genital and gastrointestinal tracts in healthy pigs (Lun et al., 2007). Among diseased pigs, the bacteria have also been isolated in the brain, lung, heart, and joint tissues (Cheung et al., 2008). Workers across all pig slaughter settings did not wear gloves when working with various parts of the swine carcass, presenting opportunities for occupational disease risks for workers considering, for instance, S. suis can enter human skin through small scratches or wounds.
4.1.2. Bleeding
Knives were not disinfected between each animal slaughtered; the opening cut presented opportunities for infected knives to potentially contaminate the blood and various tissues and subsequently pork products. Additionally, given the absence of PPE used, particularly facial protection, the exsanguination process presented an opportunity for dermal, mucosal, and ocular exposure of workers to the blood of diseased pigs. Reuse of the blood collection pan and pooling of blood sources from different animals can further propagate pathogen transmission, especially as it is also sold as a food product in Vietnam.
4.1.3. Dressing
As with other studies characterizing pig slaughterhouse practices in Vietnam, all dressing procedures took place on the floor where the processing area was not disinfected (Yokozawa et al., 2016; Chau et al., 2017). Potential contact with fecal matter and debris from the pens and processing areas was an additional opportunity for contamination of the carcass, especially because bacteria such as Salmonella spp. and S. suis can remain viable in dust and feces (Lun et al., 2007). Indeed, slaughter areas located close to lairages without hygienic measures were identified as a significant risk factor for carcass contamination with Salmonella spp. (Dang-Xuan et al., 2019).
The recommended water temperature for scalding and hair removal is 60–62°C (Heinz, 2008). While boiling water temperature was used for scalding, it was likely inadequate for sanitization—with previous studies suggesting that hot-water decontamination requires 80°C for 12–15 s (Alban and Sørensen, 2010); this excessive heat for scalding, aggravated by knife scraping, has the potential to damage swine skin through protein coagulation, resulting in increased potential for pathogen colonization of deeper tissues. Contaminated water might also have reached the swine's respiratory system during the scalding process, presenting another possible route of bacterial transmission (Marois et al., 2008). Additionally, the gastrointestinal and urogenital tracts were rinsed in the same water used for washing other parts of the carcass and equipment, another possible vector for contamination.
4.1.4. Transport to the market
Processed pork was brought directly to markets for sale without chilling considering many consumers in Vietnam prefer fresh, room-temperature pork. Consumption of undercooked pork may be particularly hazardous considering that pork products kept at high ambient temperatures can have high bacterial counts (Hoa et al., 2011).
4.2. Gaps in personal hygiene
All participants in the slaughter process had some degree of skin (arms or legs), ocular, and/or mucous membrane exposure throughout the entire process. These results are consistent with a previous study that found over two-thirds of workers at abattoirs visited in Southern and Central Vietnam never used PPE; the same study also found that over two-thirds did not know they could contract infections from healthy animals, and over a quarter did not know they could become infected through direct contact with diseased animals (Tu et al., 2019). Furthermore, in a study where researchers conducted sampling at slaughterhouses in Hanoi and Hung Yen, researchers observed that slaughterhouse workers were not wearing any PPE and suggested that this gap could pose a risk of influenza transmission from swine to humans (Baudon et al., 2018). Yet, complete coverage of hands and feet along with surfaces prone to micro-abrasions (including open wounds) are often considered necessary to prevent disease transmission (Hoa et al., 2011). Handwashing can also help to protect slaughterhouse workers against transmitted bacterial pathogens such as Salmonella spp. as well as protect the meat from contamination (Cook et al., 2017; Durmuşoglu et al., 2020). And given emerging evidence of the bio-aerosolization of pathogenic bacteria such as S. suis (Bonifait et al., 2014; Nguyen et al., 2021; Tang et al., 2021), facial shields or masks are increasingly necessary to both prevent potential inhalation of infected particles.
4.3. Gaps in equipment sanitation
During decapitation, cuts were made through the chain of pharyngeal and cervical lymph nodes as well as parts of the upper respiratory system where S. suis tends to reside (Lun et al., 2007). Infected knives could be a source of cross-contamination within and between carcasses as knives were not disinfected after instances of organ perforation (Dang-Xuan et al., 2018). Although guidance documents recommend a number of sterilizers for hand tools and knives be available (Mann et al., 1983; Heinz, 2008), the use of the same knives for different carcasses without sanitation between carcasses was observed across all abattoirs and mobile sites visited.
4.4. Gaps in facility sanitation
The separation of animals—such as pigs and dogs—was not often practical given space constraints, increasing the potential risk of disease transmission between animals. For example, beyond swine, Salmonella spp. and S. suis have been discovered in domestic animals such as dogs and cats (Wertheim et al., 2009; Wei et al., 2020). The presence of other animals on pig farms is also a risk factor for other diseases such as swine influenza and Trichinella infection by acting as “couriers” for spreading diseases within a farm (Takemae et al., 2016; Le et al., 2022a). Additionally, without a protocol for waste disposal, waste products often accumulate throughout a slaughter shift. The absence of adequate sanitation procedures for facilities could bacterial colonization within facilities (Dang-Xuan et al., 2016).
4.5. Opportunities and barriers
Our study identified potential entry points within slaughter processes to limit intra-carcass contamination, inter-carcass contamination, and direct occupational transmission of zoonoses associated with swine (Table 1). Currently, in small-scale slaughter settings, there is intimate contact between humans, live animals, and meat products; upgrades to facilities that provide better separation would be highly impactful but are not often possible in small-scale settings where profit margins are low and surplus income is insufficient to cover facility upgrades. Without external investments, more light-touch interventions would be promising.
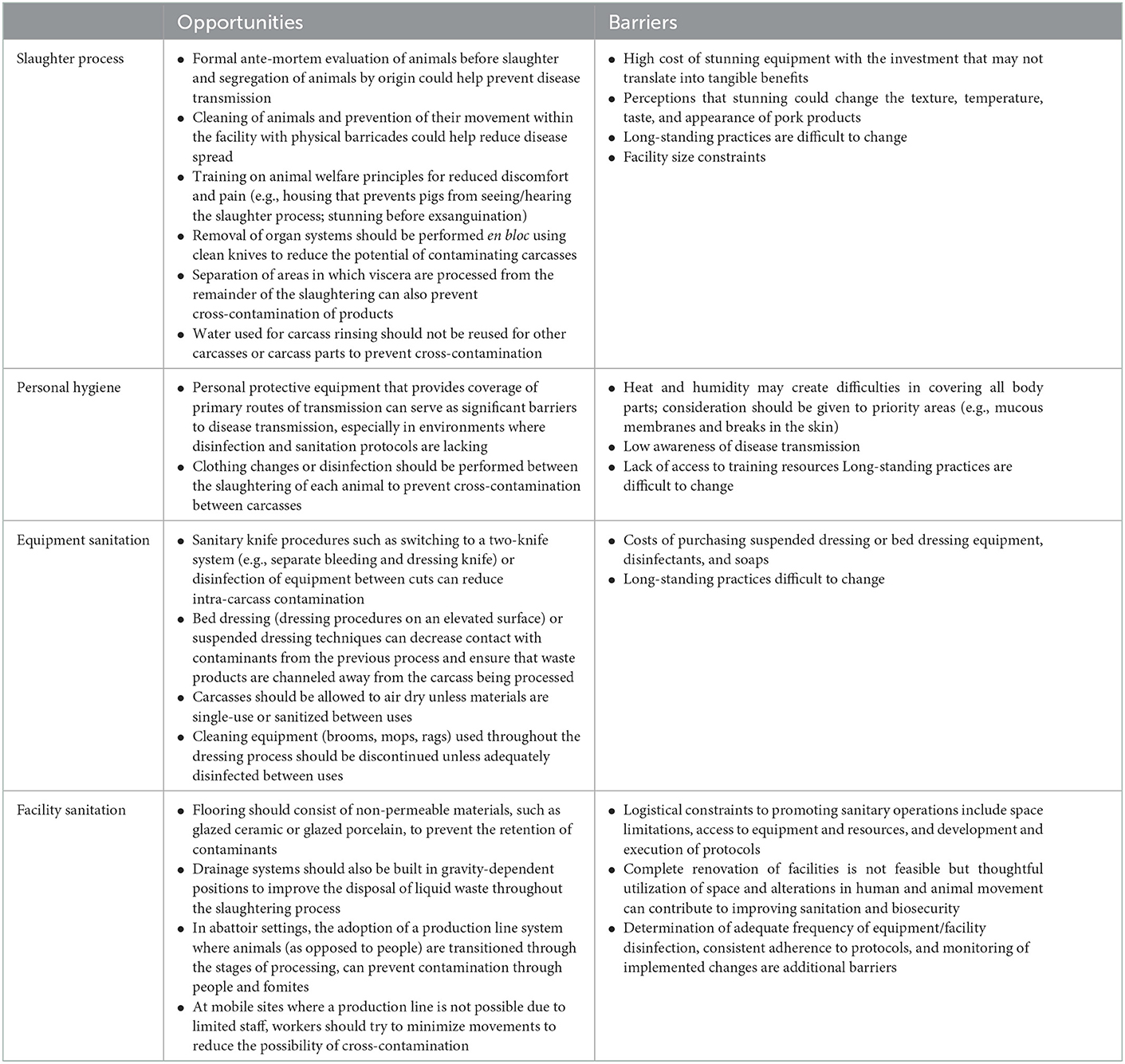
Table 1. Opportunities and barriers to improving food safety in the areas of pork processing, personal hygiene, equipment sanitation, and facility sanitation in small-scale settings.
Efforts to reduce disease transmission have previously focused on developing centralized systems for slaughtering (Lapar et al., 2012; Nguyen-Viet et al., 2017). However, focusing exclusively on centralized systems comes at a high cost to many rural families who depend on swine raising for a significant portion of their income along with consumers who prefer these pork products. Light-touch interventions that promote sanitary slaughter processes (e.g., ante-mortem evaluation of animals before slaughter) and personal hygiene (e.g., PPE providing coverage of primary routes of transmission) within smallholder slaughter settings will be important for supporting this industry and are generally seen as most feasible given the low cost and effort involved, followed by programs targeting equipment sanitation and facility sanitation. Such interventions must also consider principles of animal welfare including good housing and good health (Smith et al., 2021). Thus, continued investment in the education and training of employees will be crucial in working toward humane slaughter, a safe pork product, and a safe working environment. Additionally, considering some workers have existing food safety knowledge, training should be accompanied by improvements to the enabling environment (e.g., equipment, facilities) to facilitate knowledge application (Lam, 2022).
Importantly, there is a lack of regulation within small-scale slaughter settings. Nearly 10 years later after collecting data for this study in 2013, small-scale slaughtering practices remained largely the same, as noted by observations from the second phase of PigRISK (SafePORK: 2017–2022) (Hennessey et al., 2020; Lam et al., 2021). Strengthening regulations will be essential for supporting the sustainability of hygienic slaughtering practices. The creation of oversight committees for small-scale slaughterhouses is a promising way to ensure slaughter settings can maintain their businesses without risk to workers and the general population (Nguyen-Viet et al., 2017; Pham and Dinh, 2020). Committees should develop protocols for monitoring and enforcement with input from swine workers to ensure buy-in and practicality. It will also be essential to engage local authorities to shape these protocols and support monitoring and enforcement. Equally important to a supportive environment is the need to raise awareness of and demand for safer food among consumers (Le et al., 2022b). Applying the concept of One Health whereby multiple sectors and multiple disciplines collaborate could inform food safety strategies that also support animal and environmental health (Grace, 2017).
In addition to the specific entry points for food safety improvement presented in Table 1, we suggest several recommendations for different food safety actors. First, improvements need to be made to food safety practices to enhance worker safety and reduce the risk of meat contamination. Training programs for workers should raise awareness of risks which could lead to the adoption of better practices. Secondly, facility managers should be aware of guidelines for slaughterhouse and meat hygiene and encourage workers to adhere to such guidelines. Additionally, the lack of access to improved infrastructure is an important factor constraining the ability to substantially improve hygiene; promoting access to finance could support facility managers in improving equipment and facilities (Nguyen-Thi et al., 2021). Finally, local and national governments should consider implementing programs that raise awareness of and demand for food safety among consumers, which could drive good hygienic practices in not only food processing settings but also retail (Le et al., 2022a). Importantly, strategies that are holistic and attuned to the needs, priorities, and constraints of different food safety actors are key to the success of food safety programs.
We note a couple of limitations of our study. First, while our research focuses on observing behaviors as they were happening (rather than what participants say they do), our research did not explore motivations or rationales behind behaviors in a systematic way. Future studies should consider interviews alongside observations to enrichen the findings. Secondly, there may have been issues of observer bias, where participants adjusted behaviors when they know they are being observed. However, we expect this phenomenon to be minimal considering this research was part of a larger research program where existing relationships and trust have been established. Despite the study limitations, this observational study enhanced our understanding of small-scale pig processing and entry points for food safety improvements. Having the visits feel informal by keeping questions conversational was helpful in supporting a sense of comfort among participants. In addition to keeping conversations informal, we wrote memos quickly and expanded on them after the visits. Furthermore, we recorded photos and videos as participants were working which could then be analyzed later. These strategies helped to reduce the research burden on participants and could reduce barriers to long-term participation. Given the strengths of this approach, we suggest further integrating observational studies into One Health research to visualize and characterize behavioral risks to health.
5. Conclusion
While slaughterhouse pigs are an important reservoir for human infection from zoonotic diseases, the specific pig slaughtering practices contributing to disease transmission remain under-researched. This study identified and characterized elements of the pig slaughtering process that likely contribute to the spread of zoonotic pathogens—from infected pigs to uninfected pigs and humans—drawing on a case study of traditional pig slaughter settings in Vietnam. Areas potentially leading to zoonotic occupational and foodborne disease risks included slaughtering procedures, personal hygiene of workers, equipment sanitation, and facility sanitation. While small-scale slaughtering practices are long-standing and difficult to change, light-touch interventions that target safe handling practices show promise. Our study underscores the importance of hygiene training of workers—supported by an enabling environment—in helping to reduce the burden of zoonotic disease risks.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All slaughterhouse owners and/or workers verbally provided their informed consent to participate in this study and to be photo- and video-documented. This work received research ethics approval from the Institution Research Board of the Hanoi University of Public Health as part of the PigRISK project (IRB no. 148/2012/YTCC-HD3).
Author contributions
Conceptualization, data collection, analysis, and writing: NT. Conceptualization and revision: NN, JG, SD-X, and HN-V. Analysis and revision: SL. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Australian Centre for International Agricultural Research (ACIAR) and the International Livestock Research Institute as part of the PigRISK project. This work was partly supported by EcoZD project funded by IDRC and CGIAR initiative on One Health.
Acknowledgments
We thank Nguyen Tien Thanh and Nguyen Duy Tien from the Center for Public Health and Ecosystem Research (CENPHER), Hanoi University of Public Health for the field support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alban, L., and Sørensen, L. L. (2010). Hot-water decontamination is an effective way of reducing risk of Salmonella in pork. Fleischwirtschaft 90, 109–113.
Asan, O., and Montague, E. (2014). Using video-based observation research methods in primary care health encounters to evaluate complex interactions. Inform. Prim. Care 21, 161–170. doi: 10.14236/jhi.v21i4.72
Baudon, E., Chu, D. K. W., Tung, D. D., Thi Nga, P., Vu Mai Phuong, H., Le Khanh Hang, N., et al. (2018). Swine influenza viruses in Northern Vietnam in 2013–2014. Emerg. Microb. Infect. 7, 123. doi: 10.1038/s41426-018-0109-y
Bonifait, L., Veillette, M., Létourneau, V., Grenier, D., and Duchainea, C. (2014). Detection of Streptococcus suis in bioaerosols of swine confinement buildings. Appl. Environ. Microbiol. 81, 3296–3304. doi: 10.1128/AEM.04167-13
Braun, V., Clarke, V., Hayfield, N., and Terry, G. (2018). “Thematic analysis,” in Handbook of Research Methods in Health Social Sciences, ed P. Liamputtong (Singapore: Springer). doi: 10.1007/978-981-10-2779-6_103-1
Chau, L. T. M., Lebailly, P., and Trung, T. Q. (2017). Enhancing farmers market power and income in the pig value chain; a case study in Bac Giang province, Vietnam. Livest. Res. Rural Dev. 29, 1–2.
Cheung, P. Y., Lo, K. L., Cheung, T. T., Yeung, W. H., Leung, P. H., and Kam, K. M. (2008). Streptococcus suis in retail markets: how prevalent is it in raw pork? Int. J. Food Microbiol. 127, 316–320. doi: 10.1016/j.ijfoodmicro.2008.08.006
Cook, E. A. J., De Glanville, W. A., Thomas, L. F., Kariuki, S., Bronsvoort, B. M., de, C., et al. (2017). Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health 17, 4. doi: 10.1186/s12889-016-3923-y
Dang-Xuan, S., Nguyen-Viet, H., Meeyam, T., Fries, R., Nguyen-Thanh, H., Pham-Duc, P., et al. (2016). Food safety perceptions and practices among smallholder pork value chain actors in Hung Yen Province, Vietnam. J. Food Prot. 79, 1490–1497. doi: 10.4315/0362-028X.JFP-15-402
Dang-Xuan, S., Nguyen-Viet, H., Pham-Duc, P., Grace, D., Unger, F., Nguyen-Hai, N., et al. (2018). Simulating cross-contamination of cooked pork with salmonella enterica from raw pork through home kitchen preparation in Vietnam. Int. J. Environ. Res. Public Health. 15, 2324. doi: 10.3390/ijerph15102324
Dang-Xuan, S., Nguyen-Viet, H., Pham-Duc, P., Unger, F., Tran-Thi, N., Grace, D., et al. (2019). Risk factors associated with Salmonella spp. prevalence along smallholder pig value chains in Vietnam. Int. J. Food Microbiol. 290, 105–115. doi: 10.1016/j.ijfoodmicro.2018.09.030
Dang-Xuan, S., Nguyen-Viet, H., Unger, F., Pham-Duc, P., Grace, D., Tran-Thi, N., et al. (2017). Quantitative risk assessment of human salmonellosis in the smallholder pig value chains in urban of Vietnam. Int. J. Public Health 79, 1490–1497. doi: 10.1007/s00038-016-0921-x
Durmuşoglu, H., Incili, G. K., Demir, P., and Ilhak, O. I. (2020). Effects of workers hand washing and knife disinfection practices on microbiological quality of small animal carcasses in slaughterhouse environment. J. Food Process. Preserv. 44, e14918. doi: 10.1111/jfpp.14918
Harvey, S. A. (2018). Observe before you leap: why observation provides critical insights for formative research and intervention design that you ll never get from focus groups, interviews, or KAP surveys. Glob. Health Sci. Pract. 6, 299–316. doi: 10.9745/GHSP-D-17-00328
Heinz, G. (2008). Abattoir Development. Options and Designs for Hygienic Basic and Medium-Sized Abattoirs. Rome: Food and Agriculture Organisation.
Hennessey, M., Kim, S., Unger, F., Nguyen-Viet, H., Dang-Xuan, S., Nguyen-Thi, T., et al. (2020). Exploring the potential of using nudges to promote food hygiene in the pork value chain in Vietnam. Prev. Vet. Med. 181:105003. doi: 10.1016/j.prevetmed.2020.105003
Ho, D. T. N., Le Thi Phuong, T.u, Wolbers, M., Cao, Q. T., Nguyen, V. M. H., Tran, V. T. N., Le Thi Phuong, T., hao Nguyen, H. P., et al. (2011). Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS ONE 6, e17604. doi: 10.1371/journal.pone.0017604
Hoa, N. T., Chieu, T. T. B., Nga, T. T. T., Dung, N., Van, C., ampbell, J., Anh, P. H., et al. (2011). Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in Southern Vietnam. PLoS ONE 6, e17943. doi: 10.1371/journal.pone.0017943
Hung Yen Portal (2021). Population and Labor. Available online at: https://en.hungyen.gov.vn/portal/Pages/2014-04/Population20and20Labor-ba6838feb700d749.aspx (accessed December 14, 2022).
Lam, S. (2022). Enhancing Evaluations of Complex Food Security Programs Operating Under Climate Change. Disseration. University of Guelph, Guelph.
Lam, S., Barot, M., Nguyen-Viet, H., and Unger, F. (2016). Changes in Researcher Capacity in Assessing Food Safety Risks and Value Chains: Insights From PigRisk Team. Hanoi: ILRI.
Lam, S., Nguyen, H. T. T., Tuan, H. N. H., Nguyen, L. T., Nguyen-Viet, H., Toribio, J. A., et al. (2021). Unpacking the theory behind one health food safety programs: a Vietnam case study. Front. Vet. Sci. 8, 763410. doi: 10.3389/fvets.2021.763410
Lapar, M. L. A., Toan, N. N., Staal, S., Minot, N., Tisdell, C., Que, N. N., et al. (2012). “Smallholder competitiveness : insights from household pig production systems in Vietnam,” in International Association of Agricultural Economists (IAAE) Triennial Conference (Foz de Iguaçu, Brazil).
Le, T. T.-H., Langley, S. J., Dunham, J. G., Dang-Xuan, S., and Unger, F. (2022a). Food safety knowledge, needed and trusted information of pork consumers in different retail types in Northern Vietnam. Front. Sustain. Food Syst. 6, 1063927. doi: 10.3389/fsufs.2022.1063927
Le, T. T.-H., Vu-Thi, N., Dang-Xuan, S., Nguyen-Viet, H., Pham-Duc, P., Nguyen-Thanh, L., et al. (2022b). Seroprevalence and associated risk factors of Trichinellosis and T. Solium Cysticercosis in indigenous pigs in Hoa Binh Province, Vietnam. Trop. Med. Infect. Dis. 7, 57. doi: 10.3390/tropicalmed7040057
Lun, Z. R., Wang, Q. P., Chen, X. G., Li, A. X., and Zhu, X. Q. (2007). Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect. Dis. 7, 201–209. doi: 10.1016/S1473-3099(07)70001-4
Mann, I., Koulikovskii, A., and Matyas, Z. (1983). Guidelines on Small Slaughterhouses and Meat Hygiene for Developing Countries. Geneva: WHO.
Marois, C., Cariolet, R., Morvan, H., and Kobisch, M. (2008). Transmission of pathogenic respiratory bacteria to specific pathogen free pigs at slaughter. Vet. Microbiol. 129:325–332. doi: 10.1016/j.vetmic.2007.11.030
Nguyen Thi Thuy, M., Dorny, P., Lebailly, P., Le Thi Minh, C., Nguyen Thi Thu, H., and Dermauw, V. (2020). Mapping the pork value chain in Vietnam: a systematic review. Trop. Anim. Health Prod. 52, 2799–2808. doi: 10.1007/s11250-020-02338-y
Nguyen, N. T. T., Luu, Y. T. H., Hoang, T. D., Nguyen, H. X., Dao, T. D., Bui, V. N., et al. (2021). An epidemiological study of Streptococcus suis prevalence among swine at industrial swine farms in Northern Vietnam. One Health 13, 100254. doi: 10.1016/j.onehlt.2021.100254
Nguyen-Thi, T. T., Pham-Thi-Ngoc, L., Nguyen-Ngoc, Q., Dang-Xuan, S., Lee, H. S., Nguyen-Viet, H., et al. (2021). An assessment of the economic impacts of the 2019 African swine fever outbreaks in Vietnam. Front. Vet. Sci. 8, 686038. doi: 10.3389/fvets.2021.686038
Nguyen-Viet, H., Dang-Xuan, S., Pham-Duc, P., Roesel, K., Huong, N. M., Luu-Quoc, T., et al. (2019). Rapid integrated assessment of food safety and nutrition related to pork consumption of regular consumers and mothers with young children in Vietnam. Global Food Secur. 20, 37–44. doi: 10.1016/j.gfs.2018.12.003
Nguyen-Viet, H., Lam, S., Nguyen-Mai, H., Trang, D. T., Phuong, V. T., Tuan, N. D. A., et al. (2022). Decades of emerging infectious disease, food safety, and antimicrobial resistance response in Vietnam: the role of One Health. One Health 14:100361. doi: 10.1016/j.onehlt.2021.100361
Nguyen-Viet, H., Tuyet-Hanh, T. T., Unger, F., Dang-Xuan, S., and Grace, D. (2017). Food safety in Vietnam: where we are at and what we can learn from international experiences. Infect. Dis. Poverty 6, 39. doi: 10.1186/s40249-017-0249-7
Pham, H. V., and Dinh, T. L. (2020). The Vietnams food control system: achievements and remaining issues. Food Control 108, 106862. doi: 10.1016/j.foodcont.2019.106862
Rayanakorn, A., Goh, B. H., Lee, L. H., Khan, T. M., and Saokaew, S. (2018). Risk factors for Streptococcus suis infection: A systematic review and meta-analysis. Sci. Rep. 8, 13358. doi: 10.1038/s41598-018-31598-w
Rayanakorn, A., Katip, W., Goh, B. H., Oberdorfer, P., and Lee, L. H. (2019). Clinical manifestations and risk factors of Streptococcus suis mortality among Northern Thai population: Retrospective 13-year cohort study. Infect. Drug Resist. 12, 3955–3965. doi: 10.2147/IDR.S233326
Smith, A., Bidesi, S., Wijerathna, Y., Doyle, R., Shonara, L., Jordan, D., et al. (2021). Animal Welfare Along the Smallholder Pig Value Chain in Vietnam: Current Status, Legal Perspectives and Way Forward. Nairobi: ILRI.
Takemae, N., Harada, M., Nguyen, P. T., Nguyen, T., Nguyen, T. N., To, T. L., et al. (2017). Influenza A Viruses of Swine (IAV-S) in Vietnam from 2010 to 2015: multiple Introductions of A(H1N1)pdm09 viruses into the pig population and diversifying genetic constellations of enzootic IAV-S. J. Virol. 91, e01490–e01416. doi: 10.1128/JVI.01490-16
Takemae, N., Shobugawa, Y., Nguyen, P. T., Nguyen, T., Nguyen, T. N., To, T. L., et al. (2016). Effect of herd size on subclinical infection of swine in Vietnam with influenza A viruses. BMC Vet. Res. 12, 227. doi: 10.1186/s12917-016-0844-z
Tang, Q., Huang, K., Liu, J., Jin, X., and Li, C. (2021). Distribution characteristics of bioaerosols inside pig houses and the respiratory tract of pigs. Ecotoxicol. Environ. Saf. 212, 112006. doi: 10.1016/j.ecoenv.2021.112006
Tu, N. T. K., Tue, N. T., Vapalahti, O., Virtala, A. M. K., Van Tan, L., Rabaa, M. A., et al. (2019). Occupational animal contact in Southern and Central Vietnam. Ecohealth 16, 759–771. doi: 10.1007/s10393-019-01444-0
Van De, N., Nga, V. T., Dorny, P., Trung, N. V., Minh, P. N., Dung, D. T., et al. (2015). Trichinellosis in Vietnam. Am. J. Trop. Med. Hyg. 92, 1265–1270. doi: 10.4269/ajtmh.14-0570
Wei, L., Yang, C., Shao, W., Sun, T., Wang, J., Zhou, Z., et al. (2020). Prevalence and drug resistance of Salmonella in dogs and cats in Xuzhou, China. J. Vet. Res. 64, 263–268. doi: 10.2478/jvetres-2020-0032
Wertheim, H. F. L., Nghia, H. D. T., Taylor, W., and Schultsz, C. (2009). Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48, 617–625. doi: 10.1086/596763
Yokozawa, T., Dang-Xuan, S., Nguyen-Viet, H., Lapar, L., and Makita, K. (2016). Transition of salmonella prevalence in pork value chain from pig slaughterhouses to markets in Hung Yen, Vietnam. J. Vet. Epidemiol. 20, 51–58. doi: 10.2743/jve.20.51
Keywords: food safety, pigs, Vietnam, slaughterhouse, zoonotic disease, One Health
Citation: Ting NI, Dang-Xuan S, Gilbert J, Nguyen NTT, Lam S and Nguyen-Viet H (2023) A glance into traditional pig slaughtering practices in Vietnam and opportunities for zoonotic disease prevention. Front. Sustain. Food Syst. 7:1101282. doi: 10.3389/fsufs.2023.1101282
Received: 17 November 2022; Accepted: 09 January 2023;
Published: 21 February 2023.
Edited by:
Jens Andre Hammerl, Bundesinstitut für Risikobewertung, GermanyReviewed by:
Tamsin Sarah Barnes, The University of Queensland, AustraliaShawn Ting, Charles Darwin University, Australia
Copyright © 2023 Ting, Dang-Xuan, Gilbert, Nguyen, Lam and Nguyen-Viet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hung Nguyen-Viet,  aC5uZ3V5ZW5AY2dpYXIub3Jn
aC5uZ3V5ZW5AY2dpYXIub3Jn
 Nancy I. Ting1
Nancy I. Ting1 Sinh Dang-Xuan
Sinh Dang-Xuan Jeffrey Gilbert
Jeffrey Gilbert Nguyen Thao Thi Nguyen
Nguyen Thao Thi Nguyen Steven Lam
Steven Lam Hung Nguyen-Viet
Hung Nguyen-Viet