
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 17 April 2023
Sec. Agro-Food Safety
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1085809
This article is part of the Research Topic Food Safety in Low and Middle Income Countries View all 25 articles
 Ruhena Begum1
Ruhena Begum1 Rahima Akter1
Rahima Akter1 Sinh Dang-Xuan2
Sinh Dang-Xuan2 Shariful Islam1
Shariful Islam1 Nure Alam Siddiky1
Nure Alam Siddiky1 ASM Ashab Uddin1
ASM Ashab Uddin1 Asheak Mahmud1
Asheak Mahmud1 Md Samun Sarker1
Md Samun Sarker1 Delia Grace3,4
Delia Grace3,4 Mohammed Abdus Samad1
Mohammed Abdus Samad1 Johanna F. Lindahl2,5,6*
Johanna F. Lindahl2,5,6*Introduction: Contamination with heavy and toxic metals along the food value chain is a public health concern in Bangladesh.
Methods: In this study, 608 fish and chicken samples from traditional and modern retail outlets in urban, peri-urban, and rural areas were collected and analyzed for chromium (Cr), cadmium (Cd), and lead (Pb) contamination, using atomic absorption spectrometry method. The daily intake, target hazard quotient and the target carcinogenic risk (for lead only) as a result of fish and chicken consumption was calculated based on mean results, and by Monte Carlo simulation in @Risk with 100,000 iterations (quantitative risk assessment).
Results: Cr and Cd were detected in 80–86% of both chicken meat and fish samples, while Pb positivity found in chicken meat and fish was 54.9 and 23.3%, respectively. The mean concentration (±SD) of Cr, Cd, and Pb in chicken meat were 0.66 ± 0.93, 0.02 ± 0.03, and 0.09 ± 0.10 mg/kg, respectively; and in fish were 0.49 ± 0.62, 0.02 ± 0.03, and 0.06 ± 0.09 mg/kg, respectively. The estimated daily intakes of Cr, Cd, and Pb from chicken and fish were lower than the maximum tolerable daily intake in all studied areas. In addition, the target carcinogenic risk for Pb in chicken was lower than the negligible range, which indicated the risk of cancer due to exposure to Pb through chicken meat and fish consumption was very low.
Discussion: The present study concludes that consumption of chicken meat and fish in Bangladesh, currently at very low levels, is unlikely to constitute a major health risk for humans in respect to these metals. However, continuous market surveillance for heavy metals in food stuff is recommended, especially since consumers may increase their meat intake.
Heavy and toxic metals/metalloids are ubiquitous in the environment with natural and anthropogenic sources, including agriculture, industry, mining, land fill and transportation (Cui et al., 2005; Zheng et al., 2007; Islam et al., 2014). Heavy metals enter plant or animal-source foods through contaminated soil or water, and can accumulate along the food chain, in animals, and humans (Kachenko and Singh, 2006; Kumar Sharma et al., 2007). Environmental pollution with heavy metals is a serious threat because of their toxicity, bioaccumulation, and biomagnification in the food chain (Demirezen and Uruç, 2006). Although contamination of animal feed and water with toxic metals cannot be entirely avoided, there is a clear need for such contamination to be minimized, in order to reduce effects on animal and human health. Under the food value chain aspect, different production systems or raising locations may affect heavy metal contamination level due to different environment (water and air) and feed sources. Food at markets also may come from different areas, and therefore the final consumers may be exposed to higher levels depending on the origin of the products.
Heavy metal intoxication causes a range of adverse health effects. Some micronutrients [e.g., copper (Cu), chromium (Cr), and nickel (Ni)] are toxic at high concentration (McLaughlin et al., 1999; Rahman et al., 2014) although small quantities are essential for plant growth and human nutrition (Sankar et al., 2006; Kumar Sharma et al., 2007; Bundschuh et al., 2012; Ji et al., 2013; Rahman et al., 2014). Trace metals such as Cr, Ni, As, Cd, and Pb have been considered as the most toxic elements in the environment by the US Environmental Protection Agency (EPA) (Lei et al., 2009; Islam et al., 2014), and dietary intake is an important exposure pathway for these. For example, lead (Pb) hinders the cognitive development and intellectual performance of children, causes high blood pressure and cardiovascular disease in adults (Luckey and Venugopal, 1979; Flora et al., 2006), and is also associated with cancer (Steenland and Boffetta, 2000). Similarly, cadmium (Cd) intoxication leads to cancer, but also to impaired kidney function, poor reproductive capacity, hypertension, and hepatic dysfunction (Luckey and Venugopal, 1979; Wilbur et al., 2012). Cd, classed as a group 1 carcinogen, was estimated to cause over 2,000 deaths globally in 2015 (Gibb et al., 2019). Chromium (Cr) exposure may result in severe respiratory, cardiovascular, gastrointestinal, hematological, hepatic, renal, and neurological effects, which may ultimately lead to death (Wilbur et al., 2012). It is found in two forms, trivalent and hexavalent. The latter has been classed as a group 1 carcinogen (carcinogenic to humans) (International Agency for Research on Cancer, 2012), and causes respiratory tract cancers, often due to occupational exposure.
Animal-source foods are important sources of protein, and major components of many diets (Alonso et al., 2019), particularly in low- and middle-income countries (LMICs). Consumption of poultry and fish is rising rapidly in LMICs as the result of increasing incomes, increasing population, and urbanization (Rae, 1998). Poultry can acquire heavy metals from different sources, including feed containing tannery waste, and heavy metal residues may accumulate in body tissue as well as in eggs (Nisianakis et al., 2009), while heavy metals easily sediment in the aquatic environment from and bioaccumulate in fish and other aquatic animals (Islam et al., 2015a; Ullah et al., 2017). The extensive tannery industry in Bangladesh is a risk factor for Cr toxicity. The human health burden of disease caused by Cd and Pb has been assessed globally by the World Health Organization, which estimated that the median disability adjusted life years (DALYs) lost in South-East Asian region D, where Bangladesh belongs, was 0.01 and 52 per 100,000 inhabitants, for Cd and Pb, respectively (Gibb et al., 2019). However, the evidence and available data related to human health risk due to consumption of different food items in Bangladesh is limited. The concentration of heavy metals in fish and chicken meat has been studied before in Bangladesh across urban and peri-urban areas, indicating varying levels of contamination, some in excess of the tolerable limit (Pintaeva et al., 2011; Hasan et al., 2013; Ahmed et al., 2015; Ullah et al., 2017), but not put in context of a risk assessment.
The present study was conducted as part of a broader project which aimed to identify priority food safety hazards in animal-source foods in Bangladesh. It aimed to determine concentrations of Pb, Cd, and Cr in fish and chicken meat sold in different markets in urban, peri-urban, and rural areas in order to produce a risk assessment for the Bangladeshi population.
A cross-sectional study was conducted between November 2018 and June 2019. In total, 71 traditional markets and 41 modern markets were selected from urban (Dhaka), peri-urban (Savar) and rural (Netrokona) areas (Figure 1), purposively selected to represent areas of different degree of urbanization. In Bangladesh, traditional markets often sell different type food commodities (animal source food, fish, vegetable, dried food). Traditional markets have permanent locations close to the residential area, however, they often lack infrastructure to store food properly, and lack food quality control as well as food traceability. Modern markets are represented by convenience stores and supermarkets which are mainly available in the urban area, and have better infrastructure to keep and display food to sell. In each area, traditional markets were selected using predefined criteria, including markets that sold both chicken and fish. In addition, 41 modern markets were selected in urban area only.
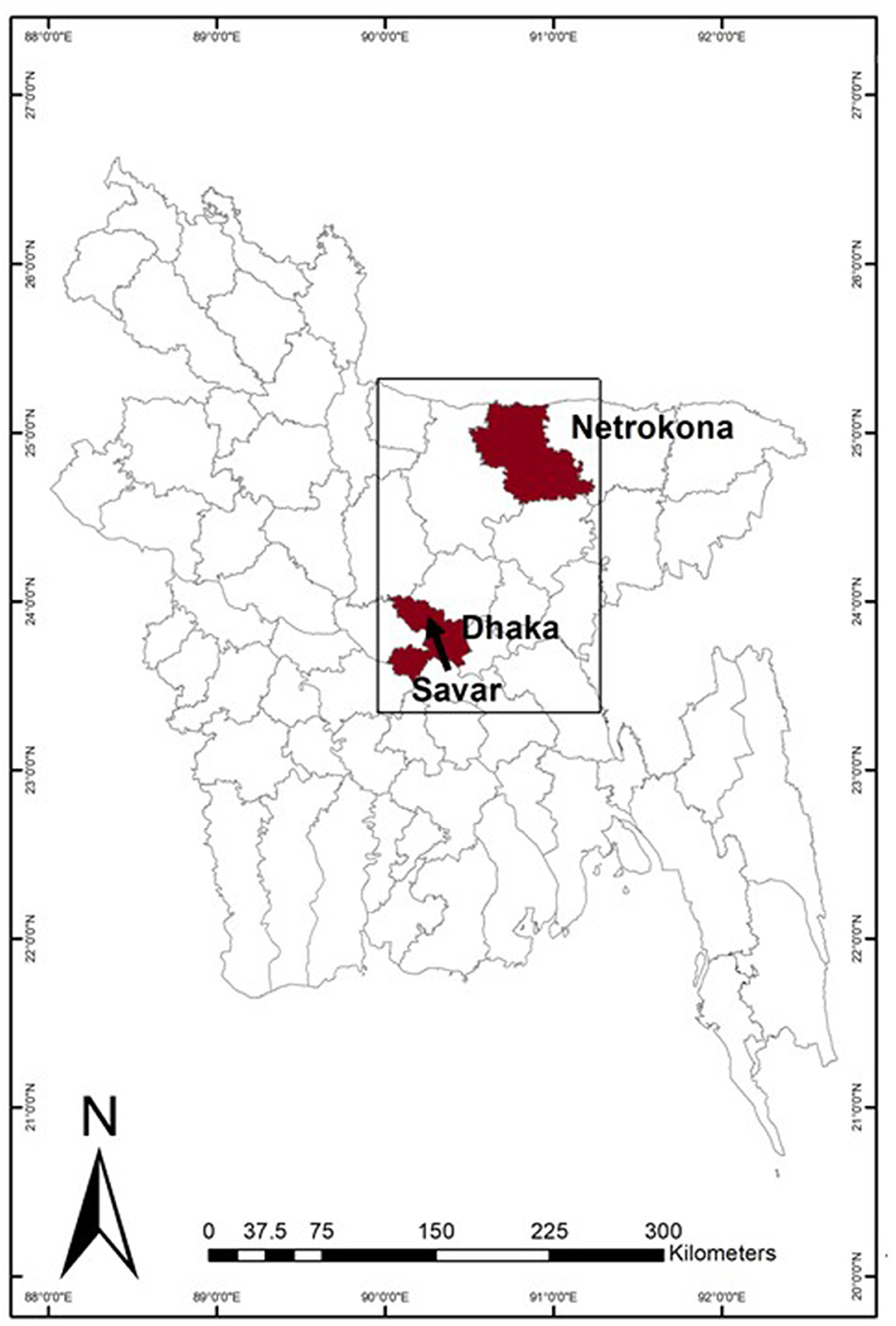
Figure 1. The study sites in Bangladesh, showing Dhaka and Netrokona district in dark, and an arrow indicating the location of Savar within the Dhaka district.
The selection of which food items to prioritize for the study was decided in collaboration with national stakeholders, including the Bangladesh Food Safety Authority (BFSA). In each selected traditional market, three to four chicken and two to three fish vendors were randomly selected, and chicken meat and fish were purchased. Each selected vendor was asked for one sample of either pangasius fish or chicken meat. Modern food retail outlets located in the same area as the traditional markets were also included, and in each modern food outlet, two chicken meat and fish (pangasius) samples were also collected. We hypothesized heavy metals would be lower in rural areas, as we considered industrial pollution to be the main source in Bangladesh and is lower in rural areas. The study aimed to collect 350 samples per site, and was designed to detect a difference of 10% between urban and rural contamination, 95% confidence and 0.8 power, with no clustering assumed. In total, 608 samples (359 chicken meat and 249 fish) were collected. All collected samples were kept in cool box (0–4°C) after sampling and transported to the laboratory for analysis within 24 h.
For each shop where a sample was taken, one to two consumers were interviewed and asked how much of this product they would usually consume in the household, and how many members in the household consumed it. A total of 675 consumers in rural, peri-urban and urban areas were interviewed. An average meat amount (gram) consumed per person per day was calculated by dividing the total amount of fish or chicken/day/household by the number of household members.
Ethical approval was given by International Livestock Research Institute (ILRI) Institutional Research Ethics Committee (IREC) with approval number 2018-27.
The selection of the prioritized heavy metals Pb, Cr, and Cd was decided in collaboration with national stakeholders, including BFSA, based on the perceived priorities and concerns of the country. Approximately 5–10 g of meat was cut from four to six (depending on size of the specimen) different parts of the whole fish or chicken carcass sample (avoiding intestinal content or giblet), cut into small pieces and mixed properly. One gram of this homogenized sample was accurately weighted in a crucible, then an oven (Nüve, Turkey) drying step was done at 120°C for 1 h, until constant weight was obtained. The dried samples were placed in a muffle furnace (Witeg, Baden-Württemberg, Germany) at 450°C for 10 h to generate ash. The ash was transferred into the digestion vessel, a conical flask, by adding 5 ml of 5 M HCl (Merck, Darmstadt, Germany) and 10 ml of 0.01 M HNO3 (Merck, Darmstadt, Germany) per sample (Idera et al., 2015). Then the vessel was heated to 120°C until the fume disappeared. After digestion step, a semi solid residue remains in the bottom of the tube that was diluted with deionized water to a final volume of 100 ml.
Atomic absorption spectrometer (AAS-7000, Shimadzu, Japan) was used for the determination of Cr, Cd, and Pb. Hollow cathode lamps used for Pb, Cd, and Cr were 283.3 nm and slit 0.5 nm; 228.8 nm and slit 0.5 nm; and 357.9 nm and slit 0.2 nm, respectively. Analysis was carried out according to the conditions recommended by the manufacturer. Atomic signals were measured for Pb, Cd, and Cr in peak area. The AAS system was standardized and validated using the limit of detection (LOD), limit of quantification (LOQ), linearity test, precision, accuracy and recovery. Calibration standards for each heavy metal were prepared each day from the certified stock solution of 1,000 mg/kg (manufactured under ISO 9001 quality assurance system). At least five different concentrations of each metal were spiked into blank matrix before digestion. At least 10 individual replicates were prepared for each concentration. The concentration range was considered to give linear analytical response when the regression coefficient, R2 > 0.995. All solutions were prepared in double-distilled water. All containers and glassware were cleaned by soaking in 0.1% HNO3 (Merck, Darmstadt, Germany) for at least 24 h and rinsed three times with deionized water before using.
Survey and laboratory data were recorded in Microsoft Excel 2007 (Microsoft Corporation, WA, USA) and imported into STATA 13 and 14.2 (StataCorp, College Station, TX 77845) for analysis. Means, standard deviations of heavy metal concentration, and percentage of samples contaminated with heavy metals calculated. Chi-square or Fisher exact tests (where appropriate) was used to compare proportions of heavy metals contamination by heavy metal types and study areas. One-way analysis of variance (ANOVA) with Bonferroni correction was applied to assess differences in values by heavy metal types and study areas. The correlation between the heavy metal in chicken and fish meat was assessed using Pearson correlation test. A p-value <0.05 was considered statistically significant. To account for variability in amount of meat consumed, heavy metal concentration and body weight, a stochastic risk assessment model was conducted using Monte Carlo simulation in @Risk 7.6 (Palisade, USA) with 100,000 iterations to estimate the distributions of the health risks.
The estimated daily intake (EDI) of metals due to consumption of contaminated food on fresh weight basis (FW) was calculated using the following formula:
Where, FIR (food ingestion rate, kg/day) was the average amount of meat (fish or chicken) (kg) eaten per day by fresh weight; C was the concentration of heavy metal in food (mg/kg FW); BW was average body weight, assumed to be 60 kg for an average adult. For the point estimate, average FIR used in the point estimate for adult consumers was 22.5 and 15.1 for meat and 67.9 and 60.6 g for fish on FW basis from urban/peri-urban and rural area respectively (HIES, 2019).
The oral reference dose (RfD) was set at 1.5 (EPA, 2000), 0.001 (EPA, 1989) and 0.0125 (FDA, 2019; Flannery et al., 2020) mg/kg/day for Cr, Cd, and Pb, respectively, following the United States Environmental Protection Agency (EPA). The EPA does not have an oral reference dose for lead so the United States Food and Drug Administration (FDA) interim reference level for lead for adults was used instead. The non-carcinogenic risk for each heavy metal through fish or meat consumption were assessed using the target hazard quotient (THQ) (EPA, 2022), the ratio of a single substance exposure level to the reference dose (RfD) for that substance. The equation used for estimating THQ is as followed:
Where, THQ is the target hazard quotient, EDI is the estimated daily intake (mg/kg/day), and RfD is an oral reference dose (mg/kg/day). Target carcinogenic risk factor (TR) was calculated by following formula:
Where, CSFo is the oral carcinogenic slope factor, which is equal to 8.5 × 10−3 (mg/kg/day) for lead phosphate (OEHHA, 2009).
A stochastic risk assessment model was built using @Risk add-in in MS Excel. The model used the same equations as in the point estimation of daily intake and non-carcinogenic risk (Section 2.5). Meat consumption, heavy metal concentration and body weight were parameterized to describe their variability by distributions. Different input parameters of relevant variables were generated from the data of consumer survey (meat consumption) and laboratory analysis (heavy metal concentration). To define body weight value of the adult, normal distribution was used, with mean of 60 kg and standard deviation of 10 kg. The RfD for Cr, Cd, and Pb, and oral carcinogenic slope factor for Pb were set as fixed values as the same as in Section 2.5. Variables, distributions, parameters and values used in the model were described in Table 1.
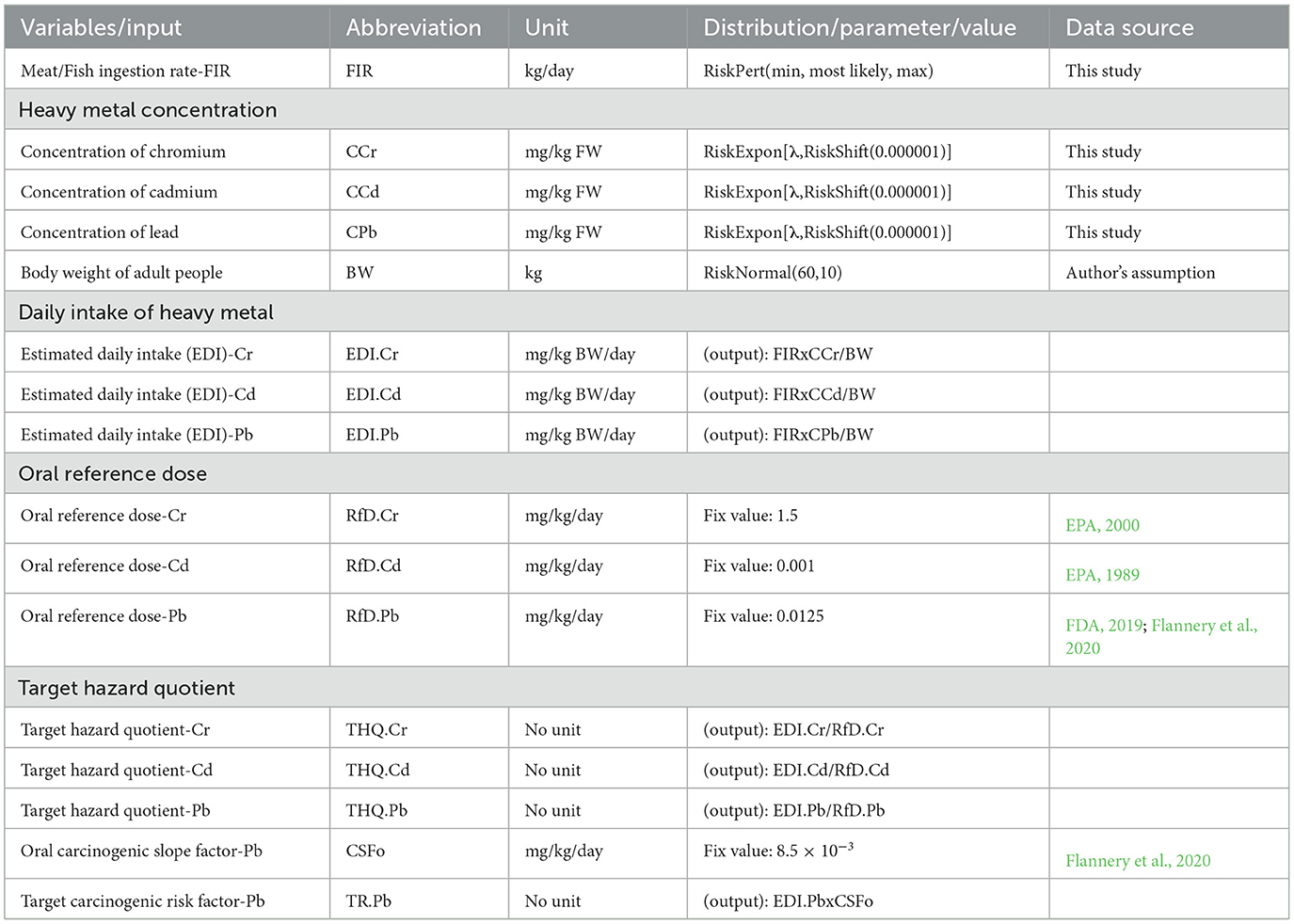
Table 1. Variables, distributions, parameters and source of data used in the stochastic risk assessment model.
In brief, meat consumption data was fitted following Pert distribution using minimum, most likely, and maximum which were derived from the dataset of a consumer survey. Concentration of Pb, Cd, and Cr data were obtained in Section 2.3 above. Samples which had concentration below detectable level (BDL) were assumed to be equal to 0.0001 mg/kg. Sample groups were categorized by areas, heavy metal and meat types, and fitted with Exponential distribution using fitting distribution function in @RISK. “RiskExpon(λ,RiskShift(0.000001))” function was used to compute concentration of heavy metal as positive values. For each sample group, lambda (λ) values were obtained from fitting distribution in @RISK accordingly. The file containing data and simulations are provided as Supplementary material 1.
Out of 359 chicken meat samples, 80.2%, 85.5%, and 55% were contaminated with Cr, Cd, and Pb, respectively; while out of 249 fish samples, 86%, 85%, and 23% were contaminated with Cr, Cd, and Pb, respectively. Least detected metal was Pb with 55% in chicken and 23% in fish, while the most detected metal was Cd in chicken, 86%, and Cr in fish, 86% (Table 2). Prevalence of heavy metal contamination in both chicken and fish samples was significantly lower in rural area compared to that in urban area (p <0.01, Table 2).
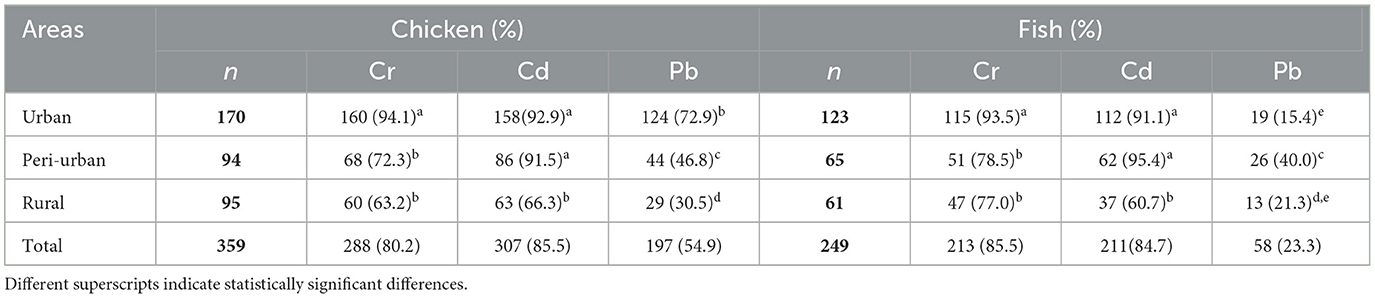
Table 2. Number of chicken and fish samples collected and tested positive for heavy metals contamination in different areas of Bangladesh.
Average concentrations of heavy metals (mean ± SD) in chicken meat and fish are presented in Table 3. Highest mean concentration was found in chicken meat sold in different areas: 0.78 ± 0.077 mg/kg, 0.02 ± 0.04 mg/kg (urban) and 0.05 ± 0.11 mg/kg (rural) for Cr, Cd, and Pb, respectively, whereas the lowest mean concentration was found in the peri-urban markets. Regarding fish samples, the highest mean concentration of Cr, Cd, and Pb was 0.68 ± 0.67 mg/kg, 0.02 ± 0.03 mg/kg (urban) and 0.02 ± 0.08 mg/kg (rural), respectively. There were significant differences (p < 0.05) in heavy metals concentration of chicken and fish samples between study sites.
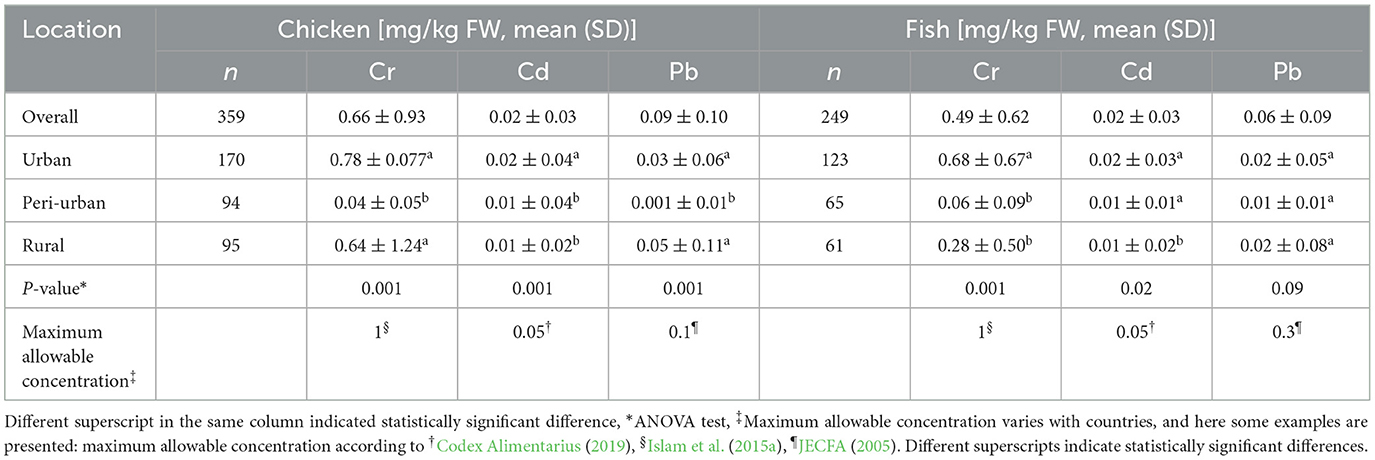
Table 3. Metal concentration [mg/kg fresh weight (FW)] in chicken meats and fish of three study sites in Bangladesh.
Correlation among the tested heavy metals in the meats tested with Pearson's correlation depicted in Table 4. Only Cr and Cd showed a clear pattern of positive correlation found in both chicken meat and fish (p = 0.003 for chicken and p < 0.0001 for fish).
The consumption rates and estimated daily intakes (EDIs) of heavy metals in adult inhabitants from eating chicken and fish were listed in Table 5. Total daily intakes of Cr, Cd, and Pb by eating chicken meat were 0.00029, 0.000008, and 0.000011 mg/kg/day BW in urban area, while in rural area intakes were 0.00016, 0.000003, and 0.000013 mg/kg/day BW, respectively. The total daily intakes of Cr, Cd, and Pb by eating fish were 0.00077, 0.000023, and 0.000023 mg/kg/day BW in urban area, and 0.000283, 0.00001, and 0.00002 mg/kg/day BW in rural area, respectively. A higher contribution of dietary intake of metals came from fish, due to these being the most highly consumed protein-based food in urban and rural area (67.9 and 60.6 g/person/day, respectively) (HIES, 2019). Metal specific point estimates for the EDIs revealed that EDI of Cr, Cd and Pb from consumption of all examined foodstuffs were lower than the maximum tolerable daily intake (MTDI, Table 5). The stochastic model which allowed for slightly higher consumption had higher estimates, but still well below the MTDI.
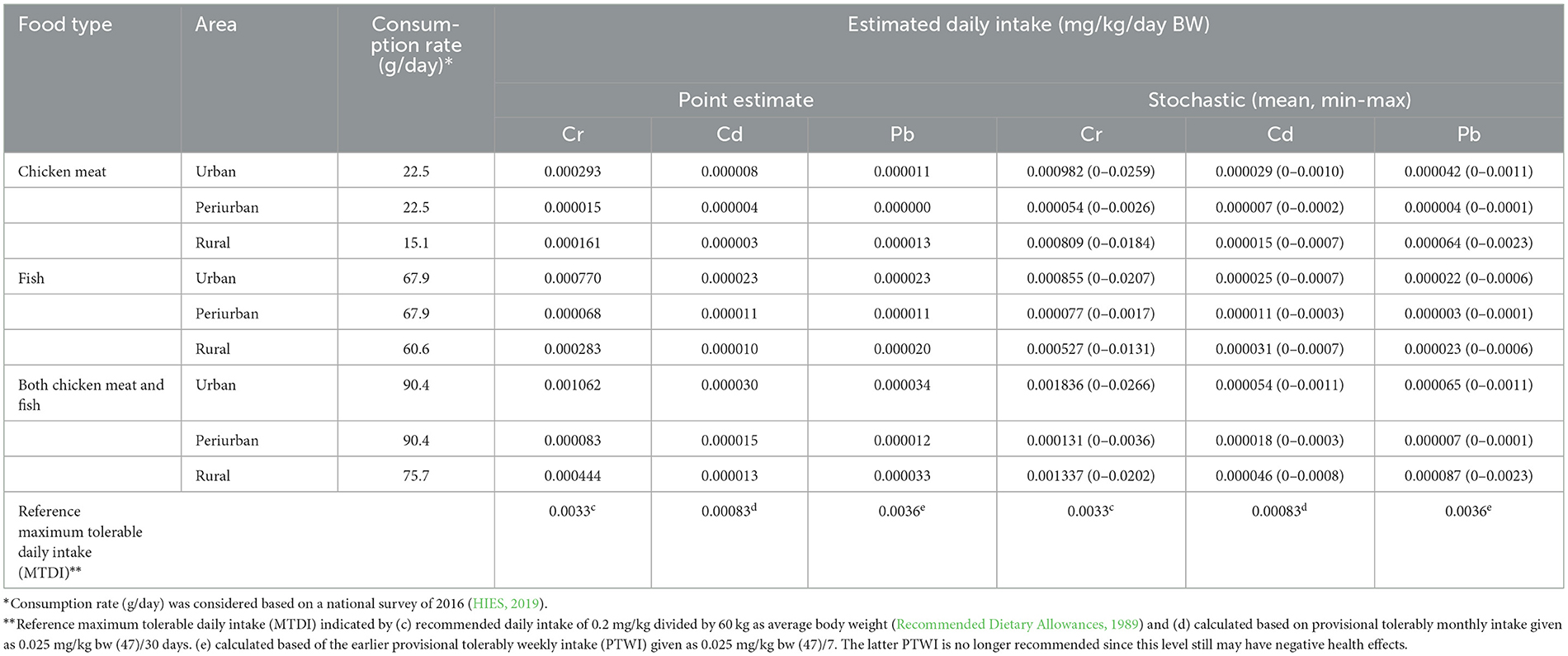
Table 5. Food consumption rate and estimated daily intake of heavy metals from eating chicken meat and fish.
THQs of individual heavy metal through chicken meat and fish consumption by average Bangladeshi adults are presented in Table 6. Average heavy metal concentration in chicken and fish (pangasius) was used to calculate THQ for the people of Bangladesh. The THQ value for the targeted heavy metals followed the order Cd > Pb > Cr in chicken and fish. Table 6 indicated that the THQ value for all three metal Cd, Cr and Pb were <1 in all study areas. The maximum THQ was the highest for Cd in chicken meat and fish across urban, peri-urban and rural areas. The average target carcinogenic risk of Pb due to exposure from the consumption of targeted chicken meat and fish samples were 9.6 × 10−8, 1.9 × 10−7 (urban area) and 1.0 × 10−7, 1.7 × 10−7 (rural area), respectively. Regarding consumption of chicken meat, the stochastic model showed 3.7 to 10 times higher average target carcinogenic risk of Pb. Whereas average target carcinogenic risk of Pb due to consumption of fish calculated between point estimate and the stochastic model was almost similar (Table 6).
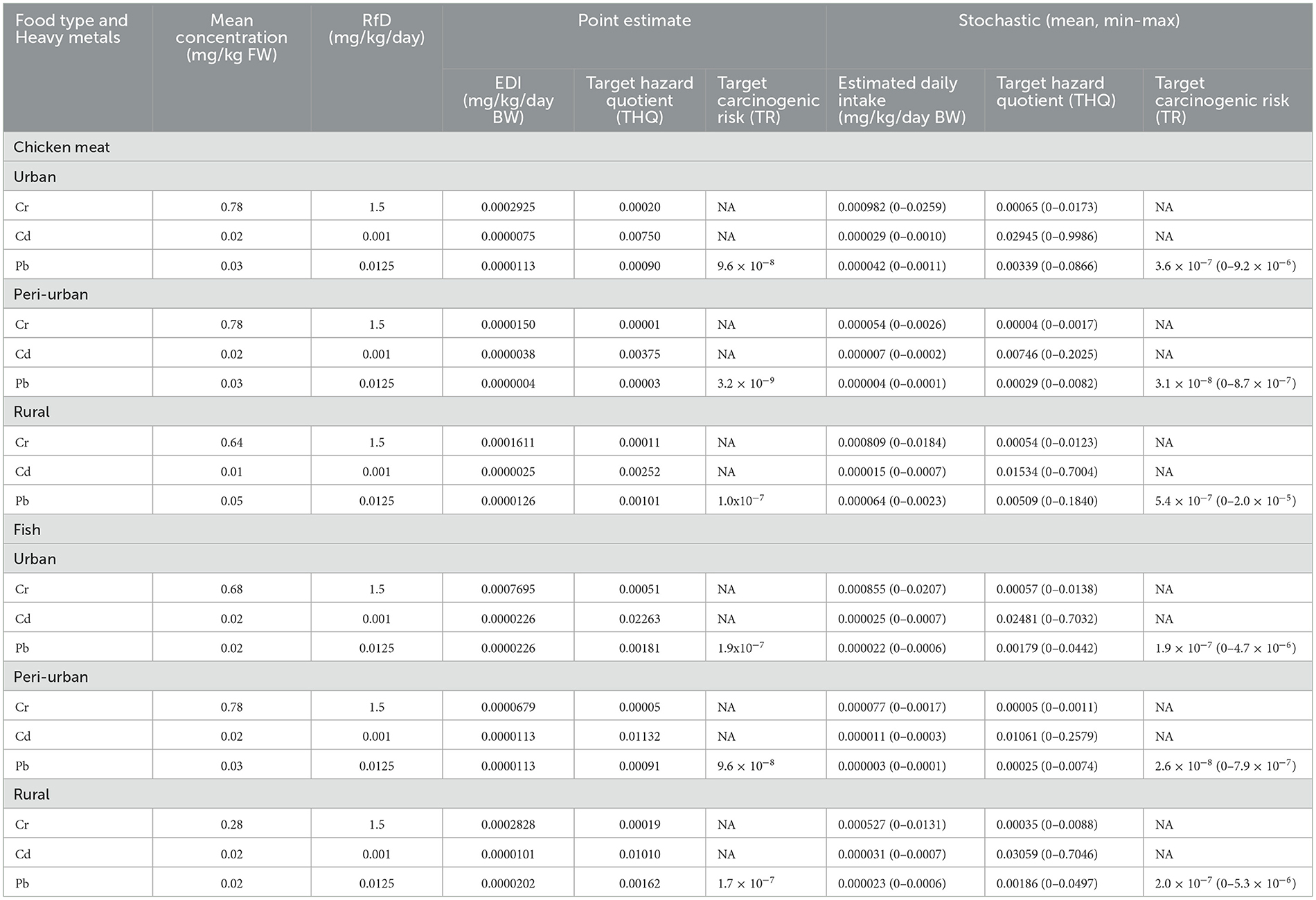
Table 6. Mean contamination levels, oral reference dose (RfD), estimated daily intake (EDI), target hazard quotient (THQ) and target carcinogenic risk (TR) of metals for adults (assumed 60 kg body weight) due to consumption of chicken meat and fish in Bangladesh.
Determination of heavy metal concentration in different food types is important from a public health aspect. This present study highlighted the heavy metal contamination in fish and chicken meat in different areas of Bangladesh, and provided an estimation of the risk for consumers. We found that the contamination levels varied both between and within the different sampling sites. These observed variations in heavy metal concentrations in foodstuffs could be due to various absorption and accumulation capabilities (Pandey and Pandey, 2008), growth period and stages during food production (Saha and Zaman, 2013) as well as climatic differences of the study areas (Santos et al., 2004).
The presence of chromium (mean ± SD) in chicken meat were found as 0.64 ± 1.24, 0.04 ± 0.05 and 0.78 ± 0.077 mg/kg in rural, peri-urban and urban, while in fish samples, mean value were 0.28 ± 0.50, 0.06 ± 0.09 and 0.68 ± 0.67 mg/kg in rural, peri-urban and urban, respectively. This could indicate a great concern for the public, but the levels observed here were generally lower than what was found in other studies. An earlier study conducted in Bangladesh stated that mean concentration of Cr in chicken was 1.4 ± 0.31 mg/kg (Islam et al., 2015a), which is almost double the levels detected in our study. In fish species caught from different rivers, much higher Cr concentration have also been reported previously in Bangladesh, such as 6.92–12.23 mg/kg dry weight (Dhaleshwari river, Ahmed et al., 2009), 0.47–2.07 mg/kg dry weight (Bangshi River, Rahman et al., 2012), 5.27–7.38 mg/kg dry weight (Buriganga River, Ahmad et al., 2010). Our finding on Cr concentration was also lower compared to fish caught from urban rivers around the Dhaka city (0.75–4.8 mg/kg wet weight) or from cultured fishes (1.054–1.349 mg/kg wet weight, Islam et al., 2015b). The differences in contamination levels to our results could be explained by different study areas, but could potentially also indicate an improvement of food production conditions and environmental pollution compared to the earlier studies.
Cadmium is a highly toxic element capable of causing severe toxicity even when it is present at a very low concentration of ~1 mg/kg (Friberg et al., 2018). The accumulation of Cd in the human body may give rise to hepatic, pulmonary, renal, skeletal, reproductive effects, and even cancer. The mean Cd concentration in both chicken and fish detected in this study were between 0.01 and 0.02 mg/kg amongst rural, peri-urban and urban areas. Islam et al. (2015a) reported 0.030 ± 0.032 mg/kg FW in chicken meat, whereas another study reported much higher levels 0.23 mg/kg FW in chicken meat (Islam, 2018). Cd concentration in fish in the present study were similar to the concentrations reported earlier in Bangladesh, which ranged from 0.51–0.73 mg/kg dry weight (fish from Dhaleshwari river, Ahmed et al., 2009), 0.09–0.87 mg/kg dry weight (fishes from Bangshi River, Rahman et al., 2012), 0.008–0.13 mg/kg wet weight (fish from urban rivers around Dhaka city, Islam et al., 2015b), 0.001–0.003 mg/kg wet weight in cultured fishes (Ahmed et al., 2015). In addition, the concentration of Cd found in the different study sites in this study was lower than the maximum allowable range (JECFA, 2005), indicating that consumption of these two commodities may rarely contribute to toxic effects of Cd.
Regarding the lead contamination in chicken meat, concentrations were 0.05 ± 0.11, 0.001 ± 0.01, and 0.03 ± 0.06 mg/kg in rural, peri-urban and urban areas, respectively. Average concentrations of lead in fish were 0.02 ± 0.08, 0.01 ± 0.01, and 0.02 ± 0.05 mg/kg collected in rural, peri-urban, and urban, respectively. Previous studies reported that lead in chicken meat was 0.17 ± 0.16 mg/kg (Islam et al., 2015a) which was higher compared to our finding. It was reported that the range of lead contamination in fish caught from different rivers were 4.25–8.17 mg/kg dry weight (Dhaleshwari river, Ahmed et al., 2009), 1.76–10.27 mg/kg dry weight (Bangshi river, Rahman et al., 2012), 8.03–13.52 mg/kg dry weight (Buriganga river, Ahmad et al., 2010), 0.052–2.7 mg/kg wet weight (urban rivers around Dhaka city, Islam et al., 2015b), 0.017–0.090 mg/kg wet weight (cultured fishes, Ahmed et al., 2015). Lead is a non-essential heavy metal and may cause many adverse health effects, including neurotoxicity and nephrotoxicity (García-Lestón Julia et al., 2010). Compared to the earlier studies, it may seem that the lead contamination in chicken meat and fish has been reduced, which shows a positive signal in improving the safety level related to lead.
In the present study, concentrations of the three heavy/toxic metals were lower than maximum tolerable daily intake in all three studied areas, which suggested that these food items alone are not contributing to significant health risks for consumer. However, the consumption amount revealed in this study was relatively low, with <100 g consumed per person per day. Growing income could lead to increased consumption of meat and fish, which, along with poor monitoring, surveillance, and implementation, could contribute to higher exposure in the future. In addition, a tendency of accumulation of metal in various organ may trigger serious health implication in a longer term. Positive correlation was shown between Cr and Cd signifying it might be common sources of these metals, such as contamination of feed (Hasan et al., 2013; Ullah et al., 2017).
The result on THQ values related Pb, Cr, and Cd in this study would suggest that on average, people would not be suffering negative health risks through the consumption of these food. In addition, TR for Pb was also lower than 10−6, which indicating the risk of cancer due to exposure to Pb through chicken meat and fish consumption was negligible, given the present amount of consumption. Our results are in line with earlier assessments of the risks from heavy metals in fish (Ullah et al., 2017), which also concluded that there were negligible risks from fish consumption at the present levels. Given the potential health benefits of consuming animal-source foods, particularly by undernourished children (Alonso et al., 2019), it may be important that people are not afraid to increase their consumption from the presently very low levels. However, this study could only cover a few of all the potential heavy metal hazards in the food, and Pb, Cr, and Cd were selected based on the priorities of the country, according to the national stakeholders consulted. Future studies are needed to more accurately assess the risks of additional hazards present in the food.
This study had some limitations. First, the risk assessment based on the total amount of metals show actual exposure, that could not cover the nature of the metal metabolism in the human body, percentage of bio-accessible, e.g., not reaching the circulatory system. Secondly, during food production and cooking processes, part of the metal may be reduced which could also influence the final exposure dose. However, using the total dietary intake, the estimates provided here would likely be overestimations, and the real risk, taking bioavailability into account would then likely be even lower. Third, due to the laboratory capacity of the national laboratory BLRI, AAS was used, which is not as accurate as some other methods. Optimally ICP-OES or ICP-MS would have been used for confirmation, but these methods were not available. Instead all precautions were done to ensure validity of the results, including the use of multiple standards for calibration. In addition, the dietary intake was based on interviews, which also may be prone to errors in the estimate. Thus, the final risk assessments should be considered taking into account that the data is limited and there are major assumptions, that may not make even the stochastic estimates applicable to the entire population. Fourth, we only found related data of Pb which we could conduct a cancer risk assessment, while for other heavy metal (Cr and Cd), we have limited access to the information which could use to carry out the cancer risk assessment.
The results of this study revealed the prevalence and concentration of Pb, Cr, and Cd in fish and chicken meat in different retailed types and study areas. Their concentrations were below the maximum allowable range which indicates acceptable level for human consumption. However, heavy metal contamination in fish and chicken meat would imply that contaminated feed and water, potentially through environmental sources like polluted sewage water, intensive use of pesticide and rapid development of industry, which could affect the quality and safety of livestock and aquaculture products, and as consequence effect to human health. Therefore, it is important to keep monitoring and implement measures to reduce heavy metals contamination in feed and water along the food chain.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Ethical Review Committee of ILRI, approval number 2018–27. The patients/participants provided their written informed consent to participate in this study.
Funding and conceptualization: JFL and DG. Study design: JFL, MAS, SD-X, and DG. Data collection and laboratory analyses: RB, SI, RA, NAS, ASMAU, MSS, and AM. Supervision: MAS and JFL. Data analyses: RB, SD-X, SI, and JFL. Drafting of manuscript: RB, SI, and JFL. Critically revising and approving the manuscript: All authors. All authors contributed to the article and approved the submitted version.
This research is an output of the CGIAR Initiative on Resilient Cities. SD-X was supported by the CGIAR Initiative on One Health (Protecting Human Health through a One Health Approach). The authors thank all funders who supported this research through their contributions to the CGIAR Trust Fund: https://www.cgiar.org/funders.
The authors would like to thank Corey Watts for her help with this study. The authors are grateful to all donors that globally support through their contribution to the Consultative Group for International Agricultural Research (CGIAR) system, France. Additionally, we are thankful for the kind help from the members of Bangladesh Food Safety Authority (BFSA), Dhaka, Bangladesh Agricultural University (BAU), Mymensingh, Bangladesh and Wazed Mia Science Research Centre, Jahangirnagar University, Savar, Bangladesh during field sampling and analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1085809/full#supplementary-material
Ahmad, M. K., Islam, S., Rahman, M. S., Haque, M. R., and Islam, M. M. (2010). Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int. J. Environ. Res. 4, 321–332. doi: 10.22059/IJER.2010.24
Ahmed, M. K., Ahamed, S., Rahman, S., Haque, M. R., and Islam, M. M. (2009). Heavy metal concentrations in water, sediments and their bio-accumulations in fishes and oyster in Dhaleswari River. Asian J. Water Environ. Pollut. 3, 33–41.
Ahmed, M. K., Shaheen, N., Islam, M. S., Habibullah-al-Mamun, M., Islam, S., Mohiduzzaman, M., and Bhattacharjee, L. (2015). Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128, 284–292. doi: 10.1016/j.chemosphere.2015.02.016
Alonso, S., Dominguez-Salas, P., and Grace, D. (2019). The role of livestock products for nutrition in the first 1,000 days of life. Anim. Front. 9, 24–31. doi: 10.1093/af/vfz033
Bundschuh, J., Nath, B., Bhattacharya, P., Liu, C. W., Armienta, M. A., Moreno López, M. V., et al. (2012). Arsenic in the human food chain: the Latin American perspective. Sci. Total Environ. 429, 92–106. doi: 10.1016/j.scitotenv.2011.09.069
Codex Alimentarius (2019). General standard for contaminants and toxins in food and feed. CXS 193–1995. Rome: Food and Agricultural Organization.
Cui, Y., Zhu, Y. G., Zhai, R., Huang, Y., Qiu, Y., Liang, J., et al. (2005). Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ. Int. 31, 784–790. doi: 10.1016/j.envint.2005.05.025
Demirezen, D., and Uruç, K. (2006). Comparative study of trace elements in certain fish, meat and meat products. Meat Sci. 74, 255–260. doi: 10.1016/j.meatsci.2006.03.012
EPA (1989). Agency Integrated Risk Information System (IRIS): Chemical Assessment Summary (Cadmium; CASRN 7440-43-9). Available online at: https://iris.epa.gov/static/pdfs/0141_summary.pdf (accessed October 03, 2022).
EPA (2000). Chromium Compounds-Health Hazard Information (Chromium III). Available online at: https://www.epa.gov/sites/default/files/2016-09/documents/chromium-compounds.pdf (accessed October 03, 2022).
EPA (2022). Risk Assessment Guidance for Superfund (RAGS): Part A. US EPA. Available online at: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed September 26, 2022).
FDA (2019). Lead in Food, Foodwares, and Dietary Supplements. Available online at: https://www.fda.gov/food/metals-and-your-food/lead-food-foodwares-and-dietary-supplements (accessed October 03, 2022).
Flannery, B. M., Dolan, L. C., Hoffman-Pennesi, D., Gavelek, A., Jones, O. E., Kanwal, R., et al. (2020). Food and Drug Administration's interim reference levels for dietary lead exposure in children and women of childbearing age. Regul. Toxicol. Pharmacol. 110, 104516. doi: 10.1016/j.yrtph.2019.104516
Flora, S. J. S., Flora, G., and Saxena, G. (2006). Environmental Occurrence, Health Effects and Management of Lead Poisoning. Amsterdam: Elsevier Science BV 158–228. doi: 10.1016/B978-044452945-9/50004-X
Friberg, L., Piscator, M., and Nordberg, G. Cadmium in the Environment, II. Boca Raton, FL: CRC Press, Taylor Francis (2018).
García-Lestón Julia, J., Méndez, J., Pásaro, E., and Laffon, B. (2010). Genotoxic effects of lead: an updated review. Environ. Int. 36, 623–636. doi: 10.1016/j.envint.2010.04.011
Gibb, H. J., Barchowsky, A., Bellinger, D., Bolger, P. M., Carrington, C., Havelaar, A. H., et al. (2019). Estimates of the 2015 global and regional disease burden from four foodborne metals—arsenic, cadmium, lead and methylmercury. Environ. Res. 174, 188–194. doi: 10.1016/j.envres.2018.12.062
Hasan, S., Rahman, M. L., Mazumder, L. T., Hasan, S., and Rahman, M. L. (2013). Hexavalent chromium in tannery solid waste based poultry feed in Bangladesh and its transfer to food chain. IOSR J. Environ. Sci. Toxicol. Food Technol. 3, 44–51. doi: 10.9790/2402-0344451
HIES (2019). Household Expenditure Survey. Dhaka. Available online at: http://data.gov.bd/dataset/household-income-and-expenditure-survey-hies-2016 (accessed September 26, 2022).
Idera, F., Omotola, O., Adedayo, A., and Paul, U. J. (2015). Comparison of acid mixtures using conventional wet digestion methods for determination of heavy metals in fish tissues. J. Sci. Res. Rep. 8, 1–9. doi: 10.9734/JSRR/2015/19717
International Agency for Research on Cancer (2012). “Arsenic, metals, fibres, and dusts,” in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
Islam, M. S. (2018). Heavy metals in meat with health implications in Bangladesh. SDRP J. Food Sci. Technol. 2, 218–227.
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M., and Masunaga, S. (2014). Trace metals in soil and vegetables and associated health risk assessment. Environ. Monit. Assess. 186, 8727–8739. doi: 10.1007/s10661-014-4040-y
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M., and Masunaga, S. (2015a). Assessment of trace metals in foodstuffs grown around the vicinity of industries in Bangladesh. J. Food Compos. Anal. 42, 8–15. doi: 10.1016/j.jfca.2014.12.031
Islam, M. S., Ahmed, M. K., Raknuzzaman, M., Habibullah-Al-Mamun, M., and Masunaga, S. (2015b). Metal speciation in sediment and their bioaccumulation in fish species of three urban rivers in Bangladesh. Arch. Environ. Contam. Toxicol. 68, 92–106. doi: 10.1007/s00244-014-0079-6
JECFA (2005). 64th Joint FAO/WHO Expert Committee on Food Additives (JECFA) Meeting—Food Contaminants. Summary and conclusions. Available online at: https://www.fao.org/documents/card/en/c/f95956a6-c4ac-4843-bd48-b6a1fbe3afac/ (accessed September 26, 2022).
Ji, K., Kim, J., Lee, M., Park, S., Kwon, H. J., Cheong, H. K., et al. (2013). Assessment of exposure to heavy metals and health risks among residents near abandoned metal mines in Goseong, Korea. Environ. Pollut. 178, 322–328. doi: 10.1016/j.envpol.2013.03.031
Kachenko, A. G., and Singh, B. (2006). Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 169, 101–123. doi: 10.1007/s11270-006-2027-1
Kumar Sharma, R., Agrawal, M., and Marshall, F. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 66, 258–266. doi: 10.1016/j.ecoenv.2005.11.007
Lei, M., Zhang, Y., Khan, S., Pu-Feng, Q., Liao, B.-H., Lei, M., et al. (2009). Pollution, fractionation, and mobility of Pb, Cd, Cu, and Zn in garden and paddy soils from a Pb/Zn mining area. Environ. Monit. Assess. 168, 215–222. doi: 10.1007/s10661-009-1105-4
Luckey, T. D., and Venugopal, B. (1979). Metal Toxicity in Mammals. Volume 1. Physiologic and Chemical Basis for Metal Toxicity. 2nd ed. New York, NY: Plenum Press.
McLaughlin, M. J., Parker, D. R., and Clarke, J. M. (1999). Metals and micronutrients—food safety issues. Food Crop Res. 60, 143–163. doi: 10.1016/S0378-4290(98)00137-3
Nisianakis, P., Giannenas, I., Gavriil, A., Kontopidis, G., and Kyriazakis, I. (2009). Variation in trace element contents among chicken, Turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Biol. Trace Elem. Res. 128, 62–71. doi: 10.1007/s12011-008-8249-x
OEHHA (2009). California Office of Environmental Health Hazard Assessment: Lead and Lead Compounds. Available online at: https://oehha.ca.gov/chemicals/lead-and-lead-compounds (accessed October 03, 2022).
Pandey, J., and Pandey, U. (2008). Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environ. Monit. Assess. 148, 61–74. doi: 10.1007/s10661-007-0139-8
Pintaeva, E. T., Bazarsadueva, S. V., Radnaeva, L. D., Petrov, E. A., and Smirnova, O. G. (2011). Content and character of metal accumulation in fish of the Kichera River (a tributary of Lake Baikal). Contemp. Probl. Ecol. 41, 64–68. doi: 10.1134/S1995425511010103
Rae, A. N. (1998). The effects of expenditure growth and urbanisation on food consumption in East Asia: a note on animal products. Agric. Econ. 18, 291–299. doi: 10.1111/j.1574-0862.1998.tb00506.x
Rahman, M. A., Rahman, M. M., Reichman, S. M., Lim, R. P., and Naidu, R. (2014). Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: health hazard. Ecotoxicol. Environ. Saf. 100, 53–60. doi: 10.1016/j.ecoenv.2013.11.024
Rahman, M. S., Molla, A. H., Saha, N., and Rahman, A. (2012). Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, <city>Dhaka</city>, Bangladesh. Food Chem. 134, 1847–1854. doi: 10.1016/j.foodchem.2012.03.099
Recommended Dietary Allowances (1989). Subcommittee on the Tenth Edition of the Recommended Dietary Allowances, 10th edition. Washington D.C: National Academies Press (US).
Saha, N., and Zaman, M. R. (2013). Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 185, 3867–3878. doi: 10.1007/s10661-012-2835-2
Sankar, T. V., Zynudheen, A. A., Anandan, R., and Viswanathan Nair, P. G. (2006). Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerala, India. Chemosphere 65, 583–590. doi: 10.1016/j.chemosphere.2006.02.038
Santos, E. E., Lauria, D. C., and Porto Da Silveira, C. L. (2004). Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci. Total Environ. 327, 69–79. doi: 10.1016/j.scitotenv.2004.01.016
Steenland, K., and Boffetta, P. (2000). Lead and cancer in humans: where are we now? Am. J. Ind. Med. 38, 295–299. doi: 10.1002/1097-0274(200009)38:3<295::AID-AJIM8>3.0.CO
Ullah, A. K. M. A., Maksud, M. A., Khan, S. R., Lutfa, L. N., and Quraishi, S. B. (2017). Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol Rep. 4, 574–579. doi: 10.1016/j.toxrep.2017.10.002
Wilbur, S., Abadin, H., Fay, M., Yu, D., Tencza, B., Ingerman, L., et al. (2012). Toxicological Profile for Chromium. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US).
Keywords: food safety, chemical hazards, quantitative risk assessment, trace metal, dietary intake, lead, cadmium, chromium
Citation: Begum R, Akter R, Dang-Xuan S, Islam S, Siddiky NA, Uddin ASMA, Mahmud A, Sarker MS, Grace D, Samad MA and Lindahl JF (2023) Heavy metal contamination in retailed food in Bangladesh: a dietary public health risk assessment. Front. Sustain. Food Syst. 7:1085809. doi: 10.3389/fsufs.2023.1085809
Received: 31 October 2022; Accepted: 22 March 2023;
Published: 17 April 2023.
Edited by:
Xiao-Hua Zhang, Xuchang Univerisity, ChinaReviewed by:
Hui-Wen Gu, Yangtze University, ChinaCopyright © 2023 Begum, Akter, Dang-Xuan, Islam, Siddiky, Uddin, Mahmud, Sarker, Grace, Samad and Lindahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna F. Lindahl, ai5saW5kYWhsQGNnaWFyLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.