
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 10 February 2023
Sec. Crop Biology and Sustainability
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1085223
This article is part of the Research Topic Biostimulants for Climate-Smart and Sustainable Agriculture View all 12 articles
Plant growth-promoting rhizobacteria (PGPR) have been shown to augment plant responses against drought and other abiotic stresses. In the present study, we isolated 27 bacteria from the rhizosphere of various plants cultivated in the Kumaon Himalayas., and to measure their abiotic stress tolerance, these 27 isolates were subjected to variations in pH, temperature, and drought. All 27 isolates were also screened for various plant growth-promoting traits. Among these, the four isolates RR1, ASC1, AFS3, and NG4 demonstrated various plant growth promotion activities including the synthesis of indole-3-acetic acid (IAA), siderophores, ammonia, and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase production, and concomitantly high tolerance to abiotic stresses. Moreover, 16S rRNA sequencing of these four isolates validated their identities as Bacillus, Pseudomonas, and Staphylococcus sp. Finally, to assess the in-vivo drought tolerance potential of these four isolates, a pot-trial experiment was undertaken in wheat cultivar VL-892. The results demonstrated that inoculating wheat plants with these four PGPR isolates greatly improved plant growth under drought circumstances by increasing root and shoot length and both fresh and dry weight of root and shoot. This study endeavors to discover the biochemical and molecular diversity of cultivable PGPR in six remotely located districts of Uttarakhand. In conclusion, the drought-tolerant PGPR strains described in this study are plant-beneficial and can effectively mobilize nutrients under drought conditions. Consequently, they could be used as bioinoculants to alleviate drought stress in wheat plants, in a sustainable manner. To the best of our knowledge, this is the first report of exploring the diversity and characterization of PGPR from the Kumaon Himalayas and their drought evaluation.
Global warming, rising population, and declining agricultural land are all going to exacerbate global food insecurity. The expected growth in the world's population will place significant pressure on food security and constitutes a substantial threat to sustainable agriculture. Similarly, chemical fertilizers and pesticides were employed to meet a major portion of this increased requirement in crop yields (Alori and Babalola, 2018). The extensive utilization of pesticides and fertilizers has already caused serious repercussions, including loss of soil quality and pollution in the agroecosystem (Meena et al., 2017). Due to this unexpected shift in ecological parameters, the plant's ability to adapt to fluctuating climates has been compromised. Plants are exposed to a variety of abiotic stresses present in their environment. Some of the most significant abiotic challenges that plants endure include desiccation, flooding, extreme temperature, salinity, and heavy metal contaminants (Gontia-Mishra et al., 2016). Among these, the most prominent abiotic factor that inhibits plant production is drought (Sati et al., 2022b). Drought is among the most widespread abiotic stress encountered by plants, and it impacts root-water dynamics and other general responses, further lowering the growth and nutrients of the plant and, consequently, global agricultural production (Ma et al., 2020). Drought exerts its effects by altering the morpho-physiological characteristics of plants by changing the water potential and turgor pressure of plants (Mukarram et al., 2021). Wheat (Triticum aestivum L.) is a key cereal crop grown worldwide. For about 35% of the global population, wheat serves as the primary food source (Poursarebani et al., 2014). In comparison to any other cereal e.g., maize, rice, etc., wheat offers greater proteins and calories (Kumar et al., 2017). Unfortunately, wheat is also among the top key cereal crops, where high temperatures and drought impede plant growth and harvest. The wheat crop is primarily grown in non-irrigated settings, having typical precipitation lower than 900 mm (Zhang et al., 2022). Wheat grown in non-irrigated circumstances in many emerging economies is vulnerable to drought in every stage of development (Rockström et al., 2009). Conventional plant breeding and genetic engineering approaches have been utilized to enhance drought resistance in crops. Using genetic engineering for making drought-tolerant cultivars is difficult since drought tolerance is a composite and multigenic character (Kumar et al., 2018). As a result, new solutions that offer higher crop yields while still ensuring ecological protection are urgently needed. One of the approaches to help plants cope with drought is by using PGPR, which has been documented to boost the nutritional, biochemical, physiological, and morphological properties of several plants in an environment-friendly and cost-effective manner (Sati et al., 2020). PGPR promotes plant growth directly by delivering the plant with substances synthesized by the bacteria or assisting the plant's absorption of soil nutrients, i.e., by phytohormone and siderophore formation, dissolution of mineral (P, Zn, K), and reducing ethylene levels in plants (Lugtenberg and Kamilova, 2009). Indirect PGP traits involve the exclusion of harmful aspects of a phytopathogen by diverse means, e.g., imparting resistance from the pathogen, production of enzymes, antibiotics, and anti-fungal chemicals (Goswami et al., 2016; Sati et al., 2022a). Plants treated with PGPR strains maintained better water stature than non-PGPR control, leading to greater production under drought (Shivakumar and Bhaktavatchalu, 2017). The Kumaon region experiences frequent extreme climatic disturbances each year. The monsoon season accounts for 60–85% of the annual precipitation rate, which ranges from 260 to 3,955 nm. Likewise, the average temperature fluctuates between below zero to −43°C due to the diverse topography of the region (Malik and Kumar, 2020). Keeping in mind the recurrent extreme climatic events and the significance of PGPR in growth enhancement, the current study was conducted to evaluate the diversity of native PGPR from the rhizosphere of various crop plants in the Kumaon region.
The bacterial isolates were collected from all six districts of the Kumaon region of Uttarakhand. The Kumaon is one of the two administrative provinces in Uttarakhand, and it encompasses six districts: Almora, Bageshwar, Champawat, Nainital, Udham Singh Nagar, and Pithoragarh (Figure 1). The Kumaon region has a geographical size of 21,313 km2 and an elevation range of 223–3,669 m above MSL. Kumaon is ensconced in the highlands of North India, bordering Chinese Tibet and Nepal. It encompasses the Himalayan Mountain range and is one of India's most secluded, scarcely inhabited, and undeveloped areas, with a static agricultural economy. Along the elevational gradient, the Kumaon region is divided into three agro-climatic zones: (i) lower elevation (up to 1,200m), (ii) intermediate elevation (between 1,200 and 2,300 m), and (iii) higher elevations (above 2,300 m). This region is excellent for the growth of multiple varieties of plants owing to its diverse climate (subtropical to alpine), height, elevation, soil types, valleys, rivers, watersheds, and forest resources.
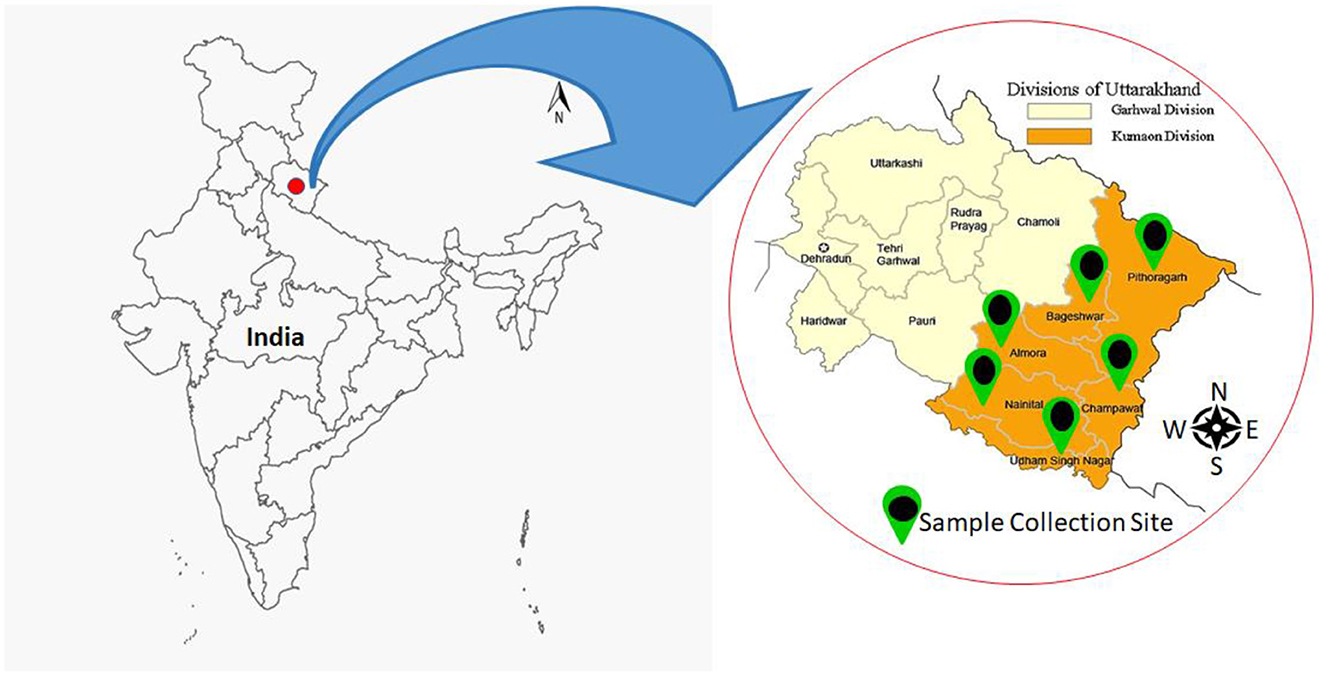
Figure 1. Map depicting the various geographical locations in the Kumaon region where samples were collected.
Soil samples were randomly obtained from the rhizosphere of diverse plants across six districts of the Kumaon region of Uttarakhand. Samples were taken from four separate places at each location. To generate the composite sample, five samples from each site were taken and mixed. The obtained soil samples were diluted serially for up to 10−6 dilutions and a 0.1 mL aliquot of this diluted soil solution was spread onto the Nutrient Agar (NA) plates. Microbial colonies began to develop on the plates during an overnight incubation at 28 ± 2°C. For further purifications, only colonies with clearly distinguishable morphologies were chosen. The pure culture was stored in a petri dish and maintained at 4°C for regular use. A solution of 20% glycerol was used to briefly store the pure isolates at −20°C.
A nutrient broth (NB) medium was prepared by amending polyethylene glycol (PEG 6000) with varying water potentials to test the drought endurance of isolated bacteria (−0.05, −0.15, −0.30, −0.49, and −0.73 MPa) (Sarma and Saikia, 2014; Gontia-Mishra et al., 2016). A 100 μL of each bacterial isolate having a concentration of 1 × 107 colony-forming unit (CFU)/mL, calculated by taking optical density (OD) at 600 nm, was added to the test tubes containing 5 ml of NB. The OD at 600 nm was measured spectrophotometrically after overnight incubation in an orbital shaker (200 rpm) at 30°C. Under varying levels of PEG, the growth of bacterial cultures was evaluated. By growing the test isolates in the nutrient broth with varying pH i.e., 4, 6, 8, and 10 with either 1N HCl or NaOH their pH tolerance was determined (Küçük et al., 2006). Isolated cultures were evaluated for salinity endurance in nutrient broth (NB) supplemented with varying percentages of NaCl (0, 2.5, 5, 7.5, and 10%) (ben Romdhane et al., 2009). A 50 μl of each bacterial inoculum having culture turbidity of 1 × 107 CFU/ml, accessed spectrophotometrically, was injected per 5 ml of media and overnight incubated in an orbital shaker (200 rpm) at 30°C. The absorbance at 600 nm was calculated with a UV-Vis spectrophotometer to quantify growth (Eppendorf) (Gontia-Mishra et al., 2016).
All the 27 isolates were tested for plant beneficial attributes e.g., indole acetic acid (IAA) generation, siderophore formation, ACC deaminase production, phosphate dissolution, HCN, and NH3 generation, were evaluated as discussed by Bakker and Schippers (1987), Schwyn and Neilands (1987), Bric et al. (1991), Cappucino and Sherman (1992), Nautiyal et al. (2000), Penrose and Glick (2003), respectively. The Salkowski colorimetric test was used to identify and estimate IAA biosynthesis using Nutrient Broth supplemented with L-tryptophan (2 mgL−1; Sigma-Aldrich, Milan, Italy). To analyze the potential of bacterial isolates to form siderophores, the isolates were spot inoculated on agar plates supplemented with Chrome-azurol S (CAS) dye, as defined by Schwyn and Neilands (1987). The appearance of an orange-yellow zone surrounding the colony after 5–7 days of incubation at 28°C revealed the siderophore synthesis by microbes. The presence of enzyme ACC deaminase (ACCd) in bacterial cultures was calculated by monitoring growth on nitrogen-free minimum medium (MM) agar (Dworkin and Foster, 1958) enriched with 3 mM ACC (Sigma-Aldrich) after incubating at 28°C in darkness for 5–7 days of incubation at, as defined by Jaemsaeng et al. (2018). 2 gL−1 (NH4)2SO4 enriched MM agar plates were employed for control. Phosphate solubilization was checked using a modified NBRIP medium defined by Nautiyal et al. (2000). The isolated strains were examined for exopolysaccharide (EPS) synthesis and biofilm production (Kavita et al., 2011). The indirect plant beneficial traits e.g., hydrogen cyanide (HCN) formation were confirmed as per (Bakker and Schippers, 1987).
Seeds of late sown wheat cultivar VL-892 were procured from VPKAS Hawalbagh, Uttarakhand, India. Robust seeds were hand-picked and surface disinfected by immersing them in 70% ethanol for 3 min and then by dipping them in 0.2 % (v/v) HgCl2. The seeds were rinsed five times in deionized water to eliminate residual ethanol. For each bacterial isolate, a 0.8 OD bacterial solution was prepared in which sterilized seeds were soaked for around 60 min. Seeds serving as control were kept in 0.5% (w/v) saline solution and the immersed seeds were laid on Petri plates coated with damp sterilized filter paper and cultured in triplicate at 25°C and under dark conditions. After incubating for 72 h, the germination percent, root, and shoot length were calculated, and an average score was determined for ten seedlings. For pot assay, cultures displaying greater impacts on seedlings (in terms of root/shoot or vitality index) were selected. In the current work, the bacterial cultures were chosen depending on their tolerance to drought, and various plant-beneficial traits e.g., ACCd synthesis, siderophore formation, IAA production, P and Zn dissolution, HCN production, and seed germination rate. Based on the above-mentioned properties, four isolates namely RR1, ASC1, AFS3, and NG4 were selected. Discrete PGPR isolates were chosen for their high drought endurance, and potential to promote root proliferation and vitality index.
The surface-sterilized, untreated (control), and PGPR-treated seeds of wheat cultivar VL-892 were grown in separate 12″ × 9″ pots, filled with three kilograms of sterile soil, sand, and peat. The pot assay experiment involved 10 treatments as follows:
Control: Unstressed, uninoculated
T1: Unstressed, inoculated with AFS3
T2: Unstressed, inoculated with ASC1
T3: Unstressed, inoculated with NG4
T4: Unstressed, inoculated with RR1
D: Drought-stressed, uninoculated
DT1: Drought-stressed, inoculated with AFS3
DT2: Drought-stressed, inoculated with ASC1
DT3: Drought-stressed, inoculated with NG4
DT4: Drought-stressed, inoculated with RR1.
The experiment was done in triplicate and ten plants were maintained per pot. Seven days old PGPR-treated seedlings were again supplemented with 1% bacterial solution (~105 CFU ml−1), while untreated control plants were given an equal amount of MS media. Both the PGPR-treated and control plants were watered every alternate day with 70% of field capacity for 3 weeks. Further, these plants were subjected to 7 days of drought stress by not providing water. The non-stressed plants were watered normally. For drought recovery purposes, plants were watered again subsequently for 3 days. Water-stressed saplings without any PGPR treatment were taken as the negative control, while regularly watered plants were taken as the positive control. The saplings were treated with PGPR strains RR1, ASC1, NG4, and AFS3 to evaluate their growth promotion potential in both non-stressed (watered every alternate day) and drought-stressed (watered after 7 days) conditions.
After the 7 days of induced drought and 3 days of drought recovery, the growth characteristics of each plant, e.g., root length, shoot length, fresh and dry weight of root and shoot were measured. To calculate the total dry weight (DW) plants were oven dried at 70°C for 72 h. The fraction of root adhering soil to root tissue (RAS/RT) was calculated by Sandhya et al. (2009). The relative water content (RWC) was determined according to Zhang and Blumwald (2001).
For PCR amplification of the 16S ribosomal DNA, the primer sets 27f (5′AGAGTTTGATCCTGGCTCAG-3′) and 1378r (5′-CGGTGTGTACAAGGCCCGGGAACG-3′) targeting the rDNA of about 1,500 bp were utilized (Gontia-Mishra et al., 2016). The PCR reaction steps were early denaturation for 5 min at 94°C, afterward 35 rounds for 1 min at 55°C, extension for 3 min at 72°C, and final extension for 10 min at 72°C. The 16S rRNA gene sequence was obtained by Biologia Pvt. Ltd. (India) via genomic sequencing of the PCR sample. The basic sequence alignment BLAST Program was used to compare the sequences, scanned against the database given on the National Center for Biotechnology Information's website (http://www.ncbi.nlm.nih.gov/BLAST). MEGA version 6 was used to perform a phylogenetic assessment of the 16S rRNA gene sequences (Tamura et al., 2013). The neighbor-joining technique (Saitou and Nei, 1987) was used to construct the phylogenetic tree.
The data from all 10 different wheat saplings was computed. All the experiments were carried out in triplicates and the results were shown as mean ± SD. The results for, bacterial drought tolerance and bacterial treatments were examined by one-way ANOVA using GraphPad Prism (version 5) software. To calculate the significant differences between the means bacterial treatment under control and stress conditions. The critical difference (C.D.) values were calculated at the p = 0.05 level. The significantly different mean values are indicated by different letters.
A total of twenty-seven bacteria were isolated from the rhizosphere of Finger millet, Linseed, Wheat, Rice, Potato, Red kidney bean, Maize, Sugarcane, Nettle grass, Barley, Tomato, Chili, Horse gram, Black soybeans, and, Hemp from all the six districts of Kumaon region of Uttarakhand, India (Table 1).
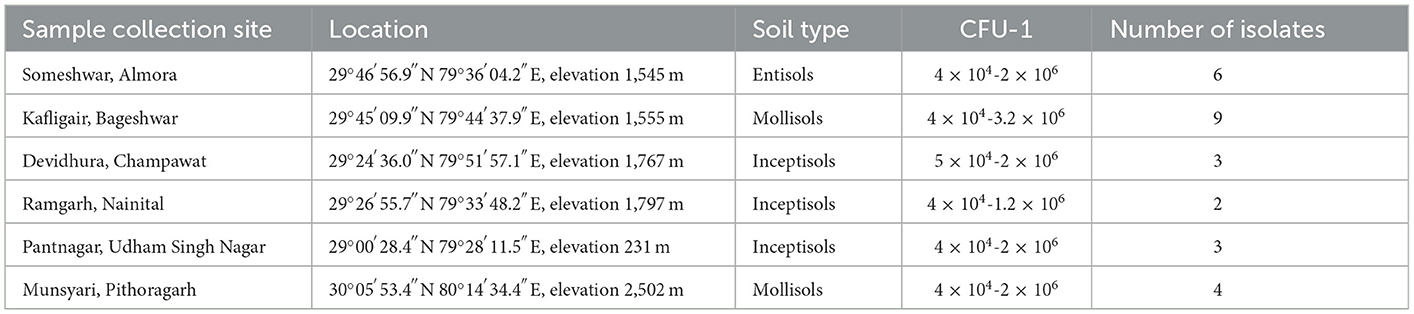
Table 1. Geographical location, type of soil, and the number of isolates collected from various districts of the Kumaon region.
The drought endurance of these PGPR isolates was tested by culturing them in varying concentrations of PEG 6000 in NB medium, and nine of them showed luxuriant growth in 15% PEG. Among these nine, only five demonstrated growth in 25% PEG. Lastly, only four isolates exhibited growth up to 35% PEG (Figure 2). These isolates were further tested for pH tolerance, and five of them demonstrated tolerance in NB medium up to pH 4.5 and only two demonstrated tolerance up to pH 9.5 (Figure 3). However, salt tolerance remains lacking in all the selected isolates.
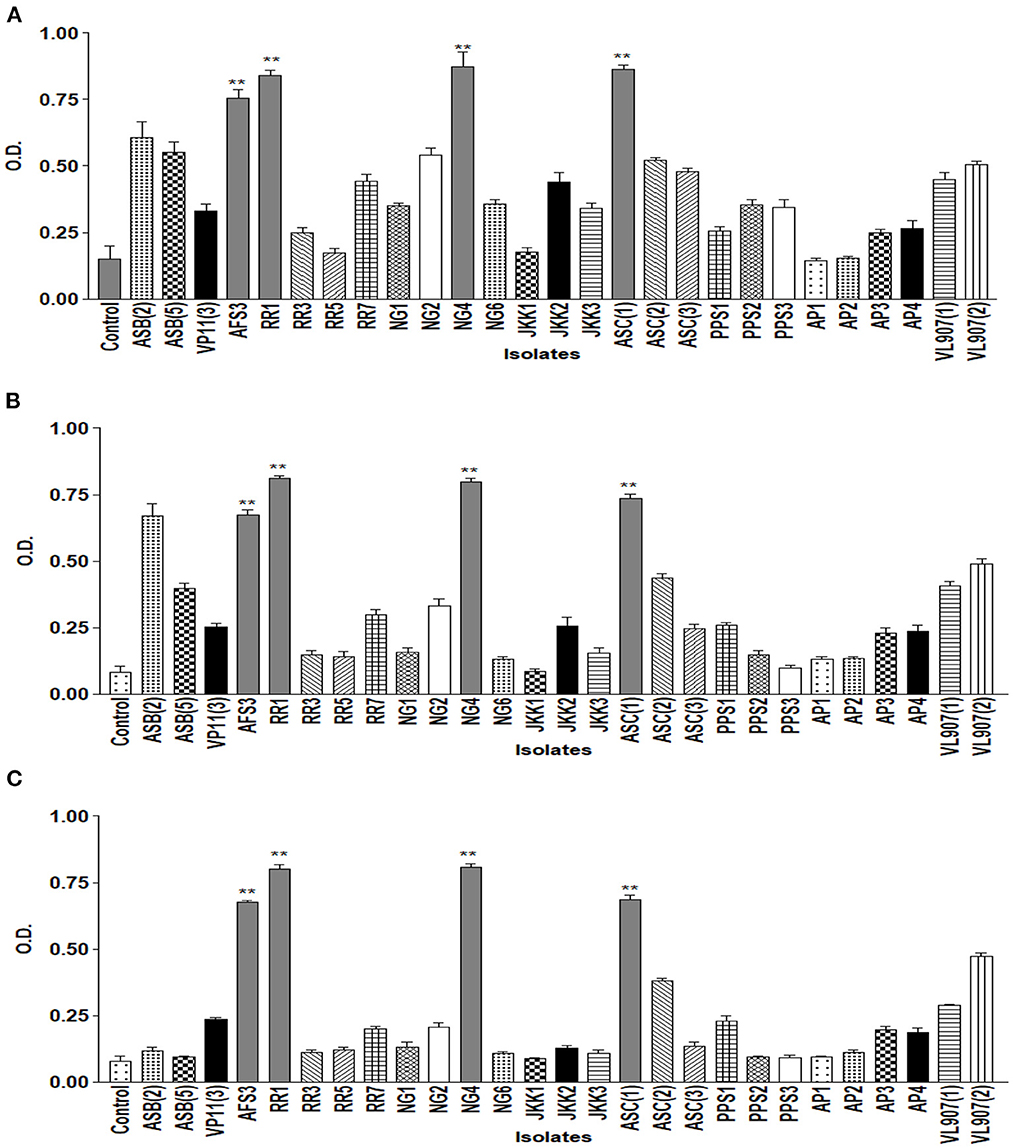
Figure 2. Graph showing growth curve at different concentrations of PEG (A) 15%; (B) 25%; (C) 35% of all 27 isolates. ** = p value < 0.01.
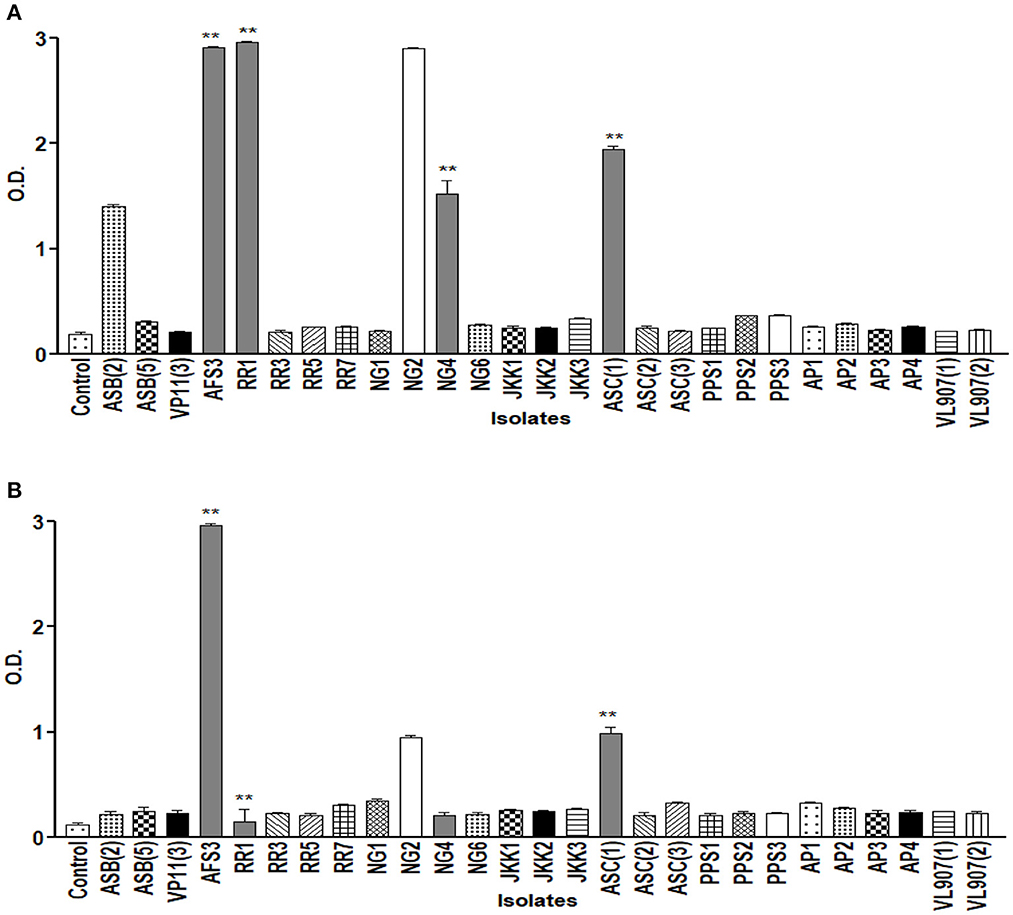
Figure 3. Graph showing growth curve at different pH (A) 4.5; (B) 9.5 of all 27 isolates. ** = p value < 0.01.
All twenty-seven isolates were analyzed for IAA generation, phosphate solubilization, and ACCd activity among other direct PGP characteristics. Out of 27 isolates, 18 isolates were IAA producers. Isolate NG 4 registered the highest IAA production (0.9 μg/ml of culture medium). Similarly, except for nine isolates (ASB5, RR5, RR7, NG1, JKK2, JKK3, PPS3, AP1, and AP3), all the other isolates showed in vitro phosphate solubilization. Isolate ASC2 showed maximum phosphate solubilization (0.83 μg/ml). The PGPR isolates were assessed for ACC deaminase activity and except for 5, all 22 isolates exhibited this trait. The PGPR isolates were also tested for many indirect PGP attributes such as NH3 production, siderophore production, HCN production, and biofilm formation. Except for 5, all other PGPR isolates exhibited NH3 production whereas all of the isolates registered HCN production (Figure 4). Twenty-one isolates showed siderophore production whereas only 16 isolates showed biofilm/EPS secretion. The direct and indirect PGP traits of the PGPR isolates are shown in Table 2.
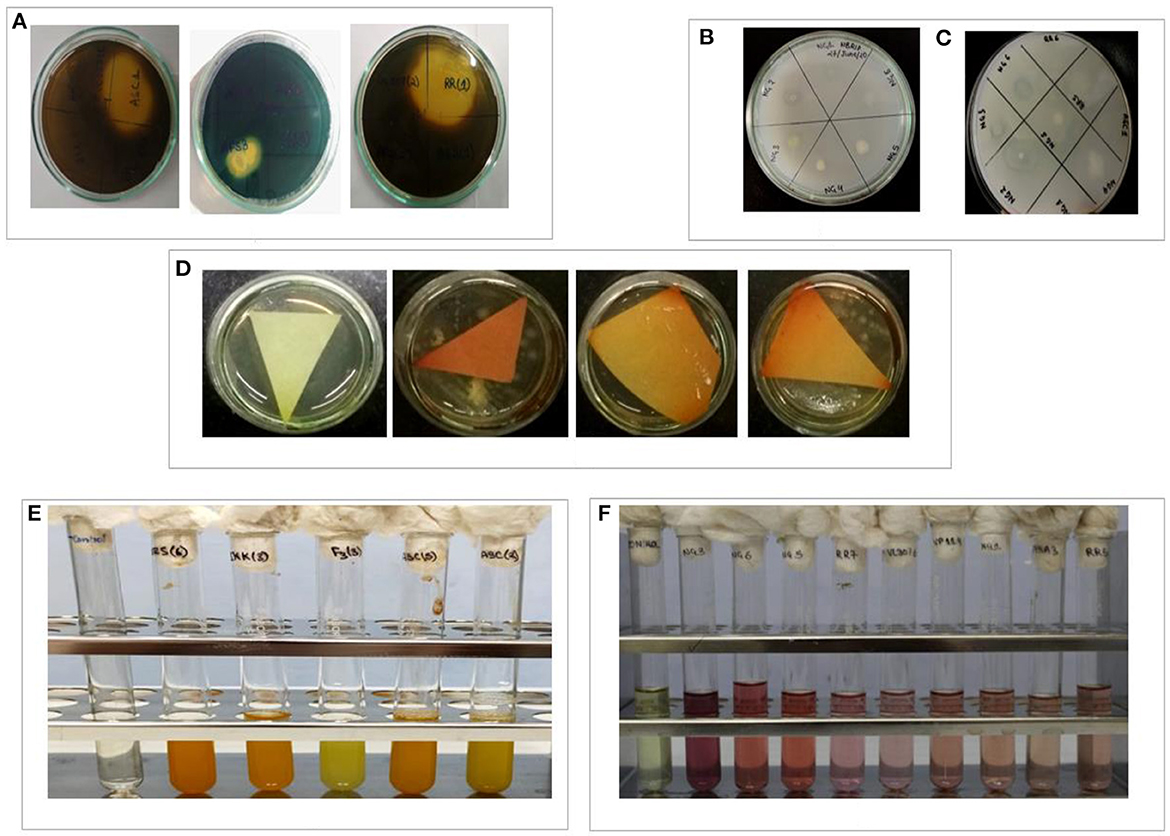
Figure 4. In-vitro depiction of different PGP parameters shown by isolated bacteria. (A) Siderophore production, (B) phosphate solubilization, (C) ACCd production, (D) HCN production, (E) NH3 production, and (F) IAA production.
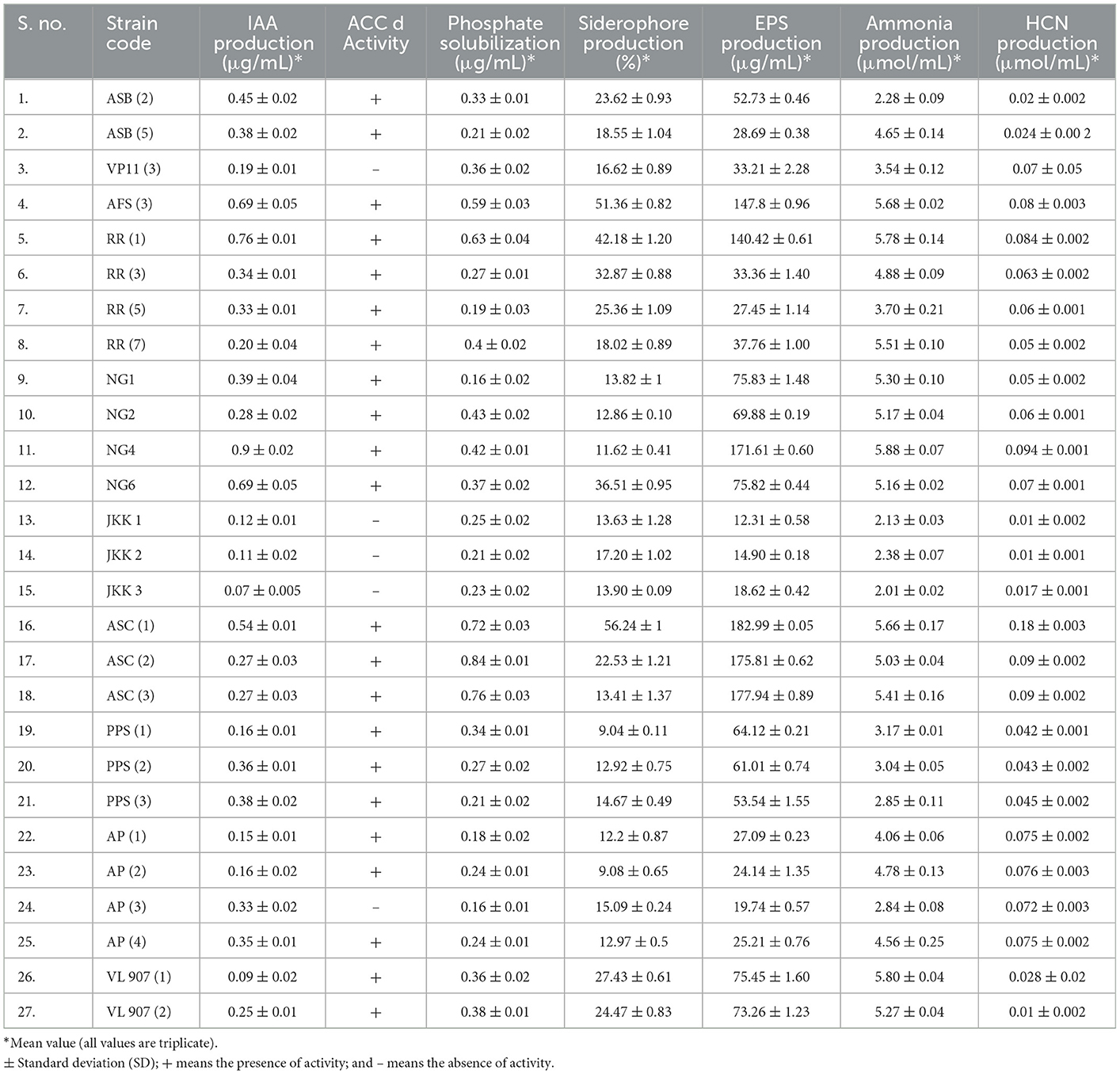
Table 2. Screening of isolated rhizobacteria for various direct and indirect plant growth-promoting traits.
To benefit plants, all 27 bacterial isolates were screened for plant promotion characteristics. Four best bacterial isolates were selected for the pot experiment, out of which three isolates namely ASC1, RR1, and AFS3 were the highest producers of siderophores, due to an orange halo around the colony. Similarly, the IAA production potential was found highest for these four isolates NG4, RR1, AFS3, and ASC1, respectively. In addition to the other PGPR traits, these 4 were also able to solubilize insoluble forms of phosphorus on the NBRIP plate as well. All four isolates also exhibited ACC deaminase activity. The PGPR isolates were also found positive for many indirect PGP attributes such as NH3 production, EPS production, and HCN production. The highest ammonia production was shown by the bacterial isolate NG4. Similarly, the greatest EPS and HCN production was recorded for isolate ASC1. Bacterial strains isolated from the rhizosphere of red kidney bean and hemp i.e., RR1 and ASC1, respectively, were able to tolerate drought for up to 35% PEG. Additionally, isolates AFS3 and ASC1 demonstrated remarkable pH tolerance with a range of 4.5–9.5. Based on variable plant growth-promoting traits AFS3, ASC1, NG4, and RR1, were selected for application as individual test subjects.
A 1,500 bp region of the the16S rRNA gene was purified and sequenced. All 4 bacterial strains were assigned to two distinct phyla based on their 16S rRNA gene sequences, including γ-proteobacteria, and Firmicutes. Out of the four test isolates, two strains belonged to the genus Bacillus, these were RR1 (Bacillus velezensis), and NG4 (Bacillus cereus), one strain belonged to the genus Pseudomonas, which was ASC1 (Pseudomonas baetica) and one strain belonged to the genus Staphylococcus, AFS3 (Staphylococcus pasteuri). A phylogenetic tree was built using 16S rDNA data to show the relationship between the tested isolates and related bacteria (Figure 5). Despite the two isolates belonging to the same genus as Bacillus sp., they exhibited sufficient variation in plant growth-promoting characteristics along with diverse seed vitality metrics. Owing to their stress adaption and nutrient solubilization potential, these PGPRs are highly effective at reducing stressful situations.
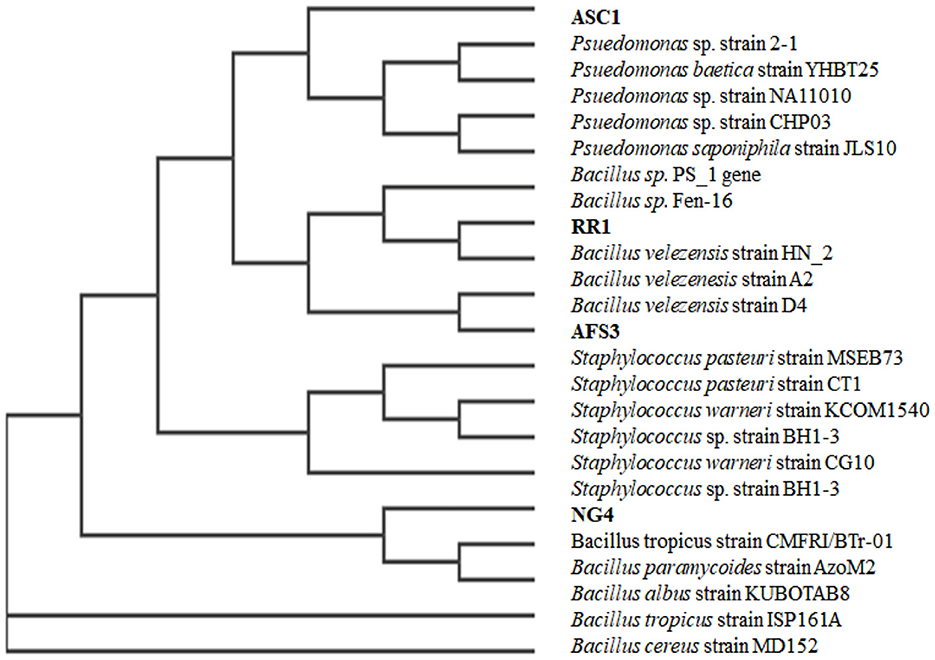
Figure 5. Phylogenetic tree constructed using the 16S rRNA gene sequences of the four selected isolates and some of their nearest phylogenetic taxa.
The final isolates AFS3, ASC1, NG4, and RR1 were chosen based on their PGP characteristics and capacity to endure high pH and higher concentrations of PEG in an NB medium. These isolates were analyzed for drought stress tolerance for seven consecutive days along with 3 days of the recovery phase. Plant root length, shoot length, and fresh and dry weights were calculated in PGPR inoculated and un-inoculated pots under normal and drought-stressed conditions (Table 3).
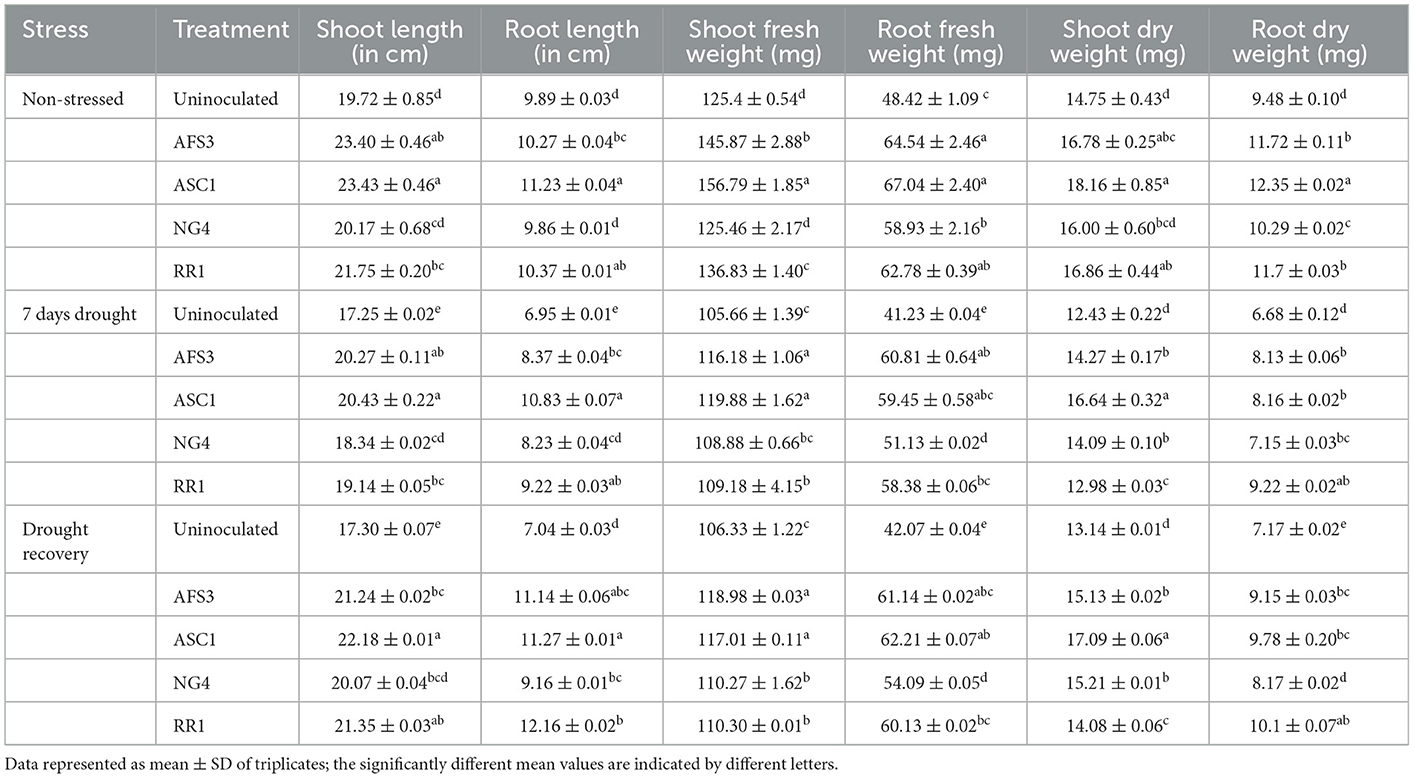
Table 3. Effects of bacterial treatments on root and shoot length and fresh, and dry weight of root and shoot of wheat under non-stress conditions, drought conditions, and during drought recovery under pot trial conditions.
During normal circumstances, ASC1-primed wheat seedlings developed considerably longer shoots, and roots, over uninoculated control and AFS3, NG4, and RR1 seedlings, and their fresh and dry weight of shoot and root were also greater. Drought suffered, control plants greatly reduced in root length and shoot fresh and dry weights relative to PGPR primed plants. Under drought stress conditions, PGPR treatment was found to be beneficial in elevating the growth of wheat seedlings. PGPR-infected plants recovered from drought stress more effectively regarding the following growth parameters such as shoot length, root length, and shoot and root fresh and dry weights. However, the recovery of uninoculated plants under drought stress was minimal. A prominent upsurge in fresh and dry weight of root and shoot was observed with all bacterial treatments in comparison to the un-inoculated control. Thus, it was observed that isolate ASC1 emerged as the most effective PGPR for boosting plant growth under drought conditions and recovery.
Wheat (Triticum sp.) is among India's major winter cereal crops. However, in hills, its production is impeded by various climatic constraints and its cultivation is mainly rain-fed. Also, wheat production is negatively affected by multiple abiotic stresses, predominantly, drought. Decreased wheat production and nutritive value in the hilly region are mostly caused by scattered agricultural lands, poor farming techniques, and insufficient nutrient availability. Hence, the present study aims at exploring rhizobacteria from Kumaon Himalayas and analyzing their drought stress resistance. A total of 27 PGPR were isolated from the rhizosphere of various plants growing in the Kumaon region. The study demonstrates the role of these isolates to alleviate salinity and drought stress in wheat plants. Variation in soil types and quality is a common attribute of the hilly region. Fluctuating elevation and temperature influence soil properties and health, which further affect soil biological mechanisms, plant growth, and yield. Therefore, to check the abiotic stress endurance of rhizospheric isolates were screened in drought, pH, and salinity conditions. Out of the 27 isolates, nine were able to grow in moderate drought and four isolates showed extreme drought tolerance by growing in 35% PEG-supplemented NB. Similarly, a total of seven isolates demonstrated extreme pH tolerance, in which five isolates were able to grow in acidic pH of 4.5 while only 2 isolates showed growth in basic pH of 9.5. Salt tolerance was not detected in any of the isolates. Further, to confirm the plant beneficial properties of these 27 isolates, an extensive PGP-traits analysis was performed including IAA production, phosphate solubilization, ACC deaminase production, NH3 production, siderophore production, HCN production, and biofilm formation. Multiple PGP characteristics, including IAA generation, phosphate solubilization, ACC deaminase, siderophore production, etc., were present in the PGPR isolates. Out of a total of 27 isolates, we chose the best four for further analysis based on the greatest drought and pH stress tolerance and the presence of the greatest number of PGP traits. IAA production is a direct PGP trait as this plant hormone help in plant growth, root development, tissue formation, cell elongation, etc. (Tsavkelova et al., 2007). In the present study, all four final isolates showed IAA production. Isolate NG 4 registered the highest IAA production (0.9 μg/ml of culture medium). The final four isolates were also capable of P solubilization. ASC1 was the highest P solubilizer among all, followed by RR1. Other similar studies of P solubilization have also been reported (Wang et al., 2020; López-Hernández et al., 2022). Additionally, all four selected isolates were noted to produce siderophores up to variable levels. A significant quantity of siderophore was observed in ASC1 and AFS3 ranging from 56.24 ± 1.0 to 51.36 ± 0.82. Production of siderophores, which aids in iron acquisition and accessibility for plants. This PGPR-triggered iron transport helps chlorophyll buildup and assists with iron retention in the edible sections of plants (López-Hernández et al., 2022). Wheat plants under drought stress were used to examine the ability of isolates AFS3, ASC1, RR1, and NG4 to promote plant growth. Under both non-stressed and drought-stressed conditions, all the isolates boosted the fresh and dry biomass of roots and shoots and significantly improved their length as well. The presence of various PGP characteristics in these four isolates, which could be the plausible explanation for their plant growth-promoting capacity, are well-known. Additionally, these isolates demonstrated adequate ACC deaminase activity, which could trigger stress-relieving effects. The 16S rRNA gene sequencing revealed RR1 as (Bacillus velezensis), NG4 as (Bacillus cereus), ASC1 as (Pseudomonas baetica), and AFS3 as (Staphylococcus pasteuri). The role of Pseudomonas baetica (González et al., 2021), Staphylococcus pasteuri (Bhattacharyya et al., 2020), Bacillus velezensis (López-Hernández et al., 2022), and Bacillus cereus (Akhtar et al., 2021) has been reported in earlier studies as PGPR with multifarious PGP activities and our observations are in line with these reports. The current study exhibited the diversity of indigenous PGPR from the Kumaon Himalayas. The PGPR isolates demonstrated multi-dimensional PGP traits along with a good tolerance range toward multiple abiotic stresses like drought and pH. The isolates are extensively capable of bio-inoculum formulations. The isolates ASC1 performed best among the four treatments with the highest growth augmentation of wheat saplings. Treating plants with PGPR is advisable in mitigating many abiotic stresses in wheat plants. To the best of our knowledge, this is the first report of exploring the diversity and characterization of PGPR from the Kumaon Himalayas and their drought evaluation. Still, advanced research on the communication of these bacterial treatments with other soil microflora and extensive field trials are required to authenticate their efficacy under real field circumstances and in curtailing the repercussions of drought stress. These indigenous rhizospheric isolates could be used as formulated as prospective biofertilizers for agroecosystem sustainability.
The present study illustrates the importance of rhizobacteria under in-vitro conditions with drought tolerance potential. These rhizospheric bacteria become crucial under agricultural droughts by not only protecting the plants from drought but also maintaining their productivity. It can be established from the above discussion that rhizobacteria have immense potential to enhance plant growth by imparting tolerance against drought stress. The present study also advocates the use of rhizobacteria as bio-inoculants for substituting chemical fertilizers to enhance the growth and productivity of plants under severe abiotic stress conditions like drought. Such bacteria can be introduced into the root system to augment their stress-tolerant potential without compromising their productivity and thus safeguarding the ecosystem. Hence, it is concluded that the isolated bacteria can be efficiently used in drought-suffered plants, and in the future, further research can be done to assess the potential of these PGPR in a consortium to combat drought and facilitate growth promotion.
The original contributions presented in the study are publicly available, with the following accession numbers NG4-Bacillus cereus-MT642947.1, RR1-Bacillus velezensis-MG727659.1, ASC1-Pseudomonas baetica- MG571730.1, and AFS3-Staphylococcus pasteuri-KP261074.1.
DS conceptualized, designed, performed the experiment, and wrote the first manuscript. VP assisted with the manuscript's writing and editing. MS supervised the research, supported data analysis, and reviewed and edited the paper. All authors contributed to the article and approved the submitted version.
The authors are thankful to the Department of Zoology, SSJ University Campus, Almora (Uttarakhand), India and DST FIST grant SR/FST/LS-I/2018/131 for providing the facility for this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akhtar, N., Ilyas, N., Yasmin, H., Sayyed, R. Z., Hasnain, Z., Elsayed, E. A., et al. (2021). Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 26, 1569. doi: 10.3390/molecules26061569
Alori, E. T., and Babalola, O. O. (2018). Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 9, 2213. doi: 10.3389/fmicb.2018.02213
Bakker, A. W., and Schippers, B. (1987). Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol. Biochem. 19, 451–457. doi: 10.1016/0038-0717(87)90037-X
ben Romdhane, S., Trabelsi, M., Aouani, M. E., de Lajudie, P., and Mhamdi, R. (2009). The diversity of rhizobia nodulating chickpea (Cicer arietinum) under water deficiency as a source of more efficient inoculants. Soil Biol. Biochem. 41, 2568–2572. doi: 10.1016/j.soilbio.2009.09.020
Bhattacharyya, C., Banerjee, S., Acharya, U., Mitra, A., Mallick, I., Haldar, A., et al. (2020). Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 10. doi: 10.1038/s41598-020-72439-z
Bric, J. M., Bostock, R. M., and Silverstone, S. E. (1991). Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 57, 535–538. doi: 10.1128/aem.57.2.535-538.1991
Cappucino, J. C., and Sherman, N. (1992). “Nitrogen cycle,” in Microbiology: A Laboratory Manual. 4th Edn (New York, NY: Benjamin-Cumming Pub. Co.), 311–312.
Dworkin, M., and Foster, J. W. (1958). Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603. doi: 10.1128/jb.75.5.592-603.1958
Gontia-Mishra, I., Sapre, S., Sharma, A., and Tiwari, S. (2016). Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 18, 992–1000. doi: 10.1111/plb.12505
González, D., Blanco, C., Probanza, A., Jiménez, P. A., and Robas, M. (2021). Evaluation of the PGPR capacity of four bacterial strains and their mixtures, tested on Lupinus albus var. dorado seedlings, for the bioremediation of mercury-polluted soils. Processes 9, 1293. doi: 10.3390/pr9081293
Goswami, D., Thakker, J. N., and Dhandhukia, P. C. (2016). Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. 2, 1127500. doi: 10.1080/23311932.2015.1127500
Jaemsaeng, R., Jantasuriyarat, C., and Thamchaipenet, A. (2018). Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agric.Nat. Resour. 52, 330–334. doi: 10.1016/j.anres.2018.09.008
Kavita, K., Mishra, A., and Jha, B. (2011). Isolation and physico-chemical characterisation of extracellular polymeric substances produced by the marine bacterium Vibrio parahaemolyticus. Biofouling 27, 309–317. doi: 10.1080/08927014.2011.562605
Küçük, Ç., Kivanç, M., and Kinaci, E. (2006). Characterization of rhizobium Sp. isolated from bean. Turk. J. Biol. 30, 127–132. Available online at: https://journals.tubitak.gov.tr/biology/vol30/iss3/2
Kumar, P., Ggsss, Y., Jhirka, F., Yadava, R. K., Gollen, B., Kumar, S., et al. (2017). Nutritional contents and medicinal properties of wheat: a review genetic analysis for spike morphology and grain yield component traits in wheat view project approaches and strategies for empowering the youth of deprived region view project nutritional contents and medicinal properties of wheat: a review. Life Sci. Med. Res. 2011, 22.
Kumar, S., Sachdeva, S., Bhat, K. V., and Vats, S. (2018). Plant responses to drought stress: physiological. biochemical and molecular basis. Biotic Abiotic Stress Toler. Plants. 1–25. doi: 10.1007/978-981-10-9029-5_1
López-Hernández, J., García-Cárdenas, E., López-Bucio, J. S., Jiménez-Vázquez, K. R., de la Cruz, H. R., Ferrera-Rodríguez, O., et al. (2022). Screening of phosphate solubilization identifies six pseudomonas species with contrasting phytostimulation properties in arabidopsis seedlings. Microb. Ecol. 1, 1–15. doi: 10.1007/s00248-022-02080-y
Lugtenberg, B., and Kamilova, F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63, 541–556. doi: 10.1146/annurev.micro.62.081307.162918
Ma, Y., Dias, M. C., and Freitas, H. (2020). Drought, and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11, 1750. doi: 10.3389/fpls.2020.591911
Malik, A., and Kumar, A. (2020). Spatio-temporal trend analysis of rainfall using parametric and non-parametric tests: case study in Uttarakhand, India. Theor. Appl. Climatol. 140, 183–207. doi: 10.1007/s00704-019-03080-8
Meena, S. K., Rakshit, A., Singh, H. B., and Meena, V. S. (2017). Effect of nitrogen levels and seed bio-priming on root infection, growth and yield attributes of wheat in varied soil type. Biocatal. Agric. Biotechnol. 12, 172–178. doi: 10.1016/j.bcab.2017.10.006
Mukarram, M., Choudhary, S., Kurjak, D., Petek, A., and Khan, M. M. A. (2021). Drought: sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 172, 1291–1300. doi: 10.1111/ppl.13423
Nautiyal, C. S., Bhadauria, S., Kumar, P., Lal, H., Mondal, R., Verma, D., et al. (2000). Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 182, 291–296. doi: 10.1111/j.1574-6968.2000.tb08910.x
Penrose, D. M., and Glick, B. R. (2003). Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 118, 10–15. doi: 10.1034/j.1399-3054.2003.00086.x
Poursarebani, N., Nussbaumer, T., Šimková, H., Šafár, J., Witsenboer, H., van Oeveren, J., et al. (2014). Whole-genome profiling and shotgun sequencing delivers an anchored, gene-decorated, physical map assembly of bread wheat chromosome 6A. Plant J. 79, 334–347. doi: 10.1111/tpj.12550
Rockström, J., Barron, J., and Fox, P. (2009). Water productivity in rain-fed agriculture: challenges and opportunities for smallholder farmers in drought-prone tropical agroecosystems. Water Prod. Agric. Limits Opport. Improv. 145–162. doi: 10.1079/9780851996691.0145
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sandhya, V., Grover, A. S. Z., Reddy, M., and Venkateswarlu, G. (2009). Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol. Fertil. Soils 46, 17–26. doi: 10.1007/s00374-009-0401-z
Sarma, R. K., and Saikia, R. (2014). Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 377, 111–126. doi: 10.1007/s11104-013-1981-9
Sati, D., Joshi, T., Pandey, S. C., Pande, V., Mathpal, S., Chandra, S., et al. (2022a). Identification of putative elicitors from plant root exudates responsible for PsoR activation in plant-beneficial Pseudomonas spp. by docking and molecular dynamics simulation approaches to Decipher plant–microbe interaction. Front. Plant Sci. 13, 875494. doi: 10.3389/fpls.2022.875494
Sati, D., Pande, V., Pandey, S. C., and Samant, M. (2022b). Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. 2021, 1–19. doi: 10.1007/s42729-021-00724-5
Sati, D., Pandey, S. C., Pande, V., Upreti, S., Gouri, V., Joshi, T., et al. (2020). Toward an enhanced understanding of plant growth promoting microbes for sustainable agriculture. Recent Adv. Microb. Divers. 87–112. doi: 10.1016/B978-0-12-821265-3.00005-0
Schwyn, B., and Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Shivakumar, S., and Bhaktavatchalu, S. (2017). Role of plant growth-promoting rhizobacteria (PGPR) in the improvement of vegetable crop production under stress conditions. Microb. Strat. Veg. Prod. 81–97. doi: 10.1007/978-3-319-54401-4_4
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis Version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tsavkelova, E. A., Cherdyntseva, T. A., Botina, S. G., and Netrusov, A. I. (2007). Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 162, 69–76. doi: 10.1016/j.micres.2006.07.014
Wang, C., Zhao, D., Qi, G., Mao, Z., Hu, X., Du, B., et al. (2020). Effects of Bacillus velezensis FKM10 for promoting the growth of Malus hupehensis Rehd. and inhibiting Fusarium verticillioides. Front. Microbiol. 10, 2889. doi: 10.3389/fmicb.2019.02889
Zhang, C., Xie, Z., Wang, Q., Tang, M., Feng, S., Cai, H., et al. (2022). AquaCrop modeling to explore optimal irrigation of winter wheat for improving grain yield and water productivity. Agric. Water Manag. 266, 107580. doi: 10.1016/j.agwat.2022.107580
Keywords: plant growth-promoting rhizobacteria (PGPR), drought stress, PEG 6000, inoculation, wheat
Citation: Sati D, Pande V and Samant M (2023) Plant-beneficial Bacillus, Pseudomonas, and Staphylococcus spp. from Kumaon Himalayas and their drought tolerance response. Front. Sustain. Food Syst. 7:1085223. doi: 10.3389/fsufs.2023.1085223
Received: 31 October 2022; Accepted: 25 January 2023;
Published: 10 February 2023.
Edited by:
Abdelilah Meddich, Cadi Ayyad University, MoroccoReviewed by:
Raja Ben-Laouane, Cadi Ayyad University, MoroccoCopyright © 2023 Sati, Pande and Samant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mukesh Samant,  bXVrZXNoc2FtYW50QGdtYWlsLmNvbQ==
bXVrZXNoc2FtYW50QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.