
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 08 March 2023
Sec. Land, Livelihoods and Food Security
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1078314
This article is part of the Research Topic Socio-Economic Evaluation of Cropping Systems for Smallholder Farmers – Challenges and Options View all 13 articles
 Shinoj Parappurathu1*
Shinoj Parappurathu1* Muktha Menon1
Muktha Menon1 Charles Jeeva1
Charles Jeeva1 Johnson Belevendran1
Johnson Belevendran1 Anuraj Anirudhan1
Anuraj Anirudhan1 P. S. Swathi Lekshmi1
P. S. Swathi Lekshmi1 C. Ramachandran1
C. Ramachandran1 Shelton Padua1
Shelton Padua1 Natarajan Aswathy1
Natarajan Aswathy1 Shubhadeep Ghosh1
Shubhadeep Ghosh1 Divu Damodaran1
Divu Damodaran1 Sekar Megarajan1
Sekar Megarajan1 Geetha Rajamanickam2
Geetha Rajamanickam2 S. V. Vinuja1
S. V. Vinuja1 Boby Ignatius1
Boby Ignatius1 Suresh Vettath Raghavan1
Suresh Vettath Raghavan1 Ramani Narayanakumar1
Ramani Narayanakumar1 Achamveetil Gopalakrishnan1
Achamveetil Gopalakrishnan1 Prem Chand3
Prem Chand3This study undertakes a comprehensive assessment of selected mariculture enterprises in the coastal regions of India, centered on long-term sustainability as the key focus. This is juxtaposed against India's ambitious blue economy targets and policy thrust that pin on the expansion of mariculture as a promising avenue for enhancing marine fish production. Farm-level, region-specific, techno-economic, and socio-cultural factors associated with, and conditional on, sustainable intensification of mariculture-based production systems are examined in detail. The Principles-Criteria-Indicators (PCI) approach is used to establish the linkage between identified farm-level indicators and various dimensions of sustainability. While the selected enterprises were assessed to be technically and economically viable in general, glaring gaps were evident on key indicators of sustainability such as the legitimacy of access over water bodies, use of quality seed and feed, institutional credit access, market access, and fair marketing practices, optimal stocking density, mechanization, use of renewable energy, adoption of environmental-friendly culture practices, farm surveillance, crew safety, and social protection. This indicates the need for taking proactive measures to ensure the long-term sustainability of mariculture, particularly in the initial stages of establishment when such interventions are easy to adopt. Based on the insights obtained from the analysis, a broad set of strategies, policy options, and institutional interventions critical to scaling-up coastal mariculture enterprises along the east and west coasts of India are presented.
The significance of the “blue economy” as a paradigm toward furthering sustainable use of ocean resources for economic growth, improved livelihoods, and jobs while preserving the health of the ocean ecosystem has been widely accepted [(World Bank and United Nations Department of Economic and Social Affairs (UN-DESA), 2017)]. Capture fisheries, finfish, and bivalve mariculture constitute the main food-producing sectors in the ocean. They form the key components of the blue economy and account for about 17 percent of global edible meat production (Costello et al., 2020). Recent studies have indicated that, through the intensification of mariculture,1 aided by commensurate technological improvements and policy reforms, it is possible to enhance food from the sea by 21–44 million tons (36–74% increase compared to current yields) by 2050 (Edwards et al., 2019; Costello et al., 2020). There are already sufficient indications to suggest that commercial farming of marine fish and shellfish species, mainly those with high export demand, will take off at unprecedented levels across the globe. For instance, farming Atlantic salmon off Norway's coast has become a global business that generates US$ 18 billion in annual revenue. This industry operates at such high levels of economies of scale that offshore marine cages at Norwegian salmon farms often have a circumference of up to 157 m, enclosing water volume of ~40,000 m3, and hold up to 200,000 individual fish, wherein each farming crew is responsible for several million animals, amounting to the biomass of up to 15,000 tons (Fore et al., 2018). Similar accounts of success have been reported from other countries such as China, Chile, the United States, and Ireland, where mariculture has been established as a commercial industry. Technological breakthroughs in breeding for disease resistance and other traits using genetic and genomic interventions, which enable farmed fish species to grow roughly twice as fast as their wild counterparts, have made such remarkable success possible (Stockstad, 2020). Precision fish farming (PFF) techniques that help maneuver environmental parameters for optimal growth are catalyzing the transition (Fore et al., 2018; Wang et al., 2021). Besides, recent assessments of biological production potential for mariculture, despite being subject to substantial constraints based on existing ocean uses, indicate the availability of vast swathes of ocean space in nearly every coastal country which are suitable for future development (Gentry et al., 2017; Troell et al., 2017; Clawson et al., 2022).
Nevertheless, ensuring holistic sustainability in the pursuit of large-scale mariculture production enhancement, mainly when driven by commercial interests, is easier said than done. Previous experiences in the commercial expansion of the shrimp aquaculture industry around the world during the 1990s are ample testimony to the far-reaching negative economic externalities of expansionist policies that are unmindful of sustainability (Primavera, 1997; Arquitt et al., 2005; Belton and Little, 2008; Davies et al., 2019; Salunke et al., 2020). Such concerns are equally applicable in mariculture as well, and therefore, the global discourse on the prospects of mariculture as a sustainable food production industry continues to remain contested. Against the worldwide euphoria driven by a recent wave of literature that promotes mariculture as a “technological and spatial fix” for apparent constraints to terrestrial food production, some studies warn of the potential adverse impacts that touch upon environmental, economic, and social dimensions of sustainability. Belton et al. (2020) note that much of the literature that projects the future mariculture potential is empirically contestable, and, neglects the potential chances of appropriation of ocean space to benefit extractive industries and conservation interests through the extension of private property rights. Though broadly optimistic about the future promises of marine aquaculture, Gentry et al. (2017) stressed that future intensification in mariculture systems would be conditional upon several market and governance-related factors. Such concerns are even more relevant in developing coastal economies where a large contingent of small-scale fishers depend on marine fisheries for their livelihood. Significant capital investments for mariculture development as part of “blue economy” and “blue growth” narratives without due concern for sustainability, equity; small scale fishers' access rights, and participation could possibly jeopardize economic stability in coastal regions (Bavinck et al., 2017; Cohen et al., 2019). Moreover, the technical, logistical, and market-related pre-conditions necessary to backstop mariculture in its initial development phases are of particular relevance.
Mariculture along the shallow marine, and internal waters2 has been a priority for fisheries development in India over the last decade. India's National Policy on Marine Fisheries, 2017 states that mariculture can play an essential role in increasing fish production from marine and coastal waters and that the Government of India will support addressing the institutional and commercial needs of this emerging sector [(Government of India (GoI), 2017)]. Presently, mariculture is predominantly small-holder-centric in India. With steady technological advancements and faster adoption among the small-scale fisher community, supposedly, there is potential for sustainable intensification (SI) of farming operations in India's coastal regions. Some promising enterprises for possible scale-up include open sea and ‘coastal water3' cage farming of fin fishes and shell fishes, seaweed farming, and integrated multi-trophic aquaculture (IMTA), among others (Gopalakrishnan et al., 2017; Parappurathu et al., 2017). The Government of India has recently floated ambitious programs to support such farming ventures, intending to provide the necessary logistics, funding, and policy support [(National Fisheries Development Board (NFDB), 2018)]. However, the success of such programs depends to a large extent on a clear understanding of the suitability of each of the above aquaculture technologies to match the specific socio-economic and demographic features of the farming community involved and the general status of the markets and institutions in the region of interest. Notably, the quality of resource endowments, entrepreneurial readiness and capital availability at the farm level, farming skills and technical prowess, the general willingness of farmers to embrace sustainable practices, community knowledge capital, and backward and forward linkages to the input and product markets, and value chain integration are important determinants (Bostock et al., 2010; Little et al., 2013; Buck and Langan, 2017). The emergence of new successful farm enterprises often results in significant changes in the rural economy, leading to mushrooming of several mutually complementary allied enterprises. Assimilation and integration of these economic units into the diversified coastal economy are equally important to positively change people's lives (Grealis et al., 2017; Seung and Kim, 2020).
Given the above context, this paper undertakes a comprehensive assessment of farm-centric and region-specific factors associated with, and conditional upon, sustainable intensification of selected mariculture enterprises in India. To set the stage, the following section deals with the potential of India's marine and coastal water aquaculture, and the efforts made so far to explore it. Further, a critical assessment of the techno-economic performance of selected mariculture enterprises and their level of alignment with key indicators of sustainable intensification is carried out based on primary surveys conducted across selected locations along India's east and west coasts. Besides, various constraints faced by mariculture entrepreneurs are discussed, and relevant technological and policy interventions to develop the sector are put forth.
The earliest known attempt at the culture of marine fish species in India was made during 1958–1959 with the farming of milkfish, Chanos chanos (Gopakumar et al., 2007). Subsequently, in the 1970s, farming trials were conducted to standardize the culture of green mussels (Perna viridis) and brown mussels (P. indica) using the rack method, long line method, and raft methods (Qasim et al., 1977; Appukuttan, 1980; Kuriakose, 1980). The culture of pearl oysters (Pinctada fucata and P. margaritifera) was also attempted along the coasts of Tamil Nadu (Alagarswami, 1974). Other budding attempts toward mariculture include seaweed culture experiments initiated for the first time in Gujarat in 1964 (Thivy, 1964), followed by farming trials and commercial exploitation along the southeast coast of Tamil Nadu for agar and algin production (Silas and Kalimuthu, 1987). Presently, mariculture in India constitutes capture and hatchery-based fin-fish and shell-fish culture, which include cage culture (in the open sea and internal waters), bivalve culture; aquaculture systems such as seaweed culture, pearl, and oyster culture, as well as ornamental fish culture. Nevertheless, the current mariculture production in India is negligible at <0.1 million tons (mt) in relation to the projected potential of 4–8 mt (Jena et al., 2022). A brief account of the status of mariculture enterprises such as cage farming, seaweed farming, and IMTA in India, which constitute key focus areas of this paper, is provided below.
Attempts on open sea cage culture were initiated in the mid-2000s with Asian sea bass (Lates calcarifer), which led to locally adapted innovations in the designing and fabrication of cages and mooring systems, standardized guidelines and farming practices, as well as the development of breeding, larval production, and grow-out technologies for several prioritized marine fin fish species (Rao et al., 2013; Ayyappan et al., 2015). So far, the ICAR-Central Marine Fisheries Research Institute (CMFRI), India, has standardized techniques for breeding and seed production, including nursery protocols for Cobia (Rachycentron canadum), Orange-spotted grouper (Epinephelus coioides), Silver pompano (Trachinotus blochii), Indian pompano (T. mookalee), Pink-ear sea bream (Lethrinus lentjan), banded grunter (Pomadasys furcatus), John's snapper (Lutjanus johnii), Vermiculated spine foot (Siganus vermiculatus) and picnic seabream (Acanthopagrus berda) (Gopalakrishnan et al., 2019; Anuraj et al., 2021; Suresh Babu et al., 2022). The culture technology for Asian Seabass was standardized by the ICAR-Central Institute of Brackishwater Aquaculture (CIBA) (Arasu et al., 2009). Apart from the above, a recent publication from ICAR-CMFRI has prioritized 76 finfish and shellfish species that could be targeted for future expansion of mariculture production in the country (Ranjan et al., 2017). Most of these technologies have either been transferred or are at various stages of farm-level demonstrations. The major candidate species used in coastal water cage farming include Asian sea bass, Silver pompano, Indian pompano, mullets (Mugil cephalus), milkfish (C. chanos), pearl spot (Etroplus suratensis), and Genetically Improved Farmed Tilapia (GIFT) (Oreochromis niloticus). Currently, cage farming has been reported to be economically viable. It is spreading rapidly along both coasts of the country, aided by the adoption of the technology by small-scale farmer entrepreneurs, self-help groups, and fisher societies (Gopalakrishnan et al., 2019; Aswathy et al., 2020; Jena et al., 2022).
Seaweed farming has been identified as one of the diversified-livelihood options for coastal fishers in India. However, enabling factors for significant commercial expansion and holistic development of allied industries are yet to take shape in the country (Johnson et al., 2017, 2020). Past studies (Kaladharan et al., 1996; Kaliaperumal and Kalimuthu, 1997; Rao and Mantri, 2006) have identified several commercially important seaweed species, which include red algae species such as Gracilaria edulis, Gelidiella acerosa, and Kappaphycus alvarezii and brown algae species such as Sargassum wightii, Turbinaria conoides, and Cystoseira spp. A number of farming techniques using floating rafts, net-tubes, long-lines, and fin fish-stocked cage-based IMTA systems have been standardized for seaweed culture. Recent literature indicated that farming of seaweed species including K. alvarezii and G. acerosa, and Gracilaria spp. is economically profitable as well as livelihood enhancing, thereby suitable for commercial scale-up (Mantri et al., 2022a,b). Moreover, the demand for seaweeds has been on the rise due to recent innovations involving their use in the production of secondary bioactive metabolite-based nutraceuticals, plant growth promoters, and fertilizers, besides their traditional industrial applications (Chakraborty et al., 2018; Cotas et al., 2020; Gopalakrishnan et al., 2020). A recent study by Johnson et al. (2020) identified a potential area of 23,970 ha suitable for seaweed cultivation along India's shallow coastal waters using a combination of a primary survey approach as well as a GIS-based site suitability model (Divu et al., 2020). The study took into account significant variations in geomorphology and demography, as well as a broad array of desirable biological and environmental parameters along the coastline. Presently, seaweed farming is practiced on a limited scale by several hundreds of farmers' groups along the Palk bay areas of Tamil Nadu supported by the carrageenan, agar, and seaweed-based fertilizer industries located in neighboring areas. Earlier, the farming of K. alvarezii experienced a boom during 2000–2013 when the local fishers along the coasts of Tamil Nadu, Gujarat, and Odisha entered into a contractual farming arrangement with PepsiCo India Holdings Ltd. followed by Aqua Agri Processing Pvt. Ltd. for carrageenan production. However, this was short-lived due to many biophysical and economic constraints (Krishnan and Narayanakumar, 2013; Johnson and Ignatius, 2020). Nevertheless, the sector is re-entering into a phase of renewed development owing to considerable policy thrust and technological and logistical advancements in recent times.
Integrated Multi-trophic Aquaculture (IMTA) is another novel mariculture practice that has been gaining momentum in recent years with its bio-mitigation potential, complementarity functions in the ecosystem, besides economic potential (Chopin et al., 2008; Barrington et al., 2009). In India, recent integrated trials carried out by ICAR-CMFRI in the Palk Bay area of Tamil Nadu involving cobia in marine cages with K. alvarezii in floating rafts set around the cage have shown encouraging results (Johnson et al., 2021). Similar trials involving different combinations of mullets (M. cephalus and Liza parsia), milkfish (Chanos chanos), pearl spot (E. suratensis), and shrimp (Penaeus monodon, P. indicus) as fed species, together with oyster (Crassostrea cuttackensis, C. madrasensis) and seaweed (Enteromorpha spp.) as extractive species were evaluated as viable aquaculture options in brackishwater ecosystems of the Indian Sundarban areas of West Bengal and Sindhudurg district of Maharashtra (Balasubramanian et al., 2018; Biswas et al., 2019). Recognizing the potential, fishers from Palk Bay and other parts of the southwest coast of India have started practicing IMTA-based farming operations in recent times (Johnson et al., 2021).
The research and development activities of mariculture in India are spearheaded mainly through public institutions and agencies functioning under the State and Union governments of India. Research on the development of culture technologies and allied areas are being led by institutions such as ICAR-CMFRI, Kochi; ICAR-CIBA, Chennai; Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Bhavnagar; National Centre for Sustainable Coastal Management (NCSCM), and National Institute of Ocean Technology (NIOT), Chennai. The initial stages of research were guided by isolated attempts on project mode limited to individual research institutes and Universities. Recently, coordinated research focus was brought about through network projects such as the “All India Network Project on Mariculture” of the Indian Council of Agricultural Research (ICAR) and other inter-institutional collaborative research efforts involving NCSCM, CSIR-CSMCRI, and State Universities. Additionally, development efforts in the form of training, funding as well as logistical support by Government organizations such as the National Fisheries Development Board (NFDB), Hyderabad, and the Marine Products Export Development Authority (MPEDA), Kochi have also contributed significantly to popularizing mariculture among the fisher folk and fish farmers. Most of the developmental programs are presently being supported by budgetary allocations under the Pradhan Mantri Matsya Sampada Yojana (PMMSY), a flagship scheme of the Union Government for fisheries development. A draft National Mariculture Policy was prepared in 2019 consequent to the constitution of an expert committee by the NFDB. The draft policy, which identified thrust areas for development and underlying policy imperatives, was subsequently incorporated as a part of the “National Fisheries Policy 2020,” which is due to be notified by the Government of India and will eventually supersede all other existing policy documents in fisheries and allied sectors. Apart from this, various maritime state governments are in the process of firming-up separate state-level policies to expedite mariculture development at the grassroots level. The Government of Goa notified “Goa State Mariculture Policy 2020” in June 2022, which is the first of its kind in the country.
The concept of sustainable intensification (SI) in aquaculture production systems aims to attain at least one of the following objectives, viz., (1) improved production and resource use efficiency, w.r.t. land, water, feed, and energy; (2) enhanced environmental benefits; (3) strengthened economic viability and farmers' resilience; and (4) improved social acceptance and equality and, to not compromise on the rest (FAO, 2016). The concept has its roots in African small-holder agriculture (Pretty, 1997) and summarily deals with producing more for less while minimizing negative environmental impacts and optimizing societal benefits (Little et al., 2018). Most of the literature on SI in aquaculture is empirical, wherein improvements in resource use efficiency and the contribution of specific technologies toward SI are measured using a set of objectively measurable indicators. These generally cut across various domains such as productivity, nutrition, economic viability/feasibility, human and animal wellbeing, environmental sustainability, biodiversity conservation, and social acceptability (Garnett et al., 2013; Smith et al., 2017) (Figure 1). On the other hand, a few recent studies have adopted life cycle assessment (LCA) as a framework for evaluating SI by covering the multiple impact pathways along the entire production chains (Cao et al., 2011; Henriksson et al., 2018).
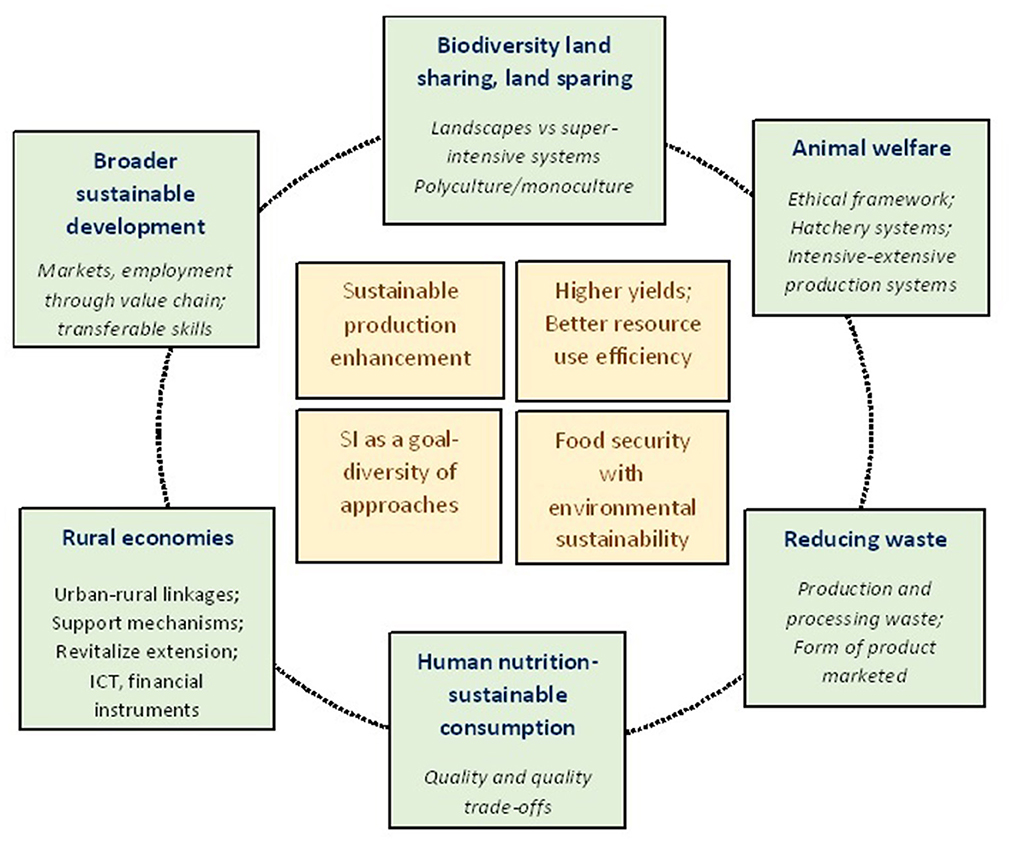
Figure 1. Main pillars of sustainable intensification (adapted from Garnett et al., 2013; Little et al., 2018).
This paper follows the basic premises and principles related to the concept of SI to assess the level of readiness of the mariculture production system in India to embark on an intensification pathway that is consistent with economic, environmental/ecological, and social dimensions of sustainability and is tuned to the country's larger commitments to ensure “sustainable blue growth.” The study is novel in proposing a suitable conceptual and methodological framework for SI assessment of small-holder mariculture enterprises and one of such early attempts in the country.
The study mainly encompasses the emerging mariculture hotspots in the country and covers five out of ten maritime states, namely, Tamil Nadu and Andhra Pradesh along the east coast; Kerala, Karnataka, and Gujarat, on the west coast of India. Apart from these, data were also collected from Diu, a Union Territory (UT) enveloped by the state of Gujarat. The specific locations are depicted in Figure 2, and their coverage in the primary surveys pertaining to various mariculture enterprises is presented in Table A1. The selection of locations for the survey was driven by predetermined criteria that include: (i) a reasonably high presence of operating mariculture units, practicing any one, or more, of the selected enterprises covered in the study, (ii) the presence of auxiliary enterprises such as seed production centers/hatcheries, fish markets, processing units, etc. in nearby locations, and (iii) known linkages of the entrepreneurs with research and development institutions and agencies dealing with marine/coastal aquaculture. Such criteria were imposed to ensure that the multiple dimensions associated with viable and sustainable mariculture, including social and institutional pre-conditions and forward and backward integration vis-a-vis fish input and product value chain nodes, can be adequately understood.
As noted above, the study was primarily inspired by the necessity to document and describe the techno-economic, techno-environmental and social dimensions associated with the proposed “blue economy” targets set by the Government of India, which envisages SI of mariculture in the near future [(Government of India (GoI), 2019)]. In this context, the “techno-economic” dimension signifies an integrated assessment of the technological performance and economic feasibility of a production system, process, or value chain to identify the underlying parameters for investment and resource allocation decisions (Kobos et al., 2020). On the other hand, the “techno-environmental” dimension relates to the trade-offs between technological interventions and associated environmental/ecological parameters for viable and sustainable management of a production system (Wan and Liu, 2010; Cossutta et al., 2022). Toward this objective, the data collection using primary sample surveys was closely guided by the SI framework and based on the detailed mapping of various mariculture facilities and associated stakeholders along the east and west coasts. The first phase of this process mainly involved in-depth discussions with scientists and practitioners related to mariculture research and development activities in each identified location. Subsequently, a set of semi-structured questionnaires were developed, specific to each identified enterprise, which was subsequently pretested and fine-tuned to suit location-specific and contextual variables. As the study covered a wide range of topics, languages, and diverse societal conventions, the data collection was facilitated by identifying enumerators from respective study locations. This process was relatively easier for the present study, as the author-investigators are attached to various research stations/centers of ICAR-CMFRI/ICAR-CIBA located on either coast and were in constant contact with the key informants in respective locations as part of their routine research activities. As such, no focus group discussions (FDGs) were carried out; nevertheless, the findings obtained from the questionnaire-based field data were later triangulated with key informants and experts (scientific and technical staff working in the study locations, State Department officials, etc.) for validation. The questionnaires used for the primary survey are annexed as Supplementary material. The selected mariculture enterprises include (i) open sea cage farming, (ii) coastal water cage farming, (iii) IMTA, and (iv) seaweed farming. Even though farming of bivalves, mainly green mussel, is another enterprise with immense potential for sustainable intensification in India (Mohamed et al., 2016), this study did not consider including it. Such a choice was primarily driven by the fact that the hotspots of green mussel farming in the northern region of Kerala are presently experiencing a phase of decline due to several biotic and abiotic factors (Parappurathu et al., 2021) and hence an SI assessment involving it could yield potentially biased results. The surveys were administered by randomly selecting farm units in purposively selected coastal regions where mariculture has been established as an alternative livelihood option in the recent past. The sample units were randomized by obtaining a list of farm units operating in each location and randomly assigning them for the surveys. However, in areas where the investigators had relatively less prior access, the enumerators were entrusted to bring about randomization to the best of their judgment. Further, care was taken to capture the diversity in farmed species and culture practices across the sample farms in a given location by following the broad principles of stratification (though no formal stratified sampling methods were adopted). The respondents were either the owner-farmer or the farm manager responsible for the day-to-day activities of the farm units. Many of the units covered in the sample, especially those from Tamil Nadu and Andhra Pradesh were operating either with the financial support of the respective State Fisheries Departments or funded by central agencies such as NFDB. In certain farms, the capital expenditure for the fabrication of the cages was met by ICAR-CMFRI as part of its extended research trials. The specific details of the sample units covered in each identified location are presented in Table A1. Apart from the above, secondary data were gathered from various sources, including published literature, online sources, and other databases, to facilitate an objective assessment of the present status of mariculture and potential future scenarios.
The data collected at the farm level include both qualitative and quantitative variables. To establish a linkage between various dimensions of sustainability with the above variables, different sets of farm-level indicators were constructed by broadly following the Principles-Criteria-Indicators (PCI) framework as proposed by Rey-Valette et al. (2008, 2010). The PCI approach establishes a cascading relationship between principles (which express the values and issues of sustainability), criteria (variables that are most appropriate to express these principles), and indicators (variables to be measured). This approach was followed by past studies such as FAO (2011), Fezzardi et al. (2013), and Valenti et al. (2018) under varying contexts. The above framework summarily draws from various national and international standards, reference materials, and recommendations for realizing SI of aquaculture (Pretty, 1997; European Commission, 2001; Parris and Kates, 2003; Liu and Ou, 2007). However, to use in this study, context-specific deviations were made to suit location-/enterprise-specific realities without compromising on the core ideas of the approach. Table 1 presents the key dimensions, criteria, and indicators used to assess the present level of economic viability, environmental sustainability, and social acceptability of the mariculture enterprises besides their future orientation for SI. Further, the extent to which the sample respondents are willing to adopt standard sustainable farming practices as well as risk-proofing mechanisms on-farm (Rey-Valette et al., 2008, 2010; Valenti et al., 2018; Carballeira Braña et al., 2021), over and above the existing level is assessed using their responses to a set of selected questions on a five-point Likert scale (Table 2). Most of the estimations pertaining to the techno-economic, techno-environmental, and social indicators of sustainability were performed using Microsoft Excel. However, statistical tests to assess the level of internal consistency (Cronbach's alpha) and the level of significance across parameters relating to willingness for sustainable farming and risk proofing (Kruskal-Wallis test), were carried out using STATA software, version 14.
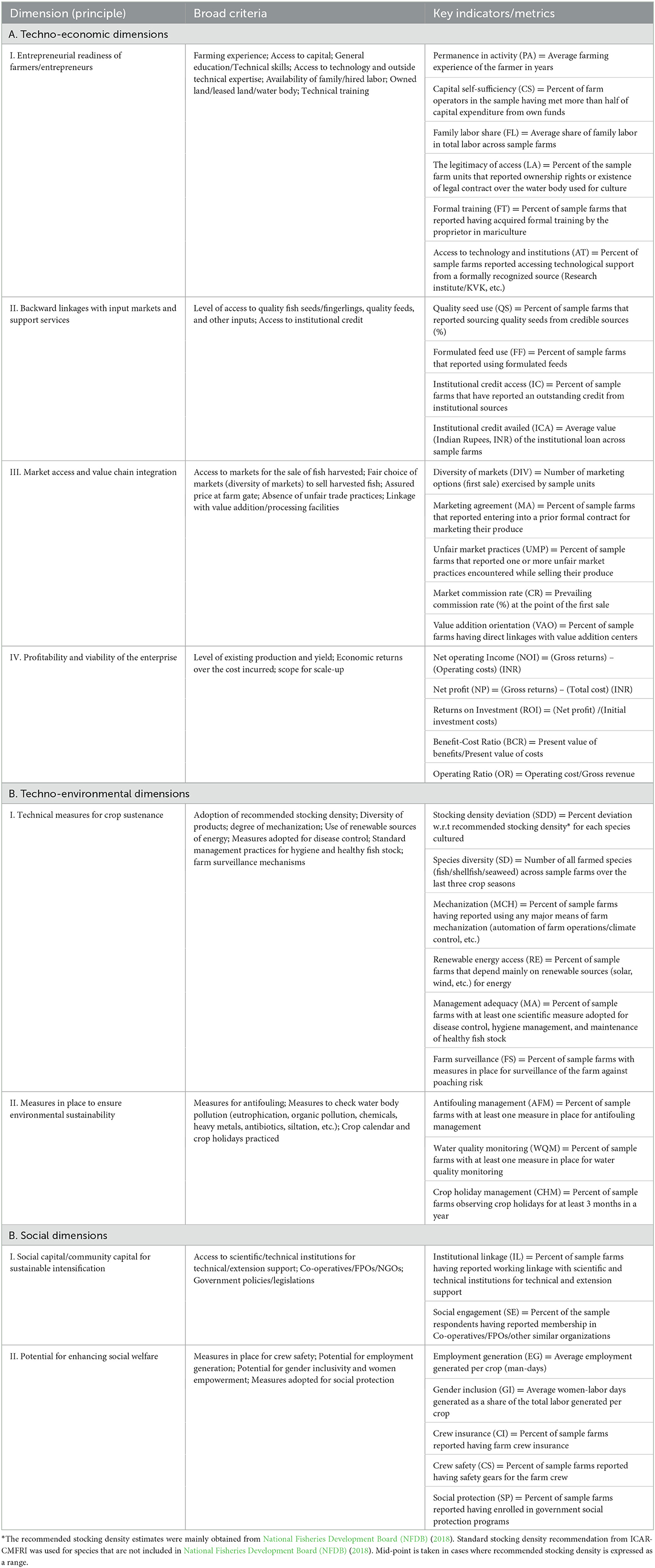
Table 1. Summary of key dimensions, criteria, and indicators as per PCI approach to assess the level of sustainability associated with selected mariculture enterprises in sample locations in India, 2022.
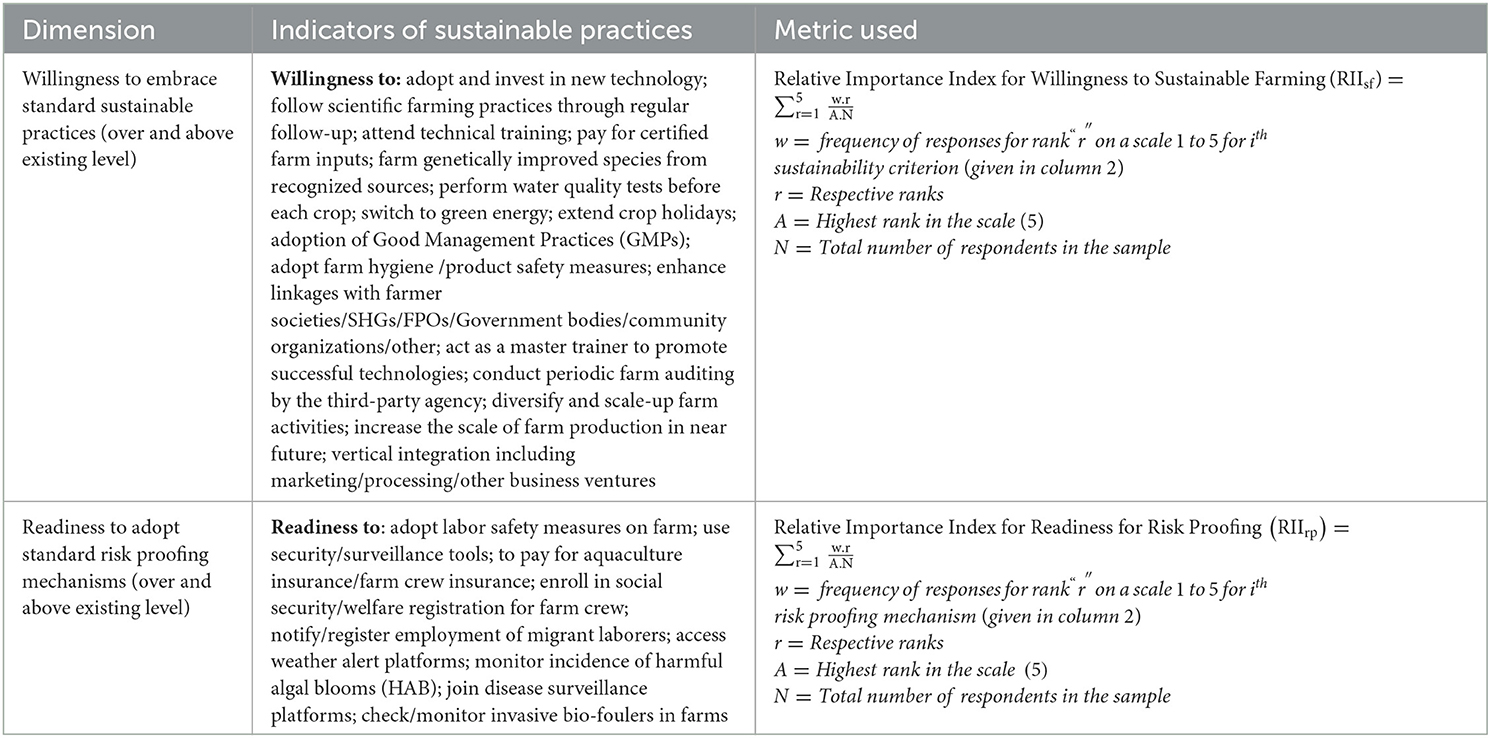
Table 2. Summary of key dimensions, criteria, and indicators as per PCI approach followed to assess future readiness of sample respondents for sustainable intensification of mariculture.
The sample farms belonging to each of the selected enterprises from respective locations were assessed and characterized based on a set of identified features and farming practices followed. These include the type, make, and size of the culture units, the average number of units per farm, the location of the farm in terms of depth of water and distance from the shore, major species farmed, average stocking density, type of the seeds, crop duration, feed type, feeding rate and Feed Conversion Ratio (FCR), measures in place for aeration management, antifouling, and disease management, as well harvest and yield particulars for the sample time frame. Such general features of farming, along with some specific details w.r.t. two major species cultured by the sample farms are presented in Table 3.
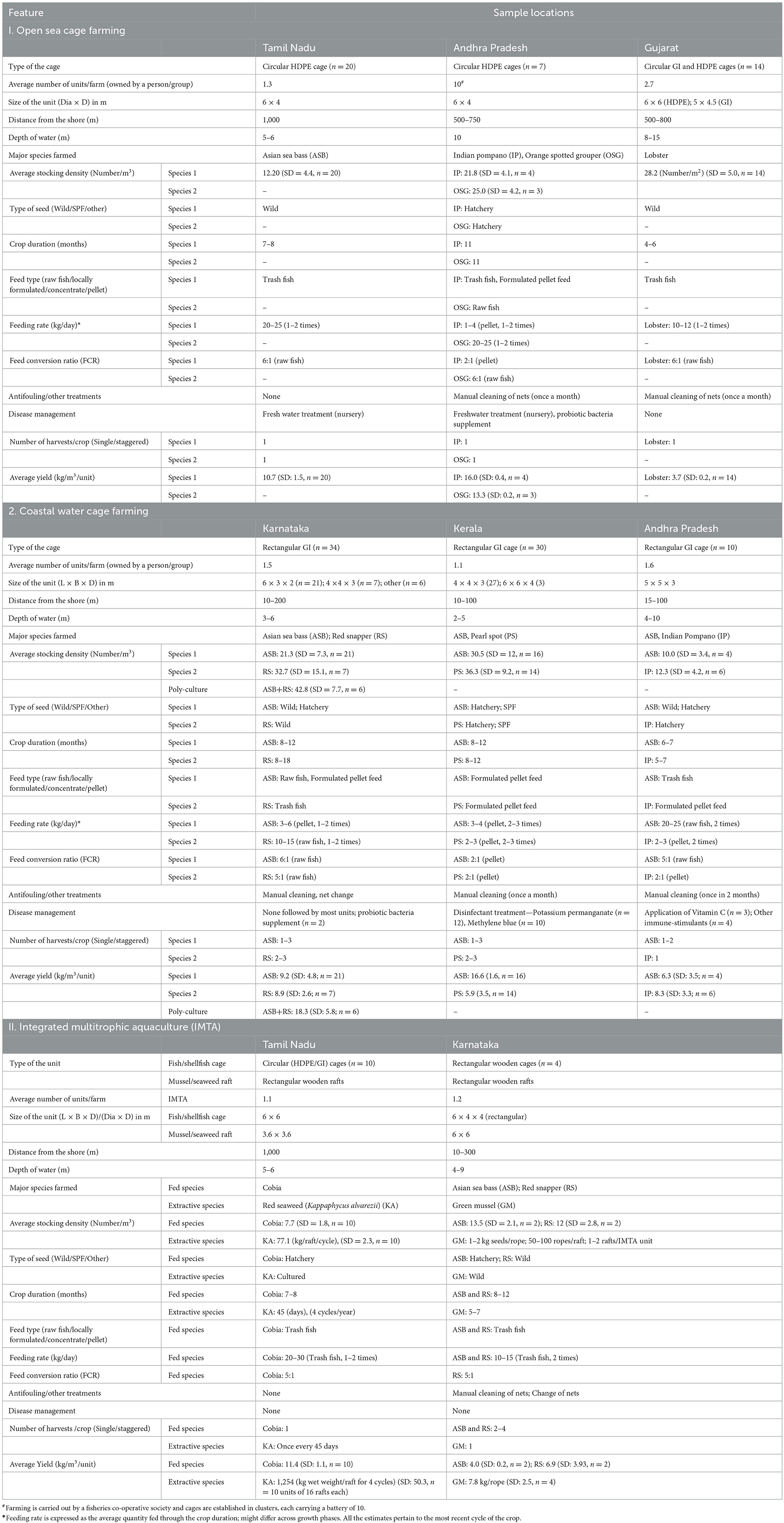
Table 3. General features of sample farms practicing mariculture in the selected coastal regions of India.
Most of the open sea cage farms were operated by small-scale fishers, consisting of 1–2 units by taking one crop per year. As an exception, a few farmers in Gujarat, Kerala, and Andhra Pradesh owned and operated a battery of 4–10 cages. The farm units were located in groups in suitable areas with low tidal activity, mostly within a distance of 1 kilometer from the shore where the depth was 10–15 m. Sea cage farming in the sample locations in the states of Tamil Nadu, Andhra Pradesh, and Gujarat was mainly carried out in circular marine cages made of high-density polyethylene (HDPE) or galvanized iron (GI) of 6-m diameter and 4-m depth (113 m3). These are cages originally designed and popularized by ICAR-CMFRI in the late 2000s and improved upon subsequently. All the sample farms in Tamil Nadu cultured Asian Sea bass sourced from the wild for 7–8 months, whereas those in Visakhapatnam of Andhra Pradesh state grew Indian pompano and Orange-spotted grouper sourced from hatcheries for an 11-month culture duration. The spiny lobster (Panulirus homarus) was grown for a relatively shorter duration of 4–6 months along the coasts of Gujarat and Diu. The stocking density adopted by farms varied from 9 to 10 fish seeds/m3 for Asian Sea bass; 20 to 25 seeds/ m3 for Indian Pompano and Orange-spotted grouper; 12 to 15 seeds/ m3 for Cobia and 20 to 30 seeds/ m2 for lobster (the observed average stocking density values are presented in Table 3). The seeds required for cage culture are either collected from the wild or sourced directly from private and public hatcheries. Public hatcheries are mainly operated by research institutions such as ICAR-CMFRI, ICAR-CIBA, Rajiv Gandhi Centre for Aquaculture (RGCA), and other state-funded agencies. Certain government agencies such as NFDB through their network of Aqua One Centres and State-level aquaculture development agencies also provide subsidized seeds sourced from certified hatcheries. Variations in feeding practices across farms were noted, including both raw fish and artificial pellet feeds with varying FCRs, depending on the farmed species. The raw fish fed were mostly low-value trash fish which are less preferred for human consumption and obtained either through fishing trips as by-catch or sourced from the local market at cheap prices. Some farmers also reported having cultured mono-sex tilapia and other similar fast-proliferating species for feed purposes. Most farmers followed a mixed feeding regime, i.e., formulated pellet feed in the initial phases of the crop, which raw fish gradually substitute in advanced growth stages. The feeding rate varied depending on the feed type and the phase of the crop. Crop management in terms of aeration, anti-fouling treatments, and disease mitigation was rather weak in most of the sampled locations. Manual cleaning of nets was carried out at regular intervals by a select few farmers in Andhra Pradesh to control fouling. Similarly, as a disease management measure, only a few farmers from Andhra Pradesh have reported using probiotic bacterial consortia as a prophylactic measure to check disease incidence. Staggered harvesting of the mature crop was not common, and most farms carried out harvesting at a single stretch. Yields varied widely depending on the location and the species cultured, with the highest being 16 kg/m3, reported by the sample farmers culturing Indian Pompano in Vishakhapatnam (Table 3).
Compared to open sea cages, coastal water cages were smaller in size and rectangular in shape, generally made of galvanized iron (GI). However, isolated cages made of bamboo wood were also observed in certain regions. Their dimensions varied from case to case, but were generally within 75 m3 in volume. As in the case of sea cage farmers, coastal water cage farmers were also smallholders, each operating 1–2 units, taking one crop of 6–12 months. The farms were mostly located in internal backwater areas or estuaries, very close to the shore (15–200 m), where the water depth ranged from 2 to 10 m. These farms are generally owned and managed by small families mainly using domestic labor. The Asian sea bass was the most preferred species across all sample locations, but other amenable species such as red snapper, silver pompano, and Indian pompano are also being cultured. In certain locations, brackishwater species such as pearl spot and mullets were also cultured along with the above species. However, the sample farms did not report any of them as major farmed species, except in Ernakulam and Alappuzha districts of Kerala, where farmers grew pearl spot either as the main crop or in the outer nets of the cages (100–200 seeds/cage) to minimize biofouling. Greater variations in stocking density were observed across sample locations, which ranged from 7 to 40 seeds/m3 for Asian Sea bass; 10 to 40 seeds/m3 for red snapper; 30 to 80 seeds/m3 for silver pompano and 8 to 10 seeds/m3 for Indian pompano. In Karnataka, where polyculture using Asian sea bass and red snapper are practiced, bigger seeds (of about 200 g) were stocked to address the problem of cannibalism. Variations in feeding practices across farms were also noted, with most of them using raw fish 1–2 times a day with FCR ranging from 6:1 to 7:1 depending on the farmed species and the type of raw fish used. Formulated pellet feed was used for feeding hatchery-based Asian sea bass grown in the sample farms of Karnataka and Kerala and Indian pompano and silver pompano, respectively in Kerala and Andhra Pradesh. In many cases, a combination of raw fish and pellet feeds was used depending on the growth phase of the crop at varying feeding rates. Owing to the proximity of the cages from the shore, the coastal water cage farmers were seen to follow better crop management practices than the sea cage farmers. Manual cleaning and regular changing of nets were common practices to minimize fouling of the cages. Most farmers in Kerala and Andhra Pradesh applied chemicals such as potassium permanganate, methylene blue, vitamin C, etc. to reduce the incidence of diseases. A limited number of farmers in Karnataka and Andhra Pradesh (two each) reported using probiotic bacterial consortia as a prophylactic measure to minimize disease incidence in their cages. The usage of antibiotics was not reported in any of the farms, except for random instances of applying them by a couple of backwater cage farmers. The sample farmers generally harvested grown fish 1–2 times per crop. However, a few reported having practiced staggered farming (3–5 times) extended over a period, either as a measure to capitalize on price variations or as a distress measure to meet operating costs. As in the case of marine cage farms, crop yields varied widely, and ranged from 8 to 16 kg/m3 on average across sample locations and farmed fish species. The highest yield (18.3 kg/m3 on average) was reported by farmers practicing polyculture of Asian sea bass and red snapper for an extended crop duration ranging from 8 to 18 months in the Karnataka state (Table 3).
The IMTA farms covered in the study were of two types: (i) open sea cage farming of cobia integrated with red seaweed (K. alvarezii) in the Mandapam region of Tamil Nadu state and (ii) coastal water cage farming of Asian sea bass and red snapper integrated with green mussel in the Byndoor region of Karnataka state. In the former case, each unit consisted of one HDPE circular cage, encircled by about 16 seaweed rafts nearby. The units were located about 1 km from the shore at a water depth of 5–6 m. The cages were stocked with cobia seeds sourced mainly from hatcheries at an average density of 5–6/m3 and maintained for 7–8 months by feeding raw fish (FCR: 7:1). Seaweeds were raised in rectangular rafts in four cycles of 45 days each during a cropping season. The respondent farmers practicing this system reported having realized an average yield of 11.4 kg/m3 of cobia and 1,254 kg K. alvarezii per raft after harvest of the crop. The coastal water IMTA units were located very close to the shore and each unit, consisted of one rectangular cage surrounded by 1–2 green mussel rafts. Each raft carried 50–100 seeded ropes suspended into the water body. The crop duration ranged from 8 to 12 months for the fed species and 5–7 months for the extractive species (green mussel). The fish in the cage was fed with raw trash fish with an FCR of 5:1. At the end of the harvest season, the average fish yield realized by the sample farmers was 4.0 kg/m3 in the case of Asian sea bass and 6.9 kg/m3 for red snapper. The average green mussel yield recorded for the four sample units was 7.8 kg/rope with a standard deviation of 2.5. In either type of IMTA unit, the farmers did not report any notable aeration, anti-fouling, or disease management approaches being followed (Table 3).
As noted earlier, the sample seaweed farms were mainly located in adjoining areas along the Mandapam and Rameswaram coasts of Tamil Nadu. They were operated primarily by women-centric self-help groups (SHGs) or independent smallholder farm families. All farmers grew K. alvarezii, the red seaweed species in floating bamboo rafts of 3.6 × 3.6 dimension at a distance of 10–30 m from the shore. Each operator owned 10–20 rafts and raised 5–6 cycles of the crop for 45 days a year. About 50–60 kg of planting material from previous crops was used to stock each raft. The farmers generally did not follow any management practices to prevent fouling or disease incidence. The respondents reported that grazing seaweeds by fish and other aquatic species was a major problem. An average wet yield of 1,177 kg/raft (SD: 104, n = 30) was obtained per raft per year from the sample units, which translates to 14.0 tons of wet yield per farm unit per year (SD: 4,542, n = 30) on average.
Techno-economic viability of aquaculture units depends mainly on farm-level factors, the local economy's degree of openness, and general economic development status (Boyd et al., 2020). The estimates of techno-economic indicators in respect of the sample farms are presented in Table 4. Among all, experience in mariculture (permanence in activity, PA) was highest for cage farmers operating in the Vishakhapatnam region of Andhra Pradesh state (11.4 years on average), followed by IMTA farmers in Karnataka (8.8 years) and seaweed farmers in Tamil Nadu (7.8 years). Indicators for self-sufficiency in capital and labor, which are important determinants of economic viability in smallholder farms, showed mixed results. Legitimacy of access (LA), which indicates whether a farm possesses legal farming rights over the water body, was reported in the coastal waters of Karnataka and some parts of Kerala only. In other locations, farming was taken up without any authorization from the government agencies concerned. The majority of the respondents across states reported having acquired necessary technological inputs and formal training from recognized sources such as research institutes, Krishi Vigyan Kendras (KVK), or other state government agencies. Similarly, access to quality seeds was fairly good in most locations except in Gujarat and Karnataka, where <50% of farmers only received good quality seeds for culture. On the other hand, the use of formulated feeds depended upon the species cultured and the level of market access to feeds in adequate quantities. Survey data suggested that, while most of the farmers in Andhra Pradesh and Kerala and some of them (23.5%) in Karnataka fed formulated pellet feeds to standing fish stock, others relied on low-value trash fish for the purpose. Access to institutional credit to meet capital and operational expenses were reported to be a major limiting factor for mariculture farmers across the board, except for a few, in the states of Karnataka and Kerala. The harvested fish was sold mostly in local markets as indicated by the indicators, Diversity of Markets (DIV). The coastal water farmers in Karnataka reported having access to up to five domestic market formats, whereas most others depended on farm gate and wholesale only. Prior marketing contract for fish was reported only by 20 percent of the coastal water farmers in Tamil Nadu while a few respondents in Andhra Pradesh and Karnataka incurred commission charges (ranging from 3.5 to 7.0%) for the first sale of fish to the local market agents. Notably, a significant proportion of sample farmers engaged in coastal water cage farming, IMTA, and seaweed farming in Karnataka, Kerala, Andhra Pradesh, and Tamil Nadu reported having encountered various forms of unfair market practices such as under-pricing, weight manipulation, and excess market commission.

Table 4. Estimated sustainability indicators associated with selected mariculture enterprises in sample locations in India, 2022.
The results on financial viability and profitability of sample farms reflected through indicators such as net profit (NP), net operating income (NOI), returns on investment (ROI), benefit-cost ratio (BCR), and operating ratio (OR) are presented in Figures 3, 4. While Figure 3 indicates the absolute level of profitability adjusted for costs of the farm units, Figure 4 shows the relative viability of the units based on ratios. Among open sea cage culture units, those from Andhra Pradesh realized greater profitability than those from other locations. Similarly, coastal water cage units operating from Kerala displayed much higher profitability in relation to others, with all sample units realizing NOI greater than INR 100,000 per crop. Though marine cage farmers generally fared better in terms of absolute indicators of profitability owing to the greater size of culture units, they were trailing behind other enterprises in terms of relative profitability indicators such as ROI, BCR, and OR. While most of the units in the entire sample were observed to be profitable on all indicators, a few of them, especially those practicing open sea cage culture in Tamil Nadu, as well as coastal water cage culture and IMTA in Karnataka and Andhra Pradesh reported having incurred losses during the period under study.
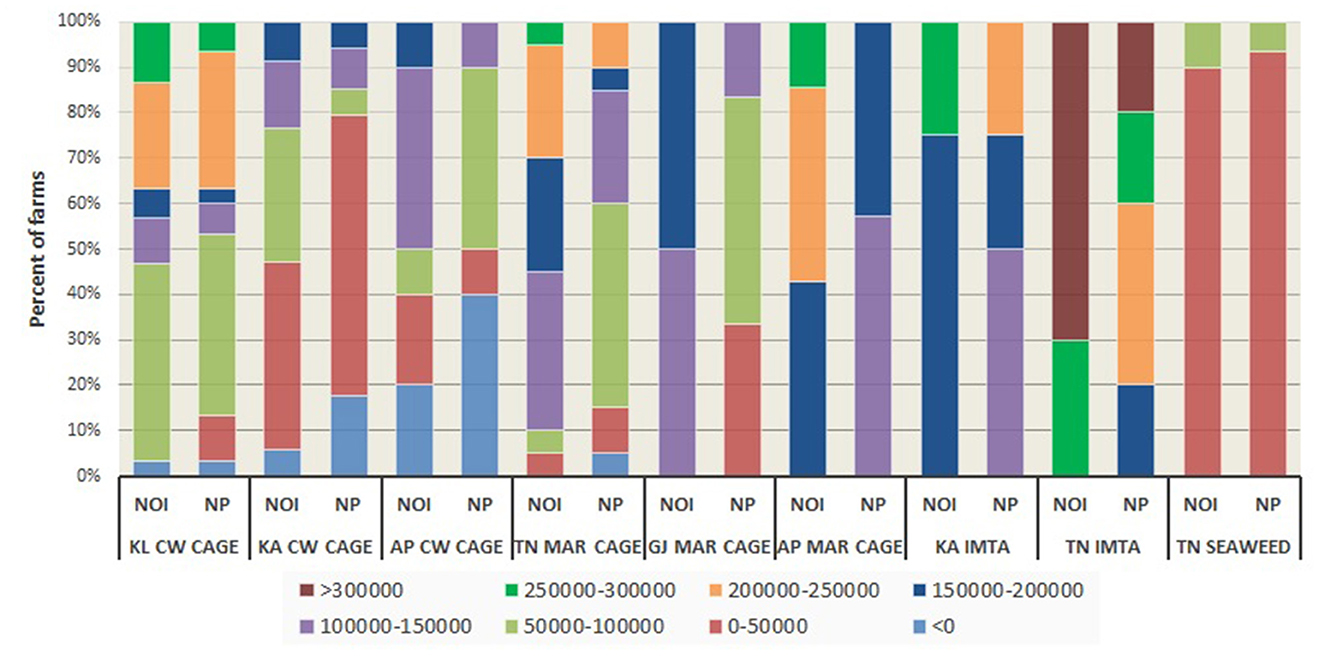
Figure 3. Distribution of net operating income (NIO) and net profit (NP) of sample farms. For seaweeds, income estimates are reported for a batch of 10 rafts each for the sample farmers. Profitability is expressed in Indian rupees [1 Indian Rupee (INR) = 0.012 US Dollars]. KL CW CAGE: Coastal water cage, Kerala; KA CW CAGE: Coastal water cage, Karnataka; AP CW CAGE: Coastal water cage, Andhra Pradesh; TN MAR CAGE: Marine cage, Tamil Nadu; GJ MAR CAGE: Marine cage, Gujarat; AP MAR CAGE: Marine cage, Andhra Pradesh; KA IMTA: IMTA, Karnataka; TN IMTA: IMTA, Tamil Nadu; TN SEAWEED: Seaweed, Tamil Nadu.
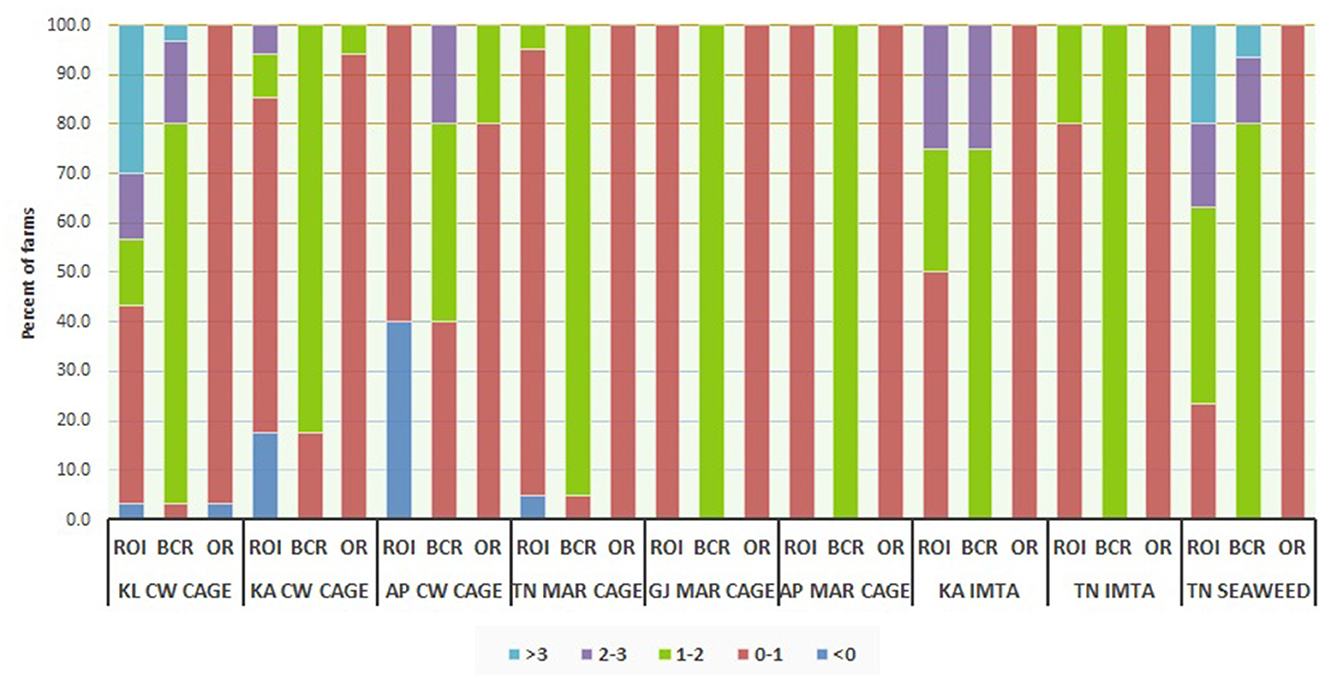
Figure 4. Distribution of economic viability indicators of sample farms. For seaweeds, profitability indicators are reported for a batch of 10 rafts each for the sample farmers. KL CW CAGE: Coastal water cage, Kerala; KA CW CAGE: Coastal water cage, Karnataka; AP CW CAGE: Coastal water cage, Andhra Pradesh; TN MAR CAGE: Marine cage, Tamil Nadu; GJ MAR CAGE: Marine cage, Gujarat; AP MAR CAGE: Marine cage, Andhra Pradesh; KA IMTA: IMTA, Karnataka; TN IMTA: IMTA, Tamil Nadu; TN SEAWEED: Seaweed, Tamil Nadu.

Figure 5. Box plot depicting percent deviation concerning recommended stocking density in sample farms, India. KL CW CAGE: Coastal water cage, Kerala; KA CW CAGE: Coastal water cage, Karnataka; AP CW CAGE: Coastal water cage, Andhra Pradesh; TN MAR CAGE: Marine cage, Tamil Nadu; GJ MAR CAGE: Marine cage, Gujarat; AP MAR CAGE: Marine cage, Andhra Pradesh; KA IMTA: IMTA, Karnataka; TN IMTA: IMTA, Tamil Nadu; TN SEAWEED: Seaweed, Tamil Nadu.
The results of techno-environmental indicators are presented in Table 4. In general, most of the sample farms were found to understock their culture units mainly because of a shortage of quality seeds and their relatively high cost. Only the lobster cage farmers in Gujarat followed greater stocking density than recommended, as they are capture-based aquaculture (CBA) units. Many farmers practicing coastal water cage farming in Kerala were also reported over-stocking their cages. Species diversity (SD) that indicates the number of all farmed species in a sample location during the last three cropping seasons ranged between 1 and 6, the highest reported in IMTA farms in Karnataka (Table 4). None of the farm units, except those in Visakhapatnam (71.4%) reported any means of mechanization or automation in their farming operations. In the latter case, farmers reported attempting automated feeding in their cages on a trial basis with technical support from the Visakhapatnam Centre of ICAR-CMFRI. The use of solar energy in the farms for lighting, surveillance and, to power other minor farm operations is gradually becoming common in cage farms with varying levels of adoption across locations. Management adequacy (MA), a measure to determine the level of adoption of disease control, hygiene management, and general health management of farm stock was observed to be relatively higher among marine cages in Andhra Pradesh (57.1%) and coastal water cage farms in Kerala (81.8%). In other locations, the farmers were either non-adopters or at the initial stages of adoption. Almost all coastal water cage farms were observed to adopt farm surveillance measures like closed circuit cameras or watch and ward mechanisms, still, marine-based enterprises (except in Vishakhapatnam), were low on this aspect. The low incidence of poaching in the open sea has been cited as a reason.
The estimates of environmental sustainability indicators such as aeration management (AM) and water quality monitoring (WQM) were nil in all sample coastal water farms, while in contrast, anti-fouling management was adopted by all coastal water farms. Open sea cage farmers in Visakhapatnam also adopted anti-fouling measures such as cleaning and changing the cage nets at regular intervals. As most of the farms across sample locations were taking only one crop (6–8 months) per season, crop holidays were in place as a matter of course. However, nearly half of the practicing IMTA and coastal water cage farmers who raised Asian sea bass and red snapper for 9 months or more did not follow any crop holidays in between two consecutive crops.
The indicators of social sustainability in selected mariculture enterprises, as observed from the sample farms are presented in Table 4. Though inter-farm variations existed, the farms in general scored high on institutional linkage (IL) and social engagement (SE), as they maintained close linkages with research institutions, aquaculture development agencies of Union and State Governments, training organizations, farmers' associations/societies and non-governmental organizations in the area. They used such linkages mainly to acquire technological updates on farming, to gain access to financial, technical, and extension assistance, skill development through training programs, and to enhance their farm management skills. The indicators also suggested that mariculture has augmented employment and gender inclusion in the study areas. Employment estimates varied across enterprises, and locations, and ranged from 94 to 396 man-days/unit/crop. The highest average man-day requirement was for the IMTA (395.7 man-days/unit) and marine cage farms (321.4 man-days/unit) in Tamil Nadu, whereas coastal water IMTA in Karnataka (90.2 man-days/crop) and seaweed farming (98.7 man-days/crop) in Tamil Nadu scored low on employment. The results corresponding to crew insurance (CI) and social protection (SP) were nil in all sample farms across locations, suggesting wide gaps in the social dimensions of sustainability in sample farms. The farm units in Andhra Pradesh and Karnataka were, however, maintaining notably good measures to ensure crew safety at work like the use of floaters, life jackets, hand gloves, rubber shoes, etc.
Apart from the current level of adoption of sustainable practices, the study also assessed the farmers' general inclination and the likelihood of adopting sustainable farming as well as readiness to implement standard risk-proofing solutions. The estimates of the relative importance index (RII) on the above two dimensions are presented in Tables 5, 6. They ranged from 0 to 1, and values closer to 1 indicated a greater orientation to adopt sustainable farming practices and risk solutions. The RII estimate of a particular parameter, however, cannot be compared across enterprises/locations, but only makes sense if interpreted in relation to that of other parameters. The results suggested that the farmers were positively oriented toward most of the sustainable farming practices irrespective of sample locations, except for a few specific aspects. For instance, the open sea cage farmers in Andhra Pradesh and Tamil Nadu; coastal water cage farmers in Kerala and Andhra Pradesh, as well as seaweed farmers in Tamil Nadu, were relatively less inclined to pay for certified farm inputs such as seed or feed. Similarly, the open sea cage farmers in Andhra Pradesh were particularly reluctant to extend crop holidays, beyond what is being practiced presently. The respondents, cutting across enterprises, also showed their general unwillingness to engage any third-party auditing agencies in their farms. Likewise, varying levels of readiness were exhibited for vertical integration of value chain activities, diversification/scale-up of existing farm activities, use of genetically improved fish species, development of linkage with local government agencies, and so on in the future. Among the risk-proofing solutions, the sample respondents were relatively less inclined to pay for/arrange for insurance cover and social security/welfare registration of farm crew. Readiness for registration of migrant laborers was also low across the board. Coastal water cage farmers from Kerala and Andhra Pradesh were notably averse to accessing any disease surveillance platforms. Nevertheless, future measures to strengthen labor safety measures, surveillance tools, use of weather alert platforms, measures to check for invasive biofoulers, etc. scored relatively high among the sample respondents, irrespective of the enterprises and farming locations. The statistical tests indicated reasonable levels of internal consistency in all samples as indicated by the respective estimates of Cronbach's alpha. The χ2 values from the Kruskal-Wallis test across indicators were insignificant in any of the cases. The results of Dunn's test on the pair-wise significance of indicators were performed, and are available upon request.
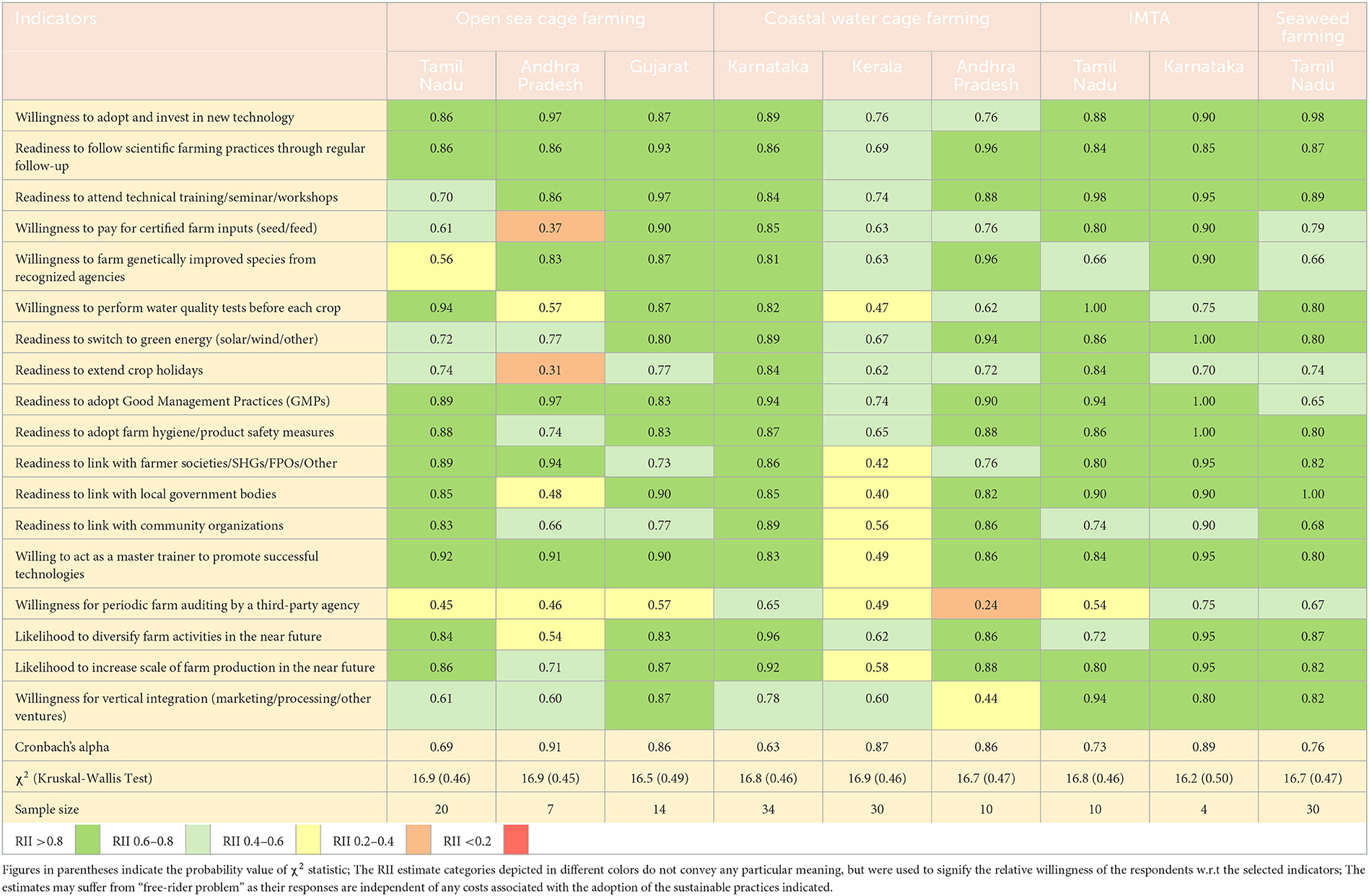
Table 5. Estimates of Relative Importance Index (RII) indicating the willingness to embrace standard sustainable practices in the future by the sample respondents practicing the selected mariculture enterprises (over and above existing level).
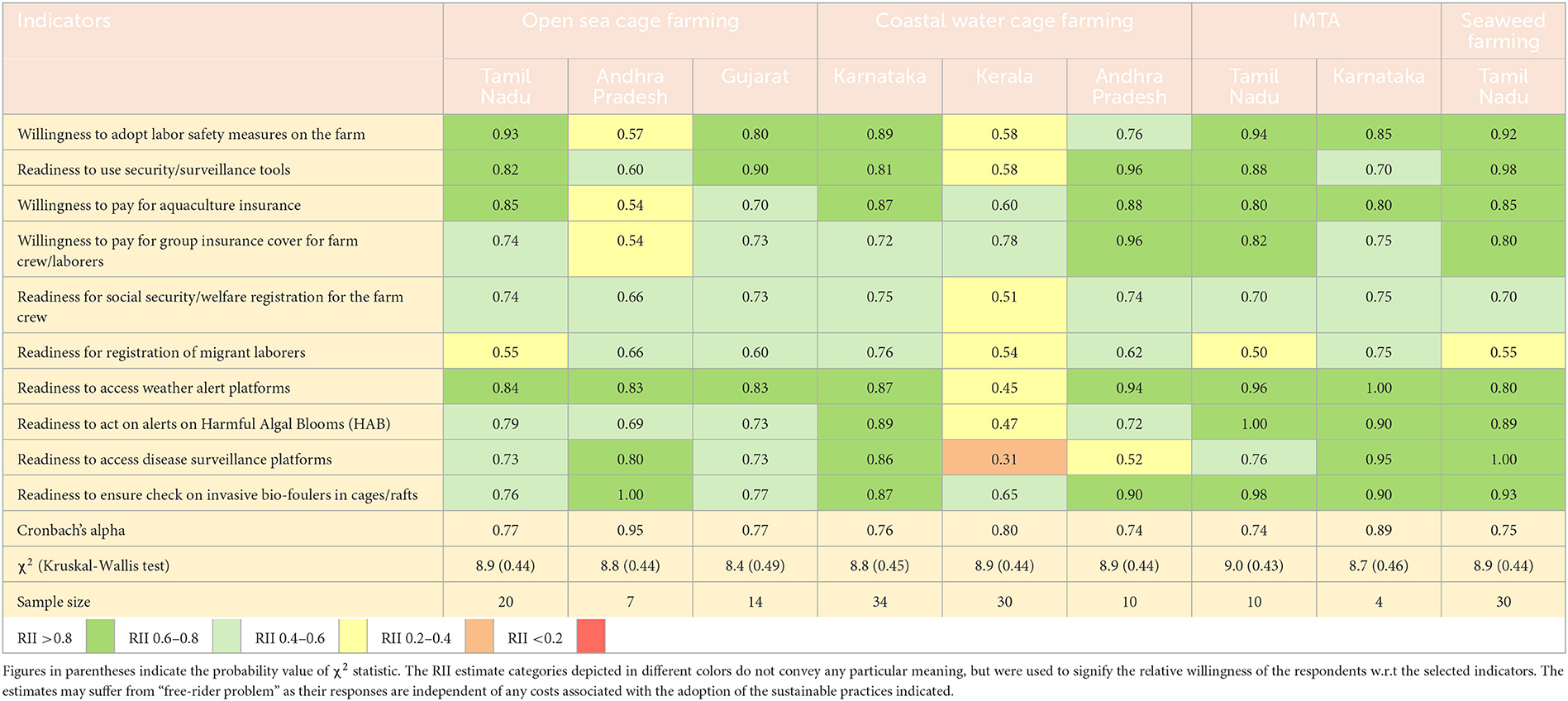
Table 6. Estimates of relative importance index (RII) indicating the readiness to adopt standard risk proofing mechanisms in the future by the sample respondents practicing the selected mariculture enterprises (over and above existing level).
This section analyses the major gaps in the study areas, viewed through the prism of sustainable intensification, stressing its major pillars and dimensions. It has been observed that the sample farms constitute either individual family-operated units (mainly backwater cages and a few open sea cages or IMTA units), or a cluster of units jointly operated by SHGs or fisher societies. In general, all the enterprises examined were relatively labor-intensive, with family labor as a major source. A significant part of the labor is consumed for feeding and other routine management practices. Though a source of gainful employment for the farm families and local labor community, the higher labor requirement of these enterprises adds to the cost of production, thereby affecting input-use efficiency and profitability. The efficiency of the systems can be enhanced through the gradual introduction of cost-effective mechanization and automation solutions for routine management practices so that the labor thus released can be utilized for broad-basing and intensifying culture activities. Similarly, capital self-sufficiency was found to be low in several sample locations, which is to be read in conjunction with the abysmally low availability of institutional credit. This indicates the dependence of farmers on informal credit sources to meet capital and operational expenditure. Previous literature suggests that the rural non-institutional credit market is generally unorganized, exploitative, and devoid of transparency (Inoue, 2011; Parappurathu et al., 2019), often leading to a perpetual debt burden on the farming community. Enhancements in financial inclusion in coastal areas therefore can potentially improve access to capital, reduce capital costs and boost the entrepreneurial capacity of mariculture farmers. Another notable feature associated with mariculture farms in most of the study areas is the lack of any legally valid use rights for culture. This is mainly due to the regulatory vacuum on mariculture governance in the internal waters, territorial waters, or beyond, which constrains the local government institutions from taking appropriate actions to issue leases or licenses for culture in open-access water bodies. The lack of any serious conflicts with other users of internal and marine waters presently, given the early stage of mariculture development, is another reason for low concern about such legal rights. However, this is subject to change with the greater intensification of culture activities and will be more obvious in the open ocean where access rights are contentious and subject to intense debates (Percy et al., 2013; Cohen et al., 2019; Davies et al., 2019).
Both marine and coastal water cage farmers have reported using wild collected seed, which is notably on the flip side from the sustainability angle, as there is a severe shortage of cultured seeds. Lobster farming in Gujarat is a CBA enterprise with complete dependence on wild sources for seed, as commercial hatchery production facilities for lobsters are yet to be established. For most species, the local availability of hatchery-produced seed becomes a limiting factor often due to value chain constraints, leading to delays in the establishment of the crop. The need for the development of broodstock centers and hatcheries across the coastal belt is increasingly being felt, given the growing acceptance of mariculture among the prospective entrepreneurs and fisher folk community. A wide variety of aquaculture feed, mainly artificial floating pellet feeds are being used by the sample farm units. At the same time, a significant proportion of sample farmers also depend on low-value trash fish sourced from the wild as by-catch. The choice of feed depended considerably on the type of fish farmed, and the market availability of pellet feeds at affordable prices. For instance, farmers growing pompano mainly opted for pellet feeds throughout the culture duration, as raw fish is not a desirable option in such farms. However, those farmers using hatchery-based Asian sea bass and Orange-spotted grouper seeds administer artificial pellet feeds in the initial stages of the crop, before switching to raw fish subsequently. Farmers from almost all sample locations indicated that a shortage of good quality feed is a major constraint in mariculture, and excessive feed prices push the cost of production up. As in the case of the shrimp farming sector, private entrepreneurs in India presently have greater opportunities to capitalize on the growing demand for feeds and specialized growth promoters in the mariculture sector. There is also immense scope to diversify and broad-base value chains associated with mariculture farms to overcome their relative recentness. Most of the existing farms depend on limited formats of local markets with very few market linkages, as the results indicate. There is also a need to modernize the existing aqua-fish markets and minimize the prevalence of reported unfair marketing practices. Social dimensions of sustainability such as social engagement, gender inclusion, crew insurance, crew safety, social protection, etc. also need significant attention to ensure the long-term welfare of both the owner-operators as well as farm crew associated with mariculture units. However, much of the change is possible only through enhanced institutional and policy interventions over and above farm-level attention, given their overarching nature cutting across sectors and regions.
The sample units in general performed low on technical and environmental indicators. Major gaps were noticed in mechanization, use of renewable energy, disease, and hygiene management, farm surveillance, aeration management, anti-fouling, water quality monitoring, etc., with negative impacts on the environmental sustainability of the farms. Notably, most of the farms, except cage farms engaged in lobster fattening in Veraval (in Gujarat state), and coastal water cage farms in Kerala were predominantly observed to under-stock their farm units with fish seeds, leading to sub-optimal crop yields. Plugging this gap by enhancing seed availability and extension interventions can substantially improve the economic viability of the units. The profitability of farms at many locations was also found to be affected by several input-side constraints and other extraneous factors. For instance, the coastal water cage farmers in Andhra Pradesh indicated that delay in obtaining fish seeds on time resulted in the late start of culture activities thereby curtailing the culture period. Some farmers in the same location also indicated mortality due to wastewater infusion in the water body from neighboring industrial units. Yield enhancement, being one of the primary pillars of SI, thus needs concerted attention in all the study areas, and can be achieved through the optimal stocking of seeds, enhancing culture intensity through polyculture of suitable species, and the adoption of scientific management of various biotic and abiotic constraints. Some of the prospective interventions on the latter dimension include carrying capacity and water quality assessment at regular intervals, use of disease-free SPF seeds, surveillance mechanisms for disease incidence, adoption of aquatic animal health codes applicable for open water bodies, measures to prevent siltation and bio-fouling, checking incidence of invasive species, re-alignment of crop schedules to suit salinity and temperature of water body through regular monitoring, and so on (OIE, 2019; Fox et al., 2020; Wanja et al., 2020). IMTA, being a novel practice introduced recently in Tamil Nadu, has limited adoption presently. Nevertheless, enhanced growth and higher yields of the extractive species and the potential for mitigation of biofouling around the cages, it has considerable future scope in India. Similarly, the seaweed farming sector needs a greater supply of planting materials either through genetic improvement and mass multiplication programs or the introduction of suitable exotic species after due screening for any negative ecological consequences (Johnson et al., 2021).
Given the early stage of development, sustainability problems associated with the expansion of mariculture activities are yet to unfold fully in India. As obvious, the role of public policy is much more pronounced in ensuring the SI of mariculture compared to that of inland aquaculture. This is mainly because of the complexities associated with property rights, equity, and social justice; investment necessities for standardization of hatchery production and culture protocols of non-domesticated marine species, cumbersome management requirements in the marine environment, technological challenges associated with production scale-up and precision mariculture, as well as the emerging challenges posed by climate change and associated extreme weather events. The technical and human-resource prerequisites for empowering and enabling resource-poor coastal dwellers to take-up capital intensive mariculture activities are also high. The Government of India has recently floated ambitious programs to support prospective farming ventures, intending to provide the necessary logistic, funding, and policy support. However, there are glaring gaps that include the lack of a comprehensive mariculture policy at national and state levels and the lack of clarity on property rights in the open ocean and internal waters. Apart from these, executive and policy actions are needed to address the emerging requirements to enable the development of a self-sustaining mariculture sector in the country. Some of the specific recommendations in this regard include (i) development of marine spatial plans (MSP) for optimal allocation of available ocean space, (ii) introduction of legislations at appropriate levels to support leasing and licensing arrangements, (iii) measures to ensure adequate supply of seed and feed through channelizing public funding and by incentivising the private sector, (iv) strengthening of food safety and health management in mariculture farms, (v) developing mandatory guidelines on good farming practices (e.g., measures for anti-fouling, water quality monitoring, crop holiday management, safety and security measures, etc.) to obtain farm registration, (vi) enhancing multi-disciplinary research on mariculture systems, (vii) bring about market reforms for the development of competitive value chains, (viii) introduction of specialized schemes to support auxiliary pre-requisites such as credit, insurance, and other support services, and (ix) promoting group farming, co-operative farming and farmer producer companies among mariculture farmers (Bostock et al., 2010; FAO, 2016; Gopalakrishnan et al., 2017, 2019). Governance of mariculture is equally convoluted, given the existence of diverse stakeholders with competing interests, besides the concerns about equity and the challenges of enforcement. There are innumerable debatable issues related to the ownership and operatorship formats (cooperative/corporate/private/other), engagement within the varied social and political realms, alignment with cross-cutting sectors, and so on, which need early resolution (Percy et al., 2013; Davies et al., 2019). Above all, there have to be appropriate institutions and governance arrangements in place to ensure that future expansion of mariculture development in the country is fully consistent with a precautionary approach to environmental sustainability and guided by Ecosystem Approach to Aquaculture (EAA) to ensure the resilience of interlinked social-ecological systems.
Though predominantly smallholder-centric, mariculture can be a potential future source of marine fish production in India. Over the past one and half decades, there have been notable achievements in terms of technological breakthroughs in breeding, seed production, and grow-out of marine finfish and shellfish species in artificial enclosures/structures, thereby aiding their profitable farming in the open sea as well as coastal and estuarine waters. Some of the potential enterprises for future scale-up include open sea cage farming, coastal water cage farming, seaweed farming, and integrated multi-trophic aquaculture, among others. This paper evaluates the present status of selected mariculture enterprises in their very cradles situated along India's east and west coasts, followed by a critical assessment of their future potential for sustainable intensification. The study follows the Principles-Criteria-Indicator (PCI) approach to assess the sustainability status and scope for intensification based on primary field data, pinning on a set of objectively measurable indicators on techno-economic, techno-environmental as well as social dimensions of sustainability. Further, the extent to which the sample respondents are willing to adopt sustainable farming practices as well as risk-proofing mechanisms on-farm, over and above the existing level is assessed using their responses to a set of selected questions on a five-point Likert scale. Of particular relevance for the above analysis include the quality of resource endowments, entrepreneurial readiness and capital availability, farming skills and technical prowess of the farmers, farm-level profitability, on-farm interventions to ensure environmental sustainability, community knowledge capital, backward and forward linkages w.r.t. input and product markets respectively, level of value chain integration, and so on. All the selected enterprises were assessed to be technically and economically viable in general; nevertheless, glaring gaps were evident on key indicators of sustainability such as the legitimacy of access over water bodies, use of quality seed and feed, institutional credit access, market access and fair marketing practices, optimal stocking density, mechanization, use of renewable energy, adoption of environment-friendly culture practices, farm surveillance, crew safety, and social protection. The study takes due cognizance of the fact that the development of several auxiliary economic enterprises, directly or indirectly related to mariculture, and their assimilation and integration into the diversified coastal economy are necessary to realize transformational changes. The findings underscore the need for greater technological, policy, and institutional intercessions, as India gears toward the sustainable and inclusive expansion of its blue economy in the years to come.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SPar: conceptualization, methodology, investigation, and original draft preparation. MM, CJ, JB, and AA: investigation, data, and reviewing and editing. CR: conceptualization and methodology. PL and NA: data and reviewing and editing. SPad: study area map and reviewing and editing. SG, DD, SM, GR, and SV: investigation and review and editing. BI, SR, RN, AG, and PC: conceptualization, reviewing, and overall guidance. All authors contributed to the article and approved the submitted version.
The authors gratefully acknowledge the financial support obtained from the Indian Council of Agricultural Research (through ICAR National Network Project No. #1013464 entitled, Production Systems, Agribusiness, and Institutions—Component 1: Impact of Agricultural Technology hosted by ICAR-NIAP, New Delhi).
The insightful comments and overall guidance provided by Dr. Prathap Singh Birthal, Director, NIAP and Dr. J. Jayasankar, Head, Fishery Resource Assessment, Economics and Extension Division of ICAR-CMFRI is thankfully acknowledged. The support provided by Dr. Vinaya Kumar Vase in data collection, review, and editing of the manuscript is sincerely appreciated. The technical assistance from Dr. Suresh Kumar Mojjada in validating some of the results is also appreciated. We place on record, our sincere gratitude to all the mariculture farmers, entrepreneurs, experts and other government officials who provided valuable information for use in the manuscript through primary surveys and direct/telephonic interviews. The views expressed are personal.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KP declared a past co-authorship with the author SP to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1078314/full#supplementary-material
1. ^Mariculture or marine farming is a specialized branch of aquaculture involving the cultivation of marine organisms for food and other animal products, in enclosed sections of the open ocean (offshore mariculture), fish farms built on near-shore waters (inshore mariculture), or in artificial tanks, ponds or raceways which are filled with seawater (onshore mariculture).
2. ^As per United Nations Convention on the Law of the Sea (UNCLOS), internal waters are “those waters which lie landward of the baseline from which the territorial sea is measured.” This include (i) parts of the sea along the coast down to the low water mark, (ii) ports and harbours, (iii) estuaries and landward waters from the closing line of bays, and (v) waters enclosed by straight baselines.
3. ^The term “Coastal water” farming is used in this paper to refer to fish farming operations carried out in internal water areas.
Alagarswami, K. (1974). “Development of pearl culture in India and scope for a pearl culture industry,” in Proceedings of the Group Discussion On Pearl Culture, ed R. V. Nair (Cochin: CMFRI), 4–19.
Anuraj, A., S. B., Loka, J., Ignatius, B., Santhosh, B., Ramudu, K. R., et al. (2021). Induced breeding and larval rearing of vermiculated spinefoot, Siganus vermiculatus (Valenciennes, 1835) in indoor conditions. Aquaculture 539, 736600. doi: 10.1016/j.aquaculture.2021.736600
Appukuttan, K. K. (1980). Culture of brown mussel Perna indica at Vizhinjam. Mar. Fish. Info. Serv. 16, 11–13.
Arasu, A. R. T., Kailasam, M., and Sundaray, J. K. (2009). Asian Seabass, Fish Seed Production and Culture. CIBA Special Publication 42. Chennai: Central Institute of Brackishwater Aquaculture, 158.
Arquitt, S., Honggang, X., and Johnston, R. (2005). A system dynamics analysis of boom and bust in the shrimp aquaculture industry. Syst. Dyn. Rev. 21, 305–324. doi: 10.1002/sdr.313
Aswathy, N., Joseph, I., Ignatius, B., and Joseph, S. (2020). Economic Viability of Cage Fish Farming in India. CMFRI Special Publication 134. Kochi: Central Marine Fisheries Research Institute.
Ayyappan, S., Jena, J. K., Lakra, W. S., Gopal, T. K. S., Gopalakrishnan, A., Vass, K. K., et al. (2015). “Fisheries sciences,” in 100 Years of Agricultural Sciences in India, ed R. B. Singh (New Delhi: National Academy of Agricultural Sciences).
Balasubramanian, C. P., Mhaskar, S. S., Sukumaran, K., Panigrahi, A., Vasagam, K., Kumararaja, P., et al. (2018). Development of integrated multi-trophic aquaculture (IMTA) for tropical brackishwater species in Sindhudurg district, Maharashtra, west coast of India. Ind. J. Fish. 65, 59–64. doi: 10.21077/ijf.2018.65.1.70128-10
Barrington, K., Chopin, T., and Robinson, S. (2009). “Integrated multi-trophic aquaculture (IMTA) in marine temperate waters,” in Integrated Mariculture: A Global Review, ed D. Soto. FAO Fisheries and, Aquaculture Technical Paper 529 (Rome: FAO), 7–46.
Bavinck, M., Berkes, F., Charles, A., Dias, A. C. E., Doubleday, N., Nayak, P., et al. (2017). The impact of coastal grabbing on community conservation—a global reconnaissance. Mar. Stud. 16, 8. doi: 10.1186/s40152-017-0062-8
Belton, B., and Little, D. C. (2008). The development of aquaculture in Central Thailand : domestic demand versus export-led production. J. Agric. 8, 123–143. doi: 10.1111/j.1471-0366.2007.00165.x
Belton, B., Little, D. C., Zhang, W., Edwards, P., Skladany, M., and Thilsted, S. H. (2020). Farming fish in the sea will not nourish the world. Nat. Commun. 11, 5804. doi: 10.1038/s41467-020-19679-9
Biswas, G., Kumar, P., Kailasam, M., Ghoshal, T. K., Bera, A., and Vijayan, K. K. (2019). “Application of integrated multi-trophic aquaculture (IMTA) concept in brackishwater ecosystem: the first exploratory trial in the Sundarban, India,” in BRAQCON 2019: World Brackishwater Aquaculture Conference, Special Issue No. 86. eds K. P. Jithendran, R. Saraswathy, C. P. Balasubramanian, K. P. Kumaraguru Vasagam, V. Jayasankar, R. Raghavan, et al. (FL: Coconut Creek), 49–55. doi: 10.2112/SI86-007.1
Bostock, J., McAndrew, B., Richards, R., Jauncy, K., Telfer, T., Lorenzen, K., et al. (2010). Aquaculture: global status and trends. Philos. Trans. R. Soc. B 365, 2897–2912. doi: 10.1098/rstb.2010.0170
Boyd, C. E., D'Abramo, L. R., Glencross, B. D., Huyben, D. C., Juarez, L. M., Lockwood, G. S., et al. (2020). Achieving sustainable aquaculture: historical and current perspectives and future needs and challenges. J. World Aqua. Soc. 51, 578–633. doi: 10.1111/jwas.12714
Buck, B. H., and Langan, R., (eds.). (2017). Aquaculture Perspective of Multi-Use Sites in the Open Ocean. Geneva: Springer Nature. doi: 10.1007/978-3-319-51159-7
Cao, L., Diana, J. S., Keoleian, G. A., and Lai, Q. (2011). Life cycle assessment of Chinese shrimp farming systems targeted for export and domestic sales. Environ. Sci. Technol. 45, 6531–6538. doi: 10.1021/es104058z
Carballeira Braña, C. B., Cerbule, K., Senff, P., and Stolz, I. K. (2021). Towards environmental sustainability in marine finfish aquaculture. Front. Mar. Sci. 8, 666662. doi: 10.3389/fmars.2021.666662
Chakraborty, K., Vijayagopal, P., and Gopalakrishnan, A. (2018). Nutraceutical products from seaweeds-wonder herbs of the oceans. Mar. Fish. Info. Serv. 237, 7–12.
Chopin, T., Robinson, S. M. C., Troell, M., Neori, A., Buschmann, A. H., and Fang, J. (2008). “Multitrophic integration for sustainable marine aquaculture,” in The Encyclopedia Ecolological Engineering, Vol 3, ed S. E. Jørgensen and B. D. Fath (Oxford: Elsevier), 2463–2475. doi: 10.1016/B978-008045405-4.00065-3
Clawson, G., Kuempel, C. D., Frazier, M., Blasco, G., Cottrell, R. S., Froehlich, H. E., et al. (2022). Mapping the distribution of global mariculture production. Aquaculture 553, 738066. doi: 10.1016/j.aquaculture.2022.738066
Cohen, P. J., Allison, E. H., Andrew, N. L., Cinner, J., Evans, L. S., Fabinyi, M., et al (2019). Securing a just space for small-scale fisheries in the blue economy. Front. Mar. Sci. 6, 171. doi: 10.3389/fmars.2019.00171
Cossutta, M., Pholboon, S., McKechnie, J., and Sumner, M. (2022). Techno-economic and environmental analysis of community energy management for peak shaving. Energy Conv Manage. 251, 114900. doi: 10.1016/j.enconman.2021.114900
Costello, C., Cao, L., Gelcich, S., Cisneros-Mata, M. A., Free, C. M., Froehlich, H. E., et al. (2020). The future of food from the sea. Nature 588, 95–100. doi: 10.1038/s41586-020-2616-y
Cotas, J., Leandro, A., Pacheco, D., Gonçalves, A. M. M., and Pereira, L. A. (2020). Comprehensive review of the nutraceutical and therapeutic applications of Red Seaweeds (Rhodophyta). Life (Basel) 10, 19. doi: 10.3390/life10030019
Davies, I. P., Carranza, V., Froehlich, H. E., Gentry, R. R., Kareiva, P., and Halpern, B. S. (2019). Governance of marine aquaculture: Pitfalls, potential, and pathways forward. Mar. Pol. 104, 29–36. doi: 10.1016/j.marpol.2019.02.054
Divu, D. N., Mojjada, S. K., Pokkathappada, A. A., Sukhdhane, K., Menon, M., Mojjada, R. K., et al. (2020). Decision-making framework for identifying best suitable mariculture sites along north-east coast of Arabian Sea, India: a preliminary GIS-MCE based modelling approach. J. Clean Prod. 284, 124760. doi: 10.1016/j.jclepro.2020.124760
Edwards, P., Zhang, W., Belton, B., and Little, D. C. (2019). Misunderstandings, myths, and mantras in aquaculture: its contribution to world food supplies has been systematically over-reported. Mar. Pol. 106, 103547. doi: 10.1016/j.marpol.2019.103547
European Commission (2001). A Framework for Indicators for the Economic and Social Dimension of Sustainable Agriculture and Rural Development. Brussels: EC.
FAO (2011). Indicators for the Sustainable Development of Finfish Mediterranean Aquaculture: Highlights From the InDAM Project. Studies and Reviews. General Fisheries Commission for the Mediterranean. No. 90. Rome: Food and Agricultural Organization of the United Nations.
FAO (2016). “Sustainable intensification of aquaculture in the Asia-Pacific region,” in Documentation of Successful Practices, eds W. Miao and K. K. Lal (Bangkok: Food and Agricultural Organization of the United Nations).
Fezzardi, D., Massa, F., Àvila-Zaragoza, P., Rad, F., Yuzel-Gier, G., Deniz, H., et al. (2013). Indicators for Sustainable Aquaculture in Mediterranean and Black Sea Countries. Guide for the Use of Indicators to Monitor Sustainable Development of Aquaculture, Studies and Reviews, No. 93. Rome: UNFAO.
Fore, M., Frank, K., Norton, T., Svendsen, E., Alfredsen, J. A., Dempster, T., et al. (2018). Precision farming: a new framework to improve production in aquaculture. Biosyst. Eng. 173, 176–193. doi: 10.1016/j.biosystemseng.2017.10.014
Fox, M., Christley, R., Lupo, C., Morre, H., Service, S., and Campbell, K. (2020). Preventing and mitigating farmed bivalve disease: a Northern Ireland case study. Aquac. Int. 28, 2397–2417. doi: 10.1007/s10499-020-00597-y
Garnett, T., Applebey, M. C., Balmford, A., Bateman, J., Benton, G., Bloomer, P., et al. (2013). Sustainable intensification in agriculture: premises and policies. Science 341, 33–34. doi: 10.1126/science.1234485
Gentry, R. R., Froehlich, H. E., Grimm, D., Kereiva, P., Parke, M., Rust, M., et al. (2017). Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 1, 1317–1324. doi: 10.1038/s41559-017-0257-9
Gopakumar, G., Nair, K. R. M., and Kripa, V. (2007). “Mariculture research in India—status, constraints and prospects,” in Status and Perspectives in Marine Fisheries Research in India, eds M. J. Modayil and N. G. K. Pillai (Kochi: CMFRI Diamond Jubilee Publication, Central Marine Fisheries Research Institute), 316–361.
Gopalakrishnan, A., Ignatius, B., and George, G. (2017). India's largest fisheries research body turns 70-CMFRI's legacy and a few recent achievements. Fish. Chim. 37, 38–44.
Gopalakrishnan, A., Kirubagaran, R., John, G., Ponniah, A. G., Gopakumar, G., Mohamed, K. S., et al. (2019). Draft National Mariculture Policy 2019, Report of the Committee Constituted by the National Fisheries Development Board. CMFRI Mar. Fish. Pol. Ser. No. 17/2020. New Delhi: Ministry of Fisheries, Animal Husbandry and Dairying, Government of India.
Gopalakrishnan, A., Ravishankar, C. N., Pravin, P., and Jena, J. K. (2020). ICAR-Technologies: High-Value Nutraceutical and Nutritional Products From Seaweeds. New Delhi: Indian Council of Agricultural Research.
Government of India (GoI) (2017). National Policy on Marine Fisheries. New Delhi: Ministry of Agriculture and Farmers Welfare, Government of India.
Government of India (GoI) (2019). Report of Blue Economy Working Group-3, Fisheries, Aquaculture and Fish Processing. New Delhi: Economic Advisory Council to the Prime Minister of India, Government of India.
Grealis, E., Hynes, S., O'Donoghue, C., Vega, A., and Osch, S. V. (2017). The economic impact of aquaculture expansion: an input-output approach. Mar. Pol. 81, 29–36. doi: 10.1016/j.marpol.2017.03.014
Henriksson, P. J. G., Belton, B., Murshed-e-Jahan, K., and Rico, A. (2018). Measuring the potential for sustainable intensification of aquaculture in Bangladesh using life cycle assessment. PNAS 115, 2958–2963. doi: 10.1073/pnas.1716530115
Inoue, T. (2011). “Financial inclusion and poverty alleviation in India: an empirical analysis using state-wise data,” in Inclusiveness in India: A Strategy for Growth and Equality, IDE-JETRO Series, ed S. Hirashima, H. Oda, and Y. Tsujitha (London: Palgrave Mcmillan). doi: 10.1057/9780230304956_4
Jena, J. K., Gopalakrishnan, A., Ravisankar, C. N., Lal, K. K., Das, B. K., Das, P. C., et al. (2022). “Achievements in fisheries and aquaculture in independent India,” in Indian Agriculture after Independence, eds H. Pathak, J. P. Mishra and T. Mohapatra (New Delhi: Indian Council of Agricultural Research), 426.
Johnson, B., Divu, D., Jayasankar, R., Ranjith, L., Dash, G., Megarajan, S., et al. (2020). Preliminary estimates of potential areas for seaweed farming along the Indian coast. Mar. Fish. Inf. Serv. Ser. 246, 14–28.
Johnson, B., and Ignatius, B. (2020). Seaweed farming in India: progress and prospects. Ind. Farm. 70, 42–45.
Johnson, B., Jayakumar, A., Abdul Nazar, A. K., Tamilmani, G., Sakthivel, M., Rameshkumar, P., et al. (2021). “Climate resilient mariculture technologies for food and nutritional security,” in Exploring Synergies and Trade-offs Between Climate Change and the Sustainable Development Goals, ed V. Venkatramanan (Berlin: Springer Nature). doi: 10.1007/978-981-15-7301-9_4
Johnson, B., Narayanakumar, R., Abdul Nazar, A. K., Kaladharan, P., and Gopakumar, G. (2017). Economic analysis of farming and wild collection of seaweeds in Ramanathapuram District, Tamil Nadu. Ind. J. Fish. 64, 94–99. doi: 10.21077/ijf.2017.64.4.61828-13
Kaladharan, P., Vijayakumaran, K., and Chennubhotla, V. S. K. (1996). Optimization of certain physical parameters for the mariculture of Gracilaria edulis (Gmelin) Silva in Minicoy lagoon (Luccadive Archipelago). Aquaculture 139, 265–270. doi: 10.1016/0044-8486(95)01160-9
Kaliaperumal, N., and Kalimuthu, S. (1997). Seaweed potential and its exploitation in India. Seaweed Res. Util. 19, 33–40.
Kobos, P. H., Drennen, T. E., Outkin, A. S., Webb, E. K., Paap, S. M., and Wiryadinata, S. (2020). Techn-economic analysis, best practices and assessment tools. Sandia Rep. California 2020, 94550. doi: 10.2172/1738878
Krishnan, M., and Narayanakumar, R. (2013). Social and Economic Dimensions of Carrageenan Seaweed Farming. FAO Fisheries and Aquaculture Technical Paper 580. Rome: FAO, 163–184.
Kuriakose, P. S. (1980). “Opensea raft culture of green mussel at Calicut,” in Coastal Aquaculture: Mussel Farming-Progress and Prospects, eds K. N. Nayar, S. Mahadevan, K. Alagarswami, and P. T. Meenakshisundaram (Cochin: Bull. Cent. Mar. Fish. Res. Inst).
Little, D. C., Murray, F. J., Leschen, W., and Waley, D. (2013). “Socio-economic factors affecting aquaculture site selection and carrying capacity,” in Site Selection and Carrying Capacities for Inland and Coastal Aquaculture, eds L. G. Ross, T. C. Telfer, L. Falconer, D. Soto and J. Aguilar-Manjarrez. FAO/Institute of Aquaculture, University of Stirling, Expert Workshop, 6-8 December 2010. Stirling, the United Kingdom of Great Britain and Northern Ireland. FAO Fisheries and Aquaculture Proceedings No. 21 (Rome: FAO), 282.
Little, D. C., Young, J. A., Zhang, W., Newton, R. W., Mamun, A. A., and Murray, F. J. (2018). Sustainable intensification of aquaculture value chains between Asia and Europe: a framework for understanding impacts and challenges. Aquaculture 493, 338–354. doi: 10.1016/j.aquaculture.2017.12.033
Liu, W. H., and Ou, C. H. (2007). A comparative analysis of sustainable fishery development indicator system in Australia and Canada. Sustain. Dev. 15, 28–40. doi: 10.1002/sd.291
Mantri, V. A., Dineshkumar, R., Yadav, A., Easwaran, K., Shanmugam, M., and Gajaria, T. K. (2022a). Projections for profitability assessment parameters under short-term, medium-term and long-term evaluation for three farming techniques of Kappaphycus alvarezii along eastern coast of India. Aquaculture 551, 737912. doi: 10.1016/j.aquaculture.2022.737912
Mantri, V. A., Dineshkumar, R., Yadav, A., Veeragurunathan, V., Ganesan, M., Eswaran, K., et al. (2022b). How profitability assessment parameters score under large-scale commercial cultivation of different agarophyte seaweeds along the south-eastern coast of India. Aquac. Int. 30, 1505–25. doi: 10.1007/s10499-022-00866-y
Mohamed, K. S., Kripa, V., Asokan, P. K., Sasikumar, G., Venkatesan, V., Jenni, B., et al. (2016). “Development of bivalve farming as a source of income generation for women's self-help groups in costal India,” in Sustainable Intensification of Aquaculture in the Asia-Pacific Region. Documentation of Successful Practices, eds W. Miao and K. K. Lal (Bangkok: UN FAO).
National Fisheries Development Board (NFDB) (2018). Guidelines for Sea Cage Farming in India: Towards Blue Revolution. New Delhi: Ministry of Agriculture and Farmers Welfare, Government of India.
OIE (2019). The Aquatic Animal Health Code, Twenty-Second Edition. Paris: World Organization for Animal Health. Available online at: http://www.oie.int/standard-setting/aquatic-code/acces-online/ (accessed March 20, 2021).
Parappurathu, S., George, G., Narayanakumar, R., Aswathy, N., Ramachandran, C., and Gopalakrishnan, A. (2017). Priorities and strategies to boost incomes of marine fisher folk in India. Agric. Econ. Res. Rev. 30, 205–216. doi: 10.5958/0974-0279.2017.00035.0
Parappurathu, S., Ramachandran, C., Baiju, K. K., and Xavier, A. K. (2019). Formal versus informal: insights into the credit transactions of small-scale fishers along the south west coast of India. Mar. Pol. 103, 101–122. doi: 10.1016/j.marpol.2019.02.032
Parappurathu, S., Sanil, N. K., Asokan, P. K., Sharma, S. R. K., Pradeep, M. A., Padua, S., et al. (2021). Green mussel (Perna viridis L.) farming in India: an analysis of major growth milestones, recent decline due to disease incidence, and prospects for revival. Aquac. Int. 29, 1813–1828. doi: 10.1007/s10499-021-00716-3
Parris, T. M., and Kates, R. W. (2003). Characterizing and measuring sustainable development. Annu. Rev. Environ. Resour. 28, 559–586. doi: 10.1146/annurev.energy.28.050302.105551
Percy, D. R., Hishamunda, N., and Kuemlangan, B. (2013). “Governance in marine aquaculture: the legal dimension,” in Expanding Mariculture Farther Offshore: Technical, Environmental, Spatial and Governance Challenges, eds A. Lovatelli, J. Aguilar-Manjarrez and D. Soto. FAO Technical Workshop, 22–25 March 2010, Orbetello, Italy. FAO Fisheries and Aquaculture Proceedings No. 24. Rome: FAO, 245–262.
Pretty, J. (1997). The sustainable intensification of agriculture. Nat. Res. For. 21, 247–256. doi: 10.1111/j.1477-8947.1997.tb00699.x
Primavera, J. H. (1997). Socio-economic impacts of shrimp culture. Aqua. Res. 28, 815–827. doi: 10.1111/j.1365-2109.1997.tb01006.x
Qasim, S. Z., Parulekar, A. H., Harkantra, S. N., Ansari, Z. A., and Nair, A. (1977). Aquaculture of green mussel Mytilus viridis L. cultivation on ropes from floating rafts. Indian J. Mar. Sci. 4, 189–197.
Ranjan, R., Muktha, M., Ghosh, S., Gopalakrishnan, A., Gopakumar, G., and Joseph, I. (2017). Prioritized Species for Mariculture in India. Kochi: ICAR-Central Marine Fisheries Research Institute.
Rao, G. S., Joseph, I., Philipose, K. K., and Mojjada, S. K. (2013). Cage Aquaculture in India. Kochi: Central Marine Fisheries Research Institute.
Rao, P. V. S., and Mantri, V. A. (2006). Indian seaweed resources and sustainable utilization: scenario at the dawn of a new century. Curr. Sci. 91, 164–174.
Rey-Valette, H., Clement, O., Aubin, J., Mathe, S., Chia, E., Legendre, M., et al. (2008). Guide to the Co-construction of Sustainable Development Indicators in Aquaculture. Montpellier: EVAD.
Rey-Valette, H., Clement, O., Aubin, J., Mathe, S., Chia, E., Legendre, M., et al. (2010). “An approach to co-construct sustainable development indicators in aquaculture,” in IIFET 2010 (Montpellier, France).
Salunke, M., Kalyankar, A., Khedkar, C. D., Shingare, M., and Khedkar, G. D. (2020). A review on shrimp aquaculture in India: historical perspective, constraints, status and future implications for impacts on aquatic ecosystem and biodiversity. Rev. Fish. Sci. Aquac. 28, 283–302. doi: 10.1080/23308249.2020.1723058
Seung, C. K., and Kim, D.-H. (2020). Examining supply chain for seafood industries using structural path analysis. Sustainability 12, 2061. doi: 10.3390/su12052061
Silas, E. G., and Kalimuthu, S. (1987). Commercial exploitation of seaweeds in India. CMFRI Bull. 41, 55–59.
Smith, A., Snapp, S., Chikowo, R., Thorne, P., Bekunda, M., and Glover, J. (2017). Measuring sustainable intensification in smallholder agro-ecosystems: a review. Glob. Food Sec. 12, 127–138. doi: 10.1016/j.gfs.2016.11.002
Suresh Babu, P. P., Anuraj, A., Shilta, M. T., Asokan, P. K., Vinod, K., Praveen, N. D., et al. (2022). Broodstock development, breeding and larval rearing of Acanthopagrus berda (Forsskal, 1775), a suitable species for farming in tropical waters. Aquacult. Res. 53, 6439–6453. doi: 10.1111/are.16114
Troell, M., Jonell, M., and Henriksson, P. J. G. (2017). Ocean space for seafood. Nat. Ecol. Evol. 1, 1224–1225. doi: 10.1038/s41559-017-0304-6
Valenti, W. C., Kimpara, J. M., Preto, B. L., and Moraes-Valenti, P. (2018). Indicators of sustainability to assess aquaculture systems. Ecol. Ind. 88, 402–413. doi: 10.1016/j.ecolind.2017.12.068
Wan, Z., and Liu, H. (2010). “On environmental impact assessment (EIA) of technology,” in Proceedings of the 2010 International Conference of Information Science and Management Engineering, 450–452. doi: 10.1109/ISME.2010.241
Wang, C., Li, Z., Wang, T., Xu, X., Zhang, X., and Li, D. (2021). Intelligent fish farm: the future of aquaculture. Aquac. Int. 29, 2681–2711. doi: 10.1007/s10499-021-00773-8
Wanja, D. W., Mbuthia, P. G., Waruiru, R. M., Mwadime, J. M., Bebora, L. C., Nyaga, P. N., et al. (2020). Fish husbandry practices and water quality in central Kenya: potential risk factors for fish mortality and infectious diseases. Vet. Med. Int 2020, 6839354. doi: 10.1155/2020/6839354
World Bank and United Nations Department of Economic and Social Affairs (UN-DESA) (2017). The Potential of the Blue Economy: Increasing Long-Term Benefits of the Sustainable Use of Marine Resources for Small Island Developing States and Coastal Least Developed Countries. Washington DC: World Bank.
Keywords: mariculture, cage culture, small-holder aquaculture, sustainable intensification, diversified coastal economy, production system assessment, blue economy, India
Citation: Parappurathu S, Menon M, Jeeva C, Belevendran J, Anirudhan A, Lekshmi PSS, Ramachandran C, Padua S, Aswathy N, Ghosh S, Damodaran D, Megarajan S, Rajamanickam G, Vinuja SV, Ignatius B, Raghavan SV, Narayanakumar R, Gopalakrishnan A and Chand P (2023) Sustainable intensification of small-scale mariculture systems: Farm-level insights from the coastal regions of India. Front. Sustain. Food Syst. 7:1078314. doi: 10.3389/fsufs.2023.1078314
Received: 24 October 2022; Accepted: 16 February 2023;
Published: 08 March 2023.
Edited by:
Subhasis Mandal, Central Soil Salinity Research Institute (ICAR), IndiaReviewed by:
Abdullah-Al Mamun, Noakhali Science and Technology University, BangladeshCopyright © 2023 Parappurathu, Menon, Jeeva, Belevendran, Anirudhan, Lekshmi, Ramachandran, Padua, Aswathy, Ghosh, Damodaran, Megarajan, Rajamanickam, Vinuja, Ignatius, Raghavan, Narayanakumar, Gopalakrishnan and Chand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinoj Parappurathu, cHNoaW5vakBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.