- 1Animal and Human Health Programme, International Livestock Research Institute, Addis Ababa, Ethiopia
- 2Animal and Human Health Programme, International Livestock Research Institute, Nairobi, Kenya
- 3Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden
- 4Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 5College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
- 6Veterinary Public Health Institute, University of Bern, Bern, Switzerland
- 7School of Environment and Natural Resources, Ohio State University, Columbus, OH, United States
- 8Department of Environmental and Occupational Health, and Safety, University of Gondar, Gondar, Ethiopia
- 9Animal and Human Health Programme, International Livestock Research Institute, Dakar, Senegal
- 10Animal and Human Health Programme, International Livestock Research Institute, Ouagadougou, Burkina Faso
- 11Natural Resources Institute, University of Greenwich, Chatham, United Kingdom
Background: Foodborne disease (FBD) affects millions of people each year, posing a health burden similar to malaria, tuberculosis or HIV. A recent World Bank study estimated the productivity losses alone attributed to unsafe food within Africa at $20 billion in 2016, and the cost of treating these illnesses at an additional $3.5 billion. Ethiopia faces multiple food safety challenges due to lack of infrastructure and basic pre-requisites for food safety such as clean water and environment, washing facilities, compounded by limited implementation of food safety regulations, and a lack of incentives for producers to improve food safety. A consolidation of our understanding and evidence of the source, nature and scale of FBD in Ethiopia is needed to inform policy and future research. We performed a Systematic Literature Review (SLR) of publications on FBD occurrence in Ethiopia including hazard presence and impact.
Method: The SLR followed Cochrane and PRISMA guidelines. We searched PubMed and CAB-Direct for relevant publications between 1990 and 2019 (inclusive). Observational studies and reviews were included. Two reviewers screened titles and abstracts, and retained publications were reviewed in full for quality and data extraction.
Result: In total 128 articles met the inclusion criteria. Most articles focused on the identification of biological hazards in food. High levels of microbial contamination in different food value chains were often found in the small, ad hoc, observational studies that dominated the literature. Raw milk (22/128, 17.0%) and raw beef (21/128, 16.4%) were the most studied food products. Foodborne (FB) parasites were often found at higher rates in food than bacterial and viral pathogens, possibly due to differences in ease of identification. High levels of bacterial contamination on the hands of food handlers were widely reported. There were no reports on the incidence of human FBDs or resulting health and economic impacts.
Conclusion: Our findings reflect existing concerns around food safety in Ethiopia. A lack of substantial, coordinated studies with robust methodologies means fundamental gaps remain in our knowledge of FBD in Ethiopia, particularly regarding FBD burden and impact. Greater investment in food safety is needed, with enhanced and coordinated research and interventions.
1. Introduction
Foodborne diseases (FBDs) are illnesses caused by contaminated, or naturally harmful, food. A foodborne (FB) hazard is anything present in food that can harm consumers' health. They are usually categorized as: biological hazards, which are pathogenic organisms and the toxins they produce; chemical hazards, which may be artificial or natural; and physical hazards, such as foreign objects in food (Grace et al., 2018). The most comprehensive estimates of the health burden of FBDs in African countries are those provided by the World Health Organization (WHO) Foodborne Disease Epidemiology Reference Group (FERG) (Havelaar et al., 2015), which estimated that FBD burden is comparable to that of malaria, HIV/AIDS or tuberculosis. Nearly all of this burden (98%) is borne by low-income countries and most of it (97%) is due to biological hazards (Havelaar et al., 2015), with diarrheal disease agents being the most important contributor to overall FBD burden in African region E (which includes Ethiopia), followed by helminths and invasive bacteria (Havelaar et al., 2015).
Accurate information on the health and burden associated with FBDs is critical for countries to guide food safety risk management strategies and to justify allocation of resources. The lack of reliable data from surveillance systems for FBD in low and middle income countries limits the ability to conduct risk-based food safety management. FBD burden is thought to be high in Ethiopia (Pieracci et al., 2016; Keba et al., 2020; Belina et al., 2021; Mekonnen et al., 2021). Salmonella, Listeria monocytogenes, Escherichia coli (E. coli), Campylobacter spp. and Shigella are among the most common FB pathogens reported from Ethiopia and food-producing animals are the major reservoirs (Belina et al., 2021). Reports of food poisoning outbreaks in Ethiopia are often linked to consumption of raw meat and milk (Kassahun and Wongiel, 2019), However, cases of FB illnesses are underreported and are rarely investigated in detail (Ayana et al., 2015).
Given the lack of knowledge around this vital topic, this Systematic Literature Review (SLR) was conducted to investigate the existing literature and collate the evidence on FB hazards in Ethiopia. The SLR did not look at specific pre-specified hazards or specific foods, but instead explored available literature on any FB hazards and any foods. This SLR is one early output of a portfolio of research projects1looking to improve understanding and control of FBD in Ethiopia. This review was used to inform these projects which then went on to examine FBD using various approaches, including modeling, molecular and field-based approaches.
2. Methods
2.1. Research question
This SLR aimed to inform the design of further studies by addressing the following research questions:
• What biological and chemical hazards have been identified in foods in Ethiopia?
• What is the prevalence (i.e., percent of contaminated products) and concentration of these hazards in foods in Ethiopia?
• What is the incidence of FBD and what is the associated health burden in Ethiopia?
2.2. Search strategy
We conducted an SLR following the methods suggested by the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009). A comprehensive search on PubMed and CAB Direct was performed for articles published in English from the 1st of January of 1990 to the 30th of September 2019, inclusive. The search was done on 30th of September 2019. The search syntax with different search terms and Boolean Operators including the following search terms:
i. foodborne OR “food borne” OR food-borne OR “food safety” OR “food related” OR “food associated” OR “food derived” OR “food* illness” OR “food* disease*” OR “food* intoxica*” OR “food pathogen” OR “food* poison*” OR “food* microb*” OR “food* vir*” OR “food parasit*” OR “food* toxin.”
ii. AND Ethiop*.
The syntax was left broad and generic to capture all the literature covering the various aspects of interest (e.g., prevalence, impact, risk factors, and control). Inclusion and exclusion criteria are shown in Table 1.
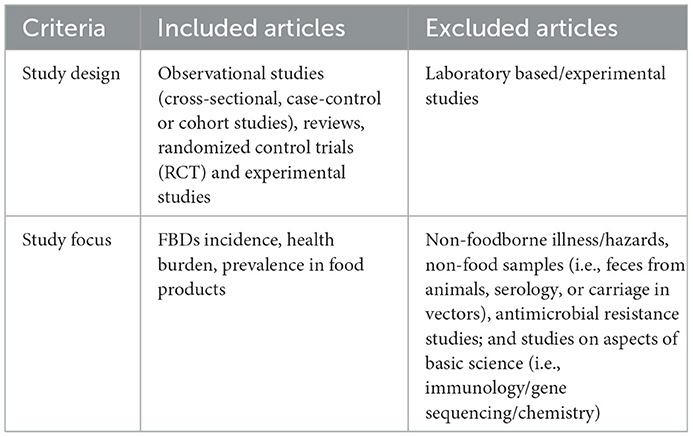
Table 1. Inclusion and exclusion criteria (inclusion also require Ethiopia focus and published on or after 1st January 1990 but not after 30th September 2019).
2.3. Article selection process and quality assessment
Lists with the identified titles and abstracts were downloaded to an excel file and checked for duplicates using Mendeley (https://www.mendeley.com/download-reference-manager/windows). After the removal of duplicates, titles and abstracts were reviewed independently by two reviewers against the inclusion and exclusion criteria. All titles considered relevant by both reviewers were included; articles considered relevant by just one reviewer were reviewed by a third reviewer who made a final decision on article inclusion.
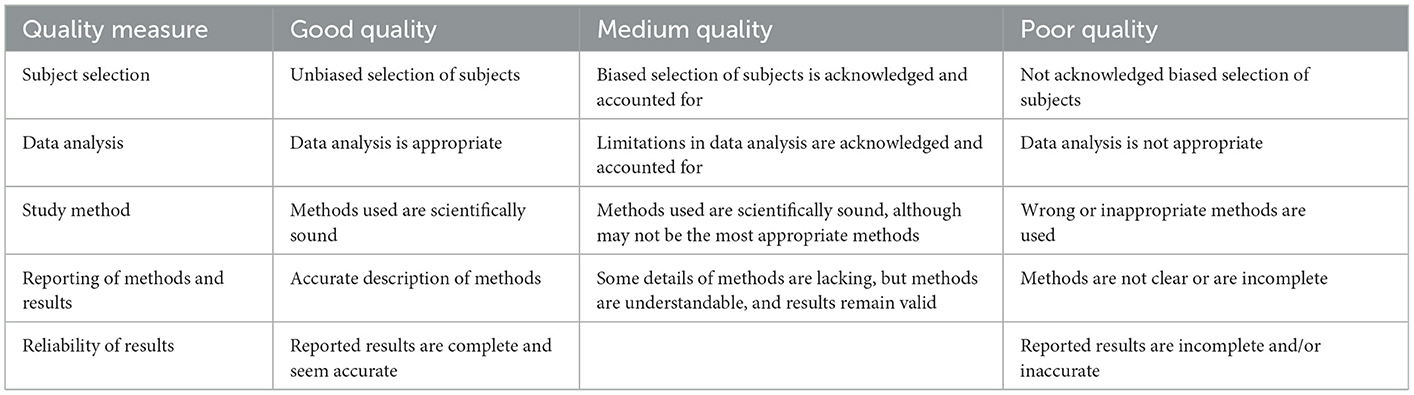
Full articles for the included titles were obtained, when available. The full articles were then subjected to a two-step review process; Articles were reviewed against (1) inclusion/exclusion criteria (as above) and (2) quality criteria. The quality criteria considered the paper's merit on five aspects: (1) selection of subjects, (2) study methods, (3) data analysis, (4) reporting of methods and results, and (5) reliability of results (Table 2). Quality of the papers was rated as “Good” (scoring positively to all five quality criteria), “Moderate” (scoring positively in three or four of the quality criteria) or “Poor” (scoring positively in two or less of the quality criteria). However, an overall reviewer's overall impression could over-ride this rating. Poor-quality articles were excluded from data synthesis.
2.4. Data extraction and analysis
An excel template was designed to allow standardized data extraction by different reviewers (Supplementary material). Ten percent (n = 13) of the full articles were systematically selected by dividing the total number of included articles by 13 so each 10th article in the list was selected. Then theses were reviewed in parallel by two reviewers and outputs were compared to ensure the review process was standardized and comparable across reviewers. After standardization, the remaining articles were reviewed by a single reviewer. Given the large diversity of foods, organisms, and studies, it was not possible to conduct any meaningful meta-analysis.
3. Results
3.1. Literature search
The database searches returned 760 and 244 records from PubMed and Cab Direct, respectively. Out of 519 unique articles, 307 were excluded at title and abstract review, and 3 were not available as full manuscripts (Figure 1). From the remaining 209 full articles, 53 were excluded based on the inclusion/exclusion criteria and 28 based on the quality criteria. These were excluded due to poor scientific quality, mostly from biased selection of subjects, inappropriate data analysis and incomplete and/or inaccurate results.

Figure 1. Flow chart of the process followed during the literature search for data extraction. The PRISMA flow diagram for the systematic review detailing the database searches, the number of abstracts screened, and the full texts retrieved.
Data was ultimately extracted from 128 selected articles.
3.2. Profile of the reviewed publications
Articles reported studies conducted in Oromia (42/128, 32.8%), Amhara (30/128, 23.4%), Addis Ababa (29/128, 22.6%), and Southern Nation Nationalities People (SNNP; 21/128, 16.4%) region. Few studies had been done in the other regions of the country. These were generally conducted in major cities and were not representative of all regional states (Figure 2).
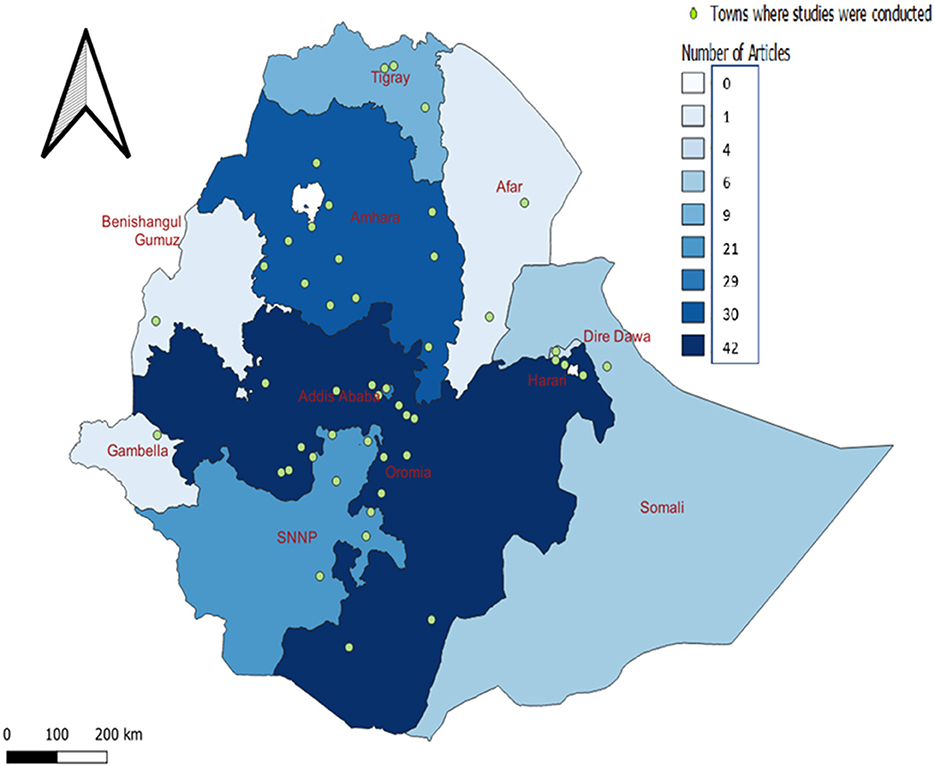
Figure 2. Spatial distribution of regions and towns where the studies were conducted. Concentration of included studies over regions in Ethiopia with dark blue color indicating the highest number of articles and light blue representing fewer number of articles. The green circle dots indicating the towns where the studies were conducted.
Most articles were conducted in the capital city, Addis Ababa (n = 29), followed by Debre Zeit (n = 14), Awassa (n = 9), Jimma (n = 9), Gondar (n = 9), Bahir Dar (n = 7), Haromaya (n = 6), and Arba Minch (n = 5) the location of well-established universities.
The number of articles relevant to the topic identified in our review increased over time (Figure 3).
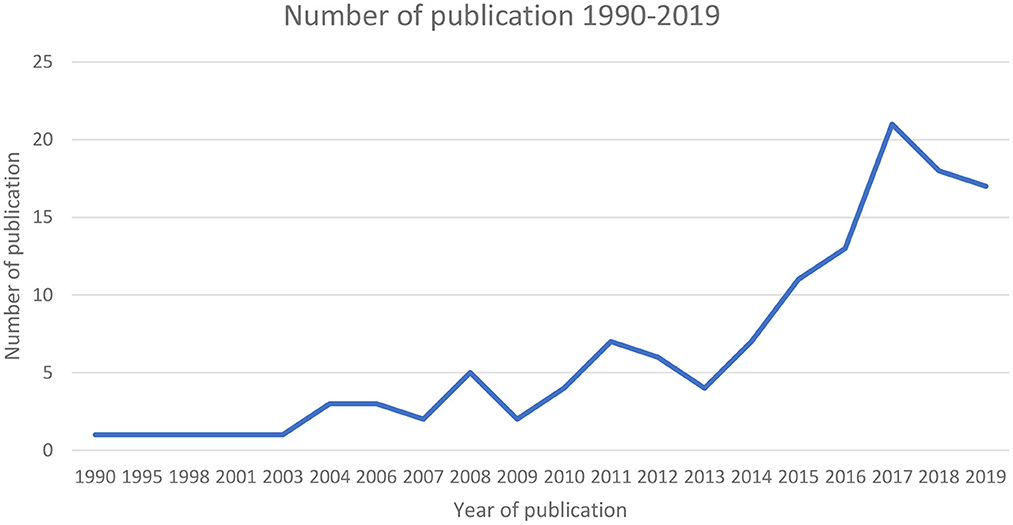
Figure 3. Number of retained publications on FBD in Ethiopia between 1990 and 2019. The solid line shows actual number of articles published in a particular year.
Majority of the articles included in the review (119/128, 93%) focused on biological hazards and six articles looked at presence of chemical hazards in food. Among the 119 articles focused on biological hazards, 82/119 (68.9%) investigated presence of pathogens in food, 48/119 (40.3%) presence in human samples (stool and hand swab samples) and 19/119 (15.9%) in food environments (i.e., beef slaughterhouses, butcheries, and milk shops). While half of the articles studied one food item and one hazard, other half covered various hazards and/or various matrices. None of the selected studies covered incidence of FBD or FBD burden.
The majority (n = 119, 92.9%) of the 128 papers examined the presence of bacteria (n = 87, 73.11%), parasites (n = 23, 19.33%), parasites and bacteria (n = 7, 5.88%), fungal toxins (n = 3, 2.52%), and viruses (n = 1, 0.84%) using a cross-sectional design. Nearly half of the articles (47.3%) incorporated risk factor analysis. The majority of these publications (n = 32, 55.7%) concentrated on evaluating risks from food handlers, feverish patients in healthcare facilities, and randomly selected school students or community members. Of the 128 retained articles, 72 (56.25%) were mainly focused on detecting pathogens in animal source foods. Bacterial contamination was reported in food products in beef, dairy, and poultry value chains while parasites were reported from apparently healthy food handlers, from stool samples of patients of health care facilities and to some extent (4/10,40%) in vegetable value chain.
Figure 4 below, describes number of articles reporting a particular bacteria species.2
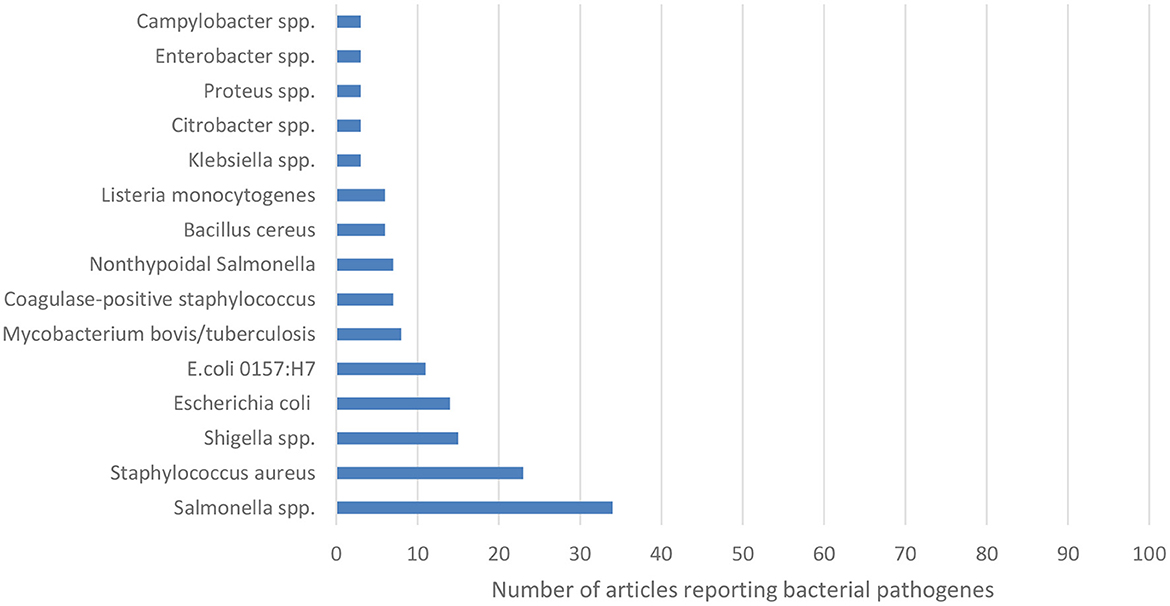
Figure 4. Number of articles reporting a particular bacterial pathogen. Numbers are derived from every article investigating on the pathogens. That is an article may report more than one bacteria species.
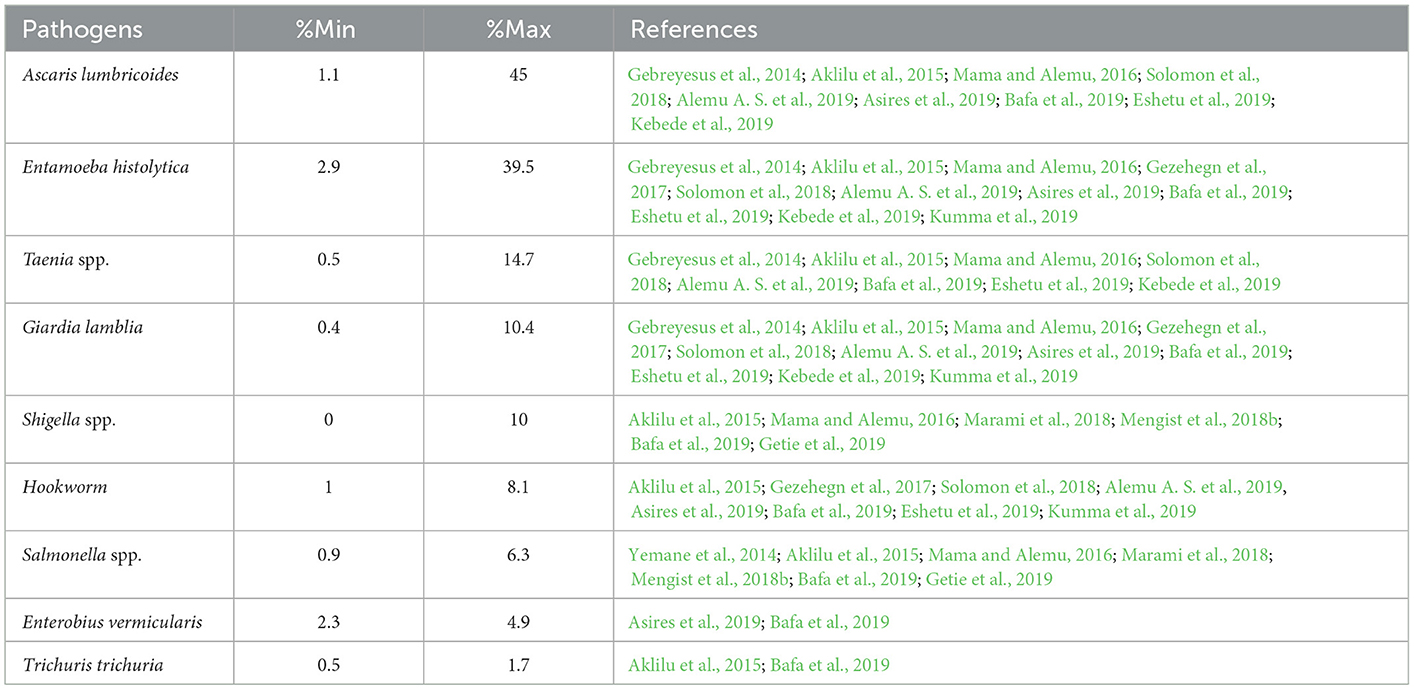
In terms of parasitic hazards, Entamoeba, Giardia and Ascaris were the most commonly studied hazards (Figure 5).
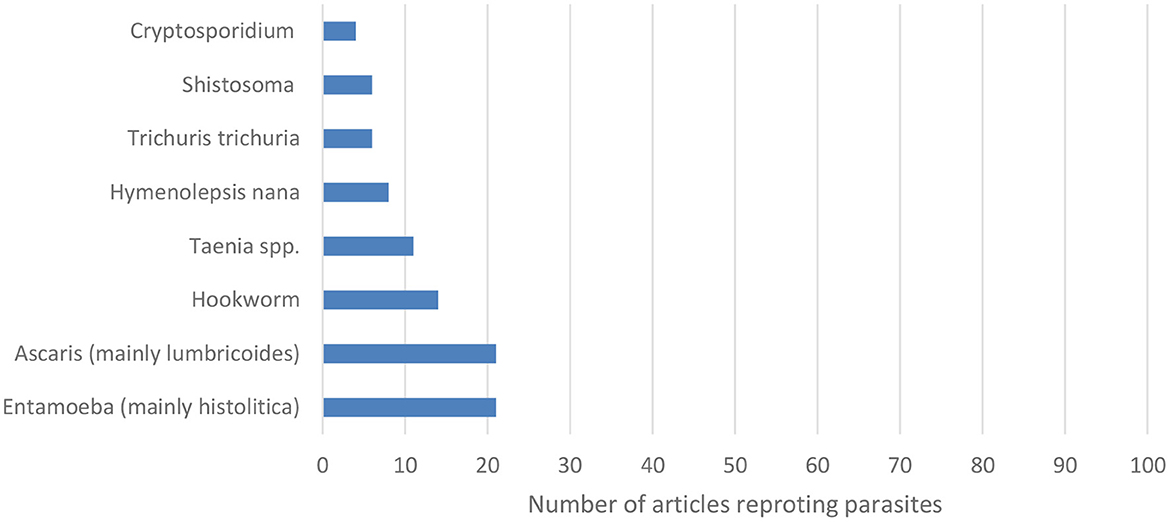
Figure 5. Number of articles reporting a particular parasite. Numbers are derived from every article investigating on parasites. That is an article may report more than one parasite.
Study designs were often duplicated, to identify similar pathogens in similar populations. For example, most of the articles investigating pathogens on food handlers were conducted in student cafeterias of public universities. However, this may be more down to convenience, rather than intended inter-study comparability or the widespread use of established, optimized methods. The parasites investigated in these articles were similar, focusing on widely known FB parasites (e.g., Ascaris lumbricoides, Giardia lamblia, Entamoeba histolytica, Taenia spp. etc.).
3.3. Prevalence of foodborne hazards in the food value chains
Of the 82 articles that investigated hazards in food, the majority (64/82, 78%) focused on beef, dairy, vegetable poultry, and eggs value chains. Other value chains, such as camel, pork, fish, mutton, goat meat, fruits, crops, and street-vended locally made food (Sambusa, Bombolino, donat, and all doughnut variants) were the focus of 18 (22%) articles, the results of which are not reported on this paper (Ashenafi, 1995; Muleta and Ashenafi, 2001; Molla et al., 2005; Abdel Gadir Atif et al., 2006; Hiko et al., 2008; Kibret and Tadesse, 2013; Dulo et al., 2015; Garedew et al., 2015b; Eromo et al., 2016; Taye et al., 2016; Messele et al., 2017; Wendwesen et al., 2017; Tegegne et al., 2019) (Supplementary material). This section presents the literature findings per value chain or host, and per pathogen investigated. Pathogens are listed from the more frequently studied to the least, for each value chain or host.
3.3.1. Beef value chain
Close to one third of the articles investigated bacterial pathogens in the beef value chain (37/128, 28.9%).
3.3.1.1. Salmonella spp.
Eighteen articles (48.6%) reported information on Salmonella spp. Salmonella spp. contamination estimates ranged from 1 to 13% in raw beef samples collected from slaughterhouses in different parts of the country (Molla et al., 2003; Alemu and Zewde, 2012; Hiko et al., 2016, 2018; Edget et al., 2017; Wabeto et al., 2017; Ketema et al., 2018; Takele et al., 2018; Bersisa et al., 2019), and between 1 and 35% in raw meat samples from butcheries and retail shops (Garedew et al., 2015b; Hiko et al., 2016, 2018; Edget et al., 2017). One study found 70% of fresh meat samples collected in butcher shops contaminated with Salmonella spp. (Azage and Kibret, 2017). Four articles investigated foodborne pathogens in “kitfo,” a traditional Ethiopian raw (or lightly cooked) minced meat dish. About 9.8–12, 8, and 30% of “kitfo” samples in supermarkets, restaurants, and street vendors, respectively, were found to carry Salmonella spp. (Muleta and Ashenafi, 2001; Molla et al., 2003; Ejo et al., 2016; Dagnachew, 2017). One article investigated Salmonella spp. in processed meat (mortadella) and found 0.8% positive samples (Hiko et al., 2015).
3.3.1.2. Escherichia coli
Eleven articles (29.7%) reported contamination of beef with E. coli. About 5.5%−35.5% of raw beef samples from slaughterhouses,13.8% from restaurants, and 6%−29.4% from supermarkets were found contaminated with E. coli (Hiko et al., 2015; Edget et al., 2017; Messele et al., 2017; Bedasa et al., 2018; Bersisa et al., 2019). Kumar et al. (2014), reported that 62.5% of raw beef samples from butcher shops were positive for E. coli. Articles showed E. coli O157:H7 detection ranged from 0.8 to 9% in raw beef samples from slaughterhouses (Hiko et al., 2016; Abdissa et al., 2017; Atnafie et al., 2017; Edget et al., 2017; Haile et al., 2017; Bedasa et al., 2018). The rate of contamination was higher in raw beef samples from butchers (2%−18%) (Kumar et al., 2014; Hiko et al., 2016; Atnafie et al., 2017; Beyi et al., 2017). Escherichia coli O157:H7 was detected in raw beef and minced meat samples in (0%−3.1%) from retailer shops, restaurants, and supermarkets (Beyi et al., 2017; Edget et al., 2017; Bedasa et al., 2018).
3.3.1.3. Mycobacterium bovis
Five articles (13.5%) looked at Mycobacterium bovis; postmortem inspection studies conducted in abattoirs reported 0.8%−10% of carcasses showing mycobacterial lesions. The sensitivity and specificity of the routine postmortem examination method was also reported to be lower compared to detailed inspection and culturing. Specifically, 0.7–7.5 and 2.7–19.4% of carcass negative in routine postmortem examination were found to be positive in detailed examination and by culture, respectively (Asseged et al., 2004; Teklu et al., 2004; Demelash et al., 2009; Biffa et al., 2010; Aylate et al., 2013).
3.3.1.4. Listeria monocytogenes
In four (10.8%) articles investigating L. monocytogenes, 1.6%–−2.6% of raw and minced meat samples at retailer shops in Addis Ababa were found to be positive (Molla et al., 2005; Gebretsadik et al., 2011; Derra et al., 2013; Garedew et al., 2015b). 6.7%−12% of raw meat and minced meat samples from restaurants in Gondar showed contamination with L. monocytogenes (Garedew et al., 2015b).
3.3.1.5. Staphylococcus spp.
According to findings in three (8.1%) articles, up to 11.6% of samples from slaughterhouses and 19.7% from butchers were contaminated with Staphylococcus aureus (Beyene et al., 2017; Adugna et al., 2018; Bersisa et al., 2019). Authors indicated continuous contamination throughout transportation from slaughterhouses to butcher shops (Tolosa et al., 2016; Beyene et al., 2017). Beyene et al. (2017) reported 10.6% carcass swabs from slaughterhouse were positive for coagulase-negative Staphylococci in beef.
3.3.1.6. Shigella spp.
Three articles (8.1%) investigated Shigella spp. in beef. None of the beef samples (“kitfo” and raw meat) collected from restaurants and slaughterhouses were positive for Shigella spp. (Muleta and Ashenafi, 2001; Bersisa et al., 2019). However, 11% of raw beef samples from butchers were found to be contaminated with this pathogen (Garedew et al., 2016).
3.3.2. Dairy value chain
Bacterial pathogens in dairy value chains were reported in 23.4% (30/128) of the articles reported.
3.3.2.1. Staphylococcus spp.
Different Staphylococcus spp. were reported in 15 articles (50%). Staphylococcus aureus was common in milk at the farm (14.3%−73.2%) and up to 80% of milk samples at collection centers carried this pathogen (Almaw et al., 2008; Lakew et al., 2009; Daka et al., 2012; Haftu et al., 2012; Makita et al., 2012; Tigabu et al., 2015; Abebe et al., 2016; Tolosa et al., 2016; Ayele et al., 2017; Beyene et al., 2017). Coagulase-negative Staphylococcus was found in 5%−15% of milk samples collected from farms (Almaw et al., 2008; Lakew et al., 2009; Beyene et al., 2017). Baby bottle (made of cow milk, powder, and cereal blend) samples collected from outpatient infants visiting public clinics in Addis Ababa were contaminated with S. aureus (64.2%−68.3%) (Erku and Ashenafi, 1998).
3.3.2.2. Listeria spp.
Six (20%) articles looked at the presence of Listeria spp. in the dairy value chain. Listeria monocytogenes was detected in 4%−13% of raw milk samples collected from retailers (Gebretsadik et al., 2011; Garedew et al., 2015b). While pasteurization should kill this bacterium, an article reported that 20% of pasteurized milk samples from retailers carried this pathogen, likely derived from cross-contamination during processing (Seyoum et al., 2015). However, another article reported that none of the pasteurized milk samples at restaurants carry L. monocytogenes (Garedew et al., 2015b). Contamination rate of L. monocytogenes in locally produced cottage cheese was low (0%−1%) (Molla et al., 2005; Gebretsadik et al., 2011; Garedew et al., 2015b). On the contrary, 27% of cheese and 5% of yogurt from supermarkets were positive for L. monocytogenes (Seyoum et al., 2015). Also, two articles reported that 15%−20% of ice cream samples from retailers were contaminated with L. monocytogenes (Molla et al., 2005; Garedew et al., 2015b).
3.3.2.3. Bacillus cereus
According to five (16.7%) articles, 0.6 to 0.8% of milk samples collected from producers were positive for B. cereus (Almaw et al., 2008; Getahun et al., 2008). Up to 63% of milk samples from markets were contaminated with B. cereus (Ashenafi, 1990; Abraha et al., 2017). Erku and Ashenafi (1998) also identified contamination of baby bottle contents with B. cereus, including 38.3% of cow's milk samples, 42.8% in cereal blend and none in powder milk.
3.3.2.4. Salmonella spp.
Five studies (16.7%) investigated Salmonella spp. in the dairy value chain. Three percent to 20% of milk samples collected at dairy farms carried Salmonella spp. (Addis et al., 2011; Tadesse and Dabassa, 2012). While Salmonella spp. was absent in dairy products (cottage cheese and cream) or pasteurized milk, 6% of raw milk samples from retailers were found to be contaminated (Ejo et al., 2016). Erku and Ashenafi (1998) also identified Salmonella contamination of baby bottle contents (3.3% of cow's milk and 7.1% of cereal blends), but not in powder milk.
3.3.2.5. Mycobacterium bovis
Mycobacterium species were the focus in four (13.3%) articles. One article reported that 13% of farms with tuberculosis reactors had milk contaminated with M. bovis (Fetene et al., 2011). Between 3%−14% of tuberculosis infected animals were reported to shed M. bovis in their milk (Ameni and Erkihun, 2007; Elias et al., 2008).
3.3.2.6. Escherichia coli
Articles on E. coli were seen in four (13.3%) recently published articles on the dairy value chain (since 2017). Articles on milk found E. coli contamination increasing from 7% on farm to above 60% at retailer milk shops selling raw milk. This increase was attributable to post-farm contamination and lack of cold chain (Disassa et al., 2017; Bedasa et al., 2018; Haftay et al., 2018; Messele et al., 2019). An article reported that none of the pasteurized milk samples collected at cafeterias, restaurants, and supermarkets were contaminated with E. coli while milk derived products, like cheese (40%) and yogurt (25.7%), contained E. coli (Bedasa et al., 2018). Two articles detected E. coli O157:H7 in pasteurized milk (5.7%) sampled from cafeterias, restaurants, open markets, and supermarket but E. coli O157:H7 was not detected in yogurt and cheese samples (Disassa et al., 2017; Bedasa et al., 2018).
3.3.3. Poultry and egg value chains
Ten (7.81%) of the included articles reported pathogens in poultry value chain. An article on chicken meat detected E. coli in 37% of samples from slaughterhouses (Messele et al., 2017). Off the 452 chicken meat samples from retailer shops, 11.5% were contaminated with Non-typhoidal Salmonella spp. (Molla et al., 2003). Listeria monocytonenes was detected in 1.9% of raw chicken meat samples collected from retailers (Molla et al., 2005). Salmonella spp. was isolated in 2.6%−18% of egg content and egg sandwich samples collected from either retailers, producers and restaurants (Muleta and Ashenafi, 2001; Bayu et al., 2013; Ejo et al., 2016; Kemal et al., 2016; Taddese et al., 2019). Listeria monocytogenes was detected in 4.3% of eggs sampled at retailer (Gebretsadik et al., 2011).
3.3.4. Vegetable value chain
Ten (7.81%) of the included articles investigated hazards in vegetable value chain. High parasite contamination rates were reported for a range of raw vegetables. Presence of at least one type of parasite (A. lumbricoides, E. histolytica/dispar, G. lamblia) in samples of raw vegetables (including green paper, carrot, tomato, cabbage, lettuce) was reported in 49%−71% samples (Bekele et al., 2017; Alemu A. S. et al., 2019; Bekele and Shumbej, 2019). Two articles looked at bacterial hazards in vegetables and reported Salmonella spp. in 0 to 10% of samples and Shigella spp. in 10%−20% of samples (Guchi and Ashenafi, 2011; Eromo et al., 2016). Contaminated irrigation water attributed to open air defecation was stated as a possible source of vegetable contamination at the farm (Alemu G. et al., 2019).
3.3.5. Prevalence of hazards in the environment
The selected articles included studies looking at food safety hazards in the environment of beef slaughterhouses (six articles), butcheries (three articles) and milk shops (two articles). However, these studies had small samples sizes (2–30 samples per study).
In slaughterhouses, Salmonella spp. was detected in workers hand swab (2%−50%), surfaces (50%), environmental pooled samples (6.7%), aprons (7.1%), knives (7.7%), room floor (23.5%), refrigerator (10%), beef transport truck (36.4%) and tap water (8.3%) used for washing (Sibhat et al., 2011; Edget et al., 2017; Hiko et al., 2018). Escherichia coli was present on 50, 23, and 50% of samples from equipment, environment pooled samples and workers hand swab, respectively (Edget et al., 2017; Bersisa et al., 2019). Escherichia coli O157:H7 was identified on knife swabs (13.3%) and in environmental pooled samples (6.6%) at slaughterhouses (Atnafie et al., 2017; Edget et al., 2017). Staphylococcus spp. (S. aureus, Staphylococcus intermedius, and Staphylococcus hyicus) were detected in swab samples from knives (16.7%−33.3%) and hanging materials (33.3%−50%) (Beyene et al., 2017).
About 0%−6.6% cutting board swabs samples from butchers were positive for E. coli O157:H7 (Atnafie et al., 2017; Beyi et al., 2017). Bersisa et al. (2019) reported 11.1% cutting board swab and 5.5% of knives swab in butchery shops positive for Salmonella species. In One article E. coli was found in 25% and 19.4% of cutting board and knives swab. This study also reported presence of other bacteria (Klebsiella, Proteus, and Shigella species) in butchery shops (Bersisa et al., 2019).
Articles found S. aureus contamination rate ranging between 12 and 25% in samples from milk buckets. Same rate was reported in milk tank samples (Ayele et al., 2017; Beyene et al., 2017).
3.4. Prevalence in clinically healthy food operators
Articles investigated (26/128, 20%) carriage of bacteria and parasites by food handlers, including workers in universities cafeteria (9/26, 34%), workers at dairy farms, abattoirs, and butchery (9/26, 34%), and workers in other food establishments (hotels, restaurants, bars, and cafeterias; 6/26, 23%). Nineteen (73%) and 12 (46%) of the 26 articles, respectively identified bacteria (mainly Salmonella spp., Shigella spp. and S. aureus) and parasites from stool samples collected from apparently health food handlers. Table 3 presents the range of contamination with different foodborne pathogens reported in the selected articles.
One article reported 6% of stool samples from abattoir staff carrying non-typhoidal Salmonella spp. (Molla et al., 2003).
Four articles reported isolation of different bacteria species on worker's hand swab samples. Results showed these swab samples were frequently positive for Salmonella spp., having been found in 24% of samples from butchers' shops operators and 30%−50% of samples of slaughterhouse personnel (Garedew et al., 2015a; Edget et al., 2017; Hiko et al., 2018). Shigella spp. was present in 13% of hand swab samples from butcher shops. Staphylococcus aureus was reported in 25%−32% of dairy farm milkers, and coagulase-negative Staphylococcus in 12%−16% of dairy and beef farm workers' hands. Escherichia coli was found in 50% of hand swabs taken from slaughterhouse workers (Garedew et al., 2016; Ayele et al., 2017; Beyene et al., 2017; Edget et al., 2017).
Swabs from fingernails examined for the presence of bacteria and internal parasites were often positive for coagulase-negative Staphylococcus (12%) and S. aureus (5%) (Mengist et al., 2018a).
3.5. Prevalence in non-food operators
Parasites were the most common foodborne hazard investigated in stool samples from children at school, patients visiting health centers and household members of community, including A. lumbricoides (4.2%−28%), G. lamblia (0.8%−10%), Entamoeba (6.7%−7.8%), Trichuris trichuria (0.4%−5.6%), hookworm (0.6%−1.3%) and other parasites (Desalegn et al., 2014; Alelign et al., 2015; Jejaw et al., 2015; Gebresilasie et al., 2018; Gizaw et al., 2018; Mekonnen and Ekubagewargies, 2019).
In stool samples from adult patients (mostly with enteric signs), Salmonella spp. (non-typhoidal Salmonella 7.18%−39.7%, typhoidal Salmonella 0.8%−39.7%, and unspecified Salmonella species 10.7%), Shigella (1.13%−13.86%), Campylobacter spp. (Campylobacter jejuni 7.3%−11.89%, C. coli 0.6%−3.5%), and internal parasites (Entamoeba, Giardia and Cryptosporidium in 24.6%−35.6% of the patients) were identified (Kassu et al., 2007; Ewnetu and Mihret, 2010; Tafa et al., 2014; Eguale et al., 2015, 2018; Lamboro et al., 2016; Berhe et al., 2018; Deksissa and Gebremedhin, 2019). Acute gastroenteritis patients were positive for norovirus (25.3%) and less commonly for sapovirus (4.2%) (Sisay et al., 2016).
Among prison inmates in north Ethiopia, intestinal parasites were detected in 40% of sample population and the dominant parasite was E. histolytica/dispar (n = 60, 22.2%) followed by G. lamblia, 39 (14.4%) (Mardu et al., 2019). In Gondar, 37.0% of apparently healthy street dwellers carried A. lumbricoides (Moges et al., 2006).
An article exploring the risk of congenital transmission of Toxoplasma gondii showed that 85% of pregnant women monitored in a hospital in Ethiopia had seroconverted by the third trimester of pregnancy (Gelaye et al., 2015). In another article, 31.3% of pregnant women attending antenatal care at Gondar were infected with one or more intestinal parasites. The most common single and mixed parasites observed were E. histolytica (38.1%) and A. lumbricoides (24.6%) (Kumera et al., 2018). Entamoeba histolytica, G. lamblia, Taenia species, A. lumbricoides, and Cryptosporidium parvum were mainly identified in one article which determine the presence of intestinal parasites and associated risk factors among HIV/AIDS patients (Gedle et al., 2017).
4. Discussion
The literature on FBDs hazards in Ethiopia is patchy, mostly consisting of small ad hoc local investigations, with no single comprehensive overview of the topic. This is not a unique feature of Ethiopia and has been reported across Africa (Alonso et al., 2016). The majority of the studies were performed in Oromia, Amhara, Addis Ababa and Southern Nation Nationalities People (SNNP) region which may reflect local outbreaks occurring more frequently in this area due to presence of many food establishments and consumers (Addis Ababa and surrounding Oromia region). It may also reflect a biased picture, with studies performed where relevant research groups happen to be based. The Ethiopian FBD literature has focused on measuring food contamination with key biological hazards, especially bacteria, which are suggested to account for much of the FBD burden (Havelaar et al., 2015).
The identified articles focusing on the prevalence of FB hazards in humans mostly focused on parasitic infection in children (sampled from schools, health centers and community households), adult patients with enteric signs, and susceptible populations (pregnant women and HIV/AIDS patients). A few studies looked at, and reported findings from, presence of Salmonella spp. (typhoidal and non-typhoidal), Campylobacter spp. and Shigella spp. in blood and stool samples.
Some of the most important bacteria in terms of public health (e.g., non-typhoidal Salmonella, L. monocytogenes, and Campylobacter spp.), compared to Salmonella, Staphylococcus and Shigella, have received, to date, little attention in the country. Assessments of the amount of these bacterial pathogens present in food and food environments are very rare. Few of the included articles which reported pathogens in environment do not take representative samples which compromises the quality of work. However, such quantitative assessments are needed to estimate consumer-pathogen exposure and health and economic risk from FBD (Zaneti et al., 2021). The outputs of such assessments are a critical part of food safety management systems and are important for countries to prioritize food safety areas of interventions.
Hazards that are harder to study such as chemicals, viruses, and certain bacteria like Campylobacter spp., were investigated less frequently. This is a well-known challenge in low- and middle-income countries, where resources and facilities for diagnostics are often limited. In our review, we observed an increase overtime in the number of articles on FBD. This could be suggestive of an increased interest in the topic over the past few years, which could have been matched with increased funding for research in this area. It could also be a result of the quality of articles having improved overtime, meaning that an increasing proportion of identified papers would have passed the review's quality assessment which is evidenced by increased number of included (five-fold) than excluded articles from 2015 to 2019. Both options are encouraging and suggest that more attention is being given to food safety and FBD among the national scientific and the international donor communities.
Foodborne pathogens, such as intestinal parasites, E. coli including O157:H7, Salmonella species and S. aureus, were commonly isolated on different foods and at different levels in various value chains. This is not surprising as the level of hygiene and the application of good practices of food quality management are highly variable across the country, but in general are limited, especially in rural areas. Even simple equipment, refrigeration, and key infrastructure, such as a reliable power and clean water supply are not available in informal food supply chains, where most people get their food (FAO, 2007; Glatzel, 2017) Even in the more up-market outlets, including supermarkets and restaurants, food safety is a challenge, given both the limited infrastructure and the relative lack of quality suppliers and quality management.
Beef and milk are widely consumed in Ethiopia and were often the target of the included articles. Although consumption of raw beef is a common practice in Ethiopia, hygiene standards in abattoirs are poor, with high levels of E. coli and Salmonella spp. For most pathogens, contamination rates are lower for samples of product collected in slaughterhouses compared to subsequent steps in the supply chain. In the case of meat, the butcher appears to be a node in the chain where levels of contamination tend to increase. Unhygienic practices, both at the slaughterhouses and retail shops, which underpin the public health risk associated with meat-borne pathogens, have been reported in Ethiopia (Gutema et al., 2021).
A variety of articles assessed hygiene and bacterial contamination of milk, typically finding high microbial contamination. Lack of cold chain and the presence of technical limitations by dairy operators were frequently reported as reasons for poor microbial quality of milk (Disassa et al., 2017; Bedasa et al., 2018; Haftay et al., 2018; Messele et al., 2019). Escherichia coli, S. aureus, and Bacillus spp. were the most prevalent bacteria identified in the milk value chain. A recent article showed that the proportion of contamination was significantly lower in milk collected from dairy farms when compared to milk from vendors (Berhe et al., 2020). Generally, presence of E. coli, E. coli 0157:H7, Bacillus spp, and Listeria spp was more likely in raw milk samples collected from retailers than those from producers, indicating that milk microbial quality may derived from contamination at various points of the value chain post-harvest, and that storage conditions are facilitating bacterial growth. The literature also showed contamination by foodborne pathogens of various milk derived products; it is worth noting that, in Ethiopia, these products are typically consumed without any further processing at home, meaning that no steps that could reduce the pathogen load are taken before consumption (Beyene et al., 2017; Amenu et al., 2019; Mebrate et al., 2020; Deneke et al., 2022). The presence of hazards in pasteurized milk reported in some of the studies is concerning. Pasteurization is a heat-treatment process used to decrease the bacterial load of milk. Presence of bacteria in pasteurized milk is indicative of failures in the pasteurization process, cross- or re-contamination post-pasteurization or inadequate storage after pasteurization (Garedew et al., 2012; Tekilegiorgis, 2018).
The importance of beef and milk processing points and practices to food safety, are highlighted in articles from beef and dairy value chains. Foodborne pathogens originating from fecal contamination during slaughter, such as Salmonella spp. and E. coli, can potentially contaminate the carcass and spread to the cut or raw meat intended for further processing, causing a major public health threat (Soepranianondo et al., 2019). This is supported by several of the included articles which show presence of different bacterial species in samples collected from different environmental surfaces at beef slaughterhouses (Hiko et al., 2015; Edget et al., 2017; Messele et al., 2017; Bedasa et al., 2018). However, extrapolation of these findings to the entire country could not be reliable due to the small sample sizes and geographical coverage in the majority of the articles reviewed.
Further evidence of sources of contamination along the food value chains is presented by the microbial investigations of apparently healthy food handlers. These records confirm the potential role of food handlers in the spread of FBD (Dagnew et al., 2012). Food handlers with poor health and hygiene may be infected with a wide range of foodborne pathogens and have already been demonstrated to play a role in transmitting disease to the public (Khurana et al., 2007). This is an important area in food safety research, and our results show that it deserves greater attention in the country.
There were no published assessments of FBD burden and incidence in humans. The SLR only included published literature but did not consider hospital records that are unpublished (gray literature), therefore, the study cannot assess the true burden of FBD. However, it is true that in Ethiopia, many foods are consumed raw (beef, milk) (Dagne et al., 2022; Deneke et al., 2022), therefore the risk of FBD is higher if the prevalence of pathogens in the product is high. Disease burden and cost estimates are critical for risk-based decision-making. Estimating the incidence of illness caused by FBD is a gap to be addressed in the future.
It is important for policy makers to know the burden of a disease in order to allocate appropriate resources for its control. However, FBD burden is harder to measure than food contamination, either requiring an effective FBDs surveillance system, which does not exist in many low- and middle-income countries, or well-designed, large epidemiological studies. These studies require complex analysis to overcome issues of under-reporting and imperfect diagnosis.
We acknowledge that the search being done only in two databases and required articles to be available electronically some articles may not have been detected. Therefore, information from gray literatures is not included. However, all quality research articles are expected to have been captured and inclusion of electronically available articles as a limitation to this review. Only 10% (n = 13) of the 128 articles were reviewed in pairs. This may also be one limitation of this review. In addition, publications since 2019 recent years are not considered due to time constraints and because the review was performed at the time to inform overarching research projects that started around 2019. Lastly, we cannot exclude the possibility that the results from our review are affected by publication bias, but we have no ability to estimate the magnitude of that potential bias.
5. Conclusion
In conclusion, little has been done to assess FBD burden in Ethiopia. The scientific literature reveals high levels of contamination, with both bacterial and parasitic pathogens, and shows fundamental gaps in food safety for many food value chains. Pathogens that are hard to assess are largely over-looked. In both beef and dairy value chains bacterial contamination was observed with increasing prevalence from farm/slaughterhouse to point of sale. Given the findings, the following recommendations are made to improve food safety in Ethiopia:
1. More systematic and ongoing evaluation of contamination should be implemented to provide a comprehensive overview of the topic including in Benishangul-gumuz, Afar, and Somali regions.
2. Chemicals, viruses and some of the most important bacteria which are of public health concern should be investigated more.
3. Future research on FBD should thoroughly investigate risk factors.
4. The potential role of food handlers and food environment should be investigated in detail by considering representative samples.
5. In addition to assessing presence or absence of hazards, quantitative assessments of the amount of hazards present in food and food environments is required.
6. Due attention should be given to vegetables, fruits, crops, fish, sheep, goats, and camel value chains.
7. Incidence of human FBDs or resulting health and economic impacts should also be center of attention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceived and designed the study protocol: DG, SA, FMu, KR, JL, KA, and MD. Carried out the screening and data extraction from records: DG, SA, FMu, KR, JL, LG, KA, FMa, PU, TG, and GI. Drafted the manuscript: LG, SA, and TK-J. All authors read, reviewed, and approved the final manuscript.
Funding
This publication is based on research funded by the Bill & Melinda Gates Foundation and the UK Government Foreign, Commonwealth & Development Office (FCDO)—UK Aid from the United Kingdom government (INV-008430-OPP1195588 and OPP1156625). The findings and conclusions are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation or the UK government. The work also received financial support from the CGIAR Research Program on Agriculture for Nutrition and Health. The funder played no role in the design or conclusion of the study.
Acknowledgments
This activity is part of the Urban food markets in Africa: incentivizing food safety using a pull-push approach and the MoreMilk: making the most of milk investments, which are supported by the Bill & Melinda Gates Foundation, the Foreign, Commonwealth and Development Office (FCDO) of the UK Government, with co-funding from the One Health Research, Education and Outreach Center in Africa (OHRECA) funded by German Federal Ministry of Economic Cooperation and Development (BMZ) and the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH) led by the International Food Policy Research Institute. We also acknowledge the CGIAR Fund Donors (https://www.cgiar.org/funders).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1058977/full#supplementary-material
Footnotes
1. ^
• Urban food markets in Africa: Incentivizing food safety using a pull-push approach (“pull-push” project), led by the International Livestock Research Institute.
• The assessment and management of risk from non-typhoidal Salmonella, diarrheagenic Escherichia coli and Campylobacter in raw beef and dairy in Ethiopia (TARTARE), led by Ohio State University.
• Foodborne disease epidemiology, surveillance and control in African LMIC (FOCAL), led by Technical University of Denmark.
• Ensuring the safety and quality of milk and dairy products across the dairy value chain in Ethiopia (ENSURE), led by Addis Ababa University.
2. ^Salmonella spp.—many studies did not provide the speciation of Salmonella, and it is possible some of these studies may or may not include Non-Typhoidal Salmonella., N.B. Salmonella spp. does not include Non-Typhoidal Salmonella, and Escherichia coli does not include E. coli O157:H7. N.B. articles may include more than one bacteria and parasite.
References
Abdel Gadir Atif, E., Hildebrandt, G., Kleer, J. N., Molla, B., Kyule, M. N., and Baumann, M. P. Comparison of California Mastitis Test (CMT), Somatic Cell Counts (SCC) and bacteriological examinations for detection of camel (Camelus dromedarius) mastitis in Ethiopia. Berl Munch Tierarztl Wochenschr. (2006) 119, 45–49.
Abdissa, R., Haile, W., Fite, A. T., Beyi, A. F., Agga, G. E., Edao, B. M., et al. (2017). Prevalence of Escherichia coli O157: H7 in beef cattle at slaughter and beef carcasses at retail shops in Ethiopia. BMC Infect. Dis. 17, 277. doi: 10.1186/s12879-017-2372-2
Abebe, R., Hatiya, H., Abera, M., Megersa, B., and Asmare, K. (2016). Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 12, 270. doi: 10.1186/s12917-016-0905-3
Abraha, A., Bikila, T., Alemu, S., and Muktar, Y. (2017). Bacillus cereus isolation and load from raw cow milk sold in markets of Haramaya district, eastern Ethiopia. Int. J. Food Contam. 4, 15. doi: 10.1186/s40550-017-0060-z
Addis, Z., Kebede, N., Worku, Z., Gezahegn, H., Yirsaw, A., and Kassa, T. (2011). Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect. Dis. 11, 222. doi: 10.1186/1471-2334-11-222
Adugna, F., Pal, M., and Girmay, G. (2018). Prevalence and antibiogram assessment of Staphylococcus aureus in beef at Municipal Abattoir and butcher shops in Addis Ababa, Ethiopia. Biomed Res. Int. 2018, 5017685. doi: 10.1155/2018/5017685
Aklilu, A., Kahase, D., Dessalegn, M., Tarekegn, N., Gebremichael, S., Zenebe, S., et al. (2015). Prevalence of intestinal parasites, Salmonella and Shigella among apparently health food handlers of Addis Ababa University student's cafeteria, Addis Ababa, Ethiopia. BMC Res. Notes 8, 17. doi: 10.1186/s13104-014-0967-x
Alelign, T., Degarege, A., and Erko, B. (2015). Prevalence and factors associated with undernutrition and anaemia among school children in Durbete Town, northwest Ethiopia. Arch. Public Health 73, 34. doi: 10.1186/s13690-015-0084-x
Alemu, A. S., Baraki, A. G., Alemayehu, M., and Yenit, M. K. (2019). The prevalence of intestinal parasite infection and associated factors among food handlers in eating and drinking establishments in Chagni Town, Northwest Ethiopia. BMC Res. Notes 12, 302. doi: 10.1186/s13104-019-4338-5
Alemu, G., Mama, M., Misker, D., and Haftu, D. (2019). Parasitic contamination of vegetables marketed in Arba Minch town, southern Ethiopia. BMC Infect. Dis. 19, 410. doi: 10.1186/s12879-019-4020-5
Alemu, S., and Zewde, B. M. (2012). Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Trop. Anim. Health Prod. 44, 595–600. doi: 10.1007/s11250-011-9941-y
Almaw, G., Zerihun, A., and Asfaw, Y. (2008). Bovine mastitis and its association with selected risk factors in smallholder dairy farms in and around Bahir Dar, Ethiopia. Trop. Anim. Health Prod. 40, 427–432. doi: 10.1007/s11250-007-9115-0
Alonso, S., Lindahl, J., Roesel, K., Traore, S. G., Yobouet, B. A., Ndour, A. P. N., et al. (2016). Where literature is scarce: observations and lessons learnt from four systematic reviews of zoonoses in African countries. Anim. Health Res. Rev. 17, 28–38. doi: 10.1017/S1466252316000104
Ameni, G., and Erkihun, A. (2007). Bovine tuberculosis on small-scale dairy farms in Adama Town, central Ethiopia, and farmer awareness of the disease. Rev. Sci. Tech. 26, 711–719. doi: 10.20506/rst.26.3.1778
Amenu, K., Wieland, B., Szonyi, B., and Grace, D. (2019). Milk handling practices and consumption behavior among Borana pastoralists in southern Ethiopia. J. Health Popul. Nutr. 38, 6. doi: 10.1186/s41043-019-0163-7
Ashenafi, M. (1990). Microbiological quality of ayib, a traditional Ethiopian cottage cheese. Int. J. Food Microbiol. 10, 263–268. doi: 10.1016/0168-1605(90)90074-F
Ashenafi, M. (1995). Bacteriological profile and holding temperatures of ready-to-serve food items in an open market in Awassa, Ethiopia. Trop. Geogr. Med. 47, 244–247.
Asires, A., Wubie, M., and Reta, A. (2019). Prevalence and associated factors of intestinal parasitic infections among food handlers at Prison, East and West Gojjam, Ethiopia. Adv. Med. 2019, 1–8. doi: 10.1155/2019/2101089
Asseged, B., Woldesenbet, Z., Yimer, E., and Lemma, E. (2004). Evaluation of abattoir inspection for the diagnosis of Mycobacterium bovis infection in cattle at Addis Ababa abattoir. Trop. Anim. Health Prod. 36, 537–546. doi: 10.1023/B:TROP.0000040934.32330.44
Atnafie, B., Paulos, D., Abera, M., Tefera, G., Hailu, D., Kasaye, S., et al. (2017). Occurrence of Escherichia coli O157:H7 in cattle feces and contamination of carcass and various contact surfaces in abattoir and butcher shops of Hawassa, Ethiopia. BMC Microbiol. 17, 24. doi: 10.1186/s12866-017-0938-1
Ayana, Z., Yohannis, M., and Abera, Z. (2015). Food-borne bacterial diseases in Ethiopia. Acad. J. Nutr. 4, 62–76. doi: 10.5829/idosi.ajn.2015.4.1.95168
Ayele, Y., Gutema, F. D., Edao, B. M., Girma, R., Tufa, T. B., Beyene, T. J., et al. (2017). Assessment of Staphylococcus aureus along milk value chain and its public health importance in Sebeta, central Oromia, Ethiopia. BMC Microbiol. 17, 1–7. doi: 10.1186/s12866-017-1048-9
Aylate, A., Shah, S. N., Aleme, H., and Gizaw, T. T. (2013). Bovine tuberculosis: prevalence and diagnostic efficacy of routine meat inspection procedure in Woldiya municipality abattoir north Wollo zone, Ethiopia. Trop. Anim. Health Prod. 45, 855–864. doi: 10.1007/s11250-012-0298-7
Azage, M., and Kibret, M. (2017). The bacteriological quality, safety, and antibiogram of Salmonella Isolates from fresh meat in retail shops of Bahir Dar City, Ethiopia. Int. J. Food Sci. 2017, 4317202. doi: 10.1155/2017/4317202
Bafa, T. A., Sherif, E. M., Hantalo, A. H., and Woldeamanuel, G. G. (2019). Magnitude of enteropathogens and associated factors among apparently healthy food handlers at Wolkite University Student's Cafeteria, Southern Ethiopia. BMC Res. Notes 12, 567. doi: 10.1186/s13104-019-4599-z
Bayu, Z., Asrade, B., Kebede, N., Sisay, Z., and Bayu, Y. (2013). Identification and characterization of Salmonella species in whole egg purchased from local markets in Addis Ababa, Ethiopia. J. Vet. Med. Anim. Health 5, 133–137. doi: 10.5897/JVMAH12.055
Bedasa, S., Shiferaw, D., Abraha, A., and Moges, T. (2018). Occurrence and antimicrobial susceptibility profile of Escherichia coli O157:H7 from food of animal origin in bishoftu town, central Ethiopia. Int. J. Food Contam. 5, 2. doi: 10.1186/s40550-018-0064-3
Bekele, F., and Shumbej, T. (2019). Fruit and vegetable contamination with medically important helminths and protozoans in Tarcha town, Dawuro zone, Southwest Ethiopia. Res. Rep. Trop. Med. 10, 19–23. doi: 10.2147/RRTM.S205250
Bekele, F., Tefera, T., Biresaw, G., and Yohannes, T. (2017). Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect. Dis. Poverty 6, 19. doi: 10.1186/s40249-016-0226-6
Belina, D., Hailu, Y., Gobena, T., Hald, T., and Njage, P. M. K. (2021). Prevalence and epidemiological distribution of selected foodborne pathogens in human and different environmental samples in Ethiopia: a systematic review and meta-analysis. One Health Outlook 3, 19. doi: 10.1186/s42522-021-00048-5
Berhe, B., Bugssa, G., Bayisa, S., and Alemu, M. (2018). Foodborne intestinal protozoan infection and associated factors among patients with watery diarrhea in Northern Ethiopia; a cross-sectional study. J. Health Popul. Nutr. 37, 5. doi: 10.1186/s41043-018-0137-1
Berhe, G., Wasihun, A. G., Kassaye, E., and Gebreselasie, K. (2020). Milk-borne bacterial health hazards in milk produced for commercial purpose in Tigray, northern Ethiopia. BMC Public Health 20, 894. doi: 10.1186/s12889-020-09016-6
Bersisa, A., Tulu, D., and Negera, C. (2019). Investigation of bacteriological quality of meat from abattoir and butcher shops in Bishoftu, Central Ethiopia. Int. J. Microbiol. 2019, 6416803. doi: 10.1155/2019/6416803
Beyene, T., Hayishe, H., Gizaw, F., Beyi, A. F., Abunna, F., Mammo, B., et al. (2017). Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res Notes. 10, 1–9. doi: 10.1186/s13104-017-2487-y
Beyi, A. F., Fite, A. T., Tora, E., Tafese, A., Genu, T., Kaba, T., et al. (2017). Prevalence and antimicrobial susceptibility of Escherichia coli O157 in beef at butcher shops and restaurants in central Ethiopia. BMC Microbiol. 17, 49. doi: 10.1186/s12866-017-0964-z
Biffa, D., Bogale, A., and Skjerve, E. (2010). Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: implications for public health. BMC Public Health 10, 462. doi: 10.1186/1471-2458-10-462
Dagnachew, D. (2017). Distribution and antimicrobial resistance of Salmonella serotypes in minced beef, calves and humans in Bishoftu and Addis Ababa, Ethiopia. J. Parasitol. Vector Biol. 9, 64–72. doi: 10.5897/JPVB2016.0276
Dagne, T., Gutema, F. D., and Terefe, Y. (2022). Zoonotic diseases risk perceptions and protective behaviors of consumers associated with consumption of meat and milk in and around Bishoftu, Ethiopia. Heliyon 8, e10351. doi: 10.1016/j.heliyon.2022.e10351
Dagnew, M., Tiruneh, M., Moges, F., and Tekeste, Z. (2012). Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia. BMC Public Health 12, 837. doi: 10.1186/1471-2458-12-837
Daka, D., Gsilassie, S., and Yihdego, D. (2012). Antibiotic-resistance Staphylococcus aureus isolated from cow's milk in the Hawassa area, South Ethiopia. Ann. Clin. Microbiol. Antimicrob. 11, 26. doi: 10.1186/1476-0711-11-26
Deksissa, T., and Gebremedhin, E. Z. (2019). A cross-sectional study of enteric fever among febrile patients at Ambo hospital: prevalence, risk factors, comparison of Widal test and stool culture and antimicrobials susceptibility pattern of isolates. BMC Infect. Dis. 19, 288. doi: 10.1186/s12879-019-3917-3
Demelash, B., Inangolet, F., Oloya, J., Asseged, B., Badaso, M., Yilkal, A., et al. (2009). Prevalence of Bovine tuberculosis in Ethiopian slaughter cattle based on post-mortem examination. Trop. Anim. Health Prod. 41, 755–765. doi: 10.1007/s11250-008-9248-9
Deneke, T. T., Bekele, A., Moore, H. L., Mamo, T., Almaw, G., Mekonnen, G. A., et al. (2022). Milk and meat consumption patterns and the potential risk of zoonotic disease transmission among urban and peri-urban dairy farmers in Ethiopia. BMC Public Health 22, 222. doi: 10.1186/s12889-022-12665-4
Derra, F. A., Karlsmose, S., Monga, D. P., Mache, A., Svendsen, C. A., Félix, B., et al. (2013). Occurrence of Listeria spp. in retail meat and dairy products in the area of Addis Ababa, Ethiopia. Foodborne Pathog. Dis. 10, 577–579. doi: 10.1089/fpd.2012.1361
Desalegn, A., Mossie, A., and Gedefaw, L. (2014). Nutritional iron deficiency anemia: magnitude and its predictors among school age children, southwest Ethiopia: a community based cross-sectional study. PLoS ONE 9, e0114059. doi: 10.1371/journal.pone.0114059
Disassa, N., Sibhat, B., Mengistu, S., Muktar, Y., and Belina, D. (2017). Prevalence and antimicrobial susceptibility pattern of E. coli O157:H7 isolated from traditionally marketed raw cow milk in and around Asosa Town, Western Ethiopia. Vet. Med. Int. 2017, 7581531. doi: 10.1155/2017/7581531
Dulo, F., Feleke, A., Szonyi, B., Fries, R., Baumann, M. P. O., and Grace, D. (2015). Isolation of multidrug-resistant Escherichia coli O157 from goats in the somali region of Ethiopia: a cross-sectional, abattoir-based study. PLoS ONE 10, e0142905. doi: 10.1371/journal.pone.0142905
Edget, A., Shiferaw, D., and Mengistu, S. (2017). Microbial safety and its public health concern of E. coli O157:H7 and Salmonella spp. in beef at Dire Dawa administrative city and Haramaya University, Ethiopia. J. Vet. Med. Anim. Health. 9, 213–227. doi: 10.5897/JVMAH2015.0381
Eguale, T., Asrat, D., Alemayehu, H., Nana, I., Gebreyes, W. A., Gunn, J. S., et al. (2018). Phenotypic and genotypic characterization of temporally related non-typhoidal Salmonella strains isolated from humans and food animals in central Ethiopia. Zoonoses Public Health 65, 766–776. doi: 10.1111/zph.12490
Eguale, T., Gebreyes, W. A., Asrat, D., Alemayehu, H., Gunn, J. S., and Engidawork, E. (2015). Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect. Dis. 15, 497. doi: 10.1186/s12879-015-1235-y
Ejo, M., Garedew, L., Alebachew, Z., and Worku, W. (2016). Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. Biomed Res. Int. 2016, 4290506. doi: 10.1155/2016/4290506
Elias, K., Hussein, D., Asseged, B., Wondwossen, T., and Gebeyehu, M. (2008). Status of Bovine tuberculosis in Addis Ababa dairy farms. OIE Rev. Sci. Tech. 27, 915–923. doi: 10.20506/rst.27.3.1850
Erku, W. A., and Ashenafi, M. (1998). Prevalence of food-borne pathogens and growth potential of Salmonella in weaning foods from Addis Ababa, Ethiopia. East Afr. Med. J. 75, 215–218.
Eromo, T., Tassew, H., Daka, D., and Kibru, G. (2016). Bacteriological quality of street foods and antimicrobial resistance of isolates in Hawassa, Ethiopia. Ethiop. J. Health Sci. 26, 533–542. doi: 10.4314/ejhs.v26i6.5
Eshetu, L., Dabsu, R., and Tadele, G. (2019). Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte Town, West Oromia, Ethiopia. Res. Rep. Trop. Med. 10, 25–30. doi: 10.2147/RRTM.S186723
Ewnetu, D., and Mihret, A. (2010). Prevalence and antimicrobial resistance of Campylobacter isolates from humans and chickens in Bahir Dar, Ethiopia. Foodborne Pathog. Dis. 7, 667–670. doi: 10.1089/fpd.2009.0433
FAO (2007). Promises and Challenges of the Informal Food Sector in Developing Countries. Rome, Italy: A joint publication by the Rural Infrastructure and Agro-industries division and the Nutrition and Consumer Protection division of the Agriculture and Consumer Protection Department of FAO. I collaboration with the Agricultural Economics and Engineering Department (University of Bologna, Italy) and the Department of Sociology and Anthropology (University of Ottawa, Canada).
Fetene, T., Kebede, N., and Alem, G. (2011). Tuberculosis infection in animal and human populations in three districts of Western Gojam, Ethiopia. Zoonoses Public Health 58, 47–53. doi: 10.1111/j.1863-2378.2009.01265.x
Garedew, L., Berhanu, A., Mengesha, D., and Tsegay, G. (2012). Identification of gram-negative bacteria from critical control points of raw and pasteurized cow milk consumed at Gondar town and its suburbs, Ethiopia. BMC Public Health 12, 950. doi: 10.1186/1471-2458-12-950
Garedew, L., Hagos, Z., Addis, Z., Tesfaye, R., and Zegeye, B. (2015a). Prevalence and antimicrobial susceptibility patterns of Salmonella isolates in association with hygienic status from butcher shops in Gondar town, Ethiopia. Antimicrob. Resist. Infect. Control 4, 21. doi: 10.1186/s13756-015-0062-7
Garedew, L., Hagos, Z., Zegeye, B., and Addis, Z. (2016). The detection and antimicrobial susceptibility profile of Shigella isolates from meat and swab samples at butchers' shops in Gondar town, Northwest Ethiopia. J. Infect. Public Health 9, 348–355. doi: 10.1016/j.jiph.2015.10.015
Garedew, L., Taddese, A., Biru, T., Nigatu, S., Kebede, E., Ejo, M., et al. (2015b). Prevalence and antimicrobial susceptibility profile of Listeria species from ready-to-eat foods of animal origin in Gondar Town, Ethiopia. BMC Microbiol. 15, 100. doi: 10.1186/s12866-015-0434-4
Gebresilasie, Y. M., Tullu, K. D., and Yeshanew, A. G. (2018). Resistance pattern and maternal knowledge, attitude and practices of suspected diarrheagenic Escherichia coli among children under 5 years of age in Addis Ababa, Ethiopia: cross sectional study. Antimicrob. Resist. Infect. Control 7, 110. doi: 10.1186/s13756-018-0402-5
Gebretsadik, S., Kassa, T., Alemayehu, H., Huruy, K., and Kebede, N. (2011). Isolation and characterization of Listeria monocytogenes and other Listeria species in foods of animal origin in Addis Ababa, Ethiopia. J. Infect. Public Health 4, 22–29. doi: 10.1016/j.jiph.2010.10.002
Gebreyesus, A., Adane, K., Negash, L., Asmelash, T., Belay, S., Alemu, M., et al. (2014). Prevalence of Salmonella typhi and intestinal parasites among food handlers in Mekelle University student cafeteria, Mekelle, Ethiopia. Food Control 44, 45–48. doi: 10.1016/j.foodcont.2014.03.040
Gedle, D., Kumera, G., Eshete, T., Ketema, K., Adugna, H., and Feyera, F. (2017). Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDS patients on HAART in Butajira, Ethiopia. J. Health Popul. Nutr. 36, 15. doi: 10.1186/s41043-017-0092-2
Gelaye, W., Kebede, T., and Hailu, A. (2015). High prevalence of anti-toxoplasma antibodies and absence of Toxoplasma gondii infection risk factors among pregnant women attending routine antenatal care in two Hospitals of Addis Ababa, Ethiopia. Int. J. Infect. Dis. 34, 41–45. doi: 10.1016/j.ijid.2015.03.005
Getahun, K., Kelay, B., Bekana, M., and Lobago, F. (2008). Bovine mastitis and antibiotic resistance patterns in Selalle smallholder dairy farms, central Ethiopia. Trop. Anim. Health Prod. 40, 261–268. doi: 10.1007/s11250-007-9090-5
Getie, M., Abebe, W., and Tessema, B. (2019). Prevalence of enteric bacteria and their antimicrobial susceptibility patterns among food handlers in Gondar town, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 8, 111. doi: 10.1186/s13756-019-0566-7
Gezehegn, D., Abay, M., Tetemke, D., Zelalem, H., Teklay, H., Baraki, Z., et al. (2017). Prevalence and factors associated with intestinal parasites among food handlers of food and drinking establishments in Aksum Town, Northern Ethiopia. BMC Public Health 17, 819. doi: 10.1186/s12889-017-4831-5
Gizaw, Z., Adane, T., Azanaw, J., Addisu, A., and Haile, D. (2018). Childhood intestinal parasitic infection and sanitation predictors in rural Dembiya, northwest Ethiopia. Environ. Health Prev. Med. 23, 26. doi: 10.1186/s12199-018-0714-3
Glatzel, K. (2017). “Why supporting Africa's informal markets could mean better nutrition for poor city dwellers,” In IFPRI Blog: Research Post (Washington, DC: International Food Policy Research Institute). Available online at: https://www.ifpri.org/blog/why-supporting-africas-informal-markets-could-mean-better-nutrition-poor-city-dwellers#:~:text=This%20is%20because%20people%20living,their%20household%20income%20on%20food
Grace, D., Alonso, S., Mutua, F., Roesel, K., Lindahl, J., and Amenu, K. (2018). Food Safety Investment Expert Advice: Burkina Faso, Ethiopia, Nigeria. Nairobi, Kenya: ILRI.
Guchi, B., and Ashenafi, M. (2011). Microbial load, prevalence and antibiograms of Salmonella and Shigella in lettuce and green peppers. Ethiop. J. Health Sci. 20, 41–48. doi: 10.4314/ejhs.v20i1.69431
Gutema, F. D., Agga, G. E., Abdi, R. D., Jufare, A., Duchateau, L., de Zutter, L., et al. (2021). Assessment of hygienic practices in beef cattle slaughterhouses and retail shops in bishoftu, ethiopia: implications for public health. Int. J. Environ. Res. Public Health 18, 1–13. doi: 10.3390/ijerph18052729
Haftay, A., Geberemedhin, H., Belay, A., Goytom, E., and Kidane, W. (2018). Antimicrobial resistance profile of Staphylococcus aureus isolated from raw cow milk and fresh fruit juice in Mekelle, Tigray, Ethiopia. J. Vet. Med. Anim. Health 10, 106–113. doi: 10.5897/JVMAH2017.0664
Haftu, R., Taddele, H., Gugsa, G., and Kalayou, S. (2012). Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 44, 1765–1771. doi: 10.1007/s11250-012-0135-z
Haile, A. F., Kebede, D., and Wubshet, A. K. (2017). Prevalence and antibiogram of Escherichia coli O157 isolated from bovine in Jimma, Ethiopia: abattoirbased survey. Ethiop. Vet. J. 21, 109. doi: 10.4314/evj.v21i2.8
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12, e1001923. doi: 10.1371/journal.pmed.1001923
Hiko, A., Ameni, G., Langkabel, N., and Fries, R. (2015). Microbiological load and zoonotic agents in beef mortadella from Addis Ababa City supermarkets. J. Food Prot. 78, 1043–1045. doi: 10.4315/0362-028X.JFP-14-395
Hiko, A., Asrat, D., and Zewde, G. (2008). Occurrence of Escherichia coli O157:H7 in retail raw meat products in Ethiopia. J. Infect. Dev. Ctries. 2, 389–393. doi: 10.3855/jidc.203
Hiko, A., Irsigler, H., Ameni, G., Zessin, K. H., and Fries, R. (2016). Salmonella serovars along two beef chains in Ethiopia. J. Infect. Dev. Ctries. 10, 1168–1176. doi: 10.3855/jidc.6354
Hiko, A., Irsigler, H., Bräutigam, L., Ameni, G., Fries, R., and Maximilian, B. (2018). Antimicrobial resistance and genotypic profiles of Salmonella Saintpaul isolated along beef processing and distribution continuum. Heliyon 4, 1–14. doi: 10.1016/j.heliyon.2018.e01025
Jejaw, A., Zemene, E., Alemu, Y., and Mengistie, Z. (2015). High prevalence of Schistosoma mansoni and other intestinal parasites among elementary school children in Southwest Ethiopia: a cross-sectional study. BMC Public Health 15, 600. doi: 10.1186/s12889-015-1952-6
Kassahun, M., and Wongiel, S. (2019). Food poisoning outbreak investigation in Dewachefa woreda, Oromia Zone, Amhara Region, Ethiopia, 2018. BMC Res. Notes 12, 377. doi: 10.1186/s13104-019-4407-9
Kassu, A., Andualem, B., van Nhien, N., Nakamori, M., Nishikawa, T., Yamamoto, S., et al. (2007). Vitamin A deficiency in patients with diarrhea and HIV infection in Ethiopia. Asia Pac. J. Clin. Nutr. 16(Suppl 1), 323–328.
Keba, A., Rolon, M. L., Tamene, A., Dessie, K., Vipham, J., Kovac, J., et al. (2020). Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. Int. Dairy J. 109, 104762. doi: 10.1016/j.idairyj.2020.104762
Kebede, E., Seid, A., and Akele, S. (2019). Prevalence and associated risk factors of intestinal parasitic infections among asymptomatic food handlers in Wollo University student's cafeteria, Northeastern Ethiopia. BMC Res. Notes 12, 139. doi: 10.1186/s13104-019-4182-7
Kemal, J., Sibhat, B., Menkir, S., and Beyene, D. (2016). Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in haramaya, Ethiopia. J. Infect. Dev. Ctries. 10, 1230–1235. doi: 10.3855/jidc.7885
Ketema, L., Ketema, Z., Kiflu, B., Alemayehu, H., Terefe, Y., Ibrahim, M., et al. (2018). Prevalence and Antimicrobial Susceptibility Profile of Salmonella serovars Isolated from Slaughtered Cattle in Addis Ababa, Ethiopia. Biomed Res. Int. 2018, 9794869. doi: 10.1155/2018/9794869
Khurana, S., Sharma, M., Taneja, N., Thapar, R., and Malla, N. (2007). Intestinal bacterial and parasitic infections among food handlers in a tertiary care hospital of North India. Trop. Gastroenterol. 4, 207–209.
Kibret, M., and Tadesse, M. (2013). The bacteriological safety and antimicrobial susceptibility of bacteria isolated from street-vended white lupin (Lupinus albus) in Bahir Dar, Ethiopia. Ethiop. J. Health Sci. 23, 19–26.
Kumar, A., Balcha, E., and Tassew, H. (2014). Evaluation of safety of beef sold in and around mekelle with special reference to enterohemorrhagic Escherichia coli O157:H7. Glob. Vet. 12, 569–572. doi: 10.5829/idosi.gv.2014.12.04.8375
Kumera, G., Gedle, D., Alebel, A., Feyera, F., and Eshetie, S. (2018). Undernutrition and its association with socio-demographic, anemia and intestinal parasitic infection among pregnant women attending antenatal care at the University of Gondar Hospital, Northwest Ethiopia. Matern. Health Neonatol. Perinatol. 4, 18. doi: 10.1186/s40748-018-0087-z
Kumma, W. P., Meskele, W., and Admasie, A. (2019). Prevalence of intestinal parasitic infections and associated factors among food handlers in Wolaita Sodo University students caterings, Wolaita Sodo, Southern Ethiopia: a cross-sectional study. Front Public Health 7, 140. doi: 10.3389/fpubh.2019.00140
Lakew, M., Tolosa, T., and Tigre, W. (2009). Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop. Anim. Health Prod. 41, 1525–1530. doi: 10.1007/s11250-009-9343-6
Lamboro, T., Ketema, T., and Bacha, K. (2016). Prevalence and antimicrobial resistance in Salmonella and Shigella species isolated from outpatients, Jimma University Specialized Hospital, Southwest Ethiopia. Canad. J. Infect. Dis. Med. Microbiol. 2016, 4210760. doi: 10.1155/2016/4210760
Makita, K., Desissa, F., Teklu, A., Zewde, G., and Grace, D. (2012). Risk assessment of staphylococcal poisoning due to consumption of informally-marketed milk and home-made yoghurt in Debre Zeit, Ethiopia. Int. J. Food Microbiol. 153, 135–141. doi: 10.1016/j.ijfoodmicro.2011.10.028
Mama, M., and Alemu, G. (2016). Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect. Dis. 16, 686. doi: 10.1186/s12879-016-2035-8
Marami, D., Hailu, K., and Tolera, M. (2018). Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella species among asymptomatic food handlers working in Haramaya University cafeterias, Eastern Ethiopia. BMC Res. Notes 11, 74. doi: 10.1186/s13104-018-3189-9
Mardu, F., Berhe, B., Tesfay, K., and Negash, H. (2019). Assessment of sanitary condition of services as implication for intestinal parasitic infections among prison inmates: institutional based cross-sectional study in eastern Tigray zonal prison, northern Ethiopia (2018). BMC Res. Notes 12, 4–9. doi: 10.1186/s13104-019-4449-z
Mebrate, G., Tewodros, A., and Derbie, Z. (2020). The milk processing: status, challenges and opportunities in Ethiopia. Int. J. Vet. Sci. Res. 6, 052–057. doi: 10.17352/ijvsr.000054
Mekonnen, H. S., and Ekubagewargies, D. T. (2019). Prevalence and factors associated with intestinal parasites among under-five children attending Woreta Health Center, Northwest Ethiopia. BMC Infect. Dis. 19, 256. doi: 10.1186/s12879-019-3884-8
Mekonnen, S. A., Gezehagn, A., Berju, A., Haile, B., Dejene, H., Nigatu, S., et al. (2021). Health and economic burden of foodborne zoonotic diseases in Amhara region, Ethiopia. PLoS ONE 16, e0262032. doi: 10.1371/journal.pone.0262032
Mengist, A., Aschale, Y., and Reta, A. (2018a). Bacterial and parasitic assessment from Fingernails in Debre Markos, Northwest Ethiopia. Canad. J. Infect. Dis. Med. Microbiol. 2018, 6532014. doi: 10.1155/2018/6532014
Mengist, A., Mengistu, G., and Reta, A. (2018b). Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. Int. J. Infect. Dis. 75, 74–79. doi: 10.1016/j.ijid.2018.08.008
Messele, Y. E., Abdi, R. D., Tegegne, D. T., Bora, S. K., Babura, M. D., Emeru, B. A., et al. (2019). Analysis of milk-derived isolates of E. coli indicating drug resistance in central Ethiopia. Trop. Anim. Health Prod. 51, 661–667. doi: 10.1007/s11250-018-1737-x
Messele, Y. E., Abdi, R. D., Yalew, S. T., Tegegne, D. T., Emeru, B. A., and Werid, G. M. (2017). Molecular determination of antimicrobial resistance in Escherichia coli isolated from raw meat in Addis Ababa and Bishoftu, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 16, 55. doi: 10.1186/s12941-017-0233-x
Moges, F., Kebede, Y., Kassu, A., Degu, G., Tiruneh, M., and Gedefaw, M. (2006). Infection with HIV and intestinal parasites among street dwellers in Gondar City, northwest Ethiopia. Jpn. J. Infect. Dis. 59, 400–403.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Altman, D., Antes, G., et al. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Molla, B., Alemayehu, D., and Salah, W. (2003). Sources and distribution of Salmonella serotypes isolated from food animals, slaughterhouse personnel and retail meat products in Ethiopia: 1997-2002. Ethiop. J. Health Dev. 17, 63–70. doi: 10.4314/ejhd.v17i1.9782
Molla, B., Yilma, R., and Alemayehu, D. (2005). Listeria monocytogenes and other Listeria species in retail meat and milk products in Addis Ababa, Ethiopia. Ethiop. J. Health Dev. 18, 208–212. doi: 10.4314/ejhd.v18i3.9962
Muleta, D., and Ashenafi, M. (2001). Salmonella, Shigella and growth potential of other food-borne pathogens in Ethiopian street vended foods. East Afr. Med. J. 78, 576–580. doi: 10.4314/eamj.v78i11.8946
Pieracci, E. G., Hall, A. J., Gharpure, R., Haile, A., Walelign, E., Deressa, A., et al. (2016). Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health 2, 131–135. doi: 10.1016/j.onehlt.2016.09.001
Seyoum, E. T., Woldetsadik, D. A., Mekonen, T. K., Gezahegn, H. A., and Gebreyes, W. A. (2015). Prevalence of Listeria monocytogenes in raw bovine milk and milk products from central highlands of Ethiopia. J. Infect. Dev. Ctries. 9, 1204–1209. doi: 10.3855/jidc.6211
Sibhat, B., Molla Zewde, B., Zerihun, A., Muckle, A., Cole, L., Boerlin, P., et al. (2011). Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health 58, 102–109. doi: 10.1111/j.1863-2378.2009.01305.x
Sisay, Z., Djikeng, A., Berhe, N., Belay, G., Gebreyes, W., Abegaz, W. E., et al. (2016). Prevalence and molecular characterization of human noroviruses and sapoviruses in Ethiopia. Arch. Virol. 161, 2169–2182. doi: 10.1007/s00705-016-2887-7
Soepranianondo, K., Wardhana, D. K., and Budiarto, D. (2019). Analysis of bacterial contamination and antibiotic residue of beef meat from city slaughterhouses in East Java Province, Indonesia. Vet World 12, 243–248. doi: 10.14202/vetworld.2019.243-248
Solomon, F. B., Wada, F. W., Anjulo, A. A., Koyra, H. C., and Tufa, E. G. (2018). Burden of intestinal pathogens and associated factors among asymptomatic food handlers in South Ethiopia: emphasis on salmonellosis. BMC Res. Notes 11, 502. doi: 10.1186/s13104-018-3610-4
Taddese, D., Tolosa, T., Deresa, B., Lakow, M., Olani, A., and Shumi, E. (2019). Antibiograms and risk factors of Salmonella isolates from laying hens and eggs in Jimma Town, South Western Ethiopia. BMC Res. Notes 12, 472. doi: 10.1186/s13104-019-4516-5
Tadesse, T., and Dabassa, A. (2012). Prevalence and antimicrobial resistance of Salmonella isolated from raw milk samples collected from Kersa district, Jimma Zone, Southwest Ethiopia. J. Med. Sci. 12, 224–228. doi: 10.3923/jms.2012.224.228
Tafa, B., Sewunet, T., Tassew, H., and Asrat, D. (2014). Isolation and antimicrobial susceptibility patterns of campylobacter species among diarrheic children at Jimma, Ethiopia. Int. J. Bacteriol. 2014, 1–7. doi: 10.1155/2014/560617
Takele, S., Woldemichael, K., Gashaw, M., Tassew, H., Yohannes, M., and Abdissa, A. (2018). Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at the Jimma municipal abattoir, south-West Ethiopia. Trop. Dis. Travel Med. Vaccines 4, 13. doi: 10.1186/s40794-018-0072-6
Taye, W., Ayalew, A., Chala, A., and Dejene, M. (2016). Aflatoxin B1 and total fumonisin contamination and their producing fungi in fresh and stored sorghum grain in East Hararghe, Ethiopia. Food Addit. Contam. Part B Surveill. 9, 237–245. doi: 10.1080/19393210.2016.1184190
Tegegne, H. A., Berhanu, A., Getachew, Y., Serda, B., Nölkes, D., Tilahun, S., et al. (2019). Microbiological safety and hygienic quality of camel meat at abattoir and retail houses in Jigjiga city, Ethiopia. J. Infect. Dev. Ctries. 13, 188–194. doi: 10.3855/jidc.9686
Tekilegiorgis, T. (2018). Microbiological quality analysis of raw and pasteurized milk samples collected from addis ababa and its surrounding in Ethiopia. Approaches Poult. Dairy Vet. Sci. 4, 374–381. doi: 10.31031/APDV.2018.04.000598
Teklu, A., Asseged, B., Yimer, E., Gebeyehu, M., and Woldesenbet, Z. (2004). Tuberculous lesions not detected by routine abattoir inspection: the experience of the Hossana municipal abattoir, southern Ethiopia. OIE Rev. Sci. Tech. 23, 957–964. doi: 10.20506/rst.23.3.1534
Tigabu, E., Asrat, D., Kassa, T., Sinmegn, T., Molla, B., and Gebreyes, W. (2015). Assessment of risk factors in milk contamination with Staphylococcus aureus in urban and peri-urban small-holder dairy farming in central Ethiopia. Zoonoses Public Health 62, 637–643. doi: 10.1111/zph.12199
Tolosa, T., Verbeke, J., Piepers, S., Tefera, M., Getachew, Y., Supré, K., et al. (2016). Milk production, quality, and consumption in Jimma (Ethiopia): facts and producers', retailers', and consumers' perspectives. Prev. Vet. Med. 124, 9–14. doi: 10.1016/j.prevetmed.2015.12.016
Wabeto, W., Abraham, Y., and Anjulo, A. A. (2017). Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J. Health Popul. Nutr. 36, 52. doi: 10.1186/s41043-017-0131-z
Wendwesen, T., Dagmar, N., Yitbarek, G., and Matusala, M. (2017). Microbiological quality of frozen raw and undercooked Nile tilapia (Oreochromis niloticus) fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. J. Vet. Med. Anim. Health 9, 55–62. doi: 10.5897/JVMAH2015.0424
Yemane, G., Mulaw, G., and Gaim, T. (2014). Prevalence and antimicrobial susceptibility of salmonella species in diarrheal children under five-years of age in Bahir Dar Town, Ethiopia. Int. J. Int sci. Inn. Tech. Sec. A. 3, 12–17.
Keywords: food borne diseases, value chains, hazards, burden, Ethiopia
Citation: Gazu L, Alonso S, Mutua F, Roesel K, Lindahl JF, Amenu K, Maximiano Sousa F, Ulrich P, Guadu T, Dione M, Ilboudo G, Knight-Jones T and Grace D (2023) Foodborne disease hazards and burden in Ethiopia: A systematic literature review, 1990–2019. Front. Sustain. Food Syst. 7:1058977. doi: 10.3389/fsufs.2023.1058977
Received: 30 September 2022; Accepted: 25 January 2023;
Published: 15 February 2023.
Edited by:
Arun K. Bhunia, Purdue University, United StatesReviewed by:
Kolawole Banwo, University of Ibadan, NigeriaJonathan Munguti, Kenya Marine and Fisheries Research Institute, Kenya
Yaohua Feng, Purdue University, United States
Copyright © 2023 Gazu, Alonso, Mutua, Roesel, Lindahl, Amenu, Maximiano Sousa, Ulrich, Guadu, Dione, Ilboudo, Knight-Jones and Grace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Gazu, 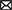 bC5tZWdvQGNnaWFyLm9yZw==
bC5tZWdvQGNnaWFyLm9yZw==
 Lina Gazu
Lina Gazu Silvia Alonso
Silvia Alonso Florence Mutua
Florence Mutua Kristina Roesel
Kristina Roesel Johanna F. Lindahl
Johanna F. Lindahl Kebede Amenu
Kebede Amenu Filipe Maximiano Sousa
Filipe Maximiano Sousa Pattama Ulrich7
Pattama Ulrich7 Michel Dione
Michel Dione Guy Ilboudo
Guy Ilboudo Theodore Knight-Jones
Theodore Knight-Jones Delia Grace
Delia Grace