
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 06 February 2023
Sec. Crop Biology and Sustainability
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1010204
This article is part of the Research Topic Novel Approaches on Conservation and Utilization of Plant Genetic Resources: Ensuring Food Availability and Diversity for Generations to Come View all 6 articles
 Nigel Maxted*
Nigel Maxted* Joana Magos Brehm
Joana Magos BrehmCrop breeders are currently facing the need to continue increasing crop production to feed the growing human population, while mitigating the negative impacts of climate change on agriculture. Taxonomic and genetic diversity, which includes taxa, genes and alleles that offer novel sources of resistance to pests, disease and abiotic factors that affect crop quality and quantity, are a key tool for crop breeders to address these challenges. Lack of access to this diversity is currently limiting crop improvement. This paper focuses on how the breeder's requirement for greater diversity may be met despite the continue challenges of growing human population, and the impacts of climate change. It is argued that gene pool diversity is largely concentrated in crop wild relatives (CWR) and their more active conservation, especially focusing on in situ conservation applications, will enable the breeding challenges to be met. Further, that the science of in situ conservation is only now coming of age but is sufficiently advanced to facilitate the establishment of integrated national, regional, and global in situ CWR conservation networks. For humankind to substantially benefit from the additional adaptive diversity made available through these collaborative networks for CWR in situ conservation for the first time, breeders need to be provided with the critical resources necessary to address the negative impacts of climate changes on food production—therefore promoting greater global food security.
Crop breeders are currently faced with two over-riding, existential challenges, to continue increasing crop production to feed the growing human population, while mitigating the expanding negative impact of climate change on agriculture. The human population is today 8.01 billion (20th December 2022), with 78% living in developing countries, and is predicted to rise to 9.7 billion by 2050, with 86% in developing countries (United Nations, 2022). To feed the global human population in 2050 we will require nutritious food supplies to increase by 60% globally, and 100% in developing countries (FAO, 2011). While climate change is predicted to reduce agricultural production by 2% each decade this century (IPCC, 2014). Crop breeders are thus facing the perfect storm: trying to boost food production for a rapidly increasing human population, within a changing and more extreme production system, with the threat of social unrest, societal collapse and human migration if they fail.
A key tool in the crop breeder's armory for addressing these challenges is diversity, which includes taxonomic and genetic diversity, and taxa, genes and alleles that offer novel sources of resistance to pests, pathogens and abiotic factors that restrict crop quality and quantity. Continually being able to overcome these production restricting factors is the key to food security. Taxonomic and genetic diversity within crop gene pools is referred to as plant genetic resources (PGR)—the “genetic material of… plants which is of value as a resource for the present and future generations of people” (IPGRI, 1993). In relation to food and agricultural production, that diversity is found in modern cultivars, obsolete cultivars, breeder's lines, genetic stocks, but particularly, in terms of resources found on-farm or in nature, in crop landraces and CWR, and they hold most crop gene pool diversity (FAO, 1998, 2010; Maxted et al., 2020). For the crop breeder to produce a constant stream of new cultivars as resistances are breached and current cultivars become obsolete, a constant stream of novel genetic diversity is required to enter the breeding cycle. Further, as the cropping environment or consumer demand changes, so the genetic diversity required must be adjusted to meet the new requirement. Also as the changes in the cropping environment become more extreme, so the genetic diversity required is likely to include previously unused diversity to meet the new requirements.
The policy context for PGR conservation and use is focused and explicit. The CBD draft post-2020 Global Biodiversity Framework (UNEP, 2022) in Milestone A.3 calls for “Genetic diversity of wild and domesticated species is safeguarded, with an increase in the proportion of species that have at least 90 per cent of their genetic diversity maintained”. While the UN Sustainable Development Goals highlighted the need of eradicating extreme poverty and hunger; goals 1, 2 and 3, but particularly goal 2.5 aims that “By 2020, maintain the genetic diversity of seeds, cultivated plants and farmed and domesticated animals and their related wild species, including through soundly managed and diversified seed and plant banks at the national, regional and international levels…”. These goals, although focused and explicit, have not yet been achieved, suggesting the need for radical action beyond the status quo is required. Another global indicator is the State of Food Security and Nutrition in the World 2021 (FAO et al., 2021) which states that in 2020, between 720 and 811 million people faced hunger (46 million in Africa, ≈57 million in Asia, and ≈14 million Latin America and the Caribbean). Globally the figures for moderate to severe food insecurity have been rising since 2014 and nearly one in three people in the world (2.37 billion) did not have access to adequate food in 2020. The crop breeder's requirement for new diversity is, thus, persistent, and if not met, will result in greater global, regional and national food insecurity, malnourishment and even starvation. Therefore, the focus of this paper is how the breeder's requirement for greater diversity may be met, despite the growing human population, their necessary requirement for more nutritious food production, and the disruptive forces of climate change.
It will be argued below that CWR contain the greatest range of diversity and therefore offer the best opportunity to supply the required novel diversity. Maxted et al. (2006) define CWR broadly as “all taxa within the same genus as a crop and more precise as wild plant taxon that has an indirect use derived from its relatively close genetic relationship to a crop; this relationship is defined in terms of the CWR belonging to gene pools 1 or 2, or taxon groups 1 to 4 of the related crop”. However, it can also be argued that one of the barriers to incorporating novel diversity into breeder's material is the breeders themselves, some think they do not need additional diversity in their breeding programmes and are put off using CWR material because of associated linkage drag. Linkage drag being the transfer of deleterious traits along with the target beneficial traits from the CWR to the crop, that then requires extensive back-crossing with the crop material to eliminate (Maxted et al., 2020). Some breeders today may still feel working with CWR is not worth the effort. However, the impact of climate change causing breeders to search more regularly for novel traits (McCouch et al., 2013; Dempewolf and Guarino, 2015), the fact that substantial funds are being devoted to the provision of pre-breed lines that already contains beneficial CWR traits for farmer and breeder usage (https://www.croptrust.org/work/projects/the-bold-project/#c4667; Dempewolf et al., 2017), the increased ease of access to CWR germplasm (Kilian et al., 2021; Eastwood et al., 2022) and the rapid progress in gene editing techniques (Hartung and Schiemann, 2014; Wang et al., 2022) are making linkage drag minimal and means breeder's reluctance to use CWR diversity to maintain food security is less readily justified.
For PGR conservationists to meet the breeders' needs requires that PGR conservation maximizes the broadest range of genetic diversity in the minimum number of accessions available to breeders. Tanksley and McCouch (1997) established that the process of crop domestication necessarily involves a significant loss of genetic diversity, because individual populations of a species are domesticated, not the whole species, farmers require uniformity during agricultural cultivation to maximize production and some natural wild traits are unsuitable for inclusion in any crop (e.g., brittle rachis, exploding fruits). Therefore, genetic diversity is lost in the transition between wild species and crop landraces, and further between landraces and cultivars. It is therefore unsurprising, as Tanksley and McCouch (1997) note, that 95% of tomato genetic diversity is found in wild Lycopersicon/Solanum spp. not the crop itself. This can be generalized to demonstrate the relative amounts of genetic diversity in different elements of the crop gene pool, as illustrated for the barley gene pool (Figure 1), where the filled circles indicate the crop, the hatched circles indicate the wild species, and the relative area of the circle indicates the quantity of genetic diversity in that constituent of the crop gene pool. The highest quantity of genetic diversity is found in the primary and secondary CWR and then crop landraces.
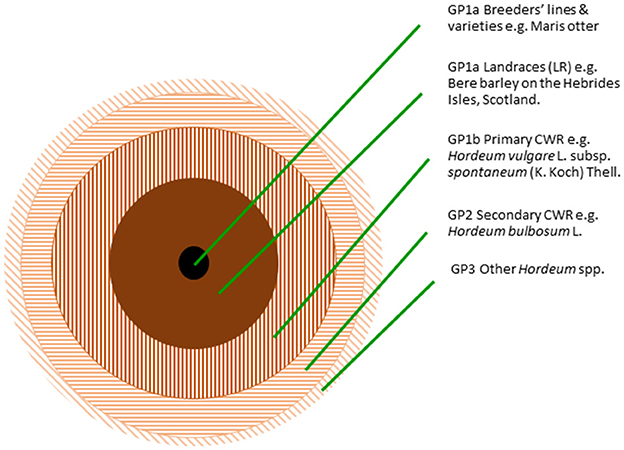
Figure 1. The relative amounts of genetic diversity in different elements of the gene pool, illustrated for the barley gene pool (Maxted et al., 2020).
If the breeders' focus is moving toward access to greater breadth of diversity, then, as Tanksley and McCouch (1997) conclude, the obvious focus would be CWR taxa. They are wild plant species relatively closely related to crops, including crop's wild ancestors that retain indirect use value as gene donors for crop improvement and high level of genetic diversity. Although initially the CWR conservation and use focus has been on the most closely related CWR to the crops and the highest value crops, in the longer term it is likely, with the easier application of gene editing (Hartung and Schiemann, 2014; Wang et al., 2022) and by applying speed breeding (Watson et al., 2018), CWR usage will be expanded providing the full breadth of CWR diversity is conserved and available to breeders.
Over the last 60 years, as the science of PGR conservation has developed, the gene banks have played a central role in that conservation: (a) to sustainably conserve the broadest range of genetic diversity found in the target species (as many alleles, or as many gene combinations as possible) held as population samples or accessions, (b) to characterize and evaluate the diversity conserved to aid selection for utilization, and (c) to make the conserved accessions available to the user community. Therefore, the role of the gene banks might be defined as being “to maximize the conservation, characterization, documentation and promoting the use of PGR diversity for the benefit of humankind” (Maxted et al., 2020), as summarized in Figure 2. Gene bank-based conservation of population samples as seed is the primary (≈85%) method for conserving diversity, because it is suitable for most species, is relatively inexpensive, facilitates characterization, evaluation and user access, and requires little routine maintenance. However, many recalcitrant species, whose seed cannot be conserved by desiccation and freezing, are very poorly conserved, except for a few major crop species (e.g., potato, banana, coffee, chocolate).
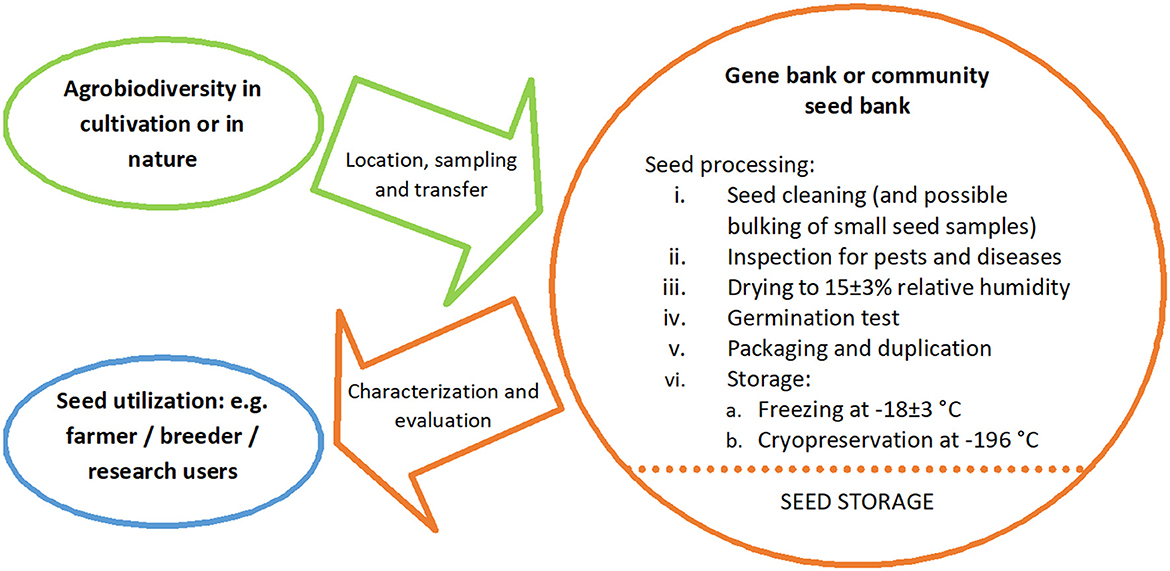
Figure 2. Key elements of gene bank conservation (Maxted et al., 2020).
As stated, the traditional role of gene banks in CWR conservation is to sample populations, maintain sample viability, characterization and evaluation, and make the accession available for use. An essential step of which is to multiply/regenerate the sample accessions when quantities or viability decline. However, unlike crop materials where the sampling/multiplication/regeneration protocols are well established, these are highly specific and vary extensively for CWR (Terry et al., 2003; Way, 2003; FAO, 2016), although the Seed Information Database (Bone et al., 2003) does provide help in suggesting protocol for many species. Nevertheless, gene bank maintenance of CWR diversity is more complicated and requires greater knowledge/skills than crop material and is, therefore, more likely to fail, which reinforces the need for complementary in situ conservation action.
Despite the 7.4 million ex situ accessions held in ≈1,750 gene banks globally (FAO, 2010), several authors draw attention to the lack of sufficient access to diversity and note it is restricting plant breeding outcomes (Volbrecht and Sigmon, 2005; Dwivedi et al., 2007; Feuillet et al., 2008; McCouch et al., 2013). While at the same time, the CWR taxa, that contain the bulk of the diversity required are suffering erosion and extinction, 16–35% of CWR are assessed as threatened using the IUCN Red List Categories and Criteria (Bilz et al., 2011; Kell et al., 2012; Goettsch et al., 2021). A global analysis of priority CWR holdings also found that about a third were unconserved, a third were poorly conserved (<10 accessions per CWR taxon) and 95% required additional collections (Castañeda-Álvarez et al., 2016). It is well established that the conservation ideal is to apply ex situ and in situ techniques in a complementary manner (FAO, 1998, 2010). In practice, the application of in situ conservation has almost completely been ignored and as yet only a handful of active genetic reserves, either in protected areas (PA) or Other Effective Area-based Conservation Measures, have been established.
It is this lack of in situ actions which offers such an opportunity now for action. It is estimated that systematically applying in situ conservation measures would at least double the diversity available to users which, in turn, would generate substantial economic gain and further underpin the utilization of genetic resources in contributing to food security.
It is foolish to place all “our conservation eggs in one basket”. To ensure breeders' requirements are met, and facilitate economic advancement and food security, dynamic in situ conservation must be promoted alongside ex situ gene banking in a fully complementary manner. The aim is to create a permanent international “network” for global in situ conservation of CWR diversity. It would include associated complementary and backup conservation ex situ and, critically, it would promote and facilitate use of the in situ conserved resources for the benefit of all society. It is anticipated that the network will comprise:
• Specific localities where CWR populations are actively conserved in in situ genetic reserves that are maintained to agreed minimum standards;
• A named custodian institution manages those populations conserved in situ;
• Each in situ genetic reserve site has a back-up in a named ex situ conservation facility, which could provide material for reintroduction or reinforcement, if the original population is diminished or lost, and provides access to the in situ conserved diversity so facilitating user access and utilization; and
• Stakeholders with a specific interest in the conservation and sustainable use of CWR.
The network would encompass organizations, other networks and individuals, who would each be able to join the in situ network.
Hawkes (1991) was summing up at the first international congress on “Dynamic in situ conservation of wild relatives of major cultivated plants” and noted that “in situ conservation is still imperfectly understood”, it was in its scientific infancy. This was undoubtedly true in 1991 but significant progress has been made subsequently, notably by a series of primarily EC funded projects focused on CWR in situ conservation: PGR Forum, AEGRO,1 PGR Secure,2 SADC Crop Wild Relatives,3 Farmer's Pride,4 GenRes Bridge,5 plus the UK Darwin Initiative funded SADC Crop Wild Relative Network project,6 and the Norwegian government funded Global Crop Diversity Trust Crop Wild Relative project.7 Each has produced a wealth of innovations that have advanced the status quo.
These recent research projects have, for example, (a) identified global areas of CWR richness (Vincent et al., 2019) (Figure 3A), and (b) hotspots for further CWR collecting for ex situ conservation (Castañeda-Álvarez et al., 2016) (Figure 3B); (c) revised the Vavilov centers of diversity (Vavilov 1926, Maxted and Vincent, 2021) (Figure 3C); and (d) identified the top 170 sites for global in situ CWR conservation (Vincent et al., 2019) (Figure 3D). The Farmer's Pride and the SADC Crop Wild Relatives projects have also particularly developed useful tools to aid conservation planning, including:
• The Interactive Toolkit for CWR Conservation Planning8;
• Various templates for documenting and managing CWR related information (checklists, occurrence data for conservation planning) and for guiding in the development of National Strategic Action Plans (or National Strategies) (see Magos Brehm et al., 2019);
• The CAPFITOGEN tools for CWR and landrace conservation planning.9 (Parra-Quijano et al., 2020);
• A Concept for an Extension of the EURISCO Structure to Include In Situ Crop Wild Relative and On-farm Landrace Data10;
• The in situ CWR population look-up tool in European protected areas11;
• The CWR Population Management Guidelines12 and associated web tool.13
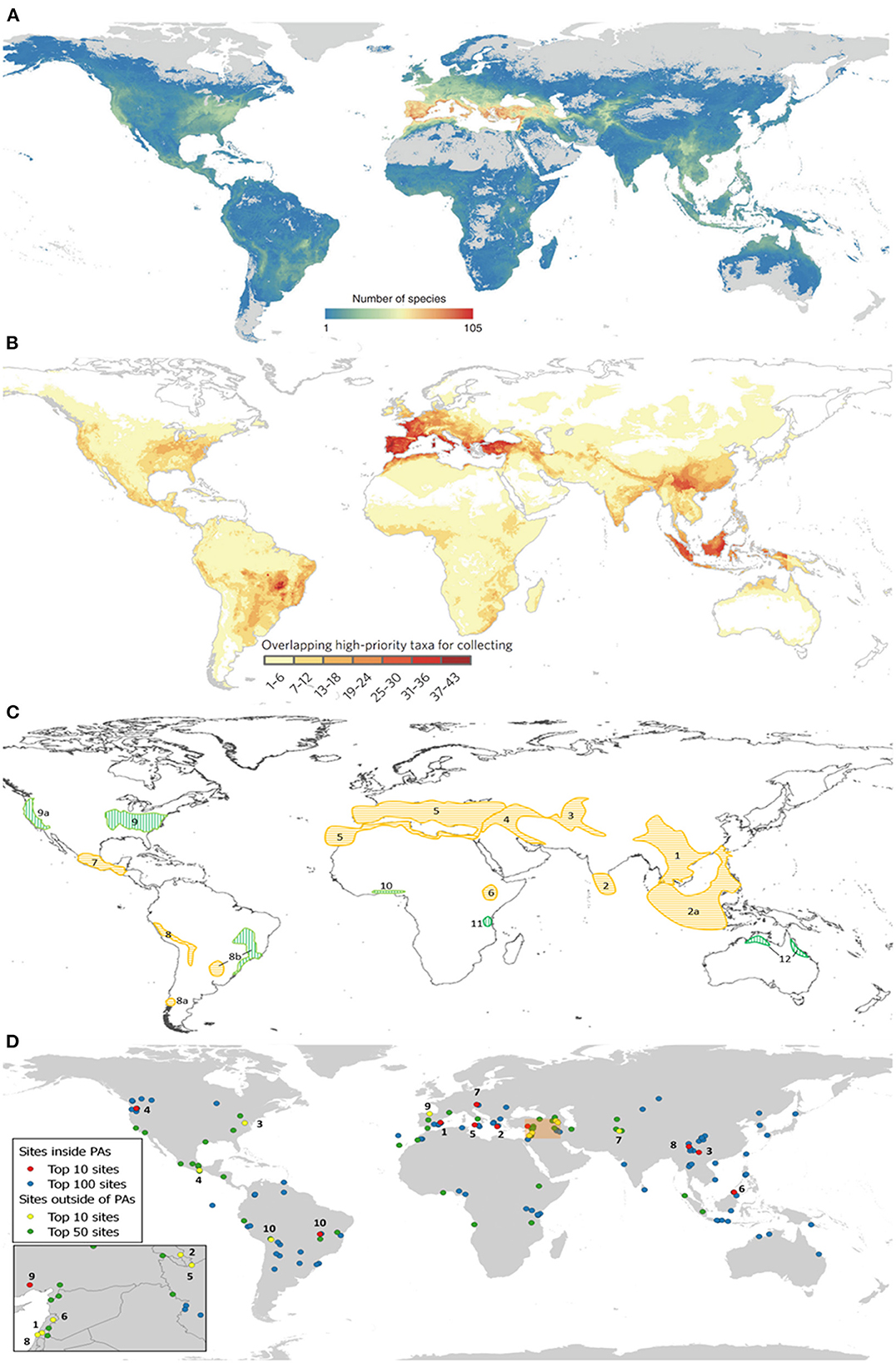
Figure 3. (A) CWR richness for 1,261 CWR species related to 167 crops (Vincent et al., 2019); (B) hotspots for further CWR collection (Castañeda-Álvarez et al., 2016); (C) revised Vavilov centers of diversity (Vavilov, 1926; Maxted and Vincent, 2021); and (D) top 170 sites for global in situ CWR conservation (Vincent et al., 2019).
Additionally, the Secretariat of the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) of the FAO together with worldwide experts, technical staff and national focal points of the Treaty elaborated the Descriptors for Crop Wild Relatives Conserved Under In Situ Conditions (Alercia et al., 2022).
The evidence base has developed significantly, and we currently have a much better idea of where CWR diversity is found, what conservation actions are needed and how they might best be implemented. Recent research has shown the disjunction between IUCN Key Biodiversity Areas and CWR distribution (Saunders et al., in prep.) and highlighted the need for specific CWR conservation initiatives as general biodiversity conservation measures will not effectively address CWR conservation requirements. Therefore, the imperative now is to move from research to the implementation of in situ conservation actions to ensure CWR diversity is available for crop improvement.
It is also increasingly recognized that the application of ex situ conservation techniques alone will not meet the breeders' requirements for diversity to mitigate the impact of climate change on agriculture. Urgent focus is therefore required on (a) actively conserving threatened CWR in globally important hotspots, (b) filling conservation gaps in existing ex situ holdings, (c) filling gaps in conserved germplasm that is unavailable for users, (d) linking with breeders' needs for more diversity to adapt cultigen improvement programmes to climate change, (e) re-focusing PGR conservation activities in a way that regional and national level conservation activities are fully integrated and complementary—possibly building on a European network composed of networks of European national networks, and on (f) meeting policy and legislative obligations (e.g., Sustainable Development Goals,14 Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture,15 Convention on Biological Diversity,16 European Green Deal,17 including the Biodiversity and Farm to Fork Strategies18 in Europe).
Most literature on CWR in situ conservation assumes CWR will be most effectively conserved in pre-existing conservation PA sites. This implies there is no fundamental difference between sites managed for CWR and for other wild plant species, though in fact different CWR may require slightly different management regimes just as any wild plant species may vary in their specific conservation management requirements (Maxted et al., 2008a). Sites where CWR populations are conserved in situ are named “genetic reserves” and they are defined as “sites established to manage and monitor the genetic diversity of natural wild populations within defined areas designated for active, long-term conservation” (Maxted et al., 1997). They may be established on private lands, roadsides, in indigenous reserves and community conserved areas, as well as officially recognized protected areas.
Initially the in situ CWR conservation communities have pragmatically focused on establishing genetic reserves within PA, the reasons being: (a) sites already have a generalized long-term conservation ethos, (b) sites are less prone to hasty management changes associated with private lands or roadsides, (c) it is relatively easy to amend the existing site management plan to facilitate CWR genetic conservation, (d) the prohibitive cost of acquiring non-conservation land is avoided, and (e) there is evidence from throughout the world that CWR populations are found in existing PA in significant numbers (Maxted et al., 2008b; Vincent et al., 2019; Magos Brehm et al., 2022). Therefore, often the simplest way forward in economic and political terms is for countries to locate genetic reserves in existing PA, ideally IUCN recognized national parks or heritage sites. However, to ensure the widest range of CWR diversity is conserved and available for use, conserving CWR populations in PAs alone will not suffice. CWR are more often found in pre-climax, anthropogenic environments outside of formal PA networks, primarily field margins, orchards, cultivated terraces, roadsides and weedy fields (Jain, 1975; Jarvis et al., 2015; Fagandini Ruiz et al., 2021).
The requirement for extra-PA site based in situ conservation for CWR taxa was first highlighted by Al-Atawneh et al. (2007), when implementing area-based conservation in the Fertile Crescent of West Asia. In this region, the global hottest spot of CWR diversity (Vincent et al., 2019), large CWR populations are often found in and around cultivated areas, in the weedy crop itself or at its margins in field edges, orchards, terraces, habitat patches or roadsides (Al-Atawneh et al., 2007; Maxted et al., 2008b; Iriondo et al., 2021). This concept of active conservation outside of established PA was also developed by IUCN, who within the IUCN-WCPA established a Task Force on Other Effective Area-based Conservation Measures (OECMs) (IUCN-WCPA Task Force on OECMs, 2019) to complement more formal PA-based conservation. If an OECM site is established as opposed to a PA, there will be no need to compromise site management objectives to facilitate overall PA vs. CWR conservation and the management can focus entirely on the CWR populations. Additionally, it is relatively easy to make the link between CWR and sustainable agriculture for rural communities as they generally see the obvious phenotypic likeness between CWR and the crops they cultivate. Therefore, they perceive the direct benefit to themselves of OECM based CWR conservation and are more likely to support conservation initiatives. As such the involvement of local communities in active in situ conservation of CWR in OECM sites may be easier than in existing PA since the latter are more rarely located near dense human populations' centers. In situ conservation of CWR in OECM sites has, therefore, significant potential to complement and possibly in time exceed PA-based CWR conservation.
Given that any in situ CWR conservation effort is starting from scratch, because it did not evolve ad hoc as happened with ex situ conservation over the last 60 years, we can structure the network in the most appropriate manner. This includes evaluating whether it is preferable to allow each in situ CWR conservation site to be independent or to be linked in some form of network. Whatever the geographic scale, it appears to be preferable to establish a network of collectively managed in situ CWR sites because: (a) it helps ensures systematic coordination and reporting of the sites and their CWR populations (e.g., to the FAO Global Plan of Action); (b) fosters stronger partnerships and mutual support between sites, populations and staff; (c) facilitates integration and complementarity of global, regional and national actions; (d) links local communities of practice with common goals; (e) helps safeguards evolving in situ CWR populations for perpetuity; (f) helps further promote integrated, long-term complementary in situ–ex situ conservation; (g) promotes access to PGR held in protected areas and farmers/farming communities via their linked Genetic Resources Centers (GRC); and (h) ensures land managers, protected areas and farmers/farming communities receive adequate help with implementing the Access and Benefit Sharing (ABS)19 mechanisms.
The benefits to individual land managers, protected areas and farmers/farming communities of network membership are expected to be numerous, including:
• Kudos of belonging to an international community of practice concerned with PGR diversity and conservation implementation;
• Satisfaction of contributing to bigger/stronger partnerships, and in knowing their PGR is important for humanity, is safeguarded and available to provide provisioning ecosystem services in perpetuity;
• Legislative protection of conservation sites;
• Assistance with adding value to the conservation work;
• Developing markets and fostering greater cross-sector collaboration;
• Increased opportunities for improved visibility through a PA certification schemes;
• Technical support and training for in situ plant genetic resources conservation and sustainable use activities;
• Guidance in seeking funds and agri-environmental schemes to support specific initiatives, such as management interventions and research;
• Provision of a platform for access to reliable expertise, information (e.g., in situ management tools, protocols, exemplars, evidence-base), knowledge sharing and collaboration;
• Ensuring that in situ PGR populations are securely backed-up in a national Genetic Resources center (gene bank) and provide an emergency repatriation service if, and when, a population is under threat;
• Assistance with the ABS legislation and its implementation, so custodians can be secure that the genetic diversity they share and is used will bring back benefit to them.
For germplasm users that belong to the network, benefits include:
• Facilitated access to a greater breadth of PGR in accordance with the requirements of the ITPGRFA and the CBD Nagoya protocol;
• Coordination of networking activities: monitoring, documentation, and reporting.
Just as there are obvious advantages of organizing CWR in situ conservation sites into networks that are managed collectively, so it is also obvious that sites and networks should be geographically and politically integrated. This means that sites are integrated like a series of Russian dolls, with some sites likely containing the highest concentration of CWR populations of multiple CWR taxa being incorporated into the national in situ network, and some of these from multiple countries forming the sub-regional or continental regional in situ network, and some of these from multiple countries and continents forming the global in situ conservation network.
In essence, effective and systematic global in situ conservation of CWR diversity will be achieved via three interrelated geographic or more precisely geopolitical levels of conservation strategy planning: (i) national (Figure 4 in light green) (ii) regional (Figure 4 in blue) and (iii) integrated global (Figure 4 in orange). National governments will be at the core of the establishment of the Network and their support will be essential to its success. Therefore, whether a particular site is provided with national only, national and regional, or national, regional and global designation as part of a CWR network, its inclusion is justified by containing significant CWR populations that have respective national, regional and/or global value, and the national authority alone has precedence over this decision, since it should be the national agency that nominates the sites/populations to join the national, regional or global networks. This is in line with what the CBD stressed that “the conservation of biological diversity is a common concern of humankind” but recognizes “that States have sovereign rights over their own biological resources” (UNEP, 1992). Figure 4 also highlights the key point that CWR conservation must be linked to utilization and germplasm utilization can only be granted via the appropriate nation agency reiterating its national sovereign rights over biological (including CWR) resources.
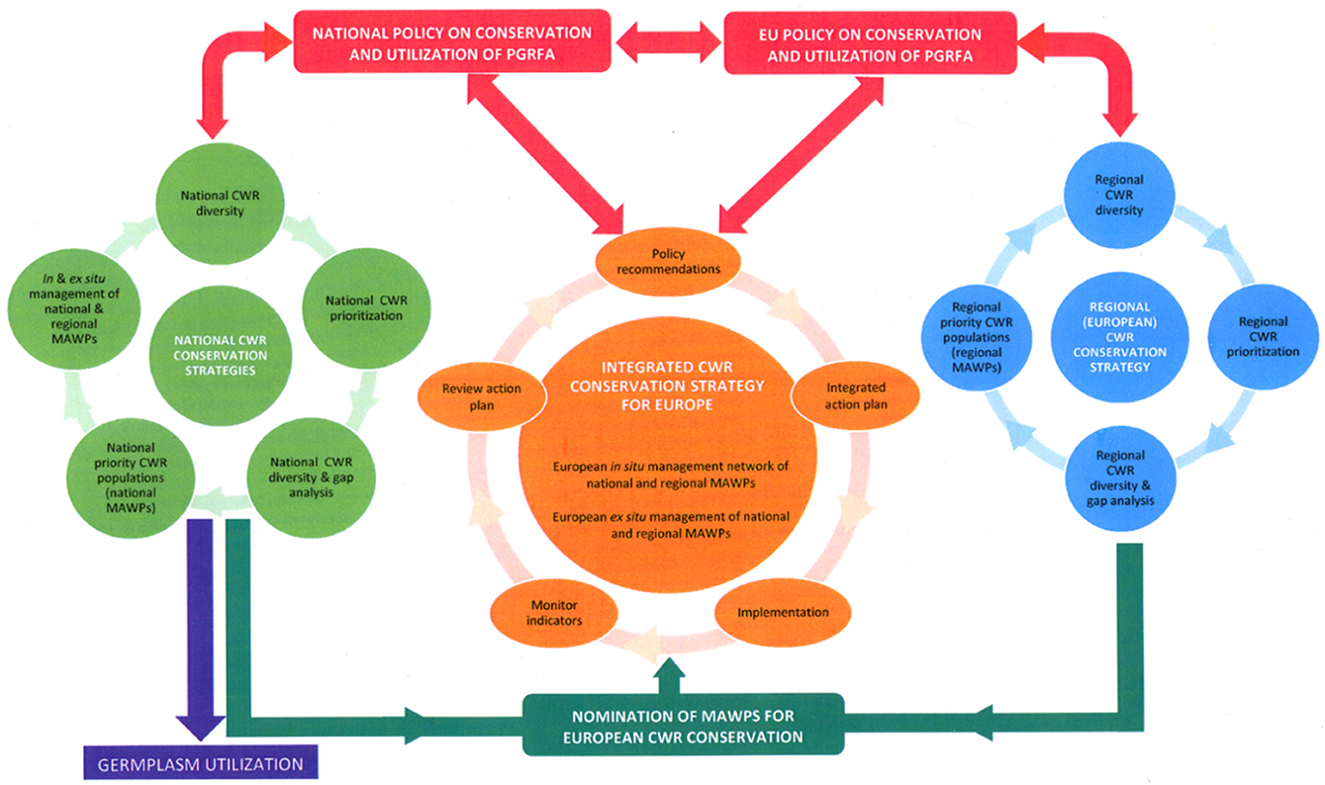
Figure 4. Integration of CWR populations from national and regional networks in one integrated in situ network (Maxted et al., 2015).
Priority sites containing CWR diversity of global importance for inclusion in the global network can be identified by national, regional and global authorities and these sites could be recommended to individual countries as sites within their borders where genetic reserves might be established (Figure 4 in dark green). The actual establishment of these regional or globally identified genetic reserves would be at the discretion of individual nations, although support to encourage their establishment could be forthcoming from the international community. The final integrated global CWR in situ network would contain both genetic reserves identified by individual countries (bottom-up) and those initially identified by regional or global research (top-down), but the latter's nomination for inclusion in the integrated global network would be by individual countries and the genetic reserve conservation planning, practical management and monitoring would necessarily be implemented at national level, though potentially with international support. The integrated global CWR in situ network is therefore integrated because it contains both the bottom-up and top-down identified priority sites.
The integrated global CWR in situ network would be driven by international, regional and national policy on conservation and utilization of plant genetic resources for food and agriculture (PGRFA) (Figure 4 in red) and it is implemented at national level (Figure 4 in dark green). Since the purpose of the integrated strategy is to preserve CWR genetic resources for use in crop improvement and to maintain cultivar development options, a fundamental element is making conserved CWR germplasm available to the user community (Figure 4 in purple) and to achieve this, the interface between in situ, ex situ and use of conserved diversity needs to be strengthened. As indicated by the cyclical flow of the related strategies in Figure 4, planning and implementing global in situ conservation of CWR will be an iterative process requiring periodic review and updating as CWR conservation and utilization policy, science and practice develops. Promoting awareness of the value of CWR to food and economic security as well as raising additional funding, will be critical to support this process and ensure long-term in situ CWR conservation.
Finally, the establishment of the network at each geopolitical level requires some form of integrated governance structure to manage and sustain the network. Governance may be defined as “the activity of governing a country or controlling a company or an organization; the way in which a country is governed or a company or institution is controlled…” (Oxford Learner's Dictionaries, 2022). One element of governance are the rules or minimum inclusion criteria for a CWR site/population to join the network. Such rules are likely to vary depending on the geopolitical level of the network, but the sort of criteria that might apply were originally proposed by Iriondo et al. (2012), amended by Maxted (2016), and further amended here.
• The genetic reserve should be located following a rigorous scientific process involving all appropriate stakeholders.
• The genetic reserves are designed to capture maximum genetic diversity of each target CWR taxon.
• There is a complete inventory of all CWR populations present.
• The genetic reserve should be located within a PA or OECM.
• The target CWR populations are native at that location, or if introduced, are believed to have existed at that location for at least fifteen generations.
• The CWR populations contain distinct or complementary genetic diversity (ecogeographic diversity may be used as a proxy for genetic diversity) or specific traits of interest that enhances the overall value of the network.
• The site is recognized by the appropriate national PA/OECM authorities as a site for conservation action.
• The population is nominated by the appropriate national PGR authority for inclusion in the network.
• A polygon of the genetic reserve containing the CWR population and natural processes should be clearly defined.
• The target CWR population sizes are large enough to sustain long-term population presence, be ideally ≥10,000 individuals.
• The population is “healthy” with a good chance of long-term survival (normally thought to mean 100 years) and so threats from development or climate change are relatively minimal in the short term.
• The population is accessible for research or utilization in accordance with the ITPGRFA via the appropriate national agencies and samples must be available on request from a specified companion ex situ facility as part of the Multilateral System (MLS).
• The site management plan acknowledges genetic diversity conservation as a priority activity and the link to access for commercial and non-commercial utilization.
• The site has some form of legal protection that prohibits population mismanagement.
• The site management plan refers to the CWR taxa and populations present and contains appropriate recommendations for individual CWR taxon management.
• The site management plan contains a CWR taxa and population genomic monitoring element, which should be implemented at appropriate intervals.
• The site management plan contains a CWR taxa and population demographic monitoring element, which should be implemented at appropriate intervals.
• The local community is encouraged to be involved in site management and monitoring.
• There are clearly defined procedures to regulate the use of genetic material of each CWR taxon present and ideally germplasm samples are available for utilization via the MLS via a partner genetic resource center.
• The population is routinely sampled and held in a backup ex situ facility every fifteen generations.
• The site governance ensures continuing commitment to in situ CWR conservation of the site/populations.
• The site undergoes periodically review as to whether it still meets minimum criteria for inclusion in the network and fulfills its reporting obligations.
The actual process of a site/population joining the Network might work as is shown in Figure 5. A site/population is identified as having rare, threatened, or high levels of genetic diversity and worthy of joining the national, regional, or global networks. The person identifying the site (e.g., local farmer, protected area manager, landowner, conservation scientist, plant breeders, etc.,) contacts the appropriate national authority and suggests the site and its CWR populations should join the network. The National PGRFA Coordinator (of the national authority) reviews the proposed site/populations and assess whether they meet the in situ network eligibility criteria. If the national authority wishes to recommend the site for inclusion and it meets the network eligibility criteria, they formally nominate the site to join the network by submitting a formal application with supporting documentation to the Secretariat of the Network Management Committee. The Network Management Committee members assess whether the nomination meets the site eligibility criteria and whether the in situ site nomination descriptors are complete. A protocol for decision-making will be established and either the inclusion of the nominated sites/populations will be endorsed, or if not deemed acceptable, the application will be sent back to the National PGRFA Coordinator for amendment. If accepted to join the network, the site joins the network and engages in network activities but is subsequently periodically re-assessed against the network eligibility criteria to ensure the site/population characteristics continue to be met. Once a site/population is within the in situ network, a user can request access to samples from the associated GRC, however, all material remains under the control of the in situ population manager, and they would or would not grant access for research or utilization. The provision of each in situ sample would need to be sanctioned by the national PGR authorities or delegated to the national plant GRC using a Standard Material Transfer Agreement (SMTA) in accordance with the ITPGRFA.
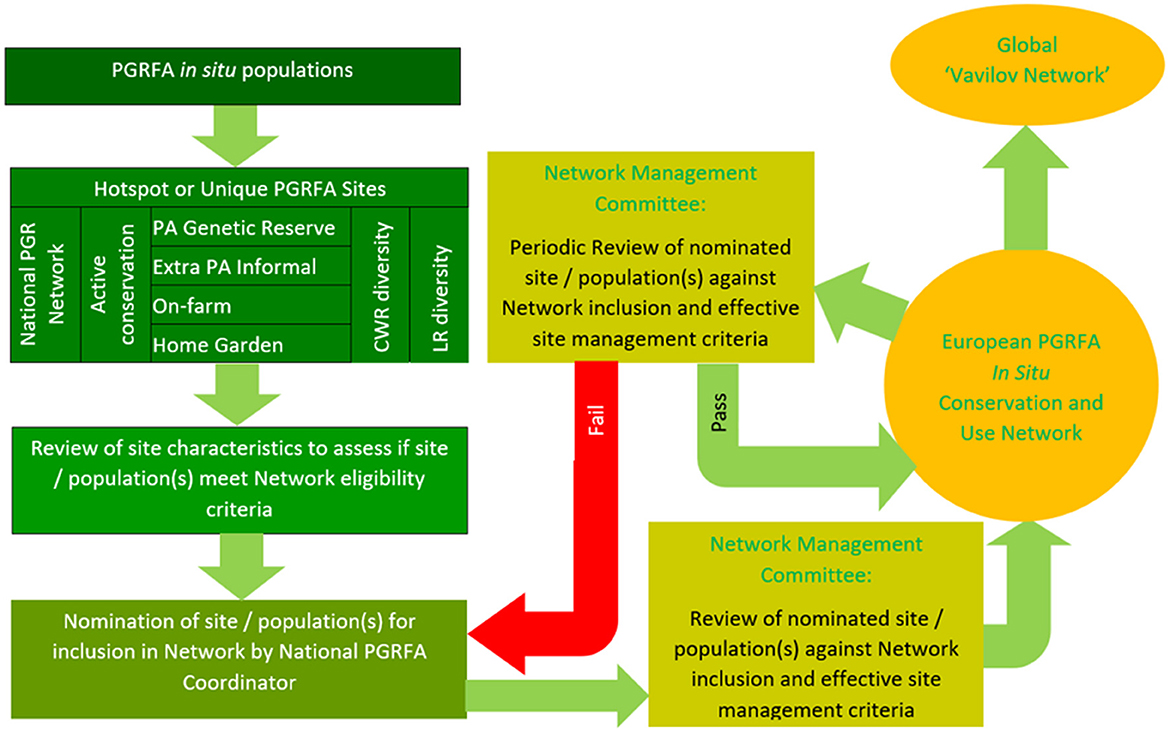
Figure 5. Methodology for CWR site/population to join the network (Maxted et al., 2015).
Instinctively, it would appear simplest and most beneficial for the protected area manager or farmer to each grant access to the genetic resources they conserve and for users to contact them directly. However, in practice, this is unlikely to ever work smoothly, because a priori we do not know which of the literally millions of in situ or on-farm conserved populations the user is likely to request samples from. Therefore, ensuring all protected area managers or farmers that manage sites containing CWR populations are sufficiently aware of their rights and obligations under the Nagoya Protocol and the ITPGRFA to supply germplasm and ensure their own ABS rights are secured is impractical. A more practical option is that the protected area manager or farmer supply population backup samples to a local/national named gene bank or rather GRC and the center supplies subsamples of these to users, securing the suppliers rights and ensuring the users' needs are met (see Figure 6). Figure 6 provides four alternative options for how the user might be linked to the in situ conserved resource via the GRC.
Option 1 shows the routine route that germplasm enters the GRC follows: population samples are either collected from the wild or from an on-farm location; on entering the gene bank the samples are registered and documented, the collection samples are cleaned and dried, the germination percentage is tested and if over 70–85% (depending on species) the sample is packaged and banked, and upon user request a viable seed sample of 30–50 seeds is made available (FAO, 2016). The sample is tested periodically for the germination level, if the seed viability is <70–85%, then the sample must be grown out and regenerated to ensure the seed viability is maintained at an appropriate level. Alternatively,
Option 2 shows that the in situ back-up sample would be treated similarly to an ex situ “black box” sample, i.e., the samples are registered and documented, cleaned and dried, the germination tested, then packaged and banked, with the banked sample tested periodically for the germination level. But the difference to Option 1 is the sample is not made available to users and is only available to the donor as part of a population reinforcement / reintroduction programme.
Option 3 involves the user identifying the in situ population they wish sampled, expressing their wish to their national GRC; then the GRC collectors collect and supply population samples on user demand.
Option 4 treats the backup samples similarly as ex situ samples but excludes the most expensive element of ex situ storage, periodic population regeneration to maintain germination levels; when the seed viability of the in situ sample stored ex situ falls below 70–85% a further sample is taken from the host in situ population.
Considering the four options, Option 1 would place significant additional workload and resource expenditure on the GRC as they are treated the same way as ex situ collections. Option 2 does not facilitate access to the in situ conserved resources, so is undesirable. Option 3 is demand and supply based and because of seasonality of population seed supply, could significantly delay provision of the conserved resources to the user but would be the cheapest for GRC to implement. Option 4 is most preferable as it would be relatively cheap to implement and would mean in situ population samples would be made accessible alongside the ex situ conserved material. The regular resampling of the host in situ populations would mean the sample would better reflect their current genetic diversity content which is continually evolving. Presence of the in situ sample in the GRC would mean it could be characterized and evaluated alongside the ex situ samples. The provision of accessibility to in situ population samples via the gene bank/GRC would acknowledge the fact that they have the appropriate expertise in user seed supply and might see this as a natural extension of their existing role.
Given the evidence above, it seems the science of in situ PGR conservation is now well advanced and that a community of practice has been established. However, to “create a permanent “network” for global in situ conservation of CWR diversity… that promotes and facilitates use of the in situ conserved resource for the benefit of all society” (Maxted et al., 2016) will require a self-sustaining governance structure and secretariat to be established at national, regional and global levels. At the national level oversight would naturally be provided by the national PGR agency; at the global level, authorities such as the FAO's Commission on Genetic Resources for Food and Agriculture (CGRFA), the FAO's ITPGRFA, and CGIAR Centers could potentially take the lead, while at the European level the potential authority providing oversight might include the European Cooperative Programme for Plant Genetic Resources (ECPGR), the European Environmental Agency (EEA), the European Parliament's Committee on Environment, Public Health and Food Safety (ENVI) (Natura 2000 network), and the EUROPARC Federation. The final governance structure at global and European continental levels have yet to be clarified, but on the 7th May 2021, the Ministers responsible for Agriculture and Food Security, Fisheries and Aquaculture of the Southern African Development Community approved the establishment of the SADC Network for In Situ Conservation of CWR, the first regional network to be formally established with an overarching governance structure.20
Despite the advance with the SADC in situ network establishment, the goal of a CWR network is to meet conservation and use (access) criteria and it remains unclear how breeders will gain access to the conserved resource. While in Europe there has been no agreement to a governance structure, the concept for establishing the European network for in situ conservation and sustainable use of plant genetic resources is well supported across the region and by many diverse groups of stakeholders (Figure 7). In Europe the route to regional network establishment may pragmatically be via the establishment of national CWR networks; there is growing implementation in Germany, Scandinavian countries, Spain, and the UK—once sufficient national networks are in place, the logic for the European network would seem inevitable, and perhaps by extension, the logic for the global network also becomes inevitable—we can hope this is the case so proceed with optimism.
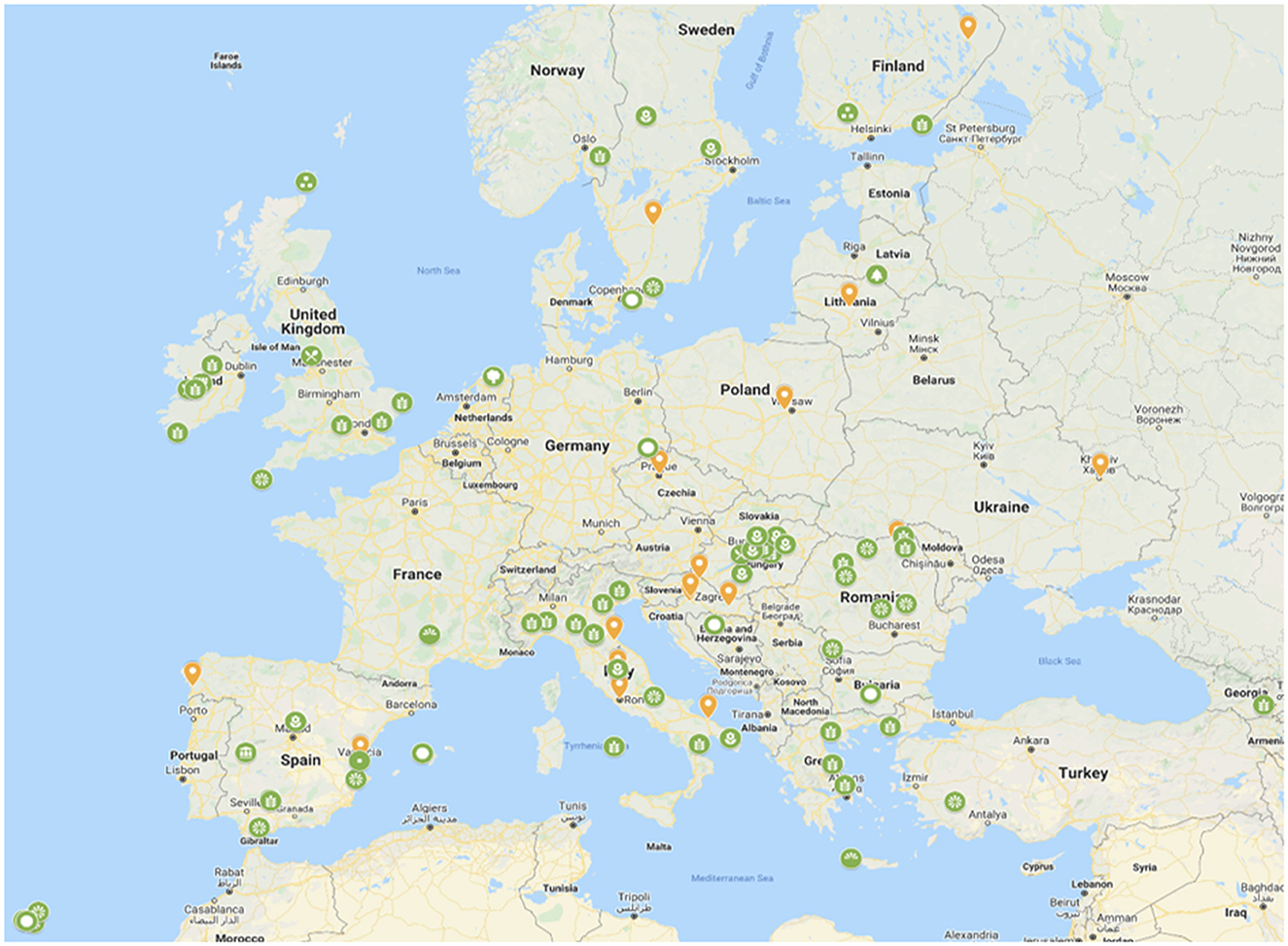
Figure 7. Stakeholders expressing their wish to join the European network for in situ conservation and sustainable use of plant genetic resourcesa. ahttps://more.bham.ac.uk/farmerspride/network/.
As a final thought, it is worth remembering Darwin comment:
“… it appears strange to me that so many of our cultivated plants should still be unknown or only doubtfully known in the wild state.” (Darwin, 1868)
Although today this is no longer true. It is negligent of the biodiversity and PGR communities, that plants with such current and potential economic and societal value and which are increasingly threatened by genetic erosion and extinction in the wild receive such little attention to their active conservation and use. The time is surely right to systematically invest in integrated national, regional and global in situ CWR conservation networks and for humankind to substantially benefit from the additional adaptive diversity made available for the first time. These resources provide us with a critical resource for addressing climate change's impact on food production, a resource humankind may depend on if it is to survive till the next millennium.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
NM wrote the draft manuscript and JM revised it. All authors have read and agreed to the published version of the manuscript.
The research reported here is meant to reflect the current status in the development of CWR in situ networks concept and its implementation. As such, this research is associated with several recent research projects, notably the EC funded PGR Secure project (Novel characterization of crop wild relative and landrace resources as a basis for improved crop breeding, grant agreement no. 266394), Farmer's Pride project (Networking, partnerships and tools to enhance in situ conservation of European plant genetic resources, grant agreement no. 774271), the SADC Crop Wild Relatives project (In situ conservation and use of crop wild relatives in three ACP countries of the SADC region, grant agreement no. FED/2013/330-210) co-funded by the European Union and implemented through ACP-EU Co-operation Programme in Science and Technology (S&T II) by the African, Caribbean and Pacific (ACP) Group of States, and the UK Darwin Initiative funded SADC CWR Network Project (Bridging agriculture and environment: Southern African crop-wild-relative regional network, project no. 26-023).
We fully acknowledge the significant contribution of numerous colleagues involved in the various projects mentioned above to the knowledge presented here.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^http://aegro.juliuskuehn.de/aegro/
2. ^https://www.pgrsecure.bham.ac.uk/
3. ^http://www.cropwildrelatives.org/sadc-cwr-project/
4. ^http://www.farmerspride.eu/
5. ^http://www.genresbridge.eu/
6. ^http://www.cropwildrelatives.org/sadc-cwr-net/
7. ^https://www.croptrust.org/project/the-crop-wild-relatives-project/
8. ^http://www.cropwildrelatives.org/conservation-toolkit/
10. ^https://more.bham.ac.uk/farmerspride/wp-content/uploads/sites/19/2021/09/D2.5_EURISCO_in_situ_extension_concept.pdf
11. ^https://www.ecpgr.cgiar.org/crop-wild-relatives-in-natura-2000
12. ^https://more.bham.ac.uk/farmerspride/wp-content/uploads/sites/19/2021/07/Crop_Wild_Relative_Population_Management_Guidelines.pdf
13. ^https://cwrpopulation-toolkit.cropwildrelatives.org/
14. ^https://sdgs.un.org/goals
15. ^https://www.fao.org/3/i2624e/i2624e00.pdf
17. ^https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en
18. ^https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en
20. ^http://www.cropwildrelatives.org/sadc-cwr-net/latest-news/
Al-Atawneh, N., Amri, A., Assi, R., and Maxted, N. (2007). “Management plans for promoting in situ conservation of local agrobiodiversity in the west Asia centre of plant diversity,” in Crop Wild Relative Conservation and Use, eds N. Maxted, B. Ford-Lloyd, S. Kell, M. Dulloo, and J. Turok (CAB International, Wallingford). p. 340–63. doi: 10.1079/9781845930998.0340
Alercia, A., López, F., Marsella, M., and Cerutti, A. L. (2022). Descriptors for Crop Wild Relatives conserved in situ (CWRI v.1, 1.) Revised Version. Rome, FAO on behalf of the International Treaty on Plant Genetic Resources for Food and Agriculture.
Bilz, M., Kell, S. P., Maxted, N., and Lansdown, R. V. (2011). European Red List of Vascular Plants. Luxembourg: Publications Office of the European Union.
Bone, J., Turner, R., and Tweddle, J. (2003). in Seed Conservation: Turning Science into Practice, eds. R. D. Smith, J. B. Dickie, S. H. Linington, H. W. Pritchard, and R. J. Probert (Kew: Royal Botanic Gardens). p. 305–25.
Castañeda-Álvarez, N. P., Khoury, C. K., Achicanoy, H. A., Bernau, V., Dempewolf, H., Eastwood, R. J., et al. (2016). Global conservation priorities for crop wild relatives. Nat. Plants. 2, 15091022A. doi: 10.1038/nplants.2016.22
Dempewolf, H., Baute, G., Anderson, J., Kilian, B., Smith, C., and Guarino, L. (2017). Past and future use of wild relatives in crop breeding. Crop Sci. 57, 1070–1082. doi: 10.2135/cropsci2016.10.0885
Dempewolf, H., and Guarino, L. (2015). Reaching back through the domestication bottleneck: tapping wild plant biodiversity for crop improvement. Acta Horticulturae. 101, 165–168. doi: 10.17660/ActaHortic.2015.1101.25
Dwivedi, S. L., Crouch, J. H., Mackill, D. J., Xu, Y., Blair, M. W., Ragot, M., et al. (2007). The molecularization o public sector crop breeding: Progress, problems, and prospects. Adv. Agronomy 95, 163–318. doi: 10.1016/S0065-2113(07)95003-8
Eastwood, R. J., Tambam, B. B., Aboagye, L. M., Akparov, Z. I., Aladele, S. E., Allen, R., et al. (2022). Adapting agriculture to climate change: a synopsis of coordinated national crop wild relative seed collecting programs across five continents. Plants. 11, 1840. doi: 10.3390/plants11141840
Fagandini Ruiz, F., Bazile, D., Drucker, A. D., Tapia, M., and Chura, E. (2021). Geographical distribution of quinoa crop wild relatives in the Peruvian andes: a participatory mapping initiative. Environ. Develop. Sustain. 23, 6337–6358. doi: 10.1007/s10668-020-00875-y
FAO (1998). The State of the World's Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations.
FAO (2010). The Second Report on the State of the World's Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations. Available online at: https://www.fao.org/plant-treaty/tools/toolbox-for-sustainable-use/details/en/c/1373627/ (accessed July 2022).
FAO (2011). Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations. Available online at: https://www.fao.org/3/i2624e/i2624e00.pdf (accessed July 2022).
FAO (2016). Gene bank Standards for Plant Genetic Resources for Food and Agriculture. Rev. Ed. Food and Agriculture Organization of the United Nations, Rome, Italy. Available online at: http://www.fao.org/3/a-i3704e.pdf (accessed March 6, 2018).
FAO, IFAD, UNICEF, WFP, and WHO. (2021). The State of Food Security and Nutrition in the World. Rome: Food and Agriculture Organization of the United Nations. Available online at: https://www.fao.org/documents/card/en/c/cb4474en/ (accessed July 2022).
Feuillet, C., Langridge, P., and Waugh, R. (2008). Cereal breeding takes a walk on the wild side. Trends Genet. 24, 24–32. doi: 10.1016/j.tig.2007.11.001
Goettsch, B., Urquiza-Haas, T., Koleff, P., Gasman, F. A., Aguilar-Meléndez, A., Alavez, V., et al. (2021). Extinction risk of mesoamerican crop wild relatives. Plants People Planet 3, 775–795. doi: 10.1002/ppp3.10225
Hartung, F., and Schiemann, J. (2014). Precise plant breeding using new genome editing techniques: opportunities, safety and regulation in the EU. Plant J. 78, 742–52. doi: 10.1111/tpj.12413
Hawkes, J. (1991). International workshop on dynamic in-situ conservation of wild relatives of major cultivated plants: summary of final discussion and recommendations. Israel J. Botany 40, 529–536.
IPCC (2014). “Summary for policymakers,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge: Cambridge University Press).
Iriondo, J. M., Magos Brehm, J., Dulloo, M. E., and Maxted, N. (2021). Crop wild relative population management guidelines. Farmer's Pride: Networking, partnerships and tools to enhance in situ conservation of European plant genetic resources. Available online at http://www.farmerspride.eu/ (accessed July 2022).
Iriondo, J. M., Maxted, N., Kell, S. P., Ford-Lloyd, B. V., Lara-Romero, C., Labokas, J., et al. (2012). “Identifying quality standards for genetic reserve conservation of CWR,” in Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces, eds N. Maxted, M. E. Dulloo, B. V. Ford-Lloyd, L. Frese, J. M. Iriondo, and M. A. A. Pinheiro de Carvalho (Wallingford: CAB International). p. 72–77. doi: 10.1079/9781845938512.0072
IUCN-WCPA Task Force on OECMs (2019). Recognising and Reporting Other Effective Area-Based Conservation Measures. Gland, Switzerland: IUCN. doi: 10.2305/IUCN.CH.2019.PATRS.3.en
Jain, S. K. (1975). “Genetic reserves,” in Crop Genetic Resources for Today and Tomorrow, eds O. H. Frankel, and J. G. Hawkes (Cambridge: Cambridge University Press). p. 379–396.
Jarvis, S. G., Fielder, H., Hopkins, J., Maxted, N., and Smart, S. (2015). Distribution of crop wild relatives of conservation priority in the UK landscape. Biol Conserv. 191, 444–451. doi: 10.1016/j.biocon.2015.07.039
Kell, S. P., Maxted, N., and Bilz, M. (2012). “European crop wild relative threat assessment: Knowledge gained and lessons learnt,” in Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces, eds N. Maxted, M. E. Dulloo, B. V. Ford-Lloyd, L. Frese, J. M. Iriondo, and M. A. A. Pinheiro de Carvalho (Wallingford: CAB International). p. 218–242. doi: 10.1079/9781845938512.0218
Kilian, B., Dempewolf, H., Guarino, L., Werner, P., Coyne, C., Warburton, M. L., et al. (2021). Crop Science special issue: Adapting agriculture to climate change: a walk on the wild side. Crop Sci. 61, 32–36. doi: 10.1002/csc2.20418
Magos Brehm, J., Gaisberger, H., Kell, S., Parra-Quijano, M., Thormann, I., Dulloo, M. E., et al. (2022). Planning complementary conservation of crop wild relative diversity in southern Africa. Diversity Distributions. 28, 1358–1372. doi: 10.1111/ddi.13512
Magos Brehm, J., Kell, S., Thormann, I., Gaisberger, H., Dulloo, E., Maxted, N., et al. (2019). New tools for crop wild relative conservation planning. Plant Genet. Resour. 17, 208.−212. doi: 10.1017/S1479262118000527
Maxted, N. (2016). “European governance structure for crop wild relative conservation and use,” in Conference: Plant genetic resources for food security and ecosystem services: Planning and implementing national and regional conservation strategies (Vilnius, Lithuania).
Maxted, N., Amri, A., Castañeda-Álvarez, N. P., Dias, S., Dulloo, M. E., Fielder, H., et al. (2016). “Joining up the dots: a systematic perspective of crop wild relative conservation and use,” in Enhancing Crop Gene pool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement, eds Maxted, N., M. E. Dulloo, and B. V. Ford-Lloyd, B.V. (Wallingford: CAB International). p. 87–124. doi: 10.1079/9781780646138.0000
Maxted, N., Avagyan, A., Frese, L., Iriondo, J. M., Magos Brehm, J., Singer, A., et al. (2015). Preserving Diversity: A Concept for In Situ Conservation of Crop Wild Relatives in Europe, Version 2. Rome: In Situ and On-farm Conservation Network, European Cooperative Programme for Plant Genetic Resource. Available online at: http://www.ecpgr.cgiar.org/fileadmin/templates/ecpgr.org/upload/WG_UPLOADS_PHASE_IX/WILD_SPECIES/Concept_for_in__situ_conservation_of_CWR_in_Europe.pdf (accessed July 2022).
Maxted, N., Ford-Lloyd, B. V., and Hawkes, J. G. (1997). Plant Genetic Conservation: The In Situ Approach. (London: Chapman and Hall). doi: 10.1007/978-94-009-1437-7
Maxted, N., Ford-Lloyd, B. V., Jury, S. L., Kell, S. P., and Scholten, M. A. (2006). Toward a definition of a crop wild relative. Biodiversity Conserv. 15, 2673–2685. doi: 10.1007/s10531-005-5409-6
Maxted, N., Hunter, D., and Ortiz Rios, R. O. (2020). Plant Genetic Conservation. Cambridge: Cambridge University Press. doi: 10.1017/9781139024297
Maxted, N., Iriondo, J., De Hond Dulloo, L., Lefèvre, E., Asdal, F., and Guarino, L. (2008b). “Genetic reserve management,” in Plant Genetic Population Management, eds J. M. Iriondo, N. Maxted, and E. Dulloo (Wallingford: CAB International). p. 65–87. doi: 10.1079/9781845932824.0065
Maxted, N., Iriondo, J., Dulloo, E., and Lane, A. (2008a). “Introduction: The integration of PGR conservation with protected area management,” in Plant Genetic Population Management, eds J. M. Iriondo, N. Maxted, and E. Dulloo, 1–22 (Wallingford: CAB International). doi: 10.1079/9781845932824.0001
Maxted, N., and Vincent, H. (2021). Review of congruence between global crop wild relative hotspots and centres of crop origin/diversity. Genetic Resources Crop Evolut. 68, 1283–1297. doi: 10.1007/s10722-021-01114-7
McCouch, S., Baute, G. J., Bradeen, J., Bramel, P., Bretting, P. K., Buckler, E., et al. (2013). Agriculture: Feeding the future. Nature 499, 23–24. doi: 10.1038/499023a
Parra-Quijano, M., Iriondo, J. M., Torres, M. E., López, F., Maxted, N., Kell, S., et al. (2020). CAPFITOGEN3. A Toolbox for the Conservation and Promotion of the use of Agricultural Biodiversity. Bogotá, Colombia: Universidad Nacional de Colombia. Bogotá campus—Faculty of Agricultural Sciences. Available online at: http://www.capfitogen.net/es/acceso/manuales/ (accessed July 2022).
Tanksley, S. D., and McCouch, S. R. (1997). Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 277, 1063–1066. doi: 10.1126/science.277.5329.1063
Terry, J., Probert, R. J., and Linington, S. H. (2003). Processing and maintenance of the Millennium Seed Bank Collections. In: Seed Conservation: turning science into practice, eds R. D. Smith, J. B. Dickie, S. H. Linington, H. W. Pritchard, and R. J. Probert (Royal Botanic Gardens, Kew). p. 305–25.
UNEP (2022). Draft post-2020 Global Biodiversity Framework. United Nations Environment Programme, Nairobi, Kenya. Available online at: https://www.unep.org/resources/publication/1st-draft-post-2020-global-biodiversity-framework (accessed December 2022).
United Nations (2022). World Population Prospects 2022. Department of Economic and Social Affairs Population Dynamics. New York. Available online at: https://population.un.org/wpp/ (accessed December 2022).
Vavilov, N. I. (1926). Centres of origin of cultivated plants. Bulletin Appl. Botany Genet. Plant Breed. 16, 248.
Vincent, H., Amri, A., Castañeda-Álvarez, N. P., Dempewolf, H., Dulloo, M. E., Guarino, L., et al. (2019). Modelling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2, 136. doi: 10.1038/s42003-019-0372-z
Volbrecht, E., and Sigmon, B. (2005). Amazing grass: developmental genetics of maize domestication. Biochem. Soc. Trans. 33, 1502–1506. doi: 10.1042/BST0331502
Wang, Y., Zafar, N., Ali, Q., Manghwar, H., Wang, G., Yu, L., et al. (2022). CRISPR/Cas genome editing technologies for plant improvement against biotic and abiotic stresses: advances, limitations, and future perspectives. Cells 11, 3928. doi: 10.3390/cells11233928
Watson, A., Ghosh, S., Williams, M. J., Cuddy, W. S., Simmonds, J., Rey, M. D., et al. (2018). Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants. 4, 23–29. doi: 10.1038/s41477-017-0083-8
Keywords: crop improvement, crop wild relatives (CWR), ex situ conservation, in situ conservation, networks, Other Effective Area-based Conservation Measure (OECM), plant genetic resources (PGR), protected areas (PA)
Citation: Maxted N and Magos Brehm J (2023) Maximizing the crop wild relative resources available to plant breeders for crop improvement. Front. Sustain. Food Syst. 7:1010204. doi: 10.3389/fsufs.2023.1010204
Received: 02 August 2022; Accepted: 11 January 2023;
Published: 06 February 2023.
Edited by:
Vânia Cristina Rennó Azevedo, International Potato Center, PeruReviewed by:
Didier Bazile, Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), FranceCopyright © 2023 Maxted and Magos Brehm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nigel Maxted,  Ti5NYXh0ZWRAYmhhbS5hYy51aw==
Ti5NYXh0ZWRAYmhhbS5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.