- 1Laboratory of Entomology International Center for Agricultural Research in the Dry Areas (ICARDA), Rabat Institutes, Rabat, Morocco
- 2Laboratory of Biology and Health, Department of Biology, Faculty of Science, Ibn-Tofail University, Kenitra, Morocco
- 3Biodiversity and Plant Sciences Program, AgroBioSciences, Mohammed VI Polytechnic University, Ben Guerir, Morocco
- 4Regional Center of Agricultural Research of Agadir, National Institute of Agricultural Research, Rabat, Morocco
The cactus Opuntia ficus-indica L. is widly cultivated in Morocco and has a very an important economic role as a source of food, livestock feed and not forgetting that it is considered to be an income for people in rural communities. This crop is subject to the attack of a serious insect pest, the Dactylopius opuntiae (Cockerell), which sucks the sap from the plant causing huge production losses since its introduction to the country in 2014. The present study investigated the entomopathogenic effect of six fungal isolates {Beauveria bassiana [HASS; RFSL10; SPT 011(a)]; Akanthomyces lecanii [RFSLV; SPT R 215] and Cordyceps farinosa [SPSBI4]} for controlling of both nymphs and adult females of D. opuntiae in laboratory and greenhouse bioassays. Under laboratory conditions, the highest mortality of female D. opuntiae was registered by B. bassiana strain HASS at 108 conidia ml−1 with 100%, followed by B. bassiana strain RFSL10, A. lecanii RFSLV, and C. farinosa SPSBI4 isolates with 98%, respectively, 10 days after treatments. Similarly, the highest level of nymph mortality (100%) was recorded by B. bassiana RFSL10 and HASS isolates at 108 conidia ml1, respectively, 4 and 5 days after application. Under greenhouse conditions, B. bassiana (HASS and RFSL10) and A. lecanii (RFSLV) isolates sprayed alone expressed a higher toxicity on nymphs with 75, 68.5 and 58%, respectively, 12 days after treatments. However, no significant difference was observed in adult female's mortality between different fungal isolates, where B. bassiana (HASS) at 108 conidia ml−1 presented a moderate mortality rate with 55%, 12 days after application. In fact, the combination of black soap (60 g/L) with B. bassiana HASS and RFSL10 and A. lecanii (RFSLV) isolates at 108 conidia ml−1 caused the highest toxic activity on D. opuntiae adult females, with 70.5, 68.75 and 67.65%, respectively. These findings showed that entomopathogenic fungi are promising for developing a biopesticide formulation for the management of D. opuntiae as an adequate and safe alternative to chemical pesticides.
Introduction
The prickly pear Opuntia ficus-indica (L.) Mill. belongs to the family of Cactaceae which is composed of 178 genera, with about 2,000 known species typically distributed in arid and semi-arid zones. The cactus is native to Mexico, which is an important reservoir of several species of cacti of the genus opuntia, and currently widely cultivated worldwide (Bravo-Hollis, 1978; Griffith, 2004; Silva Neto et al., 2008; Chávez-Moreno et al., 2009).
The cactus is a very polyvalent crop that plays an ecological and economical important role. Cactus provides human food in the form of fruits and vegetables, fodder for animals, in the pharmaceutical and cosmetics industry, to produce natural dyes through Carminic acid extracted from cochineal insects (Gebreegziabher and Tsegay, 2015). Furthermore, the cactus pear is recognized for its great nutritional importance due to its high content of soluble carbohydrates, copper, iron, calcium, phenolic acids, vitamins, sodium, potassium (Santos et al., 2006; Chougui et al., 2013; Pretti et al., 2014). On the other hand, the physiological and morphological characteristics of prickly pears allow them to tolerate high temperatures, and low water availability (Arba, 2009).
Currently, the cochineal scale (Dactylopius opuntiae) (Hemiptera: Dactylopiidae) has come to be a serious pest of prickly pear crops Opuntia ficus-indica (L.) in a large number of countries, particularly in the Mediterranean basin (Mazzeo et al., 2019). It was described for the first time in Mexico by Cockerell in 1896 (De Lotto, 1974), and then in Australia (Dodd, 1940), South Africa (Pettey, 1950; Annecke and Moran, 1978), India, Sri Lanka and Brazil (Pérez Guerra and Kosztarab, 1992), and other countries (Foldi, 2001; Moussa et al., 2017). In Morocco, D. opuntiae was introduced in 2015 in Sidi Benour region, 70 km from El Jadida city (Bouharroud et al., 2016), then the insect has spread quickly several regions in the country. Nymphs and adult females of D. opuntiae feed on cladode plants by sucking the sap, and causing chlorosis, desiccation, weakening of the plants and eventually death in in case of heavy infestations (Vanegas-Rico et al., 2010).
The excessive use of chemical insecticides in the control of the wild cochineal can lead to the accumulation of residues in the cladodes (Ramírez-Bustos et al., 2018). However, a number of interesting studies have been conducted recently on the biological control of D. opuntiae as safe, and effective tools using botanical extracts, plant essential oils, detergents and entomopathogenic fungi (EPF), natural enemies and the use of resistant ecotypes (Vigueras et al., 2009; Carneiro-Leão et al., 2017; Bouharroud et al., 2018; Lopes et al., 2018; El Aalaoui et al., 2019a,b; Ramírez-Sánchez et al., 2019; Sbaghi et al., 2019; Ramdani et al., 2021a,b). Indeed, entomopathogenic fungi used as biopesticides are respectful of the environment and provide multiple advantages in the control of insects pests, such as by minimizing the risk of developing resistance (Gao et al., 2017), and offers more safety for humans and other non-target vertebrate or invertebrate species (Hajek and Meyling, 2018). They kill insects at various life cycle stages of due to their biopersistence (Gul et al., 2014). Species such as Beauveria bassiana, Metarhizium anisopliae, Cordyceps fumosorosea, and Akanthomyces lecaniiare the most common and commercial entomopathogenic fungi with potentials as biocontrol agents for different insect pests (Shia and Feng, 2004; Wright et al., 2004; Panyasiri et al., 2007; Mantzoukas and Grammatikopoulos, 2020).
The objective of this work was to investigate the insecticidal effect of six entomopathogenic fungal isolates at three different concentrations (106, 107 and 108 conidia ml−1) applied alone or in combination with detergent black soap to control nymphs and females of D. opuntiae under laboratory and greenhouse conditions.
Materials and methods
Insect rearing
Non-infested cladodes of cactus pear (Opuntia ficus indica) were cultivated with a mixture of one third soil, one third sand and one third peat in plastic pots (27 cm diameter and 24 cm height) under greenhouse at 30°C. The plants were exposed to strongly infested cladodes collected in the Marchouch area (33°56′10″ N 6°69′21″ W).
Each infested cladode was put between two pots, and after 20 days of exposition, a successful artificial infestation took place with colonies showing mature females with wax covering their body that are carefully selected for use in the experiments.
Laboratory bioassays
Fungal isolates
To carry out the study, six isolates of entomopathogenic fungi from 3 species: Beauveria bassiana [HASS, RFSL10, SPT 011(a)], Akanthomyces lecanii (RFSLV, SPT R 215) and Cordyceps farinosa (SPSBI4). These fungi were obtained from the Fungal Culture Collection of ICARDA Terboul, Lebanon (Table 1).

Table 1. Six isolates of entomopathogenic fungi used in the laboratory bioassay on D. opuntiae nymphs and females.
Preparation and production of isolates fungal spores
The six fungal isolates were inoculated into Potato Dextrose Agar medium (PDA) in sterile Petri dishes and then incubated at 25 ± 1°C temperature, and 14:10 L/D photoperiod for 14 days. 10 ml of sterile distilled water containing Tween-80 (0.01%) was put into each Petri dish and the spores were scratched with a sterile scalpel. The spore suspensions obtained were adjusted to a concentration of 1 × 108 conidia ml−1 using Neubauer haemocytometer counting chamber under microscope (Motic, BA410E).
Entomopathogenic test
The entomopathogenic effect of six fungal isolates was evaluated at constant laboratory conditions of 25 ± 1°C temperature, 75 ± 5% relative humidity and a photoperiod of 14:10 (L:D). Three concentrations (106, 107 and 108 conidia ml−1) were used, mixed with sterile distilled water containing 0.01% Tween-80. The control nymphs and females of D. opuntiae were sprayed with distilled water containing Tween-80 (0.01%). The biological trials were performed using a completely randomized design (CRD) with five replicates per concentration for each treatment.
A total of ten females of similar age and ten first instar nymphs of D. opuntiae were separately placed on cladodes of the same size with an entomological brush deposited in Petri dishes (9 cm diameter).
The adult females mortality was recorded for 10 days, while the mortality of nymphs was registered for 5 days after treatment using the binocular microscope (Motic DM-143). Dead females presented dark brown color and deshydratation of their bodies, while no movement and no color change was observed in the dead nymphs.
Mortality was calculated according to Abbott's (1925) formula:
Greenhouse assay
Toxicity of fungal isolates used singly and in combination with black soap for control of D. opuntiae nymphs and adult females
Fungal entomopathogenic isolates that showed high toxicity against both females and nymphs of D. opuntiae under laboratory conditions were selected for evaluation of their efficacy in the greenhouse at temperatures ranging from 24 to 30°C during April–July 2021. Healthy young cladodes of O. fícus-indica were placed in plastic pots (27 cm in diameter by 24 cm in height), and maintained in the greenhouse for a period of 2 months. After this period, the cladodes were infested artificially using the method described above and conserved for an additional 20 days for the development of adult females.
The experiment was performed using a randomized complete block design with four replicates for each treatment. The most effective treatments included fungal isolates selected from laboratory trials: B. bassiana (HASS); B. bassiana (RFSL10); A. lecanii (RFSLV); A. lecanii (SPTR 215); B. bassiana (SPT 011 a); C. farinosa (SPSBI4). The six conidial suspensions containing Tween-80 (0.01%) were applied alone on cladodes at concentration of 108 conidia ml−1 and on cladodes pre-sprayed with the detergent black soap (60 g/L) with the detergent black soap at (60 g/L) over a 2-h spray interval which served to remove the cuticular wax and exposed the females and nymphs to fungal isolates using a 1 L hand sprayer (Ramdani et al., 2021a). The cladodes treated with distilled sterile water suspended in 0.01% Tween-80 as a control. The mortalities of nymphs and females were registered at 3, 6, 9 and 12 days after spraying using a binocular magnifier. The treatments were applied on selected cladodes at medium infestation levels (26–50%) using a modified Silva (1991) rating scale.
Data analyses
The percentages of mortality were converted to angular values (arcsine √P) before the statistical analysis. Under laboratory conditions, Transformed percentages were analyzed by two-way analysis of variance ANOVA (concentration and isolate). Under greenhouse conditions, the transformed percentages were analyzed by one-way analysis of variance. Means were separated by Tukey's test (p < 0.05) with Genstat (20th edition, VSN International, Hemel Hempstead, UK).
Results
Laboratory bioassays
Effect of the fungal isolates on D. opuntiae adult females and nymphs
Mortality of D. opuntiae nymphs and adult females after treatment with different fungal isolates is shown in Tables 2, 3. The results revealed that the efficacy of entomopathogenic fungi varies considerably according to the isolates tested and concentration. There was a significant difference (p < 0.001) in nymphs and adult females mortality of D. opuntiae, due to the six fungal isolates at different tested concentrations during various periods of time exposure.
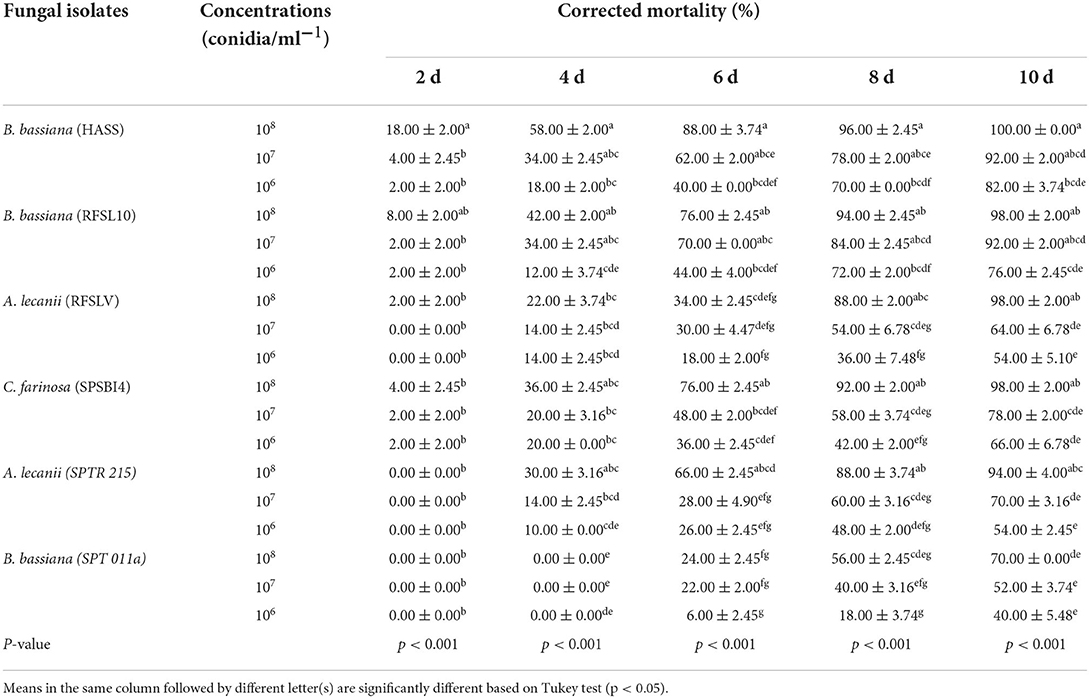
Table 2. Mean percentage mortality ±SE of D. opuntiae adult females after exposure to six tested entomopathogenic fungi isolates at 3 concentrations.
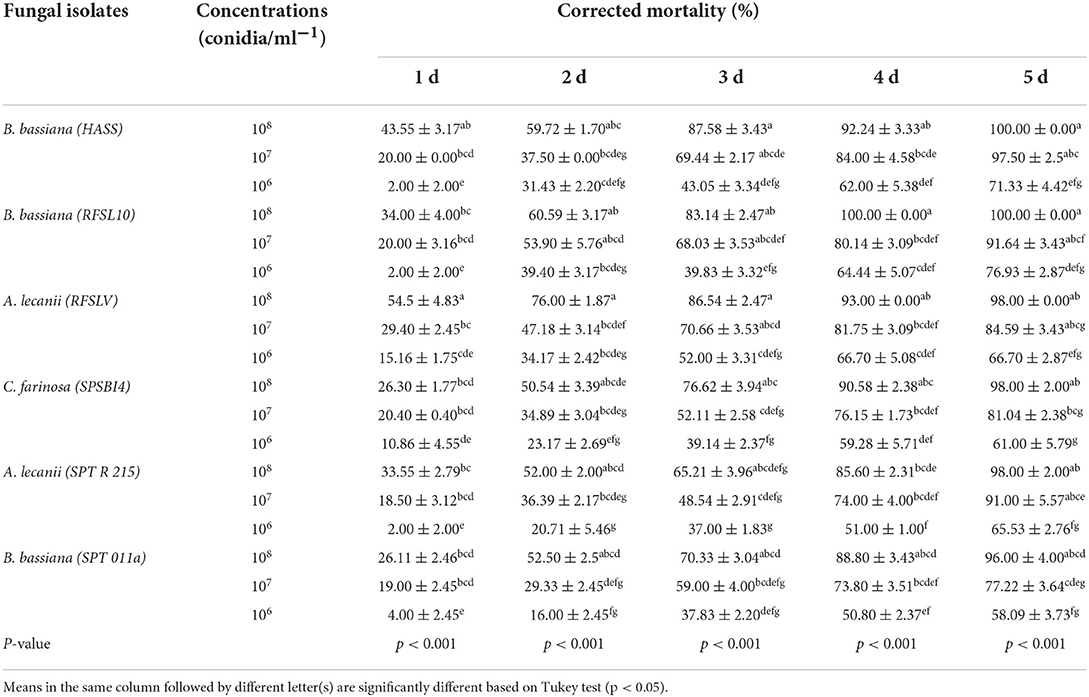
Table 3. Mean percentage of D. opuntiae nymphs mortality after exposure to different entomopathogenic fungi isolates at 3 concentrations.
Among all the tested isolates, B. bassiana (HASS) was the most virulent than the other isolates. At 6 after treatment, the highest percentage of mortality (88.00%) of adult females was registered for B. bassiana (HASS) strain followed by B. bassiana (RFSL10) and C. farinosa (SPSBI4) with 76.00% at 108 conidia ml−1, respectively. However, 10 days after treatments, the maximum mortality of D. opuntiae females was recorded by B. bassiana (HASS) with 100%, followed by B. bassiana (RFSL10) and A. lecanii (RFSLV) and C. farinosa (SPSBI4) isolates at 108 conidia ml−1 with a mortality percentage of 98.00%, respectively.
Data analysis indicated a significant difference in the percentage of nymph mortality of all fungal isolates tested during various time periods of exposure (p < 0.001). The mortality of nymphs for different fungal isolates increased with increasing concentrations for different exposure times.
At 3 days after treatment, the highest percentage mortality (87.58%) of nymphs was registered for B. bassiana (HASS) at 108 conidia ml−1, followed by A. lecanii (RFSLV) and B. bassiana (RFSL10) isolates with (86.54%) and (83.14%) at 108 conidia ml−1, respectively. At 5 days post-application, B. bassiana (HASS and RFSL10) isolates showed the highest levels of nymph mortality (100%) at 108 conidia ml−1, respectively. The second most effective fungal isolates were A. lecanii (RFSLV), C. farinosa (SPSBI4), A. lecanii (SPTR 215), causing 98.00% of nymphal mortality at 108 conidia ml−1, respectively.
Greenhouse assay
Insecticidal effects of fungal isolates applied on nymphs and adult females of D. opuntiae
The ANOVA revealed a significant difference in the mortality of adult females of D. opuntiae induced by different fungal isolates at 6 days after treatments (p < 0.001; Table 4), while no significant difference was observed in mortality between treatment groups after 9 and 12 days after application (P > 0.001). At 6 days, B. bassiana (RFSL10 and HASS) isolates caused highest mortality rates compared to other isolates, with 27.50 and 20.00%, respectively. Mortalities of D. opuntiae females have increased at 9 days after treatment for B. bassiana (HASS) strain with 52.50% and for both isolates B. bassiana (RFSL10) and A. lecanii (RFSLV) with 40.00% at 108 conidia ml−1, respectively. At 12 days post application, B. bassiana (HASS) reached 55.00% as a maximum mortality.
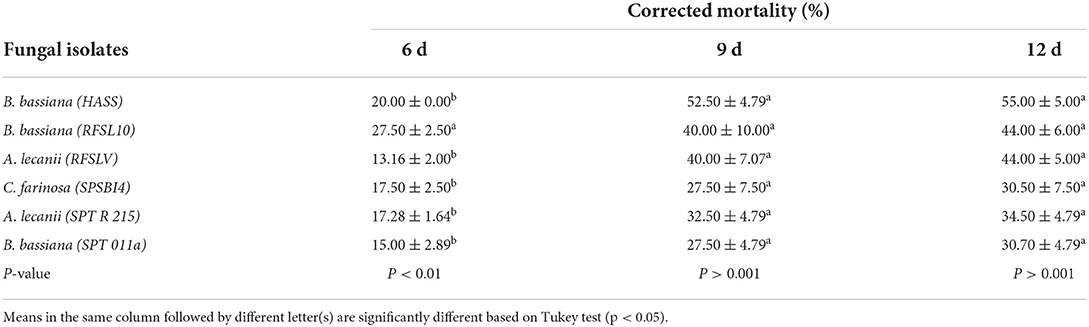
Table 4. Mean percentage of D. opuntiae adult females mortality ±SE after exposure to six tested entomopathogenic fungi isolates at 108 conidia ml−1 under greenhouse conditions.
The mortality of D. opuntiae nymphs treated by the six fungal isolates is shown in Table 5. Analysis of the data indicated a significant difference in nymph mortality caused by fungal isolates applied alone at 108 conidia ml−1 after 6 d (p < 0.001). The toxicity of different isolates was low during the first week after application. However, 9 days after sprays, the toxicity of B. bassiana (HASS and RFSL10) and A. lecanii (RFSLV) isolates sprayed alone expressed a higher toxicity with 70.00, 67.50 and 52.00%, respectively. The high percentage mortality of D. opuntiae nymphs was observed 12 days after treatment by B. bassiana (HASS and RFSL10) and A. lecanii (RFSLV) with 75.00, 68.00 and 58.00%, respectively.
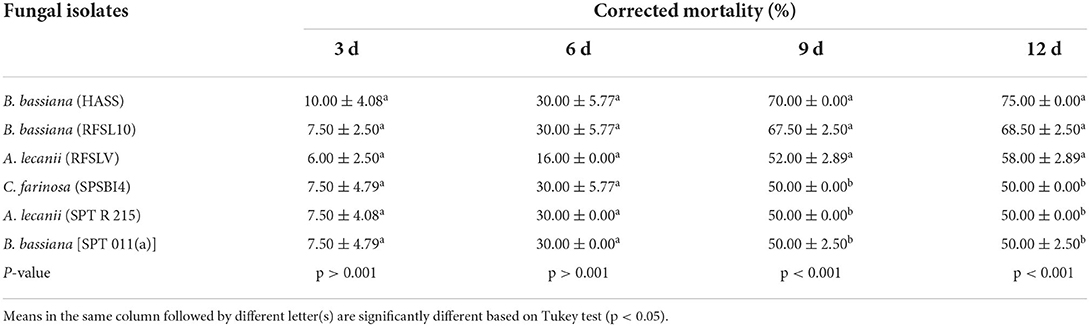
Table 5. Mean percentage of D. opuntiae nymphs mortality ±SE after exposure to six tested entomopathogenic fungi isolates at 108 conidia ml−1 under greenhouse conditions.
Insecticidal effects of fungal isolates combined with black soap on nymphs and adult females of D. opuntiae
Mortality of nymphs and adult females of D. opuntiae after exposure to entomopathogenic fungi isolates at 108 conidia ml−1 in combination with black soap (60 g/L) for different exposure times is shown in Tables 6, 7.
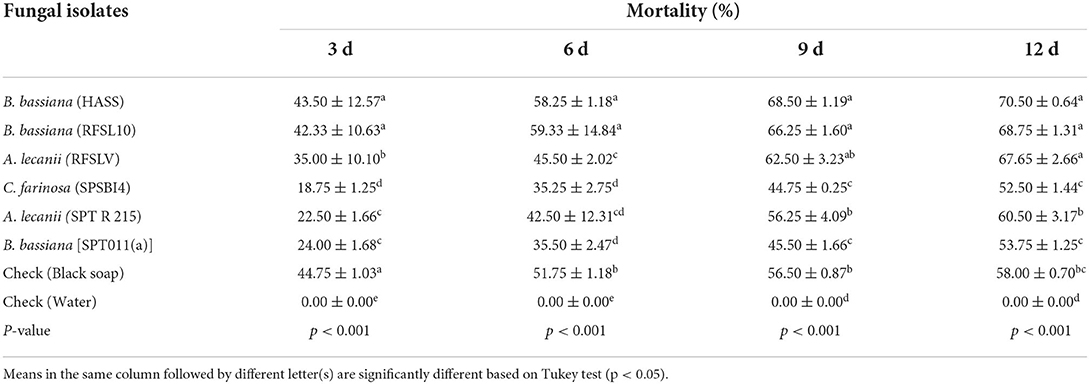
Table 6. Mean percentage mortality ±SE of D. opuntiae adult females after exposure to six tested entomopathogenic fungi isolates at 108 conidia ml−1 in combination with black soap (60 g/L).
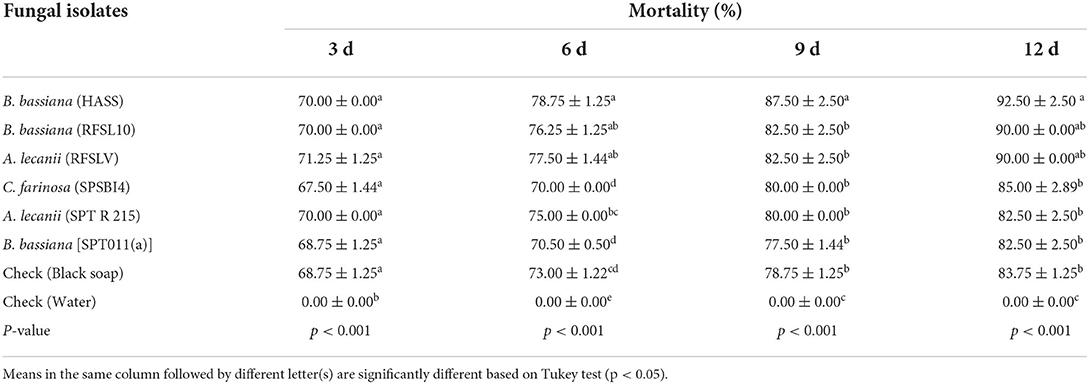
Table 7. Mean percentage mortality ±SE of D. opuntiae nymphs' mortality after exposure to six tested entomopathogenic fungi isolates at 108 conidia ml−1 in combination with black soap (60g/L).
The ANOVA demonstrated significant differences in adult females mortality resulted by the six isolates at different periods of exposure (p < 0.001, Table 6). According to these observations, both isolates of B. bassiana (HASS and RFSL10) applied in combination with black soap revealed a significant difference in percentage of nymph mortality among all isolates tested, 3 days of application, with 43.50 and 42.33%, respectively. A moderate increase in adult females mortality was recorded 6 days after application for all tested isolates. At 12 d after treatments, three fungal isolates caused the highest toxicity, B. bassiana (HASS and RFSL10) isolates and A. lecanii (RFSLV) at 108 conidia ml−1 combined with black soap (60 g/L), with a percentage mortality of 70.50, 68.75% and 67.65%, respectively.
Analysis of the data indicated a significant difference in percentage of nymph mortality among all the tested isolates for different periods of exposure (p < 0.001, Table 7). The entomopathogenic fungal isolates of A. lecanii (RFSLV) and B. bassiana (HASS and RFSL10) at a concentration of 108 conidia ml−1, and in combination with black soap (60 g/L), presented the highest insecticidal activity with a mortality of 71.25, 70.00 and 70.00%, respectively, 3 days after treatment. The mortality rate of nymphs increased significantly as exposed time was increased. At 12 days after treatments, the use of black soap (60 g/L) followed by B. bassiana (HASS) at 108 conidia ml−1 showed the highest mortality of nymphs with 92.00%, followed by both B. bassiana (RFSL10) and A. lecanii (RFSLV) with 90.00% nymphs' mortality, respectively.
Discussion
The current study was carried out in laboratory and greenhouse conditions to assess the potential effect of six entomopathogenic fungal isolates alone or in combination with a black soap for controlling of nymphs and adult females of D. opuntiae. Of all the fungal isolates tested, B. bassiana (HASS and RFSL10) and A. lecanii (RFSLV) applied at 108 conidia ml−1 in combination with 60 g/L of black soap, provided the highest toxicity on D. opuntiae nymphs and adult females under greenhouse conditions. The toxicity of the six fungal isolates against adult females of D. opuntiae was higher under laboratory bioassays compared to the greenhouse trials; the females under laboratory conditions did not have the filamentous layer of wax, which could reduce fungal penetration. The nymphs were also exposed to the various treatments without any protection. The potential of entomopathogenic fungi as biopesticides has been shown against a broad spectrum of insects (Van Den Berg et al., 2001; Pedrini et al., 2006; Fang et al., 2008). The variation in potency of fungal isolates may be influenced by many parameters including temperature, humidity, experimental conditions, and the concentrations of the isolates used, the site of collection and the origin of the isolate (Meyling and Eilenberg, 2007; Herrero et al., 2012).
Fungal species of tested genera have been previously evaluated as entomopathogenic on several insect pests from different orders with a strong potential for use as biocontrol agent The present study corroborates the findings of Andaló et al. (2004) using B. bassiana isolates, which caused a mortality rate of 50–65% against Dysmicoccus texensis (Hemiptera: Pseudococcidae), while, Metarhizium anisopliae isolates caused mortalities between 30 and 50% against Praelongorthezia praelonga (Hemiptera: Ortheziidae). Likewise, a study conducted by Kulkarni and Patil (2013) confirmed the effectiveness of A. lecanii at the rate of 6 x 105 spore/g and B. bassiana at 106 spores/g in controlling the mealybug Planococcus citri (Hemiptera: Pseudococcidae), in two sprays applied at an interval of 15 days. Also, Mohamed (2016) reported that B. bassiana at 5 x 107 conidia/ml in a dipping bioassay, was the most effective fungus causing 98% mortality of the vine adults mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Similarly, Mantzoukas and Grammatikopoulos (2020) confirmed the good efficacy of B. bassiana at 108 conidial concentration with 87% against third instar Sesamia nonagrioides (Lepidoptera: Noctuidae), 7 days after treatment. For the control of the wild cochineal, Menezes et al. (2008) proved that B. bassiana (LCB62) strain was found to be the most effective against the nymphs of D. opuntiae with 96.80% mortality, 15 days after inoculation. While, Oliveira et al. (2019) found that ten isolates of B. bassiana were not pathogenic to D. opuntiae at 108 (conidia/ ml) causing low mortality rates ranging from 2 to 10% in the laboratory conditions.
Other studies have highlighted the use of entomopathogenic fungi isolates alone, as B. bassiana to control D. opuntiae (Santos et al., 2011) or the use of other species like Fusarium incarnatum-equiseti combined with botanical extracts (Da Silva et al., 2016) with promising results. According to a recent study by Diniz et al. (2020) confirmed the effectiveness of Fusarium caatingaense isolates (URM 6779 w) combined with the 10% aqueous extract of Nicotiana tabacum (w/v), causing 98.7% of the mortality of adult females of D. opuntiae under greenhouse conditions, 10 days after application. Similarly, Santos et al. (2016) reported that the combination of Fusarium incarnatum-equiseti species complex (FIESC) 20-b (URM6778) with a 5% aqueous extract of Ricinus communis caused 100% adult females of D. opuntiae, 10 days after application, while, this mortality decreased by 83.89% using the isolate only.
Likewise the Potential use of F.incarnatum-equisetispecies complex isolates URM6779 + 5% aqueous extract of Enterolobium contortisiliquum and URM6777 + 5% aqueous extract of E. contortisiliquum induced the highest mortality rates with 73.64 and 70.34%, respectively, 10 days after application (Velez et al., 2019). However, the use of the Fusarium isolates URM6779 and URM6777 alone gives 50.60 and 51.69%, respectively. The same study showed that the mortality of the wild cochineal caused by different FIESC 20 isolates was not affected when extracts of C. ambrosioides or E. contortisiliquum were added.
The current study showed that the insecticidal effect of fungal isolates on nymphs and females of D. opuntiae was enhanced by the application of the detergent black soap at 60 g/L. The first application of black soap is applied to remove the thicker wax, leading to exposure of D. opuntiae females and nymphs to the contact toxicity of the fungal isolates tested. The present study corroborates the findings of Ramdani et al. (2021a) using the black soap solution at 60 g/L combined with Capsicum annuum fruit extract at 200 g/L. This combination exhibited great control of D. opuntiae females with 87.31% at 7 days after treatment.
This study reported that the black soap (60 g/L) used in combination with Mentha pulegium and Origanum vulgare at 5% gave the highest mortality of adult female mortalities with 96.33 and 92.56%, respectively, 7 days after the first spraying (Ramdani et al., 2021b). The mechanisms implicated in the use of soaps and detergents are elimination of waxes, repellency or the disruption of the cell membrane, dislodging of arthropod, and drowning (Butler et al., 1993; Curkovic, 2016).
Fungi have a unique mode of infection; they get to the hemocoel via the cuticle or mouthparts. Infection results after contact with a virulent inoculum and the cuticle of a susceptible insect, the spores germinate and the germ tubes penetrate eventually the pathogen spreads through the tissues of the host. After penetrating the integument, the fungi spread to the internal tissues. Entomopathogenic fungi produced mycotoxins, which cause the death of the host by causing a progressive degeneration of their tissues dehydration (Ferron, 1981).
The results of the present study proved that the application of B. bassiana (HASS and RFSL10) and A. lecanii (RFSLV) applied at 108 conidia ml−1 in combination with black soap (60 g/L) may be used as one of the components of integrated pest management against D. opuntiae as a good alternative to chemical pesticides. However, additional are necessary to establish adequate fungal formulations, their compatibility with other bio pesticide (Botanical extracts or Oils). Laboratory and greenhouse studies should be supported by field experiments to find the optimal use of the most effective fungal isolates on the wild cochineal, in addition to determine the most effective method of application under field conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization: CR, KE, and ME. Methodology: CR, KE, and RBoul. Visualization: KE and ME. Software and writing: CR and KE. Formal analysis: CR, RBoul, RBouh, ME, AM, and MA-J. Review of the article: ME, KE, RBoul, RBouh, and MA-J. Supervision: ME. Project administration: ME and KE. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267.
Andaló, V., Moino Júnior, A., Santa-Cecilia, L. V. C., and Souza, G. C. (2004). Seleção de isolados de fungos e nematóides entomopatogênicos para a cochonilha-da-raiz-do-cafeeiro Dysmicoccus texensis (Tinsley). Arq. Inst. Biol. 71, 181–187. Available online at: http://www.biologico.agricultura.sp.gov.br/uploads/docs/arq/V71_2/andalo.PDF (accessed August 16, 2022).
Annecke, D. P., and Moran, V. C. (1978). Critical reviews of biological pest control in South Africa. 2. The prickly pear, Opuntia ficus-indica (L) Miller. J. Entomol. Soc. South. Afr. 41, 161–188
Arba, M. (2009). Le cactus opuntia, une espèce fruitière et fourragère pour une agriculture durable au Maroc. Available online at: http://agrimaroc.net/agdumed2009/Arba_cactus_opuntia_espece_fruitiere_fourragere.pdf (accessed August 16, 2022).
Bouharroud, R., Amarraque, A., and Qessaoui, R. (2016). First report of the Opuntia cochineal scale Dactylopius opuntiae (Hemiptera: Dactylopiidae) in Morocco. EPPO Bull 46, 308–310. doi: 10.1111/epp.12298
Bouharroud, R., Sbaghi, M., Boujghagh, M., and El Bouhssini, M. (2018). Biological control of the prickly pear cochineal Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae). EPPO Bull 48, 300–306. doi: 10.1111/epp.12471
Bravo-Hollis, H. (1978). Las Cactáceas de México. Volumen 1, Segunda Edición. Universidad Nacional Autónoma de México, 743.
Butler, G. D., Henneberry, T. J., Stansly, P. A., and Schuster, D. J. (1993). Insecticidal effects of selected soaps, oils and detergents on the sweet potato whitefly. Fla Entomol. 76, 161–167. doi: 10.2307/3496023
Carneiro-Leão, M. P., Tiago, P. V., Medeiros, L. V., Costa, A. F., and Oliveira, N. T. (2017). Dactylopius opuntiae: control by the Fusarium incarnatum–equiseti species complex and confirmation of mortality by DNA fingerprinting. J. Pest Sci. 90, 925–933. doi: 10.1007/s10340-017-0841-4
Chávez-Moreno, C. K., Tecante, A., and Casas, A. (2009). The Opuntia (Cactaceae) and Dactylopius (Hemiptera: Dactylopiidae) in Mexico: a historical perspective of use, interaction and distribution. Biodivers. Conserv. 18, 3337–3355. doi: 10.1007/s10531-009-9647-x
Chougui, N., Tamendjari, A., Hamiidj, W., Hallal, S., Barras, A., Richard, T., et al. (2013). Oil composition and characteristics of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 139, 796–803. doi: 10.1016/j.foodchem.2013.01.054
Curkovic, T. (2016). “Detergents and soaps as tools for IPM in agriculture,” in Integrated Pest management (IPM): Environmentally Sound Pest Management,eds H. K. Gill, G. Goyal (London, UK: IntechOpen), 155–189. doi: 10.5772/64343
Da Silva, S. A. C., Soares, O. R. L., Da Costa, A. F., Vieira, T. P., and de Oliveira, N. T. (2016). Controlling Dactylopius opuntiae with Fusarium incarnatum-equiseti species complex and extracts of Ricinus communis and Poincianella pyramidalis. J. Pest Sci. 89, 539–547. doi: 10.1007/s10340-015-0689-4
De Lotto, G. (1974). On the status and identity of the cochineal insects (Homoptera: Coccoidea: Dactylopiidae). J. Entomol. Soc. South. Afr. 37, 167–193.
Diniz, G. A., Barbosa, L. F. S., Santos, A. C. D. S., Oliveira, N. T. D., Costa, A. F. D., Carneiro-Leão, M. P., et al. (2020). Bio-insecticide effect of isolates of Fusarium caatingaense (Sordariomycetes: Hypocreales) combined to botanical extracts against Dactylopius opuntiae (Hemiptera: Dactylopiidae). Biocontrol Sci. Technol. 30, 384–395. doi: 10.1080/09583157.2020.1720601
Dodd, A. P. (1940). The Biological Campaign Against Prickly Pear. Common Wealth Prickly Pear Board Bulletin. Brisbane, Australia: Government Printer, 177.
El Aalaoui, M., Bouharroud, R., Sbaghi, M., El Bouhssini, M., and Hilali, L. (2019b). Predatory potential of eleven native Moroccan adult ladybird species on different stages of Dactylopius opuntiae (Cockerell) (Hemiptera: Dactylopiidae). EPPO Bull. 49, 374–379. doi: 10.1111/epp.12565
El Aalaoui, M., Bouharroud, R., Sbaghi, M., El Bouhssini, M., Hilali, L., and Dari, K. (2019a). Comparative toxicity of different chemical and biological insecticides against the scale insect Dactylopius opuntiae and their side effects on the predator Cryptolaemus montrouzieri. Arch. Phytopathol. Pflanzenschutz. 52, 155–169. doi: 10.1080/03235408.2019.1589909
Fang, W., Scully, L. R., Zhang, L., Pei, Y., and Bidochka, M. J. (2008). Implication of a regulator of G protein signalling (BbRGS1) in conidiation and conidial thermotolerance of the insect pathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 279, 146–156. doi: 10.1111/j.1574-6968.2007.00978.x
Ferron, P. (1981). “Pest control by the fungi Beauveria and Metarhizium,” in Microbial Control of Pests and Plant Diseases 1970–1980, ed H. D. Burges (London: Academic Press), 465–482.
Foldi, I. (2001). Liste des cochenilles de France (Hemiptera, Coccoidea). Bull. Soc. Entomol. Fr. 106, 303–308. doi: 10.3406/bsef.2001.16768
Gao, T., Wang, Z., Huang, Y., Keyhani, N. O., and Huang, Z. (2017). Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 7, 1–11. doi: 10.1038/srep42727
Gebreegziabher, Z., and Tsegay, B. A. (2015). Efficacy of cactus pear (Opuntia ficus-indica) varieties as a source of food and feed in endamehoni district, Northern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 15, 10406–10427. doi: 10.18697/ajfand.72.14090
Griffith, M. P. (2004). The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): new molecular evidence. Am. J. Bot. 91, 1915–1921. doi: 10.3732/ajb.91.11.1915
Gul, H. T., Saeed, S., and Khan, F. A. (2014). Entomopathogenic fungi as effective insect pest management tactic: A review. Appl. Sci. Bus. Econ. 1, 10–18.
Hajek, A. E., and Meyling, N. V. (2018). “Fungi,” in Ecology of Invertebrate Diseases, ed A. E. Hajek (Chichester, UK: John Wiley and Sons, Ltd.), 327–377. doi: 10.1002/9781119256106.ch9
Herrero, N., Dueñas, E., Quesada-Moraga, E., and Zabalgogeazcoa, I. (2012). Prevalence and diversity of viruses in the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol. 78, 8523–8530. doi: 10.1128/AEM.01954-12
Kulkarni, S. R., and Patil, S. K. (2013). Efficacy of different biopesticides and insecticides against mealy bugs on custard apple. Pest Manage. Horticult. Ecosys. 19, 113–115. Available online at: http://aapmhe.in/index.php/pmhe/article/download/178/170 (accessed August 16, 2022).
Lopes, R. S., Oliveira, L. G., Costa, A. F., Correia, M. T. S., Lima, E. A. L. A., and Lima, V. L. (2018). Efficacy of Libidibia ferrea var. ferrea and Agave sisalana extracts against Dactylopius opuntiae (Hemiptera: Coccoidea). J. Agric. Sci. 10, 255–267. doi: 10.5539/jas.v10n4p255
Mantzoukas, S., and Grammatikopoulos, G. (2020). The effect of three entomopathogenic endophytes of the sweet sorghum on the growth and feeding performance of its pest, Sesamia nonagrioides larvae, and their efficacy under field conditions. J. Crop Prot. 127, 104952. doi: 10.1016/j.cropro.2019.104952
Mazzeo, G., Nucifora, S., Russo, A., and Suma, P. (2019). Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: an overview. Entomol. Exp. Appl. 167, 59–72. doi: 10.1111/eea.12756
Menezes, M. E. L., Brito, E S, Malheiro, M. G., Lopes, A. C. R., Santos, P., and de S Gava, C. A. T. (2008). “Seleção de fungos entomopatogênicos para o controle de Dactylopius opuntiae Cockerel (Hemiptera: Dactylopiidae) no Semi-Árido nordestino,” in Jornada de Iniciação Científica da Embrapa Semi-Arido, 2010, Petrolina : Jornada de Iniciação Científica da Embrapa Semi-Árido, 83–89.
Meyling, N. V., and Eilenberg, J. (2007). Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol. Control 43, 145–155. doi: 10.1016/j.biocontrol.2007.07.007
Mohamed, G. S. (2016). Virulence of entomopathogenic fungi against the vine mealy bug, planococcus ficus (signoret)(hemiptera: pseudococcidae). Egypt. J. Biol. Pest Control. 26, 47-51.
Moussa, Z., Yammouni, D., and Azar, D. (2017). Dactylopius opuntiae (Cockerell, 1896), a newinvasive pest of the cactus plants Opuntia ficus-indica in the south of Lebanon (Hemiptera, Coccoidea, Dactylopiidae). Bull. Soc. Entomol. Fr. 122, 173–178. doi: 10.3406/bsef.2017.3194
Oliveira, L. G., Lopes, R. S., Santos, V., Luna-Alves, E. A. L., Maranhão, E. A., and Costa, A. (2019). Efficacy of biocontrol agents beauveria bassiana and plant extracts on Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae). J. Agric. Sci. 12, 171. doi: 10.5539/jas.v12n1p171
Panyasiri, C., Attathom, T., and Poehling, H. M. (2007). Pathogenicity of entomopathogenic fungi-potential candidates to control insect pests on tomato under protected cultivation in Thailand. J. Plant Dis. Prot. 114, 278–287. doi: 10.1007/BF03356230
Pedrini, N., Crespo, R., Juárez, M. P., and Tacconi de Alaniz, M. J. (2006). Clues on the role of Beauveria bassiana catalases in alkane degradation events. Mycologia. 98, 528–534. doi: 10.1080/15572536.2006.11832655
Pérez Guerra, G., and Kosztarab, M. (1992). Biosystematics of the family Dactylopiidae (Homoptera: Coccinea) with emphasis on the life cycle of Dactylopius coccus Costa. Bull. Virginia Agric. Exp. Station 92, 1–90.
Pettey, F. W. (1950). The cochineal insect (Dactylopius opuntiae), and the problem of its control in spineless cactus plantations. Part I. Its history, distribution, biology and achievements in the control of prickly pear in South Africa. Union of South Africa, Department of Agriculture, Entomology Series, Science Bulletin 296, 1–12.
Pretti, L., Bazzu, G., Serra, P. A., and Nieddu, G. (2014). A novel method for the determination of ascorbic acid and antioxidant capacity in Opuntia ficus-indica usang in vivo microdialysis. Food Chem. 147, 131–137. doi: 10.1016/j.foodchem.2013.09.120
Ramdani, C., Bouharroud, R., Sbaghi, M., Mesfioui, A., and El Bouhssini, M. (2021a). Field and laboratory evaluations of different botanical insecticides for the control of Dactylopius opuntiae (Cockerell) on cactus pear in Morocco. Int. J. Trop. Insect Sci. 41, 1623–1632. doi: 10.1007/s42690-020-00363-w
Ramdani, C., El-Fakhouri, K., Sbaghi, M., Bouharroud, R., Boulamtat, R., Aasfar, A., et al. (2021b). Chemical composition and insecticidal potential of six essential oils from Morocco against Dactylopius opuntiae (Cockerell) under field and laboratory conditions. Insects 12, 1007. doi: 10.3390/insects12111007
Ramírez-Bustos, I. I., López-Martínez, V., Juárez-López, P., Guillén-Sánchez, D., Alia-Tejacal, I., Rivera-León, I., et al. (2018). Identificación de envases vacíos de plaguicidas en plantaciones de nopal verdura, Opuntia ficus-indica (L.) Mill. (Cactaceae), en Morelos, México. Acta agríc. Pec. 4, 18–25. doi: 10.30973/aap/2018.4.1/3
Ramírez-Sánchez, C. J., Morales-Flores, F. J., Alatorre-Rosas, R., Mena-Covarrubias, J., and Méndez Gallegos, S. J. (2019). Efectividad de hongos entomopatógenos sobre la mortalidad de Dactylopius opuntiae (Hemiptera: Dactylopiidae) en condiciones de laboratório. Rev. Mexicana cienc. Agric. 22, 1–14. doi: 10.29312/remexca.v0i22.1854
Santos, A. C. S., Oliveira, R. L. S., Costa, A. F., Tiago, P. V., and Oliveira, N. T. (2016). Controlling Dactylopius opuntiae with Fusarium incarnatum-equiseti species complex and extracts of Ricinus communis and Poincianella pyramidalis. J. Pest Sci. 89, 539–547.
Santos, D. C., Farias, I., Lira, M. A., Santos, M. V., Arruda, G. P., Coelho, R. S. B., et al. (2006). Manejo e utilização da palma forrageira (Opuntia e Nopalea). Empresa Pernambucana de Pesquisa Agropecuária. Recife, IPA, (Documento 30). Brazil, 48. Available online at: http://www.ipa.br/publicacoes_tecnicas/Pal01.pdf(accessed August 16, 2022).
Santos, P. D. S., Silva, M. A. Q., Monteiroa, A. C., and Gava, C. A. T. (2011). Improving photoprotection of Beauveria bassiana conidia for biological control of the cactus pest Dactylopius opuntiae in the semiarid region northeast of Brazil. Biocontrol. Sci. Technol. 21, 893–902. doi: 10.1080/09583157.2011.586022
Sbaghi, M., Bouharroud, R., Boujghagh, M., and El Bouhssini, M. (2019). Sources de résistance d'Opuntia spp. contre la cochenille à carmin, Dactylopius opuntiae, au Maroc. EPPO Bull. 49, 585–592. doi: 10.1111/epp.12606
Shia, W. B., and Feng, M. G. (2004). Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol. Control. 30, 165–173. doi: 10.1016/j.biocontrol.2004.01.017
Silva Neto, F. L., Andrade, R. L., Souto, J. S., Bezerra, D. M., Silva, A. L. N., Ferreira, S. D., et al. (2008). Crescimento da palma forrageira (Opuntia fícus- indica Mill.) em função do espaçamento e doses de fósforo. Anais. ZOOTEC, João Pessoa. 4.
Silva, S. Q. (1991). Proposta Para Avaliação do Controle Biológico da Cochonilha Diaspis Echinocacti (Bouché, 1833) (Homoptera, Diaspididae) da Palma Forrageira em Pernambuco (Mater's Thesis). Universidade Federal Rural de Pernambuco, Recife, Brazil.
Van Den Berg, J. D., Sandvol, L. E., Jaronski, S. T., Jackson, M. A., Souza, E. J., and Halbert, S. E. (2001). Efficacy of fungi for control of Russian wheat aphid (Homoptera: Aphididae) in irrigated wheat. Southwest. Entomol. 26, 73–85.
Vanegas-Rico, J. M., Lomeli-Flores, J. R., Rodríguez-leyva, E., Mora-aguilera, G. Y., and Valdez, J. M. (2010). Enemigos naturales de Dactylopius opuntiae (Cockerell) en Opuntia ficus-indica (L.) Miller en el centro de México. Acta Zool. Mex. 26, 415–433. doi: 10.21829/azm.2010.262718
Velez, B. A. D. A., Diniz, A. G., Barbosa, L. F. S., Santos, A. C. D. S., da Costa, A. F., and Tiago, P. V. (2019). Potential of Fusarium incarnatum-equiseti species complex isolates with Chenopodium ambrosioides and Enterolobium contortisiliquum extracts to control Dactylopius opuntiae. Int. J. Trop. Insect Sci. 39, 131–138. doi: 10.1007/s42690-019-00014-9
Vigueras, A. L., Cibrían-Tovar, J., and Pelayo-Ortiz, C. (2009). Use of botanicals extracts to control wild cochineal (Dactylopius opuntiae Cockerell) on cactus pear. Acta Hortic. 811, 229–234. doi: 10.17660/ActaHortic.2009.811.28
Keywords: Dactylopius opuntiae, entomopathogenic fungi, Beauveria bassiana, Akanthomyces lecanii, Cordyceps farinosa, black soap, cactus pear
Citation: Ramdani C, El Fakhouri K, Boulamtat R, Bouharroud R, Mesfioui A, Al-Jaboobi M and El Bouhssini M (2022) Entomopathogenic fungi as biological control agents of Dactylopius opuntiae (Hemiptera: Dactylopiidae) under laboratory and greenhouse conditions. Front. Sustain. Food Syst. 6:997254. doi: 10.3389/fsufs.2022.997254
Received: 27 July 2022; Accepted: 11 August 2022;
Published: 02 September 2022.
Edited by:
Everlon Cid Rigobelo, São Paulo State University, BrazilReviewed by:
Fátima Gandarilla, Autonomous University of Nuevo León, MexicoIsela Quintero, Autonomous University of Nuevo León, Mexico
Copyright © 2022 Ramdani, El Fakhouri, Boulamtat, Bouharroud, Mesfioui, Al-Jaboobi and El Bouhssini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaimae Ramdani, Y2gucmFtQGhvdG1haWwuY29t
†ORCID: Chaimae Ramdani orcid.org/0000-0002-7971-3649
 Chaimae Ramdani
Chaimae Ramdani Karim El Fakhouri
Karim El Fakhouri Rachid Boulamtat
Rachid Boulamtat Rachid Bouharroud
Rachid Bouharroud Abdelhalim Mesfioui2
Abdelhalim Mesfioui2 Muamar Al-Jaboobi
Muamar Al-Jaboobi