- Department of Biodiversity, School of Molecular and Life Sciences, Faculty of Science and Agriculture, University of Limpopo, Polokwane, South Africa
The enhanced growth and productivity of soybeans during the past decades were possible due to the application of agrichemicals such as bio-fertilizers, chemical fertilizers, and the use of high yielding, as well as disease resistant transgenic and non-transgenic varieties. Agrichemicals applied as seed primers, plant protectants, and growth regulators, however, had a diminutive significance on growth and productivity improvements across the globe. The utilization of plant growth regulators (PGRs) for vegetative growth, reproduction and yield quality improvements remains unexplored, particularly, the use of cytokinins such as 6-benzyl adenine (6-BAP) to improve soybean response to abiotic stresses. Therefore, an understanding of the role of 6-BAP in the mediation of an array of adaptive responses that provide plants with the ability to withstand abiotic stresses must be thoroughly investigated. Such mitigative effects will play a critical role in encouraging exogenous application of plant hormones like 6-BAP as a mechanism for overcoming drought stress related effects in soybean. This paper discusses the evolving role of synthetic cytokinin 6-bezyl adenine in horticulture, especially the implications of its exogenous applications in soybean to confer tolerance to drought stress.
Introduction
Soybean (Glycine max L. Merr.) is one of the most important leguminous crops belonging to the Fabaceae family, and cultivated globally as a primary source of food, feed, oil, and proteins. In the past, soybean mainly constituted the crucial components of the traditional popular diets in China and other East and South-eastern Asian countries. Currently, it serves as a major industrial crop accounting for roughly more than 50% of the total oil and 40% protein (Yao et al., 2020). Soybean oil contained in seeds is mainly composed primarily of triacylglycerides, thinly dispersed quantities of mono- and di-acylglycerols, as well as significant amounts of essential fatty acids as indicated in Figure 1B. The quality of foods and feeds derived from this crop also depend on the composition of globulin (2S, 7S, 11S, and 15S) proteins (Figure 1A), oil fatty acids (Figure 1B), carbohydrates, lipids and lesser amounts of anti-nutritional factors. Overall, soybean seeds contain 25% of carbohydrates, 20% lipids (with 95–98% triglycerides, 1.5–2.5% phospholipids, and 0.5–1.6% unsaponifiable matter), 5% crude fiber, and at least 1.5% phytic acid (Qin et al., 2017; Li et al., 2020). According to Yao et al. (2020), the unsaturated fatty acids shown in Figure 1B play a critical role in human health by regulating blood clotting, cholesterol metabolism, neurotransmission problems, and significantly contribute to the structure of membranes and membrane-phospholipids found in the brain and the retina. However, the nutritional contents in soybean may be reduced by fatty acid oxidation, including anti-nutritional factors such as phytic acid, and susceptibility of cultivated soybean varieties to various abiotic stress factors, especially water shortage or water logging. Soybeans are undergoing breeding to confer tolerance to drought and the various types of abiotic stresses that this crop has endured for decades. Many studies in genetic engineering have been conducted by introducing Bacillus thuringiensis (Bt) genes such as Cry4Aa, Cry4Ba, Cry10Aa, and Cry11Aa, encoding Cry toxins. Trypsin inhibitors, serine (Kunitz-type) protease inhibitors and cystatins (Oryzacystatins I and II) genes have also been investigated for abiotic and biotic stress resistance (Parmar et al., 2017; Abbas, 2018; Nair et al., 2018). Although, not as widely reported as genetic engineering, the use of agrichemicals such as primers, osmoprotectants, and plant growth regulators (PGRs) like auxins and cytokinins also offer a myriad of applications in the improvement of horticultural crops for trait and yield improvements, as well as for stress resistance. In the demand for increased cereals and pulses, including reduced pesticides use, chemical fertilizers and carbon footprint, plant modifications through exogenous application of PGRs may also increase crop yields. In view of the fact that, auxins inhibit formation of axillary shoot bubs while cytokinins such as 6-BAP efficiently regulate bud proliferation, shoot branching, flowering, fruiting and tissue/organ senescence. In cereal grains, exogenous application of cytokinins contributed to the development of spikelets to mature grains after fertilization in rice. The reported cytokinin effects included increased cell number and filling in seed endosperms (Panda et al., 2018). Apart from seed setting, grain yields in cereals and other crops is determined by the number of branches on the stem, which is influenced by exogenous levels of 6-BAP used. In soybean, based on two-year field trials conducted to assess the impact of different irrigation regimes on plant growth, net photosynthesis and yield, El–Metwally et al. (2021) reported increased plant height, net assimilation rates and enhanced pod number per plant using 100–200 mgL−1 6-BAP under low water supply conditions. This study recommended the use of 150 mgL−1 6-BAP as part of the irrigation program to compensate reduced water supply of up to 80% of crop evapotranspiration in soybean. Other studies that have exploited 6-BAP due to its crucial role in soybean plant growth and development include Abdallah (2020), Mangena (2020), Ibrahim et al. (2021), and Amoanimaa-Dede et al. (2022).

Figure 1. Composition of protein (A) and oil (fatty acids) (B) in soybean seeds. Data sourced from Yao et al. (2020) and Guan et al. (2021).
During the past two decades, a large number of transgenic soybean lines have been developed, including those still being optimized through genome editing (CRISPR/Cas9), but only a limited number of varieties were enhanced using plant growth regulators as major role players during the crop improvement (Liu et al., 2017; Parmar et al., 2017). Although, a large body of information is widely available on the role of plant growth regulators such as cytokinins on improving root and shoot growth, biomass, antioxidant capacity, and other physiological processes. Additional work still need to be done to elucidate growth and developmental responses of plants treated with these PGRs like 6-BAP under environmentally constraining conditions. Therefore, the role of 6-BAP in the mediation of an array of adaptive responses that provide plants with the ability to withstand abiotic drought stress is discussed in this review. Such mitigative effects may also play a critical role in encouraging exogenous application of plant hormones as alternative mechanisms for overcoming drought stress related effects in soybean. In summary, this paper discusses the evolving role of 6-bezyl adenine (6-BAP) in horticulture, paying particular attention to the implications of exogenously optimizing 6-BAP levels in soybean treatments to confer tolerance to drought stress.
Effect of drought stress in soybean
Drought, together with other non-living environmental constraints are adverse abiotic stress factors affecting soybean crop production worldwide in numerous ways (Table 1). Drought stress reduces soybean's survival rates, growth, and development by interfering with complex networks of coupled anabolic and catabolic pathways that direct the flow of energy and resources within, as well as between cells. Drought stress in plants is characterized by cell dehydration, reduced water potential, altered carbon partitioning, membrane and protein destabilization, production of reactive oxygen species (ROS), and tissue senescence (Abid et al., 2018; Zhang et al., 2018; Bashir et al., 2021). Plants exposed to moderate and severe water deficit stress experiences oxidative stress, which damages protein molecules, macromolecules such as nucleic acids and lipids, subsequently increasing the number of harmful reactive oxygen species (ROS) by-products, aggregated and misfolded proteins. In crops such as soybean, cowpea (Vigna unguiculata), chickpea (Cicer arietinum), common bean (Phaseolus vulgaris), and lentil (Lens culinaris), oxidative stress also rapidly induces protein damage by the activity of proteolytic enzymes which are mostly localized in the nodules, leaves, and roots.

Table 1. General biochemical and physiological growth parameters influenced by different abiotic stress factors in plants.
Proteolytic enzymes, which form a diverse group of proteases capable of cleaving peptide bonds, are implicated in various essential processes including fine control of protein catabolism, selective degradation of damaged proteins during stress and bulk hydrolysis of dietary proteins (Mangena, 2020). Similarly, in monocot grains such as wheat (Triticum aestivum L.), small ubiquitin-like modifier proteases (SUMO) were reported to play a vital role in regulating pathway fluxes during plant growth and development under drought stress conditions. According to Le Roux et al. (2019), wheat plants transformed with a vector expression of Arabidopsis thaliana cysteine protease inhibitor (OVERLYTOLERANT TO SALT-1, OTS1) showed enhanced stress tolerance and delayed tissue senescence compared to other lines of this species that only contained SUMO proteases. In soybean, drought negatively influences the various parameters shown in Table 1, in addition to causing significant changes in the shoot and root morphology, reduction of cortical tissue in stems, and causes a decrease in the number and size of flowers, fruit pods, and seeds (Du et al., 2020; Arya et al., 2021). Apart from these diminished vegetative and reproductive growth, soybeans also show serious inhibitions on the formation of cell protuberance containing nitrogen-fixing Gram-negative bacteria in the roots, discussed later in this paper. However, vegetative and reproductive soybean traits, including nodulation could be enhanced by treating seeds with cytokinins before sowing, followed by the exposure of plants to drought stress and higher temperatures as indicated by Kempster et al. (2020) and Zhang et al. (2022). These growth aspects still need to be thoroughly researched amid the criticism and challenges suffered by animals, humans and the environment as post-impacts of using chemical pesticides and chemical fertilizers to improve crop productivity as highlighted by Isman (2019).
Role of 6-BAP in soybean
The 6-benzyl adenine, also known as 6-benzylamino purine (Figure 2), is a synthetic N6-substituted adenine derived from the N6-aromatic ring compounds (Wang et al., 2016). The conversion or rearrangement of 3-bezyladenine to N6-benzyl adenine involves ring opening of both the imidazole and pyrimidine rings (Figure 2A) to produce the 6-benzyl adenine presented as 3D and 2D conformational chemical structures depicted in Figures 2B,C (SpectraBase, 2022). Generally, 6-BAP regulates various aspects of cell division, apical dominance, tissue senescence and translocation of photosynthates from the source to the sinks. 6-BAP in cooperation with other cytokinins or auxins can further control fundamental growth and developmental processes, including direct/indirect somatic embryogenesis, lateral shoot formation and callus initiation under controlled in vitro cell culture conditions (Solorzano-Cascante et al., 2018; Srilestari et al., 2020). However, the 6-BAP levels used in plant tissue culture are generally applied at concentration ranging from 0.01 to 10.0 mgL−1 to stimulate axillary and adventitious shoot proliferation, regulate cell differentiation, activate RNA synthesis, and stimulate protein/enzyme activity (Naito et al., 2006). In vitro growth of seeds and explants is more sensitive to exogenous application of PGRs which induce significant stimulatory effects due to increased rate of absorption and endogenous hormone content in the cells. Moreover, differences exist among in vivo and ex vitro studies in the levels of 6-BAP used, at a range of 50–500 mgL−1 and 0.01–10 mgL−1, respectively. Physiological characteristics and growth regulation measures, however, indicated marked concentration differences because exogenously applied PGRs cannot enter plant tissues rapidly to directly promote or inhibit growth. Booker et al. (2003) and Cai et al. (2018) reported that indole-3-acetic acid (IAA) did not directly inhibit the growth of lateral buds because IAA applied at shoot apices could not enter inside the lateral buds. Although, information on the mechanism of hormonal absorption and translocation in plant tissues has not been widely reported, many studies nevertheless, found a positive correlation between growth and enhanced exogenous levels of 6-BAP which had tremendous effects on endogenous cytokinin content (Yuan et al., 2019; Li et al., 2021; Zhang et al., 2021).
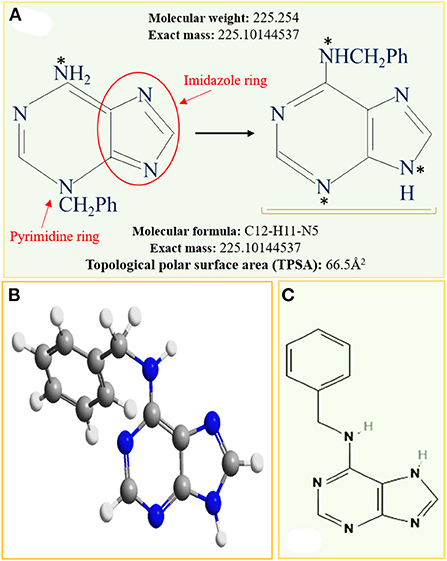
Figure 2. Depictions of 6-benzyl adenine biosynthesis with some important chemical properties (A), and the chemical structural appearance in 3D (B), and 2D (C) structural conformations (SpectraBase, 2022).
Liu and Zhang (2017), reported foliar application of exogenous 6-BAP to improve plant drought stress resistance by increasing the rate of photosynthesis, stomatal conductance, and transpiration rates, reducing yield losses up to 46% in soybean. In another study, foliar spraying with 20 mg.L−1 6-BAP improved soybean growth and yield which were negatively influenced by maize (Zea mays L.) shading during maize-soybean relay strip intercropping systems (Kai et al., 2020). According to Kai et al. (2020), maize shading inhibited soybean by influencing morphological characteristics, biomass, and physiological responses of this crop, including chlorophyll content, net photosynthetic rates, and leaf thickness. Furthermore, the results indicated that the application of suitable PGRs could alleviate the detrimental effects caused by maize shading, and other growth inhibitory factors serving as both biotic and abiotic stress.
For instance, exogenous application of 6-BAP delayed the degradation of photosynthetic pigments by increasing the activities of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) enzymes during seed filling and production in maize (Ahmad et al., 2019). Kempster et al. (2020) also reported seed priming and foliar application of cytokinins to improve yield and cold stress tolerance in soybean, cultivar DM5OI17 and DM4OR16 in field trials with early (September and early November) and conventional (late November) sowing dates in Argentine. However, apart from lack of irrigation or rainfall, water deficit stress in plants can be caused by cold/chilling and salt/salinity stress. Therefore, cytokinins interactions, especially with other hormones, play a significant role in counteracting cellular dehydration and turgor pressure losses suffered by plants exposed to drought, salinity, and cold stress, especially frost (Beck et al., 2007). Although, several reports such as those highlighted above are widely interrogate these effects, only a handful of studies have emphasized the role of these growth regulators on vegetative growth, yield components and nodulation in numerous crop plants, such as leguminous pigeonpea, cowpea, and soybean.
Modulation of shoot and root architecture
Growth modulation of the root and shoot systems are among the most essential adaptations under abiotic drought stress conditions. Although, more literature is available that describe the immediate physiological and molecular pathways coordinating the changes in root and shoot growth, biomass (Figure 3) and nodulation (Figure 4), including branching because of ABA and auxin expressions in plants during their response to drought stress. Not much reported work is available regarding the role of cytokinins on growth adjustments in response to stress. Cytokinins, including 6-BAP, play a pivotal role in plant signaling and adaptation to the fluctuating availability of nutrients and water from different soil horizons. To cope with fluctuating temperatures and water availability, soybean plants integrate their systemic signals pertaining to their water status into developmental pathways that coordinate changes in the architecture of both roots and shoots. Typically, any change perceived by the plant concerning plant nutritional and water status, in relation to the external supply firstly modulate the root system. As reported by Giehl et al. (2013), such changes occur over time and they determine the degree of root plasticity based on the individual root system components, including root phenotype, extension, placement, and the direction of root growth.
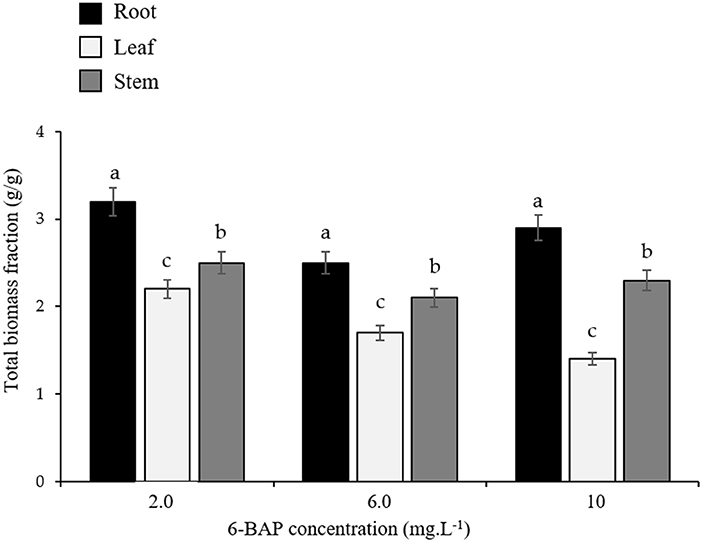
Figure 3. Variations in total biomass fractions of root, stem, and leaf following seed priming (16 h) with different concentrations of 6-BAP (Mangena, 2020).

Figure 4. Soybean plants with nodulated roots. (A) Healthy nodules on soybean plants used as a control plants. (B) Roots of moderated water-stressed plant with numerous mature nodule structures. (C) Severe water-stressed plant root showing poor nodulation. (D) Nitrogen (N) fixing nodules with Rhizobia as observed in the control. (E) Green less-effective nodules from moderately water-stressed roots. (F) Decomposing root nodule of severely water-stressed plants (Mangena, 2018).
Furthermore, some organ modifications may be stress specific and acted upon by local specific signals. The role of root system modulation in soybean, like many other crop plants can be demonstrated by cell proliferation and differentiation that take place when root growth is observed in response to drought stress. In general, increased root growth biomass (like as exemplified in Figure 3 in relation to 6-BAP treatments) and depth are predominantly observed in water-stressed soybean plants. Müller et al. (2021) reported greater proportions of root growth in the first centimeters of soil-depth in response to soil water-related properties. Many studies also concurred with findings by Dong et al. (2019), Hanum and (2020), and Amoanimaa-Dede et al. (2022) who reported that drought tolerance mechanism in soybean is closely related to cytokinin activities in combination with other hormones, which closely regulate the rooting and shooting patterns. Furthermore, the lack of high-level cytokinins in plant tissues also immediately inhibit root extension because of halted cell cycle and other cellular activities. In terms of shoots, drought affect nutrient uptake and availability to soybean plants, which completely diminishes their physiological, and biochemical growth responses as indicated previously in Table 1.
Low water content in the cells causes leaves to wilt and drop (Wang et al., 2022), have a negative impact on plant's architecture by affecting stem elongation, branching and subsequently blossoming as well as fruit production (Du et al., 2020; Arya et al., 2021). All of these growth effects appears to be modulated or regulated by cytokinins as illustrated later in Figure 5. Root and shoot growth was inhibited by the enhanced concentrations of 6-BAP (2–10 mgL−1) applied through seed priming. The decrease in leaf, stem, and root biomass was inversely proportional to the increase in 6-BAP levels during a 16 h priming period of soybean seeds (Figure 3). These results confirms the inhibitory role of 6-BAP during seed germination, seedling development, and plant establishment as reported by Gu et al. (2022) in which 6-BAP concentration of 5 mgL−1 and less promoted germination, while higher levels caused seedling retardation. In contrast, foliar application of 6-BAP solutions at concentration ranging between 20 and 200 mgL−1 presented maximum plant establishment and net assimilation rates, together with higher yields in soybean plants subjected to induced drought stress. 6-BAP was reported to improve water use efficiency progressively with decreased irrigation and gradually increasing the amounts of the hormone (Bozso and Barna, 2021; El–Metwally et al., 2021; Amoanimaa-Dede et al., 2022; Gu et al., 2022). Therefore, more scientific evidence is required to strengthen exogenous application of 6-BAP and other synthetic and naturally occurring hormones in playing a pivotal role in the modulation of shoot and root development, especially in response to biotic and abiotic stress.
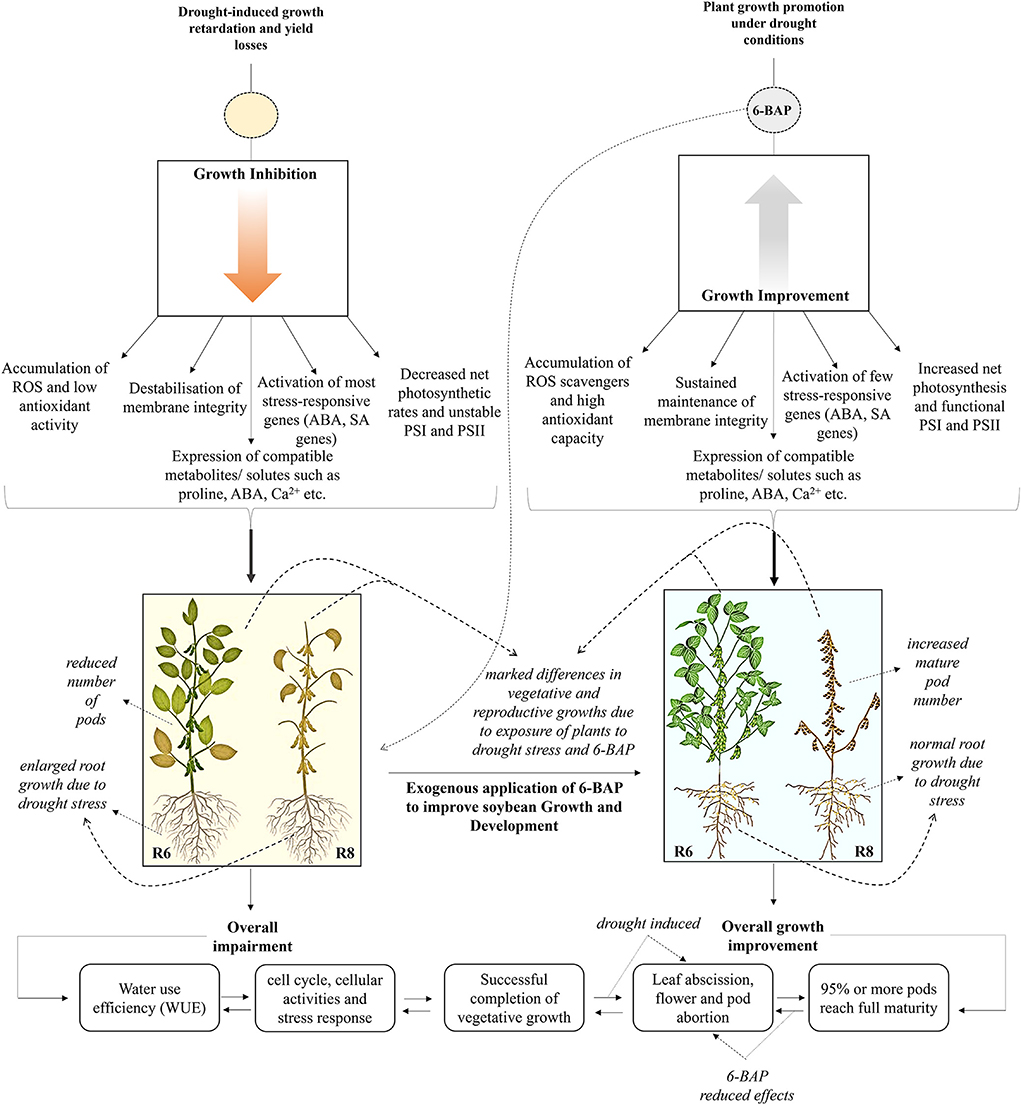
Figure 5. Summary of the effects of 6-BAP on the growth and development of soybean plants following the exposure of the plants to drought stress.
Effect of 6-BAP on lateral bud break
The control of changes in bud dormancy along stems may as well be attributed to the variations in endogenous hormonal concentrations. Generally, cessation of branching or lateral shoot growth, and frequent occurrence of abscission of both leaves and apices may result from the apical dominance, but the precise hormone regulated mechanisms still need to be elucidated. Although, the regulation of lateral bud formation is not extensively studied, numerous reports have demonstrated the role of drought on the inhibition of apical/lateral buds during shoot formation. In soybean, Dong et al. (2019) showed that drought stress inhibited increases in plant height and leaf area by halting bud activity. Other shoot related characteristics that develop from lateral buds that are affected by drought include stem diameter, leaf formation, and node density (Gao et al., 2020; Carrera et al., 2021). Stem elongation in plants is usually controlled by the intercalary meristems. However, cytokinins such as 6-BAP and kinetin play a significant role in regulating the activities of apical meristem, lateral meristem (vascular and cork cambium) and intercalary meristem that are responsible for shoot growth (Wu et al., 2021; Yang et al., 2021).
Cytokinins influence lateral bud growth by promoting nuclear shuttling of transcriptional factors that activate mitotic gene expression (Yang et al., 2021). Under drought conditions, high cytokinin activity was reported in the veins of poplars (Populus x canescens), with areas of leave scars exhibiting low cytokinin activity. Other high cytokinin activities were observed in the differentiation zones of stem pith, mature phloem, and primary meristematic tissues in drought-stressed plants (Paul et al., 2017). As expected, cytokinin concentrations will be high in the bud meristematic tissues as these hormones support primary and secondary growth through bud initiation, a process predetermined to take place regardless of drought stress (Prerostova et al., 2018). Furthermore, failure of lateral buds on the shoots of soybean plants to grow into lateral branches may reduce various yield components and yield quality (Yoshihira and Shiraiwa, 2016; Xu et al., 2020).
Influence of cytokinins on soybean sex expression
Male sterility constitutes the most common type of reproductive mutations observed in soybean. Previous studies suggested that the high frequencies of male sterility reported in soybean result from the numerous genes that are involved in mega- and micro- gametogenesis (Thu et al., 2019; Zhao et al., 2019). However, in agricultural breeding, such sex mutations are very valuable for the production of hybrid seeds. These seeds tend to perform much better compared to their normal counterparts, particularly in terms of yield quantity, quality, disease, and pest resistance, as well as tolerance to abiotic stress factors (Liu et al., 2021). The use of hybrid seeds has greater commercial value since male sterility increases the effectiveness of first filial generation (F1) hybrid seed production. This takes place without pollination and reduces production costs for many farmers (Zhao et al., 2019). Huang et al. (2003) reported the involvement of cytokinins in male reproductive development of all flowering plants. Although, elucidating the role of specific hormones on particular tissue types or developmental stages remains a daunting task due to the fact that these hormones operate in interaction/ cooperation with each other. Furthermore, no reports have been found on the role of 6-BAP on sex expressions in soybean. Seesangboon et al. (2018) reported that the application of 6-BAP on Jatropha curcas L. flower buds increased the number of female flowers and seed yield. This crop had initially exhibited dwindling number of female flowers which led to insufficient seed yields.
All of the abovementioned studies clearly indicated that exogenous applications of cytokinins and increased accumulations in reproductive tissues of transgenic maize and Jatropha curcas plants resulted in altered sex expressions. In maize plants, this further enhanced male-sterility whose restoration was also achieved by further application of this class of PGRs. According to Huang et al. (2003), exogenous application of kinetin at 0, 20, and 100 mg restored male sterility when used with or without 3 mg thidiazuron (TDZ). The results obtained in this study, together with other uncited reports provided the evidence of direct involvement of cytokinin in sex expressions, and further showed that combined effects of multiple hormones control this phenomenon. In Jatropha curcas L. again, similar observations of a strong dependence on cytokinin 6-BAP on flowering, which increased the number of male and female flowers per inflorescence, were also reported by Fröschle et al. (2017). Other specific sex expressions related to traits reported in species such as Cucumis sativus (cucumber) and Momordica charantia L. (bitter gourd) included improved female to male flower ratio, number of seeds per fruit, seed size, seed weight, and seed oil content (Amiriam et al., 2019; Abbas et al., 2020).
Role of 6-BAP on flowering, fruit development, and yield
The increase in crop production during the past years, until currently, were made possible by the application of agrichemicals like fertilizers and cultivation of genetically improved transgenic varieties. However, exogenous chemicals like plant growth regulators such as ethylene, jasmonic acid, ABA, GA3, and 6-BAP had little to no use in the promotion of crop growths, especially for the enhancement of flowering, fruiting, and yield. Increments in the utilization of PGRs for reproductive growth, yield increases and stress tolerance could offer an optimistic view of the future beneficial effects of these agrichemicals to farmers. Jasmonic acid (JA) for instance, was attributed to several positive effects in response to biotic and abiotic stress. To accurately demonstrate the beneficial role of high JA levels in plant tissues, Wang et al. (2020) reported the synergistic and antagonistic effects of exogenously applied JA with ABA, ethylene (ET), salicylic acid (SA), and other synthetic hormones in the process of resisting environmental stress. Although, soybean produces a large number of flowers per plant, one of its major challenges is that the plant also experiences a great deal of flower abortions (Cho et al., 2019). Fruit and flower abortions under unfavorable water deficit conditions has been a serious problem in commercial production of soybean and other leguminous and non-legume crops. Nagel et al. (2001) indicated that exogenous application of cytokinin (6-BAP) to raceme tissues stimulated flower production and prevented abortions in soybean, SD-87001 line that has been proved to be highly sensitive to external treatment of cytokinins.
Exogenous application of 6-BAP (300 mgL−1) at end of flowering stage significantly reduced flower abortion, thus increasing productivity by increasing the number of seeds per plant, seed weight, and seed diameter (Larrisa et al., 2014). In another study, El–Metwally et al. (2021) reported enhanced pod number per plant, and increased overall yield when 100 and 150 mgL−1 6-BAP interacted with 80 and 100% of crop evapotranspiration water regimes, respectively. The findings indicated that lowering water supply up to 80% of crop evapotranspiration (saving 20% of irrigation water) could be compensated by folia spraying of plants with 6-BAP. This study provides significant insights showing that exogenous 6-BAP application could serve as a potential practice for reducing flower abortion, improving fruit and productivity on fields cultivated with soybean.
Role of 6-BAP on nodulation
All leguminous crop species, including soybean play a critical role toward sustainable agriculture and maintenance of soil fertility through a biological nitrogen fixation via a highly specialized symbiotic relationship with Rhizobia bacteria (Figure 4). A sophisticated signaling exchange mediated by glycopeptides and phytohormones regulate soybean root infection by Rhizobia and induce the formation of novel organs termed nodules by cytokinin mediated cell proliferation (Figures 4A,D). A high nitrogen supply is required to achieve high yields in soybean. Kempster et al. (2020) increased early biological nitrogen fixation and total nodule area by priming soybean seeds with 2 mL of 10−7 (high) and 10−9 (low) molL−1 kinetin solution for 4 h in cultivar DM50I17 and DM40R16. Root drenching and petiole feeding technique were also used to increase nodule number in soybean using 6-BAP and trans-zeatin (Mens et al., 2018). The isopentenyl transferase (IPT) genes and their homologous duplicate gene partners (GmIPT5 and GmIPT6) involved in cytokinin biosynthesis for controlling nodule number were also identified in soybean genome (Ye et al., 2006). The proteins encoded by GmIPT1 gene are responsible for the production of active cytokinins during nodulation. IPT genes, especially the constitutive expression of the GmIPT gene in roots, act as key regulator of cytokinin homeostasis and phytohormones crosstalk in soybean under both biotic and abiotic stress together with delaying senescing of nodules as indicated in Figures 4B,C,E,F (Nguyen et al., 2021). Recent research has revealed other regulatory functions of cytokinins during nodulation such as nodule cell proliferation and tissue differentiation, root system architecture and regulation of auxins expression during this process (Dolgikh et al., 2020). Therefore, both cytokinins and auxin are essential for regulating rhizobial infection and nodule organogenesis, while synthetic hormones such as 6-BAP plays a fundamental role in ensuring that specific recognition of symbiotic partners occur, initiate root cells infection by symbiont, and inception of nodules in the root cortex of all legumes, including soybeans (Gamas et al., 2017). However, more research is still required to elucidate the role of exogenous 6-BAP treatments on nodulation in soybean and other legume genotypes as reported by Mens et al. (2018), Kempster et al. (2020), and Amoanimaa-Dede et al. (2022).
Role of 6-BAP on delayed cell, tissue and organ senescence
Cytokinins have become important chemicals not only as yield or growth enhancers as shown in Figure 5 but also as quality improvers. These plant hormones play a crucial role in delaying the development of cell, tissue and organ senescence, and eventually plant death. Senescence of plant cells, tissues or organs is often understood as an evolutionary acquired process that is critical for plant fitness (Woo et al., 2018). Under natural circumstances, plant regulate senescence by timing initiation, rate of progression and nature of senescence. This is more evident on hormonal regulation of leaf and petal abscission by plants for the reason of enhancing yield, biomass and maintaining high nutritional value (Ma et al., 2018; Patharkar and Walker, 2018). In this case, the process proceeds naturally to shed some leaves, flowers, floral organs, and fruits that are no longer required. In soybean, flower abscission of about 50% or more is considered a natural process since the crop profusely produces flowers, but only set a limited number of pods and seeds. Furthermore, the agronomic significance of this phenomenon is still yet to be fully understood. Different soil water level regimes and elevated temperatures influence senescing of nodules (Figures 4E,F) and abscission of flowers and pods in soybean. Hoque et al. (2016) and Kim et al. (2020) reported some of these effects in flower and pod abscission caused by both hot temperatures and drought. Ethylene-induced leaf and petiole abscission revealed the expression of several families of cell wall modifying enzymes. The enzymes were produced by about 188 abscission-specific transcription factors (TFs) encoded by pathogenesis-related (PR) genes in soybean. These TFs are involved in determining cell separation, balancing organ polarity within the abscission zone and regulating plant hormones, which plays a key role in cell differentiation (Kim et al., 2016). The TF regulated hormones act as chemical messengers that coordinate signaling pathways and many cellular processes, including facilitating the adaptation of plants under environmental stress conditions.
According to Amoanimaa-Dede et al. (2022), exogenous application of cytokinins before or during the exposure of plants to stress modulates endogenous hormone levels activating systemic response to stress. When 6-BAP was investigated for the formation of senescence on intact leaves, like in many herbaceous plants, this hormone was found to retard senescence in the detached leaves (Kai et al., 2020; El–Metwally et al., 2021; Wu et al., 2021). However, modulation of senescence by cytokinins in intact soybean cells or tissues could be easily demonstrated using plant materials or explants grown under in vitro plant tissue culture conditions (Aremu et al., 2012; Jablonska-Trypuc et al., 2016; Damanik et al., 2018; Solorzano-Cascante et al., 2018; Teixeira da Silva et al., 2020; Desta and Amare, 2021). The application of 10–4 M 6-BAP to Phaseolus vulgaris germinated seeds under etiolating conditions markedly delayed initiation of cotyledon senescence (Gilbert et al., 2011). Furthermore, Jablonska-Trypuc et al. (2016) also reported stimulatory effects of 6-BAP and kinetin on antioxidant enzyme activity, reduced glutathione and thiol group content that influence tissue and organ senescence, not in plant cell culture, but in in vitro cell culture of fibroblast cells. This evidence warrant further studies on 6-BAP's signaling role in mediating cell senescence and response to environmental stresses which may lead to improved nitrogen accumulation through nodulation and increased yield, especially in soybean genotypes that are more sensitive to hot temperatures and drought stress conditions.
Overall signaling and regulation of drought stress by 6-BAP
As already discussed, cytokinins are plant-specific chemical messengers that play a critical role in the regulation of numerous plant developmental processes. Although, many studies focused on the role that these hormones play on processes involving physiological and metabolic activities, a limited number of reports attempted to unravel the understanding of cytokinin signal transduction. Recent studies proposed that calmodulin and the G-protein-linked receptors were involved in cytokinin signaling. However, calmodulin (CaM, Ca2+ sensing protein) has been implicated on the regulation of metal ions, ROS and modulation of transcription factors such as CAMTA3, GTL1, and WRKY39 as reported by Virdi et al. (2015) and Kolling et al. (2019). Furthermore, the interactions between cytokinins and CaM cannot be denied, particularly, given the effects of these hormones on regulating cytosolic Ca2+ levels. Hooley (1998) earlier showed that G-proteins signaling in plants implicated their heterotrimeric forms on influencing gibberellins and possibly auxin signaling. Moreover, the antisense suppression of the Arabidopsis putative G-protein-coupled receptor (GCR1) revealed the influence of these proteins on cytokinin signaling (Hooley, 1998; Pandey and Assman, 2004). It should, however, be noted that scientific evidence describing the involvement of these factors on cytokinins signaling is very scant. This is similar to the comparison of cytokinin signaling with auxin, ABA and ethylene signaling frequently reported in literature due to their versatility on germination, plant growth and stress responses.
In fact, according to Feng et al. (2017), major obstacles still exist in determining responses that mainly or specifically involve cytokinin signal transduction pathways, and this is because exogenous cytokinin applications evoke ethylene biosynthesis and most components of cytokinin signaling pathways are deemed highly redundant. Indeed, these issues have made it difficult to use biochemical, physiological or genetic approaches to investigate and elucidate cytokinin signaling, even under different environmental conditions, let alone signaling of individual hormones such as 6-BAP. Even though, the biochemical and physiological phytohormonal crosstalk occurring during the exposure of plants to drought stress are still not fully understood, exogenous treatment of plants with 6-BAP reveals widespread effects on morphological, physiological, and biochemical indices that ameliorated drought stress symptoms in plants (Ghaleh et al., 2020). Regulation was also observed in waterlogged maize, cultivar DengHai, following the application of 100 mgdm−3 6-BAP that resulted in changes on leaf protein abundance levels at the tasselling stage (Hu et al., 2020). Plant tissues take up 6-BAP actively as free-hormone in the cells with the exception of only small portions of it being transformed into small nucleotide forms (Centeno et al., 1998). The free 6-BAP remains active in the cells to trigger the induction and initiation of plant cell cycle, cell maturation and differentiation states of the basal and apical meristematic cells. Specifically, the presence of 6-BAP in water-stressed soybean plant tissues is necessary to induce antioxidant activity (El–Metwally et al., 2021) depending on the amount of exogenously applied 6-BAP. Furthermore, Hurny and Benková (2017), Amoanimaa-Dede et al. (2022), and Wang et al. (2022) reported that the application of appropriate amounts of 6-BAP at suitable growth periods relieves abiotic stress such as drought by modulating senescence associated with chlorophyll breakdowns, oxidative cell damage and inhibition of important enzyme activities.
Conclusions and future perspectives
A great deal of work involving other cytokinins and 6-BAP have been reported, particularly, on cell division and differentiation, bud formation, shoot proliferation and direct/indirect somatic embryogenesis under controlled in vitro cell culture conditions in soybean (Phat et al., 2015; Raza et al., 2017; Mishra et al., 2020). In this review, an attempt was made to present current knowledge and improve the state of understanding of the role of synthetic cytokinin 6-BAP in modulating different drought-stress related cellular processes and its applications in horticulture for abiotic stress tolerance. 6-BAP regulate numerous cellular processes that influence the morphology, physiology, and yield of many plants, including recalcitrant crops such as soybean, with or without drought stress. Similar to other methods of crop improvement, seed priming or plant treatments with 6-BAP have to be encouraged to exploit its crucial influence on stress response during plant growth and development. The current trend clearly indicates that cytokinins remain less used as primers or in foliar applications despite the demand for low cost, environmentally friendly methods of improvement, and their natural influence on almost all of the developmental stages of plant life.
Some of those stages already discussed above include vasculature formation, embryonic cell differentiation, shoot formation, and delays in leaf senescence (Saleiman et al., 2021). Although, the discovery of this distinguished class of growth regulators by Gottlieb Haberlandt precisely 109 year ago, now well-known as cytokinins have been widely used in plant tissue culture, plant biotechnology (including in vitro cell genetic engineering), pharmacology, and cosmetic industry, more still need to be done particularly, on commercially targeted traits in horticultural crops such as soybean. Nevertheless, as the various biotechnological strategies remain targeted toward the manipulation of soybean tolerance to drought stress, the application of plant growth regulators as an evolving strategy for potential engineering of soybean plants showing tolerance to abiotic stress must also be developed (Figure 5). Presently, this cytokinin-based compound (6-BAP) has also been widely tested for conferring resistance against temperature and salinity stress in grain crops, such as maize, rice, and wheat (Liu et al., 2020; Saleiman et al., 2021). The use of 6-benzyl adenine in plant growth and development plays an important role as an alternative approach for inducing abiotic stress resistance (Figure 5). This cytokinin enhances stress resistance by effectively functioning as a regulatory hormone, directing cell division, elongation, controlling dormancy, and influencing other biological responses necessary for plants to cope with adverse growth conditions. All above-mentioned biological functions of cytokinins, particularly 6-BAP, were revealed through research progress made thus far, and the fact that auxin and cytokinin crosstalk remains a critical regulatory function for these hormones in differentiation of plant organs and response to stress (Hurny and Benková, 2017). Furthermore, it is primarily on the best interest of farmers and consumers that plant biotechnologists and crop breeders continue their work on deciphering the roles played by cytokinins in general. These must take place at a physiological and molecular level to provide insights into mechanisms of action, regulation, and to develop cost-effective approaches needed to best regulate plant growth and development in soybean under drought stress conditions.
Author contributions
The author wrote the manuscript and verified the submitted version.
Acknowledgments
The author acknowledges APC financial support of the Department of Research Administration and Development at the University of Limpopo and the National Research Foundation of the Republic of South Africa (TTK200210502915).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABA, Abscisic acid; 6-BAP, 6-Benzyl adenine; CaM, Calmodulin; CAT, Catalase; ET, Ethylene; GA3, Gibberellic acid; IPT, Isopentenyl transferase; JA, Jasmonic acid; PGR, Plant growth regulators; POD, Peroxidase; ROS, Reactive oxygen species; SA, Salicylic acid; SOD, Superoxide dismutase, SUMO, Small ubiquitin-like modifier proteases.
References
Abbas, M., Imran, F., Khan, R. I., Zafar-ul-Hye, M., Rafique, T., Khan, M. J., et al. (2020). Gibberellic acid induced changes on growth, yield, superoxide dismutase, catalase and peroxidase in fruits of bitter gourd (Momordica charantia L.). Horticulturae 6:72. doi: 10.3390/horticulturae6040072
Abbas, M. S. T. (2018). Genetically engineered (modified) crops (Bacillus thuringiensis crops) and the world controversy on their safety. Egypt J. Biol. Pest Control 28, 1–12. doi: 10.1186/s41938-018-0051-2
Abdallah, H. R. (2020). Effect of foliar application with urea benzyladenine and dry yeast on flowering and fruiting of Minneola tangelo trees to reduce the severity of alternate bearing phenomena. Mid-East J. Agric. Res. 9, 893–933. doi: 10.36632/mejar/2020.9.4.70
Abid, M., Ali, S., Qi, L. K., Zahoor, R., Tian, Z., Jiang, D., et al. (2018). Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 8:4615. doi: 10.1038/s41598-018-21441-7
Ahmad, I., Kamran, M., Meng, X., Ali, S., Bilegjargal, B., Cai, T., et al. (2019). Effects of plant growth regulators on seed filling, endogenous hormone contents and maize production in semiarid regions. J. Plant Growth Regul. 38, 1467–1480. doi: 10.1007/s00344-019-09949-2
Amiriam, R., Hojati, Z., and Azadi, P. (2019). Male flower induction significantly affect androgenesis in cucumber (Cucumis sativum L.). J. Hortic. Sci. Biotechnol. 95, 183–191. doi: 10.1080/14620316.2019.1655488
Amoanimaa-Dede, H., Su, C., Yeboah, A., Zhou, H., Zheng, D., and Zhu, H. (2022). Growth regulators promote soybean productivity: a review. PeerJ 10:e12556. doi: 10.7717/peerj.12556
Aremu, A. O., Bairu, M. W., DoleŽal, K., Fannie, J. F., and van Staden, J. (2012). Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 108, 1–16. doi: 10.1007/s11240-011-0007-7
Arya, H., Singh, M. B., and Bhalla, P. L. (2021). Towards developing drought-smart soybeans. Front. Plant Sci. 12:750664. doi: 10.3389/fpls.2021.750664
Baccari, S., Elloumi, O., Chaari-Rkhis, A., Fenollosa, E., Morales, M., Drira, N., et al. (2020). Linking leaf water potential, photosynthesis and chlorophyll loss with mechanisms of photo- and antioxidant protection in juvenile olive trees subjected to severe drought. Front. Plant Sci. 11:614144. doi: 10.3389/fpls.2020.614144
Bashir, S. S., Hussain, A., Hussain, S. J., Wani, O. A., Nabi, S. Z., Dar, N. A., et al. (2021). Plant drought stress tolerance: understanding its physiological, biochemical and molecular mechanism. Biotechnol. Biotechnol. Equip. 35, 1912–1925. doi: 10.1080/13102818.2021.2020161
Beck, E. H., Fettig, S., Knake, C., Hartig, K., and Bhattarai, T. (2007). Specific and unspecific responses of plants to cold and drought stress. J. Biosci. 32, 501–510. doi: 10.1007/s12038-007-0049-5
Booker, J., Chatfield, S., and Leyser, O. (2003). Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15, 495–507. doi: 10.1105/tpc.007542
Bozso, Z., and Barna, B. (2021). Diverse effects of two cytokinins, kinetin and benzyladenine, on plant development, biotic stress tolerance and gene expression. Life 11:1404. doi: 10.3390/life11121404
Cai, T., Meng, X., Liu, X., Liu, T., Wang, H., Jia, Z., et al. (2018). Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 9:1886. doi: 10.3389/fpls.2018.01886
Carrera, C. S., Solis, S. M., Ferrucci, M. S., Vega, C. C. R., Galati, B. G., Ergo, V., et al. (2021). Leaf structure and ultrastructure changes induced by heat stress and drought during seed filling in field-grown soybean and their relationship with grain yield. Crop Sci. 93:e20191388. doi: 10.1590/0001-3765202120191388
Centeno, M. L., Rodriquez, A., Albuerne, M. A., Feitol, I., and Fernandez, B. (1998). Uptake, distribution and metabolism of 6-benzyadenine and cytokinin content during callus initiation from Actinidia deliciosa tissues. J. Plant Physiol. 154, 480–486. doi: 10.1016/S0176-1617(98)80267-8
Cho, H. S., Lee, H. D., Wang, H. W., Oh, S.-W., Kim, H. J., and Chung, Y.-S. (2019). Evaluation of yield components from transgenic soybean overexpressing chromatin architecture-controlling ATPG8 and ATPG10 genes. Plant Breed Biotech. 7, 34–41. doi: 10.9787/PBB.2019.7.1.34
Damanik, R. I., Manurung, B. H., and Bayu, E. S. (2018). Effects of hypoxia condition in embryogenic callus growth of soybean cell culture. IOP Conf. Ser. Earth Environ. Sci. 122:012056. doi: 10.1088/1755-1315/122/1/012056
Desta, B., and Amare, G. (2021). Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 8:1. doi: 10.1186/s40538-020-00199-z
Dolgikh, E. A., Kusakin, P. G., Kitaeva, A. B., Tsyganova, A. V., Kirienko, A. N., Leppyaven, I. V., et al. (2020). Mutational analysis indicates that abnormalities in rhizobial infection and subsequent plant cell and bacteroid differentiation in pea (Pisum sativum) nodules coincide with abnormal cytokinin responses and localisation. Ann. Bot. 125, 905–923. doi: 10.1093/aob/mcaa022
Dong, S., Jiang, Y., Dong, Y., Wang, L., Wang, W., Ma, Z., et al. (2019). A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 26, 2006–2017. doi: 10.1016/j.sjbs.2019.08.005
Du, Y., Zhao, Q., Chen, L., Yao, X., Zhang, H., Wu, J., et al. (2020). Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. Int. J. Mol. Sci. 21:618. doi: 10.3390/ijms21020618
El–Metwally, I. M., Saudy, H. S., and Abdelhamid, M. T. (2021). Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital. J. Agrometeorol. 2, 81–90. doi: 10.36253/ijam-872
Feng, J., Shi, Y., Yang, S., and Zhao, J. (2017). “Cytokinins,” in Hormone Metabolism and Signalling in Plants, eds J. Li, C. Li, and S. M. Smith (London: Academic Press), 77–106. doi: 10.1016/B978-0-12-811562-6.00003-7
Fröschle, M., Horn, H., and Spring, O. (2017). Effects of the cytokinins 6-benzyladenine and forchlorfenuron on fruit, seed and yield parameters according to developmental stages of flowers of the biofuel plant Jatropha curcas L. (Euphorbiaceae). Plant Growth Regul. 81, 293–303. doi: 10.1007/s10725-016-0206-7
Gamas, P., Brault, M., Jardinaud, M.-F., and Frugier, F. (2017). Cytokinins in symbiotic nodulation: when, where, what for? Trends Plant Sci. 22, 792–802. doi: 10.1016/j.tplants.2017.06.012
Gao, X.-B., Guo, C., Li, F.-M., Li, M., and He, J. (2020). High soybean yield and drought adaptation being associated with canopy architecture, water uptake and root traits. Agronomy 10:608. doi: 10.3390/agronomy10040608
Ghaleh, Z., Sarmast, M. K., and Atashei, S. (2020). 6-Benzylaminopurine (6-BA) ameliorates drought stress response in tall fescue via the influencing of biochemical and strigolactone-signaling genes. Plant Physiol. Biochem. 155, 877–887. doi: 10.1016/j.plaphy.2020.08.009
Giehl, R. F. H., Gruber, B. D., and van Wiren, N. (2013). It's time to make changes: modulation of shoot system architecture by nutrient signals. J. Exp. Bot. 65, 769–778. doi: 10.1093/jxb/ert421
Gilbert, M. L., Thompson, J. E., and Dumbroff, E. B. (2011). Delayed cotyledon senescence following treatment with a cytokinin, an effect at the level of membranes. Can. J. Bot. 58, 1797–1803. doi: 10.1139/b80-208
Gu, Y., Chen, Y., Yue, X., Xiong, P., Pan, D., Song, P., et al. (2022). LF-NMR/MRI determination of different 6-benzylaminopurine concentrations and their effects on soybean moisture. Front. Plant Sci. 13:885804. doi: 10.3389/fpls.2022.885804
Guan, X., Zheng, X., Lu, Y., Du, X., Jia, R., Li, H., et al. (2021). Changes of soybean protein during tofu processing. Foods 10:1594. doi: 10.3390/foods10071594
Hanum, C., and Meiriani (2020). Characteristics of root growth and soybean yield on drought stress. IOP Conf. Ser. Earth Environ. Sci. 454:012183. doi: 10.1088/1755-1315/454/1/012183
Honório, A. B. M., De-la-Cruz-Chacón, I., Martínez-Vázquez, M., da Silva, M. R., Campos, F. G., Martin, B. C., et al. (2021). Impact of drought and flooding on alkaloid production in Annona crassiflora Mart. Horticulturae 7:414. doi: 10.3390/horticulturae7100414
Hooley, R. (1998). Plant hormone perception and action: a role for G-protein signal transduction. Philos. Trans. R. Soc. Lond. B 353, 1425–1430. doi: 10.1098/rstb.1998.0297
Hoque, A., Hassan, M., Khan, M. M. K., Khatun, R., and Baten, M. A. (2016). Effect of temperature on flower and pod abscission and yield of three soybean genotypes. J. Environ. Sci. Nat. Res. 8, 89–92. doi: 10.3329/jesnr.v8i2.26872
Hu, J., Ren, B., Dong, S., Liu, P., Zhao, B., and Zhang, D. (2020). Comparative proteomic analysis reveals that exogenous 6-benzyladenine (6-BA) improves the defense system activity of waterlogged summer maize. BMC Plant Biol. 20:44. doi: 10.1186/s12870-020-2261-5
Huang, S., Cerny, R. E., Qi, Y., Bhat, D., Aydt, C. M., Hanson, D. D., et al. (2003). Transgenic studies in the involvement of cytokinin and gibberellin male development. Plant Physiol. 131, 1270–1282. doi: 10.1104/pp.102.018598
Hurny, A., and Benková, E. (2017). Methodological advances in auxin and cytokinin biology. Methods Mol. Biol. 1569, 1–29. doi: 10.1007/978-1-4939-6831-2_1
Ibrahim, H. M., Ali, B., El-Keblawy, A., Ksiksi, T., E. I., Esawi, M. A., Josko, I., et al. (2021). Effect of source-sink ratio manipulation on growth, flowering and yield potential of soybean. Agriculture 11:926. doi: 10.3390/agriculture11100926
Iqbal, N., Hussain, S., Raza, M. A., Yang, C.-Q., Safdar, M. E., Brestic, M., et al. (2019). Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme activities under a split-root system. Front. Physiol. 10:786. doi: 10.3389/fphys.2019.00786
Isman, M. B. (2019). Challenges of pest management in the twenty first century: new tools and strategies to combat old and new foes alike. Front. Agron. 1:2. doi: 10.3389/fagro.2019.00002
Jablonska-Trypuc, A., Matejczyk, M., and Czerpak, R. (2016). N6-benzyladenine and kinetin influence antioxidantive stress parameters in human skin fibroblasts. Mol. Cell Biochem. 413, 97–107. doi: 10.1007/s11010-015-2642-5
Kai, L., Chen, X., Wengu, Y., and Taiwen, Y. (2020). Plant growth regulators increase soybean yields by delaying leaf senescence in maize (Zea mays L.)- soybean [Glycine max (L.) Merr] relay strip intercropping system. Legume Res. 43, 794–799. doi: 10.18805/LR-521
Kapoor, D., Bhardwaj, S., Landi, M., Sharma, A., Ramakrishnan, M., and Sharma, A. (2020). The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl. Sci. 10:5692: doi: 10.3390/app10165692
Kempster, R., Barat, M., Bishop, L., Rufino, M., Borras, L., and Dodd, C. I. (2020). Genotype and cytokinin effects on soybean yield and biological nitrogen fixation across soil temperatures. Ann. Appl. Biol. 178, 341–345. doi: 10.1111/aab.12652
Khaleghi, A., Naderi, R., Brunetti, C., Maserti, B. E., Selami, S. A., and Babalar, M. (2019). Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 9:19250. doi: 10.1038/s41598-019-55889-y
Kim, J., Yang, J., Yang, R., Sicher, R. C., Chang, C., and Tucker, M. L. (2016). Transcriptome analysis of soybean leaf abscission identifies transcriptional regulators of organ polarity and cell fate. Front. Plant Sci. 7:125. doi: 10.3389/fpls.2016.00125
Kim, Y.-V., Choi, D.-H., Ban, H.-Y., Seo, B. S., Kim, J., and Lee, B.-W. (2020). Temporal patterns of flowering and pod set of determinate soybean in response to high temperature. Agronomy 10:414. doi: 10.3390/agronomy10030414
Kolling, M., Kumari, P., and Burstenbinder, K. (2019). Calcium- and calmodulin- regulated microtubule-associated proteins as signal-integration hubs at the plasma membrane-cytoskeleton nexus. J. Exp. Bot. 70, 387–386. doi: 10.1093/jxb/ery397
Larrisa, P. B., Hilton, D. T. J., and de Freitas Santos Priscilla, G. (2014). Does benzyladenine application increase soybean productivity? Afr. Agric. Res. 9, 2799–2804. doi: 10.5897/AJAR2014.8960
Le Roux, M. L., Kunert, K. J., van der Vyver, C., Cullis, C. A., and Botha, A.-M. (2019). Expression of a small ubiquitin-like modifier protease increases drought tolerance in wheat (Triticum aestivum L.). Front. Plant Sci 10:266. doi: 10.3389/fpls.2019.00266
Li, J., Feng, X., and Xie, J. (2021). A simple method for the application of exogenous phytohormones to the grass leaf base protodermal zone to improve grass leaf epidermis development research. Plant Methods 17:128. doi: 10.1186/s13007-021-00828-0
Li, M., Dong, H., Hu, D., Chen, H., Qin, W., Liu, W., et al. (2020). Nutritional evaluation of whole soybean curb made from different soybean materials based on amino acid profiles. Food Qual. Saf. 4, 41–50. doi: 10.1093/fqsafe/fyaa011
Liu, J., Gunapati, S., Mihelich, N. T., Stec, A. O., Michno, J.-M., and Stupar, R. M. (2017). Genome editing in soybean with CRISPR/Cas9. Methods Mol. Biol. 1917, 217–234. doi: 10.1007/978-1-4939-8991-1_16
Liu, J., and Zhang, J. (2017). Effects of seed treatment with uniconazole powder on soybean morphological characteristics and yield under drought stress. Adv. Eng. Res. 135, 344–348. Available online at: https://www.atlantis-press.com/article/25878870.pdf
Liu, J. Y., Sheng, Z. W., Hu, Y. Q., Liu, Q., Qiang, S., Song, X. L., et al. (2021). Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgenic Res. 30, 105–119. doi: 10.1007/s11248-020-00230-x
Liu, Y., Zhang, M., Meng, Z., Wang, B., and Chen, M. (2020). Research progress on the roles of cytokinins in plant response to stress. Int. J. Mol. Sci. 21:6574. doi: 10.3390/ijms21186574
Ma, N., Ma, C., Liu, Y., Shahid, M. O., Wang, C., and Gao, J. (2018). Petal senescence: a hormone view. J. Exp. Bot. 69, 719–732. doi: 10.1093/jxb/ery009
Mangena, P. (2018). “Water stress: morphological and anatomic changes in soybean (Glycine max L.) plants,” in Plant, Abiotic Stress and Responses to Climate Change, ed V. Andjelkovic (London: Intech Open), 9–31. doi: 10.5772/intechopen.72899
Mangena, P. (2020). Role of benzyladenine seed priming on growth and physiological and biochemical response of soybean plants grown under salinity stress condition. Int. J. Agron. 2020:8847098. doi: 10.1155/2020/8847098
Mansoor, S., Wani, O. A., Lone, J. K., Manhas, S., Kour, N., Alam, P., et al. (2022). Reactive oxygen species in plants: from source to sink. Antioxidants 11:225. doi: 10.3390/antiox11020225
Mens, C., Li, D., Haaima, L. E., Gresshoff, P. M., and Ferguson, B. J. (2018). Local and systemic effect of cytokinins on soybean nodulation and regulation of their isopentenyl transferase (IPT) biosynthesis genes following Rhizobia inoculation. Front. Plant Sci. 9:1150. doi: 10.3389/fpls.2018.01150
Mishra, N., Tripathi, M. K., Tiwani, S., Tripathi, N., Sapre, S., Ahuja, A., et al. (2020). Cell suspension culture and in vitro screening for drought tolerance in soybean using poly-ethylene glycol. Plants 10:517. doi: 10.3390/plants10030517
Müller, M., Schneider, J. R., Klein, V. A., da Silva, E., da Silva Júnior, J. P., Souza, A. M., et al. (2021). Soybean root growth in response to chemical, physical, and biological soil variations. Front. Plant Sci. 12:602569. doi: 10.3389/fpls.2021.602569
Nagel, l., Brewster, R., Riedell, W.E., and Reese, R.N. (2001). Cytokinin regulation of flower and pod set in soybeans (Glycine max (L.) Merr.). Ann. Bot. 88, 27–31. Available oline at: http://www.jstor.org/stable/42771016
Nair, K., Al-Thani, R., Al-Thani, F., Ahmed, T., and Jaoua, S. (2018). Diversity of Bacillus thuringiensis strains from Qatar as shown by crystal morphology, δ-endotoxins and Cry gene content. Front. Microbiol 9:708. doi: 10.3389/fmicb.2018.00708
Naito, K., Tsuji, H., and Hatakeyama, I. (2006). Effect of benzyladenine on DNA, RNA, protein and chlorophyll contents in intact bean leaves: differential responses to benzyladenine according to leaf age. Physiol Plant 43, 367–371. doi: 10.1111/j.1399-3054.1978.tb01596.x
Nguyen, H. N., Lai, N., Kisiala, A. B., and Neil Emery, R. J. (2021). Isopentenyl transferases as master regulators of crop performance: their function, manipulation and genetic potential for stress adaptation and yield improvement. Plant Biotechnol. J. 19, 1279–1313 doi: 10.1111/pbi.13603
Panda, B. B., Sekhar, S., Dash, S. K., Behera, L., and Shaw, B. P. (2018). Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice. BMC Plant Biol. 18, 89. doi: 10.1186/s12870-018-1279-4
Pandey, S., and Assman, S. M. (2004). The Arabidopsis putative G protein coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16, 1616–1632. doi: 10.1105/tpc.020321
Parmar, N., Singh, K. H., Sharma, D., Singh, L., Kumar, P., Nanjundani, J., et al. (2017). Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3 Biotech 7, 1–35. doi: 10.1007/s13205-017-0870-y
Patharkar, O. R., and Walker, J. C. (2018). Advances in abscission signaling. J. Exp. Bot. 69, 733–740. doi: 10.1093/jxb/erx256
Paul, S., Wildhagen, H., Janz, D., and Polle, A. (2017). Drought effects on the tissue- and cell- specific cytokinin activity in poplar. AoB Plants 10:plx067. doi: 10.1093/aobpla/plx067
Phat, P., Rehman, S., and Ho-jong, J. (2015). Optimisation of soybean (Glycine max L.) regeneration for Korean cultivars. Pak. J. Bot. 47, 2379–2385. Available online at: https://www.pakbs.org/pjbot/abstracts/47(6)/42.html
Prerostova, S., Dobrev, P. I., Gaudinova, A., Knirsch, V., Körber, N., Pieruschka, R., et al. (2018). Cytokinins: their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 9:655. doi: 10.3389/fpls.2018.00655
Qin, Y., Park, S.-Y., Oh, S.-W., Lim, M.-H., Shin, K.-S., Cho, H.-S., et al. (2017). Nutritional compositional analysis for beta-carotene-enhanced transgenic soybeans (Glycine max L.). Appl. Biol. Chem. 60, 299–309. doi: 10.1007/s13765-017-0282-z
Raza, G., Singh, M., and Bhalla, P. L. (2017). In vitro plant regeneration from commercial cultivars of soybean. BioMed Res. Int. 2017:7379693. doi: 10.1155/2017/7379693
Saleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. doi: 10.3390/plants10020259
Sarker, U., and Oba, S. (2018). Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 8:16496. doi: 10.1038/s41598-018-34944-0
Seesangboon, A., Pokawattana, T., Eungwanichayapant, P. D., Tovaranonte, J., and Popluechai, S. (2018). Effects of 6-benzyladenine on Jatropha gene expression and flower development. Russ. J. Plant Physiol. 65, 345–356. doi: 10.1134/S1021443718030135
Solorzano-Cascante, P., Sanchez-Chiang, N., and Jimenez, V. M. (2018). Explant type, culture system, 6-benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front. Plant Sci. 9:1769. doi: 10.3389/fpls.2018.01769
SpectraBase (2022). Compound ID: 617hyvu7LnB. John Wiley and Sons, Inc. N6-Benzyladenine. Available online at: https://spectrabase.com/compound/617hyvu7LnB (accessed June 5, 2022).
Srilestari, R., Wijayani, A., and Supriyanta, B. (2020). In vitro addition of benzyladenine (BA) and thiamine on growth of Abaca banana shoots. Sys. Rev. Pharm. 11, 960–962. doi: 10.31838/srp.2020.6.135
Teixeira da Silva, J. A., Nezami-Alanagh, E., Barreal, M. E., Kher, M. M., Wicaksono, A., Gulyas, A., et al. (2020). Shoot tip necrosis of in vitro plant cultures: a reappraisal of possible causes and solutions. Planta 252:47. doi: 10.1007/s00425-020-03449-4
Thu, S. W., Rai, K. M., Sandhu, D., Rajangam, A., Balasubramanian, V. K., Palmer, R. G., et al. (2019). Mutation in a PHD-finger protein MS4 causes male sterility in soybean. BMC Plant Biol. 19:378. doi: 10.1186/s12870-019-1979-4
Virdi, A. S., Singh, S., and Singh, P. (2015). Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front. Plant Sci. 6:809. doi: 10.3389/fpls.2015.00809
Vosnjak, M., Sircelj, H., Hudina, M., and Usenik, V. (2021). Response of chloroplast pigments, sugars and phenolics of sweet cherry leaves to chilling. Sci. Rep. 11:7210. doi: 10.1038/s41598-021-86732-y
Wang, J., Song, L., Gong, X., Xu, J., and Li, M. (2020). Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 21:1446. doi: 10.3390/ijms21041446
Wang, X., Wu, Z., Zhou, Q., Wang, X., Song, S., and Dong, S. (2022). Physiological response of soybean plants to water deficit. Front. Plant Sci. 12:809692. doi: 10.3389/fpls.2021.809692
Wang, Y., Letham, D. S., John, P. C. L., and Zhang, R. (2016). Synthesis of a cytokinin linked by a spacer to dexamethasone and biotin conjugates to detect cytokinin-binding proteins. Molecules 21:576. doi: 10.3390/molecules21050576
Wang, Z., Li, G., Sun, H., Ma, L., Guo, Y., Zhao, Z., et al. (2018). Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 7:bio035279. doi: 10.1242/bio.035279
Woo, H. R., Masclaux-Daubresse, C., and Lim, P. O. (2018). Plant senescence: how plants know when and how to die. J. Exp. Bot. 69, 715–718. doi: 10.1093/jxb/ery011
Wu, W., Du, K., Kang, X., and Wei, X. (2021). The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 8:118. doi: 10.1038/s41438-021-00558-3
Xu, C., Li, R., Song, W., Wu, T., Sun, S., Hu, S., et al. (2020). Responses of branch number and yield component of soybean cultivars tested in different planting densities. Agriculture 11:69. doi: 10.3390/agriculture11010069
Yang, W., Cortijo, S., Korsbo, H., Roszak, P., Schiessl, K., Gurzadyan, A., et al. (2021). Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 371, 1350–1355. doi: 10.1126/science.abe2305
Yao, Y., You, Q., Duan, G., Ren, J., Chu, S., Zhao, J., et al. (2020). Quantitative trait loci analysis of seed oil content and composition of wild and cultivated soybean. BMC Plant Biol. 20, 1–13. doi: 10.1186/s12870-019-2199-7
Ye, C., Wu, S., Kong, F., Zhou, C., Yang, Q., Sun, Y., et al. (2006). Identification and characterization of an isopentenyl transferase (IPT) gene in soybean (Glycine max L.). Plant Sci. 170, 542–550. doi: 10.1016/j.plantsci.2005.10.008
Yoshihira, A. T., and Shiraiwa, T. (2016). Branch development responses to planting density and yield stability in soybean cultivars. Plant Prod. Sci. 19, 331–339. doi: 10.1080/1343943X.2016.1157443
You, J., Zhang, Y., Liu, A., Li, D., Wang, X., Dossa, R., et al. (2019). Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 19:267. doi: 10.1186/s12870-019-1880-1
Yuan, H., Zhao, L., Guo, W., Yu, Y., Tao, L., Zhang, L., et al. (2019). Exogenous application of phytohormones promotes growth and regulates expression of weed-formation-related genes in Populus simonii x P. nigra. Int. J. Mol. Sci. 20:792. doi: 10.3390/ijms20030792
Zhang, H., Sun, X., and Dai, M. (2022). Improving crop drought resistance with plant growth regulators and rhizobacteria: mechanisms, applications, and perspectives. Plant Commun. 3:100228. doi: 10.1016/j.xplc.2021.100228
Zhang, J., Vrieling, K., Klinklamer, P. G. L., and Bezemer, M. T. (2021). Exogenous application of plant defense hormones alters the effects of live soils on plant performance. Basic Appl. Ecol. 56, 144–155. doi: 10.1016/j.baae.2021.07.011
Zhang, X., Lei, L., Lai, J., Zhao, H., and Song, W. (2018). Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 18, 1–16. doi: 10.1186/s12870-018-1281-x
Keywords: 6-benzyl adenine, drought stress, foliar application, reactive oxygen species, leaf senescence, soybean
Citation: Mangena P (2022) Evolving role of synthetic cytokinin 6-benzyl adenine for drought stress tolerance in soybean (Glycine max L. Merr.). Front. Sustain. Food Syst. 6:992581. doi: 10.3389/fsufs.2022.992581
Received: 12 July 2022; Accepted: 07 September 2022;
Published: 27 September 2022.
Edited by:
Praveen Guleria, DAV University, IndiaReviewed by:
Saroj Kumar Sah, Brookhaven National Laboratory (DOE), United StatesGayacharan, National Bureau of Plant Genetic Resources (ICAR), India
Copyright © 2022 Mangena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phetole Mangena, UGhldG9sZS5NYW5nZW5hJiN4MDAwNDA7dWwuYWMuemE=; bWFuZ2VuYS5waGV0b2xlJiN4MDAwNDA7Z21haWwuY29t
 Phetole Mangena
Phetole Mangena