
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 15 August 2022
Sec. Crop Biology and Sustainability
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.988319
This article is part of the Research Topic Biotechnology for Agricultural Sustainability View all 7 articles
Drought is one of the major abiotic stress factors limiting soybean growth and yield, and it frequently occur globally. Therefore, exploring resistant varieties from soybean germplasm is important under climate change. To screen drought resistant spring soybean varieties at seedling stage, pot experiment was used to detect the Survival percentage after drought stress of 60 soybean varieties at seedling stage, twice drought rehydration treatments on seedlings, to evaluate the drought tolerance of spring soybean. The results showed that at the seedling stage, seven varieties were considered drought tolerant, 17 varieties were considered drought sensitive, and 36 varieties were considered to be moderately drought tolerant. Based on this experiment, number 44 (heinong37), 48 (heinong44), 49 (heinong45), 52 (heinong48) is considered the best drought resistant, and number 3 (dongnong48), 4 (dongnong52), 27 (suinong25), 60 (heinong65) is the most sensitive. These varieties provide a reference for further study on drought tolerance and stress resistance gene screening of soybean at the molecular level. The selected soybean varieties can be planted in areas with suitable climates and frequent drought to meet the local soybean demand. In other regions, although cannot be directly grown, they can still be used as parents of selected varieties or as materials for gene screening and extraction, to assist crop breeding at the molecular level in response to increasingly severe drought stress problems under the current climate trends.
Soybean originated in China and has been cultivated for more than 4,000 years. After years of domestication and cultivation, now soybeans are important oil and cash crops. Providing rich protein and fat for people's daily diet (Muhammad et al., 2009; Chinakwe et al., 2019), as well as having an irreplaceable role in animal husbandry and industry (Tyczewska et al., 2016). However, the current soybean production is still unable to meet people's needs, especially during the growth and development of soybeans are extremely vulnerable to various abiotic stresses, resulting in a significant decline in yield (Feng et al., 2020).
Drought is the most common abiotic stress that affects plant growth globally (Ahanger et al., 2021), and as climate change becomes more and more complex, the problem of reduced food production caused by drought is becoming increasingly serious, posing a serious challenge to world food security and food sustainability. Moreover, the effects of drought on plants vary due to the uncertainty of the occurrence time (Shen et al., 2022). Therefore, previous studies have been conducted on the various stages of plant growth subjected to drought stress (Lotfi et al., 2019; Teixido and Valladares, 2019). The purpose is to analyze the impact mechanism of drought and explore ways to cope with it.
For soybeans, adequate moisture is necessary, and drought stress at all periods of soybean growth and development can affect growth or lead to yield reduction. The developmental status of soybean in the seedling stage determines the quality of individual and population development (Hua et al., 2018). In production, only by mastering the laws of water metabolism during the soybean seedling stage can we effectively avoid drought damage and lay a solid foundation for high-yielding cultivation (Liu et al., 2017). Soybean yield formation is closely related to moisture, previous studies have explored the relationship between water content and yield (Grassini et al., 2015; Prince et al., 2016; Anda et al., 2020). Therefore, drought resistance is an important indicator in the selection and planting of soybean varieties.
Breeders have bred a large number of varieties to cope with the crises that may be encountered during plant growth. In breeding efforts, germplasm resources with excellent traits such as high yield (Fukushima et al., 2011), resistance to specific diseases or insects (Mir et al., 2022), and tolerance to abiotic adversity are crucial (Vahdati et al., 2009). Studying the excellent traits possessed by these varieties, analyzing their physiological and molecular mechanisms, mining the key genes, and breeding or transforming them so that the excellent traits are stably inherited to future generations are often the keys to breakthroughs in breeding efforts (Timerbaev et al., 2019; Mou and Zhao, 2022).
Northeast China is a major soybean producing region, but frequent and severe drought in spring has a seriously impact on soybean growth and development, so it is especially important to identify and screen spring soybeans for drought tolerance at the seedling stage. In this study, we compared the drought tolerance of 60 soybean varieties through twice drought rehydration treatments at the seedling stage and observed plant survival to screen stable drought-tolerant and drought-sensitive materials, select a number of excellent soybean varieties for the soybean production area in northeastern China, and tap new germplasm resources for drought-tolerant soybean breeding so as to breed new varieties to cope with the increasingly complex future of climate change.
A total of 60 materials were collected from Soybean Research Institute of Northeast Agricultural University, Heilongjiang Bayi Agricultural Reclamation University, Heilongjiang Academy of Agricultural Reclamation Sciences, Suihua Branch of Heilongjiang Academy of Agricultural Sciences, Jiamusi Branch of Heilongjiang Academy of Agricultural Sciences, and Soybean Research Institute of Heilongjiang Academy of Agricultural Sciences (Table 1).
In the experiment, screen drought resistance at seedling stage by twice consecutive drought rehydration methods. Sow full seeds to plastic pots after disinfected (upper mouth diameter 10 cm, bottom diameter 8 cm, high 8 cm). The total amount of soil is 1 kg, sowing three holes at an equal distance in each pot, sowing two seeds in each hole, ensure three seedlings of similar growth per pot. Repeat three groups, five pots in each group. Set up the control group with the same number of pots as the drought treatment. As shown in Figure 1, treatment group irrigate normally before the first drought stress treatment, and pay attention to rain protection during the whole experiment. The water supply was stopped when the seedlings grew to the first triple compound leaf, until soil moisture content decreases to 15–20% of field capacity, then water supply was restored, so that the soil moisture reached (85 ± 5)% of the field capacity. The number of survival seedlings was investigated 72 h after rehydration, and consider all leaves to remain green as surviving seedlings. In the second drought stress-rehydration treatment, the water supply was stopped after the first statistics of seedling survival rate, same as the first time. Drought persisted until soil moisture content decreased to 15–20% of field capacity. Then rehydration, the soil moisture reached (85 ± 5)% of the field capacity. The number of survival seedlings was investigated after 72 h. The control group maintained (85 ± 5)% of field capacity during the whole experiment. The number of surviving seedlings in the drought treatment group compared to the number in the control group, which is the survival rate. The mean of the three replicates was calculated as the final survival rate of the drought treatment. Real-time monitoring of soil moisture by Soil moisture meter ECH2O-TE/EC-TM (EM-50, Decagon, Washington DC, USA). Ensures the accuracy of drought or rehydration treatment.

Figure 1. Blue lines indicate adequate water supply, red lines indicate drought treatment, and dashed lines indicate the time of measurement to calculate seedling survival.
At the seedling stage, the survival percentage after the first drought stress, the survival percentage after the second drought stress, the average survival rate after the first drought stress, the number of fixed seedlings before the first drought treatment, the average number of survival after the first rehydration and the average number of survival after the second rehydration. The calculation formula is as follows:
In the formula:
DS: Average survival rate of drought stress, %
DS1: Survival percentage after first drought stress, %
DS2: Survival percentage after second drought stress, %
XTT: Number of surviving seedlings in the control group
XDS1: Survival numbers after first drought stress
XDS2: Survival numbers after second drought stress
All data were processed with Microsoft Office Excel 2021, statistical analysis of the data was performed using IBM SPSS software (version 23.0: IBM Corporation, Armonk, NY, USA).
It can be seen from Table 2 that the variation of seedling survival number is larger after the first drought stress and the second drought stress, and the second drought stress is greater than the first drought stress, indicating that drought stress has a greater impact on seedling survival, that is, the seedling survival number has a large range of changes after the first drought stress and the second drought stress. The average survival number of seedlings after the first drought stress was significantly higher than that under the second drought stress, in which some varieties survived completely after the first drought stress, and no varieties survived completely after the second drought stress. However, some varieties died after the second drought stress, and no varieties died after the first drought stress.
As can be seen from Table 3 and Figure 2, the seedling survival rate of each variety is different after the first drought stress, and most of the materials can resume growth after rehydration, the seedling survival rate is 87.22%. The results showed that there was a great difference in seedling survival rate among varieties after the first drought stress. The survival rate of 19 materials, such as Heinong44, Suinong4 and Hefeng42, reached 100% after the first drought stress, and the survival rate of all tested materials reached more than 60% after the first drought stress.
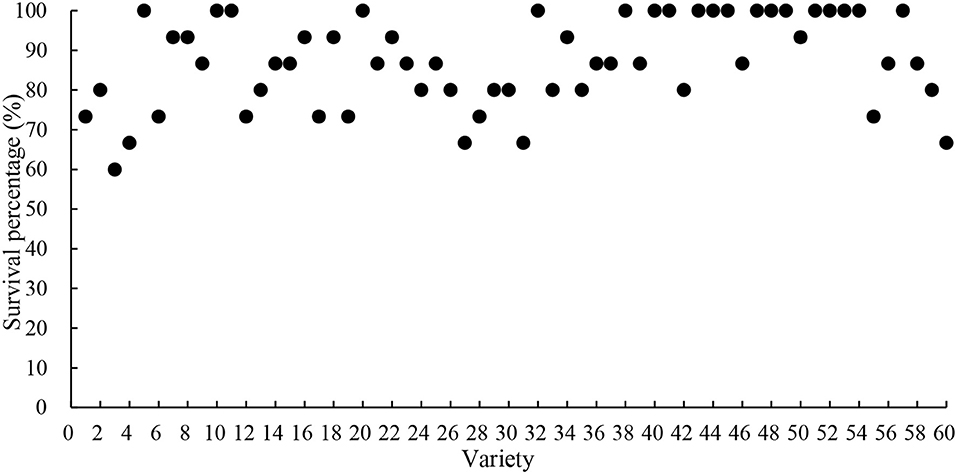
Figure 2. Survival percentage after first drought stress (the horizontal coordinate serial number indicates the corresponding varieties. The specific correspondence is shown in Table 1).
It can be seen from Table 3 and Figure 3 that after two consecutive drought stresses, the seedling survival rate of each variety decreased significantly, and the decreasing range of different varieties was different, which reflected that the drought resistance of each variety was different. The results showed that the drought tolerance of each variety after the second drought stress was more different than that of the first drought stress. After the second drought stress, the average survival rate of seedlings decreased from 87.22% after the first drought stress to 21.44%, of which 14 varieties had a survival rate of 0, indicating that most of the tested materials were not tolerant to continuous repeated drought stress. There were only five varieties with survival rate of more than 60% (including 60%), which were Suinong30, Heinong44, Heinong37, Heinong45, and Heinong48, respectively, indicating that these five varieties showed good tolerance to continuous and repeated drought stress.
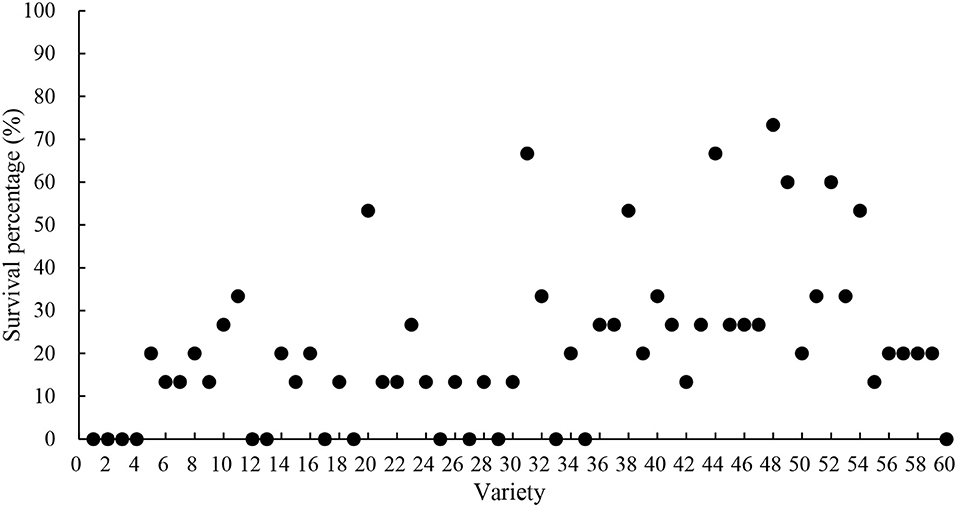
Figure 3. Survival percentage after second drought stress (the horizontal coordinate serial number indicates the corresponding varieties The specific correspondence is shown in Table 1).
Figure 4 show that the average survival rate of the seedlings after two consecutive drought stresses, reflecting the ability of seedlings to survive after two consecutive drought stresses. The higher the average survival rate of the tested seedlings under drought stress, the stronger their survival ability under drought stress, that is, the stronger their drought tolerance. After two consecutive drought stresses, the average survival rate of Heinong44 under drought stress was 86.67%, and there were only four varieties with an average survival rate of more than 80% (including 80%) under drought stress. They were Heinong37, Heinong44, Heinong45, and Heinong48. In addition, they were the varieties with a survival rate of more than 60% under the second drought stress, indicating that these four varieties had good survival ability under continuous and repeated drought stress.

Figure 4. Average survival rate of drought stress (the horizontal coordinate serial number indicates the corresponding varieties. The specific correspondence is shown in Table 1).
To group the studied soybean varieties according to their dissimilarity; Based on the survival rate under drought treatments, all the soybean varieties were clustered into four groups, all drought tolerant varieties were grouped in cluster IV and sensitive varieties in cluster I. Cluster II and cluster III are considered moderately tolerant genotypes (Figure 5). Comparison in terms of survival rate, the order of drought tolerance from strong to weak is cluster IV, cluster II, cluster III, and cluster I. There were seven varieties were classified in Cluster IV, accounting for 11.67% of the total materials tested, they were Heinong37, Heinong44, Heinong45, Heinong48, Heinong57, Suinong4, and Hefeng42. In the cluster I, there were 17 varieties were considered drought sensitive, accounting for 28.33% of the total materials tested.
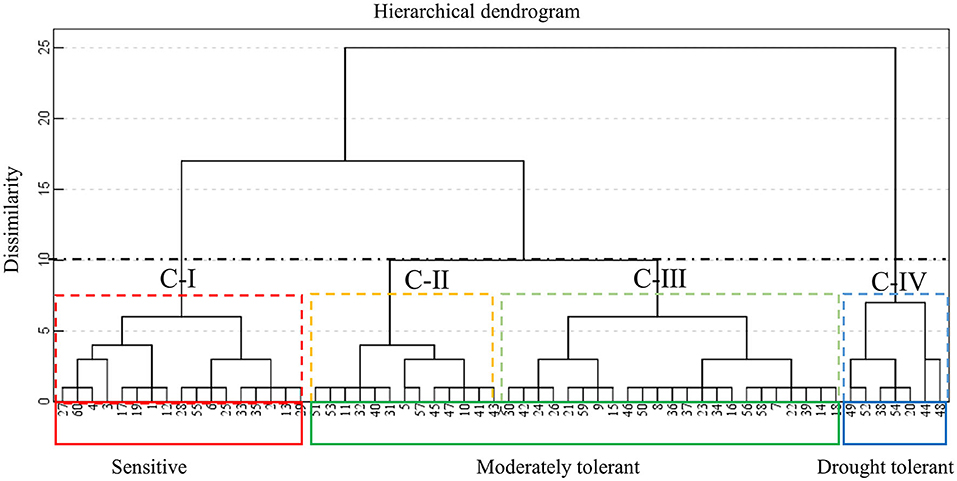
Figure 5. Hierarchical cluster analysis of 60 soybean varieties based on euclidean distance of the average survival rate under twice drought rehydration treatments. Abbreviations of varieties are found in Table 1. Cluster IV (blue) (drought tolerant), Cluster I (red) (drought sensitive), Cluster II (yellow), and Cluster III (green) (moderately tolerant).
Table 4 showed that the correlation coefficients between the average survival rate of drought stress and the survival rate of the first drought stress and the second drought stress were 0.875 and 0.943, respectively, which reached a very significant level. The correlation coefficient between the survival rate of the second drought stress and the survival rate of the first drought stress is higher than that of the first drought stress.
There have been many studies focusing on the physiological responses of plants under drought stress, including the production of reactive oxygen species (Zhanassova et al., 2021), changes in enzyme activity (Moloi et al., 2016), accumulation of osmoregulatory substances (Lotfi et al., 2010; Lu et al., 2019). changes in pigment content (Saha et al., 2020) and related molecular processes (Wang et al., 2022), etc. Resistant and sensitive varieties may differ in several of these indexes, however, the ultimate difference is either survival or death at a certain critical moisture or a certain critical stress time. Therefore, although several different physiological indicators have been reported previously to evaluate the tolerance of germplasm resources in an integrated manner (Belay et al., 2021; Xie et al., 2021), this experiment did not measure any physiological parameter but used twice drought rehydration treatments to observe plant survival. The varieties with strong drought resistance have higher survival rate under drought stress, and vice versa (Denton et al., 2018). The drought resistance of varieties was evaluated by the percentage of surviving seedlings. This method is simple, economical, and reliable. Ability to screen a large amount of plant material in a short period of time. Researchers proposed to identify the drought tolerance of plants at seedling stage by repeated drought stress, and many scholars used this method to identify the drought tolerance of sorghum and cotton, respectively (Nour et al., 1978; Longenberger et al., 2006).
In this study, all leaves remained green after drought stress and were considered as surviving seedlings. The leaf is an important organ for photosynthesis in plants (Khan et al., 2022). As long as the leaf remains green, it means that the assimilation process is still going on, i.e., the plant is still alive. And under drought stress, leaves lose water and wilt, chlorophyll content decreases and a series of other physiological changes (Song et al., 2021), which eventually lead to the loss of green and death of the leaves and hinder the normal physiological activities.
The results of seedling experiment showed that the survival rate of tested materials after the first drought stress was significantly higher than that after the second drought stress, which may be that the time of the first drought stress was short. The degree of drought stress is not enough to reach the wilting coefficient of most of the tested materials, but it also causes varying degrees of damage to the plants, resulting in yellow leaves and death. After the second drought stress, only a few of the tested materials showed relatively high survival rate, because the degree of drought stress for a long time reached the wilting coefficient of most of the tested materials, resulting in their withering. Rehydration can not alleviate the damage caused by drought (Martinez-Fernandez et al., 2015). In addition, there is a possible reason that soybean plants had only one compound leaf at the time of the first drought stress, while after experiencing drought and rehydration, the plants had grown new leaves at the time of the second drought treatment, and the number of leaves may imply a different transpiration effect, thus leading to an apparent difference in plant survival after the first and second drought stress (Gu et al., 2019). Our results indicate that the ability of plants to tolerate the second drought stress seems to be more important, while the damage of the second drought stress to plants is significantly higher than that of the first. However, in many previous studies, recurrent drought stress led to stress memory, causing relevant physiological changes or gene expression (Li et al., 2019; Kim et al., 2020), which eventually improved tolerance to subsequent stress. The key to the difference may be the rehydration issue, this experiment was rehydrated and treated for 3 days, which is similar to the first drought time. This may lead to changes in gene expression levels caused by stress memory, resulting in heavier damage to the second drought stress. Of course, this needs further exploration.
According to the screening and identification of drought tolerance of spring soybean at seedling stage, each variety was evaluated comprehensively by calculating the survival rate of seedlings under repeated drought stress. According to cluster analysis, 60 varieties were divided into four groups, all drought tolerant varieties were grouped in cluster IV, Including Heinong37, Heinong44, Heinong45, Heinong48, Heinong57, Suinong4, and Hefeng42. A total of 17 sensitive varieties were grouped into Group I. accounting for 28.33% of the total materials tested. The remaining 36 varieties were considered to be moderately drought tolerant, accounting for 60% of the total materials tested.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
XW wrote the draft of the manuscript. XW and XL contributed to data curation, analysis, and visualization. SD contributed to manuscript revision. All authors approved the submitted version.
This work was supported by the National Key R&D Program of China (No. 2018YFD1000903). This work was also by Natural Science Foundation of Heilongjiang Province of China (No. LH2021C023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahanger, M. A., Siddique, K. H. M., and Ahmad, P. (2021). Understanding drought tolerance in plants. Physiol. Plantarum 172, 286–288. doi: 10.1111/ppl.13442
Anda, A., Soos, G., Menyhart, L., Kucserka, T., and Simon, B. (2020). Yield features of two soybean varieties under different water supplies and field conditions. Field Crops Res. 245:107673. doi: 10.1016/j.fcr.2019.107673
Belay, G. A., Zhang, Z., and Xu, P. (2021). Physio-morphological and biochemical trait-based evaluation of Ethiopian and Chinese wheat germplasm for drought tolerance at the seedling stage. Sustainability 13:4605. doi: 10.3390/su13094605
Chinakwe, E. C., Ibekwe, V. I., Nwogwugwu, U. N., Ofoegbu, J., Mike-Anosike, E., Nwachukwu, I. N., et al. (2019). Evaluation of plant growth promoting potentials exhibited by rhizobacteria associated with beans plant. Malays. J. Sustainable Agric. 3, 20–22 doi: 10.26480/mjsa.01.2019.20.22
Denton, E. M., Smith, B. S., Hamerlynck, E. P., and Sheley, R. L. (2018). Seedling defoliation and drought stress: variation in intensity and frequency affect performance and survival. Rangeland Ecol. Manage. 71, 25–34. doi: 10.1016/j.rama.2017.06.014
Feng, Z., Ding, C. Q., Li, W. H., Wang, D. C., and Cui, D. (2020). Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 310:125914. doi: 10.1016/j.foodchem.2019.125914
Fukushima, A., Shiratsuchi, H., Yamaguchi, H., and Fukuda, A. (2011). Varietal differences in morphological traits, dry matter production and yield of high-yielding rice in the Tohoku region of Japan. Plant Prod. Sci. 14, 47–55. doi: 10.1626/pps.14.47
Grassini, P., Torrion, J. A., Yang, H. S., Rees, J., Andersen, D., Cassman, K. G., et al. (2015). Soybean yield gaps and water productivity in the western US Corn Belt. Field Crops Res. 179, 150–163. doi: 10.1016/j.fcr.2015.04.015
Gu, D. X., He, W., Huang, K. C., Otieno, D., Zhou, C. M., He, C. X., et al. (2019). Transpiration of moso bamboo in southern China is influenced by ramet age, phenology, and drought. For. Ecol. Manage. 450:117526. doi: 10.1016/j.foreco.2019.117526
Hua, L., Challa, G. S., Subramanian, S., Gu, X. Y., and Li, W. L. (2018). Genome-wide identification of drought response genes in soybean seedlings and development of biomarkers for early diagnoses. Plant Mol. Biol. Rep. 36, 350–362. doi: 10.1007/s11105-018-1085-z
Khan, A., Yan, L., Wang, W., Xu, K., Zou, G. W., Liu, X. D., et al. (2022). Leaf traits and leaf nitrogen shift photosynthesis adaptive strategies among functional groups and diverse biomes. Ecol. Indic. 141:109098. doi: 10.1016/j.ecolind.2022.109098
Kim, Y. K., Chae, S., Oh, N. I., Nguyen, N. H., and Cheong, J. J. (2020). Recurrent drought conditions enhance the induction of drought stress memory genes in Glycine max L. Front. Genet. 11:576086. doi: 10.3389/fgene.2020.576086
Li, P., Yang, H., Wang, L., Liu, H. J., Huo, H. Q., Zhang, C. J., et al. (2019). Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front. Genet. 10:55. doi: 10.3389/fgene.2019.00055
Liu, Z. X., Li, H. H., Fan, X. H., Huang, W., Yang, J. Y., Wen, Z. X., et al. (2017). Phenotypic characterization and genetic dissection of nine agronomic traits in Tokachi nagaha and its derived cultivars in soybean (Glycine max (L.) Merr.). Plant Science 256, 72–86. doi: 10.1016/j.plantsci.2016.11.009
Longenberger, P. S., Smith, C. W., Thaxton, P. S., and McMichael, B. L. (2006). Development of a screening method for drought tolerance in cotton seedlings. Crop Sci. 46, 2104–2110. doi: 10.2135/cropsci2006.01.0026
Lotfi, N., Soleimani, A., Vahdati, K., and Cakmakci, R. (2019). Comprehensive biochemical insights into the seed germination of walnut under drought stress. Sci. Hortic. 250, 329–343. doi: 10.1016/j.scienta.2019.02.060
Lotfi, N., Vahdati, K., Amiri, R., and Kholdebarin, B. (2010). Drought-induced accumulation of sugars and proline in radicle and plumule of tolerant walnut varieties during germination phase. Acta Hortic. 861, 289–296. doi: 10.17660/ActaHortic.2010.861.39
Lu, X. P., Gao, H. J., Zhang, L., Wang, Y. P., Shao, K. Z., Zhao, Q., et al. (2019). Dynamic responses of haloxylon ammodendron to various degrees of simulated drought stress. Plant Physiol. Biochem. 139, 121–131. doi: 10.1016/j.plaphy.2019.03.019
Martinez-Fernandez, J., Gonzalez-Zamora, A., Sanchez, N., and Gumuzzio, A. (2015). A soil water based index as a suitable agricultural drought indicator. J. Hydrol. 522, 265–273. doi: 10.1016/j.jhydrol.2014.12.051
Mir, R. R., Rustgi, S., Zhang, Y. M., and Xu, C. W. (2022). Multi-faceted approaches for breeding nutrient-dense, disease-resistant, and climate-resilient crop varieties for food and nutritional security. Heredity 128, 387–390. doi: 10.1038/s41437-022-00542-0
Moloi, M. J., Mwenye, O. J., and van der Merwe, R. (2016). Differential involvement of ascorbate and guaiacol peroxidases in soybean drought resistance. S. Afr. J. Sci. 112, 100–103. doi: 10.17159/sajs.2016/20160028
Mou, Z. M., and Zhao, D. K. (2022). Gene rational design: the dawn of crop breeding. Trends Plant Sci. 27, 633–636. doi: 10.1016/j.tplants.2022.03.007
Muhammad, A., Khalil, S. K., Marwat, K. B., Khan, A. Z., Khalil, I. H., and Amanullah Arifullah, S. (2009). Nutritional quality and prduction of soybean land races and improved varieties as affected by planting dates. Pak. J. Bot. 41, 683–689.
Nour, A. E. M., Weibel, D., and Todd, G. (1978). Effect of repeated drought periods on the survival of sorghum seedlings. Agron. J. 70, 509–510. doi: 10.2134/agronj1978.00021962007000030037x
Prince, S. J., Murphy, M., Mutava, R. N., Zhang, Z. Z., Nguyen, N., Kim, Y. H., et al. (2016). Evaluation of high yielding soybean germplasm under water limitation. J. Integr. Plant Biol. 58, 475–491. doi: 10.1111/jipb.12378
Saha, S., Begum, H. H., Nasrin, S., and Samad, R. (2020). Effects of drought stress on pigment and protein contents and antioxidant enzyme activities in five varieties of rice (Oryza sativa L.). Bangladesh J. Bot. 49, 997–1002. doi: 10.3329/bjb.v49i4.52516
Shen, X., Liu, Y., Liu, B., Zhang, J., Wang, L., Lu, X., et al. (2022). Effect of shrub encroachment on land surface temperature in semi-arid areas of temperate regions of the northern hemisphere. Agric. For. Meteorol. 320:108943. doi: 10.1016/j.agrformet.2022.108943
Song, X. Y., Zhou, G. S., and He, Q. J. (2021). Critical leaf water content for maize photosynthesis under drought stress and its response to rewatering. Sustainability 13:7218. doi: 10.3390/su13137218
Teixido, A. L., and Valladares, F. (2019). Heat and drought determine flower female allocation in a hermaphroditic mediterranean plant family. Plant Biol. 21, 1024–1030. doi: 10.1111/plb.13031
Timerbaev, V., Mitiouchkina, T., Pushin, A., and Dolgov, S. (2019). Production of marker-free apple plants expressing the supersweet protein gene driven by plant promoter. Front. Plant Sci. 10:388. doi: 10.3389/fpls.2019.00388
Tyczewska, A., Gracz, J., Kuczynski, J., and Twardowski, T. (2016). Deciphering the soybean molecular stress response via high-throughput approaches. Acta Biochim. Pol. 63, 631–643. doi: 10.18388/abp.2016_1340
Vahdati, K., Lotfi, N., Kholdebarin, B., Hassani, D., Amiri, R., Mozaffari, M. R., et al. (2009). Screening for drought-tolerant genotypes of persian walnuts (Juglans regia L.) during seed germination. Hortscience 44, 1815–1819. doi: 10.21273/HORTSCI.44.7.1815
Wang, X. Y., Song, S., Wang, X., Liu, J., and Dong, S. K. (2022). Transcriptomic and metabolomic analysis of seedling-stage soybean responses to PEG-simulated drought stress. Int. J. Mol. Sci. 23:6869. doi: 10.3390/ijms23126869
Xie, H. Y., Li, M. R., Chen, Y. J., Zhou, Q. P., Liu, W. H., Liang, G. L., et al. (2021). Important physiological changes due to drought stress on oat. Front. Ecol. Evol. 9:644726. doi: 10.3389/fevo.2021.644726
Keywords: spring soybean, seedling stage, drought tolerance, identification, genetic resources
Citation: Wang X, Li X and Dong S (2022) Screening and identification of drought tolerance of spring soybean at seedling stage under climate change. Front. Sustain. Food Syst. 6:988319. doi: 10.3389/fsufs.2022.988319
Received: 07 July 2022; Accepted: 29 July 2022;
Published: 15 August 2022.
Edited by:
Praveen Guleria, DAV University, IndiaReviewed by:
Syed Srfraz Shah, Forman Christian College, PakistanCopyright © 2022 Wang, Li and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shoukun Dong, ZHNrc2tkMjAyMEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.