- 1Department of Animal Science, University of Connecticut, Storrs, CT, United States
- 2Department of Nutritional Sciences, University of Connecticut, Storrs, CT, United States
Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7 are the major foodborne pathogens that have been implicated in outbreaks related to consumption of contaminated cantaloupes. Current chlorine-based decontamination strategies are not completely effective for inactivating the aforementioned pathogens on cantaloupes, especially in the presence of organic matter. This study investigated the efficacy of eugenol nanoemulsion (EGNE) wash treatments in inactivating L. monocytogenes, Salmonella spp., and E. coli O157:H7 on the surface of cantaloupes. In addition, the efficacy of EGNE in inhibiting the growth of the three pathogens on cantaloupes during refrigerated and room temperature storage of 5 days was investigated. Moreover, the effect of EGNE wash treatment on cantaloupe color was assessed using a Miniscan® XE Plus. The EGNE was prepared with either Tween 80 (TW) or a combination of Gum arabic and Lecithin (GA) as emulsifiers. The cantaloupe rind was washed with EGNE (0.3, 0.6, and 1.25%), in presence or absence of 5% organic load, for 1, 5, or 10 min at 25°C. Enumeration of surviving pathogens on cantaloupe was performed by serial dilution and plating on Oxford, XLD or SMA agar followed by incubation at 37°C for 24–48 h. EGNE-GA and EGNE-TW wash significantly reduced all three pathogens by at least 3.5 log CFU/cm2 as early as 5 min after treatment. EGNE-GA at 1.25% inactivated L. monocytogenes, E. coli O157:H7 and S. Enteritidis on cantaloupes to below the detectable limit within 5 and 10 min of treatment, respectively (~4 log CFU/cm2, P < 0.05). EGNE treatments significantly reduced the survival of L. monocytogenes, S. Enteritidis, and E. coli O157:H7 on cantaloupe by at least 6 log CFU/cm2 at day 5 of storage at 25 and 4°C (P < 0.05). Presence of organic matter did not modulate the antimicrobial efficacy of nanoemulsion treatments (P > 0.05). EGNE treatments did not affect the rind color of cantaloupes (P > 0.05). In conclusion, eugenol nanoemulsions could potentially be used as a natural sanitizer to inactivate foodborne pathogens on cantaloupes. Further investigations in an industry setting are warranted.
Introduction
Fresh produce constitutes an integral part of the American diet and the demand has surpassed domestic production for more than a decade. Improvements in infrastructure, household income, market access, and an understanding of the tremendous health benefits of produce has fueled this trend. The inflation-adjusted value of U.S fresh fruits imports increased from ~$5 billion in 2009 to $14 billion in 2019 (ERS, 2020). Currently, fresh produce is the largest portion of horticulture imports, amounting to 21% in value (ERS, 2020). Therefore, maintaining the microbiological safety of fresh produce is very important. This is especially significant since fresh produce is often consumed raw and there is no heat inactivation step as encountered with cooked food. In the US, fresh produce has been implicated in ~13% of foodborne outbreaks (Doyle and Erickson, 2008; Centers for Disease Control and Prevention, 2012; Callejón et al., 2015). Among the foodborne outbreaks in fresh produce, cantaloupe (Cucumis melo L. var. reticulatus) ranks fourth after leafy greens, sprouts, and tomatoes. The United States is one of the world's leading consumers of melon with an average cantaloupe consumption of about 6.11 pounds per capita/year in 2019 (ERS, 2020). There has been at least 34 foodborne outbreaks reported in cantaloupe in the United States between 1973 and 2011 (Danyluk et al., 2014). Proximity of the fruit to the soil, contact with irrigation water, insects, animals, or humans during harvesting and processing can result in surface contamination with pathogens (Shewfelt, 1987; Nguyen and Carlin, 1994). The major foodborne pathogens associated with cantaloupe outbreaks have been Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes (Scallan et al., 2011; Centers for Disease Control and Prevention (CDC), 2018; USDA, 2022). The deadliest outbreak in recent US history occurred in 2011 due to the consumption of cantaloupes contaminated with L. monocytogenes (Centers for Disease Control and Prevention, 2011). This outbreak affected 28 states and more than 1.5 million cantaloupes were recalled. The outbreak resulted in twenty-nine deaths and one miscarriage.
According to FDA code of federal regulation title 21, current decontamination strategies employed to reduce pathogen load on cantaloupes include chlorine-based sanitizers, quaternary ammonium compounds, and peracetic acid (Dharmarha et al., 2020). Chlorine based sanitizers are used in the range of 50–200 ppm with 1–2 min of contact time (Beuchat, 1992; FDA, 1998). However, several researchers reported that treatment with both chlorine or peracetic acid have resulted in a pathogen reduction of ~1.5 log CFU/sample (Ukuku and Sapers, 2001; Fan et al., 2009; Upadhyay et al., 2014). Moreover, presence of organic load (soil residues, farm debris, plant leaves and tissue fractions etc.) in the wash water further reduces the antimicrobial efficacy of the aforementioned compounds (Teng et al., 2018). The microscopic lenticellar netting surface of the cantaloupe facilitates the attachment and growth of bacteria thus reducing the efficiency of aqueous disinfectants (Annous et al., 2004; Wang et al., 2012). In addition, the netted surface protects the bacteria from complete exposure to sanitizers, further reducing the antimicrobial potential of these compounds. The organic matter present in the wash water reacts with free chlorine, which further reduces the antimicrobial efficacy of chlorine-based treatments (Li et al., 2019). Moreover, offensive odor, eye and respiratory irritation have been reported with the use of ammonia-based compounds (Mrvos et al., 1993; White and Martin, 2010). Furthermore, use of chlorine-based sanitizers poses significant health safety concerns in humans due to the formation of carcinogenic byproducts such as chloramines, trihalomethanes and other organochlorine compounds (Zhang and Farber, 1996; Richardson et al., 1998; Donato and Zani, 2010). Recently, quaternary ammonium compounds have been detected in breast milk, raising concerns for the safety of infants and newborns (Zheng et al., 2022). Therefore, there is a need for an alternative strategy, that is safe, effective, and easy to use, to control foodborne pathogens on cantaloupes.
Since ancient times, plant extracts have been used in many cultures as food preservatives or in traditional medicine for treating various diseases. Plant extracts contain several phytochemicals that exhibit significant anti-cancer, anti-parasitic, or antibacterial properties (Burt, 2004; Holley and Patel, 2005; Upadhyay et al., 2014; Nirmala et al., 2019; Niksic et al., 2021). Phytochemicals are secondary metabolites produced as a result of reciprocal interactions between plants and their environment and contribute to the natural defense system of plants (Jones and Dangl, 2006; Reichling, 2010). Due to the diverse mechanism of antibacterial action of phytochemicals, the probability of resistance development in bacteria is low (Apolónio et al., 2014; Borges et al., 2015). The majority of phytochemicals are poorly soluble in water, yet highly soluble in organic solvents (Vergis et al., 2015; Dhifi et al., 2020; Falleh et al., 2020). Eugenol is a Generally Recognized as Safe (GRAS) phytochemical obtained from clove (Eugenia caryophyllus) (Code of Federal Regulations 21 Part 172). Eugenol exerts significant antibacterial activity against L. monocytogenes (Upadhyay et al., 2013, 2015, 2016b), Salmonella (Devi et al., 2010; Mattson et al., 2011; Upadhyaya et al., 2013) and E. coli O157:H7 (Pei et al., 2009; Ghosh et al., 2014; Niu et al., 2019). However, eugenol, like most phytochemicals, has low solubility in water (2,460 mg/L at 25°C), which thwarts its application as a water-soluble disinfectant (Yalkowsky and Banerjee, 1992). To overcome this challenge, we prepared eugenol nanoemulsion using food grade emulsifiers and high-energy sonication method. This technique enables the dispersion of phytochemicals in the aqueous phase by forming nanoscale oil droplets in surfactants-containing aqueous phase. Small particle between 20 and 200 nm provide high thermodynamic stability against flocculation and coalescence, thus increasing the distribution of the antimicrobial agent in food matrices (Ezhilarasi et al., 2013; Yildirim et al., 2017; Chen et al., 2018).
The objective of this study was to investigate the efficacy of two types of eugenol nanoemulsions (prepared with tween-80 or a combination of gum arabic and lecithin) in inactivating L. monocytogenes, Salmonella spp., and E. coli O157:H7 on cantaloupe surface at 25°C. In addition, the efficacy of eugenol nanoemulsions in inhibiting the growth of the three pathogens on cantaloupes during refrigerated and room temperature storage of 5 days was investigated. Moreover, the effect of eugenol nanoemulsions on cantaloupe color was studied under storage conditions at refrigerated temperature.
Materials and methods
Bacterial strains, growth conditions, preparation of cantaloupe and inoculum
Five strains of L. monocytogenes (Scott A, AT19115, LM1, LM2, LM3), four strains of S. Enteritidis (SE28, SE31, SE12 and SE90) and four strains of E. coli O157:H7 (RM4688, RM4407, RM1918 and RM4406) were used in this study. AT19115 was procured from ATCC, Scott A is a human outbreak strain. LM1, LM2, LM3 are wild type strains isolated from produce. Similarly, E. coli O157:H7 strains were wild type strains isolated from spinach and lettuce. The Salmonella strain SE 90 is of human origin while SE28, 31, 12 were isolated from poultry. The organisms were streaked from their corresponding glycerol stock on Oxford, Xylose Lysine Deoxycholate (XLD), or Sorbitol MacConkey (SMA) agar plates, respectively, followed by incubation at 37°C for 48 h. Individual colonies were selected and cultured in 10 ml of tryptic soy broth (Fisher Scientific Co LLC, Hanover Park, IL) at 37°C for 24 h. For inoculum preparation, the individual overnight culture was centrifuged at 7,000 rpm for 15 min at 25°C. The bacterial pellet was washed three times and resuspended in 10 ml of sterile 1X PBS. Equal portion of the washed cultures were mixed together and diluted appropriately to yield a final inoculum concentration of 6 log CFU/ml. The average inoculum on the cantaloupe was ~5–5.5 log CFU/cm2. The concentration of the bacterial population in the initial culture was confirmed by plating on the Oxford, XLD, SMA agar followed by incubation at 37°C for 24–48 h.
Fresh cantaloupes, free of any visual blemishes, were procured from the local store in Storrs, Connecticut and were used within 12 h for experiments. Circular cantaloupe rind plugs (~2 cm diameter, ~0.5 cm thickness) were prepared using a steel corer as described previously (Sapers et al., 2001; Upadhyay et al., 2014, 2016a). Cocktail of L. monocytogenes, S. Enteritidis or E. coli O157:H7 was spot inoculated separately (200 μl volume with 20 spots of 10 μl each; ~5.5 log CFU/cm2) on cantaloupe rind plugs followed by incubation for 2 h at 25°C in the biosafety cabinet, to facilitate bacterial attachment. The uninoculated rind plugs were used as the negative control.
Preparation and characterization of eugenol nanoemulsions
Two types of nanoemulsion were prepared as described previously (Bhargava et al., 2015; Hu et al., 2021). For type 1 nanoemulsion (EGNE-GA), gum arabic and lecithin were used as emulsifiers. A 4% stock solution of gum arabic or lecithin was prepared separately in water (w/v) followed by mixing equal volumes to prepare a gum arabic-lecithin mix solution. Eugenol (Sigma Aldrich, Germany) stock prepared in ethanol was added to appropriate volume of the solution containing gum arabic and lecithin drop by drop to attain a final concentration of 1.25%. The formulation (total volume of 120 ml) was sonicated using high speed homogenizer Q 700 (QSonica L.L.C, Newtown, CT, USA) for 10 min with 10 s On and 5 s Off cycle and amplitude of 60 Watts. The final formulation of nanoemulsion had 1.25% eugenol, 0.5% of gum arabic and 0.5% lecithin and ethanol as co-surfactant (16.67%). The final formulation was appropriately diluted to prepare the various wash treatments.
For the second type of nanoemulsion, Tween 80 (Sigma Aldrich, Germany) was used as an emulsifier. For preparing a 5% eugenol nanoemulsion stock, 5 ml of eugenol and 2.5 ml of Tween 80 were mixed using a magnetic stir plate for 30 min at a constant speed of approx. 500 rpm. Deionized water (92.5 ml) was added to the mixture drop by drop and was mixed for 30 min on a magnetic stir plate with a constant speed of 500 rpm. The formulation was sonicated using high speed homogenizer Q 700 for 20 min with 5 s On and 3 s Off cycle and an amplitude of 60 Watts. The final concentration of the eugenol in the nanoemulsion was 5% (w/v). The final formulation was appropriately diluted to prepare the various wash treatments.
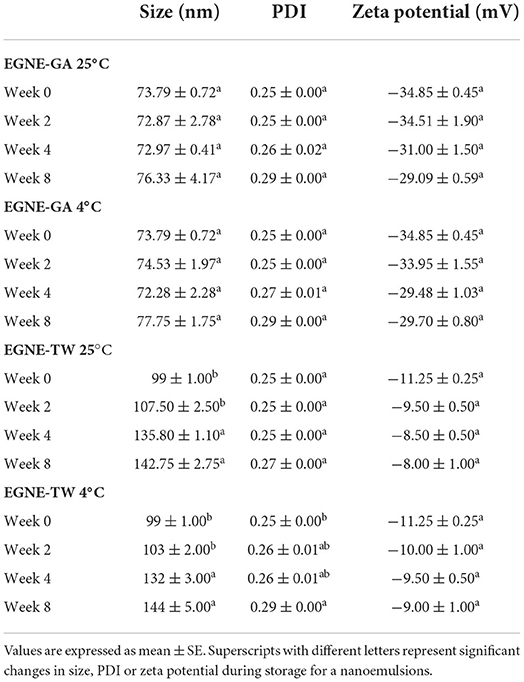
The prepared nanoemulsions were stored at 25 and 4°C for a period of 8 weeks. The stability of the nanoemulsions was measured by characterizing the size, PDI, and zeta potential on week 0, 2, 4 and 8 using Nano Zetasizer. The antibacterial efficacy was measured by estimating minimum bactericidal concentration (CLSI, 2019).
ABTS antioxidant activity assay
The antioxidant activity of eugenol in water, eugenol in ethanol and eugenol nanoemulsion was determined by 2,2′-azinobis-(3-ethylenebenzothiazoline)-6-sulfonic acid (ABTS) method as described before (Xue et al., 2019). The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS∙+) is reduced by a test antioxidant to colorless ABTS. The assay measures the relative ability of antioxidants to neutralize ABTS generated in aqueous phase and is a reflection of the encapsulation efficiency/dispersion of the oil in the system. The antioxidant activity was measured at regular intervals for a period of 8 weeks. The ABTS working solution was prepared by dissolving 68.5 mg of ABTS and 13.5 mg of 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH) into 100 mL of 10 mM pH 7.4 PBS buffer. The prepared mixture was heated at 70°C for 30 min and filtrated through 0.45 μm filter after cooling down to room temperature. The working solution was diluted with PBS until the absorbance at 734 nm achieved 0.64 ± 0.37. Then, 20 μL of test solution was added into 96 cell plates followed by the addition of 280 μL of ABTS working solution. The mixture was incubated at 37°C for 10 min and the absorbance at 734 nm was recorded using Biotek microplate reader (Synergy H1M, Thermo Scientific, Waltham, MA, USA). Final antioxidant activity was expressed as μg vitamin C equivalent antioxidant capacity per micro liter sample (Xue et al., 2021).
Transmission electron microscopy
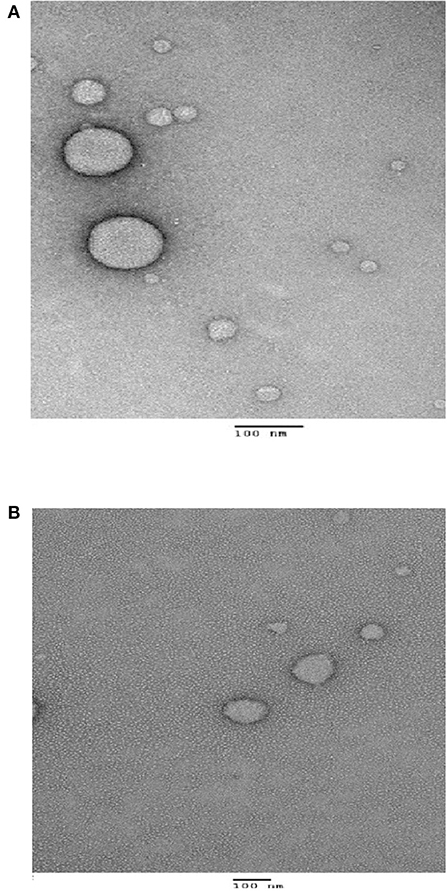
The morphology of obtained nanocomplexes was determined using transmission electron microscope (TEM) as reported before (Xue et al., 2019). Briefly, 3 μL sample was added on a copper coated 400 mesh grid and dried for 2 min, followed by staining with 0.5% uranyl acetate solution. After the sample was completely dried, the grid was loaded into the sample chamber of Tecnai T12 and the images were obtained by a CCD camera (AMT 2k XR40).
Inactivation of foodborne pathogens with eugenol nanoemulsion wash treatments on cantaloupe rind plugs in absence or presence of 5% organic load
Cantaloupe rind plugs inoculated with either L. monocytogenes, Salmonella or E. coli O157:H7 were washed with DI water (control) or DI water containing eugenol (0.3, 0.6, 1.25%), eugenol nanoemulsion type 1 (GA, 0.3, 0.6, 1.25%), eugenol nanoemulsion type 2 (TW, 0.3, 0.6, 1.25%) or chlorine (80, 200 ppm; industry control) in a Whirl-PakTM bag (Nasco, Fisher Scientific; 25 ml treatment volume). The inoculated rind plug without any treatment was used as a baseline to determine the pathogen load on the rind. The treatment was carried out at 25°C for 1, 5 or 10 min. The aforementioned treatments along with corresponding dose and time are summarized in Table 1.
The efficacy of the most effective treatments in inactivating L. monocytogenes, Salmonella spp., and E. coli O157:H7 on cantaloupe rind plugs was tested in presence of 5% organic load (Yang et al., 2012; Zhang et al., 2016). Potting soil procured from local store was used for preparing the organic load. Appropriate amount of sterile potting soil was added to Whirl-PakTM bags containing 25 ml of DI water followed by addition of respective treatments and inoculated cantaloupe rind plugs. The samples were treated for 10 min at 25°C.
Effect of eugenol nanoemulsions on the survival of foodborne pathogens on cantaloupe rind during refrigerated or room temperature storage
The inoculated cantaloupe rind plugs were washed with the most effective treatment from 2.3. The control and treated plugs were stored in a sterile container at 4 or 25°C for a period of 5 days. The survival of the pathogens was enumerated at 24 h interval by plating onto Oxford, XLD, or SMA agar plates, respectively.
Effect of eugenol nanoemulsion wash treatments on cantaloupe color
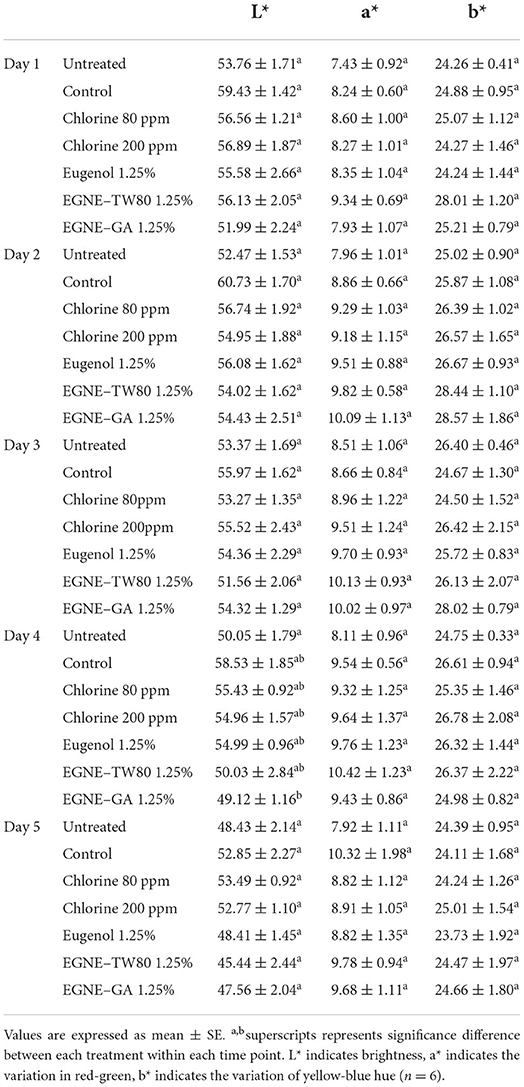
The effect of the highest dose of eugenol or nanoemulsion treatments on the color of cantaloupe was studied as described previously (Shrestha et al., 2019). A separate batch of cantaloupe rind plug samples not inoculated with pathogens were allocated for color analysis using a HunterLab MiniScan XE Plus colorimeter (HunterLab Associates, Reston, VA, USA) with illuminant A, 2.54-cm diameter aperture, and 10° standard observer. L* (lightness), a* (redness), and b* (yellowness) values for each sample were recorded every day for 5 days. The instrument was calibrated as per manufacturer's instructions using a custom white tile before the measurements. Three readings were recorded on each sample, averaged, and analyzed.
Statistical analysis
The experiment was done in a completely randomized design with duplicate samples and replicated three times. For antioxidant activity assay, the experiment had triplicates and was repeated two times. The data was analyzed in R (version 4.0.2), using ANOVA followed by Tukey HSD. P < 0.05 was considered statistically significant.
Results
Preparation and characterization of eugenol nanoemulsions
Particle size, polydispersity index, zeta potential
The average particle size, PDI and zeta potential of the eugenol nanoemulsions on the day of preparation and during storage at 25 or 4°C for a period of 8 weeks is presented in Table 2. The average particle size of EGNE-GA was ~73 nm, whereas the average particle size of EGNE-TW was ~99 nm on day 0. Storing the nanoemulsions at 25 or 4°C for 8 weeks did not change the particle size in EGNE-GA (P > 0.05). However, an increase in particle size of EGNE-TW was observed on week 4 and 8 (P < 0.05). Polydispersity index (PDI) represents the uniformity of the particle size distribution in nanoemulsions. A reduction in uniformity in the particle size increases the PDI value. The PDI of EGNE-GA and EGNE-TW was ~0.25. Storing the nanoemulsion for 8 weeks did not significantly modulate the PDI in both types of nanoemulsion (P < 0.05). Zeta potential (ZP) is a measure of surface charge of the particle in colloidal system. ZP in the range of ±30 mV indicates good stability against coalescence of the particles. EGNE-GA and EGNE-TW exhibited a zeta potential of ~ (-34) and ~ (-11) mV, respectively (Table 2).
ABTS antioxidant activity assay
The antioxidant activity of eugenol in water, eugenol in ethanol, and eugenol nanoemulsions, estimated over a storage period of 8 weeks, is presented in Figure 1. The antioxidant activity of eugenol oil was the lowest when compared with eugenol in ethanol and eugenol nanoemulsions (GA and TW) during refrigerated or room temperature storage for 8 weeks (P < 0.05). Since eugenol has a high solubility in ethanol, an increase in antioxidant activity was observed in ethanol treatment as compared with eugenol in water (P < 0.05). Both types of eugenol nanoemulsion displayed antioxidant activity either higher (P < 0.05) or comparable (P > 0.05) to eugenol in ethanol treatment, over a period of 8 weeks at 25 or 4°C, indicating uniform dispersal of the essential oil in the nanoemulsion.
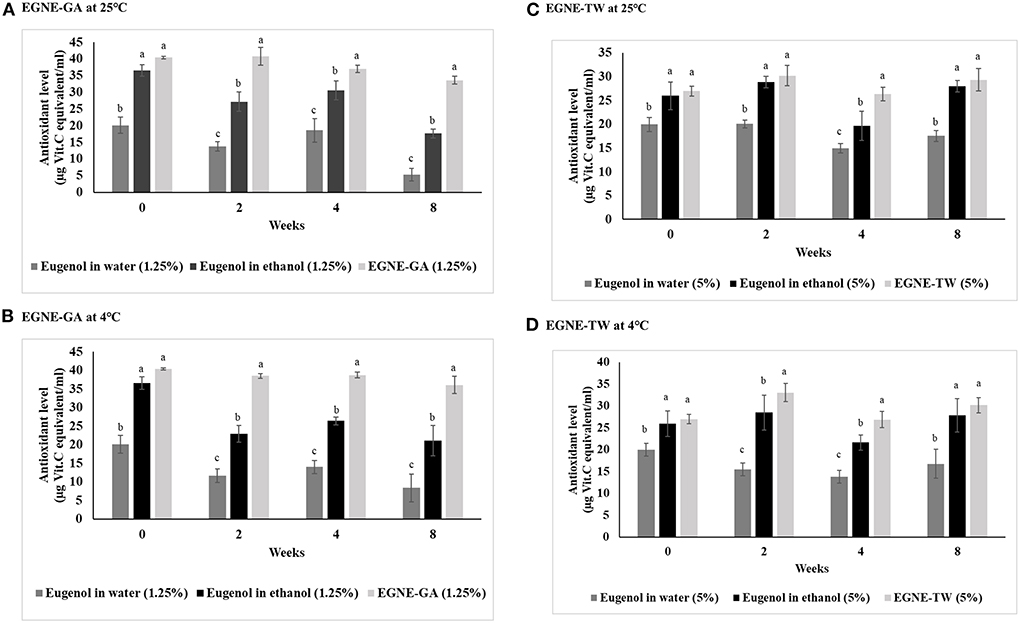
Figure 1. Antioxidant activity (ug Vit. C equivalent/ml) of eugenol in water, eugenol in ethanol, EGNE-GA, and EGNE-TW stored at 25 (A,C) or 4°C (B,D) for a period of 8 weeks. The error bar represents mean ± SE. a−csuperscripts represents significance difference between each treatment within each time point. Error bar represents SEM (n = 6).
Surface morphology of eugenol nanoemulsions by TEM
The surface morphology of EGNE-GA and EGNE-TW as observed by TEM is presented in Figure 2. The droplet of both the nanoemulsions were spherical in shape with size ranging between 70 and 100 nm. The interfacial layer of nanoemulsion droplet was composed of surfactant, and the eugenol was encapsulated in the droplet core.
Inactivation of foodborne pathogens with eugenol nanoemulsion wash treatments on cantaloupe rind plugs in absence or presence of 5% organic load
The efficacy of eugenol nanoemulsions in inactivating foodborne pathogens on cantaloupe rind plugs is shown in Table 3. The presence of inherent L. monocytogenes, S. Enteritidis and E. coli O157:H7 on the cantaloupe plug was ruled out by plating representative samples on Oxford, XLD and SMA plates, respectively (Data not shown). The average L. monocytogenes, S. Enteritidis and E. coli O157:H7 recovered from the inoculated cantaloupe plug (Baseline) was ~5.5, 5 and 5.5 log CFU/cm2, respectively (Data not shown). In case of cantaloupe plugs washed with sterile DI water (control) ~5–5.3 log CFU/cm2 of L. monocytogenes, S. Enteritidis or E. coli O157:H7 was recovered suggesting that washing with water is not effective in reducing pathogen load significantly as compared to baseline. Increase in treatment time of washing with DI water from 1 to 5 or 10 min showed no significant increase in the reduction of L. monocytogenes, S. Enteritidis or E. coli O157:H7 on the cantaloupes as compared to baseline (P > 0.05).
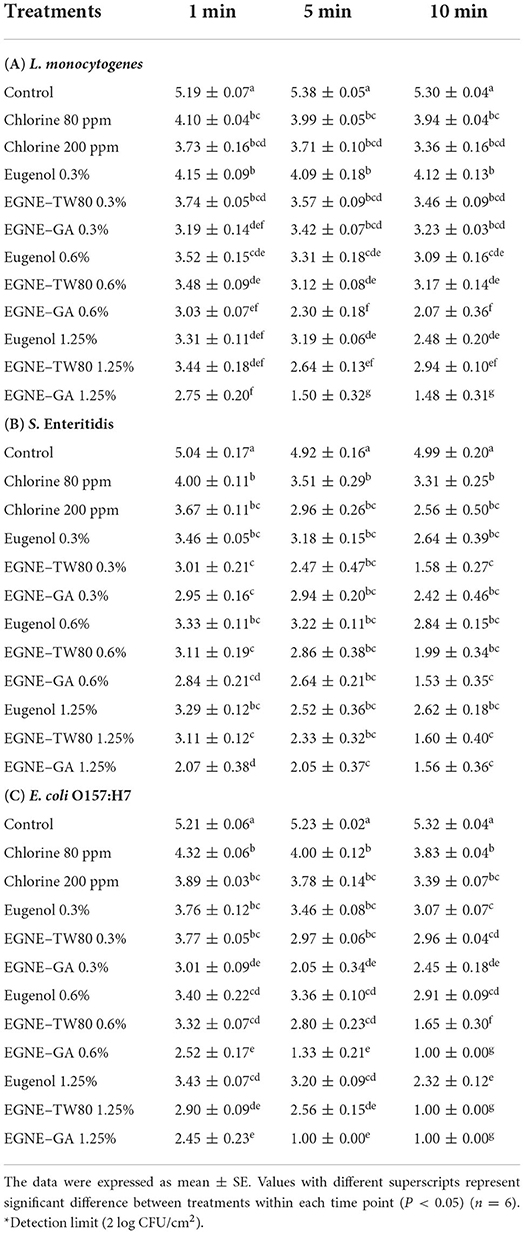
Table 3. Inactivation of L. monocytogenes, S. Enteritidis, or E. coli O157:H7 on cantaloupe rind by eugenol or eugenol nanoemulsions wash at 25°C.
Washing the cantaloupe rind with chlorine (80 and 200 ppm) for 1 min significantly reduced L. monocytogenes, S. Enteritidis and E. coli O157:H7 by ~1–1.5 log CFU/cm2 as compared with control (P < 0.05). Increasing treatment time to 5 or 10 min did not enhance antimicrobial efficacy significantly except against E. coli O157:H7 where after washing with chlorine 200 ppm for 10 min, the pathogen load reduced to ~3.3 log CFU/cm2 (P < 0.05).
The wash treatments containing eugenol oil, EGNE-TW80, and EGNE-GA significantly reduced L. monocytogenes, Salmonella spp. and E. coli O157:H7 on cantaloupe rind, by 1–2 log CFU/cm2, as early as 1 min of treatment time when compared to control (P < 0.05). A dose dependent increase in antimicrobial efficacy of eugenol oil (0.3 vs. 1.25%) was observed against L. monocytogenes at 1, 5 or 10 min washing (P < 0.05). For example, eugenol oil 0.3% treatment at 1 min, reduced L. monocytogenes by ~1 log CFU/cm2. Eugenol oil 1.25% treatment reduced L. monocytogenes by ~1.9 log CFU/cm2 after 1 min of treatment time (P < 0.05). However, this increase was not observed against S. Enteritidis or E. coli O157:H7 (except Eugenol 0.3%, 1.25% at 10 min). In case of EGNE-GA and EGNE-TW, a dose dependent increase in antimicrobial efficacy was observed at 10 min of washing between 0.3 and 1.25% treatments against L. monocytogenes and E. coli O157:H7 (P < 0.05).
Several treatments of EGNE-GA were found to be more effective than eugenol oil in inactivating L. monocytogenes, Salmonella and E. coli O157:H7 on cantaloupe rind. For example, all EGNE-GA treatments, at all timepoints, were more effective in inactivating E. coli O157:H7 than their corresponding eugenol oil treatments (P < 0.05). EGNE-GA 0.6% and 1.25% wash reduced E. coli O157:H7 on cantaloupes by more than 5 log CFU/cm2 at 10 min of treatment time (P < 0.05). Similarly, EGNE-GA 1.25% treatment was more effective than 1.25% eugenol oil treatment in inactivating S. Enteritidis at a treatment time of 1 min. By 10 min of wash time, EGNE-GA 0.6 and 1.25% treatments reduced S. Enteritidis counts by >4 log CFU/sample (P < 0.05). In case of L. monocytogenes, EGNE-GA 0.3, 0.6, and 1.25% were found to be more effective than eugenol oil treatments at 1 and 10 min, respectively (P < 0.05). By 10 min of wash time, EGNE-GA 1.25% reduced L. monocytogenes by ~ 4 log CFU/cm2 (P < 0.05). No significant difference in the antimicrobial efficacy of EGNE-TW and corresponding eugenol oil treatments was observed at any of the washing timepoints (P > 0.05) except by 0.6 and 1.25% EGNE-TW against E. coli O157:H7 at 10 min of wash time (P < 0.05).
The efficacy of the highest dose of EGNE-TW (1.25%) and EGNE-GA (1.25%) in reducing pathogen load in presence of 5% organic matter is presented in Table 4. The efficacy of eugenol was significantly reduced at 1 and 5 min of treatment time against L. monocytogenes and at 1 min and 5 min against S. Enteritidis and E. coli O157:H7, respectively (P < 0.05). Similarly, several of the chlorine-based treatments also reduced in their efficacy in the presence of organic load. However, the presence of organic load did not change the efficacy of EGNE-TW or EGNE-GA treatments at 1, 5 or 10 min against the three pathogens (P > 0.05).
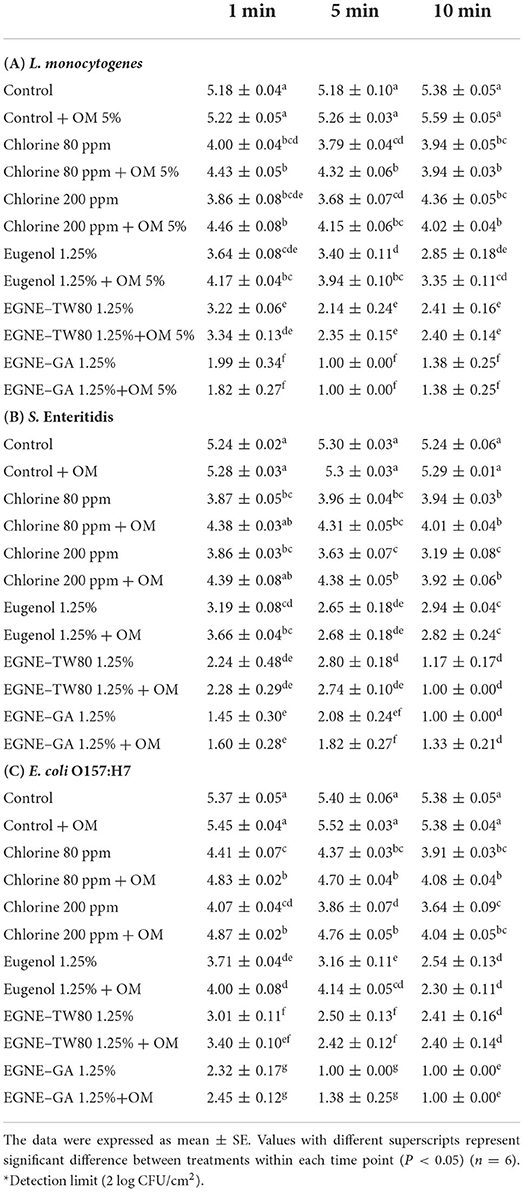
Table 4. Inactivation of L. monocytogenes, S. Enteritidis, or E. coli O157:H7 by eugenol or eugenol nanoemulsions in presence of 5% organic matter (OM).
Effect of eugenol nanoemulsions on the survival of foodborne pathogens on cantaloupe rind during refrigerated or room temperature storage
Figure 3 shows the efficacy of EGNE-GA and EGNE-TW (1.25%) wash treatments in reducing the survival of the three pathogens on cantaloupe rind during storage at 25 or 4°C for 5 days. The pathogen inoculum on the cantaloupes before the wash treatments was ~5.5–6 log CFU/cm2. In the control group, after washing with water, the surviving L. monocytogenes population on the cantaloupe significantly increased from ~6 to 8 log CFU/cm2 as early as day 3 at 25°C and day 5 at 4°C (Figures 3A,B). Similar observations were made with S. Enteritidis (Figure 3C) and E. coli O157:H7 (Figure 3E), at 25°C storages, where the surviving pathogens in the control grew on the fruit surface after washing. At 4°C, ~0.5 log increase in S. Enteritidis (Figure 3D) and E. coli O157:H7 (Figure 3F) counts was observed.
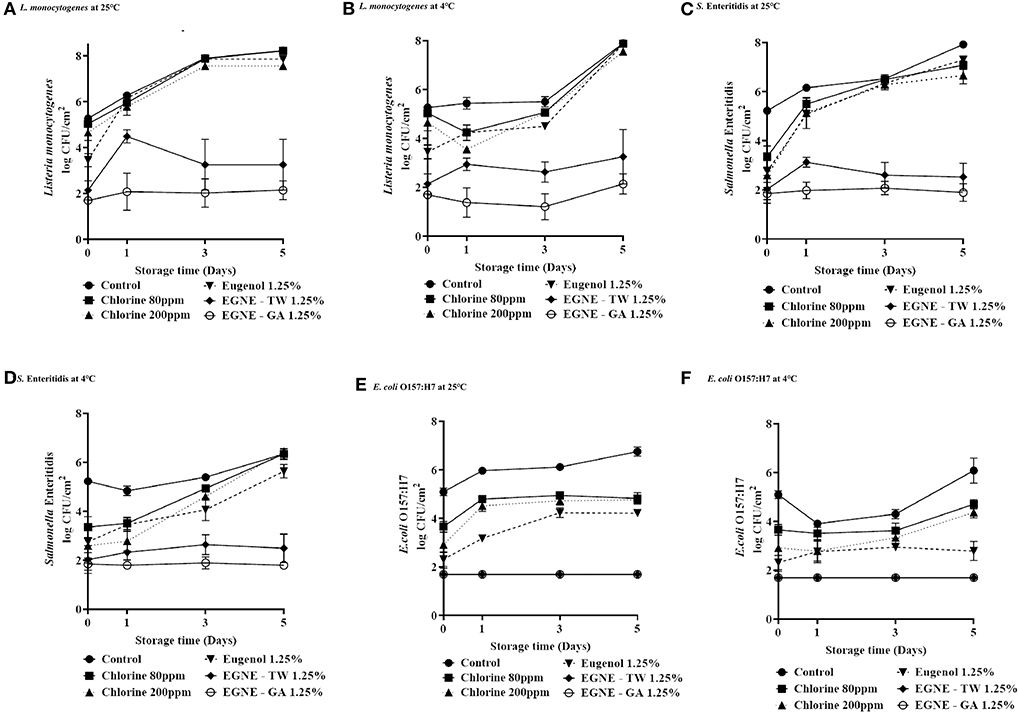
Figure 3. Survival of L. monocytogenes (A,B), S. Enteritidis (C,D) and E. coli O157:H7 (E,F) (log CFU/cm2) on cantaloupe washed with eugenol nanoemulsions during the 5-day storage at 25 and 4°C (A,B). Error bar represents SEM (n = 6). The treatment groups are control, chlorine 80 ppm, chlorine 200 ppm, Eugenol 1.25%, EGNE-GA 1.25%, and EGNE-TW 1.25%.
Washing with chlorine and eugenol treatments reduced pathogen loads of L. monocytogenes, S. Enteritidis and E. coli O157:H7 on day 0. However, the surviving pathogens recovered during room temperature and refrigerated storage. For example, after storage for 5 days at 25 or 4°C, no difference in the counts of L. monocytogenes or S. Enteritidis was observed on control, chlorine, or eugenol oil treated cantaloupes (P > 0.05; Figures 3A–D). In case of E. coli O157: H7, although cantaloupes washed with chlorine and eugenol had lower counts than control on day 5 (P < 0.05), the majority of the chlorine and eugenol oil treated groups did increase the number of pathogens compared to the corresponding counts on day 0 (P < 0.05). However, no significant increase in pathogen load was observed on cantaloupes washed with EGNE treatments upon storage for 5 days at 25 or 4°C compared with day 0 (P > 0.05).
Effect of eugenol nanoemulsion wash treatments on cantaloupe color
Table 5 shows the effect of nanoemulsion wash treatments on the color of cantaloupe during refrigerated storage. No significant change in cantaloupe surface color was observed after the wash treatments and subsequent storage for 5 days at 4°C (P > 0.05).
Discussion
We prepared eugenol nanoemulsions with two types of emulsifiers (Type 1 with gum arabic and lecithin combination and type 2 with Tween-80) and tested their efficacy in inactivating three major foodborne pathogens on cantaloupes at 25°C. Gum arabic and lecithin are food grade emulsifiers and are used extensively in the food industry (Patel and Goyal, 2015; Sethuraman and Rajendran, 2019). Gum arabic is a polysaccharide which has both hydrophobic and hydrophilic ends. Hydrophobic end attaches to the droplet surfaces whereas hydrophilic end provides stability against droplet aggregation through steric and electrostatic repulsion (Anarjan and Tan, 2013). Lecithin is a mixture of glycerophospholipids and is obtained from various sources such as soybean. The emulsification functionality of lecithin is primarily attributed to amphiphilic phospholipids (Bot et al., 2021). Tween-80 is a hydrophilic non-ionic compound, widely used as a surfactant in the food industry (Zhang et al., 2003; Tan and McClements, 2021). Several researchers have previously used Tween-80 or gum-arabic and lecithin for preparing phytochemical nanoemulsions (Ghosh et al., 2014; Esmaeili et al., 2016; Ahmad et al., 2018; Shrestha et al., 2019; Wagle et al., 2019; Hu et al., 2021) followed by characterizing the formulations using size, PDI and zeta potential. Size refers to the average dimensions of the particles in the suspension. PDI represents the homogeneity of the particle size distribution and correlates to the stability of the nanoemulsion (Preetz et al., 2010; Tan et al., 2015; Narawi et al., 2020). An emulsion with a particle size of <200 nm is referred as nanoemulsion (Jaiswal et al., 2015). A PDI <0.08 is considered as monodispersed whereas a PDI in the range of 0.08–0.7 is mid-range value. A PDI >0.7 indicates a very broad size distribution of droplets in nanoemulsion (Tang et al., 2013; Asmawati et al., 2014). The size and PDI of the nanoparticles in the aforementioned studies ranged from (70 to 200 nm) and (0.2–0.3), respectively. In our study, EGNE-GA and EGNE-TW had size in the range of 70–100 nm with a PDI <0.3, which shows that the size and homogeneity of the nanoemulsion was acceptable. Nanoemulsion prepared using gum arabic and lecithin as emulsifiers had smaller average particle size as compared to the tween 80 type nanoemulsion. This could be attributed to the hydrophilic part of gum arabic that provides stability against droplet aggregation through steric and electrostatic repulsion thereby reducing the particle size (Anarjan and Tan, 2013). Zeta potential is a measure of the surface charge of particles and plays a major role in flocculation, dispersion, aggregation and stability of the colloidal system (Sapsford et al., 2011; Pinto and Buss, 2020). In this study, nanoemulsion prepared with gum arabic and lecithin exhibited higher negative charge when compared to tween 80. This is primarily due to the ionic nature of gum arabic and lecithin used for preparing nanoemulsions (Wang et al., 2012; McClements and Jafari, 2018). Ozturk et al. (2015) reported that gum arabic stabilized the colloidal system against temperature, pH and salt by steric repulsion due to the formation of thick interfacial layers that inhibit droplet aggregation. Zhang et al. (2021) demonstrated that presence of negatively charged phosphate groups in lecithin impart a high negative zeta potential on the particles. Since tween 80 is non-ionic, the zeta potential observed in the nanoemulsion was low as compared to gum arabic and lecithin.
ABTS antioxidant assay was performed to evaluate the encapsulation efficiency/dispersion of the eugenol oil in the system. An increase in ABTS value would directly correspond to an increased dispersion of the oil in the system which in turn supports its antimicrobial efficiency. Several researchers have reported significant antioxidant and radical scavenging activity of eugenol (Donsı̀ et al., 2011; Gülçin, 2011; Nam and Kim, 2013; Chen et al., 2017; Zhang et al., 2017). Moreover, an increase in the antioxidant effect after nanoencapsulation has been reported previously. For example, Hadidi et al. (2020) reported that antioxidant activity of clove essential oil-loaded chitosan nanoparticle was significantly higher than that of free oil. Similarly, curcumin, resveratrol and grape extracts when presented in lipid-based nanostructures also showed increased antioxidant activity (Spigno et al., 2013; Coradini et al., 2014). In line with the above findings, our results indicated that eugenol nanoemulsion had higher antioxidant activity during the storage period of 8 weeks at 25 or 4°C as compared to eugenol oil. The increased antioxidant activity after encapsulation may be due to a combination of reduced evaporation rate of the volatile compounds and increased dispersion in the system that facilitates enhanced interaction with the test substrates (Woranuch and Yoksan, 2013).
Water is often used in dump tanks or flotation flumes to transport cantaloupes from field to the packing facility. In addition, water also is used for sanitation and washing of the fruit. The amount of chlorine in the wash water is usually up to 200 ppm as indicated in the Federal regulations (21 CFR-Part 173 and 21 CFR Part 178 permit). However, chlorine wash only achieves ~1–3 log reductions depending on type and method of application and presence of organic matter (Ukuku et al., 2018). The organic particles may be introduced in wash water from soil, plant leaves, or farm debris. Organic load has been previously shown to reduce sanitizer efficacy and also assist in cross-contamination (Shen et al., 2013; Huang et al., 2020). Therefore, we tested the efficacy of wash treatments both in the presence or absence of organic load. Internalization of the pathogen in cantaloupes is also a concern, especially if there is a temperature differential between the fruit and the wash water (Macarisin et al., 2017). Therefore, we selected a wash temperature of 25°C and the cantaloupes were maintained at the same temperature. Washing the cantaloupe with water for 1, 5 or 10 min did not reduce pathogen load. Similar results were observed by Upadhyay et al. (2014) and Bhargava et al. (2015) that washing with water had no effect in reducing the pathogens on cantaloupe and lettuce surface.
In this study, several of EGNE-GA and EGNE-TW wash treatments were found to be effective in reducing pathogen load on cantaloupes, both in the absence (Table 3) or presence (Table 4) of organic load. Similar results have been reported previously by other researchers who prepared nanoemulsions with oregano oil (Bhargava et al., 2015), clove/cinnamon mixture (Majeed et al., 2016; Zhang et al., 2017), and thyme (Hu et al., 2021). In our study, majority of EGNE-GA treatments were found to be more effective in reducing the three test pathogens (especially E. coli O157:H7) on cantaloupes than the corresponding oil control (Table 3). The superior antimicrobial efficacy of EGNE-GA over its oil control was also observed in the presence of organic load (Table 4) suggesting that the wash treatment could tolerate organic matter without losing antimicrobial efficacy. This may be due to the smaller particle size of EGNE-GA that increases the contact area with the bacteria. EGNE-GA and TW 1.25% treatment was also more effective in inhibiting the recovery and growth of surviving L. monocytogenes, S. Enteritidis and E. coli O157:H7 on cantaloupes during refrigerated or room temperature storage as compared to control, chlorine, and eugenol treatments (Figure 3). This could be due to potential cell injury following wash treatment (Yuan et al., 2019). Even though chlorine significantly reduced the bacterial population initially, the survival of the pathogen was similar to control during storage suggesting that chlorine treatment is not completely effective in eradicating the pathogens.
In conclusion, this study demonstrated that nanoencapsulation of eugenol increased the dispersion and antimicrobial efficacy of the compound against the three major foodborne pathogens on cantaloupes without affecting rind color. In the long term, eugenol nanoemulsions could be a viable alternative for enhancing the microbiological safety of fresh produce in the industry. However, further investigations in an industry setting are warranted to develop the aforementioned treatments into natural and effective sanitizers for industry application.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
BB and AU conceptualized the study and designed the experiments. BB, JA, KR, TS, and JX conducted the experiments. BB collected and analyzed the data and wrote the manuscript. AU, RM, and YL critically analyzed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, through the Northeast Sustainable Agriculture Research and Education program under subaward number LNE20-412R and partially supported by A1601 program of USDA-NIFA, Grant No. 2020-69006-31684 with project accession number 1022281.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor H-BY declared a past co-authorship with the authors YL and AU.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, N., Alam, M. A., Ahmad, F. J., Sarafroz, M., Ansari, K., Sharma, S., et al. (2018). Ultrasonication techniques used for the preparation of novel Eugenol-Nanoemulsion in the treatment of wounds healings and anti-inflammatory. J. Drug. Deliv. Sci. Technol. 46, 461–473. doi: 10.1016/j.jddst.2018.06.003
Anarjan, N., and Tan, C. (2013). Physico-chemical stability of astaxanthin nanodispersions prepared with polysaccharides as stabilizing agents. Int. J. Food Sci. Technol. 64, 744–748. doi: 10.3109/09637486.2013.783001
Annous, B. A., Burke, A., and Sites, J. E. (2004). Surface pasteurization of whole fresh cantaloupes inoculated with salmonella poona or escherichia coli. J. Food Prot. 67, 1876–1885. doi: 10.4315/0362-028X-67.9.1876
Apolónio, J., Faleiro, M. L., Miguel, M. G., and Neto, L. (2014). No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS. Microbiol. Let. 354, 92–101. doi: 10.1111/1574-6968.12440
Asmawati, M. W. A. W., Yusop, S. M., Maskat, M. Y., and Shamsuddin, A. F. (2014). “Characteristics of cinnamaldehyde nanoemulsion prepared using APV-high pressure homogenizer and ultra turrax,” in AIP Conference Proceedings, Malaysia, Vol. 1614 (American Institute of Physics), 244–250. doi: 10.1063/1.4895203
Bhargava, K., Denise, S. C., Sandro, R. P., Rocha, da., and Zhang, Y. (2015). Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food. Microbiol. 47, 69–73. doi: 10.1016/j.fm.2014.11.007
Borges, A., Saavedra, J. M., and Simoes, M. (2015). Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 22. doi: 10.2174/0929867322666150530210522
Bot, F., Cossuta, D., and O'Mahony, J. A. (2021). Inter-relationships between composition, physicochemical properties and functionality of lecithin ingredients. Trends Food Sci. Technol. 111, 261–270. doi: 10.1016/j.tifs.2021.02.028
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in food—a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Callejón, R. M., Rodríguez-Naranjo, M. I., Ubeda, C., Hornedo-Ortega, R., Garcia-Parrilla, M. C., and Troncoso, A. M. (2015). Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne. Pathog and Dis. 12, 32–38. doi: 10.1089/fpd.2014.1821
Centers for Disease Control Prevention (CDC). (2018). National Outbreak Reporting System (NORS). Available online at: https://wwwn.cdc.gov/norsdashboard/ (accessed May 30, 2022).
Centers for Disease Control and Prevention. (2011). Multistate outbreak of Listeriosis associated with jensen farms cantaloupe—United States, August–September 2011. MMWR 60, 1357–1358.
Centers for Disease Control Prevention. (2012). Multistate outbreak of Salmonella Typhimurium and Salmonella Newport Infections Linked to Cantaloupe (final update). Available online at: https://www.cdc.gov/salmonella/typhimurium-cantaloupe-08-12/index.html (accessed May 29, 2022).
Chen, E., Cao, L., McClements, D. J., Liu, S., Li, B., and Li, Y. (2018). Enhancement of physicochemical properties of whey protein-stabilized nanoemulsions by interfacial cross-linking using cinnamaldehyde. Food Hydrocoll. 77, 976–985. doi: 10.1016/j.foodhyd.2017.11.047
Chen, X., Ren, L. L. M., Qian, J., and Fan, B. (2017). Du Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. J. Agric. Food Chem. 214, 432–439. doi: 10.1016/j.foodchem.2016.07.101
CLSI. (2019). Performance Standards for Antimicrobial Susceptibility Testing. 29th edition CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
Coradini, K., Lima, F. O., Oliveira, C. M., Chaves, P. S., Athayde, M. L., Carvalho, L. M., et al. (2014). Co-encapsulation of resveratrol and curcuminin lipid-core nanocapsules improves their in vitro antioxidant effects. Eur J Pharm Biopharm. 88, 178–185. doi: 10.1016/j.ejpb.2014.04.009
Danyluk, M. D., Friedrich, L. M., and Schaffner, D. W. (2014). Modeling the growth of Listeria monocytogenes on cut cantaloupe, honeydew and watermelon. Food Microbiol. 38, 52–55. doi: 10.1016/j.fm.2013.08.001
Devi, K. P., Nisha, S. A., Sakthivel, R., and Pandian, P. K. (2010). Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 130, 107–115. doi: 10.1016/j.jep.2010.04.025
Dharmarha, V., Boyer, R. R., Strawn, L. K., Drape, T. A., Eifert, J., Vallotton, A. D., et al. (2020). An assessment of produce growers' sanitizer knowledge and practices on the correct use of sanitizers. Food Prot. Trends. 40, 140–146.
Dhifi, W., Jazi, S., El Beyrouthy, M., Sadaka, C., and Mnif, W. (2020). Assessing the potential and safety of Myrtus communis flower essential oils as efficient natural preservatives against Listeria monocytogenes growth in minced beef under refrigeration. Food Sci. Nutr. 8, 2076–2087. doi: 10.1002/fsn3.1497
Donato, F., and Zani, C. (2010). Chronic exposure to organochlorine compounds and health effects in adults: cancer, non-Hodgkin lymphoma. Review of literature. Ann. Ig. Med. Prev. Comunita. 22, 357–367.
Donsì, F., Annunziata, M., Sessa, G., and Ferrari, G. (2011). Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci Technol. 44, 1908–1914. doi: 10.1016/j.lwt.2011.03.003
Doyle, M. P., and Erickson, M. C. (2008). Summer meeting 2007 - the problems with fresh produce: an overview. J. Appl. Microbiol. 105:317–330. doi: 10.1111/j.1365-2672.2008.03746.x
ERS. (2020). Available online at: https://www.ers.usda.gov/amber-waves/2020/september/us-fruit-imports-grew-by-89-billion-over-the-last-decade-to-meet-rising-demand/ (accessed May 29, 2022).
Esmaeili, F., Rajabnejhad, S., Partoazar, A. R., Mehr, S. E., Faridi-Majidi, R., Sahebgharani, M., et al. (2016). Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 21, 887–893. doi: 10.3109/10837450.2015.1078353
Ezhilarasi, P. N., Karthik, P., Chhanwal, N., and Anandharamakrishnan, C. (2013). Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 6, 628–647. doi: 10.1007/s11947-012-0944-0
Falleh, H., Ben Jemaa, M., Saada, M., and Ksouri, R. (2020). Essential oils: a promising eco-friendly food preservative. Food Chem 330, 127268. doi: 10.1016/j.foodchem.2020.127268
Fan, X., Annous, B. A., Keskinen, L. A., and Matthe, J. P. (2009). Use of chemical sanitizers to reduce microbial populations and maintain quality of whole and fresh-cut cantaloupe. J. Food Protect. 72, 8. doi: 10.4315/0362-028X-72.12.2453
FDA. (1998). Federal Register: June 11, 1998 (Volume 63. Number 112). From the Federal Register Online via GPO Access [wais.access.gpo.gov]. p. 32013–32014.
Ghosh, V., Mukherjee, A., and Chandrasekaran, N. (2014). Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces. 114, 392–397. doi: 10.1016/j.colsurfb.2013.10.034
Gülçin, I. (2011). Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food. 14, 975–985. doi: 10.1089/jmf.2010.0197
Hadidi, M., Pouramin, S., Adinepour, F., Haghani, S., and Jafari, S. M. (2020). Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr Polym. 15,236. doi: 10.1016/j.carbpol.2020.116075
He, Q., Guo, M., Jin, T. Z., Arabi, S. A., and Liu, D. (2021). Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 337, 108936 doi: 10.1016/j.ijfoodmicro.2020.108936
Holley, R. A., and Patel, D. (2005). Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 4, 273–292. doi: 10.1016/j.fm.2004.08.006
Huang, K., Tian, Y., Tan, J., Salvi, D., Karwe, M., and Nitin, N. (2020). Role of contaminated organic particles in cross-contamination of fresh produce during washing and sanitation. Postharvest Biol. Technol. 168,111283. doi: 10.1016/j.postharvbio.2020.111283
Jaiswal, M., Dudhe, R., and Sharma, P. K. (2015). Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5, 123–127. doi: 10.1007/s13205-014-0214-0
Jones, J. D., and Dangl, J. L. (2006). The plant immune system. Nature. 444, 323–329. doi: 10.1038/nature05286
Li, J., Teng, Z., Weng, S., Zhou, B., Turner, E. R., and Vinyard, B. T. (2019). Dynamic changes in the physicochemical properties of fresh-cut produce wash water as impacted by commodity type and processing conditions. PLoS ONE. 14:e0222174. doi: 10.1371/journal.pone.0222174
Macarisin, D., Wooten, A., De Jesus, A., Hur, M., Bae, S., Patel, J., et al. (2017). Internalization of Listeria monocytogenes in cantaloupes during dump tank washing and hydrocooling. Int. J. Food Microbiol. 257, 65–175. doi: 10.1016/j.ijfoodmicro.2017.06.018
Majeed, H., Liu, F., Hategekimana, J., Sharif, H. R., Qi, J., Ali, B., et al. (2016). Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 197, 75–83. doi: 10.1016/j.foodchem.2015.10.015
Mattson, T. E., Johny, A. K., Amalaradjou, M. A. R., More, K., Schreiber, D. T., Patel, J., et al. (2011). Inactivation of Salmonella spp. on tomatoes by plant molecules. Int. J. Food Microbiol. 144,464–468. doi: 10.1016/j.ijfoodmicro.2010.10.035
McClements, D. J., and Jafari, S. M. (2018). Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 251,55–79. doi: 10.1016/j.cis.2017.12.001
Mrvos, R., Dean, B. S., and Krenzelok, E. P. (1993). Home exposures to chlorine/chloramine gas: review of 216 cases. South Med J. 86, 654–657. doi: 10.1097/00007611-199306000-00013
Nam, H., and Kim, M. M. (2013). Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem Toxicol. 55, 106–112. doi: 10.1016/j.fct.2012.12.050
Narawi, M. M., Chiu, H. I., Yong, Y. H., Zain, N. N. M., Ramachandran, M. R., Tham, C. L., et al. (2020). Biocompatible nutmeg oil-loaded nanoemulsion as phyto-repellent. Front. Pharmacol. 11, 214. doi: 10.3389/fphar.2020.00214
Nguyen, C., and Carlin, F. (1994). The microbiology of minimally processed fresh fruits and vegetables. Crit Rev Food Sci Nutr. 34, 371–401. doi: 10.1080/10408399409527668
Niksic, H., Becic, F., and Koric, E. (2021). Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep. 11, 13178. doi: 10.1038/s41598-021-92679-x
Nirmala, M. J., Durai, L. G. V., and Nagarajan, R. (2019). Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int J Nanomedicine. 14, 6439–6450. doi: 10.2147/IJN.S211047
Niu, D., Wang, Q. Y., Ren, E. F., Zeng, X., Wang, L. H., He, T. F., et al. (2019). Multi-target antibacterial mechanism of eugenol and its combined inactivation with pulsed electric fields in a hurdle strategy on Escherichia coli. Food Control. 106:106742. doi: 10.1016/j.foodcont.2019.106742
Ozturk, B., Argin, S., Ozilgen, M., and McClements, D. J. (2015). Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem.188, 256–263. doi: 10.1016/j.foodchem.2015.05.005
Patel, S., and Goyal, A. (2015). Applications of natural polymer gum arabic: a review. Int. J. Food Prop. 18, 986–998. doi: 10.1080/10942912.2013.809541
Pei, R. S., Zhou, F., Ji, B. P., and Xu, J. (2009). Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 74,379–383. doi: 10.1111/j.1750-3841.2009.01287.x
Pinto, I., and Buss, A. (2020). ζ Potential as a measure of asphalt emulsion stability. Energy Fuels. 34, 2143–2151. doi: 10.1021/acs.energyfuels.9b03565
Preetz, C., Hauser, A., Hause, G., Kramer, A., and Mäder, K. (2010). Application of atomic force microscopy and ultrasonic resonator technology on nanoscale: distinction of nanoemulsions from nanocapsules. Eur J Pharm Sci. 39, 141–151. doi: 10.1016/j.ejps.2009.11.009
Reichling, J. (2010). Plant-microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Annu plant reviews 39, 214–347. doi: 10.1002/9781444318876.ch4
Richardson, S. D., Thurston, A. D. Jr., Caughran, T. V., Collette, T. W., Patterson, K. S., and Lykins, B. W. Jr. (1998). Chemical by products of chlorine and alternative disinfectants. Food Technol. 52, 58–61.
Sapers, G. M., Miller, R. L., Pilizota, V., and Mattrazzo, A. M. (2001). Antimicrobial treatments for minimally processed cantaloupe melon. J. Food Sci. 66, 345–349. doi: 10.1111/j.1365-2621.2001.tb11344.x
Sapsford, K. E., Tyner, K. M., Dair, B. J., Deschamps, J. R., and Medintz, I. L. (2011). Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem. 83, 4453–4488. doi: 10.1021/ac200853a
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15. doi: 10.3201/eid1701.P11101
Sethuraman, S., and Rajendran, K. (2019). Is Gum Arabic a Good Emulsifier Due to CH… π Interactions? How Urea Effectively Destabilizes the Hydrophobic CH… π Interactions in the Proteins of Gum Arabic than Amides and GuHCl? ACS Omega. 4:16418-16428. doi: 10.1021/acsomega.9b01980
Shen, C., Luo, Y., Nou, X., Wang, Q., and Millner, P. (2013). Dynamic effects of free chlorine concentration, organic load, and exposure time on the inactivation of Salmonella, Escherichia coli O157: H7, and non-O157 Shiga toxin–producing E. coli. J Food Prot. 76, 386–393. doi: 10.4315/0362-028X.JFP-12-320
Shewfelt, R. L. (1987). Quality of minimally processed fruits and vegetables J. Food Qual. 10:143–156. doi: 10.1111/j.1745-4557.1987.tb00855.x
Shrestha, S., Wagle, B. R., Upadhyay, A., Arsi, K., Upadhyaya, I., Donoghue, D. J., et al. (2019). Edible coatings fortified with carvacrol reduce Campylobacter jejuni on chicken wingettes and modulate expression of select virulence genes. Front. Microbiol. 10:583. doi: 10.3389/fmicb.2019.00583
Spigno, G., Dons,ì, F., Amendola, D., Sessa, M., Ferrari, G., and De Faveri, D. M. (2013). Nanoencapsulation systems to improve solubility andantioxidant efficiency of a grape marc extract into hazelnut paste. J Food Eng.114, 207–214. doi: 10.1016/j.jfoodeng.2012.08.014
Tan, C., and McClements, D. J. (2021). Application of advanced emulsion technology in the food industry: a review and critical evaluation. Foods 10:812. doi: 10.3390/foods10040812
Tan, L. S., Stanslas, J., Basri, M., Karjiban, R. A. A., Kirby, P. B., Sani, D., et al. (2015). Nanoemulsion-based parenteral drug delivery system of carbamazepine: preparation, characterization, stability evaluation and blood-brain pharmacokinetics. Curr. Drug Deliv. 12, 795–804. doi: 10.2174/1567201812666150901112544
Tang, S. Y., Shridharan, P., and Sivakumar, M. (2013). Impact of process parameters in the generation of novel aspirin nanoemulsions–comparative studies between ultrasound cavitation and microfluidizer. Ultrason Sonochem. 20, 485–497. doi: 10.1016/j.ultsonch.2012.04.005
Teng, Z., Luo, Y., Alborzi, S., Zhou, B., Chen, L., Zhang, J., et al. (2018). Investigation on chlorine-based sanitization under stabilized conditions in the presence of organic load. Int. J. Food Microbiol. 266,150–157. doi: 10.1016/j.ijfoodmicro.2017.11.027
Ukuku, D. O., Mukhopadhyay, S., and Olanya, M. (2018). Reducing transfer of Salmonella and aerobic mesophilic bacteria on melon rinds surfaces to fresh juice by washing with chlorine: effect of waiting period before refrigeration of prepared juice. Front Sustain. Food Syst. 78. doi: 10.3389/fsufs.2018.00078
Ukuku, D. O., and Sapers, G. M. (2001). Effect of sanitizer treatments on Salmonella stanley attached to the surface of cantaloupe and cell transfer to fresh-cut tissues during cutting practices. J. Food Prot. 64, 1286–1291. doi: 10.4315/0362-028X-64.9.1286
Upadhyay, A., Chen, C. H., Yin, H., Upadhyaya, I., Fancher, S., Liu, Y., et al. (2016a). Inactivation of Listeria monocytogenes, Salmonella spp. and Escherichia coli O157: H7 on cantaloupes by octenidine dihydrochloride. Food microbiol. 58,121-127. doi: 10.1016/j.fm.2016.04.007
Upadhyay, A., Upadhyaya, I., Karumathil, D. P., Yin, H. B., Nair, M. S., Bhattaram, V., et al. (2015). Control of Listeria monocytogenes on skinless frankfurters by coating with phytochemicals. LWT. Food Sci. Technol. 63, 37–42. doi: 10.1016/j.lwt.2015.03.100
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., and Venkitanarayanan, K. (2013). Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 36, 79–89. doi: 10.1016/j.fm.2013.04.010
Upadhyay, A., Upadhyaya, I., Mooyottu, S., Kollanoor-Johny, A., and Venkitanarayanan, K. (2014). Efficacy of plant-derived compounds combined with hydrogen peroxide as antimicrobial wash and coating treatment for reducing Listeria monocytogenes on cantaloupes. Food Microbiol. 44,47–53. doi: 10.1016/j.fm.2014.05.005
Upadhyay, A., Upadhyaya, I., Mooyottu, S., and Venkitanarayanan, K. (2016b). Eugenol in combination with lactic acid bacteria attenuates Listeria monocytogenes virulence in vitro and in invertebrate model Galleria mellonella. J. Med. Microbiol. 65, 443–455. doi: 10.1099/jmm.0.000251
Upadhyaya, I., Upadhyay, A., Kollanoor-Johny, A., Darre, M. J., and Venkitanarayanan, K. (2013). Effect of plant derived antimicrobials on Salmonella enteritidis adhesion to and invasion of primary chicken oviduct epithelial cells in vitro and virulence gene expression. Int J Mol Sci. 14, 10608–10625. doi: 10.3390/ijms140510608
USDA (2022). Available online at: https://www.fsis.usda.gov/food-safety/foodborne-illness-and-disease/outbreaks/outbreak-investigations-response (accessed June 01, 2022).
Vergis, J., Gokulakrishnan, P., Agarwal, R. K., and Kumar, A. (2015). Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr 55, 1320–1323. doi: 10.1080/10408398.2012.692127
Wagle, B. R., Arsi, K., Shrestha, S., Upadhyay, A., Upadhyaya, i., Bhargava, K., et al. (2019). Eugenol as an antimicrobial wash treatment reduces campylobacter jejuni in postharvest poultry. J. Food Saf. 39, 12704. doi: 10.1111/jfs.12704
Wang, B., Han, Y., Song, K., and Zhang, T. (2012). The use of anionic gum arabic as a dispersant for multi-walled carbon nanotubes in an aqueous solution. . J. Nanosci. Nanotechnol. 12, 4664–4669. doi: 10.1166/jnn.2012.6425
White, C. W., and Martin, J. G. (2010). Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7, 257–263. doi: 10.1513/pats.201001-008SM
Woranuch, S., and Yoksan, R. (2013). Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation carbohydrate. Polymers 96, 578–585. doi: 10.1016/j.carbpol.2012.08.117
Xue, J., Inzero, J., Hu, Q., Wang, T., and Luo, Y. (2019). Development of easy, simple and low-cost preparation of highly purified phytoglycogen nanoparticles from corn. Food Hydrocoll. 95, 256–261. doi: 10.1016/j.foodhyd.2019.04.041
Xue, J., Luo, Y., Balasubramanian, B., Upadhyay, A., Li, Z., and Luo, Y. (2021). Development of novel biopolymer-based dendritic nanocomplexes for encapsulation of phenolic bioactive compounds: a proof-of-concept study. Food Hydrocoll. 120, 106987. doi: 10.1016/j.foodhyd.2021.106987
Yalkowsky, S. H., and Banerjee, S. (1992). Aqueous Solubility, in: Methods of Estimation for Organic Compounds. New York, NY: Marcel Dekker.
Yang, Y., Luo, G., Millner, P., Shelton, D., and Nou, X. W. (2012). Enhanced chlorine efficacy against bacterial pathogens in wash solution with high organic loads. J. Food Process. Preserv., 36, 560–566. doi: 10.1111/jfpp.12000
Yildirim, S. T., Oztop, M. H., and Soyer, Y. (2017). Cinnamon oil nanoemulsions by spontaneous emulsification: Formulation, characterization and antimicrobial activity. LWT Food Sci. Technol. 84, 122–128. doi: 10.1016/j.lwt.2017.05.041
Yuan, W.H. G Yuan, et al. (2019). Effects of sublethal thymol, carvacrol, and transcinnamaldehyde adaptation on virulence properties of Escherichia coli O157: H7. Appl. Environ. Microbiol. 85, 00271–00219. doi: 10.1128/AEM.00271-19
Zhang, H., Yao, M., Morrison, R. A., and Chong, S. (2003). Commonly used surfactant, Tween 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch, Pharm. Res. 26,768–772. doi: 10.1007/BF02976689
Zhang, L. L., Zhang, L. F., Xu, J. G., and Hu, Q. P. (2017). Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr Res. 61:1353356. doi: 10.1080/16546628.2017.1353356
Zhang, S., and Farber, J. M. (1996). The effects of various disinfectants against Listeria monocytogenes on fresh-cut vegetables. Food Microbiol. 13, 311–321. doi: 10.1006/fmic.1996.0037
Zhang, X., Qi, B., Xie, F., Hu, M., Sun, Y., Han, L., et al. (2021). Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 113, 106391. doi: 10.1016/j.foodhyd.2020.106391
Zhang, Y., Ma, Q., Critzer, F., Davidson, P. M., and Zhong, Q. (2016). Organic thyme oil emulsion as an alternative washing solution to enhance the microbial safety of organic cantaloupes. Food Control. 67, 31–38. doi: 10.1016/j.foodcont.2016.02.032
Keywords: foodborne pathogens, cantaloupe, eugenol nanoemulsions, inactivation, rind
Citation: Balasubramanian B, Shah T, Allen J, Rankin K, Xue J, Luo Y, Mancini R and Upadhyay A (2022) Eugenol nanoemulsion inactivates Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7 on cantaloupes without affecting rind color. Front. Sustain. Food Syst. 6:984391. doi: 10.3389/fsufs.2022.984391
Received: 02 July 2022; Accepted: 15 August 2022;
Published: 07 September 2022.
Edited by:
Hsin-Bai Yin, Agricultural Research Service (USDA), United StatesReviewed by:
Cangliang Shen, West Virginia University, United StatesReha Azizoglu, Akdeniz University, Turkey
Copyright © 2022 Balasubramanian, Shah, Allen, Rankin, Xue, Luo, Mancini and Upadhyay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhinav Upadhyay, YWJoaW5hdi51cGFkaHlheUB1Y29ubi5lZHU=
 Brindhalakshmi Balasubramanian
Brindhalakshmi Balasubramanian Trushenkumar Shah
Trushenkumar Shah Jodie Allen1
Jodie Allen1 Yangchao Luo
Yangchao Luo Abhinav Upadhyay
Abhinav Upadhyay