- 1Faculty of Agronomy and Veterinary Medicine, University of Brasília, Brasília, Brazil
- 2Department of Animal Science, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
- 3Laboratory of Poultry Science, Federal Institute of Brasília, Brasília, Brazil
This work aimed to evaluate the eggshell microbiota and the internal egg quality after coatings with cassava starch biopolymer enriched with different essential oils during 35 days of storage at 20°C. A total of 369 brown table eggs were used and distributed in the following treatments: uncoated eggs, coated with cassava starch + Ginger essential oil (CS+GIN), cassava starch + Lemongrass essential oil (CS+LEM), and cassava starch + Tahiti lemon essential oil (CS+TAH). The count of total aerobic mesophilic bacteria on coated eggshells at 0 and 35 days of storage were similar to each other (mean 0.70 ± 0.37 and 0.91 ± 0.22 log10 CFU/mL) and significantly lower compared to uncoated eggs (2.21 ± 0.17 and 3.17 ± 0.22 log10 CFU/mL), in that order. On the 35th day, coated eggs showed similar Haugh unit (HU) values between them (mean 70.61 ± 5.35; classified as A - high quality) and significantly higher than uncoated eggs (51.60 ± 4.28; classified as B - average quality). Cassava starch coatings added with essential oils preserved the internal quality of the eggs during storage for 5 weeks at 20°C, reducing the eggshell microbiota and effectively keeping it at low levels during storage.
Introduction
The beneficial effects of eggs for any purpose are linked to their quality. During storage, an increase in egg weight loss (Sariyel et al., 2022), can be seen, together with a decrease in Haugh unit (Pires et al., 2020), a decrease in egg yolk index (Yüceer and Caner, 2014), and an increase in albumen and yolk pH (Espíndola et al., 2019). According to Oliveira et al. (2020), egg quality is lost faster at room temperature (25°C) than during refrigeration (5°C) which also more quickly affects its value in terms of safety, nutrition, and applicability. Therefore, efficient, safe, and cost-effective approaches, such as egg coatings are a solution to reduce spoilage of eggs when there is no possibility to provide them with a cold environment (Eddin et al., 2019). As refrigeration of eggs is not mandatory in Brazil, they are usually maintained at room temperature until their final destination (Menezes et al., 2012). Different coatings may be used to extend shelf life such as gums (Sariyel et al., 2022), proteins (Biladeau and Keener, 2009; Pires et al., 2020), starch (Eddin et al., 2019) and pectin (Oliveira et al., 2020). These coatings are thin, edible layers from food sources (Ananey-Obiri et al., 2018).
Biopolymers are structurally complex, renewable, biodegradable and biocompatible biomolecular materials (Dassanayake et al., 2018). Starch is an abundant biopolymer and shows promise for coating stored eggs. Lima et al. (2020) demonstrated that cassava starch is a coating that can control water loss and gas transport, preserving eggs longer during storage at 25°C. Starch-based coatings can be combined with natural antimicrobial hydrophobic substances (such as essential oils) to prolong the shelf life of the food and protect it from unwanted microbiological damage (Syafiq et al., 2020). However, the joint applicability of these biopolymer coatings and essential oils on eggs is still not common. The addition of essential oils into coating materials can be through techniques such as emulsification (Tangpao et al., 2022).
Essential oils are volatile, safe, biodegradable, and food preservative liquids extracted from different plants (Pandey et al., 2017). They have multiple natural ingredients [those that occur in nature and are unprocessed or only processed, for example, by manual, gravitational or mechanical means (Dini and Laneri, 2021)] with antimicrobial characteristics. This may be rooted in the ability of essential oils to act detrimentally on the permeability of the cell membrane of microorganisms due to their lipophilic constituents (Macwan et al., 2016). Andrade et al. (2014) studied the efficacy of twenty-seven essential oils against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa strains [microorganisms that can be found in eggshells as reviewed by Oliveira et al. (2022)], with Gram-positive species being more sensitive to natural products than Gram-negative bacteria. Three of these essential oils were chosen for the present study.
Ginger essential oil (Zingiber officinale; Zingiberaceae family) is mainly extracted from the ginger rhizomes, and its complex chemical configuration includes gingerol, aromatic alcohol, and terpenoids (Wang et al., 2021). Lemongrass essential oil (Cymbopogon citratus; Poaceae family) is usually extracted from lemongrass leaves and its chemical arrangement is versatile and includes citral, ketones, alcohols and esters (Shah et al., 2011). Tahiti lemon essential oil (Citrus aurantifolia; Rutaceae family) can be extracted from the lemon fruit peels, and its chemical structure includes monoterpene hydrocarbons such as limonene and myrcene (Lemes et al., 2018).
Given the importance of incorporating natural antimicrobial substances from plants in coatings as an alternative to maintaining microbial safety and food quality over a longer period of time, this work aimed to evaluate the eggshell microbiota and the internal quality of eggs after coatings with cassava starch biopolymer enriched with different essential oils during 35 days of storage at 20°C.
Materials and methods
A total of 369 brown table eggs (single collection) from 71-wk-old Embrapa 051 laying hens, from a free-range rearing system that offers all the necessary management to maintain the birds in good health, were used and distributed in the following treatments: uncoated eggs, coated with cassava starch + Ginger essential oil (CS+GIN), cassava starch + Lemongrass essential oil (CS+LEM), and cassava starch + Tahiti lemon essential oil (CS+TAH). Cassava starch (Yoki®, São Bernardo do Campo, São Paulo, Brazil) and essential oils (Phytoterápica®, Rio de Janeiro, Brazil) were purchased comercially. Essential oils of ginger (rhizomes) and lemongrass (leaves) were extracted by steam distillation. The essential oil of Tahiti lemon (fruit peels) was extracted by cold pressing. In addition to the perfect combination of antimicrobial safety and efficiency, cost and easy availability were key factors in choosing the three essential oils used in this study.
Based on gas chromatography-mass spectrometry analysis, zingiberene, citral, and limonene are commonly the main compounds in the essential oils of ginger, lemongrass, and Tahiti lemon, respectively (Sasidharan and Nirmala Menon, 2010; Ali et al., 2017; Lemes et al., 2018). This analysis is normally performed in a gas chromatograph equipped primarily with an autoinjector, capillary column, and mass spectrometer with detector. The structures can be defined through the retention times, calculation of the Kovats index and databases of the system itself.
Preliminary test: Antimicrobial efficiency of essential oils from ginger, lemongrass and Tahiti lemon
The antimicrobial efficiency of essential oils was investigated before the coatings were prepared by a procedure adapted from Bauer et al. (1966). Sterile filter paper discs (4 mm in diameter) were prepared with 10 μL of essential oil at a concentration of 1% (v/v), and the other discs were made with 5% dimethylsulfoxide (negative control) and 30 ug of cefadroxil (positive control). All discs were placed in Petri dishes (5 per dish) with Mueller-Hinton agar containing 100 μL of bacteria (Escherichia coli [ATCC 25.922], or Staphylococcus aureus [ATCC 25.923]). These standard bacterial strains were purchased commercially. Plates were incubated at 36°C for 24 h, and, subsequently, the diameter of the inhibition zones was measured.
In this antimicrobial test, the essential oils of ginger, lemongrass and Tahiti lemon had an inhibitory effect at a concentration of 1% against Escherichia coli and Staphylococcus aureus (Figure 1). This result was important to ensure the antimicrobial effects of essential oils, as one of the objectives was that they could add these effects to the coating. Studies carried out by Andrade et al. (2014) and Silva et al. (2014) corroborate our results, as they found that the same essential oils have antimicrobial potential in vitro against the same microorganisms tested in this study. Therefore, the essential oils (concentration of 1%—this was chosen considering the concentration that was already intended to be added to the coating) evaluated were added to the biopolymer (cassava starch) for the elaboration of the coatings. As mentioned earlier, Escherichia coli (mesophilic bacterium of the Enterobacteriaceae family) and Staphylococcus aureus (mesophilic bacterium) are microorganisms that can proliferate eggshells.
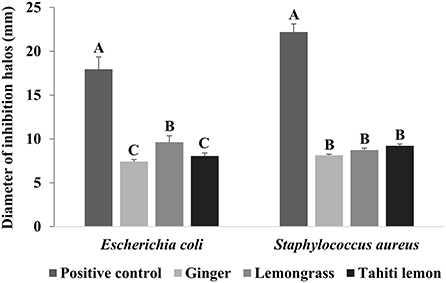
Figure 1. Antimicrobial action of essential oils (ginger, lemongrass, and Tahiti lemon; concentration 1%) against Escherichia coli and Staphylococcus aureus by the disk diffusion method. (A–C) Means with different uppercase letters differ significantly (P < 0.05).
Preparation and spraying of coatings on brown eggs
The coatings were prepared by dissolving 3% (w/w) of cassava starch, 1.5% (w/w) of glycerol, 1% (w/w) of essential oils (ginger, lemongrass, or Tahiti lemon) and 0.5% (w/w) of Tween 80 in 400 mL of distilled water with the aid of a mechanical magnetic stirrer at 70°C for 40 min (Figure 2). Prior to coating application, all eggs were individually weighed to ensure treatments were homogeneous (mean weight = 61.85 ± 0.85 g). The coatings were cooled and then applied homogeneously (spray method; ~2 mL/egg) on the eggs individually. The eggs were placed in sterile trays and stored in a fridge with a thermostat (programmed to maintain a constant temperature at 20°C) for five successive weeks, 1 h after application. The trays were sterilized by ultraviolet light for 30 min in a laminar flow cabinet (OptiMair, ESCO, Horsham, PA, USA).
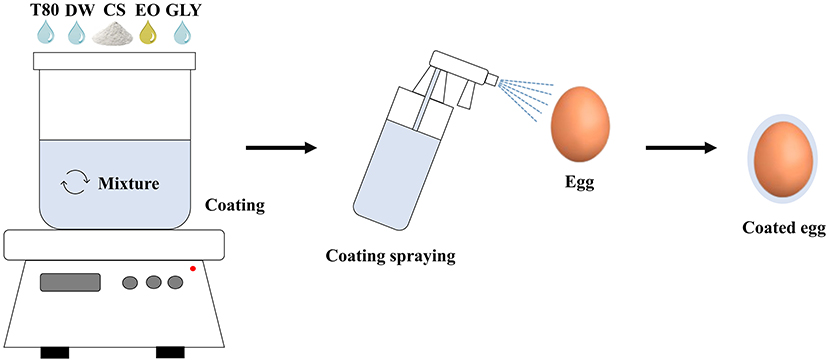
Figure 2. Preparation and application of coatings on eggs. CS, cassava starch; DW, distilled water; GLY, glycerol; EO, essential oil; T80, Tween 80.
Count of total aerobic mesophilic bacteria and enterobacteriaceae in eggshells
A method adapted from Wells et al. (2010) was used to count eggshell microorganisms. On days 0 and 35, fifteen eggs from each treatment were placed in sterile bags (group of 3 eggs/bag) containing 180 mL of 0.1% peptone saline solution and massaged for 1 min. The control treatment rinsing solutions required serial dilution. The solutions from the other treatments were not serially diluted. One milliliter of each dilution or rinse was plated on a standard plate count agar (total aerobic mesophilic bacteria count) and violet red bile glucose agar (Enterobacteriaceae count). Plates were incubated at 36°C for 48 h. The results were transformed by log10 CFU/mL.
Parameters used to assess internal egg quality
Haugh unit (HU), yolk index (YI), and pH of albumen and yolk of 25 eggs were evaluated to determine the initial quality of eggs. On days 7, 14, 21, 28, and 35, analyses were repeated (including egg weight loss) on 10 eggs per treatment to determine egg quality. Yolk color was evaluated, in 6 eggs per treatment, only on day 35.
Egg weight loss
Egg weight was measured weekly on an analytical scale with 0.0001 g precision (Gehaka, São Paulo, São Paulo, Brazil) and the recorded values were used to calculate egg weight loss based on the difference between the initial and final egg weight divided by the initial egg weight and multiplied by 100.
Haugh unit (HU)
In addition to egg weight, albumen height was measured on a glass table using a digital caliper with 0.001-mm precision (Mitutoyo, Suzano, São Paulo, Brazil) and the data obtained were used to calculate HU, which is HU = 100 log (albumen height + 7.57–1.7 egg weight0.37) (Haugh, 1937).
Yolk index (YI)
After separating it from the albumen, still on the glass table, the height and diameter of the yolk were measured with a digital caliper with 0.001-mm precision (Mitutoyo, Suzano, São Paulo, Brazil) and the recorded data were used to calculate the YI, which is to divide the height by the diameter of the yolk (Funk, 1948).
Albumen and yolk pH
After the previous analyses, the yolk and albumen were placed in a beaker (one for the albumen and one for the yolk) for individual pH analysis using a calibrated digital pH meter (206–pH2, Testo®, Lenzkirch, Baden-Württemberg, Germany).
Yolk color
The color was analyzed, at three different points of the yolk, using a calibrated colorimeter (Delta Vista, Delta Color®, São Leopoldo, Rio Grande do Sul, Brazil) that expresses the results as L* (lightness [white/black coordinate]), a* (red/green coordinate) and b* (yellow/blue coordinate).
Statistical analysis
The experiment was carried out in a completely randomized design. Egg quality analysis was based on 10 replicates per treatment, where each egg was a replicate. To analyse microorganism counts, each pool of 3 eggs was considered a replicate, totaling 5 replicates per treatment. PROC UNIVARIATE and PROC GLM, from SAS Studio University Edition software (SAS Inst. Inc., Cary, NC), were used to test normality and perform analysis of variance on the data, respectively. The significant differences (P <0.05) between treatment means were tested by the Tukey test.
Results
Count of total aerobic mesophilic bacteria and enterobacteriaceae in eggshells
The count of total aerobic mesophilic bacteria on coated eggshells (CS+GIN, CS+LEM, and CS+TAH) at 0 and 35 days of storage were similar to each other (mean 0.70 ± 0.37 and 0.91 ± 0.22 log10 CFU/mL) and significantly lower (P <0.05) compared to uncoated eggs (2.21 ± 0.17 and 3.17 ± 0.22 log10 CFU/mL), in that order (Figure 3). There was no growth of Enterobacteriaceae family bacteria on the eggshells.
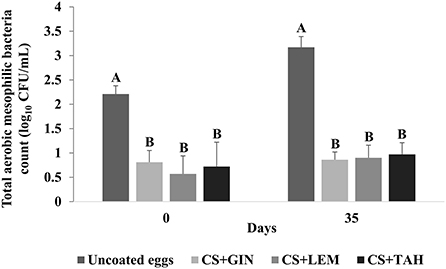
Figure 3. Counts of total aerobic mesophilic bacteria on eggshell surfaces of the different treatments at 0 and 35 days of storage at 20°C. A−BMeans with different uppercase letters differ significantly (P < 0.05). CS+GIN, cassava starch + Ginger essential oil; CS+LEM, cassava starch + Lemongrass essential oil; CS+TAH, cassava starch + Tahiti lemon essential oil.
Egg weight loss
Eggs on day zero had HU, YI, albumen, and yolk pH of 87.08 ± 2.97 (classified as AA, excellent quality), 0.44 ± 0.02, 7.92 ± 0.15, 5.96 ± 0.10, respectively. On the 7th day of storage, there was no difference (P > 0.05) in weight loss (mean 0.86 ± 0.19%) between eggs coated with CS+GIN, CS+LEM, and CS+TAH and uncoated eggs (Figure 4). However, from day 14 to day 35, coated eggs showed significantly (P <0.05) less weight loss (mean ranging from 1.49 ± 0.33 to 5.08 ± 0.61%) than uncoated eggs (ranging from 2.92 ± 0.18 to 8.08 ± 0.44%), respectively (Figure 4).
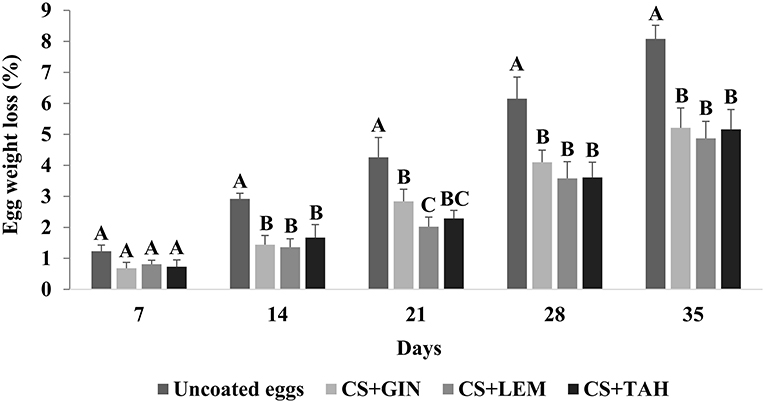
Figure 4. Egg weight loss (%) of the different treatments during the 35 days of storage at 20°C. (A–C) Means with different uppercase letters differ significantly (P < 0.05). CS+GIN, cassava starch + Ginger essential oil; CS+LEM, cassava starch + Lemongrass essential oil; CS+TAH, cassava starch + Tahiti lemon essential oil.
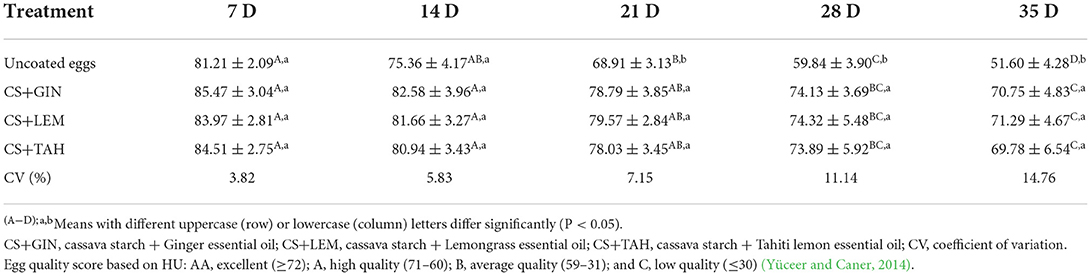
Haugh unit (HU)
HU differed significantly (P <0.05) between treatments from day 21 of storage (Table 1). On the 35th day, coated eggs with CS+GIN, CS+LEM, and CS+TAH showed similar values between them (mean 70.61 ± 5.35; classified as A - high quality) and significantly higher than uncoated eggs (51.60 ± 4.28; classified as B - average quality) (Table 1).
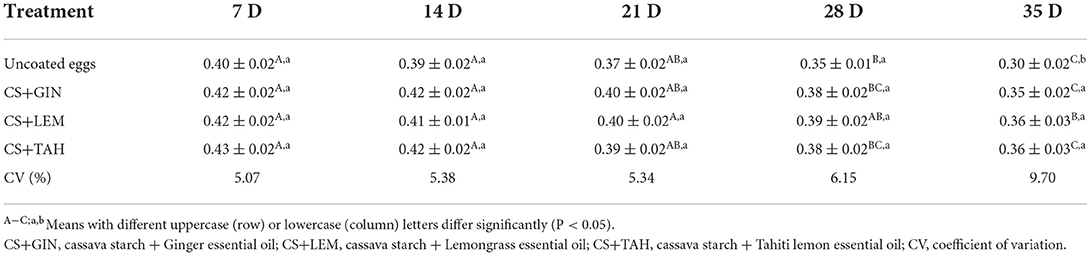
Yolk index (YI)
The YI decreased during egg storage and at the end was similar between eggs treated with CS+GIN, CS+LEM and CS+TAH (mean 0.36 ± 0.03), being less pronounced (P <0.05) compared to uncoated eggs (0.30 ± 0.02) (Table 2).
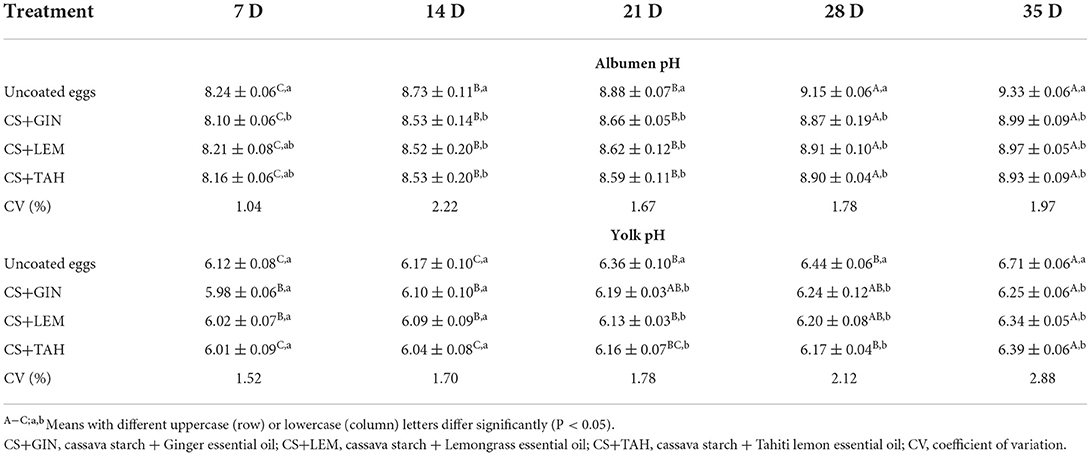
Albumen and yolk pH
Albumen and yolk pH values between eggs coated with CS+GIN, CS+LEM, or CS+TAH and uncoated eggs were significantly different (P <0.05) and increased during the storage period (Table 3). However, the final value was higher for uncoated (9.33 ± 0.06 and 6.71 ± 0.06) when compared to coated eggs (mean 8.96 ± 0.08 and 6.33 ± 0.06), respectively (Table 3). Coated treatments did not differ from each other.
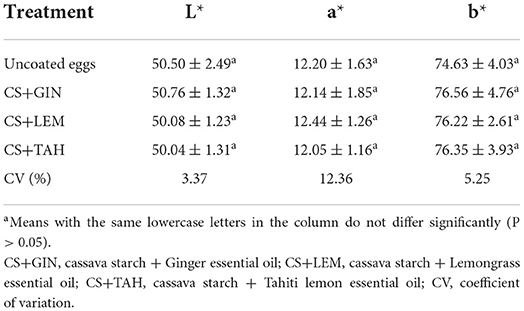
Yolk color
No significant difference (P > 0.05) was observed between coated with CS+GIN, CS+LEM, and CS+TAH and uncoated eggs for yolk color (Table 4). The mean for L*, a* and b* was 50.35 ± 1.59 (very slight tendency toward white), 12.21 ± 1.48 (red color trend), 75.94 ± 3.83 (yellow color trend), respectively.
Discussion
Essential oils have demonstrated functionalities necessary for the composition of biopolymeric coatings for table eggs, contributing to promising results in terms of shelf life and microbiological quality (Upadhyaya et al., 2016; Pires et al., 2020). Thus, continuous studies are advised to fill gaps, optimize and strengthen its use in egg management.
Count of total aerobic mesophilic bacteria and enterobacteriaceae in eggshells
Gram-negative and Gram-positive bacteria and fungi contaminate eggshells (see review by Oliveira et al., 2022). Strategies to control eggshell contamination must be among the main commitments of all stages of the poultry farm-consumer interface to ensure the viability of the egg for consumption. One of these strategies is the use of coatings that contain safe antimicrobial agents in their chemical arrangement. In this study, for example, the lower load of total aerobic mesophilic bacteria on the eggshell for coated eggs on both days evaluated (Figure 3) may reflect the presence of the essential oils of ginger, lemongrass and Tahiti lemon. These natural compounds have potent antimicrobial activity, which in the case of ginger essential oil is due to the presence of constituents such as zingiberene, eugenol, shogaols, and zingerone (Singh et al., 2008). In the lemongrass essential oil this same reaction is due to components such as citral, limonene, geranyl acetate and β-caryophyllene (Vazirian et al., 2012) and in Tahiti lemon essential oil it can be associated with compounds such as limonene, linalool, citronellal, citronellol (Lemes et al., 2018). Furthermore, it appears that these essential oils played a significant long-term antimicrobial residual role in the eggshells. At 35 days of storage, the microbial load of coated eggs remained low and lower than that of uncoated eggs. This residual activity is important to avoid rapid recontamination and is probably associated with its chemical constituents (Oliveira et al., 2021). The absence of bacteria from the Enterobacteriaceae family in the eggshells shows that the hygienic conditions of the laying hen rearing system in which the eggs were collected are ideal (Musgrove et al., 2014). This study demonstrated the beneficial effect of adding essential oils to coatings to reduce eggshell contamination and, consequently, keep them microbiologically safer. In this sense, Sun et al. (2021) reported that the chitosan-beeswax-basil essential oil composite coating has an excellent antibacterial effect on Escherichia coli and Staphylococcus aureus. These authors suggested that extending the time period of eggs by employing a multi-component coating with active substances, such as essential oils, is of great interest. Pectin and gum arabic coatings with the inclusion of carvacrol and eugenol have shown potential to reduce Salmonella Enteritidis on eggshells (Upadhyaya et al., 2016).
Egg weight loss
Egg weight loss (%) can be used to analyse, monitor, and track the viability of an egg. Along the way until it is consumed, the egg will lose weight and, at the same time, its viability. This weight loss is associated with the phenomenon of H2O and CO2 evaporation, intensified at room temperature (Feddern et al., 2017). Thus, occluding the eggshell pores with some coating can be a solution to optimize the control of this phenomenon (Nongtaodum et al., 2013; Ezazi et al., 2021). In the present study, cassava starch associated with essential oils may have been able to form an efficient and long-lasting sealing structure in the eggshell, which is supported by the lower weight loss in coated compared to uncoated eggs at the end of storage at 20°C. There are recent reports on the effectiveness of coatings combined with essential oils on egg weight loss during storage (Eddin and Tahergorabi, 2019; Sun et al., 2021), which is possibly due to the consistent and oily nature of the essential oils that contributes to lower porosity of the eggshell surface (Pires et al., 2020).
Haugh unit (HU)
The reduction of the egg HU occurs in sync with the decrease in its internal quality (Yüceer and Caner, 2020). This is due to the strong correlation (r = 0.98) that HU has with the height of the albumen (Oliveira et al., 2020), which undergoes biochemical reactions such as ovomucin proteolysis (protein linked to gelatinous albumen) (Robinson and Monsey, 1971; Alleoni, 2006), resulting in its height and HU value reduction. Therefore, cassava starch coatings mixed with essential oils may have delayed the protein degradation process that occurred in the albumen during the 35 days evaluated at 20°C. This is corroborated by the high HU values (A, high quality) obtained in coated eggs compared to uncoated eggs (B - average quality) on the last day of analysis (Table 1). Other studies with HU of eggs stored for 5 or 6 weeks have shown that coatings composed of rice protein or sweet potato starch plus essential oil are another means of prolonging undesirable alterations in egg quality (Eddin and Tahergorabi, 2019; Pires et al., 2020).
Yolk index (YI)
The responsibility in eggs handling is probably one of the most critical issues to avoid a sudden reduction in egg quality and, consequently, in YI. The reduction in YI (i.e., reduced yolk quality), which occurs due to the movement of H2O from albumen to yolk after chemical processes (Obanu and Mpieri, 1984), is significantly higher at room temperature than under refrigeration (Oliveira et al., 2020). Therefore, the temperatures at which eggs are laid on farms, markets, and homes require strict monitoring and conservation strategies. In general, in fresh eggs, the YI ranges from 0.30 to 0.50 (Santo et al., 2017). In this scenario, the tested coatings were effective in minimizing the loss of yolk quality throughout the study. Espíndola et al. (2019) observed that eggs coated with cassava starch at concentrations 3 and 5% and stored for 28 days at 20°C had higher YI (mean = 0.36 ± 0.01), that is, better yolk quality than uncoated eggs (0.31 ± 0.01) and stored under the same conditions. These results reinforce the importance of using egg coatings.
Albumen and yolk pH
The output of H2O and CO2 from the egg contents to the external environment promotes an increase in the pH of the albumen by unbalancing the carbonic acid (H2CO3) buffering system (Cedro et al., 2009). Therefore, the tested coatings may have contributed to the conservation of the buffer system by reducing the release of H2O and CO2 from the interior of the eggs. This was evidenced by the lower pH values of albumen in coated eggs. As well as the reduction in YI, the slight increase in yolk pH is also due to the movement of H2O from the albumen to the yolk (Soares et al., 2021). As the coated eggs had lower yolk pH values, this study assumes that the coatings reduced the movement of H2O to the yolk. Based on albumen and yolk pH, eggs coated with cassava starch plus essential oils had better internal quality than uncoated eggs during the experimental period. These findings agree with the results of Yüceer and Caner (2020).
Yolk color
In this study, the coatings did not influence the yolk color. Caner (2005) also reported that yolk color did not differ between coated (whey protein isolate, chitosan, and shellac) and uncoated eggs during 4 weeks of storage at 25°C. The different values of b* obtained between this study and Caner (2005) may be associated with the rearing system and the poultry feed. Suresh et al. (2015) also found no difference in yolk color between uncoated and chitosan-coated eggs during storage for 5 weeks at 22 ± 1 and 32 ± 1°C. The temperature and storage time also apparently do not affect yolk color when comparing the present study with the studies by Caner (2005) and Suresh et al. (2015). Research is encouraged to investigate the intrinsic and extrinsic causes that affect yolk color during egg storage to truly understand the relationship between coating use and yolk color.
Conclusions
Cassava starch coatings added with essential oils preserved the internal quality of the eggs during storage for 35 days at 20°C, which were classified as excellent quality (AA) (until day 28) and high quality (A) (only on the 35th day) according to the degree of freshness. In addition, the coatings effectively reduced the eggshell microbiota, keeping it at low levels during storage. Therefore, these coatings are promising materials that can somehow replace or compensate for the lack of refrigeration, but it is important for future studies to verify if they affect the sensory quality of eggs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GO: conceptualization, methodology, formal analysis, investigation, writing-original draft preparation, and writing—review and editing. CM: validation, writing—review and editing, and supervision. PP: validation and writing—review and editing. VS: conceptualization, methodology, validation, formal analysis, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, M. M., Yusuf, M. A., and Abdalaziz, M. N. (2017). GC-MS Analysis and antimicrobial screening of essential oil from lemongrass (Cymbopogon citratus). Int. J. of Pharm. Chem. 3, 72–76. doi: 10.11648/j.ijpc.20170306.11
Alleoni, A. C. C.. (2006). Albumen protein and functional properties of gelation and foaming. Sci. Agric. 63, 291–298. doi: 10.1590/S0103-90162006000300013
Ananey-Obiri, D., Matthews, L., Azahrani, M. H., Ibrahim, S. A., Galanakis, C. M., and Tahergorabi, R. (2018). Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 80, 167–174. doi: 10.1016/j.tifs.2018.08.012
Andrade, B. F. M. T., Barbosa, L. N., Probst, I. S., and Fernandes, A. Jr (2014). Antimicrobial activity of essential oils. J. Essent. Oil Res. 26, 34–40. doi: 10.1080/10412905.2013.860409
Bauer, A. W., Kirby, W. M., Sherris, J. C., and Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. doi: 10.1093/ajcp/45.4_ts.493
Biladeau, A. M., and Keener, K. M. (2009). The effects of edible coatings on chicken egg quality under refrigerated storage. Poult. Sci. 88, 1266–1274. doi: 10.3382/ps.2008-00295
Caner, C.. (2005). The effect of edible eggshell coatings on egg quality and consumer perception. J. Sci. Food Agric. 85, 1897–1902. doi: 10.1002/jsfa.2185
Cedro, T. M. M., Calixto, L. F. L., Gaspar, A., Curvello, F. A., and Hora, A. S. (2009). Internal quality of conventional and omega-3-enriched commercial eggs stored under different temperatures. Braz. J. Poult. Sci. 11, 181–185. doi: 10.1590/S1516-635X2009000300007
Dassanayake, R. S., Acharya, S., and Abidi, N. (2018). “Biopolymer-based materials from polysaccharides: properties, processing, characterization and sorption applications,” in Advanced Sorption Process Applications, ed S. Edebali (London: IntechOpen), 1–24. doi: 10.5772/intechopen.80898
Dini, I., and Laneri, S. (2021). The new challenge of green cosmetics: natural food ingredients for cosmetic formulations. Molecules 26:3921. doi: 10.3390/molecules26133921
Eddin, A. S., Ibrahim, S. A., and Tahergorabi, R. (2019). Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 296, 29–39. doi: 10.1016/j.foodchem.2019.05.182
Eddin, A. S., and Tahergorabi, R. (2019). Efficacy of sweet potato starch-based coating to improve quality and safety of hen eggs during storage. Coatings 9:205. doi: 10.3390/coatings9030205
Espíndola, M. H. M., Rodrigues, G. D., Dahlke, F., Hauptli, L., Cristofolini, A. C., Pires, P. G. S., et al. (2019). Use of coating based on cassava starch on the quality of freerange eggs stored at room temperature. Bol. Cent. Pesq. Proc. Aliment. 37, 59–67. doi: 10.5380/bceppa.v37i2.78307
Ezazi, A., Javadi, A., Jafarizadeh-Malmiri, H., and Mirzaei, H. (2021). Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens' eggs: optimization and characteristics edible coating of egg using chitosan and propolis. Food Biosci. 40:100894. doi: 10.1016/j.fbio.2021.100894
Feddern, V., De Prá, M. C., Mores, R., Nicoloso, R. S., Coldebella, A., and de Abreu, P. G. (2017). Egg quality assessment at different storage conditions, seasons and laying hen strains. Cien. Agrotec. 41, 322–333. doi: 10.1590/1413-70542017413002317
Funk, E.. (1948). The relation of the yolk index determined in natural position to the yolk index as determined after separating the yolk from the albumen. Poul. Sci. 27, 367–367. doi: 10.3382/ps.0270367
Lemes, R. S., Alves, C. C. F., Estevam, E. B. B., Santiago, M. B., Martins, C. H. G., dos Santos, T. C. L., et al. (2018). Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. An. Acad. Bras. Cienc. 90, 1285–1292. doi: 10.1590/0001-3765201820170847
Lima, V. V. C., Gomes, B. O., Pereira, M., Barros, E. K. C., Pereira, A. L. F., Silva, D. S., et al. (2020). Coating of cassava and yam starches in egg conservation. Res. Soc. Dev. 9:e23984949. doi: 10.33448/rsd-v9i8.4949
Macwan, S. R., Dabhi, B. K., Aparnathi, K. D., and Prajapati, J. B. (2016). Essential oils of herbs and spices: their antimicrobial activity and application in preservation of food. Int. J. Curr. Microbiol. App. Sci. 5, 885–901. doi: 10.20546/ijcmas.2016.505.092
Menezes, P. C. D., Lima, E. R., Medeiros, J. P., Oliveira, W. N. K., and Evêncio-Neto, J. (2012). Egg quality of laying hens in different conditions of storage, ages and housing densities. Rev. Bras. Zootec. 41, 2064–2069. doi: 10.1590/S1516-35982012000900014
Musgrove, M. T., Stephens, C. B., Bourassa, D. V., Cox, N. A., Mauldin, J. M., Berrang, M. E., et al. (2014). Enterobacteriaceae and Salmonella recovered from nonsanitized and sanitized broiler hatching eggs. J. Appl. Poult. Res. 23, 516–522. doi: 10.3382/japr.2014-00975
Nongtaodum, S., Jangchud, A., Jangchud, K., Dhamvithee, P., No, H. K., and Prinyawiwatkul, W. (2013). Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food Sci. 78, S329–S335. doi: 10.1111/1750-3841.12035
Obanu, Z. A., and Mpieri, A. A. (1984). Efficiency of dietary vegetable oils in preserving the quality of shell eggs under ambient tropical conditions. J. Sci. Food Agr. 35, 1311–1317. doi: 10.1002/jsfa.2740351207
Oliveira, G. D. S., dos Santos, V. M., and McManus, C. (2022). Propolis: effects on the sanitisation of hatching eggs. Worlds Poult. Sci. J. 78, 261–272. doi: 10.1080/00439339.2022.2003173
Oliveira, G. D. S., dos Santos, V. M., and Nascimento, S. T. (2021). Essential oils as sanitisers for hatching eggs. Worlds Poult. Sci. J. 77, 605–617. doi: 10.1080/00439339.2021.1959276
Oliveira, G. S., dos Santos, V. M., Rodrigues, J. C., and Santana, Â. P. (2020). Conservation of the internal quality of eggs using a biodegradable coating. Poult. Sci. 99, 7207–7213. doi: 10.1016/j.psj.2020.09.057
Pandey, A. K., Kumar, P., Singh, P., Tripathi, N. N., and Bajpai, V. K. (2017). Essential oils: sources of antimicrobials and food preservatives. Front. Microbiol. 7:2161. doi: 10.3389/fmicb.2016.02161
Pires, P. G. S., Leuven, A. F. R., Franceschi, C. H., Machado, G. S., Pires, P. D. S., Moraes, P. O., et al. (2020). Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poult. Sci. 99, 604–611. doi: 10.3382/ps/pez546
Robinson, D. S., and Monsey, J. B. (1971). Studies on the composition of egg-white ovomucin. Biochen. J. 121, 537–547. doi: 10.1042/bj1210537
Santo, L. S. E., Correa, G. S. S., Vieites, F. M., Romani, A., Tavares, J. M. N., Silva, G. M. M., et al. (2017). Performance and egg quality parameters of laying hens submitted to different rearing densities. Semin. Ciênc. Agrár. 38, 831–842. doi: 10.5433/1679-0359.2017v38n2p831
Sariyel, V., Aygun, A., Coklar, H., Narinc, D., and Akbulut, M. (2022). Effects of prestorage application of gum arabic coating on the quality of table eggs during storage. Kafk. Univ. Vet. Fak. Derg. 28, 363–370. doi: 10.9775/kvfd.2022.27077
Sasidharan, I., and Nirmala Menon, A. (2010). Comparative chemical composition and antimicrobial activity fresh and dry ginger oils (Zingiber officinale Roscoe). Int. J. Curr. Pharm. Res. 2, 40–43.
Shah, G., Shri, R., Panchal, V., Sharma, N., Singh, B., and Mann, A. S. (2011). Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemongrass). J. Adv. Pharm. Technol. Res. 2, 3–8. doi: 10.4103/2231-4040.79796
Silva, A. A., Bergamo, L., Camargo, L. P., Fernandes, C., Mussato, D., Canazart, D., et al. (2014). Atividade microbiológica de óleos essenciais obtidos por arraste a vapor [Microbiological activity of essential oils obtained by steam drag]. Uningá Rev. 20, 33–39.
Singh, G., Kapoor, I. P. S., Singh, P., de Heluani, C. S., de Lampasona, M. P., and Catalan, C. A. N. (2008). Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 46, 3295–3302. doi: 10.1016/j.fct.2008.07.017
Soares, R. A., Borges, S. V., Dias, M. V., Piccoli, R. H., Fassani, E. J., and da Silva, E. M. C. (2021). Impact of whey protein isolate/sodium montmorillonite/sodium metabisulfite coating on the shelf life of fresh eggs during storage. LWT 139:110611. doi: 10.1016/j.lwt.2020.110611
Sun, R., Song, G., Zhang, H., Zhang, H., Chi, Y., Ma, Y., et al. (2021). Effect of basil essential oil and beeswax incorporation on the physical, structural, and antibacterial properties of chitosan emulsion-based coating for eggs preservation. LWT 150:112020. doi: 10.1016/j.lwt.2021.112020
Suresh, P. V., Raj, K. R., Nidheesh, T., Pal, G. K., and Sakhare, P. Z. (2015). Application of chitosan for improvement of quality and shelf life of table eggs under tropical room conditions. J. Food Sci. Technol. 52, 6345–6354. doi: 10.1007/s13197-015-1721-7
Syafiq, R., Sapuan, S. M., Zuhri, M. Y. M., Ilyas, R. A., Nazrin, A., Sherwani, S. F. K., et al. (2020). Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: a review. Polymers 12:2403. doi: 10.3390/polym12102403
Tangpao, T., Charoimek, N., Teerakitchotikan, P., Leksawasdi, N., Jantanasakulwong, K., Rachtanapun, P., et al. (2022). Volatile organic compounds from basil essential oils: plant taxonomy, biological activities, and their applications in tropical fruit productions. Horticulturae 8:144. doi: 10.3390/horticulturae8020144
Upadhyaya, I., Yin, H. -B., Surendran Nair, M., Chen, C. -H., Lang, R., Darre, M. J., et al. (2016). Inactivation of Salmonella enteritidis on shell eggs by coating with phytochemicals. Poult. Sci. 95, 2106–2111. doi: 10.3382/ps/pew152
Vazirian, M., Kashania, S. T., Ardekania, M. R. S., Khanavia, M., Jamalifarb, H., Fazeli, M. R., et al. (2012). Antimicrobial activity of lemongrass (Cymbopogon citratus (DC) Stapf.) essential oil against food-borne pathogens added to cream-filled cakes and pastries. J. Essent. Oil Res. 24, 579–582. doi: 10.1080/10412905.2012.729920
Wang, H. H., Li, M. Y., Dong, Z. Y., Zhang, T. H., and Yu, Q. Y. (2021). Preparation and characterization of ginger essential oil microcapsule composite films. Foods 10:2268. doi: 10.3390/foods10102268
Wells, J. B., Coufal, C. D., Parker, H. M., and McDaniel, C. D. (2010). Disinfection of eggshells using ultraviolet light and hydrogen peroxide independently and in combination. Poult. Sci. 89, 2499–2505. doi: 10.3382/ps.2009-00604
Yüceer, M., and Caner, C. (2014). Antimicrobial lysozyme-chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 94,153–162. doi: 10.1002/jsfa.6322
Keywords: coating, egg quality, egg storage, essential oils, ginger, lemongrass, Tahiti lemon
Citation: Oliveira GdS, McManus C, Pires PGdS and dos Santos VM (2022) Combination of cassava starch biopolymer and essential oils for coating table eggs. Front. Sustain. Food Syst. 6:957229. doi: 10.3389/fsufs.2022.957229
Received: 30 May 2022; Accepted: 04 July 2022;
Published: 22 July 2022.
Edited by:
Sergio Torres-Giner, Universitat Politècnica de València, SpainReviewed by:
Ali Aygun, Selcuk University, TurkeySarana Rose Sommano, Chiang Mai University, Thailand
Copyright © 2022 Oliveira, McManus, Pires and dos Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vinícius Machado dos Santos, dmluaWNpdXMuc2FudG9zQGlmYi5lZHUuYnI=
 Gabriel da Silva Oliveira
Gabriel da Silva Oliveira Concepta McManus
Concepta McManus Paula Gabriela da Silva Pires
Paula Gabriela da Silva Pires Vinícius Machado dos Santos
Vinícius Machado dos Santos