
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 28 September 2022
Sec. Nutrition and Sustainable Diets
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.929308
This article is part of the Research TopicIndigenous Food Systems to Address Food Security and Nutritional StatusView all 6 articles
In many rural farming societies, wild plant foods (WPFs) continue to play an important role in everyday diets as well as in coping with hunger during food shortages. However, WPF collection and consumption may pose challenges to biodiversity conservation efforts (e.g., in protected areas), and some “famine foods,” foods not typically eaten under normal conditions, may have deleterious health impacts. Using data from a cross-sectional survey of 328 smallholder farmers and fisherfolk living in 15 villages surrounding Manombo Special Reserve on the southeastern coast of Madagascar, we examine the relationship between food security, dietary diversity, and consumption of WPFs, specifically giant aquatic arrowhead or via (Typhonodorum lindleyanum) and Polynesian arrowroot or tavolo (Tacca leontopetaloides), during the region's main lean season. We complement survey findings with focus group interviews to document traditional ecological knowledge and perceptions of these WPFs, including how tavolo and via are rendered edible, as well as human health effects from collecting, preparing, and eating them. Using multilevel logistic regression modeling, we found that consumption of these WPFs were significantly associated with inadequate nutrition among farmers. Wealthier households were less likely to consume these WPFs as a coping strategy during food insecure periods, while larger and more food insecure households were more likely to consume them. These findings reaffirm the importance of access to natural areas and support the design of protected area conservation strategies that honor local foodways and consider WPFs that serve as food safety nets for more vulnerable populations.
Smallholder farmers, generally cultivating multiple small plots totaling ten hectares or less, provide an estimated one-third of the world's food supply (Herrero et al., 2017; Ricciardi et al., 2018; Lowder et al., 2021), and up to 90% of the food in some parts of sub-Saharan Africa (SSA; Kaur, 2021). However, in what has been termed the “hungry farmer paradox” (Bacon et al., 2014), many smallholder farmers, despite growing crops for both subsistence and sale, remain impoverished, undernourished (Wiggins and Keats, 2013), and experience seasonal and/or chronic food insecurity (Mazoyer and Roudart, 2006; Alpízar et al., 2020). Food (in)security has been defined as not having “physical, social and economic access to sufficient, safe, and nutritious food that meets their dietary needs and food preferences for an active and healthy life” (World Food Summit, 1996).
During periods of food shortage (“hunger season”), when stores of staple crops have run low or are entirely depleted, traditional coping strategies among rural farming populations have included, in addition to eating seed stocks intended for future plantings (Minnis, 2021), increased collection and consumption of seasonally available wild, or uncultivated, foods (Erskine et al., 2015; Paumgarten, 2018). Specifically, when sufficient quantities of preferred staple foods become (temporarily) unavailable, farmers may turn to certain wild plant foods (henceforth WPFs) not regularly consumed, known as “famine foods” or “emergency foods” (Minnis, 2021). Indeed, wild foods are important for the diets of more than a billion people across the globe (Burlingame, 2000), including farmers. For example, consumption of WPFs has been extensively documented in agricultural environments (e.g., fields, fallows, pastures, etc.) throughout South and Southeast Asia (Price, 1997, 2006; Ogle, 2001; Cruz-Garcia and Price, 2011; White, 2014; Ray and Ray, 2022), as well as across Africa (Bharucha and Pretty, 2010). Powell et al. (2013) found that farmers in Tanzania gathered more wild foods from their farmland than from the forest. Thus, despite some dominant ideas on how food insecurity is addressed among farming communities, supplementing agricultural production with foraging as a short-term solution to hunger remains an important function of many rural societies and should not be overlooked (Hickey et al., 2016). Furthermore, an ability to obtain foodstuffs outside of subsistence production and markets contributes to indigenous food sovereignty, and also ensures a level of agency to manage food security challenges without solely relying on external assistance (e.g., food aid).
Despite the potential importance of WPFs in the food security and nutrition (FSN) of smallholder farmers, there is limited understanding of their role. This is largely due to agricultural and household nutritional surveys which have historically failed to collect information on wild food consumption (Erskine et al., 2015). Even the 2019 report on sustainable diets from the EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems omitted wild foods (Sax, 2019), highlighting how western assumptions on what constitutes everyday diets have colored the research and analysis on food acquisition and consumption. Furthermore, notwithstanding the continued existence of mixed foraging-farming subsistence modes, the major assumption has been that food security is achieved through cultivated food production (Tucker et al., 2010). And while there has been an uptick in studies on the contribution of wild foods to human diets in recent years (Minnis, 2021; Pieroni, 2021), research on rural populations, food security and dietary diversity has predominantly focused on agricultural systems (Bharucha and Pretty, 2010; Sunderland et al., 2013). Relatively little food insecurity work has looked at the “overlapping, interdependent, coequal and complementary” (Sponsel, 1989; cited in Bharucha and Pretty, 2010) roles of both farming and foraging on FSN in agricultural communities.
In addition, research has shown that living near forests and protected areas (PAs) is associated with greater dietary diversity and improved nutritional status (e.g., Blaney et al., 2009; Fungo et al., 2016; Rasolofoson et al., 2018). However, though buffer zones and “zones of utilization” within and around PAs have become increasingly common, many fortress-style PAs still forbid the collection of wild plants from within their boundaries. Despite evidence that reduced access to wild foods can negatively affect FSN, specifically micronutrient consumption (Johnson et al., 2013; Powell et al., 2015; Galway et al., 2018; Rasolofoson et al., 2020), the impact of PAs on food security, and human wellbeing in general, remains under-examined in the literature (Pullin et al., 2013; Jouzi et al., 2020). Of the studies focusing on wild food access in these spaces, most has focused on wildlife (e.g., Golden et al., 2011; Mavah et al., 2018). Thus, more research is needed to understand the role that access to WPFs plays in the FSN of communities living in and along the boundary of PAs.
Using a case study based on survey data and focus group interviews collected from smallholder farmers in rural Madagascar, we contribute to filling these gaps by documenting the two main WPFs important for populations living within proximity of a PA, as well drivers of their consumption and barriers to accessing them, during food insecure periods.
Madagascar, one of the most biodiverse countries on earth and a top global conservation priority (Mittermeier et al., 2011), is also one of the most impoverished and food insecure, with 30,000 people in near-famine conditions (World Bank, 2020). It has the fourth highest rate of chronic malnutrition in the world (IFAD, n.d.) and 42% of children under age five suffer from stunting [Institut National de la Statistique (INSTAT) and United Nations Children's Fund (UNICEF), 2019]. Most of the population (80%) are considered to be farmers (World Bank, 2020)—the vast majority of which are smallholders (Rakotobe et al., 2016), who are among the world's most vulnerable to climate change (Harvey et al., 2014). However, despite the prevalence of agriculture across the island, wild foods remain a prominent approach for coping with food insecurity (Golden et al., 2016; Randrianarison et al., 2020). Indeed, even before farming and herding came to the island, foraging was an important food procurement strategy for the Malagasy (Dewar et al., 2013). Thus, we argue that most of today's population actually falls along a forager-farmer continuum.
While the primary focus of this study is on WPFs consumed as famine foods, it is important to highlight that wild plants and animals are also important components of everyday Malagasy diets. For example, in the southwest, 77% of interviewed households had collected wild yam (Andriamparany et al., 2014), and across three eastern rainforest sites, Styger et al. (1999) documented 150 different wild fruit species being consumed both regularly as well as during periods of food shortage. Furthermore, it is still common to see a family walking home from a long day in their fields carrying wild greens along with small fish and crustaceans gleaned from the rice paddies, a practice which has also been documented among other rice cultures (e.g., Cruz-Garcia and Price, 2011; Ray and Chakraborty, 2021). Therefore, as we assess consumption of WPFs in Madagascar as an indication of food insecurity, we also recognize the role of WPFs in providing important micronutrients (Ray and Chakraborty, 2021; Cantwell-Jones et al., 2022), as well as its ties to ancestral food pathways (Campbell et al., 2021), and cultural food identity (Tucker et al., 2010; Ghosh-Jerath et al., 2021).
Of the substantial body of research on wild food consumption in Madagascar, the majority has been on aquatic animal-source foods (Le Manach et al., 2012; Golden et al., 2019a; Taylor et al., 2019; AASFs) and wild terrestrial animals, or bushmeat (Golden et al., 2011, 2016), such as lemurs (Golden, 2009; Borgerson et al., 2017, 2018, 2022), tenrecs (Stiles, 1991; Golden et al., 2014b), small carnivores (Farris et al., 2015), bats (Jenkins and Racey, 2008; Golden et al., 2014a), and frogs (Jenkins et al., 2009). And while rural communities in Madagascar still depend heavily on wild plants, not only for the provisioning of food, but also for fuel and fiber (Ingram and Dawson, 2006; Brown et al., 2011), most of the ethnobotanical research has been limited to their use in traditional medicine (e.g., Rasoanaivo, 1990; Novy, 1997; Golden et al., 2012; Razafindraibe et al., 2013; Rabearivony et al., 2015; Riondato et al., 2019; Tida et al., 2020). Furthermore, as researchers have observed a distinct loss in traditional ecological knowledge (TEK) of WPFs from older to younger generations of Malagasy (Styger et al., 1999), there is an increasing need to document TEK on “neglected” plant species important for FSN (Baldermann et al., 2016), including their identification and preparation (Mbhenyane, 2017; Pawera et al., 2020; Minnis, 2021).
In our study area, the two main wild plants that are typically consumed during the hungry season are the giant aquatic arrowhead or water banana, locally known as via (Typhonodorum lindleyanum, also called T. madagascariense; family Araceae) (Figure 1A), and the Polynesian arrowroot, locally known as tavolo (Tacca leontopetaloides; family Taccaceae) (Figure 1B). Little is understood of their nutritional value, though all parts of the via plant are known to contain calcium oxalate crystals, making it toxic if not cooked or fully dried, and particularly concerning for people suffering from conditions related to the buildup of uric acid, such as rheumatoid arthritis and gout (Bown, 1995). Tavolo, which has been naturalized in Madagascar, is typically found in tropical forest openings and grasslands (Missouri Botanical Gardens, n.d.). Via, a wild aroid native to Madagascar and South Africa (Croat and Ortiz, 2020), grows in marshy areas. Both are used as medicinal plants: tavolo to treat malnutrition, and via to aid in placental evacuation, alleviate hip problems (Razafindraibe et al., 2013), treat burns and wounds (Rabearivony et al., 2015), and as a remedy against venomous bites (USDA Bureau of Plant Industry, 1919). There is also evidence of via being consumed as a famine food in both Zanzibar (Walsh, 2009; Freedman, 2019) and Zimbabwe (Manduna and Vibrans, 2018). Additionally, via leaves are used in woven handicrafts across eastern Madagascar.
In this paper, a mixed methods approach is taken to examine the relationship of tavolo and via to food security and dietary diversity (a proxy for nutritional status) among farming and fishing communities situated around Manombo Special Reserve in southeastern Madagascar, as well as predictors of their consumption. The research was guided by the following questions: (1) What is the relationship of WPF (tavolo and via) consumption to food security and nutrition (FSN) outcomes?; (2) To what extent are WPFs consumed as a food insecurity coping strategy, and what factors predict the consumption of tavolo and via as a coping strategy?; and (3) How are these WPFs perceived by farmers, and what are their implications for health and biodiversity conservation?
Based on previous research findings (e.g., Niles and Salerno, 2018), we hypothesized that larger, poorer households would be more likely to consume WPFs as a food insecurity coping strategy. We also predicted that household food insecurity and consumption of wild plant famine foods will be associated with low dietary diversity/inadequate nutrition, while greater farm crop diversity and household wealth will be associated with higher dietary diversity levels/adequate nutrition (Faber et al., 2009).
Our study population consisted of rural smallholder farmers (growing on 10 Ha of land or less) and fisherfolk, who self-identified as being primarily of the Antaifasy (People of the Sand) ethnolinguistic group and sub-groups (e.g., Antevatobe, Rabakara, Zaravalala, Zaramanampy), living within 2 km of Manombo Special Reserve (5320 Ha) in southeastern Madagascar (Figure 2), which is among the most food insecure regions of the island (Randrianarison et al., 2020). Manombo, established in 1962, is an International Union for Conservation of Nature (IUCN) category IV protected area (PA). Managed by Madagascar National Parks (MNP), human entry into Manombo is regulated and extraction (hunting, tree cutting, etc.) is strictly prohibited. There is no buffer zone or “zone of utilization” associated with this PA. Communities are highly reliant on local food production and fisheries, as both market access, and agricultural extension services are extremely limited, and farmers employ traditional methods to grow rice and other crops, such as cassava, jackfruit, banana, breadfruit, etc. They also engage in cash crop production of coffee and cloves to a lesser extent.
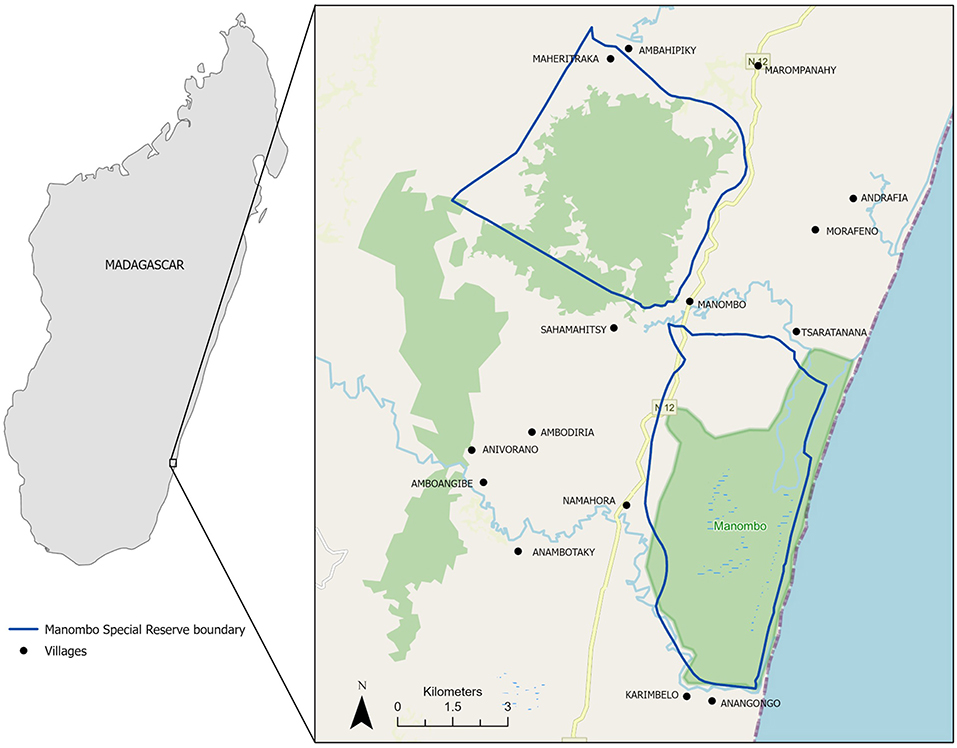
Figure 2. Map of study area with 15 survey villages, southeastern Madagascar. Black dots are villages with village names. Blue outline denotes Manombo PA boundaries. Green shaded area is remaining forest. Yellow line is RN12.
Data was used from a cross-sectional survey of male and female adult rice farmers (n = 328), each representing a separate and distinct household, living in 15 villages and sub-villages surrounding Manombo Special Reserve. Each village was assigned to one of three groups indicating increasing distance (Km) from coast (see Table 1). As our initial sample (n = 204) consisted of participants in a 2020–2021 rice-growing training, we wanted to ensure that we also sampled farmers that had not self-selected to participate in the training. Therefore, probability proportional to size (PPS) sampling (Skinner, 2014) was used to estimate the target number of remaining households within a given village of which to randomly select additional respondents—one per household (n = 124). To randomly select households and within-household respondents (i) a number was assigned to each remaining eligible household and a random number table was used to select households (World Health Organization, 2000), and (ii) to select the respondent when more than one adult (over age 18) in the household was present at the time of the interview, a “lottery method” of drawing a name from a hat was employed (Yadav et al., 2019).
Fieldwork took place over 3 weeks in February 2021, during the start of the region's main “hungry season” (sakave), as collecting data during this period is recommended to capture acute food insecurity (Coates et al., 2007). Exemption for this study was received from the University of Vermont's Institutional Review Board (IRB; study #00001290). Verbal informed consent was received from all participants and documented as per the IRB protocol. A trained team of five Malagasy enumerators conducted face-to-face interviews in Malagasy and recorded data on standardized paper surveys. The questionnaire consisted predominantly of closed-ended questions, took 60–90 min to complete, and collected household and farm characteristic information, as well as data on agricultural practices, household food insecurity and dietary diversity from a 24-h open-ended dietary recall (see Supplementary Table 1 for a subset of questions from the questionnaire). A team of Malagasy and American research assistants entered the responses from paper surveys into a digital format and conducted translations of qualitative data from the survey.
To better assess famine food consumption patterns and document traditional ecological knowledge (TEK) in the Manombo area, communities in which participants had reported consumption of tavolo and/or via, the only two wild plant species specifically mentioned in the dietary recall, were identified, and four focus group (FG) interviews were conducted in October 2021. In particular, we were interested to learn if tavolo and via, as well as other WPFs, were harvested from within the reserve or elsewhere, local opinions on consumption of these plants, which parts of the plants were edible, if there were any local taboos or stigmas surrounding their consumption, how they make people feel physically, and any other issues or concerns related to accessing WPFs. Each FG lasted 45–60 min and consisted of eight participants (four men and four women) selected by ampanjaka (village elders). Interviews were voice recorded, transcribed verbatim into Malagasy, and then translated into English by a native Malagasy speaker. Two of the FGs also included narrative walks to view plant habitats; short demonstrations of processing techniques were also filmed (see in Supplementary Videos 1–3).
Data on food consumption was gathered using an open-ended dietary intake over 24-h, following standard dietary diversity questionnaire procedures (Swindale and Bilinsky, 2006; FAO, 2010). Enumerators asked respondents to list the food items that they had eaten for breakfast, lunch and dinner, as well as any snacks eaten between meals, during the previous day. Food consumption information from those that responded positively to a question as to whether the previous day was a feast day, celebration or holiday was omitted from the analysis (n = 5). Responses were recorded in Malagasy and then translated into English. Translated data was then coded and used to generate food variety scores (FVS) from the number of unique food items consumed, and individual dietary diversity scores (IDDS) from the number of food groups in which consumed foods were classified under (Ruel, 2003), using IBM SPSS Statistics for Macintosh, Version 28.0. Both FVS and DDS are useful, simple indicators of micronutrient adequacy (Steyn et al., 2006).
Foods were classified into 13 out of 16 possible food groups suggested by FANTA (Swindale and Bilinsky, 2006; see Supplementary Table 2 for a list of food groups). Based on the findings of Moursi et al. (2008), we omitted group 14 (fats and oils). We also omitted groups 15 and 16 (sweets, spices, condiments, and beverages) as they are typically consumed in quantities too small to be nutritionally important (Faber et al., 2009), although when available, likely contribute greatly to palatability and food enjoyment. A score of 1 was entered if the respondent ate one or more foods within a food group, and a score of 0 was used to indicate an absence of any foods consumed within that group. We then calculated an individual dietary diversity score (IDDS) for each respondent from 0 to 13 (out of the 13 food categories), as well as generated a binary variable to represent dietary diversity scores of four and above or lower than four (1 = 4 and above, 0 = below 4), as consuming from four different food groups per day is generally accepted as the critical value for adequate nutrition (Steyn et al., 2006).
Respondents were surveyed on various food insecurity coping strategies, including famine food consumption (see Supplementary Table 1). Specifically, respondents were asked if they had eaten any plant foods (such as tavolo or via) as a food insecurity coping strategy in the last 12 months. Given the custom of liquidating assets, such as large livestock, as a food insecurity coping strategy (e.g., Dercon, 2002), and the high socio-cultural and economic value of zebu cattle (Bos indicus) in Malagasy society (Fauroux et al., 1990), we also examined the interaction between the number of zebu owned and household food insecurity as it relates to WPF consumption.
To gauge the complexity of the local agri-food system, survey respondents were asked about their farming practices, including the types of crops grown, and type and number of livestock owned. Farm production diversity was calculated by counting the number of food crops that respondents reported growing on their farm, out of a list of 12 possible crops (see Supplementary Table 1). Data was also collected on distance (measured in minutes walking) of nearest and farthest rice fields, cash crop (vanilla, coffee and cloves) engagement, as well as household size and type (female-headed or not).
Following methods developed by Demographic and Health Surveys (DHS; Rutstein and Johnson, 2004) to assess household wealth, an asset index was created by asking questions regarding ownership of durable assets such as radio, cellphone, bicycle, dugout canoe, etc. as well as ownership of specific agricultural tools such as machete, spade, and ox cart (see Supplementary Table 1 for a complete list). The presence of each of these assets was aggregated as a count variable from 0 to 30. In addition to the asset index, the number of large livestock owned—in this case, zebu—as a continuous variable, and land ownership as a binary variable, were also included.
To measure household food insecurity, respondents were asked five yes-no questions about their experience with food insecurity in the last 30 days (see Supplementary Table 1). Affirmative responses were used to generate a food (in)security score expressed in numerical values ranging from 0 to 5. The first question, “In the past 30 days, have household members ever had to eat meals without rice?” was included because of the cultural significance of eating rice, Madagascar's staple food.1 The second question, “In the past 30 days, have you ever feared that your food supply would run out?” comes from FAO's Food Insecurity Experience Scale (FIES) and is used to measure concern or anxiety over having sufficient food. The last three questions comprise the three-question Household Hunger Scale (HHS), a subset of USAID's Household Food Insecurity Access Scale (HFIAS) which has been validated across seven countries (Deitchler et al., 2011). A recall period of 30 days is a standard way to capture food security and has been validated for HFIAS (Coates et al., 2007). As is standard in many food security assessments (e.g., USDA six-item food security module; Bickel et al., 2000), we then categorized households into three food (in)security categories based on the number of affirmative responses to our questions: food secure (answering “yes” to zero or one of the five insecurity questions), moderately food insecure (answering “yes” to two or three questions), and very food insecure (answering “yes” to four or five questions).
Using a generalized linear mixed (GLM) model function in IBM SPSS Statistics for Macintosh, Version 28.0, we fitted two multilevel mixed effects logistic regression models to analyze the relationship between food security, dietary diversity and WPF consumption where all households are clustered at the village level. Correlation analysis using Spearman's rho was used to determine relationships between variables to be included in the models. Lowest Akaike Information Criteria (AIC) score and highest percent correct were used to select the most parsimonious, best fitting models.
Table 1 lists both the outcome and predictor variables included in the two models. The first model used adequate nutrition based on independent dietary diversity scores (IDDS; IDDS of 4 and above) as a binary dependent variable to analyze the relationship of consuming WPFs and food security on the odds of having adequate nutrition. The second model was fitted with WPF consumption as a binary dependent variable. Both models included four variable types to document geographic, socioeconomic, agricultural, and diet/food insecurity variables following what has commonly appeared in the food security literature. WPF consumption was also included as a predictor variable in the first model; IDDS and the interaction term between zebu ownership and food insecurity were included as predictor variables in the second model. Engaging in cash crop production and selling crops were significantly correlated with farm production diversity and therefore, not included in the models. All continuous variables were standardized before analysis (z-score transformation). To account for variation across villages, we treated them as a random effect.
Both logit models take the same basic form:
where p is the probability of the outcome variable being equal to 1, or p = P{Y = 1}, β0 is the overall intercept, and β1 and β2 are the coefficients for the fixed predictor effects, V is village predictor, HH is household predictor, eV is the random variation from village to village, and e(HH) is household, or residual, variability that cannot be explained by any other factor. The model assumption is that deviation from overall mean is the same for all households on average. We can make this assumption because we have already accounted for the fact that some combinations of household measurements are more similar by including the extra village error term.
Transcript-based analysis was used to analyze the word-for-word written record from the audio recordings of the focus group (FG) interviews. Using classic analysis strategy for analyzing FG results (SAGE, 2015), responses were organized according to themes, and compiled into a written descriptive summary including English translations of direct quotes from FG participants.
Our sample was 64.5% (n = 211) female and 35.5% (n = 116) male, with ages ranging from 18 to 71 years (mean age of 35.7 years). Table 2 provides details on household type and size. Households were primarily male-headed (81%, n = 262), and size ranged from one to 20 individuals (adults and children), with an average of 6.2 individuals per household. Three-quarters of all respondents (75.6%, n = 248) identified farming/agriculture as their primary occupation. Weaving mats was a primary occupation for 13.7% (n = 45), while only 4% (n = 13) of respondents identified fishing as their primary occupation.
Manombo area households exhibited low household wealth, as evidenced by the low number of assets owned (see Table 2). The most commonly owned household items were a bed, radio, and cellphone. The most commonly owned agricultural tools were a spade (“his spade is a matter of pride to every [Malagasy] farmer,” Linton, 1927, p. 655), machete, and ax.
Most respondents (88.3%; n = 287) reported owning multiple small parcels of land (10 Ha or less) under traditional tenure (see Table 2), as opposed to growing on rented cropland. However, of those with land, just over half (53.3%; n = 153) had a title deed for the land. Distance to rice fields averaged between 30 and 60 min walking time to nearest and furthest fields, respectively.
Farmers grew 8.13 (s.d. 2.64) different food crops on average (see Table 2). All respondents grew rice, with 95.7% (n = 314) practicing rice paddy cultivation and 43.9% (n = 144) practicing upland rice production. After rice, cassava was the most commonly grown food crop, followed by jackfruit, bananas, breadfruit, pineapple, avocado and litchi. Many respondents also engaged in cash crop production (71.3%; n = 234), with most growing coffee (63.7%; n = 209), cloves (52.4%; n = 172), and vanilla (29.3%; n = 97) to a lesser extent. While growing predominantly for subsistence, 53.9% (n = 174) of respondents reported selling crops locally; only 0.6% (n = 2) reporting selling crops at the national level or for international export.
There are two rice growing seasons in this area. Half of all respondents (50.6%, n = 166) only grow rice during vary vatomandry, the primary rice-growing season (see Supplementary Figure 1); 46% (n = 150) grow in both vary vatomandry and varihosy.2 Very few respondents (1.8%, n = 6) grew rice during varihosy season alone. On average, respondents reported harvesting 24.9 and 11.2 daba3 of rice during vary vatomandry and varihosy, respectively.
In general, area rice production falls short of meeting farmers' needs. Figure 3 shows the percentage of farmers buying and selling rice per month. Nearly three-quarters of respondents (73.8%; n = 239) indicated that they had not sold any of their rice harvest in the previous year, and almost all reported needing to buy rice at some point during the year (98.5%, n = 322). Furthermore, as is common practice across Madagascar, farmers reported selling rice at a lower price (471 Ariary/kapoaka on average)4 during harvest season and then buying it back later at a higher price (544 Ariary/kapoaka on average).
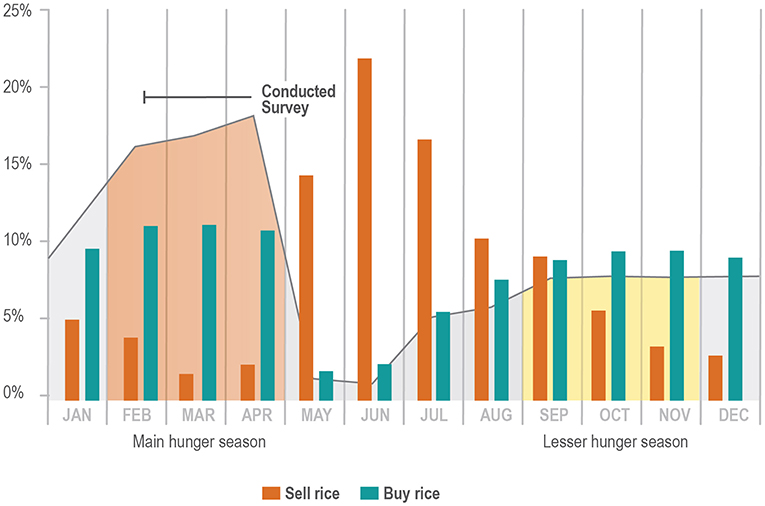
Figure 3. Percentage of farmers buying and selling rice per month; Percentage of households reporting food insecurity by month. Black line indicates 3-week time period in which survey was conducted, which is the beginning of the “hunger season” in which rice stores run low or are completely depleted.
Poultry was the most commonly owned livestock type (74.7% of households; n = 242), followed by pigs (35.8%; n = 116) and zebu (25.9%; n = 84). The number of zebu owned varied by household, from zero to 40, with an average of 1.19 zebu per household (s.d. 3.38; see Table 2).5 Livestock was more important for income generation than home consumption, with 82.3% (n = 219) of respondents reporting selling livestock compared to 64.7% (n = 172) of respondents reporting livestock used for feeding the family.
A total of 31 distinct foods were listed as being consumed over a single 24-h period during the month of February (see Supplementary Table 2),6 with a mean food variety score (FVS) of 3.68 (s.d. 1.27; see Table 2). Typical of the Malagasy diet and in line with what Randrianarison et al. (2020) previously reported in two Manombo area villages, respondents consumed a monotonous diet predominantly consisting of starchy staples (rice, breadfruit, and cassava), and low in animal and plant protein and non-starchy vegetables (see Supplementary Table 2). As surveys were conducted during breadfruit season, breadfruit was a common substitute for rice.7 Cassava (tuber) was also consumed frequently but to a lesser extent. As February is the beginning of the ~3-month hungry season, only 9.6% (n = 31) reported eating no rice in the 24-h period, indicating that rice stocks had not been depleted at the time of the survey.
The main source of protein was from aquatic animal-source foods (AASFs) or blue foods (19.3%; n = 62). Just 2.8% (n = 9) of respondents reporting consumption of domesticated animal protein (zebu, pork, chicken, and eggs). There were no reports of bushmeat consumption, despite recent alerts raised by American researchers of increased lemur hunting within the reserve (M. Donohue, personal communication, Feb. 2020), and previous documented cases of subsistence hunting of lemurs and tenrecs (Johnson and Overdorff, 1999). Though entomophagy (insect consumption) is also part of Malagasy culinary tradition (Borgerson et al., 2021; Conti et al., 2021b), our data does not reflect this.
Foods collected from the wild comprised five of the 31 (16.1%) distinct food items listed in the 24-h recall—three (9.7%) were blue foods and two (6.5%) were wild plants (tavolo and via). However, while consumption of one or both of these plants was reported by only 2.5% (n = 8) of respondents over the 24-h period (see Supplementary Table 2), 55.3% (n = 177) of households reported consuming tavolo and/or via as a food insecurity coping strategy during the last 12 months (see Figure 4). In February, at the start of the main hunger season, many households still have access to breadfruit (see Supplementary Figure 1) and tavolo tubers may not yet be mature. Therefore, reliance on via and tavolo likely increases later in the season, as rice stocks become depleted.
Overall, dietary diversity of individual respondents was nutritionally inadequate, with an average individual dietary diversity score (IDDS) of 3.22 (s.d. 0.99; see Table 2); 65.9% of respondents (n = 216) had an IDDS below four (see Table 2). Figure 5 shows the percentage of respondents consuming foods from each of the 13 food groups. The majority of respondents consumed foods from three food groups (cereals, white roots & tubers, dark green leafy vegetables). Consumption of foods from several food groups were not reported by any respondents (dairy, organ meats) or were reported by a single respondent (eggs, vitamin A-rich vegetables).
Results from the five-item food insecurity experience questionnaire indicated a high level of food insecurity among Manombo area households in the 30 days preceding the survey (see Table 3), with 91.1% (n = 296) of respondents fearing running out of food, 82.2% (n = 267) having gone without eating rice, and 66.7% (n = 217) having gone to bed hungry in the previous month. As shown in Table 4, nearly half of households were categorized as being very food insecure (47.3%, n = 155) in the previous month, with 36.9% as moderately food insecure (n = 121), and just 14.9% (n = 49) as food secure. Mean IDDS also decreased with increasing levels of household food insecurity, and on average, moderately and very food insecure households owned less zebu than more food secure households (see Table 4).
Figure 3 shows the percentage of respondents experiencing food insecurity by month, with the period from February—April having the highest prevalence of food insecurity.8 There was also a marked decrease in food insecurity reported in May and June which coincides with the May/June vatomandry rice harvest. Higher rates of food insecurity are also evidenced by the percentage of farmers reporting needing to buy rice, vs. the periods of time when rice is plentiful enough to sell (see Figure 3).
Out of a list of 10 pre-coded responses selected based on past experience and literature (see Supplementary Table 1), respondents reported employing a mean of 3.43 (s.d. 2.0) coping strategies in the previous 12 months (see Table 2). As shown in Figure 4, the most commonly reported strategy was working as an agricultural day laborer, followed by eating foods not normally eaten, such as tavolo and via. Of those that consumed WPFs, 38.1% (n = 67) and 50.0% (n = 88) were moderately and very food insecure, respectively, whereas only 11.9% (n = 21) of food secure households reported having consumed them (see Table 4), indicating that the consumption of tavolo and via are important coping strategies for households dealing with greater food insecurity. Other commonly cited strategies included obtaining food from relatives, reducing the number of meals per day and eating less food per meal. Weaving baskets and mats, using natural materials such as mahampy (Lepironia micronata) to generate additional income, was another common strategy cited as an open-ended response by female respondents.
In the first model, we analyzed the relationship between consuming WPFs and household food (in)security on the probability of having adequate nutrition (consuming foods from four food groups or more in a 24-h period). Overall, households reporting greater food insecurity (as compared to the baseline of more food secure households) and those that had consumed WPFs in the last 12 months as a food security coping strategy were both significant predictors of inadequate nutrition (p < 0.05; Figure 6). Specifically, the odds of having adequate nutrition were lower for both individuals from very food insecure households (OR = 0.338; CI [0.154, 0.739]; Table 5), and for individuals in households consuming WPFs (OR = 0.526; CI [0.299, 0.923]).
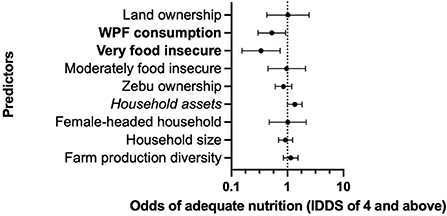
Figure 6. Standardized effects of predictor variables on adequate nutrition based on individual dietary diversity scores (IDDS) of four and above. Points represent the odds ratio estimates with upper and lower 95% confidence intervals. Bolded predictors indicate significance at the p < 0.05 level. Italicized predictors indicate significance at the p < 0.10 level. The value of 1 on the x-axis (dashed line) is equivalent to no effect. Results based on a multilevel random effects model of data from 302 respondents.
We also found that household wealth (measured using the household asset index) was a significant predictor of adequate nutrition (p < 0.10; Figure 6). Wealthier households had greater odds of having adequate nutrition than households with fewer assets (OR = 1.349; CI [0.995, 1.828]; Table 5). While not significant, farm production diversity was also associated with greater odds of adequate nutrition (OR = 1.145; CI [0.847, 1.547]). Lastly, as we would expect, we did not find any significant interaction effects between zebu ownership and household food insecurity on diet diversity outcomes (e.g., consuming foods from additional food groups) because zebus are not typically slaughtered for home consumption, even during periods of food insecurity.
In the second model, we examined predictors of consuming WPFs during periods of food shortage as a binary dependent variable, and found that larger households, female-headed households and households growing more types of crops were more likely to consume WPFs (Figure 7). Specifically, the model estimates that wealthier households are significantly less likely to consume WPFs (OR = 0.608, CI [0.432, 0.854]; Table 6), while farms with more diversified production (OR = 1.434, CI [1.056, 1.947]) and larger households are significantly more likely to (OR = 1.353; CI [1.001, 1.829]). Female-headed households were also associated with greater odds of WPF consumption than male-headed households (OR = 1.885; CI [0.879, 4.004]), while greater individual dietary diversity scores (IDDS) were negatively associated with WPF consumption (OR = 0.929; CI [0.695, 1.242]). Though not significant, both moderately food insecure (OR = 2.051; CI [0.828, 5.083]) and very food insecure households (OR = 1.302; CI [0.564, 3.009]) were more likely to consume WPFs than food secure households.
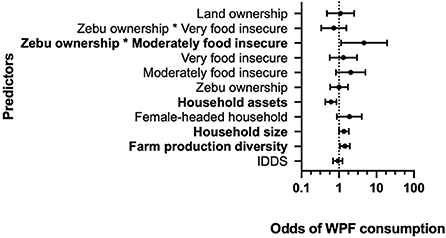
Figure 7. Standardized effects of predictor variables on consumption of wild plant foods (WPFs). Points represent the odds ratio estimates with upper and lower 95% confidence intervals. Bolded predictors indicate significance at the p < 0.05 level. The value of 1 on the x-axis (dashed line) is equivalent to no effect. Results based on a random effects model of data from 302 respondents.
In addition to the main effect estimates, there was a significant positive interaction effect between moderately food insecurity households that also owned zebu on WPF consumption, compared to food secure, zebu-owning households (OR = 4.661; CI [1.139, 19.083]; Table 6). This suggests non-linear effects, where zebu ownership itself is not a predictor of WPF consumption, but instead moderates the effect of household food (in)security in predicting the odds of WPF consumption (Figure 8).
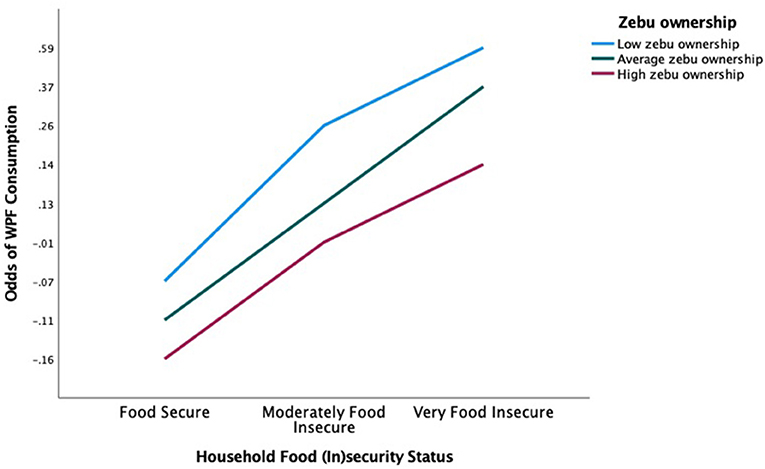
Figure 8. Interaction of number of zebus owned and food (in)security level on odds of WPF consumption. This plot of simple slopes displays the odds of WPF consumption among the three food (in)security categories on the x-axis, and a separate line for each level of zebu owned (mean, 1 SD above, 1 SD below).
As indicated by the results of the surveys, focus group participants articulated their struggles with food security and the niche that WPFs, specifically tavolo and via, fill when preferred staple foods (i.e., rice) are not available. Therefore, while these WPFs are part of the overall diet, they do not function as dietary or culinary staples. Ultimately, the complexity of harvesting and processing, the implications for health, the hierarchy of famine foods, and changing access to certain lands emerged as crucial.
There are customary practices governing the timing of WPF collection. Specifically, as Randrianarison et al. (2020) also found, local regulations dictate when via harvest may occur. It is fady (taboo) to collect via during the May-June rice harvest period because, we were told, doing so will cause the rice crop to be destroyed by hail. Similar restrictions on harvesting tavolo were not uncovered. Collection of via may start as early as November, but tavolo is typically harvested starting in late February/early March.9
As via grows in marshy areas, its collection can be demanding, often necessitating crossing waist- or even chest-deep water, and that collecting it makes the body itchy. Therefore, men are primarily responsible for digging it up. Tavolo is less challenging to collect, growing in joliky (coffee plantations around village) and roanga (grassy area or bush). However, respondents shared that by May/June, tavolo plants are already dried out, making it difficult to spot the plant and know where to dig for the tuber.
In the interviews, a tension surfaced between the existence of WPFs that are important for community food security, and yet, because of local conservation efforts, made inaccessible to them. One respondent evoked the collective memory of the long-standing prohibition of extracting WPFs, like oviala (generic vernacular for wild yam spp.), from the PA, as well as the frustration that much needed WPFs are “off limits” within its boundaries:
Before [the creation of the PA], people were able to enter the forest. Now it is not allowed anymore. Before there were a lot of oviala, enough for food. Now in this lean season, we should collect oviala but [Madagascar National Parks (MNP)] owns it now, forbidden. It's banned to enter the forest.
Similarly, while FG participants were aware of tavolo growing within Manombo Reserve, they also knew that they were not allowed to collect it, and expressed fear of retribution:
I work there in the reserve, but we don't collect it from there…We will get into trouble if [MNP] learns that we said there is no tavolo in the reserve. There is [a] cleared area in the reserve, and there are tavolo in that area.
At the same time, community members indicated a growing struggle to find sufficient amounts of WPFs outside of the reserve. In what Hardin (1968) famously termed the “Tragedy of the Commons,” tavolo and via are becoming increasingly scarce as greater numbers of people collect them. To cope, respondents reported initiating some limited management of these resources, e.g., transplanting the sauvageons (wild seedlings) of tavolo into their cassava fields and joliky. Via is also occasionally planted—for food as well as to prevent soil erosion around earthen irrigation dams—in marshy areas not under rice cultivation. Thus, while blurring the line between “wild” and “semi-domesticated” plants (see Bharucha and Pretty, 2010), these practices also speak to human resilience and ability to innovate when overharvesting of a common resource poses a problem.
Some WPFs have toxic, anti-nutritive factors that cause adverse effects on human health and thus require significant processing to make palatable (Ocho et al., 2012; Minnis, 2021). This is the case with via tuber, which must first be peeled and cut into pieces, then crushed, dried in the sun, and pounded into a powder. The powder is then made into bonoky (mixture wrapped in leaves of banana, ravinala or longoza—Aframomum augustifolium—and boiled in a pot of water). To eat the seeds of the via fruit, the fruit bunch (Figure 9A) is peeled and the grains are removed (“your hand becomes itchy because of the liquid from it”). The first layer of the seeds is removed using wood ash; then the “eye” of the seed is removed (see Supplementary Video 1). The remaining “core” is then washed and boiled in water until all of the water evaporates. It is then either dried and stored for several days, or more water is added, and the process is repeated two to three times until the bitterness is removed. The resulting seed mush is then eaten immediately.

Figure 9. (A) Peeled via flower with seeds (B) Immature tavolo tuber, (C) Tavolo tuber being processed, (D) Tavolo “pancake”, (E) Tavolo powder sold in market.
After digging up the tuber (Figure 9B), tavolo is washed and then crushed using a rock or fandra (local crushing tool). It is then mixed with water and placed in a piece of cloth to strain out the water (see Figure 9C; see Supplementary Video 2). The solid residue is discarded, and the remaining solution is placed in either a plastic bucket, or a local “bucket” made from ravinala (Ravenala madagascariensis). Once the water separates from the solid, the water is poured out again and replaced with clean water. This process is repeated several times, depending on how mature the tuber is, to remove the bitterness. The resulting paste is then pressed thin onto the inner side of a pot lid and placed over the fire (see Supplementary Video 3). The tavolo “pancake” (Figure 9D) is often eaten immediately but can last up to 1 week by drying it in the sun. One respondent said, “It's good if we eat them for 3 days or sell…then collect again.” Men are mainly responsible for crushing the tubers, while women do the “twisting” to wring out the water.
While we did not find any social stigmas related to local consumption of either of these two WPFs, we were told that there is shame associated with preparing via for guests, especially foreigners (both Malagasy and non-Malagasy from other parts of the island). One FG participant told us, “I am shy [to serve you via] because I should give you rice with chicken, but between us [community members], there is no shame to share it.” This corresponds with a marked preference for tavolo over via, as well as a preference for via seeds over via tuber (“we don't hope to eat it”).
Furthermore, while both WPFs may be found in the market, tavolo is more commonly bought and sold. As one farmer explained: “People rarely sell via … people are shy… people don't sell via around here, because no one will buy it here.” Respondents reported selling tavolo powder (Figure 9E) for 200–300 Ariary per kapoaka in order to buy basic necessities such as rice, salt and sugar. Another FG participant told us, “We sell them because our kids are hungry—kids get hungry even eating tavolo—so you have to sell it to get rice.”
Respondents shared that, unlike tavolo which they feel makes them “stronger” because of its “vitamins” (“even a baby can eat it, the baby gains weight even if they don't breastfeed”), they only eat via tuber when they are “afraid to die,” “it's real starvation,” and when they “don't have [any other] choice.” Reported side effects of collecting and consuming via include contact dermatitis (from collecting and preparing) and itchy throat (from consuming).
Not only do nutritional and anti-nutritional qualities vary between plant species, but they can also differ among parts of the same plant (Read, 1946; cited in Minnis, 2021), such that certain parts of a plant are more preferable than others and involve different techniques to remove toxins. Thus, respondents told us that they preferred via seeds to via tuber. Consumption of via tuber reportedly results in extreme weakness and trembling, facial swelling, stomachache and diarrhea. One respondent described the horrible outcomes of consuming via: “You can't even ride a bicycle…because it sucks your blood. It's not a real food…it has no vitamins, so sad!” Another respondent explained the potential consequences of consuming via:
Only hard-working people can eat via [tuber]. If you eat too much via but you [are] just sitting, not working, your stomach is heavy. It's ok if you go to the field and plant cassava for example. If you just sit, you will have digestion problems.
Nevertheless, despite its deleterious effects on human health, via serves as an important stopgap measure to save communities from hunger and starvation when desirable foods are unavailable.
Forest fringe communities living around Manombo Protected Area had very low food variety (FVS) and dietary diversity scores (DDS) during the main hungry season, indicating micronutrient inadequacy in the diet (Steyn et al., 2006). Individuals had lower average FVS and DDS than has been reported elsewhere in sub-Saharan Africa (SSA; Supplementary Table 3), with the exception of children from rural Burundi and Rwanda (Custodio et al., 2019). As many of these studies have focused on children's diets, comparisons are difficult. Nonetheless, our results (mean DDS of 3.22) match the average DDS reported by Niles et al. (2021) for children five and under in 19 low and middle income countries. Our findings are also in line with the Global Food Security Index (GFSI) which, based on consumption of non-starchy foods, classifies Madagascar as having “very weak” dietary diversity (GFSI, 2021).10
The causes of low dietary diversity are complex. As Farris et al. (2019) found among primary caregivers in the Betampona area of eastern Madagascar, dietary diversity was not a major driver for food selection (either purchasing or preparing), underlining how different cultural beliefs surrounding what constitutes “nutritious” and “healthy” foods affect dietary diversity and nutrition outcomes. In Tanzania, Keding et al. (2012) found that dietary diversity was more dependent on the purchase of foods than from on-farm production diversity. Thus, the underlying causes of the low dietary diversity scores recorded in Manombo may be a combination of low farm productivity, seasonality, limited market access and financial capital to purchase a variety of foods, as well as cultural views on adequate meal composition.
In addition to having very low FVS and DDS, Manombo area communities are heavily reliant on natural resources for their food security needs, mixing foraging with farming in order to cope with hunger. In between harvests, when rice stocks have run low or are completely depleted, WPFs clearly fill a gap in the local food environment, evidenced by over half of surveyed households eating WPFs in the last 12 months as a food insecurity coping strategy. Similarly, among subsistence farming communities in Timor-Leste, Erskine et al. (2015) document that 50% of households foraged for WPFs during periods of food insecurity. Moreover, our finding that weaving baskets and mats made of reeds growing in marshy areas in and around the reserve, to sell during periods of food shortage, further highlights the importance of access to natural resources in coping with depleted stores of staple foods. However, based on the qualitative data from our focus group interviews, we find evidence that some wild plants are becoming increasingly scarce, which may compound food insecurity severity.
The early twentieth-century American anthropologist, Ralph Linton, recorded that “poor [Malagasy] people eat boiled greens with their rice while the rich have meat or fish” (Linton, 1927, p. 658). A century later, we find that Linton's observation of WPFs being “poor man's food” (Andriamparany et al., 2014) persists. In our study population, wealthier households were significantly less likely to consume WPFs. Consistent with extensive findings in the literature (e.g., Faber et al., 2009), wealthier households were also significantly more likely to have adequate nutrition, indicating that poorer households are consuming a more limited variety of foods. Indeed, we found that Manombo households had very monotonous diets high in starchy carbohydrates, owned very few durable assets, and had extremely high levels of food insecurity.
Furthermore, while many researchers argue that ownership of large assets, such as land and large livestock (e.g., Niles and Salerno, 2018; Anderzén et al., 2020) lowers food insecurity among smallholder farmers, we did not find these variables alone to significantly explain consumption of WPFs as a food insecurity coping strategy. Despite nearly 90% of respondents reporting owning their land in the customary sense (with over half having a title deed for the land), land ownership was not a significant predictor of adequate nutrition or WPF consumption; nor did we find owning large livestock (zebu cattle) to have a significant main effect on these two outcomes. Similarly, in a study among smallholder farmers in Central America, Alpízar et al. (2020) found no significant effect of land ownership on food security, and that selling livestock as a food insecurity coping strategy was used only infrequently. In addition, Bogale and Shimelis (2009) report that the number of oxen owned did not have a statistically significant effect on household food security in Ethiopia. Thus, as other studies have shown, zebu ownership alone is not protective against food insecurity and famine food consumption.
However, while zebu ownership alone has no significant main effect on the odds of WPF consumption, we found a more nuanced relationship in which food insecure households that own a larger zebu herd are less likely to eat WPFs than households that own less zebu (Figure 8). Indeed, in Madagascar, zebus are typically only slaughtered for ritual celebrations, or to pay for large expenses (e.g., school fees, purchasing land). They are not for home consumption. Thus, households with more zebu (wealthier, more food secure) are more likely to sell or slaughter a zebu during periods of food shortage than those with just one or two. This supports the buffer stock hypothesis, in which large livestock, such as zebu, are kept in reserve as a form of insurance to be sold off in times of hardship. This has been documented elsewhere in Madagascar (Hänke and Barkmann, 2017), as well as in other countries in SSA (e.g., Miura et al., 2012; Karanja Ng'ang'a et al., 2016).
Despite having diversified farming systems, Manombo area farmers still rely on WPFs to meet their food needs. Furthermore, while much of the literature indicates that diversified farming systems (i.e., cultivating a large variety of crops and/or raising multiple livestock types) is associated with food security (e.g., Silvestri et al., 2015; Adjimoti and Kwadzo, 2018) and greater dietary diversity/improved nutritional status (e.g., Jones et al., 2014; Makate et al., 2016; Luna-González and Sørensen, 2018; Sibhatu and Qaim, 2018; Nkonde et al., 2021), we find that crop diversity does not necessarily equate to better FSN outcomes, as the results of the second model show increasing on-farm crop diversity to be associated with increased likelihood of WPF consumption. As consumption of certain WPFs can be considered an indicator of food insecurity (Ocho et al., 2012), this finding adds nuance to the crop diversification conversation and highlights the complexity of the food environment due to seasonality (see Supplementary materials for a seasonal crop calendar).
While our analysis did not capture data on all of the underlying causes limiting local crop production, existing research documents three main reasons: low yields, small farm sizes and distant plots. Indeed, there exists a substantial “yield gap11” across all of Madagascar (e.g., Tucker et al., 2010). One of the reasons for this agricultural underperformance has been attributed to farm size. Herrera et al. (2021) report that over half of respondents in northeastern Madagascar ascribed their experiences of food insecurity to the limited size of their agricultural plots. Small farm size has also been found to have a significant negative effect on food security in other contexts as well (e.g., Ethiopia, Bogale and Shimelis, 2009). Alpízar et al. (2020) found that smallholders in Central America farming on “microplots,” many small plots spaced apart, faced more food insecurity than farmers with one larger plot. In Madagascar, as land is traditionally passed down from parents to children, smaller and smaller parcels are typically carved out in the process (Laney and Turner, 2015), and as we have demonstrated in our case study of Manombo, often involve substantial amounts of time to reach by foot.
In addition to the constraints of “microplots,” lack of productive land and appropriate technologies (e.g., climate-adapted seeds, organic fertilizers, grain storage), climatic conditions (e.g., moisture-stress; Waha et al., 2018), frequent natural hazards, such as cyclones (Harvey et al., 2014), fear of crop and livestock theft, and virtually no agricultural extension services all contribute to low yields. Limited access to—or embeddedness in—local and national markets also affects food shortage responses (Minnis, 2021). For example, in their study of smallholder farmers in Malawi, Koppmair et al. (2016) suggest that access to markets and inputs to increase productivity may be more important to dietary diversity than growing a diverse array of crops.
Considering our finding that larger households were significantly more likely to consume WPFs, constraints in the Manombo area may be “forcing” farmers, to grow a greater diversity of crops for food (harvest) security; yet they are unable to produce enough to meet their needs and must “resort” to wild harvesting, even when the food (e.g., via) is undesirable. Thus, rather than focus their limited resources (e.g., land, labor) on cultivating crops alone, Manombo area farmers engage in what we refer to as a “scattershot approach,” casting a wide net in terms of their food procurement options as a food insecurity coping mechanism under extremely risky conditions (Harvey et al., 2014). This scattershot approach is further evidenced by the average number of coping strategies employed (3.43), which is greater than has been reported elsewhere (e.g., average of 1.7 food insecurity coping strategies used after extreme weather events in Guatemala and Honduras; Alpízar et al., 2020). Therefore, where resources are extremely limited, traditional mixed farmer-forager approaches may be the best option, as foraging is more opportunistic (e.g., farmers can forage on their way to and from their fields) and often requires less time investment than farming. In their study among farming and foraging groups in southwestern Madagascar, Tucker et al. (2010) conclude that “more is not always better” (p. 384) and that farming is a riskier activity than foraging due to the uncertainty of agricultural harvests. They point out that, while agricultural harvests are limited to a certain number of days per year, one can hypothetically get up to 365 “harvest days” per year by foraging.
Contrary to adopting a scattershot approach, others have found that, in Madagascar, focusing more intensively on a smaller number of crops led to better food insecurity outcomes, particularly when those crops were sold. For example, Herrera et al. (2021) found that among smallholder farmers in northeastern Madagascar, half of their study population grew just two of the top crops (rice and vanilla) and that the probability of household food insecurity was lower when both of these crops had high yields. However, it is important to note that, as their study area is the leading region of vanilla production in Madagascar with a well-established international export market, these findings are not applicable to all parts of the country. It could also indicate that food security in this region is “market exposed” and subject to the vagaries of the global market. Indeed, there have been anecdotal reports of farmers in the northeast pulling up their vanilla vines to plant rice when the price of vanilla plummeted. Thus, as we have seen in our study of Manombo area farmers, increased crop diversity is not always protective from an FSN standpoint, though relying on too few crops may also be precarious.
There are several limitations to this study based on time constraints and other logistical considerations. As has been noted in other studies (e.g., Alpízar et al., 2020), the reporting of food insecurity experience can be subjective. However, the method used has been widely validated as a tool suitable for rapid assessment. Furthermore, we understand that a single 24-h dietary recall provides only a snapshot into an individual's dietary diversity and that recalls repeatedly collected over many months or seasons would provide a fuller understanding of variations in the diet (see Keding et al., 2012). In addition, no visual aids were used to assist respondents/enumerators, which might have alleviated any potential memory-related difficulties with recall. However, we feel confident that our results represent an accurate summary of dietary diversity for the study population during this time of year.
As Stone and Campbell (1984) lay out in their seminal work, there are always possibilities for misinterpreting the meaning of questions and responses in cross-cultural research, especially when using a survey developed by a Western researcher in one language (English) translated into another (Malagasy). We remain reflective about our translations (Helmich et al., 2017) and have attempted to reduce error by working closely with bilingual team members (two of the co-authors are fluent in both Malagasy and English) who are particularly familiar with the culture and context, and by employing Malagasy enumerators to conduct the survey. Furthermore, we recognize the role that social desirability bias may play in shaping some responses (e.g., not reporting certain behaviors, such as bushmeat consumption or collecting WPFs from within the PA). Lastly, while we did gather detailed information on land dedicated to paddy rice, our general assessment of diversified farm systems was limited to the number of different crops grown. A more robust understanding of the system would be gained through additional data on the extent to which each crop is cultivated (e.g., number of carreaux of cassava, avocado trees, or vanilla vines, etc.) and annual production/harvest amounts for all crops. Future data collection will more completely capture the underlying factors contributing to low yields, such as land size.
This paper provides additional evidence of the reliance on WPFs during periods of food shortage for certain forest-edge farming and fishing communities, and is the first to document the consumption of both nutritious (tavolo) and anti-nutritious (via) WPFs and the challenges experienced obtaining these foods—including inability to access certain WPFs from within protected areas (PAs)—in Madagascar. These results have policy implications for improving food security in Madagascar as well as in other countries where smallholders mix foraging with farming to meet their food security and nutritional (FSN) needs. Most notably, wild foods should be more fully integrated into FSN policies, such that agriculture is no longer the sole food procurement strategy considered, and WPFs are not denigrated as the “weeds of agriculture” (Grivetti and Ogle, 2000), but recognized for their important role in indigenous foodways (e.g., Barreau Daly, 2014; Huambachano, 2019). While much more research is needed to better understand the nutrient profile and preparation requirements of these often neglected plant foods, the Brazilian national Plants for the Future program is a successful example of how the creation of a nutritional composition database of native edible plants can be used to inform policies aimed at improving FSN while protecting biodiversity (Hunter and de Oliveira Beltrame, 2015).
However, as we have documented with via, not all WPFs are healthy to eat. Some may even be deadly, as is the recent case of five deaths attributed to consumption of toxic veoveo (Dioscorea sansibarensis) in the Manakara area of southeastern Madagascar after back-to-back cyclones decimated area food crops. Therefore, consumption of WPFs with deleterious health effects can be used to rapidly identify at-risk households and target interventions. Additionally, education campaigns informing communities about the dangers of consuming certain WPFs without proper preparation should be launched.
Furthermore, rather than adding new (and exotic) crops to a farmer's portfolio for better FSN outcomes, programs should support agriculturalists in increasing the yield potential of crops that they are already cultivating (Koppmair et al., 2016). This will, on the one hand, help diminish the need to eat foods that are harmful to human health, and on the other, prevent ecological damage like that described by Cheban et al. (2009) resulting from excavation of wild Dioscorea spp. We also recommend that programs promote cultivation of micronutrient-dense indigenous vegetables (Conti et al., 2021a), rather than more recently introduced vegetables (carrots, cabbage, etc.) that may not be adapted to local growing conditions or be culturally suitable. For example, nonprofit organizations in Madagascar are assisting in the cultivation of indigenous yams such as bodoa (Dioscorea sp.) which are good sources of fiber, potassium and other micronutrients (Jeannoda et al., 2010).
Other strategies to improve community FSN include national food policies supporting school-based food and nutrition programs sourcing local foods, which have been shown to improve FSN status of youth, while also providing support to farmers and increasing comprehension on components of nutritious diets. Successful examples of incorporating indigenous plants and local produce into school lunch programs include the Biodiversity for Food and Nutrition Project in Kenya (Hunter et al., 2017) and the Home-Grown School Feeding model developed in Ethiopia and expanding to neighboring countries in SSA (Wineman et al., 2022). Furthermore, access to veterinary medicine has been associated with reduced odds of food insecurity (Niles and Salerno, 2018). This coupled with improved animal husbandry techniques can ameliorate FSN outcomes by increasing animal protein (meat and eggs) availability from healthier livestock, as well as augment an important source of household income. In Madagascar, projects are actively working to introduce nutrient-rich insects, already traditionally consumed, into more diets (e.g., Borgerson et al., 2021).
Not only is continued access to natural resources (including fisheries) important for FSN outcomes and preservation of indigenous foodways and food agency, but conservation efforts must work to ensure that WPF biodiversity is protected and that WPF harvesting is sustainable. This may be achieved by supporting the development and enforcement of community-designed, self-governed rules and regulations regarding the management of common-pool resources (Ostrom, 1990), as seen with via. Indeed, a substantial body of evidence indicates that community involvement in conservation projects has greater potential for achieving “win-win” outcomes for both biodiversity conservation and food security (e.g., Oldekop et al., 2016; Nielsen et al., 2018; Naidoo et al., 2019).
Future research directions include looking more granularly at how various types of cultivated crops (e.g., seasonal, perennial, cash crops) contribute to food security and dietary diversity. In addition, as Lachat et al. (2018) describe, using dietary recall to document dietary species richness, a count of the number of different species consumed, would complement our understanding of the contribution of WPF biodiversity in diets among rural populations living near PAs, and their potential for semi-domesticated use. For example, Madagascar is thought to have more than 30 endemic species of wild yam (Columbus, 2017), with new ones still being described that are already threatened or endangered due to overharvesting and habitat degradation (Wilkin et al., 2008, 2009). In an age in which much of the world's agrobiodiversity has already been lost, this salvaged knowledge would equip conservation efforts with a deeper understanding of the array of nutritious wild plant species that rural populations are consuming, providing additional direction on which genetic resources are important to preserve for now and the future (Cantwell-Jones et al., 2022). Lastly, as there appears to be a general decline in the abundance of wild, indigenous plant foods in Madagascar (Rasoanaivo, 1990), as well as worldwide (FAO, 2019), documenting traditional ecological knowledge of WPFs is more crucial than ever.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Vermont IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MM developed the concept for the paper, designed the methods, wrote the original draft, conducted the quantitative and qualitative data analysis, and made revisions based on feedback. MA assisted with paper concept, data management and analysis, and provided feedback on early drafts. KR oversaw fieldwork, conducted interviews and focus groups, and validated findings. AT provided guidance on framing and feedback. MN provided guidance on methods, concepts, data analysis, and feedback on multiple drafts. All authors contributed to the article and approved the submitted version.
This study was funded by the Bridge Spark Fund (Bridge Collaborative) and the Gund Institute for Environment at the University of Vermont.
We are grateful to the Manombo community members who graciously spent time answering our many questions and provided us with insight into their lived experiences, as well as to our partner organization, Health in Harmony, for helping to facilitate our work. We are also thankful for financial support from the Bridge Spark Fund (Bridge Collaborative) and the Gund Institute for Environment, and especially to Tim Trueur for securing this funding. We thank our Madagascar research team led by KR, Lynne Gaffikin for assistance in sampling and questionnaire design, Alisha Farris for sharing examples of nutrition questionnaires, Christoph den Biggelaar for helping to puzzle through unexpected results, Maria Sckolnick for statistical support, Hannah Dekker and Ashleigh Angle for data entry, Ashley McCarthy for map development, Christopher Golden and Janica Anderzén for their insightful feedback on earlier versions of the paper, as well as the two reviewers for providing suggestions which strengthened the quality of the manuscript.
Author KR was the founder of Kimmerling Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.929308/full#supplementary-material
1. ^In cultures where one food dominates the diet, hunger is often associated with decreased availability of that staple (Minnis, 2021). Indeed, one of the Malagasy words for “to eat” (mihinim-bary) translates as “to eat rice,” and in a form of culinary discontent, many Malagasy do not consider having eaten if they have not had rice, even if they have eaten less preferred staples known collectively as haninkotrana (e.g. cassava, sweet potato, taro, etc.). Furthermore, there is a Malagasy belief that if you go to bed without eating rice (mandry fotsy; Richardson, 1885), then you will not sleep well (Conti et al., 2021b). These expressions are important in terms of the politics of (food) adequacy, which considers the social and emotional “dimensions of food consumption…beyond the caloric content” (Garth, 2020, p. 158).
2. ^There are two rice-growing seasons locally known as vary vatomandry and varihosy or vary kitra (off-season rice), which correspond to a warmer season (Vatomandry) from December - May, and a drier season (Hosy) from June - November. Vary vatomandry is the main harvest in May/June and the start of a roughly four-month “period of abundance.” Varihosy is harvested in December and supplies last about one month (Randrianarison et al., 2020).
3. ^Daba is a local unit of measurement made from 18-20 liter metal coconut oil containers used to measure rice seed (with hull still intact), which equates to roughly 15-20 kilograms).
4. ^Kapoaka is a local unit of measurement using an empty condensed milk can to measure hulled rice.
5. ^Locally, zebu numbers may have reduced in recent years due to frequent attacks by dahalo (“zebu thieves”; Randrianarison et al., 2020).
6. ^This contrasts to the 250 distinct foods consumed by households in the more verdant northeastern area of Madagascar over a nine-month period as reported by Golden et al. (2019b), demonstrating that a 24-hour dietary recall only provides a snapshot of the full range of foods available to a community throughout the year.
7. ^Breadfruit is typically boiled for kadaky (cut into small pieces then boiled and mixed with salt to get a savory porridge) or sambaiky (whole breadfruit is cut into four pieces and boiled).
8. ^Though Madagascar is highly cyclone-prone and cyclones routinely exacerbate food insecurity by destroying crops, no major cyclones made direct landfall with the southeast coast during the 2019-2020 and 2020-2021 cyclone seasons (December – March/April).
9. ^A survey conducted during this period would likely uncover higher consumption levels of tavolo than was found in February.
10. ^The 2021 GFSI scores Madagascar’s dietary diversity as 1.5 (out of 100) and lists it as 100th out of 113 countries in terms of its food security score.
11. ^Arouna et al. (2021) found the average rice yield per hectare in irrigated lowland systems in Madagascar to be 2.5 tons per hectare compared to an average of 4.1 tons per hectare across 12 other countries in SSA.
Adjimoti, G. O., and Kwadzo, G. T.-M. (2018). Crop diversification and household food security status: evidence from rural Benin. Agric. Food Secur. 7, 82. doi: 10.1186/s40066-018-0233-x
Alpízar, F., Saborío-rodríguez, M., Martínez-rodríguez, M. R., Viguera, B., Vignola, R., and Harvey, C. A. (2020). Determinants of food insecurity among smallholder farmer households in Central America: recurrent versus extreme weather-driven events. Reg. Environ. Change 20, 1–16. doi: 10.1007/s10113-020-01592-y
Anderzén, J., Luna, A. G., Luna-González, D. V., Merrill, S. C., Caswell, M., Méndez, V. E., et al. (2020). Effects of on-farm diversification strategies on smallholder coffee farmer food security and income sufficiency in Chiapas, Mexico. J. Rural Stud. 77, 33–46. doi: 10.1016/j.jrurstud.2020.04.001
Andriamparany, J. N., Brinkmann, K., Jeannoda, V., and Buerkert, A. (2014). Effects of socio-economic household characteristics on traditional knowledge and usage of wild yams and medicinal plants in the Mahafaly region of south-western Madagascar. J. Ethnobiol. Ethnomed. 10, 82. doi: 10.1186/1746-4269-10-82
Arouna, A., Devkota, K. P., Yergo, W. G., Saito, K., Frimpong, B. N., Adegbola, P. Y., et al. (2021). Assessing rice production sustainability performance indicators and their gaps in twelve sub-Saharan African countries. Field Crops Res. 271, 108263. doi: 10.1016/j.fcr.2021.108263
Bacon, C. M., Sundstrom, W. A., Flores Gómez, M. E., Ernesto Méndez, V., Santos, R., Goldoftas, B., et al. (2014). Explaining the “hungry farmer paradox”: smallholders and fair trade cooperatives navigate seasonality and change in Nicaragua's corn and coffee markets. Glob. Environ. Change 25, 133–149. doi: 10.1016/j.gloenvcha.2014.02.005
Baldermann, S., Blagojević, L., Frede, K., Klopsch, R., Neugart, S., Neumann, A., et al. (2016). Are neglected plants the food for the future? Crit. Rev. Plant Sci. 35, 106–119. doi: 10.1080/07352689.2016.1201399
Barreau Daly, A. (2014). Narrating Changing Foodways: Wild Edible Plant Knowledge and Traditional Food Systems in Mapuche Lands of the Andean Temperate Forests, Chile (T). University of British Columbia. Available online at: https://open.library.ubc.ca/collections/ubctheses/24/items/1.0135637
Bharucha, Z., and Pretty, J. (2010). The roles and values of wild foods in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 365, 2913–2926. doi: 10.1098/rstb.2010.0123
Bickel, G., Nord, M., Price, C., Hamilton, W., and Cook, J. (2000). Guide to Measuring Household Food Security, Revised 2000. U.S. Department of Agriculture, Food and Nutrition Service, Alexandria, VA.
Blaney, S., Beaudry, M., and Latham, M. (2009). Contribution of natural resources to nutritional status in a protected area of Gabon. Food Nutr. Bull. 30, 49–62. doi: 10.1177/156482650903000105
Bogale, A., and Shimelis, A. (2009). Household level determinants of food insecurity in rural areas of Dire Dawa, Eastern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 9, 1917–1919. doi: 10.18697/ajfand.30.3740
Borgerson, C., Fisher, B. L., Razafindrapaoly, B. N., Rasolofoniaina, B. J. R., Randriamanetsy, J. M., Razafindrapaoly, B. L., et al. (2021). A nutrient-rich traditional insect for improving food security and reducing biodiversity loss in Madagascar and sub-Saharan Africa. Conserv. Sci. Pract. 3, e480. doi: 10.1111/csp2.480
Borgerson, C., Johnson, S. E., Hall, E. Jr., and Golden, C. D. (2022). A national-level assessment of lemur hunting pressure in Madagascar. Int. J. Primatol. 43, 92–113. doi: 10.1007/s10764-021-00215-5
Borgerson, C., Rajaona, D., Razafindrapaoly, B., Rasolofoniaina, B. J. R., Kremen, C., and Golden, C. D. (2017). Links between food insecurity and the unsustainable hunting of wildlife in a UNESCO world heritage site in Madagascar. Lancet 389, S3. doi: 10.1016/S0140-6736(17)31115-7
Borgerson, C., Vonona, M. A., Vonona, T., Anjaranirina, E. J. G., Lewis, R., Ralainasolo, F., et al. (2018). An evaluation of the interactions among household economies, human health, and wildlife hunting in the Lac Alaotra wetland complex of Madagascar. Madag. Conserv. Dev. 13, 25–33. doi: 10.4314/mcd.v13i1.5
Bown, D. (1995). The Royal Horticultural Society Encyclopedia of Herbs and Their Uses. Dorling Kindersley Limited.
Brown, K. A., Flynn, D. F. B., Abram, N. K., Ingram, J. C., Johnson, S. E., and Wright, P. (2011). Assessing natural resource use by forest-reliant communities in Madagascar using functional diversity and functional redundancy metrics. PLoS ONE 6, e24107. doi: 10.1371/journal.pone.0024107
Burlingame, B. (2000). Wild nutrition. J. Food Composition Anal. 2, 99–100. doi: 10.1006/jfca.2000.0897
Campbell, D., Moulton, A. A., Barker, D., Malcolm, T., Scott, L., Spence, A., et al. (2021). Wild food harvest, food security, and biodiversity conservation in Jamaica: a case study of the Millbank Farming Region. Front. Sustain. Food Syst. 5, 663863. doi: 10.3389/fsufs.2021.663863
Cantwell-Jones, A., Ball, J., Collar, D.C., Diazgranados, M., Douglas, R., Forest, F., et al. (2022). Global plant diversity as a reservoir of micronutrients for humanity. Nat. Plants 8, 225–232. doi: 10.1038/s41477-022-01100-6
Cheban, S. A., Rejo-Fienena, F., and Tostain, S. (2009). Etude ethnobotanique des ignames (Dioscorea spp.) dans la foret Mikea et le couloir d'Antseva (sud-ouest de Madagascar). Malagasy Nat. 2, 111–126. Available online at: http://www.vahatra.mg/volume2/mn02_06.pdf
Coates, J., Swindale, A., and Bilinski, P. (2007). Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3). Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development. doi: 10.1037/e576842013-001
Columbus, C. (2017). The Yam That Deserves to Win the Internet. NPR. Available online at: https://www.npr.org/sections/goatandsoda/2017/11/23/565935375/the-yam-that-deserves-to-win-the-internet (accessed August 30, 2022).
Conti, M. V., De Giuseppe, R., Monti, M. C., Mkindi, A. G., Mshanga, N. H., Ceppi, S., et al. (2021a). Indigenous vegetables: a sustainable approach to improve micronutrient adequacy in Tanzanian women of childbearing age. Eur. J. Clin. Nutr. 75, 1475–1482. doi: 10.1038/s41430-021-00865-x
Conti, M. V., Kalmpourtzidou, A., Lambiase, S., De Giuseppe, R., and Cena, H. (2021b). Novel foods and sustainability as means to counteract malnutrition in Madagascar. Molecules 26, 2142. doi: 10.3390/molecules26082142
Croat, T. B., and Ortiz, O. O. (2020). Distribution of araceae and the diversity of life forms. Acta Soc. Botanicorum Pol. 89, 1–23. doi: 10.5586/asbp.8939
Cruz-Garcia, G. S., and Price, L. L. (2011). Ethnobotanical investigation of 'wild' food plants used by rice farmers in Kalasin, Northeast Thailand. J. Ethnobiol. Ethnomed. 7, 33. doi: 10.1186/1746-4269-7-33
Custodio, E., Herrador, Z., Nkunzimana, T., Weziak-Białowolska, D., Perez-Hoyos, A., and Kayitakire, F. (2019). Children's dietary diversity and related factors in Rwanda and Burundi: a multilevel analysis using 2010 Demographic and Health Surveys. PLoS ONE 14, e0223237. doi: 10.1371/journal.pone.0223237
Deitchler, M., Ballard, T., Swindale, A., and Coates, J. (2011). Introducing a Simple Measure of Household Hunger for Cross-Cultural Use 2010. Washington, DC: Food and Nutrition Technical Assistance IIProject, FHI 360.
Dercon, S. (2002). Income risk, coping strategies and safety. World Bank Res. Obs. 17, 141–166. doi: 10.1093/wbro/17.2.141
Dewar, R. E., Radimilahy, C., Wright, H. T., Jacobs, Z., Kelly, G. O., and Berna, F. (2013). Stone tools and foraging in northern Madagascar challenge Holocene extinction models. Proc. Nat. Acad. Sci. U.S.A. 110, 12583–12588. doi: 10.1073/pnas.1306100110
Erskine, W., Ximenes, A., Glazebrook, D., Williams, R., and Nesbitt, H. (2015). The role of wild foods in food security : the example of Timor-Leste. Food Secur. 7, 55–65. doi: 10.1007/s12571-014-0406-9
Faber, M., Schwabe, C., and Drimie, S. (2009). Dietary diversity in relation to other household food security indicators. Int. J. Food Saf. Nutr. Public Health 2, 1–15. doi: 10.1504/IJFSNPH.2009.026915
FAO (2019). “The State of the World's biodiversity for food and agriculture,” in FAO Commission on Genetic Resources for Food and Agriculture Assessments, eds J. Bélanger and D. Pilling (Rome), 572. Available online at: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed August 30, 2022).
FAO (2010). Guidelines for Measuring Household and Individual Dietary Diversity. Available online at: https://www.fao.org/3/i1983e/i1983e.pdf (accessed August 30, 2022).
Farris, A. R., Misyak, S., O'Keefe, K., VanSicklin, L., and Porton, I. (2019). Understanding the drivers of food choice and barriers to diet diversity in Madagascar. J. Hunger Environ. Nutr. 15, 388–400. doi: 10.1080/19320248.2019.1566110
Farris, Z. J., Golden, C. D., Karpanty, S., Murphy, A., Stauffer, D., Ratelolahy, F., et al. (2015). Hunting, exotic carnivores, and habitat loss: anthropogenic effects on a native carnivore community, Madagascar. PLoS ONE 10, e0136456. doi: 10.1371/journal.pone.0136456
Fauroux, E., Rakotomalala, L., Delcroix, F., Nerine, E., Randriaminoda, P., and Telolahy, A. (1990). Le bœuf et le riz dans la vie économique et sociale Sakalava de la vallée de la Maharivo. (E. Fauroux, Ed.). Paris: ORSTOM.
Freedman, R. L. (2019). Famine Food Database. Available online at: www.purdue.edu/hla/sites/famine-foods (accessed February 10, 2022).
Fungo, R., Muyonga, J., Kabahenda, M., Kaaya, A., Okia, C. A., Donn, P., et al. (2016). Contribution of forest foods to dietary intake and their association with household food insecurity: a cross-sectional study in women from rural Cameroon. Public Health Nutr. 19, 3185–3196. doi: 10.1017/S1368980016001324
Galway, L. P., Acharya, Y., and Jones, A. D. (2018). Deforestation and child diet diversity: a geospatial analysis of 15 Sub-Saharan African countries. Health Place 51, 78–88. doi: 10.1016/j.healthplace.2018.03.002
Ghosh-Jerath, S., Kapoor, R., Barman, S., Singh, G., Singh, A., Downs, S., et al. (2021). Traditional food environment and factors affecting indigenous food consumption in munda tribal community of Jharkhand, India. Front. Nutr. 7, 600470. doi: 10.3389/fnut.2020.600470
Global Food Security Index (2021). Madagascar. Available online at: https://impact.economist.com/sustainability/project/food-security-index/Country/Details#Madagascar (accessed August 30, 2022).
Golden, C. D. (2009). Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. Oryx 43, 386–392. doi: 10.1017/S0030605309000131
Golden, C. D., Bonds, M. H., Brashares, J. S., Rodolph Rasolofoniaina, B. J., and Kremen, C. (2014a). Economic valuation of subsistence harvest of wildlife in Madagascar. Conserv. Biol. 28, 234–243. doi: 10.1111/cobi.12174
Golden, C. D., Borgerson, C., Rice, B. L., Allen, L. H., Anjaranirina, E. J. G., Barrett, C. B., et al. (2019a). Cohort description of the Madagascar Health and Environmental Research–Antongil (MAHERY–Antongil) Study in Madagascar. Front. Nutr. 6, 109. doi: 10.3389/fnut.2019.00109
Golden, C. D., Fernald, L. C. H., Brashares, J. S., Rasolofoniaina, B. J. R., and Kremen, C. (2011). Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc. Nat. Acad. Sci. U.S.A. 108, 19653–19656. doi: 10.1073/pnas.1112586108
Golden, C. D., Gupta, A. C., Vaitla, B., and Myers, S. S. (2016). Ecosystem services and food security: assessing inequality at community, household and individual scales. Environ. Conserv. 43, 381–388. doi: 10.1017/S0376892916000163
Golden, C. D., Rabehatonina, J. G. C., Rakotosoa II, A., and Moore, M. (2014b). Socio-ecological analysis of natural resource use in Betampona Strict Natural Reserve. Madag. Conserv. Dev. 9, 83–89. doi: 10.4314/mcd.v9i2.4
Golden, C. D., Rasolofoniaina, B. J. R., Anjaranirina, E. J. G., Nicolas, L., Ravaoliny, L., and Kremen, C. (2012). Rainforest pharmacopeia in Madagascar provides high value for current local and prospective global uses. PLoS ONE 7, e41221. doi: 10.1371/journal.pone.0041221
Golden, C. D., Vaitla, B., Ravaoliny, L., Vonona, M. A., Gasta Anjaranirina, E. J., Randriamady, H. J., et al. (2019b). Seasonal trends of nutrient intake in rainforest communities of north-eastern Madagascar. Public Health Nutr. 22, 2200–2209. doi: 10.1017/S1368980019001083
Grivetti, L. E., and Ogle, B. M. (2000). Value of traditional foods in meeting macro-and micronutrient needs: the wild plant connection. Nutr. Res. Rev. 13, 31–46. doi: 10.1079/095442200108728990
Hänke, H., and Barkmann, J. (2017). Insurance function of livestock, farmers coping capacity with crop failure in southwestern Madagascar. World Dev. 96, 264–275. doi: 10.1016/j.worlddev.2017.03.011
Hardin, G. (1968). The tragedy of the commons. Science 162, 1243–1248. doi: 10.1126/science.162.3859.1243
Harvey, C. A., Rakotobe, Z. L., Rao, N. S., Dave, R., Razafimahatratra, H., Rabarijohn, R. H., et al. (2014). Extreme vulnerability of smallholder farmers to agricultural risks and climate change in Madagascar. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130089. doi: 10.1098/rstb.2013.0089
Helmich, E., Cristancho, S., Diachun, L., and Lingard, L. (2017). How would you call this in English? Perspect. Med. Educ. 6, 127–132. doi: 10.1007/s40037-017-0329-1
Herrera, J. P., Rabezara, J. Y., Ravelomanantsoa, N. A. F., Metz, M., France, C., Owens, A., et al. (2021). Food insecurity related to agricultural practices and household characteristics in rural communities of northeast Madagascar. Food Secur. 13, 1393–1405. doi: 10.1007/s12571-021-01179-3
Herrero, M., Thornton, P. K., Power, B., Bogard, J. R., Remans, R., Fritz, S., et al. (2017). Farming and the geography of nutrient production for human use: a transdisciplinary analysis. Lancet Planet. Health 1, e33–e42. doi: 10.1016/S2542-5196(17)30007-4
Hickey, G. M., Pouliot, M., Smith-Hall, C., Wunder, S., and Nielsen, M. R. (2016). Quantifying the economic contribution of wild food harvests to rural livelihoods: a global-comparative analysis. Food Policy 62, 122–132. doi: 10.1016/j.foodpol.2016.06.001
Huambachano, M. (2019). Traditional ecological knowledge and indigenous foodways in the Andes of Peru. Rev. Int. Am. Stud. 12, 87–110. doi: 10.31261/rias.6866
Hunter, D., and de Oliveira Beltrame, M. D. (2015). Biodiversity for Food and Nutrition in Brazil. A4HN Outcome Note. IFPRI 3 p. Available online at: https://hdl.handle.net/10568/72416
Hunter, D., Giyose, B., Pologalante, A., Tartanac, F., Bundy, D., Mitchell, A., et al. (2017). Schools as a System to Improve Nutrition: A New Statement for School-Based Food and Nutrition Interventions. United Nations System Standing Committee on Nutrition. P44
IFAD (n.d.). Madagascar. Available online at: https://www.ifad.org/en/web/operations/w/country/madagascar (accessed April 25, 2022).
Ingram, J. C., and Dawson, T. P. (2006). Forest cover, condition, and ecology in human-impacted forests, south-eastern Madagascar. Conserv. Soc. 4, 194–230. Available online at: https://dlc.dlib.indiana.edu/dlc/bitstream/handle/10535/2430/Forest_Cover,_Condition,_and_Ecology.pdf?sequence=1
Institut National de la Statistique (INSTAT) and United Nations Children's Fund (UNICEF) (2019). Enquête par grappes à indicateurs multiples-MICS Madagascar, 2018, Rapport final. Antananarivo, Madagascar: INSTAT and UNICEF.
Jeannoda, V. H., Razanamparany, J. L., Rajaonah, T., Monneuse, M., Hladik, A., Marcel, C., et al. (2010). The yams (Dioscorea spp.) of Madagascar: wild endemic and cultivated species; diversity,perception, nutritional value, and sustainable management. Rev. Ecol. 62, 191–207. Available online at: https://www.researchgate.net/publication/296936764_The_yams_Dioscorea_spp_of_Madagascar_wild_endemic_and_cultivated_species_diversity_perception_nutritional_value_and_sustainable_management
Jenkins, R. K. B., Rabearivelo, A., Andre, C. T. C. W. M., Randrianavelona, R., and Randrianantoandro, J. C. (2009). The harvest of endemic amphibians for food in eastern Madagascar. Trop. Conserv. Sci. 2, 25–33. doi: 10.1177/194008290900200105
Jenkins, R. K. B., and Racey, P. A. (2008). Bats as bushmeat in Madagascar. Madag. Conserv. Dev. 3, 22–30. doi: 10.4314/mcd.v3i1.44132
Johnson, K. B., Jacob, A., and Brown, M. E. (2013). Forest cover associated with improved child health and nutrition: evidence from the Malawi Demographic and Health Survey and satellite data. Glob. Health Sci. Pract. 1, 237–248. doi: 10.9745/GHSP-D-13-00055
Johnson, S. E., and Overdorff, D. J. (1999). Census of brown lemurs (Eulemur fulvus sspp.) in southeastern Madagascar: Methods-testing and conservation implications. Am. J. Primatol. 47, 51–60. doi: 10.1002/(SICI)1098-2345(1999)47:1<51::AID-AJP6>3.0.CO;2-O
Jones, A. D., Shrinivas, A., and Bezner-Kerr, R. (2014). Farm production diversity is associated with greater household dietary diversity in Malawi: findings from nationally representative data. Food Policy 46, 1–12. doi: 10.1016/j.foodpol.2014.02.001
Jouzi, Z., Leung, Y., and Nelson, S. (2020). Terrestrial protected areas and food security: a systematic review of research approaches. Environments 7, 83. doi: 10.3390/environments7100083
Karanja Ng'ang'a, S., Bulte, E. H., Giller, K. E., Ndiwa, N. N., Kifugo, S. C., Mcintire, J. M., et al. (2016). Livestock wealth and social capital as insurance against climate risk: a case study of Samburu County in Kenya. Agric. Syst. 146, 44–54. doi: 10.1016/j.agsy.2016.04.004
Kaur, M. (2021). Smallholder Farmers: The Backbone of Food Security. World Food Program. Available online at: https://www.wfp.org/publications/smallholder-farmers-backbone-food-security (accessed August 30, 2022).
Keding, G. B., Msuya, J. M., Maass, B. L., and Krawinkel, M. B. (2012). Relating dietary diversity and food variety scores to vegetable production and socio-economic status of women in rural Tanzania. Food Secur. 4, 129–140. doi: 10.1007/s12571-011-0163-y
Koppmair, S., Kassie, M., and Qaim, M. (2016). Farm production, market access and dietary diversity in Malawi. Public Health Nutr. 20, 325–335. doi: 10.1017/S1368980016002135
Lachat, C., Raneri, J. E., Smith, W. K., Kolsteren, P., Van Damme, P., Verzelen, K., et al. (2018). Dietary species richness as a measure of food biodiversity and nutritional quality of diets. PNAS 115, 127–132. doi: 10.1073/pnas.1709194115
Laney, R., and Turner, B. L. (2015). The persistence of self-provisioning among smallholder farmers in northeast Madagascar. Hum. Ecol. 43, 811–826. doi: 10.1007/s10745-015-9791-8
Le Manach, F., Gough, C., Harris, A., Humber, F., Harper, S., and Zeller, D. (2012). Unreported fishing, hungry people and political turmoil: the recipe for a food security crisis in Madagascar? Mar. Policy 36, 218–225. doi: 10.1016/j.marpol.2011.05.007
Linton, R. (1927). Rice, a Malagasy tradition. Am. Anthropol. 29, 654–660. doi: 10.1525/aa.1927.29.4.02a00060
Lowder, S. K., Sánchez, M. V., and Bertini, R. (2021). Which farms feed the world and has farmland become more concentrated? World Dev. 142, 105455. doi: 10.1016/j.worlddev.2021.105455
Luna-González, D. V., and Sørensen, M. (2018). Higher agrobiodiversity is associated with improved dietary diversity, but not child anthropometric status, of Mayan Achí people of Guatemala. Public Health Nutr. 21, 2128–2141. doi: 10.1017/S1368980018000617
Makate, C., Wang, R., Makate, M., and Mango, N. (2016). Crop diversification and livelihoods of smallholder farmers in Zimbabwe: adaptive management for environmental change. Springerplus 5, 1135. doi: 10.1186/s40064-016-2802-4
Manduna, I., and Vibrans, H. (2018). Consumption of wild-growing vegetables in the Honde Valley, Zimbabwe. Econ. Bot. 72, 436–449. doi: 10.1007/s12231-019-9441-y
Mavah, G., Funk, S., Child, B., Swisher, M., Nasi, R., and Fa, J. (2018). Food and livelihoods in park-adjacent communities: the case of the Odzala Kokoua National Park. Biol. Conserv. 222, 44–51. doi: 10.1016/j.biocon.2018.03.036
Mazoyer, M., and Roudart, L. (2006). A History of World Agriculture: From the Neolithic Age to the Current Crisis. NYU Press.
Mbhenyane, X. G. (2017). Indigenous foods and their contribution to nutrient requirements. S. Afr. J. Clin. Nutr. 30, 5–7. Available online at: http://hdl.handle.net/10019.1/104799
Minnis, P. E. (2021). Famine Foods: Plants We Eat to Survive. Tucson: University of Arizona Press. doi: 10.2307/j.ctv1k13b98
Missouri Botanical Gardens (n.d.). Tacca Leontopetaloides. Available online at: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=287291andisprofile=0andletter=T (accessed August 30, 2022).
Mittermeier, R. A., Turner, W. R., Larsen, F. W., Brooks, T. M., and Gascon, C. (2011). “Global biodiversity conservation: the critical role of hotspots,” in Biodiversity Hotspots (Heidelberg, Berlin: Springer), 3–22. doi: 10.1007/978-3-642-20992-5_1
Miura, K., Kanno, H., and Sakurai, T. (2012). Shock and livestock transactions in rural Zambia: a re-examination of the buffer stock hypothesis. Jpn. J. Rural Econ. 14, 20–34. doi: 10.18480/jjre.14.20
Moursi, M. M., Arimond, M., Dewey, K. G., Trèche, S., Ruel, M. T., and Delpeuch, F. (2008). Dietary diversity is a good predictor of the micronutrient density of the diet of 6- to 23-month-old children in Madagascar. J. Nutr. 138, 2448–2453. doi: 10.3945/jn.108.093971
Naidoo, R., Gerkey, D., Hole, D., Pfaff, A., Ellis, A. M., Golden, C. D., et al. (2019). Evaluating the impacts of protected areas on human well-being across the developing world. Sci. Adv. 5, eaav3006. doi: 10.1126/sciadv.aav3006
Nielsen, M. R., Meilby, H., Smith-Hall, C., Pouliot, M., and Treue, T. (2018). The importance of wild meat in the global south. Ecol. Econ. 146, 696–705. doi: 10.1016/j.ecolecon.2017.12.018
Niles, M. T., Emery, B. F., Wiltshire, S., Brown, M. E., Fisher, B., and Ricketts, T. H. (2021). Climate impacts associated with reduced diet diversity in children across nineteen countries. Environ. Res. Lett. 16, 015010. doi: 10.1088/1748-9326/abd0ab
Niles, M. T., and Salerno, J. D. (2018). A cross-country analysis of climate shocks and smallholder food insecurity. PLoS ONE 13, e0192928. doi: 10.1371/journal.pone.0192928
Nkonde, C., Audain, K., Kiwanuka-Lubinda, R. N., and Marinda, P. (2021). Effect of agricultural diversification on dietary diversity in rural households with children under 5 years of age in Zambia. Food Sci. Nutr. 9, 6274–6285. doi: 10.1002/fsn3.2587
Novy, J. W. (1997). Medicinal plants of the eastern region of Madagascar. J. Ethnopharmacol. 55, 119–126. doi: 10.1016/S0378-8741(96)01489-4
Ocho, D. L., Struik, P. C., Price, L. L., Kelbessa, E., and Kolo, K. (2012). Assessing the levels of food shortage using the traffic light metaphor by analyzing the gathering and consumption of wild food plants, crop parts and crop residues in Konso, Ethiopia. J. Ethnobiol. Ethnomed. 8, 7. doi: 10.1186/1746-4269-8-30
Ogle, B. M. (2001). Wild Vegetables and Micronutrient Nutrition: Studies on the Significance of Wild Vegetables in Women's Diets in Vietnam (Ph.D. dissertation, Acta Universitatis Upsaliensis).
Oldekop, J. A., Holmes, G., Harris, W. E., and Evans, K. L. (2016). A global assessment of the social and conservation outcomes of protected areas. Conserv. Biol. 30, 133–141. doi: 10.1111/cobi.12568
Ostrom, E. (1990). Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge: Cambridge University Press.
Paumgarten, F., Locatelli, B., and Witkowski, E. T. F. (2018). Wild foods: safety net or poverty trap? A South African case study. Hum. Ecol. 46, 183–195. doi: 10.1007/s10745-018-9984-z
Pawera, L., Khomsan, A., Zuhud, E. A. M., Hunter, D., Ickowitz, A., and Polesny, Z. (2020). Wild food plants and trends in their use: From knowledge and perceptions to drivers of change in West Sumatra, Indonesia. Foods 9, 1240. doi: 10.3390/foods9091240
Pieroni, A. (2021). Wild foods: a topic for food pre-history and history or a crucial component of future sustainable and just food systems? Foods 10, 827. doi: 10.3390/foods10040827
Powell, B., Maundu, P., Kuhnlein, H. V., and Johns, T. (2013). Wild foods from farm and forest in the East Usambara Mountains, Tanzania. Ecol. Food Nutr. 52, 451–478. doi: 10.1080/03670244.2013.768122
Powell, B., Thilsted, S. H., Ickowitz, A., Termote, C., Sunderland, T., and Herforth, A. (2015). Improving diets with wild and cultivated biodiversity from across the landscape. Food Secur. 7, 535–554. doi: 10.1007/s12571-015-0466-5
Price, L. (1997). Wild plant food in agricultural environments: a study of occurrence, management, and gathering rights in Northeast Thailand. Hum. Organ. 56, 209–221. doi: 10.17730/humo.56.2.6572033n03673640
Price, L. (2006). Wild food plants in farming environments with special reference to Northeast Thailand, food as functional and medicinal, and the social roles of women. Eating and Healing: Traditional food as medicine, 65–99.
Pullin, A. S., Bangpan, M., Dalrymple, S., Dickson, K., Haddaway, N. R., Healey, J. R., et al. (2013). Human well-being impacts of terrestrial protected areas. Environ. Evid. 2, 19. doi: 10.1186/2047-2382-2-19
Rabearivony, A. D., Kuhlman, A. R., Razafiariso, Z. L., Raharimalala, F., Rakotoarivony, F., Randrianarivony, T., et al. (2015). Ethnobotanical study of the medicinal plants known by men in Ambalabe, Madagascar. Ethnobotany Res. Appl. 14, 123–138. doi: 10.17348/era.14.0.123-138
Rakotobe, Z. L., Harvey, C. A., Rao, N. S., Dave, R., Rakotondravelo, J. C., Randrianarisoa, J., et al. (2016). Strategies of smallholder farmers for coping with the impacts of cyclones: a case study from Madagascar. Int. J. Disaster Risk Reduct. 17, 114–122. doi: 10.1016/j.ijdrr.2016.04.013
Randrianarison, N., Nischalke, S., and Andriamazaoro, H. (2020). The role of biodiversity and natural resource management in food security in south-eastern Madagascar. Acta Hortic. 1267, 267–273. doi: 10.17660/ActaHortic.2020.1267.40
Rasoanaivo, P. (1990). Rain forests of Madagascar: sources of industrial and medicinal plants. Ambio 19, 421–424.
Rasolofoson, R. A., Hanauer, M. M., Pappinen, A., Fisher, B., and Ricketts, T. H. (2018). Impacts of forests on children's diet in rural areas across 27 developing countries. Sci. Adv. 4, eaat2853. doi: 10.1126/sciadv.aat2853
Rasolofoson, R. A., Ricketts, T. H., Jacob, A., and Johnson, K. B. (2020). Forest conservation: a potential nutrition-sensitive intervention in low- and middle-income countries. Front. Sustain. Food Syst. 4, 20. doi: 10.3389/fsufs.2020.00020
Ray, A., and Chakraborty, A. (2021). The edible biota in irrigated, deepwater, and rainfed rice fields of Asia: a neglected treasure for sustainable food system. Environ. Dev. Sustain. 23, 17163–17179. doi: 10.1007/s10668-021-01386-0
Ray, A., and Ray, R. (2022). The leafy greens of India - their diversity, pattern of consumption, and overriding implication on food and nutrition security. Agroecol. Sustain. Food Syst. 46, 431–451. doi: 10.1080/21683565.2022.2025987
Razafindraibe, M., Kuhlman, A. R., Rabarison, H., Rakotoarimanana, V., Rajeriarison, C., Rakotoarivelo, N., et al. (2013). Medicinal plants used by women from Agnalazaha littoral forest (Southeastern Madagascar). J. Ethnobiol. Ethnomed. 9, 73. doi: 10.1186/1746-4269-9-73
Read, B. E. (1946). Famine Foods Listed in the Chiu Huang Pen Ts'ao. Henry Lester Institute of Medical Research, Shanghai.
Ricciardi, V., Ramankutty, N., Mehrabi, Z., Jarvis, L., and Chookolingo, B. (2018). How much of the world's food do smallholders produce? Glob. Food Secur. 17, 64–72. doi: 10.1016/j.gfs.2018.05.002
Riondato, I., Donno, D., Roman, A., Razafintsalama, V. E., Petit, T., Mellano, M. G., et al. (2019). First ethnobotanical inventory and phytochemical analysis of plant species used by indigenous people living in the Maromizaha forest, Madagascar. J. Ethnopharmacol. 232, 73–89. doi: 10.1016/j.jep.2018.12.002
Ruel, M. T. (2003). Is dietary diversity an indicator of food security or dietary quality? A review of measurement issues and research needs. Food Nutr. Bull. 24, 231–232. doi: 10.1177/156482650302400217
Rutstein, S. O., and Johnson, K. (2004). The DHS Wealth Index. DHS Comparative Reports No. 6. Claverton, Maryland.
SAGE (2015). Analyzing Focus Group Results. Available online at: https://us.sagepub.com/sites/default/files/upm-assets/65005_book_item_65005.pdf (accessed August 30, 2022).
Sibhatu, K. T., and Qaim, M. (2018). Farm production diversity and dietary quality: linkages and measurement issues. Food Secur. 10, 47–59. doi: 10.1007/s12571-017-0762-3
Silvestri, S., Sabine, D., Patti, K., Wiebke, F., Maren, R., Ianetta, M., et al. (2015). Households and food security: lessons from food secure households in East Africa. Agric. Food Secur. 4, 23. doi: 10.1186/s40066-015-0042-4
Skinner, C. J. (2014). Probability Proportional to Size (PPS) Sampling. Wiley StatsRef: Statistics Reference Online, 1–5. doi: 10.1002/9781118445112.stat03346
Sponsel, L. E. (1989). Farming and foraging: a necessary complementarity in Amazonia? In Farmers as Hunters: The Implications of Sedentism, ed S. Kent (Cambridge, UK: Cambridge University Press), 37–45.
Steyn, N., Nel, J., Nantel, G., Kennedy, G., and Labadarios, D. (2006). Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 9, 644–650. doi: 10.1079/PHN2005912
Stiles, D. (1991). Tubers and tenrecs: the Mikea of southwestern Madagascar. Ethnology 30, 251–263. doi: 10.2307/3773634
Stone, L., and Campbell, J. (1984). The use and misuse of surveys in international development: an experiment from Nepal. Hum. Organ. 43, 27–37. doi: 10.17730/humo.43.1.6wl5k01724878166
Styger, E., Rakotoarimanana, J. E. M., and Rabevohitra, R. (1999). Indigenous fruit trees of Madagascar : potential components of agroforestry systems to improve human nutrition and restore biological diversity. Agroforestry Syst. 46, 289–310. doi: 10.1023/A:1006295530509
Sunderland, T., Powell, B., Ickowitz, A., Foli, S., Pinedo-vasquez, M., Nasi, R., et al. (2013). Food Security and Nutrition: The Role of Forests. Bogor: Indonesia.
Swindale, A., and Bilinsky, P. (2006). Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide (v.2). Washington, DC: FHI 360/FANTA.
Taylor, S. F. W., Roberts, M. J., Milligan, B., and Ncwadi, R. (2019). Measurement and implications of marine food security in the Western Indian Ocean: an impending crisis? Food Secur. 11, 1395–1415. doi: 10.1007/s12571-019-00971-6
Tida, M. M. A., Nanjarisoa, O., Rabearivony, J., Ranarijaona, H. L. T., and Fenoradosoa, T. A. (2020). Ethnobotanical survey of plant species used in traditional medicine in Bekaraoka Region, northeastern Madagascar. Int. J. Adv. Res. Publ, 4, 107–114. Available online at: http://www.ijarp.org/published-research-papers/mar2020/Ethnobotanical-Survey-Of-Plant-Species-Used-In-Traditional-Medicine-In-Bekaraoka-Region-Northeastern-Madagascar.pdf
Tucker, B., Humber, F., Benbow, S., and Iida, T. (2010). Foraging for development: a comparison of food insecurity, production, and risk among farmers, forest foragers, and marine foragers in Southwestern Madagascar. Hum. Organ. 69, 375–386. doi: 10.17730/humo.69.4.m1n76k5272632873
USDA Bureau of Plant Industry (1919). Inventory of Seeds and Plants Imported (Vol. 46). Washington, DC: USDA Government Printing Office. Available online at: http://biodiversitylibrary.org. (accessed August 30, 2022).
Waha, K., Van Wijk, M. T., Fritz, S., See, L., Thornton, P. K., Wichern, J., et al. (2018). Agricultural diversification as an important strategy for achieving food security in Africa. Glob. Change Biol. 24, 3390–3400. doi: 10.1111/gcb.14158
Walsh, M. (2009). The use of wild and cultivated plants as famine foods on Pemba Island, Zanzibar. Études Océan Indien 42–43, 217–241. doi: 10.4000/oceanindien.793
White, J. C. (2014). “Ethnoecological observations on wild and cultivated rice and yams in northeastern Thailand,” in Foraging and Farming: The Evolution of Plant Exploitation, eds D. R. Harris, and G. C. Hillman (London: Routledge), 152–158.
Wiggins, S., and Keats, S. (2013). Smallholder agriculture's contribution to better nutrition. Afr. J. Food Agric. Nutr. Dev. 13, S24–S57. Available online at: https://odi.org/en/publications/smallholder-agricultures-contribution-to-better-nutrition/
Wilkin, P., Hladik, A., Weber, O., Marcel Hladik, C., and Jeannoda, V. (2009). Dioscorea orangeana (Dioscoreaceae), a new and threatened species of edible yam from northern Madagascar. Kew Bull. 64, 461–468. doi: 10.1007/s12225-009-9126-2
Wilkin, P., Rajaonah, M. T., Jeannoda, V. H., Jeannoda, V. L., Hladik, C. M., Wilkin, P., et al. (2008). An endangered new species of edible yam (Dioscorea, Dioscoreaceae) from western Madagascar and its conservation. Kew Bull. 63, 113–120. doi: 10.1007/s12225-007-9000-z
Wineman, A., Ekwueme, M.C., Bigayimpunzi, L., Martin-Daihirou, A., Rodrigues, E.L.D.G.V., Etuge, P., et al. (2022). School meal programs in Africa: Regional results from the 2019 Global Survey of School Meal Programs. Front. Public Health 10, 871866. doi: 10.3389/fpubh.2022.871866
World Bank (2020). The World Bank in Madagascar: Overview. Available online at: https://www.worldbank.org/en/country/madagascar/overview#1 doi: 10.1596/978-1-4648-1503-4_ov (accessed August 30, 2022).
World Food Summit (1996). World Food Summit Plan of Action, paragraph 32 (g). In Rome Declaration on World Food Security and World Food Summit Plan of Action; World Food Summit, 13-17 November 1996, Rome, Italy (p. 43).
World Health Organization (2000). Management of Nutrition in Major Emergencies (The): 2nd Edn. Geneva: World Health Organization.
Keywords: wild plant foods, famine foods, food security, dietary diversity, Madagascar, smallholder farmers, protected areas (PAs), hunger season
Citation: Moore M, Alpaugh M, Razafindrina K, Trubek AB and Niles MT (2022) Finding food in the hunger season: A mixed methods approach to understanding wild plant foods in relation to food security and dietary diversity in southeastern Madagascar. Front. Sustain. Food Syst. 6:929308. doi: 10.3389/fsufs.2022.929308
Received: 26 April 2022; Accepted: 15 August 2022;
Published: 28 September 2022.
Edited by:
Suparna Ghosh-Jerath, Indian Institute of Public Health Delhi, IndiaReviewed by:
Avik Ray, Center for Studies in Ethnobiology, Biodiversity, and Sustainability (CEiBa), IndiaCopyright © 2022 Moore, Alpaugh, Razafindrina, Trubek and Niles. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maya Moore, bWF5YS5tb29yZUB1dm0uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.