
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 09 December 2022
Sec. Nutrition and Sustainable Diets
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.870147
This article is part of the Research Topic Insights in Nutrition and Sustainable Diets: 2021 View all 9 articles
 Piyanut Sridonpai1
Piyanut Sridonpai1 Pichakorn Kongprapun1
Pichakorn Kongprapun1 Nongnuch Sungayuth2
Nongnuch Sungayuth2 Monruedee Sukprasansap1
Monruedee Sukprasansap1 Chanika Chimkerd1
Chanika Chimkerd1 Kunchit Judprasong1*
Kunchit Judprasong1*Indigenous edible plants are important components of food systems that are linked to food security and are important sources of nutrients with potential health benefits. Since knowledge about Thailand's various indigenous plants is limited, this study determined the nutritive values and bioactive compounds contained in eight edible indigenous plants growing within the conservation area of the Electricity Generating Authority of Thailand, Srinakarind Dam, Kanchanaburi province. Plant samples were analyzed as fresh or cooked (blanched or boiled) depending on customary preparation and consumption habits. Results showed that shoots and young leaves of Jang (Maerua siamensis Kurz), Ta-Kuk (Albizia lebbeck (L.) Benth), Pak-Wan-Pa (Melientha suavis Pierre), and Som-kob (Hymenodictyon exelsum Wall.) have potential health benefits in terms of nutritive values (vitamin C, dietary fiber, protein) and bioactive compounds (carotenoids, phenolic compounds, antioxidant activity). Shoots and young leaves of Jang were highest in protein, dietary fiber, phenolic compounds, and antioxidant activity. Shoots and young leaves of Ta-Kuk had the highest vitamin C level and considerable amounts of protein, dietary fiber, phenolic compounds, antioxidant activity, and carotenoids. Loss of vitamin C and bioactive compounds occurred most often in boiled plants rather than those that were blanched, though carotenoids increased with either boiling or blanching. This study's important findings should be translated into practical knowledge and disseminated to local communities and at the national level to encourage plant conservation, nutrition education, and the increased consumption of these indigenous plants.
Food and nutrition security is attained when all individuals access sufficient quantities of affordable, safe, and nutritious foods that are utilized to maintain healthy and active lives. One of several indigenous foods which have a potential to enhance the food and nutrition security in countries around the world, including Thailand, is the indigenous plants. Numerous studies suggest that the indigenous herbs and plants are able to prevent or cure various human diseases in both non-communicable and infectious diseases (Aziz et al., 2018; Chaachouay et al., 2022; Eddouks et al., 2014; Gras et al., 2021; Phumthum and Balslev, 2020). Furthermore, some of these plants can be used for cooking and are beneficial to health (ESCOP (European Scientific Cooperative on Phytotherapy), 2021).
Based on this above-mentioned information and relevant to this present study, some indigenous plants, such as Albizia lebbeck (L.) Benth has a long history within Indian traditional medicine (Pal et al., 1995), in which its leaves and pods are used to treat cancer (Bobby and Wesely, 2012; Bobby et al., 2012; Desai and Joshi, 2019). A. lebbeck (L.) Benth. is found in dense deciduous forests in tropical and subtropical Asian countries, such as Cambodia, Indonesia, Laos, Malaysia, and Vietnam, Africa (Dy Phon, 2000), and Thailand, as well as being widely cultivated. It is a famous plant due to its high nutrients, phytochemicals, and medicinal values (Vasanthi et al., 2014; Sharma and Nishtha, 2015). The various parts of this plant have a wide range of pharmacological activities such as antimicrobial, anti-inflammatory, antioxidant, antitumor, antiulcer, antidiarrhoeal, and immunomodulatory capabilities (Saha and Ahmed, 2009; Vasanthi et al., 2014; Praengam et al., 2017). Additionally, some research found that the ethanolic extract of its leaves has been shown to ameliorate the effects of depression in animals (Velraj et al., 2010). Maerua siamensis (Kurz) Pax. is native to Southeast Asia. Extracts from its leaves and roots have demonstrated antioxidant activities and total phenolic content (Phosri, 2017; Chanthasri et al., 2018; Issuriya et al., 2019), while extracts from leaves and twigs of M. siamensis also have shown acetylcholinesterase inhibitory activity (Phosri, 2017). Melientha suavis Pierre is an edible local plant found in Southeast Asia, including Thailand. Previous reports have shown a radical scavenging effect and a strong antioxidant correlation between the FRAP scavenging activities and the flavonoid contents of extracts from its leaves and stems (Charoenchai et al., 2015; Sansomchai et al., 2021). Hymenodictyon exelsum Wall. is the indigenous plant found in South Asia, including India, Burma, and Thailand. It contains various phytochemicals and has been used in traditional medicine to treat ulcers, fever, tumors, and cancer. Some studies revealed that leaves and bark extracts of H. exelsum exhibited fantioxidant, anti-inflammatory, antimicrobial, antitumor, and anticancer properties (Kar et al., 2013; Rahman, 2015; Paramita et al., 2017).
In Thailand, indigenous plants are important food sources for communities living in and around forested areas. Consequently, data on the nutritive values and bioactive compounds of these plants are needed for dietary assessment, nutrition education, and health improvement. An earlier study reported 10 years ago provided information on nutrient content, bioactive compounds, and the health benefits of 40 species of indigenous plants based on 118 samples (Charoenkiatkul et al., 2012). This previous study was conducted within the conservation area of the Plant Genetic Conservation Project under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn in Kanchanaburi province, Western Thailand. This previous study's information had the potential to be distributed to communities to encourage the plants' cultivation and consumption, improve nutrition and health, and promote the conservation of these plant species. However, it had limitations in terms of sample collection and analysis (n = 1). Moreover, very little information exists about the biodiversity of Thailand's forests in terms of the health and nutritional benefits of many indigenous plants. Thereby, the present study was carried out in 2019 to explore indigenous plant foods within the conservation area of the Plant Genetic Conservation Project under the Royal initiative of Her Royal Highness Princess Maha Chakri Sirindhorn in the area of the Electricity Generating Authority of Thailand, Srinakarind Dam, Kanchanaburi province. This new survey's objective was to determine the nutritional potential of Thai indigenous plants in terms of nutrients, bioactive compounds, as well as antioxidant activities and carotenoid contents.
This study was conducted along five routes (Figure 1) within the Plant Genetic conservation area at four separate time periods from January to September 2019. The eight commonly consumed indigenous plants collected for this study were identified and confirmed by local people and botanists. These plants are selected based on their being commonly consumed by local people and available in the study's areas. Information about these plants and photographs are presented in Table 1 and Figure 2. Plant samples were kept in dark plastic bags to protect the sunlight and then transported via an ice-box to the ISO/IEC 17025 laboratory at the Institute of Nutrition, Mahidol University.

Figure 2. Photographs of Pak-Wan-Pa (A), Ta-Kuk (B), Ka-Pee-Jun (C), Ka-Tur (D), Jang (E), Pak-God (F), Wan-Pro (G), and Som-Kob (H).
At the laboratory, the edible part of each sample was collected and recorded. Each sample was washed with tap water, followed by washing twice with deionized water. After cleaning and removing excess water, cooked samples were blanched (plant to water ratio about 1:2) or boiled (plant to water ratio about 1:1) depending on each plant's commonly eaten method (Table 1). Weights before and after cooking (about 10 min post-heat treatment) were recorded. Thereafter, both fresh and cooked samples were homogenized using a food processor (Mara®, Thailand) and divided into two portions. For the first portion, each sample was stored in an acid-washed screw-capped plastic bottle at −20°C for nutrient analyses. For vitamin C analysis, each sample was weighed to about 20 g in a plastic bottle, then about 20 g 10% MPA was added, mixed together, and then kept in a freezer. For the second portion, each sample was dried using a freeze dryer (Heto powerdry PL 9000), ground, vacuum-packed in laminated aluminum foil bags, and stored at−20°C for determination of bioactive compound and antioxidant activities.
Nutrient analysis was conducted using the standard AOAC method (2019) (Saha and Ahmed, 2009). All samples were analyzed at the ISO/IEC 17025:2017 accredited laboratory of the Institute of Nutrition, Mahidol University, which analyzed proximate compositions, vitamins, and minerals in duplicate analyses. All values were presented per 100 g fresh sample.
Total nitrogen was determined using the Kjeldahl method, methods nos. 910.20 and 981.10 (Saha and Ahmed, 2009), and calculated into protein content using specific (Jones) factors (N × 6.25). Moisture content was determined by drying each sample in a hot air oven at 100 ± 2°C until a constant weight was obtained based on method nos. 990.19 and 952.23 [Association of Official Analytical Chemists (AOAC), 2016]. Crude fat content was determined by acid hydrolysis and extraction using petroleum ether in the Soxtec system (Avanti 2055, Foss Tecator, Sweden), method nos. 922.32 and 945.16 [Association of Official Analytical Chemists (AOAC), 2016]. Ash content was analyzed by incinerating all organic matter at 550 ± 5°C, method nos. 945.46 and 930.30 [Association of Official Analytical Chemists (AOAC), 2016]. Available carbohydrate was calculated using the following formula: 100-moisture-protein-fat-ash-dietary fiber; energy was calculated by Atwater factors (4 for protein and available carbohydrate, 2 for dietary fiber, and 9 for total fat). Total dietary fiber was analyzed using the enzymatic gravimetric method, method no. 985.29 [Association of Official Analytical Chemists (AOAC), 2016].
Based on the previous report of Charoenkiatkul et al. (2012), the potential of vitamin for the studied plants is only vitamin C. For vitamin C, high pressure liquid chromatography (HPLC) with a UV/Vis detector was used for analysis (Sánchez-Mata et al., 2000). Samples were extracted in 3% metaphosphoric acid and filtered to homogenate. Ascorbic acid was separated by reversed-phase HPLC with UV detection at 248 nm and quantified against an external ascorbic acid standards. For minerals, microwave digestion was used for sample decomposition to determine magnesium, iron, copper, and zinc using an inductively coupled plasma optical emission spectrophotometer (ICP-OES), method no. 984.27 [Association of Official Analytical Chemists (AOAC), 2016]. The acid solution dissolved from the ash residue was used for calcium, sodium, and potassium analyses by flame atomic absorption spectrophotometer (AAS), method no. 985.35 [Association of Official Analytical Chemists (AOAC), 2016]. Phosphorus was determined by the gravimetric method [Association of Official Analytical Chemists (AOAC), 2016].
Samples were saponified and extracted according to the procedures of Sungpuang et al. (Sungpuang et al., 1999) with slight modification. In brief, freeze-dried samples were boiled with ethanolic potassium hydroxide with the addition of 10% (w/v) ascorbic acid, before being extracted with hexane, and then evaporated to dryness. The residue was reconstituted with mobile phase and then filtered through a 0.2 lm PTFE syringe filter. Chromatographic separation of individual carotenoids was modified slightly from the method described by Chitchumroonchokchai et al. (2017). The HPLC system for determining carotenoid content was carried out by Agilent 1100 series (Agilent Technologies, U.S.A.) and a photodiode array detector. The column used was an analytical YMC™ C30 column (4.6 × 150 mm, 5 μm, Waters, Milford, MA). The mobile phase consisted of 98% (v/v) methanol with 2% (w/v) ammonium acetate (solvent A) and methyl tert-butyl ether (MtBE) (solvent B). Isocratic system with 80% solvent A and 20% solvent B was used at a constant flow rate at 0.6 ml/min at ambient temperature. Qualitative and quantitative evaluations of chromatograms were monitored at 450 and 470 nm using ChemStation (Agilent Technologies, USA). Individual carotenoids (lutein, zeathantin, β-cryptoxanthin, α-carotene, and β-carotene) were identified based on comparing retention time and the UV spectrum of unknown peaks to the authentic standards. Carotenoid contents were expressed as mg per 100 g sample.
Total phenolic contents were determined by spectrophotometry using Folin–Ciocalteau reagent method (Lu et al., 2007) with slight modification. The absorbance was measured against the gallic acid standard at 760 nm. Total phenolic contents were expressed as mg gallic acid equivalent (GAE) per 100 g sample.
The method for performing ORAC assay based on fluorescence probe and fluorescein has been described by Ou et al. (2002). Each sample's ethanolic extract was mixed with fluorescein solution and AAPH solution at 37°C. Fluorescence was recorded using a spectrofluorometer (Luminescence spectrophotometer, Perkin Elmer LS55, Maryland, USA) with an excitation wavelength of 493 nm and an emission wavelength of 515 nm. Results were calculated using the differences of areas under the curve between samples or standard (trolox) and blank. The ORAC values were expressed as μmol trolox equivalent (TE) per 100 g sample.
Samples were weighed (4 significant digits) before and after cooking. The data obtained, combined with the amounts of nutrients in raw and cooked plants, were used to calculate true retention (Murphy et al., 1975) as per the formula
Each nutrient in the plant samples was reported as mean values ± standard deviation from three individual samples. The paired samples t-test compares the means of two measurements (fresh and cooked) taken from the same individual sample was used to indicate significance difference (p ≤ 0.05) of effect of cooking of each parameter. Statistical analysis was performed using IBM® (Hampshire, UK) SPSS Statistics for Window, Version 19.0.
The edible portions of shoots and young leaves of Jang, Pak-Wan-Pa, Ta-Kuk, Ka-Pee-Jun, Pak-God, and Som-Kob ranged from 52 to 66%, while these portions for Ka-Tur and Wan-Pro ranged from 72 to 95%, respectively.
The proximate compositions of the eight indigenous plant varieties is shown in Table 2 below. Approximately 69–96% of composition was water. Cooked indigenous plant samples had an increased moisture content of 1–5%, which may be due to the water used for blanching or boiling. The protein content of samples ranged from 0.8 to 9.5 g/100 g fresh weight (FW). Protein content was greatest in Jang, Ta-Kuk, and Pak-Wan-Pa. Dietary fiber was the main nutrient contained in the samples, ranging from 2.6 to 10.3 g/100 g FW. The shoots and young leaves of Jang contained the highest amount of dietary fiber, followed by Som-Kob, Ta-Kuk, Pak-Wan-Pa, Ka-Tur, and Wan-Pro. Available carbohydrate content ranged from 2.2 to 9.3 g/100g FW. Fat content in all samples was <1 g/100g FW. Protein, dietary fiber, and fat contents in this study were similar and showed no significant differences (p ≥ 0.05) with those previously reported (Charoenkiatkul et al., 2012), i.e., Pak-Wan-Pa (protein 5.97 vs. 6.5 g/100g, dietary fiber 4.95 vs. 6.5 g/100 g, fat 0.83 vs. 1.2 g/100 g), Ta-Kuk (protein 7.70 vs. 7.30 g/100 g, dietary fiber 5.35 vs. 5.6 g/100 g, fat 0.68 vs. 0.60 g/100 g), Wan-Pro (protein 0.80 . 0.80 g/100 g, dietary fiber 2.81 vs. 3.20 g/100 g, fat 0.07 . 0.10 g/100 g), and Ka-Tur (protein 1.08 vs. 0.90 g/100 g, dietary fiber 3.86 vs. 4.80 g/100 g, fat 0 vs. 0.10 g/100 g), respectively. Protein content in this study was lower than that reported from Egypt, which was 15.14 g/100 g for Ta-Kuk (Hawary et al., 2011). For cooked vegetables, most proximate compositions decreased significantly (p < 0.05) compared to those of the same fresh vegetables. A wide range of decreasing protein content was observed (2.5% in Pak-Wan-Pa to 39.6% in Jang). A similar decreasing pattern was also found in ash after cooking (25.3% in Som-Kob to 55.4% in Jang). Nutrient amounts decreased slightly after cooking, which may have been caused by the dissolution of nutrients into the water used for cooking (Lyimo et al., 1990).
Vitamin C and mineral contents in the eight varieties of indigenous plants are presented in Table 3 below. The shoots and young leaves of Ta-Kuk contained the highest amount of vitamin C (212 mg/100 g FW), followed by Pak-Wan-Pa, Jang, and Ka-Pee-Jun (146 ± 19, 126 ± 7, and 27 ± 9 mg/100 g FW, respectively). The vitamin C contents of Pak-God (7 ± 1 mg/100g), Wan-Pro, Ka-Tur, and Som-Kob were < 10 mg/100 g FW. A previous report (Charoenkiatkul et al., 2012) showed lower levels of vitamin C in Pak-Wan-Pa (146 vs. 99 mg/100 g) and Ta-Kuk (126 vs. 161 mg/100 g), while Wan-Pro was higher (2 vs. 3 mg/100 g). There were significant vitamin C losses (p < 0.05) in cooked vegetables compared to that of fresh vegetables (ranging from 34.9% in Jang to 73.6% in Ta-Kuk) except for Wan-Pro due to no vitamin C being detected in the previous study (Charoenkiatkul et al., 2012). After cooking, the amount of vitamin C probably decreased due to its instability at high temperatures (Oboh, 2005).
The highest macro mineral content was potassium, ranging from 231 to 560 mg/100 g FW, with the highest being contained in the shoots and young leaves of Jang. All indigenous plant samples also provided calcium and phosphorus (39–186 and 31–137 mg/100 g FW, respectively), but only a small amount of magnesium (21–62 mg/100 g FW) and sodium (<40 mg/100 g FW), which were mainly found in the shoots and young leaves of Pak-Wan-Pa. Consistent with previous studies, Pak-Wan-Pa was high in vitamin C, phosphorus, and calcium [99, 133, and 108 mg/100 g FW, respectively (Charoenkiatkul et al., 2012); 83.6, 68, and 24 mg/100 g FW, respectively (Somsub et al., 2008)]. For trace elements, all indigenous plant samples contained iron, copper, and zinc at <3 mg/100 g FW. The shoots and young leaves of Wan-Pro contained the highest amount of iron (2.79 mg/100 g FW), while Jang contained the highest amount of copper and zinc (0.30 and 1.41 mg/100 g FW, respectively). After cooking, the amount of minerals in most vegetables significantly decreased (p < 0.05) probably due to their migrating into the boiling water (Oboh, 2005). Shoots and young leaves of Jang have a major characteristic being consumed as softened leaves as opposed to other vegetables which may play a role in loss of several minerals (63.6% for K, 54.8% for Mg, 50.0% for Cu, 47.9% for Ca, 43.3% for Zn, 25.2% for Fe and 22.8% for P).
Carotenoid contents of the eight indigenous plant varieties are shown in Table 4 below. The highest total carotenoid contents were found in the shoots and young leaves of Wan-Pro, followed by Ta-Kuk, Som-Kob, Pak-Wan-Pa (142.9, 42.8, 32.2, 28.0 mg/100 g FW, respectively) (Figure 3), while the lowest was in the flowers of Ka-Tur (2.0 mg/100 g FW). The carotenoid contents of indigenous plants in this study were significantly higher (p > 0.05) than in the original previous study (Charoenkiatkul et al., 2012), such as Pak-Wan-Pa (28 vs. 5.4 g/100g), Ta-Kuk (1.97 vs. 0.72 g/100 g), Wan-Pro (142.9 vs. 3.8 g/100 g), and Ka-Tur (1.97 vs. 0.72 g/100 g). All indigenous plant varieties contained predominantly β-carotene (55–89%), followed by α-carotene (10–45%), except for Wan-Pro which contained α-carotene (51–54%) followed by β-carotene (46–49%). Lutein, zeathantin, and β-cryptoxanthin were not detected in all plants in this study, except for Pak-Wan-Pa where β-cryptoxanthin was detected at 3 mg/100 g FW. Comparing carotenoid content between cooked and raw plant, the carotenoid contents of the cooked plant samples were significantly higher (p < 0.05) than that of the corresponding raw samples, except for Pak-Wan-Pa and Pak-God. Thermal food processes, such as boiling and blanching, can cause partial degradation of carotenoid pigments and promote isomerization of β-carotene content in the samples (Bernhardt and Schlich, 2006; D'evoli et al., 2013).
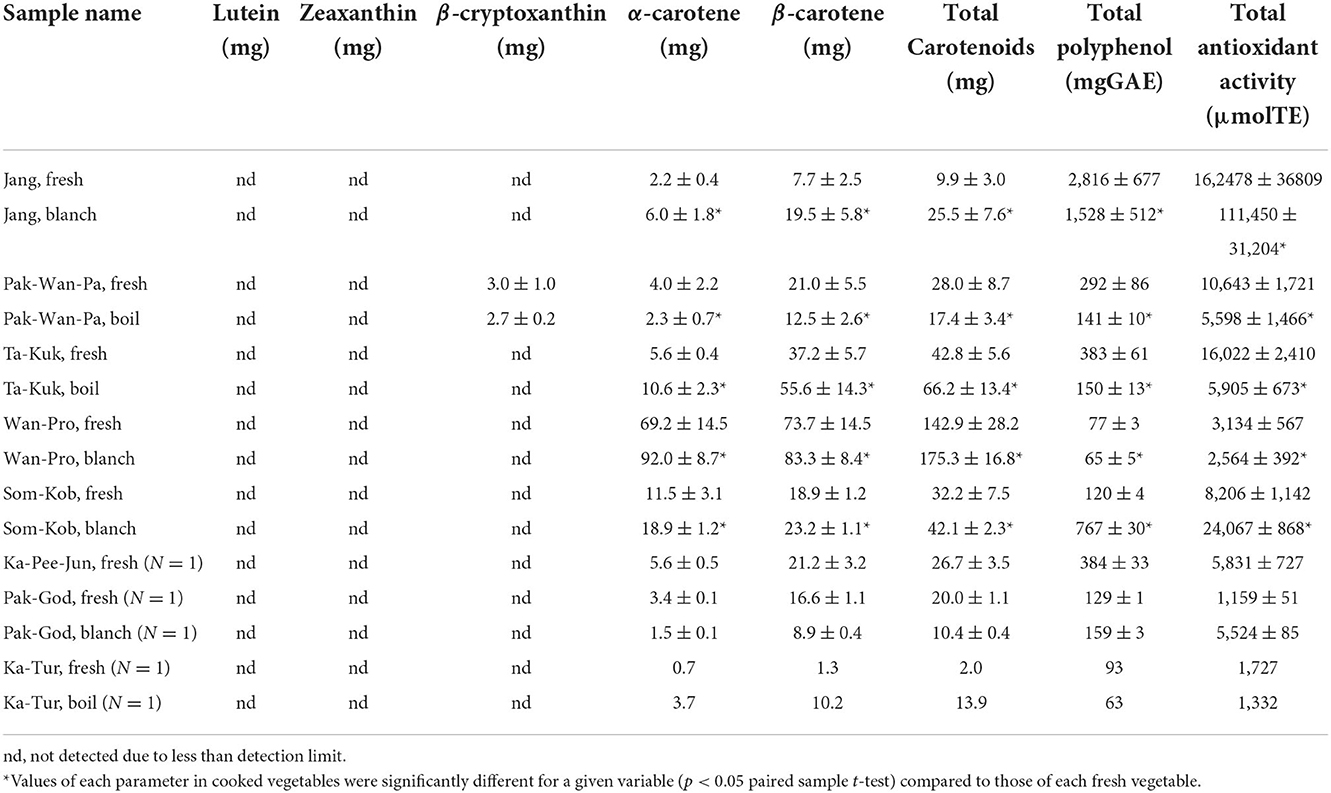
Table 4. Bioactive compounds and antioxidant activities of the indigenous plants (per 100 g fresh weight, N = 3).
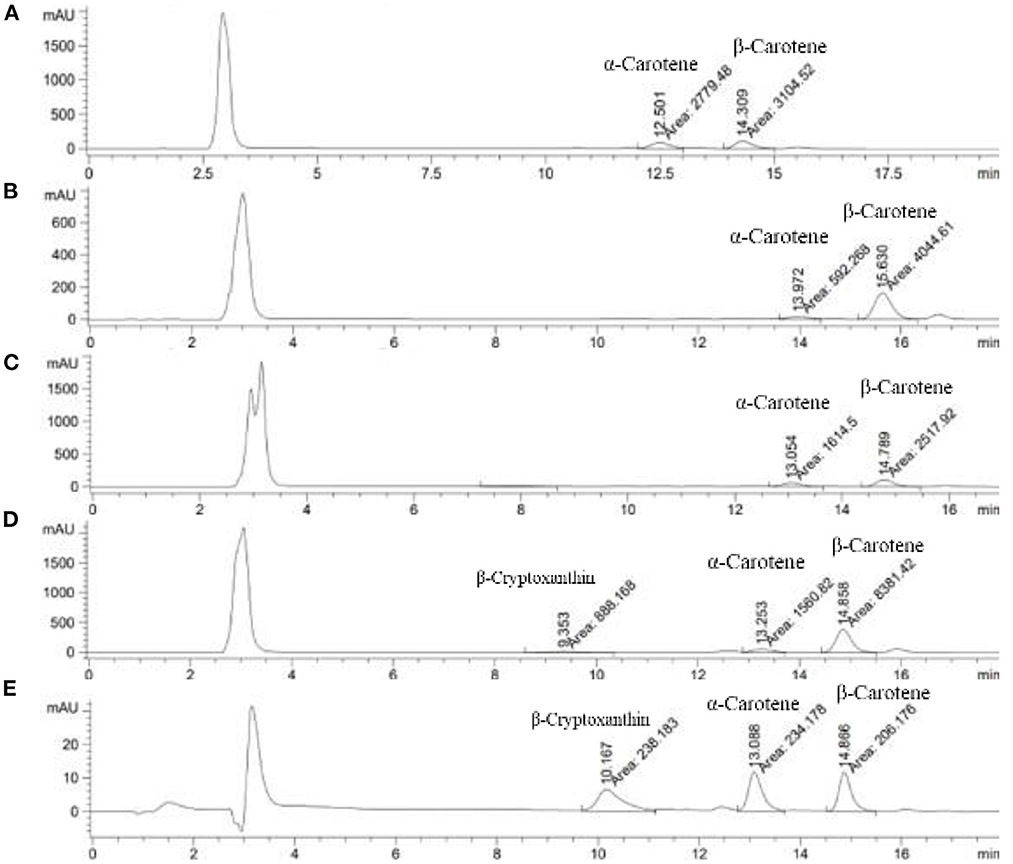
Figure 3. Chromatogram of identifying the peaks of carotenoid compounds of Wan-Pro (A), Ta-Kuk (B), Som-Kob (C), Pak-Wan-Pa (D), Standard carotenoids (E).
Total phenolic contents of the eight indigenous plant varieties varied widely, ranging from 77 to 2816 mg GAE/ 100 g FW as shown in Table 4 below. Shoots and young leaves of Jang had the highest total phenolic contents, followed by Ta-Kuk and Pak-Wan-Pa (2816, 383, 292 mg GAE/ 100 g FW, respectively), and the lowest content was in Wan-Pro (77 mg GAE/ 100 g FW). The wide variation in total polyphenol contents in plants might occur from the variety of the plant, stage of ripening, climate conditions, sample collection, transportation, and sample preparation (Rop et al., 2011). Comparison of total phenolic contents of some indigenous plants in this study, such as Pak-Wan-Pa and Ta-Kuk, with results of previous studies indicate similar contents, i.e., Pak-Wan-Pa from Saraburi and Uthai Thani provinces (296.44 and 265.63 mg GAE/100 g, respectively) (Charoenchai et al., 2015), Pak-Wan-Pa and Ta-Kuk from Kanchanaburi province (296 and 451 mg GAE/100 g, respectively) (Charoenkiatkul et al., 2012).
The results of antioxidant activities as measured by ORAC assay were accordance with the result of total phenolic contents, which are shown in Table 4 below. The shoots and young leaves of Jang (162,478 μmol TE/100 g FW), Ta-Kuk (16,022 μmol TE/100 g FW), and Pak-Wan-Pa (10,643 μmol TE/100 g FW) had the greatest ORAC values, while the lowest was in Wan-Pro (3,134 μmol TE/100 g FW). A strong consistency between total phenolic content and ORAC values was observed in many other studies, which confirmed that phenolic was the major contributor to antioxidant activities (Maisuthisakul and Suttajit, 2007; Han and Yang, 2008; Maisuthisakul and Pasuk, 2008). ORAC values in this study were less than those previously reported (Charoenkiatkul et al., 2012), but there were no significant differences (p ≥ 0.05), such as Pak-Wan-Pa (10,643 vs. 8,000 μmol TE/100 g FW), Ta-Kuk (16,022 vs. 14,400 μmol TE/100 g FW), Wan-Pro (3,134 vs. 3,800 μmol TE/100 g FW), and Ka-Tur (1,727 vs. 1,400 μmol TE/100 g FW), respectively.
The percentage true retention (%TR) of nutrients in the cooked processes is presented in Table 5 below. The retention of protein, dietary fiber, and minerals (calcium, phosphorus, magnesium, iron, copper, and zinc) were almost wholly retained (closed to 100%TR), while potassium was the least retained (49.9–77.10%TR). Blanched plants retained higher concentrations of vitamin C than those that had been boiled (97.8–109.8 and 35.8–42.5%TR for blanching and boiling, respectively). Vitamin C retention in cooked vegetables in a previous study ranged from 34.1 to 79.4% among the various vegetables and cooking methods, but was the least in boiling (Mellova et al., 1996). This result was consistent with the lowest vitamin C retention values shown in the boiling of spinach (33.9%TR) and green beans (63.7%TR) (Warthesen et al., 1984). The main mechanisms of vitamin C loss appear to be due to the vitamin's water solubility and sensitivity to temperature, so it is easily degraded during cooking. Elevated temperatures and long cooking times have been found to cause particularly severe losses of vitamin C (Rumm-Kreuter and Demmel, 1990; Oboh, 2005; Tian et al., 2016). The retention of total polyphenol and antioxidant in this study's boiled plants (52.6 and 50.0%TR in Ta-Kuk, 60.0 and 62.5%TR in Pak-Wan-Pa, respectively) was lower than in blanched plants (81.7 and 102.0% TR in Jang, 98.1 and 96.2% TR in Wan-Pro, respectively). The retention of carotenoids increased in boiled or blanched plants (>100%TR), except for Pak-Wan-Pa (74.3%TR). A previous study of cooked vegetables showed β-carotene retention was in the range of 40.02–125.37% (Lee et al., 2018). Carotenoids extractability may be influenced by cooking. Additionally, the difference in the retention of carotenoids in cooked plants might be attributed to the loss of carotene caused by dripping during the cooking process (Bureau et al., 2015).
Nutrients, bioactive compounds, as well as antioxidant activities and carotenoid contents of eight commonly consumed indigenous plants in the conservation area were analyzed. The major nutrients found in all of the study's indigenous plants were protein and dietary fiber, while there were low levels of available carbohydrate and fat. The highest macro mineral content in all of the plants was potassium, followed by calcium and phosphorus. However, only small amounts of magnesium and sodium were evident. Trace elements found in small amounts in all of the plants were iron, copper, and zinc. The shoots and young leaves of fresh Ta-Kuk and Pak-Wan-Pa contained high level of vitamin C however it dramatically lost after cooking. Antioxidant activity in all of the plants was in accordance with phenolic compounds. Greater retentions of vitamin C, phenolic contents, and antioxidant activities were achieved by blanching compared to boiling, while retention of carotenoids increased when boiled or blanched. Shoots and young leaves of three indigenous plants [Jang (Maerua siamensis (Kurz), Ta-Kuk (Albizia lebbeck (L.) Benth), and Pak-Wan-Pa (Melientha suavis Pierre)] showed the greatest amounts of nutrients, bioactive compounds, and antioxidant activity which are recommended for consumption and promotion.
The data generated from this study are important for highlighting the nutritional value of these indigenous plants, their potential role in different food products, and their wider application for the food system overall. As such, the conservation of these indigenous plants should be a major food security concern and initiative. Sustainable extensive cultivation should also be undertaken to increase availability and diversity of the foods for community consumption. Further studies on health promotion and disease prevention as well as development of value-added products for trade should be undertaken.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
KJ and NS contributed to the conception and design of the study. PS, PK, NS, MS, and KJ did the survey and collect all samples in the conservation areas. PS and CC analyzed nutritive values and bioactive compounds. PK, CC, and KJ performed the statistical analysis and wrote a draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was supported by Mahidol University (Research budget of the year 2019).
We would like to thank the Plant Genetic Conservation Project initiated by Her Royal Highness Princess Maha Chakri Sirindhorn for allowing access to the study area and to the Electricity Generating Authority of Thailand at Srinakarind Dam for providing staff, accommodation, facilities, and cooperation during field work. We also would like to thank Mr. Philipda Suthipibul and Miss Sang-Arun Meepho for collecting indigenous plants in the study area.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Association of Official Analytical Chemists (AOAC) (2016). Official methods of Analysis (20th ed.). Gaithersburg, MD: AOAC International.
Aziz, M. A., Khan, A. H., Adnan, M., and Ullah, H. (2018). Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J Ethnobiol Ethnomed. 14, 11. doi: 10.1186/s13002-018-0212-0
Bernhardt, S., and Schlich, E. (2006). Impact of different cooking methods on food quality: retention of lipophilic vitamins in fresh and frozen vegetables. J. Food Eng. 77, 327–333. doi: 10.1016/j.jfoodeng.2005.06.040
Bobby, M. D. N., Wesely, E. G., and Johnson, M. (2012). High performance thin layer chromatography profile studies on the alkaloids of Albizia lebbeck. Asian Pac. J. Trop. Biomed. 2, S1–S6. doi: 10.1016/S2221-1691(12)60119-1
Bobby, M. N., and Wesely, E. G. (2012). In vitro anti-bacterial activity of leaves extracts of Albizia lebbeck Benth against some selected pathogens. Asian Pac. J. Trop. Biomed. 2, S859–S862. doi: 10.1016/S2221-1691(12)60324-4
Bureau, S., Mouhoubi, S., Touloumet, L., Garcia, C., Moreau, F., Be'douet, V., et al. (2015). Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT-Food Sci. Technol. 64, 735–741. doi: 10.1016/j.lwt.2015.06.016
Chaachouay, N., Douira, A., and Zidane, L. (2022). Herbal medicine used in the treatment of human diseases in the rif, northern morocco. Arab. J. Sci. Eng. 47, 131–153. doi: 10.1007/s13369-021-05501-1
Chanthasri, W., Puangkeaw, N., Kunworarath, N., Jaisamut, P., Limsuwan, S., Maneenoon, K., et al. (2018). Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complement Altern. Med. 18, 73. doi: 10.1186/s12906-018-2131-y
Charoenchai, L., Settharaksa, S., Madaka, F., and Sueree, L. (2015). Evaluation of the antioxidant activities, total phenolic and flavonoid contents of Melientha suavis Pierre. Bull. Health Sci. Technol. 12, 29–37.
Charoenkiatkul, S., Judprasong, K., Sukprasansap, M., Thiyajai, P., and Kettawan, A. (2012). Survey and Database Development of Nutrients, Bioactive Compounds and Health Benefit of Local Foods at Conserve Area of Plant Genetic Conservation Project under the Royal Initiative of Her Royal Highness Princess Maha Chakri, Sirindhorn, Kanchanaburi, Province., Final report (2012).
Chitchumroonchokchai, C., Gianfranco, D., Bruno, P., Giovanni, G., and Mark, L. F. (2017). Potential of golden potatoes to improve vitamin A and vitamin E status in developing countries. PLoS ONE. 2017, 1–13. doi: 10.1371/journal.pone.0187102
Desai, T. H., and Joshi, S. V. (2019). Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol. 231, 494–502. doi: 10.1016/j.jep.2018.11.004
D'evoli, L., Lombardi-Boccia, G., and Lucarini, M. (2013). Influence of heat treatments on carotenoid content of cherry tomatoes. Foods. 2, 352–363. doi: 10.3390/foods2030352
Eddouks, M., Chattopadhyay, D., De Feo, V., and Cho, W. C. S. (2014). Medicinal plants in the prevention and treatment of chronic diseases 2013. Evidence Based Complement. Alternative Med. 3, 2014. doi: 10.1155/2014/180981
ESCOP (European Scientific Cooperative on Phytotherapy) (2021). ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. Available at: https://escop.com/ (accessed November 16, 2022).
Gras, A., Parada, M., Vallès, J., and Garnatje, T (2021). The role of traditional plant knowledge in the fight against infectious diseases: a meta-analytic study in the catalan linguistic area. Front. Pharmacol. 12, 744616. doi: 10.3389/fphar.2021.744616
Han, J., and Yang, Y. (2008). Content of phytosterols in vegetables and fruits commonly consumed in China. Biomed. Environ. Sci. 21, 449–453. doi: 10.1016/S0895-3988(09)60001-5
Hawary, S. E. I., Fouly, K. E. I., Sokkar, N. M., and Talaat, Z. (2011). A phytochemical profile of Albizia lebbeck (L.) Benth. cultivated in Egypt. Asian J. Biochem. 6, 122–141. doi: 10.3923/ajb.2011.122.141
Issuriya, A., Puangkeaw, N., Choochana, P., Jaisamut, P., Kunworarath, N., Maneenoon, K., et al. (2019). Safety and antioxidant potential of traditional Thai poly-herbal tea “phy-blica-d” used as a rejuvenation formula. Phcog. Res. 11, 295–303. doi: 10.4103/pr.pr_5_19
Kar, B., Nepal, A., Kumar, R. B., Dolai, N., Bhattacharya, S., Mazumder, U. K., et al. (2013). Antioxidant and anti-inflammatory properties Hymenodictyon excelsum bark. Orient Pharm. Exp. Med. 13, 103–111. doi: 10.1007/s13596-012-0077-z
Lee, S., Choi, Y., Jeong, H. S., and Lee, J. (2018). Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 27, 333–342. doi: 10.1007/s10068-017-0281-1
Lu, J., Zhao, H., Chen, J., Fan, W., Dong, J., Kong, W., et al. (2007). Evaluation of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 55, 10994–11001. doi: 10.1021/jf0722710
Lyimo, M. H., Nyagwegwe, S., and Mnkeni, A. P. (1990). Investigations on the effect of traditional food processing, preservation and storage methods on vegetable nutrients: a case study in Tanzania. J. Plant Foods Hum. Nutr. 41, 53–57. doi: 10.1007/BF02196382
Maisuthisakul, P., and Pasuk, S. (2008). Relationship between antioxidant properties and chemical composition of some Thai plants. J. Food Compos. Anal. 21, 229–240. doi: 10.1016/j.jfca.2007.11.005
Maisuthisakul, P., and Suttajit, M. (2007). Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 100, 1409–1418. doi: 10.1016/j.foodchem.2005.11.032
Mellova, A. M., David, W. G., and Jydy, A. D. (1996). Retention of vitamin C, iron, and β-carotene in vegetables prepared using different cooking methods. J. Food Quality. 20, 403–418. doi: 10.1111/j.1745-4557.1997.tb00483.x
Murphy, E. W., Criner, P. E., and Gray, B. C. (1975). Comparisons of methods for calculating retentions of nutrients in cooked foods. J. Agr. Food Chem. 23, 1153–1157. doi: 10.1021/jf60202a021
Oboh, G. (2005). Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Sci. Technol. 38, 513–517. doi: 10.1016/j.lwt.2004.07.007
Ou, B., Huang, D., Hampsch-Woodili, M. H., Flanagan, J. A., and Deemer, E. K. (2002). Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing power (FRAP) assays: a comparative study. J. Agric. Food Chem. 50, 3122–3128. doi: 10.1021/jf0116606
Pal, B. C., Achari, B., Yoshikawa, K., and Arihara, S. (1995). Saponins from Albizia lebbeck. Phytochemistry 38, 1287–1291. doi: 10.1016/0031-9422(94)00796-V
Paramita, C., Sajeesha, S., Anuja, A. Nair Nishat, A., Tripathi, Y. C. (2017). Medicinal applications, phytochemistry and pharmacology of hymenodictyon excelsum (Roxb) wall: a review. Organic Med. Chem. J. 2, 555589. doi: 10.19080/OMCIJ.2017.02.555589
Phosri, R. (2017). Chemistry and Biological Activity Studies of Maerua siamensis. (Master's thesis, Burapha University, Chonburi, Thailand). Available onlinea at: http://digital_collect.lib.buu.ac.th/dcms/files/57920931.pdf (accessed April 10, 2022).
Phumthum, M., and Balslev, H. (2020). Anti-infectious plants of the thai karen: a meta-analysis. Antibiotics 9, 298. doi: 10.3390/antibiotics9060298
Praengam, K., Muangnoi, C., Charoenkiatkul, S., and Thiyajai, P. (2017). Antioxidant and anti-inflammatory activity of aqueous fraction from Albizia lebbeck leaves. Int. Food Res. J. 24, 1174–1185. Available online at: http://www.ifrj.upm.edu.my/24%20(03)%202017/(37).pdf
Rahman, M. M. (2015). Evaluation of Hymenodictyon excelsum phytochemical's therapeutic value against prostate cancer by molecular docking study. Jundishapur J. Nat. Pharmaceut. Prod. 10, e18216. doi: 10.17795/jjnpp-18216
Rop, O., Sochor, J., Jurikova, T., Zitka, O., and Skutkova, H. (2011). Effect of five different stages of ripening on chemical compounds in Medlar (Mespilus germanica L.) Molecules. 16, 74–91. doi: 10.3390/molecules16010074
Rumm-Kreuter, D. R., and Demmel, I. (1990). Comparison of vitamin losses in vegetables due to various cooking methods. J. Nutr. Sci. Vitaminol. 36, S7–S15. doi: 10.3177/jnsv.36.4-SupplementI_S7
Saha, A., and Ahmed, M. (2009). The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pak. J. Pharm. Sci. 22, 74–77.
Sánchez-Mata, M. C., Cámara-Hurtado, M., Díez-Marqués, C., and Torija-Isasa, M. E. (2000). Comparison of high-performance liquid chromatography and spectrofluorimetry for vitamin C analysis of green beans (Phaseolusvulgaris L.). Eur. Food Res. Technol. 210, 220–225. doi: 10.1007/PL00005516
Sansomchai, P., Jumpatong, K., Lapinee, C., and Utchariyajit, K. (2021). Melientha suavis Pierre. Extract: antioxidant and sunscreen properties for future cosmetic development. CMUJ. Nat. Sci. 20, e2021008. doi: 10.12982/CMUJNS.2021.008
Sharma, G. K., and Nishtha, D. (2015). Review of Shirish (Albizia lebbeck) therapeutic properties. Int. J. Ayurvedic Herbal Med. 5, 1683–1688. Available online at: https://www.semanticscholar.org/paper/Review-of-Shirish-(-Albizia-lebbeck-)-therapeutic-Sharma-Dubey/77d78ae2262164e48d5799c56e576c1c039d167c
Somsub, W., Kongkachuichai, R., Sungpuag, P., and Charoensiri, R. (2008). Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. J. Food Compos. Anal. 21, 187–197. doi: 10.1016/j.jfca.2007.08.002
Sungpuang, P., Tangchitpianvit, S., and Chittchang, U. (1999). Retinal and beta carotene content of indigenous raw and home-prepared foods in Northeast Thailand. Food Chem. 64, 163–167. doi: 10.1016/S0308-8146(98)00154-X
Tian, J., Chen, J., Lv, F., Chen, S., Chen, J., Liu, D., et al. (2016). Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 197, 1264–1270. doi: 10.1016/j.foodchem.2015.11.049
Vasanthi, P., Evanjelene, G. M., Ayyavuv, N., Angamuthu, J. (2014). Phytochemical screening and antioxidant activity of extracts of the leaf and bark of Albizzia lebbeck (Benth). Acad. J. Med. Plants 2, 26–31. doi: 10.15413/ajmp.2013.0138
Velraj, M., Vijayalakshmi, A., Jayakumari, S., Ramamoorthy, S. K., Ravichandiran, V., and Srikanth, J. (2010). Antidepressant-like effects of the ethanolic extract of Albizzia lebbeck (Linn) leaves in animal models of depression. Res. J. Pharmacognosy Phytochemistry 2, 30–33. Available online at: https://www.indianjournals.com/ijor.aspx?target=ijor:rjpp&volume=2&issue=1&article=008
Keywords: indigenous plants, nutrients, bioactive compound, cooking method, true retention
Citation: Sridonpai P, Kongprapun P, Sungayuth N, Sukprasansap M, Chimkerd C and Judprasong K (2022) Nutritive values and phytochemical compositions of edible indigenous plants in Thailand. Front. Sustain. Food Syst. 6:870147. doi: 10.3389/fsufs.2022.870147
Received: 06 February 2022; Accepted: 22 November 2022;
Published: 09 December 2022.
Edited by:
Shivraj Hariram Nile, Zhejiang Chinese Medical University, ChinaReviewed by:
C. K. Sunil, Indian Institute of Food Processing Technology, IndiaCopyright © 2022 Sridonpai, Kongprapun, Sungayuth, Sukprasansap, Chimkerd and Judprasong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunchit Judprasong, a3VuY2hpdC5qdWRAbWFoaWRvbC5hYy50aA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.