- 1Food Microbiology and Biotechnology Unit, Department of Microbiology, University of Ibadan, Ibadan, Nigeria
- 2Department of Plant Sciences, North Dakota State University, Fargo, ND, United States
- 3Department of Pharmaceutical Chemistry, University of Ibadan, Ibadan, Nigeria
Phenolics- enriched plant food sources are excellent dietary and therapeutic targets to combat the increasing prevalence of diet and lifestyle-influenced non-communicable chronic diseases (NCDs), such as type 2 diabetes (T2D). Among plant sources, edible flowers rich in health protective phenolic compounds provide novel opportunities as ingredient and nutraceutical sources. Roselle (Hibiscus sabdariffa Linn.) is a popular edible flower and consumed as part of traditional cuisines and processed foods in several countries of Asia and Africa. Red calyces of Roselle are rich in phenolic compounds, which potentially have high antioxidant and anti-hyperglycemic properties. Therefore, there is merit in screening of dried Roselle calyces as sources for functional food ingredients or nutraceuticals to counter chronic oxidative stress and chronic hyperglycemia using in vitro assays. This has led to this study to investigate and compare phenolic compounds associated antioxidant and anti-hyperglycemic functions of different organic solvent-extracted fractions of dried Roselle calyces using rapid in vitro assays-based screening strategy. Total soluble phenolic content, profile of phenolic compounds, free radical scavenging assay-based total antioxidant activity, and anti-hyperglycemic function linked α-amylase and α-glucosidase inhibitory activities of four different organic solvents (chloroform, hexane, ethyl acetate, and initial crude extraction in 100% methanol) extracted fractions of calyces of Roselle were determined using in vitro assays. Studies indicated high phenolic-linked antioxidant and anti-hyperglycemic relevant properties in red Roselle calyces, specifically in ethyl acetate and methanol solvent-based extracted fractions. Major phenolic compounds in extracted fractions of Roselle calyces were chlorogenic acid, caffeic acid, gallic acid, catechin, rutin, benzoic acid, and cinnamic acid. Additionally, moderate α-amylase (30–92%) and very high α-glucosidase (81–98%) inhibitory activities were confirmed in undiluted samples of organic solvent-extracted fractions of Roselle calyces in the in vitro assays. Taken together these in vitro screening results indicated that calyces of Roselle are excellent sources of health protective phenolic compounds with high antioxidant and anti-hyperglycemic functions and organic solvent (ethyl acetate and methanol) extracted fractions of this edible flower can be strategically utilized to design functional food ingredients and nutraceuticals.
Introduction
Improving the access and availability of nutritious, human health relevant, safe, and inexpensive plant-based food sources is essential to counter the rapid rise in the prevalence of poor diet (higher daily intake of hyper-processed and calorie dense food) and lifestyle-influenced non-communicable chronic diseases (NCDs) worldwide. To find solutions to these NCD-linked challenges, it is essential to diversify contemporary dietary sources by targeting and integrating underutilized plant-based edible sources for wider human health protective functional benefits. Edible flowers offer opportunities with balanced nutrition and rich health protective bioactive profile which are safe dietary sources with therapeutic potential that can be targeted as functional food ingredients or nutraceuticals to prevent and mitigate NCD-related health risks. Among major NCDs, type 2 diabetes (T2D) is a chronic and complex syndrome of interlinked metabolic conditions caused by a systemic breakdown of glucose homeostasis (Ademiluyi and Oboh, 2013a; Gondokesumo et al., 2017). Low- and middle-income countries are disproportionately (3 in 4 adults) affected by this metabolic breakdown-linked disease as they account for 79% of the adults with T2D, with evidence linking between nutrition patterns and epidemiological transition (International Diabetes Federation, 2019). Therefore, there is an urgent need to improve nutritional needs with higher daily intake of more diverse and bioactive compounds enriched plant-based foods to address the emerging T2D epidemic-related public health challenges.
The pathophysiology of T2D involves insulin resistance in specific tissues leading to chronic hyperglycemia, which subsequently leads to excessive production of reactive oxygen species (ROS) and an alteration in endogenous antioxidant system (Negre-Salvayre et al., 2009; Ademiluyi and Oboh, 2013a; Gondokesumo et al., 2017). Therefore, countering chronic hyperglycemia and the associated chronic oxidative stress is critical to prevent and delay the progression of T2D. Plant-based foods as part of diets provide rich source of phenolic compounds and other antioxidants, which potentially offer protection against T2D-associated chronic oxidative stress and chronic hyperglycemia (Sarkar and Shetty, 2014). Phenolic antioxidants are secondary metabolites of plants with one or more hydroxyl units on the aromatic rings. These stress-inducible and oxidative stress protective compounds range extensively from simple to complex and polymerized phenolic compounds. Due to their unique chemical structure the most common bioactive function of phenolics that are widely considered having potent antioxidant activity which can be targeted in dietary and therapeutic strategies to counter chronic oxidative stress-induced diseases like T2D (Manach et al., 2004; Bandyopadhyay et al., 2012; Karaaslan, 2019). Additionally, phenolics can interact with other nutritional components like proteins via hydrophobic or hydrophilic interactions, which leads to the formation of soluble or insoluble complexes. The development of such complexes may influence overall bioavailability, bioaccessibility, and health relevant functional properties of phenolics and can alter the health protective functional qualities of plant foods and food ingredients (Bandyopadhyay et al., 2012).
However, this human health relevant bioactive profile and associated functional benefits of food and other botanicals vary widely between different tissues, plant parts, different species and cultivars, and based on the growing condition and environment (Mollica et al., 2018; Stefanucci et al., 2018; Bibi Sadeer et al., 2019). Previously, Sadeer et al. (2019) reported high bioactive linked antioxidant and anti-hyperglycemic relevant functional benefits in leaves and stem extracts of select African medicinal plants. Like leaves, stems, and fruits, flowers are also rich sources of human health protective bioactive compounds with wider health benefits. Specifically, flowers are the reproductive organs of plants and part of their natural adaptive responses making them rich source of abiotic and biotic stress associated protective bioactives such as phenolics (Stefanucci et al., 2018). The effect of different geographical locations and growing environment on varying phenolic bioactive-linked functional qualities were previously observed in extracts of Caper (Capparis spinosa L.) buds (Stefanucci et al., 2018). Similarly, variations in different extraction procedures also resulted in varying bioactive linked anti-diabetic and anti-cholesterol functional qualities in two different broccoli species (Mollica et al., 2018). This provides opportunities to screen and target optimized extracts of edible flowers rich phenolic compound profile as dietary and therapeutic sources supporting T2D-linked health benefits.
Hibiscus sabdariffa (L.) is an edible flower widely distributed in different continents with resilience to extreme climate and is popular in diverse cuisines. It has been traditionally used in herbal medicine and is a good target as dietary and therapeutic source to complement T2D solutions (Zhang et al., 2015; Gondokesumo et al., 2017; Peredo Pozos et al., 2020). This edible flower is commonly known as Roselle/Red sorrel (English), Bissap in Senegal, Sobolo in Ghana, Folera in Cameroon, Zobo in Nigeria (Yoruba), Jamaica in Spanish, Karkadeh in Arabic and Zoborodo in Northern Nigeria, Gongura/Lal-ambari/Patwa in Hindi, Pulachakiri in Kannada, and Polechi in Malayalam (Anokwuru et al., 2011; Shruthi et al., 2016; Izquierdo-Vega et al., 2020) and belongs to the family Malvaceae. This annual/perennial shrub is thought to be of tropical Asia (Indian to Malaysia) or African origin (Anokwuru et al., 2011). The plant is widely grown in tropics and is found throughout the Caribbean, Central America, India, Africa, Brazil, Australia, Southern United States, Hawaii, and Philippines. In botanical terms, it is a thick red plant with fleshly cup-shaped calyces (Anokwuru et al., 2011; Izquierdo-Vega et al., 2020; Peredo Pozos et al., 2020). The species is widely and commercially grown for its fibers and calyces, of which there are three types: green, red, and dark red. However, the red calyces are most common and widely used in traditional cuisines, especially in different parts of Asia and Africa.
The red calyces of Roselle are rich sources of diverse phenolic compounds such as simple phenolics, flavonoids, and anthocyanins (Riaz and Chopra, 2018; Peredo Pozos et al., 2020). Additionally, they are also rich in vitamin C, riboflavin, carotene, niacin, calcium, and iron (Anokwuru et al., 2011). The fleshy red calyces are commonly used in making wine, juice, jam, syrup, pudding, pickle, cakes, ice cream, or herbal tea. Beyond its use in traditional foods and as natural food color and additives by food industries, red calyces of Roselle have also been used in traditional herbal and folk medicines. One previous study found that Roselle is effective against reduced glutathione and protects against Low Density Lipoprotein (LDL)-oxidation and has hypolipidemic effects in vivo (Hirunpanich et al., 2006). Additionally, Roselle flowers and calyces have been reported to possess antiseptic, diuretic, antioxidant and antimutagenic properties (Mahadevan and Pradeep, 2009; Carvajal-Zarrabal et al., 2012). Furthermore, potential antioxidant, hypercholesteremic, antihypertensive, anti-diabetic, antimicrobial, and anti-inflammatory activities were observed in Roselle (Riaz and Chopra, 2018; Izquierdo-Vega et al., 2020). Anti-hyperglycemic property relevant, α-glucosidase, and α-amylase inhibitory activities were also reported previously (Ademiluyi and Oboh, 2013a; Zulfiqar et al., 2019). All these above studies indicated that red calyces of Roselle are rich sources of diverse human health protective phenolic compounds with significant antioxidant and anti-hyperglycemic potentials.
However, the potential relationship between phenolic compounds-linked to antioxidant and anti-hyperglycemic functionalities of calyces of Roselle is not well-understood. Additionally, prior to integrating them into functional food ingredient and nutraceutical design supporting T2D benefits, it is also important to optimize different organic solvent extracted fractions based on their phenolic content and associated antioxidant and anti-hyperglycemic functionalities using rapid in vitro assays-based screening strategy. Such rapid in vitro assays-based screening strategy would provide foundation to select organic fraction of Roselle with optimum phenolic compounds-linked functional qualities, which can be targeted for future in vivo and animal model-based studies for high value ingredients and nutraceuticals design. Therefore, the objective of this study was to investigate and compare phenolic bioactive linked antioxidant and anti-hyperglycemic properties of red Roselle calyces of four different organic solvents including methanol, which was further partitioned into chloroform, hexane, and ethyl acetate fractions. Following extraction, soluble phenolic content, phenolic profile, antioxidant, and anti-hyperglycemic functional activities of fractions of red Roselle calyces were determined using in vitro assays to investigate wider T2D health benefits.
Materials and Methods
Collection of Samples
Red calyces of Roselle (Hibiscus sabdariffa) (Figure 1) were purchased from a local retail market in Oyo State, South-West Nigeria. The dried calyces were sorted to remove debris and stones and stored for sample extractions and biochemical analysis.
Preparation of Samples
Crude Methanolic Extract Preparation
The modified method of Hossain et al. (2014) was used in the crude extraction of calyces of Hibiscus sabdariffa. The dried samples of Hibiscus sabdariffa calyces were finely ground into powder (~2.0 mm) by a grinder. During the first step of the extraction, dried and ground samples (500 g) were extracted with pure methanol (1,000 mL) and using continuous stirring for 72 h. Following extraction, the filtrate was obtained using a muslin cloth and filtered through Whatman filter paper No. 1. The filtrate (methanol solvent) was then evaporated using rotary evaporator (Heidolph, Laborata 4000 efficient, Schwabach, Germany) set at 40°C and the evaporated samples were further concentrated using a vacuum oven (Thermo Fisher Scientific, United Kingdom) set at 40°C with a pressure of 700 mm Hg to obtain the crude methanolic extract.
Liquid—Liquid Extraction
The crude extract of methanol was employed in the second stage of the fractionation procedure. The fractionation was done using the liquid-to-liquid extraction method. Three organic solvents, namely hexane, chloroform and ethyl acetate, respectively, were selected for the partitioning of the crude extract into individual fractions. The concentrated crude methanolic extract (13 g) was reconstituted in methanol (50 mL) and distilled water (50 mL). Then the mixture was carefully added and dynamically extracted by hexane (100 mL) in a 500 mL separating funnel. The solution was left as it is to phase separate following extraction. After the first round of extraction, the organic phase was withdrawn, and another 100 mL of the hexane was added for a second round of extraction until a clear phase was obtained for the hexane fraction. The same procedure as followed in hexane fractionation was applied to obtain chloroform and ethyl acetate fractions leaving behind the methanol fraction. The organic fractions of hexane, chloroform, ethyl acetate and methanol were mixed separately, evaporated using rotary evaporator (Heidolph, Laborata 4000 efficient, Schwabach, Germany) set at 40°C and further concentrated using a vacuum oven (Thermo Fisher Scientific, United Kingdom) set at 40°C with a pressure of 700 mmHg (Zahradníková et al., 2008; Hossain et al., 2014).
Preparation of the Sample Extracts
All four fractionated samples were then prepared by mixing 9 mg of the chloroform and hexane, and 1 g of ethyl acetate and methanol fractions, respectively, with 10 mL of absolute ethanol for miscibility. The solution was thoroughly mixed using a vortex. The mixture was transferred two times into polypropylene tubes and centrifuged at 4,038 g for 20 and 15 min, respectively. The supernatant obtained from the second centrifugation were transferred into a new polypropylene tube and used for the in vitro biochemical assays.
Total Soluble Phenolic Assay
The modified method of Shetty et al. (1995) was used for the determination of total soluble phenolics content using Folin-Ciocalteau assay. In this assay, one milliliter of supernatant of the extracts from the fractions of Roselle were transferred into a test tube and mixed with 5 mL of distilled water. A volume of 0.5 mL of 50% (v/v) Folin-Ciocalteau reagent was added to each sample and mixed. After 5 min, 1 mL of 5% Na2CO3 was added to the reaction mixture and left to stand for 60 min. The absorbance was read using a UV-Vis spectrophotometer (Genesys UV–vis, Rochester, NY, USA) at 725 nm. The absorbance values were converted to TSP and expressed as milligram equivalent of gallic acid per gram of dry weight (D.W). Standard curves were established using various concentrations of gallic acid (10–300 μg/mL) in 95% ethanol.
2,2-diphenyl-1-picrylhydrazyl (DPPH•) Free Radical Scavenging Assay
The antioxidant activity of the extracts of the different fractions of Roselle were determined with DPPH• based free radical scavenging assay as described by Kwon et al. (2006). Due to the dark color of the sample and to avoid potential interference with DPPH• stock solution, 100 μL of the sample extract was diluted in 900 μL of distilled water, where an aliquot of 4.16 μL/mL of the sample was added into a test-tube and mixed with 2 mL of 0.1 mM DPPH• dissolved in 95% ethanol. The mixture was vortexed and left to stand in the dark at room temperature for 5 min. The absorbance of the resulting solution was measured using a UV-vis spectrophotometer (Genesys UV–vis, Rochester, NY) at 517 nm. The scavenging percentage of DPPH• was calculated using the following equation:
2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS•+) Based Free Radical Scavenging Assay
The antioxidant activity of the extracts from the different organic solvent fractions of Roselle were measured using ABTS•+ (2,2 – azinobis (3 – ethylbenzothiazoline – 6 – sulfonic acid) based free radical scavenging assay (Re et al., 1999). One mL of ABTS•+ was mixed with 50 μL of the extracts of the different fractions, the mixture was thoroughly mixed with a vortex, incubated at room temperature for 3 min and the absorbance was read at 734 nm using a UV-vis spectrophotometer (Genesys, Rochester, NY). The activities of the extracts were expressed in percentage inhibition of ABTS•+ free radical formation and calculated using the formula:
α-Amylase Inhibitory Activity Assay
The α-amylase inhibitory activity relevant to know the anti-hyperglycemic activity potential of different fractions of red calyces of Roselle was carried out according to the method described by Fujita et al. (2017). The buffer used in this assay was 0.1 M sodium phosphate (pH 6.9). A volume of 500 μL of the extracts from the different fractions of Roselle were added to test tubes while the control tubes having 500 μL of buffer only. Then 500 μL of porcine pancreatic amylase (0.5 mg/mL buffer) was added to all the tubes except the sample blank and blank tubes and then incubated at 25°C for 10 min. After incubation, 500 μL of 1% starch (1 g/100 mL buffer) was added to all the tubes and incubated for 10 min. The reaction was then stopped by the addition of 1 mL of 3, 5 dinitro salicylic acid, and the tubes were placed in a boiling water bath (> 90°C) for 10 min. After cooling to room temperature, the reference point reading of the control was optimized to 1.0 ± 0.2 (with only enzyme and substrate) by diluting with distilled water and absorbance was measured at 540 nm using a UV-VIS Genesys spectrophotometer. The percentage of inhibition of a-amylase activity was calculated based on the following formula:
α-Glucosidase Inhibitory Activity Assay
The α–glucosidase inhibitory activity of the extracts from the different fractions of Roselle were carried out according to the method described by McCue and Shetty (2005). An aliquot of 50 μL of the extracts were added into a 96—well-microplates, 100 μL of α- glucosidase enzyme (1 U/mL), which was made in 0.1 M phosphate buffer (pH 6.9) was then added and incubated at room temperature (25 °C) for 10 min. After incubation, 50 μL of 5 mM p—nitrophenyl—α–D—glucopyranoside solution made in 0.1 M phosphate buffer (pH 6.9) was added into the wells at a timed—interval. The mixture was incubated at 25°C for 5 min. The absorbance was read and recorded at 405 nm at 0 min and after 5 min using a microplate reader (Spectra Max 190, Molecular Device Co., Sunnyvale, CA, USA). The results were compared to control with 50 μL of buffer solution in place of the sample extracts. The results were determined as percentage of α- glucosidase inhibition and calculated using the formula:
Determination of Major Phenolic Compounds Using High Performance Liquid Chromatography (HPLC)
The extracts from the different fractions of Roselle were centrifuged using a micro-centrifuged at 8,000 g for 10 min while an aliquot of 5 μL volume of the sample was used for the chromatographic analysis using a reverse phase high performance liquid chromatography (HPLC) (Agilent 1260 Infinity series equipped with DAD 1100 diode array detector, Palo Alto, CA, USA). The gradient method of elution involving 10 mM phosphoric acid (pH 2.5; Solvent A) and 100% methanol (Solvent B) were used. The extracts of the fractions of calyces of Roselle were eluted using a C-−18 analytical columns (Agilent Supelco SB—C18250 × 4.6 mm internal diameter) with a packing material particle with size 5 μm, at 0.7 mL/min flow rate at ambient temperature with 25 min as the total run time. The absorbance was recorded at 230, 234, 250, and 258 nm for each run and both retention time and UV spectrum analysis of phenolic acid standards (from the library) were used to identify and match the peaks. The pure standards of ellagic acid, gallic acid, catechin, chlorogenic acid, caffeic acid, quercetin, rutin, benzoic acid, cinnamic acid, and o, p—coumaric acid in 100% methanol were used to calibrate the retention times on the standard curve. The chromatograms were analyzed using Agilent Chemstation integration software (Fujita et al., 2017).
Statistical Analysis
Each biochemical parameter was analyzed in triplicates, and all the in vitro assays were repeated two times (n = 6). The data obtained were evaluated with analysis of variance using Statistical Analytical Software (SAS, version 9.4; SAS Institute, Cary, NC, USA). The mean and standard error was determined using Xcel software. The statistically significant differences among the samples were determined using Tukey's least mean square test at 95% confidence level (P < 0.05).
Results
Percentage Yield
Understanding overall yield in different organic solvent fractions is critical for their effective utilization and integration in further dietary and therapeutic strategies or to utilize optimized fractions in future in vivo and animal model-based studies for designing red Roselle calyces-based functional food ingredients and nutraceuticals. The yield of the respective fractions was obtained after further concentration using the vacuum oven. The ethyl acetate fraction had the highest yield of 8.163 g (67.39%) followed by methanol (32%), hexane (0.28%), and chloroform (0.12%) (Table 1).
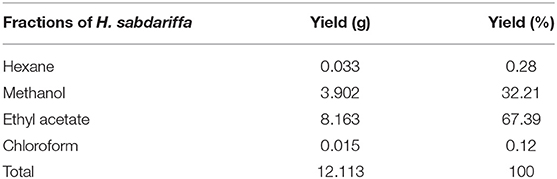
Table 1. Yield of the respective organic solvent extracted fractions of Roselle (Hibiscus sabdariffa).
Total Soluble Phenolic Content and Profile of Phenolic Compounds
In this study, very high total soluble phenolic content (7.25 mg GAE/g DW) was observed in methanol extracted fraction of Roselle calyces (Figure 2). Significant statistical differences (P < 0.05) in total soluble phenolic content were also observed between different organic solvent fractions of Roselle calyces. Following methanol fraction, high total soluble phenolic content was observed in ethyl acetate fraction (5.57 mg GAE/g DW), while hexane (0.73 mg GAE/g DW) and chloroform (1.12 mg GAE/g DW) fractions had significantly low (P < 0.05) total soluble phenolic content (Figure 2).
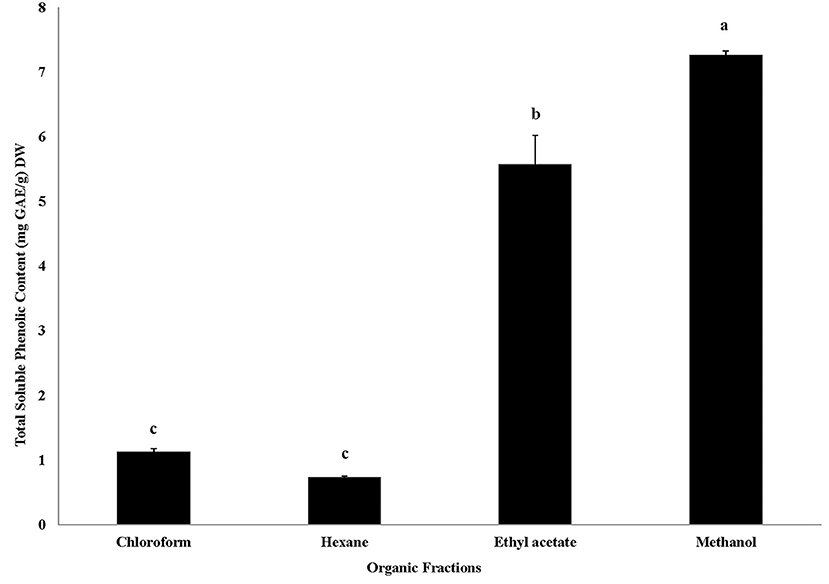
Figure 2. Total soluble phenolic content (mg GAE/g DW) of four organic solvent extracted fractions of red dried calyces of Roselle. Different lower-case letters represent statistically significant differences in total soluble phenolic content between different organic solvent extracted fractions at 95% confidence level (Tukey's test).
In addition to the total soluble phenolic content, individual phenolic compounds present in four different fractions of Roselle calyces were also identified and quantified using high performance liquid chromatography (HPLC) analysis. Overall, major phenolic compounds observed in all four fractions of Roselle calyces were chlorogenic acid, caffeic acid, catechin, benzoic acid, and cinnamic acid (Figure 3). Additionally, gallic acid and rutin were also found in chloroform, hexane, and ethyl acetate fractions, but was not present in methanol fraction (Table 2). Among all detected phenolic compounds, significantly (P < 0.05) high chlorogenic, caffeic, and cinnamic acid were observed in methanol extracted fraction of calyces of Roselle and it was positively correlated with high total soluble phenolic content of the same fraction. Interestingly, methanol fraction had significantly low catechin and benzoic acid content when compared with other three fractions. High gallic acid and catechin content were observed in hexane fraction, and the values were statistically (P < 0.05) different from other three fractions. No statistically significant differences in rutin content were observed between hexane, chloroform, and ethyl acetate fractions. The phenolic profile results of this study indicated that different phenolic compounds of Roselle calyces have organic solvent specific affinity and solubility based on their polarity and binding property in tissues of Roselle calyces. Variations in concentrations and composition of phenolic compounds might have impacted in varying antioxidant and anti-hyperglycemic functional activities of different organic solvent based fractions of Roselle calyces. Such differences in structure-function relationship of phenolic compounds of Roselle calyces are potentially relevant for rationally targeting them in functional food ingredient and nutraceutical design as it influences their health protective functions such as antioxidant and anti-hyperglycemic properties.
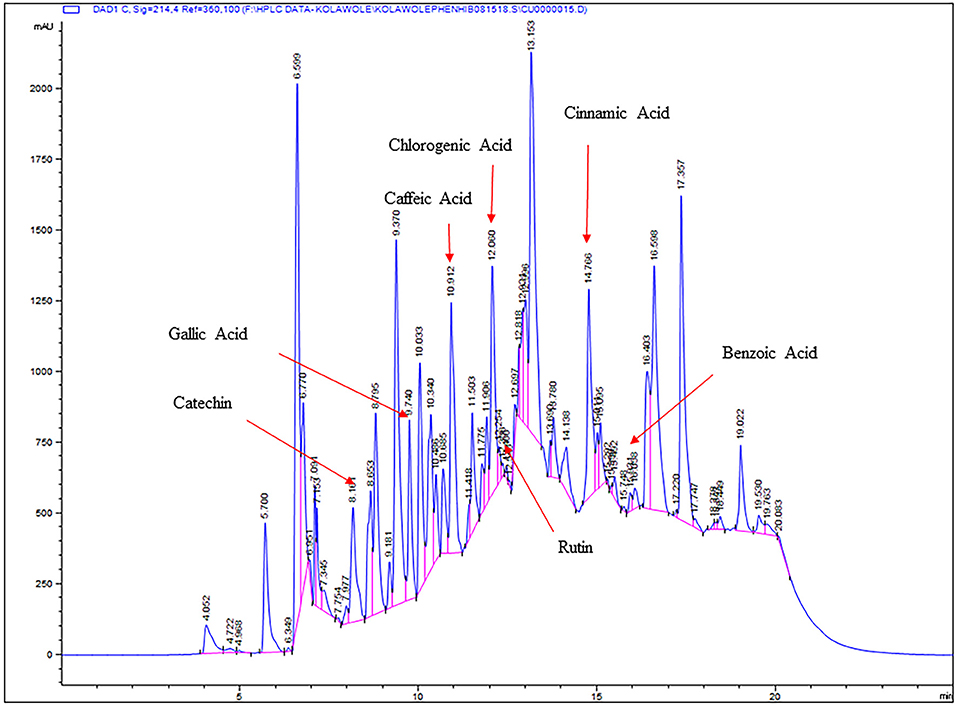
Figure 3. Chromatogram (high performance liquid chromatography) of detected phenolic compounds of chloroform fraction of Roselle calyces.
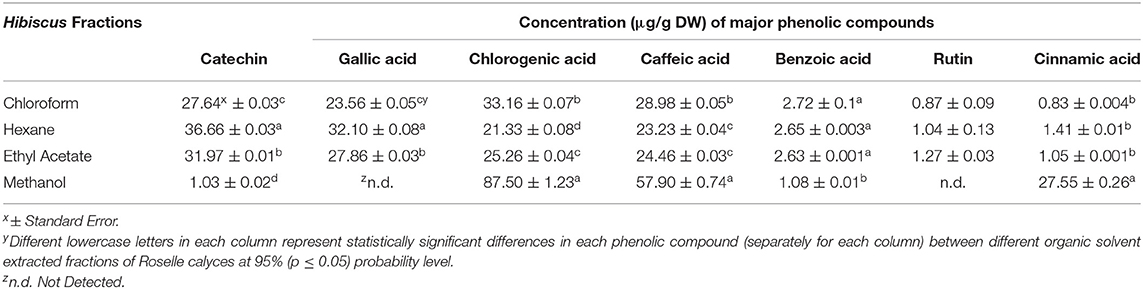
Table 2. Major phenolic compounds (μg/g DW) in four different organic solvent extracted fractions of calyces of Hibiscus sabdariffa (Roselle).
Total Antioxidant Activity
The total antioxidant activity of four organic solvent-based fractions of Roselle calyces were determined using two different free radicals (DPPH• and ABTS•+) scavenging assays. In this study, very high antioxidant activity (89% inhibition in DPPH• and 98% inhibition in ABTS•+-based assays) was observed in ethyl acetate fraction of Roselle calyces (Figures 4A,B). Overall, higher mean antioxidant activity (30–98% inhibition) was observed in ABTS•+ free radical scavenging assay, when compared with the DPPH•-free radical scavenging assay-based results (9–89% inhibition) of same fractions. Differences in baseline antioxidant activity between ABTS•+ and DPPH•-based free radical scavenging assays might be due to their different affinity toward hydrophilic and hydrophobic antioxidants that were present in organic fractions. Significant statistical differences in total antioxidant activity among four different fractions of Roselle calyces (P < 0.05) were also found in both ABTS•+ and DPPH•-based assays. The antioxidant activity of methanol extracted fraction (97% inhibition) of Roselle calyces based on ABTS•+ free radical scavenging assay was statistically at par with ethyl acetate fraction, while it was significantly higher when compared with hexane and chloroform fractions. Overall, low antioxidant activity was observed in hexane and chloroform fractions in both ABTS•+ and DPPH•-based assays, and the result of antioxidant activity positively correlated with soluble phenolic content of Roselle fractions.
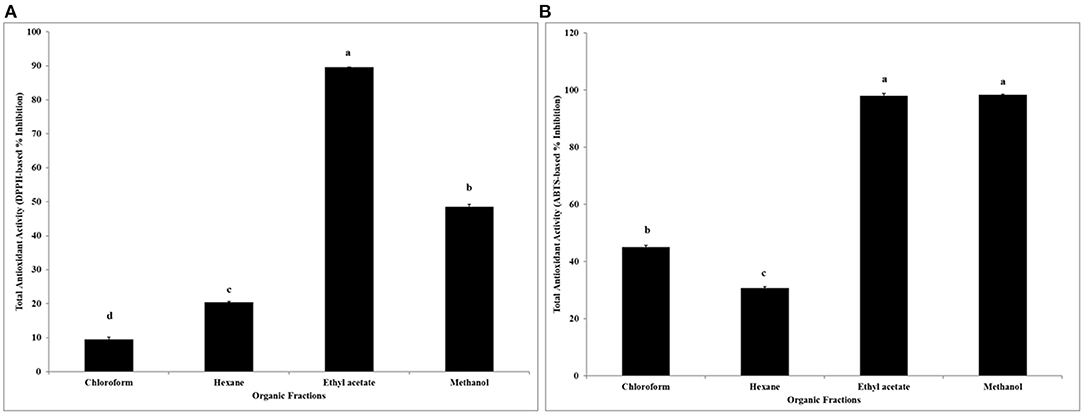
Figure 4. Total antioxidant activity (% inhibition) of four organic solvent extracted fractions of red dried calyces of Roselle based on DPPH (A) and ABTS (B) free radical scavenging assays. Different lower-case letters represent statistically significant differences in total antioxidant activity between different organic solvent extracted fractions at 95% confidence level (Tukey's test) separately for two antioxidant assays.
α–Amylase Inhibitory Activity
To determine the potential anti-hyperglycemic relevant functional qualities of different fractions of red and dried Roselle calyces, α-amylase inhibitory activity was determined using in vitro assay method. Like the result of the antioxidant activity, very high α-amylase inhibitory activity (93% inhibition) was observed in undiluted samples of ethyl acetate fraction, which also had high phenolic content, rich phenolic profile and high antioxidant activity (Figure 5). The differences in α-amylase inhibitory activity among four different fractions of Roselle calyces were statistically significant (P < 0.05), with only exception between methanol and chloroform fractions (statistically at par). In this in vitro assay model-based study, undiluted samples of methanol and chloroform fractions also showed moderate α-amylase inhibitory activity (65 & 62% inhibition, respectively), while hexane fraction had significantly low (30%) inhibitory activity against α-amylase.
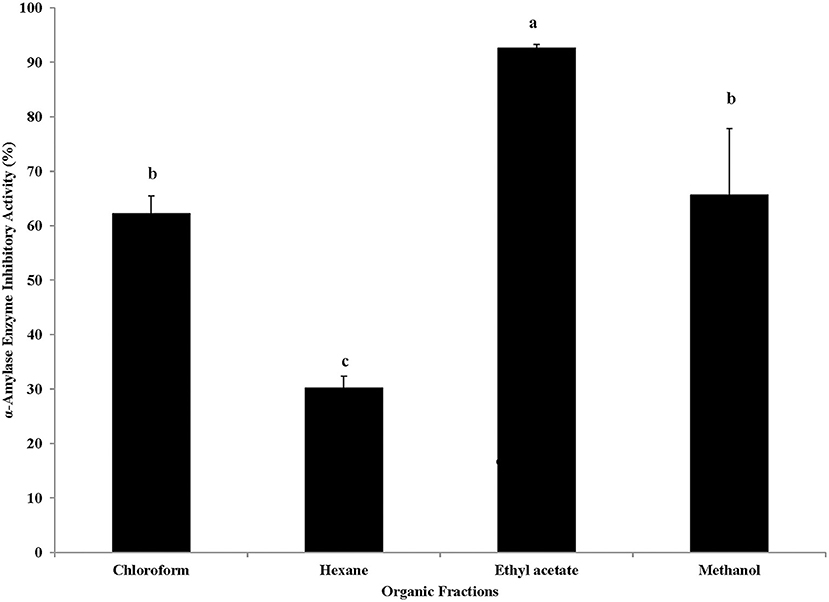
Figure 5. α-Amylase enzyme inhibitory activity (% inhibition) of undiluted samples of four organic solvent extracted fractions of red dried calyces of Roselle. Different lower-case letters represent statistically significant differences in α-amylase enzyme inhibitory activity between different organic solvent extracted fractions at 95% confidence level (Tukey's test).
α-Glucosidase Inhibitory Activity
In vitro inhibitory activity of Roselle calyces' fractions against another key enzyme, α-glucosidase, which is involved in glucose metabolism and relevant for managing post-prandial glucose homeostasis was also determined. Extracts of all four fractions were diluted (half and one-fifth) to determine the dose dependent response against α-glucosidase (Figure 6). Overall, very high α-glucosidase inhibitory activity (81–98% inhibition) was observed in all four fractions of calyces of Roselle that were targeted in this study. Additionally, significant dose dependent (undiluted, half-diluted, and one-fifth diluted) responses in α-glucosidase inhibitory activity was also found. Like the results of antioxidant activity and α-amylase inhibitory activity, significantly high α-glucosidase inhibitory activity was observed in undiluted (98% inhibition) and diluted (87% in inhibition in half-diluted and 66% inhibition in one-fifth diluted) samples of ethyl acetate fraction, which was statistically significant (P < 0.05). Following the result of ethyl acetate fraction, chloroform (62–91% inhibition), and methanol (60–90% inhibition) fractions of calyces of Roselle also had high α-glucosidase inhibitory activity. In this study, hexane fraction had comparatively lower α-glucosidase inhibitory activity (44–81% inhibition). The results of this in vitro assay model-based study indicated that organic solvent fractions, specifically ethyl acetate and methanol fractions of Roselle calyces are good dietary and therapeutic sources that can be targeted for improving antioxidant and anti-hyperglycemic protective functions of food, functional food ingredients, beverages, and nutraceuticals with T2D benefits.
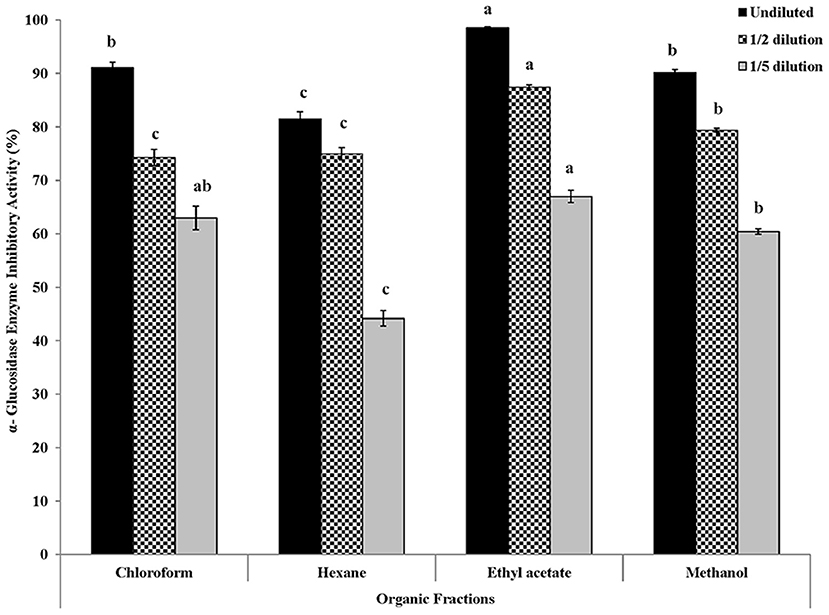
Figure 6. α-Glucosidase enzyme inhibitory activity (% inhibition) of undiluted, half-diluted, and one-fifth diluted samples of four organic solvent extracted fractions of red dried calyces of Roselle. Different lower-case letters represent statistically significant differences in α-glucosidase enzyme inhibitory activity between different organic solvent extracted fractions at 95% confidence level (Tukey's test).
Discussion
Edible flowers enriched in human health protective phenolic compounds and other functional components are potentially safe dietary and therapeutic targets to counter chronic oxidative stress and chronic hyperglycemia commonly associated with T2D. However, the availability and bioactivity of health protective phenolic compounds of edible flowers and their overall yield in extracted fractions are largely dependent on the composition of phenolics (free vs. bound fractions), location of the phenolic compounds within the cell wall and other cellular compartments, and their specific solubility and polarity toward the organic and inorganic solvents and based on extraction protocols (Peredo Pozos et al., 2020). Similarly, different growing environment, storage conditions, and different extraction methods also significantly influence phenolic compounds-linked functional qualities of plant foods and plant-based ingredients (Mollica et al., 2018; Stefanucci et al., 2018; Bibi Sadeer et al., 2019). Therefore, it is important to optimize different solvent-based extracted fractions based on their phenolic bioactive-linked antioxidant and anti-hyperglycemic properties using comparatively inexpensive and rapid in vitro assays. Therefore, in this in vitro assays-based screening study, dried edible calyces of Roselle were initially extracted (crude) in 100% methanol and further extracted and partitioned using three different organic solvents (chloroform, hexane, and ethyl acetate). All four extracted fractions were investigated for their phenolic compounds-linked antioxidant and anti-hyperglycemic functional activities using rapid in vitro assays.
In this study, the final yield (%) of the fractions of Roselle calyces varied significantly between four different organic solvent-based extracts, which also influenced their antioxidant and anti-hyperglycemic relevant functional properties. The varied yield obtained for the fractions of the different organic solvents-based Roselle extracts might be due to the differences in solubility and polarity of major phenolic compounds present in red calyces and based on the composition of phenolic compounds in tissues of calyces. This indicates why ethyl acetate and methanol fractions had higher yields than the hexane and chloroform fractions, as ethyl acetate and methanol are more polar solvents (Abdille et al., 2005; Peredo Pozos et al., 2020). Additionally, the phenolic compounds of plant tissues including calyces of Roselle are often bound to and interact with proteins, carbohydrate, terpenes, chlorophyll, and inorganic compounds, which potentially aids in the dissolution of endogenous compounds with phenolics (Koffi et al., 2010; Anokwuru et al., 2011; Peredo Pozos et al., 2020). In this study, percent yield and total soluble phenolic content decreased significantly in the chloroform and hexane fractions. Solvents employed in extraction of biomolecules from plants are mostly selected based on their polarity. Therefore, the use of multiple solvents can be exploited to limit the quantity of analogous compounds in the expected yield (Altemimi et al., 2017). This may have led to the reduced yield of total soluble phenolic content observed in the hexane and chloroform fractions of the Roselle calyces. Previously, Jung et al. (2013) reported similar concentration of total phenolic content in aqueous and ethanol extracts of Roselle. Higher phenolic content was also reported in complete calyces and decoction residues of Roselle (Mercado-Mercado et al., 2015). The high soluble phenolic content of the different organic solvent extracted fractions (ethyl acetate and methanol) of Roselle found in this in vitro assays-based screening study are relevant for their specific health-targeted application in the targeting of edible flowers as sources of functional food ingredients and nutraceuticals to support solutions against chronic oxidative stress and chronic hyperglycemia, which are common health risks of T2D and can be further validated through future in vivo model or animal model-based studies.
In addition to the variability and value of total soluble phenolic content, the concentration of individual phenolic compounds also varied significantly between different organic solvent extracted fractions of Roselle calyces. Like the results of the total phenolic content, high chlorogenic acid, caffeic acid, and cinnamic acid content were observed in methanol extracted fractions. The variations in phenolic profile found in different organic solvent extracts of Roselle calyces were based on their solubility and affinity toward specific organic solvent and polarity-associated effectiveness of the organic solvent to pull free and bound phenolic fractions from dry Roselle calyces. Previously, Mercado-Mercado et al. (2015) reported higher concentration of chlorogenic acid, gallic acid, syringic acid, and catechin in calyces of Roselle. In another study, higher concentration of chlorogenic acid was found in extracts of Mexican Roselle (Borrás-Linares et al., 2015). Additionally, Karaaslan (2019) found the presence of gallic, caffeic and chlorogenic acids, catechin, and rutin in extracts of H. sabdariffa, which were obtained from acidified water, methanol, ethanol, acetone, and acetonitrile. Some of the phenolics identified in the different fractions of the extracts have shown antioxidant and anti-hyperglycemic activities (Yang et al., 2013). Caffeic acid, which was significantly higher in methanol fractions, potentially stimulates glycolysis and inhibits gluconeogenesis in insulin resistant hepatocytes. Furthermore, caffeic acid and caffeic acid enriched plant foods can protect kidney from diabetic induced cellular injuries, by assisting in the reduction of blood glucose and urine nitrogen and enhances the removal of creatinine. Gallic and chlorogenic acids are also potent dietary antioxidants and have exhibited specific anti-diabetic functions like the reduction of elevated glucose induced epithelial to mesenchymal transition of the renal tubular cells, stimulation of the secretion of insulin, which increases plasma insulin and glucose tolerance (Latha and Daisy, 2011; Punithavathi et al., 2011; Yang et al., 2013). Previous studies with phenolic compounds revealed that phenolic enriched plant foods can be beneficial in the prevention of chronic oxidative stress induced vascular and renal damages (Yang et al., 2013). Beyond these phenolic compounds found in this present study, red Roselle calyces are also rich sources of anthocyanin and several flavonoids with diverse human health relevant functional benefits. More systematic mass-spectroscopy (LC-MS) based analytical techniques can be targeted in the future for detailed and specific characterization of phenolic compounds of red Roselle calyces targeting improved understanding and wider health-focused ingredient and nutraceutical applications.
In the current study, gallic acid, catechin, benzoic acid, rutin, and cinnamic acids were also found in ethyl acetate, chloroform, and hexane extracted fractions of red Roselle calyces. All these phenolic compounds found in extracted fractions of Roselle calyces are potent antioxidants and can be used as dietary antioxidant sources to target solutions against chronic oxidative stress-associated metabolic breakdowns, a common health risk factor of T2D.The in vitro antioxidant activities of plant-based foods and botanical medicine extracts are generally analyzed using free radical scavenging assays, specifically to determine their dietary and therapeutic potentials to counter chronic oxidative stress (Tsai et al., 2002; Tang and Tsao, 2017; Peredo Pozos et al., 2020). Therefore, the antioxidant capacities of the hexane, chloroform, ethyl acetate and methanol extracted fractions of Roselle calyces were investigated using DPPH• and ABTS•+ free radical scavenging assays. The variation in antioxidant activity between ABTS•+ and DPPH• assays found in this study might be due to their differences in affinity toward hydrophilic and hydrophobic antioxidants that varied widely in different organic fractions of Roselle calyces (Re et al., 1999). Additionally, higher baseline antioxidant activity in ABTS•+ free radical scavenging assay when compared to the result of DPPH•-based assay, might be attributable to DPPH• radicals being more stable than ABTS•+ cation and a resultant delay is attained in achieving a steady state due to the slow quenching of DPPH• by the hydrophilic antioxidants (Shalaby and Shanab, 2013; Ramakrishna et al., 2017). Furthermore, the differences in phenolic composition and their relative concentrations potentially contributed to different antioxidant activity found among select organic solvent-based fractions of Roselle calyces (Xiong et al., 2014; Karaaslan, 2019).
The results of this in vitro screening study indicated a significantly higher antioxidant activity in the ethyl acetate and methanol fractions, when compared to the hexane and chloroform fractions. The use of polar organic solvents had been reported to result in higher antioxidant activities in comparison to the non-polar counterparts (Orhan et al., 2007; Anokwuru et al., 2011; Altemimi et al., 2017). These findings agree with the studies of Orhan et al. (2007) who observed that the antioxidants activities of the Arnebia densiflora fruit was higher in polar than the non-polar solvent-based extracts. This is also in accordance with the results of the study by Abdille et al. (2005), who reported that the highest antioxidant activity of Dillenia indica fruit was observed in the ethanol extracts followed by ethyl acetate and water extracts. Previous studies have shown that phenolic compounds found in Roselle possess significant free radical scavenging abilities to quench DPPH• and ABTS•+ (Anokwuru et al., 2011; Peredo Pozos et al., 2020). The presence of diverse phenolic compounds, including simple phenolics, flavonoids, and anthocyanin of Roselle calyces influence the antioxidant activities against free radicals (Da-Costa-Rocha et al., 2014; Riaz and Chopra, 2018; Izquierdo-Vega et al., 2020; Peredo Pozos et al., 2020). High chlorogenic acid, caffeic acid and cinnamic acid content in methanolic fraction might have resulted in its higher antioxidant activity, while high antioxidant function in ethyl acetate fraction might be due to the wider distribution of different phenolic compounds including gallic acid and catechin and their additive or synergistic effects to quench free radicals. High antioxidant capacity of these individual phenolic compounds from plant food sources was widely studied and reported previously (Amić et al., 2018; Szewczyk et al., 2018; Tajner-Czopek et al., 2020). The high antioxidant activity found in ethyl acetate and methanol extracted fractions of the Roselle calyces have significant dietary and therapeutic relevance to counter chronic oxidative stress and related pathophysiology of chronic diseases such as T2D. However, for the effective integration of Roselle calyces in T2D benefit supporting strategies, it is also important to define their glycemic control relevant anti-hyperglycemic functions.
Therefore, in this study, inhibitory activity of different organic solvent extracted fractions of Roselle calyces against key enzymes of carbohydrate metabolism, which are potentially relevant for maintaining glucose homeostasis at post-prandial stages were also investigated using in vitro assays. Inhibition of two specific enzymes targeted for understanding anti-hyperglycemic functions of extracted fractions of Roselle calyces were α-amylase and α-glucosidase. In vitro screening strategy targeting 2 key starch-hydrolyzing enzymes α-amylase and α-glucosidase is an inexpensive and effective approach to find functional ingredients with optimum anti-hyperglycemic benefits from large number of samples or for comparing different extraction methods, which will be difficult and expensive to investigate using in vivo or animal model-based strategies. These rapid in vitro assays-based enzyme inhibitory activities provide potential anti-hyperglycemic benefits of novel plant extracts, especially the further targeting the ability of the best extracts to reduce post-prandial blood glucose spike and countering chronic hyperglycemia (Mollica et al., 2018; Stefanucci et al., 2018; Bibi Sadeer et al., 2019) in subsequent in vivo models. The results of this rapid in vitro assay model-based study showed that undiluted sample of ethyl acetate fraction had the highest α-amylase inhibitory activity, while hexane fraction exhibited low inhibitory activity against α-amylase. The same organic fraction also had high phenolic content and high antioxidant activity. Methanol and chloroform fractions of Roselle calyces also showed moderate to high α-amylase inhibitory activity. Previously, Ademiluyi and Oboh (2013b), observed similar α-amylase inhibitory activity in aqueous extract of Hibiscus sabdariffa. Furthermore, the extracts of the different fractions of Roselle also displayed inhibitory activity against α–amylase as observed in Hibiscus sabdariffa and other plant extracts (Adisakwattana et al., 2012; Da-Costa-Rocha et al., 2014; Alegbe et al., 2019). Therefore, corroborated results of the current and previous in vitro assay model-based studies suggested that ethyl acetate, methanol, and chloroform extracted fractions of Roselle calyces are good dietary and therapeutic sources to slow down the breakdown of complex carbohydrates during post-meal period and with additional in vivo studies could be potentially targeted for managing blood glucose homeostasis, which is essential for countering T2D-associated chronic hyperglycemia. The phenolic composition and their relative interaction with protein molecules might also have contributed to difference of α-amylase and α-glucosidase inhibitory activities among different organic solvent-based fractions of Roselle calyces (Domínguez Avila et al., 2017; Foegeding et al., 2017).
In addition to the α-amylase inhibitory activity, very high α-glucosidase inhibitory activity was also observed in all four extracted fractions of Roselle calyces in the targeted in vitro assay models. Significant dose dependent responses in α-glucosidase inhibitory activity were also observed in all four extracted organic fractions. The results indicated that red Roselle calyces enriched in phenolic compounds is relevant for anti-hyperglycemic protection and can be targeted for anti-diabetic benefits. The results of the current study confirmed that like other phenolic enriched plant-based food extracts, Roselle calyces also have high α-glucosidase and moderate α-amylase inhibitory activities, which is essential to avoid distention of the abdomen, gassiness, meteorism, and diarrhea commonly associated with synthetic enzyme inhibitors (Tsai et al., 2002; Ademiluyi and Oboh, 2013a; Uchida-Maruki et al., 2015; Alegbe et al., 2019). The in vitro inhibitory activity against α-amylase and α-glucosidase enzymes found in this study may indicate the probable mechanism by which extracts of Roselle calyces modulate hypoglycemic activity (Ademiluyi and Oboh, 2013a). Such metabolically-linked function potentially influences the anti–glycemic properties of Roselle calyces and are relevant toward integrating this edible flower in dietary support strategies targeting T2D benefits like with other Hibiscus and plant extracts (Gondokesumo et al., 2017; Alegbe et al., 2019; Vinh et al., 2019).
Results of the in vitro assays-based current study indicated that the ethyl acetate fraction of H. sabdariffa was more effective at inhibiting the carbohydrate-hydrolyzing enzymes (α-amylase and α-glucosidase) due to the extraction of diverse phenolic compounds like catechin, gallic acid, chlorogenic acid, caffeic acid, benzoic acid, rutin and cinnamic acid, which was also coupled with their high antioxidant properties. Significant α-amylase and α-glucosidase inhibitory activities were previously observed with individual and combination of different phenolic compounds (Rasouli et al., 2017). The antioxidant and anti-hyperglycemic results of Roselle extracts also corroborated with the previous report of the antioxidant potentials of ethyl acetate extract of six Algerian propolis extracts and the potency of ethyl acetate fraction from H. sabdariffa in attenuating diabetes associated cognitive impairment in mice (Boufadi et al., 2014; Seung et al., 2018). Previously, Zheoat et al. (2017) reported that crude extracts of Roselle and the hibiscus acids obtained from the extracts had vasorelaxant effect on the aorta of Sprague-Dawley rats. They concluded that the hibiscus acid was more effective in the vasorelaxant action by inhibition of Ca2+ influx through the dependent on voltage Ca2+ channels (Zheoat et al., 2017; Izquierdo-Vega et al., 2020). The results of previous and this current in vitro assays-based study indicated that inhibition of starch-hydrolyzing enzymes along with other health protective functions of Roselle phenolic compounds may contribute to the overall glycemic control by improving glucose metabolism and by providing protection against chronic oxidative stress-induced breakdowns (Wang et al., 2000; Alegbe et al., 2019; Vinh et al., 2019; Izquierdo-Vega et al., 2020). Therefore, edible calyces of Roselle are promising and inexpensive source that can be targeted as functional food ingredients and nutraceuticals to counter chronic hyperglycemia and chronic oxidative stress, which are major risk factors of T2D. However, though the present study is primary screening study determining promising extract targets, further studies based on in vivo models and animal studies are required to advance the findings of this study, specifically for effective integration of Roselle calyces in dietary and therapeutic interventions supporting wider T2D benefits based on clinical relevance.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
KB, DS, and KS: conceptualization and project administration. KB and DS: data curation, formal analysis, and writing—original draft. KB, AS, DS, and OA: investigation. KB, DS, and OA: methodology. KS, KB, DS, and OA: writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank the University of Ibadan, Oyo State for research leave granted to KB to carry out the research at the Department of Plant Sciences, North Dakota State University, Fargo, ND.
References
Abdille, M. H., Singh, R. P., Jayaprakasha, G. K., and Jena, B. S. (2005). Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 90, 891–896. doi: 10.1016/j.foodchem.2004.09.002
Ademiluyi, A. O., and Oboh, G. (2013a). Aqueous extracts of roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-Amylase and α-glucosidase activities in vitro. J. Med. Food 16, 88–93. doi: 10.1089/jmf.2012.0004
Ademiluyi, A. O., and Oboh, G. (2013b). Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 65, 305–309.
Adisakwattana, S., Ruengsamran, T., Kampa, P., and Sompong, W. (2012). In vitro inhibitory effects of plant-based foods and their combinations on intestinal inverted question mark-glucosidase and pancreatic inverted question mark amylase. BMC Compl. Alt. Med. 12:110. doi: 10.1186/1472-6882-12-110
Alegbe, E. O., Teral,i, K., Olofinsan, K. A., Surgun, S., Ogbaga, C. C., and Ajiboye, T. O. (2019). Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 43:e12927. doi: 10.1111/jfbc.12927
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D., and Lightfoot, D. (2017). Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6:42. doi: 10.3390/plants6040042
Amić, A., Marković, Z., Klein, E., Marković, J. M. D., and Milenković, D. (2018). Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 246, 481–489. doi: 10.1016/j.foodchem.2017.11.100
Anokwuru, C. P., Esiaba, I., Ajiboye, O., and Adesuyi, A. O. (2011). Polyphenolic content and antioxidant activity of Hibiscus sabdariffa Calyces. Res. J. Med. Plant. 5, 557–566. doi: 10.3923/rjmp.2011.557.566
Bandyopadhyay, P., Ghosh, A. K., and Ghosh, C. (2012). Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 3:592. doi: 10.1039/c2fo00006g
Bibi Sadeer, N., Llorent-Martínez, E. J., Bene, K., Fawzi Mahomoodally, M., Mollica, A., Ibrahime Sinan, K., et al. (2019). Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 174, 19–33. doi: 10.1016/j.jpba.2019.05.041
Borrás-Linares, I., Fernández-Arroyo, S., Arráez-Roman, D., Palmeros-Suárez, P. A., Del Val-Díaz, R., Andrade-Gonzáles, I., et al. (2015). Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Indust. Crops Products 69, 385–394. doi: 10.1016/j.indcrop.2015.02.053
Boufadi, Y., Soubhye, J., Riazi, A., Rousseau, A., Vanhaeverbeek, M., Nève, J., et al. (2014). Characterization and antioxidant properties of six algerian propolis extracts: ethyl acetate extracts inhibit myeloperoxidase activity. Int. J. Mol. Sci. 15, 2327–2345. doi: 10.3390/ijms15022327
Carvajal-Zarrabal, O., Barradas-Dermitz, D. M., Orta-Flores, Z., Hayward-Jones, P. M., Nolasco-Hipolito, C., and Aguilar- Uscanga, M. G. (2012). Hibiscus sabdariffa L., roselle calyx, from ethnobotany to pharmacology. J. Exp. Pharmacol. 4, 25–39. doi: 10.2147/JEP.S27974
Da-Costa-Rocha, I., Bonnlaender, B., Sievers, H., Pischel, I., and Heinrich, M. (2014). Hibiscus sabdariffa L. – a phytochemical and pharmacological review. Food Chem. 165, 424–443. doi: 10.1016/j.foodchem.2014.05.002
Domínguez Avila, J. A., Rodrigo García, J., González Aguilar, G. A., and De la Rosa, L. A. (2017). The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules. 22, 903.
Foegeding, E. A., Plundrich, N., Schneider, M., Campbell, C., and Lila, M. A. (2017). Protein-polyphenol particles for delivering structural and health functionality. Food Hydrocoll. 72, 163–173. doi: 10.1016/j.foodhyd.2017.05.024
Fujita, A., Sarkar, D., Genovese, M. I., and Shetty, K. (2017). Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem. 59, 133–140, doi: 10.1016/j.procbio.2017.05.017
Gondokesumo, M. E., Kusuma, H. S. W., and Widowati, W. (2017). α-/β-glucosidase and α-amylase inhibitory activities of roselle (Hibiscus sabdariffa L.) ethanol extract. Mol. Cellular Biomed. Sc. 1:34. doi: 10.21705/mcbs.v1i1.3
Hirunpanich, V., Utaipat, A., Molales, N. P., Bunyapraphtsala, N., Sato, H., and Herunsale, A. (2006). Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 103, 252–260. doi: 10.1016/j.jep.2005.08.033
Hossain, M. A., Al-Hdhrami, S. S., Weli, A. M., Al-Riyami, Q., and Al-Sabahi, J. N. (2014). Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in Sultanate of Oman. Asian Pacific J. Trop. Biomed. 4, S368–S372. doi: 10.12980/APJTB.4.2014C1051
International Diabetes Federation (2019). IDF Diabetes Atlas, 9th Edn. Available online at: https://www.diabetesatlas.org/en/ (accessed December 16, 2020).
Izquierdo-Vega, J., Arteaga-Badillo, D., Sánchez-Gutiérrez, M., Morales-González, J., Vargas-Mendoza, N., Gómez-Aldapa, C., et al. (2020). Organic acids from roselle (Hibiscus sabdariffa L.)—a brief review of its pharmacological effects. Biomedicines 8:100. doi: 10.3390/biomedicines8050100
Jung, E., Kim, Y., and Joo, N. (2013). Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). J. Sci. Food Agric. 93, 3769–3776. doi: 10.1002/jsfa.6256
Karaaslan, N. M (2019). A comprehensive study about Hibiscus sabdariffa leaves: antioxidant activity, polyphenol profile and macro- and micro-element content. Chem. Papers 73, 791–799. doi: 10.1007/s11696-018-0629-x
Koffi, E., Sea, T., Dodehe, Y., and Soro, S. (2010). Effect of solvent type on extraction of polyphenols from twenty-three Ivorian plants. J. Anim. Plant Sci. 5, 550–558. Available online at: http://www.biosciences.elewa.org/JAPS/2010/5.3/3.pdf
Kwon, Y. I., Vattem, D. A., and Shetty, K. (2006). Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr.15, 107–118.
Latha, R. C. R., and Daisy, P. (2011). Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 189, 112–118. doi: 10.1016/j.cbi.2010.11.005
Mahadevan, S., and Pradeep, K. (2009). Hibiscus sabdariffa Linn: an overview. Natural Prod. Radiance. 8, 77–83.
Manach, C., Scalbert, A., Morand, C., Rémésy, C., and Jiménez, L. (2004). Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79, 727–747.
McCue, P., and Shetty, K. (2005). “Principles of biochemistry and molecular biology food biotechnology,” in Food Biotechnology, 2nd edn, eds K. Shetty, G. Paliyath, A. L. Pometto, III R.E. Levin (Boca Raton, FL: CRC Press Taylor and Francis Co), 19–32.
Mercado-Mercado, G., Blancas-Benitez, F. J., Velderrain-Rodríguez, G. R., Montalvo-González, E., González-Aguilar, G. A., Alvarez-Parrilla, E., et al. (2015). Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 18, 171–181. doi: 10.1016/j.jff.2015.07.001
Mollica, A., Stefanucci, A., Zengin, G., Locatelli, M., Macedonio, G., Orlando, G., et al. (2018). Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 107, 129–138. doi: 10.1016/j.biopha.2018.07.169
Negre-Salvayre, A., Salvayre, R., Auge, N., Pamplona, R., and Portero-Otin, M. (2009). Hyperglycemia and glycation in diabetic complications. Antioxid. Redox Signal. 11, 3071–3109. doi: 10.1089/ars.2009.2484
Orhan, I., Kartal, M., Naz, Q., Ejaz, A., Yilmaz, G., Kan, Y., et al. (2007). Antioxidant and anticholinesterase evaluation of selected Turkish salvia species. Food Chem. 103, 1247–1254. doi: 10.1016/j.foodchem.2006.10.030
Peredo Pozos, G. I., Ruiz-López, M. A., Zamora Nátera, J. F., Álvarez Moya, C., Barrientos Ramírez, L., Reynoso Silva, M., et al. (2020). Antioxidant capacity and antigenotoxic effect of Hibiscus sabdariffa L. extracts obtained with ultrasound-assisted extraction process. App. Sci. 10:560. doi: 10.3390/app10020560
Punithavathi, V. R., Prince, P. S. M., Kumar, R., and Selvakumari, J. (2011). Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Europ. J. Pharmacol. 650, 465–471. doi: 10.1016/j.ejphar.2010.08.059
Ramakrishna, R., Sarkar, D., Schwarz, P., and Shetty, K. (2017). Phenolic linked anti-hyperglycemic bioactives of barley (Hordeum vulgare L.) cultivars as nutraceuticals targeting type 2 diabetes. Indust. Crops Products 107, 509–517. doi: 10.1016/j.indcrop.2017.03.033
Rasouli, H., Hosseini-Ghazvini, S. M. B., Adibi, H., and Khodarahmi, R. (2017). Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 8, 1942–1954. doi: 10.1039/C7FO00220C
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant capacity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Riaz, G., and Chopra, R. A. (2018). Review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 102, 575–586. doi: 10.1016/j.biopha.2018.03.023
Sadeer, N. B., Llorent-Martínez, E. J., Bene, K., Mahomoodally, M. F., Mollica, A., Sinan, K. I., et al. (2019). Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. 174, 19–33.
Sarkar, D., and Shetty, K. (2014). Metabolic stimulation of plant phenolics for food preservation and health. Ann Rev Food Sci Technol. 5, 395–413. doi: 10.1146/annurev-food-030713-092418
Seung, T. W., Park, S. K., Kang, J. Y., Kim, J. M., Park, S. H., Kwon, B. S., et al. (2018). Ethyl acetate fraction from Hibiscus sabdariffa L. attenuates diabetes-associated cognitive impairment in mice. Food Res. Int. 105, 589–598. doi: 10.1016/j.foodres.2017.11.063
Shalaby, E. A., and Shanab, S. M. M. (2013). Comparison of DPPH and ABTS assays fordetermining antioxidant potential of water and methanol extracts of Spirulinaplatensis. Indian J. Mar. Sci. 42, 556–564.
Shetty, K., Curtism, O. F., Levin, R. E., Witkowsky, R., and Ang, W. (1995). Prevention of vitrification associated with in vitro Shoot Culture of Oregano (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 147, 447–451. doi: 10.1016/S0176-1617(11)82181-4
Shruthi, V. H., Ramachandra, C. T., Nidoni, U., Hiregoudar, S., Naik, N., and Kurubar, A. R. (2016). Roselle (Hibiscus sabdariffa L.) as a source of natural colour: a review. Plant Arch. 16, 515–522.
Stefanucci, A., Zengin, G., Locatelli, M., Macedonio, G., Wang, C.-K., Novellino, E., et al. (2018). Impact of different geographical locations on varying profile of bioactives and associated functionalities of caper (Capparis spinosa L.). Food Chem. Toxicol. 118, 181–189. doi: 10.1016/j.fct.2018.05.003
Szewczyk, M., Morawiak, M., Narczyk, A., Pakulski, Z., and Urbanczyk-Lipkowska, Z. (2018). Chlorogenic acids mimics-synthesis, structure and antioxidant activity. J. Chem. 6, 28–37.
Tajner-Czopek, A., Gertchen, M., Rytel, E., Kita, A., Kucharska, A. Z., and Sokół-Łetowska, A. (2020). Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 9:412. doi: 10.3390/antiox9050412
Tang, Y., and Tsao, R. (2017). Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: a review. Mol. Nutr. Food Res. 61:1600767. doi: 10.1002/mnfr.201600767
Tsai, P. J., Mcintosh, J., Pearce, P., Camden, B., and Jordan, B. R. (2002). Anthocyanin and antioxidant capacity in roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 35, 351–356. doi: 10.1016/S0963-9969(01)00129-6
Uchida-Maruki, H., Inagaki, H., Ito, R., Kurita, I., Sai, M., and Ito, T. (2015). Piceatannol lowers the blood glucose level in diabetic mice. Biol. Pharm. Bull. 38, 629–633. doi: 10.1248/bpb.b15-00009
Vinh, L. B., Nguyen, T. M. N., Chu, D. T., Tran, T. H., Le Huyen, T., Thong, N. V., et al. (2019). Chemical constituents of Vietnamese mangrove Hibiscus tiliaceus with antioxidant and alpha-glucosidase inhibitory activity. Natural Product Res. 35, 2899–2904. doi: 10.1080/14786419.2019.1672065
Wang, C. J., Wang, J. M., Lin, W. L., Chu, C. Y., Chau, F. P., and Tseng, T. H. (2000). Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 38, 411–416. doi: 10.1016/S0278-6915(00)00011-9
Xiong, L., Yang, J., Jiang, Y., Lu, B., Hu, Y., Zhou, F., et al. (2014). Phenolic compounds and antioxidant capacities of 10 common edible flowers from China. J. Food Sci. 79, C517–C525. doi: 10.1111/1750-3841.12404
Yang, Y.-S., Wang, C.-J., Huang, C.-N., Chen, M.-L., Chen, M.-J., and Peng, C.-H. (2013). Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via attenuating renal epithelial mesenchymal transition. J. Agric. Food Chem. 61, 7545–7551. doi: 10.1021/jf4020735
Zahradníková, L., Schmidt, Š., Sékelyová, Z., and Sekretár, S. (2008). Fractionation and identification of some phenolics extracted from evening primrose seed meal. Czech J. Food Sci. 26, 58–64. doi: 10.17221/1135-CJFS
Zhang, Y. J., Gan, R. Y., Li, S., Zhou, Y., Li, A. N., Xu, D. P., et al. (2015). Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20, 21138–21156. doi: 10.3390/molecules201219753
Zheoat, A. M., Gray, A. I., Igoli, J. O., Kennedy, A. R., and Ferro, V. A. (2017). Ferro crystal structures of hibiscus acid and hibiscus acid dimethyl ester isolated from Hibiscus sabdariffa (Malvaceae). Acta Crystallogr. E Crystallogr. Commun. 73, 1368–1371. doi: 10.1107/S2056989017011902
Keywords: antioxidant, anti-hyperglycemic, Roselle calyces, Type 2 diabetes, phenolic compounds
Citation: Banwo K, Sanni A, Sarkar D, Ale O and Shetty K (2022) Phenolics-Linked Antioxidant and Anti-hyperglycemic Properties of Edible Roselle (Hibiscus sabdariffa Linn.) Calyces Targeting Type 2 Diabetes Nutraceutical Benefits in vitro. Front. Sustain. Food Syst. 6:660831. doi: 10.3389/fsufs.2022.660831
Received: 29 January 2021; Accepted: 06 January 2022;
Published: 28 January 2022.
Edited by:
Valentina Scariot, University of Turin, ItalyReviewed by:
Chayon Goswami, Bangladesh Agricultural University, BangladeshAdriano Mollica, University “G. d'Annunzio” of Chieti-Pescara, Italy
Gopinadhan Paliyath, University of Guelph, Canada
Emmanuel Anyachukwu Irondi, Kwara State University, Nigeria
Copyright © 2022 Banwo, Sanni, Sarkar, Ale and Shetty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kolawole Banwo, a29sYWJhbndvQHlhaG9vLmNvbQ==; Kalidas Shetty, a2FsaWRhcy5zaGV0dHlAbmRzdS5lZHU=
 Kolawole Banwo
Kolawole Banwo Abiodun Sanni
Abiodun Sanni Dipayan Sarkar
Dipayan Sarkar Oluwatosin Ale3
Oluwatosin Ale3 Kalidas Shetty
Kalidas Shetty