- 1Biology Study Program, Mathematics, and Natural Sciences, Udayana University, Badung Regency, Bali, Indonesia
- 2Biopesticide Laboratory, Agriculture Faculty, Udayana University, Badung Regency, Bali, Indonesia
- 3Faculty of Veterinary Medicine, Udayana University, Badung Regency, Bali, Indonesia
- 4PGPR Society for Sustainable Agriculture and Auburn Ventures, Department of Plant Pathology and Entomology, Auburn University, Auburn, AL, United States
- 5Department of Agrotechnology, Faculty of Agriculture, Stiper Agricultural Institute Yogyakarta, Yogyakarta, Indonesia
- 6Department of Soil Science, Faculty of Agriculture, Universitas Pembangunan Nasional Veteran Yogyakarta, Yogyakarta, Indonesia
- 7National Research and Innovation Agency, Jakarta, Indonesia
- 8Research Center for Food Crops, Research Organization for Agriculture and Food, National Research and Innovation Agency (BRIN), Cibinong, Indonesia
- 9Faculty of Science and Natural Resources, University Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia
- 10Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia
- 11Department of Botany, Hindu College Moradabad, (Mahatma Jyotiba Phule Rohilkhand University Bareilly), Moradabad, India
Under the guise of enhancing productivity, using pesticides and artificial fertilizers in agriculture affects both the environment and living things. High chemical residues in food and the environment disrupt the health of consumers. One of the solutions that can bring about a reduction in the use of pesticides and chemicals is switching to organic fertilizers. The application of biopesticides originating from biological sources such as plant extracts and the use of microbes is gaining global acceptance. Therefore, this study aimed to obtain the best biopesticides and biostimulants that could suppress the leaf spot pathogen, Nigrospora oryzae, and increase the growth and yield of Bali red rice. The study contained four treatments, namely untreated control (F0), Piper caninum leaf extract (F1), Brevibacillus agri (F2), and fermented P. caninum leaf extract plus B. agri (F3). The treatments were arranged in a randomized complete block design, and each treatment was replicated three times. The parameters measured were the number of tillers per plant, number of leafs per plant, chlorophyll content, number of grains per panicle, grain weight, and grain yield. Furthermore, antimicrobial and antioxidants were assayed using SEM. GC-MS. At the end of the experiment, the disease index of the leaf spot was measured. The results showed that F3 significantly suppressed leaf spots caused by N. oryzae compared to other treatments, including untreated control in red rice. Additionally, the F3 significantly increased the number of productive tillers, number of grains per panicle, and grain yield compared to all other treatments. The F3 enhanced the crop yield at 6.19 tons/ha, an increase of 50% compared to the untreated control. The SEM.GC-MS results showed the presence of 2.3 butanediol, tetra-decanoic acid, butanoic acid, ethyl ester, benzene propanal, 3-(1,1-dimethylethyl)-a-methyl, a-N-Normethadol in treated plants with P. canicum plus B. agri.
Introduction
Rice is one of the staple food in Indonesia, and various varieties are being developed worldwide. It has been extensively studied due to the importance of production and consumption and its vast distribution and destructiveness worldwide (Mir et al., 2022). Several management practices are being followed to increase rice yields, including chemical fertilizers and pesticides (Sagar et al., 2020). However, these products are toxic to human health and the environment (Ghatak et al., 2013). Leaf spot of rice caused by Nigrospora oryzae is a severe fungal disease of rice (Oryza sativa L.), threatening global food security.
The most prevalent method for rice leaf spot disease is using chemical pesticides. For instance, chlorpyrifos, an organophosphate insecticide, can inhibit insects and pests and is found in various commercial pesticides (Shabbir et al., 2021). Agricultural scientists developed fertilizers from natural materials known as organic fertilizers and pesticides to overcome pesticides. Organic fertilizers and pesticides are very safe for the environment and humans due to their decomposition by microorganisms and not impacting non-target organisms (Moustafa et al., 2022).
Many organic fertilizers and biopesticides are developed from microbes and plant extracts (Reshma et al., 2018; Kumar et al., 2022). Biostimulants are one such category of rhizobacteria that can increase plant growth due to their ability to produce hormones (Khan et al., 2020) and other growth-promoting metabolites (Tembo et al., 2018; Kalam et al., 2020; Basu et al., 2021; Hamid et al., 2021; Kusale et al., 2021a,b; Lyu et al., 2022). Furthermore, they are an essential aspect of the integrated crop management (ICM) system to make agriculture more sustainable and resilient. When given to plants or the rhizosphere, these inoculants stimulate natural processes to improve nutrient uptake (Jabborova et al., 2020, 2021a,b; Moradzadeh et al., 2021; Nithyapriya et al., 2021; Saboor et al., 2021; Sarkar et al., 2021), usage efficiency, abiotic stress tolerance (Ilyas et al., 2020; Sagar et al., 2020; Khan et al., 2021), biocontrol (Sukmawati et al., 2021; Khumairah et al., 2022), and crop quality (Hamid et al., 2021; Kannepalli et al., 2021; Manasa et al., 2021). Plant extracts have been used to suppress diseases, such as spotting in rice caused by Pyricularia oryzae, Nigrospora oryzae, and other fungi.
Extracts from Piper caninum were reported earlier to suppress spotting disease caused by P. oryzae and contribute to increased rice growth and production (Suriani et al., 2020a). However, certain drawbacks exist in such studies either related to the over dosage of such extracts or shorter shelf-life (Suriani et al., 2020a). Therefore, there is a need for a new approach to overcoming the above problems by combining plant-based extract and selected bacterial isolate. The study combines P. caninum leaf extract with Bacillus agri, where B. agri is a fermented bacterium that uses P. caninum extract as a food source. New compounds from the fermentation in coordination with B. agri, which has integral antifungal properties, could serve as a more powerful biopesticide to effectively suppress spotting disease in rice. This approach aids to contribute for disease control, growth, and production of red Bali rice (O. sativa L).
Materials and methods
Time and location of research
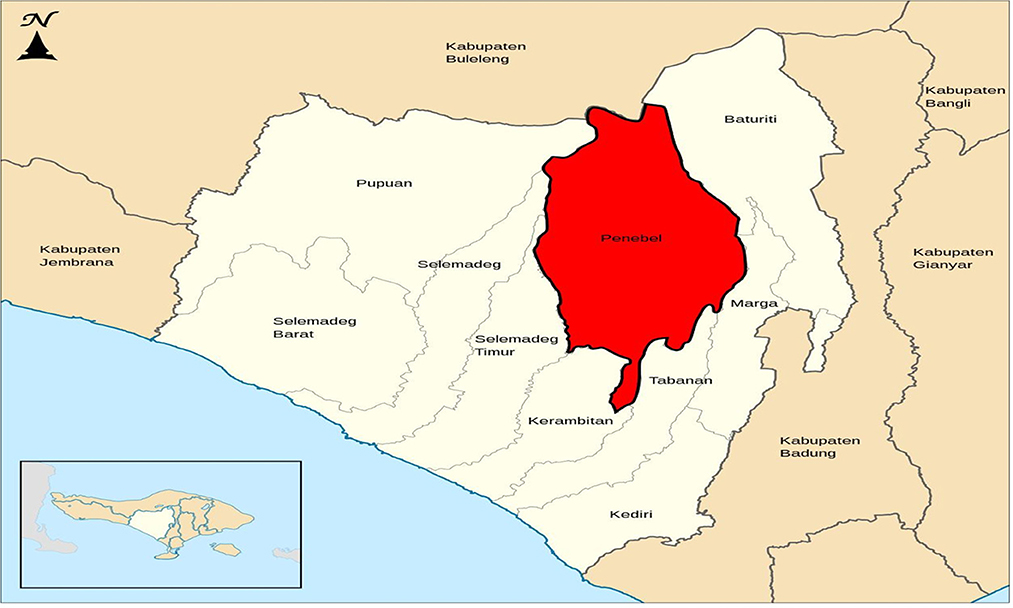
The present study was conducted from February 2021 to February 2022 at two locations, including the biopesticide laboratory of Udayana University, Bali, Indonesia, and the greenhouse at Munduk Paku Village, Senganan Penebel (Figure 1), Tabanan, Bali, Indonesia (8°22'49.3“S 115°09'43.2”E) and elevation 249 m above sea level).According to Schmidt & Ferguson's classification of climates, the climate in this area is a Type A climate. The average annual rainfall is between 2,000 and 2,800 mm, and there are typically 155.6 wet days. The average number of wet months is 4–10, and the average number of dry months is 0–5. The range of the average air temperature is 25–28°C. Rice, horticultural crops (vegetables, fruits, and flowers), and flora kepodang birds, earthworms, and dragonflies (Sri Widari, 2021).
Isolation and identification of rice leaf spot pathogen and rhizobacteria
The leaf spot pathogen was discovered in a rice field in Bali, Indonesia's Senganan Village, Tabanan regency. After removing the diseased rice leaves (with the brown spot), they are thoroughly washed under running water, washed with 70% alcohol, and then rinsed under running water. They are then dried and cut into small pieces and placed in a petri dish with PDA medium, incubated for 2 days at room temperature, and the growth of pathogenic fungi is then observed under a microscope. Following its purification, the pathogen is spread and examined for the following step (Parwanayoni et al., 2021).
Sequencing of ITS regions and computer analysis of DNA sequences. The fungus pathogen was cultivated on PDA at ambient temperature (28°C) for 3 days. In Jakarta, PT Genetic Science, sequencing was carried out. Internal Transcriptional Spacer (ITS) 1 (5- TCCGTAGGTGAACCTGCGG-3) primer and ITS 4 were used in PCR to amplify the 18S rRNA gene (5- TCCTCCGCTTATTGATATGC-3). Takara PCR thermal cycler Personal (Takara Bio, Otsu, Japan) with Ex Tag (Takara Bio, Otsu, Japan) was used to perform the PCR in this study. The pre-denaturation step took 4 min, and then there were 35 cycles of denaturation at 94°C for 35 s, annealing at 52°C for 55 s, elongation at 72°C for 2 min, and post-elongation. The nucleotide sequences were determined using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) according to the guidelines of the tool and with the PE Applied Biosystems Automated DNA Sequencer (3130xl model, Applied Biosystems). Using Genetyx-ATSQ (version 4.0) software (Genetyx, Tokyo, Japan), compared with the same DNA sequence from DDBJ / EMBL / GenBank through the NCBI BLAST program (Thompson et al., 1997). Phylogeny analysis was carried out using the MEGA 6.0 program (Stecher et al., 2020), the Maximum Parsimony (MP) method with bootstrap1000x, with the following steps: (1). Search for similarities between sequences. Sequence data stored in the notes in the FASTA format is analyzed using the Blast-WU facility available online at www.ebi.ac.uk/Clustalw. (2). Making a tree of phylogeny with the MEGA program. Data from processing using the ClustalW facility will then be used as basic data to make phylogeny trees using MEGA data facilities (Darmadi et al., 2019).
The rhizobacteria were isolated from Senganan Village, Tabanan regency, Bali, Indonesia. For SRJL1 isolates, information was gathered from earlier research in which these isolates had IAA, protease, nitrogen. One strain—this isolate—was acquired from the 20 examined isolates in the earlier investigation. This isolate tested positive for all parameters. Therefore, this isolates and a squensing test were used in this study. Rhizobacteria were identified using an analysis of 16S rRNA gene sequences. First, the GeneJET Genomic DNA Purification Kit was used to extract and purify the DNA from rhizobacteria (Thermo Fisher Scientific). Using the primer pairs 16S (63F 5′ - CAG GCC TAA CAC ATG CAA GTC-3′) and 1387R (5′ - GGG CGG WGT GTA CAA GGC-3′), the 16S rRNA gene was amplified by PCR with the help of 2 Kappa PCR ReadyMix (Kappa Biosystem) at 94°C for 5 min, followed by 30 successive cycles at 94°C for 30 s, 55°C for nucleotide sequences were determined using the ABI PRISM 3100-Avant Genetic Analyzer. The DNA sequences were trimmed and assembled using the ChromasPro version 1.5 program. The assembled data was then processed for BLAST analysis with data registered in the National Center for Biotechnology Information (NCBI) through the website http://www.ncbi.nlm.nih.gov/BLAST. Several homologous sequence data from BLAST results, which are the closest species, were taken from GenBank data at the NCBI. The data were then analyzed again by aligning the sequences using the MEGA version 6.0 program. Furthermore, the data were analyzed using the PAUP 4.0b program with the maximum parsimony method with 1,000 replicates bootstrap (Vasseur-Coronado et al., 2021).
P. caninum extract
A total of 5 kg of mature P. caninum leaf (i.e., leaf number 3 from the top of the shoot of the plant were collected from Munduk Paku Village, Senganan, Penebel, and Tabanan Bali to make P. caninum leaf extract. The leafs were cleaned with running water, twisted, cut into small pieces, dried in a shaded place without sunlight until the weight was constant, and made into powder. Subsequently, the powder was macerated with methanol in a 1:10 (weight/volume) ratio for 48 h. The filtrate was filtered using Whatman filter paper (No. 2) then the obtained filtrate was evaporated using an evaporator (IWAKI, Japan) at a temperature of 40°C until the methanol evaporated, and the crude extract obtained was ready for use (Suriani, 2018).
Crude extract test against N. oryzae (MIC test)
Petri dishes were filled with 200 μl of N. oryzae spores for this test and then added with 10 ml of diluted PDA, shaken horizontally to mix spores and PDA evenly. After solidification, diffusion wells were made with a cork borer (5 mm diameter) in each petri dish. Each diffusion well was filled with 20 ml of crude extract of P. caninum leaf. The plates were incubated in the dark at room temperature (Suriani et al., 2020a). The diameter of the resistance zone created around the diffusion well was measured. Meanwhile, the well method was also used to determine the minimum inhibitory concentration (MIC). There were five concentrations of crude extract of P. caninum leafs; 0% as a control, 0.5; 1; 1.5; and 2%. All tests were carried out in PDA, and each treatment was replicated five times. PDA was poured into a petri dish filled with extracts of 20 μl of each concentration and allowed to solidify, and then filled with discs of N. oryzae. Furthermore, the plates were incubated at ±25°C, and observations were recorded daily until the control fungal colony growth reached the Petri dish's edges. The plant's inhibitory effect of crude extract was calculated as control diameter- treatment diameter/ control diameter x 100% (Suriani et al., 2021).
Production of B. agri
B. agri was propagated on sterile potato dextrose agar, one liter of substrate is created from 200 grams of small-grained potatoes that have been cooked for 20 min with 1 liter of aquades, filtered, and then filled to capacity with 70 grams of dextrose and 20 grams of water. After that, the media is poured into each test tube, which is then compacted and autoclaved to make it sterile. Each test tube contains 10 ml of medium. The bacterial isolates were transferred with an ose needle to the PDA media and incubated at 27 ± 2°C for 3 days. Fermentation (room temperature 28–30°C) of P. caninum leafs extract: a mixture of 20 mg of P caninum extract, 1 one liter of potato boiled water, and 1 ml suspension of B. agri was transferred into a glass bottle and incubated for 30 days at 27 ± 2°C) before application to rice plants (Suprapta, 2022).
Application of treatments to rice seeds
Bali red rice What is obtained from senganan village, penebel, Tabanan bali is local balinese rice was soaked for 48 h with P. caninum extract and P. caninum plus B. agri. There were four treatments, and each was replicated six times. The treatments were: F0 = untreated control; F1=P. caninum extract at 2%; F2 = B. agri at 2%; F3= Fermentation of P. caninum extract with B. agri at 2%. Approximately 20 g of each treatment was mixed with 100 ml for 30 min. It was then twisted, put in Petri disc dial tissue, and watered with soaking water daily. After 2 weeks of growth, the parameters recorded were root growth, wet weight, and dry weight of Bali red rice seedlings (Suriani et al., 2021).
Seed treatment
Rice seeds were immersed in water for 48 h. After soaking, the seeds were grown in a tray filled with soil, and periodic watering schedules were maintained. After germination, young seedlings were transferred to another tray filled with planting media. The planting medium used in the experiment was the top layer of soil collected from rice fields, precisely 20 cm from the surface. Furthermore, the seedlings were maintained in trays, ensuring a proper watering schedule for 2 weeks. After 15 days of growth, they were carefully removed and re-planted into pots (Suriani et al., 2020a).
Greenhouse trials
A randomized complete block design was used for the greenhouse trials. The treatments were as follows: F0 = untreated control; F1 = P. caninum extract at 2%; F2 = B. agri at 2%; F3 = Fermentation of P. caninum extract with B. agri at 2%. Each treatment was replicated six times. Therefore, there were 20 experimental units, each consisting of 10 clumps planted with two rice seedlings, totaling 200 trials. The implementation of the experiment includes sowing seeds, preparing planting media, planting seeds, maintaining plants, fertilizing, and harvesting (Suriani et al., 2019).
Rice is planted in a greenhouse using a 30-centimeter-diameter bucket as a container for the media. The media is created by mixing soil (Soil permeability calls for low to moderate bulk density, medium to outstanding porosity conditions, and moderately quick air or water movement through the soil. Typical prerequisites for soil infiltration include. Terrain type: clay loam) and compost (material from chicken waste and rice husk) (trade name- Surya compost, procured from the local market in Bali, Indonesia) to create a mud-like consistency. This planting is ready for use after receiving 1 component of compost (Suriani, 2019).
Planting
The pest and disease-free seedlings of uniform size (15 cm tall) and aged 15 days after planting. They were removed and planted in the morning, and a rice seed was sown in each pot. Planting is conducted perpendicular to the ground at a depth of 3 cm (IRRI, 2015).
Fungus inoculation
After planting rice for 30 days (HST), N. oryzae isolates are inoculated by spraying 20 ml of mold spore solution per clump with a hand sprayer, then covering it with plastic for 12 h to keep the moisture intact. A pure fungal culture is harvested with 10 ml of sterile water over an oblique medium. Subsequently, it is harvested with a nose needle and filtered with Whatman Filter Paper No. 2 to separate the mold spores from their hyphae. The collected fungal spores are diluted in sterile water till the volume is 20 ml. Meanwhile, the mold spores utilized have a density of 25 × 104 spores/ml (Hoesain et al., 2021).
Treatment application
Application of treatment is conducted by spraying on rice plants after 1-month-old. This application should be conducted the day after the inoculation of the fungus. Each rice plant is sprayed with 20 ml of treatment and repeated 4 times with an interval of 1 week (Suriani et al., 2021).
Maintenance
Feeding, watering, and weeding rice plants are part of the maintenance process. Weeding keeps plants from being disturbed and prevents weeds and rice plants from competing for nutrients. Fertilization is carried out with compost on the substrate's preparation. Furthermore, watering begins with the planting and continues once a day (morning or evening).
Harvest
Harvesting occurs after the rice has turned yellow after 4.5 months. Rice is dried entirely before being ground in the drain.
Growth and production parameters
The observed data was in the form of growth, including plant height, number of leafs, number of tillers and chlorophyll, number of grains per panicle, the weight of grain per clump, the percentage of empty grain, and yield. The formula for calculating the yield is ton/ha per treatment. Chlorophyll content was measured according to the method of Aron (1949) with the help of a chlorophyll meter (Konika-Minolta, Inc, Osaka, Japan-SPAD 502) (Zhang et al., 2022).
The intensity of leaf spot disease
The intensity of leaf spot disease was measured with the following formula:
IP = The intensity of leaf spot disease
Observations were recorded as follows (Suriani, 2019)-
0 - No attack,
1 - very mild attack (0–10% damage to leaf surface),
2 - mild attack (10–30% damage to leaf surface),
3- moderate attack (30–50% damage to leaf surface),
4- severe attacks (50–75% damage to the leafs' surface) and
5 -heavy attack (75–100% damage to the leafs' surface)
Gas chromatography–mass spectrophotometry (GC-MS) analysis
Analysis of GC-MS (QP2010SE, Shimadzu, Japan) was performed to identify active chemicals. The structure of the separated compounds was determined by comparing their molecular weight and fragmentation pattern by the GC-MS database library (Reshma et al., 2018). Additionally, this method was conducted on P. caninum extract fermented along with B. agri. This experiment was conducted at UNUD's Joint Mathematics and Natural Sciences Laboratory.
Data analysis
The data were analyzed by analysis of variance (ANOVA), and the treatment differences were separated by Duncans Multiple Range Test (DMRT) at a 5% significance level (Febrianna et al., 2018).
Results and discussion
Identification of bacterial isolate
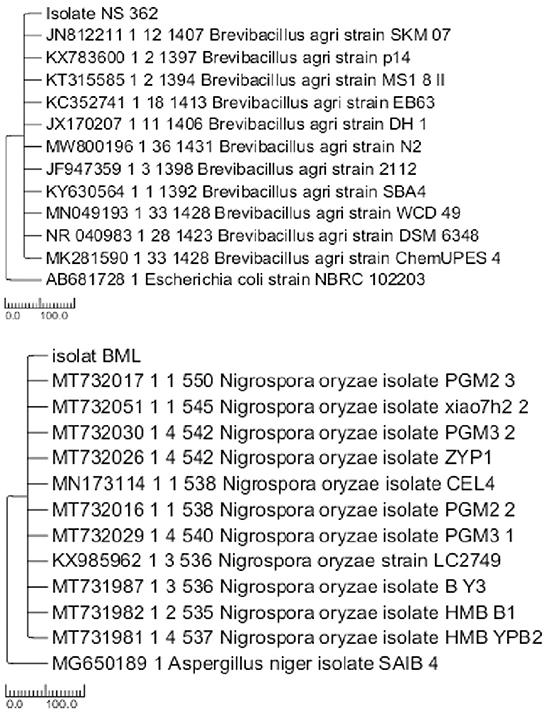
The identification results showed that the SRJL1 isolate was a rhizobacteria B. agri shown in Figure 3 with an assessment number: OM510267. The synthesis of organic acids, phosphatase activity, and concentration of P-dissolved in Pikovskaya media indicated the phosphate solubilizing capacity of the isolates (Table 1). Analysis of the organic acids production revealed that both isolates, Burkholderia sp. and Penicillium sp. produced organic acids, including lactate acid, oxalate acid, citric acid, and acetate acid. Lactic acid was produced in more amounts compared to other organic acids. In comparison, glutamic acid was produced in the least amount. The capacity of these bacteria to dissolve phosphate will differ depending on their ability to produce organic acids (Sharma et al., 2013; Serna-Posso et al., 2017). Osmolovskaya et al. (2018) claimed that each organic acid has a different capacity to chelate metal ions. Two factors, including the stability constant of complex organic acids with metal ions and the structure of the hydroxyl and carboxyl molecules in the primary carbon chain, affect this variance. Yang et al. (2022) reported that the capacity of phosphate-solubilizing fungi to produce organic acids and a decrease in the pH of the medium is closely related to the ability of phosphate solubilizing to produce organic acids.
Identification of leafs spot pathogenic fungal isolates
Isolation of SUR fungus from rice plants in Senganan Village, Penebel Tabanan, with brown patches on rice leaf (Figure 2A). Spores of N. oryzae were black and round (Figure 2B). The 16S rDNA sequencing results matched with Nigrospora oryzae (Figure 3). Thus the fungus was identified as N. oryzae. The fungus's 16S rRNA nucleotide gene sequence was deposited in the NCBI gene bank under accession No. OP035911. N. oryzae can cause panicle branch rot disease in rice plants, cause decreased yields and decreased rice quality, is the first study in china (Liu et al., 2021).
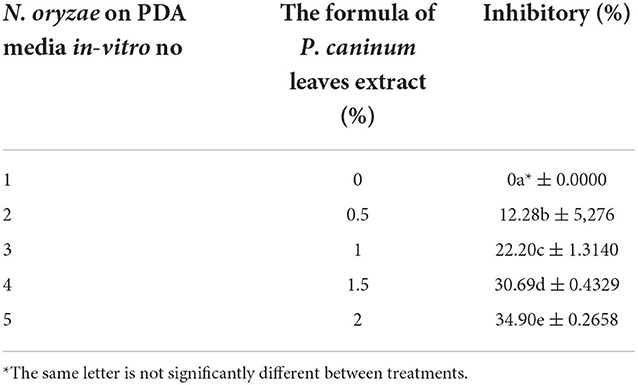
The inhibition of crude extracts of P. caninum against N. oryzae under in-vitro condi-tions
The inhibition of crude extract of P. caninum against N. oryzae was with an average diameter of 40 mm (Figure 4 and Table 1). The highest inhibition was found with P. caninum leaf extract at 2% (34.90%). The concentration of the extract is directly proportional to the inhibitory capacity against N. oryzae. Using P. caninum extract in-vitro can suppress leaf spotting disease in Bali red rice caused by N. oryzae. The test of the crude extract can inhibit N. oryzae with a diameter of 40 mm, classified as having powerful inhibitory properties. Tests of P. caninum leaf extract against N. oryzae fungal colonies showed the most significant inhibitory properties at a concentration of 2 at 34.90%. The higher the concentration of the extract, the greater the inhibition. The content of phytochemicals in P. caninum extracts, such as alkaloids, flavonoids, and phenols, can damage the fungus N. oryzae. The cell fluid flows out, and the cell becomes lysis. The extract can damage the cell wall of P. oryzae, causing blast disease, which can result in death. P. caninum also contains phytochemical substances such as Benzene, xylene, Tetradecane, dodecanoic acid, heptadecane, hexadecenoic acid, octadecamethyl cyclononasiloxa ne, Phthaic acid, and 8.11, 14-dococatrinic acid serves as fungicidal and bactericidal [33,34]. Essential oil from stems [Major compound: safrole (25.5%)] and leafs [Major compound: safrole (17.1%)] have antimicrobial and antioxidant activities (Salleh et al., 2015; Najafi et al., 2021; Gowtham et al., 2022). Plant extracts offer antifungal benefits (Arora et al., 2022) as they activate typical defense-related responses such as the production of H2O2, the up-regulation of genes encoding pathogenesis-related proteins, stilbene synthase, and the accumulation of resveratrol or its derivative piceid. This is consistent with additional experiments on cell suspensions and plants (Krzyzaniak et al., 2018).
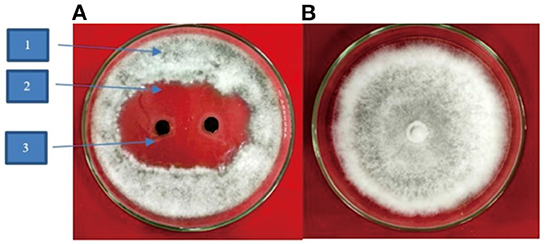
Figure 4. (A) Inhibitory of crude leaves extract of P. caninum against N. oryzae; 1-N. oryzae, 2-crude leaf extract, 3-clear zone (B) Control.
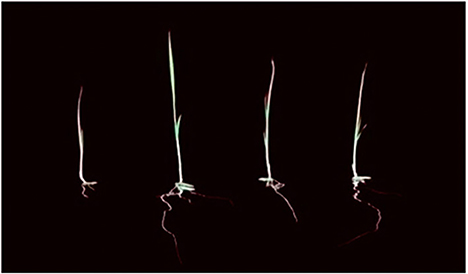
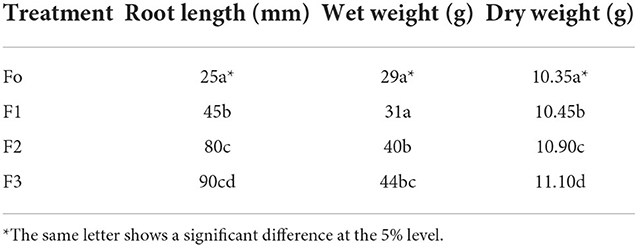
Effect of treatment in Bali red rice plant growth under in-vitro conditions
The study of the impact of treatment on the length of the roots grown on Petri dishes in the laboratory showed a difference in root length (Figure 5). The best treatment for long and compact development will be the F3 (fermentation of P. caninum leaf extract with B. agri), followed by F2 (B. agri) and F1 (P. caninum leaf extract), as described in Table 2. The use of P. caninum extract (F1), B. agri (F2), and fermentation extract of P. caninum with B. agri (F3) gave different results from the control. The in-vitro experiments prove they can increase the number of roots and compactness. Root length data show that control differs markedly from treatment, and the best roots are found in the F3 treatment (Table 2). Growth in rice plants is heavily influenced by the IAA hormone produced by rhizobacteria B. agri, where the qualitative test results in B. agri are positive. Additionally, there are growth hormones produced by P. caninum leaf extract in synergy with metabolites produced by rhizobacteria, which spurs the growth of rice plants. The results are supported by the research of P. caninum extract combined with rhizobacteria and compost. It provides the best barrier to Pyricularia oryzae, causing spotting disease in rice. It also gives Balinese red rice the best growth and production response compared to control or without combination treatment (Suriani et al., 2020b). B. agri and Brevibacillus formosus produce various antagonistic metabolites, such as HCN, chitinase, and siderophore, and suppress Salvia officinalis wilt and root rot infections. IAA formation of Brevibacillus spp. has been postulated as a method for plant growth promotion. Some Brevibacillus spp. could synthesize 3.8 mgL−1 indole-3-acetic acid (IAA) in vitro, contributing to the favorable effects (Ahmed et al., 2018). Bacteria and smoke-water extract improve growth and induce the synthesis of volatile defense mechanisms in Vitis vinifera (Salomon et al., 2017). The combination of plant growth-promoting bacteria and botanical pesticides increases organic red rice yield and reduces the Leptocorisa acuta population in Indonesia (Hoesain et al., 2021). Bacillus and Cyanobacteria isolates showed positive effects on rice seed growth compared to controls
Effect of treatment on Bali red rice plant growth in-vivo
The results showed that the treatment's growth parameters, plant height, number of tillers, number of leafs, and chlorophyll content significantly differed from the controls. All treatments are significantly different from the controls for the high parameters of red rice plants. The best influence on the number of leafs, chlorophyll content, and several tillers is found in the treatment of P. caninum leafs extract fermented with B. agri, followed in a row by treatment B. agri, with P. caninum leafs extract (Figure 6). The highest chlorophyll content parameters are found in the treatment with the fermentation of P. caninum leaf extract and B. agri. However, using P. caninum leaf extract and fermentation treatment with B. agri is shorter. In the treatment using B. agri, when compared to control then, the height of the plant is higher. This is very profitable for red Bali rice, considering it has a plant height of up to 250 cm. The harvest obtained will also be reduced (Suriani, 2019) with a decrease in rice. The amount of chlorophyll influences rice yields as it regulates the photosynthetic rate, plant growth, and grain yield. Nitrogen-fixing bacteria in the rhizosphere are non-symbiotic microorganisms that can boost N availability for rice plants (Sagar et al., 2022a,b). The synthesis of growth hormones by these bacteria also aids growth (Bhat et al., 2022). Meanwhile, the environment and their relationship with plants significantly impact these skills (Dutta and Gachhui, 2006).
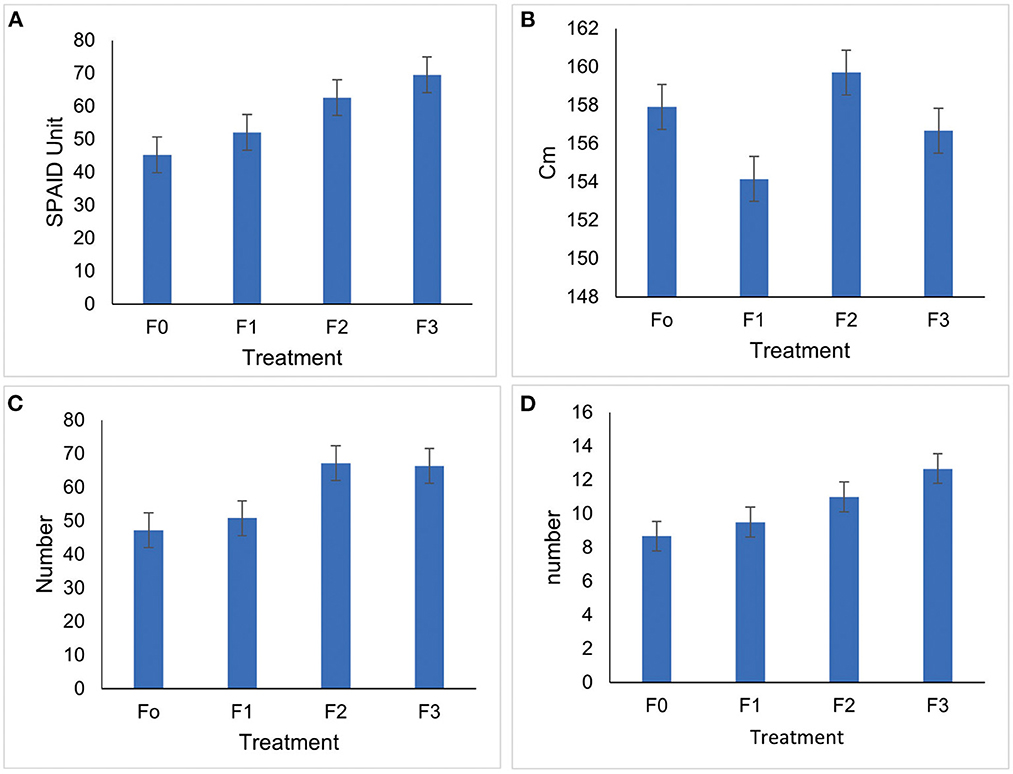
Figure 6. Average growth of Bali red rice plants (A) Chlorophyll content, (B) Height, (C) No. of leaves, (D) No. of tillers.
The parameters of the number of tillers and the highest number of leafs are also found in the treatment with the fermentation of P. caninum leaf extract with B. agri. A large number of tillers will increase the amount of dry grain in rice plants (Suriani et al., 2021). Furthermore, the control has an average number of tillers of 8.67, while in the treatment with extract of P. caninum fermented by B. agri, the number increased to 9.5 and 11. The highest number of tillers in the treatment using extracts of P. caninum leaf with B. agri is 12. The best treatment for the average growth of Balinese red rice uses the leaf extracts of P. caninum fermented with B. agri. Over the approved fertilizer dose, grain yield increased by 4.15–9.14%. Furthermore, grain yields improved significantly beyond the prescribed fertilizer dose when seaweed bio-stimulants were applied, ranging from 5.31 to 5.58 t/ha (T7). As a result, the bio-stimulant LBS6 S can be used to improve the growth and yield of transplanted rice (Raklami et al., 2019). The use of PGPR Bacillus megaterium CCMMB583 and Bacillus subtilis KJB06 can increase plant height growth, dry weight of plants and also increase chlorophyll in rice plants (Abd El-Mageed et al., 2022).
Effect of treatment on Bali red rice crop production
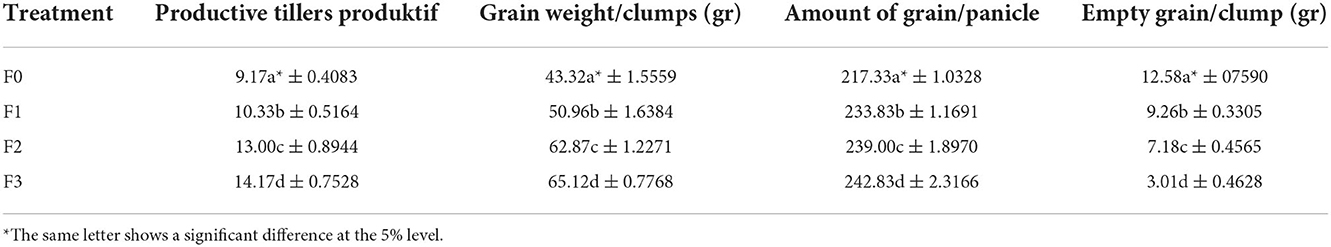
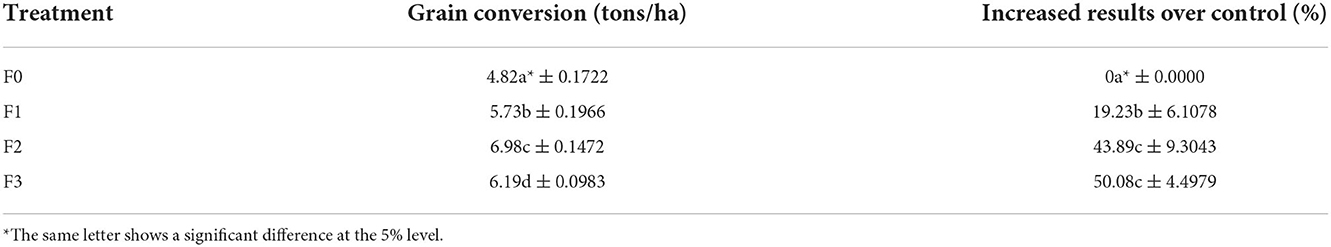
The results showed that the data on the highest number of productive tillers was found in the fermentation treatment of P. caninum leaf extracts with B. agri (F3), followed by B. agri treatment (F2) and the treatment of P. caninum leafs extract (F1)with 10.33, 13.00, and 14.17, respectively. This indicates that there was a significant difference between the control and treatments. The most important number of threads are found in the F3 treatment, amounting to 242.83. Similarly, there was a significant difference in data on grain weight/clumps between treatments and controls, where the increased grain/clump yield is found in the treatment of F3 with 65.12 gr. The empty grain/clump parameters between treatment and control also significantly differed; the lowest weight was found in the treatment of F3 with 3.01 gr, as presented in Table 3. The yield of grain tons/ha and the addition of results between treatment and control were also significantly different (Table 3). The highest grain yield of tons/ha was found in F3 silverware at 6.19 tons/ha, with the highest crop yield of 50.08% (Table 4).
Combining 3 Piper extract with rhizobacteria and compost can increase the number of tillers, the number of leafs, and rice productivity. This is achieved by reducing the amount of empty grain, increasing the amount of grain/panicle, grain weight/clumps, increasing the yield of grain tons/ha, and also affecting the growth of tons/ha (Suriani et al., 2019). The increase in grain yield is due to the decrease in the intensity of spotting disease, where the intensity of spot leaf disease is inhibited by P. caninum leafs extract, and B. agri., rhizobacteria Pseudomonas isolated from rice rooting in California can suppress spotting diseases caused by Magnophore oryzae, 90% of appressoria, and 78% M. oryzae fungus because it contains antifungal phytochemical substances (Spence et al., 2014). N. oryzae causes panicle branch rot, and this disease reduces yields and lowers milling quality (Liu et al., 2021). It is also reported to cause spots on rice leafs in China (Organisms, 2018). Additionally, N. oryzae can also rod Brassica juncea in India (Sharma et al., 2013).
The colony of M. oryzae was inhibited by 68, 65, and 48% by Pseudomonas sp., Burkholderia sp., and Bacillus spp., respectively. The bacterial suspension filtrates inhibited leafs spotting by 81.0, 79.2, and 66.3%, respectively. They were reported to solubilize phosphate, generate siderophores and cellulose, form biofilms, and reduce spot leaf when tested as M. oryzae antagonists (Martins et al., 2020; Nithyapriya et al., 2021).
The F3 treatment gives the best results for growth, covering the number of tillers, number of leafs, and chlorophyll content as well as crop parameters comprising of a number of productive tillers, number of grains/panicles, grain weight/clumps, grain yield tons/ha, and addition of yields; while reducing the weight of empty grain/clumps. This is due to the low intensity of the disease in F3 treatment (Table 5). The intensity of the disease is inversely proportional to the inhibition of the leaf spot. The chlorophyll content in all treatments, which is highest in the F3 treatment, is directly proportional to photosynthesis, growth, and components of the yield. A consortium of Bacillus sp., Pantoea agglomerans, and mycorrhiza has been reported to increase chlorophyll, biomass amount, and proline of crops in saline soil (Diagne et al., 2020). PGPR in rice plants can increase plant height, the number of saplings, chlorophyll content of leafs, yield of tons/ha, and water use efficiency (Abd El-Mageed et al., 2022). PGPR isolates from aromatic local rice rhizosphere can be used as biostimulants, biofertilizers, and biocontrol agents to boost plant development (Ali et al., 2022). Furthermore, it can promote the high growth of plants, chlorophyll, and biomass. It also improved the yield and health of rice plant genotypes Jaya, PA6444, and Pusa basmati-1, in India (Sharma et al., 2013). It provides attractive alternatives to synthetic fertilizers that are both environmentally benign and financially effective (Cen et al., 2020). Eucalyptus camaldulensis leafs extract (LE), Citrus sinensis LE, Ficus benghalensis fruit extract (FE), and two microbial antagonists were used as plant extracts and bioagents. The botanical pesticides from Azadirachta indica, Aglaia odorata, and Ageratum conyzoides combined with rhizobacteria (Bacillus sp. and Pseudomonas) can increase the growth and production of rice and suppress Leptocorisa acuta disease in organic red rice in Jember, Indonesia (Hoesain et al., 2021).
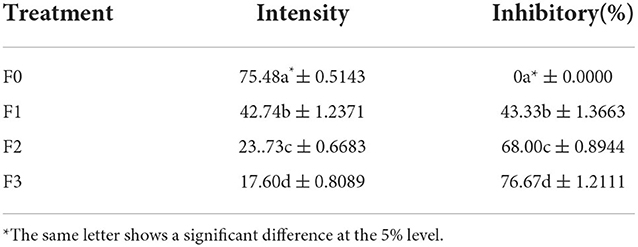
The intensity of leafs spot disease in greenhouse
The leaf spot disease studied in this study is spotting caused by the fungus N. oryzae. The intensity of leafs spot disease between treatment and control showed a noticeable difference, and data between treatments also reported a significant difference. The lowest intensity was found in the treatment of F3, followed by F2 and F1, with data on the minor inhibitory disease in F3, followed by F2 and F1 (Table 5). Furthermore, data on the intensity of spotting disease with the resistance is inversely proportional. The higher the intensity of spotting disease, the smaller the inhibition. Phytochemical content in the fermentation of P. caninum extract with B. agri can suppress leafs spotting diseases caused by N. oryzae fungus because it is anti-fungicidal (Table 5). Growth hormones such as IAA produced by B. agri and found in the leaf extract of P. caninum can increase growth. Additionally, new phytochemical substances result from the fermentation of P. caninum extract with B. agri to produce 2.3 butanadiol, tetradecanoic acid, Butanoic acid, ethyl ester, Benzenepropanal, 3-(1,1-dimethylethyl)-a-methyl, a-N-Normethadol strengthens the durability of rice plant tillers and increase growth. These findings contribute to comprehending bacterial volatiles in the rhizosphere and their functions (Yi et al., 2016). The bioactive components of tetradecanoic acid have been employed as additives in insecticides, pest control, insect repellents, and insecticidal agents (Bharathithasan et al., 2021).
GC-MS results
Gc-MS results showed that fermentation of P. caninum leafs extract with B. agri produced seven peak compounds (Figure 7). However, it showed different results for the extract of P. caninum without fermentation with B. agri. It revealed the presence of benzene, xylene, tetradecane, dodecanoic acid, heptadecane, hexadecenoic acid, octadecamethyl cyclononasiloxane, phthaic acid, and 8.11, 14- dococatrinic acid (Suriani et al., 2021). After the P. caninum leafs extract is fermented using B.agri, 2.3 butanadiol, tetradecanoic acid, Butanoic acid, ethyl ester, benzenepropanal, 3-(1,1-dimethylethyl)-a-methyl, a-N-Normethadol will be obtained. These compounds function as anti-fungicidal, and some as antioxidants such as a-N-Normethadol (Table 6). Meanwhile, 2,3-butanediol causes the release of root exudates, which influence soil fungus and bacteria. Tagetes erecta plant extract with phytochemical combined with rhizobacteria (Bacillus sp.) more effectively suppresses pathogenic fungi such as Monilinia laxa, Fusarium gramin earum, Aspergillus niger (Perisoara et al., 2022).
Conclusions
The treatment of P. caninum leafs extract, B. agri, and the fermentation of P. caninum with B. agri affects the intensity of leaf spot disease caused by N. oryzae. The best influence on the growth and production of Bali red rice is the treatment of F3, which is the fermentation of P. caninum extract using B. agri. The new phytochemical substances produced possess antifungal, antimicrobial, and antioxidant properties. These compounds are 2,3 butanediol, tetradecanoic acid, Butanoic acid, ethyl ester, Benzenepropanal, 3-(1,1-dimethylethyl)-a-methyl, and a-N-Normethadol.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/-~OP035911.
Author contributions
Conceptualization, methodology, and writing original draft: NS. Data analysis: DS. Writing, review and editing: NR, EP, SG, MR, SA, MA, and MS. Formal analysis: IS, NS, EP, SG, and MR. Fund acquisition: SA and MS. All authors contributed to the article and approved the submitted version.
Acknowledgments
This project was supported by Researchers Supporting Project Number (RSP-2023R7), King Saud University, Riyadh, Saudi Arabia and Research Management Center (RMC), University Malaysia Sabah (UMS), Malaysia through grant Number SKIM DANA NICHE SDN0020-2019 and SP DIPA-023.17.2.677526/2022 Udayana University, Bali, Indonesia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Mageed, T. A., Abd El-Mageed, S. A., El-Saadony, M. T., Abdelaziz, S., and Abdou, N. M. (2022). Plant growth-promoting rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of rice plants under full and deficit drip irrigation. Rice. 15, 1–15. doi: 10.1186/s12284-022-00564-6
Ahmed, A. I., Omer, A. M., Ibrahim, A. I., and Agha, M. K. (2018). Brevibacillus Spp. in agroecology: the beneficial impacts in biocontrol of plant pathogens and soil bioremediation. Fungal Genom. Biol. 8, 4–9. doi: 10.4172/2165-8056.1000157
Ali, S. A. M., Sayyed, R. Z., Mir, M. I., Hameeda, B., Khan, Y., Alkhanani, M. F., et al. (2022). Production, characterization and gene expression of Surfactin of Bacillus velezensis MS20 and evaluation of its induced systemic resistance and antibiofilm activity. Front. Microbiol. 13, 879739. doi: 10.3389/fmicb.2022.879739
Aron, D. (1949). Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15.
Arora, H., Sharma, A., Poczai, P., Sharma, S., Haron, F. F., Gafur, A., et al. (2022). Plant-derived protectants in combating soil-borne fungal infections in tomato and chilli. J. Fungi. 8, 1–18. doi: 10.3390/jof8020213
Basu, A., Prasad, P., Das, S. N., Kalam, S., Sayyed, R. Z., Reddy, M. S., et al. (2021). Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability, 13, 1140. doi: 10.3390/su13031140
Bharathithasan, M., Ravindran, D. R., Rajendran, D., Chun, S. K., Abbas, S. A., Sugathan, S., et al. (2021). Analysis of chemical compositions and larvicidal activity of nut extracts from Areca catechu Linn against Aedes (Diptera: Culicidae). PLoS ONE, 16, 1–14. doi: 10.1371/journal.pone.0260281
Bhat, B. A., Tariq, L., Nissar, S., Islam, S. T., Islam, S. U., Mangral, Z., et al. (2022). Plant-associated rhizobacteria in plant growth and metabolism as a tool for sustainable agriculture. J. Appl. Microbiol. 1–25. doi: 10.1111/jam.157962022
Cen, Y., Guo, L., Liu, M., Gu, X., Li, C., Jiang, G., et al. (2020). Using organic fertilizers to increase crop yield, economic growth, and soil quality in a temperate farmland. PeerJ 8, e9668. doi: 10.7717/peerj.9668
Darmadi, A. A. K., Suriani, N. L., Sudirga, S. K., and Khalimi, K. (2019). First study on fusarium equiseti: causes fusarium wilt in tomato crop in Bali, Indonesia. Sabrao J. Breed. Genet. 51, 442–450.
Diagne, N., Ndour, M., Djighaly, P. I., Ngom, D., Ngom, M. C. N., Ndong, G., et al. (2020). Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of casuarina obesa (Miq.). Front. Sustain. Food Syst. 4, 1–8. doi: 10.3389/fsufs.2020.601004
Dutta, D., and Gachhui, R. (2006). Novel nitrogen-fixing Acetobacter nitrogenifigens sp. nov., isolated from Kombucha tea. Int. J. Syst. Evolut. Microbiol. 56, 1899–1903. doi: 10.1099/ijs.0.64101-0
Febrianna, M., Prijono, S., and Kusumarini, N. (2018). The use of liquid organic fertilizer to increase nitrogen uptake and growth and yield of mustard (Brassica juncea L.) on sandy soil. J. Tanah Dan Sumberdaya Lahan 5, 1009–1018.
Ghatak, S., Muthukumaran, R. B., and Nachimuthu, S. K. (2013). A simple method of genomic DNA extraction from human samples for PCR-RFLP analysis. J. Biomol. Tech. 24, 224–231. doi: 10.7171/jbt.13-2404-001
Gowtham, H. G., Singh, S. B., Shilpa, N., Aiyaz, M., Nataraj, K., Udayashankar, A. C., et al. (2022). Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: a review. Antioxidants 11, 1763. doi: 10.3390/antiox11091763
Hamid, B., Zaman, M., Farooq, S., Fatima, S., Sayyed, R. Z., Baba, Z. A., et al. (2021). Bacterial plant biostimulants: a sustainable way towards improving growth, productivity, and health of crops. Sustainability 13, 1–24. doi: 10.3390/su13052856
Hoesain, M., Prastowo, S., Suharto, S., Pradana, A. P., Asyiah, I. N., Alfarizy, F. K., et al. (2021). Combination of plant growth-promoting bacteria and botanical pesticide increases organic red rice yield and reduces the leptocorisa acuta population. Biodiversitas 22, 1686–1694. doi: 10.13057/biodiv/d220411
Ilyas, N., Mumtaz, K., Akhtar, N., Yasmin, H., Sayyed, R. Z., Khan, W., et al. (2020). Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 12, 8876. doi: 10.3390/su12218876
Jabborova, D., Sayyed, R. Z., Azimov, A., Jabbarov, Z., Matchanov, A., and Enakiev, Y. (2021a). Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi J of Biol Sci. 28, 5268–5274. doi: 10.1016/j.sjbs.2021.05.037
Jabborova, D., Sulaymanov, K., Sayyed, R. Z., Alotaibi, S. H., Enakiev, Y., Azimov, A., et al. (2021b). Effect of different mineral fertilizers on turmeric rhizome mineral nutrients and soil properties under field condition. Sustainability 13, 9437. doi: 10.3390/su13169437
Jabborova, D., Wirth, S., Kannepalli, A., Narimanov, A., Desouky, S., Davranov, K., et al. (2020). Co-inoculation of rhizobacteria and biochar application improves growth and nutrient in soybean and enriches soil nutrients and enzymes, Agronomy 10, 1142. doi: 10.3390/agronomy10081142
Kalam, S., Basu, A., Ahmad, I., Sayyed, R. Z., El-Enshasy, H. A., Dailin, D. J., et al. (2020). Recent understanding of soil acidobacteria and their ecological significance: a critical review. Front. Microbiol. 11, 580024. doi: 10.3389/fmicb.2020.580024
Kannepalli, A., Davranov, K., Narimanov, A., Enakiev, Y., Syed, A., Elgorban, A. M., et al. (2021). Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 11, 22081. doi: 10.1038/s41598-021-01337-9
Khan, I., Awan, S. A., Ikram, R., Rizwan, M., Akhtar, N., Yasmin, H., et al. (2020). 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiologia Plantarum 172, 2, 696–706. doi: 10.1111/ppl.13237
Khan, N., Ali, S., Shahid, M. A., Mustafa, A., Sayyed, R. Z., and Curá, J. A. (2021). Insights into the Interactions among roots, rhizosphere and Rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10, 1551. doi: 10.3390/cells10061551
Khumairah, F. H., Setiawati, M. R., Fitriatin, B. N., Simarmata, T., Alfaraj, S., Ansari, M. J., et al. (2022). Halotolerant plant growth promoting rhizobacteria isolated from saline soil improve nitrogen fixation and alleviate salt stress. Front. Microbiol. 13, 905210. doi: 10.3389/fmicb.2022.905210
Krzyzaniak, Y., Trouvelot, S., Negrel, J., Cluzet, S., Valls, J., Richard, T., et al. (2018). A plant extract acts both as a resistance inducer and an oomycide against grapevine downy mildew. Front. Plant Sci. 9, 1–14. doi: 10.3389/fpls.2018.01085
Kumar, S., Sindhu, S. S., and Kumar, R. (2022). Biofertilizers: an ecofriendly technology for nutrient recycling and environmental sustainability. Current Res. Microbial Sci. 3, 100094. doi: 10.1016/j.crmicr.2021.100094
Kusale, S. P., Attar, Y. C., Sayyed, R. Z., Enshasy, H. E., Hanapi, Z., Ilyas, N., et al. (2021a). Inoculation of Klebsiella variicola alleviated slat stress salinity and improved growth and nutrients in wheat and maize. Agronomy 8, 11.927. doi: 10.3390/agronomy11050927
Kusale, S. P., Attar, Y. C., Sayyed, R. Z., Malek, R. A., Ilyas, N., Suriani, N. L., et al. (2021b). Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules 26, 1894. doi: 10.3390/molecules26071894
Liu, L. M., Zhao, K. H., Zhao, Y., Zhang, Y. L., and Fu, Q. (2021). Disease Note. 105, 2724. doi: 10.1094/PDIS-11-20-2423-PDN
Lyu, D., Backer, R., and Smith, D. L. (2022). Three plant growth-promoting rhizobacteria alter morphological development, physiology, and flower yield of Cannabis sativa L. Ind. Crops Prod. 178, 114583. doi: 10.1016/j.indcrop.2022.114583
Manasa, M., Ravinder, P., Gopalakrishnan, S., Srinivas, V., Sayyed, R. Z., El Enshasy, H. A., et al. (2021). Co-inoculation of Bacillus spp. for growth promotion and iron fortification in sorghum. Sustainability 13, 12091. doi: 10.3390/su132112091
Martins, B. E. D. M., Chaibub, A. A., Cortês, M. V. D. C. B., Lobo, V. L. D. S., and Filippi, M. C. C. (2020). Characterization of bacterial isolates for sustainable rice blast control. Revista Caatinga, 33, 702–712. doi: 10.1590/1983-21252020v33n313rc
Mir, M. I., Bee, H., Quadriya, H., Kumar, B. K., Ilyas, I., Kasem, H. S., et al. (2022). Multifarious indigenous diazotrophic rhizobacteria of rice (Oryza sativa L.) rhizosphere and their effect on plant growth promotion. Front. Nutr. 8, 781764. doi: 10.3389/fnut.2021.781764
Moradzadeh, S., Moghaddam, S. S., Rahimi, A., Pourakbar, L., Enshasy, H. A. E., Sayyed, R. Z., et al. (2021). Bio-chemical fertilizer improves the oil yield, fatty acid compositions, and macro-nutrient contents in Nigella sativa L. Horticulturae 7, 345. doi: 10.3390/horticulturae7100345
Moustafa, M. A. M., Amer, A., Al-Shuraym, L. A., Ibrahim, E. D. S., El-Hefny, D. E., Salem, M. Z. M., et al. (2022). Efficacy of chemical and bio-pesticides on cowpea aphid, Aphis craccivora, and their residues on the productivity of fennel plants (Foeniculum vulgare). J. King Saud Univ. Sci. 34, 101900. doi: 10.1016/j.jksus.2022.101900
Najafi, S., Nasi, H. N., Tuncturk, R., Tuncturk, M., Sayyed, R. Z., and Amirnia, R. (2021). Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 7, 588. doi: 10.3390/horticulturae7120588
Nithyapriya, S., Lalitha, S., Sayyed, R. Z., Reddy, M. S., Dailin, D. J., Enshasy, H. A. E., et al. (2021). Iand Herlambang S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability, 13, 5394. doi: 10.3390/su13105394
Osmolovskaya, N., Dung, V. V., and Kuchaeva, L. (2018). The role of organic acids in heavy metal tolerance in plants. Biol. Commun. 63, 1–8. doi: 10.21638/spbu03.2018.103
Parwanayoni, N. M. S., Suprapta, D. N., Darsini, N., and Sudirga, S. K. (2021). Isolation and molecular identification of fungi that cause stem rot disease in Bali's local legumes. Biogenesis J. Ilmiah Biologi 9, 73–80. doi: 10.24252/bio.v9i1.20426
Perisoara, A., Marinas, I. C., Geana, E. I., Constantin, M., Angheloiu, M., Pirvu, L., et al. (2022). Phytostimulation and synergistic antipathogenic effect of tagetes erecta extract in presence of rhizobacteria. Horticulturae, 8, 8090779. doi: 10.3390/horticulturae8090779
Raklami, A., Bechtaoui, N., Tahiri, A., Anli, M., Meddich, A., Oufdou, K., et al. (2019). Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 10, 1106. doi: 10.3389/fmicb.2019.01106
Reshma, P., Naik, M. K., Aiyaz, M., Niranjana, S. R., Chennappa, G., Shaikh, S. S., et al. (2018). Induced systemic resistance by 2, 4di.acetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J. Exp. Biol. 56, 207–12. Available online at: http://nopr.niscpr.res.in/handle/123456789/43660
Saboor, A., Muhammad, A. A., Hussain, S., El Enshasy, H. E., Hussain, S., Ahmed, N., et al. (2021). Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi J. Biol Sci. 28, 6339–6351. doi: 10.1016/j.sjbs.2021.06.096
Sagar, A., Rai, S., Ilyas, N., Sayyed, R. Z., Al-Turki, A. I., El Enshasy, H. A., et al. (2022a). Halotolerant rhizobacteria for salinity stress mitigation: diversity, mechanism and molecular approaches. Sustainability 14, 490. doi: 10.3390/su14010490
Sagar, A., Sayyed, R. Z., Ramteke, P. W., Ramakrishna, W., Poczai, P., Obaid, S. A., et al. (2022b). Synergistic effect of Azotobacter nigricans and NPK fertilizer on agronomic and yield traits of Maize (Zea mays L.). Front. Plant Sci. 13, 952212. doi: 10.3389/fpls.2022.952212
Sagar, A., Sayyed, R. Z., Ramteke, P. W., Sharma, S., Marraiki, N., Elgorban, A. M., et al. (2020). ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants 26, 1847–54. doi: 10.1007/s12298-020-00852-9
Salleh, W. M. N. H. W., Ahmad, F., and Yen, K. H. (2015). Chemical constituents from Piper caninum and antibacterial activity. J. Appl. Pharmaceut. Sci. 5, 020–025. doi: 10.7324/JAPS.2015.50604
Salomon, M. V., Piccoli, P., Funes Pinter, I., Stirk, W. A., Kulkarni, M., van Staden, J., et al. (2017). Bacteria and smoke-water extract improve growth and induce the synthesis of volatile defense mechanisms in Vitis vinifera L. Plant Physiol. Biochem. 120, 1–9. doi: 10.1016/j.plaphy.2017.09.013
Sarkar, D., Sankar, A., Devika, O. S., Singh, S., Parihar, M., Rakshit, A., et al. (2021). Optimizing nutrient use efficiency, productivity, energetics, and economics of red cabbage following mineral fertilization and biopriming with compatible rhizosphere microbes. Sci. Rep. 11, 15680. doi: 10.1038/s41598-021-95092-6
Serna-Posso, E. J., Prager, M. S., and Cisneros-Rojas, A. D. (2017). Organic acids production by rhizosphere microbes isolated from a typic melanudands and its effects on the inorganic phosphates solubilization. Acta Agronómica. 66, 241–247. doi: 10.15446/acag.v66n2.56148
Shabbir, M., Singh, M., Maiti, S., and Saha, S. K. (2021). Organophosphate pesticide (Chlorpyrifos): environmental menace; study reveals genotoxicity on plant and animal cells. Environ. Challenges 5, 100313. doi: 10.1016/j.envc.2021.100313
Sharma, P., Meena, P. D., and Chauhan, J. S. (2013). First report of nigrospora oryzae (berk. and broome) petch causing stem blight on brassica juncea in India. J. Phytopathol. 161, 439–441. doi: 10.1111/jph.12081
Spence, C., Alff, E., Johnson, C., Ramos, C., Donofrio, N., Sundaresan, V., et al. (2014). Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 14, 1–17. doi: 10.1186/1471-2229-14-130
Sri Widari, D. A. D. (2021). Dampak pengelolaan subak jatiluwih sebagai warisan budaya terhadap Lingkungan. J. Kajian Dan Terapan Pariwisata 2, 38–50. doi: 10.53356/diparojs.v2i1.48
Stecher, G., Tamura, K., and Kumar, S. (2020). Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol, 37, 1237–1239. doi: 10.1093/molbev/msz312
Sukmawati, D., Family, N., Hidayat, I., Sayyed, R. Z., Elsayed, E. A., Dailin, D. J., et al. (2021). Biocontrol activity of Aureubasidium pullulans and Candida orthopsilosis isolated from Tectona grandis L. Phylloplane against Aspergillus sp. in post-harvested citrus fruit. Sustainability 13, 7479. doi: 10.3390/su13137479
Suprapta, D. N. (2022). Biocontrol of anthracnose disease on chili pepper using a formulation containing paenibacillus polymyxa C1. Front. Sustain. Food Syst. 5, 1–7. doi: 10.3389/fsufs.2021.782425
Suriani, N. L. (2018). Bioactive substance use of leaf extract of piper caninum blume pressing for blas disease and increase production in rice. Int. J. Life Sci. 2, 42–50. doi: 10.29332/ijls.v2n2.156
Suriani, N. L. (2019). Piper caninum blume leaf extract and compost to suppress blast disease and increase the production of bali red rice (oryza sativa) in green house. Int. Res. J. Eng. IT Sci. Res. 5, 46–54. doi: 10.21744/irjeis.v5n4.693
Suriani, N. L., Darmadi, A. A. K., Parwanayoni, N. M. S., Hamid, M. H. N. A., and Yamin, B. M. (2019). The combination of piper Caninum Blume leaf extract and compost fertilizer for pressing blast disease and improving growth of bali red rice (Oryza Sativa Linn). Int. J. Adv. Sci. Eng. Inf. Technol. 9, 518–525. doi: 10.18517/ijaseit.9.2.7449
Suriani, N. L., Suprapta, D. N., Indrayani, A. W., Herlambang, S., Resiani, N. M. D., AL-Shwaiman, H. A., et al. (2021). The synergistic action of three piper plant extracts and biofertilizer for growth promotion and biocontrol of blast disease in red rice. Sustainability 13, 10412. doi: 10.3390/su131810412
Suriani, N. L., Suprapta, D. N., Nazir, N., Darmadi, A. A. K., Parwanayoni, N. M. S., Sudatri, N. W., et al. (2020a). Inhibitory activity of piper caninum leaf extract against curvularia spotting disease on rice plants. Indian J. Agric. Res. 54, 411–419. doi: 10.18805/IJARe.A-560
Suriani, N. L., Suprapta, D. N., Nazir, N., Parwanayoni, N. M. S., Darmadi, A. A. K., Dewi, D. A., et al. (2020b). A mixture of piper leaves extracts and rhizobacteria for sustainable plant growth promotion and bio-control of blast pathogen of organic bali rice. Sustainability 12, 1–18. doi: 10.3390/su12208490
Tembo, Y., Mkindi, A. G., Mkenda, P. A., Mpumi, N., Mwanauta, R., Stevenson, P. C., et al. (2018). Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 9, 1–10. doi: 10.3389/fpls.2018.01425
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Vasseur-Coronado, M., du Boulois, H. D., Pertot, I., and Puopolo, G. (2021). Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 245, 126672. doi: 10.1016/j.micres.2020.126672
Yang, T., Li, L., Wang, B., Tian, J., Shi, F., and Zhang, S. (2022). Isolation, mutagenesis, and organic acid secretion of a highly efficient phosphate-solubilizing fungus. Front. Microbiol. 13, 793122. doi: 10.3389/fmicb.2022.793122
Yi, H. S., Ahn, Y. R., Song, G. C., Ghim, S. Y., Lee, S., Lee, G., et al. (2016). Impact of a bacterial volatile 2,3-butanediol on Bacillus subtilis rhizosphere robustness. Front. Microbiol. 7, 1–11. doi: 10.3389/fmicb.2016.00993
Keywords: antimicrobial products, biopesticides, rice growth, green-house, GC-MS, IAA, piper caninum, SEM
Citation: Suriani NL, Suprapta DN, Suarsana IN, Reddy MS, Gunawan S, Herlambang S, Resiani NMD, Pratiwi E, Sabullah MK, Alfarraj S and Ansari MJ (2022) Piper caninum extract and Brevibacillus agri mixture suppresses rice leaf spot pathogen; Nigrospora oryzae and improves the production of red rice (Oryza sativa L). Front. Sustain. Food Syst. 6:1080481. doi: 10.3389/fsufs.2022.1080481
Received: 26 October 2022; Accepted: 15 November 2022;
Published: 07 December 2022.
Edited by:
Javid A. Parray, Department of Environmental Science GDC Eidgah Srinagar, IndiaReviewed by:
Nakkeeran S., Tamil Nadu Agricultural University, IndiaHuma Naz, MANFDC, India
Lubna Ansari, Pir Mehr Ali Shah Arid Agriculture University, Pakistan
Copyright © 2022 Suriani, Suprapta, Suarsana, Reddy, Gunawan, Herlambang, Resiani, Pratiwi, Sabullah, Alfarraj and Ansari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ni Luh Suriani, bmlsdWhzdXJpYW5pQHVudWQuYWMuaWQ=; Mohd. Khalizan Sabullah, a2hhbGl6YW5AdW1zLmVkdS5teQ==
 Ni Luh Suriani
Ni Luh Suriani Dewa Ngurah Suprapta
Dewa Ngurah Suprapta I. Nyoman Suarsana3
I. Nyoman Suarsana3 M. S. Reddy
M. S. Reddy Etty Pratiwi
Etty Pratiwi Mohd. Khalizan Sabullah
Mohd. Khalizan Sabullah