
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 09 January 2023
Sec. Crop Biology and Sustainability
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1062449
This article is part of the Research TopicElectrophysiology as a Path to Sustainable Crop and Food ProductionView all 4 articles
 Lyubov Yudina
Lyubov Yudina Ekaterina Sukhova
Ekaterina Sukhova Alyona Popova
Alyona Popova Yuriy Zolin
Yuriy Zolin Karina Abasheva
Karina Abasheva Kseniya Grebneva
Kseniya Grebneva Vladimir Sukhov*
Vladimir Sukhov*Electrical signals (ESs), which are generated in irritated zones of plants and propagate into their non-irritated parts, are hypothesized to be an important mechanism of a plant systemic response on the local action of adverse factors. This hypothesis is supported by influence of ESs on numerous physiological processes including expression of defense genes, production of stress phytohormones, changes in photosynthetic processes and transpiration, stimulation of respiration and others. However, there are several questions, which require solution to support the hypothesis. Particularly, the non-physiological stimuli (e.g., strong heating or burning) are often used for induction of ESs; in contrast, the ES induction under action of physiological stressors with moderate intensities requires additional investigations. Influence of long-term environmental factors on generation and propagation of ESs is also weakly investigated. In the current work, we investigated ESs induced by local action of the moderate heating and illumination in wheat plants under irrigated and drought conditions. It was shown that combination of the moderate heating (40°C) and illumination (blue light, 540 μmol m−2s−1) induced electrical signals which were mainly depolarization electrical signals near the irritation zone and hyperpolarization electrical signals (HESs) on the distance from this zone. The moderate soil drought did not influence HESs; in contrast, the strong soil drought significantly decreased amplitude of HESs. Finally, it was shown that the moderate heating could induce HESs without additional action of illumination. It was hypothesized that both hyperpolarization and depolarization ESs could be caused by the hydraulic wave.
Local action of stressors on plants requires specific stress signals providing a systemic adaptation response. Electrical signals (ESs) are considered to be an important mechanism of this response (Fromm and Lautner, 2007; Gallé et al., 2015; Choi et al., 2016; Hedrich et al., 2016; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021). There are three main types of ESs in higher plants including action potential, variation potential and system potential (Szechyńska-Hebda et al., 2017).
Action potential is a short-term pulse depolarization electrical signal (DES) (Trebacz et al., 2006); i.e., signal with the initial depolarization and following repolarization of the electrical potential across the plasma membrane. It is caused by non-damaging stressors (e.g., cooling, illumination, or touch) and mainly related to transient activation of calcium channels, short-term Ca2+ flux into the cytoplasm, and subsequent activation of anion and potassium channels (Trebacz et al., 2006; Felle and Zimmermann, 2007); however, the Ca2+-dependent transient inactivation of H+-ATP-ase in the plasma membrane can also participate in generation of this signal (Sukhova and Sukhov, 2021). Generation of action potential is with the “all-or-none law”; it is the self-propagating signal (Trebacz et al., 2006).
Variation potential is the long-term DES which has an irregular shape including the long-term slow wave and, possibly, short-term “action potential-like spikes” (Sukhova and Sukhov, 2021). It is considered that this signal is caused by local damages (e.g., burning, extremal heating, and crushing) and mainly related to the activation of calcium channels, Ca2+ flux into the cytoplasm and subsequent Ca2+-dependent inactivation of the H+-ATP-ase (Stahlberg et al., 2006; Fromm and Lautner, 2007; Sukhova and Sukhov, 2021); short-term activation of anion and potassium channels can also participate in variation potential generation providing forming the action potential-like spikes (Sukhova and Sukhov, 2021). It is important that variation potential is often considered as a local electrical response induced by propagation of non-electrical chemical (Fromm and Lautner, 2007; Toyota et al., 2018) or hydraulic (Stahlberg and Cosgrove, 1997; Mancuso, 1999) signals from the damaged zone; these signals are possible to activate ligand-dependent or mechano-sensitive Ca2+ channels, respectively (Sukhova and Sukhov, 2021). The propagation of interacted chemical and hydraulic signals is also potentially possible (Malone, 1994; Sukhova and Sukhov, 2021).
System potential is a long-term hyperpolarization electrical signal (HES) (Zimmermann et al., 2009, 2016); i.e., signal with the initial hyperpolarization and following repolarization of the electrical potential across the plasma membrane. This electrical signal can be caused by actions of various stressors (including variation potential-inducing stressors; Lautner et al., 2005; Yudina et al., 2022); it is probably to be related to activation of the H+-ATP-ase in the plasma membrane (Zimmermann et al., 2009, 2016). Mechanisms of propagation of system potential are actively discussed (Zimmermann et al., 2009; Yudina et al., 2022). Considering relations between generations of variation potential and system potential (Zimmermann et al., 2009), it cannot be excluded that system potential is also the local electrical response on propagation of the hydraulic wave (Yudina et al., 2022).
It is known that electrical signals can strongly influence physiological processes in non-irritated parts of plant (Fromm and Lautner, 2007; Gallé et al., 2015; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021); moreover, ESs can transmit between plants and influence physiological processes in non-irritated plants (Szechyńska-Hebda et al., 2022). Targets of influence of ESs can include the expression of defense genes (Wildon et al., 1992; Stanković and Davies, 1996; Fisahn et al., 2004; Mousavi et al., 2013), production of phytohormones (Dziubinska et al., 2003; Hlavácková et al., 2006; Hlavinka et al., 2012; Krausko et al., 2017; Farmer et al., 2020), photosynthesis (Fromm and Lautner, 2007; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021), phloem mass flow (Fromm and Bauer, 1994; Furch et al., 2009, 2010; van Bel et al., 2014), respiration (Filek and Kościelniak, 1997; Pavlovič et al., 2011; Lautner et al., 2014), transpiration (Kaiser and Grams, 2006; Grams et al., 2007; Vuralhan-Eckert et al., 2018), growth (Shiina and Tazawa, 1986; Stahlberg and Cosgrove, 1996), leaf reflectance (Sukhova and Sukhov, 2021) and many others. It should be noted that the influence of ESs on these parameters is mainly shown for DESs (variation potential and action potential); the influence of HES on plants is weakly investigated (Sukhova and Sukhov, 2021).
Many mechanisms of the ESs influence on physiological processes in plants require further investigations (Sukhova and Sukhov, 2021), but some ways of this influence are shown. For example, the fast influence of DESs on photosynthesis is mainly caused by decrease of the CO2 mesophyll conductance (Gallé et al., 2013) and next suppression of photosynthetic CO2 assimilation (Pavlovič et al., 2011; Sukhova and Sukhov, 2021); decreasing the quantum yield of photosystems and photosynthetic linear electron flow and increasing the non-photochemical quenching of chlorophyll fluorescence and cyclic electron flow are results of this suppression. However, DESs-induced changes in photosynthetic light reactions can be observed after excluding changes in the photosynthetic CO2 assimilation (Sukhova and Sukhov, 2021); i.e., the direct influence of DESs on photosynthetic light reactions is also possible. Earlier, we hypothesized (Sukhova and Sukhov, 2021) that both influences are caused by the DESs-related inactivation of H+-ATP-ase in the plasma membrane. This inactivation provides alkalization of the apoplast and acidification of the cytoplasm, stroma, and lumen of chloroplasts (Grams et al., 2009; Sukhova and Sukhov, 2021) and, thereby, suppresses photosynthetic processes. The long-term influence of DESs on photosynthetic processes can be related to other mechanisms (Sukhova and Sukhov, 2021); particularly, a DESs-induced long-term photosynthetic inactivation can be caused by stimulation of production of abscisic and jasmonic acids (Hlavácková et al., 2006; Hlavinka et al., 2012).
Stimulation of plant tolerance to actions of stressors is considered as the result of the physiological systemic responses (Choi et al., 2017; Sukhova and Sukhov, 2021). Particularly, it is known that ESs can decrease damage of photosynthetic machinery (Retivin et al., 1999; Sukhova and Sukhov, 2021) and increase stability of biological membranes and photosynthetic pigment content in plants under stress conditions (Zandalinas et al., 2020a,b). It is considered that this tolerance is rather non-specific (Retivin et al., 1997; Choi et al., 2017; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021); however, forming the ESs-induced plant tolerance to specific stressors cannot be excluded (Zandalinas et al., 2020a). ESs-induced photosynthetic changes and subsequent increasing the photosynthetic machinery tolerance to stressors are probable to participate in the stimulation of the total plant tolerance (Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021) because damages of photosynthetic machinery can be directly dangerous for plant life through decrease of productivity, these damages can also induce ROS production and cell death. Additionally, increase of ATP content, which is caused by ESs-induced photosynthetic changes (Sukhova and Sukhov, 2021), can participate in the plant reparation after action of stressors.
Thus, ESs are strongly related to the plant tolerance to stressors. These relations can be basis of (i) development of methods of modification of the plant tolerance (Sukhova and Sukhov, 2021) and (ii) development of methods of estimation of plant processes under action of stressors with using measurements of electrical activity (Chatterjee et al., 2015, 2018; Saraiva et al., 2017; Simmi et al., 2020; Parise et al., 2021); both directions of investigation are potentially important for the plant cultivation and supporting sustainable food systems. However, there are some questions and problems requiring further investigations in this field; in the current work we focused on two points.
(i) The most of noted results were shown with using local extremal damages (e.g., burning or heating to 55°C and more) which are typical inductors of variation potential (Sukhova and Sukhov, 2021). In contrast, induction of action potentials in higher plants by stressors with weak and moderate intensity requires their long-term adaptation (at least several hours) under very stable and favorable conditions that is not probable in environment (Sukhova and Sukhov, 2021). It means that it is not clear: Can ESs be induced by stressors with physiological intensities without this multi-hours adaptation? The negative response on this question can strongly limit perspective of investigation of ESs for the plant cultivation. There are several works showing propagation of variation potential-like ESs induced by local illumination (Szechyńska-Hebda et al., 2010) and induction of systemic physiological changes under combined local action of illumination and moderate heating (Zandalinas et al., 2020a). As a result, the first task of the current work was investigation of ESs induced by combination of the local illumination and moderate heating because the local action of this combination is probable under environmental conditions.
(ii) Plants can be often affected by a long-term action of adverse factors (e.g., drought) under environment; however, induction of electrical signals is weakly investigated in these unfavorable conditions. Our previous work (Yudina et al., 2022) showed that the moderate water deficit did not influence burning-induced DESs (variation potentials). In contrast, the strong water deficit decreased amplitudes of DESs; moreover, HESs were observed in some cases. As a result, the second task of the current work was investigation of ESs induced by combination of illumination and moderate heating under imitation of the long-term moderate and strong soil drought because combination of the illumination, moderate heating, and drought widely acts on plants under environmental conditions.
As a result, the general aim of our work was analysis of possibility of induction of ESs by action of “physiological” stressors (on the example of local illumination and moderate heating) and investigation of parameters of these signals under favorable and adverse conditions. To achieve this goal, we investigated directions and amplitudes of the electrical signals on different distance from the irritated zone in well-irrigated plants and plants under the moderate and strong soil drought.
Spring wheat plants (Triticum aestivum L., cultivar “Daria”) were used in the current investigation because wheat is the key agricultural crop with investigated electrical signals (Sukhova and Sukhov, 2021). The cultivar “Daria” is characterized by moderate tolerance to drought; it means that this cultivar is perspective object for investigation of influence of water deficit on ESs in plants.
Plant cultivation (in the vegetation room of Department of Biophysics of N. I. Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia) and experimental measurements were carried out from October 2021 to February 2022. Wheat seeds, which were provided by Federal Research Center N. I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR) (St. Petersburg, Russia), were planted after two days of soaking. Plants were cultivated in pots with the universal soil in the vegetation room under 16/8 h (light/dark) photoperiod at 24°C. Luminescent lamps FSL YZ18RR (Foshan Electrical And Lighting Co., Ltd, Foshan, China) were used as a light source. Thirteen to fourteen days old plants were used for electrophysiological measurements in the most of experiments excluding investigation of influence of the soil drought on ESs in plants.
In the case of the soil drought, 14 days old wheat plants were divided into two groups: with periodical irrigation every 2 days (control) and without this irrigation (experiment). Electrophysiological measurements were performed in 8–10 and 15–17 days after termination of the soil irrigation. The relative water content (RWC) in wheat shoots was estimated in control and experimental plants on basis of the fresh (FW) and dry (DW) weights which were measured in 10 and 17 days after termination of the soil irrigation. DW was measured after 2 h of high temperature action in a TV-20-PZ-K thermostat (Kasimov Instrument Plant, Kasimov, Russia) (about 100°C). RWC was calculated as .
Extracellular measurements of a surface potential are effective tool for investigation of ESs propagation in plants because this method is simple and relatively stable; multi-channel measurement (with large distance between electrodes) can be used. Thus, a system including extracellular Ag+/AgCl electrodes (RUE Gomel Measuring Equipment Plant, Gomel, Belarus), a high-impedance IPL-113 amplifier (Semico, Novosibirsk, Russia), and a personal computer was used for measurements of surface electrical potentials. These electrodes were contacted to plant via Uniagel conductive gel (Geltek-Medica, Moscow, Russia). There were two levels of localization of electrodes: (i) E1 was placed on 0 cm from the irritated zone (on border of this zone), E2 was placed on 2 cm from the irritated zone, and E3 was placed on 5 cm from the irritated zone, and (ii) E1 was placed on 2 cm from the irritated zone, E2 was placed on 5 cm from the irritated zone, and E3 was placed on 9 cm from the irritated zone. ER was placed on the wheat stem (near the soil). Figure 1A shows a scheme of localization of measuring (E1, E2, and E3) and reference (ER) surface electrodes on wheat plants for the second level of localization. Measurements were carried out on the second wheat leaf (excluding coleoptile).
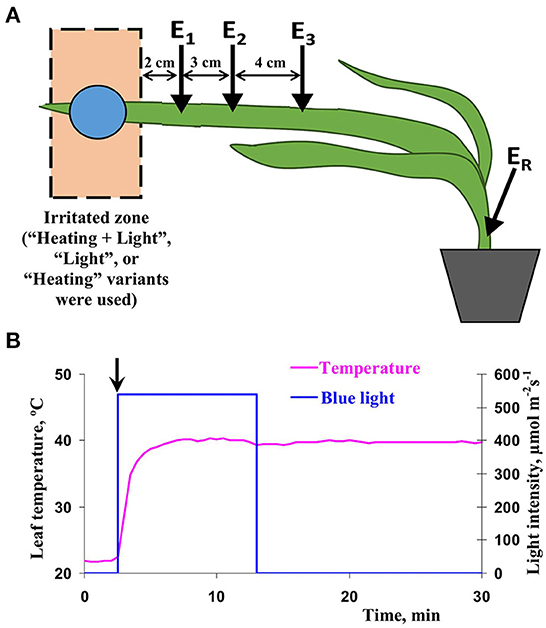
Figure 1. (A) Scheme of localization of measuring (E1, E2, and E3) and reference (ER) surface electrodes on wheat plants. There were two levels of localization of electrodes: (i) E1 was placed on 0 cm from the irritated zone (on border of this zone), E2 was placed on 2 cm from the irritated zone, and E3 was placed on 5 cm from the irritated zone, and (ii) E1 was placed on 2 cm from the irritated zone, E2 was placed on 5 cm from the irritated zone, and E3 was placed on 9 cm from the irritated zone. ER was placed on the wheat stem (near the soil). Figure show the second variant of electrode localization and distance between measuring electrodes. Combination of the blue light (540 μmol m−2s−1) and the heating (40°C), which locally acted the top of the second wheat leaf, was used as the main irritation (“Heating + Light”). In separate experiments, only the blue light (“Light”) or only the heating (“Heating”) were used. (B) Dynamics of leaf temperature and intensity of blue light during the leaf irritation by the combination of heating and illumination. Initiation of irritation is marked by arrow.
A combination of blue light (540 μmol m−2s−1) and heating (40°C), which locally acted the top of the second wheat leaf, was used as the main irritation; in separate experiments, only the blue light or only the heating were used. Total size of the irritated zone was about 4 cm from the top of leaf. Duration of the blue light action was 10 min after initiation of this irritation (Figure 1B); duration of the heating was 30 min (all duration of electrical measurements after the irritation). A self-manufactured system including blue light LED TDS-P003L4C04, 460 nm, 40 lm, 3 W (TDS Lighting Co., Huishan district, Wuxi city, Jiangsu Province, China) and the Peltier element STORM-71, 3.6 A, 36 W (Kryotherm, St.Petersburg, Russia) was used for illumination and heating; the light intensity and heating were regulated. The LED was equipped by black tube; it prevented illumination of other parts of plant. Intensity of light on leaf level was measured by the light flux meter PM100D with sensor S120C (Thorlabs Ultrafast Optoelectronics, Ann Arbor, Michigan, United States); the leaf temperature was measured by the thermometer monitor ATE-9380 (Aktakom, Moscow, Russia).
As a whole, there were three groups of experiments in the current work.
(i) Well-irrigated 13–14 days old plants were fixed and adapted (60 min) in the system for extracellular measurements of the surface potential. After that, top of the second wheat leaf was irritated by the combination of the blue light (540 μmol m−2s−1) and moderate heating (40°C); parameters of ESs were measured for 30 min after the leaf irritation. Both levels of localization of electrodes (with electrodes placed on 0, 2, and 5 cm from the irritated zone and on 2, 5, and 9 cm from the irritated zone) were used in this group of experiments.
(ii) Using similar procedure, ESs were measured in the control wheat (irrigated plants) and in wheat under the moderate (8–10 days without irrigation) and strong (15–17 days without irrigation) soil drought. The combination of local action of the blue light and moderate heating was used for induction of electrical signals in this variant of experiments. The second level of localization of electrodes (2, 5, and 9 cm from the irritated zone) was only used in this group of experiments. It should be noted that 22–24 days old plants (the moderate soil drought) and 29–31 old days plants (the strong soil drought) were used in this group of experiments. We did not reveal differences in parameters of ESs of control plants after 22–24 and 29–32 days of cultivation; therefore, results of all control plants in this experimental variant were combined into a common group of repetitions.
(iii) Using similar procedure, well-irrigated 13–14 days old plants were treated by local action of the combination of the blue light and moderate heating (control), by the moderate heating only (40°C), or by the blue light only (540 μmol m−2s−1). Parameters of electrical signals induced by all types of local stressors were analyzed. The first level of localization of electrodes (0, 2, and 5 cm from the irritated zone) was only used in this group of experiments.
Each measurement was performed on a separate plant. From 5 to 17 separate plants were used for different experiments; quantities of plants were shown in caption of figures. Representative records, mean values, and standard errors were calculated and presented in the figures. Numbers of replicates are shown in the figures. Significant differences were determined according to the Student's t-test.
In the first stage of investigation, we analyzed question: Could combination of moderate local illumination and heating induce ESs in wheat plants? It was shown (Figures 2, 3A) that this combined local irritation mainly induced DESs on border of the irritated zone (about 70% of signals). This DESs were typical variation potentials (Sukhova and Sukhov, 2021) and included the fast depolarization (the shift of the surface potential to negative direction) and subsequent irregular repolarization (the shift of the surface potential to positive direction) (Figure 2A). It should be also noted that about 30% of ESs in this zone were HESs (Figure 3A).
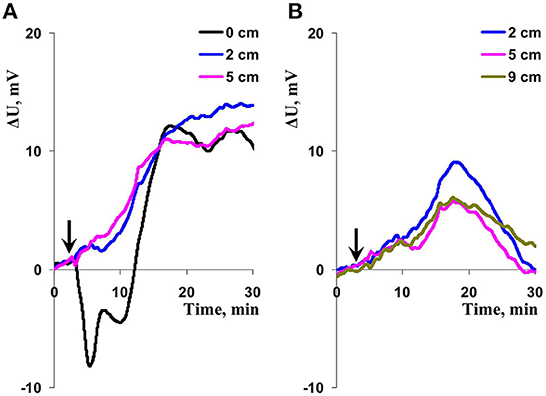
Figure 2. Records of changes in the surface potential (ΔU) induced by action of combination of local illumination and heating on wheat plants. (A) Extracellular measuring electrodes contacted to the second wheat leaf on border of the irritated zone (0 cm) and to this leaf on 2 and 5 cm from this zone (n = 10). (B) The extracellular measuring electrodes contacted to second wheat leaf on 2, 5, and 9 cm from the irritated zone (n = 17). Combination of the blue light (540 μmol m−2s−1) and heating (40°C) locally acted the top of the second wheat leaf; initiation of this action is marked by arrow.

Figure 3. Dependences of percentages of hyperpolarization electrical signals (HESs) and depolarization electrical signals (DESs) (A) and amplitudes of these signals (B) on distance from the zone of the local action of illumination and heating. Total dataset of electrical records (see Figure 2) was used for this analysis; n shows total quantity of repetitions for each distance. Average amplitude of DES at the 5 cm distance was not calculated because only one DES was observed. Vertical bars represent standard errors.
In contrast, ESs, which were observed on distance from border of the irritated zone, were mainly HESs (Figures 2, 3A). Percentage of HESs was about 85% of ESs on 2 cm from the border of the irritated zone, about 96% of ESs on 5 cm, and 100% of ESs on 9 cm (Figure 3A). Measured HESs (Figure 2) included the initial slow hyperpolarization which reached to maximal values for 10–20 min after initiation of irritation. Hyperpolarization of the surface potential could be observed for all time of measurement (30 min); alternatively, the slow repolarization could be observed. These signals seemed to be similar with long-term system potentials (Zimmermann et al., 2009, 2016).
Figure 3B shows amplitudes of measured DESs and HESs. Amplitude of DESs was strongly decreased with increasing distance from the irritated zone. Amplitude of HESs was maximal on 2 cm from border of the irritated zone and was slowly decreased with increasing this distance. It was interesting that amplitude of HESs on border of the irritated zone was lower than the maximal HESs amplitude on 2 cm from the irritated zone.
Thus, results of the first stage of investigation showed that combination of local illumination and heating could induce ESs in wheat plants; at that, both stressors (heating to 40°C, 540 μmol m−2s−1 light intensity) could be observed under environmental conditions. Revealed ESs mainly were DESs near the irritated zone and HESs on the distance from this zone.
Influence of the long-term soil drought, which is the important environmental stressor, on ESs was investigated in the next stage of our work. It was shown (Figure 4A) that shape of HESs under the moderate soil drought (8–10 days after termination of irrigation) was similar with shape of HESs under control conditions. This result was in accordance with the absence of changes in RWC in wheat shoots under this moderate soil drought (Figure 5A). Significant changes in amplitude of HESs were absent under the moderate drought (Figure 5B); only weak decrease of this amplitude in comparison to control one was observed. It should be additionally noted that DESs were not revealed in this case.
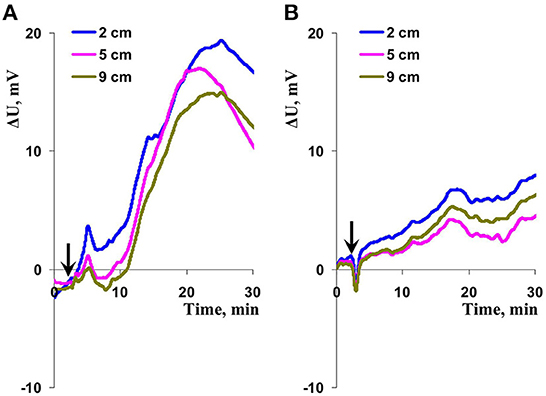
Figure 4. Records of changes in the surface potential (ΔU) induced by action of combination of local illumination and heating in wheat plants after 8–10 days (A) and 15–17 days (B) of absence of irrigation. The extracellular measuring electrodes contacted to the second wheat leaf on 2, 5, and 9 cm from the irritated zone. Combination of the blue light (540 μmol m−2s−1) and heating (40°C) locally acted the top of the second wheat leaf; initiation of this action is marked by arrow.
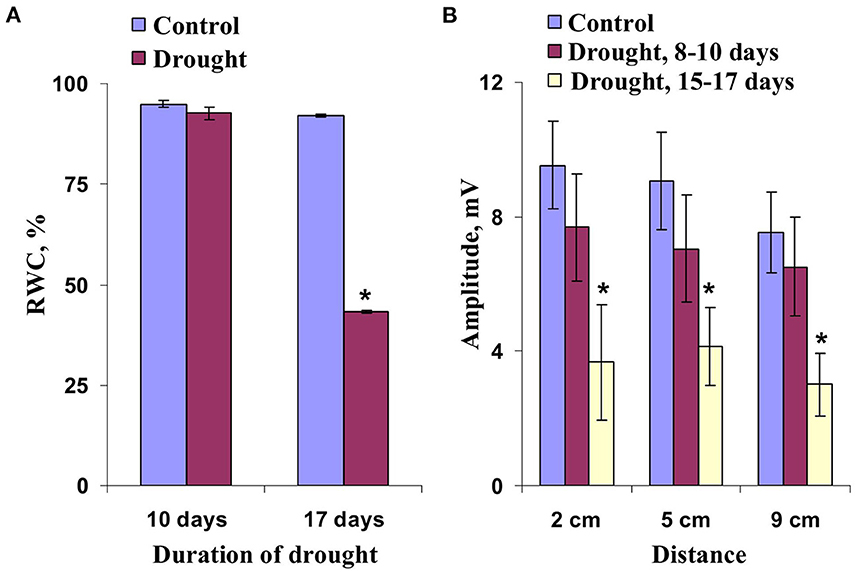
Figure 5. Dependence of the relative water content (RWC) in wheat leaves on duration of the soil drought (n = 5–9) (A) and dependence of the amplitude of hyperpolarization electrical signals on distance from the zone of the local action of combination of illumination and heating under control and drought conditions (n = 5–11) (B). The soil drought was provided by termination of irrigation of plants. *, values were significantly differed from ones in the control (p < 0.05). Vertical bars represent standard errors.
In contrast, the strong soil drought decreased RWC in wheat shoots from about 92–43% (Figure 5A); i.e., this drought should influence plant signaling. It was shown that the strong soil drought modified shape of HESs in wheat plants (a weak slow hyperpolarization was only observed for time of measurement, Figure 4B) and decreased amplitude of this signals (Figure 5B). DESs were also absent in this case.
Thus, induction of HESs by combination of local illumination and heating was not significantly influenced by the moderate soil drought; in contrast, the strong soil drought decreasing the shoot RWC suppressed these hyperpolarization signals.
The final stage of our experiments was devoted to analysis of the question: Was combined action of illumination and heating necessary for induction of ESs? It was shown (Figures 6A, 7) that ESs induced by only local heating were very similar with electrical signals induced by combination of local illumination and heating. Particularly, typical variation potentials were often observed on border of the irritated zone and large hyperpolarization electrical signals were revealed on 2 and 5 cm from this zone (Figure 6A). Amplitude of HESs induced by only heating and the amplitude of HESs induced by combination of illumination and heating were not significantly distinguished (Figure 7). It should be additionally noted that amplitude of heating-induced HESs on the 2 cm distance from the irritation zone could be lower than this amplitude on the 5 cm distance (Figure 6A); however, this effect was not significant for average amplitudes (Figure 7). Finally, it was shown the heating-induced DESs were absent on 2 and 5 cm distances from the irritated zone.
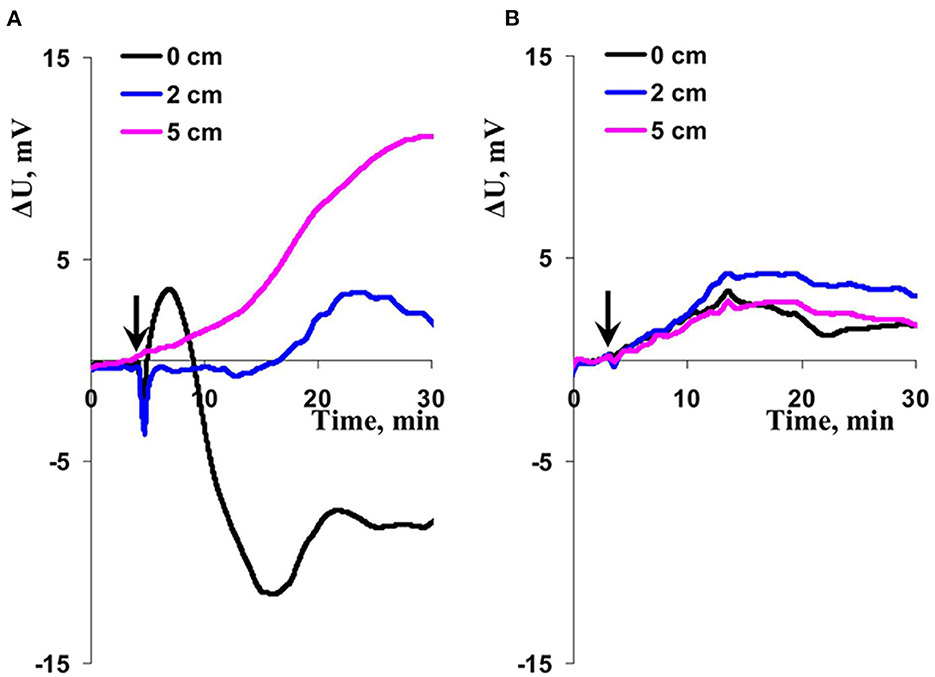
Figure 6. Records of changes in the surface potential (ΔU) induced by action of local illumination (A) and heating (B) in wheat plants. The extracellular measuring electrodes contacted to the second wheat leaf on 0 (border of this zone), 2, and 5 cm from the irritated zone. The blue light (540 μmol m−2s−1) or the heating (40°C) locally acted the top of the second wheat leaf; initiation of this action is marked by arrow.
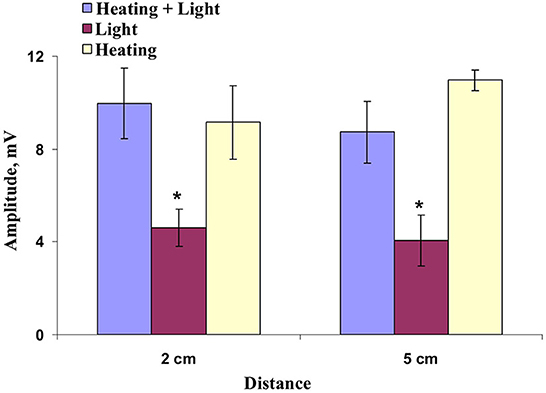
Figure 7. Dependence of the amplitude of hyperpolarization electrical signals in wheat plants on distance from the zone of the local action of combination of illumination and heating (“Heating + Light”), only illumination (“Light”), and only heating (“Heating”) (n = 6–10). Depolarization electrical signals were not analyzed. The 0 cm distance was not analyzed because both depolarization and hyperpolarization electrical signals were generated on this distance. *, values were significantly differed from ones in the “Heating + Light” variant (p < 0.05). Vertical bars represent standard errors.
The local action of the blue light without heating could also induced weak HESs (Figure 6B); however, amplitudes of these hyperpolarization signals were significantly lower than ones of HESs induced by the moderate heating or combination of the blue light and moderate heating (Figure 7). It was interesting that light-induced DESs were not revealed in this experiment on all investigated distances from the irritated zone (0, 2, and 5 cm).
Thus, results of this stage of our work showed that the local action of moderate heating was sufficient condition for induction of propagation of hyperpolarization electrical signal through plant body: moreover, this heating could provide generation of variation potential-like depolarization electrical signals in the irritated zone. The last point was in a good accordance with effective induction of variation potentials by action of high temperatures (burning or strong heating) (Sukhova and Sukhov, 2021).
Potentially, ESs, which are induced by local irritations and propagate through a plant body, can be the important mechanism of the systemic adaptation response in higher plants (Sukhova and Sukhov, 2021). There are numerous results (see, e.g., reviews by Fromm and Lautner, 2007; Gallé et al., 2015; Choi et al., 2016; Hedrich et al., 2016; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021; and many others) which support influence of ESs (mainly, DESs) on physiological processes and plant tolerance to action of stressors. However, there are several problems which require solution for understanding of role of ESs in plants under environmental conditions. (i) Induction of ESs under the local action of moderate stressors (or their combinations) requires further investigation because variation potential, which can be considered as the key ES in higher plants (Sukhova and Sukhov, 2021), is mainly induced by strong damages (particularly, burning and extremal heating); these damages can be rare events under natural conditions (e.g., they can be observed at wildfires). Action potentials can be induced by the local action of weak and moderate stressors; however, their propagation in higher plants requires the long-term adaptation under stable and favorable conditions that is not also typical for environment (Sukhova and Sukhov, 2021). (ii) Long-term action of stressors (e.g., the soil drought) can be often observed under environmental conditions. It can be expected that generation and propagation of ESs should be observed under the weak and moderate intensity of these “chronic” stressors; in contrast, the strong intensity of these stressors should suppress ESs because their induction and consequent development of physiological responses under these conditions seem to be rather dangerous (Sukhova and Sukhov, 2021). We earlier showed results supporting this proposition for burning-induced variation potentials (Yudina et al., 2022): these signals were not affected by the moderate water deficit and were changed by the strong water deficit; however, this problem requires further investigations. (iii) It is not clear: Can ESs induced by the local action of moderate stressors (or their combinations) induce physiological changes in non-irritated parts of plants? There are only few works devoted to analysis of this question (e.g., Szechyńska-Hebda et al., 2010; Suzuki et al., 2013). The current work was devoted to experimental investigation of the first and second problems; there are following important results.
First, the local action of combination of moderate heating (40°C) and illumination (540 μmol m−2s−1) can induce electrical signals in higher plants (wheat) (Figures 2, 3). Moreover, similar ESs are induced by the local action of only heating (Figures 6A, 7); i.e., the heating is probable to be the main reason of ESs induction under action of combined heating and illumination. Type of these signals are dependent on the distance from the irritated zone: DESs, which can be identified as variation potentials (Sukhova and Sukhov, 2021), are generated near this zone and HESs, which can be identified as system potentials (Zimmermann et al., 2009), are generated on the distance from the irritated zone. The local illumination can also induce HESs (Figures 6B, 7), but their amplitude is lower than this amplitude of heating-induced HESs.
The extremal heating (Sukhova and Sukhov, 2021) and illumination (Szechyńska-Hebda et al., 2010, 2017) are known to can induce DESs (variation potentials); however, induction of HESs (system potentials) by action of moderate heating and/or illumination shows that (i) propagation of ESs through plant body can be caused by widespread stressors under environmental conditions and (ii) system potentials can play important role in induction of the systemic adaptive response in higher plants. The last hypothesis is preliminary and requires future investigations because influence of DESs on physiological processes in plants is well-investigated (Fromm and Lautner, 2007; Gallé et al., 2015; Choi et al., 2016; Hedrich et al., 2016; Szechyńska-Hebda et al., 2017; Sukhova and Sukhov, 2021), but influence of HESs on physiological processes is not clear.
Second, it is shown that the moderate soil drought does not significantly influence parameters of HESs induced by combination of illumination and heating; in contrast, the strong drought significantly decreases amplitude of HESs (Figures 4, 5B). The first point means that system potentials can participate in the plant physiological regulation under moderate adverse conditions which are widespread in the environment. It is important because propagation of action potential (another ES, which can be induced in higher plants by weak and moderate stressors) can be strongly suppressed under adverse conditions (Sukhova and Sukhov, 2021). The decrease of amplitude of HESs under chronic action of strong stressors can be also important for plant protection because the ESs-dependent induction of additional adaptive changes (e.g., decreasing of photosynthetic dark reactions, Pavlovič et al., 2011; Gallé et al., 2013) is probable to be dangerous for damaged plants (Sukhova and Sukhov, 2021).
In the previous work (Yudina et al., 2022), we proposed a potential mechanism of induction and propagation of both DESs (variation potentials) and HESs (system potentials) in plants (Figure 8). This mechanism is based on classical hydraulic hypothesis of the variation potential propagation (Stahlberg and Cosgrove, 1997; Mancuso, 1999). This hypothesis assumes that burning, extremal heating, or crushing induce increase of a hydrostatic pressure in the irritated zone; the pressure increase is caused by physical processes (steam formation and increasing the volume of water under high temperatures; mechanical compression of tissues under crushing), by efflux of osmotically active compounds from cells through damaged membranes, and by their efflux related to the local electrical responses (Yudina et al., 2022). The increased pressure can be propagated through xylem bundles in plant body (the hydraulic wave); its magnitude is decreased with increasing the distance from the irritated zone (Stahlberg and Cosgrove, 1997). The classical hydraulic hypothesis supposes that the increased hydrostatic pressure inactivates H+-ATP-ase in the plasma membrane and provides generation of variation potential (Stahlberg and Cosgrove, 1996; Mancuso, 1999; Stahlberg et al., 2006; Sukhova and Sukhov, 2021). In contrast, we hypothesize (Yudina et al., 2022) that the dependance of the H+-ATP-ase activity on the pressure value can have maximum: weak increasing the pressure stimulates this activity and strong increasing the pressure suppresses H+-ATP-ase. There are some arguments supporting this hypothesis (Yudina et al., 2022). (i) It is known that the increased hydrostatic pressure induces depolarization of the plasma membrane (Stahlberg and Cosgrove, 1996, 1997); however, increasing pressure can also stimulate H+-ATP-ase (Okamoto et al., 2022). (ii) Ca2+ influx through mechanosensitive Ca2+ channels is considered to be the main mechanism of influence of hydraulic signal on activity of H+-ATP-ase (Sukhova and Sukhov, 2021); however, the increased Ca2+ concentration can both inactivate (Kinoshita et al., 1995) and activate (Yang Y. et al., 2019; Yang Z. et al., 2019) H+-ATP-ase.
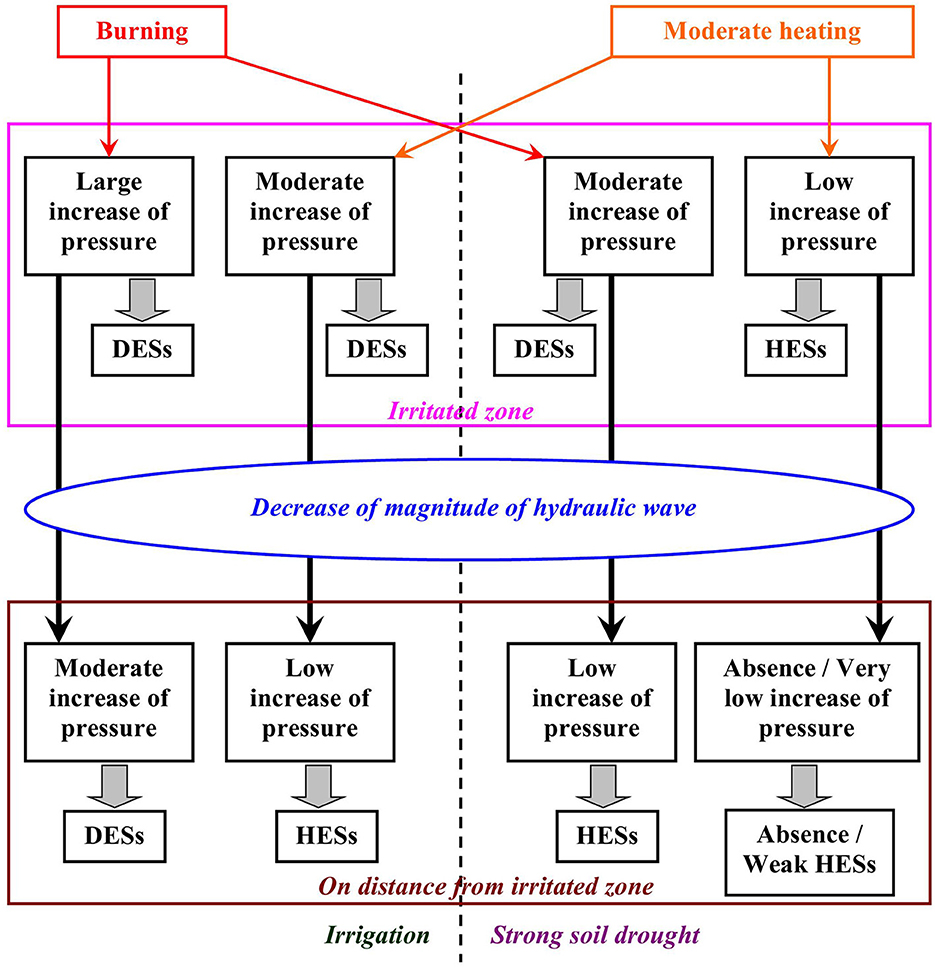
Figure 8. Hypothetical scheme of propagation of depolarization electrical signals (DESs) and hyperpolarization electrical signals (HESs) induced by local burning and moderate heating in the plants under irrigated conditions and drought conditions. The scheme is based on the current results and results of the previous work (Yudina et al., 2022). The ESs induced by local illumination are not shown in this scheme; it is probable that ways of induction of HESs by local illumination are similar to ones under the local moderate heating, but magnitude of the hydrostatic pressure increase is lower.
Our hypothesis predicts generation of HESs under low magnitudes of the hydraulic wave (e.g., as result of decreasing the magnitude of the pressure increase in the irritated zone or as result of increasing the distance from this zone) or full suppression of ESs generation after strong decreasing of pressure changes in the irritated zone. We previously showed (Yudina et al., 2022) that the strong water deficit, which should decrease hydrostatic pressure in plants (Christmann et al., 2013; Huber and Bauerle, 2016), increases probability of the HESs generation on the long distance from the irritated zone (the zone of local burning).
In the current work, magnitude of increasing the hydrostatic pressure in the irritated zone, which are caused by the moderate heating (40°C), should be lower than this magnitude induced by burning because increasing the volume of water is weak and destruction of plasma membranes in plants is absent (it requires 55°C temperature or more, Ilík et al., 2018). In contrast, local electrical responses can be observed in higher plants under heating to 30°C (Sukhov et al., 2017); it means that this mechanism of the efflux of osmotically active compounds from cells should be active under the moderate heating. Using this moderate heating shows predicted results: mainly HESs are generated on the distance from the irritated zone, and additional decreasing the hydrostatic pressure changes in the irritated zone (through the strong soil drought) completely suppresses generation of ESs.
Potentially, mechanisms of HESs induction and propagation under action of illumination can be similar to ones under heating. It is known that illumination induces local electrical responses (e.g., see Bulychev and Vredenberg, 1995; Trebacz and Sievers, 1998; Szechyńska-Hebda et al., 2010); i.e., illumination can also increase the hydrostatic pressure in the irritated zone. However, these light-induced ESs have low duration (from several seconds to several minutes); it means that the light-induced efflux of osmotically active compounds from cells is probable to be lower than the moderate heating-induced efflux because duration of local electrical responses induced by this heating are at least about 10 min (Sukhov et al., 2017). Experimental results are in a good accordance with this proposition because only HESs are observed in this experimental variant.
Thus, our current results are in a good accordance with the modified hydraulic hypothesis of propagation of variation and system potentials (Yudina et al., 2022) and support this hypothesis. The common mechanism, which is probable to be basis of long-term DESs (variation potentials) and HESs (system potentials), can explain relations between variation potentials and system potentials which are shown in the current work and in literature (Zimmermann et al., 2009). However, the modified hydraulic hypothesis makes very important the following question: Do variation potential and system potential influence physiological process in plants in similar or specific manner? This question is crucial for understanding of role of these signals in induction of physiological changes in non-irritated parts of plant and the systemic adaptation response. Investigation of the burning-induced HESs under the strong water deficit preliminary show that HESs can activate the photosynthetic CO2 assimilation (Yudina et al., 2022); in contrast, the heating-induced DESs (variation potentials) inactivate this assimilation under irrigated conditions. However, this difference can be related to different photosynthetic processes in plants under irrigated conditions and plants under the strong water deficit. Method of induction of HESs shown in the current work can be used as tool for future investigations of influence of hyperpolarization electrical signals on physiological processes (particularly, photosynthesis) in plants under the control conditions.
Results of the current work show several important points. First, physiological stressors (local action of the illumination and moderate heating, local action of the moderate heating) can induce electrical signals which propagate on long distances from the irritated zone and have large duration (tens of minutes). It means that ESs can be induced by action of environmental factors and, therefore, can participate in plant adaptation under natural conditions. Second, these signals are hyperpolarization electrical signals, which can be identified as system potentials. It shows that weakly-investigated system potentials can participate in long-distance signaling in plants; moreover, these signals are probable to play main role under natural conditions because action potentials require long-term adaptation under stable and favorable environmental conditions and variation potentials require action of non-physiological damaging stressors (mainly, burning or extremal heating). Thirdly, the moderate soil drought weakly influences parameters of hyperpolarization electrical signals; in contrast, the strong soil drought suppressed hyperpolarization electrical signals. This result seems to be expected because additional physiological responses can be dangerous for damaged plants under the strong soil drought. Finally, we hypothesis that mechanisms of these signals can be related to the two-phase response of H+-ATPase in the plasma membrane on increasing the hydraulic pressure (stimulation under the low increase of pressure and suppression under the high increase of pressure).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LY, ES, and VS: conceptualization and writing—original draft preparation. LY, AP, YZ, KA, and KG: methodology and investigation. ES and VS: formal analysis. VS: writing—review and editing and supervision. LY: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Investigation was funded by the Russian Science Foundation, grant number 21-74-10088.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bulychev, A. A., and Vredenberg, W. J. (1995). Enhancement of the light-triggered electrical response in plant cells following their de-energization with uncouplers. Physiol. Plant. 94, 64–70. doi: 10.1034/j.1399-3054.1995.940110.x
Chatterjee, S. K., Das, S., Maharatna, K., Masi, E., Santopolo, L., Mancuso, S., et al. (2015). Exploring strategies for classification of external stimuli using statistical features of the plant electrical response. J. R. Soc. Interface. 12, 20141225. doi: 10.1098/rsif.2014.1225
Chatterjee, S. K., Malik, O., and Gupta, S. (2018). Chemical sensing employing plant electrical signal response-classification of stimuli using curve fitting coefficients as features. Biosensors 8, 83. doi: 10.3390/bios8030083
Choi, W. G., Hilleary, R., Swanson, S. J., Kim, S. H., and Gilroy, S. (2016). Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 67, 287–307. doi: 10.1146/annurev-arplant-043015-112130
Choi, W. G., Miller, G., Wallace, I., Harper, J., Mittler, R., and Gilroy, S. (2017). Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 90, 698–707. doi: 10.1111/tpj.13492
Christmann, A., Grill, E., and Huang, J. (2013). Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 16, 293–300. doi: 10.1016/j.pbi.2013.02.011
Dziubinska, H., Filek, M., Koscielniak, J., and Trebacz, K. (2003). Variation and action potentials evoked by thermal stimuli accompany enhancement of ethylene emission in distant non-stimulated leaves of Vicia faba minor seedlings. J. Plant Physiol. 160, 1203–1210. doi: 10.1078/0176-1617-00914
Farmer, E. E., Gao, Y. Q., Lenzoni, G., Wolfender, J. L., and Wu, Q. (2020). Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 227, 1037–1050. doi: 10.1111/nph.16646
Felle, H. H., and Zimmermann, M. R. (2007). Systemic signaling in barley through action potentials. Planta 226, 203–214. doi: 10.1007/s00425-006-0458-y
Filek, M., and Kościelniak, J. (1997). The effect of wounding the roots by high temperature on the respiration rate of the shoot and propagation of electric signal in horse bean seedlings (Vicia faba L. minor). Plant Sci. 123, 39–46. doi: 10.1016/S0168-9452(96)04567-0
Fisahn, J., Herde, O., Willmitzer, L., and Peña-Cortés, H. (2004). Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant Cell Physiol. 45, 456–459. doi: 10.1093/pcp/pch054
Fromm, J., and Bauer, T. (1994). Action potentials in maize sieve tubes change phloem translocation. J. Exp.Bot. 45, 463–469. doi: 10.1093/jxb/45.4.463
Fromm, J., and Lautner, S. (2007). Electrical signals and their physiological significance in plants. Plant Cell Environ. 30, 249–257. doi: 10.1111/j.1365-3040.2006.01614.x
Furch, A. C., van Bel, A. J., Fricker, M. D., Felle, H. H., Fuchs, M., and Hafke, J. B. (2009). Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell. 21, 2118–2132. doi: 10.1105/tpc.108.063107
Furch, A. C., Zimmermann, M. R., Will, T., Hafke, J. B., and van Bel, A. J. (2010). Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J. Exp. Bot. 61, 3697–3708. doi: 10.1093/jxb/erq181
Gallé, A., Lautner, S., Flexas, J., and Fromm, J. (2015). Environmental stimuli and physiological responses: the current view on electrical signaling. Environ. Exp. Bot. 114, 15–21. doi: 10.1016/j.envexpbot.2014.06.013
Gallé, A., Lautner, S., Flexas, J., Ribas-Carbo, M., Hanson, D., Roesgen, J., et al. (2013). Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ. 36, 542–552. doi: 10.1111/j.1365-3040.2012.02594.x
Grams, T. E., Koziolek, C., Lautner, S., Matyssek, R., and Fromm, J. (2007). Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ. 30, 79–84. doi: 10.1111/j.1365-3040.2006.01607.x
Grams, T. E., Lautner, S., Felle, H. H., Matyssek, R., and Fromm, J. (2009). Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 32, 319–326. doi: 10.1111/j.1365-3040.2008.01922.x
Hedrich, R., Salvador-Recatalà, V., and Dreyer, I. (2016). Electrical wiring and long-distance plant communication. Trends Plant Sci. 21, 376–387. doi: 10.1016/j.tplants.2016.01.016
Hlavácková, V., Krchnák, P., Naus, J., Novák, O., Spundová, M., and Strnad, M. (2006). Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 225, 235–244. doi: 10.1007/s00425-006-0325-x
Hlavinka, J., NoŽková-Hlaváčková, V., Floková, K., Novák, O., and Nauš, J. (2012). Jasmonic acid accumulation and systemic photo-synthetic and electrical changes in locally burned wild type tomato, ABA-deficient sitiens mutants and sitiens pre-treated by ABA. Plant Physiol. Biochem. 54, 89–96. doi: 10.1016/j.plaphy.2012.02.014
Huber, A. E., and Bauerle, T. L. (2016). Long-distance plant signaling pathways in response to multiple stressors: the gap in knowledge. J. Exp. Bot. 67, 2063–2079. doi: 10.1093/jxb/erw099
Ilík, P., Špundová, M., Šicner, M., Melkovičová, H., Kučerová, Z., Krchnák, P., et al. (2018). Estimating heat tolerance of plants by ion leakage: a new method based on gradual heating. New Phytol. 218, 1278–1287. doi: 10.1111/nph.15097
Kaiser, H., and Grams, T. E. (2006). Rapid hydropassive opening and subsequent active stomatal closure follow heat-induced electrical signals in Mimosa pudica. J. Exp. Bot. 57, 2087–2092. doi: 10.1093/jxb/erj165
Kinoshita, T., Nishimura, M., and Shimazaki, K. (1995). Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of Fava bean. Plant Cell 7, 1333–1342. doi: 10.2307/3870106
Krausko, M., Perutka, Z., Šebela, M., Šamajová, O., Šamaj, J., Novák, O., et al. (2017). The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytol. 213, 1818–1835. doi: 10.1111/nph.14352
Lautner, S., Grams, T. E. E., Matyssek, R., and Fromm, J. (2005). Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 138, 2200–2209. doi: 10.1104/pp.105.064196
Lautner, S., Stummer, M., Matyssek, R., Fromm, J., and Grams, T. E. E. (2014). Involvement of respiratory processes in the transient knockout of net CO2 uptake in Mimosa pudica upon heat stimulation. Plant Cell Environ. 37, 254–260. doi: 10.1111/pce.12150
Malone, M. (1994). Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 128, 49–56. doi: 10.1111/j.1469-8137.1994.tb03985.x
Mancuso, S. (1999). Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust. J. Plant Physiol. 26, 55–61. doi: 10.1071/PP98098
Mousavi, S. A., Chauvin, A., Pascaud, F., Kellenberger, S., and Farmer, E. E. (2013). Glutamate receptor-like genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426. doi: 10.1038/nature12478
Okamoto, H., Kitamura, S., and Masaki, N. (2022). Activation of the root xylem proton pump by hydraulic signals from leaves under suppressed transpiration. J. Plant Res. 135, 211–322. doi: 10.1007/s10265-022-01368-x
Parise, A. G., Reissig, G. N., Basso, L. F., Senko, L. G. S., Oliveira, T. F. C., de Toledo, G. R. A., et al. (2021). Detection of different hosts from a distance alters the behaviour and bioelectrical activity of Cuscuta racemosa. Front. Plant Sci. 12, 594195. doi: 10.3389/fpls.2021.594195
Pavlovič, A., Slováková, L., Pandolfi, C., and Mancuso, S. (2011). On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J. Exp. Bot. 62, 1991–2000. doi: 10.1093/jxb/erq404
Retivin, V. G., Opritov, V. A., and Fedulina, S. B. (1997). Generation of action potential induces preadaptation of Cucurbita pepo L. stem tissues to freezing injury. Russ. J. Plant Physiol. 44, 432–442.
Retivin, V. G., Opritov, V. A., Lobov, S. A., Tarakanov, S. A., and Khudyakov, V. A. (1999). Changes in the resistance of photosynthesizing cotyledon cells of pumpkin seedlings to cooling and heating, as induced by the stimulation of the root system with KCl solution. Russ. J. Plant Physiol. 46, 689–696
Saraiva, G. F. R., Ferreira, A. S., and Souza, G. M. (2017). Osmotic stress decreases complexity underlying the electrophysiological dynamic in soybean. Plant Biol. 19, 702–708. doi: 10.1111/plb.12576
Shiina, T., and Tazawa, M. (1986). Action potential in Luffa cylindrica and its effects on elongation growth. Plant Cell Physiol. 27, 1081–1089.
Simmi, F. Z., Dallagnol, L. J., Ferreira, A. S., Pereira, D. R., and Souza, G. M. (2020). Electrome alterations in a plant-pathogen system: toward early diagnosis. Bioelectrochemistry 133, 107493. doi: 10.1016/j.bioelechem.2020.107493
Stahlberg, R., Cleland, R. E., and van Volkenburgh, E. (2006). Slow wave potentials–a propagating electrical signal unique to higher plants. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in Plants. Neuronal Aspects of Plant Life. New York, NY: Springer-Verlag (2006), 291–308.
Stahlberg, R., and Cosgrove, D. J. (1996). Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 200, 416–425. doi: 10.1007/BF00231397
Stahlberg, R., and Cosgrove, D. J. (1997). The propagation of slow wave potentials in pea epicotyls. Plant Physiol. 113, 209–217. doi: 10.1104/pp.113.1.209
Stanković, B., and Davies, E. (1996). Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 390, 275–279. doi: 10.1016/0014-5793(96)00672-2
Sukhov, V., Gaspirovich, V., Mysyagin, S., and Vodeneev, V. (2017). High-temperature tolerance of photosynthesis can be linked to local electrical responses in leaves of pea. Front. Physiol. 8, 763. doi: 10.3389/fphys.2017.00763
Sukhova, E., and Sukhov, V. (2021). Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants 10, 1704. doi: 10.3390/plants10081704
Suzuki, N., Miller, G., Salazar, C., Mondal, H. A., Shulaev, E., Cortes, D. F., et al. (2013). Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 25, 3553–3569. doi: 10.1105/tpc.113.114595
Szechyńska-Hebda, M., Kruk, J., Górecka, M., Karpińska, B., and Karpiński, S. (2010). Evidence for light wavelength-specific photoelec-trophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell. 22, 2201–2218. doi: 10.1105/tpc.109.069302
Szechyńska-Hebda, M., Lewandowska, M., and Karpiński, S. (2017). Electrical signaling, photosynthesis and systemic acquired acclimation. Front. Physiol. 8, 684. doi: 10.3389/fphys.2017.00684
Szechyńska-Hebda, M., Lewandowska, M., Wito,ń, D., Fichman, Y., Mittler, R., and Karpiński, S. M. (2022). Aboveground plant-to-plant electrical signaling mediates network acquired acclimation. Plant Cell. 34, 3047–3065. doi: 10.1093/plcell/koac150
Toyota, M., Spencer, D., Sawai-Toyota, S., Jiaqi, W., Zhang, T., Koo, A. J., et al. (2018). Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115. doi: 10.1126/science.aat7744
Trebacz, K., Dziubinska, H., and Krol, E. (2006). Electrical signals in long-distance communication in plants. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in Plants. Neuronal Aspects of Plant Life. Berlin, Heidelberg, New York, NY: Springer-Verlag, 277–290.
Trebacz, K., and Sievers, A. (1998). Action potentials evoked by light in traps of Dionaea muscipula Ellis. Plant Cell Physiol. 39, 369–372. doi: 10.1093/oxfordjournals.pcp.a029379
van Bel, A. J., Furch, A. C., Will, T., Buxa, S. V., Musetti, R., and Hafke, J. B. (2014). Spread the news: systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J. Exp. Bot. 65, 1761–1787. doi: 10.1093/jxb/ert425
Vuralhan-Eckert, J., Lautner, S., and Fromm, J. (2018). Effect of simultaneously induced environmental stimuli on electrical signalling and gas exchange in maize plants. J. Plant Physiol. 223, 32–36. doi: 10.1016/j.jplph.2018.02.003
Wildon, D. C., Thain, J. F., Minchin, P. E. H., Gubb, I. R., Reilly, A. J., Skipper, Y. D., et al. (1992). Electrical signalling and systemic proteinase inhibitor Induction in the wounded plant. Nature. 360, 62–65. doi: 10.1038/360062a0
Yang, Y., Wu, Y., Ma, L., Yang, Z., Dong, Q., Li, Q., et al. (2019). The Ca2+ sensor SCaBP3/CBL7 modulates plasma membrane H+-ATPase activity and promotes alkali tolerance in Arabidopsis. Plant Cell. 31, 1367–1384. doi: 10.1105/tpc.18.00568
Yang, Z., Wang, C., Xue, Y., Liu, X., Chen, S., Song, C., et al. (2019). Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 10, 1199. doi: 10.1038/s41467-019-09181-2
Yudina, L., Gromova, E., Grinberg, M., Popova, A., Sukhova, E., and Sukhov, V. (2022). Influence of burning-induced electrical signals on photosynthesis in pea can be modified by soil water shortage. Plants 11, 534. doi: 10.3390/plants11040534
Zandalinas, S. I., Fichman, Y., Devireddy, A. R., Sengupta, S., Azad, R. K., and Mittler, R. (2020a). Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA. 117, 13810–13820. doi: 10.1073/pnas.2005077117
Zandalinas, S. I., Fichman, Y., and Mittler, R. (2020b). Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell. 32, 3425–3435. doi: 10.1105/tpc.20.00453
Zimmermann, M. R., Maischak, H., Mithöfer, A., Boland, W., and Felle, H. H. (2009). System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 149, 1593–1600. doi: 10.1104/pp.108.133884
Keywords: hyperpolarization electrical signals, system potential, local illumination, local moderate heating, soil drought
Citation: Yudina L, Sukhova E, Popova A, Zolin Y, Abasheva K, Grebneva K and Sukhov V (2023) Local action of moderate heating and illumination induces propagation of hyperpolarization electrical signals in wheat plants. Front. Sustain. Food Syst. 6:1062449. doi: 10.3389/fsufs.2022.1062449
Received: 05 October 2022; Accepted: 12 December 2022;
Published: 09 January 2023.
Edited by:
Vasile Stoleru, Ion Ionescu de la Brad University of Agricultural Sciences and Veterinary Medicine of Iaşi, RomaniaReviewed by:
Agnieszka Maria Sekara, University of Agriculture in Krakow, PolandCopyright © 2023 Yudina, Sukhova, Popova, Zolin, Abasheva, Grebneva and Sukhov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimir Sukhov,  dnNzdWhAbWFpbC5ydQ==
dnNzdWhAbWFpbC5ydQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.