- Institute of Aquaculture, College of Fisheries and Ocean Sciences, University of the Philippines Visayas, Miagao, Iloilo, Philippines
The low omega-3 content of tilapia flesh, when compared to marine fish, affects its marketability. In marine animals, the highly unsaturated fatty acids (HUFAs) can be linked to the oil produced by marine diatoms. Among the marine diatoms, the genus Thalassiosira is known to exhibit high content of HUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Thus, in this study, the use of marine diatom Thalassiosira weissflogii as a dietary additive in the seawater-tolerant Nile Tilapia strain was evaluated. One hundred ninety-two, 1.40 ± 0.05g seawater tilapia were randomly allocated into 4 treatment groups in 4 replicates. The first treatment group was fed with a control diet (D0), without the diatoms while treatments 1, 2, and 3 were each fed with diets supplemented with T. weissflogii paste at 2.55% (D1), 6% (D2), and 12% (D3), respectively for 60 days. The diets were isonitrogenous, isolipodic and the omega-3 and omega-6 requirements were satisfied. Results demonstrated that D1 had the highest percent weight gain among treatments. Although not significantly different, other parameters such as percent survival, specific growth rate (SGR), protein efficiency ratio (PER), feed conversion ratio (FCR), and feed intake had desirable results in D1. The proximate composition of seawater tilapia showed that % crude protein was highest in D0 but % crude lipid was highest in D1. The fatty acid composition of tilapia in D1 had the highest omega-3 content at 9.29 mg/g tissue and also had the highest n3:n6 at 2.19. Muscle growth-related genes (MyoD and MYG) were up-regulated while liver genes involved in long-chain polyunsaturated fatty acid synthesis (oni-fads2 and elvol5) were down-regulated in D1 as compared to D0. Feeding the diatom-supplemented diet to tilapia had no significant effects on hepatic cells and intestinal morphology. The results suggested that a 2.55% supplementation dose of T. weissflogii could promote growth and enhance the tissue content of omega-3 fatty acids of the seawater strain Oreochromis niloticus.
Introduction
The Tilapia aquaculture in the Philippines plays a significant role in the overall economy of the country and the Nile tilapia (Oreochromis niloticus) is the dominant species that contribute to the country's tilapia production (Romana-Eguia et al., 2013). In 2018, the farmed tilapia production in the Philippines was at 277,006 metric tons and valued at Php 21.5B (PSA, 2019). However, the industry's average annual production rate was only at 0.7% from 2007 to 2016 (Guerrero, 2019). The expansion of Nile tilapia aquaculture is limited due to the availability of freshwater resources. Brackishwater ponds and marine coastal water cages are seen to have a high potential for the growth and development of farmed tilapia (Guerrero, 2019). For the past years, several seawater-tolerant strains had been developed for the expansion of tilapia culture toward the sea (Rosario et al., 2004; Watanabe et al., 2006; Jaspe and Caipang, 2011; Verdal et al., 2014; El-Dakar et al., 2015; Intoy and Traifalgar, 2021).
Another issue that affects the marketability of tilapia is the low omega-3 content of tilapia flesh. The level of omega-3 fatty acids in tilapia is comparatively lower than that of marine fish. This condition sometimes lowers the price of tilapia as compared to marine fish. However, in contrast to marine fish, it is a well-established fact that tilapia can synthesize long-chain omega-3 fatty acids from precursors of short-chain fatty acids. Tilapia is considered an ecologically friendly species for aquaculture since these fish are not dependent on fish oil to attain optimal growth.
Omega-3 (n-3) fatty acids include, among others, alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). Benefits of omega-3 fatty acids to humans include prevention of cardiovascular disease, improvement of visual acuity, and fortification of mental health. Hence, the American Heart Association (AHA) recommends two 4 oz (113 g) servings of fatty fish that are high in omega-3 fats per week (Kris-Etherton et al., 2002). However, freshwater tilapia contains only 200 mg/100 g of omega-3 and a higher amount of omega-6 (30–600 mg/100 g; Young, 2009). In particular, for some people with heart disease, arthritis, asthma, and other allergic and autoimmune illnesses, the ratio of omega-3 to omega-6 is not ideal for human health (Holub and Holub, 2004; Young, 2009). Therefore, increasing the content of omega-3 in tilapia meat is an important breeding target for tilapia. However, traditional breeding to increase omega-3 concentration is challenging, expensive, time-consuming and the molecular and genetic markers and factors related to the control and modulation of fatty acid synthesis in tilapia is not yet fully established (Xia et al., 2014).
Microalgae biomass was reported to be rich in polyunsaturated fatty acids (PUFA) and could be an important source of essential fatty acids for aquatic animals (Becker, 2004). Marine diatoms are known for their high content of biologically important highly unsaturated fatty acids (HUFA) including EPA and DHA. These long chains HUFA's are known to be absent in terrestrial sources of feed oils including soybean oil and other seed oils. It has been demonstrated that the HUFAs detected in marine animal flesh can be linked to the oil produced by marine diatoms (Brown, 2002). Marine diatoms such as the genus Chaetoceros, Thalassiosira exhibited the highest EPA and DHA fatty acid contents ranging from 11 to 19% of the total fatty acids (Volkman et al., 1989). Thalassiosira weissflogii has been known to improve the nutrient profile as well as the fatty acid composition when used as a live feed to shrimp larvae and copepod (Arendt et al., 2005; Kiatmetha et al., 2011; Sandeep et al., 2021). The use of T. weissflogii as a feed additive to cultured shrimp in pond improved the growth and also increased the fatty acid and astaxanthin contents of shrimp tail muscles (Ju et al., 2009). However, the use of diatoms as a feed supplement to influence the fatty acid composition of tilapia tissue has not been evaluated until the present.
The present study aims to optimize the dosage of T. weissflogii supplementation in tilapia diets to increase the levels of n-3 PUFA such as EPA and DHA incorporation to tilapia flesh. The effect of the T. weissflogii supplemented diet on the growth performance and morphology of seawater tilapia was also evaluated.
Materials and methods
Experimental diets
Thalassiosira weissflogii pastes were procured from Algacon Aquaculture Manufacturing and were stored at −20°C prior to use. Four diets were formulated including the control diet with zero T. weissflogii and three diets containing increasing levels of T. weissflogii at 2.55%, 6%, and 12%. Experimental diets were formulated to be isonitrogenous and isolipidic. Also, the diets were designed to satisfy the omega-3 and omega-6 requirements of tilapia (Tables 1, 2).
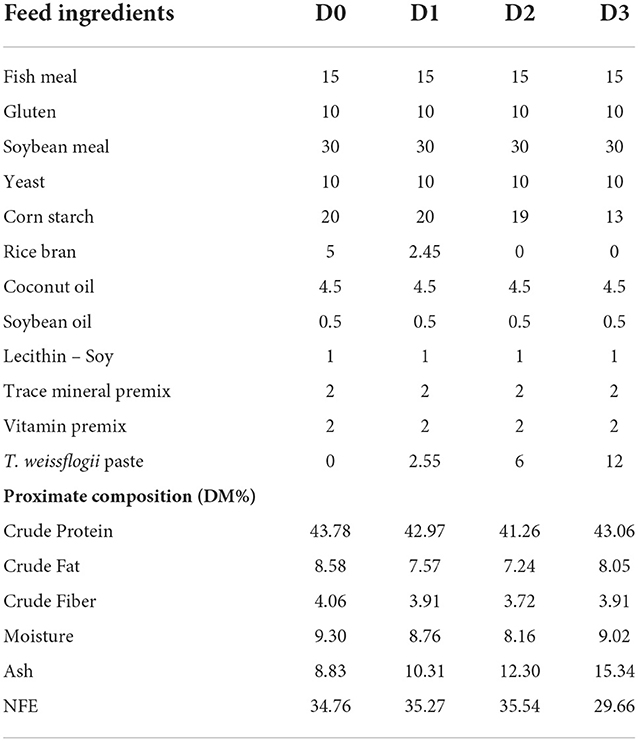
Table 1. Feed formulation (%) and proximate composition (% dry matter) of different experimental diets.
The diets were prepared by mixing all the dry ingredients separately in an electric mixer before adding oils, lecithin, and T. weissflogii paste. The moist dough was pelleted to a 2 mm diameter electric pelletizer and was oven-dried at 60°C for 13–15 h. After drying, the strands were ground and sieved to the appropriate size for feeding. The diets were packed in a sealed plastic bag and were stored at −20°C until use.
Feeding trial
The experiment was conducted in the rearing facilities of the Institute of Aquaculture, College of Fisheries and Ocean Sciences at the University of the Philippines, Miagao, Iloilo, Philippines. Juvenile seawater Nile tilapia (O. niloticus) strain were collected from the production tanks and were acclimatized to laboratory conditions for 2 weeks. Tilapia were fed with a commercial diet containing 45% crude protein during acclimation. Twelve tilapia (1.40 ± 0.05 g) were stocked in a 50-L rearing tank supplied with seawater and aeration. The seawater was filtered using two units of a 1-micron (μ) cartridge filter and the flow rate was maintained at 300 ml/min allowing up to 400% percent water change per day. A total of 16 rearing tanks were used representing 4 treatments in 4 replicates and were arranged in a complete randomized design. Feeding was done 3x a day to satiation. Water temperature and salinity were maintained at 28 ± 0.17°C and 34.31 ± 0.12 ppt, respectively. Uneaten feeds were collected daily, dried and weighed to quantify the feed intake.
At the end of the 60-day feeding trial, all fish were weighed and growth parameters such as percent weight gain (%WG), specific growth rate (SGR), protein efficiency ratio (PER), feed conversion ratio (FCR), and percent survival (%S) were calculated using the equation stated by Hardy and Barrows (2003).
Proximate analysis, nutrient retention, and fatty acid analysis
To assess the influence of the dietary treatments on the composition of the fish carcass, proximate analyses of the experimental diet and the fish carcass were performed. The fish samples were oven-dried at the end of the 60-day feeding trial. Crude protein was determined through Kjeldahl method, using Block Digestion and Steam Distillation (Foss Tecator™ Digestion and Foss Kjeltec™ 8200 Auto Distillation) while crude fat was quantified through Soxhlet Extraction using Foss Soxtec™ 2050 Automatic System. The ceramic fiber filter method using Foss Fibertec ™2010 System was used to determine crude fiber. Moisture content was determined by moisture analyzer (Mettler Toledo Halogen) and ash content was analyzed by furnace combustion following the official method of AOAC (14th edition) (AOAC, 1984). Nitrogen-free extract (NFE) was calculated following the procedure of Aksnes and Opstvedt (1998). The nutrient retention was also computed using the formula;
The fatty acid analysis of the experimental diets and the fish carcass was conducted to evaluate the influence of the dietary treatments on the fatty acid profile of seawater tilapia. Prior to analysis, the fish samples were freeze-dried. The experimental diets and fish carcass were analyzed for fatty acid analysis using the AOAC 996.0 (AOAC, 2000) method through Gas chromatography with Flame Ionization Detector (GC-FID).
Gene expression
At the end of the feeding trial best treatment group and the control group were evaluated for the transcription of muscle growth related genes and the fatty acid synthesis related genes. Liver tissues and dorsal muscles of seawater tilapia collected in the best treatment group and in the control, groups were collected for gene expression analysis. Total RNA was extracted from the liver and muscle of tilapia using Trizol Reagent (Life Technologies) following the manufacturer's protocol. Tissues from three fish per replicate tanks per treatment groups were used in the analysis. Complementary DNA was synthesized from the extracted RNA using SuperScript™ IV VILO™ Master Mix (ThermoFisher Scientific) following the manufacturer's instruction. The resulting cDNA was diluted five times. Quantitative real-time PCR analysis was carried out in CFX Connect Real-Time PCR Detection System (Biorad) using SYBR green PCR master mix ViPrimePLUS Taq qPCR Green MasterMix I (Vivantis). Primers specific to fatty acid delta-6 desaturase (oni-fads2) gene, elongase of very long-chain fatty acids 5 (elovl5), insulin-like growth factor (IGF), myostatin or growth and differentiation factor-8 (GDF-8), MyoD and myogenin (MyG) were used in the analysis (Table 3). Beta-actin served as the reference gene. A post-amplification melt-curve analysis was performed to confirm the specificity of the PCR product. No reverse transcription control and no template control were included in every qPCR run. Efficiencies of PCR reactions were determined for each primer set by running a set of 5 serial dilutions of a pool of all sample RNAs (Pfaffl, 2007). The expression levels of relative genes were calculated using the 2−ΔΔCT method of Livak and Schmittgen (2001). Each analysis was replicated five times.
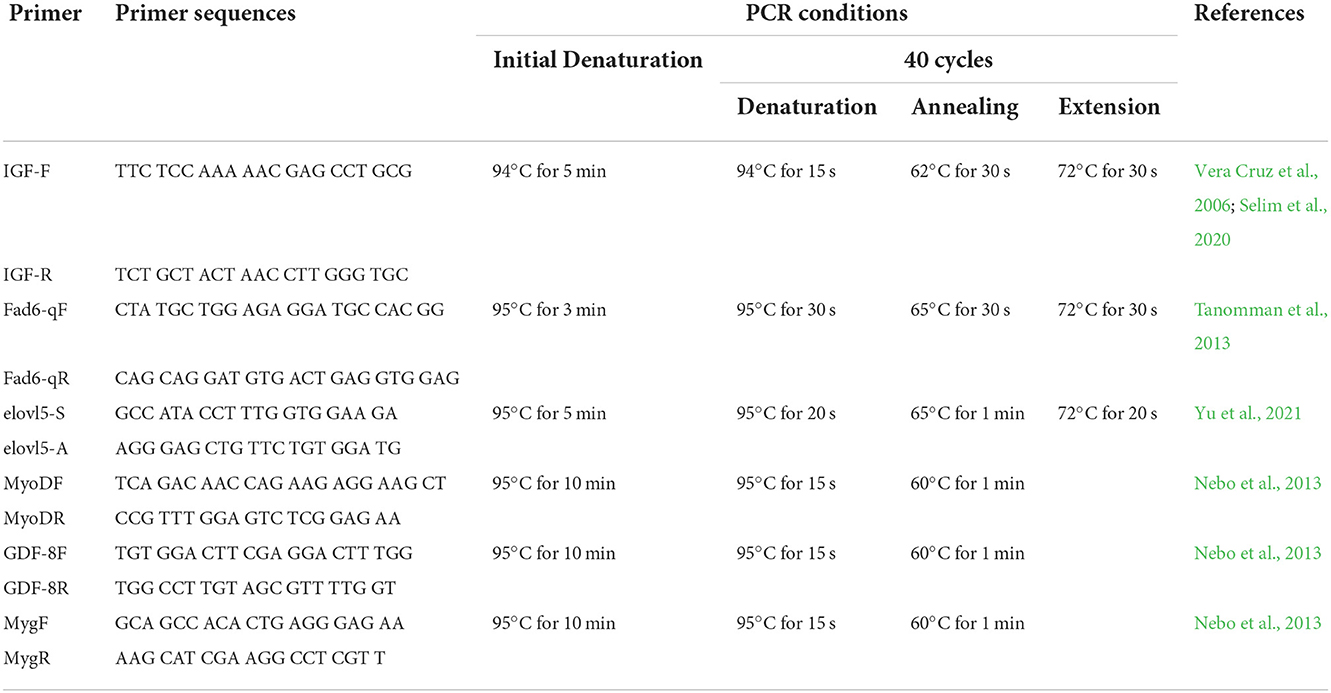
Table 3. Primers and conditions used in real-time reverse transcription polymerase chain reaction for muscle and liver genes.
Hepatosomatic index and histology of liver and intestine
Three fish from each experimental treatment replicate tank were dissected for histology of the liver and intestine. The dissected tissues were placed on a glass plate and stained with hematoxylin and eosin after being fixed in Bouin's solution, dehydrated with isopropanol, cleaned in xylene infiltrated with paraffin, and sectioned to a thickness of 5 m. Histological tissues were examined using a compound microscope (Motic®, Hong Kong) with images recorded using Motic Images Plus 2.0 (Motic®, Hong Kong). Measurements of the histological images were done using Image J 1.52i (National Institute of Health, USA). The hepatosomatic index was also calculated by measuring the weight of the liver over the total body weight of the fish.
Statistical analysis
All values were expressed as mean ± standard error of the mean (SEM). The results were analyzed by one-way ANOVA with a significance level of P ≤ 0.05 followed by Tukey's test to determine the difference between treatments. All statistical analyses were carried out with IBM SPSS version 26.0.
Results
Growth performance and feed efficiency
The growth performance and feed efficiency of seawater tilapia fed with a diet supplemented with T. weissflogii paste are shown in Table 4. The addition of T. weissflogii paste to the diet had no significant effect on the survival of seawater tilapia for the 60-day culture duration. However, seawater-tilapia fed with D1 had a significantly high percent weight gain among treatments (Figure 1). Specific growth rate (SGR) and protein efficiency ratio (PER) was also highest in tilapia fed with D1 but not significantly higher than in the control. Moreover, the lowest FCR was observed in D1 and the highest in D3. There was no significant difference among treatments in terms of feed intake but was observed to be decreasing with the increasing dose of T. weissflogii paste.
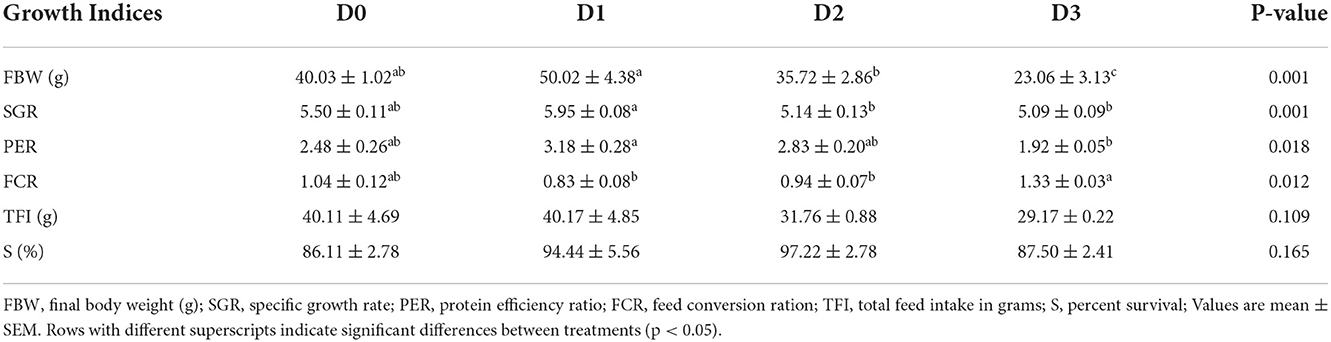
Table 4. Growth performance indices, survival, feed efficiency, and nutrient retention of seawater-tolerant tilapia fed with diets supplemented with T. weissflogii paste.
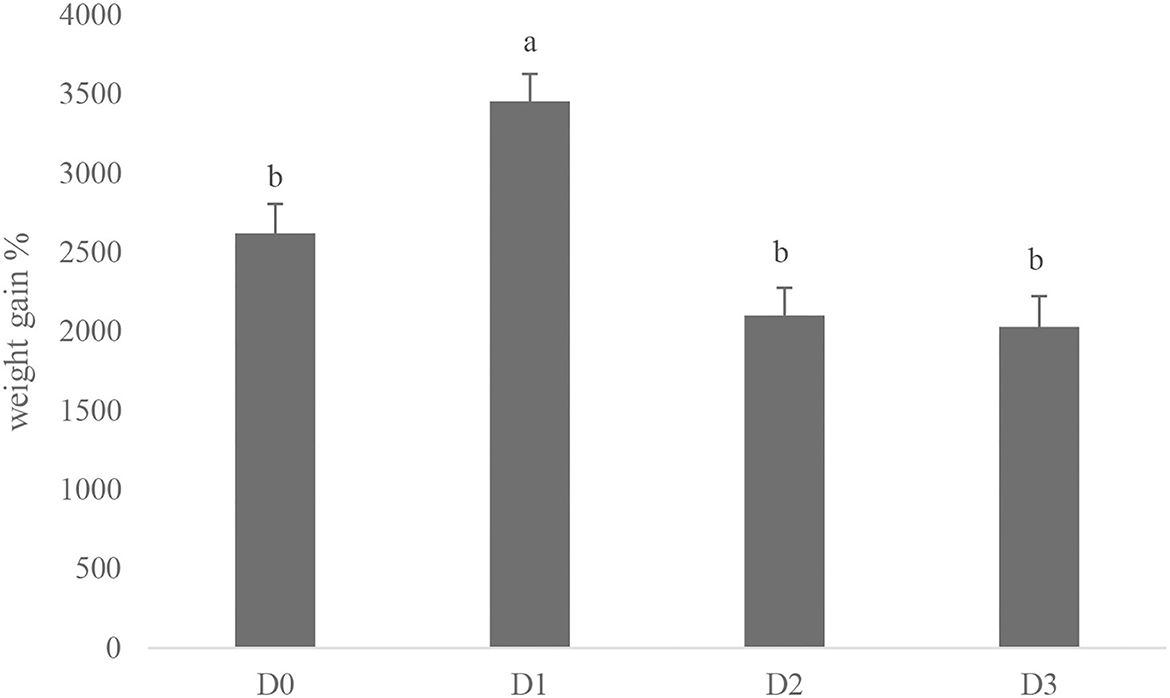
Figure 1. Percent weight gain of seawater-tolerant tilapia fed with diets supplemented with T. weissflogii paste. Bars with different superscripts indicate significant differences between treatments (P < 0.05).
Proximate composition and nutrient retention
The proximate composition of seawater tilapia fed with different levels of T. weissflogii showed significant effect (P < 0.05) on crude fat with the highest observed in D1 (Table 5). However, crude protein was significantly decreased when fed with the diatom-supplemented diet. There was no significant difference among treatments in terms of crude fiber and nitrogen-free extract.
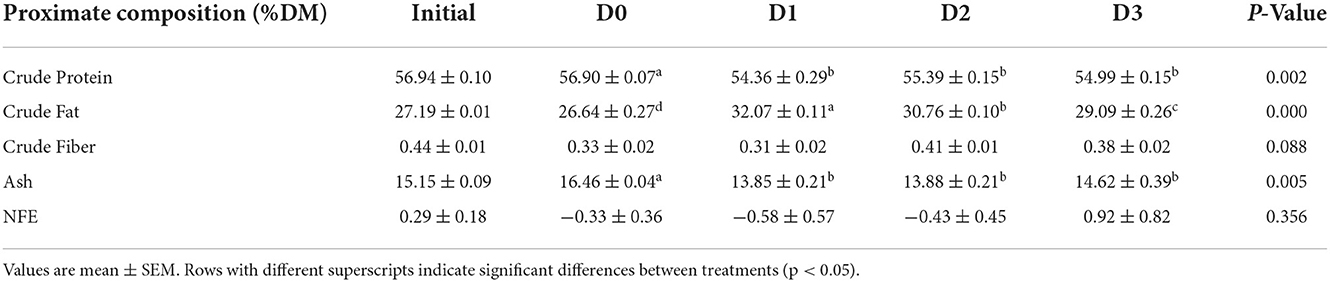
Table 5. Proximate composition (%) of seawater-tolerant tilapia fed with diets supplemented with T. weissflogii paste.
The nutrient retention of the seawater tilapia fed with different doses of T. weissflogii was found to be significantly different (Figure 2). The highest protein retention was observed in D1 followed by D2 but not significantly higher than the D0. Lipid retention was also found highest in D1. The lowest protein and lipid retention was observed in D3.
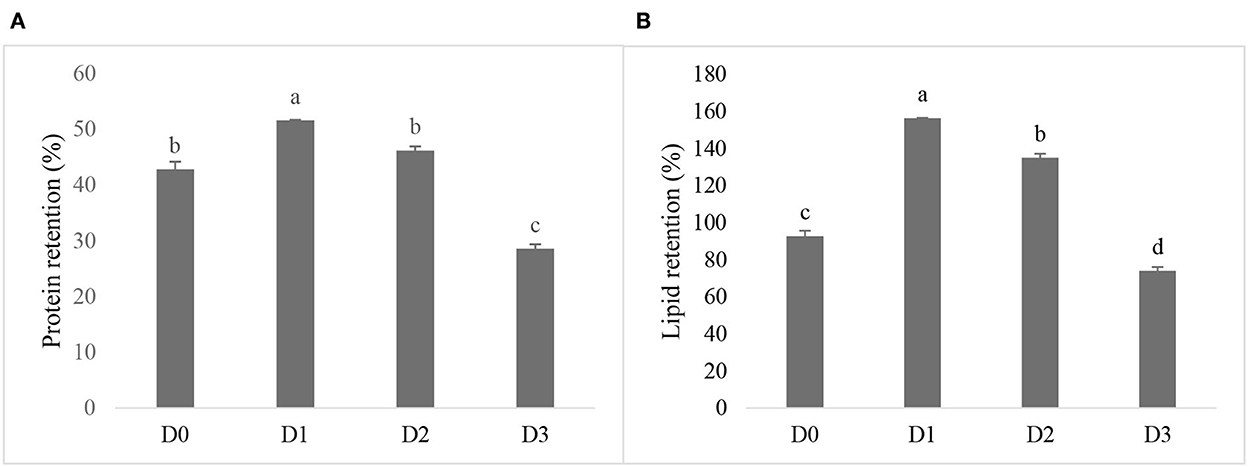
Figure 2. Protein retention (A) and lipid retention (B) of seawater-tolerant tilapia fed with diet supplemented with different levels of T. weissflogii paste. Bars with different superscripts indicate significant differences between treatments (P < 0.05).
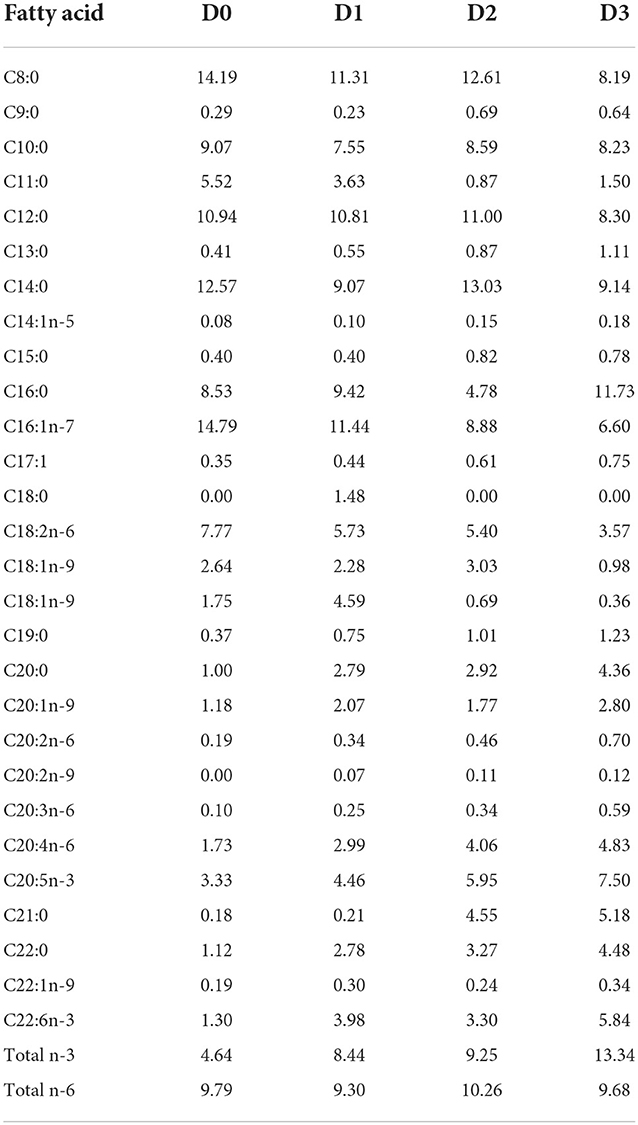
Fatty acid composition
The fatty acid composition of seawater tilapia fed with different experimental diets is shown in Table 6. Significantly high omega-3 was observed in D1 while the lowest was observed in D3. The highest omega-6 was also detected in D1, however, with regards to the omega-3 to omega-6 ratio, D1 had the significantly highest omega-3 to omega-6 ratio. A decreasing trend of omega-3 to omega-6 ratio was observed from the lowest dose of T. weissflogii paste at 2.55% to the highest dose at 12% T. weissflogii paste. Moreover, omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) such as EPA and DHA were significantly higher in diatom supplemented diet compared to the control with the highest detected in D1.
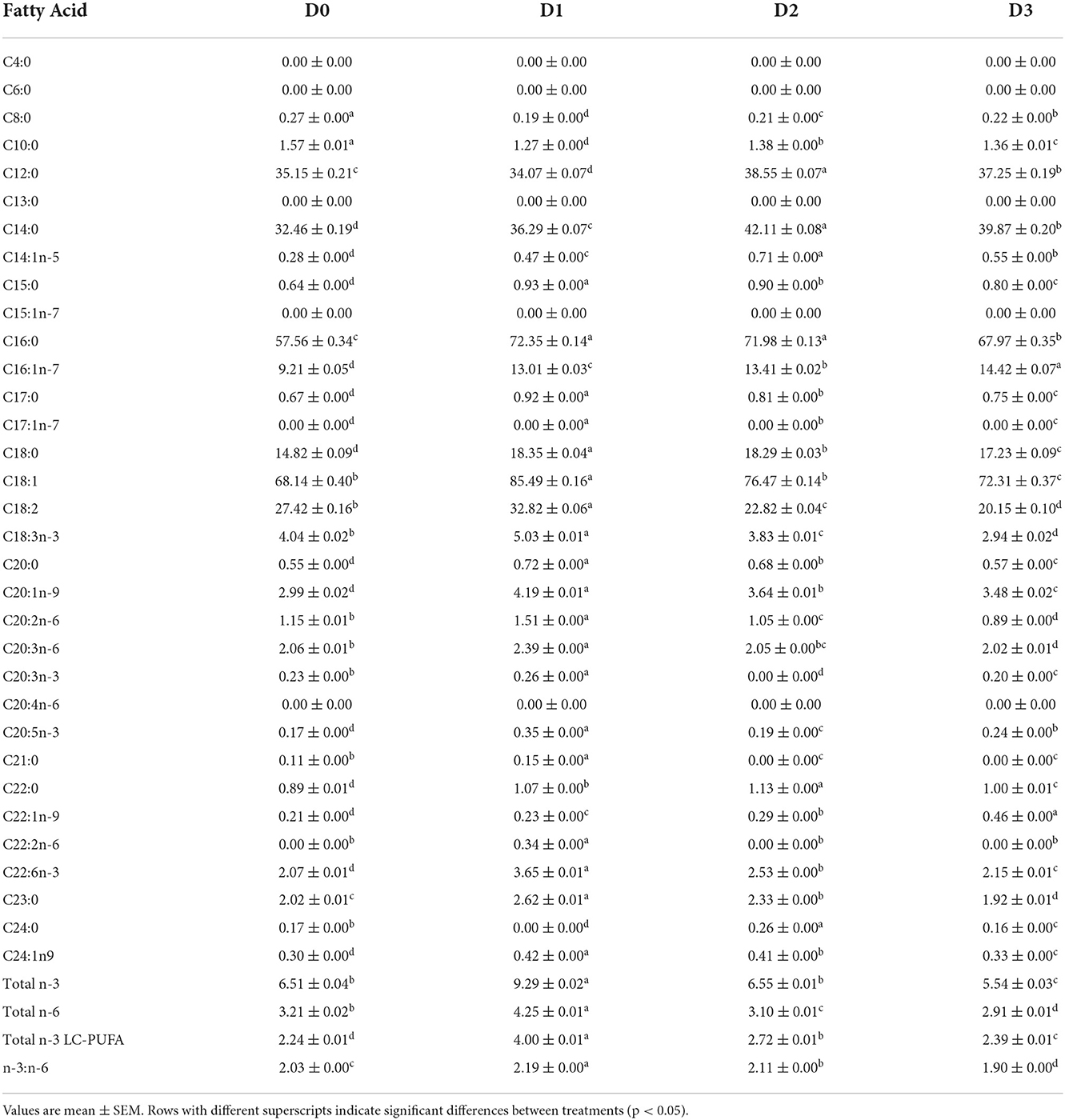
Table 6. Fatty acid composition (mg/g tissue) of seawater-tolerant tilapia fed with diets supplemented with T. weissflogii paste.
Muscle and liver gene expression levels
Gene expression levels in muscle and liver of the seawater tilapia fed with a 2.55% dose of T. weissflogii are shown in Figure 3. Muscle growth-related genes such as MyoD, myogenin (MYG), and GDF-8 were upregulated in D1. Conversely, the genes expressed in the liver of Nile tilapia such as oni-fads2, elovl5, and IGF were downregulated.
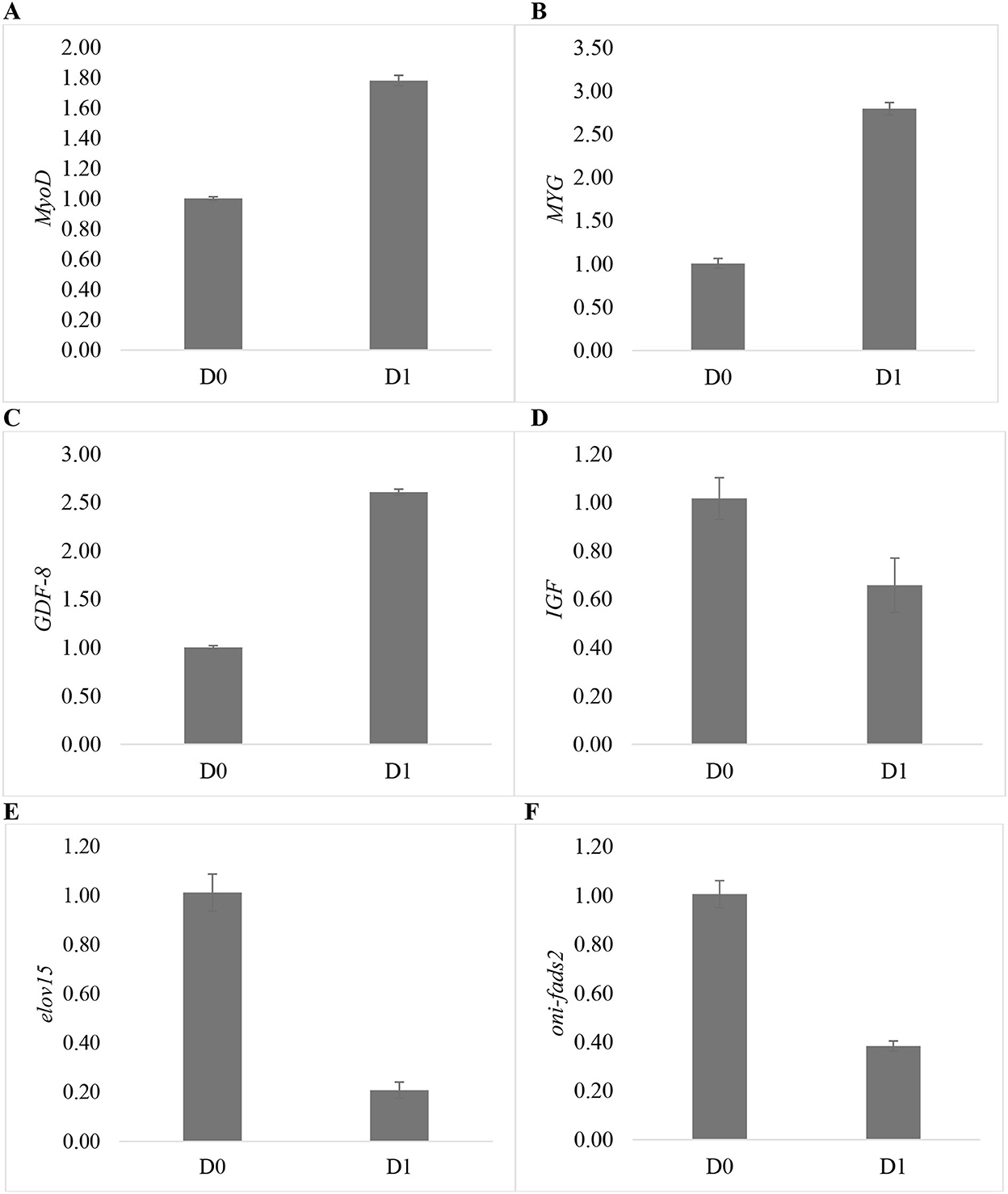
Figure 3. Relative mRNA levels of different genes expressed in the muscle and liver of seawater-tolerant tilapia fed with 2.55% T. weissflogii paste. (A) MyoD, (B) MyG, (C) GDF-8, (D) IGF, (E) elovl5, and (F) oni-fads2.
Histology of liver and intestine
There were no morphological abnormalities detected in the liver of seawater tilapia fed with different dosages of T. weissflogii (Figure 4). The hepatosomatic index of seawater tilapia showed statistically similar results among treatments (Figure 5). No significant difference (P>0.05) was also observed in the intestinal villi length and enterocyte height of seawater tilapia in all treatments (Figures 6, 7).

Figure 4. Liver morphology of seawater-tolerant tilapia fed with different levels of T. weissflogii paste; (A) D0, (B) D1, (C) D2, (D) D3. Scale bar = 100μm.
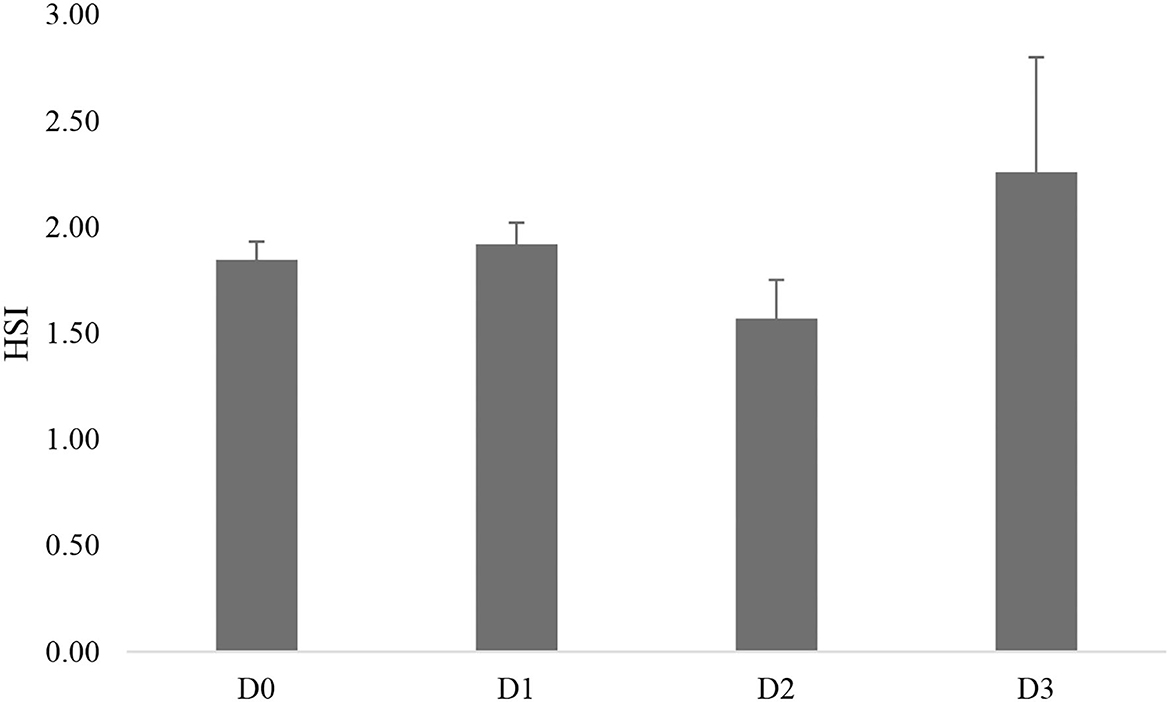
Figure 5. Hepatosomatic index (HSI) of seawater-tolerant tilapia fed with different levels of T. weissflogii paste.

Figure 6. Intestinal morphology of seawater-tolerant tilapia fed with different levels of T. weissflogii paste. (A) D0, (B) D1, (C) D2, (D) D3. Scale bar = 100μm.
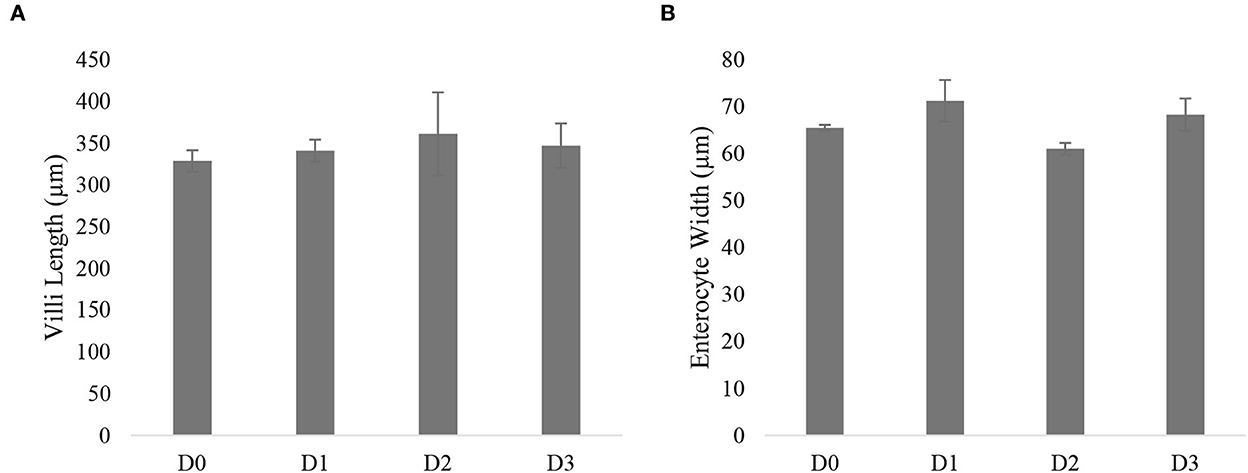
Figure 7. Villi length (A) and enterocyte height (B) of seawater-tolerant tilapia Nile tilapia fed with different supplementation levels of T. weissflogii paste.
Discussion
The use of marine diatoms as a feed supplement to freshwater strain of Nile tilapia was previously evaluated using the diatom Amphora coffeaeformis (Ayoub et al., 2019, 2022; Saleh et al., 2020). However, the use of T. weissflogii, as a dietary supplement to seawater tilapia strain in the present study, to our knowledge is the first time to be reported. Our results indicate that the growth performance of tilapia was improved (P < 0.05) in treatment with 2.55% T. weissflogii supplement. However, a higher dose of 6% to 12% of T. weissflogii did not affect the growth performance of seawater tilapia. This indicates an optimum dose at 2.55% T. weissflogii supplementation. Similar to our findings previous work on the supplementation of the diatom A. coffeaformis in the Nile tilapia diet also showed improved growth performance and feed utilization at the supplementation level of 3% (Ayoub et al., 2019, 2022). Also, no significant improvement was observed when the fish were fed higher supplementation dose at 2.5% to 10% of A. coffeaformis (Saleh et al., 2020). Spirulina platensis supplementation were also documented to improve the growth related parameters when added at low dosage of 0.5 and 1% in the diet of juvenile Nile tilapia (Abd El-Daim et al., 2021). Similar to our findings, it was also reported that supplementation at 5% to 10% of Ulva rigida to the diet of O. niloticus elicited no significant effects on the growth performance and growth inhibition was evident when the supplementation level was increased to 15% (Güroy et al., 2007). The significant growth improvement in tilapia in the present study may be due to different nutrients contents and availability of these nutrients including vitamins and minerals in the different species of algae used as dietary supplements (Belay et al., 1996). T. weissflogii contains high levels of vitamins and minerals which could attribute to the enhanced growth performance of tilapia (Kiatmetha et al., 2011; Tam et al., 2021).
The observed improvement in fish growth in the diatom-supplemented diet is linked to the enhancement in nutrient retention. Protein retention of tilapia fed with the diet supplemented with 2.55% T. weissflogii was significantly higher (P < 0.05) than in the control diet. Moreover, lipid retention was also significantly higher (P < 0.05) in the 2.55% supplementation level than in the control. A similar observation was reported by Nandeesha et al. (1998) and Gbadamosi and Lupatsch (2018) suggesting that adding Nannochloropsis salina and S. platensis to the fish diet improved the weight gain as well as the protein retention of Nile tilapia and common carp, respectively. When seaweed Porphyra spheroplasts were used as a feed additive to the diet of red sea bream, an increase in weight gain and protein and lipid retention was also observed (Kalla et al., 2008). These earlier findings suggested that the growth promoting activity of algae as a dietary supplement could be attribute to the better utilization of dietary energy that spare protein for growth and promoted tissue protein accumulation. It is tempting to speculate that these mechanisms may be at work in the present study. However, this aspect requires detailed investigation in future studies.
The present study also found that supplementation of T. weissflogii in the diet influenced the fatty acid profile of seawater tilapia. The fish's fatty acid composition is influenced by the types and sources of fatty acids it consumes (Webster and Lim, 2006). The diatom additive, T. weissflogii contains substantial levels of n-3 fatty acids, particularly EPA and DHA (Kiatmetha et al., 2011). Evidently, the supplementation of T. weissflogii in the diet significantly improved the fatty acid profile with higher levels of n-3 as well as higher n3:n6 ratio in seawater tilapia. Similar results have been previously reported in studies of Nile tilapia when microalgae Schizochytrium sp. (Watters et al., 2013; Sarker et al., 2016; Stoneham et al., 2018) and N. salin (Gbadamosi and Lupatsch, 2018) were used as a feed supplement. Moreover, the addition of T. weissflogii to the diet improved the n-3 LC-PUFA of seawater tilapia. The health benefits of n-3 LC-PUFA to humans include neurological development, improved cognition as well as cardiovascular disease benefits (Meyer and De Groot, 2017). The improvement of n-3 LC-PUFA content could be a way in improving the market acceptability of Nile tilapia.
The growth-related genes in the muscle of saline tolerant tilapia were upregulated. This verifies the improvement in the growth performance of tilapia when 2.55% supplementation was added to the diet. During the development of fish muscle, MyoG regulates cell differentiation while MyoD controls satellite cell activation and proliferation (Watabe, 2000). Similar to the present study, upregulation of MyoD and MyG correlates with growth improvement in Nile tilapia when algae was supplemented in the diet (Asaduzzaman et al., 2017; Prabu et al., 2021). GDF-8 or myostatin expression regulates muscle growth as well. However, it functions as a negative regulator that prevents the proliferation of satellite cells during the development and expansion of muscles (Nebo et al., 2013). In contrast with the present study, in the study of Asaduzzaman et al. (2017), GDF-8 expression in Nile tilapia was downregulated using inosine monophosphate as a dietary supplement, thus indicating higher satellite cell proliferation activity. Nevertheless, myostatin regulation in muscle growth is dependent on fish species, nutritional conditions, growth phase, and muscle type (Patruno et al., 2008).
On the other hand, genes expressed in the liver including oni-fads2 and elovl5 were significantly down-regulated. Fads2 and elovl5 genes play important roles in the long chain poly unsaturated fatty acid synthesis (LC-PUFA) synthesis pathway (Ma et al., 2018). These encoding products contain the most important lipogenic enzymes, and modifications to their activity can alter how quickly fatty acids are generated (Yu et al., 2022). Similar to the present result, high n-3 LC-PUFA content in the diet of fish led to the downregulation of fads2 in euryhaline fish Lateolabrax japonicus. Further the modulation and down regulation of these genes are associated with the presence of n-3 LC-PUFA (Xu et al., 2014). It is known that tilapia are able to synthesize LC-PUFA if the biological supply is limiting. In the present study it could be noted that T. weissflogii supplement has high content of n-3 LC-PUFA and the presence of these fatty acid may have triggered low the down regulation of the Fads2 and elovl5 genes that are involved in the synthesis of n-3 LC-PUFA. Moreover, though the fatty acid synthesis related genes were inhibited but the high n-3 LC-PUFA tissue content of fish in treatment with T. weissflogii supplement could be explained in terms of dietary nutrient bioaccumulation.
No morphological abnormalities were observed in the intestine of seawater tilapia fed with T. weissflogii supplemented diet with no significant effect on the villi length and enterocyte height. The structure of villi and enterocyte is related to the number of intestinal epithelial cells which acts as the first line of defense against potentially harmful agents, while also ensuring adequate nutrient utilization, immune defense, and growth of fish (Silva et al., 2015; Ma et al., 2018). The normal intestinal morphological structure of seawater tilapia implies an efficient nutrient absorption that led to improved fish growth. Additionally, normal morphological attributes were observed in the liver of seawater tilapia fed with a diet containing T. weissflogii. The supplementation of 15% of microalgae Chlorella to the diet of Nile tilapia had no pathological changes to the liver (El-Habashi et al., 2019). Abdelrhman et al. (2022) also reported normal hepatic morphology when Sargassum dentifolium was added to the diet of hybrid red tilapia at supplementation levels up to 30 g/kg−1.
With the dwindling supply and unsustainable use of fish meal, utilization of marine diatoms was seen to be a practical and sustainable way to improve the omega-3 composition of tilapia. In the present study, the use of T. weissflogii with supplementation dose of 2.55% in the diet of saline tolerant Nile tilapia not only improved its growth performance but also increased its n-3 LC-PUFA content with no negative effect on its hepatic and intestinal morphology. Further investigation on the period and frequency of feeding the diatom supplemented diet is recommended. The findings of this research might pave the way for increased market acceptability of tilapia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the animal study because this study adheres to the Philippine National Standard (PNS) on the Code of Good Aquaculture Practices (GAqP) for Milkfish and Tilapia (BAFS, 2017). Protocols on rearing, handling and animal welfare, were strictly followed based on the guidelines stipulated in Philippine Republic Act Number 8485, known as the Animal Welfare Act of 1998.
Author contributions
FH and RT conceived and designed the experiment, wrote, reviewed, and edited the manuscript. CC reviewed and edited the manuscript. CD performed lab analyses, gathered data, interpreted results, and wrote the manuscript. ET performed lab analyses and monitored feeding experiments. All authors read and approved the final manuscript.
Funding
This study was funded by the Department of Science and Technology- Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD).
Acknowledgments
The authors would like to acknowledge the Department of Science and Technology- Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) for funding this study. Institute of Aquaculture, College of Fisheries and Ocean Sciences, University of the Philippines Visayas are acknowledged for the technical help, use of facilities, and all the support that made this project possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Daim, A., El Asely, A., Kandiel, M., Abd El-Gawad, E., Elabd, H., Shaheen, A., et al. (2021). Research article: effect of Spirulina platensis and Azolla nilotica as feed additives on growth performance, antioxidant enzymes and fecundity of Oreochromis niloticus. Iran. J. Fish. Sci. 20, 846–862. doi: 10.22092/ijfs.2021.124136
Abdelrhman, A. M., Ashour, M., Al-Zahaby, M. A., Sharawy, Z. Z., Nazmi, H., Zaki, M. A. A., et al. (2022). Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquacult. Rep. 25, 101212. doi: 10.1016/j.aqrep.2022.101212
Aksnes, A., and Opstvedt, J. (1998). Content of digestible energy in fish feed ingredients determined by the ingredient-substitution method. Aquaculture 161, 45–53. doi: 10.1016/S0044-8486(97)00255-X
AOAC (2000). Official Method 996, 06. Fat (Total, Saturated, and Unsaturated) in Foods: Hydrolytic Extraction Gas Chromatographic Method. AOAC Official Methods of Analysis, Rockville, MD, USA.
Arendt, K. E., Jónasdóttir, S. H., Hansen, P. J., and Gärtner, S. (2005). Effects of dietary fatty acids on the reproductive success of the calanoid copepod Temora longicornis. Marine Biol. 146, 513–530. doi: 10.1007/s00227-004-1457-9
Asaduzzaman, M. D., Ikeda, D., Abol-Munafi, A. B., Bulbul, M., Ali, M. E., Kinoshita, S., et al. (2017). Dietary supplementation of inosine monophosphate promotes cellular growth of muscle and upregulates growth-related gene expression in Nile tilapia Oreochromis niloticus. Aquaculture 468, 297–306. doi: 10.1016/j.aquaculture.2016.10.033
Ayoub, F. H. F, Abdelghany, M. B., and El-Sayed, A. E. -K. (2019). Effects of Diatoms Amphora coffeaeformis on growth parameters, non specific immunity and protection of the Nile tilapia (Oreochromis niloticus) to Aeromonas hydrophila infection. Egypt. J. Aqu. Biol. Fish. 23, 413–426. doi: 10.21608/ejabf.2019.37466
Ayoub, H. F., Abdelghany, M. F., Alsaiad, S. M., and El Asely, A. M. (2022). Amphora coffeaeformis diatom involved in reducing the susceptibility of Nile tilapia (Oreochromis niloticus) fingerlings to Aeromonas hydrophila infection by boosting immune and antioxidant responses and improving growth performance indicators. Aquacult. Res. 53, 3626–3636. doi: 10.1111/are.15866
Becker, W. (2004). Microalgae for Aquaculture. Handbook of microalgal culture: biotechnology and applied phycology. New York, NY: John Wiley and Sons.
Belay, A., Kato, T., and Ota, Y. (1996). Spirulina (Arthrospira): potential application as an animal feed supplement. J Appl Phycol 8, 303–311. doi: 10.1007/BF02178573
Brown, M. R. (2002). Nutritional value and use of microalgae in aquaculture. in Avances en Nutricion Acuicola VI. (Cancun, Quintana Roo, Mexico), 281–292. Available online at: http://hdl.handle.net/102.100.100/199007?index=1
El-Dakar, A. Y., Shalaby, S. M. A., and Elmonem, A. I. A. (2015). Growth performance and feed utilization of hybrid red tilapia, Oreochromis niloticus (Linnaeus) x Oreochromis mossambicus (Peters) fed different dietary protein and energy levels under rearing in seawater conditions. Mediterr. Aquacult. J. 7, 12–21. doi: 10.21608/maj.2015.4629
El-Habashi, N., Fadl, S. E., Farag, H. F., Gad, D. M., Elsadany, A. Y., El Gohary, M. S., et al. (2019). Effect of using Spirulina and Chlorella as feed additives for elevating immunity status of Nile tilapia experimentally infected with Aeromonas hydrophila. Aquacult. Res. 50, 2769–2781. doi: 10.1111/are.14229
Gbadamosi, O. K., and Lupatsch, I. (2018). Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia, Oreochromis niloticus. Algal Res. 33, 48–54. doi: 10.1016/j.algal.2018.04.030
Guerrero, R. D. (2019). Farmed tilapia production in the Philippines is declining: What has happened and what can be done. Philippine J. Sci. 148, 11–15. Available online at: https://philjournalsci.dost.gov.ph/87-current-issue/vol-148-no-2-june-2019/1062-farmed-tilapia-production-in-the-philippines-is-declining-what-has-happened-and-what-can-be-done-2
Güroy, B. K., Cirik, S., Güroy, D., Sanver, F., and Tekinay, A. A. (2007). Effects of Ulva rigida and Cystoseira barbata meals as a feed additive on growth performance, feed utilization, and body composition of Nile Tilapia, Oreochromis niloticus. Turkish J. Vet. Anim. Sci. 31, 91–97. Available online at: https://journals.tubitak.gov.tr/veterinary/vol31/iss2/2
Hardy, R. W., and Barrows, F. T. (2003). “9 - Diet formulation and manufacture,” in Fish Nutrition, eds. J. E. Halver and R. W. Hardy (San Diego, CA: Academic Press), 505–600.
Holub, D. J., and Holub, B. J. (2004). Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell Biochem. 263, 217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d
Intoy, M. M., and Traifalgar, R. (2021). Feed value of fermented copra meal as a sustaianble feed ingredient in the diet of saline-tolerant nile tilapia Oreochromis niloticus (Linnaeus,1758). J. Sust. Sci. Manage. 16, 28–43. doi: 10.46754/jssm.2021.12.003
Jaspe, C. J., and Caipang, C. M. (2011). Small-scale hatchery and larval rearing techniques for local strains of saline-tolerant tilapia, Oreochromis spp. Anim. Biol. Anim. Husbandry 3, 71–77. Available online at: http://www.abah.bioflux.com.ro/docs/2011.3.71-77.pdf
Ju, Z. Y., Forster, I. P., and Dominy, W. G. (2009). Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture 292, 237–243. doi: 10.1016/j.aquaculture.2009.04.040
Kalla, A., Yoshimatsu, T., Araki, T., Zhang, D. M., Yamamoto, T., Sakamoto, S., et al. (2008). Use of Porphyra spheroplasts as feed additive for red sea bream. Fish Sci. 74, 104–108. doi: 10.1111/j.1444-2906.2007.01501.x
Kiatmetha, P., Siangdang, W., Bunnag, B., Senapin, S., and Withyachumnarnkul, B. (2011). Enhancement of survival and metamorphosis rates of Penaeus monodon larvae by feeding with the diatom Thalassiosira weissflogii. Aquacult. Int. 19, 599–609. doi: 10.1007/s10499-010-9375-y
Kris-Etherton, P. M., Harris, W. S., and Appel, L. J. (2002). Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106, 2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, H., Jin, M., Zhu, T., Li, C., Lu, Y., Yuan, Y., et al. (2018). Effect of dietary arachidonic acid levels on growth performance, fatty acid profiles and lipid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture 486, 31–41. doi: 10.1016/j.aquaculture.2017.11.055
Meyer, B. J., and De Groot, R. H. M. (2017). Effects of omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: the importance of the dose of DHA. Nutrients 9, 1305. doi: 10.3390/nu9121305
Nandeesha, M. C., Gangadhar, B., Varghese, T. J., and Keshavanath, P. (1998). Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio L. Aquacult. Res. 29, 305–312. doi: 10.1111/j.1365-2109.1998.tb01135.x
Nebo, C., Portella, M. C., Carani, F. R., Almeida, d. e., Padovani, F. L. A., Carvalho, C. R., et al. (2013). Short periods of fasting followed by refeeding change the expression of muscle growth-related genes in juvenile Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Biochem. Mol. Biol. 164, 268–274. doi: 10.1016/j.cbpb.2013.02.003
Patruno, M., Sivieri, S., Poltronieri, C., Sacchetto, R., Maccatrozzo, L., Martinello, T., et al. (2008). Real-time polymerase chain reaction, in situ hybridization and immunohistochemical localization of insulin-like growth factor-I and myostatin during development of Dicentrarchus labrax (Pisces: Osteichthyes). Cell Tissue Res. 331, 643–658. doi: 10.1007/s00441-007-0517-0
Pfaffl, M. W. (2007). “Relative quantification,” in Real-time PCR, eds M. T. Dorak (New York, NY: Taylor and Francis), 89–108.
Prabu, E., Felix, N., and Uma, A. (2021). Dietary arginine requirement in diets of GIFT strain of Nile tilapia, Oreochromis niloticus: effects on growth performance, whole-body composition, growth-related gene expression and haemato-biochemical responses. Aquaculture Res. 52, 4816–4828. doi: 10.1111/are.15315
PSA (2019). Fisheries Statistics of the Philippines 2016-2018. Quezon: Philippines Statistics Authority.
Romana-Eguia, M. R. R., Laron, M. A., and Catacutan, M. R. (2013). On-farm feed management practices for Nile tilapia (Oreochromis niloticus) in the Philippines in On-farm feeding and feed management in aquaculture (Food and Agriculture Organization), 131–158. Available online at: https://repository.seafdec.org.ph/handle/10862/2162 (accessed July 25, 2022).
Rosario, W. R., Georget, C., Chevassus-Au-Louis, B., Morissens, P., Muyalde, N. C., de la Cruz, A. E., et al. (2004). “Selection from an interspecific hybrid population of two strains of fast growing and salinity tolerant tilapia,” in Remedios B. Bolivar, Graham C. Mair, and Kevin Fitzsimmons. Tilapia 6th International Symposium on Tilapia in Aquaculture Philippine International Convention Center Roxas Boulevard, Manila, Philippines, September 12–16.
Saleh, N. E., Ismail, R. F., Sayed, A. E.-. D. H, Zaghloul, E. H., and Saleh, H. (2020). Comprehensive assessment of benthic diatom (Amphora coffeaeformis) as a feed additive in Nile tilapia (Oreochromis niloticus) diet. Aquaculture Res. 51, 3506–3519. doi: 10.1111/are.14686
Sandeep, K. P., Avunje, S., Dayal, J. S., Balasubramanian, C. P., Sawant, P. B., Chadha, N. K., et al. (2021). Efficiency of different microalgae as monospecific and bispecific diets in larval rearing of Penaeus indicus with special reference to growth, nutrient composition and antimicrobial activity of microalgae. Aquaculture Res. 52, 5146–5154. doi: 10.1111/are.15382
Sarker, P. K., Gamble, M. M., Kelson, S., and Kapuscinski, A. R. (2016). Nile tilapia (Oreochromis niloticus) show high digestibility of lipid and fatty acids from marine Schizochytrium sp. and of protein and essential amino acids from freshwater Spirulina sp. feed ingredients. Aquaculture Nutr. 22, 109–119. doi: 10.1111/anu.12230
Selim, K. M., Reda, R. M., Mahmoud, R., and El-Araby, I. E. (2020). Effects of nucleotides supplemented diets on growth performance and expressions of ghrelin and insulin-like growth factor genes in Nile tilapia, Oreochromis niloticus. J. Appl. Aquacult. 32, 157–174. doi: 10.1080/10454438.2019.1696911
Silva, D. M., Valente, L. M. P., Sousa-Pinto, I., Pereira, R., Pires, M. A., Seixas, F., et al. (2015). Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J Appl Phycol. 27, 1671–1680. doi: 10.1007/s10811-014-0453-9
Stoneham, T. R., Kuhn, D. D., Taylor, D. P., Neilson, A. P., Smith, S. A., Gatlin, D. M., et al. (2018). Production of omega-3 enriched tilapia through the dietary use of algae meal or fish oil: Improved nutrient value of fillet and offal. PLoS ONE 13, e0194241. doi: 10.1371/journal.pone.0194241
Tam, L. T., Van Cong, N., Thom, L. T., Ha, N. C., Hang, N. T. M., Van Minh, C., et al. (2021). Cultivation and biomass production of the diatom Thalassiosira weissflogii as a live feed for white-leg shrimp in hatcheries and commercial farms in Vietnam. J. Appl. Phycol. 33, 1559–1577. doi: 10.1007/s10811-021-02371-w
Tanomman, S., Ketudat-Cairns, M., Jangprai, A., and Boonanuntanasarn, S. (2013). Characterization of fatty acid delta-6 desaturase gene in Nile tilapia and heterogenous expression in Saccharomyces cerevisiae. Comp. Biochem. Physiol. 166, 148–156. doi: 10.1016/j.cbpb.2013.07.011
Vera Cruz, E. M., Brown, C. L., Luckenbach, J. A., Picha, M. E., Bolivar, R. B., Borski, R. J., et al. (2006). Insulin-like growth factor-I cDNA cloning, gene expression and potential use as a growth rate indicator in Nile tilapia, Oreochromis niloticus. Aquaculture 251, 585–595. doi: 10.1016/j.aquaculture.2005.06.039
Verdal, D. E., Rosario, H., Vandeputte, W., Muyalde, M., Morissens, N., Baroiller, P., et al. (2014). Response to selection for growth in an interspecific hybrid between Oreochromis mossambicus and O. niloticus in two distinct environments. Aquaculture 430, 159–165. doi: 10.1016/j.aquaculture.2014.03.051
Volkman, J. K., Jeffrey, S. W., Nichols, P. D., Rogers, G. I., and Garland, C. D. (1989). Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 128, 219–240. doi: 10.1016/0022-0981(89)90029-4
Watabe, S. (2000). Myogenic regulatory factors. Fish Physiol. 18, 19–41. doi: 10.1016/S1546-5098(01)18003-9
Watanabe, W. O., Fitzsimmons, K., and Yi, Y. (2006). Farming tilapia in saline waters. Biol. Cult. Nutr. 347–447. Available online at: https://www.crcpress.com/Tilapia-Biology-Culture-and-Nutrition/Webster-Lim/p/book/9781560228882
Watters, C. A., Rosner, L. S., Franke, A. A., Dominy, W. G., Klinger-Bowen, R., Tamaru, C. S., et al. (2013). Nutritional enhancement of long-chain omega-3 fatty acids in tilapia (Oreochromis honorum). Isr. J. Aquacult. 65, 1–7. Available online at: https://www.researchgate.net/profile/Clyde-Tamaru/publication/287341834_Nutritional_Enhancement_of_Long-Chain_Omega-_Fatty_Acids_in_Tilapia_Oreochromis_honorum/links/56e74ef308ae85e780cff796/Nutritional-Enhancement-of-Long-Chain-Omega-3-Fatty-Acids-in-Tilapia-Oreochromis-honorum.pdf
Webster, C. D., and Lim, C. (2006). Tilapia: Biology, Culture, and Nutrition. Boca Raton, FL: CRC Press.
Xia, J. H., Lin, G., He, X., Yunping, B., Liu, P., Liu, F., et al. (2014). Mapping quantitative trait loci for omega-3 fatty acids in Asian seabass. Marine Biotechnol. 16, 1–9. doi: 10.1007/s10126-013-9524-1
Xu, H., Dong, X., Ai, Q., Mai, K., Xu, W., Zhang, Y., et al. (2014). Regulation of tissue LC-PUFA contents, Δ6 Fatty Acyl desaturase (FADS2) gene expression and the methylation of the putative FADS2 gene promoter by different dietary fatty acid profiles in Japanese Seabass (Lateolabrax japonicus). PLoS ONE 9, e87726. doi: 10.1371/journal.pone.0087726
Young, K. (2009). Omega-6 (n-6) and omega-3 (n-3) fatty acids in tilapia and human health: a review. Int. J. Food Sci. Nutr. 60, 203–211. doi: 10.1080/09637480903140503
Yu, J., Wen, X., You, C., Wang, S., Chen, C., Tocher, D. R., et al. (2021). Comparison of the growth performance and long-chain polyunsaturated fatty acids (LC-PUFA) biosynthetic ability of red tilapia (Oreochromis mossambicus♀ × O. niloticus♂) fed fish oil or vegetable oil diet at different salinities. Aquaculture 542, 736899. doi: 10.1016/j.aquaculture.2021.736899
Yu, L., Tian, J., Zhang, C., Xue, Z., Lu, X., Wen, H., et al. (2022). Acetylferulic paeonol ester: a new feed additive reduces lipid accumulation in the liver of Nile tilapia (Oreochromis niloticus) by modulating lipid and glucose metabolism. Aquaculture 561, 738671. doi: 10.1016/j.aquaculture.2022.738671
Keywords: feed additive, Thalassiosira weissflogii, seawater-strain, Nile tilapia, omega-3 fatty acid
Citation: Huervana FH, Dionela CS, de la Torre EDS, del Castillo CS and Traifalgar RFM (2022) Utilization of marine diatom Thalassiosira weissflogii as a feed additive in seawater-tolerant Nile tilapia (Oreochromis niloticus, Linnaeus 1758) strain. Front. Sustain. Food Syst. 6:1052951. doi: 10.3389/fsufs.2022.1052951
Received: 24 September 2022; Accepted: 21 November 2022;
Published: 16 December 2022.
Edited by:
Janice Alano Ragaza, Ateneo de Manila University, PhilippinesReviewed by:
Abdullah-Al Mamun, Noakhali Science and Technology University, BangladeshDomitila Kyule, Kenya Marine and Fisheries Research Institute, Kenya
Copyright © 2022 Huervana, Dionela, de la Torre, del Castillo and Traifalgar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fredson H. Huervana, ZmhodWVydmFuYUB1cC5lZHUucGg=
 Fredson H. Huervana
Fredson H. Huervana Cleresa S. Dionela
Cleresa S. Dionela Eirene Dorothy S. de la Torre
Eirene Dorothy S. de la Torre Carmelo S. del Castillo
Carmelo S. del Castillo Rex Ferdinand M. Traifalgar
Rex Ferdinand M. Traifalgar