- 1Institute of Aquaculture, College of Fisheries and Ocean Sciences, University of the Philippines Visayas, Iloilo, Philippines
- 2College of Fisheries and Marine Sciences, Zamboanga State College of Marine Sciences and Technology, Zamboanga, Philippines
The poor growth of aquatic animals fed with diets containing high plant proteins has been attributed to low diet acceptability and feed value. Supplementation of protein hydrolysate, with high contents of free amino acids and soluble low molecular weight peptides, may increase the acceptability and feed value of a plant protein-based diet. In the present work, squid processing by-products were enzymatically hydrolyzed and used as a supplement in a plant protein-based diet, without fish meal, of Penaeus monodon to fully maximize the utilization of this marine resource. The hydrolysate was incorporated at 0, 0.5, and 1% levels in P. monodon diets containing 0 and 10% fish meal levels. Growth, digestive enzyme activities, muscle growth-, gut pro-inflammatory and immune-related gene expressions, and muscle morphometric measurements were evaluated as biological indices in an 8-week feeding trial. The squid by-product hydrolysate produced in the present study contains 90.25% protein, 5.84% lipid, and 3.91% ash, and has a molecular weight of 3.76 kDa. Supplementation at 1% hydrolysate in the experimental shrimp diet without fish meal resulted in the highest growth performance associated with increased feed intake, efficient feed and nutrient conversion and retention, enhanced digestive enzyme activities, upregulation of muscle growth- and immune-related genes, and suppression of the gut pro-inflammatory gene. The growth promotion is also linked with a significant increase in muscle mean fiber area, which suggests hypertrophic growth in shrimp. Generally, the supplementation of 1% squid by-product hydrolysate supported the growth of P. monodon fed on a plant protein-based diet without fish meal.
Introduction
Fish meal is considered the ideal protein source for aquaculture feeds since it contains a balanced amount of indispensable amino acids, essential fatty acids, vitamins, and minerals and has a high palatability and attractability for cultured aquatic animals (Samocha et al., 2004). However, in recent years, the fish meal supply has become unstable and its market price has increased. The supply and price issues associated with fish meals have been linked to the rapid growth and demand of the aquaculture industry, and the declining fish stocks that are used in the production of fishmeal (FAO., 2016). This erratic supply of fish meals and the increased utilization of small pelagic fishes for human consumption (FAO., 2020) led to intensive research on alternative protein sources such as plant proteins for aquaculture feeds (Samocha et al., 2004).
Currently, there has been a significant number of published works elucidating the potential and success of plant proteins to replace fish meals in aquaculture feeds. However, the plant protein ingredients have been found inadequate to fully replace fish meal in the diet of most carnivorous aquatic animals including shrimp. High inclusion levels of plant protein sources have been observed to depress the growth rate, lower feed intake, increase the feed conversion ratio (FCR), lower digestive enzyme activities, and decrease nutrient retention efficiencies in aquatic animals (Bulbul et al., 2016; Li et al., 2018). In Penaeus monodon, abnormalities in the hepatopancreas and the midgut have been reported to be associated with high plant protein inclusion in the diets (Kumaraguru-Vasagam et al., 2007). These negative effects of feeding shrimp with plant proteins are due to the presence of anti-nutritional factors, imbalanced amino acids, and low palatability and digestibility of the diets (Kumaraguru-Vasagam et al., 2007; Bulbul et al., 2016).
In attempts to develop a practical solution to alleviate the concerns linked to the use of plant protein-based ingredients, strategies have been evaluated including the use of fish protein hydrolysates to supplement the plant protein-based diets for aquatic animals (Zhou et al., 2016; Li et al., 2018; Nunes et al., 2019). These fish protein hydrolysates are produced from low value fish and fishing by-catch and the majority are supplied from fish processing industry by-products. The maximum utilization of these industry by-products would enhance the fish resource's economic value and reduce the problem in waste production and disposal (Xu et al., 2008). It has also been projected that the production of fish meal from fish by-products would gradually increase toward 2030. At present, around 25–35% of fish by-products are used in the production of fish meal and fish oil (FAO., 2020).
Other than the fish processing industry, the squid processing industry has also the potential to contribute processing by-products that could be developed to support the requirement of the aquaculture industry. The squid fishery landings have been documented to increase starting in 2018 (FAO., 2020). With the increase in global squid production, squid processing also expanded and the generation of large volumes of squid by-products is also expected to rise. Therefore, the conversion of these processing industry by-products has been viewed as an efficient way to maximize the utilization of these resources. Squid industry by-products can also be developed into a hydrolysate that can be incorporated or used as functional bioactive additives in the diets of several aquaculture species (Novriadi et al., 2017; Costa et al., 2020).
Earlier reports had shown that fish protein hydrolysates could improve growth by enhancing protein and lipid gain, digestive enzyme activities, and muscle growth brought about by the regulation of muscle growth-related genes (Canada et al., 2018; Shao et al., 2018; Wei et al., 2020a,b). Improvement of the immune responses and downregulation of gut pro-inflammatory genes have also been reported (Li et al., 2018). However, most of these earlier works were using a hydrolysate produced from fish and there have been limited works on the development and bioconversion of squid industry by-products into a functional feed ingredient for aquaculture. The previous report on the utilization of squid by-product hydrolysate has shown to completely replace fish meal (15% of the diet) in a plant protein-based diet of P. monodon (Pan and Traifalgar, 2022). It has been also shown that fish meal replacement at 25% elicited growth promotion in P. monodon. Another report showed that dietary supplementation of squid by-product hydrolysate at 0.5% in combination with 5% dietary fish meal could promote the growth of P. monodon similar to the growth of the shrimp fed a diet with a full fish meal at 22%. The growth improvement has been attributed to the enhancement in muscle growth-related gene transcription activities. It was also shown that the growth improvement was not influenced by the activation of the digestive amylase, lipase, and protease enzymes (Pan et al., 2022). Although the influence of squid by-product hydrolysate in a low fish meal-based diet has been shown to influence muscle growth-related gene transcription, the actual evidence of muscle growth in terms of muscle morphometric growth in shrimp has not yet been fully elucidated. In addition, the influence of squid by-product hydrolysate supplementation on the gut pro-inflammatory and immune-related gene transcriptions in P. monodon fed with a low fish meal diet has not been reported to date. Therefore, the present study reports the development of squid industry by-product hydrolysate and further evaluation of the functionality of this ingredient as a supplement in the plant protein-based diet of P. monodon and its effect on muscle morphometry, gut pro-inflammatory, and immune-related genes. The present work will provide additional information on the functional properties of squid by-product hydrolysate as a dietary supplement to decrease the utilization of fish meal in a plant protein-based diet of the carnivore shrimp, P. monodon.
Materials and methods
Enzymatic hydrolysis of squid by-products to generate hydrolysate
Squid by-products including the head, tentacles, viscera, fins, and skin were obtained from a local squid processing plant in Zamboanga, Philippines and were used for the production of the hydrolysate. The quality and freshness index of the raw material was evaluated by its ammonia content (LeBlanc and Gill, 1984), urea content (Rahmatullah and Boyde, 1980), and expressible drip (Hasegawa, 1987).
where x - is the weight of the sample prior to pressing it with a 5-kg standard weight and z - is the weight of the sample after pressing it with a 5-kg standard weight.
A hydrolysis trial using 0.25 and 1% enzyme-to-substrate ratio has been conducted prior to the production of squid by-product hydrolysate to be used in the feed formulation. The trial was conducted to assess the optimum enzyme-to-substrate ratio to be used in the present study. Prior to hydrolysis, squid by-products were homogenized in a blender with distilled water at a 1:1 (w/v) ratio. The homogenates were hydrolyzed using 0.25 and 1% bromelain (enzyme-to-substrate ratio) following Haslaniza et al. (2010) and Soufi-Kechaou et al. (2012) with modifications. Hydrolysis was carried out at 60°C, pH 6.0 for 5 h with constant stirring. Then, the hydrolyzed samples were heated at 85°C for 15 min to terminate the hydrolysis. The slurry was filtered using a muslin cloth and the liquid fraction was collected. The collected filtrate was spray-dried into powdered form in a Buchi Mini Spray Dryer B-290 with an inlet temperature of 80°C, outlet temperature of 29–32°C, pump rate of 25%, and an average filter pressure of 50 to 70 mbar. The dried hydrolysate was stored at −20°C until use.
The degree of hydrolysis was determined according to Choi et al. (2014) with modifications. Yield and proximate composition were also determined. The amino acid profile of the squid by-product hydrolysate was also analyzed using ortho-phthalaldehyde in a high-performance liquid chromatography LC-10A/C-R7A amino acid analysis system (Shimadzu, Japan) according to Llames and Fontane (1994). The chemical score index of the squid by-product hydrolysate was determined according to Traifalgar et al. (2019) using the essential amino acid P. monodon tissue protein as the reference (Peñaflorida, 1989). The essential amino acid index (EAAI) of the hydrolysate was also computed (Teruel, 2002).
Where a, b,…j - percent of essential amino acids in the hydrolysate protein ar, br, jr - percent of respective essential amino acids in P. monodon tissue protein.
Molecular weight determination
Determination of the molecular weight of samples was carried out with sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to Laemmli (1970) with modifications. Samples were dissolved in 2 × sample buffer (0.5 M Tris-HCl, pH 6.8, glycerol, 10% (w/v) SDS, 0.5% (w/v) bromophenol blue with deionized water) at a different sample to buffer ratios (w/v) and were loaded into the wells of 5% stacking gel and 10% resolving gel. The electrophoresis was run at 100 V for 30 min, and gels were stained with Coomassie Brilliant Blue R-250 overnight and also destained overnight with a destaining solution (45% methanol and 10% acetic acid with distilled water). After destaining, the gels were scanned and the obtained images were analyzed using ImageJ (Schneider et al., 2012) to determine the molecular weight of the samples.
Test diets
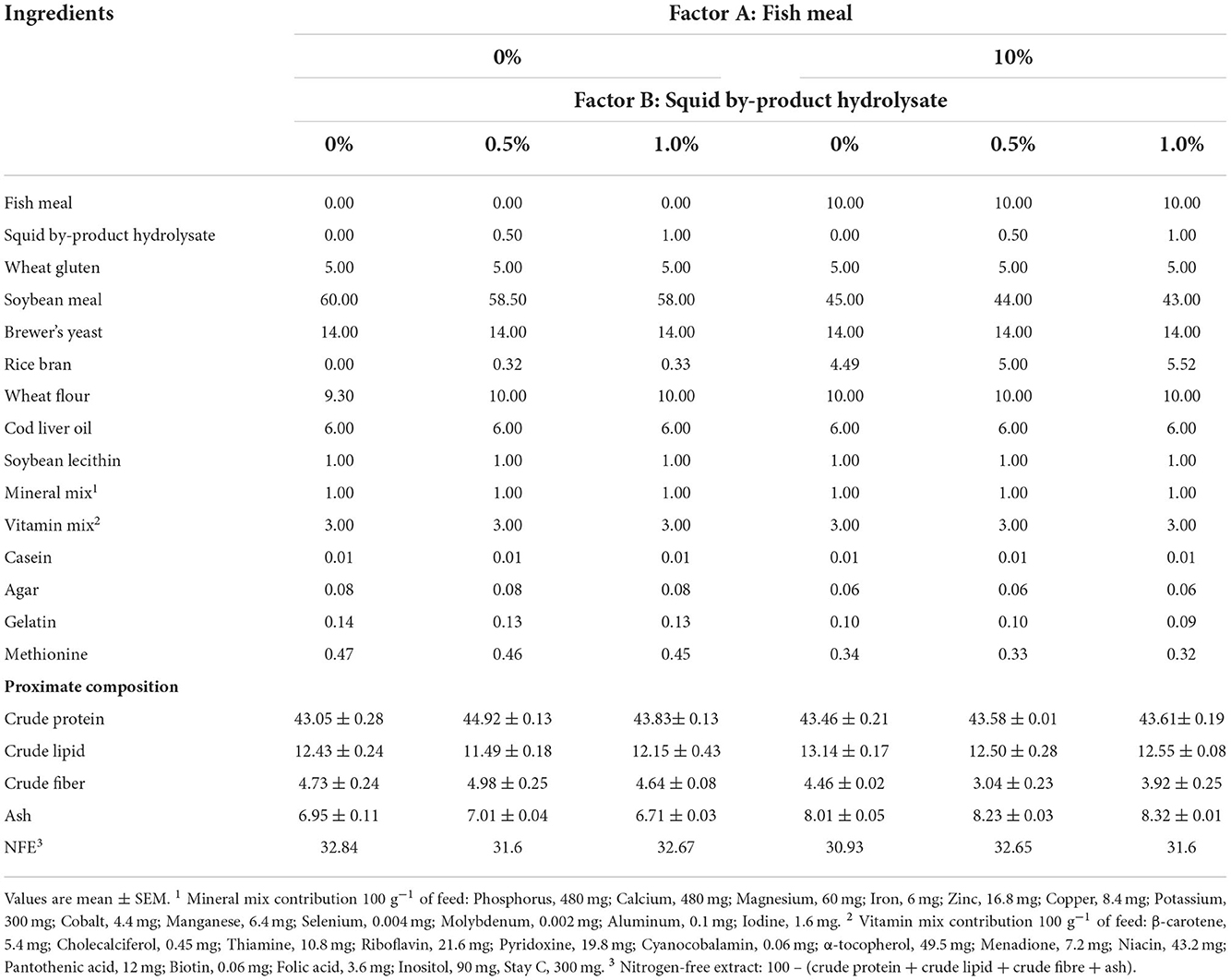
Six isonitrogenous and isolipidic plant protein-based diets were formulated with two levels of fish meal (10 and 0%) supplemented with three different concentrations of squid by-product hydrolysate (0, 0.5, and 1%). The formulation with 10% fish meal without squid by-product hydrolysate supplementation served as the basal diet [modified from González-Félix et al. (2014)]. These diets were formulated to meet the amino acid and other nutrient requirements of P. monodon. The composition of the test diets is shown in Table 1.
In the preparation of the diet, fish oil was added to the mixture of dry ingredients. Then, an adequate amount of water was added and the mixture was mashed into a dough. The resulting dough was passed through a pelletizer and the spaghetti-like strings were dried in an oven at 60°C until the desired moisture was achieved. The dried pellets were ground to appropriate sizes and stored at −20°C prior to feeding.
Test animals and maintenance
Specific pathogen-free P. monodon postlarvae were sourced from a commercial hatchery in Guimbal, Iloilo, Philippines and transported to the Multi-Species Hatchery of the Institute of Aquaculture, College of Fisheries and Ocean Sciences, Miag-ao, Iloilo, Philippines. The shrimp were acclimated to laboratory conditions and fed with a commercial diet for 2 weeks. Water physico-chemical parameters were monitored and maintained throughout the culture period (ammonia - 0.13 ± 0.03 ppm, nitrite - 0.00 ± 0.00 ppm, pH - 8.17 ± 0.03, salinity - 23.13 ± 0.22 ppt, temperature - 29.34 ± 0.28 °C, dissolved oxygen - >5 ppm).
Growth trial
A total of 450 P. monodon postlarvae (ABW: 0.11 ± 0.1 g) were utilized in this experiment. After acclimation, each group of 25 shrimp was bulk weighed and randomly stocked in 60-L tanks. The test diets were given to triplicate tanks which followed a 2 × 3 factorial in a Completely Randomized Design. The shrimp were given the test diets for 56 days at equal rations every 0800, 1,200, and 1,600 h. Excess feeds were siphoned out 1 h after every feeding. The collected uneaten feed was oven-dried, weighed, and subtracted from the given feed to account for the total feed intake. The feeding trial was carried out in a recirculating set-up and the water in the reservoir was changed every week to maintain good water quality. The bulk weight of shrimp per tank was monitored every 15 days to adjust the feeding ration.
Upon the termination of the feeding trial, shrimp growth was determined based on weight gain and specific growth rate (SGR). Feed conversion ratio (FCR), protein efficiency ratio (PER), and nutrient retention were also computed (Hardy and Barrows, 2003). Survival was also determined.
Biochemical analysis
Squid by-product hydrolysate, feed, and carcass proximate composition of the remainder shrimp of each treatment from all the analyses, which include moisture, protein, fiber, and ash contents were analyzed following AOAC (1990) while crude lipid was determined using the Bligh and Dyer (1959) method.
Digestive enzyme activity assays
Shrimp (n = 6 shrimp per treatment) were excised, hepatopancreas was collected, and homogenized in 50 mM sodium phosphate buffer, pH 8.0 for the determination of protease, α-amylase, and lipase activities. Protease activity assay was carried out following Buroker-Kilgore and Wang (1993) using casein as a substrate. The α-amylase activity was analyzed using Bernfeld (1951) method and lipase activity was determined according to Pinsirodom and Parkin (2001). The protein content of the shrimp hepatopancreas extracts was analyzed according to Bradford (1976) using bovine serum albumin as standard. One enzyme unit activity is equivalent to the amount of product produced min−1 mg−1 protein. Results of the digestive enzyme assay are expressed as units of mg−1 protein.
Gene expression analysis
The first abdominal segment of 6 shrimp per treatment (2 shrimp per replicate tank) was used in the analysis of muscle growth-related genes [α-actin, GLUT1, myosin heavy chain, tor signaling pathway genes (tor, 4e-bp, and s6k)] while the whole shrimp gut was utilized in the determination of RAB-6A (gut pro-inflammatory gene) and prophenoloxidase (immune-related gene). Only shrimp exhibiting the highest growth and those given the basal diet were subjected to gene expression analyses.
Tissue samples that were previously stored in RNAlater were blot-dry quickly and homogenized in TRIzol Reagent (Life Technologies) for RNA extraction. Then, cDNA was generated using the GoScript™ Reverse Transcription System (Promega). Gene expressions were determined in CFX Connect (Bio-Rad) using 1 μl cDNA and 200 nM of each primer in 1X SYBR Green Master Mix (Vivantis). Primers and conditions used in the real-time reverse-transcription polymerase chain reaction are presented in Table 2. Relative gene expression was computed as 2−ΔΔCT (Livak and Schmittgen, 2001) using shrimp elongation factor-1 alpha as the housekeeping gene.

Table 2. Primers and conditions utilized in real-time reverse transcription polymerase chain reaction.
Shrimp muscle histological evaluation
The abdominal muscles of 6 shrimp per treatment (2 shrimp per replicate tank) were fixed in Davidson's solution and processed for histological evaluation according to Bell and Lightner (1988). Then, morphometric measurements of the muscle cross-section area (CSA), mean fiber area, and muscle fiber density were done according to Valente et al. (2016) and Wei et al. (2020a). Muscle CSA and mean fiber area were determined at 100 × objective magnification while fiber density was determined at 40 × objective magnification (Figure 1). All images were analyzed using ImageJ (Schneider et al., 2012).
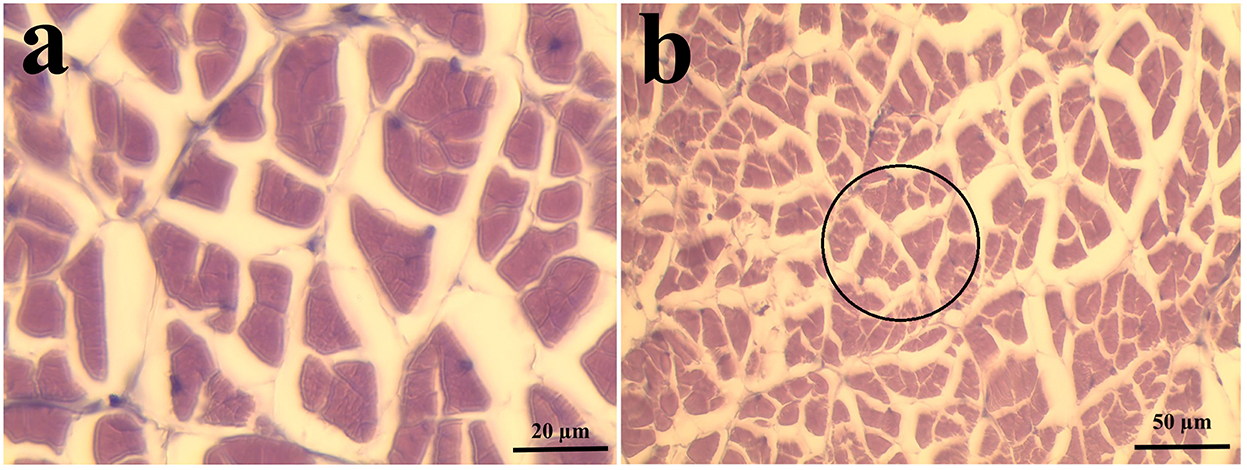
Figure 1. Representative samples of shrimp muscle used in the morphometric measurements. (a) Sample section used in the determination of muscle cross-section area and mean fiber area/size (n = 6 shrimp, 5 random frames per replicate; 1,000 × magnification; bar length = 20 μm). (b) Sample section used in the determination of muscle fiber density (n = 6 shrimp, 5 random circles per replicate, 400 × magnification; bar length = 50 μm). Transverse, 4–5 μm paraffin section, H and E stain, Davidson's fixative.
Statistical analysis
Data on the degree of hydrolysis, the proximate composition of the squid by-product and hydrolysate, muscle growth-, gut pro-inflammatory, and immune-related gene expressions were analyzed using a t-test. On the other hand, results on the growth, survival, digestive enzyme assays, and muscle morphometry were analyzed using a two-way analysis of variance and Tukey's honest significant difference test. All probability values were set at a 0.05 level of significance.
Results
Quality of the squid by-products
The squid by-products in the present study had an ammonia concentration of 27.30 ± 0.00 mg 100 g−1 squid sample and a urea content of 10.66 ± 0.10 mg 100 g−1 squid sample. In addition, the squid sample had an expressible drip of 18.14 ± 0.70 %.
Degree of hydrolysis
A high degree of hydrolysis has been observed during the trial even at the first h (Figure 2) upon hydrolyzing the squid by-products. A higher degree of hydrolysis has been observed upon using a 1% bromelain enzyme to substrate ratio in comparison with 0.25% and it had reached a peak at the 4th h (57.89 ± 1.61%). These results served as the basis for the production of hydrolysate to be used in the feed formulation.
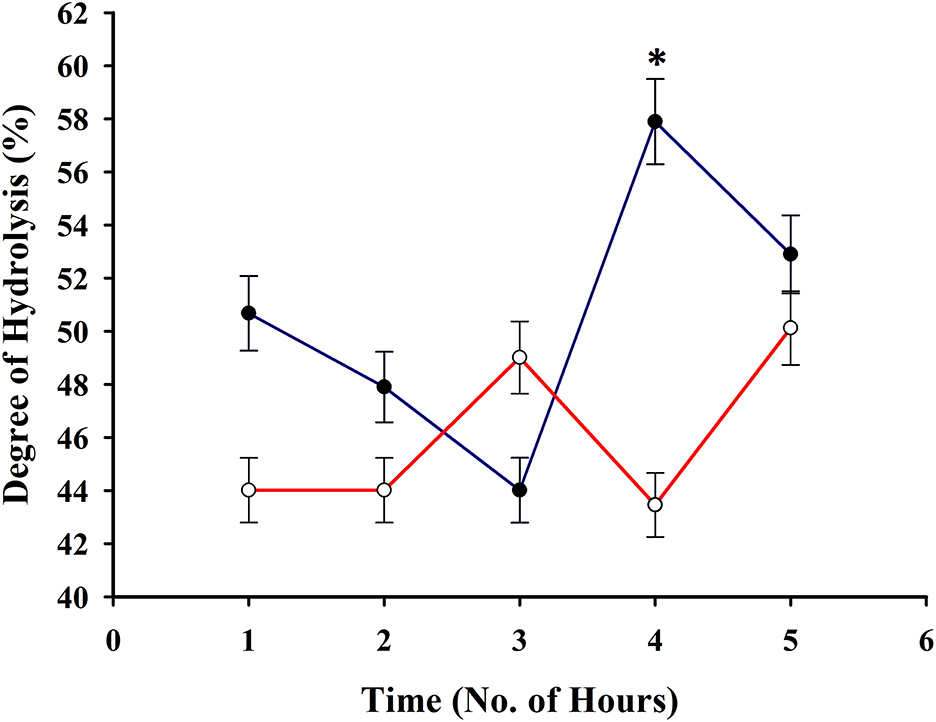
Figure 2. Degree of hydrolysis of squid by-products at 0.25% (red line) and 1% (blue line) enzyme to substrate ratio. The asterisk indicates a significant difference between treatments.
Yield and proximate composition
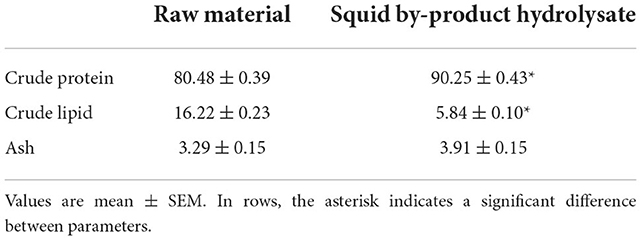
The conversion of squid by-products into hydrolysate had an average yield of 19.16 ± 5.16%. In terms of proximate composition (Table 3), the hydrolysis led to an increase in the crude protein content of the raw material by almost 10%. An opposite result has been noted in the lipid content of the raw material and hydrolysate, which decreased from 16.24 ± 0.23% to 5.84 ± 0.10%. No significant difference was observed between the ash content of the raw material and hydrolysate. The quality of hydrolysate has been further evaluated by determining its amino acid content (Table 4). The squid by-product hydrolysate utilized in this study had a chemical score index of 6.16 wherein the limiting amino acids are arginines followed by tryptophan, with an EAAI value of 0.74 and an EAA/NEAA ratio of 0.86.

Table 4. Amino acid content, chemical score, chemical score index, EAAI, EAA/NEAA ratio of the squid by-product hydrolysate.
The molecular weight of squid by-product hydrolysate
The squid by-product hydrolysate used in the present study had a molecular weight of 3.76 kDa based on the SDS-PAGE results (Figure 3).
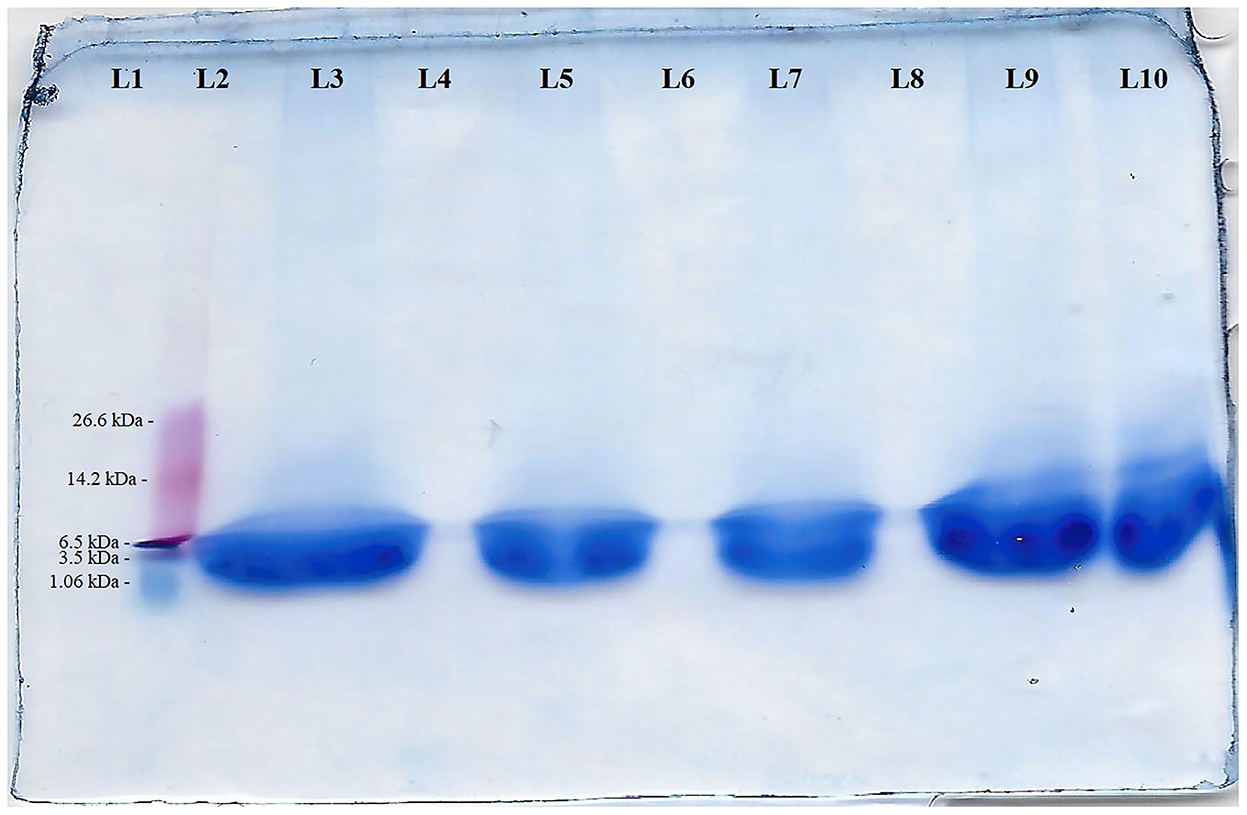
Figure 3. SDS-PAGE of squid by-product hydrolysate in 10% resolving gel of SDS-PAGE of squid by-products hydrolyzed using bromelain for 4 h. L1- Blank; L2- protein ladder (marker - 26.6 - 1.06 kDa from top to bottom); L3 - 17.33 μg protein 15 μl−1; L4 - Blank; L5 - 11.55 μg protein 15 μl−1; L6 - Blank; L7 - 7.70 μg protein 15 μl−1; L8- Blank; L9 - 17.33 μg protein 15 μl−1, L10 - 11.55 μg protein 15 μl−1.
Growth
After 56 days of the feeding trial, shrimp growth in terms of weight gain and SGR, FCR, and PER were found to be significantly influenced by the interaction of the fishmeal levels and the squid by-product hydrolysate inclusion (Table 5). The highest weight gain was recorded in the treatment maintained in 0% fish meal with 1% squid by-product hydrolysate. This was followed by the treatment that was also fed with 0% fish meal but with 0.5% hydrolysate inclusion, and this was not significantly different from the other treatments except for those shrimp that were given with 10% fish meal and 1% hydrolysate. The 10 (1) diet group exhibited the lowest weight gain. Similar to weight gain, SGR was also found to be higher in 0 (1) treatment compared to other treatments except for the full plant protein-based diet with 0.5% squid by-product hydrolysate. The shrimp fed with a 10 (1) diet also exhibited the lowest SGR. In terms of FCR, it was found to be lowest in shrimp given with 10% fish meal and 0.5% hydrolysate. This was not significantly different from other treatments except for the group provided with 0% fish meal without hydrolysate supplementation, which recorded the highest FCR. The protein efficiency ratio was found to be highest in the group fed with 0% fish meal with 1% hydrolysate and it was not significantly different from 10 (0), 10 (0.5), and 0 (0.5). The lowest PER was recorded in 0 (0) and 10 (1) diet groups. The total feed intake of shrimp in the present study was found to be affected by the fishmeal levels only, the inclusion of squid by-product hydrolysate had not influenced this index and no interaction has been observed between the two factors. It has been noted that lower feed intake can be observed in the shrimp given with 10% fish meal in comparison to those fed with high plant protein-based diets. Survival rates were not influenced by the fish meal levels and hydrolysate supplementation, or their interaction.
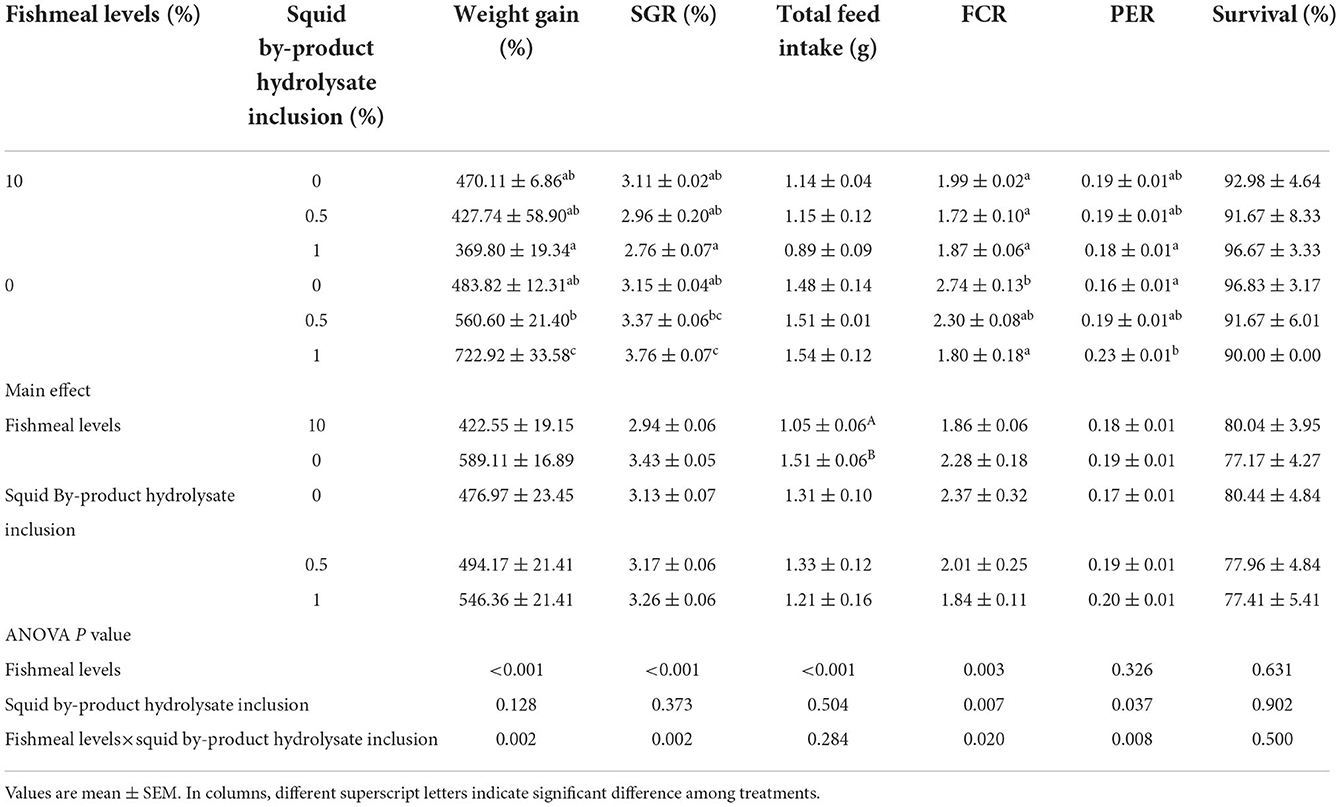
Table 5. Growth performance of shrimp fed with plant protein-based diets supplemented with squid by-product hydrolysate.
Proximate composition of shrimp carcass and nutrient retention
Interactive effects of the fish meal levels and squid by-product hydrolysate inclusion have been observed in the proximate composition in terms of protein, lipid, and ash of the shrimp carcass (Table 6). Carcass protein of shrimp was found to be highest in diets with 10% fish meal without hydrolysate and this was not significantly different from those shrimp given with similar fish meal levels with 1% hydrolysate inclusion. The lowest protein was observed in shrimp given a 0% fish meal diet with 1% hydrolysate supplementation. However, the highest lipid content was noted in this diet group. Ash content, on the other hand, was found to be higher in shrimp given with the basal diet.

Table 6. Carcass composition of shrimp fed with plant protein-based diets supplemented with squid by-product hydrolysate (% dry weight).
Protein retention was also found to be influenced by the interaction between the fish meal inclusion and the hydrolysate supplementation (Table 7). The highest protein retention was observed in shrimp given a plant protein-based diet with 1% squid by-product hydrolysate. In contrast, the lowest value was recorded on the diet with 0% fish meal without hydrolysate supplementation. A similar pattern can be observed in lipid retention wherein the group fed with 0% fish meal supplemented with 1% hydrolysate recorded the highest value while the lowest was noted in 0% fish meal without hydrolysate. Lipid retention in shrimp in the present study was also found to be affected by the interaction between the fishmeal levels and squid by-product hydrolysate inclusion.
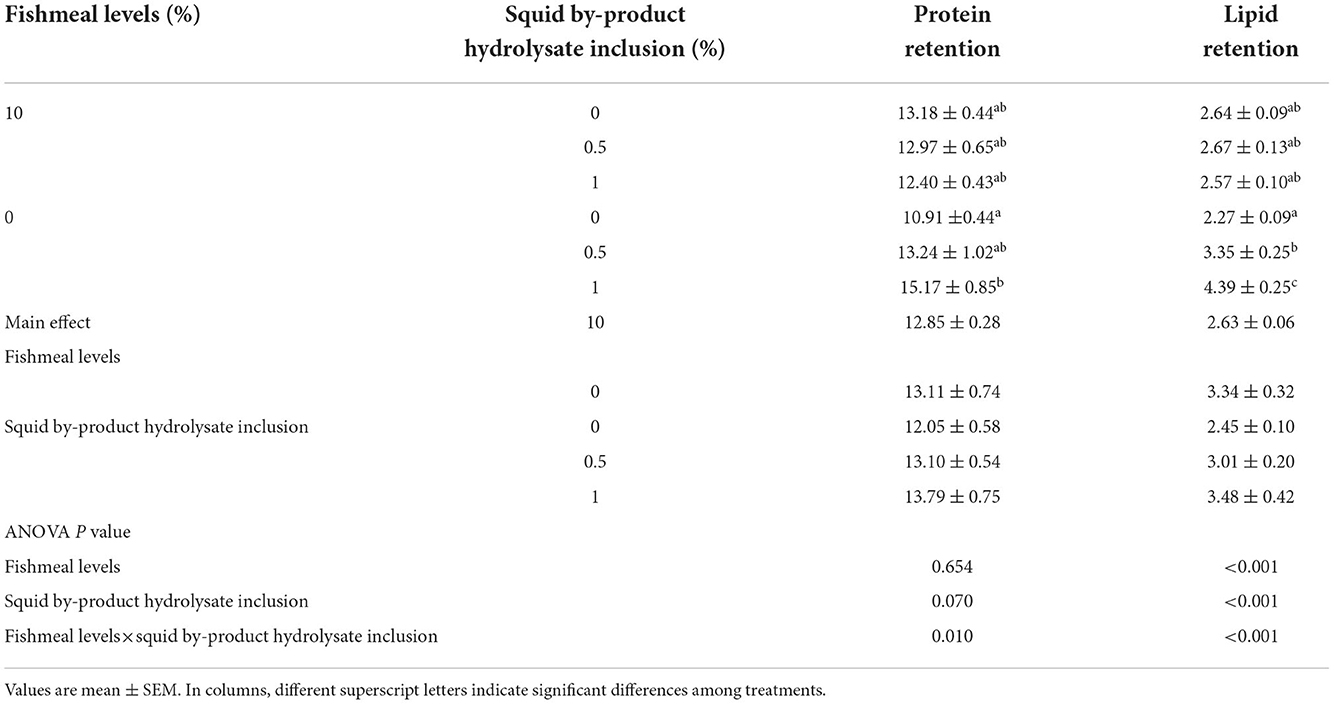
Table 7. Nutrient retention of shrimp fed with plant protein-based diets supplemented with squid by-product hydrolysate (% dry weight).
Digestive enzyme activity
The digestive protease activity of shrimp was not influenced by the hydrolysate inclusion in the diets but by the fish meal levels only and no interaction between these two factors was observed. Higher activity had been observed in those shrimp given with plant protein-based diets (Table 8). Moreover, α-amylase and lipase activities were affected by the interaction between fish meal levels and squid by-product hydrolysate supplementation. The highest α-amylase activity was exhibited by the shrimp fed on a diet of 10% fish meal supplemented with 0.5% hydrolysate, followed by 0 (1) and 10 (0) groups. The lowest α-amylase activity was recorded in the treatment given with a 0 (0.5) diet. In terms of lipase activity, the highest digestive enzyme activity was found in the diet with 10% fishmeal supplemented with 0.5% squid by-product hydrolysate, followed by treatments 0 (1), 0 (0.5), and 10 (0). The lowest lipase activities were recorded in 0 (0) and 10 (1) groups.
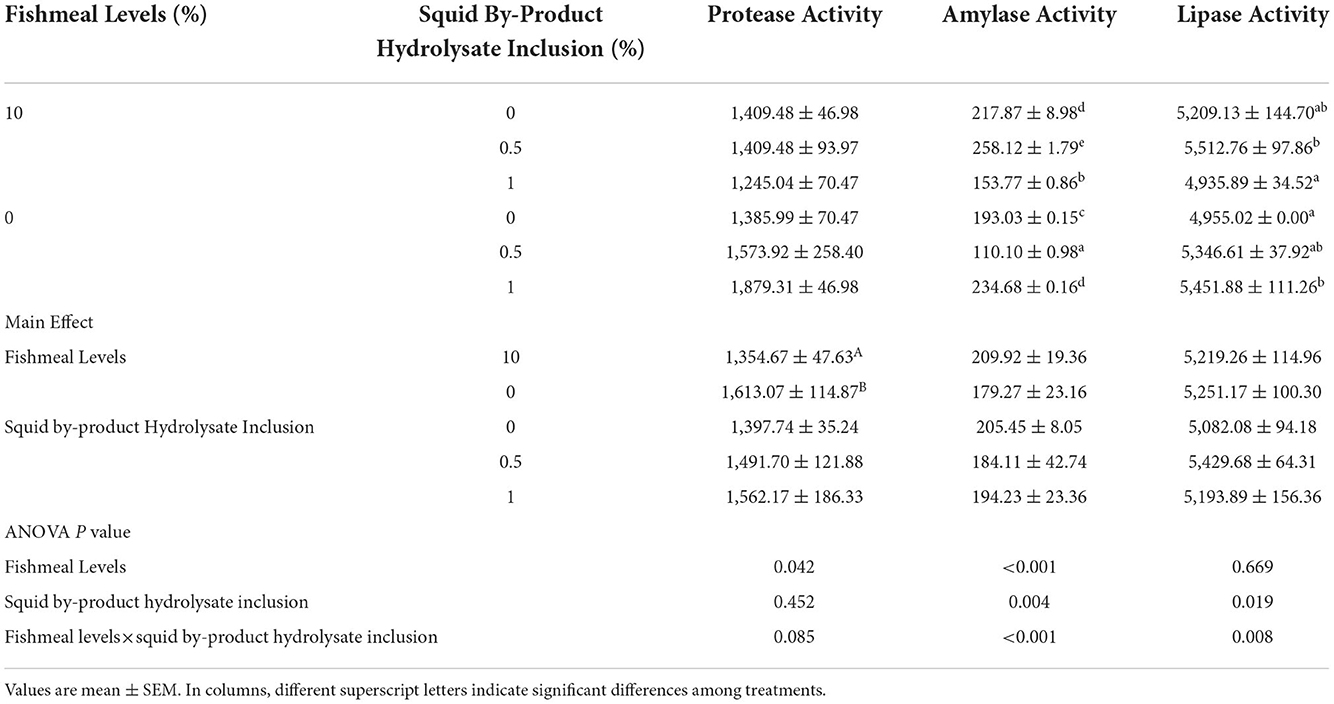
Table 8. Digestive enzyme activities of shrimp fed with plant protein-based diets supplemented with squid by-product hydrolysate (units mg−1 protein).
Muscle growth related genes
Upregulation of muscle growth-related genes in shrimp given with a 0% fish meal diet supplemented with 1% squid by-product hydrolysate has been observed except for α-actin (Figures 4A–F).
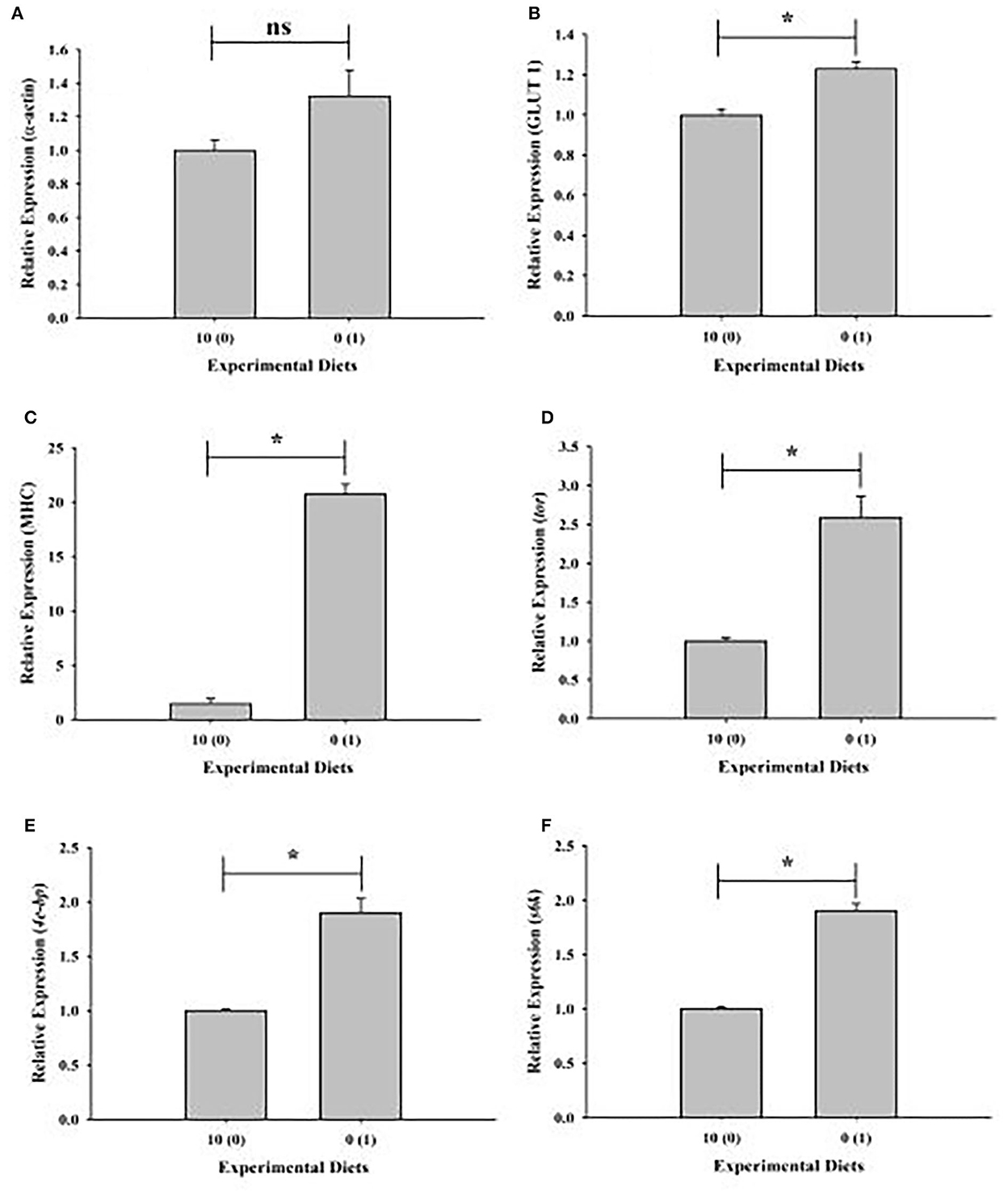
Figure 4. Relative expression of muscle growth-related genes of shrimp fed with plant-based diets supplemented with squid by-product hydrolysate. (A) α-actin, (B) GLUT1, (C) MHC, (D) tor, (E) 4e-bp, (F) s6k. The asterisk indicates a significant difference between treatments.
Gut pro-inflammatory and immune-related genes
Expression of the RAB-6A gene was found to be highest in shrimp given with 10% fish meal without squid by-product hydrolysate supplementation while it was downregulated in the group maintained in 0% fish meal with 1% squid by-product hydrolysate (Figure 5). Moreover, opposite to the RAB-6A gene expression, the prophenoloxidase gene was upregulated in the shrimp fed with 0% fish meal and 1% hydrolysate (Figure 6).
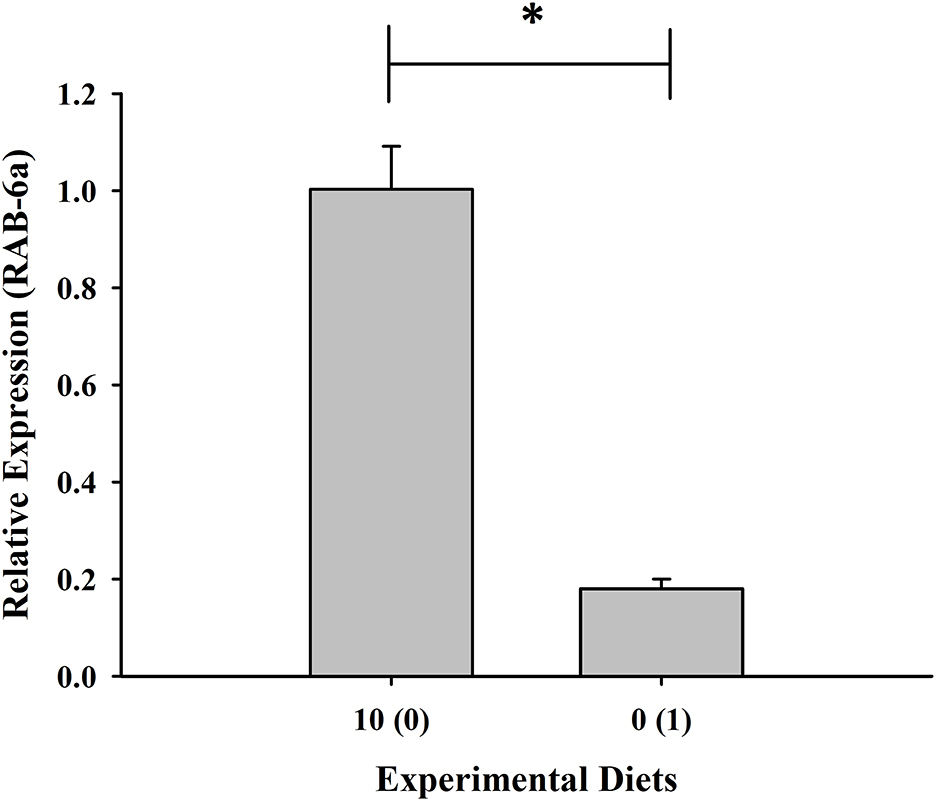
Figure 5. Relative expression of RAB-6a, a gut pro-inflammatory gene, of shrimp fed with plant-based diets supplemented with squid by-product hydrolysate. The asterisk indicates a significant difference between treatments.
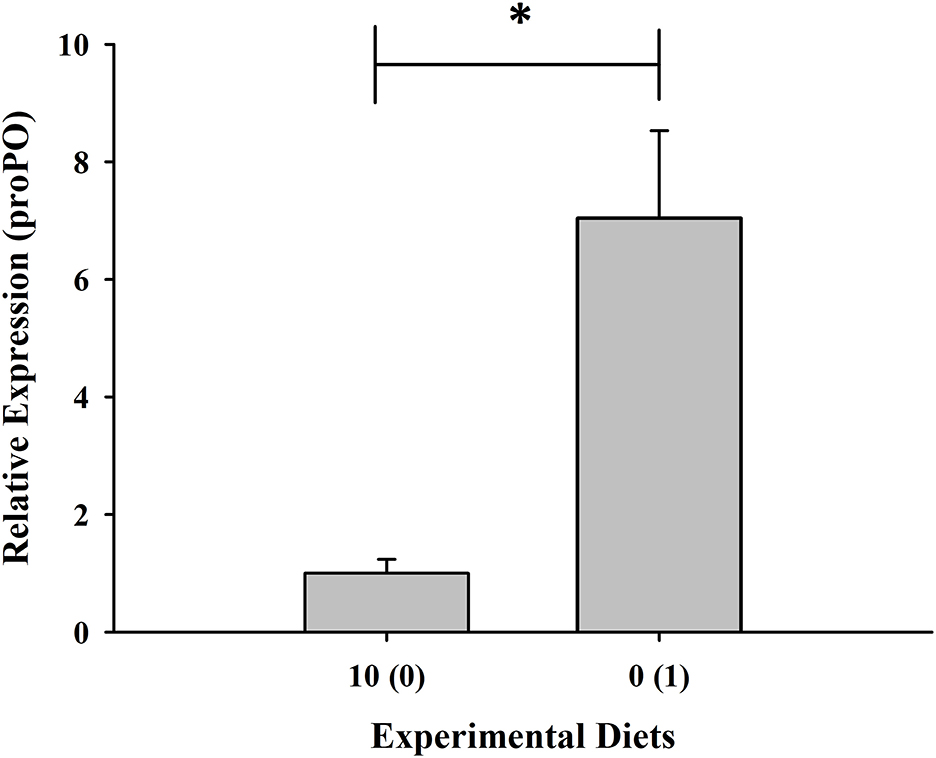
Figure 6. Relative expression of prophenoloxidase gene of shrimp fed with plant-based diets supplemented with squid by-product hydrolysate. Values are mean ± SEM. The asterisk indicates a significant difference between treatments.
Muscle cross-section area and fiber density
The muscle cross-section area was not influenced by the fish meal levels but was only affected by the supplementation of squid by-product hydrolysate (Table 9). The two factors (fishmeal and squid by-product hydrolysate) had not shown any interactive effects on this index. The highest muscle CSA was observed in those shrimp given diets supplemented with 0.5% squid by-product hydrolysate. This was comparable to the muscle CSA of shrimp given with 1% squid by-product hydrolysate supplementation. The lowest muscle CSA was recorded in shrimp maintained in diets without hydrolysate supplementation. Whereas mean fiber area was found to be significantly influenced by the interaction between fish meal levels and squid by-product hydrolysate supplementation. It was found to be highest in shrimp given with 0% fish meal with 1% hydrolysate supplementation. Shrimp muscle fiber density was found to be affected by the squid by-product hydrolysate supplementation only and was not influenced by the fish meal levels in the diets or the interaction of these factors. Fiber density was found to be highest in shrimp fed on diets without hydrolysate and was statistically similar to diets with 0.5% hydrolysate supplementation. The lowest fiber density was recorded in diets with 1% squid by-product hydrolysate inclusion.
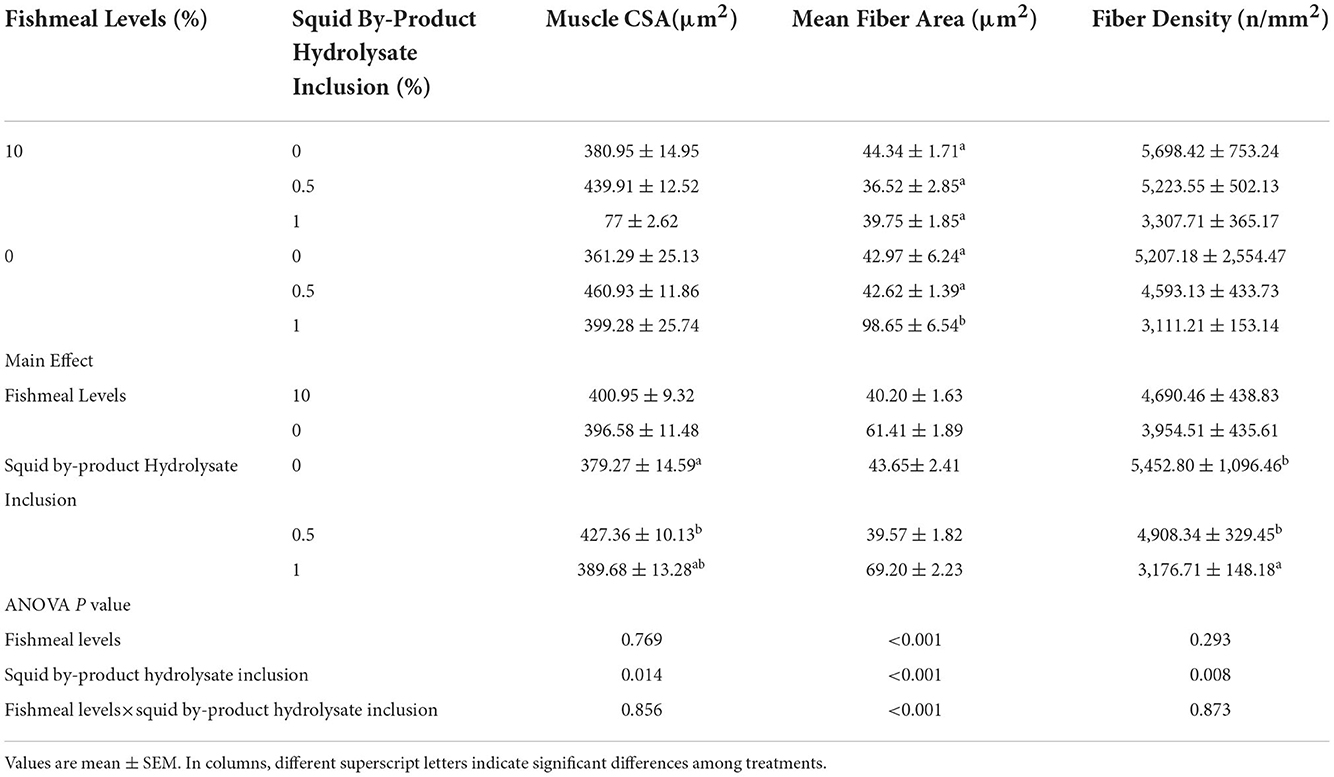
Table 9. Morphometric measurements of muscle cross-section area (CSA), mean fiber area, and fiber density of P. monodon fed with plant protein-based diets supplemented with squid by product hydrolysate.
Discussion
The conversion of squid processing by-products into a hydrolysate and its supplementation in the plant protein-based diet of P. monodon has been elucidated. The results of the present study show that hydrolysate from squid by-products was of good quality and promoted the growth of shrimp upon its supplementation in the diet.
The hydrolysate produced has ammonia and urea contents that were lower or within the reported values indicating squid spoilage. Paarup et al. (2002) reported that the ammonia concentration at the end of the shelf-life of whole and gutted squid was 33 and 24 mg 100 g−1, respectively. The ammonia content of the material in the present study is lower than the value obtained from the whole squid stored in ice for 10 days given the fact that the by-products were also composed of viscera. Several authors reported that ammonia is a good indicator of squid quality as it increased with longer storage duration. Lapa-Guimarães et al. (2013) reported that the ammonia content of the whole squid stored in ice for 16 days ranged from 10.1 mg 100 g−1 to 32.8 mg 100 g−1. However, Tantasuttikul et al. (2011) reported a much lower value around 0.13 to 0.34 mg 100 g−1. It has also been indicated that ammonia production in the squid mantle is sensitive to storage temperature, physical abuse, and handling method (LeBlanc and Gill, 1984). In addition, Paarup et al. (2002) reported that urea remained at 10 mg 100 g−1 samples until the 12th day of storage. Comparable findings have been also reported by several authors on various squid species. Vaz-Pires et al. (2008) reported a urea content of 5.4 to 18.8 mg 100 g−1 for 13 days of ice storage, which is also similar to the present study that urea content was around 10 mg 100 g−1 at the end of its shelf-life. Moreover, Lapa-Guimarães et al. (2013) noted the urea content from 1.6 to 15.7 mg 100 g−1 for 16 days of storage, which can be observed to be increasing toward the end of the experiment. The expressible drip of the raw materials in the present study is higher than in the observation of Tantasuttikul et al. (2011) with a value of around 4 to 13% for 16 days of iced storage wherein they concluded that squid with shelf-life up to 6 days only had 6–7% expressible drip. This increase in expressible drip indicates that squid muscle losses its water-holding capacity which can be related to loser structure due to partial degradation of proteins or autolysis. Based on earlier studies, ammonia, urea, and expressible drip are also good indicators of squid quality (Lapa-Guimarães et al., 2005; Tantasuttikul et al., 2011). However, it is difficult to compare the present results on the quality with the findings of several authors on the shelf-life and quality of squid since whole or gutted materials have been used in the analysis. In contrast, the present study utilized by-products which include viscera undergo several processing steps. However, it can still be stated that the squid by-products used in the present study are still of good quality since their ammonia and urea concentrations are below or within the limit of the reported levels indicating the spoilage of squid. The expressible drip of the squid by-products may have been higher; however, it had not significantly affected the chemical composition of the raw materials.
During the hydrolysis production, the high degree of hydrolysis in the first hour implies that a large number of proteins were being broken down into peptides and free amino acids during this period. Quinto et al. (2018) reported a similar rapid initial hydrolysis reaction in hydrolyzing tilapia processing residues using Alcalase. As the hydrolysis progresses, a declining trend in the hydrolysis rate could be observed as almost all peptide bonds have been hydrolyzed. It was also stated that the higher the enzyme to substrate ratio, the higher the degree of hydrolysis (Kristinsson and Rasco, 2000). A similar trend was also observed in the present study. González-Félix et al. (2014) also stated that prolonged duration and higher temperatures greatly improved squid by-product hydrolyzation. Varying degree of hydrolysis has been reported by several authors upon hydrolyzing squid by-products (Choi et al., 2014; González-Félix et al., 2014; Suárez-Jiménez et al., 2015; Lee et al., 2018). These differences might be due to several factors such as the enzyme to substrate ratio, squid species used, hydrolysis conditions, and the squid parts used in the production of hydrolysate. Nevertheless, the production of fish protein hydrolysate utilizing commercial enzymes similar to the present study is much preferable since it is more predictable and repeatable, and the separation of soluble and insoluble nitrogen compounds is milder (Hevrøy et al., 2005), preserving the natural properties of the product while improving its biological activity (Kristinsson and Rasco, 2000).
Moreover, the average yield of hydrolysate recovered from the present study was higher than those previously reported using bromelain in hydrolyzing fish by-products (Elavarasan et al., 2014; Gajanan et al., 2016; Raftani Amiri et al., 2016). Several authors have noted that with the increase in hydrolysis and duration, the hydrolysate yield also increases (Gajanan et al., 2016; Slizyte et al., 2016). This may be the reason for the high yield of the squid by-product hydrolysate obtained in the present study since the squid by-products were hydrolyzed for 4 h.
Given the present results of the proximate composition of the squid by-product hydrolysate, it is evident that enzymatic hydrolysis has improved the protein content of squid by-products. This value is much higher than squid by-product hydrolysate's previously reported protein content (Lian et al., 2005; Choi et al., 2014; González-Félix et al., 2014; Lee et al., 2018). Similar to the degree of hydrolysis, these differences in the proximate composition of the hydrolysate produced in the present study in comparison with the previous results might be due to the squid species used, degree of hydrolysis, hydrolysis duration, enzyme to substrate ratio, and the enzyme used to hydrolyze the raw materials.
Further assessment of the quality of the hydrolysate was done by determining its amino acid content and computing its chemical score and EAAI, which are considered practical ways of evaluating proteins in fish diets (Hepher, 1988). Based on the relative amount of the essential amino acids of proteins used in feed formulations, a chemical score is used to predict its biological values. This index is also based on the assumption that whole egg protein had a high biological value and can support the growth of an organism, while growth would be limited by the essential amino acid in the test protein with the lowest ratio relative to whole egg protein (Hepher, 1988). In the present study, chemical scores of most essential amino acids of squid by-product hydrolysate were close to or above 100%. This indicates that these amino acids suffice the requirement of P. monodon. The most limiting amino acid of squid by-product hydrolysate is arginine, followed by tryptophan based on its chemical score index. However, the literature on the chemical scores of squid by-product hydrolysate is nil, but it was found to be higher than the reported chemical scores of squid meal as a feed ingredient in Macrobrachium species (Montoya-Martinez et al., 2016). Montoya-Martinez et al. (2016) also stated that the low chemical score for squid meal might be due to its manufacture and source quality, however, squid meal is still considered an excellent protein source comparable to fish meal in the formulation of aquaculture feeds. They also reported that the most limiting amino acidon Macrobrachium americanum upon using squid meal as a feed ingredient is arginine, which is similar to the results of the present study. However, upon comparing with other Macrobrachium species and yellowtail (Tomas-Vidal et al., 2019), different essential amino acids appear to be limiting for each species. Therefore, it can be stated that limiting amino acid or chemical score index for different species varies despite using similar protein sources since their nutrient requirement are also different. Moreover, the quality of the squid by-product hydrolysate has also been assessed by its EAAI value which indicated that the generated hydrolysate material could be classified as a useful quality feed protein. EAAI is also used in the evaluation of the nutritional value of proteins based on their amino acid composition (Peñaflorida, 1989). This index has been considered in the evaluation of proteins aside from the determination of the limiting amino acid since other essential amino acids might also have some effect on protein quality (Hepher, 1988). Peñaflorida (1989) rated feed ingredients based on the method of Oser (1959) wherein protein materials with an EAAI value of 0.90 as good protein materials, around 0.80 as useful protein, and below 0.70 are considered inadequate. The squid by-product hydrolysate produced in the present study has lowerEAAI values than shrimp meal, squid meal, fish meal, and soybean meal (Teruel, 2002). The EAA/NEAA ratio of squid by-product hydrolysate in the present study is slightly higher than the EAA/NEAA ratio of whole chicken egg protein which is considered of high biological value based on the chemical scores. They reported a range of 0.75–0.82 (Attia et al., 2020). However, upon comparing the present results with other aquaculture feed ingredients, its EAA/NEAA ratio is lower than that of fishmeals, shrimp meal, and squid meal which were computed based on its amino acid composition as reported by Peñaflorida (1989). On the other hand, in comparison with other studies on hydrolyzing squid by-products, its EAA/NEAA ratio is higher than the values (0.51–0.70) reported by Lian et al. (2005) and González-Félix et al. (2014), stating that it was still a good source of high-quality protein.
The squid by-product hydrolysate used in the present study indicates that it is primarily composed of low molecular weight peptides. This is much lower than those reported in earlier studies on hydrolyzing squid by-products by endogenous enzymes or commercially available proteases (Lian et al., 2005; González-Félix et al., 2014). However, it is within the range of the molecular weight of squid hydrolysate reported by Suárez-Jiménez et al. (2015), from 16 to 2.5 kDa. These differences are due to the factors that also influenced the degree of hydrolysis, particularly the hydrolysis duration. It has been documented in fish (Aksnes et al., 2006) and shrimp (Li et al., 2018) that small or low molecular weight peptides from fish protein hydrolysates had beneficial effects on the growth and immunity of the cultured organism. It has been stated by Bauchart et al. (2006) that peptides with a molecular weight of <5 kDa may exhibit diverse biological activities. In addition, it has been reported that peptides with a molecular size of <3 kDa showed antioxidant potential (Unnikrishnan et al., 2020).
The observed improvement in the growth of P. monodon in the present study is associated with its increase in feed intake coupled with better feed conversion efficiency and an increase in the protein efficiency ratio. The high survival in all treatments observed in the present study indicates that the shrimp was in good health condition and its nutritional needs had been provided (Suárez et al., 2009). Similar observations on growth enhancement have been reported in P. vannamei (Forster et al., 2011; González-Félix et al., 2014; Li et al., 2018) and L. schmitti (González et al., 2007), upon feeding with plant protein-based diets supplemented with fish protein hydrolysate. This improvement in growth may have been influenced by the molecular weight of the supplemented hydrolysate. Quinto et al. (2018) reported better growth in shrimp fed with tilapia residue hydrolyzed for 2 h composed of 10-250 kDa proteins than those given with hydrolyzed for 1 hour and 2 hours. This conforms to the results obtained by Li et al. (2018) upon the inclusion of low molecular weight fish hydrolysate in a high soybean meal diet for P. vannamei. However, a further increase in hydrolysate inclusion depressed the growth of the organism. This observation has been also reported by Córdova-Murueta and García-Carreño (2002) upon incorporating 9–15% squid meal in a shrimp diet wherein the improvement of growth was evident only at 3% inclusion. They attributed this to the high quantities of small peptides under 14.4 kDa and larger bands between 29 and 45 kDa. Therefore, the low molecular weight of the squid by-product hydrolysate in the present study, although incorporated at lower levels than the previous studies, might have contributed to the enhancement of the growth of the organism. Whereas the negative effect of feeding shrimp with a plant protein-based diet without squid by-product hydrolysate supplementation is evident in its high feed conversion ratio and low protein efficiency ratio. This observation has also been reported by several authors on P. vannamei (Alvarez et al., 2007; Suárez et al., 2009; Bulbul et al., 2016).
The results in the present study indicate that feed intake of P. monodon has not been affected by the supplementation of squid by-product hydrolysate but by the difference in the sources of proteins. This conforms to the report of Zhou et al. (2016) that feeding shrimp with a plant-based diet still supported its normal growth and was not significantly different from those diets incorporated with squid and scallop hydrolysates. Furthermore, the inclusion of 3% squid by-product hydrolysate in a plant-based diet had not improved the feed intake of P. vannamei in comparison to the basal diet without hydrolysate. A similar observation has been reported upon supplementing tuna by-products (Hernández et al., 2011) and poultry by-product plus swine liver (Soares et al., 2020) hydrolysates in P. vannamei diets. This observation of the feed intake of shrimp in the present study could be due to the lower inclusion level or the type of hydrolysate used in the diets. An increased feed intake of a shrimp plant-based diet had been observed with the addition of 1% krill and 2% fish hydrolysate (Smith et al., 2005; Li et al., 2018).
Moreover, the growth of shrimp given with a plant protein-based diet incorporated with 1% squid by-product hydrolysate can be also attributed to the enhancement in its feed conversion ratio. This improved feed conversion ratio might be due to the enhancement of the amino acid profile brought about by the inclusion of 1% squid by-product hydrolysate in the plant protein-based diet of shrimp. Thus, the nutrient present in the aquatic feed has been utilized efficiently. Furthermore, upon the improvement of the amino acid profile, optimal protein synthesis may have occurred since all amino acids which were derived from whole proteins or amino acid supplements were simultaneously presented to the tissue (Tacon, 1987). Better FCR upon protein hydrolysate supplementation or comparable to the fishmeal-based diet has been observed in Pacific white shrimp (Hernández et al., 2011), red sea bream (Kondo et al., 2017), and Asian sea bass (Chotikachinda et al., 2018) which agrees with the present results.
Furthermore, this improvement in the amino acid profile of the 1% hydrolysate supplemented plant protein-based diet is evident in the significantly higher protein efficiency ratio. The improved protein efficiency ratio of P. monodon given this diet indicates that they were provided with good protein quality in an aquatic feed (Nguyen et al., 2012). The negative effects of the high plant protein inclusion in the diet might have been offset by the squid by-product hydrolysate through the improvement of its amino acid profile and protein digestibility (Hernández et al., 2011). Similar observations have been noted by Valle et al. (2015) wherein even at 40% replacement of fishmeal with biofloc flour and fish protein hydrolysate, growth, survival, and protein efficiency ratio were not affected.
A significant influence of the diets has been observed in nutrient retention. The increase in protein retention might have contributed to the enhancement of growth in P. monodon given a plant protein-based diet with 1% squid by-product hydrolysate which is also not significantly different from the control group. The improvement of protein retention can be associated with the enhancement of protein synthesis due to the better amino acid profile and protein digestibility (Hernández et al., 2011) of the plant protein-based diet with 1% squid by-product hydrolysate supplementation. It has been also observed in Atlantic salmon that fish protein hydrolysate supplementation was positively correlated with muscle protein deposition without any influence on its growth (Hevrøy et al., 2006).
In addition, an increase in lipid retention has also been observed upon the supplementation of 1% hydrolysate in the plant protein-based diet for P. monodon which had also contributed to its growth improvement. Similar observations have been reported by Niu et al. (2014) wherein carcass lipid deposition increased with increasing supplementation of fish hydrolysate. This has also been documented in Nile tilapia fed with shrimp head hydrolysates (Leal et al., 2010). This influence of protein hydrolysate supplementation in the lipid deposition of aquatic organisms has not yet been fully elucidated and warrants further investigation.
The increase in protein and lipid retention might have been aided by the improvement of digestive enzyme activities of shrimp fed with a plant protein-based diet supplemented with 1% squid by-product hydrolysate. Similar observations that fish protein hydrolysate can influence digestive enzyme activities have been documented earlier. Niu et al. (2014) observed an increase in trypsin activity upon supplementation of fish protein hydrolysate in the diet of P. vannamei. Similar findings have been reported by Shao et al. (2018) on the trypsin activity of shrimp upon feeding with protein hydrolysate. However, α-amylase and lipase activities were found to be not significant. Córdova-Murueta and García-Carreño (2002) reported the enhancement of total proteolytic activity in shrimp fed on diets supplemented with 3% squid meal, and trypsin and chymotrypsin activities at 3 and 9% squid meal similar to fish and krill hydrolysates. However, digestive enzyme activities were depressed upon the higher supplementation of squid meal (15%) in the shrimp diet. The supplementation of the protein hydrolysate in the diet of shrimp might have promoted intestinal maturation in shrimp and reduced the negative impact of feeding on plant protein-based diets. Similar observations have been reported by Cahu and Infante (1995a,b) that feeding protein hydrolysates affected the intestinal maturation and pancreatic and intestinal enzyme activities of sea bass larvae. It has also been documented that 19% protein hydrolysate in sea bass larvae diet helped in the occurrence of adult mode digestion (Cahu et al., 1999). This might be also true for the shrimp in the present study upon feeding with plant protein-based diets supplemented with 1% squid by-product hydrolysate.
The increase in the muscle growth-related genes of shrimp maintained in plant protein-based diets with 1% hydrolysate could be related to the increase in protein synthesis that supports the results of its growth enhancement due to muscle protein accretion. GLUT 1, a glucose transporter, has been upregulated upon the 1% supplementation of squid by-product hydrolysate in a plant protein-based diet. The enhancement of this gene may have also contributed to the increase in the protein retention of the shrimp in the present study since the increase of this glucose transporter gene would lead to the increase in the metabolic fuels for muscle growth (Richter et al., 2001). Similar to our findings, significant upregulation of glucose transporter proteins from the intestinal cells and overall growth enhancement in European seabass has been observed upon feeding it with a low fish meal diet supplemented with 5% mixed tilapia and shrimp protein hydrolysate (Leduc et al., 2018). Moreover, it was also reported in Wistar rats provided with whey protein hydrolysates that glucose transporter-4 genes were upregulated. An increase in the expression of this gene leads to the availability of glucose and the enhancement of glycogen synthesis. It was stated that the upregulation of the glucose transporter gene expression is due to the peptide and amino acid composition of the whey protein hydrolysate (Morato et al., 2013). Moreover, upon the supplementation of 1% squid by-product hydrolysate in the plant protein-based diet of P. monodon, upregulation of the myosin heavy chain has been observed. This upregulation in the myosin heavy chain might have resulted in the enhancement of protein accretion observed in the present study. This is in agreement with the report of Hevrøy et al. (2006) that the expression of myosin heavy chain is strongly correlated with muscle protein accretion than growth in Atlantic salmon fed with fish protein hydrolysate. It has also been stated that diets supplemented with high levels of fish protein hydrolysate contained high amounts of small peptides below 10 kDa and free and peptide amino acids (Espe et al., 1993; Hevrøy et al., 2005). In addition, it has also been observed in vitro that digestible macromolecules such as proteins or peptides (<5 kDa) from soybean-germ protein hydrolysate upregulated myosin heavy chain expression (Saneyasu et al., 2018). It is speculated that this myosin heavy chain activation system also works for the shrimp fed with 1% squid by-product hydrolysate (about 4 kDa) supplementation in its plant protein-based diet in the present study. The enhancement of protein retention upon the inclusion of 1% squid by-product hydrolysate can be also linked to the increase in the TOR signaling pathway which regulates cell growth and metabolism in response to environmental cues (Wullschleger et al., 2006) through the phosphorylation of s6k and 4e-bp genes (Song et al., 2016). Regulation of growth-related genes in fish has been associated with dietary protein levels (Gao et al., 2019) and the amino acid pattern of its protein source (Wu et al., 2017). Previous studies have shown that upregulation of the TOR pathway genes is related to the enhanced growth performance of several aquatic species such as black sea bream (Irm et al., 2020), grouper (Wu et al., 2017), and turbot (Song et al., 2016). Furthermore, downregulation of tor encoding genes and growth depression has been exhibited by fish given a low fish meal diet and high plant protein source (Yuan et al., 2019). Similar to our findings, it was reported that a mixture of marine protein hydrolysates which include fish soluble, squid paste, and shrimp paste (Dai et al., 2020) and a composite mixture of shrimp hydrolysate and plant proteins (Li et al., 2021) in the diets of largemouth bass upregulated its tor and s6k genes and enhanced its growth performance, feed utilization, and protein and amino acid digestibility. In addition, the upregulation of 4e-bp genes has been also linked to the enhancement of growth in tiger puffers fed with plant protein-based diets upon the supplementation of 12 g fish protein hydrolysate kg−1 fish meal (Wei et al., 2021). Whereas, the downregulation of tor signaling pathway genes due to feeding on a high plant protein diet has been shown to be overcome in turbot upon the supplementation of fish protein hydrolysate which was evident in its increase in growth and protein accretion associated with the increase in 4e-bp gene expression (Wei et al., 2020b). In shrimp, it has been reported by Shao et al. (2018) that expression of tor and s6k genes in the group fed with hydrolysate were not significant with those given the fish meal diet. Furthermore, it was significantly higher than the commercial diet. The upregulation of these genes has been also linked to the growth and carcass protein composition of the shrimp. These earlier findings support the present results indicating that the growth improvement and higher protein retention in P. monodon fed a plant protein-based diet with 1% squid by-product hydrolysate is associated with the upregulation of muscle growth-related genes.
Canada et al. (2018) also observed the upregulation of muscle growth-related and de novo DNA methyltransferases encoding genes in Senegalese sole fed with diets containing high levels of protein hydrolysate although it has not greatly impacted its growth. It was also reported that a 1% supplementation of yeast hydrolysate in the diet of P. vannamei increased the motor signaling pathway, inhibit the expression of inflammatory-related genes, and enhanced the expression of immune-related genes such as prophenoloxidase (Jin et al., 2018). These reports agree with the observations in the present study.
It has been reported that soybean meal and soybean protein concentrate can induce enteritis in aquaculture species such as turbot and grouper (Gu et al., 2016; Zhang et al., 2022) and increased the expression of gut pro-inflammatory genes. It has also been reported by Kumaraguru-Vasagam et al. (2007) that feeding shrimp with raw plant ingredients caused hemocytic enteritis which they associated with anti-nutritional factors. In shrimp, He et al. (2017) and Li et al. (2018) evaluated gut inflammation in shrimp through the expression of pro-inflammatory genes which include RAB-6A. The suppression of the RAB-6A gene in shrimp fed with a plant protein-based diet with 1% squid by-product hydrolysate inclusion indicates that gut inflammatory responses might have been prevented. He et al. (2017) accounted the decrease in gut pro-inflammatory gene expression in shrimp to the proliferation of lactic acid bacteria in the gut, thus, preventing the growth of pathogenic bacteria and maintaining homeostasis in shrimp. The promotion of the growth of lactic acid bacteria in shrimp gut fed with squid by-product hydrolysate had been previously reported by Pan and Traifalgar (2022). Li et al. (2018) stated that the composition of the diets can influence intestinal health, therefore, it can affect inflammatory gene expression. RAB-6A was also evaluated in P. vannamei upon the supplementation of low molecular weight fish hydrolysate, however, no significant difference has been observed (Li et al., 2018). Shrimp gut's main functions include food digestion, nutrient absorption, and storage (Kumaraguru-Vasagam et al., 2007). In addition, they also act as a barrier and first line of shrimp defense against pathogenic bacteria, which are associated with intestinal integrity, gut microbiota, and mucus immune compounds (Duan et al., 2018).
Aside from influencing gut inflammatory gene expression, innate immunity has been also found to be affected by the supplementation of protein hydrolysate in low fishmeal diets (Khosravi et al., 2015). Therefore, the present study also evaluated the expression of an immune-related gene in P. monodon such as prophenoloxidase in the hepatopancreas which is a part of the midgut (Holt et al., 2021) and was found to also play an important role in shrimp innate immune response (Ji et al., 2009; Rahimnejad et al., 2018; Li et al., 2019). The present finding indicates that supplementation of 1% squid by-product hydrolysate in the shrimp diet enhanced its prophenoloxidase activity. This observation concurs with the report of Li et al. (2018) on P. vannamei upon the inclusion of 1 to 1.5% low molecular weight fish hydrolysate in a high soybean meal diet. They stated that the activation of this immune-related gene would result in the improvement of the innate immune response and antioxidant activity of the shrimp.
The increase in the muscle mean fiber area implies that squid by-product hydrolysate induced hypertrophic growth in shrimp which is a response to the upregulation of several muscle growth related genes. In vitro and in vivo studies have shown that supplementation of protein hydrolysate also influences muscle growth and muscle growth-related genes (Muthuramalingam et al., 2017; Kim et al., 2019; Wei et al., 2020a). The observed decline in fiber density in the present study may be the consequence of the increase in the mean fiber area of the shrimp muscle. The results of the present study concur with the report of Wei et al. (2020a) on turbot. Canada et al. (2018) also associated muscle growth in Senegalese sole to hypertrophy upon feeding it with hydrolysate-incorporated diets containing small peptides with a molecular weight of 5–20 kDa in high proportion and below 5 kDa but they added that this also lowers the growth potential of the organism due to lower recruitment of small-sized fibers. In addition, Cruz-Suarez et al. (1987) suggested that growth promotion in P. japonicus determined by the RNA to DNA ratio is due to cell hypertrophy upon the inclusion of squid protein fraction in the diet. However, they attributed it to an unknown growth factor in the squid protein fraction but not to the amino acid composition. Whereas hyperplastic growth in Senegalese sole has been reported by Valente et al. (2016) upon feeding with a 100 % plant protein-based diet without hydrolysate supplementation. The results of the present study are in agreement with the previous reports; thus, it can be stated that the growth of P. monodon fed with a plant-based diet supplemented with 1% squid by-product hydrolysate is due to the upregulation of muscle growth-related genes which promote nutrient accretion leading to hypertrophic muscle growth.
Conclusion
The supplementation of 1% squid by-product hydrolysate in the plant protein-based diet of P. monodon supported its growth requirements which led to its enhancement. This growth enhancement can be attributed to its increase in feed intake, better feed conversion ratio, high protein efficiency ratio, and nutrient retention. These results can be also linked to the improvement of its digestive enzyme activities, regulation of muscle growth-, gut pro-inflammatory, and immune-related genes. The growth in shrimp given a plant protein-based diet with 1% squid by-product supplementation is evident in its increase in muscle mean fiber area which suggests hypertrophic growth. Given the beneficial effects of squid by-product supplementation, it is suggested that the digestive histomorphology of shrimp should also be evaluated. In addition, the conversion of these by-products into hydrolysate is an efficient way of maximizing the utilization of this resource and promoting the use of a plant protein-based diet for sustainable aquaculture. Furthermore, it would also help alleviate the problems in waste disposal.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the animal study because the conduct of this research does not need approval from the Animal Ethics Committee of the Agency.
Author contributions
MP designed the study, conducted the experiment, analyzed the data, and wrote the manuscript draft. RC and EM conducted the experiment, analyzed the data, and wrote the manuscript draft. RT designed the study, analyzed the data, and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Department of Science and Technology-Science Education Institute Accelerated Science and Technology Human Resource and Development Program: Student Research Support Fund.
Acknowledgments
The DOST-Science Education Institute and the DOST-PCAARRD-Institute of Aquaculture, College of Fisheries and Ocean Sciences, UP Visayas Project on the Improvement of Philippine Penaeus vannamei for Enhanced Growth and White Spot Syndrome Virus Resistance through Selective Breeding are acknowledged for the financial and technical support on this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aksnes, A., Hope, B., Jönsson, E., Björnsson, B. T., and Albrektsen, S. (2006). Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. I: Growth, growth regulation and feed utilization. Aquaculture 261, 305–317. doi: 10.1016/j.aquaculture.2006.07.025
Alvarez, J. S., Hernández-Llamas, A., Galindo, J., Fraga, I., García, T., and Villarreal, H. (2007). Substitution of fishmeal with soybean meal in practical diets for juvenile white shrimp Litopenaeus schmitti (Pérez-Farfante and Kensley 1997). Aquac. Res. 38, 689–695. doi: 10.1111/j.1365-2109.2007.01654.x
AOAC. (1990). “Official Methods of Analysis 15th edition, vol. 1,” in Association of Official Analytical Chemists, Inc., ed. K. Helrich (Virginia, USA: Association of Official Analytical Chemists, Inc.) 77.
Attia, Y., Al-Harthi, M., Korish, M., and Shiboob, M. (2020). Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals 10, 406. doi: 10.3390/ani10030406
Bauchart, C., Remond, D., Chambon, C., Patureau Mirand, P., Savary-Auzeloux, I., Reynes, C., et al. (2006). Small peptides (<5 kDa) found in ready-to-eat beef meat. Meat Sci. 74, 658–666. doi: 10.1016/j.meatsci.2006.05.016
Bell, T., and Lightner, D. (1988). A Handbook of Normal Penaeid Shrimp Histology. Lawrence, Kansas: The World Aquaculture Society, Allen Press, Inc.
Bernfeld, P. (1951). Enzymes of starch degradation and synthesis. Adv. Enzymol. Relat. Subj. Biochem. 12, 379–428. doi: 10.1002/9780470122570.ch7
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/y59-099
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bulbul, M., Kader, M. A., Asaduzzaman, M., Ambak, M. A., Chowdhury, A. J. K., Hossain, M. S., et al. (2016). Can canola meal and soybean meal be used as major dietary protein sources for kuruma shrimp, Marsupenaeus japonicus? Aquaculture 452, 194–199. doi: 10.1016/j.aquaculture.2015.10.036
Buroker-Kilgore, M., and Wang, K. (1993). A Coomassie Brilliant Blue G-250-based colorimetric assay for measuring activity of calpain and other proteases. Anal. Biochem. 208:387–392. doi: 10.1006/abio.1993.1066
Cahu, C. L., and Infante, J. L. Z. (1995a). Maturation of the pancreatic and intestinal digestive functions in sea bass (Dicentrarchus labrax): effect of weaning with different protein sources. Fish. Physiol. Biochem. 14, 431–437. doi: 10.1007/BF00004343
Cahu, C. L., and Infante, J. L. Z. (1995b). Effect of the molecular form of dietary nitrogen supply in sea bass larvae: Response of pancreatic enzymes and intestinal peptidases. Fish. Physiol. Biochem. 14, 209–214. doi: 10.1007/BF00004311
Cahu, C. L., Infante, J. L. Z., Quazuguel, P., and Le Gall, M. M. (1999). Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass Dicentrarchus labrax larvae. Aquaculture 171, 109–119. doi: 10.1016/S0044-8486(98)00428-1
Canada, P., Engrola, S., Mira, S., Teodósio, R., Yust, M., del, M., et al. (2018). Larval dietary protein complexity affects the regulation of muscle growth and the expression of DNA methyltransferases in Senegalese sole. Aquaculture 491, 28–38. doi: 10.1016/j.aquaculture.2018.02.044
Cesar, J. R. O., and Yang, J. (2007). Expression patterns of ubiquitin, heat shock protein 70, α-actin and β-actin over the molt cycle in the abdominal muscle of marine shrimp Litopenaeus vannamei. Mol. Reprod. Dev. 74, 554–559. doi: 10.1002/mrd.20605
Choi, J. H., Kim, K. T., and Kim, S. M. (2014). Optimization and biochemical characteristics of an enzymatic squid hydrolysate for manufacture of a squid complex seasoning. Food Sci. Biotechnol. 23, 417–423. doi: 10.1007/s10068-014-0057-9
Chotikachinda, R., Tantikitti, C., Benjakul, S., and Rustad, T. (2018). Tuna viscera hydrolysate products prepared by different enzyme preparations improve the feed intake and growth of asian seabass, Lates calcarifer, fed total fishmeal replacement diets. Songklanakarin J. Sci. Technol. 40, 167–177. Available online at: https://www.thaiscience.info/Journals/Article/SONG/10988612.pdf
Córdova-Murueta, J. H., and García-Carreño, F. L. (2002). Nutritive value of squid and hydrolyzed protein supplement in shrimp feed. Aquaculture 210, 371–384. doi: 10.1016/S0044-8486(02)00011-X
Costa, M., Costas, B., Machado, M., Teixeira, C., Fernández-Boo, S., Sá, T., et al. (2020). Anchovy and giant squid hydrolysates can enhance growth and the immune response of European seabass (Dicentrarchus labrax) fed plant-protein-based diets. Aquaculture 523, 735182. doi: 10.1016/j.aquaculture.2020.735182
Cruz-Suarez, L. E., Guillaume, J., and Wormhoudt, A. V. (1987). Effect of various levels of squid protein on growth and some biochemical parameters of Penaeus japonicus juveniles. Nippon Suisan Gakkaishi 53, 2083–2088. doi: 10.2331/suisan.53.2083
Dai, M., Li, S., Fu, C., Qiu, H., and Chen, N. (2020). The potential role of marine protein hydrolyzates in elevating nutritive values of diets for largemouth bass, Micropterus salmoides. Front. Mar. Sci. 7, 1–11. doi: 10.3389/fmars.2020.00197
Duan, Y., Liu, Q., Wang, Y., Zhang, J., and Xiong, D. (2018). Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 78, 279–288. doi: 10.1016/j.fsi.2018.04.050
Elavarasan, K., Naveen Kumar, V., and Shamasundar, B. A. (2014). Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Preserv. 38, 1207–1214. doi: 10.1111/jfpp.12081
Espe, M., Lied, E., and Torrissen, K. (1993). Changes in plasma and muscle free amino acids in Atlantic salmon (Salmo salar) during absorption of diets containing different amounts of hydrolysed cod muscle protein. Comp. Biochem. Physiol. 105A, 555–562. doi: 10.1016/0300-9629(93)90434-6
FAO. (2016). The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All. Rome: FAO.
FAO. (2020). The State of World Fisheries and Aquaculture 2020: Sustainability in Action. Rome: FAO.
Forster, I. P., Bechtel, P., Dominy, W. G., Lane, S., Avena, R., Ju, Z. Y., et al. (2011). Use of fish hydrolysates and fish meal byproducts of the Alaskan fishing industry in diets for Pacific white shrimp Litopenaeus vannamei. N. Am. J. Aquac. 73, 288–295. doi: 10.1080/15222055.2011.598371
Gajanan, P. G., Elavarasan, K., and Shamasundar, B. A. (2016). Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 23, 24901–24911. doi: 10.1007/s11356-016-7618-9
Gao, Y., Lu, S., Wu, M., Yao, W., Jin, Z., and Wu, X. (2019). Effects of dietary protein levels on growth, feed utilization and expression of growth-related genes of juvenile giant grouper (Epinephelus lanceolatus). Aquaculture 504, 369–374. doi: 10.1016/j.aquaculture.2019.02.023
González, D., Córdoba, J., and Esperanza Buitrago, F. I. (2007). Estudios preliminares en la formulación de dietas para camarón blanco (Litopenaeus schmitti) utilizando ensilado de pescado. Rev. Cient. 17, 166–172.
González-Félix, M. L., Perez-Velazquez, M., Ezquerra-Brauer, J. M., Bringas-Alvarado, L., Sánchez-Sánchez, A., and Torres-Arreola, W. (2014). Evaluation of jumbo squid (Dosidicus gigas) byproduct hydrolysates obtained by acid-enzymatic hydrolysis and by autohydrolysis in practical diets for Pacific white shrimp (Litopenaeus vannamei). Food Sci. Technol. 34, 552–558. doi: 10.1590/1678-457x.6414
Gu, M., Bai, N., Zhang, Y., and Krogdahl, A. (2016). Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 464, 286–295. doi: 10.1016/j.aquaculture.2016.06.035
Hardy, R., and Barrows, F. (2003). “9-Diet Formulation and Manufacture”, in Fish Nutrition 3rd edition, eds. J. Halver, R. Hardy (California, USA: Academic Press) 505–600. doi: 10.1016/B978-012319652-1/50010-0
Hasegawa, H. (1987). Laboratory Manual on Analytical Methods and Procedures for Fish and Fish Products. Singapore: Marine Fisheries Research Department, Southeast Asian Development Center. A4.1–A4.2.
Haslaniza, H., Maskat, M. Y., Wan Aida, W. M., and Mamot, S. (2010). The effects of enzyme concentration, temperature and incubation time on nitrogen content and degree of hydrolysis of protein precipitate from cockle (Anadara granosa) meat wash water. Int. Food Res. J. 17, 147–152. Available online at: http://www.ifrj.upm.edu.my/17%20(01)%202010/(16)%20IFRJ-2010-147-152%20Maskat%20malaysia.pdf
He, W., Rahimnejad, S., Wang, L., Song, K., Lu, K., and Zhang, C. (2017). Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 70, 164–173. doi: 10.1016/j.fsi.2017.09.007
Hepher, B. (1988). Nutrition of Pond Fishes. New York: Cambridge University Press, 404. doi: 10.1017/CBO9780511735455
Hernández, C., Olvera-Novoa, M. A., Smith, D. M., Hardy, R. W., and Gonzalez-Rodriguez, B. (2011). Enhancement of shrimp Litopenaeus vannamei diets based on terrestrial protein sources via the inclusion of tuna by-product protein hydrolysates. Aquaculture 317, 117–123. doi: 10.1016/j.aquaculture.2011.03.041
Hevrøy, E. M., Espe, M., Waagb,ø, R., Sandnes, K., Ruud, M., and Hemre, G. I. (2005). Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 11, 301–313. doi: 10.1111/j.1365-2095.2005.00357.x
Hevrøy, E. M., Jordal, A. E. O., Hordvik, I., Espe, M., Hemre, G. I., and Olsvik, P. A. (2006). Myosin heavy chain mRNA expression correlates higher with muscle protein accretion than growth in Atlantic salmon, Salmo salar. Aquaculture 252, 453–461. doi: 10.1016/j.aquaculture.2005.07.003
Holt, C., Bass, D., Stentiford, G., and Giezen, M. (2021). Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 186, 107387. doi: 10.1016/j.jip.2020.107387
Irm, M., Taj, S., Jin, M., Timothée Andriamialinirina, H. J., Cheng, X., and Zhou, Q. (2020). Influence of dietary replacement of fish meal with fish soluble meal on growth and TOR signaling pathway in juvenile black sea bream (Acanthopagrus schlegelii). Fish Shellfish Immunol. 101, 269–276. doi: 10.1016/j.fsi.2020.03.053
Ji, P.-F., Yao, C.-L., and Wang, Z.-Y. (2009). Immune response and gene expression in shrimp (Litopenaeus vannamei) hemocytes and hepatopancreas against some pathogen-associated molecular patterns. Fish Shellfish Immunol. 27, 563–570. doi: 10.1016/j.fsi.2009.08.001
Jin, M., Xiong, J., Zhou, Q. C., Yuan, Y., Wang, X. X., and Sun, P. (2018). Dietary yeast hydrolysate and brewer's yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 82, 121–129. doi: 10.1016/j.fsi.2018.08.020
Khosravi, S., Rahimnejad, S., Herault, M., Fournier, V., Lee, C.-R., Bui, H. T. D., et al. (2015). Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 45, 858–868. doi: 10.1016/j.fsi.2015.05.039
Kim, S. Y., Kim, H. S., Cho, M., and Jeon, Y. J. (2019). Enzymatic hydrolysates of Hippocampus abdominalis regulates the skeletal muscle growth in C2C12 cells and zebrafish model. J. Aquat. Food Prod. Technol. 28, 264–274. doi: 10.1080/10498850.2019.1575940
Kondo, F., Ohta, T., Iwai, T., Ido, A., Miura, C., and Miura, T. (2017). Effect of the squid viscera hydrolysate on growth performance and digestion in the red sea bream Pagrus major. Fish. Physiol. Biochem. 43, 1543–1555. doi: 10.1007/s10695-017-0391-y
Kristinsson, H. G., and Rasco, B. A. (2000). Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 40, 43–81. doi: 10.1080/10408690091189266
Kumaraguru-Vasagam, K. P., Balasubramanian, T., and Venkatesan, R. (2007). Apparent digestibility of differently processed grain legumes, cow pea and mung bean in black tiger shrimp, Penaeus monodon Fabricius and associated histological anomalies in hepatopancreas and midgut. Anim. Feed Sci. Technol. 132, 250–266. doi: 10.1016/j.anifeedsci.2006.03.022
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. doi: 10.1038/227680a0
Lapa-Guimarães, J., Felício, P. E., and Contreras Guzmán, E. (2005). Chemical and microbial analyses of squid muscle (Loligo plei) during storage in ice. Food Chem. 91, 477–483. doi: 10.1016/j.foodchem.2004.04.038
Lapa-Guimarães, J., Felício, P. E., and Contreras Guzmán, E. (2013). An alternative approach for improving freshness indices for squid (Loligo plei). Acta Aliment. 42, 437–450. doi: 10.1556/AAlim.42.2013.3.17
Leal, A. L. G., Castro, P. F., Lima, J. P. V., Souza Correia, E., and Souza Bezerra, R. (2010). Use of shrimp protein hydrolysate in Nile tilapia (Oreochromis niloticus L.) feeds. Aquac. Int. 18, 635–646. doi: 10.1007/s10499-009-9284-0
LeBlanc, R. J., and Gill, T. A. (1984). Ammonia as an objective quality index in squid. Can. Inst. Food Sci. Technol. J. 17, 195–201. doi: 10.1016/S0315-5463(84)72557-0
Leduc, A., Hervy, M., Rangama, J., Delépée, R., Fournier, V., and Henry, J. (2018). Shrimp by-product hydrolysate induces intestinal myotropic activity in European seabass (Dicentrarchus labrax). Aquaculture 497, 380–388. doi: 10.1016/j.aquaculture.2018.08.009
Lee, C. M., Volson, B., Kang, B., Karayannakidis, P. D., Gamez, E., Miller, J., et al. (2018). Effects of dietary scallop and squid hydrolysates on growth of European seabass, Dicentrarchus labrax; California yellowtail, Seriola lalandi; and barramundi, Lates calcarifer. J. World Aquac. Soc. 49, 971–984. doi: 10.1111/jwas.12572
Li, H., Tian, X., Zhao, K., Jiang, W., and Dong, S. (2019). Effect of Clostridium butyricum in different forms on growth performance, disease resistance, expression of genes involved in immune responses and mTOR signaling pathway of Litopenaeus vannamei. Fish Shellfish Immunol. 87, 13–21. doi: 10.1016/j.fsi.2018.12.069
Li, S., Dai, M., Qiu, H., and Chen, N. (2021). Effects of fishmeal replacement with composite mixture of shrimp hydrolysate and plant proteins on growth performance, feed utilization, and target of rapamycin pathway in largemouth bass, Micropterus salmoides. Aquaculture 533, 736185. doi: 10.1016/j.aquaculture.2020.736185
Li, X., Wang, L., Zhang, C., Rahimnejad, S., Song, K., and Yuan, K. (2018). Effects of supplementing low-molecular-weight fish hydrolysate in high soybean meal diets on growth, antioxidant activity and non-specific immune response of Pacific white shrimp (Litopenaeus vannamei). Turkish J. Fish. Aquat. Sci. 18, 717–727. doi: 10.4194/1303-2712-v18_5_07
Lian, P. Z., Lee, C. M., and Park, E. (2005). Characterization of squid-processing byproduct hydrolysate and its potential as aquaculture feed ingredient. J. Agric. Food Chem. 53, 5587–5592. doi: 10.1021/jf050402w
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Llames, C., and Fontane, J. (1994). Determination of amino acids in feeds: collaboraive study. J. AOAC Int. 77, 1362–1402. doi: 10.1093/jaoac/77.6.1362
Montoya-Martinez, C., Nolasco-Soria, H., Carrillo-Farnes, O., Civera-Cerecedo, R., Alvarez-Gonzalez, C., and Vega-Villasante, F. (2016). Chemical score of different protein sources to four Macrobrachium species. Lat. Am. J. Aquat. Res. 44, 835–844. doi: 10.3856/vol44-issue4-fulltext-19
Morato, P. N., Lollo, P. C. B., Moura, C. S., Batista, T. M., Camargo, R. L., Carneiro, E. M., et al. (2013). Whey protein hydrolysate increases translocation of GLUT-4 to the plasma membrane independent of insulin in Wistar rats. PLoS ONE 8, e71134. doi: 10.1371/journal.pone.0071134
Muthuramalingam, K., Kim, J. H., Jeon, Y. J., Rho, S., Kim, Y. M., and Cho, M. (2017). Effects of sea horse (Hippocampus abdominalis)-derived protein hydrolysate on skeletal muscle development. J Appl. Biol. Chem. 60, 373–381. doi: 10.3839/jabc.2017.058
Nguyen, C., Nguyen, T. G., Nguyen, L., Van Pham, H. Q., Nguyen, T. H., Pham, H. T., et al. (2016). De novo assembly and transcriptome characterization of major growth-related genes in various tissues of Penaeus monodon. Aquaculture 464, 545–553. doi: 10.1016/j.aquaculture.2016.08.003
Nguyen, H. T. M., Perez-Galvez, R., and Berge, J. P. (2012). Effects of diets containing tuna head hydrolysates on the survival and growth of shrimp Penaeus vannamei. Aquaculture 325, 127–134. doi: 10.1016/j.aquaculture.2011.11.014
Niu, J., Zhang, Y., Liu, Y., Tian, L., Lin, H., Chen, X., et al. (2014). Effects of graded replacement of fish meal by fish protein hydrolysate on growth performance of early post-larval Pacific white shrimp (Litopenaeus vannamei). J. Appl. Animal Res. 42, 6–15. doi: 10.1080/09712119.2013.795897
Novriadi, R., Spangler, E., Rhodes, M., Hanson, T., and Allen Davis, D. (2017). Effects of various levels of squid hydrolysate and squid meal supplementation with enzyme-treated soy on growth performance, body composition, serum biochemistry and histology of Florida pompano Trachinotus carolinus. Aquaculture 481, 85–93. doi: 10.1016/j.aquaculture.2017.08.032
Nunes, A. J. P., Sabry-Neto, H., Oliveira-Neto, S., and Burri, L. (2019). Feed preference and growth response of juvenile Litopenaeus vannamei to supplementation of marine chemoattractants in a fishmeal-challenged diet. J. World Aquac. Soc. 50, 1048–1063. doi: 10.1111/jwas.12648
Okumura, T. (2007). Effects of lipopolysaccharide on gene expression of antimicrobial peptides (penaeidins and crustin), serine proteinase and proteinase and prophenoloxidase in haemocytes of the Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 22, 68–76. doi: 10.1016/j.fsi.2006.03.013
Oser, B. (1959). “An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins,” in Protein and Amino Acid Nutrition, eds. A. Albanese (New York, USA: Academic Press Inc.) 281–295. doi: 10.1016/B978-0-12-395683-5.50014-6
Paarup, T., Sanchez, J. A., Moral, A., Christensen, H., Bisgaard, M., and Gram, L. (2002). Sensory, chemical and bacteriological changes during storage of iced squid (Todaropsis eblanae). J. Appl. Microbiol. 92, 941–950. doi: 10.1046/j.1365-2672.2002.01604.x
Pan, M. V., and Traifalgar, R. F. M. (2022). Growth enhancing effects of squid by-product hydrolysate in plant protein-based diet fed to black tiger shrimp, Penaeus monodon Fabricius, 1798. Asian Fish. Sci. 35, 56–67. doi: 10.33997/j.afs.2022.35.1.005
Pan, M. V., Traifalgar, R. F. M., Ferriols, V. M. E., Laureta, L. V., and Peralta, J. P. (2022). Efficacy of squid by-product hydrolysate supplementation on the growth performance, digestive enzyme activities and muscle growth-related genes expression of Penaeus monodon fed on plant protein-based diet. Cogent Food Agric. 8, 2103067. doi: 10.1080/23311932.2022.2103067
Peñaflorida, V. (1989). An evaluation of indigenous protein sources as potential component in the diet formulation for tiger prawn, Penaeus monodon, using essential amino acid index (EAAI). Aquaculture 83, 319–330. doi: 10.1016/0044-8486(89)90043-4
Pinsirodom, P., and Parkin, K. (2001). “Lipase Assays” in: Current Protocols in Food Analytical Chemistry, eds. J. Whitaker (New York, USA: John Wiley and Sons, Inc.), C3.1.1–C3.1.13. doi: 10.1002/0471142913.fac0301s00
Quinto, B. P. T., Albuquerque, J. V., Bezerra, R. S., Peixoto, S., and Soares, R. (2018). Replacement of fishmeal by two types of fish protein hydrolysate in feed for postlarval shrimp Litopenaeus vannamei. Aquac. Nutr. 24, 768–776. doi: 10.1111/anu.12605
Raftani Amiri, Z., Safari, R., Bakhshandeh, T., and Ahmadi Vavsari, F. (2016). Functional properties of fish protein hydrolysates from cuttlefish (Sepia pharaonis) muscle produced by two commercial enzymes. Iran. J. Fish. Sci. 15, 1485–1499. Available online at: http://hdl.handle.net/1834/12127
Rahimnejad, S., Yuan, X., Wang, L., Lu, K., Song, K., and Zhang, C. (2018). Chitooligosaccharide supplementation in low-fish meal diets for Pacific white shrimp (Litopenaeus vannamei): Effects on growth, innate immunity, gut histology, and immune-related genes expression. Fish Shellfish Immunol. 80, 405–415. doi: 10.1016/j.fsi.2018.06.025
Rahmatullah, M., and Boyde, T. R. C. (1980). Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 107, 3–9. doi: 10.1016/0009-8981(80)90407-6
Richter, E. A., Derave, W., and Wojtaszewski, J. F. P. (2001). Glucose, exercise and insulin: Emerging concepts. J. Physiol. 535, 313–322. doi: 10.1111/j.1469-7793.2001.t01-2-00313.x
Samocha, T. M., Davis, D. A., Saoud, I. P., and DeBault, K. (2004). Substitution of fish meal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 231, 197–203. doi: 10.1016/j.aquaculture.2003.08.023
Saneyasu, T., Shindo, H., Honda, K., and Kamisoyama, H. (2018). The extract of soybean protein increases slow-myosin heavy chain expression in C2C12 myotubes. J. Nutr. Sci. Vitaminol. 64, 296–300. doi: 10.3177/jnsv.64.296
Schneider, C., Rasband, W., and Eliceiri, K. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Shao, J., Zhao, W., Liu, X., and Wang, L. (2018). Growth performance, digestive enzymes, and TOR signaling pathway of Litopenaeus vannamei are not significantly affected by dietary protein hydrolysates in practical conditions. Front. Physiol. 9, 1–8. doi: 10.3389/fphys.2018.00998
Slizyte, R., Rommi, K., Mozuraityte, R., Eck, P., and Five, K. (2016). Bioactivities of fi sh protein hydrolysates from defatted salmon backbones. Biotechnol. Reports 11, 99–109. doi: 10.1016/j.btre.2016.08.003
Smith, D. M., Tabrett, S. J., Barclay, M. C., and Irvin, S. J. (2005). The efficacy of ingredients included in shrimp feeds to stimulate intake. Aquac. Nutr. 11, 263–272. doi: 10.1111/j.1365-2095.2005.00349.x
Soares, M., Rezende, P. C., Corrêa, N. M., Rocha, J. S., Martins, M. A., Andrade, T. C., et al. (2020). Protein hydrolysates from poultry by-product and swine liver as an alternative dietary protein source for the Pacific white shrimp. Aquac. Reports 17, 100344. doi: 10.1016/j.aqrep.2020.100344
Somboonwiwat, K., Supungul, P., Rimphanitchayakit, V., Aoki, T., Hirono, I., and Tassanakajon, A. (2006). Differentially expressed genes in hemocytes of Vibrio harveyi-challenged shrimp Penaeus monodon. J. Biochem. Mol. Biol. 39, 26–36. doi: 10.5483/BMBRep.2006.39.1.026
Song, F., Xu, D., Mai, K., Zhou, H., Xu, W., and He, G. (2016). Comparative study on the cellular and systemic nutrient sensing and intermediary metabolism after partial replacement of fishmeal by meat and bone meal in the diet of turbot (Scophthalmus maximus L.). PLoS ONE 11, 1–19. doi: 10.1371/journal.pone.0165708
Soonthornchai, W., Rungrassamee, W., Karoonuthaisiri, N., Jarayabhand, P., Klinbunga, S., Söderhäll, K., et al. (2010). Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 34, 19–28. doi: 10.1016/j.dci.2009.07.007
Soufi-Kechaou, E., Jaouen, P., Ben Amar, R., and Bergé, J. P. (2012). Influence of hydrolysis time on protein recovery and amino acid composition of hydrolysates from Sepia officinalis viscera. Sci. Res. Report. 2, 115–129. Available online at: https://archimer.ifremer.fr/doc/00114/22508/20191.pdf
Suárez, J. A., Gaxiola, G., Mendoza, R., Cadavid, S., Garcia, G., Alanis, G., et al. (2009). Substitution of fish meal with plant protein sources and energy budget for white shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture 289, 118–123. doi: 10.1016/j.aquaculture.2009.01.001
Suárez-Jiménez, G. M., Robles-Sánches, R. M., and Yépiz-Plascencia, G. (2015). In vitro antioxidant, antimutagenic and antiproliferative activities of collagen hydrolysates of jumbo squid (Dosidicus gigas) byproducts. Food Sci. Technol. 35, 421–427. doi: 10.1590/1678-457X.6658
Tacon, A. (1987). The Nutrition and Feeding of Farmed Fish and Shrimp - A Training Manual 1. The Essential Nutrients. Brazil: Food and Agriculture Organization of the United Nations.
Tantasuttikul, A., Kijroongrojana, K., and Benjakul, S. (2011). Quality indices of squid (Photololigo duvaucelii) and cuttlefish (Sepia aculeata) stored in ice. J. Aquat. Food Prod. Technol. 20, 129–147. doi: 10.1080/10498850.2010.548114
Teruel, M. B. (2002). “Evaluation of feedstuffs and aquafeeds,” in Nutrition In Tropical Aquaculture: Essentials of Fish Nutrition, Feeds, and Feeding of Tropical Aquatic Species, eds. O. Millamena, R. Coloso, F. Pascual (Tigbauan, Iloilo, Philippines: Aquaculture Department, Southeast Asian Fisheries Development Center) 149–168.
Tomas-Vidal, A., Monge-Ortiz, R., Jover-Cerda, M., and Martinez-Llorens, S. (2019). Apparent digestibility and protein quality evaluation of selected feed ingredient in Seriola dumerili. J. World Aquac. Soc. 50, 842–855. doi: 10.1111/jwas.12597
Traifalgar, R. F., Pagapulan, J., Valdez, M. T., Ellamar, J. B., Ocampo, E. J. C., and Ilag, L. L. (2019). Fermented sweet potato meal, a sustainable dietary protein ingredient for juvenile Penaeus vannamei Boone 1931. Isr. J. Aquac. - Bamidgeh 11. doi: 10.46989/001c.20973
Unnikrishnan, P., Kizhakkethil, B. P., George, J. C., Abubacker, Z. A., Ninan, G., and Nagarajarao, R. C. (2020). Antioxidant peptides from dark meat of yellowfin tuna (Thunnus albacares): process optimization and characterization. Waste Biomass Valor. 12, 1845–1860. doi: 10.1007/s12649-020-01129-8
Valente, L. M. P., Cabral, E. M., Sousa, V., Cunha, L. M., and Fernandes, J. M. O. (2016). Plant protein blends in diets for Senegalese sole affect skeletal muscle growth, flesh texture and the expression of related genes. Aquaculture 453, 77–85. doi: 10.1016/j.aquaculture.2015.11.034
Valle, B. C. S., Dantas, E. M., Silva, J. F. X., Bezerra, R. S., Correia, E. S., Peixoto, S. R. M., et al. (2015). Replacement of fishmeal by fish protein hydrolysate and biofloc in the diets of Litopenaeus vannamei postlarvae. Aquac. Nutr. 21, 105–112. doi: 10.1111/anu.12149
Vaz-Pires, P., Seixas, P., Mota, M., Lapa-Guimarães, J., Pickova, J., Lindo, A., et al. (2008). Sensory, microbiological, physical and chemical properties of cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii) stored in ice. LWT - Food Sci. Technol. 41, 1655–1664. doi: 10.1016/j.lwt.2007.10.003
Wei, Y., Li, B., Xu, H., and Liang, M. (2020a). Fish protein hydrolysate in diets of turbot affects muscle fibre morphometry, and the expression of muscle growth-related genes. Aquac. Nutr. 26, 1780–1791. doi: 10.1111/anu.13129
Wei, Y., Liang, M., and Xu, H. (2020b). Fish protein hydrolysate affected amino acid absorption and related gene expressions of IGF-1/AKT pathways in turbot (Scophthalmus maximus). Aquac. Nutr. 26, 145–155. doi: 10.1111/anu.12976
Wei, Y., Wang, J., Zhang, X., Duan, M., Jia, L., Xu, H., et al. (2021). Fish protein hydrolysate supplementationin plant protein based diets for tiger puffer (Takifugu rubripes) is an effective strategy of fish meal sparing. Aquac. Reports 20, 100720. doi: 10.1016/j.aqrep.2021.100720
Wu, M., Lu, S., Wu, X., Jiang, S., Luo, Y., Yao, W., et al. (2017). Effects of dietary amino acid patterns on growth, feed utilization and hepatic IGF-I, TOR gene expression levels of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 468, 508–514. doi: 10.1016/j.aquaculture.2016.11.019
Wullschleger, S., Loewith, R., and Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. doi: 10.1016/j.cell.2006.01.016
Xu, W., Yu, G., Xue, C., Xue, Y., and Ren, Y. (2008). Biochemical changes associated with fast fermentation of squid processing by-products for low salt fish sauce. Food Chem. 107, 1597–1604. doi: 10.1016/j.foodchem.2007.10.030
Yuan, X. Y., Liu, M. Y., Cheng, H. H., Huang, Y. Y., Dai, Y. J., Liu, W., et al. (2019). Replacing fish meal with cottonseed meal protein hydrolysate affects amino acid metabolism via AMPK/SIRT1 and TOR signaling pathway of Megalobrama amblycephala. Aquaculture 510, 225–233. doi: 10.1016/j.aquaculture.2019.05.056
Zhang, W., Tan, B., Deng, J., Yang, Q., Chi, S., Pang, A., et al. (2022). Soybean protein concentrate causes enteritis in juvenile pearl gentian groupers (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Anim Nutr. (in press). doi: 10.1016/j.aninu.2022.08.006
Keywords: alternative feed, black tiger shrimp, digestive enzymes, muscle growth, gut inflammation
Citation: Pan MV, Cadiz RE, Mameloco EJG and Traifalgar RFM (2022) Squid industry by-product hydrolysate supplementation enhances growth performance of Penaeus monodon fed plant protein-based diets without fish meal. Front. Sustain. Food Syst. 6:1027753. doi: 10.3389/fsufs.2022.1027753
Received: 25 August 2022; Accepted: 07 November 2022;
Published: 28 November 2022.
Edited by:
Janice Alano Ragaza, Ateneo de Manila University, PhilippinesReviewed by:
Indra Suharman, Riau University, IndonesiaSaichiro Yokoyama, Kagoshima University, Japan
Copyright © 2022 Pan, Cadiz, Mameloco and Traifalgar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rex Ferdinand M. Traifalgar, c2VwaWF6YW1ib2FuZ2FAZ21haWwuY29t
 Maila V. Pan
Maila V. Pan Rowena E. Cadiz1
Rowena E. Cadiz1 Rex Ferdinand M. Traifalgar
Rex Ferdinand M. Traifalgar