
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 22 November 2022
Sec. Sustainable Food Processing
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1023123
This article is part of the Research TopicSustainable Processing and Preservation of Underutilized Indigenous FoodsView all 7 articles
Chocolate is a well-liked and popular food product made from the cocoa bean. The objective of this research was to evaluate the effects of box fermentation and solar drying of cocoa bean on chocolate quality. Fermentation was carried out in a perforated wooden box for 168 h with periodic turning after every 48 h. The succession of microorganisms during fermentation and total microbial count were monitored. Both the fermented and unfermented samples were solar dried. During drying the change in weight of the beans, amount of solar radiation, and ambient wind speed of the atmosphere were measured. The approximate nutrient (crude protein, crude fat, total ash, crude fiber, and carbohydrate) and phytochemical (phenol) contents of the dried cocoa beans were evaluated. Sensory properties of chocolate, produced from the two samples (box fermented and dried as well as unfermented and dried) were compared. The initial temperature of the fermenting cocoa mash was 30°C. It rose to 46°C by the 96th h of fermentation and sharply declined to 38°C by the 120th h. Candida, Pseudomonas and Staphylococcus spp were probable organisms identified with the fermenting mass at the initial stage of the fermentation. Lactic acid bacteria dominated after 72 h. Bacillus spp was also observed until the 168th h. Solar drying of unfermented bean lasted for 4 days, while that of fermented bean lasted for 3 days. Fermentation increased the crude protein, total ash and carbohydrate contents of the cocoa beans. It also improved the appearance, and aroma of chocolate from the cocoa beans.
Chocolate is the most important universally popular product made from cocoa (Theobroma cacao). Cocoa is produced in Ivory Coast, Ghana, Indonesia, Nigeria, Cameroon, Brazil, Ecuador, and Mexico (Wood and Lass, 1985; ICCO, 2018). Ivory Coast and Ghana are responsible for 60% of the global production. Seventy percent of the world cocoa production is undertaken by small holder farmers in West Africa (Wessel et al., 2015). Despite its enormous potentials for improving the economy, the challenges facing the chocolate industry are numerous and include: low production of cocoa which is the main raw material (b) poor quality cocoa beans due to non-standardized fermentation and drying processes (c) infection by molds and other pests (d) long periods of natural fermentation (5–7 days) (González-Ríos et al., 2019), (e) cocoa farming is still a small holder concern in many developing countries and quality of the processed bean which is the raw material for chocolate production is poor. During on-farm processing, the pulp surrounding the seed naturally ferments (referred to as sweating) to produce a running liquid pulp. The methods of fermentation and drying determine the quality of the finished product.
Efforts to improve cocoa processing into chocolate have focused on (a) pulp reduction (Schwan and Wheals, 2004), (b) addition of enzymes during fermentation (Ganda-Putra et al., 2010) (c) optimizing the activities of intracellular enzymes during the natural fermentation of cocoa seeds (Jinap et al., 2003) (d) use of inoculums (Batista et al., 2016). The aroma of chocolate from cocoa bean depends on genotype (Qin et al., 2017), geographic origin (Marseglia et al., 2020), and postharvest processing (fermentation, drying, and roasting) (Mota-Gutierrez et al., 2018). The fermentation of cocoa is characterized by a microbial succession initiated by yeasts (Ardhana and Fleet, 2003), followed by lactic acid bacteria eventually, the acetic acid bacteria. Spore forming bacilli and filamentous fungi also participate (González-Ríos et al., 2019). It is the balance in the activities of the endogenous enzymes from these microorganisms (which include invertase, polyphenol oxidase, carboxypeptidase, α-galactosidase etc.) that are responsible for flavor characteristics of the chocolate produced. Despite the availability of many fermentation and drying technologies, there is limited research on improving the fermentation and drying methods of cocoa for good quality chocolate production targeted for small scale farmers who dominate the cocoa production industry in developing countries. Many cocoa farmers in developing countries still use fermentation and drying methods that do not encourage proper development of cocoa quality. They may keep the cocoa seeds in heaps on the ground and sundry the decorticated seeds. Such inappropriate treatments lead to poor quality cocoa products with decreased exportation rate and low domestic income (GDI). Box fermentation and solar drying have been selected for the present research since they are processing methods and technologies that could be easily adopted by small holder farmers in developing countries. The present research will explore the combined effects of box fermentation and solar drying of cocoa on chocolate quality. Figure 1A shows the cocoa pods used for experiments and Figure 1B shows the nature of the cocoa pulp that covers the seeds.
Freshly harvested ripe cocoa pods (mixed varieties of Trinitario and Forastero commonly known as DK39 and DK47) were purchased from a cocoa farm located at Umuariaga village, Ikwuano Local Government Area, Umudike, Abia State, Nigeria. Other materials used in the production and analysis were obtained from Ariara International market Aba, Abia State, Nigeria.
Wholesome cocoa pods obtained from the market were cut with a knife, weighed and poured into a wooden box (50 × 42 × 28) m. The box was covered with plantain leaves and jute bag to prevent heat loss and was perforated at the bottom. Fermentation of the cocoa beans took place for eight (8) days during which the beans were turned at intervals of every two (2) days to aid homogenization and distribution of heat within the fermenting mass.
The temperature of the fermenting mass was determined with a precision thermometer. The pH was determined according to the method of Senanayake et al. (1997), using a pH meter.
The method of Monica (2006) was used. Microbial sampling (2.5 g portions of the cocoa seed) was conducted on the initial, first, third, fifth, and seventh days of fermentation. Man Rogosa Sharpe (MRS) Agar, Nutrient Agar (NA) and Sabouraud Dextrose Agar (SDA) were prepared according to the manufacturer's instructions.
A quantity of 0.85 g of Sodium Chloride (NaCl) was put in 1,000 ml of distilled water, mixed vigorously and sterilized by autoclaving at 121°C for 15 min and was used as the saline solution. The stock solution for subsequent dilution was prepared from 2.5 g of the fermenting cocoa homogenized in 22.5 ml of normal saline. It was serially diluted to a strength of 10−1. Further serial dilution was carried out: 10−2, 10−3, 10−4, 10−5, 10−6, 10−7, 10−8, 10−9.
The prepared agars (Nutrient agar, Man Rogosa and Sharpe and Sabouraud Dextrose Agar), were poured aseptically in petri dishes, prepared in triplicates and labeled. One (1) ml of test extract from the different dilutions was taken and inoculated into each of the Petri dishes (containing the different types of agars) and spread using an L- shaped glass. The plate was covered and incubated at 37°C for 24-48 h. The process was repeated until the 8th fermentation day.
At the end of incubation each day, the number of colonies of microbes growing in the plates was counted using a colony counter. The morphological characteristics of the organisms were recorded. Identified organisms were isolated by sub culturing. Biochemical tests were carried out to confirm the probable organism present in the fermenting mass. This included the gram stain reaction; indole test; citrate test; catalase test and carbohydrate fermentation test.
The unfermented and fermented cocoa beans were dried using a solar dryer fabricated by the Department of Agricultural Engineering, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria (Eke, 2013) (Figure 2). The dryer was fabricated with aluminum rails, extended stainless steel sheets, air blower, solar panel, deep circle battery, marbles, aluminum and black oil paints. It has a dimension of 600 × 400 × 250 mm for the solar collector and 600 × 400 mm for the crop trays. The air plenum between the crop trays was 250 mm except for the topmost tray which had an air plenum of 300 mm between the blower and the tray. The dryer had a height of 1,500 mm and stood on a base 200 mm high (Figure 2).
The cocoa bean portions were weighed and placed in different layers in the drying chamber. The solar radiation was measured using a solar photometer, ambient temperature and wind speed were measured using an Anemometer, percentage relative humidity, chamber temperature and humidity were measured using an Arklon thermometer.
Atmospheric pressure and humidity were measured with a barometer. The results and the sample weights were recorded at 2 h intervals.
Proximate analysis was carried out for fermented and unfermented dried cocoa seeds using standard AOAC methods [Association of Official Analytical Chemist (AOAC), 2012]. The moisture content: crude protein, fat, crude fiber, total ash, and carbohydrate contents were determined. Carbohydrate content was determined by difference.
Where: M = moisture; P = protein; F = fat; A = ash.
The total phenolic content was analyzed using the Folin-Ciocalteu colorimetric method (Chlopicka et al., 2012).
Chocolate was produced from cocoa, using the method of Ndife et al. (2013) as shown in Figure 3. Cocoa bean seeds were removed from the pod, sorted, fermented for 8 days, dried (3–4 h), and roasted (120–140°C), for 45–90 min. After roasting, the beans were dehulled, and winnowed. The cotyledon was milled with sugar, milk and nutmeg as additives. It was conched for 45 min at 80°C, tempered and put in a mold (see Figure 3).

Figure 3. Production of chocolate bars from cocoa bean (Ndife et al., 2013).
The prepared chocolate was evaluated using a 9-point Hedonic scale (9 = like extremely; 1 = dislike extremely). Twenty semi-trained panelists comprising of students from the College of Applied Food Science and Tourism (CAFST), Michael Okpara University of Agriculture, Umudike, were randomly selected for the sensory evaluation. The appearance, flavor, texture, taste and general acceptability of the chocolate samples were evaluated. The samples were presented in a dish and labeled with 3-digit number codes. Each judge was given a glass of water at room temperature (28–30°C) to rinse the mouth after tasting to avoid interfering with the taste of the preceding product.
The design of the experiment was a Factorial in completely randomized design.
All data generated in this study were subjected to statistical analysis using the Statistical Package for Social Sciences (SPSS) version 21. The P-value was considered significant at 95% confidence level using Duncan New Multiple Range Test.
The highest mean temperature of the cocoa mass (46°C) was observed at the 96th h (Figure 4). The initial temperature of the fermenting mass was 30°C. There was a rise in temperature and a sudden decline at the 120th h. The fermentation process lasted for 168 h. This is in accordance with the work of Pedro et al. (2016) on cocoa fermentation. These researchers noticed a final fermentation temperature of 47–70°C after 168 h. Ganeswari et al. (2015), observed that early in the fermentation process the cocoa mass temperatures normally vary between 45 and 50°C. The steady increase in temperature is associated with the release of heat from cocoa biomass throughout the process. Initially, the yeasts were the dominant species that utilized the available fermentable substrate (sugar) transforming them into ethanol and eventually to acetic acid via microbial succession (Ganeswari et al., 2015). This conversion of fermentable sugars is exothermic and leads to increase of temperature (Schwan and Wheals, 2004). The mean cocoa mass temperature decreased after the 96th h due to inactivation of predominant bacteria at temperatures greater than 40°C and embryonic death caused by the penetration of acetic acid into the bean, favoring the development of chocolate flavor precursors (Kongor et al., 2016). This result was slightly different from that recorded by Liliane et al. (2015). Generally, the turning increases the temperature inside the bean mass during cocoa bean fermentation. The beans fermented using box covered with leaves had the highest temperature (48°C) due to the growth of microorganisms that assisted the conversion of sugar into ethanol. This explains why the temperature increases faster to optimum (48°C) for the turned beans compared to those were not turned. The observed result was in line to those reported by Guehi et al. (2010).
Table 1a showed that yeasts, initiated fermentation and were succeeded by lactic acid bacteria, followed by acetic acid bacteria. These microorganisms are responsible for the synthesis of related metabolites such as ethanol, lactate, acetate and volatile precursors (Misnawi and Teguh, 2010). Five major groups of micro-organisms have been observed to participate in cocoa fermentation (Binh et al., 2017). They include filamentous fungi, yeast, lactic acid bacteria, acetic acid bacteria and Bacillus spp (Binh et al., 2017). Table 1b shows the morphological characteristics of the microorganisms while Table 1c shows results of the biochemical tests using sugars. At the initial stage of fermentation yeast (Candida spp.), Pseudomonas spp. and Staphylococcus spp. were the probable organisms found in the fermenting mass. After 72 h they were succeeded by Lactic acid bacteria (e.g., Lactobacillus plantarum). Lactobacillus spp. was seen up till 120 h of fermentation, while Bacillus spp. was observed from the third day till the end of the fermenting period of 168 h. Liliane et al. (2015) made similar observations but did not detect Acetic Acid Bacteria (AAB) probably because they are non-tolerant to high temperatures (Schwan, 1995). The increase in population of Bacillus spp. is due to the increase in aeration, pH value of cocoa pulp and a rise in temperature to about 40°C in the cocoa mass during the later stages of fermentation (Schwan and Wheals, 2004). Cocoa fermentation removes mucilage and facilitates drying (Thompson et al., 2012). Fermenting cocoa beans attract many flying insects such as fruit flies (Drosophila melanogaster), beetles, bees, and wasps, which are potential vectors of microorganisms. Studies of box fermentations in Belize, Brazil, Côte d'Ivoire, Malaysia and Indonesia have confirmed that the yeast community associated with cocoa fermentations is complex involving several species like Saccharomyces cerevisiae and various Candida species (Ardhana and Fleet, 2003; Thompson et al., 2012). According to Nielsen et al. (2005), Camu et al. (2008) microorganisms that colonize the fermenting mass come from cocoa surface, hands of workers, knives, and fermenting boxes. This indicates the reason for the detection of Pseudomonas and Staphylococcus spp. in the fermenting mass. Many strains of Lactic acid bacteria and Bacillus species are generally regarded as safe (GRAS substances) and would not constitute any problem to food safety. Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans show pathogenicity of varying degrees. These organisms would not survive the subsequent drying and roasting treatments following the fermentation process. While it would not be safe to consume the fermenting beans, the end-product after roasting should be safe for consumption. Quality checks should be carried out at all critical control points to ensure microbial safety and wholesomeness of the products.
Microorganisms found in Sabouraud Dextrose Agar (SDA) and Rogosa Sharpe (MRS) Agar cultures, on the first day of fermentation were too numerous to count. Phenotypic identification showed that Candida and Pseudomonas species were predominant. Staphylococcus species was detected only in nutrient agar.
Figures 5, 6 show the graphs of time changes in relation to the temperature, loss in weight, ambient wind speed and relative humidity for unfermented and fermented cocoa beans. The initial drying rate was faster for the unfermented cocoa bean than the fermented one (within the first 1 h), while the drying rate was faster in the fermented cocoa beans within the 3rd and 4th h. Drying period of the fermented cocoa beans was shorter than that of the unfermented beans. The initial weight of the fermented cocoa was about 800 g and reduced to 500 g while that of the unfermented reduced from 500 to 230 g. Drying causes heat transfer into the cocoa beans making water and acetic acid to evaporate to the exterior of the seeds. By the time the moisture content is reduced to about 7% browning reactions occur and flavor compounds are developed (Irie et al., 2010). The solar dryer enhances the drying process and improves hygiene when compared with sun-drying.
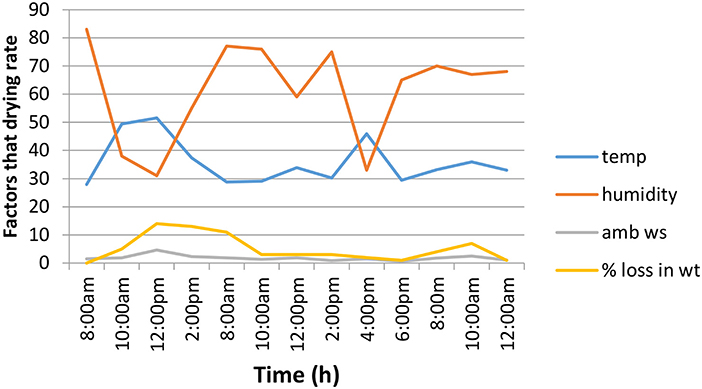
Figure 5. Changes during the solar drying of fermented cocoa beans. Temp, temperature (oC); Ambws, ambient wind speed (m/s); % loss in wt, % loss in weight; humidity (g/m3).
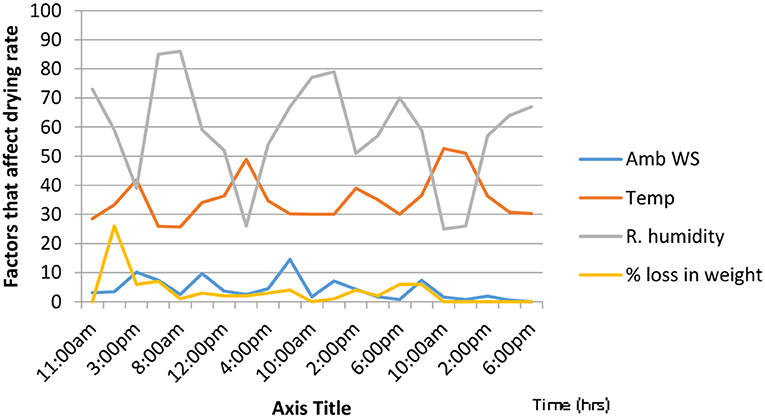
Figure 6. Changes during the solar drying of unfermented cocoa beans. Temp, temperature (oC); Ambws, ambient wind speed (m/s); % loss in wt, % loss in weight; humidity (g/m3).
The temperature, humidity and ambient wind speed of the atmosphere affected the rate of drying. The highest mean temperature of the cocoa mass (46°C) was observed at the 96th h (Figure 4). The initial temperature of the fermenting mass was 30°C. The result shows that the higher the temperature, the lower the relative humidity, the higher the ambient wind speed and the faster the drying rate as shown in Figure 6 in which the highest temperature of 51.5°C, observed at 12:00 noon had the lowest relative humidity of 31%, the highest weight loss of 14% and the highest ambient wind speed of 4.6 m/s. This effect of temperature, relative humidity and ambient wind speed on solar drying is in accordance with the findings of Ajadi and Sanusi (2013) and has been shown to affect the drying efficiency. Pictures of the cocoa beans at different processing stages are shown in Figure 7.
The pH of unfermented cocoa bean (Sample A) was 5.64 and was lower than that of the fermented sample (pH 6.59), showing a transformation from acidic to neutral condition. According to Guehi et al. (2010), an increase in pH (5.34–6.31) has been observed in cocoa fermentation worldwide. However, the acid pH at the beginning of fermentation depends primarily on the presence of citric acid in cocoa beans mucilage (Lagunes-Galvez et al., 2007). The alkaline pH reached at the end of process was also reported by Ouattara et al. (2008).
The phenol content of fermented cocoa beans (2.765 mM gallic acid equivalent) was lower than that of the unfermented (4.61 mM GAE) bean (Figure 8). Similar results were obtained by Djikeng et al. (2018). This decrease is because of hydrolysis and oxidation by the enzyme polyphenol oxidase during fermentation (Abdularmir et al., 2012). The European Food Safety Authority (EFSA) (2012) has opined that “cocoa flavanols help maintain endothelium-dependent vasodilation, thereby promoting normal blood flow when consumed at 200 mg of cocoa daily [European Food Safety Authority (EFSA), 2012]. Cocoa polyphenols possess antioxidant, antiradical and anti-carcinogenic properties (Othman et al., 2007; Belscak et al., 2009). Cacao consumption promotes heart health.
The moisture contents of dried fermented and unfermented cocoa beans are shown in Figure 9. Fermentation time affected the moisture content of the processed cocoa beans. Asiedu (1989) had observed that the moisture content of processed cocoa beans fluctuates depending on the fermentation and drying conditions. The moisture content was in line with the acceptable limit of 6–7 % although sample B (dried box fermented cocoa bean) was slightly higher. The crude protein content of sample A (dried unfermented cocoa bean) (3.76%) was significantly lower than that of sample B (8.93%). Similar results were recorded by Ndife et al. (2013) and this could be as a result of fermentation.
The fat content of sample A (dried unfermented cocoa bean) (20. 35%) was significantly higher than that of sample B (dried box fermented cocoa bean) (11.54%) (Figure 9). This result was in line with that observed by Ndife et al. (2013). The crude fiber of sample A (dried unfermented cocoa bean) (14.83%) was significantly higher than that of sample B (dried box fermented cocoa bean) (1.97%), this was also observed by Adegunloye and Famolu (2016). Carbohydrate content was significantly higher in sample B (dried box fermented cocoa bean) (61.72%) compared to sample A (48.29%). Similar results were given by Adegunloye and Famolu (2016), indicating that fermentation increases the carbohydrate content of cocoa beans. The ash content of sample B was greater than that of sample A. These results agree with the work of Adegunloye and Famolu (2016). The nutritional benefits of cocoa and chocolate are numerous. It is a natural storehouse of nutrients, when compared with many other food items. The cocoa fruit contains 40% pulp on fresh weight basis and is made up of a spongy parenchymatous tissue containing cell sap, which is rich in sugars (10–13% glucose + fructose; 0.7% sucrose), salts (8–10%), pentosans (2–3% pectin), organic acids (1–2%) and 0.6% proteins (Schwan and Wheals, 2004).
The results in Table 2 show the sensory properties of chocolate produced from solar dried box fermented (B) and unfermented (A) cocoa beans. The result showed that sample B was preferred in all the attributes. Sample B was “Liked Very Much” on the 9-point hedonic scale while sample A was “Neither Liked, Nor Disliked” Flavor compounds are often properly developed during the turning of fermenting cocoa beans (Velasquez-Reyes et al., 2021). Figures 10A,B show that the chocolate bars produced from properly fermented and dried cocoa beans are darker in color, had a more appealing aroma and were more homogenous in texture than those produced from unfermented cocoa beans.
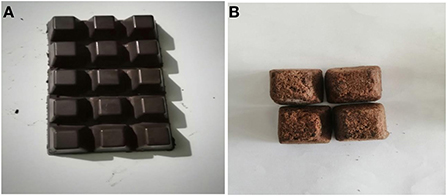
Figure 10. (A) Chocolate from solar-dried, box fermented cocoa beans. (B) Chocolate from unfermented cocoa bean.
The effect of box fermentation and solar drying on the quality of chocolate from cocoa beans were evaluated in this research. The major microorganisms involved in cocoa fermentation were fungi, lactic acid bacteria and bacillus spp. Box fermented solar dried cocoa bean had better nutritional attributes than unfermented cocoa beans and resulted in the production of better-quality chocolate. Fermentation increased the crude protein, carbohydrate and ash contents, but reduced the crude fiber, fat and phenol contents of the cocoa beans. Drying caused 37.5% reduction in weight of the unfermented cocoa bean and 54% weight reduction in the fermented cocoa bean. Chocolate produced from the fermented cocoa bean was dark and had a strong aroma while that from unfermented cocoa bean was lighter in color and had a less desirable flavor. The use of wooden box fermentation, complemented with solar drying (which is cost effective) should be adopted in developing countries in order to produce good quality chocolate from cocoa.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
SO carried out the laboratory analyses and wrote the original draft as thesis. PO conceptualized the research and its methodology, participated in the analyses, data curation, and writing of the manuscript. BE fabricated the local solar dryer and handled some aspects of the methodology and data analysis. All authors contributed to the article and approved the submitted version.
Some experiments in this research were financially supported by TETFund a research grant from the Tertiary Education Fund, Nigeria. Personal funds were used to carry out other aspects of the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdularmir, D. N. M., Khatib, A., and Sabu, M. C. (2012). Antioxidant activity of different extracts from leaves of Pereskia Bleo (cactaceac), J. Med. Plant Res. 6, 2932–2293. doi: 10.5897/JMPR11.760
Adegunloye, D. V., and Famolu, O. F. (2016). Influence of fermentation on the nutritional content of cocoa pods. (Theobroma cacao L). J. Global. Biosci. 5, 3408–3413.
Ajadi, D. A., and Sanusi, Y. K. (2013). Effect of relative humidity on oven temperature of locally designed solar cabinet dryer. Global J. Sci. Frontier Res. Physics Space Sci. 13, 2249–4626. https://journalofscience.org/index.php/GJSFR/article/view/713
Ardhana, M. M., and Fleet, G. H. (2003). The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 86, 87–99. doi: 10.1016/S0168-1605(03)00081-3
Asiedu, J. J. (1989). Processing Tropical Crop-a-Technological Approach. London and Basingstoke: Macmillian Education Ltd., 24–42.
Association of Official Analytical Chemist (AOAC) (2012). Official Methods of Analysis. 20th Edn. Washington, DC.
Batista, N. N., de Andrade, D. P., Ramos, C. L., Dias, D. R., and Schwan, R. F. (2016). Antioxidant capacity of cocoa beans and chocolate assessed by FTIR, Food. Res. Intl. 90, 313–319. doi: 10.1016/j.foodres.2016.10.028
Belscak, A., Komes, D., Horzie, D., Kovacevic, G., and Karlovic, D. (2009). Comparative study of commercially available cocoa products in terms of their bioactive composition. J. Food Res. Intl. 42, 707–716. doi: 10.1016/j.foodres.2009.02.018
Binh, P. T., Tru, N. V., Dung, V. T. T., Thoa, N. T., and Thao, P. V. (2017). Bacteria in wooden box fermentation of Cocoa in Daklak, Vietnam. J. Microbiol. 5, 21–64. doi: 10.15406/jmen.2017.05.00176
Camu, N., Gonzalez, A., DeWinter, T., Van Schoor, A., De Bruyne, K, Vandamme, P., et al. (2008). Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. J. Appl. Environ. Microbiol. 74, 86–95. doi: 10.1128/AEM.01512-07
Chlopicka, J., Pasko, P., Gorinstein, S., Jedryas, A., and Zagrodzki, P. (2012). Total phenolic and flavonoid content, antioxidant activity and sensory evaluation. J. Food Sci. Technol. 46, 548–555. doi: 10.1016/j.lwt.2011.11.009
Djikeng, F. T., Teyomnou, W. T., Tenyang, N., Tiencheu, B., Morfor, A. T., Touko, B. A. H., et al. (2018). Effect of traditional and ovenroasting on thephysicochemical properties offermented cocoa beans. Heliyon 4, e00533. doi: 10.1016/j.heliyon.2018.e00533
Eke, B. A. (2013). Development of small-scale direct mode natural convection solar dryer for tomato, okra and carrot. Intl. J. Eng. Tech. 3, 199–204.
European Food Safety Authority (EFSA) (2012). Panel on dietetic products, nutrition and allergies (NDA). Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 10, 2809. doi: 10.2903/j.efsa.2012.2809
Ganda-Putra, G. P., Wrasiati, L. P., and Wartini, N. M. (2010). Fermentation process of cocoa based on optimum condition of pulp pectin depolymerization by endogenous pectolityc enzymes. Pelita Perkebunan. 26, 122–132. doi: 10.22302/iccri.jur.pelitaperkebunan.v26i2.130
Ganeswari, I., Khairul, B. S., Amizi, M. A., and Sim, K. Y. (2015). Effects of different fermentation approaches on the microbiological and physicochemical changes during cocoa bean fermentation. Int. Food Res. J. 22, 70–76.
González-Ríos, O., Cocolin, L., and Suárez-Quiroz, M. L. (2019). (2019). The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 301, 41–50. doi: 10.1016/j.ijfoodmicro.2019.05.002
Guehi, S. T., Zahoulli, I. B., Bankotti, L., Fae, M. A., and Namlin, G. J. (2010). Performance of different drying methods and their effects on the chemical quality attributes of raw cocoa material. Int. J. Food Sci. Technol. 45, 1564–1571. doi: 10.1111/j.1365-2621.2010.02302.x
ICCO (2018). International Cocoa Organization0 Abidjan, Cote d'Ivore. International Cocoa Organization
Irie, B. Z., Guehi, S. T., Fae, A. M., Ban-Koffi, I. L., and Nemlin, J. G. (2010). Effect of drying methods on the chemical quality traits of cocoa raw material. Adv. J. Food Sci. Technol. 2, 184–190.
Jinap, S., Jamilah, B., and Nazamid, S. (2003). Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. Int. J. Food Sci. Technol. 38, 285–295. doi: 10.1046/j.1365-2621.2003.00674.x
Kongor, J., Hinneh, M., Walle, D., Afoakwa, E., Boeckx, P., and Dewettinck, K. (2016). Factors influencing quality variation in cocoa (Theobroma cacao) bean Flavour profile. J. Food Res. Int. 82, 44–52. doi: 10.1016/j.foodres.2016.01.012
Lagunes-Galvez, S., Loiseau, G., Paredes, J. L., Barel, M., and Giraud, J. P. (2007). Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 114, 124–130. doi: 10.1016/j.ijfoodmicro.2006.10.041
Liliane, M. K. G., Goualie, J. N., Adom, G. K. H., Ouattara, G., and Sebastien, L. N. (2015). Diversity of microbial strain involved in cocoa fermentation rom Sud-Comoe (Cote D' Ivoire) Based on biochemical properties. European Sci. J. 11, 1857–7881. https://eujournal.org/index.php/esj/article/view/5822
Marseglia, A., Musci, M., Rinaldi, M., Palla, G., and Caligiani, A. (2020). Volatile fingerprint of unroasted and roasted cocoa beans (Theobroma cacao L.) from different geographical origins. Food Res. Int. 132, 109101. doi: 10.1016/j.foodres.2020.109101
Monica, C. (2006). District Laboratory Practice in Tropical Countries. 2nd Edn. Hong kong: SheckWah Tong Printing Press Ltd.
Mota-Gutierrez, J., Botta, C., Ferrocino, I., Giordano, M., Bertolino, M., Dolci, P., et al. (2018). Dynamics and biodiversity of bacterial and yeast communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 84, 1–17. doi: 10.1128/AEM.01164-18
Ndife, J., Bolaji, P., Atoyebi, D., and Umezurike, C. (2013). Production and quality evaluation of cocoa. Amer. J. Food Nutr. 3, 31–38.
Nielsen, D. S., Hønholt, S., Tano-Debrah, K., and Jespersen, L. (2005). Yeast populations associated with Ghanaian cocoa fermentations analysed using denaturing gradient gel electrophoresis (DGGE). Yeast 22, 271–284. doi: 10.1002/yea.1207
Othman, A., Ismail, A, Abdul, G., and Adenan, I. (2007). Antioxidant capacity and phenolic content of cocoa beans. J. Food Chem. 4, 1523–1530. doi: 10.1016/j.foodchem.2005.12.021
Ouattara, H. G., Koffi, B. L, Karou, G. T., Sangare, A., Niamke, S. L., and Diopoh, J. K. (2008). Implication of Bacillus sp. in the production of pectinolytic enzymes during cocoa fermentation. World J. Microbiol. Biotech. 24, 1753–1760. doi: 10.1007/s11274-008-9683-9
Pedro, P. P., Gueras, S., and Contreras, D. (2016). Changes in physical and chemical characteristics of fermented cocoa (Theobroma cacao) beans with manual and semi-mechanized transfer, between fermentation boxes. Scientia Agropecuaria 07, 111–119. doi: 10.17268/sci.agropecu.2016.02.04
Qin, X. W., Lai, J. X., Tan, L. H., Hao, C. Y., Li, F. P., He, S. Z., et al. (2017). Characterization of volatile compounds in Criollo, Forastero, and Trinitario cocoa seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 20, 2261–2275. doi: 10.1080/10942912.2016.1236270
Schwan, R. F. (1995). Microbial fermentation of cocoa beans, with emphasis on enzymatic degradation of the pulp. J. Appl. Bacteriol. 79, 96–107.
Schwan, R. F., and Wheals, A. E. (2004). The microbiology of cocoa fermentation and its role in chocolate quality. Critical Rev. Food Sci. Nutr. 44, 205–221. doi: 10.1080/10408690490464104
Senanayake, M., Jansz, E., and Buckle, K. (1997). Effect of different mixing intervals on the fermentation of cocoa beans. J. Sci. Food Agric. 74, 42–48.
Thompson, S. S., Miller, K. B., Lopez, A. S., and Camu, N. (2012). “Cocoa and coffee,” in Food Microbiol. Fundamentals and Frontiers, eds M. J. Doyle and L. R. Beuchanan (Washington, D.C.: ASM Press), 721–733. doi: 10.1128/9781555818463.ch35
Velasquez-Reyes, D., Gschaedler, A., Kirchmayr, M., Avendano, A. C., Rodríguez-Campos, J., Calva-Estrada, S. J., et al. (2021). Cocoa bean turning as a method for redirecting the aroma compound profile in artisanal cocoa fermentation. Heliyon 7, e07694. doi: 10.1016/j.heliyon.2021.e07694
Wessel, M., Foluke, P.M, and Wessel, Q. (2015). Cocoa production in West Africa, a review and analysis of recent developments. NJAS - Wageningen J. Life Sci. 75, 1–7. doi: 10.1016/j.njas.2015.09.001
Keywords: cocoa beans (Theobroma cacao L.), box fermentation, solar drying, succession of microorganisms, nutrient composition and phenolic content, sensory properties, chocolate
Citation: Obinze S, Ojimelukwe PC and Eke BA (2022) Box fermentation and solar drying improve the nutrient composition and organoleptic quality of chocolate from cocoa beans. Front. Sustain. Food Syst. 6:1023123. doi: 10.3389/fsufs.2022.1023123
Received: 19 August 2022; Accepted: 24 October 2022;
Published: 22 November 2022.
Edited by:
Samuel Ayofemi Olalekan Adeyeye, Hindustan University, IndiaReviewed by:
Abiodun Aderoju Adeola, Federal University of Agriculture, Abeokuta, NigeriaCopyright © 2022 Obinze, Ojimelukwe and Eke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippa C. Ojimelukwe, cGhpbGlwcGFjbzYwQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.