
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 19 January 2023
Sec. Crop Biology and Sustainability
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1015315
This article is part of the Research Topic Elucidating the roles of plant-microbe interactions, biological control and plant breeding in promotion of soil health and sustainable crop production systems View all 5 articles
 Florence Mutave Munguti1,2*
Florence Mutave Munguti1,2* Evans Nyaega Nyaboga3
Evans Nyaega Nyaboga3 Dora Chao Kilalo1
Dora Chao Kilalo1 Hillary Kipkoech Yegon2
Hillary Kipkoech Yegon2 Isaac Macharia2
Isaac Macharia2 Agnes Wakesho Mwango'mbe1
Agnes Wakesho Mwango'mbe1Cassava productivity is threatened by viral diseases which have become the main phytosanitary problems in cassava farmers. Cassava brown streak disease (CBSD) is a devastating viral disease caused by Cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) which are transmitted by whitefly vectors and mainly disseminated through the use of infected stem cuttings as planting materials. The aim of the current study was to: (1) assess farmers' knowledge, perceptions on spread, causes and current management practices of CBSD; (2) determine the factors associated with farmers' satisfaction with cassava planting material; and (3) determine the distribution, incidence, and severity of CBSD and association of factors influencing the disease epidemics in smallholder cassava cropping systems in coastal Kenya. Information was collected using semi-structured questionnaire administered to 250 smallholder farmers through face-to-face interviews coupled with field visits to assess the incidence, severity and distribution of CBSD. Symptomatic and asymptomatic cassava leaf samples were collected for reverse transcription-polymerase chain reaction (RT-PCR) analysis of the causal viruses of CBSD. The results revealed that majority of the farmers (96.6%) could recognize CBSD symptoms on the roots, and only 11.5% could recognize the foliar symptoms of the disease. The cause of the disease was unknown to the farmers, with no effective management methods available to them. Majority of farmers (82.5%) recycled own cassava cuttings from previous season's crop as planting material followed by exchanging/borrowing from neighbors (67.5%). The field incidence of CBSD was highest in Kilifi (27.9%) followed by Kwale (24.7%) and Taita Taveta (10.8%), with severities ranging from 2 to 3 in the three Counties. RT-PCR analysis indicated that 91% of the symptomatic samples tested positive for either of the two viruses occurring either singly or as dual infection. Approximately 3.2% of the asymptomatic samples tested positive for only CBSV. Findings from this study demonstrates the need for awareness creation of farmers on the causes, spread and management practices to control CBSD and the importance of strengthening certified cassava seed systems to reduce the impact of the disease. The study provides base-line information imperative for development of management strategies of CBSD.
Cassava (Manihot esculanta Crantz), is an important food security crop for close to one billion people in the tropical and sub-tropical countries especially sub-Saharan Africa where it is grown mostly by smallholder farmers [Food and Agricultural Organization of the United Nations (FAOSTAT), 2018]. Africa produces more than 60% of global cassava production (http://faostat3.fao.org/). In Kenya, cassava is the second most important crop after maize especially in western and coastal regions of Kenya where it accounts for 63 and 30% of the total production, respectively (Mwango'mbe et al., 2013; Were et al., 2016). Cassava farming in Kenya is practiced on ~90,394 hectares of land throughout the country producing on average about 1,112 000 MT per year [Food and Agricultural Organization of the United Nations (FAOSTAT), 2018]. Cassava has high productivity per unit land area than other staple food crops such as maize and wheat and can stay in soil during adverse climatic conditions (Jarvis et al., 2012; Tumuhimbise et al., 2014). This makes it a food security crop and provides income for many smallholder farmers. The current cassava yield in Kenya stands at 12.3 metric tons per hectare compared to the potential yield estimates of 40 metric tons per hectare [Food and Agricultural Organization of the United Nations (FAOSTAT), 2020]. This yield gap is due to an array of biotic and abiotic constraints (Ntawuruhunga et al., 2013).
Cassava production is constrained by viral diseases, the main one and economically important is cassava brown streak diseases (CBSD) (Legg et al., 2015; Neuenschwander and Tamò, 2019). Cassava brown streak disease (CBSD) has been so far reported in sub-Saharan Africa, with recent studies indicating a widespread of the disease in Uganda, Kenya, Malawi, Burundi, Rwanda, and some parts of Democratic Republic of Congo (Hillocks and Maruthi, 2015; Chipeta et al., 2016; Koima and Orek, 2018; Munganyinka et al., 2018). The disease is caused by two genetically distinct virus species namely Cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) belonging to Genus Ipomovirus of the family Potyviridae (Monger et al., 2010; Winter et al., 2010; Mbanzibwa et al., 2011). The viruses are transmitted by whitefly (Bemisia tabaci) and spiraling whitefly in a semi-persistent manner (Maruthi et al., 2005; Mware et al., 2009; Wosula et al., 2017; Chen et al., 2019). The disease is majorly disseminated through movement and exchange of infected cassava planting material over long distances and by the whitefly vectors over short distances (Maruthi et al., 2005, 2017; Wosula et al., 2017; Chen et al., 2019).
Cassava production and productivity in Kenya is adversely impacted by cassava brown streak disease (Masinde et al., 2016). The disease causes economic losses resulting from damage to the aboveground parts of the plant characterized by leaf chlorosis and elongated necrotic lesions on stems (Winter et al., 2010). The major economic damage arises from the necrotic rotting of cassava roots, which reduces nutritional and industrial quality and renders the roots unpalatable and unmarketable (Hillocks and Jennings, 2003; Winter et al., 2010). Yield losses associated with CBSD infection was previously estimated at 70% per plant (Hillocks et al., 2001), but recent records indicated yield losses of up to 100% in susceptible cultivars (Rwegasira and Rey, 2009; Mbanzibwa et al., 2011). The disease has been reported to affect all the cassava mosaic disease (CMD)-resistant varieties that have been widely adopted for the management of the CMD epidemics in Kenya (Masinde et al., 2016).
The effectiveness of any disease management strategy depends on adequate information on the occurrence, distribution, patterns of spread, impact and awareness of the disease amongst the farmers in a particular region. Previous surveys on occurrence and distribution of viral diseases of cassava in Kenya have reported limited information on the distribution of the two Ipomoviruses occurring either singly or as a co-infection (Mware et al., 2009). In addition, these surveys did not incorporate farmers' awareness and perceptions on CBSD and other factors that could be associated with the disease spread. A survey conducted by Kathurima et al. (2016) in the major cassava growing regions in Kenya revealed the occurrence of dual infection of CBSV and UCBSV although the study was based on relatively few samples and did not cover Taita Taveta County in the Coastal region. With the changes in farming practices, recent reports on dymamics of the spread of the viruses especially UCBSV from highlands to low altitude areas (Mbanzibwa et al., 2011), as well as increased cases of co-infection of the two viruses, there is need for frequent surveys to establish the status of the distribution of the viruses in order to inform control and management strategies to curb the disease. This study was therefore conducted to: (1) assess farmers' knowledge, perception and current management practices of CBSD, and (2) determine the distribution, incidence, severity of CBSD and association of factors influencing disease epidemics in smallholder cassava cropping systems of coastal Kenya.
The survey was conducted between May and July 2018 in three counties (Kilifi, Taita-Taveta, and Kwale) in coastal region of Kenya. These three counties are among the 29 Arid and Semi-Arid Land (ASAL) counties in Kenya. In general, the coastal region of Kenya has rainfall ranging from 500 to 100 mm annually, temperature ranges between 22.4 and 30.3°C, altitude of 900–1,800 m and savanna grassland (https://infonet-biovision.org/agro_ecologic_zones). Taita-Taveta County is among the Kenya's ASAL regions with 89% of the area characterized by semi-arid and arid conditions. The county altitude ranges from 500 to 2,228 m above sea level and receives bimodal rainfall ranging between 440 mm per annum in lowlands and over 1,900 mm per annum in highland areas.
Kilifi County covers a geographical area of 12,539.7 km2 and has a temperature range of 21°C during the coldest months (June—July) and 32°C during the hottest months (January–February). It has two rainy seasons, April–June (long rains) and October–December (short rains) with annual rainfall ranging between 900 mm and 1,000 mm per annum. The attitude of Kilifi County ranges from 0 to 450 m above sea level. Kwale County is divided into different agro-ecological zones and has moderately hot and dry climate throughout the year. The average temperature is >23°C throughout the county with areas along the coast generally above 25°C annually. There has been significant changes and variations in climatic conditions over the past years in the county, affecting agricultural production and livelihoods in most Counties in the coastal region of Kenya.
The baseline survey to assess farmers' awareness, perceptions and management of cassava brown steak disease was conducted in Kilifi and Taita Taveta counties between the months of May–July in 2018. The study employed three stage sampling design targeting Kilifi and Taita Taveta due to their different agroecological zones as well as different farming patterns with the sub-counties as the administrative units. The interviewed farmers were purposively sampled focusing on those who cultivated cassava. The sample size was calculated following a formula adapted from Krejcie and Daryle (1970). The sample size used in this study was also based on other related studies (Tirra et al., 2019). Smallholder farmers were selected for each county through a stratified random sampling whereby a stratum was cassava farmers with at least cassava crop at the time of the survey.
A semi-structured questionnaire was administered to the respondents through face-to face interviews. The information captured included socioeconomic characteristics such as age, gender, level of education as well as household size. Data was also collected on the cassava varieties grown in the area in the order of preference, the acreage, the sources of cassava planting materials, general challenges faced by the farmers, whether the farmer had received information on cassava and the sources of the information. A total of 250 cassava farmers were chosen at random, 125 from each of the two counties (Kilifi and Taita-Taveta). Colored photographs showing CBSD symptoms on leaves, roots and stem as well as on different pests and symptoms of pest infestation were shown to the farmers to determine their ability to recognize the disease symptoms and the farmers' knowledge and perceptions on the cause of the disease, spread and management.
County Agricultural extension officers who had clear information about the study sites identified the farmers or farmer groups interviewed. Prior to the baseline survey, the questionnaire had been pre-tested on a small group of farmers during Focus Group Discussions and adjustments were made to ensure the content was clear and valid. Data was captured in the questionnaires and recorded for analysis using SPSS and Microsoft Excel. Field visits were undertaken for incidence and severity assessment and sample collection for laboratory confirmation of its causal viruses. A total of 250 respondents were interviewed in the two counties of Kilifi and Taita-Taveta.
During the survey, semi-structured interviews were followed by fields' visits to assess the disease incidence, severity and distribution. The sampled farms were selected at regular intervals of 5–10 km along motorable roads. Once in the field, the farm was assessed in an “X”-shaped transect sampling pattern for assessment of foliar symptoms for incidence and severity of cassava brown streak disease. Thirty cassava plants per farm along the diagonals of the “X” transect were selected for assessment of presence or absence of CBSD symptoms. The targeted symptoms ranged from slight leaf feathery chlorotic blotches along the margins of veins to severe chlorosis on leaves and stem lesions. Where possible, cassava plants showing foliar symptoms were uprooted for assessment of any root necrosis. Disease severity was recorded using a scale of 1–5 according to Gondwe et al. (2003), where; 1 = no apparent symptoms; 2 = slight leaf feathery chlorosis with no stem lesions; 3 = pronounced leaf feathery chlorosis, mild stem lesions and no dieback; 4 = severe leaf feathery chlorosis, severe stem lesions and no dieback; and 5 = defoliation, severe stem lesions and dieback.
Incidence of the CBSD was recorded as the percentage of plants showing CBSD symptoms to the total number of plants inspected in the field using a scoring scale adopted from Nono-Womdim et al. (1996) where; 1%−20% was rated as low incidence, 21%−49% as moderate incidence and 50%−100% as high incidence. The information on incidence, severity scores, the name of the cultivars, size of the farm, the crop intercropped where necessary as well as any insect vectors observed was captured using Online Data Kit (ODK) software on a smart phone as per a preset questionnaire already input in the Application software. Photographs of samples showing symptoms as well as asymptomatic samples were taken in the farms visited. GPS coordinates for the farms were also recorded as part of the data filled in the ODK online Collect application.
In every cassava farm visited, leaf samples with viral symptoms where possible were collected for laboratory analysis to confirm the presence of CBSD infection. Sampling for the cassava leaf materials for disease diagnosis in the fields incorporated Kwale County due to its significance in cassava production in coastal region of Kenya. Leaf samples with feathery chlorosis, typical of cassava brown streak disease viral symptoms were collected. Where possible, the roots were harvested to check for any necrotic lesions. Clear labeling of the sample details was indicated per each sample collected. A total of 256 leaf samples (100 symptomatic and 156 asymptomatic) were collected in the three counties (Kwale, Kilifi, and Taita-Taveta). The leaf samples were pressed in between sheets of newsprint (in herbarium press) and shipped to the laboratory for analysis.
Cassava leaf samples previously collected in herbarium press were kept at room temperature until sufficiently dry for RNA extraction. Cetyltrimethyl ammonium bromide (CTAB) method for total nucleic acid extraction as described by Xu et al. (2010) and modified from Lodhi et al. (1994) was used for RNA extraction from the dried leaf samples. One hundred milligram of each of the cassava leaf samples were ground in 2 ml of CTAB extraction buffer (pre-heated at 65°C). Eight hundred microliter of the liquid sap was transferred into a micro tube and incubated for 30 min at 65°C with mixing by inversion after every 10 min. The tubes were left to stand at room temperature for 10 min. Eight hundred microliter chloroform was added to each tube followed by gentle voltexing.
The samples were centrifuged for 10 min at 13,000 rpm at 4 °C. The upper aqueous phase was transferred into a clean 1.5 ml microfuge-tube placed on ice. Chloroform: isoamyl-alcohol (24:1; 650 μl) was added vortexed and centrifuged at 13,000 rpm at 4°C for 10 min. Five hundred microliters of the supernatant was transferred to another clean tube and 0.7 volume of cold isopropanol (pre-stored at −20°C) added and inverted gently for 1 min. The RNA was precipitated at −20°C for 15 min. Centrifuging at 13,000 r.p.m for 10 min, 4°C followed and the isopropanol was decanted. Five hundred microliters of 70% ethanol was added and washed by tapping the tubes. This was followed by centrifuging at 16,000 r.p.m for 1 min at 4°C. Excess ethanol was decanted and the pellet dried for 15 min under a clean fume hood.
The pellet was re-suspended in 50 μl RNAse-free water. Genomic DNA contamination was removed by digestion with DNAse 1 from Quick-RNA Plant Miniprep Kit (Catalog No. R2024) following the manufacturer's instructions. The quantity and concentration of the RNA extracted from the cassava leave samples was estimated using NanodropONE (Thermo Scientific, Wilmington, DE, USA). These measurements were based on the optical density (OD) readings ratio at 260 and 280 nm. Samples with the ration of 2 and above were selected. The integrity of RNA was also assessed using 1% denaturing agarose gel electrophoresis. The gels were visualized under UV-transiluminator (Azure biosystems (C200). The RNA was stored at −80°C awaiting further downstream analysis.
Complementary DNA synthesis was carried out using CBSV and UCBSV-specific primers. Briefly, Complementary DNA synthesis was carried out in 20 μl reactions containing 2 μl total RNA, 1 × M-MLV reaction buffer (Promega, WI, USA), 0.5 mM each dNTP, 0.5 μM oligo (dT) primer, 0.5 μM random N primer and 200 units M-MLV reverse transcriptase (Promega). A mixture of RNA and primer was heated to 70°C for 5 min then placed on ice, after which the remaining components were added and the reactions incubated at 25°C for 5 Min, 42°C for 60 min and finally at 80°C for 3 min.
Reverse transcription polymerase chain reaction (RT-PCR) was carried out using the protocol described by Winter et al. (2010). This was done to detect the viruses in the samples using the specific primers to distinguish the two strains namely (CBSV; Forward primer; 5′GTACGTGCCTCCATCACAT3′, Reverse primer 5′CTCAACAGCTCTCCACGATTT3′ amplifying a 493 base pair region and for (UCBSV; Forward primer 5′AACAGACATACGTGTGCAT3′ with Reverse primer 5′ATTTCCAGGTTCCTTTGTCACT3′ with expected band size of 176 base pair targeting the coat protein region.
The cycling conditions were initial denaturation at 95°C for 3 min followed by 35 cycles of 30 s at 95°C, 60 s at 53°C and 60 s at 72°C and a final extension step of 72°C for 10 min. The PCR amplifications were done in an Applied Biosystems 9700 thermocycler (Applied Biosystems, Foster City, CA, USA). The PCR products were electrophoresed in 1.5% agarose gel pre-stained with Gel red (Biotium Hayward, CA, and USA) in a 1 × TAE buffer. A DNA ladder (ThermoFisher Scientific) was added to estimate the expected amplicon size. Positive and negative controls were included in every assay. Visualization of electrophoresed products was done under C280 gel doc (Azure Biosytems).
Data on baseline survey collected from the farmer interviews were encoded and analyzed using Statistical Package for Social Sciences version 20.0 statistical software (SPSS, Inc. 2011). Statistics (frequencies, percentages and means) were used to generate summaries and tables per the counties. The significance level was set at p = 0.05 and means separated using least significant difference (LSD). The data was summarized using cross tabulations. The bivariate Pearson correlation was done to show the strength and direction (i.e., positive/negative) of linear relationship between the socio-economic factors (i.e., age, farm size education and sex) and CBSD awareness in the different counties surveyed. The information was analyzed using descriptive statistics and presented in tables and bar graphs. Disease severity data was analyzed by computing the percentages of samples testing positive to CBSV, UCBSV (%) and a combination of both CBSV and UCBSV.
Of the 250 cassava farmers surveyed, 133 (53.4%) were females and 117 (46.6%) were males (Table 1). The gender of the farmers surveyed varied significantly (p < 0.001) between females and males. Kilifi County recorded significantly (p < 0.001) higher female (66.9%) respondents compared to male (33.1%), whereas in Taita-Taveta County, the female respondents (60%) were significantly higher than male respondents (40%). Majority (43%) of the farmers were between 36 and 50 years of age. About one out of five (20.8%) of the farmers had no formal education. Among the respondents with formal schooling were at various education levels: 53.6, 20.4, and 5.2% at the primary, secondary and college levels, respectively. Majority of the respondents were small-scale farmers as evidenced with farm size ranging from 2 to 5 ha as per information received from 48% of respondents. On average, the size of the farm under cassava production ranged from 0.5 to 1 ha as per 39.2% of respondents.
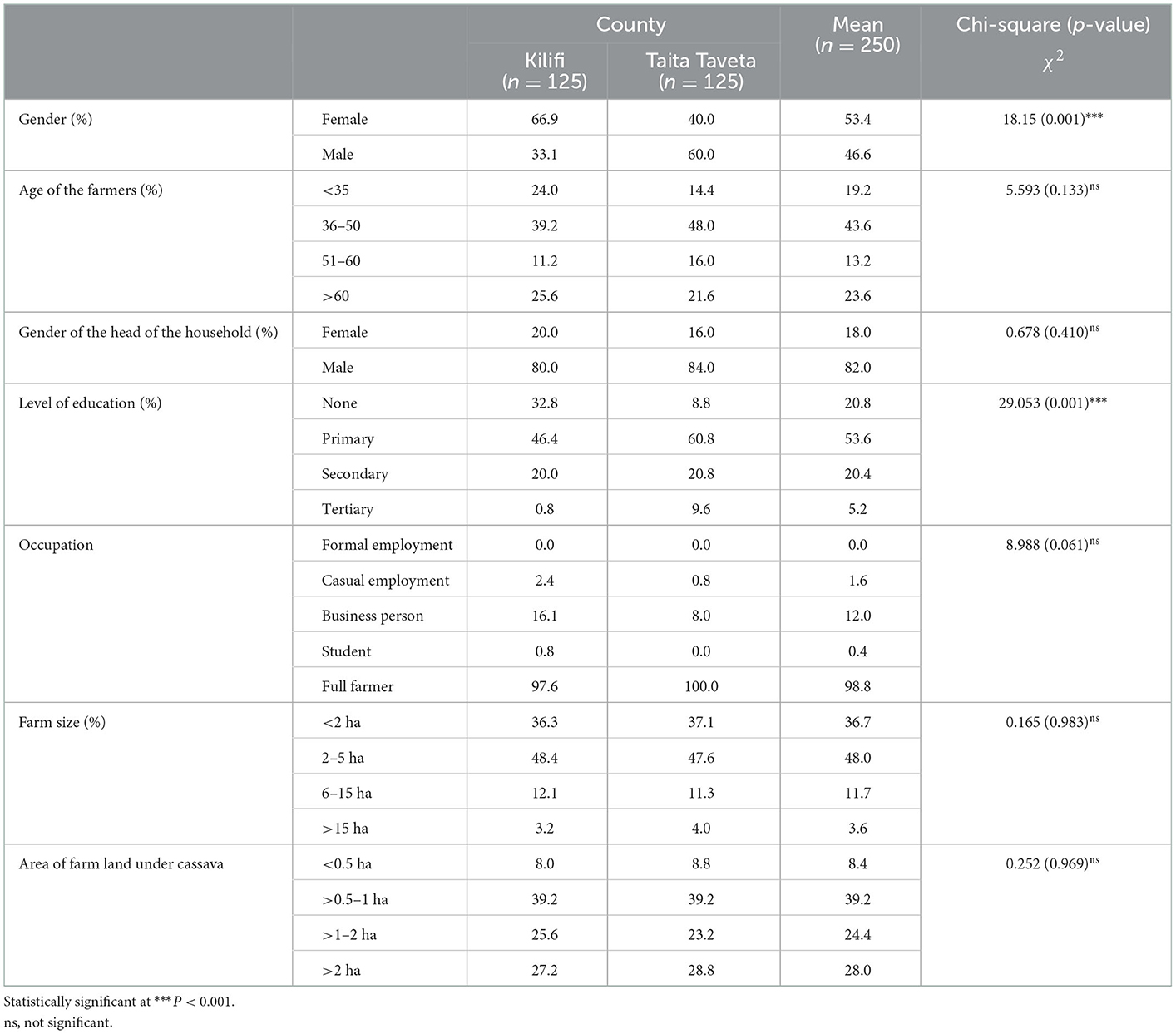
Table 1. Characteristics of the respondents (farmers surveyed; n = 250) in Kilifi and Taita Taveta counties.
Farmers gave their opinion on constraints affecting cassava production including inadequate clean planting material, pests and diseases, drought, low market price among others (Table 2). The opinions significantly varied across the two Counties. The highest ranked constraint to cassava production as reported in the two counties was pests and diseases, followed by drought and inadequate planting material. Overall, pests and diseases were reported by 67.2% of the farmers in both counties with Taita Taveta County having 77.6 % of respondents and Kilifi 56.7% of the respondents. Drought was a significant constraint in Kilifi County (51%) than in Taita-Taveta County (44%). The third ranked constraint to cassava was inadequate planting material reported by 49.4% of respondents in the two Counties. Other challenges reported by smallholder farmers in order of importance were low market price, wild animals, theft, perishability of cassava and floods (Table 2).
The major sources of information mentioned by farmers during the interview were from extension staff, research organizations and from fellow farmers (Table 3). The information on cassava obtained from these sources include planting material, new cassava varieties, general crop husbandry issues, pest and disease management, marketing and cassava utilization and processing. On average, the major sources of information were obtained from extension officers followed by research organizations. The information on cassava pests and diseases management obtained from extension staff was significantly (p < 0.001) higher than from other farmers and research organizations. The information mostly sought by farmers in the two Counties was on crop husbandry as reported by 82.8% of the respondents, followed by information on new varieties, marketing, pests and disease management, planting material and cassava utilization as reported by 70.6, 70.4, 59.5, 56.8, and 42.6%, respectively (Table 3).
Majority of the of the surveyed farmers (86.8%; Table 4) in both Counties were able to recognize cassava brown streak disease based on observation of CBSD symptoms on colored photographs presented to them. However, significantly higher farmers (96.6%) could recognize CBSD symptoms on the roots (root necrotic lesions), only 11.5% of them were able to recognize the foliar symptoms of CBSD (Table 4). Farmers had various perceptions about the possible causes of CBSD on cassava. Approximately 29 and 27% of the respondents reported poor soils and heavy rains, respectively, to be associated with the disease. About one out of five (20%) of the interviewed smallholder farmers did not know the cause of CBSD. Farmers also perceived and associated other factors including poor weather, insect vectors and over maturity at 14, 14, and 12%, respectively, to be responsible for CBSD (Table 4). Only 7.5% of the farmers associated the disease with planting material.
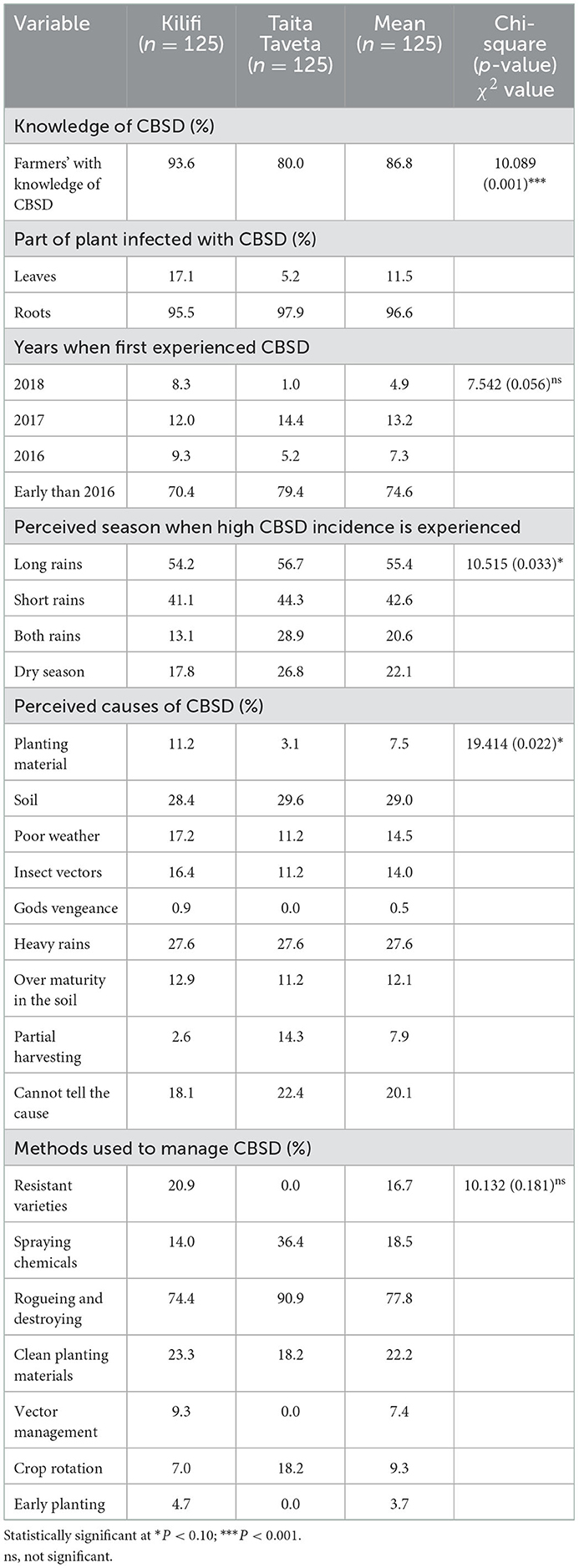
Table 4. Farmers' knowledge of CBSD, perceptions of spread, season of appearance, causes and management practices of CBSD.
Farmers reported to apply different management options for the control of cassava brown streak disease in the two Counties. Rogueing and destroying of the infected plants was reported by the respondents as the most commonly applied management practice (77.8%), with Kilifi and Taita Taveta Counties having 74.4 and 90.9%, respectively. Use of clean planting material was reported by 22.2% of the respondents as a management option for CBSD. A minority of farmers reported vector management and early planting as a method used for management of CBSD with 9.3 and 3.7% of respondents, respectively (Table 4). Use of resistant varieties as a management option for CBSD was reported by 16.7% of the respondents. Most of the farmers (55.4%) associated long rain season with high CBSD incidences, followed by short rain season (42.6%). Dry season was least associated with CBSD as reported by 22.1% of the respondents on from the two Counties. Farmers reported to have had experienced CBSD symptoms in their farms earlier than 2016 and reported to have experienced the disease in all the subsequent years (Table 4).
The bivariate Pearson correlation was done to show the strength and direction (i.e., positive/negative) of linear relationship between the socio-economic factors (i.e., age, farm size education and sex) and CBSD awareness in the different counties surveyed. Results indicated a positive correlation between the CBSD awareness and the farm size (0.018), whereas there was a negative correlation between awareness of CBSD with other factors including gender (−0.029) and the level of education (−0.083; Table 5).
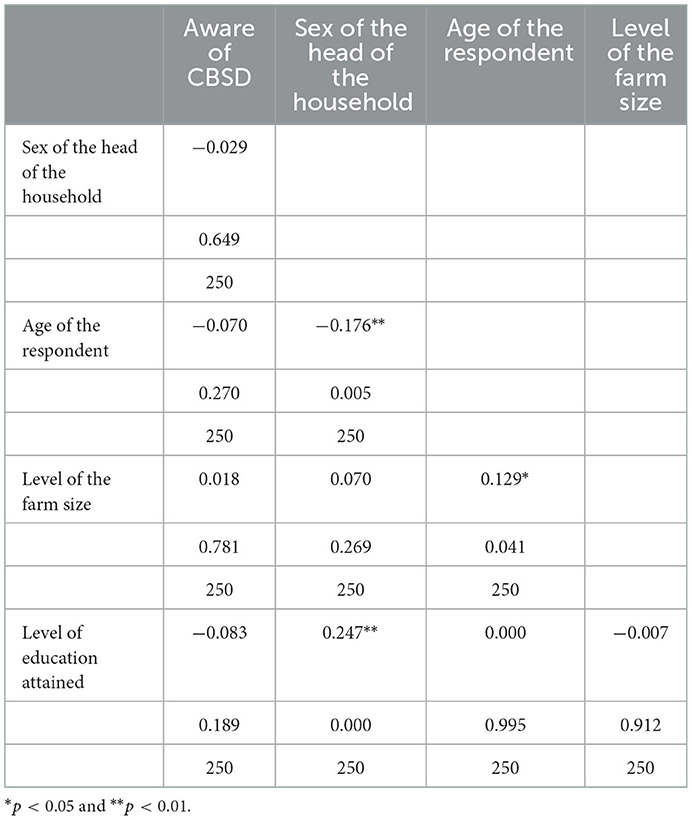
Table 5. Analysis of association between farmers' awareness on levels of CBSD and socio-economic factors.
On average 82.5% of farmers in the two Counties reported to recycle their own cassava cuttings from previous crop as the source of planting material. This was as reported by 88.6 and 76.4% of the respondents from Taita-Taveta and Kilifi Counties, respectively (Figure 1A). Borrowing or exchanging cassava planting material with neighbors or from one farmer to another or from one region to another was reported to be practiced by 67.5% of farmers on average in both Counties. On average, 11 and 5.3% of interviewed farmers in both Counties reported to source planting material from research centers (KALRO) and purchase of cuttings from the market, respectively. On the frequency of sourcing for planting material, majority of respondent farmers (61.1%), followed by 36.5% who sourced after more than one season and by 2.45% of respondents who reported to source for planting material every season (Figure 1B).
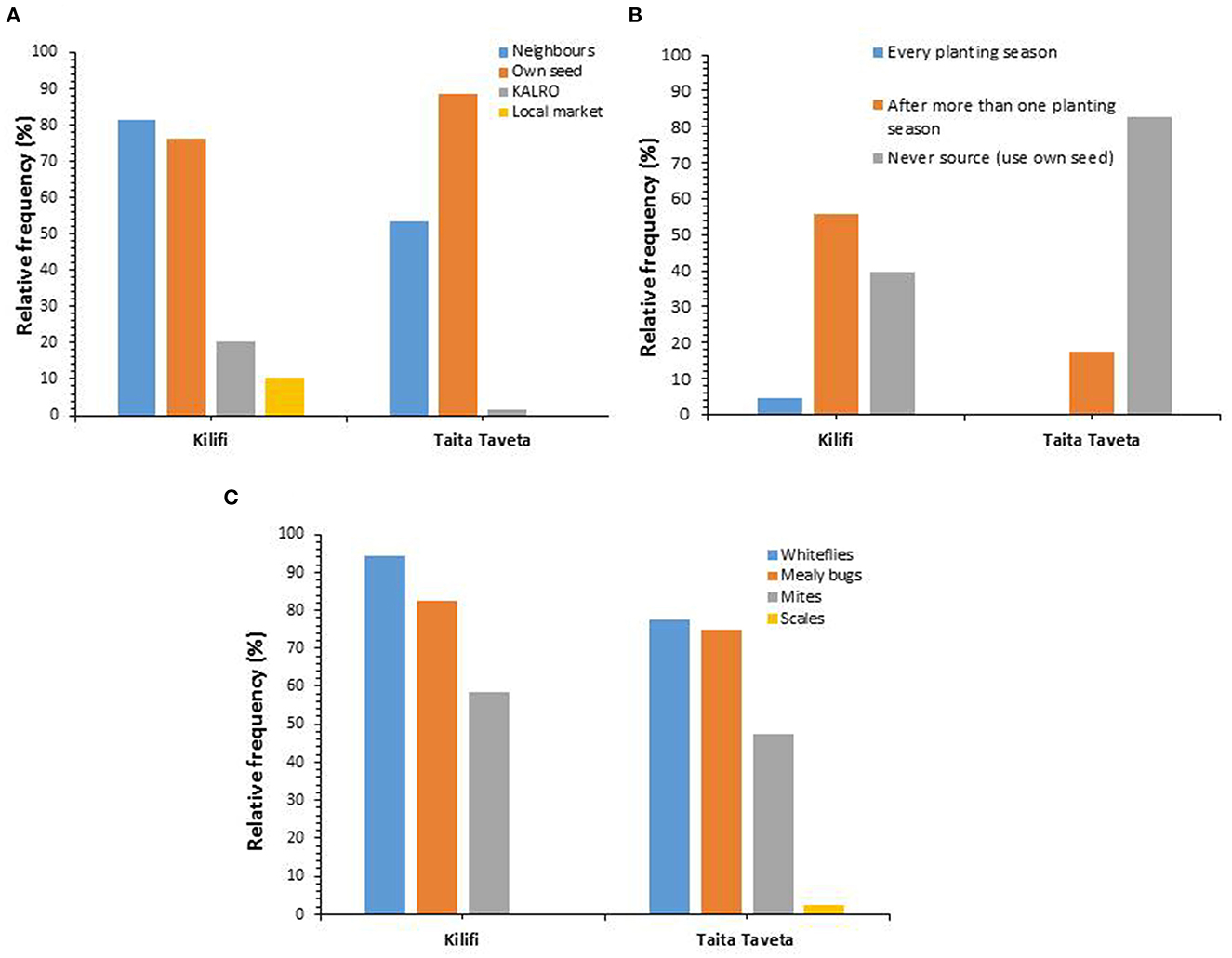
Figure 1. Farmers knowledge on: (A) sources of cassava planting material, (B) frequency of sourcing cassava planting material, and (C) presence of pests on cassava planting material in Kilifi and Taita-Taveta Counties.
Farmers reported experience of pests on planting materials based on the colored pest cards that were presented to them. Majority of farmers (85.5%) reported whiteflies as the major pests observed on cassava planting materials with 94.2% in Kilifi County and 77.5% in Taita-Taveta County (Figure 1C). Mealybugs were reported as the second most abundant pest observed on cassava by 78.8% of respondents with majority of the farmers (82.5%) observing the insect pest on planting material in Kilifi County than in Taita-Taveta County which had 75% of the respondents reporting the insect pest. Other pests and diseases mentioned were scale insects as well as mites.
Based on the survey data, farmers in the two Counties cultivate different cassava cultivars in the same field. These varieties include Kibandameno, Tajirika, Kaleso, and Girikacha among others (Supplementary Table 1). The variety Kibandameno was the most popular as reported by 36.8 and 34.2% of the interviewed farmers in Taita-Taveta and Kilifi Counties, respectively. On average, 69% of farmers in the two counties believed that CBSD does not affect all cassava cultivars while 31% of them believed that the disease affects all the cultivars cultivated. On variety susceptibility, 84.8% of the farmers reported Kibandameno as the most highly affected variety by CBSD while Tajirika was stated by 64.5% as the least affected variety by CBSD. Only 6.5% of farmers from both counties rated Kibandameno as least affected by CBSD. Farmers in both Kilifi and Taita Taveta Counties preferred Kibandameno variety due to its good taste, cooking ability and availability of cuttings while Tajirika variety was preferred due to its high yielding and disease tolerant traits (Supplementary Table 2).
During the survey, most commonly observed cassava brown streak disease symptoms in the fields were severe chlorosis on leaves (Figures 2A, B) and slight leaf feathery chlorotic blotches along the margins of veins (Figure 2C) and stem lesions (Figure 2D). Where possible, plants with foliar symptoms were uprooted and necrosis manifesting as brown lesions were observed on the roots (Figures 2E, F).

Figure 2. Symptoms of cassava brown streak disease (CBSD) observed in the field during the surveys in coastal region in Kenya and RT-PCR analysis from representative samples that amplified for CBSV, UCBSV and dual infection. The observed foliar symptoms include severe chlorosis on leaves (A, B), feathery vein chlorosis (C). Affected plants may also show brown streaks on stems (D) and root necrosis (E, F). A gel image of PCR products (G); sample 3A, 1A, and W3A represents samples that amplified for CBSV at 176 bp of coat protein region; samples 11A and 26 A represents positive samples for UCBSV at 493 bp while samples 206 and 199 represent positive samples for both CBSV and UCBSV indicating dual infection. L; indicates 50 bp Ladder, NT indicates no-template (negative control), +ve indicates a positive control.
The average field incidence of CBSD ranged from low (for Taita Taveta County, 10.8%) to moderate (for Kilifi and Kwale Counties with 27.9 and 24.7%, respectively). The symptom severity scores recorded in the fields were 2 (slight leaf feathery chlorosis with no stem lesions) and 3 (pronounced leaf feathery chlorosis, mild stem lesions and no dieback). Based on the cassava varietal effects, different cassava varieties grown by farmers had significant different levels of reaction to CBSD. For instance, the highest foliar incidence (40%) and severity (3.13) was recorded in Kibandameno variety, followed by landraces with incidence and severity of 16.38% and 1.97, respectively. Tajirika variety recorded the lowest CBSD foliar incidence and severity (1.80; Table 6).
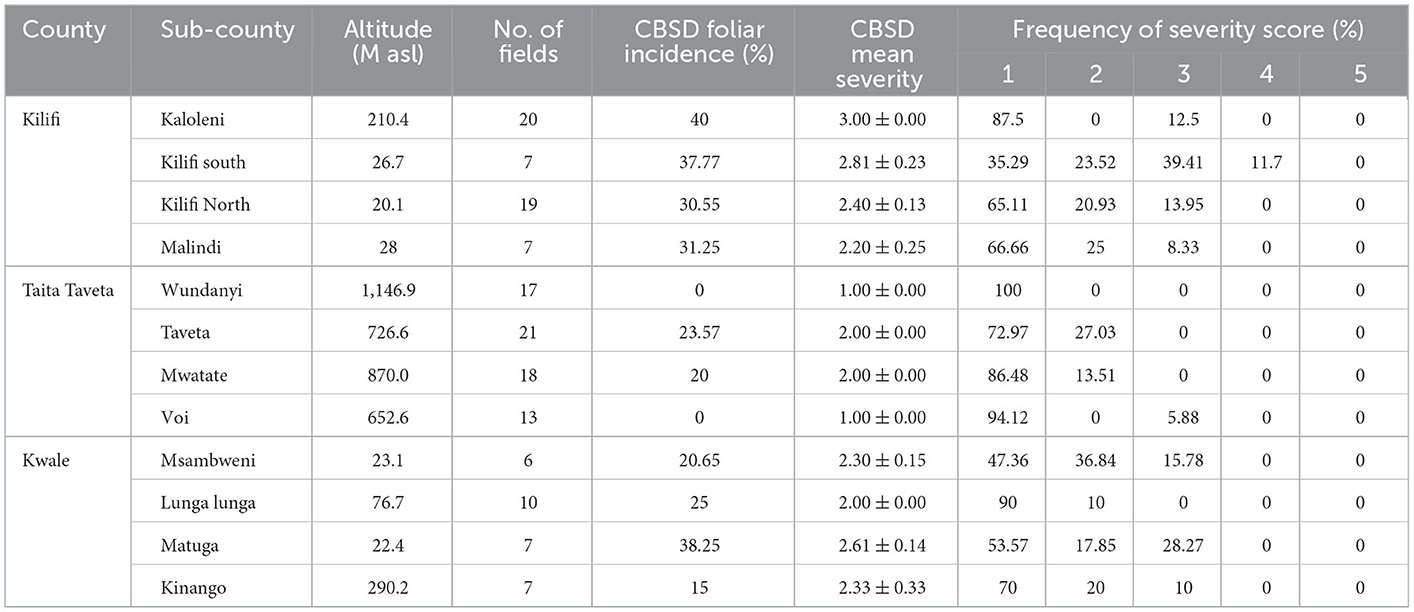
Table 6. Incidence and severity of CBSD in Kwale, Kilifi and Taita Taveta Counties of coastal Kenya.
Results from RT-PCR analysis indicated that 91% of the symptomatic samples tested positive for either of the two viruses (UCBSV and CBSV) either occurring singly or as dual infections. Amplicons of expected band sizes of 493 and 176 base pairs for UCBSV and CBSV, respectively were obtained (Figure 2G). Approximately 3.2% of the samples that were asymptomatic tested positive for only CBSV. The most predominant virus based molecular analysis was CBSV (59%) which was detected in all the Counties (Kilifi, Kwale and Taita Taveta) followed by UCBSV (54.2%). On average, co-infection by both CBSV and UCBSV was low at 8.4% and was detected in Kilifi and Kwale Counties (Table 7).
The distribution of the two viruses (UCBSV and CBSV) causing CBSD in the surveyed Counties of coastal region is presented in Figure 3. The presence of Ugandan cassava brown streak virus (UCBSV) in the three Counties was predominant in Kilifi (58.8%), followed by Kwale (46.6%) and Taita-Taveta (5.26%). In Taita Taveta County, CBSV was the most prevalent virus detected in three of the four sub-counties and the highest number of samples testing positive was in Taveta sub-County (40.54%) followed by Mwatate (13.51%) and Voi (6.25%) sub-Counties. All the 31 samples collected in Wundanyi sub-county in Taita-Taveta County tested negative for both CBSV and UCBSV. Only one sample tested positive for UCBSV in Taita Taveta County and this was in Taveta sub-County near the border of Kenya and Tanzania. There was no co-infection of CBSV and UCBSV recorded in Taita Taveta County based on RT-PCR analysis.
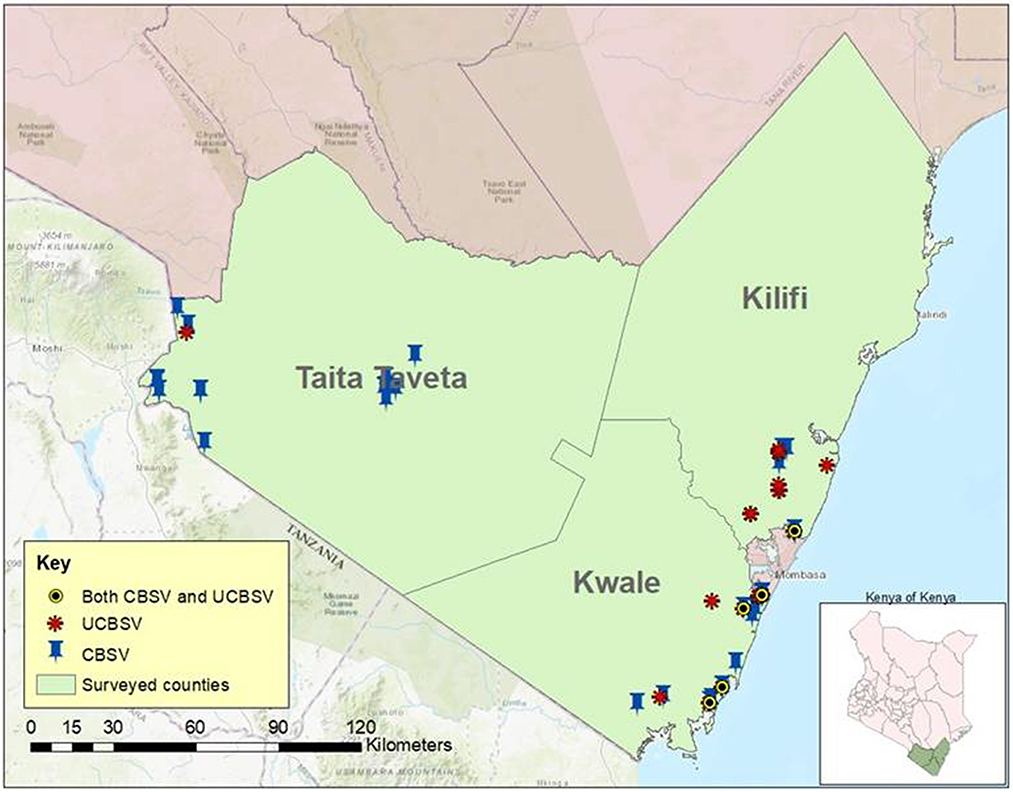
Figure 3. Distribution of cassava brown streak viruses mapped based on 153 cassava fields surveyed in Kilifi, Kwale and Taita Taveta counties of coastal region in Kenya.
The two viruses (UCBSV and CBSV) were detected and found both as single infections and as co-infections in Kwale County (Figure 3). CBSV was most prevalent in the County as found in 56.6% of the leaf samples while UCBSV was 46.6% and the two viruses were present in all the four sub-counties in Kwale County. Dual infection was found 16.6% of the samples in the County and was detected two sub-Counties namely Matuga and Msambweni. In terms of distribution of the disease incidence in Kwale County, Msambweni sub-County had the highest CBSD incidence (73.68%), followed by Matuga sub-County (64.28%), Kinango (30%) and Lungalunga (10%) sub-Counties and this incidence was based on RT-PCR analysis.
Kilifi County had the highest incidence of CBSD in the coastal Kenya. In terms of the distribution of viruses causing CBSD, UCBSV was dominant (58.8%), which was detected in all the four sub-Counties (Kilifi North, Kilifi South, Kaloleni, and Malindi) and CBSV (32%) was detected in all the sub-Counties except Kaloleni sub-County. Dual infection (5.8%) was only detected in Kilifi south sub-County based on the samples analyzed.
The current study assessed smallholder farmers' awareness on cassava brown streak virus disease (CBSD) and the distribution of the disease in coastal Kenya. Findings on socio-demographic characteristics of the 250 surveyed smallholder farmers in both Kilifi and Taita-Taveta Counties revealed more female respondents than male. This shows that they are significant in agricultural development in Coastal Kenya. Many smallholder cassava farmers were organized in groups most of which were women groups and this contributed to the significant number of women carrying out cassava farming activities. The findings from the current study are similar to those reported by Mwango'mbe et al. (2013), Diiro et al. (2018), and Sell and Minot (2018). Majority of the respondents (43%) were adults aged between 36 and 50 years and majority of them having attained primary school level. Educated farmers have the ability to interpret and respond to new information on uptake of technologies and knowledge on the management and control of CBSD by the larger population and can play a positive role in reducing the impact of the disease in the region. Most of the farmers in the area under study had farm sizes of ranging from 2 to 5 ha, indicating that majority of the respondents were small-scale farmers. The area under cassava production on average was ranging from 0.5 to 1 ha as reported by 39.2% of respondents and this in agreement with previous reports by Chikoti et al. (2016) and Kidasi et al. (2021). Therefore, for farmers to realize the benefits of high cassava productivity and utilization potential, there is need to promote large-scale cultivation of cassava in many cassava-growing regions (Shirima et al., 2019).
The findings from this study identified the major challenges that affect cassava production and productivity including inadequate clean planting material, pests and diseases, drought, low market price among other factors, similar to previous studies in major cassava-growing regions in Kenya (Kathurima et al., 2016; Chege et al., 2017; Tirra et al., 2019; Livoi et al., 2021; Onyango et al., 2021). Pests and diseases especially viral diseases were mentioned as a serious constraint whose effects led to the unavailability of clean planting material. Majority of farmers (85.5%) mentioned whiteflies as the major pests observed on cassava farms with 94.2% and 77.5% in Kilifi and Taita Taveta Counties, respectively, in coastal Kenya. The whitefly (Bemisia tabaci) has been reported to transmit viruses that cause the devastating cassava brown streak disease (Maruthi et al., 2005; Legg et al., 2014). Munguti et al. (2021) reported increased diversity of whitefly species from cassava farms at the coastal region of Kenya including the Bemisia tabaci haplotypes. Future studies need to investigate the possible link of the increasingly reported diverse whitefly species and the continued spread of CBSD in the coastal region of Kenya. Mealybugs were reported as the second most abundant pest observed on cassava farms. Similarly, papaya mealybugs (Paracoccus marginatus Williams) has been reported along the coastal belts of Kenya especially in Kwale, Kilifi, Mombasa, and Taita-Taveta counties on papaya, cassava, chili pepper, amaranth, coconut, and other fruits, ornamental plants and weeds (Macharia et al., 2017). Mealy bugs have been reported as vectors of many species of viruses (Selvarajan et al., 2016) including the recently identified novel amplelovirus infecting cassava in Central Africa and South West Indian Ocean islands (Kwibuka et al., 2021). Future studies therefore recommend investigative studies on the possible role played by the mealybug species observed on cassava in the coastal region of Kenya in the spread of CBSD during their feeding patterns on cassava.
Although the findings in the current study demonstrated that majority of the farmers (96.6%) in Kilifi and Taita Taveta Counties could recognize CBSD symptoms on the roots (root necrotic lesions), only 11.5% were aware and could correctly recognize foliar symptoms of the disease. Similar findings were reported in by Legg et al. (2011) and Chipeta et al. (2016). Farm management practices including regouing of diseased plants is a practice by many famers. However, the inability to recognize CBSD foliar or symptoms which are often subtle and in some cases asymptomatic infections based on many factors including weather conditions and genetic characteristics of cassava varieties means that farmers continue to exchange or recycle infected cuttings as planting material. This scenario leads to the disease easily manifesting in the crop plants in the field until the harvesting stage and such a time is too late to implement any management intervention measures by the farmers (Legg et al., 2011, 2014; Tomlinson et al., 2018). The ability to recognize the root symptoms by majority of the farmers is a demonstration that the disease is a major problem in the region. There is need to intensify awareness of the disease among farmers, extension workers on the disease symptoms, causes, mode of transmission and the available management options.
Farmers' perception on causes and management of CBSD demonstrated that farmers lack accurate information on the cause, spread and management of the disease. Soils and heavy rains were reported and perceived to be associated with the disease as reported by 29 and 27% respondents, respectively. These findings are similar to reports in Malawi by Chipeta et al. (2016) that indicated that farmers attributed cassava root necrosis symptoms to poor soil fertility. This is due to the fact that cassava root necrosis caused by CBSD is often observed on the roots during harvesting and therefore the possible association of the disease with poor soils. Farmers also perceived and associated the CBSD infections with other factors including poor weather, insect vectors and over maturity at 14, 14, and 12%, respectively. However, only 7.5% of the farmers associated the disease with planting material and this is a clear indication that farmers were not aware that exchange of cassava stem cuttings contributes to the spread of the disease. The current study therefore puts emphasize on the need to enhance awareness creation on CBSD, its causes as well as on control options. Previous studies also emphasized the need for intensified farmer's awareness on the cause, spread and management strategies (Chipeta et al., 2016; Bentley et al., 2017; Munguti et al., 2018; Kidasi et al., 2021). Rogueing and destroying of infected plants was reported by the respondents as the most commonly applied management practice in Kilifi and Taita Taveta Counties, with 74.4 and 90.9%, respectively. However, most of the rogueing was done at harvesting stage after observing the root symptoms to due to the challenge of identification of foliar symptoms.
The major source of cassava planting materials as reported by respondents/farmers in both Kilifi and Taita Taveta Counties was recycling own cassava cuttings from previous season's crop and exchange/borrowing from neighbors. These results demonstrate that farmers lack knowledge of the importance of clean planting materials as a critical factor that determines the success of cassava production. The farmers' inability to recognize the foliar symptoms coupled with significant amounts of infections, results to increased risk of transmission of the disease in their own farms and yield reduction over time (Legg et al., 2011; Shirima et al., 2019). These practices of recycling and exchange of cassava cuttings are common in many parts of sub-Saharan Africa where cassava is grown (Chipeta et al., 2016; Munguti et al., 2018; Shirima et al., 2019; Kidasi et al., 2021). These practices have been reported to be the main source of virus inoculum in cassava farms within the subsequent crop cycles as per the reports from previous studies (Mbewe et al., 2015; Maruthi et al., 2017). In addition, since cassava is a vegetatively propagated crop the quality of recycled planting material gradually degenerates due to pests and disease infections, agronomic and other factors (Calvert and Thresh, 2002; Shirima et al., 2019).
The practice of recycling and exchange of cassava cuttings with neighbors or region is a typical of informal seed system that is marred with planting material. These planting materials often consist of mixed cassava varieties, which are low yielding and highly susceptible to viral diseases including CBSD. The ideal solution in Kenya is to encourage farmers to plant certified cassava seeds (stem cuttings). Therefore, there is need to strengthen cassava seed certification systems using virus-indexed disease-free starting material and establishment of a sustainable seed system for multiplication and distribution of disease-free cassava planting material as a method to mitigate the rapid spread of CBSD (McQuaid et al., 2016; Maruthi et al., 2017; Munganyinka et al., 2018). Based on our findings, minority of farmers sourced cassava stem cuttings for planting from research institutions and purchase from markets. This could be attributed to unaffordability of the planting material by most farmers as well as lack of sufficient quantities of certified seed material hindering efforts to use certified cassava planting material. There is need for the promotion and adoption of seed business models for sustainable supply of disease-free cassava planting material for farmers (Kilwinger et al., 2021). In addition appropriate incentives need to be implemented to attract more private investors into cassava seed production businesses.
The use of host resistance against CBSD might be an effective method to control this disease. However, the use of host resistance is also complicated by the qualities of the preferred cassava varieties. For instance in the coastal region of Kenya, Kibandameno is a local cassava variety preferred by farmers due high dry matter and sweetness, however, it is highly susceptible to CBSD and this possibly explains the high prevalence of the disease in the region. Similar findings have been reported by Jones (2020) that most farmer-preferred cassava varieties are highly susceptible to viral diseases. Farmers rated Tajirika cassava variety as the least affected by CBSD and other diseases, which explains the CBSD foliar symptoms on Tajirika variety but the roots were not affected (showed no root necrosis). However, this can be a source of inoculum for accelerated transmission of the viruses to susceptible cassava cultivars. Therefore, this study shows that the main cassava cultivars grown in the coastal region of Kenya were infected with viruses causing CBSD. Use of released, improved and disease-resistant varieties in order to reduce the losses incurred due to CBSD is highly encouraged to farmers in the coastal region of Kenya.
The current study reports wide spread of the two viruses that cause CBSD in the coastal region of Kenya occurring either singly or as a co-infection (CBSV + UCBSV). The study revealed that cassava brown streak virus (CBSV) was the most predominant virus at incidence of 59% and was detected in all the three Counties (Kilifi, Kwale, and Taita-Taveta) followed by UCBSV at 54.2%. These findings are consistent with previous studies by Kathurima and Ateka (2019), who reported CBSV to be the predominant virus in the coastal region of Kenya. However, the incidence of CBSV was significantly lower compared with our study. Multiple viral infections that result in devastating disease often occur when two or more unrelated viruses infect a plant simultaneously (Munguti et al., 2018). Co-infection of the two viruses was recorded in all the surveyed areas in Kilifi and Kwale Counties except in Taita-Taveta County. However, our findings reported a lower incidence of co-infection than previously reported by Kathurima and Ateka (2019). These findings are contrary to previous studies that had associated CBSV as restricted to coastal lowlands and UCBSV regarded a highland epidemic virus (Alicai et al., 2007; Monger et al., 2010; Winter et al., 2010). Measures need to be put in place to manage the increasing incidence of CBSV and UCBSV in the coastal region of Kenya which has continued to impact negatively on cassava production, productivity and utilization and becoming a threat to food security. There is need for further investigative studies to understand the synergistic interactions that occur between CBSV and UCBSV isolates.
In this study, cassava plants with symptoms indicative of CBSD showed negative results for the viruses analyzed by RT-PCR. These symptoms and negative RT-PCR results could possibly be due to presence of new variants of the virus isolates or the possible lack of the existing diagnostic assay to accurately detect the viruses or possible new variants. Similar findings were reported by Shirima et al. (2022). Inversely, there were cassava plants without CBSD symptoms which tested positive for CBSV. This implies the difficulty when establishing control strategies for CBSVs. CBSD foliar symptoms have been reported to be depended on weather conditions or varietal characteristics and in many cases the disease remain undetected on the leaves until the crop is mature and the symptoms noticed in the roots (Alicai et al., 2007; Ntawuruhunga and Legg, 2007). This supports the need for a rapid field-based diagnostic tool to compliment the visual inspection of the diseased planting material especially during the multiplication stages and seed certification programs (Shirima et al., 2019).
In all the surveyed regions, only Wundanyi Sub-county-in Taita Taveta County was found to be free from the viruses based on symptomatology and negative RT-PCR results. This is an indication that the sub-County could be a possible preferred area for setting up multiplication sites for disease-free cassava planting materials for supply to the rest of the other sub-Counties in the coastal region where CBSD is endemic. Previous study by McQuaid et al. (2016) also recommended setting up multiplication sites for cassava planting material in areas of low disease pressure and low vector population density.
The majority of cassava growers are small-scale farmers and therefore effective traditional control strategies are critical for the management and control of cassava brown streak disease. The currently used methods for management of CBSD include use of clean planting materials, cultural practices (rouging, eradication of diseased plants, intercropping) and certification of seeds (Legg at al., 2015). However, these methods only reduce the virus inoculum in the field without completely eliminating the disease. Farmers who reuse stem cuttings from their own fields will not reduce the effects of the disease as this tends to maintain 30%−50% of CBSD infection particularly in disease hotspots (Patil et al., 2015). Limited natural resistance to CBSD has been identified in a few cassava genotypes and demonstrated for one of the viral species (UCBSV) causing CBSD (Winter et al., 2010). Furthermore, continued screening and characterization of cassava germplasm for responses to CBSD infection is important to identify more genotypes with CBSD tolerance or resistance traits. Deploying such tolerant or resistant cultivars can help in the effective control of the disease spread and reduction in losses associated with it. Since, resistance to CBSD has not been found in cassava genotypes traditionally grown by farmers in Africa, therefore there is need for development of transgenic and gene-editing approaches to reduce the increasing impact of CBSD.
The study reveals the continued threat to cassava production and productivity in the coastal region of Kenya due to the continued spread of cassava brown streak disease. The increased CBSD presence and spread could be attributed many factors including the inability of the farmers to recognize the disease thereby contributing to its dissemination through recycling and exchange of diseased stem cuttings, or possible genetic variants of the CBSD virus strains among other factors. Lack of clean cassava planting material in the surveyed areas contributed to the recycling of cuttings, borrowing or exchange of infected material among farmers and regions as well as use of highly susceptible cassava varieties which play a significant role in the spread of CBSD. The findings points to the need to strengthen cassava seed certification systems for farmers to reap the benefits of cassava to address the food security challenges. Our findings demonstrate that farmers lack sufficient knowledge of the importance of plant health as a critical factor in the success of cassava production. There is need to sensitize farmers and enhance awareness on the spread and management of CBSD. The study also provides a basis for interventions to control CBSD. Opportunities for establishment sustainable source of disease-free cassava planting materials through formalized cassava seed systems involving use of officially released and improved cassava varieties should be priotized. Therefore, with the increasing spread of CBSD epidemics, there is need for further genome characterization of the identified viruses and possible genetic variants to inform better genome targets for development of markers for better detection and as well as to inform breeding strategies. There is need for successive assessments of the CBSD spread in all cassava growing areas over time and further identify sources of resistance to CBSD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
FM, EN, DK, IM, and AM contributed to the conception and designed the study. FM, EN, DK, HY, IM, and AM contributed to the methodology design. FM collected the survey data, analyzed data, and wrote the first original draft of manuscript. EN validated the data and contributed to data analysis. EN, DK, IM, and AM revised and edited the manuscript and supervised the work. AM provided the resources. All authors contributed to the article and approved the submitted version of the manuscript.
This work was financed by the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM) through the Transforming African Agricultural Universities to meaningfully contribute to Africa's growth and development (TAGDEv) through the Mastercard Foundation (Grant ID: RU/2018/CARP+/04).
The authors acknowledge Kenya Plant Health Inspectorate Service and the University of Nairobi for their support. We thank all the farmers where the sampling was conducted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.1015315/full#supplementary-material
Alicai, T., Omongo, C. A., Maruthi, M. N., Hillocks, R. J., Baguma, Y., Kawuki, R., et al. (2007). Re-emergence of cassava brown streak disease in Uganda. Plant Dis. 91, 24–29. doi: 10.1094/PD-91-0024
Bentley, J. W., Olanrewaju, A. S., Madu, T., Olaosebikan, O., Abdoulaye, T., Assfaw Wossen, T., et al. (2017). Cassava Farmers' Preferences for Varieties and Seed Dissemination System in Nigeria: Gender and Regional Perspectives. Ibadan, Nigeria: IITA Monograph.
Calvert, L. A., and Thresh, J. M. (2002). The Viruses and Virus Diseases of Cassava. In: R. J. Hillocks, J. M. Thresh, and A. C. Bellotti, eds (Wallingford: Cassava: Biology, Production and Utilization, CAB International), pp. 237–260. doi: 10.1079/9780851995243.0237
Chege, M. N., Wamunyokoli, F., Kamau, J., and Nyaboga, E. N. (2017). Phenotypic and genotypic diversity of Xanthomonas axonopodis pv. manihotis causing bacterial blight disease of cassava in Kenya. J. Appl. Biol. Biotechnol. 5, 038–044. doi: 10.7324/JABB.2017.50206
Chen, W., Wosula, E. N., Hasegawa, D. K., Casinga, C., Shirima, R. R., Fiaboe, K. K., et al. (2019). Genome of the African cassava whitefly Bemisia tabaci and distribution and genetic diversity of cassava-colonizing whiteflies in Africa. Insect Biochem. Mol. Biol. 110, 112–120. doi: 10.1016/j.ibmb.2019.05.003
Chikoti, P. C., Melis, R., and Shanahan, P. (2016). Farmer's perception of cassava mosaic disease, preferences and constraints in Lupaula Province of Zambia. Am. J. Plant Sci. 7, 1129. doi: 10.4236/ajps.2016.77108
Chipeta, M. M., Shanahan, P., Melis, R., Sibiya, J., and Benesi, I. R. (2016). Farmers' knowledge of cassava brown streak disease and its management in Malawi. Int. J. Pest Manag. 62, 175–184. doi: 10.1080/09670874.2016.1167268
Diiro, G. M., Seymour, G., Kassie, M., Muricho, G., and Muriithi, B. W. (2018). Women's empowerment in agriculture and agricultural productivity: evidence from rural maize farmer households in western Kenya. PLoS ONE 13, e0197995. doi: 10.1371/journal.pone.0197995
Food Agricultural Organization of the United Nations (FAOSTAT) (2018). Food and Agricultural Organization of the United Nations (FAO), Rome. Available from http://faostat.fao.org (accessed August 2022).
Food and Agricultural Organization of the United Nations (FAOSTAT) (2020). Production Quantities of Cassava in the World. Rome: Food and Agricultural Organization of the United Nations.
Gondwe, F. M. T., Mahungu, N. M., Hillocks, R. J., Raya, M. D., Moyo, C. C., Soko, M. M., et al. (2003). “Economic losses experienced by small-scale farmers in Malawi due to cassava brown streak virus disease,” in: Cassava Brown Streak Virus Disease: Past, Present and Future, eds J. P. Legg, and R. J. Hillocks (Aylesford: Natural Resources International), 28–35.
Hillocks, R. J., and Jennings, D. L. (2003). Cassava brown streak disease: a review of present knowledge and research needs. Int. J. Pest Manage. 49, 225–234. doi: 10.1080/0967087031000101061
Hillocks, R. J., and Maruthi, M. N. (2015). Post-harvest impact of cassava brown streak disease in four countries in eastern Africa. Food Chain. 5, 116–122. doi: 10.3362/2046-1887.2015.008
Hillocks, R. J., Raya, M. D., and Mtunda, K. (2001). Effects of brown streak virus disease on yield and quality of cassava in Tanzania. J. Phytopathol. 149, 389–394. doi: 10.1046/j.1439-0434.2001.00641.x
Jarvis, A., Ramirez-Villegas, J., Campo, B. V. H., and Navarro-Racines, C. (2012). Is cassava the answer to African climate change adaptation? Trop. Plant Biol. 5, 9–29. doi: 10.1007/s12042-012-9096-7
Jones, R. A. (2020). Disease pandemics and major epidemics arising from new encounters between indigenous viruses and introduced crops. Viruses 12, 1388. doi: 10.3390/v12121388
Kathurima, T. M., and Ateka, E. M. (2019). Diversity and phylogenetic relationships of full genome sequences of cassava brown streak viruses in Kenya. Biotechnol. J. Int. 23, 1–11. doi: 10.9734/bji/2019/v23i330080
Kathurima, T. M., Nyende, A. B., Kiarie, S. M., and Ateka, E. M. (2016). Genetic diversity and distribution of cassava brown streak virus and ugandan cassava brown streak virus in major cassava-growing regions in Kenya. Annu. Res. Rev. Biol. 10, 1–9. doi: 10.9734/ARRB/2016/26879
Kidasi, P. C., Chao, D. K., Obudho, E. O., and Mwang'ombe, A. W. (2021). Farmers' sources and varieties of cassava planting materials in coastal Kenya. Front. Sustain. Food Syst. 5, 611089. doi: 10.3389/fsufs.2021.611089
Kilwinger, F., Mugambi, S., Manners, R., Schut, M., Tumwegamire, S., Nduwumuremyi, A., et al. (2021). Characterizing cassava farmer typologies and their seed sourcing practices to explore opportunities for economically sustainable seed business models in Rwanda. Outlook Agric. 50, 441–454. doi: 10.1177/00307270211045408
Koima, I. N., and Orek, C. O. (2018). Distribution of cassava mosaic and cassava brown streak diseases in agro-ecological zones of lower eastern Kenya. Int. J. Innovative Sci. Res. Technol. 3, 391–399.
Krejcie, R. V., and Daryle, W. M. (1970). Determining sample size for research activities. Educ. Psychol. Meas. 30, 607–610. doi: 10.1177/001316447003000308
Kwibuka, Y., Bisimwa, E., Blouin, A. G., Bragard, C., Candresse, T., Faure, C., et al. (2021). Novel ampeloviruses infecting cassava in central africa and the South-West Indian Ocean Islands. Viruses 13, 1030. doi: 10.3390/v13061030
Legg, J., Somado, E. A., Barker, I., Beach, L., Ceballos, H., Cuellar, W., et al. (2014). A global alliance declaring war on cassava viruses in Africa. Food Secur. 6, 231–248. doi: 10.1007/s12571-014-0340-x
Legg, J. P., Jeremiah, S. C., Obiero, H. M., Maruthi, M. N., Ndyetabula, I., Okao-Okuja, G., et al. (2011). Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 159, 161–170. doi: 10.1016/j.virusres.2011.04.018
Legg, J. P., Kumar, P. L., Makeshkumar, T., Tripathi, L., Ferguson, M., Kanju, E., et al. (2015). Cassava virus diseases: biology, epidemiology, and management. Adv. Virus Res. 91, 85–142. doi: 10.1016/bs.aivir.2014.10.001
Livoi, A., Mwangombe, A. W., Nyaboga, E., Kilalo, D., and Obutho, E. (2021). Prevalence and distribution of cassava bacterial blight in the Kenyan Coast. Agric. Sci. 3, 2021. doi: 10.30560/as.v3n1p7
Lodhi, M. A., Ye, G. N., Weeden, N. F., and Reisch, B. I. (1994). A simple and efficient method for DNA extraction from grapevine cultivars and vitis species. Plant Mol. Biol. Rep. 12, 6–1. doi: 10.1007/BF02668658
Macharia, I., Kimani, E., Koome, F., Kosiom, T., Heya, H., Otipa, M., et al. (2017). First report and distribution of the papaya mealybug, Paracoccus marginatus, in Kenya. J. Agric. Urban Entomol. 33, 142–150. doi: 10.3954/JAUE17-02.1
Maruthi, M. N., Hillocks, R. J., Mtunda, K., Raya, M. D., Muhanna, M., Kiozia, H., et al. (2005). Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 155, 307–312. doi: 10.1111/j.1439-0434.2005.00974.x
Maruthi, M. N., Jeremiah, S. C., Mohammed, I. U., and Legg, J. P. (2017). The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 165, 707–717. doi: 10.1111/jph.12609
Masinde, E. A., Ogendo, J. O., Maruthi, M. N., Hillocks, R., Mulwa, R. M. S., Arama, P. F., et al. (2016). Occurrence and estimated losses caused by cassava viruses in Migori County, Kenya. Afric. J. Agric. Res. 11, 2064–2074. doi: 10.5897/AJAR2016.10786
Mbanzibwa, D. R., Tian, Y. P., Tugume, A. K., Patil, B. L., Yadav, J. S., Bagewadi, B., et al. (2011). Evolution of cassava brown streak disease-associated viruses. J. Gen Virol. 92, 974–987. doi: 10.1099/vir.0.026922-0
Mbewe, K. W., Kumar, L. P., Changadeya, W., Ntawuruhunga, P., and Legg, J. P. (2015). Diversity, distribution and effects on cassava cultivars of cassava brown streak viruses in Malawi. J. Phytopathol. 163, 433–443. doi: 10.1111/jph.12339
McQuaid, C. F., Sseruwagi, P., Pariyo, A., and Van den Bosch, F. (2016). Cassava brown streak disease and the sustainability of a clean seed system. Plant Pathol. 65, 299–309. doi: 10.1111/ppa.12453
Monger, W. A., Alicai, T., Ndunguru, J., Kinyua, Z. M., Potts, M., Reeder, R. H., et al. (2010). The complete genome sequence of the Tanzanian strain of Cassava brown streak virus and comparison with the Ugandan strain sequence. Arch. Virol. 155, 429–433. doi: 10.1007/s00705-009-0581-8
Munganyinka, E., Ateka, E. M., Kihurani, A. W., Kanyange, M. C., Tairo, F., Sseruwagi, P., et al. (2018). Cassava brown streak disease in Rwanda, the associated viruses and disease phenotypes. Plant Pathol. 67, 377–387. doi: 10.1111/ppa.12789
Munguti, F., Kilalo, D., Macharia, I., Nyaboga, E. N., and Mwang'ombe, A. (2018). RUFORUM Working Document Series (ISSN 1607-9345), No. 17(1), 739–746. Available online at: http://repository.ruforum.org (accessed July, 2022).
Munguti, F. M., Kilalo, D. C., Nyaboga, E. N., Wosula, E. N., Macharia, I., and Mwango'mbe, A. W. (2021). Distribution and molecular diversity of whitefly species colonizing cassava in Kenya. Insects 12, 875. doi: 10.3390/insects12100875
Mwango'mbe, A. W., Mbugua, S. K., Olubayo, F. O., Ngugi, E. K., Mwinga, R., Munga, T., et al. (2013). Challenges and opportunities in cassava production among the rural households in Kilifi County in the Coastal region of Kenya. J. Biol. Agric. Healthcare. 3, 2013.
Mwango'mbe, A. W., Mbugua, S. K., Olubayo, F. O., Ngugi, E. K., Mwinga, R., Munga, T., and Muiru, W. M. (2013). Challenges and opportunities in cassava production among the rural households in Kilifi County in the Coastal region of Kenya. J. Biol. Agric. Healthcare 10, 2224–3208.
Mware, B., Narla, R., Amata, R., Olubayo, F., Songa, J., Kyamanyua, S., et al. (2009). Efficiency of cassava brown streak virus transmission by two whitefly species in coastal Kenya. J. Gen. Mol. Virol. 1, 040–045.
Neuenschwander, P., and Tamò, M. (2019). Critical Issues in Plant Health: 50 Years of Research in African Agriculture. Cambridge: Burleigh Dodds Science Publishing. doi: 10.19103/AS.2018.0043
Nono-Womdim, R., Swai, I. S., Green, S. K., Gebre-Selassie, K., Laterrot, H., Marchoux, G. R. T., et al. (1996). Tomato viruses in Tanzania: identification, distribution and disease incidence. J. Southern Afri. Soc. Hortic. Sci. 6, 41–44.
Ntawuruhunga, P., Kanju, E., Ssemakula, G., Okechukwu, R., Whyte, J., Schofield, J., et al. (2013). Successful innovations and lessons learnt in cassava improvement and deployment by IITA in Eastern African Region. AJRTC 10, 41–51.
Ntawuruhunga, P., and Legg, J. (2007). New Spread of Cassava Brown Streak Virus Disease and its Implications for the Movement of Cassava Germplasm in the East and Central African Region. Kampala: International Institute of Tropical Agriculture-Uganda and Eastern Africa Root Crops Research Network Report.
Onyango, S. O., Abong, G. O., Okoth, M. W., Kilalo, D. C., and Mwang'ombe, A. W. (2021). Effect of pre-treatment and processing on Nutritional composition of Cassava roots-millet-cowpea leaves composite flours. Front. Sustain. Food Syst. 5, 176. doi: 10.3389/fsufs.2021.625735
Patil, B. L., Legg, J. P., Kanju, E., and Fauquet, C. M. (2015). Cassava brown streak disease: a threat to food security in Africa. J. Gen. Virol. 96, 956–968. doi: 10.1099/jgv.0.000014
Rwegasira, G. M., and Rey, M. E. (2009). Response of selected cassava varieties to the incidence and severity of cassava brown streak disease in Tanzania. J. Agric. Sci. 4, 237–245. doi: 10.5539/jas.v4n7p237
Sell, M., and Minot, N. (2018). What factors explain women's empowerment? Decision-making among small-scale farmers in Uganda. Women's Stud. Int. Forum 71, 46–55. doi: 10.1016/j.wsif.2018.09.005
Selvarajan, R., Balasubramanian, V., and Padmanaban, B. (2016). “Mealybugs as vectors,” in Mealybugs and their Management in Agricultural and Horticultural Crops, eds C. Shivaraju, and M. Mani (New Delhi: Springer), 123–130. doi: 10.1007/978-81-322-2677-2_10
Shirima, R. R., Maeda, D. G., Kanju, E. E., Tumwegamire, S., Ceasar, G., Mushi, E., et al. (2019). Assessing the degeneration of cassava under high-virus inoculum conditions in coastal Tanzania. Plant Dis. 103, 2652–2664. doi: 10.1094/PDIS-05-18-0750-RE
Shirima, R. R., Wosula, E. N., Hamza, A. A., Mohammed, N. A., Mouigni, H., Nouhou, S., et al. (2022). Epidemiological analysis of cassava mosaic and brown streak diseases, and Bemisia tabaci in the Comoros islands. Viruses 14, 2165. doi: 10.3390/v14102165
Tirra, A. N., Oluoch-Kosura, W., Nyanganga, H., and Mwang'ombe, A. W. (2019). Determinants of participation decision in cassava marketing by smallholder farmers in Taita-Taveta and Kilifi Counties, Kenya. J. Agric. Sci. 11, 98–109. doi: 10.5539/jas.v11n17p98
Tomlinson, K. R., Bailey, A. M., Alicai, T., and Seal, S. (2018). Cassava brown streak disease: historical timeline, current knowledge and future prospects. Mol. Plant Pathol. 19, 1282–1294. doi: 10.1111/mpp.12613
Tumuhimbise, R., Paul, S., Rob, M., and Kawuki, R. (2014). Combining ability analysis of storage root yield and related traits in cassava at the seedling evaluation stage of breeding. J. Crop Improv. 28, 530–546. doi: 10.1080/15427528.2014.923798
Were, M. N., Mukoye, B., Osogo, A. K., Mangeni, B. C., Nyamwamu, P. A. A., Ogemah, V. K., et al. (2016). Occurrence and distribution of begomoviruses infecting cassava in Western Kenya. Plant 4, 108–113. doi: 10.11648/j.plant.20160406.18
Winter, S., Koerbler, M., Stein, B., Pietruszka, A., and Paape, M. (2010). Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J. Gen. Virol. 91, 1365–1372. doi: 10.1099/vir.0.014688-0
Wosula, E. N., Chen, W., Fei, Z., and Legg, J. P. (2017). Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biol. E9, 2958–2973. doi: 10.1093/gbe/evx219
Keywords: cassava, farmers' knowledge, disease-free planting material, CBSD field survey, virus control, Kenya
Citation: Munguti FM, Nyaboga EN, Kilalo DC, Yegon HK, Macharia I and Mwango'mbe AW (2023) Survey of cassava brown streak disease and association of factors influencing its epidemics in smallholder cassava cropping systems of coastal Kenya. Front. Sustain. Food Syst. 6:1015315. doi: 10.3389/fsufs.2022.1015315
Received: 16 September 2022; Accepted: 30 December 2022;
Published: 19 January 2023.
Edited by:
Dr Abe Shegro Gerrano, Agricultural Research Council of South Africa (ARC-SA), South AfricaReviewed by:
Amadou Sidibé, Agriculture and Agri-Food Canada (AAFC), CanadaCopyright © 2023 Munguti, Nyaboga, Kilalo, Yegon, Macharia and Mwango'mbe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence Mutave Munguti,  bXVuZ3V0aS5mbG9yZW5jZUBnbWFpbC5jb20=
bXVuZ3V0aS5mbG9yZW5jZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.