
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 17 October 2022
Sec. Nutrition and Sustainable Diets
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1010556
This article is part of the Research TopicDietary Change Strategies for Sustainable Diets and their Impact on Human Health - Volume 1View all 44 articles
Background: Raw hawthorn (RH) is a traditional Chinese medicine commonly used to treat indigestion. Charred hawthorn (CH) is obtained from RH by charring. It is reported that the effect of CH treatment on dyspepsia is stronger than RH. However, this has not been fully proven. The purpose of this study was to compare the effects of RH and CH on functional dyspepsia (FD) model rats. And contribute to the development of dietary therapy for dyspepsia.
Methods: SPF-grade male SD rats were divided into 5 groups: the control group, the model group, the Mos group, the RH group, and the CH group. The FD rat model was established by using the methods of water restriction, fasting, tilting cage restraint, day and night upside down, swimming, and tail damping. The body weight of rats in each group was recorded. And the gastric emptying rate, intestinal propulsive rate, and the levels of motilin (MTL), gastrin (GAS), and 5-HT in serum were compared in each group. The expression of C-kit in the stomach and small intestine of each group was compared by immunofluorescence and PCR.
Results: RH and CH could increase weight, improve the gastric emptying rate and intestinal propulsive rate, and promote the secretion of motilin (MTL), gastrin (GAS), and 5-HT in the serum of FD rats. RH and CH can upregulate the expression of the characteristic protein c-kit of ICC in the stomach and small intestine of FD model rats, and the effect of CH is stronger than RH.
Conclusion: RH and CH may increase the number of interstitial cells of Cajal (ICC) in the gastrointestinal tract by upregulating c-kit expression, thus improving gastrointestinal motility in FD model rats. And compared with RH, CH has certain advantages.
Functional dyspepsia (FD) is one of the clinics' most common functional gastrointestinal diseases, but its etiology and pathogenesis are still unclear. It refers to a series of symptoms of dyspepsia in the stomach and duodenum, including epigastric pain, epigastric distention, early satiety, belching, loss of appetite, nausea, and vomiting (Tack et al., 2006). In the Roman type III diagnostic criteria, FD is divided into two subtypes, namely, postprandial discomfort syndrome and epigastric pain syndrome. Usually, in the general population, the incidence of symptoms of dyspepsia is 20%. In addition, 80% of patients were not found to have organic lesions during other tests such as endoscopy. The disease is called functional dyspepsia because there's no explanation for its symptoms (Ford et al., 2015). The overall prevalence of FD is 16% in the general population (Ford et al., 2020). FD is a difficult disease to cure clinically, and its influencing factors are many and complex. Although FD is usually not life-threatening, the quality of life, social behavior, and mental health of patients would be severely affected (Aro et al., 2011). In one survey, there was no significant difference in the effect of FD and organic dyspepsia on work efficiency (Sander et al., 2011). This suggests that FD is as harmful as organic dyspepsia.
Although FD is a common disease, the pathophysiological basis is not clear, and it may be related to gastrointestinal motility disorders, hypersensitivity reactions in the stomach, Helicobacter pylori infection, and psychosocial factors (Wauters et al., 2020). In response to the possible pathogenesis, many common drugs have been used to treat FD. For example, anti-Helicobacter pylori drugs, proton pump inhibitors (PPI), and prokinetic drugs (Tack and Camilleri, 2018). However, these drugs are difficult to show high effectiveness in the treatment of FD, especially since it is difficult to solve the problem of recurrent episodes of FD. Finding new targets for the treatment of FD and developing targeted drugs have become the current research hotspots (Chiarioni et al., 2018). Interstitial cells of Cajal (ICC) are a class of cells located in the muscle tissue of the gastrointestinal tract (GIT). Although they make up only 5% of the cells in the muscle tissue of the gastrointestinal tract, they play a key role in regulating smooth muscle function and gastrointestinal movement in harmony with the enteric nervous system (Huizinga et al., 2021). ICC mediates signal transmission of the gastrointestinal motor nervous system to smooth muscle. ICC is the pacemaker of gastrointestinal motility, which stimulates the rhythmic peristalsis of the gastrointestinal tract by spontaneously generated electrical slow waves. Therefore, structural and functional abnormalities of ICC are closely related to gastrointestinal motility disorders and the cause of many gastrointestinal diseases (Sanders et al., 2014; Zhang et al., 2016). FD is a representative disease of gastrointestinal motility disorder. ICC will be a possible direction to respond to FD in the future (Zhang et al., 2018).
Hawthorn refers to the ripe fruit of Crataegus pinnatifida Bge in the Rosaceae family (Figure 1). As a widely used Chinese herbal medicine and medicinal herb, many drugs related to hawthorn are included in the pharmacopeias of many countries (Martinelli et al., 2021). It has a long history of being used in indigestion, blood circulation and stasis, and cardiovascular diseases. The charred hawthorn (CH) was prepared from raw hawthorn (RH) by the stir-frying method which is a thermal processing method of traditional Chinese medicine. Modern studies have shown that RH has a certain role in improving digestive function. The traditional Chinese medicine records that compared with RH, CH has a stronger effect on improving digestive function. Related studies have investigated this effect, but more studies are needed to research the mechanism (Wei et al., 2019). As hawthorn, many foods have special pharmacological effects. They are useful for preventing and treating disease. Dietary therapy refers to the use of this kind of food to regulate bodily functions. In recent years, many studies have paid attention to the importance of dietary therapy because of the close relationship between food and gastrointestinal diseases (Liu et al., 2015; Pearlman and Akpotaire, 2019).
In this study, we compared the effects of RH and CH in the intervention of FD and elucidated the possible pathways for improving digestive function from the perspective of ICC. It will provide a possible solution for developing a CH-based dietary therapy for functional dyspepsia.
50 g of RH and CH (Tongrentang, China) were weighed and placed in the flask. They were extracted three times with 500 mL boiling water for 1.5 h each time. After filtration, the filtrate was combined and concentrated at 45°C. Add pure water and dilute to 0.3 g/ml. Mosapride citrate dispersible tablets (Mos, Kanghong, China) were dissolved in pure water to make a solution of 0.15 mg/ml.
The Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine approved the experimental program and followed the international animal studies guidelines to minimize the pain and discomfort of the animals. Fifty SPF-grade male SD rats (180 ± 20 g) were purchased from Chengdu Dasuo Experimental Animal Co., Ltd. Forty rats were randomly selected to receive the following stimuli to establish the model: fasting and not prohibiting water for 24 h, water deprivation and not fasting for 24 h, tilting the cage for 24 h, the fixing for 2 h, day and night upside down, swimming for 5 min in 4°C water, and clamping the tail for 1 min. The rats were stimulated in random order. And the same stimulation does not occur for 2 consecutive days. After 21 consecutive molding days, they were divided into the model group, Mos group, RH group, and CH group. During the 7-day administration period, the rats in the model group were still subjected to random stimuli to maintain the model. The dosage of the Mos group as a positive drug group was 1.5 mg/kg/d. The dosage of RH and CH groups as therapeutic drug groups was 3 g/kg/d. The Control group and model group were given equal amounts of normal saline. The weight of the rats was recorded daily throughout the experiment.
All rats fasted for 12 h (free water) before the last dose. After the last dose for 40 min, they were fed a black semisolid paste (Including sodium carboxymethyl cellulose, milk powder, soluble starch, and activated carbon). About 2 ml each and the weight is recorded (W3). After 20 min, a blood sample was taken from the abdominal aorta. The serum was separated from blood by centrifugation at 4°C 3,000 r/min, and stored at −80°C. After the pylorus and cardia were ligated, the entire stomachs of the rats were separated. Then they were dried on filter paper and weighed (total weight of the stomach, W1). Cut the stomach along the great curvature of the stomach and remove the contents. Then the stomachs were weighed again (the net of the stomach, W2). After the small intestine is isolated, the length of the small intestine (L1) and the movement distance of black solid paste (L2) were measured by a ruler. Then, the stomach and small intestine tissues were fixed in the centrifuge tubes with 4% paraformaldehyde.
First, the stomach and small intestine tissues of rats were fixed in 4% paraformaldehyde solution for 48 h. After the tissues were dehydrated and embedded in paraffin, they were cut into 4 μm slices and sealed with neutral resin after hematoxylin and eosin (H and E) staining. Then the pathological changes in the stomach and small intestine tissues in each group were observed under a microscope.
The data of W1, W2, L1, and L2 can be obtained from item 2.3. The gastric emptying rate and small intestinal propulsion rate were calculated according to the formula (1-1) and (1-2) (Xiao et al., 2021).
The contents of motilin (MTL, Elabscience Biotechnology, China), gastrin (GAS, Elabscience Biotechnology, China), and 5-hydroxytryptamine (5-HT, Elabscience Biotechnology, China) in the serum of rats in the control group, model group, Mos group, RH group, and CH group were determined by enzyme-linked immunosorbent assay (ELISA). According to the determination method of the commercial rat ELISA kit, the samples were incubated in 96-well-plates at 37°C for 30 min. And the washed solution was added to each well and then discarded after incubation for the 30s. The samples were operated in 96-well-plates according to the commercial rat ELISA kit. The optical density (OD) value of the sample was measured at 450 nm with a microplate reader.
Paraffin-embedded slides of the stomach and small intestine tissues were heated in an oven at 60°C for 1 hour. Then the slides were deparaffinized in xylene and rehydrated in ethanol series. Next, the slides were incubated in blocking buffer (1x PBS, 0.3% Triton X-100, 10% normal goat serum) for 1 h at room temperature. Then, the slides were incubated with c-kit primary antibody (1:100, A0357, ABclonal Technology, China) overnight at 4°C, washed, and incubated with Cy3-coupled goat anti-rabbit fluorescent secondary antibody for 1 h at room temperature. Images were obtained using a fluorescence microscope. Five fields were randomly selected for each sample. And the image was semi-quantized by Image J software.
The mRNA expression of C-kit protein in the stomach and small intestine was detected by RT-qPCR. The total RNA was isolated from the stomach and small intestine by using the Total RNA Extraction Kit (R1200, Solarbio, China). The total RNA was reverse-transcribed to cDNA with the SuperReal PreMix Plus (FP205, TIANGEN, China). The 2 × SYBR Green PCR Mastermix (KR118, TIANGEN, China) was used for the amplification of cDNA. The primer sequences are shown in Table 1. The conditions of thermal cycling were 95°C for 5 min in the start cycle, 95°C for 10 s, and then a circulation was processed 40 times at 55°C for 20 s and 72°C for 30 s. A melt curve was gained by raising the temperature. In the end, cycle threshold (CT) values were obtained according to fluorescent signals. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control for normalization. The expression of c-kit mRNA was calculated with the 2−ΔΔCt methods.
All experimental data are shown as mean ± standard deviation (SD). Statistical comparisons were made by one-way analysis of variance (ANOVA) using SPSS software (version 18.0, USA), followed by the Dunnet t multiple comparison test. The P < 0.05 was considered the significance level.
Before modeling, the rats had normal behaviors such as feeding and sleeping. After modeling, there was no significant change in the control group, but the rats in the model group showed a gradual loss of appetite, no increase or even decrease in body weight, and the fur color of the rats was messy and dim. The rats in the model group showed decreased sensitivity to external stimuli such as capture and drive.
During modeling (Figure 2A), the weight of rats in the control group showed an increasing trend, while that in the model group showed a decreasing trend. At the end of the modeling, there was a significant difference in weight between the two groups. During the administration period (Figure 2B), the weight of rats in the control group remained unchanged, while the weight of rats in the other groups showed an increasing trend. The weight increase of rats in the Mos, RH, and CH groups was better than that in the model group, but there was still a significant difference from that in the control group.
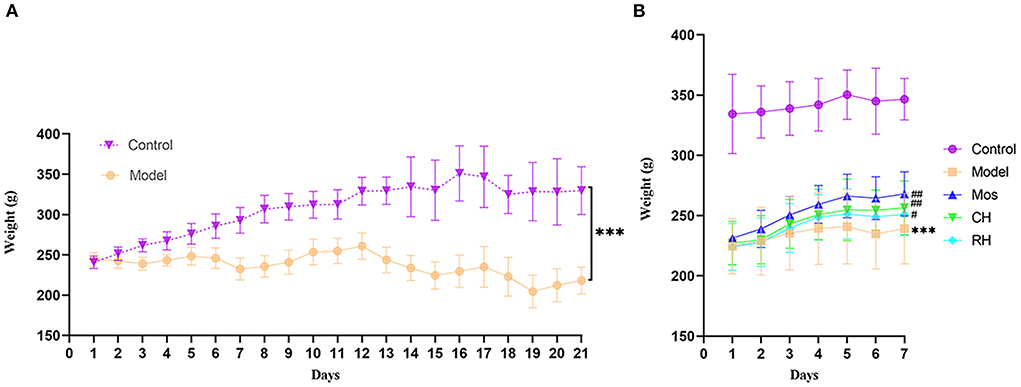
Figure 2. Weight changes in FD rats during modeling (A) and weight change during administration (B) (*** represents comparison with the control group: P < 0.001. # represents comparison with the model group: P < 0.05. ## represents comparison with the model group: P < 0.01. ### represents the comparison with the model group: P < 0.01).
According to the histological observation of the model group and the control group, the morphology of the stomach and small intestine of rats in each group were normal without obvious ulcers, bleeding, and erosion. Histological sections of each tissue sample showed that the histological structure of the stomach and small intestine was normal without obvious inflammatory cell infiltration and other pathological changes (Figure 3). The results showed that there was no organic lesion of the gastrointestinal tract in rats, indicating that the model met the requirements.
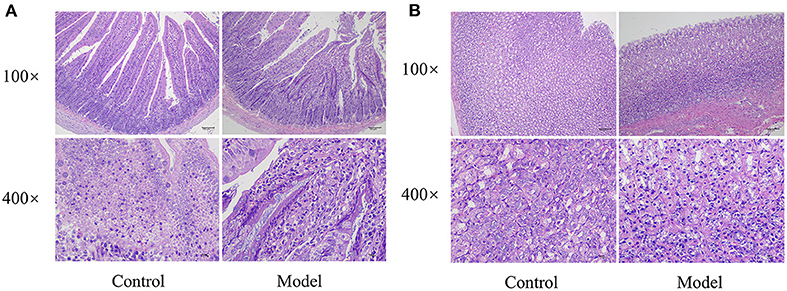
Figure 3. Micrographs of the small intestine (A) and stomach (B) of rats in the control group and FD model group (H and E staining).
As shown in Figure 4 and Table 2, there were significant differences between the control group and the model group (P < 0.01). It indicates successful modeling. Compared with the model group, the intestinal propulsion rate and gastric emptying rate of rats in the CH and Mos groups were significantly increased (P < 0.05). RH significantly promoted intestinal propulsion in FD rats (P < 0.05). However, there was no significant difference between the RH group and the model group in promoting gastric emptying.
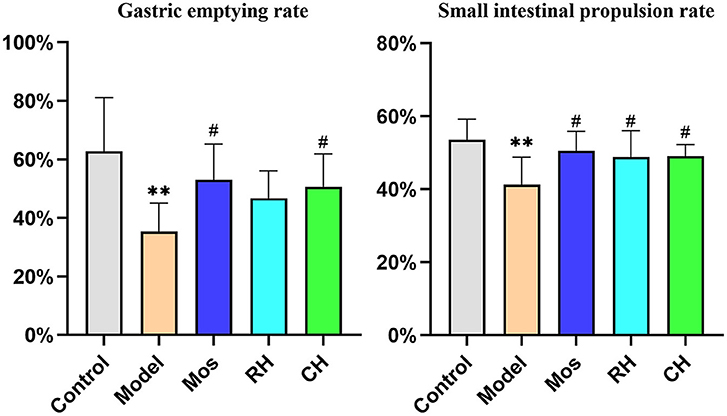
Figure 4. Comparison of gastric emptying rate and small intestine propulsion rate between the control, FD model, Mos group, RH, and CH group. (** represents comparison with the control group: P < 0.01. *represents comparison with the control group: P < 0.05. # represents comparison with the model group: P < 0.05).
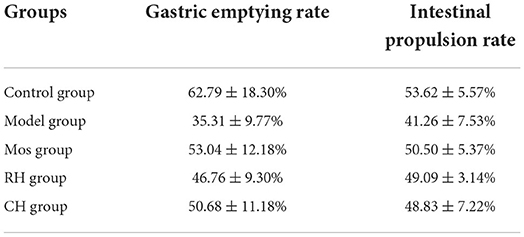
Table 2. Gastric emptying rate and intestinal propulsion rate in the control, FD model, Mos group, RH, and CH group.
As shown in Figure 5 and Table 3, the contents of MTL, GAS, and 5-HT in the serum of the model group were significantly lower than the control group (P < 0.001). However, Mos, RH, and CH can interfere with their contents in the serum of FD rats. Compared with the model group, the contents of GAS increased significantly in the Mos group and CH group (P < 0.05), but there was no obvious upward trend in the RH group. For MTL, only the Mos group had a significant increase compared with the model group (P < 0.05), and there was an insignificant increase in the RH group and CH group. Compared with the model group, 5-HT increased significantly in the CH group and the Mos group (P < 0.01), while the RH group had an insignificant increasing trend.
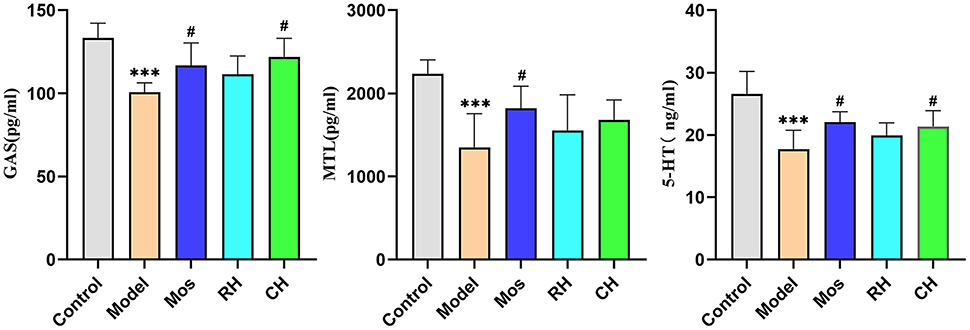
Figure 5. Comparison of the contents of 5-HT, GAS, and MTL in serum of rats in the control, FD model, Mos group, RH, and CH group. (*** represents comparison with the control group: P < 0.001. ** represents comparison with the control group: P < 0.01. * represents comparison with the control group: P < 0.05. # represents comparison with the model group: P < 0.05).
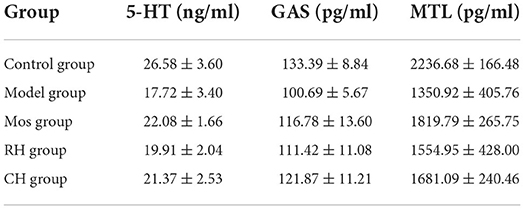
Table 3. Contents of 5-HT, GAS, and MTL in serum of rats in the control, FD model, Mos group, RH, and CH group.
The results are shown in Table 4, Figures 6, 7. Compared with the control group, the mean fluorescence density of c-kit protein in the stomach and small intestine of the model group was significantly decreased (P < 0.001). RH and CH can significantly increase the mean fluorescence density of c-kit protein in the small intestine of FD model rats (P < 0.001), but Mos has no obvious effect. The mean fluorescence density in the CH group was higher than that in the RH group. In gastric tissues, compared with the model group, the mean fluorescence density of the Mos group and CH group was significantly different (P < 0.001). The mean fluorescence density in gastric tissues of the RH group was also significantly different from that in the model group (P < 0.01), but significantly lower than that in the CH group (P < 0.05).
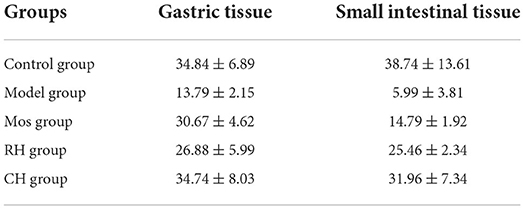
Table 4. Expression of the c-kit protein in the stomach and small intestine of rats in the control, FD model group, Mos group, RH, and CH group.
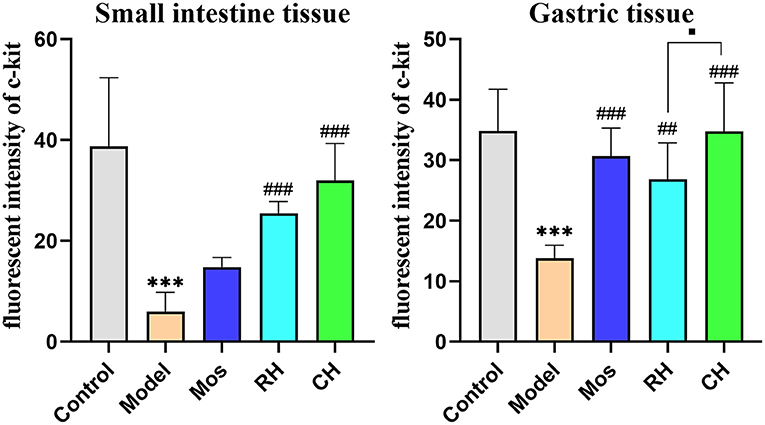
Figure 6. Comparison of c-kit protein expression in the stomach and small intestine of rats in the control, FD model group, Mos group, RH, and CH group. (*** represents comparison with the control group: P < 0.001. ## represents comparison with the model group: P < 0.01. ### represents the comparison with the model group: P < 0.01. ■ represents the comparison with the RH group: P < 0.05).
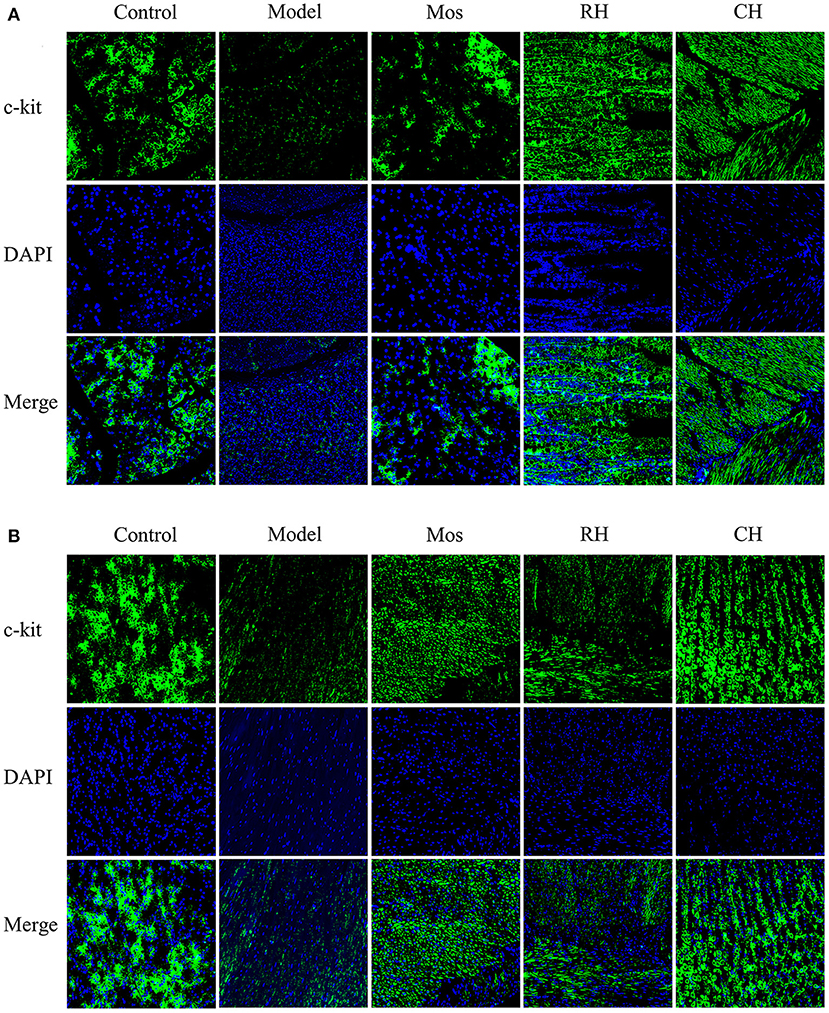
Figure 7. Immunofluorescence images of Characteristic protein c-kit of Cajal interstitial cells in the stomach (B) and small intestine (A) of rats in the control, FD model, Mos group, RH, and CH group.
As shown in Figure 8 and Table 5, compared with the control group, the expression of c-kit mRNA in the stomach and small intestine of the FD model group was significantly decreased (P < 0.001). RH, CH, and Mos could interfere with c-kit mRNA expression in the stomach and small intestine of FD rats. In small intestine tissue, CH had the most significant effect in upregulating c-kit mRNA expression (P < 0.001), followed by hawthorn (P < 0.01), and Mos was weaker than the former two (P < 0.05). In the gastric tissue, the effect of CH is still the most significant (P < 0.001), followed by Mos (P < 0.01), and RH had the weakest effect (P < 0.05).
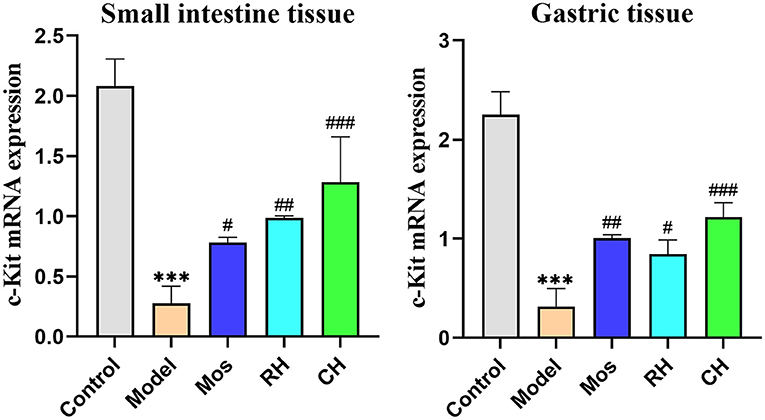
Figure 8. The expression of c-kit mRNA in the control, FD model, Mos group, RH, and CH group. (*** represents comparison with the control group: P < 0.001. # represents the comparison with the model group: P < 0.05. ## represents comparison with the model group: P < 0.01. ### represents the comparison with the model group: P < 0.01).
The traditional theory of Chinese medicine holds that “fried to brown” helps to improve digestive function and promote gastrointestinal motility. Many herbal medicines that are fried to brown are thought to promote digestion (Liu et al., 2021). Hawthorn has a long history of being used to treat indigestion, and it often appears in prescriptions related to digestive function. Some studies have shown that CH is more effective in improving digestive function than RH (Wang et al., 2019). Our study investigated the effects of RH and CH in the intervention of FD and elucidated the possible mechanism.
There are many methods for establishing the FD model. The method of establishing the FD model in this study is improved based on the existing research (Liang et al., 2018; Zhu et al., 2020). In this study, multiple factors were used for chronic induction modeling, which is determined by the complex pathogenesis of FD. During the modeling process, the weight of rats in the control group increased gradually, while the weight of rats in the model group increased slowly or decreased occasionally. This is consistent with the clinical features of FD patients (Tack et al., 2016). No obvious pathological changes were found in the stomach and small intestine sections of the control group and the model group after H&E staining. This indicated that no organic lesions of the digestive system were caused by modeling. This is in line with the characteristics of FD and indicates that the FD rat model in this study is reasonable.
Gastric emptying is the process by which food is pushed from the stomach into the duodenum. The gastric emptying rate can reflect the quality of gastric motility (Al-Saffar et al., 2019). The delay of gastric emptying is closely related to gastrointestinal motility (Kusano et al., 2014). The small intestine propulsion rate can reflect the peristaltic performance of the intestine. Reduced intestinal peristalsis is consistent with the clinical symptoms of FD. In this study, gastric emptying and intestinal propulsion in FD model rats were significantly weakened after modeling. After 7 days of continuous administration, gastric emptying rate and intestinal propulsion rate were improved in Mos, RH, and CH groups. CH showed significant effects on improving intestinal propulsion and gastric emptying in FD rats. RH can also improve intestinal propulsion in FD rats, but the effect of promoting gastric emptying is weak.
Gastrointestinal hormones secreted by gastrointestinal mucosal cells are closely related to gastrointestinal motility disorders (Mori et al., 2022). MTL is produced by endocrine cells in the intestinal mucosa. It is an important regulator of gastrointestinal motility through a specific motilin receptor (MLN-R) (Kitazawa and Kaiya, 2021). GAS is a peptide hormone mainly existing in G cells of the gastric antrum. Its main physiological function is to stimulate the secretion of gastric acid (Schubert and Rehfeld, 2019). 5-HT is synthesized, stored, and released by a subset of intestinal endocrine cells called enterochromaffin (EC) cells in the intestinal mucosa, which has been recognized as an important signaling molecule in the intestine (Mawe and Hoffman, 2013). In this study, the three gastrointestinal hormones in the FD model group showed a downregulation trend. As a positive drug, Mos can upregulate three gastrointestinal hormones in FD rats. RH and CH also have the effect to upregulate partial gastrointestinal hormones. However, in general, the effect of RH and CH on gastrointestinal hormones in FD rats was not strong, and there was no significant difference between them.
C-Kit protein is a quantitative marker of ICC. C-kit is a proto-oncogene that encodes receptor tyrosine kinase (kit) (Huizinga et al., 1995). The emergence of c-kit protein antibodies provides a variety of effective methods to identify ICC in pathological tissue sections (Streutker et al., 2007). The number of ICC was consistent with the expression trend of c-kit protein (Li et al., 2019). That is, the expression of c-Kit protein reflects the number of ICC changes. The results of immunofluorescence and PCR showed that the number of ICC in the small intestine and stomach of the FD model group was significantly decreased, while the three therapeutic drugs showed different effects on the number of ICC in gastrointestinal tissue. In general, the effect of hawthorn on the upregulation of ICC in the gastrointestinal tract was better than RH and Mos. RH and CH may improve gastrointestinal motility in FD model rats by regulating the number of ICC cells in the gastrointestinal tract. However, the limitation of this study is that more research is needed to prove this pathway and explain the mechanism.
We have proved that hawthorn decoction had an effect on improving the digestive function of FD rats, and the effect of CH was stronger than RH. CH could increase weight, improve intestinal propulsion and gastric emptying, and promote the secretion of motilin (MTL), gastrin (GAS), and 5-HT in the serum of FD rats. And the possible pathway is to improve gastrointestinal motility by increasing the number of ICC in the stomach and small intestine of FD rats. Our study will help to develop a functional food based on hawthorn and provide a possible dietary therapy for dyspepsia.
The data used to support the findings of this study are available from the first author upon request.
The experimental program was approved by the Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine.
LA and LZ conceived the project and wrote the article. CW provided supervision. LZ, QL, YT, and TC performed the research. LA, LZ, QL, YT, and CW analyzed the data. All authors have read and approved the manuscript for publication.
This study was financially supported by Sichuan Applied Basic Research Fund (No: 2021YJ0112).
We thank Chengdu University of Traditional Chinese Medicine for providing the laboratory conditions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Saffar, A., Takemi, S., Saaed, H. K., Sakata, I., and Sakai, T. (2019). Utility of animal gastrointestinal motility and transit models in functional gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 40–41, 101633. doi: 10.1016/j.bpg.2019.101633
Aro, P., Talley, N. J., Agréus, L., Johansson, S. E., Bolling-Sternevald, E., Storskrubb, T., et al. (2011). Functional dyspepsia impairs quality of life in the adult population. Aliment. Pharmacol. Ther. 33, 1215–1224. doi: 10.1111/j.1365-2036.2011.04640.x
Chiarioni, G., Pesce, M., Fantin, A., and Sarnelli, G. (2018). Complementary and alternative treatment in functional dyspepsia. United European Gastroenterol. J. 6, 5–12. doi: 10.1177/2050640617724061
Ford, A. C., Mahadeva, S., Carbone, M. F., Lacy, B. E., and Talley, N. J. (2020). Functional dyspepsia. Lancet 396, 1689–1702. doi: 10.1016/S0140-6736(20)30469-4
Ford, A. C., Marwaha, A., Sood, R., and Moayyedi, P. (2015). The global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 64, 1049–1057. doi: 10.1136/gutjnl-2014-307843
Huizinga, J. D., Hussain, A., and Chen, J. H. (2021). Interstitial cells of Cajal and human colon motility in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 321, G552–G575. doi: 10.1152/ajpgi.00264.2021
Huizinga, J. D., Thuneberg, L., Klüppel, M., Malysz, J., Mikkelsen, H. B., and Bernstein, A. (1995). W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349. doi: 10.1038/373347a0
Kitazawa, T., and Kaiya, H. (2021). Motilin comparative study: structure, distribution, receptors, and gastrointestinal motility. Front. Endocrinol. 12, 700884. doi: 10.3389/fendo.2021.700884
Kusano, M., Hosaka, H., Kawada, A., Kuribayashi, S., Shimoyama, Y., Zai, H., et al. (2014). Gastrointestinal motility and functional gastrointestinal diseases. Curr. Pharm. Des. 20, 2775–2782. doi: 10.2174/13816128113199990572
Li, L., Zou, C., Zhou, Z., Wang, X., and Yu, X. (2019). Phenotypic changes of interstitial cells of Cajal after intestinal obstruction in rat model. Braz. J. Med. Biol. Res. 52, e8343. doi: 10.1590/1414-431x20198343
Liang, Q., Yan, Y., Mao, L., Du, X., Liang, J., Liu, J., et al. (2018). Evaluation of a modified rat model for functional dyspepsia. Saudi J. Gastroenterol. 24, 228–235. doi: 10.4103/sjg.SJG_505_17
Liu, X., Cao, S., and Zhang, X. (2015). Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 63, 7885–7895. doi: 10.1021/acs.jafc.5b02404
Liu, Y., Liao, W., Liu, X., Hu, Y., Zhu, X., Ju, L., et al. (2021). Digestive promoting effect and mechanism of Jiao Sanxian in rats. J. Ethnopharmacol. 278, 114334. doi: 10.1016/j.jep.2021.114334
Martinelli, F., Perrone, A., Yousefi, S., Papini, A., Castiglione, S., Guarino, F., et al. (2021). Botanical, phytochemical, anti-microbial and pharmaceutical characteristics of hawthorn (Crataegusmonogyna Jacq.), Rosaceae. Molecules 26, 7266. doi: 10.3390/molecules26237266
Mawe, G. M., and Hoffman, J. M. (2013). Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486. doi: 10.1038/nrgastro.2013.105
Mori, H., Verbeure, W., Schol, J., Carbone, F., and Tack, J. (2022). Gastrointestinal hormones and regulation of gastric emptying. Curr. Opin. Endocrinol. Diabetes Obes. 29, 191–199. doi: 10.1097/MED.0000000000000707
Pearlman, M., and Akpotaire, O. (2019). Diet and the role of food in common gastrointestinal diseases. Med. Clin. North Am. 103, 101–110. doi: 10.1016/j.mcna.2018.08.008
Sander, G. B., Mazzoleni, L. E., Francesconi, C. F., Balbinotto, G., Mazzoleni, F., Wortmann, A. C., et al. (2011). Influence of organic and functional dyspepsia on work productivity: the HEROES-DIP study. Value Health 14(5 Suppl 1), S126–S129. doi: 10.1016/j.jval.2011.05.021
Sanders, K. M., Ward, S. M., and Koh, S. D. (2014). Interstitial cells: regulators of smooth muscle function. Physiol. Rev. 94, 859–907. doi: 10.1152/physrev.00037.2013
Schubert, M. L., and Rehfeld, J. F. (2019). Gastric peptides-gastrin and somatostatin. Compr. Physiol. 10, 197–228. doi: 10.1002/cphy.c180035
Streutker, C. J., Huizinga, J. D., Driman, D. K., and Riddell, R. H. (2007). Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology 50, 176–189. doi: 10.1111/j.1365-2559.2006.02493.x
Tack, J., and Camilleri, M. (2018). New developments in the treatment of gastroparesis and functional dyspepsia. Curr. Opin. Pharmacol. 43, 111–117. doi: 10.1016/j.coph.2018.08.015
Tack, J., Ly, H. G., Carbone, F., Vanheel, H., Vanuytsel, T., Holvoet, L., et al. (2016). Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin. Gastroenterol. Hepatol. 14, 385–392.e4. doi: 10.1016/j.cgh.2015.09.043
Tack, J., Talley, N. J., Camilleri, M., Holtmann, G., Hu, P., Malagelada, J. R., et al. (2006). Functional gastroduodenal disorders. Gastroenterology 130, 1466–1479. doi: 10.1053/j.gastro.2005.11.059
Wang, Y., Lv, M., Wang, T., Sun, J., Wang, Y., Xia, M., et al. (2019). Research on mechanism of charred hawthorn on digestive through modulating “brain-gut” axis and gut flora. J. Ethnopharmacol. 245, 112166. doi: 10.1016/j.jep.2019.112166
Wauters, L., Talley, N. J., Walker, M. M., Tack, J., and Vanuytsel, T. (2020). Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 69, 591–600. doi: 10.1136/gutjnl-2019-318536
Wei, Z., Ai, L., Chen, X., Li, L., Wang, L., Fan, W., et al. (2019). Comparative studies on the regulatory effects of raw and charred hawthorn on functional dyspepsia and intestinal flora. Trop. J. Pharm. Res. 18, 333–339. doi: 10.4314/tjpr.v18i2.16
Xiao, H. L., Xiao, Y. J., Wang, Q., Chen, M. L., and Jiang, A. L. (2021). Moxibustion regulates gastrointestinal motility via HCN1 in functional dyspepsia rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 27, e932885. doi: 10.12659/MSM.932885
Zhang, G., Xie, S., Hu, W., Liu, Y., Liu, M., Liu, M., et al. (2016). Effects of electroacupuncture on interstitial cells of Cajal (ICC) ultrastructure and connexin 43 protein expression in the gastrointestinal tract of functional dyspepsia (FD) rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 22, 2021–2027. doi: 10.12659/MSM.899023
Zhang, L. M., Zeng, L. J., Deng, J., Zhang, Y. Q., Wang, Y. J., Xie, T. Y., et al. (2018). Investigation of autophagy and differentiation of myenteric interstitial cells of Cajal in the pathogenesis of gastric motility disorders in rats with functional dyspepsia. Biotechnol. Appl. Biochem. 65, 533–539. doi: 10.1002/bab.1635
Keywords: charred hawthorn, raw hawthorn, interstitial cells of Cajal, functional dyspepsia, gastrointestinal motility
Citation: Ai L, Zhang L, Liang Q, Tian Y, Chen T and Wu C (2022) Investigation of the improving effect of raw and charred hawthorn on functional dyspepsia based on interstitial cells of Cajal. Front. Sustain. Food Syst. 6:1010556. doi: 10.3389/fsufs.2022.1010556
Received: 03 August 2022; Accepted: 30 September 2022;
Published: 17 October 2022.
Edited by:
Monica Trif, Centre for Innovative Process Engineering, GermanyReviewed by:
Chongbo Zhao, Shaanxi University of Chinese Medicine, ChinaCopyright © 2022 Ai, Zhang, Liang, Tian, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunjie Wu, d3VjamNkdGNtQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.