- Laboratory of Food Analysis, Department of Food Technology, Faculty of Agriculture and Food Technology, Tien Giang University, My Tho City, Vietnam
Garlic (Allium sativum L.) has been used as a functional food and medicine in traditional prescriptions for centuries. The extract of garlic husks contains phytonutrients and antioxidant capacity, which can be applied in the food, nutraceutical, cosmetics, and pharmaceutical industries. However, garlic husks, a by-product of the food industry, are considered agricultural wastes. Hence, this research aims at evaluating the content of several compounds in the extract of garlic husks and determining the appropriate temperature and time for the extraction processing of bioactive compounds from garlic husks. In this research, garlic husk powder was extracted at different temperatures from 40 to 80oC during time durations of 30–120 min. This study found that the optimum temperature was from 60 to 70oC and the time duration was from 60 to 90 min for the extraction process. The optimal content of total polyphenols content of 8.93 ± 0.252 mg gallic acid equivalent/g, total flavonoids content of 0.028 ± 0.002 (mg quercetin equivalent/g), total thiosulfinates content of 9.73 ± 0.071 (μmol/g), and total anthocyanins content of 0.0047 ± 0.0001 (mg/g) of dried garlic husk. Based on the finding, the study suggests that garlic husk should be utilized as a potential source of natural antioxidants in garlic extract, a food supplement, that contains antioxidants to support the cardiovascular and immune systems+ and odorless garlic products.
Introduction
Garlic (Allium sativum L.) has been used for disease treatments due to the existence of multiple natural antioxidant compounds in the bulb, cloves, leaves, and flowers. The garlic plant's bulb is the most widely used part of the plant. Apart from the single clove type, garlic bulbs are divided into numerous fleshy sections called cloves. Along with leaves and flowers, garlic cloves are used for food consumption or medicinal purposes. Garlic is grown in various regions, including Asia (i.e. Vietnam, Thai, Myanmar, Lao, Cambodia, Singapore, China, Japan, and Korea), the Middle East, northern Africa, southern Europe, and parts of Latin America. Garlic is easy to grow and can be grown year-round in mild climates and the price is normally affordable for most people (Sikandar et al., 2022).
Garlic contains more sulfur (at least four times) than other high sulfur vegetables: onions, broccoli, and cauliflower. Garlic is also rich in carbohydrates containing fructose, protein, fiber, saponins, phosphorus, potassium, zinc, moderate amounts of selenium and vitamin C, steroid glycosides, lectins, prostaglandins, essential oils, adenosine, vitamins B1, B2, B6, and E, biotin, nicotinic acid, fatty acids, glycolipids, phospholipids, anthocyanins, flavonoids, phenols, and essential amino acids (Borek, 2006).
There are many studies showing the health benefits and anti-aging effects of garlic. Due to being rich in antioxidant compounds and organic sulfur, garlic has been shown to help prevent many chronic diseases and conditions related to aging, especially cardiovascular and cerebrovascular disease, cancer, and Alzheimer's disease. Garlic reduces risk factors for disease and aging, including reducing oxidative stress and inflammation, lowering cholesterol, triglycerides, and homocysteine, inhibiting coronary plaque formation, and preventing platelet aggregation. Garlic treatment increases vasodilation and blood circulation, lowers blood pressure, prevents nerve cell death and increases memory and cognitive ability, boosts immunity, boosts glutathione, promotes internal antioxidants, increases physical endurance, and improves fatigue (Watson and Preedy, 2010).
For thousands of years, garlic has been used for the treatment of many diseases thanks to the existence of several bioactive compounds with proven therapeutic effects, such as antimicrobial, antioxidant, hypolipidaemic, anticoagulant, anti-hypertensive, antitherosclerotic. and hypoglycaemic (Bautista et al., 2005). Allicin (diallylthiosulfinate) is the main thiosulfinate with two allyl groups as carbon chains in fresh garlic. Diallylthiosulfinate is responsible for the characteristic flavor of garlic (Salazar et al., 2008). Besides, anthocyanins are compounds belonging to the flavonoid, common water-soluble pigments, often exhibiting purple, burgundy, or green colors in vegetables, fruits, flowers, and roots of plants.
These compounds have antioxidant attributes, cancer prevention, and anti-aging properties for consumers. However, recent studies have found that garlic husk extraction contains phenolic compounds with high antioxidant and antibacterial activity (Kallel et al., 2014; Kimthet et al., 2017; Mohamed et al., 2022; Paula et al., 2022). Considering the huge benefits of garlic for human wellbeing, all the components of garlic should be utilized to enhance people's livelihoods and reduce the waste of agricultural products.
The wastes incurred from crop cultivation and processing are generally referred to as agricultural crop residues and food industry, respectively. The quintessentially lignocellulosic biomass is considered an important sustainable raw material for bio-refineries, the production of fuels, fertilizer, and chemicals (Balogun et al., 2018; Vivek et al., 2019; Singh et al., 2021, 2022). Out of this, garlic husks from the food processing industry made up ~25% garlic, thereby it was considered agricultural waste (Kallel et al., 2016; Kimthet et al., 2017; Paula et al., 2022). On the other hand, currently, there are many techniques to obtain phytochemicals from plants, such as soxhlet extraction, immersion, supercritical liquid extraction, supercritical water extraction, and ultrasonic extraction (Kallel et al., 2014). However, the extraction efficiency and antioxidant activity depended not only on the extraction method but also on the extraction temperature and time. According to Spigno et al. (2007), temperature and time extraction affect the solubility, mass-to-transfer rate (diffusion coefficient), and stability of phenolic compounds. It is highly unstable at a near-neutral pH level, at elevated temperatures, or in the presence of lipids. It also rapidly degrades during processing or storage which limits its bioavailability (Kim et al., 2012). Hence, it is essential to investigate the appropriate temperature and time for the extraction processing of bioactive compounds from garlic husks by using a solvent extraction method.
To achieve sustainable development goals, the utilization of the by-products from garlic is the focus of many stakeholders in the food and medical industries. However, there are few studies focusing on the potential safe sources of natural additives with antibacterial and/or antioxidant properties for the food industry. In addition, different compounds have been extracted and isolated, some of them are known as polyphenols. There is limited reported data about the extraction of bioactive compounds from garlic husk (Kimthet et al., 2017). Therefore, this study is expected to fill the research gap.
Therefore, the objective of this research was twofold. Firstly, this study aims to determine the appropriate temperature and time for the extraction processing of bioactive compounds from garlic husks by using a solvent extraction method. Secondly, this research aims at evaluating the content of several compounds in the extract of garlic husks.
Materials and methods
Materials
Fresh garlic bulbs were harvested and selected at the maturity of 130–135 days (after planting) at Van Hai ward, Phan Rang—Thap Cham city, Ninh Thuan province, Vietnam. The dried garlic husks were separated from the garlic bulbs. Dried garlic husks were collected and dried to obtain a moisture content of 3%−5%. Next, the dried garlic husk samples were finely ground and passed through a 1 mm sieve to obtain homogeneous powder particles. The garlic husk powder was packed in aluminum-coated plastic bags and stored at 4oC before extracting.
Methods
Garlic husk powder (5 g) was mixed with 250 ml of distilled water and extracted at 40–80oC for 30–120 min. The mixture extracts were filtered through a Whatman® 41 filter paper. All extracts were kept at 4oC prior to the analysis.
Total polyphenols content [mg gallic acid equivalent (GAE)/g dry matter]
Total polyphenols content (TPC) was determined by the Folin-Ciocalteu method (Wolfe et al., 2003). Phenol reacts with phosphomolybdic acid in the Folin-Ciocalteau reagent. A blue complex is formed in an alkaline medium. The absorbance of the sample was measured at wavelength 765 nm by using a UV spectrophotometer. To determine TPC in the samples, the color intensity was measured on the spectrophotometer and the gallic acid standard curve.
Total flavonoid content
The total flavonoid content (TFC) was determined through the coloration method with AlCl3 (Zhu et al., 2010). The absorbance of the reaction solution was measured at a wavelength of 415 nm. To determine TFC in the samples, the quercetin standard curve was employed. The results were expressed by using mg of quercetin equivalent (QE) per gram of dry weight (mg QE/g).
Total thiosulfinate content (μmol/g; TTC)
The measurement of absorbance was conducted at a wavelength of 412 nm of 2-nitro-5-thiobenzoate by adopting the method of Kinalski and Norena (2014).
Total anthocyanin content (mg/g)
Total anthocyanin content (TAC) was analyzed by using differential pH methods based on the color change due to a change in the structure of anthocyanins between pH = 1.0 and pH = 4.5 (The Association of Analytical Communities (AOAC) International., 2003). At pH = 1, anthocyanins exist in the form of oxonium or flavium with maximum absorption, and they are in the form of colorless carbinol at pH = 4.5. The differential pH method allows for the fast and exact determination of the TAC, even in the presence of other interfering compounds.
Statistical analysis
The data was recorded in triplicates. The data were analyzed by two-way ANOVA followed by using Fisher's Least Significant Difference (LSD) test. The reference equation was chosen based on the R2 obtained from the multiple regression analysis, the equation obtains higher R2 as follows:
The significance or non-significance of results was considered statistically significant at p < 0.05. Moreover, the Statgraphics Centurion XVI software (version 16.0; Statpoint Technologies, Inc., USA) was used for response surface plots in this study.
Results and discussion
The experimental research results show that regression equations were constructed, using the influence of temperature (X: 40–80oC) and time (Y: 30–120 min) extraction on the TAC, TFC, TPC, and TTC (Equations 1, 2, 3, and 4, respectively) with a rather high coefficient of determination (R2 > 0.7).
R2 = 0.91
R2 = 0.72
R2 = 0.86
R2 = 0.92.
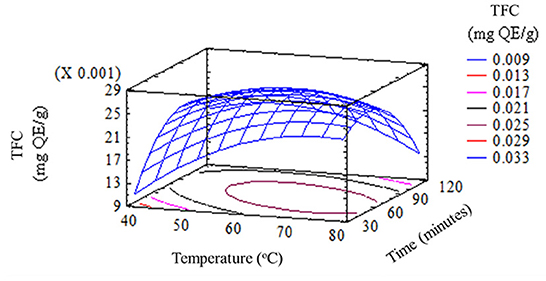
The results of the experiments were shown in Figures 1–4. The response surface models indicated that the effects of temperature and time on TAC, TFC, TPC, and TTC could help to predict extraction results, respectively. In the different garlic husk extracts, the content of total flavonoid was notably higher than it was in mature garlic bulbs (5.78 and 4.16 μg QE/g, respectively) (Bozin et al., 2008). That difference between the total polyphenol and flavonoid in the bulb and the husk could be explained by the accumulation of polyphenol compounds in the outer parts of plants such as husks, skins, and shells (Lecumberri et al., 2007). Generally, in immersion extraction, the extraction processes consist of desorption from the solid surface and diffusion of the solid matrix to the extraction phase. Therefore, the extraction rate is slow. Anthocyanins are a well-water-soluble compound. The results in Figure 1 recommended that temperature and time of extraction had significant effects (p < 0.05) on TAC in garlic husk extract. Garlic husk was extracted at 80oC for 120 min, indicating the lowest TAC (0.0010 mg/g). As the extraction temperature increased, TAC also increased. However, anthocyanins would be degraded if extraction time was prolonged. TAC was 0.0055 ± 0.0003 mg/g, with the suitable temperature and time for extracting anthocyanins from garlic husks being 70oC and 60 min, respectively.
Temperature is one of the important parameters which significantly influence the total polyphenol compound content of extraction. At high temperatures, the solubility of compounds could be increased because of decreasing the polarity of the solvent, which facilitated the solvent penetration into the material (Williams et al., 2009). In addition, the cell tissues of the material may be disrupted during extraction processing which makes the mass transfer rate increase (Maran et al., 2013; Mokrani and Madani, 2016). Besides, the lignin (this is the component of the plant cell wall) might be decomposed into polyphenol compounds at high temperatures (Wahyu et al., 2008; Brebu and Vasile, 2010). Brebu and Vasile (2010) trusted that the functional group of lignin was cleaved at high temperatures. The results expressed the low molecular weight product. Rearrangement of the backbone is complete when it leads to the release of the volatile product. Besides, the cleavage of the aryl-ether linkages results in the formation of highly reactive and unstable free radicals. Then these products have further reactions through rearrangement, electron abstraction, or radical–radical interaction to form stable products. So, the decomposition of the polymer structures in lignin results in the formation of aromatic hydrocarbons, polyphenol, hydroxyphenolics, and guaiacyl-syringyl type compounds (Brebu and Vasile, 2010).
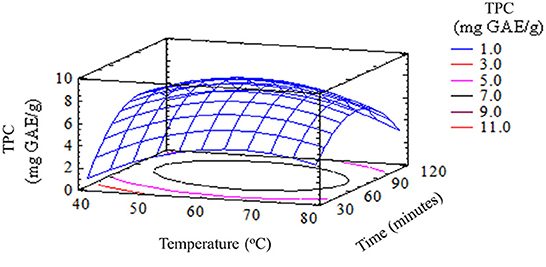
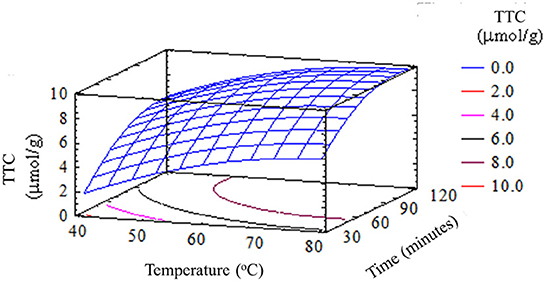
Similarly, the temperature and time of extraction also had a significant effect on the remaining three compounds (polyphenols, flavonoids, and thiosulfinates) in garlic husks by (p < 0.05). The TPC in garlic husks extracted at 60oC for 90 min was 8.93 ± 0.25 (mg GAE/g), which was higher than other extraction conditions. It was lower than the content present in the dry skin of red onion (80 mg GAE/g) (Nuutila et al., 2003), but was similar to or higher than the contents in several vegetables and plants, such as garlic bulb extracts (0.05–0.98 mg GAE/g) (Bozin et al., 2008), onion (2.5 ± 0.1 mg GAE/g), carrot leaf and peel (7.4 ± 0.1 and 6.6 ± 0.1 mg GAE/g, respectively) and potato peel (4.3±0.2 mg GAE/g) (Kähkönen et al., 1999; Meneses et al., 2013). Therefore, the results implied that garlic husk is a raw material with a significant content of polyphenol compounds. In parallel, water dissolves a wide range of compounds and is inexpensive, nontoxic, and nonflammable which is commonly used in food processing.
On the other hand, thanks to the polarity and good solubility of polyphenol components from various plant materials, methanol was a better solvent than others in extracting phenolic compounds (Siddhuraju and Becker, 2003). For example, the extraction of phenolic compounds from walnut green husk (Fernández-Agulló et al., 2013), cacao bean husk (Kim et al., 2004), and wild rice hulls (Asamarai et al., 1996).
To a limited extent, high temperatures increased the efficiency of garlic husk extraction by enhancing the diffusivity and solubility of the substance. Beyond this limit, high extraction temperature would reduce the content of polyphenols, flavonoids, and thiosulfinates in extracts. The time of extraction affected the extraction of active ingredients. When the extraction time was short, the bioactive compounds were not completely extracted. On the contrary, when the time was too long, some bioactive compounds would be oxidized and decomposed. The quality and quantity of the phytonutrients would also decrease because most of these compounds were sensitive to high temperatures. Besides that, total polyphenol content (14.47 mg GAE/g of dried weight garlic husk) and antioxidant activity (1.13 mg/ml) were attained by using methanol rather than using ethanol soxhlet as the solvent in soxhlet extraction. This result can be explained by the fact that methanol could extract more polar compounds as well as polyphenol compounds than ethanol (Lapornik et al., 2005).
Conclusions
The results obtained in this work showed that garlic husks can be used as a source of phytonutrients. The extraction of polyphenol compounds and various bioactive compounds could be considered as an alternative of great interest for the change in the value of this food processing by-product. Research results expressed that both factors, namely extraction temperature and time affected the extraction of healthful compounds from garlic husks. The high amount of TPC of 8.93 ± 0.252 mg GAE/g, TFC of 0.028 ± 0.002 (mg QE/g), TTC of 9.73 ± 0.071 (μmol/g), and TAC of 0.0047 ± 0.0001 (mg/g) of dried garlic husk which was achieved at a temperature of 60–70oC for a period of 60–90 min. This suggested that garlic husks could be a potential source of antioxidants to be used for different biological applications, health promotion, and disease prevention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NT contributed to conception and design of the study who organized the database, performed the statistical analysis, wrote the manuscript, contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asamarai, A. M., Addis, P. B., Epley, R. J., and Krick, T. P. (1996). Wild rice hull antioxidants. J. Agric. Food Chem. 44, 126–130. doi: 10.1021/jf940651c
Balogun, A. O., Lasode, O. A., and Mcdonald, A. G. (2018). Thermochemical and pyrolytic analyses of Musa spp. residues from the rainforest belt of Nigeria. Environ. Prog. Sustain. Energy 37, 1–10. doi: 10.1002/ep.12869
Bautista, D. M., Movahed, P., Hinman, A., Axelsson, H. E., Sterner, O., Hogestatt, E. D., et al. (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA. 102, 12248–12252. doi: 10.1073/pnas.0505356102
Borek, C. (2006). Garlic reduces dementia and heartdisease risk. J. Nutr. 136, 810S−812S. doi: 10.1093/jn/136.3.810S
Bozin, B., Mimica-Dukic, N., Samojlik, I., Goran, A., and Igic, R. (2008). Phenolics as antioxidants in garlic (A. sativum L. Alliaceae). Food Chem. 111, 925–929. doi: 10.1016/j.foodchem.2008.04.071
Brebu, M., and Vasile, C. (2010). Thermal degradation of lignin – a review. Cellulose Chem. Technol. 44, 353–363.
Fernández-Agulló, A., Pereira, E., Freire, M. S., Valentão, P., Andrade, P. B., González-Álvareza, J., et al. (2013). Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 42, 126–132. doi: 10.1016/j.indcrop.2012.05.021
Kähkönen, M. P., Hopia, A. I., Vuorela, H. J., Rauha, J.-P., Pihlaja, K., Kujala, T. S., et al. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47, 3954–3962. doi: 10.1021/jf990146l
Kallel, F., Bettaieb, F., Khiari, R., Garcia, A., Bras, J., Chaabouni, S. E., et al. (2016). Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 87, 287–296. doi: 10.1016/j.indcrop.2016.04.060
Kallel, F., Driss, D., Chaari, F., Belghith, L., and Bouaziz, F. (2014). Garlic (Allium ativum L.) husk wasted as a potential source of phenolic compounds: influence of extracting solvents on its antimicrobial and antioxidant activity. Ind. Crops Prod. 62, 34–41. doi: 10.1016/j.indcrop.2014.07.047
Kim, J. H., Nam, S. H., Rico, C. W., and Kang, M. Y. (2012). A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 47, 1176–1182. doi: 10.1111/j.1365-2621.2012.02957.x
Kim, K. H., Lee, K. W., Kim, D. Y., Park, H. H., Kwon, I. B., Lee, H. J., et al. (2004). Extraction and fractionation of glucosyltransferase inhibitors from cacao bean husk. Process Biochem. 39, 2043–2046. doi: 10.1016/j.procbio.2003.10.006
Kimthet, C., Chiho, U., Wahyu, D., Hideki, K., and Motonobu, G. (2017). Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chem. Eng. Process. 117, 113–119. doi: 10.1016/j.cep.2017.03.023
Kinalski, T., and Norena, C. P. Z. (2014). Effect of blanching treatments on antioxidant activity and thiosulfinate degradation of garlic (Allium sativum L.). Food Bioproc. Technol. 7, 2152–2157. doi: 10.1007/s11947-014-1282-1
Lapornik, B., Prošek, M., and Wondra, A. G. (2005). Comparison of extracts prepared from plant by-product using solvents and extraction time. J. Food Eng. 71, 214–222. doi: 10.1016/j.jfoodeng.2004.10.036
Lecumberri, E., Mateos, R., Izquierdo-Pulido, M., Ruperez, P., Goya, L., Bravo, L., et al. (2007). Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 104, 948–954. doi: 10.1016/j.foodchem.2006.12.054
Maran, J. P., Manikandan, S., Thirugnanasambandham, K., Nivetha, C. V., and Dinesh, R. (2013). Box–Behnken design based statistical modeling for ultrasonic assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 92, 604–611. doi: 10.1016/j.carbpol.2012.09.020
Meneses, N. G. T., Martins, S., Teixeira, J. A., and Mussatt, S. I. (2013). Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer's spent grains. Sep. Purif. Technol. 108, 152–158. doi: 10.1016/j.seppur.2013.02.015
Mohamed, H. S., Zineb, K., Youness, A., Ariel, G. C., Abdoulaye, S., El-houssaine, A., et al. (2022). Exploration of multifunctional properties of garlic skin derived cellulose nanocrystals and extracts incorporated chitosan biocomposite films for active packaging application. Int. J. Biol. Macromol. 210, 639–653. doi: 10.1016/j.ijbiomac.2022.04.220
Mokrani, A., and Madani, K. (2016). Effect of solvent, time and temperature on extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 162, 68–76. doi: 10.1016/j.seppur.2016.01.043
Nuutila, A. M., Puupponen-Pimia, R., Aarmi, M., and Oksman-Caldentey, K. M. (2003). Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 81, 485–493. doi: 10.1016/S0308-8146(02)00476-4
Paula, C. M. S., Larissa, M. R. S., Francisco, E. A. M., Fernando, E. T. C., Maria, J. G. F., Evania, A. T. F., et al. (2022). Garlic (Allium sativum L.) peel extracts: from industrial by-product to food additive. Appl. Food Res. 2 100186. doi: 10.1016/j.afres.2022.100186
Salazar, H., Llorente, I., Jara-oseguera, A., Gacia-villegas, R., Munari, M., Gordon, S. E., et al. (2008). A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 11, 255–261. doi: 10.1038/nn2056
Siddhuraju, P., and Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves. J. Agric. Food Chem. 51, 2144–2155. doi: 10.1021/jf020444+
Sikandar, H., Ammar, A., Husain, A., Kashif, H., Muhammad, A. K., Tian, R., et al. (2022). Garlic, from medicinal herb to possible plant bioprotectant: a review. Sci. Horticult. 304, 111296. doi: 10.1016/j.scienta.2022.111296
Singh, R., Das, R., Sangwan, S., Rohatgi, B., Khanam, R., Peera, S. K., et al. (2021). Utilisation of agro-industrial waste for sustainable green production: a review. Environ. Sustain. 4, 619–636. doi: 10.1007/s42398-021-00200-x
Singh, R., Langyan, S., Sangwan, S., Gaur, P., Khan, F. N., Yadava, P., et al. (2022). Optimization and production of alpha-amylase using Bacillus subtilis from apple peel: comparison with alternate feedstock. Food Biosci. 101978. doi: 10.1016/j.fbio.2022.101978
Spigno, G., Tramelli, L., and De Faveri, D. M. (2007). Effects of extraction time, temperature, and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81, 200–208. doi: 10.1016/j.jfoodeng.2006.10.021
The Association of Analytical Communities (AOAC) International. (2003). AOAC Official Methods Program Manual. Available online at: www.aoac.org/vmeth/omamanual/omamanual.htm (accessed June 3, 2021).
Vivek, N., Nair, L. M., Mohan, B., Nair, S. C., Sindhu, R., Pandey, A., et al. (2019). Bio-butanol production from rice straw – recent trends, possibilities, and challenges. Bioresour. Technol. Rep. 7, 100224. doi: 10.1016/j.biteb.2019.100224
Wahyu, D., Sasaki, M., and Goto, M. (2008). Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process. 47, 1609–1619. doi: 10.1016/j.cep.2007.09.001
Watson, R. R., and Preedy, V. R. (2010). Bioactive Foods in Promoting Health Fruits and Vegetables. New York, NY: Elsevier Inc, USA.
Williams, J. R., Al-Nabhani, F., and Al-Hamdi, A. (2009). The microware-assisted solvent extraction of propranolol from Tablets. Can. J. Pure Appl. Sci. 3, 911−915.
Wolfe, K., Wu, X., and Liu, L. H. (2003). Antioxidant activity of apple peels. J. Agric. Food Chem. 51, 609–614. doi: 10.1021/jf020782a
Keywords: bioactive compound, extraction, garlic husk, temperature, time
Citation: Thach NA (2022) Investigation of the effects of extraction temperature and time on bioactive compounds content from garlic (Allium sativum L.) husk. Front. Sustain. Food Syst. 6:1004281. doi: 10.3389/fsufs.2022.1004281
Received: 27 July 2022; Accepted: 11 November 2022;
Published: 06 December 2022.
Edited by:
Taku Tsusaka, Ostrom, ThailandReviewed by:
Kshirod Kumar Dash, Ghani Khan Choudhury Institute of Engineering & Technology (GKCIT), IndiaSapna Langyan, National Bureau of Plant Genetic Resources (ICAR), India
Phan Ke Son, Vietnam Academy of Science and Technology, Vietnam
Copyright © 2022 Thach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nguyen Ai Thach, bmd1eWVuYWl0aGFjaEB0Z3UuZWR1LnZu
 Nguyen Ai Thach
Nguyen Ai Thach