
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst., 04 February 2022
Sec. Sustainable Food Processing
Volume 5 - 2021 | https://doi.org/10.3389/fsufs.2021.799431
This article is part of the Research TopicSustainable Postharvest Management Practices for Fresh ProduceView all 5 articles
 Truong Dang Le1,2
Truong Dang Le1,2 Thanh Viet Nguyen1,2*
Thanh Viet Nguyen1,2* Nguyen Van Muoi3
Nguyen Van Muoi3 Ha Thanh Toan4
Ha Thanh Toan4 Nguyen Mai Lan5,6*
Nguyen Mai Lan5,6* Tri Nhut Pham1,2*
Tri Nhut Pham1,2*Mango (Mangifera indica L.) is a widely consumed fruit in tropical/subtropical regions around the world due to its excellent flavor and taste, and valuable source of nutrients and phytochemical compounds. As a climacteric fruit, mango is easily perishable after harvesting due to the ripening process, environmental conditions, and improper postharvest handling, leading to significant quality losses as well as economic loss throughout a supply chain. Postharvest losses are attributed to harvesting at an improper maturity stage, poor postharvest pretreatment, improper packing and packaging, inappropriate storage temperature and distribution conditions. These caused mechanical damage, sap burn, spongy tissue, weight loss, fruit softening, decay, chilling injury, and postharvest diseases. Currently, each step in the supply chain has been applied many postharvest technologies to reduce the quality losses of mango fruits as well as improving their marketability with the highest retention of quality. This review documented available possible causes for the quality losses and observed the physicochemical changes of mango fruit when applying postharvest technologies at each critical step in the mango supply chain from harvesting, pre-treatment, packaging, storage, to distribution. The summarized information is expected to provide comprehensive quality changes of mango fruits and point out the proper technology at each step of the supply chain.
Mango (Mangifera indica L.), belonging to the Anacardiaceae family, is one of the most vital fruit worldwide in both producing and consuming countries due to its characteristic flavor, taste, and nutritional aspects (Tharanathan et al., 2006; Ntsoane et al., 2019). However, mango is a climacteric fruit that ripens rapidly at ambient temperature (Singh et al., 2013). During ripening, mangoes experience various physicochemical changes which are susceptible to perishability under environmental conditions such as microorganism infection, climacteric respiration, postharvest handling, and storage (Wei et al., 2021). This inherent nature of mango leads to a noticeable amount of postharvest losses during the supply chain (Ntsoane et al., 2019). Mango quality losses are mainly attributed to improper harvesting, handling, packaging, and storage, leading to some typical deterioration such as mechanical damage, sap burn, discoloration, spongy tissue, chilling injury, softening, decay, or fungal and pest damage (Sivakumar et al., 2011). The postharvest losses primarily occur as mango production is in tropical and subtropical regions, mostly in developing countries where postharvest technology is limited along with poor and weak infrastructures (Ntsoane et al., 2020). The postharvest losses could reach a value of 50% depending on mango varieties, postharvest handling, and technologies (Janisiewicz and Korsten, 2002; Perumal et al., 2021). Postharvest technologies, packhouse management were noted to show significant impacts on the retention of mango quality throughout the supply chain (Sivakumar et al., 2011).
A supply chain is defined as the movement of materials-related activities, information, and resources from the producer to the consumer. It requires cohesive coordination and alignment of activities and processes of material, financial, and information flows. Supply chain management aims to reduce waste and cut down costs while maximizing overall values. In general, the supply chain in developing countries has undergone basic postharvest processing steps before entering the stage of transportation to consumers. Figure 1 generally describes the value chain flowchart of the mango supply in developing countries. The common steps in the mango supply chain including harvesting, pretreatment, packaging, storage, and distribution are noted with their applied postharvest technologies. Postharvest technologies in each processing step have been observed to influence the quality of mango fruits. A supply chain study in Sierra Leone (West Africa) has shown that the mango deterioration is due to limited production conditions followed by the influence of external variables throughout the stages (Arinloye et al., 2017). At the same time, the inconsistency in the packaging process from harvest to transportation is also considered a cause that significantly affects the fruit quality (Orjuela-Castro et al., 2017). Each critical step in the supply chain such as harvesting, pre-treatment, packaging, storage, and distribution may serve a key role in contributing to the quality changes of mango. Currently, a vast number of postharvest studies on mango fruit were conducted to observe the physicochemical changes of mango fruits but usually focus only on one specific stage in the supply chain. Meanwhile, mango quality changes in a whole supply chain, from harvesting to the distribution stage, are rarely discussed. Hence, this review aimed to describe an overview of postharvest qualities of mango fruit affected by different environmental conditions and current postharvest technologies at each critical step of the mango supply chain. Moreover, further research prospective, after a comprehensive observation, can be suggested in this review.
Determination of maturity of fruit at harvest is a crucial step that decides how mango quality is and its postharvest shelf-life (Sivakumar et al., 2011). Mango maturity is evaluated upon the visual appearance, fruit size, shape, total soluble solids (TSS) content, total acid, etc. (Subedi et al., 2007). A recent study showed that tree-ripen mangoes resulted in the best quality characteristics. Generally, tree-ripe mangoes attain better quality than less ripe mangoes, but they showed the shelf-life limitation due to the susceptibility to bruising, decay, and over-ripening during a supply chain (Ullah et al., 2010; Siddiq et al., 2017). Therefore, it is highly recommended that mangoes should be harvested at a mature green state (commercial maturity). This ensures that mango quality will be fully developed at the stage of consumption in the supply chain (Tian et al., 2010; Ullah et al., 2010; Gianguzzi et al., 2021). However, the standard green maturity indices are difficultly characterized due to the diversity of mango varieties and cultivation conditions, thus it should be determined in practice for each specific cultivar (Sivakumar et al., 2011). Generally, unripe fruits are characterized by their green color, high firmness, high starch and organic acids, and low pH and carotenoids (Narayana et al., 2012). It was previously noted that mangoes should be harvested at a soluble solid content of 9–10% for most markets (Sivakumar et al., 2011). Quality of mangoes including TSS, pH, carotenoids content, and sugar/acid ratio is highly dependent on cultivars, maturity state, and the environmental conditions that mangoes experience during the supply chain (Doreyappa Gowda and Huddar, 2001; Ueda et al., 2005; Gentile et al., 2021). The quality of mangoes with different cultivars, maturity at harvest, and locations are noted in Table 1. Green mature mangoes showed very high firmness (>100 N) and total acid (16–20%) with low TSS (5–8%) (Lawson et al., 2019). On the contrary, ripe-mature mango, which was only suitable for a short supply chain, exhibits a low firmness (1–16 N) and total acid with high TSS content (14–20%) (Gentile et al., 2019; Haseeb et al., 2020). The high variations in the firmness, TSS, total acid, vitamin C, carotenoid, and total polyphenols content (TPC) are highly dependent on the cultivar, maturity stage, and country of origin, shown in Table 1.
At the harvesting step, postharvest losses are attributed to improper harvesting approaches, disease pathogens, sap burn, bruising, etc. (Tian et al., 2010; Sivakumar et al., 2011). The harvest time should be carried out at a cool temperature in order to hinder field heat in harvested mangoes, alleviating metabolism in mangoes (Sivakumar et al., 2011). Picking poles are usually in mango harvesting with the bags on and the bags are immediately removed at the packing house to avoid the contact of mango injury-inducing sap to the mango peel (Crane et al., 2009). Besides, the stem end of mango fruits should be positioned down to allow the sap to drain (Siddiq et al., 2017). In developing countries, allowing detached fruits to drop to the ground causes mechanical injury which is a major postharvest loss of mango fruits (Siddiq et al., 2017). Harvesting that causes bruising and cuts on the mango surface should be avoided as these damaged fruits are easily susceptible to disease pathogens such as Aspergillus sp. and Botryodiplodia, Diplodia natalensis, causing a rapid decay for mango fruit (Yahia, 1998). Postharvest diseases also contribute to the main quality losses of mangoes due to microbial contamination (Prabakar et al., 2005). The predominant diseases found in mango fruits include anthracnose (Colletotrichum gloeosporioides), stem-end rot (Lasiodiplodia theobromae), and soft rots (Alternaria sp. and Aspergillus sp.) which cause rapid deterioration of mango during the supply chain (Sivakumar et al., 2011; Siddiq et al., 2017). Anthracnose symptoms are described as dark, sunken lesions on ripe fruit while stem-end rot is characterized by a dark rot from the stem-end of the fruit (Arauz, 2000). The contamination of disease pathogens was previously reported via injured or weakened mango, brown circular spot on the mango surface (Al-Najada and Al-Suabeyl, 2014). Alam et al. (2020) showed that stem-end rot disease fungal already infected at bloom stage, being endophytic within twigs and branches, or its airborne spores priorly invaded the pedicels and xylem then grew in the ripening mango. The pathogenic spores can disperse in the air, then come to attach and develop on the mango surface as soon as environmental conditions facilitate its germination and growth (Arauz, 2000). After harvesting, green mature mangoes experience biochemical changes including changes in carbohydrates, organic acids, development of yellow color, phenolics, and aroma volatile compounds (Singh et al., 2013). A good practice at the harvesting step could promote the harvested mango with high quality. However, the fast-ripening process and the presence of disease pathogens may cause the serve losses of mango quality. Postharvest technologies at later steps in the supply chain, which are discussed in later sections, are necessary to maintain the quality of mango fruits. In summary, the harvesting stage is considered a critical stage as it determines the quality of mango. It is essential to strictly comply with good practices in harvesting to avoid mechanical damage which negatively affects the quality of mango fruits. The stage of maturity for each specific variety in practice should be further studied to avoid noticeable postharvest losses in later steps.
After harvesting, mangoes are easily perishable due to postharvest disease pathogens (Perumal et al., 2021) and rapid ripening of mango leads to high susceptibility to post harvest losses (Sivakumar et al., 2011). Therefore, postharvest pretreatment of mango fruits before the packaging step is one of the critical points in the supply chain to prevent postharvest diseases and delay the ripening process. Postharvest pretreatment can be mainly divided into two groups: non-chemical pretreatment and chemical pretreatment.
Non-chemical pretreatment is the use of thermal treatment or irradiation treatment. Thermal treatments in postharvest pathogens disinfection to prolong the shelf-life of mangoes have been widely used around the world. Hot air treatment (HAT) and hot water treatment (HWT), which are commercially used in mango pretreatment, are considered low-cost approaches, high efficiency with acceptable quality (Sivakumar et al., 2011; Ntsoane et al., 2019). The obvious efficiency of thermal treatment has been demonstrated by the potential in the inactivation of intracellular enzymes which cause the quality deterioration. Typically, thermal treatment was found to inactivate aminocyclopropane-1-carboxylic acid oxidase (ACO) for the ethylene biosynthesis from 1-aminocyclopropane-1-carboxylic acid (ACC) (an ethylene precursor), leading to a delay in the ripening process by the attenuated ethylene production (Bender et al., 2003). The effects of HAT on the quality aspects of mango are listed in Table 2. HAT at 47°C for 180 min was found to inactivate 99% of fruit fly eggs and larvae on the skin, and accelerate the ripening process of Cat Hoa Loc mango regarding color changes, firmness loss but these changes were insignificantly different compared to the control sample after 7 days of storage (Hoa et al., 2010). It was noted that the HAT did not noticeably affect the vitamin C and β-carotene content after the treatment (Hoa et al., 2010; Ornelas-Paz and Yahia, 2014).
HWT has been widely used to control postharvest diseases. The general principle is the immersion of mango fruits in hot water at 48–55°C for 4–180 min depending on cultivars, types of disease pathogens. Besides, HWT also shows the improvement in such mango qualities as color, pH, total acid, TSS content (Luria et al., 2014; Dautt-Castro et al., 2018). Chok Anan mango treated by HWT at 55°C for 5 min showed a high reduction in disease pathogens while mango qualities including color, firmness, TSS, total acid did not differ from non-treated mangoes (Ding and Mijin, 2013). Hernández et al. (2018) also reported that HWT at 46.1°C for 75 min could completely inactivate A. ludens and A. seekqua with the retention of visual appearance and nutritional properties. HWT at 52°C for 5 min reduced the weight loss after 15 days of storage (Shahin et al., 2020). Besides, the chilling injury tolerance of mango fruits after HWT, which was evidenced by the low chilling incidence of symptoms, was also enhanced along with the elevated TPC, total flavonoids content (TFC), and carotenoids content (López-López et al., 2018; Vega-Alvarez et al., 2020). HWT for 24 h was also reported to affect gene expression which upregulated cell wall metabolism, transcription, defense response, and heat shock in mango mesocarp (Dautt-Castro et al., 2018). Moreover, HWT combined with HAT could promote better efficiency in delaying the ripening process evidenced by the retention of the green color of mango peel as well as its physicochemical properties compared to the single method (Shiesh and Lin, 2010; Oladele and Fatukasi, 2020). In comparison with other methods, HWT (55°C, 5 min) showed better inhibitory effect against disease pathogens, whereas treatment with 6-benzyladenine (100 ppm) was found to better alleviate the weight and firmness loss of mangoes (Thokchom and Mandal, 2019). Treatment with CaCl2 (2%) combined with polyethylene packaging was found to reduce the weight loss and decay level, and to keep the acceptable mango quality compared to HWT (Shahin et al., 2020). Besides, mangoes packed in shrink film were observed with lower decay and weight loss, and higher retention of vitamin C content compared to HWT (Ezz and Awad, 2011). It can be seen that HWT is a simple and cost-effective method for pre-treatment of mango fruits after harvesting which is highly dependent on the treatment parameters (temperature and treatment time). An extended time or elevated processing temperature of HWT could induce heat-damage, firmness loss, or weight loss. Therefore, suitable process parameters of HWT for each mango variety should be considered for preventing postharvest losses of mango fruits. Besides, other pre-treatment methods associated with HWT could be an option to better maintain the quality of mango fruits.
The irradiation method has been currently employed as an effective alternative to control postharvest losses. The irradiation method is a non-thermal treatment in which UV-C light (190–280 nm) irradiation produces a cytotoxic effect against disease pathogens on the surface of the fruit. Rotting disease fungi such as Botryosphaeria dothidea, Lasiodiplodia theobromae, Alternaria alternata, and Colletotrichum gloeosporioides were eradicated during 15 days of storage by applying an UV-C irradiation (253 nm) of < 3 kJ/m2 (González-Aguilar et al., 2007). This was attributed to the accelerated formation of phenylalanine ammonia-lyase—a catalyst for the biosynthesis of cytotoxic compounds (mostly phenols) against disease pathogens. Besides, mangoes treated with UV-C (310 nm, 5 min) light irradiation were found to increase the antioxidants content such as α-tocopherol, β-carotene, and ascorbic acid (González-Aguilar et al., 2007). UV-C irradiation (254 nm, 60 min) showed better efficiency than HWT in terms of vitamin retention, microbial decontamination, and visual appearance after 15 days of storage (George et al., 2015). Tommy Atkins mangoes exposed to UV-C irradiation (250–280 nm) for 10 min were observed with attenuated decay as well as maintaining the firmness, sugar, and organic acid levels compared to the non-irradiated mangoes (González-Aguilar et al., 2001). However, it should be noted that the approved dosage for the irradiation treatment should be <1 kGy due to safety concerns (Sivakumar et al., 2011). The efficacy of irradiation treatment is highly dependent on the cultivars, dosage, and fruit maturity stage. A low dosage cannot show the effective cytotoxic effect against disease pathogens or a high dosage may induce fruit damage (Mitcham and Yahia, 2008). Besides, the implementation and maintenance costs for irradiation facilities are quite expensive, thus it may be infeasible to be adopted in developing countries (Sivakumar et al., 2011).
In this chemical pre-treatment, synthetic reagents or naturally derived reagents as edible coatings can be used to preserve mango quality. Utilization of edible coating has been considered an environmental-friendly approach to prolong the shelf-life, reduce weight loss, delay the ripening process, inhibit microbial growth, and maintain physicochemical properties of mango fruits (Ntsoane et al., 2019). Some commonly used chemical reagents are noted.
1-Methylcyclopropene (1-MCP) is a cyclic alkene that binds to the ethylene receptor, inhibiting ethylene production and delaying the ripening process (Sisler, 2006; Liu et al., 2013). 1-MCP is a useful reagent in commercial use for controlling the ripening process of tropical fruits while maintaining their properties and prolonging shelf-life (Ntsoane et al., 2019). A previous study showed that 1-MCP could maintain the firmness and color and delay the sugar biosynthesis during ripening for up to 3 weeks of transportation followed by more than 1 week in the commercialization phase (Osuna-García et al., 2017). 1-MCP was reported to suppress anthracnose-induced fruit damage and reduce the rotting rate of mango fruit. 1-MCP could produce reactive oxygen species (ROS) to damage mitochondria and the integrity of the plasma membrane of C. gloeosporioides, preventing the growth of these fungi (Xu et al., 2017). 1-MCP was also evidenced that it served a role in inhibiting the accumulation of genes and enzymatic activities involved in cell wall transformation, resulting in the retention of firmness and delaying the ripening process (Razzaq et al., 2016). Nitric oxide is also a gaseous compound that is highly reactive at extremely low concentrations (Bambalele et al., 2021). Its mechanism of action is also correlated to the inhibition of ethylene biosynthesis, resulting in a delay in the ripening process and prolonging the shelf-life of mangoes (Tran et al., 2013). Postharvest pre-treatment of nitric oxide at varied concentrations (10–40 μL/L) was reported to alleviate chilling injury when exposed to the 5°C storage, delay fruit coloration changes, softening, and ripening as well as maintaining the mango quality during 4 weeks of cold storage (Zaharah and Singh, 2011). Many other impacts from nitric oxide treatment on the mango quality such as reducing weight and firmness loss, or disease pathogens inhibition were also reported in previously reported studies (Zaharah and Singh, 2010; Sakimin Siti and Singh, 2011; Hu et al., 2014; Zerbini et al., 2015). In addition, salicylic acid is also a reagent in regulating the ripening process, tolerance to chilling injury, and postharvest disease pathogens inactivation (He et al., 2017). Salicylic acid was found to mitigate the deposition of free radicals such as hydrogen peroxide (H2O2) and superoxide radicals () which are induced by the low-temperature storage and caused oxidative stress, chilling injury, and firmness loss (Junmatong et al., 2015). Salicylic acid has been also well-recognized to effectively decontaminate disease pathogens, thereby it reduces the disease incidence (Zeng et al., 2006; He et al., 2017). Although synthetic reagents show effectiveness in preventing quality deterioration of mango fruits, their adverse effects in long-term use should be a concerning issue regardless of their high efficiency at very low dosage.
Arabic gum is commonly used in coating techniques. It was reported that 10% Arabic gum coating reduced the biosynthesis of ethylene, firmness, respiration, or ripening rate as well as maintaining vitamin C content in mango fruit after 28 days storage (Khaliq et al., 2015). Daisy et al. (2020) reported that Arabic gum coating at 15% could extend the shelf-life and alleviate the weight loss during storage of “Apple” mango. Mango treated by Arabic gum coating showed better antioxidant activity during the storage period by maintaining vitamin C content—a strong antioxidant (Khaliq et al., 2015).
Chitosan has been considered an effective coating material to prevent postharvest losses due to its antioxidant and antimicrobial activities. It showed the inhibitory effect against postharvest disease pathogens such as gloeosporioides, Alternaria, and Dothoriella spp. (Jitareerat et al., 2007; Bambalele et al., 2021). According to recent research, using chitosan at 25–75 ml/L was highly effective in delaying ripening and preserving the visual appearance and quality of HindiBesennara mango after 2 weeks of storage (Awad et al., 2017). The microbial growth inhibition and attenuation of weight loss by using 0.8% chitosan coating in Falun cultivar was also reported (Nongtaodum and Jangchud, 2009). A similar finding was also observed in a study done by Jitareerat et al. (2007). The effectiveness of chitosan coating to prevent postharvest losses due to microbial spoilage could stem from its positive charges that interact with negatively charged cell membranes, affecting bacterial cell permeability (Bambalele et al., 2021). Furthermore, chitosan coating can reduce water loss and fruit weight by inhibiting endogenous respiration in mango, thereby maintaining product quality and appearance (Zhu et al., 2008).
Carboxymethyl cellulose (CMC) is a linear chain of β (1–4) glycosidic units with carboxyl substituent, hydroxypropyl, and methyl. CMC coating acts as a semi-permeable barrier to limit gas exchange, thereby reducing respiration rate and delaying the ripening process (Salinas-Roca et al., 2018; Bambalele et al., 2021). Treatment with CMC (10 g/kg mango) helped prevent appearance deterioration, retain the firmness in Tommy Atkins and Kent mango varieties as well as hindering the growth of microorganisms during 14 days of storage (Plotto et al., 2010). The use of 1% CMC was found to effectively impair mango respiration rate, reduce mass loss, and enhance the TSS in mango fruit (Phaiphan and Rattanapanone, 2008). When comparing the use of CMC and chitosan, mangoes treated with CMC experienced fewer color changes and higher TSS than chitosan-coated fruits (Phuangto et al., 2019). Recently, there has been increasingly gaining interest in combining coating reagents with antimicrobial, antioxidant compounds such as moringa, curcumin, aloe vera gel from plant extracts to enhance the efficiency in extending the shelf-life of fruits (Tesfay and Magwaza, 2017; Nicolau-Lapena et al., 2020; Ghosh et al., 2021). Therefore, the combination of common edible coating materials with antimicrobial, antioxidant compounds from natural resources will be a promising “green” technology to prevent the postharvest losses in mango fruit. Moreover, the combined effect of naturally-derived coating materials with other chemical reagents could be further studied to give an ideal approach to enhance the effectiveness in maintaining the quality aspects of mango fruits.
It has been seen that mango quality could be prolonged via applying such postharvest pretreatments as chemical pretreatments, low-temperature storage, etc. (Hoque et al., 2018). Proper packing and packaging are also strictly required for maintaining acceptable quality to the consumer stage (Anwar and Malik, 2007). Orjuela-Castro et al. (2017) noted that packaging is highly considered a critical point contributing to the physicochemical changes of mango fruit during the ripening stage. The main functionalities of packaging are to ensure protection against mechanical damage, microorganism contamination, weight loss, and mitigating respiration rate, or delaying ripening (Verghese et al., 2015; Giuggioli et al., 2018). However, improper handling in packing and packaging may lead to quality deterioration. For example, tight fruit packing (Sivakumar et al., 2011) or over-packing of mangoes in wooden crates was noted to induce significant quality losses (Malik et al., 2015). This mechanical damage can lead to the spongy tissue phenomenon which is only visualized when the mango is openly cut. The spongy region is an unripe flesh due to biochemical disruption and accumulation of unhydrolyzed starch during storage (Amin, 1967). Besides, this affected section was reported to have low pH, sugar, vitamin C, and β-carotene content (Amin, 1967).
As a climacteric fruit, mango is quickly subjected to the ripening stage after harvesting for 3–9 days (Sapkota et al., 2021). During ripening, some physicochemical changes in mango fruits have been evaluated. Normally, the weight loss of mangoes occurs over the storage time due to the continuous catabolism during the ripening stage (Ff et al., 2011). The TSS shows an increment while the total acid content tends to decrease (Tharanathan et al., 2006). A decrease in firmness of mango is highly associated with an increase in the TSS (Boonruang et al., 2012). In terms of visual appearance, the apparently noticeable change during ripening is the color change from green to yellowish-green, reddish, orangish-yellow, or complete yellow depending on types of cultivars (Sivakumar et al., 2011). These color conversions are ascribed to the disappearance of chlorophyll and the rise of other pigments such as carotenoids, anthocyanins (Tharanathan et al., 2006). Aroma and taste also are developed during ripening due to transformational changes in fatty acid profile (from palmitic acids to palmitoleic acids) (Lalel et al., 2002).
Any mechanical or chemical impacts on the mango fruits will induce ethylene—a ripening agent in fruit and accelerate the ripening process, leading to faster decay, or reducing the shelf-life of mango before reaching the consumer level. Hence, good packaging is considered an essential step for delaying ripening as well as extending the shelf-life of mango fruit (Siddiq et al., 2017). Types of packaging have been previously observed to have noticeable influences on the physicochemical changes in mango quality. Normally, mangoes wrapped with paper are packed in multi-layer in wooden crates, bamboo baskets, gunny bags, plastic polypropylene sacks, high or low density polyethylene, carton liners, etc. (Singh et al., 2001; Defraeye et al., 2015; Orjuela-Castro et al., 2017). Wooden boxes and cardboard boxes are commonly used for packaging and distribution in the domestic market (Bernstad Saraiva et al., 2016; Patel et al., 2019). These materials were found to cause physical damage and bruises to mango fruits during transportation (Anwar et al., 2006). Some other packaging materials influencing the mango quality during storage are also documented (Table 3). Types of packaging materials contributed to the variation in the weight loss and physiochemical properties of mango fruits during ripening. Roy and Pal (1989) indicated that the use of corrugated fiber box with partition showed the attenuation of weight loss (2.38%), ripening (15.33%), and bruising (1.33%) percentage compared to the wooden box with straw and paper with respect to 2.54, 21.33, and 5%. Low-density polyethylene (LDPE) packaging showed better efficiency than plastic boxes in the alleviation of weight loss, remaining firmness (Srinivasa et al., 2004). Vacuum packaging was reported to better delay ripening compared to the use of Cryovac PD-941 bags with plastic trays (Martínez-Ferrer et al., 2002). Mangoes packaged in perforated polythene bags were observed with a decrease in weight loss by 2.3% compared to unpacked mangoes after 6 days of storage at 31°C (Ff et al., 2011). Mangoes packaged in cartons lined with intact polyethylene bags showed the highest efficiency in reducing weight loss followed by that in cartons lined with perforated polyethylene bags and unpacked mangoes (Elkashif et al., 2003). A special box was designed to be sturdy and stackable so that it could not induce mechanical damage to mango fruits, leading to the reduction in the postharvest loss by 10–15% (Subramanian et al., 2018). Currently, in developing countries, the postharvest loss is inevitable, the choice of packaging type for retaining high mango quality to make the successful sale is a crucial step in the supply chain (Giuggioli et al., 2018). Improper packaging types or packing techniques will accelerate the ripening process which causes the fast deterioration of mango quality. A good packaging stage requires an appropriate package and proper technique in packing to minimize the mechanical damage as well as to delay the ripening process.
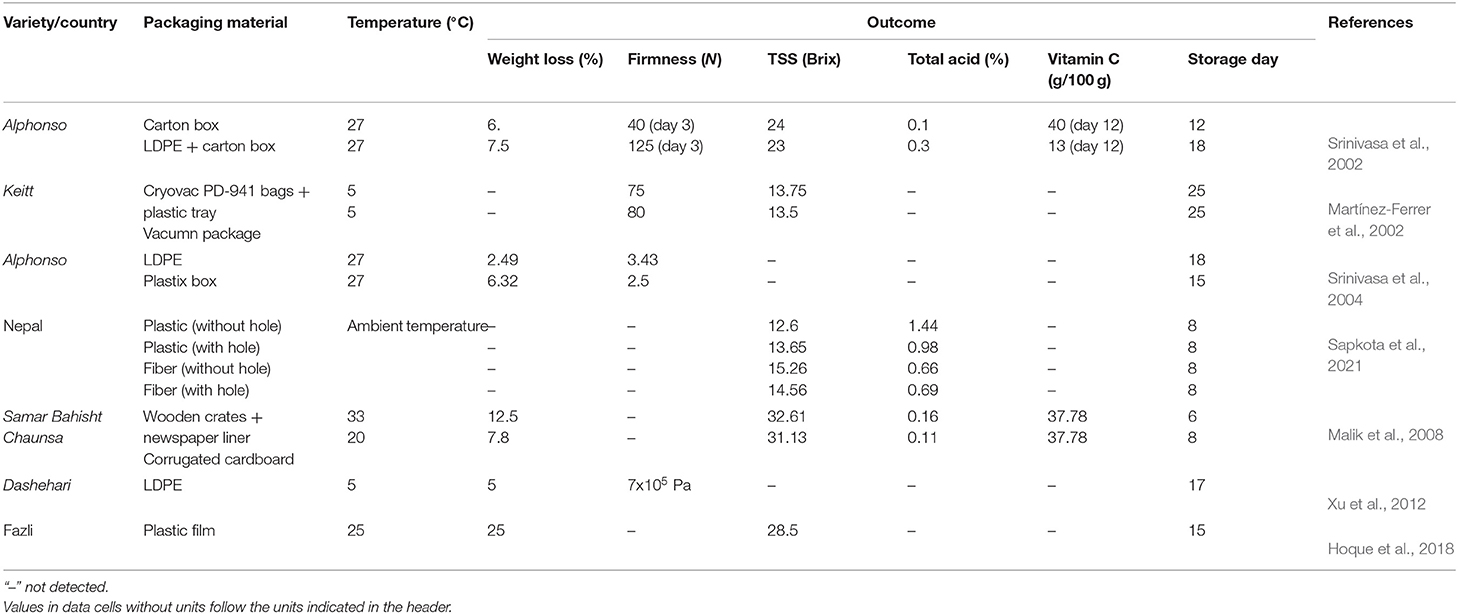
Table 3. Physicochemical changes of mango fruit introduced to different types of packaging material.
For delaying ripening, an advanced technology used in packaging is the use of modified atmosphere packaging (MAP). MAP, which has been considered an effective approach to preserve the high quality of fruit with extended shelf-life, is the introduction of high CO2 and low O2 surrounding fruit inside the package (Ntsoane et al., 2019; Wei et al., 2021). As a consequence, MAP delays the ripening process of mango fruit due to the elevated CO2 level and attenuated O2 level which mitigate the respiration rate of mango and ethylene production (Singh et al., 2013). A rapid respiration rate leads to a loss of mango quality before reaching the consumer stage (Martínez-Ferrer et al., 2002). MAP has been demonstrated to show a positive effect on retaining the high quality of mango fruit during the supply chain. The quality changes of mango during ripening are dependent on types of packaging and CO2: O2 ratios in the package (Martínez-Ferrer et al., 2002; Wei et al., 2021). Mango, in the study of Boonruang et al. (2012), was observed to have an extended shelf-life up to 40 days with HPNE packaging (4 kPa O2 + 5 kPa CO2), 35 days with HNP packaging (5 kPa O2 + 5 kPa CO2) and 30 days with HMP packaging (9 kPa O2 + 17 kPa CO2) compared to the unpacked mango, shown in Table 4. MAP with 10% O2 and 5% CO2 showed 8.55% weight loss and the firmness of ~1.9 N, whereas the non-packed mango was observed with 11.36% weight loss and ~1.7 N in firmness (Perumal et al., 2021). A lower TSS was obtained in MAP packed mangoes compared to the unpacked one, indicating the lower respiration rate during storage (Martínez-Ferrer et al., 2002). Perumal et al. (2021) also indicated that MAP retained the TPC and TFC in mango fruits due to their alleviated oxidation in MAP (Wang et al., 2015). The reduction of TPC in mango fruit was due to the ripening process (Kim et al., 2007; Boonruang et al., 2012). Modified atmosphere (5% CO2 and 10% CO2) in micro-perforated polyethylene was reported to tackle off-flavors during storage or in Xtend® film-lined cartons was also found to reduce the sap burn effect by reducing the level of sap inside the package (Pesis et al., 2000). A slower color conversion from green to yellow in MAP packed mango also indicated the delay in maturation and ripening (Boonruang et al., 2012). The effectiveness of MAP in fruit preservation was confirmed by many previous studies. The efficacy of using MAP for mango preservation is highly dependent on the gas compositions which determine the shelf-life of mango during storage. In developing countries, however, the added costs for gases, packaging, and MAP system installation as well as maintenance costs should be taken into consideration.
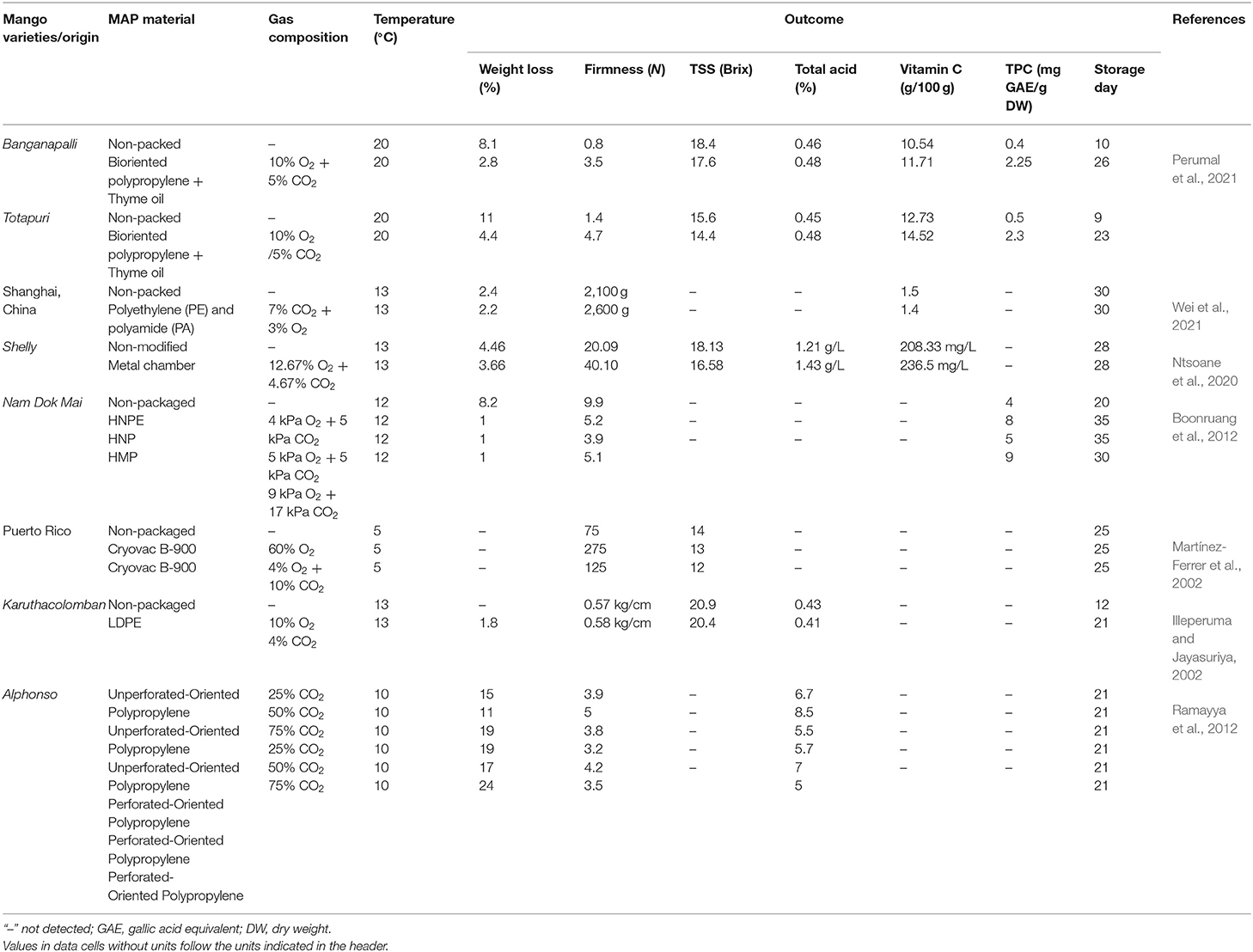
Table 4. Physicochemical changes of mangoes packed in different types of modified atmosphere packaging material with different gas compositions.
Storage is also a vital step in a supply chain that contributes to controlling the quality of mango fruit. It has been suggested that mango quality is noticeably maintained in low-temperature storage and starts decreasing with an increase in storage temperature (Ezz and Awad, 2011). Low temperature has been found to attenuate the mango catabolism and delay ripening (Sudhakar Rao and Gopalakrishna Rao, 2008). Cold storage with a suitable temperature range of 10–13°C depending on mango cultivars could extend the shelf-life for 2–3 weeks (Singh and Zaharah, 2013; Singh et al., 2013; Wei et al., 2021). However, in developing countries, weak infrastructure and low affordability in constructing cold storage rooms may hinder the retention of mango quality during storage. Hence, a low-cost alternative technique, which shows positive outcomes in extending the shelf-life of fresh fruit, is applied by the use of evaporative cooling with simple installation and not requiring external supply (Liberty et al., 2013, 2014). Evaporative cooling technique namely coolbotTM cold storage has been considered an effective approach for preserving Apple and Ngowe mango cultivars with an extended shelf-life of 35 days compared to that stored in ambient room conditions (Karithi, 2016). However, it should be noted that mango fruits are easily susceptible to chilling injury (Nair and Singh, 2003; Zaharah and Singh, 2011; Singh et al., 2012). Chilling injury is a result of dysfunction or disruption of cell wall membrane, causing negative impacts on the flow rate of cellular fluid inside and outside of plant tissues and abnormal metabolites (Sivakumar et al., 2011). The chilling injury level is highly dependent on the maturity stage of mango, duration of storage at chilling temperature, and packaging materials (Sivakumar et al., 2011). Matured green mangoes when exposed to storage temperature below 10°C were observed with an abnormal ripening, discoloration of the peel, skin pitting, reduction in carotenoid content, flavor and aroma, and were easily susceptible to fungal decay (Singh et al., 2012, 2013). These phenomena are even more severe when mangoes are transferred from cold storage to ambient storage (Watanawan et al., 2014).
Controlled atmosphere storage has been also applied to ease the chilling injury. In this technique, similar to modified atmosphere packaging, mango fruits are introduced to the storage room with the modified gas composition of low O2 content which is reported to reduce the ethylene production, respiration rate, and the ripening process (Ntsoane et al., 2019). Controlled atmosphere storage (2% O2 and 6% CO2) at 13°C was considered a potent approach for extending the shelf-life and maintaining the high quality of mango while the gas constituents of 2% O2 and 2% CO2 showed better efficiency in maintaining the aroma compounds of ripe mangoes (Lalel et al., 2002). Controlled atmosphere storage (3% CO2 and 8% CO2) associated with the effect of 1-MCP (500 nl/L) was found to extend the shelf-life of mangoes by reducing the incidence of anthracnose, weight and firmness loss, delaying the development of color, TSS/total acid ratio, retaining the carotenoid, vitamin C, TPC, TFC, and antioxidant activity (Gil et al., 2000). The ideal gas compositions for the storage of Shelly mango varied from 5–8% O2, 5–9% CO2, 86–91% N2 were reported to maintain the quality and prolong the shelf-life of this variety by retarding ripening and weight loss, softening and chlorophyll decomposition (Ntsoane et al., 2020). Similar findings were found in many previously reported studies in the use of controlled atmosphere storage (Nair and Singh, 2003; Lalel and Singh, 2006; Singh and Zaharah, 2013; Wei et al., 2021). However, a very low O2 concentration or very high CO2 concentration in controlled atmosphere storage could lead to adverse effects such as discoloration of the peel, uneven ripening, higher susceptibility to decay, and development of off-flavor (Sudhakar Rao and Gopalakrishna Rao, 2008; Singh and Zaharah, 2015). The gas composition of CO2 >25% and O2 < 2% in controlled atmosphere storage was found to induce unfavorable aroma and discoloration of mango peel due to the formation of ethanol during storage (Sudhakar Rao and Gopalakrishna Rao, 2008). Controlled atmosphere storage of 1.5% O2 and 6% CO2, or 1.5–2% O2 and 8% CO2 was reported to produce ethanol, acetaldehyde, and ester from anaerobic metabolism, leading to off-flavor in R2E2 mangoes (Lalel and Singh, 2006).
Cold temperature storage is an effective approach to preserve the mango quality. However, a suitable temperature should be pointed out to prevent the chilling injury during the cold storage. Besides, further studies on the physicochemical changes of mango fruits when transferring from cold temperature to ambient temperature are needed to provide great insights into the behavior of mango fruits after cold temperature storage. In terms of controlled atmosphere storage, it may be not feasible in developing countries due to high requirements in the system installation and high-cost operation as well maintenance.
The quality of mango during distribution should be a crucial point in physicochemical changes of mangoes. Mangoes can be transported by truck, ship, or airplane depending on the distance and their maturity. Major quality losses of mango during distribution are attributed to improper packing in container and distribution temperature, and poor road (Siddiq et al., 2017). Malik et al. (2015) noted that the overloading of mangoes in trucks contributed to significant quality losses (Malik et al., 2015). Vibration conditions during transport also affected mango quality reported in a study of E. Yasunaga et al. (2012). For a short distance such as transportation to the local market, mangoes can be transported by trucks at ambient temperature if the weather is not very warm. In the contrast, if it is warm weather, distribution can be carried out at night due to lower temperature (Siddiq et al., 2017). The quality of mangoes such as carotenoid and vitamin C content was unchangeable during transportation from a local market to the commercial restaurant at 25°C by trucks (Oliveira et al., 2010). For a long-distance, mangoes could be transported by a 2-tons refrigerator van at 12°C (Maekawa, 1990) or by ship at below 12.5°C but the major concern is the occurrence of chilling injury over a long period of shipping at low temperature (Siddiq et al., 2017). The airplane is a fast but expensive approach that is only used when the fruits are extremely perishable (Siddiq et al., 2017). Physicochemical changes of mangoes during distribution to different destinations seem to be rarely reported. For a long period of shipping around 2–3 weeks, mangoes were observed with a decrease in firmness and vitamin C, an increase in TSS content, and changes in the peel color due to the ripening process. These changes were accelerated when the temperature was elevated during the distribution process (Yasunaga et al., 2012, 2018). So far, the distribution stage is also crucial to maintaining the quality of mango fruits. To better maintain the high quality of mango fruits during shipping, it requires a low-temperature distribution to delay the ripening process and stability during distribution to avoid mechanical damage. To date, this stage has been less focused compared to the others; therefore, the study in investigating the changes of mango quality during distribution can be a prospective approach to provide a good basis of physicochemical changes during distribution.
Each processing step in the supply chain poses very high risks in postharvest losses of mango fruit when applying improper handling techniques. Therefore, it is highly necessary to employ a suitable approach at each processing step in the supply chain to maintain the quality of mango fruits, resulting in a constructed quality assurance system to maintain the high quality of mango until reaching the consumer level. The harvesting date should be selected at the green maturity stage with a proper technique to avoid mechanical damage to mango fruits. In developing countries, HWT is highly recommended due to its cost-effectiveness and simplicity to run at a grower level. Besides, newly adopted technologies of coating could be a promising approach to prevent disease pathogens, ripening rate, and extend the shelf-life of the product. Besides, combining pre-treatment methods could give effective means of enhancing the efficiency in maintaining mango quality. Modified atmosphere packaging and modified atmosphere storage are advanced technologies that reveal high efficiency in maintaining the mango quality to prevent weight loss, delay ripening, pathogenic disease, and chilling injury but may require modern facilities, high-cost production, and maintenance. Storage temperature should be highly concerned depending on cultivars, currently applied technologies, and facilities. High storage temperature will accelerate the ripening process, causing rapid mango decay before reaching the consumer, whereas low temperature (<13°C) will facilitate chilling injury, leading to mango deterioration. Vehicles and storage temperature should be considered to avoid mechanical damage and chilling injury during long-distance transportation.
So far, recent studies have focused on only one single processing step in the supply chain to optimize postharvest technology at that step. Further studies can turn to the detailed quality assessments of mango fruit at each processing step in the supply chain applied for a specific mango cultivar and its current postharvest technologies. This will provide great insights into the quality changes of mango fruit in the supply chain. From this point of view, it allows to give an actual evaluation to consider which critical step is determined to cause the most postharvest losses in the supply chain and further improvement could be studied to reduce postharvest losses. Besides, to our best knowledge, due to the lack of published data in evaluating variations in the phytochemicals content of mango fruit during the supply chain, further studies in this prospective aspect can draw the attention of researchers as well as health-conscious consumers.
NM, HT, and NL contributed to conception and design of the study. TL, TV, and TP organized the database, performed the statistical analysis, wrote the first draft of the manuscript, and wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by grants from Department of Science and Technology, Ben Tre Province, Vietnam; and Nguyen Tat Thanh University, Ho Chi Minh City 700000, Vietnam.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2021.799431/full#supplementary-material
Alam, M. W., Rehman, A., Malik, A. U., Ahmad, S., Haider, M. S., Amin, M., et al. (2020). Dynamics of stem end rot disease of mango fruit and its management. Pakistan J. Agric. Sci. 57, 63–71. doi: 10.21162/PAKJAS/20.8336
Al-Najada, A. R., and Al-Suabeyl, M. S. (2014). Isolation and classification of fungi associated with spoilage of post-harvest mango (Mangifera indica L.) in Saudi Arabia. Afr. J. Microbiol. Res. 8, 685–688. doi: 10.5897/AJMR12.1898
Amin, H. D. (1967). Development of white corky tissue in a mango fruit (Mangifera indica). Navsari Agric. Coll. Mag. 6, 14–17.
Anwar, M. R., Zahoor, M. H., Saeed, A. I., and Fakharuddin, M. R. (2006). Vegetative and reproductive physiology of April flush in mango (Mangifera indica L.) cv. Dusehri. Int. J. Agri. Biol. 8, 452–454.
Anwar, R., and Malik, A. U. (2007). Hot water treatment affects ripening quality and storage life of mango (Mangifera indica L.). Pakistan J. Agric. Sci. 44, 304–311.
Anwar, R., and Malik, A. U. (2008). Effect of hot water treatment on storage life and quality of mango (Mangifera indica L.). Acta Hortic. 768, 201–207. doi: 10.17660/ActaHortic.2008.768.24
Arauz, L. F. (2000). Mango anthracnose: economic impact and current options for integrated managaement. Plant Dis. 84, 600–611. doi: 10.1094/PDIS.2000.84.6.600
Arinloye, A. D. D., Degrande, A., Fassinou Hotegni, V. N., Asaah, E., Bockarie, R., Nyemeck, J. B., et al. (2017). Value chain development for mango (Mangifera indica) around Outamba Kilimi National Park in Sierra Leone: constraints and opportunities for smallholders. Agric. Food Secur. 6, 1–13. doi: 10.1186/s40066-017-0092-x
Awad, M. A., Al-Qurashi, A. D., Mohamed, S. A., and El-Shishtawy, R. M. (2017). Quality and biochemical changes of ‘Hindi-Besennara’ mangoes during shelf life as affected by chitosan, gallic acid and chitosan gallate. J. Food Sci. Technol. 54, 4139–4148. doi: 10.1007/s13197-017-2762-x
Bambalele, N. L., Mditshwa, A., Magwaza, L. S., and Tesfay, S. Z. (2021). Recent advances on postharvest technologies of mango fruit: a review. Int. J. Fruit Sci. 21, 565–586. doi: 10.1080/15538362.2021.1918605
Bender, R. J., Seibert, E., and Brecht, J. K. (2003). Heat treatment effects on ACC oxidase activity of'Keitt'mangoes. Brazil. J. Plant Physiol. 15, 145–148. doi: 10.1590/S1677-04202003000300003
Bernstad Saraiva, A., Pacheco, E. B., Gomes, G. M., Visconte, L. L. Y., Bernardo, C. A., Simões, C. L., et al. (2016). Comparative lifecycle assessment of mango packaging made from a polyethylene/natural fiber-composite and from cardboard material. J. Clean. Prod. 139, 1168–1180. doi: 10.1016/j.jclepro.2016.08.135
Boonruang, K., Chonhenchob, V., Singh, S. P., Chinsirikul, W., and Fuongfuchat, A. (2012). Comparison of various packaging films for mango export. Packag. Technol. Sci. 25, 107–118. doi: 10.1002/pts.954
Crane, J. H., Salazar, G. S., Lin, T. S., and de Queiroz Pinto, A. C. (2009). Crop production: management, in The Mango, Botany, Production and Uses, 2nd Edn (Wallingford: CAB International), 432–483.
Daisy, L. L., Nduko, J. M., Joseph, W. M., and Richard, S. M. (2020). Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 57, 79–85. doi: 10.1007/s13197-019-04032-w
Dautt-Castro, M., Ochoa-Leyva, A., Contreras-Vergara, C. A., Muhlia-Almazán, A., Rivera-Domínguez, M., Casas-Flores, S., et al. (2018). Mesocarp RNA-Seq analysis of mango (Mangifera indica L.) identify quarantine postharvest treatment effects on gene expression. Sci. Hortic. (Amsterdam). 227, 146–153. doi: 10.1016/j.scienta.2017.09.031
de Cassia Mirela Resende Nassur, R., González-Moscoso, S., Crisosto, G. M., de Oliveira Lima, L. C., de Barros Vilas Boas, E. V., and Crisosto, C. H. (2015). Describing quality and sensory attributes of 3 mango (Mangifera indica L.) cultivars at 3 ripeness stages based on firmness. J. Food Sci. 80, 2055–2063. doi: 10.1111/1750-3841.12989
Defraeye, T., Cronjé, P., Berry, T., Opara, U. L., East, A., Hertog, M., et al. (2015). Towards integrated performance evaluation of future packaging for fresh produce in the cold chain. Trends Food Sci. Technol. 44, 201–225. doi: 10.1016/j.tifs.2015.04.008
Ding, P., and Mijin, S. (2013). Physico-chemical characteristics of chok anan mango fruit after hot water treatment. Pertanika J. Trop. Agric. Sci. 36, 359–372.
Doreyappa Gowda, I. N., and Huddar, A. G. (2001). Studies on ripening changes in mango (Mangifera indica L.) fruits. J. food Sci. Technol. 38, 135–137.
Elkashif, E. M., Elamin, O. M., and Mohamed, T. B. (2003). Effects of ethrel application and packaging on mango fruit quality. Gezira J. Agric. Sci. 1, 52–62.
Ezz, T. M., and Awad, R. M. (2011). Effect of some post harvest treatments under different low temperature on two mango cultivars. Aust. J. Basic Appl. Sci. 5, 1164–1174.
Ff, I., Oa, O., Ar, O., Mo, O., Ko, Z., Ao, A., et al. (2011). Effect of polythene packaging on the shelf life of mango fruits. J. Stored Prod. Postharvest Res. 2, 148–150.
Gentile, C., Di Gregorio, E., Di Stefano, V., Mannino, G., Perrone, A., Avellone, G., et al. (2019). Food quality and nutraceutical value of nine cultivars of mango (Mangifera indica L.) fruits grown in Mediterranean subtropical environment. Food Chem. 277, 471–479. doi: 10.1016/j.foodchem.2018.10.109
Gentile, C., Mannino, G., Palazzolo, E., Gianguzzi, G., Perrone, A., Serio, G., et al. (2021). Pomological, sensorial, nutritional and nutraceutical profile of seven cultivars of cherimoya (Annona cherimola Mill). Foods 10:35. doi: 10.3390/foods10010035
George, D. S., Razali, Z., Santhirasegaram, V., and Somasundram, C. (2015). Effects of ultraviolet light (UV-C) and heat treatment on the quality of fresh-cut chokanan mango and josephine pineapple. J. Food Sci. 80, S426–S434. doi: 10.1111/1750-3841.12762
Ghosh, T., Nakano, K., and Katiyar, V. (2021). Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 184, 936–945 doi: 10.1016/j.ijbiomac.2021.06.098
Gianguzzi, G., Farina, V., Inglese, P., and Rodrigo, M. G. L. (2021). Effect of harvest date on mango (Mangifera indica L. cultivar osteen) fruit's qualitative development, shelf life and consumer acceptance. Agronomy. 11:811. doi: 10.3390/agronomy11040811
Gil, A. M., Duarte, I. F., Delgadillo, I., Colquhoun, I. J., Casuscelli, F., Humpfer, E., et al. (2000). Study of the compositional changes of mango during ripening by use of nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 48, 1524–1536. doi: 10.1021/jf9911287
Giuggioli, N. R., Briano, R., and Peano, C. (2018). Packaging in the fresh fruit and vegetable supply chain: innovation and sustainability. Italus Hortus. 25, 23–38. doi: 10.26353/j.itahort/2018.1.2338
González-Aguilar, G. A., Villegas-Ochoa, M. A., Martínez-Téllez, M. A., Gardea, A. A., and Ayala-Zavala, J. F. (2007). Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 72, S197–S202. doi: 10.1111/j.1750-3841.2007.00295.x
González-Aguilar, G. A., Wang, C. Y., Buta, J. G., and Krizek, D. T. (2001). Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe “Tommy Atkins” mangoes. Int. J. Food Sci. Technol. 36, 767–773. doi: 10.1046/j.1365-2621.2001.00522.x
Hasan, M. U., Malik, A. U., Khan, A. S., Anwar, R., Amjad, A., Shah, M. S., et al. (2020). Impact of postharvest hot water treatment on two commercial mango cultivars of pakistan under simulated air freight impact of postharvest hot water treatment on two commercial mango cultivars of pakistan under simulated air freight conditions for china. Pakistan J. Agric. Sci. 57, 1381–1391. doi: 10.21162/PAKJAS/20.9930
Haseeb, G. M., Ghounim, I. E.-S., Hmmam, I., and Mustafa, M. R. (2020). Evaluation of four newly introduced mango (Mangifera indica L.) Cultivars grown under el-giza conditions. Plant Arch. 20, 9405–9410.
He, J., Ren, Y., Chen, C., Liu, J., Liu, H., and Pei, Y. (2017). Defense responses of salicylic acid in mango fruit against postharvest anthracnose, caused by Colletotrichum gloeosporioides and its possible mechanism. J. Food Saf. 37:e12294. doi: 10.1111/jfs.12294
Hernández, E., Aceituno-Medina, M., Toledo, J., Gómez-Simuta, Y., Villarreal-Fuentes, J. M., Carrasco, M., et al. (2018). Generic irradiation and hot water phytosanitary treatments for mango fruits cv.‘Ataulfo’niño infested by Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J. Econ. Entomol. 111, 2110–2119. doi: 10.1093/jee/toy198
Hoa, T. T., Hien, D. M., Self, G., and Ducamp, M. N. (2010). Effects of hot air treatment on postharvest quality of “cat Hoa loc” mangoes. Fruits 65, 237–244. doi: 10.1051/fruits/2010019
Hoque, M., Chowhan, S., and Kamruzzaman, M. (2018). Physiological changes and shelf life of mango (Mangifera indica L.) influenced by post harvest treatments. SAARC J. Agric. 15, 219–226. doi: 10.3329/sja.v15i2.35164
Hu, M., Yang, D., Huber, D. J., Jiang, Y., Li, M., Gao, Z., et al. (2014). Reduction of postharvest anthracnose and enhancement of disease resistance in ripening mango fruit by nitric oxide treatment. Postharvest Biol. Technol. 97, 115–122. doi: 10.1016/j.postharvbio.2014.06.013
Illeperuma, C. K., and Jayasuriya, P. (2002). Prolonged storage of “Karuthacolomban” mango by modified atmosphere packaging at low temperature. J. Hortic. Sci. Biotechnol. 77, 153–157. doi: 10.1080/14620316.2002.11511472
Janisiewicz, W. J., and Korsten, L. (2002). Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 40, 411–441. doi: 10.1146/annurev.phyto.40.120401.130158
Jitareerat, P., Paumchai, S., Kanlayanarat, S., and Sangchote, S. (2007). Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. New Zeal. J. Crop Hortic. Sci. 35, 211–218. doi: 10.1080/01140670709510187
Junmatong, C., Faiyue, B., Rotarayanont, S., Uthaibutra, J., Boonyakiat, D., and Saengnil, K. (2015). Cold storage in salicylic acid increases enzymatic and non-enzymatic antioxidants of Nam Dok Mai No. 4 mango fruit. Sci. Asia 41, 12–21. doi: 10.2306/scienceasia1513-1874.2015.41.012
Karithi, E. M. (2016). Evaluation of the Efficacy of coolbotTM Cold Storage Technology to Preserve Quality and Extend Shelf Life of Mango Fruits. Nairobi: University of Nairobi.
Khaliq, G., Muda Mohamed, M. T., Ali, A., Ding, P., and Ghazali, H. M. (2015). Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 190, 187–194. doi: 10.1016/j.scienta.2015.04.020
Kim, Y., Brecht, J. K., and Talcott, S. T. (2007). Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 105, 1327–1334. doi: 10.1016/j.foodchem.2007.03.050
Lalel, H. J. D., and Singh, Z. (2006). Controlled atmosphere storage of “Delta R2E2” mango fruit affects production of aroma volatile compounds. J. Hortic. Sci. Biotechnol. 81, 449–457. doi: 10.1080/14620316.2006.11512087
Lalel, H. J. D., Singh, Z., and Tan, S. C. (2002). Elevated levels of CO2 in controlled atmosphere storage affects shelf life, fruit quality and aroma volatiles of mango, in XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture, Toronto, Vol. 628, 407–413. doi: 10.17660/ActaHortic.2003.628.51
Lawson, T., Lycett, G. W., Ali, A., and Chin, C. F. (2019). Characterization of Southeast Asia mangoes (Mangifera indica L.) according to their physicochemical attributes. Sci. Hortic. 243, 189–196. doi: 10.1016/j.scienta.2018.08.014
Liberty, J. T., Agidi, G., and Okonkwo, W. I. (2014). Predicting storability of fruits and vegetables in passive evaporative cooling structures. Int. J. Sci. Eng. Technol. 3, 518–523.
Liberty, J. T., Ugwuishiwu, B. O., Pukuma, S. A., and Odo, C. E. (2013). Principles and application of evaporative cooling systems for fruits and vegetables preservation. Int. J. Curr. Eng. Technol. 3, 1000–1006.
Liu, R., Lai, T., Xu, Y., and Tian, S. (2013). Changes in physiology and quality of Laiyang pear in long time storage. Sci. Hortic. 150, 31–36. doi: 10.1016/j.scienta.2012.10.017
López-López, M. E., López-Valenzuela, J. Á., Delgado-Vargas, F., López-Angulo, G., Carrillo-López, A., Ayón-Reyna, L. E., et al. (2018). A treatment combining hot water with calcium lactate improves the chilling injury tolerance of mango fruit. HortScience 53, 217–223. doi: 10.21273/HORTSCI12575-17
Luria, N., Sela, N., Yaari, M., Feygenberg, O., Kobiler, I., Lers, A., et al. (2014). De-novo assembly of mango fruit peel transcriptome reveals mechanisms of mango response to hot water treatment. BMC Genomics 15, 1–15. doi: 10.1186/1471-2164-15-957
Maekawa, T. (1990). On the Mango Ca Storage and Transportation From Subtropical To Temperate Regions in Japan. Acta Hortic. 269, 367–374. doi: 10.17660/ActaHortic.1990.269.47
Malik, A. U., Amin, M., Jabbar, A., and Saleem, B. A. (2008). Packaging material and ripening methods affect mango fruit quality. Int. J. Agri. Biol. 10, 35–41.
Malik, A. U., Amin, M., Mazhar, M. S., Johnson, P. J., Hofman, P., Campbell, J., et al. (2015). Mango value chain improvement through postharvest research and development: a developing country case study, in XI International Mango Symposium, Darwin, NT, Vol. 1183, 411–420.
Martínez-Ferrer, M., Harper, C., Pérez-Muñoz, F., and Chaparro, M. (2002). Modified atmosphere packaging of minimally processed mango and pineapple fruits. J. Food Sci. 67, 3365–3371. doi: 10.1111/j.1365-2621.2002.tb09592.x
Mitcham, E., and Yahia, E. (2008). Alternative Treatments to Hot Water Immersion for Mango Fruit Report to the National Mango Board.
Nair, S., and Singh, Z. (2003). Pre-storage ethrel dip reduces chilling injury, enhances respiration rate, ethylene production and improves fruit quality of ‘Kensington’mango. Food Agric. Environ. 1, 93–97.
Narayana, C. K., Rao, D. S., and Roy, S. K. (2012). Mango production, postharvest physiology and storage, in Tropical and Subtropical Fruits: Postharvest Physiology, Processing and Packaging (Ames, IA: John Wiley & Sons), 259–276.
Nicolau-Lapena, I., Colas-Meda, P., Alegre, I., Aguilo-Aguayo, I., Muranyi, P., and Vinas, I. (2020). Aloe vera gel: an update on its use as a functional edible coating to preserve fruits and vegetables. Prog. Org. Coatings 151:106007. doi: 10.1016/j.porgcoat.2020.106007
Nongtaodum, S., and Jangchud, A. (2009). Effects of edible chitosan coating on quality of fresh-cut mangoes (Fa-lun) during storage. Kasetsart J. Nat. Sci. 43, 282–289.
Ntsoane, M. L., Sivakumar, D., and Mahajan, P. V. (2020). Optimisation of O2 and CO2 concentrations to retain quality and prolong shelf life of ‘shelly’ mango fruit using a simplex lattice mixture design. Biosyst. Eng. 192, 14–23. doi: 10.1016/j.biosystemseng.2020.01.009
Ntsoane, M. L., Zude-Sasse, M., Mahajan, P., and Sivakumar, D. (2019). Quality assesment and postharvest technology of mango: a review of its current status and future perspectives. Sci. Hortic. 249, 77–85. doi: 10.1016/j.scienta.2019.01.033
Oladele, O. O., and Fatukasi, O. I. (2020). Effect of pre-storage hot air and hot water treatments on post-harvest quality of mango (Mangifera indica Linn.) fruit. Not. Sci. Biol. 12, 842–851. doi: 10.15835/nsb12410634
Oliveira, D. D. S., Lobato, A. L., Ribeiro, S. M. H. R., Santana, Â. M. C., Chaves, J. B. P., and Pinheiro-SantAna, H. M. (2010). Carotenoids and vitamin C during handling and distribution of guava (Psidium guajava L.), mango (Mangifera indica L.), and papaya (Carica papaya L.) at commercial restaurants. J. Agric. Food Chem. 58, 6166–6172. doi: 10.1021/jf903734x
Orjuela-Castro, J. A., Herrera-Ramírez, M. M., and Adarme-Jaimes, W. (2017). Warehousing and transportation logistics of mango in Colombia: a system dynamics model. Rev. Fac. Ing. 26, 73–86. doi: 10.19053/01211129.v26.n44.2017.5773
Ornelas-Paz, J., de, J., and Yahia, E.M. (2014). Effect of the moisture content of forced hot air on the postharvest quality and bioactive compounds of mango fruit (Mangifera indica L. cv. Manila). J. Sci. Food Agric. 94, 1078–1083. doi: 10.1002/jsfa.6384
Osuna-García, J. A., Nolasco-González, Y., Pérez-Barraza, M. H., Gómez-Jaimes, R., and Urías-López, M. A. (2017). Aqueous 1-methylcyclopropene (1-Mcp) to delay ripening of Keitt mango fruit with quarantine hot water treatment. Rev. Fitotec. Mex. 40, 349–357. doi: 10.35196/rfm.2017.2.199-209
Patel, K. K., Khan, M. A., Kumar, Y., and Yadav, A. K. (2019). Novel techniques in post harvest management of mango-an overview. Environment 5, 821–835. doi: 10.46370/sajfte.2019.v05i02.01
Perumal, A. B., Nambiar, R. B., Sellamuthu, P. S., and Emmanuel, R. S. (2021). Use of modified atmosphere packaging combined with essential oils for prolonging post-harvest shelf life of mango (cv. Banganapalli and cv. Totapuri). LWT. 148:111662. doi: 10.1016/j.lwt.2021.111662
Pesis, E., Aharoni, D., Aharon, Z., Ben-Arie, R., Aharoni, N., and Fuchs, Y. (2000). Modified atmosphere and modified humidity packaging alleviates chilling injury symptoms in mango fruit. Postharvest Biol. Technol. 19, 93–101. doi: 10.1016/S0925-5214(00)00080-6
Phaiphan, A., and Rattanapanone, N. (2008). Effect of edible coatings on quality of mango fruit (Mangifera indica) “Chok-Anan” during storage. Acta Hortic. 773, 227–232. doi: 10.17660/ActaHortic.2008.773.33
Phuangto, S., Chandee, O., Subsomboon, T., and Wattanakaroon, W. (2019). Post-harvest shelf life extension of mango using chitosan and carboxymethyl cellulose-based coatings. Key Eng. Mater. 824, 81–86. doi: 10.4028/www.scientific.net/KEM.824.81
Plotto, A., Narciso, J. A., Rattanapanoneb, N., and Baldwina, E. A. (2010). Surface treatments and coatings to maintain fresh-cut mango quality in storage. J. Sci. Food Agric. 90, 2333–2341. doi: 10.1002/jsfa.4095
Prabakar, K., Raguchander, T., Parthiban, V. K., Muthulakshmi, P., and Prakasam, V. (2005). Post harvest fungal spoilage in mango at different levels marketing. Madras Agric. J. 92, 42–48.
Ramayya, N., Niranjan, K., and Duncan, E. (2012). Effects of modified atmosphere packaging on quality of ‘Alphonso’mangoes. J. Food Sci. Technol. 49, 721–728. doi: 10.1007/s13197-010-0215-x
Razzaq, K., Singh, Z., Khan, A. S., Khan, S. A. K. U., and Ullah, S. (2016). Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. 78, 401–411. doi: 10.1007/s10725-015-0101-7
Roy, S. K., and Pal, R. K. (1989). Multilocational studies to reduce post harvest losses during harvesting, handling, packaging, transportation and marketing of mango in India, in III International Mango Symposium, Darwin, NT, Vol. 291, 499–507. doi: 10.17660/ActaHortic.1991.291.57
Sakimin Siti, Z., and Singh, Z. (2011). Post-harvest fumigation with nitric oxide at the pre-climacteric and climacteric-rise stages influences ripening and quality in mango fruit. J. Hortic. Sci. Biotechnol. 86, 645–653. doi: 10.1080/14620316.2011.11512817
Salinas-Roca, B., Guerreiro, A., Welti-Chanes, J., Antunes, M. D. C., and Martín-Belloso, O. (2018). Improving quality of fresh-cut mango using polysaccharide-based edible coatings. Int. J. Food Sci. Technol. 53, 938–945. doi: 10.1111/ijfs.13666
Sapkota, S., Kc, D., Giri, H., Saud, M., Basnet, M., and Gautam, S. (2021). Effect of ethephon and packaging practices on ripening of mango cv. Maldah. SAARC J. Agric. 19, 155–163. doi: 10.3329/sja.v19i1.54786
Shahin, N., Kumar, R., Karuna, K., and Mankar, A. (2020). Postharvest application of calcium, packaging material and hot water treatment on quality of mango (Mangifera indica L.) cv. Zardalu. J. Postharvest Technol. 8, 77–85.
Shiesh, C., and Lin, H. (2010). Effect of vapor heat and hot water treatments on disease incidence and quality of Taiwan native strain mango fruits. Int. J. Agric. Biol. 12, 673–678.
Siddiq, M., Brecht, J. K., and Sidhu, J. S. (2017). Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition. Chichester: John Wiley & Sons.
Singh, Z., Singh, R. K., Sane, V. A., and Nath, P. (2013). Mango-postharvest biology and biotechnology. CRC Crit. Rev. Plant Sci. 32, 217–236. doi: 10.1080/07352689.2012.743399
Singh, Z., and Zaharah, S. S. (2015). Controlled atmosphere storage of mango fruit: challenges and thrusts and its implications in international mango trade. Acta Hortic. 92, 481–492. doi: 10.17660/ActaHortic.2015.1066.21
Singh, Z., and Zaharah, S. S. (2013). Controlled atmosphere storage of mango fruit - an overview. Acta Hortic. 1066, 179–191. doi: 10.17660/ActaHortic.2013.992.59
Singh, N. P., Jerath, N., Singh, G., and Gill, P. P. S. (2012). Physico-chemical characterization of unexploited mango diversity in sub-mountane zone of Northern India. Indian J. Plant Genet. Resour. 25, 261–269.
Singh, Z., Janes, J., and Nair, S. (2001). Packaging materials affect physiological weight loss fruit colour and quality of mango during storage. Acta Hortic. 553, 603–604. doi: 10.17660/ActaHortic.2001.553.142
Sisler, E. C. (2006). The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol. Adv. 24, 357–367. doi: 10.1016/j.biotechadv.2006.01.002
Sivakumar, D., Jiang, Y., and Yahia, E. M. (2011). Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 44, 1254–1263. doi: 10.1016/j.foodres.2010.11.022
Srinivasa, P. C., Baskaran, R., Ramesh, M. N., Harish Prashanth, K. V., and Tharanathan, R. N. (2002). Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 215, 504–508. doi: 10.1007/s00217-002-0591-1
Srinivasa, P. C., Susheelamma, N. S., Ravi, R., and Tharanathan, R. N. (2004). Quality of mango fruits during storage: effect of synthetic and eco-friendly films. J. Sci. Food Agric. 84, 818–824. doi: 10.1002/jsfa.1760
Subedi, P. P., Walsh, K. B., and Owens, G. (2007). Prediction of mango eating quality at harvest using short-wave near infrared spectrometry. Postharvest Biol. Technol. 43, 326–334. doi: 10.1016/j.postharvbio.2006.09.012
Subramanian, K. S., Sekar, C., Meneka, S. S., and Prakash, L. V. (2018). Reducing Postharvest Losses in Mango in South Asia. Available online at: https://www.idrc.ca/en/research-in-action/reducing-post-harvest-losses-south-asias-mango-orchards
Sudhakar Rao, D. V., and Gopalakrishna Rao, K. P. (2008). Controlled atmosphere storage of mango cultivars “Alphonso” and “Banganapalli” to extend storage-life and maintain quality. J. Hortic. Sci. Biotechnol. 83, 351–359. doi: 10.1080/14620316.2008.11512391
Terao, D., de Carvalho Campos, J. S., Benato, E. A., and Hashimoto, J. M. (2015). Alternative strategy on control of postharvest diseases of mango (Mangifera indica L.) by use of low dose of ultraviolet-C irradiation. Food Eng. Rev. 7, 171–175. doi: 10.1007/s12393-014-9089-4
Tesfay, S. Z., and Magwaza, L. S. (2017). Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 11, 40–48. doi: 10.1016/j.fpsl.2016.12.001
Tharanathan, R. N., Yashoda, H. M., and Prabha, T. N. (2006). Mango (Mangifera indica L.), “The king of fruits”—an overview. Food Rev. Int. 22, 95–123. doi: 10.1080/87559120600574493
Thokchom, R., and Mandal, G. (2019). Effect of postharvest treatments on physical characteristics of mango. Int. J. Pharmacogn. Phytochem. 8, 2239–2243.
Tian, S., Liu, J., Zhang, C., and Meng, X. (2010). Quality properties of harvested mango fruits and regulating technologies. Fresh Prod. 4, 49–55.
Tran, T. T. L., Aiamla-Or, S., Srilaong, V., Jitareerat, P., Wongs-Aree, C., and Uthairatanakij, A. (2013). Application of nitric oxide to extend the shelf life of mango fruit, in II Southeast Asia Symposium on Quality Management in Postharvest Systems, Vientiane, Vol. 1088, 97–102.
Ueda, M., Sasaki, K., Utsunomiya, N., Inaba, K., and Shimabayashi, Y. (2005). Effect of temperature and time on some properties during storage of mango fruit (Mangifera indica L.'Irwin') cultured in plastic house. J. Jpn. Soc. Food Sci. Technol. 48, 349–355. doi: 10.3136/nskkk.48.349
Ullah, H., Ahmad, S., Anwar, R., Nafees, M., and Thompson, A. K. (2010). Effect of'oxygen and carbon-dioxide'on the post-harvest management in tree-ripe mango storage. J. Chem. Soc. Pakistan 32, 485–491.
Vega-Alvarez, M., Salazar-Salas, N. Y., López-Angulo, G., Pineda-Hidalgo, K. V., López-López, M. E., Vega-García, M. O., et al. (2020). Metabolomic changes in mango fruit peel associated with chilling injury tolerance induced by quarantine hot water treatment. Postharvest Biol. Technol. 169:111299. doi: 10.1016/j.postharvbio.2020.111299
Verghese, K., Lewis, H., Lockrey, S., and Williams, H. (2015). Packaging's role in minimizing food loss and waste across the supply chain. Packag. Technol. Sci. 28, 603–620. doi: 10.1002/pts.2127
Wang, Y., Bai, J., and Long, L. E. (2015). Quality and physiological responses of two late-season sweet cherry cultivars ‘Lapins’ and ‘Skeena’to modified atmosphere packaging (MAP) during simulated long distance ocean shipping. Postharvest Biol. Technol. 110, 1–8. doi: 10.1016/j.postharvbio.2015.07.009
Watanawan, C., Wasusri, T., Srilaong, V., Wongs-Aree, C., and Kanlayanarat, S. (2014). Near infrared spectroscopic evaluation of fruit maturity and quality of export Thai mango (Mangifera indica L. var. Namdokmai). Int. Food Res. J. 21:1073. doi: 10.17660/ActaHortic.2013.989.12
Wei, S., Mei, J., and Xie, J. (2021). Effects of different carbon dioxide-modified atmosphere packaging and low-temperature storage at 13?c on the quality and metabolism in mango (Mangifera indica L.). Agriculture. 11:636. doi: 10.3390/agriculture11070636
Xu, X., Lei, H., Ma, X., Lai, T., Song, H., Shi, X., et al. (2017). Antifungal activity of 1-methylcyclopropene (1-MCP) against anthracnose (Colletotrichum gloeosporioides) in postharvest mango fruit and its possible mechanisms of action. Int. J. Food Microbiol. 241, 1–6. doi: 10.1016/j.ijfoodmicro.2016.10.002
Xu, W., Li, D., Fu, Y., and Wang, Y. (2012). The effect of active packaging film on the quality of Dashehari mango fruits. Adv. Mater. Res. 399, 1181–1185. doi: 10.4028/www.scientific.net/AMR.399-401.1881
Yahia, E. M. (1998). Modified and Controlled Atmospheres for Tropical Fruits, Horticultural Reviews-Westport Then New York. New Jersey:John Wiley & Sons, Inc.
Yasunaga, E., Fukuda, S., Takata, D., Spreer, W., Sardsud, V., and Nakano, K. (2018). Quality changes in fresh mango fruits (Mangifera indica L. ‘nam dok mai’) under actual distribution temperature profile from Thailand to Japan. Environ. Control Biol. 56, 45–49. doi: 10.2525/ecb.56.45
Yasunaga, E., Sardsud, V., Wanwarang, P., Spreer, W., Yuge, K., and Fukuda, S. (2012). Effect of post-harvest distribution environment on quality deterioration of mango fruits. Acta Hortic. 934, 921–927. doi: 10.17660/ActaHortic.2012.934.123
Zaharah, S. S., and Singh, Z. (2011). Postharvest nitric oxide fumigation alleviates chilling injury, delays fruit ripening and maintains quality in cold-stored ‘Kensington Pride’mango. Postharvest Biol. Technol. 60, 202–210. doi: 10.1016/j.postharvbio.2011.01.011
Zaharah, S. S., and Singh, Z. (2010). Nitric oxide fumigation delays mango fruit ripening, in IX International Mango Symposium, Sanya, Vol. 992, 543–550. doi: 10.17660/ActaHortic.2013.992.67
Zeng, K., Cao, J., and Jiang, W. (2006). Enhancing disease resistance in harvested mango (Mangifera indica L. cv.‘Matisu’) fruit by salicylic acid. J. Sci. Food Agric. 86, 694–698. doi: 10.1002/jsfa.2397
Zerbini, P. E., Vanoli, M., Rizzolo, A., Grassi, M., de Azevedo Pimentel, R. M., Spinelli, L., et al. (2015). Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 101, 58–65. doi: 10.1016/j.postharvbio.2014.11.008
Keywords: supply chain, postharvest losses, mango quality, postharvest technologies, mango storages
Citation: Le TD, Viet Nguyen T, Muoi NV, Toan HT, Lan NM and Pham TN (2022) Supply Chain Management of Mango (Mangifera indica L.) Fruit: A Review With a Focus on Product Quality During Postharvest. Front. Sustain. Food Syst. 5:799431. doi: 10.3389/fsufs.2021.799431
Received: 21 October 2021; Accepted: 31 December 2021;
Published: 04 February 2022.
Edited by:
Asanda Mditshwa, University of KwaZulu-Natal, South AfricaCopyright © 2022 Le, Viet Nguyen, Muoi, Toan, Lan and Pham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanh Viet Nguyen, bnR2aWV0QG50dC5lZHUudm4=; Nguyen Mai Lan, bm1sYW5AbnR0LmVkdS52bg==; Tri Nhut Pham, cHRuaHV0QG50dC5lZHUudm4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.