- 1Sustainable Agriculture, Teri Deakin Nano Biotechnology Centre, Gurugram, India
- 2Chemistry Department, Deakin University, Geelong, VIC, Australia
- 3Environmental Toxicology, Amity Institute of Environmental Toxicology, Safety & Management, Uttar Pradesh, India
This review article highlights alternative innovative uses of soil, brown coal, and composted solid city waste. The latter leads to environmental pollution, which can be addressed by using these materials to generate value-added products. Humic substances present there can be isolated in large amounts and used in diverse fields like sustainable agriculture, horticulture, biomedicine, and materials science. These have been shown to be non-toxic and safe for humans and serve as growth promotants for plants and to cure stomach ailments. The recent discovery of their antiviral/anti-HIV-AIDS activity is described here in some detail. The use of humic substances for making dye-sensitized solar cells (DSSCs) and for preparing a catalyst for reduction and for oxidation processes is also highlighted. Such innovative uses of humic substances can lead to environmental cleaning and positively impact climate change.
New planets are not discovered every day, neither are new amino acids
Introduction
Value-added products are expected to be available from different natural resources like soil, compost, brown coal, and city solid waste (Bruna et al., 2016; Eladia et al., 2005). Most of these, as humic substances, are available in the market as brown or black in color. As part of organic farming, these help grow better vegetables, quality fruits (like peas, potato, tomato, pomegranate, mangoes) cereals, and pulses. Humic acids serve as growth promotants. Their mode of action is through chelating cations [Mg (II), Zn (II), Cu (II), Al (III), and Fe (III)] and water retention (Khanna et al., 2010). There is much interest in the market for such products esp. for use in organic farming. The combined use of humic substances with Mycorrhizae (Ekin, 2019) leading to increased growth in cereals, pulses, tomatoes, beans, garlic, etc., has been demonstrated. There is much interest in exploiting the commercial aspects in the energy sector of the economy, as these new technologies could help in cleaning the environment, as well.
Focus has to be on establishing innovative uses of humic substances. These include making humic substance-based solar cells (Rohit et al., 2016). Based on reverse polymerization of humic substances, zirconium-humic acid (Zr-HA) and copper-humic acid (Cu-HA) catalysts, respectively for reduction and oxidation processes, have been developed recently (Hao et al., 2021)1. However, in these pandemic times, what catches attention most is the antiviral activity, in particular the reported anti-HIV-AIDS activity of humic substances (Zhernov et al., 2020), which has been shown to be superior to Himalayan Shilajit, an immune booster available from Dabur Co., Ghaziabad, India2.
Humic substances are obtained from diverse sources like soil, compost, brown coal, and city solid waste. Extraction with aqueous alkali, filtration, and acidification leads to humic and fulvic acids, of which the latter is more soluble, while the alkali-insoluble portion is referred to as humin. Even after nearly a century of work (Baveye and Wander, 2019), there are many very complicated structures proposed for this class of compounds and every other group seems to have a different proposed structure. Two examples are shown below, but it must be pointed out that none of the plethora of structures available in literature have been established conclusively and are at best only proposed structures.
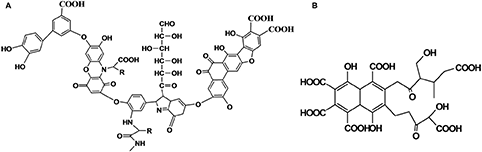
Based on mass (Wang et al., 2017) and NMR spectral studies, it is becoming increasingly clear that humic/fulvic acids are relatively small molecules which masquerade as large supramolecular assemblies (Nebbioso et al., 2014). The other reason for this is that all these starting materials, be it soil, compost, brown coal, or city solid waste, arise from different plant and animal sources, which decompose over long periods of time, in some cases over centuries, and which could differ from country to country. However, despite this, the world over there is an agreement and great expectation that, in future, novel technological products can be derived from such sources.
Techniques Commonly Used for Characterization of Humic Acids
Characterization of humic acids is done using thin layer chromatography (TLC), melting point, optical rotation, water solubility, FT-IR, UV-visible, 1H-NMR, 2D-NMR, and mass spectrometry which help establish their structures. A 2011 paper (Helal, 2011) describes the different analytical techniques used for characterization of different humic materials.
Described in This Review Are Only a few Such Techniques Which Require Special Mention
A typical FT-IR spectrum of a humic acid (de Souza and Braganc, 2017) is shown in Figure 1. The broad absorption from 3,750 to 2,400 cm−1 shows the presence of strong H-bonding and carboxylic acid groups, also evidenced by the presence of bands in the 1,700-cm−1 region.
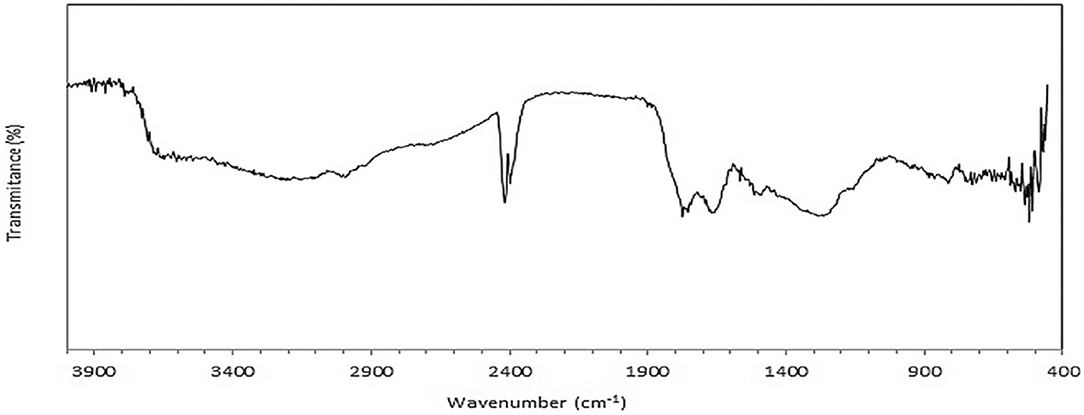
Figure 1. FT-IR spectrum of a typical humic acid (de Souza and Braganc, 2017).
Different NMR techniques then help establish whether the organic material is predominantly aromatic/quinonoid or aliphatic/alicyclic in nature. The relatively newer technique, solid-state NMR spectra (Chemical Polarization-Magic Angle Spinning, CP-MAS-SS-NMR), is often cited in this field. Unlike the 1H-NMR spectra where sharp signals are observed, the solid-state NMR spectra are broad, yet these help identify the nature of functionalities present in the material. Thus, CPMAS 13C-NMR was used to characterize humic acid from the composted Saudi agricultural waste (Faiyaz, 2017) (Figure 2).
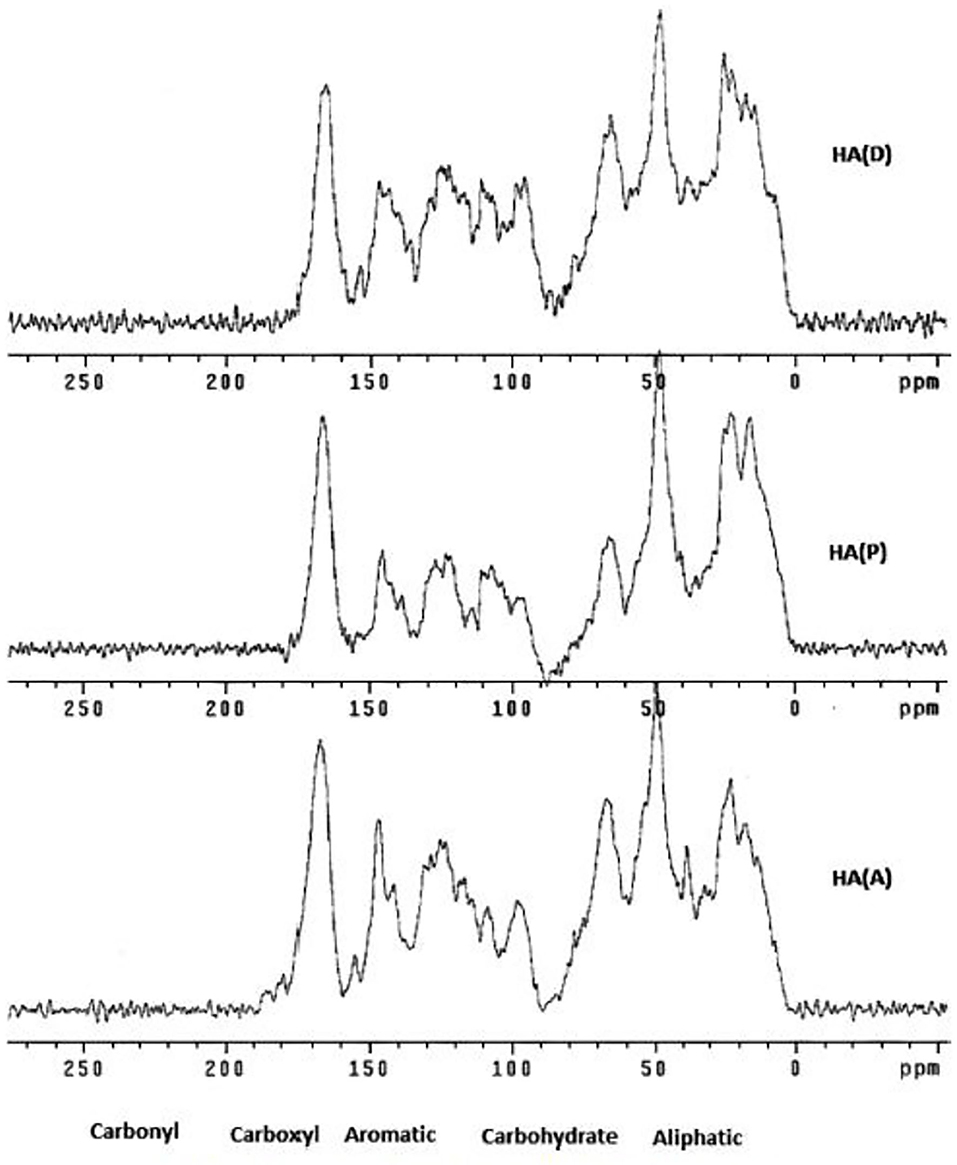
Figure 2. CP-MAS-SS NMR spectrum of a humic acid (Faiyaz, 2017).
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) studies (Prakash et al., 2014) show the range of temperatures over which humic acids are stable and the weight loss these undergo over 50–650°C (Figure 3). These also give information over the kind of functionalities present which affect stability and loss of units from them.
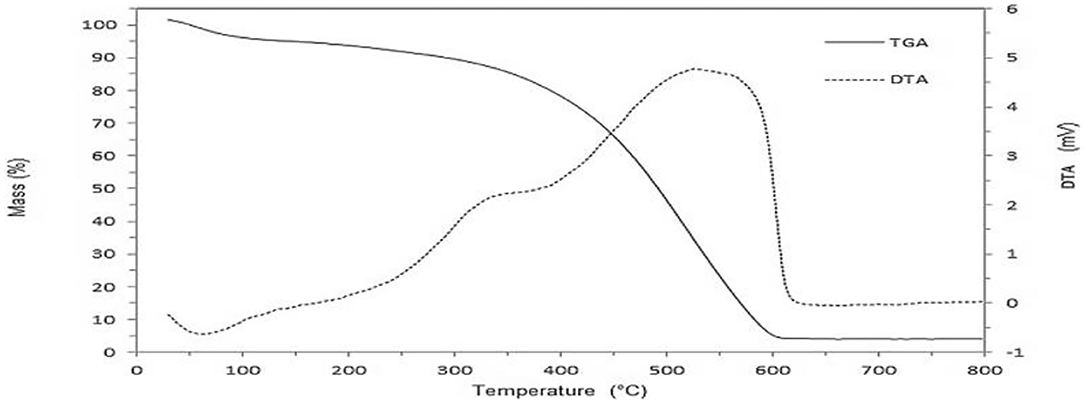
Figure 3. DTA and TGA studies on a humic acid (Prakash et al., 2014).
Determination of elemental composition plays an important role in the early part of the study. Field emission scanning electron microscopy–energy-dispersive X-ray analysis (FESEM-EDAX) spectral studies are a more recent addition to the repertoire of the investigator, and they help quantitatively establish the elements present in the sample.
Humic acid was subjected to FESEM-EDAX analysis, and the results (Figure 4) show the presence of potassium 42.53%, oxygen 34.67%, carbon 19.27%, chlorine 2.72%, and sulfur 0.28%, by weight % (atomic%: 32.33, 43.61, 0.45, 0.17, and 21.89%, resp.).
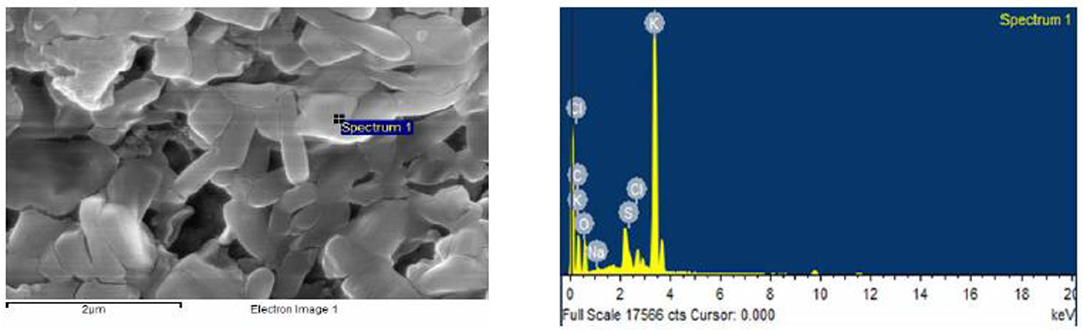
Figure 4. FESEM-EDAX spectrum of a humic acid (Prakash et al., 2014).
Molecular Dynamics Studies on Humic Acids
The earlier DOM model for humic acids was refined (Orsi, 2014) to get a molecular formula C447 H421O272N15S2 and a molecular weight of 10,419 g mol−1. This was then hydrated with water, and hydration conditions in soil were simulated.
Using the TNB (Temple–Northeastern–Birmingham) model (Figure 5), the Schulten DOM 3D model shown in Figure 6 was generated:
Figure 5. The TNB (Temple–Northeastern–Birmingham) model (Orsi, 2014).
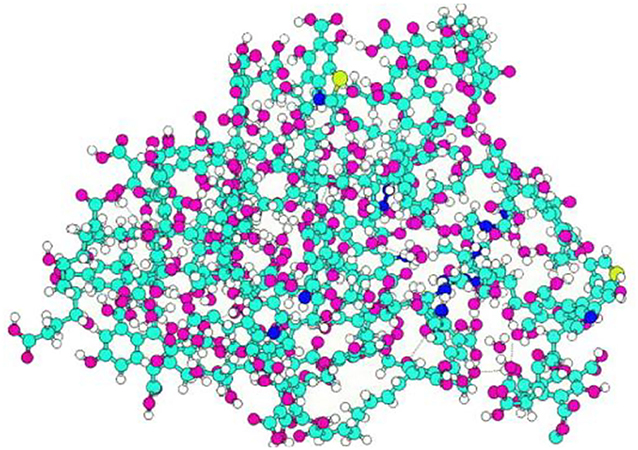
Figure 6. Schulten DOM 3D model using the TNB (Temple–Northeastern–Birmingham) model (Orsi, 2014).
Similarly, interactions between DOM and fullerene C60 have been studied (Figure 7) using molecular simulations (Wang et al., 2011). In another report, it was shown that aromatic acids interact more strongly than aliphatic acids.
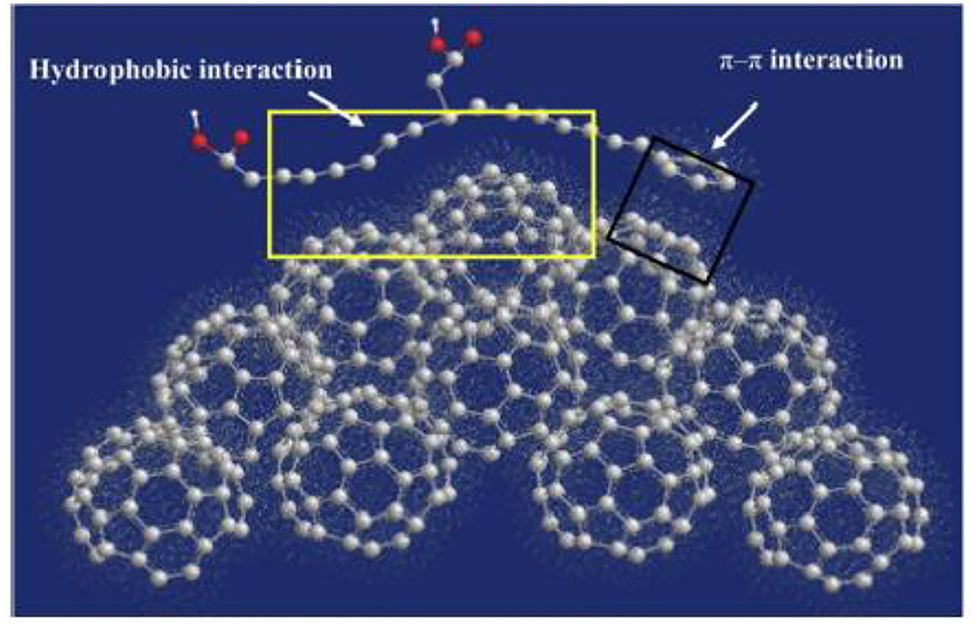
Figure 7. A snapshot of the interaction between a small humic substance molecule and fullerene C60 (carbon, gray; oxygen, red). (Wu et al., 2013).
Most recent reports point to the fact that unlike what has been believed in the past, humic acids are relatively simple small molecules. Most papers show different spectra but refrain from putting down a precise chemical structure. This is because these form large assemblies and supramolecular structures (Figure 8), whose formation is critically dependent on pH (Piccolo, 2002).
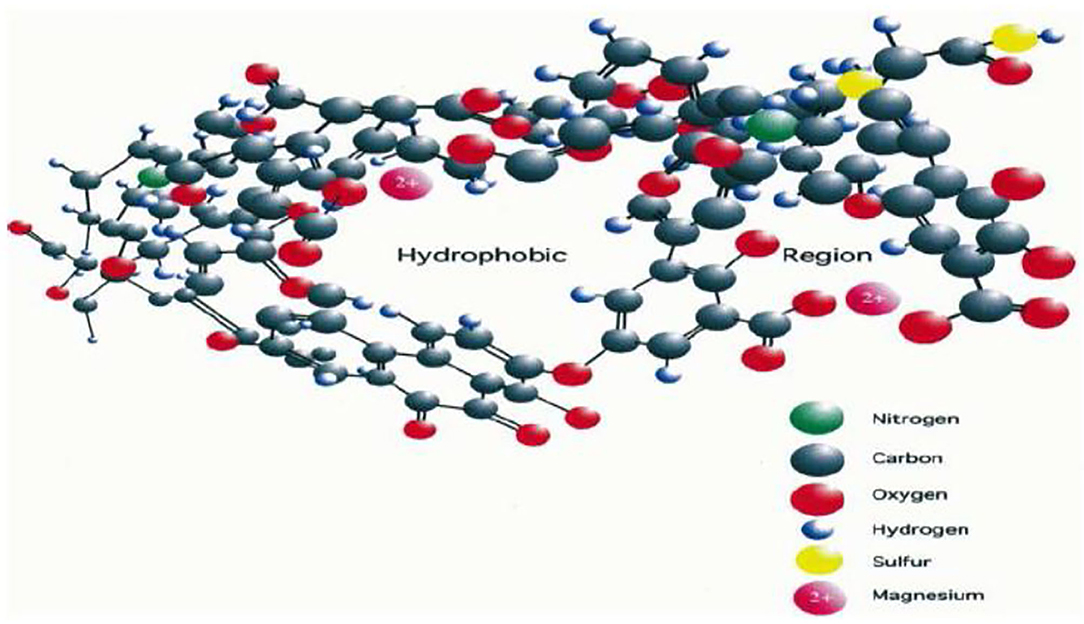
Figure 8. Portion of the “pseudomicelle type” structure of humic acids. (Salati et al., 2011).
Humic acids may be considered as a membrane-like structure with a hydrophobic interior and charged-exterior surfaces (Figure 9).
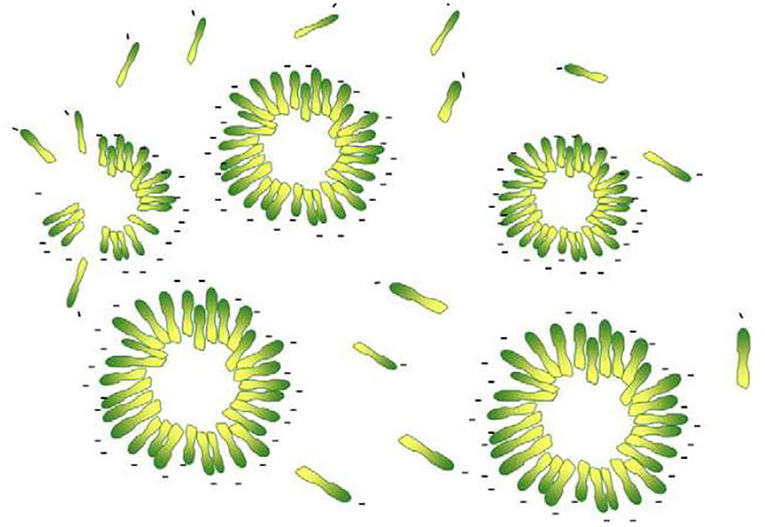
Figure 9. Humic acid micelles (Salati et al., 2011).
Cross-Linking of Humic Acids for Increasing Water Retention in Soil
Increased water retention of humic acid by cross-linking with formaldehyde (Cihlár et al., 2014) has been demonstrated. Particularly useful are the measurement of t1 and t2 NMR relaxation times, before and after addition of water to humic acid. DOSY-NMR (Asmejkalova and Piccolo, 2008) has been used in these studies. Water retention can be crucial for plants under water distress conditions, especially in the deserts.
Applications in Agriculture and Horticulture
These natural compounds serve as growth promotants and have uses in agriculture and horticulture. Shown below are the effects of the use of humic acid preparations on the luscious growth of pomegranate (Figure 10) as well as growth of roots (Figure 11).
Similarly, humic acid preparations have been successfully used in horticulture. Shown below are the effects on Chrysanthemum flower growth (Figure 12) 10 days after treatment with a humic acid preparation.
Use of humic acid in sustainable agriculture has been advocated, and it has been stated that “Highly significant increases have been reported recently in potato growth, tuber yields, and quality in PGPR (Plant Growth Promoting Rhizobacteria; Bacillus megatorium and Bacillus subtilis) and Humic Acid inoculated crops. Tuber size, weight, specific gravity, dry matter, starch, protein, and mineral contents (except Cu) were improved with PGPR treatments and further increased when administered with humic acids.”
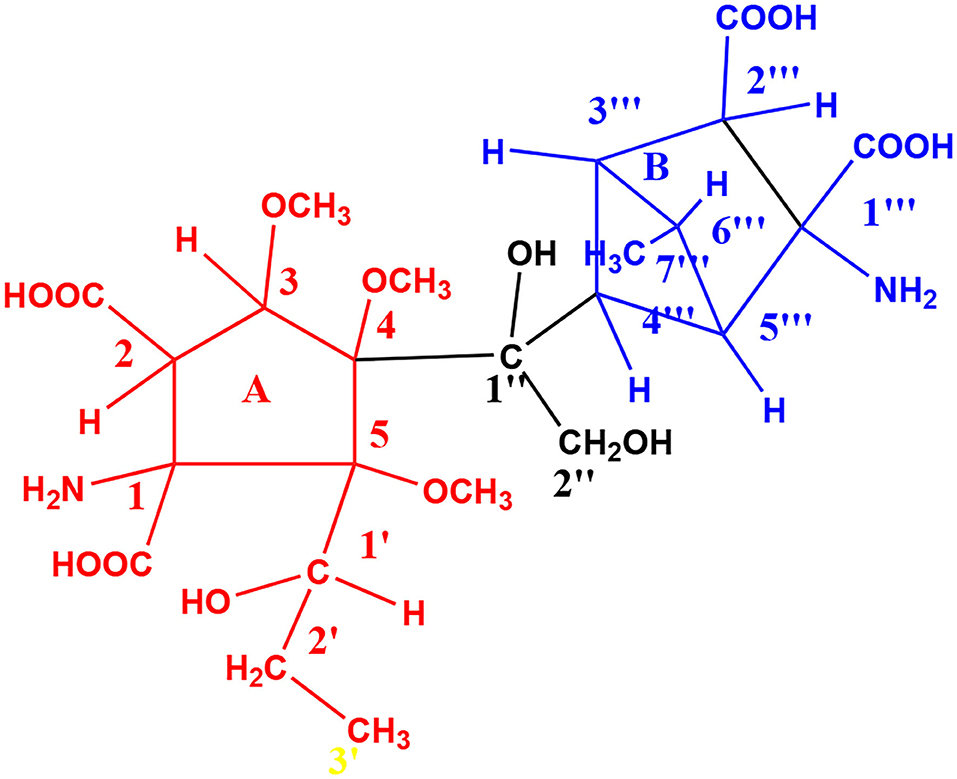
From the author's own laboratory, the isolation of “Gwal Pahari Acid,” from the soil of Gwal Pahari, Teri Gram, Gurugram, Haryana, India, which shows good growth promotant activity in tomatoes, has been reported. This chiral, ninhydrin-positive amino acid has the molecular weight of m/z 578 a.m.u. corresponding to the molecular formula, C24 H38 N2O14. A chemical structure has been proposed by us based on Figure 13 various spectral studies, including FT-IR, UV-visible, 1H-NMR, 2D-NMR, and mass spectrometry.
Ring A in the above proposed that the structure is relatively flexible, while ring B is more rigid and pseudorotation raises many conformational possibilities, where the conformers differ from each other by low-energy barriers allowing many of these conformations to be populated, making especially NMR studies non-trivial in nature. We are carrying out similar studies on the inert soil from, for example, the Ghazipur landfill in East Delhi, India, to obtain value-added products for the targeted uses highlighted in this review.
Lignite has been described as a yet untapped rich source of carbochemicals. In 2006, a process for production of “Humi Gold” (A salt of Humic acid) has been patented by the Neyveli Lignite Corporation, India (Neyveli Lignite Corporation, 2006). This patent describes a process for the preparation of potassium salt of humic acid by extraction using aqueous potassium hydroxide of lignite. The potassium humate thus obtained has been named by them as “Humi Gold,” which is obtained as black flakes and is used for promoting growth on groundnuts, maize, and bhindi (ladies finger). (Indian patent number 201577, August 1, 2006, 626/MAS/2000; Mukherjee et al., 2010; Kandasamy et al., 2011). Lignite from Neyveli has been identified as a potential source of new chemicals with possible technological significance but no chemical has been characterized by these authors.
In many countries, lignite, leonardite, and brown coal have been used in thermal power plants. Germany for example has decided to shut down these plants by the year 2030, as there is much opposition due to the environmental pollution caused by them. Only in USA, e.g., in North Dakota, did the plants continue to produce the cheapest electricity in America, and there is thus there is no proposal to shut these plants down. Alternative nonpolluting technologies based on lignite are thus eagerly awaited for welcome use of this natural abundant material the world over.
Uses in Biomedicine
Humic substances are known to be generally safe for use in humans, as these cause no toxicity issues. Preparations of humic acid have been used for treatment of stomach (gastroenteritis) (Swidsinski et al., 2017) diseases and skin ailments. Activomin®, a commercial preparation, is already available in the market in Germany.
Recently, humic acid has been used for the delivery of an anti-epileptic drug (Mirza et al., 2011). Thus, the drug carbamazepine was coated with humic acid preparation, and its safe delivery and efficacy in epileptic patients were demonstrated.
Antiviral Activity of Humic Acids
A 2004 US patent (Zanetti, 2004) states, “The Humic Acid preparations hereof are thus useful in the treatment of HIV-AIDS and are described as particularly useful alone or as adjuvants to be co-administered in immunevaccinations against HIV.” It has also been pointed out that humic acid is an IL-2 immunostimulant.
A recent report (2019) describes studies of a new anti-HIV humic substance-based preparation, a solubilized butanol fraction of humic substances (SBF-HS) (Kornilaeva et al., 2019). Mass spectrometry data and the results of an elemental analysis indicate that the fraction contains 52.7% (w/w) and 37.1% (w/w) carbon and oxygen, respectively, but considerably lower amounts of nitrogen (4.3% w/w) and sulfur (2.1% w/w). It has been stated that, “It should be noted that an elemental analysis of humic substances isolated from the same source using the same isolation and fractionation methods yields reproducible results. High-performance liquid chromatography (HPLC) data demonstrate that the preparation is characterized by a high content of hydrophobic aromatic fragments (74%), which was confirmed by 13C NMR. The hydrogen-to-carbon atomic ratio (H/C) is 0.8; the oxygen-to-carbon atomic ratio (O/C) is 0.53.” Cytotoxicity studies in three independent experiments demonstrated that SBF-HS preparations were nontoxic to both the CEM-SS cell line and the primary cells (PBMC). The infection induced in Mφ by the R5 strain HIV-1Ba-L exhibited maximum sensitivity to SBF-HS: 90 and 50% inhibition were observed in the presence of relatively low amounts of the preparation.
It is further stated that, “It is known that humic substances form as a result of the decay of dead organisms and are among the vast reservoirs of organic carbon. SBF-HS is an ecologically sound and safe product exhibiting antitumor, antifungal, antibacterial, and antiviral activities. In addition, it stimulates hemopoiesis and acts as an immunomodulator, a powerful antioxidant, and an efficient hepatoprotector. SBF-HS stimulates cell-mediated immunity (particularly in inflammatory foci), accelerates the regeneration of wounds, burns, and ulcers of skin and mucosa, and lacks toxicity and allergenicity.” The authors point out that “only two companies (one in the United States and one in Russia) have so far succeeded in developing humic substance-based drugs and in completing all the requisite preclinical and clinical trials of their safety and efficacy.”
A 2020 report compared a set of humic materials with Himalayan Shilajit the immunobooster marketed by Dabur Co., India. In MTT assays, it was shown that even at 1,000 mg l−1 both the humic materials and Shilajit showed no cytotoxicity to CEM-SS cells. The obtained EC50 values for humic materials varied from 0.37 to 1.4 mg·l−1, whereas the values for Shilajit were much higher at 54 to 103 mg·l−1.
In S. Africa, a “wellness drink” (Botes et al., 2018) based on fulvic acid is available for HIV-AIDS patients, and the same can also be consumed by the normal populace. The authors stated that “the CHD-FA containing wellness F0210 demonstrated no detrimental effects on the progression of disease in HIV-1 infected pre-ART patients” and that organ function was maintained and “no serious adverse events occurred.”
The use of humic acids in sunscreens and antiaging and skin care products has been suggested. These could also be considered for use in functional lipsticks (Klöcking et al., 2013) and in facial masks (Wollina, 2009). Mud baths in spas and nature cure centers is very common, as these are believed to have rejuvenating effects. In this context, the use of humic and fulvic acids in cosmetics and skin care is also becoming popular, as evidenced by the number of such products available on the internet.
Uses in Material Sciences
Use of Humic Substances for DSSCs
Dr. Michael Graetzel, 1991 developed the dye-sensitized solar cells (DSSCs; Graetzel Cells) (Sharma et al., 2018; Mariotti et al., 2020) with ~13% photoconversion efficiency (PCE). Since DSSCs utilize low-cost materials, these offer a low-cost alternative to the expensive Si solar cells (Figure 14). The favorable economics has encouraged the European Union (EU) to set a target of 200 MW of electricity generation from DSSCs by the year 2020, with an anticipated annual growth of 51%.
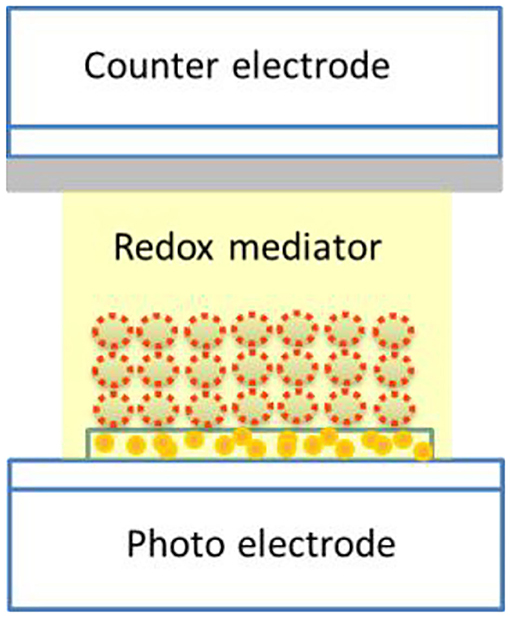
Figure 14. Photoelectrode ITO; nano-TiO2 layer and then the dye sensitizer, e.g., humic substance; and counter Pt electrode and a redox mediator /I– sandwiched between the two electrodes in a Graetzel solar cell. Illumination from the bottom and the electric current was tapped.
Recently, humic acid has been successfully used for making DSSCs (Rohit et al., 2016), where it serves as a photosensitizer. This discovery was made after it was reported in 2012 that humic acid binds to titanium dioxide (TiO2). These workers observed that a 12-nm hypsochromic shift was observed when humic acid was coated on TiO2. This could be due to the interaction of the carboxylic acid/quinone groups with the surface of TiO2. The PCE achieved is better than that obtained using many other natural substances like vegetable dyes based on flowers. In the presence of a deoxycholic acid, the value of PCE rises to 2.1%.The authors stated that, “The DSSC sensitized with humic acid produced Jsc = 5.3 mA/cm2, Voc = 0.472 V, and FF = 56.6% corresponding to a P.C.E. of 1.4%. The data reveal that HA is efficient as a sensitizer in the DSSC and the entity responsible for binding in HA is considered to be quinone and/or carboxylic acid groups. The P.C.E. was further improved to 2.1% (Jsc = 6.6 mA/cm2, Voc = 0.524 V, and FF = 60.7%) using the co-adsorbent, CDCA, under 100 mW/cm2 (1 sun) illumination. The same device delivered 2.4% P.C.E. under 50 mW/cm2 illumination.”
Zr-HA and Cu-HA Catalysts for Reduction and Oxidation Processes
A very recent report in 2021 from China describes the use of Zr-HA and Cu-HA catalysts (Hao et al., 2021). The former zirconium-based compound has been demonstrated as a suitable reusable catalyst for carrying out the reduction of organic substrates, while the latter copper-containing compound is useful for oxidation. Many substrates have been included, and the conversion yields are consistently high. The catalysts could be used in aqueous phase or in organic phase using, in that case, DMF as the solvent for the preparation of the catalyst. These workers use a ruthenium-catalyzed depolymerized version of humic acid and cite a report where the raw humic acid itself has been employed for this purpose. Reduction of furfural has been cited as an example with different catalyst preparations. Other substrates used include different aliphatic aldehydes, cyclohexanone, and 2-hexanone. For reduction, isopropanol is used along with the Zr-HA catalyst in a sealed container. The Cu-HA catalyst has been used successfully to oxidize benzyl alcohol, cinnamyl alcohol, veratryl alcohol, p-methyl-phenyl ethanol, 2-hydroxy-naphthalene, p-chloro-phenylethanol, and furfuryl alcohol. In this case, there is no requirement for the use of a sealed container.
Environmental Cleaning and Climate Change
It is known that in humic acids, gel-like formation occurs when the pH is changed from alkaline toward mildly acidic pH, and a supramolecular self-assembly results, which is capable of capturing metals and pollutants. This is a very important and sensitive topic today because climate change (Yang and Antonietti, 2020) is impacted greatly by such environmental factors. The question that is raised is: Can soil matter serve the purpose of clearing large masses of environmental pollutants captured in small volumes so that these can be disposed of relatively easily?
Conclusion
Humic substances, like humic/fulvic acids, are products of degradation of plant, animal, forest, and city waste composted over long periods of time, sometimes over centuries. In this review, it is shown that humic substances are useful as growth promotants in agriculture and horticulture. These have also been demonstrated to contribute to biomedicine and materials science (DSSC solar cells and as catalysts). The discovery of the antiviral (anti-HIV-AIDS) activity of humic acids is a most noteworthy development. Thus, not only looking for value-added products from sources like soil, brown coal, and composted city solid waste attractive for adding to the economy, but also in the long run these could lead to environmental cleaning and containing the adverse effects of climate change.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I thank Dr Alok Adholeya, Director, TDNBC for providing facilities.
Footnotes
1. ^Delivered online as a Valedictory address at the Centre for Professional Development in Higher Education, University of Delhi, Nov., 2020.
2. ^https://www.kapiva.in/product/kapiva-himalayan-shilajit-resin-20g/?utm_campaignname=%20shilajit&adgroupid=1354599454698550&msclkid=1b3bd5c931ca142d9571b529a41331.
References
Asmejkalova, D., and Piccolo, A. (2008). Aggregation and disaggregation of humic supramolecular assemblies by nmr diffusion ordered spectroscopy (DOSY-NMR). Environ. Sci. Technol. 42, 699–706. doi: 10.1021/es071828p
Baveye, P. C., and Wander, M. (2019). The (Bio) chemistry of soil humus and humic substances: why is the “new view” still considered novel after more than 80 years? Frontiers in Environ. Sci. 7:27. doi: 10.3389/fenvs.2019.00027
Botes, M. E., Gilada, I. S. J. R., and Labuschange, J. P. L. (2018). Carbohydrate derived fulvic acid wellness drink: its tolerability, safety and effect on disease markers in pre-ART HIV-AIDS positive subjects. South Afr. Farm Prac. 60, 91–96. doi: 10.1080/20786190.2017.1397381
Bruna, A. G., Melo, d. e., Motta, F. L., and Santana, M. H. A. (2016). Materials Humic acids: Structural properties and multiple functionalities for novel technological developments. Sci. Eng. C. 62, 967–974. doi: 10.1016/j.msec.2015.12.001
Cihlár, Z., Vojtov,á, L., Conte, P., Nasir, S., and Kučerík, J. (2014). (2014) Hydration and water holding properties of cross-linked lignite humic acids. Geoderma. 230–231, 151–160. doi: 10.1016/j.geoderma.2014.04.018
de Souza, F., and Braganc, S. R. (2017). FT-IR Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders. J. Mater. Res. Technol. 7:8. doi: 10.1016/j.jmrt.2017.08.008
Ekin, Z.. (2019). Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability. 11:3417. doi: 10.3390/su11123417
Eladia, M., Peña-Méndez Havel, J., and Patočka, J. (2005). Humic substances-compounds of still unknown structure: applications in agriculture, industry, environment, and biomedicine. J. Appl. Biomed. 3, 13–24. doi: 10.32725/jab.2005.002
Faiyaz, A. S. S. (2017). CPMAS 13C-NMR characterization of humic acids from composted agricultural waste. Arabian J. Chem. 10, S839–S853. doi: 10.1016/j.arabjc.2012.12.018
Hao, J., Han, L., Yang, K., Li, N., He, R., Zhi, K., et al. (2021). A general route of using lignite depolymerized derivatives for catalyst construction: insights into the effects of the derivative structures and solvents. ACS Omega. 6,14926–14937. doi: 10.1021/acsomega.1c00766
Helal, A. A. (2011) Characterization of different humic acids by various analytical techniques. Arabian J Chem. 4, 51–54. doi: 10.1016/j.arabjc.2010.06.018
Kandasamy, R., Sivagnanam, B., Santhanam, S., and Manoharan, V. (2011). Development and Production of Eco-Friendly Humic Acid from Neyveli Lignite for Sustainable Agriculture, Productivity. A Q. J. Nat. Productiv. Council. 51:4.
Khanna, R., Agarwal, S. P., and Khar, R. K. (2010). Fulvic acids and humic acids as novel complexing agents and a process Indian, Patent number 239574. Avaiable online at: https://www.health-solution.eu/human/
Klöcking, R., Felber, Y., Guhr, M., Meyer, G., Schubert, R., et al. (2013). Development of an innovative peat lipstick based on the UV-B protective effect of humic substances. Mires Peat. 11, 1–9.
Kornilaeva, G. V., Siniavin, A. E., Schultz, A., Germann, A., Moog, C., von Briesen, H., et al. (2019). The differential anti-hiv effect of a new humic substance-derived preparation in diverse cells of the immune system. Acta Nature. 41, 68–76. doi: 10.32607/20758251-2019-11-2-68-76
Mariotti, N., Bonomo, M., Fagiolari, L., Barbero, N., Gerbaldi, C., Bella, F., et al. (2020). Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 22, 7168–7218.
Mirza, M. A., Agarwal, S. P., Rahman, M. A., Rauf, A., Ahmad, N., Alam, A., et al. (2011). Role of humic acid on oral drug delivery of an antiepileptic drug, Drug Dev. Ind. Pharm. 37, 310–319. doi: 10.3109/03639045.2010.512011
Mukherjee, J., Adak, A. K., Khan, S., Kumar, S., and Sarkar, A. (2010). Solubilization of Neyveli lignite by oxidative degradation: a potential source of carbochemicals. Solid Fuel Chemistry. 44, 299–304. doi: 10.3103/S0361521910050034
Nebbioso, A., Mazzei, P., and Savy, D. (2014). Reduced complexity of multidimensional and diffusion NMR spectra of soil humic fractions as simplified by Humeomics. Chem. Biol. Technol. Agric. 1:24. doi: 10.1186/s40538-014-0024-y
Neyveli Lignite Corporation (2006). A Process for Production of Humi Gold (A salt of Humic acid) Indian patent number 201577, 1st. Aug., 2006, 626/MAS/2000 Indian patent number. 201577.
Orsi, M.. (2014). Molecular dynamics simulation of humic substances. Chem. Biol. Technol. Agricult. 1:10. doi: 10.1186/s40538-014-0010-4
Piccolo, A.. (2002). The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 75, 57–134. doi: 10.1016/S0065-2113(02)75003-7
Prakash, P., Chitradevi, A., Anand, T. A., Arasu, R., Narendrakumar, G., and Masilamani, S. M. (2014). A novel humic acid extraction procedure from Tunisian lignite. Int. J. Chem Tech Res. 6, 1531–1537.
Rohit, L. V., Fadadu, J.ayraj, V., Vaghasiya, and Soni, S. S. (2016). Humic acid as a sensitizer in highly stable dye solar cells: energy from an abundant natural polymer soil component. ACS Omega. 1, 14–18. doi: 10.1021/acsomega.6b00010
Salati, S., and Papa, G. A. (2011). Perspective on the use of humic acids from biomass as natural surfactants for industrial applications. Biotechnol. Adv. 29, 913–922. doi: 10.1016/j.biotechadv.2011.07.012
Sharma, K., Sharma, V., and Sharma, S. S. (2018). Dye-sensitized solar cells: fundamentals and current status. Nanoscale Res. Lett. 3:381. doi: 10.1186/s11671-018-2760-6
Swidsinski, A., Doerffel, Y., Baucke, V. L., Gille, C., Reisshauer, A., Goktas, O., et al. (2017). Impact of humic acids on the colonic microbiome in healthy Volunteers. World J. of Gastroenerol. 23, 885–890. doi: 10.3748/wjg.v23.i5.885
Wang, C. F., Faw, X., Zhang, F., Wang, S. Z., Zhao, Y-. P., et al. (2017). Characterization of Humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 17:20677. doi: 10.1039/C7RA01497J
Wang, Z., Chen, J., Sun, Q., and Peijnenburg, W. J. (2011). C60-dom interactions and effects on C60 apparent solubility A molecular mechanics and density functional theory study. Environ Int. 37, 1078–1082. doi: 10.1016/j.envint.2011.02.016
Wollina, U.. (2009). Peat: a natural source for dermatocosmetics and dermatotherapeutics. J. Cutan. Aesthet. Surg. 2, 17–20. doi: 10.4103/0974-2077.53094
Wu, F., Bai, Y., Mu, Y., Pan, B., Xing, B., and Lin, Y. (2013). Fluorescence quenching of fulvic acids by fullerene in water. Environ Pollut. 172, 100–107. doi: 10.1016/j.envpol.2012.08.005
Yang, F., and Antonietti, M. (2020). Artificial Humic Acids: Sustainable Materials against Climate Change, Adv. Sci. 190, 2992. doi: 10.1002/advs.201902992
Zanetti, M.. (2004). Treatment of HIV Infection with Humic acid U. S. patent Application Publication US 2004O137085A1.
Keywords: humic substances, sustainable agriculture, anti-HIV-AIDS, catalysts, DSSCs
Citation: Eswaran SV (2021) Value-Added Products From Soil, Brown Coal, and Composted City Solid Waste. Front. Sustain. Food Syst. 5:738899. doi: 10.3389/fsufs.2021.738899
Received: 16 July 2021; Accepted: 08 November 2021;
Published: 08 December 2021.
Edited by:
Engracia Madejon, Institute of Natural Resources and Agrobiology of Seville, Spanish National Research Council (CSIC), SpainReviewed by:
Emmanuel Amagu Echiegu, University of Nigeria, Nsukka, NigeriaR. Pandiselvam, Central Plantation Crops Research Institute (ICAR), India
Copyright © 2021 Eswaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. V. Eswaran, c3YuZXN3YXJhbkBnbWFpbC5jb20=; Q19zdi5lc3dhcmFuQHRlcmkucmVzLmlu; ZXN3YXJhbi5zQGRlYWtpbi5lZHUuYXU=
 S. V. Eswaran
S. V. Eswaran