- Agriculture and Livestock Research Enterprise of Minas Gerais (EPAMIG), Viçosa, Brazil
Coffee plants host several herbivorous species, but only few are considered pests. Brazil is the largest coffee producer of the world, and the two key coffee pests of the crop in the country are the coffee leaf miner Leucoptera coffeella and the coffee berry borer Hypothenemus hampei. However, in some regions or on specific conditions, species of mites and scales can also cause damage to coffee plants. Conventional management of coffee pests relies on chemical pesticides, and it is the most commonly used strategy in Brazil, but environmental problems, pest resistance, and toxicity-related issues have led coffee growers to search for alternatives for pest control. Agro-ecological strategies suitable to coffee cultivation can be adopted by farmers, based on plant diversification, in order to provide resources for natural enemies, such as nectar, pollen, shelter, microclimate conditions, and oviposition sites, thereby promoting conservation biological control. Here I revise these strategies and report the results from research in Brazil. I include results on agroforestry, use of cover crops, and non-crop plant management. These are complemented by curative measures based on the use of organic farming-approved pesticides that can be employed when the agro-ecological practices are not yet consolidated. I also present the cultural control method used by several coffee producers in Brazil to decrease coffee berry borer damage.
Introduction
Coffee (Coffee arabica L. and Coffee canephora L) (Rubiaceae) can host at least 850 insect species, but only few are considered major pests (Le Pelley, 1968). Among those, the coffee leaf miner Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae) and the coffee berry borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae) stand out as key pests in Brazil, the largest coffee producer of the world (Le Pelley, 1968; Reis et al., 2002; Vega et al., 2009). Pest attack on coffee plants causes losses of hundreds of millions of dollars every year (Oliveira et al., 2013; Milligan et al., 2016; Avelino et al., 2018; Cure et al., 2020). The coffee leaf miner (CLM) is disseminated throughout the American continent (Pantoja-Gomez et al., 2019). Its development and reproduction are favored by hot and dry climate conditions, which are found in most regions where coffee is cultivated in Brazil (Silva et al., 2014; Giraldo-Jaramillo et al., 2019; Leite et al., 2020). Females of CLM lay their eggs on the adaxial leaf surface of coffee plants, and the larvae feed on the cells of palisade parenchyma, causing leaves to dry and to prematurely fall (Reis and Souza, 1996; Reis et al., 2002). At high population levels, CLM may cause defoliation up to 70%, which decreases photosynthesis and results in up to 50% decrease in coffee yield (Reis and Souza, 1996).
The coffee berry borer (CBB) is a cosmopolitan pest currently present in all coffee producer countries except in Australia and Nepal (Johnson et al., 2020; Sun et al., 2020). It is considered the most damaging pest of coffee worldwide (Damon, 2000; Vega et al., 2009; Cure et al., 2020). Females of CBB bore into the berries and oviposit inside the coffee berry endosperm. The hatched larvae feed on the seeds, resulting in losses of quality and quantity of the marketable coffee (Damon, 2000; Jaramillo et al., 2006; Vega et al., 2009). It is the only species that can feed and complete its cycle on coffee seeds due to the bacterial symbiotes in its gut that degrade caffeine (Ceja-Navarro et al., 2015; Vega et al., 2021). In addition to CBB and CLM, mites and scales can also cause damage to coffee plants, leading to yield reduction (Reis et al., 2002). The red mite, Oligonychus ilicis (McGregor) (Acari: Tetranychidae), is found on the upper coffee leaf surface where it punctures the epidermis and mesophyll cells of the leaf and absorbs the cell contents, resulting in bronze-colored leaves, which can reduce the photosynthesis rate by up to 50% (Franco et al., 2008). The infestation usually occurs in patches, but it may spread over the entire crop, mainly in the dry periods. Scales from Coccidae [Coccus viridis (Green)] and Pseudococcidae [Planococcus citri (Risso)] families cause damage to coffee plants due to their feeding on the plant sap and to the injection of toxins into the vascular system (Santa-Cecília et al., 2002; Fernandes et al., 2009; Rosado et al., 2013).
The use of pesticides is the most common control measure for coffee pests in Brazil. For instance, at higher infestations of CLM, the number of sprayings can reach up to 20 per year (Leite et al., 2020). The use of pyrethroids for CLM control has been related to increased phytophagous mite outbreaks, mainly due to their deleterious effects on predatory mites (Reis et al., 2007) and to pest-induced hormesis (a stimulating and beneficial effect to living organisms of a harmful substance) under low doses (Cordeiro et al., 2013). Despite the overuse of synthetic pesticides, CBB continues to cause major economic losses (Oliveira et al., 2013; Infante et al., 2014; Johnson et al., 2020). There are many concerns associated with the reliance on pesticide applications, such as harmful effects to human health and to the environment, pest resistance, outbreaks of secondary pest, and loss of beneficial insects (Fragoso et al., 2003; Reis et al., 2015; Hutter et al., 2018; Leite et al., 2020, 2021), which signal the need for developing alternative strategies and discovering new sustainable pest controls. Considering the biology and feeding habits of coffee pests, integrative measures are key to manage them (Damon, 2000; Vega et al., 2009; Infante, 2018; Johnson et al., 2020).
Here I revise the main agro-ecological strategies, focusing on conservation biological control, that could be used for coffee pest management in Brazil. I also report curative measures based on the use of organic farming-approved biopesticides that can be employed when agro-ecological measures are not yet consolidated. Finally, I present and discuss about cultural practices for managing coffee berry borer population. The agro-ecological coffee pest strategies proposed here are based on scientifically reported results. The strategies aim not only coffee productivity but also to recover from the environmental and social harms caused by conventional agriculture and to reduce the dependency on external inputs such as pesticides (Cardoso et al., 2001; Sales et al., 2013). Most of the presented strategies can be adopted and adapted for a range of coffee size farms, but they are especially suitable for small-scale coffee farmers that, in Brazil, are responsible for 80% of coffee production.
Conservation Biological Control
Coffee crops naturally harbor a great diversity of natural enemy species, such as predatory and parasitoid wasps, green lacewings, ants, ladybugs, predatory mites, and entomopathogens (Reis et al., 2002; Fernandes et al., 2008; Amaral et al., 2010; Rodrigues-Silva et al., 2017; Moreira et al., 2019; Botti et al., 2021; Rosado et al., 2021). However, their abundance in coffee monocultures is not always enough to keep pest populations below economic injury levels; for instance, predators and parasitoids, although carnivores, need to feed on plant-derived food, such as nectar and pollen, to supplement or complement their diet or during a non-carnivorous life stage (Olson et al., 2005; Venzon et al., 2006, 2019). In coffee monocultures, these resources are not available throughout the year, as coffee blooms for a limited period and the flowers last only a few days, when they are already visited by pollinators (Peters and Carroll, 2012). Besides alternative food, predators and parasitoids need microclimate conditions, shelter, and place to oviposit and build their nests (e.g., predatory wasps), which are not available in coffee monocultures. These resource provisions can be done by keeping forest fragments near to crop area (Aristizábal and Metzger, 2019; Medeiros et al., 2019), by increasing the plant diversity on it, and on the surroundings by adding trees (i.e., agroforestry), intercropping cover crops, and managing non-crop plants.
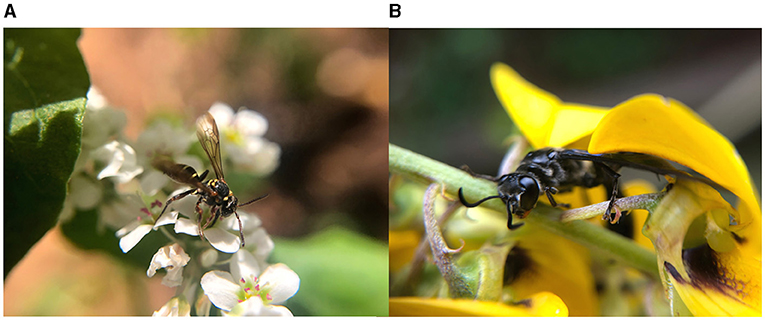
More diversified agroforestry systems are usually related to positive effects on coffee pest control (Philpott and Armbrecht, 2006; Philpott et al., 2008; Teodoro et al., 2009; Perfecto et al., 2014; Pumariño et al., 2015). Moreover, selecting tree species for biological control purposes can be optimized based on their interaction with pests and natural enemies (Heil, 2015; Peters et al., 2016). A remarkable example is the coffee agroforestry system in the Atlantic Rainforest Biome (a South American forest that extends along the Atlantic coast of Brazil) where small stakeholders associated coffee to several trees and, among them, nitrogen-fixing species that bear extrafloral nectaries (EFN) (Souza et al., 2010; Rezende et al., 2014) (Figure 1). EFN are nectar-secreting glands located outside the flowers, and their presence is common in tropical plants (Koptur, 2005). Nectar from EFN is available along the year, and it is more accessible to natural enemies of pests that usually have short mouthparts. In return to the food provided by EFN, the natural enemies protect the plants against herbivory. Rezende et al. (2014) showed an associational resistance provided by EFN-possessing Inga trees (Inga spp.) to coffee plants in agroforestry systems. The EFN of inga attract predators and parasitoids of CLM (Figures 2A,B). The parasitism of CLM increased significantly with the abundance of nectary visitors, and the proportion of mined leaves decreased significantly with this abundance. Later, in a replicated field experiment, Rezende et al. (2021) confirmed that the damage caused by CLM and CBB was lower in coffee consorted with inga trees than in plots with coffee only. Moreover, the authors show that coffee plants consorted with inga trees produced heavier fruits than unconsorted coffee plants. Furthermore, inga trees mediated CBB predation by hosting a predatory thrips of the genus Trybomia (Thysanoptera: Phlaeothripidae) that feeds on CBB eggs, larvae, and pupae (Rezende et al., 2014; Pantoja, 2018). The predator benefits from EFN feeding (Figure 3) as its survival increases but still depends on a protein food source to complete its development (Rezende, 2014; Coffler, 2020).

Figure 1. Agroforestry coffee system in the Atlantic Rainforest Biome, Araponga, state of Minas Gerais, Brazil (photo from Maíra Queiroz Rezende).
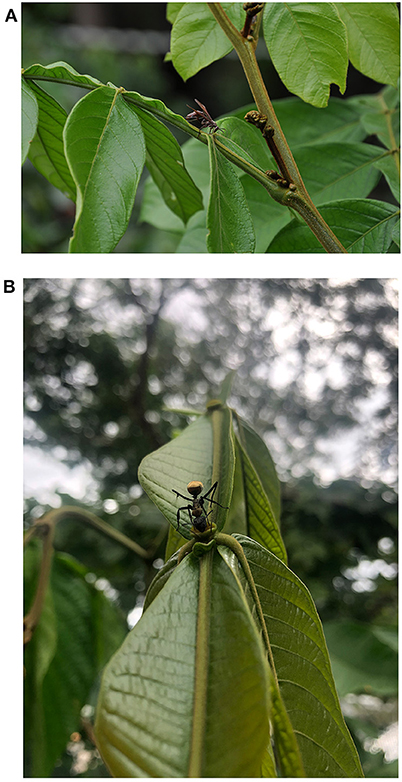
Figure 2. Predators feeding on extrafloral nectar of inga trees: (A) Vespidae (photo from Maíra Q. Rezende). (B) Formicidae (photo from Madelaine Venzon).
The diversified landscape, microclimatic stability, and reduced soil disturbance in agroforestry coffee systems in the Atlantic Rainforest Biome had a positive effect on the activity and abundance of insect-pathogenic fungi when compared with the full-sun conventional coffee production system (Moreira et al., 2019). Soil from coffee plots diversified with Inga edulis Mart. (Fabaceae), Senna macranthera (Collad.) Irwin et Barn. (Fabaceae), Varronia curassavica Jacq. (Cordiaceae), and non-crop plants at Cerrado (a Savanna-like vegetation and one of the Brazilian biomes) of Minas Gerais harbor a more abundant and diverse community of entomopathogenic fungi species than plots with conventional monoculture (Franzin, M.L, personal communication) (Figure 4).

Figure 4. Coffee plot diversified with Inga edulis, Senna macranthera, Varronia curassavica, and non-crop plants at Cerrado, Patrocínio, state of Minas Gerais, Brazil (photo from Mayara Loss Franzin).
Intercropping coffee with cover crops is another viable strategy to increase the availability of plant-provided food, refuges, and favorable microclimate for predators and parasitoids, enhancing their survival and performance and thereby resulting in increased effectiveness for pest control (Venzon et al., 2006; Amaral et al., 2010; Rosado et al., 2021). Cover crops improve the chemical, physical, and biological characteristics of the soil and contribute to the reduction of the diseases and weeds in coffee crops (Colozzi Filho and Cardoso, 2000; Paulo et al., 2001; Mendonça et al., 2017). Regarding the biological control of coffee pests, Venzon et al. (2006) evaluated the suitability of leguminous cover crop pollens to the green lacewing Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae), a common predator species found in coffee agro-ecosystems (Ribeiro et al., 2014; Rodrigues-Silva et al., 2017). Both the adults and larvae of C. externa can feed on plant material, while the larvae feed on a variety of soft-bodied arthropods, including the CLM, CBB, mites, and scales (Ecole et al., 2002; Venzon et al., 2009; Rodrigues-Silva et al., 2017; Carvalho et al., 2019; Botti et al., 2021). The presence of alternative plant food sources for lacewings is especially important in times of prey scarcity, allowing their presence in crops even when prey is temporarily unavailable. The pollens of pigeon pea (Cajanus cajan (L) Millsp., Fabaceae) and sunn hemp (Crotalaria juncea L., Fabaceae) were equally suitable for C. externa, especially when they were complemented with a carbohydrate source (Venzon et al., 2006). Combining a plant providing pollen (sunn hemp) and a plant providing nectar (buckwheat, Fagopyrum esculentum Moench, Polygonaceae) increased the chances of predator survival (Rosado, 2007) (Figures 5A,B). Buckwheat also affords pollen, but it is known by the high nectar productivity of its flowers.
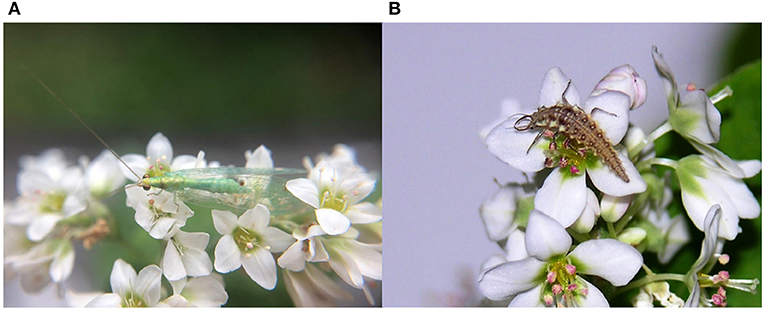
Figure 5. Chrysoperla externa feeding on buckwheat nectar and pollen: (A) adult and (B) larvae (photos from Madelaine Venzon).
Intercropping buckwheat and sunn hemp with coffee increased the activities of predation and of parasitism, respectively, promoted by wasps on CLM (Rosado et al., 2021) (Figures 6A,B). The predation rate of Vespidae on CLM larvae was higher in intercropped plots compared to monoculture, where no other plant food resource was available. Adults of Vespidae forage for nectar and pollen to satisfy their nutritional demands and to feed their larvae (Klein et al., 2002). Both foods can be found in the large sunn hemp papilionaceous flowers that can be easily accessed by adults of Vespidae (Amaral et al., 2010; Meagher Jr et al., 2019). Buckwheat intercropped with coffee increased the parasitism by Eulophidae and Braconidae in CLM (Rosado et al., 2021) (Figure 7). Feeding on buckwheat nectar enhances the survival and reproduction of some parasitoid species of these families (Rosado, 2007; Nafziger and Fadamiro, 2011) (Figure 8). Intercropping sunn hemp with coffee increased the population of predatory mites of the Phytoseiidae family and lowered the herbivorous Tetranychidae population compared to coffee monoculture (Rosado et al., 2021). The pollen of sunn hemp is nutritionally suitable to Phytoseiidae (Rodríguez-Cruz et al., 2013).

Figure 7. Buckwheat intercropped with coffee in the Atlantic Rainforest Biome, São Miguel do Anta, state of Minas Gerais, Brazil (photo from Maria da Consolação Rosado).

Figure 8. Coffee leaf miner parasitoid feeding on buckwheat nectar (photo from Jéssica Mayara Coffer Botti).
Non-crop plants (i.e., spontaneous plants) can also be managed for conservation biological control purposes in agro-ecosystems, as they provide resources and conditions that allow natural enemy survival, growth, and reproduction, even when their prey are scarce or absent (Venzon et al., 2019). One of the main advantages of using non-crop plants to habitat manipulation is that they grow rapidly and spontaneously, and generally farmers know them well. However, the effectiveness of this strategy depends on finding the functional role of each plant to specific biological control agents. Chrysopidae larvae can benefit by feeding on flower resources of non-crop plants during periods of prey scarcity. The larvae of C. externa had higher survival when tropical ageratum (Ageratum conyzoides L., Asteraceae) was offered (Figure 9), and the same happens to the larvae of Ceraeochrysa cubana (Hagen) (Neuroptera: Chrysopidae) when A. conyzoides or beggar tick (Bidens pilosa L., Asteraceae) was provided (Salgado, 2014). Coccinellidae was more abundant when aphids were present on non-crop plants, but they were also observed foraging on flowers, EFN, and using the plants as refuge (Amaral et al., 2013) (Figure 10). Feeding on flowers of B. pilosa increased Coccinellidae survival in the absence of prey (Fonseca et al., 2017). Spiders use non-crop plants as substrate to build webs, mainly on taller and ramified plants (Amaral et al., 2016) (Figure 11). Keeping and managing non-crop plants contribute to the maintenance of ants that nest in the ground and are important predators of CBB and CLM (Lomelí-Flores et al., 2009; Larsen and Philpott, 2010; Piato et al., 2021).
Besides the top-down effect on coffee pests mediated by vegetational diversity, as shown above, increasing plant diversity can have effects on pest populations by modifying the local abiotic parameters that affect pest dynamics (Teodoro et al., 2008; Lomelí-Flores et al., 2010; Avelino et al., 2012; Rice, 2018). Temperature affects CBB infestation (Jaramillo et al., 2009), and trees can effectively reduce the temperature in coffee fields (Mariño et al., 2016; Gomes et al., 2020). Jaramillo et al. (2009) modeled CBB reproduction with temperature and concluded that a 1°C rise in temperature results in 8.5% of average increase on pest population. However, the effect of shade on CBB infestation is not yet clear, as variable results are reported and may partly reflect the varying conditions in the country and areas where the studies have been done (Mariño et al., 2016). In Brazil, there are some preliminary reports about CBB infestation on agroforestry coffee systems, and the effects on CBB range from negative, neutral, and positive (Campanha et al., 2004; Lopes et al., 2012; Figueiredo et al., 2016). Long-term studies in different biomes where coffee is cultivated in Brazil are thus needed. For CLM, intercropping rubber trees with coffee lowered the pest infestation due to microclimate conditions unfavorable to CLM (Androcioli et al., 2018). The authors pointed out that the shade of coffee leaves may result in changes in the leaf structure that may impair CLM survival rate. The use of border crops which act as physical barriers to CBB and CLM flight between coffee areas could also represent a strategy to reduce pest movement (DeClerck et al., 2015).
Several criteria are used when selecting plants for coffee crop diversification. The trees used for diversification of coffee agroforesty systems in Brazil are chosen by family farmers based on compatibility with coffee, biomass production, nitrogen fixation, labor intensity, and diversification of the production (Cardoso et al., 2001; Souza et al., 2010), but as shown above, research has been carried out to select more plant species to be used in coffee agro-ecosystems, with the additional aim of reducing coffee pest populations by increasing the natural enemy populations. One important trait when selecting plants that provide food to predators and parasitoids is that they should not benefit the herbivores that attack the coffee plants. This is important considering that CLM can feed on nectar. Thus, an assessment of accessibility and suitability of floral nectar is necessary. For buckwheat, laboratory experiments confirmed that its nectar does not benefit the CLM (Rosado, 2007). Furthermore, plants should be employed according to the production system and biome where coffee is cultivated. It is also important to point out that plant diversification in coffee crops enhances pollinator populations, leading to increasing yield (Saturni et al., 2016; Hipólito et al., 2018). Understanding the ecosystem services provided by individual plant species will help in unraveling the mechanisms which enhance pest control in diversified systems and can also help in the design of pest-suppressive coffee systems (Staver et al., 2001; Rezende et al., 2021). The final aim is to diversify coffee agro-ecosystems while ensuring food security, healthy environment, and the economy support of coffee growing families.
Curative Pest Control Measures
Curative measures are applied when the preventive tactics fail to restrain the pest population growth (Zehnder et al., 2007). These measures include the use of biopesticides and other non-synthetic products approved by national organic standard organizations.
Biopesticides
Releases of the parasitoid wasp species Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae), Prorops nasuta Wat., and Phymastichus coffea LaSalle (Hymenoptera: Eulophidae) showed a variable action on CBB populations, lack of establishment in some of the new world coffee areas and challenges in their mass rearing. Comprehensive reviews about their origin, introduction, and constrains related to their use can be found on revisions done by Vega et al. (2009), Aristizábal et al. (2016), and Johnson et al. (2020). A successful example is from Colombia, where Aristizábal et al. (2012) show that the release of CBB parasitoids in areas without pesticide applications reduced the CBB populations. Possibly, a combination of such releases with conservation biological control strategies would provide a long-lasting CBB population control, but it has to be tested. In Brazil, despite past efforts (Benassi, 1995, 2007), currently there are no reports about rearing and releasing of parasitoids for CBB control.
Predators are the least-studied natural enemies of coffee pests for augmentative control. Notwithstanding the important role of ants, coffee farm workers typically have a negative view of ants due to their aggressiveness during harvesting (Philpott and Armbrecht, 2006; Offenberg, 2015). Other CBB predators reported are species from Thysanoptera (Phlaeothripidae) (Jaramillo et al., 2010; Rezende et al., 2014), Hemiptera (Anthocoridae) (Bustillo et al., 2002), and Coleoptera (Silvanidae, Laemophloeidae, Cucujidae) (Vega et al., 1999; Bustillo et al., 2002; Follett et al., 2016; Sim et al., 2016). Except for bark beetles (Follett et al., 2016), there are no reports about the rearing and releasing of predators for CBB control, but they are important for conservation biological control purposes. Recently, a Chrysopidae species (C. externa) was reported to prey on CBB in Brazil. First-instar larvae were able to access CBB galleries, remove pest immature stages, and prey on them (Figure 12). Predation by the third instar larvae on CBB adults was also observed (Botti et al., 2021). Additionally, this species and C. cubana prey on CLM immature stages (Figure 13), on mites, and on scales (Ecole et al., 2002; Venzon et al., 2009; Martins et al., 2021). A reference specification (Togni et al., 2019) needed for the registration process of C. externa as a biopesticide was recently published by the Ministry of Agriculture Livestock and Food Supply and will represent a useful tool for coffee pest management (MAPA-Ministério da Agricultura, 2021).

Figure 12. First-instar larvae of Chrysoperla externa feeding on coffee berry borer egg (photo from Jéssica Mayara Coffer Botti).

Figure 13. Second-instar larvae of Ceraeochrysa cubana feeding on coffee leaf miner egg (photo from Elem Fialho Martins).
Among biopesticides, the entomopathogenic fungi B. bassiana is one of the widespread biopesticides for CBB in Brazil. There are several formulated products that are commercially available. It is now currently used in medium and large coffee farms, and it is beginning to be used in small-scale farms. The efficiency of B. bassiana-formulated products is extremely variable and dependent on environmental conditions and on the strain of the pathogen, among other factors (Aristizábal et al., 2016; Johnson et al., 2020). According to Mascarin and Jaronski (2016), Beauveria sprayable formulations can be applied for CBB control as conidia that are sprayed onto CBB female founders during migration from refuges, at the peak of their flight activity, and onto fallen infested berries on the ground. An autoinoculation trap for CBB management with B. bassiana was proposed by Mota et al. (2017). A B. bassiana fungal strain was grown on a synthetic fabric that was incorporated in a trap baited with ethanol and methanol. The trapped CBB females are contaminated by the fungus before they leave the trap, and they act as reservoirs for pathogen dissemination in the crop. The autoinoculation trap provided high levels of CBB mortality in the field, but they attract a small portion of the pest (Mota et al., 2017). Small farmers in Brazil routinely use ethanol–methanol traps to monitor and to mass collect CBB (Silva et al., 2006; Fernandes et al., 2014). Thus, it is possible that using the B. bassiana bait trap would increase CBB control, but controlled experiments are necessary. Hollingsworth et al. (2020) showed the importance of using threshold-based B. bassiana sprays for CBB control in order to keep the control efficiency but with reduced costs as opposite calendar-based spray programs. Recently, Macedo et al. (2020) discussed the possibilities of disseminating B. basssiana spores by bees during coffee blooming and its contributition to the regulation of CBB populations. The main advantages of using Beauveria formulations are their low toxicity to workers and low impact on some beneficial insects (Mingotti Dias et al., 2020), but their high cost and variable efficiency should be considered. Finally, is worth to mention that the propagule viability and infection of B. bassiana on CBB are favored in coffee plantations under managed shade compared to full sun exposure, possibly due to the interception of solar radiation and higher humidity (Edgington et al., 2000; Turro et al., 2013; Mariño et al., 2016).
Organic Farming-Compatible Products
Among the products allowed in organic coffee production are sulfur-based and botanicals. Lime sulfur, a mixture of calcium polysulfides obtained by boiling calcium hydroxide and sulfur, has toxic effects on some insects and mites. The control of mites (O. ilicis) on coffee can be achieved by spraying lime sulfur at a concentration of 0.5% (Tuelher et al., 2014). Higher concentrations are unnecessary for mite control and should be avoided due to deleterious effects on natural enemies, especially on phytoseiid mite predators (Venzon et al., 2013a; Tuelher et al., 2014). Although lime sulfur is used by some farmers aiming to control other pests, such as CLM, it has only ovicidal effect on this pest and at a higher concentration (>1.6%) and has no significant effect on CLM larvae mortality (Venzon et al., 2013b). No reported data about the control of scales on coffee with lime sulfur is available, but considering its efficiency in controlling these insects in other crops (Afonso et al., 2007; Venzon et al., 2016), we expect a negative effect on scale infestations.
Neem (Azadiracta indica A. Juss)-derived products are the most commonly used botanical pesticides in Brazil. Martinez and Meneguim (2003) reported a reduction on CLM oviposition when coffee seedlings were either treated with neem oil (0.125–2.5%) or with neem leaf extract (20–40%). Coffee seedlings sprayed with 0.1 g/L of azadirachtin did not prevent CLM female oviposition, but mine development stopped when leaves with eggs or larvae of CLM were treated with 0.025–0.1 g/L of azadirachtin (Venzon et al., 2005). The neem seed extract has a systemic and translaminar effect that permeated into the leaves, stopped the CLM development, drastically reduced the pupation, and prevented adult emergence (Venzon et al., 2005). Plants treated with neem products are expected to have a lower CLM infestation, either because treated plants repel ovipositing females or because CLM development is negatively affected by neem. For CBB, some laboratory studies show a different mortality rate (Depieri and Martinez, 2010). Concentrations of azadirachtin above 0.065 g/L reduced the population growth rate of O. ilicis (Venzon et al., 2005). By carefully choosing the formulation and concentration, based on research data and technical information, the side effects on natural enemies can be minimized (Depieri et al., 2005; Venzon et al., 2005). Several other plant extracts were tested and have promising results, under laboratory and/or greenhouse conditions, for CLM and CBB control (Santos et al., 2010; Alves et al., 2011; Fanela et al., 2020).
Cultural Practices
The main cultural practices for pest control in coffee crops are related to CBB and consist of harvesting dry overripe fruit on trees and cleaning up of abscised fruits on the ground to reduce CBB reservoir in the inter-crop season (Aristizábal et al., 2016; Johnson et al., 2020). A bioeconomic analysis considering the multitude of factors that influence coffee production was performed by Cure et al. (2020). In their analysis, the authors used a system model that incorporates realistic field models based on considerable new field data and models for coffee plant growth, CBB development, and dynamics on CBB control strategies, including biological control. Their analysis estimated the potential of each CBB control tactic singly and in combination. Their conclusions, based on the analysis, were that the periodic harvest of fruit and the cleaning up were the major control practices that reduced the CBB infestation levels both in Colombia and in Brazil. They also added that the efficacy of the practice decreases as the time between harvests and cleanup increased from 15 to 60 days. It is important to point out that this cultural measure is more feasible in regions with one and short harvesting season, as in Brazil, than in places with two or long harvesting season, such as Colombia. In fact, this is a common practice in Brazil, especially adopted by small farmers, and it was intensified after some pesticide restriction to CBB control. At lower slopes in the Cerrado, Brazilian coffee farmers use a mechanized set for the collection of coffee berries that have fallen on the ground (Tavares et al., 2015; Alvarenga et al., 2018). Other post-harvesting control for CBB is the use of alcohol-based traps around the processing facility to capture any CBB that escapes from the processing facility (Aristizábal et al., 2016).
Concluding Remarks
Coffee agro-ecosystems are managed by human labor, which means that providing ecosystem service of biological pest control depends on a co-work between human and nature (Bengtsson, 2015). This revision reports a variety of strategies based on habitat manipulation for conservation biological control of coffee pests that have also the potential of conserving biodiversity. This approach has other benefits such as protection of soil from erosion, enhanced soil fertility and moisture, prevention of weed growth, carbon sequestration, and nutrient cycling. Plant diversification in coffee agro-ecosystems is also important for mitigating the effects of rising temperatures on coffee production due to climate change. The associated plants, being either fruit and wood trees, cover crops, or non-crop plants, have the potential of not only providing direct and indirect income to coffee farmer families but also aggregate value to coffee that will be produced with low external inputs and following regenerative practices. Production of such coffee will allow farmers to enter in specialty coffee marketing, a growing market where a high price for good-quality, high-biodiversity, and sustainable coffee is paid. There are knowledge gaps that need to be filled in the adoption of conservation biological control of coffee pests, but there are also plenty of opportunities to use the reported techniques and implement them for scaling regenerative coffee production. The importance of Brazil in global coffee production and the fact that it is the most biodiverse country in the world open up an opportunity for its prominence in the adoption of agrobiodiversity, fulfilling the dual role of agricultural production and environmental conservation. Finally, to achieve such goal, a collaborative work supported by public policies among farmers, researchers, field extensionists, industry, coffee traders, and consumers is necessary.
Author Contributions
The author confirms being the sole contributor of this publication and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES), and Consórcio Brasileiro de Pesquisa e Desenvolvimento do Café (CBP & D-Café) for financial support and scholarships. I especially thank all the students, researchers, and farmers involved in the reported studies and José Geraldo Silva (in memoriam) for his invaluable technical support. Angelo Pallini contributed to the reported research and revised the manuscript. I also thank the reviewers for their valuable and constructive comments.
References
Afonso, A. P. S., Faria, J. L. C., Botton, M., and Zanardi, O. Z. (2007). Avaliação da caldasulfocálcica e do óleo mineral no controle da cochonilha-parda Parthenolecanium persicae (Hemiptera: Coccidae) na cultura da videira. Arq. Inst. Biol. 74, 167–169.
Alvarenga, C. B., de, Júnior, N. Â. V., Val, V. L. P., Zampiroli, R., and Rinaldi, P. C. N. (2018). Perdas nas operações de colheita e recolhimento mecanizados do café arábica na região do cerrado mineiro. Acta Iguazu 7, 35–46. doi: 10.48075/actaiguaz.v7i4.17856
Alves, D. S., Oliveira, D. F., Carvalho, G. A., dos Santos Jr, H. M., Carvalho, D. A., Santos, M. A. I., et al. (2011). Plant extracts as an alternative to control Leucoptera coffeella (Guérin-Mèneville)(Lepidoptera: Lyonetiidae). Neotrop. Entomol. 40, 123–128. doi: 10.1590/S1519-566X2011000100019
Amaral, D. S., Venzon, M., dos Santos, H. H., Sujii, E. R., Schmidt, J. M., and Harwood, J. D. (2016). Non-crop plant communities conserve spider populations in chili pepper agroecosystems. Biol. Control 103, 69–77. doi: 10.1016/j.biocontrol.2016.07.007
Amaral, D. S., Venzon, M., Duarte, M. V., Sousa, F. F., Pallini, A., and Harwood, J. D. (2013). Non-crop vegetation associated with chili pepper agroecosystems promote the abundance and survival of aphid predators. Biol. Control 64, 338–346. doi: 10.1016/j.biocontrol.2012.12.006
Amaral, D. S., Venzon, M., Pallini, A., Lima, P. C., and Souza, O. (2010). A diversificação da vegetação reduz o ataque do bicho-mineiro-do-cafeeiro Leucoptera coffeella (Guérin-mèneville) (Lepidoptera: Lyonetiidae)? Neotrop. Entomol. 39, 543–548. doi: 10.1590/S1519-566X2010000400012
Androcioli, H. G., Hoshino, A. T., Menezes Júnior, A. O., Morais, H., Bianco, R., and Caramori, P. H. (2018). Coffee leaf miner incidence and its predation bay wasp in coffee intercropped with rubber trees. Coffee Sci. 13, 389–400. doi: 10.25186/cs.v13i3.1487
Aristizábal, L. F., Bustillo, A. E., and Arthurs, S. P. (2016). Integrated pest management of coffee berry borer: Strategies from Latin America that could be useful for coffee farmers in Hawaii. Insects 7, 1–24. doi: 10.3390/insects7010006
Aristizábal, L. F., Lara, O., and Arthurs, S. P. (2012). Implementing an integrated pest management program for coffee berry borer in a specialty coffee plantation in Colombia. J. Int. Pest Maneg. 3, G1–G5. doi: 10.1603/IPM11006
Aristizábal, N., and Metzger, J. P. (2019). Landscape structure regulates pest control provided by ants in sun coffee farms. J. Appl. Ecol. 56, 21–30. doi: 10.1111/1365-2664.13283
Avelino, J., Cerda, R., Willocquet, L., and Savary, S. (2018). Multiple-disease system in coffee: from crop loss assessment to sustainable management. Annu. Rev. Phytopat. 56, 611–635. doi: 10.1146/annurev-phyto-080417-050117
Avelino, J., Romero-Gurdián, A., Cruz-Cuellar, H. F., and Declerck, F. A. (2012). Landscape context and scale differentially impact coffee leaf rust, coffee berry borer, and coffee root-knot nematodes. Ecol. Appl. 22, 584–596. doi: 10.1890/11-0869.1
Benassi, V. L. R. M. (1995). “Introdução da espécie Cephalonomia stephanoderis Betrem, 1961 (Hymenoptera: Bethylidae), parasitóide da broca-do-café, Hypothenemus hampei (F., 1867) (Coleoptera: Scolytidae),” in Proceedings, 15th Congresso Brasileiro de Entomologia Caxambu, MG, Brazil, 336.
Benassi, V. L. R. M. (2007). Biologia em diferentes temperaturas e ocorrência de Prorops nasuta Wat. e Cephalonomia stephanoderis Betr. (Hymenoptera: Bethlydae) parasitando Hypothenemus hampei (Ferr.) (Coleoptera: Scolytidae). Thesis (Doctorate) - Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias, Brazil, 90.
Bengtsson, J. A. N. (2015). Biological control as an ecosystem service: partitioning contributions of nature and human inputs to yield. Ecol. Entomol. 40, 45-55. doi: 10.1111/een.12247
Botti, J. M. C., Martins, E. F., Franzin, M. L., and Venzon, M. (2021). Predation of coffee berry borer by a green lacewing. Neotrop. Entomol. doi: 10.1007/s13744-021-00884-0. [Epub ahead of print].
Bustillo, A. E., Cárdenas, R., and Posada, F. J. (2002) Natural enemies competitors of Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) in Colombia. Neotrop. Entomol. 31, 635–639. doi: 10.1590/S1519-566X2002000400018
Campanha, M. M., Santos, R. H. S., De Freitas, G. B., Martinez, H. E. P., Finger, F. L., and Garcia, S. L. R. (2004). Incidência de pragas e doenças em cafeeiros cultivados em sistema agroflorestal e em monocultivo. Rev. Ceres 51, 391–396.
Cardoso, I. M., Guijt, I., Franco, F. S., Carvalho, A. F., and Ferreira Neto, P. S. (2001). Continual learning for agroforestry system design: university, NGO and farmer partnership in Minas Gerais, Brazil. Agric. Syst. 69, 235–257. doi: 10.1016/S0308-521X(01)00028-2
Carvalho, C. F., Carvalho, S. M., and Souza, B. (2019). “Coffee,” in Natural Enemies of Insect Pests in Neotropical Agroecosystems, eds B. Souza, L. L. Vázquez, and R. C. Marucci (Cham: Springer), 277–291. doi: 10.1007/978-3-030-24733-1_23
Ceja-Navarro, J. A., Vega, F. E., Karaoz, U., Hao, Z., Jenkins, S., Lim, H. C., et al. (2015). Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Comm. 6:8618. doi: 10.1038/ncomms8618
Coffler, T. (2020). Interações ecológicas do tripes predador Trybomia sp. (Thysanoptera: Phlaeothripidae) com Hypothenemus hampei (Ferrari, 1987) (Coleoptera: Scolytidae) em sistemas agroflorestais. Master Thesis, Federal University of Viçosa, Brazil, 54.
Colozzi Filho, A., and Cardoso, E. J. B. N. (2000). Detecção de fungos micorrízicos arbusculares em raízes de cafeeiro e de crotalária cultivada na entrelinha. Pesq. Agrop. Brasil. 35, 2033–2042. doi: 10.1590/S0100-204X2000001000015
Cordeiro, E. M. G., Moura, I. L. T., Fadini, M. A. M., and Guedes, R. N. C. (2013). Beyond selectivity: are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere 93, 1111–1116. doi: 10.1016/j.chemosphere.2013.06.030
Cure, J. R., Rodríguez, D., Gutierrez, A. P., and Ponti, L. (2020). The coffee agroecosystem: bio-economic analysis of coffee berry borer control (Hypothenemus hampei). Sci. Rep. 10:12262. doi: 10.1038/s41598-020-68989-x
Damon, A. (2000). A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Ent. Res. 90, 453–465. doi: 10.1017/S0007485300000584
DeClerck, F., Estrada-Carmona, N., Garbach, K., and Martinez-Salinas, A. (2015). “Biodiversity and ecosystem services of agricultural landscapes: reversing agriculture's externalities,” in Agroecology for Food Security and Nutrition-Proceedings of the FAO International Symposium on Agroecology for Food Security and Nutrition (Rome).
Depieri, R. A., and Martinez, S. S. (2010). Reduced survival and infestation of coffee borer, Hypothenemus hampei (Ferrari)(Coleoptera: Scolytidae), on coffee fruits, in response to neem sprays in laboratory. Neotrop. Entomol. 39, 632–637. doi: 10.1590/S1519-566X2010000400026
Depieri, R. A., Martinez, S. S., and Menezes Jr, A. O. (2005). Compatibility of the fungus Beauveria bassiana (Bals.) Vuill. (Deuteromycetes) with extracts of neem seeds and leaves and the emulsible oil. Neotrop. Entomol. 34, 601–606. doi: 10.1590/S1519-566X2005000400010
Ecole, C. C., Silva, R. A., Louzada, J. N. C., Moraes, J. C., Barbosa, L. R., and Ambrogi, B. G. (2002). Predação de ovos, larvas e pupas do bicho-mineiro-do-cafeeiro, Leucoptera coffeella (Guérin-Menèville and Perrottet, 1842) (Lepidoptera: Lyonetiidae) por Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae). Cienc. Agrotec. 26, 318–324.
Edgington, S., Segura, H., De la Rosa, W., and Williams, T. (2000). Photoprotection of Beauveria bassiana: testing simple formulations for control of the coffee berry borer. Int. J. Pest Manag. 46, 169–176. doi: 10.1080/096708700415490
Fanela, T. L. M., Bentivenha, J. P. F., Baldin, E. L. L., and Santana, A. S. (2020). Assessing potential plants extracts to reduce Leucoptera coffeella (Lepidoptera: Lyonetiidae) attack in coffee. Coffee Sci. 15, e151653–e151653. http://www.coffeescience.ufla.br/index.php/Coffeescience/article/view/1653 (accessed August 29, 2021).
Fernandes, F. L., Picanço, M. C., Fernandes, M. E., Galdino, T. V., and Tomaz, A. C. (2009). Perdas causadas por Coccus viridis (Green) (Hemiptera: Coccidae) em mudas de Coffea arabica L. EntomoBrasilis 2, 49–53. doi: 10.12741/ebrasilis.v2i2.43
Fernandes, F. L., Picanço, M. C., Silva, R. S. D., Silva, Í. W. D., Fernandes, M. E. D. S., and Ribeiro, L. H. (2014). Controle massal da broca-do-café com armadilhas de garrafa Pet vermelha em cafeeiro. Pesq. Agropec. Bras. 49, 587–594. doi: 10.1590/S0100-204X2014000800002
Fernandes, F. L., Picanço, M. C., Zambolim, L., Queiroz, R. B., Pereira, R. M., Benevenute, J. S., et al. (2008). Spatial and temporal distributions of predator wasps and indirect effects of the irrigation. Sociobiology. 52, 543–551.
Figueiredo, V. G., de Araújo Gomes, M., and Carrero, G. C. (2016). “Sistemas agroflorestais e a produção de café agroflorestal na Amazônia,” in Governança Local para a Amazônia Sustentável, eds Almeida, M. C. S., and May, P. H. Gestão e (Rio de Janeiro: IBAM). 1–11.
Follett, A. P., Kawabata, A., Nelson, R., Asmus, G., Burt, J., Goschke, K., et al. (2016). Predation by flat bark beetles (Coleoptera: Silvanidae and Laemophloeidae) on coffee berry borer (Coleoptera: Curculionidae) in Hawaii coffee. Biol. Control 101, 152–158. doi: 10.1016/j.biocontrol.2016.07.002
Fonseca, M. M., Lima, E., Lemos, F., Venzon, M., and Janssen, A. (2017). Non-crop plant to attract and conserve an aphid predator (Coleoptera: Coccinellidae) in tomato. Biol. Control 115, 129–134. doi: 10.1016/j.biocontrol.2017.10.005
Fragoso, D. B., Guedes, R. N. C., and Ladeira, J. A. (2003). Seleção na evolução de resistência a organofosforados em Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae). Neotrop. Entomol. 32, 329–334. doi: 10.1590/S1519-566X2003000200020
Franco, R. A., Reis, P. R., Zacarias, M. S., Altoé, B. F., and Pedro Neto, M. (2008). Dinâmica populacional de Oligonychus ilicis (McGregor, 1917) (Acari: Tetranychidae) em cafeeiro e de fitoseídeos associados a ele. Coffee Sci. 3, 38–46.
Giraldo-Jaramillo, M., Garcia-Gonzalez, J., and Rugno, J. B. (2019). Fertility life table of Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae) at seven temperatures in coffee. Am. J. Entom. 3, 70–76. doi: 10.11648/j.aje.20190304.12
Gomes, L. C., Bianchi, F. J. J. A., Cardoso, I. M., Fernandes, R. B. A., Fernandes Filho, E. I., and Schulte, R. P. O. (2020). Agroforestry systems can mitigate the impacts of climate change on coffee production: a spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 294:106858. doi: 10.1016/j.agee.2020.106858
Heil, M. (2015). Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Ann. Rev. Entomol. 60, 213–232. doi: 10.1146/annurev-ento-010814-020753
Hipólito, J., Boscolo, D., and Viana, B. F. (2018). Landscape and crop management strategies to conserve pollination services and increase yields in tropical coffee farms. Agric. Ecosyst. Environ. 256, 218–225. doi: 10.1016/j.agee.2017.09.038
Hollingsworth, R. G., Aristizábal, L. F., Shriner, S., Mascarin, G. M., Moral, R. D. A., and Arthurs, S. P. (2020). Incorporating Beauveria bassiana into an integrated pest management plan for coffee berry borer in Hawaii. Front. Sustain. Food Syst. 4:22. doi: 10.3389/fsufs.2020.00022
Hutter, H.-P., Kundi, M., Lemmerer, K., Poteser, M., Weitensfelder, L., Wallner, P., et al. (2018). Subjective symptoms of male workers linked to occupational pesticide exposure on coffee plantations in the Jarabacoa region, Dominican Republic. Int. J. Environ. Res. Public Health 15:2099. doi: 10.3390/ijerph15102099
Infante, F. (2018). Pest management strategies against the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food Chem. 66, 5275–5280. doi: 10.1021/acs.jafc.7b04875
Infante, F., Pérez, J., and Vega, F. E. (2014). The coffee berry borer: the centenary of a biological invasion in Brazil. Braz. J. Biol. 74, 125–126. doi: 10.1590/1519-6984.15913
Jaramillo, J., Borgemeister, C., and Baker, P. (2006). Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): searching for sustainable control strategies. Bull. Ent. Res. 96, 223–233. doi: 10.1079/BER2006434
Jaramillo, J., Chabi-Olaye, A., Kamonjo, C., Jaramillo, A., Vega, F. E., Poehling, H. M., et al. (2009). Thermal tolerance of the coffee berry borer Hypothenemus hampei: predictions of climate change impact on a tropical insect pest. PLoS ONE 4:e6487. doi: 10.1371/journal.pone.0006487
Jaramillo, J., Chapman, E. G., Vega, F. E., and Harwood, J. D. (2010). Molecular diagnosis of a previously unreported predator–prey association in coffee: Karnyothrips flavipes Jones (Thysanoptera: Phlaeothripidae) predation on the coffee berry borer. Naturwissenschaften 97, 291–298. doi: 10.1007/s00114-009-0641-7
Johnson, M. A., Ruiz-Diaz, C. P., Manoukis, N. C., and Rodrigues, J. C. V. (2020). Coffee berry borer (Hypothenemus hampei), a global pest of coffee: Perspectives from historical and recent invasions, and future priorities. Insects 882, 2–35. doi: 10.3390/insects11120882
Klein, A. M., Steffan-Dewenter, I., Buchori, D., and Tsharntke, T. (2002). Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv. Biol. 16, 1003–1014. doi: 10.1046/j.1523-1739.2002.00499.x
Koptur, S. (2005). “Nectar as fuel for plant protectors,” in Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications, eds F. L. Wäckers, P. C. J. van Rijn, and J. Bruin (Cambridge: Cambridge University Press), 75–108. doi: 10.1017/CBO9780511542220.004
Larsen, A., and Philpott, S. M. (2010). Twig-nesting ants: the hidden predators of the coffee berry borer in Chiapas, Mexico. Biotropica 42, 342–347. doi: 10.1111/j.1744-7429.2009.00603.x
Leite, S. A., Santos, M. P., Costa, D. R., Moreira, A. A., Guedes, R. N. C., and Castellani, M. A. (2021). Time-concentration interplay in insecticide resistance among populations of the Neotropical coffee leaf miner, Leucoptera coffeela. Agric. For. Entomol. 23, 232–241. doi: 10.1111/afe.12425
Leite, S. A., Santos, M. P., Resende-Silva, G. A., Costa, D. R., Moreira, A. A., Lemos, O. L., et al. (2020). Area-wide survey of chlorantraniliprole resistance and control failure likelihood of the Neotropical coffee leaf miner Leucoptera coffeella (Lepidoptera: Lyonetiidae). J. Econ. Entomol. 113, 1399–1410. doi: 10.1093/jee/toaa017
Lomelí-Flores, J. R., Barrera, J. F., and Bernal, J. S. (2009). Impact of natural enemies on coffee leaf miner Leucoptera coffeella (Lepidoptera: Lyonetiidae) population dynamics in Chiapas, Mexico. Biol. Control. 51, 51–60. doi: 10.1016/j.biocontrol.2009.03.021
Lomelí-Flores, J. R., Barrera, J. F., and Bernal, J. S. (2010). Impacts of weather, shade cover and elevation on coffee leafminer Leucoptera coffeella (Lepidoptera: Lyonetiidae) population dynamics and natural enemies. Crop. Prot. 29, 1039–1048. doi: 10.1016/j.cropro.2010.03.007
Lopes, P. R., Araújo, K. C. S., Ferraz, J. M. G., Lopes, I. M., and Fernandes, L. G. (2012). Produção de café agroecológico no sul de Minas Gerais: sistemas alternativos à produção intensiva em agroquímicos. Rev. Bras. Agroec. 7, 25–38.
Macedo, J., Viana, B., Freitas, B., Medeiros, A., Kevan, P. G., and Vergara, C. H. (2020). “The potential of bee vectoring on coffee in Brazil,” in Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops, eds G. Smagghe, O. Boecking, B. Maccagnani, M. Mänd, P. Kevan (Springer: Cham), 165–181. doi: 10.1007/978-3-030-18917-4_10
MAPA-Ministério da Agricultura Pecuária e Abastecimento. (2021). PORTARIA N° 363. Available online at: https://www.in.gov.br/en/web/dou/-/portaria-n-363-de-14-dejulho-de-2021-333280433 (accessed August 01, 2021).
Mariño, Y. A., Pérez, M.-E., Gallardo, F., Trifilio, M., Cruz, M., and Bayman, P. (2016). Sun vs. shade affects infestation, total population and sex ratio of the coffee berry borer (Hypothenemus hampei) in Puerto Rico. Agric. Ecosyst. Environ. 222, 258–266. doi: 10.1016/j.agee.2015.12.031
Martinez, S. S., and Meneguim, A. M. (2003). Redução da oviposição e da sobrevivência de ovos de Leucoptera coffeella causadas pelo óleo emulsionável de nim. Manejo integrado de plagas y Agroecología 67, 58–62.
Martins, E. F., Franzin, M. L., Perez, A. L., Schmidt, J. M., and Venzon, M. (2021). Is Ceraeochrysa cubana a coffee leaf miner predator? Biol. Control 160:104691. doi: 10.1016/j.biocontrol.2021.104691
Mascarin, G. M., and Jaronski, S. T. (2016). The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 32:177. doi: 10.1007/s11274-016-2131-3
Meagher, R. L. Jr, Watrous, K. M., Fleischer, S. J., Nagoshi, R. N., Brown, J. T., Bowers, K., et al. (2019). Documenting potential sunn hemp (Crotalaria juncea L.)(Fabaceae) pollinators in Florida. Environ. Entomol. 48, 343–350. doi: 10.1093/ee/nvy190
Medeiros, H. R., Martello, F., Almeida, E. A. B., Mengual, X., Harper, K. A., Grandinete, Y. C., et al. (2019). Landscape structure shapes the diversity of beneficial insects in coffee producing landscapes. Biol. Conserv. 238:108193. doi: 10.1016/j.biocon.2019.07.038
Mendonça, E. S., Lima, P. C., Guimarães, G. P., Moura, W. M., and Andrade, F. V. (2017). Biological nitrogen fixation by legumes and n uptake by coffee plants. Rev. Bras. Ciênc. Solo. 41:e0160178. doi: 10.1590/18069657rbcs20160178
Milligan, M. C., Johnson, M. D., Garfinkel, M., Smith, C. J., and Njoroge, P. (2016). Quantifying pest control services by birds and ants in Kenyan coffee farms. Biol. Conserv. 194, 58–65. doi: 10.1016/j.biocon.2015.11.028
Mingotti Dias, P., de Souza, L. E., Amorim, L. G. P., Reis, G. L. D., Barbosa Junior, G. B., Werner, A. M., et al. (2020). Selectivity of entomopathogenic fungi to Chrysoperla externa (Neuroptera: Chrysopidae). Insects 11:716. doi: 10.3390/insects11100716
Moreira, C. C., Celestino, D., Guerra Sobrinho, T., Cardoso, I. M., and Elliot, S. L. (2019). Agroforestry coffee soils increase the insect-suppressive potential offered by entomopathogenic fungi over full-sun soils: a case proposing a “bait survival technique.” Ecol. Evol. 9, 10777–10787. doi: 10.1002/ece3.5598
Mota, L. H. C., Silva, W. D., Sermarini, R. A., Demétrio, C. G. B., Bento, J. M. S., and Delalibera, I. Jr (2017). Autoinoculation trap for management of Hypothenemus hampei (Ferrari) with Beauveria bassiana (Bals.) in coffee crops. Biol. Control 111, 32–39. doi: 10.1016/j.biocontrol.2017.05.007
Nafziger, T. D. Jr., and Fadamiro, H. Y. (2011). Suitability of some farmscaping plants as nectar sources for the parasitoid wasp, Microplitis croceipes (Hymenoptera: Braconidae): effects on longevity and body nutrients. Biol. Control. 56, 225–229. doi: 10.1016/J.BIOCONTROL.2010.11.005
Offenberg, J. (2015). Ants as tools in sustainable agriculture. J. App. Ecol. 52, 1197–1205. doi: 10.1111/1365-2664.12496
Oliveira, C. M., Auad, A. M., Mendes, S. M., and Frizzas, M. R. (2013). Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Entomol. 137:1–15. doi: 10.1111/jen.12018
Olson, D. M., Takasu, K., and Lewis, W. J. (2005). “parasitoids: Behavioral Adaptations and Consequences,” in Plant-Provided Food for Carnivorous Insects: a Protective Mutualism and Its Applications, eds F. L. Wäckers, P. C. J. van Rijn, and J. Bruin (Cambridge: Cambridge University Press), 137–147. doi: 10.1017/CBO9780511542220.006
Pantoja, G. M. (2018). Artrópodes predadores da broca-do-café associados ao ingá. Master Thesis, Federal University of Viçosa, Brazil. 68 p.
Pantoja-Gomez, L. M., Corrêa, A. S., Oliveira, L. O., and Guedes, R. N. C. (2019). Common origin of Brazilian and Colombian populations of the Neotropical coffee leaf miner, Leucoptera coffeella (Lepidoptera: Lyonetiidae). J. Econ. Entomol. 112, 924–931. doi: 10.1093/jee/toy416
Paulo, E. M., Berton, R. S., Cavichioli, J. C., Bulisani, E. A., and Kasai, F. S. (2001). Produtividade do café apoatã em consórcio com leguminosas na região da alta paulista. Bragantia 60, 195–199. doi: 10.1590/S0006-87052001000300006
Perfecto, I., Vandermeer, J., and Philpott, S. M. (2014). Complex ecological interactions in the coffee agroecosystem. Ann. Rev. Ecol. Evol. Syst. 45, 137–158. doi: 10.1146/annurev-ecolsys-120213-091923
Peters, V. E., Carlo, T. A., Mello, M. A., Rice, R. A., Tallamy, D. W., Caudill, S. A., et al. (2016). Using plant–animal interactions to inform tree selection in tree-based agroecosystems for enhanced biodiversity. BioScience 66, 1046–1056. doi: 10.1093/biosci/biw140
Peters, V. E., and Carroll, C. R. (2012). Temporal variation in coffee flowering may influence the effects of bee species richness and abundance on coffee production. Agroforestry Syst. 85, 95–103. doi: 10.1007/s10457-011-9476-2
Philpott, S. M., Arendt, W. J., Armbrecht, I., Bichier, P., Diestch, T. V., Gordon, C. M., et al. (2008). Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv. Biol. 22, 1093–1105. doi: 10.1111/j.1523-1739.2008.01029.x
Philpott, S. M., and Armbrecht, I. (2006). Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Environ. Entomol. 31, 369–377. doi: 10.1111/j.1365-2311.2006.00793.x
Piato, K., Subía, C., Pico, J., Calderón, D., Norgrove, L., and Lefort, F. (2021). Organic farming practices and shade trees reduce pest infestations in robusta coffee systems in Amazonia. Life 11:413. doi: 10.3390/life11050413
Pumariño, L., Sileshi, G. W., Gripenberg, S., Kaartinen, R., Barrios, E., Muchane, M. N., et al. (2015). Effects of agroforestry on pest, disease and weed control: a meta-analysis. Basic Appl. Ecol. 16, 573–582. doi: 10.1016/j.baae.2015.08.006
Reis, M. R., dos Fernandes, F. L., Lopes, E. A., Gorri, J. E. R., and Alves, F. M. (2015). “Pesticide residues in coffee agroecosystems,” in Coffee in Health and Disease Prevention, ed V. R. Preedy (London: Academic Press), 235–244. doi: 10.1016/B978-0-12-409517-5.00026-7
Reis, P. R., and Souza, J. C. (1996). Manejo integrado do bicho-mineiro, Perileucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae) e seus reflexos na produção de café. An. Soc. Entomol. Bras. 25, 77–82. doi: 10.37486/0301-8059.v25i1.1093
Reis, P. R., Souza, J. C., and Venzon, M. (2002). Manejo ecológico das principais pragas do cafeeiro. Inf. Agropec. 23, 83–99.
Reis, P. R., Teodoro, A. V., Pedro Neto, M., and Silva, E. A. D. (2007). Life history of Amblyseius herbicolus (Chant)(Acari: Phytoseiidae) on coffee plants. Neotrop. Entomol. 36, 282–287. doi: 10.1590/S1519-566X2007000200016
Rezende, M. Q. (2014). Extrafloral Nectary-Bearing Trees Enhance Pest Control and Increase Fruit Weight in Associated Coffee Plants. Doctorate Thesis, Federal University of Viçosa, Brazil, 109.
Rezende, M. Q., Venzon, M., Perez, A. L., Cardoso, I. M., and Janssen, A. (2014). Extrafloral nectaries of associated trees can enhance natural pest control. Agric. Ecosyst. Environ. 188, 198–203. doi: 10.1016/j.agee.2014.02.024
Rezende, M. Q., Venzon, M., Santos, P. S., Cardoso, I. M., and Janssen, A. (2021). Extrafloral nectary-bearing leguminous trees enhance pest control and increase fruit weight in associated coffee plants. Agric. Ecosyst. Environ. 319:107538. doi: 10.1016/j.agee.2021.107538
Ribeiro, A. E. L., Castellani, M. A., Pérez-Maluf, R., Moreira, A. A., Leite, A. S., and Costa, D. R. (2014). Occurrence of green lacewings (Neuroptera: Chrysopidae) in two coffee cropping systems. Afr. J. Agric. Res. 9, 1597–1603. doi: 10.5897/AJAR2013.7841
Rice, R. A. (2018). Coffee in the crosshairs of climate change: agroforestry as abatis. Agroecol. Sustain. Food Syst. 42, 1058–1076. doi: 10.1080/21683565.2018.1476428
Rodrigues-Silva, N., de Oliveira Campos, S., de Sá Farias, E., de Souza, T. C., Martins, J. C., and Picanço, M. C. (2017). Relative importance of natural enemies and abiotic factors as sources of regulation of mealybugs (Hemiptera: Pseudococcidae) in Brazilian coffee plantations. An. Appl. Biol. 171, 303–315. doi: 10.1111/aab.12373
Rodríguez-Cruz, F. A., Venzon, M., and Pinto, C. M. F. (2013). Performance of Amblyseius herbicolus on broad mites and on castor bean and sunn hemp pollen. Exp. Appl. Acarol. 60, 497–507. doi: 10.1007/s10493-013-9665-y
Rosado, J. F., Bacci, L., Martins, J. C., Silva, G. A., Gontijo, L. M., and Picanço, M. C. (2013). Natural biological control of green scale (Hemiptera: Coccidae): a field life-table study. Bio. Sci. Tech. 24, 190–202. doi: 10.1080/09583157.2013.855165
Rosado, M. C. (2007). Plantas favoráveis a agentes de controle biológico. Universidade Federal de Viçosa. Master Thesis, Federal University of Viçosa, Brazil.
Rosado, M. C., Araújo, G. J., Pallini, A., and Venzon, M. (2021). Cover crop intercropping increases biological control in coffee crops. Biol. Control 160:104675. doi: 10.1016/j.biocontrol.2021.104675
Sales, E. F., Méndez, V. E., Caporal, F. R., and Faria, J. C. (2013). Agroecological transition of conilon coffee (Coffea canephora) agroforestry systems in the State of Espírito Santo, Brazil. Agroec. Sustain. Food Syst. 37, 405–429. doi: 10.1080/10440046.2012.712633
Salgado, N. D. (2014). Plantas espontâneas favorecem crisopídeos em plantio de pimenta malagueta. Master Thesis, Federal University of Viçosa, Brazil, 59.
Santa-Cecília, L. V., Reis, P. R., and Souza, J. C. (2002). Sobre a nomenclatura das espécies de cochonilhas-farinhentas do cafeeiro nos Estados de Minas Gerais e Espírito Santo. Neotrop. Entomol. 31, 333–334. doi: 10.1590/S1519-566X2002000200024
Santos, M. R. A. D., Silva, A. G., Lima, R. A., Lima, D. K. S., Sallet, L. A. P., Teixeira, C. A. D., et al. (2010). Atividade inseticida do extrato das folhas de Piper hispidum (Piperaceae) sobre a broca-do-café (Hypothenemus hampei). Braz. J. Bot. 33, 319–324. doi: 10.1590/S0100-84042010000200012
Saturni, F. T., Jaffe, R., and Metzger, J. P. (2016). Landscape structure influences bee community and coffee pollination at different spatial scales. Agric. Ecosyst. Environ. 235, 567–575. doi: 10.1016/j.agee.2016.10.008
Silva, F. C. D., Ventura, M. U., and Morales, L. (2006). Capture of Hypothenemus hampei Ferrari (Coleoptera, Scolytidae) in response to trap characteristics. Sci. Agric. 63, 567–571 doi: 10.1590/S0103-90162006000600010
Silva, R. A., Souza, J. C., Reis, P. R., Carvalho, T. A. F., and Alves, J. P. (2014). Pragas do cafeeiro: bioecologia e manejo integrado. Inf. Agropec. 35, 7–13.
Sim, S. B., Yoneiishi, N. M., Brill, E., Geib, S. M., and Follett, P. A. (2016). Molecular markers detect cryptic predation on coffee berry borer (Coleoptera: Curculionidae) by Silvanid and Laemophloeid flat bark beetles (Coleoptera: Silvanidae, Laemophloeidae) in coffee beans. J. Econ. Entomol. 109, 100–105. doi: 10.1093/jee/tov284
Souza, H., Cardoso, I., Fernandes, J., Garcia, F., Bonfim, V., Santos, A., et al. (2010). Selection of native trees for intercropping with coffee in the Atlantic Rainforest biome. Agroforest. Syst. 80, 1–16. doi: 10.1007/s10457-010-9340-9
Staver, C., Guharay, F., Monterroso, D., and Muschler, R. (2001). Designing pest-suppressive multistrata perennial crop systems: shade-grown coffee in Central America. Agrofor. Syst. 53, 151–170. doi: 10.1023/A:1013372403359
Sun, S., Wang, Z., Liu, A., Lai, S., Wang, J., Meng, Q., et al. (2020). First record of the coffee berry borer, Hypothenemus hampei (Ferrari)(Coleoptera: Curculionidae: Scolytinae) on Hainan Island, China. Coleopterists Bull. 74, 710–713. doi: 10.1649/0010-065X-74.4.710
Tavares, T. O., Santinato, F., Silva, R. P., Voltarelli, M. A., Paixão, C. S. S., and Santinato, R. (2015). Qualidade do recolhimento mecanizado do café. Coffee Sci. 10, 455–463.
Teodoro, A., Klein, A. M., Reis, P. R., and Tscharntke, T. (2009). Agroforestry management affects coffee pests contingent on season and developmental stage. Agric. Forest Entomol. 11, 295–300. doi: 10.1111/j.1461-9563.2008.00417.x
Teodoro, A., Klein, A. M., and Tscharntke, T. (2008). Environmentally mediated coffee pest densities in relation to agroforestry management, using hierarchical partitioning analyses. Agric. Ecosyst. Environ. 25, 120–126. doi: 10.1016/j.agee.2007.12.004
Togni, P. H. B., Venzon, M., Lagôa, A. C. G., and Sujii, E. R. (2019). Brazilian legislation leaning towards fast registration of biological control agents to benefit organic agriculture. Neot. Entomol. 48, 175–185. doi: 10.1007/s13744-019-00675-8
Tuelher, E. S., Venzon, M., Guedes, R. N. C., and Pallini, A. (2014). Toxicity of organic-coffee-approved products to the southern red mite Oligonychus ilicis and to its predator Iphiseiodes zuluagai. Crop Prot. 55, 28–34. doi: 10.1016/j.cropro.2013.09.011
Turro, A. F., Hidalgo, E. C., Ricardo, F. S., Fernandes, E. K. K., Cos, J. I. D., and Fuentes, O. B. (2013). Contribution of different agronomic practices and the fungus Beauveria bassiana on the coffee berry borer. J. Life Sci. 7, 980–992.
Vega, F. E., Emche, S., Shao, J., Simpkins, A., Summers, R. M., Mock, M. B., et al. (2021). Cultivation and genome sequencing of bacteria isolated from the coffee berry borer (Hypothenemus hampei), with emphasis on the role of caffeine degradation. Front Microbiol 12:644768. doi: 10.3389/fmicb.2021.644768
Vega, F. E., Infante, F., Castillo, A., and Jaramillo, J. (2009). The coffee berry borer Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae):a short review, with recent findings and future research directions. Terr. Arthropod. Rev. 22, 129–147. doi: 10.1163/187498209X12525675906031
Vega, F. E., Mercadier, G., Damon, A., and Kirk, A. (1999). Natural enemies of the coffee berry borer Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) in Togo and Côte d'Ivoire, and other insects associated with coffee beans. Afr. Entomol. 7, 243–248
Venzon, M., Amaral, D. S. S. L., Togni, P. H. B., and Chiguachi, J. A. M. (2019). “Interactions of natural enemies with non-cultivated plants,” in Natural Enemies of Insect Pests in Neotropical Agroecosystems, eds B. Souza, L. Vázquez, R. Marucci (Cham: Springer), 15–26. doi: 10.1007/978-3-030-24733-1_2
Venzon, M., Diez-Rodríguez, G. I., Ferraz, C. S., Lemos, F., Nava, D. E., and Pallini, A. (2016). Manejo agroecológico das pragas das fruteiras. Inf. Agropec. 37, 94–103.
Venzon, M., Krüger, R. F., Soto, A., Tuelher, E. D. S., Bonomo, I. S., Fadini, M. A. M., et al. (2013b). Toxicity of organic farming-compatible products to the coffee leaf miner. Pesq. Agropec. Bras. 48, 241–248. doi: 10.1590/S0100-204X2013000300001
Venzon, M., Lemos, F., Sarmento, R. A., Rosado, M. C., and Pallini, A. (2009). Predação por coccinelídeos e crisopídeo influenciada pela teia de Tetranychus evansi. Pesq. Agropec. Bras. 44, 1086–1091. doi: 10.1590/S0100-204X2009000900003
Venzon, M., Oliveira, R. M., Perez, A. L., Rodríguez-Cruz, F. A., and Martins Filho, S. (2013a). Lime sulfur toxicity to broad mite, to its host plants and to natural enemies. Pest Manag. Sci. 69, 738–743. doi: 10.1002/ps.3431
Venzon, M., Rosado, M. C., Euzébio, D. E., Souza, B., and Schoereder, J. H. (2006). Suitability of leguminous cover crop pollens as food source for the green lacewing Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotrop. Entomol. 35, 371–376. doi: 10.1590/S1519-566X2006000300012
Venzon, M., Rosado, M. C., Fadini, M. A. M., Ciociola, A. I. Jr, and Pallini, A. (2005). The potential of NeemAzal for the control of coffee leaf pests. Crop Prot. 24, 213–219. doi: 10.1016/j.cropro.2004.07.008
Keywords: conservation biological control, coffee berry borer, coffee leaf miner, biopesticides, cultural control
Citation: Venzon M (2021) Agro-Ecological Management of Coffee Pests in Brazil. Front. Sustain. Food Syst. 5:721117. doi: 10.3389/fsufs.2021.721117
Received: 06 June 2021; Accepted: 16 August 2021;
Published: 27 September 2021.
Edited by:
Paulo Mazzafera, State University of Campinas, BrazilReviewed by:
Luis F. Aristizabal, Consultant, Kailua-Kona, United StatesMarisol Giraldo-Jaramillo, National Research Center of Coffee (CENICAFE), Colombia
Copyright © 2021 Venzon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madelaine Venzon, bWFkZWxhaW5lQGVwYW1pZy5icg==
 Madelaine Venzon
Madelaine Venzon