
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 08 November 2021
Sec. Crop Biology and Sustainability
Volume 5 - 2021 | https://doi.org/10.3389/fsufs.2021.680862
This article is part of the Research TopicOrphan Plant Species for Food Security and Nutrition: Successes, Challenges, and a Way ForwardView all 13 articles
Centella asiatica is a medicinal herb commonly known as Asiatic pennywort or gotu kola. The species is valued for its medicinal and nutritional properties. It is a perennial plant with leaves and stems that can be consumed as a green leafy vegetable. It is used as a folk remedy to cure various mild and chronic diseases due to its anti-rheumatic, antipyretic, antibacterial, antiviral, and anti-inflammatory effects, and as a mental rejuvenator. Although the species is widely distributed throughout the tropics and subtropics, its recognition was limited. The morphological description of C. asiatica is not well-documented in Myanmar, in Burmese or other local languages. Plant assessment via morphological markers is one of the ultimate methods for the primary description and characterization of their phenotypic traits. The objectives of this study were focused on the description of Myanmarese C. asiatica grown in Japan through morphological markers and a brief overview of its ethnobotanical use in Asia. Morphological characterization revealed the quantitative and qualitative differences regarding several traits among assessed C. asiatica samples. Furthermore, the study can provide information on the primary C. asiatica cultivation system in Japan, as it can be a potentially new and economically important crop for the Japanese market.
Consumers are paying more attention to proper nutrition and the health effect of food. The green leafy vegetables (GLVs) make up a great portion of this diet and play a vital role to ensure food security and fulfill daily needs. The growing interest in GLVs is mainly due to health benefits associated with bioactive nutrients such as minerals, vitamins, especially antioxidant vitamins (vitamins A, C and E), dietary fibers, and non-nutritive phytochemicals (Septembre-Malaterre et al., 2018).
Increased migration and ethnic diversity in many regions and countries around the world stimulates the introduction of new plant species for daily consumption. Centella asiatica is one of the prominent medicinal herbs that can be introduced for Japanese consumers as a GLV for its nutritional and medicinal values. It is one of the most consumed GLV in Myanmar, Thailand, Northern Laos, Indonesia, Malaysia, Bangladesh, and Sri Lanka (Peiris and Kays, 1996; Hashim, 2011; DeFilipps and Krupnick, 2018; Parker, 2020), prized for its slightly pungent flavor and aroma. Although the species is widespread throughout the tropics and subtropics (Brinkhaus et al., 2000; Parker, 2020), it can be considered orphaned. In Japan, the local people are little aware about this species for daily consumption.
C. asiatica (L.) Urb. (syn. Hydrocotyle asiatica L.) is a stoloniferous plant belonging to the genus Centella, family Apiaceae (Umbelliferae). It is known as “Asiatic or Indian pennywort,” “gotu kola,” “penny weed” in English, but it also has over 60 common indigenous names (Engels and Brinckmann, 2011). In Burmese, the species is better known as “myin-hkwa,” “myin-khwar pin,” “ranjneh-hnah” (Chin State), and “hlahnip-chai” (Mon State), (DeFilipps and Krupnick, 2018). It has found wide application in the orient as a potential remedy to cure-all and the list of its therapeutic properties are exhaustive (Gohil et al., 2010). C. asiatica is used in traditional medicine for treating gastrointestinal diseases, skin disorders (burn wounds, rashes, and itchy), sleep disorders, tuberculosis, and diabetes; as an anti-rheumatic, antipyretic, diuretic, anti-bacterial, antiviral, and anti-inflammatory agent; as a memory enhancer; and as a mental rejuvenator (Brinkhaus et al., 2000; Kimura et al., 2008; Gohil et al., 2010; Bylka et al., 2013; Prasad et al., 2014; DeFilipps and Krupnick, 2018).
C. asiatica prefers relatively shady and damp habitats such as wetlands, riversides, ponds, wet meadows, and forests from a 300-to 1,800-m altitude (Gohil et al., 2010; Lansdown, 2019; Parker, 2020). The species is characterized as a perennial, flowering, faintly aromatic, low-growing, creeping herbaceous plant, with prostrate or semi-erect stems and rooting at the nodes, with a height varying between 10.0 and 45.0 cm (Engels and Brinckmann, 2011; Udumalagala et al., 2015; Parker, 2020). The leaves are reniform, shovel-shaped, emerging alternately in rosettes at the nodes (Uddin et al., 2017; Ravi et al., 2019). It is an entomophilous plant and can be reproduced through seeds or vegetative reproduction by far-reaching runners. It has been reported that plants derived from cuttings are more competitive than those developing from seeds (Peiris and Kays, 1996). In Myanmar, it is widely distributed to the cooler regions and found all year near the water's edge (DeFilipps and Krupnick, 2018).
Recently, increasing attention is being given to the nutritional value, medicinal function, and biological activities of C. asiatica (Brinkhaus et al., 2000; Matsuda et al., 2001; Siddiqui et al., 2007; Zainol et al., 2008; Chong and Aziz, 2011; Hashim et al., 2011; Bylka et al., 2013; Paudel et al., 2017; Sardrood et al., 2019). Fresh leaves consist of 87.7% moisture, 0.49% soluble dietary fiber, and 5.4% insoluble dietary fiber (Peiris and Kays, 1996; Udumalagala et al., 2015). The plant is also low in the content of fats (0.2%), proteins (2.4%), and carbohydrates (6.7%), but rich in the content of vitamins: vitamin C (7 mg/100 g), vitamin A (738 IU), vitamin B1 (0.09 mg/100 g), and minerals such as Ca (171 mg/100 g), P (32 mg/100 g), K (468.59 mg/100 g) and Fe (5.6 mg/100 g). Numerous conducted studies have pointed out that major bioactive constituents attributing its medicinal value are triterpenes (asiaticoside, asiatic acid, madecassic acid, madecassoside), (Engels and Brinckmann, 2011; Hashim et al., 2011; Belwal et al., 2019), which have also been regarded as its biomarker components (Zheng and Qin, 2007).
The plant genotype integration with different environments (genotype × environment interactions, GEI) can produce a wide range of phenotypes, which leads to its phenotypic plasticity (Baye et al., 2011; Osei et al., 2018). Habitat-related populations of C. asiatica show marked variation in morphological traits, such as leaf area, stolon production, petiole length, leaf color, and other traits (Peiris and Kays, 1996). About 20 morphotypes of C. asiatica are reported depending on habitat (elevation, terrain), (Gohil et al., 2010). The geographical location, climatic condition, cultural diversity of different tribes, and agricultural approaches led to plant diversification in Myanmar (Jatoi et al., 2008). C. asiatica originally from Myanmar is not documented well in Burmese and other local languages. This study is a step toward future studies and promotion of C. asiatica originating from Myanmar.
The objectives of this study were focused on the morphological description of the obtained Myanmar C. asiatica plant material growing in Japan and a brief overview of its ethnobotanical use. The study will provide information on the cultivation system of C. asiatica in Japan, as well as its further promotion and commercialization. It can be a potentially new GLV for Japanese daily consumption, especially during winter when demand for GLVs is particularly high.
The original C. asiatica plant material was provided by the Vegetables and Fruit Research and Development Center (VFRDC) of Myanmar. The obtained plant material was transferred to Japan via the Standard Material Transfer Agreement (SMTA) in 2009 with Myanmar Agriculture Service. The plant material was introduced to the collection of the Gene Research Center of the University of Tsukuba (Japan), (GRC UT). The plant material was cultivated in the pots and maintained as a living collection in the greenhouse of the GRC UT to preserve germplasm.
To conduct this study, C. asiatica plant samples were derived from the vegetative multiplication of the mother plant by stem cutting and cultivated in 10.5 × 9.0 cm pots in the greenhouse of GRC UT. The rooted cuttings were then transplanted to open ground in a vinyl house (36°7′28.40″ north latitude and 140°5′44.28″ east longitude) of a local farmer (Tsukuba, Japan). To evaluate the morphological characteristics, a common field test was carried out according to a randomized complete block design with three replications. The observation, visualization (photographing), scoring, and measurement of morphological characters were carried out from August 10 to 15, 2020, when the plant was in its full vegetative and flowering stages.
C. asiatica originating from Myanmar was characterized using the developed list of descriptors after cultivation at the homogeneous condition in Japan. The qualitative morphological data of this study were compared with herbarium specimen records for C. asiatica available on the Flora of Myanmar Database (Flora of Myanmar Database, 2020).
Morphological evaluation was recorded on 10 randomly selected rosettes from each plot, for a total of 30 plant samples. Fifteen qualitative and quantitative markers were chosen for morphological evaluation, as shown in Supplementary Table 1. Four quantitative characters, i.e., leaf length, leaf width, leaf area (per one leaf), and petiole length, were measured using a ruler and a graph paper. Eleven qualitative characters were identified visually and recorded directly from measurement using a 1–4 scale. The color of the leaf, petiole, stolon, flowers, and the petiole color at the base was identified according to the RHS color chart and recorded using a 1–4 scale.
The mean values for each quantitative morphological trait were calculated and used for statistical data analysis. The mean value, range value, and standard deviation (SD) were measured for each quantitative character. Single-factor analysis of variance (ANOVA) was calculated using SPSS software package version 24.0 (IBM Corp., Armonk, NY, USA) to determine the significance of variation for all quantitative morphological characters. The quantitative data (Table 1) are expressed as a mean ± SD value, range value, and with probability level of significance of 5% (P ≤ 0.05).
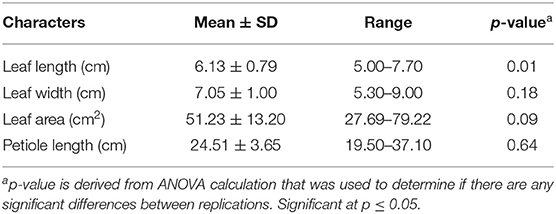
Table 1. Means, standard deviations, ranges and p-values of four quantitative characters assessed on C. asiatica.
The developed quantitative morphological markers included four traits as follows: leaf length (cm), leaf width (cm), leaf area (cm2), and petiole length (cm). Mean, standard deviation, range, and ANOVA were calculated to determine the significance of the variation accordingly (Table 1). The quantitative data were then categorized based on the descriptor list and combined with the qualitative data (Supplementary Table 1). Leaf size varied from 5.00 to 7.70 cm in length and from 5.30 up to 9.00 cm in width. Leaf area ranged between 27.69 and 79.22 cm2. Petioles were long and their length ranged between 19.50 and 37.10 cm. The leaf length showed significant variation compared to four other measured quantitative traits. The variation in leaf width, leaf area and petiole length were not significant, but the range of minimum and maximum values varied considerably for the leaf area and petiole length (Table 1).
In this study, C. asiatica exhibited a semi-erect and prostrate growth habit (Figure 1A). The plant stem is weak and glabrous, and consists of long creeping stolons rooting at the nodes (Figure 1B). The thin and fragile stem grew along the surface. The stolons are long internodes of mixed colors: from milky-white in young developing stolons, to light-green, green-pinkish, or dark-pink (purplish) in developed ones (Figures 1B,D, 2A). The roots are adventitious and hairy, growing vertically down and white-creamish in color. Leaves are simple, emerging alternately on slender petioles and arranged solitary or in a cluster of two to three at each node (Figures 1D, 2A). The leaf lamina is wide, peltate, with orbicular shape (Figure 1C), and cordate at base. Lamina's margin is slightly crenate with shallow lateral sinuses, glabrous abaxially and adaxially. The leaf margin is brought together and united at the lamina base, although there are sometimes leaves with the margin cleft at the base of the leaf blade. The flowers are actinomorphic, inconspicuous, <3.0 mm in size, white color, and arranged in small rounded whorls (umbels) that are born on slender pedicels with different lengths (19.50–37.10 cm), (Figures 3A,B). Flowers contain five corolla lobes, with five stamens per flower, which are attached to the glandular disc and bent inside the flower when young (Figure 3C). The gynoecium is syncarpous and consists of two carpels; the lower two-celled ovary at the apex passes into the sub-column (or stylopodia), often called the glandular or nectar disc. The calyx teeth are invisible and underdeveloped. Seeds have not been observed.

Figure 1. (A) General view (habit) of C. asiatica. (B) Stem of C. asiatica plant with the creeping stolons. (C) Leaf lamina. (D) Part of stem with young leaf and stolon of white color.
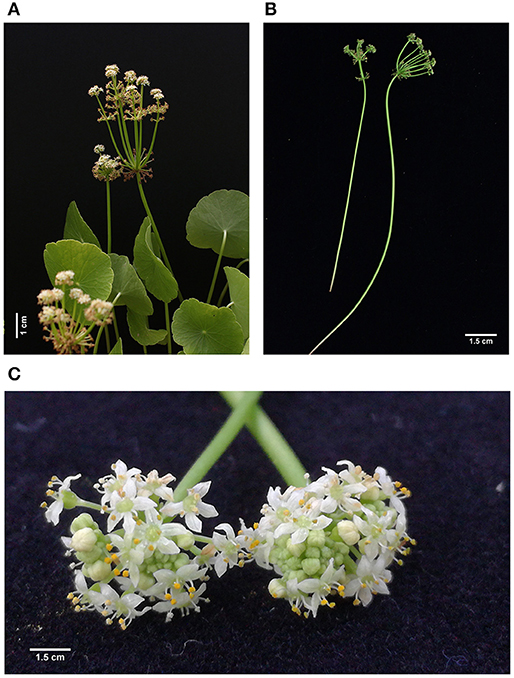
Figure 3. (A) General view of C. asiatica inflorescence. (B) Inflorescence with pedicel. (C) Close-up view of flowers.
This study was conducted on morphological characterization of C. asiatica plant originating from Myanmar to overview plant adaptation and growth habits in Japanese environmental condition.
C. asiatica (L.) Urb. is reported as a very variable species and having over 40 synonyms but only Hydrocotyle asiatica L. is still occasionally used (Parker, 2020). The leaf size is a quite unstable character and considerably varies in width and length (Alfarra and Omar, 2013). In Myanmar, C. asiatica is widely distributed to the cooler regions and found all year near the water's edge, paddy fields (DeFilipps and Krupnick, 2018). It grows wild but it is also widely cultivated as it is much used. It is characterized as a slender herbaceous plant with long creeping stems coming off from the leaf axils (Medicinal Plants of Myanmar, 2007).
C. asiatica is poorly recorded in Myanmar. We used the records of herbarium specimens on C. asiatica available at the Flora of Myanmar Database (2020) for taxonomic identification and comparative morphological assessment. In total, Flora of Myanmar Database (2020) contains five records on specimens for the Centella genus collected from Myanmar and deposited in herbaria of the National Museum of Nature and Science, Tokyo (TNS) and the University of Tokyo (TI) for the period 2000–2017.
On closer inspection of the images available for these specimens, the phenotypic differences were detected regarding leaf shape compared with plant samples used in this study. Four herbarium specimens (TI00072253–TI00072256) possess reniform or orbicular-reniform leaves and are determined as C. asiatica. One specimen (TNS01300836) displays peltate leaves and was determined as Centella species (specific epithet absent). The leaves clustered simultaneously at the nodes and visually different in size. The lamina margins are crenate. Two specimens—TI00072253 (Shan State) and TI00072255 (Chin State)—visually possess long internodes, whereas specimens TI00072254 and TI00072256 (Mandalay Division) are characterized with short internodes and dense leaf arrangement.
Among four quantitative morphological traits, the significant variation was observed only in leaf length (P < 0.05). The other three morphological traits, i.e., leaf width, leaf area, and petiole length, did not display any significant differences.
Upon close observation and characterization, we found phenotypic differences and similarities in qualitative traits such as plant's growth habit, leaf shape, leaf margin, and leaf color from those that have been previously reported (Peiris and Kays, 1996; Prasad et al., 2014; Alqahtani et al., 2017; Ravi et al., 2019; Flora of Myanmar Database, 2020). In the current study, plants displayed a semi-erect growth habit with thin and fragile horizontally grown stem along the soil surface (Figure 1), peltate shaped leaves, cordate at the base (Figures 1, 2), glabrous and glossy, corresponding with those that have been reported from India by Ravi et al. (2019) and from Australia by Alqahtani et al. (2017). The erect growing plants are ideal for maximum and uniform exposure to sunlight resulting in increased dry matter production and, subsequently, yield, compared with semi-erect and prostate types (Uddin et al., 2017). According to the leaf shape characteristic, and petiole and pedicel length, only one C. asiatica herbarium specimen (TNS01300836, Shan State) from Myanmar (Flora of Myanmar Database, 2020) matched our result.
Leaf color was another phenotypic trait that displayed notable variation ranging from light green to dark green. Plants growing under full sun, in dry non-enriched soil with low moisture, had thin, mat light-green colored leaves (data has not shown), whereas the plants that were growing under the shade, in a sufficiently moist and enriched soil, had fleshy, glossy, and dark green colored leaves (Figure 1). According to Peiris and Kays (1996), plants growing under the shade develop a profuse canopy of leaves with long petioles, while high light intensity produces more leaves, clonal offspring, a greater leaf area, and dry matter content. The leaf size itself directly determines the photosynthetic efficiency of the plant, influencing its growth, yield, synthesis, and accumulation of secondary metabolites. At the same time, the nutritional composition in C. asiatica can be varied somewhat with growth location and environmental conditions (Peiris and Kays, 1996).
Since the phenotypic traits are genetically attributed, a set of environmental conditions (soil nutrition, moisture supply, and light), cultivation method, cultivation duration (mutation collection caused by the vegetative reproduction), and plant interaction with the environment may influence the phenotypic expression of plants during growth and development. There are different morphotypes of C. asiatica reported from Sri Lanka (Udumalagala et al., 2015; Dissanayake et al., 2016), Madagascar (Rahajanirina et al., 2012), India (Prasad et al., 2014; Ravi et al., 2019), and Australia (Alqahtani et al., 2017) based on leaf morphology and growth pattern. In this regard, we assume that observed phenotypic expression in leaf color, termed phenotypic plasticity, was determined by the environmental conditions, i.e., soil nutrition, moisture, and light intensity. Although C. asiatica grows easily under full sun, it is beneficial to provide plants with adequate soil nutrients and moisture for their initial establishment and subsequent growth (Peiris and Kays, 1996).
The practical uses of plants in particular regions and indigenous cultures found their reflection in different ethnobotanical studies and are well-described in ancient oriental medicinal tracts such as Indian Ayurveda, Chinese herbology medicine, and Japanese Kampo medicine. Nearly 1,500 plant species are incorporated into official Indian ayurvedic pharmacopeia for medicinal purposes (Kumar et al., 2017). About 11,000 plant species are listed in Chinese pharmacopeia, medicinal botany textbooks, and ancient Chinese medical texts (Zhang and Yang, 2012). A total of 148 different herbal formulations are approved to be used as official Kampo remedies, and 294 formulations are approved for non-prescription Kampo products in Japan (Arai and Kawahara, 2019; Watanabe et al., 2019). Plant-based medications and additives are synchronized with the food and extensively used in both developing and developed countries for daily healthcare. It is evidenced by numerous accumulated studies highlighting the enormous potential of herbs utilized for medicinal and nutritional purposes (Gohil et al., 2010; Engels and Brinckmann, 2011).
C. asiatica has a long history of use as a GLV and medicinal herb in oriental medicine in Asian countries (Stafford et al., 2008; Zainol et al., 2008; Gohil et al., 2010; Hashim, 2011) including Myanmar (DeFilipps and Krupnick, 2018). It is cultivated all over the world for consumption as a fresh vegetable (Kosaka et al., 2013). All parts of the plant are used for medicinal purposes in ethnomedicine in Asian countries (Gohil et al., 2010). The leaves are slightly bitter and eaten raw or cooked as a vegetable and/or as a soft drink. It is also available as a dried herb or extract, in teas, tablets, capsules, ointments, tinctures, and cosmetic preparations (Engels and Brinckmann, 2011). Most of all, C. asiatica is valuable due to its healing effects to cure various mild and chronic disorders such as hepatitis, anemia, skin diseases (Kimura et al., 2008; Gohil et al., 2010), diarrhea, ulcers, fever, and amenorrhea (Brinkhaus et al., 2000; Gohil et al., 2010; Engels and Brinckmann, 2011), and as an antioxidant, antibacterial, antiviral, and anti-cancer agent (Bylka et al., 2013; Belwal et al., 2019). In Western medicine, it is popular as a “brain tonic” agent to revitalize the brain and nervous system, increase attention span and concentration, and combat aging, as well as for its neuroprotective activities (Matsuda et al., 2001; Gohil et al., 2010). The list of therapeutic properties of C. asiatica is huge and it has been used in ancient cultures and tribal groups with diverse prescriptions (Alfarra and Omar, 2013).
Known as a “myin-hkwa,” in Myanmar (DeFilipps and Krupnick, 2018), C. asiatica is utilized both as a leafy vegetable and a medicinal herb. Information on the ethnobotanical use of C. asiatica in Myanmar was provided in personal communication with Daisy Myint and according to DeFilipps and Krupnick (2018). Myin-hkwa young leaves are more valuable as a leaf vegetable and consumed raw in salads, deep-fried (“tenpura”), as a separate soup, and in combination with Roselle (Hibiscus sabdariffa) leaves fried or in a soup. The plant is used to treat diabetes, skin diseases (eczema, leprosy, itching, rashes, and sores), and nervous system and blood problems; to control phlegm; and to treat dysentery and urine retention, painful urination, blood in the urine, and syphilitic affections; it is also used as a poison neutralizer. The leaf extracts, together with sugar and honey, are consumed daily as a restorative product to treat colds and fever. The dried leaves are used as a tea to relieve hypertension and to treat severe sore eyes and hypersensitivity to strong light. The fresh green leaves, crushed and wrapped in a thin cloth, are used as an eye mask. The dried powdered leaves mixed with an equal amount of honey are used against insomnia, and mixed with water used to treat coughs and tuberculosis in children or applied to the chest as a warm compress.
C. asiatica is known as “snow plant” (Engels and Brinckmann, 2011) for its cooling properties. Historically, it has been called one of the “miracle elixirs of life” and for the first time was mentioned in the Chinese Shennong Herbal (1st−2nd century of CE) because of a Chinese herbalist named Li Ching-Yun, who some believe lived to the age of 197 and used gotu kola regularly (Engels and Brinckmann, 2011). In traditional Chinese medicine, dried gotu kola, known as ji xue cao (at a dosage of 15–30 g), or fresh plant (at a dosage of 30–60 g) is prescribed to use for treatment various disorders (Emboden, 1985). The plant possesses diuretic properties and is used for treating looseness of the bowels, heaving, jaundice and scabies, Hansen's ailment (disease), urinary troubles, nosebleeds, breaks, tonsillitis, measles, and tuberculosis (Menglan and Watson, 2005).
C. asiatica was incorporated into the Indian Pharmacopeia in the 19th century (Engels and Brinckmann, 2011). In Ayurveda, C. asiatica is known as a mental rejuvenator, or medhya rasayana, a tonic used to reduce mental fatigue and improve mental clarity (Premila, 2006). In Ayurveda and Unani ethnomedicine systems, it has been used to cure different ailments like ulcers and body aches, stomach disorders, asthma, leprosy, leukorrhea and urethritis, loose bowels, and dysentery, and for enhancing memory power, in maternal healthcare (Sidhu et al., 2006; Das et al., 2009; Uddin et al., 2017). C. asiatica is an important part of Ayurvedic formulation known as Brahma Rasayana, a complex mixture of herbs and fruits in a paste form, taken with warm milk as a cerebral tonic for mental exhaustion, nervous weakness, insomnia, and memory loss (Engels and Brinckmann, 2011).
In Nepal, all parts of C. asiatica are used in the form of teas for diarrhea and hypertension, as a detoxicant and diuretic, and for urinary tract infections and poor memory (Ahmad and Ismail, 2003). Smashed leaf and root concentrate are used to eliminate germs from wounds. Around four teaspoons of leaf juice obtained from 50 leaves by squeezing is taken orally in the morning during 2–3 weeks to cool the body and stomach (Mahato and Chaudhary, 2005). Crushed leaf and root extract are applied to kill germs from wounds (Uddin et al., 2017).
Kavirajes, traditional medicinal practitioners from the Chalna area of Bangladesh, use the whole plant of thankhai (local name of C. asiatica) to treat multiple ailments like dog bite, asthma, carminative, itching, leucorrhea, malaria, tumors, and wounds (Rahmatullah et al., 2010).
C. asiatica is commonly known as pegaga in Malaysia and pegagan or kaki kuda in Indonesia (Alfarra and Omar, 2013). In Malaysia and Indonesia, the whole plant is eaten fresh as a vegetable in a salad, soup, or appetizer (Hashim et al., 2011). It is always cooked with the addition of coconut milk or shredded coconut to soften the plant's mild bitterness. Kadazandusun communities (Malaysia) use the plant as a tea for treating hypertension, diarrhea, and urinary tract infections; as a detoxicant and diuretic; and to lower blood pressure and decrease heart rate (Uddin et al., 2017).
In Thailand, the fresh leaves are eaten with northern food such as sour chopped meat salad or fried noodles (JIRCAS, 2020). The fresh prepared green juice is rich in vitamin A and is commonly taken for thirst quenching purposes or as a cooling drink to reduce “inner heat” (Hashim, 2011). For medicinal purposes, the fresh plants are used to soothe headaches or heat-burns, and as a diuretic, anti-syphilitic, astringent, and expectorant.
Although the therapeutic potential of C. asiatica is obvious, some precautions are needed to avoid side effects occurring with high doses of the herb. Reported side effects may include skin allergy, burning sensations (with external use), headache, stomach upset, nausea, dizziness, and extreme drowsiness (Orhan, 2012). No toxicity was reported within the recommended doses as well as negative drug–herb interactions between C. asiatica with other medications. The reported daily dose is about 600 mg of dried herb in the form of an infusion, single-dose capsules (300–680 mg, thrice daily), or a 10-mg concentrated extract, for a period of time up to 6 weeks (Gohil et al., 2010).
The ethnic communities are custodians of the traditional knowledge of plant consumption, plant's healing properties, and formulations accumulated empirically and passed down from generation to generation. Their knowledge can be implemented in modern science and serve as a base for the investigation of the new biologically active compounds from important orphan crop species that can cure a variety of diseases.
As part of our future research promotion, we have conducted a preliminary evaluation of one of the important phytonutrients—carotenoids in C. asiatica. The result revealed the high intensity of the lutein and beta-carotene constituents (data unpublished). Lutein and beta-carotene are carotenes found in many fruits and vegetables, most notably in GLVs like kale and spinach (Ranard et al., 2017). Beta-carotene is the most common carotene found in plants and acts as a precursor for vitamin A in humans and animals (Grune et al., 2010). Lutein and its isomer zeaxanthin are the only carotenoids that accumulate in the fovea of the human retina that play a role in the visual system (Ranard et al., 2017; Alvarado-Ramos et al., 2018).
There are no data on the market-oriented production of C. asiatica in Japan. The plant possesses unique nutritional and health attributes and can be a potentially new and economically important plant for the Japanese market, especially during the winter season when there is a huge demand for leafy vegetables. Generally, C. asiatica is easy to cultivate. With the correct provision of the necessary conditions for growth, the first harvest can be possible in about 90 days from planting with subsequent 60-day intervals of harvesting over 2–3 years (Peiris and Kays, 1996). As a first step, a consumer acceptance test in Japan may be worth considering to help to define subsequent steps for future market production. Combined with molecular and tissue culture techniques, the nutritional and medicinal values of the plant can be increased as well as new varieties promoted and conserved in the future.
This study is the first to report on the morphological description of C. asiatica originating from Myanmar, which is not well-documented in Burmese or other local languages. The evaluation revealed that C. asiatica originating from Myanmar is different considering its morphological characteristics. The morphological diversity of the foliar traits and growth pattern can serve as a taxonomic marker for primary C. asiatica differentiation/identification in Myanmar. It is assumed that different morphotypes of C. asiatica may exist in Myanmar. Moreover, different morphotypes can considerably differ by both morphological characteristics and biochemical composition. Future studies are needed to elucidate this assumption.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The experimental idea was based on KW with a future contribution on guidance, project administration, funding acquisition, review, and editing of the original draft. MS prepared the original draft. KW, MS, and DM contributed to conceptualization and including the design of the experiment. MS and DM equally contributed to methodology and data curation, including formal and statistical analysis, investigation, and visualization. Plant material resources and validation of the obtained data were carried out by KW, SY, and OS. All authors have read and agreed to the publication of this version of the manuscript.
This research was funded by the KAKENHI, Grant in Aid (17H01682) from the Japanese Society for Promotion of Science (JSPS) and by the Plant Transgenic Design Initiative (PTraD) at the University of Tsukuba, Tsukuba, Japan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are thankful to the KAKENHI of the Japanese Society for Promotion of Science (JSPS) and the Plant Transgenic Design Initiative (PTraD) of the University of Tsukuba for the financial support to conduct the current research. Authors MS and DM were supported by the Special Program Global Food Security Partnership Program under the Ministry of Education, Culture, Supports, Science and Technology (MEXT) Scholarship, of which they are very grateful.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2021.680862/full#supplementary-material
Ahmad, F. B., and Ismail, G. (2003). Medicinal plants used by kadazandusun communities around crocker range. ARBEC 1, 1–10.
Alfarra, H. Y., and Omar, M. N. (2013). Centella asiatica: from folk remedy to the medicinal biotechnology–a state revision. Int. J. Biosci. 3, 49–67. doi: 10.12692/ijb/3.6.49-67
Alqahtani, A., Cho, J.-L., Wong, K. H., Li, K. M., Razmovski-Naumovski, V., and Li, G. Q. (2017). Differentiation of three Centella species in Australia as inferred from morphological characteristics, ISSR molecular fingerprinting and phytochemical composition. Front. Plant Sci. 8:1980. doi: 10.3389/fpls.2017.01980
Alvarado-Ramos, K. E., De Leon, L., Fontes, F., and Rios-Castillo, I. (2018). Dietary consumption of Lutein and Zeaxanthin in Panama: a cross-sectional study. Curr. Dev. Nutr. 2, 1–7. doi: 10.1093/cdn/nzy064
Arai, I., and Kawahara, N. (2019). Kampo pharmaceutical products in the japanese health-care system: legal status and quality assurance. Tradit. Kampo Med. 6, 3–11. doi: 10.1002/tkm2.1204
Baye, T. M., Abebe, T., and Wilke, R. A. (2011). Genotype–environment interactions and their translational implications. Pers. Med. 8, 59–70. doi: 10.2217/pme.10.75
Belwal, T., Andola, H. C., Atanassova, M. S., Joshi, B., Suyal, R., Thakur, S., et al. (2019). “Gotu kola (Centella asiatica),” in Non-vitamin and Non-mineral Nutritional Supplements, eds. S. M. Nabavi, and A. S. Silva (Amsterdam: Elsevier), 265–275. doi: 10.1016/B978-0-12-812491-8.00038-2
Brinkhaus, B., Lindner, M., Schuppan, D., and Hahn, E. G. (2000). Chemical, pharmacological and clinical profile of the east Asian medical plant Centella asiatica. Phytomedicine 7, 427–448. doi: 10.1016/S0944-7113(00)80065-3
Bylka, W., Znajdek-Awizeń, P., Studzińska-Sroka, E., and Brzezińska, M. (2013). Centella asiatica in cosmetology. Postep. Derm. Alergol. 30, 46–49. doi: 10.5114/pdia.2013.33378
Chong, N. J., and Aziz, Z. (2011). A systematic review on the chemical constituents of Centella asiatica. Res. J. Pharm. Biol. Chem. Sci. 2, 445–459.
Das, S., Khan, M. L., Rabha, A., and Bhattacharjya, D. K. (2009). Ethnomedicinal plants of Manas National Park, Assam, Northeast India. Indian J. Tradit. Know. 8, 514–517.
DeFilipps, R. A., and Krupnick, G. A. (2018). The medicinal plants of Myanmar. PhytoKeys 341, 1–341. doi: 10.3897/phytokeys.102.24380
Dissanayake, T. D. C. K., Abeysekera, A. M., Salim, N., Chandrika, U. G., and Padumadasa, C. (2016). Evaluation of Centella asiatica morphotypes for high yields of asiaticoside. J. Pharmacogn. Phytochem. 5, 451–454.
Emboden, W. A. (1985). The ethnopharmacology of Centella asiatica (L.) Urban (Apiaceae). J. Ethnobiol. 5, 101–107.
Engels, G., and Brinckmann, J. (2011). Gotu kola. Centella asiatica. Family: Apiaceae. HerbalGram 90, 1–5.
Flora of Myanmar Database (2020). Available online at: www.kahaku.go.jp/research/db/botany/myanmarflora/index.php (accessed August 19, 2021).
Gohil, K. J., Patel, J. A., and Gajjar, A. K. (2010). Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J. Pharm. Sci. 72, 546–556. doi: 10.4103/0250-474X.78519
Grune, T., Lietz, G., Palou, A., Ross, A. C., Stahl, W., Tang, G., et al. (2010). β-carotene is an important vitamin a source for humans. J. Nutr. 140, 2268-2285. doi: 10.3945/jn.109.119024
Hashim, P. (2011). MiniReview Centella asiatica in food and beverage applications and its potential antioxidant and neuroprotective effect. Int. Food Res. J. 18, 1215–1222.
Hashim, P., Sidek, H., Helan, M. H. M., Sabery, A., Palanisamy, U. D., and Ilham, M. (2011). Triterpene composition and bioactivities of Centella asiatica. Molecules 16, 1310–1322. doi: 10.3390/molecules16021310
Jatoi, S. A., Kikuchi, A., Mimura, M., San-San-Yi, and Watanabe, K. N. (2008). Relationships of Zingiber Species, and genetic variability assessment in ginger (Zingiber officinale) accessions from ex-situ Genebank, on-farm and rural markets. Breed. Sci. 58, 261–270. doi: 10.1270/jsbbs.58.261
JIRCAS (2020). Centella asiatica: Local Vegetables of Thailand. Available online at: https://www.jircas.affrc.go.jp/project/value_addition/Vegetables/027.html. (accessed October 3, 2020).
Kimura, Y., Sumiyoshi, M., Samukawa, K., Satake, N., and Sakanaka, M. (2008). Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur. J. Pharmacol. 584, 415–423. doi: 10.1016/j.ejphar.2008.02.036
Kosaka, Y., Xayvongsa, L., Vilayphone, A., Chanthavong, H., Takeda, S., and Kato, M. (2013). Wild edible herbs in paddy fields and their sale in a mixture in Houaphan Province, the Lao people's Democratic Republic. Econ. Bot. 67, 335–349. doi: 10.1007/s12231-013-9251-6
Kumar, S., Dobos, G. J., and Rampp, T. (2017). The Significance of ayurvedic medicinal plants. Evid. Based Complement. Alternat. Med. 22, 494–501. doi: 10.1177/2156587216671392
Lansdown, R. V. (2019). Centella asiatica. The IUCN Red List of Threatened Species 2019. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T168725A88308182.en (accessed December 12, 2020). doi: 10.2305/IUCN.UK.2019-2.RLTS.T168725A88308182.en
Mahato, R. B., and Chaudhary, R. P. (2005). Ethnomedicinal study and antibacterial activities of selected plants of Palpa District, Nepal. Sci. World 3, 26–31.
Matsuda, H., Morikawa, T., Ueda, H., and Yoshikawa, M. (2001). Medicinal foodstuffs. XXVII. Saponin constituents of gotu kola (2): structures of new ursane-and oleanane-type triterpene oligoglycosides, centellasaponins B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chem. Pharm. Bull. 49, 1368–1371. doi: 10.1248/cpb.49.1368
Medicinal Plants of Myanmar (2007). Apiaceae. Available online at: https://www.tuninst.net/MMPD/TIL/famA/Apiaceae.htm#Centella-asiatica (accessed September 3, 2021).
Menglan, S., and Watson, M. F. (2005). Centella Linnaeus. Flora of China 14. Available online at: http://www.efloras.org/florataxon.aspx?flora_id=2andtaxon_id=106022 (accessed August 19, 2021).
Orhan, I. E. (2012). Centella asiatica (L.) urban: from traditional medicine to modern medicine with neuroprotective potential. Evid. Based Complement. Altern. Med. 2012:946259. doi: 10.1155/2012/946259
Osei, K., Annor, B., Adjebeng-Danquah, J., Danquah, A., Danquah, E., Blay, E., et al. (2018). “Genotype × environment interaction: a prerequisite for tomato variety development,” in Recent Advances in Tomato Breeding and Production, eds. S. T. Nyaku, and A. Danquah (London: IntechOpen), 71–91.
Parker, C. (2020). Centella asiatica [original text by Chris Parker]. Invasive Species Compendium. CAB International. Available online at: https://www.cabi.org/isc/datasheet/12048#tocontributors (accessed October 6, 2020).
Paudel, P., Satyal, P., Dosoky, N. S., and Setzer, W. N. (2017). Chemical composition and biological activity of Centella asiatica essential oil from Nepal. AJEONP 5, 5–8.
Peiris, K. H. S., and Kays, S. J. (1996). Asiatic pennywort [Centella asiatica (L.) Urb.]: a little-known vegetable crop. HortTechnology 6, 13–18. doi: 10.21273/HORTTECH.6.1.13
Prasad, A., Dhawan, S. S., Mathur, A. K., Prakash, O., Gupta, M. M., Verma, R. K., et al. (2014). Morphological, chemical and molecular characterization of Centella asiatica germplasms for commercial cultivation in the Indo-Gangetic Plains. Nat. Prod. Commun. 9, 779–784. doi: 10.1177/1934578X1400900612
Premila, M. S. (2006). Ayurvedic Herbs: A Clinical Guide to the Healing Plants of Traditional Indian Medicine. Binghamton, NY: Haworth Press.
Rahajanirina, V., Rakotondralambo Raoseta, S. O., Roger, E., Razafindrazaka, H., Pirotais, S., Boucher, M., et al. (2012). The influence of certain taxonomic and environmental parameters on biomass production and triterpenoid content in the leaves of Centella asiatica (L.) Urb. from Madagascar. Chem. Biodivers. 9, 298–308. doi: 10.1002/cbdv.201100073
Rahmatullah, M., Ferdausi, D., Mollik, M. A. H., Jahan, R., Chowdhury, M. H., and Haque, W. M. (2010). A survey of medicinal plants used by kavirajes of Chalna Area, Khulna District, Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 7, 91–97. doi: 10.4314/ajtcam.v7i2.50859
Ranard, K. M., Jeon, S., Mohn, E. S., Griffiths, J. C., Johnson, E. J., and Erdman, J. W. (2017). Dietary guidance for lutein: consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 56, 37–42. doi: 10.1007/s00394-017-1580-2
Ravi, C. S., Umesha, K., HimaBindu, K., Raviraja Shetty, G., and Anil Kumar, G. S. (2019). Collection and morphological variability in ecotypes of Indian pennywort (Centella asiatica L.) of hill zone of Karnataka, India. Int. J. Curr. Microbiol. Appl. Sci. 8, 994–1008. doi: 10.20546/ijcmas.2019.809.117
Sardrood, S. G., Saadatmand, S., Assareh, M. H., and Satan, T. N. (2019). Chemical composition and biological activity of essential oils of Centella asiatica (L.). J. Toxicol. Environ. Health Sci. 11, 125–131. doi: 10.1007/s13530-019-0397-1
Septembre-Malaterre, A., Remize, F., and Poucheret, P. (2018). Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res. Int. 104, 86–99. doi: 10.1016/j.foodres.2017.09.031
Siddiqui, B. S., Aslam, H., Ali, S. T., Khan, S., and Begum, S. (2007). Chemical constituents of Centella asiatica. J. Asian Nat. Prod. Res. 9, 407–414. doi: 10.1080/10286020600782454
Sidhu, K., Kaur, R., and Pannu, K. (2006). For managing editor indigenous way to maternal health care within the social system. J. Soc. Sci. 13, 79–81. doi: 10.1080/09718923.2006.11892534
Stafford, G. I., Pedersen, M. E., van Staden, J., and Jäger, A. K. (2008). Review on plants with cns-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 119, 513–537. doi: 10.1016/j.jep.2008.08.010
Uddin, S., Alam, K., Hoq, O., and Nuri, Z. N. (2017). The therapeutic use of Centella asiatica. Int. J. Chem. Stud. 1, 21–26.
Udumalagala, G. C., Peramune, A. A. S., and Prasad, K. (2015). “Gotu kola (Centella asiatica): nutritional properties and plausible health benefits,” in Advances in Food and Nutrition Research, ed. J. Henry (Amsterdam: Elsevier), 125–157. doi: 10.1016/bs.afnr.2015.08.001
Watanabe, S., Toyama, T., Sato, T., Suzuki, M., Morozumi, A., Sakagami, H., et al. (2019). Kampo therapies and the use of herbal medicines in the dentistry in Japan. Medicines 6, 2–31. doi: 10.3390/medicines6010034
Zainol, N. A., Voo, S. C., Sarmidi, M. R., and Aziz, R. A. (2008). Profiling of Centella Asiatica (L.) urban extract. MJAS 12, 322–327.
Zhang, H. F., and Yang, X. H. (2012). Asian medicine: protect rare plants. Nature 482:35. doi: 10.1038/482035e
Keywords: Centella asiatica, myin-hkwa, Asiatic pennywort, gotu kola, underutilized orphan crop, medicinal species, ethnobotanical use, morphological characterization
Citation: Shukurova MK, Myint D, Yi SS, Saw OM and Watanabe KN (2021) Morphological Description and Ethnobotanical Review of the Orphan Crop Myin-Hkwa (Centella asiatica L.) From Myanmar. Front. Sustain. Food Syst. 5:680862. doi: 10.3389/fsufs.2021.680862
Received: 15 March 2021; Accepted: 28 September 2021;
Published: 08 November 2021.
Edited by:
Katherine Steele, Bangor University, United KingdomReviewed by:
Ayodeji B. Oyenihi, Cape Peninsula University of Technology, South AfricaCopyright © 2021 Shukurova, Myint, Yi, Saw and Watanabe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuo N. Watanabe, d2F0YW5hYmUua2F6dW8uZmFAdS50c3VrdWJhLmFjLmpw
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.