
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst. , 28 June 2021
Sec. Sustainable Food Processing
Volume 5 - 2021 | https://doi.org/10.3389/fsufs.2021.678045
This article is part of the Research Topic Sustainable Production of Ethnic Alcoholic Beverages View all 10 articles
In this review, the relevance of diversity of yeasts and their interactive association in household ethnic fermentation are discussed. The longstanding traditional household fermentation practice involves preparation of fermented product such as alcoholic beverages from various indigenous agricultural products with the help of microorganisms cultivated from local environment and perpetuated for hundreds of years through generations indoctrinating an indigenous knowledge system. Northeast India is known for its rich physiographic and geo-demographic diversity and is home to several ethnicities who follow unique practices of household traditional fermentation. The diversity of yeasts present within the microbial inoculum used for fermentation by different indigenous communities has been keenly studied and reported to be unique in spite of their common source for starter substrates. Saccharomyces yeasts are primarily involved in alcoholic fermentation, whereas non-Saccharomyces yeasts, which are reportedly confined to a particular geographical region, have been reported to contribute toward the final outcome of fermentation produce. During fermentation, interaction among these large microbial communities and their resulting physiological expression within the fermentation micro-environment is believed to affect the final quality of the product. Mechanism of quorum sensing plays an important role in these interactions in order to maintain proportionality of different yeast populations wherein the quorum sensing molecules not only regulate population density but also effectively aid in enhancement of alcoholic fermentation. Additionally, various secondary metabolites, which are secreted as a result of inter-species interactions, have been found to affect the quality of beverages produced. This review concludes that diverse species of yeasts and their interaction within the fermentation micro-environment influence the sustainability and productivity of household ethnic fermentation.
Yeasts are single-celled eukaryotic microorganisms known to be the key players in the age-old practice of alcoholic fermentation for thousands of years. The practice of fermentation involving yeasts is one of the oldest human technologies dating back to the Neolithic period when spontaneous fermentation was carried out from plant sources such as grape must (Compagno et al., 2014). Due to their ability to ferment sugary substances into ethanol, yeasts are incorporated during formulation of inoculum or starter materials, which are used for preparation of fermented beverages in household fermentation worldwide (Hesseltine et al., 1988). This ancient practice of household ethnic fermentation in Southeast Asia involving yeasts is believed to have been originated from China, which spread across most of the neighboring countries with time (Limtong et al., 2002). Apart from the Asiatic region, the tradition of fermented product preparation is widespread among most of the indigenous communities belonging to different regions all over the world (Kabak and Dobson, 2011). These longstanding systems of alcoholic beverage preparation from various agricultural products with the aid of locally perpetuated fermentative agents have been in practice for generations indoctrinating indigenous knowledge system (Parasar et al., 2017; Nath et al., 2020a).
Methods of preparation of starter culture cakes among various communities is almost similar with minor variations observed in the methods followed and plant condiments added (Anupama et al., 2018). Household fermentation is recognized to be a popular practice among home brewers of indigenous communities in the entire Southeast Asia (Tsuyoshi et al., 2005) that reflect the ethnic heritage of existing communities, and northeast India is not an exception. Interestingly, in spite of a common source of ancestry of the indigenous communities of northeast India and usage of similar methods for ethnic fermentation, the diversity of yeasts perpetuated through those natural and spontaneous fermentation processes and maintained by the communities have been found to be unique (Buragohain et al., 2013; Tiwari et al., 2014; Parasar et al., 2017). Furthermore, since each community produces fermented beverage of distinct quality, the role of these genotypically diversified yeasts in both intra- and intercommunication is a question to be inquired, and their precise role in attributing quality characteristics in the final products of the fermented beverages needs to be understood. In addition to the interaction among yeasts in a consortia, the role of added plant condiments during starter material preparation may also play an important role in generating quality attributes as well as resuscitation of microbes within the starter cake/material propagated generation after generation (Nath et al., 2019).
In a fermentative micro-environment, intra- and interspecies interactions occur between co-existing yeasts and other microbes (Fleet, 2003) where the final quality of fermented beverage is believed to be affected due to the interaction among these large microbial communities and their resulting physiological expression (Ciani et al., 2016). Apart from the formation of ethanol, certain yeast species produce secondary metabolites such as fatty acids and killer toxins that act as growth inhibitors for other yeast species that eventually kill vulnerable yeasts (Pérez-Nevado et al., 2006). Direct physical interaction among different yeasts or with other microbes was also believed to be the limiting factor for the growth of certain non-Saccharomyces yeasts that spawn and proliferate during initial stages of fermentation (Nissen and Arneborg, 2003; Nissen et al., 2003; Renault et al., 2013). Noticeably, death of desirable yeast cells is triggered when unwanted yeast population reaches high cell density threshold during progressive fermentation (Nissen and Arneborg, 2003; Pérez-Nevado et al., 2006). These findings therefore suggest the importance of yeast interactions during both spontaneous and induced fermentation.
Apart from the above, it has been confirmed that quorum sensing molecules (QSMs) expressed by yeasts also play a key role in the expression of various population density-dependent characteristics that can affect the quality of a fermented product. QSMs are hormone-like molecules that accumulate in the external environment in proportionality to cell density population (Sprague and Winans, 2006) and either activates or represses certain genes when a particular threshold level of the molecules is obtained (Fuqua et al., 1994). The two fungal QSMs, viz., tryptophol and phenylethyl alcohol, were first recognized in Candida albicans (Lingappa et al., 1969) followed by the discovery of three additional QSMs, viz., farnesol, tyrosol, and farnesoic acid (Hornby et al., 2001; Oh et al., 2001; Chen et al., 2004; Hornby and Nickerson, 2004). These QSMs have further been examined by several researchers and their multifarious role in controlling morphogenic transition of yeast to filamentous form and vice versa has been established in the preceding years. Moreover, the QSM tyrosol has been reported to induce the upregulation of genes associated with DNA replication (Chen et al., 2004), thereby implying the probable enhancing effect of the molecule on cellular growth. This assumption was found to be true as was noted in our recent investigation where tyrosol significantly increased the population density of Candida tropicalis, Wickerhamomyces anomalus, and Saccharomyces cerevisiae (Nath et al., 2020b) during alcoholic fermentation.
Due to the involvement of diverse yeast species in the production of fermented goods, it is necessary to explore the link between their interactions and product outcome. There has been extensive research on determining the diversity of yeast conglomerates in fermentative cakes from northeast India, but unfortunately no clear explanation of yeast–yeast interferences or intercommunication within these starter cakes as well as fermented products could be inferred until the preparation of this report. As mentioned earlier, each indigenous community of northeast India produces fermented beverages with distinct quality attributed by specific yeast lineages tamed and cultivated by the individuals of these communities who exclusively preserve and perpetuate starter materials that are unique to each community type. It therefore becomes important to document the diversity of yeasts persisting within the starter materials, and it is pertinent that subtle differences in genotypes and phenotypes of diverse yeasts and the subsequent interactions among them are key factors that determine the oenological properties of final output in fermented beverages (Granchi et al., 2002). Keeping in view about the importance of cell–cell interactions during fermentations, we extrapolate that the diversity of yeast species in starter cultures of northeast India should be explored and the inherent oenological properties be evaluated to understand the complexity of traits unique to the region. The volatile profiles of higher alcohols, esters, carbonyls, and sulfur compounds have not been studied and the prospects of delineating yeasts that produce secondary alcohols, acetic acid, acetaldehyde, ethyl acetate, acetoin, and glycerol have never been accessed (Steward, 2017). We assume that the quality traits need appreciation so that the prospective yeasts can be commercialized for large-scale production of chemicals and metabolites. Earlier, we had examined the factors that determine the persistence of yeast communities within the fermentation micro-environment but a larger view of the entire repertoire of yeasts from the region is still lacking. Moreover, the role of QSMs during fermentation does interfere and alter the quality of final products (Nath et al., 2020b), and henceforth, the array of QSMs expressed by the native yeasts of the region has to be deciphered to validate unique combination of yeasts with profound benefits.
The importance of traditional and sustainable food has received tremendous attention in recent years due to several reasons including their benefits to health, environment friendly methods of preparation, natural origin, absence of toxic effects, and prospects of commercialization. The exploration of indigenous knowledge systems inherent in cultures and traditions practiced through ages have helped in the conservation of innumerable diversity of both conventional and non-conventional food that have not been fully explored in the context of the Indian subcontinent. India is home to 550 ethnic communities (Schedule Tribes in India, jagranjosh.com) with a major proportion residing in the northeastern part of the country. Traditional brewing and household preparation of fermented food and drinks has been a major source of activity inculcated in their customs and traditions. The diversity of yeasts and other microbes inherent in these traditional brewing methods have been studied only recently, and we could find literature from 1994 onwards. In this review, all bibliographic literature available in PubMed, Web of Science, and Scopus indexed journals were consulted (1994–2020). A good number of research publications on the diversity and identity of yeasts during fermentation have been published since 1990 to date (PubMed 1,008 papers). To get a clear view of the extent of work conducted on the subject, we searched various scientific engines and found that the approximate number of papers published for the last 10 years stands at 1,432, which were retrieved from PubMed using various keywords/phrases [(1) Yeasts in traditional fermentation, (2) Traditional fermentation from northeast India, (3) Yeasts from northeast India, (4) Quorum sensing in yeasts, (5) Quorum sensing in traditional fermented yeasts, (6) Quorum sensing of Saccharomyces and non-Saccharomyces yeasts, (7) Quorum sensing in non-conventional yeasts, etc.], which reflected that the extent of research is not very pronounced. The search query found that the maximum number of documents (1,008) were related to the general term “yeasts in traditional fermentation” where a lot of literature on a global scale was available. This was followed by the term “quorum sensing in yeasts” where an appreciable number of publications (382) could be retrieved and 80% of the publications were related to Candida and Cryptococcus spp. that cause fungal infections in human. We could retrieve only nine publications related to the term “quorum sensing of Saccharomyces and non-Saccharomyces yeasts,” which suggests that the area remains unexplored and very little attention is drawn toward quorum sensing among yeasts and their subsequent impact on fermentation/traditional fermentation, which therefore remains as a major gap. Bibliometric analysis enabled us to understand the global context of the subject, themes that have not been addressed yet and also the major drawbacks of the extent of research impact (Ashraf et al., 2021). The data, when analyzed, showed that the available knowledge on the subject is still limited and the milestones achieved are yet to be acknowledged, which has hindered the prospects of commercialization of ethnic foods on a scientific basis.
The northeastern part of India includes a biogeographic realm with a characteristic biome and encompasses unique features in terms of rich biodiversity and multiple ethnicities of its people. The indigenous communities follow their own practice of household traditional fermentation. Each community harbors unique distribution of yeast species, which have been perpetuated throughout generations (Buragohain et al., 2013; Tiwari et al., 2014; Parasar et al., 2017) and this has resulted in the variety of alcoholic beverages assorted and produced by the communities through traditional fermentation practice. In household fermentation practiced by the communities of northeast India, solid-state starter cultures in the form of “flat cakes” or “spherical balls” are prepared from starchy substances such as rice, millets, maize, or wheat that are powdered and mixed with microbial inoculum (Nath et al., 2020a). These “cakes” supplement carbon constituents for the growth of yeasts that initiate fermentation. In the Rabha community, fermentative starter cake (known as bakhaor) is prepared in the form of “flat globules” by mixing soaked rice granules (Oryza sativa L., land variety sali and ahu) with considerable amount of old starter cakes (containing fermentation yeasts) and various plant condiments (Deka and Sarma, 2010). In order to prepare a consumable beverage, rice is tightly cooked and air cooled over bamboo mats, which act as substrate for rice beer. Starter cake is powdered (inoculum) and mixed with boiled rice. Later, this mixture is placed inside earthen pots while a special cylindrical structure made of bamboo (janthi) is placed inside and the mouth of the pots are tightly closed with banana leaves. After a few days (4–5 days in summer, 7–8 days in winter), fermented rice beer accumulates inside the janthi. This beer is further fermented using water and starter cakes, followed by distillation using series of earthen or metallic pots. Finally, a strong, consumable fermented beverage is produced (Deka and Sarma, 2010).
The presence of non-Saccharomyces species along with chief fermentative yeasts in starter cakes of various indigenous communities has been observed universally. The microbial inoculum in starter materials has been maintained for generations. However, in spite of extensive perpetuation for hundreds of years, the quality and consistency of the fermented produce have not varied much and appear to be constant across continued generations without any noticeable change in their fermentation efficiency. This consistency in fermentation outcome indicates the steady maintenance of yeasts and their stable genetic features. Amylolytic yeasts presumably play a crucial role in fermentation by enabling the breakdown of high carbon materials such as starch into simpler glucose molecules while fermentative yeasts thrive and carry out fermentation on those ready-made energy sources. Competition over common resources arises when two or more species of yeasts compete to thrive within a specified fermentation environment. However, cooperation between amylolytic and non-amylolytic yeasts always prove to be a beneficial trait when the population has to rely on starchy carbon source (Nath et al., 2020a). To note, non-Saccharomyces yeasts such as W. anomalus produces volatile compounds that enhance the aroma characteristics of wines, thereby enabling better quality yield of fermented beverage (Ye et al., 2014).
Fermented products are categorized into solid or semi-solid fermented food and fermented beverages. The diversity of yeasts in fermentative sources has been well-documented from Southeast Asia (Boekhout and Robert, 2003). The documentation of diversified yeasts from fermentative foods of northeast India have also been done from time to time. Buragohain et al. (2013) had reported the presence of S. cerevisiae, Debaryomyces hansenii, W. anomalous, and Candida glabrata in starter cakes collected from the tea ethnic community, Ahom, Nepali, Bodo, Adivasi, Karbi, and Dimasa communities of Assam, Meitei community of Manipur, Angami community of Nagaland, Apatani community of Arunachal Pradesh, and the Khasi community of Meghalaya. The presence of Candida parapsilosis and Geotrichum candidum in kinema fermented food of the Gorkha and Nepali communities was reported from the state of Sikkim (Sarkar et al., 1994). Dewan and Tamang (2006) had reported about the presence of Saccharomycopsis fibuligera and Candida spp. from chhu or sheden ethnic fermented milk products of the Bhutias, the Lepchas, the Monpas, the Sherdukpen, the Khambas, the Membas, and the Tibetan people living in the higher altitudes of the eastern Himalayas. The persistence of the yeasts representing Saccharomycopsis and Candida have also been reported from dahi (curd) from the entire northeast India (Dewan and Tamang, 2007); Candida spp. from goyang fermentation of the Sherpa community in Sikkim (Tamang and Tamang, 2007); S. cerevisiae, Pichia burtonii, and Candida castellii from maseura or masyaura fermentations (Chettri and Tamang, 2008); and S. cerevisiae, Saccharomyces kluyveri, D. hansenii, P. burtonii, as well as Zygosaccharomyces rouxii from selroti fermented product of the Gorkha community (Yonzan and Tamang, 2010). Chettri (2012) had described about the presence of S. cerevisiae, D. hansenii, and P. burtonii in tungrymbai fermented food of the Khasi community from Meghalaya as well as from bekang fermentation of Mizoram. Tamang et al. (2016) reportedly marked about the presence of Saccharomyces and Torulopsis yeasts in soibum fermentation of the Meitei community from Manipur. On the contrary, Thapa et al. (2004) reported the predominance of Candida and Saccharomycopsis yeasts in ngari and hentak fermented drinks of Manipur, Candida chiropterorum, Candida bombicola, and Saccharomycopsis spp. from gnuchi of the Lepcha community of Sikkim while the presence of same yeasts had been reported from suka ko maccha and sidra fermentations prepared by the Gorkha community from Sikkim (Thapa et al., 2006).
Since solid-state starter cultures are primarily used as inoculum for fermented beverage production, these have a special mention in most of the published reports from the region, such as starter cake marcha/murcha, which is used to produce the alcoholic drink called jaanr in Sikkim and Himalayan terrains (Shrestha et al., 2002; Tamang, 2005, Thapa and Tamang, 2020). Incidentally, the persistence of the yeasts S. fibuligera, Saccharomycopsis capsularis, Pichia anomala, P. burtonii, S. cerevisiae, Saccharomyces bayanus, W. anomalus, and C. glabrata seem to be ubiquitously present along the eastern Himalayan landscape (Tamang and Sarkar, 1995; Thapa, 2001; Tsuyoshi et al., 2005; Sha et al., 2018). The ecology and distribution of yeasts in fermented products from the rest of the seven northeastern states have also been reviewed by several researchers. The supremacy of S. cerevisiae over other yeasts in consortia was observed in a starter material called piazu, which is used to prepare alcoholic brew called zutho by the Angami community of Nagaland (Teramoto et al., 2002). Similar observations were noted for the alcoholic brew called litchumsu, which is prepared by the Ao community of Nagaland (Das et al., 2012). Zutho in the state of Nagaland is also produced from another starter culture named Khekhrii, wherein the dominance of W. anomalus and P. anomala was observed (Sha et al., 2018). Similarly, existence of S. cerevisiae, P. anomala, Trichosporon spp., C. tropicalis, Pichia guilliermondi, C. parapsilosis, Torulaspora delbrueckii, Pichia fabianii, Pichia kudriavzevi, Candida montana, and C. glabrata was reported from hamei starter culture, which is employed in the production of atingba and yu alcoholic beverages by Meitei and Kabui community of the state of Manipur (Jeyaram et al., 2008, Sha et al., 2018, Wahengbam et al., 2020). In Meghalaya, starter cultures called wanti and thiat are used in preparation of alcoholic beverages (Anupama et al., 2018; Mishra et al., 2018). The starters of wanti are primarily composed of the yeasts S. cerevisiae, Rhodotorula mucilaginosa, and W. anomalus, which is used to prepare chubitchi beverage by the Garo community, kyiad beverage by the Khasi community, and sadhiar beverage by the Jaintia communitiy of Meghalaya (Mishra et al., 2018). The starters of thiat contain yeasts belonging to S. fibuligera, W. anomalus, and Pichia terricola, which is used to prepare alcoholic beverages by the Khasi community (Sha et al., 2018). Earlier investigations revealed the presence of W. anomalus in epo starter culture perpetuated by the Apatani community and phut starter cake of the Monpa community of Arunachal Pradesh (Tanti et al., 2010; Buragohain et al., 2013; Anupama et al., 2018; Sha et al., 2018). In the state of Mizoram, beverages such as zupui, zufâng, and rakzu are prepared by Mizo community from a starter culture called dawdim, where the occurrence of W. anomalus, C. glabrata, P. anomala, and S. fibuligera were found to coexist as a consortia (Sha et al., 2018; Thanzami and Lalhlenmawia, 2020). In the state of Tripura, production of local alcoholic drinks like gora bwtwk and chuwak are customary traditions carried out by the Kalai, Jamatia, Debbarma, and Molsom communities who conserve and perpetuate a starter culture called chowan, where the dominance of C. glabrata and W. anomalus was reported (Anupama et al., 2018; Sha et al., 2018; Majumdar, 2020). In our previous investigations (Parasar et al., 2017), we analyzed the starter cultures conserved by the Bodo, Karbi, Rabha, Mishing, Ahom, and Kachari communities of Assam. The starter culture emao/amao of the Bodo community was found to be dominated by P. burtonii, P. anomalus, and S. cerevisiae while another starter culture called thap of the Karbi community was dominated by S. cerevisiae (Parasar et al., 2017). The Ahoms perpetuate a starter culture called xajar pitha, which is enriched with C. tropicalis and C. glabrata, an unusual observation with a great deal of research interest since no Saccharomyces isolates could be retrieved from the starters (Parasar et al., 2017). The Mishing community of Assam prepare and preserve apong kusure/apop pitha starter cake where the unusual yeast Rhodotorula taiwanensis was found to predominate (Parasar et al., 2017). The Rabhas, another major ethnic community, perpetuate starter cultures called bakhor where the persistence of Meyerozyma carribica was reported (Tanti et al., 2010; Narzary et al., 2016; Parasar et al., 2017; Barooah et al., 2020). The Kachari community of Assam prepare modor pitha starter cultures that contain a consortia of S. cerevisiae, W. anomalus, and C. glabrata (Parasar et al., 2017; Anupama et al., 2018). To our utter surprise, the entire spectrum of starter cultures studied from the region revealed the presence of W. anomalous except for the starter cultures collected from the Ahom community (Parasar et al., 2017; Nath et al., 2020a). Occurrence of dominant yeasts in starter cultures are summarized in Table 1. Additionally, the distribution of ethnic communities of the region, the starter materials used in traditional brewing, and the names of resultant beverages are summarized in Figure 1. Here, we have mentioned only those starter cultures whose detailed studies are available in literature and which have been investigated by researchers from the region. The repertoire of cultures is colossal and shall require far more intricate research plans to reach a final conclusion regarding the origin, distribution, and diversity of yeast types and the array of traditional fermentation procedures practiced in the region (Parasar et al., 2017; Nath et al., 2020a). Our recent findings regarding distribution of yeasts in starter cakes (Parasar et al., 2017; Nath et al., 2020a) are illustrated in Figure 2.
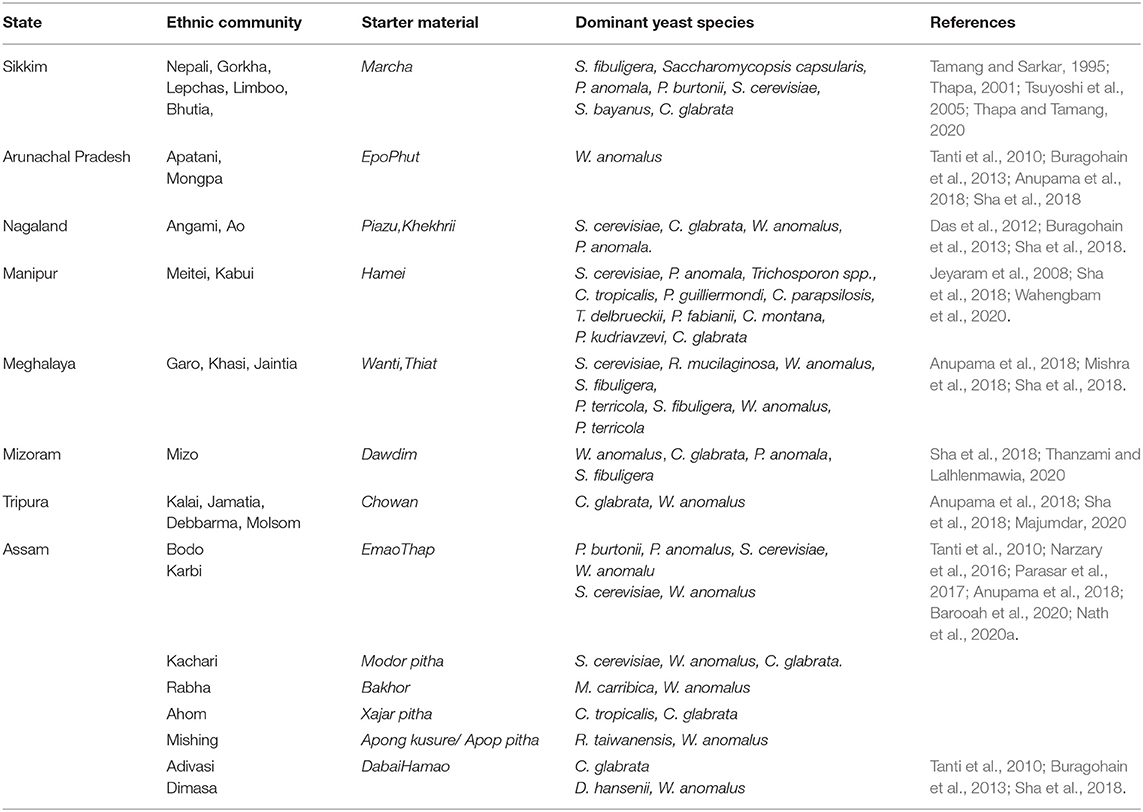
Table 1. Dominant yeast species associated with starter materials from ethnic communities of northeast India.
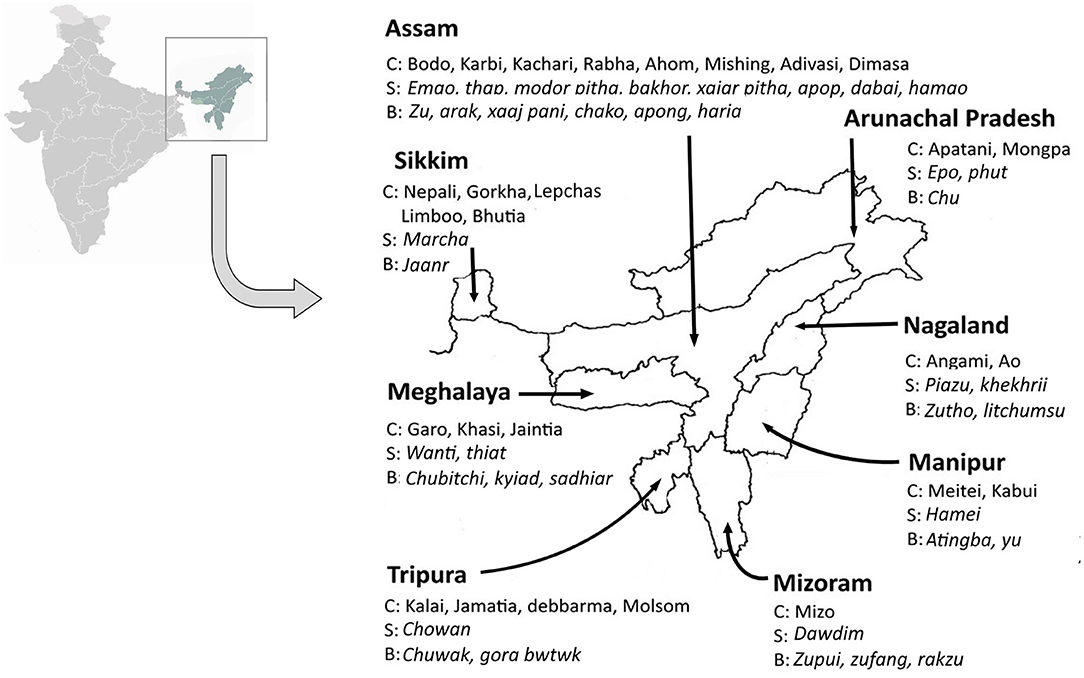
Figure 1. Map of northeast India indicating ethnic communities (C), starter material/cake (S), and fermented beverages (B) associated with ethnic culture of the seven states. This illustration is based on reports by various researchers on yeast population harbored in the starter cakes and their beverages (Tamang and Sarkar, 1995; Thapa, 2001; Tsuyoshi et al., 2005; Jeyaram et al., 2008; Tanti et al., 2010; Das et al., 2012; Buragohain et al., 2013; Narzary et al., 2016; Parasar et al., 2017; Anupama et al., 2018; Mishra et al., 2018; Sha et al., 2018; Barooah et al., 2020; Majumdar, 2020; Nath et al., 2020a; Thanzami and Lalhlenmawia, 2020; Thapa and Tamang, 2020; Wahengbam et al., 2020).
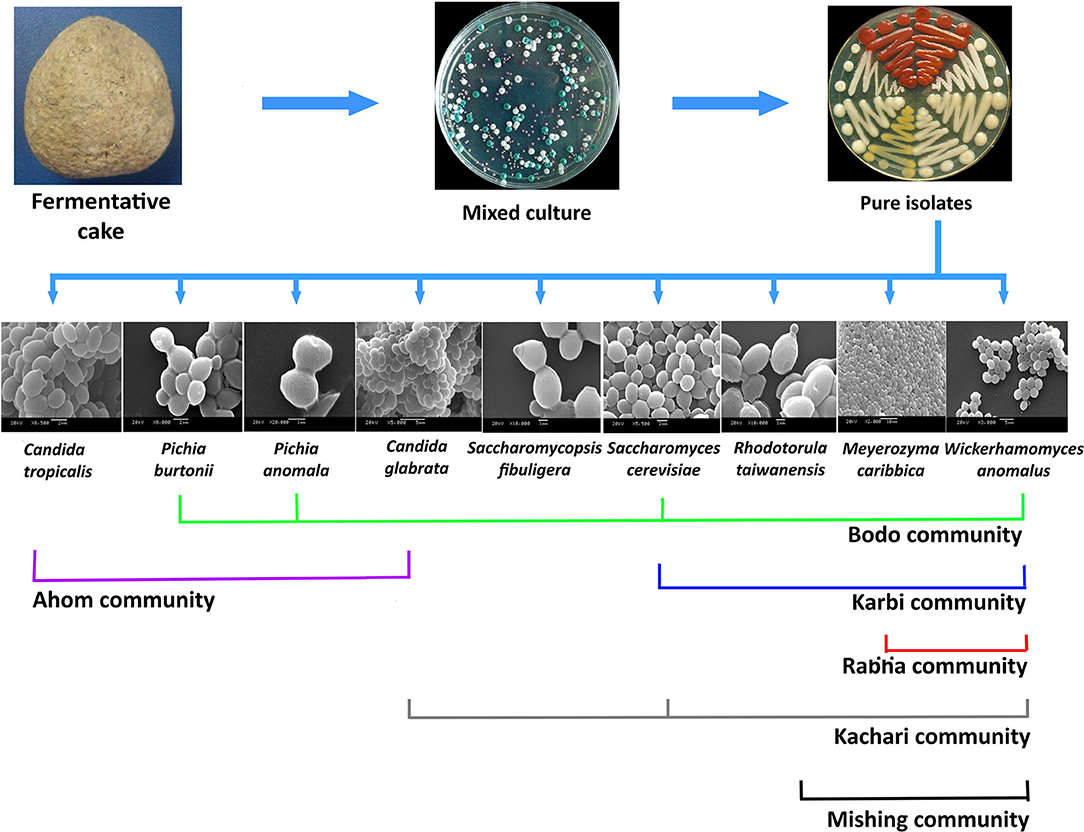
Figure 2. Graphical depiction of distribution of yeasts in various starter cultures of indigenous community of Assam. The experimental data are derived from Parasar et al. (2017) and Nath et al. (2020a).
Microorganisms exist in diverse communities in both natural and man-made ecosystems. Interactions among these microbes define the complexity of food and beverages (Avbelj et al., 2016). Such intra- and interspecies interference could be observed between yeasts and other microbes that coexist in wine micro-environment (Fleet, 2003). Essential metabolic interactions and complementation physiognomies have been reviewed in food ecosystems (Ivey et al., 2013). Positive interactions such as mutualism and synergism allow the participating microbes to derive benefit from the interactions. For example, during alcoholic fermentation of starchy material, two types of yeasts have been found to play the principal role of fermentation that include amylolytic yeasts that degrade starch into simpler carbohydrates while alcohol-fermenting yeasts grow on the resulting simpler carbohydrates to yield ethanol (Tsuyoshi et al., 2005). This was further analyzed in our recent experiments and was observed that utilization of starch and subsequent ethanol production was higher when S. cerevisiae (non-amylolytic) was combined with C. tropicalis (amylolytic) in co-culture combination compared to tested combinations of S cerevisiae with other non-amylolytic yeasts (Nath et al., 2020a). Cooperation of yeasts with other species has also been found to be beneficial for the production of quality fermented product. For example, production of Japanese alcoholic drink sake employs fermentation by S cerevisiae var. sake along with Aspergillus oryzae, wherein Aspergillus break down rice starch into simpler sugars while yeasts convert resultant sugars into ethanol (Jay, 1991; Azumi and Goto-Yamamoto, 2001). Furthermore, such interactions also do enhance unique characteristics by producing secondary metabolites, higher alcohols, and ethers that produce pleasant aroma and quality texture (Hosaka et al., 1998).
Contrarily, negative interactions such as amensalism adversely affect the growth of competitive microbes. Two good examples can be cited for amensalism wherein the production of ethanol by S. cerevisiae eliminate all non-Saccharomyces yeasts present in the must during fermentation while nisin production by Lactobacillus lactis inhibits proliferation of other competing lactic acid bacteria (LAB) (Hutkins, 2007). Competition is another aspect of negative interaction, where the microbes compete with one another for nutrient uptake and for space to survive. Such examples can be seen during alcoholic fermentation, where S. cerevisiae engage in defensive mechanisms such as physical cell contact and secretion of antimicrobial peptides against other microbes that impart significant impact on wine profiles (Albergaria and Arneborg, 2016). Such microbial interactions influence the quality of the final product and impact critical parameters such as sensory quality, low toxicity, and shelf life of the fermented food or beverage (Rul and Monnet, 2015).
Application of the QSMs 2-phenylethanol, tryptophol, and tyrosol have already been reported in various biotechnological techniques, which include evaluation of wine quality (Garde-Cerdán et al., 2007; González-Marco et al., 2010) and enhancement of aroma in foods and drinks (Etschmann et al., 2003; Wang et al., 2011). In addition to enhancing food value, quorum sensing mechanisms have the potential to control food spoilage (Avbelj et al., 2016). Such observation has been reported during wine fermentation, where K2 and Klus-type yeast killer toxins were found to be responsible for controlling proliferation of spoilage yeasts (Comitini et al., 2004; Rodríguez-Cousiño et al., 2011). Alternatively, quorum sensing inhibitors (QSIs) can be employed to control the proliferation of spoilage microbes thereby inhibiting the expression of undesired virulent traits (Sharma and Jangid, 2015). Recent studies reveal that different plant extracts harboring phytochemical constituents and essential oils inhibit biofilm formation of harmful yeasts/microbes, thereby rendering the product suitable for human consumption and henceforth these substances may be considered as effective QSI during wine fermentation (Avbelj et al., 2016). Likewise, a QS mechanism has been observed to control food spoilage by regulating the expression of enzymes such as cellulases, lipases, chitinases, nucleases, pectate lyase, and various proteases, thereby facilitating the management of quality control of processed foods (Skandamis and Nychas, 2012).
In traditional fermentations practiced by the communities of northeast India, different plant condiments are added during the preparation of starter cultures (Tanti et al., 2010). Metabolites and constituents from such plant additives/parts are believed to play an important role during fermentation (Nath et al., 2019). The phytohormone indole-3-acetic acid (IAA) is one of several metabolites that are known to promote rapid and long-term responses in plants (Cleland, 2010) and incidentally has also been observed to be expressed by certain yeasts inherent in traditional starters from the region (Nath et al., 2019). It is noteworthy that reports of yeasts capable of producing IAA are already available in published literature, which contemplates the importance of traditional yeasts to be exploited for producing phytohormones other than conventional metabolites (Nakamura et al., 1991; El-Tarabily, 2004; Nassar et al., 2005). Parenthetically, previous studies had reported that IAA stimulates growth and promotion of filamentous morphogenesis in Saccharomyces (Prusty et al., 2004), which was also supported by our recently published findings (Nath et al., 2019). From such observations, it can be presumed that IAA contributes toward the growth of yeasts while the addition of plant condiments supplements the necessary phytohormone for perpetuation and coexistence of the varied yeast species in starter cakes.
It is to be mentioned that tyrosol, the chief QSM expressed in yeasts, was reported to confer protection from oxidative stress induced by respiratory burst of neutrophils (Cremer et al., 1999) and ethanol (Nath et al., 2020b) apart from morphogenic transition and other QS-related activities. In an earlier report, tyrosol was reported to influence germ tube formation through upregulation of DNA replication and cell cycle maintenance (Chen et al., 2004). Such upregulations can presumably exert the growth-enhancing effect in yeasts concurrent with the increase in cell density. Evidently, our recently published work also demonstrated an increase in cell number under the influence of self-expressed and exogenously introduced tyrosol in both Saccharomyces and non-Saccharomyces yeasts (Nath et al., 2020b), and since ethanol production was found to be proportional to cellular density, an indirect involvement of tyrosol during fermentation could be well-justified. Nevertheless, such QS activity is believed to be limited by ethanol concentration and addition of external ethanol was found to inhibit the production of tyrosol by S. cerevisiae, a strong indication of an antagonistic role of ethanol on QS activity (Avbelj et al., 2015).
This review was an attempt to provide an insight into yeast–yeast interrelation and the role of quorum sensing mechanism on the production of ethnic beverages during traditional fermentations. As mentioned earlier, diverse species of yeasts in fermentative cakes impart substantive effect on the shelf life and oenological properties in wines and beverages when cultured in consortium. Survival experiments of yeast isolates under co-culture conditions revealed the usefulness of non-Saccharomyces yeasts during fermentation and is now well-understood that apart from the enhancement of ethanol content, other metabolites also play important roles in the enhancement of quality attributes of the fermented products (Nath et al., 2020a). The unusual yeast W. anomalus was found to be ubiquitously present and dominant in most of the fermentative cakes representing several indigenous communities of Assam (Parasar et al., 2017), and is not a chief fermenting yeast, and yet, the species has been reported to produce volatile compounds that enhance the aroma profile of traditional wines (Clemente-Jimenez et al., 2005). Several investigations reported earlier stated that high-quality balanced wines can be produced when W. anomalus is mixed and co-cultured with S. cerevisiae, which often leads to the production of acetate esters, ethyl propanoate, phenyl ethanol, and 2-phenylethyl acetate that are known to enhance the aroma profile of wines (Rojas et al., 2003; Clemente-Jimenez et al., 2005; Swangkeaw et al., 2011, Passoth et al., 2006). Similarly, other unusual non-Saccharomyces yeasts like Zygosaccharomyces bailii (Gueguen et al., 1995), D. hansenii (Yanai and Sato, 1999), P. anomala (Manzanares et al., 2001; Swangkeaw et al., 2011), and Hanseniaspora (Swangkeaw et al., 2011) have been reported to express and produce several valuable metabolites, volatile compounds, and enzymes like β-glucosidase during fermentation of grape must (Rodríguez et al., 2004). It is now well-known that non-Saccharomyces yeasts saturate the fermentation culture during early stages of fermentation, and with an increase in ethanol concentration, the dominance of ethanol tolerant S. cerevisiae persists, which continues the process till the end of fermentation (Xufre et al., 2006; Lee et al., 2012a,b). Meanwhile, although the precise role of QSMs during the fermentation process has not received much attention, considering the effects of QSMs studied to date, the importance of molecules like tyrosol has proved to be beneficial, not only because of its cell growth-enhancing capability but also because it provides nutritional value to fermented products with quality traits that confers health benefits as tyrosol possesses incredible antioxidant property (Giovannini et al., 1999).
From the preceding discussion, it is evident that association of yeasts and their productive communication results in the production of beverages with distinct enological qualities inherent in fermented beverages/drinks prepared by various indigenous communities of northeast India. These unique characteristics are presumably observed due to the presence of distinct dominant species of yeasts and the addition of plant concoctions/condiments into the starter materials for perpetuation and cultivation of fermenting principle. However, several limitations such as bad odor, turbidity, inconsistency, presence of toxic metabolites, and variable texture contribute to uninvited traits rendering the fermented products unsuitable for commercialization (Tsuyoshi et al., 2005). Although genetic or chemical engineering approaches can eliminate such limitations, natural methods of co-culturing or sequential culture procedures could prove to be more cost-effective and user-friendly. To conclude, the aforementioned yeast–yeast association experiments and studies pertaining to the role of chemical communication by QSMs like tyrosol in controlling the quality of fermented products need to be thoroughly investigated, but unfortunately, records of such investigations are scanty from northeast India, which has an abundant microflora distribution and rich in cultural heritage of a huge congregation of ethnic indigenous people. Considering the immense potential of these traditional products, more and more applications of such natural interactions in fermentation should be prioritized so that the indigenous people could benefit from these household preparations and get an opportunity for choosing sustenance through commercialization of these indigenous products that will impart sustainable livelihood options.
The mechanism of QS in both gram-positive and gram-negative bacteria are well-established (Rutherford and Bassler, 2012). This understanding has resulted in efforts to inhibit bacterial pathogenesis and design novel antimicrobial therapeutics. Unlike bacteria, where the role of autoinducing molecules in QS like lactones and oligo-peptides and the corresponding genes that express them have been deciphered, the fungal QS system is yet to be studied in detail, particularly with reference to the effects of QS on fermentation. Processes in QS include other activities like bioluminescence, sporulation, competence, antibiotic production, biofilm formation, and virulence factor secretion (Rutherford and Bassler, 2012) and such effects cannot be nullified in fungal quorum sensing too. Research on autoinducing molecules and transporters that release or transport these molecules in and out of cells shall complement their effects on the formation of final products as well as adduct secondary molecules that could prove to be industrially beneficial. Similarly, the effect of QSMs in starter culture perpetuation needs to be appreciated and should be studied to explicate the benefits of QS among yeasts in solid-state fermentation. At the same time, the differences of expression and activity of QSMs in starter cultures and during submerged fermentation need to be investigated. In starter materials, yeasts remain in dormant dry state that are activated upon contact with substrates. Proliferation of yeasts within starter materials without the involvement of fermentation is a question to ponder. Notwithstanding the fact that a large number of studies pertaining to the probable role of plant material concoctions in the enhancement of attributes of fermented beverages in spontaneous or traditional fermentation from starter cultures have been discussed (Nath et al., 2019), an authentic explanation to the definite role of these concoctions on QS activity concomitant to fermentation process has never been addressed. Besides these, the necessary information on the accessibility and roles of other QSMs other than tyrosol on fermentation (Nath et al., 2020b) is still missing and thus our knowledge on the array of QSMs remains limited, which needs to be addressed. More importantly, the ecology and behavior of traditional yeasts in fermentation have not been studied much and henceforth the queries on the key ecological aspects of these beneficial fungi in response to change in parameters and composition of substrates need to be studied. It has been reported that QSMs in yeasts like farnesol suppress the pathogenicity of certain pathogenic yeasts like C. albicans through morphogenesis (Hornby et al., 2001). Similarly, 2-phenylethanol and tryptophol regulates phenotypic changes in S. cerevisiae (Smukalla et al., 2008; Avbelj et al., 2016). The regulatory mechanisms of these QSMs are well-explained (Avbelj et al., 2016), but their direct involvement in fermentation is yet to be thoroughly explored. In addition, assessing the role of these QSMs during inter- and intra-species competition in a fermentation microenvironment needs validation. The role of QSMs in traditional fermentation is important to check the changes in parameters and the expression/production of both desirable and undesirable metabolites in culture. There are several future prospects of QS mechanism in the production of fermented foods. QSMs discovered to date have various health benefits (Nath et al., 2020b). As QSMs are produced in minute quantities, purification of these shall not be an economically viable option. Therefore, understanding the mechanism to develop fortified fermented food enriched with beneficial molecules could be one useful alternative. In anaerobic condition, increased production of 2-phenylethanol, tryptophol, and tyrosol was observed in C. albicans (Ghosh et al., 2008) and S. cerevisiae (Avbelj et al., 2016), implying the role of QSMs in the enhancement of fermentation during anaerobic conditions. Anaerobic condition suppresses biomass proliferation and increases more carbon conversion to end product (Huang and Tang, 2007). It is therefore envisioned that proper exploration and application of the regulating mechanisms of fermentation through QSMs can open new insights that will be beneficial in food and beverage industries. Furthermore, to the above, the interactions among yeasts, their presence, and their metabolic exchanges are key to the formation of products that will have economic value. As QS mechanism is a density-dependent phenomenon, regulation of cell population during expression of QSMs is anticipated (Nath et al., 2020b). It is evident that more experiments shall have to be conducted to reach to a conclusion as to how a fermented product is modified through QS mechanism. Co-culturing techniques could possibly help in determining the competitiveness among species, while genetic engineering approaches can facilitate in understanding the underlying molecular mechanisms of such interaction during fermentation.
All the authors have contributed equally in conceiving, designing, and writing the manuscript.
A part of this article has been comprehended from our previous work supported by a project (BT/303/NE/TBP/2012 dated 04/01/2013) funded by the Department of Biotechnology, Government of India. Some observations have also been generated from our ongoing Department of Biotechnology, Government of India-funded project on pigmented yeasts from northeast India (BT/PR25652/NER/95/1326/2017 dated 26/07/2018). BN was a recipient of NFOBC-UGC fellowship (F./2017-18/NFO-2017-18-OBC-ASS-61070/SA-III/Website) for PhD under HS. DP was a recipient of BSR-UGC fellowship (GU/UGC/BSR/P.J.Handique/2012/1183-87 dated 6.7.12) for PhD under HS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Albergaria, H., and Arneborg, N. (2016). Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 100, 2035–2046. doi: 10.1007/s00253-015-7255-0
Anupama, A., Pradhan, P., Sha, S. P., and Tamang, J. P. (2018). Traditional skill of ethnic people of the Eastern Himalayas and North East India in preserving microbiota as dry amylolytic starters. Indian J. Tradit. Knowl. 17, 184–190. Available online at: http://nopr.niscair.res.in/handle/123456789/43133.
Ashraf, S. A., Siddiqui, A. J., Elkhalifa, A. E. O., Khan, M. I., Patel, M., Alreshidi, M., et al. (2021). Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 768:144990. doi: 10.1016/j.scitotenv.2021.144990
Avbelj, M., Zupan, J., Kranjc, L., and Raspor, P. (2015). Quorum-sensing kinetics in Saccharomyces cerevisiae : a symphony of aro genes and aromatic alcohols. J. Agric. Food Chem. 63, 8544–8550. doi: 10.1021/acs.jafc.5b03400
Avbelj, M., Zupan, J., and Raspor, P. (2016). Quorum-sensing in yeast and its potential in wine making. Appl. Microbiol. Biotechnol. 100, 7841–7852. doi: 10.1007/s00253-016-7758-3
Azumi, M., and Goto-Yamamoto, N. (2001). AFLP analysis of type strains and laboratory and industrial strains of Saccharomyces sensu stricto and its application to phenetic clustering. Yeast 18, 1145–1154. doi: 10.1002/yea.767
Barooah, M., Bora, S. S., and Goswami, G. (2020). “Ethnic fermented foods and beverages of Assam,” in Ethnic Fermented Foods and Beverages of India: Science History and Culture, ed J. P. Tamang (Singapore: Springer Nature), 85–104. doi: 10.1007/978-981-15-1486-9_3
Boekhout, T., and Robert, V. (2003). Yeast in Food: Beneficial and Detrimental Aspects. Sawston: Woodhead Publishing, CRC Press.
Buragohain, A. K., Tanti, B., Sarma, H. K., Barman, P., and Das, K. (2013). Characterization of yeast starter cultures used in household alcoholic beverage preparation by a few ethnic communities of Northeast India. Ann. Microbiol. 63, 863–869. doi: 10.1007/s13213-012-0537-1
Chen, H., Fujita, M., Feng, Q., Clardy, J., and Fink, G. R. (2004). Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 101, 5048–5052. doi: 10.1073/pnas.0401416101
Chettri, R. (2012). Microbiological evaluation of turangbai and bekang ethnic fermented soybean foods of North East India. Doctoral thesis. University of North Bengal, India.
Chettri, R., and Tamang, J. P. (2008). Microbiological evaluation of Maseura, an ethnic fermented legume-based condiment of Sikkim. J. Hill Res. 21, 1–7. Available online at: http://14.139.206.50:8080/jspui/handle/1/1369.
Ciani, M., Capece, A., Comitini, F., Canonico, L., Siesto, G., and Romano, P. (2016). Yeast interactions in inoculated wine fermentation. Front. Microbiol. 7:555. doi: 10.3389/fmicb.2016.00555
Cleland, R. E. (2010). “Auxin and Cell Elongation,” in Plant Hormones, ed P. J. Davies (Dordrecht: Springer Netherlands), 204–220. doi: 10.1007/978-1-4020-2686-7_10
Clemente-Jimenez, J. M., Mingorance-Cazorla, L., Martínez-Rodríguez, S., Las Heras-Vázquez, F. J., and Rodríguez-Vico, F. (2005). Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 98, 301–308. doi: 10.1016/j.ijfoodmicro.2004.06.007
Comitini, F., Di Pietro, N., Zacchi, L., Mannazzu, I., and Ciani, M. (2004). Kluyveromyces phaffii killer toxin active against wine spoilage yeasts: purification and characterization. Microbiology 150, 2535–2541. doi: 10.1099/mic.0.27145-0
Compagno, C., Dashko, S., and Piškur, J. (2014). “Introduction to carbon metabolism in yeast,” in Molecular Mechanims in Yeast Carbon Metabolisms, eds J. Piškur and C. Compagno (Berlin, Heidelberg: Springer), 1–19. doi: 10.1007/978-3-662-45782-5_1
Cremer, J., Vatou, V., and Braveny, I. (1999). 2,4-(hydroxyphenyl)-ethanol, an antioxidative agent produced by Candida spp., impairs neutrophilic yeast killing in vitro. FEMS Microbiol. Lett. 170, 319–325. doi: 10.1111/j.1574-6968.1999.tb13390.x
Das, A. J., Deka, S. C., and Miyaji, T. (2012). Methodology of rice beer preparation and various plant materials used in starter culture preparation by some tribal communities of north-east India: a survey. Int. Food Res. J. 19, 101–107. Available online at: http://www.ifrj.upm.edu.my/19%20(01)%202011/(14)IFRJ-2011-137%20Deka.pdf.
Deka, D., and Sarma, G. C. (2010). Traditionally used herbs in the preparation of rice-beer by the Rabha tribe of Goalpara district, Assam. Indian J. Tradit. Knowl. 9, 459–462. Available online at: http://nopr.niscair.res.in/handle/123456789/9773.
Dewan, S., and Tamang, J. P. (2006). Microbial and analytical characterization of Chhu - A traditional fermented milk product of the Sikkim Himalayas. J. Sci. Ind. Res. 65, 747–752. Available online at: http://nopr.niscair.res.in/handle/123456789/30803.
Dewan, S., and Tamang, J. P. (2007). Dominant lactic acid bacteria and their technological properties isolated from the Himalayan ethnic fermented milk products. Antonie Van Leeuwenhoek 92, 343–352. doi: 10.1007/s10482-007-9163-5
El-Tarabily, K. A. (2004). Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 96, 69–75. doi: 10.1046/j.1365-2672.2003.02043.x
Etschmann, M. M. W., Sell, D., and Schrader, J. (2003). Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium. Biotechnol. Lett. 25, 531–536. doi: 10.1023/A:1022890119847
Fleet, G. H. (2003). Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22. doi: 10.1016/S0168-1605(03)00245-9
Fuqua, W. C., Winans, S. C., and Greenberg, E. P. (1994). Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176, 269–275. doi: 10.1128/jb.176.2.269-275.1994
Garde-Cerdán, T., Marsellés-Fontanet, A. R., Arias-Gil, M., Martín-Belloso, O., and Ancín-Azpilicueta, C. (2007). Influence of SO2 on the consumption of nitrogen compounds through alcoholic fermentation of must sterilized by pulsed electric fields. Food Chem. 103, 771–777. doi: 10.1016/j.foodchem.2006.09.018
Ghosh, S., Kebaara, B. W., Atkin, A. L., and Nickerson, K. W. (2008). Regulation of aromatic alcohol production in Candida albicans. Appl. Environ. Microbiol. 74, 7211–7218. doi: 10.1128/AEM.01614-08
Giovannini, C., Straface, E., Modesti, D., Coni, E., Cantafora, A., De Vincenzi, M., et al. (1999). Tyrosol, the major olive oil biophenol, protects against oxidized-LDL- induced injury in Caco-2 cells. J. Nutr. 129, 1269–1277. doi: 10.1093/jn/129.7.1269
González-Marco, A., Jiménez-Moreno, N., and Ancín-Azpilicueta, C. (2010). Influence of nutrients addition to nonlimited-in-nitrogen must on wine volatile composition. J. Food Sci. 75, 206–211. doi: 10.1111/j.1750-3841.2010.01578.x
Granchi, L., Ganucci, D., Messini, A., and Vincenzini, H. (2002). Oenological properties of Hanseniaspora osmophilla and Kloeckera corticis from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res. 2, 403–407. doi: 10.1016/S1567-1356(02)00089-2
Gueguen, Y., Chemardin, P., Arnaud, A., and Galzy, P. (1995). Comparative study of extracellular and intracellular β-glucosidases of a new strain of Zygosaccharomyces bailii isolated from fermenting agave juice. J. Appl. Bacteriol. 78, 270–280. doi: 10.1111/j.1365-2672.1995.tb05026.x
Hesseltine, C. W., Rogers, R., and Winarno, F. G. (1988). Microbiological studies on amylolytic oriental fermentation starters. Mycopathologia 101, 141–155. doi: 10.1007/BF00437031
Hornby, J. M., Jensen, E. C., Lisec, A. D., Tasto, J., Jahnke, B., Shoemaker, R., et al. (2001). Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67, 2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001
Hornby, J. M., and Nickerson, K. W. (2004). Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48, 2305–2307. doi: 10.1128/AAC.48.6.2305-2307.2004
Hosaka, M., Komuro, Y., Yamanaka, M., Yamaura, T., Nakata, H., and Sakai, T. (1998). Fermentability of Sake Moromi Prepared with Shochu, Wine, Brewer's, Alcohol, or Sake strains of Saccharomyces cerevisiae. J. Brew. Soc. Japan 93, 833–840. doi: 10.6013/jbrewsocjapan1988.93.833
Huang, W. C., and Tang, H. C. (2007). “Bacterial and yeast cultures – process characteristics, products, and applications,” in Bioprocessing for Value-Added Products from Renewable Resources, ed S. T. Yang (Amsterdam: Elsevier), 185–223. doi: 10.1016/B978-044452114-9/50009-8
Hutkins, R. W. (2007). Microbiology and Technology of Fermented Foods. Glasgow: Blackwell Publishing. doi: 10.1002/9780470277515
Ivey, M., Massel, M., and Phister, T. G. (2013). Microbial interactions in food fermentations. Annu. Rev. Food Sci. Technol. 4, 141–162. doi: 10.1146/annurev-food-022811-101219
Jay, J. M. (1991). “High-temperature food preservation and characteristics of thermophilic microorganisms” in Modern Food Microbiology. ed J. M. Jay (New York, NY: Van Nostrand, Remheld), 362–407. doi: 10.1007/978-94-011-6480-1_14
Jeyaram, K., Singh, W., Capece, A., and Romano, P. (2008). Molecular identification of yeast species associated with ‘Hamei' - a traditional starter used for rice wine production in Manipur, India. Int. J. Food Microbiol. 124, 115–125. doi: 10.1016/j.ijfoodmicro.2008.02.029
Kabak, B., and Dobson, A. D. (2011). An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 51, 248–260. doi: 10.1080/10408390903569640
Lee, P. R., Chong, I. S. M., Yu, B., Curran, P., and Liu, S. Q. (2012a). Effects of sequentially inoculated Williopsis saturnus and Saccharomyces cerevisiae on volatile profiles of papaya wine. Food Res. Int. 45, 177–183. doi: 10.1016/j.foodres.2011.10.011
Lee, P. R., Saputra, A., Yu, B., Curran, P., and Liu, S. Q. (2012b). Effects of pure and mixed-cultures of Saccharomyces cerevisiae and Williopsis saturnus on the volatile profiles of grape wine. Food Biotechnol. 26, 307–325. doi: 10.1080/08905436.2012.723606
Limtong, S., Sintara, S., and Suwannarit, P. (2002). Yeast diversity in Thai traditional alcoholic starter (Loog-pang). Kasetsart J. 36, 149–158. Available online at: http://kasetsartjournal.ku.ac.th/abstractShow.aspx?param=YXJ0aWNsZUlEPTk2OHxtZWRpYUlEPTc4MQ==.
Lingappa, B. T., Prasad, M., Lingappa, Y., Hunt, D. F., and Biemann, K. (1969). Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans. Science 163, 192–194. doi: 10.1126/science.163.3863.192
Majumdar, R. K. (2020). “Ethnic fermented foods and beverages of Tripura,” in Ethnic Fermented Foods and Beverages of India: Science History and Culture, ed J. P. Tamang (Singapore: Springer Nature), 583–619. doi: 10.1007/978-981-15-1486-9_21
Manzanares, P., Rojas, V., Genovés, S., and Vallés, S. (2001). A preliminary search for anthocyanin-β-D-glucosidase activity in non-Saccharomyces wine yeasts. Int. J. Food Sci. Technol. 35, 95–103. doi: 10.1046/j.1365-2621.2000.00364.x
Mishra, K. B., Hati, S., Brahma, J., Patel, M., and Das, S. (2018). Identification and characterization of yeast strains associated with the fermented rice beverages of Garo Hills, Meghalaya, India. Int. J. Curr. Microbiol. Appl. Sci. 7, 3079–3090. doi: 10.20546/ijcmas.2018.702.371
Nakamura, T., Murakami, T., Saotome, M., Tomita, K., Kitsuwa, T., and Meyers, S. P. (1991). Identification of indole-3-acetic acid in Pichia spartinae, an Ascosporogenous Yeast from Spartina alterniflora marshland environments. Mycologia 83, 662–664. doi: 10.1080/00275514.1991.12026067
Narzary, Y., Brahma, J., Brahma, C., and Das, S. (2016). A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. J. Ethn. Foods 3, 284–291. doi: 10.1016/j.jef.2016.11.010
Nassar, A. H., El-Tarabily, K. A., and Sivasithamparam, K. (2005). Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 42, 97–108. doi: 10.1007/s00374-005-0008-y
Nath, B. J., Mishra, A. K., and Sarma, H. K. (2020b). Assessment of quorum sensing effects of tyrosol on fermentative performance by chief ethnic fermentative yeasts from northeast India. J. Appl. Microbiol. doi: 10.1111/jam.14908
Nath, B. J., Parasar, D. P., Verma, E., Sarma, H. K., and Mishra, A. K. (2019). Assessing the stimulatory effect of indole-3-acetic acid on growth and sustenance of yeasts isolated from traditional fermentative sources maintained by six ethnic communities of Asssam, north-east India. J. Pure Appl. Microbiol. 13, 905–914. doi: 10.22207/JPAM.13.2.27
Nath, B. J., Verma, E., Sarma, H. K., Mishra, A. K., Tanti, B., and Jha, D. K. (2020a). Evaluation of basic fermentation parameters and effective combinations of predominant yeasts from traditional starter materials of indigenous communities from northeast India. J. Am. Soc. Brew. Chem. 78, 219–230. doi: 10.1080/03610470.2020.1739601
Nissen, P., and Arneborg, N. (2003). Characterization of early deaths of non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Arch. Microbiol. 180, 257–263. doi: 10.1007/s00203-003-0585-9
Nissen, P., Nielsen, D., and Arneborg, N. (2003). Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell - cell contact-mediated mechanism. Yeast 20, 331–341. doi: 10.1002/yea.965
Oh, K. B., Miyazawa, H., Naito, T., and Matsuoka, H. (2001). Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 98, 4664–4668. doi: 10.1073/pnas.071404698
Parasar, D. P., Sarma, H. K., and Kotoky, J. (2017). Exploring the genealogy and phenomic divergences of indigenous domesticated yeasts cultivated by six ethnic communities of Assam, India. J. Biol. Sci. 17, 91–105. doi: 10.3923/jbs.2017.91.105
Passoth, V., Fredlund, E., Druvefors, U. Ã., and Schnürer, J. (2006). Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 6, 3–13. doi: 10.1111/j.1567-1364.2005.00004.x
Pérez-Nevado, F., Albergaria, H., Hogg, T., and Girio, F. (2006). Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 108, 336–345. doi: 10.1016/j.ijfoodmicro.2005.12.012
Prusty, R., Grisafi, P., and Fink, G. R. (2004). The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 101, 4153–4157. doi: 10.1073/pnas.0400659101
Renault, P. E., Albertin, W., and Bely, M. (2013). An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 97, 4105–4119. doi: 10.1007/s00253-012-4660-5
Rodríguez, M. E., Lopes, C. A., van Broock, M., Valles, S., Ramón, D., and Caballero, A. C. (2004). Screening and typing of Patagonian wine yeasts for glycosidase activities. J. Appl. Microbiol. 96, 84–95. doi: 10.1046/j.1365-2672.2003.02032.x
Rodríguez-Cousiño, N., Maqueda, M., Ambrona, J., Zamora, E., Esteban, R., and Ramírez, M. (2011). A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded RNA virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl. Environ. Microbiol. 77, 1822–1832. doi: 10.1128/AEM.02501-10
Rojas, V., Gil, J., Piñaga, F., and Paloma, M. (2003). Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 86, 181–188. doi: 10.1016/S0168-1605(03)00255-1
Rul, F., and Monnet, V. (2015). How microbes communicate in food: a review of signaling molecules and their impact on food quality. Curr. Opin. Food Sci. 2, 100–105. doi: 10.1016/j.cofs.2015.03.003
Rutherford, S. T., and Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. doi: 10.1101/cshperspect.a012427
Sarkar, P. K., Tamang, J. P., Cook, P. E., and Owens, J. D. (1994). Kinema - a traditional soybean fermented food - proximate composition and microflora. Food Microbiol. 11, 47–55. doi: 10.1006/fmic.1994.1007
Sha, S. P., Suryavanshi, M. V., Jani, K., Sharma, A., Shouche, Y., and Tamang, J. P. (2018). Diversity of yeasts and molds by culture-dependent and culture-independent methods for mycobiome surveillance of traditionally prepared dried starters for the production of indian alcoholic beverages. Front. Microbiol. 9:2237. doi: 10.3389/fmicb.2018.02237
Sharma, R., and Jangid, k. (2015). “Fungal quorum sensing inhibitors,” in Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight, ed V. C. Kalia (New Delhi: Springer), 237–257. doi: 10.1007/978-81-322-1982-8_20
Shrestha, H., Nand, K., and Rati, E. R. (2002). Microbiological profile of murcha starters and physico-chemical characteristics of Poko, a rice based traditional fermented food product of Nepal. Food Biotechnol. 16, 1–15. doi: 10.1081/FBT-120004198
Skandamis, P. N., and Nychas, G. J. E. (2012). Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 78, 5473–5482. doi: 10.1128/AEM.00468-12
Smukalla, S., Caldara, M., Pochet, N., Beauvais, A., Guadagnini, S., Yan, C., et al. (2008). FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell 135, 726–737. doi: 10.1016/j.cell.2008.09.037
Sprague, G. F. Jr, and Winans, S. C. (2006). Eukaryotes learn how to count: quorum sensing by yeast. Genes Dev. 20, 1045–1049. doi: 10.1101/gad.1432906
Steward, G. G. (2017). The production of secondary metabolites with flavour potential during brewing and distilling wort fermentations. Fermentation 3:63. doi: 10.3390/fermentation3040063
Swangkeaw, J., Vichitphan, S., Butzke, C. E., and Vichitphan, K. (2011). Characterization of β-glucosidases from Hanseniaspora sp. and Pichia anomala with potentially aroma-enhancing capabilities in juice and wine. World J. Microbiol. Biotechnol. 27, 423–430. doi: 10.1007/s11274-010-0474-8
Tamang, B., and Tamang, J. P. (2007). Role of lactic acid bacteria and their functional properties in goyang, a fermented leafy vegetable product of the Sherpas. J. Hill Res. 20, 53–61. Available online at: http://14.139.206.50:8080/jspui/handle/1/5995.
Tamang, J. P. (2005). Food Culture of Sikkim. Sikkim Study Series Volume IV. Information and Public Relations Department, Government of Sikkim, Gangtok, 120.
Tamang, J. P., and Sarkar, P. K. (1995). Microflora or murcha: an amylolytic fermentation starter. Microbios 81, 115–122.
Tamang, J. P., Watanabe, K., and Holzapfel, W. H. (2016). Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 7:377. doi: 10.3389/fmicb.2016.00377
Tanti, B., Gurung, L., Sarma, H. K., and Buragohain, A. K. (2010). Ethnobotany of starter cultures used in alcohol fermentation by a few ethnic tribes of Northeast India. Indian J. Tradit. Knowl. 9, 463–466. Available online at: http://nopr.niscair.res.in/handle/123456789/9774.
Teramoto, Y., Yoshida, S., and Ueda, S. (2002). Characteristics of a rice beer (zutho) and a yeast isolated from the fermented product in Nagaland, India. World J. Microbiol. Biotechnol. 18, 813–816. doi: 10.1023/A:1021293804327
Thanzami, K., and Lalhlenmawia, H. (2020). “Ethnic fermented foods and beverages of Mizoram,” in Ethnic Fermented Foods and Beverages of India: Science History and Culture, ed J. P. Tamang (Singapore: Springer Nature), 435–457. doi: 10.1007/978-981-15-1486-9_16
Thapa, N., Pal, J., and Tamang, J. P. (2004). Microbial diversity in ngari, hentak and tungtap, fermented fish products of North-East India. World J. Microbiol. Biotechnol. 20:599. doi: 10.1023/B:WIBI.0000043171.91027.7e
Thapa, N., Pal, J., and Tamang, J. P. (2006). Phenotypic identification and technological properties of lactic acid bacteria isolated from traditionally processed fish products of the Eastern Himalayas. Int. J. Food Microbiol. 107, 33–38. doi: 10.1016/j.ijfoodmicro.2005.08.009
Thapa, N., and Tamang, J. P. (2020). “Ethnic fermented foods and beverages of Sikkim and Darjeeling Hills (Gorkhaland Territorial Administration)” in Ethnic Fermented Foods and Beverages of India: Science History and Culture, ed J. P. Tamang (Singapore: Springer Nature), 479–537. doi: 10.1007/978-981-15-1486-9_18
Thapa, S. (2001). Microbiological and biochemical studies of indigenous fermented cereal-based beverages of the Sikkim Himalayas. Doctoral thesis. Sikkim Government college, North Bengal University, India.
Tiwari, S. C., Singh, T. G., and Sarma, H. K. (2014). Occurrence and diversity of phenotypically distinct yeast strains isolated from starter cultures used in alcoholic fermentation by two ethnic tribes of Arunachal Pradesh. Int. J. Biosci. 4, 212–219. doi: 10.12692/ijb/4.1.212-219
Tsuyoshi, N., Fudou, R., Yamanaka, S., Kozaki, M, Tamang, N., Thapa, S., et al. (2005). Identification of yeast strains isolated from marcha in Sikkim, amicrobial starter for amylolytic fermentation. Int. J. Food Microbiol. 99, 135–146. doi: 10.1016/j.ijfoodmicro.2004.08.011
Wahengbam, R., Thangjam, A. S., Keisam, S., Asem, I. D., Ningthoujam, D. S., Jeyaram, et al. (2020). “Ethnic fermented foods and alcoholic beverages of Manipur,” in Ethnic Fermented Foods and Beverages of India: Science History and Culture, ed J. P. Tamang (Singapore: Springer Nature), 349–419. doi: 10.1007/978-981-15-1486-9_14
Wang, H., Dong, Q., Guan, A., Meng, C., Shi, X., and Guo, Y. (2011). Synergistic inhibition effect of 2-phenylethanol and ethanol on bioproduction of natural 2-phenylethanol by Saccharomyces cerevisiae and process enhancement. J. Biosci. Bioeng. 112, 26–31. doi: 10.1016/j.jbiosc.2011.03.006
Xufre, A., Albergaria, H., Inácio, J., Spencer-Martins, I., and Gírio, F. (2006). Application of fluorescence in situ hybridisation (FISH) to the analysis of yeast population dynamics in winery and laboratory grape must fermentations. Int. J. Food Microbiol. 108, 376–384. doi: 10.1016/j.ijfoodmicro.2006.01.025
Yanai, T., and Sato, M. (1999). Isolation and properties of β-glucosidase produced by Debaryomyces hansenii and its application in winemaking. Am. J. Enol. Vitic. 50, 231–235.
Ye, M., Yue, T., and Yuan, Y. (2014). Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 14, 873–882. doi: 10.1111/1567-1364.12175
Keywords: ethnic fermentation, yeast, diversity, quorum sensing, alcoholic beverage
Citation: Nath BJ, Parasar DP and Sarma HK (2021) Linking the Diversity of Yeasts Inherent in Starter Cultures to Quorum Sensing Mechanism in Ethnic Fermented Alcoholic Beverages of Northeast India. Front. Sustain. Food Syst. 5:678045. doi: 10.3389/fsufs.2021.678045
Received: 08 March 2021; Accepted: 24 May 2021;
Published: 28 June 2021.
Edited by:
Avinash Sharma, National Centre for Cell Science, IndiaReviewed by:
Mangesh Vasant Suryavanshi, Yenepoya University, IndiaCopyright © 2021 Nath, Parasar and Sarma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hridip Kumar Sarma, aHJpZGlwQGdhdWhhdGkuYWMuaW4=
†ORCID: Bhaskar Jyoti Nath orcid.org/0000-0002-6536-8063
Deep Prakash Parasar orcid.org/0000-0002-0692-2861
Hridip Kumar Sarma orcid.org/0000-0002-0786-9222
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.