- 1Noble Research Institute, LLC, Ardmore, OK, United States
- 2Department of Crop Sciences, University of Illinois, Urbana, IL, United States
- 3Department of Agronomy, Iowa State University, Ames, IA, United States
- 4Environmental Defense Fund, Raleigh, NC, United States
- 5Plant Production Systems group, Department of Plant Sciences, Wageningen University, Wageningen, Netherlands
- 6Department of Plant Sciences, University of California, Davis, Davis, CA, United States
- 7College of Resources and Environmental Sciences, China Agricultural University, Beijing, China
- 8Department of Tropical Plant and Soil Sciences, University of Hawaii at Manoa, Honolulu, HI, United States
- 9School of Biotechnology, Guru Gobind Singh Indraprastha University, New Delhi, India
- 10W. K. Kellogg Biological Station and Department of Plant, Soil, and Microbial Sciences, Michigan State University, Hickory Corners, MI, United States
- 11School of Agriculture and Food Sciences, The University of Queensland, Brisbane, QLD, Australia
- 12School of Biological Sciences, Washington State University, Pullman, WA, United States
- 13Institute of Biological Chemistry, Washington State University, Pullman, WA, United States
Nitrogen (N) is an essential but generally limiting nutrient for biological systems. Development of the Haber-Bosch industrial process for ammonia synthesis helped to relieve N limitation of agricultural production, fueling the Green Revolution and reducing hunger. However, the massive use of industrial N fertilizer has doubled the N moving through the global N cycle with dramatic environmental consequences that threaten planetary health. Thus, there is an urgent need to reduce losses of reactive N from agriculture, while ensuring sufficient N inputs for food security. Here we review current knowledge related to N use efficiency (NUE) in agriculture and identify research opportunities in the areas of agronomy, plant breeding, biological N fixation (BNF), soil N cycling, and modeling to achieve responsible, sustainable use of N in agriculture. Amongst these opportunities, improved agricultural practices that synchronize crop N demand with soil N availability are low-hanging fruit. Crop breeding that targets root and shoot physiological processes will likely increase N uptake and utilization of soil N, while breeding for BNF effectiveness in legumes will enhance overall system NUE. Likewise, engineering of novel N-fixing symbioses in non-legumes could reduce the need for chemical fertilizers in agroecosystems but is a much longer-term goal. The use of simulation modeling to conceptualize the complex, interwoven processes that affect agroecosystem NUE, along with multi-objective optimization, will also accelerate NUE gains.
Introduction
Protein availability has shaped human settlement across the globe for millennia (Diamond, 1997). The development of agriculture as a reliable, localized source of protein and other foodstuffs enabled civilization as we know it, including the scientific enterprise that now underpins it. Nitrogen (N) is a crucial element of proteins and other essential biomolecules, and soil N availability is a key limiting factor for both natural and agricultural productivity in terrestrial ecosystems (O'Neill et al., 2004; Elser et al., 2007). Development of the Haber-Bosch process to convert atmospheric dinitrogen (N2) to ammonia (NH3) in the early twentieth century gave rise to the N fertilizer industry. This synthetic N fertilizer, together with improved crop genetics and agronomy, fueled the Green Revolution that led to several-fold increases in crop productivity in many parts of the world, averting starvation for large numbers of people (Smil, 2002; Godfray et al., 2010). Today, over 120 Tg (million metric tons) of synthetic N fertilizer are used in agriculture each year (FAO, 2019). In 2010, total N inputs from synthetic N fertilizers, biological N fixation (BNF) by leguminous crops, atmospheric deposition, and manure amounted to 174 Tg N, yet only 74 Tg N were captured in harvested products (Zhang et al., 2015). Much of the remaining N is lost from agricultural land to the surrounding environment, where it damages sensitive ecosystems, reduces air quality, and contributes to climate change, with costs to biodiversity, fisheries, human health, and societal infrastructure (Sutton et al., 2013). Anthropogenic reactive-N compounds are readily assimilated by plants and other organisms, and have doubled the flux of N in the global N cycle, taking us beyond what is considered a safe operating space for humanity (Rockstrom et al., 2009; Canfield et al., 2010; Steffen et al., 2015; Kanter et al., 2020). The United Nations (UN) Environment Programme has identified excessive reactive N as one of five emerging threats facing the planet (Sutton et al., 2019), such that the fourth UN Environment Assembly (March 2019) adopted a resolution on “Sustainable N management.”
Fertilizer N is a “double-edged sword” that ensures food security for much of humanity, while having enormous negative impacts on the environment and human health (Sutton et al., 2011). Globally, it is also the single largest source of nitrous oxide (N2O), a potent (~300-times the global warming potential of carbon dioxide) and long-lived greenhouse gas (Winiwarter et al., 2018) that has increased by 0.8 parts per billion (ppb) per annum from 300 ppb in 1980 to 332 ppb in 2020 (NOAA, 2021). Thus, in many agricultural systems too much N is used and there is a need to rein-in the amounts of N fertilizer used to reduce environmental pollution. In contrast, in regions such as the sub-Saharan Africa, limited access to N fertilizer results in poor crop yields and food insecurity (Mueller et al., 2012), requiring increased N inputs from either biological or industrial sources. Overall, cropping systems worldwide would benefit from practices that increase agricultural N use efficiency (NUE) while sustaining or building soil organic matter and soil fertility.
Nitrogen use efficiency is an umbrella term to broadly compare agronomic, physiological, and environmental effects of N use in agroecosystems. At least 18 numerical permutations of NUE appear in the literature (Ladha et al., 2005), which highlights that there is no standard definition. Three NUE terms are widely used to quantify efficiency: (1) partial factor productivity (PFP), defined as the ratio of yield to N inputs from fertilizer, BNF, crop residues, manure, and atmospheric deposition (Figure 1); (2) N removal efficiency, defined as the ratio of harvested plant N to N inputs; and (3) N utilization efficiency (NUtE), defined as the ratio of yield to plant N. Other terms such as system NUE (Martinez-Feria et al., 2018) and N surplus or balance (McLellan et al., 2018; Basso et al., 2019) have also proved useful for assessing environmental and production outcomes. We focus on N removal efficiency as it is amongst the easiest to measure and calculate from data available from many crop species and countries. This definition of NUE allows for global comparisons, trend analysis, identification and diffusion of best practices, and provides the impetus for this article. The average NUE of cropping systems has been estimated to be only 42% globally (Zhang et al., 2015), and further losses of N occur along the food chain before it is consumed. Boosting NUE is one of four major areas of intervention identified to reduce N losses to the environment; the other three are dietary shifts toward more plant-based foods in high-income countries, reductions in food loss and waste, and reduced biofuel production from human-edible foods (Bodirsky et al., 2014; Cassman and Grassini, 2020). Here, we focus on opportunities to increase NUE by improving technologies and agricultural management strategies for high-input and low-input cropping systems.
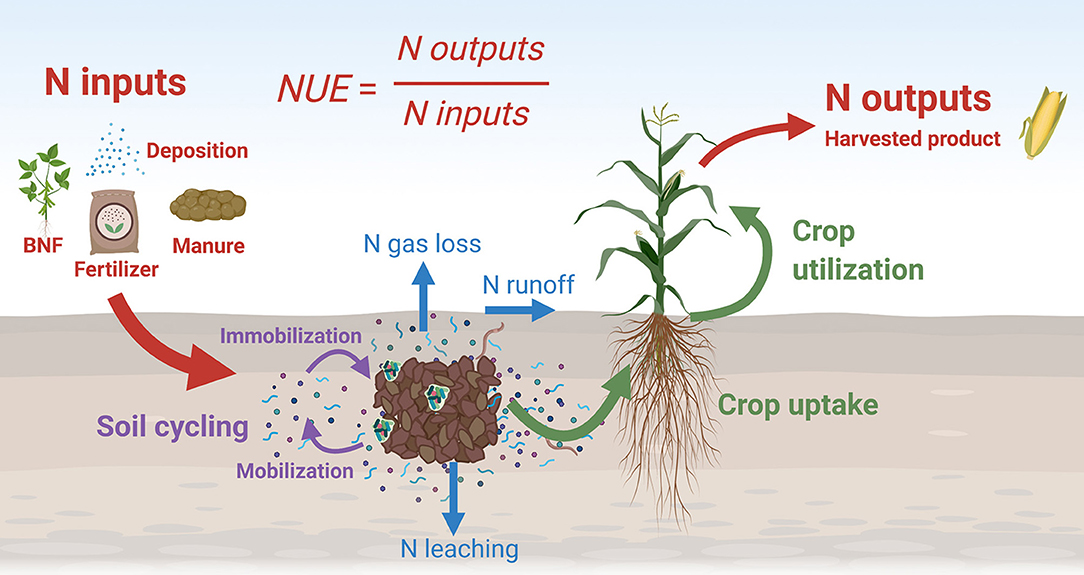
Figure 1. Nitrogen use efficiency (NUE) is the ratio of N outputs in the harvested product to N inputs, including from fertilizer and natural processes, and has also been called N removal efficiency. Nitrogen inputs to the system (red arrow, BNF is biological nitrogen fixation) will cycle through the soil (purple arrows), are susceptible to environmental losses (blue arrows), and are eventually used by crops (green arrows) with a portion harvested or removed (red arrow). We propose simultaneously targeting these different processes to increase NUE and agricultural sustainability.
Current Status of NUE From a Global Perspective
Globally, NUE for major crops averaged between 30% and 50% from 1970 to 2010 (Smil, 1999; Sheldrick et al., 2002; Tilman et al., 2002; Ladha et al., 2005; Liu et al., 2010; Robinson et al., 2011). While NUE improved significantly in parts of Europe and the USA from 1980 to 2010 due to increases in productivity for a given N rate (e.g., USA) or coupled with decreases in N use (e.g., Europe; Lassaletta et al., 2014), it decreased (with high N surplus) in many developing countries, notably China and India, from 1960 to 2014 due to increases in N fertilizer use exceeding N harvest rates (Lassaletta et al., 2014; Zhang et al., 2015; He et al., 2018). Trends of NUE in other regions with low yields and low N fertilization rates have been inconsistent, such as in many countries in sub-Saharan Africa (Lassaletta et al., 2014).
The NUE of cropping systems ranges from as little as 14% for fruits and vegetables, because they are relatively low-protein but high-value foods (motivating farmers to use large amounts of fertilizer), to as high as 80% for legumes, as exemplified by soybean (Zhang et al., 2015). Most of the N assimilated by legumes is derived from BNF by symbiotic bacteria (rhizobia) within plant root nodules, and much of this N is transferred eventually to harvested grains rather than lost to the surrounding environment (Córdova et al., 2019). Cereal NUE falls between these extremes, with the three primary cereals, wheat, rice, and maize, exhibiting global averages of 42%, 39%, and 46% NUE, respectively, in 2010. Because cereal production accounts for approximately half of all N fertilizer use and half of all agricultural N pollution, it represents a high priority target for NUE improvement. For individual cropping systems, NUE varies widely, and highly mechanized, broad-acre, precision agriculture generally achieves higher NUE than some small-holder farms in countries such as China and India that are over-supplied with highly-subsidized synthetic N fertilizer (Abrol et al., 2017). Thus, there are opportunities to increase NUE in many agricultural systems by applying up-to-date best practices with appropriate support from social, economic, and environmental policies (Kanter et al., 2020).
Agricultural N Flow and Soil Cycling
The view of soil as principally a support medium for plants, rather than a complex biogeochemical system driven by soil biota, dominates soil management decisions in agronomic practice globally. To a large extent, the success of the Green Revolution is based on new technologies that provide, via inputs external to the system, ecological services traditionally supplied by soil—N supply among them (Robertson and Grandy, 2006). The result has been an agricultural enterprise that often values soil largely as a porous media that supports plants and drains excess rainfall, ignoring its crucial role in nutrient cycle regulation. Agricultural N flows are illustrated in Figure 1, in which nitrogen inputs enter the system through synthetic fertilizer, biological nitrogen fixation, manure, or atmospheric deposition. This nitrogen, in various chemical forms, enters the soil N cycle with pools and fluxes from soil organic matter, microbes, and dissolved N molecules in soil water. Plant uptake of N is mostly from inorganic forms in the soil water. This N is utilized by the plant for growth and seed production, and some amount is harvested with the agricultural product and removed from the system.
Less than 50% of the N taken up by most cereal crops is derived from N inputs from that planting season, a percentage unchanged from the 1930s to 2010 (Allison, 1955; Cassman et al., 2002; Gardner and Drinkwater, 2009). The remaining N is derived from soil organic matter, crop residues or residual inorganic N present in the system, itself the product of soil biota partially consuming plant inputs dating from last year to past millennia. Soil organic N is a significant contributor to plant nutrition, satisfying much of the plant N demand (Olson et al., 1979; Sowers et al., 1994; Corbeels et al., 1999; López-Bellido et al., 2006; Jayasundara et al., 2007). Even high-yielding soybean can derive a significant amount of its N from the soil (Salvagiotti et al., 2008, 2009), particularly where high amounts of residual inorganic N limit BNF. Although soil N mineralization can provide sufficient N to support the N demands of modern cropping systems in some individual years (Lory and Scharf, 2003), over the long-term, it is apparent from using simple mass balance calculations that a highly productive maize crop with an annual grain yield of 16 Mg ha−1 removes ~184 kg N ha−1 (Tenorio et al., 2020) or ~3.68 Mg N over 20 years of cropping. The N stores of many highly productive, rainfed arable soils can be as high as 10 Mg N ha−1. Thus, continuous cropping has the potential to remove, within 20 years, 1/3 of the N in the original soil organic N stock, demonstrating the potential for rapid soil organic matter depletion and the consequent dependence of cropping systems on external N sources in order to mitigate such risks (Poffenbarger et al., 2017). This is why 'zero budget natural farming', as promoted by the Indian government and some international agencies, carries a serious risk of long-term soil nutrient mining and soil health decline (Smith et al., 2020).
Regardless of the source of N, whether derived from N2 fixation, fertilizer, or other exogenous source, N cycles through soil, and the rate and manner in which it cycles matters fundamentally to its availability and loss (Figure 1). Soil has the capacity to not only provide plant-available N through soil organic matter turnover, but also to buffer its supply to plants—whether internal N or exogenous N—and control the loss of unused N to the environment by storing N as organic matter or binding N in the soil mineral matrix. As a result, accounting for soil N is integral to optimizing N use (Stanford, 1973; Cassman et al., 2002). Perhaps the single most important impact of the soil N cycle on NUE is the soil's capacity for helping to match the timing of soil N availability with periods of plant N demand. In natural ecosystems, the presence of diverse plant species having different life histories, including perenniality, means that at least some species will be actively demanding N whenever soil conditions permit N release from soil organic matter. The result is a relatively tight N cycle: when N available for loss is instead taken up by plants, loss is at least partially averted. For example, fertilized perennial biofuel crops lose little-to-no nitrate-()-N to drainage, yet loss in drainage of fertilized corn (maize) is no different from unfertilized soybean (Christianson et al., 2012; Daigh et al., 2015). This highlights that a lack of synchrony between crop N demand and soil N availability is the primary reason for environmental N losses.
Strategies to Increase NUE
How can the agricultural N flow be managed to improve NUE? The various processes depicted in Figure 1 serve as reminders of the major interventions that can be used as levers to adjust the flow of N through the system in such a way as to minimize inputs and losses while maximizing N capture and output. We held a workshop in 2019 to discuss problems associated with N in different agricultural cropping systems and to examine promising research and development (R&D) avenues to solve these problems. Four broad areas of needed endeavor were identified: soil N cycling, systems agronomy, BNF, and plant breeding as shown in Figure 2. Strategies in each of these areas were organized into a matrix from low-to-high risk and low-to-high reward (Table 1). In the sections below, we review briefly the state-of-the art in these areas, consider current frontiers in R&D, and propose an integrated set of strategies to increase by 2050 global cropping system NUE and protein yield by 50%, while simultaneously reducing N losses from crop production by 50%.
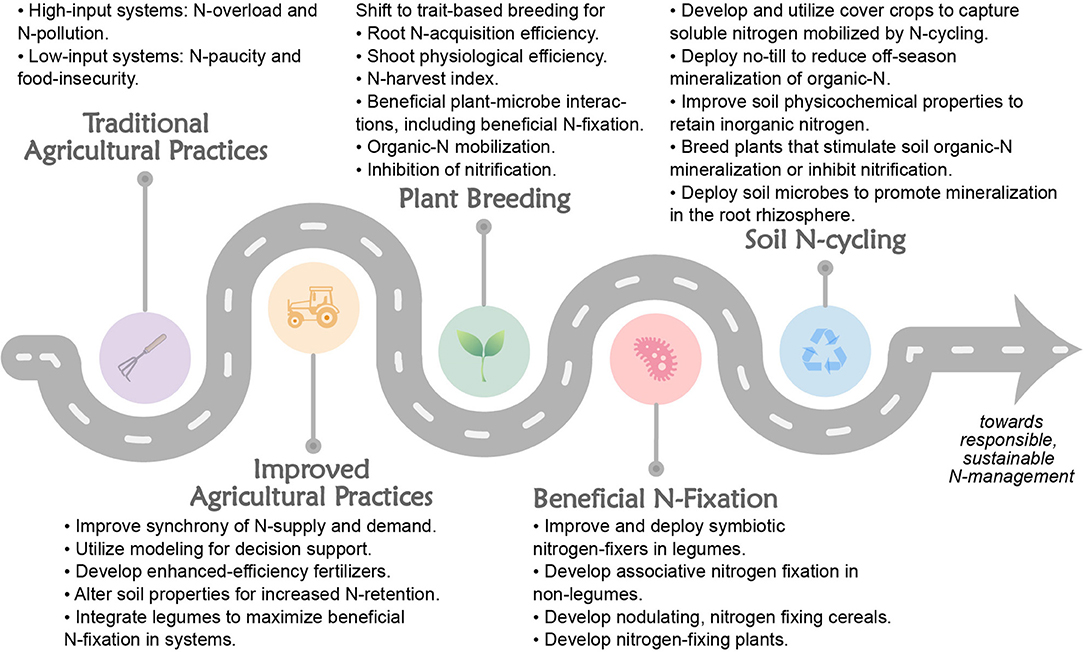
Figure 2. We propose a systematic roadmap for improving NUE using improved agronomy (i.e., agricultural practices), plant breeding, biological N fixation, and controlled soil N cycling.
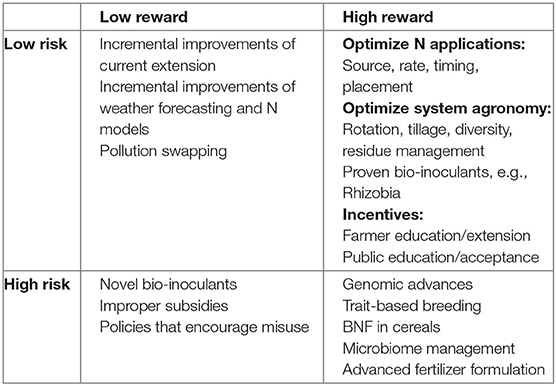
Table 1. A risk-reward matrix organizes possible solutions to the nitrogen problem in four groups based on high or low risk and high or low reward.
Improving Synchrony of Soil N Supply and Crop Demand
As discussed above, N is used most efficiently when its availability in soil is synchronized with crop demand (Robertson, 1997; Ladha et al., 2005). Nitrogen synchrony is rare and difficult to achieve in annual monocultures typical of most high-productivity agriculture. Most grain crops, for example, have a 90–100 day growing season and accumulate biomass and N at a significant rate only for 30–40 days mid-season. In the maize example above, N uptake can reach the astonishing rate of 5 kg N ha−1 day−1 (Osterholz et al., 2017). This high rate is sustained for only 3–4 weeks and it falls to nil quickly. Meanwhile, soil microbial processes that cycle N between various organic and inorganic forms are active whenever soils are not too dry or too cold to support biological activity, i.e., much of the rest of the year. This asynchrony between when N is available and when N is needed creates windows of N loss and is a principal cause of low NUE in most cropping systems (Robertson, 1997; Robertson and Vitousek, 2009).
Depending upon cropping system and environment, achieving synchrony can be challenging as a result of variable weather, timing of equipment and labor availability, and other limitations and sources of uncertainty. First, plant N demand can be difficult to predict based on the data available at fertilizer decision time points and possible future weather scenarios. Nitrogen demand can strongly vary across complex landscapes in a given year and even from year-to-year in a single location (Basso et al., 2019). Second, estimating the N-supplying power of the system is difficult, particularly in mesic climates where N inputs from mineralization of soil organic matter and N outputs/losses to denitrification (reduction of and other oxides of N to N2) and leaching into groundwater exhibit large variation from year-to-year and field-to-field. Addressing these issues inherent to cropping systems remains challenging. The most commonly used N fertilizers readily dissolve into soil solution as mobile N ions (ammonium or ; ) that are subject to loss if not acquired by crops or retained by soils. Low NUE characterizes crop systems where (i) transient or permanent N supply exceeds crop demand (for example when low crop N demand coincides with large amounts of soluble N in soil); (ii) soil has a low capacity for N retention due to low organic matter content, coarse texture, and/or presence of weathered clay minerals with low ion exchange capacity; and (iii) climate and agronomic management promote N loss when there is high rainfall, heavy irrigation, or temporary waterlogging.
The difficulty in synchronizing N supply and demand is exemplified by mechanized sugarcane cropping, where farmers apply all fertilizer early in the crop season because crop height and summer rain prevent field access later. Sugarcane grows over 10 months or longer. Large pools of soluble N, high rainfall and/or irrigation, and an initial low crop N demand drive N losses from sugarcane soils in the first months (Allen et al., 2010). To compensate for this risk, sugarcane farmers in the main producer countries apply, on average, twice as much N fertilizer as is required by the crop (Robinson et al., 2011). The range of N fertilizer use spans from near perfect use of fertilizer N at 100% (Thailand), 60% (Brazil) to only 10% (China) (Robinson et al., 2011). Fertilizer timing with most or all fertilizer applied before the cropping season is also common with maize in the US Corn Belt, where crop height and wet fields can also hamper in-season applications.
There are many management options available for increasing NUE through matching N supply with crop demand and thereby mitigating loss of N from agroecosystems. Many tools and best management practices are intended to help farmers apply nutrients in a “4R” management framework—using the right N source at the right rate, right time and in the right place. Basic, or low-tech, approaches involve adjusting timing or rates without needing different equipment. Beyond that, a range of precision N tools that detect chlorophyll and other crop vigor-related measures (e.g., hand-held and remote spectral sensors) and agronomic techniques (e.g., variable rate fertilizer applications, direct drilling, deep placement of fertilizer, and fertigation) combined with weather forecasting are now available to support improved nutrient management.
Spatial synchrony can be as important as temporal synchrony for matching soil N availability to plant demand. This is true both at the plant scale, ensuring soil N is most available close to growing plants, as occurs with furrow mulching and fertilizer banding, and at the field scale, where erosion and other geomorphological processes have created subfield regions of low fertility. Using satellite images with 30 × 30 m subfield resolution in 8 M cornfields across 30 Mha of the U.S. Midwest, Basso et al. (2019) identified that low-yield subfield areas covering over half of the region had low NUE (as little as 48% N removed with harvest), in contrast with high-yield, high-NUE areas (up to 88% fertilizer N removed with harvest). The N losses from low-yield areas could explain a major portion of the average annual 1.12 Tg -N loss from the Mississippi Basin (Basso et al., 2019). Spatial synchrony can be partially optimized using precision agriculture techniques, discussed below.
Although much progress has been made in developing technologies for efficient N management, good systems agronomy is critical to enhance crop N harvest and NUE with less surplus N (Chen et al., 2011, 2014; Grassini and Cassman, 2012). While certain approaches have relatively higher relevance and impact in different regions and crops, the most successful are those that adopt a “systems approach” that considers the complexity and interactions between practices, weather, and outcomes. Holistic management approaches will maximize crop N uptake, minimize N losses, and optimize endogenous soil N supply by maintaining soil health.
Fertilizer Type, Inhibitors, and Manure Management
New fertilizer formulations are a target for improving NUE of crop systems, primarily aiming to slow solubilization and the conversion of fertilizer N to more mobile forms while plant N demand is low. Globally, efforts are accelerating to improve N fertilizers, from nanotechnology formulations to achieve targeted release profiles, to supplying N as part of organic matter to slow the N release (Bindraban et al., 2015). Fertilizers and application technologies are being designed to take the physiological needs of crops (such as nutrient uptake, redistribution, and utilization) as an entry point for fertilizer development (Bindraban et al., 2020). Enhanced efficiency fertilizers (EEFs) are formulations with coatings that consist of polymers or other materials that prevent immediate solubilization, or with added inhibitors to temporarily slow the activity of urease enzymes and that of nitrifying microbes. Several meta-analyses have reported small, but consistent, positive yield responses to N fertilizers treated with urease inhibitors, nitrification inhibitors, or a combination of both (Linquist et al., 2013; Abalos et al., 2014; Burzaco et al., 2014; Qiao et al., 2015; Thapa et al., 2016; Li et al., 2018). The variability in yield response to these treatments has been attributed to interactions among genetics, environment, and management (Hatfield and Walthall, 2015). EEFs containing urease inhibitors were successful in paddy rice systems, increasing average NUE by 29% and reducing N losses by 41%, while the various types of EEFs in wheat and maize systems are generally less effective (Li et al., 2018), and yield responses may be site-specific (Linquist et al., 2013; Abalos et al., 2014; Thapa et al., 2016). However, meta-analyses have also exposed possible pollution swapping when applying nitrification inhibitors, where a decrease in N2O emissions (Qiao et al., 2015; Pan et al., 2016; Li et al., 2018) coincides with an increase in NH3 volatilization (Lam et al., 2017; Li et al., 2018). Of additional concern is that some enzyme inhibitors can be transported to surface waters (Woodward et al., 2016) and non-biodegradable polymer coatings can impact soil biota such as earthworms (Huerta Lwanga et al., 2016). Alternative slow-release fertilizer formulations are being developed, for example with biodegradable polymers that soil microbes can consume (Zumstein et al., 2018).
Crop N physiology must be considered in all N fertilizer regimes. While and NH are considered the main N sources for crops, all plants that have been examined can use organic N (e.g., amino acids, oligopeptides). The exact proportion of inorganic and organic N acquired by plants remains unknown (reviewed by Näsholm et al., 2009), but the presence of soluble organic N in soils is well-documented. Soluble organic N is associated with reduced losses, which might motivate the development of alternative, organic N-based fertilizers, nutrient management, and crop breeding (reviewed by Paungfoo-Lonhienne et al., 2012). Comparing the fluxes of inorganic and organic N forms in differently fertilized sugarcane soil, the estimated root intake rate for amino acids matched soil fluxes, while fluxes of NH and exceeded the root intake rate (Brackin et al., 2015). To maximize NUE, the release rate and forms of N should match the crop's N acquisition capacity (discussed below).
Organic matter, including recycled organic wastes (i.e., manures, crop residues, green waste, humanure), has potential as an N source, and is widely used, though not always with the aim to supply nutrients (Bindraban et al., 2015, 2020). The overall effects of organic fertilizers are difficult to disentangle because soil physical, chemical, and biological properties are altered. Compared to inorganic fertilizer only, field experimentation often shows benefits when organic and inorganic fertilizers are supplied together due to interacting effects of improved micronutrient and soil microbial community status (e.g., Adediran et al., 2007). In a global meta-analysis, Xia et al. (2017) found that substituting up to 50% of the mineral fertilizer N with fresh or composted manure increased grain crop yields, crop N uptake, and N use efficiency, but substituting more than 75% of mineral fertilizer N with manure negatively impacted yields. The authors also reported environmental benefits of integrative management, including a reduction in N losses (e.g., leaching, runoff, and NH3 emission) and improvement in soil organic carbon content. On the other hand, regional trends for NUE in the USA are negatively associated with the proportion of total N from livestock excreted N, largely because manure is treated as waste rather than a nutrient (Swaney et al., 2018). Net anthropogenic N balances for these regions are also high, indicating elevated risk of environmentally-concerning losses (Howarth et al., 2012). Thus, there is both need and opportunity to repurpose nutrient-rich wastes as fertilizers, which requires formulating suitable nutrient stoichiometry and N release profiles to avoid N over- or under-supply of target crops.
Soil Management for Controlling N Processes
Managing the release of N from crop residue and as well from endogenous soil organic matter stores during the growing season is a difficult proposition. Tillage, developed primarily for weed control, has been traditionally used for this purpose but inefficiently so—tillage occurs 6–8 weeks prior to high plant demand for N, leaving a significant intervening window for N loss as accelerated microbial activity mineralizes soil organic N stores. A purported advantage of no-till is to avoid this quick release of N, and while subsequently slower mineralization rates avoid the early pulses of N associated with tillage, there is little evidence that no-till reduces exogenous N needs and, by inference, improves NUE. No-till does, however, appear to reduce off-season N losses (e.g., Syswerda et al., 2012 and references therein) as more of the N immobilized in crop residues persists in accumulating soil organic matter until a new equilibrium is met. Improvements to soil porosity and other physical properties related to soil structure can also keep inorganic N from being easily leached (Hess et al., 2019), though beneficial effects on gaseous N losses are less clear (van Kessel et al., 2013; Gelfand et al., 2016).
Improving the adsorption capacity of soils, or the ability to bind ions to soil components, is another approach used to control soil N cycling. NUE of maize and rice systems improved substantially when adding clay, such as zeolite, most likely due to enhanced retention (Perrin et al., 1998; Kavoosi, 2007). Whether these ameliorants, or sorbents, such as zeolites or biochar, are effective agronomic treatments will depend on the system's vulnerability to N losses. Experiments in controlled conditions have shown that sorbents can optimize release considerably (Chin et al., 2018).
Other technological interventions currently available include plastic mulch, now in widespread use in parts of China (Liu et al., 2003; Yang et al., 2015). While developed as a water conservation measure, plastic also heats soil and thereby accelerates N mineralization as the growing season progresses (Fan et al., 2005). However, the long-term application of plastic mulching can lead to pollution that damages soil health and threatens long-term food security (Zhang et al., 2019).
Manipulating the microbes responsible for soil N cycling could be a fruitful frontier for future research. We are only now learning the functional importance of the myriad plant-microbe associations that form the plant microbiome, and emerging evidence that plants can stimulate rhizosphere (the soil adjacent to and influenced by roots) microbes to oxidize soil carbon (Kumar et al., 2016) implies that plants might also be bred or engineered to harness the microbiome for N release. Or that the microbiome might be intentionally composed to include microbes capable of mineralizing soil organic N when triggered by plant N demand. Additionally, slow-release N fertilizers or even cover crops might be created with an enhanced ability to release organic N when similarly triggered.
N Management in Flooded Soils
Management of fertilizer N in paddy soils is notoriously difficult due to loss of N through NH3 volatilization from the floodwaters. The deep placement of urea granules is one technology to enhance N capture by rice and reduce losses, although demanding in terms of labor (Giller et al., 2004; Liu et al., 2005). Climate mitigation practices to reduce methane emissions from paddy rice call for periodic drainage (Wassmann et al., 2004), which will make N management all the more difficult as organic N mineralizes to NH that will then undergo nitrification to during drained periods with subsequent loss via denitrification upon re-wetting. Losses can be mitigated with management that avoids the presence of excessive mineral N pools in the soil at these high risk time periods, such as split N applications, retaining crop residue, and keeping N balances in check.
Precision Agriculture and NUE
Precision agriculture is a farm management approach that seeks to identify practices that optimize the use of farm inputs (Mulla, 2013). As a result, precision management relies on technologies that enable intensive data collection, processing, and evaluation needed to properly characterize and synthesize temporal and spatial variability. Theoretically, the variance in yield and environmental outcomes is attributable to measurable climatic, edaphic, and management factors. Precision agriculture is not exclusively focused upon N management, but improving NUE is a common goal given the potential variance of crop N demand across the landscape and with time. Site-specific management can help tailor N applications, improve NUE, increase profits, and/or minimize risk of N loss (Balafoutis et al., 2017; Cao et al., 2017; Muschietti-Piana et al., 2018; McNunn et al., 2019; Wang et al., 2020). NUE, as a performance outcome, can also be used to evaluate management decisions in fields characterized by high spatial and temporal variability in biophysical conditions (Li et al., 2019).
The calculation of field or subfield level NUE requires spatial tools to estimate crop N content. Coincidently, a cornerstone of tactical N management is fine-tuning in-season N management to meet crop N needs based upon the status of the plant. For example, the N nutritional index can be used to determine whether the crop N concentration is suboptimal relative to the critical N dilution curve at maximum yield (Justes et al., 1994), while the N sufficiency index can assess status by referencing a well-fertilized area (Varvel et al., 1997). A rapid, non-destructive assessment of field or subfield NUE depends upon the remote or proximal sensing tools and algorithms that reliably monitor N concentrations in the crop (Pinter et al., 2003; Ladha et al., 2005; Yao et al., 2015; Magney et al., 2017). The working assumption is that crop N sufficiency status is functionally related to plant N either expressed as a concentration (%) or accumulation (kg N ha−1) in the leaf or plant.
Though not always consistent across growth stage and fertilizer rate, chlorophyll or protein indicators can be used as proxies for N status due to the strong relationship between N-containing compounds and N content (Fu et al., 2021). Many different vegetation indices are widely used to estimate crop N content or accumulation, alleviating confounding factors from soils or water, which are generally calculated from the leaf or canopy reflectance values of wavebands in the visible and near-infrared regions (Zhang et al., 2018; Caballero et al., 2020; Fu et al., 2021). Rapid developments in sensing technologies coupled with machine learning (and other techniques) have increased our abilities to accurately predict yield and non-destructively estimate plant N status (Yao et al., 2015; Chlingaryan et al., 2018). However, challenges persist for practitioners (Fu et al., 2021), including the influence of growth stage, cultivars, and N management across space and time, as well as the limitation imposed as indices approach saturation levels. Furthermore, canopy sensing data is often instantaneous, infrequent, and does not capture the N status of the entire plant (i.e., vertical distribution), thus potentially missing dynamic N behavior in the plant pertinent to making timely recommendations. To combat these limitations, Fu et al. (2021) recommends that hyperspectral data be integrated with crop growth models and radiative transfer models to improve assessments.
The variance in crop and soil data can also be used to delineate subfield management zones through the combination of sensing, geostatistical, and interpolation techniques (Chlingaryan et al., 2018; Basso et al., 2019). However, when developing site-specific N recommendations, precision agriculture tools must also account for the dynamic nature of soil N and crop uptake efficiencies across landscapes (Chlingaryan et al., 2018; Morris et al., 2018; Qin et al., 2018). Furthermore, recommendations that rely primarily on vegetation indices cannot guide pre-planting or pre-emergent N decisions. Therefore, an integrative site-specific N management approach links georeferenced decision support models to dynamic biogeochemical models that simulate outcomes based upon relevant crop, soil, weather, management, and enterprise factors (Morris et al., 2018; Sela and van Es, 2018; Sharma and Bali, 2018; Schroeck et al., 2019). Models that simulate N status can then be validated through field measurements collected throughout the growing season. Therefore, precision agriculture technologies are compatible within an adaptive N management framework, in which site-specific empirical data is used to improve model accuracy on a field or subfield level (Morris et al., 2018). Ultimately, data from these various sources can be fused through machine learning or other techniques to provide on-the-go assessments and automated recommendations (Chlingaryan et al., 2018).
Crop sensing and georeferenced management data could be used to calculate and map NUE spatially and temporally for assessment purposes. As a performance indicator, NUE can help evaluate fertilizer management within the context of yield and crop quality goals, and even diagnose factors contributing to inefficiencies of fertilizer use (Maaz et al., 2018). As an environmental indicator, NUE estimates can help farmers assess the risk of N losses from farms or fields, or relative to regional or supply chain estimates (Lassaletta et al., 2014, 2016; Erisman et al., 2018). Variable rate N fertilizer technology could substantially reduce N losses by matching the low plant N demand in low fertility subfield areas with appropriately reduced fertilizer rates, as could planting these areas to perennial conservation or bioenergy species. With current technology, the best way to capture unused N after the main crop's growing season is by using cover crops planted to grow quickly following senescence of the main crop. The N that cover crops remove from the soil solution is N that is not lost to the environment and instead can be remobilized from cover crop biomass to provide N to the following year's main crop.
Biological N Fixation (BNF) and Incorporation of Legumes
Although plants cannot use atmospheric N2 directly, it has been known for over a century that diverse bacteria and archaea, known as diazotrophs, can convert atmospheric N2 to NH3 through BNF and that the NH3 produced can be utilized directly or indirectly by plants for growth (Leigh, 2004; Gorman, 2013; Bottomley and Myrold, 2015). Diazotrophs can be found in bulk soil, within the rhizosphere of plants, physically-associated with plant roots and other organs, and even inside plants within specialized, N fixing organs called nodules (Roy et al., 2020). Rates of BNF by free-living diazotrophs in soil are typically low, between 1 and 20 kg N ha−1 yr−1 (Vadakattu and Paterson, 2006), although associative N fixation by microbes in the rhizosphere or on plant surfaces may contribute significantly to plant growth in low-N input systems (e.g., Ladha and Reddy, 2003; Martins et al., 2020). In contrast, BNF in nodules is highly efficient, and in high-yield environments can exceed 300 kg N ha−1yr−1 (Giller, 2001; Peoples et al., 2009; Cafaro La Menza et al., 2020) as nutrient exchange between plants and their intracellular bacterial endosymbionts is highly targeted (Udvardi and Poole, 2013), avoiding losses of plant-C and bacterial-ammonia to the soil and associated microbiome. However, BNF in nodules is confined largely to legumes and a few non-legume plant families (Santi et al., 2013), while most crop species, including cereals, are unable to access atmospheric N2 in this way.
The escalating global N problem has sparked renewed interest in BNF as a partial solution, deployable through: development and use of legumes and rhizobia (their natural symbionts) with increased BNF potential; development of more-effective associative N fixation in non-legumes, especially the major cereals; and potentially through the engineering of nodule symbioses or even plants capable of fixing their own N (Ladha and Reddy, 2003; Beatty and Good, 2011; Rogers and Oldroyd, 2014). BNF in grain legumes remains an important source of N in many cropping systems, where it contributes to higher NUE, although the relative contribution of legumes in agriculture has declined with the increase in N fertilizer use. This was partly due to the emphasis on cereal production in the policies of the Green Revolution, which replaced traditional cereal-legume crop rotations in countries like India, leading to scarcity of grain legumes and even imports from Africa (Raghuram, 2020). Therefore, there is tremendous scope to increase the contribution of legume BNF to agriculture, via systems agronomy and plant breeding approaches (see below, see also Franke et al., 2018; Liu et al., 2020), and by improving the effectiveness and resilience of rhizobium strains used as inoculants (Meghvansi et al., 2010; Koskey et al., 2017; Santos et al., 2019).
Growing legumes, as grain or green manure crops, and recycling shoot biomass to the soil generally improves soil fertility, increases the yield of the subsequent crop, and reduces the requirement for synthetic N. Many reports cite more grain production in cereals grown after a legume, than after a non-legume or after a fallow (Giller, 2001; Franke et al., 2018). Legumes are often used in short-term rotation, such as corn-soybean, or in continuous corn with a legume winter cover crop. These systems offer farmers many benefits, and help to solve environmental problems associated with N use in agriculture. In many developing regions of the world, legumes are used extensively to meet protein requirements. Nevertheless, in past decades, the widespread availability of synthetic N fertilizer and low yields of legumes relative to cereals have resulted either in a stagnation or a decrease in the area under cultivation of legumes in different regions (FAO, 2019). There are several environmental (e.g., temperature and excess rainfall) and economic constraints (e.g., inadequate purchasing power of farmers to buy seed) limiting legume yield and profitability (Giller, 2001) that may be responsible for a decrease in legume cultivation. In a recent review, Vanlauwe et al. (2019) argued that although considerable progress has been made in understanding grain legume agronomy, the relationship between legumes and rhizobia populations, the benefits of BNF to farming systems, and the spatial and temporal integration of legumes in these systems are important knowledge gaps that prevent the formulation of recommendations that would further enhance the contributions of legumes to farming systems in Sub-Saharan Africa (Vanlauwe et al., 2019). They recommend integration of BNF in breeding programs and improvements in overall agronomy to maximize the potential of symbiosis through eliminating various soil and other environmental constraints.
Legumes are also an attractive option for mixed-crop systems where two crop species are grown simultaneously in the same field. But despite advantages of intercropping that include greater resource use efficiency, including NUE, intercropping remains ‘at the fringes of modern intensive agriculture' (Brooker et al., 2015). This may change when the benefits of intercropping are realized, with a recent estimate that globally increased NUE of cereal-legume intercropping reduces the requirements for fossil-based fertilizer N by about 26% (reviewed by Jensen et al., 2020). Challenges will include planting using the same implements, weed management, and harvesting.
Engineering BNF Through Microbial Association and Direct Genetic Manipulation
There is growing interest in the development of effective associative N fixation for cereal crops, especially maize, rice, and wheat (Mus et al., 2016; Bloch et al., 2020; Mahmud et al., 2020), and as well for perennial forage and bioenergy grasses (Roley et al., 2019; Bahulikar et al., 2020). These range from simply isolating, testing, and deploying the most effective natural plant-associated diazotrophs of target plant species, based primarily on plant growth promotion (Fox et al., 2016), to current attempts to edit the genomes of such bacteria to remove genetic controls that prevent N fixation and NH3 release in agricultural soils containing potentially high levels of mineral and organic-N (Barney et al., 2017; Bueno Batista and Dixon, 2019). Decades of genetic, genomic, and biochemical research and technology development provide a basis for attempts to edit or engineer diazotrophs for optimal association with non-legumes (Bueno Batista and Dixon, 2019). However, it is difficult to estimate small amounts of BNF in the field and many recent claims of large amounts of fixed N in cereals result from flawed application of measurement methods (Unkovich et al., 2020). Further research is required to understand and minimize trade-offs between N fixation with NH3 loss and growth of diazotrophs, to optimize symbiotic interactions and nutrient exchanges between diazotrophs and plants, and to understand interactions between diazotrophs and the microbiome at large. For instance, arbuscular mycorrhizal fungi may facilitate NH3 transfer between diazotrophs and the plant (El-Shanshoury et al., 1989; Frey-Klett et al., 2007; Guether et al., 2009; Meenakshisundaram and Santhaguru, 2011; Sabannavar and Lakshman, 2011).
Parallels between N fixing symbioses in legumes and arbuscular mycorrhizal (AM) symbioses that occur in most plant species, including common signaling components/genes involved in establishing these distinct beneficial symbioses, have spurred efforts to engineer nodulation and BNF in plant species that form AM symbioses, including the major cereals (Rogers and Oldroyd, 2014; Bailey-Serres et al., 2019). In principle, current efforts are focused on engaging cereal AM signaling components to recognize rhizobial signals and transducing these signals into altered gene expression to promote plant cell division and nodule formation, while allowing bacterial entry into plant cells (Radhakrishnan et al., 2020). These processes are complex, with over 200 genes known to be required to establish and maintain effective BNF in legumes (Roy et al., 2020). However, recent evidence suggests that evolution of one or a few genes was sufficient to put the ancestors of modern legumes on the pathway to effective BNF (van Velzen et al., 2018), so the hope is that the same key genes may set the stage for engineering of effective BNF in cereals (Rogers and Oldroyd, 2014; Bailey-Serres et al., 2019). Further investments in this line of strategic and applied research will, at the very least, test our current understanding of the development and maintenance of BNF, while further basic research on the genetics, cell biology and biochemistry of legume BNF will advance knowledge and continue to inform efforts to engineer BNF in non-legumes.
The most audacious approach to solving the two-sided N-problem is to engineer N fixation into plants directly by transferring genes responsible for BNF into plant genomes and expressing active nitrogenase enzymes in an appropriate plant compartment (Burén et al., 2017; Yang et al., 2018). There are many features of nitrogenase that make this venture a high barrier including coordinating the expression of the numerous genes involved in the assembly of the unique metal cofactors, and extreme oxygen sensitivity and high energy demand of the active enzyme complex (Rubio and Ludden, 2008; Burén and Rubio, 2018; Yang et al., 2018). These features may explain why the process was never co-opted from microbes during plant evolution. Nonetheless, progress has been made toward this objective (Burén et al., 2017; Burén and Rubio, 2018; Eseverri et al., 2020; Xiang et al., 2020), and such work is testing and extending our understanding of BNF. Given the current state of knowledge and technology, the greatest impediment to solving the N problems of agriculture through N fixing plants may turn out to be public acceptance of such technology rather than our ability to develop it. At this stage, however, this approach, like the nodulating, N fixing cereals approach, remains in the high-risk, high-reward category (Table 1).
Plant Breeding for Improved NUE
Breeding programs have traditionally focused on yield-based selection to achieve genetic gains, and comparisons among crop varieties representing historic and modern materials often show associated increases of mass yield but not of harvested N (Duvick, 2005). This indicates that increasing NUE on a grain yield basis is not the same as increasing NUE on a N recovery basis. However, yield-based selection may have problems in efficiently overcoming possible barriers to improvement relative to trait-based breeding (Messina et al., 2011). Therefore, ideotype breeding (Donald, 1968) centered on identifying and selecting traits affecting NUE may be an opportunity for breeding strategies that are based on understanding important processes rather than treating them as a black box, as done with yield-based selection. This opportunity is particularly attractive now because the rise of genomics and cheap DNA sequencing, functional phenomics, and high-throughput phenotyping allows the simultaneous identification of phenotypic variation, genetic mapping and marker identification, and understanding of underlying physiological processes (Mandal et al., 2018; York, 2019).
Natural variation for NUE has been observed among and within many crop species, including maize, rice, wheat, and soybeans. This variation implies that breeding programs may be able to select for high NUE, although many traits influence NUE and they are all under complex genetic control. For relevance to crop breeding, Moll et al. (1982) partitioned a NUE variant into N uptake efficiency (NUpE), the fraction of N inputs found in the shoot of the plant at maturity, and NUtE, the weight of grain produced per unit of acquired N (Moll et al., 1982). Traits that contribute to plant NUE range from N uptake, assimilation, partitioning processes and transient storage, to N remobilization and utilization in source and sink organs (Tegeder and Masclaux-Daubresse, 2018; Raghuram and Sharma, 2019; The et al., 2021). We propose synergistic breeding activities to improve underlying traits in the NUE functional hierarchy. NUpE can be partitioned into root and shoot processes, while NUtE centers around shoot processes related to grain production. While these efficiency measures are especially relevant for grain crops, others may be more relevant for forage crops where we expect high NUE due to greater synchrony of crop growth and N availability.
Plant roots are responsible for acquiring N from soil. The acquisition efficiency of the root system has been defined in terms of the amount of N acquired per unit carbon investment to the root system (Nielsen et al., 1994). Root system architecture determines the spatial arrangement of roots, even at a given investment of carbon. Architectural features that increase topsoil foraging may be useful earlier in the season when N is applied, while deep rooting has been hypothesized to be beneficial for catching N before it leaches (Lynch, 2013). Increasing acquisition efficiency may be possible by focusing on root traits that reduce the metabolic burden of individual root segments, or of the entire root system (reviewed by Lynch, 2015; Mandal et al., 2018). For example, allocation among root classes may be optimized by increasing the number of lateral roots, which are thinner with less construction costs than axial roots. Research in maize has shown that increased lateral relative to axial rooting can enhance N acquisition and shoot growth in soils with N limitation (Zhan and Lynch, 2015; Guo and York, 2019). Besides construction costs, maintenance costs such as respiration must also be considered (Guo et al., in press). Anatomical traits including increased aerenchyma or reduced cortical cell area reduce respiration and have been shown to improve N acquisition, and in some cases to allow N remobilization from senescing root tissue (Chimungu et al., 2014; Saengwilai et al., 2014). Recently, N-responsive differences in germination, respiration and crop maturity have been associated with NUE in rice (Sharma et al., 2018). Specific uptake rate may be defined as the instantaneous potential rate of N uptake by a short root segment, but has rarely been considered as a root trait (reviewed by Griffiths and York, 2020). However, variation among maize lines (Pace and McClure, 1986) and maize root classes (York et al., 2016) for nitrate uptake rates indicate that there may be a genetic basis that could be harnessed for plant breeding. Recent developments in high throughput phenotyping of multiple nutrient uptake by maize roots highlights this opportunity (Griffiths et al., 2021). Molecular biologists have identified many genes involved in root system architecture or transport of various types of N compounds and targeted some of these for genetic manipulation to improve NUpE, with varying success (Mandal et al., 2018; Tegeder and Masclaux-Daubresse, 2018). Multiple approaches exist to increase uptake efficiency by the root system that could be included in pre-breeding programs screening for genetic variation.
Once N is acquired by the plant, physiological processes in source organs that assimilate N and carbon, as well as in sinks that use or store the assimilates, will determine how well the plant can perform to increase growth and acquire more N. Photosynthesis is responsible for fixing carbon from the atmosphere and several strategies exist to decrease the N requirement of photosynthesis. Shoot architecture determines the overall arrangement of leaves, which has substantial impact of light interception efficiency (Wang et al., 2018). On a leaf area basis, photosynthetic N use efficiency may be related to chloroplast size (Li et al., 2013) and early seedling vigor (Pang et al., 2014). Recent work has demonstrated the importance of reducing photorespiration (South et al., 2019) and accelerating photosynthetic induction after light changes (Acevedo-Siaca et al., 2020) for increasing overall photosynthetic efficiency. Steps involved in N assimilation, including reduction to nitrite then , and assimilation of this into amino acids could be targeted to increase NUtE. Given the dependency of plant metabolism on enzymes composed of N-containing amino acids, substantial work could be done on the overall physiological efficiency of N anabolism and catabolism. However, it has been suggested that such strategies may only be successful if N metabolism and transport processes, including source-to-sink N partitioning, are coordinated or modified to avoid end-product inhibition or substrate limitation (Fernie et al., 2020).
For many years, breeders have focused on increasing the crop harvest index (HI), which reflects the ratio of harvested grain to total shoot dry matter (Donald and Hamblin, 1976). However, increasing the HI on a mass basis may not necessarily increase NUtE (i.e., seed N yield relative to total shoot N content), as indicated by the trade-off between grain mass and N concentration (Oury and Godin, 2007). HI may also be reaching a theoretical maximum because the remainder of the shoot including leaves is necessary to support grain production (Hütsch and Schubert, 2017). However, altering remobilization and translocation of N from vegetative shoot organs to grains has been shown to affect NUtE (c.f. Perchlik and Tegeder, 2017; Tegeder and Masclaux-Daubresse, 2018; Tegeder and Perchlik, 2018). Delayed leaf senescence (“stay-green”) can be important for prolonging grain filling as well as N uptake from the soil, but it may also hinder partitioning of remobilized N from leaves to seeds (Kosgey et al., 2013). More research is needed on the timing and interrelationship of these various traits and how they may be co-optimized to increase NUtE.
Plant-microbe interactions that influence biological N fixation, soil N mineralization, and nitrification could also be the targets of plant breeding. Most directly, legume crops form nodules with symbiotic rhizobia bacteria that fix N to share with the plant in exchange for C-compounds. More efficient fixation (i.e., N fixed per C supplied) could substantially improve overall NUE. Legumes have the capacity to “autoregulate” nodulation and BNF, reducing these when abundant soil N is available, which prevents “luxury” fixation. Selecting or producing legume varieties that continue N fixation despite the presence of soil N could potentially result in enhanced release of biologically-fixed N into agricultural soils, thereby increasing available soil N pools for subsequent crops (Santachiara et al., 2018). Promoting external root-microbiota that fix N, mobilize soil organic-N, or inhibit nitrification that drives N leaching from soil and gaseous N losses to the atmosphere, is another opportunity to make N gains for plant nutrition. Such knowledge of crop genotype-microbe interactions is increasingly important in plant breeding, for example where beneficial bacterial communities are associated with particular crop genotype and N processes (Zhang et al., 2019). Therefore, it is plausible to select for plants that promote associative N fixation within the rhizosphere. Another attractive, if elusive, alternative is for crops to exude nitrification inhibitors directly from roots (Subbarao et al., 2015; Sun et al., 2016).
Over the past 70 years, selection of crops has primarily been performed under high N fertilization, which may have limited gains in NUE. In some cases, NUE of modern genotypes is greater in both high and low N soils (York et al., 2015). However, multiple traits influence NUE, most probably leading to considerable variations in improvements of NUE between genotypes. We propose breeding programs that select genotypes under sub-optimal N as a way to increase NUE under these conditions and to substantially reduce N losses to the environment (c.f. Perchlik and Tegeder, 2018). Genetic gains could be accelerated with marker assisted selection, genomic selection, and gene editing technologies (Mandal et al., 2018; Raghuram and Sharma, 2019). New phenotyping technologies will need to be incorporated, also, to increase the number of lines evaluated and to improve precision of measurements to maximize selection pressure. A promising method in the context of N would be the use of unmanned aerial vehicles (UAVs) that can estimate biomass, height, chlorophyll content, and yield throughout the growth season. Using high-throughput phenotyping with UAVs to select for both N status and plant mass on greatly expanded breeding populations in low-input fields has substantial promise for quick gains.
Frontiers of plant breeding for NUE will include perennialization of agriculture using crop rotations, mixed crops, and perennial species. The perennial grain crop intermediate wheatgrass (popular as Kernza®) was shown to decrease N leaching substantially compared to maize, for example (Jungers et al., 2019), because roots are already in place when soil N mineralization proceeds as soils warm in the spring. Introgression of wild perenniality traits may be possible through the creation of interspecies hybrids, such as in sorghum (Foster et al., 2020). Seeding summer annuals like maize into perennial covers can have similar effects, but the agronomic management is difficult. Synchronizing crop demand with soil N availability (discussed above) can be partially accomplished through plant phenology, like stay-green, and also increased early vigor even in hot or cold planting conditions. Therefore, advances in breeding for perennial crops or for polycultures of annual crops within perennial systems could have substantial impact on the NUE of cropping systems.
Knowledge Integration With Cropping Systems Models
Next-generation computational frameworks can examine the complex N interactions in crop systems to inform management, prioritize research, and increase understanding of the complexities. These computational frameworks include statistical models, process-based mechanistic simulation models, and hybrids of the two (Jones et al., 2017; Morris et al., 2018). Such decision-support tools explore all facets of N in the soil-crop interface—from gene expression, crop physiology, and phenology to soil processes and predictions of N behavior. For example, cropping systems modeling frameworks (e.g., APSIM, DSSAT, etc.) consider critical N concentration for crop growth in the context of genetic, environment, and management factors (G × E × M) that control interactions among soil N availability, crop phenology, and crop N partitioning and yield (Holzworth et al., 2014), including BNF (Chen et al., 2014). Cropping systems models are integrated assemblies of individual component models that address specific biophysical components (e.g., water balance, crop growth and soil N mineralization). They can be used to develop hypotheses, test hypotheses, and generate management-focused decision support tools that improve productivity, profitability, and environmental quality. Although statistical models are relatively easy-to-use and well-suited for decision support tools, unlike process-based models, they cannot extrapolate beyond the G × E × M context in which they were developed. Hence, they cannot predict the response of NUE to unobserved combinations of G × E × M. Yet, such capacity is critical for two reasons. First, experiments alone are insufficient to address the many potential G × E × M combinations that arise from interactions between farmer decisions and weather. In a given field and year, cropping systems outcomes result from billions of potential combinations of hundreds of variables. Some of these are chosen by the farmer (e.g., cultivar, planting date, soil and N fertilizer management) while others are subject to variations in weather and climate. Second, new N fertilizer and cropping systems management strategies may be addressed in silico, to increase the efficiency of field experiments and to prioritize research based on sensitivity analysis that reveals scenarios with major impact. Simultaneously, field experiments will find and fill knowledge gaps of the models.
The Path Forward
Historically, NUE in agricultural systems has shifted from high NUE in low-input, low-output systems through low NUE in high-input, high-output systems, to moderate NUE in moderate-input, high-output systems (Zhang et al., 2015). In fact, some existing low-input, low-output systems, e.g., in Benin, exhibit NUE >1, signifying net N extraction and soil fertility decline (Lassaletta et al., 2014). While many countries experience a dramatic decline in agricultural NUE as N fertilizers are adopted and overused (e.g., NUE in China fell below 0.3), it has been argued that this is not inevitable and that countries experiencing a downward trend in NUE could learn from those that have been able to “bend” their NUE curve toward higher NUE (>0.6 in the USA and France), through government policy, education, careful management, etc. (Zhang et al., 2015).
As the historical trajectory shows, simply increasing NUE alone will not be adequate if it leads to low-output systems and food insecurity amongst the growing world population. Thus, we are faced with a complex, multi-objective problem, which is further complicated by dynamic economic and environmental factors. Profitability can be relatively insensitive to N fertilizer rate. For example, in Midwest US maize, budgets based on return on investment to N fertilizer (i.e., the ratio of the cost of N fertilizer and return on grain) demonstrate that the economic optimum N rate varies by as much as 50 kg N ha−1 based only on realistic differences in N fertilizer: grain price ratios (e.g., 0.05–0.20; Camberato et al., 2017). Fifty kg N ha−1 is ~30% of the mean economic optimum N rate for these systems. Hence, while there is economic incentive to optimize N fertilizer rates, the optimum N rate is highly dependent on grain and fertilizer markets. Together, these challenges demand a robust, interdisciplinary approach to increase NUE using multi-objective optimization that considers social and biophysical sciences.
Multi-objective optimization is a computational framework that searches for optimal solutions and takes into account trade-offs among potentially-conflicting objectives, such as minimizing N inputs while maximizing outputs. Such trade-offs are captured by cropping systems simulations, which are powerful integrators for using multi-objective optimization techniques. While the simulations could be used only to maximize the NUE ratio, instead yield and economics can be maximized and N losses minimized simultaneously. Multi-objective methods have been used to optimize parametrization of a maize system simulation to match empirical results (Harrison et al., 2019), but could also be applied to optimize the objectives for NUE. At the regional scale, these optimization methods have been used to allocate rainfed and irrigation areas in order to maximize yield and minimize environmental impact (Galán-Martín et al., 2017), so similar concepts could be used to maximize NUE across regions or the globe. Trade-offs in objectives have also been identified in crop breeding, such as between total grain yield and concentration of N in grain, but recent work with multi-trait genomic selection offers a path forward (Moeinizade et al., 2020). Therefore, we propose that explicit consideration of multiple objectives in optimization frameworks is crucial for future progress to increase NUE while meeting food security and economic needs.
Conclusion
Ultimately, improved management options for agricultural producers will help to bend the NUE curve more quickly to higher NUE for responsible use of N in agriculture. We have described opportunities to increase agricultural NUE through R&D in several areas: agronomy, plant breeding, biological N fixation, and soil N cycling. Maximal impact R&D will likely involve advances in all these areas, although we anticipate that short-term impacts (within 5–10 years) will result from advances in agronomy, decision support tools, and greater use of existing legume cultivars; followed in the medium-term (10-20 years) by improved cereal and other crop varieties, selected specifically for high NUE traits, and more-effective microbes; and in the long-term (>20 years) by entirely new N fixing symbioses in plants or plants engineered to fix N without bacterial partners (Figure 2). Of course, the risks associated with some of the long-term R&D opportunities described above are relatively high, as are the potential returns (Table 1). This research roadmap for improving NUE along with the risk-reward matrix will be useful to policy makers when deciding how to address these pressing global challenges.
Finally, we recognize that achieving responsible use of N for food security and environmental health requires more than technical solutions to biophysical problems. Ultimately, the problem stems from society and the solution must involve the social dimension. This includes expanding the farming objectives from the primary focus on production-for-profit, which of course is essential for livelihoods, to include stewardship-of-the-land and surrounding environment, which is crucial for sustainability of agriculture and society. Understanding what motivates individuals and societies will help to develop educational and other activities to remove barriers to the responsible use of N in agriculture.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
This work was primarily supported by the NSF Research Coordination Network, PlaNNet, grant awarded to MU and JP (grant # IOS-1444832). Additional support for individual authors includes grants from the US National Science Foundation (grant # IOS-1457183 to MT, # 1832042 to GR), and the US Department of Agriculture/National Institute of Food and Agriculture (NIFA; Grant no. 2017-67013-26158 to MT and grant no. 2017-67007-25948 to LY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Figure 1 was created with BioRender.com.
References
Abalos, D., Jeffery, S., Sanz-Cobena, A., Guardia, G., and Vallejo, A. (2014). Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 189, 136–144. doi: 10.1016/j.agee.2014.03.036
Abrol, Y. P., Adhya, T. K., Aneja, V. P., Raghuram, N., Pathak, H., Kulshrestha, U., et al. (2017). The Indian Nitrogen Assessment: Sources of Reactive Nitrogen, Environmental and Climate Effects, Management Options, and Policies. Elsevier.
Acevedo-Siaca, L. G., Coe, R., Wang, Y., Kromdijk, J., Quick, W. P., and Long, S. P. (2020). Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol. 227, 1097–1108. doi: 10.1111/nph.16454
Adediran, J. A., Taiwo, L. B., Akande, M. O., Sobulo, R. A., and Idowu, O. J. (2007). Application of Organic and inorganic fertilizer for sustainable maize and cowpea yields in Nigeria. J. Plant Nutr. 27, 1163–1181. doi: 10.1081/PLN-120038542
Allen, D. E., Kingston, G., Rennenberg, H., and Dalal, R. C. S S. (2010). Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 136, 209–217. doi: 10.1016/j.agee.2009.11.002
Bahulikar, R. A., Chaluvadi, S. R., Torres-Jerez, I., Mosali, J., Bennetzen, J. L., and Udvardi, M. Nitrogen fertilization reduces nitrogen fixation activity of diverse diazotrophs in switchgrass roots. Phytobiomes J. (2020) 5. 10.1094/PBIOMES-09-19-0050-FI.
Bailey-Serres, J., Parker, J. E., Ainsworth, E. A., Oldroyd, G. E., and Schroeder, J. I. (2019). Genetic strategies for improving crop yields. Nature 575, 109–118. doi: 10.1038/s41586-019-1679-0
Balafoutis, A., Beck, B., Fountas, S., Vangeyte, J., Wal, T. V., der, Soto, I., et al. (2017). Precision agriculture technologies positively contributing to GHG emissions mitigation, farm productivity and economics. Sustainability 9:1339. doi: 10.3390/su9081339
Barney, B. M., Plunkett, M. H., Natarajan, V., Mus, F., Knutson, C. M., and Peters, J. W. (2017). Transcriptional analysis of an ammonium-excreting strain of Azotobacter vinelandii deregulated for nitrogen fixation. Appl. Environ. Microbiol. 83:20. doi: 10.1128/AEM.01534-17
Basso, B., Shuai, G., Zhang, J., and Robertson, G. P. (2019). Yield stability analysis reveals sources of large-scale nitrogen loss from the U.S. Midwest. Sci. Rep. 9:5774. doi: 10.1038/s41598-019-42271-1
Beatty, P. H., and Good, A. G. (2011). Future prospects for cereals that fix nitrogen. Science 333, 416–417. doi: 10.1126/science.1209467
Bindraban, P. S., Dimkpa, C., Nagarajan, L., Roy, A., and Rabbinge, R. (2015). Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 51, 897–911. doi: 10.1007/s00374-015-1039-7
Bindraban, P. S., Dimkpa, C. O., White, J. C., Franklin, F. A., Melse-Boonstra, A., Koele, N., et al. (2020). Safeguarding human and planetary health demands a fertilizer sector transformation. Plants People Planet 2, 302–309. doi: 10.1002/ppp3.10098
Bloch, S. E., Ryu, M. H., Ozaydin, B., and Broglie, R. (2020). Harnessing atmospheric nitrogen for cereal crop production. Curr. Opin. Biotechnol. 62, 181–188. doi: 10.1016/j.copbio.2019.09.024
Bodirsky, B. L., Popp, A., Lotze-Campen, H., Dietrich, J. P., Rolinski, S., Weindl, I., et al. (2014). Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 5, 1–7. doi: 10.1038/ncomms4858
Brackin, R., Näsholm, T., Robinson, N., Guillou, S., Vinall, K., Lakshmanan, P., et al. (2015). Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertilizer supply and sugarcane root uptake capacity. Sci. Rep. 5:15727. doi: 10.1038/srep15727
Brooker, R. W., Bennett, A. E., Cong, W. F., Daniell, T. J., George, T. S., Hallett, P. D., et al. (2015). Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 206, 107–117. doi: 10.1111/nph.13132
Bueno Batista, M., and Dixon, R. (2019). Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 47, 603–614. doi: 10.1042/BST20180342
Burén, S., Jiang, X., López-Torrejón, G., Echavarri-Erasun, C., and Rubio, L. M. (2017). Purification and in vitro activity of mitochondria targeted nitrogenase cofactor maturase NifB. Front. Plant Sci. 8:1567. doi: 10.3389/fpls.2017.01567
Burén, S., and Rubio, L. M. (2018). State of the art in eukaryotic nitrogenase engineering. FEMS Microbiol. Lett. 365:fnx 274. doi: 10.1093/femsle/fnx274
Burzaco, J. P., Ciampitti, I. A., and Vyn, T. J. (2014). Nitrapyrin iImpacts on maize yield and nitrogen use efficiency with spring-applied nitrogen: Field studies vs. meta-analysis comparison. Agron. J. 106, 753–760. doi: 10.2134/agronj2013.0043
Caballero, D., Calvini, R., and Amigo, J. M. (2020). “Chapter 3.3 - Hyperspectral imaging in crop fields: precision agriculture,” in Data Handling in Science and Technology, ed J. M. Amigo (Elsevier), 32, 453–473. doi: 10.1016/B978-0-444-63977-6.00018-3
Cafaro La Menza, N., Monzon, J. P., Lindquist, J. L., Arkebauer, T. J., Knops, J. M., Unkovich, M., et al. (2020). Insufficient nitrogen supply from symbiotic fixation reduces seasonal crop growth and nitrogen mobilization to seed in highly productive soybean crops. Plant Cell Environ. 43, 1958–1972. doi: 10.1111/pce.13804
Camberato, J., Nielsen, R., and Joern, B. (2017). Nitrogen Management Guidelines for Corn in Indiana. Purdue Nitrogen Management Update. Available online at: www.agry.purdue.edu/ext/corn/news/timeless/nitrogenmgmt.pdf
Canfield, D. E., Glazer, A. N., and Falkowski, P. G. (2010). The evolution and future of earth's nitrogen cycle. Science 330, 192–196. doi: 10.1126/science.1186120
Cao, Q., Miao, Y., Feng, G., Gao, X., Liu, B., Liu, Y., et al. (2017). Improving nitrogen use efficiency with minimal environmental risks using an active canopy sensor in a wheat-maize cropping system. Field Crops Res. 214, 365–372. doi: 10.1016/j.fcr.2017.09.033
Cassman, K. G., Dobermann, D., and Walters, D. T. (2002). Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31, 132–140 doi: 10.1579/0044-7447-31.2.132
Cassman, K. G., and Grassini, P. (2020). A global perspective on sustainable intensification research. Nat. Sustain. 3, 262–268. doi: 10.1038/s41893-020-0507-8
Chen, X., Cui, Z., Fan, M., Vitousek, P., Zhao, M., Ma, W., et al. (2014). Producing more grain with lower environmental costs. Nature 514, 486–489. doi: 10.1038/nature13609
Chen, X.-P., Cui, Z.-L., Vitousek, P. M., Cassman, K. G., Matson, P. A., Bai, J.-S., et al. (2011). Integrated soil–crop system management for food security. Proc. Natl. Acad. Sci. U.S.A. 108, 6399–6404. doi: 10.1073/pnas.1101419108
Chimungu, J. G., Brown, K. M., and Lynch, J. P. (2014). Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol. 166, 1943–1955. doi: 10.1104/pp.114.249037
Chin, A., Schmidt, S., Buckley, S., Pirie, R., Redding, M., Laycock, B., et al. (2018). Sorbents can tailor nitrogen release from organic wastes to match the uptake capacity of crops. Sci. Total Environ. 645, 1474–1483. doi: 10.1016/j.scitotenv.2018.07.135
Chlingaryan, A., Sukkarieh, S., and Whelan, B. (2018). Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: a review. Comp. Electronics Agric. 151, 61–69. doi: 10.1016/j.compag.2018.05.012
Christianson, L., Castellano, M., and Helmers, M. (2012). “Nitrogen and phosphorus balances in Iowa cropping systems: Sustaining Iowa's soil resource,” in Proceedings of the Integrated Crop Management Conference (Ames, IL: Iowa State University Extension).
Corbeels, M., Hofman, G., and Van Cleemput, O. (1999). Soil mineral nitrogen dynamics under bare fallow and wheat in vertisols of semi-arid Mediterranean Morocco. Biol. Fertil. Soils 28, 321–328. doi: 10.1007/s003740050500
Córdova, C. S., Castellano, M. J., Dietzel, R., Licht, M. A., Togliatti, K., Martinez-Feria, R., et al. (2019). Soybean nitrogen fixation dynamics in Iowa, USA. Field Crops Res. 236, 165–176. doi: 10.1016/j.fcr.2019.03.018
Daigh, A. L., Zhou, X., Helmers, M. J., Pederson, C. H., Horton, R., Jarchow, M., et al. (2015). Subsurface drainage nitrate and total reactive phosphorus losses in bioenergy-based prairies and corn systems. J. Environ. Qual. 44, 1638–1646. doi: 10.2134/jeq2015.02.0080
Diamond, J. (1997). Guns, Germs and Steel: A Short History of Everybody for the Last 13,000 Years. New York, NY: W. W. Norton and Company.
Donald, C. M. (1968). The breeding of crop ideotypes. Euphytica 17, 385–403. doi: 10.1007/BF00056241
Donald, C. M., and Hamblin, J. (1976). The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Adv. Agron. 28, 361–405. doi: 10.1016/S0065-2113(08)60559-3
Duvick, D. N. (2005). The contribution of breeding to yield advances in maize (Zea Mays L.). Adv. Agron. 86, 83–145. doi: 10.1016/S0065-2113(05)86002-X
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
El-Shanshoury, A., Hassan, M., and Abdel-Ghaffar, B. (1989). Synergistic effect of vesicular-arbuscular mycorrhizas and Azotobacter chroococcum on the growth and the nutrient contents of tomato plants. Phyton 29, 203–212.
Erisman, J. W., Leach, A., Bleeker, A., Atwell, B., Cattaneo, L., and Galloway, J. (2018). An integrated approach to a nitrogen use efficiency (NUE) indicator for the food production–consumption chain. Sustainability 10:925. doi: 10.3390/su10040925
Eseverri, Á., López-Torrejón, G., Jiang, X., Burén, S., Rubio, L. M., and Caro, E. (2020). Use of synthetic biology tools to optimize the production of active nitrogenase Fe protein in chloroplasts of tobacco leaf cells. Plant Biotechnol. J. 27, 1–15. doi: 10.1111/pbi.13347
Fan, M. S., Jiang, R. F., Liu, X. J., Zhang, F. S., Lu, S. H., Zeng, X. Z., et al. (2005). Interactions between non-flooded mulching cultivation and varying nitrogen inputs in rice-wheat rotations. Field Crops Res. 91, 307–318. doi: 10.1016/j.fcr.2004.08.006
Fernie, A. R., Bachem, C. W. B., Helariutta, Y., Neuhaus, H.-E., Pratt, S., Ruan, Y.-L., et al. (2020). Synchronization of developmental, molecular and metabolic aspects of source–sink interactions. Nat. Plants 6, 55–66. doi: 10.1038/s41477-020-0590-x
Foster, T. L., Baldi, H. D., Shen, X., Burson, B. L., Klein, R. R., Murray, S. C., et al. (2020). Development of novel perennial S. bicolor × S. propinquum hybrids. Crop Sci. 60, 863–872. doi: 10.1002/csc2.20136
Fox, A. R., Soto, G., Valverde, C., Russo, D., Lagares, A. Jr, Zorreguieta, Á., et al. (2016). Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen-fixing bacterium Pseudomonas protegens Pf-5 X940. Environ. Microbiol. 18, 3522–3534. doi: 10.1111/1462-2920.13376
Franke, A. C., van den Brand, G. J., Vanlauwe, B., and Giller, K. E. (2018). Sustainable intensification through rotations with grain legumes in sub-Saharan Africa: A review. Agric. Ecosyst. Environ. 261, 172–185. doi: 10.1016/j.agee.2017.09.029
Frey-Klett, P., Garbaye, J., and Tarkka, M. (2007). The mycorrhiza helper bacteria revisited. New Phytol. 176, 22–36. doi: 10.1111/j.1469-8137.2007.02191.x
Fu, Y., Yang, G., Pu, R., Li, Z., Li, H., Xu, X., et al. (2021). An overview of crop nitrogen status assessment using hyperspectral remote sensing: Current status and perspectives. Eur. J. Agron. 124, 126241. doi: 10.1016/j.eja.2021.126241
Galán-Martín, Á., Vaskan, P., Antón, A., Esteller, L. J., and Guillén-Gosálbez, G. (2017). Multi-objective optimization of rainfed and irrigated agricultural areas considering production and environmental criteria: a case study of wheat production in Spain. J. Clean. Prod. 140, 816–830. doi: 10.1016/j.jclepro.2016.06.099
Gardner, J. B., and Drinkwater, L. E. (2009). The fate of nitrogen in grain cropping systems: a meta-analysis of 15N field experiments. Ecol. Appl. 19, 2167–2184. doi: 10.1890/08-1122.1
Gelfand, I., Shcherbak, I., Millar, N., Kravchenko, A. N., and Robertson, G. P. (2016). Long-term nitrous oxide fluxes in annual and perennial agricultural and unmanaged ecosystems in the upper Midwest USA. Glob. Change Biol. 22, 3594–3607. doi: 10.1111/gcb.13426
Giller, K. E., Chalk, P., Dobermann, A., Hammond, L., Heffer, P., Ladha, J. K., et al. (2004). “Emerging technologies to increase the efficiency of use of fertilizer nitrogen,” in Agriculture and the Nitrogen Cycle, eds A.R. Mosier, J.K. Syers, and J.R. Freney. Washington: Island Press, 35–51DC.
Godfray, H. C. J., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., et al. (2010). Food security: the challenge of feeding 9 billion people. Science 327, 812–818. doi: 10.1126/science.1185383
Gorman, H. S. (2013). The story of N: A Social History of the Nitrogen Cycle and the Challenge of Sustainability. New Brunswick, NJ: Rutgers University Press. doi: 10.36019/9780813554396
Grassini, P., and Cassman, K. G. (2012). High-yield maize with large net energy yield and small global warming intensity. Proc. Natl. Acad. Sci. U.S.A. 109, 1074–1079. doi: 10.1073/pnas.1116364109
Griffiths, M., Roy, S., Guo, H., Seethepalli, A., Huhman, D., Ge, Y., et al. (2021). A multiple ion-uptake phenotyping platform reveals shared mechanisms affecting nutrient uptake by roots. Plant Physiol. 185, 781–795 doi: 10.1093/plphys/kiaa080
Griffiths, M. G., and York, L. M. (2020). Targeting root uptake kinetics for increasing plant productivity and nutrient use efficiency. Plant Physiol. 182, 1854–1868. doi: 10.1104/pp.19.01496
Guether, M., Neuhäuser, B., Balestrini, R., Dynowski, M., Ludewig, U., and Bonfante, P. (2009). A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 150, 73–83. doi: 10.1104/pp.109.136390
Guo, H., Ayalew, H., Seethepalli, A., Dhakal, K., Griffiths, M., Ma, X., and York, L. M. (in press). Functional phenomics genetics of the root economics space in winter wheat using high-throughput phenotyping of respiration architecture. N. Phytol. 10.1111/nph.17329
Guo, H., and York, L. M. (2019). Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. J. Exp. Bot. 70, 5299–5309. doi: 10.1093/jxb/erz258
Harrison, M. T., Roggero, P. P., and Zavattaro, L. (2019). Simple, efficient and robust techniques for automatic multi-objective function parameterisation: Case studies of local and global optimisation using APSIM. Environ. Model Softw. 117, 109–133. doi: 10.1016/j.envsoft.2019.03.010
Hatfield, J. L., and Walthall, C. L. (2015). Meeting global food needs: Realizing the potential via genetics × environment × management interactions. Agron. J. 107, 1215–1226. doi: 10.2134/agronj15.0076
He, W., Jiang, R., He, P., Yang, J., Zhou, W., Ma, J., et al. (2018). Estimating soil nitrogen balance at regional scale in China's croplands from 1984 to 2014. Agric. Syst. 167, 125–135. doi: 10.1016/j.agsy.2018.09.002
Hess, M. C., Mesléard, F., and Buisson, E. (2019). Priority effects: Emerging principles for invasive plant species management. Ecol. Eng. 127, 48–57. doi: 10.1016/j.ecoleng.2018.11.011
Holzworth, D. P., Huth, N. I., deVoil, P. G., Zurcher, E. J., Herrmann, N. I., McLean, G., et al. (2014). APSIM - Evolution towards a new generation of agricultural systems simulation. Environ. Model Softw. 62, 327–350. doi: 10.1016/j.envsoft.2014.07.009
Howarth, R., Swaney, D., Billen, G., Garnier, J., Hong, B., Humborg, C., et al. (2012). Nitrogen fluxes from the landscape are controlled by net anthropogenic nitrogen inputs and by climate. Front. Ecol. Environ. 10, 37–43. doi: 10.1890/100178
Huerta Lwanga, E., Gertsen, H., Gooren, H., Peters, P., Salánki, T., Van Der Ploeg, M., et al. (2016). Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 50, 2685–2691. doi: 10.1021/acs.est.5b05478
Hütsch, B. W., and Schubert, S. (2017). Harvest Index of Maize (Zea mays L.): Are there possibilities for improvement? Adv. Agron. 146, 37–82. doi: 10.1016/bs.agron.2017.07.004
Jayasundara, S., Wagner-Riddle, C., Parkin, G., von Bertoldi, P., Warland, J., et al. (2007). Minimizing nitrogen losses from a corn–soybean–winter wheat rotation with best management practices. Nutr. Cycl. Agroecosyst. 79, 141–159 doi: 10.1007/s10705-007-9103-9
Jensen, E. S., Carlsson, G., and Hauggaard-Nielsen, H. (2020). Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: a global-scale analysis. Agron. Sustain. Dev. 40:5. doi: 10.1007/s13593-020-0607-x
Jones, J. W., Antle, J. M., Basso, B., Boote, K. J., Conant, R. T., Foster, I., et al. (2017). Brief history of agricultural systems modeling. Agric. Syst. 155, 240–254. doi: 10.1016/j.agsy.2016.05.014
Jungers, J. M., DeHaan, L. H., Mulla, D. J., Sheaffer, C. C., and Wyse, D. L. (2019). Reduced nitrate leaching in a perennial grain crop compared to maize in the Upper Midwest, USA. Agric. Ecosyst. Environ. 272, 63–73. doi: 10.1016/j.agee.2018.11.007
Justes, E., Mary, B., Jean-Marc, M., Machet, J., and Huché-Thélier, L. (1994). Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 74, 397–407. doi: 10.1006/anbo.1994.1133
Kanter, D., Winiwarter, W., Bodirsky, B., Bouwman, L., Boyer, E., Buckle, S., et al. (2020). A framework for nitrogen futures in the shared socioeconomic pathways. Glob. Environ. Change 61:102029. doi: 10.1016/j.gloenvcha.2019.102029
Kavoosi, M. (2007). Effects of zeolite application on rice yield, nitrogen recovery, and nitrogen use efficiency. Commun Soil Sci. Plant Anal. 38, 69–76. doi: 10.1080/00103620601093652
Kosgey, J. R., Moot, D. J., Fletcher, A. L., and McKenzie, B. A. (2013). Dry matter accumulation and post-silking N economy of ‘stay-green' maize (Zea mays L.) hybrids. Eur. J. Agron. 51, 43–52. doi: 10.1016/j.eja.2013.07.001
Koskey, G., Mburu, S. W., Njeru, E. M., Kimiti, J. M., Ombori, O., and Maingi, J. M. (2017). Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of Eastern Kenya. Front. Plant Sci. 8:443. doi: 10.3389/fpls.2017.00443
Kumar, A., Kuzyakov, Y., and Pausch, J. (2016). Maize rhizosphere priming: field estimates using 13C natural abundance. Plant Soil 409, 87–97. doi: 10.1007/s11104-016-2958-2
Ladha, J. K., Pathak, H., Krupnik, T. J., Six, J., and van Kessel, C. V. (2005). Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Adv. Agron. 87, 85–156. doi: 10.1016/S0065-2113(05)87003-8
Ladha, J. K., and Reddy, P. M. (2003). Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252, 151–167. doi: 10.1023/A:1024175307238
Lam, S. K., Suter, H., Mosier, A. R., and Chen, D. (2017). Using nitrification inhibitors to mitigate agricultural N2O emission: a double-edged sword? Glob. Change Biol. 23, 485–489. doi: 10.1111/gcb.13338
Lassaletta, L., Billen, G., Garnier, J., Bouwman, L., Velazquez, E., Mueller, N. D., et al. (2016). Nitrogen use in the global food system: past trends and future trajectories of agronomic performance, pollution, trade, and dietary demand. Environ. Res. Lett. 11:095007. doi: 10.1088/1748-9326/11/9/095007
Lassaletta, L., Billen, G., Grizzetti, B., Anglade, J., and Garnier, J. (2014). 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 9:105011. doi: 10.1088/1748-9326/9/10/105011
Leigh, G. J. (2004). The World's Greatest Fix: A History of Nitrogen and Agriculture. Oxford: Oxford University Press.
Li, T., Zhang, W., Yin, J., Chadwick, D. D, Norse, D., et al. (2018). Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Change Biol. 24, e511–e521. doi: 10.1111/gcb.13918
Li, T., Zhang, X., Gao, H., Li, B., Wang, H., Yan, Q., et al. (2019). Exploring optimal nitrogen management practices within site-specific ecological and socioeconomic conditions. J. Clean. Prod. 241:118295. doi: 10.1016/j.jclepro.2019.118295
Li, Y., Ren, B., Ding, L., Shen, Q., Peng, S., and Guo, S. (2013). Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS ONE 8:e62036. doi: 10.1371/journal.pone.0062036
Linquist, B. A., Liu, L., van Kessel, C., and van Groenigen, K. J. (2013). Enhanced efficiency nitrogen fertilizers for rice systems: meta-analysis of yield and nitrogen uptake. Field Crops Res. 154, 246–254. doi: 10.1016/j.fcr.2013.08.014
Liu, J., You, L., Amini, M., Obersteiner, M., Herrero, M., Zehnder, A. J., et al. (2010). A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. U.S.A. 107, 8035–8040. doi: 10.1073/pnas.0913658107
Liu, J., Yu, X., Qin, Q., Dinkins, R. D., and Zhu, H. (2020). The impacts of domestication and breeding on nitrogen fixation symbiosis in legumes. Front. Genet. 11:973. doi: 10.3389/fgene.2020.00973
Liu, X. J., Ai, Y. W., Zhang, F. S., Lu, S. H., Zeng, X. Z., and Fan, M. S. (2005). Crop production, nitrogen recovery and water use efficiency in rice-wheat rotation as affected by non-flooded mulching cultivation (NFMC). Nutri. Cycl. Agroecosyst. 71, 289–299. doi: 10.1007/s10705-004-6801-4
Liu, X. J., Wang, J. C., Lu, S. H., Zhang, F. S., Zeng, X. Z., Ai, Y. W., et al. (2003). Effects of non-flooded mulching cultivation on crop yield, nutrient uptake and nutrient balance in rice-wheat cropping systems. Field Crops Res. 83, 297–311. doi: 10.1016/S0378-4290(03)00079-0
López-Bellido, L., LópezLopez-Bellido, R. J., and LópezLopez-Bellido, F. J. (2006). Fertilizer nitrogen efficiency in durum wheat under rainfed Mediterranean conditions: Effect of split application. Agron. J. 98, 55–62. doi: 10.2134/agronj2005.0017
Lory, J. A., and Scharf, P. C. (2003). Yield goal versus delta yield for predicting fertilizer nitrogen need in corn. Agron. J. 95, 994–999. doi: 10.2134/agronj2003.9940
Lynch, J. P. (2013). Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 112, 347–357. doi: 10.1093/aob/mcs293
Lynch, J. P. (2015). Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell Environ. 38, 1775–1784. doi: 10.1111/pce.12451
Maaz, T. M., Brown, T., Glover, A., and Brooks, E. (2018). Nitrogen balances can help fine-tune nitrogen management. Crops Soils 51, 4–52. doi: 10.2134/cs2018.51.0503
Magney, T. S., Eitel, J. U. H., and Vierling, L. A. (2017). Mapping wheat nitrogen uptake from RapidEye vegetation indices. Precis. Agric. 18, 429–451. doi: 10.1007/s11119-016-9463-8
Mahmud, K., Makaju, S., Ibrahim, R., and Missaoui, A. (2020). Current progress in nitrogen fixing plants and microbiome research. Plants 9:97. doi: 10.3390/plants9010097
Mandal, V., Sharma, N., and Raghuram, N. (2018). “Molecular targets for improvement of crop nitrogen-use efficiency: current and emerging options,” in Engineering Nitrogen Utilization in Crop Plants, eds. A.K. Shrawat, A. Zayed & D.A. Lightfoot (Springer), 77–93. doi: 10.1007/978-3-319-92958-3_5
Martinez-Feria, R. A., Castellano, M. J., Dietzel, R. N., Helmers, M. J., Liebman, M., Huber, I., et al. (2018). Linking crop-and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 256, 131–143. doi: 10.1016/j.agee.2018.01.002
Martins, D. S., Reis, V. M., Schultz, N., Alves, B. J. R., Urquiaga, S., Pereira, W., et al. (2020). Both the contribution of soil nitrogen and of biological N2 fixation to sugarcane can increase with the inoculation of diazotrophic bacteria. Plant Soil 35, 1–15. doi: 10.1007/s11104-020-04621-1
McLellan, E. L., Cassman, K. G., Eagle, A. J., Woodbury, P. B., Sela, S., Tonitto, C., et al. (2018). The nitrogen balancing act: tracking the environmental performance of food production. Bioscience 68, 194–203. doi: 10.1093/biosci/bix164
McNunn, G., Heaton, E., Archontoulis, S., Licht, M., and VanLoocke, A. (2019). Using a crop modeling framework for precision cost-benefit analysis of variable seeding and nitrogen application rates. Front. Sustain. Food Syst. 3:108. doi: 10.3389/fsufs.2019.00108
Meenakshisundaram, M., and Santhaguru, K. (2011). Studies on association of arbuscular mycorrhizal fungi with Gluconacetobacter diazotrophicus and its effect on improvement of Sorghum bicolor (L.). Int. J. Sci. Res. (IJCSR) 1, 23–30.
Meghvansi, M. K., Prasad, K., and Mahna, S. K. (2010). Symbiotic potential, competitiveness and compatibility of indigenous Bradyrhizobium japonicum isolates to three soybean genotypes of two distinct agro-climatic regions of Rajasthan, India. Saudi J. Biol. Sci. 17, 303–310. doi: 10.1016/j.sjbs.2010.06.002
Messina, C. D., Podlich, D., Dong, Z., Samples, M., and Cooper, M. (2011). Yield-trait performance landscapes: from theory to application in breeding maize for drought tolerance. J. Exp. Bot. 62, 855–868. doi: 10.1093/jxb/erq329
Moeinizade, S., Kusmec, A., Hu, G., Wang, L., and Schnable, P. S. (2020). Multi-trait genomic selection methods for crop improvement. Genetics 215, 931–945. doi: 10.1534/genetics.120.303305
Moll, R. H., Kamprath, E. J., and Jackson, W. A. (1982). Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 74, 562–564. doi: 10.2134/agronj1982.00021962007400030037x
Morris, T. F., Murrell, T. S., Beegle, D. B., Camberato, J. J., Ferguson, R. B., et al. (2018). Strengths and limitations of nitrogen rate recommendations for corn and opportunities for improvement. Agron. J. 110, 1–37. doi: 10.2134/agronj2017.02.0112
Mueller, N. D., Gerber, J. S., Johnston, M., Ray, D. K., Ramankutty, N., and Foley, J. A. (2012). Closing yield gaps through nutrient and water management. Nature 490, 254–257. doi: 10.1038/nature11420
Mulla, D. J. (2013). Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Engg. 114, 358–371. doi: 10.1016/j.biosystemseng.2012.08.009
Mus, F., Crook, M. B., Garcia, K., Costas, A. G., Geddes, B. A., Kouri, E. D., et al. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82, 3698–3710. doi: 10.1128/AEM.01055-16
Muschietti-Piana, M., del, P., Cipriotti, P. A., Urricariet, S., Peralta, N. R., and Niborski, M. (2018). Using site-specific nitrogen management in rainfed corn to reduce the risk of nitrate leaching. Agric. Water Manag. 199, 61–70. doi: 10.1016/j.agwat.2017.12.002
Näsholm, T., Kielland, K., and Ganeteg, U. (2009). Uptake of organic nitrogen by plants. New Phytol. 182, 31–48. doi: 10.1111/j.1469-8137.2008.02751.x
Nielsen, K. L., Lynch, J. P., Jablokow, A. G., and Curtis, P. S. (1994). Carbon cost of root systems: an architectural approach. Plant Soil 165, 161–169. doi: 10.1007/BF00009972
NOAA (2021). Global Monitoring Laboratory, Dataset for nitrous oxide. Available online at: https://www.esrl.noaa.gov/gmd/hats/combined/N2O.html (accessed April 9, 2021).
Olson, R. V., Murphy, L. S., Moser, H. C., and Swallow, C. W. (1979). Fate of tagged fertilizer nitrogen applied to winter wheat. Soil Sci. Soc. Am. J. 43, 973–975. doi: 10.2136/sssaj1979.03615995004300050032x
O'Neill, P. M., Shanahan, J. F., Schepers, J. S., and Caldwell, B. (2004). Agronomic responses of corn hybrids from different eras to deficit and adequate levels of water and nitrogen. Agron. J. 96, 1660–1667. doi: 10.2134/agronj2004.1660
Osterholz, W. R., Rinot, O., Liebman, M., and Castellano, M. J. (2017). Can mineralization of soil organic nitrogen meet maize nitrogen demand? Plant Soil 415, 73–84. doi: 10.1007/s11104-016-3137-1
Oury, F. X., and Godin, C. (2007). Yield and grain protein concentration in bread wheat: how to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 157, 45–57. doi: 10.1007/s10681-007-9395-5
Pace, G. M., and McClure, P. R. (1986). Comparison of nitrate uptake kinetics parameters across maize inbred lines. J. Plant Nutr. 9, 1095–1112. doi: 10.1080/01904168609363512
Pan, B., Lam, S. K., Mosier, A., Luo, Y., and Chen, D. (2016). Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric. Ecosyst. Environ. 232, 283–289. doi: 10.1016/j.agee.2016.08.019
Pang, J., Palta, J. A., Rebetzke, G. J., and Milroy, S. P. (2014). Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth. Funct. Plant Biol. 41, 215–222 doi: 10.1071/FP13143
Paungfoo-Lonhienne, C., Visser, J., Lonhienne, T. G., and Schmidt, S. (2012). Past, present and future of organic nutrients. Plant Soil 359, 1–18. doi: 10.1007/s11104-012-1357-6
Peoples, M. B., Brockwell, J., Herridge, D. F., Rochester, I. J., Alves, B. J. R., Urquiaga, S., et al. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48, 1–17. doi: 10.1007/BF03179980
Perchlik, M., and Tegeder, M. (2017). Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 175, 235–247. doi: 10.1104/pp.17.00608
Perchlik, M., and Tegeder, M. (2018). AAP2 amino acid transporter function in photosynthetic and plant nitrogen use efficiency in Arabidopsis. Plant Physiol. 178, 174–188. doi: 10.1104/pp.18.00597
Perrin, T. S., Drost, D. T., Boettinger, J. L., and Norton, J. M. (1998). Ammonium-loaded clinoptilolite: a slow-release nitrogen fertilizer for sweet corn. J. Plant Nutr. 21, 515–530. doi: 10.1080/01904169809365421
Pinter, J.r, P. J., Hatfield, J. L., Schepers, J. S., Barnes, E. M., Moran, M. S., et al. (2003). Remote sensing for crop management. Photogramm. Eng. Rem. S 69, 647–664. doi: 10.14358/PERS.69.6.647
Poffenbarger, H. J., Barker, D. W., Helmers, M. J., Miguez, F. E., Olk, D. C., Sawyer, J. E., et al. (2017). Maximum soil organic carbon storage in Midwest US cropping systems when crops are optimally nitrogen-fertilized. PLoS ONE 12:e0172293. doi: 10.1371/journal.pone.0172293
Qiao, C., Liu, L., Hu, S., Compton, J. E., Greaver, T. L., and Li, Q. (2015). How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob. Change Biol. 21, 1249–1257. doi: 10.1111/gcb.12802
Qin, Z., Myers, D. B., Ransom, C. J., Kitchen, N. R., Liang, S.-Z., Camberato, J. J., et al. (2018). Application of machine learning methodologies for predicting corn economic optimal nitrogen rate. Agron. J. 110, 2596–2607. doi: 10.2134/agronj2018.03.0222
Radhakrishnan, G. V., Keller, J., Rich, M. K., Verni,é, T., Mbadinga, D. L. M., Vigneron, N., et al. (2020). An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants 6, 280–289. doi: 10.1038/s41477-020-0613-7
Raghuram, N. (2020). “Zeroing in on farm budgets or zero budget natural farming? A perspective from India,” in Perspectives 37. (Nairobi: UN Environment Programme).
Raghuram, N., and Sharma, N. (2019). “Improving crop nitrogen use efficiency,” in Comprehensive Biotechnology, ed. M. Moo-Young (Elsevier: Pergamon), 211–220. doi: 10.1016/B978-0-444-64046-8.00222-6
Robertson, G. P. (1997). “Nitrogen use efficiency in row crop agriculture: crop nitrogen use and soil nitrogen loss,” in Ecology in Agriculture, ed. L.E. Jackson. (New York, New York, USA: Academic Press), 347–365. doi: 10.1016/B978-012378260-1/50011-7
Robertson, G. P., and Grandy, A. S. (2006). “Soil system management in temperate regions,” in Biological Approaches to Sustainable Soil Systems, eds. N. Uphoff, A.S. Ball, E. Fernandes, H. Herren, O. Husson, M. Laing, C. Palm, J. Pretty, P. Sanchez, N. Snanginga, and J. Thies. (Boca Raton, FL: CRC Press), 27–39.
Robertson, G. P., and Vitousek, P. M. (2009). Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 34, 97–125. doi: 10.1146/annurev.environ.032108.105046
Robinson, N., Brackin, R., Vinall, K., Soper, F., Holst, J., Gamage, H., et al. (2011). Nitrate paradigm does not hold up for sugarcane. PLoS ONE 6:e19045. doi: 10.1371/journal.pone.0019045
Rockstrom, J., Steffen, W., Noone, K., Persson, A., Chapin, F. S., III, Lambin, E. F., et al. (2009). A safe operating space for humanity. Nature 461, 472–475. doi: 10.1038/461472a
Rogers, C., and Oldroyd, G. E. D. (2014). Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. J. Exp. Bot. 65, 1939–1946. doi: 10.1093/jxb/eru098
Roley, S. S., Xue, C., Hamilton, S. K., Tiedje, J. M., and Robertson, G. P. (2019). Isotopic evidence for episodic nitrogen fixation in switchgrass (Panicum virgatum L.). Soil Biol. Biochem. 129, 90–98. doi: 10.1016/j.soilbio.2018.11.006
Roy, S., Liu, W., Nandety, R. S., Crook, A., Mysore, K. S., Pislariu, C. I., et al. (2020). Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. The Plant Cell 32, 15–41. doi: 10.1105/tpc.19.00279
Rubio, L. M., and Ludden, P. W. (2008). Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu. Rev. Microbiol. 62, 93–111. doi: 10.1146/annurev.micro.62.081307.162737
Sabannavar, S. J., and Lakshman, H. (2011). Synergistic interactions among Azotobacter, Pseudomonas, and arbuscular mycorrhizal fungi on two varieties of Sesamum Indicum L. Commun. Soil Sci. Plant Anal. 42, 2122–2133. doi: 10.1080/00103624.2011.596241
Saengwilai, P., Nord, E. A., Chimungu, J., Brown, K. M., and Lynch, J. P. (2014). Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize (Zea mays L.). Plant Physiol. 166, 726–735. doi: 10.1104/pp.114.241711
Salvagiotti, F., Cassman, K. G., Specht, J. E., Walters, D. T., Weiss, A., and Dobermann, A. (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 108, 1–13. doi: 10.1016/j.fcr.2008.03.001
Salvagiotti, F., Specht, J. E., Cassman, K. G., Walters, D. T., Weiss, A., and Dobermann, A. (2009). Growth and nitrogen fixation in high-yielding soybean: Impact of nitrogen fertilization. Agron. J. 101, 958–970. doi: 10.2134/agronj2008.0173x
Santachiara, G., Salvagiotti, F., Gerde, J. A., and Rotundo, J. L. (2018). Does biological nitrogen fixation modify soybean nitrogen dilution curves? Field Crops Res. 223, 171–178. doi: 10.1016/j.fcr.2018.04.001
Santi, C., Bogusz, D., and Franche, C. (2013). Biological nitrogen fixation in non-legume plants. Ann. Bot. 111, 743–767. doi: 10.1093/aob/mct048
Santos, M. S., Nogueira, M. A., and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9:205. doi: 10.1186/s13568-019-0932-0
Schroeck, A. M., Gaube, V., Haas, E., and Winiwarter, W. (2019). Estimating nitrogen flows of agricultural soils at a landscape level – A modelling study of the Upper Enns Valley, a long-term socio-ecological research region in Austria. Sci. Total Environ. 665, 275–289. doi: 10.1016/j.scitotenv.2019.02.071
Sela, S., and van Es, H. M. (2018). Dynamic tools unify fragmented 4Rs into an integrative nitrogen management approach. J. Soil Water Conserv. 73, 107A–112A. doi: 10.2489/jswc.73.4.107A
Sharma, L. K., and Bali, S. K. (2018). A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 10:51. doi: 10.3390/su10010051
Sharma, N., Sinha, V. B., Gupta, N., Rajpal, S., Kuchi, S., Sitaramam, V., et al. (2018). Phenotyping for nitrogen use efficiency: rice genotypes differ in N-responsive germination, oxygen consumption, seed urease activities, root growth, crop duration, and yield at low N. Front. Plant Sci. 9:1452. doi: 10.3389/fpls.2018.01452
Sheldrick, W. F., Syers, J. K., and Lingard, J. (2002). A conceptual model for conducting nutrient audits at national, regional, and global scales. Nutr. Cycl. Agroecosyst. 62, 61–72. doi: 10.1023/A:1015124930280
Smil, V. (1999). Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cy. 13, 647–662. doi: 10.1029/1999GB900015
Smil, V. (2002). Nitrogen and food production: Proteins for human diets. Ambio 31, 126–131. doi: 10.1579/0044-7447-31.2.126
Smith, J., Yeluripati, J., Smith, P., and Nayak, D. (2020). Potential yield challenges to scale-up of zero budget natural farming. Nat. Sustain. 3, 247–252. doi: 10.1038/s41893-019-0469-x
South, P. F., Cavanagh, A. P., Liu, H. W., and Ort, D. R. (2019). Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363:6422 doi: 10.1126/science.aat9077
Sowers, K. E., Miller, B. C., and Pan, W. L. (1994). Optimizing Yield and Grain Protein in Soft White Winter Wheat with Split Nitrogen Applications. Agron J. 86, 1020–1025 doi: 10.2134/agronj1994.00021962008600060017x
Stanford, G. (1973). Rationale for optimum nitrogen fertilization in corn production. J. Environ. Qual. 2, 159–166. doi: 10.2134/jeq1973.00472425000200020001x
Steffen, W., Richardson, K., Rockstrom, J., Cornell, S. E., Fetzer, I., Bennett, E. M., et al. (2015). Planetary boundaries: Guiding human development on a changing planet. Science 347:6223. doi: 10.1126/science.1259855
Subbarao, G. V., Yoshihashi, T., Worthington, M., Nakahara, K., Ando, Y., Sahrawat, K. L., et al. (2015). Suppression of soil nitrification by plants. Plant Sci. 233, 155–164. doi: 10.1016/j.plantsci.2015.01.012
Sun, L., Lu, Y., Yu, F., Kronzucker, H. J., and Shi, W. (2016). Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 212, 646–656. doi: 10.1111/nph.14057
Sutton, M. A., Bleeker, A., Howard, C. M., Bekunda, M., Grizzetti, B., de Vries, W., et al. (2013). Our Nutrient World: The Challenge to Produce More Food and Energy With Less Pollution. Edinburgh: Centre for Ecology and Hydrology.
Sutton, M. A., Oenema, O., Erisman, J. W., Leip, A., van Grinsven, H., and Winiwarter, W. (2011). Too much of a good thing. Nature 472, 159–161. doi: 10.1038/472159a
Sutton, M. A., Raghuram, N., Adhya, T., Baron, J., Cox, C., de Vries, W., et al. (2019). “The nitrogen fix: from nitrogen cycle pollution to nitrogen circular economy,” in Frontiers 2018/19: Emerging Issues of Environmental Concern (Nairobi: United Nations Environment Programme), 52–65.
Swaney, D. P., Howarth, R. W., and Hong, B. (2018). Nitrogen use efficiency and crop production: Patterns of regional variation in the United States, 1987–2012. Sci. Total Environ. 635, 498–511. doi: 10.1016/j.scitotenv.2018.04.027
Syswerda, S., Basso, B., Hamilton, S., Tausig, J., and Robertson, G. (2012). Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA. Agric. Ecosyst. Environ. 149, 10–19. doi: 10.1016/j.agee.2011.12.007
Tegeder, M., and Masclaux-Daubresse, C. (2018). Source and sink mechanisms of nitrogen transport and use. New Phytol. 217, 5–53. doi: 10.1111/nph.14876
Tegeder, M., and Perchlik, M. (2018). “The importance of organic nitrogen transport processes for plant productivity and nitrogen use efficiency,” in Engineering Nitrogen Utilization in Crop Plants, eds. A.K. Shrawat, A. Zayed, and D.A. Lightfoot (New York, NY: Springer), 233–253. doi: 10.1007/978-3-319-92958-3_13
Tenorio, F. A., McLellan, E. L., Eagle, A. J., Cassman, K. G., Andersen, D., Krausnick, M., et al. (2020). Benchmarking impact of nitrogen inputs on grain yield and environmental performance of producer fields in the western US Corn Belt. Agric. Ecosyst. Environ. 294:106865. doi: 10.1016/j.agee.2020.106865
Thapa, R., Chatterjee, A., Awale, R., McGranahan, D. A., and Daigh, A. (2016). Effect of enhanced efficiency fertilizers on nitrous oxide emissions and crop yields: a meta-analysis. Soil Sci. Soc. Am. J. 80, 1121–1134. doi: 10.2136/sssaj2016.06.0179
The, S. V., Snyder, R., and Tegeder, M. (2021) Targeting nitrogen metabolism transport processes to improve plant nitrogen use efficiency. Front. Plant Sci. 11:628366. 10.3389/fpls.2020.628366
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R., and Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature 418, 671–677. doi: 10.1038/nature01014
Udvardi, M., and Poole, P. S. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64, 781–805. doi: 10.1146/annurev-arplant-050312-120235
Unkovich, M., Herridge, D., James, E. K., Giller, K. E., and Peoples, M. B. (2020). Reliable quantification of N2 fixation by non-legumes remains problematic. Nut. Cycl. Agroecosyst. 35, 1–3. doi: 10.1007/s10705-020-10083-9
Vadakattu, G., and Paterson, J. (2006). Free-living bacteria lift soil nitrogen supply. Farm. Ahead 169:40.
van Kessel, C., Venterea, R., Six, J., Adviento-Borbe, M. A., Linquist, B. J., et al. (2013). Climate, duration, and N placement determine N2O emissions in reduced tillage systems: a meta-analysis. Glob. Change Biol. 19, 33–44. doi: 10.1111/j.1365-2486.2012.02779.x
van Velzen, R., Holmer, R., Bu, F., Rutten, L., van Zeijl, A., Liu, W., et al. (2018). Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses. Proc. Natl. Acad. Sci. U.S.A. 115, E4700–E4709. doi: 10.1073/pnas.1721395115
Vanlauwe, B., Hungria, M., Kanampiu, F., and Giller, K. E. (2019). The role of legumes in the sustainable intensification of African smallholder agriculture: lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 284:106583. doi: 10.1016/j.agee.2019.106583
Varvel, G. E., Schepers, J. S., and Francis, D. D. (1997). Ability for in-season correction of nitrogen deficiency in corn using chlorophyll meters. Soil Sci. Soc. Am. J. 61, 1233–1239. doi: 10.2136/sssaj1997.03615995006100040032x
Wang, B., Smith, S. M., and Li, J. (2018). Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 69, 437–468. doi: 10.1146/annurev-arplant-042817-040422
Wang, X., Miao, Y., Dong, R., Chen, Z., Kusnierek, K., Mi, G., et al. (2020). economic optimal nitrogen rate variability of maize in response to soil and weather conditions: implications for site-specific nitrogen management. Agronomy 10:1237. doi: 10.3390/agronomy10091237
Wassmann, R., Neue, H. U., Ladha, J. K., and Aulakh, M. S. (2004). “Mitigating greenhouse gas emissions from rice-wheat cropping systems in Asia,” in Tropical Agriculture in Transition—Opportunities for Mitigating Greenhouse Gas Emissions? (Dordrecht: Springer), 65–90. doi: 10.1007/978-94-017-3604-6_4
Winiwarter, W., Höglund-Isaksson, L., Klimont, Z., Schöpp, W., and Amann, M. (2018). Technical opportunities to reduce global anthropogenic emissions of nitrous oxide. Environ. Res. Lett. 13:014011. doi: 10.1088/1748-9326/aa9ec9
Woodward, E. E., Hladik, M. L., and Kolpin, D. W. (2016). Nitrapyrin in Streams: The first study documenting off-field transport of a nitrogen stabilizer compound. Environ. Sci. Technol. Lett. 3, 387–392. doi: 10.1021/acs.estlett.6b00348
Xia, L., Lam, S. K., Yan, X., and Chen, D. (2017). How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses, and soil carbon balance? Environ. Sci. Technol. 51, 7450–7457. doi: 10.1021/acs.est.6b06470
Xiang, N., Guo, C., Liu, J., Xu, H., Dixon, R., Yang, J., et al. (2020). Using synthetic biology to overcome barriers to stable expression of nitrogenase in eukaryotic organelles. Proc. Natl. Acad. Sci. U.S.A. 117, 16537–16545. doi: 10.1073/pnas.2002307117
Yang, J., Xie, X., Xiang, N., Tian, Z. X., Dixon, R., and Wang, Y. P. (2018). Polyprotein strategy for stoichiometric assembly of nitrogen fixation components for synthetic biology. Proc. Natl. Acad. Sci. U.S.A. 115, E8509–E8517. doi: 10.1073/pnas.1804992115
Yang, N., Sun, Z., Feng, L., Zheng, M., Chi, D., Meng, W., et al. (2015). Plastic film mulching for water-efficient agricultural applications and degradable films materials development research. Mater. Manuf. Process. 30, 143–154. doi: 10.1080/10426914.2014.930958
Yao, X., Huang, Y., Shang, G., Zhou, C., Cheng, T., Tian, Y., et al. (2015). Evaluation of six algorithms to monitor wheat leaf nitrogen concentration. Remote Sens. 7, 14939–14966. doi.10.3390/rs71114939 doi: 10.3390/rs71114939
York, L. M. (2019). Functional phenomics: An emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. J. Exp. Bot. 70, 379–386. doi: 10.1093/jxb/ery379
York, L. M., Galindo-Castaneda, T., Schussler, J. R., and Lynch, J. P. (2015). Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. J. Exp. Bot. 66, 2347–2358. doi: 10.1093/jxb/erv074
York, L. M., Silberbush, M., and Lynch, J. P. (2016). Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architectural phenes. J. Exp. Bot. 67, 3763–3775. doi: 10.1093/jxb/erw133
Zhan, A., and Lynch, J. P. (2015). Reduced frequency of lateral root branching improves N capture from low N soils in maize. J. Exp. Bot. 66, 2055–2065. doi: 10.1093/jxb/erv007
Zhang, H.-Y., Ren, X.-X., Zhou, Y., Wu, Y.-P., He, L., Heng, Y.-R., et al. (2018). Remotely assessing photosynthetic nitrogen use efficiency with in situ hyperspectral remote sensing in winter wheat. Eur. J. Agron. 101, 90–100. doi: 10.1016/j.eja.2018.08.010
Zhang, J., Liu, Y. X., Zhang, N., Hu, B., Jin, T., Xu, H., et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37, 676–684. doi: 10.1038/s41587-019-0104-4
Zhang, X., Davidson, E. A., Mauzerall, D. L., Searchinger, T. D., Dumas, P., and Shen, Y. (2015). Managing nitrogen for sustainable development. Nature 528, 51–59. doi: 10.1038/nature15743
Keywords: agronomy, biological nitrogen fixation, breeding, microbiome, nitrogen use efficiency, policy, soil health, roots
Citation: Udvardi M, Below FE, Castellano MJ, Eagle AJ, Giller KE, Ladha JK, Liu X, Maaz TM, Nova-Franco B, Raghuram N, Robertson GP, Roy S, Saha M, Schmidt S, Tegeder M, York LM and Peters JW (2021) A Research Road Map for Responsible Use of Agricultural Nitrogen. Front. Sustain. Food Syst. 5:660155. doi: 10.3389/fsufs.2021.660155
Received: 28 January 2021; Accepted: 26 April 2021;
Published: 31 May 2021.
Edited by:
Xinhua Yin Yin, The University of Tennessee, United StatesReviewed by:
Nirmalendu Basak, Central Soil Salinity Research Institute (ICAR), IndiaDr. Tarik Mitran, Indian Space Research Organisation, India
Euan James, The James Hutton Institute, United Kingdom
Copyright © 2021 Udvardi, Below, Castellano, Eagle, Giller, Ladha, Liu, Maaz, Nova-Franco, Raghuram, Robertson, Roy, Saha, Schmidt, Tegeder, York and Peters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Udvardi, bXVkdmFyZGlAbm9ibGUub3Jn; John W. Peters, ancucGV0ZXJzQHdzdS5lZHU=
†Present address: Sonali Roy, Tennessee State University, Nashville, TN, United States
 Michael Udvardi
Michael Udvardi Frederick E. Below
Frederick E. Below Michael J. Castellano
Michael J. Castellano Alison J. Eagle4
Alison J. Eagle4 Ken E. Giller
Ken E. Giller Jagdish Kumar Ladha
Jagdish Kumar Ladha Xuejun Liu
Xuejun Liu Tai McClellan Maaz
Tai McClellan Maaz Barbara Nova-Franco
Barbara Nova-Franco G. Philip Robertson
G. Philip Robertson Sonali Roy
Sonali Roy Susanne Schmidt
Susanne Schmidt Mechthild Tegeder
Mechthild Tegeder Larry M. York
Larry M. York John W. Peters
John W. Peters