
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 29 April 2021
Sec. Nutrition and Sustainable Diets
Volume 5 - 2021 | https://doi.org/10.3389/fsufs.2021.639619
This article is part of the Research Topic Food and Nutrition Security: Underutilized Plant and Animal-Based Foods View all 20 articles
 Kaliyaperumal Ashokkumar1*
Kaliyaperumal Ashokkumar1* Sampathrajan Vellaikumar2
Sampathrajan Vellaikumar2 Muthusamy Murugan1
Muthusamy Murugan1 M. K. Dhanya1
M. K. Dhanya1 Gunasekaran Ariharasutharsan2
Gunasekaran Ariharasutharsan2 Shaji Aiswarya1
Shaji Aiswarya1 Manoharan Akilan2
Manoharan Akilan2 Thomas D. Warkentin3
Thomas D. Warkentin3 Adhimoolam Karthikeyan4*
Adhimoolam Karthikeyan4*The essential oil of cardamom capsules is a high-value ingredient in foods, beverages, perfumery, and traditional medicines. It is responsible for the characteristic aroma of cardamom. The present study aimed to evaluate essential oil yield and chemical constituents of 22 diverse accessions of cardamom. A total of 20 g of the cured capsules were hydrodistilled in a Clevenger apparatus for 3 h in three replications. The amount of essential oil yield ranged from 4.5 to 9.5%, indicating a substantial variation in this feature among the accessions. The GC/MS analysis results discovered 24 constituents that constituted 98.1–100% of total essential oil. The main fractions were found to be oxygenated monoterpenes (40.7–66.7%), monoterpene hydrocarbons (23.1–58.6%), and sesquiterpenes (0.1–2.0%). Among the monoterpenoids, the predominant constituents were α-terpinyl acetate (29.9–61.3%) followed by 1,8-cineole (15.2–49.4%), α-terpineol (0.83–13.2%), β-linalool (0.44–11.0%), and sabinene (1.9–4.9%). Two sesquiterpene constituents, cardinen and nerolidol and p-cresol (a phenol derivative) were also identified. The compositional data were subjected to euclidean-distance-based similarity analysis, which showed two major clusters. The major constituents of cardamom essential oil (CEO) are 1,8-cineole, α-terpinyl acetate, sabinene, and β-linalool that can be used in food, aroma, and pharmaceutical applications.
Elettaria cardamomum (L.) Maton, commonly known as small cardamom, Indian cardamom, green cardamom, or true cardamom, is a herbaceous perennial plant that belongs to the Zingiberaceae family. It is also called the “Queen of spices” as it is the third most valuable spice after vanilla and saffron. Cardamom is grown mainly in India, Guatemala, Sri Lanka, Nepal, and can also be found in Tanzania, Indonesia, Vietnam, Thailand, Papua New Guinea, and El Salvador (Garg et al., 2016).
Middle Eastern countries constitute major global consumers of cardamom. The wide variety of national dishes that use cardamom as a major component ingredient and the age-old practice of using cardamom spices in medicine clarifies the spice's popularity. According to data collected, Saudi Arabia was the largest importer of cardamom in 2017, accounting for 19.3% of the global market. UAE, Syria, Jordan, India, Bangladesh, and Singapore are the next countries (UN Comtrade, 2017). Cardamom is widely used as a flavoring ingredient in whole and ground form in the Middle East, especially Saudi Arabia. It is used extensively in the preparation of “kahwa”–a drink that is a sign of hospitality in any home. It gives a lingering flavor in most Asian cuisines. Cardamom is used in baked goods and confectioneries in Scandinavian countries. Curry powder and some sausage products in Europe and North America contain it (https://cardamomassociation.com/report/cardamom/).
Despite cardamom as a spice and in the pharmaceutical and cosmetic industries, the European market remains limited. The crushed cardamom capsules are boiled with tea and water in south India to add a good fragrance to tea, which is popularly known as “Elakkai tea” and has been used to alleviate tiredness and depression (Ashokkumar et al., 2020a). Some people believe that excessive cardamom capsules' excessive use could cause impotence in humans (Nair, 2011). However, to date, there is no scientific evidence reported that the daily consumption limit of cardamom. The cardamom preparation, “Eladigana chooranm” is commonly used to cure arthritis, congestion, and itching in south Indian Ayurvedic medicine (Nair, 2011). Cardamom capsule extracts and cardamom essential oil (CEO) have numerous potential therapeutic activities. The use of this plant as a source of various natural products has a great interest in many parts of the world. According to Hamzaa and Osman (2012) and Khan et al. (2011), cardamom capsules have been used in traditional medicine for controlling asthma, nausea, diarrhea, cataracts, teeth, and gum infections, digestive, kidney, and cardiac disorders. Besides folk medicinal uses, potential applications in modern medicine have been explored (Saeed et al., 2014; Elguindy et al., 2018).
Cardamom capsules are a storehouse of several bioactive metabolites like flavonoids, carotenoids, and terpenes, etc. (Ashokkumar et al., 2019, 2020a,b). The primary bioactive metabolites of CEO contribute to its characteristic strong aromatic aroma. The CEO's concentration in cardamom capsules ranges from 6 to 14%, depending upon the extraction and processing methods (Nirmala Menon, 2000). The CEO's composition could rely on the origin of the sample, varieties, and parts used (Ashokkumar et al., 2020a). The CEO is rich in monoterpene constituents like α-terpinyl acetate, 1,8-cineole (28.94–34.91%), α-terpineol (12.47–14.89%), sabinene (11.17–13.50%), nerol (3.69–6.10%), β-linalool (1.43–2.97%), and α-pinene (1.15–2.42%) (Murugan et al., 2005, 2019; Yashin et al., 2017; Ashokkumar et al., 2019). These predominant constituents have potential pharmacological and therapeutic properties such as antioxidant, anti-inflammatory, antidiabetic, anticancerous, antimicrobial, antiviral, and gastroprotective activities (Hamzaa and Osman, 2012; Winarsi et al., 2014).
Several studies showed substantial variation in the essential oil from cardamom capsules; however, these samples' origin has generally not been sufficiently defined. In several cases, commercial samples from unknown original habitat and bulk samples obtained from the mixture of various cardamom genotypes, which do not truly represent the individual chemo-types. Besides, the CEO's major constituents can be affected by several factors like origin, soil types, seasonal influence, storage, processing conditions, and extraction methods. Hence, most of the studies have not adequately elucidated the true chemo-diversity of CEO. The variability in essential oil constituents among cardamom types is very high (Murugan et al., 2005; Ashokkumar et al., 2019). The chemo-profiling of elite cardamom types has been done for the first time. Thus far, there has been limited information on the chemical composition of essential oils extracted from south Indian E. cardamomum accession. Identifying cardamom accessions with higher essential oil may offer future breeding activities. In this context, the present study's objective was to evaluate essential oil yield and chemical constituents of 22 diverse accessions of E. cardamomum, which will aid the selection of suitable accessions to address consumer and manufacture demand.
The Cardamom Research Station, Pampadumpara, Idukki, Kerala, India located at 9°45′ N latitude, 77°10′ E longitude, and altitude is 1,100 m above mean sea level. This station is maintained 187 germplasm collections. Among them, 22 cardamom accessions were grown under uniform field conditions, and each accession had 12 plants. The plants were planted at spacing 2.5 × 2.5 m, and fertilizer was applied 100:100:250 Kg NPK ha−1, yr−1 in two splits before (May–June) and after (September–October) the first primary season. The selected 22 accessions were chosen based on the observation made by the previous year's high yield, pest, and disease resistance potential. The matured capsules were harvested from 5-year-old plants during June 2019 and cured as per the standard procedure of Kerala Agricultural University (KAU, 2011) to evaluate CEO composition. Sufficient quantities (100 g) of cured capsules of each accession were stored at room temperature (24°C). The diagrammatic representation of usable parts of cardamom is presented in Figure 1.
Cured capsules collected from each accession were ground individually into a fine powder (20 μ). Twenty grams of powdered sample was placed in a 500 ml distillation flask separately, to which 250 ml deionized water was added. Several studies from cardamom capsules have been utilized a similar sample weight of 20 g and produced 0.8–1.5 ml of essential oil (Murugan et al., 2005, 2019; Ashokkumar et al., 2019). A hydro distillation run time of 3 h was used to obtain the optimum yield (Ashokkumar et al., 2019). Oil yield was estimated with an average of three replications. The obtained essential oil was dried over anhydrous sodium sulfate, weighed, and then stored at 4°C in the dark until use. The essential oil yield was calculated as a volume by weight basis using the following formulae: Essential oil (%, v/w) = volume of oil collected (ml)/weight of the sample (g) × 100 (AOAC, 2000).
The qualitative analysis of CEO was carried out through gas chromatography (GC) coupled with a mass spectrometer (MS) (GC-MS—QP2020 NX SHIMADZU). The GC was equipped with a fused silica capillary column, Rxi®-5 Sil MS (20 m, 0.18 mm ID), with a film thickness of 0.18 μM. The EO was injected by split mode (1:20). The helium gas flow rate was constantly maintained at 1 ml/min (Ashokkumar et al., 2019). The oven temperature was programmed at 70°C for 15 min and then gradually increased at 6°C/min to 200°C and then 30°C/min to 280°C (10 min). The detector and injector temperature was maintained at 290°C. The MS conditions were electron energy 70 eV, electron impact (EI) ion source temperature 260°C, and transmission line temperature 280°C. The mass scan range (m/z) was 50–650 amu, data acquired at full scan mode with solvent delay for 3 min. The qualitative analysis of volatile oil was carried using Shimadzu GC/MS solution™ Ver.4 software. The CEO constituents were identified by comparing retention indices (RI) under programmed similar oven temperature conditions for homologous series of n-alkanes (C8–C24). Identification of individual essential oil constituents was based on comparing mass spectra with those present in NIST and Wiley libraries and literature data (Adams, 2007). Identification of certain compounds (1,8-cineole, α-terpineol, β-linalool, α-pinene, β-pinene, sabinene, α-terpinyl acetate, α-citral, nerol, and geranyl acetate) was further confirmed by co-injection of their authentic standards (Sigma-Aldrich, Mumbai, India) under same chromatographic conditions mentioned above.
Hierarchical clustering was used to understand the relationship between the cardamom accessions based on essential oil composition and determine the chemotypes. Euclidean distance was selected to measure the similarity and the nearest-neighbor method used for cluster definition (Gwari et al., 2016). SPSS software (version 24.0) used for cluster analysis (Cor, 2016) (IBM Corp 2016).
The extraction of CEO was performed by hydrodistillation method, and an average yield of three separate analyses was ranged from 4.5 to 9.6%. Among the accessions, the highest essential oil content was observed in accession HY 3 (9.6%) followed by SAM 5 (9.3%), which were higher than previously reported in four different cardamom types viz., malabar, mysore, vazhukka, and guatemala (7.9–8.8%) (Padmakumari et al., 2010). CEO varied with various extraction methods, varieties, and plant parts (Ashokkumar et al., 2020b). GC/MS examined the CEO extracted from these 22 accessions. The list of constituents identified among the accessions is presented in Table 1. The constituents found in the greatest quantity were 1,8-cineole, α-terpinyl acetate, α-terpineol, sabinene, and β-linalool. The typical CEO chromatograms of major bioactive constituent-rich chemotypes are shown in Figure 2.
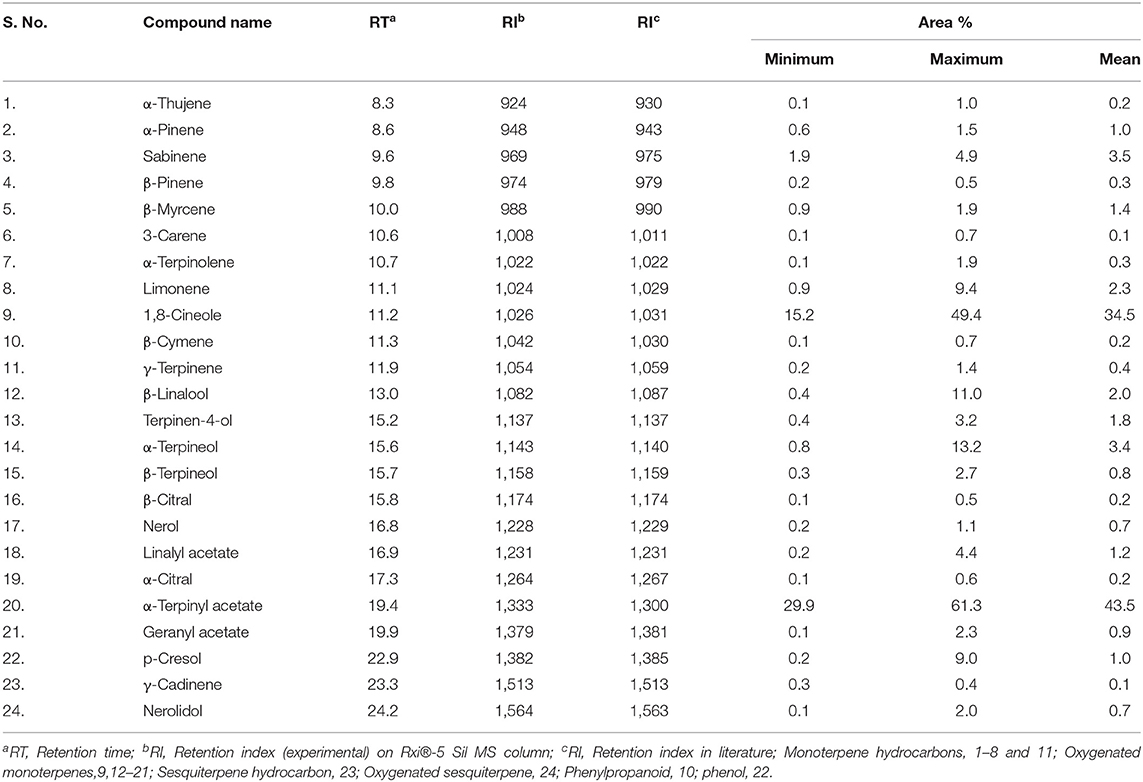
Table 1. Minimum and maximum range, retention time, retention index of essential oil compounds in 22 cardamom accessions.
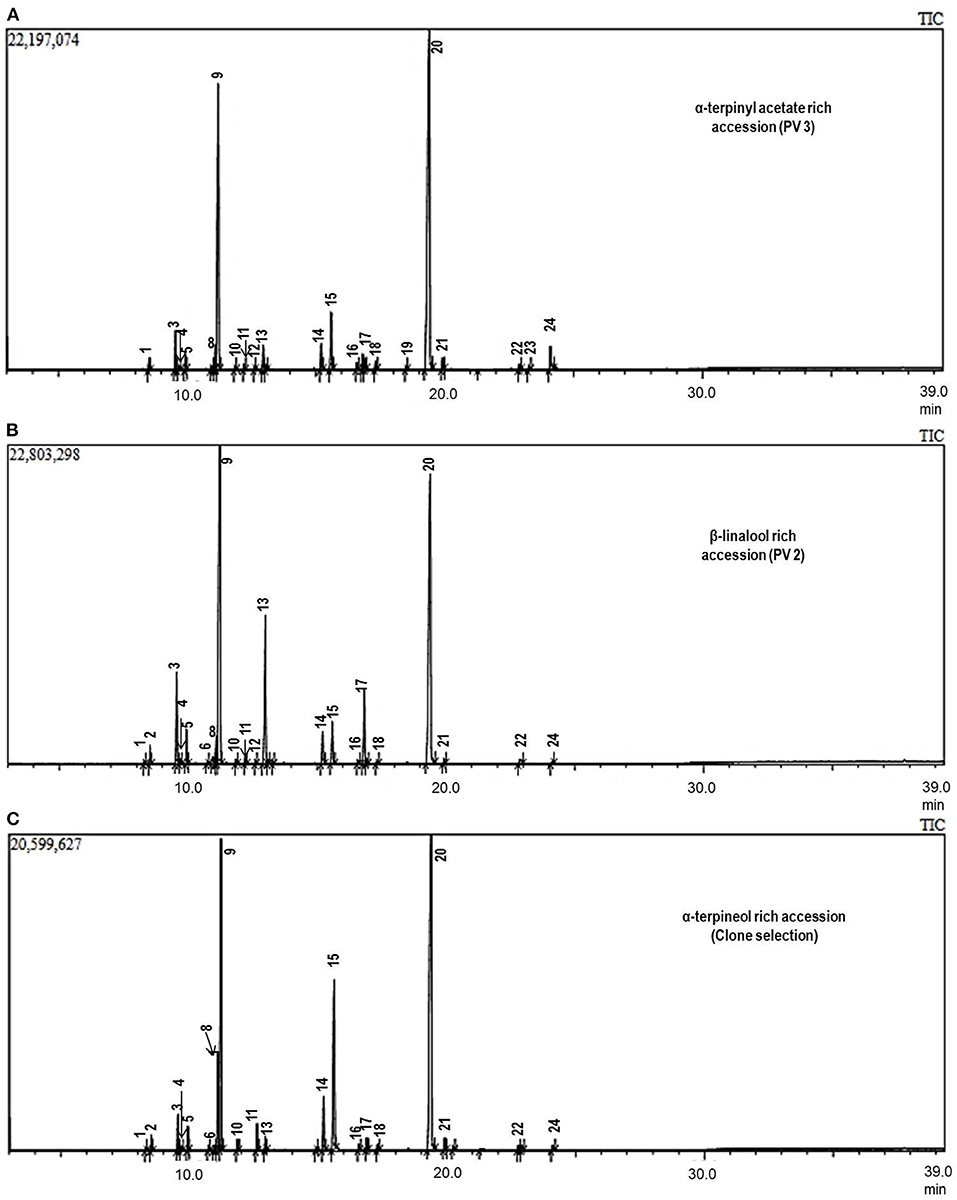
Figure 2. Essential oil chromatogram of chemovariants of Elettaria cardamomum (L.) Maton: (A) α-terpinyl acetate-rich chemotype, (B) β-linalool rich chemotype, and (C) α-terpineol rich chemotype.
The chemical profile of 22 cardamom diverse accessions essential oil showed the presence of 24 volatile constituents, which comprised about 98.1–99.9% of the total essential oil. Oxygenated monoterpenes dominated oil composition with 81.6–92.5%, followed by monoterpene hydrocarbons (6.2–14.5%), oxygenated sesquiterpenes (0.1–2.0%), and sesquiterpene hydrocarbon (0.3–0.4%) (Table 1). Among the monoterpenes, α-terpinyl acetate (29.9–61.3%) is the predominant constituent and was found in CEO of all the cardamom accessions evaluated. Similar results were observed in earlier studies (Ashokkumar et al., 2019), based on that highest α-terpinyl acetate (61.3%) was observed in accession PV3. The second most major monoterpene constituent, 1,8-cineole (15.2–49.4%), also presents all the cardamom accession's essential oil. According to a recent study conducted by Alagupalamuthirsolai et al. (2019), cardamom variety Appangala-1 had a 1,8-cineole concentration of 41.8% under 75% shade condition. In our research, the accession HY15 had recorded the highest 1,8-cineole (49.4%) compared with previous reports. This higher concentration could depend on varietal difference and environmental effects (Ashokkumar et al., 2020a). However, the present study shows that a higher concentration of α-terpinyl acetate in some cardamom accessions had a lesser concentration of 1,8-cineole and vice versa.
The amount of several monoterpene compounds accumulated substantially viz., sabinene (1.9–4.9%), β-terpineol (0.3–2.7%), β-linalool (0.4–11.0%), terpinen-4-ol (0.4–3.2%), α-terpineol (0.8–13.2%), geranyl acetate (0.1–2.3%), linalyl acetate (2.2–4.4%), D-limonene (0.9–9.4%), and nerol (0.2–1.1%) have varied among the accessions. Furthermore, the higher concentration of sabinene (4.9%), β-linalool (11.0%), α-terpineol (13.2%), and nerol (1.1%) was present in accessions PV1, PV2, Clone selection, and Kaniparamban, respectively (Table 2).
Two sesquiterpene constituents, cardinen and nerolidol, and a phenol derivative of p-cresol were also identified (Table 2). Different chemotypes have been reported from CEO richness of 1,8-cineole, α-terpinyl acetate, α-terpineol, β-terpineol, β-myrcene, sabinene, β-linalool, α-terpinyl acetate, geranyl acetate, limonene, nerol, and linalyl acetate was reported by several workers (Miniraj et al., 2000; Murugan et al., 2002, 2005; Kumar et al., 2005; Kaskoos et al., 2006; Goudarzvand Chegini and Abbasipour, 2017; Alagupalamuthirsolai et al., 2019; Ashokkumar et al., 2019). To find out the similarity of oil composition among the 22 cardamom accessions, a hierarchical cluster analysis was carried out based on the composition of its major constituents (Figure 3). Two major clusters were identified, namely, clusters 1 and 2. Cluster 1 is composed of 12 accessions, while cluster 2 is composed of 10 accessions. Cluster 1 formed four sub-clusters: Green gold (GG), SAM5, PV4, HY9, and Elarajan; PV34, HY11, PV1, PV8, and Kaniparamban; Clone selection and PV3. Cluster 2 formed four sub-clusters, namely HY15 and Pink base; PS1 x GG Type-1, Minipink, HY12, HY14, HY13, and HY3; PV5; and PV2. Accessions in the same sub-cluster were similar in essential oil composition. In brief, accessions in cluster-1 had higher α-terpinyl acetate concentration, and cluster 2 accessions had higher 1,8-cineole concentration. Accessions having the highest α-terpinyl acetate concentration (61.3%) were in cluster 1, and accessions with the highest 1,8-cineole (49.4%) concentration were in cluster 2. Furthermore, Clone selection had the highest D-limonene concentration (9.4%), and PV3 had the highest p-cresol concentration (9.0%) and is separately sub clustered in cluster 1. Accession PV2 had the greatest concentration of β-linalool (11.0%) and sub-clustered in cluster 2.
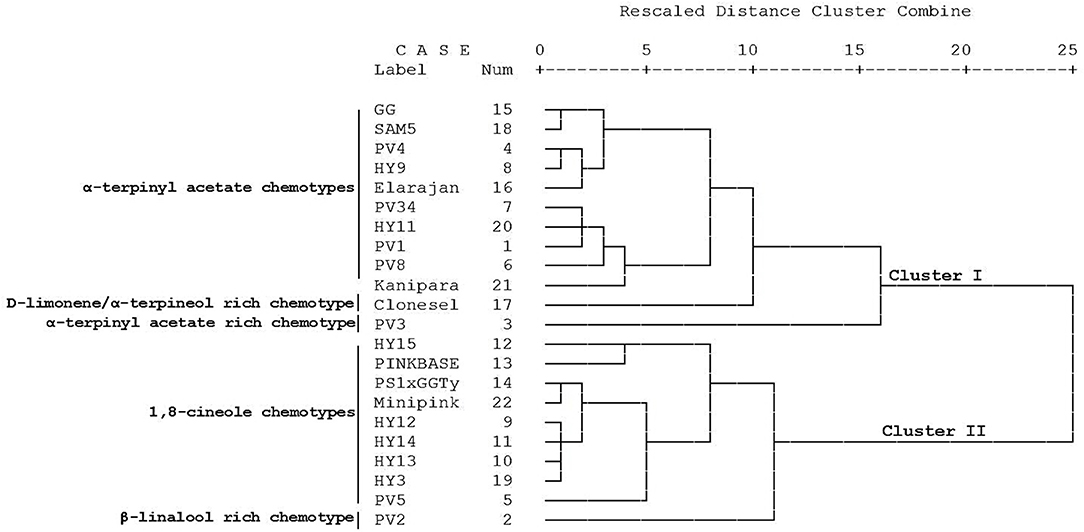
Figure 3. Clustering (nearest-neighbor method using the Euclidean distance) based on oil constituents for 22 cardamom accessions.
These chemotypes offer unlimited opportunity to produce cardamom to meet the market supplies of essential oil and individual constituents. Further, the present study revealed new essential oils/chemotypes (γ-cadinene) of the cardamom (PV3, PV8, PV34, HY11, and Kaniparamban), which were not described before from southern India. Also, new essential oils/chemotypes (3-carene) of the Cardamom were not described in this region. The results of this research will be useful for cardamom breeders to choose accessions for developing new varieties with greater CEO concentration and a higher concentration of specific pharmaceutically desired CEO constituents.
The present study investigated the essential oil yield and chemical composition from 22 promising cardamom accessions from south India. This research demonstrated substantial variation in essential oil concentration and its composition of available cardamom accessions. Since all accessions were grown under the same environmental conditions, the effects of the environmental factors such as soil type, shade, and location have been excluded from the compositional variation of CEO. In this study, we have identified that cardamom accessions had predominantly oxygenated monoterpene compounds followed by monoterpene hydrocarbons, and sesquiterpenes. The CEO compositional data were subjected to similarity analysis, which showed two major clusters. Cluster 1 and 2 is composed of 12 and 10 accessions, respectively. In overall observation, Cluster-1 accessions had higher α-terpinyl acetate concentration, and cluster 2 accessions had higher 1,8-cineole concentration. The accession PV 3 had the highest α-terpinyl acetate (61.3%), and accession HY 15 had the highest 1, 8-cineole (49.4%) were in clusters 1 and 2, respectively. These outcomes implied that there is a large potential for domestication, cultivation, and selective breeding programs. Also, these results could be used as a database for trading and pharmaceutical sectors engaged with cardamom processing. Farmers will also benefit from the study results by adopting suitable varieties with superior quality for cultivation. Furthermore, the two major constituents of CEO were α-terpinyl acetate, and 1,8-cineole can serve as a new potential natural source, which can be used in the food, aroma, cosmetics, and pharmaceutical domains.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
KA and MM conceptualized the study. KA, SV, GA, and MA performed the experiment. SA, MKD, and AK collected the samples and performed the data analyses. KA, TW, and AK drafted the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors express their gratefulness to the AICRP on Spices, Indian Council of Agricultural Research, and New Delhi, India.
Adams, R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL: Allured Publishing Corporation.
Alagupalamuthirsolai, M., Ankegowda, S., Murugan, M., Sivaranjani, R., Rajkumar, B., and Akshitha, H. (2019). Influence of light intensity on photosynthesis, capsule yield, essential oil and insect pest incidence of small cardamom (Elettaria cardamomum (L.) Maton). J. Essent. Oil Bear. Plants 22, 1172–1181. doi: 10.1080/0972060X.2019.1690587
AOAC (2000). Official Methods of Analysis of AOAC International, 17th Edition. Gaithersburg, MD: AOAC.
Ashokkumar, K., Murugan, M., Dhanya, M., Raj, S., and Kamaraj, D. (2019). Phytochemical variations among four distinct varieties of Indian cardamom Elettaria cardamomum (L.) Maton. Nat. Prod. Res. 34, 1919–1922. doi: 10.1080/14786419.2018.1561687
Ashokkumar, K., Murugan, M., Dhanya, M., and Warkentin, T. (2020a). Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton] - a critical review. J. Ethnopharmacol. 246:112244. doi: 10.1016/j.jep.2019.112244
Ashokkumar, K., Pandian, A., Murugan, M., Dhanya, M., Sathyan, T., Sivakumar, P., et al. (2020b). Profiling bioactive flavonoids and carotenoids in select south Indian spices and nuts. Nat. Prod. Res. 34, 1306–1310. doi: 10.1080/14786419.2018.1557179
Elguindy, N. M., Yacout, G. A., and El Azab, E. F. (2018). Amelioration of DENA-induced oxidative stress in rat kidney and brain by the essential oil of Elettaria cardamomum. Beni Suef Univ. J. Basic Appl. Sci. 7, 299–305. doi: 10.1016/j.bjbas.2018.02.005
Garg, G., Sharma, S., Dua, A., and Mahajan, R. (2016). Antibacterial potential of polyphenol rich methanol extract of Cardamom (Amomum subulatum). J. Innov. Biol. 3, 271–275.
Goudarzvand Chegini, S., and Abbasipour, H. (2017). Chemical composition and insecticidal effects of the essential oil of cardamom, Elettaria cardamomum on the tomato leaf miner, Tuta absoluta. Toxin Rev. 36, 12–17. doi: 10.1080/15569543.2016.1250100
Gwari, G., Lohani, H., Bhandari, U., Haider, S. Z., Singh, S., Andola, H., et al. (2016). Chemical diversity in the volatiles of Perilla frutescens (L.) Britt. populations from Uttarakhand Himalaya (India). J. Essent. Oil Res. 28, 49–54. doi: 10.1080/10412905.2015.1081415
Hamzaa, R. G., and Osman, N. N. (2012). Using of coffee and cardamom mixture to ameliorate oxidative stress induced in γ-irradiated rats. Biochem. Anal. Biochem. 1, 2161–1009. doi: 10.4172/2161-1009.1000113
Kaskoos, R. A., Ali, M., Kapoor, R., Akhtar, M. M. S., and Mir, S. R. (2006). Essential oil composition of the fruits of Eletteria cardamomum. J. Essent. Oil Bear. Plants 9, 81–84. doi: 10.1080/0972060X.2006.10643475
KAU (2011). Package of Practices Recommendations: Crops, 14th Edn. Thrissur: Kerala Agricultural University. 360 pages.
Khan, A.-U., Khan, Q. J., and Gilani, A.-H. (2011). Pharmacological basis for the medicinal use of cardamom in asthma. Bangl. J. Pharmacol. 6, 34–37. doi: 10.3329/bjp.v6i1.8133
Kumar, A., Tandon, S., Ahmad, J., Yadav, A., and Kahol, A. (2005). Essential oil composition of seed and fruit coat of Elettaria cardamomum from South India. J. Essent. Oil Bear. Plants 8, 204–207. doi: 10.1080/0972060X.2005.10643446
Miniraj, N., Murugan, M., and Joseph, C. R. (2000). Evaluation of cardamom (Elettaria cardamomum Maton) germplasm. J. Spices Aromat. Crops 9, 55–56.
Murugan, M., Dhanya, M. K., Ashokkumar, K., Thiravidamani, S., and Raj, S. (2019). Changes of enzyme activities and phytochemical constituents in small cardamom capsules caused by the infestation of thrips, Sciothrips cardamomi (Ramk.). Res. J. Biotechnol. 14, 113–116.
Murugan, M., Josephrajkumar, A., Backiyarani, S., and Sainamole Kurian, P. (2002). Essential oil of cardamom (Elettaria cardamomum M): effect of infestation by thrips (Sciothrips cardamomi Ramk.). Ind. Perfum. 46, 321–323.
Murugan, M., Josephrajkumar, A., Sheeba, B., Vasanthakumar, K., and Ambikadevi, D. (2005). Essential oil profile of elite small cardamom (Elettaria cardamomum M.) accessions and their interaction with thrips (Sciothrips cardamomi Ramk.) infestation. Ind. Perfum. 49, 219–224.
Nair, K. P. P. (2011). The agronomy and economy of cardamom (Elettaria cardamomum M.):the “queen of spices”. in Agronomy and Economy of Black Pepper and Cardamom; The King and Queen of Spices, ed K. P. P. Nair (London: Elsevier), 109–366.
Nirmala Menon, A. (2000). Studies on the volatiles of cardamom (Elleteria cardamomum). J. Food Sci. Technol. 37, 406–408.
Padmakumari, K., Rani, M. P., Sasidharan, I., Nair, V., and Nisha, P. (2010). Chemical composition, flavonoid-phenolic contents and radical scavenging activity of four major varieties of cardamom. Int. J. Biol. Med. Res. 1, 20–24.
Saeed, A., Sultana, B., Anwar, F., Mushtaq, M., Alkharfy, K. M., and Gilani, A.-H. (2014). Antioxidant and antimutagenic potential of seeds and pods of green cardamom (Elettaria cardamomum). Int. J. Pharmacol. 10, 461–469. doi: 10.3923/ijp.2014.461.469
UN Comtrade (2017). Available online at: https://comtrade.un.org/ (accessed March 29, 2021).
Winarsi, H., Sasongko, N., Purwanto, A., and Nuraeni, I. (2014). Effect of cardamom leaves extract as antidiabetic, weight lost and hypocholesterolemic to alloxan-induced Sprague Dawley diabetic rats. Int. Food Res. J. 21, 2253–2261.
Keywords: cardamom, essential oil, GC/MS analysis, 1,8-cineole, α-terpinyl acetate
Citation: Ashokkumar K, Vellaikumar S, Murugan M, Dhanya MK, Ariharasutharsan G, Aiswarya S, Akilan M, Warkentin TD and Karthikeyan A (2021) Essential Oil Profile Diversity in Cardamom Accessions From Southern India. Front. Sustain. Food Syst. 5:639619. doi: 10.3389/fsufs.2021.639619
Received: 09 December 2020; Accepted: 06 April 2021;
Published: 29 April 2021.
Edited by:
Olivia Renee Louise Wright, The University of Queensland, AustraliaReviewed by:
Tânia Gonçalves Albuquerque, Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA), PortugalCopyright © 2021 Ashokkumar, Vellaikumar, Murugan, Dhanya, Ariharasutharsan, Aiswarya, Akilan, Warkentin and Karthikeyan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaliyaperumal Ashokkumar, YmlvdGVjaC5hc2hva0BnbWFpbC5jb20=; Adhimoolam Karthikeyan, a2FydGhpazIzNzNAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.