- 1Laboratory of Soil Microbiology, Division of Crop Production, Central Rice Research Institute, Cuttack, India
- 2School of Biotechnology, KIIT University, Bhubaneswar, India
Submergence stress slows seed germination, imposes fatalities, and delays seedling establishment in rice. Seeds of submergence susceptible rice variety IR 42 were inoculated with four 1-aminocyclopropane-1-carboxylic acid (ACC) utilizing isolates viz., Bacillus sp. (AR-ACC1), Microbacterium sp. (AR-ACC2), Methylophaga sp. (AR-ACC3), and Paenibacillus sp. (ANR-ACC3) and subjected to submergence stress under controlled conditions for 7 days. Seeds treated with Microbacterium sp. AR-ACC2, Paenibacillus sp. ANR-ACC3, and Methylophaga sp. AR-ACC3 significantly enhanced the germination percentage (GP), seedling vigor index (SVI), and other growth parameters like root and shoot length and total chlorophyll contents, when compared with nonbacterized seeds submerged similarly. However, the values were statistically at par when control seeds were treated with L-α-(2-aminoethoxyvinyl) glycine hydrochloride (AVG), a known inhibitor of ethylene production. Results suggest that stress ethylene production was significantly reduced by around 85% in seedlings treated with Microbacterium sp. AR-ACC2 as compared with untreated control seeds under submergence. Paenibacillus sp. ANR-ACC3 and Methylophaga sp. AR-ACC3 were the next effective strains. Ethylene synthesis in seedlings was statistically at par with seeds treated with AVG suggesting ACC deaminase can effectively reduce ethylene levels in plants subjected to submergence. Bacillus sp. (AR-ACC1) was neither able to significantly promote seedling growth parameters nor inhibit ethylene production as compared with control seeds. Results suggest that flooded soil planted to rice harbor microorganisms with plant growth-promoting properties that can be used effectively to alleviate submergence stresses in susceptible rice varieties under field conditions.
Introduction
Soil flooding is one of the most important abiotic constraints for rice yields, with complete submergence of plants being particularly serious for rice farmers in the rainfed lowlands of humid and semihumid tropics of Asia (Jackson and Ismail, 2015). Indeed, submergence affects more than 5 million ha of rainfed lowlands in India resulting into drastic reduction in rice growth and yield (Sandhu et al., 2019). Heavy rainfall and poor drainage causing accumulation of water in the fields shortly after sowing, leads to poor crop establishment and causes hindrance to widespread acceptance of direct-seeded rice practices. This is due to the inability of most rice varieties to geminate and reach water surface under complete submergence. Submergence is also an acute problem at the early growth stages which causes serious damages of plants by uprooting of the seedlings particularly at coastal lowland where traditional semidwarf varieties are cultivated (Kato et al., 2020). Plants tolerant to submergence stress exhibit morphological adaptations like shoot elongation, formation of aerenchyma and adventitious roots, and metabolic adaptations like activation of fermentation process, induction of anaerobic proteins (ANPs), and hormonal regulation (Magneschi and Perata, 2009; Jackson and Ismail, 2015).
Ethylene is an important plant growth hormone mediating growth and development of plant only at optimal concentration. Both indole acetic acid (IAA) and ethylene have ability to promote plant growth in a coordinate fashion. IAA-induced ethylene at lower concentration promotes initiation of root but at higher concentration, inhibiting root growth (Ali and Kim, 2018). Complete submergence induces ethylene biosynthesis and ethylene accumulation in most plant species (Khan et al., 2020). In contrast to other stresses, gas diffusion is inhibited under submergence because the diffusion coefficient of ethylene in water is 10,000 times lower than in air. The excess ethylene entrapped in the plant tissue and its effect under submergence is crop species specific. Rice is the most studied crop for submergence-tolerant species because it is an important crop in humid tropic and areas affected by high rainfall (Jackson, 2008; Khan et al., 2020).
During submergence, the increase in ethylene production is due to an increase in the activity of both 1-aminocyclopropane-1-carboxylic acid (ACC) synthase in the submerged roots and ACC oxidase in the shoots. Induction of ACC synthase enhances ACC level in root where as its oxidation to ethylene is blocked due to anoxic condition. This accumulated and unmetabolized ACC transport to the stem by the transpiration stream and convert to ethylene by ACC oxidase enzyme in the presence of oxygen. This boost in ACC oxidase activity in stem initiates the adventitious root formation during submergence (Fukao and Bailey-Serres, 2008).
The growth inhibitory effect of the submergence stress on crop plants can be alleviated by lowering the stress ethylene level. Beside several harmful chemical ethylene inhibitors, plant growth-promoting rhizobacteria (PGPR) having ACC deaminase enzyme alleviate stress ethylene levels by cleaving ACC to α-ketobutyrate and ammonia (Oleńska et al., 2020). It has been the subject of much research due to its biochemical properties, action, substrate specificity, and even its genetic regulation and mode of heredity. Lowering of stress ethylene level facilitates the crop to substantially tolerate different environmental stresses, all of which induce the plant to increase its endogenous level of ethylene (Ali and Kim, 2018).
Grichko and Glick (2001a) studied the effect of inoculation with ACC deaminase producing PGPR on tomato subjected to flooding. Seeds of wild-type tomato plants were inoculated either with Pseudomonas putida UW4, Enterobacter cloacae CAL2, P. putida (ATCC17399/pRKACC), or P. putida (ATCC17399/pRK415); the first three of these bacterial strains were carrying and expressing the gene for ACC deaminase. The inoculation of ACC deaminase containing PGPR resulted in statistically significant differences in overall plant growth, leaf chlorophyll content, and substantially decreased ethylene production in leaf petiole tissue, thereby ameliorating some of the damages to plants caused by flooding. Thus, the “protective effect” of ACC deaminase-containing plant growth-promoting bacteria on flooded tomato plants results from these bacteria acting as a sink for ACC, thereby lowering the level of ethylene that can be formed in the shoots.
Rice is the second most widely consumed cereal in the world next to wheat and more often than not is subjected to submergence stress during its growing period (Oladosu et al., 2020). In the present study, we examined the efficiency of ACC deaminase containing PGPR, isolated from rice field soils, in alleviating the inhibitory effect of submergence stress in germinating rice seeds and on seedling growth. Various parameters of rice growth at the seedling stage were thoroughly evaluated to understand their possible growth-promoting activities on rice. We also assessed whether inoculation with select PGPR would modulate the ethylene level in plant to enhance its growth.
Materials and Methods
Bacterial Strains and Their Plant Growth-Promoting Traits
Bacillus sp. AR-ACC1 (HM063033), Microbacterium sp. AR-ACC2 (HM063034), Methylophaga sp. AR-ACC3 (HQ222610), and Paenibacillus sp. ANR-ACC3 (HM063032) were isolated from soil samples of Ganjam district of Orissa (19° 11′ 04.7″ to 20° 06′ 48.7″ N and 84° 48′ 06.3″ to 85° 12′ 49.5″ E) (Bal et al., 2013). These bacterial strains were isolated based upon their ability to utilize ACC, present in sterile DF salt minimal medium, as the sole nitrogen source (Dworkin and Foster, 1958). All the four strains were screened positive for IAA and ammonia production and only two strains (Microbacterium sp. AR-ACC2 and Paenibacillus sp. ANR-ACC3) produced siderophore but none of them were phosphate solubilizer and HCN producer (Bal et al., 2013). All the four isolates were believed to be plant growth-promoting rhizobacteria based on their ability to promote the elongation of rice roots under gnotobiotic conditions (Bal et al., 2013). The isolates were grown on either solid or liquid tryptic soy broth (TSB) medium at 30°C.
Alleviation of Submergence Stress in Rice by PGPR Inoculation
Plant Materials and Experimental Set-Up
The seeds of two rice cultivars contrasting in their tolerance to submergence stress namely, cv. IR42 which is a susceptible cultivar and cv. Panikekua, resistant to submergence stress, were collected from the Germplasm collection center of Central Rice Research Institute, Cuttack. Seeds were surface sterilized by dipping in 95% ethanol and in 0.2% HgCl2 solution for 3 min followed by rinsing five times with sterile distilled water (Glick, 2020). Fifty seeds of each cultivar were placed on the field soil (150 g) surface in tall beakers (1,000 ml) and submerged with sterile distilled water to a depth of 10 cm. The beakers were maintained in the laboratory (28 ± 2 C) in diffuse light. Germination was measured daily up to 7 days.
PGPR Inoculation to Rice Under Submergence Stress
Bacterial suspensions of four isolates were prepared by growing the strains in 250 ml conical flask containing DF salt minimal medium at 28°C for 24 h in an orbital shaking incubator at 100 rpm. The cultures were centrifuged at 8,000 × g, and the bacterial cell pellets of the strains were suspended in 0.5 ml sterile 0.03 M MgSO4 and the absorbance was adjusted to OD = 1 at 600 nm (Penrose and Glick, 2003). Surface sterilized rice seeds (cv. IR42) were incubated for 1 h at room temperature with the appropriate treatment: sterile 0.03 M MgSO4 (negative controls), 10−4M AVG (Sigma, India) in 0.03 M MgSO4 (positive control), and fresh bacterial suspensions in sterile 0.03 M MgSO4 (Penrose and Glick, 2001).
The experiment was carried out in sterile 1,000 ml tall glass beaker for 7 days. After incubation with each treatment, 25 seeds were planted by sterilized forceps on the soil (150 g) surface in tall beakers and submerged with sterile distilled water to a depth of 10 cm inside laminar airflow. The beakers were placed in a growth chamber in completely randomized design with five replications for each treatment. Maximum and minimum temperatures were maintained at 28 and 20°C, respectively with a cycle of 12 h dark/light (Sapsirisopa et al., 2009). The number of seeds that sprouted and germinated was counted daily up to 7 days. After final count, germination percentage (GP) and seedling vigor index (SVI) were calculated using the equations described by Long et al. (2008). Plant growth parameters like root length (RL), shoot length (SL), root fresh weight (RFW), shoot fresh weight (SFW), root dry weight (RDW), and shoot dry weight (SDW) of 10 randomly selected seedlings from each replication were measured at the time of harvest. Chlorophyll concentrations were determined by spectrophotometry in 80% acetone extracts following the equation described by Porra (2002).
Estimation of Ethylene Level in Plant Tissue
For ethylene estimation in rice seedlings by gas chromatography (GC), five seedlings each from different treatments were kept in tightly sealed vials in the dark for 1 h at 30°C. Headspace gas (1 ml) was drawn by airtight syringe (2 ml) and injected into GC (Model-Ceres 800 plus, Thermo-Scientific) packed with a Porapak-Q column (183 cm length and 0.3 cm internal diameter, 80/100 mesh) and equipped with flame ionization detector (FID). The GC was adjusted to 100, 300, and 150°C for oven, injection, and detection temperature, respectively. The carrier gas was N2 at a flow rate of 30 ml min−1, and the combustion gas was H2 at a flow rate of 30 ml min−1 with air at the flow rate of 300 ml min−1. The amount of ethylene emission was expressed as nmol of ethylene gfw (fresh weight)−1 h−1 by comparing the standard curve of pure ethylene (9.12 ppm in nitrogen, Matheson Tri-Gas) (Fišerová et al., 2008; Siddikee et al., 2011).
Statistical Analysis
Data on various character sets were subjected to statistical analysis by using a statistical package (IRRISTAT version 3.1: International Rice Research Institute, Los Banos, Philippines). The mean difference comparison between the treatments was analyzed by analysis of variance wherever necessary and subsequently by Duncan's multiple range test (DMRT) at P < 0.05.
Results
Screening Submergence Tolerance of Rice Cultivars
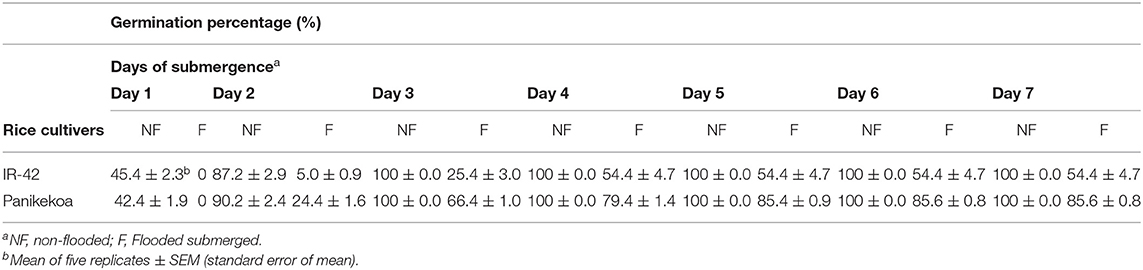
The submergence susceptible rice cultivar, IR42, and tolerant cultivar Panikekua had the same germination efficiency (100%) after 3 days of growth under normal conditions, but GP of both the cultivar decreased under submergence, with a substantially greater reduction in the susceptible cultivar (54%) (Table 1). Moreover, the germinated seeds of the susceptible variety became brown in color and died after 6 days of submergence (Figure 1). Hence, for this study, susceptible variety, IR42 was selected to screen the effect of ACC deaminase producing PGPR strains on rice seedling growth under submergence.

Figure 1. Root and shoot growth of 7-day-old rice (cv. IR-42) seedlings exposed to submergence stress under gnotobiotic conditions. (A) 0.03 M MgSO4 (negative control); (B) Bacillus sp. (AR-ACC1); (C) Methylophaga sp. (AR-ACC3); (D) Paenibacillus sp. (ANR-ACC3); (E) Microbacterium sp. (AR-ACC2); (F) AVG (positive control).
Effect of PGPR Strains on Seed Germination Under Submergence Stress
All the four ACC utilizing isolates identified as Bacillus sp., Microbacterium sp., Methylophaga sp., and Paenibacillus sp. respectively, enhanced the overall plant growth under normal condition (Bal et al., 2013) and were subsequently investigated to quantify the effect of these rhizobacteria in ameliorating the damage caused by submergence stress.
The effects of the bacterial strains on seed germination, seedling vigor, and ethylene synthesis are summarized in Table 2. Seeds treated with Microbacterium sp. AR-ACC2 and Paenibacillus sp. ANR-ACC3 enhanced the GP by 48.15 and 40.74% over the negative control. These effects were, however, statistically at par with that of the positive control of amendment with AVG (Sigma, India), a known inhibitor of ethylene production (44.4% more than negative control). Inoculation with Microbacterium sp. AR-ACC2 also increased seedling vigor when compared with the negative control. It was followed by the vigor of seeds treated with Paenibacillus sp. ANR-ACC3 and Methylophaga sp. AR-ACC3 and was statistically at par with the treatment by AVG (Table 2). However, the isolate Bacillus sp. AR-ACC1 was not able to enhance the seedling vigor under submergence.
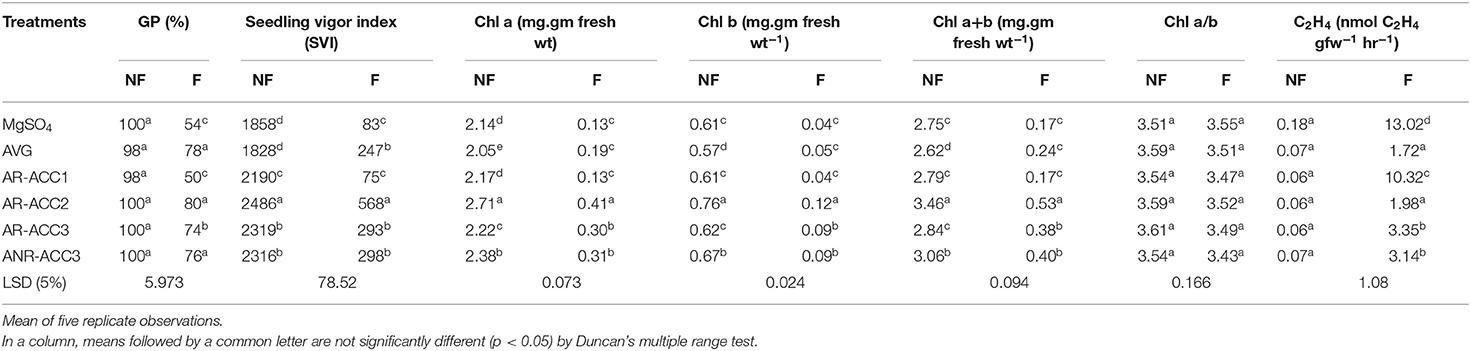
Table 2. Effect of select PGPR inoculation on Germination percentage (GP), Seedling Vigor Index (SVI), chlorophyll a and b contents and ethylene production by rice (cv IR-42) seedlings under submergence stress for 7 days.
The effect of inoculation of ACC deaminase producing PGPR on growth of flooded rice plants was assessed after 7 days of submergence (Figure 1). Seven days of continuous submergence resulted in a decrease in all the growth parameters studied (Table 3). However, inoculation with the ACC deaminase containing PGPR strains noticeably stimulated the growth of the plants both under normal and submerged conditions. Under submerged condition, seedlings treated with all bacterial strains except Bacillus sp. AR-ACC1 significantly (P ≤ 0.05) enhanced all the plant growth parameters studied, as compared with the negative control.
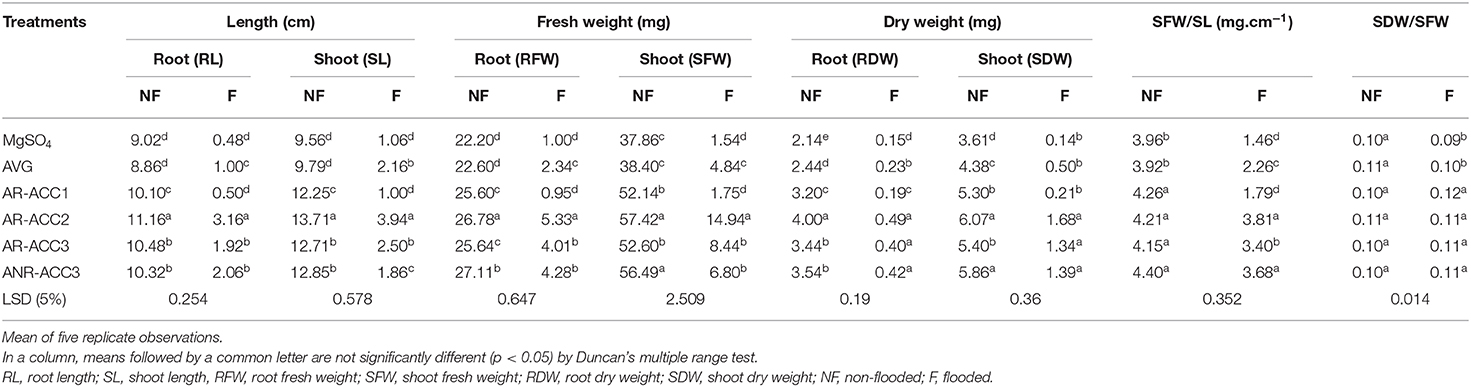
Table 3. Effect of select PGPR on different growth parameters of submergence susceptible rice (cv. IR-42) seedlings exposed to submergence stress for 7 days.
Seedlings treated with Microbacterium sp. AR-ACC2 showed highest root elongation to 3.16 cm. The bacterium also caused maximum shoot elongation (3.94 cm) followed by Methylophaga sp. AR-ACC3 and Paenibacillus sp. ANR-ACC3. Data regarding SFW and RFW showed that Microbacterium sp. AR-ACC2 caused maximum promotion to 14.94 and 5.33 mg, respectively. Strain Methylophaga sp. AR-ACC3 and Paenibacillus sp. ANR-ACC3 were the next effective strain to enhance fresh weights. In the case of SDW and RDW, seedlings treated with the three strains Microbacterium sp. AR-ACC2, Methylophaga sp. AR-ACC3, and Paenibacillus sp. ANR-ACC3 showed maximum promotion. These were significantly (P ≤ 0.05) higher than the negative control but statistically at par with each other. The ratio of SFW and SL of submerged plants was in the following comparative order of Microbacterium sp. AR-ACC2 > Paenibacillus sp. ANR-ACC3 > Methylophaga sp. AR-ACC3 > AVG > Bacillus sp. AR-ACC1 > negative control. The ratios of SDW and SFW of rice seedlings treated with Microbacterium sp. AR-ACC2, Methylophaga sp. AR-ACC3, and Paenibacillus sp. ANR-ACC3 were statistically at par (Table 3).
Under submergence stress, the chlorophyll concentration of rice seedlings significantly decreased compared with the normal plants (Table 2). However, significant increase in total chlorophyll and both Chl a and Chl b contents were observed in the submerged plants that were treated with the rhizobacteria excepting Bacillus sp. AR-ACC1 when compared with flooded nonbacterized plants. Treatment with Microbacterium sp. AR-ACC2 caused maximum increase in the concentration of total chlorophyll (0.53 mg) and also both Chl a (0.41 mg) and Chl b (0.12 mg), followed by Methylophaga sp. AR-ACC3 and Paenibacillus sp. ANR-ACC3 as the next effective strains. However, seedlings treated with Bacillus sp. AR-ACC1 did not show any significant enhancement of chlorophyll content under submergence.
Effect of PGPR on Ethylene Production
The submergence stress resulted in a significant increase in the production of ethylene as compared with the nonflooded plants (Table 2). Highest amount of ethylene production was observed in the negative control plants as compared with plants from the positive control (treated with AVG) under submergence. Ethylene synthesis was reduced by around 85% in seedlings treated with Microbacterium sp. AR-ACC2 and was statistically at par with the positive control plants. Paenibacillus sp. ANR-ACC3, Methylophaga sp. AR-ACC3, and Bacillus sp. AR-ACC1 were the next effective strains to reduce ethylene level by around 76, 74, and 21%, respectively (Table 2).
Discussion
The underlying postulate that was tested in this study was to lower the stress ethylene level by the oxidation of ACC with the help of ACC deaminase enzyme and subsequent colonization of roots by PGPR strains having multiple plant growth-promoting traits to stimulate the plant growth under submergence stress. The four bacterial strains used in this investigation belonged to Bacillus sp., Microbacterium sp., Methylophaga sp., and Paenibacillus sp. that were tested as PGPR in earlier studies under normal conditions (Bal et al., 2013). To study their role in the alleviation of submergence stress in rice seedlings, the performance of the four ACC deaminase-producing PGPR strains was monitored in plant growth chamber under submergence stress.
Submergence stress slows seed germination, imposes mortality, and delays seedling establishment in direct-seeded rice (Kato et al., 2020). The primary experiment for selection of rice cultivar to study the inhibitory effect of submergence stress on rice seedling showed that the GP was decreased by around 50% in the case of the susceptible variety, IR-42 whereas the GP decreased by only 15% in the case of the tolerant variety (Table 2) as reported earlier (Das et al., 2004). Hence, the susceptible cultivar, IR-42 was selected for submergence stress study.
The inhibitory effect of submergence stress on plant growth is directed by accelerated synthesis of ethylene (Kumar et al., 2020). Hypoxia during submergence causes an increase in the synthesis of ACC in roots due to both induction of ACC synthase genes and arrest of ACC oxidation (Houben and Van de Poel, 2019). During flooding, the concentration of ACC in roots increases and roots release high amount of ACC to the soil. The enzyme-substrate relationship demonstrates that ACC deaminase does not have a particularly high affinity for ACC (Gamalero and Glick, 2015). Moreover, ACC levels in plants are typically in micromolar range; therefore, in most plant tissues, the ACC concentration will be dramatically below the Km of ACC deaminase for this substrate. Hence, based on the Michaelis–Menten rate equation, an increase in the ACC concentration due to flooding caused parallel increase in the rate of ACC cleavage (Olanrewaju et al., 2017). In this way, the conversion of ACC to α-ketobutyrate and ammonia is favored over its oxidation to ethylene despite the fact that ACC oxidase binds ACC with a much higher affinity than does ACC deaminase (Glick et al., 1998).
The results obtained in the present investigation agree with the abovementioned predictions. A simple correlation analysis between in vitro ACC deaminase production and ethylene synthesis reduction by the isolates indicated a positive correlation (r = 0.92, n = 4), suggesting a direct impact of ACC deaminase activity on ethylene content under submergence. Similar to an earlier report by Grichko and Glick (2001b) on flooded tomato plants, this study revealed that inoculation with all the four ACC deaminase-containing PGPR strains caused a significant reduction in ethylene production compared with the negative control plants under submergence (Table 2). The ethylene level in plant tissue had significant negative correlation with GP (r = −0.96, P = 0.05) and seedling vigor (r = −0.78, P = 0.05) which suggested that ethylene content has direct negative impact on germination rate. Inoculation with strain Microbacterium sp. AR-ACC2 caused maximum reduction (around 85%) in ethylene synthesis which was statistically at par with the plants treated with ethylene inhibitor AVG (Table 2).
Waterlogging accelerates the synthesis of stress ethylene and higher concentrations of ethylene have inhibitory effects on root growth that may lead to significant reduction in plant height, plant fresh and dry weights, and chlorophyll content (Loreti et al., 2016). Hence, it is imperative to regulate the ethylene production in the close vicinity of plant roots for normal growth and development of the plants (Kumar et al., 2020). Earlier studies (Grichko and Glick, 2001a; Ali and Kim, 2018) on inoculation of tomato and Ocimum sanctum plants with ACC deaminase producing PGPR showed substantial tolerance to flooding stress implying that bacterial ACC deaminase lowered the effects of stress-induced ethylene. Present study on rice plant treated with ACC deaminase containing PGPR strains also revealed that rice seedlings exhibited alleviation of stress ethylene production and significantly increased tolerance to submergence stress than the negative control plants (Figure 1). Except Bacillus sp. AR-ACC1 other three strains exhibited significant (P ≤ 0.05) growth-promoting activities in rice seedlings under gnotobiotic conditions, including increased rate of germination, root and shoot length, fresh and dry weight of root and shoot, and total chlorophyll content. Inoculation with Microbacterium sp. AR-ACC2 enhanced the RL maximum up to fivefold over the negative control plants. It was the most promising strains to enhance other plant growth parameters: SL (~3-fold), RFW (~4-fold), SFW (~9-fold), RDW (~2-fold), SDW (~11-fold), SFW/SL (~2-fold), and SDW/SFW (~0.2-fold) (Table 3). Inoculation with ACC deaminase-containing bacteria promotes root growth of developing seedlings of various crops (Glick, 2020). The differences in plant growth promotion among the isolates are also attributed to their individual rhizospheric competencies and hydrolyzing the ACC synthesized in roots. The elongation of root system in submerged plant might be due to the alleviation of ethylene inhibitory effect due to the ACC deaminase producing PGPR treatment.
Regulation of stress ethylene level is not the only trait of PGPR strains to enhance plant growth but other growth-promoting mechanisms also contribute to growth promotion. IAA produced by most of the PGPR strains play an imperative role as a direct mechanism of plant growth enhancement (Kochar and Srivastava, 2012). It is likely that IAA and ACC deaminase stimulated root growth in a coordinated fashion (Nascimento et al., 2018). The complex cross-talk between IAA and ethylene in plant growth promotion by PGPR suggested that ACC deaminase producing PGPR might decrease the extent of IAA signal transduction inhibition by ethylene (Glick et al., 1998; Nascimento et al., 2018). Use of PGPR strains having multiple plant growth-promoting traits is expected to help increase crop productivity on a sustainable basis. All the bacteria used in the present study enhancing seed germination and seedling growth had the ability to produce IAA and ammonia (Bal et al., 2013) which might have helped the plants to withstand the submergence stress. Microbacterium sp. and Paenibacillus sp. were also siderophore producers.
Enhanced ethylene synthesis in submerged plants could promote chlorophyll degradation and leaf senescence that may reduce photosynthetic carbon fixation during and after submergence resulting in the depletion of carbohydrate reserves with a consequent increase in plant mortality (Adak et al., 2011). Leaf chlorophyll content decreased in submerged plants as compared with plants without submergence stress. However, treatment with ACC deaminase containing strains (except Bacillus sp. AR-ACC1) significantly increased the concentration of total chlorophyll and both Chl a and b in submerged plants in comparison with negative control plant (Table 2). Microbacterium sp. AR-ACC2 enhanced the total chlorophyll content by twofold over negative control plants. The increased chlorophyll content and expanded root architecture resulting from inoculation of PGPR strains would likely have improved photosynthetic capacity and higher nutrient uptake efficiency, respectively which in turn would have favored higher ratio of SFW to SL and SDW to SFW and provide some protection against submergence stress (Table 3) (Biswas et al., 2000; Grichko and Glick, 2001b). Thus, the microbial treatments provided to the plants were found beneficial under submerged condition as it reduced the inhibitory effect of stress ethylene and consequently enhanced the plant growth parameters of inoculated plants to withstand the stress.
Tolerance to anaerobic conditions during germination, often referred to as anaerobic germination is a complex genetically controlled process (Yang et al., 2019). Accordingly, breeding direct seeded rice varieties with anaerobic germination has been difficult. Most rice varieties fail to germinate and thrive under anaerobic conditions in waterlogged fields, leading to poor seedling establishment. ACC deaminase can significantly decrease ACC levels in plants, especially plants subjected to flooding stress, thereby decreasing the amount of stress ethylene and the subsequent damage to the plant that might occur as a consequence of that stress ethylene. This can be achieved either through the interaction of ACC deaminase-containing plant growth-promoting bacteria with plant roots or by the development of transgenic plants expressing this enzyme (Grichko and Glick, 2001b). Our study shows that flooded soil planted to rice harbor several microorganisms with plant growth-promoting properties that can be used effectively to alleviate such stresses due to submergence under field conditions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
HB carried out the research under the supervision of TA and also HB wrote the manuscript. TA reviewed and edited the manuscript. Both authors approved the submitted version.
Funding
This work was supported in part by the ICAR Networking Project, Application of Microorganisms in Agriculture and Allied Sciences [AMAAS]—Theme Microbial Diversity and Identification by the Indian Council of Agricultural Research, New Delhi. Results presented in the communication forms part of the Ph.D. dissertation submitted by the senior author to Utkal University, Bhubaneswar, India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adak, M. K., Ghosh, N., Dasgupta, D. K., and Gupta, S. (2011). Impeded carbohydrate metabolism in rice plants under submergence stress. Rice Sci. 18, 116–126. doi: 10.1016/S1672-6308(11)60017-6
Ali, S., and Kim, W. (2018). Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing Bacteria. Front. Microbiol. 9:1096. doi: 10.3389/fmicb.2018.01096
Bal, H. B., Das, S., Dangar, T. K., and Adhya, T. K. (2013). ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 53, 972–984. doi: 10.1002/jobm.201200445
Biswas, J. C., Ladha, J. K., Dazzo, F. B., Yanni, Y. G., and Rolfe, B. G. (2000). Rhizobial inoculation influences seedling vigor and yield of rice. Agron. J. 92, 880–886. doi: 10.2134/agronj2000.925880x
Das, K. K., Panda, D., Nagaraju, M., Sharma, S. G., and Sarkar, R. K. (2004). Antioxidant enzynes and aldehyde releasing capacity of rice cultivars (Oryza sativa L.) as determinants of anaerobic seedling establishment capacity. Bulg. J. Plant Physiol. 30, 34–44. Available online at: http://www.bio21.bas.bg/ipp/gapbfiles/v-30/04_1-2_34-44.pdf
Dworkin, M., and Foster, J. W. (1958). Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603. doi: 10.1128/JB.75.5.592-603.1958
Fišerová, H., Mikušová, Z., and Klemš, M. (2008). Estimation of ethylene production and 1-aminocyclopropane1-carboxylic acid content in plants by means of gas chromatography. Plant Soil Environ. 54, 55–60. Available online at: https://www.agriculturejournals.cz/publicFiles/00736.pdf (accessed August 8, 2020).
Fukao, T., and Bailey-Serres, J. (2008). Ethylene-A key regulator of submergence responses in rice. Plant Sci. 175, 43–51. doi: 10.1016/j.plantsci.2007.12.002
Gamalero, E., and Glick, B. R. (2015). Bacterial modulation of plant ethylene levels. Plant Physiol. 169, 13–22. doi: 10.1104/pp.15.00284
Glick, B. R. (2020). “Introduction to plant growth-promoting bacteria,” in Beneficial Plant-Bacterial Interactions, ed B. R. Glick (Cham: Springer International Publishing), 1–37. doi: 10.1007/978-3-030-44368-9_1
Glick, B. R., Penrose, D. M., and Li, J. (1998). A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 190, 63–68. doi: 10.1006/jtbi.1997.0532
Grichko, V. P., and Glick, B. R. (2001a). Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol. Biochem. 39, 11–17. doi: 10.1016/S0981-9428(00)01212-2
Grichko, V. P., and Glick, B. R. (2001b). Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35S, rolD or PRB-1b promoter. Plant Physiol. Biochem. 39, 19–25. doi: 10.1016/S0981-9428(00)01217-1
Houben, M., and Van de Poel, B. (2019). 1-aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 10:695. doi: 10.3389/fpls.2019.00695
Jackson, M. B. (2008). Ethylene-promoted elongation: an adaptation to submergence stress. Ann. Bot. 101, 229–248. doi: 10.1093/aob/mcm237
Jackson, M. B., and Ismail, A. M. (2015). Introduction to the special issue: electrons, water and rice fields: plant response and adaptation to flooding and submergence stress. AoB Plants 7:plv078. doi: 10.1093/aobpla/plv078
Kato, Y., Collard, B. C. Y., Septiningsih, E. M., and Ismail, A. M. (2020). Increasing flooding tolerance in rice: combining tolerance of submergence and of stagnant flooding|Annals of Botany|Oxford Academic. Ann. Bot. Available online at: https://academic.oup.com/aob/article-abstract/124/7/1199/5532391 (accessed August 8, 2020).
Khan, M. I. R., Trivellini, A., Chhillar, H., Chopra, P., Ferrante, A., Khan, N. A., et al. (2020). The significance and functions of ethylene in flooding stress tolerance in plants. Environ. Exp. Bot. 179, 104188. doi: 10.1016/j.envexpbot.2020.104188
Kochar, M., and Srivastava, S. (2012). Surface colonization by Azospirillum brasilense SM in the indole-3-acetic acid dependent growth improvement of sorghum. J. Basic Microbiol. 52, 123–131. doi: 10.1002/jobm.201100038
Kumar, A., Singh, S., Gaurav, A. K., Srivastava, S., and Verma, J. P. (2020). Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 11, 1–15. doi: 10.3389/fmicb.2020.01216
Long, H. H., Schmidt, D. D., and Baldwin, I. T. (2008). Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE 3:2702. doi: 10.1371/journal.pone.0002702
Loreti, E., van Veen, H., and Perata, P. (2016). Plant responses to flooding stress. Curr. Opin. Plant Biol. 33, 64–71. doi: 10.1016/j.pbi.2016.06.005
Magneschi, L., and Perata, P. (2009). Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 103, 181–196. doi: 10.1093/aob/mcn121
Nascimento, F. X., Rossi, M. J., and Glick, B. R. (2018). Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant Sci. 9, 1–17. doi: 10.3389/fpls.2018.00114
Oladosu, Y., Rafii, M. Y., Arolu, F., Chukwu, S. C., Muhammad, I., Kareem, I., et al. (2020). Submergence tolerance in rice: review of mechanism, breeding and, future prospects. Sustainability 12:1632. doi: 10.3390/su12041632
Olanrewaju, O. S., Glick, B. R., and Babalola, O. O. (2017). Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 33, 1–16. doi: 10.1007/s11274-017-2364-9
Oleńska, E., Małek, W., Wójcik, M., Swiecicka, I., Thijs, S., and Vangronsveld, J. (2020). Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci. Total Environ. 743:140682. doi: 10.1016/j.scitotenv.2020.140682
Penrose, D. M., and Glick, B. R. (2001). Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can. J. Microbiol. 47, 368–372. doi: 10.1139/w01-014
Penrose, D. M., and Glick, B. R. (2003). Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 118, 10–15. doi: 10.1034/j.1399-3054.2003.00086.x
Porra, R. J. (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156. doi: 10.1023/A:1020470224740
Sandhu, N., Dixit, S., Swamy, B. P. M., Raman, A., Kumar, S., Singh, S. P., et al. (2019). Marker assisted breeding to develop multiple stress tolerant varieties for flood and drought prone areas. Rice 12, 1–16. doi: 10.1186/s12284-019-0269-y
Sapsirisopa, S., Chookietwattana, K., Maneewan, K., and Khaengkhan, P. (2009). Effect of salt-tolerant Bacillus inoculum on rice KDML 105 cultivated in saline soil. J. Food Agro. Ind. 69–74. Available online at: www.ajofai.info (accessed August 8, 2020).
Siddikee, M. A., Glick, B. R., Chauhan, P. S., Yim, W., and jong, Sa, T. (2011). Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 49, 427–434. doi: 10.1016/j.plaphy.2011.01.015
Keywords: ACC deaminase, ethylene production, growth promoting rhizobacteria, rice, seed germination, seedling growth, submergence stress
Citation: Bal HB and Adhya TK (2021) Alleviation of Submergence Stress in Rice Seedlings by Plant Growth-Promoting Rhizobacteria With ACC Deaminase Activity. Front. Sustain. Food Syst. 5:606158. doi: 10.3389/fsufs.2021.606158
Received: 14 September 2020; Accepted: 25 January 2021;
Published: 19 March 2021.
Edited by:
Duraisamy Saravanakumar, The University of the West Indies St. Augustine, Trinidad and TobagoReviewed by:
Amarendra Narayan Misra, Central University of Jharkhand, IndiaIfigeneia Mellidou, Hellenic Agricultural Organisation (HAO), Greece
Copyright © 2021 Bal and Adhya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tapan Kumar Adhya, YWRoeWFzQHlhaG9vLmNvbQ==
 Himadri Bhusan Bal
Himadri Bhusan Bal Tapan Kumar Adhya
Tapan Kumar Adhya