
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst. , 10 February 2021
Sec. Agroecology and Ecosystem Services
Volume 4 - 2020 | https://doi.org/10.3389/fsufs.2020.585685
This article is part of the Research Topic Grazing in Future Multi-scapes: From Thoughtscapes to Landscapes, Creating Health from the Ground Up View all 46 articles
The productive performance of large ungulates in extensive pastoral grazing systems is modulated simultaneously by the effects of climate change and human intervention independent of climate change. The latter includes the expansion of private, civil and military activity and infrastructure and the erosion of land rights. We used Saami reindeer husbandry in Norway as a model in which to examine trends in, and to compare the influence of, both effects on a pastoral grazing system. Downscaled projections of mean annual temperature over the principal winter pasture area (Finnmarksvidda) closely matched empirical observations across 34 years to 2018. The area, therefore, is not only warming but seems likely to continue to do so. Warming notwithstanding, 50-year (1969–2018) records of local weather (temperature, precipitation and characteristics of the snowpack) demonstrate considerable annual and decadal variation which also seems likely to continue and alternately to amplify and to counter net warming. Warming, moreover, has both positive and negative effects on ecosystem services that influence reindeer. The effects of climate change on reindeer pastoralism are evidently neither temporally nor spatially uniform, nor indeed is the role of climate change as a driver of change in pastoralism even clear. The effects of human intervention on the system, by contrast, are clear and largely negative. Gradual liberalization of grazing rights from the 18th Century has been countered by extensive loss of reindeer pasture. Access to ~50% of traditional winter pasture was lost in the 19th Century owing to the closure of international borders to the passage of herders and their reindeer. Subsequent to this the area of undisturbed pasture within Norway has decreased by 71%. Loss of pasture due to piecemeal development of infrastructure and to administrative encroachment that erodes herders' freedom of action on the land that remains to them, are the principal threats to reindeer husbandry in Norway today. These tangible effects far exceed the putative effects of current climate change on the system. The situation confronting Saami reindeer pastoralism is not unique: loss of pasture and administrative, economic, legal and social constraints bedevil extensive pastoral grazing systems across the globe.
The productive performance of free-living large ungulates, including wild populations and domestic herds managed in extensive pastoral grazing systems, is modulated by two kinds of drivers: those associated with variation in the natural environment and those associated with human intervention independent of the natural environment (Godde et al., 2018). These act simultaneously and together constitute the holistic climate of change that governs the performance of animals and hence the well-being of people—in particular pastoralists—whose livelihoods depend on them. The two kinds are nevertheless commonly considered separately: environmental interactions are principally modelled and reported in ecological literature while the influence of socio-economic and other anthropogenic developments is explored mainly in anthropological and geographical literature. The disciplinary divide sharpens the focus of analyses but constrains interpretation of their results. The growth and performance of large ungulates, and the dynamics of the (socio-)ecological systems of which they are a part, obviously reflect the integrated effect of all the drivers that impinge on them, not just those of one particular kind. The partial effect of drivers of one kind likewise necessarily depends on the partial effect of those of the other but this relationship, too, is lost across the disciplinary divide. In this paper we use Saami reindeer husbandry in Norway as a model in which to examine how environmental variation and human intervention impinge jointly on a pastoral grazing system and from which to assess the relative impact of each on such a system. Several of the drivers we examine are specific in their character or their settings to the boreal region and even particular to Saami reindeer husbandry in Norway: our approach, however, is entirely general in its application and our conclusion reflects the situation in many, perhaps even most, extensive pastoral grazing systems.
Ecological studies of the dynamics of extensive grazing systems are primarily concerned with the influence of natural variation in conditions and resources on the performance of animals or on the ecosystem processes that modulate it. ‘Conditions’ in this respect include abiotic factors that influence organisms such as temperature, precipitation, wind, photoperiod and, for chionophile organisms like reindeer/caribou (Rangifer tarandus; Box 1), the characteristics of the snowpack. Conditions also include biotic components such as the density of conspecifics, competitors, predators and parasites. Resources are things required by and also reduced by the activity of organisms (or by the activity of other organisms): food, shelter and mates are examples (Begon et al., 2006). World attention is currently directed increasingly and often passionately toward the effects of climate variation on conditions, on levels of resources and hence on the performance of animals and the function of the ecosystems of which they are a part. Climate effects include the degradation of grazing lands though desertification, encroachment of bush and woodland and deforestation (Asner et al., 2004), the modulation of the phenology, growth and the nutritional quality of herbage (Herrero et al., 2016; Thackeray et al., 2016), the modulation of the phenology, growth and patterns of migration of animals (Forchhammer et al., 1998; Ozgul et al., 2009; Robinson et al., 2009; Sheridan and Bickford, 2011; Thackeray et al., 2016) and, arising from these, the modulation of the dynamics of animal populations (Coulson et al., 2001; Post and Forchhammer, 2002; Post et al., 2009a; Marshal et al., 2011; see also IPCC, 2019).
Box 1. Reindeer and the northern grazing system
Reindeer, Rangifer tarandus, is a boreal to super-boreal species complex within the monospecific genus Rangifer (family Cervidae [deer]). The species has a circumpolar boreal (Arctic and sub-Arctic) distribution. The animals are called—in English—‘caribou’ in North America and ‘reindeer’ in Eurasia. Distinction is also generally made between wild and domesticated reindeer, the latter being herded by indigenous peoples (Figure 1). The term ‘semi-domesticated’ is frequently applied to herded reindeer (e.g., Colman et al., 2013; Meng et al., 2014; Uboni et al., 2016) but the prefix is superfluous. The distinction between ‘domestic’ and ‘domesticated’ animals is clear, comprehensive and sufficient (see Clutton-Brock, 1987, p. 104). All three forms (caribou, wild and domesticated reindeer), of course, are the same species (Flagstad and Røed, 2003; Røed et al., 2008, 2011). There are ~5–6 million Rangifer worldwide, including 3–4 million caribou and wild reindeer and ~2.5 million domesticated reindeer of which 650,000 are in Fennoscandia (CAFF, 2013; Gunn, 2016; Government of Norway, 2017; Norwegian Agriculture Agency, 2019a).
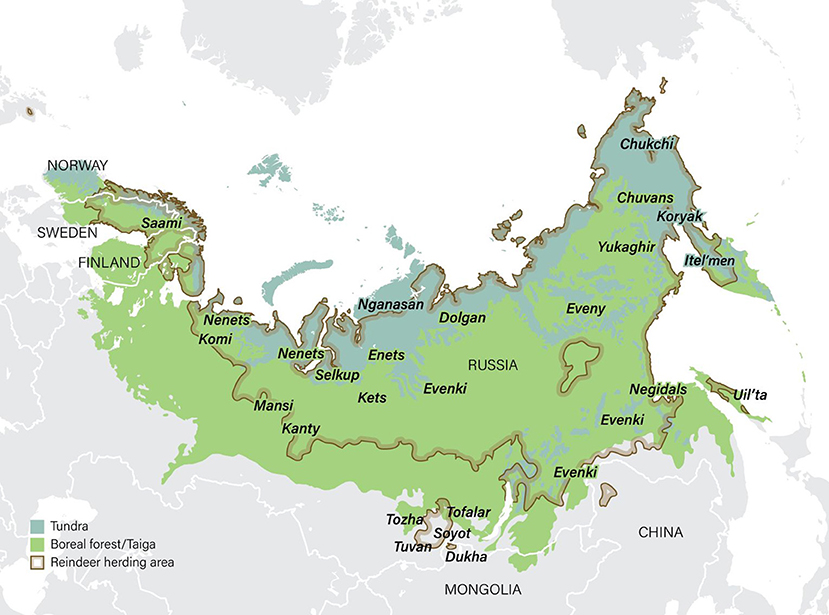
Figure 1. Some 24 indigenous peoples graze some 2.5 million reindeer (Rangifer tarandus) across 10 million km2 of mountain, forest, taiga and tundra in northern Eurasia. This is an area equivalent to 7% of the land surface of the globe.
Rangifer belong to the intermediate feeder type (Hoffman, 1989). The animals select a species rich diet of browse and non-woody plants, and unusually for ruminants, they may take a considerable amount of lichens (especially, but not exclusively, in winter; Trudell and White, 1981; Boertje, 1984; Adamczewski et al., 1988; Mathiesen et al., 2000; Sundset et al., 2010). Their supply of forage is highly seasonal. In the boreal zone plant growth is restricted to the period from late May to early September when the daily mean ambient temperature is >0°C: for the rest of the year plants are frozen and therefore inert. The animals therefore normally have access to fresh green forage only for 3–4 months annually when, during the boreal summer, they grow, fatten and rear their young. In winter, by contrast, the available biomass of green material is reduced because plants enter dormancy and access to them is restricted by snow. Rangifer display a suite of adaptations to this situation, the most conspicuous being migration between spatially distinct summer and winter pastures.
Barren-ground caribou in northern North America and wild reindeer in Siberia migrate north in spring to tundra pastures around the rim of the Arctic Ocean. Here they spend the summer before returning hundreds of kilometers south in autumn to winter pastures in the taiga and boreal forest inland (Kelsall, 1968; Parker, 1972; Chernov, 1985; Fancy et al., 1989). Northwards migration in spring is in part a response to the progressive emergence of fresh herbage which appears at the edge of the retreating snowline. This has been likened to a ‘green wave’ which the animals track as it spreads northwards across the landscape (Skogland, 1984, 1989; see also Aikens et al., 2017; Middleton et al., 2018). Rangifer trade quantity for quality, the small size of new shoots being compensated by their high nitrogen content and digestibility (Russell et al., 1993; Van der Wal et al., 2000; Johnstone et al., 2002).
Feeding conditions in winter when plants are inert are influenced by the quality of the snowpack. Wind, and in some areas, recurring cycles of thawing and re-freezing associated with interludes of mild weather sometimes accompanied by rain, increases the density and the hardness of snow consequently making it difficult for the animals to dig down to reach the plants beneath (Schnitler [circa 1751] in Hansen and Schmidt, 1985, p. 24; Woo et al., 1982; Bartsch et al., 2010; Tyler, 2010; Forbes et al., 2016; Langlois et al., 2017). Forests provide shelter from wind and thaw-freeze cycles are less frequent inland where the climate is generally colder and drier than at the coast where, where there is open water, it is warmer and wetter. Both factors contribute to easier snow conditions, and hence better grazing, and the animals therefore move inland to the forest zone where they spend the winter. Domesticated reindeer follow the same pattern as their wild conspecifics, resulting in the spectacular seasonal migration of herds and herders—usually hundreds and sometimes of more than 1,000 km each way—which are a prominent feature of reindeer peoples everywhere (Manker, 1935; Krupnik, 1993; Paine, 1994; Vitebsky, 2005; Dwyer and Istomin, 2009; Degteva and Nellemann, 2013).
Effects of human intervention on the abundance and performance of free-living large ungulates are readily apparent, often negative and not infrequently dramatic. Unrestrained hunting for meat, hides and bone in the latter half of the 19th Century, for instance, reduced bison (Bison bison) in North America from around 60 million to some few dozen animals and deer (Odocoileus spp.) from 50 million to some few thousands (Soper, 1941; Isenberg, 2000; VerCauteren, 2003; Webb, 2018). At the same time saiga antelope (Saiga tatarica tatarica) in Central Asia were driven, it is thought, to the verge of extinction by hunting for meat, hides and horns (Bekenov et al., 1998; Milner–Gulland et al., 2001). An estimated half million Canadian barren-ground caribou (R. t. groenlandicus) were killed by hunters between 1949 and 1954 (Kelsall, 1968, p. 201) and, in the following two decades, half a million wildebeest (Connochaetes taurinus), deemed a threat to domestic cattle in Botswana, died in extermination programmes and as a result of the construction of veterinary cordon fences which excluded the animals from dry season access to water (Williamson and Williamson, 1984; Spinage, 1992; Gadd, 2012). These instances, directly or indirectly, were deliberate acts of destruction. By contrast, the introduction of the rinderpest virus (Rinderpest morbillivirus) from Arabia or India in 1889, which led to devastation of buffalo (Syncercus caffer), wildebeest and the death of around five million cattle in Southern and East Africa, was presumably an accident, albeit one on a monumental scale (Sinclair, 1977; Phoofolo, 1993; Van den Bossche et al., 2010). Examples of positive effects of human intervention on large ungulate grazing systems include the maintenance (as opposed to the deterioration) of the conservation status of many species of ungulates worldwide (Hoffmann et al., 2015; Barnes et al., 2016), the enhancement of primary and secondary production through grazing management (Odadi et al., 2017; Crawford et al., 2019; McDonald et al., 2019), and the successful—at least in numerical terms—introduction of species such as horse (Equus caballus) to North America (current population 9 million; McKnight, 1959; American Horse Council Foundation, 2018) and sheep (Ovis aries) to Australia (current population 93 million; FAO, 2019).
Reindeer pastoralism, practiced across some 10 million km2 of northern Eurasia, constitutes the largest contiguous ungulate grazing system on Earth (Box 1). The performance of these animals and this system is influenced by both effects, i.e., by variation in the natural environment and, the remoteness of the system notwithstanding, also by human intervention. Considering the former, the mean annual temperature of the Arctic has increased by about 2°C since 1960 (Figure 2). This is more than twice the mean global increase and considerable attention has been directed toward examining the effect of this on the species, the ecosystems and the peoples of the North (ACIA, 2005; Overland et al., 2017; Post et al., 2019). Not surprisingly, large scale warming influences the tundra, taiga and boreal forests where reindeer and caribou, their North American conspecifics (Box 1), graze. The effects of warming include the stimulation and an advance of the timing (phenology) of photosynthetic activity (Xu et al., 2013; Fauchald et al., 2017; Park et al., 2019) and the modulation of snow cover (AMAP, 2017), all of which are associated with variation in individual and population rates of growth (Tyler et al., 2008; Post et al., 2009b; Mallory and Boyce, 2018). Considering the latter (i.e., human intervention), the principal negative effects on reindeer pastoralism seem not to have arisen primarily through deliberate, large-scale slaughter of animals, as in the case of the other species of large ungulates given above, but as a consequence of legislation developed and imposed for political, economic and other reasons (although see Vitebsky, 2005, p. 406). The compulsory organization of reindeer pastoralism in collective (kolkhoz) and State (sovkhoz) farms in the Soviet Union from the 1920s until the 1990s (or even, in some cases, to the present day: see Kumpula et al., 2011) is a conspicuous example. Collectivization not only disrupted the lifestyles and the cultural, economic and spiritual values of herding peoples throughout the region, it also anticipated the demise of herds following the abandonment of this form of organization at the fall of Russian Communism in 1991 (Vitebsky, 2005; Anderson, 2006; Povoroznyuk, 2007; Klokov, 2011; Konstantinov, 2015). Less conspicuous but no less pervasive were—and still are—the effects on reindeer pastoralism of the loss of pasture and the disruption of movement of herds and herders owing to the expansion of infrastructure and commercial, military and private activity into reindeer pasture areas. Modern examples include the direct and cumulative impact of oil, gas, mining, wind- and hydro-electricity and other infrastructure developments in northern pasture areas since the 1970s (Dwyer and Istomin, 2009; Forbes et al., 2009; Degteva and Nellemann, 2013; Tolvanen et al., 2019). We return to this below.
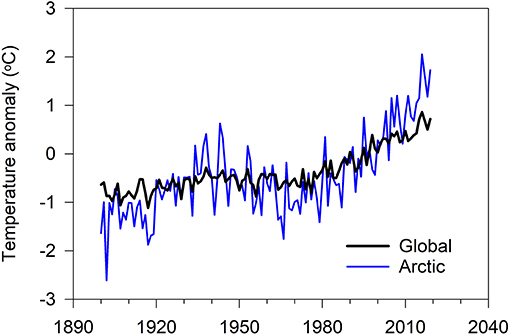
Figure 2. ‘Arctic’ (land stations north of 60° North latitude) and global annual surface air temperature anomalies 1900–2019 (°C) relative to the 1981–2010 regional mean values. Updated from Overland et al. (2017).
The problem of range loss and the disruption of reindeer herding is not new. Disagreements between Saami reindeer herders and other users over rights of access and rights of use of reindeer pasture (utmark; Box 2) in Norway can be traced back at least 150 years (Strøm Bull, 2015). Domesticated reindeer in Norway graze and are grazed exclusively in utmark but herders' rights of usufruct have repeatedly been challenged (below). Solutions have been sought in the courts and through legislation aimed at regulating and, through regulation, at managing Saami reindeer pastoralism. This has been done with the specific intention of addressing problems—real or perceived—associated with it, including disputes over grazing rights, low productivity, poor animal welfare associated with the use of traditional methods and the environmental impact of reindeer pastoralism. The addressing of such issues has resulted in Saami reindeer pastoralism in Norway becoming an administrative and economic burden for national and local legislatures, in addition to which an unrelenting focus on issues deemed problems has led to profoundly negative political and public discourse: Saami reindeer pastoralism in Norway is perceived as persistently problematic (Box 2).
Box 2. The pastoral system: Saami reindeer herding in Norway
There are ~215,000 domesticated reindeer in Norway (data for 2019: Norwegian Agriculture Agency, 2019a). The majority (94%) of these are herded by Saami pastoralists who graze their animals on 141,000 km2 of utmark designated as Saami reindeer pasture (Government of Norway, 2017; Figures 3, 4). (Utmark, pronounced ‘oot-mark’, is a Norwegian word for uncultivated land, including forests, meadows, moorland and mountains). The majority (80%) of reindeer in this area live in the Troms, East Finnmark and West Finnmark reindeer pasture areas (which together constitute the single country of ‘Troms and Finnmark’; Figure 3). A minority (6%) of reindeer in Norway are herded by Saami and non-Saami Norwegians in utmark in the south of the country outside the Saami reindeer pasture area (Government of Norway, 2017; marked as ‘Other areas’ in Figure 3).
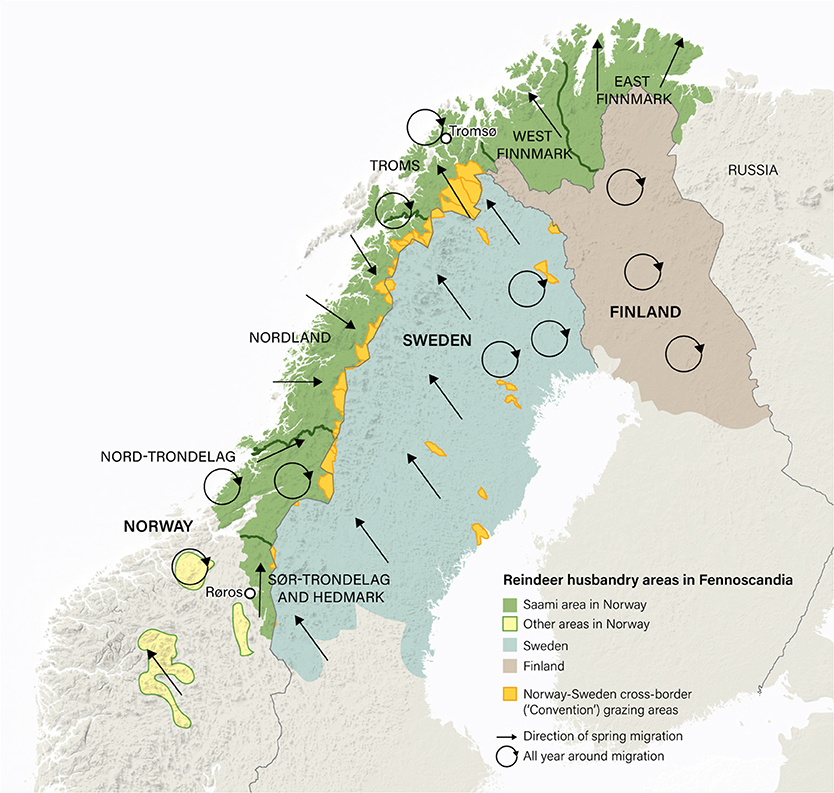
Figure 3. Saami reindeer pastoralism in Norway, Sweden and Finland. The Saami reindeer husbandry area in Norway (green shading) constitutes 141,000 km2 of utmark (uncultivated land, including forests, meadows, moorland and mountains). This area is equivalent to 40% of the entire country. It is in turn divided into six ‘reindeer pasture areas’ (Troms, East Finnmark, West Finnmark, Nordland, Nord-Trøndelag and Sør-Trøndelag and Hedmark). The map also shows the reciprocal cross-border (‘Convention’) grazing areas used by Norwegian Saami in Sweden and vice versa (orange shading). These constitute an area of ~14,000 km2. The Convention on cross-border grazing between Norway and Sweden is currently in abeyance (see text). Sources: Government of Norway (2010) and Pape and Löffler (2016).

Figure 4. Saami reindeer pastoralism in Norway. (a) The late Mathis Aslaksen Sara with his family's reindeer in a temporary paddock at Cuovddatmohkki (Figure 7). This paddock was erected in April (2002) to enable his and another family to separate their herds which, after wintering together, were about to move independently to their summer pasture on the island of Magerøya (Figure 7). (b) The same site 5 months later (September 2002): virtually no trace remains either of the paddock or of the presence of the hundreds of reindeer which had been gathered in it. Grazing rights accrue through the legal principle of ‘use since time immemorial’ (Norwegian: alders tids bruk) but, as photograph (b) shows, it may be no simple matter for pastoralists to document their use of an area. (c) A pregnant reindeer on winter pasture in northern Norway. The snow all around the animal has been excavated by reindeer which have been feeding on the plants beneath. (d) Reindeer herder and her son inspecting their herd in the same area. (e) Ear marks (yellow arrows) permanently and indelibly identify the ownership of every reindeer in a herd. Each owner has his or her own unique pattern of marks which are cut into the left and right ears of animals in their first summer and which they bear for life (see Paine, 1994, p. 24; Beach, 2007). (f) Transhumant pastoralism: 3,000 reindeer swim in September (2004) from their summer pasture on the island of Magerøya to the mainland at the start of their 200 km autumn migration to winter pasture south of Cuovddatmohkki on Finnmarksvidda (Figure 7). Magerøya Sound is ~1,200 m wide at this point. Photographs: Nicholas Tyler.
The Saami reindeer pasture area in Norway is divided into six ‘reindeer pasture areas’ (of which Troms, East Finnmark and West Finnmark are the most northerly; Figure 3). These six areas are in turn divided into altogether 82 ‘grazing districts’. These administrative divisions are government, not Saami, constructs. Within each district, groups of reindeer owners—members of one or more families—keep their reindeer in combined herd(s) which they manage collectively. Herding alliances (‘siida’ and ‘sijte’ in northern and southern Saami language, respectively) may persist across all or just part of the year. A particular summer siida may, for instance, routinely divide in autumn, with some herders (and their reindeer) joining another siida for winter. Currently there are around 100 and 150 different summer and winter siida, respectively, in Norway (Government of Norway, 2017). This dynamic is possible because every reindeer is the property of a particular owner, not a particular siida. Ownership is established by a pattern of ear marks, each unique to an owner, that provide permanent identification of animal ownership (Figure 4).
Saami reindeer pastoralism in Norway is typically, but not invariably, transhumant. The Saami enjoy the right of usufruct throughout the Saami reindeer pasture area. Siida with anything from 100–10,000 reindeer of mixed age and sex normally move between discrete summer and winter pastures. Summer pastures are usually, although not invariably, at the coast where mild, humid weather favors plant growth. Winter pastures are usually, although not invariably, at higher elevation inland where winters are colder and the snow tends to remain dry and friable and hence easier for the animals to dig through to reach the plants beneath. The reverse pattern of migration occurs where low lying coastal pastures remain largely free of snow in winter and where inland mountains provide mild, humid conditions in summer. There are also places where reindeer remain in the same area all the year round, largely performing only altitudinal migration (Figure 3).
In contrast to domestic species, which in Norway remain indoors all winter, reindeer remain outdoors, grazing natural pasture all year round. The animals usually receive minimal attention in summer. This is especially the case where herds are swum or ferried to islands or led onto peninsulas before calving spring, and where they remain, undisturbed, until they are gathered for the return journey in autumn (Figure 4). Close herding is normally practiced only during migration and throughout winter when herders move their animals frequently in response to snow conditions and to the presence of other herds.
Reindeer pastoralism has considerable economic, social and cultural significance for Norwegian Saami. Its principal economic product now, although not historically, is meat. The level of production of reindeer meat is the same today as it was in 1960 (1960: 1,600 tons; 2018/19: 1,683 tons; Government of Norway, 1992; Norwegian Agriculture Agency, 2019a; Figure 6). In 2018 meat and other products had a value of NOK 123 million and NOK 67.5 million (~US$ 18 and 10 million, respectively), equivalent to 49% of the total income of reindeer pastoralism that year (NOK 387 million). Income also derives from government subsidies (NOK 92.5 million, 24%) and compensation (NOK 104 million, 27%) for animals lost to predators (NOK 92 million) and for pasture lost through encroachment (NOK 12 million; Norwegian Agriculture Agency, 2019b, p. 2). Saami reindeer husbandry in Norway is beset by conflict and criticism (Figure 5). Pastoralists' rights of usufruct, ultimately confirmed following 100 years' tortuous passage through the courts (see main text), are still challenged, albeit informally but no less bitterly (e.g., Anonymous, 2009; Lysvold, 2017). Public opinion is divided. Claims of ‘overpopulation’ and ‘overgrazing’ (e.g., Government of Norway, 1992; Office of the Auditor General, 2004; Vogt, 2007; Anonymous, 2012, 2014, 2015a; Hætta, 2018; Enoksen, 2019) are met with counter-claims of misunderstanding and political bias (Benjaminsen et al., 2015, 2016a,b; Benjaminsen, 2018; Benjaminsen et al., 2019).

Figure 5. Divided discourse: a selection of cuttings illustrating contrasting opinions about reindeer pastoralism in Norway. Translations are as follows. On the left: Reports reindeer herders [to the police]; Finnmarksvidda is being destroyed—abolish reindeer husbandry; Senior member of the Progress Party on reindeer husbandry: economic swindle and animal cruelty; Reindeer starving to death; Marginal loss… [of reindeer pasture owing to mining at] Repparfjord; Thin reindeer fall prey to predators; Fear catastrophic starvation; Serious animal cruelty exposed in reindeer husbandry; The anticipated tragedy; Reindeer eat up garden [plants]; This reindeer calf is starving to death; Demands removal of reindeer from island [pastures]; The myth of lost pasture land in Finnmark; Sustainable reindeer husbandry has not been achieved. On the right: School book criticized for stigmatizing reindeer husbandry; Let the mountain live!; Beginning of the end of reindeer husbandry; Norwegian herder ordered to put down dozens of reindeer in controversial cull; Rudolf's relatives given a death sentence: Norway orders mass reindeer slaughter before Christmas; Scientists evasive about reindeer husbandry; Threaten Mikkel Mathis with a colossal fine to make him slaughter his reindeer; In Norway, fighting the culling of reindeer with a macabre display; Reindeer pastoralism considers court action; ‘Celebrity’ herd threatened by the Nussir mine; No one listens to reindeer herders—I don't know what we'll do now; The State defeated Jovsset Ánte in the Supreme Court; Swedish Saami institute legal proceedings against the [Norwegian] State [over]… grazing rights… aim to stop social Darwinism; Saami culture must be prioritized!; Not surprising that the Saami are furious.
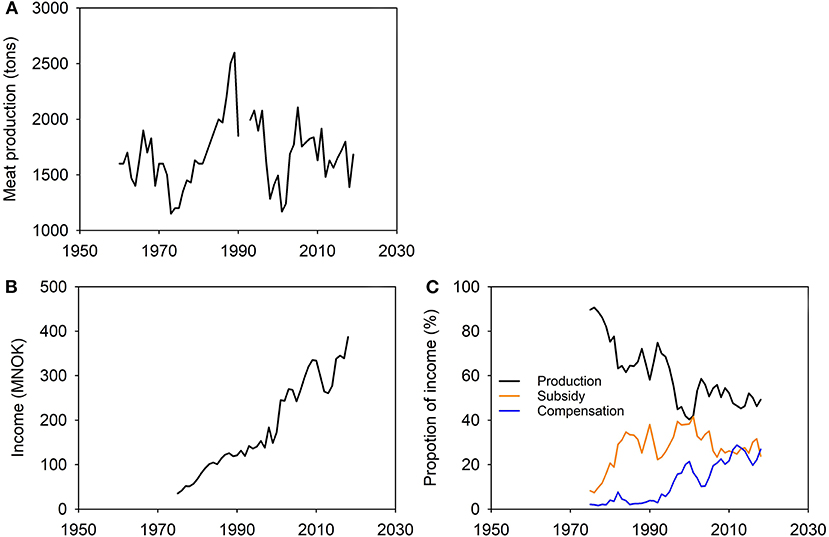
Figure 6. Production and income in reindeer pastoralism in Norway (Saami and non-Saami combined). (A) Annual production of meat (tons). (B) Annual income (MNOK). (C) Proportion of income from each of three sources: production, State subsidies and State compensation. Sale of meat accounts on average for 90% of production income while on average 83% of compensation is paid for reindeer lost to predators. Sources: Government of Norway (1992), Norwegian Agriculture Agency (2019a), and Norwegian Reindeer Husbandry Administration (2006).
Poor rates of production are attributed to high stocking density not predators (Kintisch, 2014) and to high levels of predation not stocking density (Berg, 2018). Allegations of poor animal welfare (e.g., Gauslaa, 2001; Grøndahl and Mejdell, 2012; Lund, 2017; see also Anonymous, 2015b) are symptomatic of dissatisfaction with reindeer pastoralism (e.g., Salvesen, 2009; Ringjord, 2016; Bergersen, 2017) that is anathema to its adherents (e.g., Sara, 2001; Anti, 2017; Fjellheim, 2020).
A decade ago it was suggested that the effects of human intervention and, in particular, of the reduction of herders' freedom of action resulting from loss of pasture through various forms of encroachment and from aspects of governance related to this, dwarfed the putative effects of climate change on reindeer pastoralism in Norway (Tyler et al., 2007). The model was specific but its conclusion appears to be general: there is increasing evidence that the effects of various forms of human intervention not unusually far exceed the effects of climate change on pastoral systems (e.g., Hobbs et al., 2008; Havlík et al., 2015; Ahmed et al., 2016; López-i-Gelats et al., 2016). Here we review the conclusion of the reindeer model. First, we extend parts of the analysis upon which it was based by externally validating and testing the predictive power of current projections of regional climate change. (For the difference between projections and predictions of climate, see Box 3). Second, we examine spatial and temporal trends in local weather conditions around Finnmarksvidda, which is the principle reindeer winter pasture area in Norway (Figure 7). Third, we review the gradual but erratic liberalization of Saami grazing rights since the mid-18th Century and the current administrative curtailment of herders' rights and freedom of action in herding and herd management. Finally, we review avoidance behavior and the effects of infrastructure on the use of habitat by reindeer. We conclude that the role of climate change as a driver of change in grazing conditions—and by extension as a driver of change in reindeer pastoralism—is unclear except insofar as it is spatially and temporally highly diverse. The effects of human intervention on reindeer pasture in northern Norway, by contrast, are consistently negative. Saami pastoralists struggle with loss of pasture resulting from encroachment and with restrictions and reorganizations that erode their independence and constrain their freedom of action on the pasture areas that remain available to them. The effects of human intervention seem far to exceed the effects of climate change on the system. This situation is not unique: loss of pasture and myriad administrative, economic legal and social constraints are a feature not only of Saami reindeer husbandry but of extensive pastoral grazing systems across the globe.
Box 3. Meteorological terms, models and data
Climate and Weather
Weather is the day-to-day state of the atmosphere. It is, generally speaking, the combination of temperature, humidity, precipitation, cloudiness, visibility and wind that we experience instantaneously at a given place at a given time.
Climate is a description of the probability of particular kinds of weather at a given place at a given time. It is a statistical norm calculated over a period of time, usually 30 years, and includes not only middle values but also the characteristic level of deviation around statistical middle values. The climate of a particular location, region or zone is thus defined in terms of the long-term averages and the frequencies of different kinds of weather conditions observed within it. The popular aphorism is apt: Climate is what you expect: weather is what you get.
Spatial variation in climate arises as a consequence of latitude, topography and the distribution of land and water (sea or lake). Ambient temperature, for instance, normally decreases with increasing latitude and altitude (although cold air tends to descend and fill depressions in the terrain when the sun is below the horizon and the air pressure field connected to the large-scale circulation, and hence wind, is weak). Inland areas tend to be warmer in summer and colder in winter than coastal areas. When the large-scale circulation is strong, the windward side of mountain areas may be exposed for orographic enhanced precipitation while the leeward side experiences a ‘rain shadow’ with low precipitation and few clouds.
Temporal variation in climate is in part a product of external and internal forcing at various time scales. Forcing may be natural or anthropogenic. External forcing, such as variation in solar radiation or in the concentration of greenhouse gases, lead to changes in the total energy budget of the Earth-atmosphere system. Internal forcing mainly affects the distribution of energy within the Earth-atmosphere system such as, for instance, between the atmosphere and the ocean. Some of the temporal variation in climate is random, while some of it seems to be relatively regular with distinct patterns and phases of temperature and other weather variables. Such patterns, captured and quantified in ‘climate indices,’ may be quasi-periodic: i.e., they oscillate at more or less distinct frequencies measurable at annual, multi-annual, decadal or multi-decadal timescales. Examples include the El Niño–Southern Oscillation (ENSO), the Madden–Julian Oscillation (MJO), the North Atlantic Oscillation (NAO), the Northern Annular Mode (NAM) or Arctic Oscillation (AO) and the Pacific Decadal Oscillation (PDO). For Norway, the NAO and AO are the most important. Positive values of the NAO and AO indices indicate stronger-than-average westerlies over middle latitudes, leading to mild winters and, especially in western regions, abundant precipitation while negative values indicate the reverse (Hanssen-Bauer et al., 2005) [For details about the various indices see Anonymous (no date)].
Climate Projections and Climate Predictions
Climate models and weather forecast models are numerical systems based on equations that attempt to capture principal features of the climate system: they are, however, used in different ways. A weather forecast is a prediction. It aims to predict the weather a few days ahead at specific sites as accurately and reliably as possible. Weather forecasting is therefore based on detailed descriptions of the current weather that are fed into models that calculate the development of the weather day by day and even hour by hour. Climate models, by contrast, are used to calculate weather statistics under different boundary conditions (such as the concentrations of greenhouse gases in the atmosphere). Such models do not aim to predict the weather on a particular day or even the average weather for a particular season or year; rather, they aim to calculate the long-term weather statistics under given boundary conditions. They generate climate projections, not climate predictions, because they are based on boundary conditions which may or may not ever actually arise.
Projections of global climate change under different emission scenarios are based on global numerical models of the climate system. Results from different climate models are compared in the Coupled Model Intercomparison Project (CMIP). The fifth assessment report from the Intergovernmental Panel on Climate Change (IPCC, 2013) is based on results from the 5th phase of this project, CMIP5. The results are projections of change in global and continental scale climate under four scenarios called ‘Representative Concentration Pathways’ (RCPs; IPCC, 2013). The moderate RCP4.5 scenario, which is applied in the estimates in this paper, lies between the low emission RCP2.6 scenario and the ‘business as usual’ RCP8.5 scenario. Average (mean or median) values from the CMIP5 ensemble exemplify potential changes in large-scale climate under particular RCPs. The 10 and 90 percentiles of the ensemble are often used to indicate the level of uncertainty in the projections.
Meteorological Data in the Present Study
Meteorological Stations
The data presented here are drawn from the six meteorological stations that are within or near the reindeer winter pasture area of Finnmarksvidda, Norway (Figure 7). All were originally manned stations but Suolovuopmi, Karasjok and Sihccajarvi were automated in 2005, 2005 and 2009, respectively. The station at Kautokeino has been relocated twice during the last 50 years and the temperature data have been adjusted to compensate for this, thereby ensuring the homogeneity of the data time-series.
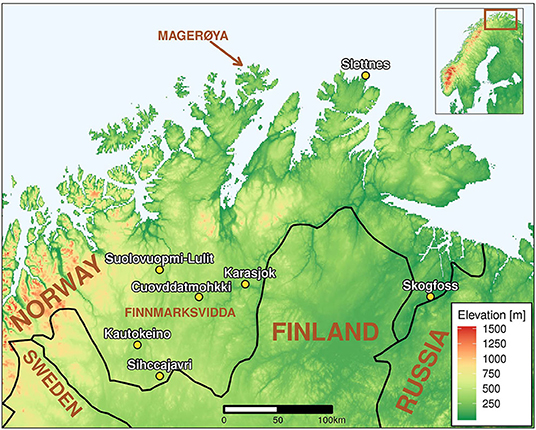
Figure 7. Meteorological stations within the reindeer winter pasture area of Finnmarkvidda at which the data presented here were collected. These are Cuovddatmohki (station number 97350, 286 m a.s.l.), Kautokeino (93700, 307 m a.s.l.), Karasjok (97250, 131 m a.s.l.), Sihccajarvi (93900, 382 m a.s.l.), Skogfoss (99500, 55 m a.s.l.) and Suolovuopmi (93300, 381 m a.s.l.). Data for weather conditions during calving time (May) were collected at Slettnes Lighthouse (96400, 8 m a.s.l.).
Study Period
Most weather data have a large stochastic component and long-term trends in weather (the signal) are therefore liable to be obscured by short-term random variation (noise). Detecting trends therefore requires analysis of long time-series, especially where the trends are weak. Weather records collected at remote stations, however, are frequently incomplete which reduces the number of datasets available if very long length is an absolute requirement. Here we have used a period of 50 years (1969–2018). This is a compromise but it spans the period in which significant anthropogenic influence on the climate has been recognized (IPCC, 2013) and it proved long enough to reveal both decadal variation and significant trends in the weather.
The influence of environmental variation—specifically, the supply of water and forage—on the productive performance of large ungulates has been recognized and recorded for millennia. The effect of drought on domestic animals in the semi-arid grasslands of the Middle East is vividly described in the Book of Joel, parts of which date from the early 8th Century B.C. (Allen, 1976) and in Hittite mythology of even greater antiquity (Bryce, 2002). Large annual and multi-annual fluctuations in the performance of animals in response to corresponding fluctuation in the weather is a feature of extensive grazing systems everywhere (e.g., Clutton–Brock and Pemberton, 2004; Thornton et al., 2009; Megersa et al., 2014; de Araujo et al., 2018).
Interest in the influence of environmental variation on animal performance has increasingly focussed on the role of climate change as an ecological driver. Indeed, the biological basis of the dynamics of wild populations and of production in extensive grazing systems is now rarely considered in any other context. This is a paradox because organisms—specifically, grazing animals and the plants on which they feed—do not respond to large-scale climate per se. Rather, they respond explicitly to those features of the thermal environment that impinge on them, their resources, their competitors, their predators and their parasites. The growth, survival and productive performance of grazing animals and the plants they eat are modulated by ambient temperature, radiation, wind speed, precipitation and other factors which together constitute the physical conditions of their immediate environment or, more simply, the weather (Mount, 1979; WMO, 2010). Weather and climate are different concepts (Box 3) and large-scale climate has no bearing on the performance of organisms except insofar as it influences the conditions to which they are exposed and to which they respond.
Indices of large-scale climate such as the North Atlantic Oscillation (NAO) or the El Niño–Southern Oscillation (ENSO; Box 3) are nevertheless routinely incorporated within analytical models of animal performance. There are several reasons for this. First, they are regularly updated and are available free of charge on the web. Second, unlike local weather data, they are spatially extensive and therefore afford investigators a common numerator with which to evaluate ecological responses to variation in environmental (meteorological) conditions over large spatial scales (e.g., Post and Forchhammer, 2002, 2004; Stige et al., 2006; Post et al., 2009a; Ascoli et al., 2017; Hagen et al., 2017). Climate indices also represent convenient environmental metrics for use at remote locations where there are no weather stations, and hence no weather data, for the same reason (e.g., Forchhammer et al., 2002). Finally, climate represents an integration of the thermal environment and indices of climate may therefore capture associations between environmental conditions and ecological processes better than more precise metrics (e.g., monthly averages of local weather variables; Hallett et al., 2004; Knape and de Valpine, 2011). The usefulness of indices of large-scale climate in post hoc accounting of variation in the growth and performance of organisms has been demonstrated many times in many taxa, and the expediency of incorporating such indices in analyses which aim to determine the consequences of climate change for species and ecosystems has repeatedly been emphasized (e.g., Raynor et al., 2020).
It is nevertheless also clear that this approach has limited predictive power. The impact of global warming on particular species of large ungulates varies widely across space and time. Effects of climate change on the physical growth of individuals, and on the numerical growth of populations, vary from positive to negative and from weak to strong across the distributional range of species (i.e., between populations) and over time (Mysterud et al., 2001; Tyler et al., 2008; Post et al., 2009a; Joly et al., 2011; Uboni et al., 2016; see also Krebs and Berteaux, 2006). Spatial and temporal variation in the strength and form of responses of a species to variation in large-scale climate reflects spatial and temporal variation in the relationship between large-scale climate and the weather (Post, 2005; Zuckerberg et al., 2020) and in local ecological settings (Martínez–Jauregui et al., 2009). Predicting the magnitude and the sign of responses of large ungulates to changes in climate therefore requires more information than is contained in summary indices. It is necessary, for instance, to confirm that components of the weather which actually influence the metabolic state of focal species (plants or animals) correlate with, and hence may reasonably be assumed to be a function of, indices of large-scale climate. It is also necessary to confirm that climate related variation in local weather conditions is physiologically relevant. Heat loads (hot or cold) imposed by statistical extremes of ambient temperature, for instance, are likely to have a measurable impact on the performance of an animal only where they fall outside its thermoneutral range (Mount, 1979; Blaxter, 1989). The omission of either step from analyses that aim to explore the consequences of climate change for a particular population of a species confines results within the realm of attractive but inconclusive association (Seebacher and Franklin, 2012; Cooke et al., 2013).
Effects of weather conditions on the performance of animals derive from situations in which meteorological factors like solar radiation, ambient temperature, rainfall and wind speed modulate the flow of energy to or from them and hence also the amount of energy they retain and can allocate to growth and production (Box 4). Effects of weather conditions on energy flow may be either direct or indirect. Direct effects involve the modulation, by the weather, of any one of four channels of heat flow from the animal to the environment (i.e., convection, conduction, radiation or evaporation; Mount, 1979). Indirect effects are chiefly associated with variation in energy supply which, for herbivores, normally means modulation of the growth and chemical composition of forage plants and, at high latitudes or altitudes, of the availability of forage beneath snow.
Box 4. How weather influences performance: relationship between the intake, loss and retention of energy
Energy in the food animals eat may be used to fuel chemical and mechanical work, whence it is lost to the environment, or it may be retained in body tissue. Retention of energy, realized as growth and fattening, influences survival and, where energy is exported as offspring and milk, also production.
The relationship between the intake, retention and loss of food energy is:
where MEI is metabolizable energy intake, ER is energy retained in body tissue and H is energy (heat) lost to the surroundings. When an animal is in thermal equilibrium (i.e., when there is no change in its mean body temperature), its rates of heat production and heat loss are necessarily equal. From Equation 1 it follows that if the animal's rate of intake of metabolizable energy in this state equals its rate of heat loss (i.e., MEI = H), then energy retention is zero (ER = 0). This level of intake is known as ‘maintenance’. Super-maintenance intake, where metabolizable energy intake exceeds the rate of heat loss (MEI > H) results in net retention of energy (ER > 0) and hence growth and production. Sub-maintenance intake (MEI < H) results correspondingly in weight loss (ER < 0) as the deficiency in energy is made good through mobilization of body tissue, including fat reserves.
The boreal region is cold: the mean ambient winter (October to April) temperature throughout the distributional range of Rangifer is 40–50°C below the species' body temperature. (The rectal and brain temperatures of Rangifer resting or standing at ambient temperature within their thermoneutral zone ≈38°C; Blix and Johnsen, 1983; Mercer et al., 1985; Blix et al., 2011.) The large temperature gradient between the animals and the environment renders them potentially susceptible to hypothermia. However, low temperatures and high wind speeds have only a small effect on the rate of heat loss—and hence on performance—in this species because the animals are exceedingly well adapted to the cold. Their thick winter coat, with hollow guard hairs filled with a honeycomb of air-filled cavities separated by thin septa (Timisjarvi et al., 1984; Blix et al., 2015), provides superb insulation (Nilssen et al., 1984) even in strong wind (Cuyler and Øritsland, 2002). Consequently, with the exception of newborn calves, it is most unlikely that Rangifer ever suffer hypothermia except perhaps under the most severe weather conditions or when starving (Blix, 2016; Tyler, 2019). Newborn Rangifer, by contrast, are highly susceptible to windchill and hence hypothermia. Calves are born in May and early June at which time cold, wet, windy conditions, coincidental with the spring melt, generally prevail. For example, the mean May temperature and precipitation at Slettnes Lighthouse (station 96400, Figure 7), representative of coastal calving areas for reindeer in northern Norway, are +3.4°C (SD 2.8°C) and 35 mm (SD 18 mm), respectively (temperature data for 1969–2019 and precipitation data for 1969–2003 from the Norwegian Meteorological Institute). Calves are precocious (Blix and Steen, 1979) but their light brown natal coat provides poor thermal protection especially when wet (Markussen et al., 1985). Their principal defense against cold is to increase heat production by mobilizing deposits of thermogenic brown adipose tissue with which they are born (Soppela et al., 1986, 1991, 1992; Blix, 2016) but harsh weather at calving may result in substantial mortality from hypothermia (Kelsall, 1968, p. 238; Miller et al., 1988).
Indirect effects of weather conditions on the performance of Rangifer are remarkable for their heterogeneity: seasonal warming, and the increase in precipitation associated with it, can have both positive and negative effects on the animals.
Warm weather in spring and summer in a region where summers are usually cold encourages earlier and faster growth of tundra plants (Elmendorf et al., 2012; Myers-Smith et al., 2019; but see Gustine et al., 2017) and warming across the last four decades has consequently resulted in widespread greening of the Arctic (Pattison et al., 2015; Zhu et al., 2016; but see Lara et al., 2018). Consistent with this, mild spring weather and earlier snow melt are associated at some sites with increased availability of forage, earlier onset of plant growth, increased primary production and, in turn, earlier calving (an advance of ~7 days over 45 years in Finland: Paoli et al., 2018) and increased body mass of animals in autumn (Norway: Pettorelli et al., 2005; Tveraa et al., 2013; Albon et al., 2017; Canada: Couturier et al., 2009). At other sites, however, warming has negative effects. Mild weather in spring (May and June) has been associated with heavy mortality of caribou, owing to the formation of ground (basal) ice that restricts the animals' access to forage (Canadian high-Arctic: Miller et al., 1982; Woo et al., 1982), and to trophic mismatch (i.e., the uncoupling of phenological events within food chains; see Visser et al., 2010; Kerby et al., 2012). The negative effect of trophic mismatch on cervids has been attributed to disruption, by an advance in the emergence of forage, of the phase relationship between the seasonal pulse of primary production and the seasonal demand for nutrients in lactating females (Kerby and Post, 2013; Plard et al., 2014). Thus, an advance in the spring emergence of plants of ~10 days over 5 years was associated with declines in the rates of production and survival of caribou calves in West Greenland of ~75% (Post and Forchhammer, 2008; Post et al., 2008, 2009b).
Winter warming, likewise, has both positive and negative effects on performance in Rangifer. These derive from the different ways in which warm weather modulates the snowpack and, hence, the animals' access to forage. This especially important for Rangifer because females are pregnant throughout winter (the animals mate in October and give birth in May or early June) and therefore have to meet the metabolic requirements of gestation at a time when access to forage is restricted by snow (LaPerriere and Lent, 1977; Skogland, 1978). Warming stimulates the hydrological cycle and has led to an increase in the average level of precipitation at mid-to-high northern latitudes across the last century (Stocker et al., 2014). Enhanced poleward moisture transport at high latitudes amplifies this trend (Zhang et al., 2013). Increased precipitation in the boreal zone can lead to increased accumulation of snow which, in turn, is associated with reduced body mass of calves at birth and also subsequently at weaning (Adams, 2005; Couturier et al., 2009; Hendrichsen and Tyler, 2014). The negative effect of snow on birth weight reflects reduction of dams' food intake and increased energy expenditure during pregnancy owing to restriction of their access to forage and to the high energy cost of walking through and digging snow to find food, respectively (Thing, 1977; Fancy and White, 1985; see also Ossi et al., 2015). This may lead to reduced allocation of nutrients to placental and fetal growth and hence to reduced birth weight (Redmer et al., 2004; Wu et al., 2006; Wallace et al., 2010). The negative effect of snow on weaning weight is presumably a result of fetal programming (Lucas, 1991; Rhind et al., 2001) and to reduced growth of forage plants (above). Increased accumulation of snow may also, however, enhance early postnatal growth. This occurs where the prolonged duration of the melt, reflecting the greater mass of snow that has to melt, extends the period of emergence of plants and hence the length of time in which the animals find and feed on freshly emerging highly nutritious shoots (Mårell et al., 2006, Leffler et al., 2016). That this positive effect of increased accumulation of snow in winter on growth of animals in summer, evident in red deer Cervus elaphus and sheep (Mysterud and Austrheim, 2014), has not been detected in Rangifer (e.g., Pettorelli et al., 2005) presumably reflects the complexity of the spatio-temporal dynamics of forage and foraging on the floral mosaic of tundra-taiga pastures (Skogland, 1980, 1984, 1989; White, 1983; Mårell and Edenius, 2006; Mårell et al., 2006; Gustine et al., 2017).
Interludes of warm weather and rain in winter that modulate the availability of forage by restructuring the snowpack are another feature of weather conditions which has both positive and negative effects on the performance of Rangifer. Warming that results in the formation of layers of ice in the snowpack or on the ground beneath it may reduce the availability of forage causing weight loss and starvation (Albon et al., 2017; Eira et al., 2018). Such ‘icing’ is held to cause of heavy mortality in Rangifer (e.g., Putkonen and Roe, 2003; Bartsch et al., 2010) although the empirical evidence for the generality of this effect is surprisingly weak (Tyler, 2010; Hansen et al., 2011; Forbes et al., 2016). By contrast, the intensity of such interludes, and the thawing they cause, is on occasion sufficient to melt snow away, exposing the vegetation and, by thus increasing the availability of forage (Vibe, 1967; Damman, 1983; Mahoney and Schaefer, 2002), enhancing survival and reproduction and stabilizing the dynamics of populations (Tyler et al., 2008; Hansen et al., 2019a).
Weather conditions thus influence the performance of Rangifer in different ways and through effects that can be measured at many different scales: locally, regionally, even continentally. Usually what matters to people and animals most, however, is the weather local to where they are, will or might wish to be. The central issue for the present study is the extent to which trends in climate that are conventionally assessed at regional, zonal or global scale, actually influence weather conditions locally within Saami herding areas. This is the topic of the next section.
The boreal zone is currently warming. Climate projections (Box 3) indicate that the warming is likely to continue for the foreseeable future (Christensen et al., 2014). Such projections, based on global climate models, have coarse spatial resolution (typically 100–200 km between the grid-points). Pastoralists and biologists alike, however, are chiefly interested in the conditions which affect plants and animals locally. Local conditions are a product of interaction between large-scale climate and local topography and generating local climate projections consequently requires a further stage of analysis. Results from the global models are downscaled by taking account of climate-landscape interactions through procedures known as Empirical Statistical Downscaling (ESD; Benestad et al., 2008) and Regional Climate Modeling (RCM; Anonymous, 2019).
The median projection for the mean annual temperature of Finnmarksvidda (Figure 7), based on 10 RCMs and modelled under the RCP4.5 emission scenario (see Box 3), indicates an increase of 2.5°C across the period 1971–2000 to 2030–2060, equivalent to a rate of warming of 0.4°C · decade−1 (Figure 8A, Table 1). This projection closely matches the trend of warming observed across the region since the 1980s (Figures 8A,C). The corresponding projection for annual precipitation indicates an increase of 40 mm (7%) across the same period, equivalent to 7 mm · decade−1 (1.5% · decade−1, Figure 8B). The trend in precipitation actually observed across the region, however, is currently more than twice this (about 3.5% · decade−1) and exceeds all but the upper part of the ensemble of projections (Figures 8B,D).
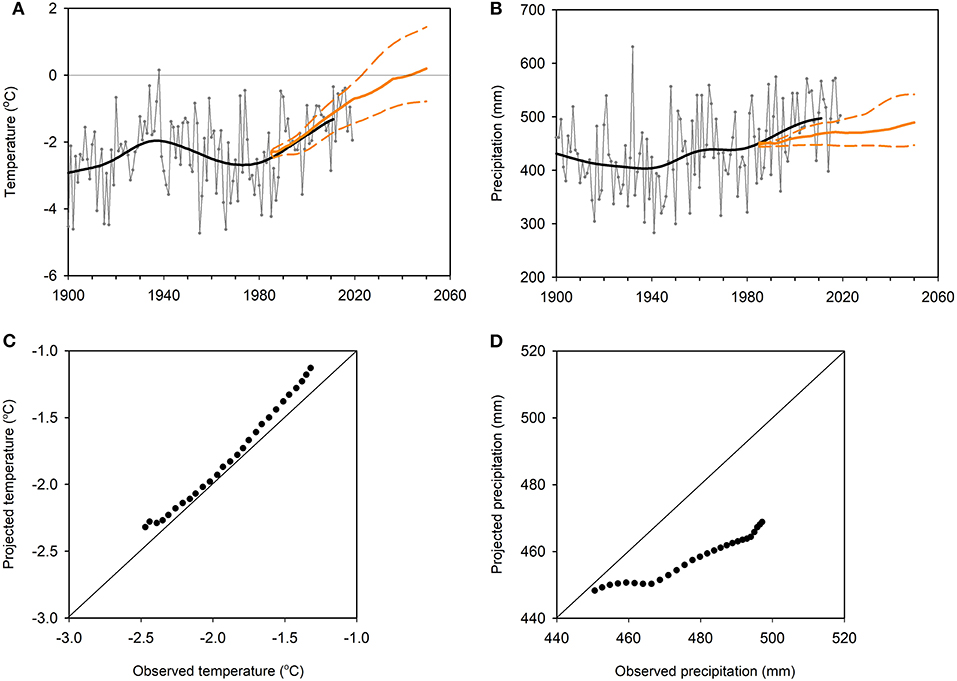
Figure 8. Weather conditions over the reindeer winter pasture area of Finnmarksvidda (Figure 7) 1900–2018: observations and projections. (A) Mean annual temperature. (B) Mean annual precipitation. Observations: data (gray) and low-pass filtered series (black; window ~30 years). Data are averages from 1 × 1 km gridded datasets covering the entire region, based in turn on observations of temperature and precipitation from around 30 meteorological stations within it. Projections (RCP4.5 scenario): median (brown line) and 10 (lower) and 90 (upper) percentiles (dashed brown lines) of ten RCM projections (Hanssen-Bauer et al., 2015, 2017). The percentiles describe the spread of the mean values of all the different models. They illustrate the uncertainty of the mean projections under the RCP4.5 scenario (Box 3) not the projected inter-annual variability. (C,D) Comparison of observations and projections for median annual temperature and precipitation for the period 1985–2011 [data from (A,B), respectively]. In each case the diagonal lines represent the position of perfect prediction. Expected (projected) and observed data have been plotted on the ordinate and abscissa, respectively, for ease of comparison with (A,B).
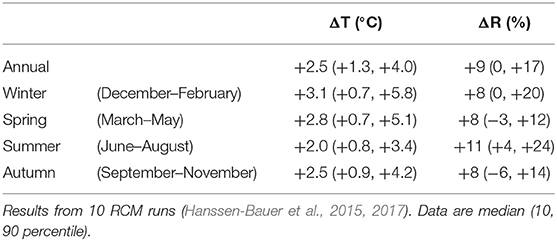
Table 1. Projected change in annual and seasonal 30-year averages of temperature (ΔT°C) and precipitation (ΔR %) over the reindeer winter pasture area of Finnmarksvidda (Figure 7) from the reference period 1971–2000 toward the middle of the 21st Century (2031–2060) under the RCP4.5 scenario (Box 3).
Projections for the duration and depth of snow cover, produced by running a hydrological model with input from the projections for temperature and precipitation (Hanssen-Bauer et al., 2015, 2017), show a reduction in the length of the snow season all over Norway. The effect is most marked over coastal lowlands but is also apparent over inland mountain areas (Hanssen-Bauer et al., 2015, 2017). For Finnmarksvidda, the RCP4.5 scenario typically gives a reduction in the period of snow cover of 1–2 months from 1971–2000 to 2071–2100 which, if the trend were linear, would be equivalent to a rate of 3–6 days · decade−1.
Projections for maximum snow depth (measured as ‘water equivalent,’ mm) show only small changes over Finnmarksvidda toward the end of the 21st Century (Hanssen-Bauer et al., 2015, 2017). These include a small reduction for most of the area but also minor increases at some sites (which vary from model to model). In neither case are the trends likely to be linear because snow depth is a function of both precipitation and temperature. Hence, snow depth is likely initially to increase with increasing precipitation in cold areas but then to decrease where increased temperature causes rain or melting (Hanssen-Bauer et al., 2015, 2017).
The mean temperature over the reindeer winter pasture area of Finnmarksvidda during the snow season (October to April, O-A) increased by 2.3°C across the last 50 years, from regression estimates of −10.4°C in 1969 to −8.1°C in 2018 (Figure 9A). The average rate of warming was therefore 0.46°C · decade−1. The observed increase is slightly less than the median projections for the corresponding period under a medium scenario (2.5–3.1°C; Table 1). The pattern of warming has been remarkably consistent across the region: the data from five weather stations spread across 120 km (Figure 7) are closely correlated (correlation coefficients of inter-annual variation between the stations range from 0.95 to 0.99; Figure 9A). The temperature varied considerably from year to year at every station. The mean annual O-A temperature (all stations combined) deviated from the regression model by, on average, |1.1|°C (range: −2.9 to 2.7°C; Figure 9B) which is equivalent to half the linear trend over the entire period 1969–2018. There was also conspicuous decadal variation in temperature: winters in the early 1970s and 1990s were consistently warmer than indicated by the 50-year regression line while the 1980s and late 1990s were consistently colder than indicated by the line (Figures 9A,B).
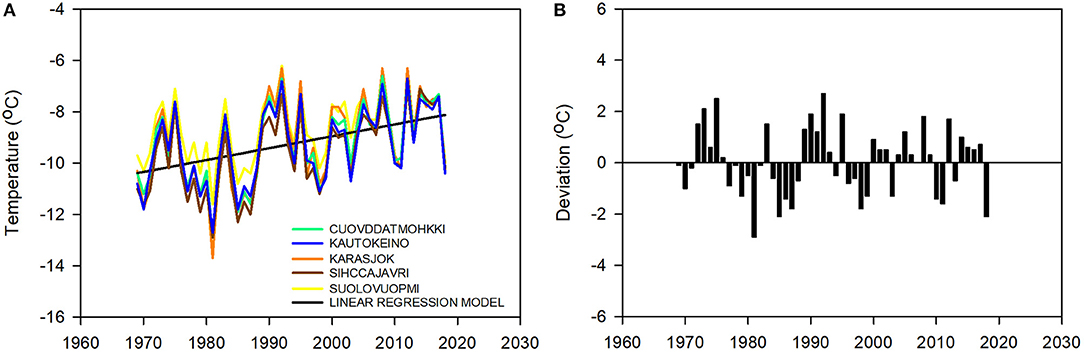
Figure 9. Winter warming. (A) Mean annual ambient temperature (October to April, O-A) and linear trend in temperature (°C) over the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. Data from five weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri and Suolovuopmi (Figure 7). The linear regression model (straight black line), O-A(°C)year = [(0.05 · year) – 101.75], r2 = 0.21, p < 0.001), is based on data combined from all five stations. (B) Annual deviations (°C) from the regression model.
Annual and decadal variability in temperature is connected to variation in atmospheric circulation patterns such as the North Atlantic Oscillation index (NAO; see Box 3; Hanssen-Bauer and Førland, 2000; Hanssen-Bauer, 2005). Thus, the annual mean temperature in the reindeer winter pasture area of Finnmarksvidda is strongly correlated with the NAO annual index (Figure 10). The NAO seems to influence trends in regional weather conditions over several decades, alternately countering and then amplifying trends related to increases in concentrations of greenhouse gases (Deser et al., 2017). There is still no consensus concerning how global warming may affect the NAO: Rind et al. (2005) have argued that it may lead to more frequent positive values of the NAO. Such an effect would potentially amplify the warming of the reindeer winter range of Finnmarksvidda consistent with the positive correlation between temperature and the NAO index (Figure 10). It might also increase precipitation although the correlation between precipitation and the NAO index in this region is quite weak (Hanssen-Bauer, 2005). The effects of more frequent positive values of the NAO on the depth and cover of snow are likely to be complex. Increased temperature and precipitation would potentially result in more snow but only so long as the temperature stayed below 0°C, while warmer temperatures that reduce the duration of the frost season would potentially result in a shorter snow season.
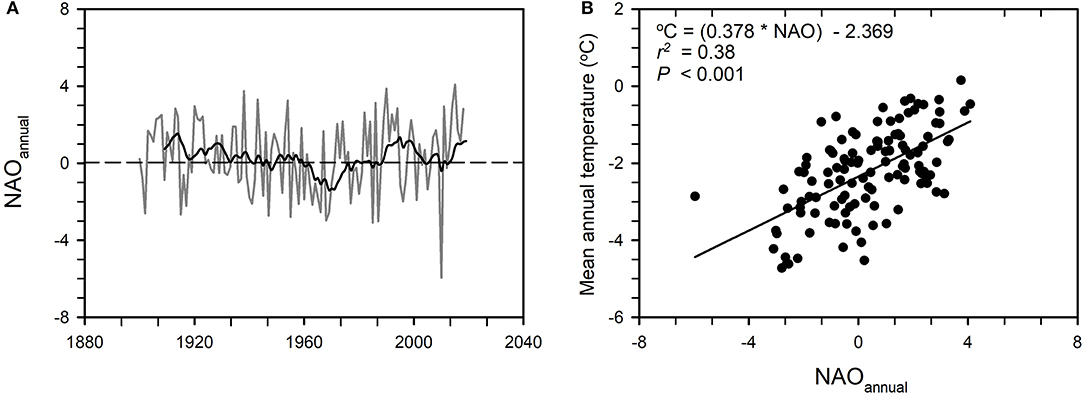
Figure 10. Influence of large-scale climate on local weather. (A) Annual values of the North Atlantic Oscillation annual index 1900–2018 (NAOannual, gray curve). The trend is indicated by a 10-year running mean (black curve). (B) Relationship between NAOannual and the mean annual temperature over the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1900–2018. Each point represents one year. Data: Hurrell and National Center for Atmospheric Research Staff (2020), Norwegian Meteorological Institute.
Consistent with warming, winters are becoming shorter. The onset of the frost season on Finnmarksvidda has occurred progressively later, and the offset of the frost season (i.e., the spring melt) progressively earlier, across the last five decades. The onset of the frost season has delayed by 9.8 days, from a regression estimate of 8th October [day of year (DoY) = 280.9] in 1969 to 18th October (DoY = 290.7) in 2018; the average rate of delay has therefore been 2.0 days · decade−1. The end of the frost season has advanced by 9.3 days, from a regression estimate of 27th May (DoY = 116.9) in 1969 to 6th May (DoY 126.4) in 2018; the average rate of advance has therefore been 1.9 days · decade−1 (Figure 11A). Both effects have been consistent across the region: the data from five weather stations are closely correlated (correlation coefficients of inter-annual variation between the stations ranges from 0.68 to 0.86 for the start of the frost season and from 0.37 to 0.83 for the end of the frost season; Figure 11A). The dates of each, however, varied considerably from year to year at all stations. The date of the start of the frost season deviated from the regression model by, on average, 7.4 days (range: 52 days); corresponding values for the end of the frost season were 7.5 days (range: 44 days; Figure 11B).
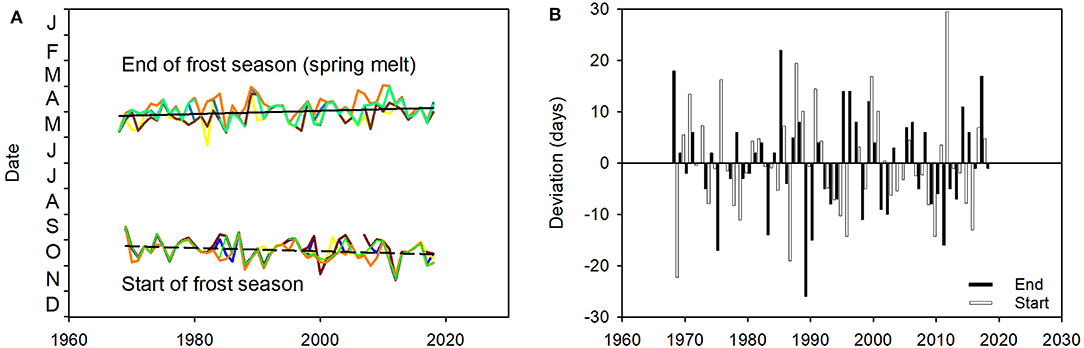
Figure 11. Date of the start of the frost season in autumn and the end of the frost season in spring on the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. Data from five weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri and Suolovuopmi (Figure 7). (A) Annual date of start and end, and trends in dates from linear regression models: Datestart = [(0.199 · year) – 110.9], r2 = 0.08, p < 0.05 (straight dashed line) and Dateend = [(−0.188 · year) + 497.2], r2 = 0.08, p < 0.05 (straight solid line). (B) Annual deviations from the regression models (days; note expanded ordinate scale). For color codes, see Figure 9.
The number of days in winter (O-A) with middle ambient temperature above 0°C (‘thaw days’) increased by 13 (58%) across the last 50 years, from regression estimates of 22 days in 1969 to 35 days in 2018; the average rate of increase was therefore 2.6 days · decade−1 (Figure 12A). The period October to April counts 212 days (213 in leap years), and 35 thaw days therefore represent 16.5% of the total period. The effect was consistent across the region: the data from five weather stations are closely correlated (correlation coefficients of inter-annual variation between the stations ranges from 0.77 to 0.96; Figure 12A). The number of thaw days varied considerably from year to year at all stations. The annual mean deviated from the regression model, on average, by 5.8 days (range: 30 days; Figure 12B). The average number and average duration of periods of thawing have increased only slightly across the last 50 years, from regression estimates of 9 in 1969 to 11 in 2018 and from 2.3 to 3.1 days, respectively. Annual values for the former, in particular, deviate substantially around the 50-year trend (Figure 13).
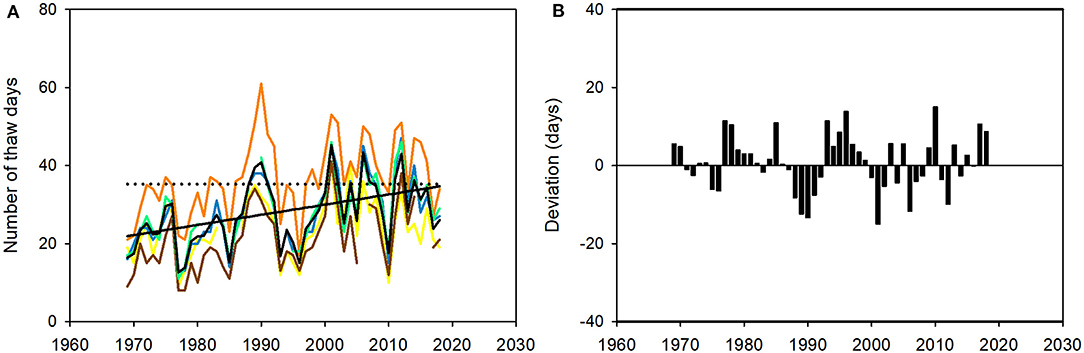
Figure 12. The number of days in winter (October to April, O-A) on the reindeer winter pasture area of Finnmarksvidda (Figure 7) with middle ambient temperature >0°C (‘thaw days’), 1969–2018. Data from six weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri, Skogfoss and Suolovuopmi (Figure 7). (A) Annual mean value for all stations combined (black curve) and at each station (colored curves; days), and trend in numbers of days from a linear regression model O-Ayear thaw days = [(0.26 · year) – 491.45], r2 = 0.22, p < 0.01 (straight black line). The dotted black line shows how the regression estimate for 2018 (35 days) was exceeded by the observed annual average in only 15.2% (n = 5) of years before 2001 but in 47.1% (n = 8) of years after 2001. (B) Annual deviations from the linear models (days). For color codes, see Figure 9.

Figure 13. The average number (n) and duration (days) of periods of thawing in winter (October to April, O-A) on the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. Data combined from five weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri and Suolovuopmi (Figure 7). Trend lines derive from linear regression models: Number of daysyear = [(0.043 · year) – 74.993], r2 = 0.08, p = 0.05 and Average durationyear = [(0.017 · year) – 30.695], r2 = 0.095, p < 0.05.
Precipitation in winter (O-A) on Finnmarksvidda increased by 66 mm (52%) across the last 50 years, from a sum of 127 mm in 1969 to 193 mm in 2018 (linear regression estimates; data from six station combined), yielding an average rate of increase of 13.4 mm · decade−1. The observed annual mean deviated, on average, by |13.1|% (range: −36.4 to 44.0%) around the linear trend. In contrast to the previous parameters, the linear rate of increase varied substantially across the region, ranging from 8.6 mm · decade−1 at Suolovuopmi in the north to 22.3 mm · decade−1 at Sihccajarvi in the south from initial (1969) estimates of 175 and 131 mm, respectively (Figure 14). Moreover, a conspicuously wetter-than-average period was evident during the 1990s in the west of the region (Kautokeino and Suolovuopmi) but not in the east (Karasjok and Skogfoss), and there was a conspicuous decrease in the level of variation in precipitation in the east of the region during the decade up to 2018 (Skogfoss; Figure 14).
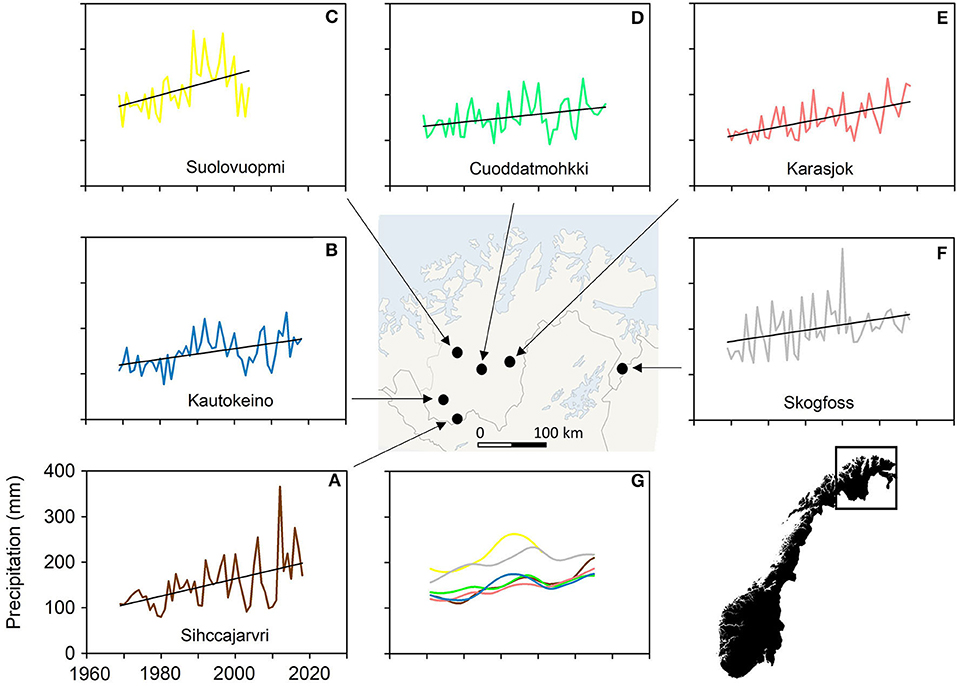
Figure 14. Total precipitation in winter (October to April, O-A; mm) on the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. (A–F) Raw data from six weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri, Skogfoss and Suolovuopmi (Figure 7). (G) Low-pass filtered data (window ~30 years: the first and last three years in the time series from each station are excluded). Precipitation increased at all stations over the last 50 years. Linear regression coefficients (mm · year−1) are Cuovddatmohkki 0.860, p = 0.1 NS; Karasjok 1.561, p < 0.001; Kautokeino 1.157, p < 0.001; Sihccajavri 1.190, p < 0.001; Skogfoss 1.226, p < 0.01; Suolovuopmi 2.233, p < 0.01. The level and pattern of change in precipitation was generally uniform across the region but with some exceptions. Skogfoss and Suolovuopmi, for instance, were consistently wetter than all other stations (G).
The average depth of snow in March (the snowiest month) on Finnmarksvidda increased by 14 cm (31%) across the last 50 years, from regression estimates of 45 cm in 1969 to 59 cm in 2018; the average rate of increase was therefore 3 cm · decade−1 (Figure 15B). This value, however, disguises considerable variation in snow depth from year to year at the five weather stations (Figure 15A). Annual March depth of snow deviated, on average, by |6.9| cm (range: −25.5 to 18.1 cm or −45.3 to 37.6%) around the linear trend (Figure 15C). There was conspicuous decadal variation in snow depth: thus, the 1980s and 1990s were characterised by greater depth of snow than predicted by the regression model, while snow depths in the first decade of the present century were consistently lower than predicted (Figure 15C). The pattern and the rate of increase in snow depth also varied across the region. The annual coefficient of variation of snow depth among the five weather stations varied seven-fold, from 4.6 to 31.4%, while the average rate of increase in snow depth ranged from 2 cm · decade−1 (Karasjok) to 6 cm · decade−1 (Skogfoss; Figure 15B).
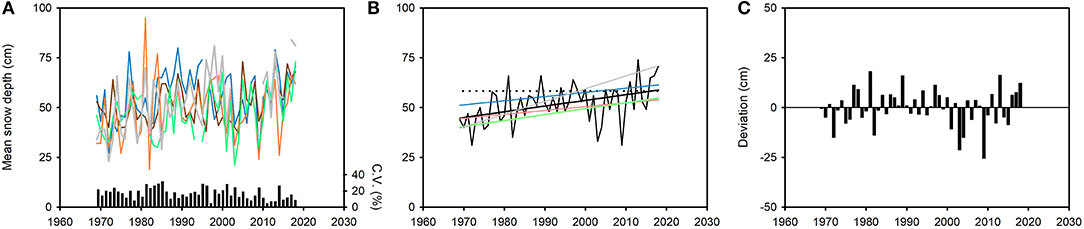
Figure 15. Average depth of snow in March (the snowiest month) on the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. Data from five weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri and Skogfoss (Figure 7). (A) Annual mean values at each station (cm) and annual coefficient of variation (C.V.) in mean depth across all stations (%). (B) Trends in snow depth from linear regression models. The model for the combined dataset (black curve) is Average depth (cm)year = [(0.288 · year) – 522.45], r2 = 0.18, p < 0.01(solid black line). The dotted black line shows how the regression estimate for 2018 (58.8 cm) was exceeded by the observed annual average only six times prior to 2011. Regression lines (but not data) for each station (excepting the regression line for Sihccajarvi which is indistinguishable from the line for the combined data set). (C) Annual deviations from the regression model for the combined dataset (cm). For color codes, see Figure 9; gray: Skogfoss.
There were on average 21 (9.5%) fewer days with snow cover in winter (O-A) on Finnmarksvidda in 2018 (n = 198) compared to 1969 (n = 219); the average rate of decrease was therefore 4 days · decade−1 (Figure 16A). There was, however, considerable annual variation at all stations (Figure 16A). The observed annual mean deviated, on average, by |9.5| days (range: −31.4 to 19.0 days or −14.5 to 9.2%) around the linear trend (Figure 16B).
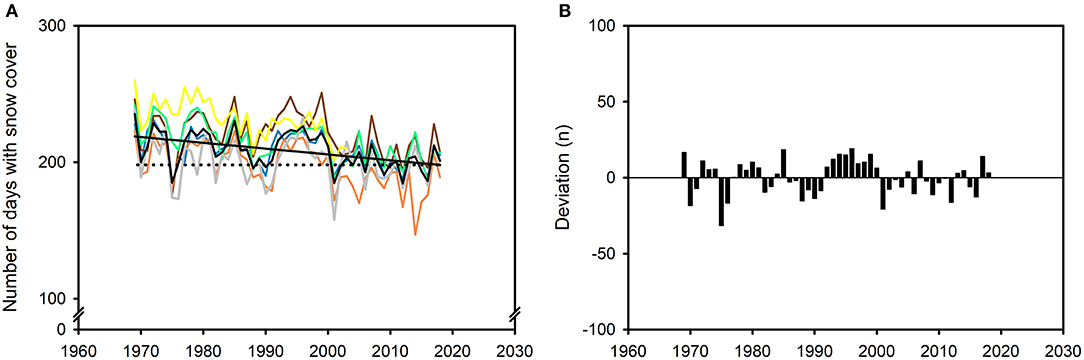
Figure 16. Number of days with snow cover in winter (October to April, O-A) on the reindeer winter pasture area of Finnmarksvidda (Figure 7), 1969–2018. Data from six weather stations: Cuovddatmohkki, Karasjok, Kautokeino, Sihccajavri, Skogfoss and Suolovuopmi (Figure 7). (A) Annual mean value for all stations (black curve) and for at each station (colored curves; days) and the trend in number of days derived from a linear regression model (data combined for all stations): Number of daysyear = [(0.43 · year) + 1058.5], r2 = 0.23, p < 0.001 (black line). The average number of days with snow cover exceeded the regression value for 2018 (198 days) in 29 (91%) of the first 32 years of the series (1969–2000) but in only 10 (56%) of the last 18 years (2001–2018; dotted line). (B) Annual deviations from the model for the combined dataset (n). For color codes, see Figure 9; gray: Skogfoss.
The climate of the north of Norway is changing and with it, this analysis has shown, weather conditions that influence the level of resources on reindeer pasture there. The dynamic, however, includes another dimension: human intervention can alter the resource base in ways entirely independent of climate change and to which the analysis now turns.
Loss of land area is the greatest threat to future viable reindeer husbandry [in Norway today].
(Government of Norway, 2016, p. 69)
Reindeer husbandry is an extensive form of land use. Approximately 40% (141,000 km2) of Norway's mainland is designated reindeer pasture (Box 2) and within this area Saami herders have—in principle—the right to graze their animals on uncultivated land (utmark) irrespective of ownership (below). Herders' right of usufruct (Box 2), however, affords them neither exclusive access to land nor protection from the activities of other land users. Conflicts of interest are common. For herders the principle issue is the securing of pasture on which to graze their reindeer. Indeed, the progressive and effectively irreversible loss of grazing land is recognized as the single greatest threat to reindeer husbandry in Norway today (Government of Norway, 2016, p. 69).
Herders lose pasture principally in two ways: physical loss and non-physical loss (Tyler et al., 2007). Physical loss occurs where pasture is either physically destroyed, transformed into another biotope (such as water or agri- or silviculture), or rendered unavailable by the erection of barriers that physically exclude reindeer from it. Non-physical loss occurs either where herders are individually or collectively denied the right to graze pasture that is otherwise available, or where their access to pasture is reduced by disruption of animals' mobility (including obstructing migration routes), or where the value of pasture as a resource is reduced as a result of human activity, the latter manifest as avoidance behavior (below).
Expansion of agriculture was historically the principal cause of loss of prime lowland reindeer pasture in Norway. Ethnic Norwegian (i.e., non-Saami) people moved north and east into remote parts of the country throughout the 18th and early 19th Centuries and settled areas that had previously largely been unoccupied save for Saami. Settlement was encouraged by the government through legislation designed to stimulate agriculture by affording farmland legal protection from grazing by reindeer which was mandated by the imposition of substantial fines on transgressors (Hætta et al., 1994; Strøm Bull, 2015). Today, by contrast, the principal physical cause of loss of pasture is construction. Reindeer can graze a field even if they are not supposed to (Figure 17) but pasture covered by asphalt, concrete, wood or water leaves them nothing. The effect is absolute and effectively irreversible.

Figure 17. Illegal grazing: herders are not allowed by law to graze reindeer on actively cultivated ground (Government of Norway, 2007, §19). Are these male reindeer, enjoying a lawn on Kvaløysletta just outside Tromsø, Norway, encroaching on cultivated ground or has cultivation encroached on traditional reindeer pasture? Photograph: Bjørn Lockertsen.
Domestic and commercial building and infrastructure expanded across Norway during the 20th Century (Figure 18). The physical loss of pasture resulting from this, however, was and is small and localized. In 2019 just 365 km2 or 0.5% of the northernmost county of Troms and Finnmark (74,830 km2) were classified as built upon (data: Statistics Norway https://www.ssb.no/statbank/table/09594). Agriculture likewise currently represents only small-scale encroachment, albeit on the best land: in 2017 just 331 km2 or 0.4% of the same area was under cultivation. Altogether 3,544 km2 or 2.5% of the whole Saami reindeer husbandry area is currently under cultivation (not including forestry; data: Statistics Norway https://www.ssb.no/statbank/table/11506). The most extensive components of infrastructure, in terms of area covered, are technical installations associated with transport (e.g., roads, airports, energy and water facilities); the most rapidly increasing component has been building associated with industry and other forms of commercial activity (Figure 19). Recreational cabins/huts (Norwegian: hytter) and their grounds, though small in extent (occupying in 2017 just 199 km2 or 0.1% of the Saami reindeer husbandry area), are a significant feature of encroachment because they are invariably situated in the mountains and along the coastal strip where reindeer graze. On average, 1,450 (range 1,231–2,135) huts have been built in the Saami reindeer husbandry area annually during the last 20 years and in 2017 there were some 135,000 huts, almost 1 per km2, there (Data: 1998–2017 from Statistics Norway https://www.ssb.no/statbank/table/06952).
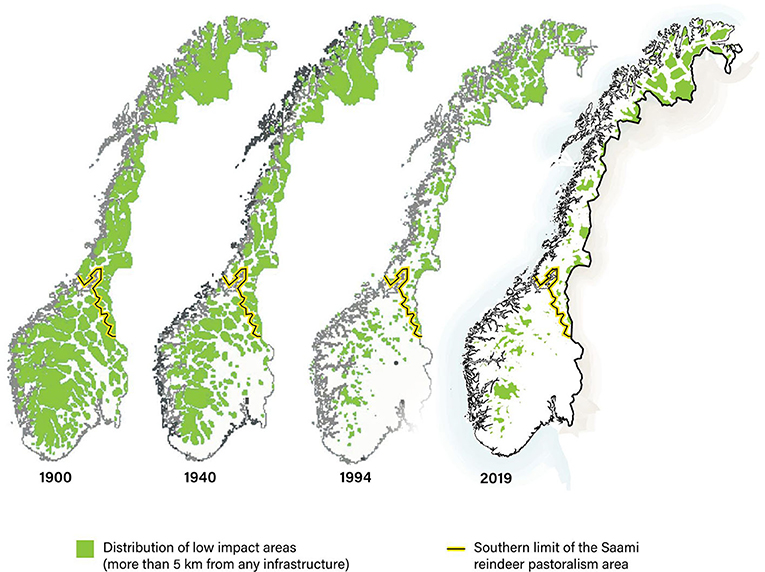
Figure 18. Loss of habitat: distribution of areas of Norway >5 km from any infrastructure (green shading) 1900–2019. The yellow line marks the southern boundary of the Saami reindeer husbandry area (see Figure 3). Sources: Nellemann et al. (2003), Norwegian Mapping Authority.
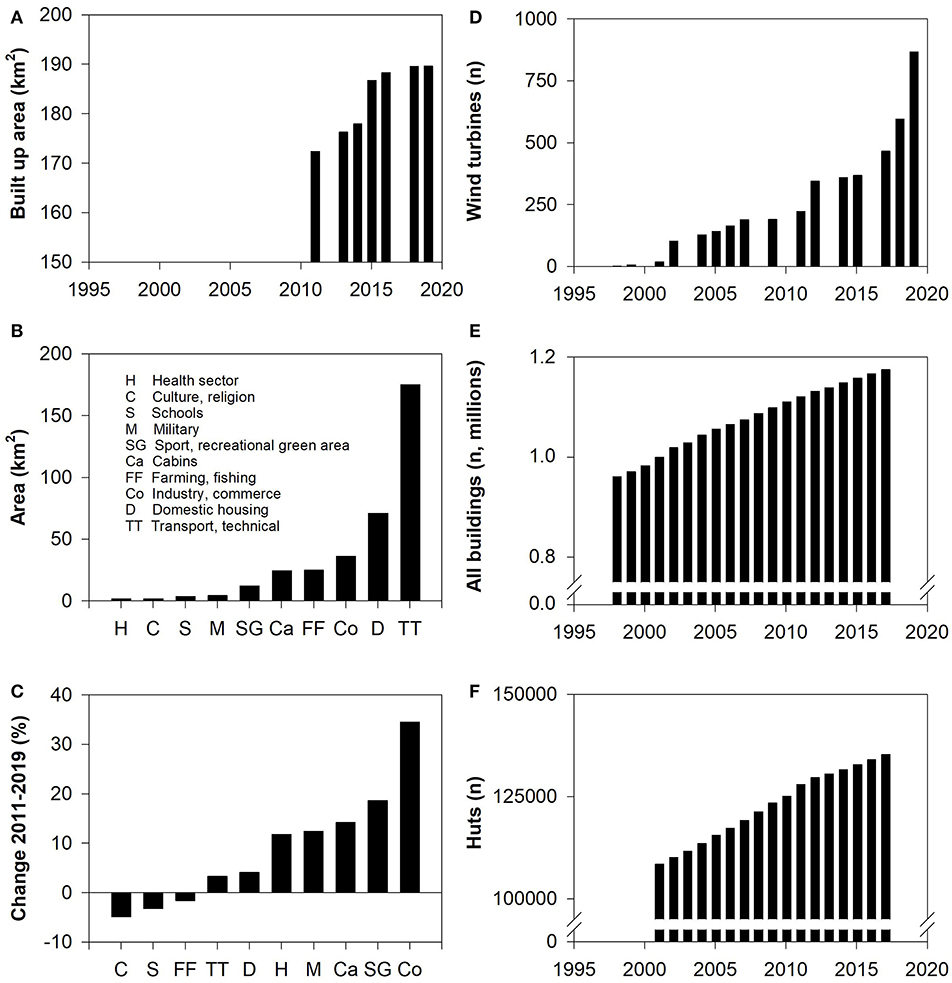
Figure 19. Expansion of infrastructure since 1998 across the Saami reindeer husbandry area. (A) Total area of infrastructure (km2) according to the categories shown in (B). (B) Area of infrastructure (km2) in 2019 by category. (C) Proportional change in the area of infrastructure (km2) from 2011 to 2019 by category (%). (D) Number of wind turbines. (E) Number of buildings. (F) Number of recreational huts and summer houses (Norwegian: hytter og fritidsbolig). (A–C) Data for the Troms, East and West Finnmark husbandry areas (Figure 3); (D–F) data for the entire the Saami reindeer husbandry area. Sources: All data except (D) Statistics Norway; (D) Water Resources and Energy Directorate (NVE).
Saami reindeer herders in Norway, like indigenous pastoralists throughout the world, generally do not own the land they use. The utmark on which they graze their animals—and cut timber, collect fuel, gather berries, catch fish and hunt—is generally owned by the State or by corporate or private non-pastoralists. Historically, however, it served as de facto commons. The right of herders to use such land derived from unwritten customary law and subsequently achieved legal recognition on the principle that rights accrue where there has been ‘use since time immemorial’ (Norwegian: alders tids bruk; Ravna, 2010a; Strøm Bull, 2015). The right to graze utmark, formally codified in the Lapp Codicil of 1751 (Pedersen, 1987; Hætta et al., 1994; Mazzullo, 2009; Ravna, 2010b) seems generally to have been accepted until the late 19th Century when, however, it was challenged on several grounds. (Note: ‘Lapp’ was a then contemporary word for ‘Saami’).
In 1889 Professor Yngvar Nielsen confronted the conventional view—that Norwegians encroached on land to which the Saami, as the original inhabitants, had precedence—with evidence that the former were in fact the original occupiers. He argued, in particular, that Saami people did not settle the area around the town of Røros, in southern part of what is today the reindeer herding area of Norway (Figure 3), until after it had been occupied by Norwegians in the 18th Century. From this it followed that Saami herders encroached on Norwegian farm and hill pasture rather than the other way around. A Lapp Commission, convened later that year to investigate Professor Nielsen's claim, concurred and, in doing so, legitimized an interpretation which constrained Saami grazing rights for the next 100 years (Strøm Bull, 2015).
Professor Nielsen's historical appraisal of patterns of settlement resulted in a fundamental challenge to herders' use of utmark. Thus, in 1926 the view was advanced that the Saami right of usufruct was an instance not of ‘use since time immemorial’ but of ‘tolerated use’ (Norwegian: tålt bruk). It followed that their use was subject to statutory legislation on the basis that a right conferred by the State might equally be withdrawn at any time by the State (Strøm Bull, 2015). This interpretation was refined by a judgement in 1955 that herders' rights of hunting and fishing applied only to State commons and not to private land. Private landowners desiring to forbid Saami herders access to their land prevailed before the Land Consolidation Court and subsequently at the Court of Appeal before, in 1968, both the decisions were reversed by the Norwegian Supreme Court. The Supreme Court ruled that Saami reindeer herders' historic use of land might on occasion be so grounded in custom that it could not summarily be equated with usufruct or any common right of access. In the opinion of the court such use represented an independent legal basis from which, furthermore, stemmed the right of compensation for expropriation (Strøm Bull, 2015).
It remained unclear, however, exactly to which areas and to what land the 1968 ruling applied. In a series of cases, lower courts attached decisive weight to descriptions of land use drawn from the report of the Lapp Commission of 1889. This encouraged private landowners to claim that Saami herders had no right to herd reindeer outside areas specifically mentioned in the Commission's report. Their challenge, brought to court in 1995, was indirectly supported by the Royal Ministry of Agriculture which, ignoring the Supreme Court's ruling, based the 1978 Reindeer Herding Act on the premise that the right to engage in reindeer husbandry was governed exclusively by statutory regulation. The Ministry's interpretation was subsequently reversed by an amendment to the Act passed in 1996. However, it was not until 2001—exactly 250 years after the Lapp Codicil—that the Supreme Court confirmed the use of utmark for pasturing reindeer to be an independent right based on use since time immemorial and independent, therefore, of the provisions of the 1978 Act. On this occasion, the court explicitly recognized the inherent difficulty of demonstrating prolonged and continuous use of land. It therefore specified that weight should always be given to the ‘nature of the right’—a reference to the itinerant character of land use which is the hallmark of reindeer husbandry—and that lengthy interruption of the use of a particular area was insufficient to hinder the acquisition of rights of use (Strøm Bull, 2015).
Professor Nielsen's 1889 report thus spawned uncertainty and controversy which reverberated through the courts for more than a century and which continues to reverberate in the public discourse about Saami reindeer pastoralism to the present day (e.g., Larsen, 2019). It is dismaying therefore to note that his conclusion regarding the sequence of occupation of land around Røros, and hence his allegation that Saami reindeer herders encroached on land already occupied by Norwegian peasant farmers, was incorrect. In 1799 the Revd. Thomas Malthus FRS (1766–1834), the English priest and scholar best known today for his theory of population growth, travelled through the area that Nielsen explored a century later. He recorded how Mr. Knoph, the Director of the Røros Copper Words, informed him that Saami people ‘had inhabited these mountains before Røros was known.’ This, and Malthus' own observations of Saami reindeer herders there, only came to light when the latter's diaries were published in 1966 (James, 1966, p. 189–195). Knoph's observations regarding the antiquity of the Saami presence in the area have subsequently been corroborated by evidence of Saami heritage throughout the region (Strøm Bull, 2015). The situation around Røros, moreover, was not unique in this regard. Throughout the country Norwegians settled areas already used by Saami reindeer herders. Thus, the valley of Dividalen in Troms and Finnmark in the north was
‘… populated … late. The innermost farm … was first cleared in 1844-45 … The settlers’ conquest of these areas was of major importance for the use of the mountain [pastures] … Norwegian settlements restricted … Saami traditional use [Norwegian: ‘hevd’] of the land and obstructed reindeer husbandry … Saami dwelling places were occupied and herders were obliged to shift their migration routes. … The State largely supported the [settlers'] claims [to the land] …' Kalstad (1974, p. 101).
‘Reindeer husbandry … has been in turmoil since the border was closed in 1852.’
(Hætta et al., 1994, p. 23).
By far the most extensive loss of reindeer pasture in Norway occurred and occurs through the withdrawal of herders' right of access to land owing to the closure of international borders and to the reallocation of land for other purposes.
International Borders. Long distance movement of large ungulates across rangeland is a ubiquitous and defining feature of extensive grazing systems. In nomadic systems, herds and herders move continuously, opportunistically seeking transient pasture resources along paths that may vary substantially from year to year. In transhumant systems they move between established points that are likely to be regular and of ancient pedigree (Blench, 2000). Reindeer pastoralism in Norway is largely transhumant: Saami herders and their herds normally migrate between discrete summer and winter pastures with the former usually, although not invariably, at the coast and the latter usually, although not invariably, at higher elevation inland (Box 2). The animals follow an ecological imperative: they track changing snow conditions in winter and the phenological progression of forage plants across spring and summer just like their wild conspecifics (Skogland, 1984, 1989; Fancy et al., 1989): herders, of course, move with them.
Historically, reindeer herders in Fennoscandia enjoyed freedom of passage across the jurisdictionally unchartered mountains, forests and taiga of the northern landscape. This situation lasted until the 18th and 19th Centuries when borders demarcating the then kingdoms of Denmark-Norway, Sweden-Finland and subsequently Russia-Finland (now the independent countries of Norway, Sweden, Finland and the Russian Federation) were extended across the region (Kirchner, 2020).
Border with Sweden-Finland. The Commission responsible for drawing up the border between northern Norway and northern Sweden-Finland in 1751 (Figure 20A) accepted that a closed border would disrupt established patterns of grazing including the seasonal migration of reindeer across the border. It would affect Saami herders in Sweden-Finland who moved west over the mountain divide into Norway in spring before returning east to the low-lying forests of Sweden in autumn and Norwegian Saami herders who moved the other way (Figure 3). The Commission therefore proposed that reindeer herders from both countries should be permitted to cross the border with their animals according to customary practice. Herders' rights of passage across the border were secured through the medium of an Appendix (the ‘Lapp Codicil’) to the Treaty of Strømstad of 1751:
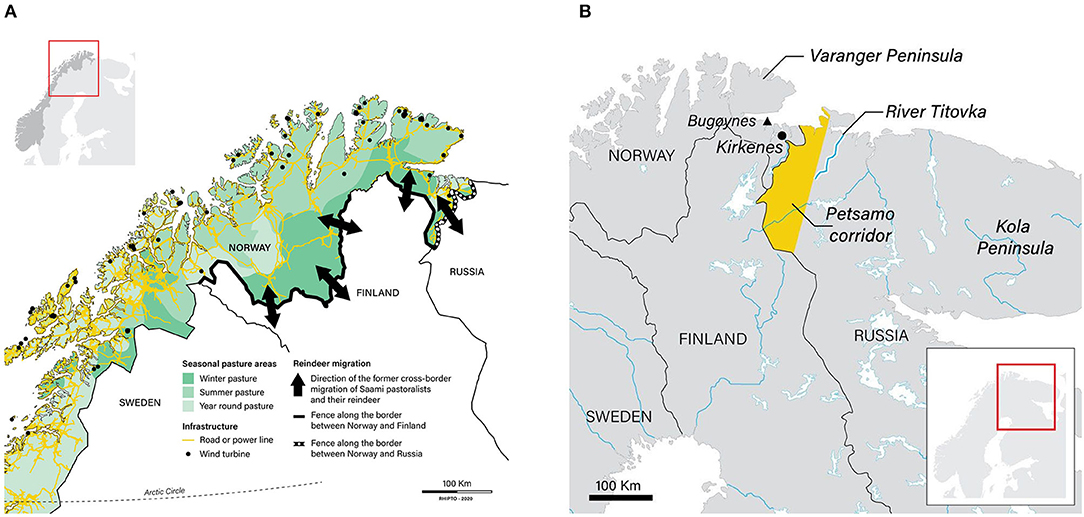
Figure 20. Legal constraints and infrastructure reduce land available for pastoralism. (A) Map showing fences (thick lines) built along the border between Norway and Russia (fence 200 km in length) and between Norway and Finland (fence 637 km in length) to prevent cross-border grazing by domesticated reindeer. Heavy black arrows indicate the direction of seasonal migration of reindeer before the borders were closed. The map also shows infrastructure (roads and wind turbines) on seasonal reindeer pasture in northern Norway. Infrastructure and related features that substantially reduce the area of reindeer pasture not shown here include agricultural land, airports, hydro-electricity plant (including flooded ground), industrial plant, military training areas, mines and recreational facilities including private huts, ski fields and walking-, dog-driving, ski- and snowmobile-trails. (B) Map showing places mentioned in the text regarding the consequences for reindeer husbandry of the closure of the border with Russia. Sources: Roto (2015) and Government of Norway (2016), Norwegian Mapping Authority.
‘Recognizing that the Saami require [pasture in] both the lands of the realm, they shall be allowed to move with their herds of reindeer across the border into the other kingdom in autumn and spring according to ancient custom. And there, as before, … they shall be allowed to use the land … to sustain their animals and themselves, and must be kindly received, protected and helped to justice just like [all] subordinates of the country [they have entered]’ (Government of Norway, 2015, §10).
The text of the Codicil, moreover, specified that the document carried the same legal weight (‘skal … være af samme Kraft’) as the Treaty itself (Government of Norway, 2015).
Freedom of passage, however, did not entail free use of pasture on each side of the border. The Codicil specified that:
‘Swedish Lapps who move across the border onto Norwegian ground with their [reindeer shall] pay a ground rent for every 20 animals, … large and small of both sexes, except for calves born in the same spring, one Danish shilling or one Swedish styver, in copper coin, not more …’
while Norwegian Saami (‘Lapps’) traveling in the opposite direction were to pay exactly twice as much (Government of Norway, 2015, §13–14). These fees still apply but, given that they are charged at the original rate, uncorrected for 250 years' inflation, the charge is minimal (Øyvind Ravna, personal communication, 8 February 2020).
Neither the Commissioners' appreciation of the function and significance of transhumance nor the legal obligation specified by the Lapp Codicil were sufficient, however, to protect herders' right of movement and cross-border grazing from developments in the organization of, and the relationships between, neighboring countries (below).
Border with Russia. In 1826 (effective from 1827; Gabrielsen, 2009) the border between Norway and Russia was closed with the stated aim of preventing disputes of the kind that arose in the absence of clarity over its exact position (‘… Grund heraf villet forebygge de Tvistigheter, som hidtil have kunne opstaae, paa Grund af, at der savnedes en nøiaktig Grændsebestemmelse imellom Norge og Russland …’ Hætta et al., 1994, p. 14). Then, in 1852, the border with Finland, which Sweden had ceded to Russia in 1809, was closed following Russian refusal to be bound by the Treaty of Strømstad to which she was not a signatory. Thus, at two strokes of the administrative pen reindeer herders in Finnmark lost access to half their traditional winter pasture (Hætta et al., 1994). Not surprisingly, reindeer continued to cross the border and to use winter pasture in Russia and Finland as they were accustomed to do and, equally not surprisingly, measures were instituted to prevent this, including the appointment of bailiffs whose task was to enforce the new legislation. For 7 years (1826–1833) Johan Henrik Cappelen served as bailiff responsible for collecting fines from Norwegian herders whose animals strayed across the border. The fine was set at one specie dollar (spesidaler) for each 50 reindeer that crossed. What proportion of the money ever reached the State coffers is unknown but Cappelen's ‘luxurious lifestyle and exuberant extravagance’ (råflotte levevis og overmodige utskeielser) suggested that it was probably not large (Gabrielsen, 2009).
The threat of fines did not, however, stop herders moving animals across the borders. Herders from Varanger in northern Norway continued to use pasture in Russia, traveling as far east as the River Titovka and sometimes beyond it onto the Kola Peninsula (Figure 20B; Leinonen, 2007; Odd Erling Smuk, personal communication, 5 February 2020). The Russians had no effective border controls and the practice of grazing animals in Russia in winter continued until 1918 when civil war broke out in Finland:
“… war [reached] Petsamo. The hospital in Kirkenes filled with casualties. … it was no longer safe to graze reindeer in the Petsamo region. … [the now independent country of] Finland closed the [formerly Russian] border … and declared that they would slaughter 10% of Norwegian reindeer that entered the Petsamo area [which it now controlled]. Actually, they slaughtered all they could and considerable business activity linked to this developed there as a result. In the spring of 1918 my great-grandfather and grandfather removed [with their herd from Petsamo] to Bugøynes where they stayed until after calving [in May]. Then, around St. Hans's Day [24th June], when the rivers had fallen, they moved [west] onto the Varanger Peninsula. In this way they saved most of their reindeer. Many of my relatives on the other hand lost many reindeer—some lost all: the animals became war food [for soldiers] in Russia.” (Odd Erling Smuk, personal communication, 6 February 2020).
Following WWII and area known as the ‘Petsamo corridor’ (Figure 20B), which had been ceded by Russia to Finland under the terms of Treaty of Tartu in1920, was returned to the U.S.S.R. (as Russia had now become). In 1949 Norway and the U.S.S.R. agreed their mutual intention of returning to their country of origin any reindeer that wandered across the border. The reality, however, was quite otherwise. Cold War passport controls and visa restrictions prevented Norwegian Saami herders from retrieving stray animals (Hætta et al., 1994). (The problem was one-sided: the Russian side of the border was heavily militarized and there were therefore no reindeer there to stray the other way.) The solution was to prevent the movement of animals and in 1961 construction commenced on a reindeer-proof fence that ultimately stretched 200 km along the Norwegian side of the border all the way south to Finland (Hætta et al., 1994). Work on the fence progressed only slowly. Animals continued to stray and, in 2010, Russian frustration led to a meeting of no less than three Norwegian Government departments (the Ministries of Agriculture and Food, Foreign Affairs and the Environment) to expedite construction (Lien, 2010). The fence was finally completed in 2018 (Directorate of Agriculture, 2019).
Border with Finland. The emergence of Finland as an independent country in 1917 necessitated re-negotiation of the agreement of 1852 whereby the border between the then kingdoms of Norway-Sweden and Russia-Finland had been closed to the passage of reindeer. A convention negotiated in 1922 set out principles for reducing cross-border grazing: it specified the duty of herders to prevent it, the rates of fines, confiscation and slaughter of reindeer which were caught on the wrong side of the border, and the reimbursement to the State of the costs of implementing such measures. The regulations proved ineffective and a new convention, signed in 1935, agreed the erection of 390 km of reindeer-proof fencing along the border, the cost of which was to be divided equally between the two countries. This seems, however, never to have been implemented (Hætta et al., 1994). The situation was aggravated during WWII. Norwegian Saami herders accused Finnish herders of crossing the border and stealing reindeer while Finns, assisted by the German occupation forces in Norway, presented the Norwegian authorities with successive demands for compensation for Finnish reindeer lost—presumably claimed stolen—on the Norwegian side of the border. A third convention on the prevention of cross-border grazing negotiated in 1948 was followed by a fourth, in 1952 (subsequently revised in 1962, 1981, and 2017), which resulted in the erection of 637 km of fencing along of the border (Hætta et al., 1994; Government of Norway, 2016, 2017, p. 26). Thus, the chronology of the judicialization of reindeer husbandry (i.e., the introduction of, and reliance on, judicial means for addressing predicaments and policy questions) at the border between Norway and Finland had three principle milestones each almost exactly 100 years apart: the border was created by the Treaty of Strømstad in 1751 was legally closed in 1852 and was physically closed in 1952. The path between these was flooded by buckets of ink drawn up from a well of legal argument and diplomatic negotiation.
Disrupting migration, whether by imposing a legal obligation on herders to prevent the passage of their animals or by erecting hundreds of kilometers of animal-proof fencing across the route (like the border fences in the present case or veterinary cordon fences in Africa [above]), is obviously a major intervention in the natural function of any extensive grazing system. The consequences of the closure of the border for reindeer husbandry in northern Norway and Finland have been conspicuous and profound:
‘Reindeer husbandry has been turmoil at the border since 1852, when it was closed, and up to the present day. … the border conventions … have influenced pasture utilization and the pattern of husbandry, and [consequently] … the central authorities have erected an expensive fence system along the border’ (Hætta et al., 1994, p. 23).
‘Reindeer herding has been greatly affected by closure of national borders to cross-border herding migration and [the resulting] foundation of the herding co-operative system … during the past decades. … these have greatly modified traditional pastoralism’ (Markkula et al., 2019).
‘… the closure of the [Finnish]-Norwegian border in 1852 revolutionized old nomadic Saami reindeer husbandry [by] preventing or shortening seasonal migrations’ (Jaakkola, 2014).
The immediate consequence of border closure was that Norwegian Saami herders in Finnmark lost access to approximately half their traditional winter pasture, which was in Finland, while Finnish Saami herders lost access to all their summer pasture in Norway. This led to conspicuous transformation of the habitat, particularly in Finland, and of the structure, organization and pattern of herding on both sides of the border.
Reindeer winter pastures typically include extensive mats of terricolous lichens (e.g., Cetraria spp. and Cladonia spp.) which, unusually among ruminants, are highly digestible in reindeer (Salgado-Flores et al., 2016) and often comprise a substantial part of the animals' winter diet (Storeheier et al., 2002 and references therein). These lichens are soft and pliant when wet but brittle, easily fragmented and rapidly eliminated by trampling when dry (Crittenden, 2000). The border fence prevented Finnish reindeer from leaving their winter pasture with the result that they trampled on the lichens in summer, when they were dry and brittle, and gradually destroyed them. The effect is evident at the border today as a stark line of demarcation owing to the presence of lichens on the Norwegian side and their absence on the Finnish side (Figure 21; see also Väre et al., 1996).
Figure 21. Aerial photograph looking from north to south across the Norway-Finland border on Finnmarksvidda (site indicated by a yellow dot on the location map). The border is evident as a line of change in the color of the ground running horizontally across the middle of the picture. The pale green color of the foreground (Norway) reflects the presence of a continuous mat of terricolous lichens; the brown color in the upper half of the picture (Finland) indicates the absence of lichens, the brown color being due to tundra plants (creeping shrubs, graminoids and mosses). Four posts of the fence erected to prevent the movement of reindeer either way across the border are visible (yellow arrows) in an insert corresponding to the yellow square in the main picture. The lichen mats on the Finnish side of the border have been destroyed by reindeer trampling them in summer when they are dry and therefore brittle. This in turn is a result of the animals having been prevented by the fence from leaving the area and moving to their traditional summer pastures near the coast of Norway. Photograph (21st August 2013): Bernt Johansen.
The closing and subsequent fencing of the border caused two major transformations in reindeer pastoralism in northern Finland. First, transhumance was replaced in the 1890s by the development of an intensive herding system in which animals organized within cooperatives were grazed all year round within defined, ultimately fenced, areas. Depletion of the range from the late 1960s, due in part to the trampling of lichens (above), however, led to intensive herding being replaced by an extensive system. In this system animals range freely within the same fenced areas throughout summer but are gathered and held in feeding corrals, where they are provided with concentrates, for several months in winter (Helle and Jaakkola, 2008; Jaakkola, 2014; Turunen and Vuojala–Magga, 2014).
Unlike in Finland, transhumance persists in Norway but the closure of the border created a series of problems for herders there. Topography is crucial to reindeer pastoralism because local relief influences the physical characteristics of the snowpack which is a major determinant of the availability of forage for reindeer in winter (Skogland, 1978; Eira et al., 2018). Loss of access to areas of favorable terrain across the border in Finland has had two principal effects. First, it has resulted in heavier grazing pressure. Second, it has reduced the options available to herders for adjusting the pattern of grazing in response to changes in snow conditions during winter. A Saami reindeer herder from the Varanger area, summarized the situation thus:
“We used to be able to move out of the forest and onto the high ground in Finland when necessary but we cannot do that now because the border is closed. Nowadays the way the ground is used is sometimes all wrong. People stay too long in one place and even use winter areas in summer. There are now no places we can set aside for a year or more to guarantee us good grazing in another year. We know this but what can we do? Where can we go? Some herders feed concentrates. Others argue. Herds mix and have to be separated. It is all very difficult” (Inger Anita Smuk, personal communication, 12 February 2020).
Border with Sweden. The legal constraints on cross-border grazing between Norway and Sweden differ fundamentally from those at the borders with Finland and Russia: this border has never been closed. The Lapp Codicil of 1751, which secures the right of herders and their animals to cross the border according to ancient custom (above), has never been revoked and therefore remains in force. However, the reciprocal rights of cross-border grazing intended and guaranteed by the Codicil have nevertheless been progressively eroded.
In addition to securing their right to cross the border, the Lapp Codicil specified a series of duties and responsibilities for transhumant herders. These included a requirement to report the numbers and the individual ownership of animals, and to adhere to itemized limits regarding the use by herders of one country of pasture and other resources in the other (Government of Norway, 2015, §§15–21). From these few rules there subsequently developed more elaborate and comprehensive regulations for reindeer pastoralism in the two countries of (from 1814 to 1905) the joint kingdom. Cross-border grazing was regulated by the Common Lapp Law (Felleslappeloven) which passed into law in 1883 after no less than 40 years' enquiry and planning. This law included a novel provision whereby land could, where necessary, be closed to reindeer specifically to protect the interests of farming and forestry (Hætta et al., 1994). Negotiations leading up to the dissolution of the union of the joint kingdom of Sweden and Norway in 1905 afforded the Norwegian authorities an opportunity to tighten this constraint by exerting pressure on the Swedes to reduce the extent of grazing by Swedish Saami on the Norwegian side. Reindeer Grazing Conventions were negotiated and agreed between the now independent countries in 1919 and 1949. The area of summer pasture in Norway available to the four northernmost Swedish herding co-operatives alone was reduced by 53%, from 17,000 to 8,053 km2 (Koch and Miggelbrink, 2011). The dissatisfaction that this caused festered but the authorities remained resolute. In 1968 a claim by Swedish Saami for compensation for pasture lost in Norway following the construction of a hydroelectricity plant at Lake Alte was rejected on the grounds that under the terms of the 1919 convention members of one country had no independent right of access in the other. This result was challenged and, later in the same year, the Norwegian Supreme Court upheld the right of Swedish Saami to graze summer pasture in Norway according to the principle of use since time immemorial (Strøm Bull, 2015). The court's decision notwithstanding, the Norwegian-Swedish Reindeer Grazing Convention of 1972 concluded 10 years' negotiation by reducing the area of pasture available to the northern Swedish group in Norway by a further 4,903 km2 to just 3,150 km2 (Koch and Miggelbrink, 2011). The herder's overall loss since 1919 was therefore 82%.
The 1972 Grazing Convention had a term of 30 years and, anticipating its expiry in 2002, a Norwegian-Swedish Reindeer Pasture Commission was convened in 1997 to ‘investigate the question of whether one country’s Saami reindeer herders will continue to require pasture in all or parts of the reindeer grazing areas covered by the Convention in the other country beyond the end of the current Convention' (Government of Norway, 2001b). The Commission's report, submitted in 2001, was heavily criticized and, in the absence of an agreed basis for a new convention, the existing one was extended for 3 years to 2005 (Government of Norway, 2017). Sweden declined further extension after that and consequently there has been no convention on cross-border grazing between Sweden and Norway—beyond the principles set out in the Lapp Codicil—since then. The Swedes consider these principles sufficient but the Norwegians take the view that additional provisions in national law are necessary. Their argument is that the Codicil refers only to customary practice, not specific areas, and is therefore incompatible with modern regulations regarding the spatial definition and the temporal and numerical pattern of use of reindeer pasture. The reciprocal cross-border grazing areas currently under negotiation are clearly delineated in a document drafted by both sides (Figure 3). The current impasse regarding their use means that Norwegian herders have in effect lost their legal right of access to pasture in Sweden. In the view of the Norwegian government:
‘Almost 12 years without a new convention is a very unfortunate situation. The Norwegian authorities have repeatedly pointed out to Sweden the importance of ratifying a new convention. Norway and Sweden have international legal obligations to Sami reindeer husbandry, including cross-border … herding. The Government aims to ratify a negotiated convention as soon as possible and will continue to exert pressure on Sweden [to this end]’ (Government of Norway, 2017, p. 60).
In the view of one herder:
“We cannot wait for the law. We, and our Swedish colleagues, agree that herding cannot stop. It must go on. So we have made our own private arrangements: we take our animals to Sweden and they bring theirs to Norway as before. Others are not so fortunate. It is now 15 years since there was a Convention on cross-border grazing. That is two generations of animals. So neither our reindeer nor our young people now have any experience or even memory of their traditional pastures across the border. How will they know how to use them if they are ever allowed to return? And how can you defend pasture from encroachment if you are never there?” (Ragnhild Sparrok Larsen, personal communication, 13 February 2020).
Withdrawal of Domestic Grazing Rights. The legal battle for the right to graze utmark which Saami reindeer herders in Norway fought across the 20th Century (above) was not won without casualties, the ghosts of some of which still walk abroad.
Concurrent with Professor Nielsen's study of rural settlement in south-eastern Norway at the close of the 19th Century (above), the government received persistent complaints from farmers about damage allegedly done by reindeer to fields, open meadows and hayracks (Supreme Court of Norway, 1981; Valstad, 1989). This resulted in the passing, in 1897, of an Act ‘containing supplementary provisions concerning the Lapps and reindeer husbandry in those parts of the country south of the county of Finnmark’ (Government of Norway, 2001a, p. 79; Fjellheim, 2012, p. 129). The new law was swiftly implemented. A series of governmental executive orders (kongelige resolusjoner literally ‘Royal resolutions’) in 1899–1902 restricted reindeer husbandry to specified areas within the region. Herders outside those areas had no option but to move or to abandon pastoralism. They were in effect outlawed. Martin Jonassen, a spokesman for the southern Saami, was twice granted audience with Haakon VII, King of newly independent Norway. In 1906 and again in 1908 he presented and explained to the King the distress these measures caused (Oppdal, 2007) although apparently to no avail (Jonassen, 2017).
Some herders ignored the law and returned with their animals to areas from which reindeer had been banned: courts imposed fines for illegal grazing in 1907, 1909, 1942, 1944, 1947, and 1975 (Supreme Court of Norway, 1981). Such was the herders' persistence and, presumably also, so remote were the areas involved, that the effect of the ban was actually quite limited for some:
‘… the ban imposed by the executive orders did not have any significant practical effects on reindeer husbandry in [the] Trollheimen [area] … [Although it] continued for several decades … [and] there is no evidence that it led to serious conflict with local people’
(Supreme Court of Norway, 1981).
Herders challenged the withdrawal of their right to graze both outside the designated reindeer husbandry areas and within such areas without landowners' permission in cases brought before the District Court (1976), the Court of Appeal (1978), and finally the Supreme Court which ultimately found against them (Supreme Court of Norway, 1981). The herders' persistence nevertheless bore fruit when, 3 years later, some of the land closed by executive order at the beginning of the century was once more opened to reindeer husbandry (Government of Norway, 1984).
‘… society has a duty to help [reindeer herders] such that they can themselves better apportion and utilize their resources.’
The fundamental right to graze utmark that emerged from the legal gyrations of the last century does not afford herders free access to pasture or complete freedom to organize grazing themselves (Government of Norway, 2016, p. 20). Herders' traditional regulatory mechanisms and systems of land tenure are explicitly subordinate to State management (Turi and Keskitalo, 2014). Each siida is obliged to maintain no more than a designated number of animals within designated seasonal ‘pasture districts,’ access to which follows a designated temporal schedule (Government of Norway, 2007, §§59–61. Siida: see Box 2). The current level and pattern of organization evolved from a system defined in the Reindeer Husbandry Act of 1933 which for the first time determined where and when reindeer might be pastured. Government bailiffs (lappefogd), appointed to enforce the regulations, had authority both to grant and to withdraw permission for herders to graze particular areas (Bjørklund, 2016). Their authority was enhanced in 1949 by the creation of a special force of ‘reindeer police’ (reinpoliti) but the bailiffs and the police were nevertheless deemed inadequate. Land use conflicts increased, both internally between siida and externally due, in particular, to encroachment—notably in the case of the damming of the Alta River (Brantenberg, 1985)—and this, together with policy makers' desire to ‘modernize, rationalize and optimise’ reindeer husbandry, led to a major revision of the entire regulatory system (Johnsen and Benjaminsen, 2017). A second Reindeer Husbandry Act (1978) broadened the scope and authority of the national Reindeer Husbandry Administration in a manner consistent with the view that ‘central management [should be] free to organize reindeer husbandry in the manner that coincides with the prevailing policies … it is up to the authorities to decide, within the framework of the law and its intentions, the division of districts, the allotment of production units, number of reindeer and so on, based on what is considered appropriate and justifiable’ (Government of Norway, 2001a, p. 124). This Act increased the breadth and complexity of government administration of reindeer pastoralism, further eroding Saami land tenure systems and management institutions and exacerbating tension between State administration and pastoralists which remains evident today (Ravna, 2011; Turi and Keskitalo, 2014; Benjaminsen et al., 2016a).
The judicialization of reindeer husbandry and, in particular, the setting of fixed boundaries resulted in ‘stiffer [administrative] structures and less room for the solutions that the situation at all times requires’ (Government of Norway, 2017, p. 20). A third Reindeer Husbandry Act (2007), more sympathetic to the traditions, aspirations and methods of pastoralists—and, in this respect, considerably more in harmony with contemporary empowering of indigenous peoples of the North (Coates and Broderstad, 2020)—therefore relaxed the role of central administration and awarded herders greater self-determination (Government of Norway, 2017, p. 39). The principle of use since time immemorial was elevated to a ‘central place in reindeer husbandry law … [and] … carries considerable weight in the setting of [seasonal pasture] boundaries …’ (Norwegian Reindeer Husbandry Administration, 2006, p. 2). The Act nevertheless retained the view that herders enjoy ‘no common right to graze their animals wherever they choose’ (Government of Norway, 2016, p. 20) and its provision for the transfer to herders of responsibility for the division and use of pasture is effectively unworkable. Under the terms of the Act, grazing within ‘pasture districts’ is regulated by district boards, composed of local herders, which are required to develop rules of usage ‘based on the traditional practice … [that] promote rational land use … [and that do not] conflict with siida rights established separately in law’ (Government of Norway, 2007, §59). The Act, however, neither clarifies these objectives nor provides any structure for the resolution of conflicts which arise where different objectives prove incompatible. The resulting frustration among herders (Turi and Keskitalo, 2014) is compounded by the fact that central authorities retain the right to reverse board decisions thereby effectively disempowering them (Government of Norway, 2017, p. 68). A herder who was involved in the drafting of the new Act summarized her experience thus:
“At first I was optimistic about this but my optimism drained away as the work progressed. They go around us and avoid the things that affect us. They do not understand our way of doing things. Sometimes it seems they do not even want to understand them. And there are no regulations on how the law should be applied: not one. This leaves people free to interpret the law however they wish. The result is chaos: it's a real mess.” (Inger Anita Smuk, personal communication, 12 February 2020).
The management of pasture and, specifically, of access to pasture is further complicated by the fact that grazing cannot be regulated independently of the aspirations and requirements of other land users. From a herders' perspective land use planning might legitimately be considered the way in which the State legitimizes loss of pasture through encroachment. This is the topic of the next section.
‘The extensive nature of land use characteristic of reindeer husbandry can lead to substantial conflict [of interest where] land [is required] for building and other commercial activities.’
(Government of Norway, 2017, p. 52)
Loss of pasture is the greatest single threat to reindeer pastoralism in Norway today and herders and the Saami Parliament alike consider the strengthening of legal protection of grazing land a priority (Government of Norway, 2017, p. 69–70). The situation is paradoxical because grazing reindeer on utmark is already explicitly protected by law. The 2007 Reindeer Herding Act states specifically that ‘The owner or [other] legitimate user must not use land … [to the] material disadvantage or inconvenience [of] reindeer husbandry’ and it grants herders the right of compensation for loss of pasture (Government of Norway, 2007, §4, §63). The protection afforded by the Act, however, is weak. Reindeer pastoralism does not have an exclusive right of access to pasture within designated reindeer grazing areas: herders are obliged to concede land to the development of infrastructure and activities including agriculture (Government of Norway, 2007, §19), local airports, hydro-electricity facilities (Nellemann et al., 2003; Bjørklund, 2016), linear structures (power lines, railways, roads [metalled and unmetalled]: Vistnes and Nellemann, 2001; Office of the Auditor General, 2004; Tyler et al., 2016), military training areas (Nellemann and Vistnes, 2002; Finn, 2019), mining operations (Johnsen, 2016; Eftestøl et al., 2019), wind farms (Skarin et al., 2015; Skarin and Alam, 2017; Strand et al., 2017), recreational facilities including private mountain huts (Lie et al., 2006; Anttonen et al., 2011), ski fields and walking, dog-driving, ski and snowmobile trails (Office of the Auditor General, 2004; Riseth and Johansen, 2018). All encroachment in reindeer husbandry areas requires a concession but planning authorities are liberal in their discretion: there is a gulf between the intention of the law and planning practice (Hanssen et al., 2018). Norwegian land management law requires consultation, participation, coordination and investigation, each stage scheduled in elaborate rules of process (Government of Norway, 2008). Regulations appended to the Planning and Building Act specify, in particular, that assessment of the potential impact of proposed measures on reindeer husbandry must evaluate the overall (i.e., cumulative) effects of encroachment and not just its specific effect(s) (Government of Norway, 2014: Appendix IV). Planning authorities are nevertheless empowered to rank different societal considerations and to disregard the interests of reindeer husbandry where other interests are afforded greater weight (Johnsen, 2016; Winge, 2016). The problem for herders is exacerbated by legal ambiguity (Ravna, 2011), extensive and burdensome bureaucracy and asymmetric negotiating procedures (Bjørklund, 2016; Winge, 2016), all compounded by a lack of consideration—or even understanding—of Saami tradition, aims and perspectives: ‘… it seems difficult to get … elected officials to recognize that Saami interests are [categorically] different [from those of] other commercial or even recreational [activities]’ (Hanssen et al., 2018, p. 491; see also Wilson, 2003; Turi and Keskitalo, 2014; Bjørklund, 2016; Lawrence and Larsen, 2017; Persson et al., 2017; Finn, 2019). All these aspects are explicitly recognized, which is progress of a kind: ‘The government sees a need to increase regional and municipal planners’ knowledge of about reindeer husbandry, [and herders'] and reindeer husbandry authorities' knowledge about the Plan and Building Act' and to facilitate, ‘through increased understanding of reindeer husbandry’s use of the land … smoother and more predictable land use planning' (Government of Norway, 2017, p. 54).
The physical loss of pasture associated with construction is usually small and localized in extent (above). The extent of non-physical losses, by contrast, can be vast. Losses due to the withdrawal of grazing rights are extensive and conspicuous: the area of pasture lost following the closure of the border with Finland (above) is an obvious example. Losses resulting from avoidance behavior, by contrast, are extensive but inconspicuous.
Avoidance is a behavioral response induced by the sight, sound, or smell of humans or human artifacts either directly perceived or associated through learning with infrastructure (Dyer et al., 2001; Barber et al., 2011; Brown et al., 2012; Shannon et al., 2014). The response is manifest as reduced abundance of the species of interest in the vicinity of stimuli—the so-called ‘zone of avoidance.’ Hesitant or re-routed passage of animals past, across or through infrastructure is symptomatic. Such responses indicate that the value of the site or area has been reduced insofar as animals are reluctant to use it. Avoidance has been reported in 234 species worldwide, among them reptiles, amphibians, birds and mammals, in nearly every type of habitat, at nearly every type of infrastructure and with or without human presence or traffic (Andrews, 1990; Forman and Alexander, 1998; Lawton et al., 1998; Trombulak and Frissell, 2000; UNEP, 2001; Nellemann et al., 2003; Fahrig and Rytwinski, 2009; Benítez-López et al., 2010; Chen and Koprowski, 2019).
Avoidance in Rangifer ranges from modest withdrawal to complete abandonment of part of the animals' normal range. Zones of avoidance, within which the density of animals is typically 50–95% lower than in control areas, typically extend 2.5–5 km from infrastructure (e.g., Wolfe et al., 2000; Vistnes and Nellemann, 2008; Skarin and Åhman, 2014; Engelien and Aslaksen, 2019). Avoidance is not usually evident within the zone: of 85 studies, only 13% detected avoidance within 2 km of infrastructure while 83% detected avoidance when comparing the density of animals <2 and >2 km from infrastructure (Vistnes and Nellemann, 2008; see also Skarin and Åhman, 2014; Plante et al., 2018). Abandonment of pasture around infrastructure, resulting in fragmentation of the range, has been observed in both wild and domesticated Rangifer. A spectacular example is the way in which the formerly contiguous range of wild reindeer in Norway has been fragmented by infrastructure to such an extent that the animals are now managed as 23 independent populations (Andersen and Hustad, 2004; Panzacchi et al., 2013a). Avoidance and barrier effects (i.e., hindrance to passage) in Rangifer have been documented at roads (Cameron et al., 1992; Nellemann and Cameron, 1996, 1998; Vistnes et al., 2008; Beyer et al., 2016; Plante et al., 2018; Serrouya et al., 2020), power lines (Tyler et al., 2016), pipelines, seismic trails, oil well and mining sites (Nellemann and Cameron, 1996; Polfus et al., 2011; Johnson and Russell, 2014; Johnson et al., 2015; MacNearney et al., 2016), dams and hydroelectric development (Mahoney and Schaefer, 2002; Nellemann et al., 2003), wind turbines (Skarin et al., 2015, 2018; Skarin and Alam, 2017), hut resorts, ski trails, paths (Helle and Särkelä, 1993; Nellemann et al., 2000, 2001; Vistnes and Nellemann, 2001; Anttonen et al., 2011; Helle et al., 2012; Lemerises et al., 2018; Gundersen et al., 2019) and at forestry sites (Schaefer, 2003; Anttonen et al., 2011; MacNearney et al., 2016; Fryxell et al., 2020; for reviews see Wolfe et al., 2000; Vistnes and Nellemann, 2008; Skarin and Åhman, 2014). Rangifer are not unique in this respect. Similar responses have been observed in other large ungulates including Arabian gazelle (Gazella arabica, Ross et al., 2019), Balkan chamois (Rupicapra rupicapra balcanica, Kati et al., 2020), Eld's deer (Cervus eldi, Yan et al., 2013), elephant (Loxodonta africana, Orrick, 2018), elk (Cervus canadensis, Paton et al., 2017; Prokopenko et al., 2017; Spitz et al., 2019), guanaco (Lama guanicoe, Cappa et al., 2019), mule deer (Odocoileus hemionus, Northrup et al., 2015), pronghorn (Antilocapra americana, Jones et al., 2019), red deer (Cervus elaphus, D'Amico et al., 2016), wild boar (Sus scrofa, D'Amico et al., 2016) and wildebeest (Connochaetes taurinus, Stabach et al., 2016).
Avoidance is a graded response. The strength of expression of the behavior varies with the type of infrastructure and with the ecological setting of each encounter. Thus, levels of avoidance vary with age, sex and life-phase (e.g., migration, post-calving, over-wintering), and hence with the animals' imperative to stay or to move, and also with the proximity of other stimuli including other types of infrastructure, human activity and predators (Wolfe et al., 2000; Vistnes et al., 2001; Vistnes and Nellemann, 2008; Fortin et al., 2013; Panzacchi et al., 2013a; Skarin and Åhman, 2014; Wilson et al., 2016; Plante et al., 2018; Skarin et al., 2018). Forestry practices, including the creation of logging roads, cuttings and transitional forests, encourage predators (e.g., wolves Canis lupus) which in turn provoke avoidance (Leech et al., 2017; Mumma et al., 2018) and even the abandonment of parts of the animals' former range (Schaefer, 2003; Anttonen et al., 2011; Fortin et al., 2015; Rudolph et al., 2017). Extensive developments and high levels of disturbance (e.g., vehicular and foot traffic) induce stronger avoidance than smaller developments and low levels of traffic (Nellemann et al., 2010; Lemerises et al., 2018; Fryxell et al., 2020). Thus, avoidance typically occurs at rate of a 0–50% reduction in use of land up to 1 km from trails, small power lines and wooden telephone poles, at around 50% 2.5–5 km from large, single power lines and roads, and at 50–95% 5–10 km from large industrial developments (e.g., dams and hydroelectricity stations, multiple power lines and pipelines, mines and oil field complexes) and hut resorts (Wolfe et al., 2000; Vistnes and Nellemann, 2008; Skarin and Åhman, 2014; Engelien and Aslaksen, 2019). Animals also practice trade-offs: adult Rangifer males in particular seem sometimes actively to seek out structures that afford dry ground, shade or exposure to breezes (e.g., buildings, underpasses, elevated concrete pads) which apparently provide relief from biting insects (Wolfe et al., 2000; Vistnes and Nellemann, 2008; Skarin and Åhman, 2014). Finally, avoidance and barrier effects may be short-lived or may persist for years or even decades after the construction of infrastructure. In Norway, for instance, the Setesdal-Ryfylke population of wild reindeer continued to avoid those parts of their range associated with dams, roads and high-voltage power lines for more than 30 years after these were built (Nellemann et al., 2003) and barrier effects and avoidance of power lines, in particular, have been observed to persist for years (Nellemann et al., 2003; Reimers et al., 2007) or even decades after construction (Vistnes and Nellemann, 2001; Vistnes et al., 2004). Caribou in Alaska, likewise, avoided the infrastructure and abandoned the area around the Prudhoe Bay-Kuparuk oil fields for up to 50 years after development started there (Nellemann and Cameron, 1998; Joly et al., 2006; Johnson et al., 2019). Responses of similar duration have been recorded in association with hydroelectric developments and with logging (Mahoney and Schaefer, 2002; Schaefer, 2003).
The area of undisturbed pasture within the Saami reindeer husbandry area (i.e., land more than 5 km of infrastructure) decreased by 71%, from ~134,000 km2 in 1,900 to 40,000 km2 in 2019 (Figure 18). Approximately 102,000 km2 (72%) of the total area (141,000 km2) now lies within the 5 km impact zones where animals' use of pasture is likely to be reduced through avoidance. A corollary is that grazing pressure within in the remaining 28% low impact areas is likely to have increased correspondingly. The situation is exacerbated by the distribution of infrastructure. Roads, power lines and recreational huts are scattered across the Saami reindeer husbandry area. Even in northern Norway, the most remote part of the country, reindeer migration routes cross infrastructure at 150 sites (Figure 22). Rangifer commonly seem reluctant to approach and, where appropriate, to cross infrastructure (i.e., roads), and intersections of this kind therefore represent obstacles that impede and delay their passage (Wolfe et al., 2000; Dyer et al., 2002; Cameron et al., 2005; Degteva and Nellemann, 2013; Panzacchi et al., 2013a,b; Muhly et al., 2015; Skarin et al., 2015; Wilson et al., 2016).
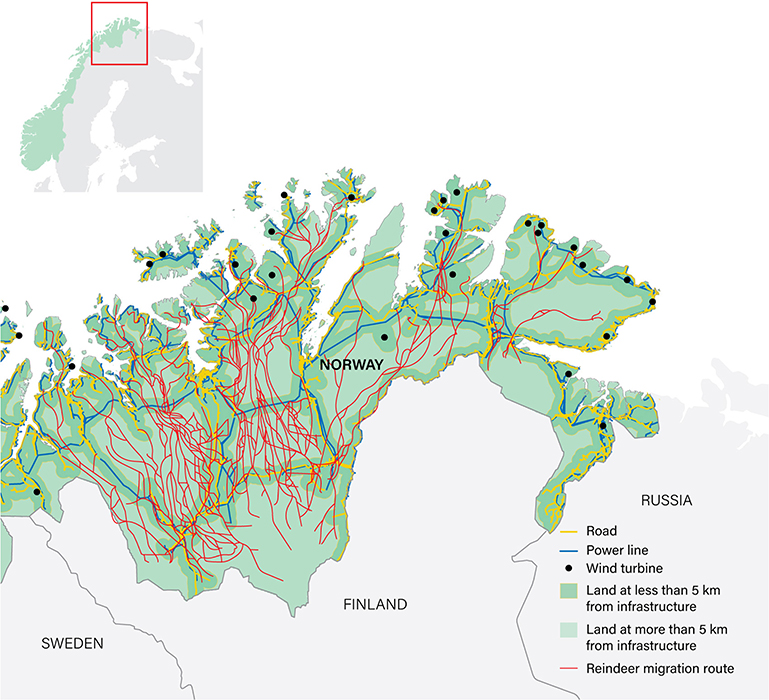
Figure 22. Encroachment: map of reindeer migration routes and infrastructure in East and West Finnmark reindeer pasture areas (Figure 3). There are 150 sites in this area where reindeer traversing their traditional migration routes between summer pastures at the coast and winter pastures inland (red lines) are obliged to cross roads or pass beneath high voltage power lines. Sources: Infrastructure from the Norwegian Mapping Authority; migration routes from Vorren (1962).
Avoidance behavior is as prevalent and pronounced as its effect on the distribution of animals is inconspicuous and technically demanding to detect. Two alternative approaches are commonly used to quantify it. The first involves infrequent observation of the distribution of a large number of animals (usually a significant proportion of a herd or population) in relation to infrastructure. This approach is necessarily spatially extensive. To confirm or refute the presence of a zone of avoidance along, say, 50 km of road requires recording the position of animals within an area of 1,000 km2, i.e., 50 km (the length of the road) × 10 km (twice the width of the suspected zone) × 2 (each side of the road). Counts are usually made from fixed wing aircraft, helicopters, or snowmobiles [or on foot along transects where the metric is animal sign (e.g., pellet groups) rather than animals themselves]. The obvious logistic constraint involved in such work means that data are normally limited to just one sample per season, effectively yielding sporadic ‘snapshots’ of the distribution of animals. This is a severe limitation in the case of a long-distance migratory species like Rangifer. The second approach involves frequent observation of the distribution of animals in relation to infrastructure made using GPS localization transmitters mounted on collars which the animals wear around their necks. This method generates vast amounts of precise data about the pattern of movement of the marked animals. Sample sizes, however, are usually small: studies typically monitor just some tens of individuals that, in turn, usually constitute a tiny fraction of the population of interest. Both approaches thus have limitations but methodology is not the only factor that influences the likelihood of detecting avoidance: source of funding is also a determinant.
In Norway, like many other countries, developers are required by law to assess the environmental impact of their activity. Assessment is not invariably independent and impartial. Many examples of such work being carried or contracted out by the developers themselves confirm the wisdom of the proverb that ‘He who pays the piper calls the tune’ (Oreskes and Conway, 2010). We examined potential sponsor bias in the conclusions of 54 studies of the effect of human activity and/or infrastructure on spatial distribution and range use in Rangifer. The studies, all published since 2007, were recovered from the Thomson Reuter ISI database using the search criterion <Rangifer* avoidance*>. We classified the principal finding of each study as either (i) negative impact or (ii) low impact (positive, minor negative, or no avoidance). We also classified each study as either (i) funded wholly or in part by a developer (usually an industrial concern responsible for the installation and subsequent use of infrastructure) or (ii) funded by government agencies or research councils. Remarkably, a significantly lower proportion of studies funded by industry detected negative impact of encroachment. Of the 54 studies, 43 (80%) concluded that Rangifer were affected negatively by infrastructure or human activity but of these just 8 (19%) were funded by industry while 35 (81%) were funded by non-industry sources (p < 0.05; Table 2). The apparent influence of source of funding on the likelihood of detecting avoidance is also evident in the subset of studies that used GPS tracking to record the position and movements of Rangifer in relation to infrastructure. Of 34 (85%) of 40 such studies that detected negative impact of infrastructure, all were funded principally by non-industry sources: none of those funded by principally by industry detected negative impact (Table 2).
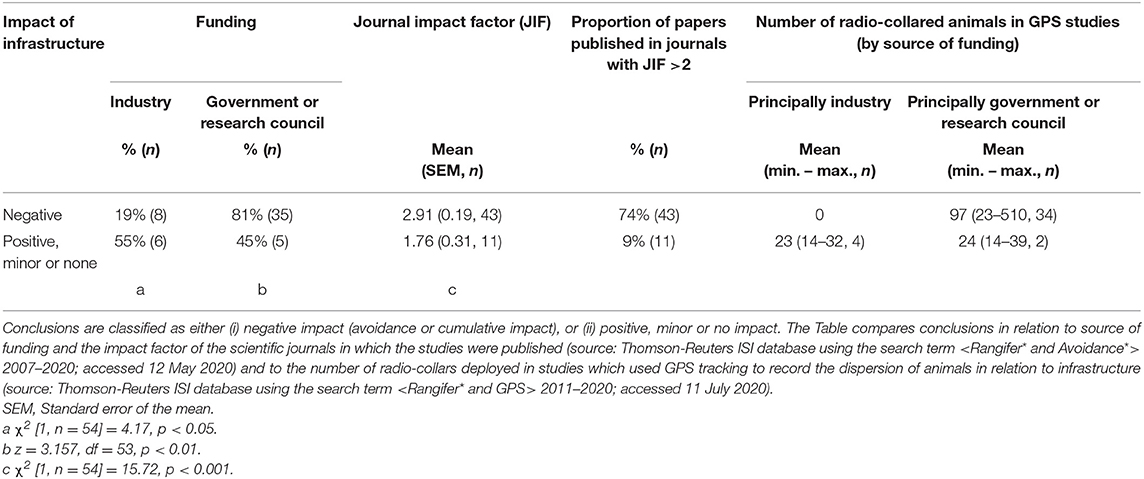
Table 2. Comparison of characteristics of studies which have drawn opposite conclusions about the effect of infrastructure on Rangifer.
It may be significant that GPS-based studies which detected significant negative impact of infrastructure on Rangifer have consistently used substantially larger sample sizes than those, including those funded by industry, which detected positive, minor or no impact (mean number of collared animals per study: negative impact 97, low impact ≤24; Table 2). GPS tracking potentially provides important insight about animal movements but may also mislead where researchers, constrained by cost, deploy too few units thereby unwittingly diminishing their ability to draw robust population-level inferences from their results (Leban et al., 2001; Lindberg and Walker, 2007; Hebblewhite and Haydon, 2010). Consistent with this, studies which have reported low impact have generally published in journals with a lower impact factor (JIF) than those which have reported negative impact [mean JIF: low impact 1.76 (0.31 SEM), high negative impact 2.91 (0.19 SEM), p < 0.01; Table 2]. Indeed, only one (9%) of 11 studies which have reported low impact was published in a journal with JIF >2 compared to 74% of those which have reported negative impact (p < 0.001; Table 2). There are several potential explanations why particular classes of results come to be associated with more or less highly rated scientific journals, respectively, but it is not our intention to examine these here. It is sufficient to note that avoidance is considerably less conspicuous in studies funded by parties which have a vested interest in being disassociated from such behavior. Such bias undoubtedly contributes to the tendency for non-physical losses of reindeer pasture to fade from public awareness. We return to this in the Discussion.
In this paper we returned to the suggestion that the effects of human intervention and, in particular, the loss of pasture through various forms of encroachment, dwarf the putative effects of climate change on Saami reindeer husbandry in Norway (Tyler et al., 2007). Our approach has been to juxtapose examination of the characteristics and influence of climate and encroachment on the resource base of this pastoral grazing system. We made five principal observations. First, northern reindeer pasture lands are warming and seem likely to continue to do so. Second, semi-centurial (50 year) trends notwithstanding, seasonal weather conditions show a high degree of annual and decadal variation: local ambient temperature, precipitation and the characteristics of the snowpack remain highly unpredictable on both time-scales. Third, warming has both positive and negative effects on ecosystem services for reindeer in Fennoscandia: the role of climate change as a driver of change in reindeer pastoralism is neither temporally nor spatially uniform, nor even clear. In contrast, fourth, the effects of human intervention on reindeer pastures throughout Norway are consistently negative. Saami reindeer pastoralists in Norway struggle with loss of pasture from physical encroachment and from administrative encroachment which erodes their independence and constrains their freedom of action on the land that remains available to them. Both are conspicuous, pervasive and continuing effects and they represent the principal threat to reindeer pastoralism in Norway today. Herders resist and, in doing so, provoke negative public discourse about their way of life: Saami reindeer pastoralism is consequently perceived as—and indeed is—problematic. It is so, fifth, because it is extensive pastoralism and, as such, it is confronted by myriad administrative, economic, legal and social constraints of a kind which bedevil extensive pastoral grazing systems across the globe.
Local data demonstrate clearly that Finnmarksvidda has become progressively warmer and wetter across the last 50 years (Figures 8, 9, 11–15). The pattern of development of the weather is not unique to this area: similar trends (i.e., generally warmer winters, increased precipitation in winter, shorter period of snow cover, earlier melt, later freeze up and hence longer plant growing season) are evident across northern Fennoscandia (Førland et al., 2004; Markkula et al., 2019). A major point of interest is whether these trends will persist. Several projections are available (Räisänen, 2012; Hanssen-Bauer et al., 2015, 2017; Benestad et al., 2016) and we chose a ‘middle of the road’ emission scenario with which to project changes in temperature and precipitation across the present century (Figure 8). Projections, however, are not forecasts (Box 3): uncertainty arises because future greenhouse gas emissions are unknown, because of flaws and deficiencies in the models, and because of internal variability on both annual and decadal scales (Hawkins and Sutton, 2009, 2011; Hanssen-Bauer et al., 2017). We therefore validated projections for Finnmarksvidda externally by comparing them with empirical field data. We found that the projection reproduced the development of mean annual temperature measured locally across the period 1985–2018 remarkably well (Figure 8C). The climate models we applied evidently performed well and the downscaling procedure nicely captured the way in which large-scale climate has influenced ambient temperature over Finnmarksvidda. On this basis, therefore, it is not unreasonable to interpret the trajectory of the projection forwards from 2018 as a prediction (Box 3) and hence to conclude that Finnmarksvidda is moving into a phase in which the weather will on average be warmer than at any time during the last 100 years.
The fit between projected and observed precipitation, on the other hand, was less convincing (Figure 8D). The model clearly underestimated the observed trend. Precipitation over Finnmarksvidda followed the 90 percentile of the model ensemble. This is curious because in other areas models overestimate observations (e.g., Svalbard; Hanssen-Bauer et al., 2019). The difference is conceivably due to internal climate variability which is a major source of uncertainty in regional precipitation models (Hawkins and Sutton, 2011). Such variability may have amplified the climate change signal of recent global warming or alternatively models may currently underestimate the local effect of the climate change on precipitation. Either way, the trajectory for precipitation from 2018 remains highly uncertain.
Having elevated the projection of temperature to a prediction, it is apposite to consider the potential consequences of warming for reindeer and hence for Saami reindeer pastoralism. The concept of ‘warming’ has beguiling simplicity in this context. The effects of warming on habitat services provided by tundra, taiga and boreal forest are diverse and complex. The boreal zone is cold and, not surprisingly, plants throughout it generally respond positively to warming during the growing season (Walker et al., 2006; Prevéy et al., 2019). Indeed, recent warming is considered a principal cause of the current greening of the Arctic (Pattison et al., 2015; Zhu et al., 2016). The effects of warming on ecosystem function and species' performance, however, are both temporally and spatially heterogeneous and, in particular, scale dependent. The positive effects of warming on plants, plant communities and biotypes are moderated and even reversed locally by a range of non-climate factors including geomorphology, surface hydrology and other features of the physical environment (Lara et al., 2018; Myers-Smith et al., 2020), species type and community type and composition (Elmendorf et al., 2012; Gruner et al., 2017), and grazing by vertebrates (Post and Pedersen, 2008; Bernes et al., 2015; Bråthen et al., 2017; Vanneste, 2017; Løkken et al., 2019; Andruko et al., 2020), invertebrates (Bjerke et al., 2017) or both (Gamm et al., 2018). The same applies in the reindeer pastures of northern Fennoscandia. The effects of warming on the performance of plants and plant communities there, too, are modulated by interactions between species and species groups (plants, lichens and herbivores), soil nutrient availability, inter-annual variation in weather conditions and human activity and consequently neither the magnitude nor even the sign of responses to warming are reliably predicted by changes in temperature alone (Olofsson et al., 2009; Grau et al., 2012; Bernes et al., 2015; Bjerke et al., 2017; Kaarlejärvi et al., 2017; Maliniemi et al., 2018; Markkula et al., 2019; Tømmervik et al., 2019).
The situation in the non-growing season (winter) is similar. Assessing the potential consequences of winter warming for reindeer is complicated by the contradictory nature of the signal and its effects. The weather on Finnmarksvidda has changed considerably over the last 50 years: there is and are, for instance, currently more precipitation (Figure 14), greater depth of snow (Figure 15) and more thaw days in winter (Figure 12) than 50 years ago. The regression estimates for 2018 for these parameters fall outside their former range and what is statistically ‘normal weather’ today would quite properly have been denoted statistically ‘extreme weather’ then. The trends are clear but their potential consequences, should they continue, are not. It is frequently suggested that warming in winter is inevitably likely to have a negative effect on reindeer pastoralism owing to reduced availability of forage in winter (through ‘icing’; above) and to the constraining of herders' options for the use of pasture (e.g., Reinert et al., 2009; Bartsch et al., 2010; Risvoll and Hovelsrud, 2016; Turunen et al., 2016). Winter warming, however, also leads to increased ablation of snow (sensu Forchhammer and Boertmann, 1993), shorter winters (fewer days of snow cover; Figure 16), and to earlier and extended growing seasons (Figure 11), all of which are conditions associated with increased body growth in Rangifer (Pettorelli et al., 2005; Couturier et al., 2009; Tveraa et al., 2013; Albon et al., 2017) and, at least in some cases, population increase (Tyler et al., 2008; Post et al., 2009a). Positive effects of warming like these, together with a projected reduction in depth of snow on Finnmarksvidda toward the end of the 21st Century (Hanssen-Bauer et al., 2015, 2017), suggest that the overall trend is toward increasingly favorable winter grazing conditions for reindeer there, at least in the long term.
The situation is further complicated by the large inter-annual and decadal variability in the climate of the boreal zone and to the relatively small signal-to-noise ratio at many sites, net warming notwithstanding (ACIA, 2005; Figures 2, 8). Winters on Finnmarksvidda, for instance, were consistently colder in the 1980s but consistently warmer in the early 1990s than indicated by the linear regression line (Figure 9). The duration of snow cover likewise was consistently longer than expected in the 1990s (Figure 16) and there was considerably less depth of snow than expected throughout the first decade of the present century (Figure 15). Projections from numerous climate models suggest that the inter-annual and decadal variability in conditions characteristic of the boreal zone will persist, albeit around a new level. The effect of this, we suggest, will be perpetuation of the current pattern whereby the effects of general warming on grazing conditions are alternately amplified and then diminished across the region.
Our analysis also revealed substantial local variation in weather conditions. We have already noted how in some areas of Norway the normal pattern of migration, in which herders and their reindeer move inland in winter to escape difficult snow conditions at the coast, is reversed where local conditions render coastal pastures snow free in winter (Box 1). Conditions also vary substantially within seasonal pasture areas. In the 1990s, for instance, the weather was markedly wetter than expected at Suolovuopmi and at Kautokeino but not at four adjacent sites (Figure 14). There were likewise on average nearly twice as many thaw days in winter at Karasjok (mean 37 days · winter−1, 7.9 SD) than just 70 km away at Sihccajarvi (mean 21 days · winter−1, 7.9 SD; data 1969–2018; Figure 12). The unstable winter temperatures at Karasjok, reflecting its relatively low altitude (140 m a.s.l.), conceivably impinge on the snowpack, rendering it denser and harder and, in turn, rendering winter grazing conditions more difficult for reindeer there compared to at Sihccajarvi (375 m a.s.l.). This could easily be tested. Local variation in weather conditions that influence the performance of wild Rangifer translate into spatial heterogeneity in individual and population responses to climate change (e.g., Post, 2005; Post et al., 2009a; Hansen et al., 2019b) and there is no reason why this should not be the case for domesticated Rangifer, too. This also could easily be tested.
Climate change is widely presented as a threat to the condition of reindeer pasture and, by extension, to reindeer pastoralism. Its potential to corrupt grazing conditions in summer or winter or in both is consistently emphasized in studies and reports, irrespective of whether this was their primary focus (e.g., Weladji and Holand, 2003; ACIA, 2005; Rees et al., 2008; CAFF, 2013) or merely their justification (e.g., Paoli et al., 2018). Emphasis solely on negative effects of climate change, however, is partial and perhaps premature. Trends in weather conditions, and the specific effects of variation in weather on ecosystem services, vary qualitatively and quantitatively, temporally and spatially around northern Fennoscandia (Markkula et al., 2019 and above). The influence of climate change on reindeer pasture there is neither uniformly positive nor uniformly negative: it is a combination of both. The chief feature of the role of human intervention on reindeer pasture, by contrast, is consistently negative.
Climate is a forcing agent: it modulates the performance of reindeer through its influence on weather and pasture conditions. Humans are also forcing agents and their intervention has eroded and is continuing to erode the resource base of the reindeer extensive grazing system. In this paper, as previously (Tyler et al., 2007), we distinguished two aspects of such erosion: physical loss and non-physical loss of pasture. Physical loss of pasture as a result of construction, especially construction of infrastructure, and of the transformation of uncultivated land (utmark) into other biotopes, is tangible, conspicuous but clearly only limited in extent. Buildings, infrastructure and agriculture cover no more than 1% of the county of Troms and Finnmark which is the principal reindeer husbandry area in Norway (above). Non-physical losses of pasture due to the withdrawal of grazing rights and to the reduction in the value of pasture, by contrast, are neither tangible nor conspicuous. Their extent, however, is vast: 50% of traditional winter pasture was lost when the border with Finland was closed; 72% of remaining pasture lies within 5 km of infrastructure and is therefore likely to be under-used by reindeer to some degree. Though potent and prevalent, such losses are prominent neither in official nor public discourse concerning the state of reindeer pastoralism. Avoidance behavior, for instance, is afforded but one sentence in the recent White Paper on reindeer husbandry (Government of Norway, 2017; but see Frostating Court of Appeal, 2020a). There are several reasons for this.
First, short-term memory. Border closures quickly fade from public awareness. This is not surprising: remote land lying beyond remote borders is literally out of sight and events of more than half a century ago (in the case of the closure of the Russian and the Finnish borders) are not surprisingly out of mind. The closure of the border with Sweden is more recent and has not been forgotten, albeit that negotiations to reopen it have stagnated (above). The area of pasture potentially available to Norwegian Saami in Sweden (14,000 km2), however, is equivalent to just 10% of the Saami reindeer husbandry area in Norway and the problem probably achieves little prominence for that reason.
Second, pragmatism. Government policy regarding the management of reindeer pastoralism in Norway is sharply focussed on the productive performance of the animals and on the environmental consequences of grazing. Management policy has been supported and encouraged by evidence of poor body growth of reindeer and of density-dependent changes in the biomass and botanical composition of reindeer pasture, especially in the north of the country (Fauchald et al., 2004; Bråthen et al., 2007; Ims et al., 2007; Tømmervik et al., 2009). These perceived evils have been attributed to overgrazing associated with their being ‘too many reindeer’ (e.g., Office of the Auditor General, 2004; Riseth and Vatn, 2009; Pape and Löffler, 2012; Benjaminsen et al., 2016b, Skonhoft et al., 2017). From this interpretation stems policy and legislation aimed specifically at reducing numbers of reindeer and thereby in some unspecified way achieving ‘ecological, economic and cultural sustainability’ of reindeer pastoralism (Government of Norway, 1992, 2007, 2017; see also Tyler et al., 2007). The terminology is unfortunate: ‘overabundance’ (‘too many’ animals) and ‘overgrazing’ are diffuse, plastic concepts in ecology. They are neither generally applicable nor, often, even meaningful outside the confines of definitions specific to the ecological settings of particular classes of cases (see Caughley, 1981; Behnke and Scoones, 1993; Behnke, 2000; Mysterud, 2006). Domesticated reindeer in Norway obviously impose heavy grazing pressure on utmark: 215,000 animals within 141,000 km2 (Box 2) constitute an average density of 1.5 reindeer · km−2. This is six times the average density of domesticated reindeer in Eurasia as a whole (0.25 reindeer · km−2; Box 1). However, where—and for whatever reason—it is considered desirable to reduce stocking rate, invoking value-laden terms like overabundance and overgrazing achieves nothing. Far from serving to enrich understanding of the biological basis of the situation, it serves only to direct attention toward the activity of pastoralists who influence animal numbers and hence the grazing system from within, and away from those parties who influence it from without. State management, however, is pragmatic: herders are less empowered than landowners (including the State) who have personal, commercial or national interests at stake. It is therefore invariably simpler to manipulate stocking rate by legislating for reduction in numbers of animals than for an increase in the area of pasture on which they may graze (but see Supreme Court of Norway, 2017).
Third, myopia. Encroachment on utmark occurs piecemeal. The number of structures (of whatever kind) and the extent of commercial and recreational activities all increase incrementally, each encroachment contributing just a fraction of the total. Recreational huts, single-track access roads and other small features scattered in the terrain are likely to pass the casual observer unnoticed and to slip easily through planning authorities' bureaucratic nets. Yet huts, though small, are built in their thousands (Figure 19), access roads carry not only workers but walkers, and infrastructure is usually aggregated where the natural relief affords convenient passage for humans and animals alike (e.g., Nellemann and Cameron, 1996; Forman and Alexander, 1998; Benítez-López et al., 2010; Panzacchi et al., 2013a; Plante et al., 2017). Even large scale infrastructure, likewise, may be rendered effectively invisible. This occurs in several ways. Ambiguity is one. The validity of the claim that ‘that reindeer husbandry’s land area in Finnmark did not change significantly [i.e., was not reduced] in the period 2001–2011' (Government of Norway, 2017, p. 54) depends on how the word ‘significantly’ (nevneverdig in the original) is construed: the rate of building there proceeded unabated through this period (Figure 19). Scale is another. Large local encroachment seems quite small when viewed in sufficiently broad perspective. The Mauken-Blåtind military training area near Tromsø (Figure 3), for instance, extends over 200 km2: this is just 1% of the Troms reindeer husbandry area (18,718 km2) but fully 12% of Mauken reindeer husbandry district (1,699 km2). Finally, courts evaluating herders' claims for damages confine their deliberations to the impact of the infrastructure for which the developers, as defendants, are responsible: other infrastructure falls outside their jurisdiction (e.g., Hålogaland Court of Appeal, 2019; Frostating Court of Appeal, 2020b). Animals, however, make no such distinction. They respond to the sum of constraints and their responses reflect the cumulative effect of encroachment (Theobald et al., 1997; Johnson et al., 2005; Krausman and Harris, 2010). By narrowing the focus such that the effect of each new intrusion is evaluated in isolation, impact is packaged and presented in doses small enough to be acceptable to the public and to planners alike and hence falls from the discourse. The concept of cumulative effects of encroachment on reindeer pasture is officially recognized; it is also officially recognized that it is not currently implemented in the planning process but remains an ideal which administrators ought to embrace (e.g., Government of Norway, 2017; Troms County Municipality, 2018).
Encroachment is not the only non-climate anthropogenic factor which influences the resource base for reindeer. Manipulation of the number of animals is another. The data and the interpretation of data on numbers, however, are contentious. From 1970 to 2010 the number of domesticated reindeer in Norway almost doubled (Tømmervik and Riseth, 2011); in the West Finnmark reindeer pasture area (Figure 3) it more than doubled, rising apparently from a long-term stable level of around 40,000 to around 100,000 animals (Figure 23). The increase has been ascribed to the combination of socio-economic factors that made reindeer herding an attractive option for young Saami and to government economic support designed to stimulate production specifically by increasing numbers (Government of Norway, 1992, p. 36). The increase had two outcomes. The first was depletion of the resource base evident as an inverse relationship between the number of reindeer and the cover and biomass of dietary lichens (Office of the Auditor General, 2004; Tømmervik et al., 2004, 2009; Riseth and Vatn, 2009). This was an entirely predictable response. The second was a reversal of government policy and the introduction of legislation aimed specifically at reducing numbers (above). This has been criticized as misguided and theoretically unsound (Benjaminsen et al., 2015, 2016a, 2019; Marin et al., 2020). Sara et al. (2016), in particular, argued that the official census data were inaccurate and that, far from irrupting from a long-term stable low level, the increase in numbers actually only restored the population to its former level (Figure 23). Cast in this light, the point of interest is not the depletion of edible biomass that accompanied a doubling of the population but the reason for the remarkable abundance of forage, in particular lichens, immediately prior to this. Perhaps the richness of the sward and abundance of forage immediately prior to the increase was an artifact of several decades of under-grazing co-incidental with low numbers of reindeer in the 1950s and 1960s? The point will probably never be settled because there is no objective way of assessing the accuracy of the two contrasting sets of estimates (Figure 23). However, the very fact that numbers are contentious indicates broad acceptance of the fact that manipulation of population size is another non-climate determinant of the resource base.
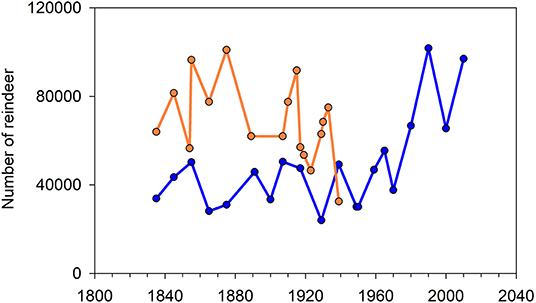
Figure 23. Contrasting accounts of the development of numbers of reindeer in West Finnmark reindeer pasture area (Figure 3) across the last two centuries. The lower curve (blue) is based on official records (Tømmervik and Riseth, 2011) while the upper curve (orange) is based on herders' estimates (Sara et al., 2016). Sara et al. (2016) attribute the difference between the level of the two curves to herders' reluctance to declare the true size of their herds, leading to chronic underestimation of numbers in the official record (blue) before relatively rigorous counting procedures were introduced toward the end of the 20th Century. The accuracy of neither herders' estimates nor official records before then is known.
The state and development of Saami reindeer husbandry are influenced by non-climate anthropogenic factors besides those that affect the resource base for reindeer. These include predation (Tveraa et al., 2014), where the intensity of predation is influenced through legislation designed to protect populations of predators (Tyler et al., 2007; Vuojala-Magga, 2012; Sjölander-Lindqvist et al., 2020), manipulation of the economic environment (Reinert, 2006, 2016; Tyler et al., 2007) and the evolution and development of the social and technological environments that influence all aspects of the pastoral way of life (Newhouse, 1952; Herbert, 1976; Government of Norway, 1992; Riseth and Vatn, 2009; Vuojala-Magga et al., 2011; Risvoll and Hovelsrud, 2016). All these in their separate ways influence the pastoral system and may therefore reasonably be assumed to reduce the proportional influence of climate variation on the dynamics of reindeer husbandry.
The influence of non-climate anthropogenic factors on reindeer pastoralism has recently received considerable attention (e.g., Brännlund and Axelsson, 2011; Vuojala-Magga, 2012; Löf, 2013; Turi and Keskitalo, 2014; Strøm Bull, 2015; Riseth et al., 2016; Tolvanen et al., 2019; du Plessis, 2020; Hausner et al., 2020; Kirchner, 2020, this study; see also López-i-Gelats et al., 2015, 2016). There is increasing recognition that the effects of human intervention may on occasion far exceed those of climate variation on reindeer pastoralism (Vitebsky, 2005; Anderson, 2006; Povoroznyuk, 2007; Tyler et al., 2007; Rees et al., 2008; Konstantinov, 2015; Uboni et al., 2016) particularly, but not exclusively, in the near-term (Kelman and Næss, 2019). We have focussed on non-climate loss of pasture which, in its various forms, is such a potent factor in Saami reindeer pastoralism in Norway. Norway is a wealthy country with highly developed and expanding infrastructure. Is the kind of human impact on pasture and on pastoralism evident there dependent on close proximity of grazing commons to industrialized society? Certainly not. No Supreme Court ruling (above), however favorable to pastoralism, can alter the fact that herders' requirements for land on which to graze their animals is fundamentally incompatible with the requirements of those who would use the same land for other purposes. Conflicts of interest, articulated in industrialized Norway in newsprint (and academic journals) and argued in meeting rooms, council chambers and courts of law, are a ubiquitous feature of this form of land use. Land use conflicts of the kinds outlined in this paper are not unique to Saami reindeer husbandry in Norway: they are a feature of extensive pastoral grazing systems across the globe (Table 3).

Table 3. Land use conflicts of the kinds outlined in this paper are not unique to Saami reindeer husbandry in Norway: they are a feature of extensive pastoral grazing systems across the globe.
Saami reindeer husbandry is problematic (Box 2) precisely because it is extensive pastoralism and, like extensive pastoralism confronted by rapidly expanding modern society elsewhere around the globe, it struggles with inexorable piecemeal diminution of pasture and has to grapple with a plethora of administrative, economic, legal, social and societal obstacles associated with this. There is meager comfort in the realization that populations of wild ungulates are subject to some of same constraints (Lutz et al., 2003; Hobbs et al., 2008; Venier et al., 2014; Gordon, 2018). Nor is the prognosis encouraging: civil, commercial, industrial, military and private activity are set to expand throughout the Eurasian Arctic and sub-Arctic and to reduce the resource base of reindeer pastoralism still further (Latola et al., 2016; McCauley et al., 2016; Karlsdottir et al., 2017; Stephen, 2018; Kröger, 2019). On the other hand, the very existence of reindeer herding today, its shrinking resource base across the 20th Century (Figure 18) notwithstanding, is a testimony to the adaptability and resilience of herds and herders alike (see Heikkinen et al., 2007; Helle and Jaakkola, 2008; Brännlund and Axelsson, 2011; Vuojala-Magga et al., 2011; Jaakkola, 2014; Risvoll and Hovelsrud, 2016). Both features are likely to be sorely tested as development expands north.
Extensive pastoralism is a system that produces food and sustains culture on land too poor and in environments too harsh for any form of agriculture. Herders and their animals, the latter physiologically adapted to local conditions, have developed ways of life that enable them to thrive and successfully to exploit grazing opportunities afforded by scattered and ephemeral pasture resources. Mobility is the key: it is means by which herders and their animals adjust and adapt to changes in conditions and in levels resources irrespective of the cause(s) of those changes. This is evident wherever pastoralism is practiced, from parched savannah to frozen tundra or steppe. The mobility of herds and herders, however, is increasingly threatened by human population pressure, by piecemeal development and by the loss of grazing rights that are inconsistent with the urban and agricultural concept of rights achieved through ownership. For herders, constraints on movement are the primary threat while securing use of traditional grazing land is the primary goal.
NT and CN developed the concept of the review. All authors contributed to analyses and to writing the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the author NT.
We are grateful to Bernt Johansen and Bjørn Lockertsen for allowing us to their photographs, Riccardo Pravettoni and Julia Lutz for their skilful cartography, Professor Kirsti Strøm Bull for clarification of matters relating to the evolution of Saami grazing rights, Ragnhild Sparrok Larsen, Øyvind Ravna, Inger Anita Smuk and Odd Erling Smuk for their information about Saami reindeer pastoralism in Norway, John and Merika Jonassen for information regarding Martin Jonassen's audiences with the King Haakon VII and Dr. Muyin Wang and Professor James Overland for updated data on Global Arctic temperature anomalies.
Abel, N. O. J. (1993). “Reducing cattle numbers on Southern Africa communal range: is it worth it?,” in Range Ecology at Disequilibrium. New Models of Natural Variability and Pastoral Adaptation in African Savannahs, eds R. H. Behnke, I. Scoones, and C. Kerven (London: Overseas Development Institute), 173–195.
Adamczewski, J. Z., Gates, C. C., Soutar, B. M., and Hudson, R. J. (1988). Limiting effects of snow on seasonal habitat use and diets of caribou (Rangifer tarandus groenlandicus) on Coats Island, Northwest Territories, Canada. Can. J. Zool. 66, 1986–1996. doi: 10.1139/z88-291
Adams, L. G. (2005). Effects of maternal characteristics and climatic variation on birth masses of Alaskan caribou. J. Mammal. 86, 506–513. doi: 10.1644/1545-1542(2005)86[506:EOMCAC]2.0.CO;2
Ahmed, A., Lawson, E. T., Mensah, A., Gordon, C., and Padgham, J. (2016). Adaptation to climate change or non-climatic stressors in semi-arid regions? Evidence of gender differentiation in three agrarian districts of Ghana. Environ. Dev. 20, 45–58. doi: 10.1016/j.envdev.2016.08.002
Aikens, E. O., Kauffman, M. J., Merkle, J. A., Dwinnell, S. P. H., Fralick, G. L., and Monteith, K. L. (2017). The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol. Lett. 20, 741–750. doi: 10.1111/ele.12772
Albon, S. D., Irvine, R. J., Halvorsen, O., Langvatn, R., Loe, L. E., Ropstad, E., et al. (2017). Contrasting effects of summer and winter warming on body mass explain population dynamics in a food–limited Arctic herbivore. Glob. Change Biol. 23, 1374–1389. doi: 10.1111/gcb.13435
Allen, L. C. (1976). The Books of Joel, Obadiah, Jonah & Micah. New International Commentary on the Old Testament. Grand Rapids, MI: Wm. B. Eerdmans Publishing, 427.
AMAP (2017). Snow, Water, Ice and Permafrost in the Arctic (SWIPA). Oslo: Arctic Monitoring and Assessment Programme (AMAP), 269.
American Horse Council Foundation (2018). The Economic Impact Study of the U.S. Horse Industry. Washington, DC: American Horse Council.
Andersen, R., and Hustad, H., (eds.). (2004). Villrein og Samfunn. En veiledning til bevaring og bruk av Europas siste villreinfjell [Wild Reindeer and Society. A Guide to Conservation and Use of the Last Wild Reindeer Mountain Pasture in Europe]. Trondheim: NINA Temahefte 27, 77.
Anderson, D. G. (2006). Is Siberian reindeer herding in crisis? Living with reindeer fifteen years after the end of State socialism. Nomad. Peoples 10, 87–103. doi: 10.3167/np.2006.100206
Andrews, A. (1990). Fragmentation of habitat by roads and utility corridors: a review. Aust. Zool. 26, 130–141. doi: 10.7882/AZ.1990.005
Andruko, R., Danby, R., and Grogan, P. (2020). Recent growth and expansion of birch shrubs across a low Arctic landscape in continental Canada: are these responses more a consequence of the severely declining caribou herd than of climate warming? Ecosystems 23, 1362–1379. doi: 10.1007/s10021-019-00474-7
Anonymous (2009). Bønder og samer krangler om reinen [Farmers and Saami argue about reindeer]. Available online at: https://www.aftenposten.no/norge/i/R9gWA/boender-og-samer-krangler-om-reinen
Anonymous (2012). Nå skal antallet rein på Finnmarksvidda ned [Now the number of reindeer on Finnmarksvidda is going to be reduced]. Available online at: https://www.nordlys.no/nyheter/na-skal-antallet-rein-pa-finnmarksvidda-ned/s/1-79-6106300
Anonymous (2014). Tragedien på Finnmarksvidda [The tragedy on Finnmarksvidda]. Available online at: https://www.midtnorskdebatt.no/meninger/leder/article10471464.ece
Anonymous (2015a). Reinsdyr sulter i hjel [Reindeer are starving to death]. Available online at: https://dyrevern.no/dyrevern/reinsdyr-sulter-i-hjel/
Anonymous (2015b). Reineiere bør akseptere moderne krav til dyrevelferd [Reindeer owners ought to accept modern standards of animal welfare]. Available online at: https://www.nrk.no/nordland/_-reineiere-bor-akseptere-moderne-krav-til-dyrevelferd-1.12640862
Anonymous (2019). Regional Climate Models. Available online at: https://www.climateprediction.net/climate-science/climate-modelling/regional-models/
Anonymous (no date). KNMI Climate Explorer. Available online at: http://climexp.knmi.nl/about.cgi?id=
Anti, N. P. (2017). Reindrift er en levemåte [Reindeer pastoralism is a way of life]. Available online at: https://www.altaposten.no/meninger/2017/08/12/%E2%80%93-Reindrift-er-en-levem%C3%A5te-15145991.ece
Anttonen, M., Kumpula, J., and Colpaert, A. (2011). Range selection by semi-domesticated reindeer (Rangifer tarandus tarandus) in relation to infrastructure and human activity in the boreal forest environment, Northern Finland. Arctic 64, 1–14. doi: 10.14430/arctic4075
Ascoli, D., Vacchiano, G., Turco, M., Conedera, M., Drobyshev, I., Maringer, J., et al. (2017). Inter-annual and decadal changes in teleconnections drive continental-scale synchronization of tree reproduction. Nat. Commun. 8:2205. doi: 10.1038/s41467-017-02348-9
Asner, G. P., Elmore, A. J., Olander, L. P., Martin, R. E., and Harris, A. T. (2004). Grazing systems, ecosystem responses, and global change. Annu. Rev. Environ. Resour. 29, 261–99. doi: 10.1146/annurev.energy.29.062403.102142
Barber, J. R., Burdett, C. L., Reed, S. E., Warner, K. A., Formichella, C., Crooks, K. R., et al. (2011). Anthropogenic noise exposure in protected natural areas: estimating the scale of ecological consequences. Landsc. Ecol. 26, 1281–1295. doi: 10.1007/s10980-011-9646-7
Barnes, M. D., Craigie, I. D., Harrison, L. B., Geldmann, J., Collen, B., Whitmee, S., et al. (2016). Wildlife population trends in protected areas predicted by national socio–economic metrics and body size. Nat. Commun. 7:12747. doi: 10.1038/ncomms12747
Bartsch, A., Kumpula, T., Forbes, B. C., and Stammler, F. (2010). Detection of snow surface thawing and refreezing in the Eurasian Arctic with QuikSCAT: implications for reindeer herding. Ecol. Appl. 20, 2346–2358. doi: 10.1890/09-1927.1
Basupi, L. V., Quinn, C. H., and Dougill, A. J. (2017). Pastoralism and land tenure transformation in Sub-Saharan Africa: conflicting policies and priorities in Ngamiland, Botswana. Land 6:89. doi: 10.3390/land6040089
Beach, H. (2007). “Reindeer ears: calf marking during the contemporary era of extensive herding in Swedish Saamiland,” in Årsbok 2007 Kungl. Humanistiska Vetenskaps-Samfundet i Uppsala. Annales Societatis Litterarum Humaniorum Regiae Upsaliensis (Uppsala), 91–118.
Begon, M., Townsend, C. R., and Harper, J. L. (2006). Ecology: From Individuals to Ecosystems, 4th Edn. Oxford: Blackwell Publishing Ltd.
Behnke, R. (2000). Equilibrium and non-equilibrium models of livestock population dynamics in pastoral Africa: their relevance to Arctic grazing systems. Rangifer 20, 141–152. doi: 10.7557/2.20.2-3.1509
Behnke, R. H., and Scoones, I. (1993). “Rethinking range ecology: implications for rangeland management in Africa,” in Range Ecology at Disequilibrium: New Models of Natural Variability and Pastoral Adaptation in African Savannas, eds R. H. Behnke, I. Scoones, and C. Kerven (London: Overseas Development Institute), 1–30.
Bekenov, A. B., Grachevand, I. U. A., and Milner-Gulland, E. J. (1998). The ecology and management of the saiga antelope in Kazakhstan. Mammal Rev. 28, 1–52. doi: 10.1046/j.1365-2907.1998.281024.x
Benestad, R. E., Hanssen-Bauer, I., and Chen, D. (2008). Empirical-Statistical Downscaling. Singapore: World Scientific Publishing Company Pte. Ltd., 228.
Benestad, R. E., Parding, K. M., Isaksen, K., and Mezghani, A. (2016). Climate change and projections for the Barents region: what is expected to change and what will stay the same? Environ. Res. Lett. 11:054017. doi: 10.1088/1748-9326/11/5/054017
Benítez-López, A., Alkemade, R., and Verweij, P. A. (2010). The impacts of roads and other infrastructure on mammal and bird populations: a meta-analysis. Biol. Conserv. 143, 1307–1316. doi: 10.1016/j.biocon.2010.02.009
Benjaminsen, T. A. (2018). Skandaløst av Høyesterett – Debatt [Scandalous (decision of) the Supreme Court - Debate]. Available online at: https://www.dagsavisen.no/debatt/skandalost-av-hoyesterett-1.1081695 (accessed February 9, 2020).
Benjaminsen, T. A., Borgenvik, J., Sjaastad, E., and Marin, A. (2016a). “Reintetthet, produktivitet og effektivisering: om modeller, forskning og politikk [Reindeer density, productivity and effectivization: on models, research and politics],” in Samisk Reindrift Norske Myter [Saami Reindeer Pastoralism Norwegian Myths], eds T. A. Benjaminsen, I. M. G. Eira, and M. N. Sara (Fagbokforlaget: Bergen), 69–85.
Benjaminsen, T. A., Mathiesen, S. D., and Reinert, E. (2019). Reindriften – hva slags krise? [Reindeer husbandry – what sort of crisis?]. Available online at: https://www.dagsavisen.no/debatt/reindriften-hva-slags-krise-1.437789
Benjaminsen, T. A., Reinert, H., Sjaastad, E., and Sara, M. N. (2015). Misreading the Arctic landscape: a political ecology of reindeer, carrying capacities, and overstocking in Finnmark, Norway. Norsk Geografisk Tidsskrift–Norwegian. J. Geogr. 69, 219–229. doi: 10.1080/00291951.2015.1031274
Benjaminsen, T. A., Sara, M. N., and Sjaastad, E. (2016b). “Myten om overbeiting på finnmarksvidda [The myth of overgrazing on Finnmarksvidda],” in Samisk Reindrift Norske Myter [Saami Reindeer Pastoralism Norwegian Myths], eds T. A. Benjaminsen, I. M. G. Eira, and M. N. Sara (Fagbokforlaget: Bergen), 87–105.
Berg, R. (2018). Ikke ta Livet av Reindrifta i Nordland [Don't kill off reindeer husbandry in Nordland]. Available online at: https://www.nationen.no/debatt/ikke-ta-livet-av-reindrifta-i-nordland/
Bergersen, G. (2017). Inviterer til møte for å redusere konflikter mellom grunneiere og reineiere. Available online at: https://www.retten.no/nyheter/os/reindrift/inviterer-til-mote-for-a-redusere-konflikter-mellom-grunneiere-og-reineiere/s/5-44-195788
Bernes, C., Bråthen, K. A., Forbes, B. C., Speed, J. D. M., and Moen, J. (2015). What are the impacts of reindeer/caribou (Rangifer tarandus L.) on Arctic and Alpine vegetation? A systematic review. Environ. Evid. 4:4. doi: 10.1186/s13750-014-0030-3
Beyer, H. L., Gurarie, E., Boerger, L., Borger, L., Panzacchi, M., Basille, M., et al. (2016). ‘You shall not pass!’: quantifying barrier permeability and proximity avoidance by animals. J. Anim. Ecol. 85, 45–53. doi: 10.1111/1365-2656.12275
Bjerke, J. W., Treharne, R., Vikhamar-Schuler, D., Karlsen, S. R., Ravolainen, V., Bokhorst, S., et al. (2017). Understanding the drivers of extensive plant damage in boreal and Arctic ecosystems: insights from field surveys in the aftermath of damage. Sci. Tot. Environ. 599–600, 1965–1976. doi: 10.1016/j.scitotenv.2017.05.050
Bjørklund, I. (2016). “Fra formynder til forhandler: Om inngrep, konsekvensanalyser og ‘balansert sameksistens’ [From custodian to negotiator: on intervention, impact assessments and ‘balanced coexistence’],” in Samisk Reindrift Norske Myter, eds T. A. Benjaminsen, I. M. G. Eira, and M. N. Sara (Fagbokforlaget: Bergen), 177–194.
Blaxter, K. L. (1989). Energy Metabolism in Animals and Man. Cambridge: Cambridge University Press, 336.
Blench, R. (1996). Aspects of Resource Conflict in Semi-Arid Africa. ODI Natural Resource Perspectives No. 15. Available online at: https://www.odi.org/publications/2153-aspects-resource-conflict-semi-arid-africa
Blench, R. (2000). Extensive Pastoral Livestock Systems: Issues and Options for the Future. FAO–Japan Cooperative Project GCP/JPN/005/JPN. Rome: Food and Agriculture Organization of the United Nations.
Blix, A. S. (2016). Adaptations to polar life in mammals and birds. J. Exp. Biol. 219, 1093–1105. doi: 10.1242/jeb.120477
Blix, A. S., and Johnsen, H. K. (1983). Aspects of nasal heat exchange in resting reindeer. J. Physiol. 340, 445–454. doi: 10.1113/jphysiol.1983.sp014772
Blix, A. S., Kvadsheim, P. H., Kholodova, M. V., Sokolov, V. E., Messelt, E. B., and Tyler, N. J. C. (2015). Superb winter fur insulation in the small Siberian musk deer (Moschus moschiferus). Rangifer 35, 53–60. doi: 10.7557/2.35.1.3575
Blix, A. S., and Steen, J. B. (1979). Temperature regulation in newborn polar homeotherms. Physiol. Rev. 59, 285–304. doi: 10.1152/physrev.1979.59.2.285
Blix, A. S., Walløe, L., and Folkow, L. P. (2011). Regulation of brain temperature in winter-acclimatized reindeer under heat stress. J. Exp. Biol. 214, 3850–3856. doi: 10.1242/jeb.057455
Boertje, R. D. (1984). Seasonal diets of the Denali Caribou Herd, Alaska. Arctic 37, 161–165. doi: 10.14430/arctic2182
Boles, O. J. C., Shoemaker, A., Courtney Mustaphi, C. J., Petek, N., Ekblom, A., and Lane, P. J. (2019). Historical ecologies of pastoralist overgrazing in Kenya: long-term perspectives on cause and effect. Hum. Ecol. 47, 419–434. doi: 10.1007/s10745-019-0072-9
Bråthen, K. A., Ims, R. A., Yoccoz, N. G., Fauchald, P., Tveraa, T., and Hausner, V. H. (2007). Induced shift in ecosystem productivity? Extensive scale effects of abundant large herbivores. Ecosystems 10, 773–789. doi: 10.1007/s10021-007-9058-3
Bråthen, K. A., Ravolainen, V. T., Stien, A., Tveraa, T., and Ims, R. A. (2017). Rangifer management controls a climate-sensitive tundra state transition. Ecol. Appl. 27, 2416–2427. doi: 10.1002/eap.1618
Brännlund, I., and Axelsson, P. (2011). Reindeer management during the colonization of Sami lands: a long-term perspective of vulnerability and adaptation strategies. Glob. Environ. Change 21, 1095–1105. doi: 10.1016/j.gloenvcha.2011.03.005
Brantenberg, O. T. (1985). “The alta–kautokeino conflict: saami reindeer herding and ethnopolitics,” in Native Power: The Quest for Autonomy and Nationhood of Indigenous Peoples, eds J. Brøsted, J. Dahl, A. Gray, H. C. Gulløv, G. Henriksen, and J. B. Jørgensen (Bergen: Universitetsforlaget), 23–48.
Brown, C. L., Hardy, A. R., Barber, J. R., Fristrup, K. M., Crooks, K. R., and Angelloni, L. M. (2012). The effect of human activities and their associated noise on ungulate behavior. PLoS ONE 7:e40505. doi: 10.1371/journal.pone.0040505
CAFF (2013). Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Akureyri: Conservation of Arctic Flora and Fauna.
Cameron, R. D., Reed, D. J., Dau, J. R., and Smith, W. T. (1992). Redistribution of calving caribou in response to oil field development on the Arctic Slope of Alaska. Arctic 45, 338–342. doi: 10.14430/arctic1412
Cameron, R. D., Smith, W. T., White, R. G., and Griffith, B. (2005). Central Arctic caribou and petroleum development: distributional, nutritional, and reproductive implications. Arctic 58, 1–9. doi: 10.14430/arctic382
Cappa, F. M., Borghi, C. E., and Giannoni, S. M. (2019). How roads affect the spatial use of the guanaco in a South American protected area: human connectivity vs animal welfare. Diversity 11, 110–120. doi: 10.3390/d11070110
Caughley, G. (1981). “Overpopulation,” in Problems in Management of Locally Abundant Wild Animals, eds P. A. Jewell, S. Holt, and D. Hart (New York, NY: Academic Press), 7–19. doi: 10.1016/B978-0-12-385280-9.50008-1
Chen, H. L., and Koprowski, J. L. (2019). Can we use body size and road characteristics to anticipate barrier effects of roads in mammals? A meta-analysis. Hystrix It. J. Mammal. 30, 1–7. doi: 10.4404/hystrix-00185-2019
Christensen, J. H., Kanikicharla, K. K., Aldrian, E., An, S. I., Cavalcanti, I. F. A., de Castro, M., et al. (2014). “Climate phenomena and their relevance for future regional climate change,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, et al. (Cambridge: Cambridge University Press), 1217–1308. Available online at: https://www.tfb.no/db/trondheimturistforening/arbok1988/1988%20SCAN0008.pdf
Clutton-Brock, J. (1987). A Natural History of Domesticated Mammals. London: British Museum (Natural History).
Clutton–Brock, T. H., and Pemberton, J. M. (2004). Soay Sheep: Dynamics and Selection in an Island Population. Cambridge: Cambridge University Press, 408. doi: 10.1017/CBO9780511550669
Coates, K. S., and Broderstad, E. G. (2020). “Indigenous peoples of the Arctic: re–taking control of the far North,” in The Palgrave Handbook of Arctic Policy and Politics, eds K. S. Coates and C. Holroyd (London: Palgrave Macmillan), 9–25. doi: 10.1007/978-3-030-20557-7_2
Colman, J. E., Eftestøl, S., Tsegaye, D., Flydal, K., and Mysterud, A. (2013). Summer distribution of semi–domesticated reindeer relative to a new wind–power plant. Eur. J. Wildl. Res. 59, 359–370. doi: 10.1007/s10344-012-0682-7
Cooke, S. J., Sack, L., Franklin, C. E., Farrell, A. P., Beardall, J., Wikelski, M., et al. (2013). What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv. Physiol. 1:cot001. doi: 10.1093/conphys/cot001
Coulson, T., Catchpole, E. A., Albon, S. D., Morgan, B. J. T., Pemberton, J. M., Clutton–Brock, T. H., et al. (2001). Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292, 1528–1531. doi: 10.1126/science.292.5521.1528
Couturier, S., Côté, S. D., Otto, R. D., Weladji, R. B., and Huot, J. (2009). variation in calf body mass in migratory caribou: the role of habitat, climate, and movements. J. Mammal. 90, 442–452. doi: 10.1644/07-MAMM-A-279.1
Crawford, C. L., Volenec, Z. M., Sisanya, M., Kibet, R., and Rubenstein, D. I. (2019). Behavioral and ecological implications of bunched, rotational cattle grazing in East African savanna ecosystem. Rangel. Ecol. Manage. 72, 204–209. doi: 10.1016/j.rama.2018.07.016
Crittenden, P. D. (2000). Aspects of the ecology of mat–forming lichens. Rangifer 20, 127–139. doi: 10.7557/2.20.2-3.1508
Cuyler, C., and Øritsland, N. A. (2002). Effect of wind on Svalbard reindeer fur insulation. Rangifer 22, 93–99. doi: 10.7557/2.22.1.694
D'Amico, M., Periquet, S., Roman, J., and Revilla, E. (2016). Road avoidance responses determine the impact of heterogeneous road networks at a regional scale. J. Appl. Ecol. 53, 181–190. doi: 10.1111/1365-2664.12572
Damman, A. W. H. (1983). “An ecological subdivision of the Island of Newfoundland,” in Biogeography and Ecology of the Island of Newfoundland, ed G. R. South (The Hague: Dr. W. Junk Publishers), 163–206.
Dangwal, D. D. (2009). The lost mobility: pastoralism and modernity in Uttarakhand Himalaya (India). Nomad. Peoples 13, 84–101. doi: 10.3167/np.2009.130206
Davies, R., Kosec, K., Nkonya, E., and Song, J. (2020). Global Land Reform Experiences: a Review for South Africa. Southern Africa–Towards Inclusive Economic Development (SA-TIED) Working Paper Nr. 98. Washington, DC: International Food Policy Research Institute, 41.
de Araujo, A. G. J., Obregón, G. O., Sampaio, G., Monteiro, A. M. V., da Silva, L. T., Soriano, B., et al. (2018). Relationships between variability in precipitation, river levels, and beef cattle production in the Brazilian Pantanal. Wetlands Ecol. Manage. 26, 829–848. doi: 10.1007/s11273-018-9612-0
Dean, W. R. J., Seymour, C. L., and Joseph, G. S. (2018). Linear structures in the Karoo, South Africa, and their impacts on biota. Afr. J. Range For. Sci. 35, 223–232. doi: 10.2989/10220119.2018.1514530
Degteva, A., and Nellemann, C. (2013). Nenets migration in the landscape: impacts of industrial development in Yamal peninsula, Russia. Pastoralism Res. Policy Pract. 3:15. doi: 10.1186/2041-7136-3-15
Deser, C., Hurrell, J. W., and Phillips, A. S. (2017). The role of the North Atlantic Oscillation in European climate projections. Clim. Dyn. 49, 3141–3157. doi: 10.1007/s00382-016-3502-z
Din, J. U., Ali, H., Ali, A., Younus, M., Mehmood, T., Norma-Rashid, Y., et al. (2017). Pastoralist-predator interaction at the roof of the world: conflict dynamics and implications for conservation. Ecol. Soc. 22:32. doi: 10.5751/ES-09348-220232
Directorate of Agriculture (2019). Landbruksdirektoratet årsrapport 2018 [Directorate of Agriculture Annual Report 2018]. Available online at: https://www.landbruksdirektoratet.no/no/om-landbruksdirektoratet/virksomhet/arsrapporter/%C3%A5rsrapporter
du Plessis, G. (2020). Killing reindeer: a spatial analysis of Nordic States and nomadic forms of life in the Arctic. Int. Polit. Soc. 14, 347–365. doi: 10.1093/ips/olaa008
Dukhan, H. (2014). ‘They talk to us but never listen to us’: development-induced displacement among Syria's Bedouin. Nomad. Peoples 18, 61–79. doi: 10.3197/np.2014.180105
Dwyer, M. J., and Istomin, K. V. (2009). Komi reindeer herding: the effects of socialist and post–socialist change on mobility and land use. Polar Res. 28, 282–297. doi: 10.1111/j.1751-8369.2009.00108.x
Dyer, S. J., O'Neill, J. P., Wasel, S. M., and Boutin, S. (2001). Avoidance of industrial development by woodland caribou. J. Wildlife Manage. 65, 531–542. doi: 10.2307/3803106
Dyer, S. J., O'Neill, J. P., Wasel, S. M., and Boutin, S. (2002). Quantifying barrier effects of roads and seismic lines on movements of female woodland caribou in northeastern Alberta. Can. J. Zool. 80, 839–845. doi: 10.1139/z02-060
Eftestøl, S., Flydal, K., Tsegaye, D., and Colman, J. E. (2019). Mining activity disturbs habitat use of reindeer in Finnmark, Northern Norway. Polar Biol. 42, 1849–1858. doi: 10.1007/s00300-019-02563-8
Eira, I. M. G., Oskal, A., Hanssen–Bauer, I., and Mathiesen, S. D. (2018). Snow cover and the loss of traditional indigenous knowledge. Nat. Clim. Change 8, 924–936. doi: 10.1038/s41558-018-0319-2
Elmendorf, S. C., Henry, G. H., Hollister, R. D., Björk, R. G., Bjorkman, A. D., Callaghan, T. V., et al. (2012). Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175. doi: 10.1111/j.1461-0248.2011.01716.x
Engelien, E., and Aslaksen, I. (2019). “Fysiske inngrep i samiske reinbeiteområder: beregning av påvirkningssoner [Physical encroachment in saami reindeer pasture areas: calculating zones of effect],” in Samiske Tall Forteller 12 (Guovdageaidnu: Sámi allaskuvla/Sámi University of Applied Sciences), 51–75.
Ennsa, C., and Bersagliob, B. (2016). Pastoralism in the time of oil: youth perspectives on the oil industry and the future of pastoralism in Turkana, Kenya. Extract. Ind. Soc. 3, 160–170. doi: 10.1016/j.exis.2015.11.003
Enoksen, O. (2019). SV i lomma på Sametinget? [The Socialist Left Party in the pocket of the Saami Parliament?]. Available online at: https://nordnorskdebatt.no/article/sv-i-lomma-pa-sametinget-0
Fahrig, L., and Rytwinski, T. (2009). Effects of roads on animal abundance: an empirical review and synthesis. Ecol. Soc. 14:21. doi: 10.5751/ES-02815-140121
Fancy, S. G., Pank, L. F., Whitten, K. R., and Regelin, W. L. (1989). Seasonal movements of caribou in Arctic Alaska as determined by satellite. Can. J. Zool. 67, 644–650. doi: 10.1139/z89-093
Fancy, S. G., and White, R. G. (1985). Energy expenditures by caribou while cratering snow. J. Wildl. Manage. 49, 987–993. doi: 10.2307/3801384
FAO (2019). Available online at: http://www.fao.org/faostat/en/#data/QA/visualize (accessed October 2019).
Fauchald, P., Park, T., Tømmervik, H., Myneni, R., and Hausner, V. H. (2017). Arctic greening from warming promotes declines in caribou populations. Sci. Adv. 3:e1601365. doi: 10.1126/sciadv.1601365
Fauchald, P., Tveraa, T., Yoccoz, N. G., and Ims, R. A. (2004). En økologisk bærekraftig reindrift - Hva begrenser naturlig produksjon og høsting? [Ecological sustainable reindeer husbandry – What limits natural production and harvesting?] NINA Fagrapport 76. Trondheim: Norwegian Institute for Nature Research, 35.
Finn, K. E. (2019). Tensions within nature management in inner Troms, Norway: Different narratives on power and decision–making (Master's thesis). Norwegian University of Life Sciences, Norway. Available online at: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/11250/2608470/Master%27s%20Thesis%20%5bKarina%20Finn%5d.pdf?sequence=1&isAllowed=y
Fjellheim, E. M. (2020). Ærede Lagmannsrett [Honorable Court of Appeal]. Available online at: https://www.harvestmagazine.no/pan/aerede-lagmannsrett
Fjellheim, S. (2012). Gåebrien sijte – En sameby i Rørostraktene [Gåebrien herding group – a Saami community in the Røros area]. Røros: Sverre Fjellheim forlag.
Flagstad, Ø., and Røed, K. (2003). Refugial origins of reindeer (Rangifer tarandus L.) inferred from mitochondrial DNA sequences. Evolution 57, 658–670. doi: 10.1111/j.0014-3820.2003.tb01557.x
Forbes, B. C., Kumpula, T., Meschtyb, N., Laptander, R., Macias-Fauria, M., Zetterberg, P., et al. (2016). Sea ice, rain–on–snow and tundra reindeer nomadism in Arctic Russia. Biol. Lett. 12:20160466. doi: 10.1098/rsbl.2016.0466
Forbes, B. C., Stammler, F., Kumpula, T., Meschtyb, N., Pajunen, A., and Kaarlejarvia, E. (2009). High resilience in the Yamal–Nenets social–ecological system, West Siberian Arctic, Russia Proc. Natl Acad. Sci. U.S.A. 106, 22041–8. doi: 10.1073/pnas.0908286106
Forchhammer, M. C., and Boertmann, D. M. (1993). The muskoxen Ovibos moschatus in north and northeast Greenland: population trends and the influence of abiotic parameters on population dynamics. Ecography 16, 299–308. doi: 10.1111/j.1600-0587.1993.tb00219.x
Forchhammer, M. C., Post, E., and Stenseth, N. C. (1998). Breeding phenology and climate. Nature 391, 29–30. doi: 10.1038/34070
Forchhammer, M. C., Post, E., Stenseth, N. C., and Boertmann, D. M. (2002). Long-term responses in Arctic ungulate dynamics to changes in climatic and trophic processes. Popul. Ecol. 44, 113–120. doi: 10.1007/s101440200013
Førland, E. J., Skaugen, T. E., Benestad, R. E., Hanssen-Bauer, I., and Tveito, O. E. (2004). Variations in thermal growing, heating, and freezing indices in the Nordic Arctic, 1900–2050. Arctic Antarctic Alpine Res. 36, 347–356. doi: 10.1657/1523-0430(2004)036[0347:VITGHA]2.0.CO;2
Forman, R. T. T., and Alexander, L. E. (1998). Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 29, 207–231. doi: 10.1146/annurev.ecolsys.29.1.207
Fortin, D., Buono, P. L., Fortin, A., Courbin, N., Tye Gingras, C., Moorcroft, P. R., et al. (2013). Movement responses of caribou to human-induced habitat edges lead to their aggregation near anthropogenic features. Am. Nat. 181, 827–836. doi: 10.1086/670243
Fortin, D., Buono, P. L., Schmitz, O. J., Courbin, N., Losier, C., St-Laurent, M. H., et al. (2015). A spatial theory for characterizing predator–multiprey interactions in heterogeneous landscapes. Proc. R. Soc. B 282:20150973. doi: 10.1098/rspb.2015.0973
Fratkin, E. (2014). Ethiopia's pastoralist policies: development, displacement and resettlement. Nomad. Peoples 18, 94–114. doi: 10.3197/np.2014.180107
Frostating Court of Appeal (2020a). Frostating Lagmannsrett, Trondheim, Norway, case 18-150314SKJ-FROS, 18-150323SKJ-FROS,18-150327SKJ-FROS.
Frostating Court of Appeal (2020b). Frostating Lagmannsrett, Trondheim, Norway, case 19-007266SKJ-FROS.
Fryxell, J. M., Avgar, T., Liu, B., Baker, J. A., Rodgers, A. R., Shuter, J., et al. (2020). Anthropogenic disturbance and population viability of woodland caribou in Ontario. J. Wildl. Manage. 84, 636–650. doi: 10.1002/jwmg.21829
Gabrielsen, T. (2009). Reinbeiteproblemer i ‘fellesdistriktene’ etter 1826 [Grazing problems in the 'commons' after 1826] Ságat 170:12. Available online at: http://finnmarkforlag.no/09_10.html
Gadd, M. E. (2012). “Barriers, the beef industry and unnatural selection: a review of the impact of veterinary fencing on mammals in Southern Africa,” in Fencing for Conservation: Restriction of Evolutionary Potential or a Riposte to Threatening Processes?, eds M. J. Somers and M. W. Hayward (Berlin; Heidelberg: Springer Science+Business Media, LLC.), 153–186. doi: 10.1007/978-1-4614-0902-1_9
Gamm, C. M., Sullivan, P. F., Buchwal, A., Dial, R. J., Young, A. B., Watts, D. A., et al. (2018). Declining growth of deciduous shrubs in the warming climate of continental western Greenland. J. Ecol. 106, 640–654. doi: 10.1111/1365-2745.12882
Gauslaa, J. (2001). Ny sultkatastrofe truer reindrifta [Reindeer husbandry threatened with a new starvation catastrophe]. Available online at: https://bellona.no/nyheter/ukategorisert/2001-01-reinen-trues-av-sult-igjen
Godde, C. M., Garnett, T., Thornton, P. K., Ash, A. J., and Herrero, M. (2018). Grazing systems expansion and intensification: drivers, dynamics, and trade-offs. Glob. Food Secur. 16, 93–105. doi: 10.1016/j.gfs.2017.11.003
Gordon, I. J. (2018). Review: livestock production increasingly influences wildlife across the globe. Animal 12, s372–s382. doi: 10.1017/S1751731118001349
Government of Norway (1984). Lov om reindrift i kommunene Meldal, Midtre Gauldal, Oppdal, Rennebu, Rindal, Sunndal og Surnadal (Trollheimenloven) [Act on Reindeer Husbandry in the Municipalities of Meldal, Midtre Gauldal, Oppdal, Rennebu, Rindal, Sunndal og Surnadal (Trollheim Act)]. Available online at: https://lovdata.no/dokument/NL/lov/1984-12-21-101?q=Trollheimen (accessed February 1, 2020).
Government of Norway (1992). En bærekraftig reindrift. Melding til Stortinget 28 (1991–1992) [Sustainable Reindeer Husbandry. Norwegian Government White Paper 28 (1991–1992)]. Oslo.
Government of Norway (2001a). Forslag til Endringer i Reindriftsloven. Norges Offentlige Utredninger 2001:35 [Proposal for Changes in the Reindeer Husbandry Act. Norwegian Government Green Paper 2001:35]. Available online at: https://www.regjeringen.no/no/dokumenter/nou−2001–35/id145307/?ch=1 (accessed January 20, 2020).
Government of Norway (2001b). Norsk-Svensk Reinbeitekommisjon av 1997 Innstilling [Norwegian-Swedish Reindeer Pasture Commission of 1997 Recommendation]. Available online at: https://www.regjeringen.no/globalassets/upload/kilde/ld/rap/2001/0006/ddd/pdfv/132470-sa-no.pdf (accessed December 12, 2019).
Government of Norway (2007). Lov om reindrift [Reindeer Husbandry Act]. Available online at: https://lovdata.no/dokument/NL/lov/2007-06-15-40 (accessed January 7, 2020).
Government of Norway (2008). Lov om planlegging og byggesaksbehandling [Planning and Building Act]. Available online at: https://lovdata.no/dokument/NL/lov/2008-06-27-71 (accessed January 20, 2020).
Government of Norway (2010). Høring - Ny konvensjon mellom Norge og Sverige om grenseoverskridende reindrift [Consultation (Document) - New Convention Between Norway and Sweden on cross-Border Reindeer Husbandry]. Available online at: https://www.regjeringen.no/no/dokumenter/horing—ny-konvensjon-mellom-norge-og-s/id611197/?expand=horingsnotater (accessed February 11, 2020).
Government of Norway (2014). Forskrift om konsekvensutredninger for planer etter plan– og bygningsloven [Regulations on impact assessments for plans under the Planning and Building Act]. Available online at: https://lovdata.no/dokument/LTI/forskrift/2014-12-19-1726 (accessed January 16, 2020).
Government of Norway (2015). Første Codicill og Tillæg til Grendse-Tractaten Imellem Kongerigerne Norge og Sverrig Lapperne Betreffende (Lappekodisillen) [First Codicil and Supplement to the Border Treaty Between the Kingdom of Norway and Sweden Concerning the Lapps (Lapp Codicil)]. Available online at: https://lovdata.no/dokument/NL/lov/1751-10-02 (accessed February 7, 2020).
Government of Norway (2016). Proposisjon til Stortinget (forslag til stortingsvedtak). Samtykke til inngåelse av konvensjon mellom Norge og Finland om oppføring og vedlikehold av reingjerder og andre tiltak for å hindre at rein kommer inn på det andre rikets område av 9. desember 2014. Prop. 49 S (2015–2016) [Parliamentary Proposal (Proposal for a Parliamentary Decision). Consent to enter into a Convention between Norway and Finland on the construction and maintenance of reindeer fences and other measures to prevent reindeer from entering the second kingdom's territory of 9 December 2014]. Available online at: https://www.regjeringen.no/contentassets/bf51648f86b34319b609199f4e0b373f/no/pdfs/prp201520160049000dddpdfs.pdf (accessed February 3, 2020).
Government of Norway (2017). Reindrift. Melding til Stortinget 32 (2016–2017) [Reindeer Husbandry. Norwegian Government White Paper 32 (2016–2017)]. Oslo.
Grau, O., Ninot, J. M., Blanco-Moreno, J. M., van Logtestijn, R. S. P., Cornelissen, J. H. C., and Callaghan, T. V. (2012). Shrub-tree interactions and environmental changes drive treeline dynamics in the subarctic. Oikos 121, 1680–1690. doi: 10.1111/j.1600-0706.2011.20032.x
Grøndahl, A. M., and Mejdell, C. M. (2012). Beiteforhold og tap i reindriften sett fra et Dyrevelferdsperspektiv [Pasture conditions and losses in reindeer husbandry in the perspective of animal welfare]. Norsk Veterinærtidsskrift 124, 631–639.
Gruner, D. S., Bracken, M. E. S., Berger, S. A., Eriksson, B. K., Gamfeldt, L., Matthiessen, B., et al. (2017). Effects of experimental warming on biodiversity depend on ecosystem type and local species composition. Oikos 126, 8–17. doi: 10.1111/oik.03688
Gundersen, V., Vistad, O. I., Panzacchi, M., Strand, O., and van Moorter, B. (2019). Large-scale segregation of tourists and wild reindeer in three Norwegian national parks: management implications. Tourism Manage. 75, 22–33. doi: 10.1016/j.tourman.2019.04.017
Gunn, A. (2016). Rangifer tarandus. The IUCN Red List of Threatened Species 2016: e.T29742A22167140.
Gustine, D., Barboza, P., Adams, L., Griffith, B., Cameron, R., and Whitten, K. (2017). Advancing the match–mismatch framework for large herbivores in the Arctic: evaluating the evidence for a trophic mismatch in caribou. PLoS ONE 12:e0171807. doi: 10.1371/journal.pone.0171807
Hætta, J. I., Sara, O. K., and Rushfeldt, I. (1994). Reindriften in Finnmark: Lovgivning og distrikstinndeling [Reindeer Husbandry in Finnmark: Legal Legislation and the Division and Spatial Arrangement of Husbandry Areas] Reindriftsadministrasjonen. Bjørkmanns Trykkeri: Alta, 124.
Hætta, L. B. (2018). Et spørsmål om bærekraft og felles ansvar [A question of sustainability and joint responsibility]. Available online at: https://www.altaposten.no/meninger/2018/12/18/%E2%80%93-Et-sp%C3%B8rsm%C3%A5l-om-b%C3%A6rekraft-og-felles-ansvar-18089100.ece
Hagen, R., Heurich, M., Storch, I., Hanewinkel, M., and Kramer-Schadt, S. (2017). Linking annual variations of roe deer bag records to large-scale winter conditions: spatio-temporal development in Europe between 1961 and 2013. Eur. J. Wildl. Res. 63:97. doi: 10.1007/s10344-017-1155-9
Hallett, T. B., Coulson, T., Pilkington, J. G., Clutton-Brock, T. H., Pemberton, J. M., and Grenfell, B. T. (2004) Why large-scale climate indices seem to predict ecological processes better than local weather. Nature 430, 71–75. doi: 10.1038/nature02708
Hansen, B. B., Aanes, R., Herfindal, I., Kohler, J., and Sæther, B.–E. (2011). Climate, icing, and wild Arctic reindeer: past relationships and future prospects. Ecology 92, 1917–1923. doi: 10.1890/11-0095.1
Hansen, B. B., Gamelon, M., Albon, S. D., Lee, A. M., Stien, A., Irvine, R. J., et al. (2019a). More frequent extreme climate events stabilize reindeer population dynamics. Nat. Commun. 10:1616. doi: 10.1038/s41467-019-09332-5
Hansen, B. B., Pedersen, Å. Ø., Peeters, B., Le Moullec, M., Albon, S. D., Herfindal, I., et al. (2019b). Spatial heterogeneity in climate change effects decouples the long-term dynamics of wild reindeer populations in the high Arctic. Glob. Change Biol. 25, 3656–3668. doi: 10.1111/gcb.14761
Hansen, L. I., and Schmidt, T. (1985). Major Peter Schnitlers grenseeksaminasjonsprotokoller 1742–1745 Bind 3 [Major Peter Schnitler's Border Examination Protocols 1742–1745 Volume 3]. Oslo: Kjedseskriftfondet.
Hanssen, G. S., Aarsæther, N., Hofstad, H., Anker, H. T., Kalbro, T., Buanes, A., et al. (2018). “En operativ lov? Spenningen mellom lovens intensjoner og planpraksis – behov for forbedring? [An operative law? The tension between the intentions of the law and planning practice – need for improvement?],” in Plan– og Bygningsloven 2008 – En lov for vår tid?, eds G. S. Hanssen and N. Aarsæther (Oslo: Universitetsforlaget), 481–521.
Hanssen-Bauer, I. (2005). Regional Temperature and Precipitation Series for Norway: Analyses of Timeseries Updated to 2004. Met.no Report 15/2005. Oslo: The Norwegian Meteorological Institute, 34. Available online at: https://www.met.no/publikasjoner/met-report/met-report-2005/_/attachment/download/e5ccf079-6722-4f43-a0c5-fb8b7338c1e2:2660d2fbcff5b94bad1c675078dd173cbdcaaaac/MET-report-15-2005.pdf
Hanssen-Bauer, I., Achberger, C., Benestad, R. E., Chen, D., and Førland, E. J. (2005). Statistical downscaling of climate scenarios over scandinavia. Clim. Res. 29, 255–268. doi: 10.3354/cr029255
Hanssen-Bauer, I., and Førland, E. J. (2000). Temperature and precipitation variations in Norway 1900–1994 and their links to atmospheric circulation. Int. J. Climatol. 20, 1693–1708. doi: 10.1002/1097-0088(20001130)20:14<1693::AID-JOC567>3.0.CO
Hanssen-Bauer, I., Førland, E. J., Haddeland, I., Hisdal, H., Lawrence, D., Mayer, S., et al. (2017). Climate in Norway 2100 - A Knowledge Base for Climate Adaptation. The Norwegian Centre for Climate Services: Report 1/2017.
Hanssen-Bauer, I., Førland, E. J., Haddeland, I., Hisdal, H., Mayer, S., Nesje, A., et al. (2015). Klima i Norge 2100 – Kunnskapsgrunnlag for Klimatilpasning, Oppdatert i 2015. NCCS Report no. 2/2015. Available online at: www.klimaservicesenter.no
Hanssen-Bauer, I., Førland, E. J., Hisdal, H., Mayer, S., Sandø, A. B., and Sorteberg, A. (2019). Climate in Svalbard 2100. NCCS Report 1/2019. Available online at: https://cms.met.no/site/2/klimaservicesenteret/rapporter-og-publikasjoner/_attachment/14442?_ts=168a8d63a73
Hausner, V. H., Engen, S., Brattland, C., and Fauchald, P. (2020). Sámi knowledge and ecosystem-based adaptation strategies for managing pastures under threat from multiple land uses. J. Appl. Ecol. 57, 1656–1665. doi: 10.1111/1365-2664.13559
Havlík, P., Leclère, D., Valin, H., Herrero, M., Schmid, E., Soussana, J., et al. (2015). “Global climate change, food supply and livestock production systems: a bioe- conomic analysis,” in Climate Change and Food Systems: Global Assessments and Implications for Food Security and Trade, ed A. Elbehri [Rome: Food Agriculture Organization of the United Nations (FAO)].
Hawkins, E., and Sutton, R. (2009). The potential to narrow uncertainty in regional climate predictions. Bull. Am. Meteorol. Soc. 90, 1095–1107. doi: 10.1175/2009BAMS2607.1
Hawkins, E., and Sutton, R. (2011). The potential to narrow uncertainty in projections of regional precipitation change. Clim. Dyn. 37, 407–418. doi: 10.1007/s00382-010-0810-6
Hebblewhite, M., and Haydon, D. T. (2010). Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Biol. Sci. 365, 2303–2312. doi: 10.1098/rstb.2010.0087
Heikkinen, H. I., Lakomäki, S., and Baldridge, J. (2007). The dimensions of sustainability and the neo-entrepreneurial adaptation strategies in reindeer herding in Finland. J. Ecol. Anthropol. 11, 25–42. doi: 10.5038/2162-4593.11.1.2
Heikkinen, H. I., Moilanen, O., Nuttall, M., and Sarkki, S. (2011). Managing predators, managing reindeer: contested conceptions of predator policies in Finland's southeast reindeer herding area. Polar Rec. 47, 218–230. doi: 10.1017/S0032247410000513
Helle, T., Hallikainen, V., Sarkela, M., Haapalehto, M., Niva, A., and Puoskari, J. (2012). Effects of a holiday resort on the distribution of semi-domesticated reindeer. Ann. Zool. Fennici 49, 23–35 doi: 10.5735/086.049.0103
Helle, T. P., and Jaakkola, L. M. (2008). Transitions in herd management of semi–domesticated reindeer in northern Finland. Ann. Zool. Fennici 45, 81–101. doi: 10.5735/086.045.0201
Helle, T., and Särkelä, M. (1993). The effects of outdoor recreation on range use by semi-domesticated reindeer. Scand. J. For. Res. 8, 123–133. doi: 10.1080/02827589309382761
Hendrichsen, D. K., and Tyler, N. J. C. (2014). How the timing of weather events influences early development in a large mammal. Ecology 95, 1737–1745. doi: 10.1890/13-1032.1
Herrero, M., Addison, J., Bedelian, C., Carabine, E., Havlík, P., Henderson, B., et al. (2016). Climate change and pastoralism: impacts, consequences and adaptation. Rev. Sci. Tech. Int. Epiz. 35, 417–433. doi: 10.20506/rst.35.2.2533
Herrmann, T. M., Sandström, P., Granqvist, K., D'Astous, N., Vannar, J., Asselin, H., et al. (2014). Effects of mining on reindeer/caribou populations and indigenous livelihoods: community-based monitoring by Sami reindeer herders in Sweden and First Nations in Canada. Polar J. 4, 28–51. doi: 10.1080/2154896X.2014.913917
Higazi, A. (2016). Farmer-pastoralist conflicts on the Jos Plateau, central Nigeria: security responses of local vigilantes and the Nigerian State. Conflict Secur. Dev. 16, 365–385. doi: 10.1080/14678802.2016.1200314
Ho, P. (2016). Empty institutions, non-credibility and pastoralism: China's grazing ban, mining and ethnicity. J. Peasant Stud. 43, 1145–1176. doi: 10.1080/03066150.2016.1239617
Hobbs, N. T., Andrén, H., Persson, J., Aronsson, M., and Chapron, G. (2012). Native predators reduce harvest of reindeer by Sámi pastoralists. Ecol. Appl. 22, 1640–1654. doi: 10.1890/11-1309.1
Hobbs, N. T., Reid, R. S., Galvin, K. A., and Ellis, J. E. (2008). “Fragmentation of arid and semi-arid ecosystems: implications for people and animals,” in Fragmentation in Semi-Arid and Arid Landscapes: Consequences for Human and Natural Systems, eds K. A. Galvin, R. S. Reid, R. H. Behnke, and N. T. Hobbs (Dordrecht: Springer), 25–44. doi: 10.1007/978-1-4020-4906-4_2
Hoffman, R. R. (1989). Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457. doi: 10.1007/BF00378733
Hoffmann, M., Duckworth, J. W., Holmes, K., Mallon, D. P., Rodrigues, A. S. L., and Stuart, S. N. (2015). The difference conservation makes to extinction risk of the world's ungulates. Conserv. Biol. 29, 1303–1313. doi: 10.1111/cobi.12519
Hurrell, J., and National Center for Atmospheric Research Staff (2020). The Climate Data Guide: Hurrell North Atlantic Oscillation (NAO) Index (Station-Based). Available online at: https://climatedataguide.ucar.edu/climate-data/hurrell-north-atlantic-oscillation-nao-index-station-based (accessed January 09, 2020).
Ims, R. A., Yoccoz, N. G., Bråthen, K. A., Fauchald, P., Tveraa, T., and Hausner, V. (2007). Can reindeer overabundance cause a trophic cascade? Ecosystems 10, 607–622. doi: 10.1007/s10021-007-9060-9
IPCC (2013). “Climate change 2013: the physical science basis,” in Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker et al. (Cambridge, United Kingdom: Cambridge University Press), 1535. Available online at: http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_ALL_FINAL.pdf
IPCC (2019). Climate Change and Land. An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Available online at: https://www.ipcc.ch/site/assets/uploads/sites/4/2020/02/SPM_Updated-Jan20.pdf
Isenberg, A. C. (2000). The Destruction of the Bison: An Environmental History, 1750–1920. New York, NY: Cambridge University Press. doi: 10.1017/CBO9780511549861
Jaakkola, L. (2014). Can learning from the past help to predict the future in the environmental impact assessment on reindeer husbandry? (Ph.D. dissertation), University of Jyväskylä, Jyväskylä, Finland.
James, P. (1966). The Travel Diaries of Thomas Robert Malthus. Cambridge: Cambridge University Press, 316.
Johnsen, K. I. (2016). Land–use conflicts between reindeer husbandry and mineral extraction in Finnmark, Norway: contested rationalities and the politics of belonging. Polar Geogr. 39, 58–79. doi: 10.1080/1088937X.2016.1156181
Johnsen, K. I., and Benjaminsen, T. A. (2017). The art of governing and everyday resistance: ‘rationalization’ of Sámi reindeer husbandry in Norway since the 1970s. Acta Borealia 34, 1–25. doi: 10.1080/08003831.2017.1317981
Johnson, C. J., Boyce, M. S., Case, R. L., Cluff, H. D., Gau, R. J., Gunn, A., et al. (2005). Cumulative effects of human developments on Arctic wildlife. Wildlife Monogr. 160, 1–36. doi: 10.2193/0084-0173(2005)160[1:CEOHDO]2.0.CO;2
Johnson, C. J., Ehlers, L. P. W., and Seip, D. R. (2015). Witnessing extinction - cumulative impacts across landscapes and the future loss of an evolutionarily significant unit of woodland caribou in Canada. Biol. Conserv. 186, 176–186. doi: 10.1016/j.biocon.2015.03.012
Johnson, C. J., and Russell, D. E. (2014). Long-term distribution responses of a migratory caribou herd to human disturbance. Biol. Conserv. 177, 52–63. doi: 10.1016/j.biocon.2014.06.007
Johnson, H. E., Golden, T. S., Adams, L. G., Gustine, D. D., and Lenart, E. A. (2019). Caribou use of habitat near energy development in Arctic Alaska. J. Wildl. Manage. 84, 401–412. doi: 10.1002/jwmg.21809
Johnstone, J., Russell, D. E., and Griffith, B. (2002). Variations in plant forage quality in the range of the Porcupine Caribou Herd. Rangifer 22, 83–91. doi: 10.7557/2.22.1.693
Joly, K., Klein, D. R., Verbyla, D. L., Rupp, T. S., and Chapin, F. S. (2011). Linkages between large–scale climate patterns and the dynamics of Arctic caribou populations. Ecography 34, 345–352. doi: 10.1111/j.1600-0587.2010.06377.x
Joly, K., Nellemann, C., and Vistnes, I. (2006). A reevaluation of caribou distribution near an oilfield road on Alaska's North Slope. Wildl. Soc. Bull. 34, 866–869. doi: 10.2193/0091-7648(2006)34[866:AROCDN]2.0.CO;2
Jonassen, M. K. (2017). Daniel Mortensson (f. 1860) [Daniel Mortensson (born 1860)]. Available online at: https://rorosnytt.no/daniel-mortenson-f-1860/
Jones, P. F., Jakes, A. F., Telander, A. C., Sawyer, H., Martin, B. H., and Hebblewhite, M. (2019). Fences reduce habitat for a partially migratory ungulate in the Northern Sagebrush Steppe. Ecosphere 10, 1–25. doi: 10.1002/ecs2.2782
Kaarlejärvi, E., Eskelinen, A., and Olofsson, J. (2017). Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8:419. doi: 10.1038/s41467-017-00554-z
Kalstad, J. A. (1974). “Samene og Dividalen [The Saami in Dividalen],” in Norges Nasjonaleparker: Øvre Dividalen. 2nd. edn, ed K. D. Vorren (Luther Forlag AS), 91–104.
Karlsdottir, A., Olsen, L. S., Harbo, L. G., Jungsberg, L., and Rasmussen, R. O. (2017). Future Regional Development Policy for the Nordic Arctic: Foresight Analysis 2013–2016. Nordregio Report 2017:1. Stockholm: Nordregio, 67.
Kati, V., Kassara, C., Vassilakis, D., and Papaioannou, H. (2020). Balkan chamois (Rupicapra rupicapra balcanica) avoids roads, settlements, and hunting grounds: an ecological overview from Timfi Mountain, Greece. Diversity 12, 1–18. doi: 10.3390/d12040124
Kelman, I., and Næss, M. W. (2019). Climate change and migration for Scandinavian Saami: a review of possible impacts. Climate 7:47. doi: 10.3390/cli7040047
Kelsall, J. P. (1968). The Migratory Barren Ground caribou of Canada. Ottawa, ON: Queen's Printer, 340.
Kerby, J., and Post, E. (2013). Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Phil. Trans. R. Soc. B 368:20120484. doi: 10.1098/rstb.2012.0484
Kerby, J. T., Wilmers, C. C., and Post, E. (2012). “Climate change, phenology and the nature of consumer–resource interactions: advancing the match/mismatch hypothesis,” in Trait–Mediated Indirect Interactions: Ecological and Evolutionary Perspectives, eds T. Ohgushi, O. J. Schmitz, and R. D. Holt (Cambridge: Cambridge University Press), 508–525. doi: 10.1017/CBO9780511736551.032
Kintisch, E. (2014). What's killing the reindeer? Science 346:685. doi: 10.1126/science.346.6210.685
Kirchner, S. (2020). “Cross-border forms of animal use by indigenous peoples,” in Studies in Global Animal Law, ed A. Peters (Berlin; Heidelberg: Springer Open), 57–69. doi: 10.1007/978-3-662-60756-5_6
Klokov, K. B. (2011). National fluctuations and regional variation in domesticated reindeer numbers in the Russian north: possible explanations. Sibirica 10, 23–47. doi: 10.3167/sib.2011.100102
Knape, J., and de Valpine, P. (2011). Effects of weather and climate on the dynamics of animal population time series. Proc. R. Soc. B 278, 985–992. doi: 10.1098/rspb.2010.1333
Koch, P., and Miggelbrink, J. (2011). Being in the frontline of a Sámi culture and a private business: cross-border reindeer herding in northern Norway and Sweden. Nomad. People 15, 114–143. doi: 10.3167/np.2011.150106
Konstantinov, Y. (2015). Conversations with power: Soviet and post–Soviet developments in the reindeer husbandry part of the Kola Peninsula. Uppsala Stud. Cult. Anthropol. 56, 1–417.
Krausman, P. R., and Harris, L. K. (2010). Cumulative Effects in Wildlife Management: Impact Mitigation. Boca Raton, FL; London, New York, NY: CRC Press. doi: 10.1201/b10788
Krebs, C. J., and Berteaux, D. (2006). Problems and pitfalls in relating climate variability to population dynamics. Clim. Res. 32, 143–149. doi: 10.3354/cr032143
Kreutzmann, H. (2013). The tragedy of responsibility in high Asia: modernizing traditional pastoral practices and preserving modernist worldviews. Pastoralism 3:7. doi: 10.1186/2041-7136-3-7
Kröger, M. (2019). “The global land rush and the Arctic,” in The Global Arctic Handbook, eds. M. Finger and L. Heininen (Cham: Springer International Publishing AG), 27–43. doi: 10.1007/978-3-319-91995-9_3
Krupnik, I. (1993). Arctic Adaptations: Native Whalers and Reindeer Herders of Northern Russia. Hanover, NH: University Press of New England, 355. doi: 10.1349/ddlp.781
Kumpula, T., Pajunen, A., Kaarlejärvi, E., Forbes, B. C., and Stammler, F. (2011). Land use and land cover change in Arctic Russia: ecological and social implications of industrial development. Glob. Environ. Change 21, 550–562. doi: 10.1016/j.gloenvcha.2010.12.010
Lange, S. (2008). Land Tenure and Mining in Tanzania. CMI Report R 2008: 2. Chr. Bergen: Michelsen Institute, 34.
Langlois, A., Johnson, C. A., Montpetit, B., Royer, A., Blukacz-Richards, E. A., Neave, E., et al. (2017). Detection of rain–on–snow (ROS) events and ice layer formation using passive microwave radiometry: a context for Peary caribou habitat in the Canadian Arctic. Remote Sens. Environ. 189, 84–95. doi: 10.1016/j.rse.2016.11.006
LaPerriere, A. J., and Lent, P. C. (1977). Caribou feeding sites in relation to snow characteristics in northeastern Alaska. Arctic 30, 101–108. doi: 10.14430/arctic2690
Lara, M. J., Nitze, I., Grosse, G., Martin, P., and McGuire, A. D. (2018). Reduced Arctic tundra productivity linked with landform and climate change interactions. Sci. Rep. 8:2345. doi: 10.1038/s41598-018-20692-8
Larsen, D. L. (2019). Reineier: – Vi har et godt forhold til grunneierne i Selbu [Reindeer Herders: – We have a good relationship with landowners in Selbu]. Available online at: https://www.nrk.no/sapmi/reineier_–_–vi–har–et–godt–forhold–til–grunneierne–i–selbu-1.14589915 (accessed January 2, 2020).
Latola, K., Sarkki, S., Stepień, A., and Jokinen, M. (2016). “Activities affecting land use in the European Arctic,” in The Changing Arctic and the European Union. A Book Based on the Report ‘Strategic Assessment of Development of the Arctic: Assessment Conducted for the European Union’. Nijhoff Law Specials Nr. 89, eds A. Stepień, T. Koivurova, and P. Kankaanpää (Leiden; Boston, MA: Brill Nijhoff), 186–212.
Lawrence, R., and Larsen, R. K. (2017). The politics of planning: assessing the impacts of mining on Sami lands. Third World Q. 38, 1164–1180. doi: 10.1080/01436597.2016.1257909
Lawton, J. H., Bignell, D. E., Bolton, B., Bloemers, G. F., Eggleton, P., Hammond, P. M., et al. (1998). Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391, 72–76. doi: 10.1038/34166
Leban, F. A., Wisdom, M. J., Garton, E. O., Johnson, B. K., and Kie, J. G. (2001). “Effect of sample size on the performance of resource selection analyses,” in Radio Tracking and Wildlife Populations, eds J. J. Millspaugh and J. M. Marzluff (New York, NY: Academic Press), 291–307. doi: 10.1016/B978-012497781-5/50012-8
Leech, H., Jelinski, D. E., DeGroot, L., and Kuzyk, G. (2017). The temporal niche and seasonal differences in predation risk to translocated and resident woodland caribou (Rangifer tarandus caribou). Can. J. Zool. 95, 809–820. doi: 10.1139/cjz-2016-0076
Leffler, A. J., Klein, E. S., Oberbauer, S. F., and Welker, J. M. (2016). Coupled long–term summer warming and deeper snow alters species composition and stimulates gross primary productivity in tussock tundra. Oecologia 181, 287–297. doi: 10.1007/s00442-015-3543-8
Lemerises, F., Dery, F., Johnson, C., and St-Laurent, M. H. (2018). Spatio-temporal response of mountain caribou to the intensity of backcountry skiing. Biol. Conserv. 217, 149–156. doi: 10.1016/j.biocon.2017.10.030
Lesorogol, C. K. (2008). Land privatization and pastoralist well-being in Kenya. Dev. Chang. 39, 309–331. doi: 10.1111/j.1467-7660.2007.00481.x
Lie, I., Vistnes, I., and Nellemann, C. (2006). Bit for bit utbygging av hytter reduserer reindriftens beitearealer. UTMARK Tidsskrift Utmarksforskning 2, 1–7. Available online at: https://utmark.org/portals/utmark/utmark_old/utgivelser/pub/2006-2/art/Lie_Vistnes_Nellemann_Utmark_2_2006.html
Lien, K. S. (2010). Russerne lei av norsk rein [The Russians are tired of Norwegian reindeer]. Available online at: https://www.nrk.no/tromsogfinnmark/russerne–raser–over–norsk–rein−1.7434166 (accessed February 5, 2020).
Lindberg, M. S., and Walker, J. (2007). Satellite telemetry in avian research and management: sample size considerations. J. Wildl. Manage. 71, 1002–1009. doi: 10.2193/2005-696
Linnell, J. D. C., Andersen, R., Kvam, T., Andrén, H., Liberg, O., Odden, J., et al. (2001). Home range size and choice of management strategy for lynx in scandinavia. Environ. Manage 27, 869–879. doi: 10.1007/s002670010195
Löf, A. (2013). Examining limits and barriers to climate change adaptation in an indigenous reindeer herding community. Clim. Dev. 5, 328–339. doi: 10.1080/17565529.2013.831338
Løkken, J. O., Hofgaard, A., Dalen, L., and Hytteborn, H. (2019). Grazing and warming effects on shrub growth and plant species composition in subalpine dry tundra: an experimental approach. J. Veg. Sci. 30, 698–708. doi: 10.1111/jvs12752
López-i-Gelats, F., Fraser, E. D. G., Morton, J. F., and Rivera-Ferre, M. G. (2016). What drives the vulnerability of pastoralists to global environmental change? A qualitative meta-analysis. Glob. Environ. Change 39, 258–274. doi: 10.1016/j.gloenvcha.2016.05.011
López-i-Gelats, F., Paco, J. L. C., Huayra, R. H., Robles, O. D. S., Peña, E. C. Q., and Filella, J. B. (2015). Adaptation strategies of Andean pastoralist households to both climate and non-climate changes. Hum. Ecol. 43, 267–282. doi: 10.1007/s10745-015-9731-7
Løvschal, M., Bøcher, P. K., Pilgaard, J., Amoke, i., Odingo, A., Thuo, A., et al. (2016). Fencing bodes a rapid collapse of the unique Greater Mara ecosystem. Sci. Rep. 7:41450. doi: 10.1038/srep41450
Lucas, A. (1991). “Programming by early nutrition in man,” in The Childhood Environment and Adult Disease. Ciba Foundation Symposium 156, eds G. R. Bock and J. Whelan (Chichester; New York, NY: John Wiley and Sons), 38–55. doi: 10.1002/9780470514047.ch4
Lund, J. (2017). Vi må snakke om Rudolf. Bevaring av samisk kultur er viktig. Men må det innebære å påføre dyr lidelser? [We must talk about Rudolf. Protecting Saami culture is important. But does it have to involve inflicting suffering on the animals?]. Available online at: https://www.aftenposten.no/meninger/kommentar/i/MgR90J/vi-maa-snakke-om-rudolf-joacim-lund
Lutz, D. W., Cox, M., Wakeling, B. F., McWhirter, D., Carpenter, L. H., Rosenstock, S., et al. (2003). “Impacts and changes to mule deer habitat,” in Mule Deer Conservation: Issues and Management Strategies, eds J. C. de Vos Jr., M. R. Conover, and N. E. Headrick (Logan, UT: Berryman Institute Press, Utah State University), 13–61.
Lyamuya, R. D., Masenga, E. H., Fyumagwa, R. D., Mwita, M. N., and Røskaft, E. (2016). Pastoralist herding efficiency in dealing with carnivore-livestock conflicts in the eastern Serengeti, Tanzania. Int. J. Biodiversity Sci. Ecosyst. Serv. Manage. 12, 202–211. doi: 10.1080/21513732.2016.1163735
Lysvold, S. S. (2017). Oppfordrer naboer til å skyte rein på innmark [Encourage Neighbours to Shoot Reindeer (Observed) on Cultivated Ground]. Available online at: https://www.nrk.no/nordland/oppfordrer-naboer-til-a-skyte-rein-pa-innmark-1.13381181
Mårell, A., and Edenius, L. (2006). Spatial heterogeneity and hierarchical feeding habitat selection by reindeer. Arctic Antarctic Alpine Res. 38, 413–420. doi: 10.1657/1523-0430(2006)38[413:SHAHFH]2.0.CO;2
Mårell, A., Hofgaard, A., and Danell, K. (2006). Nutrient dynamics of reindeer forage species along snowmelt gradients at different ecological scales. Basic Appl. Ecol. 71, 13–30. doi: 10.1016/j.baae.2005.04.005
MacNearney, D., Pigeon, K., Stenhouse, G., Nijland, W., Coops, N. C., and Finnegan, L. (2016). Heading for the hills? Evaluating spatial distribution of woodland caribou in response to a growing anthropogenic disturbance footprint. Ecol. Evol. 6, 6484–6509. doi: 10.1002/ece3.2362
Mahoney, S. P., and Schaefer, J. A. (2002). Hydroelectric development and the disruption of migration in caribou. Biol. Conserv. 107, 147–153. doi: 10.1016/S0006-3207(02)00052-6
Maliniemi, T., Kapfer, J., Saccone, P., Skog, A., and Virtanen, R. (2018). Long-term vegetation changes of treeless heath communities in northern Fennoscandia: links to climate change trends and reindeer grazing. J. Veg. Sci. 29, 469–479. doi: 10.1111/jvs.12630
Mallory, C. D., and Boyce, M. S. (2018). Observed and predicted effects of climate change on Arctic caribou and reindeer. Environ. Rev. 26, 13–25. doi: 10.1139/er-2017-0032
Marin, A., Sjaastad, E., Benjaminsen, T. A., Sara, M. N. M., and Borgenvik, E. J. L. (2020). Productivity beyond density: a critique of management models for reindeer pastoralism in Norway. Pastoralism 10:9. doi: 10.1186/s13570-020-00164-3
Markkula, I., Turunen, M., and Rasmus, S. (2019). A review of climate change impacts on the ecosystem services in the Saami homeland in Finland. Sci. Tot. Environ. 692, 1070–1085. doi: 10.1016/j.scitotenv.2019.07.272
Markussen, K. A., Rognmo, A., and Blix, A. S. (1985). Some aspects of thermoregulation in newborn reindeer calves (Rangifer tarandus tarandus). Acta Physiol. Scand. 123, 215–220. doi: 10.1111/j.1748-1716.1985.tb07580.x
Marshal, J. P., Owen-Smith, N., Whyte, I. J., and Stenseth, N. C. (2011). The role of El Niño–Southern Oscillation in the dynamics of a savanna large herbivore population. Oikos 120, 1175–1182. doi: 10.1111/j.1600-0706.2010.19155.x
Martin, P. (2012). “Conflicts between pastoralists and farmers in tana river district,” in Spaces of Insecurity: Human Agency in Violent Conflicts in Kenya. African Studies Collection Vol. 45. eds K. Witsenburg and F. Zaal (Leiden: African Studies Centre), 167–193.
Martínez–Jauregui, M., San Miguel-Ayanz, A., Mysterud, A., Rodríguez–Vigal, C., Clutton–Brock, T., Langvatn, R., et al. (2009). Are local weather, NDVI and NAO consistent determinants of red deer weight across three contrasting European countries? Glob. Chang. Biol. 15, 1727–1738. doi: 10.1111/j.1365-2486.2008.01778.x
Mathiesen, S. D., Haga, Ø. E., Kaino, T., and Tyler, N. J. C. (2000). Diet composition, rumen papillation and maintenance of carcass mass in female Norwegian reindeer (Rangifer tarandus tarandus) in winter. J. Zool. Lond. 251, 129–138. doi: 10.1111/j.1469-7998.2000.tb00598.x
Mazzullo, N. (2009). “Sápmi: a symbolic re–appropriation of Lapland as Saamiland,” in Máttut – Máddagat: The Roots of Saami Ethnicities, Societies and Spaces/Places, ed T. Äikäs (Oulu: Giellagas Institute, University of Oulu), 174–85.
McCauley, D., Heffron, R., Pavlenko, M., Rehner, R., and Holmes, R. (2016). Energy justice in the Arctic: implications for energy infrastructural development in the Arctic. Energy Res. Soc. Sci. 16, 141–146. doi: 10.1016/j.erss.2016.03.019
McDonald, S. E., Reid, N., Smith, R., Waters, C. M., Hunter, J., and Rader, R. (2019). Rotational grazing management achieves similar plant diversity outcomes to areas managed for conservation in a semi–arid rangeland. Rangel. J. 41, 135–145. doi: 10.1071/RJ18090
McGahey, D. J. (2011). Livestock Mobility and Animal Health Policy in Southern Africa: the Impact of Veterinary Cordon Fences on Pastoralists. Pastoralism, 1:14. Available online at: http://www.pastoralismjournal.com/content/1/1/14
McKnight, T. L. (1959). The feral horse in Anglo-America. Geogr. Rev. 49, 506–525. doi: 10.2307/212210
Megersa, B., Markemann, A., Angassa, A., Ogutu, J. O., Piepho, H.–P., and Zaráte, A. V. (2014). Impacts of climate change and variability on cattle production in southern ethiopia: perceptions and empirical evidence. Agric. Syst. 130, 23–34. doi: 10.1016/j.agsy.2014.06.002
Meng, X., Aryalb, A., Taitb, A., Raubenhiemerbc, D., Wua, J., Ma, Z., et al. (2014). Population trends, distribution and conservation status of semi–domesticated reindeer (Rangifer tarandus) in China. J. Nat. Conserv. 22, 539–546. doi: 10.1016/j.jnc.2014.08.008
Mercer, J. B., Johnsen, H. K., Blix, A. S., and Hotvedt, R. (1985). Central control of expired air temperature and other thermoregulatory effectors in reindeer. Am. J. Physiol. 248, R679–R685. doi: 10.1152/ajpregu.1985.248.6.R679
Middleton, A. D., Merkle, J. A., McWhirter, D. E., Cook, J. G., Cook, R. C., White, P. J., et al. (2018). Green–wave surfing increases fat gain in a migratory ungulate. Oikos 127, 1060–1068. doi: 10.1111/oik.05227
Miller, F. L., Broughton, E., and Gunn, A. (1988). Mortality of Migratory Barren–Ground Caribou on the Calving Grounds of the Beverly herd, Northwest Territories, 1981–83. Canadian Wildlife Service Occasional Paper 66. Ottawa, ON, 26.
Miller, F. L., Edmonds, E. J., and Gunn, A. (1982). Foraging Behaviour of Peary Caribou in Response to Springtime Snow and Ice Conditions. Canadian Wildlife Service Occasional Paper No. 48. Ottawa, ON, 41.
Milner–Gulland, E. J., Kholodova, M. V., Bekenov, A., Bukreeva, O. M., Grachev, Iu. A., Amgalan, L., et al. (2001). Dramatic declines in saiga antelope populations. Oryx 35, 340–345. doi: 10.1046/j.1365-3008.2001.00202.x
Mishra, C. (2001). High altitude survival: conflicts between pastoralism and wildlife in the trans-himalaya (Doctoral thesis), Wageningen University, Wageningen, The Netherlands.
Mount, L. E. (1979). Adaptation to Thermal Environment: Man and his Productive Animals. London: Edward Arnold (Publishers) Limited, 333.
Muhly, T., Serrouya, R., Neilson, E., Li, H. T., and Boutin, S. (2015). Influence of in-situ oil sands development on caribou (Rangifer tarandus) movement. PLoS ONE. 10:e0136933. doi: 10.1371/journal.pone.0136933
Mumma, M. A., Gillingham, M. P., Parker, K. L., Johnson, C. J., and Watters, M. (2018). Predation risk for boreal woodland caribou in human-modified landscapes: evidence of wolf spatial responses independent of apparent competition. Biol. Conserv. 228, 215–223. doi: 10.1016/j.biocon.2018.09.015
Myers-Smith, I. H., Kerby, J. T., and Wipf, S. (2020). Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 10, 106–117.
Myers-Smith, I. H., Thomas, H. J. D., and Bjorkman, A. D. (2019). Plant traits inform predictions of tundra responses to global change. N. Phytol. 221, 1742–1748. doi: 10.1111/nph.15592
Mysterud, A. (2006). The concept of overgrazing and its role in management of large herbivores. Wildl. Biol. 12, 129–141. doi: 10.2981/0909-6396(2006)12[129:TCOOAI]2.0.CO;2
Mysterud, A., and Austrheim, G. (2014). Lasting effects of snow accumulation on summer performance of large herbivores in alpine ecosystems may not last. J. Anim. Ecol. 83, 712–719. doi: 10.1111/1365-2656.12166
Mysterud, A., Stenseth, N. C., Yoccoz, N. G., Langvatn, R., and Steinheim, G. (2001). Nonlinear effects of large–scale climatic variability on wild and domestic herbivores. Nature 410, 1096–1099. doi: 10.1038/35074099
Nautiyal, S., Rao, K. S., Maikhuri, R. K., and Saxena, K. G. (2003). Transhumant pastoralism in the Nanda Devi Biosphere Reserve, India: a case study in the buffer zone. Mt. Res. Dev. 23, 255–262. doi: 10.1659/0276-4741(2003)023[0255:TPITND]2.0.CO;2
Nellemann, C., and Cameron, R. D. (1996). Effects of petroleum development on terrain preferences of calving caribou. Arctic 49, 23–28. doi: 10.14430/arctic1180
Nellemann, C., and Cameron, R. D. (1998). Cumulative impacts of an evolving oilfield complex on the distribution of calving caribou. Can. J. Zool. 76, 425–1430. doi: 10.1139/z98-078
Nellemann, C., Henriksen, R., Pravettoni, R., Jesperson, S., and Sandei, P. (2019). “New times, new opportunities: grasping the future with the World's youngest population in the Sahel,” in A RHIPTO Assessment Prepared for the Special Advisor for the Sahel to the Secretary-General (Dakar; Oslo; Paris; London; Rome).
Nellemann, C., Jordhøy, P., Støen, O.-G., and Strand, O. (2000). Cumulative impacts of tourist resorts on wild reindeer (Rangifer tarandus tarandus) during winter. Arctic 53, 9–17. doi: 10.14430/arctic829
Nellemann, C., and Vistnes, I. (2002). Halkavarre-Porsangmoen Bombing and Firing Range. Impacts and Opportunities for the Saami Reindeer Herders and the National Defence. NINA Report 750. Trondheim: Norwegian Institute for Nature Research, 32.
Nellemann, C., Vistnes, I., Jordhøy, P., Støen, O.-G., Kaltenborn, B. P., Hanssen, F., et al. (2010). Effects of recreational cabins, trails and their removal for restoration of reindeer winter ranges. Restor. Ecol. 18, 873–881. doi: 10.1111/j.1526-100X.2009.00517.x
Nellemann, C., Vistnes, I., Jordhøy, P., and Strand, O. (2001). Winter distribution of wild reindeer in relation to power lines, roads and resorts. Biol. Conserv. 101, 351–360. doi: 10.1016/S0006-3207(01)00082-9
Nellemann, C., Vistnes, I., Jordhoy, P., Strand, O., and Newton, A. (2003). Progressive impact of piecemeal infrastructure development on wild reindeer of piecemeal infrastructure development on wild reindeer. Biol. Conserv. 113, 307–317. doi: 10.1016/S0006-3207(03)00048-X
Neudert, R., Etzold, J., Münzner, F., Manthey, M., and Busse, S. (2013). The opportunity costs of conserving pasture resources for mobile pastoralists in the greater caucasus. Landsc. Res. 38, 499–522. doi: 10.1080/01426397.2012.728204
Nilssen, K. J., Sundsfjord, J. A., and Blix, A. S. (1984). Regulation of metabolic rate in Svalbard and Norwegian reindeer. Am. J. Physiol. 16, R837–R841. doi: 10.1152/ajpregu.1984.247.5.R837
Northrup, J. M., Anderson, C. R., and Wittemyer, G. (2015). Quantifying spatial habitat loss from hydrocarbon development through assessing habitat selection patterns of mule deer. Glob. Chang. Biol. 21, 3961–3070. doi: 10.1111/gcb.13037
Norwegian Agriculture Agency (2019a). Ressursregnskap for reindriftsnæringen for reindriftsåret 1. April 2018 – 31. mars 2019 [Resource Inventory for the Reindeer Husbandry Year 1 April 2018 – 31 March 2019] Report 34/2019. Oslo: Landbruksdirektoratet, 112. Available online at: https://www.landbruksdirektoratet.no/no/dokumenter/publikasjoner/_attachment/77880?_ts=16fb3f2d2e8&download=true (accessed February 20, 2020).
Norwegian Agriculture Agency (2019b). Totalregnskap for Reindriftsnæringen. Regnskap 2018 og budsjett 2019 [Annual Inventory for Reindeer Husbandry. Inventory 2018 and Budget 2019]. Report 31/2019. Oslo: Landbruksdirektoratet, 175. Available online at: https://www.landbruksdirektoratet.no/no/reindriften/reindriftsavtalen/totalregnskapet/_attachment/77126?_ts=16f18bdc6c0 (accessed February 24, 2010).
Norwegian Reindeer Husbandry Administration (2006). Ressursregnskap for reindriftsnæringenfor reindriftsåret 1. april 2004 – 31. mars 2005 [Resource Inventory for the Reindeer Husbandry Year 1 April 2004 – 31 March 2005]. Alta: Reindriftsforvaltningen, 161.
Odadi, W. O., Fargione, J., and Rubenstein, D. I. (2017). Vegetation, wildlife, and livestock responses to planned grazing management in an African pastoral landscape. Land Degrad. Dev. 28, 2030–2038 doi: 10.1002/ldr.2725
Office of the Auditor General (2004). Riksrevisjonens undersøkelse av bærekraftig bruk av reinbeiteressursene i Finnmark. Dokument nr. 3:12 (2003–2004) [The Office of the Auditor General's Study of Sustainable Use of Reindeer Grazing Resources in Finnmark. Document nr. 3:12 (2003–2004)]. Available online at: https://www.stortinget.no/globalassets/pdf/dokumentserien/2003-2004/dok_3_12_2003_2004.pdf
Okech, R. N. (2010). Wildlife-community conflicts in conservation areas in Kenya. Afr. J. Conflict Resol. 10, 65–80. doi: 10.4314/ajcr.v10i2.63311
Oli, M. K., Taylor, I. R., and Rogers, M. E. (1994). Snow leopard Panthera uncia predation of livestock-an assessment of local perceptions in the Annapurna Conservation area, Nepal. Biol. Conserv. 68, 63–68. doi: 10.1016/0006-3207(94)90547-9
Olofsson, J., Oksanen, L., Callaghan, T., Hulme, P. E., Oksanen, T., and Suominen, O. (2009). Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x
Oppdal, S. S. (2007). “Hellaugsetra i Åmotsdalen, og familien Martin Jonassen [Hellaugsetra in Åmotsdalen, and the family of Martin Jonassen],” in 2007 Litt Historisk om Tamreindriften i Åmotsdalen og Sunndalsfjella, [Some History of Reindeer Husbandry in Åmotsdalen og Sunndalsfjella], ed S. S. Oppdal. Published privately.
Oreskes, N., and Conway, E. M. (2010). Merchants of Doubt: How a Handful of Scientists Obscured the truth on Issues From Tobacco Smoke to Global Warming. London: Bloomsbury Press, 355.
Orrick, K. D. (2018). Range size and drivers of African elephant (Loxodonta africana) space use on Karongwe private game reserve, South Africa. Afr. J. Ecol. 56, 52–581. doi: 10.1111/aje.12500
Ossi, F., Gaillard, J.–M., Hebblewhite, H., and Cagnacci, F. (2015). Snow sinking depth and forest canopy drive winter resource selection more than supplemental feeding in an alpine population of roe deer. Eur. J. Wildl. Res. 61, 111–124. doi: 10.1007/s10344-014-0879-z
Overland, J., Walsh, J., and Kattsov, V. (2017). “Trends and feedbacks,” in Snow, Water, Ice and Permafrost in the Arctic 2019, 9–23 [Oslo: Arctic Monitoring and Assessment Programme (AMAP)].
Ozgul, A., Tuljapurkar, S., Benton, T. G., Pemberton, J. M., Clutton–Brock, T. H., and Coulson, T. (2009). The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467. doi: 10.1126/science.1173668
Paine, R. (1994). Herds of the Tundra: A Portrait of Saami Reindeer Pastoralism. Washington, DC; London: Smithsonian Institution Press, 242.
Pantuliano, S. (2010). Oil, land and conflict: the decline of Misseriyya pastoralism in Sudan. Rev. Afr. Polit. Econ. 37, 7–23. doi: 10.1080/03056241003637847
Panzacchi, M., van Moorter, B., Jordhøy, P., and Strand, O. (2013a). Learning from the past to predict the future: using archaeological findings and GPS data to quantify reindeer sensitivity to anthropogenic disturbance in Norway. Landsc. Ecol. 28, 847–859. doi: 10.1007/s10980-012-9793-5
Panzacchi, M., van Moorter, B., and Strand, O. (2013b). A road in the middle of one of the last wild reindeer migration routes in Norway: crossing behaviour and threats to conservation. Rangifer 33, 15–26. doi: 10.7557/2.33.2.2521
Paoli, A., Weladji, R. B., Holand, Ø., and Kumpula, J. (2018). Winter and spring climatic conditions influence timing and synchrony of calving in reindeer. PLoS ONE 13:e0195603. doi: 10.1371/journal.pone.0195603
Pape, R., and Löffler, J. (2012). Climate change, land use conflicts, predation and ecological degradation as challenges for reindeer husbandry in northern Europe: what do we really know after half a century of research? Ambio 41, 421–434. doi: 10.1007/s13280-012-0257-6
Pape, R., and Löffler, J. (2016). Towards a process-based biogeography of reindeer—scaling space, time, and organizational levels of space use. Norsk Geografisk Tidsskrift Norwegian J. Geogr. 70, 230–246. doi: 10.1080/00291951.2016.1203018
Park, T., Chen, C., Macias-Fauria, M., Tømmervik, H., Choi, S., Winkler, A., et al. (2019). Changes in timing of seasonal peak photosynthetic activity in northern ecosystems. Glob. Chang. Biol. 25, 2382–2395. doi: 10.1111/gcb.14638
Parker, G. R. (1972). Biology of the Kaminuriak Population of Barren–Ground Caribou. Part 1: Total Numbers, Mortality, Recruitment and Seasonal Distribution. Canadian Wildlife Service Report Series Nr. 20. Ottawa, ON, 95.
Paton, D. G., Ciuti, S., Quinn, M., and Boyce, M. S. (2017). Hunting exacerbates the response to human disturbance in large herbivores while migrating through a road network. Ecosphere 8:e01841. doi: 10.1002/ecs2.1841
Pattison, R. R., Jorgenson, J. C., Raynolds, M. K., and Welker, J. M. (2015). Trends in NDVI and tundra community composition in the Arctic of NE Alaska between 1984 and 2009. Ecosystems 18, 707–719. doi: 10.1007/s10021-015-9858-9
Pedersen, S. (1987). The Lappcodicil of 1751 – Magna Charta of the Sami? Mennesker Rettigheter 5, 33–38.
Persson, S., Harnesk, D., and Islar, M. (2017). What local people? Examining the Gállok mining conflict and the rights of the Sámi population in terms of justice and power. Geoforum 86, 20–29. doi: 10.1016/j.geoforum.2017.08.009
Pettorelli, N., Weladji, R. B., Holand, Ø., Mysterud, A., Breie, H., and Stenseth, N. C. (2005). The relative role of winter and spring conditions: linking climate and landscape–scale plant phenology to alpine reindeer body mass. Biol. Lett. 1, 24–26. doi: 10.1098/rsbl.2004.0262
Phoofolo, P. (1993). Epidemics and revolutions: the rinderpest epidemic in late nineteenth–century southern Africa. Past Present. 138, 112–143. doi: 10.1093/past/138.1.112
Plante, S., Dussault, C., and Côté, S. D. (2017). Landscape attributes explain migratory caribou vulnerability to sport hunting. J. Wildl. Manage. 81, 238–247. doi: 10.1002/jwmg.21203
Plante, S., Dussault, C., Richard, J. H., and Côté, S. D. (2018). Human disturbance effects and cumulative habitat loss in endangered migratory caribou. Biol. Conserv. 224, 129–143. doi: 10.1016/j.biocon.2018.05.022
Plard, F., Gaillard, J.–M., Coulson, T., Hewison, A. J. M., Delorme, D., Warnant, C., et al. (2014). Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biol. 12:e1001828. doi: 10.1371/journal.pbio.1001828
Polfus, J. L., Hebblewhite, M., and Heinemeyer, K. (2011). Identifying indirect habitat loss and avoidance of human infrastructure. Biol. Conserv. 144, 2637–2646. doi: 10.1016/j.biocon.2011.07.023
Post, E. (2005). Large–scale spatial gradients in herbivore population dynamics. Ecology 86, 2320–2328. doi: 10.1890/04-0823
Post, E., Alley, R. B., Christensen, T. R., Macias-Fauria, M., Forbes, B. C., Gooseff, M. N., et al. (2019). The polar regions in a 2°C warmer world. Sci. Adv. 5:eaaw9883. doi: 10.1126/sciadv.aaw9883
Post, E., Brodie, J., Hebblewhite, M., Anders, A. D., Maier, J. A. K., and Wilmers, C. C. (2009a). Global population dynamics and hot spots of response to climate change. Bioscience 59, 489–497. doi: 10.1525/bio.2009.59.6.7
Post, E., and Forchhammer, M. C. (2002). Synchronization of animal population dynamics by large–scale climate. Nature 420, 168–171. doi: 10.1038/nature01064
Post, E., and Forchhammer, M. C. (2008). Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B. 363, 2369–2375. doi: 10.1098/rstb.2007.2207
Post, E., Forchhammer, M. C., Bret-Harte, S., Callghan, T. V., Chirtsensen, T. R., Elberling, B., et al. (2009b). Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358. doi: 10.1126/science.1173113
Post, E., and Pedersen, C. (2008). Opposing plant community responses to warming with and without herbivores. Proc. Natl. Acad. Sci. U.S.A. 105, 12353–12358. doi: 10.1073/pnas.0802421105
Post, E., Pedersen, C., Wilmers, C. C., and Forchhammer, M. C. (2008). Warming, plant phenology and the spatial dimension of trophic mismatch for large herbivores. Proc. R. Soc. B 275, 2005–2013. doi: 10.1098/rspb.2008.0463
Povoroznyuk, O. (2007). “Reindeer herders and hunters of Eastern Siberia: life of Kalar Evenks,” in International Handbook of Research on Indigenous Entrepreneurship, eds L.–P. Dana and R. B. Anderson (Edward Elgar Publishing Limited: Cheltenham, UK), 137–154.
Prevéy, J. S., Rixen, C., Rüger, N., Høye, T. T., Bjorkman, A. D., Myers-Smith, I. H., et al. (2019). Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 3, 45–52. doi: 10.1038/s41559-018-0745-6
Prokopenko, C. M., Boyce, M. S., and Avgar, T. (2017). Extent-dependent habitat selection in a migratory large herbivore: road avoidance across scales. Landsc. Ecol. 32, 313–325. doi: 10.1007/s10980-016-0451-1
Putkonen, J., and Roe, G. (2003). Rain–on–snow events impact soil temperatures and affect ungulate survival. Geophys. Res. Lett. 30:1188. doi: 10.1029/2002GL016326
Räisänen, J. (2012). “Enhanced greenhouse effect and climate change in northern Europe,” in From the Earth's Core to Outer Space. Lecture Notes in Earth Sciences, ed I. Haapala (Berlin; Heidelberg: Springer), 227–239. doi: 10.1007/978-3-642-25550-2_16
Ravna, Ø. (2010a). Alders tids bruk og hevd som ervervsgrunnlag i samiske områder—særlig med vekt på kravet til aktsom god tro ved rettsidentifiseringen i Finnmark [Use since time immemorial and land claim as the basis for land acquisition in Sami areas—with emphasis on the requirement of good faith for legal recognition [of such claims] in Finnmark]. Tidsskrift Rettsvitenskap 123, 464–504.
Ravna, Ø. (2010b). Lappekodisillen av 1751 og dens rettslige betydning i dag [The legal significance today of the Lapp Codicil of 1751]. Lov. Rett. 49, 392–406.
Ravna, Ø. (2011). Bruksordning av reindriftsrettigheter i utkast til ny jordskiftelov [Reindeer husbandry rights in the draft of the new Land Consolidation Act]. Kart Plan 71, 22–34.
Raynor, E. J., Derner, J. D., Hoover, D. L., Parton, W. J., and Augustine, D. J. (2020). Large-scale and local climatic controls on large herbivore productivity: implications for adaptive rangeland management. Ecol. Appl. 30:e02053. doi: 10.1002/eap.2053
Redmer, D. A., Wallace, J. M., and Reynolds, L. P. (2004). Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 27, 199–217. doi: 10.1016/j.domaniend.2004.06.006
Rees, W. G., Stammler, F. M., Danks, F. S., and Vitebsky, P. (2008). Vulnerability of European reindeer husbandry to global change. Clim. Change 87, 199–217. doi: 10.1007/s10584-007-9345-1
Reimers, E., Dahle, B., Eftestøl, S., Colman, J. E., and Gaare, E. (2007). Effects of a power line on migration and range use of wild reindeer. Biol. Conserv. 134, 484–494. doi: 10.1016/j.biocon.2006.08.034
Reinert, E. S. (2006). The economics of reindeer herding: Saami entrepreneurship between cyclical sustainability and the powers of State and oligopolies. Br. Food J. 108, 522–540. doi: 10.1108/00070700610676352
Reinert, E. S. (2016). “Årsaker til at planøkonomien er blitt varig ødeleggende for verdiskapningen i samisk reindrift [Reasons why planned economy has been persistently destructive for the economic growth of Saami reindeer husbandry],” in Samisk Reindrift Norske Myter [Saami Reindeer Pastoralism Norwegian Myths], eds T. A. Benjaminsen, I. M. G. Eira, and M. N. Sara (Bergen: Fagbokforlaget), 145–174.
Reinert, E. S., Aslaksen, J., Eira, I. M. G., Mathiesen, S. D., Reinert, H., and Turi, E. I. (2009). “Adapting to climate change in Sami reindeer herding: the nation-state as problem and solution,” in Adapting to Climate Change: Thresholds, Values, Governance, eds W. N. Adger, I. Lorenzoni, and K. O'Brien (Cambridge: Cambridge University Press), 417–432.
Rhind, S. M., Rae, M. T., and Brooks, A. N. (2001). Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 122, 205–214. doi: 10.1530/rep.0.1220205
Rind, D., Perlwitz, J., and Lonergan, P. (2005). AO/NAO response to climate change: 1. Respective influences of stratospheric and tropospheric climate changes. J. Geophys. Res. 110:D12107. doi: 10.1029/2004JD005103
Ringjord, K. (2016). Reinsdyrplager på Kvaløya [The nuisance of reindeer on Kvaløya]. Available online at: https://www.itromso.no/meninger/debatt/2016/07/25/Reinsdyrplager-p%C3%A5-Kval%C3%B8ya-13084617.ece
Riseth, J. Å., and Johansen, B. (2018). Inngrepskartlegging for reindrifta i Troms fylke. Report to Troms County Council, 174.
Riseth, J. Å., Tømmervik, H., and Bjerke, J. W. (2016). 175 years of adaptation: North Scandinavian Sámi reindeer herding between government policies and winter climate variability (1835–2010). J. For. Econ. 24, 186–204. doi: 10.1016/j.jfe.2016.05.002
Riseth, J. Å., and Vatn, A. (2009). Modernization and pasture degradation: a comparative study of two Sámi reindeer pasture regions in Norway. Land Econ. 85, 87–106. doi: 10.3368/le.85.1.87
Risvoll, C., and Hovelsrud, G. K. (2016). Pasture access and adaptive capacity in reindeer herding districts in Nordland, Northern Norway. Polar J. 6, 87–111. doi: 10.1080/2154896X.2016.1173796
Robinson, R. A., Crick, H. Q. P., Learmonth, J. A., Maclean, I. M. D., Thomas, C. D., Bailein, F., et al. (2009). Travelling through a warming world: climate change and migratory species. Endanger. Species Res. 7, 87–99. doi: 10.3354/esr00095
Røed, K. H., Flagstad, Ø., Bjørnstad, G., and Hufthammer, A. K. (2011). Elucidating the ancestry of domestic reindeer from ancient DNA approaches. Q Int. 238, 83–88. doi: 10.1016/j.quaint.2010.07.031
Røed, K. H., Flagstad, Ø., Nieminen, M., Holand, Ø., Dwyer, M. J., Røv, N., et al. (2008). Genetic analyses reveal independent domestication origins of Eurasian reindeer. Proc. R. Soc. B Biol. Sci. 275:1849.e1855. doi: 10.1098/rspb.2008.0332
Ross, S., Al Zakwani, W. H., Al Kalbani, A. S., Al Rashdi, A., Al Shukailli, A. S., and Al Jandhami, M. H. (2019). Combining distance sampling and resource selection functions to monitor and diagnose declining Arabian gazelle populations. J. Arid Environ. 164, 23–28. doi: 10.1016/j.jaridenv.2019.02.003
Roto, J. (2015). Reindeer Herding Areas and Districts in Norway. Nordregio. Available online at: https://www.nordregio.org/maps/reindeer-herding-areas-and-districts-in-norway/
Rudolph, T. D., Drapeau, P., Imbeau, L., Brodeur, V., Legare, S., and St-Laurent, M. H. (2017). Demographic responses of boreal caribou to cumulative disturbances highlight elasticity of range-specific tolerance thresholds. Biodiversity Conserv. 26, 1179–1198. doi: 10.1007/s10531-017-1292-1
Russell, D. E., Martell, A. M., and Nixon, W. A. C. (1993). Range ecology of the Porcupine Caribou Herd in Canada. Rangifer Special Issue 8, 1–168. doi: 10.7557/2.13.5.1057
Said, M. Y., Ogutu, J. O., Kifugo, S. C., Makui, O., Reid, R. S., and de Leeuw, J. (2016). Effects of extreme land fragmentation on wildlife and livestock population abundance and distribution. J. Nat. Conserv. 34, 151–164. doi: 10.1016/j.jnc.2016.10.005
Salgado-Flores, A., Hagen, L. H., Ishaq, S. L., Zamanzadeh, M., Wright, A.–D. G., Pope, P. B., et al. (2016). Rumen and cecum microbiomes in reindeer (Rangifer tarandus tarandus) are changed in response to a lichen diet and may affect enteric methane emissions. PLoS ONE 11:e0155213. doi: 10.1371/journal.pone.0155213
Salvesen, G. (2009). Iskalde Fronter på Vidda [Ice Cold Political Perspectives on the Vidda]. Available online at: https://www.aftenposten.no/norge/politikk/i/mBqGE/iskalde-fronter-paa-vidda
Sara, M. N. (2001). Reinen - et Gode fra Vinden: Reindriftens Tilpasningsformer i Kautokeino [Reindeer - a Blessing from the Wind: Forms of Adaptation in Reindeer Husbandry in Kautokeino]. Karasjok: Davvi Girji.
Sara, M. N., Eira, I. M. G., Bjørklund, I., and Oskal, A. (2016). “Hvordan skal vi forstå reintall? [How should we interpret reindeer numbers?],” in Samisk Reindrift Norske Myter [Saami Reindeer Pastoralism Norwegian Myths], eds T. A. Benjaminsen, I. M. G. Eira, and M. N. Sara (Bergen: Fagbokforlaget), 51–67.
Schaefer, J. A. (2003). Long-term range recession and the persistence of caribou in the Taiga. Conserv. Biol. 17, 1435–1439. doi: 10.1046/j.1523-1739.2003.02288.x
Seebacher, F., and Franklin, C. E. (2012). Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614. doi: 10.1098/rstb.2012.0036
Serrouya, R., Dickie, M., DeMars, C., Wittmann, M. J., and Boutin, S. (2020). Predicting the effects of restoring linear features on woodland caribou populations. Ecol. Modell. 416:108891. doi: 10.1016/j.ecolmodel.2019.108891
Shannon, G., Angeloni, L. M., Wittemyer, G., Fristrup, K. M., and Crooks, K. R. (2014). Road traffic noise modifies behaviour of a keystone species. Anim. Behav. 94, 135–141. doi: 10.1016/j.anbehav.2014.06.004
Sheridan, J. A., and Bickford, D. (2011). Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406. doi: 10.1038/nclimate1259
Sinclair, A. R. E. (1977). The African Buffalo. A Study in Resource Limitation of Populations. Chicago, IL: University of Chicago Press, 355.
Sjölander-Lindqvist, A., Risvoll, C., Kaarhus, R., Lundberg, A. K., and Sandström, C. (2020). Knowledge claims and struggles in decentralized large carnivore governance: insights from Norway and Sweden. Front. Ecol. Evol. 8:120. doi: 10.3389/fevo.2020.00120
Skarin, A., and Åhman, B. (2014). Do human activity and infrastructure disturb domesticated reindeer? The need for the reindeer's perspective. Polar Biol. 37, 1041–1054. doi: 10.1007/s00300-014-1499-5
Skarin, A., and Alam, M. (2017). Reindeer habitat use in relation to two small wind farms, during preconstruction, construction, and operation. Ecol. Evol. 7, 3870–3882. doi: 10.1002/ece3.2941
Skarin, A., Nellemann, C., Rönnegård, L., Sandström, P., and Lundqvist, H. (2015). Wind farm construction impacts reindeer migration and movement corridors. Landsc. Ecol. 30, 1527–1540. doi: 10.1007/s10980-015-0210-8
Skarin, A., Sandström, P., and Alam, M. (2018). Out of sight of wind turbines—reindeer response to wind farms in operation. Ecol. Evol. 8, 9906–9919. doi: 10.1002/ece3.4476
Skogland, T. (1978). Characteristics of the snow cover and its relationship to wild mountain reindeer (Rangifer tarandus tarandus L.) feeding strategies. Arctic Alpine Res. 10, 569–580. doi: 10.2307/1550680
Skogland, T. (1980). Comparative summer feeding strategies of Arctic and Alpine Rangifer. J. Anim. Ecol. 49, 81–89. doi: 10.2307/4278
Skogland, T. (1984). Wild reindeer foraging–niche organization. Holarctic Ecol. 7, 345–379. doi: 10.1111/j.1600-0587.1984.tb01138.x
Skogland, T. (1989). Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv. Ethol. 29, 1–74.
Skonhoft, A., Johannesen, A. B., and Olaussen, J. O. (2017). On the tragedy of the commons: when predation and livestock loss may improve the economic lot of herders. Ambio 46, 644–654. doi: 10.1007/s13280-017-0910-1
Soper, J. D. (1941). History: range and home life of the northern bison. Ecol. Monogr. 11, 347–412. doi: 10.2307/1943298
Soppela, P., Nieminen, M., Saarela, S., and Hissa, R. (1986). The influence of ambient temperature on metabolism and body temperature of newborn and growing reindeer calves. Comp. Biochem. Physiol. A 83, 371–386. doi: 10.1016/0300-9629(86)90592-X
Soppela, P., Nieminen, M., Saarela, S., Keith, J. S., Morrison, J. N., Macfarlane, F., et al. (1991). Brown fat–specific mitochondrial uncoupling protein in adipose tissues of newborn reindeer. Am. J. Physiol. 260, R1229–R1234. doi: 10.1152/ajpregu.1991.260.6.R1229
Soppela, P., Sormunen, R., Saarela, S., Huttunen, P., and Nieminen, M. (1992). Localization, cellular morphology and respiratory capacity of “brown” adipose tissue in newborn reindeer. Comp. Biochem. Physiol. A 101, 281–293. doi: 10.1016/0300-9629(92)90534-W
Spinage, C. A. (1992). The decline of the Kalahari wildebeest. Oryx 26, 147–150. doi: 10.1017/S0030605300023577
Spitz, D. B., Rowland, M. M., Clark, D. A., Wisdom, M. J., Smith, J. B., Brown, C. L., et al. (2019). Behavioral changes and nutritional consequences to elk (Cervus canadensis) avoiding perceived risk from human hunters. Ecosphere 10:e02864. doi: 10.1002/ecs2.2864
Stabach, J. A., Wittemeyer, G., Boone, R. B., Reid, R. S., and Worden, J. S. (2016). Variation in habitat selection by white-bearded wildebeest across different degrees of human disturbance. Ecosphere 7:e01428. doi: 10.1002/ecs2.1428
Stephen, K. (2018). Societal impacts of a rapidly changing Arctic. Curr. Clim. Change Rep. 4, 223–237. doi: 10.1007/s40641-018-0106-1
Stige, L. C., Stave, J., Chan, K.–S., Ciannelli, L., Pettorelli, N., Glantz, M., et al. (2006). The effect of climate variation on agro–pastoral production in Africa. Proc. Natl. Acad. Sci. U.S.A. 103, 3049–3053. doi: 10.1073/pnas.0600057103
Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M. M. B., Allen, S. K., Boschung, J., . (eds.). (2014). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. doi: 10.1017/CBO9781107415324
Storeheier, P. V., Mathiesen, S. D., Tyler, N. J. C., and Olsen, M. A. (2002). Nutritive value of terricolous lichens for reindeer in winter. Lichenologist 34, 247–257. doi: 10.1006/lich.2002.0394
Strand, O., Colman, J. E., Eftestøl, S., Sandström, P., Skarin, A., and Thomassen, J. (2017). Wind–Power and Reindeer – A Synthesis of Knowledge. NINA Report 1305. Trondheim: Norsk Institutt for Naturforskning, 62.
Strøm Bull, K. (2015). “Sami reindeer herders' herding rights in Norway from the nineteenth century to the present day,” in Indigenous Rights in Scandinavia. Autonomous Sami Law, eds C. Allard and S. F. Skogvang (Farnham: Ashgate Publishing Limited), 79–94. doi: 10.4324/9781315588353-7
Sundset, M. A., Barboza, P. S., Green, T. K., Folkow, L. P., Blix, A. S., and Mathiesen, S. D. (2010). Microbial degradation of usnic acid in the reindeer rumen. Naturwissenschaften 97, 273–278. doi: 10.1007/s00114-009-0639-1
Supreme Court of Norway (1981). Rt-1981-1215. Available online at: http://folk.uio.no/olavt/Dommer/hr/Rt-1981-1215.htm (accessed February 11, 2020).
Supreme Court of Norway (2017). The State Represented by the Ministry of Agriculture and Food vs. Jovsset Ante Iversen Sara. HR-2017-2428-A (Case No. 2017/981), Civil Case, Appeal Against Judgment. Available online at: https://www.domstol.no/globalassets/upload/hret/decisions-in-english-translation/hr-2017-2428-a.pdf (accessed June 3, 2020).
Thackeray, S. J., Henrys, P. A., Hemming, D., Bell, J. R., Botham, M. S., Burthe, S., et al. (2016). Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. doi: 10.1038/nature18608
Theobald, D. M., Miller, J. R., and Hobbs, N. T. (1997). Estimating the cumulative effects of development on wildlife habitat. Landsc. Urban Plan 39, 25–36. doi: 10.1016/S0169-2046(97)00041-8
Thing, H. (1977). Behavior, Mechanics and Energetics Associated with Winter Cratering by Caribou in Northwestern Alaska. Biological Papers of the University of Alaska No. 18. Fairbanks, AK, 41.
Thornton, P. K., van de Steeg, J., Notenbaert, A., and Herrero, M. (2009). The impacts of climate change on livestock and livestock systems in developing countries: a review of what we know and what we need to know. Agric. Syst. 101, 113–127. doi: 10.1016/j.agsy.2009.05.002
Timisjarvi, J., Nieminen, M., and Sippola, A.-L. (1984). The structure and insulation properties of the reindeer fur. Comp. Biochem. Physiol. 79, 601–609. doi: 10.1016/0300-9629(84)90456-0
Tolvanen, A., Eilu, P., Juutinen, A., Kangas, K., Kivinen, M., Markovaara-Koivisto, M., et al. (2019). Mining in the Arctic environment – a review from ecological, socioeconomic and legal perspectives. J. Environ. Manage. 233, 832–844. doi: 10.1016/j.jenvman.2018.11.124
Tømmervik, H., Bjerke, J. W., Park, T., Hanssen, F., and Myneni, R. B. (2019). Legacies of historical exploitation of natural resources are more important than summer warming for recent biomass increases in a Boreal–Arctic transition region. Ecosystems 22, 1512–1529. doi: 10.1007/s10021-019-00352-2
Tømmervik, H., Johansen, B., Riseth, J. Å., Karlsen, S. R., Solberg, B., and Høgda, K. A. (2009). Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, Northern Norway, in the period 1957-2006. For. Ecol. Manage. 257, 244–257. doi: 10.1016/j.foreco.2008.08.038
Tømmervik, H., Johansen, B., Tombre, I., Thannheiser, D., Høgda, K. A., Gaare, E., et al. (2004). Vegetation changes in the Nordic mountain birch forest, the influence of grazing and climate change. Arctic Antarctic Alpine Res. 36, 323–332. doi: 10.1657/1523-0430(2004)036[0323:VCITNM]2.0.CO;2
Tømmervik, H., and Riseth, J. Å. (2011). Historiske tamreintall i Norge fra 1800-tallet fram til i dag [Historical Number of Domesticated Reindeer in Norway From the 1800s Until Today]. NINA Rapport 672. Trondheim: Norsk Institutt for Naturforskning, 36.
Trombulak, S. C., and Frissell, C. A. (2000). Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30. doi: 10.1046/j.1523-1739.2000.99084.x
Troms County Municipality (2018). Regional plan for reindrift i Troms 2018–2030 [Regional plan for reindeer husbandry in Troms 2018–2030]. Tromsø: Troms fylkeskommune, 54.
Trudell, J., and White, R. G. (1981). The effect of forage structure and availability on food intake, biting rate, bite size and daily eating time of reindeer. J. Appl. Ecol. 18, 63–81. doi: 10.2307/2402479
Tumenta, P. N., de Iongh, H. H., Funston, P. J., and Udo de Haes, H. A. (2013). Livestock depredation and mitigation methods practised by resident and nomadic pastoralists around Waza National Park, Cameroon. Oryz 47, 237–242. doi: 10.1017/S0030605311001621
Turi, E. I., and Keskitalo, E. C. H. (2014). Governing reindeer husbandry in western Finnmark, barriers for incorporating traditional knowledge in local–level policy implementation. Polar Geogr. 37, 234–251. doi: 10.1080/1088937X.2014.953620
Turunen, M., and Vuojala–Magga, T. (2014). Past and present winter feeding of reindeer in Finland, herders' adaptive learning of feeding practices. Arctic 67, 173–188. doi: 10.14430/arctic4385
Turunen, M. T., Rasmus, S., Bavay, M., Ruosteenoja, K., and Heiskanen, J. (2016). Coping with difficult weather and snow conditions, reindeer herders' views on climate change impacts and coping strategies. Clim. Risk Manage. 11, 15–36. doi: 10.1016/j.crm.2016.01.002
Tveraa, T., Stien, A., Bårdsen, B.-J., and Fauchald, P. (2013). Population densities, vegetation green-up, and plant productivity, impacts on reproductive success and juvenile body mass in reindeer. PLoS ONE 8:e56450. doi: 10.1371/journal.pone.0056450
Tveraa, T., Stien, A., Brøseth, H., and Yoccoz, N. G. (2014). The role of predation and food limitation on claims for compensation, reindeer demography and population dynamics. J. Appl. Ecol. 51, 1264–1272. doi: 10.1111/1365-2664.12322
Tyler, N. J. C. (2010). Climate, snow, ice, crashes and declines in populations of reindeer and caribou. Ecol. Monogr. 80, 197–219. doi: 10.1890/09-1070.1
Tyler, N. J. C. (2019). “Adaptations to the Arctic environment,” in Svalbard Reindeer (Rangifer tarandus platyrhynchus) A Status Report. Norwegian Polar Institute Report Series 151, eds G. S. Jaklin and Å. Ø. Pedersen, 55, pp 10–15.
Tyler, N. J. C., Forchhammer, M. C., and Øritsland, N. A. (2008). Non–linear effects of climate and density in the dynamics of a fluctuating population of reindeer. Ecology 89, 1675–1686. doi: 10.1890/07-0416.1
Tyler, N. J. C., Stokkan, K.-A., Hogg, C. R., Nellemann, C., and Vistnes, A.-I. (2016). Cryptic impact, visual detection of corona light and avoidance of power lines by reindeer. Wildl. Soc. Bull. 40, 50–58. doi: 10.1002/wsb.620
Tyler, N. J. C., Turi, J. M., Sundset, M. A., Strøm Bull, K., Sara, M. N., Reinert, E., et al. (2007). Saami reindeer pastoralism under climate change, applying a generalised framework for vulnerability studies to a sub–Arctic social–ecological system. Glob. Environ. Change 17, 191–206. doi: 10.1016/j.gloenvcha.2006.06.001
Uboni, A., Horstkotte, T., Kaarlejärvi, E., Sévêque, A., Stammler, F., Olofsson, J., et al. (2016). Long–term trends and role of climate in the population dynamics of Eurasian reindeer. PLoS ONE 11:e0158359. doi: 10.1371/journal.pone.0158359
UNEP (2001). “GLOBIO—global methodology for mapping human impacts on the biosphere,” in United Nations Environment Programme, UNEP/DEWA/TR.01-3, eds C. Nellemann, L. Kullerud, I. Vistnes, B. C. Forbes, G. P. Kofinas, B. P. Kaltenborn, et al., 47. Available online at: www.globio.info
Valstad, O. (1989). “Omstridt tamreindrift [Controversial reindeer husbandry],” in Trondhjems Turistforening Årbok 1988, 51–60. Available online at: https://www.tfb.no/db/trondheimturistforening/arbok1988/1988%20SCAN0008.pdf
Van den Bossche, P., de La Rocque, S., Hendrickx, G., and Bouyer, J. (2010). A changing environment and the epidemiology of tsetse–transmitted livestock trypanosomiasis. Trends Parasitol. 26, 236–243. doi: 10.1016/j.pt.2010.02.010
Van der Wal, R., Madan, N., van Lieshout, S., Dormann, C., Langvatn, R., and Albon, S. D. (2000). Trading forage quality for quantity? Plant phenology and patch choice by Svalbard reindeer. Oecologia 123, 108–115. doi: 10.1007/s004420050995
Vanneste, T. (2017). Impact of climate change on alpine vegetation of mountain summits in Norway. Ecol. Res. 32, 579–593. doi: 10.1007/s11284-017-1472-1
Väre, H., Ohtonen, R., and Mikkola, K. (1996). The effect and extent of heavy grazing by reindeer in oligotrophic pine heaths in northeastern Fennoscandia. Ecography 19, 245–253. doi: 10.1111/j.1600-0587.1996.tb01251.x
Venier, L. A., Thompson, I. D., Fleming, R., Malcolm, J., Aubin, I., Trofymow, J. A., et al. (2014). Effects of natural resource development on the terrestrial biodiversity of Canadian boreal forests. Environ. Rev. 22, 457–490. doi: 10.1139/er-2013-0075
VerCauteren, K. C. (2003). The Deer Boom, Discussions on Population Growth and Range Expansion of the White–Tailed Deer. USDA National Wildlife Research Center – Staff Publications, 281. Available online at: https://digitalcommons.unl.edu/icwdm_usdanwrc/281
Vibe, C. (1967). Arctic animals in relation to climatic fluctuations. Meddelelser Grønland 170, 1–227.
Visser, M. E., Caro, S. P., van Oers, K., Schaper, S. V., and Helm, B. (2010). Phenology, seasonal timing and circannual rhythms, towards a unified framework. Philos. Trans. R. Soc. B 365, 3113–3127. doi: 10.1098/rstb.2010.0111
Vistnes, I., and Nellemann, C. (2001). Avoidance of cabins, roads, and power lines by reindeer during calving. J. Wildl. Manage. 65, 915–925. doi: 10.2307/3803040
Vistnes, I., and Nellemann, C. (2008). The matter of spatial and temporal scales, a review of reindeer and caribou response to human activity. Polar Biol. 31, 399–407. doi: 10.1007/s00300-007-0377-9
Vistnes, I., Nellemann, C., Jordhøy, P., and Støen, O.-G. (2008). Summer distribution of wild reindeer in relation to human activity and insect stress. Polar Biol. 31, 1307–1317. doi: 10.1007/s00300-008-0468-2
Vistnes, I., Nellemann, C., Jordhoy, P., and Strand, O. (2001). Wild reindeer: impacts of progressive infrastructure development on distribution and range use. Polar Biol. 24, 531–537. doi: 10.1007/s003000100253
Vistnes, I., Nellemann, C., Jordhoy, P., and Strand, O. (2004). Effects of infrastructure on migration and range use of wild reindeer. J. Wildl. Manage. 68, 101–108. doi: 10.2193/0022-541X(2004)068[0101:EOIOMA]2.0.CO;2
Vitebsky, P. (2005). Reindeer People. Living with Animals and Spirits in Siberia. London: HarperCollins Publishers, 464.
Vogt, Y. (2007). Finnmarksvidda ødelegges – Nedlegg reindriften! [Finnmarksvidda is being destroyed - Abolish reindeer husbandry!]. Available online at: https://www.apollon.uio.no/artikler/2007/reindrift.html (accessed March 9, 2020).
Vorren, Ø. (1962). Finnmarksamenes nomadisme 1 Kartmessig fremstilling av Finnmarksamenes flyttinger, driftsområder, bosteder og leirplasser m. m. i tida 1953–1957. Oslo: Universitetsforlaget.
Vuojala-Magga, T. (2012). “Adaptation of Sámi reindeer herding, EU regulation and climate change,” in Governing the Uncertain, Adaptation and Climate in Russia and Finland, ed M. Tennberg (Berlin; Heidelberg: Springer Science+Business Media BV), 101–122. doi: 10.1007/978-94-007-3843-0_6
Vuojala-Magga, T., Turunen, M., Ryyppö, T., and Tennberg, M. (2011). Resonance strategies of Sámi reindeer herders in northernmost Finland during climatically extreme years. Arctic 64, 227–241. doi: 10.14430/arctic4102
Walker, M. D., Wahren, C. H., Hollister, R. D., Henry, G. H. R., Ahlquist, L. E., Alatalo, J. M., et al. (2006). Plant community responses to experimental warming across the tundra biome. Proc. Natl. Acad. Sci. U.S.A. 103, 1342–1346. doi: 10.1073/pnas.0503198103
Wallace, J. M., Milne, J. S., and Aitken, R. P. (2010). Effect of weight and adiposity at conception and wide variations in gestational dietary intake on pregnancy outcome and early postnatal performance in young adolescent sheep. Biol. Reprod. 82, 320–330. doi: 10.1095/biolreprod.109.080069
Webb, G. K. (2018). Searching the internet to estimate deer population trends in the U.S.: California, and Connecticut. Issues Inform. Syst. 19, 163–173.
Weladji, R. B., and Holand, Ø. (2003). Global climate change and reindeer, effects of winter weather on the autumn weight and growth of calves. Oecologia 136, 317–323. doi: 10.1007/s00442-003-1257-9
White, R. G. (1983). Foraging patterns and their multiplier effects on productivity of northern ungulates. Oikos 40, 377–384. doi: 10.2307/3544310
Williamson, D., and Williamson, J. (1984). Botswana's fences and the depletion of Kalahari wildlife. Oryx 18, 218–222. doi: 10.1017/S0030605300019268
Wilson, E. (2003). Freedom and loss in a human landscape, multinational oil exploitation and the survival of reindeer herding in north–eastern Sakhalin, the Russian Far East. Sibirica 3, 21–47. doi: 10.1080/1361736032000168012
Wilson, R. R., Parrett, L. S., Joly, K., and Dau, J. (2016). Effects of roads on individual caribou movements during migration. Biol. Conserv. 195, 2–8. doi: 10.1016/j.biocon.2015.12.035
Winge, N. K. (2016). Konsekvensutredning i reindriftsområder [Impact assessment in reindeer husbandry areas]. Tidsskrift Eiendomsrett 12, 101–124. doi: 10.18261/issn.0809-9529-2016-02-02
WMO (2010). Guide to Agricultural Meteorological Practices. WMO–No. 134 (2012 update). Geneva: World Meteorological Organization.
Wolfe, S. A., Griffith, B., and Wolfe, C. A. G. (2000). Response of reindeer and caribou to human activities. Polar Res. 19, 63–73. doi: 10.1111/j.1751-8369.2000.tb00329.x
Woo, M.-K., Heron, R., and Marsh, P. (1982). Basal ice in high Arctic snowpacks. Arctic Alpine Res. 14, 251–260.
Wu, G., Bazer, F. W., Wallace, J. M., and Spencer, T. E. (2006). Intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84, 2316–2337. doi: 10.2527/jas.2006-156
Wu, Z., and Du, W. (2008). Pastoral nomad rights in Inner Mongolia. Nomadic Peoples 12, 13–33. doi: 10.3167/np.2008.120202
Xu, L., Myneni, R. B., Chapin, F. S. III, Callaghan, T. V., Pinzon, J. E., Tucker, C. J., et al. (2013). Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang 3, 581–586. doi: 10.1038/nclimate1836
Yan, W. B., Zeng, Z. G., Pan, D., Wang, T. J., Zhang, Q., Fu, Y. N., et al. (2013). Scale-dependent habitat selection by reintroduced Eld's deer (Cervus eldi) in a human-dominated landscape. Wildl. Res. 40, 217–227. doi: 10.1071/WR12131
Zhang, X., He, J., Zhang, J., Polaykov, I., Gerdes, R., Inoue, J., and Wu, P. (2013). Enhanced poleward moisture transport and amplified northern high-latitude wetting trend. Nat. Clim. Change 3, 47–51. doi: 10.1038/nclimate1631
Zhu, Z., Piao, S., Myneni, R. B., Huang, M., Zeng, Z., Canadell, J. G., et al. (2016). Greening of the earth and its drivers. Nat. Clim. Change 6, 791–795. doi: 10.1038/nclimate3004
Keywords: Arctic, climate change, encroachment, grazing rights, infrastructure, pastoralism, reindeer, Saami
Citation: Tyler NJC, Hanssen-Bauer I, Førland EJ and Nellemann C (2021) The Shrinking Resource Base of Pastoralism: Saami Reindeer Husbandry in a Climate of Change. Front. Sustain. Food Syst. 4:585685. doi: 10.3389/fsufs.2020.585685
Received: 21 July 2020; Accepted: 27 November 2020;
Published: 10 February 2021.
Edited by:
Pablo Gregorini, Lincoln University, New ZealandReviewed by:
Roy Behnke, University College London, United KingdomCopyright © 2021 Tyler, Hanssen-Bauer, Førland and Nellemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas J. C. Tyler, bmljaG9sYXMudHlsZXJAdWl0Lm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.