- 1School of Nutrition and Food Sciences, Louisiana State University, Baton Rouge, LA, United States
- 2Apis Group, Limited Liability Company, Colbert, GA, United States
- 3School of Animal Sciences, Louisiana State University, Baton Rouge, LA, United States
Shelf-stable, ready-to-eat shrimp offer a convenient, portion-controlled option for a highly desirable seafood commodity. The perishability of shrimp requires tight cold-temperature control during distribution and handling. Thermal processing of shrimp was evaluated in a multimode retort with automated control of come-up, heat, and cool times and with addition of high-speed reciprocal agitation. Reciprocal agitation was compared with static retort for thermal process and quality parameters, including cook time, process time to achieve target lethality (F0), cook yield, texture, and appearance of shelf-stable shrimp. Total retort thermal processing times decreased from 30 min at 0 shakes per minute (SPM) to 17.1 min at 180 SPM to achieve same F0. The time to reach targeted F0 value of 6 min decreased by 29, 37, and 42% for 45, 90, and 180 SPM, respectively, compared with static retort thermal processing. The shrimp blanch yield was about 90% and retort yield was 70–75% after retort thermal processing for all retort runs (0, 45, 90, and 180 SPM). Shear force texture was significantly higher at all agitation speeds compared with static retort processing with values of 384, 422, and 475 g-F with increasing agitation and 294 g-F at static conditions. Increase surface sloughing and sedimentation was observed at higher agitation speeds. Oxygen headspace was under 1% for containers flushed with nitrogen, and the rigid plastic containers used for retort packaging were not adversely affected by either static or by reciprocal agitation up to 180 SPM. Thermal processing with reciprocal agitation at 90 or 180 SPM achieves similar (p > 0.05) shear force. Reciprocal agitation at 90 SPM is recommended for improved retort efficiency and increased textural quality of retorted shrimp. A sustainable, high-quality canned shrimp that can be stored at ambient temperature can be produced by reciprocal agitation retorts.
Introduction
Global food waste is estimated at 33% or 1.3 billion tons per year and impacts the entire food system, with waste of land, water, and energy resources and preventable carbon dioxide emissions (FAO, 2015). Consumers in developed countries account for 28% of food waste, compared with 7% waste by consumers in developing countries (FAO, 2011a). In the USA, about 31% of the 430 billion pounds of food produced in 2010 was lost at the retail level, and the greatest loss was in the meat, poultry, and fish category at 30% (Buzby et al., 2014), which points to critical need for interventions to reduce waste of animal-based foods. To reduce food waste, multistep recommendations across food systems include greater transparency, innovations in food manufacturing and packaging that extend shelf life, as well as intervention at the retail level with development of foods that meet consumer needs (www.FoodWasteAlliance.org; www.fao.org; www.ReFed.org).
Fisheries account for 17% of animal protein intake, and per capita consumption is over 20 kg fish. World fishery and aquaculture supplies are expected to increase to 200 million tons by 2030, pushing the need for sustainable production, processing, and distribution strategies (FAO, 2011b). Farmed shrimp operations in Asia provide a global shrimp supply, and China, India, and Viet Nam are the top 3 largest shrimp producers in the world (FAO, 2019a). Shrimp and prawns are in the top 3 imports in the USA (National Fisheries Institute (NFI), 2018). The harvest of brown shrimp (Farfantepenaeus aztecus) from the Gulf of Mexico was about 98.3 million kilograms and in Louisiana, the harvest was 42.1 million kilograms, valued at $137.5 million (Louisiana Agriculture, 2017; National Marine Fisheries Service, 2018). The frozen and preserved values of shrimp are estimated at $5.2 and $1.3 billion, respectively (FAO, 2019b).
Innovations in processing and packaging of foods, especially animal-based foods, that retain high levels of nutrients and other quality attributes, with long shelf life, contributes to a balance between food security and food sustainability. Reliable and efficient processing improves operational efficiency within the food supply chain and reduces food waste (FAO, 2015). Chilled and frozen food products dominate the world market but require tight control of the cold distribution chain for food safety, quality, and shelf life. Preserved foods that do not rely on cold preservation offer the opportunity for lower energy costs during distribution and storage, more food security, and greater consumer convenience.
Thermal processing represents a significant proportion of the processed foods industry, allows extended shelf life of 1–5 years and energy savings associated with ambient temperature distribution and storage (Awuah et al., 2007). Shelf-stable foods are typically processed in cans, glass, flexible pouches, or rigid plastic, hermetically sealed, and processed with high temperature and pressure for a specified time to ensure destruction of target pathogens. Once processed, food safety risks associated with temperature fluctuations during storage are minimal for shelf-stable foods.
The global canned fisheries and seafood market, including tuna, salmon, sardines, shrimp, prawns, and other fisheries, were $21.5 billion in 2016 and expected to grow due to greater interest in ready-to-eat foods and health benefits (www.grandviewresearch.com). The market size of the US canned food industry was estimated at $16.35 billion in 2017; sustainable farming practices, ease of cooking, and increased shelf life contribute to the expected growth to $22.23 billion by 2025 (www.grandviewresearch.com).
Shelf-stable foods are historically a staple in food service and after natural disasters and recognition of the convenience factor of in-container thermally processed foods is accelerating. At the food service level, availability of shelf-stable, ready-to-eat shrimp would improve on demand response in back-of-the-house operations, avoid waste associated with overestimation of need and excess thawed shrimp or underestimation and need to rapidly thaw material. The development of ready-to-eat meals in single-served, portion-controlled, and calorie-controlled packages are popular. With the availability of high-quality, microwaveable, shelf-stable meal kits, meal planning, and preparation time are greatly reduced.
A severe limitation of any thermally processed food is the loss of quality attributes, such as texture, color, and flavor and nutritive value. Improved heat penetration compared with static processing was observed with oscillation at a 15° angle with a 15 s hold (MacNaughton et al., 2018). The localized overprocessing at the package surface, high-energy costs, high water usage, and thermal process deviations have increased efforts for process and equipment optimization (Banga et al., 2003) and use of zero, first, biphasic, and Weibull mathematical models of quality to achieve food safety with minimal quality loss (Ling et al., 2015).
Reciprocal agitating retorts are a promising thermal process technology, which reduces process times, results in energy savings, and improves product quality (Singh and Ramaswamy, 2015). Reciprocal agitation in thermal processing is capable of extremely high longitudinal frequencies that rapidly shake the package back and forth; product movement in the package is much higher than observed with rotary or oscillating retorts (Walden, 2008). The package orientation and potential advantages of reciprocal agitation, vs. axial rotation, oscillation, or end-over-end agitation was described (Singh et al., 2018). For liquid foods or purees, reciprocal agitation increases heat penetration, reduces quality loss, and uses less energy (Walden, 2008). In tomato puree, a 63–81% reduction in process time with an improved retention of antioxidant activity, nutritive content, and color was obtained at 2 Hz (Singh and Ramaswamy, 2015). However, high-frequency reciprocal agitation severely disrupted the texture of potato and radish cubes in brine (You et al., 2016), and reduced frequency was needed to minimize integrity loss, leaching, and quality loss in green beans (Singh et al., 2016). Heat penetration of solid foods dispersed in fluid, heat slower than fluids or purees. Canned shrimp are expected to grow with compounded annual growth rate of 4.1% from 2017 to 2025, attributed to high nutritional value, easily digestible protein, and use of shrimp in foodservice. Increasing quality of particulate muscle seafood, such as shrimp, would be advantageous. In this study, the objectives were to compare heat penetration and process times of reciprocal agitation and static retort thermal processing of brown shrimp packed in water. The cook yield and texture of brown shrimp were determined to assess quality of shrimp. In addition, shrimp were packaged in an oxygen barrier rigid plastic containers.
Materials and Methods
Shrimp and Container Preparation
Louisiana Gulf Coast (LGC) brown shrimp (Farfantepenaeus azecus), which were phosphate treated, beheaded, peeled, and individually quick frozen in 2 kg bags, were obtained from Gulf Crown Seafood (Delcambre, LA, USA). Prior to using, shrimp were thawed for 3 days at 4°C. Post-thaw, raw shrimp (7–9 shrimp/100 g) were drained and raw shrimp weight was measured before blanching. The shrimp were blanched in boiling water for 3 min at a ratio of 1:2, shrimp:water. Blanched shrimp were drained of excess water and immediately submerged in an ice slurry for 3 min. Excess water from ice slurry was drained and blanched shrimp weight was measured to determine blanch shrimp yield. Sufficient shrimp were blanched to process two probed and two non-probed rigid plastic containers, each at 0, 45, 90, and 180 SPM in retort. Shrimp were thermally processed in quadruplicate.
The retort packaging was polypropylene injection in-mold-label (IML) oxygen barrier rigid plastic containers with a permeation rate of <0.5 cc/m2/day (Sonoco, Hartsville, South Carolina USA). Each package contained 10 blanched shrimp and water was added to adjust the final weight to 150 g and headspace of ~0.85 cm:
Retort and total yield was calculated post-retort thermal processing. Retort yield represents the amount of shrimp shrinkage from retort processing, and total yield represents the combined yield after blanching and retort processing. Only non-probed shrimp were used to make quality assessments.
Probed containers were hole punched at the geometric center with a C-12 lever-type can punch to insert 3.81 cm CNS needle thermocouples. For each probed container, one shrimp was attached in a particulate cage and lodged onto thermocouple to secure the needle thermocouple in shrimp at the center of the largest segment of the largest shrimp. Copper-constantan wires were used to connect the thermocouples to data logger. Thermocouple parts were purchased from Ecklund-Harrison Technologies Inc. (Fort Myers, FL USA).
Retort containers were sealed with a semiautomatic Control GMC (CGMC) PL200G (Boucherville, QC CA) with Bemis retort-grade L7288 film (Neenah, WI USA). The seal duration was set to 1.5 s (150 × 0.01 s). The nitrogen gas flush was set to 1 s (100 × 0.01 s) with a gas pressure of 207 kPa and a film length of 10.2 cm. Nitrogen gas flush was used on half of the 64 rigid plastic containers and the other half did not have a nitrogen gas flush.
Retort Thermal Processing Settings
All samples were processed with a multimode Allpax R&D Retort 2402 Series with Shaka® technology (Covington, LA USA) in water spray-mode under static (0 SPM) and reciprocal agitation speeds of 45, 90, and 180 SPM. TechniCAL CALSoft 5 software and CALPLex data logger (Metairie, LA USA) were used to track heat penetration (time and temperature data throughout retort processing) and F values (time in minutes required under specified conditions for a given food to destroy a known population of microorganisms, which is based on a reference temperature of 121.1°C and z = 10°C in current study) to determine lethality with the general and Ball's formula method (Goff, 2020).
The time to reach F0 of 6.0 min was set to ensure commercial sterility as described in the literature and provided a control to analyze the effectiveness of each reciprocal agitation speed. Thermal process variables were based on averaged heat penetration data from four probed containers at each of the four shake speeds. Come-up was set to 10 min. Pressure and atmospheric cooling were set to 10 min each. The overpressure was set to 241 kPa during cook and ramped to atmospheric pressure during cooling. Only cook time to target F0 of 6.0 and reciprocal agitation speeds were varied in the scheduled process.
Due to Ball's formula flexibility, Ball's formula parameters were compared for the target F0 of 6.0, obtained from the literature, with an initial product temperature of 4°C and retort temperature of 121.1°C, to a target F0 at 3.5 min after review of the NFPA guidelines recommending these conditions (National Food Processors Association, 1982). For Ball's formula method, the slowest heat penetration data of the probed samples, at each agitation speed was used to determine Ball's formula method variables and cook times (BB: Ball's cook time and Pt [BB – (0.42*CUT)]: Ball's cook time with 42% come-up time credit) (Ramaswamy, 1993) using CalSoft5 “evaluate data/set a process” feature. Broken-line heating was used to improve fit of heat penetration data on semilogarithmic plot constructed in CalSoft5 software. Ball's formula method invariables include jh, f h, f 2, xbh, and f c, where jh is the heating rate lag factor; f h is the first slope heating rate index and f 2 is the second slope heating rate index of the semilogarithmic plotted heat penetration curve; xbh is time difference between the change in heating rate and corrected zero start time; f c is the heating rate index during the cooling phase of processing. The cooling lag factor (jc) was held constant at 1.41 (Stoforos, 2010; Singh et al., 2016). The critical factors included a maximum product fill weight of 150 g, a horizontal orientation along axis of reciprocation of retort rigid plastic containers, and thermocouple position at geometric center of container and largest shrimp in container.
Texture Analysis
Shear force of shrimp was determined using a Warner-Bratzler shear 3 mm attachment blade (Stable Micro Systems TA.HD Plus texture analyzer, (TA) and Exponent software, Godalming, Surrey UK). Because the cooked shrimp had a curvature, the tail end of shrimp was trimmed to facilitate consistent placement of the second segment of the shrimp between Warner-Bratzler “V cut” slot and to avoid interference of last segments near the tail. Shrimp were placed on the texture analyzer mounting plate with head facing front and shrimp curving to the right. The texture analyzer settings were test mode, compression; pre-test speed, 4.00 mm/s; test speed, 4.00 mm/s; post-test speed, 10.00 mm/s; distance, 13.500 mm; and trigger force, 10.0 g. Texture of shrimp was analyzed on packages with and without nitrogen flush.
Controls From Commercial Sources
Blanched LGC shrimp blanched on day of retorting was one control. Bumblebee's tiny canned shrimp (more than 65 shrimp/100 g), containing salt, sugar, citric acid, sodium acid, pyrophosphate, calcium disodium EDTA, and sodium metabisulfite, was a second control. Bumblebee's medium canned shrimp (20–34 shrimp/100 g), containing salt, sugar, citric acid, sodium acid, pyrophosphate, calcium disodium EDTA, and sodium metabisulfite was a third control. The shrimp count for the controlled canned products were based on FAO guidelines for tiny and medium canned shrimp (FAO, 2013). A fourth control included Fisherman's Wharf ready-to-eat (RTE) cooked, frozen shrimp (5–7 shrimp/100 g). The RTE product contained salt and sodium tripolyphosphate. A fifth control was retail shrimp (5–7 shrimp/100 g), which was shrimp that was previously frozen and thawed before sale. For phosphate and other mineral analysis, individually quick frozen (IQF), no phosphate-treated Louisiana Gulf Coast brown shrimp (LGCnp, 7–9 shrimp/100 g), was also included.
Oxygen and Carbon Dioxide Headspace Analysis
Residual oxygen and carbon dioxide in headspace of retort containers were measured using a 5 s draw through a rubber septum (Systech Illinois GS6600 O2 and CO2, Illinois, IL USA). Containers were tilted to avoid solution contamination of needle.
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) Mineral Analysis
The LSU Agricultural Chemistry lab was used to determine phosphorous (P) and other minerals in retorted shrimp and controls. Shrimp tails were removed, prior to analysis. Shrimp was freeze dried and ground (6875D Freezer/Mill® Dual Chamber Cryogenic Grinder, Metuchen, NJ USA) in a pre-cooled freezer/mill in three grinding cycles of 2 min each and a 1 min cool time at a rate of 10 cycles/s. For the canned shrimp (Bumblebee's tiny and medium canned shrimp) extraction, shrimp were drained and followed the same procedure (6875D Freezer/Mill operating manual for fish/seafood). Post-extraction, approximately 0.5 g of sample was placed in a Teflon microwave tube; 8 ml of nitric acid and 2 ml of hydrochloric acid were added. Sample was microwaved (CEM Mars 230 Microwave Digestor, Matthews, North Carolina USA) under a 20 min ramp up to 200°C and held for 20 min. A Perkin Elmer 8300 ICP-OES analyzer (Waltham, MA USA) was used to determine mineral content.
Statistical Analysis
Mean, standard deviation, and coefficient of variation were computed; ANOVA analyses was conducted with Tukey HSD post-hoc test for measuring agitation significance with cook yield (N = 32) and texture analysis (N = 32) using JMP statistical software.
Results and Discussion
The general method determines the lethality from the time/temperature profile for a specific food under the conditions utilized during the study. The Ball's formula method calculation of lethality allows extrapolation of heat penetration data from direct heat penetration data calculations. The method allows more flexibility in adjusting a scheduled process when retort temperature or initial product temperature are adjusted.
Reciprocal Agitation, Processing Time, and F0 – General Formula Method
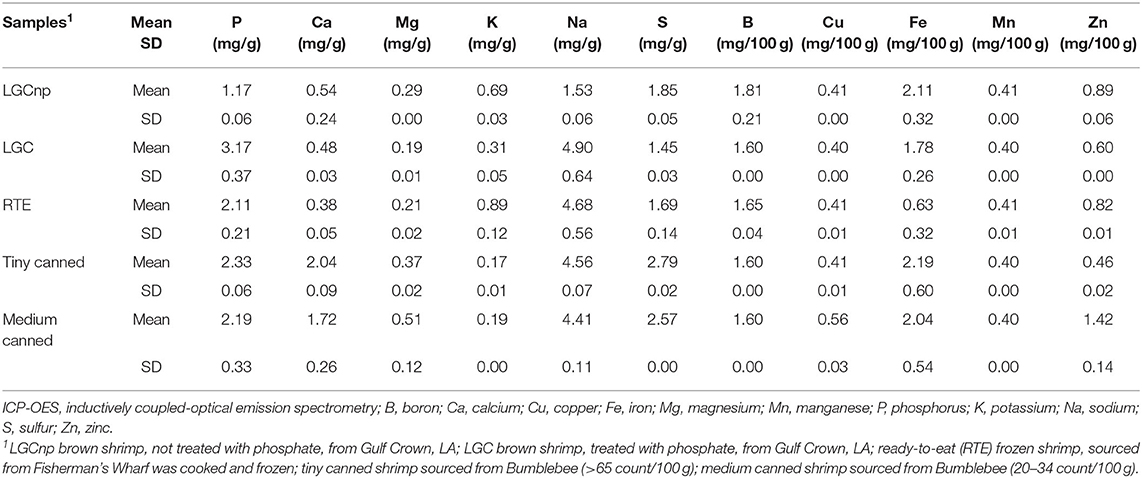
Average heat penetration curves, average retort temperature, and average F0 values are depicted at agitation speeds of 0, 45, 90, and 180 SPM (Figures 1, 2, 3, 4). The heat penetration curves truncate to shorter processing times as agitation increased from 0 to 180 SPM. Under static conditions, the retort temperature and shrimp heat penetration crossover after 25 min process time (Figure 1). Under 45 SPM, the time of crossover was >15 min (Figure 2); at 90 and 180 SPM (Figures 3, 4), the times of crossover were <15 min. As agitation increased, heat penetration curves and retort temperature were more superimposable. At 180 SPM, temperature of probed shrimp was nearly the same temperature as the free retort lead.
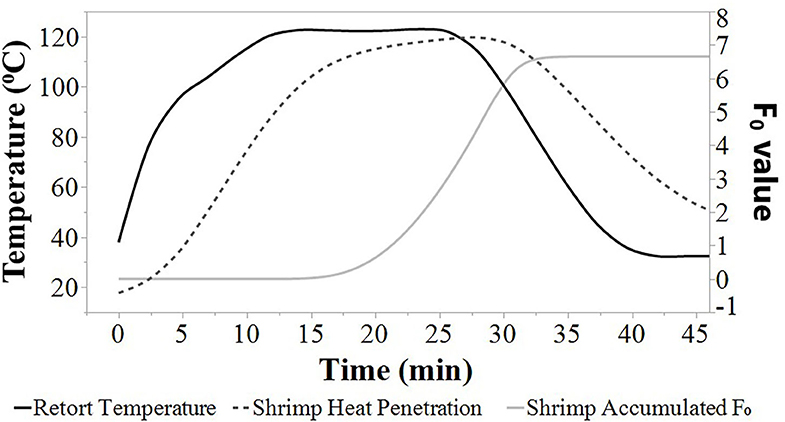
Figure 1. Average retort temperatures (solid black), heat penetration curves (dotted black), and accumulated F0 (solid gray) values for Louisiana Gulf Coast (LGC) brown shrimp during retort thermal processing at 0 SPM. JMP smoother function was used to construct all figure lines.
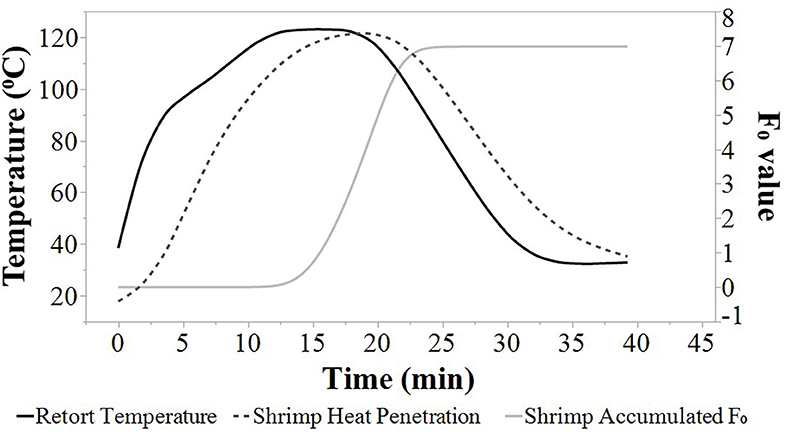
Figure 2. Average retort temperatures (solid black), heat penetration curves (dotted black), and accumulated F0 (solid gray) values for Louisiana Gulf Coast (LGC) brown shrimp during retort thermal processing at 45 SPM. JMP smoother function was used to construct all figure lines.
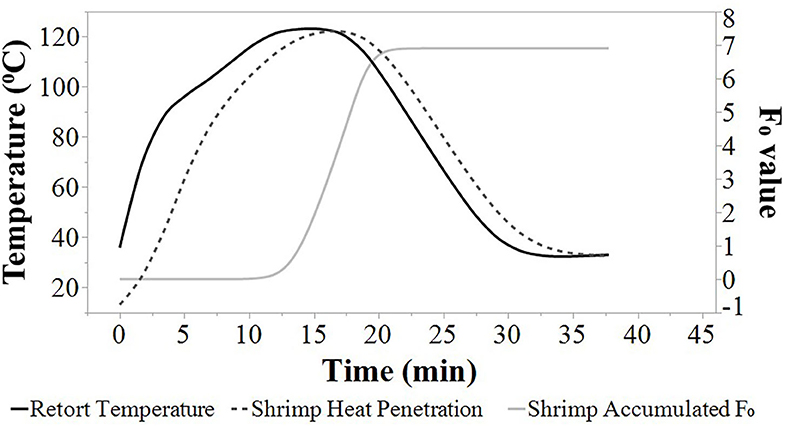
Figure 3. Average retort temperatures (solid black), heat penetration curves (dotted black), and accumulated F0 (solid gray) values for Louisiana Gulf Coast (LGC) brown shrimp during retort thermal processing at 90 SPM. JMP smoother function was used to construct all figure lines.

Figure 4. Average retort temperatures (solid black), heat penetration curves (dotted black), and accumulated F0 (solid gray) values for Louisiana Gulf Coast (LGC) brown shrimp during retort thermal processing at 180 SPM. JMP smoother function was used to construct all figure lines.
Additionally, the accumulated F0 value increased at a higher rate as the agitation speed increased from 0 to 180 SPM (Figures 1, 2, 3, 4). The time to achieve an F0 value of 6.0 was about 60% shorter at 180 SPM than static (Figure 1). The times to achieve F0 values of 6.0 decreased non-linearly from 30.0, 21.3, 19.0 to 17.3 min at 0, 45, 90, and 180 SPM, respectively (Table 1).
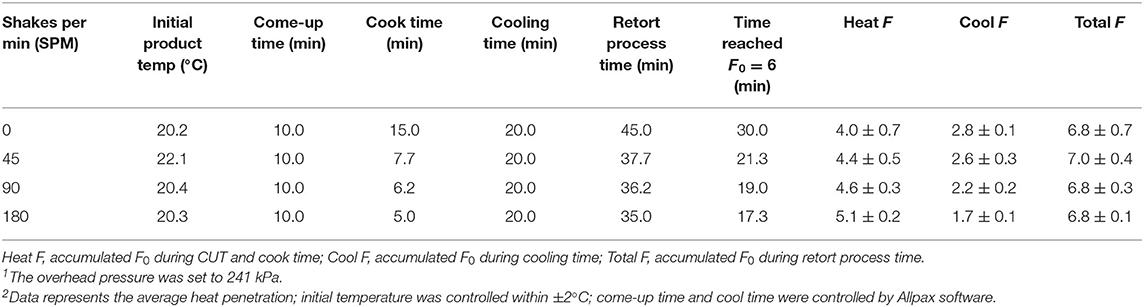
Table 1. Retort settings and accumulated F0 values at 0, 45, 90, and 180 SPM of retorted Louisiana Gulf Coast (LGC) brown shrimp using the general formula method1,2.
Times associated with the CUT, cook time, cooling time, and total retort process time for each agitation speed are presented in Table 1. The CUT was set to 10 min. Pressure and atmospheric cooling was set to 10 min each totaling to a cooling time of 20 min to ensure that final product temperature was <40°C after cooling. The cook time decreased from 15.0, 7.7, 6.2 to 5.0 min, as agitation increased from 0, 45, 90 to 180 SPM, respectively. The accumulated F0 achieved during heating and cooling as well as the total F0 during the process are summarized (Table 1). Note that at higher agitation speeds, lethality was achieved primarily during cook with heat F0 ranging between 63 and 75% of total F0 for reciprocal agitation runs and 58% for static runs. At lower agitation speeds, a higher proportion of lethality of 42% was achieved during cooling for static runs, compared with the 25 and 37% range for reciprocal agitation runs. Total lethality was achieved 1.4 to 1.7 times faster with reciprocal agitation compared with static.
Similar decreases in time to achieve lethality was reported for béchamel sauce with sterilization methods that included static, rotary, and reciprocal agitation; reciprocal agitation achieved a target F0 of 6 at 90 and 96% quicker than rotary and static, respectively (Walden and Emanuel, 2010). Improvements in process time were also observed in a canned tomato puree processed in a vertical steam reciprocating retort. The process time decreased from 43.4 min under static conditions to 8.1 min at 3 Hz, which is equivalent to 180 SPM (Singh et al., 2017). Overall, reciprocal agitation technology provided shorter process time than static retorts at all agitation speeds for process time and achieving target F0 value.
Ball's Formula Method
The Ball's formula was implemented to determine heat penetration parameters with slowest heat penetration data and to compare Ball's parameters at F0 values of 6.0 and 3.5. An F0 of 3.5 was selected for further analysis based on referenced literature provided by a thermal process authority that provided a lethality F0 value of 3.5 (National Food Processors Association, 1982). The cook times (BB) were 25.7, 16.3, 13.6, and 11.8 min for a F0 value of 6.0 at 0, 45, 90, and 180 SPM, respectively; cook times were 22.0, 13.5, 11.0, and 9.2 min for a F0 value of 3.5 at 0, 45, 90, and 180 SPM, respectively (Table 2). All Ball's formula factors decreased as agitation increased, except for Ball's heating factor (f h), which increased from 45 to 90 SPM and decreased from 90 to 180 SPM; however, 180 SPM f h was higher than 45 SPM. The shorter CUT between 45 SPM compared with 90 and 180 SPM almost certainly influenced the results by increasing the heating rate index to reach retort temperature. The total F0 for all replications ranged from 6.28 to 7.93 min. The jh and f h factors for all replications showed similar trends as the slowest heating probe. The jh decreased with agitation at 0, 45, 90, and 180 SPM and mean values were 0.81 ± 0.10, 0.49 ± 0.05, 0.38 ± 0.03, and 0.29 ± 0.01, respectively, indicating a favorable change in heating rate lag factor. At the same agitation speeds, the f h factors were 11.84 ± 0.38, 11.21 ± 0.58, 10.02 ± 0.38, 9.42 ± 0.38, respectively. Both BB and Pt process times decreased with agitation. The BB and Pt values were 20.53 ± 0.69 and 15.70 ± 0.69, respectively, at 0 SPM and decreased to 11.69 ± 0.12 and 686 ± 0.12, respectively at 180 SPM.
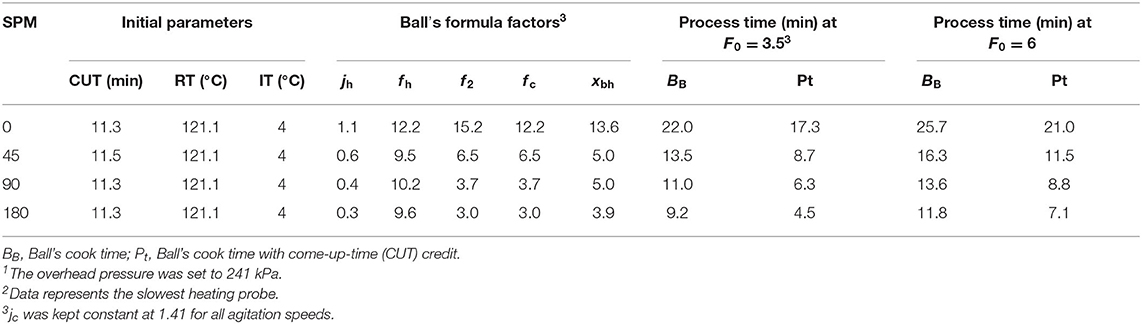
Table 2. Ball's formula method parameters, factors, and cook time using slowest heat penetration data for 0, 45, 90, and 180 SPM of retorted Louisiana Gulf Coast (LGC) brown shrimp1,2.
Additionally, applying Ball's formula method (Table 2) showed a similar trend as the general formula method (Table 1) of decreased cook time as reciprocal agitation increased. Compared with Table 1 cook times, the Ball's formula method had higher cook times (BB) for all agitation speeds at a F0 value of 6.0 and 3.5. When come-up-time credit was considered during cook (Pt), only three of the four agitation speeds had higher general method cook times than the Ball's formula method at a F0 value of 3.5. Overall, the processor could reduce the cook time by 2 to 4 min to achieve an F0 of 3.5; the reduced time at retort temperature can have a significant impact on improving product quality (Stoforos, 2010; Erdogdu et al., 2016; Singh et al., 2017, 2018).
Blanched and Retort Shrimp Yield
Shrimp yield was evaluated after blanching and after retorting and total yield was reported (Figure 5). The average blanch yield was 90% for blanch batches. The retort yield averaged 73% for all agitation speeds and was not different (p > 0.05) for different reciprocal agitation speeds. The average total yield of 63% was also not different (p > 0.05). In comparison, yield of white shrimp after cooking in boiling water ranged between 70 and 85% (Erdogdu et al., 2004), 77 and 90% (Niamnuy et al., 2007), and 59 and 87% (Carneiro et al., 2013). The LGC brown shrimp blanch yield was within the range of earlier studies.
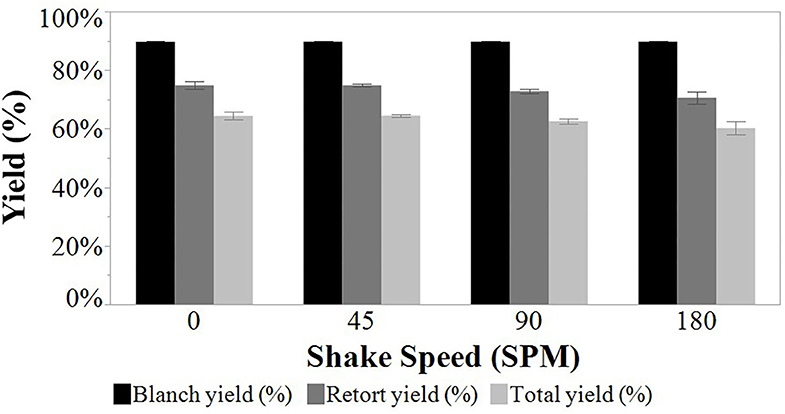
Figure 5. Yield measured by weight relative to starting material. Louisiana Gulf Coast (LGC) brown shrimp used in the study. Blanched (LGC shrimp yield post-blanching raw LGC shrimp), retort (LGC shrimp yield post-retort processing blanch LGC shrimp), and total (LGC shrimp yield from blanch and retort shrinkage combined) yield of all non-probed LGC brown shrimp at 0, 45, 90, and 180 SPM.
In post-retort processed shrimp in the rigid plastic package, intact pieces of shrimp were observed, with some evidence of increasing surface sloughing with reciprocal agitation (Figures 6, 7). Marked differences were observed in shrimp that were drained (Figure 8); shrimp size decreased from blanched, non-retorted shrimp, to retorted shrimp with increased reciprocal agitation. Additionally, the amount of sedimentation increased with reciprocal agitation speeds from 0 to 180 SPM (Figure 9). Overall, the information collected provided additional visual confirmation of the impact of agitation speed and yield.

Figure 6. Post-retort thermally processed Louisiana Gulf Coast (LGC) brown shrimp in retort containers at 0 and 45 SPM.

Figure 7. Post-retort thermally processed Louisiana Gulf Coast (LGC) brown shrimp in retort containers at 90 and 180 SPM.

Figure 8. Unprocessed, blanched Louisiana Gulf Coast (LGC) brown shrimp (control 1) compared with retort thermally processed LGC brown shrimp at 0, 45, 90, and 180 SPM.
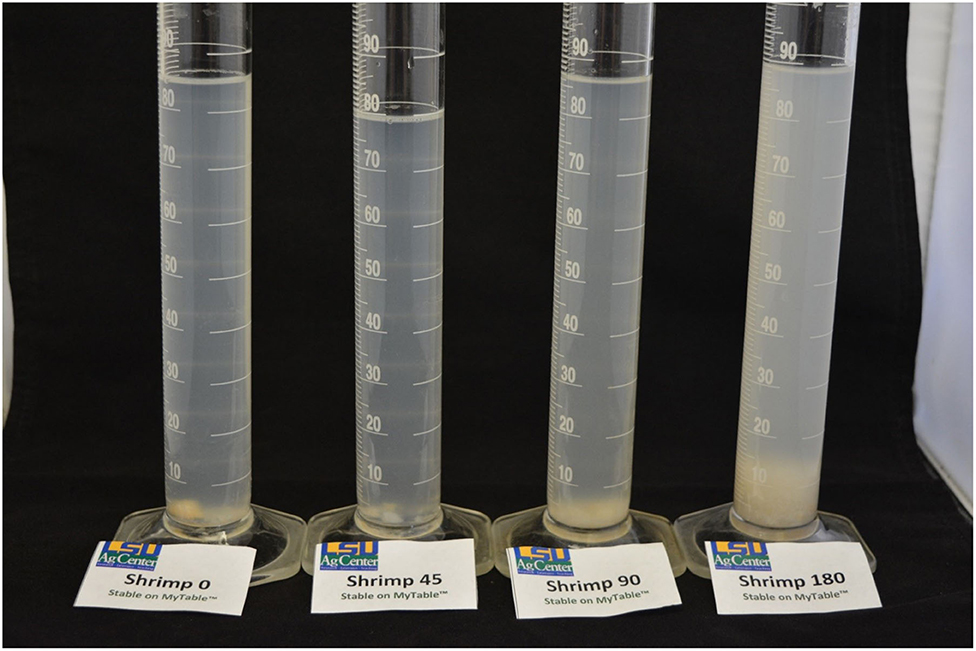
Figure 9. Louisiana Gulf Coast (LGC) brown shrimp filtrate and sediment, retort thermally processed at 0, 45, 90, and 180 SPM.
Texture Analysis
The shear force values of commercially available canned or frozen shrimp and shrimp evaluated in this study are presented in Table 3. The shear force of shrimp processed at four agitation speeds ranged between 294 and 475 g-F and increased with increased agitation (Table 3). Shear force was significantly (p < 0.05) higher from shrimp processed at 0 or 45 SPM and shrimp processed at 45 or 180 SPM. There was no difference (p > 0.05) in shrimp processed at 90 and 180 SPM. Shear force of shrimp processed under any agitation speed was significantly higher than no agitation. Although slightly smaller shrimp size, more pieces, and more sedimentation were observed with higher agitation speeds, the data showed that 90 SPM agitation speed achieve increased shear force and minimal changes in shrimp appearance.
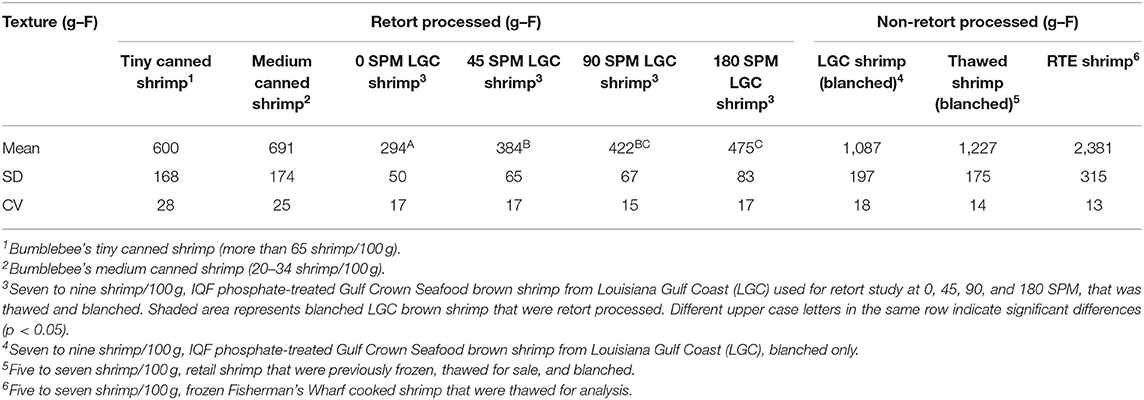
Table 3. Firmness of commercial shrimp and in-house retort-processed Louisiana Gulf Coast (LGC) brown shrimp.
Shear force is a common textural analysis method used for determining firmness that is noted in many studies (Sreenath et al., 2008; Mallick et al., 2010; Majumdar et al., 2017);(Shah et al., 2017). In retorted shrimp, a shear force range between 1,000 and 1,300 g-F was observed when processing shrimp in pouches in a static retort at 6, 8, and 9 min F0 values (Majumdar et al., 2017). Lower values between 700 and 900 g-F were reported when shrimp were processed in pouches with an autoclave at 5, 7, and 9 min F0 values (Mallick et al., 2010). Additionally, between 510 and 663 g-F was reported in shrimp processed in steel cans in a static retort at 6, 7, and 8 min F0 values (Sreenath et al., 2008). Although absolute values of firmness varied, lower F0 values resulted in higher shear force in all studies. In the current study, shear force was lower than reported in the literature results. Variation in the shear force across studies likely results from variables such as equipment used, target F0, type, size, and post-harvest age of shrimp, pre-treatment of shrimp, such as blanching, presence and concentration of phosphate, sulfites, or other ingredients (Byun et al., 2010; Hassan and Ramaswamy, 2013).
Shear force of commercially available shrimp products are reported (Table 3). As a blanched only, before retort control, the shear force of LGC shrimp was 1,087 g-F, about four times firmer than shrimp processed in static retort and about twice as firm as shrimp processed with reciprocal agitation at 180 SPM (Table 3). Thawed, blanched shrimp obtained from retail stores were similar in shear force at 1,227 g-F as blanched LGC shrimp control, 1,087 g-F. A fully cooked, ready-to-eat thawed shrimp had a shear force value of 2,381 g-F, which is more than double the value of the blanched, LGC shrimp control. The difference cannot be attributed to phosphate content as phosphate levels are higher in LGC shrimp than RTE shrimp (Table 4). It may be that toughening resulted from supply chain handling, multiple freeze/thaw cycles prior to cooking, refreezing, and distribution of this fully cooked product. Shear force of commercial, tiny and medium size, retorted shrimp was 600 and 691 g-F, respectively (Table 3). The commercial canned shrimp were smaller with a shrimp count between 20 and more than 65 shrimp/100 g, compared with seven to nine shrimp count/100 g for LGC shrimp. By qualitative visual and tactile assessment, the commercial retort shrimp showed obvious disintegration and failed in handling for sample analysis. In addition, the commercial retort shrimp and the ready-to-eat, cooked shrimp had an extensive ingredients list that may have contributed to the texture values. While the comparisons with commercial products cannot be used directly, the data clearly shows the benefit of reciprocal agitation to texture in this study.
Headspace Analysis
The headspace was ~0.85 cm for all containers. Headspace is a crucial critical factor in reciprocal agitating retorts in regard to heat penetration, especially at high agitation speeds (Singh and Ramaswamy, 2015). The oxygen content was 80 ± 13.2 mg/g and 4.7 ± 2.7 mg/g for control, no nitrogen flush, and nitrogen flush, respectively. Carbon dioxide was 3.6 ± 1.2 mg/g and 5.5 ± 0.9 mg/g for control, no nitrogen flush, and nitrogen flush, respectively. A target of <10 mg/g oxygen is desired in retort foods. High oxygen levels negatively impacts food quality making it crucial to control during packaging (Bonilla et al., 2012).
Mineral Analysis
Mineral analysis (B, Ca, Cu, Fe, Mg, Mn, P, K, Na, S, and Zn) of five types of shrimp evaluated in this study is shown in Table 4. The amount of phosphorous in LGCnp and LGC brown shrimp with phosphate (LGC) was essential data to estimate phosphate levels of the LGC brown shrimp used for this study. LGC brown shrimp were treated with a phosphate brine during chilling on ship at sea. The type, concentration, solubility, and time of treatment with phosphate were not known. With access to LGC and LGCnp brown shrimp, the phosphorous levels were determined. LGCnp had 1.17 mg/g compared with LGC of 3.17 mg/g, respectively. Although there can be a wide variety of natural occurring phosphorus in shrimp (Heitkemper et al., 1993), the mineral analysis confirmed that phosphate treatment resulted in a measurable increase in phosphorus content in the LGC brown shrimp compared with LGCnp brown shrimp.
Additionally, the amount of phosphorous in tiny and medium canned shrimp was 2.33 and 2.19 mg/g, respectively. The RTE shrimp had 2.11 mg/g. The mineral profiles were similar except that calcium was higher in tiny and medium canned shrimp; higher calcium may result in increased shear force. Zinc is higher in medium canned shrimp compared with other products.
Conclusions
Real-time heat penetration data for shrimp in water to a target F0 value of 6.0 min with and without reciprocal agitation was developed. In conclusion, reciprocal agitation thermal processing of canned shrimp resulted in superior product compared with static retort processing, improved process parameters, and potential energy savings. The time to reach the targeted F0 value of 6 was achieved 42% faster at 180 SPM than 0 SPM. In addition, firmness of retort processed shrimp increased by 62% when processed at 180 SPM, compared with static retort thermal processing. Yield of retort processed shrimp remained high at 73%. Integrity remained high with minimal, visible damage, and sloughing not impacting yield. Subjective color and overall appearance indicated that the shrimp processed with reciprocal agitation were superior to existing commercial, canned shrimp and comparable with frozen thawed products. Since firmness of shrimp processed at 90 or 180 SPM were similar, reciprocal agitation at 90 SPM is recommended to achieve high retort efficiency and to provide a high-quality, canned shrimp that can be distributed and stored at ambient temperature. Additionally, the polypropylene injection IML oxygen barrier rigid plastic containers were not adversely affected by either static or reciprocal agitation thermal processing. Thermal processing of shrimp with reciprocal agitation offers an alternative preservation method to cold chain distribution, to provide high-quality, convenient, long-shelf, ready-to-eat shrimp. Future work should evaluate the sensory and analytical flavor profile, and other quality changes determined by an extended shelf life study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
WD: methodology, data curation, statistical analysis, original draft preparation, and reviewing. EW: methodology, conceptualization, and reviewing. XF: conceptualization, methodology, and reviewing. JK: conceptualization, reviewing, editing, and funding. LW: conceptualization, writing, reviewing, editing, funding, and supervision. All authors contributed to the article and approved the submitted version.
Funding
The authors greatly acknowledged the partial funding and contributions of LSU AgCenter Hatch Project No. LAB94334 and Board of Regents Project No. 037 ENH-19.
Conflict of Interest
JK was employed by company Apis Group, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Awuah, G. B., Ramaswamy, H. S., and Economides, A. (2007). Thermal processing and quality: principles and overview. Chem. Eng. Process. 46, 584–602. doi: 10.1016/j.cep.2006.08.004
Banga, J. R., Balsa-Canto, E., Moles, C. G., and Alonso, A. A. (2003). Improving food processing using modern optimization methods. Trends Food Sci. Technol. 14:13. doi: 10.1016/S0924-2244(03)00048-7
Bonilla, J., Atarés, L., Vargas, M., and Chiralt, A. (2012). Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. J. Food Eng. 110, 208–213. doi: 10.1016/j.jfoodeng.2011.05.034
Buzby, J. C., Wells, H. F., and Hyman, J. (2014). The Estimated Amount, Value and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States. USDA Economic Research Service. Available online at: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/food-loss/ (accessed June 20, 2020).
Byun, Y., Hong, S. I., Mangalassary, S., Bae, H. J., Cooksey, K., Park, H. J., and Whiteside, S. (2010). The performance of organic and inorganic coated retort pouch materials on the shelf life of ready-to-eat rice products. Food Sci. Technol. 43, 862–866. doi: 10.1016/j.lwt.2010.01.009
Carneiro, C. D., Mársico, E. T., Ribeiro, R. D., Júnior, C. D., Álvares, T. S., and Oliveira De Jesus, E. F. (2013). Quality attributes in shrimp treated with polyphosphate after thawing and cooking: a study using physicochemical analytical methods and low-field H NMR. J. Food Process. Eng. 36, 492–499. doi: 10.1111/jfpe.12011
Erdogdu, F., Balaban, M. O., Otwell, W. S., and Garrido, L. (2004). Cook-related yield loss for pacific white (Penaeus vannamei) shrimp previously treated with phosphates: effects of shrimp size and internal temperature distribution. J. Food Eng. 64, 297–300. doi: 10.1016/j.jfoodeng.2003.10.012
Erdogdu, F., Tutar, M., Oines, S., Barreno, I., and Skipnes, D. (2016). Determining the optimal shaking rate of a reciprocal agitation sterilization system for liquid foods: a computational approach with experimental validation. Food Bioproducts Process. 100, 512–524. doi: 10.1016/j.fbp.2016.07.012
FAO (2011a). Global Food Losses and Food Waste. Extent, Causes and Prevention. Available online at: http://www.fao.org/3/a-i2697e.pdf (accessed May 29, 2020).
FAO (2011b). Food Loss and Waste in Fish Value Chains. Available online at: http://www.fao.org/flw-in-fish-value-chains/overview/objective/en/ (accessed June 4, 2020).
FAO (2013). “Codex standard for canned shrimps or prawns,” in Codex Standard for Canned Shrimps or Prawns, ed Codex Alimentarius. Available online at: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B37-1991%252FCXS_037e.pdf
FAO (2015). Save Food: Global Initiative on Food Loss and Waste Reduction. Available online at: http://www.fao.org/3/a-i4068e.pdf (accessed June 14, 2020).
FAO (2019a). Globefish Highlights. April 2019. Available online at: http://www.fao.org/3/ca5307en/ca5307en.pdf (accessed June 4, 2020).
FAO (2019b). United States of America GLOBEFISH Profile. Available online at: http://www.fao.org/3/ca5212en/ca5212en.pdf (accessed June 4, 2020).
Goff, H. D. (2020). “Thermal destruction of microorganisms,” in Thermal Destruction of Microorganisms, Dairy Science and Technology Education Series, 8. University of Guelph. Available online at: https://www.uoguelph.ca/foodscience/industry/dairy-education-ebook-series
Hassan, H. E., and Ramaswamy, H. S. (2013). Bio-validation of bi-axial rotary thermal processing. Food Sci. Technol. 53, 418–425. doi: 10.1016/j.lwt.2013.02.009
Heitkemper, D. T., Kaine, L. A., Jackson, D. S., and Wolnik, K. A. (1993). “Determination of tripolyphosphate and related hydrolysis products in processed shrimp,” in 18th Annual Tropical and Subtropical Fisheries Technological Conference (Williamsburg, VA), 92–100.
Ling, B., Tang, J., Kong, F., Mitcham, E. J., and Wang, S. (2015). Kinetics of food quality changes during thermal processing: a review. Food Bioprocess Technol. 8, 343–358. doi: 10.1007/s11947-014-1398-3
Louisiana Agriculture (2017). “Louisiana summary agriculture and natural resources,” in Louisiana Summary Agriculture and Natural Resources, ed Louisiana Cooperative Extension Service (Baton Rouge: LSU AgCenter), 11.
MacNaughton, M., Whiteside, W. S., Rieck, J. R., and Thomas, R. L. (2018). The effects of static, oscillating, and oscillating with dwell time retort motions on the rate of heat penetration of a food simulant. J. Food Process. Preserv. 42:e13410. doi: 10.1111/jfpp.13410
Majumdar, R. K., Deepayan, R., and Apurba, S. (2017). Textural and sensory characteristics of retort-processed freshwater prawn (Macrobrachium rosenbergii) in curry medium. Int. J. Food Prop. 20, 2487–2498. doi: 10.1080/10942912.2016.1242139
Mallick, A. K., Srinivasa Gopal, T. K., Ravishankar, C. N., Vijayan, P. K., and Geethalakshmi, V. (2010). Changes in instrumental and sensory properties of indian white shrimp in curry medium during retort pouch processing at different F0 values: changes in instrumental and sensory properties of shrimp. J. Texture Stud. 41, 611–632. doi: 10.1111/j.1745-4603.2010.00243.x
National Fisheries Institute (NFI) (2018). “Top 10 list shows significant increase in seafood consumption,” in: Top 10 List Shows Significant Increase in Seafood Consumption. Washington, DC: National Fisheries Institute. Available online at: https://www.aboutseafood.com/press_release/top-10-list-shows-significant-increase-in-seafood-consumption/
National Food Processors Association (1982). Thermal Processes for Low-Acid Foods in Metal Containers. Westport, CT: AVI Publishing Co.
National Marine Fisheries Service (2018). “Fisheries of the United States,” in Fisheries of the United States, ed U.S. Department of Commerce (Washington, DC: NOAA Current Fishery Statistics No. 2017), xxvi.
Niamnuy, C., Devahastin, S., and Soponronnarit, S. (2007). Quality changes of shrimp during boiling in salt solution. J. Food Sci. 72, 289–297. doi: 10.1111/j.1750-3841.2007.00349.x
Ramaswamy, H. S. (1993). Come-up time effectiveness for process calculations involving thin-profile packages. J. Food Eng. 19, 109–117. doi: 10.1016/0260-8774(93)90037-K
Shah, M. A., Bosco, S. J. D., Mir, S. A., and Sunooj, K. V. (2017). Evaluation of shelf life of retort pouch packaged Rogan josh, a traditional meat curry of Kashmir, India. Food Packag. Shelf Life 12, 76–82. doi: 10.1016/j.fpsl.2017.04.001
Singh, A. P., and Ramaswamy, H. S. (2015). Effect of can orientation on heat transfer coefficients associated with liquid particulate mixtures during reciprocation agitation thermal processing. Food Bioprocess Technol. 8, 1405–1418. doi: 10.1007/s11947-015-1500-5
Singh, A. P., Singh, A., and Ramaswamy, H. S. (2016). A controlled agitation process for improving quality of canned green beans during agitation thermal processing. J. Food Sci. 81, 1399–1411. doi: 10.1111/1750-3841.13308
Singh, A. P., Singh, A., and Ramaswamy, H. S. (2017). Effect of reciprocating agitation thermal processing (RA-TP) on quality of canned tomato (Solanum lycopersicum) puree. J. Sci. Food Agric. 97, 2411–2418. doi: 10.1002/jsfa.8054
Singh, A. P., Yen, P. P. L., Ramaswamy, H. S., and Singh, A. (2018). Recent advances in agitation thermal processing. Curr. Opin. Food Sci. 23, 90–96. doi: 10.1016/j.cofs.2018.07.001
Sreenath, P. G., Abhilash, S., Ravishankar, C. N., and Gopal, T. K. S. (2008). Standardization of process parameters for ready-to-eat shrimp curry in tin-free steel cans. J. Food Process. Preserv. 32, 247–269. doi: 10.1111/j.1745-4549.2008.00177.x
Stoforos, N. G. (2010). Thermal process calculations through Ball's original formula method: a critical presentation of the method and simplification of its use through regression equations. Food Eng. Rev. 2, 1–16. doi: 10.1007/s12393-010-9014-4
Walden, R. (2008). “The Zinetec Shaka™ retort and product quality,” in In-Pack Processed Foods, ed P. Richardson (Sawston: Woodhead Publishing), 86–101. doi: 10.1533/9781845694692.2.86
Walden, R., and Emanuel, J. (2010). “Developments in in-container retort technology: the Zinetec Shaka® process,” in Case Studies in Novel Food Processing Technologies, eds C. J. Doona, K. Kustin, and F. E. Feeherry (Sawston: Woodhead Publishing), 389–406. doi: 10.1533/9780857090713.4.389
Keywords: shear force, shelf stable, canned, thermal processing, Farfantepenaeus aztecus, reciprocal agitation
Citation: Dixon WR, Watts EG, King JA, Fu X and Wicker L (2020) Shelf-Stable Sustainable Shrimp Thermally Processed With Reciprocal Agitation. Front. Sustain. Food Syst. 4:569790. doi: 10.3389/fsufs.2020.569790
Received: 05 June 2020; Accepted: 26 August 2020;
Published: 06 October 2020.
Edited by:
Lourdes Maria Correa Cabral, Brazilian Agricultural Research Corporation (EMBRAPA), BrazilReviewed by:
Ravishankar Chandragiri Nagarajarao, Central Institute of Fisheries Technology (ICAR), IndiaAngela Aparecida Lemos Furtado, Brazilian Agricultural Research Corporation (EMBRAPA), Brazil
Copyright © 2020 Dixon, Watts, King, Fu and Wicker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louise Wicker, bHdpY2tlciYjeDAwMDQwO2FnY2VudGVyLmxzdS5lZHU=
 William R. Dixon1
William R. Dixon1 Louise Wicker
Louise Wicker