
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 11 January 2021
Sec. Nutrition and Sustainable Diets
Volume 4 - 2020 | https://doi.org/10.3389/fsufs.2020.561820
This article is part of the Research Topic Food Security and Nutrition: Plant Diseases and Food Contamination View all 4 articles
 Rachel V. Wilkins1*
Rachel V. Wilkins1* James F. Campbell2
James F. Campbell2 Kun Yan Zhu1
Kun Yan Zhu1 Laura A. Starkus3
Laura A. Starkus3 Tanja McKay3
Tanja McKay3 William R. Morrison III2
William R. Morrison III2At any point along the post-harvest supply chain, commodities are vulnerable to insect infestation. This is due to a variety of factors, but includes landscape-scale movement of stored product insects to and from food facilities and natural refugia. Long-lasting insecticide-incorporated netting (LLIN) is an innovative tactic that may be used to intercept immigrating insects. LLIN can be used to cover gaps in architecture (e.g., vents, windows, eaves, or over pallets of goods) at food facilities. Another novel approach would be to use LLIN as a kill mechanism in attract-and-kill inspired interception traps on the perimeter of facilities. Furthermore, employing these two LLIN-based approaches together would create multiple protective barriers to reduce infestation in commodities. Therefore, the goal of the current study was to (1) examine the ability of interception traps to capture stored product insects at commercial wheat and rice food facilities, (2) assess whether LLIN deployment method affected efficacy in preventing infestation by stored product insects in pilot-scale warehouses, and (3) determine the success of using LLIN alone, interception traps alone, or both together to prevent infestations. Over 2 years, interception traps deployed for 48-h periods on the perimeter of commercial food facilities captured over 3,000 insects, representing 14 stored product insect taxa. Warehouses deploying LLIN exhibited an 89–93% and 98–100% reduction in insects reaching and progeny production in commodities, even after the release of 3,600 insects of three species over 12 weeks. The combined use of LLIN and interception traps did not improve control above LLIN alone, but this may be because insects could fly unencumbered, highlighting the importance of covering gaps with LLIN on food facilities.
Stored product integrated pest management (IPM) ideally attempts to holistically combine different management tactics to control insects as commodities are harvested, transported, stored, processed, and marketed to end consumers. At any point along this supply chain, commodities are vulnerable to insect infestation (Kumar and Kalita, 2017). Globally, insect feeding and damage accounts for approximately $100 billion USD in postharvest losses (Wacker, 2018). Stored product insects may also act as allergens and present health hazards in the postharvest environment (Hubert et al., 2018). Thus, developing an effective management strategy to reduce these economic losses is crucial. Fumigation is the most common chemical control tactic once insects have entered and infested commodities, and food facilities, including bulk storage, are routinely fumigated on a calendar basis (Espino et al., 2014). Methyl bromide, historically one of the most common structural fumigants, was banned in 2005 by the Montreal Protocol after being declared an ozone-depleting substance (Fields and White, 2002). Phosphine has remained a commonly used fumigant for product fumigations, but it is highly corrosive against electrical equipment which limits its application for structural fumigations. Meanwhile, insects are becoming increasingly resistant to phosphine worldwide (Zhao et al., 2015; Huang et al., 2018; Schlipalius et al., 2018). On the other side of the post-harvest supply chain, there is a high demand for organic or low-insecticide products by consumers, who are willing to pay a price premium (Batte et al., 2006). Thus, a central drive in stored product IPM has been increasing the efficiency of preventative management tactics to avoid insect infestations, while reducing the need for remedial chemical control tactics.
Insect movement and dispersal in the landscape around food facilities presents a serious challenge to existing chemical control tactics. For example, fumigation only kills insects currently present in a grain mass or structure, but efficacy may be short-lived as insects quickly disperse into the facility from refugia in the surrounding landscape (Roesli et al., 2003; Campbell and Arbogast, 2004). Insect abundance outside storage bins full of grain has been found to be greater than bins that are empty, suggesting that the abundant quantity of commodities in a spatially circumscribed space makes bulk storage and food facilities a strong attractant for insects in the landscape (Vela-Coiffier et al., 1997). Prior trapping studies have indicated high species diversity outside facilities, yet due to the numerous landscape features present and commodities handled, the patchy distribution of these species makes monitoring for them particularly challenging (Semeao et al., 2013a; McKay et al., 2017).
Prior work has made it clear that stored product insects are highly mobile. For example, release-recapture studies have found that Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) have an average dispersal capacity of 380 m in the field (Ching'oma et al., 2006). Other work documenting dispersal in different landscapes found R. dominica dispersed 337–375 m and were more often recaptured in wooded sites, whereas they typically dispersed 261–333 m in open sites (Mahroof et al., 2010). In Australia, R. dominica was found in traps throughout the landscape, while Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) was localized around grain storage facilities (Daglish et al., 2017). Even where resources are abundant, T. castaneum apparently leaves and returns to the same area, suggesting regular, and frequent exchange of individuals between food facilities and the surrounding landscape (Rafter et al., 2019). Even mites appear able to disperse and distribute well among grain patches (Hubert et al., 2006). Further, pheromone trap captures of T. castaneum outside a food facility were reduced after fumigation treatments, and mark-recapture data found that P. interpunctella (Hübner) (Lepidoptera: Pyralidae) from outside had immigrated inside facilities, further suggesting that inside populations are connected to those outside (Campbell and Arbogast, 2004; Buckman et al., 2013). Additionally, T. castaneum had low genetic differentiation between field and storage facility captures, indicating that populations inside facilities are readily dispersing outside (Ridley et al., 2011). Thus, it appears that insect movement to and from food facilities may be common, and it may occur over long distances.
Even inside a food facility, there may be significant movement of stored product insects. For instance, Trogoderma variabile Ballion (Coleoptera: Dermestidae) is capable of moving between floors and can travel an average distance of 21 m (Campbell et al., 2002). Tribolium castaneum is highly mobile between floors as well, typically moving downward even in a relatively well-sealed facility (Semeao et al., 2013b). Therefore, developing and optimizing methods to intercept these insects as they disperse and move into and around facilities is key for preventative stored product IPM.
Long-lasting insecticide-incorporated netting (LLIN) may be a particularly efficacious tactic for intercepting stored product insects prior to entering food facilities or even halting movement of insects between different parts of facilities, which may contribute to decreased infestation of commodities. Historically, insecticide-treated netting has been successfully used as bed nets to kill mosquitoes and reduce the spread of malaria in tropical regions of Africa (Alonso et al., 1991; Martin et al., 2007). The efficacy of the netting has typically spanned multiple years and is relatively inexpensive to replace (Dev et al., 2010; Hailu et al., 2018). Recent studies in apple production have incorporated LLIN as a kill mechanism in monitoring traps against the invasive Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) (Kuhar et al., 2017; Peverieri et al., 2017).
The netting has also been used as a preventative measure against pests of specialty crops and ornamental trees (Marianelli et al., 2018; Ranger et al., 2020). These studies support the idea that LLIN can be used to intercept insects prior to reaching their destination and causing damage. In a postharvest setting, recent work has shown that LLIN significantly causes mortality of stored product insects, including Lasioderma serricorne (F.) (Coleoptera: Ptinidae) (Rumbos et al., 2018), Sitophilus oryzae (L.) (Coleoptera: Curculionidae) (Anaclerio et al., 2018), R. dominica, T. castaneum (Morrison et al., 2018), T. variabile, and other species (Paloukas et al., 2020), including different life stages (Wilkins et al., 2020). Further, LLIN has been documented to reduce movement and dispersal ability by multiple-fold over long periods even after just brief exposure times compared to untreated netting controls (Morrison et al., 2018). Importantly, only a single field study with LLIN has been performed in the postharvest environment, which showed that an α-cypermethrin-incorporated LLIN could be successfully deployed as a cube encasing tobacco to protect against Ephestia elutella (Hübner) (Lepidoptera: Pyralidae) and L. serricorne (Rumbos et al., 2018). Thus, there has been relatively little semi-field or field research regarding the application method of LLIN for intercepting stored product insects.
There are several possible ways to imagine LLIN being deployed in the post-harvest environment. One method may be to cover external vents and openings on a building, which would provide a large distance between the LLIN deployment location and the commodity. Another deployment method may be as a wrap for a pallet of goods in a warehouse, which would necessitate close contact with the product. A final alternative method may be as a screen on windows, partitions, and doors between different parts of a facility, which may provide an intermediate amount of distance between the LLIN and the commodity. However, to date, no study has assessed whether deployment in these different ways affects LLIN efficacy at preventing infestation and/or progeny production in stored products.
One concern with the use of LLIN at food facilities is that insects that are exposed may be insufficiently dosed to induce knockdown or kill. However, Gerken et al. (2020) found that multiple, brief exposures to LLIN had the same effect on T. castaneum as a single, longer exposure. Thus, this limitation may be potentially circumvented by exploiting kairomones, pheromones, and other semiochemicals to attract stored product insects and help ensure prolonged or repeated exposures (e.g., multiple dosings) to LLIN. However, food facility managers are generally concerned about deploying attractive compounds adjacent to where commodities are stored, so an ideal option would be to include an efficient kill mechanism (e.g., LLIN) and attractive stimuli in an interception trap that can be deployed on the perimeter of the facility to divert immigrating insects from the landscape. An interception trap may provide an added layer of protection to a food facility if used in combination with LLIN on vents and openings along with other ongoing IPM protocols. Previous research has already documented a plethora of volatile compounds that elicit positive responses from multiple stored product species, including T. castaneum, R. dominica, Trogoderma spp., Sitophilus spp. (Coleoptera: Curculionidae), mite, and moth pests (Burkholder and Ma, 1985; Cox, 2004; Wakefield and Dunn, 2005; Balakrishnan et al., 2017; Morrison et al., 2020). These interception traps may function as miniature attract-and-kill (AK) traps, whereby the pest population is attracted to a spatially circumscribed area and removed from the foraging population with a kill mechanism (Gregg et al., 2018). In other agricultural systems, AK traps have been employed to intercept a multitude of pest insect species before they contact valuable specialty crops (Charmillot et al., 2000; Kroschel and Zegarra, 2010; Morrison et al., 2016b, 2019a). Whether used alone or in combination with other control tactics, AK traps may be successful in reducing insect damage to commodities (El-Sayed et al., 2009). AK traps are most efficient when used against small to moderate population sizes (Charmillot et al., 2000; El-Sayed et al., 2009), which is likely the case for diffuse populations of stored product insects in the landscape. As a result, interception traps, like attract-and-kill, may be a strong perimeter management tool that pairs well with other uses of LLIN in food facilities, as part of a comprehensive IPM program.
Therefore, the goal of the current study was to (1) examine the ability of interception traps to capture stored product insects at commercial wheat and rice food facilities, (2) assess whether LLIN deployment method affected efficacy in preventing infestation by stored product insects in pilot-scale warehouses, and (3) determine the success of using LLIN alone, interception traps alone, or both together to prevent infestations.
Insects used for these experiments were reared in an environmental chamber held under constant conditions (27.5°C, 60% RH, 14:10 L:D). In particular, T. castaneum (field-derived strain from central Kansas in 2012) was reared on 95% organic flour and 5% brewer's yeast, while R. dominica (field-derived strain from north-central Kansas in 2012) was reared on organic whole wheat, and T. variabile (field-derived strain from eastern KS in 2016) was reared on ground dog food (Lamb & Rice, Purina One, St. Louis, MO) and whole grain oats with a crumpled, damp paper towel placed on the surface of the diet in 828-ml containers filled two-thirds full with diet. For the assays below, 1–8-week old individuals were used.
A suite of potential attractants was tested in the laboratory in order to determine which were the most promising for inclusion in an interception trap to deploy at the perimeter of commercial food facilities. The behavioral response of T. castaneum and R. dominica to two kairomones and one commercial pheromone lure was assessed in two separate assays. Trogoderma variabile was not used in these assays because of their strong startle response. In particular, dried distiller's grains with solubles (DDGS) was included as a kairomone, because prior research has suggested it is attractive to T. castaneum (Fardisi, 2015). Wheat germ oil has also been found to be attractive to stored product insects (Nara et al., 1981; Phillips et al., 1993), as have a variety of commercial lures (Morrison et al., 2020). Thus, the specific treatments included: 0.76 g of dried distillers' grains (DDGS), 950 μl wheat germ oil (WGO), and one, two, or three Stored Product Beetle tab lures (SPB tab; a broad-spectrum attractant for 20 species of stored product beetles, IL-2800-10, Insects Limited, Westfield, IN, USA) and were tested in the wind tunnel and release-recapture assays as described below. Based on the results from these two issues, the most attractive stimulus was chosen for final inclusion in interception traps for the field-based assay.
To assess attraction among potential interception trap attractants, a laminar flow wind tunnel assay was conducted in an environmental chamber set at constant conditions (25°C; 65% RH). Either DDGS, WGO, or a single SPB tab were placed in a 100 mm × 10 mm petri dish without a lid and located 13.5 cm upwind of a 21.6 cm × 27.9 cm release arena. An empty dish with no attractant was designated as the negative control. A single adult insect was released in the center of the arena and given 2 min to exit the arena. Insects leaving on the edge of the arena closest to the stimulus source (e.g., upwind edge) were denoted as a positive stimulus response, while insects exiting from one of the other three sides of the arena were denoted as a non-stimulus response. Insects that did not exit the arena within the allotted time were excluded from the analysis. For each treatment, there were a total of n = 30 replicate individuals tested.
To evaluate whether the deployment of the attractants above would result in higher captures in a trap, we employed a release-recapture assay conducted in large walk-in environmental chambers under constant conditions (4.88 × 5.81 × 2.43 m; 25°C, 65% RH, 16:8 L:D). Treatments were placed in commercial pitfall traps (e.g., Storgard® Dome™ Trap, Trécé Inc., Adair, OK). An empty trap acted as a negative control. For T. castaneum, four pitfall traps were placed equidistant along the perimeter of the chamber. A total of 300 T. castaneum adults (1:1 M:F) were removed from colony jars 24 h before release and allowed to settle in an 8 × 8 cm square of corrugated cardboard. The following day, the cardboard refuge was placed in the center of the chamber and the adults were given 24 h to respond to the traps. The number of insects captured per trap treatment was recorded. There were a total of n = 8 replicates per treatment. Because R. dominica are not as mobile (e.g., Morrison et al., 2018), an altered release-recapture assay was performed as follows. In the same large walk-in chambers, each treatment was placed in a large individual plastic bin (86.3 × 39.4 × 30.5 cm L:W:H; Sterilite Corp., Townsend, MA, USA) whose bottom surface had been systematically scuffed up with sandpaper to allow for easy movement by insects. R. dominica adults were pulled from colony jars 24 h prior to the beginning of the experiment and allowed to settle in an 8 × 8 cm square of cardboard. A single pitfall trap was placed in a randomized opposite corner from where 20 R. dominica adults (mixed sex) were released. The adults were given 24 h to respond to the trap, and at the end, the number of insects captured by each trap was recorded. A total of n = 2 replicates with all treatments represented were run at a time, and over the course of the experiment there were a total n = 12 replicates per treatment.
Following the assessment of attractants above, the SPB tab was deemed the most attractive lure for T. castaneum and R. dominica and a strong candidate for use as the primary attractant in interception traps. A follow-up experiment was conducted to assess whether increasing the number of SPB tabs would result in a dose-dependent increase in attraction by stored product insects to interception traps. For these assays, one, two, or three SPB tab lures were incorporated into petri dishes (wind tunnel assay) or commercial pitfall traps (release-recapture experiment) as described above. There were n = 30 replicate individuals tested for each treatment and species in the wind tunnel assay, and there were n = 24 replicates per treatment and species for the release-recapture assay.
To understand whether interception traps could be developed to prevent insects from immigrating into food facilities, spillage traps from prior work (e.g., Campbell et al., unpublished data) were constructed and modified as follows. The spillage traps (henceforth termed interception traps or AK-based interception traps) were constructed with PVC pipe (5 cm length × 5.2 cm I.D.) and filled with 60 g of crimped wheat kernels (Kansas) or a mixed variety of brown rice (Arkansas) as a kairomone source. These kairomonal sources were included, based on prior work describing them as attractive (Phillips et al., 1993), and to provide a refuge so as to increase retention of insects in traps. LLIN (0.4% deltamethrin-incorporated, Vestergaard-Frandsen Inc., Lusanne, Switzerland) or control netting without insecticide acted as the kill mechanism in the interception traps. Prior work has shown that the LLIN remains effective in excess of 12 months (Scheff et al., unpublished data). The attractant included in the interception traps was a single SPB tab (based on prior laboratory assays) placed on the surface of the grain mass. Two pieces of perforated metal plates held together by a screw, washer, and wingnut were arranged on both openings of the PVC pipe to hold in the grain (Figure 1). Two pieces of control netting or LLIN (6.4 cm diameter) were placed between the metal plates and the openings of the PVC pipe. Both the netting (2 mm I.D.; 49 holes/cm2) and perforated metal plates (2 mm I.D.; 9 holes /cm2) had openings large enough for insects from the surrounding refugia to enter the trap. Each trap corresponded to one of four treatments: control netting only (no insecticide) without attractant, control netting with attractant, LLIN only without attractant, LLIN with attractant. These four treatments made up one transect, and three transects were placed around the perimeters of each of the six food facilities in Kansas (n = 3) and Arkansas (n = 3) (Table 1). Traps were left out for 48 h, then retrieved, and the number of stored product insects found in each trap and their health conditions (alive, affected, or dead) were recorded according to prior definitions in the literature (Morrison et al., 2018). In brief, alive were individuals consisted of those walking unimpeded, able to right themselves, while dead individuals were those completely motionless even after prodding; affected individuals fell between these two extremes. Trapping occurred roughly once every 2 weeks from 17 August 2018 to 21 September 2018 and 25 April 2019 to 18 September 2019 in Kansas, and from 31 May 2019 to 30 September 2019 in Arkansas. There were a total of n = 8–12 deployments at each site. In some cases, trapping dates were altered to account for adverse weather conditions. All captured stored product insects were individually placed on 20 g of fresh wheat or brown rice, held at the above environmental chamber conditions, and their health condition was recorded a week later. The original wheat in the trap was held for 6 weeks under the same conditions and checked for progeny production by any stored product insect species.
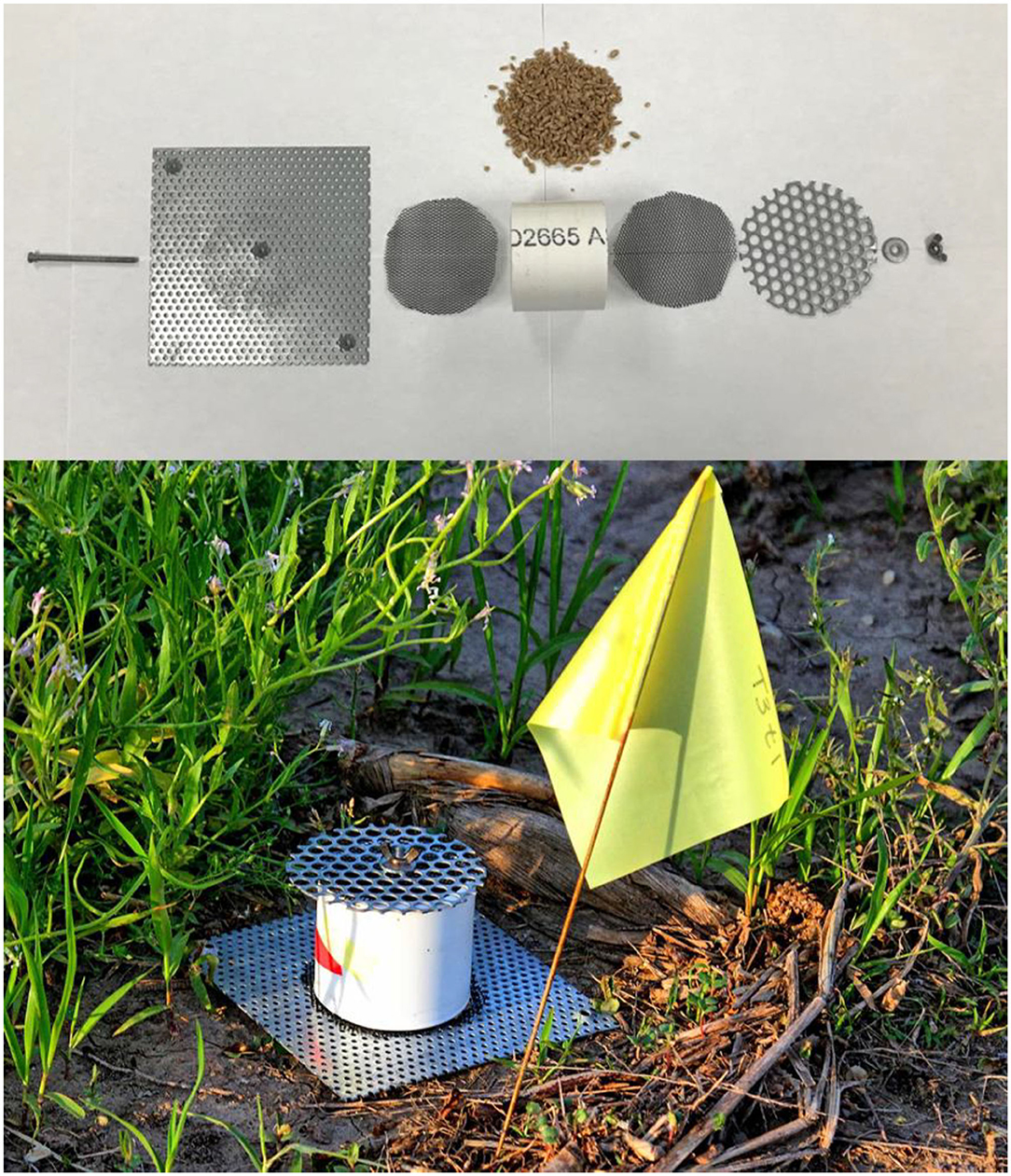
Figure 1. Exploded-view picture of interception traps (top), and field-deployed interception trap (bottom). In order from left to right, each trap included a screw (to hold all parts together), a bottom perforated metal plate, a piece of netting (control or LLIN), cut PVC pipe holding 60 g of whole wheat kernels as kairomone and SPB tab lure, a second piece of netting, a top perforated metal plate, and a washer and wingnut on the end of the screw to tightly hold each piece together (top). Interception trap assembled and deployed in the field (bottom).
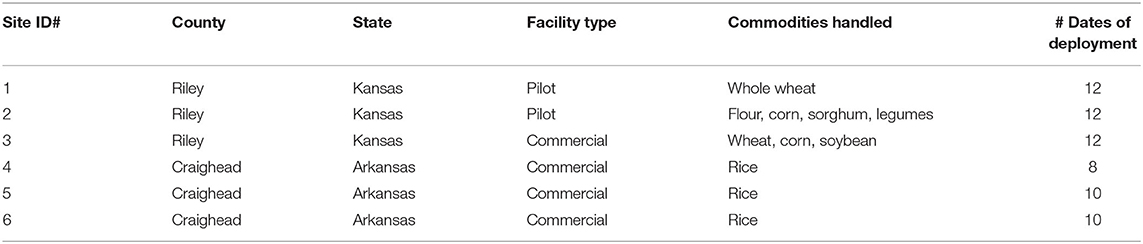
Table 1. Summary of field sites used for the interception trap assay in 2018 and 2019 in Kansas and Arkansas, USA.
To understand whether the method by which LLIN was deployed affected subsequent commodity infestation and progeny production, pilot-scale warehouses (5.85 × 2.81 m) in Manhattan, KS were used. The temperature of the warehouse was monitored with a datalogger (HOBO UX-100, Onset Computers, Bourne, MA) at hourly intervals, with average temperature and RH at 24.5 ± 0.13°C and 62.3 ± 0.57%, respectively, during the course of the experiment. At the far end of the warehouse against the back wall, a commodity consisting of a mixture of 210 mL organic, whole wheat kernels and 210 mL of organic, unbleached flour was placed in a lid-less plastic container (14 × 24 cm) with eight holes (0.32 cm diameter) drilled and equally placed around the bottom circumference of the container to allow for insect dispersal into the commodity. A total of 100 individuals each of T. castaneum, R. dominica, and T. variabile were released at the opposite end of the warehouse (~5.25 m away). There were n = 12 replicate releases per treatment from 26 April 2019 to 16 August 2019, comprising a total of 3,600 released insects.
There were four LLIN deployment methods that were tested (Figure 2). In the “hanging” treatment, LLIN (2.72 × 2.41 m) was affixed to the warehouse ceiling and allowed to hang down to the floor, completely bisecting the room. This represented deployment of LLIN to partition two areas of a food facility, or as a screen for doors and windows. In the “cover” deployment method, LLIN was directly laid over the commodity, representing LLIN application on a pallet as a wrap to protect final products. In the “pipe” deployment method, a PVC pipe (91 cm length, 5.1 cm I.D.) was bisected halfway with LLIN to represent insects immigrating into a food facility through small openings such as vents, eaves, or crevices. These were compared with a control that used the same PVC pipe design, but without netting. For the pipe treatments, insects were released directly into one end of the pipe, and the release end was sealed off with parafilm. For the hanging and cover treatments, insects were released at an identical location but on the floor of the warehouse, 0.5 m away from the netting.
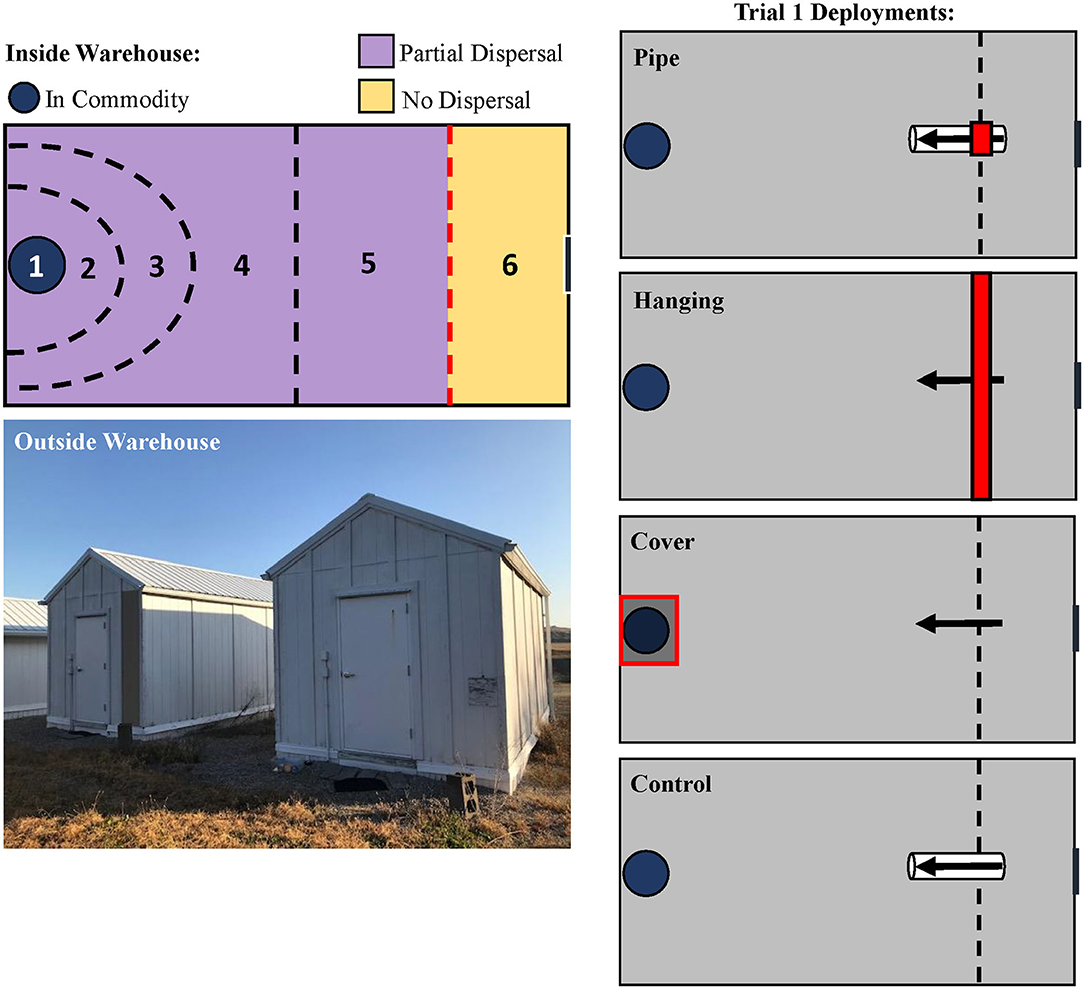
Figure 2. Schematic of the designated recapture zones inside the pilot-scale warehouses where insects were released (top, left) in the LLIN Deployment Assay. Insects were released in Zone 6, given 72-h to travel across the warehouse through Zones 2–5, and collected in the commodity (Zone 1). Outside habitus image of the pilot-scale warehouses used for the LLIN and interception trap deployment assays (bottom, left). Treatments are schematically represented on the right, showing the three LLIN deployment methods for Trial 1, including a control treatment with no LLIN. The red lines indicate deployment of LLIN.
Insects were given 72 h to disperse across the warehouse to the commodity. After this period, insects were collected by pre-designated zones in the warehouse (Figure 2). The zones were noted respective to the location of the commodity, and included Zone 1 (inside the commodity), Zone 2 (0.5 m radius around commodity), Zone 3 (1 m radius around the commodity), Zone 4 (1–2.7 m), Zone 5 (2.7–4.5 m; approx. halfway), and Zone 6 (4.5 m−5.6 m, e.g., the release zone). For statistical analysis and discussion, the zones were reclassified as in commodity (e.g., Zone 1), partial dispersal (Zones 2–5), and no dispersal (Zone 6). The insects were retrieved, and then brought back to the lab where their health condition was assessed as alive, affected, or dead. The commodity was sieved (#10 sieve, 2.0 × 2.0 mm mesh, W.S. Tyler, Mentor, OH; then #25 sieve, 0.71 × 0.71 mm mesh, Fisher Scientific Co., Hampton, NH) for adult insects, whose number and health condition were recorded. The commodity was held for 6 weeks after deployment under the previously described environmental chamber conditions to evaluate progeny production. The species and health conditions of the progeny were recorded.
The final assay in this study also occurred in pilot-scale warehouses as described above and was intended to evaluate the efficacy of LLIN deployment and interception traps (as described in section Field Interception Trap Assay) alone or together. There were four treatments in total applied to warehouses for this experiment: LLIN alone, AK-based interception trap alone, both together (AK + LLIN), or neither (e.g., a control treatment that had no netting or interception trap) (Figure 3). The zones were similar to the descriptions above, but a Zone 7 was introduced which described insects captured inside the interception traps (Figure 3). For analysis, the zones were collapsed to their new definitions as above (e.g., in commodity, partial dispersal, no dispersal). White butcher paper was affixed to the floor of the warehouse to encourage mobility of the insects. To simulate the inside and outside environment of a food facility at a single warehouse, two wooden planks (1.7 m long) projected into the warehouse from the two corners of Zone 6 at a 50° angle in a funnel arrangement, leaving a 10 cm-wide gap between them in the center of the warehouse floor (Figure 3). No netting (e.g., control, or AK alone) or LLIN (for LLIN alone, or AK + LLIN) bridged the two wooden planks to create an unprotected or protected entrance for released insects to pass through. The boards were affixed to the floor and fluon (polytetrafluoroethylene, MilliporeSigma, Burlington, MA) was applied to the vertical sides of the wooden planks to prevent circumventing the netting by climbing insects. This presents a realistic scenario of an imperfectly sealed food facility, because flying insects in the experiment could circumvent the LLIN. In the interception trap-only treatment and treatment with both tactics, the interception traps (as described above with LLIN, whole wheat grain, and SPB tab lure) were placed 0.76 m in front of the simulated entrance, giving the insects the option to enter the warehouse or be diverted to the interception trap, as may happen under field conditions. For each treatment, insects were released in the two corners of Zone 6, approximately 1.5 m from the netting, and given 72 h to reach the commodity. Afterwards, the insects were collected by zone and their health condition was recorded. There were n = 12 replicate releases per treatment from 23 August 2019 to 8 November 2019. As in the previous assay, the commodity was sieved for adults and held for 6 weeks to check for progeny production.
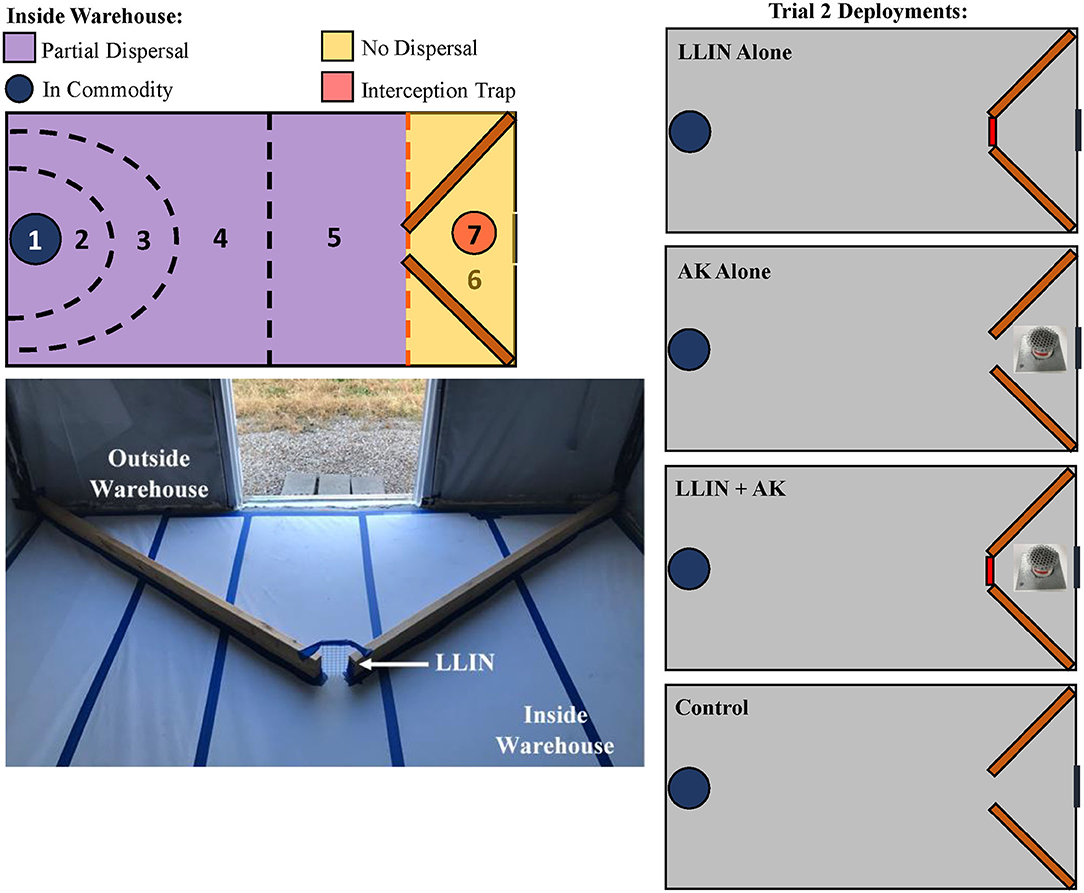
Figure 3. Schematic of the designated recapture zones inside the pilot-scale warehouses where insects were released in Trial 2 (the combined AK and LLIN Assay), including a Zone 7 for the interception trap deployment (top, left). Insects were released “outside” the warehouse in the two corners of Zone 6, given 72-h to travel “inside” the warehouse through Zones 2–5, and collected in the commodity Zone 1 Two wooden planks acted as a funnel for the insects to enter inside the warehouse; the gap (see arrow) between the two planks was the point of entrance with control netting (without insecticide) or LLIN bridging the gap between the two planks (bottom, left). Treatments are schematically represented on the right, showing the three LLIN and interception trap deployment methods for Trial 2, including a control treatment with no LLIN or interception trap. The red lines indicate deployment of LLIN.
A generalized linear model with exit edge (stimulus or non-stimulus) for the wind tunnel or percentage of adults recaptured in the release-recapture assay was used as the response variables. Models were checked for overdispersion, which was found to be a problem, thus a quasibinomial (wind tunnel) or quasipoisson (release-recapture) with a logit-link function was used as the underlying distribution. The R package multcomp was used for multiple comparisons with a call to the glht function (Bretz et al., 2010). R software was used for this and all analyses (R Core Team, 2019) with α = 0.05, unless otherwise specified.
The total captures from interception traps were expressed as a percentage of total captures and compared using a χ2-test with a Bonferroni correction to the α-threshold for significance. These were based on the main explanatory factors, including year (2018 or 2019), state of collection (KS or AR), interception trap configuration (LLIN only, Ctrl only, LLIN + lure, and Ctrl + lure). The null hypothesis assumed equal percentages among levels within categories. If preliminary analysis indicated no significant differences between years or states, the data were collapsed for the final analysis.
The number of adults found in each collapsed dispersing zone (in commodity, partial dispersal, and no dispersal) from the LLIN deployment assay were analyzed with a multivariate analysis of variance (MANOVA) using the health status (alive, affected, or dead) and treatment (control, hanging, cover, or pipe) as fixed, explanatory factors. Release date was used as a random blocking variable. Upon a significant result from the overall model, sequential ANOVAs were performed with the same model structure followed by Tukey HSD for multiple comparisons. In addition, a generalized linear model based on a quasipoisson distribution (to account for overdispersion) was used to determine changes in progeny production among the LLIN deployment treatments, followed by Tukey HSD upon a significant result from the overall model for multiple comparisons. The data from the combined tactic assay was analyzed in a similar manner with the exception of using tactic (LLIN alone, interception trap alone, both, or neither) as a fixed explanatory variable. Inspection of residuals and quantile-quantile plots confirmed that there were no significant deviations from normality or homogeneity of variances for normality-based tests.
In the wind tunnel, lures had a significant effect on attraction by T. castaneum (χ2 = 27.5; df = 3; P < 0.0001), with 2.2-fold more adults exiting on the stimulus edge of the SPB tab than for the negative control (Figure 4). Fewer than 3% of the tested T. castaneum did not respond and had to be excluded from the statistical analysis.
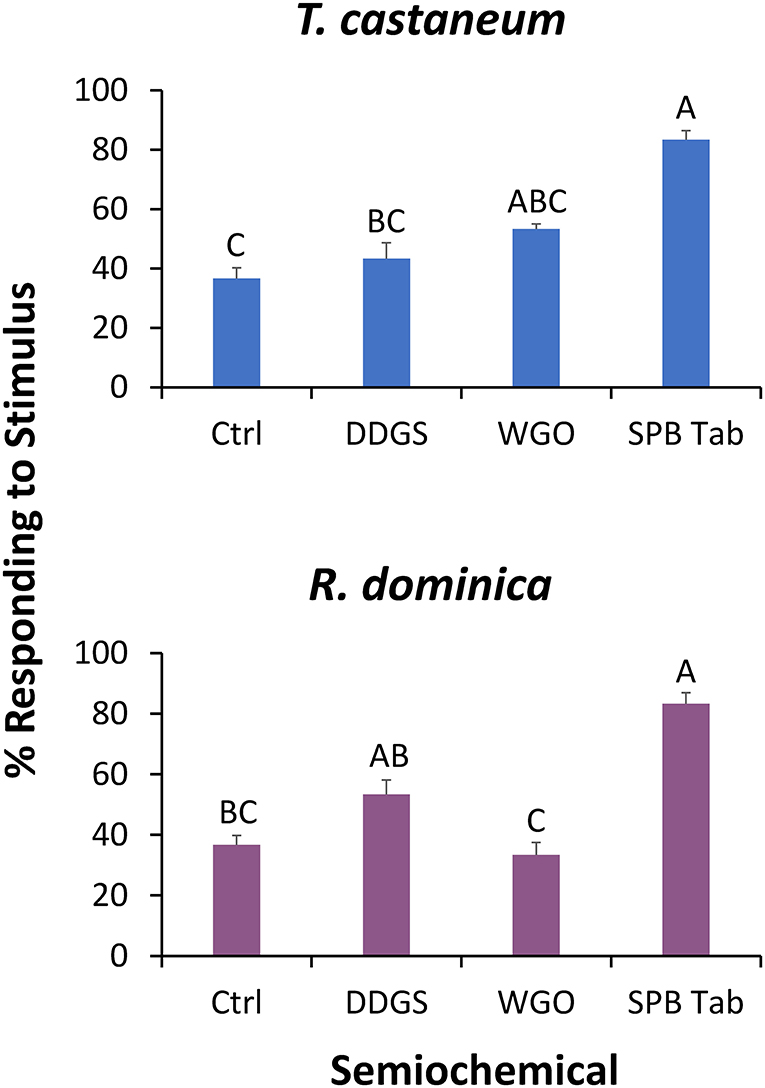
Figure 4. The percentage of T. castaneum (top) and R. dominica (bottom) exiting the release arena on the stimulus (upwind) edge in a wind tunnel assay. These stimuli included dried distillers' grains with solubles (DDGS), wheat germ oil (WGO), the commercial lure Stored Product Beetle Tab (SPB Tab), and ambient air (Ctrl). Bars with shared letters are not significantly different from each other (χ2-tests, Bonferroni correction).
Similar to T. castaneum, the lures had a significant effect on attraction by R. dominica in the wind tunnel (χ2 = 27.1; df = 3; P < 0.0001). In particular, the SPB tab resulted in 2.2-fold more R. dominica adults exiting on the stimulus edge compared to the negative control (Figure 4). Fewer than 20% of the tested R. dominica did not respond and had to be excluded from the statistical analysis.
Likewise, the traps baited with different lures had a significant effect on recapture of T. castaneum (χ2 = 24.8; df = 3; P < 0.0001), with the SPB tab-baited traps capturing 2.8-fold more adults in the release-recapture experiment than control traps (Figure 5). The same pattern was observed for R. dominica, where traps with lures had a significant effect on recapture of conspecifics (χ2 = 54.3; df = 3; P < 0.0001), and the greatest recapture was in traps baited with the SPB tab lure (Figure 5). Based on the results from the wind tunnel and release-recapture, we used the SPB tab lure for all subsequent assays with the interception traps.
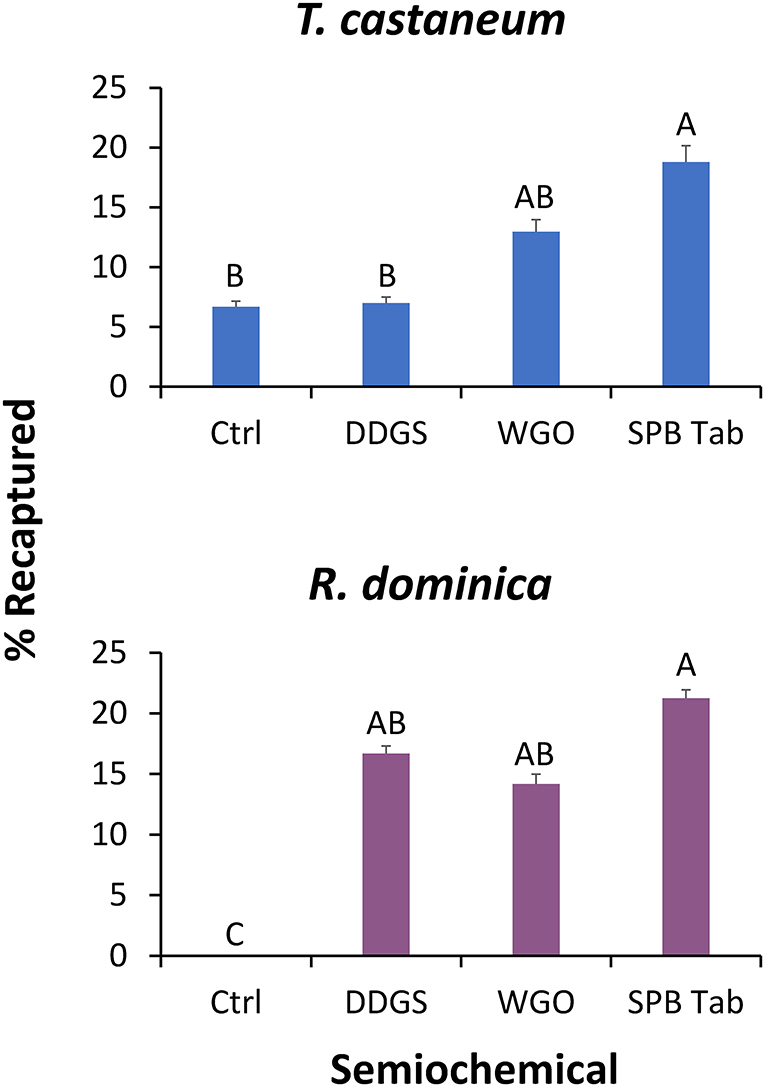
Figure 5. The percentage of T. castaneum (top) and R. dominica (bottom) captured in Trécé Storgard Dome® pitfall traps in a release-recapture assay. Each dome trap contained the dried distillers' grains with solubles (DDGS), wheat germ oil (WGO), the commercial lure Stored Product Beetle Tab (SPB Tab), or ambient air (Ctrl). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
When including the negative control with no lures, the number of lures significantly affected attraction by T. castaneum (χ2 = 13.6; df = 3; P < 0.01) and R. dominica (χ2 = 30.8; df = 3; P < 0.0001) adults in the wind tunnel. In particular, between the negative control and a single lure, there were 1.5 and 2.2-fold increases in attraction by T. castaneum and R. dominica, respectively (Table 2). Importantly, there was no statistically significant benefit of adding additional lures beyond a single one (Table 2). Fewer than 1% of the T. castaneum and fewer than a third of the R. dominica were considered non-responders.
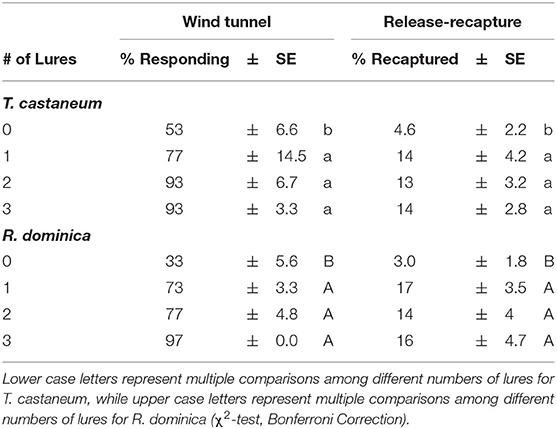
Table 2. Assessing lure number-dependent attraction to Stored Product Beetle (SPB) tab lures in the wind tunnel by individuals exiting on the stimulus edge of the arena and recapture in traps in a release-recapture assay by T. castaneum and R. dominica adults.
Likewise, the number of lures had a significant effect on capture of T. castaneum (χ2 = 9.07; df = 3; P < 0.05) and R. dominica (χ2 = 10.0; df = 3; P < 0.05) in baited traps in a release-recapture experiment. There were 3-fold and almost 6-fold more T. castaneum and R. dominica adults captured, respectively, in traps baited with a single lure compared to no lures. Importantly, there was no significant benefit from adding more lures to a trap (Table 2).
Captures of stored product insects in interception traps at the perimeter of facilities were significantly different by state (χ2 = 6.55; df = 1; P < 0.05). Thus, each state was analyzed separately for the main analysis. However, there was no significant effect of year on captures in interception traps (χ2 = 0.82; df = 1; P = 0.37), as a result the sampling years were collapsed for the final analysis. In total, over 3,000 insects were collected over the 2 years, representing 14 stored product insect taxa (Table 3). The interception trap configuration had a significant effect on captures in both Arkansas (χ2 = 46.6; df = 3; P < 0.0001) and Kansas (χ2 = 94.5; df = 3; P < 0.0001; Figure 6). In Arkansas, there were 2.5- to 2.8-fold more stored products insects captured in interception traps with lures than without lures, while there were 89- to 100-fold more insects in Kansas interception traps. The use of LLIN appeared not to impede the colonization of traps by stored product insects. From 2018 to 2019, there were 20 weeks of insect captures, with average numbers of captures more variabile on a week-to-week basis in Arkansas than Kansas (Supplementary Figure 1).
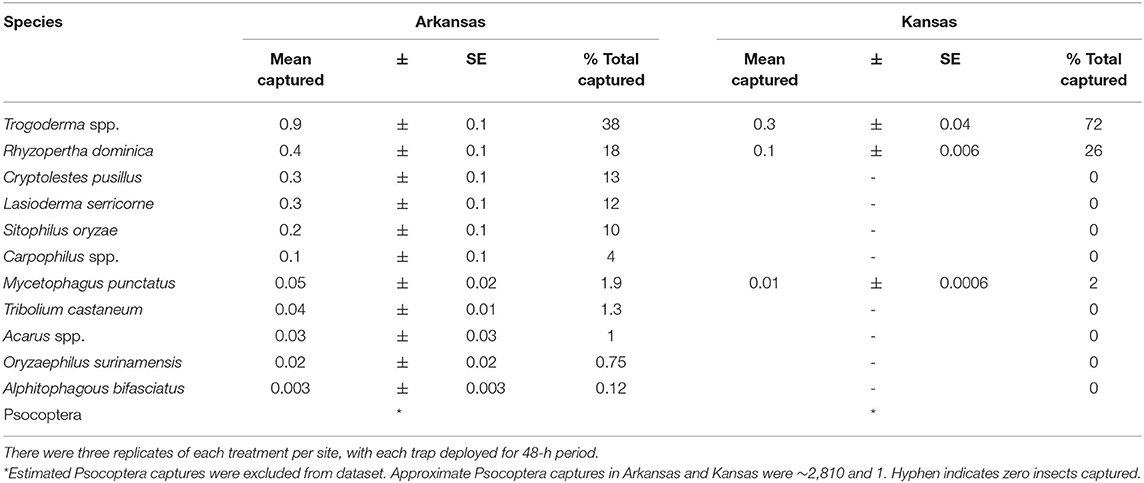
Table 3. The community composition of stored product arthropods captured in interception traps deployed from 17 August 2018 at three sites in KS, and from 25 April 2019 to 18 September 2019 at three commercial sites in KS and 31 May 2019 to 30 September 2019 at three commercial sites in AR.
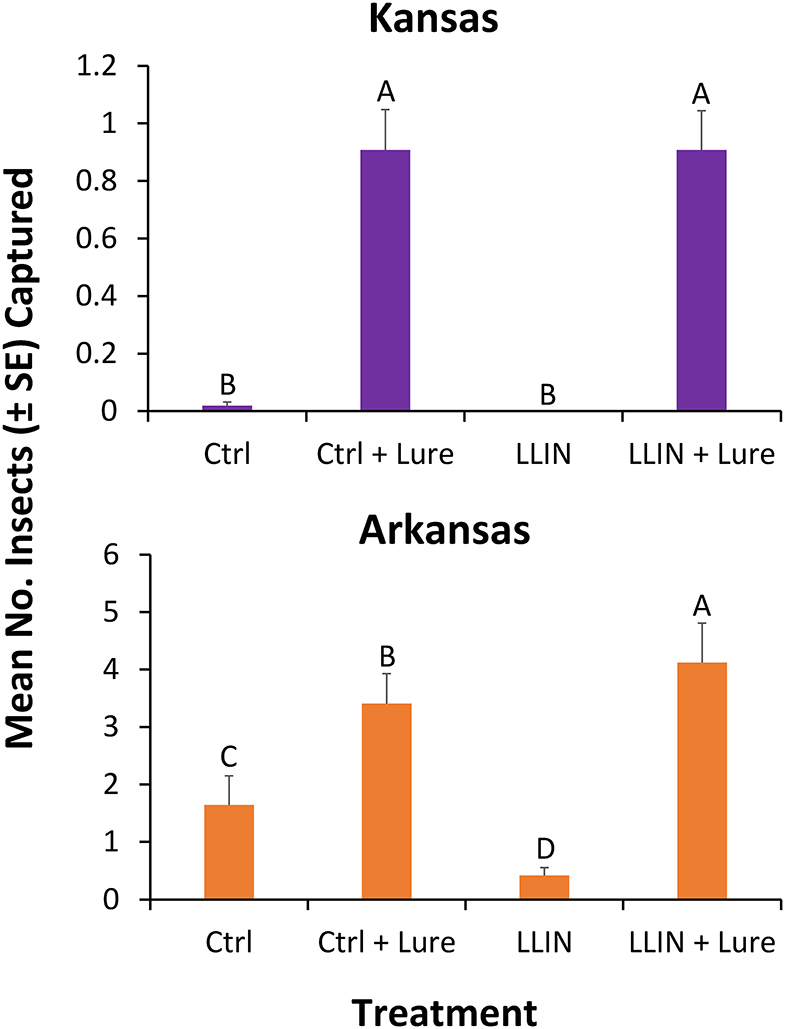
Figure 6. Mean (±SE) number of stored product insects captured by interception configuration. Traps were deployed for 48-h periods once every other week at six sites during 2018 and 2019 in in Kansas (top) and Arkansas (bottom) at commercial food facilities. Treatments included interception traps (1) with control netting and no lure (Ctrl), (2) control netting + SPB Tab (Ctrl + Lure), (3) with LLIN and no SPB Tab (LLIN), (4) with LLIN + SPB Tab (LLIN + Lure). Bars with shared letters are not significantly different from each other (χ2-tests, Bonferroni correction).
Progeny production after 6 weeks in interception traps by stored product insects after capture was significantly different by state (χ2 = 8.33; df = 1; P < 0.01), so each was analyzed separately for the final analysis. Sampling year did not significantly affect progeny production in interception traps (χ2 = 3.83; df = 1; P = 0.06), thus year was collapsed for the final analysis. The configuration of the interception trap significantly affected progeny production for those deployed in both Kansas (χ2 = 93.0; df = 3; P < 0.0001), and Arkansas (χ2 = 33.2; df = 3; P < 0.0001). In Kansas, deployment of LLIN reduced progeny production by 99% in traps with stimuli compared to when control netting was used that lacked insecticide (Figure 7). In Arkansas, LLIN deployment in interception traps reduced progeny production by 57% in traps with lures compared to when interception traps contained control netting.
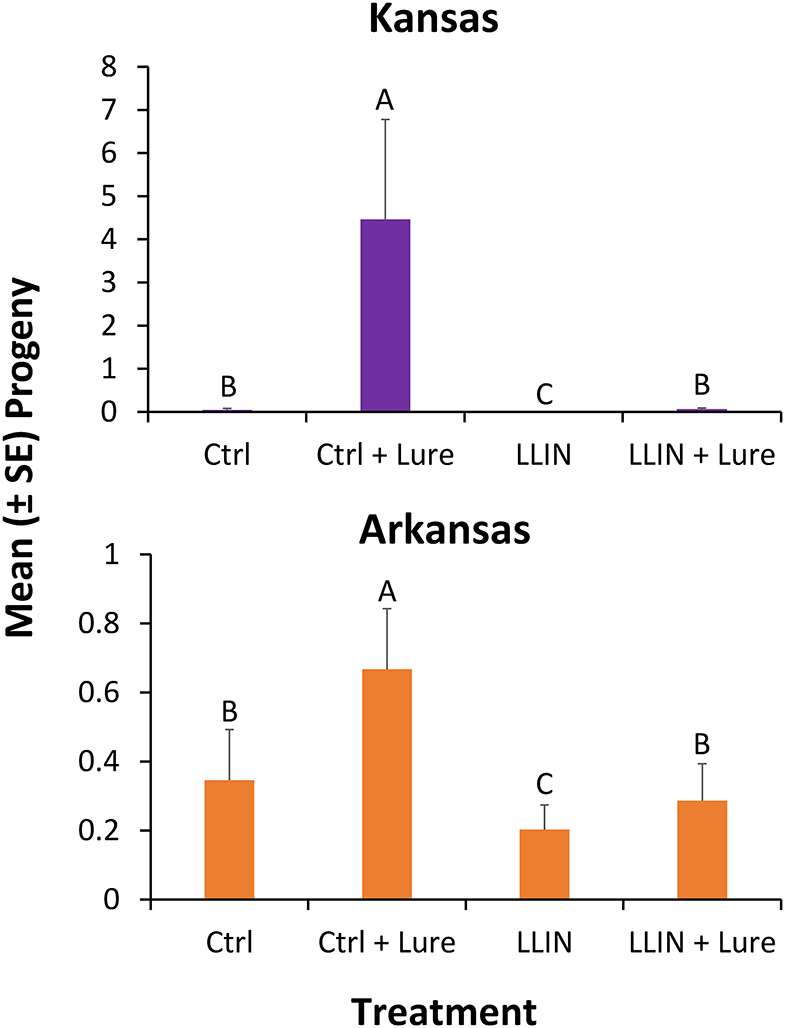
Figure 7. Mean number progeny (±SE) produced by stored product insects ovipositing prior to succumbing to the treatments after 6 weeks from grain inside traps deployed for 48-h periods once every other week at six sites during 2018 and 2019 in in Kansas (top) and Arkansas (bottom) at commercial food facilities. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction).
Overall, the deployment of long-lasting insecticide netting in pilot-scale warehouses had a significant effect on the percentage of insects that were able to disperse (Table 4 and Figure 8). The released insect species also affected dispersal (Table 4). Warehouses that employed LLIN demonstrated an 89–93% reduction in the number of insects making it to the commodity compared to the control warehouses, which lacked LLIN. There was a significant interaction between treatment and species type, with far more T. castaneum infesting the commodity in controls, and more individuals partially dispersing in the hanging and pipe deployments than either of the other two species.
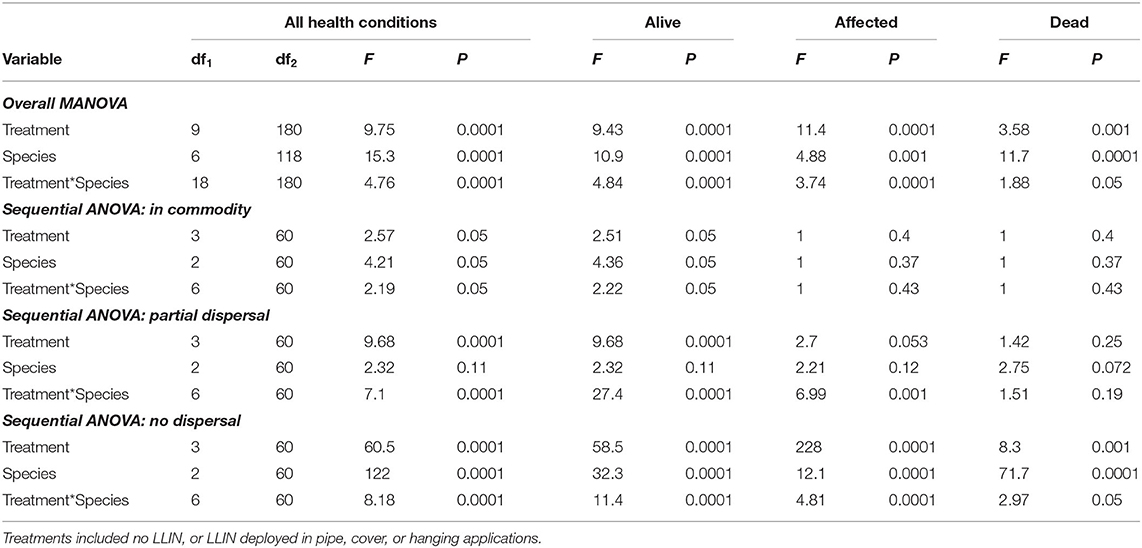
Table 4. Statistical model results for recapture of individuals in the commodity, partially dispersing, and not dispersing in Trial 1 examining efficacy of LLIN deployment method in a pilot-scale warehouse release-recapture assay deployed in 2019 in Manhattan, KS.
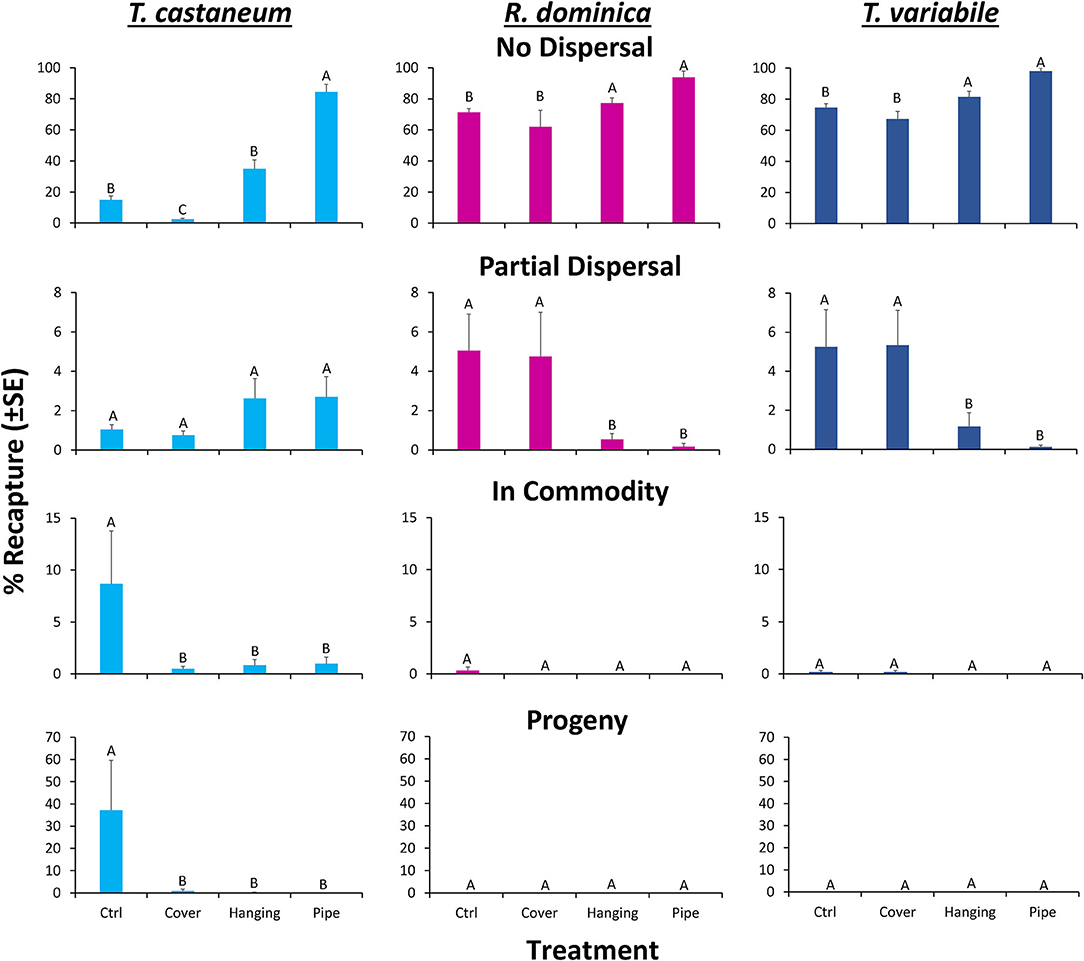
Figure 8. Mean (±SE) percentage of 100 T. castaneum (light blue bars), R. dominica (pink bars), and T. variabile (dark blue bars) adults released in pilot-scale warehouses in Manhattan, KS during 2019 recaptured after 72 h for Trial 1 to assess relative efficacy of different LLIN deployment methods. Individuals were recorded as not dispersing (Zone 6, top row), partially dispersing (Zones 2–5, second row), infesting the commodity (Zone 1, third row), or producing progeny after a 6-week holding period (bottom row). Deployment methods included: Cover—covering the commodity directly with LLIN, Hanging—a single piece of LLIN bisecting the warehouse, Pipe—a piece of LLIN bisecting a PVC pipe with adults released in the pipe, and Ctrl—no deployment of LLIN. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
A sequential ANOVA indicated that species significantly affected the percentage of insects reaching the commodity (Figure 8). In particular, there were 33-fold more T. castaneum that made it to the commodity than either R. dominica or T. variabile. In addition, while the treatment did not have an overall effect, there was a significant species by treatment interaction (Table 4). Regardless of method, deployment of LLIN resulted in an 88–94% reduction in the percentage of T. castaneum making it to the commodity, while it had no significant effect for the other two species, which both had uniformly low success in reaching the commodity regardless of treatment.
By contrast, species did not significantly affect the number of insects partially dispersing, but the LLIN deployment method did (Table 4). There were 60–74% fewer individuals that partially dispersed across the pilot-scale warehouse in the hanging and pipe deployment of LLIN compared to the control without LLIN and the cover treatment. The cover treatment likely did not have as much impact on individuals partially dispersing because it was located so close to the commodity (e.g., far from the release point of the insects). Further, there was a significant interaction between species and LLIN deployment method (Table 4). The hanging and pipe deployment of LLIN reduced the percentage of partially dispersing R. dominica and T. variabile by 89–96% and 78–98%, respectively, while it increased the percentage of partially dispersing T. castaneum, a much stronger walker, by 152–160% (Figure 8).
Species, LLIN deployment method, and the interaction between the two all significantly affected the percentage of insects that did not disperse. There were 2.2- to 2.3-fold more R. dominica and T. variabile that did not disperse compared to T. castaneum. Furthermore, there were 20–72% more individuals that did not disperse across the pilot-scale warehouse in the hanging and pipe deployment of LLIN compared to the control without LLIN, likely because the release point was so close to the plane of deployed LLIN. Additionally, the cover treatment allowed a greater number of individuals to disperse, because the LLIN was located so close to the commodity (e.g., far from the insect release point in the warehouse), with 1.5–2.1-fold more individuals dispersing compared to the hanging and pipe treatments. The LLIN deployment method had a much stronger effect on the percentage of T. castaneum that did not disperse compared to either R. dominica or T. variabile.
The LLIN deployment method significantly affected progeny production 6 weeks after bringing the commodity back to an environmental chamber from the pilot-scale warehouse (Table 4 and Figure 8). Warehouses that deployed LLIN had a 98–100% reduction in T. castaneum progeny production compared to control warehouses without LLIN (χ2 = 21.4; df = 3; P < 0.0001). The only appreciable number of progeny from the commodities was from T. castaneum, but was confined to the controls (Figure 8). There were no significant differences between the three types of LLIN deployments, suggesting that infestation and contamination of commodities could be reduced through multiple different LLIN application methods.
Overall, the MANOVA indicated that treatment, species, and their interaction had a significant effect on the percentage of affected insects recaptured throughout a warehouse (Table 4). However, neither the species, LLIN deployment method, nor their interaction significantly changed the percentage of affected individuals in the commodity. In addition, the released species did not significantly alter the percentage of affected individuals found to be partially dispersing in the warehouse, but the LLIN deployment method and its interaction with species did (Table 4). For instance, there was a 44% reduction in the percentage of insects partially dispersing when LLIN was deployed in the pipe treatment compared to the control treatment. However, species responded differently to the LLIN deployment method, with the percentage of partially dispersing R. dominica reduced by 7.7–9.3-fold compared to controls without LLIN, while partially dispersing T. castaneum and T. variabile increased by 9–15 and 3–5-fold.
The LLIN deployment method, species, and their interaction significantly changed the number of individuals that did not disperse in pilot-scale warehouses that were affected (Table 4). There were 8–13-fold more affected individuals among the insects that did not disperse in the pipe and hanging LLIN deployment compared to the control, likely due to the proximity of treated netting to the release location. On average, 1.4-fold more R. dominica did not disperse compared to either of the other two species. The interaction between the two variables was likely quantitative, as there were 5–7, 20–49, and 14–18-fold fewer affected R. dominica, T. castaneum, and T. variabile, respectively, that did not disperse in the hanging and pipe deployment of LLIN compared to controls without LLIN.
By contrast, the LLIN deployment method, species, and their interaction strongly affected the number of recaptured dead insects within the group of insects that did not disperse (Table 4). For example, there was a 1.7–1.8-fold more dead insects found in the no dispersal group in the hanging and pipe deployments compared to controls, again likely a result of the proximity of the LLIN to the release point. There were 3.9–20-fold more dead T. variabile that did not disperse than either R. dominica or T. castaneum, respectively. While there were 6–10-fold and 1.4–1.5-fold more dead R. dominica and T. variabile, respectively, recaptured in the no dispersal group for the hanging and pipe deployments than the controls, there was 8–67% fewer T. castaneum (Figure 9). However, none of the variables affected the percentage of dead insects in the commodity at the far end of the warehouse, nor the percentage of dead insects that were able to partially disperse prior to mortality (Figure 9).
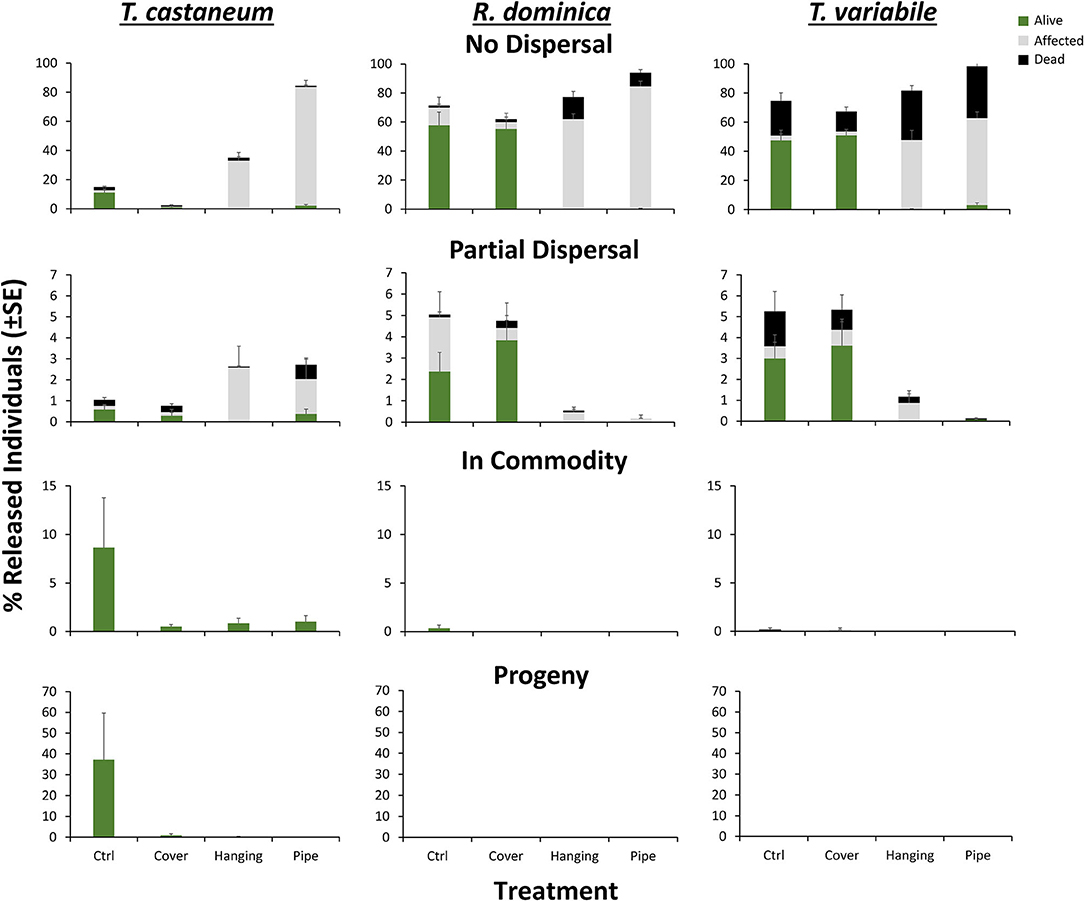
Figure 9. Mean (±SE) percentage of 100 T. castaneum (left column), R. dominica (middle), and T. variabile (right) recaptured that were classified as alive (green), affected (gray), or dead (black) after dispersing 72 h in pilot-scale warehouses with different methods of LLIN deployment in Manhattan, KS during 2019. Individuals were recorded as not dispersing (Zone 6, top row), partially dispersing (Zones 2–5, second row), infesting the commodity (Zone 1, third row), or producing progeny after a 6-week holding period (bottom row). Deployment methods included: Cover—covering the commodity directly with LLIN, Hanging—a single piece of LLIN bisecting the warehouse, Pipe—a piece of LLIN bisecting a PVC pipe with adults released in the pipe, and Ctrl—no deployment of LLIN. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
An overall MANOVA demonstrated no significant effect of single or combined tactics on the dispersal dynamics of insects in pilot scale warehouses, while species significantly altered dispersal, though not the interaction between the two (Table 5). Species affected the percentage of individuals making it to the commodity, partially dispersing, and not dispersing at all. Single or combined tactics, by contrast, had no effect on the dispersal of insects in warehouses, nor did its interaction with species. For example, only T. castaneum were able to reach the commodity (Figure 10), while there were 2.9–3.3-fold more T. variabile that did not disperse compared to R. dominica and T. castaneum, respectively. By contrast, there were 5- and 7-fold more T. variabile and T. castaneum, respectively, that partially dispersed compared to R. dominica. Only R. dominica and T. castaneum were captured by interception traps, but at low levels (Figure 11).
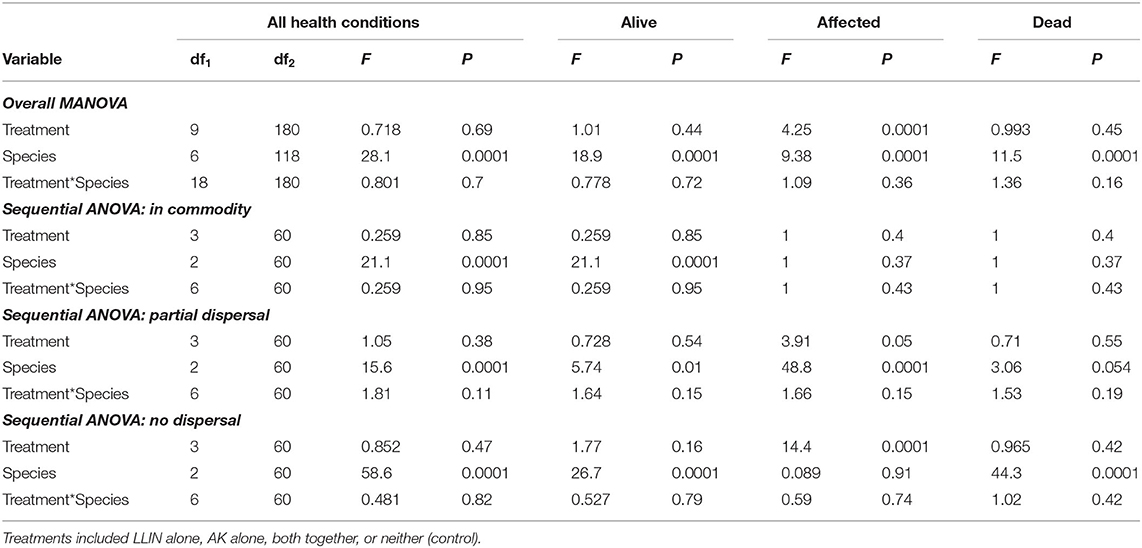
Table 5. Statistical model results for recapture of individuals in the commodity, partially dispersing, and not dispersing in the Trial 2 examining management tactic efficacy in a pilot-scale warehouse release-recapture assay deployed in 2019 in Manhattan, KS.
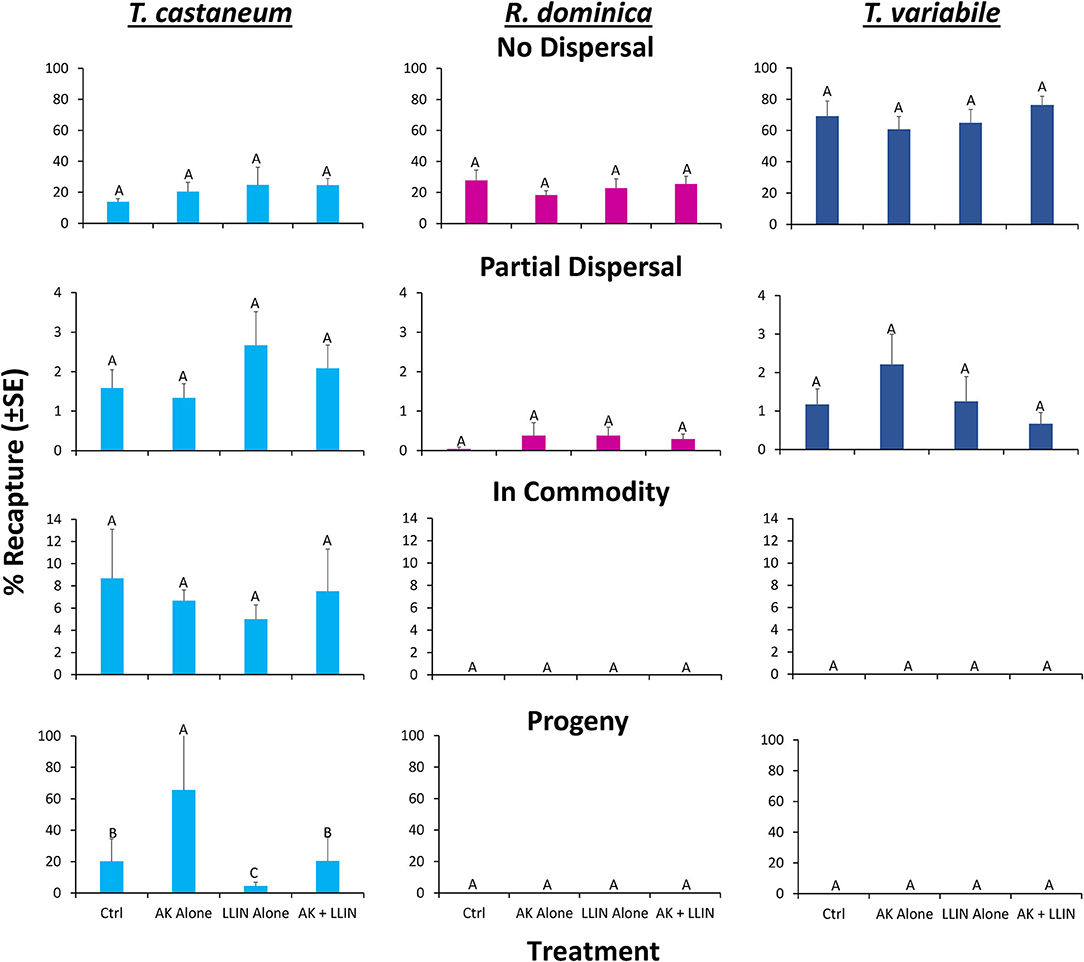
Figure 10. Mean (±SE) percentage of 100 T. castaneum (light blue bars), R. dominica (pink bars), and T. variabile (dark blue bars) adults released in pilot-scale warehouses in Manhattan, KS during 2019 recaptured after 72 h for Trial 2 to assess relative efficacy of LLIN alone (LLIN alone), AK-based interception traps alone (AK alone), both together (AK + LLIN), or neither (Ctrl). Individuals were recorded as not dispersing (Zone 6, top row), partially dispersing (Zones 2–5, second row), infesting the commodity (Zone 1, third row), or producing progeny after a 6-week holding period (bottom row). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
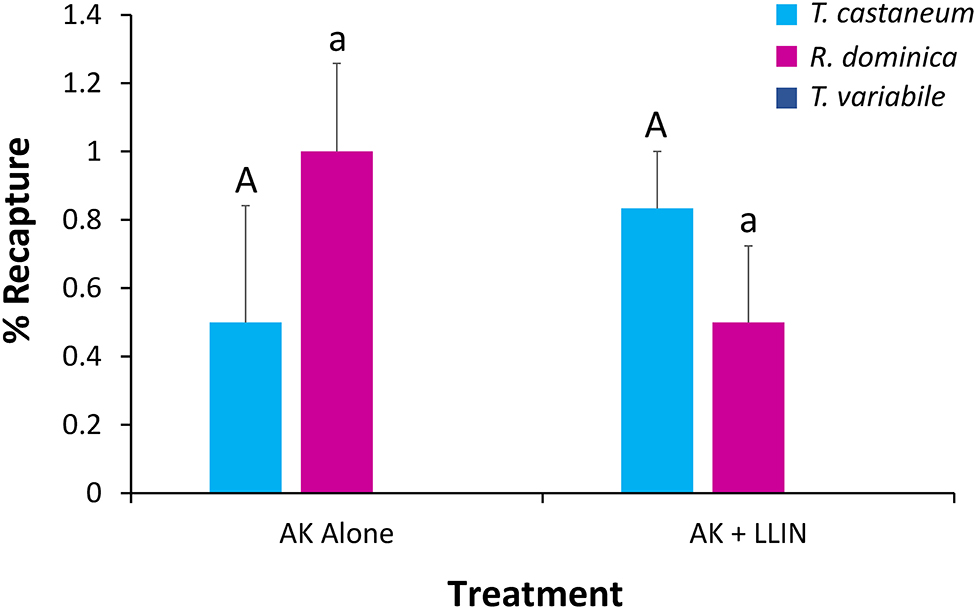
Figure 11. Mean (SE) percentage of 100 T. castaneum (light blue bars), R. dominica (pink bars), and T. variabile (dark blue bars) recaptured in AK-based interception traps deployed in pilot-scale warehouses during Trial 2 in Manhattan, KS in 2019. Lower case letters represent pairwise comparisons within R. dominica, while upper case letters represent pairwise comparisons within T. castaneum. Bars with shared letters are not significant different from each other (Tukey HSD, α = 0.05).
Only T. castaneum progeny were recorded in the commodities after 6 weeks (χ2 = 57.0; df = 2; P < 0.0001). The management tactic significantly affected progeny production (χ2 = 17.2; df = 3; P < 0.001), with an 83 and 19% reduction in the number of progeny produced in the commodity when LLIN alone or both LLIN and interception traps together were used, respectively (Figure 10). Conversely, there was 2.3-fold more progeny in the commodity where AK-based interception traps alone were used (Figure 10). There was no significant interaction between tactic and species (χ2 = 0.01; df = 6; P = 0.99). Therefore, LLIN, alone or with other IPM tools such as interception traps, can effectively reduce progeny production within stored products.
The management tactic and species changed the percentage of affected insects recaptured in the partial dispersal area of warehouses, but not their interaction (Table 5). On average, there was an 82% increase in the number of affected individuals recaptured in the partial dispersal area with interception traps alone compared to the control. For LLIN alone or both LLIN and interception traps, there were 1.6- and 1.5-fold more affected individuals recaptured in the partial dispersal area relative to the control where no tactics were deployed (Figure 12). There were 5.6 and 13.4-fold more affected T. castaneum than T. variabile or R. dominica recaptured in the partial dispersal area of the warehouse. Only the management tactic changed the percentage of affected individuals that did not disperse in warehouses, with 3, 5-, 2-, and 5-fold more affected individuals recaptured in the no dispersal group when using the interception trap only, LLIN only, or both combined, respectively, compared to warehouses without either. However, there were no affected insects recorded from the commodity, thus none of the variables had a significant effect on the percentage recaptured.
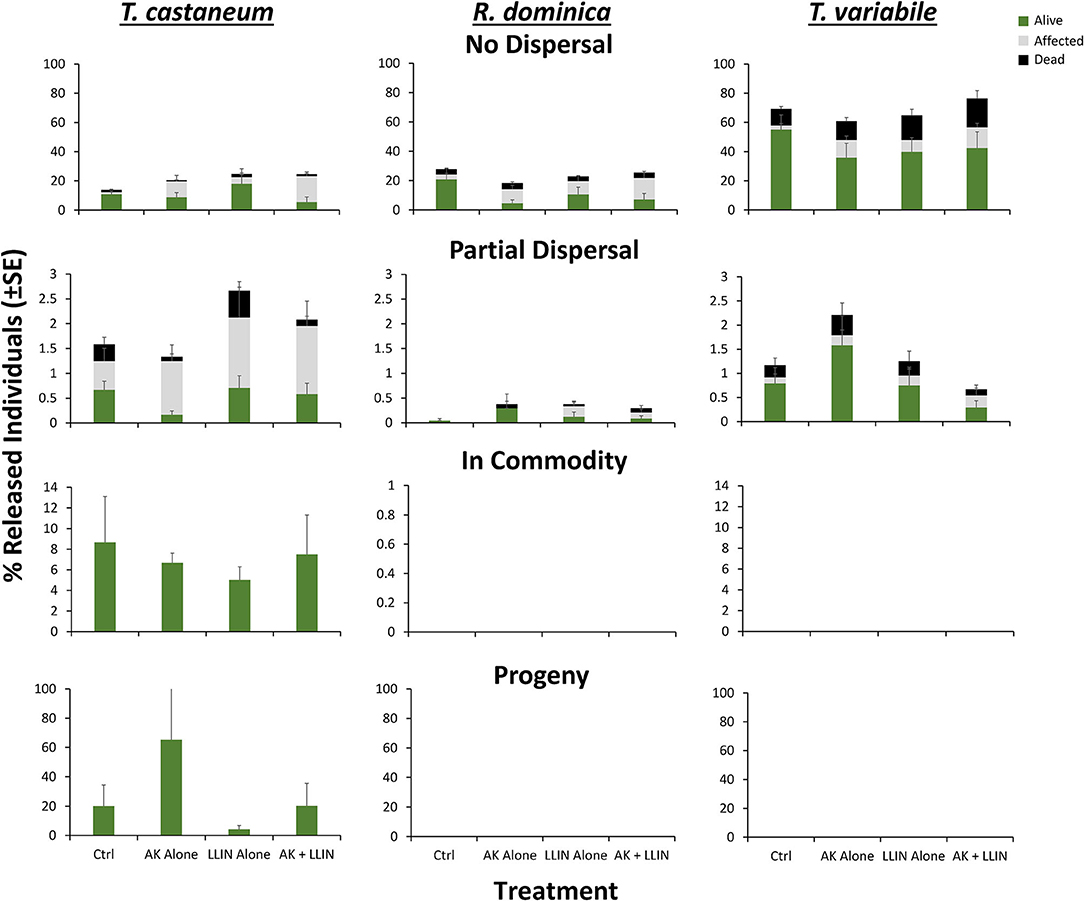
Figure 12. Mean (±SE) percentage of 100 T. castaneum (left column), R. dominica (middle), and T. variabile (right) recaptured that were classified as alive (green), affected (gray), or dead (black) after dispersing 72 h for Trial 2 in pilot-scale warehouses in Manhattan, KS during 2019 to assess the relative efficacy of LLIN alone (LLIN alone), AK-based interception traps alone (AK alone), both together (AK + LLIN), or neither (Ctrl). Individuals were recorded as not dispersing (Zone 6, top row), partially dispersing (Zones 2–5, second row), infesting the commodity (Zone 1, third row), or producing progeny after a 6-week holding period (bottom row). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
By contrast, only species identity affected the percentage of dead insects recaptured in the partial or no dispersal zones, but not the management tactic used, nor their interaction (Table 5). No dead insects were found in the commodity (Figure 12). There was a 5.2-fold greater percentage of dead T. castaneum and T. variabile that partially dispersed prior to death compared to R. dominica. There were 3- and 11-fold more dead R. dominica and T. variabile, respectively, that did not disperse compared to T. castaneum.
Overall, we have found that LLIN was highly effective when used alone or in interception traps to halt immigrating stored product insects. It appears likely that food facilities with LLIN deployed will have less insect colonization and fewer infestations. Many insects that contact LLIN while moving through facilities will be affected, through reduced mobility and increased mortality, and the net result is that individuals will be unable to successfully infest commodities.
While the findings from this study found no dose-dependency using the commercial SPB tab lure in AK-based interception traps, interception traps were still able to intercept naturally-occurring insects immigrating toward commercial food facilities. This aligns with previous literature, where AK traps were used to successfully intercept insects and monitor for or reduce infestations in other agricultural settings (Navarro-Llopis et al., 2013; Camelo et al., 2014; Morrison et al., 2016b). Deploying these traps around the perimeters of food facilities could be effective at capturing insects, but it is currently unknown what percentage of immigrating insects in the vicinity would be ensnared by the traps (e.g., their trapping efficiency). In the wind tunnel assays, a very small fraction of T. castaneum and a somewhat larger proportion of R. dominica did not respond to stimuli; however, this likely is related to the propensity of T. castaneum to orient in an upwind direction with air movement (Campbell, 2012) and the poor mobility of R. dominica (Morrison et al., 2019b) rather than reflecting on the suitability of the stimuli in the interception traps. Future work should investigate the density of traps needed and the distance these traps should be placed from each other and the food facility for optimal effectiveness. Optimizing deployment could increase the efficiency of trapping for stored product pests and avoid unnecessary costs or loss of product (Hossain et al., 2010; Sargent et al., 2014). Additionally, effectiveness of AK could be improved through the identification of more effective attractants and different trap designs. Other novel cues should be investigated that may elicit a stronger attraction from insects than the lure or trap design used in this study. For example, in other work, aggregation pheromones or other sensory stimuli deployed in AK settings are commonly synergized by the presence of host plant volatiles (Morrison et al., 2016a; Wallingford et al., 2018), while trap type for another agricultural pest, H. halys, significantly affected successful capture (Morrison et al., 2015). In stored products, traps that combined pheromonal cues and food (e.g., cracked wheat) resulted in greater captures of S. zeamais and S. oryzae than traps with either cue alone (Likhayo and Hodges, 2000). Ideally, in AK-based approaches, the goal is to attract a large number of insects to a circumscribed area; however, we were not able to increase captures by increasing the number of lures in the trap. Despite the very short deployment periods (~48 h) over the course of two summers, these interception traps were able to attract 3,800 insects, suggesting that these traps were fairly effective. Further, the community of pests captured in our traps were indicative of the typical pest species found in the wheat and rice growing regions of Kansas and Arkansas, which comprise the primary wheat and rice production areas of the U.S. (Campbell and Arbogast, 2004; McKay et al., 2017; USDA-NASS, 2020). Other parts of the world may have different pest communities, thus, the response of these insects to our interception traps would need to be investigated against other pest communities if considered for adoption in an area with a significantly different pest community. Importantly, the inclusion of LLIN eliminated progeny production of captured stored product insects, but did not hinder their entrance into interception traps, suggesting that it acted as an effective kill mechanism for insects that were captured while not reducing trap attractiveness. Finally, perhaps the largest limitation of using interception traps as they are currently designed is the fact that they cannot be deployed for long periods. This may be improved if provided with a small rain shelter or overhang built into the top of the trap, or by using a more durable kairomone source than grain, which easily molds under unprotected field conditions.
Furthermore, we found that deploying LLIN in pilot-scale warehouses significantly reduces the dispersal ability and commodity colonization by three species of stored product insects. These findings expand on a previous study that showed a significant decrease in the movement and dispersal of adult and immature T. castaneum and R. dominica after exposure to LLIN in the laboratory (Morrison et al., 2018; Wilkins et al., 2020). Regardless of deployment method, infestations and progeny production by T. castaneum decreased by 89–93% and 98–100%, respectively, in warehouses that incorporated the netting. The impact of LLIN deployment primarily affected T. castaneum; R. dominica and T. variabile exhibited low dispersal and colonization in the pilot-scale warehouse, possibly due to the nature of the control, which consisted of a PVC pipe. This may have worsened R. dominica and T. variabile's walking abilities more than they inherently are, while preventing them from dispersal by flight. The PVC pipe, however, was necessary for use in the control treatment to rule out additional effects the pipe may have had on the dispersal of the three species in other treatments. A follow-up experiment evaluates this hypothesis by using a control where insects are simply released in the warehouse on the floor. Another factor may have been the duration of the experiment; a longer dispersal period may have improved colonization. Nevertheless, we found that even laying LLIN directly over a commodity reduced the ability of T. castaneum to infest the product. This is consistent with an earlier study that used an α-cypermethrin-based LLIN against L. serricorne and E. elutella to surround a carton of tobacco in a commercial tobacco facility (Athanassiou et al., 2019). Trees wrapped with LLIN also exhibited significantly reduced beetle attacks (Ranger et al., 2020). Another study found that LLIN reduced S. oryzae infestations of maize in mini-bag bioassays by 98–100%, however, there were varying amounts of permethrin residues on the maize (Anaclerio et al., 2018). Thus, applications of LLIN deployed farther from the commodity, like the hanging and pipe treatments used in this study, may be preferred for commercial implementation.
Interestingly, when interception traps were deployed together with LLIN, there was actually a significant decrease in efficacy and an increase in progeny production inside the commodity relative to deploying LLIN alone in pilot scale warehouses or even nothing at all. This may have arisen as a result of attractive stimuli in the traps promoting flight initiation (e.g., Cox and Dolder, 1995); thus, individuals may have been able to circumvent the LLIN at ground-level, which did not reach to the ceiling. Further, due to the size constraints of the pilot-scale warehouses, the area of arrestment around the trap may have been large enough to attract insects to the opening of the warehouse where they may have wandered in the vicinity of the trap until “accidentally” entering the warehouse. While sex pheromones typically attract individuals to a point-source emission, aggregation pheromones, by contrast, attract individuals only until they reach some threshold level of pheromone, after which they wander in a delimited area of arrestment around the odor source in multiple systems (Halliday and Blouin-Demers, 2016; Morrison et al., 2016b). Combined with the tight spatial arrangements, the interception trap was not actually placed in a realistic location if the setup were adjusted to scale. Instead of being placed on the equivalent of the perimeter of a food facility where the area of arrestment would not intersect buildings, the interception trap was placed at the equivalent of the front door for the facility in our experiment. Thus, the net result was greater commodity colonization when interception traps were used alone compared to other treatments. Nonetheless, we have successfully shown the utility of LLIN at pilot-scale, and future work should assess both the specific area of arrestment around the interception traps and the use of LLIN against insect immigration in commercial-scale food facilities and in bulk storage commodity bins. While our results suggest that LLIN is an effective, preventative IPM tool that can work along with other tactics in a comprehensive IPM program, it is unlikely to completely replace the need for fumigations. However, it could reduce the number of treatments required. Therefore, the ability of LLIN to reduce fumigation events should also be further evaluated in future studies to confirm these predictions.
Exposure to LLIN does not always result in mortality, but may instead manifest as indirect toxicity through reduced movement and dispersal. In this study, there was an extensive number of affected individuals recaptured inside the warehouses. These were most often the insects that contacted the netting as they attempted to move through the warehouse toward the commodity. However, it was clear that the netting was successfully acting as a barrier to dispersal because most of these insects were found near the LLIN, but situated on the opposite side of the LLIN relative to the food source. Thus, while insects can physically pass through the netting, the knockdown effects are immediate enough that most insects are unable to crawl through the netting and advance farther into the warehouse to colonize commodities. Importantly, this portends success in the use of LLIN in the ways that we are describing here for food facilities. These results are also in line with a previous study that found both brief, multiple and continuous, longer exposures to LLIN by T. castaneum resulted in equally poor recovery (Arthur et al., 2020; Gerken et al., 2020). Additionally, in the AK-based interception trap field study and Trial 1 semi-field study, progeny production in the traps with LLIN and the commodities of pilot-scale warehouse where LLIN was deployed was minimal, which is consistent with past research on the sublethal effects of deltamethrin on progeny production (Athanassiou et al., 2004). Accounting for both these lethal and non-lethal effects of insecticide-incorporated netting provides a fuller picture of the efficacy of the netting as an IPM tool (Guedes et al., 2016).
Finally, there is more research needed on the efficacy of LLIN and interceptions traps together at a commercial level, especially if improvements to AK-based interception trap design or stimuli are made. For example, it would be of interest to know from what distance stored product insects are attracted to interception traps, and whether the use of LLIN can result in decreased numbers of insects inside facilities or bins and thus reduce the need for suppressive management tactics such as fumigation. Previous studies show that integrating additional tools like AK improved the overall efficacy of pest management programs by lowering total crop loss (Hafsi et al., 2016; Rahman and Broughton, 2016). Additionally, deploying an interception trap incorporating LLIN will likely result in multiple exposures to the netting, decreasing the chances of insects like T. castaneum recovering (Gerken et al., 2020). Furthermore, incorporating novel insecticide active ingredients into the netting will help mitigate insecticide resistance in insect pest populations and should also be investigated. Building upon and implementing these novel tactics and IPM programs will help conserve the efficacy of current fumigant tools for years to come.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Wilkins, R. V., Campbell, J. F., Zhu, K. Y., Starkus, L., McKay, T., Morrison, W. R. (unpublished). Data from: “Long-lasting insecticide-incorporated netting and interception traps at pilot-scale warehouses and commercial facilities prevents infestation by stored product beetles.” Ag Data Commons. https://data.nal.usda.gov/dataset/data-long-lasting-insecticide-incorporated-netting-and-interception-traps-pilot-scale-warehouses-and-commercial-facilities-prevents-infestation-stored-product-beetles. (accessed May 12, 2020).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded, in part, by a United States Department of Agriculture, National Institute of Food and Agriculture, Crop Protection and Pest Management Grant No. 2017-70006-27262.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the excellent technical assistance of Chloe Albin (USDA), Brian Barnett (USDA), Alexander Bruce (USDA), Ken Friesen (USDA), Robert Grosdidier (USDA), Matt Hamblin (KSU), Rich Hammel, Kathy Leonard, and Hannah Quellhorst (KSU). The use of trade names is for the purposes of providing scientific information only, and does not constitute endorsement by the United States Department of Agriculture. The USDA is an equal opportunity employer.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2020.561820/full#supplementary-material
Alonso, P. L., Lindsay, S. W., Armstrong, J. R. M., Conteh, M., Hill, A. G., David, P. H., et al. (1991). The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet 337, 1499–1502. doi: 10.1016/0140-6736(91)93194-E
Anaclerio, M., Pellizzoni, M., Todeschini, V., Kane, D., Hanafi, A., Trevisan, M., et al. (2018). Efficacy and residues of permethrin-incorporated nets used to protect maize grains postharvest. Pest. Manag. Sci. 74, 240–245. doi: 10.1002/ps.4709
Arthur, F. H., Athanassiou, C. G., and Morrison, W. R. (2020). Mobility of stored product beetles after exposure to a combination insecticide containing deltamethrin, methoprene, and a piperonyl butoxide synergist depends on species, concentration, and exposure time. Insects 11:151. doi: 10.3390/insects11030151
Athanassiou, C. G., Papagregoriou, A. S., and Buchelos, C. (2004). Insecticidal and residual effect of three pyrethroids against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on stored wheat. J. Stor. Prod. Res. 40, 289–297. doi: 10.1016/S0022-474X(03)00025-0
Athanassiou, C. G., Rumbos, C. I., Stephou, V. K., Sakka, M., Schaffert, S., Sterz, T., et al. (2019). Field evaluation of Carifend® net for the protection of stored tobacco from storage insect pests. J. Stor. Prod. Res. 81, 46–52. doi: 10.1016/j.jspr.2018.12.005
Balakrishnan, K., Holighaus, G., Weißbecker, B., and Schütz, S. (2017). Electroantennographic responses of red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to volatile organic compounds. J. Appl. Entomol. 141, 477–486. doi: 10.1111/jen.12366
Batte, M. T., Hooker, N. H., Haab, T. C., and Beaverson, J. (2006). Putting their money where their mouths are: consumer willingness to pay for multi-ingredient, processed organic food products. Food Policy 32, 145–159. doi: 10.1016/j.foodpol.2006.05.003
Bretz, F., Hothorn, T., and Westfall, P. (2010). Multiple Comparisons Using R, Version 1.4. Boca Raton, FL: CRC Press, 208. Available online at: http://multcomp.R-forge.R-project.org (accessed November 15, 2020).
Buckman, K. A., Campbell, J. F., and Subramanyam, B. (2013). Tribolium castaneum (Coleoptera: Tenebrionidae) associated with rice mills: fumigation efficacy and population rebound. J. Econ. Entomol. 106, 499–512. doi: 10.1603/EC12276
Burkholder, W. E., and Ma, M. (1985). Pheromones for monitoring and control of stored product insects. Ann. Rev. Entomol. 30, 257–272. doi: 10.1146/annurev.en.30.010185.001353
Camelo, L. D. A., Landolt, P. J., and Zack, R. S. (2014). A kairomone based attract-and-kill system effective against alfalfa looper (Lepidoptera: Noctuidae). J. Econ. Entomol. 100, 366–374. doi: 10.1093/jee/100.2.366
Campbell, J. F. (2012). Attraction of walking Tribolium castaneum adults to traps. J. Stor. Prod. Res. 51, 11–22. doi: 10.1016/j.jspr.2012.06.002
Campbell, J. F., and Arbogast, R. T. (2004). Stored-product insects in a flour mill: population dynamics and response to fumigation treatments. Entomol. Exp. Appl. 112, 217–225. doi: 10.1111/j.0013-8703.2004.00197.x
Campbell, J. F., Mullen, M. A., and Dowdy, A. K. (2002). Monitoring stored-product pests in food processing plants with pheromone trapping, contour mapping, and mark-recapture. J. Econ. Entomol. 95, 1089–1101. doi: 10.1093/jee/95.5.1089
Charmillot, P. J., Hofer, D., and Pasquier, D. (2000). Attract and kill: a new method for control of the codling moth Cydia pomonella. Entomol. Exp. Appl. 94, 211–216. doi: 10.1046/j.1570-7458.2000.00621.x
Ching'oma, G. P., Campbell, J. F., Toews, M. D., and Ramaswamy, S. B. (2006). “Spatial distribution and movement patterns of stored-product insects,” in Proceedings, 9th International Working Conference on Stored Product Protection (Brazilian Post-harvest Association–ABRAPOS: Passo Fundo), 361–370.
Cox, P. D. (2004). Potential for using semiochemicals to protect stored products from insect infestation. J. Stor. Prod. Res. 40, 1–25. doi: 10.1016/S0022-474X(02)00078-4
Cox, P. D., and Dolder, H. S. (1995). A simple flight chamber to determine flight activity in small insects. J. Stor. Prod. Res. 31, 311–316. doi: 10.1016/0022-474X(95)00023-Z
Daglish, G. J., Ridley, A. W., Reid, R., and Walter, G. H. (2017). Testing the consistency of spatio-temporal patterns of flight activity in the stored grain beetles Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). J. Stor. Prod. Res. 72, 68–74. doi: 10.1016/j.jspr.2017.03.005
Dev, V., Raghavendra, K., Barman, K., Phookan, S., and Dash, A. P. (2010). Wash-resistance and field efficacy of OlysetTM net, a permethrin-incorporated long-lasting insecticidal netting, against Anopheles minimus-transmitted malaria in Assam, northeastern India. Vector-borne Zoonot 10, 403–410. doi: 10.1089/vbz.2008.0191
El-Sayed, A. M., Suckling, D. M., Byers, J. A., Jang, E. B., and Wearing, C. H. (2009). Potential of “lure and kill” in long-term pest management and eradication of invasive species. J. Econ. Entomol. 102, 815–835. doi: 10.1603/029.102.0301
Espino, L., Greer, C. A., Mutters, R., and Thompson, J. F. (2014). Survey of rice storage facilities identifies research and education needs. Calif. Agric. 68, 38–46. doi: 10.3733/ca.v068n01p38
Fardisi, M. (2015). Investigation of dried distillers grains with solubles (DDGS) susceptibility to red flour beetle (Tribolium castaneum (Herbst)) infestation (Doctoral dissertation). Department of Entomology, Purdue University, West Lafayette, IN, USA.
Fields, P. G., and White, N. D. G. (2002). Alternatives to methyl bromide treatments for stored-product and quarantine insects. Ann. Rev. Entomol. 47, 331–359. doi: 10.1146/annurev.ento.47.091201.145217
Gerken, A. R., Scully, E. D., Campbell, J. F., and Morrison, W. R. (2020). Effectiveness of long-lasting insecticide netting on Tribolium castaneum is modulated by multiple exposures, biotic, and abiotic factors. Pest Manag. Sci. doi: 10.1002/ps.6134
Gregg, P. C., Del Socorro, A. P., and Landolt, P. J. (2018). Advances in attract-and-kill for agricultural pests: beyond pheromones. Annu. Rev. Entomol. 63, 453–470. doi: 10.1146/annurev-ento-031616-035040
Guedes, R. N. C., Smagghe, G., Stark, J. D., and Desneux, N. (2016). Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Ann. Rev. Entomol. 61, 43–62. doi: 10.1146/annurev-ento-010715-023646
Hafsi, A., Abbes, K., Harbi, A., Duyck, P. F., and Chermiti, B. (2016). Attract-and-kill systems efficiency against Ceratitis capitata (Diptera: Tephritidae) and effects on non-target insects in peach orchards. J. Appl. Entomol. 140, 28–36. doi: 10.1111/jen.12259
Hailu, A., Lindtjørn, B., Deressa, W., Gari, T., Loha, E., and Robberstad, B. (2018). Cost-effectiveness of a combined intervention of long lasting insecticidal nets and indoor residual spraying compared with each intervention alone for malaria prevention in Ethiopia. Cost. Eff. Resour. Alloc. 16:61. doi: 10.1186/s12962-018-0164-1
Halliday, W. D., and Blouin-Demers, G. (2016). Male aggregation pheromones inhibit ideal free habitat selection in red flour beetles (Tribolium castaneum). J. Insect Behav. 29, 355–367. doi: 10.1007/s10905-016-9565-1
Hossain, M. S., Hossiain, M. A., Williams, D. G., and Chandra, S. (2010). Potential to reduce the spatial density of attract and kill traps required for effective control of Carpophilus spp. (Coleoptera: Nitidulidae) in stone fruit in Australia. Aust. J. Entomol. 49, 170–174. doi: 10.1111/j.1440-6055.2010.00748.x
Huang, Y., Li, F., Liu, M., Wang, Y., Shen, F., and Tang, P. (2018). Susceptibility of Tribolium castaneum to phosphine in China and functions of cytochrome P450s in phosphine resistance. J. Pest Sci. 92, 1239–1248. doi: 10.1007/s10340-019-01088-7
Hubert, J., Münzbergová, Z., Kučerová, Z., and Stejskal, V. (2006). Comparison of communities of stored product mites in grain and grain residues in the Czech Republic. Exp. Appl. Acarol. 28:149. doi: 10.1007/s10493-006-0026-y
Hubert, J., Stejskal, V., Athanassiou, C. G., and Throne, J. E. (2018). Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol. 63, 553–573. doi: 10.1146/annurev-ento-020117-043218
Kroschel, J., and Zegarra, O. (2010). Attract-and-kill: a new strategy for the management of the potato tuber moths Phthorimaea operculella (Zeller) and Symmetrischema tangolias (Gyen) in potato: laboratory experiments towards optimizing pheromone and insecticide concentration. Pest Manag. Sci. 66, 490–496. doi: 10.1002/ps.1898
Kuhar, T. P., Short, B. D., Krawczyk, G., and Leskey, T. C. (2017). Deltamethrin-incorporated nets as an integrated pest management tool for the invasive Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 110, 543–545. doi: 10.1093/jee/tow321
Kumar, D., and Kalita, P. (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing counties. Foods 6:8. doi: 10.3390/foods6010008
Likhayo, P. W., and Hodges, R. J. (2000). Field monitoring Sitophilus zeamais and Sitophilus oryzae (Coleoptera: Curculionidae) using refuge and flight traps baited with synthetic pheromone and cracked wheat. J. Stor. Prod. Res. 36, 341–353. doi: 10.1016/S0022-474X(99)00052-1
Mahroof, R. M., Edde, P. A., Robertson, B., Puckette, J. A., and Phillips, T. W. (2010). Dispersal of Rhyzopertha dominica (Coleoptera: Bostrichidae) in different habitats. J. Econ. Entomol. 39, 930–938. doi: 10.1603/EN09243
Marianelli, L., Paoli, F., Peverieri, G. S., Benvenuti, C., Barzanti, G. P., Bosio, G., et al. (2018). Long-lasting insecticide-treated nets: a new integrated pest management approach for Popillia japonica (Coleoptera: Scarabaeidae). Integr. Environ. Asses. 15, 259–265. doi: 10.1002/ieam.4107
Martin, T., Chandre, F., Chabi, J., Guillet, P. F., Akogbeto, M., and Hougard, J. M. (2007). A biological test to quantify pyrethroid in impregnated nets. Trop. Med. Int. Health. 12, 245–250. doi: 10.1111/j.1365-3156.2006.01777.x
McKay, T., White, A. L., Starkus, L. A., Arthur, F. H., and Campbell, J. F. (2017). Seasonal patterns of stored-product insects at a rice mill. J. Econ. Entomol. 110, 1366–1376. doi: 10.1093/jee/tox089
Morrison, I. I. I. W. R, Grosdidier, R. F., Arthur, F. H., Myers, S. W., and Domingue, M. J. (2020). Attraction, arrestment, and preference by immature Trogoderma variabile and Trogoderma granarium to food and pheromonal stimuli. J. Pest Sci. 93, 135–147. doi: 10.1007/s10340-019-01171-z
Morrison, W. R., Blaauw, B. R., Short, B. D., Nielsen, A. L., Bergh, J. C., et al. (2019a). Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag. Sci. 75, 104–114. doi: 10.1002/ps.5156
Morrison, W. R., Cullum, J. P., and Leskey, T. C. (2015). Evaluation of trap designs and deployment strategies for capturing Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 108, 1683–1692. doi: 10.1093/jee/tov159
Morrison, W. R., Larson, N. L., Brabec, D. L., and Zhang, A. (2019b). Methyl benzoate as a putative alternative, environmentally-friendly fumigant for the control of stored product insects. J. Econ. Entomol. 112, 2458–2468. doi: 10.1093/jee/toz179
Morrison, W. R., Lee, D. H., Reissig, W. H., Combs, D., Leahy, K., Tuttle, A., et al. (2016a). Inclusion of specialist and generalist stimuli in attract-and-kill programs: their relative efficacy in apple maggot fly (Diptera: Tephritidae) pest management. Environ. Entomol. 45, 974–982. doi: 10.1093/ee/nvw043
Morrison, W. R., Lee, D. H., Short, B. D., Khrimian, A., and Leskey, T. C. (2016b). Establishing the behavioral basis for an attract-and-kill strategy to manage the invasive Halyomorpha halys in apple orchards. J. Pest Sci. 89, 81–96. doi: 10.1007/s10340-015-0679-6
Morrison, W. R., Wilkins, R. V., Gerken, A. R., Scheff, D. S., Zhu, K. Y., Arthur, F.H., et al. (2018). Mobility of adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after exposure to long-lasting insecticide-incorporated netting. J. Econ. Entomol. 111, 2443–2453. doi: 10.1093/jee/toy173
Nara, J. M., Lindsday, R. C., and Burkholder, W. E. (1981). Analysis of volatile compounds in wheat germ oil responsible for an aggregation response in Trogoderma glabrum larvae. J. Agric. Food Chem. 29, 68–72. doi: 10.1021/jf00103a018
Navarro-Llopis, V., Primo, J., and Vacas, S. (2013). Efficacy of attract-and-kill devices for the control of Ceratitis capitata. Pest Manag. Sci. 69, 478–482. doi: 10.1002/ps.3393
Paloukas, Y. Z., Agrafioti, P., Rumbos, C. I., Schaffert, S., Sterz, T., Bozoglou, C., et al. (2020). Evaluation of Carifend® for the control of stored-product beetles. J. Stor. Prod. Res. 85:101534. doi: 10.1016/j.jspr.2019.101534
Peverieri, G. S., Binazzi, F., Marianelli, L., and Roversi, P. F. (2017). Lethal and sublethal effects of long-lasting insecticide-treated nets on the invasive bug Halyomorpha halys. J. Appl. Entomol. 142, 141–148. doi: 10.1111/jen.12428
Phillips, T. W., Jiang, X.-L., Burkholder, W. E., Phillips, J. K., and Tran, H. Q. (1993). Behavioral responses to food volatiles by two species of stored-product Coleoptera, Sitophilus oryzae (Curculionidae), and Tribolium castaneum (Tenebrionidae). J. Chem. Ecol. 19, 723–734. doi: 10.1007/BF00985004
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Rafter, M. A., Muralitharan, V., Chandrasekaran, S., Mohankumar, S., Daglish, G. J., Loganathan, M., et al. (2019). Behaviour in the presence of resource excess—flight of Tribolium castaneum around heavily infested grain storage facilities. J. Pest Sci. 92, 1227–1238. doi: 10.1007/s10340-019-01085-w
Rahman, T., and Broughton, S. (2016). Suppressing Mediterranean fruit fly (Diptera: Tephritidae) with an attract-and-kill device in pome and stone fruit orchards in Western Australia. Crop Prot. 80, 108–117. doi: 10.1016/j.cropro.2015.11.005
Ranger, C. M., Werle, C. T., Scultz, P. B., Addesso, K. M., Oliver, J. B., and Reding, M. E. (2020). Long-lasting insecticide netting for protecting tree stems from attack by ambrosia beetles (Coleoptera: Curculionidae: Scolytinae). Insects 11:8. doi: 10.3390/insects11010008
Ridley, A. W., Hereward, J. P., Daglish, G. J., Raghu, S., Collins, P. J., and Walter, G. H. (2011). The spatiotemporal dynamics of Tribolium castaneum (Herbst): adult flight and gene flow. Mol. Ecol. 20, 1635–1646. doi: 10.1111/j.1365-294X.2011.05049.x
Roesli, R., Subramanyam, B., Fairchild, F. J., and Behnke, K. C. (2003). Trap catches of stored-product insects before and after heat treatments in a pilot feed mill. J. Stor. Prod. Res. 39, 521–540. doi: 10.1016/S0022-474X(02)00058-9
Rumbos, C. I., Sakka, M., Schaffert, S., Sterz, T., Austin, J. W., Bozoglou, C., et al. (2018). Evaluation of Carifend®, an alpha-cypermethrin-coated polyester net, for the control of Lasioderma serricorne and Ephestia elutella in stored tobacco. J. Pest Sci. 91, 751–759. doi: 10.1007/s10340-017-0947-8
Sargent, C., Martinson, H. M., and Raupp, M. J. (2014). Traps and trap placement may affect location of brown marmorated stink bug (Hemiptera: Pentatomidae) and increase injury to tomato fruits in home gardens. Environ. Entomol. 43, 432–438. doi: 10.1603/EN13237
Schlipalius, D. I., Tuck, A. G., Jagadeesan, R., Nguyen, T., Kaur, R., Subramanian, S., et al. (2018). Variant linkage analysis using de novo transcriptome sequencing identifies a conserved phosphine resistance gene in insects. Genetics 209, 281–290. doi: 10.1534/genetics.118.300688
Semeao, A. A., Campbell, J. F., Hutchinson, J. M. S., Whitworth, R. J., and Sloderbeck, P. E. (2013a). Spatio-temporal distribution of stored-product insects around food processing and storage facilities. Agr. Ecosyst. Environ. 165, 151–162. doi: 10.1016/j.agee.2012.11.013
Semeao, A. A., Campbell, J. F., Whitworth, R. J., and Sloderbeck, P. E. (2013b). Movement of Tribolium castaneum within a flour mill. J. Stor. Prod. Res. 54, 17–22. doi: 10.1016/j.jspr.2013.03.004
USDA-NASS (2020). Quickstats v. 2.0. Washington, DC: U.S. Department of Agriculture, National Agricultural Statistics Service. Available online at: https://quickstats.nass.usda.gov/ (accessed December 14, 2020).
Vela-Coiffier, E. L., Fargo, W. S., Bonjour, E. L., Cuperus, G. W., and Warde, W. D. (1997). Immigration of insects into on-farm stored wheat and relationships among trapping methods. J. Stor. Prod. Res. 33, 157–166. doi: 10.1016/S0022-474X(96)00043-4
Wacker, F. (2018). “Food waste and food losses—importance of international partnerships and research,” in Proceedings, 12th International Working Conference on Stored Product Protection (Berlin: Julius-Kühn Institut), 31.
Wakefield, M. E., and Dunn, J. A. (2005). Effectiveness of the BT mite trap for detecting the storage mite pests, Acarus siro, Lepidoglyphus destructor, and Tyrophagus longior. Exp. Appl. Acarol. 35:17. doi: 10.1007/s10493-004-2678-9
Wallingford, A. K., Kuhar, T. P., and Weber, D. C. (2018). Avoiding unwanted vicinity effects with attract-and-kill tactics for harlequin bug, Murgantia histrionica (Hahn) (Hemiptera: Pentatomidae). J. Econ. Entomol. 111, 1780–1787. doi: 10.1093/jee/toy109
Wilkins, R. V., Zhu, K. Y., Campbell, J. F., and Morrison, W. R. (2020). Mobility and dispersal of two cosmopolitan stored product insects are adversely affected by long-lasting netting in a life stage-dependent manner. J. Econ. Entomol. 113, 1768–1779. doi: 10.1093/jee/toaa094
Keywords: stored product insect, postharvest, chemical ecology, attract-and-kill, integrated pest management
Citation: Wilkins RV, Campbell JF, Zhu KY, Starkus LA, McKay T and Morrison WR III (2021) Long-Lasting Insecticide-Incorporated Netting and Interception Traps at Pilot-Scale Warehouses and Commercial Facilities Prevents Infestation by Stored Product Beetles. Front. Sustain. Food Syst. 4:561820. doi: 10.3389/fsufs.2020.561820
Received: 07 August 2020; Accepted: 03 December 2020;
Published: 11 January 2021.
Edited by:
Marcello Iriti, University of Milan, ItalyReviewed by:
Jan Hubert, Crop Research Institute (CRI), CzechiaCopyright © 2021 Wilkins, Campbell, Zhu, Starkus, McKay and Morrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel V. Wilkins, cnZ3QGtzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.