- 1Environmental Microbial and Food Safety Laboratory, Beltsville Agricultural Research Center, U.S. Department of Agriculture, Beltsville, MD, United States
- 2Department of Nutrition and Food Science, University of Maryland, College Park, MD, United States
Treated wastewater (TW) and roof-collected rain water (RW) that meet the required microbial quality as per Food Safety Modernization Act (FSMA) regulation may serve as alternative irrigation water sources to decrease the pressure on the current water scarcity. Alternative water sources may have different water characteristics that influence the survival and transfer of microorganisms to the irrigated produce. Further, these water sources may contain pathogenic bacteria such as Shiga-toxigenic Escherichia coli. To evaluate the risk associated with TW and RW irrigation on the fresh produce safety, the effect of TW and RW irrigation on the transfer of two non-pathogenic E. coli strains as surrogates for E. coli O157:H7 to different lettuce cultivars grown in the field was investigated. Lettuce cultivars “Annapolis,” “Celinet,” and “Coastline” were grown in the field at the Fulton farm (Chambersburg, PA). Approximately 10 days before harvest, lettuce plants were spray-irrigated with groundwater (GW), TW, or RW containing 6 log CFU ml−1 of a mixture of nalidixic acid-resistant E. coli O157:H12 and chloramphenicol-resistant E. coli K12 in fecal slurry as non-pathogenic surrogates for E. coli O157:H7. On 0, 1, 3, 7, and 10 days post-irrigation, four replicate lettuce leaf samples (30 g per sample) from each group were collected and pummeled in 120 ml of buffered peptone water for 2 min, followed by spiral plating on MacConkey agars with antibiotics. Results showed that the recovery of E. coli O157:H12 was significantly greater than the populations of E. coli K12 recovered from the irrigated lettuce regardless of the water sources and the lettuce cultivars. The TW irrigation resulted in the lowest recovery of the E. coli surrogates on the lettuce compared to the populations of these bacteria recovered from the lettuce with RW and GW irrigation on day 0. The difference in leaf characteristics of lettuce cultivars significantly influenced the recovery of these surrogates on lettuce leaves. Populations of E. coli O157:H12 recovered from the RW-irrigated “Annapolis” lettuce were significantly lower than the recovery of this bacterium from the “Celinet” and “Coastline” lettuce (P < 0.05). Overall, the recovery of specific E. coli surrogates from the RW and TW irrigated lettuce was comparable to the lettuce with the GW irrigation, where GW served as a baseline water source. E. coli O157:H12 could be a more suitable surrogate compared to E. coli K12 because it is an environmental watershed isolate. The findings of this study provide critical information in risk assessment evaluation of RW and TW irrigation on lettuce in Mid-Atlantic area.
Introduction
The World Health Organization encourages people to consume at least 400 g of fresh produce such as fruits and vegetables per day to prevent chronic disease including heart disease, diabetes, and obesity (World Health Organization, 2018). Lettuce is the most consumed leafy green vegetables in the United States with an annual consumption of 5.9 kg per person [U.S. Department of Agriculture (USDA), 2016]. As the consumption of fresh produce increased during the past decades, incidences for foodborne outbreaks linked to fresh produce have also increased [Sivapalasingam et al., 2004; European Food Safety Authority (EFSA), 2012; Painter et al., 2013]. In European Union, fresh produce-associated outbreaks accounted for 8.7% of all reported outbreaks in 2010 compared to 2.1% in 2009 [European Food Safety Authority (EFSA), 2011, 2012]. A total of 110 outbreaks in the U.S. between 2010 and 2017 were associated with the consumption of the contaminated lettuce; pathogenic Escherichia coli was responsible for 24 of these outbreaks that resulted in 506 illnesses, 159 hospitalizations, and one death [National outbreak reporting system (NORS), 2019].
Contamination of produce may occur at any point during the production of fresh produce through soil, animal manures, farming equipment, and post-harvest processes (Holvoet et al., 2015; Weller et al., 2017); however, agricultural water has been identified as a major risk factor in the contamination of leafy greens [Steele and Odumeru, 2004; Food and Drug Administration (FDA), 2008; Gerba, 2009; Jung et al., 2014; Araújo et al., 2017]. Cross-contamination of field-grown vegetables by the foodborne pathogens through the contaminated irrigation water with fecal waste or improperly composted manure is suggested to be a possible source of pathogen at the pre-harvest level (Oliveira et al., 2012). Attachment of human bacterial pathogens on fresh produce during spray irrigation can be affected by the bacterial strains, bacterial population, stress tolerance, and their biofilm formation ability (Carey et al., 2009; Ge et al., 2012; Yaron and Römling, 2014; Kljujev et al., 2018). Other factors such as produce growing conditions, plant development stage, and the leaf characteristics of cultivars also affect bacterial attachment on the fresh produce leaf (Barak et al., 2011; Ge et al., 2012; Hirneisen et al., 2012; Quilliam et al., 2012).
Traditionally, groundwater and surface water are commonly used for irrigation in the United States, where groundwater withdrawals at 57.2 billion gallons per day account for 48% of total water usage for irrigation (Dieter et al., 2018). In Pennsylvania, nearly 58 million gallons per day of water were used for irrigation and livestock purposes, which constituted more than 10% of the total water consumption in the state [Penn State Extension (PSE), 2007]. The water scarcity has become an issue due to the unpredictable climate and the increased food demands for the growing world populations (Cabera, 2017), requiring approaches to mitigate the scarcity of irrigation waters or supplement more common water sources. Several water management strategies have been employed to overcome the water scarcity issue, including the selection of water-use efficient crops and the use of water-conservation practices such as mulching and sensible irrigation technologies (Pereira et al., 2012). Additionally, research studies have been conducted on the use of alternative water sources such as treated domestic wastewater and roof-collected rain water for fresh produce irrigation (Ahmed et al., 2011; Mizyed, 2013; Yin et al., 2018, 2019).
Domestic wastewater that contains household sewage and industrial wastewater could be a reliable alternative water source for agriculture once treated at the wastewater treatment plant [Xie, 2009; Pedrero et al., 2010; European Commission (EC), 2016]. Reuse of domestic wastewater reduces the release of nutrient-rich wastewater from the wastewater treatment plants into surface water sources such as stream and river (Scott et al., 2004). In Pennsylvania, treated domestic wastewater can be reused for the irrigation of crops intended for human consumption that would be peeled, skinned, cooked, or thermally processed before consumption or commercially processed foods [Department of environmental protection (DEP), 2012]. However, such wastewater used as irrigation water for edible crops should undergo at least a secondary treatment with filtration and disinfection steps at the wastewater treatment plants and should meet the requirements for Class B or better. For spray irrigation of edible crops, Class B wastewater should contain fecal coliforms <2.2 log CFU per 100 ml during the biweekly monitoring process and apply for a period of 15 days prior to harvest as per the wastewater reuse guidance in Pennsylvania [Department of environmental protection (DEP), 2012]. Rain water harvested from the rooftop is another strategy that may serve to cope with current water shortages; however, such water could be contaminated by the bird and rodent droppings on the roof (Zhu et al., 2004; Fletcher et al., 2008; Yin et al., 2019). Although the use of treated wastewater and roof-collected rain water has been increasingly seen as alternative agricultural water sources to overcome water scarcity, these water sources may contain bacterial pathogens including E. coli O157:H7 and Salmonella spp. (Steele and Odumeru, 2004; Yin and Patel, 2018). Potential transfer of pathogens from contaminated irrigation waters to fresh produce could pose significant risk as fresh produce is often consumed raw (Beuchat, 2002).
Previous studies have confirmed the transfer of fecal microorganisms from the irrigation water to the irrigated vegetables (De Roever, 1998; Yin et al., 2018, 2019). Water characteristics such as total organic carbon, hardness, ion composition, pH, and indigenous microflora influenced the survival of E. coli O157:H7 in different water sources (Williams et al., 2007; Avery et al., 2008). Spray irrigation of spinach with wastewater and roof-collected rain water significantly altered its microbiome compositions (Gu et al., 2019). Effects of alternative irrigation waters on the transference and persistence of non-pathogenic E. coli surrogates from the water to the lettuce cultivars at farm levels need further investigation. This study investigated the impact of secondary-treated wastewater and roof-collected rain water on the transfer and persistence of E. coli O157:H12 and E. coli K12 as non-pathogenic surrogates for E. coli O157:H7 on three lettuce cultivars in the field. Lettuce cultivars “Annapolis,” “Celinet,” and “Coastline,” with different leaf structures and ~35 days of maturity time were selected. “Annapolis” cultivar has wavy red leaves, and “Celinet” variety has medium green leaves and thin petioles (Skrsyniarz, 2016; U.S. Patent No. 9,392,765). “Coastline” lettuce is a type of Batavian lettuce with medium green leaves.
Materials and Methods
Preparation of Bacteria Inoculum
Non-pathogenic E. coli strains E. coli O157:H12 and E. coli K12 (ER2420/pBeloBAC11) were used as surrogates for E. coli O157:H7 in water for lettuce irrigation (Fonseca et al., 2011; Ingram et al., 2011). E. coli O157:H12 isolated from the environment was spontaneously resistant to nalidixic acid (NA; Sigma-Aldrich, St. Louis, MO, USA) as previously described by Ingram et al. (2011). E. coli K12 (New England BioLabs, Ipswich, MA) contained the plasmid pBeloBAC11, which carried the chloramphenicol-resistant gene (CP; Sigma-Aldrich). The antibiotic-resistant properties enabled the differentiation of these E. coli surrogates from the generic E. coli present in the water and lettuce plants (Ingram et al., 2011).
E. coli O157:H12 and E. coli K12 were individually grown in 10 ml of tryptic soy broth (TSB; Fisher Scientific, Waltham, MA) that contained NA at 50 ng ml−1 or CP at 200 ng ml−1 at 37°C for 24 h. After incubation, overnight cultures were centrifuged at 5,000 × g for 15 min, followed by re-suspending bacterial pellets with 10 ml of phosphate-buffered saline (PBS, Fisher Scientific). Two milliliter portions of each bacterial suspension were transferred to 100 ml of fecal slurry and incubated at 37°C for 48 h. Bovine fecal slurry was prepared as described by Patel et al. (2010), in which dairy manure solids were obtained from the USDA-ARS Holstein dairy herd (Beltsville, MD). After 48 h of incubation, equal amount of E. coli O157:H12 and E. coli K12 cultures was mixed (8 log CFU ml−1) and supplied as 1:100 ratio to each type of irrigation waters to obtain final bacterial populations at 6 log CFU ml−1 in irrigation water samples.
Irrigation Water on Lettuce Grown in Field
Field experiment was conducted at the Fulton farm in Chambersburg, PA, in October 2018. Seeds of lettuce cultivars “Annapolis,” “Celinet,” and “Coastline” were planted into flats with 128 cells and then manually transplanted into the field after the growth of true leaves was observed. Approximately 150 plants of each lettuce cultivar were planted in each 4 × 2-m plot.
Three irrigation waters were used in this experiment including groundwater (GW), secondary-treated wastewater (TW), and roof-collected rain water (RW). Prior to the experimental irrigation treatments, lettuce plants were drip irrigated weekly with GW without the supplementation of E. coli surrogates. The GW collected from the Fulton farm (Chambersburg, PA) was chosen as the baseline water source in this study because it is the main water source used for farm irrigation, and it is also commonly used in the Mid-Atlantic area. The TW was obtained from the stage of secondary treatment that removed ~80% of organic matter in the sewage with denitrification by microbial process, followed by the filtration and ultraviolet disinfection at Chambersburg Wastewater Treatment Plant (Chambersburg, PA). RW was collected from the rain barrels located at the Fulton farm. Microbiological properties of these irrigation waters (GW, TW, and RW) were determined by using the membrane filtration method as previously described by Yin and Patel (2018) for analyzing the populations of indicator bacteria including total coliforms, fecal coliforms, and generic E. coli. The pH and electrical conductivity of these irrigation waters were measured with a pH meter (Orion, St. Louis, MO, USA) and a conductivity meter (HM Digital, Culver City, CA, USA) as described by Castro et al. (2009).
The irrigation treatments were initiated at 25 days of lettuce transplant in the field soil. Freshly collected irrigation treatment waters including GW, RW, and TW were inoculated with E. coli O157:H12 and E. coli K12 as previously described and used for irrigation. A total of nine lettuce plots (three plots per cultivar) were used in this study, and each cultivar plot was separately irrigated with each type of irrigation treatment water. Each lettuce plot (4 × 2 m) was spray irrigated with ~5 L of treatment water by using handheld sprayers (Forestry suppliers, Jackson, MS, USA) and lettuce plants were allowed to dry in the field for 2 h. After irrigation, four lettuce leaf samples (~30 g per sample) from each plot were randomly collected on days 0, 1, 3, 7, 10 post-irrigation. Lettuce leaf samples were aseptically harvested by cutting ~2 cm above the soil with sterile scissors and forceps and transferred to sterile Whirl-Pak bags. Lettuce samples were stored on ice and transported to the USDA laboratory for microbiological analysis.
Microbiological Analysis
The lettuce leaf sample (~30 g) collected in sterile Whirl-Pak bag was homogenized with 120 ml of buffered peptone water (BPW) by pummeling for 2 min in a stomacher (Interscience Lab Inc., Woburn, MA, USA). Serial 10-fold dilutions of the homogenate were made with PBS and the homogenate or its appropriate dilution was spiral plated onto the MacConkey agar (MAC; Neogen) with NA and CP for the enumeration of E. coli O157:H12 and E. coli K12, respectively. Agar plates were then incubated at 37°C for 24 h, and the bacterial populations were determined and expressed as log CFU g−1. Prior to the experiment, irrigation water samples and lettuce leaves were analyzed by spiral plating onto MAC agar supplemented with NA or CP to ensure that the natural bacteria in irrigation waters and lettuce leaves were unable to grow on these agar media. The lettuce-BPW homogenate samples were incubated at 37°C for 24 h, followed by streaking a loopful of the enrichment onto MAC with NA and CP to determine the presence of the applied E. coli surrogates. Lettuce samples, in which E. coli surrogates were undetectable by the direct plating method but positive by the enrichment procedure, were reported as 1 log CFU g−1 in this study.
To detect the presence of the pathogenic bacteria including Salmonella, L. monocytogenes, and E. coli O157:H7, the homogenates of lettuce samples with BPW were incubated at 37°C for 24 h as primary enrichment [American Public Health Association (APHA), 2005]. A portion of primary enriched sample (5 ml) was transferred to 45 ml of tetrathionate broth (Neogen), Fraser broth (Neogen), and mEHEC broth (Biocontrol, Bellevue, WA, USA) and incubated at 37°C for 24 h, followed by streaking a loopful of these enrichment broths on xylose lysine tergitol-4 agar (XLT4; Neogen), rapid Lmono agar (BioRad), and sorbitol MacConkey agar with CT supplement (CT-SMAC; Neogen), for the detection of Salmonella, L. monocytogenes, and E. coli O157:H7, respectively. Presumptive isolates of the target pathogens on selective media (XLT4, rapid Lmono, or CT-SMAC) were further confirmed by real-time quantitative PCR (RT-qPCR) as previously described (Yin and Patel, 2018).
Statistical Analysis
Completely randomized design was used to study the effect of irrigation treatment waters (GW, RW, and TW) on the persistence of E. coli surrogates on different lettuce cultivars. A three-way factorial repeated measurement ANOVA model with PROC MIXED procedure of Statistical Analysis Software (SAS 9.4, Cary, NC) was used for each of the two E. coli surrogates (E. coli O157:H12 and E. coli K12). Four individual samples of lettuce collected from each plot were analyzed at each sampling time point. The factors included water (GW, RW, and TW), lettuce cultivar (“Annapolis,” “Celinet,” and “Coastline”), and time (0, 1, 3, 7, and 10 days after irrigation). The standardized skewness was verified to assure normal distribution of the data. Differences among the means were analyzed at P < 0.05 using Fisher's least significance difference test.
Results
Prior to the supplementation of E. coli surrogates and fecal slurry, microbiological analysis of the irrigation waters revealed that GW sample contained 1.8 log, 1.7 log, and 1.0 log CFU per 100 ml of total coliforms, fecal coliforms, and generic E. coli, respectively. Further, 0.3 log CFU per 100 ml of total coliforms was recovered from the TW and RW samples, whereas fecal coliforms and generic E. coli were not detected. Bacterial pathogens including Salmonella, L. monocytogenes, and E. coli O157:H7 were not detected from lettuce and water samples throughout the entire experiment. The values of pH and electrical conductivity of all three irrigation waters met irrigation water quality standards according to FAO's guideline. The pH values of GW, TW, and RW were at 7.08, 7.77, and 8.21, respectively. The GW contained the highest electrical conductivity at 371 μs/cm, followed by TW (60 μs/cm) and RW (4 μs/cm).
Figure 1 presents the recovery of E. coli O157:H12 on the three lettuce cultivars “Annapolis,” “Celinet,” and “Coastline” with three types of irrigation waters including GW, RW, and TW. After irrigation, E. coli O157:H12 populations recovered from the irrigated lettuce leaves were in the range of 4.1 ± 0.1 and 4.6 ± 0.0 log CFU g−1 on day 0. Results of the ANOVA table showed that lettuce cultivars significantly affected the recovery of E. coli O157:H12 on the irrigated lettuce leaves (Cultivar P = 0.0013; Table 1). On 0 and 1 day after irrigation with GW, RW, and TW, populations of E. coli O157:H12 recovered from the “Annapolis” lettuce samples were lower than the “Celinet” and “Coastline” lettuce samples that received the same type of irrigation water. Precisely, when three lettuce cultivars were irrigated with RW, E. coli O157:H12 populations recovered from the “Annapolis” lettuce (2.9 ± 0.5 log CFU g−1) on day 1 were significantly lower than the bacterial populations recovered from “Celinet” and “Coastline” lettuce samples (4.0 ± 0.2 log and 3.9 ± 0.2 log CFU g−1). Likewise, significantly lower E. coli O157:H12 populations were recovered from the GW-irrigated “Annapolis” lettuce on day 1 (2.8 ± 0.4 log CFU g−1) and day 7 (1.1 ± 0.1 log CFU g−1) compared to GW-irrigated “Coastline” lettuce samples on day 1 (3.6 ± 0.3 log CFU g−1) and day 7 (1.9 ± 0.2 log CFU g−1), respectively. Additionally, recovery of E. coli O157:H12 from the irrigated lettuce leaves was also significantly affected by the interaction effect of the irrigation water and the lettuce cultivar (water × cultivar P = 0.0218; Table 1). Populations of E. coli O157:H12 recovered from different lettuce cultivars were dependent on the source of irrigation water. The recovery of E. coli O157:H12 was similar in the TW and GW-irrigated lettuce irrespective of the lettuce cultivars on day 3 (P > 0.05); however, irrigation with RW resulted in variable bacterial populations in different cultivars of lettuce when compared to the GW irrigation.
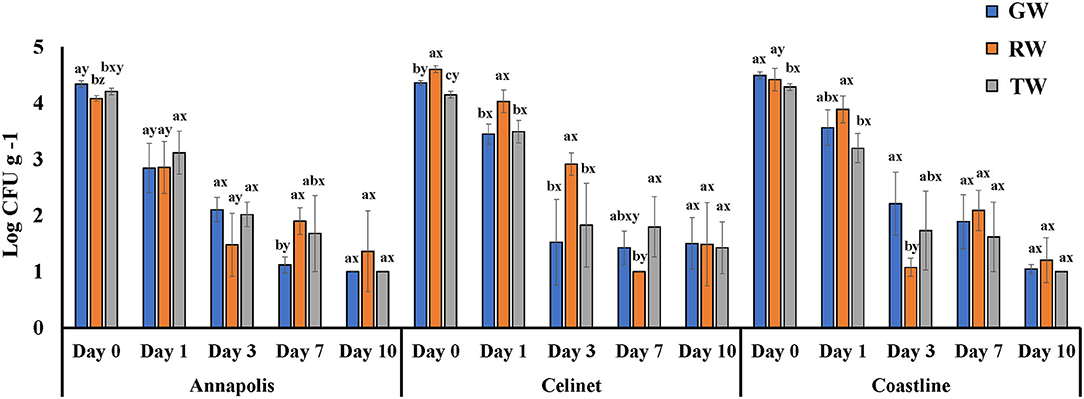
Figure 1. Populations of E. coli O157:H12 recovered from three cultivars of lettuce irrigated with groundwater (GW), roof-collected rain water (RW), and secondary-treated wastewater (TW). Three cultivars included “Annapolis,” “Celinet,” and “Coastline,” and sampling time points were 0, 1, 3, 7, and 10 days post-irrigation. Four replicate samples were analyzed per treatment group at each sampling time point (n = 4). Error bars indicate the SD. abMeans of bacterial populations in the same sampling time point for the same lettuce cultivar with different letters are significantly different (P < 0.05). xyMeans of bacterial populations in the same time point on different lettuce cultivars irrigated with the same type of irrigation water with different letters are significantly different (P < 0.05).
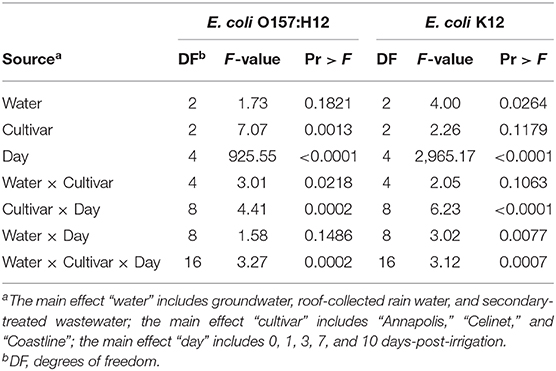
Table 1. Analysis of variance (ANOVA) table for identifying the significant effects and their interactions with lettuce data on E. coli O157:H12 and Escherichia K12.
Recovery of E. coli K12 on the lettuce cultivars with alternative irrigation waters is shown in Figure 2. E. coli K12 populations were recovered at the lower levels than E. coli O157:H12 from the lettuce leaves on day 0 after irrigation (P < 0.05); the populations of this bacterium were in the range of 3.2 ± 0.2 and 3.8 ± 0.2 log CFU g−1. Populations of E. coli K12 on the lettuce leaves were significantly affected by the source of irrigation waters (water effect P = 0.0264; Table 1). Higher recovery of E. coli K12 from RW to the leaves of “Celinet” and “Coastline” lettuce was observed (3.7 ± 0.1 and 3.8 ± 0.1 log CFU g−1) compared to the lettuce samples irrigated with TW (3.3 ± 0.0 and 3.4 ± 0.1 log CFU g−1) on day 0 (P < 0.05).
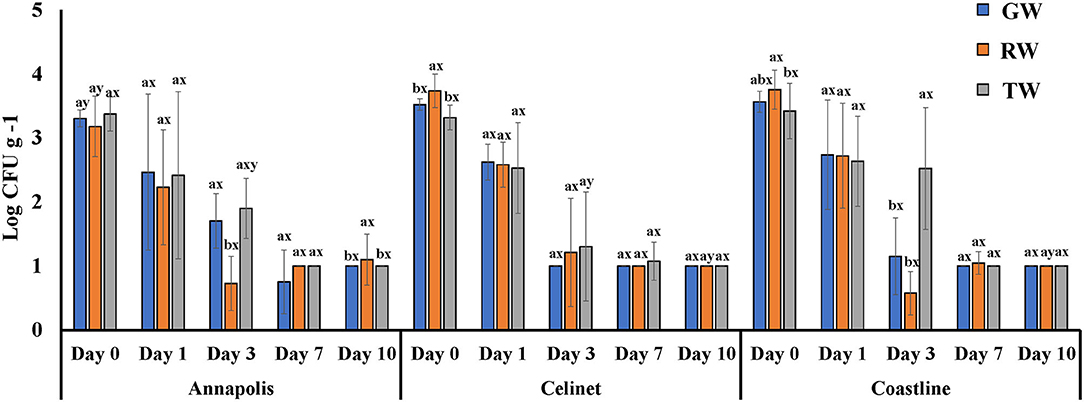
Figure 2. Populations of E. coli K12 recovered from three cultivars of lettuce irrigated with groundwater (GW), roof-collected rain water (RW), and secondary-treated wastewater (TW). Three cultivars included “Annapolis,” “Celinet,” and “Coastline” and sampling time points were 0, 1, 3, 7, and 10 days-post-irrigation. Four replicate samples were analyzed per treatment group at each sampling time point (n = 4). Error bars indicate the SD. abMeans of bacterial populations in the same sampling time point for the same lettuce cultivar with different letters are significantly different (P < 0.05). xyMeans of bacterial populations in the same time point on different lettuce cultivars irrigated with the same type of irrigation water with different letters are significantly different (P < 0.05).
Populations of E. coli K12 recovered from the lettuce samples irrigated with the TW and GW were similar throughout the 10-day experimental period (P > 0.05). Similar results were observed with the recovery of E. coli O157:H12 in TW- and GW-irrigated lettuce samples. Lettuce irrigation with alternative TW irrigation did not significantly increase the transfer of E. coli O157:H12 compared to the control GW irrigation, irrespective of lettuce cultivar. For example, irrigation of TW resulted in the transfer of E. coli O157:H12 at ~4.2 log CFU g−1 on the lettuce compared to ~4.4 log CFU g−1 of E. coli O157:H12 from the GW irrigated lettuce leaves immediately after irrigation (P > 0.05).
Regardless of the type of the irrigation waters and the lettuce cultivars, populations of these E. coli surrogates on the irrigated lettuce leaves gradually decreased during the 10-day experimental period. The recovery of E. coli O157:H12 and E. coli K12 from the irrigated lettuce was not significantly different 10 days after irrigation as populations of both E. coli strains on the lettuce leaves had become non-culturable by direct plating method (detection limit 1.0 log CFU g−1), except for RW irrigation, which resulted in higher E. coli K12 populations on “Annapolis” lettuce in comparison to other two types of irrigation.
Discussion
Our previous reports investigated the impact of RW and TW irrigation on the microbiological quality of spinach and lettuce by monitoring the transfer of indicator bacteria including total coliforms, fecal coliforms, and generic E. coli from the irrigation water to the produce under different growing conditions (Yin et al., 2018, 2019, 2020), and the results of these studies support the use of alternative waters for fresh produce irrigation in the Mid-Atlantic area provided that these waters contained acceptable levels of bacterial indicator populations. In the current study, the potential usage of RW and TW as irrigation water sources for fresh produce was further investigated by inoculating known concentrations of E. coli surrogates to the irrigation water and then determining the transfer and persistence of these surrogates on the irrigated lettuce.
The use of water contaminated with fecal materials for irrigation could be a direct route of produce contamination (Wood et al., 2010; Gorman, 2014). The TW and RW could contain fecal materials as human waste could present in TW and roof could be contaminated by bird and rodent droppings (Ahmed et al., 2011; Madoux-Humery et al., 2013). Further, as manure application is commonly used to fresh produce fields, it has been noted that leafy greens could be contaminated by fecal materials owing to fecal splash subsequent to spray irrigation. Atwill et al. (2015) reported that E. coli O157:H7 in simulated wildlife feces could be transferred to field-grown lettuce via splash during irrigation. Fecal slurry has been used to mimic the potential fecal splash by wild and farm animals during foliar irrigation (Chase et al., 2019). Weller et al. (2017) used rabbit fecal slurry to examine the transfer of E. coli on lettuce once splash contaminated by wild animal waste, and the populations of E. coli decreased from 8.9 log to 3.6 log MPN per lettuce head during the 10-day study between the inoculation and the harvest. It has been previously reported that E. coli could adapt better on the fresh produce and spinach when applied with fecal slurry (Patel et al., 2010; Chase et al., 2017).
Several factors affect the likelihood of fresh produce becoming contaminated during irrigation including the frequency of irrigation, the microbial quality of irrigation water, the characteristic of the leaf surface, and the type of irrigation method (Gerba, 2009; Uyttendaele et al., 2015). The U.S. Food and Drug Administration (FDA) (2009) reported that the irrigation of edible crops by flooding and spraying method represents the highest risk as any bacteria in the water can be transferred to the leaf of these crops during the contact with water. Persistence of E. coli on irrigated produce increased by ~50% through spray (overhead) irrigation system compared to a drip irrigation system (Allende et al., 2017). In the current study, a direct contact of irrigation water with lettuce via spray irrigation method was employed to maximize the potential for microbial contamination (Solomon et al., 2002), and yet irrigation of TW did not significantly affect the recovery of E. coli surrogates from the irrigated leaves compared to the GW irrigation.
In this study, lettuce cultivars influenced the recovery of E. coli surrogates on the lettuce leaves; lower populations of E. coli O157:H12 were recovered from the “Annapolis” lettuce than from “Celinet” and “Coastline” lettuce. Plant variety may have different leaf surface properties including morphology, chemical constituent, and metabolic activity that could affect the bacterial colonization on phyllosphere (Heaton and Jones, 2008; Leveau, 2009; Quilliam et al., 2012). In addition, leaf surfaces harbored microbiota communities that were well-adapted to the nutrient-scarce environment, and the competition of microbiota to the invading human pathogens on the leaves could affect the persistence of these pathogens (Monier and Lindow, 2004; Poza-Carrion et al., 2013). Leaf structural morphology of different cultivars of fresh produce have played an important role in the fate of enteric pathogens residing in lettuce foliage (Lopez-Velasco et al., 2015; Van der Linden et al., 2016; Erickson et al., 2019).
The recovery of E. coli surrogates from the irrigated lettuce varied with E. coli strains. E. coli O157:H12 populations recovered from the irrigated lettuce leaves were ~1 log CFU g−1 greater than the recovery of E. coli K12 on the lettuce leaves on day 0 following irrigation. Previous reports have suggested that bacterial attachment to produce surfaces varied depending on the sources of the bacteria being isolated (Patel et al., 2010; Sharma et al., 2016). It is possible that E. coli O157:H12 strain attached at a higher rate to lettuce leaves than E. coli K12 because of its environmental origin characteristic.
Plant surfaces are considered harsh environments for the enteric organisms and the pre-harvest conditions in the field such as the competition of natural flora, solar radiation, and temperature pose additional challenges for the survival of microorganisms (Aruscavage et al., 2006). The survival profile of E. coli surrogates on the lettuce observed in this study was similar to the results of other previous ones, where spinach and lettuce were directly spray-inoculated with E. coli surrogates (Moyne et al., 2011; Gutiérrez-Rodríguez et al., 2012; Lopez-Velasco et al., 2015). In the current study, ~2–3 log reductions in E. coli surrogate populations were observed during the 10-day experimental period. The mean E. coli O157:H12 levels decreased from ~4.5 log CFU g−1 on day 0 to below detection limit (1.0 log CFU g−1) after 10 days of irrigation treatments. Decline in E. coli populations on fresh produce surface between the inoculation and the harvest was also previously reported; E. coli populations on “Green Tower” lettuce leaves grown in the field reduced from 8.9 log to 3.6 log MPN per sample after 10 days of inoculation (Weller et al., 2017).
The current study supports the use of alternative RW and TW as irrigation waters for fresh produce since the recovery of E. coli surrogates from the irrigated lettuce leaves were mostly comparable to the GW irrigation. RW is commonly collected by the rooftops of the households, temporarily stored in barrels, and used as small-scale practices in the United States (Campisano et al., 2017). Large-scale rainwater harvesting systems may be used as demonstrated by Lani et al. (2018) and Zhang et al. (2020); however, the performance of these harvesting systems may be affected by the rainfall pattern, the roof area, and the tank size (Morales-Pinzón et al., 2014). Unlike GW and RW, TW could be considered as a reliable and stable water source since it is not significantly affected by the season, climatic condition, or precipitation level (Zhang and Shen, 2019). There were no pathogenic bacteria including L. monocytogenes, Salmonella, and E. coli O157:H7 found in water samples and the irrigated lettuce in the current study; however, microbiological quality of these alternative irrigation waters must be confirmed prior to use as irrigation water sources for fresh produce.
Conclusion
The current study investigated the applicability of RW and TW as alternative agricultural water sources for fresh produce irrigation. The transfer of two non-pathogenic E. coli surrogates from GW, RW, and TW to “Annapolis,” “Celinet,” and “Coastline” cultivars of lettuce grown in the field was examined. The recovery of E. coli on the irrigated lettuce was affected by bacterial strains, irrigation water types, and lettuce cultivars. The recovery of E. coli surrogates from the RW and TW irrigated lettuce samples was comparable to the populations of these bacteria recovered from the GW-irrigated lettuce. The E. coli surrogates transferred via irrigation waters persisted for at least 7 days on the lettuce leaves irrespective of water sources and lettuce cultivars, which suggests that incidental contamination of lettuce with irrigation water during the later stage of harvest could pose potential health concern and may require additional mitigation strategies to remove bacteria attached to the lettuce leaves. The findings of the current study provide data on the transfer of E. coli surrogates from RW and TW to the lettuce leaves that can be used in quantitative risk assessments of fresh produce grown in the Mid-Atlantic area.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
H-BY and JP developed the project idea. H-BY, NG, C-HC, AB, and JP designed and performed the experiment. H-BY, NG, C-HC, and AB collected and organized the data. H-BY initiated the manuscript, and JP revised the manuscript. All authors contributed to manuscript revision, and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YL declared a shared affiliation, though no other collaboration, with several of the authors H-BY, C-HC, AB, JP to the handling editor.
Acknowledgments
The authors gratefully thank Dr. Bryan Vinyard for assistance with statistical analysis and Christine Myers and Jessica Larkin at the Wilson College for farm management. Furthermore, the authors thank Suyeun Byun for the technical assistance.
References
Ahmed, W., Gardner, T., and Toze, S. (2011). Microbiological quality of roof-harvested rainwater and health risks: a review. J. Environ. Qual. 40, 13–21. doi: 10.2134/jeq2010.0345
Allende, A., Castro-Ibáñez, I., Lindqvist, R., Gil, M. I., Uyttendaele, M., and Jacxsens, L. (2017). Quantitative contamination assessment of Escherichia coli in baby spinach primary production in Spain: effects of weather conditions and agricultural practices. Int. J. Food Microbiol. 257, 238–246. doi: 10.1016/j.ijfoodmicro.2017.06.027
American Public Health Association (APHA) (2005). Standard Methods for the Examination of Water and Wastewater. Washington, D.C: American Public Health Association.
Araújo, S., Silva, I. A., Tacão, M., Patinha, C., Alves, A., and Henriques, I. (2017). Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 257, 192–200. doi: 10.1016/j.ijfoodmicro.2017.06.020
Aruscavage, D., Lee, K., Miller, S., and LeJeune, J. T. (2006). Interactions affecting the proliferation and control of human pathogens on edible plants. J. Food Sci. 71, 89–99. doi: 10.1111/j.1750-3841.2006.00157.x
Atwill, E. R., Chase, J. A., Oryang, D., Bond, R. F., Koike, S. T., Cahn, M. D., et al. (2015). Transfer of Escherichia coli O157: H7 from simulated wildlife scat onto romaine lettuce during foliar irrigation. J. Food Prot. 78, 240–247. doi: 10.4315/0362-028X.JFP-14-277
Avery, L. M., Williams, A. P., Killham, K., and Jones, D. L. (2008). Survival of Escherichia coli O157: H7 in waters from lakes, rivers, puddles and animal-drinking troughs. Sci. Total Environ. 389, 378–385. doi: 10.1016/j.scitotenv.2007.08.049
Barak, J. D., Kramer, L. C., and Hao, L.-Y. (2011). Colonization of tomato plants by Salmonella enterica is cultivar dependent, and type 1 trichomes are preferred colonization sites. Appl. Environ. Microbiol. 77, 498–504. doi: 10.1128/AEM.01661-10
Beuchat, L. R. (2002). Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microb, Infect. 4, 413–423. doi: 10.1016/S1286-4579(02)01555-1
Cabera, R. (2017). Considerations on Alternative Water Source for Urban Irrigation. Available online at: https://gpnmag.com/article/considerations-on-alternative-water-sources-for-urban-irrigation/ (accessed April 13, 2020).
Campisano, A., Butler, D., Ward, S., Burns, M. J., Friedler, E., DeBusk, K., et al. (2017). Urban rainwater harvesting systems: research, implementation and future perspectives. Water Res. 115, 195–209. doi: 10.1016/j.watres.2017.02.056
Carey, C. M., Kostrzynska, M., and Thompson, S. (2009). Escherichia coli O157: H7 stress and virulence gene expression on Romaine lettuce using comparative real-time PCR. J. Microbiol. Methods 77, 235–242. doi: 10.1016/j.mimet.2009.02.010
Castro, E., Manas, P., and De las Heras, J. (2009). A comparison of the application of different waste products to a lettuce crop: effects on plant and soil properties. Sci. Hortic-Amsterdam. 123, 148–155. doi: 10.1016/j.scienta.2009.08.013
Chase, J. A., Atwill, E. R., Partyka, M. L., Bond, R. F., and Oryang, D. (2017). Inactivation of Escherichia coli O157: H7 on romaine lettuce when inoculated in a fecal slurry matrix. J. Food Protect. 80, 792–798. doi: 10.4315/0362-028X.JFP-16-307
Chase, J. A., Partyka, M. L., Bond, R. F., and Atwill, E. R. (2019). Environmental inactivation and irrigation-mediated regrowth of Escherichia coli O157: H7 on romaine lettuce when inoculated in a fecal slurry matrix. PeerJ 7:e6591. doi: 10.7717/peerj.6591
De Roever, C. (1998). Microbiological safety evaluations and recommendations on fresh produce. Food Control 9, 321–347. doi: 10.1016/S0956-7135(98)00022-X
Department of environmental protection (DEP) (2012). Reuse of Treated Wastewater Guidance Manual. Available online at: http://www.depgreenport.state.pa.us/elibrary/PDFProvider.ashx?action=PDFStreamanddocID=7415andchksum=andrevision=0anddocName=385-2188-002.pdfandnativeExt=pdfandPromptToSave=FalseandSize=426042andViewerMode=2andoverlay=0 (accessed April 13, 2020).
Dieter, C. A., Maupin, M. A., Caldwell, R. R., Harris, M. A., Ivahnenko, T. I., et al. (2018). Estimated Use of Water in the United States in 2015 U.S. Available online at: https://pubs.usgs.gov/circ/1441/circ1441.pdf (accessed April 13, 2020).
Erickson, M. C., Liao, J. Y., Payton, A. S., Cook, P. W., Den Bakker, H. C., Bautista, J., et al. (2019). Pre-harvest internalization and surface survival of Salmonella and Escherichia coli O157: H7 sprayed onto different lettuce cultivars under field and growth chamber conditions. Int. J. Food Microbiol. 291, 197–204. doi: 10.1016/j.ijfoodmicro.2018.12.001
European Commission (EC) (2016). Guidelines on Integrating Water Reuse into Water Planning and Management in the context of the WFD. Available online at: http://ec.europa.eu/environment/water/pdf/Guidelines_on_water_reuse.pdf (accessed April 13, 2020).
European Food Safety Authority (EFSA) (2011). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2009. Available online at: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=253411 (accessed December 10, 2019).
European Food Safety Authority (EFSA) (2012). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2010. Available online at: http://nepis.epa.gov/Adobe/PDF/P100FS7K.pdf (Accessed April 13, 2020).
Fletcher, T. D., Deletic, A., Mitchell, V. G., and Hatt, B. E. (2008). Reuse of urban runoff in Australia: a review of recent advances and remaining challenges. J. Environ. Qual. 37, 116–127. doi: 10.2134/jeq2007.0411
Fonseca, J. M., Fallon, S. D., Sanchez, C. A., and Nolte, K. D. (2011). Escherichia coli survival in lettuce fields following its introduction through different irrigation systems. J. Appl. Microbiol. 110, 893–902. doi: 10.1111/j.1365-2672.2011.04942.x
Food Drug Administration (FDA) (2008). Guide for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-Cut Fruits and Vegetables. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-guide-minimize-microbial-food-safety-hazards-fresh-cut-fruits-and-vegetables (accessed February 20, 2020).
Food Drug Administration (FDA) (2009). Draft Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Leafy Greens; Draft; Availability. Available online at: https://www.federalregister.gov/documents/2009/08/03/E9-18451/draft-guidance-for-industry-guide-to-minimize-microbial-food-safety-hazards-of-leafy-greens (accessed February 20, 2020).
Ge, C., Lee, C., and Lee, J. (2012). The impact of extreme weather events on Salmonella internalization in lettuce and green onion. Food Res. Int. 45, 1118–1122. doi: 10.1016/j.foodres.2011.06.054
Gerba, C. P. (2009). “The role of water and water testing in produce safety,” in Microbial Safety of Fresh Produce, eds X. Fan, B. A. Niemira, C. J. Doonam, F. E. Feeherty, and R. B. Gravani (Indianapolis, IN: Blackwell Publishing), 129–142. doi: 10.1002/9781444319347.ch7
Gorman, S. (2014). Transfer and survival of microorganisms to produce from surface irrigation water (M.S. thesis). Knoxville: University of Tennessee. Available online at: http://trace.tennessee.edu/utk_gradthes/2819/ (accessed July 13, 2020).
Gu, G., Yin, H. B., Ottesen, A., Bolten, S., Patel, J., Rideout, S., et al. (2019). Microbiomes in ground water and alternative irrigation water, and spinach microbiomes impacted by irrigation with different types of water. Phytobiomes J. 3, 137–147. doi: 10.1094/PBIOMES-09-18-0037-R
Gutiérrez-Rodríguez, E., Gundersen, A., Sbodio, A. O., and Suslow, T. V. (2012). Variable agronomic practices, cultivar, strain source and initial contamination dose differentially affect survival of Escherichia coli on spinach. J. Appl. Microbial. 112, 109–118. doi: 10.1111/j.1365-2672.2011.05184.x
Heaton, J. C., and Jones, K. (2008). Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104, 613–626. doi: 10.1111/j.1365-2672.2007.03587.x
Hirneisen, K. A., Sharma, M., and Kniel, K. E. (2012). Human enteric pathogen internalization by root uptake into food crops. Foodborne Pathog. Dis. 9, 396–405. doi: 10.1089/fpd.2011.1044
Holvoet, K., Sampers, I., Seynnaeve, M., Jacxsens, L., and Uyttendaele, M. (2015). Agricultural and management practices and bacterial contamination in greenhouse versus open field lettuce production. Int. J. Environ. Res. Public Health. 12, 32–63. doi: 10.3390/ijerph120100032
Ingram, D. T., Callahan, M. T., Ferguson, S., Hoover, D. G., Shelton, D. R., Millner, P. D., et al. (2011). Use of zero-valent iron biosand filters to reduce Escherichia coli O157: H12 in irrigation water applied to spinach plants in a field setting. J. Appl. Microbiol. 112, 551–560. doi: 10.1111/j.1365-2672.2011.05217.x
Jung, Y., Jang, H., and Matthews, K. R. (2014). Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 7, 517–527. doi: 10.1111/1751-7915.12178
Kljujev, I., Raicevic, V., Vujovic, B., Rothballer, M., and Schmid, M. (2018). Salmonella as an endophytic colonizer of plants-A risk for health safety vegetable production. Microb. Pathog. 115, 199–207. doi: 10.1016/j.micpath.2017.12.020
Lani, N. H. M., Syafiuddin, A., Yusop, Z., and bin Mat Amin, M. Z. (2018). Performance of small and large scales rainwater harvesting systems in commercial buildings under different reliability and future water tariff scenarios. Sci. Total Environ. 636, 1171–1179. doi: 10.1016/j.scitotenv.2018.04.418
Lopez-Velasco, G., Tomas-Callejas, A., Sbodio, A. O., Pham, X., Wei, P., Diribsa, D., et al. (2015). Factors affecting cell population density during enrichment and subsequent molecular detection of Salmonella enterica and Escherichia coli O157: H7 on lettuce contaminated during field production. Food Control. 54, 165–175. doi: 10.1016/j.foodcont.2015.01.041
Madoux-Humery, A. S., Dorner, S., Sauvé, S., Aboulfadl, K., Galarneau, M., Servais, P., et al. (2013). Temporal variability of combined sewer overflow contaminants: evaluation of wastewater micropollutants as tracers of fecal contamination. Water Res. 47, 4370–4382. doi: 10.1016/j.watres.2013.04.030
Mizyed, N. (2013). Challenges to treated wastewater reuse in arid and semi-arid areas. Environ. Sci. Policy. 25, 186–195. doi: 10.1016/j.envsci.2012.10.016
Monier, J. M., and Lindow, S. E. (2004). Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70, 346–355. doi: 10.1128/AEM.70.1.346-355.2004
Morales-Pinzón, T., Lurueña, R., Gabarrell, X., Gasol, C. M., and Rieradevall, J. (2014). Financial and environmental modelling of water hardness—Implications for utilising harvested rainwater in washing machines. Sci. Total Environ. 470, 1257–1271. doi: 10.1016/j.scitotenv.2013.10.101
Moyne, A. L., Sudarshana, M. R., Blessington, T., Koike, S. T., Cahn, M. D., and Harris, L. J. (2011). Fate of Escherichia coli O157: H7 in field-inoculated lettuce. Food Microbiol. 28:1417–1425. doi: 10.1016/j.fm.2011.02.001
National outbreak reporting system (NORS) (2019). NORS Dashboard. Available online at: https://wwwn.cdc.gov/norsdashboard/ (accessed April 05, 2020).
Oliveira, M., Viñas, I., Usall, J., Anguera, M., and Abadias, M. (2012). Presence and survival of Escherichia coli O157: H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int. J. Food Microbiol. 156, 133–140. doi: 10.1016/j.ijfoodmicro.2012.03.014
Painter, J. A., Hoekstra, R. M., Ayers, T., Tauxe, R. V., Braden, C. R., Angulo, F. J., et al. (2013). Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerging Infect. Dis. 19, 407–415. doi: 10.3201/eid1903.111866
Patel, J., Millner, P., Nou, X., and Sharma, M. (2010). Persistence of enterohaemorrhagic and nonpathogenic E. coli on spinach leaves and in rhizosphere soil. J. Appl. Microbiol. 108, 1789–1796. doi: 10.1111/j.1365-2672.2009.04583.x
Pedrero, F., Kalavrouziotis, I., Alarcón, J. J., Koukoulakis, P., and Asano, T. (2010). Use of treated municipal wastewater in irrigated agriculture—review of some practices in Spain and Greece. Agric. Water Manag. 97, 1233–1241. doi: 10.1016/j.agwat.2010.03.003
Penn State Extension (PSE) (2007). Access and allocation of water in Pennsylvania. Available online at: https://extension.psu.edu/access-and-allocation-of-water-in-pennsylvania (accessed March 20, 2020).
Pereira, L. S., Oweis, T., and Zairi, A. (2012). Irrigation management under water scarcity. Agric. Water Manag. 57, 175–206. doi: 10.1016/S0378-3774(02)00075-6
Poza-Carrion, C., Suslow, T., and Lindow, S. (2013). Resident bacteria on leaves enhance survival of immigrant cells of Salmonella enterica. Phytopathology 103, 341–351. doi: 10.1094/PHYTO-09-12-0221-FI
Quilliam, R. S., Williams, A. P., and Jones, D. L. (2012). Lettuce cultivar mediates both phyllosphere and rhizosphere activity of Escherichia coli O157: H7. PLoS ONE 7:33842. doi: 10.1371/journal.pone.0033842
Scott, C. A., Faruqui, N. I., and Raschid-Sally, L. (2004). “Wastewater use in irrigated agriculture: management challenges in developing countries,” in Wastewater Use in Irrigated Agriculture: Confronting the Livelihood and Environmental Realities, eds C. A. Scott, N. I. Faruqui, and L. Raschid-Sally (Wallingford: CAB International), 1–208. doi: 10.1079/9780851998237.0000
Sharma, M., Millner, P. D., Hashem, F., Camp, M., Whyte, C., Graham, L., et al. (2016). Survival and persistence of nonpathogenic Escherichia coli and attenuated Escherichia coli O157: H7 in soils amended with animal manure in a greenhouse environment. J. Food Prot. 79, 913–921. doi: 10.4315/0362-028X.JFP-15-421
Sivapalasingam, S., Friedman, C. R., Cohen, L., and Tauxe, R. V. (2004). Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Protect. 67, 2342–2353. doi: 10.4315/0362-028X-67.10.2342
Skrsyniarz, M. (2016). Lettuce Variety “Celinet.” U.S. Patent No. 9,392,765. Washington, DC: U.S. Patent and Trademark Office.
Solomon, E. B., Potenski, C. J., and Matthews, K. R. (2002). Effect of irrigation method on transmission to and persistence of Escherichia coli O157: H7 on lettuce. J. Food Prot. 65, 673–676. doi: 10.4315/0362-028X-65.4.673
Steele, M., and Odumeru, J. (2004). Irrigation water as source of foodborne pathogens on fruits and vegetables. J Food Protect. 67, 2839–2849. doi: 10.4315/0362-028X-67.12.2839
U.S. Department of Agriculture (USDA) (2016). Food Availability (Per Capita) Data System. Available online at: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed April 05, 2020).
Uyttendaele, M., Jaykus, L. A., Amoah, P., Chiodini, A., Cunliffe, D., Jacxsens, L., et al. (2015). Microbial hazards in irrigation water: standards, norms, and testing to manage use of water in fresh produce primary production. Compr. Rev. Food Sci. Food Safety 14, 336–356. doi: 10.1111/1541-4337.12133
Van der Linden, I., Eriksson, M., Uyttendaele, M., and Devlieghere, F. (2016). Is there a relation between the microscopic leaf morphology and the association of Salmonella and Escherichia coli O157: H7 with iceberg lettuce leaves? J. Food Protect. 79, 1784–1788. doi: 10.4315/0362-028X.JFP-15-590
Weller, D. L., Kovac, J., Roof, S., Kent, D. J., Tokman, J. I., Kowalcyk, B., et al. (2017). Survival of Escherichia coli on lettuce under field conditions encountered in the northeastern United States. J. Food Protect. 80, 1214–1221. doi: 10.4315/0362-028X.JFP-16-419
Williams, A. P., Avery, L. M., Killham, K., and Jones, D. L. (2007). Persistence, dissipation, and activity of Escherichia coli O157: H7 within sand and seawater environments. FEMS Microbiol. Ecol. 60, 24–32. doi: 10.1111/j.1574-6941.2006.00273.x
Wood, J. D., Bezanson, G. S., Gordon, R. J., and Jamieson, R. (2010). Population dynamics of Escherichia coli inoculated by irrigation into the phyllosphere of spinach grown under commercial production conditions. Int. J. Food Microbiol. 143, 198–204. doi: 10.1016/j.ijfoodmicro.2010.08.022
World Health Organization (2018). Global Strategy on Diet, Physical Activity and Health-Promoting Fruit and Vegetable Consumption Around the World. Available online at: http://www.who.int/dietphysicalactivity/fruit/en/index2.html (accessed April 13, 2020).
Xie, J. (2009). Addressing China's Water Scarcity: A Synthesis of Recommendations for Selected Water Resources Management Issues. Washington, DC: The World Bank. doi: 10.1596/978-0-8213-7645-4
Yaron, S., and Römling, U. (2014). Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb. Biotechnol. 7, 496–516. doi: 10.1111/1751-7915.12186
Yin, H. B., Chen, C. H., Karanth, S., Byun, S., Mayer, C., Harriger, D., et al. (2020). Effect of cultivars and irrigation waters on persistence of indicator bacteria on lettuce grown in high tunnel. J. Food Safety 40:e12795. doi: 10.1111/jfs.12795
Yin, H. B., Gu, G., Nou, X., and Patel, J. (2019). Comparative evaluation of irrigation waters on microbiological safety of spinach in field. J. Appl. Microbiol. 127, 1889–1900. doi: 10.1111/jam.14436
Yin, H. B., Nou, X., Gu, G., and Patel, J. (2018). Microbiological quality of spinach irrigated with reclaimed wastewater and roof-harvest water. J. Appl. Microbial. 125, 133–141. doi: 10.1111/jam.13746
Yin, H. B., and Patel, J. (2018). Comparison of methods to determine the microbial quality of alternative irrigation waters. Agric. Water Manag. 201, 38–45. doi: 10.1016/j.agwat.2018.01.012
Zhang, S., Jing, X., Yue, T., and Wang, J. (2020). Performance assessment of rainwater harvesting systems: Influence of operating algorithm, length and temporal scale of rainfall time series. J. Clean. Prod. 253:120044. doi: 10.1016/j.jclepro.2020.120044
Zhang, Y., and Shen, Y. (2019). Wastewater irrigation: past, present, and future. WIRES Water 6:e1234. doi: 10.1002/wat2.1234
Keywords: cultivar, irrigation water, food safety, E. coli, pre-harvest, fresh produce
Citation: Yin H-B, Gupta N, Chen C-H, Boomer A, Pradhan A and Patel J (2020) Persistence of Escherichia coli O157:H12 and Escherichia coli K12 as Non-pathogenic Surrogates for O157:H7 on Lettuce Cultivars Irrigated With Secondary-Treated Wastewater and Roof-Collected Rain Water in the Field. Front. Sustain. Food Syst. 4:555459. doi: 10.3389/fsufs.2020.555459
Received: 24 April 2020; Accepted: 08 October 2020;
Published: 24 November 2020.
Edited by:
Karl Matthews, Rutgers, The State University of New Jersey, United StatesReviewed by:
Keith Warriner, University of Guelph, CanadaYanhong Liu, United States Department of Agriculture (USDA), United States
Copyright © 2020 Yin, Gupta, Chen, Boomer, Pradhan and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitendra Patel, aml0dS5wYXRlbEB1c2RhLmdvdg==
 Hsin-Bai Yin
Hsin-Bai Yin Nidhi Gupta2
Nidhi Gupta2 Abani Pradhan
Abani Pradhan Jitendra Patel
Jitendra Patel