- 1Gund Institute for Environment, University of Vermont, Burlington, VT, United States
- 2Rubenstein School of Environment and Natural Resources, University of Vermont, Burlington, VT, United States
- 3School of Forest Sciences, University of Eastern Finland, Joensuu, Finland
- 4BRIDGE Project, Smithsonian Conservation Biology Institute, Smithsonian Institution, Washington, DC, United States
- 5Bureau for Food Security, United States Agency for International Development, Washington, DC, United States
- 6Foreign Agriculture Service, United States Department of Agriculture, Washington, DC, United States
- 7Environmental Program, University of Vermont, Burlington, VT, United States
Childhood undernutrition yearly kills 3.1 million children worldwide. For those who survive early life undernutrition, it can cause motor and cognitive development problems that translate into poor educational performance and limited work productivity later in life. It has been suggested that nutrition-specific interventions (e.g., micronutrient supplementation) that directly address the immediate determinants of undernutrition (e.g., nutrient intake) need to be complemented by nutrition-sensitive interventions that more broadly address the underlying determinants of undernutrition (e.g., food insecurity). Here, we argue that forest conservation represents a potentially important but overlooked nutrition-sensitive intervention. Forests can address a number of underlying determinants of undernutrition, including the supply of forest food products, income, habitat for pollinators, women's time allocation, diarrheal disease, and dietary diversity. We examine the effects of forests on stunting—a debilitating outcome of undernutrition—using a database of household surveys and environmental variables across 25 low- and middle-income countries. Our result indicates that exposure to forest significantly reduces child stunting (at least 7.11% points average reduction). The average magnitude of the reduction is at least near the median of the impacts of other known nutrition interventions. Forest conservation interventions typically cover large areas and are often implemented where people are vulnerable, and thus could be used to reach a large number of the world's undernourished communities that may have difficult access to traditional nutrition programs. Forest conservation is therefore a potentially effective nutrition-sensitive intervention. Efforts are needed to integrate specific nutrition goals and actions into forest conservation interventions in order to unleash their potential to deliver nutritional benefits.
Introduction
Childhood undernutrition is a global problem, a factor responsible for the death of 3.1 million children under the age of five annually, or roughly 45% of all child deaths (Black et al., 2013). Prevalence is higher in low and middle-income countries than elsewhere (Perez-Escamilla et al., 2018). Childhood undernutrition, particularly from conception to a child's second birthday, has been related to motor and cognitive development problems that have adverse effects later in life, such as poor school performance, limited learning, and work capacity, decreased economic productivity, and shorter adult stature [Almond and Currie, 2011; Currie and Vogl, 2013; United Nations Children's Fund (UNICEF), 2013]. In addition to the high prevalence and detrimental consequences of childhood undernutrition, the fight against it is only growing more difficult as growing human population, volatile food and oil prices, conflicts and governance crises, and the increasing human perturbation of Earth's natural systems (e.g., climate, land cover) all threaten the food system (Godfray et al., 2010).
Given these challenges, it has been suggested that nutrition-specific interventions—those addressing the immediate causes of undernutrition—(e.g., nutrient supplementation, food fortification) need to be complemented by nutrition-sensitive interventions that address the underlying determinants of undernutrition and incorporate specific nutrition goals and actions (e.g., agriculture, social safety nets; Ruel and Alderman, 2013). Underlying determinants of nutrition include household income, food security, and access to services affecting nutritional status (i.e., anthropometry, micronutrient status). Nutrition-sensitive interventions often are implemented at large scale with intention to reach vulnerable populations. They therefore can also serve as vehicles to improve both the coverage and targeting of delivery of nutrition-specific interventions (Ruel and Alderman, 2013). A recent systematic review conducted by Hossain et al. (2017) indicates that greater effectiveness has been observed when programs combine nutrition-specific and nutrition-sensitive interventions. Investments in development and implementation of nutrition-sensitive interventions have increased in the latest decade (Ruel and Alderman, 2013).
Here, we investigate whether forest conservation represents a potentially important but overlooked nutrition-sensitive intervention. We first describe the underlying determinants of undernutrition that can be addressed by forests. We then use a unique multi-country database to examine effects of forests on nutritional status, particularly stunting (i.e., low height-for-age), which is a common manifestation of long-term childhood undernutrition (Hossain et al., 2017). Finally, we discuss the features of forest conservation interventions that make them potentially effective nutrition-sensitive interventions and suggest ways to increase their nutrition sensitivity.
Underlying Determinants of Undernutrition Addressed By Forests
A number of studies examine effects of forests on underlying determinants of undernutrition, forming intermediate outcomes along the pathways between forests and nutritional status (Figure 1). Forests supply ecosystem services important to nutrition. Among these services, forest foods are collected by a large number of rural forest households in low- and middle-income countries. A study covering 24 countries indicates that over 55% of rural households with moderate-to-good access to forest resources collect forest food products (e.g., diverse species of animals, plants, and mushrooms) for subsistence (Hickey et al., 2016). For the top forest dependent communities across these countries, forest food products provide nearly 15% of the recommended quantities of fruits and vegetables, and 106% for meat and fish (Rowland et al., 2017). Fungo et al. (2016) report that forest foods contribute 93% of daily vitamin A intake of women in rural forest-dependent communities in Cameroon.
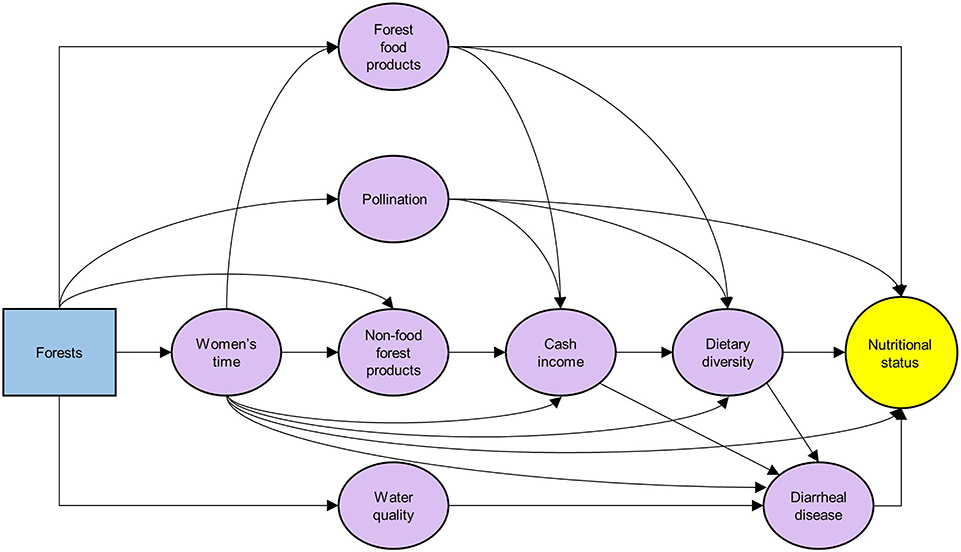
Figure 1. Mechanistic pathways linking forests and nutritional status (purple oval boxes: underlying determinants of nutrition addressed by forests).
Forest food and non-food products (e.g., timber and non-timber forest products) form a significant portion of the income (in-kind and cash) of rural forest households across low- and middle-income countries. A synthesis of 51 case studies suggests that, on average, forest products compose 22% of forest household total income (Vedeld et al., 2007), a percentage similar to that found by a more recent study across 24 low- and middle-income countries (Angelsen et al., 2014). In addition to being used for subsistence, forest products are also sold for cash income (Angelsen et al., 2014), which can be invested in household nutrition through food purchase, protection against or treatment for diseases (e.g., diarrhea, measles) that affect nutritional status.
Another nutrition-relevant service that forests provide is habitat for pollinators. Seventy-five percent of leading food crops, accounting for 35% of the world's crop production, depend to varying extents on pollinators (Klein et al., 2007). Pollination is also crucial for the provision of essential micronutrients. For example, 98, 70, and 55% of the available vitamin C, vitamin A, and folate, respectively, in the world's leading crops are produced by pollinated plants (Eilers et al., 2011). In addition to pollinators' roles in subsistence agriculture, they substantially contribute to the cash income of millions of rural and poor people [Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), 2017]. For example, many of the world's leading export crop products from rural low- and middle-income countries are pollinator-dependent (e.g., coffee and cocoa) [Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), 2017]. The additional cash income arising from pollination service can be used to improve household nutrition.
Women's empowerment is a key underlying determinant of childhood nutrition that could be addressed by many nutrition-sensitive interventions (Ruel and Alderman, 2013), including forest conservation. Particularly, in many low- and middle-income countries, women are the primary collectors of non-timber forest products (e.g., forest food, firewood, fodder; Sunderland et al., 2014). Reduced access to these products due to deforestation and forest degradation increases time and energy women spend collecting them, shifting their time and energy away from food preparation, more careful child feeding behaviors, income generation, and health care (Agarwal, 2009; Johnson et al., 2013). For example, Wan et al. (2011) reported that in India, women used to walk 1–2 km every day to gather sufficient firewood for cooking. Eight years later, after deforestation, they needed to walk 8–10 km for the same activity. Such a shift in the use of time and energy by women can negatively affect the nutrition of household members (Ruel and Alderman, 2013).
Forests are also linked to reduced risk of diarrheal disease (Pattanayak and Wendland, 2007; Johnson et al., 2013; Herrera et al., 2017), which is a strong underlying determinant of stunting in children (Checkley et al., 2008). For example, a study across 35 low- and middle-income countries indicates that, in rural areas, a 30% increase in upstream tree cover is associated with 4% reduction in the probability of downstream incidence of diarrheal disease (Herrera et al., 2017). The reduced diarrheal disease could be at least partly due to the improvement of drinking water quality by forests. Forests have been shown to remove pathogens and sediments from water (Ensign and Mallin, 2001; Cunha et al., 2016). Water filtration by forests is likely to be particularly valuable for the 663 million people, living primarily in low- and middle-income countries, who use unimproved drinking water sources [World Health Organization (WHO), 2017].
Dietary diversity is another underlying determinant of undernutrition affected by forests (Ickowitz et al., 2014; Galway et al., 2018; Rasolofoson et al., 2018; Rasmussen et al., in press). In a study across 27 low- and middle-income countries, Rasolofoson et al. (2018) estimate that exposure to forest leads to at least 25% greater dietary diversity in children exposed to forest than non-exposed children. Rasmussen et al. (in press) indicate, in a study across five African countries, that forest configuration across landscapes, not just forest coverage, influences dietary diversity. High dietary diversity correlates significantly with better nutritional status in several low- and middle-income countries (Arimond and Ruel, 2004; Steyn et al., 2006). Forests could therefore improve childhood nutritional status through their effects on dietary diversity. Increased dietary diversity can also affect nutritional status via reducing risk for diarrheal disease. More diverse diets are more likely to provide adequate levels of micronutrients (Moursi et al., 2008; Zhao et al., 2017), which can shield children against infectious diseases. In particular, through its role in the immune system, vitamin A—the consumption of which is positively affected by exposure to forest (Johnson et al., 2013; Rasolofoson et al., 2018)—decreases susceptibility to diarrheal disease (Semba, 1999; Villamor and Fawzi, 2005) and thus lowers the probability of stunting (Checkley et al., 2008).
The weight of evidence therefore tilts toward forests addressing underlying determinants of nutritional status. However, effects on underlying determinants do not necessarily translate into effects on actual measures of nutritional status (Ruel and Alderman, 2013). Empirical evidence about effects of forests on nutritional status is therefore needed, but unfortunately such evidence is rare (e.g., Golden et al., 2011; Johnson et al., 2013). To strengthen the evidence about effects of forests on nutritional status, we examine effects of exposure to forest on prevalence of child stunting across 25 low and middle-income countries in Africa, South America, and Southeast Asia (Figure 2). We also explore effects of forests in view of the impacts of different nutrition interventions on stunting, in order to shed light on the potential of forest conservation as a nutrition-sensitive intervention.
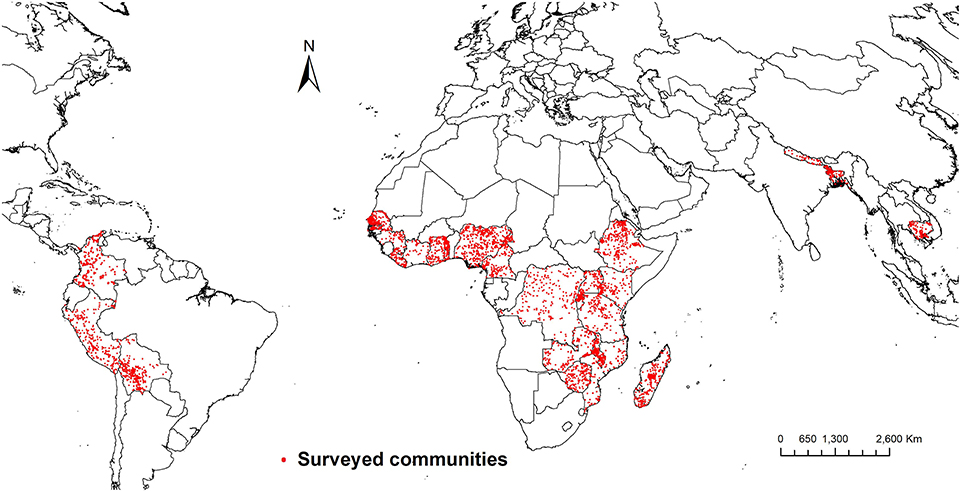
Figure 2. Communities included in the estimation of effects of forest on child stunting. Data are from the Demographic and Health Surveys (DHS) program of the [United States Agency for International Development (USAID), 2018].
Materials and Methods
Stunting
Height-for-age represents the linear growth achieved at the age of measurement. Prevalence of child stunting is the percentage of children whose height-for-age values fall below two standard deviations (−2 Z-scores) from the median height-for-age of a reference population [World Health Organization (WHO), 2006]. In 2011, stunting affected 165 million children across the globe (Black et al., 2013). In a synergistic association with infectious diseases (diarrhea, pneumonia, measles), stunting is responsible for a third of child deaths due to undernutrition, making it one the deadliest manifestations of undernutrition, particularly in South Asia and sub-Saharan Africa (Black et al., 2013).
Our data come from the Demographic and Health Surveys (DHS) program of the [United States Agency for International Development (USAID), 2018]. The DHS program has collected demographic and health information across more than 90 developing countries. We used the DHS stunting information that is based on the Centers for Disease Control and Prevention (CDC) reference population [United States Agency for International Development (USAID), 2013]. Our dataset comprises 59,378 children under the age of five across 25 low- and middle-income countries surveyed in different years between 2006 and 2013 (Supplementary Material Table S1).
Exposure to Forest
We defined exposure to forest following and using the same data as Rasolofoson et al. (2018). Our forest data are from the MODIS Vegetation Continuous Field products at 250 m spatial resolution (DiMiceli et al., 2011). Forests are areas with at least 40% tree coverage [United Nations Environmental Programme (UNEP), Food and Agriculture Organization of the United Nations (FAO), and United Nations Forum on Forests Secretariat (UNFF), 2008]. The georeferenced communities surveyed by the DHS program (referred to as “clusters” in DHS documents) were integrated with the spatial forest data. Each child was assigned to the forest cover of the year when they were surveyed or to the 2010 forest cover when the survey took place in or after 2010 as the MODIS Vegetation Continuous Field products ended in 2010.
We defined children exposed to forest as those living in communities within 3 km of the nearest forest edge and with at least 30% of the land within 5 km buffer around the community centers covered by forests. We defined children not exposed to forests as those living farther than 8 km from of the nearest forest edge. The criteria for the definitions of exposure and non-exposure to forests are based on the average distance forest people in low- and middle-income countries walk to come to the closest forest to collect forest products, foraging distance of pollinators (a mechanism through which forests affect nutrition), and the uncertainty associated with the locations of communities in our data (see full details and data description in Materials and Methods of Rasolofoson et al., 2018). In fact, the locations of communities in DHS were randomly displaced up to 5 km to protect anonymity of survey respondents. This is the reason why we used the 5-km buffer around the community centers in our definition of exposure to forest. Moreover, this displacement also means that communities located between 3 and 8 km from forest edges could actually be within 3 km of forest edges and thus their children could be exposed to forest according to our definition of exposure to forest. This is why we defined children not exposed to forest as those living further than 8 km from forest edge and we excluded children of communities located between 3 and 8 km from forest edges. We identified 13,927 children exposed and 45,451 children non-exposed to forest.
Identification of the Effect of Forests on Prevalence of Stunting: Partial Identification
The effect of exposure to forest on stunting prevalence is the difference between the prevalence of stunting for children exposed to forest and the counterfactual prevalence of stunting had these same children not been exposed to forest. The former is the observed percentage of stunted children among those exposed to forest. The latter, i.e., the counterfactual, is not observed. We must thus assume that percentage of stunted children among a comparison group not exposed to forest represents the counterfactual. The credibility of our effect estimate depends on the plausibility of the assumptions invoked to identify the counterfactual. A precise point estimate of effect (e.g., regression coefficients) often requires non-transparent and strong identifying assumptions about the counterfactual and thus, is of limited credibility (Manski, 2011; Ferraro and Hanauer, 2014; McConnachie et al., 2016). We used the partial identification approach (Manski, 2003), which considers observed data on the characteristics of children exposed and non-exposed to forest and invokes weak, but plausible, identifying assumptions to generate ranges—delimited by lower and upper bounds—within which the counterfactual and thus, the estimate of effects of exposure to forest on stunting can occur.
Without making any assumptions, we know that the counterfactual prevalence of stunting for the children exposed to forest, had they not been exposed to forest, would be greater than 0% (no stunted child) and smaller than 100% (all children stunted). The difference between the prevalence of stunting for children exposed to forest and these two extreme counterfactual values, respectively, give the upper and lower “no-assumption” bounds of the effect of exposure to forests.
We then invoked the monotone treatment selection (MTS) assumption (Manski and Pepper, 2000). MTS posits that either positive or negative selection bias is plausible (McConnachie et al., 2016). Positive selection bias occurs when children exposed to forest, had they not been exposed to forest, would have stunting prevalence (counterfactual) greater than that for children not exposed to forest. Negative selection bias occurs when the counterfactual stunting prevalence for children exposed to forest is smaller than the stunting prevalence for children not exposed to forest. For our study, positive selection is plausible. Forests are often located in marginal lands with low agricultural potential, far from infrastructure (e.g., roads, markets), and with high poverty (Sunderlin et al., 2005). These forest related characteristics are not favorable for nutrition (Rasolofoson et al., 2018). Children exposed to forest are therefore likely to have characteristics less favorable for nutrition than children not exposed to forest (Rasolofoson et al., 2018)—as generally confirmed in our data (Table 1). It is thus plausible to assume that the counterfactual stunting prevalence for children exposed to forest, had they not been exposed to forests, would be greater than the stunting prevalence for children not exposed to forest. Therefore, we moved the lower bound of the counterfactual from 0% (no assumption) to the stunting prevalence for children not exposed to forest. In turn, the upper bound of the range of the effect estimate becomes the difference between the prevalence of stunting for children exposed to forest and that for children not exposed to forest.
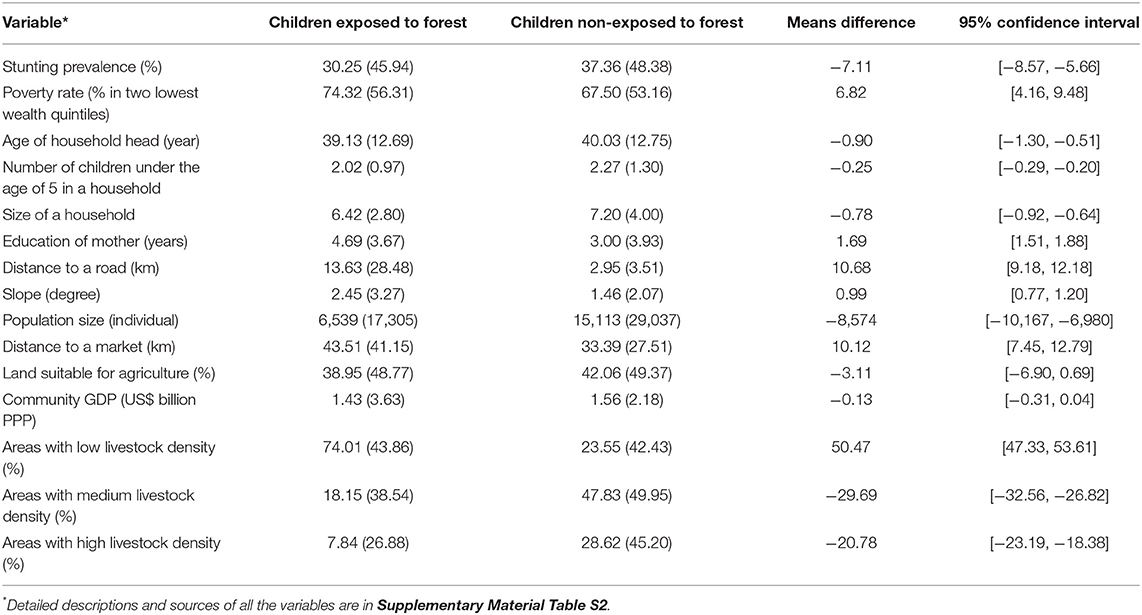
Table 1. Means and standard deviations (in parentheses) of characteristics of children exposed vs. non-exposed to forest.
To test the statistical significance of the upper and lower bounds of the effect estimate (i.e., the differences between the prevalence of stunting for children exposed to forest and the upper or lower bounds of the counterfactual), we used linear regressions with stunting statuses (stunted or not stunted) of children as dependent variable and forest exposure (exposed or not exposed to forest) as independent variable. These regressions are equivalent to using independent t-tests to compare the prevalence of stunting for children exposed to forest and the upper or lower bounds of the counterfactual (Pandis, 2016). Comparing means of a binary variable (stunted or not stunted) between two groups (exposed or not exposed to forest) with t-test is similar to comparing proportions (percentages) with proportion z-test when the sample size is large (Park, 2009), as in the case of our analyses. We clustered the standard errors at the community level. We computed bootstrap confidence intervals. We did the analyses with the R “clusterSEs” package (Esarey, 2017).
Effects of Forests and Impacts of Different Nutrition Interventions on Child Stunting
To put the result of the partial identification approach into perspective, we plotted the conservative bound of the estimate of effects of exposure to forest on stunting with the estimated impacts of different interventions on stunting investigated in studies systematically reviewed in Hossain et al. (2017). Hossain et al. (2017) estimated the impacts of the interventions they reviewed as the average annual rate of reduction (AARR). We perused the reviewed studies. We extracted the raw numbers of stunting prevalence from each reviewed study. We then calculated impacts as the total changes in stunting prevalence (in percent points) brought by the interventions (Supplementary Material Table S3). Nevertheless, these studies are not directly comparable to ours for a variety of reasons. They investigate bundles of nutrition (specific or sensitive) interventions instead of one at a time, and study designs and scales differ from ours. Our plot therefore needs to be interpreted with caution.
Results
Partial Identification
The percentage of stunted children among those exposed to forest is 30.25% (SD: 45.94%). The no-assumption lower bound of the effect of exposure to forest on stunting is therefore 30.25–100 = −69.75% points (95% CI [−70.86%; −68.64%]). The no-assumption upper bound is 30.55–0 = 30.25% points (95% CI [29.10%; 31.40%]). Without invoking any assumption then, we can identify the estimate of effects of exposure to forest on stunting to be within the range of [−69.75%; 30.25%].
The positive selection bias posited by the monotone treatment selection assumption implies that counterfactual stunting prevalence for children exposed to forest, had they not been exposed to forests, would be greater than 37.36% (SD: 48.38%), which is the stunting prevalence for children not exposed to forest. Therefore, we can move the upper bound (conservative) estimate of effects of exposure to forest on stunting to 30.25–37.36 = −7.11% points (95% CI [−8.57%; −5.66%]), thus narrowing our range of effect estimate to [−69.75%; −7.11%].
Effects of Forests and Impacts of Different Nutrition Interventions on Child Stunting
Figure 3 plots our conservative (upper bound) estimate of effects of exposure to forest on stunting (−7.11% points) and the estimated impacts of different interventions on stunting reviewed in Hossain et al. (2017). The interventions reviewed in Hossain et al. (2017) include combinations of nutrition education, growth monitoring and promotion, micronutrient supplementation, immunization, health and family planning, access to health facilities, women's empowerment, social safety net, poverty, and food security alleviation, food fortification, integrated management of childhood illness, infant and young child feeding, water, sanitation and hygiene, deworming, child psychological stimulation, community kitchen and garden, telemedicine, feeding practices, and diarrhea and malaria prevention and treatment (Supplementary Material Table S3). The impacts of different combinations of these interventions span from a reduction of 30% points to an increase of 6.3% points in stunting prevalence (see Supplementary Material Table S3). Our conservative estimate of forest effects falls near the median impact of these other interventions (−10.05% point) (Figure 3).
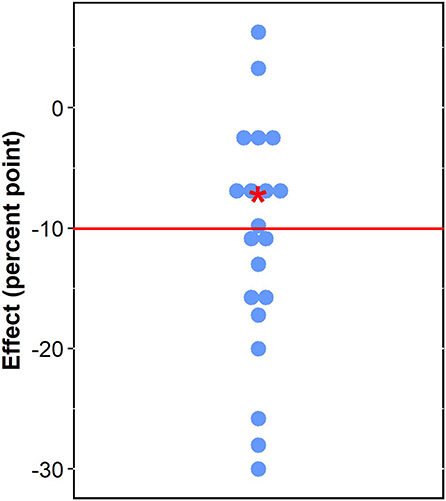
Figure 3. Effects of forests and nutrition interventions on child stunting (red star: conservative effect of forests; blue circles: effects of interventions reviewed in Hossain et al., 2017; red line: median of blue circles).
Discussion
Forests address a number of underlying determinants of undernutrition, including the supply of forest food products, income, habitat for pollinators, women's time allocation, prevalence of diarrheal disease, and dietary diversity. As a likely result of these mechanisms, our analysis across 25 low- and middle-income countries suggests that, on average, exposure to forest leads to lower child stunting prevalence compared to non-exposure. Further, the average magnitude of the effect of exposure to forest on child stunting prevalence is at least near the median of the estimated impacts of different nutrition (specific and sensitive) interventions.
Our study confirms the increasingly recognized beneficial effects of forests on quality of human diet (Ickowitz et al., 2014; Galway et al., 2018; Rasolofoson et al., 2018; Rasmussen et al., in press). We moved beyond effects on diet to actual measure of nutritional status (stunting). While we did not identify a precise point estimate of effects of forests on stunting, based on a plausible assumption, we were able to indicate that, on average, forests reduce the prevalence of child stunting and that this average reduction is at least comparable to the impacts of other known nutrition interventions. Our results thus suggest that forest conservation can be a promising nutrition-sensitive intervention.
Different levels of restrictions on use of forest resources by different types of forest conservation interventions can block, to a various degree, some of the mechanisms through which forests affect nutritional status. These restrictions, for example, include limited access to forest food products and non-food products important for income (Poudyal et al., 2018) and therefore may negatively affect nutritional status. On the other hand, forest conservation interventions can also generate benefits through improved ecosystem services, tourism and infrastructure development (Andam et al., 2010). These benefits could lead to improvement in the nutritional status of affected communities (Naidoo et al., 2019). Therefore, the net impact of forest conservation interventions on nutritional status is an empirical question.
A number of studies capture promising actions to enhance the nutrition sensitivity of forest conservation interventions (e.g., volume 13, special issue 3 in International Forest Review; Vira et al., 2015). Some studies suggest that multifunctional landscapes that integrate diversity of agricultural production systems and forests deliver both nutritional and conservation benefits by maintaining key ecosystem services (Sunderland, 2011; Vira et al., 2015). Similar to the cases of other nutrition-sensitive interventions (Ruel and Alderman, 2013), other studies indicate that actions promoting gender equity can increase the nutrition benefits of forest conservation interventions (Sunderland, 2011; Wan et al., 2011). Jamnadass et al. (2011) advocate that actions improving yield, quality, and market access for forest food products can enhance nutrition in rural communities by supplying ample nutritious food products of good quality and raising income. Education, particularly nutrition education, which is shown to enhance the impact of different nutrition-sensitive interventions on nutritional status (Berti et al., 2004; Leroy et al., 2009; Girard et al., 2012), also has great potential to improve the effect of forests on nutrition (Vira et al., 2015; Rasolofoson et al., 2018).
To further determine the merit of recognizing forest conservation among nutrition-sensitive interventions, it helps to examine the key features specified in their definition. One key feature of nutrition-sensitive interventions is that they are often implemented at large scale and can effectively target disadvantaged populations with high rates of undernutrition. Nutrition-sensitive interventions can therefore serve as delivery platforms for nutrition-specific interventions (e.g., nutrition behavior-change communications, food fortification) in efforts to increase their scale, coverage and effectiveness (Ruel and Alderman, 2013). Another key feature is that nutrition-sensitive interventions incorporate specific nutrition goals and actions to achieve these goals (Ruel and Alderman, 2013).
One of the most widespread measures to conserve forests is the designation and management of protected areas [Millennium Ecosystem Assessment (MEA), 2005]. Protected areas currently cover 14.7% of the globe's land area (Jones et al., 2018). Forest conservation can also be advanced through community forest management. Local communities, to a various extent, manage 15.5% of the world's forests [Rights and Resources Initiative (RRI), 2014]. Protected forests and community managed forests are often located in lands with higher elevations, steeper slopes, greater distances to roads and cities, less suitable for agriculture, and high poverty rates (though protected areas may be more remote and less developed relative to community forest areas; Sunderlin et al., 2005; Joppa and Pfaff, 2009; Rasolofoson et al., 2015). Hence, people living in or around protected areas or community managed forests often lack access to sufficient agricultural products, markets, and health services, and thus are likely to have high rates of undernutrition. Using protected areas or community forest management, in different ways, as delivery platforms for nutrition-specific interventions will therefore ensure that these interventions reach large numbers of the world's undernourished communities.
Combination of nutrition-sensitive and nutrition-specific interventions is one of the elements of success of nutrition programs (Hossain et al., 2017; Perez-Escamilla et al., 2018). Therefore, using forest conservation interventions as delivery platforms for nutrition-specific interventions may better deliver nutrition benefits than either of them alone. Examples of such combination could include addition of nutrition behavior-change communications, micronutrient supplementation, food fortification, or disease prevention programs to forest conservation initiatives. Where local communities are involved in forest conservation (e.g., community forest management), the experience and external support they receive in managing their forests can develop social (e.g., community associations, network), human (e.g., skills, expertise), and institutional (e.g., community rules and regulations) assets that constitute a good foundation upon which nutrition interventions can build to reach their goals (Pailler et al., 2015). These community assets are important, given that nutrition interventions are more likely to be successful where there are community-based delivery platforms accompanied with active community engagement (Hossain et al., 2017).
Addition of explicit nutrition goals and actions to nutrition-sensitive interventions help boost their potential to deliver on nutrition outcomes (Ruel and Alderman, 2013). International funding for forest conservation increasingly links conservation and poverty alleviation goals (Miller, 2014). Forest conservation projects therefore increasingly include activities aiming to compensate local communities for benefits forgone due to restrictions on access to forest resources and to improve their livelihoods in order to win their support for conservation (Tabor et al., 2017). Nevertheless, forest conservation initiatives rarely consider health issues (Wan et al., 2011)—including the integration of nutrition goals and actions. Adding nutrition-specific interventions and nutrition goals to forest conservation interventions may be challenging. However, cases of collaboration between health and conservation experts have promoted positive health and conservation outcomes (Wan et al., 2011).
In conclusion, given that forests address a number of underlying determinants of undernutrition, lead to lower child stunting prevalence, and that forest conservation interventions cover large areas and are often implemented where people are vulnerable, policy makers, and public health practitioners might consider forest conservation as a potential nutrition-sensitive intervention. Such interventions might be particularly useful in contexts where implementation of standard nutrition interventions is challenging and where forest conservation interventions might be feasible. This suggests that public health and conservation practitioners should work together to identify, design, and implement projects that help achieve both forest conservation and nutritional goals. Nutrition benefits of forest conservation would not only be of interest to those trying to improve public health, but also those concerned with biodiversity conservation. Co-benefits for nutrition could help to incentivize local communities to participate in conservation, a key factor in determining the success of conservation interventions (Wan et al., 2011). There is unlikely to be a single bundle of nutrition interventions that is effective across all contexts (Hossain et al., 2017) and future research should test different combinations of forest and nutrition interventions in various contexts. Nonetheless, our growing understanding of the potential nutritional benefits of forest conservation is promising.
Data Availability Statement
The primary data on stunting, household, and individual socio-economic characteristics analyzed in this study were obtained from the Demographic and Health Surveys (DHS) Program of the United States Agency for International Development. Information about each DHS survey used in the analysis is included in the Supplementary Material. Requests to access these datasets should be directly submitted at https://dhsprogram.com/Data/. The spatial data (forests, road, market, agriculture suitability, livestock density, slope, population, GDP) are available from the corresponding author upon request. Complete metadata is available at https://dx.doi.org/10.6084/m9.figshare.7264658.
Author Contributions
All authors contributed to the design of the study and writing of the manuscript. RR organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. All authors read and approved the submitted version.
Funding
This study was funded by the National Socio-Environmental Synthesis Center (SESYNC) under funding from the National Science Foundation DBI-1052875, the Gordon and Betty Moore Foundation and The Rockefeller Foundation as part of the Health and Ecosystems: Analysis of Linkages (HEAL) program, the Luc Hoffmann Institute at WWF International under funding provided by the Mava Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the constructive feedback from the reviewers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2020.00020/full#supplementary-material
References
Agarwal, B. (2009). Rule making in community forestry institutions: the difference women make. Ecol. Econ. 68, 2296–2308. doi: 10.1016/j.ecolecon.2009.02.017
Almond, D., and Currie, J. (2011). Killing me softly: the fetal origins hypothesis. J. Econ. Perspect. 25, 153–172. doi: 10.1257/jep.25.3.153
Andam, K. S., Ferraro, P. J., Sims, K. R. E., Healy, A., and Holland, M. B. (2010). Protected areas reduced poverty in Costa Rica and Thailand. Proc. Natl. Acad. Sci. U.S.A. 107, 9996–10001. doi: 10.1073/pnas.0914177107
Angelsen, A., Jagger, P., Babigumira, R., Belcher, B., Hogarth, N. J., Bauch, S., et al. (2014). Environmental income and rural livelihoods: a global-comparative analysis. World Dev. 64, 12–28. doi: 10.1016/j.worlddev.2014.03.006
Arimond, M., and Ruel, M. T. (2004). Dietary diversity is associated with child nutritional status: evidence from 11 Demographic and Health Surveys. J. Nutr. 134, 2579–2585. doi: 10.1093/jn/134.10.2579
Berti, P. R., Krasevec, J., and FitzGerald, S. (2004). A review of the effectiveness of agriculture interventions in improving nutrition outcomes. Public Health Nutr. 7, 599–609. doi: 10.1079/PHN2003595
Black, R. E., Victora, C. G., Walker, S. P., Bhutta, Z. A., Christian, P., de Onis, M., et al. (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451. doi: 10.1016/S0140-6736(13)60937-X
Checkley, W., Buckley, G., Gilman, R. H., Assis, A. M. O., Guerrant, R. L., Morris, S. S., et al. (2008). Multi-country analysis of the effects of diarrhoea on childhood stunting. Int. J. Epidemiol. 37, 816–830. doi: 10.1093/ije/dyn099
Cunha, D. G. F., Sabogal-Paz, L. P., and Dodds, W. K. (2016). Land use influence on raw surface water quality and treatment costs for drinking supply in São Paulo State (Brazil). Ecol. Eng. 94, 516–524. doi: 10.1016/j.ecoleng.2016.06.063
Currie, J., and Vogl, T. (2013). Early-life health and adult circumstance in developing countries. Annu. Rev. Econ. 5, 1–36. doi: 10.1146/annurev-economics-081412-103704
DiMiceli, C. M., Carroll, M., Sohlberg, R., Huang, M. C., Hansen, M. C., and Townsend, J. R. G. (2011). Annual Global Automated MODIS Vegetation Continuous Fields (MOD44B) at 250 m Spatial Resolution for Data Years Beginning Day 65, 2000-2010, Collection 5, Percent Tree Cover. College Park, MD: Global Land Cover Facility, University of Maryland.
Eilers, E. J., Kremen, C., Greenleaf, S. S., Garber, A. K., and Klein, A. (2011). Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 6:e21363. doi: 10.1371/journal.pone.0021363
Ensign, S. H., and Mallin, M. A. (2001). Stream water quality changes following timber harvest in a coastal plain swamp forest. Water Res. 35, 3381–3390. doi: 10.1016/S0043-1354(01)00060-4
Esarey, J. (2017). clusterSEs: Calculate Cluster-Robust p-Values and Confidence Intervals. R Package Version 2.3.3. Available online at: https://CRAN.R-project.org/package=clusterSEs (accessed June 6, 2017).
Ferraro, P. J., and Hanauer, M. M. (2014). Advances in measuring the environmental and social impacts of environmental programs. Annu. Rev. Environ. Resour. 39, 495–517. doi: 10.1146/annurev-environ-101813-013230
Fungo, R., Muyonga, J., Kabahenda, M., Kaaya, A., Okia, C. A., Donn, P., et al. (2016). Contribution of forest foods to dietary intake and their association with household food insecurity: a cross-sectional study in women from rural Cameroon. Public Health Nutr. 19, 3185–3196. doi: 10.1017/S1368980016001324
Galway, L. P., Acharya, Y., and Jones, A. D. (2018). Deforestation and child diet diversity: a geospatial analysis of 15 sub-Saharan African countries. Health Place 51, 78–88. doi: 10.1016/j.healthplace.2018.03.002
Girard, A. W., Self, J. L., McAuliffe, C., and Olude, O. (2012). The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatr. Perinatal. Epidemiol. 26, 205–222. doi: 10.1111/j.1365-3016.2012.01282.x
Godfray, H. C. J., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., Nisbett, N., et al. (2010). The future of the global food system. Philos. Trans. R. Soc. B Biol. Sci. 365, 2769–2777. doi: 10.1098/rstb.2010.0180
Golden, C. D., Fernald, L. C. H., Brashares, J. S., Rasolofoniaina, B. J. R., and Kremen, C. (2011). Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A. 108, 19653–19656. doi: 10.1073/pnas.1112586108
Herrera, D., Ellis, A., Fisher, B., Golden, C. D., Johnson, K. B., Mulligan, M., et al. (2017). Upstream watershed condition predicts rural children's health across 35 developing countries. Nat. Commun. 8:811. doi: 10.1038/s41467-017-00775-2
Hickey, G. M., Pouliot, M., Smith-Hall, C., Wunder, S., and Nielsen, M. R. (2016). Quantifying the economic contribution of wild food harvests to rural livelihoods: a global-comparative analysis. Food Policy 62, 122–132. doi: 10.1016/j.foodpol.2016.06.001
Hossain, M., Choudhury, N., Abdullah, K. A. B., Mondal, P., Jackson, A. A., Walson, J., et al. (2017). Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Arch. Dis. Childhood 102, 903–909. doi: 10.1136/archdischild-2016-311050
Ickowitz, A., Powell, B., Salim, M. A., and Sunderland, T. C. H. (2014). Dietary quality and tree cover in Africa. Glob. Environ. Change 24, 287–294. doi: 10.1016/j.gloenvcha.2013.12.001
Intergovernmental Science-Policy Platform on Biodiversity Ecosystem Services (IPBES) (2017). The Assessment Report on Pollinators, Pollination and Food Production. Bonn: Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Available online at: https://www.ipbes.net/assessment-reports/pollinators (accessed October 30, 2018).
Jamnadass, R. H., Dawson, I. K., Franzel, S., Leakey, R. R. B., Mithöfer, D., Akinnifesi, F. K., et al. (2011). Improving livelihoods and nutrition in sub-Saharan Africa through the promotion of indigenous and exotic fruit production in smallholders' agroforestry systems: a review. Int. Forestry Rev. 13, 338–354. doi: 10.1505/146554811798293836
Johnson, K. B., Jacob, A., and Brown, M. E. (2013). Forest cover associated with improved child health and nutrition: evidence from the Malawi Demographic and Health Survey and satellite data. Glob. Health Sci. Pract. 1, 237–248. doi: 10.9745/GHSP-D-13-00055
Jones, K. R., Venter, O., Fuller, R. A., Allan, J. R., Maxwell, S. L., Negret, P. J., et al. (2018). One-third of global protected land is under intense human pressure. Science 360, 788–791. doi: 10.1126/science.aap9565
Joppa, L. N., and Pfaff, A. (2009). High and far: biases in the location of protected areas. PLoS ONE 4:e8273. doi: 10.1371/journal.pone.0008273
Klein, A. M., Vaissiere, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Leroy, J. L., Ruel, M., and Verhofstadt, E. (2009). The impact of conditional cash transfer programmes on child nutrition: a review of evidence using a programme theory framework. J. Dev. Effect. 1, 103–129. doi: 10.1080/19439340902924043
Manski, C. F. (2011). Policy analysis with incredible certitude. Econ. J. 121, F261–F289. doi: 10.1920/wp.cem.2011.0411
Manski, C. F., and Pepper, J. V. (2000). Monotone instrumental variables: with an application to the returns to schooling. Econometrica 68, 997–1010. doi: 10.1111/1468-0262.00144
McConnachie, M. M., Romero, C., Ferraro, P. J., and van Wilgen, B. W. (2016). Improving credibility and transparency of conservation impact evaluations through the partial identification approach. Conserv. Biol. 30, 371–381. doi: 10.1111/cobi.12610
Millennium Ecosystem Assessment (MEA) (2005). Ecosystems and Human Well-being. Synthesis. Washington, DC: Island Press. Availble online at: https://www.millenniumassessment.org/documents/document.356.aspx.pdf (accessed October 30, 2018).
Miller, D. C. (2014). Explaining global patterns of international aid for linked biodiversity conservation and development. World Dev. 59, 341–359. doi: 10.1016/j.worlddev.2014.01.004
Moursi, M. M., Arimond, M., Dewey, K. G., Treche, S., Ruel, M. T., and Delpeuch, F. (2008). Dietary diversity is a good predictor of the micronutrient density of the diet of 6- to 23-month-old children in Madagascar. J. Nutr. 138, 2448–2453. doi: 10.3945/jn.108.093971
Naidoo, R., Gerkey, D., Hole, D., Pfaff, A., Ellis, A. M., Golden, C. D., et al. (2019). Evaluating the impacts of protected areas on human well-being across the developing world. Sci. Adv. 5:eaav3006. doi: 10.1126/sciadv.aav3006
Pailler, S., Naidoo, R., Burgess, N. D., Freeman, O. E., and Fisher, B. (2015). Impacts of community-based natural resource management on wealth, food security and child health in Tanzania. PLoS ONE 10:e0133252. doi: 10.1371/journal.pone.0133252
Pandis, N. (2016). Using linear regression for t tests and analysis of variance. Am. J. Orthod. Dentofacial Orthop. 149:769. doi: 10.1016/j.ajodo.2016.02.007
Park, H. M. (2009). Comparing Group Means: T-tests and One-way ANOVA Using STATA, SAS, R, and SPSS. Bloomington: University Information Technology Services Center for Statistical and Mathematical Computing, Indiana University. Available online at: https://scholarworks.iu.edu/dspace/handle/2022/19735 (accessed January 15, 2020).
Pattanayak, S. K., and Wendland, K. J. (2007). Nature's care: diarrhea, watershed protection, and biodiversity conservation in Flores, Indonesia. Biodivers. Conserv. 16, 2801–2819. doi: 10.1007/s10531-007-9215-1
Perez-Escamilla, R., Bermudez, O., Buccini, G. S., Kumanyika, S., Lutter, C. K., Monsivais, P., et al. (2018). Nutrition disparities and the global burden of malnutrition. BMJ 361:k2252. doi: 10.1136/bmj.k2252
Poudyal, M., Jones, J. P. G., Rakotonarivo, O. S., Hockley, N., Gibbons, J. M., Mandimbiniaina, R., et al. (2018). Who bears the cost of forest conservation? PeerJ 6:e5106. doi: 10.7717/peerj.5106
Rasmussen, L. V., Fagan, M. E., Ickowitz, A., Wood, S. L. R., Kennedy, G., Powell, B., et al. (in press). Forest pattern, not just amount, influences dietary quality in five African countries. Global Food Security. doi: 10.1016/j.gfs.2019.100331.
Rasolofoson, R. A., Ferraro, P. J., Jenkins, C. N., and Julia, P. G. (2015). Effectiveness of community forest management at reducing deforestation in Madagascar. Biol. Conserv. 184, 271–277. doi: 10.1016/j.biocon.2015.01.027
Rasolofoson, R. A., Hanauer, M. M., Pappinen, A., Fisher, B., and Ricketts, T. H. (2018). Impacts of forests on children's diet in rural areas across 27 developing countries. Sci. Adv. 4:eaat2853. doi: 10.1126/sciadv.aat2853
Rights Resources Initiative (RRI) (2014). What Future for Reform? Progress and Slowdown in Forest Tenure Reform since 2002. Washington, DC: Rights and Resources Initiative. Available online at: https://rightsandresources.org/en/publication/view/what-future-for-reform/ (accessed October 30, 2018).
Rowland, D., Ickowitz, A., Powell, B., Nasi, R., and Sunderland, T. C. H. (2017). Forest foods and healthy diets: quantifying the contributions. Environm. Conserv. 44, 101–114. doi: 10.1017/S0376892916000151
Ruel, M. T., and Alderman, H. (2013). Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet 382, 536–551. doi: 10.1016/S0140-6736(13)60843-0
Semba, R. D. (1999). Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 58, 719–727. doi: 10.1017/S0029665199000944
Steyn, N. P., Nel, J. H., Nantel, G., Kennedy, G., and Labadarios, D. (2006). Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 9, 644–650. doi: 10.1079/PHN2005912
Sunderland, T. C. H. (2011). Food security: why is biodiversity important? Int. Forestry Rev. 13, 265–274. doi: 10.1505/146554811798293908
Sunderland, T. C. H., Achdiawan, R., Angelsen, A., Babigumira, R., Ickowitz, A., Paumgarten, F., et al. (2014). Challenging perceptions about men and women, and forest product use: a global comparative study. World Dev. 64, S56–S66. doi: 10.1016/j.worlddev.2014.03.003
Sunderlin, W. D., Angelsen, A., Belcher, B., Burgers, P., Nasi, R., Santoso, L., et al. (2005). Livelihoods, forests, and conservation in developing countries: an overview. World Dev. 33, 1383–1402. doi: 10.1016/j.worlddev.2004.10.004
Tabor, K., Jones, K. W., Hewson, J., Rasolohery, A., Rambeloson, A., Andrianjohaninarivo, T., et al. (2017). Evaluating the effectiveness of conservation and development investments in reducing deforestation and fires in Ankeniheny-Zahemena corridor, Madagascar. PLoS ONE 12:e0190119. doi: 10.1371/journal.pone.0190119
United Nations Children's Fund (UNICEF) (2013). Improving Child Nutrition: The Achievable Imperative for Global Progress. New York, NY: The United Nations Children's Fund. Available online at: https://www.unicef.org/gambia/Improving_Child_Nutrition_-_the_achievable_imperative_for_global_progress.pdf (accessed October 30, 2018).
United Nations Environmental Programme (UNEP), Food and Agriculture Organization of the United Nations (FAO), and United Nations Forum on Forests Secretariat (UNFF) (2008). Vital Forest Graphics. Nairobi: UNEP/GRID-Arendal.
United States Agency for International Development (USAID) (2013). Standard Recode Manual for DHS 6. Washington, DC: United States Agency for International Development. Available online at: https://www.dhsprogram.com/pubs/pdf/DHSG4/Recode6_DHS_22March2013_DHSG4.pdf (accessed November 11, 2019).
United States Agency for International Development (USAID) (2018). Demographic and Health Surveys Program. Washington, DC: United States Agency for International Development. Available online at: https://dhsprogram.com/Data/
Vedeld, P., Angelsen, A., Bojö, J., Sjaastad, E., and Berg, G. K. (2007). Forest environmental incomes and the rural poor. Forest Policy Econ. 9, 869–879. doi: 10.1016/j.forpol.2006.05.008
Villamor, E., and Fawzi, W. W. (2005). Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 18, 446–464. doi: 10.1128/CMR.18.3.446-464.2005
Vira, B., Wildburger, C., and Mansourian, S. (2015). Forests, Trees and Landscapes for Food Security and Nutrition. Contributing to the “Zero Hunger Challenge”. Vienna: International Union of Forest Research Organizations. Available online at: http://www.iufro.org/science/gfep/forests-and-food-security-panel/report/ (accessed October 30, 2018).
Wan, M., Colfer, C. J. P., and Powell, B. (2011). Forests, women and health: opportunities and challenges for conservation. Int. Forestry Rev. 13, 369–387. doi: 10.1505/146554811798293854
World Health Organization (WHO) (2006). WHO Child Growth Standards. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Methods and Development. Geneva: World Health Organization. Available online at: https://www.who.int/childgrowth/standards/technical_report/en/ (accessed November 05, 2019).
World Health Organization (WHO) (2017). Safely Managed Drinking Water. Geneva: World Health Organization. Available online at: https://data.unicef.org/wp-content/uploads/2017/03/safely-managed-drinking-water-JMP-2017-1.pdf (accessed October 30, 2018).
Keywords: demographic and health surveys, ecosystem services, food security, height-for-age, malnutrition, planetary health, partial identification, stunting
Citation: Rasolofoson RA, Ricketts TH, Jacob A, Johnson KB, Pappinen A and Fisher B (2020) Forest Conservation: A Potential Nutrition-Sensitive Intervention in Low- and Middle-Income Countries. Front. Sustain. Food Syst. 4:20. doi: 10.3389/fsufs.2020.00020
Received: 05 December 2019; Accepted: 17 February 2020;
Published: 04 March 2020.
Edited by:
Bronwen Powell, Pennsylvania State University (PSU), United StatesReviewed by:
Yubraj Acharya, Pennsylvania State University (PSU), United StatesLaura Vang Rasmussen, University of British Columbia, Canada
Copyright © 2020 Rasolofoson, Ricketts, Jacob, Johnson, Pappinen and Fisher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranaivo A. Rasolofoson, cnJhc29sb2ZAZ21haWwuY29t
†Present address: Ranaivo A. Rasolofoson, Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
 Ranaivo A. Rasolofoson
Ranaivo A. Rasolofoson Taylor H. Ricketts
Taylor H. Ricketts Anila Jacob4
Anila Jacob4 Kiersten B. Johnson
Kiersten B. Johnson Ari Pappinen
Ari Pappinen