- 1National Centre for Epidemiology and Population Health, Research School of Population Health, The Australian National University, Canberra, ACT, Australia
- 2OzFoodNet, South Australian Department for Health and Wellbeing, Adelaide, SA, Australia
- 3OzFoodNet, Department of Health and Human Services Victoria, Melbourne, VIC, Australia
- 4OzFoodNet, Health Protection Service, ACT Health, Canberra, ACT, Australia
- 5OzFoodNet, Queensland Health, Brisbane, QLD, Australia
- 6OzFoodNet, NSW Ministry of Health, Sydney, NSW, Australia
- 7Microbiological Diagnostic Unit Public Health Laboratory, Department of Microbiology and Immunology, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, VIC, Australia
Salmonella enterica is an important cause of foodborne illness in Australia, regularly causing high-profile outbreaks involving commercially-available foods. We used the national register of foodborne outbreaks to review the transmission pathways, settings, serotypes, and food vehicles of Salmonella outbreaks in Australia between 2001 and 2016. We examined trends over time of implicated food vehicles in outbreaks where there was statistical, microbiological, or descriptive evidence. Of the 990 Salmonella outbreaks reported, 79% (778/990) were suspected or confirmed to have been transmitted through contaminated food. Of these, 61% (472/778) occurred in food premises and 84% (656/778) were caused by Salmonella Typhimurium. Eggs and egg-containing foods were the most frequently identified food vehicle. Outbreaks due to egg-based sauces and Vietnamese style sandwiches, which often contain pâté and raw egg butter, increased, while outbreaks due to poultry meat, beef, pork, other sandwiches, and other desserts had a decreasing trend from 2001 to 2016. Identifying food vehicles and the Salmonella serotypes causing outbreaks in Australia provides important evidence for food regulation strategies and control measures.
Introduction
Non-typhoidal Salmonella enterica spp. infection from contaminated food is an important cause of both sporadic gastroenteritis and outbreaks internationally. In Australia, the incidence of infection due to Salmonella spp. in the community is estimated to be 185 infections per 100,000 population per year (Kirk et al., 2014). While only a small proportion of Salmonella cases are epidemiologically linked to outbreaks [6.6% of notifications in 2011 (OzFoodNet Working Group, 2015)], outbreaks can be widespread and expensive for regulators and industry (Scharff et al., 2016). OzFoodNet—Australia's enhanced foodborne disease surveillance network, has published annual surveillance reports between 2001 and 2011, which report an annual median of 36 (range 26–61) salmonellosis outbreaks nationally that are confirmed or suspected to be caused by contaminated food, with the majority due to Salmonella Typhimurium (OzFoodNet Working Group, 2003, 2015).
The investigation of outbreaks to identify and control the source of infection is an essential public health action to prevent further cases. Review of the Salmonella serotypes and associated food vehicles identified during outbreaks can assist with the development of targeted population-level interventions and prevention strategies to reduce the incidence of Salmonella serotypes associated with these food vehicles. International studies have attributed Salmonella outbreaks to food commodities, finding that eggs were the most commonly implicated food vehicle in the United States of America (USA) (28% of outbreaks), poultry in the United Kingdom (UK) (20.5% of outbreaks), and all meats in Latin America (24% of outbreaks) (Gormley et al., 2011; Pires et al., 2012; Jackson et al., 2013; Painter et al., 2013). In Australia, outbreaks of salmonellosis have been associated with a variety of foods including eggs, chicken and other poultry, pork, beef, lamb, fish, fresh produce, and nuts (Ashbolt et al., 2002; OzFoodNet Working Group, 2012). While there is evidence that outbreaks associated with eggs in Australia have increased (Moffatt et al., 2016), the food vehicles responsible for outbreaks of salmonellosis have not been systematically assessed before.
In this study, we used the national foodborne disease outbreak register data to describe the epidemiology of foodborne Salmonella outbreaks in Australia between 2001 and 2016.
Materials and Methods
We used the OzFoodNet outbreak register, the national register of foodborne disease outbreaks in Australia, to examine all reported outbreaks from 1 January 2001 to 31 December 2016 where non-typhoidal Salmonella spp. was listed as the etiological agent. Australian states and territories who investigate the outbreaks enter standardized outbreak data into this national repository, including information on: the setting of the outbreak; where the food was prepared (if applicable and defined in Supplementary Information 1); number of symptomatic cases; number of cases with laboratory-confirmed Salmonella spp., number of cases hospitalized, number who died during the outbreak (the relative contribution of illness due to salmonellosis to each death is unknown); median incubation period of cases; median duration of illness; geographical location of exposures (local government area, multiple local government areas, multiple health department regions, state-wide, multi-state); mode of transmission; food vehicle; type of investigation (case series, cohort, case-control) and evidence (statistical, microbiological, descriptive); and Salmonella spp. serotype classified in accordance with the White-Kauffmann-Le Minor scheme (Grimont and Weill, 2007).
An outbreak was defined as ≥2 cases of Salmonella spp. orientated by person, place or time, or an increase in the number of salmonellosis cases above what is normally expected and where an investigation was undertaken to try to determine the source of illness. A foodborne or suspected foodborne outbreak was defined as an outbreak where cases had consumed a common food or meal that was implicated in causing their illness. We defined statistical, microbiological, and descriptive evidence as in Moffatt et al. (2016). If an analytical epidemiological study was undertaken with a statistically significant association observed between a food vehicle and illness, the food vehicle was considered to have statistical evidence. Microbiological evidence was obtained when Salmonella was detected or cultured from the implicated food vehicle, food premises, processing, or primary production environment. Microbiological investigations and analytical studies were not undertaken in all outbreaks. An outbreak was considered to have descriptive evidence if there was information collected from the epidemiological and/or the environmental health investigations that implicated the food vehicle, which the outbreak investigation team considered compelling. This would include, for example, if an unsafe food preparation practice of a food reportedly eaten by at least some outbreak cases was observed during the environmental health investigation.
Outbreaks where < 2 people had laboratory-confirmed Salmonella spp. infection and there was no microbiological evidence implicating a food were excluded (n = 35). Duplicate entries of multi-state outbreaks using OzFoodNet outbreak reports (Ashbolt et al., 2002; OzFoodNet Working Group, 2007) (n = 2) and outbreaks where another pathogen besides Salmonella spp. was detected or isolated in cases (n = 3) or in the food vehicle (n = 2) were excluded. There was one outbreak in the outbreak register included in our analysis where a serotype was unable to be determined.
We compared the number of outbreaks per year to the national salmonellosis notification rate using data from the National Notifiable Disease Surveillance System (Department of Health, 2018). Food vehicles were grouped into food categories (Supplementary Information 1). As the food vehicle field only contained the name of the implicated food or meal, ingredient lists were obtained through internet-based recipe searches. Our food categories included egg-based sauce, desserts containing raw or lightly cooked eggs, other desserts, eggs (other), poultry, beef, pork, lamb, fish, crustacean/mollusc, fruits, vegetables, nuts, sprouts, bánh mì (Vietnamese style sandwiches), other sandwiches, salads, sushi/kimbap/gimbap, tahini or helva, dips, and mixed dishes. If there were insufficient details in the food vehicle field to categorize the food, we categorized it as undetermined. Food vehicle categories were examined by (i) evidence type and (ii) trend over time.
Descriptive analyses were performed in Stata SE 14 (StataCorp 2014) and graphs were created in Microsoft Excel 2013. Due to the importance of S. Typhimurium in Australia, we used chi-square tests for homogeneity to assess differences between outbreaks due to S. Typhimurium and non-Typhimurium serotypes. We used the ptrend command to calculate a chi-square statistic for trend to assess trends in food vehicles over time. Permission for data access was granted by OzFoodNet and ethics approval was obtained through the Australian National University Human Research Ethics Committee (2017/494).
Results
From 1 January 2001 to 31 December 2016, there were 990 outbreaks due to Salmonella spp. reported by Australian state and territory health authorities. The number of outbreaks reported per year ranged from 29 outbreaks in 2001 to 116 outbreaks in 2014 (Figure 1).
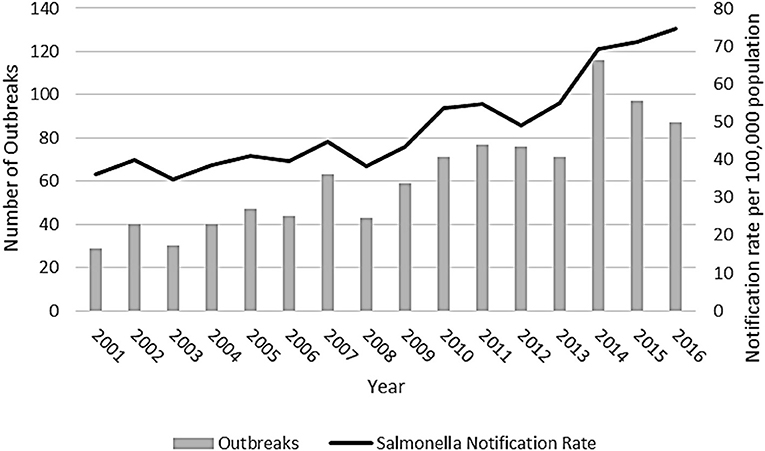
Figure 1. Salmonellosis outbreaks reported in the OzFoodNet outbreak register and National Notifiable Disease Surveillance System Salmonella notification rate, Australia, 2001–2016.
Transmission
Most Salmonella spp. outbreaks were of foodborne or suspected foodborne transmission (79%; 778/990) (Table 1). In these 778 outbreaks, a total of 14,708 people were reported to be ill, with a median of 9 people per outbreak (range 2–442). Overall, 66% (9,683/14,708) of people affected in these outbreaks had laboratory-confirmed Salmonella spp., 15% (2,191/14,708) were hospitalized, and 0.3% (48/14,708) died. The median incubation period was available for 64% (500/778) of outbreaks, with a median of 24 h (range of medians 7–192 h). Median illness duration was available for 72% (560/778), with a median of 168 h (range of medians 10–504 h).
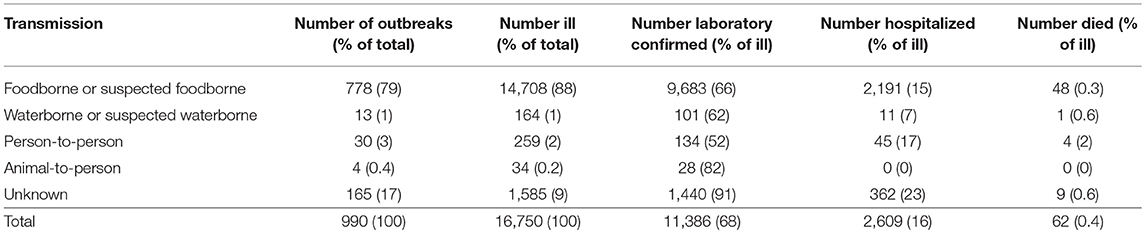
Table 1. Number of reported Salmonella spp. outbreaks, ill persons, laboratory-confirmed infections, hospitalizations, and deaths by transmission pathway, Australia, 2001–2016.
Setting
Implicated meals were prepared in food premises for the majority of foodborne or suspected foodborne outbreaks (Table 2), including 40% (314/778) in restaurants, 8% (63/778) in take-away stores, 7% (51/778) in bakeries, 4% (29/778) in commercial caterers, 1% (8/778) in national franchised fast food stores, 0.5% (4/778) in grocery stores/delicatessens, and 0.4% (3/778) on cruises or airlines. Home kitchens (18%, 142/778) were the next most common setting. In 10% (80/778) of outbreaks, food was prepared in institutional settings, including 5% (39/778) in aged care facilities, 0.8% (6/778) in schools, 0.8% (6/778) in hospitals, 0.6% (5/778) in child care centers, 0.6% (5/778) in camps, and 2% (19/778) in other institutional settings such as correctional or military facilities. In the remaining 11% (84/778) of outbreaks, food was prepared at a market, fair/festival, or mobile service; commercially manufactured food; imported food; primary produce; prepared in other settings; or prepared in unknown settings (Table 2).
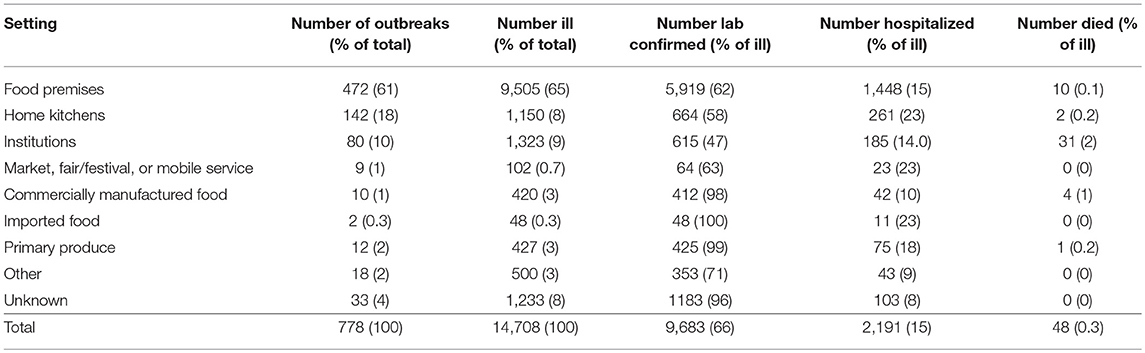
Table 2. Number of reported foodborne or suspected foodborne Salmonella spp. outbreaks, ill persons, laboratory-confirmed infections, hospitalizations, and deaths by setting food prepared, Australia, 2001-2016.
Serotype
Thirty-nine different non-typhoidal Salmonella serotypes were identified among the 778 foodborne or suspected foodborne outbreaks. Most outbreaks were due to Salmonella Typhimurium (84%, 656/778). Salmonella Saintpaul was the next most common cause of outbreaks (2%, 15/778), followed by Salmonella Virchow (2%, 12/778), Salmonella Singapore (1%, 9/778), Salmonella subsp I (1%, 8/778), and Salmonella Infantis (1%, 8/778) (Table 3). No other serotype caused more than five outbreaks over the 16-year period assessed.
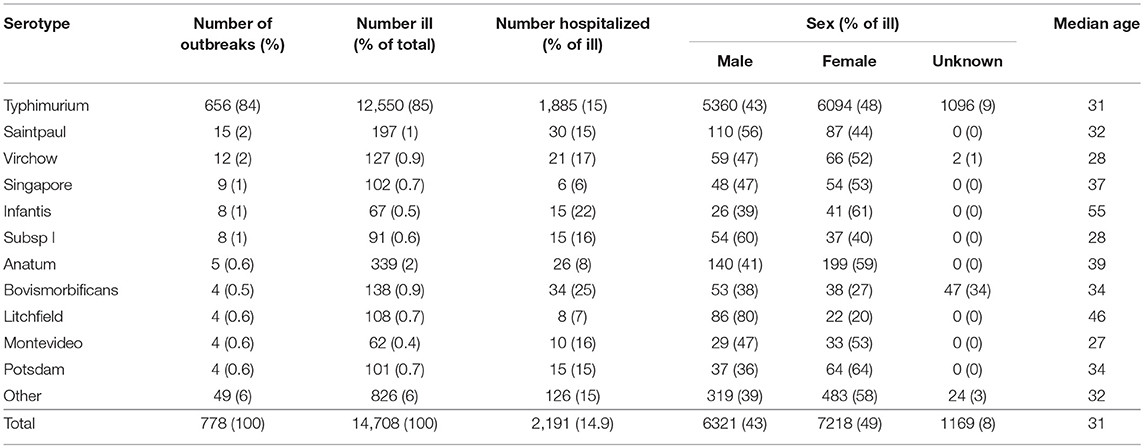
Table 3. Number of reported foodborne or suspected foodborne Salmonella spp. outbreaks, ill persons, hospitalizations, sex, and median of outbreak median age by serotype, Australia, 2001-2016.
A higher proportion of outbreaks where food was prepared in food premises were due to S. Typhimurium (63%) than to non-Typhimurium Salmonella (p < 0.01). There was no difference in the reported number of S. Typhimurium and non-Typhimurium Salmonella outbreaks where food was prepared in private kitchens or in institutions. There was no difference in the number of people ill, the number of people hospitalized, the male-to-female ratio, or the median age of those affected in foodborne or suspected foodborne outbreaks caused by S. Typhimurium compared to non-Typhimurium Salmonella in all settings.
The number of S. Typhimurium outbreaks generally increased over time, with 15 foodborne or suspected foodborne S. Typhimurium outbreaks reported in 2001, peaking at 88 outbreaks reported in 2014. In comparison to the previous year, in 2016, the number of S. Typhimurium outbreaks and the associated number of cases in these outbreaks decreased, whereas, the number of associated cases in non-Typhimurium Salmonella outbreaks increased. This was largely due to four multi-state non-Typhimurium Salmonella outbreaks in 2016 (Figure 2).
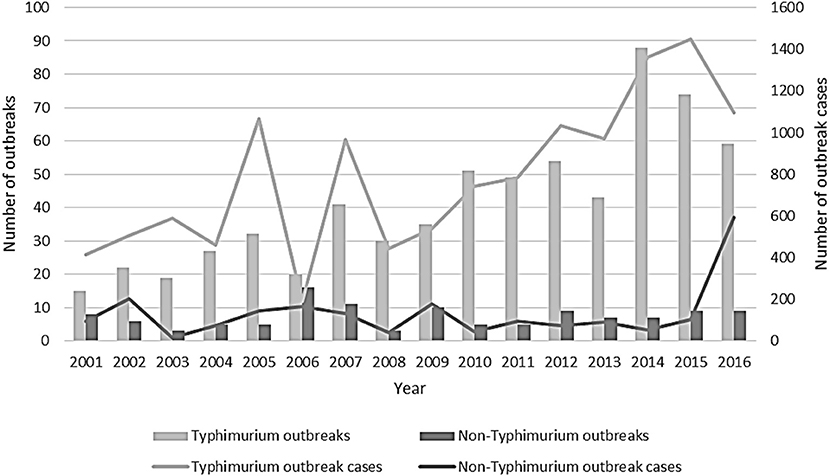
Figure 2. Number of outbreaks and outbreak cases due to Salmonella Typhimurium and non-Typhimurium Salmonella reported by state and territory health authorities, Australia, 2001–2016.
Food Vehicles
A food vehicle was listed in the OzFoodNet outbreak register for 69% (537/778) of foodborne or suspected foodborne outbreaks. Of these, there was statistical and/or laboratory evidence to support the food vehicle as the cause of the outbreak for 50% (271/537) of outbreaks. Where there was no statistical or laboratory evidence, the food vehicle was supported by descriptive evidence in 38% (205/537) of outbreaks, and there was no evidence to support the food vehicle in the remaining 11% (61/537) of outbreaks. We excluded the 61 outbreaks with no evidence from further analysis, leaving 476 outbreaks with a food vehicle listed.
In our food categories, frequently identified food vehicles in outbreaks were eggs and egg-containing foods (Table 4). Eggs, egg-based sauces (e.g., mayonnaise, aioli, hollandaise, tartare), desserts containing raw or lightly cooked eggs (e.g., tiramisu, fried ice cream, ice cream, mousse, custard), and fresh pasta eaten lightly cooked or with a lightly cooked egg based sauce, were the identified food vehicle in 238/476 (50%) of Salmonella spp. outbreaks. S. Typhimurium was the responsible serotype in 95% (226/238) of these outbreaks (see Supplementary Information 2 for all serotypes for all food categories). Chicken and other poultry were the implicated food vehicle for 34/476 (7%) and pork for 15/476 (3%) outbreaks (Table 4), with 28/34 (82%) and 9/15 (60%) due to S. Typhimurium, respectively (Supplementary Information 2). Other food source animals, including beef, lamb, fish, and crustaceans/molluscs, were each identified as the food vehicle for 1% of these outbreaks. Fruits, vegetables and sprouts were each responsible for around 1% of outbreaks, but unlike outbreaks involving animal-derived food vehicles, these were more common for non-Typhimurium serotypes (p < 0.001) (Table 4; Supplementary Information 2).
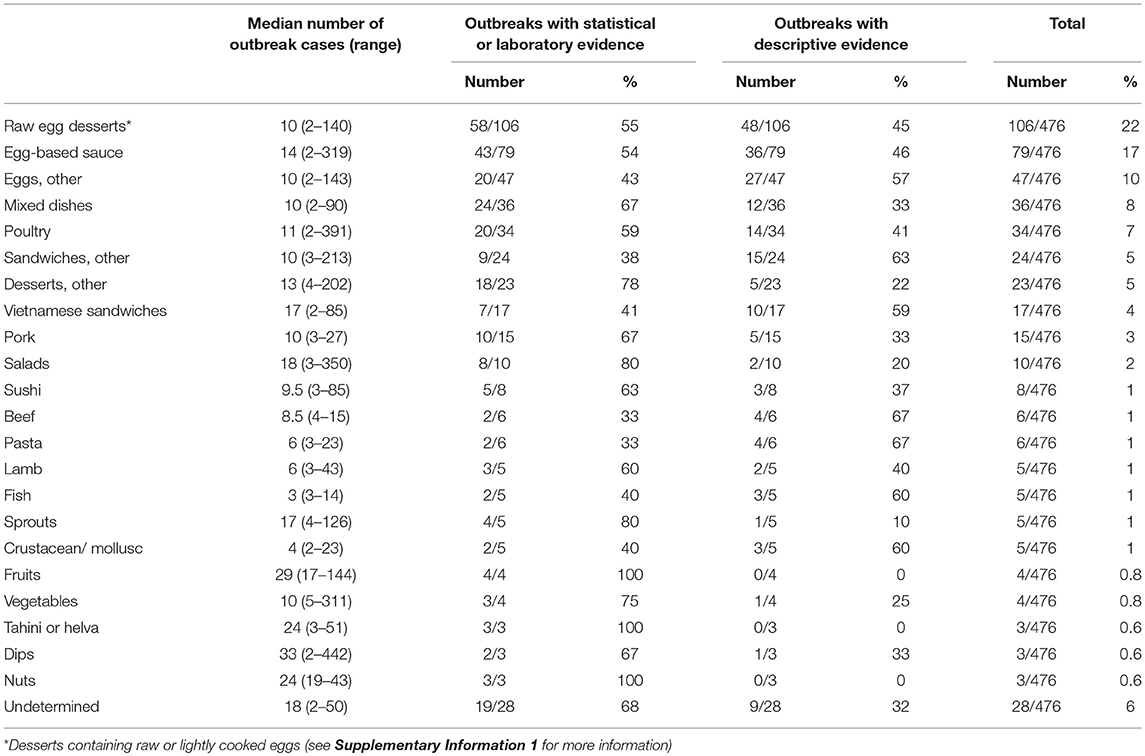
Table 4. Median number of cases affected and number of outbreaks by type of evidence due to food vehicles, Australia, 2001–2016.
Outbreaks due to sprouts, salads, Vietnamese sandwiches, fruits, nuts, tahini/helva, and dips tended to affect more people, with a median outbreak size of more than 15 cases (Table 4). Between 2001 and 2016, there was an increase in the proportion of outbreaks due to egg-based sauces (p < 0.001) and Vietnamese style sandwiches (p < 0.001), while poultry (p = 0.033), beef (p = 0.046), pork (p = 0.047), other sandwiches (p = 0.048), and other desserts (p = 0.017) had a decreasing trend over this time period (Supplementary Information 3).
Discussion
Outbreaks of non-typhoidal Salmonella spp. are a significant and high-profile cause of foodborne illness in Australia. We found an increasing trend of outbreaks in Australia, particularly between 2008 and 2014, beginning before the introduction of culture-independent diagnostic testing (May et al., 2017). This is similar to the trend in the USA, where there was an increase in the number of outbreaks reported between 2008 and 2013 (Centers for Disease Control Prevention, 2018). The increasing trend of outbreaks in Australia, together with comparatively high and increasing Salmonella spp. notification rates (Ford et al., 2016), emphasizes the continued importance of identifying food vehicles in outbreaks and implementing control strategies throughout the food chain to prevent illness.
Similar to the USA and Canada, eggs and foods containing eggs were the most commonly reported food vehicle in Australian foodborne or suspected foodborne Salmonella spp. outbreaks (Jackson et al., 2013; Belanger et al., 2015). Eggs were the responsible food vehicle in 50% of these outbreaks in Australia, compared to 28% in the USA (Jackson et al., 2013) and 39% in Canada (Belanger et al., 2015). We took a conservative approach in attributing outbreaks to eggs, as eggs may have also been the responsible food vehicle where there was more than one high risk ingredient (e.g., Vietnamese style rolls, sandwiches, and salads). While the proportion of outbreaks associated with poultry meat, beef and pork in Australia declined from 2001 to 2016, egg-based sauces, desserts containing raw or lightly cooked eggs, and Vietnamese style sandwiches, which usually contain a raw-egg butter and/or pork or chicken liver pâté, were increasingly associated with Salmonella outbreaks over the time period.
As most foodborne and suspected foodborne Salmonella outbreaks, including most S. Typhimurium outbreaks were linked to commercial food premises, additional interventions targeted at Salmonella control measures in these settings are required, particularly around the preparation of foods containing raw or lightly cooked eggs (Moffatt et al., 2016). While there are state-based guidelines, there are no rules or restrictions on the use of raw eggs in ready-to-eat foods in Australia (Moffatt et al., 2016). As Australian states and territories have oversight of food safety regulation, some states have implemented control measures across the supply chain to try to reduce the burden of egg-related salmonellosis, including targeted communication and education for bakeries, mandatory training at retail level, industry food safety plans, and the vaccination of many laying flocks against S. Typhimurium (NSW Food Authority, 2007; Groves et al., 2016; Ford et al., 2018).
Unlike in other countries where S. Enteritidis is more prevalent in eggs, S. Typhimurium caused most (84%) foodborne or suspected foodborne Salmonella spp. outbreaks between 2001 and 2016 (Gormley et al., 2011; Pires et al., 2012; Jackson et al., 2013; Belanger et al., 2015), with only 3 outbreaks of S. Enteritidis reported during this time period. The majority of fresh produce outbreaks associated with fruits, vegetables and sprouts were caused by non-Typhimurium serotypes, with S. Saintpaul, S. Litchfield, and S. Oranienburg causing more than half of the outbreaks associated with these food vehicles. While few in number, outbreaks associated with sprouts, nuts, fruit and some fresh salad produce, which are usually consumed raw, were generally large due to the wide distribution of these food products. Food vehicle identification is often long and difficult for these types of fresh produce outbreaks as these foods are frequently poorly recalled and reported by outbreak cases during food history interviews. Also, as most horticulture products are not packaged and labeled and there is high product turnover, food trace-back investigations are difficult and associated public health interventions can be delayed for these reasons (Munnoch et al., 2009). In Australia, there is currently no national horticulture primary production and processing standard, except for seed sprouts (Food Standards Australia New Zealand, 2011), so it is important to identify interventions to target these food vehicles.
While there have been multiple cohort or case-control studies undertaken during outbreaks to identify the food vehicle causing illness, there have been few published case-control studies of sporadic Salmonella illness in Australia. Without this evidence, it is difficult to compare whether the food vehicles that cause outbreaks identified in this study are the same as those that cause sporadic illness. Also, while individual risk factors, such as age and sex, have been identified to be important risk factors for sporadic illness in Australia (Ford et al., 2016), in this study, there was little difference in demographic characteristics of cases in foodborne or suspected foodborne S. Typhimurium and non-Typhimurium Salmonella outbreaks. This suggests that the individual risk factors of age and sex are not as important in outbreaks as they are with sporadic illness. While there was no data on the immune status of people exposed to outbreaks, the highest number of deaths occurred in outbreaks in institutional settings, including aged care facilities and hospitals where large numbers of immunocompromised people are expected.
A limitation of this study is that while OzFoodNet commenced in 2001, not all states and territories were routinely reporting data into the outbreak register until 2003, so some outbreaks may have been missed prior to 2003. In this study, 17% of Salmonella spp. outbreaks had an unknown mode of transmission. While it is likely that illness in many of these outbreaks was transmitted through contaminated food, there was insufficient evidence available to conclude this, so data presented in this paper is likely to under-represent the true number of foodborne outbreaks of salmonellosis in Australia. In addition to the 17% of outbreaks with an unknown mode of transmission there were: 25% of outbreaks that had a suspected foodborne/foodborne mode of transmission identified with an unknown food vehicle; 6% of suspected foodborne/foodborne outbreaks that had no evidence to support the food vehicle; and the food vehicle in an additional 6% of suspected foodborne/foodborne outbreaks could not be categorized because the meal listed lacked sufficient details (undetermined) or contained multiple foods. In addition, 38% of outbreaks were attributed to a food vehicle with descriptive evidence alone. This may be because not all outbreaks are conducive to an analytical investigation (Moffatt et al., 2016), food or environmental sampling is not always routinely performed, or because cross-contamination can make it difficult to identify a single food vehicle. While cross-contamination is likely to be an important aspect in many outbreaks (OzFoodNet Working Group, 2015; Osimani et al., 2016), another limitation of this study is that there was insufficient data to quantify the importance of cross-contamination in these Australian outbreaks.
As Australia moves toward a national strategy to reduce foodborne illness from Salmonella, data on Salmonella outbreaks, significant serotypes, and the food vehicles that cause illness are important for monitoring and surveillance, which can be used to provide evidence for effective control measures (Food Regulation Secretariat, 2018). Consistent with other studies, eggs and egg-containing foods were the most common cause of outbreaks in Australia over the period 2001–2016 causing significant morbidity in the population. Control measures have been, and continue to be implemented around preparation of egg-containing foods, particularly in food premises. Additional interventions focused on fresh produce items, such as a horticulture primary production and processing standard, could be strengthened since these vehicles can cause larger outbreaks. While collecting evidence about the food vehicles that cause outbreaks is important in the implementation of control measures, it can be challenging and further research on causes of sporadic illness and the importance of cross-contamination for Salmonella outbreaks in Australia could identify important public health interventions.
Author Contributions
LF conceived the paper with feedback from all authors and conducted the analysis and drafted the paper. All authors made contributions to the final manuscript.
Funding
LF is supported by an Australian Government Research Training Program (RTP) Scholarship. MK is supported by a National Health & Medical Research Council fellowship (APP1145997). DW is supported by a National Health & Medical Research Council fellowship (APP1123854).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the OzFoodNet Network, which is funded by the Australian Government Department of Health. We thank state public health reference laboratories who performed the Salmonella serotyping and state public health officers who investigated these outbreaks.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2018.00086/full#supplementary-material
References
Ashbolt, R., Givney, R., Gregory, J. E., Hall, G., Hundy, R., Kirk, M., et al. (2002). Enhancing foodborne disease surveillance across Australia in 2001: the OzFoodNet working group. Commun. Dis. Intell. Q. Rep. 26, 375–406.
Belanger, P., Tanguay, F., Hamel, M., and Phypers, M. (2015). An overview of foodborne outbreaks in Canada reported through outbreak summaries: 2008-2014. Canada Commun. Dis. Rep. 41, 254–262. doi: 10.14745/ccdr.v41i11a01
Centers for Disease Control Prevention (2018). National Outbreak Reporting System (NORS) Dashboard [Online]. Atlanta, GA: CDC. Availableonline at: https://wwwn.cdc.gov/norsdashboard/ (Accessed August 25, 2018).
Department of Health (2018). National Notifiable Disease Surveillance System. Notification Rate of Salmonellosis, Received From State and Territory Health Authorities in the Period of 1991 to 2017 [Online]. Canberra, ACT: Australian Government. Available online at: http://www9.health.gov.au/cda/source/cda-index.cfm (Accessed July 5, 2018).
Food Regulation Secretariat (2018). Australia's Foodborne Illness Reduction Strategy 2018-2021+ [Online]. Canberra ACT: Australian Government Department of Health. Available online at: http://health.gov.au/internet/fr/publishing.nsf/Content/australia-foodborne-illness-reduction-strategy-2018%E2%80%932021 (Accessed June 18, 2018).
Food Standards Australia New Zealand (2011). Proposal P1004 - Primary Production & Processing Standard for Seed Sprouts [Online]. Available online at: http://www.foodstandards.gov.au/code/proposals/pages/proposalp1004primary4361.aspx (Accessed November 10, 2018).
Ford, L., Glass, K., Veitch, M., Wardell, R., Polkinghorne, B., Dobbins, T., et al. (2016). Increasing incidence of Salmonella in Australia, 2000-2013. PLoS ONE 11:e0163989. doi: 10.1371/journal.pone.0163989
Ford, L., Wang, Q., Stafford, R., Ressler, K. A., Norton, S., Shadbolt, C., et al. (2018). Seven Salmonella Typhimurium outbreaks in Australia linked by trace-Back and whole genome sequencing. Foodborne Path. Dis. 15, 285–292. doi: 10.1089/fpd.2017.2353
Gormley, F. J., Little, C. L., Rawal, N., Gillespie, I. A., Lebaigue, S., and Adak, G. K. (2011). A 17-year review of foodborne outbreaks: describing the continuing decline in England and Wales (1992-2008). Epidemiol. Infect. 139, 688–699. doi: 10.1017/S0950268810001858
Grimont, P. A., and Weill, F.-X. (2007). Antigenic Formulae of the Salmonella serovars, 9th edn. Paris: WHO Collaborating Centre for Reference and Research on Salmonella.
Groves, P. J., Sharpe, S. M., Muir, W. I., Pavic, A., and Cox, J. M. (2016). Live and inactivated vaccine regimens against caecal Salmonella Typhimurium colonisation in laying hens. Aust. Vet. J. 94, 387–393. doi: 10.1111/avj.12490
Jackson, B. R., Griffin, P. M., Cole, D., Walsh, K. A., and Chai, S. J. (2013). Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998-2008. Emerg. Infect. Dis. 19, 1239–1244. doi: 10.3201/eid1908.121511
Kirk, M., Ford, L., Glass, K., and Hall, G. (2014). Foodborne illness, Australia, circa 2000 and circa 2010. Emerg. Infect. Dis. 20, 1857–1864. doi: 10.3201/eid2011.131315
May, F. J., Stafford, R. J., Carroll, H., Robson, J. M., Vohra, R., Nimmo, G. R., et al. (2017). The effects of culture independent diagnostic testing on the diagnosis and reporting of enteric bacterial pathogens in Queensland, 2010 to 2014. Commun Dis Intell Q Rep. 41, E223–E230.
Moffatt, C. R., Musto, J., Pingault, N., Miller, M., Stafford, R., Gregory, J., et al. (2016). Salmonella Typhimurium and outbreaks of egg-associated disease in Australia, 2001 to 2011. Foodb. Path. Dis. 13, 379–385. doi: 10.1089/fpd.2015.2110
Munnoch, S. A., Ward, K., Sheridan, S., Fitzsimmons, G. J., Shadbolt, C. T., Piispanen, J. P., et al. (2009). A multi-state outbreak of Salmonella Saintpaul in Australia associated with cantaloupe consumption. Epidemiol. Infect. 137, 367–374. doi: 10.1017/S.0950268808000861
NSW Food Authority (2007). Microbiological Quality of High Risk Bakery Products. A Survey to Determine the Microbiological Quality of Bakery Products Sold in NSW. Sydney, NSW: NSW Food Authority.
Osimani, A., Aquilanti, L., and Clementi, F. (2016). Salmonellosis associated with mass catering: a survey of European Union cases over a 15-year period. Epidemiol. Infect. 144, 3000–3012. doi: 10.1017/S0950268816001540
OzFoodNet Working Group (2003). Foodborne disease in Australia: incidence, notifications and outbreaks. Annual report of the OzFoodNet network, 2002. Commun. Dis. Intell. Q. Rep. 27, 209–243.
OzFoodNet Working Group (2007). Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the Ozfoodnet network, 2006. Commun. Dis. Intell. Q. Rep. 31, 345–365.
OzFoodNet Working Group (2012). Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2010. Commun. Dis. Intell. Q. Rep. 36, E213–E241.
OzFoodNet Working Group (2015). Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2011. Commun. Dis. Intell. Q. Rep. 39, E236–E264.
Painter, J. A., Hoekstra, R. M., Ayers, T., Tauxe, R. V., Braden, C. R., Angulo, F. J., et al. (2013). Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998-2008. Emerg. Infect. Dis. 19, 407–415. doi: 10.3201/eid1903.111866
Pires, S. M., Vieira, A. R., Perez, E., Lo Fo Wong, D., and Hald, T. (2012). Attributing human foodborne illness to food sources and water in Latin America and the Caribbean using data from outbreak investigations. Int. J. Food Microbiol. 152, 129–138. doi: 10.1016/j.ijfoodmicro.2011.04.018
Keywords: disease outbreaks, eggs, Australia, foodborne disease, Salmonella Typhimurium
Citation: Ford L, Moffatt CRM, Fearnley E, Miller M, Gregory J, Sloan-Gardner TS, Polkinghorne BG, Bell R, Franklin N, Williamson DA, Glass K and Kirk MD (2018) The Epidemiology of Salmonella enterica Outbreaks in Australia, 2001–2016. Front. Sustain. Food Syst. 2:86. doi: 10.3389/fsufs.2018.00086
Received: 18 September 2018; Accepted: 27 November 2018;
Published: 12 December 2018.
Edited by:
Joshua B. Gurtler, Agricultural Research Service, United States Department of Agriculture, United StatesReviewed by:
Aparna Tatavarthy, United States Food and Drug Administration, United StatesM. Leonor Faleiro, University of Algarve, Portugal
Cheleste Thorpe, Tufts University School of Medicine, United States
Copyright © 2018 Ford, Moffatt, Fearnley, Miller, Gregory, Sloan-Gardner, Polkinghorne, Bell, Franklin, Williamson, Glass and Kirk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martyn D. Kirk, TWFydHluLktpcmtAYW51LmVkdS5hdQ==
 Laura Ford
Laura Ford Cameron R. M. Moffatt
Cameron R. M. Moffatt Emily Fearnley1,2
Emily Fearnley1,2 Robert Bell
Robert Bell Neil Franklin
Neil Franklin Deborah A. Williamson
Deborah A. Williamson Kathryn Glass
Kathryn Glass Martyn D. Kirk
Martyn D. Kirk