- 1Department of Animal Science, University of Connecticut, Storrs, CT, United States
- 2Department of Nutritional Sciences, University of Connecticut, Storrs, CT, United States
Salmonellosis associated with consumption of mangoes have been traced back to the use of contaminated wash water. This highlights the critical role of wash water disinfection in mango processing, affecting its quality, and safety. Moreover, steps unique to the post-harvest handling of mangoes also create a conducive environment for internalization of pathogens into the fruit pulp. Currently, no effective treatment exists to eliminate internalized pathogens from mangoes. Therefore, it is critical to prevent contamination on the fruit to avert pathogen internalization. So the present study evaluated the efficacy of chlorine (200 ppm), peroxyacetic acid (80 ppm), and chlorine dioxide (5 ppm) for reducing Salmonella populations on mangoes and in wash water under simulated mango packing house conditions. Nalidixic-acid resistant isolates of Salmonella Montevideo, S. Newport, S. Baildon, S. Braenderup, and S. Poona were used in this study. Disinfectants were added to inoculated wash water (ca. 7 log CFU/ml) and mangoes (var. Atualfo and Tommy Atkins) were washed under simulated dump tank wash (24°C for 2 min), hot water treatment (46°C for 75 and 110 min), and hydrocooling conditions (21°C for 30 min). Wash water and mangoes were collected at different times for microbiological analysis. Additionally, residual disinfectant concentration was monitored throughout the study. All the three disinfectant tested were effective in significantly reducing Salmonella populations in wash water and on mangoes during dump tank wash, hot water treatment, and hydrocooling (p ≤ 0.05). Specifically, no Salmonella was detected from samples treated with 200 ppm chlorine and 80 ppm PAA. On the other hand, Salmonella was consistently recovered from mango and water samples treated with chlorine dioxide (5 ppm). This reduced antimicrobial efficacy can be attributed to the sharp decline in residual chlorine dioxide concentrations in wash water. Further, reductions in residual chlorine and PAA concentrations were also observed over time. Therefore, to ensure the sustained antimicrobial activity of chlorine and PAA, it is critical to regularly monitor and replenish the disinfectant in wash water. However, given the laboratory scale of these experiments, further validation of these results on a commercial scale are warranted.
Introduction
Over the last decade, the increase in consumption of fruits and vegetables has been associated with a concomitant increase in fresh-produce associated foodborne outbreaks (Lynch et al., 2009; Luo et al., 2012). Toward this end, the Centers for Disease Control and Prevention (CDC, 2014) has identified that ~46% of all foodborne illnesses in the US from the years 1998–2008 originated from contaminated fresh produce including fruits, nuts and vegetables (CDC, 2014). Among the different foodborne etiologies, Salmonella is responsible for ~18% of all foodborne outbreaks associated with fresh produce, including mangoes (Hanning et al., 2009; Callejón et al., 2015). In the US, mangoes have been associated with seven multistate Salmonellosis outbreaks with 268 illnesses and 40 hospitalizations so far (CDC, 2017). Interestingly, all these outbreaks were traced back to the use of contaminated wash water during the post-harvesting processing of mangoes (Sivapalasingam et al., 2003; Beatty et al., 2004; Hanning et al., 2009).
At the commercial packing house, mangoes are subjected to three separate washing procedures. The first wash is in the dump tank where incoming mangoes are washed for 30 s to not more than 2 min using potable water. Following the dump tank wash, mangoes are sized and immersed in hot water that is held at 46.1°C. The duration of the treatment which can vary from 65 to 110 min is determined based on the size and shape of the fruit. After hot water treatment, fruit is moved into the hydrocooling process where they are washed in water held at 21–22°C for at least 30 min to reduce flesh temperature and hot water injury (NMB, 2014). Throughout these washing steps, it is essential to maintain water quality. For instance, water quality of the dump tank can quickly deteriorate due to the accumulation of pathogens, dirt, fruit latex, and agricultural chemicals from the field. Hence, it is critical to include an effective antimicrobial in the dump tank to prevent pathogen carryover into the hot water and hydrocooling treatments. Additionally, wash water in the hot water and hydrocooling tank can serve as a medium for the potential contamination of mangoes. Therefore, in the absence of practical technologies that provide a necessary kill step for pathogens, mango producers must rely on wash water disinfectants to enhance product safety.
The commonly used wash water disinfectants in the mango industry include chlorine, peroxyacetic acid, and chlorine dioxide (NMB, 2014). As with other fresh produce, chlorine (50–200 ppm) is the most frequently used chemical disinfectant in the mango industry (Beuchat, 1999; Li et al., 2001; Shen et al., 2013; NMB, 2014). Although an effective antimicrobial agent, the efficacy of chlorine is quickly compromised in the presence of organic matter, as is commonly the case with routine produce washing (Keskinen et al., 2009; Shen et al., 2013; NMB, 2014). In fact, inadequately chlorinated wash water is thought to be a contributing factor to the Salmonella outbreaks associated with the consumption of whole mangoes (Beatty et al., 2004). Another chlorine-based disinfectant used in mango wash water is chlorine dioxide. Similar to chlorine, chlorine dioxide also oxidizes organic matter eventually leading to a reduction in its effective concentration and antimicrobial activity (Goodburn and Wallace, 2013). When employed as a wash water disinfectant, chlorine dioxide has been applied at 5 ppm in water to keep the residue concentration on washed produce within the approved 3-ppm limit [US Food Drug Administration (USFDA) and Center for Food Safety Applied Nutrition, 1998; Pao et al., 2009; 21 CFR 173.300]. Peroxyacetic acid (PAA) has been shown to be effective against foodborne pathogens on different produce surfaces (Hilgren et al., 2007; Vandekinderen et al., 2009). Further, it is approved for use in produce wash water at a maximum permissible limit of 80 ppm (21CFR173.315). Commercially available peracetic acid-based disinfectants contain a considerable amount of hydrogen peroxide that also exhibits antimicrobial activity. PAA is not susceptible to peroxidases and is known to retain its antimicrobial activity in the presence of organic loads or food residues when compared to chlorine (Fatemi and Frank, 1999; Hilgren et al., 2007; Small et al., 2007). Although antimicrobial, the oxidizing ability of these disinfectants, particularly chlorine dioxide and PAA are also associated with discoloration and negative impact on fruit color (Wang et al., 2007; Du et al., 2009; Joshi et al., 2013; Van de Velde et al., 2016; Chen, 2017).
Given the multiple foodborne outbreaks associated with the consumption of mangoes and the critical role that wash water can play in fruit contamination, this study evaluated the antimicrobial efficacy of commonly used mango wash water disinfectants (200 ppm chlorine, 80 ppm peroxyacetic acid, and 5 ppm chlorine dioxide) to mitigate Salmonella populations from wash water and mangoes under simulated packing house washing operations. Additionally, their effect on mango fruit color was investigated. Furthermore, since Salmonella can gain entry in to produce wash water either via the water itself or through contaminated fruit (Allende et al., 2009; Hanning et al., 2009, Fatica and Schneider, 2011), this study investigated disinfectant efficacy under both contamination scenarios. Thus, the main objectives of the study were (i) to determine the efficacy of water disinfectants to inactivate Salmonella in wash water and prevent water-to-mango cross contamination (scenario 1-contaminated wash water) and (ii) to determine the efficacy of water disinfectants to inactivate Salmonella on the surface of artificially inoculated mangoes and prevent mango-to-water cross-contamination (scenario 2- contaminated mangoes).
Materials and Methods
Bacterial Cultures
Five different serovars of Salmonella enterica (S. Montevideo, S. Newport, S. Baildon, S. Braenderup, and S. Poona) were used for the study. These produce isolates were kindly provided by Dr. Venkitanarayanan (Department of Animal Science, University of Connecticut, Storrs, CT). All the five isolates were induced for resistance to nalidixic acid (NA; Sigma-Aldrich, St. Louis, MO, USA; 50 μg/ml) to facilitate selective enumeration of the inoculated pathogens (Harris et al., 2001).
Preparation of Inoculum
Each isolate was cultured separately in 10 ml of sterile Tryptic soy broth (TSB, BD Difco, Becton, Dickson, and Company, Sparks, MD, USA) containing nalidixic acid (NA; 50 μg/ml) at 37°C for 24 h with agitation (100 rpm). Cultures were then transferred for two consecutive 24 h period onto xylose lysine deoxycholate (XLD; Difco) agar plates containing NA (50 μg/ml; XLDN) to produce a bacterial lawn. To prepare the inoculum, growth from the bacterial lawn was transferred to 0.1% buffered peptone water (BPW, Difco) and the approximate bacterial count in each culture was determined spectrophotometrically. Equal portions from each of the five isolates were combined to make the pathogen cocktail. The bacterial population in the cocktail was determined by plating 0.1 ml portions of appropriate dilutions on XLDN followed by incubation at 37°C for 24 h (Danyluk et al., 2005).
Mangoes
Mango varieties (Ataulfo and Tommy Atkins) used in the study were selected based on their popularity, difference in size, surface characteristics, and duration of hot water treatment. Unwashed, unwaxed, unripened mature mangoes (Ataulfo: yellow, average weight: 200–250 g; Tommy Atkins: Red–Green, average weight: 600–800 g) were procured from mango packing houses in coordination with the National Mango Board. Upon receipt, fruits were visually inspected for defects (bruises, moldy growth, breaks in peel) and any defective mangoes were discarded. All the fruits were maintained at 4°C until use. A day before the experiment, the required number of fruits were transferred to room temperature (21°C) for tempering prior to use (Penteado et al., 2004).
Wash Solutions
Chlorine wash solution (200 ppm) was prepared by diluting 6% sodium hypochlorite solution (Fisher Scientific, Waltham, MA, USA) in autoclaved tap water and adjusting the pH to 7.0 ± 0.1 with citric acid (NMB, 2014). Peroxyacetic acid wash solution (80 ppm) was prepared by diluting 35% stock solution (Fisher Scientific). Chlorine dioxide wash solution (5 ppm) was prepared by dissolving chlorine dioxide water tablets (Wisconsin Pharmacal Company LLC, Jackson, WI, USA) in autoclaved tap water as per the manufacturer's instructions. Autoclaved tap water was used as the negative control (Harris et al., 2001). Disinfectant solutions were prepared fresh prior to each experiment and residual disinfectant concentrations were determined at the start of each washing procedure. The disinfectant concentrations used in the study were selected based on preliminary studies that were conducted using varying concentrations of chlorine (50, 100, 150, and 200 ppm), PAA (30, 50, and 80 ppm), and chlorine dioxide (2.5, 3, and 5 ppm) and maximum allowable limits set by the FDA.
Preparation of Organic Load
The chemical oxygen demand (COD) was chosen as an estimate of organic load in the wash water and was measured using the dichromate reactor digestion method (Gonzalez et al., 2004; Davidson et al., 2014; Gombes et al., 2017; Teng et al., 2018). In order to simulate commercial process water, wash water samples were collected from a commercial packing house and COD was estimated. Based on these assays, organic load in the dump tank, hot water treatment and hydrocooling wash water was estimated to be 315–320, 25–30, and 15–20 ppm, respectively. Using these values as a guideline, wash water for the experiments was prepared by washing whole mangoes in tap water (to simulate latex contamination) and mixing sterile clay-loam soil in order to obtain the desired organic load.
Preparation of Contaminated Wash Water (Scenario 1)
In order to mimic wash water contamination that could occur at the mango packing facility, wash water (5 L) was inoculated with the Salmonella cocktail to obtain a starting bacterial load of ~7 log CFU/ml. Additionally, to simulate washing by immersion at the packing house, wash water (5 L) was held in sterile containers and mangoes were immersed in the different wash solutions for specified periods of time as described below.
Dump Tank Wash
Mangoes were washed in inoculated wash solutions containing 200 ppm chlorine, 80 ppm PAA, or 5 ppm chlorine dioxide in the presence and absence of organic matter (315–320 ppm) and held at 24°C for 2 min. Autoclaved tap water inoculated with the Salmonella cocktail served as the wash control. During the dump tank wash, water, and mangoes were sampled at 30 s, 1 and 2 min of washing for microbiological analysis. At each time point, three 50-ml wash water samples and three mangoes were sampled from the different treatment groups and the entire experiment was repeated four times.
Hot Water Treatment
To simulate hot water treatment, mangoes were washed in inoculated wash solutions that were maintained at 46°C in the presence and absence of organic matter (25–30 ppm). These wash solutions contained 200 ppm chlorine, 80 ppm PAA, or 5 ppm chlorine dioxide. Autoclaved water inoculated with the Salmonella cocktail served as the wash control. As per the USDA APHIS requirement, hot water treatment was performed for a duration of 75 and 110 min for Ataulfos and Tommys, respectively [U.S. Department of Agriculture (USDA), Animal Plant Health Inspection Service and Plant Protection Quarantine, 2010]. Following immersion in the hot wash solutions, wash water and mangoes were sampled at 65 and 75 min for Ataulfo and 75, 90, and 110 min for Tommy Atkins mangoes (NMB, 2014). Three 50-ml wash water samples and three mangoes from the different treatment groups were sampled at each time point and the entire experiment was repeated four times. Mangoes and wash water solutions were processed as for microbiological analysis as described below.
Hydrocooling
For hydrocooling, inoculated wash solutions containing the different disinfectants or sterile water were maintained at 21°C and mangoes were washed for 30 min in the presence and absence of organic matter (15–20 ppm). Mangoes and wash water were sampled during hydrocooling for microbiological analysis. At each time point, three 50-ml wash water samples and three mangoes were sampled from the different treatment groups and the entire experiment was repeated four times.
Preparation of Artificially Contaminated Mangoes (Scenario 2)
Salmonella cocktail was prepared as described earlier. Mangoes were spot inoculated with 7 log CFU/mango by placing 100 μl of the inoculum around the stem end. In order to prevent the inoculum from running off the side of the mango, the inoculum was applied in small approximately equal volumes to 10 different locations (Lang et al., 2004; Baskaran et al., 2013). After inoculation, mangoes were held at room temperature in a biosafety hood for 4 h to allow for the inoculum to dry. Staggered inoculation of the mangoes was performed to allow for not more than 4 h of drying time prior to washing (Parnell and Harris, 2003). Inoculated mangoes were then subjected to dump tank wash, hot water treatment, and hydrocooling as previously described. Following exposure to the different wash solutions, the surviving Salmonella population was enumerated in wash water and on mango surface as described below.
S. enterica Internalization Into Mangoes
To evaluate the efficacy of disinfectants to prevent Salmonella internalization in mangoes, fruits were subjected to hot water treatment in sterile hot water and then hydrocooled with inoculated wash water containing the disinfectants (200 ppm chlorine/80 ppm PAA/5 ppm chlorine dioxide) in the presence and absence of organic matter (15–20 ppm). After 30 min of hydrocooling, three mangoes were sampled from each treatment group and the entire experiment was repeated four times. In order to enumerate internalized Salmonella, all hydrocooled mangoes were sanitized by immersion in 1 L of 2 g/l of sodium hypochlorite solution (Fisher Scientific) for 1 min and air dried in a laminar flow hood. Each mango was then surface sanitized using 70% ethanol. Following surface sanitization, mangoes were cut and processed as previously described (Penteado et al., 2004). Briefly, pulp from each mango was transferred to individual stomacher bags with 200 ml BPW, mashed by stomaching for 2 min and Salmonella population/presence was assayed as described below.
Microbiological Analysis
At each sampling time, mangoes were individually transferred to sterile stomacher bags containing 200 ml of Dey-Engley neutralizing broth (DE broth, Difco). Each mango was hand rubbed for 1 min, and the DE broth was analyzed for presence (by enrichment) and/or Salmonella population (Harris et al., 2001; Baskaran et al., 2013). Similarly, at each sampling time, wash water samples were transferred to individual stomacher bags containing 200 ml DE broth for further microbiological analysis. Briefly, DE broth from each sample was centrifuged (1,880 X g for 5 min) and the upper portion was removed and sample concentration was performed by further centrifugation (16,000 X g for 10 min; Fukushima et al., 2007). The resulting pellet was resuspended in 25 ml of DE broth followed by serial dilution in BPW, plating on XLDN and incubation at 37°C for 24–48 h. Additionally, serially diluted samples were surface plated on bismuth sulfite agar (BSA, Difco) containing 50 ug/ml NA and 0.1% sodium pyruvate (BSANP; Harris et al., 2001; Strawn and Danyluk, 2010). In addition to plating, DE broth (25 ml) from the different samples was enriched in Rappaport-Vassiliadis broth (RVB, Difco; 225 ml) and incubated at 43°C for 16–24 (Parnell et al., 2005). When counts for the respective samples were negative by direct plating, enrichment broths were streaked on XLDN/BSANP agar and incubated at 37°C for 48 h before being examined for presumptive S. enterica colonies. The presumptive colonies isolated from the enrichment broths were confirmed by agglutination assays (Salmonella latex agglutination test, Microgen Bioproducts Ltd., Surrey, UK).
Wash Water Quality Measurements
Disinfectant concentrations were monitored throughout the experimental period. Chlorine concentration was monitored using Chlor—Test strips (MQuant™; Millipore Sigma, Burlington, MA, USA) and visual kit (CHEMetrics Inc, Midland, VA, USA). Chlorine dioxide concentration was monitored using a colorimetric assay (CHEMetrics Inc.). Peracetic acid concentration was monitored using test strips (Hydrion®; Microessential Laboratory, Brooklyn, NY, USA) and visual kit (ChemWorld, Kennesaw, GA, USA). In addition to monitoring residual disinfectant concentrations for the duration of the washing experiment, chlorine, chlorine dioxide, and PAA were also monitored over an extended period of 72 h to simulate reuse of wash water. Additionally, wash water temperatures were monitored throughout the course of the experiments.
Measurement of Mango Color
Instrumental color measurements were done to determine the effect of the disinfectant treatments on mango color. Using a HunterLab MiniScan XE Plus colorimeter (HunterLab Associates, Reston, VA, USA) with illuminant A, 2.54-cm diameter aperture, and 10° standard observer, a* and b* values were measured on mangoes before and after dump tank wash, hot water treatment and hydrocooling (Ayala-Silva et al., 2005; Adams and Brown, 2007). Washing in autoclaved tap water served as the control. a* values are representative of the redness (Tommy Atkins) of the surface and b* indicate the yellowness (Ataulfo) of the mango skin. At each time point, three mangoes were sampled from the different treatment groups and the entire experiment was repeated four times.
Statistical Analysis
Three samples (mangoes and wash water samples) for each treatment and control were collected per time point in each experiment and the experiment was repeated four times. Each wash water container served as an experimental unit and a completely randomized treatment structure was followed. The factors included 8 treatments (autoclaved water, chlorine, PAA, chlorine dioxide in the presence and absence of organic load) and time points [3 for dump tank washing (30 s, 1 and 2 min); 3 for hot water treatment (75, 90, and 110 min); and 1 for hydrocooling (30 min)] per trial. A similar analysis was performed for the wash water samples. For mango color measurement, a completely randomized treatment structure was followed with repeated measures. Three samples for each treatment and control were collected per time point in each experiment and four independent trials were conducted. Pooled samples were averaged and data were analyzed using the GLM procedure of SAS ver. 9.4. Differences among the means were considered significant at p ≤ 0.05, and detected using Fisher's least significance difference test with appropriate corrections for multiple comparisons.
Results
Results of our study revealed that 200 ppm chlorine, 80 ppm PAA, and 5 ppm Chlorine dioxide were found to be equally effective in reducing Salmonella populations on Ataulfo and Tommy Atkins in the presence and absence of organic contaminants. Further, no significant difference in disinfectant efficacy was observed under the different contamination scenarios tested namely (i) contaminated wash water and (ii) contaminated mangoes. Therefore, only data on the antimicrobial efficacy of disinfectants in reducing Salmonella populations in wash water and preventing cross-contamination of fruits (var. Tommy Atkins; Scenario 1) are presented here.
Dump Tank Wash
The efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in reducing Salmonella populations in dump tank wash water and preventing water-to-mango cross contamination is shown in Table 1. Approximately 7.2 log CFU of Salmonella was recovered from the untreated wash water and mango samples (control; Table 1). However, incorporation of disinfectants including 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide significantly inhibited Salmonella survival in wash water and on mangoes (p < 0.05). Specifically, Salmonella was not detected from water samples treated with 200 ppm chlorine or 80 ppm PAA immediately upon testing (initial sampling time point; Table 1A). Further, Salmonella was also not detected on mangoes sampled at all-time points following washing (Enrichment negative; Table 1B). Additionally, the presence of organic matter did not have any significant effect on the antimicrobial efficacy of chlorine and PAA. However, in case of chlorine dioxide (5 ppm), water and mangoes were positive for Salmonella at the initial sampling time particularly in presence of organic contaminants (Table 1).
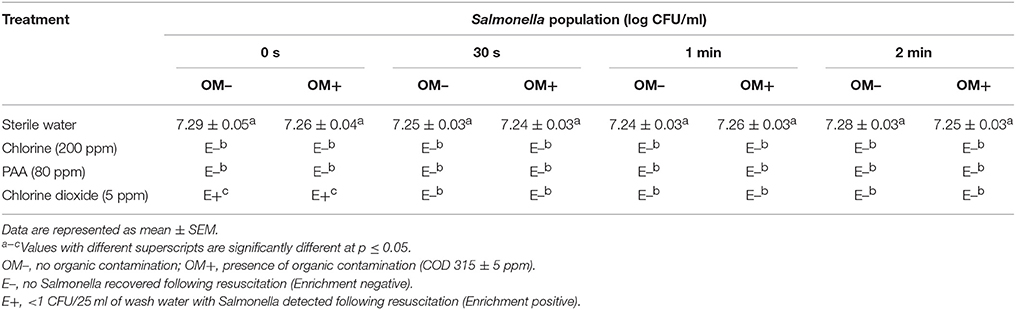
Table 1A. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in reducing Salmonella populations in dump tank wash water (Tommy Atkins mangoes).
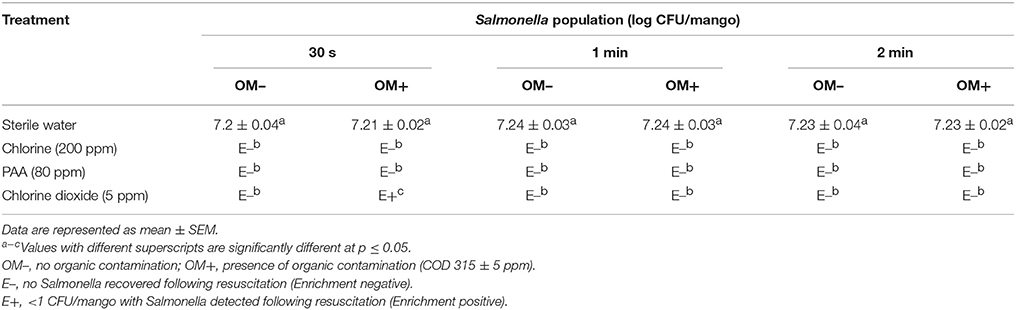
Table 1B. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in preventing transfer of Salmonella from dump tank wash water to mangoes (Tommy Atkins mangoes).
Hot Water Treatment
Exposure of the pathogen to wash water held at 46°C in the absence of a disinfectant did not have any significant effect on surviving Salmonella populations. Approximately 7.2 log CFU of Salmonella was recovered from the control wash water and mangoes throughout the experiment (Table 2). On the other hand, incorporation of 200 ppm chlorine and 80 ppm PAA resulted in a >7 log reduction in Salmonella populations in wash water and effectively inhibited Salmonella transfer onto mango surface irrespective of the presence and absence of organic matter. However, with chlorine dioxide, Salmonella was recovered (enrichment positive) from water samples and mangoes until the end of the experiment (110 min; Table 2).
Hydrocooling
Hydrocooling of mangoes in the absence of disinfectant did not result in any significant reduction in pathogen population. At the end of hydrocooling, ~7.1 log CFU of Salmonella was recovered from the wash water and mangoes (Table 3) On the other hand, when chlorine (200 ppm) or PAA (80 ppm) was added to the hydrocooling water, Salmonella was not detected in the wash water and on mangoes following direct plating and enrichment. However, in case of chlorine dioxide, samples (water and mangoes) were found to be positive for Salmonella till the end of the experiment (30 min) both in the presence and absence of organic matter (Table 3).
Salmonella Internalization in Mangoes
As can be seen from Table 4, transfer of fruits from hot water tanks into the hydrocooling containers led to significant internalization of Salmonella into the mango pulp. With respect to the control samples, ~2.3 log CFU of Salmonella/mango was recovered from the pulp. With reference to the disinfectant treatments, incorporation of 200 ppm chlorine and 80 ppm PAA significantly (p ≤ 0.05) reduced Salmonella internalization in mangoes irrespective of the presence or absence of organic matter (Table 4). However, when employing chlorine dioxide, the presence of organic matter in the wash water significantly (p ≤ 0.05) compromised its antimicrobial efficacy. In fact, application of 5 ppm chlorine dioxide to wash water with COD of 15 ± 5 ppm did not result in any significant reduction in Salmonella internalization when compared to the control. Approximately 2.3 log CFU of Salmonella was recovered from the pulp of chlorine dioxide washed mangoes at the end of the experiment (Table 4).
Change in Residual Disinfectant Concentrations Over Time in Each Washing Step
A steady reduction in the effective concentration of the three disinfectants was observed over time (Figures S1–S3). For instance, with the dump tank wash, chlorine and PAA concentrations remained unchanged at the end of the washing period (2 min) irrespective of the presence of organic load. However, chlorine dioxide concentration declined from 5 to 2.5 ppm and 2 ppm in the absence and presence of organic load, respectively (Figure S1C). Moreover, this loss was more apparent in the presence of an organic load and in the hot water tank. During hot water treatment, chlorine dioxide concentrations dropped from 5 ppm (at the start of the experiment) to 1 and 0.8 ppm (by 110 min) in the absence and presence of organic matter, respectively. In the presence of organic matter, the final concentration was 0.8 ppm. Similarly, by 110 min, PAA concentrations and chlorine concentrations reduced to 60 and 80 ppm in the presence of organic contamination, respectively. With respect to hydrocooling, by 30 min chlorine dioxide concentration reduced to 2.5 and 2 ppm in the absence and presence of organic matter, respectively. However, no change in the residual concentration of chlorine and PAA were observed (Figures S3A,B). Further, with the extended sampling over 72 h, we observed a similar decrease in the effective concentration of all the three disinfectants (72 h; Figures S1–S3). This loss was more apparent in the presence of an organic load and in the hot water tank. In the absence of organic load, chlorine concentration declined to 60 ppm by 72 h. In the presence of an organic load, the concentration dropped to undetectable levels by 24 h. PAA concentrations fell to 60 and 45 ppm by 72 h in the absence and presence of organic matter, respectively. Overall, chlorine dioxide was found to be the least stable with residual concentrations falling below detection limits by 8 and 24 h in the presence and absence of organic matter, respectively (Figures S1–S3).
Effect of Disinfectant Treatments on Mango Color
Washing mangoes (Ataulfo—yellow skin color; Tommy Atkins—red-green skin color) either in plain water or in water containing disinfectants did not significantly affect fruit color (Figures 1, 2). In other words, mango fruit color estimated before wash was not significantly different from measurements made on washed fruit. Similar results were obtained under all of the three washing conditions; namely, dump tank wash, hot water treatment, and hydrocooling. Overall, Ataulfos had a b* value (yellow) of 32–34 (Figure 1) and Tommy Atkins had an a* (green-red) value of 22–24 (Figure 2). Further, we also did not observe any visual discoloration of the fruit following washing.
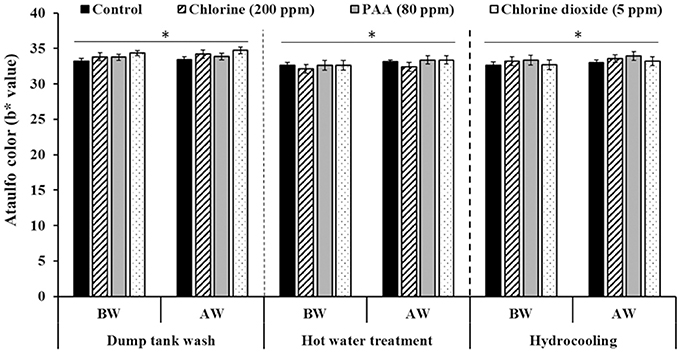
Figure 1. Effect of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide on mango (var. Ataulfo) color following dump tank wash, hot water treatment and hydrocooling. BW, Fruit color before wash; AW, Fruit color after wash. *No significant difference in fruit color before and after wash within each washing process.
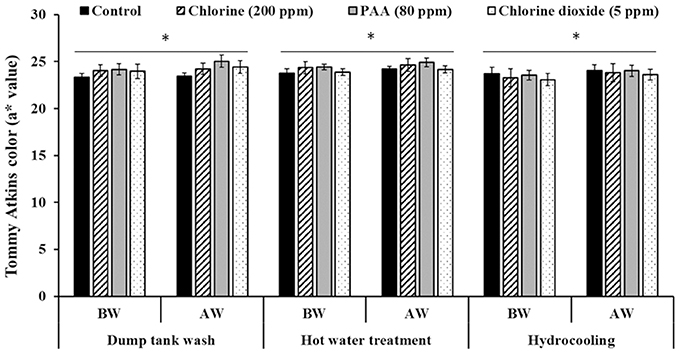
Figure 2. Effect of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide on mango (var. Tommy Atkins) color following dump tank wash, hot water treatment and hydrocooling. BW, Fruit color before wash; AW, Fruit color after wash. *No significant difference in fruit color before and after wash within each washing process.
Discussion
Fresh produce can become contaminated at any point throughout the production process (Fatica and Schneider, 2011). Therefore, in addition to incorporation of good agricultural practices (GAPs), the produce industry relies on decontamination strategies employed at the packing house (Goodburn and Wallace, 2013). In this regard, washing is one of the most commonly employed methods to reduce pathogen contamination on fresh produce. Water used for washing not only helps remove microorganisms but also eliminates soil and dirt from the produce surface (Tian et al., 2013). However, water also serves as an ideal medium for the potential spread of pathogens during produce processing (Luo et al., 2012). Hence, wash water, if not properly disinfected, can act as a source of contamination for every fruit or vegetable that passes through the packing house (López-Gálvez et al., 2009). Therefore, improperly chlorinated water has been implicated in foodborne outbreaks associated with fresh produce including tomatoes and mangoes (Zhuang et al., 1995; Beatty et al., 2004).
Given the Salmonella outbreaks and its association with wash water, water quality has been a major concern for the mango industry (Hanning et al., 2009; Nieto-Montenegro and Almanza, 2014). In addition to water quality, steps unique to the post-harvest handling of mangoes including hot water treatment followed by hydrocooling, create a conducive environment for internalization of pathogens into the fruit pulp (Parnell and Harris, 2003). In fact, washing of warm mangoes (46°C) in water held at a lower temperature (22°C) led to Salmonella internalization into mangoes resulting in foodborne outbreaks (Sivapalasingam et al., 2003; Penteado et al., 2004). Currently, no effective treatment exists to eliminate internalized pathogens from mangoes. Therefore, it is critical to prevent contamination on the fruit surface in order to avert pathogen internalization (Nieto-Montenegro and Almanza, 2014). Toward this end, research has demonstrated that incorporation of effective disinfectants in wash water could control pathogens in water and thereby prevent internalization (Penteado et al., 2004; Soto et al., 2007). Hence, the present study was undertaken to test the efficacy of commonly used disinfectants in promoting the microbial safety of mangoes under simulated packing house washing operations.
Under all of the three washing conditions tested, chlorine (200 ppm) and PAA (80 ppm) were effective in reducing Salmonella (no Salmonella detected upon enrichment) in wash water thereby preventing cross-contamination of mangoes. However, chlorine dioxide (5 ppm) was found to be the least effective, with Salmonella being recovered from wash water and mangoes particularly during hot water treatment and hydrocooling (Tables 2, 3). Further, the observed antimicrobial efficacy was found to be dependent on the organic load, disinfectant type and residual disinfectant concentration. For example, while Salmonella was not detected in wash water following treatment with 200 ppm chlorine and 80 ppm PAA during all three washing steps, samples were found to be positive for Salmonella following treatment with 5 ppm chlorine dioxide (Tables 1A, 2A, 3A). Further, the reduced antimicrobial efficacy of chlorine dioxide was found to be associated with a corresponding reduction in the residual concentration of the disinfectant in the wash water (Figures S1C, S2C, S3C). Moreover, the reduced antimicrobial efficacy was more evident in the presence of organic matter (Tables 2B, 3B). These findings are in line with previous research that demonstrates that a reduction in residual free chlorine particularly in the presence of organic contamination is primarily responsible for the observed loss in the antimicrobial efficacy of the disinfectant (Pao et al., 2007; Joshi et al., 2013; Chen and Hung, 2017; Sreedharan et al., 2017).
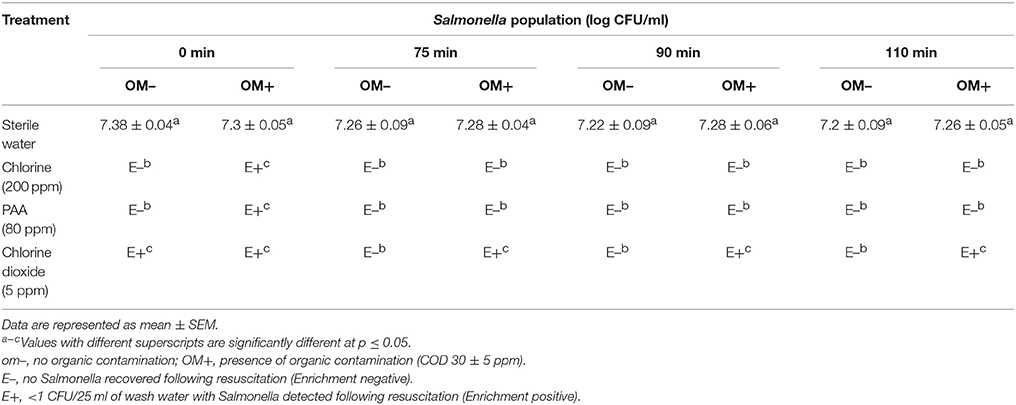
Table 2A. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in reducing Salmonella populations in hot water tank (Tommy Atkins mangoes).
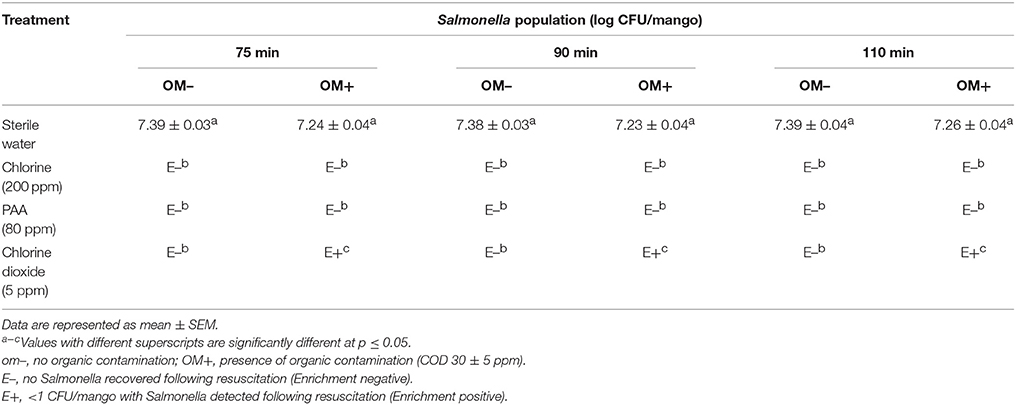
Table 2B. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in preventing transfer of Salmonella from hot wash water to mangoes (Tommy Atkins mangoes).

Table 3A. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in reducing Salmonella populations in hydrocooling tank (Tommy Atkins mangoes).
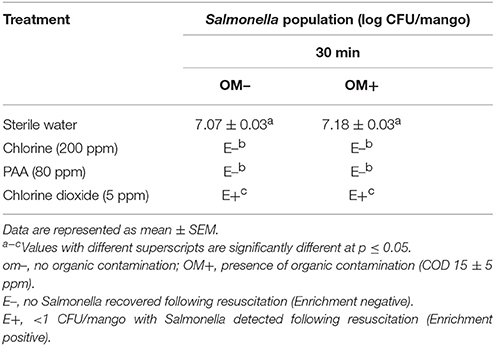
Table 3B. Efficacy of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in preventing transfer of Salmonella from hydrocooling water to mangoes (Tommy Atkins mangoes).
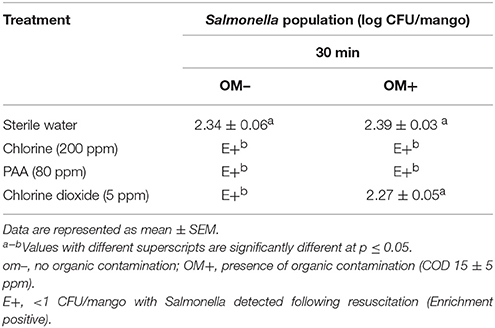
Table 4. Effect of 200 ppm chlorine, 80 ppm PAA, and 5 ppm chlorine dioxide in preventing Salmonella internalization in mangoes following hydrocooling.
Since mangoes imported to the United States can serve as a vector for tephritid flies, USDA APHIS requires that these mangoes be subjected to hot water disinfestation [U.S. Department of Agriculture (USDA), Animal Plant Health Inspection Service and Plant Protection Quarantine, 2010]. However, owing to the detrimental effect of high temperature on mango pulp, fruits are hydrocooled following hot water treatment (NMB, 2014). This rapid transition of warm fruit to a cooler environment has been shown to favor pathogen internalization in produce including mangoes, tomatoes, apples and oranges (Bartz and Showalter, 1980; Buchanan et al., 1999; Eblen et al., 2004; Penteado et al., 2004; Bordini et al., 2007). As previously reported, ~2.3 log CFU/g of internalized Salmonella was recovered from the pulp of hot water treated mangoes that were hydrocooled in contaminated wash water in the absence of a disinfectant (Bordini et al., 2007; Soto et al., 2007). Treatment with 5 ppm chlorine dioxide particularly in the presence of organic matter did not result in any significant reduction in internalized Salmonella populations when compared to the control. On the other hand, incorporation of 200 ppm chlorine and 80 ppm PAA in the hydrocooling wash water significantly inhibited Salmonella internalization. Therefore, by controlling Salmonella in wash water and preventing pathogen contamination on mangoes, chlorine, and PAA were able to inhibit Salmonella internalization into the pulp of intact fruit. Similar reduction in Salmonella populations in wash water and mango pulp was reported by Soto et al. (2007) following the incorporation of chlorine (8 mg/L) in the hydrocooling water.
Wash water quality parameters including organic load and water temperature have a significant influence on disinfectant efficacy (Goodburn and Wallace, 2013; Banach et al., 2015). These parameters primarily reduce the residual concentration of the disinfectant and therefore reduce its antimicrobial activity over time. In effect, studies have demonstrated that there is an inverse relationship between antimicrobial efficacy and organic load (Chaidez et al., 2003; López-Gálvez et al., 2009; Meireles et al., 2016). Similar to these findings, we observed a significant reduction in disinfectant over time, particularly in the hot water tank and in the presence of organic contamination (Figure S2). Overall, disinfectant stability was found to be highest with PAA > chlorine > chlorine dioxide, with steeper declines observed in the presence of organic contamination (Figures S1–S3). Therefore, to ensure sustained antimicrobial activity, it is critical to regularly monitor and replenish disinfectants in the dump tank, hot water and hydrocooling tanks.
The attractiveness and appearance of the fruit particularly its color is an important parameter that dictates consumer acceptability. Hence maintenance of an appealing color is critical to promote fruit sales. In this regard, PAA and chlorine-based disinfectants including chlorine dioxide are strong oxidizing agents and therefore can react with phytochemicals on fruits and vegetables, thereby affecting their appearance (Joshi et al., 2013). In fact, researchers have demonstrated that prolonged washing with chlorine dioxide causes darkening and loss of visual quality (Du et al., 2009; Chen, 2017). Exposure of apples to gaseous chlorine dioxide (4.32 ppm) for an hour was found to significantly reduce the visual quality of the fruits (Lee et al., 2006). Along the same lines, Mahmoud and Linton (2008) observed a reduction in visual quality of lettuce following treatment with chlorine dioxide. Similarly, hydrogen peroxide, a component of PAA, has been shown to oxidize anthocyanins and other phytochemicals leading to detrimental quality changes such as browning in apples and strawberries (Wang et al., 2007; Van de Velde et al., 2016). However, in the present study, washing fruit in water containing 200 ppm chlorine, 80 ppm PAA, or 5 ppm chlorine dioxide was not found to exert a significant impact on fruit color. Further, no significant difference in color of Ataulfos and Tommy Atkins was observed before and after washing following dump tank wash, hot water treatment and hydrocooling (p >0.05; Figures 1, 2). The absence of a significant effect on mango color could be primarily due to the difference in phytochemical composition and stability of pigments such as anthocyanins among different fruits and varieties (Laleh et al., 2006; Adams and Brown, 2007). Further, the observed rapid loss in effective concentration of chlorine dioxide could have resulted in reduced contact time with the fruit surface, thereby preventing discoloration.
In summary, 200 ppm chlorine and 80 ppm PAA were found to be the most effective in reducing Salmonella populations in wash water, preventing cross-contamination and inhibiting pathogen internalization in mangoes under simulated packing house washing operations. However, reduction in their effective residual concentrations can severely impact their antimicrobial efficacy. Hence in order to obtain sustained antibacterial activity, it is critical to continuously monitor and replenish disinfectants in the wash water tanks. Further, to prevent Salmonella carryover from one washing operation to the other, it is recommended that disinfectants are incorporated in all three washing steps; namely, dump tank wash, the hot water treatment, and the hydrocooling. However, given the laboratory scale of these experiments, further validation of the antimicrobial efficacy of these disinfectants on a commercial scale is warranted.
Author Contributions
MA conceived and designed the experiments. EM, MM, and CB performed the experiments. EM and MM performed the statistical analysis. EM and MA wrote sections of the manuscript. MA revised the manuscript. All authors read and approved the submitted version.
Funding
This work was funded by the Center for Produce Safety (CPS) and the National Mango Board (NMB) under award #2015CPS02. Any opinions, findings, conclusions, and recommendations expressed in this manuscript are those of the author(s) and do not necessarily reflect the views of CPS or NMB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the National Mango Board for supplying mangoes for use in this study. Results of this study were presented at the 2017 International Association for Food Protection Annual Meeting held at Tampa, Florida (Mathew et al., 2017; https://doi.org/10.4315/0362-028X-80.sp1.1).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2018.00018/full#supplementary-material
References
Adams, J. B., and Brown, H. M. (2007). Discoloration in raw and processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 47, 319–333. doi: 10.1080/10408390600762647
Allende, A., McEvoy, J., Tao, Y., and Luo, Y. (2009). Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite and citric acid on E. coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 20, 230–234. doi: 10.1016/j.foodcont.2008.05.009
Ayala-Silva, T., Schnell, R. J., Meerow, A., Winterstein, M., Cervantes, C., and Steven, B. J. (2005). Determination of color and fruit traits of half-sib families of mango (Magnifera indica L.). Proc. Fla. State Hort. Soc. 118, 253–257.
Banach, J. L., Sampers, I., Van Haute, S., and Van der Fels-Klerx, H. J. (2015). Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health. 12, 8658–8677. doi: 10.3390/ijerph120808658
Bartz, J., and Showalter, R. (1980). Infiltration of tomatoes by aqueous suspensions. J. Series. 71, 515–518.
Baskaran, S. A., Upadhyay, A., Kollanoor-Johny, A., Upadhyaya, I., Mooyottu, S., Roshni Amalaradjou, M. A., et al. (2013). Efficacy of plant-derived antimicrobials as antimicrobial wash treatments for reducing enterohemorrhagic Escherichia coli O157:H7 on apples. J. Food Sci. 78, M1399–M1404. doi: 10.1111/1750-3841.12174
Beatty, M. E., LaPorte, T. N., Phan, Q., Van Duyne, S. V., and Braden, C. (2004). A multistate outbreak of Salmonella enterica serotype Saintpaul infections linked to mango consumption: a recurrent theme. Clin. Infect. Dis. 38, 1337–1338. doi: 10.1086/383156
Beuchat, L. R. (1999). Survival of enterohemorrhagic Escherichia coli O157:H7 in bovine feces applied to lettuce and the effectiveness of chlorinated water as a disinfectant. J. Food Prot. 62, 845–849. doi: 10.4315/0362-028X-62.8.845
Bordini, M. E. B., Ristori, C. A., Jakabi, M., and Gelli, D. S. (2007). Incidence, internalization and behavior of Salmonella in mangoes, var. tommy atkins. Food Control 18, 1002–1007. doi: 10.1016/j.foodcont.2006.06.003
Buchanan, R. L., Edelson, S. G., Miller, R. L., and Sapers, G. M. (1999). Contamination of intact apples after immersion in an aqueous environment containing Escherichia coli O157: H7. J. Food Prot. 62, 444–450.
Callejón, R. M., Rodríguez-Naranjo, M. I., Ubeda, C., Hornedo-Ortega, R., Garcia-Parrilla, M. C., and Troncoso, A. M. (2015). Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog. Dis. 12, 32–38. doi: 10.1089/fpd.2014.1821
Centers for Disease Control Prevention (CDC) (2014). Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data. United States, 1998–2008. Available online at: https://www.cdc.gov/foodborneburden/attribution-1998-2008.html (Accessed December 17, 2017).
Centers for Disease Control Prevention (CDC) (2017). Foodborne Outbreak Online Database. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention. Available online at: http://wwwn.cdc.gov/foodborneoutbreaks (Accessed February 02, 2018).
Chaidez, C., Moreno, M., Rubio, W., Angulo, M., and Valdez, B. (2003). Comparison of the disinfection efficacy of chlorine-based products for inactivation of viral indicators and pathogenic bacteria in produce wash water. Int. J. Environ. Res. Public Health 13, 295–302. doi: 10.1080/0960312031000122442
Chen, X., and Hung, Y. C. (2017). Effects of organic load, sanitizer pH and initial chlorine concentration of chlorine-based sanitizers on chlorine demand of fresh produce wash waters. Food Control 77, 96–101. doi: 10.1016/j.foodcont.2017.01.026
Chen, Z. (2017) A focus on chlorine dioxide: the promising food preservative. J. Exp. Food Chem. 3:e107. doi: 10.4172/2472-0542.1000e107
Danyluk, M. D., Uesugi, A. R., and Harris, L. J. (2005). Survival of Salmonella Enteritidis PT 30 on inoculated almonds after commercial fumigation with propylene oxide. J. Food Prot. 68, 1613–1622. doi: 10.4315/0362-028X-68.8.1613
Davidson, G. R., Kaminski, C. N., and Ryser, E. T. (2014). Impact of organic load on Escherichia coli O157:H7 survival during pilot scale processing of iceberg lettuce with acidified sodium hypochlorite. J. Food Prot. 77, 1669–1681. doi: 10.4315/0362-028X.JFP-14-067
Du, G., Li, M., Ma, F., and Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 113, 557–562. doi: 10.1016/j.foodchem.2008.08.025
Eblen, B. S., Walderhaug, M. O., Edelson-Mammel, S., Chirtel, S. J., De Jesus, A., Merker, R. I., et al. (2004). Potential for internalization, growth, survival of Salmonella and Escherichia coli O157:H7 in oranges. J. Food Prot. 67, 1578–1584. doi: 10.4315/0362-028X-67.8.1578
Fatemi, P., and Frank, J. F. (1999). Inactivation of Listeria monocytogenes/Pseudomonas biofilms by peracid sanitizers. J. Food Prot. 62, 761–765. doi: 10.4315/0362-028X-62.7.761
Fatica, M. K., and Schneider, K. R. (2011). Salmonella and produce: survival in the plant environment and implications in food safety. Virulence 2, 573–579. doi: 10.4161/viru.2.6.17880
Fukushima, H., Katsube, K., Hata, Y., Kishi, R., and Fujiwara, S. (2007). Rapid separation and concentration of foodborne pathogens in food samples prior to quantification by viable-cell counting and real-time PCR. Appl. Environ. Microbiol. 73, 92–100. doi: 10.1128/AEM.01772-06
Gombes, D., Luo, Y., Brennan, J., Shergill, G., Petran, R., Walsh, R., et al. (2017). Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Prot. 80, 312–330. doi: 10.4315/0362-028X.JFP-16-258
Gonzalez, R. J., Luo, Y., Ruiz-Cruz, S., and McEvoy, J. L. (2004). Efficacy of sanitizers to inactivate Escherichia coli O157:H7 on fresh-cut carrot shreds under simulated process water conditions. J. Food Prot. 67, 2375–2380. doi: 10.4315/0362-028X-67.11.2375
Goodburn, C., and Wallace, C. A. (2013). The microbiological efficacy of decontamination methodologies for fresh produce: a review. Food Control 32, 418–427. doi: 10.1016/j.foodcont.2012.12.012
Hanning, I. B., Nutt, J. D., and Ricke, S. C. (2009). Salmonellosis outbreaks in the United States due to fresh produce: sources and potential intervention measures. Foodborne Pathog. Dis. 6, 635–648. doi: 10.1089/fpd.2008.0232
Harris, L. J., Beuchat, L. R., Kajs, T. M., Ward, T. E., and Taylor, C. H. (2001). Efficacy and reproducibility of a produce wash in killing Salmonella on the surface of tomatoes assessed with a proposed standard method for produce sanitizers. J. Food Prot. 64, 1477–1482. doi: 10.4315/0362-028X-64.10.1477
Hilgren, J., Swanson, K. M., Diez-Gonzalez, F., and Cords, B. (2007). Inactivation of Bacillus anthracis spores by liquid biocides in the presence of food residue. Appl. Environ. Microbiol. 73, 6370–6377. doi: 10.1128/AEM.00974-07
Joshi, K., Mahendran, R., Alagusundaram, K., Norton, T., and Tiwari, B. K. (2013). Novel disinfectants for fresh produce. Trends Food Sci. Technol. 34, 54–61. doi: 10.1016/j.tifs.2013.08.008
Keskinen, L. A., Burke, A., and Annous, B. A. (2009). Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from lettuce leaves. Int. J. Food Microbiol. 132, 134–140. doi: 10.1016/j.ijfoodmicro.2009.04.006
Laleh, G. H., Frydoonfar, H., Heidary, R., Jameei, R., and Zare, S. (2006). The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pakistan J. Nutr. 5, 90–92. doi: 10.3923/pjn.2006.90.92
Lang, M. M., Harris, L. J., and Beuchat, L. R. (2004). Evaluation of inoculation method and inoculum drying time for their effects on survival and efficiency of recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes inoculated on the surface of tomatoes. J. Food Prot. 67, 732–741. doi: 10.4315/0362-028X-67.4.732
Lee, S. Y., Dancer, G. I., Chang, S. S., Rhee, M. S., and Kang, D. H. (2006). Efficacy of chlorine dioxide gas against Alicyclobacillus acidoterrestris spores on apple surfaces. Int. J. Food Microbiol. 108, 364–368. doi: 10.1016/j.ijfoodmicro.2005.11.023
Li, Y., Brackett, R. E., Chen, J., and Beuchat, L. R. (2001). Survival and growth of Escherichia coli O157:H7 inoculated onto cut lettuce before or after heating in chlorinated water, followed by storage at 5 or 15 degrees C. J. Food Prot. 64, 305–309. doi: 10.4315/0362-028X-64.3.305
López-Gálvez, F., Allende, A., Selma, M. V., and Gil, M. I. (2009). Prevention of Escherichia coli cross-contamination by different commercial sanitizers during washing of fresh-cut lettuce. Int. J. Food Microbiol. 133, 167–171. doi: 10.1016/j.ijfoodmicro.2009.05.017
Luo, Y., Nou, X., Millner, P., Zhou, B., Shen, C., Yang, Y., et al. (2012). A pilot plant scale evaluation of a new process aid for enhancing chlorine efficacy against pathogen survival and cross-contamination during produce wash. Int. J. Food Microbiol. 158, 133–139. doi: 10.1016/j.ijfoodmicro.2012.07.008
Lynch, M. F., Tauxe, R. V., and Hedberg, C. W. (2009). The growing burden if foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137, 307–315. doi: 10.1017/S0950268808001969
Mahmoud, B. S., and Linton, R. H. (2008). Inactivation kinetics of inoculated Escherichia coli O157: H7 and Salmonella enterica on lettuce by chlorine dioxide gas. Food Microbiol. 25, 244–252. doi: 10.1016/j.fm.2007.10.015
Mathew, E. N., Muyyarikkandy, M. S., and Amalaradjou, M. A. (2017). Efficacy of wash water disinfectants in reducing water-to-mango cross contamination with Salmonella under simulated mango packing house operations. J. Food Prot. 80(Suppl. 1), 40. doi: 10.4315/0362-028X-80.sp1.1
Meireles, A., Giaouris, E., and Simões, M. (2016). Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 82, 71–85. doi: 10.1016/j.foodres.2016.01.021
National Mango Board (NMB) (2014). National Mango Board. Mango Postharvest Best Management Practices Manual. Available online at: http://www.mango.org/Mangos/media/Media/Documents/Research%20And%20Resources/Downloads/Industry/Research%20Support/Best_Practices_Manual_Eng.pdf (Accessed January 21, 2018).
Nieto-Montenegro, S., and Almanza, J. L. (2014). The Mango Industry Food Safety Training Kit. Available onlinne at: http://mangofoodsafety.org/english/Farm/Mango_FSTK/FSTK_Farm.pdf (Accessed February 02, 2018).
Pao, S., Kelsey, D. F., Khalid, M. F., and Ettinger, M. R. (2007). Using aqueous chlorine dioxide to prevent contamination of tomatoes with Salmonella enterica and Erwinia carotovora during fruit washing. J. Food Prot. 70, 629–634. doi: 10.4315/0362-028X-70.3.629
Pao, S., Kelsey, D. F., and Long, W. III. (2009). Spray washing of tomatoes with chlorine dioxide to minimize Salmonella on inoculated fruit surfaces and cross-contamination from revolving brushes. J. Food Prot. 72, 2448–2452. doi: 10.4315/0362-028X-72.12.2448
Parnell, T. L., and Harris, L. J. (2003). Reducing Salmonella on apples with wash practices commonly used by consumers. J. Food Prot. 66, 741–747. doi: 10.4315/0362-028X-66.5.741
Parnell, T. L., Harris, L. J., and Suslow, T. V. (2005). Reducing Salmonella on cantaloupes and honeydew melons using wash practices applicable to postharvest handling, foodservice, and consumer preparation. Int. J. Food Microbiol. 99, 59–70. doi: 10.1016/j.ijfoodmicro.2004.07.014
Penteado, A. L., Eblen, B. S., and Miller, A. J. (2004). Evidence of Salmonella internalization into fresh mangos during simulated postharvest insect disinfestation procedures. J. Food Prot. 67, 181–184. doi: 10.4315/0362-028X-67.1.181
Shen, C., Luo, Y., Nou, X., Wang, Q., and Millner, P. (2013). Dynamic effects of free chlorine concentration, organic load, and exposure time on the inactivation of Salmonella, Escherichia coli O157:H7, and non-O157 Shiga toxin-producing E. coli. J. Food Prot. 76, 386–393. doi: 10.4315/0362-028X.JFP-12-320
Sivapalasingam, S., Barrett, E., Kimura, A., Van Duyne, S., De Witt, W., Ying, M., et al. (2003). A multistate outbreak of Salmonella enterica Serotype Newport infection linked to mango consumption: impact of water-dip disinfestation technology. Clin. Infect. Dis. 37, 1585–1590. doi: 10.1086/379710
Small, D. A., Chang, W., Toghrol, F., and Bentley, W. E. (2007). Comparative global transcription analysis of sodium hypochlorite, peracetic acid, and hydrogen peroxide on Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 76, 1093–1105. doi: 10.1007/s00253-007-1072-z
Soto, M., Chavez, G., Baez, N., Martinez, C., and Chiadez, C. (2007). Internalization of Salmonella Typhimurium into mango pulp and prevention of fruit pulp contamination by chlorine and copper ions. Int. J. Environ. Health Res. 17, 453–459. doi: 10.1080/09603120701695593
Sreedharan, A., Li, Y., De, J., Gutierrez, A., Silverberg, R., and Schneider, K. R. (2017). Determination of optimum sanitizer levels for prevention of Salmonella cross-contamination of mature round tomatoes in a laboratory model flume system. J. Food Prot. 80, 1436–1442. doi: 10.4315/0362-028X.JFP-17-032
Strawn, L. K., and Danyluk, M. D. (2010). Fate of Escherichia coli O157:H7 and Salmonella spp. on fresh and frozen cut mangoes and papayas. Int. J. Food Microbiol. 138, 78–84. doi: 10.1016/j.ijfoodmicro.2009.12.002
Teng, Z., Luo, Y., Alborzi, S., Zhou, B., Chen, L., Zhang, J., et al. (2018). Investigation on chlorine-based sanitization under stabilized conditions in the presence of organic load. Int. J. Food Microbiol. 266, 150–157. doi: 10.1016/j.ijfoodmicro.2017.11.027
Tian, J. Q., Bae, Y. M., and Lee, S. Y. (2013). Survival of foodborne pathogens at different relative humidities and temperatures and the effect of sanitizers on apples with different surface conditions. Food Microbiol. 35, 21–26. doi: 10.1016/j.fm.2013.02.004
US Food Drug Administration (USFDA) Center for Food Safety Applied Nutrition (1998). Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. Available online at: https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM169112.pdf (Accessed April 15, 2018).
U.S. Department of Agriculture (USDA) Animal Plant Health Inspection Service Plant Protection Quarantine. (2010). Treatment Manual. Available online at: http://www.aphis.usda.gov/import_export/plants/manuals/ports/downloads/treatment.pdf (Accessed January 03, 2018).
Vandekinderen, I., Devlieghere, F., De Meulenaer, B., Ragaert, P., and Van Camp, J. (2009). Optimization and evaluation of a decontamination step with peroxyacetic acid for fresh-cut produce. Food Microbiol. 26, 882–888. doi: 10.1016/j.fm.2009.06.004
Van de Velde, F., Vaccari, M. C., Piagentini, A. M., and Pirovani, M. É. (2016). Optimization of strawberry disinfection by fogging of a mixture of peracetic acid and hydrogen peroxide based on microbial reduction, color and phytochemicals retention. Food Sci. Technol. Int. 22, 485–495. doi: 10.1177/1082013215625696
Wang, H., Feng, H., and Luo, Y. (2007). Control of browning and microbial growth on fresh-cut apples by sequential treatment of sanitizers and calcium ascorbate. J. Food Sci. 72, M001–M007. doi: 10.1111/j.1750-3841.2006.00210.x
Keywords: Salmonella, mango packing house, wash water disinfectants, chlorine, chlorine dioxide, peroxyacetic acid
Citation: Mathew EN, Muyyarikkandy MS, Bedell C and Amalaradjou MA (2018) Efficacy of Chlorine, Chlorine Dioxide, and Peroxyacetic Acid in Reducing Salmonella Contamination in Wash Water and on Mangoes Under Simulated Mango Packinghouse Washing Operations. Front. Sustain. Food Syst. 2:18. doi: 10.3389/fsufs.2018.00018
Received: 01 March 2018; Accepted: 09 May 2018;
Published: 29 May 2018.
Edited by:
Joshua B. Gurtler, Agricultural Research Service (USDA), United StatesReviewed by:
Govindaraj Dev Kumar, University of Maryland, United StatesPatricia Margaret Desmarchelier, Independent Consultant, Brisbane, Australia
Copyright © 2018 Mathew, Muyyarikkandy, Bedell and Amalaradjou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary Anne Amalaradjou, bWFyeV9hbm5lLmFtYWxhcmFkam91QHVjb25uLmVkdQ==
 Elza N. Mathew
Elza N. Mathew Muhammed S. Muyyarikkandy
Muhammed S. Muyyarikkandy Carley Bedell2
Carley Bedell2 Mary Anne Amalaradjou
Mary Anne Amalaradjou